Abstract
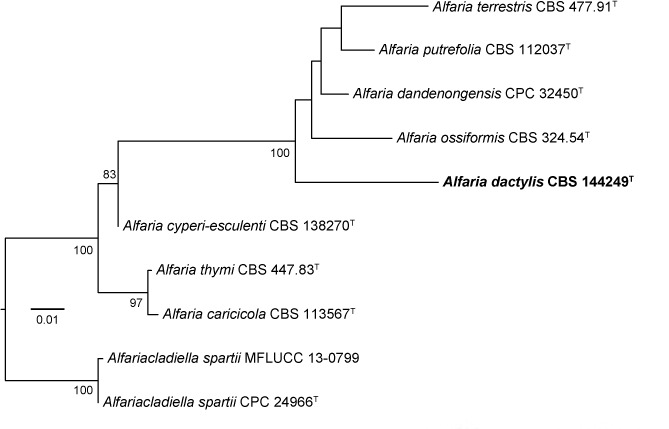
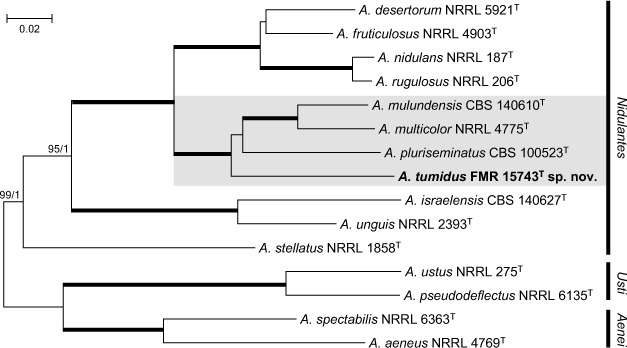
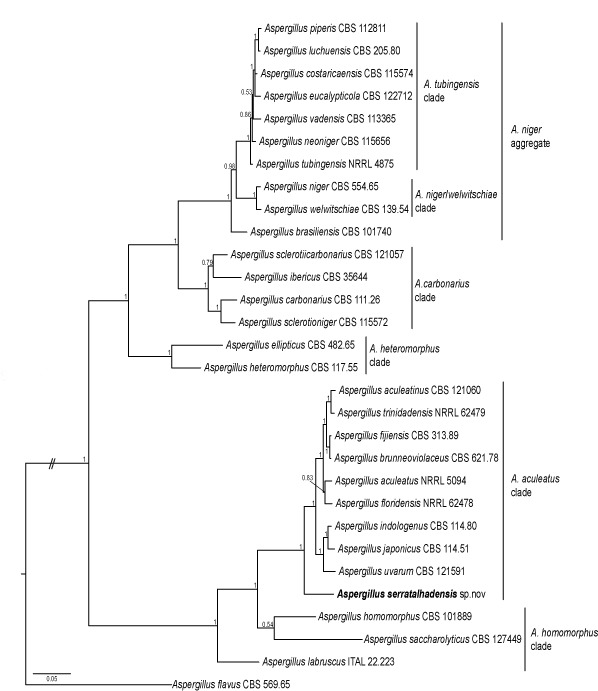
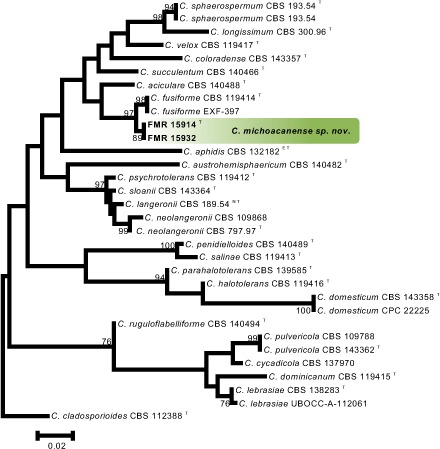
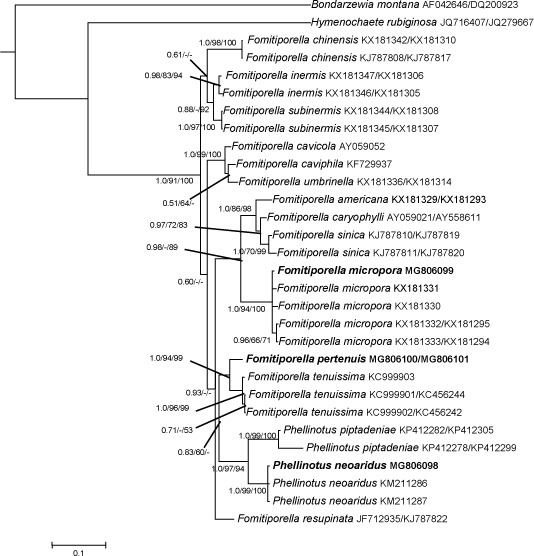
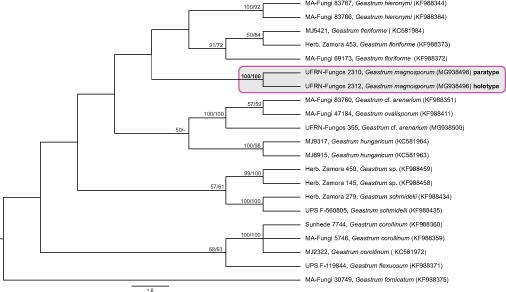
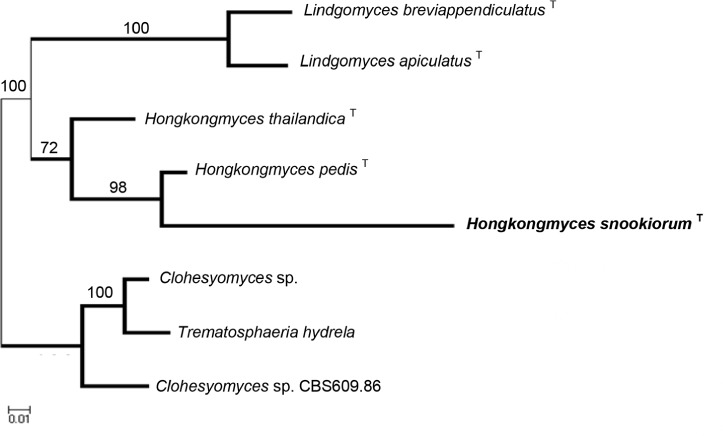
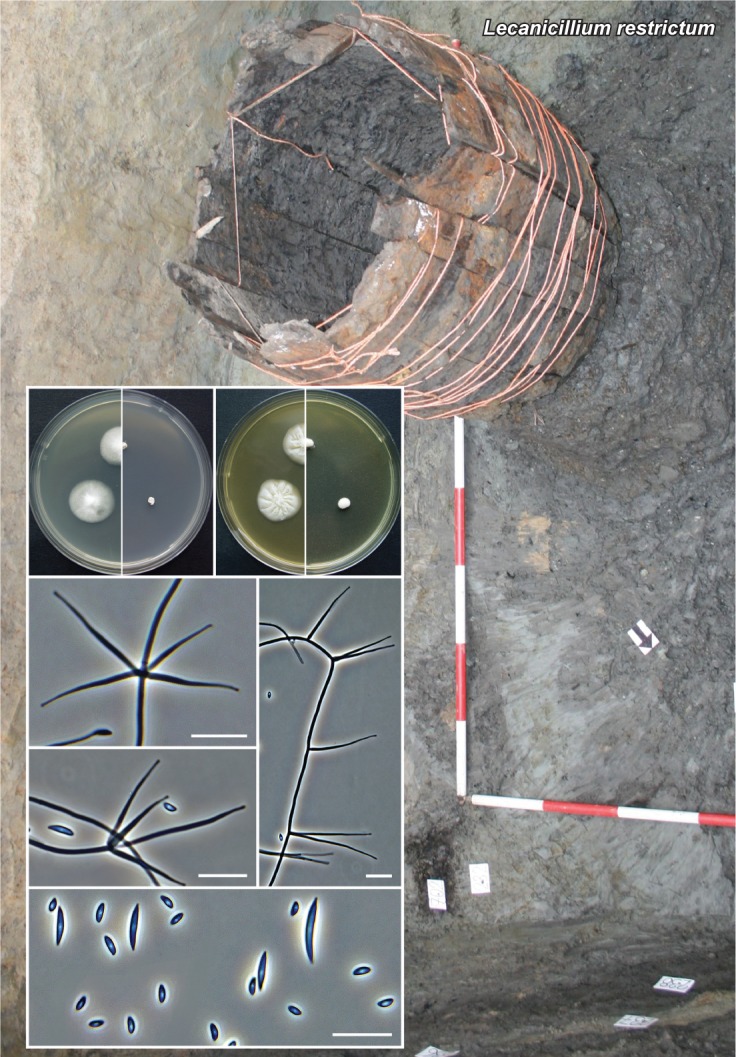
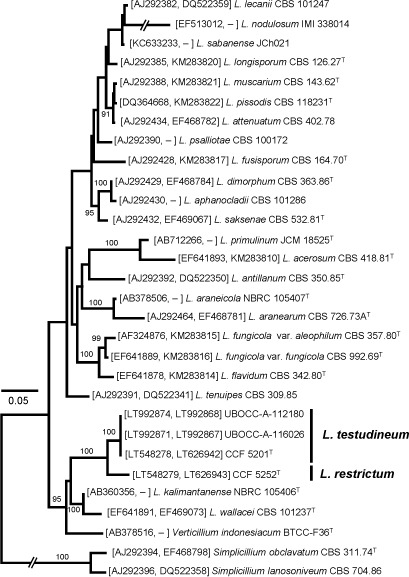
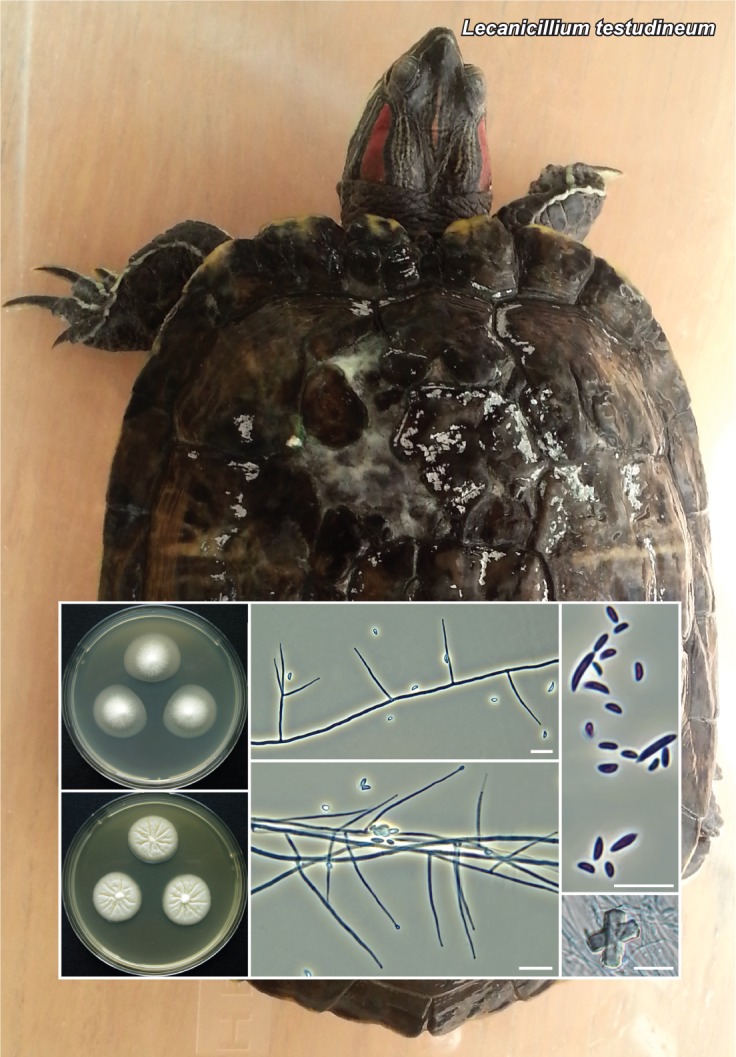
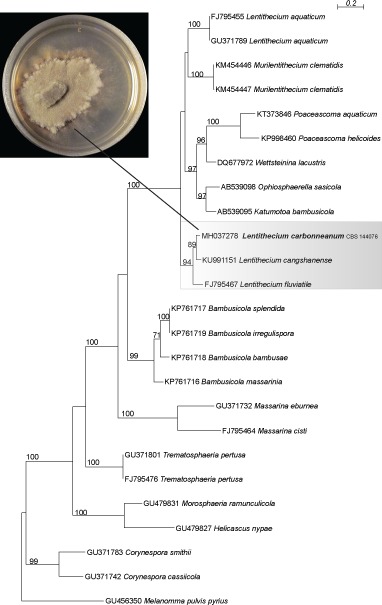
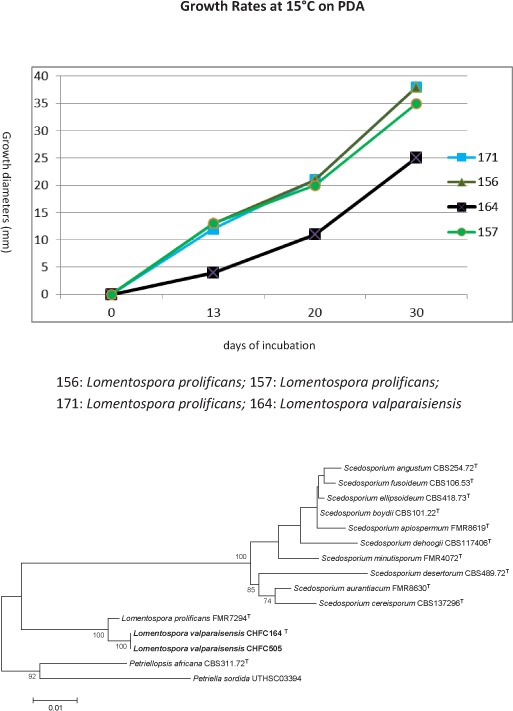
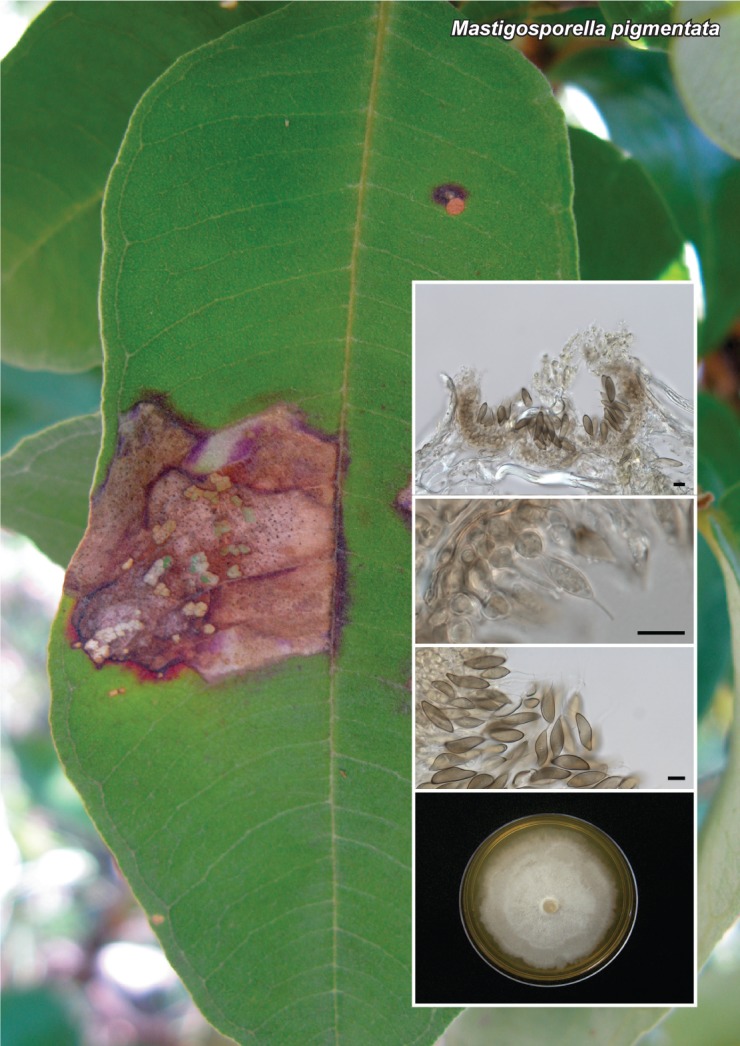
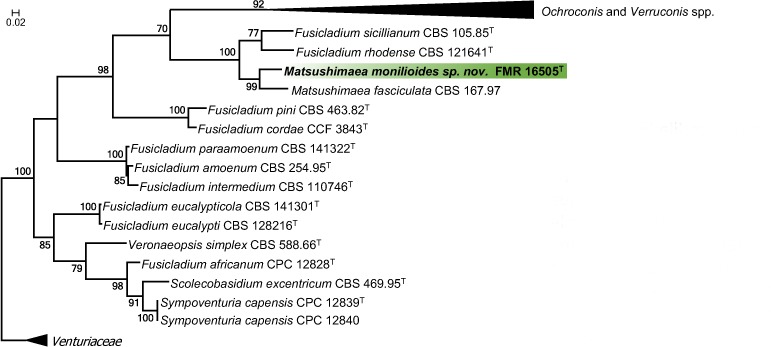
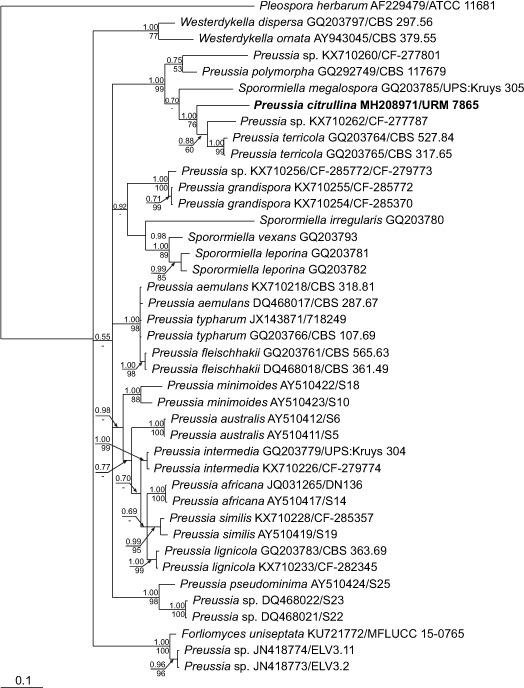
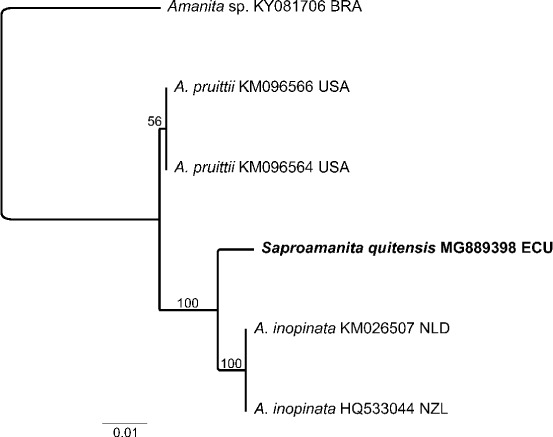
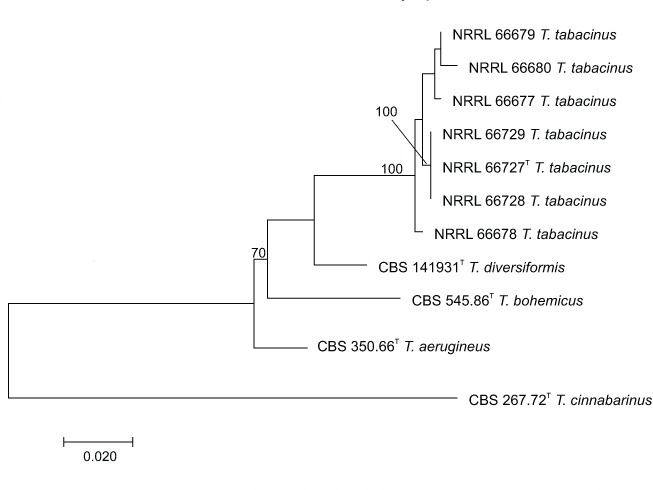
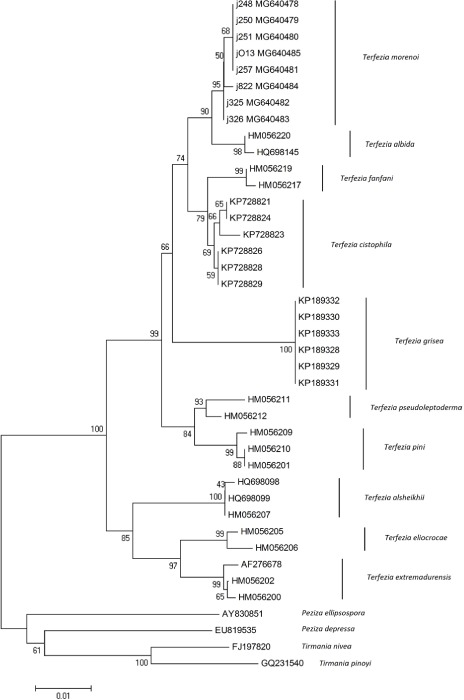
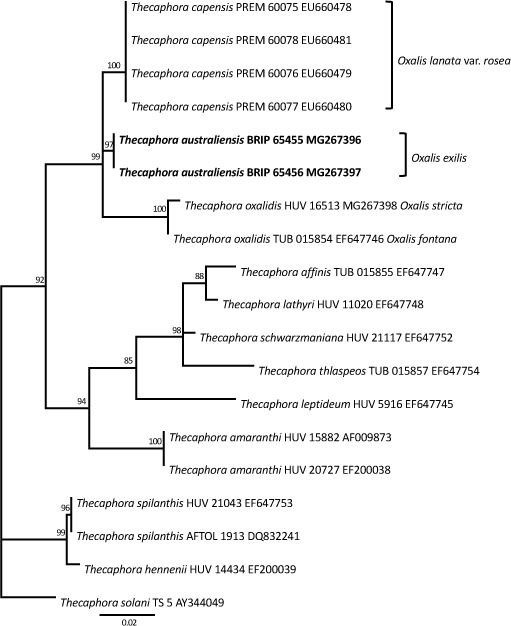
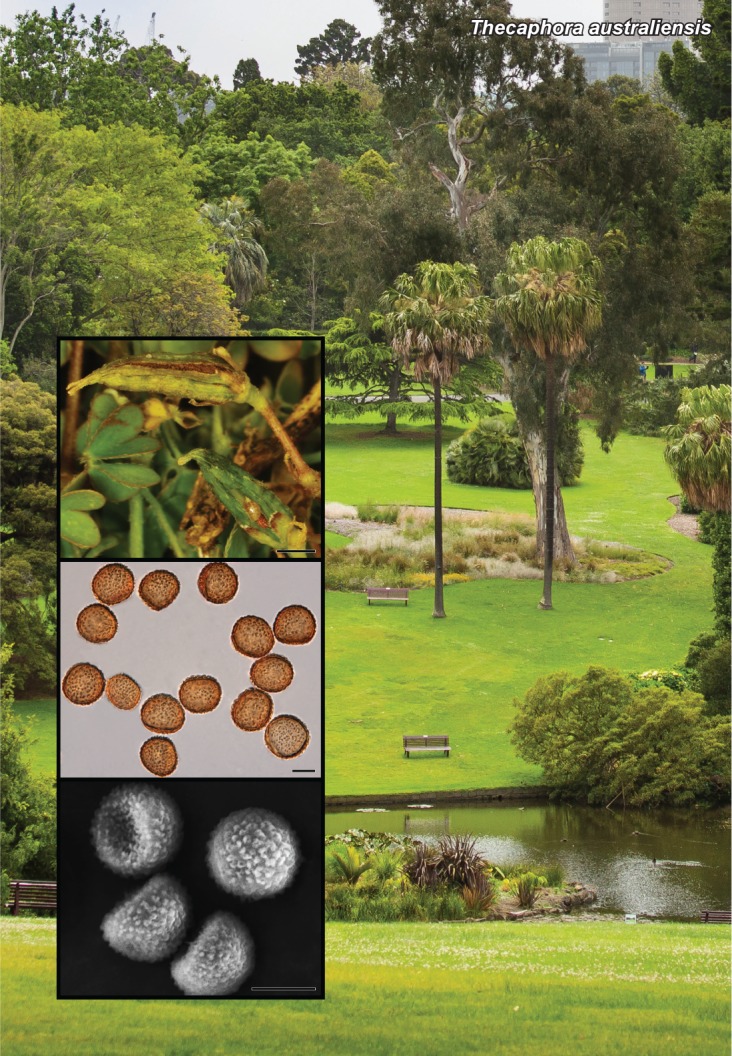
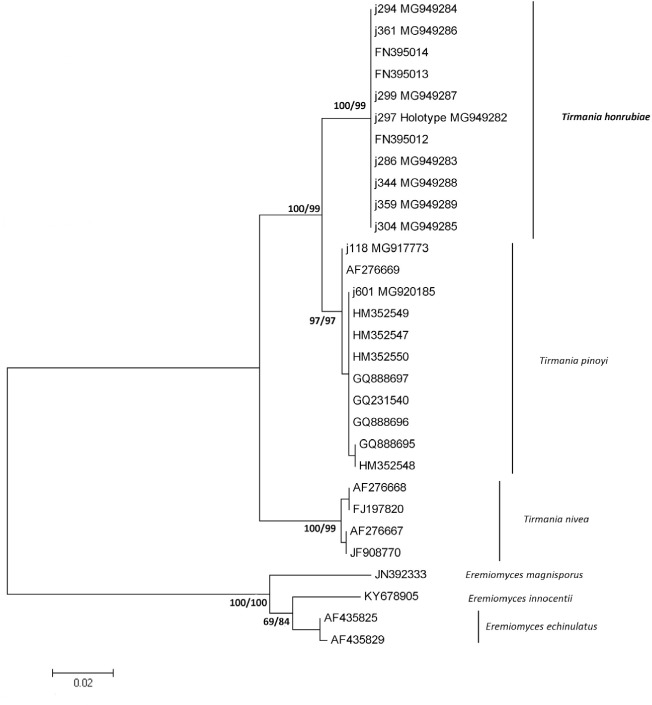
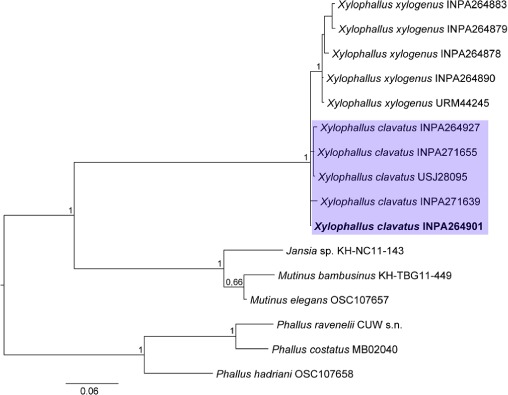
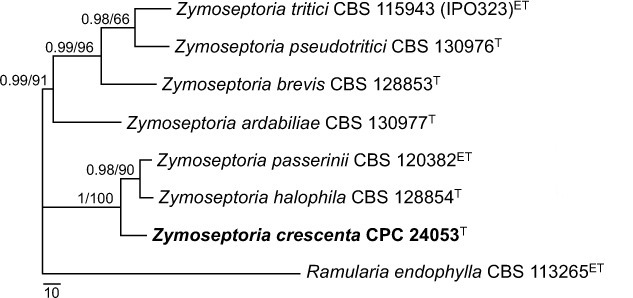
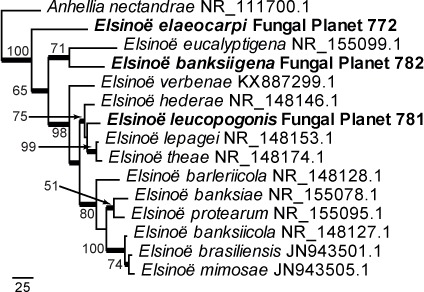
Novel species of fungi described in this study include those from various countries as follows: Australia, Chaetopsina eucalypti on Eucalyptus leaf litter, Colletotrichum cobbittiense from Cordyline stricta × C. australis hybrid, Cyanodermella banksiae on Banksia ericifolia subsp. macrantha, Discosia macrozamiae on Macrozamia miquelii, Elsinoë banksiigena on Banksia marginata, Elsinoë elaeocarpi on Elaeocarpus sp., Elsinoë leucopogonis on Leucopogon sp., Helminthosporium livistonae on Livistona australis, Idriellomyces eucalypti (incl. Idriellomyces gen. nov.) on Eucalyptus obliqua, Lareunionomyces eucalypti on Eucalyptus sp., Myrotheciomyces corymbiae (incl. Myrotheciomyces gen. nov., Myrotheciomycetaceae fam. nov.), Neolauriomyces eucalypti (incl. Neolauriomyces gen. nov., Neolauriomycetaceae fam. nov.) on Eucalyptus sp., Nullicamyces eucalypti (incl. Nullicamyces gen. nov.) on Eucalyptus leaf litter, Oidiodendron eucalypti on Eucalyptus maidenii, Paracladophialophora cyperacearum (incl. Paracladophialophoraceae fam. nov.) and Periconia cyperacearum on leaves of Cyperaceae, Porodiplodia livistonae (incl. Porodiplodia gen. nov., Porodiplodiaceae fam. nov.) on Livistona australis, Sporidesmium melaleucae (incl. Sporidesmiales ord. nov.) on Melaleuca sp., Teratosphaeria sieberi on Eucalyptus sieberi, Thecaphora australiensis in capsules of a variant of Oxalis exilis. Brazil, Aspergillus serratalhadensis from soil, Diaporthe pseudoinconspicua from Poincianella pyramidalis, Fomitiporella pertenuis on dead wood, Geastrum magnosporum on soil, Marquesius aquaticus (incl. Marquesius gen. nov.) from submerged decaying twig and leaves of unidentified plant, Mastigosporella pigmentata from leaves of Qualea parviflorae, Mucor souzae from soil, Mycocalia aquaphila on decaying wood from tidal detritus, Preussia citrullina as endophyte from leaves of Citrullus lanatus, Queiroziella brasiliensis (incl. Queiroziella gen. nov.) as epiphytic yeast on leaves of Portea leptantha, Quixadomyces cearensis (incl. Quixadomyces gen. nov.) on decaying bark, Xylophallus clavatus on rotten wood. Canada, Didymella cari on Carum carvi and Coriandrum sativum. Chile, Araucasphaeria foliorum (incl. Araucasphaeria gen. nov.) on Araucaria araucana, Aspergillus tumidus from soil, Lomentospora valparaisensis from soil. Colombia, Corynespora pseudocassiicola on Byrsonima sp., Eucalyptostroma eucalyptorum on Eucalyptus pellita, Neometulocladosporiella eucalypti (incl. Neometulocladosporiella gen. nov.) on Eucalyptus grandis × urophylla, Tracylla eucalypti (incl. Tracyllaceae fam. nov., Tracyllalales ord. nov.) on Eucalyptus urophylla. Cyprus, Gyromitra anthracobia (incl. Gyromitra subg. Pseudoverpa) on burned soil. Czech Republic, Lecanicillium restrictum from the surface of the wooden barrel, Lecanicillium testudineum from scales of Trachemys scripta elegans. Ecuador, Entoloma yanacolor and Saproamanita quitensis on soil. France, Lentithecium carbonneanum from submerged decorticated Populus branch. Hungary, Pleuromyces hungaricus (incl. Pleuromyces gen. nov.) from a large Fagus sylvatica log. Iran, Zymoseptoria crescenta on Aegilops triuncialis. Malaysia, Ochroconis musicola on Musa sp. Mexico, Cladosporium michoacanense from soil. New Zealand, Acrodontium metrosideri on Metrosideros excelsa, Polynema podocarpi on Podocarpus totara, Pseudoarthrographis phlogis (incl. Pseudoarthrographis gen. nov.) on Phlox subulata. Nigeria, Coprinopsis afrocinerea on soil. Pakistan, Russula mansehraensis on soil under Pinus roxburghii. Russia, Baorangia alexandri on soil in deciduous forests with Quercus mongolica. South Africa, Didymocyrtis brachylaenae on Brachylaena discolor. Spain, Alfaria dactylis from fruit of Phoenix dactylifera, Dothiora infuscans from a blackened wall, Exophiala nidicola from the nest of an unidentified bird, Matsushimaea monilioides from soil, Terfezia morenoi on soil. United Arab Emirates, Tirmania honrubiae on soil. USA, Arxotrichum wyomingense (incl. Arxotrichum gen. nov.) from soil, Hongkongmyces snookiorum from submerged detritus from a fresh water fen, Leratiomyces tesquorum from soil, Talaromyces tabacinus on leaves of Nicotiana tabacum. Vietnam, Afroboletus vietnamensis on soil in an evergreen tropical forest, Colletotrichum condaoense from Ipomoea pes-caprae. Morphological and culture characteristics along with DNA barcodes are provided.
Keywords: ITS nrDNA barcodes, LSU, new taxa, systematics
Overview Mucoromycotina and Basidiomycota phylogeny.

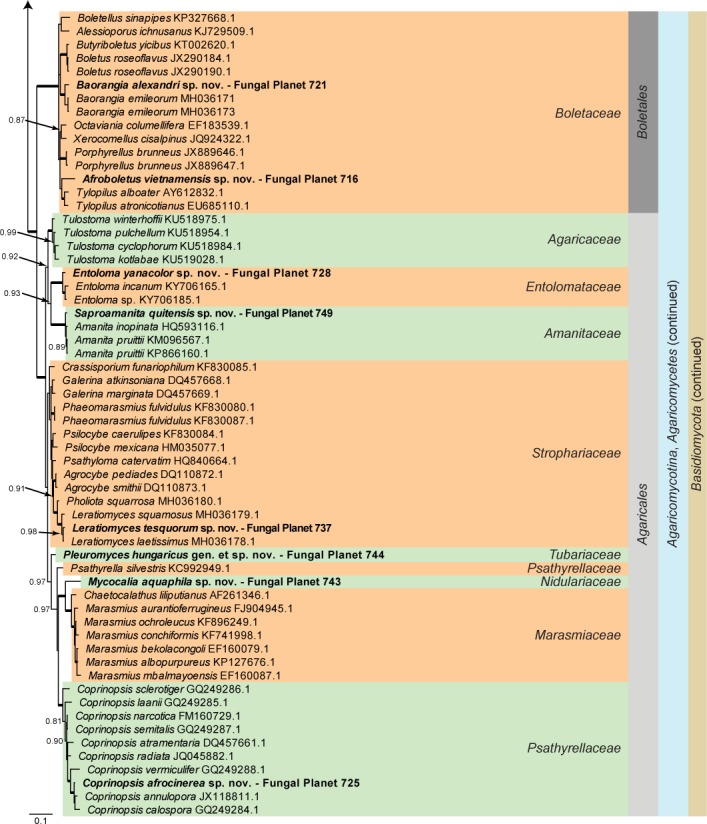
Consensus phylogram (50 % majority rule) of 57 752 trees resulting from a Bayesian analysis of the LSU sequence alignment (118 taxa including outgroup; 862 aligned positions; 551 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders, classes, subdivisions and phyla are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Phytophthora moyootj (GenBank KP004499.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S22745).
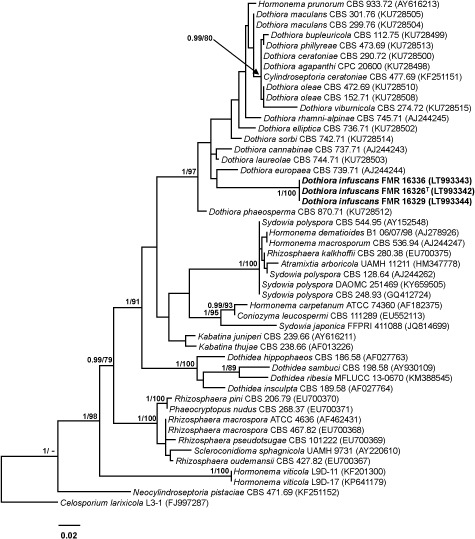
Overview Dothideomycetes phylogeny – part 1.
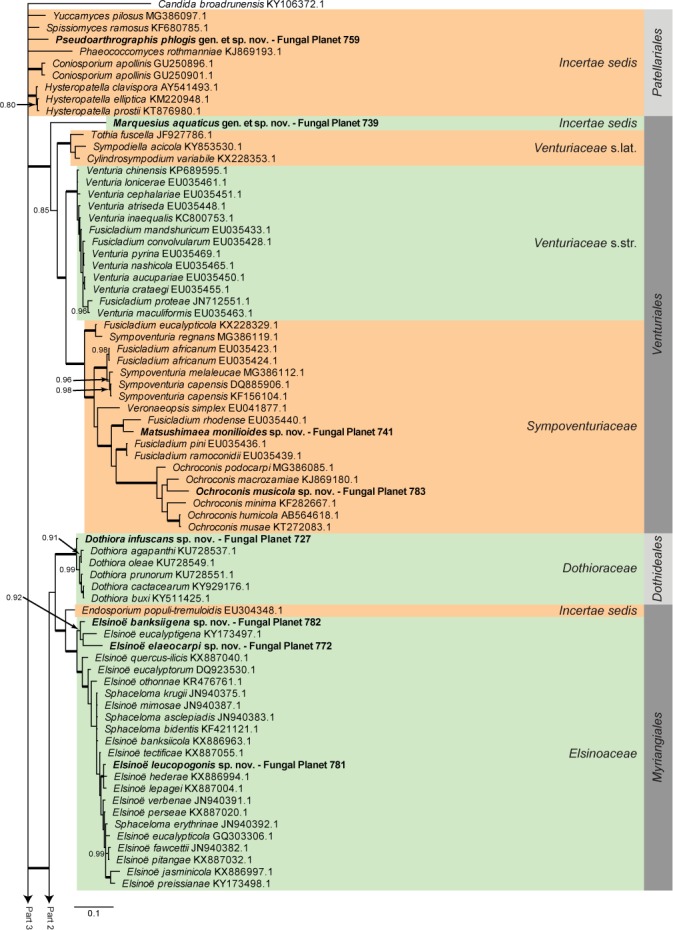
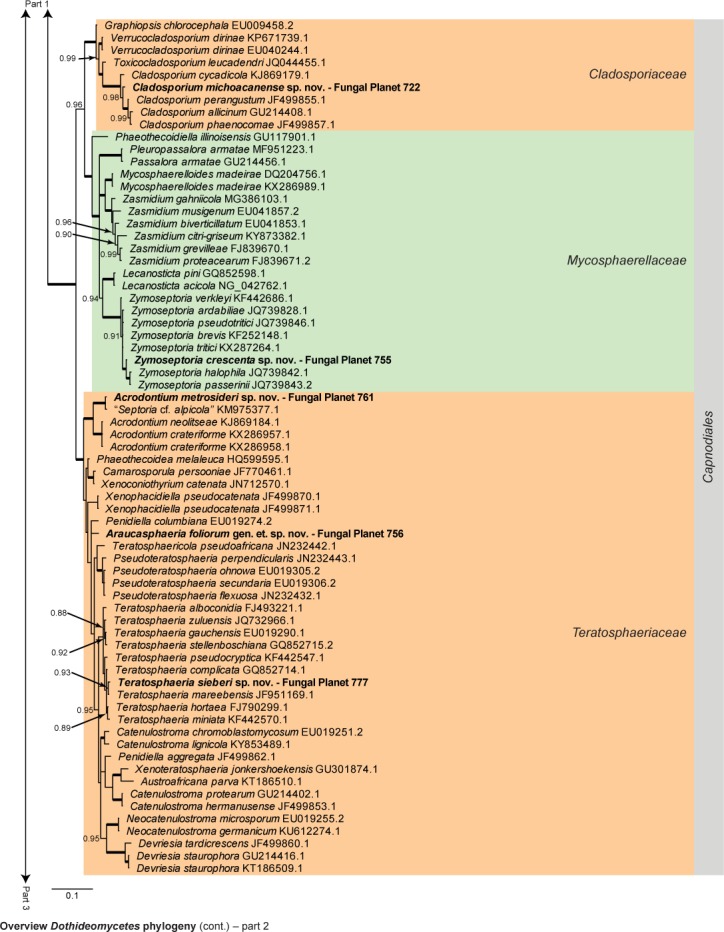
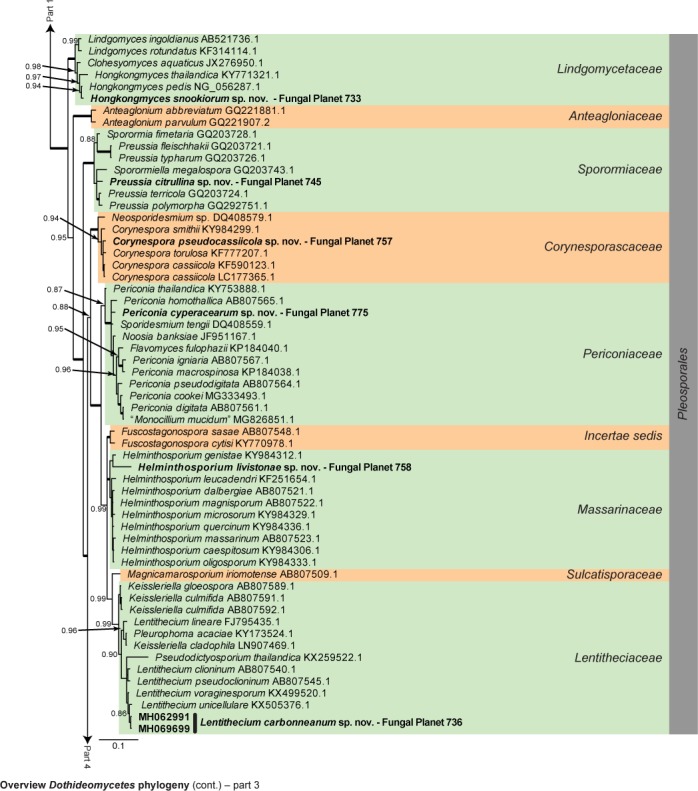
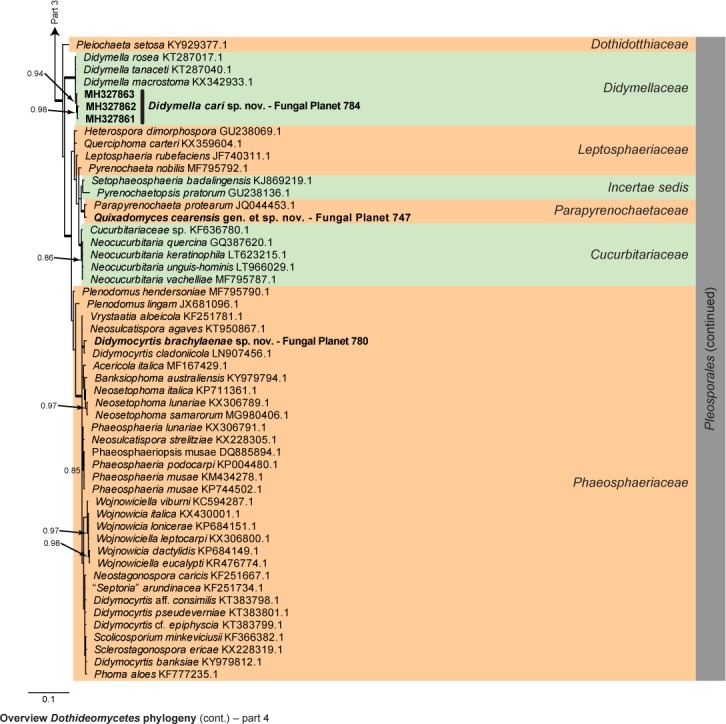
Consensus phylogram (50 % majority rule) of 23 402 trees resulting from a Bayesian analysis of the LSU sequence alignment (255 taxa including outgroup; 805 aligned positions; 450 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S22745).
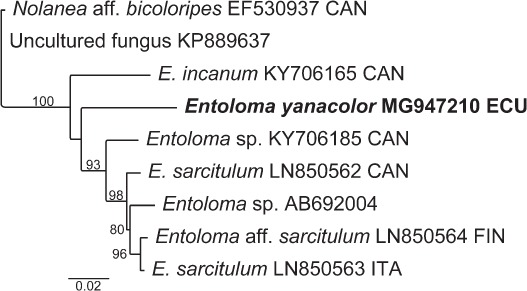
Overview Pezizomycetes, Lecanoromycetes and Eurotiomycetes phylogeny.
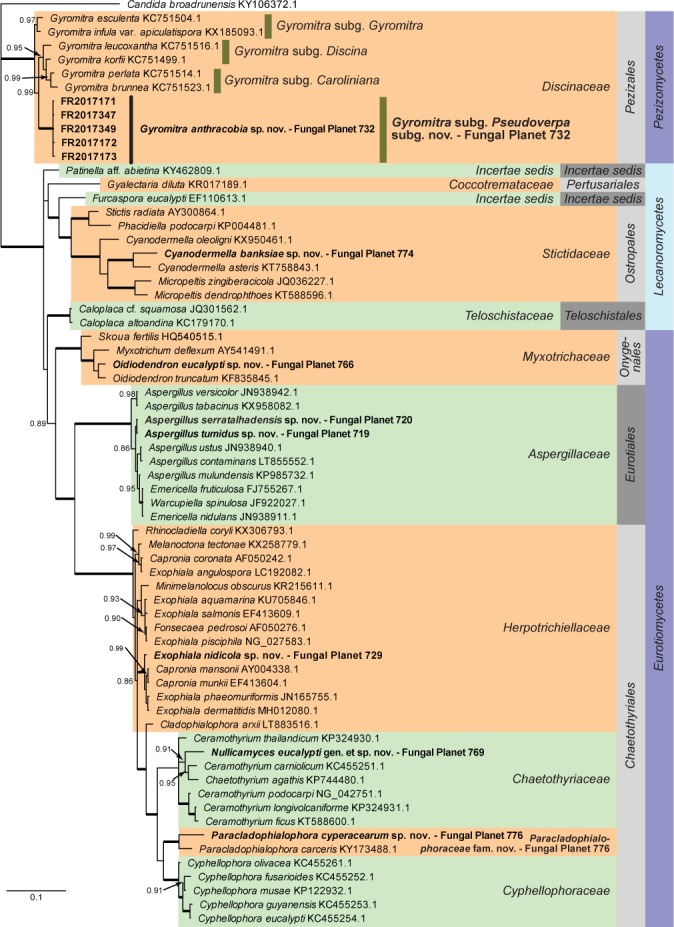
Consensus phylogram (50 % majority rule) of 6 602 trees resulting from a Bayesian analysis of the LSU sequence alignment (67 taxa including outgroup; 805 aligned positions; 380 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S22745).
Overview Leotiomycetes phylogeny.
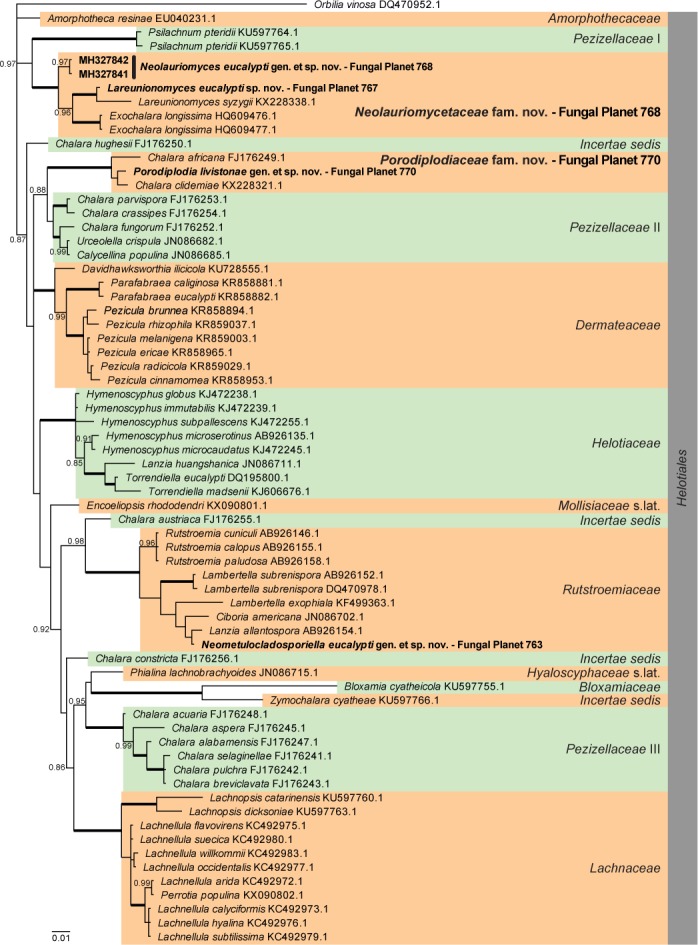
Consensus phylogram (50 % majority rule) of 18 152 trees resulting from a Bayesian analysis of the LSU sequence alignment (68 taxa including outgroup; 781 aligned positions; 227 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Orbilia vinosa (GenBank DQ470952.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S22745).
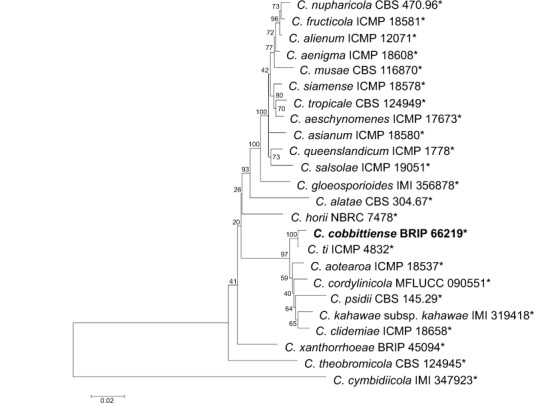
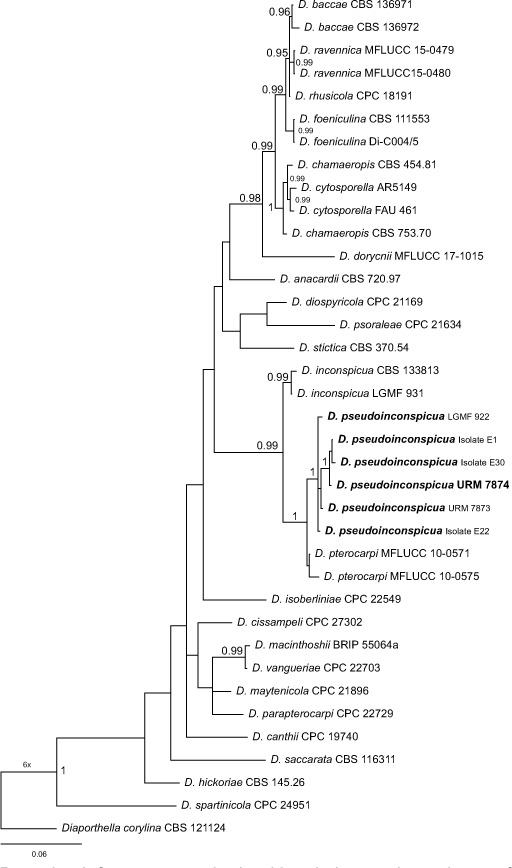
Overview Sordariomycetes phylogeny – part 1.
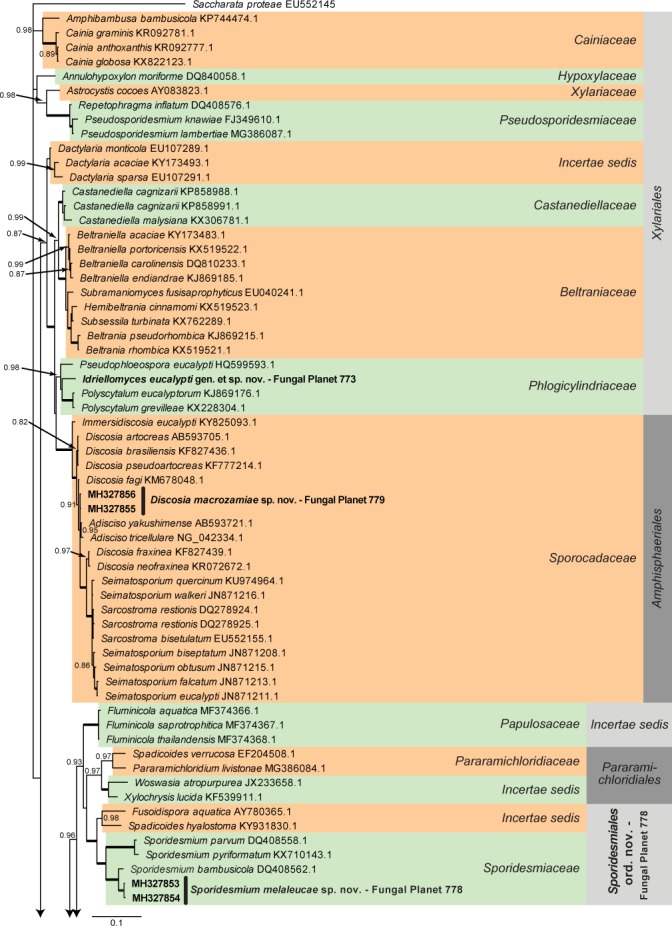
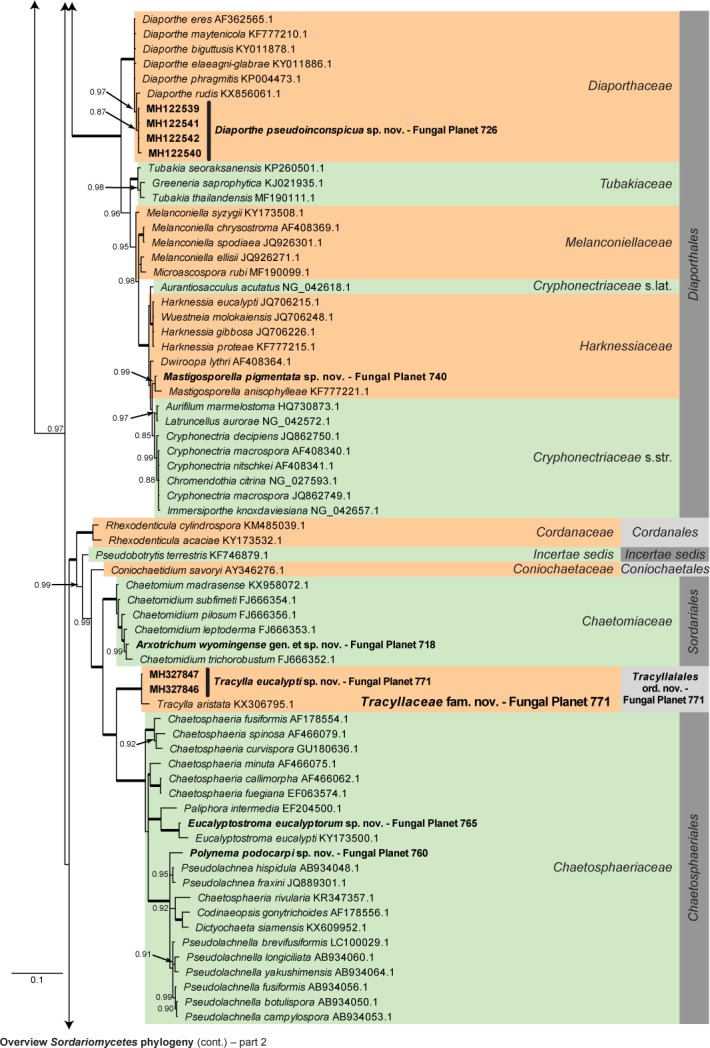
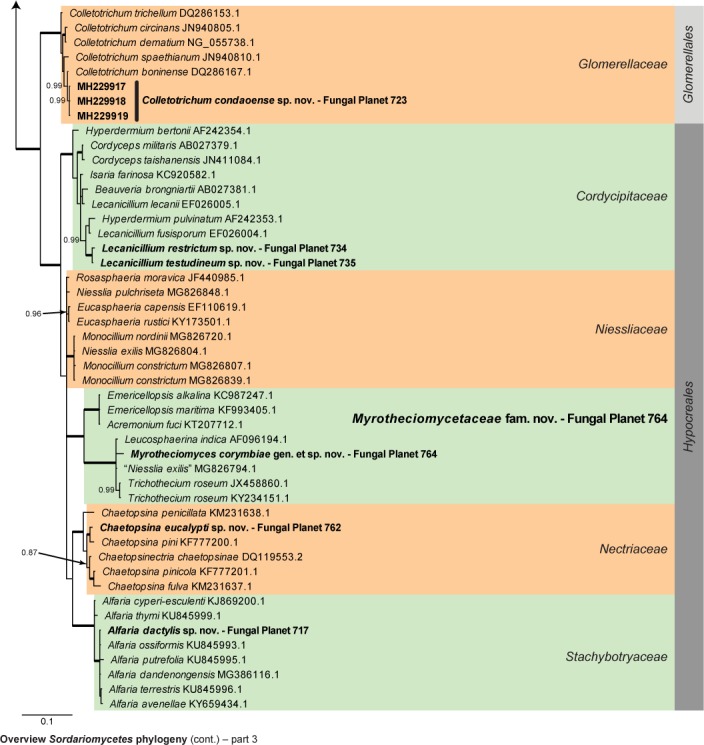
Consensus phylogram (50 % majority rule) of 115 202 trees resulting from a Bayesian analysis of the LSU sequence alignment (179 taxa including outgroup; 785 aligned positions; 340 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S22745).
Acknowledgments
The study of Olga V. Morozova and Tatiana Yu. Svetasheva was carried out within the framework of an institutional research project of the Komarov Botanical Institute RAS ‘Biodiversity and spatial structure of fungi and myxomycetes communities in natural and anthropogenic ecosystems’ (AAAA-A18-118031290108-6) using equipment of its Core Facility Center ‘Cell and Molecular Technologies in Plant Science’. Alina V. Alexandrova acknowledges financial support from the Russian Science Foundation (project N 14-50-00029).
Daniela de A. Viana Marques acknowledges Universidade de Pernambuco for financial support. Jan Borovička is thanked for providing the Portuguese collection of Baorangia emileorum and its ITS and LSU sequences, and Alessia Tatti for sending the Sardinian collections of B. emileorum. Taimy Cantillo, Luis F.P. Gusmão, Luana T. do Carmo, Lucas B. Conceição, Julieth O. Sousa, Luiz F. de Oliveira, Renan N. Barbosa, Rhudson H.S.F. Cruz, André L.C.M. de A. Santiago, Carlos A.F. de Souza, Diogo X. Lima, Rafael J.V. de Oliveira and Thalline R.L. Cordeiro, Olinto L. Pereira, Rejane M.F. Silva, Rafael J.V. Oliveira, José L. Bezerra, Gladstone A. Silva Ciro R. Félix, Melissa F. Landell, Thays G.L. Oliveira, Jadson D.P. Bezerra, Alexandre R. Machado, Cristina M. Souza-Motta and Oliane M. C. Magalhães, Tatiana B. Gibertoni, Vitor Xavier de Lima and José R. C. Oliveira-Filho acknowledge financial support and/or scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE); the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), Parque Memorial Zumbi dos Palmares and Usina Caeté – Grupo Carlos Lyra and Nordesta AS for suport during field trips. Maria E. Ordoñez and colleagues acknowledge the Secretaria de Educación Superior, Ciencia, Tecnología e Innovación del Ecuador (SENESCYT), Arca de Noé Initiative, and the Pontificia Universidad Católica del Ecuador, project N13415 for financial support. Hugo Madrid was partially funded by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, project no. 11140562. Vit Hubka and colleagues express their gratitude to Marek Kiecoň, Pavel Malík and Tereza Krasnokutská (National Heritage Institute) for providing information on archaeological research; Hana Rajhelová (Silesian University in Opava) and Jitka Koubková (Veterinary Laboratory Labvet) for providing photo documentation and material for mycological examinations; Czechoslovak Microscopy Society for support (CSMS scholarship 2016). The research of V. Hubka was supported by Charles University Research Centre program No. 204069 and the grant of the Czech Ministry of Health (AZV 17-31269A). Alfredo Vizzini and colleagues thank Jan Holec for administering of the loan of European material from PRM herbarium (Prague, Czech Republic). Soňa Jančovičová helped with the line drawings. Jozef Šibík and David Cooper are acknowledged for the support during the field collections in Colorado (USA) that was financed by the Slovak American Foundation. The sequencing of samples was funded by the Slovak national project Vega 02/0018/18. Željko Jurjević acknowledges Filomena Epifani and Sammy Sedky for their excellent technical support. Malka Saba acknowledges the Higher Education Commission (HEC), Islamabad, Pakistan, for financial assistance during field trips in Pakistan and the Slovak national project APVV-15-0210 for sequencing of Russula mansehraensis. The research of Alena Nováková and Miroslav Kolařík was supported by the Ministry of Education, Youth and Sports of the Czech Republic (grant number LO1509). Asunción Morte, Juan Julián Bordallo and Antonio Rodríguez were supported by projects 19484/PI/14 (FEDER and Fundación Séneca - Agencia de Ciencia y Tecnología de la Región de Murcia, Spain) and CGL2016-78946-R (AEI and FEDER, UE); they also thank Aurelio Garcia Blanco, Andries Gouws, Tom de Wet, Ali Hassan and Faisal Abdullab for their observations and assistance with field work. Daniel B. Raudabaugh and colleagues thank the Commonwealth of Pennsylvania, Pennsylvania Department of Conservation and Natural Resources, Pennsylvania Bureau of State Parks, and Black Moshannon State Park for research support, the Mycological Society of America and University of Illinois Urbana-Champaign School of Integrative Biology for financial support, and Michael Woodley for field support. Cheryl Armstrong-Cho and Sabine Banniza acknowledge funding and support by the Saskatchewan Ministry of Agriculture, the Western Grains Research Foundation, the Herb, Spice and Specialty Agriculture Association and the Saskatchewan Crop Insurance Corporation. Shuming Luo and colleagues thank Mui-keng Tan for helpful advice during this study.
The USDA is an equal opportunity provider and employer.
REFERENCES
- Adamčík S, Caboň M, Eberhardt U, et al. 2016. A molecular analysis reveals hidden species diversity within the current concept of Russula maculata (Russulaceae, Basidiomycota). Phytotaxa 270: 71–88. [Google Scholar]
- Álvarez E, Cano J, Stchigel AM, et al. 2011. Two new species of Mucor from clinical samples. Medical Mycology 49: 62–72. [DOI] [PubMed] [Google Scholar]
- Anonymous. 1964. ISCC-NBS centroid color charts. U.S. Department of Commerce, National Bureau of Standards, Washington. [Google Scholar]
- Aptroot A. 2006. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1–231. [Google Scholar]
- Bates ST. 2004. Arizona members of the Geastraceae and Lycoperdaceae (Basidiomycota, Fungi). Master Thesis, Arizona State University, USA. [Google Scholar]
- Bender H. 2017. Pilze in und um Mönchengladbach mit Schwerpunkt Volksgarten und Coprinus Spezial. http://www.bender-biotop.de. Retrieved 16 Nov. 2017.
- Bensch K, Braun U, Groenewald JZ, et al. 2012. The genus Cladosporium. Studies in Mycology 72: 1–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Meijer M, et al. 2018. Cladosporium species in indoor environments. Studies in Mycology 89: 177–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette AE, Bessette AR, Fischer DE. 2010. North American boletes. Syracuse University Press, Syracuse, New York. [Google Scholar]
- Bessette AE, Roody WC, Bessette AR. 2016. Boletes of Eastern North America. Syracuse University Press, Syracuse, New York. [Google Scholar]
- Bezerra JDP, Machado AR, Firmino AL, et al. 2018. Mycological diversity description I. Acta Botanica Brasilica. doi: https://doi.org./10.1590/0102-33062018abb0154. [Google Scholar]
- Boccardo D, Traverso M, Vizzini A, et al. 2008. Funghi d’Italia. Zaichelli, Bologna, Italia. [Google Scholar]
- Boerema GH, De Gruyter J, Noordeloos ME, et al. 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI publishing, Wallingford, UK. [Google Scholar]
- Bordallo JJ, Rodríguez A, Kaounas V, et al. 2015. Two new Terfezia species from Southern Europe. Phytotaxa 230: 239–249. [Google Scholar]
- Bordallo JJ, Rodríguez A, Muñoz-Mohedano JM, et al. 2013. Five new Terfezia species from the Iberian Peninsula. Mycotaxon 124: 189–208. [Google Scholar]
- Boudier E. 1909. Icones mycologicae ou iconographie des champignons de France principalement discomycètes avec texte descriptif. Tome II, pl. 194–421. Librairie des Sciences Naturelles, Paris. [Google Scholar]
- Braun U, Crous PW, Nakashima C. 2014. Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae). IMA Fungus 5: 203–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach J, Kränzlin F. 1984. Fungi of Switzerland, Vol. 1: Ascomycetes. Verilog Mykologia, Luzern. [Google Scholar]
- Breitenbach J, Kränzlin F. 1991. Fungi of Switzerland, Vol. 3. Sticher Printing, Lucern, Switzerland. [Google Scholar]
- Brodie HJ. 1975. The Bird’s Nest fungi. University of Toronto Press, Toronto, Canada. [Google Scholar]
- Butler EJ, Khan AH. 1913. Some new sugarcane diseases. Memoirs of the Department of Agriculture in India 6: 181–208. [Google Scholar]
- Calonge FD. 1998. Gasteromycetes: Lycoperdales, Nidulariales, Phallales, Sclerodermatales, Tulostomatales. Flora Micológica Ibérica. [Google Scholar]
- Cannon PF, Kirk PM. 2007. Fungal families of the world. CABI, Wallingford. [Google Scholar]
- Castañeda-Ruiz RF, Guarro J, Cano J. 1996. Notes on conidia fungi. Two new dematiaceous hyphomycetes from Cuba. Mycotaxon 57: 463–469. [Google Scholar]
- Cejp K, Palmer JT. 1963. The genera Nidularia Fr. and Mycocalia sphagneti J.T. Palmer sp. nov. from England. Česká Mykologie 17: 113–126. [Google Scholar]
- Chan HT. 2010. Diversity of Boletaceae in Peninsular Malaysia. Dissertation submitted in fulfilment of the requirements for the degree of Master of Science. Kuala Lumpur. [Google Scholar]
- Chen AJ, Frisvad JC, Sun BD, et al. 2016. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Studies in Mycology 84: 1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Hou LW, Duan WJ, et al. 2017. Didymellaceae revisited. Studies in Mycology 87: 105–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jiang JR, Zhang GZ, et al. 2015. Resolving the Phoma enigma. Studies in Mycology 82: 137–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citerin M. 1994. Clé du genre Coprinus. Révision des sections Farinosi, Lanatuli et Picacei. Documents Mycologiques 95: 1–13. [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, et al. 2009. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. 2016. They seldom occur alone. Fungal Biology 120: 1392–1415. [DOI] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. 2017. The genera of fungi – G 4: Camarosporium and Dothiora. IMA Fungus 8: 131–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, et al. 2011a. Fungal Planet description sheets: 69–91. Persoonia 26: 108–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Knox-Davies PS, Wingfield MJ. 1989. A list of Eucalyptus leaf fungi and their potential importance to South African forestry. South African Forestry Journal 149: 17–29. [Google Scholar]
- Crous PW, Schroers H-J, Groenewald JZ, et al. 2006. Metulocladosporiella gen. nov. for the causal organism of Cladosporium speckle disease of banana. Mycological Research 110: 264–275. [DOI] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2011b. Fungal Planet description sheets: 92–106. Persoonia 27: 130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Christensen M, et al. 2012. How important are conidial appendages? Persoonia 28: 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2016a. Fungal Planet description sheets: 469–557. Persoonia 37: 218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017. Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2013. Fungal Planet description sheets: 154–213. Persoonia 31: 188–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016b. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012. The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Sato T, Alizadeh A, et al. 2019. (online 2018). The Colletotrichum draecaenophilum, C. magnum and C. orchidearum species complexes. Studies in Mycology 92: 1–46. doi: https://doi.org/10.1016/j.simyco.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Woudenberg JHC, Cannon PF, et al. 2009. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39: 45–87. [Google Scholar]
- De Hoog G, Guarro J, Gené J, et al. 2013. Atlas of Clinical Fungi, 3rd edn. (e-version) Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: / Universitat Rovira i Virgili, Reus, Spain. [Google Scholar]
- De Hoog GS, Vicente V, Caligiorne RB, et al. 2003. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. Journal of Clinical Microbiology 41: 4767–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Vicente VA, Najafzadeh MJ, et al. 2011. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27: 46–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Zeng JS, Harrak MJ, et al. 2006. Exophiala xenobiotica, sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie van Leeuwenhoek 90: 257–268. [DOI] [PubMed] [Google Scholar]
- De Sousa da Câmara ME. 1931. Mycetes aliquot novi aliique in Mycoflora Lusitaniae ignoti III. In Laboratorio Pathologiae V egetalis lnstituti Agronomici Olisipponensis Observata. Extracti ex Annaes do Instituto Superior de Agronomia 4: 6. [Google Scholar]
- Dennis RWG. 1978. Bristish Ascomycetes. Ed. Cramer, Vaduz. [Google Scholar]
- Dinerstein E, Olson D, Joshi A, et al. 2017. An ecoregion-based approach to protecting half the terrestrial realm. BioScience. 67: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LJ, Schlub RL, Pernezny K, et al. 2009. Host specialization and phylogenetic diversity of Corynespora cassiicola. Phytopathology 99: 1015–1027. [DOI] [PubMed] [Google Scholar]
- Doveri F. 2004. Fungi fimicoli italici. A.M.B., Vicenza. [Google Scholar]
- Doveri F. 2013. An additional update on the genus Chaetomium with descriptions of two coprophilous species, new to Italy. Mycosphere 4: 820–846. [Google Scholar]
- Drechsler-Santos ER, Robledo G, Lima-Junior NC, et al. 2016. Phellinotus, a new neotropical genus in the Hymenochaetaceae (Basidiomycota, Hymenochaetales). Phytotaxa 261: 218–239. [Google Scholar]
- Egger KN, Paden JW. 1986. Biotrophic associations between lodgepole pine seedlings and postfire ascomycetes (Pezizales) in monoxenic culture. Canadian Journal of Botany 64 (11): 1719–1725. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. CMI, Kew, England. [Google Scholar]
- Enderle M. 2004. Die Pilzflora des Ulmer Raumes. Verein für Naturwissenschaft und Mathematik in Ulm e.V. Dietzenbach, Germany. [Google Scholar]
- Eriksson O. 1967. On graminicolous pyrenomycetes from Fennoscandia II. Phragmosporous and scolecosporous species. Arkiv før Botanik 6: 381–440. [Google Scholar]
- Ertz D, Diederich P, Lawrey JD, et al. 2015. Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Diversity 74: 53–89. [Google Scholar]
- Fan XL, Barreto RW, Groenewald JZ, et al. 2017. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Studies in Mycology 87: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr DF, Rossman AY. 2018. Fungal databases, U.S. National Fungus Collections, ARS, USDA. Retrieved 11 April 2018, from https://nt.ars-grin.gov/fungaldatabases/.
- Fell JW, Statzell-Tallman A, Scorzetti G, et al. 2011. Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Antonie van Leeuwenhoek 99: 533–549. [DOI] [PubMed] [Google Scholar]
- Fuckel L. 1867. Fungi Rhenani Cent. 18 (1), no 1702–1764. Hedwigia 6: 174–175. [Google Scholar]
- Fungaro MH, Ferranti LS, Massi FP, et al. 2017. Aspergillus labruscus sp. nov., a new species of Aspergillus section Nigri discovered in Brazil. Scientific Reports 7: 6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams W, Holubová-Jechová V. 1976. Chloridium and some other Dematiaceous Hyphomycetes growing on decaying wood. Studies in Mycology 13: 1–99. [Google Scholar]
- Gierczyk B, Kujawa A, Szczepkowski A. 2014. New to Poland species of the broadly defined genus Coprinus (Basidiomycota, Agaricomycotina). Acta Mycologica 49: 159–188. [Google Scholar]
- Giraldo A, Gené J, Sutton DA, et al. 2014. Phylogenetic circumscription of Arthrographis (Eremomycetaceae, Dothideomycetes). Persoonia 32: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzar H, Lanoiselet V, Wang C, et al. 2015. First report of Phoma multirostrata in Australia. Australasian Plant Disease Notes 10: 8. [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, et al. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Menendez V, Martin J, Siles JA, et al. 2017. Biodiversity and chemotaxonomy of Preussia isolates from the Iberian Peninsula. Mycological Progress 16: 713–728. [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Harmaja H. 1979. Notes on Gyromitra esculenta coll. and G. recurva, a noteworthy species of western North America. Karstenia 19: 46–49. [Google Scholar]
- Hawkes CV, Belnap J, D’Antonio C, et al. 2006. Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant and Soil 281: 369–380. [Google Scholar]
- Hennebert GL, Desai BG. 1974. Lomentospora prolificans, a new hyphomycete from greenhouse soil. Mycotaxon 1: 45–50. [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Crous PW. 2016a. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 36: 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Lombard L, et al. 2016b. Fungal Systematics and Evolution: FUSE 2. Sydowia 68: 193–230. [Google Scholar]
- Hipol RM. 2012. Molecular identification and phylogenetic affinity of two growth promoting fungal endophytes of sweet potato (Ipomea batatas (L.) Lam.) from Baguio City, Philippines. Electronic Journal of Biology 8: 57–61. [Google Scholar]
- Hornby D. 1984. Akenomyces costatus sp. nov. and the validation of Akenomyces Arnaud. Transactions of the British Mycological Society 82: 653–664. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hunter GC, Crous PW, Carnegie AJ, et al. 2011. Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Diversity 50: 145–166. [Google Scholar]
- Hyde KD, Hongsanan S, Jeewon R, et al. 2016. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80: 1–270. [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, et al. 2013. Families of Dothideomycetes. Fungal Diversity 63: 1–313. [Google Scholar]
- Isola D, Zucconi L, Onofri S, et al. 2016. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Diversity 76: 75–96. [Google Scholar]
- Jurjevic Z, Peterson SW, Stea G, et al. 2012. Two novel species of Aspergillus section Nigri from indoor air. IMA Fungus 3: 159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan-Zur V, Roth-Bejerano N, Sitrit Y, et al. 2014. Desert truffles. Phylogeny, physiology, distribution and domestication. Soil Biology, Volume 38. Springer-Verlag Berlin, Heidelberg. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich MA. 2002. Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands. [Google Scholar]
- Kornerup A, Wanscher JH. 1967. Methuen handbook of colour. Methuen & Co. Ltd., UK. [Google Scholar]
- Kornerup A, Wanscher JH. 1974. Farver i Farver. Politikens, København. [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour. 3rd ed. London, Eyre Methuen, London, England. [Google Scholar]
- Kotlaba F, Pouzar Z. 1974. Dalši lokality svazčitého – Gyromitra fastigiata (Krombh.) Rehm – Čechaách spoznaámkami k rodové příslušnosti uchačú a destic. Česka Mykologie 28: 84–95. [Google Scholar]
- Kuhar F, Castigli V, Papinutti L. 2012. Geastrum specie of the La Rioja province, Argentina. Mycotaxon 122: 145–156. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers H. 1979. Atlas de los colores. Editorial Blume, Barcelona. [Google Scholar]
- Kurtzman CP, Fell JW, Boekhout T. 2011. The yeasts, a taxonomic study. Vol. 3 Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- Laich F, Vaca I, Chávez R. 2013. Rhodotorula portillonensis sp. nov., a basidiomycetous yeast isolated from Antarctic shallow-water marine sediment. International Journal of Systematic and Evolutionary Microbiology 63: 3884–3891. [DOI] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SY, et al. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. [DOI] [PubMed] [Google Scholar]
- Libkind D, Sampaio JP, Van Broock M. 2010. Cystobasidiomycetes yeasts from Patagonia (Argentina): Description of Rhodotorula meli sp. nov. from glacial meltwater. International Journal of Systematic and Evolutionary Microbiology 60: 2251–2256. [DOI] [PubMed] [Google Scholar]
- Lombard L, Houbraken J, Decock C, et al. 2016. Generic hyper-diversity in Stachybotriaceae. Persoonia 36: 156–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machowicz-Stefaniak Z, Zimowska B, Zalewska E. 2008. The occurrence and pathogenicity of Phoma exigua Desm. var. exigua for selected species of herbs. Acta Agrobotanica 61: 157–166. [Google Scholar]
- Madden AA, Stchingel AM, Guarro J, et al. 2012. Mucor nidicola sp. nov., a fungal species isolated from an invasive paper wasp nest. International Journal of Systematic and Evolutionary Microbiology 62: 1710–1714. [DOI] [PubMed] [Google Scholar]
- Madrid H, Hernández-Restrepo M, Gené J, et al. 2016. New and interesting chaetothyrialean fungi from Spain. Mycological Progress 15: 1179–1201. [Google Scholar]
- Malençon G. 1973. Champignon hypogés du Nord de l’ Afrique - I Ascomycètes. Persoonia 7: 261–288. [Google Scholar]
- Mapperson RR, Kotiw M, Davis RA, et al. 2014. The diversity and antimicrobial activity of Preussia sp. endophytes isolated from Australian dry rainforests. Current Microbiology 68: 30–37. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, et al. 2019. (online 2018). Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology. doi: https://doi.org/10.1016/j.simyco.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Stchigel AM, Miller AN, et al. 2015. A re-evaluation of the genus Myceliophthora (Sordariales, Ascomycota): Its segregation into four genera and description of Corynascus fumimontanus sp. nov. Mycologia 107: 619–632. [DOI] [PubMed] [Google Scholar]
- Matsushima T. 1975. Icones microfungorum a Matsushima lectorum. Published by author, Kobe, Japan. [Google Scholar]
- Matsushima T. 1996. Matsushima Mycological Memoirs 9: 1–30. [Google Scholar]
- Medardi G. 2006. Atlante fortografico degli Ascomiceti d’Italia. Grafica Sette, Bagnolo mella, Brescia, Italia. [Google Scholar]
- Melzer A. 2017. Key to coprinoid species (Coprinellus, Coprinopsis, Parasola). http://www.vielepilze.de/coprinus/copkey/ecopkey.pdf. Retrieved 16 Nov. 2017.
- Mendes MAS, Da Silva VL, Dianese JC, et al. 1998. Fungos em Plants no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia. [Google Scholar]
- Methven AS, Zelski SE, Miller AN. 2013. A molecular phylogenetic assessment of the genus Gyromitra in North America. Mycologia 105: 1306–1314. [DOI] [PubMed] [Google Scholar]
- Milne I, Wright F, Rowe G, et al. 2004. TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20: 1806–1807. [DOI] [PubMed] [Google Scholar]
- Muñoz JA. 2005. Boletus s.l. (excl. Xerocomus). Fungi Europaei 2. Edizioni Candusso, Alassio. [Google Scholar]
- Munsell AH. 1975. Munsell soil color charts. Munsell Color Corporation, Baltimore. [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario, Canada. [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvall RF. 2013. Field crop diseases handbook. Springer Science & Business Media. [Google Scholar]
- Obase K, Douhan GW, Matsuda Y, et al. 2014. Culturable fungal assemblages growing within Cenococcum sclerotia in forest soils. Federation of European Microbiological Societies 90: 708–717. [DOI] [PubMed] [Google Scholar]
- Ondrej M. 1983. Occurrence of the genus Ascochyta Lib. on plants of the family Apiaceae. Ceská Mykologie 37: 77–82. [In Czech.] [Google Scholar]
- Palmer JT. 1961. Observations on Gasteromycetes IX. The conservation of Nidularia Fr. and the separation of Mycocalia J.T. Palmer, gen. nov. Taxon 10: 54–60. [Google Scholar]
- Parra LA, Della Maggiora M, Simonini G, et al. 2017. Nomenclatural study and current status of the names Boletus emileorum, Boletus crocipodius and Boletus legaliae (Boletales), including typification of the first two. Czech Mycology 69: 163–192. [Google Scholar]
- Partridge EC, Baker WA, Morgan-Jones G. 2001. Notes on hyphomycetes. LXXXIII. Castanedaea, a new genus in which to accommodate Alysidium minus. Mycotaxon 78: 175–180. [Google Scholar]
- Pegler DN, Young TWK. 1981. A natural arrangement of the Boletales, with reference to spore morphology. Transactions of the British Mycological Society 76: 103–146. [Google Scholar]
- Phillips AJL, Alves A, Abdollahzadeh J, et al. 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras-Alfaro A, Herrera J, Sinsabaugh RL, et al. 2008. Novel root fungal consortium associated with a dominant desert grass. Applied and Environmental Microbiology 74: 2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. 2008. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, et al. 2011. Zymoseptoria gen. nov.: a new genus to accommodate septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VG. 1963. Some new records of foliicolous fungi imperfecti from India. Bulletin of the Botanical Society, College of Science, Nagpur 4: 54–57. [Google Scholar]
- Réblová M. 1999. Studies in Chaetosphaeria sensu lato III. Umbrinosphaeria gen. nov. and Miyoshiella with Sporidesmium anamorphs. Mycotaxon 71: 13–43. [Google Scholar]
- Redhead SA, Vizzini A, Drehmel DC, et al. 2016. Saproamanita, a new name for both Lepidella E.-J. Gilbert and Aspidella E.-J. Gilbert (Amaniteae, Amanitaceae). IMA Fungus 7: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H. 1901. Beiträge zur pilzflora von Südamerika. XII. Sphaeriales. Hedwigia 40: 100–124. [Google Scholar]
- Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clinical Microbiology Reviews 23: 884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AV, Currah RS. 2005. Oidiodendron: A survey of the named species and related anamorphs of Myxotrichum. Studies in Mycology 53: 83–120. [Google Scholar]
- Ridgway R. 1912. Color standards and color nomenclature. Published by the author; Washington, DC. [Google Scholar]
- Roets F, Dreyer LL, Wingfield MJ, et al. 2008. Thecaphora capensis sp. nov., an unusual new anther smut on Oxalis in South Africa. Persoonia 21: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice, across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Adams GC, Cannon PF, et al. 2015. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz JA, Nassar M, Morales MI. 1972. Contribution to the study of Xylophallus xylogenus. Mycologia 64: 510–520. [Google Scholar]
- Salter TM. 1944. The genus Oxalis in South Africa: a taxonomic revision. Cape Times Ltd., Cape Town. [Google Scholar]
- Samerpitak K, Van der Linde E, Choi HJ, et al. 2013. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Diversity 65: 89–126. [Google Scholar]
- Samson RA, Visagie CM, Houbraken J, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78: 141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheelings TF, Dobson EC, Hooper C, et al. 2015. Cutaneous and systemic mycoses from infection with Lecanicillium spp. in captive Guthega skinks (Liopholis guthega). Australian Veterinary Journal 93: 248–251. [DOI] [PubMed] [Google Scholar]
- Schipper MAA. 1973. A study on variability in Mucor hiemalis and related species. Studies in Mycology 4: 1–40. [Google Scholar]
- Senanayake IC, Crous PW, Groenewald JZ, et al. 2017. Families of Diaporthales based on morphological and phylogenetic evidence. Studies in Mycology 86: 217–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedmousavi S, Guillot J, De Hoog GS. 2013. Phaeohyphomycoses, emerging opportunistic diseases in animals. Clinical Microbiology Reviews 26: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas RG, Tan YP, Edwards J, et al. 2016. Colletotrichum species in Australia. Australasian Plant Pathology 45: 447–464. [Google Scholar]
- Sigler L, Dunn MT, Carmichael JW. 1982. Arthrocristula and Arthropsis, two new Hyphomycetes with dematiaceous arthroconidia. Mycotaxon 15: 409–419. [Google Scholar]
- Soto MK, Wright JE. 2000. Taxonomia del genero Geastrum (Basidiomycetes, Lycoperdales) em la Provincia de Buenos Aires, Argentina. Boletin de la Sociedad Argentina de Botanica 34: 185–201. [Google Scholar]
- Sousa JO, Baracho GS, Baseia IG. 2015. Geastrum laevisporum: a new earthstar fungus with uncommonsmooth spores. Mycosphere 6: 501–507. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A toll for phylogenetic analysis and post-analysis of large phylogenenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Alachiotis N. 2010. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26: i132–i139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stchigel AM, Guarro J. 1997. A new species of Emericella from Indian soil. Mycologia 89: 937–941. [Google Scholar]
- Steiner U, Ahimsa-Muller MA, Markert A, et al. 2006. Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae). Planta 224: 533–544. [DOI] [PubMed] [Google Scholar]
- Streiblova E, Gryndlerova H, Gryndler M. 2012. Truffle brûlé: an efficient fungal life strategy. FEMS Microbiology and Ecology 80: 1–8. [DOI] [PubMed] [Google Scholar]
- Stukenbrock EH, Quaedvlieg W, Javan-Nikhah M, et al. 2012. Zymoseptoria ardabiliae and Z. pseudotritici, two progenitor species of the septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola). Mycologia 104: 1397–1407. [DOI] [PubMed] [Google Scholar]
- Su HY, Luo ZL, Liu XY, et al. 2016. Lentithecium cangshanense sp. nov. (Lentitheciaceae) from freshwater habitats in Yunnan Province, China. Phytotaxa 267: 61–69. [Google Scholar]
- Subramanian CV. 1977. Revisions of Hyphomycetes I. Kavaka 5: 93–98. [Google Scholar]
- Sukarno N, Kurihara Y, Ilyas M, et al. 2009. Lecanicillium and Verticillium species from Indonesia and Japan including three new species. Mycoscience 50: 369–379. [Google Scholar]
- Sunhede S. 1989. Geastraceae (Basidiomycotina). Morphology, ecology and systematics with special emphasis on the North European species. Synopsis Fungorum 1: 1–534. [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. CMI, Kew, England. [Google Scholar]
- Sutton BC, Hodges CS. 1976. Eucalyptus microfungi: some setose hyphomycetes with phialides from Malaysia. Nova Hedwigia 27: 343–347. [Google Scholar]
- Svrček M, Moravec J. 1972. O druhu Helvella fastigiata Krombholz. Česka Mykologie 26: 1–8. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and their methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tamura K, Dudley J, Nei M, et al. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Endo M, Hirayama K, et al. 2011. Phylogeny of Discosia and Seimatosporium, and introduction of Adisciso and Immersidiscosia genera nova. Persoonia 26: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82: 75–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakunyingcharoen T, Lombard L, Groenewald JZ, et al. 2014. Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5: 391–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trierveiler-Pereira L, Da Silveira RMB. 2012. Notes on Xylophallus xylogenus (Phallaceae, Agaricomycetes) based on Brazilian specimens. Mycotaxon 120: 309–316. [Google Scholar]
- Tsang CH, Chan JFW, Trendell-Smith NJ, et al. 2014. Subcutaneous phaeohyphomycosis in a patient with Ig G4-related sclerosing disease caused by a novel ascomycete, Hongkongmyces pedis gen. et sp. nov.: first report of human infection associated with the family Lindgomycetaceae. Medical Mycology 52: 736–747. [DOI] [PubMed] [Google Scholar]
- Tulasne LR, Tulasne C. 1851. Fungi Hipogaei. Histoire et Monographie des Champignons Hypogés; París. [Google Scholar]
- Tulloss RE. 1998. Amanita prairiicola – alive and well and living under an assumed name. Kansas Mycolog 13: 1–5. [Google Scholar]
- Tulloss RE. 2003. Seminar on Amanita. Appendix A11: Draft keys to species of Amanita subsection Vittadiniae. Available at www.amanitaceae.org/content/uploaded/pdf/vittadin.pdf.
- Tulloss RE. 2009. Determining an Amanita to section without a microscope – a synopsis. Roosevelt, New Jersey, US. [Google Scholar]
- Tulloss RE, Lindgren JE, Arora D, et al. 2014. Amanita pruittii – a new, apparently saprotrophic species from US Pacific coastal states. Amanitaceae 1: 1–9. [Google Scholar]
- Udagawa S. 1997. A new species of Staphylotrichum from Chile. In: Janardhanan KK, Rajiendran C, Natarajan K, et al. (eds), Tropical mycology: 149–155. Science Publishers, New Hampshire. [Google Scholar]
- Udayanga D, Liu X, McKenzie EHC, et al. 2012. Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptogamie Mycologie 33: 295–309. [Google Scholar]
- Uljé CB. 2005. Coprinus Pers. In: Noordeloos ME, Kuyper TW, Vellinga EC. (eds), Flora Agaricina Neerlandica 6: 22–109. Taylor & Francis, Boca Raton. [Google Scholar]
- Van den Brink J, Samson RA, Hagen F, et al. 2012. Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Diversity 52: 197–207. [Google Scholar]
- Van Nieuwenhuijzen EJ, Miadlikowska JM, Houbraken JAMP, et al. 2016. Wood staining fungi revealed taxonomic novelties in Pezizomycotina: new order Superstratomycetales and new species Cyanodermella oleoligni. Studies in Mycology 85: 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vooren N, Moreau P-A. 2009. Essai taxinomique sur le genre Gyromitra Fr. sensu lato (Pezizales). 1. Le genre Gyromitra Fr., sous-genre Gyromitra. Ascomycete.org 1: 7–14. [Google Scholar]
- Vánky K. 2011 ‘2012’. Smut fungi of the world. American Phytopathological Society Press, St Paul, Minnesota. [Google Scholar]
- Vánky K, Lutz M, Bauer R. 2008. About the genus Thecaphora (Glomosporiaceae) and its new synonyms. Mycological Progress 7: 31–39. [Google Scholar]
- Vánky K, Shivas RG. 2008. Fungi of Australia, the smut fungi. Australian Biological Resources Study, Canberra. [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. 2016. All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. 2017. Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2017. Corynespora, Exosporium and Helminthosporium revisited – New species and generic reclassification. Studies in Mycology 87: 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Krisai-Greilhuber I. 1997. Akenomyces costatus, an interesting basidiomycetous anamorph with unknown affinities. Österreichische Zeitschrift für Pilzkunde 6: 61–66. [Google Scholar]
- Von Arx JA. 1958. Über einige ascomyceten aus Südamerika. Acta Botanica Neerlandica 7: 503–518. [Google Scholar]
- Von Arx JA, Guarro J, Figueras MJ. 1986. The ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia 84: 1–162. [Google Scholar]
- Von Arx JA, Storm PK. 1967. Über einige aus dem Erdbodenisolierte, zu Sporormia, Preussia und Westerdykella gehörende Ascomyceten. Persoonia 4: 407–415. [Google Scholar]
- Wang Q-M, Yurkov AM, Göker M, et al. 2015. Phylogenetic classification of yeasts and related taxa within. Studies in Mycology 81: 149–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Houbraken J, Groenewald JZ, et al. 2016. Diversity and taxonomy of Chaetomium and Chaetomium-like fungi from indoor environments. Studies in Mycology 84: 145–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling R. 1969. Colour identification chart. Her Majesty’s Stationary Office, Edinburgh. [Google Scholar]
- Weir B, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Zhao K, Li Y-C, et al. 2016. Four new genera of the fungal family Boletaceae. Fungal Diversity 81: 1–24. [Google Scholar]
- Wu WP, Zhuang WY. 2005. Sporidesmium, Endophragmiella and related genera from China. Fungal Diversity Research Series. Fungal Diversity Press, Thailand. [Google Scholar]
- Yang T, Groenewald JZ, Cheewangkoon R, et al. 2017. Families, genera, and species of Botryosphaeriales. Fungal Biology 121: 322–346. [DOI] [PubMed] [Google Scholar]
- Yu HY, Zhao CL, Dai YC. 2013. Inonotus niveomarginatus and I. tenuissimus spp. nov. (Hymenochaetales), resupinate species from tropical China. Mycotaxon 124: 61–68. [Google Scholar]
- Yurkov AM, Kachalkin AV, Daniel HM, et al. 2015. Two yeast species Cystobasidium psychroaquaticum f.a. sp. nov. and Cystobasidium rietchieii f.a. sp. nov. isolated from natural environments, and the transfer of Rhodotorula minuta clade members to the genus Cystobasidium. Antonie van Leeuwenhoek 107: 173–185. [DOI] [PubMed] [Google Scholar]
- Yurlova NA, De Hoog GS. 2002. Exopolysaccharides and capsules in human pathogenic Exophiala species. Mycoses 45: 443–448. [DOI] [PubMed] [Google Scholar]
- Zalar P, De Hoog GS, Schroers KJ, et al. 2007. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R, Gams W. 2001. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73: 1–50. [Google Scholar]
- Zeng JS, Sutton DA, Fothergill AW, et al. 2007. Spectrum of clinically-relevant Exophiala species in the United States. Journal of Clinical Microbiology 45: 3713–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, et al. 2009a. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang HK, Fournier J, et al. 2009b. Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Diversity 38: 225–251. [Google Scholar]