Abstract
Obesity and the metabolic syndrome are characterized by chronic, low-grade inflammation mainly originating from expanding adipose tissue and resulting in inhibition of insulin signaling and disruption of glycemic control. Transgenic mice expressing human interleukin 37 (IL-37), an anti-inflammatory cytokine of the IL-1 family, are protected against metabolic syndrome when fed a high-fat diet (HFD) containing 45% fat. Here, we examined whether treatment with recombinant IL-37 ameliorates established insulin resistance and obesity-induced inflammation. WT mice were fed a HFD for 22 weeks and then treated daily with IL-37 (1 μg/mouse) during the last 2 weeks. Compared with vehicle only–treated mice, IL-37–treated mice exhibited reduced insulin in the plasma and had significant improvements in glucose tolerance and in insulin content of the islets. The IL-37 treatment also increased the levels of circulating IL-1 receptor antagonist. Cultured adipose tissues revealed that IL-37 treatment significantly decreases spontaneous secretions of IL-1β, tumor necrosis factor α (TNFα), and CXC motif chemokine ligand 1 (CXCL-1). We also fed mice a 60% fat diet with concomitant daily IL-37 for 2 weeks and observed decreased secretion of IL-1β, TNFα, and IL-6 and reduced intracellular levels of IL-1α in the liver and adipose tissue, along with improved plasma glucose clearance. Compared with vehicle treatment, these IL-37–treated mice had no apparent weight gain. In human adipose tissue cultures, the presence of 50 pm IL-37 reduced spontaneous release of TNFα and 50% of lipopolysaccharide-induced TNFα. These findings indicate that IL-37's anti-inflammatory effects can ameliorate established metabolic disturbances during obesity.
Keywords: adipose tissue, inflammation, cytokine, drug development, insulin resistance, glucose metabolism, mouse, type 2 diabetes, cellular research, mouse model
Introduction
The incidence of obesity has dramatically increased worldwide during recent decades (1). The presence of obesity leads to an increased risk for the development of the metabolic syndrome, cardiovascular disease, and type 2 diabetes (2, 4) (https://www.nhlbi.nih.gov/health-topics/overweight-and-obesity; accessed July 24, 2018) and is directly associated with all-cause mortality (5). However, despite advocated lifestyle changes and efforts to reduce obesity and its effects on morbidity and mortality, the number of overweight and obese individuals continues to increase globally (1). Therefore, developing new approaches to resolve the detrimental metabolic effects of obesity and type 2 diabetes remains a high priority. During the development of obesity and the concomitant metabolic stress, adipose tissue develops a state of chronic low-grade inflammation. Inflammation in adipose tissue is characterized by increased production of pro-inflammatory cytokines, such as IL-1β,8 IL-6, and TNFα, and an increase in macrophage numbers (6, 7). These pro-inflammatory cytokines directly interfere with insulin signaling (8–10), resulting in insulin resistance.
The interleukin-1 family of cytokines plays a critical role in the regulation of metabolic inflammation (11), particularly by pro-inflammatory members IL-1α (12–14) and IL-1β (15, 16). In contrast, the IL-1 receptor antagonist (IL-1Ra), also a member of the IL-1 family, represents an endogenous mechanism to reduce IL-1–driven inflammation (17–19). In clinical studies of recombinant IL-1Ra (anakinra) in patients with type 2 diabetes, hemoglobin A1C, a measure of elevated blood sugar, is reduced compared with placebo-treated subjects (20). Similar reductions have been reported in clinical trials of neutralizing antibodies to IL-1β (21–23) and to IL-1α (24). Another member of the IL-1 family, IL-37, has broad anti-inflammatory properties and also plays a role in obesity (25). For example, weight loss in morbidly obese humans is associated with an increase in IL-37 levels (26). Mice carrying the human gene for IL-37 are protected from the development of obesity-induced inflammation and insulin resistance when challenged with a high-fat diet (HFD) (25). Additionally, liver and plasma lipid levels of these mice improve compared with WT mice on a HFD. Importantly, mice transgenic for human IL-37 and subjected to HFD are also protected against glucose intolerance after only 4 days of HFD.
In the present study, we administered recombinant human IL-37 to WT mice that had been subjected to HFD feeding for 22 weeks. We tested the hypothesis that intervention with IL-37 can ameliorate established metabolic inflammation and insulin resistance in the presence of obesity.
Results
Treatment with recombinant IL-37 reverses impaired insulin signaling
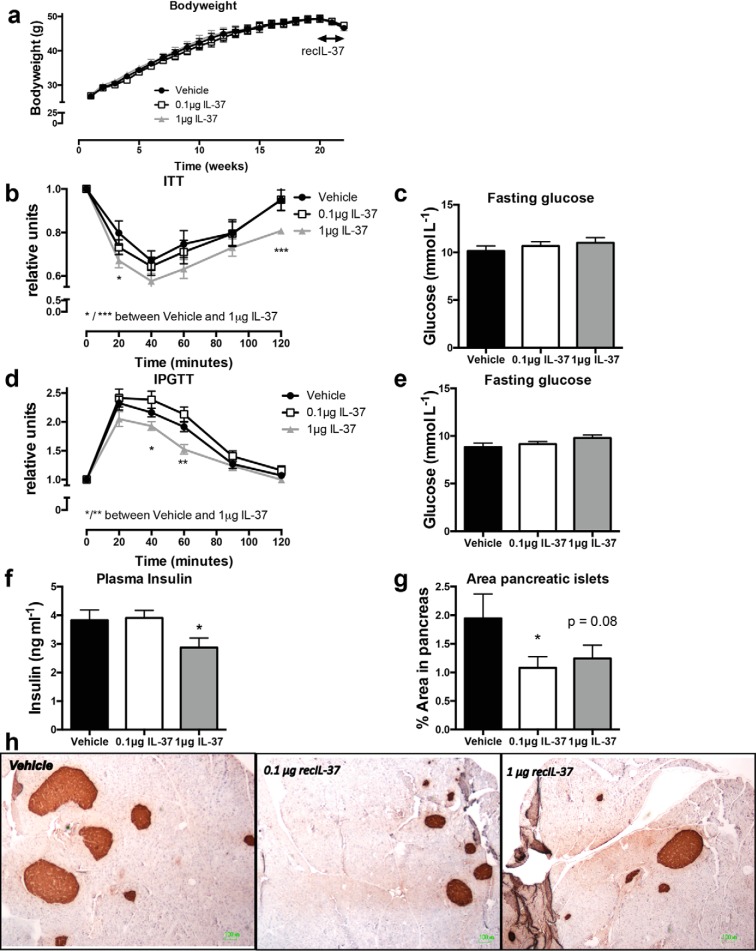
To test whether treatment with IL-37 has therapeutic value to improve insulin and glycemic control in established obesity, mice were fed a HFD for 22 weeks (Fig. 1a). Only during the last 2 weeks, mice were given vehicle or IL-37 intraperitoneally, at a concentration of either 0.1 μg or 1 μg/mouse/day. As shown in Fig. S1 (a–c), neither treatment had an effect on liver, epididymal adipose tissue, or total body weight. However, in response to insulin, animals treated with a daily dose of 1 μg showed a greater reduction in glucose levels, compared with vehicle-treated mice, consistent with improved insulin sensitivity (Fig. 1b); no differences were observed in fasting glucose levels (Fig. 1c). Moreover, we observed a higher glucose clearance in mice receiving the 1-μg dose of IL-37, suggestive of improved glucose tolerance compared with vehicle-treated mice (Fig. 1, d and e). Furthermore, there was a significant decrease in fasting insulin levels (25% reduction) in mice treated with the 1-μg dose of IL-37 (Fig. 1f), in support of improved insulin sensitivity (27).
Figure 1.
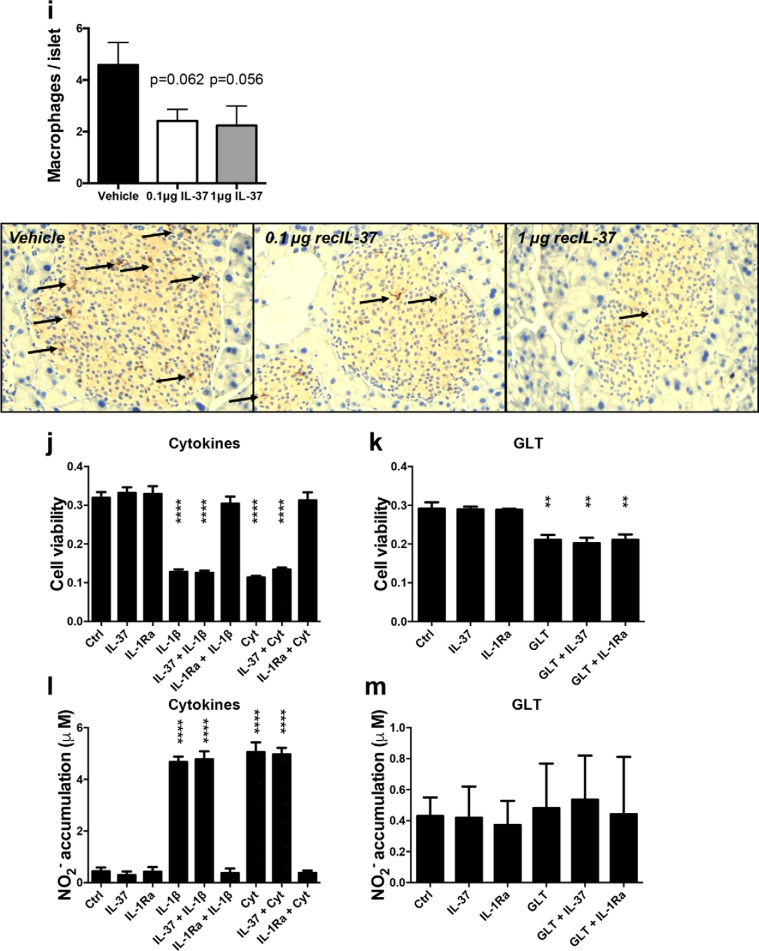
Treatment with recombinant human IL-37 ameliorates glucose and insulin tolerance in obese mice. WT mice were subjected to 22 weeks of HFD. During the last 14 days the mice were injected with recombinant IL-37 or vehicle. a, mean body weight over time. b, ITT, relative to t = 0. c, fasting glucose levels before ITT. d, glucose tolerance test (IPGTT), relative to t = 0; e, fasting glucose levels before GTT. f, fasting insulin levels at sacrifice. g, mean proportion of the insulin-producing islets in the pancreas, compared with the total area of the pancreas. h, representative histological insulin-stained sections of pancreata. i, representative histological F480-stained sections of pancreata; macrophages were quantified per islet. INS-1 cells were cultured and pre-exposed to IL-37 or vehicle overnight, or to IL-1Ra for 1 h. j and k, INS-1 cell viability after IL-1β alone or together with TNFα (cytokine mixture, Cyt) (j) or challenged with GLT (k). Cells were cultured for 24 h. Cell viability was assessed using the alamarBlue assay (n = 4 (cytokines) and n = 3 (GLT)). l and m, nitrite production in response to IL-1β alone or together with TNFα (l) or GLT (m), n = 3. *, p < 0.05; **, p < 0.01, as tested with ANOVA. n = 10 mice/group. Data are shown as means ± S.E. (error bars).
We next investigated whether 2 weeks of IL-37 treatment affected the insulin-producing pancreatic islets. Pancreatic islets were stained for insulin content, and the area of insulin staining was quantified in a blinded fashion. Although there were a similar number of pancreatic islets in the animals (Fig. S1d), the total area of the insulin-producing islets was less (40% reduction) in IL-37–treated mice (Fig. 1, g and h), similar to our earlier observation in IL-37tg mice (25). Additionally, staining for the macrophage marker F480 revealed that islets from IL-37–treated mice contained lower numbers of macrophages (Fig. 1i). Although the reduced number of islet macrophages did not reach statistical significance, the reduction of nearly 50% was observed at the 1-μg dose (p = 0.056) as well at the 0.1-μg dose (p = 0.062).
To investigate whether IL-37 had direct effects on pancreatic beta-cells, INS-1 cells were pre-exposed to IL-37 overnight before exposure to either cytokines (IL-1β alone or in combination with TNFα or glucolipotoxic conditions (GLT). IL-37 or IL-1Ra pre-exposure did not affect the cell viability in the absence of cytokines or GLT (Fig. 1, j and k). As expected, IL-1β in the absence or presence of TNFα robustly reduced cell viability, also significantly although to a lesser extent than reduced by GLT. Whereas IL-1Ra completely blocked cytokine but not GLT toxicity, IL-37 exhibited no effect on cytokine- or GLT-induced cell death.
Because cell death can be an insensitive measure of IL-37 bioactivity, we also determined accumulated nitrite in the cell supernatants as a surrogate for NO production. Being produced as a consequence of NF-κB–dependent expression of inducible NO synthase, accumulated nitrite is a validated proxy for NF-κB signaling. Neither IL-37 nor IL-1Ra increased NO production (Fig. 1l). As expected, cytokines but not GLT strongly induced NO production. Whereas IL-1Ra completely prevented cytokine-induced NO formation, IL-37 was ineffective. Neither IL-37 nor IL-1Ra affected NO production in conjunction with GLT (Fig. 1, l and m). Together, these results suggest that IL-37 changes the pancreatic islet inflammatory environment without direct effects on the beta-cells.
We also compared the effects of IL-37 administered to WT mice with that in IL-37tg mice after 8 weeks of HFD. We observed similar improvements in insulin sensitivity between recombinant IL-37–treated WT mice and IL-37tg mice when compared with control mice (Fig. S1e). A mild reduction was observed in glucose levels after a 4-h fast in IL-37tg mice when compared with control mice (Fig. S1f). These data suggest that treatment with IL-37 improves insulin and glucose homeostasis.
IL-37 reduces adipose tissue inflammation in vivo in mice and ex vivo in humans
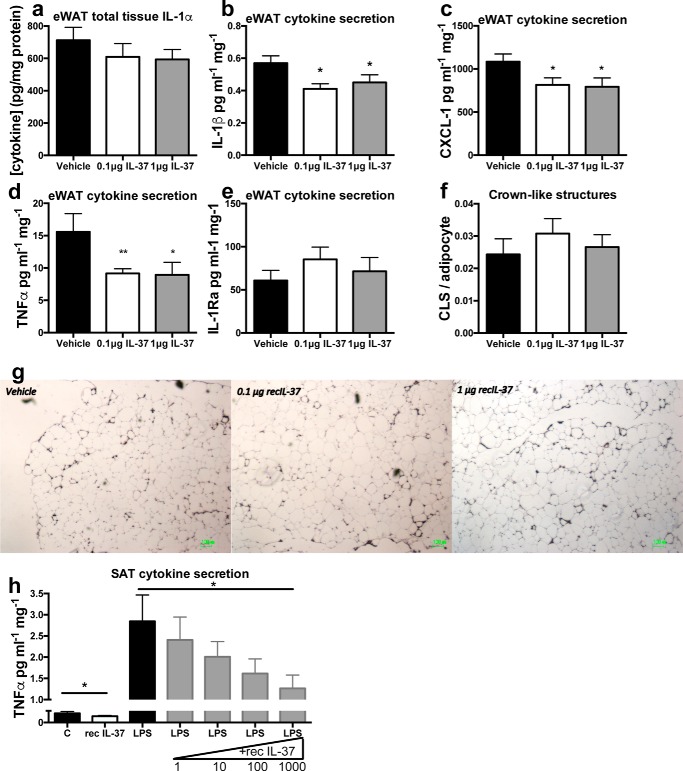
Insulin resistance and glucose intolerance are strongly linked to adipose tissue inflammation (11, 28, 29). Therefore, we determined whether administration of IL-37 affected adipose tissue secretion of cytokines after 22 weeks of HFD. As shown in Fig. 2a, total tissue levels of IL-1α were not significantly reduced in the adipose tissue from mice treated with IL-37. However, the spontaneous release of the pro-inflammatory cytokines IL-1β, CXCL-1 (KC), and TNFα was significantly reduced in the adipose tissue of the IL-37–treated mice, compared with vehicle-treated mice (Fig. 2, b–d). In contrast, the levels of anti-inflammatory cytokine IL-1Ra were not reduced (Fig. 2e).
Figure 2.
IL-37 inhibits the secretion of inflammatory cytokines in epididymal white adipose tissue. Mice were fed 22 weeks of HFD and received 2 weeks of daily IL-37 or vehicle. a, IL-1α levels in adipose tissue. 24-h spontaneous secretion of cytokines was measured from cultured adipose tissue. b, IL-1β; c, CXCL-1; d, TNFα; e, IL-1Ra. f, number of crown-like structures in adipose tissue sections stained for F480. g, representative images per group are shown. h, human subcutaneous adipose tissue was incubated with increasing concentrations of IL-37 (pg/ml) as indicated as well as with 50 ng/ml LPS. TNFα secretion was determined in the supernatants by a specific human ELISA. *, p < 0.05; **, p < 0.01, as tested with ANOVA. n = 10 mice/group for all experiments. Data are shown as means ± S.E. (error bars).
Adipose tissue macrophages are major contributors to obesity-induced inflammation (4). Therefore, we evaluated whether IL-37 affected the number of macrophages in the eWAT of the HFD-challenged mice. Using F480 staining, there were no differences in the presence of crownlike structures (multiple macrophages surrounding an adipocyte) (Fig. 2f) or in the number of macrophages (Fig. S2 (a) and Fig. 2g). Moreover, mRNA analyses of eWAT did not reveal differences in known monocyte/macrophage markers (Fig. S2, b–e). Together, this suggests that IL-37 decreased the spontaneous secretion of pro-inflammatory cytokines without affecting the number of macrophages in the adipose tissue.
To expand on this finding, we incubated human adipose tissue explants in vitro for 24 h with IL-37. Similar to the data in mice, IL-37 reduced spontaneous as well as lipopolysaccharide (LPS)-stimulated secretion of TNFα (Fig. 2h). Together, these data demonstrate that IL-37 shifts the pro-inflammatory status in the adipose tissue toward a more anti-inflammatory state.
IL-37 treatment improves metabolic markers
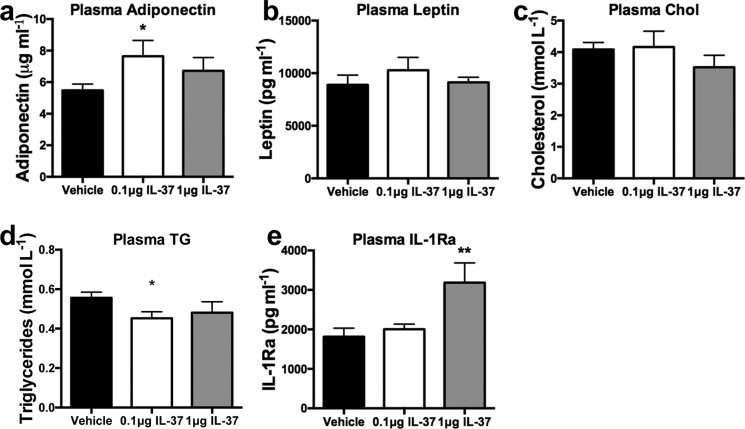
We next assessed whether the effects of IL-37 treatment on metabolic and inflammatory markers are associated with changes in circulating parameters related to metabolism or adipose tissue health. First, we investigated circulating adiponectin levels, as these were markedly elevated in the circulation of IL-37tg mice subjected to HFD (25). As shown in Fig. 3a, serum adiponectin levels increased, and this increase was statistically significant in the low-dose IL-37 treatment group. No differences were found in circulating leptin levels (Fig. 3b) or in plasma cholesterol levels (Fig. 3c). However, levels of triglycerides were reduced after IL-37 treatment (Fig. 3d). We also observed a 75% increase in plasma levels of the anti-inflammatory cytokine IL-1Ra in mice receiving the high-dose IL-37 treatment compared with vehicle-injected mice (Fig. 3e). These data suggest that IL-37 improves several metabolic and anti-inflammatory markers in the circulation.
Figure 3.
Effect of IL-37 treatment of obese mice on blood metabolites. After 22 weeks of HFD and 2 weeks of daily IL-37 or vehicle administration, metabolic markers were determined in plasma. a, adiponectin; b, leptin; c, cholesterol; d, triglycerides; e, IL-1Ra. *, p < 0.05; **, p < 0.01, as tested with ANOVA. n = 10 mice/group. Data are shown as means ± S.E. (error bars).
Short-term lipotoxicity-induced insulin resistance is not improved by IL-37 treatment
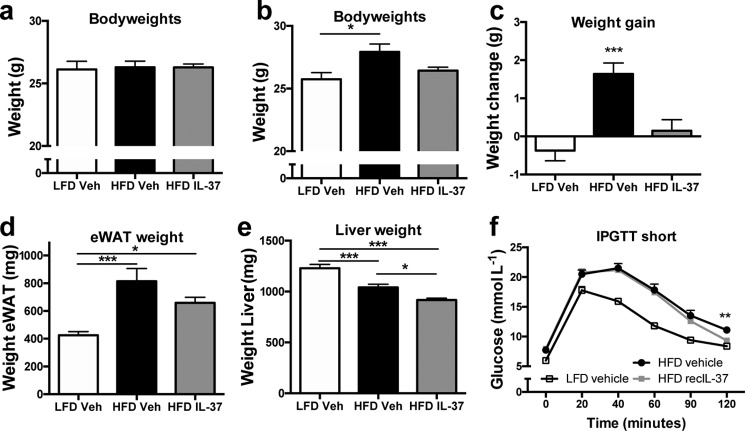
Next, we investigated whether the administration of IL-37 could prevent detrimental effects on insulin signaling and glycemic control in a short-term model of HFD feeding that impairs glucose tolerance and hepatic insulin sensitivity (30). For this study, we fed mice a very high-fat diet with 60% of energy from fat (vHFD). During this 14-day vHFD, IL-37 was administered daily (1 μg/mouse/day). Compared with mice receiving daily vehicle, mice injected with IL-37 did not gain weight during this period (Fig. 4, a–c). Consistent with this observation, the weights of epididymal adipose tissue were lower in mice treated with IL-37 (Fig. 4d). In addition, we observed that the liver weights of both groups on the vHFD were lower than for the livers of the LFD group (Fig. 4e), suggesting that the lipid overload increases adipose tissue mass rather than liver deposits in this short-term model. To assess whether intervention with IL-37 treatment could improve glucose clearance similar to the 22-week HFD intervention, mice fed the vHFD for 10 days were then subjected to a glucose tolerance test. As shown in Fig. 4f, there was a small but statistically significant improvement in glucose clearance after 2 h in the IL-37–treated mice.
Figure 4.
IL-37 limits weight gain in a model of short-term lipid overload. Mice were subjected to a LFD or very HFD and injected with recombinant IL-37 or vehicle over the course of 14 days. a, body weights at the start of diets; b, body weights at the end of diets; c, weight gain; d, epididymal adipose tissue weight; e, liver weight; f, intraperitoneal glucose tolerance test. *, p < 0.05; **, p < 0.01; ***, p < 0.001 as tested with ANOVA. n = 10 mice/group. Data are shown as means ± S.E. (error bars).
Additionally, we evaluated circulating metabolic markers. Although plasma cholesterol levels remained unchanged (Fig. S3a), both groups on the short-term HFD displayed lower triglyceride levels, whereas glucose levels in the plasma increased (Fig. S3, b and c). No differences were observed in adiponectin or leptin levels (Fig. S3, d and e). Similar to the findings after 22 weeks of HFD, IL-1Ra levels in the plasma increase after IL-37 treatment, although the increase was not statistically significant (Fig. S3f). Cholesterol, triglycerides, and glucose levels in liver lysates of the vHFD study are shown in Fig. S4. Together, these data suggest that IL-37 prevents weight gain but has limited effects on metabolic markers in the acute model of lipid overload.
Short-term lipotoxicity-induced inflammation is responsive to IL-37 treatment
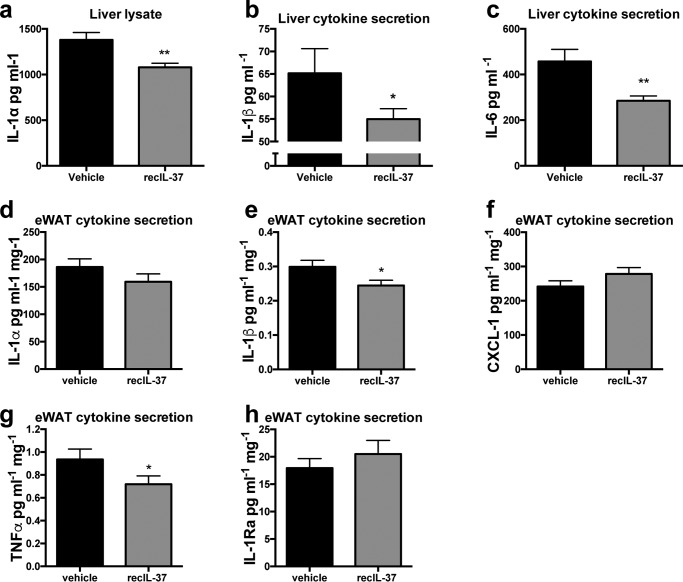
We then evaluated the anti-inflammatory effects of IL-37 on the liver and adipose tissue, both crucial organs in regulating short-term lipid overload. We compared secretion of cytokines in these tissues from both treatments after vHFD. Intracellular content of IL-1α was reduced by 22% in cultured liver tissue isolated from IL-37–treated mice (Fig. 5a). Moreover, the levels of secreted of IL-1β and IL-6 were reduced when compared with vehicle-treated mice (Fig. 5, b and c). We also assessed cytokine secretion from the cultured adipose tissue. Although the levels of IL-1α and CXCL-1 were not different, the 24-h secretion of IL-1β and TNFα was significantly reduced in epididymal adipose tissue from IL-37–treated mice (Fig. 5, d–g). Similar to mice fed the standard HFD, there was no decrease in the secretion of IL-1Ra in mice treated for 14 days with IL-37 while being fed the vHFD (Fig. 5h).
Figure 5.
The effect of recombinant IL-37 during short-term lipid overload on liver and adipose tissue inflammation. Mice were subjected to a HFD and treated daily with IL-37 or vehicle for 14 days. Liver cells were isolated and cultured, and cytokine levels were measured. a–c, intracellular IL-1α (a), secreted IL-1β (b), and IL-6 (c) after 24 h. In adipose tissue, intracellular IL-1α (d) and secretion of cytokines was measured after 24 h: IL-1β (e), CXCL-1 (f), TNFα (g), and IL-1Ra (h). *, p < 0.05; **, p < 0.01, as tested with ANOVA. n = 10 mice/group. Data are shown as means ± S.E.
Discussion
Inflammation that occurs during obesity is detrimental for several metabolic pathways, particularly insulin signaling and glucose metabolism (31), thereby increasing the risk for the development of type 2 diabetes. Using mice fed 22 weeks of a HFD, the present study shows that treatment during the last 2 weeks with recombinant human IL-37 ameliorates established insulin resistance and glucose intolerance. These effects of IL-37 treatment were associated with restoring the balance in the production of pro- and anti-inflammatory cytokines systemically as well as in the adipose tissue. In addition, we identified beneficial changes in insulin sensitivity. These effects identify the administration of IL-37 as a potential treatment strategy in obesity-associated metabolic diseases. Our previous studies in transgenic mice expressing human IL-37 (25) revealed a role for this cytokine in preventing obesity-driven metabolic derangement; however, the current studies provide a proof of concept for the efficacy of IL-37 treatment once insulin resistance has been established.
Compared with mice receiving vehicle, mice treated with a daily dose of 1 μg of IL-37 for the last 2 weeks of a 22-week HFD intervention exhibited improvement in insulin and glucose tolerance challenges, reduced fasting insulin levels, and smaller insulin-producing islets, as seen previously in the IL-37tg mice on a HFD (25). Reduced plasma insulin levels indicate normalized insulin sensitivity (27).
IL-37 broadly suppresses inflammation (32) by recruiting the anti-inflammatory IL-1 receptor 8 (IL-1R8, formally known as TIR8 or SIGIRR). Mice deficient in IL-1R8 are not protected against inflammation when treated with IL-37 (33–36). IL-37 binding to IL-1R8 decoys MyD88 and limits signaling downstream to IL-1 family and Toll-like receptors (37). By binding to IL-18Rα and IL-1R8, IL-37 has broad anti-inflammatory effects, as has been shown in several studies (33, 35, 38). In the context of obesity, IL-37 induces pseudostarvation effects by activating AMPK (25, 32) and by inhibiting mTOR (32, 33), two important energy sensors in the cell. Metabolic stress induced by higher glucose levels (16) can stimulate proliferation of beta-cells, increased insulin secretion, and production of IL-1β (39) via mechanisms involving reduced AMPK and increased mTOR activation in beta-cells (40–42). In addition, AMPK activators have recently been shown to improve glucose disposal and homeostasis in rodents and nonhuman primates (43, 44). Because IL-37 has been shown to activate AMPK and inhibit mTOR in other cells (25, 32, 33, 45), this property of IL-37 may provide a hypothesis to explain why administration of this cytokine decreases plasma insulin levels and pancreatic islet mass.
Our data reveal that IL-37 does not directly act upon beta-cells, suggesting that indirect pathways are involved. IL-18 receptor is not constitutively expressed in islet beta cells and may therefore explain the lack of direct effects of IL-37 on these cells (46). In type 2 diabetes, IL-1β plays an important role in the dysfunction of the insulin-producing beta cell (47). Moreover, IL-1β induces BMP2 and BMP4 that have been shown to reduce beta-cell function. By reducing levels of IL-1β and therefore BMP2, IL-37 may therefore improve beta-cell function (48). Together, this suggests that administration of IL-37 can reverse HFD-induced metabolic stress upon the islets, probably via a reduction of infiltrating macrophages and thereby production of beta-cell cytotoxic IL-1 and induction of BMP2, resulting in improved insulin homeostasis. Nevertheless, because IL-1Ra improves both beta-cell function and insulin sensitivity in most animal models (49–51), the increase in IL-1Ra (Fig. 3e) may contribute to the anti-inflammatory and metabolic effects of IL-37.
We found that mice receiving 1 μg of IL-37 have smaller islets than vehicle-treated mice fed a HFD (Fig. 1, g and h). These findings were also observed in previous studies in IL-37tg mice (25). Humans with type 2 diabetes and rats with insulin resistance have increased numbers of islet-associated macrophages (51), resulting in an increased inflammatory environment (50). In parallel, metabolic stress increases the size of pancreatic islets (52). It is likely that a decrease in the secretion of IL-1β from adipose tissue and an increase in systemic levels of IL-1Ra would offset inflammation in the pancreatic islets and contribute to a reduction in compensatory beta-cell hyperplasia (16, 47). Indeed, mice that received IL-37 had lower numbers of macrophages in their pancreatic islets. Thus, together with the improved insulin tolerance test (ITT) and glucose tolerance test (GTT), these data suggest that intervention with IL-37 improves insulin homeostasis in established obesity. However, it remains unknown whether IL-37 could directly influence glucose-stimulated insulin secretion. It is known that another anti-inflammatory cytokine IL-1Ra plays an important role in beta-cell insulin secretion and proliferation, as deletion of beta-cell–derived IL-1Ra impairs glucose homeostasis, beta-cell proliferation, and insulin secretion (53). Interestingly, IL-37–treated mice fed with HFD in this study presented a decrease in adipose tissue inflammatory cytokines and an increase in the anti-inflammatory IL-1Ra. This is consistent with what is observed in IL-37tg mice with HFD (25). In addition, we observed that both IL-37tg mice (25) and recombinant IL-37–treated mice fed with HFD showed a decreased area of insulin-producing pancreatic islets. Future studies would be required to study glucose-stimulated insulin secretion in these models.
As adipose tissue inflammation is a key contributor to obesity-induced insulin resistance, a relevant observation in this study is that IL-37 globally reduces inflammation in adipose tissue, as reflected by cytokine release. Decreased spontaneous IL-1β, CXCL-1, and TNFα secretion from the epididymal adipose tissue of IL-37–treated mice is relevant to improved metabolic changes. The reduced spontaneous TNFα secretion in mouse adipose tissue was also observed in human adipose tissue. Secretion of pro-inflammatory cytokines by adipose tissue leads to local and whole-body insulin resistance (9, 29, 54, 55). Also, increased IL-1β secretion from the adipose tissue induces hepatic insulin resistance (56). These observations indicate that IL-37 administration restores established obesity-induced metabolic disturbances, at least by dampening local and systemic inflammation, thus contributing to improved systemic insulin sensitivity. Interestingly, the reduction of pro-inflammatory cytokine secretion was not achieved by reducing the number of macrophages. It is possible that IL-37 skewed the development of infiltrating macrophages toward a more anti-inflammatory phenotype, as has been reported by others (32, 38). IL-37 has been shown to reduce macrophage inflammatory activity, especially when polarized toward a pro-inflammatory phenotype (38). Because macrophages in adipose tissue are part of the inflammation and result in insulin resistance (4, 57), IL-37 is an attractive novel therapeutic in obesity and diabetes.
We also investigated whether IL-37 restores metabolic abnormalities resulting from a short-term (2-week) lipid overload that results in glucose intolerance and hepatic insulin resistance (30, 58). A key finding was that mice simultaneously challenged with vHFD and treated with IL-37 did not gain weight as compared with vehicle-injected mice on the same vHFD. This finding may be due to energy expenditure in adipose tissue and muscle (25, 45), adipose tissue browning, or appetite change, which are known to regulate body weight and high-fat diet–induced obesity in mice (59–61). In addition, as discussed above, it would be intriguing to further study the effect of IL-37 on glucose-stimulated insulin secretion in this vHFD model as well. Also, in the liver as well as adipose tissue of IL-37–treated mice, the levels of secreted IL-1β and TNFα and intracellular IL-1α were reduced. Although IL-37 treatment reduced inflammatory cytokine production in liver and adipose tissue, cytokine levels in mice on the short-term diet were 2–10 times lower compared with the 22-week intervention. These findings suggest that the model of short-term HFD and lipid overload is not highly inflammatory (62). We speculate that the inflammatory trait in this model, as opposed to the lipid toxicity that causes glucose intolerance (63), might not have been sufficiently severe for IL-37 to fully exert its anti-inflammatory effects. Interestingly, IL-37tg mice showed an improvement when challenged with vHFD for 2 weeks (25). Because IL-37 in the IL-37tg model is also present intracellularly, as compared with the exogenous administration of IL-37 used in the current studies, this difference may account for the observed differences.
We also examined the effects of a lower dose of IL-37 (0.1 μg/mouse/day) because low picomolar concentrations of IL-37 suppressed LPS-induced IL-1β, IL-6, and TNFα from human M1 differentiated macrophages in vitro (38). Also, in a model of acute lung inflammation, a single dose of 0.1 μg protected mice against damaging neutrophil infiltration and resulted in lower lung cytokine levels (34). A dose of 0.1 μg/mouse corresponds to 2 μg/kg, whereas a dose of 1 μg/mouse corresponds to 20 μg/kg. Of note, these low concentrations confirm that IL-37 is effective at very low doses (34, 38). By comparison, leptin requires dosing of 100 μg/kg (64), and rapamycin is used at 1.5 mg/kg (65) to reduce fasting serum insulin and glucose levels in genetically obese mice (ob/ob) and body weight of HFD-fed WT mice, respectively. Pretreatment with IL-37 effectively reduced inflammation in acute experimental models (3). These data on mice subjected to HFD indicate great promise of IL-37 in settings in the treatment of established chronic inflammation, such as obesity and diabetes.
Together, these findings indicate that treatment with IL-37 counteracts inflammation during obesity and ameliorates glucose and insulin homeostasis in mice. These results highlight IL-37 as a potential therapeutic to ameliorate metabolic disturbances during obesity. Therefore, follow-up studies in humans are warranted, to gain more insight into the role of IL-37 in type 2 diabetes.
Materials and methods
Human samples
Subcutaneous adipose tissues were obtained from consenting healthy donors (25). The studies were approved by the ethical committee of the Radboud University Medical Centre at Nijmegen and abide by the Declaration of Helsinki principles.
Mice and diets
Eight-week-old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were housed in a pathogen-free facility of the University of Colorado Denver Anschutz Medical Campus. Five mice were housed per cage, with water and food available ad libitum. The mice were randomly allocated to a group. Animal protocols were approved by the University of Colorado animal care and use committee.
Models for high-fat diet
In each experiment, mice first received a run-in period for 2 weeks of a LFD (10% of energy derived from fat, D12450B; Research Diets, Inc., New Brunswick, NJ). Mice were then randomized to a LFD or a HFD, and body weight was recorded weekly. The HFD for the 8- and 20-week diet studies contained 45% energy derived from fat (D12451; Research Diets, Inc.). For the short-term (2-week) diet study, mice received the same LFD run-in period but were subsequently challenged with LFD or vHFD (containing 60% energy from fat, D12492; Research Diets) for 14 days. After sacrifice under anesthesia, liver, pancreas, and epididymal adipose tissue were removed, and blood was collected. Tissue weight was measured and, unless stated otherwise, tissue was snap-frozen in liquid nitrogen within 5 min of sacrifice. During the last 2 weeks, mice received intraperitoneal injections of PBS vehicle or IL-37, at a concentration of either 0.1 μg/mouse/day or 1 μg/mouse/day.
Recombinant human interleukin-37
The recombinant IL-37 used for these studies was obtained from Bio-Techne (Minneapolis, MN). The recombinant IL-37 was expressed in Escherichia coli with an N terminus at valine 46 (residues 46–218), as reported previously (38).
Insulin/glucose tolerance test
Insulin and glucose tolerance tests were performed at least 2 days before sacrifice and with at least 3 days between both tolerance tests. Before the ITT, mice were fasted for 4 h, and insulin (0.75 units/kg, NovoLog®FlexPen®, Novo Nordisk Inc., Princeton, NJ) was administered intraperitoneally. Before the GTT, mice were fasted for 6 h, and glucose (2 g/kg d-glucose, Sigma-Aldrich) was given intraperitoneally. All tests started between 9:00 and 10:00 a.m., and mice were treated/assessed randomly. Accu-Chek glucometers (Roche Diagnostics) were used to determine glucose levels during the ITT and GTT, using the Accu-Chek Smartview strips (Roche Diagnostics).
Adipose tissue culture
Fresh mouse epididymal adipose tissue was washed in ice-cold DMEM (32430, Thermo Fisher Scientific) under sterile conditions and cut into small ∼1-mm3 pieces. 100 mg of the adipose tissue was incubated in a 48-well plate for 24 h at 37 °C in 500 μl of DMEM (Thermo Fisher Scientific) in the presence of 10% FCS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). After 24 h, the supernatant and adipose tissue were collected and stored at −80 °C.
The human subcutaneous adipose tissue was cultured anonymously in a similar culture condition as above and stimulated with 50 ng/ml LPS (from E. coli serotype O55:B5, Sigma) without or with increasing concentrations of IL-37. After 24 h, the supernatants were collected and stored at −80 °C.
Liver cell culture
After sacrifice, livers were harvested and weighed. A small piece of liver was cut, weighed, and minced with a sterile surgical scalpel in RPMI 1640 (Thermo Fisher Scientific) containing 1% penicillin/streptomycin. The minced liver tissue was then macerated through a sterile 70-nm cell filter with the plunger end of a sterile plastic syringe. Additional RPMI was added to collect the cell suspension in a 50-ml tube. Cells were washed twice in RPMI (350 × g for 10 min) and suspended in RPMI with 1% penicillin/streptomycin and 5% FCS at a concentration of 12.5 mg/ml. One ml was added to each well of a 24-well flat bottom plate and cultured at 37 °C in a CO2-enriched environment for 24 h. Supernatants were collected for cytokine determinations, and cells were lysed with 250 μl of 0.5% Triton X-100.
Insulin-producing cells
INS-1 cells were a gift from Claes Wollheim (Department of Cell Physiology and Metabolism, University Medical Center, Geneva, Switzerland). Cells were maintained in RPMI 1640 medium with GlutaMAX (Gibco catalog no. 61870-010) supplemented with 10% fetal bovine serum (Life Technologies catalog no. 26140079), 100 units/ml penicillin, 100 μg/ml streptomycin (Gibco, catalog no. 15140-122), and 50 μm β-mercaptoethanol (Gibco, catalog no. 31350-010) (complete medium) at 37 °C in a humidified atmosphere containing 5% CO2.
INS-1 cell exposures
Cytokines
INS-1 cells were pre-exposed to 1 ng/ml IL-37 or vehicle for overnight incubation or 10 mg/ml IL-1Ra for 1 h. Subsequently, equal volumes of vehicle, 150 pg/ml recombinant mIL-1β (R&D Systems catalog no. 401-ML-005), or cytokine mixture (150 pg/ml recombinant mIL-1β plus 10 ng/ml recombinant hTNFα (Peprotech catalog no. 300-01A) were added for 24 h.
GLT
The fatty acid palmitate (Invitrogen) was dissolved in 80% ethanol and conjugated to BSA for at least 4 h at 37 °C in a 3:1 molar ratio of fatty acids to BSA in complete medium. Complete medium with BSA-conjugated palmitate (0.5 mm palmitate) was to added endotoxin-free glucose (Invitrogen) to obtain a final concentration of 25 mm. Control vehicle contained BSA and ethanol in concentrations identical to the final concentrations in the glucolipotoxic conditions.
INS-1 cell viability assay
Fifty thousand INS-1 cells were seeded in a 96-well plate for 24 h before exposure to either cytokines, GLT, or respective vehicles for 24 h. Next, the wells were washed in 200 μl of PBS before the addition of 110 μl of PBS containing 10% alamarBlueTM (Invitrogen, catalog no. DAL1100). The plate was then wrapped in aluminum foil and incubated for 3 h at 37 °C in a humidified atmosphere containing 5% CO2 before reading in a microplate reader at 570/595 nm.
Nitrite accumulation
Nitrite (NO2−), a surrogate measure of NO, was measured in INS-1 cell supernatants by mixing Griess reagent (0.1% (w/v) naphthylethene diamine hydrochloride; Sigma) in H2O2 with 1% (w/v) sulfanilamide (Bie and Berntsen) and diluted to 5% (v/v) with culture medium (1:1, v/v) before the absorbance was measured at 550 nm. Accumulated nitrite was calculated using a NaNO2 standard curve.
ELISA
Levels of insulin (Crystal Chem Inc.) and levels of adiponectin, leptin, IL-1α, IL-1β, IL-1Ra, IL-6, CXCL-1, and TNFα were measured with an ELISA (Bio-Techne) according to the manufacturer's protocols.
Quantitative PCR
Total mRNA was isolated from adipose tissue using TRIzol (Invitrogen), according to the manufacturer's instructions. mRNA was reverse-transcribed (iScript cDNA synthesis kit, Bio-Rad). RT-PCR was performed using specific primers with power SYBR Green master mix (Applied Biosystems, Foster City, CA) using the Step-one real-time PCR system (Applied Biosystems). 36B4 was used as a housekeeping gene. Mouse species primers used were as follows: 36B4, AGCGCGTCCTGGCATTGTGTGG (forward) and GGGCAGCAGTGGTGGCAGCAGC (reverse); CD68, CCAATTCAGGGTGGAAGAAA (forward) and CTCGGGCTCTGATGTAGGTC (reverse); F480, CTTGGCTATGGGCTTCCAGTC (forward) and GCAAGGAGGACAGAGTTTATCGTG (reverse); caspase-1, GGGACCCTCAAGTTTTGCC (forward) and GACGTGTACGAGTGGTTGTATT (reverse).
Lipids and glucose
Cholesterol, triglycerides, and glucose were measured enzymatically (Liquicolor, Human GmbH, Wiesbaden, Germany) using the manufacturer's protocols.
Immunohistochemistry
Immunohistochemistry for adipose tissue and pancreas was performed as described previously (25). Briefly, paraffin-embedded epididymal white adipose tissue was cut at 8 μm and deparaffinized and rehydrated in Clearene before ethanol and PBS rehydration. F480 antibody was used for detection of macrophages/monocytes (Serotec, Puchheim, Germany). Visualization of the proteins was done using 3,3′-diaminobenzidene for 5 min. Negative controls were stained for the primary antibody. In adipose tissue, the total adipocytes, macrophages, and crownlike structures were counted in ≥3 images per tissue. The images made with a light microscope were 1.6 × 2.0 mm, and ∼1000 adipocytes were counted per animal.
Sections of the pancreas were cut at 5 μm and stained for insulin (sc9168, Santa Cruz Biotechnology, Heidelberg, Germany) to visualize pancreatic islets. F480 antibody was used for detection of macrophages/monocytes (Serotec). Five pictures of 1.6 × 2.0 mm were taken and used to quantify total pancreatic area with ImageJ or the number of macrophages in the sections. Also, the number and (total) area of insulin-producing islets were determined with ImageJ. Total area of islets was divided by the total area of the pancreas per animal to determine the relative area of insulin-producing islets. Researchers were blinded for the experimental conditions when scoring the samples.
Statistical analysis
Data are shown as mean ± S.E. Differences between groups were analyzed with Student's t test, and differences among more than two groups were tested with ANOVA, with post hoc Bonferroni tests in GraphPad Prism version 6.0.
Author contributions
D. B. B. performed experiments, analyzed data, and wrote the manuscript. S. L. performed the experiments, analyzed data, and reviewed the manuscript. G. C., J. L. S., I. W. T., V. K., B. S., and T. A. performed experiments. J. A. D., T. M.-P., and R. S. supervised the study, performed experiments, and reviewed the manuscript. C. J. T. reviewed the manuscript. D. R. S. supervised the study and reviewed the manuscript. C. A. D. supervised the entire study.
Supplementary Material
These studies are supported by National Institutes of Health (NIH) Grant AI-15614 (to C. A. D.) and the Interleukin Foundation. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4.
- IL
- interleukin
- TNF
- tumor necrosis factor
- IL-1Ra
- IL-1 receptor antagonist
- HFD
- high-fat diet
- GLT
- glucolipotoxic conditions
- vHFD
- very high-fat diet
- LFD
- low-fat diet
- IL-1R8
- IL-1 receptor 8
- AMPK
- AMP-activated protein kinase
- mTOR
- mechanistic target of rapamycin
- ITT
- insulin tolerance test
- GTT
- glucose tolerance test
- LPS
- lipopolysaccharide
- ANOVA
- analysis of variance.
References
- 1. Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E. C., Biryukov S., Abbafati C., Abera S. F., Abraham J. P., Abu-Rmeileh N. M., Achoki T., AlBuhairan F. S., Alemu Z. A., et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donath M. Y., and Shoelson S. E. (2011) Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 3. Dinarello C. A., Nold-Petry C., Nold M., Fujita M., Li S., Kim S., and Bufler P. (2016) Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 46, 1067–1081 10.1002/eji.201545828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olefsky J. M., and Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 10.1146/annurev-physiol-021909-135846 [DOI] [PubMed] [Google Scholar]
- 5. Abdullah A., Wolfe R., Stoelwinder J. U., de Courten M., Stevenson C., Walls H. L., and Peeters A. (2011) The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int. J. Epidemiol. 40, 985–996 10.1093/ije/dyr018 [DOI] [PubMed] [Google Scholar]
- 6. Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., and Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. Clin. J. Invest. 112, 1796–1808 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao D., Madi M., Ding C., Fok M., Steele T., Ford C., Hunter L., and Bing C. (2014) Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol Metab. 307, E289–E304 10.1152/ajpendo.00430.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., and Tanti J. F. (2007) Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148, 241–251 10.1210/en.2006-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotamisligil G. S., Murray D. L., Choy L. N., and Spiegelman B. M. (1994) Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. U.S.A. 91, 4854–4858 10.1073/pnas.91.11.4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ballak D. B., Stienstra R., Tack C. J., Dinarello C. A., and van Diepen J. A. (2015) IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine 75, 280–290 10.1016/j.cyto.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J., Usui I., Ishizuka K., Kanatani Y., Hiratani K., Iwata M., Bukhari A., Haruta T., Sasaoka T., and Kobayashi M. (2006) Interleukin-1α inhibits insulin signaling with phosphorylating insulin receptor substrate-1 on serine residues in 3T3-L1 adipocytes. Mol. Endocrinol. 20, 114–124 10.1210/me.2005-0107 [DOI] [PubMed] [Google Scholar]
- 13. Um J. Y., Rim H. K., Kim S. J., Kim H. L., and Hong S. H. (2011) Functional polymorphism of IL-1 alpha and its potential role in obesity in humans and mice. PLoS One 6, e29524 10.1371/journal.pone.0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tynan G. A., Hearnden C. H., Oleszycka E., Lyons C. L., Coutts G., O'Connell J., Corrigan M. A., Lynch L., Campbell M., Callanan J. J., Mok K. H., Geoghegan J., O'Farrelly C., Allan S. M., Roche H. M., et al. (2014) Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1α-dependent inflammation. Diabetes 63, 2037–2050 10.2337/db13-1476 [DOI] [PubMed] [Google Scholar]
- 15. Stienstra R., Joosten L. A., and Koenen T., van Tits B., van Diepen J. A., van den Berg S. A., Rensen P. C., Voshol P. J., Fantuzzi G., Hijmans A., Kersten S., Müller M., van den Berg W. B., van Rooijen N., Wabitsch M., et al. (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12, 593–605 10.1016/j.cmet.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H. I., Spinas G. A., Kaiser N., Halban P. A., and Donath M. Y. (2002) Glucose-induced beta cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 10.1172/JCI200215318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juge-Aubry C. E., Somm E., Giusti V., Pernin A., Chicheportiche R., Verdumo C., Rohner-Jeanrenaud F., Burger D., Dayer J. M., and Meier C. A. (2003) Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 52, 1104–1110 10.2337/diabetes.52.5.1104 [DOI] [PubMed] [Google Scholar]
- 18. Sauter N. S., Schulthess F. T., Galasso R., Castellani L. W., and Maedler K. (2008) The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 149, 2208–2218 10.1210/en.2007-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Somm E., Henrichot E., Pernin A., Juge-Aubry C. E., Muzzin P., Dayer J. M., Nicklin M. J., and Meier C. A. (2005) Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes 54, 3503–3509 10.2337/diabetes.54.12.3503 [DOI] [PubMed] [Google Scholar]
- 20. Larsen C. M., Faulenbach M., Vaag A., Vølund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., and Donath M. Y. (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 10.1056/NEJMoa065213 [DOI] [PubMed] [Google Scholar]
- 21. Hensen J., Howard C. P., Walter V., and Thuren T. (2013) Impact of interleukin-1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab. 39, 524–531 10.1016/j.diabet.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 22. Ridker P. M., Howard C. P., Walter V., Everett B., Libby P., Hensen J., Thuren T., and CANTOS Pilot Investigative Group (2012) Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126, 2739–2748 10.1161/CIRCULATIONAHA.112.122556 [DOI] [PubMed] [Google Scholar]
- 23. Cavelti-Weder C., Babians-Brunner A., Keller C., Stahel M. A., Kurz-Levin M., Zayed H., Solinger A. M., Mandrup-Poulsen T., Dinarello C. A., and Donath M. Y. (2012) Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care 35, 1654–1662 10.2337/dc11-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timper K., Seelig E., Tsakiris D. A., and Donath M. Y. (2015) Safety, pharmacokinetics, and preliminary efficacy of a specific anti-IL-1α therapeutic antibody (MABp1) in patients with type 2 diabetes mellitus. J. Diabetes Complications 29, 955–960 10.1016/j.jdiacomp.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 25. Ballak D. B., van Diepen J. A., Moschen A. R., Jansen H. J., Hijmans A., Groenhof G. J., Leenders F., Bufler P., Boekschoten M. V., Müller M., Kersten S., Li S., Kim S., Eini H., Lewis E. C., et al. (2014) IL-37 protects against obesity-induced inflammation and insulin resistance. Nat. Commun. 5, 4711 10.1038/ncomms5711 [DOI] [PubMed] [Google Scholar]
- 26. Moschen A. R., Molnar C., Enrich B., Geiger S., Ebenbichler C. F., and Tilg H. (2011) Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 17, 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ter Horst K. W., Gilijamse P. W., Koopman K. E., de Weijer B. A., Brands M., Kootte R. S., Romijn J. A., Ackermans M. T., Nieuwdorp M., Soeters M. R., and Serlie M. J. (2015) Insulin resistance in obesity can be reliably identified from fasting plasma insulin. Int. J. Obes. (Lond.) 39, 1703–1709 10.1038/ijo.2015.125 [DOI] [PubMed] [Google Scholar]
- 28. Gregor M. F., and Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 29. Osborn O., and Olefsky J. M. (2012) The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 10.1038/nm.2627 [DOI] [PubMed] [Google Scholar]
- 30. Wiedemann M. S., Wueest S., Item F., Schoenle E. J., and Konrad D. (2013) Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. Am. J. Physiol. Endocrinol Metab. 305, E388–E395 10.1152/ajpendo.00179.2013 [DOI] [PubMed] [Google Scholar]
- 31. Hotamisligil G. S. (2006) Inflammation and metabolic disorders. Nature 444, 860–867 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 32. Nold M. F., Nold-Petry C. A., Zepp J. A., Palmer B. E., Bufler P., and Dinarello C. A. (2010) IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 11, 1014–1022 10.1038/ni.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nold-Petry C. A., Lo C. Y., Rudloff I., Elgass K. D., Li S., Gantier M. P., Lotz-Havla A. S., Gersting S. W., Cho S. X., Lao J. C., Ellisdon A. M., Rotter B., Azam T., Mangan N. E., Rossello F. J., et al. (2015) IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 16, 354–365 10.1038/ni.3103 [DOI] [PubMed] [Google Scholar]
- 34. Moretti S., Bozza S., Oikonomou V., Renga G., Casagrande A., Iannitti R. G., Puccetti M., Garlanda C., Kim S., Li S., van de Veerdonk F. L., Dinarello C. A., and Romani L. (2014) IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 10, e1004462 10.1371/journal.ppat.1004462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lunding L., Webering S., Vock C., Schröder A., Raedler D., Schaub B., Fehrenbach H., and Wegmann M. (2015) IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy 70, 366–373 10.1111/all.12566 [DOI] [PubMed] [Google Scholar]
- 36. Cavalli G., Koenders M. I., Kalabokis V., Kim J., Tan A. C., Garlanda C., Mantovani A., Dagna L., Joosten L. A., and Dinarello C. A. (2016) Treating experimental arthritis with the innate immune inhibitor IL-37 reduced joint and systemic inflammation. Rheumatology (Oxford) 55, 2220–2229 10.1093/rheumatology/kew325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wald D., Qin J., Zhao Z., Qian Y., Naramura M., Tian L., Towne J., Sims J. E., Stark G. R., and Li X. (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4, 920–927 10.1038/ni968 [DOI] [PubMed] [Google Scholar]
- 38. Li S., Neff C. P., Barber K., Hong J., Luo Y., Azam T., Palmer B. E., Fujita M., Garlanda C., Mantovani A., Kim S., and Dinarello C. A. (2015) Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. U.S.A. 112, 2497–2502 10.1073/pnas.1424626112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donath M. Y., Schumann D. M., Faulenbach M., Ellingsgaard H., Perren A., and Ehses J. A. (2008) Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31, Suppl. 2, S161–S164 10.2337/dc08-s243 [DOI] [PubMed] [Google Scholar]
- 40. Sun G., Tarasov A. I., McGinty J., McDonald A., da Silva Xavier G., Gorman T., Marley A., French P. M., Parker H., Gribble F., Reimann F., Prendiville O., Carzaniga R., Viollet B., et al. (2010) Ablation of AMP-activated protein kinase α1 and α2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 53, 924–936 10.1007/s00125-010-1692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kulkarni R. N., Mizrachi E. B., Ocana A. G., and Stewart A. F. (2012) Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61, 2205–2213 10.2337/db12-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bernal-Mizrachi E., Kulkarni R. N., Scott D. K., Mauvais-Jarvis F., Stewart A. F., and Garcia-Ocaña A. (2014) Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 63, 819–831 10.2337/db13-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Myers R. W., Guan H. P., Ehrhart J., Petrov A., Prahalada S., Tozzo E., Yang X., Kurtz M. M., Trujillo M., Gonzalez Trotter D., Feng D., Xu S., Eiermann G., Holahan M. A., Rubins D., et al. (2017) Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 357, 507–511 10.1126/science.aah5582 [DOI] [PubMed] [Google Scholar]
- 44. Cokorinos E. C., Delmore J., Reyes A. R., Albuquerque B., Kjøbsted R., Jørgensen N. O., Tran J. L., Jatkar A., Cialdea K., Esquejo R. M., Meissen J., Calabrese M. F., Cordes J., Moccia R., Tess D., et al. (2017) Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metab. 25, 1147–1159.e10 10.1016/j.cmet.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 45. Cavalli G., Justice J. N., Boyle K. E., D'Alessandro A., Eisenmesser E. Z., Herrera J. J., Hansen K. C., Nemkov T., Stienstra R., Garlanda C., Mantovani A., Seals D. R., Dagna L., Joosten L. A., Ballak D. B., and Dinarello C. A. (2017) Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. U.S.A. 114, 2313–2318 10.1073/pnas.1619011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong T. P., Andersen N. A., Nielsen K., Karlsen A. E., Fantuzzi G., Eizirik D. L., Dinarello C. A., and Mandrup-Poulsen T. (2000) Interleukin-18 mRNA, but not interleukin-18 receptor mRNA, is constitutively expressed in islet beta-cells and up-regulated by interferon-γ. Eur. Cytokine Netw. 11, 193–205 [PubMed] [Google Scholar]
- 47. Dinarello C. A., Donath M. Y., and Mandrup-Poulsen T. (2010) Role of IL-1β in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 314–321 [DOI] [PubMed] [Google Scholar]
- 48. Zeng Q., Song R., Fullerton D. A., Ao L., Zhai Y., Li S., Ballak D. B., Cleveland J. C. Jr., Reece T. B., McKinsey T. A., Xu D., Dinarello C. A., and Meng X. (2017) Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 1631–1636 10.1073/pnas.1619667114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donath M. Y., Dalmas É., Sauter N. S., and Böni-Schnetzler M. (2013) Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 17, 860–872 10.1016/j.cmet.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 50. Ehses J. A., Lacraz G., Giroix M. H., Schmidlin F., Coulaud J., Kassis N., Irminger J. C., Kergoat M., Portha B., Homo-Delarche F., and Donath M. Y. (2009) IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. U.S.A. 106, 13998–14003 10.1073/pnas.0810087106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ehses J. A., Perren A., Eppler E., Ribaux P., Pospisilik J. A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M. K., Biollaz G., Fontana A., Reinecke M., Homo-Delarche F., and Donath M. Y. (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 10.2337/db06-1650 [DOI] [PubMed] [Google Scholar]
- 52. He M., Su H., Gao W., Johansson S. M., Liu Q., Wu X., Liao J., Young A. A., Bartfai T., and Wang M. W. (2010) Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS One 5, e14205 10.1371/journal.pone.0014205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Böni-Schnetzler M., Häuselmann S. P., Dalmas E., Meier D. T., Thienel C., Traub S., Schulze F., Steiger L., Dror E., Martin P., Herrera P. L., Gabay C., and Donath M. Y. (2018) Beta cell-specific deletion of the IL-1 receptor antagonist impairs beta cell proliferation and insulin secretion. Cell Rep. 22, 1774–1786 10.1016/j.celrep.2018.01.063 [DOI] [PubMed] [Google Scholar]
- 54. Hotamisligil G. S., Peraldi P., Budavari A., Ellis R., White M. F., and Spiegelman B. M. (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271, 665–668 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 55. Kroder G., Bossenmaier B., Kellerer M., Capp E., Stoyanov B., Mühlhöfer A., Berti L., Horikoshi H., Ullrich A., and Häring H. (1996) Tumor necrosis factor-α- and hyperglycemia-induced insulin resistance: evidence for different mechanisms and different effects on insulin signaling. J. Clin. Invest. 97, 1471–1477 10.1172/JCI118569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nov O., Kohl A., Lewis E. C., Bashan N., Dvir I., Ben-Shlomo S., Fishman S., Wueest S., Konrad D., and Rudich A. (2010) Interleukin-1β may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology 151, 4247–4256 10.1210/en.2010-0340 [DOI] [PubMed] [Google Scholar]
- 57. McNelis J. C., and Olefsky J. M. (2014) Macrophages, immunity, and metabolic disease. Immunity 41, 36–48 10.1016/j.immuni.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 58. Lee Y., Ryu J. W., Chang H., Sohn J. Y., Lee K. W., Woo C. W., Kang H. J., Jeong S. Y., Choi E. K., and Lee J. S. (2010) In vivo MR evaluation of the effect of the CCR2 antagonist on macrophage migration. Magn. Reson. Med. 64, 72–79 10.1002/mrm.22409 [DOI] [PubMed] [Google Scholar]
- 59. Boon M. R., van den Berg S. A., Wang Y., van den Bossche J., Karkampouna S., Bauwens M., De Saint-Hubert M., van der Horst G., Vukicevic S., de Winther M. P., Havekes L. M., Jukema J. W., Tamsma J. T., van der Pluijm G., van Dijk K. W., and Rensen P. C. (2013) BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One 8, e74083 10.1371/journal.pone.0074083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zorrilla E. P., Sanchez-Alavez M., Sugama S., Brennan M., Fernandez R., Bartfai T., and Conti B. (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc. Natl. Acad. Sci. U.S.A. 104, 11097–11102 10.1073/pnas.0611523104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Netea M. G., Joosten L. A., Lewis E., Jensen D. R., Voshol P. J., Kullberg B. J., Tack C. J., van Krieken H., Kim S. H., Stalenhoef A. F., van de Loo F. A., Verschueren I., Pulawa L., Akira S., Eckel R. H., et al. (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 12, 650–656 10.1038/nm1415 [DOI] [PubMed] [Google Scholar]
- 62. Lee Y. S., Li P., Huh J. Y., Hwang I. J., Lu M., Kim J. I., Ham M., Talukdar S., Chen A., Lu W. J., Bandyopadhyay G. K., Schwendener R., Olefsky J., and Kim J. B. (2011) Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60, 2474–2483 10.2337/db11-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shiwa M., Yoneda M., Okubo H., Ohno H., Kobuke K., Monzen Y., Kishimoto R., Nakatsu Y., Asano T., and Kohno N. (2015) Distinct time course of the decrease in hepatic AMP-activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS One 10, e0135554 10.1371/journal.pone.0135554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levin N., Nelson C., Gurney A., Vandlen R., and de Sauvage F. (1996) Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc. Natl. Acad. Sci. U.S.A. 93, 1726–1730 10.1073/pnas.93.4.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leontieva O. V., Paszkiewicz G. M., and Blagosklonny M. V. (2014) Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 13, 616–622 10.1111/acel.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.