Abstract
Background: Thyroid cancer is an emerging health problem in the United States and worldwide. With incidence rates of thyroid cancer rapidly rising, the need to develop new treatment options is becoming a priority, and understanding the molecular mechanisms of this disease is crucial to furthering these efforts. Thyroid growth is driven by the TSH/cAMP/PKA signaling pathway, and it has previously been shown that activation of PKA through genetic ablation of the regulatory subunit Prkar1a (Prkar1a KO) is sufficient to cause follicular thyroid cancer in mouse models. cAMP also activates the Epac proteins and their downstream effectors, Rap1a and Rap1b.
Methods: Previously, the authors' laboratory generated a mouse model of follicular thyroid cancer by conferring thyroid-specific deletion of Prkar1a (R1a-TpoKO). To probe the roles of other components of the PKA signaling system in the development of thyroid cancer, this study deleted Rap1 and Epac1 in the setting of the Prkar1a knockout.
Results: Deletion of Rap1 significantly decreases thyroid size and cancer incidence in Prkar1a KO thyroids. Further, isoform-specific ablation of Rap1a and Rap1b implicates Rap1b as the downstream effector of PKA during thyroid carcinogenesis. In vivo modeling provides definitive evidence that Epac1 plays little role in thyroid proliferation and is dispensable for thyroid carcinogenesis arising from the deletion of Prkar1a.
Conclusions: This study demonstrate that PKA signaling to Rap1b is a key signaling node for follicular thyroid carcinogenesis, while Epac1 activity is not required for tumor development. This work sheds new light on the pathways involved in FTC development and identifies a possible target for the development of new therapies in the treatment of FTC.
Keywords: : PKA, PRKAR1A, Epac, Rap1, follicular thyroid cancer
Introduction
Thyroid cancer is the most common endocrine malignancy, with an estimated 45,000 new cases diagnosed per year in the United States. Further, incidence rates of thyroid carcinoma continue to rise at the fastest rate of all malignancies. While improved diagnostic tools and widespread access to care may contribute in part to the rapidly rising incidence rates, studies indicate that it is a true epidemic (1). While many thyroid cancers have a good prognosis with early detection, certain subtypes, including follicular thyroid cancer (FTC), have a poorer prognosis because of the more aggressive nature of this tumor and the tendency to develop early metastasis (2). Once the disease has metastasized, no curative therapies exist. Therefore, deeper understanding of the molecular pathways contributing to disease onset and progression is critical to the development of novel therapeutics and to aid in disease management and improve clinical outcomes.
In humans, loss-of-function mutations in PRKAR1A, which encodes the type 1a regulatory subunit of the cAMP dependent protein kinase A (PKA), cause the inherited tumor predisposition syndrome Carney Complex (3). Carney Complex is characterized by tumors in multiple tissues, including FTC, as a part of the disease spectrum (3,4). In mice, thyroid-specific deletion of Prkar1a (Tpo-R1aKO) leads to locally invasive thyroid carcinoma as a result of over-activation of the PKA pathway (5). This mouse model of FTC serves as a useful tool in the study of PKA-mediated thyroid carcinogenesis, as previous mouse models of increased PKA signaling through adenylyl cyclase have not developed cancer (6–9).
In the thyroid, PKA is known to signal downstream of thyrotropin (TSH) through the production of cAMP, and increased levels of TSH are associated with thyroid carcinogenesis in humans (10,11). As such, cAMP has been a popular object of research for targeted drug therapies. However, given the wide range of physiological responses regulated by cAMP, pharmacological manipulation of cAMP levels can have an array of side effects, including emesis and cardiac dysfunction (12). In an attempt to circumvent this problem, recent research has focused on downstream targets of cAMP for a more targeted approach to cAMP pathway manipulation. Rap1 is a small GTPase containing two isoforms, each encoded by a separate gene, Rap1a and Rap1b. Rap1 activity has been shown to be regulated by both cAMP and PKA through signaling by TSH (13). Increased Rap activity is associated with many cancers, including thyroid cancer, and it has been suggested that Rap1 dysregulation can contribute to malignancy (9,14–17).
Exchange protein directly activated by cAMP (Epac) is another intracellular receptor that serves to mediate the effects of cAMP to activate the common PKA/Epac target Rap1 (18,19). Epac has two isoforms: Epac1 and Epac2. Epac1 is ubiquitously expressed, with particularly high expression in tissues such as the kidney, ovary, and thyroid, while Epac2 is selectively expressed in a limited number of tissues, with no detectable expression in the thyroid (19,20). Epac has been shown to regulate Rap activity both in concert with PKA and independently, and the effects—whether stimulatory or inhibitory—seem to be dependent on cellular context and the type of stimuli (13,18,21,22). Further, Epac1 has been shown to play a role in cell migration and invasion in other cancers (21,23). Thus far, discriminating between PKA- and Epac-dependent Rap1 activation in vitro has proved difficult, as they have similar affinities for many cAMP analogs, although analogs with selective binding properties have been developed. However, these analogs are not usable in vivo. Therefore, in vivo gene- and isoform-specific knockout systems are needed to study the detailed interactions among these proteins further.
This study used murine knockout models to probe the roles of Rap1 and Epac1 in Prkar1a-associated thyroid carcinogenesis. The study demonstrates that loss of Rap1 in Prkar1a KO thyroids significantly reduces the risk of developing thyroid cancer, highlighting a central role of PKA-Rap1 signaling in the development of FTC. Further, it shows that Epac1 does not appear to play a significant role in mediating cAMP-PKA-Rap1 signaling in thyroid tumorigenesis. These studies further our understanding of the molecular mechanisms of FTC progression and may help direct future research into the development of novel therapeutics.
Methods
Animal studies
TPO-cre;Prkar1aloxP/loxP mice (5) were bred with Rap1aloxP/loxP;Rap1bloxP/loxP animals (24) to generate thyroid-specific deletions of total Rap1 and individual Rap1 isoforms. For studies involving Epac1, TPO-cre;Prkar1aloxP/loxP mice were crossed with global Epac1–/– (25) mice.
Three-dimensional thyroid ultrasonography was performed at three-month intervals on live animals using a VisualSonics Vevo2100 Ultrasound equipped with an MS550D transducer. Images were acquired in 3D mode and volumes calculated using Vevolab v2.1.
Goitrogen treatment was given to Epac–/– and wild-type (WT) littermate mice for six months. Treatment consisted of methimazole (0.5 g/L) and sodium perchlorate (5 g/L) in drinking water ad libitum changed twice weekly (9). Mice were also maintained on a low-iodine diet (Envigo Teklad TD.120460).
Histology
Dissected mouse thyroid tissues were fixed in 10% buffered formalin solution and embedded in paraffin. Tissue sections were cut in 5 μm sections and stained with hematoxylin and eosin. Immunohistochemistry was performed, as previously described (26), with the following antibodies: Epac1 (ab21236; Abcam), Ki-67 (ab15580; Abcam), and cleaved Caspase-3 (9661; Cell Signaling Technology). Quantification of cleaved Caspase-3 staining was performed using ImageJ software to measure the area of positive staining divided by the total area in pixels. Ki-67 staining is represented as a ratio of number of Ki-67+ cells per field divided by total number of cells per field in 40 × images.
Western blotting
Proteins from mouse tissues were prepared and run on sodium dodecyl sulfate polyacrylamide gels, followed by transfer to nitrocellulose membrane. Membranes were blocked with 2% bovine serum albumin and probed with antibodies against Rap1 (07-916; Millipore) and β-actin (3700S; Cell Signaling Technology)
Thyroid hormone assay
Blood was collected from 10 animals per genotype and centrifuged for 20 min at 4°C to separate serum from whole blood. Serums were shipped on dry ice to Vanderbilt University Medical Center Hormone Assay and Analytical Services Core (part of the Mouse Metabolic Phenotyping Center) for thyroxine (T4) concentration determination using double antibody radioimmunoassay.
Statistics
All data, except cancer incidence rates, were analyzed via unpaired t-test using Prism GraphPad software. p-Values of <0.05 were considered significant. Tumor incidence rates were compared using a logistic regression and controlled for multiple comparisons using Holm's procedure. Incidence rates are represented as a percent incidence with confidence intervals.
Results
Thyroid specific deletion of Rap1 decreases incidence of Prkar1a-mediated FTC formation
This project began as an effort to assess the role of Rap1 in Prkar1a-mediated thyroid carcinogenesis. However, before addressing this point, it was first necessary to examine the effect of Rap1 KO on normal thyroid function. To this end, first a thyroid-specific deletion of Rap1 isoforms was generated by crossing thyroid peroxidase (TPO)-cre mice with mice harboring conditional null alleles of Rap1a and Rap1b (24) to obtain TPO-cre;Rap1aloxP/loxP and TPO-cre;Rap1bloxP/loxP animals, henceforth referred to as Tpo-Rap1A and Tpo-Rap1B. Conditional deletion of either Rap isoform in the thyroid had no effect on thyroid morphology or histology (Supplementary Fig. S1A and B; Supplementary Data are available online at www.liebertpub.com/thy).
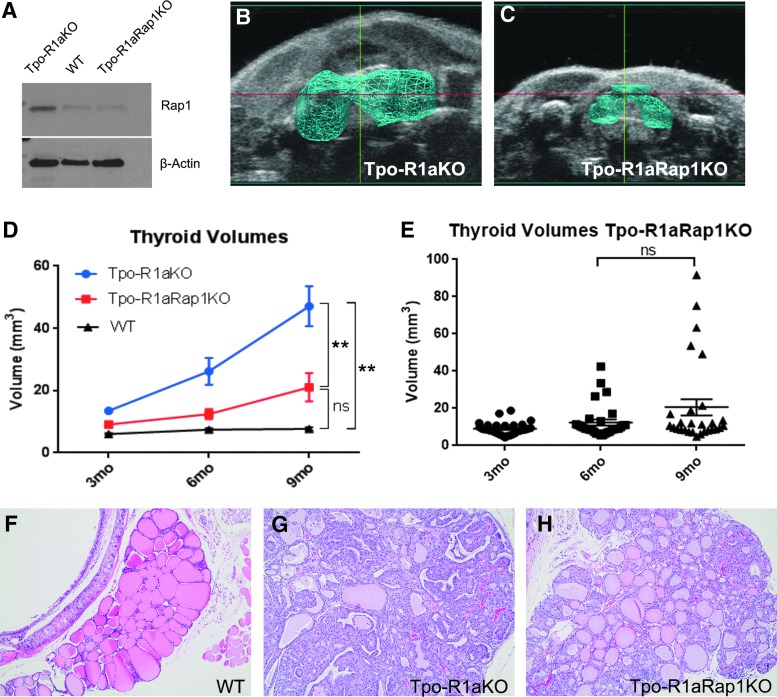
To determine if Rap1 plays a role in Prkar1a-mediated thyroid tumorigenesis, Tpo-Cre;Prkar1aloxP/loxP mice were crossed with Rap1aloxP/loxP;Rap1bloxP/loxP animals to generate Tpo-cre;Prkar1aloxP/loxP;Rap1aloxP/loxP;Rap1bloxP/loxP mice, henceforth referred to as Tpo-R1aRap1KO. Knockout was confirmed by Western blot (Fig. 1A), showing decreased Rap1 protein relative to Tpo-R1aKO mouse thyroids. As the Tpo-cre driver is highly efficient, residual Rap1 detected in the blots most likely arises from cells outside of the thyroid follicular lineage. Tpo-R1aRap1KO mice and WT littermates were observed for nine months (the time after which mice began dying), and subjected to ultrasonography at three-month intervals to record thyroid growth over time. At the study endpoint, Tpo-R1aRap1KO thyroids were significantly smaller than age-matched Tpo-R1aKO thyroids (20.584 ± 23.13 mm3 vs. 47.07 ± 24.72 mm3, respectively; p = 0.0011; Fig. 1B–E). Size differences between Tpo-R1aRap1KO and WT littermate controls showed a trend toward mild enlargement, which did not reach statistical significance (7.66 ± 1.84 mm3 for WT, p = 0.063, compared to Tpo-R1aRap1KO; Fig. 1D). No sex differences were found in the thyroid sizes of Tpo-R1aRap1KO mice (p = 0.945; data not shown). Phenotypically, this genetic model of Prkar1a-induced thyroid cancer exhibits complete involvement of the thyroid gland. As such, the entire thyroid is considered tumorous in Tpo-R1aKO mice (Fig. 1G). Histological analysis of Tpo-R1aRap1KO thyroids revealed that while many thyroids exhibited increased cellularity, the majority of Tpo-R1aRap1KO thyroids had regained substantial macrofollicular structures and lacked indicators of a carcinogenic phenotype, including capsular fibrosis and local invasion (Fig. 1F–H). Incidence rates of thyroid carcinoma in Tpo-R1aRap1KO were significantly lower than incidences in age-matched Tpo-R1aKO mice (27.78% incidence vs. 81.8%, respectively; p = 0.0091), indicating that deletion of Rap1 significantly reduces the risk of developing thyroid tumors in the setting of Prkar1a KO.
FIG. 1.
Thyroid-specific deletion of Rap1 in Tpo-R1aKO mice reduces thyroid size. (A) Western blot showing total Rap1 expression in wild-type (WT), Tpo-R1aKO, and Tpo-R1aRap1KO thyroids. Three-dimensional (3D) rendering of ultrasonographic images of (B) Tpo-R1aKO and (C) Tpo-R1aRap1KO thyroids at nine months of age. (D) Thyroid volumes determined by 3D ultrasonography at three-month intervals of Tpo-R1aKO (blue line; n = 18), Tpo-R1aRap1KO (red line; n = 30), and Cre-negative littermate control (black line; n = 25) mice. (E) Scatter plot showing the range of Tpo-R1aRap1KO thyroid volumes over time (p = 0.077, ns). (F–H) 10 × images of hematoxylin and eosin–stained thyroids from Tpo-R1aKO (G), Tpo-R1aRap1KO (H), and cre-negative littermate controls (F) at nine months of age. **p ≤ 0.001 at both six- and nine-month time points. Graphs show mean and standard error of the mean (SEM).
Rap1b is the isoform that confers resistance to thyroid tumor formation in Tpo-R1aKO mice
To determine which isoform of Rap1 is responsible for the reduction in cancer incidence, single isoform knockouts were generated in the setting of Prkar1a deletion. Tpo-R1aRap1AKO (Tpo-Cre;Prkar1aloxP/loxP;Rap1aloxP/loxP) had thyroid volumes similar to Tpo-R1aKO mice (61.24 ± 4.12 mm3, p = 0.35, compared to Tpo-R1aKO; Fig. 2A, C, and E). Due to decreased survival of the single isoform deleted Tpo-R1aRap1AKO mice (due to a nonthyroidal effect of the conditional allele), it was necessary to combine the six- and nine-month time point for phenotyping purposes, resulting in comparable incidence rates of carcinoma between Tpo-R1aRap1AKO mice at six to nine months of age compared to 9-month-old Tpo-R1aKO mice (80%; p = 0.9075 vs. Tpo-R1aKO). Conversely, selectively deleting the 1B isoform of Rap in Tpo-R1aRap1BKO thyroids resulted in a reduction in thyroid size relative to Tpo-R1aKO mice (18.93 ± 10.60 mm3 vs. 47.07 mm3, respectively; p = 0.0039; Fig. 2B, D, and F).
FIG. 2.
Decreases in thyroid size in Tpo-R1aRap1KO mice is mediated by Rap1b. (A) Thyroid volumes determined by ultrasonography of Tpo-R1aRap1AKO (red line; n = 25, 22, and 3), Cre-negative littermate control (black line; n = 15), and age-matched Tpo-R1aKO (blue line; n = 18) mice at indicated time points. (B) Thyroid volumes of Tpo-R1aRap1BKO (red line; n = 18, 17, and 9), Cre-negative littermate control (black line; n = 20), and age-matched Tpo-R1aKO (blue line; n = 18) mice at the indicated time points. (C and D) Scatter plots showing thyroid volumes of Tpo-R1aRap1AKO (C) (p = 0.0024) and Tpo-R1aRap1BKO (D) (p = 0.0021) mice. (E and F) 3D renderings of ultrasonographic images from Tpo-R1aRap1AKO (E) and Tpo-R1aRap1BKO (F) mouse thyroids. **p ≤ 0.001 at six and nine months of age. *p ≤ 0.05 at six and nine months of age. Graphs show mean and SEM.
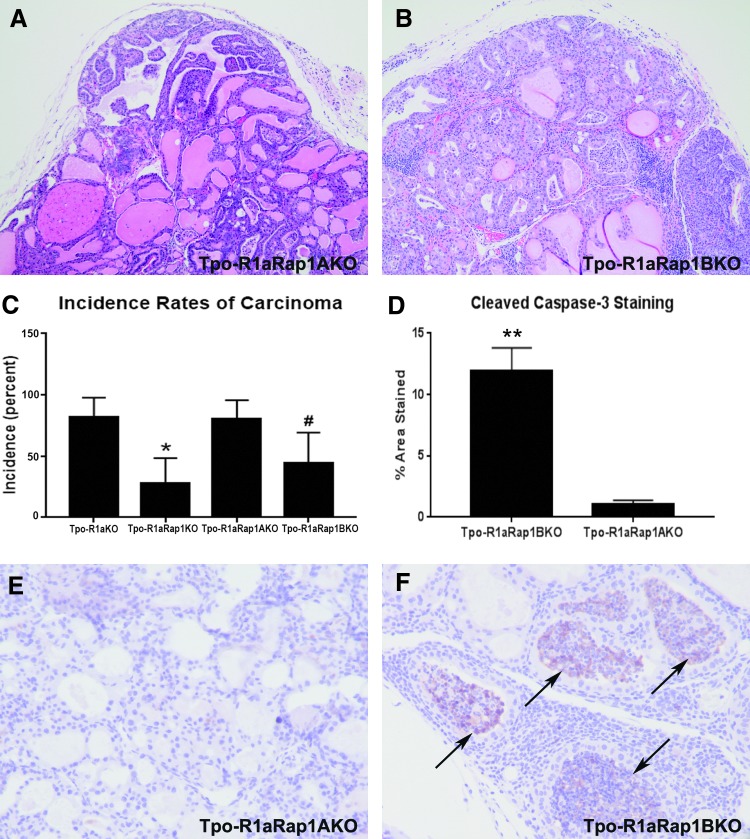
At the histologic level, the range of features of Tpo-R1aRap1AKO thyroids was comparable to that of Tpo-R1aRap1BKO thyroids (Fig. 3A and B), although Tpo-R1aRap1KO and Tpo-R1aRap1BKO mice exhibited a clear decrease in carcinoma incidence relative to Tpo-R1aRap1A mice(44% carcinomas for Tpo-R1aRap1BKO vs. 80% carcinomas for Tpo-R1aRap1AKO; p = 0.044; Fig. 3C), indicating that Rap1b is the predominant isoform required for Prkar1a-mediated tumorigenesis. To understand better the role Rap1b plays in reduction of tumor size, expression of apoptosis and proliferation markers were examined. While proliferation was unchanged between models (4.03 ± 2.55 Ki-67+ cells Tpo-R1aRap1A vs. 3.78 ± 1.38 Ki-67+ cells Tpo-R1aRap1B; p = 0.79; data not shown), Tpo-R1aRap1B thyroids expressed significantly higher levels of cleaved Caspase 3 relative to Tpo-R1aRap1AKO tumors (Fig. 3F, quantified in Fig. 3D), implicating an increase in cell death as the driving force behind the lack of excessive tumor growth in Tpo-R1aRap1KO or Tpo-R1aRap1BKO thyroids. In fact, caspase staining tended to involve entire follicles, which exhibited a solid appearance with caspase staining throughout.
FIG. 3.
Tpo-R1aRap1BKO mice have a lower incidence rate of thyroid carcinoma due to increases in apoptosis relative to Tpo-R1aRap1A mice. (A and B) 20 × images of hematoxylin and eosin–stained thyroid sections from Tpo-R1aRap1A (A) and Tpo-R1aRap1B (B) mice. (C) Percent incidence of thyroid carcinoma in Tpo-R1aKO (n = 11 at nine months of age), Tpo-R1aRap1KO (n = 18 at nine months of age), Rap isoform-specific Tpo-R1aRap1AKO (n = 15 at six to nine months of age), and Tpo-R1aRap1BKO (n = 18 at nine months of age) mice. *p ≤ 0.05 compared to Tpo-R1aKO; #p ≤ 0.05 compared to Tpo-R1aRap1AKO. (D) Quantification of cleaved caspase-3 staining represented as a percentage of the total area of the image (n = 8 mice per genotype). **p ≤ 0.001 relative to Tpo-R1aRap1AKO. (E and F) Diaminobenzidine immunohistochemistry for cleaved caspase-3 (black arrows) on thyroid tissue sections from Tpo-R1aRap1AKO (E) and Tpo-R1aRap1BKO (F) at 40 × magnification.
Epac1 is not a major component in the PKA-Rap1 thyroid tumorigenic cascade
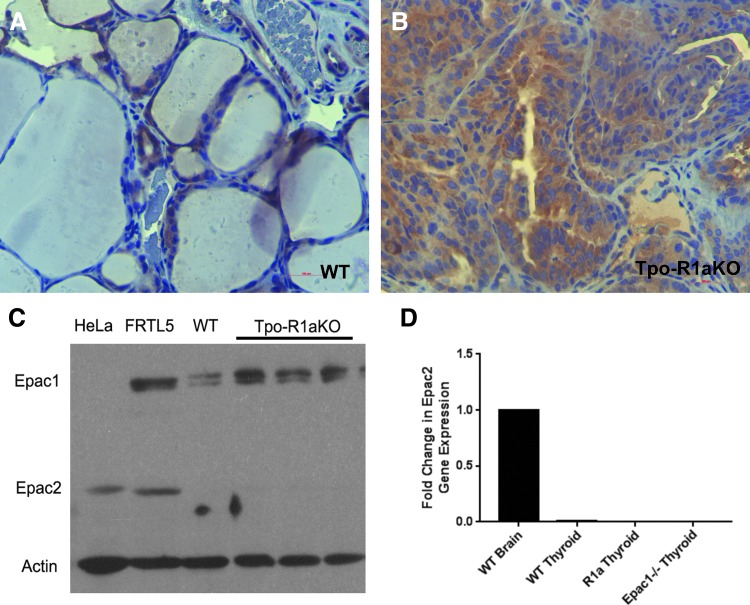
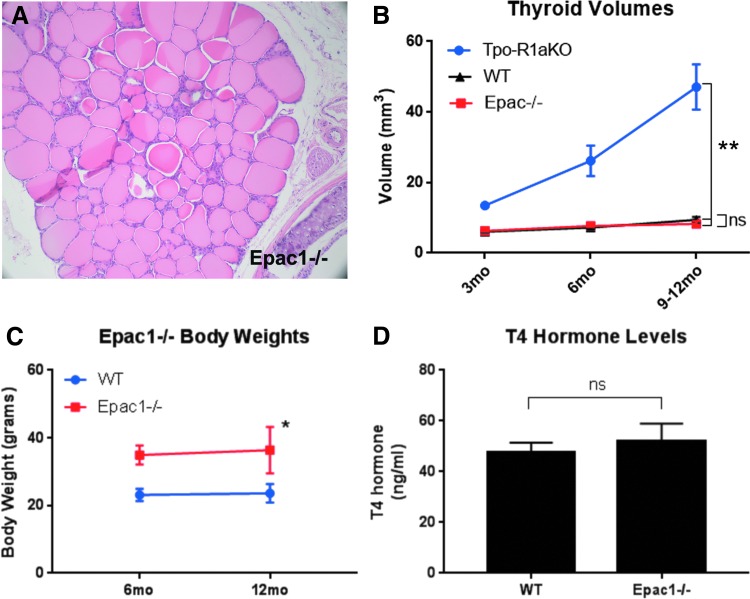
Epac1 is expressed in follicular cells of WT and Tpo-R1aKO thyroid tumors, as shown by immunohistochemistry and Western blot (Fig. 4A–C). Global Epac1–/– mice (25) were obtained to aid in the elucidation of signaling mediators in the PKA-Rap1 carcinogenic cascade. Epac2 was not found to be expressed at detectable levels in the tumors (Fig. 4C and D), nor did Epac2 expression increase upon deletion of Epac1 (Fig. 4D). Epac1–/– thyroids exhibit normal histological features (Fig. 5A) and growth patterns, with volumes similar to WT littermate thyroids (8.26 mm3 for Epac1–/– vs. 9.41 mm3 for WT; p = 0.3915; Fig. 5B). Although Epac1–/– females (n = 4) were on average larger than their WT littermates (n = 4; 36.38 vs. 23.6 g at one year of age; p = 0.013; Fig. 5C), T4 levels were similar to WT animals (Fig. 5D). Therefore, the increased body weight did not seem to be related to altered thyroid function.
FIG. 4.
Epac1 is expressed in Tpo-R1aKO tumors. (A and B) Diaminobenzidine immunohistochemistry for Epac1 on thyroid tissue sections from WT (A) and Tpo-R1aKO (B) mice at 40 × magnification. (C) Western blot showing Epac1 and Epac2 expression in WT and Tpo-R1aKO mouse thyroids; Hela cells are shown as a positive control for Epac2 expression, FRTL5 cells are shown as a positive control for both Epac1 and Epac2 expression. (D) Quantitative polymerase chain reaction showing fold change in Epac2 gene expression in WT, Tpo-R1aKO, and Epac1–/– thyroids; WT mouse brain tissue was used as a positive control.
FIG. 5.
Epac1–/– mice have normal thyroids. (A) Hematoxylin and eosin staining of Epac1–/– thyroid at 20 × magnification. (B) Comparison of thyroid volumes from age-matched WT (black line), Epac1–/– (red line), and Tpo-R1aKO (blue line) mice measured by 3D ultrasound. (C) Body weights of Epac1–/– (red line) and WT (blue line) mice at 6 and 12 months of age. (D) T4 hormone levels of WT and Epac1–/– mice (p = 0.571). **p ≤ 0.001 at six and nine months of age Tpo-R1aKO versus Epac1KO or WT. *p ≤ 0.05 at both 6 and 12 months of age.
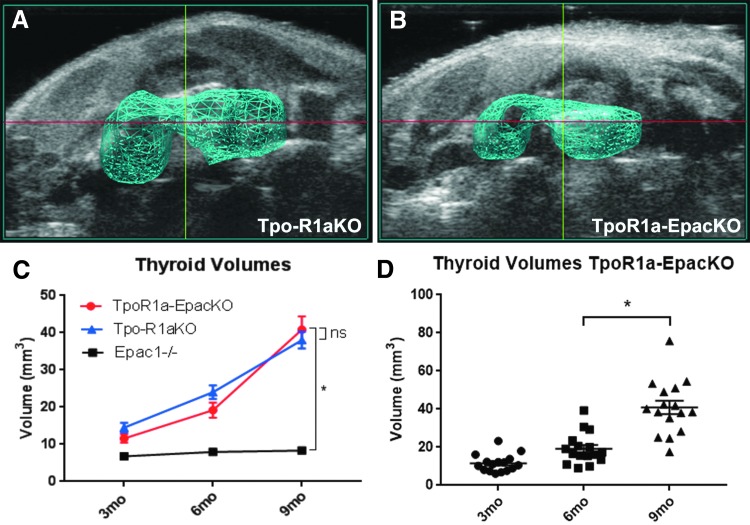
To determine whether Epac1 is involved in activation of Rap1 in Tpo-R1aKO mice, Epac1–/– mice were crossed with Tpo-R1aKO animals to produce Tpo-Cre;Prkar1aloxP/loxP;Epac1–/– mice, hereafter referred to as TpoR1a-EpacKO. Ultrasonography revealed that thyroids from TpoR1a-EpacKO mice had volumes comparable to littermate Tpo-R1aKO mice at all time points studied (Fig. 6A–D; 40.87 mm3 for TpoR1a-EpacKO vs. 38.02 mm3 for Tpo-R1aKO at nine months of age; p = 0.6397). Histological analysis also revealed similar incidences of carcinomas between the two groups (81.82% for Tpo-R1aKO vs. 85.71% for TpoR1a-EpacKO at nine months of age; p = 0.7737). These data suggest that Rap1 is functioning downstream of PKA independent of Epac1.
FIG. 6.
Epac1 is not required for tumor formation in TpoR1aKO mice. (A and B) 3D rendering of thyroids based on volumes determined by ultrasonography of Tpo-R1aKO (A) and TpoR1a-EpacKO (B) mice at nine months of age. (C) Thyroid volumes determined by ultrasound of TpoR1a-EpacKO (red line; n = 27), and littermate Tpo-R1aKO (blue line; n = 6) and cre-negative Prkar1aloxP/loxP;Epac1–/– control mice (black line; n = 23). (D) Scatter plot depicting thyroid volumes of TpoR1a-EpacKO mice at the indicated time points (p < 0.0001). *p ≤ 0.001. Graphs show mean and SEM.
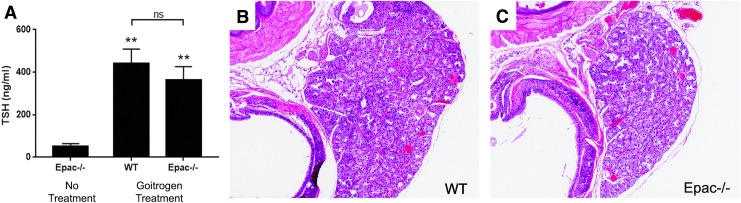
To probe the relative roles of Epac1 and PKA in this model further, Epac1–/– and WT littermates were treated with a goitrogenic protocol (9) to cause hypersecretion of TSH (Fig. 7A) and activation of the cAMP pathway upstream of PKA. After six months of treatment, the animals were sacrificed, and thyroids were collected for histological analysis. It was found that both groups developed thyroid hyperplasia that was histologically indistinguishable from one another (Fig. 7B and C), indicating that Epac1 likewise does not play a role in chronic TSH-mediated thyroid cell proliferation.
FIG. 7.
Epac1 is not involved in thyrotropin (TSH)-mediated thyroid hyperplasia. (A) Serum TSH levels in untreated and methimazole-treated mice (p = 0.0002 WT treated vs. untreated; p = 0.0009 Epac–/– treated vs. untreated; p = 0.415 WT treated vs. Epac–/– treated). (B and C) Hematoxylin and eosin–stained thyroid sections from WT (B) and Epac1–/– (C) mice after six months of continuous methimazole treatment. **p ≤ 0.001 compared to no treatment.
Discussion
As incidence rates of thyroid cancer have continued to rise at the fastest rate of all malignancies, the study of signaling pathways involved in thyroid tumorigenesis remain relevant to aid in the development of novel therapeutics. This study observed that Rap1 plays an important role in thyroid carcinogenesis arising from over-activation of the PKA pathway. Using targeted-deletion mouse modeling, it was determined that ablation of Rap1, specifically the Rap1b isoform, reduces the risk of developing thyroid cancer as a result of Prkar1a KO.
Previous work has established an oncogenic role of Rap1b resultant from cAMP stimulation. Although Rap1b activation alone is not sufficient to produce thyroid cancer in mice, it is still thought to be a major player in mitogenic signal transduction, as its activity is associated with many human cancers, including thyroid cancer (9,14–17). Using an established mouse model of PKA-dependent follicular thyroid cancer, the function of Rap1 in in vivo tumor development was probed. It was found that deletion of Rap1a and Rap1b in the setting of PKA over-activation resulted in a 65% reduction in incidence of thyroid carcinoma. Further, isoform specific knockout revealed that Rap1b deletion alone produced similar results to deleting both isoforms together, indicating that Rap1b is important for tumor formation. Although Rap1 ablation was sufficient to suppress tumor formation significantly, the carcinogenic phenotype was not completely rescued, implying that more complex signaling interactions may be at play.
Previously, interactions between PKA, Rap1, and Epac1 during thyroid carcinogenesis were unclear. Some studies have shown that both PKA and Epac signal to Rap1 downstream of TSH (13,18). However, the nature of these interactions seems to be cell context-dependent (21,22). Here, it was demonstrated that PKA is acting on downstream target Rap1 to promote in vivo thyroid carcinogenesis independently of Epac1, as deletion of Epac1 in a Prkar1a KO background had no effect on tumor growth. To discern further whether Epac1 may have an antagonistic relationship with PKA, the cAMP pathway was stimulated upstream of PKA in WT and Epac–/– mice by inducing secretion of excess TSH through treatment with antithyroid drugs. As no significant differences were observed between groups, it was concluded that Epac1 is not directly involved in PKA-Rap1-associated thyroid tumorigenesis. These findings are supported by others who have shown that PKA can directly regulate Rap proteins using a specific phosphorylation site at serine 180 on Rap1a and serine 179 on Rap1b (27). Phosphorylation of Rap by PKA has been shown to regulate its subcellular localization, as well as its downstream action, including activation of certain downstream effectors such as ERK and Rap-dependent regulation of cell migration (28,29). These previously published data show that PKA has a direct mechanism of controlling Rap action and downstream cellular processes and, taken together with the present results, suggest that the PKA-Rap1 pathway can operate independently of Epac1 in thyroid cancer initiation and progression.
The functional role of Epac1 in tumorigenesis remains elusive. Despite robust upregulation of Epac1 expression in Tpo-R1aKO mice, these studies do not reveal a causative role for Epac in PKA-mediated thyroid tumor formation. Numerous in vitro studies have also noted increased Epac1 expression under tumor promoting conditions. However, similarly to the present observations in vivo, Rap1 activation was not dependent on Epac1 in most thyroid cancer cell lines (20,30). These findings do not exclude an indirect or supportive role of Epac1 in tumor growth, rather the often-opposing results of PKA/Epac-Rap pathway activation in different cell types is consistent with this theory.
In conclusion, the data indicate that PKA signaling to Rap1b is a key signaling node for follicular thyroid carcinogenesis. Epac has been shown to activate Rap proteins in multiple systems, although the data presented here indicate that Epac activity is dispensable for thyroid tumorigenesis in vivo. Interestingly, although they are both signaling molecules implicated in the TSH/cAMP/PKA pathway, neither Epac nor Rap1 appear to be required for normal thyroid morphology or function. These studies should help shed further light on oncogenic signaling.
Supplementary Material
Acknowledgments
We would like to thank Alexei Morozov for sharing the Rap1aloxP/loxP;Rap1bloxP/loxP mouse model, and Xiaoli Zhang for statistical analyses. This work was supported by NIH R01CA170249, P01CA124570 (L.S.K.)m and a Pelotonia Postdoctoral Fellowship Award (D.H.). A.A. and A.M. were also supported Pelotonia fellowships for graduate students and undergraduates, respectively. The Hormone Assay and Analytical Services Core at Vanderbilt University is supported by NIH DK059637 and DK020593.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besic N, Auersperg M, Golouh R. 1999. Prognostic factors in follicular carcinoma of the thyroid—a multivariate survival analysis. Eur J Surg Oncol 25:599–605 [DOI] [PubMed] [Google Scholar]
- 3.Carney JA, Hruska LS, Beauchamp GD, Gordon H. 1986. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc 61:165–172 [DOI] [PubMed] [Google Scholar]
- 4.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pringle DR, Yin Z, Lee AA, Manchanda PK, Yu L, Parlow AF, Jarjoura D, La Perle KM, Kirschner LS. 2012. Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocr Relat Cancer 19:435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledent C, Dumont JE, Vassart G, Parmentier M. 1992. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. EMBO J 11:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiger MA, Saji M, Gusev Y, Westra WH, Takiyama Y, Dooley WC, Kohn LD, Levine MA. 1997. Thyroid-specific expression of cholera toxin A1 subunit causes thyroid hyperplasia and hyperthyroidism in transgenic mice. Endocrinology 138:3133–3140 [DOI] [PubMed] [Google Scholar]
- 8.Michiels FM, Caillou B, Talbot M, Dessarps-Freichey F, Maunoury MT, Schlumberger M, Mercken L, Monier R, Feunteun J. 1994. Oncogenic potential of guanine nucleotide stimulatory factor alpha subunit in thyroid glands of transgenic mice. Proc Natl Acad Sci U S A 91:10488–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro-Neto F, Leon A, Urbani-Brocard J, Lou L, Nyska A, Altschuler DL. 2004. cAMP-dependent oncogenic action of Rap1b in the thyroid gland. J Biol Chem 279:46868–46875 [DOI] [PubMed] [Google Scholar]
- 10.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. 2008. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 93:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargadine HR, Lowenstein JM, Greenspan FS. 1970. Elevated serum TSH in human thyroid cancer. Oncology 24:172–180 [DOI] [PubMed] [Google Scholar]
- 12.Parnell E, Palmer TM, Yarwood SJ. 2015. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci 36:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. 2001. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol 21:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutmann DH, Saporito-Irwin S, DeClue JE, Wienecke R, Guha A. 1997. Alterations in the rap1 signaling pathway are common in human gliomas. Oncogene 15:1611–1616 [DOI] [PubMed] [Google Scholar]
- 15.Chevillard S, Ugolin N, Vielh P, Ory K, Levalois C, Elliott D, Clayman GL, El-Naggar AK. 2004. Gene expression profiling of differentiated thyroid neoplasms: diagnostic and clinical implications. Clin Cancer Res 10:6586–6597 [DOI] [PubMed] [Google Scholar]
- 16.Hattori M, Minato N. 2003. Rap1 GTPase: functions, regulation, and malignancy. J Biochem 134:479–484 [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Chuang HC, Huang CC, Fang FM, Huang HY, Tsai HT, Su LJ, Shiu LY, Leu S, Chien CY. 2013. Overexpression of Rap-1A indicates a poor prognosis for oral cavity squamous cell carcinoma and promotes tumor cell invasion via Aurora-A modulation. Am J Pathol 182:516–528 [DOI] [PubMed] [Google Scholar]
- 18.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. 1998. A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- 20.Dremier S, Milenkovic M, Blancquaert S, Dumont JE, Døskeland SO, Maenhaut C, Roger PP. 2007. Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology 148:4612–4622 [DOI] [PubMed] [Google Scholar]
- 21.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. 2013. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Ji Z, Tsalkova T, Mei F. 2008. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 40:651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Luo C, Cheng X, Lu M. 2017. Lithium and an EPAC-specific inhibitor ESI-09 synergistically suppress pancreatic cancer cell proliferation and survival. Acta Biochim Biophys Sin (Shanghai) 49:573–580 [DOI] [PubMed] [Google Scholar]
- 24.Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. 2008. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci 28:2089–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. 2010. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem 285:24248–24259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones GN, Tep C, Towns WH, 2nd, Mihai G, Tonks ID, Kay GF, Schmalbrock PM, Stemmer-Rachamimov AO, Yoon SO, Kirschner LS. 2008. Tissue-specific ablation of Prkar1a causes schwannomas by suppressing neurofibromatosis protein production. Neoplasia 10:1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi M, Li Y, Dillon TJ, Stork PJ. 2017. Phosphorylation of Rap1 by cAMP-dependent protein kinase (PKA) creates a binding site for KSR to sustain ERK activation by cAMP. J Biol Chem 292:1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Dillon TJ, Liu C, Kariya Y, Wang Z, Stork PJ. 2013. Protein kinase A-dependent phosphorylation of Rap1 regulates its membrane localization and cell migration. J Biol Chem 288:27712–27723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. 2006. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol 26:2130–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broecker-Preuss M, Baten J, Sheu-Grabellus SY, Görges R, Bockisch A, Schmid KW, Führer D, Mann K. 2015. Expression of the cAMP binding protein EPAC1 in thyroid tumors and growth regulation of thyroid cells and thyroid carcinoma cells by EPAC proteins. Horm Metab Res 47:200–208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.