Supplemental Digital Content is available in the text.
Keywords: cardiomyopathies, electrocardiography, ethnic groups, exercise, genetic testing, mass screening
Abstract
Background:
T-wave inversion (TWI) is common in patients with cardiomyopathy. However, up to 25% of athletes of African/Afro-Caribbean descent (black athletes) and 5% of white athletes also have TWI of unclear clinical significance despite comprehensive clinical evaluation and long-term follow-up. The aim of this study was to determine the diagnostic yield from genetic testing, beyond clinical evaluation, when investigating athletes with TWI.
Methods:
We investigated 50 consecutive asymptomatic black and 50 white athletes 14 to 35 years of age with TWI and a normal echocardiogram who were referred to a UK tertiary center for cardiomyopathy and sports cardiology. Subjects underwent exercise testing, 24-hour ambulatory ECG, signal-averaged ECG, cardiac magnetic resonance imaging, and a blood-based analysis of a comprehensive 311-gene panel for cardiomyopathies and ion channel disorders associated with TWI, including hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, dilated cardiomyopathy, left ventricular noncompaction, long-QT syndrome, and Brugada syndrome.
Results:
In total, 21 athletes (21%) were diagnosed with cardiac disease on the basis of comprehensive clinical investigations. Of these, 8 (38.1%) were gene positive (myosin binding protein C[MYBPC3], myosin heavy chain 7 [MYH7], galactosidase alpha [GLA], and actin alpha, cardiac muscle 1 [ACTC1] genes) and 13 (61.9%) were gene negative. Of the remaining 79 athletes (79%), 2 (2.5%) were gene positive (transthyretin [TTR] and sodium voltage-gated channel alpha subunit 5 [SCN5A] genes) in the absence of a clinical phenotype. The prevalence of newly diagnosed cardiomyopathy was higher in white athletes compared with black athletes (30.0% versus 12%; P=0.027). Hypertrophic cardiomyopathy accounted for 90.5% of all clinical diagnoses. All black athletes and 93.3% of white athletes with a clinical diagnosis of cardiomyopathy or a genetic mutation capable of causing cardiomyopathy exhibited lateral TWI as opposed to isolated anterior or inferior TWI; the genetic yield of diagnoses from lateral TWI was 12.3%.
Conclusions:
Up to 10% of athletes with TWI revealed mutations capable of causing cardiac disease. Despite the substantial cost, the positive diagnostic yield from genetic testing was one half that from clinical evaluation (10% versus 21%) and contributed to additional diagnoses in only 2.5% of athletes with TWI in the absence of a clear clinical phenotype, making it of negligible use in routine clinical practice.
Clinical Perspective.
What Is New?
The yield of testing for pathogenic disease-causing genetic mutations in athletes exhibiting T-wave inversion is low (10%), despite the use of a comprehensive genetic panel.
Gene testing in selected athletes referred to a tertiary referral center contributes to additional diagnoses beyond comprehensive clinical evaluation in only 2.5% of athletes with T-wave inversion without a clear clinical phenotype.
The overall diagnostic yield from genetic testing in athletes with T-wave inversion is one half that of comprehensive clinical evaluation (21% versus 10%).
The prevalence of cardiomyopathy in white athletes with T-wave inversion is higher compared with black athletes with T-wave inversion (30% versus 12%).
What Are the Clinical Implications?
Genetic testing is rarely useful in the routine investigation of athletes with T-wave inversion who have undergone comprehensive clinical evaluation.
In contrast, comprehensive clinical evaluation can identify a clinical diagnosis in more than one fifth of athletes with T-wave inversion on initial evaluation, with a higher probability of a clinical diagnosis in white compared with black athletes (30.0% versus 12%).
This study demonstrates that lateral T-wave inversion in an athletic population referred to a specialized athletic center is associated with cardiac disease in 20% of athletes.
Editorial, see p 1206
A small proportion of apparently healthy adult athletes exhibit marked repolarization abnormalities in the absence of detectable structural heart disease. Of these repolarization patterns, most interest has focused on T-wave inversion (TWI),1–9 which is a common manifestation of several inherited cardiomyopathies and some ion channel diseases implicated in sudden cardiac death.
Although rare in adult white athletes,10–14 TWI is observed in up to one quarter of athletes of African/Afro-Caribbean descent (black athletes) and most commonly confined to the anterior leads (V1–V4).3,5 Observational data suggest that this specific repolarization pattern represents a benign, ethnic manifestation of the athlete’s heart.3,5,15 In contrast, the clinical significance of inferior and lateral TWI is less certain. Recent studies using cardiac magnetic resonance imaging (CMR)4 and longitudinal follow-up1,3,6 in athletes with inferior or lateral TWI have reported an association with these repolarization changes and cardiomyopathy, risk of sudden cardiac arrest, or the subsequent development of cardiomyopathy over time. The majority of athletes in these studies, however, failed to reveal any demonstrable cardiac pathology. None of these studies incorporated genetic testing to help determine whether TWI represented an early, subtle, or concealed manifestation of cardiomyopathy or ion channel disease. Genetic testing was previously expensive and impractical, but next-generation sequencing16 has now offered major advances in the molecular genetics of inherited cardiac diseases and the development of relatively inexpensive genetic panels to investigate a broad spectrum of recognized mutations in genes implicated in cardiomyopathy and ion channel disorders.
The aim of this study was to examine the additional diagnostic yield for these genetic diseases beyond standard comprehensive clinical evaluation in highly trained black and white athletes with TWI using a wide genetic panel.
Methods
Setting
The charitable organization Cardiac Risk in the Young (CRY) provides a national preparticipation screening service for several elite national sporting bodies and regional teams, including Aviva Premiership Rugby, British Rowing, English cricket, English Institute of Sport, Lawn Tennis Association, Rugby Football Union, and several Premiership soccer clubs. Athletes with abnormal findings undergo further investigations, of which >80% are conducted in the Sports Cardiology Unit at St. George’s Hospital. The principal investigator (S.S.) also receives referrals directly to the sports cardiology unit from other elite professional sporting bodies and other medical institutions throughout the United Kingdom. Since 2010, we have also received referrals from Qatar Orthopedic and Sports Medicine Hospital (Doha, Qatar), which has a highly active cardiovascular screening program that serves the entire Persian Gulf region. All elite athletes receive a 12-lead ECG and 2-dimensional echocardiography as part of these screening programs.
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Recruitment of Athletes
Between April 2012 and April 2014, a total of 2039 athletes (260 black and 1779 white) were evaluated. Fifty consecutive black and 50 consecutive white athletes 14 to 35 years of age with TWI ≥−0.1 mV in ≥2 contiguous leads (excluding aVR, V1, and lead III in isolation) were prospectively recruited into the study. Twenty-seven athletes were recruited through preparticipation cardiac evaluation performed by CRY in the United Kingdom and 14 through preparticipation cardiac evaluation performed by Qatar Orthopedic and Sports Medicine Hospital in Doha. The remainder (n=59) were recruited after direct referral to St. George’s Hospital for evaluation of TWI on clinical grounds from another institution or a professional sporting body in the United Kingdom. Exclusion criteria for recruitment into the study included cardiac symptoms or a family history of cardiomyopathy or ion channel disease, a cardiac history, a medical history of hypertension, use of anabolic steroids or performance-enhancing drugs, and a structurally abnormal heart or wall motion abnormalities on echocardiography.
Ethics Approval and Informed Consent
Ethics approval was obtained from the local ethics committee in accordance with the Declaration of Helsinki.17 Written consent for enrollment of participants was obtained from individuals ≥16 years of age and from a parent or guardian for those <16 years of age. Genetic testing is not routinely performed for the evaluation of athletes in the United Kingdom, and current European guidelines recommend disqualification of genotype-positive, phenotype-negative individuals from competitive sports.13 Therefore, the results of genetic testing were not given to the athlete or sports organization unless the athlete specifically requested the result after appropriate counseling and informed consideration of a positive test on the athlete’s career.
Clinical Investigations in Athletes
All athletes were also investigated with 12-lead ECG, signal-averaged ECG, cardiopulmonary exercise stress testing, 24-hour ambulatory electrocardiographic monitoring, and CMR.
12-Lead ECG
Standard 12-lead ECG was performed with a MAC 5000 or MAC 5500 digital resting electrocardiographic recorder (GE Medical Systems, Milwaukee, WI) and analyzed as previously described.18 TWI of ≥−0.1 mV in ≥2 contiguous leads was considered significant, other than in leads V1, aVR, and III. The distribution of TWI was classified into 3 groups: TWI confined to the anterior leads (V1–V4); TWI involving the inferior leads (II, III, aVF), with or without anterior TWI; or TWI involving the lateral leads (I, aVL, V5, V6), regardless of TWI in the other leads.
Signal-Averaged ECG
Signal-averaged ECG was acquired according to accepted methodology19 with a MAC 5000 or MAC 5500 digital resting ECG recorder.
Cardiopulmonary Exercise Stress Testing
Cardiopulmonary exercise testing was performed in an upright position with a COSMED E100w cycle ergometer (Rome, Italy) as previously described20 using an incremental ramp protocol of 30 W/min in a quiet, air-conditioned room with an average temperature of 21°C and full resuscitation facilities. Subjects were encouraged to exercise to the point of exhaustion. Breath-by-breath gas exchange analysis was performed with a dedicated COSMED Quark CPEX metabolic cart. Blood pressure was measured before the test and then at 3-minute intervals with an automated cuff. Signals from a 12-lead ECG were displayed continuously and recorded at 2-minute intervals with a COSMED Quark C12x electrocardiographic recorder.
24-Hour Ambulatory Electrocardiographic Monitoring
Twenty-four-hour ambulatory electrocardiographic monitoring (Lifecard CF Holters, Spacelabs Healthcare, Snoqualmie, WA) was performed specifically to detect supraventricular and ventricular arrhythmias.21 Nonsustained ventricular tachycardia was defined as ≥3 consecutive ventricular beats at a rate of >120 bpm with a duration of <30 seconds.
Cardiac Magnetic Resonance Imaging
CMR was performed with previously described methods and analyzed with semiautomated software.7 All volumes and masses were indexed for age and body surface area according to the Du Bois and Du Bois22 formula. Late gadolinium images were acquired after intravenous gadolinium-DTPA administration.7 The presence or absence of late gadolinium enhancement (LGE) was recorded as a binary variable.
Candidate Disease and Gene Selection for Genetic Testing
Genetic testing was performed for priority genes responsible for 6 inherited cardiac conditions most commonly associated with TWI, namely hypertrophic cardiomyopathy (HCM),23 arrhythmogenic right ventricular cardiomyopathy,24 dilated cardiomyopathy,25 left ventricular (LV) noncompaction (LVNC),26 long-QT syndrome (LQTS),27,28 and Brugada syndrome.29 To ensure that comprehensive genetic evaluation was performed, a large number of potential candidate genes were also tested after a systematic literature review. Overall, a total of 104 genes for HCM (including phenocopies), 21 genes for arrhythmogenic right ventricular cardiomyopathy, 96 genes for dilated cardiomyopathy, 37 genes for LVNC, 28 genes for LQTS, and 25 genes for Brugada syndrome were tested (Table I in the online-only Data Supplement).
Definitions of Clinical Diagnoses
The diagnosis of a cardiomyopathy (HCM, arrhythmogenic right ventricular cardiomyopathy, dilated cardiomyopathy, and LVNC) or an ion channel disorder (LQTS and Brugada syndrome) was made in accordance with internationally recognized guidelines.23,29–31 In particular, the diagnosis of HCM was based on LV hypertrophy (LVH) ≥15 mm in any myocardial segment, as assessed on CMR, in the absence of another cardiac disorder or systemic condition capable of producing the same magnitude of LVH.32,33 In cases of mild LVH, HCM was diagnosed in the context of a combination of features, including nonconcentric patterns of LVH; LGE on CMR; an established or likely pathogenic gene mutation; the presence of broader phenotypic features of the condition such as nonsustained ventricular tachycardia or a blunted blood pressure response to exercise; and, in the case of apical HCM, the appearance of relative apical hypertrophy and cavity obliteration out of keeping with athletic training in combination with typical lateral deep TWI.34–36 Phenocopies of HCM were diagnosed on the basis of the above criteria and confirmation by a relevant pathogenic genetic test. The diagnosis of LVNC was based on increased trabeculation of the LV myocardium fulfilling recognized CMR criteria37 and the concurrent presence of wall thinning or LV systolic dysfunction.
Genetic Testing in Athletes
Sample Preparation, Genetic Sequencing, and Analysis
Genomic DNA was extracted from 1 mL peripheral blood samples. Sequencing of all coding exons and intronic flanking regions was performed through massive parallel sequencing technology. Targeted enrichment of the genes associated with each condition was performed. Sample preparation was conducted with the SureSelect Target Enrichment Kit (Agilent, California, Santa Clara, CA) for Illumina (San Diego, CA) paired-end multiplexed sequencing method, following the manufacturer’s instructions. Regions of interest were captured with a custom probe library. Sequencing was performed on an Illumina HiSeq 1500 with 2×100 base read length following Illumina protocols. Low-coverage regions (defined as every base with depth of coverage <15×) in those genes related to the diseases in question were resequenced through the Sanger method. Bioinformatic analysis was performed by means of a custom pipeline that included Novoalign (Novocraft, Selangor, Malaysia), Samtools (Genome Research Ltd, Wellcome Trust Sanger Institute, Hinxton, UK), Genome Analysis Toolkit (Broad Institute, Cambridge, MA), and bcftools (Genome Research Ltd) for variant calling and genotyping and Annovar for variant annotation.
Determination of Variant Frequency
Information on the frequency of identified genetic variants in different populations was analyzed from (1) the Exome Variant Server, National Heart, Lung, and Blood Institute GO Exome Sequencing Project, Seattle, WA (http://evs.gs.washington.edu/EVS/); (2) the 1000 Genomes Project (www.1000genomes.org/); (3) the Database of Single Nucleotide Polymorphisms, National Center for Biotechnology Information, US National Library of Medicine, Bethesda, MD (https://www.ncbi.nlm.nih.gov/snp/); (4) the Human Gene Mutation Database38 (www.hgmd.cf.ac.uk); (5) ClinVar39; and (6) the Exome Aggregation Consortium, Cambridge, MA, and the Genome Aggregation Database (http://exac.broadinstitute.org, and http://gnomad.broadinstitute.org).
Definitions and Pathogenicity of Identified Variants
Identified variants were classified as mutations if absent in control populations, rare variants if present in <1% of control populations, and polymorphisms if present in ≥1% of control populations. The pathogenicity of the identified variants was classified according to current recommendations from the American College of Medical Genetics and Genomics.40 In summary, variants were considered potentially pathogenic if they were absent or rare in healthy control subjects, previously associated with disease development, and functionally relevant variants in genes previously associated with the identified phenotype (eg, in-frame or frameshift-causing insertions or deletions, variants affecting splice sites, or missense variants likely to be pathogenic as identified by software models such as SIFT,41 PolyPhen,42 and MutationTaster243). A detailed description of the methods used to determine pathogenicity of identified variants is summarized in Table II in the online-only Data Supplement.
Statistical Analysis
The Kolmogorov-Smirnov test was used to evaluate whether each continuous parameter followed a gaussian distribution. Values are expressed as absolute numbers and percentages for categorical data and mean±SD for continuous data. Comparisons were performed with the χ2 test or Fisher exact test for categorical variables, unpaired t test for normally distributed continuous variables, and Mann-Whitney U test for nonnormally distributed continuous variables. A 2-tailed value of P<0.05 was considered significant throughout. All analyses were performed with SPSS software, version 20.0 (IBM Analytics).
Results
Athlete Demographics
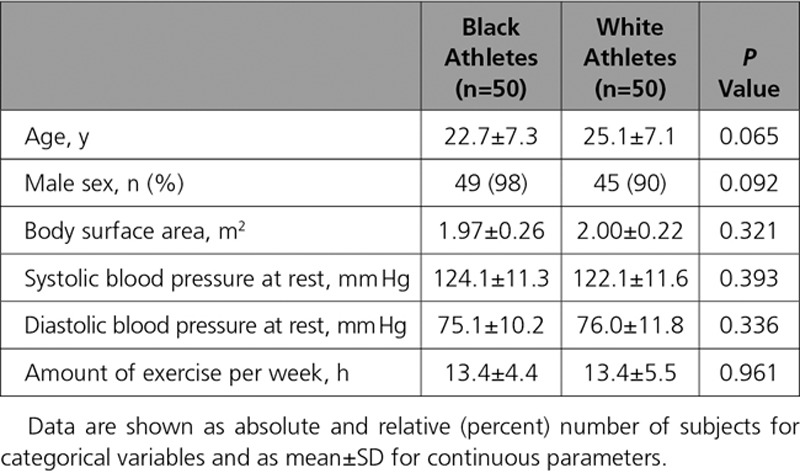
There were no differences between black and white athletes with respect to age, sex, body surface area, resting blood pressure, and hours of exercise per week (Table 1). All athletes had a resting blood pressure of <140/90 mm Hg. The majority of athletes (≥90%) in both groups were men.
Table 1.
Demographic Characteristics of Black and White Athletes
Electrocardiographic Characteristics
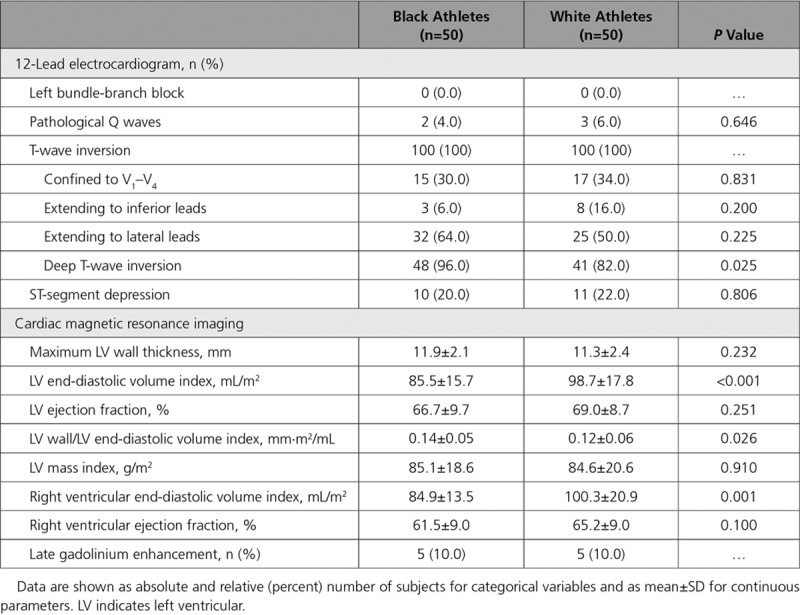
All athletes were in sinus rhythm. TWI extending into the lateral leads was the most common pattern of TWI in both black and white athletes, followed by TWI confined to the anterior leads V1 through V4 (Table 2). Of note, there was a relatively high prevalence of pathological Q waves and ST-segment depression in both groups; ST-segment depression was observed in at least one fifth (Table 2). Most athletes revealed 1 abnormal parameter on signal-averaged ECG, and 7 black (14%) and 6 white (12%) athletes revealed ≥2 abnormal parameters.
Table 2.
Electrocardiographic and Cardiac Magnetic Resonance Imaging Characteristics of Black and White Athletes
Structural Characteristics on CMR
Consistent with previous studies, white athletes had greater LV and right ventricular volumes on CMR compared with black athletes, whereas black athletes had a greater mean maximal LV wall thickness (Table 2). Five black athletes (10%) and 5 white athletes (10%) revealed LGE.
Cardiopulmonary Exercise Testing and Holter Monitoring
Three athletes with HCM demonstrated an abnormal blood pressure response to exercise. Two athletes with HCM had a short run of nonsustained ventricular tachycardia. One athlete without a diagnosis of cardiomyopathy demonstrated transient asymptomatic atrioventricular reentrant tachycardia.
Diagnoses Based on Clinical Investigations
In total, 21 athletes (21%) were diagnosed with cardiac disease on the basis of comprehensive clinical investigation. HCM was the most common diagnosis and affected 19 of the 21 athletes (90.5%). The diagnosis of HCM was based predominately on the degree and segmental nature of LVH on CMR and LGE. One athlete (4.8%) with extracardiac features was diagnosed with Fabry disease (later confirmed on genetic testing), and 1 athlete (4.8%) was diagnosed with LVNC. A clinical diagnosis of cardiomyopathy was more common in white athletes compared with black athletes (30% versus 12%; P=0.027). One black athlete (2%) revealed LGE on CMR in the absence of a clear diagnosis.
Diagnostic Yield of Genetic Testing
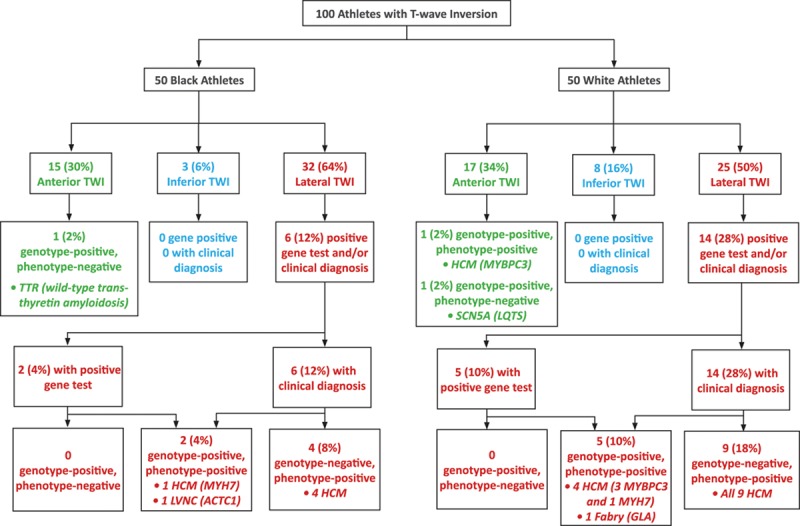
A genetic variant was identified in 63 athletes (63%); however, only 10 athletes revealed a disease-causing variant (pathogenic mutation [n=4] or variant of likely pathogenicity [n=6]), including 7 (14%) white athletes and 3 (6%) black athletes (Figure 1 and Table 3). The remaining 53 athletes were considered to have variants of unknown significance.
Figure 1.
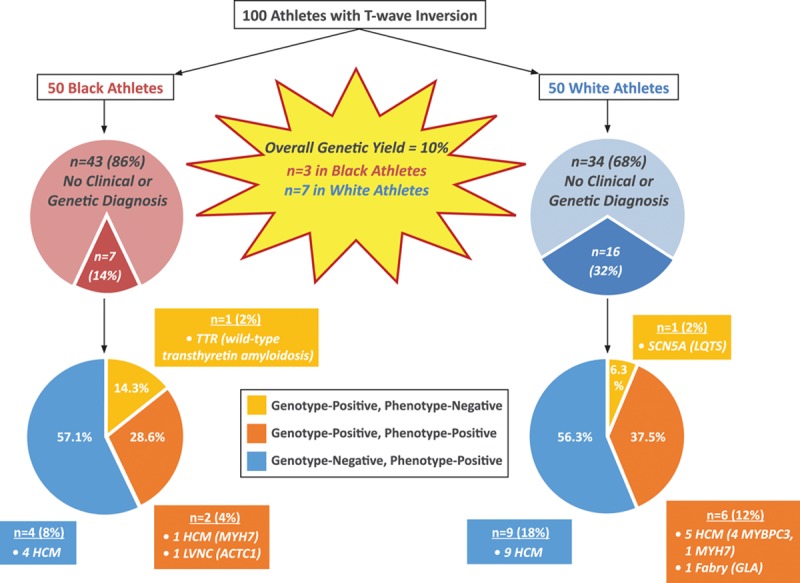
Breakdown of athletes with clinical and genetic diagnoses. The diagnostic yield with comprehensive clinical investigation was 21% compared with 10% using genetic testing. Of the 21 athletes diagnosed with cardiac disease on the basis of clinical investigation, 8 (38.1%) were gene positive (myosin binding protein C[MYBPC3], myosin heavy chain 7 [MYH7], galactosidase alpha[GLA], and actin, alpha, cardiac muscle 1 [ACTC1] genes) and 13 (61.9%) were gene negative. Of the remaining 79 athletes without a clinical diagnosis, 2 (2.5%) were gene positive (transthyretin[TTR] and sodium voltage-gated channel alpha subunit 5 [SCN5A] genes) in the absence of a clinical phenotype. HCM indicates hypertrophic cardiomyopathy; LQTS, long-QT syndrome; and LVNC, left ventricular noncompaction.
Table 3.
Clinical Diagnoses Made in Athletes
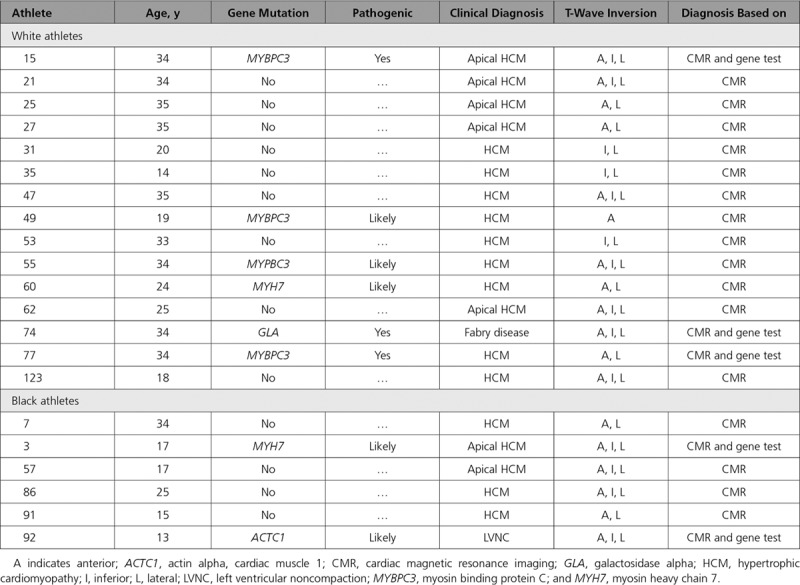
Of the 21 athletes with a clinical diagnosis, 8 individuals (38.1%; 6 white and 2 black) had a positive gene test consistent with the diagnosis (Figure 1 and Table 3). Of these 8 athletes, 6 athletes (5 white and 1 black) were diagnosed with HCM; 1 white athlete was diagnosed with Fabry disease; and 1 black athlete was diagnosed with LVNC (Figure 1 and Table III in the online-only Data Supplement).
Among the remaining 79 athletes without a clinical diagnosis, 2 (2.5%) were identified with a disease-causing variant in the absence of a clinical phenotype. Specifically, 1 white athlete had a likely pathogenic rare variant in the sodium voltage-gated channel alpha subunit 5 (SCN5A) gene previously reported to be associated with LQTS, and 1 black athlete had a pathogenic polymorphism in the transthyretin (TTR) gene that is found in up to 4% of black individuals and associated with the development of wild-type transthyretin amyloidosis.
Electric Changes in Athletes With a Cardiac Diagnosis
Pathological Q waves and ST-segment depression were more common in athletes with cardiac disease compared with those without cardiac disease (Table 4). Almost all athletes diagnosed with cardiac disease (n=20, 95.2%) revealed TWI in the lateral leads (Figure 2). TWI limited to the anterior leads was detected in just 1 white athlete (4.8%) with cardiac pathology (Figure 2). The same athlete also revealed coexisting pathological Q waves. None of the black athletes with anterior TWI was diagnosed with a cardiomyopathy or an ion channel disorder. One 23-year-old black athlete with anterior TWI harbored a pathogenic mutation in the TTR gene associated with wild-type transthyretin amyloidosis. None of the athletes with TWI confined to the inferior leads was diagnosed with cardiac disease or revealed a pathogenic genetic mutation.
Table 4.
Electrical, Structural, and Functional Characteristics of Athletes Diagnosed With Structural Cardiac Disease Compared With Those Without Cardiac Disease
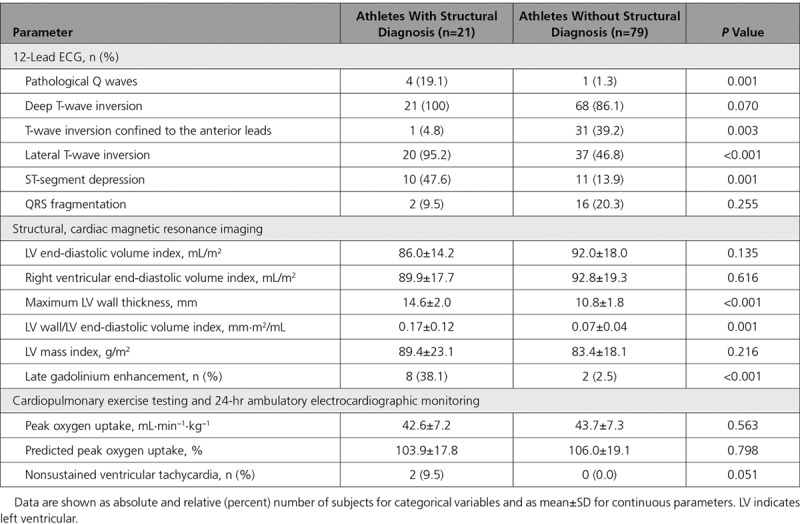
Figure 2.
Comparison of clinical and genetic diagnoses in black and white athletes in relation to the distribution of T-wave inversion (TWI). ACTC1indicates actin, alpha, cardiac muscle 1; GLA, galactosidase alpha; HCM, hypertrophic cardiomyopathy; LQTS, long-QT syndrome; LVNC, left ventricular noncompaction; MYBPC3, myosin binding protein C; MYH7, myosin heavy chain 7; SCN5A, sodium voltage-gated channel alpha subunit 5; and TTR, transthyretin.
Costs Per Diagnosis Associated With Clinical and Genetic Testing
The cost of clinical evaluation amounted to US$1084 per athlete using standard National Health Service Tariffs and an exchange rate of £1 to US$1.28. This figure equated to a cost of US$5162 per athlete diagnosed with cardiac disease. Addition of genetic testing increased the cost of evaluation by 3-fold to US$3267 per athlete, equating to a cost of US$14 204 per athlete with a clinical or genetic diagnosis and a cost of US$32 670 per genetic diagnosis alone. The cost of making additional diagnoses beyond clinical evaluation based on genetic testing (2 genotype-positive individuals without a clear clinical phenotype) was US$109 150 per athlete.
Discussion
The present study investigated whether genetic testing for mutations capable of causing cardiomyopathy and ion channel diseases has a potential role in determining the clinical significance of TWI in both black and white athletes over and above standard clinical evaluation.18 The cohort of athletes is unique, and recruitment was possible only through the assessment of a large number of athletes from several different referral sources. Although a total of 2039 athletes were evaluated in our own sports cardiology clinic over the 2-year study period, these individuals were referred from a pool of >5400 athletes assessed by CRY and >3000 athletes assessed by Qatar Orthopaedic and Sports Medicine Unit during the same study period. A significant proportion of athletes were also referred to us after being assessed at different institutions throughout the United Kingdom. Although it is more challenging to provide the precise denominator for this referral group, the majority of athletes in the present study (57%) revealed lateral TWI. On the basis of our own screening experience that only 4% of black3,5 and 0.3% of white2,3,5 athletes in the United Kingdom reveal lateral TWI, we estimate that the total number of athletes required to derive a cohort of athletes with the TWI patterns described in this study would exceed 11 000.
Diagnostic Yield From Genetic Testing
The overall diagnostic yield from genetic testing for a pathogenic or likely pathogenic mutation in athletes with TWI was 10% compared with 21% after comprehensive clinical evaluation. Genetic testing was positive in just 8 athletes (38.1%) with a clinical diagnosis of cardiomyopathy despite a very comprehensive panel of genes being tested. Of these, 6 athletes (75.0%) were white. Genetic testing identified an additional 2 athletes (2.5%) with TWI but no clear clinical phenotype who harbored potential cardiac disease. Compared with a recent study by Kadota et al44 in which 5 of 102 Japanese athletes (4.9%) with electrocardiographic abnormalities screened for mutations in 4 sarcomeric genes (myosin heavy chain 7 [MYH7], myosin binding protein C[MYBPC3], troponin T2, cardiac type [TNNT2], and troponin 13, cardiac type [TNNI3]) showed a positive result, our yield was significantly higher and likely reflects the comprehensive genetic panel used.
The yield for pathogenic mutations in our athletes clinically diagnosed with HCM was half that expected from the published literature (32% versus ≈60%–70%). One third of the athletes diagnosed with HCM exhibited the apical variant, which has been shown to have a lower-than-usual genetic yield.45 The current false-negative rate of genetic testing in our cohort indicates that routine genetic testing in athletes with TWI who have undergone comprehensive clinical evaluation will support a possible diagnosis of cardiomyopathy in a few cases at a substantial cost. These observations do not support the routine use of genetic testing for the evaluation of asymptomatic athletes with TWI in the absence of a family history of an inherited cardiac condition.
Although genetic testing identified a potentially serious SCN5A mutation implicated in LQTS in a white female athlete with TWI, she did not show any features of the disorder, including a prolonged corrected QT interval. Similarly, gene testing identified a definitive transthyretin mutation in a black athlete without evidence of cardiac amyloidosis at this young age, and it is known that this variant is detected in up to 4% of the black population. These observations also highlight that routine gene testing without appropriate clinical indications may be confusing, cause unnecessary concern, and be problematic for decision making.
Association Between Pattern of TWI and Cardiac Pathology
In both black and white athletes, TWI extending into the lateral leads was the most common pattern encountered (64% versus 50%, respectively; Figure 2). Although previous studies in both black and white athletes report anterior TWI (V1–V4) to be the most common pattern observed,3 our cohort almost certainly reflects a referral bias in favor of individuals with lateral TWI in whom suspicion of a cardiomyopathy is higher. Furthermore, given that anterior TWI is now widely recognized as a normal, ethnicity-specific training variant in black athletes, referral bias may also explain the similar prevalence of anterior TWI observed in our black and white cohorts, with fewer black athletes exhibiting anterior TWI being referred for evaluation.
ST-segment depression was found exclusively in athletes with lateral TWI, regardless of ethnicity. All but 1 of our 21 athletes (95%) exhibiting structural disease revealed lateral TWI (Table 3 and Figure 2). Indeed, the diagnostic yield of lateral TWI for a clinical diagnosis of cardiomyopathy in black and white athletes was 18.8% and 56%, respectively. In comparison, the yield for mutations associated with cardiomyopathy in athletes with lateral TWI was 12.3%.
Compared with white athletes, a smaller proportion of black athletes with TWI (n=6, 12%) were diagnosed with a cardiomyopathy (Table 3). Of these individuals, all exhibited TWI in the lateral leads, reinforcing the notion that this particular repolarization pattern should be viewed with caution, even in the black athletic population in whom its prevalence approaches 5%.3 Anterior TWI in black athletes was not associated with overt cardiomyopathy, ion channel disorders, or pathogenic genetic mutations, suggesting that this pattern is likely benign.46
Overall Number of Diagnoses Made and Yield From CMR
Significantly fewer black athletes were diagnosed with a cardiomyopathy compared with white athletes (12% versus 30%; P=0.027), suggesting that TWI may be more representative of subtle forms of cardiomyopathy in white individuals. The only other study to comprehensively investigate athletes with TWI with CMR evaluated 155 athletes.4 The authors diagnosed 37 athletes with a cardiomyopathy (predominantly HCM) on initial echocardiography. Of the remaining 118, a further 24 athletes (20.3%) were diagnosed with CMR, a figure comparable to the 21 athletes (21.0%) diagnosed in the present study, and a further 3 athletes (2.5%) were diagnosed on the basis of Holter monitoring and exercise testing. In this study, Holter monitoring and exercise stress testing revealed the broader phenotypic features of HCM in over 26% of affected athletes.
Study Limitations
The present study has several limitations. Inherited cardiomyopathies exhibit an age-related penetrance; thus, it is possible that cardiac disease was underdiagnosed in our cohort. Our population consisted of predominately young male athletes. Therefore, any conclusions about the significance of TWI in female or master athletes cannot be extrapolated from this study. It was also not possible to perform familial evaluation of first-degree family members, which would have been a valuable source of information to help determine the genetic significance of TWI in borderline cases, even in the absence of a pathogenic mutation. Cosegregation studies were not performed and would have been important to determine the pathogenicity of several of the identified variants. Finally, the absence of a pathogenic mutation does not exclude the possibility of underlying disease, and several athletes revealed variants of undetermined clinical significance; thus, long-term clinical follow-up is warranted.
Conclusions
Up to 10% of athletes with TWI show definitive or likely pathogenic mutations for cardiomyopathy or ion channel disease. Compared with standard clinical practice, the relatively low diagnostic yield and high cost of genetic testing make it of negligible use in routine clinical practice. Although genetic testing may help identify individual athletes with TWI and a potential cardiac disorder in the absence of a clear clinical phenotype, our results suggest that it is not indicated in the routine evaluation of asymptomatic athletes with TWI in the absence of a family history of an inherited cardiac condition.
Acknowledgments
The authors acknowledge the support of the British Heart Foundation, Aspetar, and Cardiac Risk in the Young.
Sources of Funding
Drs Sheikh and Sharma received a research project grant (grant PG/11/122/29310) from the British Heart Foundation to evaluate repolarization changes in athletes and from Aspetar (Doha, Qatar) for genetic testing in athletes with repolarization changes. Drs Sheikh and Malhotra were funded by research grants from the charitable organization CRY for screening and clinical evaluation of athletes.
Disclosures
Dr Monserrat is the chief executive officer and a stakeholder of Health in Code SL. The other authors report no conflicts.
Supplementary Material
Footnotes
Sources of Funding, see page 1193
The online-only Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.118.034208.
References
- 1.Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, De Luca R, Spataro A, Biffi A, Thiene G, Maron BJ. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358:152–161. doi: 10.1056/NEJMoa060781. doi: 10.1056/NEJMoa060781. [DOI] [PubMed] [Google Scholar]
- 2.Papadakis M, Basavarajaiah S, Rawlins J, Edwards C, Makan J, Firoozi S, Carby L, Sharma S. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J. 2009;30:1728–1735. doi: 10.1093/eurheartj/ehp164. doi: 10.1093/eurheartj/ehp164. [DOI] [PubMed] [Google Scholar]
- 3.Papadakis M, Carre F, Kervio G, Rawlins J, Panoulas VF, Chandra N, Basavarajaiah S, Carby L, Fonseca T, Sharma S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J. 2011;32:2304–2313. doi: 10.1093/eurheartj/ehr140. doi: 10.1093/eurheartj/ehr140. [DOI] [PubMed] [Google Scholar]
- 4.Schnell F, Riding N, O’Hanlon R, Axel Lentz P, Donal E, Kervio G, Matelot D, Leurent G, Doutreleau S, Chevalier L, Guerard S, Wilson MG, Carré F. Recognition and significance of pathological T-wave inversions in athletes. Circulation. 2015;131:165–173. doi: 10.1161/CIRCULATIONAHA.114.011038. doi: 10.1161/CIRCULATIONAHA.114.011038. [DOI] [PubMed] [Google Scholar]
- 5.Sheikh N, Papadakis M, Carre F, Kervio G, Panoulas VF, Ghani S, Zaidi A, Gati S, Rawlins J, Wilson MG, Sharma S. Cardiac adaptation to exercise in adolescent athletes of African ethnicity: an emergent elite athletic population. Br J Sports Med. 2013;47:585–592. doi: 10.1136/bjsports-2012-091874. doi: 10.1136/bjsports-2012-091874. [DOI] [PubMed] [Google Scholar]
- 6.Sheikh N, Papadakis M, Ghani S, Zaidi A, Gati S, Adami PE, Carré F, Schnell F, Wilson M, Avila P, McKenna W, Sharma S. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129:1637–1649. doi: 10.1161/CIRCULATIONAHA.113.006179. doi: 10.1161/CIRCULATIONAHA.113.006179. [DOI] [PubMed] [Google Scholar]
- 7.Rawlins J, Carre F, Kervio G, Papadakis M, Chandra N, Edwards C, Whyte GP, Sharma S. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121:1078–1085. doi: 10.1161/CIRCULATIONAHA.109.917211. doi: 10.1161/CIRCULATIONAHA.109.917211. [DOI] [PubMed] [Google Scholar]
- 8.Zaidi A, Ghani S, Sharma R, Oxborough D, Panoulas VF, Sheikh N, Gati S, Papadakis M, Sharma S. Physiological right ventricular adaptation in elite athletes of African and Afro-Caribbean origin. Circulation. 2013;127:1783–1792. doi: 10.1161/CIRCULATIONAHA.112.000270. doi: 10.1161/CIRCULATIONAHA.112.000270. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi A, Sheikh N, Jongman JK, Gati S, Panoulas VF, Carr-White G, Papadakis M, Sharma R, Behr ER, Sharma S. Clinical differentiation between physiological remodeling and arrhythmogenic right ventricular cardiomyopathy in athletes with marked electrocardiographic repolarization anomalies. J Am Coll Cardiol. 2015;65:2702–2711. doi: 10.1016/j.jacc.2015.04.035. doi: 10.1016/j.jacc.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D, Pelliccia A, Bjørnstad HH, Vanhees L, Biffi A, Borjesson M, Panhuyzen-Goedkoop N, Deligiannis A, Solberg E, Dugmore D, Mellwig KP, Assanelli D, Delise P, van-Buuren F, Anastasakis A, Heidbuchel H, Hoffmann E, Fagard R, Priori SG, Basso C, Arbustini E, Blomstrom-Lundqvist C, McKenna WJ, Thiene G Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol: consensus statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516–524. doi: 10.1093/eurheartj/ehi108. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Zipes DP. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:1312–1375. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM, Jr, Krauss MD, Maron MS, Mitten MJ, Roberts WO, Puffer JC American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643–1455. doi: 10.1161/CIRCULATIONAHA.107.181423. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- 13.Pelliccia A, Fagard R, Bjørnstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carrè F, Corrado D, Delise P, Dorwarth U, Hirth A, Heidbuchel H, Hoffmann E, Mellwig KP, Panhuyzen-Goedkoop N, Pisani A, Solberg EE, van-Buuren F, Vanhees L, Blomstrom-Lundqvist C, Deligiannis A, Dugmore D, Glikson M, Hoff PI, Hoffmann A, Hoffmann E, Horstkotte D, Nordrehaug JE, Oudhof J, McKenna WJ, Penco M, Priori S, Reybrouck T, Senden J, Spataro A, Thiene G Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology; Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–1445. doi: 10.1093/eurheartj/ehi325. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 14.Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, Biffi A, Buja G, Delise P, Gussac I, Anastasakis A, Borjesson M, Bjørnstad HH, Carrè F, Deligiannis A, Dugmore D, Fagard R, Hoogsteen J, Mellwig KP, Panhuyzen-Goedkoop N, Solberg E, Vanhees L, Drezner J, Estes NA, 3rd, Iliceto S, Maron BJ, Peidro R, Schwartz PJ, Stein R, Thiene G, Zeppilli P, McKenna WJ Section of Sports Cardiology, European Association of Cardiovascular Prevention and Rehabilitation. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. doi: 10.1093/eurheartj/ehp473. doi: 10.1093/eurheartj/ehp473. [DOI] [PubMed] [Google Scholar]
- 15.Calore C, Zorzi A, Sheikh N, Nese A, Facci M, Malhotra A, Zaidi A, Schiavon M, Pelliccia A, Sharma S, Corrado D. Electrocardiographic anterior T-wave inversion in athletes of different ethnicities: differential diagnosis between athlete’s heart and cardiomyopathy. Eur Heart J. 2016;37:2515–2527. doi: 10.1093/eurheartj/ehv591. doi: 10.1093/eurheartj/ehv591. [DOI] [PubMed] [Google Scholar]
- 16.Metzker ML. Sequencing technologies: the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, Asif IM, Owens DS, Chung EH, Emery MS, Froelicher VF, Heidbuchel H, Adamuz C, Asplund CA, Cohen G, Harmon KG, Marek JC, Molossi S, Niebauer J, Pelto HF, Perez MV, Riding NR, Saarel T, Schmied CM, Shipon DM, Stein R, Vetter VL, Pelliccia A, Corrado D. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057–1075. doi: 10.1016/j.jacc.2017.01.015. doi: 10.1016/j.jacc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Cain ME, Anderson JL, Arnsdorf MF, Mason JW, Scheinman MM, Waldo AL. Signal-averaged electrocardiography: ACC expert consensus document. J Am Coll Cardiol. 1996;27:238–249. [PubMed] [Google Scholar]
- 20.Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:217–221. doi: 10.1152/jappl.1981.50.1.217. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- 21.Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873–879. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 22.Du Bois D, Du Bois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [PubMed] [Google Scholar]
- 23.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 24.Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NA, 3rd, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–1152. doi: 10.1161/CIRCULATIONAHA.109.913871. doi: 10.1161/CIRCULATIONAHA.109.913871. [DOI] [PubMed] [Google Scholar]
- 25.Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö T, Linhart A, Mogensen J, Pinto Y, Ristic A, Seggewiss H, Sinagra G, Tavazzi L, Elliott PM. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis: a position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448–1458. doi: 10.1093/eurheartj/ehs397. doi: 10.1093/eurheartj/ehs397. [DOI] [PubMed] [Google Scholar]
- 26.Hussein A, Karimianpour A, Collier P, Krasuski RA. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol. 2015;66:578–585. doi: 10.1016/j.jacc.2015.06.017. doi: 10.1016/j.jacc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome: an update. Circulation. 1993;88:782–784. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Timothy KW, Vincent GM, Lehmann MH, Fox J, Giuli LC, Shen J, Splawski I, Priori SG, Compton SJ, Yanowitz F, Benhorin J, Moss AJ, Schwartz PJ, Robinson JL, Wang Q, Zareba W, Keating MT, Towbin JA, Napolitano C, Medina A. Spectrum of ST-T-wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation. 2000;102:2849–2855. doi: 10.1161/01.cir.102.23.2849. [DOI] [PubMed] [Google Scholar]
- 29.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, Pahl E, Vasan RS, Ramasubbu K, Rasmusson K, Towbin JA, Yancy C American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 31.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 33.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 34.Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes. Insights into methods for distinguishing athlete’s heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation. 1995;91:1596–1601. doi: 10.1161/01.cir.91.5.1596. [DOI] [PubMed] [Google Scholar]
- 35.Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- 36.Flett AS, Maestrini V, Milliken D, Fontana M, Treibel TA, Harb R, Sado DM, Quarta G, Herrey A, Sneddon J, Elliott P, McKenna W, Moon JC. Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int J Cardiol. 2015;183:143–148. doi: 10.1016/j.ijcard.2015.01.054. doi: 10.1016/j.ijcard.2015.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1–9. doi: 10.1007/s00439-013-1358-4. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue):D980–D985. doi: 10.1093/nar/gkt1113. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 42.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 44.Kadota C, Arimura T, Hayashi T, Naruse TK, Kawai S, Kimura A. Screening of sarcomere gene mutations in young athletes with abnormal findings in electrocardiography: identification of a MYH7 mutation and MYBPC3 mutations. J Hum Genet. 2015;60:641–645. doi: 10.1038/jhg.2015.81. doi: 10.1038/jhg.2015.81. [DOI] [PubMed] [Google Scholar]
- 45.Gruner C, Care M, Siminovitch K, Moravsky G, Wigle ED, Woo A, Rakowski H. Sarcomere protein gene mutations in patients with apical hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2011;4:288–295. doi: 10.1161/CIRCGENETICS.110.958835. [DOI] [PubMed] [Google Scholar]
- 46.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]


