Abstract
A minimum amount of energy is required for basic physiological processes, such as protein biosynthesis, thermoregulation, locomotion, cardiovascular function, and digestion. However, for reproductive function and survival of the species, extra energy stores are necessary. Production of sex hormones and gametes, pubertal development, pregnancy, lactation, and parental care all require energy reserves. Thus the physiological systems that control energy homeostasis and reproductive function coevolved in mammals to support both individual health and species subsistence. In this review, we aim to gather scientific knowledge produced by laboratories around the world on the role of the brain in integrating metabolism and reproduction. We describe essential neuronal networks, highlighting key nodes and potential downstream targets. Novel animal models and genetic tools have produced substantial advances, but critical gaps remain. In times of soaring worldwide obesity and metabolic dysfunction, understanding the mechanisms by which metabolic stress alters reproductive physiology has become crucial for human health.
I. INTRODUCTION
Animal life requires sufficient energy for basic physiological processes (e.g., protein biosynthesis, thermoregulation, locomotion, cardiovascular and brain function, digestion). However, reproducing requires additional energy use. The production of sex hormones and gametes, pubertal development, searching and competing for sex mates, territoriality, sexual behavior, pregnancy, lactation, and parental care all demand extra energy stores. For this reason, the physiological systems that control energy homeostasis and reproductive function coevolved to support successful reproduction and the survival of the species (138, 144, 378, 442).
Different species have developed distinct reproductive strategies to adapt to specific environmental constraints (83, 140, 399). These strategies synchronize reproduction with the best environmental conditions for bearing and feeding offspring. While metabolic signals are crucial for fertility in many species, for others, photoperiods, temperature, and humidity play stronger roles. In this review, we will not discuss these alternative environmental limitations. Instead, we will emphasize the scientific literature focusing on the brain circuitry that coordinates energy availability with reproduction and the mechanisms by which metabolic stress may alter fertility.
The modulatory effect of nutritional status on reproductive physiology has been known for centuries, from Aristotle to Darwin and among farmers. This idea originated from observations that domesticated animals with regular access to food show early puberty and have improved reproductive health. In seminal experimental studies, Kennedy and Mitra (240) found that the time of puberty onset in rats correlates with body size, not chronological age. Subsequently, Frisch (157, 158) proposed that a critical threshold of body fat is necessary for sexual maturation, based on epidemiological studies in humans.
In the past decade, the use of murine models and genetic tools has rapidly expanded our understanding of the key mechanisms associated with energy homeostasis and reproduction. The brain plays a critical role in sensing the internal environment, integrating these complex systems, and producing the appropriate neuroendocrine and behavioral responses. Following a comprehensive analysis of findings from laboratories around the world, we will propose a framework for the brain circuitry engaged in the metabolic control of reproductive physiology. Despite substantial advances, many questions remain unanswered. By identifying these gaps, we hope to inform future research. Finally, we will highlight the relevance of the scientific data collected in animal models to human reproductive health.
II. NEURAL SUBSTRATES OF REPRODUCTIVE PHYSIOLOGY
The neural control of reproductive physiology depends on sensory signals from the internal and external environment, brain integrative centers, and motor outputs. Sensory signals include sex steroids, metabolites, and peptide hormones that convey information about the individual’s reproductive status (e.g., age, estrous/menstrual stage, pregnancy) and environmental cues (e.g., presence of sex mates, nutrition, and photoperiod in seasonal breeders) to specific brain nuclei housing first-order neurons. These physiological signals are then processed in integrative sites that orchestrate a series of coordinated motor responses via the neuroendocrine system and behavioral output.
A. Neuroendocrine Control
The ultimate goal of sexual reproduction is gamete contact (oocytes and spermatocytes). In viviparous females, oogenesis starts during embryonic development, is arrested around birth, and resumes at puberty; in males, spermatogenesis is initiated during pubertal development (363, 420, 441). Puberty is defined as the transition to sexual maturity, conferring the ability to reproduce. It is a complex physiological process triggered by the activation of the hypothalamo-pituitary-gonadal (HPG) axis (338, 363, 420). The gonadotropin releasing hormone (GnRH) neurons are the final neural path for activation and coordinated control of the neuroendocrine axis during pubertal maturation and in the adult life. Numbering ~800 in rodents and 2,000 in humans, GnRH neurons are widespread in the hypothalamus of most species and in the medial septum of rodents (244, 414, 416).
GnRH neurons originate in the olfactory epithelium and migrate into the brain during embryonic development (387, 404, 446, 499). Migrating GnRH neurons follow the vomeronasal nerves caudally into the hypothalamus (and medial septum, in rodents), extending processes into the vascular organ of the lamina terminalis and the median eminence (446, 506). Before birth, GnRH cell bodies reach their final destination (the hypothalamus and medial septum), and GnRH fibers densely innervate the external layer of the median eminence, ending in the portal vasculature.
GnRH neurons exhibit several prenatal and early postnatal periods of activity, and then become relatively quiescent (361) until pubertal maturation, when the “reactivation” of GnRH pulsatile secretion triggers puberty in both rodents and humans (338, 363, 420). The influence of steroid hormones during the period of the central reactivation of GnRH release differs between species. Gonadal hormones are not required for GnRH pulse generator “reactivation” in primates, since puberty can occur despite gonadal dysgenesis or castration (95, 362, 365). In contrast, in rodents, gonadotropin secretion is suppressed by small amounts of gonadal steroids after birth through the juvenile period. In peripubertal mice and rats, low levels of steroids are no longer inhibitory (15, 16, 338, 458). The increase in GnRH pulsatile release induces gonadotropin [luteinizing hormone (LH) and follicle stimulating hormone (FSH)] synthesis and secretion from the gonadotropes in the anterior pituitary gland (adeno-hypophysis). Higher levels of gonadotropins stimulate the full development of the gonads, the synthesis and secretion of gonadal steroids, and the maturation of the gametes. Thus the precise and coordinated stimulation of GnRH neuronal activity and GnRH secretion triggers sexual maturation. However, the signals or physiological changes that increase GnRH pulsatile release at the onset of puberty are not completely known. For example, whether prepubertal GnRH neurons are under a high inhibitory restraint or low excitatory drive is still debated (86, 338). Several studies have shown that an increase in excitatory input and/or in the sensitivity of GnRH neurons to neurotransmitters (e.g., GABA, glutamate) or neuropeptides (e.g., kisspeptin) is crucial for puberty onset (51, 191, 338). Additionally, decreased sensitivity to the negative-feedback actions of estradiol and the removal of inhibitory forces also play a crucial role (86, 299, 338, 363, 441). Whether changes in neuronal inputs, receptor sensitivity, epigenomic or neuronal/glial remodeling are the drivers of these physiological changes and, if so, what triggers them is unknown. A variety of internal and external signals act as permissive factors for pubertal maturation (338, 368). Among them, nutrition and energy sufficiency are critical factors in this process (138, 144, 378).
Electron microscopy studies have shown that GnRH occupies dense core vesicles in most cellular compartments, including in cell bodies, dendrites, axons, and terminals (179, 270, 367). Therefore, dendrites and axons may release GnRH not only in the median eminence but also in the preoptic area, medial septum, and areas where neuronal processes reside (159, 178). Because of this combined input/output function of GnRH processes (dendrites and axons), investigators in the GnRH field have dubbed them “dendrons” (203). Neuromodulatory peptides such as kisspeptin and gonadotropin inhibitory hormone (GnIH) differentially regulate the release of GnRH in the median eminence and preoptic area (178, 255). GnRH may therefore be released in response to inputs arriving at the soma or directly at the terminals in the median eminence. Whether these differential effects are components of the same physiological output is still unknown.
GnRH is released into the median eminence in distinct modes depending on reproductive stage, such as puberty, phase of the estrous/menstrual cycle, and aging. Experiments using immortalized GnRH neurons and primary cell culture have shown that GnRH neurons maintain intrinsic activity sufficient for the release of GnRH in a pulsatile manner (257, 305, 486). Burst firing, synchronized calcium oscillations, and GnRH release continue to occur in GnRH neurons isolated from the embryonic olfactory placode of most mammals (131, 160, 443). These observations indicate that GnRH neurons have an intrinsic pulsatile activity and suggest that the coordination of the GnRH network controls gonadotropin secretion. In fact, neuroanatomical studies have shown that cell-to-cell contact between GnRH neurons creates an interconnected network that coordinates the activity of GnRH neurons as a whole (64, 65). Although these in vitro and anatomical findings are solid, several pieces of the puzzle are still missing. For example, it is unclear whether GnRH neurons express the GnRH receptor (482). In rhesus monkeys, the intracerebroventricular administration of GnRH or an antagonist (antide) failed to change multi-unit activity at the mediobasal hypothalamus that was synchronized with LH pulses, despite having an effect on LH secretion (341). In rats, whereas central GnRH induces LH secretion, it has no effect on LH pulsatility (44). Furthermore, the role of other neurotransmitters or neuromodulators coexpressed by GnRH cells in the synchronization of GnRH activity and release has not been demonstrated.
In most rodents and primates, changing levels of sex steroids modulate the activity of GnRH neurons across the estrous/menstrual cycle. In the initial phase of follicular growth, estradiol is low, exerting a negative feedback that maintains low GnRH and LH levels. With the maturation of the follicles, increased secretion of estradiol stimulates GnRH and LH secretion (positive feedback) (183, 202, 274, 311, 312). In concert with the actions of estradiol on the pituitary gland, this abrupt outpouring of GnRH triggers the preovulatory gonadotropin surge, ultimately leading to ovulation (183, 202, 274, 311, 312). The LH surge in rodents is triggered by the concurrence of a circadian signal with high levels of estradiol observed in the late follicular phase (79–81, 267) While progesterone has been implicated in increasing the size of the LH surge, it is not required in rodents; instead, it coordinates mating and ovulation (46, 53, 184, 185, 306). FIGURE 1 illustrates the main components of the HPG axis in humans.
FIGURE 1.
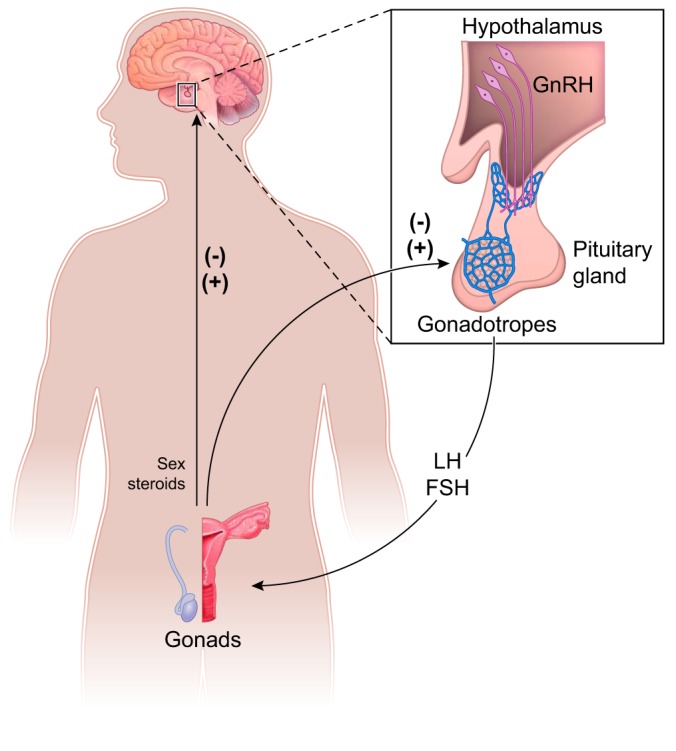
Schematic illustration showing the basic organization of the hypothalamo-pituitary-gonadal (HPG) axis of humans. Gonadotropin releasing hormone (GnRH) neurons located in the hypothalamus project to the median eminence where GnRH is released from the terminals into portal vessels. GnRH acts in gonadotropes located in the anterior pituitary gland inducing synthesis and secretion of gonadotropins, i.e., luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The gonadotropins induce production of sex steroids that feed back into the pituitary gland and hypothalamus. -, Negative feedback; +, positive feedback in females.
The negative- and positive-feedback actions of estradiol on GnRH pulsatile release are achieved mainly via binding to estrogen receptor α (ERα), encoded by the Ers1 gene (99). Because Ers1 expression is undetectable in GnRH neurons, interneurons are essential for controlling the female reproductive cycle (333, 492). Kisspeptin is the most potent GnRH secretagogue identified in mammals. Several excellent reviews of the role of kisspeptin in the neuroendocrine control of reproductive function have been published in recent years and should be consulted for details (93, 269, 335, 359). Loss-of-function mutations in KISS1/Kiss1 or kisspeptin receptor (GPR54/Gpr54, also known as Kiss1r) genes cause infertility in humans and mice due to a lack of pubertal development and hypogonadotropic hypogonadism (107, 119, 262, 408, 450). A steady increase in hypothalamic Kiss1 and Gpr54 gene expression is observed across pubertal development, and administration of kisspeptin to juvenile rodents advances puberty, increases LH secretion, and induces ovulation (328, 411, 440). Kisspeptin acts directly on GnRH neurons to control their activity and neuropeptide release via two distinct neuronal populations: 1) kisspeptin neurons in the anteroventral periventricular and preoptic periventricular nuclei (AVPV/PVpo, also called the rostral periventricular area of the third ventricle, or RP3V); and 2) kisspeptin neurons in the arcuate nucleus (ARH) (202, 425, 426). Both populations coexpress Esr1 and are tightly regulated by estradiol (155, 170, 269, 356, 424). Generally, AVPV/PVpo neurons are believed to convey the positive-feedback actions of estradiol, while ARH neurons convey the negative-feedback actions of estradiol to GnRH neurons in rodents. AVPV/PVpo kisspeptin neurons predominate in females, project to GnRH neurons, have ion channels and Kiss1 expression modulated by estradiol levels throughout the estrous cycle, and are activated at the time of the surge (1, 85, 357, 427, 473).
Kisspeptin neurons in the ARH coexpress neurokinin B (NKB) along with its receptor NK3R (116, 329). NKB appears to serve as an autoregulatory signal for ARH kisspeptin neurons, stimulating kisspeptin output to GnRH neurons (373, 374). Humans with inactivated mutations of the genes encoding NKB (TAC3, whose ortholog is Tac2 in rodents) or its receptor, NK3R (TACR3), suffer a lack of pubertal maturation and central hypogonadism (449), a phenotype reminiscent of patients with mutations of KISS1 or KISS1R (GPR54) genes. ARH kisspeptin neurons also express dynorphin (57, 269), an endogenous opioid peptide that selectively binds the κ-opioid receptor (KOR). The administration of a KOR antagonist advances pubertal development and increases LH pulse frequency in juvenile and adult rats (316, 326). Because of the coexpression of these three neuropeptides, ARH kisspeptin neurons have been named KNDy neurons. Double immunoelectron microscopy has revealed that NKB, dynorphin, and kisspeptin are contained in individual neurosecretory vesicles within kisspeptin neurons, suggesting that the synthesis of individual KNDy peptides is differentially regulated (321). It has been proposed that, when synthesized, NKB primarily contributes to the onset of kisspeptin activation and, subsequently, the preferential production of dynorphin terminates that action in KNDy neurons.
Similar to estradiol, metabolic cues affect GnRH function primarily by acting on afferent neurons. This evolutionary choice increases complexity and redundancy, allowing a more integrated and precise control of motor/endocrine responses and support in case of disruption of one path. In this review, we will describe the neural circuitry engaged in transmitting to GnRH neurons information on the immediate nutritional status and the amount of available energy stores. We will also discuss the role of kisspeptin as one of the best-known conveyors of metabolic status to the HPG axis.
B. Behavioral Output
The production of sex steroids induced by the neuroendocrine reproductive axis allows or facilitates behavioral responses in both sexes. Reproductive behavior encompasses numerous processes including courtship, sexual, aggression, and parental behaviors, all of which are vital to ensuring the survival of the species.
Research using rodents as a model species has yielded quantifiable parameters of sexual behavior in males and females. Once the opposite sex is introduced, the cycle of copulatory activity begins with an arousal phase, during which the sex and physiological competence of the new animal is assessed through anogenital sniffing and increased locomotor activity (354). This chemosensory input is essential for initiating copulatory behavior (120). Sensory neurons in the olfactory epithelium sense volatile odorants and send projections to the main olfactory bulbs. Damage to this system eliminates copulation in mice, independent of sexual experience (238, 289, 390). In addition, sensory neurons in the vomeronasal organ along the ventral midline of the nasal cavity perceive nonvolatile pheromones during physical contact. Evidence shows that the accessory olfactory system motivates males to seek female urinary pheromones and augments their sexual arousal to promote intromission (258). Females also require continued accessory olfactory signaling for maximum sexual responsiveness (300).
Both estrogens and progesterone facilitate female mating behavior in many species including rodents and primates (114, 428, 471, 511). In rodents, female sexual arousal and receptivity correlate with a rise in estradiol and progesterone before ovulation. Estrogens (via aromatization of testosterone) are also the main regulators of male sexual behavior, although the degree to which they impact behavior is species-dependent (217, 388).
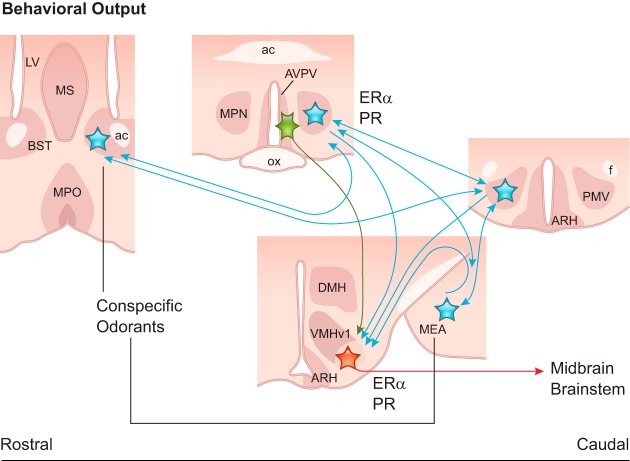
Main and accessory olfactory systems convey odorant signals (arousal cues) to sex steroid-primed brain circuits to the medial subdivision of the bed nucleus of the stria terminalis (BST), the medial nucleus of the amygdala (MEA), that in turn target the hypothalamic medial preoptic nucleus (MPN) and the ventral premammillary nucleus (PMV) (35, 96, 98, 126, 272, 396, 495). These areas are highly interconnected and converge into the ventrolateral subdivision of the hypothalamic ventromedial nucleus (VMHvl) that is essential for mating behavior in both sexes (32, 47, 48, 264) (FIGURE 2). Stimulation of the VMHvl facilitates lordosis and receptivity in females (253, 352), and VMHvl lesions abolish the lordotic response in females and the ability to mount in males (351). The VMHvl projects to the lateral and ventrolateral columns of the periaqueductal gray matter, hindbrain areas that integrate sensorial, endocrine, and autonomic inputs and modulate the motor responses of sexual behavior (66, 418).
FIGURE 2.
Schematic illustration showing coronal sections of the brain sites associated with the basic behavioral output in rodents. The presence of a potential sex mate is conveyed by odorants via the main and accessory olfactory systems to the medial subdivision of the bed nucleus of the stria terminalis (BST) and to the medial nucleus of the amygdala (MEA). These nuclei innervate hypothalamic sites associated with reproductive control including the medial preoptic nucleus (MPN) and the ventral premammillary nucleus (PMV). These areas share reciprocal connections and innervate (blue) the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl), the main output (red) to midbrain and brainstem sites and behavioral control. Kisspeptin neurons (green) in the anteroventral periventricular nucleus (AVPV) also target the VMHvl conveying information about the reproductive status. ac, anterior commissure; ARH, arcuate nucleus; DMH, dorsomedial nucleus; ERα, estrogen receptor α; f, fornix; LV, lateral ventricle; MPO, medial preoptic area; MS, medial septum; PR, progesterone receptor.
The activation of the neuronal circuitry responsible for copulatory behaviors depends on the normal functioning of the HPG axis. Notably, AVPV kisspeptin projections to VMHvl are crucial for the function of this circuitry (198). Without stimulation of these circuits by sex steroids, copulation fails. Therefore, the behavioral aspects of fertility depend on the normal functioning of the HPG axis, and the factors that alter GnRH release are likely to modify sexual behavior. These factors include nutrition and energy stored as fat.
III. CNS INTEGRATION OF METABOLIC AND REPRODUCTIVE FUNCTION
A. Sensing Metabolic Cues
To integrate metabolism and reproduction, the central nervous system (CNS) relies primarily on inputs carrying information on the immediate nutritional state and the status of energy stores. These inputs, generally called “metabolic cues,” originate either from the breakdown of nutrients into metabolites and oxidizable fuels acting directly on the CNS (e.g., amino acids, glucose, free fatty acids) or from hormones secreted into the circulation by metabolic tissues. Among them, white adipocytes (e.g., leptin, adiponectin, resistin), the pancreatic islets (e.g., insulin, glucagon), and the digestive tract and accessory organs [e.g., ghrelin, glucagon-like peptide 1 (GLP1), fibroblast growth factor 21 (FGF21), insulin-like growth factor 1 (IGF1)] are the main sources of metabolically relevant hormones (FIGURE 3). In most cases, these hormones directly target first-order neurons in hypothalamic and brainstem nuclei upstream of GnRH neurons.
FIGURE 3.
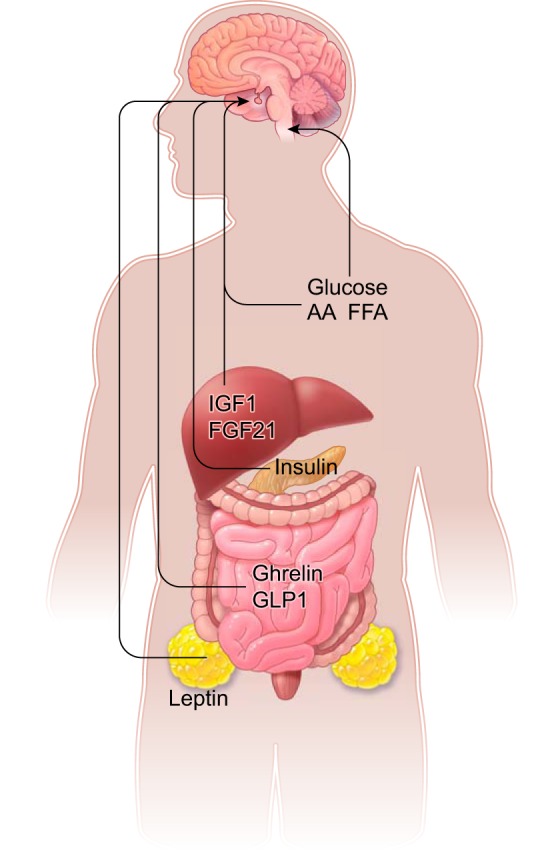
Schematic illustration showing the metabolic cues and associated organs with function in the control of reproduction via actions in the central neurons system. Leptin (produced by white adipocytes), insulin (secreted by pancreatic islets), ghrelin and glucagon-like peptide 1 (GLP1, produced by the gastrointestinal tract), fibroblast growth factor 21 (FGF21) and insulin-like growth factor 1 (IGF1), both produced by the liver, and circulating metabolites [glucose, amino acids (AA), and free fatty acids (FFA)] target neurons in the hypothalamus or caudal medulla (glucose).
To exert their effects, the metabolic hormones must cross the blood-brain barrier (BBB) and access cognate receptors in the brain parenchyma. The BBB is a dynamic structure that regulates the influx and efflux of ions, nutrients, and hormones and restricts the access of pathogens (26, 236). It consists of distinct cells from the brain parenchyma (i.e., neurons, astrocytes, pericytes, glia) and specialized endothelial cells, together named the “neurovascular unit.” The BBB exists throughout the CNS with the exception of the circumventricular organs. The circumventricular organs contain fenestrated blood vessels that allow the diffusion and interchange of large molecules (i.e., peptides and hormones) between the neural tissue and the circulation, possibly without the need for active transport (52, 165, 228). Among the well-described circumventricular organs, the vascular organ of the lamina terminalis and the median eminence are of particular interest given their proximity to and functional relationship with reproductive regulatory neurons in the MPO, AVPV/PVpo, and ARH (202, 335, 359). At these sites, metabolic cues have access to the adjacent neural tissue and bind to their receptors. Outside the circumventricular organs, metabolic cues cross the BBB via two known mechanisms: 1) lipid-mediated free diffusion or 2) carrier- or receptor-mediated active transport. Most metabolic hormones (e.g., leptin, insulin and ghrelin) have carriers or transporters that facilitate their access to deeper brain sites (23, 25–27).
B. Metabolic Cues With Primary Targets in the CNS
The metabolic control of the reproductive system is ultimately exerted by the modulation of the activity of GnRH neurons and changes in the pulsatile release of GnRH into the portal vessels of the median eminence. Altered GnRH pulsatile release (exacerbated or insufficient) disrupts gonadotropin secretion and, consequently, sex steroid production impairing gametogenesis and sexual behavior (294, 487). Metabolic cues modulate GnRH neuronal activity, GnRH release, and sexual behavior via distinct mechanisms and brain circuitry.
1. Adipocyte-derived hormones: leptin
The adipose tissue secretes hormones (adipokines) that are key for whole body physiology (4, 167, 431). The imbalance of these hormones due to excess, absent, or dysfunctional adipocytes disrupts homeostasis and has deleterious consequences for individual health. Several adipokines directly affect the reproductive function. However, only leptin has been implicated in reproductive control via actions in the CNS.
Seminal findings showed that loss-of-function mutations in leptin (Lep/LEP) or leptin receptor (Lepr/LEPR) genes cause metabolic disorders and infertility in rodents and humans (88, 92, 147, 218, 222, 513). These autosomal recessive mutations were initially identified in mice at the Jackson Laboratories (named ob/ob or Lepob for obese and db/db or Leprdb for diabetes) (218, 222). Mice homozygous for Lep or Lepr mutations are morbidly obese due to hyperphagic behavior, decreased energy expenditure, and multiple neuroendocrine dysfunctions (92, 218, 222). At peripubertal stages, the reproductive organs of mutant mice resemble those of wild types at the same developmental stage, but sexual maturation is arrested (28, 75, 317). Leptin-deficient mice have a typical distribution of GnRH somata and terminals and high hypothalamic GnRH content but low circulating levels of gonadotropins (30, 437). Exogenous GnRH administration induces LH secretion, suggesting that leptin-deficient mice have functional gonadotropes but deficient GnRH release (30, 488). Indeed, in vitro and in vivo studies have shown that leptin acts primarily in the brain by stimulating GnRH secretion (140, 493, 509).
The administration of leptin at doses that do not decrease body weight advances the onset of puberty in female wild-type mice (5, 168). In prepubertal female rats, leptin increases the secretion of gonadotropins and the expression of ovarian steroidogenesis enzymes, contributing to pubertal development (12). In doses associated with suppressed food intake and decreased body weight, leptin does not advance the age of puberty onset. However, control pair-fed mice showed delayed sexual maturation, suggesting that higher leptin levels in leptin-treated animals neutralized the delay in puberty caused by a state of negative energy balance (76, 77, 187). Moreover, the overexpression of leptin in transgenic skinny mice induced early puberty onset with uterine and ovarian development, indicative of accelerated activity of the HPG axis (510). The skinny female mice also showed early reproductive senescence, indicating that hyperleptinemia induces premature reproductive failure. Whether this mechanism explains the advanced puberty in obese girls or the dysfunctional activity of the HPG axis in obese individuals remains to be directly addressed (10, 43, 59, 133, 138, 227, 442).
Circulating levels of leptin correlate with stored white adipose tissue mass and follow a circadian pattern in rodents and primates (7, 94, 275, 276, 284, 513). However, during acute fasting or calorie restriction, unknown mechanisms cause leptin levels to fall before the depletion of adipocyte stores. First-order neurons perceive the decrease in leptin levels as a state of energy insufficiency. As a result, counter-regulatory responses are triggered to retain the energy necessary for basic physiological needs (8, 73, 151). Examples of adaptive responses are decreases in thermogenesis and locomotor activity, inhibition of the thyroid axis, and activation of the hypothalamo-pituitary-adrenal (HPA) axis (6, 8, 9, 72, 197, 268, 275, 323). Because of the high energetic costs of reproduction, the HPG axis is inhibited during fasting or in conditions of low leptin levels. Rodents and primates in a negative energy balance exhibit decreases in pulsatile LH secretion, sex steroids, and fertility (60, 62, 63, 140, 292). In several species, following distinct experimental paradigms, leptin administration during states of negative energy balance increases LH pulsatile release, restores female cyclicity, and improves fertility (8, 69, 181, 324, 478). The effect of leptin to increase LH was not observed in calorie-restricted rats under constant leptin administration using osmotic pumps (453). Because of the pulsatile nature of neuroendocrine control and the circadian patter of leptin secretion, it is not clear if the lack of fluctuations in circulating levels disrupted the physiological effects of leptin on the reproductive axis. In women with hypothalamic amenorrhea due to extreme exercise and weight loss, leptin increases LH pulsatility, sex steroid levels, ovarian volume, and the number of maturing follicles (73, 276, 309, 476, 481). Therefore, leptin serves as a signal of energy sufficiency for sexual maturation and maintenance of fertility in humans and rodents. In seasonal breeders, however, the role of leptin becomes secondary, as the main environmental signals are conveyed by changes in photoperiod (399, 400).
Leptin’s actions on reproductive function are mediated by LepR expressed in the CNS (11, 91, 118). Studies using different approaches have determined that these effects are exerted by neurons upstream of GnRH cells (127, 280, 371). Since LepR expression only in calmodulin-dependent protein kinase IIα (CaMKIIα) neurons can maintain reproductive function, the forebrain is believed to be the main player in leptin’s effect on the HPG axis (371). Work from several groups has identified the PMV, the medial preoptic area (MPO), and the ARH as the main targets of leptin action in the neuroendocrine reproductive axis (33, 125, 127, 137, 272, 280, 290, 345, 412) (FIGURE 4).
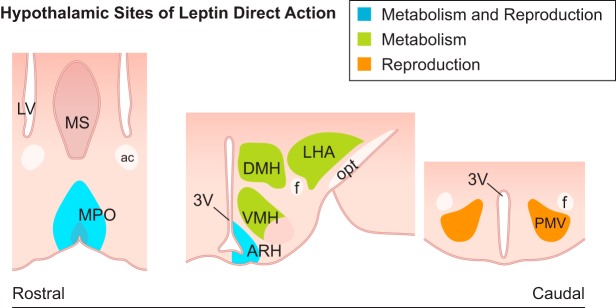
FIGURE 4.
Schematic illustration showing coronal sections of hypothalamic sites directly targeted by leptin in mice. The neuronal populations associated with leptin action in metabolic and reproductive control, i.e., the medial preoptic area (MPO) and the arcuate nucleus (ARH), are represented in blue. Green highlights the sites associated with distinct aspects of the metabolic control, including the dorsomedial subdivision of the ventromedial nucleus (VMH), the dorsomedial nucleus (DMH), and the lateral hypothalamic area (LHA). In orange is represented the ventral premammillary nucleus (PMV), associated with reproductive control. 3V, third ventricle; ac, anterior commissure; f, fornix; LV, lateral ventricle; MS, medial septum; opt, optic tract.
The role of the PMV as a primary site of leptin action in reproductive control was demonstrated by studies using lesions and viro-genetic manipulation in rodents (125, 127). Obese and infertile LepR-null female mice showed pubertal development and improved fertility after the reexpression of LepR in PMV neurons. Thus leptin action in the PMV is a sufficient stimulus for the reproductive axis (127). Excitotoxic lesions of the PMV of rats decreased the activation of GnRH and AVPV neurons and the circulating levels of LH and estradiol during proestrus. They also blunted the ability of leptin to increase LH during fasting (125). In ob/ob mice, PMV lesions caused a significant delay in pubertal maturation following leptin administration. Because puberty was delayed but not arrested, these findings indicate that leptin action in the PMV is not required for sexual maturation and that alternative pathways exist.
PMV LepR neurons directly innervate GnRH and kisspeptin neuronal populations. Approximately 75% of these neurons depolarize after acute leptin administration (49, 127, 272, 280, 386, 490). The classic excitatory amino acid glutamate (51, 288) and the gaseous neurotransmitter nitric oxide (NO) are the main neurotransmitters in PMV projections (127, 129, 272) (See note added in proof).
The effects of NO on GnRH secretion are well established. Neuronal NO synthase (nNOS; Nos1 gene) knockout mice are infertile. In addition, leptin-induced GnRH/LH secretion is blocked following the global deletion or inhibition of NO production (33, 87, 188, 235, 376, 477, 508, 509). Leptin administration increases the activity of nNOS, subsequently increasing NO production in LepR neurons of the PMV and the MPO (33, 129, 347). In the MPO, the inhibition of NO prevents leptin from inducing LH secretion in fasted mice (33). Notably, the deletion of LepR from nNOS cells disrupts metabolic function and pubertal development (271). Together, these findings indicate that the PMV and the MPO are two primary sites where leptin may promote reproduction via NO neurotransmission (FIGURE 5).
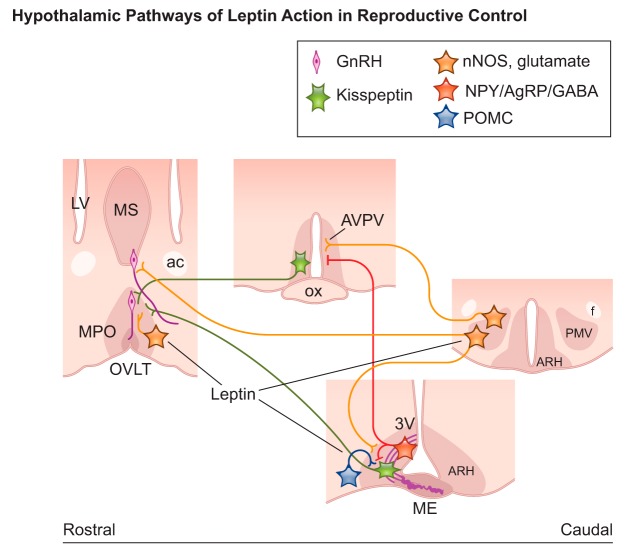
FIGURE 5.
Schematic illustration showing coronal sections of brain sites and neural pathways associated with leptin action in reproductive control in mice. Neurons in the medial preoptic area (MPO) and in the ventral premammillary nucleus (PMV) coexpress nitric oxide synthase (nNOS) and glutamate and alter gonadotropin releasing hormone (GnRH) neuronal activity via direct projections or innervation of kisspeptin neurons. Neurons in the arcuate nucleus (ARH) expressing neuropeptide Y (NPY), agouti-related protein (AgRP), and GABA as well as those expressing proopiomelanocortin (POMC) project to kisspeptin. Thick purple lines in the median eminence (ME) represent GnRH terminals at the portal circulation. ac, anterior commissure; AVPV, anteroventral periventricular nucleus; f, fornix; LV, lateral ventricle; ME, median eminence; MS, medial septum; OVLT, vascular organ of the lamina terminalis.
The ability of leptin to facilitate lordosis is reduced when NO production is blocked (61, 172, 180, 291, 470). The brain pathways involved in this effect are not known, but strong interconnections between either the PMV or the MPO and the VMHvl, a key site in the control of lordosis in female rodents, have been reported (66, 67, 386). The physiological relevance of these reciprocal connections requires further investigation. Because leptin’s behavioral effects require GnRH signaling, NO and leptin may jointly alter sex steroid levels via actions on GnRH neurons, indirectly affecting behavioral responses.
The melanocortin system in the ARH is an alternative site through which leptin modulates reproduction. A distinct subset of first-order neurons expressing agouti-related protein (AgRP), neuropeptide Y (NPY), and GABA, as well as another expressing proopiomelanocortin (POMC) and predominantly glutamate have well-described actions in metabolism and leptin physiology (139, 189, 254, 489, 498, 502). The posttranslational cleavage of POMC generates melanocortins [e.g., α-melanocyte stimulating hormone (α-MSH)], adrenocorticotropic hormone (ACTH), opioids (β-endorphin), and lipotrophins (332). Of those, the neuropeptides α-MSH and β-endorphin are the main players in reproductive control. While previous studies have shown that the deletion of LepR from neurons expressing AgRP, POMC, or both does not disrupt reproduction (24, 462), the ablation of AgRP/NPY neurons or the blockade of melanocortin receptors improved fertility in leptin-signaling deficient mice (141, 224, 412, 502). By suppressing the activity of AgRP neurons, leptin can induce puberty and improve fertility in mice. However, leptin action on AgRP neurons is sufficient but not required for reproductive function, as deletion of LepR from AgRP neurons does not preclude reproduction (137, 144). The effects of AgRP/NPY neurons seem to be attained mainly by restraining kisspeptin neuronal activity via GABAergic neurotransmission (345). This is in line with studies showing that the deletion of LepR from GABAergic neurons delays sexual maturation and disrupts the estrous cycle in mice (297, 515) (FIGURE 5). On the other hand, mice lacking both LepR and insulin receptor (IR) in POMC cells (LepR/IRPOMC mice) exhibited systematic insulin resistance as well as reduced fertility, suggesting cooperative actions by leptin and other metabolic signals.
The melanocortin system may also act downstream of leptin to affect sexual behavior. Male LepR/IRPOMC mice showed reduced α-MSH production and decreased reproductive success due to dramatic changes in sexual behavior (207). In pubertal rats, melanocortin receptor agonists activate the HPG axis by stimulating kisspeptin release (290). Kisspeptin neurons of mice coexpress melanocortin receptors, and the inhibition of KNDy neurons using designed receptors exclusively activated by designed drugs (DREADDs technology) blocks the ability of melanocortins to stimulate the reproductive axis (290). The disruption of the melanocortin system in humans, including POMC and MC4R genes, has been associated with obesity with virtually no effect on reproductive function (146, 459). On the other hand, individuals with the deletion of the prohormone convertase 1 (PCSK1) gene, an enzyme that processes distinct prohormones including POMC, show obesity and hypogonadotropic hypogonadism (432, 463). Whether this phenotype is associated with the posttranslational processing of POMC or other prohormones is undefined.
The melanocortin system has a relevant role in sexual arousal and penile erection in rodents and humans (68, 457, 464, 467). High rates of sexual dysfunction including disorders of libido, decreased sexual desire, and reduced intercourse satisfaction have been reported in obese and type 2 diabetes (T2D) patients (344, 389, 412), in whom hypothalamic leptin and insulin resistance are likely. Thus leptin signaling in POMC neurons may play a role in supporting the necessary release of α-MSH to maintain reproductive behavior.
2. Pancreatic islet-derived hormones: insulin
The pancreatic islets produce hormones with key actions in homeostasis, including insulin, glucagon, and somatostatin. While the effects of circulating glucagon and somatostatin in the central control of the reproductive function are understudied, insulin is well recognized as having a substantial impact on reproduction.
Insulin is a polypeptide hormone that islet beta cells secrete in response to high blood glucose levels in the short term and elevated body adiposity in the long term (466, 496). Insulin regulates the metabolism of carbohydrates and fat by promoting glucose uptake from the circulation to skeletal muscle, liver, and adipose tissues. IR are found at all levels of the HPG axis, allowing insulin to modulate reproductive function and behavior in a tissue-specific manner. In the ovaries of animal models and women, insulin potentiates LH action by increasing steroidogenesis (3, 327, 503).
Although apparently not required for glucose uptake by neurons, IR are located throughout the brain, with the highest concentrations in the olfactory bulb, cerebral cortex, cerebellum, hippocampus, and hypothalamus (195, 293, 364, 484). In rodents, the highest concentrations of IR are found in the MPO, ARH, and VMH (56, 293).
Circulating insulin levels correlate positively with body weight and adiposity, indicating that insulin resistance increases with weight gain (497). Intracerebroventricular or intranasal application of insulin decreases body weight and food intake in rats, mice, and primates, including humans (55, 74, 190, 496). Additionally, the blockade of insulin signaling in the hypothalamus increases food intake and body weight (336).
Insulin also plays a significant role in the neuroendocrine control of the reproductive function (56, 186, 336). Brain-specific deletion of insulin receptors results in diet-sensitive obesity and HPG axis dysregulation (56). In obese female mice, insulin increases GnRH activity and LH secretion, potentially contributing to obesity-induced reproductive dysfunction (123). However, the specific cellular targets associated with insulin action on reproductive neuroendocrine axis have not been determined. Studies using conditional deletions of IR in distinct neuronal populations (CaMIIKα, AgRP, POMC, GnRH, and kisspeptin) have not been very informative, as these mouse models failed to display changes in reproductive phenotype or the GnRH network dysregulation (124, 143, 145, 207, 252). However, redundant pathways may exist, and the deletion of IR from one population of neurons early during development may result in compensation from other cell populations or metabolic pathways. Alternatively, the hypothalamic hypogonadism observed in brain-specific IR knockout mice may have resulted from the absence of insulin signaling in nonneuronal cells (e.g., glia). This hypothesis is in line with findings showing that obesity and T2D induce signs of hypothalamic inflammation and mitochondrial dysfunction associated with cellular insulin resistance (444, 460). Notably, IR in astrocytes assist glucose transport via the BBB, thus altering the signals of glucose availability to relevant neurons (166). Whether this function also affects reproduction is unknown.
3. Gastrointestinal tract and accessory organs: ghrelin, GLP1, FGF21, and IGF1
Enteroendocrine cells in the gastrointestinal (GI) tract and hepatocytes communicate signals of immediate energy availability to the reproductive system. These cells function as nutrient sensors that respond to GI luminal content (the amount and composition of food) and release hormones to inform downstream targets about the immediate nutritional state. These nutritional signals reach the CNS via direct release into the circulation (hormones) or via afferent neurons to the nucleus of the solitary tract (39). Some of these hormones modulate reproductive physiology.
a) ghrelin.
Ghrelin is secreted by gastric mucosal cells during conditions of undernutrition (206, 318). Originally recognized for its growth hormone-stimulating action, ghrelin has potent effects on energy balance independent of the somatotropic axis. Ghrelin promotes the storage of lipids as fat, induces glucagon release, and suppresses insulin secretion and sensitivity (319). In addition to its effects on the growth axis and energy balance, ghrelin also modulates reproductive function (319, 439). In mice, the functional ghrelin receptor GHSR1a is widespread in the hypothalamus, with high expression in the AVPV, MPO, and mediobasal hypothalamus (156, 514). No reproductive phenotype has been described in mice with the global deletion of ghrelin (Ghrl gene knockout) (433). However, compensatory mechanisms may be in place, as the inhibitory actions of ghrelin on reproduction have been shown in several species including sheep, monkeys, and humans (439). For example, the intracerebroventricular or peripheral administration of ghrelin suppresses gonadotropin secretion in rats. Likewise, ghrelin inhibits GnRH release from hypothalamic explants (149, 150, 161).
The neural basis for ghrelin action in the HPG axis is not completely known, but studies have suggested that the kisspeptin neurons are a potential target. GHSR1a is expressed in AVPV/PVpo kisspeptin and KNDy neurons in mice (156). Ghsr expression is regulated by estradiol, i.e., higher expression and colocalization was observed in KNDy neurons of ovariectomized mice treated with estradiol. The higher colocalization was not caused by changes in Kiss1 mRNA expression, as the procedure was performed in Kiss1-Cre mice crossed with GFP reporter under the R26 promoter, and therefore GFP immunoreactivity was stable across estrous cycles and under different sex steroids milieu. Contrary to expectations, KNDy neurons depolarize in response to ghrelin (156). Because higher Kiss1/GHSR1a coexpression is observed when Kiss1 mRNA levels are low (in circumstances of high circulating E2) (425), it is possible that the KNDy neurons preferentially release neuropeptides or neurotransmitters other than kisspeptin when activated by ghrelin. While this model could plausibly explain the unexpected findings, it requires verification.
Ghrelin may also inhibit reproduction by increasing the activity of AgRP/NPY neurons (100). As discussed in previous sections, the stimulation of this pathway may decrease the activaton of the HPG axis by inhibiting kisspeptin neurons (345) (FIGURE 6). In female rats, peripheral ghrelin had no effect on ARH Kiss1 mRNA levels, but it decreased Kiss1 expression in the AVPV (153). Because Ghsr is not expressed in the AVPV of rats (514), upstream interneurons (e.g., AgRP neurons) may have caused this effect (345).
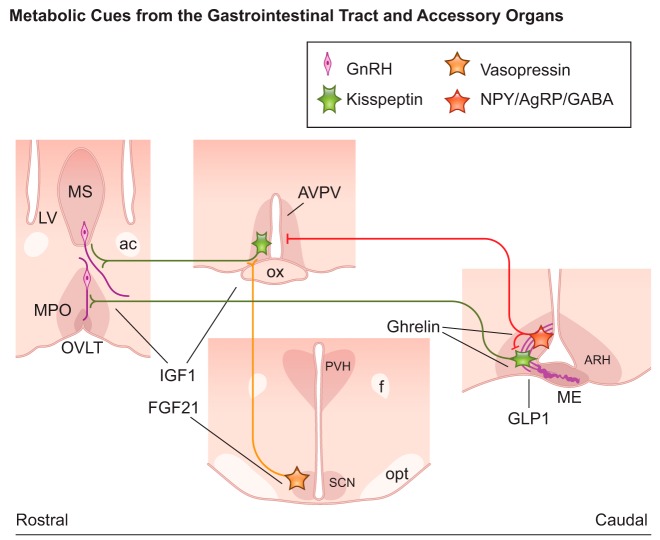
FIGURE 6.
Schematic illustration showing coronal sections of brain sites and neural pathways targeted by hormones from the gastrointestinal tract and liver with action in reproductive control in mice. Ghrelin receptor is coexpressed in agouti-related protein (AgRP) and kisspeptin neurons. Glucagon-like peptide 1 (GLP1) modulates Kiss1 expression in the arcuate nucleus (ARH). Fibroblast growth factor 21 (FGF21) acts on FGF receptor 1/βKlotho expressed in vasopressin neurons of the suprachiasmactic nucleus (SCN) upstream of kisspeptin neurons of the anteroventral periventricular nucleus (AVPV). Insulin-like growth factor 1 (IGF1) targets gonadotropin releasing hormone (GnRH) neurons and kisspeptin neurons in the AVPV. Thick purple lines in the median eminence (ME) represent GnRH terminals at the portal circulation. ac, anterior commissure; f, fornix; LV, lateral ventricle; ME, median eminence; MS, medial septum; opt, optic tract; OVLT, vascular organ of the lamina terminalis; ox, optic chiasm; PVH, paraventricular nucleus of the hypothalamus.
In male rats, ghrelin increases the latency to first mount, intromission, ejaculation and the length of postejaculatory intervals. Coadministration of ghrelin with the ghrelin receptor antagonist (d-Lys3)-GHRP6 attenuated ghrelin’s effects (19). Ghrelin may also inhibit female sexual behavior and receptivity during fasting conditions, when circulating ghrelin levels are high. Ovariectomized steroid-primed females subjected to acute food restriction and ghrelin administration showed decreased sexual receptivity. Administration of a ghrelin receptor antagonist to these females restored sexual behavior (40). However, chronic ghrelin administration did not reduce sexual receptivity. The mechanisms underlying the differential effects of acute and chronic ghrelin treatments are not completely known, but a compensatory response involving receptor downregulation is a potential explanation (40). Whether ghrelin exerts its effects on sexual behavior via direct actions on brain nuclei associated with behavioral control is unknown.
Notably, one feature common to individuals with Prader-Willi syndrome (PWS) is high levels of circulating ghrelin. PWS affected individuals are obese, hyperphagic, and show lack or delay in pubertal development and low gonadotropin levels (415). It is possible that high ghrelin levels in PWS have an effect to inhibit the HPG axis contributing to the disrupted fertility observed in affected individuals. Further studies are necessary to test this model.
b) glp1.
GLP1 is an incretin member of the structurally related glucagon family of peptide hormones, with actions in diverse physiological systems including feeding, gastric motility, and cardiovascular function (130). GLP1 is primarily synthesized from the proglucagon gene by the ileum L cells in response to food intake (29, 130). Studies have shown that the peripheral or central administration of a GLP1 receptor agonist acts on cognate receptors in the CNS to suppress feeding and decrease body weight (142).
GLP1 also modulates the reproductive physiology of rodents (201, 342). The acute administration of GLP1 increases the amplitude of the preovulatory LH surge, and chronic GLP1 treatment of prepubertal rats advances the onset of puberty (342). GLP1 also enhances LH secretion in male rats and promotes GnRH release in hypothalamic explants (31). Similarly, mice with a global deletion of GLP1 receptors show delayed onset of puberty and low gonadal weight (283). The sites of GLP1 action in reproduction are unclear, but part of these effects may involve kisspeptin neurons (200). In mice, GLP1 immunoreactive fibers are in close apposition with KNDy neurons, and electrophysiological recordings showed that GLP1 receptor agonists depolarize and increase the firing rate of those neurons (200) (FIGURE 6). GLP1 is also expressed in neurons of the brain stem (263, 468), but whether its effects on reproductive physiology are mediated by neuronal or humoral actions have not been demonstrated.
c) fgf21.
FGF21 is secreted from the liver, pancreas, and white adipocytes and acts as a hormone in several endocrine systems, with broad actions in metabolic control (247). FGF21 signals through the FGF receptor 1 (FGFR1) complexed with a membrane protein or co-receptor named βKlotho. Whereas FGFR1 is widespread, βKlotho has a restricted distribution. Other FGFs also bind to FGFR1; therefore, the sites of βKlotho expression are likely key for FGF21 actions. After a prolonged fast, FGF21 levels rise, inducing hepatic gluconeogenesis and fatty acid oxidation (22). Because of the effects of negative energy balance on fertility, studies have assessed the direct role of FGF21 as a signal of energy insufficiency to the HPG axis. Mice exposed to FGF21 show infertility secondary to a negative energy balance, an effect attributed to actions in the hypothalamic suprachiasmatic nucleus (SCN) upstream of the vasopressin-kisspeptin pathway (343). βKlotho is highly expressed in SCN vasopressinergic neurons, suggesting that FGF21 binding may inhibit the LH surge by disrupting circadian control (343) (FIGURE 6). Mice lacking βKlotho in the SCN are protected against the inhibitory effect of FGF21 on reproductive function. However, following a ketogenic diet, mice displayed high levels of circulating FGF21 but remained fertile, and the intracerebroventricular administration of FGF21 had no effect on the reproductive axis (419). Loss-of-function mutations in the KLB gene (coding for βKlotho) in humans cause congenital hypogonadotropic hypogonadism, and mice lacking the Klb gene show delayed puberty, deficient estrous cycles, and subfertility (504). These effects seem to be caused by a lack of response of GnRH neurons to FGF21 stimulation. Together, these findings suggest that FGF21 affects GnRH physiology, but whether it conveys signals of energy sufficiency remains to be independently assessed.
d) igf1.
IGF1, also called somatomedin C, is a protein hormone that in humans is encoded by the IGF1 gene (213, 225). IGF1, IGF2, and insulin are members of the same family of insulin-like peptides (97). IGF1 plays an important role in childhood growth and continues to have anabolic effects in adults (237). A large proportion of IGF1 is synthesized in the liver and released into the plasma, but IGF1 is also expressed in many tissues at low levels. Studies with rat models of low circulating IGF1 have shown that brain IGF1 is regulated independently of plasma IGF1 levels (2).
IGF1’s primary action is mediated by binding to its specific tyrosine kinase receptor, the IGF1 receptor (IGF1R), which is expressed in a wide range of tissues, including the brain. Both IR and IGF1R exist as heterotetramers composed of two dimers (alpha and beta) (325), although hybrids exist consisting of combinations of IR and IGF1R subunits (36). Previous evidence showed that insulin and IGF1 bind to their noncognate receptor with an order of magnitude lower than their cognate receptors (173, 246) and to hybrid receptors with varying affinities (36, 393). The overlap between the signaling pathways for insulin and IGF1 indicate that the temporal and spatial expression of the receptors underlies the specificity of the responses.
IGF1 of both peripheral and central origin can interact with hypothalamic IGF1R. Levels of IGF1 in the brain have been shown to play a role in puberty, estrous cyclicity, the preovulatory gonadotropin surge, and the decline of reproductive neuroendocrine function during reproductive senescence (109, 110, 124, 308). IGR1 and IGF1R are both found in GnRH neurons (108). Precocious puberty was observed in female mice following peripheral treatment with IGF1 (113, 124), although no change was seen in males (124). Intracerebroventricular infusion of IGF1 stimulates secretion of GnRH in prepubertal female rats and advances the onset of puberty (209, 210). Nevertheless, GnRH expression was not altered in IGF1-treated rats as assessed by RNase protection assay analysis of RNA from hypothalamic tissue (308). Conversely, brain IGF1R antagonism impairs estradiol positive feedback, reduces estradiol- and progesterone-dependent LH surges, and disrupts estrous cycling independent of changes in body weight or food intake (434, 447). Under estradiol positive feedback conditions, intracerebroventricular infusion of the IGF1R antagonist JB-1 in young adult females reduces GnRH neuron activation, as determined by the percentage of GnRH/c-Fos coexpressing cells (330). These findings suggest that IGF1R signaling functions as an energy indicator required for normal hypothalamic control of reproduction. Mice lacking the IGF1R in GnRH neurons (IGF1RGnRH) exhibited a delay in the onset of puberty, but showed normal reproductive function as adults (124). These results are similar to those observed when IR was specifically deleted in the mouse kisspeptin neurons (IR∆Kiss) (370) underscoring the diversity of metabolic signals that likely contribute to the pubertal process.
IGF1R signaling in neurons is also important for neuronal progenitor proliferation, neurite outgrowth, and synaptogenesis necessary for interneuronal communication (334). Reduced levels of hypothalamic IGF1 have been proposed to underlie the decline in neuroendocrine function associated with reproductive aging. During pubertal progression, hypothalamic IGF1 levels are increased, while a significant reduction in hypothalamic IGF1 was reported in older rats undergoing reproductive senescence (308). Moreover, IGF1 signaling was required for proper timing and magnitude of the estradiol induction of the GnRH surge (434, 448). Indirect effects on GnRH release can also be mediated by kisspeptin neurons (FIGURE 6). Both centrally perfused IGF1 and peripherally injected IGF1 stimulated the kisspeptin neurons of the AVPV (211). These data may also provide an anatomical explanation for the sexual dimorphism in the response of mice to IGF1 since males have limited AVPV kisspeptin expression.
4. Oxidizable fuels and metabolites
The brain also monitors the availability of energy using metabolic substrates and the oxidation of fuels. Among them, glucose, free fatty acids, and amino acids act as immediate signals of the nutritional state to the CNS (399, 407). Sensors for these metabolic substrates and oxidizable fuels reside in areas also known to control reproduction.
a) glucose.
Glucose is a primary fuel source for the CNS and is fundamental for neuronal function (13). Neuronal glucose-sensing mechanisms allow the brain to constantly monitor intracellular glucose levels and, in turn, control energy homeostasis in peripheral organs (273). Glucose also acts as a signaling molecule in glucose-sensitive neurons that may be subdivided into glucose-excited (GE) and glucose-inhibited (GI) neurons (233, 474). The firing rate of GE neurons increases and that of GI neurons decreases as glucose levels rise (134). Most GE neurons express anorexigenic peptides (e.g., α-MSH from POMC neurons), whereas GI neurons express appetite-stimulating peptides (e.g., NPY and AgRP) (220, 322).
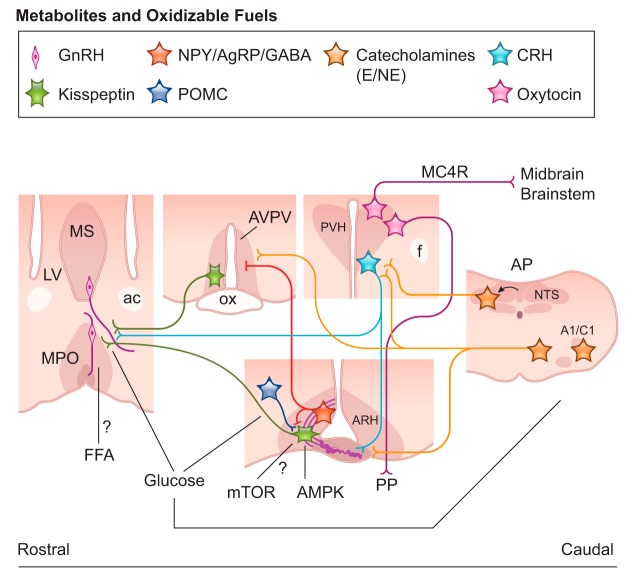
Changing glucose levels are a key signal to the neuroendocrine reproductive axis. For example, treatment with a competitive inhibitor of intracellular glucose utilization (i.e., 2-deoxy-d-glucose or 2DG, and 5-thio-glucose or 5TG) inhibits pulsatile LH secretion and pulse frequency (245, 259, 320, 337). Administration of glucose to hypoglycemic rats restores LH secretion (382). The effects of glucose on the modulation of the HPG axis may engage three potential pathways: 1) actions in neurons of the mediobasal hypothalamus (including the melanocortin system) (220, 322), 2) glucose sensing in the area postrema and in catecholaminergic neurons of the caudal medulla (71, 219, 320), and 3) direct actions in GnRH neurons (383, 512) (FIGURE 7).
FIGURE 7.
Schematic illustration showing coronal sections of brain sites and neural pathways responsive to metabolites and oxidizable fuels with action in reproductive control in mice. Glucose directly affects the proopiomelanocortin (POMC) and agouti-related protein (AgRP) neurons of the arcuate nucleus (ARH), the area postrema (AP), and the catecholaminergic neurons of the caudal medulla. These sites innervate the paraventricular nucleus of the hypothalamus (PVH), the main output to behavioral control (via oxytocin and MC4R neurons), and neuroendocrine output (via corticotropin releasing hormone/CRH and oxytocin neurons). Glucose and free fatty acid (FFA) potentially alter gonadotropin releasing hormone (GnRH) neurons activity. AMP kinase (AMPK) and mTOR change kisspeptin response to metabolic challenges. Thick purple lines in the median eminence (ME) represent GnRH terminals at the portal circulation. A1/C1 and A2, noradrenergic and adrenergic neuronal groups in the caudal medulla; ac, anterior commissure; ARH, arcuate nucleus; AVPV, anteroventral periventricular nucleus; E, epinephrine; f, fornix; LV, lateral ventricle; ME, median eminence; MS, medial septum; NE, norepinephrine; NTS, nucleus of the solitary tract; ox, optic chiasm; PP, posterior pituitary gland; PVH, paraventricular nucleus of the hypothalamus.
Ablation of hypothalamic projecting catecholaminergic neurons of the caudal medulla (A1/C1 and A2 groups) disrupts the adaptive responses of the reproductive axis to low glucose levels (219). This finding was obtained using selective deletion of catecholaminergic neurons that innervate the paraventricular nucleus of the hypothalamus (PVH), suggesting that the medulla-PVH projection plays an important role conveying signals of glucose levels to the reproductive neuroendocrine axis. However, as discussed by the investigators, deletion of A1/C1 and A2 groups of neurons also decreased cathecolaminergic innervation of ARH and MPO. Therefore, the lack of response to changes in glucose levels may be associated with decreased adrenergic/noradrenergic innervation of any one or of all the hypothalamic sites compromised by the procedure.
The PVH houses the corticotropin releasing hormone (CRH) neurons of the HPA axis. In general, stressors of different origins, including those triggered by a negative energy balance, activate the HPA axis by inducing the secretion of CRH into the hypophysial portal system (8, 111, 285, 456). The neural path or mechanisms by which the HPA axis is activated in conditions of nutritional stress are not completely known, but changes in glucose and leptin levels seem to exert a crucial role. Paradigms of 2DG administration to decrease glucose utilization activate the HPA axis and inhibit LH secretion (456, 479). Pharmacological inhibition of CRH signaling in 2DG-treated female rats normalizes LH levels, potentially via direct actions on GnRH neuronal activity (355, 456). Leptin administration to fasted mice blocks the activation of the HPA axis and the suppression of the HPG axis (8, 197).
A subpopulation of GnRH neurons has its firing rate reduced following exposure to low concentrations of glucose (383, 512). This effect seems to be mediated in part by ATP-sensitive potassium channels (KATP) and the enzyme glucokinase. When transported into cells, glucose serves as a substrate for ATP production via the actions of glucokinase. High levels of intracellular ATP block KATP channels, decreasing potassium efflux and causing depolarization of GE neurons. Alternatively, changing levels of glucose may also alter the intracellular AMP-to-ATP ratio, modulating the enzyme AMP kinase (AMPK). Low extracellular glucose reduces intracellular ATP production, raising the AMP-to-ATP ratio and AMPK activation (194). AMPK acts at the intracellular level to inhibit ATP utilization and stimulate ATP production (430). In female rats, the intracerebroventricular infusion of an AMPK activator disrupted estrous cycles, and the acute application of an AMPK agonist into hypothalamic slices hyperpolarizes GnRH neurons (101). Likewise, AMPK antagonists attenuate glucose sensing in GnRH neurons (383, 384).
The non-neuronal actions of AMPK may also play a role in GnRH suppression. Malnutrition-induced AMPK activation of glial cells suppresses GnRH/LH release (310). Interestingly, AMPKα1-knockout mice are subfertile, but exhibit no apparent metabolic phenotype, indicating that subfertility in these mice is not secondary to metabolic dysregulation (230). However, the conditional deletion of AMPKα2 in Kiss1 neurons causes neither metabolic nor reproductive deficits but does disrupt the ability of the female mice to adapt to metabolic challenges (451).
b) free fatty acids.
Although glucose is the primary energy source for the CNS, the oxidation of free fatty acids (FFA) may also provide a fraction of energy to the brain (135, 346). The limited use of FFA compared with glucose is likely due to the low β-oxidation by brain mitochondria (403). Nevertheless, the CNS may perceive changes in FFA levels and alter physiology (152, 261). In Syrian hamsters, availability of FFA (via fat oxidation) maintains the reproductive cycle in food-restricted females (401, 402). While minimal data have connected these sensors to the CNS in reproductive regulation, one study found that FFA change GnRH mRNA expression in a GnRH-synthesizing cell line (452) (FIGURE 7). Additional studies will be necessary to assess the physiological relevance of these findings.
c) amino acids.
Changes in dietary protein influence feeding behavior. Rats placed on a low-protein diet exhibit increased food intake and AgRP expression. Intracerebroventricular injection of an amino acid mixture, or just leucine, suppresses food intake (315). Amino acids (l-leucine) along with adequate levels of ATP activate the mammalian target of rapamycin (mTOR) pathway, which induces protein synthesis and growth (193, 215, 398). A loss-of-function mutation in the Mtor gene is embryonically lethal, whereas increased activity of mTOR induces metabolic dysfunctions including obesity and diabetes (164, 199, 215). The mTOR pathway also controls the HPG axis and the onset of puberty (379, 381). The blockade of mTOR signaling by rapamycin inhibits puberty, decreases LH and estradiol levels, and causes ovarian and uterine atrophy. The central activation of mTOR stimulates LH secretion. These actions may result from the mTOR control of KNDy neurons, since the inhibition of mTOR preferentially decreases Kiss1 transcription levels in the ARH (379) (FIGURE 7).
C. Integrative Sites and Neuronal Networks
The metabolic control of reproductive function is exerted ultimately by changing the activity of GnRH neurons. This effect requires direct actions on GnRH somata and terminals or the modulation of kisspeptin neurons (FIGURE 8). Most of the first-order neurons that sense changes in energy availability are located in the ARH. This is not surprising as the ARH neighbors a circumventricular organ highly relevant for neuroendocrine control, the median eminence. Therefore, most of the ARH neurons are located outside the BBB.
FIGURE 8.
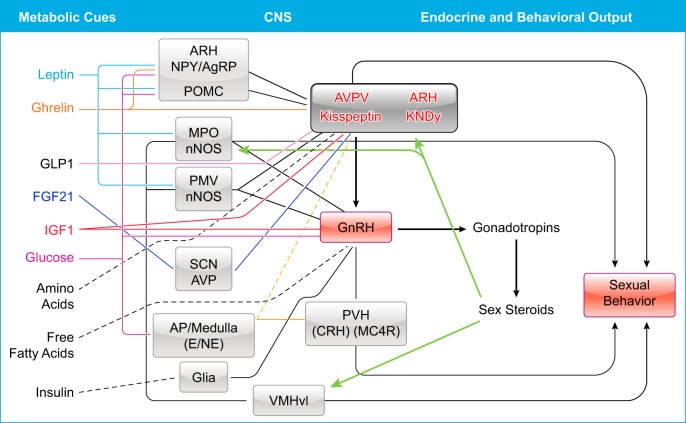
Summary of the neuronal network associated with the metabolic control of reproductive function described in rodents. Metabolic cues are listed in the left column, specific sites in the central nervous system (CNS) are distributed in the middle, and the endocrine and behavioral outputs are presented in the right. Black lines represent well-defined networks, and dashed lines represent proposed, still undefined, projections. Key sex steroid targets are represented in green arrows. AgRP, agouti-related protein; AP, area postrema; ARH, arcuate nucleus; AVP, vasopressin; AVPV, anteroventral periventricular nucleus; CRH, corticotropin releasing hormone; E, epinephrine; f, fornix; GnRH, gonadotropin releasing hormone neurons; KNDy, kisspeptin neurokinin B dynorphin; LV, lateral ventricle; MC4R, melanocortin 4 receptor; MPO, medial preoptic area; NE, norepinephrine; nNOS, nitric oxide synthase; NPY, neuropeptide Y; POMC, proopiomelanocortin; PVH, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus; PMV, ventral premammillary nucleus; VMHvl, ventrolateral subdivision of the ventromedial nucleus of the hypothalamus.
ARH neurons coexpress the orexigenic neuropeptides NPY and AgRP, both critical mediators of energy homeostasis (189, 489, 498). NPY/AgRP neurons are stimulated by signals of low energy and are inhibited in conditions of excess energy (100, 252). The suppression of basal LH levels by fasting fails in NPY knockout mice (208). However, pharmacological studies in rodents and primates (106, 494) have suggested that the NPY has a dual action on the HPG axis according to sex steroids milieu. NPY has suppressive effects on LH pulse amplitude and frequency when sex steroids are low and a robust activational effect following steroid replacement in gonadectomized rats (38, 106, 301). Moreover, LH surges are attenuated after NPY immunoneutralization or Y1 receptor blockade, and NPY-knockout female mice show reduced LH surges (505). AgRP/NPY neurons modulate reproduction by acting on both GnRH and kisspeptin neurons (224, 380, 412, 501, 502). The effects of NPY on GnRH neurons rely on the specific receptors recruited by the ligand. NPY inhibits ~10% of GnRH neurons through Y1 receptors but stimulates 25% of GnRH neurons through Y4 receptors (380). Its higher affinity for the Y1 receptor suggests an inhibitory net effect upon GnRH neurons. However, the genetic deletion of the Y4 receptor in obese infertile ob/ob mice restores fertility in males and improves the reproductive phenotype of females (392). Whether NPY modulates the HPG axis via Y4 receptors apart from those receptors on GnRH neurons has not been demonstrated. NPY/AgRP neurons also innervate kisspeptin cells. This input seems to be exerted primarily via melanocortin receptors or by GABAergic neurotransmission (20, 345). According to this model, NPY neurons are activated under conditions of negative energy balance, leading to an increase in NPY release in the ARH and AVPV.
ARH POMC neurons are also highly responsive to metabolic cues, including leptin, insulin, and glucose (207). In conditions of negative energy balance, such as fasting, POMC mRNA expression drops, and the opposite is seen when energy is abundant (445, 489, 498). POMC neurons in the ARH coexpress the neuropeptide cocaine- and amphetamine-regulated transcript (CART) and project to brain sites associated with metabolic and reproductive control (139, 386, 454).
The melanocortin system modulates reproductive function via melanocortin receptors (MC3R and MC4R). The MC4R-knockout mice are morbidly obese, but their reproductive deficits are mild (397). However, females show reduced ovulation and develop subfertility later in life (21, 290, 454). Whether this effect is caused by increased adiposity has not been demonstrated. A direct stimulation of GnRH neurons by α-MSH via MC3R and MC4R signaling has been demonstrated (380), suggesting the GnRH neurons are the melanocortin preferential targets. However, melanocortin receptors are also expressed in kisspeptin neurons (21, 104), and POMC/CART neurons directly regulate kisspeptin output (21, 290, 454). Due to the sensitivity of POMC/CART neurons to metabolic cues, the POMC-Kiss1-GnRH circuit may function as a crucial neuronal path by which signals of energy sufficiency modulate the HPG axis (290).
Obese MC4R null males show decreased motivation to engage in sexual activity as well as reduced erectile function and take longer to reach ejaculation (148, 409, 464). Blockade of the MC4R reduces mounting and increases latency to intromission, and the administration of α-MSH into the MPN facilitates sexual behavior and penile erection in male rats (68, 216, 464). In females, α-MSH stimulates sexual receptivity and lordosis, which is blocked in a dose-dependent manner by an MC3/4R antagonist (102, 182, 405). Drugs that target the melanocortin system have also been tested in humans as a treatment for male erectile dysfunction and female sexual dysfunction (83, 391, 485). A MSH analog, melanotan-II (MT-II), has been found to induce erections in men (485), and one study found that a melanocortin 3/4 receptor agonist, bremelanotide, increased satisfaction levels in men who were also taking Viagra (391). Increased understanding of the mechanisms underlying these actions may assist with sexual deficits in both men and women.
Recent work has begun to shed light on the neural pathways responsible for these effects. We recently examined the sexual behavior of mice expressing MC4R only on Sim1-positive neurons (found in the medial amygdala and PVH) in comparison with reactivable (tb)MC4R null mice. While tbMC4R null mice showed a longer latency to mount, a reduced intromission efficiency, and an inability to reach ejaculation, expression of MC4R only on Sim1 neurons reversed these sexual deficits. The amygdala is well known for its involvement in penile erection, sexual sensation, and orgasm in humans (176, 226, 438). Another possible location for the relevant POMC projections underlying these effects is the PVH. Viral tracing has established connections between the corpus cavernosus of rats and the PVH (295). In addition, lesions of PVH neurons delayed ejaculation and reduced intromission ratios (279). Oxytocin neurons in the PVH, besides having a critical role in lactation and parturition, also stimulate GnRH release (105, 163, 229, 375, 395). When injected centrally, oxytocin induces penile erection in rats, whereas the antagonist inhibits both sexual motivation and copulation (17, 303, 304). Another level of complexity and modulation of oxytocin in the PVH may be attained via projections from MC4R neurons in the PMV, a crucial olfactory relay in rodents (174). Establishing which pathway underlies the ability of melanocortins to improve sexual function has important clinical implications.
β-Endorphin also plays a critical, though opposing, role in the release of GnRH and sexual behavior. While α-MSH reduces food intake and stimulates sexual behavior and function, β-endorphin promotes food consumption and inhibits GnRH secretion (177, 182, 214, 266, 406). In line with these findings, the opioid antagonist naloxone increases LH secretion in mouse models and humans, probably due to blockade of β-endorphin inhibition of GnRH neurons (45, 82, 136, 177, 380). Opioid peptides inhibit the sexual behavior of females via projections to the VMHvl and downstream targets in the MPO and brainstem (353). This inhibitory role has also been observed in women. For example, opiate addicts often suffer from anorgasmia, amenorrhea, anovulation, and loss of libido. As in males, some of these effects result from the opioid inhibition of GnRH release, which leads to decreased production of gonadotropins and gonadal steroid hormones (41, 232, 360). Because of the dissociated effects of the two POMC neuropeptides (α-MSH and β-endorphin), investigators have proposed that a posttranslational mechanism or divergent neuronal circuits coordinate their physiological actions. In fact, electron microscopy has revealed that α-MSH and β-endorphin are located in separate pools of vesicles within the same neuron (250). These two pools of vesicles may be released with different time courses or by different regulatory stimuli (250).
Neurons expressing galanin-like peptide (GALP) comprise another ARH subpopulation associated with the metabolic control of reproduction. A negative energy balance decreases Galp expression. GALP administration induces LH secretion and restores the timing of pubertal maturation in calorie-restricted rats (154, 234, 265, 313). The effects of GALP seem to occur via direct actions on GnRH neurons (410). GALP administration also increases Kiss1 mRNA expression, but its effect on reproductive function does not require kisspeptin action (171). The metabolic cues associated with the control of GALP activity have not been clearly identified.
In addition to the ARH, the mediobasal hypothalamus consists of several nuclei housing first-order neurons involved in metabolic sensing. PMV neurons express receptors for leptin, insulin, ghrelin, and melanocortins (128, 174). They also project to brain areas associated with neuroendocrine and behavioral regulation (67, 174, 272, 386). These projections include direct innervation of GnRH and kisspeptin neurons and strong innervation of the VMHvl and MPO. The PMV has a crucial role as an integrative and modulatory site as it gathers signals from external environmental cues (i.e., odorants in rodents and photoperiod in seasonal breeders), reproductive status (sex steroids milieu), and energy availability (126, 251, 417). NO and glutamate are the main neurotransmitters, with the potential to stimulate downstream targets via glutamate and to coordinate adjacent neuronal activity via NO (34, 127, 129). Neurons in the MPO also express nNOS and respond to leptin. Because of the proximity to GnRH neurons and the volatile property of NO, the MPO nNOS neurons are well positioned to modulate the activity of GnRH neurons in response to leptin and, potentially, other metabolic cues (33).
Several other neuropeptides and hypothalamic sites may mediate the metabolic control of reproduction. For example, neurons expressing steroidogenic factor 1 (SF1), exclusively located in the VMH, play a key role in energy homeostasis (121, 242, 249). The lack of SF1 in the brain disrupts fertility, an effect that is likely due to developmental disruption of the VMH anatomical organization caused by this specific deletion (241). Melanin-concentrating hormone (MCH), orexin/hypocretin, and nesfatin in the lateral hypothalamic area play a role in energy balance and are also potential neuromodulators of the reproductive neuroendocrine axis (21, 169, 413, 422, 491, 500). However, the input and output of these neuropeptide systems and their interactions with metabolic cues have not been demonstrated. Similarly, RF-amide related peptide (RFRP, the ortholog of GnIH)-expressing neurons appear to play an important role, but the neuronal circuitry is still undefined (83, 84, 248, 377). Further studies are necessary to substantiate a position for these neuropeptides in the neuroanatomical framework of the metabolic control of reproductive function.
IV. METABOLIC DYSFUNCTION AND REPRODUCTIVE DEFICITS IN HUMANS
In addition to genetic mutations, metabolic dysfunctions such as eating disorders, common obesity, diabetes, and lipodystrophy in humans are associated with disruption of the reproductive axis, potentially via changes in the actions of metabolic signals in the HPG axis.
Approximately 20 million women and 10 million men in the United States suffer from anorexia nervosa, bulimia, binge-eating, or other unspecified feeding or eating disorders (423). Eating disorders in general are associated with amenorrhea, sexual dysfunction, infertility, miscarriage, and preterm delivery (14, 483). Pregnancy and childbirth rates are also reportedly lower in women with lifetime eating disorders compared with unaffected individuals (278). The menstrual irregularity observed in patients with anorexia nervosa likely results from suppression of GnRH release and impairments in the patterns of gonadotropin secretion (175). The suppressed secretion of LH and FSH leads to low levels of circulating estrogens and the cessation of ovulation. In adolescents with anorexia nervosa, the onset of secondary amenorrhea is usually observed after a 22% decrease in initial weight. In most cases, leptin levels predict amenorrhea and oligomenorrhea, and both are resolved when the body mass index (BMI) returns to 92.6% of the initial weight (461). Similarly, a general decrease in sexual libido and activity has been described in individuals with eating disorders (243, 358). The restoration of BMI in anorexic patients yields an increase in sexual drive, suggesting that the causal factor is hypothalamic hypogonadism secondary to energy insufficiency and decreased levels of leptin (243, 358).
Calorie restriction, which usually refers to a reduction in food intake without malnutrition, also impairs reproductive function in humans (331, 429). These deleterious effects vary according to the degree of caloric restriction. Mild and moderate reductions in calorie intake have no detrimental effect on fertility, whereas severe restriction suppresses reproductive function (296, 302, 507). A decrease in leptin levels seems to be the main signal for reproductive arrest, as leptin administration to women with hypothalamic amenorrhea due to extreme weight loss restores LH pulsatility and sex steroid levels (73, 276, 309, 476, 481).
On the other side of the spectrum, excess energy, as in obesity, also negatively affects human reproductive physiology. In girls, high adiposity is correlated with early puberty onset (42, 59, 204, 340, 472), which causes deleterious consequences for physical and psychological health. For example, earlier menarche and exposure to high levels of sex steroids increase the risk of adult obesity, T2D, and breast cancer (37, 115, 260, 350). Overweight and obese women have irregular menstrual cycles and ovulatory dysfunction. All these parameters are aggravated by a state of hyperleptinemia and leptin resistance. A blunted response to leptin leads to decreased activation of the HPG axis, low gonadotropin levels and impaired ovulation (3, 54, 307, 436).
Although obesity may cause reproductive deficits, high percentage of obese women are able to conceive. Obesity during pregnancy increases the risk of pregnancy complications, including maternal and fetal morbidity and mortality (70, 205). Obese women have higher risks of early and recurrent miscarriages, preterm births, stillbirths, and neonatal deaths (256, 372, 394). The effects of obesity on pregnancy complications are potentially associated with placental dysfunction not with HPG axis dysregulation (18, 349, 372, 394). In rodents, obesity induces placental necrosis, defective angiogenesis, and fetal resorption (287).
In obese men, testosterone is inversely correlated with fat mass and leptin due to a disruption of the HPG axis and increased aromatization of testosterone in adipocytes (223). Elevated body weight increases the risk of erectile dysfunction, potentially due to decreases in testosterone and disruptions of the melanocortin system, and impairs spermatogenesis and sperm motility (314, 457). However, obesity in men has also an effect on germ cells. Sperm from obese men have altered patterns of DNA methylation and higher rates of DNA fragmentation (103). In rodents, obesity decreases sperm motility and fertilization capacity (50). Following assisted reproductive technology, sperm from obese men resulted in deficient blastocyst development and decreased pregnancy rates (239).
In addition to having a direct impact on fertility, obesity often leads to high glucose levels, hyperinsulinemia, and diabetes. Both type 1 diabetes (T1D) and T2D have a negative impact on fertility (307). Up to 40% of females with T1D display menstrual disturbances (i.e., amenorrhea, oligomenorrhea, and menorrhagia), hyperandrogenism, and/or early menopause (480). In addition, late age at menarche is observed in girls with T1D (89, 162). The incidence of menstrual irregularities and menarche delays is higher in individuals with poor glucose control and higher BMI (162). Severe hypogonadism and low fertility rates have also been observed in T1D women (90). The few studies conducted in T1D men have revealed testosterone deficiencies in ~10% of these patients (78, 212), and its prevalence bears a direct relationship with waist circumference, triglyceride levels, and insulin requirements (78). It is possible that altered levels of glucose, FFA, and insulin disrupt the HPG axis in T1D patients. In addition, glucose metabolism in the testes, necessary for spermatogenesis and sperm quality and motility, may also be affected in T1D men (122).
Hypogonadotropic hypogonadism is the most common reproductive deficit of obesity and T2D (348). Individuals with T2D show relative decreases in LH pulsatility and disrupted sex steroid feedback, resulting in low gonadotropin levels (112). In women, T2D may induce early age at menopause (162, 480), and in men, low levels of testosterone may lead either directly or indirectly to erectile dysfunction (281). Men with erectile dysfunction are more likely to have undiagnosed diabetes (421). Whether this association results from decreased testosterone is not fully resolved, but studies in animal models have shown that neurite sprouting and length as well as the expression of growth factors are reduced in diabetic rats (298). It is also possible that in T2D and prediabetic states, insulin and leptin resistance in melanocortin neurons promotes erectile dysfunction by reducing α-MSH production, as observed in mice (207).
Insulin resistance and altered GLP1 secretion in obese and lean women have been associated with polycystic ovary syndrome (PCOS), one of the most common endocrine disorders, affecting 20% of women of reproductive age (132, 339, 366, 435, 469). Hyperinsulinemia increases LH secretion, cytochrome P-450 expression, and androgen production by the ovary as well as ACTH-mediated adrenal androgen production. High androgen levels in turn impair fertility by inducing follicle atresia and inhibiting meiotic maturation of germ cells (369), and increasing GnRH and LH pulsatility, potentially generating an uncontrolled feedforward cycle (58, 385). Metformin, an insulin-sensitizing medication, significantly reduces the production of hydroxyl-progesterone, testosterone, and androstenedione, improving the symptoms of PCOS (117).
PCOS is diagnosed by the presence of two of the following criteria: oligo or amenorrhea (anovulation), hyperandrogenemia (often visible as acne or hirsutism), and polycystic ovaries (117). In some cases, the ovaries develop “cysts” in the form of antral follicles that luteinize prematurely and fail to develop to a preovulatory stage. However, PCOS is a diagnosis of exclusion, and the development of a biochemical test of diagnostic criteria would be extremely useful in the clinic. The levels of anti-Mullerian hormone (221), insulin, or other circulating hormones have been suggested as potential biomarkers (475). Both genetic and environmental factors contribute to the development of PCOS, but the mechanisms are not entirely understood (117, 196). Hyperinsulinemia and hyperandrogenemia may both affect and disrupt the HPG axis.
Another metabolic disorder with a direct effect on reproductive function is lipodystrophy, characterized by the selective loss of adipose tissue. Lipodystrophy may be manifested in the whole body (generalized) or specific fat depots (partial) and may be genetic or acquired (277). The generalized form has been associated with mutations in 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT2), seipin lipid droplet associated gene (BSCL2), caveolin 1 (CAV1), and polymerase I and transcript release factor (PTRF) genes. The partial form is caused by mutations in distinct genes, among which lamin A/C (LMNA) is the most common (277). Decreased fat depots result in low leptin levels and, as expected, most lipodystrophy features resemble a state of leptin deficiency. Increased food intake, ectopic fat storage, and insulin resistance are usually observed, ultimately leading to diabetes, nonalcoholic liver disease, PCOS, and infertility (192, 231, 455, 465). Leptin therapy improves insulin sensitivity, reverses PCOS, and restores fertility (282, 286).
V. FINAL REMARKS AND FUTURE CHALLENGES
The development of new techniques in gene editing and brain mapping have produced a considerable amount of data and insight on the integration of metabolism and reproduction by specific neuronal populations. In the last decade, metabolic cues with a role in reproductive function, including hormones, metabolites, and fuels, have been found to target specific neurons in the mediobasal hypothalamus, previously known to control energy balance and integrate environmental cues. These signals preferentially converge onto kisspeptin neurons upstream of GnRH neurons, expanding and refining the control of the reproductive axis. We have also learned that the selective actions of metabolic cues in subsets of neurons are sufficient to maintain fertility. However, none of these paths appears to be required for the maintenance of reproductive function, strengthening the case for circuit redundancy to ensure species survival. Studies using CRISPR/Cas9 technology or derivatives will help dissociate the heterogeneity of the system, e.g., specific kisspeptin, GABAergic, or nitrergic neuronal populations and their potential circuitry. Inducible gene mutations to bypass developmental compensations will also be necessary to challenge the system and dissociate congenital from acquired disorders. This approach will allow the interrogation of untested questions, such as the role of SF1 in the metabolic control of adult reproduction. Finally, the development of new technologies for brain imaging coupled with the remote activation/inhibition of specific pathways with the use of DREADDs and optogenetic technologies will advance the understanding of the network engaged in the control and integration of these complex systems crucial for individual and species survival.
Note ADDED IN PROOF
A paper published during proof revision demonstrated that the peptide PACAP (pituitary adenylate cyclase activating polypeptide) coexpressed in LepRb cells of the PMV also has a role in the metabolic control of reproduction (Ross RA, Leon S, Madara JC, Schafer D, Fergani C, Maguire CA, Verstegen AM, Brengle E, Kong D, Herbison AE, Kaiser UB, Lowell BB, Navarro VM. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 7: e35960, 2018. doi: 10.7554/eLife.35960).
GRANTS
Studies in our laboratories are supported by National Institutes of Health Grants R01 HD069702 and R21 HD0900567 (to C. F. Elias) and R01 HD081792 (to J. W. Hill). We also thank the Michigan Diabetes Research Center (P30DK020572) for pilot grants (to C. F. Elias and J. W. Hill) and the Reproductive Sciences Program for pilot grants (to C. F. Elias).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Quinn Burrell for illustrations in Figures 1 AND 3. We also thank the members of our laboratories for generating data, making suggestions, and criticizing the manuscript. We are especially thankful to Yetunde Badmus, Sampath Choppara, Judy Daboul, Selin Longmire, Iyad Manaserh, Samyuktha Ravi, Alisha Sangal, Erin Semple, Sara Singhal, and Mengjie Wang in Dr. Hill's laboratory and to David Garcia-Galiano, Nicole Bellefontaine, Ally Cara, Xingfa Ha, Brenda Cisneros Larios, and Susan Allen in Dr. Elias's laboratory.
Address for reprint requests and other correspondence: C. F. Elias, 1137 E. Catherine St., 7732B Medical Science Building II, Ann Arbor, MI 48109–5622 (e-mail: cfelias@umich.edu).
REFERENCES
- 1.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53: 367–378, 2007. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 2.Adams MM, Elizabeth Forbes M, Constance Linville M, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors 27: 181–188, 2009. doi: 10.1080/08977190902863639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab 84: 1072–1076, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14, Suppl 5: 242S–249S, 2006. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 5.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 99: 391–395, 1997. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology 140: 4923–4931, 1999. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- 7.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101: 1020–1027, 1998. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21: 263–307, 2000. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab 20: 237–242, 2009. doi: 10.1016/j.tem.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Allen SJ, Garcia-Galiano D, Borges BC, Burger LL, Boehm U, Elias CF. Leptin receptor null mice with reexpression of LepR in GnRHR expressing cells display elevated FSH levels but remain in a prepubertal state. Am J Physiol Regul Integr Comp Physiol 310: R1258–R1266, 2016. doi: 10.1152/ajpregu.00529.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almog B, Gold R, Tajima K, Dantes A, Salim K, Rubinstein M, Barkan D, Homburg R, Lessing JB, Nevo N, Gertler A, Amsterdam A. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immature rats. Mol Cell Endocrinol 183: 179–191, 2001. doi: 10.1016/S0303-7207(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 13.Amiel SA. Organ fuel selection: brain. Proc Nutr Soc 54: 151–155, 1995. doi: 10.1079/PNS19950044. [DOI] [PubMed] [Google Scholar]
- 14.Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol 114: 1353–1367, 2009. doi: 10.1097/AOG.0b013e3181c070f9. [DOI] [PubMed] [Google Scholar]
- 15.Andrews WW, Advis JP, Ojeda SR. The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology 109: 2022–2031, 1981. doi: 10.1210/endo-109-6-2022. [DOI] [PubMed] [Google Scholar]
- 16.Andrews WW, Ojeda SR. A quantitative analysis of the maturation of steroid negative feedbacks controlling gonadotropin release in the female rat: the infantile-juvenile periods, transition from an androgenic to a predominantly estrogenic control. Endocrinology 108: 1313–1320, 1981. doi: 10.1210/endo-108-4-1313. [DOI] [PubMed] [Google Scholar]
- 17.Argiolas A, Melis MR, Gessa GL. Oxytocin: an extremely potent inducer of penile erection and yawning in male rats. Eur J Pharmacol 130: 265–272, 1986. doi: 10.1016/0014-2999(86)90277-3. [DOI] [PubMed] [Google Scholar]
- 18.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 311: 1536–1546, 2014. doi: 10.1001/jama.2014.2269. [DOI] [PubMed] [Google Scholar]
- 19.Babaei-Balderlou F, Khazali H. Effects of Ghrelin on Sexual Behavior and Luteinizing Hormone Beta-subunit Gene Expression in Male Rats. J Reprod Infertil 17: 88–96, 2016. [PMC free article] [PubMed] [Google Scholar]
- 20.Backholer K, Bowden M, Gamber K, Bjørbaek C, Iqbal J, Clarke IJ. Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology 91: 27–40, 2010. doi: 10.1159/000260060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 150: 5488–5497, 2009. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 22.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19: 293–301, 2014. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991, 2004. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept 149: 11–14, 2008. doi: 10.1016/j.regpep.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci 1264: 13–19, 2012. doi: 10.1111/j.1749-6632.2012.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 28.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology 137: 3144–3147, 1996. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 29.Bataille D, Tatemoto K, Gespach C, Jörnvall H, Rosselin G, Mutt V. Isolation of glucagon-37 (bioactive enteroglucagon/oxyntomodulin) from porcine jejuno-ileum. Characterization of the peptide. FEBS Lett 146: 79–86, 1982. doi: 10.1016/0014-5793(82)80709-6. [DOI] [PubMed] [Google Scholar]
- 30.Batt RAL, Everard DM, Gillies G, Wilkinson M, Wilson CA, Yeo TA. Investigation into the hypogonadism of the obese mouse (genotype ob/ob). J Reprod Fertil 64: 363–371, 1982. doi: 10.1530/jrf.0.0640363. [DOI] [PubMed] [Google Scholar]
- 31.Beak SA, Heath MM, Small CJ, Morgan DG, Ghatei MA, Taylor AD, Buckingham JC, Bloom SR, Smith DM. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J Clin Invest 101: 1334–1341, 1998. doi: 10.1172/JCI610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Been LE, Petrulis A. The role of the medial preoptic area in appetitive and consummatory reproductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters. Neuroscience 170: 1120–1132, 2010. doi: 10.1016/j.neuroscience.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellefontaine N, Chachlaki K, Parkash J, Vanacker C, Colledge W, d’Anglemont de Tassigny X, Garthwaite J, Bouret SG, Prevot V. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J Clin Invest 124: 2550–2559, 2014. doi: 10.1172/JCI65928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellefontaine N, Hanchate NK, Parkash J, Campagne C, de Seranno S, Clasadonte J, d’Anglemont de Tassigny X, Prevot V. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology 93: 74–89, 2011. doi: 10.1159/000324147. [DOI] [PubMed] [Google Scholar]
- 35.Beltramino C, Taleisnik S. Ventral premammillary nuclei mediate pheromonal-induced LH release stimuli in the rat. Neuroendocrinology 41: 119–124, 1985. doi: 10.1159/000124164. [DOI] [PubMed] [Google Scholar]
- 36.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J 403: 603–613, 2007. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and Breast Carcinoma Risk. New York: Wiley, 1999, p. 2400–2409. [DOI] [PubMed] [Google Scholar]
- 38.Berria M, Pau KY, Spies HG. Evidence for alpha 1-adrenergic involvement in neuropeptide Y-stimulated GnRH release in female rabbits. Neuroendocrinology 53: 480–486, 1991. doi: 10.1159/000125761. [DOI] [PubMed] [Google Scholar]
- 39.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertoldi ML, Luque EM, Carlini VP, Vincenti LM, Stutz G, Santillán ME, Ruiz RD, Fiol de Cuneo M, Martini AC. Inhibitory effects of ghrelin on sexual behavior: role of the peptide in the receptivity reduction induced by food restriction in mice. Horm Metab Res 43: 494–499, 2011. doi: 10.1055/s-0031-1277228. [DOI] [PubMed] [Google Scholar]
- 41.Bicknell RJ. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol 107: 437–446, 1985. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- 42.Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126: e583–e590, 2010. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biro FM, Khoury P, Morrison JA. Influence of obesity on timing of puberty. Int J Androl 29: 272–277, 2006. doi: 10.1111/j.1365-2605.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 44.Blake CA, Sridaran R, Elias KA, Ashiru OA, Rush ME. Plasma LH patterns after LHRH infusion in long-term, unanesthetized ovariectomized rats. Evidence for neural control of the pulsatile LH phenomenon. Neuroendocrinology 30: 45–51, 1980. doi: 10.1159/000122973. [DOI] [PubMed] [Google Scholar]
- 45.Blankstein J, Reyes FI, Winter JSD, Faiman C. Endorphins and the regulations of the human menstrual cycle. Clin Endocrinol (Oxf) 14: 287–294, 1981. doi: 10.1111/j.1365-2265.1981.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 46.Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci 1007: 238–250, 2003. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 47.Bloch GJ, Butler PC, Eckersell CB, Mills RH. Gonadal steroid-dependent GAL-IR cells within the medial preoptic nucleus (MPN) and the stimulatory effects of GAL within the MPN on sexual behaviors. In: Galanin: Basic Research Discoveries and Therapeutic Implications, edited by Hokfelt T, Bartfai T, Crawley J. New York: NY Acad. Sci., 1998, p. 188–205. doi: 10.1111/j.1749-6632.1998.tb10695.x. [DOI] [PubMed] [Google Scholar]
- 48.Bloch GJ, Butler PC, Kohlert JG. Galanin microinjected into the medial preoptic nucleus facilitates female- and male-typical sexual behaviors in the female rat. Physiol Behav 59: 1147–1154, 1996. doi: 10.1016/0031-9384(95)02087-X. [DOI] [PubMed] [Google Scholar]
- 49.Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell 123: 683–695, 2005. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Borges BC, Garcia-Galiano D, da Silveira Cruz-Machado S, Han X, Gavrilina GB, Saunders TL, Auchus RJ, Hammoud SS, Smith GD, Elias CF. Obesity-Induced Infertility in Male Mice Is Associated With Disruption of Crisp4 Expression and Sperm Fertilization Capacity. Endocrinology 158: 2930–2943, 2017. doi: 10.1210/en.2017-00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol 15: 3–49, 1994. doi: 10.1006/frne.1994.1002. [DOI] [PubMed] [Google Scholar]
- 52.Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 166: 257–283, 1976. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- 53.Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104: 1247–1255, 1979. doi: 10.1210/endo-104-5-1247. [DOI] [PubMed] [Google Scholar]
- 54.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril 107: 840–847, 2017. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav 89: 687–691, 2006. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 57.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498: 712–726, 2006. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 58.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 77: 332–337, 2012. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 140: 399–410, 2010. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cagampang FR, Maeda K, Yokoyama A, Ota K. Effect of food deprivation on the pulsatile LH release in the cycling and ovariectomized female rat. Horm Metab Res 22: 269–272, 1990. doi: 10.1055/s-2007-1004900. [DOI] [PubMed] [Google Scholar]
- 61.Całka J. The role of nitric oxide in the hypothalamic control of LHRH and oxytocin release, sexual behavior and aging of the LHRH and oxytocin neurons. Folia Histochem Cytobiol 44: 3–12, 2006. [PubMed] [Google Scholar]
- 62.Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology 128: 1532–1540, 1991. doi: 10.1210/endo-128-3-1532. [DOI] [PubMed] [Google Scholar]
- 63.Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab 73: 35–41, 1991. doi: 10.1210/jcem-73-1-35. [DOI] [PubMed] [Google Scholar]
- 64.Campbell RE, Gaidamaka G, Han S-K, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA 106: 10835–10840, 2009. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell RE, Suter KJ. Redefining the gonadotrophin-releasing hormone neurone dendrite. J Neuroendocrinol 22: 650–658, 2010. doi: 10.1111/j.1365-2826.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 66.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 41–79, 1994. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 67.Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol 324: 195–212, 1992. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- 68.Caquineau C, Leng G, Guan XMM, Jiang M, Van der Ploeg L, Douglas AJ. Effects of alpha-melanocyte-stimulating hormone on magnocellular oxytocin neurones and their activation at intromission in male rats. J Neuroendocrinol 18: 685–691, 2006. doi: 10.1111/j.1365-2826.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 69.Carro E, Pinilla L, Seoane LM, Considine RV, Aguilar E, Casanueva FF, Dieguez C. Influence of endogenous leptin tone on the estrous cycle and luteinizing hormone pulsatility in female rats. Neuroendocrinology 66: 375–377, 1997. doi: 10.1159/000127262. [DOI] [PubMed] [Google Scholar]
- 70.Castro LC, Avina RL. Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol 14: 601–606, 2002. doi: 10.1097/00001703-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Cates PS, O’Byrne KT. The area postrema mediates insulin hypoglycaemia-induced suppression of pulsatile LH secretion in the female rat. Brain Res 853: 151–155, 2000. doi: 10.1016/S0006-8993(99)02301-X. [DOI] [PubMed] [Google Scholar]
- 72.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111: 1409–1421, 2003. doi: 10.1172/JCI200317490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366: 74–85, 2005. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 74.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 109: 528–531, 1995. doi: 10.1037/0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 75.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320, 1996. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 76.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 77.Cheung CC, Thornton JE, Nurani SD, Clifton DK, Steiner RA. A reassessment of leptin’s role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology 74: 12–21, 2001. doi: 10.1159/000054666. [DOI] [PubMed] [Google Scholar]
- 78.Chillarón JJ, Fernández-Miró M, Albareda M, Vila L, Colom C, Fontserè S, Pedro-Botet J, Flores-Le Roux JA; TEST-DM1 Study Group . Age, insulin requirements, waist circumference, and triglycerides predict hypogonadotropic hypogonadism in patients with type 1 diabetes. J Sex Med 12: 76–82, 2015. doi: 10.1111/jsm.12748. [DOI] [PubMed] [Google Scholar]
- 79.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102: 15682–15687, 2005. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27: 1913–1921, 2007. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149: 3130–3136, 2008. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cicero TJ, Schainker BA, Meyer ER. Endogenous opioids participate in the regulation of the hypothalamus-pituitary-luteinizing hormone axis and testosterone’s negative feedback control of luteinizing hormone. Endocrinology 104: 1286–1291, 1979. doi: 10.1210/endo-104-5-1286. [DOI] [PubMed] [Google Scholar]
- 83.Clarke IJ, Arbabi L. New concepts of the central control of reproduction, integrating influence of stress, metabolic state, and season. Domest Anim Endocrinol 56, Suppl: S165–S179, 2016. doi: 10.1016/j.domaniend.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 95: 305–316, 2012. doi: 10.1159/000332822. [DOI] [PubMed] [Google Scholar]
- 85.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28: 8691–8697, 2008. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254-255: 32–38, 2006. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 87.Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149: 587–596, 2008. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
- 88.Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction Nature 392: 398–401, 1998. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 89.Codner E, Cassorla F. Puberty and ovarian function in girls with type 1 diabetes mellitus. Horm Res 71: 12–21, 2009. doi: 10.1159/000173737. [DOI] [PubMed] [Google Scholar]
- 90.Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum Reprod Update 18: 568–585, 2012. doi: 10.1093/humupd/dms024. [DOI] [PubMed] [Google Scholar]
- 91.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001. doi: 10.1172/JCI200113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14: 141–148, 1978. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 93.Colledge WH. Kisspeptins and GnRH neuronal signalling. Trends Endocrinol Metab 20: 115–121, 2009. doi: 10.1016/j.tem.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Considine RV, Caro JF. Leptin in humans: current progress and future directions Clin Chem 42: 843–844, 1996. [PubMed] [Google Scholar]
- 95.Conte FA, Grumbach MM, Kaplan SL. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab 40: 670–674, 1975. doi: 10.1210/jcem-40-4-670. [DOI] [PubMed] [Google Scholar]
- 96.Cooke BM, Simerly RB. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. J Comp Neurol 489: 42–58, 2005. doi: 10.1002/cne.20612. [DOI] [PubMed] [Google Scholar]
- 97.Cooke RM, Harvey TS, Campbell ID. Solution structure of human insulin-like growth factor 1: a nuclear magnetic resonance and restrained molecular dynamics study. Biochemistry 30: 5484–5491, 1991. doi: 10.1021/bi00236a022. [DOI] [PubMed] [Google Scholar]
- 98.Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol 399: 189–209, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 99.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol 17: 1039–1053, 2003. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 100.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661, 2003. doi: 10.1016/S0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 101.Coyral-Castel S, Tosca L, Ferreira G, Jeanpierre E, Rame C, Lomet D, Caraty A, Monget P, Chabrolle C, Dupont J. The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1-7 cells and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol 20: 335–346, 2008. doi: 10.1111/j.1365-2826.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 102.Cragnolini A, Scimonelli T, Celis ME, Schiöth HB. The role of melanocortin receptors in sexual behavior in female rats. Neuropeptides 34: 211–215, 2000. doi: 10.1054/npep.2000.0815. [DOI] [PubMed] [Google Scholar]
- 103.Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril 107: 848–859, 2017. doi: 10.1016/j.fertnstert.2017.02.115. [DOI] [PubMed] [Google Scholar]
- 104.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173: 37–56, 2011. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev 13: 33–65, 1992. [DOI] [PubMed] [Google Scholar]
- 106.Crowley WR, Hassid A, Kalra SP. Neuropeptide Y enhances the release of luteinizing hormone (LH) induced by LH-releasing hormone. Endocrinology 120: 941–945, 1987. doi: 10.1210/endo-120-3-941. [DOI] [PubMed] [Google Scholar]
- 107.D’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 104: 10714–10719, 2007. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol 13: 227–255, 1996. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- 109.Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: relationship to gonadotropin-releasing hormone neurons. Endocrinology 144: 2034–2045, 2003. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- 110.Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol 16: 160–169, 2004. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- 111.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology 140: 4015–4023, 1999. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- 112.Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 96: 2643–2651, 2011. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice: role of IGF-I. Endocrinology 140: 2637–2640, 1999. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- 114.Davidson JM, Rodgers CH, Smith ER, Bloch GJ. Stimulation of female sex behavior in adrenalectomized rats with estrogen alone. Endocrinology 82: 193–195, 1968. doi: 10.1210/endo-82-1-193. [DOI] [PubMed] [Google Scholar]
- 115.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, Ruth KS, Whalen S, Sarkar AK, Albrecht E, Altmaier E, Amini M, Barbieri CM, Boutin T, Campbell A, Demerath E, Giri A, He C, Hottenga JJ, Karlsson R, Kolcic I, Loh P-R, Lunetta KL, Mangino M, Marco B, McMahon G, Medland SE, Nolte IM, Noordam R, Nutile T, Paternoster L, Perjakova N, Porcu E, Rose LM, Schraut KE, Segrè AV, Smith AV, Stolk L, Teumer A, Andrulis IL, Bandinelli S, Beckmann MW, Benitez J, Bergmann S, Bochud M, Boerwinkle E, Bojesen SE, Bolla MK, Brand JS, Brauch H, Brenner H, Broer L, Brüning T, Buring JE, Campbell H, Catamo E, Chanock S, Chenevix-Trench G, Corre T, Couch FJ, Cousminer DL, Cox A, Crisponi L, Czene K, Davey Smith G, de Geus EJCN, de Mutsert R, De Vivo I, Dennis J, Devilee P, Dos-Santos-Silva I, Dunning AM, Eriksson JG, Fasching PA, Fernández-Rhodes L, Ferrucci L, Flesch-Janys D, Franke L, Gabrielson M, Gandin I, Giles GG, Grallert H, Gudbjartsson DF, Guénel P, Hall P, Hallberg E, Hamann U, Harris TB, Hartman CA, Heiss G, Hooning MJ, Hopper JL, Hu F, Hunter DJ, Ikram MA, Im HK, Järvelin M-R, Joshi PK, Karasik D, Kellis M, Kutalik Z, LaChance G, Lambrechts D, Langenberg C, Launer LJ, Laven JSE, Lenarduzzi S, Li J, Lind PA, Lindstrom S, Liu Y, Luan J, Mägi R, Mannermaa A, Mbarek H, McCarthy MI, Meisinger C, Meitinger T, Menni C, Metspalu A, Michailidou K, Milani L, Milne RL, Montgomery GW, Mulligan AM, Nalls MA, Navarro P, Nevanlinna H, Nyholt DR, Oldehinkel AJ, O’Mara TA, Padmanabhan S, Palotie A, Pedersen N, Peters A, Peto J, Pharoah PDP, Pouta A, Radice P, Rahman I, Ring SM, Robino A, Rosendaal FR, Rudan I, Rueedi R, Ruggiero D, Sala CF, Schmidt MK, Scott RA, Shah M, Sorice R, Southey MC, Sovio U, Stampfer M, Steri M, Strauch K, Tanaka T, Tikkanen E, Timpson NJ, Traglia M, Truong T, Tyrer JP, Uitterlinden AG, Edwards DRV, Vitart V, Völker U, Vollenweider P, Wang Q, Widen E, van Dijk KW, Willemsen G, Winqvist R, Wolffenbuttel BHR, Zhao JH, Zoledziewska M, Zygmunt M, Alizadeh BZ, Boomsma DI, Ciullo M, Cucca F, Esko T, Franceschini N, Gieger C, Gudnason V, Hayward C, Kraft P, Lawlor DA, Magnusson PKE, Martin NG, Mook-Kanamori DO, Nohr EA, Polasek O, Porteous D, Price AL, Ridker PM, Snieder H, Spector TD, Stöckl D, Toniolo D, Ulivi S, Visser JA, Völzke H, Wareham NJ, Wilson JF, Spurdle AB, Thorsteindottir U, Pollard KS, Easton DF, Tung JY, Chang-Claude J, Hinds D, Murray A, Murabito JM, Stefansson K, Ong KK, Perry JRB; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL Consortium . Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet 49: 834–841, 2017. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 154: 2750–2760, 2013. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 117.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol 14: 38, 2016. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC Jr. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115: 3484–3493, 2005. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Devor M, Murphy MR. The effect of peripheral olfactory blockade on the social behavior of the male golden hamster. Behav Biol 9: 31–42, 1973. doi: 10.1016/S0091-6773(73)80166-X. [DOI] [PubMed] [Google Scholar]
- 121.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 122.Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, Huang HF. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl 17: 948–953, 2015. doi: 10.4103/1008-682X.150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A. Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One 10: e0119995, 2015. doi: 10.1371/journal.pone.0119995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DiVall SA, Williams TR, Carver SE, Koch L, Brüning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest 120: 2900–2909, 2010. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donato J Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 29: 5240–5250, 2009. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donato J Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav 99: 67–77, 2010. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 127.Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121: 355–368, 2011. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donato J Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2: 57, 2011. doi: 10.3389/fendo.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Donato J Jr, Frazão R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology 151: 5415–5427, 2010. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology 142: 521–527, 2001. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 131.Duittoz AH, Batailler M. Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120: 391–396, 2000. doi: 10.1530/reprod/120.2.391. [DOI] [PubMed] [Google Scholar]
- 132.Duleba AJ. Medical management of metabolic dysfunction in PCOS. Steroids 77: 306–311, 2012. doi: 10.1016/j.steroids.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dunger DB, Ahmed ML, Ong KK. Effects of obesity on growth and puberty. Best Pract Res Clin Endocrinol Metab 19: 375–390, 2005. doi: 10.1016/j.beem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 134.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 135.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23: 5928–5935, 2003. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ebling FJP, Schwartz ML, Foster DL. Endogenous opioid regulation of pulsatile luteinizing hormone secretion during sexual maturation in the female sheep. Endocrinology 125: 369–383, 1989. doi: 10.1210/endo-125-1-369. [DOI] [PubMed] [Google Scholar]
- 137.Egan OK, Inglis MA, Anderson GM. Leptin Signaling in AgRP Neurons Modulates Puberty Onset and Adult Fertility in Mice. J Neurosci 37: 3875–3886, 2017. doi: 10.1523/JNEUROSCI.3138-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elias CF. Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab 23: 9–15, 2012. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998. doi: 10.1016/S0896-6273(00)80656-X. [DOI] [PubMed] [Google Scholar]
- 140.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci 70: 841–862, 2013. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y Science 274: 1704–1707, 1996. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 142.Estall JL, Drucker DJ. Glucagon and glucagon-like peptide receptors as drug targets. Curr Pharm Des 12: 1731–1750, 2006. doi: 10.2174/138161206776873671. [DOI] [PubMed] [Google Scholar]
- 143.Evans MC, Rizwan MZ, Anderson GM. Insulin Does Not Target CamkIIα Neurones to Critically Regulate the Neuroendocrine Reproductive Axis in Mice. J Neuroendocrinol 27: 899–910, 2015. doi: 10.1111/jne.12330. [DOI] [PubMed] [Google Scholar]
- 144.Evans MC, Anderson GM. Neuroendocrine integration of nutritional signals on reproduction. J Mol Endocrinol 58: R107–R128, 2017. doi: 10.1530/JME-16-0212. [DOI] [PubMed] [Google Scholar]
- 145.Evans MC, Rizwan M, Mayer C, Boehm U, Anderson GM. Evidence that insulin signalling in gonadotrophin-releasing hormone and kisspeptin neurones does not play an essential role in metabolic regulation of fertility in mice. J Neuroendocrinol 26: 468–479, 2014. doi: 10.1111/jne.12166. [DOI] [PubMed] [Google Scholar]
- 146.Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev 27: 710–718, 2006. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 147.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Faulkner LD, Dowling AR, Stuart RC, Nillni EA, Hill JW. Reduced melanocortin production causes sexual dysfunction in male mice with POMC neuronal insulin and leptin insensitivity. Endocrinology 156: 1372–1385, 2015. doi: 10.1210/en.2014-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fernández-Fernández R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362: 103–107, 2004. doi: 10.1016/j.neulet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 150.Fernández-Fernández R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology 82: 245–255, 2005. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- 151.Flier JS. Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab 83: 1407–1413, 1998. [DOI] [PubMed] [Google Scholar]
- 152.Folmes CD, Lopaschuk GD. Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res 73: 278–287, 2007. doi: 10.1016/j.cardiores.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 153.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett 460: 143–147, 2009. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 154.Fraley GS, Scarlett JM, Shimada I, Teklemichael DN, Acohido BV, Clifton DK, Steiner RA. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes 53: 1237–1242, 2004. doi: 10.2337/diabetes.53.5.1237. [DOI] [PubMed] [Google Scholar]
- 155.Frazão R, Cravo RM, Donato J Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor α. J Neurosci 33: 2807–2820, 2013. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Frazao R, Dungan Lemko HM, da Silva RP, Ratra DV, Lee CE, Williams KW, Zigman JM, Elias CF. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab 306: E606–E614, 2014. doi: 10.1152/ajpendo.00211.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frisch RE. Fatness, menarche, and female fertility. Perspect Biol Med 28: 611–633, 1985. doi: 10.1353/pbm.1985.0010. [DOI] [PubMed] [Google Scholar]
- 158.Frisch RE. The right weight: body fat, menarche and fertility. Proc Nutr Soc 53: 113–129, 1994. doi: 10.1079/PNS19940015. [DOI] [PubMed] [Google Scholar]
- 159.Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1-43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology 152: 4310–4321, 2011. doi: 10.1210/en.2011-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Funabashi T, Daikoku S, Shinohara K, Kimura F. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71: 138–144, 2000. doi: 10.1159/000054529. [DOI] [PubMed] [Google Scholar]
- 161.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun 288: 780–785, 2001. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 162.Gaete X, Vivanco M, Eyzaguirre FC, López P, Rhumie HK, Unanue N, Codner E. Menstrual cycle irregularities and their relationship with HbA1c and insulin dose in adolescents with type 1 diabetes mellitus. Fertil Steril 94: 1822–1826, 2010. doi: 10.1016/j.fertnstert.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 163.Gambacciani M, Yen SS, Rasmussen DD. GnRH release from the mediobasal hypothalamus. In vitro regulation by oxytocin. Neuroendocrinology 42: 181–183, 1986. doi: 10.1159/000124271. [DOI] [PubMed] [Google Scholar]
- 164.Gangloff Y-G, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz J-F, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 24: 9508–9516, 2004. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 27: 422–427, 2000. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 166.García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi C-X, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garcia-Galiano D, Allen SJ, Elias CF. Role of the adipocyte-derived hormone leptin in reproductive control. Horm Mol Biol Clin Investig 19: 141–149, 2014. doi: 10.1515/hmbci-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia-Galiano D, Borges BC, Donato J Jr, Allen SJ, Bellefontaine N, Wang M, Zhao JJ, Kozloff KM, Hill JW, Elias CF. PI3Kα inactivation in leptin receptor cells increases leptin sensitivity but disrupts growth and reproduction. JCI Insight 2: 96728, 2017. doi: 10.1172/jci.insight.96728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.García-Galiano D, Navarro VM, Roa J, Ruiz-Pino F, Sánchez-Garrido MA, Pineda R, Castellano JM, Romero M, Aguilar E, Gaytán F, Diéguez C, Pinilla L, Tena-Sempere M. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci 30: 7783–7792, 2010. doi: 10.1523/JNEUROSCI.5828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.García-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J Neuroendocrinol 24: 22–33, 2012. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 171.García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MAM, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153: 316–328, 2012. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 172.García-Juárez M, Beyer C, Gómora-Arrati P, Lima-Hernández FJ, Domínguez-Ordoñez R, Eguibar JR, Etgen AM, González-Flores O. The nitric oxide pathway participates in lordosis behavior induced by central administration of leptin. Neuropeptides 46: 49–53, 2012. doi: 10.1016/j.npep.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 173.Gauguin L, Klaproth B, Sajid W, Andersen AS, McNeil KA, Forbes BE, De Meyts P. Structural basis for the lower affinity of the insulin-like growth factors for the insulin receptor. J Biol Chem 283: 2604–2613, 2008. doi: 10.1074/jbc.M709220200. [DOI] [PubMed] [Google Scholar]
- 174.Gautron L, Cravo RM, Elmquist JK, Elias CF. Discrete melanocortin-sensitive neuroanatomical pathway linking the ventral premmamillary nucleus to the paraventricular hypothalamus. Neuroscience 240: 70–82, 2013. doi: 10.1016/j.neuroscience.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gendall KA, Bulik CM, Joyce PR, McIntosh VV, Carter FA. Menstrual cycle irregularity in bulimia nervosa. Associated factors and changes with treatment. J Psychosom Res 49: 409–415, 2000. doi: 10.1016/S0022-3999(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 176.Georgiadis JR, Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol 493: 33–38, 2005. doi: 10.1002/cne.20735. [DOI] [PubMed] [Google Scholar]
- 177.Gilbeau PM, Almirez RG, Holaday JW, Smith CG. Opioid effects on plasma concentrations of luteinizing hormone and prolactin in the adult male rhesus monkey. J Clin Endocrinol Metab 60: 299–305, 1985. doi: 10.1210/jcem-60-2-299. [DOI] [PubMed] [Google Scholar]
- 178.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci 34: 15060–15069, 2014. doi: 10.1523/JNEUROSCI.2200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goldsmith PC, Thind KK, Perera AD, Plant TM. Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology 134: 858–868, 1994. doi: 10.1210/endo.134.2.7905410. [DOI] [PubMed] [Google Scholar]
- 180.González-Flores O, Etgen AM. The nitric oxide pathway participates in estrous behavior induced by progesterone and some of its ring A-reduced metabolites. Horm Behav 45: 50–57, 2004. doi: 10.1016/j.yhbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 181.Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin(116-130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology 70: 213–220, 1999. doi: 10.1159/000054479. [DOI] [PubMed] [Google Scholar]
- 182.Gonzalez MI, Celis ME, Hole DR, Wilson CA. Interaction of oestradiol, alpha-melanotrophin and noradrenaline within the ventromedial nucleus in the control of female sexual behaviour. Neuroendocrinology 58: 218–226, 1993. doi: 10.1159/000126536. [DOI] [PubMed] [Google Scholar]
- 183.Goodman RL, Gibson M, Skinner DC, Lehman MN. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl 59: 41–56, 2002. [PubMed] [Google Scholar]
- 184.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107: 1286–1290, 1980. doi: 10.1210/endo-107-5-1286. [DOI] [PubMed] [Google Scholar]
- 185.Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Two effects of estradiol that normally contribute to the control of tonic LH secretion in the ewe. Biol Reprod 23: 415–422, 1980. doi: 10.1095/biolreprod23.2.415. [DOI] [PubMed] [Google Scholar]
- 186.Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, Reznikov LR, Smith A, Wilson SP, Sakai RR, Reagan LP. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav 92: 691–701, 2007. doi: 10.1016/j.physbeh.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gruaz NM, Lalaoui M, Pierroz DD, Englaro P, Sizonenko PC, Blum WF, Aubert ML. Chronic administration of leptin into the lateral ventricle induces sexual maturation in severely food-restricted female rats. J Neuroendocrinol 10: 627–633, 1998. doi: 10.1046/j.1365-2826.1998.00247.x. [DOI] [PubMed] [Google Scholar]
- 188.Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143: 2767–2774, 2002. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- 189.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1: 271–272, 1998. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 190.Hallschmid M, Benedict C, Schultes B, Fehm H-L, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 53: 3024–3029, 2004. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 191.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25: 11349–11356, 2005. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 87: 2395, 2002. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 193.Hara K, Yonezawa K, Weng Q-P, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484–14494, 1998. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 194.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272: 827–829, 1978. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 196.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network . Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun 6: 7502, 2015. doi: 10.1038/ncomms8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138: 3859–3863, 1997. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 198.Hellier V, Brock O, Candlish M, Desroziers E, Aoki M, Mayer C, Piet R, Herbison A, Colledge WH, Prévot V, Boehm U, Bakker J. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun 9: 400, 2018. doi: 10.1038/s41467-017-02797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hentges KE, Sirry B, Gingeras A-C, Sarbassov D, Sonenberg N, Sabatini D, Peterson AS. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci USA 98: 13796–13801, 2001. doi: 10.1073/pnas.241184198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heppner KM, Baquero AF, Bennett CM, Lindsley SR, Kirigiti MA, Bennett B, Bosch MA, Mercer AJ, Rønnekleiv OK, True C, Grove KL, Smith MS. GLP-1R Signaling Directly Activates Arcuate Nucleus Kisspeptin Action in Brain Slices but Does not Rescue Luteinizing Hormone Inhibition in Ovariectomized Mice During Negative Energy Balance. eNeuro 4: ENEURO.0198-16.2016, 2017. doi: 10.1523/ENEURO.0198-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 156: 255–267, 2015. doi: 10.1210/en.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Brain Res Rev 57: 277–287, 2008. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci 33: 12689–12697, 2013. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99: 505–512, 1997. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 205.Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta 32: 1–7, 2011. doi: 10.1016/j.placenta.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 206.Higgins SC, Gueorguiev M, Korbonits M. Ghrelin, the peripheral hunger hormone. Ann Med 39: 116–136, 2007. doi: 10.1080/07853890601149179. [DOI] [PubMed] [Google Scholar]
- 207.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11: 286–297, 2010. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hill JW, Levine JE. Abnormal response of the neuropeptide Y-deficient mouse reproductive axis to food deprivation but not lactation. Endocrinology 144: 1780–1786, 2003. doi: 10.1210/en.2002-221024. [DOI] [PubMed] [Google Scholar]
- 209.Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor I: a possible metabolic signal involved in the regulation of female puberty. Neuroendocrinology 54: 420–423, 1991. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- 210.Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology 137: 3717–3728, 1996. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- 211.Hiney JK, Srivastava VK, Pine MD, Les Dees W. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology 150: 376–384, 2009. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Holt SK, Lopushnyan N, Hotaling J, Sarma AV, Dunn RL, Cleary PA, Braffett BH, Gatcomb P, Martin C, Herman WH, Wessells H; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Prevalence of low testosterone and predisposing risk factors in men with type 1 diabetes mellitus: findings from the DCCT/EDIC. J Clin Endocrinol Metab 99: E1655–E1660, 2014. doi: 10.1210/jc.2014-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Höppener JW, de Pagter-Holthuizen P, Geurts van Kessel AH, Jansen M, Kittur SD, Antonarakis SE, Lips CJ, Sussenbach JS. The human gene encoding insulin-like growth factor I is located on chromosome 12. Hum Genet 69: 157–160, 1985. doi: 10.1007/BF00293288. [DOI] [PubMed] [Google Scholar]
- 214.Horton RJ, Francis H, Clarke IJ. Seasonal and steroid-dependent effects on the modulation of LH secretion in the ewe by intracerebroventricularly administered β-endorphin or naloxone. J Endocrinol 122: 509–517, 1989. doi: 10.1677/joe.0.1220509. [DOI] [PubMed] [Google Scholar]
- 215.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab 22: 94–102, 2011. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hughes AM, Everitt BJ, Herbert J. The effects of simultaneous or separate infusions of some pro-opiomelanocortin-derived peptides (beta-endorphin, melanocyte stimulating hormone, and corticotrophin-like intermediate polypeptide) and their acetylated derivatives upon sexual and ingestive behaviour of male rats. Neuroscience 27: 689–698, 1988. doi: 10.1016/0306-4522(88)90298-9. [DOI] [PubMed] [Google Scholar]
- 217.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav 52: 45–55, 2007. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 219.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 144: 4325–4331, 2003. doi: 10.1210/en.2003-0258. [DOI] [PubMed] [Google Scholar]
- 220.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144: 1331–1340, 2003. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 221.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 98: 3332–3340, 2013. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 222.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 223.Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fabbri A. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 84: 3673–3680, 1999. [DOI] [PubMed] [Google Scholar]
- 224.Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC Jr. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153: 2408–2419, 2012. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jansen M, van Schaik FM, Ricker AT, Bullock B, Woods DE, Gabbay KH, Nussbaum AL, Sussenbach JS, Van den Brande JL. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature 306: 609–611, 1983. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- 226.Janszky J, Szücs A, Halász P, Borbély C, Holló A, Barsi P, Mirnics Z. Orgasmic aura originates from the right hemisphere. Neurology 58: 302–304, 2002. doi: 10.1212/WNL.58.2.302. [DOI] [PubMed] [Google Scholar]
- 227.Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci 1135: 265–279, 2008. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 228.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 229.Johnston CA, Lopez F, Samson WK, Negro-Vilar A. Physiologically important role for central oxytocin in the preovulatory release of luteinizing hormone. Neurosci Lett 120: 256–258, 1990. doi: 10.1016/0304-3940(90)90053-C. [DOI] [PubMed] [Google Scholar]
- 230.Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 231.Joy TR, Hegele RA. Prevalence of reproductive abnormalities among women with familial partial lipodystrophy. Endocr Pract 14: 1126–1132, 2008. doi: 10.4158/EP.14.9.1126. [DOI] [PubMed] [Google Scholar]
- 232.Kalra S. Neural circuitry involved in the control of LHRH secretion: a model for preovulatory LH release. Front Neuroendocrinol 9: 31–75, 1986. [Google Scholar]
- 233.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- 234.Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav 48: 141–151, 2005. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 235.Kawakami S, Ichikawa M, Murahashi K, Hirunagi K, Tsukamura H, Maeda K. Excitatory amino acids act on the median eminence nerve terminals to induce gonadotropin-releasing hormone release in female rats. Gen Comp Endocrinol 112: 372–382, 1998. doi: 10.1006/gcen.1998.7140. [DOI] [PubMed] [Google Scholar]
- 236.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J 282: 4067–4079, 2015. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 237.Keating GM. Mecasermin. BioDrugs 22: 177–188, 2008. doi: 10.2165/00063030-200822030-00004. [DOI] [PubMed] [Google Scholar]
- 238.Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate–lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem Senses 31: 753–762, 2006. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- 239.Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet 27: 539–544, 2010. doi: 10.1007/s10815-010-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol 166: 408–418, 1963. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JK, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol Endocrinol 24: 1240–1250, 2010. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim KW, Sohn J-W, Kohno D, Xu Y, Williams K, Elmquist JK. SF-1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Mol Cell Endocrinol 336: 219–223, 2011. doi: 10.1016/j.mce.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kimmel MC, Ferguson EH, Zerwas S, Bulik CM, Meltzer-Brody S. Obstetric and gynecologic problems associated with eating disorders. Int J Eat Disord 49: 260–275, 2016. doi: 10.1002/eat.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.King JC, Tobet SA, Snavely FL, Arimura AA. LHRH immunopositive cells and their projections to the median eminence and organum vasculosum of the lamina terminalis. J Comp Neurol 209: 287–300, 1982. doi: 10.1002/cne.902090307. [DOI] [PubMed] [Google Scholar]
- 245.Kinoshita M, Moriyama R, Tsukamura H, Maeda KI. A rat model for the energetic regulation of gonadotropin secretion: role of the glucose-sensing mechanism in the brain. Domest Anim Endocrinol 25: 109–120, 2003. doi: 10.1016/S0739-7240(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 246.Kjeldsen T, Andersen AS, Wiberg FC, Rasmussen JS, Schäffer L, Balschmidt P, Møller KB, Møller NP. The ligand specificities of the insulin receptor and the insulin-like growth factor I receptor reside in different regions of a common binding site. Proc Natl Acad Sci USA 88: 4404–4408, 1991. doi: 10.1073/pnas.88.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 91: 254S–257S, 2010. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Klingerman CM, Williams WP III, Simberlund J, Brahme N, Prasad A, Schneider JE, Kriegsfeld LJ. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells Are Associated with Changes in Sexual Motivation and Food Hoarding, but Not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2: 101, 2011. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Klöckener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Brüning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci 14: 911–918, 2011. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519: 45–50, 2015. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66: 721–736, 1995. doi: 10.1016/0306-4522(94)00563-K. [DOI] [PubMed] [Google Scholar]
- 252.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 253.Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res 92: 169–180, 1998. doi: 10.1016/S0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 254.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 18: 588–595, 2013. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103: 2410–2415, 2006. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 112: 403–408, 2005. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 257.Krsmanović LZ, Stojilković SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA 89: 8462–8466, 1992. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kunkhyen T, McCarthy EA, Korzan WJ, Doctor D, Han X, Baum MJ, Cherry JA. Optogenetic Activation of Accessory Olfactory Bulb Input to the Forebrain Differentially Modulates Investigation of Opposite versus Same-Sex Urinary Chemosignals and Stimulates Mating in Male Mice. eNeuro 4: ENEURO.0010-17.2017, 2017. doi: 10.1523/ENEURO.0010-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lado-Abeal J, Clapper JA, Chen Zhu B, Hough CM, Syapin PJ, Norman RL. Hypoglycemia-induced suppression of luteinizing hormone (LH) secretion in intact female rhesus macaques: role of vasopressin and endogenous opioids. Stress 5: 113–119, 2002. doi: 10.1080/10253890290027886. [DOI] [PubMed] [Google Scholar]
- 260.Lakshman R, Forouhi N, Luben R, Bingham S, Khaw K, Wareham N, Ong KK. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 51: 781–786, 2008. doi: 10.1007/s00125-008-0948-5. [DOI] [PubMed] [Google Scholar]
- 261.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 8: 579–584, 2005. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 262.Lapatto R, Pallais JC, Zhang D, Chan Y-M, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 148: 4927–4936, 2007. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 263.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 264.Larsson K, Heimer L. Mating behaviour of male rats after lesions of the preoptic area. Nature 202: 413–414, 1964. doi: 10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- 265.Lawrence C, Fraley GS. Galanin-like peptide (GALP) is a hypothalamic regulator of energy homeostasis and reproduction. Front Neuroendocrinol 32: 1–9, 2011. doi: 10.1016/j.yfrne.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Leadem CA, Kalra SP. Effects of endogenous opioid peptides and opiates on luteinizing hormone and prolactin secretion in ovariectomized rats. Neuroendocrinology 41: 342–352, 1985. doi: 10.1159/000124199. [DOI] [PubMed] [Google Scholar]
- 267.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology 96: 57–62, 1975. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 268.Légrádi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138: 2569–2576, 1997. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 269.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151: 3479–3489, 2010. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lehman MN, Karsch FJ, Robinson JE, Silverman A-J. Ultrastructure and synaptic organization of luteinizing hormone-releasing hormone (LHRH) neurons in the anestrous ewe. J Comp Neurol 273: 447–458, 1988. doi: 10.1002/cne.902730402. [DOI] [PubMed] [Google Scholar]
- 271.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG Jr. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med 18: 820–823, 2012. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Leshan RL, Louis GW, Jo Y-H, Rhodes CJ, Münzberg H, Myers MG Jr. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29: 3138–3147, 2009. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiol Behav 89: 486–489, 2006. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 274.Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111: 1439–1448, 1982. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- 275.Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 3: 575–579, 1997. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 276.Licinio J, Negrão AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS, McCann SM, Gold PW. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci USA 95: 2541–2546, 1998. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lightbourne M, Brown RJ. Genetics of Lipodystrophy. Endocrinol Metab Clin North Am 46: 539–554, 2017. doi: 10.1016/j.ecl.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Linna MS, Raevuori A, Haukka J, Suvisaari JM, Suokas JT, Gissler M. Reproductive health outcomes in eating disorders. Int J Eat Disord 46: 826–833, 2013. doi: 10.1002/eat.22179. [DOI] [PubMed] [Google Scholar]
- 279.Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav Neurosci 111: 1361–1367, 1997. doi: 10.1037/0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- 280.Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG Jr. Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology 152: 2302–2310, 2011. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lue TF, Brant WO, Shindel A, Bella AJ. Sexual Dysfunction in Diabetes. In: Endotext, edited by De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A. South Dartmouth, MA: MDText.com, 2000. [Google Scholar]
- 282.Lungu AO, Zadeh ES, Goodling A, Cochran E, Gorden P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J Clin Endocrinol Metab 97: 563–567, 2012. doi: 10.1210/jc.2011-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, Asa SL, Drucker DJ. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141: 752–762, 2000. doi: 10.1210/endo.141.2.7326. [DOI] [PubMed] [Google Scholar]
- 284.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161, 1995. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 285.Magiakou MA, Mastorakos G, Webster E, Chrousos GP. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Ann N Y Acad Sci 816: 42–56, 1997. doi: 10.1111/j.1749-6632.1997.tb52128.x. [DOI] [PubMed] [Google Scholar]
- 286.Maguire M, Lungu A, Gorden P, Cochran E, Stratton P. Pregnancy in a woman with congenital generalized lipodystrophy: leptin’s vital role in reproduction. Obstet Gynecol 119: 452–455, 2012. doi: 10.1097/AOG.0b013e31822cecf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mahany EB, Han X, Borges BC, da Silveira Cruz-Machado S, Allen SJ, Garcia-Galiano D, Hoenerhoff MJ, Bellefontaine NH, Elias CF. Obesity and high fat diet induce distinct changes in placental gene expression and pregnancy outcome. Endocrinology 159: 1718–1733, 2018. doi: 10.1210/en.2017-03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mahesh VB, Brann DW. Regulatory role of excitatory amino acids in reproduction. Endocrine 28: 271–280, 2005. doi: 10.1385/ENDO:28:3:271. [DOI] [PubMed] [Google Scholar]
- 289.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci 8: 1660–1662, 2005. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 290.Manfredi-Lozano M, Roa J, Ruiz-Pino F, Piet R, Garcia-Galiano D, Pineda R, Zamora A, Leon S, Sanchez-Garrido MA, Romero-Ruiz A, Dieguez C, Vazquez MJ, Herbison AE, Pinilla L, Tena-Sempere M. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol Metab 5: 844–857, 2016. doi: 10.1016/j.molmet.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mani SK, Allen JM, Rettori V, McCann SM, O’Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci USA 91: 6468–6472, 1994. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Manning JM, Bronson FH. Effects of prolonged exercise on puberty and luteinizing hormone secretion in female rats. Am J Physiol Regul Integr Comp Physiol 257: R1359–R1364, 1989. [DOI] [PubMed] [Google Scholar]
- 293.Marks JL, Porte D Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127: 3234–3236, 1990. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 294.Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP. Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res 47: 155–187, 1991. [DOI] [PubMed] [Google Scholar]
- 295.Marson L, Platt KB, McKenna KE. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience 55: 263–280, 1993. doi: 10.1016/0306-4522(93)90471-Q. [DOI] [PubMed] [Google Scholar]
- 296.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 5: 332–353, 2006. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Martin C, Navarro VM, Simavli S, Vong L, Carroll RS, Lowell BB, Kaiser UB. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J Neurosci 34: 6047–6056, 2014. doi: 10.1523/JNEUROSCI.3003-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Matsui H, Musicki B, Sopko NA, Liu X, Hurley PJ, Burnett AL, Bivalacqua TJ, Hannan JL. Early-stage Type 2 Diabetes Mellitus Impairs Erectile Function and Neurite Outgrowth From the Major Pelvic Ganglion and Downregulates the Gene Expression of Neurotrophic Factors. Urology 99: 287.e1–287.e7, 2017. doi: 10.1016/j.urology.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107: 22693–22698, 2010. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McCarthy EA, Kunkhyen T, Korzan WJ, Naik A, Maqsudlu A, Cherry JA, Baum MJ. A comparison of the effects of male pheromone priming and optogenetic inhibition of accessory olfactory bulb forebrain inputs on the sexual behavior of estrous female mice. Horm Behav 89: 104–112, 2017. doi: 10.1016/j.yhbeh.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.McDonald JK, Lumpkin MD, DePaolo LV. Neuropeptide-Y suppresses pulsatile secretion of luteinizing hormone in ovariectomized rats: possible site of action. Endocrinology 125: 186–191, 1989. doi: 10.1210/endo-125-1-186. [DOI] [PubMed] [Google Scholar]
- 302.McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol Reprod 54: 70–75, 1996. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- 303.Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and yawning: site of action in the brain. Brain Res 398: 259–265, 1986. doi: 10.1016/0006-8993(86)91485-X. [DOI] [PubMed] [Google Scholar]
- 304.Melis MR, Spano MS, Succu S, Argiolas A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci Lett 265: 171–174, 1999. doi: 10.1016/S0304-3940(99)00236-0. [DOI] [PubMed] [Google Scholar]
- 305.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH by genetically targeted tumorigenesis. Neuron 5: 1–10, 1990. doi: 10.1016/0896-6273(90)90028-E. [DOI] [PubMed] [Google Scholar]
- 306.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Brain Res Rev 57: 470–480, 2008. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism 62: 457–478, 2013. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 308.Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol 13: 728–736, 2001. doi: 10.1046/j.1365-2826.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- 309.Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, Grinspoon SK. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab 83: 2309–2312, 1998. [DOI] [PubMed] [Google Scholar]
- 310.Minabe S, Deura C, Ikegami K, Goto T, Sanbo M, Hirabayashi M, Inoue N, Uenoyama Y, Maeda K, Tsukamura H. Pharmacological and Morphological Evidence of AMPK-Mediated Energy Sensing in the Lower Brain Stem Ependymocytes to Control Reproduction in Female Rodents. Endocrinology 156: 2278–2287, 2015. doi: 10.1210/en.2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130: 503–510, 1992. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- 312.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol 21: 327–333, 2009. doi: 10.1111/j.1365-2826.2009.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mohr MA, Leathley E, Fraley GS. Hypothalamic galanin-like peptide rescues the onset of puberty in food-restricted weanling rats. J Neuroendocrinol 24: 1412–1422, 2012. doi: 10.1111/j.1365-2826.2012.02351.x. [DOI] [PubMed] [Google Scholar]
- 314.Morrison CD, Brannigan RE. Metabolic syndrome and infertility in men. Best Pract Res Clin Obstet Gynaecol 29: 507–515, 2015. doi: 10.1016/j.bpobgyn.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 315.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab 293: E165–E171, 2007. doi: 10.1152/ajpendo.00675.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev 59: 266–272, 2013. doi: 10.1262/jrd.2012-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 138: 1190–1193, 1997. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 318.Muccioli G, Lorenzi T, Lorenzi M, Ghè C, Arnoletti E, Raso GM, Castellucci M, Gualillo O, Meli R. Beyond the metabolic role of ghrelin: a new player in the regulation of reproductive function. Peptides 32: 2514–2521, 2011. doi: 10.1016/j.peptides.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 319.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LHT, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab 4: 437–460, 2015. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Murahashi K, Bucholtz DC, Nagatani S, Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology 137: 1171–1176, 1996. doi: 10.1210/endo.137.4.8625886. [DOI] [PubMed] [Google Scholar]
- 321.Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett 612: 161–166, 2016. doi: 10.1016/j.neulet.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 322.Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett 264: 113–116, 1999. doi: 10.1016/S0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- 323.Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54: 255–263, 2005. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 324.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology 67: 370–376, 1998. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 325.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22: 818–835, 2001. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 326.Nakahara T, Uenoyama Y, Iwase A, Oishi S, Nakamura S, Minabe S, Watanabe Y, Deura C, Noguchi T, Fujii N, Kikkawa F, Maeda K, Tsukamura H. Chronic peripheral administration of kappa-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev 59: 479–484, 2013. doi: 10.1262/jrd.2013-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Nandi A, Wang X, Accili D, Wolgemuth DJ. The effect of insulin signaling on female reproductive function independent of adiposity and hyperglycemia. Endocrinology 151: 1863–1871, 2010. doi: 10.1210/en.2009-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Navarro VM, Fernández-Fernández R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561: 379–386, 2004. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29: 11859–11866, 2009. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Neal-Perry G, Yao D, Shu J, Sun Y, Etgen AM. Insulin-like growth factor-I regulates LH release by modulation of kisspeptin and NMDA-mediated neurotransmission in young and middle-aged female rats. Endocrinology 155: 1827–1837, 2014. doi: 10.1210/en.2013-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Newell LE, Heddle JA. The effect of dietary restriction during development in utero on the frequency of spontaneous somatic mutations. Mutagenesis 17: 289–292, 2002. doi: 10.1093/mutage/17.4.289. [DOI] [PubMed] [Google Scholar]
- 332.Nillni EA. Regulation of prohormone convertases in hypothalamic neurons: implications for prothyrotropin-releasing hormone and proopiomelanocortin. Endocrinology 148: 4191–4200, 2007. doi: 10.1210/en.2007-0173. [DOI] [PubMed] [Google Scholar]
- 333.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143: 2284–2292, 2002. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]
- 334.O’Kusky JR, Ye P, D’Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci 20: 8435–8442, 2000. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 30: 713–743, 2009. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 337.Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology 145: 3239–3246, 2004. doi: 10.1210/en.2003-1516. [DOI] [PubMed] [Google Scholar]
- 338.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent A-S, Matagne V, Mungenast AE. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 147: 1166–1174, 2006. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 339.Ollila ME, West S, Keinänen-Kiukaanniemi S, Jokelainen J, Auvinen J, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, Franks S, Piltonen TT, Morin-Papunen LC. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod 32: 423–431, 2017. doi: 10.1093/humrep/dew329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, Wells JC, Dunger DB. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab 94: 1527–1532, 2009. doi: 10.1210/jc.2008-2489. [DOI] [PubMed] [Google Scholar]
- 341.Ordög T, Chen MD, Nishihara M, Connaughton MA, Goldsmith JR, Knobil E. On the role of gonadotropin-releasing hormone (GnRH) in the operation of the GnRH pulse generator in the rhesus monkey. Neuroendocrinology 65: 307–313, 1997. doi: 10.1159/000127189. [DOI] [PubMed] [Google Scholar]
- 342.Outeiriño-Iglesias V, Romaní-Pérez M, González-Matías LC, Vigo E, Mallo F. GLP-1 Increases Preovulatory LH Source and the Number of Mature Follicles, As Well As Synchronizing the Onset of Puberty in Female Rats. Endocrinology 156: 4226–4237, 2015. doi: 10.1210/en.2014-1978. [DOI] [PubMed] [Google Scholar]
- 343.Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med 19: 1153–1156, 2013. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Owiredu WK, Amidu N, Alidu H, Sarpong C, Gyasi-Sarpong CK. Determinants of sexual dysfunction among clinically diagnosed diabetic patients. Reprod Biol Endocrinol 9: 70, 2011. doi: 10.1186/1477-7827-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Rønnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci USA 114: 2413–2418, 2017. [Correction in Proc Natl Acad Sci USA 114: E3584, 2017.] 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V. Fatty acids in energy metabolism of the central nervous system. BioMed Res Int 2014: 472459, 2014. doi: 10.1155/2014/472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Parkash J, d’Anglemont de Tassigny X, Bellefontaine N, Campagne C, Mazure D, Buée-Scherrer V, Prevot V. Phosphorylation of N-methyl-d-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle. Endocrinology 151: 2723–2735, 2010. doi: 10.1210/en.2010-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pellitero S, Olaizola I, Alastrue A, Martínez E, Granada ML, Balibrea JM, Moreno P, Serra A, Navarro-Díaz M, Romero R, Puig-Domingo M. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg 22: 1835–1842, 2012. doi: 10.1007/s11695-012-0734-9. [DOI] [PubMed] [Google Scholar]
- 349.Persson M, Cnattingius S, Villamor E, Söderling J, Pasternak B, Stephansson O, Neovius M. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ 357: j2563, 2017. doi: 10.1136/bmj.j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Petridou E, Syrigou E, Toupadaki N, Zavitsanos X, Willett W, Trichopoulos D. Determinants of age at menarche as early life predictors of breast cancer risk. Int J Cancer 68: 193–198, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 351.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol 288: 203–210, 1979. [PMC free article] [PubMed] [Google Scholar]
- 352.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol 288: 189–202, 1979. [PMC free article] [PubMed] [Google Scholar]
- 353.Pfaus JG, Everitt BJ. The psychopharmacology of sexual behavior. Psychopharmacology 743–758, 1995. [Google Scholar]
- 354.Pfaus JG, Jones SL, Flanagan-Cato LM, Blaustein JD. Female Sexual Behavior. In: Knobil and Neill’s Physiology of Reproduction (4th ed.). San Diego: Academic, 2015, chapt 50, p. 2287–2370. doi: 10.1016/B978-0-12-397175-3.00050-8. [DOI] [Google Scholar]
- 355.Phumsatitpong C, Moenter SM. Estradiol-Dependent Stimulation and Suppression of Gonadotropin-Releasing Hormone Neuron Firing Activity by Corticotropin-Releasing Hormone in Female Mice. Endocrinology 159: 414–425, 2018. doi: 10.1210/en.2017-00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase GnRH neuron activity and its effects are modulated by estradiol. Endocrinology 149: 1979–1986, 2008. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J Neurosci 33: 10828–10839, 2013. doi: 10.1523/JNEUROSCI.1021-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pinheiro AP, Raney TJ, Thornton LM, Fichter MM, Berrettini WH, Goldman D, Halmi KA, Kaplan AS, Strober M, Treasure J, Woodside DB, Kaye WH, Bulik CM. Sexual functioning in women with eating disorders. Int J Eat Disord 43: 123–129, 2010. doi: 10.1002/eat.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev 92: 1235–1316, 2012. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 360.Piva F, Limonta P, Maggi R, Martini L. Stimulatory and inhibitory effects of the opioids on gonadotropin secretion. Neuroendocrinology 42: 504–512, 1986. doi: 10.1159/000124495. [DOI] [PubMed] [Google Scholar]
- 361.Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol 38: 73–88, 2015. doi: 10.1016/j.yfrne.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology 116: 1341–1350, 1985. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 363.Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update 10: 67–77, 2004. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- 364.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16: 59–65, 2005. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 365.Pohl CR, deRidder CM, Plant TM. Gonadal and nongonadal mechanisms contribute to the prepubertal hiatus in gonadotropin secretion in the female rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab 80: 2094–2101, 1995. [DOI] [PubMed] [Google Scholar]
- 366.Pontikis C, Yavropoulou MP, Toulis KA, Kotsa K, Kazakos K, Papazisi A, Gotzamani-Psarakou A, Yovos JG. The incretin effect and secretion in obese and lean women with polycystic ovary syndrome: a pilot study. J Womens Health (Larchmt) 20: 971–976, 2011. doi: 10.1089/jwh.2010.2272. [DOI] [PubMed] [Google Scholar]
- 367.Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain JC. Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 84: 177–191, 1998. doi: 10.1016/S0306-4522(97)00537-X. [DOI] [PubMed] [Google Scholar]
- 368.Prevot V, Hanchate NK, Bellefontaine N, Sharif A, Parkash J, Estrella C, Allet C, de Seranno S, Campagne C, d'Anglemont de Tassigny X, Baroncini M. Function-related structural plasticity of the GnRH system: a role for neuronal-glial-endothelial interactions. Front Neuroendocrinol 31: 241–258, 2010. doi: 10.1016/j.yfrne.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 369.Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 17: 17–33, 2011. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Brüning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology 154: 1337–1348, 2013. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150: 2805–2812, 2009. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Racusin D, Stevens B, Campbell G, Aagaard KM. Obesity and the risk and detection of fetal malformations. Semin Perinatol 36: 213–221, 2012. doi: 10.1053/j.semperi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151: 4494–4503, 2010. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94: 237–245, 2011. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Rettori V, Canteros G, Renoso R, Gimeno M, McCann SM. Oxytocin stimulates the release of luteinizing hormone-releasing hormone from medial basal hypothalamic explants by releasing nitric oxide. Proc Natl Acad Sci USA 94: 2741–2744, 1997. doi: 10.1073/pnas.94.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Reynoso R, Cardoso N, Szwarcfarb B, Carbone S, Ponzo O, Moguilevsky JA, Scacchi P. Nitric oxide synthase inhibition prevents leptin induced Gn-RH release in prepubertal and peripubertal female rats. Exp Clin Endocrinol Diabetes 115: 423–427, 2007. doi: 10.1055/s-2007-970161. [DOI] [PubMed] [Google Scholar]
- 377.Rizwan MZ, Harbid AA, Inglis MA, Quennell JH, Anderson GM. Evidence that hypothalamic RFamide related peptide-3 neurones are not leptin-responsive in mice and rats. J Neuroendocrinol 26: 247–257, 2014. doi: 10.1111/jne.12140. [DOI] [PubMed] [Google Scholar]
- 378.Roa J, García-Galiano D, Castellano JM, Gaytan F, Pinilla L, Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Mol Cell Endocrinol 324: 87–94, 2010. doi: 10.1016/j.mce.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 379.Roa J, Garcia-Galiano D, Varela L, Sánchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, López M, Gaytan F, Diéguez C, Pinilla L, Tena-Sempere M. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150: 5016–5026, 2009. doi: 10.1210/en.2009-0096. [DOI] [PubMed] [Google Scholar]
- 380.Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 153: 5587–5599, 2012. doi: 10.1210/en.2012-1470. [DOI] [PubMed] [Google Scholar]
- 381.Roa J, Tena-Sempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab 21: 519–528, 2010. doi: 10.1016/j.tem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 382.Rodríguez M, Arias P, Refojo D, Feleder C, Moguilevsky J. Arrest of pulsatile luteinizing hormone (LH) secretion during insulin-induced hypoglycemia (IIH): improvement by intrahypothalamic perfusion with glucose. Exp Clin Endocrinol Diabetes 107: 257–261, 1999. doi: 10.1055/s-0029-1212109. [DOI] [PubMed] [Google Scholar]
- 383.Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol 25: 847–858, 2011. doi: 10.1210/me.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology 152: 618–628, 2011. doi: 10.1210/en.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Roland AV, Moenter SM. Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: insight from animal models. Front Neuroendocrinol 35: 494–511, 2014. doi: 10.1016/j.yfrne.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience 125: 735–748, 2004. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 387.Ronnekleiv OK, Resko JA. Ontogeny of gonadotropin-releasing hormone-containing neurons in early fetal development of rhesus macaques. Endocrinology 126: 498–511, 1990. doi: 10.1210/endo-126-1-498. [DOI] [PubMed] [Google Scholar]
- 388.Roselli CE, Cross E, Poonyagariyagorn HK, Stadelman HL. Role of aromatization in anticipatory and consummatory aspects of sexual behavior in male rats. Horm Behav 44: 146–151, 2003. doi: 10.1016/S0018-506X(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 389.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49: 822–830, 1997. doi: 10.1016/S0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 390.Rowe FA, Edwards DA. Olfactory bulb removal: influences on the mating behavior of male mice. Physiol Behav 8: 37–41, 1972. doi: 10.1016/0031-9384(72)90127-8. [DOI] [PubMed] [Google Scholar]
- 391.Safarinejad MR, Hosseini SY. Salvage of sildenafil failures with bremelanotide: a randomized, double-blind, placebo controlled study. J Urol 179: 1066–1071, 2008. doi: 10.1016/j.juro.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 392.Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev 16: 1077–1088, 2002. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sakai K, Clemmons DR. Glucosamine induces resistance to insulin-like growth factor I (IGF-I) and insulin in Hep G2 cell cultures: biological significance of IGF-I/insulin hybrid receptors. Endocrinology 144: 2388–2395, 2003. doi: 10.1210/en.2002-221133. [DOI] [PubMed] [Google Scholar]
- 394.Salihu HM. Maternal obesity and stillbirth. Semin Perinatol 35: 340–344, 2011. doi: 10.1053/j.semperi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 395.Samson WK, Alexander BD, Skala KD, Huang FL, Fulton RJ. Ricin-cytotoxin conjugate administration reveals a physiologically relevant role for oxytocin in the control of gonadotropin secretion. Ann N Y Acad Sci 652: 411–422, 1992. doi: 10.1111/j.1749-6632.1992.tb34371.x. [DOI] [PubMed] [Google Scholar]
- 396.Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161: 31–55, 1975. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- 397.Schiöth HB, Watanobe H. Melanocortins and reproduction. Brain Res Brain Res Rev 38: 340–350, 2002. doi: 10.1016/S0165-0173(01)00159-X. [DOI] [PubMed] [Google Scholar]
- 398.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 103: 253–262, 2000. doi: 10.1016/S0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 399.Schneider JE. Energy balance and reproduction. Physiol Behav 81: 289–317, 2004. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 400.Schneider JE, Casper JF, Barisich A, Schoengold C, Cherry S, Surico J, DeBarba A, Fabris F, Rabold E. Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Horm Behav 51: 413–427, 2007. doi: 10.1016/j.yhbeh.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 401.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 244: 1326–1328, 1989. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 402.Schneider JE, Wade GN. Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am J Physiol Regul Integr Comp Physiol 258: R750–R755, 1990. [DOI] [PubMed] [Google Scholar]
- 403.Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 33: 1493–1499, 2013. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature 338: 161–164, 1989. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 405.Scimonelli T, Celis ME. A central action of alpha-melanocyte-stimulating hormone on serum levels of LH and prolactin in rats. J Endocrinol 124: 127–132, 1990. doi: 10.1677/joe.0.1240127. [DOI] [PubMed] [Google Scholar]
- 406.Scimonelli T, Medina F, Wilson C, Celis ME. Interaction of alpha-melanotropin (alpha-MSH) and noradrenaline in the median eminence in the control of female sexual behavior. Peptides 21: 219–223, 2000. doi: 10.1016/S0196-9781(99)00191-6. [DOI] [PubMed] [Google Scholar]
- 407.Seeley RJ, York DA. Fuel sensing and the central nervous system (CNS): implications for the regulation of energy balance and the treatment for obesity. Obes Rev 6: 259–265, 2005. doi: 10.1111/j.1467-789X.2005.00193.x. [DOI] [PubMed] [Google Scholar]
- 408.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627, 2003. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 409.Semple E, Hill JW. Sim1 Neurons Are Sufficient for MC4R-Mediated Sexual Function in Male Mice. Endocrinology 159: 439–449, 2018. doi: 10.1210/en.2017-00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Seth A, Stanley S, Jethwa P, Gardiner J, Ghatei M, Bloom S. Galanin-like peptide stimulates the release of gonadotropin-releasing hormone in vitro and may mediate the effects of leptin on the hypothalamo-pituitary-gonadal axis. Endocrinology 145: 743–750, 2004. doi: 10.1210/en.2003-0873. [DOI] [PubMed] [Google Scholar]
- 411.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102: 2129–2134, 2005. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC Jr. Agouti-related peptide plays a critical role in leptin’s effects on female puberty and reproduction. Am J Physiol Endocrinol Metab 305: E1512–E1520, 2013. doi: 10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396: 670–674, 1998. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 414.Shivers BD, Harlan RE, Hejtmancik JF, Conn PM, Pfaff DW. Localization of cells containing LHRH-like mRNA in rat forebrain using in situ hybridization. Endocrinology 118: 883–885, 1986. doi: 10.1210/endo-118-2-883. [DOI] [PubMed] [Google Scholar]
- 415.Siemensma EPC, van Alfen-van der Velden AAEM, Otten BJ, Laven JSE, Hokken-Koelega ACS. Ovarian function and reproductive hormone levels in girls with Prader-Willi syndrome: a longitudinal study. J Clin Endocrinol Metab 97: E1766–E1773, 2012. doi: 10.1210/jc.2012-1595. [DOI] [PubMed] [Google Scholar]
- 416.Silverman AJ, Antunes JL, Abrams GM, Nilaver G, Thau R, Robinson JA, Ferin M, Krey LC. The luteinizing hormone-releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol 211: 309–317, 1982. doi: 10.1002/cne.902110309. [DOI] [PubMed] [Google Scholar]
- 417.Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294: 76–95, 1990. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 418.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol 270: 209–242, 1988. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 419.Singhal G, Douris N, Fish AJ, Zhang X, Adams AC, Flier JS, Pissios P, Maratos-Flier E. Fibroblast growth factor 21 has no direct role in regulating fertility in female mice. Mol Metab 5: 690–698, 2016. doi: 10.1016/j.molmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci 7: 1040–1047, 2004. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 421.Skeldon SC, Detsky AS, Goldenberg SL, Law MR. Erectile Dysfunction and Undiagnosed Diabetes, Hypertension, and Hypercholesterolemia. Ann Fam Med 13: 331–335, 2015. doi: 10.1370/afm.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Skrapits K, Kanti V, Savanyú Z, Maurnyi C, Szenci O, Horváth A, Borsay BÁ, Herczeg L, Liposits Z, Hrabovszky E. Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front Cell Neurosci 9: 348, 2015. doi: 10.3389/fncel.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Smink FRE, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 14: 406–414, 2012. doi: 10.1007/s11920-012-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Brain Res Rev 57: 288–298, 2008. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 425.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146: 3686–3692, 2005. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 426.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146: 2976–2984, 2005. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 427.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26: 6687–6694, 2006. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Södersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to sexual receptivity in intact and ovariectomized rats. J Endocrinol 89: 45–54, 1981. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- 429.Speakman JR. The physiological costs of reproduction in small mammals. Philos Trans R Soc Lond B Biol Sci 363: 375–398, 2008. doi: 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Stark R, Ashley SE, Andrews ZB. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol 366: 215–223, 2013. doi: 10.1016/j.mce.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 431.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab 23: 770–784, 2016. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JWM. PCSK1 Mutations and Human Endocrinopathies: From Obesity to Gastrointestinal Disorders. Endocr Rev 37: 347–371, 2016. doi: 10.1210/er.2015-1117. [DOI] [PubMed] [Google Scholar]
- 433.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23: 7973–7981, 2003. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology 152: 4276–4287, 2011. doi: 10.1210/en.2011-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Svendsen PF, Nilas L, Madsbad S, Holst JJ. Incretin hormone secretion in women with polycystic ovary syndrome: roles of obesity, insulin sensitivity, and treatment with metformin. Metabolism 58: 586–593, 2009. doi: 10.1016/j.metabol.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 436.Swain JE, Dunn RL, McConnell D, Gonzalez-Martinez J, Smith GD. Direct effects of leptin on mouse reproductive function: regulation of follicular, oocyte, and embryo development. Biol Reprod 71: 1446–1452, 2004. doi: 10.1095/biolreprod.104.033035. [DOI] [PubMed] [Google Scholar]
- 437.Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology 98: 1359–1364, 1976. doi: 10.1210/endo-98-6-1359. [DOI] [PubMed] [Google Scholar]
- 438.Tanuri FD, Thomaz RB, Tanuri JA. [Temporal lobe epilepsy with aura of pleasure. Case report]. Arq Neuropsiquiatr 58: 178–180, 2000. doi: 10.1590/S0004-282X2000000100028. [DOI] [PubMed] [Google Scholar]
- 439.Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm 77: 285–300, 2007. doi: 10.1016/S0083-6729(06)77012-1. [DOI] [PubMed] [Google Scholar]
- 440.Tena-Sempere M. The roles of kisspeptins and G protein-coupled receptor-54 in pubertal development. Curr Opin Pediatr 18: 442–447, 2006. doi: 10.1097/01.mop.0000236396.79580.cc. [DOI] [PubMed] [Google Scholar]
- 441.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22: 111–151, 2001. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 442.Terasawa E, Kurian JR, Keen KL, Shiel NA, Colman RJ, Capuano SV. Body weight impact on puberty: effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology 153: 1696–1705, 2012. doi: 10.1210/en.2011-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19: 5898–5909, 1999. doi: 10.1523/JNEUROSCI.19-14-05898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151: 4109–4115, 2010. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138: 5063–5066, 1997. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 446.Tobet SA, Sower SA, Schwarting GA. Gonadotropin-releasing hormone containing neurons and olfactory fibers during development: from lamprey to mammals. Brain Res Bull 44: 479–486, 1997. doi: 10.1016/S0361-9230(97)00229-3. [DOI] [PubMed] [Google Scholar]
- 447.Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM. Central insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats. Biol Reprod 77: 492–503, 2007. doi: 10.1095/biolreprod.107.060434. [DOI] [PubMed] [Google Scholar]
- 448.Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology 151: 1356–1366, 2010. doi: 10.1210/en.2009-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41: 354–358, 2009. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 366: 629–635, 2012. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 451.Torsoni MA, Borges BC, Cote JL, Allen SJ, Mahany E, Garcia-Galiano D, Elias CF. AMPKα2 in Kiss1 Neurons Is Required for Reproductive Adaptations to Acute Metabolic Challenges in Adult Female Mice. Endocrinology 157: 4803–4816, 2016. doi: 10.1210/en.2016-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Tran DQ, Ramos EH, Belsham DD. Induction of Gnrh mRNA expression by the ω-3 polyunsaturated fatty acid docosahexaenoic acid and the saturated fatty acid palmitate in a GnRH-synthesizing neuronal cell model, mHypoA-GnRH/GFP. Mol Cell Endocrinol 426: 125–135, 2016. doi: 10.1016/j.mce.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 453.True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol 23: 1099–1112, 2011. doi: 10.1111/j.1365-2826.2011.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology 154: 2821–2832, 2013. doi: 10.1210/en.2013-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Tsoukas MA, Farr OM, Mantzoros CS. Leptin in congenital and HIV-associated lipodystrophy. Metabolism 64: 47–59, 2015. doi: 10.1016/j.metabol.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 456.Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. J Neuroendocrinol 11: 101–105, 1999. doi: 10.1046/j.1365-2826.1999.00312.x. [DOI] [PubMed] [Google Scholar]
- 457.Ückert S, Bannowsky A, Albrecht K, Kuczyk MA. Melanocortin receptor agonists in the treatment of male and female sexual dysfunctions: results from basic research and clinical studies. Expert Opin Investig Drugs 23: 1477–1483, 2014. doi: 10.1517/13543784.2014.934805. [DOI] [PubMed] [Google Scholar]
- 458.Urbanski HF, Ojeda SR. A role for N-methyl-d-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology 126: 1774–1776, 1990. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- 459.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20: 113–114, 1998. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 460.Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab 26: 185–197.e3, 2017. [Correction in Cell Metab 27: 1356, 2018.] 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Vale B, Brito S, Paulos L, Moleiro P. Menstruation disorders in adolescents with eating disorders-target body mass index percentiles for their resolution. Einstein (Sao Paulo) 12: 175–180, 2014. doi: 10.1590/S1679-45082014AO2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG Jr, Schwartz GJ, Chua SC Jr. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149: 1773–1785, 2008. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell 161: 119–132, 2015. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 464.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci USA 99: 11381–11386, 2002. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Vantyghem MC, Vincent-Desplanques D, Defrance-Faivre F, Capeau J, Fermon C, Valat AS, Lascols O, Hecart AC, Pigny P, Delemer B, Vigouroux C, Wemeau JL. Fertility and obstetrical complications in women with LMNA-related familial partial lipodystrophy. J Clin Endocrinol Metab 93: 2223–2229, 2008. doi: 10.1210/jc.2007-2521. [DOI] [PubMed] [Google Scholar]
- 466.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13: 1079–1086, 2012. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Vergoni AV, Bertolini A, Mutulis F, Wikberg JES, Schiöth HB. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioral effects in rats. Eur J Pharmacol 362: 95–101, 1998. doi: 10.1016/S0014-2999(98)00753-5. [DOI] [PubMed] [Google Scholar]
- 468.Vrang N, Grove K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta). Brain Res 1397: 28–37, 2011. doi: 10.1016/j.brainres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 469.Vrbikova J, Hill M, Bendlova B, Grimmichova T, Dvorakova K, Vondra K, Pacini G. Incretin levels in polycystic ovary syndrome. Eur J Endocrinol 159: 121–127, 2008. doi: 10.1530/EJE-08-0097. [DOI] [PubMed] [Google Scholar]
- 470.Wade GN, Lempicki RL, Panicker AK, Frisbee RM, Blaustein JD. Leptin facilitates and inhibits sexual behavior in female hamsters. Am J Physiol Regul Integr Comp Physiol 272: R1354–R1358, 1997. [DOI] [PubMed] [Google Scholar]
- 471.Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus monkeys. Horm Behav 18: 431–450, 1984. doi: 10.1016/0018-506X(84)90028-X. [DOI] [PubMed] [Google Scholar]
- 472.Walvoord EC. The timing of puberty: is it changing? Does it matter? J Adolesc Health 47: 433–439, 2010. doi: 10.1016/j.jadohealth.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 473.Wang L, DeFazio RA, Moenter SM. Excitability and Burst Generation of AVPV Kisspeptin Neurons Are Regulated by the Estrous Cycle Via Multiple Conductances Modulated by Estradiol Action. eNeuro 3: ENEURO.0094–0016.2016, 2016. doi: 10.1523/ENEURO.0094-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 475.Wang R, Mol BW. The Rotterdam criteria for polycystic ovary syndrome: evidence-based criteria? Hum Reprod 32: 261–264, 2017. doi: 10.1093/humrep/dew287. [DOI] [PubMed] [Google Scholar]
- 476.Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab 84: 873–877, 1999. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- 477.Watanobe H, Schiöth HB. Nitric oxide mediates leptin-induced preovulatory luteinizing hormone and prolactin surges in rats. Brain Res 923: 193–197, 2001. doi: 10.1016/S0006-8993(01)03247-4. [DOI] [PubMed] [Google Scholar]
- 478.Watanobe H, Suda T, Wikberg JE, Schiöth HB. Evidence that physiological levels of circulating leptin exert a stimulatory effect on luteinizing hormone and prolactin surges in rats. Biochem Biophys Res Commun 263: 162–165, 1999. doi: 10.1006/bbrc.1999.1331. [DOI] [PubMed] [Google Scholar]
- 479.Weidenfeld J, Corcos AP, Wohlman A, Feldman S. Characterization of the 2-deoxyglucose effect on the adrenocortical axis. Endocrinology 134: 1924–1931, 1994. doi: 10.1210/endo.134.4.8137760. [DOI] [PubMed] [Google Scholar]
- 480.Wellons MF, Matthews JJ, Kim C. Ovarian aging in women with diabetes: an overview. Maturitas 96: 109–113, 2017. doi: 10.1016/j.maturitas.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997, 2004. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 482.Wen S, Götze IN, Mai O, Schauer C, Leinders-Zufall T, Boehm U. Genetic identification of GnRH receptor neurons: a new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology 152: 1515–1526, 2011. doi: 10.1210/en.2010-1208. [DOI] [PubMed] [Google Scholar]
- 483.Wentz E, Gillberg IC, Anckarsäter H, Gillberg C, Råstam M. Reproduction and offspring status 18 years after teenage-onset anorexia nervosa–a controlled community-based study. Int J Eat Disord 42: 483–491, 2009. doi: 10.1002/eat.20664. [DOI] [PubMed] [Google Scholar]
- 484.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121: 1562–1570, 1987. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 485.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology 56: 641–646, 2000. doi: 10.1016/S0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 486.Wetsel WC, Valença MM, Merchenthaler I, Liposits Z, López FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci USA 89: 4149–4153, 1992. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109: 376–385, 1981. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 488.Wilkinson M, Moger WH. Transpubertal modulation of pituitary and testicular function in the ob/ob mouse. Horm Res 14: 95–103, 1981. doi: 10.1159/000179366. [DOI] [PubMed] [Google Scholar]
- 489.Williams KW, Scott MM, Elmquist JK. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. Eur J Pharmacol 660: 2–12, 2011. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Williams KW, Sohn JW, Donato J Jr, Lee CE, Zhao JJ, Elmquist JK, Elias CF. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci 31: 13147–13156, 2011. doi: 10.1523/JNEUROSCI.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Williamson-Hughes PS, Grove KL, Smith MS. Melanin concentrating hormone (MCH): a novel neural pathway for regulation of GnRH neurons. Brain Res 1041: 117–124, 2005. doi: 10.1016/j.brainres.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 492.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne H-J, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52: 271–280, 2006. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Wójcik-Gładysz A, Wańkowska M, Misztal T, Romanowicz K, Polkowska J. Effect of intracerebroventricular infusion of leptin on the secretory activity of the GnRH/LH axis in fasted prepubertal lambs. Anim Reprod Sci 114: 370–383, 2009. doi: 10.1016/j.anireprosci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 494.Woller MJ, Campbell GT, Liu L, Steigerwalt RW, Blake CA. Estrogen alters the effects of neuropeptide-Y on luteinizing hormone and follicle-stimulating hormone release in female rats at the level of the anterior pituitary gland. Endocrinology 133: 2675–2681, 1993. doi: 10.1210/endo.133.6.8243291. [DOI] [PubMed] [Google Scholar]
- 495.Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience 78: 1027–1035, 1997. doi: 10.1016/S0306-4522(96)00629-X. [DOI] [PubMed] [Google Scholar]
- 496.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 497.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition 16: 894–902, 2000. doi: 10.1016/S0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]
- 498.Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 499.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86: 8132–8136, 1989. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci USA 106: 17217–17222, 2009. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483: 594–597, 2012. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA 109: 3155–3160, 2012. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, Wolfe A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 63: 1270–1282, 2014. doi: 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Xu C, Messina A, Somm E, Miraoui H, Kinnunen T, Acierno J Jr, Niederländer NJ, Bouilly J, Dwyer AA, Sidis Y, Cassatella D, Sykiotis GP, Quinton R, De Geyter C, Dirlewanger M, Schwitzgebel V, Cole TR, Toogood AA, Kirk JMW, Plummer L, Albrecht U, Crowley WF Jr, Mohammadi M, Tena-Sempere M, Prevot V, Pitteloud N. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol Med 9: 1379–1397, 2017. doi: 10.15252/emmm.201607376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Xu M, Hill JW, Levine JE. Attenuation of luteinizing hormone surges in neuropeptide Y knockout mice. Neuroendocrinology 72: 263–271, 2000. doi: 10.1159/000054595. [DOI] [PubMed] [Google Scholar]
- 506.Yoshida K, Tobet SA, Crandall JE, Jimenez TP, Schwarting GA. The migration of luteinizing hormone-releasing hormone neurons in the developing rat is associated with a transient, caudal projection of the vomeronasal nerve. J Neurosci 15: 7769–7777, 1995. doi: 10.1523/JNEUROSCI.15-12-07769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Young KA, Zirkin BR, Nelson RJ. Testicular regression in response to food restriction and short photoperiod in white-footed mice (Peromyscus leucopus) is mediated by apoptosis. Biol Reprod 62: 347–354, 2000. doi: 10.1095/biolreprod62.2.347. [DOI] [PubMed] [Google Scholar]
- 508.Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function [published erratum appears in Proc Natl Acad Sci U S A 1997 Sep 30;94(20):11108]. Proc Natl Acad Sci USA 94: 1023–1028, 1997. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 138: 5055–5058, 1997. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 510.Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest 105: 749–755, 2000. doi: 10.1172/JCI8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zehr JL, Maestripieri D, Wallen K. Estradiol increases female sexual initiation independent of male responsiveness in rhesus monkeys. Horm Behav 33: 95–103, 1998. doi: 10.1006/hbeh.1998.1440. [DOI] [PubMed] [Google Scholar]
- 512.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27: 10153–10164, 2007. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue [published erratum appears in Nature 1995 Mar 30;374(6521):479]. Nature 372: 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 514.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J Neurosci 33: 17874–17883, 2013. doi: 10.1523/JNEUROSCI.2278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]