Abstract
Bromodomain and extraterminal (BET) domain containing protein (BRD)-4 modulates the expression of oncogenes such as c-myc, and is a promising therapeutic target in diverse cancer types. We performed pre-clinical studies in myeloma models with bi-functional protein-targeting chimeric molecules (PROTACs) which target BRD4 and other BET family members for ubiquitination and proteasomal degradation. PROTACs potently reduced the viability of myeloma cell lines in a time- and concentration-dependent manner associated with G0/G1 arrest, reduced levels of CDKs 4 and 6, increased p21 levels, and induction of apoptosis. These agents specifically decreased cellular levels of downstream BRD4 targets, including c-MYC and N-MYC, and a Cereblon-targeting PROTAC showed downstream effects similar to those of an immunomodulatory agent. Notably, PROTACs overcame bortezomib, dexamethasone, lenalidomide, and pomalidomide resistance, and their activity was maintained in otherwise isogenic myeloma cells with wild-type or deleted TP53. Combination studies showed synergistic interactions with dexamethasone, BH3 mimetics, and Akt pathway inhibitors. BET-specific PROTACs induced a rapid loss of viability of primary cells from myeloma patients, and delayed growth of MM1.S-based xenografts. Our data demonstrate that BET degraders have promising activity against pre-clinical models of multiple myeloma, and support their translation to the clinic for patients with relapsed and/or refractory disease.
Keywords: PROTACs, BET protein family, BRD4, c-MYC, drug resistance, multiple myeloma
Introduction
Dysregulation of c-myc expression through chromosomal translocations was first discovered as a mechanism of tumorigenesis in Burkitt’s lymphoma1. Subsequent studies showed derangements of c-myc, and its relative N-myc, in other malignancies, including lymphomas, neuroblastomas, melanomas, and breast cancer, often through insertional mutagenesis or amplification1, 2. In multiple myeloma, translocations involving c-myc were originally detected in a small proportion of tumors3 and felt to be late events4. More recent studies, however, found c-myc rearrangements in almost half of cases, including in precursor states such as smoldering myeloma5. These rearrangements position c-myc near genes associated with super-enhancers that drive high levels of c-MYC expression5, 6, which has been associated with an adverse clinical prognosis in myeloma in at least some studies6–9, possibly due to a role for c-MYC in drug resistance10. Such data suggest that myeloma patients whose tumors bear c-myc aberrations may have high-risk disease for which novel therapies are needed11.
c-myc has been considered to be undruggable because of its role in proliferation of normal tissues, and its function through protein-protein interactions1, 2. Recent studies have identified a number of promising leads, including those targeting chromatin modifications associated with MYC-mediated transcriptional activation. Acetylation on lysine residues of nearby histone proteins is recognized and bound by Bromodomain and extra-terminal (BET) family proteins1, 2. This supported the development of JQ1, a thieno-triazolo-1,4-diazepine that displaced BRD4 from nuclear chromatin, and induced differentiation and growth arrest in BRD4-dependent tumors12. JQ1 down-regulated transcription of c-myc and its downstream targets, and produced potent anti-proliferative effects in myeloma models13. Subsequent studies identified the activity of the BET inhibitors JQ1 and OTX015 in a variety of malignancies, including leukemias, lymphomas, and NUT midline carcinomas14.
Beyond direct BRD4 inhibition, another approach could be to reduce BRD4 levels by promoting its degradation. This can be achieved through protein-targeting chimeric molecules (PROTACs), which combine an E3 ligase recognition sequence with a moiety that targets a protein of interest15. The chimera brings the target to an E3 ligase, catalyzing its ubiquitination and subsequent proteasome-mediated degradation16. Progress in this field was enabled by identification of the E3 ligase Cereblon (CRBN) as the binding partner for thalidomide17 and other immunomodulatory drugs (IMiDs)18. A phthalimide-derived moiety was linked with JQ1 to generate a molecule that directed Cereblon-dependent BET protein degradation (dBET1)19, 20. In models of human leukemia, dBET1 induced a rapid reduction of BRD4 and c-MYC, and activated apoptosis. Given the role of c-MYC in myeloma biology detailed earlier, this prompted us to test the possibility that BET-targeted PROTACs could be effective against myeloma. We therefore selected ARV-825 and ARV-763 for study, as these have been shown to potently and specifically induce BRD4 ubiquitination and degradation21. In the current report, we present data showing that such PROTACs are active against myeloma, overcome mechanisms of drug resistance, combine synergistically with conventional and novel therapeutics, and show activity in vivo.
Materials and Methods
Reagents and cell lines
Reagents and myeloma cell lines are detailed in the Supplementary Materials and Methods. Myeloma cells alone, or in co-culture with stromal cells22, were cultured as previously described23, and validated through The MD Anderson Cancer Center Characterized Cell Line Core. Drug resistant and p53 mutant counterparts were obtained, derived, and propagated as described previously24, 25.
Cell viability and apoptosis assays
These were performed as described in the Supplementary Materials and Methods.
Western blotting, RNA techniques, immunohistochemistry, immunofluorescence, and MDR1 efflux
Please consult the Supplementary Materials and Methods for details on these techniques.
Animal modeling
This was performed in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratory; Bar Harbor, ME) as detailed in the Supplementary Materials and Methods using protocols approved by the MD Anderson Institutional Animal Care and Use Committee.
Genomics and proteomics
Gene expression profiling (GEP)25 and reverse-phase protein array analyses (RPPA)26 were performed as described previously, with the latter through the MD Anderson RPPA Core Facility, and analyzed as described in the Supplementary Materials and Methods.
Statistical analyses
Wald’s chi-square test27 built in the “ESTIMATE” statement in the PROC GENMOD procedure was used to compare the differences of serum light chains between groups. The transformation of logarithm to the base 2 of light chains was used in the analyses to satisfy the normality assumption of the models. Bonferroni multiplicity adjustment was applied for multiple comparisons. Given that a total of 5 comparisons were conducted of the light chain data, a p-value <0.01 (0.05/5) was considered statistically significant. ANOVA27 was used for the comparisons of bone volume. Given that a total of 3 comparisons were conducted of bone volume, a p-value <0.017 (0.05/3) was considered statistically significant. SAS version 9.2 and S-Plus version 8.04 were used to carry out the computations for all analyses.
Results
BET-targeting PROTACs suppress proliferation and induce apoptosis
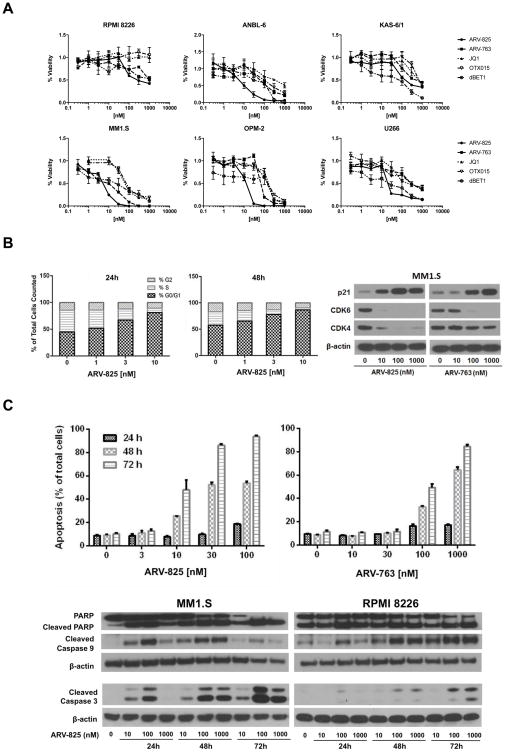
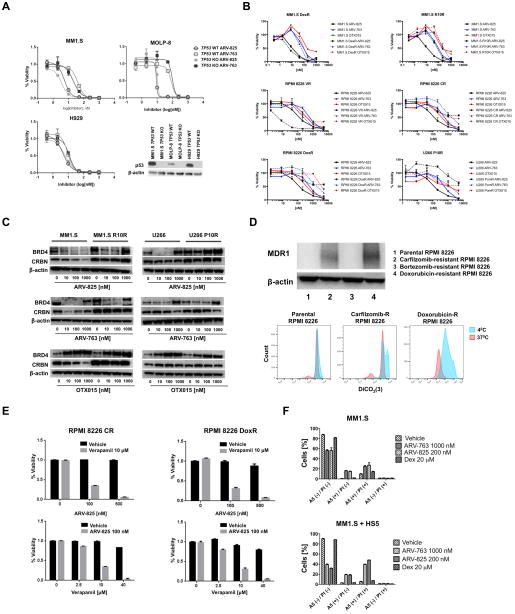
The anti-proliferative activity of the BET-targeting PROTACs ARV-825 and ARV-763 was evaluated in myeloma cell lines with c-myc translocations28. ARV-825 combines the BRD4-binding moiety of OTX015 with the CRBN-binding properties of pomalidomide20, while ARV-763 combines OTX015 with sequences that target VHL (Supplementary Figure 1). These PROTACs were active in all six lines tested, and decreased their viability in a dose-dependent manner (Figure 1A). Concurrent studies with the direct BET inhibitors JQ1 and OTX015 showed that the PROTACs were in general more potent, with lower median inhibitory concentrations (IC50’s). The same was also true, although to a lesser extent, when comparing these PROTACs to dBET1, with the exception of KAS-6/1 cells, where dBET1 demonstrated greater potency. In RPMI 8226 cells, for example, which were relatively resistant to JQ1 and OTX015 even at 10 μM, the PROTACs had an IC50 of 92 nM for ARV-825 and 1.52 μM for ARV-763, whereas the IC50 of dBET1 was 160 nM. MM1.S cells, which were more sensitive to BET-targeted agents, nonetheless showed an up to 10-fold differential effect, with an IC50 of 46.4 nM to JQ1, 59 nM to OTX015, and 84 nM for dBET1, while this was 5.7 and 13.2 nM for ARV-825 and ARV-763, respectively. Cell cycle analysis showed that ARV-825 induced a concentration- and time-dependent increase in G0/G1 phase cells, while the S-phase population dramatically decreased (Figure 1B; left, middle panels). Consistent with this finding, Cyclin-dependent kinase (CDK) 4 and CDK6 levels decreased with both PROTACs, while CDK inhibitor 1/p21 increased (Figure 1B; right panel). As part of cell cycle analysis, we found an increased proportion of sub-G0/G1 cells, suggesting the activation of apoptosis (not shown). Therefore, we performed staining with Annexin V, and detected enhanced phosphatidyl-serine externalization on MM1.S cells after exposure to ARV-825 or ARV-763 (Figure 1C; top panels), although, importantly, this was much less pronounced in human peripheral blood monocular cells from healthy donors (Supplementary Figure 2). Cell death occurred in association with increased poly-(ADP-ribose) polymerase (PARP) cleavage, and appearance of cleaved Caspases-9 and -3 (Figure 1C; bottom panels).
Figure 1. Cell cycle arrest and apoptosis induced by BET-specific PROTACs.
(A) Viability data obtained using the WST-1 assay are plotted with respect to concentrations of the CRBN- and VHL-targeted BET PROTACs ARV-825 and ARV-763, respectively, the direct BET inhibitors JQ1 and OTX015, and dBET1 after exposure to each agent for 72 hours. Data were collected in triplicate experiments and the mean ± the standard deviation (SD) were plotted for each point. (B) Left and middle panels: The concentration- and time-dependent effects of ARV-825 on cell cycle distribution were evaluated by flow cytometry after exposure of MM1.S cells to this agent under the indicated conditions. Data shown are representative from duplicate experiments. Right panel: Effects of ARV-825 and ARV-763 on the expression levels of p21, CDK4, and CDK6 were examined by Western blotting of MM1.S cell extracts 24 hours after exposure to the indicated drug concentrations of drugs, with β-actin used as a loading control. (C) Top panels: Induction of apoptosis was studied by Annexin V staining of MM1.S cells exposed to ARV-825 or ARV-763, followed by flow cytometry. Experiments were performed in triplicate and the mean ± SD is shown for the indicated conditions. Bottom panels: Programmed cell death activation was confirmed by preparing lysates of the indicated myeloma cell lines and subjecting them to Western blotting for cleaved PARP, Caspase-3 and -9, and β-actin as a loading control.
PROTACs reduce expression of BRD4, and downstream BRD4 targets
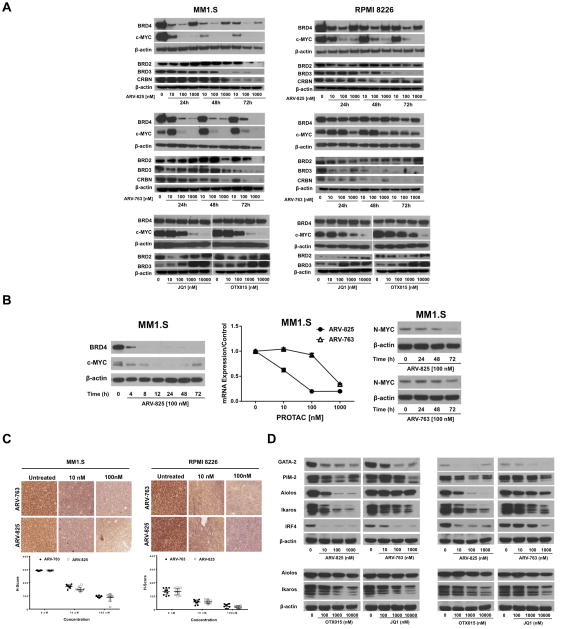
Since PROTACs were designed to target BRD4 for proteasomal degradation, we evaluated their impact on various BRD4-dependent proteins. MM1.S and RPMI 8226 cells were selected as representatives of strongly and weakly responding models, respectively. Both PROTACs induced a concentration- and time-dependent reduction of BRD4 and c-MYC expression (Figure 2A; top, middle panels), while JQ1 and OTX015 produced a modest increase in BRD4 (Figure 2A; bottom panels). BRD2 and BRD3 expression was also reduced (Figure 2A), although higher drug concentrations and longer exposures were necessary relative to BRD4. An increase in BRD4 levels was observed in cells treated with 1,000 nM PROTACs, probably due to competition for binding to their respective ligases between free and BET-bound PROTAC molecules20. JQ1 and OTX015 reduced c-MYC levels less potently, as concentrations of 1,000 nM were needed to quantitatively eliminate c-MYC expression, while the PROTACs did this at only 100 nM, though ARV-763 was less potent in RPMI 8226 cells. Notably, in MM1.S cells, ARV-825 at 100 nM reduced BRD4 and c-MYC levels by 50% after only 4 hours (Figure 2B; left panel). As expected, reduced BRD4 levels led to decreased abundance of the c-myc mRNA (Figure 2B; middle panel). Decreased intranuclear c-MYC staining was also confirmed using immunohistochemistry (Figure 2C) and immunofluorescence (Supplementary Figure 3) after 24-hour treatment with ARV-825 and ARV-763. Greater than 90% of untreated cell nuclei stained positive for c-MYC with moderate to strong intensity, while ≤50% of nuclei in drug-treated cells were c-MYC positive, and the magnitude of reduction was concentration-dependent (p<0.001).
Figure 2. BET-specific PROTACs induce degradation of BRD4 and c-MYC.
(A) The concentration- and time-dependent effects of ARV-825 (top panels) and ARV-763 (middle panels) on BRD4, c-MYC, BRD2, BRD3, and CRBN protein expression were studied by Western blotting in MM1.S and RPMI 8226 cells, with β-actin as a loading control. Also shown are data from these cell lines exposed for 24 hours to the BRD4 inhibitors JQ1 and OTX015 (bottom panels), with representative panels from one of two independent experiments shown for each. (B) Left panel: A time course experiment with earlier sampling points was performed in MM1.S cells exposed to ARV-825 at 100 nM. Middle panel: c-myc mRNA levels were determined by qRT-PCR in MM1.S cells exposed to ARV-825 or ARV-763 at the indicated concentrations for 24-hours. Right panel: N-MYC protein levels in MM1.S cells exposed to ARV-825 or ARV-763 were determined by Western blotting, with β-actin as a loading control. (C) Intranuclear c-MYC staining in MM1.S and RPMI 8226 cells exposed to vehicle, ARV-825, or ARV-763 determined by immunohistochemistry. The corresponding values of the intensity of c-MYC staining (H-score) are represented underneath the photomicrographs. (D) Impact of ARV-825 or ARV-763, and of JQ1 or OTX015, on the expression levels of other proteins of interest in MM1.S and RPMI 8226 cells examined by Western blotting, with β-actin serving as a loading control.
Since N-myc expression is influenced by BRD429, we looked at N-MYC in MM1.S cells, and this was also reduced (Figure 2B; right panel). GATA-binding protein 2 expression may suppress c-myc mRNA30, while PIM kinases inhibit c-MYC degradation31, but GATA-2 and PIM-2 levels were themselves decreased (Figure 2D), suggesting that these mechanisms did not contribute to the c-MYC changes. Finally, we looked at the impact of binding of ARV-825 to CRBN, and found that Aiolos and Ikaros levels were decreased, consistent with an activation of the CRBN E3 ligase, as was Interferon regulatory factor (IRF)-4 (Figure 2D). Interestingly, while the VHL-targeting ARV-763 was less potent than ARV-825 in reducing Ikaros, and especially Aiolos levels, it did show some activity in this regard, similar to OTX015 and JQ1.
Antagonistic effects of IMiDs and proteasome inhibitors with PROTACs
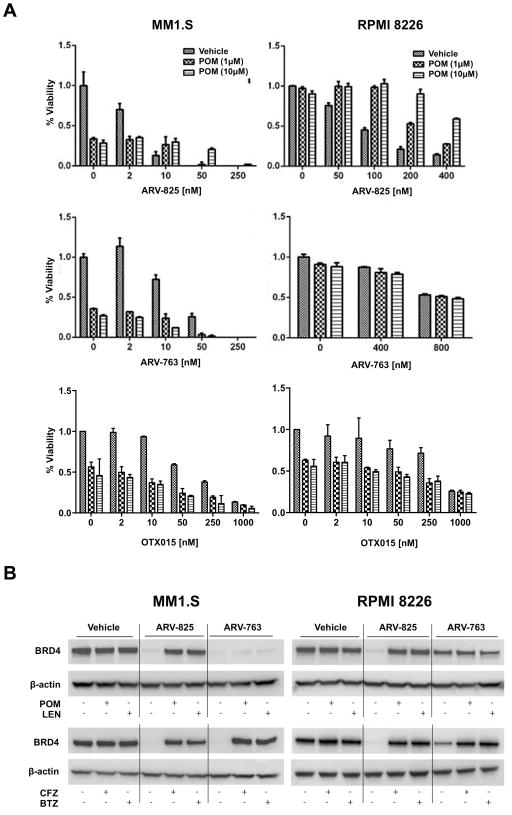
ARV-825 contains the pomalidomide Cereblon-binding moiety20, and we therefore considered the possibility that ARV-825 and pomalidomide could be antagonistic. To test this, we exposed MM1.S and RPMI 8226 cells to this PROTAC in the presence of vehicle or pomalidomide. There was no enhanced benefit when pomalidomide was combined with 2 nM ARV-825 compared to pomalidomide alone (Figure 3A; top panels). Indeed, at higher ARV-825 concentrations in MM1.S cells, and with all conditions evaluated in RPMI 8226 cells, the ARV-825/pomalidomide combinations were less active than ARV-825 alone. When the VHL-targeted PROTAC ARV-763 was used, in contrast, antagonistic effects were not seen (Figure 3A; middle panels), presumably because ARV-763 did not compete for the CRBN binding site of pomalidomide. Likewise, no antagonism was seen with OTX015 (Figure 3A; bottom panels) since it does not rely on E3 ubiquitin ligases for its efficacy. To test this further, we analyzed the effect of pomalidomide on the PROTAC-induced degradation of BRD4. As expected, pomalidomide and lenalidomide reversed ARV-825-induced degradation of BRD4 (Figure 3B; upper panels), while they had only a minimal impact on ARV-763. In contrast, the proteasome inhibitors carfilzomib and bortezomib32 antagonized the effects of both PROTACs (Figure 3B; lower panels), demonstrating their reliance on intact proteasome function.
Figure 3. Activity of PROTACs may be inhibited by immunomodulatory agents and proteasome inhibitors.
(A) The impact of ARV-825, ARV-763, OTX015, or pomalidomide (POM) alone, or in various combinations, was studied in MM1.S and RPMI 8226 cells under the indicated conditions. Cellular viability was determined after 72 hour experiments as detailed in the legend to Figure 1A. Data were collected from triplicate experiments and the mean ± SD are shown. (B) Expression levels of BRD4 in the presence of vehicle, 100 nM ARV-825, 250 nM ARV-763, or combinations of the PROTACs with either 10 μM POM or lenalidomide (LEN), or either 5 nM carfilzomib (CFZ) or bortezomib (BTZ) for 12 hours.
BET-targeted PROTACs suppress Akt/mTOR signaling
To further delineate the molecular basis of the anti-myeloma activity of these PROTACs, we performed GEP on MM1.S, U266, and RPMI 8226 cells (Gene expression omnibus accession pending). PROTAC-induced GEP changes were similar to those after treatment with the BET inhibitors (Supplementary Figure 4). The most up-regulated genes induced by ARV-825 in the three were histones (data not shown), and similar up-regulation was seen in response to ARV-763 in MM1.S and RPMI 8226 cells, though to a lesser extent in U266 cells. Gene set enrichment analysis (GSEA) performed on the three datasets revealed that c-myc-dependent signaling was the most significantly decreased (Supplementary Figure 5A). In contrast, a gene set up-regulated by mammalian target of rapamycin (mTOR) activation was one of the most enriched (Supplementary Figure 5B). Ingenuity Pathway Analysis (IPA) was then used to identify the five most perturbed gene networks by ARV-825. As expected, MYC and BRD4 were among the most common in MM1.S, RPMI 8226, and U266 cells). The remaining three were Hepatocyte nuclear factor 4 alpha (HNF4A), which has been associated with a super-enhancer33, and E2F Transcription Factor 1 (E2F1) and E2F4, E2F family members which were suppressed by BET inhibition34.
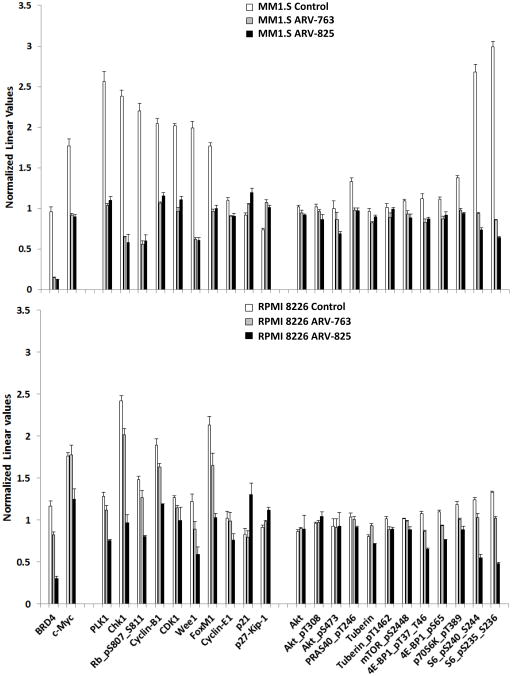
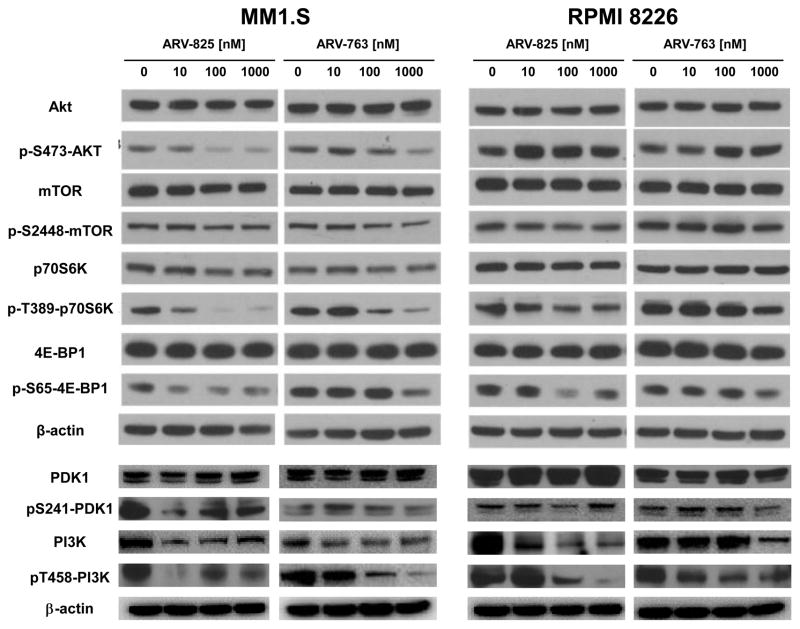
To reveal a broader effect of these PROTACs on cellular signaling networks, we performed RPPA analysis (Supplementary Figure 6). BRD4 and c-MYC were reduced by PROTACs in MM1.S and RPMI 8226 cells (Figure 4). Likely due to the role of c-MYC in cell cycle control, expression of proteins involved in cell cycle promotion, including Polo-like kinase 1 (PLK1), Checkpoint kinase 1 (CHEK1), and Cyclins B1 and E1 were also reduced. In contrast, expression of proteins involved in cell cycle inhibition, including p21 and p27, was increased. Interestingly, while the GSEA data identified a signature of mTOR activation, expression and activation of several mTOR pathway intermediates was reduced in both cell lines. Among these was phospho-Ser2481-mTOR, phospho-Thr37/Thr-46-Eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) and phospho-Ser65-4E-BP1, phospho-Thr389-Ribosomal protein S6 kinase beta-1 (p70S6K), both phospho-Ser235- and phospho-Ser236-Ribosomal protein S6 (S6), and both phospho-Ser240- and phospho-Ser244-S6. To further confirm key RPPA findings, formal Western blotting was performed and found, for example, that phospho-Ser-473-Akt, phospho-Thr389-p70S6K, and phospho-Ser65-4E-BP1 expression declined in MM1.S but not RPMI 8226 cells (Figure 5), as had been the case in the RPPAs. Further upstream in the mTOR pathway, phospho-Ser241-3-phosphoinositide-dependent protein kinase 1 (PDK1) and phospho-Tyr458-phosphoinositide 3-kinase (PI3K) expression were also decreased in MM1.S cells. These findings are consistent with prior studies suggesting that BRD4 inhibition downregulates PI3K signaling through decreased BRD4 recruitment to the regulatory domains of Epidermal growth factor and Insulin family receptor tyrosine kinases35.
Figure 4. BET-specific PROTACs affect cell cycle regulation and the Akt/mTOR pathway.
The effect of ARV-825 or ARV-763 on the expression levels of proteins are shown from reverse phase protein array (RPPA) analysis (Supplementary Figure 6) of MM1.S (top panel) or RPMI 8226 (bottom panel) cells. These were treated with either 100 nM ARV-825 or 250 nM ARV-763 for 24 hours prior to preparation for analysis. Normalized linear values of antibody signal representing levels of BRD4 and c-MYC, and of proteins involved in cell cycle regulation and the Akt/mTOR pathway, are plotted as means ± SD from triplicate experiments.
Figure 5. Influence of PROTACs on Akt and mTOR pathway signaling.
The effect of ARV-825 or ARV-763 on the expression levels of key intermediates in the PI3K-Akt-mTOR pathway signaling were probed by Western blotting of MM1.S (left panel) and RPMI 8226 (right panel) cells. These were exposed for 24 hours to the indicated drug concentrations prior to harvesting and preparation of cell lysates for analysis.
Since ARV-825 was found to inhibit mTOR signaling, it seemed possible that combinations with agents that targeted this pathway could be of interest. Therefore, we next evaluated ARV-825 with either the mTOR inhibitor everolimus, or the Akt inhibitor afuresertib, which have shown anti-myeloma activity as single agents in phase I studies36, 37. At virtually all of the concentrations tested, ARV-825 with everolimus or afuresertib showed strong synergy, as indicated by a combination index <1.0 (Table 1). In addition, a high degree of synergy was seen between ARV-825 and dexamethasone, and the BH3 mimetics obatoclax and venetoclax.
Table 1.
Combination index analysis to determine the promise of ARV-825 in combinations with other conventional and novel anti-myeloma agents
| ARV-825 (nM) | |||||||
|---|---|---|---|---|---|---|---|
| 1.25 | 2.5 | 5 | 10 | 20 | 40 | ||
| Everolimus | nM | ||||||
| 0.1 | 1.22 | 0.70 | 0.66 | 0.79 | 0.75 | ||
| 0.4 | 0.54 | 0.45 | 0.51 | 0.60 | 0.43 | ||
| 1.6 | 0.59 | 0.45 | 0.51 | 0.59 | 0.60 | ||
| 6.4 | 0.83 | 0.60 | 0.52 | 0.55 | 0.53 | ||
| Dexamethasone | μM | ||||||
| 1.25 | 0.19 | ||||||
| 2.5 | 0.25 | ||||||
| 5 | 0.37 | ||||||
| 10 | 0.65 | ||||||
| 20 | 1.24 | ||||||
| Afuresertib | nM | ||||||
| 20 | 0.95 | 1.09 | 0.83 | 0.92 | |||
| 40 | 1.11 | 0.79 | 0.64 | 0.80 | |||
| 80 | 0.68 | 0.65 | 0.56 | 0.76 | |||
| 160 | 0.56 | 0.39 | 0.46 | 0.54 | |||
| Obatoclax | nM | ||||||
| 10 | 1.10 | 1.02 | 0.76 | 0.92 | 0.97 | ||
| 50 | 0.71 | 0.63 | 0.56 | 0.79 | 0.62 | ||
| 250 | 0.34 | 0.42 | 0.52 | 0.37 | 0.35 | ||
| 1250 | 0.86 | 0.77 | 0.77 | 0.17 | 0.29 | ||
| Venetoclax | nM | ||||||
| 10 | 1.90 | 1.3 | 2.39 | 2.2 | |||
| 50 | 0.84 | 0.96 | 0.43 | 1.1 | |||
| 250 | 0.59 | 0.27 | 1.90 | 0.9 | |||
| 1250 | 0.16 | 0.07 | 0.16 | 0.23 | |||
PROTACs overcome drug resistance mechanisms
BRD4 serves as a regulator for p53 target gene transcription38, and 17p deletion with loss of p53 is a high-risk feature in myeloma associated with drug resistance11. We therefore sought to examine the impact of p53 deletion on the efficacy of these PROTACs using otherwise isogenic MM1.S, H929, and MOLP-8 models with wild-type (WT) or knockout (KO) TP53 generated using sequence-specific zinc finger nucleases39. ARV-825 was somewhat more potent than ARV-763 in all three lines (Figure 6A; top), consistent with the data in Figure 1. Comparing the KO and WT models, the viability curves for each were virtually overlapping, supporting the possibility that PROTACs work in a p53-independent manner. Cell line models of acquired resistance to conventional drugs, including dexamethasone and doxorubicin, and novel agents, including bortezomib, carfilzomib, lenalidomide, and pomalidomide were then studied. Reductions in viability from PROTACs in dexamethasone-resistant cells occurred at concentrations that were comparable to those in drug-naive cells, and at lower concentrations for bortezomib-resistance (Figure 6B). In lenalidomide- and, to a greater extent, in pomalidomide-resistant cells, cross-resistance was observed with ARV-825, but not with ARV-763. This was not explained by decreased CRBN expression in the IMiD-resistant cell lines (Figure 6C), although, notably, BRD4 expression was unchanged, particularly in the U266 P10R cells with ARV-825 treatment. This suggested impairment of CRBN E3 ubiquitin ligase activity in U266 P10R cells, with possible mechanisms including loss of function mutations in CRBN or CRBN-associated proteins that form the E3 ubiquitin ligase complex40.
Figure 6. PROTAC-mediated BRD4 degradation overcomes drug resistance.
(A) Otherwise isogenic MM1.S, H929, and MOLP-8 cell lines with either wild-type (WT) TP53 or a TP53 knockout (KO) were generated using sequence-specific zinc-finger nucleases targeting a non-specific sequence, or exon 7 of the TP53 DNA binding domain22. The anti-proliferative effects of ARV-825 and ARV-763 were then studied with viability assays performed as described earlier after a 72-hour drug exposure. Data were collected from triplicate experiments, which are represented as the mean ± SD for each concentration. (B) MM1.S, RPMI 8226, and U266 cells resistant to bortezomib (BR), carfilzomib (CR), dexamethasone (DexR), doxorubicin (DoxR), lenalidomide (R10R), and pomalidomide (P10R), and their corresponding parental cell lines, were studied to determine if there was cross-resistance to ARV-865, ARV-763, and OTX015 as described in panel A. (C) Western blotting of BRD4 and CRBN expression in MM1.S R10R and U266 P10R cells, and their corresponding parental lines after treatment with ARV-825, ARV-763, and OTX015 for 48 hours. (D) Top panel: Expression of the Multidrug resistance protein 1 (MDR1) was evaluated by Western blotting in the indicated drug-resistant and parental cell lines. Bottom panel: MDR1 efflux activity was assessed using DiCO2(3), a highly specific fluorescent substrate dye for MDR1. At 40C, MDR1 is inactive and cells retain DiCO2(3) after initial dye loading, while at 370C, MDR1 is active and cells will efflux DiCO2(3) and exhibit decreased fluorescence. (E) Carfilzomib- or doxorubicin-resistant RPMI 8226 cells were treated with the indicated concentrations of ARV-825, verapamil, or various combinations of the two. Live cell populations were assessed using the WST-1 proliferation assay after exposure to drugs for 72 hours as described in panel A. (F) MM1.S cells were treated for 48 hours with ARV-825 or ARV-763 in the presence or absence of HS5 stromal cells. Cell viability was assessed using flow cytometry with Annexin V (A5)/TO-PRO-3 (PI) staining in triplicate experiments, which are represented as the mean ± SD for each condition. Treatment with dexamethasone was used as a positive control.
Carfilzomib- and doxorubicin-resistant cells showed cross-resistance to both PROTACs, and since P-glycoprotein (Multidrug resistance protein 1 (MDR1)) is a factor in the latter41, we evaluated its expression. Consistent with the possibility of a role for MDR1, it was over-expressed in the carfilzomib- and doxorubicin-resistant cells (Figure 6D, top panel; Supplementary Figure 7), but not the parental or bortezomib-resistant cells. Increased efflux and decreased fluorescence of DiCO2(3), a highly specific substrate dye for MDR1, was also observed in carfilzomib-resistant and doxorubicin-resistant cells relative to their parental cells (Figure 6D, lower panel). We then treated carfilzomib- and doxorubicin-resistant cells with ARV-825 and verapamil, a known MDR1 inhibitor41. Verapamil alone had no influence on cell viability, but its addition to ARV-825, and vice versa, sensitized these cells to the PROTAC (Figure 6E). Verapamil also sensitized carfilzomib- and doxorubicin-resistant cells to OTX015 (Supplementary Figure 8), suggesting OTX015 is also an MDR1 substrate.
Finally, MM1.S cells were co-cultured with HS5 stromal cells to mimic the marrow microenvironment. Interestingly, the therapeutic activity of ARV-825 and ARV-763 was enhanced in the presence of stromal cells (Figure 6F), which may be explained by HS5-induced BRD4 expression in myeloma cells13.
BET-targeted PROTACs are active against primary myeloma cells and in vivo
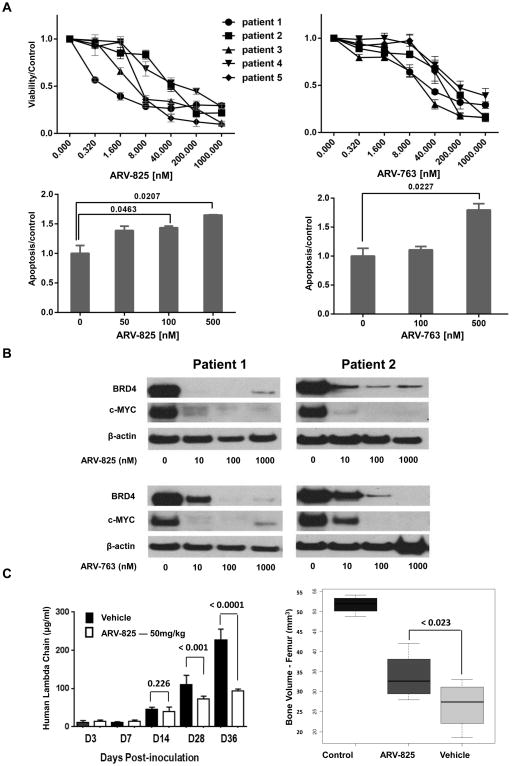
To determine if these PROTACs could potentially be useful in the clinic, their activity was evaluated against primary plasma cell samples. All five demonstrated a dose-dependent decrease in cellular viability after exposure to both agents (Figure 7A; top panels), with ARV-825 showing greater potency. Annexin V staining supported that this was associated with induction of apoptosis (Figure 7A; bottom panels). Also, reduced BRD4 and c-MYC expression were seen in the two samples for which we had sufficient cells to study by Western blotting (Figure 7B). Finally, we used MM1.S cells to establish a xenograft model of systemic myeloma in NSG mice. ARV-825 dosed intraperitoneally, and starting on day 3 after intravenous MM1.S cell injection, slowed the appearance of human lambda light chains (Figure 7C; left panel). Interestingly, a greater benefit was seen with prolonged dosing, as the differences became more dramatic on days 28 and 36 compared to earlier time points, without any apparent toxicity. Also of note, compared to vehicle-treated mice, ARV-825 helped significantly preserve femur bone volume measured at 30-days post-inoculation (p<0.023)(Figure 7C; right panel)(Supplementary Figure 9).
Figure 7. Activity of BET PROTACs against primary plasma cells and in vivo.
(A) Top: The ability of ARV-825 and ARV-763 to reduce viability in primary cells was evaluated in samples from five patients with relapsed and/or refractory myeloma, which were exposed to the indicated drug concentrations for 48 hours. Bottom: Induction of apoptosis was confirmed by staining of cells from patient 1 with Annexin V, followed by flow cytometric analysis. (B) Western blotting was performed on samples from patients 1 and 2, whose cells were treated with ARV-825 and ARV-763, to evaluate the downstream effects on BRD4 and c-MYC expression after 24 hours. (C) NSG mice injected with MM1.S cells through their tail veins were randomized to receive either vehicle (n=6) or ARV-825 (n=6), the latter at 50 mg/kg daily by intraperitoneal injection, beginning on day 3 after tumor cell inoculation. Left panel: Levels of circulating human lambda light chain immunoglobulins were measured over time in these xenograft-bearing mice using an ELISA. Right panel: Quantitation of micro-computed tomography scans of femoral bones in control, and xenograft-bearing mice treated with vehicle or ARV-825 was performed on day 30 post-inoculation. The mean femoral bone volume ± SD are shown for each treatment group.
Discussion
Our studies of these BET-targeted PROTACs showed that they induced a strong anti-proliferative effect, cell cycle arrest at G0/G1, reduced levels of CDK4 and CDK6, and activated Caspase-mediated apoptosis (Figure 1). At the protein level, they potently and rapidly induced BRD4 degradation and subsequently inhibited expression of c-MYC, as well as other down-stream BRD4 targets, including N-MYC and PIM-2. Interestingly, the CRBN-targeted ARV-825 was more potent in most of these assays than the VHL-targeted agent ARV-763. This could be because the two E3 ligases might be different in their efficiency of ubiquitinating BRD4. Also, ARV-825 contains the pomalidomide moiety, and thus can both promote BET degradation, and act to some extent like an immunomodulatory drug. In the latter guise, it can reduce Aiolos and Ikaros levels, and suppress IRF4 and c-MYC independently of its impact on BRD442, possibly explaining why it reduces c-MYC to a greater extent than ARV-763. However, we cannot exclude the possibility that other factors, such as the expression levels of CRBN and VHL, or differences in cellular uptake and efflux of the PROTACs, may contribute to this differential effect. Importantly, ARV-825, and to some extent also ARV-763, were more potent than JQ1 or OTX015, confirming prior studies in other models19, 20, and compared to dBET1, suggesting that these PROTACs could ultimately be more active in the clinic.
PROTACs rely for their activity on the availability of their target E3 ligase, and an intact ubiquitin-proteasome pathway. This was demonstrated by the finding that IMiDs interfered with ARV-825 but not of ARV-763 (Figure 3), while proteasome inhibitors antagonized both in inducing BRD4 degradation. With the goal of designing combinations with optimal clinical activity, one may therefore need to use ARV-763, or agents targeting a non-CRBN E3 ligase, with IMiDs. However, the presence of pomalidomide in ARV-825 could make it a bi-functional chemotherapeutic that would preclude the need for an IMiD. Addition of concurrent proteasome inhibitors may need to be avoided with PROTACs (Figure 3B), though some rational sequence of treatment could possibly be validated given the rapid onset of action of PROTACs.
Genomic studies suggested that BET PROTACs activated the mTOR pathway, but proteomic studies showed that they inhibited mTOR and Akt signaling (Figure 4, 5). This contradiction may indicate a compensatory increase in mTOR transcription in response to inhibition of this pathway. However, the GEP data also showed that expression of Myotubularin-related protein 3 (MTMR3), a negative mTOR complex 1 (mTORC1) regulator43, was increased, while that of mTOR-associated protein LST8 homolog (MLST8), a required subunit and positive regulator of mTORC1 and mTORC244, was decreased, supporting the possibility of mTOR inhibition. Moreover, these data are consistent with prior studies showing that BET inhibitors lowered phospho-inositide 3-kinase (PI3K) signaling, and dissociated BRD4 from chromatin at regulatory regions of the Insulin-like growth factor-1 receptor35. Also, there is cross-talk between c-MYC-mediated transcription and the PI3K/Akt pathway through MAD-145, providing several mechanisms through which PROTACs could exert this effect. mTOR-mediated regulation of metabolism is of increasing interest in myeloma46–50, and mTOR-mediated 4E-BP1 phosphorylation is required for survival of MYC-dependent hematologic malignancies51. The serine–threonine kinase mTOR can form two multi-protein complexes, mTORC1 and mTORC2. mTORC1 can activate mRNA translation through regulation of p70S6K and 4E-BP1, while mTORC2 can activate Akt through Ser473 phosphorylation52. In turn, the ability of mTORC1 to bind its substrates is regulated by a Proline-rich Akt substrate of 40 kDa (PRAS40) that is phosphorylated by Akt at Ser24653, 54. RPPA analysis revealed a significant effect of our PROTACs on mTORC1 signaling and downstream RNA translation through decreased phosphorylation of Ribosomal protein S6, a direct p70S6K target. Western blotting confirmed decreased phosphorylation/activation of p70S6K and 4E-BP1 in MM1.S cells, and to a lesser extent in RPMI 8226 cells. In MM1.S cells, RPPA showed decreased Akt phosphorylation at Ser473, and a decrease in Akt-mediated phosphorylation of PRAS40, a negative regulator of substrate binding by mTORC1, suggesting inhibition of mTORC1 binding to its substrates in PROTACs-treated MM1.S cells. In RPMI 8226 cells, the RPPA did not show any effect of our PROTACs on Akt and PRAS40, but the PROTACs decreased levels of phosphorylated Tuberin (TSC2), a negative mTORC1 regulator, consistent with the effect of PROTACs on levels of the PIM-2 kinase that can affect mTORC1 activity through phosphorylation of Tuberin55. Taken together, our data suggest that the effects of BET PROTACs on mTORC1 activity are cell line specific, and that Akt signaling could be a pathway of PROTAC resistance. Indeed, the latter hypothesis is further supported by the finding of synergistic interactions between ARV-825 and the Akt inhibitor afuresertib (Table 1). Cell-line specific effects could depend, in part, on the ability of PROTACs to suppress parallel upstream regulators of mTORC1 activity, Akt, and/or PIM-2 in myeloma cells55 by decreasing Akt and PRAS40 phosphorylation, and/or decreasing levels of PIM-2 kinase and subsequent phosphorylation of Tuberin.
BET-targeted PROTACs showed comparable activity in TP53 WT and KO cell line models, and overcame conventional and novel drug resistance, unless this was mediated by P-glycoprotein (Figure 6). These findings suggest that PROTACs should show activity against high-risk myeloma with deletion of 17p, and in the relapsed and refractory setting, which are both areas of high unmet medical need. However, expression of multi-drug resistance efflux pumps has been described to increase in the latter group56, and may influence patient outcomes to therapies such as bortezomib with pegylated liposomal doxorubicin57. Also, a subpopulation of carfilzomib-resistant myeloma cells has been noted to overexpress NIMA-related kinase 2 (NEK2), which can up-regulate MDR-related proteins including MDR1 (P-gp), and this was strongly associated with bortezomib and doxorubicin resistance58,59. Thus, additional studies will be needed to determine if the current PROTACs targeting BET proteins, or PROTACs that could be developed against other key myeloma proteins, such as Myeloid cell leukemia (MCL)-1, may need to be avoided in patients with MDR over-expression.
Finally, pre-clinical studies in primary cells, and an in vivo murine model of systemic myeloma, showed encouraging activity of ARV-825 (Figure 7). The BET inhibitor OTX015 has recently completed phase I studies with manageable dose-limiting toxicities, and a dose for phase II testing was identified60. Moreover, three diffuse large B-cell lymphoma (DLBCL) patients achieved durable responses, while six others, including two with DLBCL and four with indolent lymphomas, had evidence of clinical activity. Since the PROTACs appear to have a somewhat comparable mechanism of action but greater potency, similar toxicities, which seem to be predominantly focused on the hematopoietic and gastrointestinal systems, where some of the more proliferative normal tissues are found, would be expected. Careful titration of the dose and selection of the schedule based on pharmacokinetic and pharmacodynamic data will be needed to determine the safety and efficacy of this new, promising class of drugs.
Supplementary Material
Key Points.
Protein-targeting chimeric molecules that induce Bromodomain and extraterminal domain protein degradation are active against myeloma.
Their ability to activate apoptosis, overcome chemoresistance, and combine with conventional and novel drugs shows promise for the clinic.
Acknowledgments
This work was supported by funding from The MD Anderson Cancer Center Knowledge Gaps program, The MD Anderson Cancer Center Moon Shot in High Risk Multiple Myeloma, the National Cancer Institute (The MD Anderson Cancer Center SPORE in Multiple Myeloma (P50 CA142509) and R01s CA184464 and CA194264), the Leukemia & Lymphoma Society (New Idea Award 8994-12 and Specialized Center of Research SCOR-12206-17), the National Natural Science Foundation of China (Grant 81600170), and a Jiangsu government scholarship for overseas studies (JS-2014-195). The authors would like to thank the MD Anderson Flow Cytometry and Cellular Imaging Core Facility and the Characterized Cell Line Core Facility, which are supported by the Cancer Center Support Grant (P30 CA16672). R.Z.O. would also like to acknowledge support from the Florence Maude Thomas Cancer Research Professorship, the Brock Family Myeloma Research Fund, and the Jean Clarke High-risk Myeloma Research Fund.
Footnotes
Authorship Contributions
X.Z. planned and conducted most of the experiments, analyzed the results, and drafted the manuscript. H.C.L. planned and conducted experiments, analyzed results, and revised the manuscript. F.S. planned and conducted experiments and analyzed results. V.B. and H.L. performed statistical analyses and interpreted the data. I.K., F.S. and Y.H. performed animal experiments, while R.J.J., J.L., and Y.Q. participated in the design and analysis of experiments. S.K.T. and H.C.L. provided primary samples, B.L and H.W. with H.C.L. provided cell lines, while R.E.D. performed gene expression analysis. R.K.S performed immunohistochemistry and immunofluorescence studies. Z.B. helped analyze the data and draft the manuscript. K.R. and K.G.C. reviewed the data and the manuscript. C.M.C. helped develop the PROTACs. R.Z.O. conceived of the line of investigation, designed the experiments, analyzed the results, and edited the manuscript.
Disclosure of Conflicts of Interest
R.Z.O. has served on advisory boards for Amgen, which manufactures and distributes carfilzomib, Takeda Pharmaceuticals U.S.A., Inc., which manufactures and distributes bortezomib, and for Celgene Corporation, which manufactures and distributes lenalidomide and pomalidomide, but there was no commercial support for this research. J.L., Y.Q., K.R., and K.G.C. are employees of Arvinas, LLC, which manufactures the PROTACs studied in this work. C.M.C. is founder, consultant and shareholder of Arvinas, LLC. In addition, his lab receives research funding from Arvinas, LLC. The remaining authors have no conflicts of interest to declare.
References
- 1.Ott G. Impact of MYC on malignant behavior. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2014 Dec 5;2014(1):100–106. doi: 10.1182/asheducation-2014.1.100. [DOI] [PubMed] [Google Scholar]
- 2.Horiuchi D, Anderton B, Goga A. Taking on challenging targets: making MYC druggable. American Society of Clinical Oncology educational book/ASCO American Society of Clinical Oncology Meeting. 2014:e497–502. doi: 10.14694/EdBook_AM.2014.34.e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida K, Tamura A, Nakazawa N, Ueda Y, Abe T, Matsuda F, et al. The Ig heavy chain gene is frequently involved in chromosomal translocations in multiple myeloma and plasma cell leukemia as detected by in situ hybridization. Blood. 1997 Jul 15;90(2):526–534. [PubMed] [Google Scholar]
- 4.Shou Y, Martelli ML, Gabrea A, Qi Y, Brents LA, Roschke A, et al. Diverse karyotypic abnormalities of the c-myc locus associated with c-myc dysregulation and tumor progression in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jan 4;97(1):228–233. doi: 10.1073/pnas.97.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Affer M, Chesi M, Chen WD, Keats JJ, Demchenko YN, Tamizhmani K, et al. Promiscuous MYC locus rearrangements hijack enhancers but mostly super-enhancers to dysregulate MYC expression in multiple myeloma. Leukemia. 2014 Aug;28(8):1725–1735. doi: 10.1038/leu.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker BA, Wardell CP, Brioli A, Boyle E, Kaiser MF, Begum DB, et al. Translocations at 8q24 juxtapose MYC with genes that harbor superenhancers resulting in overexpression and poor prognosis in myeloma patients. Blood cancer journal. 2014 Mar 14;4:e191. doi: 10.1038/bcj.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo AG, Gang AO, Pedersen MO, Poulsen TS, Klausen TW, Norgaard P. Overexpression of c-myc is associated with adverse clinical features and worse overall survival in multiple myeloma. Leukemia & lymphoma. 2016 Nov;57(11):2526–2534. doi: 10.1080/10428194.2016.1187275. [DOI] [PubMed] [Google Scholar]
- 8.Sekiguchi N, Ootsubo K, Wagatsuma M, Midorikawa K, Nagata A, Noto S, et al. Impact of C-Myc gene-related aberrations in newly diagnosed myeloma with bortezomib/dexamethasone therapy. International journal of hematology. 2014 Mar;99(3):288–295. doi: 10.1007/s12185-014-1514-1. [DOI] [PubMed] [Google Scholar]
- 9.Glitza IC, Lu G, Shah R, Bashir Q, Shah N, Champlin RE, et al. Chromosome 8q24.1/c-MYC abnormality: a marker for high-risk myeloma. Leukemia & lymphoma. 2015 Mar;56(3):602–607. doi: 10.3109/10428194.2014.924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greco C, D’Agnano I, Vitelli G, Vona R, Marino M, Mottolese M, et al. c-MYC deregulation is involved in melphalan resistance of multiple myeloma: role of PDGF-BB. International journal of immunopathology and pharmacology. 2006 Jan-Mar;19(1):67–79. [PubMed] [Google Scholar]
- 11.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016 Jun 16;127(24):2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010 Dec 23;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011 Sep 16;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LL, Tian M, Li X, Li JJ, Huang J, Ouyang L, et al. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget. 2015 Mar 20;6(8):5501–5516. doi: 10.18632/oncotarget.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proceedings of the National Academy of Sciences of the United States of America. 2001 Jul 17;98(15):8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, et al. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Molecular & cellular proteomics : MCP. 2003 Dec;2(12):1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010 Mar 12;327(5971):1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2012 Nov 3;118(18):4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015 Jun 19;348(6241):1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, et al. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chemistry & biology. 2015 Jun 18;22(6):755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi AM, et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2016 Jun 28;113(26):7124–7129. doi: 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, Wang H, Baladandayuthapani V, Lin H, He J, Jones RJ, et al. RNA Polymerase I Inhibition with CX-5461 as a Novel Therapeutic Strategy to Target MYC in Multiple Myeloma. Br J Haematol. 2017 Apr;177(1):80–94. doi: 10.1111/bjh.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Fu J, Chen P, Ge X, Li Y, Kuiatse I, et al. The Nuclear Factor (Erythroid-derived 2)-like 2 and Proteasome Maturation Protein Axis Mediate Bortezomib Resistance in Multiple Myeloma. The Journal of biological chemistry. 2015 Dec 11;290(50):29854–29868. doi: 10.1074/jbc.M115.664953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn DJ, Berkova Z, Jones RJ, Woessner R, Bjorklund CC, Ma W, et al. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood. 2012 Oct 18;120(16):3260–3270. doi: 10.1182/blood-2011-10-386789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, et al. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. The Journal of biological chemistry. 2011 Apr 1;286(13):11009–11020. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Molecular cancer therapeutics. 2006 Oct;5(10):2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 27.Woolson RF, Clarke WR. Statistical methods for the analysis of biomedical data. 2. Wiley-Interscience; New York: 2002. p. xxv.p. 677. [Google Scholar]
- 28.Dib A, Gabrea A, Glebov OK, Bergsagel PL, Kuehl WM. Characterization of MYC translocations in multiple myeloma cell lines. Journal of the National Cancer Institute Monographs. 2008;(39):25–31. doi: 10.1093/jncimonographs/lgn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyce A, Ganji G, Smitheman KN, Chung CW, Korenchuk S, Bai Y, et al. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PloS one. 2013;8(8):e72967. doi: 10.1371/journal.pone.0072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezoe S, Matsumura I, Nakata S, Gale K, Ishihara K, Minegishi N, et al. GATA-2/estrogen receptor chimera regulates cytokine-dependent growth of hematopoietic cells through accumulation of p21(WAF1) and p27(Kip1) proteins. Blood. 2002 Nov 15;100(10):3512–3520. doi: 10.1182/blood-2002-04-1177. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008 Aug 14;27(35):4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 32.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nature reviews Clinical oncology. 2017 Jul;14(7):417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang H, Liu Y, Xue M, Liu H, Du S, Zhang L, et al. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic acids research. 2016 Apr 7;44(6):2514–2527. doi: 10.1093/nar/gkw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi J. Bromodomain and extraterminal domain inhibitors (BETi) for cancer therapy: chemical modulation of chromatin structure. Cold Spring Harbor perspectives in biology. 2014 Dec 01;6(12):a018663. doi: 10.1101/cshperspect.a018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratikopoulos EE, Dendy M, Szabolcs M, Khaykin AJ, Lefebvre C, Zhou MM, et al. Kinase and BET Inhibitors Together Clamp Inhibition of PI3K Signaling and Overcome Resistance to Therapy. Cancer cell. 2015 Jun 8;27(6):837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunther A, Baumann P, Burger R, Kellner C, Klapper W, Schmidmaier R, et al. Activity of everolimus (RAD001) in relapsed and/or refractory multiple myeloma: a phase I study. Haematologica. 2015 Apr;100(4):541–547. doi: 10.3324/haematol.2014.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer A, Yoon SS, Harrison SJ, Morris SR, Smith DA, Brigandi RA, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014 Oct 02;124(14):2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Molecular cell. 2013 Mar 7;49(5):843–857. doi: 10.1016/j.molcel.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HC, Wang H, Baladandayuthapani V, Lin H, He J, Jones RJ, et al. RNA polymerase I inhibition with CX-5461 as a novel therapeutic strategy to target MYC in multiple myeloma. British journal of haematology. 2016 doi: 10.1111/bjh.14525. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kortum KM, Zhu YX, Shi CX, Jedlowski P, Stewart AK. Cereblon binding molecules in multiple myeloma. Blood Rev. 2015 Sep;29(5):329–334. doi: 10.1016/j.blre.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Bellamy WT, Dalton WS, Kailey JM, Gleason MC, McCloskey TM, Dorr RT, et al. Verapamil reversal of doxorubicin resistance in multidrug-resistant human myeloma cells and association with drug accumulation and DNA damage. Cancer research. 1988 Nov 15;48(22):6365–6370. [PubMed] [Google Scholar]
- 42.Ito T, Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. International journal of hematology. 2016 Sep;104(3):293–299. doi: 10.1007/s12185-016-2073-4. [DOI] [PubMed] [Google Scholar]
- 43.Hao F, Itoh T, Morita E, Shirahama-Noda K, Yoshimori T, Noda T. The PtdIns3-phosphatase MTMR3 interacts with mTORC1 and suppresses its activity. FEBS letters. 2016 Jan;590(1):161–173. doi: 10.1002/1873-3468.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakumoto K, Ikeda J, Okada M, Morii E, Oneyama C. mLST8 Promotes mTOR-Mediated Tumor Progression. PloS one. 2015;10(4):e0119015. doi: 10.1371/journal.pone.0119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proceedings of the National Academy of Sciences of the United States of America. 2008 May 6;105(18):6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han K, Xu X, Xu Z, Chen G, Zeng Y, Zhang Z, et al. SC06, a novel small molecule compound, displays preclinical activity against multiple myeloma by disrupting the mTOR signaling pathway. Sci Rep. 2015;5:12809. doi: 10.1038/srep12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendell JC, Kelley RK, Shih KC, Grabowsky JA, Bergsland E, Jones S, et al. A phase I dose-escalation study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual mTORC1/mTORC2 kinase inhibitor CC-223 in patients with advanced solid tumors or multiple myeloma. Cancer. 2015 Oct 1;121(19):3481–3490. doi: 10.1002/cncr.29422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arcaro A. Targeting PI3K/mTOR Signaling in Cancer. Front Oncol. 2014;4:84. doi: 10.3389/fonc.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutter G, Zimmermann Y, Rieken M, Hartmann E, Rosenwald A, Hiddemann W, et al. Proteasome inhibition leads to dephosphorylation and downregulation of protein expression of members of the Akt/mTOR pathway in MCL. Leukemia. 2012 Nov;26(11):2442–2444. doi: 10.1038/leu.2012.118. [DOI] [PubMed] [Google Scholar]
- 50.Younes H, Leleu X, Hatjiharissi E, Moreau AS, Hideshima T, Richardson P, et al. Targeting the phosphatidylinositol 3-kinase pathway in multiple myeloma. Clin Cancer Res. 2007 Jul 1;13(13):3771–3775. doi: 10.1158/1078-0432.CCR-06-2921. [DOI] [PubMed] [Google Scholar]
- 51.Pourdehnad M, Truitt ML, Siddiqi IN, Ducker GS, Shokat KM, Ruggero D. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jul 16;110(29):11988–11993. doi: 10.1073/pnas.1310230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. The Journal of biological chemistry. 2007 Jul 6;282(27):20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 54.Wiza C, Nascimento EB, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab. 2012 Jun 15;302(12):E1453–1460. doi: 10.1152/ajpendo.00660.2011. [DOI] [PubMed] [Google Scholar]
- 55.Lu J, Zavorotinskaya T, Dai Y, Niu XH, Castillo J, Sim J, et al. Pim2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation. Blood. 2013 Aug 29;122(9):1610–1620. doi: 10.1182/blood-2013-01-481457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonneveld P, Lokhorst HM, Vossebeld P. Drug resistance in multiple myeloma. Seminars in hematology. 1997 Oct;34(4 Suppl 5):34–39. [PubMed] [Google Scholar]
- 57.Buda G, Ricci D, Huang CC, Favis R, Cohen N, Zhuang SH, et al. Polymorphisms in the multiple drug resistance protein 1 and in P-glycoprotein 1 are associated with time to event outcomes in patients with advanced multiple myeloma treated with bortezomib and pegylated liposomal doxorubicin. Annals of hematology. 2010 Nov;89(11):1133–1140. doi: 10.1007/s00277-010-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawley TS, Riz I, Yang W, Wakabayashi Y, Depalma L, Chang YT, et al. Identification of an ABCB1 (P-glycoprotein)-positive carfilzomib-resistant myeloma subpopulation by the pluripotent stem cell fluorescent dye CDy1. American journal of hematology. 2013 Apr;88(4):265–272. doi: 10.1002/ajh.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer cell. 2013 Jan 14;23(1):48–62. doi: 10.1016/j.ccr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. The Lancet Haematology. 2016 Apr;3(4):e196–204. doi: 10.1016/S2352-3026(16)00021-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.