Abstract
Psychomotor retardation and reduced daily activities are core features of the depressive syndrome including bipolar disorder (BD). It was the aim of this study to investigate white matter microstructure of the motor system in BD during depression and its association with motor activity. We hypothesized reduced physical activity, microstructural alterations of motor tracts and different associations between activity levels and motor tract microstructure in BD. Nineteen bipolar patients with a current depressive episode (BD) and 19 healthy controls (HC) underwent diffusion weighted magnetic resonance imaging (DW-MRI)-scans. Quantitative motor activity was assessed with 24 h actigraphy recordings. Bilateral corticospinal tracts (CST), interhemispheric connections between the primary motor cortices (M1) and between the pre-supplementary motor areas (pre-SMA) were reconstructed individually based on anatomical landmarks using Diffusion Tensor Imaging (DTI) based tractography. Mean fractional anisotropy (FA) was sampled along the tracts. To enhance specificity of putative findings a segment of the optic radiation was reconstructed as comparison tract. Analyses were complemented with Tract Based Spatial Statistics (TBSS) analyses. BD had lower activity levels (AL). There was a sole increase of fractional anisotropy (FA) in BD in the left CST. Further, there was a significant group x AL interaction for FA of the left CST pointing to a selective positive association between FA and AL in BD. The comparison tract and TBSS analyses did not detect significant group differences. Our results point to white matter microstructure alterations of the left CST in BD. The positive association between motor activity and white matter microstructure suggests a compensatory role of the left CST for psychomotor retardation in BD.
Keywords: Bipolar disorder, Depression, Motor system, Diffusion tensor imaging
Highlights
-
•
Daily physical activity is reduced in bipolar patients with a current depressive episode (BD)
-
•
The left corticospinal tract (CST) in BD shows increased fractional anisotropy (FA)
-
•
Increases of FA in the left corticospinal tract in BD are related to less pronounced psychomotor retardation
1. Introduction
Psychomotor retardation is a core feature of the depressive syndrome (Leonpacher et al., 2015). It is associated with loss of interest in previously enjoyed activities, fatigue or loss of energy (Razavi et al., 2011), which are diagnostic criteria of depression according to the Diagnostic and Statistical Manual of mental disorders DSM-IV (American Psychiatric Association, 2000). Psychomotor retardation may predict conversion to bipolar disorder (BD) in people at familial risk for BD (Frankland et al., 2017) and treatment response in depression including both pharmacological treatments and electroconvulsive therapy (Bennabi et al., 2013; Buyukdura et al., 2011). It is more pronounced in BD than in unipolar disorder (UD) and may contribute to distinguish BD from UD (Leonpacher et al., 2015).
There is increasing awareness for the relevance of the motor domain in depression e.g. (Cantisani et al., 2016; Walther et al., 2012a). Regarding diagnostic criteria for BD, DSM-5 criteria go one step beyond DSM-IV criteria by strictly requiring that during manic episodes mood changes must be accompanied by persistently increased activity or energy (Angst, 2013). Further, during depressive episodes engagement in physical activity constitutes a central and effective element of antidepressive treatment (Kvam et al., 2016). Despite the diagnostic and clinical relevance of motor behavior in BD little is known on associated neurobiological alterations.
Diffusion weighted Magnetic Resonance Imaging (DW-MRI) enables the characterization of white matter microstructure of the brain by indirectly measuring the hindrance of diffusion of water molecules (Basser et al., 1994). The most commonly used diffusion based measure is the fractional anisotropy (FA) (Basser and Pierpaoli, 1996). Automated whole brain approaches compare diffusion metrics such as FA on a voxel-by-voxel level (voxel-based analyses, VBA). The most commonly whole brain approach is tract based spatial statistics (TBSS) due to its superior spatial alignment (Smith et al., 2006)– however there is a loss of information due to the thinning of the applied white matter skeleton. In contrast, tractography allows the reconstruction of entire and specific pathways taking individual anatomical variations into account (Catani et al., 2002). Tractography approaches require a priori anatomical hypothesis and compare diffusion metrics averaged across the whole tract (Jones et al., 2013).
Meta-analyses of VBA and TBSS comparing BD with HC suggest reduced FA in BD in widespread regions of the brain such as the fronto-limbic network, parietal and temporal brain regions (Bellani et al., 2016; Nortje et al., 2013; Wise et al., 2016). Findings also include regions being associated with motor planning and execution such as the right anterior superior longitudinal fasciculus, the left genu of the corpus callosum, and parts of the corpus callosum that connect the left and right somatosensory and motor cortices (Wise et al., 2016).
In particular, findings in the corpus callosum (CC) have been repeatedly reported. (Lagopoulos et al., 2013) used TBSS and identified reduced FA in the body of the CC in young patients with BD. (Li et al., 2014) used a region of interest (ROI) approach to segment distinct regions of the CC. FA was reduced amongst other in in a segment connecting interhemispheric supplementary motor areas (SMA). Similarly, a ROI approach revealed reduced FA in anterior and middle parts of the CC (Wang et al., 2008). Findings also include tractography studies being more accurate than VBA and TBSS studies in terms of tract specificity. For instance (Mahapatra et al., 2017) reported reduced FA in remitted BD in comparison to healthy controls (HC) and healthy relatives in the CC. (Toteja et al., 2015) demonstrated lower FA and age-associated increases of mean diffusivity in the genu and the splenium of the CC, whilst (Sarrazin et al., 2014) found reduced FA in the body and the splenium of the CC.
In the present study we specifically investigate white matter microstructure of the motor system in a depressed group of BD and HC. The pre-supplementary motor area (pre-SMA) is in particularly involved in voluntary action (Nachev et al., 2008) while the primary motor cortex (M1) volitionally controls the motor output which is executed via the corticospinal tract (CST) (Ebbesen and Brecht, 2017). Thus we use tractography to specifically reconstruct segments of the CC connecting bilateral pre-SMA and bilateral M1. In addition, bilateral CST are reconstructed. To enhance specificity of putative group differences we reconstruct a comparison tract (a segment of the optic radiation) where we do not expect any group differences of FA. We complement our analyses with a whole brain TBSS approach. Motor activity is quantitatively assessed with actigraphy recordings in line with previous publications (e.g. (Bracht et al., 2012b; Bracht et al., 2016; Razavi et al., 2011; Walther et al., 2010; Walther et al., 2012b). Associations between activity levels and white matter microstructure are explored.
Based on the current literature we hypothesize (1) general motor retardation, i.e. reduced activity levels (AL) in BD, (2) alterations of motor tracts, i.e. reduced FA in motor pathways in BD and (3) differences in associations between motor behavior and motor tract diffusion properties, i.e. AL and FA between patients and controls.
2. Methods
2.1. Participants
Nineteen right-handed patients with bipolar disorder (6 males, 13 females; age = 47.6 ± 10) meeting criteria for a current depressive episode according to DSM-IV and 19 right-handed healthy controls (6 males, 13 females; age = 47.5 ± 11) matched for age, gender and years of education were recruited from the inpatient and outpatient departments of the University Hospital of Psychiatry Bern, Switzerland (for details see Table 1). The sample includes bipolar patients of a previous arterial spin labelling study (ASL) (Cantisani et al., 2016).
Table 1.
Demographics. a: including lithium, valproic acid, lamotrigine and topiramate; b: including aripiprazole, risperidone, quetiapine, olanzapine; c: including zolpideme; * significant group differences at p < .05.
| Variable | Bipolar depressed | Healthy Controls | Analyses |
|---|---|---|---|
| Gender | 13 female, 6 male | 13 female, 6 male | X2(1) = 0, p = 1 |
| Age (years) | 47.6 ± 10 | 47.5 ± 11 | T (36) = 0.04, p = .996 |
| Handedness (right, %) |
100 | 100 | |
| Duration of education (years) | 16.1 ± 5 | 13.7 ± 4 | T(36) = 1.488, p = .145 |
| Annual income (in Swiss Francs) | 43,933 ± 37,202 | 48,306 ± 18,200 | T(36) = −0.44, p = .681 |
| Activity levels (counts/h) | 14,019 ± 6200 | 19,718 ± 6576 | T(36) = −2.75, p = .009* |
| Number of depressive episodes | 5.16 ± 26 | 0 | T(36) = 5.01, p < .001* |
| Duration of illness (years) | 18.2 ± 11 | 0 | T(36) = 6.94, p < .001* |
| BDI | 22.4 ± 11 | 2 ± 2.3 | T(36) = 6.61, <0.001* |
| HAMD | 21.9 ± 8 | 0.47 ± 1.06 | T(36) = 10.26, <0.001* |
| MADRS | 24.2 ± 6 | 1.07 ± 1.62 | T(36) = 13.14, <0.001* |
| Antidepressants | 12 | 0 | |
| Mood stabilizersa | 16 | 0 | |
| Atypical antipsychoticsb | 12 | 0 | |
| Benzodiazepinesc | 12 | 0 |
Diagnoses were given according to DSM-IV following clinical interview by an experienced psychiatrist and review of case files. Depressive symptoms were assessed with the Beck Depression Inventory (BDI) (Beck et al., 1961), the Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960) and the Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979).
Inclusion criteria for patients were a diagnosis of a bipolar disorder and a current depressive episode. Further inclusion criteria for all participants were age between 18 and 65 years and right-handedness as assessed with the Edinburgh Handedness Inventory using the conventional cut-off point (Oldfield, 1971). Exclusion criteria for all participants were psychiatric comorbidities (Mini International Neuropsychiatric Interview (MINI), (Sheehan et al., 1998)) including personality disorders (Structured Clinical Interview for DSM-IV (SCID), (Wittchen et al., 1997). For exclusion of motor symptoms due to Parkinsonism participants were assessed with the Unified Parkinson's disease rating scale (Fahn et al., 1987). Furthermore, participants with neurological disorders, a history of significant head trauma, electroconvulsive therapy and substance abuse or dependence other than nicotine were excluded from analyses. Exclusion criteria specifically for controls were a lifetime history of depressive episode and first-degree relatives with any affective disorders. The study protocol was approved by the local ethics committee (KEK-BE 196/09) and was in accordance with the Declaration of Helsinki. All participants provided written informed consent.
2.2. Actigraphy
Participants wore an actigraph (Actiwatch®, Inc., UK) on the wrist of their left (non-dominant) arm continuously for 24 h directly subsequent to the MRI-scan. The actigraph detects acceleration, which corresponds to whole body spontaneous motor activity (Middelkoop et al., 1997). Motor activity was analyzed exclusively during wake time. Activity levels (AL, the cumulated activity counts during wake divided by the netto recording time in hours) thus provide information on motor activity during daytime without interference of manual work. Data were analyzed using Sleep analysis® 5 software (Cambridge Neurotechnology, Inc., UK). For detailed information on acquisition and calculation of AL, we refer to previous publications e.g. (Bracht et al., 2012a; Walther et al., 2012b).
2.3. Structural MRI scanning
All data were acquired on a 3 T Siemens MR scanner (Siemens Magnetom Trio, Erlangen, Germany, 12-channel head coil). High-resolution T1-weighted data were obtained with the MDEFT sequence (Deichmann et al., 2004) with parameters as follows: 176 sagittal slices, 256 × 224 matrix, isotropic resolution of 1 mm3, TR/TE = 7.92 ms/2.48 ms, 16° flip angle, inversion time 910 ms, and fat saturation (total acquisition time = 12 min). Identical prescription of MR images was achieved using the Siemens Autoalign sequence, which automatically sets up consistent slice orientation based on a standard MRI atlas.
2.4. Diffusion MRI scanning
For diffusion MRI measurements, we used a spin-echo EPI sequence (55 slices, FOV = 256 × 256 mm2, sampled on a 128 × 128 matrix resulting in 2 mm3 voxel size, TR/TE = 6000/78 ms) covering the whole brain (40 mT/m gradient, 5/8 partial Fourier, no acceleration factor). Diffusion-weighted images were positioned in the axial plane parallel to the AC-PC line and measured along 42 directions with a b-value = 1300 s/mm2. The sequence included four B0 images without diffusion weighting (the first and every subsequent 12th image). We used a balanced and rotationally invariant diffusion-encoding scheme over the unit sphere to generate the DTI data (Hasan et al., 2001).
2.5. Diffusion MRI data pre-processing
Data analyses were performed using ExploreDTI (Leemans et al., 2009). The data were corrected for distortions and subject motion using an affine registration to the non-diffusion-weighted images, with appropriate re-orienting of the encoding vectors. Furthermore, an echo planar imaging (EPI) correction was performed warping the diffusion images to the MDEFT images resulting in a 1 × 1 × 1 mm3 resolution for further processing (Leemans and Jones, 2009). A single diffusion tensor model was fitted (Basser et al., 1994) to the diffusion data in order to compute quantitative parameters such as FA. Diffusion properties (e.g. FA) were sampled along the tracts.
2.6. Tractography
Whole brain tractography was performed using an algorithm similar to that described by (Basser et al., 1994). Termination criteria were an angle threshold >45° and FA < 0.2.
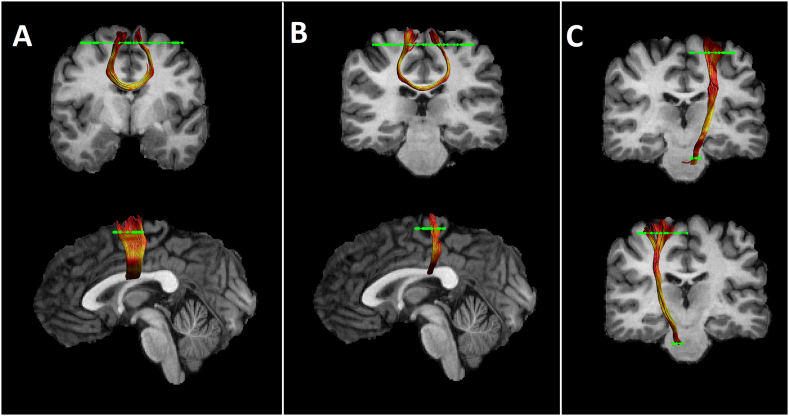
All tracts were reconstructed in horizontal sections using two anatomically defined regions of interest (ROI) per tract (see Fig. 1). For reconstruction of the CST the precentral gyrus was encircled. A second ROI was drawn at the height of the pons on colour coded DWI-images, where fibres of the corticospinal tract descend (Mole et al., 2016). In addition, bilateral ROIS were drawn for reconstruction of interhemispheric fibres between bilateral M1 and bilateral pre-SMA. The M1 corresponds to the precentral gyrus; the pre-SMA is located anterior of the precentral gyrus and posterior of the anterior commissure (Habas, 2010) (for ROIS and pathway visualisation see Fig. 1).
Fig. 1.
Reconstructed fiber tracts for an individual participant. From left to right: interhemispheric pre-SMA connections (A); interhemispheric M1 connections (B); left CST (top row), right CST (bottom row) (C) reconstructed for an individual participant. FA metrics are superimposed on the reconstructed pathways. Regions of interest are displayed in green.
ROIs of the comparison tract (a segment of the optic radiation) were drawn on two coronal sections at the height of the lateral geniculate nuclei and seven sections posterior (Counsell et al., 2007) where the optic radiation can be clearly identified on colour coded DWI-images.
Mean-FA was derived for each of the five reconstructed tracts for each subject. In addition, the average mean diffusion (MD) and the axial and radial diffusivity (AD and RD) were computed, to facilitate follow up of any group differences seen in FA, our primary outcome measure.
2.7. Statistical analyses
Statistical analyses were performed using Statistical Package for Social Sciences SPSS 24.0® (SPSS Inc., Chicago, IL, USA). Differences of demographic variables and AL were calculated using Chi-Square-tests or t-tests as appropriate (see Table 1).
In order to compare white matter microstructure between groups we calculated a MANOVA with the independent variable group (HC, BD) and the dependent variable FA for the four respective pathways (left CST, right CST, interhemispheric M1 connection, interhemispheric pre-SMA). Likewise, a MANOVA with the dependent variable group (BD, HC) and FA of the comparison tract (bilateral segments of the optic radiation) was calculated. Significant main effects of group were followed up with independent t-tests with group (HC, BD) as dependent and FA as independent variable. The p-value of these post-hoc tests was adjusted using a Bonferroni correction for multiple comparisons (0.05/4 = 0.0123). Where significant group differences of FA were found the effects on mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were explored.
In addition, we explored differences regarding the relationship of quantitative motor behavior and white matter microstructure. ANCOVAs with the independent variable group (controls vs. patients) and the dependent variables mean FA and AL were computed for each of the pathway to identify significant group x AL interactions. Correlations were reported separately for groups where there was a group x AL interaction and across groups for the remaining tracts. In addition, we report group x hemisphere interaction for the CST.
2.8. Whole brain voxel-wise analysis
Voxel-wise statistical analysis of FA data was performed using FSL TBSS software (Smith et al., 2006). FA data were projected onto a mean FA tract skeleton, before applying voxel-wise cross-subjects statistics. The tract skeleton was thinned using an FA threshold >0.2. Group comparisons between BD and HC of FA on this fiber skeleton were then performed using threshold-free cluster-enhancement (TFCE). Group comparisons were deemed to be significant at a cluster threshold of p < .05.
3. Results
3.1. Sample characteristics
Groups did not differ regarding gender, age, handedness and years of education. BD-had a mean HAMD score of 21.9 ± 8 indicating moderate to severe depressive episodes and an average of 5 previous depressive episodes. All patients were on medication at the time of scanning (for details see Table 1).
3.2. Group comparisons
BD patients had significantly lower AL than HC indicating reduced daily physical activity in BD (see Table 1).
The MANOVA revealed a main effect of group on mean-FA across the four tracts (F (4,33) = 3.87, p = .011). This main effect was followed up using separate independent t-tests for each of the four tracts. We applied a Bonferroni correction for multiple comparisons (p = .0125 (0.05/4). Post hoc t-tests indicated significant higher FA in the left CST in BD (BD = 0.60 ± 0.02; HC = 0.58 ± 0.02, T (36) = 2.84, p = .007). There were no group differences in the right CST (BD = 0.58 ± 0.02; HC = 0.57 ± 0.02, T (36) = 1.495, p = .144) and in the interhemispheric M1-connection (BD = 0.56 ± 0.02; HC = 0.55 ± 0.02, T (36) = 0.721, p = .476). However, there was a (non-significant) trend for lower FA in BD in interhemispheric pre-SMA connections (BD = 0.57 ± 0.02; HC = 0.59 ± 0.02, T (36) = −1.975, p = .058). Follow up tests for the left CST comparing mean MD (p = .362), RD (p = .077) and AD (p = .761) did not show significant differences. Neither were there differences in FA for the TBSS results nor for the comparison tract (F 4,33 = 0.33, p = .73).
Motor activity and white matter microstructure in the left CST demonstrated an interaction with group, as evidenced by a significant group x AL interaction (F 1,34) = 5.52, p = .025 (Fig. 2). In patients, we found a positive association of AL and FA (r = 0.533, p = .014), which was not found in controls (r = −0.164, p = .504). In tracts where there was no group x AL interaction correlation across groups correlations were as follows: right CST (r = −0.03, p = .857), interhemispheric M1-connection (r = 0.244, p = .139) and interhemispheric pre-SMA connection (r = 0.321, p = .05).
Fig. 2.
Association of activity levels and fractional anisotropy in the left corticospinal tract. Red dots indicate BD, blue diamonds HC.
There was a main effect of hemisphere (F (1, 36) = 27.21, p ≤ .001), but no group x hemisphere interaction (F (1,36) = 1.565, p = 0,219) for FA of the corticospinal tracts.
4. Discussion
This is the first tractography study in BD relating white matter microstructure to quantitative motor behavior. Our study has two main findings. First, we found increased FA in the left CST. In addition, there was a non-significant (p = .058) trend for reduced FA in a segment of the CC connecting left and right pre-SMA. Our finding of increased FA in the left CST complements previous studies mostly reporting reduced FA in BD (Nortje et al., 2013; Wise et al., 2016). Second, BD but not HC had a positive correlation between FA and AL. Given that AL was reduced in BD this suggests that the left CST may partially compensate for psychomotor retardation in BD.
In unipolar depression psychomotor retardation was repeatedly associated with reduced blood flow in prefrontal brain regions such as the dorsolateral prefrontal cortex (dlPFC) and the anterior cingulate cortex (Mayberg et al., 1994; Narita et al., 2004; Schrijvers et al., 2008; Videbech et al., 2002) or the orbitofrontal cortex (Walther et al., 2012a). There is also support from a DTI-tractography-study suggesting implications of the dlPFC and the ACC for psychomotor retardation (Bracht et al., 2012a) and from an EEG study linking frontal alpha asymmetry to impaired physical activity in depression (Cantisani et al., 2015). Further, selective associations between AL and FA in unipolar depression have been found in the posterior cingulum, underneath the left primary motor cortex and in proximity to the left parahippocampal gyrus (Walther et al., 2012b).
Functional MRI (fMRI) studies in BD have linked deficits of volitional motor activity to interhemispheric asymmetries in activation patterns located in pre-executive stages of motor production (Caligiuri et al., 2004; Liberg et al., 2013). For instance, an (fMRI) reaction time task study (Caligiuri et al., 2004) demonstrated disrupted activation patterns between the two hemispheres located in the SMA in depressed BD. These commissural asymmetries may well be associated with structural impairments of the CC as demonstrated in multiple DTI-studies (Bellani et al., 2016; Benedetti et al., 2011; Lagopoulos et al., 2013; Li et al., 2014; Mahapatra et al., 2017; Nenadic et al., 2017; Toteja et al., 2015; Wang et al., 2008; Wise et al., 2016).
In addition, two fMRI reaction time tasks studies in depressed BD found increased blood oxygenation dependent levels (BOLD) levels in the M1 (Caligiuri et al., 2003; Caligiuri et al., 2004). Those fMRI findings are complemented by a recent ASL study demonstrating increased perfusion in the left precentral gyrus in BD in comparison to both unipolar depressed patients and healthy controls (Cantisani et al., 2016). Higher blood flow was associated with higher AL. Thus, increases in blood flow and activation in the M1 (Caligiuri et al., 2003; Caligiuri et al., 2004; Cantisani et al., 2016) may contribute to compensate for psychomotor retardation in BD. This could very plausibly be accompanied by compensatory neuroplasticity as suggested by our findings of increased FA in the left CST. One may speculate if increases in perfusion and activation induce neuroplasticity in the CST. Alternatively, white matter microstructure alterations (such as higher density of axons, or glia cells (Andreazza et al., 2013; Bellani et al., 2016)) may require higher levels of blood flow for engaging in sufficient activation. However, methodologically it is impossible to infer such causalities based on neuroimaging studies.
Our lateralized finding of the left (but not the right CST) may be due to the fact that we investigate right handed participants. Given that neurons of the left CST cross to the right hemisphere at the pyramidal tract those pathways are likely to be more active and thus neuroplasticity may be induced (Gibson et al., 2014; Sagi et al., 2012). This assumption is also supported by the hemispheric effect demonstrating higher FA values in the left as compared to the right CST. However, given the trend for an interaction in the right CST (p = .144) it is also possible that limited sample size may have obscured an effect in the right CST.
Psychomotor retardation is a common syndrome in neuropsychiatric disorders that may stem from functional and structural alterations in different pathways. It is of interest that in Parkinson's Disease (a disorder with basal ganglia dysfunction) similar to our finding increases in FA have been reported in the CST and suggested to reflect a compensatory mechanism (Mole et al., 2016). In schizophrenia there is increasing evidence for a role of thalamo-cortical pathways for psychomotor retardation and compensatory mechanisms in the pre-SMA (Bracht et al., 2013; Walther, 2015; Walther et al., 2012b; Walther et al., 2017). Thus, disruptions in different (circumscribed) pathways in different disorders may lead to similar phenomenological behavior such as reduced daily activity.
Higher FA in the left CST could stem from decreased crossing of axons, higher axon density or from higher density of glia cells such as oligodendrocytes (Jones et al., 2013). Indeed increased subcortical oligodendrocyte density and increased cell clustering of astrocytes have been reported in BD (Hercher et al., 2014). It has been suggested that those neuropathological alterations play an important role in remyelination processes and compensate for subcortical myelin damages (Andreazza et al., 2013; Bellani et al., 2016). Thus, neuropathological studies are in line with our finding of increased FA. However, DTI-based findings do not allow for conclusions on specific neuropathological alterations (Jones et al., 2013) and therefore the neurobiological correlate of our finding of increased FA in the left CST remains unclear. This inherent limitation of DTI may in part be overcome by using advanced sub-compartment specific sequences such as McDESPOT (Bracht et al., 2016) or CHARMED (De Santis et al., 2018).
The lack of significant group differences of our TBSS analyses is consistent with increasing evidence that tractography approaches averaging diffusion properties such as FA over the whole tract may be more sensitive than isolated comparisons on a voxel-by-voxel level (Bracht et al., 2015a; Bracht et al., 2014; Kanaan et al., 2006; Keedwell et al., 2012). However, it is possible that larger sample sizes would have detected significant findings using TBSS (Benedetti et al., 2011; Lagopoulos et al., 2013).
In conclusion this is the first tractography study to link an objective measure of motor activity to white matter microstructure in bipolar disorder. In a carefully matched sample for age, gender, handedness and years of education we identify increased FA in the left CST in BD. The selective positive association between FA and AL in BD suggests a compensatory role of the left CST for psychomotor retardation in BD, which is in line with fMRI and ASL studies (Caligiuri et al., 2004; Cantisani et al., 2016). Future longitudinal studies may explore if those alterations represent a trait or a state marker in BD (Bracht et al., 2015b) and may apply advanced sub-compartment specific approaches (De Santis et al., 2018) in conjunction with tractography.
Acknowledgments
Acknowledgements
We thank Oliver Höfle for testing and recruiting some of the participants.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
Dr. Walther received honoraria from Lundbeck, Otsuka, Lilly, and Janssen. There is no conflict of interest with this work.
References
- American Psychiatric Association . Fourth Ed. 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. Washington, DC. [Google Scholar]
- Andreazza A.C., Wang J.F., Salmasi F., Shao L., Young L.T. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J. Neurochem. 2013;127:552–561. doi: 10.1111/jnc.12316. [DOI] [PubMed] [Google Scholar]
- Angst J. Bipolar disorders in DSM-5: strengths, problems and perspectives. Int J Bipolar Disord. 2013;1:12. doi: 10.1186/2194-7511-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., Lebihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bellani M., Boschello F., Delvecchio G., Dusi N., Altamura C.A., Ruggeri M., Brambilla P. DTI and Myelin Plasticity in Bipolar Disorder: Integrating Neuroimaging and Neuropathological Findings. Front Psychiatry. 2016;7:21. doi: 10.3389/fpsyt.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Yeh P.H., Bellani M., Radaelli D., Nicoletti M.A., Poletti S., Falini A., Dallaspezia S., Colombo C., Scotti G., Smeraldi E., Soares J.C., Brambilla P. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry. 2011;69:309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Bennabi D., Vandel P., Papaxanthis C., Pozzo T., Haffen E. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed. Res. Int. 2013;2013:158746. doi: 10.1155/2013/158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Federspiel A., Schnell S., Horn H., Hofle O., Wiest R., Dierks T., Strik W., Muller T.J., Walther S. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Heidemeyer K., Koschorke P., Horn H., Razavi N., Wopfner A., Strik W., Walther S. Comparison of objectively measured motor behavior with ratings of the motor behavior domain of the Bern Psychopathology Scale (BPS) in schizophrenia. Psychiatry Res. 2012;198:224–229. doi: 10.1016/j.psychres.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Bracht T., Schnell S., Federspiel A., Razavi N., Horn H., Strik W., Wiest R., Dierks T., Muller T.J., Walther S. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr. Res. 2013;143:269–276. doi: 10.1016/j.schres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Schnell S., Hofle O., Stegmayer K., Wiest R., Dierks T., Muller T.J., Walther S. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J. Affect. Disord. 2014;155:186–193. doi: 10.1016/j.jad.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Bracht T., Doidge A.N., Keedwell P.A., Jones D.K. Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol. Med. 2015;45:865–874. doi: 10.1017/S0033291714001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Jones D.K., Muller T.J., Wiest R., Walther S. Limbic white matter microstructure plasticity reflects recovery from depression. J. Affect. Disord. 2015;170:143–149. doi: 10.1016/j.jad.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Bracht T., Jones D.K., Bells S., Walther S., Drakesmith M., Linden D. Myelination of the right parahippocampal cingulum is associated with physical activity in young healthy adults. Brain Struct. Funct. 2016;221:4537–4548. doi: 10.1007/s00429-016-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukdura J.S., McClintock S.M., Croarkin P.E. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M.P., Brown G.G., Meloy M.J., Eberson S.C., Kindermann S.S., Frank L.R., Zorrilla L.E., Lohr J.B. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 2003;123:171–182. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Caligiuri M.P., Brown G.G., Meloy M.J., Eyler L.T., Kindermann S.S., Eberson S., Frank L.R., Lohr J.B. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord. 2004;6:183–196. doi: 10.1111/j.1399-5618.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Cantisani A., Koenig T., Horn H., Muller T., Strik W., Walther S. Psychomotor retardation is linked to frontal alpha asymmetry in major depression. J. Affect. Disord. 2015;188:167–172. doi: 10.1016/j.jad.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Cantisani A., Stegmayer K., Bracht T., Federspiel A., Wiest R., Horn H., Muller T.J., Schneider C., Hofle O., Strik W., Walther S. Distinct resting-state perfusion patterns underlie psychomotor retardation in unipolar vs. bipolar depression. Acta Psychiatr. Scand. 2016;134:329–338. doi: 10.1111/acps.12625. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Dyet L.E., Larkman D.J., Nunes R.G., Boardman J.P., Allsop J.M., Fitzpatrick J., Srinivasan L., Cowan F.M., Hajnal J.V., Rutherford M.A., Edwards A.D. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. NeuroImage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- De Santis S., Bastiani M., Droby A., Kolber P., Zipp F., Pracht E., Stoecker T., Groppa S., Roebroeck A. Characterizing Microstructural Tissue Properties in Multiple Sclerosis with Diffusion MRI at 7T and 3T: the Impact of the Experimental Design. Neuroscience. 2018 doi: 10.1016/j.neuroscience.2018.03.048. (in press) [DOI] [PubMed] [Google Scholar]
- Deichmann R., Schwarzbauer C., Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Ebbesen C.L., Brecht M. Motor cortex - to act or not to act? Nat. Rev. Neurosci. 2017;18:694–705. doi: 10.1038/nrn.2017.119. [DOI] [PubMed] [Google Scholar]
- Fahn S., Marsden C.D., Calne D.B., Goldstein M. The Unified Parkinson's Disease Rating Scale. Recent Developments in Parkinson's Disease. 1987;2:153–163. [Google Scholar]
- Frankland A., Roberts G., Holmes-Preston E., Perich T., Levy F., Lenroot R., Hadzi-Pavlovic D., Breakspear M., Mitchell P.B. Clinical predictors of conversion to bipolar disorder in a prospective longitudinal familial high-risk sample: focus on depressive features. Psychol. Med. 2017:1–9. doi: 10.1017/S0033291717003233. [DOI] [PubMed] [Google Scholar]
- Gibson E.M., Purger D., Mount C.W., Goldstein A.K., Lin G.L., Wood L.S., Inema I., Miller S.E., Bieri G., Zuchero J.B., Barres B.A., Woo P.J., Vogel H., Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. Functional connectivity of the human rostral and caudal cingulate motor areas in the brain resting state at 3T. Neuroradiology. 2010;52:47–59. doi: 10.1007/s00234-009-0572-1. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K.M., Parker D.L., Alexander A.L. Comparison of gradient encoding schemes for diffusion-tensor MRI. J. Magn. Reson. Imaging. 2001;13:769–780. doi: 10.1002/jmri.1107. [DOI] [PubMed] [Google Scholar]
- Hercher C., Chopra V., Beasley C.L. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. 2014;39:376–385. doi: 10.1503/jpn.130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kanaan R.A., Shergill S.S., Barker G.J., Catani M., Ng V.W., Howard R., McGuire P.K., Jones D.K. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Chapman R., Christiansen K., Richardson H., Evans J., Jones D.K. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol. Psychiatry. 2012;72:296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kvam S., Kleppe C.L., Nordhus I.H., Hovland A. Exercise as a treatment for depression: a meta-analysis. J. Affect. Disord. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J., Hermens D.F., Hatton S.N., Tobias-Webb J., Griffiths K., Naismith S.L., Scott E.M., Hickie I.B. Microstructural white matter changes in the corpus callosum of young people with Bipolar Disorder: a diffusion tensor imaging study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D.K. Proceedings of the International Society for Magnetic Resonance in Medicine 17th Annual Meeting April 18–24, 3536 Honolulu Hawaii. 2009. ExporeDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. [Google Scholar]
- Leonpacher A.K., Liebers D., Pirooznia M., Jancic D., MacKinnon D.F., Mondimore F.M., Schweizer B., Potash J.B., Zandi P.P., Consortium N.G.I.B.D., Goes F.S. Distinguishing bipolar from unipolar depression: the importance of clinical symptoms and illness features. Psychol. Med. 2015;45:2437–2446. doi: 10.1017/S0033291715000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kale Edmiston E., Chen K., Tang Y., Ouyang X., Jiang Y., Fan G., Ren L., Liu J., Zhou Y., Jiang W., Liu Z., Xu K., Wang F. A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Res. 2014;221:58–62. doi: 10.1016/j.pscychresns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Liberg B., Adler M., Jonsson T., Landen M., Rahm C., Wahlund L.O., Wiberg-Kristoffersen M., Wahlund B. Motor imagery in bipolar depression with slowed movement. J. Nerv. Ment. Dis. 2013;201:885–893. doi: 10.1097/NMD.0b013e3182a5c2a7. [DOI] [PubMed] [Google Scholar]
- Mahapatra A., Khandelwal S.K., Sharan P., Garg A., Mishra N.K. Diffusion tensor imaging tractography study in bipolar disorder patients compared to first-degree relatives and healthy controls. Psychiatry Clin. Neurosci. 2017;71:706–715. doi: 10.1111/pcn.12530. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Lewis P.J., Regenold W., Wagner H.N., Jr. Paralimbic hypoperfusion in unipolar depression. J. Nucl. Med. 1994;35:929–934. [PubMed] [Google Scholar]
- Middelkoop H.A., van Dam E.M., Smilde-Van Den Doel D.A., Van Dijk G. 45-hour continuous quintuple-site actimetry: relations between trunk and limb movements and effects of circadian sleep-wake rhythmicity. Psychophysiology. 1997;34:199–203. doi: 10.1111/j.1469-8986.1997.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Mole J.P., Subramanian L., Bracht T., Morris H., Metzler-Baddeley C., Linden D.E. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur. Radiol. 2016;26:3327–3335. doi: 10.1007/s00330-015-4178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Narita H., Odawara T., Iseki E., Kosaka K., Hirayasu Y. Psychomotor retardation correlates with frontal hypoperfusion and the Modified Stroop Test in patients under 60-years-old with major depression. Psychiatry Clin. Neurosci. 2004;58:389–395. doi: 10.1111/j.1440-1819.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- Nenadic I., Hoof A., Dietzek M., Langbein K., Reichenbach J.R., Sauer H., Gullmar D. Diffusion tensor imaging of cingulum bundle and corpus callosum in schizophrenia vs. bipolar disorder. Psychiatry Res. Neuroimaging. 2017;266:96–100. doi: 10.1016/j.pscychresns.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Razavi N., Horn H., Koschorke P., Hugli S., Hofle O., Muller T., Strik W., Walther S. Measuring motor activity in major depression: the association between the Hamilton Depression Rating Scale and actigraphy. Psychiatry Res. 2011;190:212–216. doi: 10.1016/j.psychres.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Sagi Y., Tavor I., Hofstetter S., Tzur-Moryosef S., Blumenfeld-Katzir T., Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Sarrazin S., Poupon C., Linke J., Wessa M., Phillips M., Delavest M., Versace A., Almeida J., Guevara P., Duclap D., Duchesnay E., Mangin J.F., Le Dudal K., Daban C., Hamdani N., D'Albis M.A., Leboyer M., Houenou J. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71:388–396. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- Schrijvers D., Hulstijn W., Sabbe B.G. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J. Affect. Disord. 2008;109:1–20. doi: 10.1016/j.jad.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):34–57. 22–33;quiz. [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., MacKay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Toteja N., Guvenek-Cokol P., Ikuta T., Kafantaris V., Peters B.D., Burdick K.E., John M., Malhotra A.K., Szeszko P.R. Age-associated alterations in corpus callosum white matter integrity in bipolar disorder assessed using probabilistic tractography. Bipolar Disord. 2015;17:381–391. doi: 10.1111/bdi.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P., Ravnkilde B., Pedersen T.H., Hartvig H., Egander A., Clemmensen K., Rasmussen N.A., Andersen F., Gjedde A., Rosenberg R. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr. Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233:293–298. doi: 10.1016/j.pscychresns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Walther S., Federspiel A., Horn H., Wiest R., Dierks T., Strik W., Muller T.J. White matter integrity associated with volitional motor activity. Neuroreport. 2010;21:381–385. doi: 10.1097/WNR.0b013e328337ca29. [DOI] [PubMed] [Google Scholar]
- Walther S., Hofle O., Federspiel A., Horn H., Hugli S., Wiest R., Strik W., Muller T.J. Neural correlates of disbalanced motor control in major depression. J. Affect. Disord. 2012;136:124–133. doi: 10.1016/j.jad.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Walther S., Hugli S., Hofle O., Federspiel A., Horn H., Bracht T., Wiest R., Strik W., Muller T.J. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol. Dis. 2012;47:13–19. doi: 10.1016/j.nbd.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Walther S., Stegmayer K., Federspiel A., Bohlhalter S., Wiest R., Viher P.V. Aberrant Hyperconnectivity in the Motor System at rest is Linked to Motor Abnormalities in Schizophrenia Spectrum Disorders. Schizophr. Bull. 2017;43:982–992. doi: 10.1093/schbul/sbx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kalmar J.H., Edmiston E., Chepenik L.G., Bhagwagar Z., Spencer L., Pittman B., Jackowski M., Papademetris X., Constable R.T., Blumberg H.P. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol. Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Radua J., Nortje G., Cleare A.J., Young A.H., Arnone D. Voxel-based Meta-Analytical evidence of Structural Disconnectivity in Major Depression and Bipolar Disorder. Biol. Psychiatry. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Wunderlich U., Gruschwitz S., Zaudig M. Hogrefe; Göttingen: 1997. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. [Google Scholar]