Abstract
The administration of neoadjuvant chemotherapy (NAC) preceding radical cystectomy benefits overall survival for patients with muscle-invasive bladder cancer (MIBC). However, the relationship between the genetic profiling of MIBC and NAC response remains unclear. Here, a mutation panel of six cancer-associated genes (TSC1, FGFR3, TERT, TP53, PIK3CA and ERBB2) and an immunohistochemistry (IHC) panel containing eight bladder cancer (BC) biomarkers (EGFR, RRM1, PD-L1, BRCA1, TUBB3, ERCC, ERCC1, aberrantly glycosylated integrin α3β1 (AG) and CK5/6) were developed. BC samples from patients who showed a pathologic response (n = 39) and non-response (n = 13) were applied to the panel analysis. ERBB2, FGFR3 and PIK3CA exclusively altered in the responders group (19/39, 48.7%), in which FGFR3 mutations were significantly enriched in patients with a response in the cohort (14/39, 35.9%; P = 0.01). Additionally, strong expression of ERCC1 was associated with a pathologic response (P = 0.01). However, positive lymph node metastasis (P < 0.01) and lymph-vascular invasion (LVI) (P = 0.03) were correlated with a non-response. Overall, the data show that FGFR3 mutations and elevated expression of ERCC1 in MIBCs are potential predictive biomarkers of the response to NAC.
Keywords: Bladder cancer, Neoadjuvant chemotherapy, FGFR3, ERCC1
Highlights
-
•
FGFR3 alterations are correlated with the pathologic response of MIBC patients to NAC.
-
•
Strong ERCC1 expression in MIBC patients is associated with a pathologic NAC response.
-
•
Lymph node metastasis and lymph-vascular invasion associated with non-response to NAC in MIBC patients.
Patients with muscle-invasive bladder cancers (MIBCs) are likely progressed to metastasis and have a five-year survival <50%. Neoadjuvant chemotherapy (NAC) has been demonstrated to have an overall survival benefit of 5–8% for MIBC patients. However, the relationship between genetic background and MIBC chemosensitivity remains debatable. Yang et al. identified that the mutation of FGFR3 and the elevated expression of ERCC1 were correlated with NAC response in MIBC patients.
Research in Context.
Patients with muscle-invasive bladder cancers (MIBCs) are likely progressed to metastasis and have a five-year survival <50%. Neoadjuvant chemotherapy (NAC) has been demonstrated to have an overall survival benefit of 5-8% for MIBC patients. However, the relationship between genetic background and MIBC chemosensitivity remains debatingable. Yang et al. identified that the mutation of FGFR3 and the elevated expression of ERCC1 were correlated with NAC response in MIBC patients.
1. Introduction
Bladder cancer (BC) is one of the most common urological malignancies worldwide [1] (Ferlay, 2012) with an estimated 429,800 new cases and 165,100 deaths per year [2]. BC is clinically diagnosed into two major subtypes, non-muscle-invasive bladder cancers (NMIBCs) and muscle-invasive bladder cancers (MIBCs) [3]. NMIBCs have a low rate of progression to invasion (10%–15%) but show a high rate of recurrence (50–70%), and the five-year survival is ~90% [3]. MIBCs (stage T2 and above) have a less favorable prognosis, with a five-year survival <50% and a common progression to metastasis. Radical cystectomy with pelvic lymph node dissection remains the standard treatment, which has not improved for several decades, and new approaches to systemic therapy are urgently needed [2, 3].
To address recurrence and metastasis of bladder cancer, the concept of neoadjuvant chemotherapy (NAC) has evolved. After two to four cycles of chemotherapy, patients received surgery. The results of randomized and prospective studies demonstrated an overall survival (OS) benefit of 5–8% [[4], [5], [6]] compared with surgery alone. Although NAC improves pathological down-staging and OS, approximately only 15–40% of patients achieved a pathological response, defined as the absence of muscle-invasive disease and lymph node metastasis (<pT2 and pN0) [7]. Nonresponding patients, who are unlikely to derive a clinical benefit, are exposed to substantial toxicity and the potential delay of surgery [3, 5]. The identification of predictive NAC response biomarkers is critical to providing precision medicine to patients with MIBCs. Here, we demonstrated that mutation of FGFR3 and strong expression of ERCC1 are correlated with NAC response in MIBC patients.
2. Materials and Methods
2.1. Study Design and Participants
Fifty-two MIBC patients were randomly selected for this NAC study between 2008 and 2017 from Renji Hospital, School of Medicine, Shanghai Jiao Tong University. Conventional NAC with a 21 d cycle of cisplatin and gemcitabine was administered to all patients in the study. Patients received cisplatin on d1 and gemcitabine on d1 and d8. Fifty-two patients were included in the cohort, in which 39 patients showed a pathologic response (partial response: ypT1, ypTa or ypTcis, n = 33; complete response: ypT0N0, n = 6), and 13 patients displayed a non-response (≥ ypT2).
2.2. Sample Preparation
The primary BC and matched peripheral blood samples for the cohort were obtained from Renji Hospital, School of Medicine, Shanghai Jiao Tong University with informed consent and approval by the Research Ethics Board of Shanghai Jiao Tong University. The genomic DNA from the tumor and matched peripheral blood samples was isolated according to the manufacturer's protocol (QIAGEN). Semi-quantitative PCR of TERT, FGFR3, TP53, PIK3CA, ERBB2 and TSC1 were performed using the former DNA templates with the primer sequences listed in Supplementary Table 1. All PCR products were examined by Sanger sequencing, and the putative SNPs for BC samples were selected according to the reference sequence of the matched peripheral blood samples.
2.3. Immunohistochemistry (IHC)
IHC was performed as previously described [8]. Briefly, sections (4 μm) were deparaffinized and rehydrated. After antigen retrieval, the sections were treated with 3% H2O2 solution, and incubated with 10% bovine serum albumin for 30 min. Then, the sections were incubated with the primary antibody (EGFR, RRM1, PD-L1, BRCA1, TUBB3, ERCC1, aberrantly glycosylated integrin α3β1 (AG) or CK5/6) at 4 °C overnight, and incubation with corresponding secondary antibody and subsequently staining with a DAB kit (ZSGB Bio) were performed. The nucleus was counterstained with hematoxylin. The staining intensity was assessed by two independent experienced genitourinary pathologists using a 0–3 scoring system.
2.4. FGFR3 Mutation Status in Multiple Independent Cohorts
The FGFR3 mutation status in The Cancer Genome Atlas (TCGA) urothelial bladder cancer dataset was determined using the TCGA data portal (http://cancergenome.nih.gov). The mutation frequencies of FGFR3 in the Kim et al. and Guo et al. studies were obtained from the cBioPortal for Cancer Genomics (http://www.cbioportal.org).
2.5. Statistical Analysis
The correlation between genetic alterations and NAC response was analyzed using a Fisher's exact test. Analysis of the genetic alterations found in TERT, FGFR3, TP53, PIK3CA, ERBB2 and TSC1, and the expression of EGFR, RRM1, PD-L1, BRCA1, TUBB3, ERCC1, AG and CK5/6 were performed using the Benjamini-Hochberg method and GraphPad Prism software version 5. The patient demographics, tumor characteristics and pathological findings were analyzed using the Mann-Whitney U test or Fisher's exact test. In the survival analysis, average FGFR3 expression was calculated initially. BC samples with high FGFR3 expression were defined as the high group, and the remaining samples were defined as the low group. The OS of each group was analyzed using a Kaplan-Meier analysis, and the difference between the two groups was examined using the log-rank test [9]. A P value <0.05 (*, P < 0.05; **, P < 0.01; and ***, P < 0.001) was regarded as statistically significant.
3. Results
3.1. Mutational Analysis of MIBCs Using a Six Gene Panel
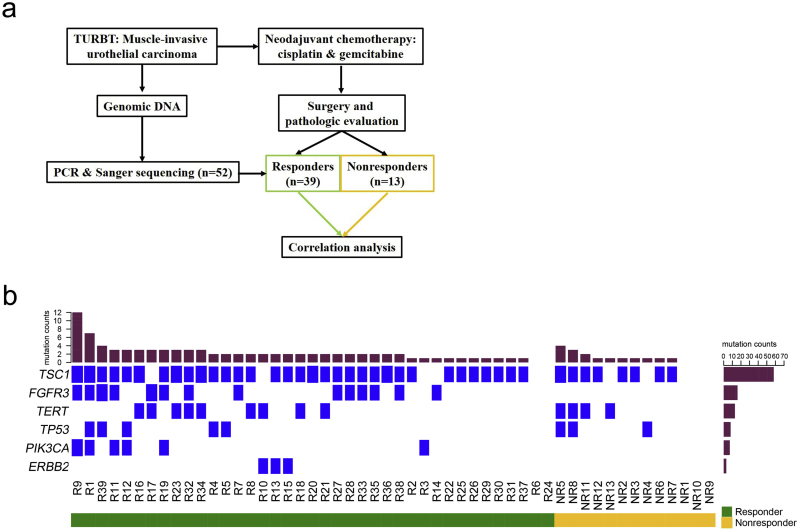
Fifty-two MIBC patients were enrolled in this study to receive NAC. Six patients showed a complete response (ypT0N0), 33 patients displayed a partial response (ypT1/a/cis) and 13 patients were resistant (≥ ypT2) to NAC (Fig. 1A, Table 1 and Supplementary Table 2). The pretreatment tumor DNA from each sample was analyzed using a mutational panel of six genes (TSC1, FGFR3, TERT, TP53, PIK3CA and ERBB2) associated with tumorigenesis and drug resistance in BC. Divided into two groups, the mutational spectrum of the response (complete response & partial response) and non-response samples were depicted in a heat map (Fig. 1B).
Fig. 1.
Study design and mutation rates of key genes in fifty-two muscle-invasive bladder cancer patients. a. Fifty-two patients were split into responders and nonresponders based on their pathologic response to neoadjuvant chemotherapy. TURBT, transurethral resection of bladder tumor. b. The alteration landscape of the aggregate cohort (n = 52 patients) are displayed in the center. Each column represents a tumor, and each row represents a gene. TERT, FGFR3, TP53, PIK3CA, ERBB2 and TSC1 are listed on the left and the center panel is divided into responders (left and green) and nonresponders (right and orange). The mutation rates (top) and mutational frequency (left) are also summarized.
Table 1.
Clinical characteristics of the bladder carcinoma patients.
| Total (52) | Nonresponder (13) | Responder (39) | P value | |
|---|---|---|---|---|
| Female | 10 | 0 | 10 | 0.175 |
| Age | 62.6 | 62.9 | 62.5 | 0.857 |
| Follow-up | 10.0 | 5.2 | 11.5 | 0.020 |
| pT > 1 | 11 | 11 | 0 | < 0.001 |
| pN > 0 | 4 | 4 | 0 | 0.003 |
| pCIS = 1 | 15 | 1 | 14 | 0.078 |
| LV1 > 0 | 6 | 4 | 2 | 0.029 |
| OS = 1 | 4 | 1 | 3 | 1.000 |
| EGFR > 1 | 50 | 12 | 38 | 1.000 |
| RRM1 > 1 | 46 | 10 | 36 | 0.580 |
| PD-L1 > 1 | 44 | 12 | 32 | 0.560 |
| BRCA1 > 1 | 48 | 10 | 38 | 0.134 |
| TUBB3 > 1 | 51 | 12 | 39 | 1.000 |
| ERCC1 > 1 | 48 | 9 | 39 | 0.011 |
| BCMab1 > 1 | 30 | 5 | 25 | 0.196 |
| CK5/6 > 1 | 0 | 0 | 0 | 1.000 |
3.2. FGFR3 Mutations Correlated with the Response to Neoadjuvant Chemotherapy
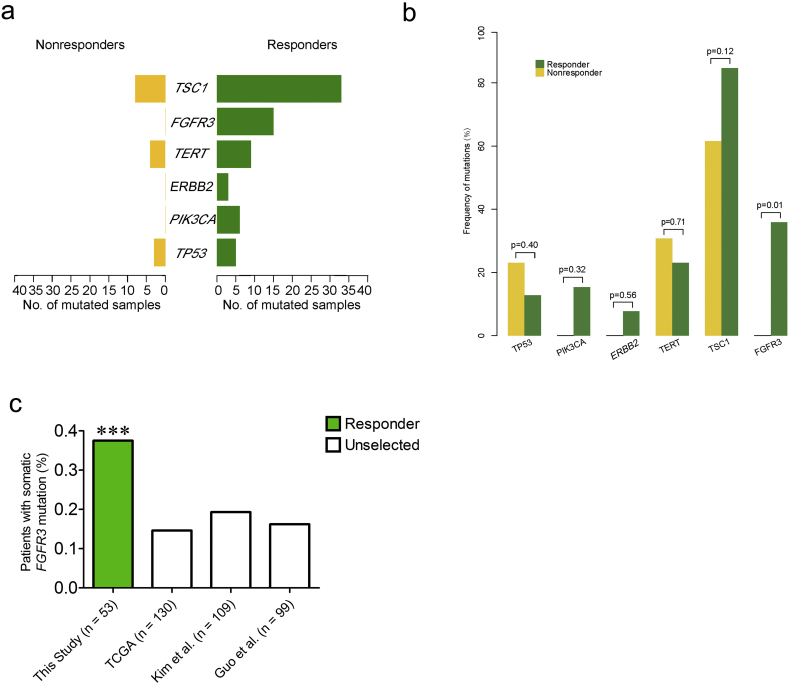
To identify the distinct altered genes between responders and nonresponders, genes with different mutation frequencies were uncovered using contrast analysis (Fig. 2A). FGFR3 (14/39, 35.9%), PIK3CA (6/39, 15.4%) and ERBB2 (3/39, 7.7%) exclusively altered in the responder group (Fig. 1B and 2A). The mutations of FGFR3, PIK3CA and ERBB2 were significantly enriched in the responder group (48.7% of cases, 19/39; Fig. 2B, P < 0.01).
Fig. 2.
FGFR3 significantly altered in the responder group of muscle-invasive bladder cancer patients. a and b. FGFR3, ERBB2 and PIK3CA somatic mutations exclusively occurred in the responder group. Only FGFR3 demonstrated significant enrichment in patients with a response in the cohort. c. FGFR3 somatic mutations were significantly enriched in the responder cohort compared with the unselected TCGA, Kim et al. and Guo et al. urothelial carcinoma cohorts.
Interestingly, FGFR3 exhibited a significant difference between two groups (Fig. 2B, P = 0.01). The somatic FGFR3 mutation frequency in the responder group was also compared with three unselected BC populations: 131 cases from TCGA [10], 109 cases from a United States patient cohort [11][10] and 99 cases from a Chinese patient cohort [12] (Fig. 2C). Compared with these unselected populations, FGFR3 mutations were significantly enriched in the responder group (14/39, 35.9% of cases; Fig. 2C, P < 0.001; binomial test). Specifically, FGFR3 mutated in 13 partial responders (13/33; nine pT1 and four pTcis) and one complete responder (1/6; pT0) in this cohort, and the mutation rate of FGFR3 showed no significant difference between the two groups (P = 0.39, Fisher test). These results suggested that FGFR3 alterations were associated with the response, especially the down-staging of BCs using NAC.
3.3. Somatic FGFR3 Mutations in Cisplatin-Based Chemotherapy Responders
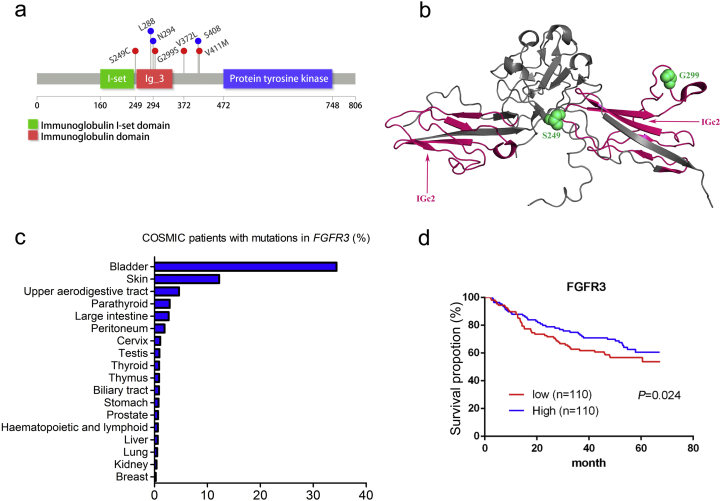
In our study, four missense FGFR3 mutations were found, including a well-known activating alteration c.746C > G (p.S249C) and three additional mutations (c.1114G > T, p.V372C; c.895G > A, p.G299S; c.1231G > A, p.V411 M) (Fig. 3A and B). To determine the relative abundance of somatic FGFR3 mutations in other tumor types, TCGA data from 19 tumor types (n = 4429) were queried [13]. Somatic FGFR3 mutations were observed at low frequencies (<5%) in 17 other tumor types except for BC and skin cancer (Fig. 3C). In the survival analysis, patients expressing higher levels of FGFR3 had a longer mean survival time than those expressing lower levels of FGFR3 (Fig. 3D). These results suggested that somatic mutations of FGFR3 could predict the pathological response of BC patients to NAC.
Fig. 3.
FGFR3 mutation mapping and distribution across tumor types. a. A stick plot of FGFR3 showing the locations of mutations in the responders. Red, somatic mutations. Blue, synonymous mutation. b. Structure of the immunoglobulin domain of FGFR3 (PDB code, 1RY7) with mutations identified in the responder cohort. c. The somatic FGFR3 mutation frequency in multiple tumor types from COSMIC. d. Kaplan-Meier curves comparing OS between BC patients expressing high or low levels of FGFR3 using the log-rank test. n, patient number.
3.4. Protein Expression Analysis of MIBCs Using an IHC Panel
The pretreatment tumor samples were analyzed with an IHC panel containing eight BC biomarkers (EGFR, RRM1, PD-L1, BRCA1, TUBB3, ERCC1, AG and CK5/6) (Supplementary Table 2). Among these biomarkers, only strong expression of ERCC1 was significantly correlated with the MIBC patient response to NAC (P < 0.05, Table 1). However, the expression of EGFR, RRM1, PD-L1, BRCA1, TUBB3, AG and CK5/6 was not associated with response (P > 0.05, Table 1).
3.5. Lymph Node Metastasis and Lymph-Vascular Invasion are Associated With Non-Response to NAC
The clinical characteristics including sex, age, OS and concomitant carcinoma in situ showed no significant differences between responders and nonresponders at baseline (P > 0.05; Mann-Whitney test) (Table 1). However, lymph node metastasis (pN) and lymph-vascular invasion (LVI) were correlated with a non-response (P < 0.05; Mann-Whitney test) (Table 1).
4. Discussion
NAC is emerging as an effective treatment for MIBCs. In the clinic, NAC can shrink tumor size and restrain tumor metastasis, as well as contributing to tumor down-staging and patient OS [2]. In this study, FGFR3, PIK3CA and ERBB2 exclusively altered in the response cohort, in which the mutation of FGFR3 was significantly enriched in the responder group. Additionally, strong expression of ERCC1 was significantly correlated with the MIBC response to NAC. However, pN and LVI were correlated with a non-response.
MIBCs are divided into basal, luminal and p53-like subtypes according to their molecular signature. The relationship between molecular subtype and chemosensitivity remains debatable [3]. McConkey et al. and Seiler et al. revealed an absolute survival benefit from NAC in patients with basal subtype tumors [14, 15]. However, Choi et al. reported that p53-like MIBCs were consistently resistant to neoadjuvant MVDC, while basal and luminal types showed no significant difference in drug sensitivity to NAC [16]. Our previous study found a significant survival benefit conferred to patients with the luminal subtype of MIBC that received NAC [17]. In this study, MIBCs with FGFR3 mutations displayed a response to NAC (cisplatin and gemcitabine) that represented the luminal type of MIBCs. Consistent with our results, Rosenberg et al. reported that the response to atezolizumab was significantly greater in the TCGA luminal cluster II subtype than in the other subtypes (34% versus 10% for subtype I, 16% for subtype III and 20% for subtype IV) in the IMvigor 210 cohort 2 trial [18]. A possible reason for this discrepancy might result from the different subtyping method of BCs and the distinct combination of the drugs applied during NAC.
Previous studies have indicated that the alteration of ERCC2 was significantly enriched in BC patients who responded to cisplatin [19]. Groenendijk et al. demonstrated that ERBB2 missense mutations exclusively occurred in responders [20]. Furthermore, Plimack et al. reported that defects in DNA repair genes (ATM, RB and FANCC) predicted the response to neoadjuvant cisplatin-based chemotherapy in MIBC [21]. FGFR3 belongs to the fibroblast growth factor receptor (FGFR) family and regulates cellular proliferation, migration and differentiation. The deregulation of FGFR3 and its receptors is correlated with the pathogenesis of many cancers originating from different tissues [22]. Activating FGFR3 mutations frequently occur in BC and were reported to correlate with drug sensitivity in lung adenocarcinoma [23]. Here, we identified that FGFR3 exclusively altered in the responder cohort (14/39, 35.9%), with one well-known activating mutation (c.746C > G) and three additional alterations (c.1114G > T, c.895G > A and c.1231G > A). Additionally, PIK3CA and ERBB2 altered exclusively in the responders, a finding that requires validation in a larger cohort. In conclusion, our findings demonstrate that FGFR3 mutations could predict the pathologic response of BC patients to NAC and provide a promising biomarker to aid clinicians in treatment decisions.
Acknowledgments
Acknowledgments
We would like to thank Professor Fengjiao Xin (Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences) for her technical supports.
Funding Sources
This work was supported by the National Natural Science Foundation of China (81602644, 81672956, 81472413, 81672514, 81660422 and 81660423), Shanghai Natural Science Foundation (16ZR1420300) and the Foundation of Shanghai Hospital Development Center (SHDC12015125).
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
C.H. and L.C. designed and supervised the study. K.X., Z.X. and W.H. searched literature. Z.R. and C.H. developed the methodology. Y.Z., Q.X., G.Y., Q.X., W.Y. and S.C. performed the experiments and analyzed the data. Y.Z., Z.R. and L.C. wrote and reviewed of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.06.011.
Contributor Information
Haige Chen, Email: chenhaige011435@renji.com.
Chong Li, Email: lichong@moon.ibp.ac.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Glaser A.P., Fantini D., Shilatifard A., Schaeffer E.M., Meeks J.J. The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol. 2017 doi: 10.1038/nrurol.2017.11. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen D.P., Thalmann G.N. Contemporary update on neoadjuvant therapy for bladder cancer. Nat Rev Urol. 2017;14:348–358. doi: 10.1038/nrurol.2017.30. [DOI] [PubMed] [Google Scholar]
- 3.Czerniak B., Dinney C., McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149–174. doi: 10.1146/annurev-pathol-012513-104703. [DOI] [PubMed] [Google Scholar]
- 4.Sherif A., Holmberg L., Rintala E., Mestad O., Nilsson J., Nilsson S. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303. doi: 10.1016/j.eururo.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Advanced Bladder Cancer Meta-Analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaborationEur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. (discussion 5-6) [DOI] [PubMed] [Google Scholar]
- 6.Grossman H.B., Natale R.B., Tangen C.M., Speights V.O., Vogelzang N.J., Trump D.L. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 7.Zargar H., Espiritu P.N., Fairey A.S., Mertens L.S., Dinney C.P., Mir M.C. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67:241–249. doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Du Y., Yang Z., He L., Wang Y., Hao L. GALNT1-mediated glycosylation and activation of sonic hedgehog signaling maintains the self-renewal and tumor-initiating capacity of bladder cancer stem cells. Cancer Res. 2016;76:1273–1283. doi: 10.1158/0008-5472.CAN-15-2309. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z., He L., Lin K., Zhang Y., Deng A., Liang Y. The KMT1A-GATA3-STAT3 circuit is a novel self-renewal signaling of human bladder cancer stem cells. Clin Cancer Res. 2017;23:6673–6685. doi: 10.1158/1078-0432.CCR-17-0882. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim P.H., Cha E.K., Sfakianos J.P., Iyer G., Zabor E.C., Scott S.N. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur Urol. 2015;67:198–201. doi: 10.1016/j.eururo.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo G., Sun X., Chen C., Wu S., Huang P., Li Z. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45:1459–1463. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConkey D.J., Choi W., Shen Y., Lee I.L., Porten S., Matin S.F. A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur Urol. 2016;69:855–862. doi: 10.1016/j.eururo.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiler R., Ashab H.A.D., Erho N., van Rhijn B.W.G., Winters B., Douglas J. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017;72:544–554. doi: 10.1016/j.eururo.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Choi W., Porten S., Kim S., Willis D., Plimack E.R., Hoffman-Censits J. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R., Chen H., Xia J., Shi O., Cao M., Jin D. The pathological and clinical response of the luminal and basal subtypes of muscle-invasive bladder cancer to neoadjuvant cisplatin-based chemotherapy and radical cystectomy depend on the immunohistochemical classification system. Eur Urol Suppl. 2017;16:e303–e304. [Google Scholar]
- 18.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Allen E.M., Mouw K.W., Kim P., Iyer G., Wagle N., Al-Ahmadie H. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–1153. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groenendijk F.H., de Jong J., Fransen van de Putte E.E., Michaut M., Schlicker A., Peters D. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2016;69:384–388. doi: 10.1016/j.eururo.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Plimack E.R., Dunbrack R.L., Brennan T.A., Andrake M.D., Zhou Y., Serebriiskii I.G. Defects in dna repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2015;68:959–967. doi: 10.1016/j.eururo.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner N., Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 23.Chandrani P., Prabhash K., Prasad R., Sethunath V., Ranjan M., Iyer P. Drug-sensitive FGFR3 mutations in lung adenocarcinoma. Ann Oncol. 2017;28:597–603. doi: 10.1093/annonc/mdw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material