Abstract
Acne is one of the most common skin diseases worldwide and results in major health care costs and significant morbidity to severely affected individuals. However, the pathophysiology of this disorder is not well understood. Host-microbiome interactions that affect both innate and adaptive immune homeostasis appear to be a central factor in this disease, with recent observations suggesting that the composition and activities of the microbiota in acne is perturbed. Staphylococcus epidermidis and Cutibacterium acnes (C. acnes; formerly Propionibacterium acnes) are two major inhabitants of the skin that are thought to contribute to the disease but are also known to promote health by inhibiting the growth and invasion of pathogens. Because C. acnes is ubiquitous in sebaceous-rich skin, it is typically labeled as the etiological agent of acne yet it fails to fulfill all of Koch’s postulates. The outdated model of acne progression proposes that increased sebum production promotes over-proliferation of C. acnes in a plugged hair follicle, thereby driving inflammation. In contrast, growing evidence indicates that C. acnes is equally abundant in both unaffected and acne-affected follicles. Moreover, recent advances in metagenomic sequencing of the acne microbiome have revealed a diverse population structure distinct from healthy individuals, uncovering new lineage-specific virulence determinants. In this article, we review recent developments in the interactions of skin microbes with host immunity, discussing the contribution of dysbiosis to the immunobiology of acne and newly emerging skin microbiome-based therapeutics to treat acne.
Keywords: Microbiome, Acne, Staphylococcus, Sebaceous, Inflammation, Commensal, Therapeutics, Skin, Metagenomics, Cutibacteria
Background
Acne is one of the most common skin diseases and affects up to 85% of adolescents and young adults worldwide [1]. Severe manifestations of acne are painful and cause disfiguration and scarring, and in some patients, profoundly reduce self-esteem and affects mental health [2, 3]. Acne is considered a disease of the pilosebaceous unit, a complex mini-organ of the body that displays considerable morphological, microbiological, and metabolic diversity depending on the skin site. The sebaceous gland in particular actively responds to fluctuations in hormonal, environmental, and immunological input. Acne development is not only individual-specific but also site-specific, with only some follicles developing inflammation even during severe manifestations of disease. Four main factors are believed to contribute to acne development: increased sebum production, follicular hyperkeratinization, colonization of skin bacteria, and inflammation.
An exciting area of recent advance in the understanding of acne has come from studies focused on the skin “microbiome,” the complex community of bacteria, viruses, and fungal organisms that inhabit all epithelial surfaces and appear to have unique functions on the skin. In sequencing studies of diverse skin sites of healthy adults, the composition of the skin bacterial microbiome was primarily dependent on the particular characteristics and chemical makeup across distinct niches [4–6]. Sebaceous sites were dominated by the lipophilic Cutibacterium species (formerly known as Propionibacterium), whereas moist areas were abundant in Staphylococcus and Corynebacterium species. C. acnes has been long thought of as a pathogenic factor for acne, yet it is a major skin commensal that prevents colonization and invasion of pathogens, via the hydrolysis of triglycerides in sebum and release of fatty acids that are antimicrobial and contribute to an acidic pH of the skin surface [7].
The increasing recognition that commensal and mutualistic microorganisms are necessary for many aspects of normal human physiology has altered the traditional pathogen-dominated view of human-bacterial interactions [8]. The classical assertion that C. acnes is the major etiological agent in acne vulgaris is still generally regarded as fact within the medical and lay community. Media advertising of acne-related cosmetic products and prescribing of antibiotics by physicians reinforce the notion of a bacterial origin. To date, some studies and articles still incorrectly attribute acne to “infection” with C. acnes, despite its ubiquitous presence on healthy skin [9–11]. Likewise, some graphical illustrations of acne development depict bacteria proliferating in the sebaceous gland [12, 13], yet observations show these structures to be sterile [14]. No convincing evidence exists to suggest bacterial overgrowth corresponds to acne development or disease severity [15–20]. C. acnes has been shown to coexist on the skin surface and in the pilosebaceous follicle with other Cutibacterium spp., including Cutibacterium granulosum and Cutibacterium avidum, as well as species belonging to Staphylococcus, Pseudomonas, Corynebacterium, and the commensal fungi Malassezia [21]. However, colonization is by no means universal. Several studies have failed to detect viable bacteria in healthy and diseased follicles [16, 17, 22]. Only recently have researchers developed the tools to visualize C. acnes colonization in the in vivo setting and observed biofilms attached to the hair shaft and follicular epithelial wall [23], in some cases extending from the stratum corneum to the base of the follicle (Fig. 1). However, this finding is still much in debate and needs to be confirmed by other groups. C. acnes biofilms were reported to be more frequent in acne lesions compared to control follicles [24]. These biofilms can exist as polymicrobial structures containing distinct populations of Staphylococcus, Cutibacterium, and Malassezia [25, 26]. The interspecies interactions in polymicrobial communities may dictate biofilm phenotype, such as enhanced antibiotic resistance and inflammatory capacity. In the pilosebaceous unit, a biofilm matrix can act as a biological glue to physically restrict sebum passage into the infundibulum, leading to comedo formation and/or promote retention and accumulation of corneocytes in the lumen, resulting in a keratinaceous plug and comedone development [27]. The in vitro and in vivo observations of biofilm structures in the follicle need further investigation and by itself support the considerations by many in the field that view acne vulgaris as a chronic disease [28].
Fig. 1.
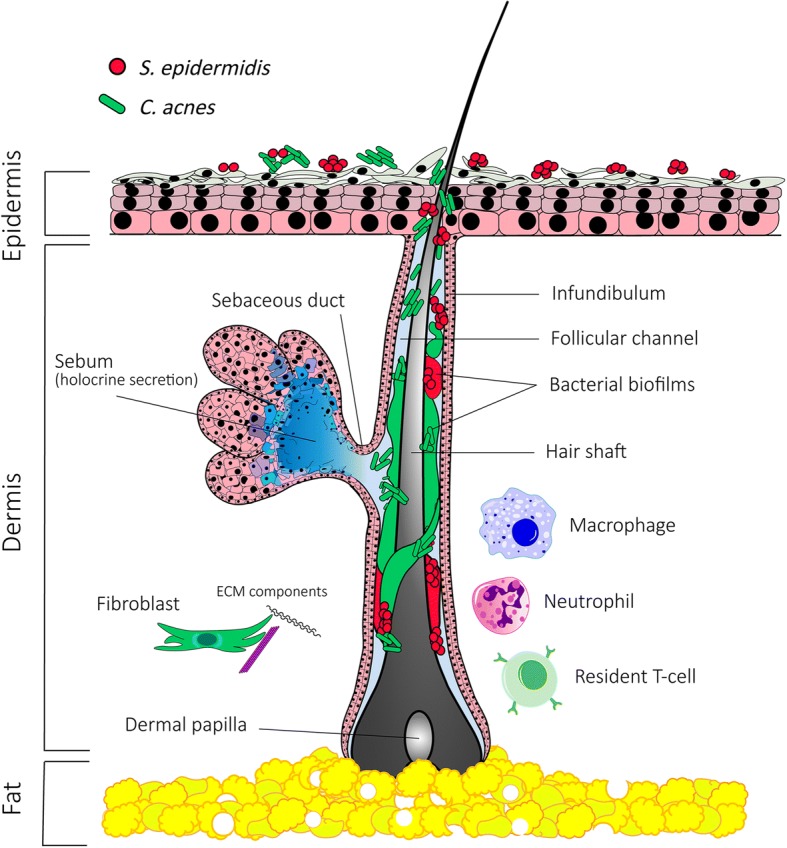
Skin organization and representation of the pilosebaceous unit. Major residents of the pilosebaceous unit, C. acnes and S. epidermidis, coexist on the skin surface and within the follicle as multiphyletic communities that can interact and coexist
In this review, we discuss recent insights into the skin resident microbial communities, including their composition and interactions with the immune system in both health and disease. We also discuss competitive and synergistic interactions between cutaneous microbes and its effect on host immune responses relevant to acne vulgaris. We end by considering important unanswered questions in the field and future research priorities. A greater understanding of host-microbiome interaction in acne is important as interest in targeting the skin microbiome for therapeutic approaches increases.
The microbiome in acne
The four dominant phyla of bacteria residing on the skin are Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes. However, depending on the skin topography and distinct chemical makeup of the site and the individual, the composition and diversity of the resident microbes can differ significantly. In a comprehensive metagenomic analyses of diverse human skin sites, including moist, dry, and sebaceous microenvironments, one study found that individuality and skin topography defined the microbiome composition [6]. Whereas, the strain-level distribution of C. acnes was more individual-specific than site-specific, the composition of S. epidermidis was less diverse, with a greater dependency on site rather than individual characteristics. In the sebaceous-rich environment, both species are frequently isolated from normal and acne-affected skin [29, 30]. However, given the unique environment and chemistry of the pilosebaceous unit, intrafollicular colonization may not correlate with the surface composition. From studies that have examined the bacterial community structure from pooled samples of extracted follicles, S. epidermidis was highly prevalent but much less abundant than C. acnes [20, 31, 32]. In contrast, a separate study examined bacterial numbers from several individual follicles and found that S. epidermidis counts can be equivalent or higher than C. acnes in some follicles [33]. Therefore, while S. epidermidis is one of the most dominant species on the skin surface, its contribution to health and follicular disease remains poorly understood.
Cutibacterium is consistently one the most abundant genus whose frequency increases as a product of enhanced sebum levels [34, 35]. This Cutibacterium enrichment coincides with a decrease in overall microbial diversity and richness. Indeed, C. acnes has the metabolic potential to substantially alter its local environment. It contains numerous biosynthetic gene clusters and lipases that together contribute to the production and release of antimicrobial and immunomodulatory molecules [36, 37]. Thus, qualitative and quantitative alterations in sebum during adolescence could have a substantial effect on the microbiome composition via interspecies and host interactions.
Despite the challenge of defining a “healthy” skin microbiome, comparing the microbiomes of diseased and healthy skin could advance our understanding of the mechanisms that can contribute to the pathophysiology of acne vulgaris. Recent studies have associated the presence of certain bacteria with specific disease states. For example, in psoriatic lesions, Cutibacterium spp. were underrepresented but Streptococcus spp. were significantly more frequent compared to healthy control skin [38]. Likewise, dysbiosis is also a hallmark of atopic dermatitis (AD). Staphylococcus aureus (S. aureus) colonization on AD skin has been directly correlated to disease severity, but the role of other bacterial members of the skin is unknown [39]. In AD lesional skin, the relative abundance of Cutibacterium species is reduced but recovers after successful treatment, suggesting an important commensal role of Cutibacterium in skin health.
Much of our current knowledge of C. acnes skin colonization is based on the biased application of culture-based methods [40, 41]. Quantitation by colony-forming units (CFU) selects for microorganisms in nutrient-rich artificial growth conditions, unlike the dry and nutrient-poor conditions of the skin, and underestimates the total diversity of the community. In culture-based surveys of the skin, Staphylococcus spp. are identified and cultivated more easily than Cutibacterium spp.—a slow growing organism that requires hypoxic conditions. Thus, to overcome bias culture practices and capture the true diversity of the bacterial microbiome, researchers have applied advanced sequencing methods to look for variable sequences in otherwise conserved taxonomic markers, like 16S rRNA for ribotyping [20], or the untargeted “shotgun” analyses of the entire collection of genetic elements to obtain the “meta” genome of all resident bacteria [31]. While 16S rRNA sequencing is advantageous to capture genetic diversity in bacterial populations, whole-genome metagenomics provide greater resolution at the strain level by capturing single nucleotide polymorphisms as well as the metabolic profile of microbial communities, which are typically altered in several skin disease states. In a study of the acne microbiome, metagenomic analyses revealed several distinct virulence-associated gene elements that encode for antimicrobial peptides, cytotoxins, and proteases that are enriched in C. acnes strains associated with disease [31]. Interestingly, the majority of these genes are localized on several genomic islands as well as a linear plasmid. Nevertheless, it is important to recognize that DNA sequencing approaches have their own set of inherent biases which can be introduced into microbiome datasets at many different stages, from the initial study design to sample collection, storage, and processing as well as the sequencing and complex computational analysis required.
Sampling methodologies for studying the acne microbiome
The choice of sampling method, anatomical location, and sequencing approach are important factors in any skin microbiome study [42]. The most common methods for sampling the skin microbiome include non-invasive methods such as swab, scrub, and tape stripping that recover microbes residing on the skin surface and within the stratum corneum. These methods have the most relevance for microbiome analysis of superficial skin diseases like psoriasis and atopic dermatitis [38, 39], and when adopted for acne studies, distinct lineages of C. acnes were found to be more associated with health or disease [43, 44]. However, acne vulgaris is generally considered a disease of the pilosebaceous unit, and an accurate representation of the acne microbiome may require sampling of the follicular environment. Common “invasive” sampling techniques include pore strips and cyanoacrylate gel biopsy that capture individual hairs or follicular “casts,” respectively. The former approach was utilized by Fitz-Gibbon et al. to characterize the microbiome of acne patients sampled from the follicular contents of the nose [20]. Crucially, to collect enough follicular material for sequencing, the authors pooled all pilosebaceous units within the same skin site, sacrificing sensitivity since uninvolved follicles vastly outnumber acne-affected follicles [45]. Many issues involving bias in sampling methods, anatomical choice, and sequencing were subject of interesting debate following publication of this study [46–48]. In light of such controversies, a recent study compared the skin microbiome of acne patients using three different sampling techniques: swab, pore strips, and gel biopsy combined with multiple sequencing approaches [32]. While greater bacterial diversity was discovered on the surface skin, the overall composition of the surface and follicular environment was comparable for the most abundant species, particularly C. acnes, with several strain-types represented in both niches. Overall, the authors concluded that surface or follicular sampling were both suitable approaches for accurate analysis of the skin microbiome in acne research, particularly in the context of C. acnes association.
Different typing methods for C. acnes
Early typing methods involving serological agglutination, cell wall sugar analysis, bacteriophage, and fermentation profiling revealed two distinct C. acnes phenotypes called type I and II [49–51]. Later, sequence analysis of the housekeeping gene recA showed that types I and II represented distinct phylogenetic lineages [52]. An additional phylogenetic group later designated as type III was included after discovery of its distinct long filamentous morphology [53]. Because C. acnes has a clonal population structure and a high degree of sequence conservation, multi locus sequence typing (MLST) is required for high-resolution strain typing. MLST is based on sequencing of internal fragments of multiple housekeeping genes, with each different sequence defined as a distinct allele that is then used to generate an allelic profile or sequence type (ST) for every bacterial isolate [54]. Presently, the C. acnes type I clade can be subdivided into closely related subtypes: IA1, IA2, IB, and IC, that contain many different clonal complexes (CC) and ST’s. The first MLST scheme developed for C. acnes, termed the Aarhus scheme, is based on nine gene loci (MLST9) and showed that a single clone ST18 within the phylotype IA1 was globally disseminated and associated with severe acne [44]. Another widely adopted but independent scheme is the MLST8 method, an expanded version of the MLST7 or Belfast scheme, that could discriminate 285 C. acnes isolates into 91 ST’s [43, 55]. This scheme also showed that ST1 (denoted as ST18 by MLST9 or ST6 by MLST7) of phylotype type IA1 was significantly enriched in acne and that several ST’s from CC72 (type II) and CC77 (type III) were associated with healthy skin. Another group utilized publicly available whole genome sequencing data of all known C. acnes strains to devise a single locus sequence typing (SLST) scheme that is advantageous in revealing C. acnes ST diversity in mixed microbial communities using pyrosequencing [56]. An alternative single-locus approach was reported by Fitz-Gibbon et al. who used the variable 16S rRNA gene called ribotyping, as well as whole genome sequencing, to demonstrate healthy- and acne-associated ribotypes (RT) in a metagenomic study of acne patients [20]. Evidently, each typing scheme utilizes different loci, and identifying which CC, ST, and/or RT is associated with health or disease can be difficult to determine [57, 58]. Nevertheless, it is now well established that C. acnes phylotype IA1 is associated with acne, while types II and III isolates are more commonly associated with healthy skin.
Metagenomic analysis of acne patients
Fitz-Gibbon et al. reported a similar relative abundance of C. acnes between both patient cohorts with the three most abundant ribotypes (RT1, RT2, RT3) evenly distributed among both acne and normal follicles [20]. However, four ribotypes including RT4 and RT5 of the phylotype IA1, (synonymous with ST3 (CC3) and ST4 (CC4) of MLST8, respectively) were significantly enriched in 30–40% of patients with acne, but rarely found in individuals with healthy skin. In contrast, RT6 which represents a subpopulation of phylotype II was found to be 99% associated with healthy skin. Interestingly, the non-acne-associated IB, types II and III, are also commonly recovered from deep tissue infections and retrieved medical devices [59]. This could indicate that certain strains can become pathogenic in different environments or simply reflect a likelihood for contamination of these strains given their ubiquitous presence on normal skin [60]. For instance, C. acnes type III was not detectable on acne skin but composed approximately 20% of isolates from healthy skin [61]. Therefore, a greater genetic, biochemical, and functional characterization is needed to establish whether type II and III strains can be classified as true commensals and IA1 acne-associated CC’s and ST’s as opportunistic pathogens. If so demonstrated, this would represent a potential new strategy for targeted antimicrobial therapy and a significant advancement for disease diagnostics in distinguishing C. acnes contamination or infection in many different medical conditions.
In a recent metagenomic analysis of acne patients, Barnard et al. suggested that their findings of a higher relative abundance of C. granulosum in healthy individuals, compared to acne, was evidence for a commensal role [31]. In contrast, early culture-based studies reported that C. granulosum is more prevalent in comedones and pustules compared to uninvolved follicles of acne patients [62]. Moreover, C. granulosum was reported to demonstrate greater lipase activity compared to C. acnes [63]. However, the limited genome data currently available for this understudied bacterium indicates a limited repertoire of virulence-associated genes, with notable absences of Christie Atkins Munch Petersen (CAMP) toxins, sialidases and hyaluronate lyases, thought to contribute to C. acnes-host interactions during disease [64]. Thus, further investigations are required to determine the potential health contribution of this minor Cutibacterium species.
Skin microbiome interactions with host immunity
Humans have coevolved with their microbiome and developed a wide range of innate immune responses to protect the body against infection, while still maintaining a bacterial presence. In contrast to the gut microbiome that is physically separated from the epithelium by a dense mucus layer in the colon [65], the skin microbiome is in constant and intimate contact with the epithelium, modulating both innate and adaptive immune cell functions [66]. For this reason, it is important that the immune response is primed to recognize and tailored to respond to an appropriate threat, as any immune reaction towards commensals could lead to chronic disease. Keratinocytes are the main cell type of the epidermis and directly participate in both innate and adaptive arms of immunity; as a source of antimicrobial peptides and cytokines that trigger inflammation when the epithelium is exposed to danger- or pathogen-associated molecular patterns (D/PAMP), including Toll-like receptor 2 and 6 (TLR2/6) ligands present on or secreted by many resident bacteria, such as C. acnes (Fig. 2) [67]. Such inflammation-causing PAMPs include major components of the coats of gram-positive bacteria such as peptidoglycan (PGN) and lipoteichoic acid (LTA). TLR2 bacterial ligands present on C. acnes have been proposed to promote inflammation in acne, stimulating interleukin-1alpha (IL-1α), and granulocyte macrophage-colony stimulating factor (GM-CSF) release [68]. Treatment of keratinocytes with LTA and PGN can trigger NFκB activation via TLR2 activation and release of the neutrophil chemoattractant cytokines TNFα and IL-8 [69]. Early acne lesions and inflammatory papules show significant infiltration of macrophages and neutrophils, which contribute to inflammation and potentially, in sporadic cases, rupture of the follicular wall via the secretion of hydrolytic lysosomal enzymes [70–72]. In one study, it is suggested that the non-acne-associated type II C. acnes can induce higher levels of IL-8 in keratinocytes than type IA [73]. In contrast, types IA and IB were found to induce greater levels of the human antimicrobial peptide β-defensin 2 (hBD2) from cultured sebocytes, than a type II isolate [74]. A long-standing question in the microbiome field is why do cells switch from a state of immunological tolerance to a chronic inflammatory state in the absence of an infection? In the case of acne development, a dynamic shift in the microenvironment of the follicle can trigger a different transcriptional response of the microbiota. For example, culturing C. acnes in a lipid-rich, hypoxic environment similar to that of an occluded hair follicle, promotes anaerobic fermentation and production of short-chain fatty acids (SCFA) that activates an epigenetic mechanism to enhance the TLR2-mediated production of IL-6, IL-8, and TNFα in human keratinocytes [75].
Fig. 2.
Interspecies interactions and host-bacterial interactions within the follicular microenvironment
Colonization by C. acnes causes activation of TLR2 in monocytes, resulting in the production of proinflammatory cytokines IL-12 and IL-8. Biopsies of acne lesions also show abundant TLR2 expression on the surface of macrophages surrounding pilosebaceous follicles [71]. In addition, gene expression profiles of inflammatory papules from acne biopsies found marked upregulation of pathways involved in inflammation and matrix remodeling, specifically gene encoding for matrix metalloproteinase 1 and 3 (MMP1, MMP3); β-defensins 1, 2, and 4; and neutrophil granzyme B [76]. There is strong evidence that host antimicrobial peptides (AMP) play a role in the pathogenesis of acne. Skin-derived AMPs comprise the family of β-defensins, S100 proteins, RNases, and the cathelicidin LL-37. While some AMPs are constitutively secreted, hBD-2 and -3 and LL-37 are upregulated in acne lesions and induced by culture supernatants of C. acnes in vitro [77, 78].
Genetic elements of the microbes associated with acne
Since the first C. acnes isolate was sequenced in 2004 (KPA171202, a type IB strain recovered from skin), a number of putative virulence genes have been identified with designated functions involved in tissue degradation, cell adhesion, inflammation, and polysaccharide biosynthesis for biofilm formation [79]. Several genetic elements specific to each lineage have since been identified, which could explain the functional differences between lineages and association with different disease states [80]. One of the most fascinating genetic differences between C. acnes lineages is the presence of clustered regularly interspaced palindromic repeats (CRISPR)/Cas locus in health-associated type II strains [81]. While this system is only partially present in type III and likely non-functional, it is completely absent from type I strains [82]. CRISPR is a bacterial adaptive immune system against viruses, phages, and foreign DNA, and its presence in C. acnes could prevent the acquisition of extra genetic elements that promote virulence and acne pathogenesis [20, 83, 84]. A case in point is the discovery of a novel linear plasmid present in acne-associated type I strains that harbors a tight adhesion (tad) locus, common among many pathogens and essential for biofilm formation, colonization, and virulence [20, 85–87]. Similarly, among the acne-associated strains of RT8, representing clade IB, is a unique genomic island (locus 4) which encode a series of enzymes capable of producing a class of biologically active natural compounds called nonribosomal peptides (NRP) [80]. Other bacteria-derived NRP metabolites have reported roles in cell-mediated toxicity and in iron sequestration as well as potential antimicrobial and antifungal activity [88]. Another plausible genetic explanation for the selectivity of type II strains with healthy skin is the discovery of potentially important mutations in several gene-encoding triacylglycerol lipases, required for the breakdown of sebum [80]. Examining the genetic repertoire of C. acnes strains recovered from acne patients revealed several large genomic islands that encode genes homologous with the streptolysin S biosynthetic cluster involved in biosynthesis and transport of bacterial toxins as well as other genes with putative roles in cell survival, virulence, and transport [31]. In contrast, genes which were more abundant on healthy skin were related to carbohydrate and lipid metabolism and nutrient biosynthesis but not virulence. Interestingly, many of these genomic islands that encode putative virulence genes were absent in C. avidum and C. granulosum. For example, gene-encoding CAMP factors camp1, camp2, and camp4, which have reported roles in hemolysis and inflammation, are absent in both species as well as other genes encoding for host-interacting factors such as hyaluronate lyase and two dermatan sulphate adhesins DsA1 and DsA2—found to be abundantly expressed in the follicular environment [64, 89]. Comparative genomic and proteomic analysis, as well as clinical observations of the three major human Cutibacteria in acne pathogenesis, seem to suggest a strict capacity of commensalism for C. avidum and C. granulosum but a flexibility for parasitism for C. acnes. Although it remains unlikely that a single genetic element drives pathogenesis of a multifactorial disease like acne, the absence of suitable tools for the genetic manipulation of C. acnes mean that the biological significance of some of these putative virulence genes remain unknown. Alternatively, some groups have tried to identify and characterize the intracellular, membrane-bound, and/or secreted proteome of distinct C. acnes strains, to determine their potential contribution to acne pathogenesis [90–93].
Role of C. acnes proteome and interaction with follicular environment
C. acnes is considered a contributing factor to the inflammation detected in follicles of patients with acne vulgaris. However, the ubiquitous distribution of C. acnes throughout the skin contrasts with the isolated occurrence of inflammatory lesions. The presence of C. acnes on healthy and acne skin has often frustrated attempts at assigning a pathogenic role in disease. However, Bek-Thomsen et al. exploited this phenomenon by analyzing and comparing the eukaryotic and prokaryotic proteome of extracted follicular casts from healthy and acne skin [89]. Not surprisingly, acne skin was enriched for host proteins associated with inflammation, wound healing, and remodeling. According to gene ontology analysis, the most significant biological process in acne skin was “response to bacterium.” Interestingly, the only bacterial proteins found belonged exclusively to C. acnes. Importantly, the authors also detected less bacterial proteins in acne casts but recorded a shift to a more virulent protein profile, with acne casts enriched for dermatan-sulphate adhesions (DsA1 and DsA2), co-hemolytic toxins CAMP factors 1 and 2 as well as several hydrolytic and lipolytic enzymes. In contrast to reports that attribute C. acnes proliferation in acne involvement, the results of this study suggest the existence of a metabolically less-active population in acne, perhaps better adapted to colonize and tolerate the harsh inflammatory microenvironment of an acne-affected follicle. Analogous to the approach adopted by Fitz-Gibbon et al., this study also had several drawbacks including the need to combine extracted casts from both affected and unaffected sites for quantitative analyses. More sensitive approaches are needed to accurately discern the acne proteome of individual follicles [89, 94].
Bacterial competition within the follicular environment
Interactions between members of the microbiota can shape the resident microbial community by acting competitively to outcompete or eliminate other species or cooperatively for mutual benefits (Fig. 2). In acne, the microbial dysbiosis could be attributed to androgen-mediated seborrhea and dysseborhea, that select for distinct strains which are genetically better adapted to exploit such an environmental shift [95]. For instance, type II strains have been shown to have reduced lipase activity compared to type I strains and could be at a growth disadvantage in a multiphyletic community competing for space and resources [80, 96]. C. acnes has been demonstrated to produce several bacteriocins and bacteriocin-like molecules which may be responsible for its successful colonization in the follicle and on the skin surface. Interestingly, the acne-associated phylogroup IA2 display direct antimicrobial activity against a range of S. epidermidis strains in vitro [36]. This could be due to the presence of a putative thiopeptide antibiotic, unique to type IA2 strains. C. acnes has several defense mechanisms to prevent pathogens that seek to establish colonization in the skin. Indirectly, the metabolic activities of C. acnes in sebaceous-rich skin can establish an inhospitable niche against many skin pathogens via the generation of antimicrobial free fatty acids (FFA), including lauric and linoleic acid, as well as SCFAs that can suppress the growth of methicillin-resistant S. aureus (MRSA) and group A Streptococcus [37, 97]. Similarly, some strains of S. epidermidis can ferment carbohydrates to produce the SCFA succinic acid that has potent anti-S. aureus and anti-C. acnes activity [98].
Genome interrogation of skin S. epidermidis strains indicate a vast array of elements involved in interspecies competition. S. epidermidis can antagonize S. aureus colonization and virulence via quorum sensing signaling—releasing an autoinducing peptide that, when recognized by S. aureus, can repress the expression of a global virulence regulator called the agr system [99]. Although one study showed strain-dependent antimicrobial activity of S. epidermidis against C. acnes, it is not yet clear whether acne-associated strains could have enhanced resistance to interspecies killing [36]. However, specific staphylococcal species on the skin secrete lantibiotics with potent antimicrobial activity against S. aureus [100]. In fact, the application of these commensal strains onto human subjects with AD was shown to reduce colonization by S. aureus [101]. In theory, a similar approach could also be utilized as a potential biotherapy for acne, with application of commensal staphylococcal strains demonstrating broad anti-C. acnes activity or even selective against those that are acne-associated. In healthy skin, C. acnes and S. epidermidis exist as stable heterogeneous communities, but in acne-affected skin, the strain-diversity and relative abundance of S. epidermidis is increased at the expense of C. acnes [29, 31]. Clearly, a greater understanding of S. epidermidis role in acne dysbiosis and pathogenesis is needed and could represent an attractive therapeutic for disease.
Current treatments that target resident skin microbes
Because acne is a multifactorial inflammatory disease, many different treatment options are available that try to target the underlying causes. Some include topical agents like benzoyl peroxide and salicylic/azelaic acids, anti-androgens, systemic antibiotics, and retinoids as well as physical modalities like laser and photodynamic therapy [102]. Knowledge of the many pathogenic factors is continuously evolving, and better knowledge of disease is crucial for effective management. Isotretinoin is a pro-drug for all-trans retinoic acid and for over 35 years has been prescribed as a “last resort” option for the most severe and recalcitrant manifestations of acne [103]. Yet surprisingly, its mode-of-action is still not fully understood. Its suppressive effect on sebaceous gland activity is well known, and recently, it was reported to normalize aberrant TLR-2-mediated innate immune responses towards C. acnes [104]. Logically, isotretinoin should have a major impact on the resident microbe population, by eliminating an essential nutrient supply and stabilizing immune hypersensitivity, but this has not been examined in detail. Interestingly, higher rates of colonization with S. aureus have been observed in patients receiving systemic isotretinoin, leading to increased incidence of minor skin infections like folliculitis and furunculosis [105]. Moreover, since relapse rates can be high, occurring in roughly 26–48% of cases [106], elucidating the microbiome composition pre- and post-treatment would be informative. Isotretinoin is a useful treatment to combat acne but has significant drawbacks including, an extended regimen, observational and laboratory monitoring, and some common and serious side effects [103].
The other major treatment options that target the underlying microbial etiology are topical and oral antibiotics. Topical antibiotics act both as antibacterial agents suppressing C. acnes and as anti-inflammatory agent. Topical clindamycin or erythromycin is also suitable, and as documented in many randomized, clinical trials, these antibiotics are more effective when combined with benzoyl peroxide (BP) or topical retinoids [102]. BP is an antibacterial agent that targets C. acnes through the release of free oxygen radicals and is also comedolytic. Oral antibiotics are indicated for moderate-to-severe disease and for patients in which topical combinations have failed or were not tolerated. Oral antibiotics are taken over a lengthy 3–6-month period [107]. However, the choice for systemic antibiotics to suppress C. acnes is counterproductive given the rise of antibiotic resistance [108]. Although it is important to focus on antimicrobial resistance in C. acnes during treatment, it is also important to consider the impact of extended antibiotic use on the hugely diverse gut microbiome. The dual approach of topical and systemic therapy does not address the issue of enrichment and transfer of resistance genes in the gut. Moreover, increasing evidence suggests that specific members of the microbiota may never recover after long term antibiotic use. Such alterations in the gut microbiome composition increases susceptibility to infections, particularly recurrent bouts of Clostridium difficile [109], increased risk of autoimmune and metabolic disorders like Chron’s disease, irritable bowel syndrome (IBD) as well as type I diabetes, and obesity [110].
Other less-popular alternatives include blue light treatment and conventional UV phototherapy [111, 112]. These approaches may benefit patients with moderate-to-severe acne by altering the skin microbiome and reducing C. acnes density in lesions. Indeed, UV-R is known to be bactericidal and can break lipopolysaccharides, LTA, and other bacterial metabolites which have immunomodulatory properties [113].
Microbiome-based therapeutic approaches to acne
Despite advances in understanding the pathophysiology and immunobiology of acne, no novel products have been brought to market in the last 10 years. Most available treatments are repurposed and still have significant drawbacks. Since 90% of new drug candidates fail to win FDA approval, resulting in average drug development costs of up to $2.6 billion, the apparent dearth of innovation in acne drug development is most likely a feature of its complex etiology [114, 115]. Moreover, to gain regulatory approval, most studies are performed on simplified human skin models under sterile conditions, which fail to accurately model the response in vivo. In the biotechnology and medical sectors, it is now appreciated that microbial dysbiosis in the gut and skin are linked to many chronic diseases and as a result, many new approaches and drug candidates are targeted towards restoring a healthy microbial community. Initial trials of fecal microbiome transplants have been demonstrated to be safe and effective for patients with Clostridium difficile infections [116, 117]. Our group is actively involved in therapeutic development of specific bacterial strains selected from the skin microbiome to treat patients suffering with AD. This approach has been shown to eliminate S. aureus and restore a balanced microbiome [101]. A similar approach is feasible for acne; however, any microbiome-targeted treatment for acne should consider that the reported dysbiosis relates to distinct C. acnes strains and not colonization or infection by a bona fide pathogen per se. Also, new approaches to employ biodegradable nanoparticles or silica microcapsules for delivery within the follicular environment are in development [118, 119]. Better delivery systems can be exploited to achieve better clinical results with classic anti-acne compounds like benzyl peroxide or work in tandem with laser treatment to achieve site-specific release of antimicrobial compounds after activation. We discuss several new microbiome-targeted strategies that are in development or clinical trial stages (Table 1).
Table 1.
Selected pipeline candidates for the next generation of acne therapeutic drugs
| Company | Drug | Formulation | Effect | Mechanism | Stage |
|---|---|---|---|---|---|
| Melinta Therapeutics | Radezolid | Topical oxazolidnone | Antibacterial | Second generation antibiotic with enhanced ribosomal binding | Phase II |
| Allergan and Paratek Pharmaceuticals | Seysara® | Oral sarecycline antibiotic | Antibacterial and anti-inflammatory | Narrow spectrum of activity against C. acnes and S. aureus | Phase III |
| Novartis | CJM112 | Anti-inflammatory | Anti-IL-17 | Phase II | |
| Sebacia | Microparticles | Topical suspension of gold microparticles | Sebostatic | Photothermolysis of sebaceous glands | Phase III |
| KLOX Technologies | Kleresca® | Cream containing chromophores | Killing of C. acnes | Excitation of bacterial porphyrins by light absorption | FDA-approved |
| Botanix | BTX 1503 | Transdermal gel containing synthetic cannabidiol | Anti-inflammatory and sebostatic | Activation of vanilloid-4 ion channels to inhibit sebocyte lipogenesis | Phase Ib |
| Foamix Pharmaceuticals | FMX101 | Topical minocycline foam (4%) | Antibacterial, anti-inflammatory, and antioxidant | Blocks inflammasome-pyroptosis and cleaved IL-1β | Phase III |
| BiophramaX | BPX-01 | Solubilized topical minocycline gel | Antibacterial, anti-inflammatory, and antioxidant | Inhibits protein synthesis in bacteria and inhibits iNOS and MMPs in cells | Phase III |
| Dermira | Olumacostat glasaretil (DRM01) | Topical small molecule prodrug | Sebostatic | Inhibitor of acetyl coenzyme A (CoA) carboxylase involved in FA biosynthesis | Phase III |
| Allergan | ANT-1207 | Topical botulinum toxin type A | Anti-inflammatory | Acetylcholine inhibitors and glutamate-antagonists | Phase II |
| Promius Pharma LLC | DFD-03 | Topical tazarotene lotion | Anti-inflammatory | Retinoid prodrug: suppress keratinocyte hyperproliferation | Phase III |
| Celtaxsys | Acebilustat (CTX-4430) | Oral capsule | Anti-inflammatory | Leukotriene A4 hydrolase inhibitors | Phase II |
| AOBiome | B244 | Topical spray | Anti-inflammatory and microbiome modulation | Production of nitrite and NO to suppress inflammation | Phase IIb |
| Novan Thereputics | SB204 | Topical gel | Antibacterial and anti-inflammatory | Release of NO that inhibits IL-1β | Phase III |
| AndroScience | ASC-J9 | Topical cream | Androgen modulation | Synthetic androgen receptor degradation enhancer | Phase III |
| Galderma | Trifarotene (CD-5789) | Topical cream | Anti-inflammatory | Retinoic acid receptor gamma agonists | Phase III |
| Seoul National University Hospital | Lupeol | Topical lupeol cream | Suppressed lipogenesis, anti-inflammatory | NF-κB and PI3K/Akt Modulator | In clinical trials |
| Sienna Biopharmaceuticals | SNA-001 | Topical silver particle suspension | Photothermolysis of sebaceous gland | Laser-excited particles that convert light energy to heat | Phase IIb |
Nitric oxide
AOBiome Therapeutics has developed a microbiome-targeted topical and intranasal formulation for the treatment of mild-to-moderate acne. The technology is based on the application of a suspension of Nitrosomonosa eutropha, an ammonia-oxidizing bacteria (AOB), isolated from organic soil samples. This therapeutic is reported to exploit the bacterium’s nitrogen cycle to convert ammonia and urea, found naturally on human skin, to nitrite and NO, which have anti-inflammatory and antimicrobial activity. Taking a NO-centric approach, Novan Therapeutics has begun phase III clinical trial for treatment of mild-to-severe acne with a topical nitric oxide-releasing drug called SB204. Results of the phase II trial reported a significant clinical improvement with reductions in number of non-inflammatory and inflammatory lesions [120]. NO exhibits broad antimicrobial activity against many cutaneous pathogens and has a demonstrated role in wound repair [121, 122]. In relation to acne pathogenesis, NO has been suggested to have a dual protective role, directly killing C. acnes and also suppressing IL-1β, IL-8, and TNFα cytokine release in keratinocytes [119].
Probiotic and prebiotics
The first clinical trial evaluating the effects of probiotics on acne was conducted by the physician Robert H. Siver in 1961 using Lactobacillus strains [123]. Although he recorded clinical improvement in his subjects, the study crucially lacked a placebo control, leaving Dr. Siver to conclude that, “interactions of skin manifestations of acne vulgaris and of metabolic processes of the intestinal tract are suggestive.” In fact, there is now a scientific merit to this suggestion and is known as the gut-brain-skin axis, which posits a mechanism that connects gastrointestinal health by oral probiotics to skin homeostasis [124]. Recent studies have shown that orally consumed pre and probiotics reduce systemic markers of oxidative stress, inflammation, and insulin resistance and also regulate inflammatory cytokine release in the skin, improving skin barrier function and hydration [125–128].
The concept of bacterial antagonism between C. acnes and S. epidermidis via fermentation could be applied to develop topical probiotics against acne and other skin disorders. Some skin commensals can produce anti-inflammatory and antimicrobial metabolites under lipid-rich, hypoxic conditions that mimic the follicular environment. S. epidermidis can ferment glycerol, a natural constituent of triglycerides in sebum, to produce succinic acid which can inhibit the growth of C. acnes and suppress C. acnes-mediated inflammation in mice [98]. A feasible approach would be to exploit this phenomenon of interspecies competition to develop a live biologic therapeutic cream containing a rationally selected commensal Staphylococcus strain with potent anti-C. acnes activity to treat acne lesions.
Phage therapy
Bacteriophages are viruses that can infect and kill bacteria but are probably the least understood component of the human microbiome [129]. The presence of C. acnes phages on human skin was first described over 50 years ago [130, 131], but advances in sequencing technology now provide us with unique insight into the role of viral communities in skin health and disease. Metagenomic analysis has revealed that C. acnes phages are more prevalent and abundant in healthy individuals compared to acne patients, consistent with other culture-based studies of C. acnes phage counts in acne [20]. Interestingly, a higher relative abundance of phage was detected in older individuals, which could explain why acne prevalence declines with increasing age. To better understand bacterial-phage interactions, Liu et al. challenged genetically distinct C. acnes strains with 15 different phages and found that strains from clades IB, II, and III were resistant to killing [132]. This suggested that antiviral strategies of some C. acnes strains may shape strain populations in healthy or diseased states, with implications for potential personalized phage-based therapy. Despite phage therapy being utilized in Eastern Europe for over a century and reportedly safe and effective, no data is available on phage therapy specifically for acne treatment [11].
Vaccines
Vaccination may be a feasible strategy to combat different types of infections caused by C. acnes. Potential application of a heat-killed C. acnes (HKCa)-based vaccine [133] as well as a vaccine targeting the cell wall-anchored sialidase [134] or secreted CAMP factor 2 have been reported [135]. This approach reduced inflammation and disease severity in a mouse ear infection model. Nevertheless, one of the major obstacles for effective vaccine design is the lack of a suitable in vivo acne model. Moreover, in light of recent metagenomic analysis of acne-associated strains, identifying distinct immunogenic proteins produced by these strains would be an attractive approach. For that purpose, several groups have made recent strides in characterizing the proteome of different C. acnes strains [90]. Nevertheless, a C. acnes vaccine would have other beneficial effects. For instance, a low dose vaccination with HKCa in an AD mouse model was shown to induce regulatory T cells (Treg) and type 1 T helper (Th1) immune responses, which improved clinical symptoms [136]. Likewise, HKCa was cross-protective against Actinobacillus pleuropneumoniae in pig and mouse models [137]. A number of attractive vaccine candidates have been reported from in vivo infection studies, including a rabbit model of implant-associated infection with C. acnes that identified 24 immunogenic proteins upregulated during infection [138].
Animal models for acne vulgaris
In the current system, any new anti-acne drug candidate requires several rounds of screening using biochemical assays, cell culture, and animal models to test for efficacy and toxicity before advancement to clinical trials. Roughly 90% of drugs entering clinical trials fail, and this high-failure rate is partly attributed to a lack of models that accurately represent the complexity and organization of human tissue and/or recapitulate human-microbiome interactions [139, 140]. Therefore, predictive and validated animal models are essential to improve the translation of drug findings from the bench to the clinic. Acne is a complex human disease of the pilosebaceous unit that currently lacks a suitable animal model. Numerous models have been tested, including the Mexican hairless dog, the rhino mouse, and the rabbit ear [141]. The rabbit ear assay is the most common model utilized to determine chemical comedogenicity but does not induce inflammation. Similarly, the hairless rhino mouse is limited as a vaccine model since it failed to produce antibodies against thymus-dependent antigens [142]. Most animals do not develop inflammatory acne-like lesions, and neither C. acnes nor S. epidermidis naturally colonize murine skin, which is histologically and biochemically different than humans and supports different microbial communities, most commonly Staphyloccous xylosus and Staphylococcus saprophyticus [143, 144]. Animal sebum does not contain sufficient amounts of triglycerides and free fatty acids to satisfy the nutritional requirements for C. acnes [145], a fact that has restricted the development of anti-acne drugs and vaccines. Instead, most studies assess sustained “acne-like” inflammation characterized by edema and cell infiltration after intradermal injection of viable or HKCa in the ears of mice. To overcome such limitations, a strategy to mimic the microenvironment of an acne lesion in mice was conducted by constructing a perforated tissue chamber scaffold (to permit influx of immune cells), containing human sebocyte cells and C. acnes, to investigate bacterial-host interactions in vivo [146]. Recently, it was proposed that HR-1 mice develop acneiform inflammation and small microcomedone-like cysts after C. acnes injection [147]. Alternatively, several ex vivo animal skin models are used to assess the cutaneous permeation of drugs, with porcine skin found to be most histologically similar to humans, in terms of thickness, hair follicle density, and lipid composition [148, 149]. Therefore, because acne is a multifactorial complex human disease, of which colonization of C. acnes is a contributing factor, no perfect animal model currently exists. Thus, without a greater focus on developing new models for acne, closing the translational gap will remain a difficult endeavor.
Human skin models for microbiome studies
Ninety percent of drugs that enter phase I clinical trials eventually fail [150]. Traditional in vitro two-dimensional (2D) cell cultures are the current standard for early drug screening but fail to recapitulate the native 3D microenvironment of in vivo tissues [151]. There are several currently available human skin equivalent (SE) models commercially available,from relatively simple reconstructed epidermis of keratinocytes, to more complex full-thickness skin models containing differentiated epidermis on a fibroblast-embedded dermis matrix [152]. The major technical hurdle of 3D SE models for research and clinical applications is how to integrate endothelial cells, immunocytes, adipocytes, and appendages together with a functional vasculature and innervation component. Nevertheless, one group has successfully incorporated Langerhans cells in a full-thickness human SE that can mount an effective innate immune response to a topical allergen [153, 154]. Likewise, another group recently reported the first SE containing a perfused vascularized network lined with endothelial cells [155]. Another emerging field with potential to revolutionize skin research is 3D bioprinting [156, 157]. A major goal for 3D skin bioprinting is the ability to print tissue that incorporates a structured microenvironment for self-organization of niche cells such as a sebaceous gland, hair follicle, or as recently demonstrated by Liu et al.—morphogenesis of epidermal progenitors to a sweat gland [158]. Given the increasing appreciation that the skin surface and associated appendages are home to a significant biomass of colonizing microorganisms, recent attempts have been made to construct 3D skin models with a microbiome that is more representative of the skin ecosystem. The Leeds model, designed at the Skin Research Centre in the United Kingdom is based on a polymerized fibrin dermal equivalent of primary human fibroblasts overlayed with primary human keratinocytes that formed a slightly acidic, fully stratified epidermis [159, 160]. This skin equivalent (SE) was successfully colonized by C. acnes and S. epidermidis as well as pathogenic S. aureus strains for up to 120 h under dry, real life conditions. Strikingly, while S. epidermidis colonization had only a minor effect on gene expression, S. aureus modulated the expression of several hundred genes, many involved in inflammation and immune defense [161]. This SE has been commercialized as Labskin® and represents a useful model for investigating microbial and host interactions.
Conclusions
For over 100 years, studies have investigated the role of C. acnes in the pathophysiology of acne vulgaris. To date, this relationship is still very much in debate. However, thanks to recent large-scale sequencing surveys of the microbial communities present on human skin, together with recent metagenomic analyses of the microbiome in multiple skin diseases, we now know that acne vulgaris can be characterized by the dominance of distinct strains of C. acnes as well as increases in S. epidermidis abundance. However, given some of the limitations and biases of the skin sampling methodologies, further research requires more robust methods and approaches. The ability to obtain reliable and quantitative analyses of the microbiome of lesional follicles from acne patients with different disease severity would represent a major breakthrough in the field. Likewise, a longitudinal metagenomic assessment of the phenotypic changes of the microbiome during treatment with antibiotics or isotretinoin would be hugely informative, particularly in patients that are treatment-resistant or relapse after treatment. Ultimately, increased knowledge of the mutualistic and antagonistic interactions between C. acnes and S. epidermidis is crucial not only to better understand the pathophysiology, but also to discover secondary metabolites that can be exploited as a therapeutic strategy. While oral and topical antibiotic therapy remains the mainstay treatment approach for acne patients, the indiscriminate targeting of the microbiome and eradication of important commensal species remains a significant drawback. Moreover, the lack of molecular tools to genetically manipulate C. acnes together with the absence of a representative in vivo model for acne, represent major obstacles for research in this field.
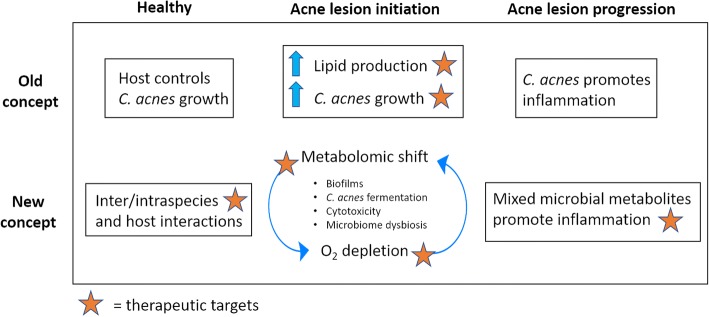
Another exciting area of investigation in the field of acne skin research is the molecular mechanisms that facilitate immune tolerance to colonization by commensal organisms in the skin epithelium and what triggers an inflammatory response in the absence of an infection. We are now beginning to understand how subtle environmental cues can prompt a switch from commensalism to parasitism in C. acnes and S. epidermidis (Fig. 3). One example is the production of immunomodulatory SCFAs generated during hypoxic conditions. SCFAs are not only antimicrobial in nature, potentially contributing to dysbiosis in the skin, but can also modulate the host epigenetic state that potentiates the epithelium towards an excessive inflammatory response against bacterial TLR ligands that under normal conditions, are well tolerated [75]. Nevertheless, the possibility exists that the microbiome dysbiosis observed in acne disease is predominantly mediated by non-microbial factors such as diet, hormones, or even genetic predisposition. However, the abundance of evidence now showing that distinct C. acnes phylotypes and strains induce differential immune responses in multiple cell types, together with new discoveries of novel host-interaction and microbe-interaction genes in C. acnes as well as S. epidermidis, suggest an important role for these organisms in mediating and promoting acne disease.
Fig. 3.
New understandings in the pathophysiology of acne vulgaris
Acknowledgments
Funding
The authors gratefully acknowledge funding from NIH R01AI05243, R01AR069653, R01AI116576, R01AR064781, and U19 AI117673.
Abbreviations
- 2D
Two dimensional
- AD
Atopic dermatitis
- AMP
Antimicrobial peptide
- AOB
Ammonia-oxidizing bacteria
- BP
Benzyl peroxide
- CAMP
Christie Atkins Munch Petersen
- CC
Clonal complex
- CFU
Colony-forming unit
- CRISPR
Clustered regularly interspaced palindromic repeats
- Dsa
Dermatan sulphate adhesion
- FFA
Free fatty acid
- GM-CSF
Granulocyte macrophage colony stimulating factor
- hBD
Human beta defensin
- HKCa
Heat-killed C. acnes
- IL
Interleukin
- LTA
Lipoteichoic acid
- MLST
Multi locus sequence typing
- MMP
Matrix metalloproteinas
- MRSA
Methicillin-resistant S. aureus
- NFκB
Nuclear factor kappa B
- NRP
Nonribosomal peptides
- PAMP
Pathogen-associated molecular pattern
- PGN
Peptidoglycan
- ROS
Reactive oxygen species
- RT
Ribotype
- SCFA
Short-chain fatty acid
- SE
Skin equivalent
- SLST
Single locus sequence typing
- Tad
Tight adhesion
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
Authors’ contributions
AON and RLG both contributed to the writing and editing of this manuscript. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RLG is a consultant and has equity interest in Sente Inc. and MatriSys Bioscience.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alan M. O’Neill, Email: aloneill@ucsd.edu
Richard L. Gallo, Email: rgallo@ucsd.edu
References
- 1.Bhate K., Williams H.C. Epidemiology of acne vulgaris. British Journal of Dermatology. 2013;168(3):474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 2.Dalgard F, Gieler U, Holm JØ, Bjertness E, Hauser S. Self-esteem and body satisfaction among late adolescents with acne: Results from a population survey. J Am Acad Dermatol. 2008;59:746–751. doi: 10.1016/j.jaad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363–370. doi: 10.1038/jid.2010.264. [DOI] [PubMed] [Google Scholar]
- 4.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J, Byrd AL, Deming C, Conlan S. NISC Comparative Sequencing Program, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gribbon EM, Cunliffe WJ, Holland KT. Interaction of Propionibacterium acnes with skin lipids in vitro. J Gen Microbiol. 1993;139:1745–1751. doi: 10.1099/00221287-139-8-1745. [DOI] [PubMed] [Google Scholar]
- 8.Neville BA, Forster SC, Lawley TD. Commensal Koch’s postulates: establishing causation in human microbiota research. Curr Opin Microbiol. 2018;42:47–52. doi: 10.1016/j.mib.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Linfante A, Allawh RM, Allen HB. The role of Propionibacterium acnes biofilm in acne vulgaris. J Clin Exp Dermatol Res. 2018;9:1–4. [Google Scholar]
- 10.Pornpattananangkul D, Fu V, Thamphiwatana S, Zhang L, Chen M, Vecchio J, et al. In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids. Adv Healthc Mater. 2013;2:1322–1328. doi: 10.1002/adhm.201300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jończyk-Matysiak E, Weber-Dąbrowska B, Żaczek M, Międzybrodzki R, Letkiewicz S, Łusiak-Szelchowska M, et al. Prospects of phage application in the treatment of acne caused by Propionibacterium acnes. Front Microbiol. 2017;8. 10.3389/fmicb.2017.00164. [DOI] [PMC free article] [PubMed]
- 12.Brandwein M, Steinberg D, Meshner S. Microbial biofilms and the human skin microbiome. Npj Biofilms Microbiomes. 2016;2:3. doi: 10.1038/s41522-016-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- 14.Jahns AC, Alexeyev OA. Three dimensional distribution of Propionibacterium acnes biofilms in human skin. Exp Dermatol. 2014;23:687–689. doi: 10.1111/exd.12482. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen B, Gonzalez M. A microbial aetiology of acne: what is the evidence? Br J Dermatol. 2011;165:474–485. doi: 10.1111/j.1365-2133.2011.10375.x. [DOI] [PubMed] [Google Scholar]
- 16.Leeming JP, Holland KT, Cuncliffe WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988;118:203–208. doi: 10.1111/j.1365-2133.1988.tb01775.x. [DOI] [PubMed] [Google Scholar]
- 17.Leeming JP, Holland KT, Cunliffe WJ. The pathological and ecological significance of microorganisms colonizing acne vulgaris comedones. J Med Microbiol. 1985;20:11–16. doi: 10.1099/00222615-20-1-11. [DOI] [PubMed] [Google Scholar]
- 18.Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98:895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- 19.Marples RR, Mcginley KJ, Mills OH. Microbiology of comedones in acne vulgaris. J Invest Dermatol. 1973;60:80–83. doi: 10.1111/1523-1747.ep12724149. [DOI] [PubMed] [Google Scholar]
- 20.Fitz-Gibbon S, Tomida S, Chiu B-H, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyden JJ, McGinley KJ, Mills OH, Kligman AM. Propionibacterium levels In patients with and without acne vulgaris. J Invest Dermatol. 1975;65:382–384. doi: 10.1111/1523-1747.ep12607634. [DOI] [PubMed] [Google Scholar]
- 23.Alexeyev OA, Lundskog B, Ganceviciene R, Palmer RH, McDowell A, Patrick S, et al. Pattern of tissue invasion by Propionibacterium acnes in acne vulgaris. J Dermatol Sci. 2012;67:63–66. doi: 10.1016/j.jdermsci.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case–control study. Br J Dermatol. 2012;167:50–58. doi: 10.1111/j.1365-2133.2012.10897.x. [DOI] [PubMed] [Google Scholar]
- 25.Jahns AC, Oprica C, Vassilaki I, Golovleva I, Palmer RH, Alexeyev OA. Simultaneous visualization of Propionibacterium acnes and Propionibacterium granulosum with immunofluorescence and fluorescence in situ hybridization. Anaerobe. 2013;23:48–54. doi: 10.1016/j.anaerobe.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Jahns AC, Golovleva I, Palmer RH, Alexeyev OA. Spatial distribution of bacterial–fungal communities in facial skin. J Dermatol Sci. 2013;70:71–73. doi: 10.1016/j.jdermsci.2012.11.592. [DOI] [PubMed] [Google Scholar]
- 27.Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57:722–724. doi: 10.1016/j.jaad.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Gollnick HPM, Finlay AY, Shear N. Global Alliance to Improve Outcomes in Acne. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9:279–284. doi: 10.2165/00128071-200809050-00001. [DOI] [PubMed] [Google Scholar]
- 29.Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seité S. Skin microbiome and acne vulgaris: Staphylococcus, a new actor in acne. Exp Dermatol. 2017;26:798–803. doi: 10.1111/exd.13296. [DOI] [PubMed] [Google Scholar]
- 30.Bek-Thomsen M, Lomholt HB, Kilian M. Acne is not associated with yet-uncultured Bacteria. J Clin Microbiol. 2008;46:3355–3360. doi: 10.1128/JCM.00799-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 2016;6:srep39491. doi: 10.1038/srep39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall Jacob B., Cong Zhaoyuan, Imamura-Kawasawa Yuka, Kidd Brian A., Dudley Joel T., Thiboutot Diane M., Nelson Amanda M. Isolation and Identification of the Follicular Microbiome: Implications for Acne Research. Journal of Investigative Dermatology. 2018;138(9):2033–2040. doi: 10.1016/j.jid.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Nakatsuji T, Chiang H-I, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013;4:1431. doi: 10.1038/ncomms2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretion. J Clin Microbiol. 1980;12:672–675. doi: 10.1128/jcm.12.5.672-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee S, Mitra R, Maitra A, Gupta S, Kumaran S, Chakrabortty A, et al. Sebum and hydration levels in specific regions of human face significantly predict the nature and diversity of facial skin microbiome. Sci Rep. 2016;6:srep36062. doi: 10.1038/srep36062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen GJM, Scholz CFP, Enghild J, Rohde H, Kilian M, Thürmer A, et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics. 2016;17. 10.1186/s12864-016-2489-5. [DOI] [PMC free article] [PubMed]
- 37.Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE. 2013;e55380:8. doi: 10.1371/journal.pone.0055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Z, Tseng C, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marples RR, Path MRC, Leyden JJ, Stewart RN, Mills OH, Kligman AM. The Skin Microflora in Acne Vulgaris. J Invest Dermatol. 1974;62:37–41. doi: 10.1111/1523-1747.ep12676718. [DOI] [PubMed] [Google Scholar]
- 41.Puhvel SM, Reisner RM, Amirian DA. Quantification of bacteria in isolated pilosebaceous follicles in normal skin. J Invest Dermatol. 1975;65:525–531. doi: 10.1111/1523-1747.ep12610239. [DOI] [PubMed] [Google Scholar]
- 42.Kong HH, Andersson B, Clavel T, Common JE, Jackson SA, Olson ND, et al. Performing skin microbiome research: A method to the madness. J Invest Dermatol. 2017;137:561–568. doi: 10.1016/j.jid.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of “pathogenic”, “commensal” and antibiotic resistant strains. PLoS ONE. 2012;e41480:7. doi: 10.1371/journal.pone.0041480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lomholt HB, Kilian M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS ONE. 2010;5:e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omer H, McDowell A, Alexeyev OA. Understanding the role of Propionibacterium acnes in acne vulgaris: The critical importance of skin sampling methodologies. Clin Dermatol. 2017;35:118–129. doi: 10.1016/j.clindermatol.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Alexeyev OA, Zouboulis CC. Shooting at skin propionibacterium acnes: to be or not to be on target. J Invest Dermatol. 2013;133:2292–2294. doi: 10.1038/jid.2013.116. [DOI] [PubMed] [Google Scholar]
- 47.Craft N, Li H. Response to the commentaries on the paper: Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2295–2297. doi: 10.1038/jid.2013.275. [DOI] [PubMed] [Google Scholar]
- 48.Eady EA, Layton AM. A distinct acne microbiome: fact or fiction? J Invest Dermatol. 2013;133:2294–2295. doi: 10.1038/jid.2013.259. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JL, Cummins CS. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972;109:1047–1066. doi: 10.1128/jb.109.3.1047-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster GF, Cummins CS. Use of bacteriophage typing to distinguish Propionibacterium acne types I and II. J Clin Microbiol. 1978;7:84–90. doi: 10.1128/jcm.7.1.84-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishishita M, Ushijima T, Ozaki Y, Ito Y. Biotyping of Propionibacterium acnes isolated from normal human facial skin. Appl Environ Microbiol. 1979;38:585–589. doi: 10.1128/aem.38.4.585-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, et al. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol. 2005;43:326–334. doi: 10.1128/JCM.43.1.326-334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDowell A, Perry AL, Lambert PA, Patrick S. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57(Pt 2):218–224. doi: 10.1099/jmm.0.47489-0. [DOI] [PubMed] [Google Scholar]
- 54.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology. 2011;157(Pt 7):1990–2003. doi: 10.1099/mic.0.049676-0. [DOI] [PubMed] [Google Scholar]
- 56.Scholz CFP, Jensen A, Lomholt HB, Brüggemann H, Kilian M. A novel high-resolution single locus sequence typing scheme for mixed populations of Propionibacterium acnes in vivo. PLoS ONE. 2014;9:e104199. doi: 10.1371/journal.pone.0104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDowell A. Over a decade of recA and tly gene sequence typing of the skin bacterium Propionibacterium acnes: what have we learnt? Microorganisms. 2017;6:1. doi: 10.3390/microorganisms6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagnelie M-A, Khammari A, Dréno B, Corvec S. Cutibacterium acnes molecular typing: time to standardize the method. Clin Microbiol Infect. 2018. 10.1016/j.cmi.2018.03.010. [DOI] [PubMed]
- 59.Achermann Y, Goldstein EJC, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mollerup S, Friis-Nielsen J, Vinner L, Hansen TA, Richter SR, Fridholm H, et al. Propionibacterium acnes: disease-causing agent or common contaminant? Detection in diverse patient samples by next-generation sequencing. J Clin Microbiol. 2016;54:980–987. doi: 10.1128/JCM.02723-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDowell Andrew, Nagy István, Magyari Márta, Barnard Emma, Patrick Sheila. The Opportunistic Pathogen Propionibacterium acnes: Insights into Typing, Human Disease, Clonal Diversification and CAMP Factor Evolution. PLoS ONE. 2013;8(9):e70897. doi: 10.1371/journal.pone.0070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gehse M, Höffler U, Gloor M, Pulverer G. Propionibacteria in patients with acne vulgaris and in healthy persons. Arch Dermatol Res. 1983;275:100–104. doi: 10.1007/BF00412883. [DOI] [PubMed] [Google Scholar]
- 63.Whiteside JA, Voss JG. Incidence and lipolytic activity of Propionibacterium acnes (Corynebacterium acnes group I) and P. granulosum (C. acnes group II) in acne and in normal skin. J Invest Dermatol. 1973;60:94–97. doi: 10.1111/1523-1747.ep12724177. [DOI] [PubMed] [Google Scholar]
- 64.Mak TN, Schmid M, Brzuszkiewicz E, Zeng G, Meyer R, Sfanos KS, et al. Comparative genomics reveals distinct host-interacting traits of three major human-associated propionibacteria. BMC Genomics. 2013;14:640. doi: 10.1186/1471-2164-14-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21:660–668. doi: 10.1016/j.tim.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol. 2013;25:370–377. doi: 10.1016/j.smim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150:421–428. doi: 10.1046/j.1365-2133.2004.05762.x. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/S1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 70.Jeremy AHT, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Ochoa M-T, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norris JF, Cunliffe WJ. A histological and immunocytochemical study of early acne lesions. Br J Dermatol. 1988;118:651–659. doi: 10.1111/j.1365-2133.1988.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 73.Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. Distinct strains of Propionibacterium acnes induce selective human β-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 74.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Sanford JA, Zhang L-J, Williams MR, Gangoiti JA, Huang C-M, Gallo RL. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016;1:eaah4609. doi: 10.1126/sciimmunol.aah4609. [DOI] [PubMed] [Google Scholar]
- 76.Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- 77.Kim JE, Kim BJ, Jeong MS, Seo SJ, Kim MN, Hong CK, et al. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J Korean Med Sci. 2005;20:649–654. doi: 10.3346/jkms.2005.20.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee D-Y, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang J-M, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill Propionibacterium acnes. J Invest Dermatol. 2008;128:1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brüggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 80.Tomida S, Nguyen L, Chiu B-H, Liu J, Sodergren E, Weinstock GM, et al. Pan-genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. mBio. 2013;4:e00003–e00013. doi: 10.1128/mBio.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brüggemann H, Lomholt HB, Tettelin H, Kilian M. CRISPR/cas loci of type II Propionibacterium acnes confer immunity against acquisition of mobile elements present in type I P. acnes. PLoS ONE. 2012;7:e34171. doi: 10.1371/journal.pone.0034171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholz CFP, Brüggemann H, Lomholt HB, Tettelin H, Kilian M. Genome stability of Propionibacterium acnes: a comprehensive study of indels and homopolymeric tracts. Sci Rep. 2016;6:20662. doi: 10.1038/srep20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- 84.Brouns SJJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJH, Snijders APL, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasimatis G, Fitz-Gibbon S, Tomida S, Wong M, Li H. Analysis of complete genomes of Propionibacterium acnes reveals a novel plasmid and increased pseudogenes in an acne associated strain. BioMed Res Int. 2013;2013:918320. doi: 10.1155/2013/918320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schreiner HC, Sinatra K, Kaplan JB, Furgang D, Kachlany SC, Planet PJ, et al. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc Natl Acad Sci. 2003;100:7295–7300. doi: 10.1073/pnas.1237223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomich M, Planet PJ, Figurski DH. The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol. 2007;5:363–375. doi: 10.1038/nrmicro1636. [DOI] [PubMed] [Google Scholar]
- 88.Strieker M, Tanović A, Marahiel MA. Nonribosomal peptide synthetases: structures and dynamics. Curr Opin Struct Biol. 2010;20:234–240. doi: 10.1016/j.sbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Bek-Thomsen M, Lomholt HB, Scavenius C, Enghild JJ, Brüggemann H. Proteome analysis of human sebaceous follicle infundibula extracted from healthy and acne-affected skin. PLoS ONE. 2014;9:e107908. doi: 10.1371/journal.pone.0107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol. 2016;136:2221–2228. doi: 10.1016/j.jid.2016.06.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230. doi: 10.1186/1471-2180-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Y, Champer J, Kim J. Analysis of the surface, secreted, and intracellular proteome of Propionibacterium acnes. EuPA Open Proteomics. 2015;9:1–7. doi: 10.1016/j.euprot.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeon J, Mok HJ, Choi Y, Park SC, Jo H, Her J, et al. Proteomic analysis of extracellular vesicles derived from Propionibacterium acnes. Proteomics Clin Appl. 2017:11. [DOI] [PubMed]
- 94.Soufi Y, Soufi B. Mass spectrometry-based bacterial proteomics: focus on dermatologic microbial pathogens. Front Microbiol. 2016;7. 10.3389/fmicb.2016.00181. [DOI] [PMC free article] [PubMed]
- 95.Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371–388. doi: 10.2147/CCID.S69135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niazi SA, Clarke D, Do T, Gilbert SC, Mannocci F, Beighton D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J Clin Microbiol. 2010;48:3859–3869. doi: 10.1128/JCM.01326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic Review Series: Skin Lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98:411–424. doi: 10.1007/s00253-013-5394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canovas J, Baldry M, Bojer MS, Andersen PS, Gless BH, Grzeskowiak PK, et al. Cross-talk between Staphylococcus aureus and other Staphylococcal species via the agr quorum sensing system. Front Microbiol. 2016;7. 10.3389/fmicb.2016.01733. [DOI] [PMC free article] [PubMed]
- 100.Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5:183–195. doi: 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakatsuji Teruaki, Chen Tiffany H., Narala Saisindhu, Chun Kimberly A., Two Aimee M., Yun Tong, Shafiq Faiza, Kotol Paul F., Bouslimani Amina, Melnik Alexey V., Latif Haythem, Kim Ji-Nu, Lockhart Alexandre, Artis Keli, David Gloria, Taylor Patricia, Streib Joanne, Dorrestein Pieter C., Grier Alex, Gill Steven R., Zengler Karsten, Hata Tissa R., Leung Donald Y. M., Gallo Richard L. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis . Science Translational Medicine. 2017;9(378):eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 103.Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162–169. doi: 10.4161/derm.1.3.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198–2205. doi: 10.1038/jid.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leyden JJ, James WD. Staphylococcus aureus infection as a complication of isotretinoin therapy. Arch Dermatol. 1987;123:606–608. doi: 10.1001/archderm.1987.01660290074019. [DOI] [PubMed] [Google Scholar]
- 106.Blasiak RC, Stamey CR, Burkhart CN, Lugo-Somolinos A, Morrell DS. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392–1398. doi: 10.1001/jamadermatol.2013.6746. [DOI] [PubMed] [Google Scholar]
- 107.Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H, et al. European recommendations on the use of oral antibiotics for acne. Eur J Dermatol EJD. 2004;14:391–399. [PubMed] [Google Scholar]
- 108.Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:e23–e33. doi: 10.1016/S1473-3099(15)00527-7. [DOI] [PubMed] [Google Scholar]
- 109.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 110.Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6. 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed]
- 111.Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC. Phototherapy in the treatment of acne vulgaris: what is its role? Am J Clin Dermatol. 2004;5:211–216. doi: 10.2165/00128071-200405040-00001. [DOI] [PubMed] [Google Scholar]
- 112.Rassai S, Rafeie E, Ramirez-Fort MK, Feily A. Adjuvant narrow band UVB improves the efficacy of oral azithromycin for the treatment of moderate to severe inflammatory facial acne vulgaris. J Cutan Aesthetic Surg. 2014;7:151–154. doi: 10.4103/0974-2077.146664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weill FS, Cela EM, Paz ML, Ferrari A, Leoni J, González Maglio DH. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br J Nutr. 2013;109:457–466. doi: 10.1017/S0007114512001225. [DOI] [PubMed] [Google Scholar]
- 114.Walter JR, Xu S. Topical drug innovation from 2000 through 2014. JAMA Dermatol. 2015;151:792–794. doi: 10.1001/jamadermatol.2015.0231. [DOI] [PubMed] [Google Scholar]
- 115.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 116.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology. 2017;152:799–811.e7. doi: 10.1053/j.gastro.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 118.Paithankar DY, Sakamoto FH, Farinelli WA, Kositratna G, Blomgren RD, Meyer TJ, et al. Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light-absorbing gold microparticles. J Invest Dermatol. 2015;135:1727–1734. doi: 10.1038/jid.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin M, Landriscina A, Rosen JM, Wei G, Kao S, Olcott W, et al. Nitric oxide–releasing nanoparticles prevent Propionibacterium acnes–induced inflammation by both clearing the organism and inhibiting microbial stimulation of the innate immune response. J Invest Dermatol. 2015;135:2723–2731. doi: 10.1038/jid.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baldwin H, Blanco D, McKeever C, Paz N, Vasquez YN, Quiring J, et al. Results of a phase 2 efficacy and safety study with SB204, an investigational topical nitric oxide-releasing drug for the treatment of acne vulgaris. J Clin Aesthetic Dermatol. 2016;9:12–18. [PMC free article] [PubMed] [Google Scholar]
- 121.Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. J Appl Microbiol. 2001;90:648–652. doi: 10.1046/j.1365-2672.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- 122.Luo J, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin. 2005;26:259–264. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 123.Bowe WP, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future? Gut Pathog. 2011;3:1. doi: 10.1186/1757-4749-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5:185–199. doi: 10.3920/BM2012.0060. [DOI] [PubMed] [Google Scholar]
- 125.Yeşilova Y, Çalka Ö, Akdeniz N, Berktaş M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24:189–193. doi: 10.5021/ad.2012.24.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J, Ko Y, Park Y-K, Kim N-I, Ha W-K, Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26:902–909. doi: 10.1016/j.nut.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 127.Hacini-Rachinel Feriel, Gheit Hanane, Le Luduec Jean-Benoit, Dif Fariel, Nancey Stéphane, Kaiserlian Dominique. Oral Probiotic Control Skin Inflammation by Acting on Both Effector and Regulatory T Cells. PLoS ONE. 2009;4(3):e4903. doi: 10.1371/journal.pone.0004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gueniche A, Philippe D, Bastien P, Reuteler G, Blum S, Castiel-Higounenc I, et al. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef Microbes. 2013;5:137–145. doi: 10.3920/BM2013.0001. [DOI] [PubMed] [Google Scholar]
- 129.Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, et al. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE. 2012;7:e38499. doi: 10.1371/journal.pone.0038499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brzin B. Studies on the Corynebacterium acnes. Acta Pathol Microbiol Scand. 1964;60:599–608. doi: 10.1111/apm.1964.60.4.599. [DOI] [PubMed] [Google Scholar]
- 131.Zierdt CH, Webster C, Rude WS. Study of the anaerobic corynebacteria. Int J Syst Evol Microbiol. 1968;18:33–47. [Google Scholar]
- 132.Liu J, Yan R, Zhong Q, Ngo S, Bangayan NJ, Nguyen L, et al. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J. 2015;9:2078–2093. doi: 10.1038/ismej.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakatsuji T, Liu Y-T, Huang C-P, Zoubouis CC, Gallo RL, Huang C-M. Antibodies elicited by inactivated Propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008;128:2451–2457. doi: 10.1038/jid.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakatsuji T, Liu Y-T, Huang C-P, Gallo RL, Huang C-M. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS ONE. 2008;3:e1551. doi: 10.1371/journal.pone.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y, Hata TR, Tong YL, Kao M-S, Zouboulis CC, Gallo RL, et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J Invest Dermatol. 2018. 10.1016/j.jid.2018.05.032. [DOI] [PubMed]
- 136.Kitagawa H, Yamanaka K, Kakeda M, Inada H, Imai Y, Gabazza EC, et al. Propionibacterium acnes vaccination induces regulatory T cells and Th1 immune responses and improves mouse atopic dermatitis. Exp Dermatol. 2011;20:157–158. doi: 10.1111/j.1600-0625.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- 137.Li L, Sun C, Yang F, Yang S, Feng X, Gu J, et al. Identification of proteins of Propionibacterium acnes for use as vaccine candidates to prevent infection by the pig pathogen Actinobacillus pleuropneumoniae. Vaccine. 2013;31:5269–5275. doi: 10.1016/j.vaccine.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 138.Achermann Y, Tran B, Kang M, Harro JM, Shirtliff ME. Immunoproteomic identification of in vivo-produced Propionibacterium acnes proteins in a rabbit biofilm infection model. Clin Vaccine Immunol. 2015;22:467–476. doi: 10.1128/CVI.00760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hay Michael, Thomas David W, Craighead John L, Economides Celia, Rosenthal Jesse. Clinical development success rates for investigational drugs. Nature Biotechnology. 2014;32(1):40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 140.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007;26:195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- 142.Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980;40:27–32. [PMC free article] [PubMed] [Google Scholar]
- 143.Lu GW, Valiveti S, Spence J, Zhuang C, Robosky L, Wade K, et al. Comparison of artificial sebum with human and hamster sebum samples. Int J Pharm. 2009;367:37–43. doi: 10.1016/j.ijpharm.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 144.Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981;41:1269–1270. doi: 10.1128/aem.41.5.1269-1270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nikkari T. Comparative chemistry of sebum. J Invest Dermatol. 1974;62:257–267. doi: 10.1111/1523-1747.ep12676800. [DOI] [PubMed] [Google Scholar]
- 146.Nakatsuji T, Shi Y, Zhu W, Huang C-P, Chen Y-R, Lee D-Y, et al. Bioengineering a humanized acne microenvironment model: Proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008;8:3406–3415. doi: 10.1002/pmic.200800044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jang YH, Lee KC, Lee S-J, Kim DW, Lee WJ. HR-1 Mice: A new inflammatory acne mouse model. Ann Dermatol. 2015;27:257–264. doi: 10.5021/ad.2015.27.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975;16:434–440. [PubMed] [Google Scholar]
- 149.Sato K, Sugibayashi K, Morimoto Y. Species differences in percutaneous absorption of nicorandil. J Pharm Sci. 1991;80:104–107. doi: 10.1002/jps.2600800203. [DOI] [PubMed] [Google Scholar]
- 150.Mullard A. Parsing clinical success rates. Nat Rev Drug Discov. 2016;15:447. doi: 10.1038/nrd.2016.136. [DOI] [PubMed] [Google Scholar]
- 151.Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FDP. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230:16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- 152.Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering — In vivo and in vitro applications. Adv Drug Deliv Rev. 2011;63:352–366. doi: 10.1016/j.addr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 153.Kosten Ilona J., Spiekstra Sander W., de Gruijl Tanja D., Gibbs Susan. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicology and Applied Pharmacology. 2015;287(1):35–42. doi: 10.1016/j.taap.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 154.Ouwehand K, Spiekstra SW, Waaijman T, Scheper RJ, de Gruijl TD, Gibbs S. Technical advance: Langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J Leukoc Biol. 2011;90:1027–1033. doi: 10.1189/jlb.0610374. [DOI] [PubMed] [Google Scholar]
- 155.Groeber F. A first vascularized skin equivalent for as an alternative to animal experimentation. ALTEX. 2016. 10.14573/altex.1604041. [DOI] [PubMed]
- 156.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 157.Zhang B, Luo Y, Ma L, Gao L, Li Y, Xue Q, et al. 3D bioprinting: an emerging technology full of opportunities and challenges. Bio-Des Manuf. 2018;1:2–13. doi: 10.1007/s42242-018-0004-3. [DOI] [Google Scholar]
- 158.Liu N, Huang S, Yao B, Xie J, Wu X, Fu X. 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Sci Rep. 2016;6:34410. doi: 10.1038/srep34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bojar RA. Studying the human skin microbiome using 3D in vitro skin models. Appl Vitro Toxicol. 2015;1:165–171. doi: 10.1089/aivt.2015.0002. [DOI] [Google Scholar]
- 160.Holland DB, Bojar RA, Jeremy AHT, Ingham E, Holland KT. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent – a novel approach. FEMS Microbiol Lett. 2008;279:110–115. doi: 10.1111/j.1574-6968.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 161.Holland DB, Bojar RA, Farrar MD, Holland KT. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol Lett. 2009;290:149–155. doi: 10.1111/j.1574-6968.2008.01402.x. [DOI] [PubMed] [Google Scholar]