Abstract
Aims/hypothesis
We investigated the association between gluten intake and long-term type 2 diabetes risk among Americans.
Methods
We followed women from the Nurses’ Health Study (NHS, n=69,276, 1984–2012) and NHS II (n=88,604, 1991–2013) and men from the Health Professionals Follow-Up Study (HPFS, n=41,908, 1986–2012). Gluten intake was estimated using a validated food frequency questionnaire every 2–4 years. Incident type 2 diabetes was defined as self-reported physician-diagnosed diabetes confirmed using a supplementary questionnaire.
Results
Gluten intake was strongly correlated with intakes of carbohydrate components, especially refined grains, starch and cereal fibre (Spearman correlation coefficients > 0.6). During 4.24 million years of follow-up, 15,947 people were confirmed to have type 2 diabetes. After multivariate adjustment, pooled HRs and 95% CIs for type 2 diabetes, from low to high gluten quintiles, were (ptrend<0.001): 1 (reference); 0.89 (0.85, 0.93); 0.84 (0.80, 0.88); 0.78 (0.74, 0.82) and 0.80 (0.76, 0.84). The association was slightly weakened after further adjusting for cereal fibre, with pooled HRs (95% CIs) of (ptrend<0.001): 1 (reference); 0.91 (0.87, 0.96); 0.88 (0.83, 0.93); 0.83 (0.78, 0.88) and 0.87 (0.81, 0.93). Dose–response analysis supported a largely linear inverse relationship between gluten intake up to 12 g/day and type 2 diabetes. The association between gluten intake and type 2 diabetes was stronger when intake of added bran was also higher (pinteraction=0.02).
Conclusions/interpretation
Gluten intake is inversely associated with type 2 diabetes risk among largely healthy US men and women. Limiting gluten in the diet is associated with lower intake of cereal fibre and possibly other beneficial nutrients that contribute to good health.
Keywords: Gluten, Type 2 diabetes
Introduction
Gluten is the major storage protein in the endosperm of wheat, barley and rye [1]. It is known that gluten triggers an autoimmune response in people with coeliac disease, a condition affecting 1% of population worldwide [2]. A concept of non-coeliac gluten sensitivity has also emerged lately, though the biological basis of this condition remains unclear [3]. The past few years have witnessed the increasing popularity of a gluten-free diet among people without apparent gluten-related disorders in the USA and many other countries, with the belief that eating a gluten-free diet is associated with health benefits [4]. Despite this perception, evidence remains lacking on the effects of gluten intake in a largely healthy population, and few studies have examined gluten intake in relation to major cardiometabolic disorders, including type 2 diabetes.
Several small intervention studies have reported that high doses of gluten (60–100 g/day) had no significant adverse effects among healthy individuals or people with dyslipidaemia [5–8]. Instead, daily intake of 60–80 g/day gluten in bread decreased blood lipids, blood pressure and oxidative stress [6–8], which are known risk factors for cardiometabolic diseases. Meanwhile, levels of gluten intake in the general population are much lower than the doses used in trials. According to two recent studies, mean gluten intake was 10.4 g/day in Danish adults [9] and 13.1 g/day among Dutch participants [10]. To examine the potential health impact of gluten at similar levels, we investigated the association between typical gluten intake and risk of type 2 diabetes among US men and women from the Nurses’ Health Study (NHS), NHS II (NHSII) and the Health Professionals Follow-Up Study (HPFS).
Methods
Study populations
The NHS, NHSII and HPFS are ongoing prospective cohort studies. The NHS includes 121,700 female registered nurses aged 30–55 years enrolled in 1976 [11], the NHSII includes 116,671 female registered nurses aged 24–44 years enrolled in 1989 and the HPFS consists of 51,529 male health professionals aged 40–75 years enrolled in 1986 [12]. Participants in all studies have been followed through posted biennial questionnaires to collect and update information on lifestyles, health-related behaviours and medical histories. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. The completion of the self-administered questionnaire was considered to imply informed consent.
Of the participants who completed a baseline food frequency questionnaire (FFQ; NHS 1984, n=79,401; NHSII 1991, n=97,605; and HPFS 1986, n=51,530), we excluded individuals if they: (1) reported a diagnosis of diabetes, cardiovascular disease or cancer at baseline (n=9,392 in NHS, n=6,155 in NHSII and n=6,933 in HPFS); (2) had daily energy intake outside the normal range (<2092 [500] or > 14644 [3,500] kJ/day [kcal/day] for NHS and NHSII; <3347 [800] or > 17572 [4,200] kJ/day[kcal/day] for HPFS) or missing gluten data (n=2,164 in NHSII and 1,275 in HPFS)[13]; (3) had a missing date of type 2 diabetes diagnosis (262 in HPFS); and (4) only completed the baseline questionnaire or had missing age at baseline (n=763 in NHS, 682 in NHSII and 1,152 in HPFS). This left 69,276 participants in NHS, 88,604 in NHSII and 41,908 in HPFS for the analysis.
Ascertainment of diet and gluten intake
In 1984, NHS participants completed a validated 118 item FFQ to assess their habitual diet in the past year. Starting in 1986 in NHS and HPFS and 1991 in NHSII, a similar but expanded questionnaire was sent to the participants every 4 years to collect and update their dietary information [14]. We calculated the cumulative averages of diet based on valid assessments from baseline to the end of follow-up, and stopped updating dietary information if participants reported a diagnosis of diabetes, cardiovascular disease or cancer [14]. Nutrient intakes were adjusted for total energy using the residual method [15]. The Alternative Health Eating Index (AHEI) was calculated as described previously without the alcohol component [14].
Gluten intake was estimated based on gluten-containing ingredients of food items in the FFQ [15]. Gluten-containing ingredients included wheat, wheat flour, wheat bran, wheat germ, wheat berries, wheat cream, wheat gluten, rye and rye flour, barley and barley malt flour, cooked cereal, bulgur, couscous, farina, beer and pasta. We identified these ingredients from food items according to product labels and ingredient information provided by manufacturers in commercially prepared foods, and recipes from cookbooks for home-prepared items. Gluten-containing ingredients in each food source were quantified by multiplying the serving size by the amounts of gluten-containing ingredients in each serving of food. The proportion of gluten in the protein portion of the ingredients was estimated to be 75–80% in previous studies, and we used a conservative estimate of 75% when calculating the gluten content of these ingredients [10, 16, 17]. We used the same conversion factor for all three grains, although the proportion of gluten in total protein may be more variable in rye and barley than in wheat [18]. We did not account for trace amounts of gluten that may be present in oats and in condiments (for example, soy sauce), as the contribution to total gluten intake would be negligible [19]. Finally, gluten from all ingredient sources was added up to estimate the total gluten consumption. Of note, the FFQ assessments of some major sources of gluten were reasonably correlated with those by 7-day diet records: deattenuated correlation coefficients ranged between 0.57 (pie) to 0.79 (cold breakfast cereal) [20]. In a more recent validation study conducted in 2010–2012, the deattenuated correlation coefficients between dietary assessments by FFQs and 7-day dietary records were in the range of 0.54 and 0.69 for protein, dietary fibre and carbohydrates [21].
Ascertainment of incident type 2 diabetes
Participants who reported a physician diagnosis of diabetes were posted a supplementary questionnaire regarding symptoms, diagnostic tests and hypoglycaemic therapy. The diagnosis of type 2 diabetes was considered confirmed if at least one of the following was reported on the supplementary questionnaire according to the National Diabetes Data Group criteria [22]: one or more classic symptoms (excessive thirst, polyuria or frequent urination, weight loss, hunger) plus fasting plasma glucose ≥ 7.8 mmol/l or random plasma glucose levels ≥ 11.1 mmol/l; ≥ 2 elevated plasma glucose concentrations on different occasions (fasting glucose ≥ 7.8 mmol/l, random plasma glucose ≥ 11.1 mmol/l and/or plasma glucose ≥ 11.1 mmol/l after ≥ 2 h shown by oral glucose tolerance testing) in the absence of symptoms; or treatment with hypoglycaemic medication. The diagnostic criteria changed in June 1998, and a fasting plasma glucose of 7.0 mmol/l was considered the threshold for the diagnosis of diabetes instead of 7.8 mmol/l according to the American Diabetes Association criteria [22]. In validation studies, 61 of 62 self-reported cases of type 2 diabetes confirmed by the supplementary questionnaire were re-confirmed after a blinded endocrinologist reviewed medical records [23]; and in the HPFS, 57 of 59 cases (97%) were re-confirmed [24].
Assessment of covariates
Information on family history of diabetes, smoking status, physical activity, menopause status and menopausal hormone use, oral contraceptive use, multivitamin use and body weight was collected in a biennial follow-up questionnaire. Physical activity was estimated by multiplying the energy expenditure in metabolic equivalent tasks (METs), measured in h/week, by time spent on that activity, and then values of all activities were summed to derive total physical activity levels. BMI was calculated as self-reported weight in kg divided by baseline height in m2.
Statistical analysis
Person-years were calculated from the return of baseline FFQ to diagnosis of type 2 diabetes, last return of a follow-up questionnaire, death or end of follow-up (2012 for NHS and HPFS and 2013 for NHSII), whichever came first. Cox proportional hazards models were used to calculate HRs and 95% CIs for the association between quintiles of gluten intake and type 2 diabetes risk in each cohort and after pooling data from three cohorts. Multivariate-adjusted models were stratified jointly by age in months and calendar year, and adjusted for ethnicity (white, African-American, Asian, and other ethnicity), family history of diabetes (yes/no), smoking status (never, former, current [1–14, 15–24, or ≥ 25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, or ≥ 15.0 in women; 0, 0.1–4.9, 5.0–29.9, or ≥ 30.0 in men; or missing), physical activity (METs: <3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥ 27.0, or missing), menopause status and menopausal hormone use (pre-menopause, postmenopause [never, former, or current hormone use], or missing, for women), oral contraceptive use (yes, no, or missing, for NHSII), multivitamin use (yes/no), BMI (kg/m2: <23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, ≥35.0, or missing), total energy intake, AHEI (in quintiles) and intakes of magnesium, folic acid and cereal fibre (in quintiles). Linear trend was tested by modelling the median gluten values in each category as a continuous variable. We performed restricted cubic spline analysis in pooled samples of three cohorts with 5 knots among participants between the 1st and 99th percentile of gluten distribution to minimise possible influence of extreme values on the curve. We repeated categorical and dose–response analysis using gluten intake as percentage of total energy.
We calculated Spearman correlation coefficients of gluten intake with whole grains, refined grains, bran, germ, starch, glycaemic index and glycaemic load, in addition to cereal fibre. We also controlled for carbohydrate variables other than cereal fibre in a secondary analysis, including: (1) refined grains; (2) whole grains; (3) glycaemic index and glycaemic load; and (4) bran, germ and starch. We also calculated gluten intake adjusted for whole grains and refined grains using the residual method for energy adjustment [15], and repeated the analysis using adjusted residuals of the gluten variable. Finally, joint analysis was performed to test potential interactions of gluten with intake of bran, added bran, cereal fibre and whole grain on risk of type 2 diabetes.
Analyses were also stratified by age (< 65 years, ≥ 65 years), BMI (< 30 kg/m2, ≥ 30 kg/m2), physical activity (< 18 METs, ≥ 18 METs) and smoking status (current smoking, or not) to determine whether any interactions existed. To assess the robustness of findings, we conducted the following sensitivity analyses: (1) adjusting for individual diet components, including trans fats, polyunsaturated fat to saturated fat ratio, fruits, vegetables and red meats (in quintiles), instead of AHEI; (2) using baseline gluten data as the exposure; and (3) using baseline BMI instead of updated BMI. Data on prevalent coeliac disease were available for NHS and HPFS, and we performed a sensitivity analysis after excluding participants who reported coeliac disease in 2014 in these two studies.
All statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA), and p values were two sided with a significance level of 0.05.
Results
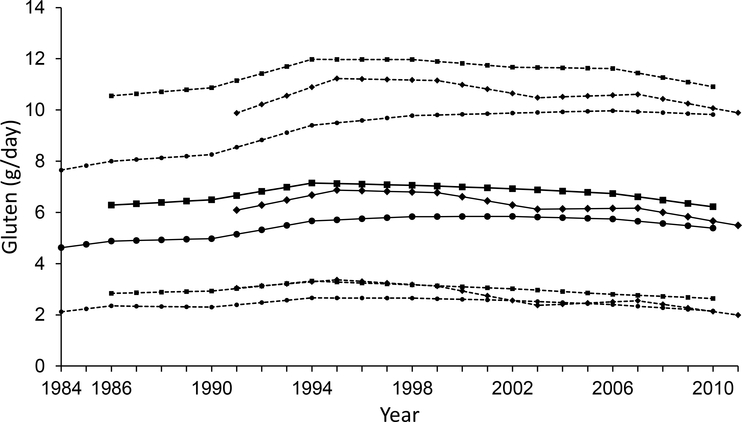
Trends in gluten intake in three cohorts are presented in Fig. 1. For most participants (between 5% and 95% distribution), gluten consumption was between 2 and 12 g/day, which reached a peak around the mid-90s. At the midpoint of follow-up, gluten intake (mean ± SD) was 5.83±2.23 (NHS 1998), 6.77±2.50 (NHSII 1999) and 7.06±2.76 (HPFS 1998) g/day. Major food sources of gluten included pasta, whole-grain bread, cold breakfast cereals, pizza, white bread and baked products made with wheat flour, including cakes, muffins, pastries, pretzels and crackers (electronic supplementary material [ESM] Table 1). Men and women with a higher gluten intake were more likely to be white, non-smokers and users of multivitamin supplements (Table 1). They had lower alcohol intake in NHS and HPFS, and higher physical activities in NHSII and HPFS. These participants also had a higher AHEI score and polyunsaturated:saturated fatty acid ratio (P/S ratio), higher intake of folic acid, and lower intake of fruit, vegetables, red meat and energy from protein. Carbohydrate intake was higher among participants with higher gluten intake, with cereal fibre intake showing the largest differences and glycaemic index showing the smallest differences between gluten quintiles.
Fig. 1.
Trends in gluten intake in three cohorts. Circles, NHS; diamonds, NHSII; squares, HPFS; solid lines, means; dashed lines show 95% CI
Table 1.
Age-standardised characteristics according to quintiles of gluten intake at the midpoint of follow-up (NHS1998, NHSII 1999, HPFS1998)
| Characteristic | NHS |
NHSII |
HPFS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| n | 12083 | 12090 | 12088 | 16630 | 16633 | 16608 | 7177 | 7181 | 7180 |
| Gluten, g/day | 3.37 | 5.08 | 7.16 | 3.90 | 6.29 | 9.27 | 3.97 | 6.48 | 9.71 |
| Age, years | 64.4 | 63.3 | 63.4 | 46.7 | 46.1 | 46.0 | 67.1 | 65.4 | 64.8 |
| White, % | 95 | 98 | 99 | 91 | 97 | 98 | 91 | 96 | 97 |
| Current smoking, % | 15 | 9 | 7 | 13 | 8 | 6 | 6 | 4 | 3 |
| Alcohol intake, g/day | 7.01 | 5.12 | 3.77 | 3.61 | 4.05 | 3.79 | 12.0 | 11.5 | 8.9 |
| Physical activity, METs | 17.6 | 17.6 | 17.7 | 19.1 | 20.5 | 22.3 | 32.2 | 32.9 | 34.6 |
| BMI, kg/m2 | 26.5 | 26.6 | 25.9 | 27.6 | 26.6 | 25.7 | 26.6 | 26.2 | 25.4 |
| Family history of diabetes, % | 23 | 24 | 25 | 17 | 15 | 15 | 16 | 19 | 18 |
| Multivitamin use, % | 52 | 56 | 59 | 44 | 49 | 51 | 45 | 54 | 57 |
| Any use of postmenopausal hormone, % | 65 | 67 | 66 | 26 | 24 | 23 | |||
| Current use of oral contraceptive, % | 7 | 8 | 8 | ||||||
| Total energy, kJ | 7296 | 7464 | 6970 | 7464 | 7665 | 7267 | 8267 | 8455 | 7949 |
| AHEI | 46.0 | 45.8 | 47.2 | 43.2 | 44.6 | 47.2 | 46.8 | 47.0 | 50.2 |
| Trans fat, % energy | 1.54 | 1.64 | 1.63 | 1.59 | 1.61 | 1.51 | 1.36 | 1.45 | 1.34 |
| P/S ratio | 0.54 | 0.57 | 0.61 | 0.49 | 0.52 | 0.58 | 0.56 | 0.59 | 0.67 |
| Fruits, servings/day | 2.42 | 2.38 | 2.12 | 1.18 | 1.23 | 1.18 | 2.48 | 2.48 | 2.44 |
| Vegetables, servings/day | 3.37 | 3.24 | 2.92 | 3.31 | 3.28 | 3.09 | 3.30 | 3.24 | 3.11 |
| Red meat, servings/day | 0.96 | 0.88 | 0.70 | 0.90 | 0.79 | 0.55 | 1.26 | 1.10 | 0.74 |
| Folic acid, μg/day | 443 | 449 | 471 | 464 | 497 | 533 | 502 | 522 | 578 |
| Magnesium, mg/day | 305 | 309 | 321 | 313 | 325 | 341 | 359 | 368 | 396 |
| Carbohydrate, % energy | 47.6 | 50.0 | 52.9 | 48.6 | 50.3 | 53.9 | 45.6 | 48.8 | 53.4 |
| Glycaemic index | 51.7 | 52.8 | 53.9 | 53.1 | 53.6 | 54.6 | 52.1 | 53.1 | 54.3 |
| Glycaemic load | 98.3 | 105.1 | 113.8 | 117.1 | 121.6 | 132.9 | 118.9 | 129.5 | 144.7 |
| Cereal fibre, g/day | 3.42 | 4.98 | 7.07 | 3.89 | 5.82 | 8.48 | 4.16 | 6.42 | 9.81 |
| Bran, g/day | 3.69 | 5.20 | 7.59 | 4.13 | 5.72 | 8.18 | 4.64 | 7.06 | 10.81 |
| Starch, g/day | 53.8 | 65.9 | 78.7 | 65.5 | 82.1 | 101.9 | 65.0 | 80.4 | 98.5 |
| Germ, g/day | 0.68 | 0.82 | 1.10 | 0.91 | 1.11 | 1.33 | 0.936 | 1.17 | 1.77 |
Values are means or number (percentages) and are standardised to the age distribution of the study population
During 4.24 million years of follow-up, 15,947 incident cases of type 2 diabetes were confirmed. As shown in Table 2, gluten intake was inversely associated with type 2 diabetes risk in the age- and multivariate-adjusted model in all three cohorts. In the combined analyses, HRs (95% CIs) for type 2 diabetes from low to high gluten quintiles were: 1 (reference); 0.89 (0.85, 0.93); 0.83 (0.80, 0.88); 0.77 (0.73, 0.81); 0.78 (0.74, 0.82); with p for trend < 0.001. The results did not change with additional adjustment for AHEI, folic acid and magnesium, although further adjustment for cereal fibre attenuated the association, with pooled HRs (95% CIs) for type 2 diabetes from low to high gluten quintiles: 1 (reference); 0.91 (0.87, 0.96); 0.88 (0.83, 0.93); 0.83 (0.78, 0.88); 0.87 (0.81, 0.93); with p for trend < 0.001.
Table 2.
Associations between gluten intake and type 2 diabetes
| Cohort/model | Gluten (g/day) |
ptrend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| NHS | ||||||
| Median intake, g/day (range) | 2.61(0.01, 3.21) |
3.67(3.22, 4.07) | 4.47(4.08, 4.87) | 5.34(4.88, 5.91) | 6.80(5.92, 23.59) | |
| Cases/person-years | 1619/330,21 1 |
1570/330,322 | 1394/330,222 | 1290/330,782 | 1215/330,914 | |
| Model 1 | 1 | 0.98 (0.91, 1.05) | 0.88 (0.82, 0.94) | 0.81 (0.75, 0.87) | 0.76 (0.70, 0.82) | <0.001 |
| Model 2 | 1 | 0.95 (0.89, 1.02) | 0.87 (0.81, 0.93) | 0.83 (0.77, 0.89) | 0.83 (0.76, 0.89) | <0.001 |
| Model 3 | 1 | 0.95 (0.88, 1.02) | 0.86 (0.80, 0.93) | 0.83 (0.77, 0.89) | 0.83 (0.77, 0.90) | <0.001 |
| Model 4 | 1 | 0.97 (0.90, 1.05) | 0.90 (0.83, 0.98) | 0.87 (0.80, 0.95) | 0.90 (0.81, 0.99) | 0.008 |
| NHSII | ||||||
| Median intake, g/day (range) | 3.62(0.01, 4.34) |
4.89(4.35, 5.37) | 5.87(5.38, 6.38) | 6.97(6.39, 7.69) | 8.83(7.70, 27.03) | |
| Cases/person-years | 1679/357,12 5 |
1168/357,596 | 997/358,121 | 830/357,727 | 786/357,611 | |
| Model 1 | 1 | 0.71 (0.66, 0.77) | 0.62 (0.57, 0.67) | 0.52 (0.47, 0.56) | 0.49 (0.45, 0.53) | <0.001 |
| Model 2 | 1 | 0.86 (0.80, 0.93) | 0.82 (0.75, 0.89) | 0.73 (0.67, 0.80) | 0.74 (0.68, 0.81) | <0.001 |
| Model 3 | 1 | 0.87 (0.81, 0.94) | 0.84 (0.77, 0.91) | 0.75 (0.69, 0.82) | 0.77 (0.71, 0.84) | <0.001 |
| Model 4 | 1 | 0.89 (0.82, 0.97) | 0.88 (0.80, 0.96) | 0.81 (0.73, 0.89) | 0.84 (0.75, 0.94) | <0.001 |
| HPFS | ||||||
| Median intake, g/day (range) | 3.49(0.01, 4.30) |
4.93(4.31, 5.48) | 6.03(5.49, 6.60) | 7.25(6.61, 8.05) | 9.35(8.06, 33.46) | |
| Cases/person-years | 904/159,813 | 730/160,276 | 668/160,392 | 575/160,293 | 522/160,334 | |
| Model 1 | 1 | 0.82 (0.74, 0.90) | 0.76 (0.69, 0.84) | 0.65 (0.58, 0.72) | 0.59 (0.53, 0.66) | <0.001 |
| Model 2 | 1 | 0.86 (0.78, 0.95) | 0.84 (0.75, 0.93) | 0.76 (0.68, 0.84) | 0.76 (0.68, 0.85) | <0.001 |
| Model 3 | 1 | 0.86 (0.78, 0.95) | 0.84 (0.76, 0.93) | 0.77 (0.69, 0.85) | 0.79 (0.71, 0.88) | <0.001 |
| Model 4 | 1 | 0.87 (0.79, 0.97) | 0.87 (0.78, 0.98) | 0.82 (0.72, 0.93) | 0.88 (0.76, 1.02) | 0.05 |
| Pooled | ||||||
| Model 1 | 1 | 0.84 (0.80, 0.88) | 0.75 (0.71, 0.78) | 0.66 (0.62, 0.69) | 0.62 (0.59, 0.65) | <0.001 |
| Model 2 | 1 | 0.89 (0.85, 0.93) | 0.83 (0.80, 0.88) | 0.77 (0.73, 0.81) | 0.78 (0.74, 0.82) | <0.001 |
| Model 3 | 1 | 0.89 (0.85, 0.93) | 0.84 (0.80, 0.88) | 0.78 (0.74, 0.82) | 0.80 (0.76, 0.84) | <0.001 |
| Model 4 | 1 | 0.91 (0.87, 0.96) | 0.88 (0.83, 0.93) | 0.83 (0.78, 0.88) | 0.87 (0.81, 0.93) | <0.001 |
HRs and 95% CIs for the association between quintiles of gluten intake and type 2 diabetes were calculated using Cox proportional hazards models Model 1, adjusted for age
Model 2, further adjusted for ethnicity (white, African-American, Asian, and other ethnicity), family history of diabetes (yes/no), smoking status (never, former, current [1–14, 15–24, or ≥ 25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, and ≥ 15.0 in women, 0, 0.1–4.9, 5.0–29.9, and ≥ 30.0 in men, or missing), physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥ 27.0 METs, or missing), menopausal status and postmenopausal hormone use (pre-menopause, postmenopause [never, former, or current hormone use], or missing, for women), oral contraceptive use (yes, no, or missing, for NHSII), multivitamin use (yes/no), BMI (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, ≥ 35.0 kg/m2, or missing), and total energy intake based on model 1
Model 3, further adjusted for AHEI (in quintiles), folic acid intake (in quintiles) and magnesium intake (in quintiles), based on model 2
Model 4, further adjusted for cereal fibre intake based on model 3
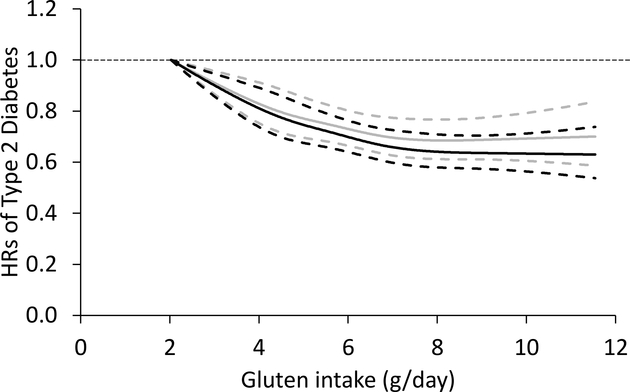
A dose–response analysis showed a monotonically lower risk of type 2 diabetes with higher gluten intake compared with the reference dose of 3.06 g/day (Fig. 2). When further adjusting for cereal fibre, the curve was flattened at higher gluten doses, but the test for a non-linear relationship was not significant (p for non-linearity = 0.09). The results were similar when gluten intake was modelled as percentage of energy (ESM Table 2). We also modelled gluten as percentage of plant protein, and found this ratio was associated with lower diabetes risk (data not shown).
Fig. 2.
Restricted cubic spline analysis of the association between gluten intake (g/day) and risk of type 2 diabetes. Adjusted for age, ethnicity (white, African-American, Asian and other ethnicity), family history of diabetes (yes/no), smoking status (never, former, current [1–14, 15–24, or ≥ 25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1– 4.9, 5.0–14.9, and ≥ 15.0 in women; 0, 0.1–4.9, 5.0–29.9, and ≥ 30.0 in men; or missing), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥ 27.0 METs, or missing), menopausal status and postmenopausal hormone use (pre-menopause, postmenopause [never, former, or current hormone use], or missing, for women), oral contraceptive use (yes, no, or missing, for NHSII), multivitamin use (yes/no), BMI (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, ≥ 35.0 kg/m2, or missing), total energy intake, AHEI, folic acid intake (in quintiles), and magnesium intake (in quintiles). Solid lines, point estimates; dashed lines, 95% CIs; black lines, estimates before further adjustment of cereal fibre; grey lines, estimates with further adjustment of cereal fibre
Gluten intake was significantly correlated with carbohydrate intake from various sources (ESM Table 3), although correlations were much stronger for refined grains, starch and cereal fibre (all Spearman correlations > 0.60, p<0.001). The association between gluten intake and type 2 diabetes tended to be stronger after controlling for refined grains (ESM Table 4), and HRs (95% CIs) from low to high gluten quintiles were: 1 (reference); 0.85 (0.81, 0.90); 0.78 (0.74, 0.83); 0.71 (0.67, 0.76); 0.71 (0.66, 0.76); with p for trend < 0.001. Alternatively, further adjusting for whole-grain intake attenuated the association between gluten and type 2 diabetes risk, and HRs (95% CIs) from low to high gluten quintiles were: 1 (reference); 0.90 (0.86, 0.95); 0.86 (0.82, 0.90); 0.80 (0.76, 0.84); 0.82 (0.78, 0.87); with p for trend < 0.001. The risk estimates did not materially change by further adjusting for glycaemic index and glycaemic load, or bran, germ and starch, or by modelling whole-grain- or refined-grain-adjusted gluten (ESM Table 4).
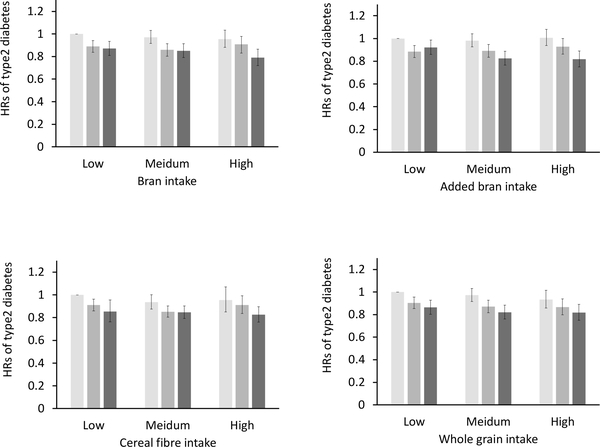
As shown in Fig. 3, a significant interaction between gluten intake and added bran was observed, with gluten intake more strongly associated with a lower type 2 diabetes risk with higher intake of added bran (p for interaction = 0.02). No significant interaction was observed for total bran, cereal fibre or whole-grain intake.
Fig. 3.
Joint analysis of gluten intake and other carbohydrate components on diabetes risk. HRs for type 2 diabetes by intake of: (a) bran; (b) added bran; (c) cereal fibre; and (d) whole grain. Adjusted for age, ethnicity (white, African-American, Asian, and other ethnicity), family history of diabetes (yes/no), smoking status (never, former, current [1– 14, 15–24, or ≥ 25 cigarettes/day], or missing), alcohol intake (g/day: 0, 0.1–4.9, 5.0–14.9, and ≥ 15.0 in women, 0, 0.1–4.9, 5.0–29.9 and ≥30.0 in men, or missing), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, ≥ 27.0 METs, or missing), menopausal status and postmenopausal hormone use (pre-menopause, postmenopause [never, former, or current hormone use], or missing, for women), oral contraceptive use (yes, no, or missing, for NHSII), multivitamin use (yes/no), BMI (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, ≥ 35.0 kg/m2, or missing), total energy intake, AHEI (in quintiles), folic acid intake (in quintiles), magnesium intake (in quintiles), and cereal fibre intake where appropriate. Statistical significance of effect modification (p for interaction) was evaluated by likelihood ratio test comparing models with and without interaction terms between quintiles of gluten and the effect modifiers. The p values for interaction were p=0.12 for bran, p=0.02 for added bran, p=0.32 for cereal fibre and p=0.97 for whole grain. Light grey bars, lowest tertile of stratification variable; dark grey, medium tertile; and black, highest tertile. Error bars show standard errors
In stratified analyses, inverse associations remained in all strata, but were stronger among participants with younger age (p for interaction = 0.002), with lower BMI (p for interaction < 0.001) and with higher physical activity (p for interaction = 0.03, ESM Table 5). In sensitivity analyses, the inverse association between gluten intake and type 2 diabetes risk remained after adjustment for individual dietary factors instead of AHEI or baseline BMI when using baseline gluten intake only in the analyses (ESM Table 6) or when participants with coeliac disease were excluded (ESM Table 7).
Discussion
In three large prospective cohort studies of US men and women, higher gluten intake was associated with a lower risk of type 2 diabetes during 20–28 years of follow-up. The associations were independent of major demographic, lifestyle and dietary factors, including cereal fibre intake. To the best of our knowledge, this is the first prospective cohort study investigating the association between gluten intake and long-term type 2 diabetes risk.
According to our study, gluten intake at the consumption level typically seen in western populations is not a risk factor for type 2 diabetes. Previously, two crossover intervention studies examined effects of high gluten dose (60–80 g/day) in hypercholesterolaemic participants (n=20–24). Both trials found that, over a month, gluten significantly reduced serum triacylglycerols, LDL-cholesterol and the oxidised LDL-cholesterol to LDL-cholesterol ratio, with no significant changes in urinary C-peptide levels [6, 11]. In another trial, body weight, blood pressure and blood lipids did not significantly change among 23 hypercholesterolaemic participants after consuming 30 g/day barley protein for a month [7]. Compared with a high carbohydrate diet (58% energy from carbohydrate and 16% energy from protein), a high-vegetable-protein diet (31% energy from protein, with 55% of protein as wheat protein) lowered LDL-cholesterol concentration, total cholesterol:HDL-cholesterol ratio and blood pressure among overweight hypercholesterolaemic men and women, but had no effect on body weight or insulin resistance [8]. Although these intervention studies were limited in sample size and study duration, the findings suggest that at least among individuals with dyslipidaemia, high-dose gluten intake does not exert significant adverse effects on cardiometabolic risk factors. These trials used much higher doses of gluten than typically consumed in general populations [9, 10, 25]. Our results extend these findings by demonstrating a monotonically inverse association with type 2 diabetes within the low-dose range of gluten intake.
Potential mechanisms linking gluten with type 2 diabetes are unknown. Among healthy participants, a gluten-free diet substantially decreased the abundance of beneficial bacteria (Bifidobacterium species, B. longum and Lactobacillus species) in the colon, increased potentially pathogenic bacteria (Escherichia coli and Enterobacteriaceae species) in the intestine, and suppressed the immune response of peripheral blood mononuclear cells to bacteria [26]. An early study found that intestinal absorption of bread carbohydrate was reduced by 10–20% when consumed with gluten [27], which may increase the availability of carbohydrate to the colonic microbiome for producing lactate and short-chain fatty acids [28]. Similarly, removing gluten from wheat products enhanced glycaemic index, starch digestion and starch absorption, and these were not restored after gluten was added back, suggesting that the starch–protein interaction is important in carbohydrate digestion and absorption [29]. This possibly explains the joint effects of wheat bran and gluten among 24 healthy participants whose serum triacylglycerol levels were reduced only when both ingredients were added to bread [6]. Consistent with this, we found a stronger inverse association between added bran and type 2 diabetes risk when gluten intake was higher, and vice versa. On the other hand, higher gluten intake was associated with higher levels of α2-macroglobulin, an inflammatory marker, among > 1,000 young participants without clinically diagnosed coeliac disease [25]. In vitro studies report that gliadin (a gluten protein) increases the permeability of the intestine and activates secretion of inflammatory cytokines in peripheral blood mononuclear cells from both people with coeliac disease and healthy control individuals, but the effects were much weaker in control individuals [30, 31]. Intervention studies failed to show that gluten intake of 100 g/day induced an acute tissue response and gastrointestinal malabsorption in healthy participants [5], nor any effects of 60–80 g/day gluten intake on C-reactive protein in people with hyperlipidaemia [7, 8].
Given the low prevalence of coeliac disease in the general population [32] and the relative obscurity of the gluten-free diet during the early follow-up period in these cohorts, the relatively lower gluten intake among our participants compared with Dutch and Danish men and women could be mainly explained by lower intake of foods containing gluten (e.g. barley and rye) among Americans [9, 10], instead of selective consumption of gluten-free foods with low fibre content. Nonetheless, a gluten-free diet is typically low in fibre and micronutrients including B vitamins, iron and calcium that are otherwise rich in wheat products [33–36]. Our participants with low gluten intake also had lower intake of other nutrients from grains (e.g. folic acid and magnesium) and lower overall diet quality (reflected by AHEI score), which may benefit long-term health [37–39]. Consistently, studies have suggested that individuals with coeliac disease have lower intake of dietary fibre and significant weight gain after following a gluten-free diet for 12–40 months [40–43]. More recent studies have observed improvement in the nutrient contents of gluten-free foods, although the overall nutritional profile of these foods is not superior to the ordinary sources of carbohydrates [44, 45].
The strengths of our study include large sample sizes, long follow-up duration with repeated measurement of exposure and extensive accounting for confounding factors. We used cumulative average intake of gluten to reflect long-term intake, thus minimising random variation and incorporating changes of diet over time. The validity of our gluten measurement is supported by validation studies showing that the food sources of gluten (such as pasta, bread, cold cereal, pie, cake, biscuits and beer) are assessed reasonably well when compared with diet records [20]. In addition, we found strong correlations of gluten intake with other components of grains, and results were consistent in three independent cohorts.
One major limitation is the possibility of residual or unmeasured confounding, which cannot be ruled out in observational studies. Second, participants are exclusively health professionals, and therefore the generalisability of findings to other populations is limited. In addition, the major dietary sources of gluten for Americans are wheat products; whether the same finding could be generalised to populations who primarily consume other dietary sources of gluten, such as rye, needs further investigation. Third, as the gluten content of food items was not given in the nutrient database, we estimated gluten intake primarily from ingredients containing wheat, rye or barley. We did not account for minor sources of gluten (e.g. trace amount of gluten in some brands of soy sauce) [2, 46], but the quantity of gluten in these sources is low and therefore relevant only when considering a strict gluten-free diet. Finally, we did not have an adequate number of participants with gluten intake close to zero for comparisons. Thus, we cannot exclude the possibility that the observed associations did not necessarily reflect the health effects of a strictly gluten-free diet.
In conclusion, we found an inverse association between gluten intake and type 2 diabetes risk, which was partly explained by high consumption of cereal fibre associated with gluten intake. Our findings suggest that gluten intake in the general population is unlikely to contribute to an elevated type 2 diabetes risk. Our observed inverse association between gluten intake and type 2 diabetes risk needs confirmation in further prospective studies and large intervention studies.
Data availability
The datasets generated during and/or analysed for the current study are not publicly accessible, but are available from the corresponding author on reasonable request.
Supplementary Material
Research in context
What is already known about this subject?
There has been growing public attention to the potential health impact of gluten intake
Gluten is the major storage protein in grains—primarily wheat, rye and barley—and triggers an autoimmune response in people with coeliac disease
What is the key question?
What is the risk of type 2 diabetes among people with low gluten intake?
What are the new findings?
In three prospective cohort studies of 199,794 US men and women, gluten intake (2–12 g/day) has been relatively stable over up to 28 years of follow-up
Compared with people in the lowest quintile of gluten intake, those in the highest quintile had a 13% lower risk of type 2 diabetes
How might this impact on clinical practice in the foreseeable future?
For most people who do not have coeliac disease or gluten sensitivity, limiting habitual gluten intake might not aid the prevention of type 2 diabetes
Acknowledgments
Funding This study was funded by research grants CA186107, CA176726, CA167552 and DK058845 from the National Institutes of Health (NIH). BL was supported by the American Gastroenterological Association Research Scholar Award. QS was supported by NIH grants ES021372, ES022981 and HL035464.
Abbreviations:
- AHE
Alternative Health Eating Index
- FFQ
Food frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- MET
Metabolic equivalent task
- NHS
Nurses’ Health Study
- NHSII
NHS II
- P/S ratio
Polyunsaturated : saturated fatty acid ratio
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
References
- [1].Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53: 947–958 [DOI] [PubMed] [Google Scholar]
- [2].Lebwohl B, Ludvigsson JF, Green PH (2015) Celiac disease and non-celiac gluten sensitivity. BMJ 351: h4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR (2013) No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145: 320–328 e321–323 [DOI] [PubMed] [Google Scholar]
- [4].Gaesser GA, Angadi SS (2015) Navigating the gluten-free boom. JAAPA 28. [DOI] [PubMed] [Google Scholar]
- [5].Levine RA, Briggs GW, Harding RS, Nolte LB (1966) Prolonged gluten administration in normal subjects. N Engl J Med 274: 1109–1114 [DOI] [PubMed] [Google Scholar]
- [6].Jenkins DJ, Kendall CW, Vuksan V, et al. (1999) Effect of wheat bran on serum lipids: influence of particle size and wheat protein. J Am Coll Nutr 18: 159–165 [DOI] [PubMed] [Google Scholar]
- [7].Jenkins DJ, Srichaikul K, Wong JM, et al. (2010) Supplemental barley protein and casein similarly affect serum lipids in hypercholesterolemic women and men. J Nutr 140: 1633–1637 [DOI] [PubMed] [Google Scholar]
- [8].Jenkins DJ, Wong JM, Kendall CW, et al. (2009) The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med 169: 1046–1054 [DOI] [PubMed] [Google Scholar]
- [9].Hoppe C, Gobel R, Kristensen M, et al. (2017) Intake and sources of gluten in 20- to 75-year-old Danish adults: a national dietary survey. Eur J Nutr 56:107–117. [DOI] [PubMed] [Google Scholar]
- [10].van Overbeek FM, Uil-Dieterman IG, Mol IW, Kohler-Brands L, Heymans HS, Mulder CJ (1997) The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol 9: 1097–1099 [DOI] [PubMed] [Google Scholar]
- [11].Jenkins DJ, Kendall CW, Vidgen E, et al. (2001) High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. The American journal of clinical nutrition 74: 57–63 [DOI] [PubMed] [Google Scholar]
- [12].van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB (2002) Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes care 25: 417–424 [DOI] [PubMed] [Google Scholar]
- [13].Willett W (1998) Chapter 13. Issues in analysis and presentation of dietary data. In: Nutritional Epidemiology, 2nd edn. Oxford University Press, New York, USA, pp 321–346 [Google Scholar]
- [14].Chiuve SE, Fung TT, Rimm EB, et al. (2012) Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142: 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition 65: 1220S–1228S [DOI] [PubMed] [Google Scholar]
- [16].Andren Aronsson C, Lee HS, Koletzko S, et al. (2016) Effects of Gluten Intake on Risk of Celiac Disease: A Case-Control Study on a Swedish Birth Cohort. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 14: 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kasarda DD (2013) Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J Agric Food Chem 61: 1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lebwohl B, Cao Y, Zong G, et al. (2017) Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ 357: j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA (2015) Practical insights into gluten-free diets. Nature reviews Gastroenterology & hepatology 12: 580–591 [DOI] [PubMed] [Google Scholar]
- [20].Salvini S, Hunter DJ, Sampson L, et al. (1989) Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology 18: 858–867 [DOI] [PubMed] [Google Scholar]
- [21].Yuan C, Spiegelman D, Rimm EB, et al. (2017) Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. American journal of epidemiology 185: 570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].(1979) Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 28: 1039–1057 [DOI] [PubMed] [Google Scholar]
- [23].Manson JE, Rimm EB, Stampfer MJ, et al. (1991) Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338: 774–778 [DOI] [PubMed] [Google Scholar]
- [24].Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB (2001) Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 161: 1542–1548 [DOI] [PubMed] [Google Scholar]
- [25].Jamnik J, Garcia-Bailo B, Borchers CH, El-Sohemy A (2015) Gluten Intake Is Positively Associated with Plasma alpha2-Macroglobulin in Young Adults. J Nutr 145: 1256–1262 [DOI] [PubMed] [Google Scholar]
- [26].Sanz Y (2010) Effects of a gluten-free diet on gut microbiota and immune function in healthy adult humans. Gut Microbes 1: 135–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anderson IH, Levine AS, Levitt MD (1981) Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med 304: 891–892 [DOI] [PubMed] [Google Scholar]
- [28].Jenkins DJ, Taylor RH, Wolever TM (1982) The diabetic diet, dietary carbohydrate and differences in digestibility. Diabetologia 23: 477–484 [DOI] [PubMed] [Google Scholar]
- [29].Jenkins DJ, Thorne MJ, Wolever TM, Jenkins AL, Rao AV, Thompson LU (1987) The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion. The American journal of clinical nutrition 45: 946–951 [DOI] [PubMed] [Google Scholar]
- [30].Lammers KM, Khandelwal S, Chaudhry F, et al. (2011) Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 132: 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Drago S, El Asmar R, Di Pierro M, et al. (2006) Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 41: 408–419 [DOI] [PubMed] [Google Scholar]
- [32].Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE (2012) The prevalence of celiac disease in the United States. Am J Gastroenterol 107: 1538–1544 [DOI] [PubMed] [Google Scholar]
- [33].Thompson T (2000) Folate, iron, and dietary fiber contents of the gluten-free diet. Journal of the American Dietetic Association 100: 1389–1396 [DOI] [PubMed] [Google Scholar]
- [34].Thompson T (1999) Thiamin, riboflavin, and niacin contents of the gluten-free diet: is there cause for concern? Journal of the American Dietetic Association 99: 858–862 [DOI] [PubMed] [Google Scholar]
- [35].Thompson T, Dennis M, Higgins LA, Lee AR, Sharrett MK (2005) Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet 18: 163–169 [DOI] [PubMed] [Google Scholar]
- [36].Hallert C, Grant C, Grehn S, et al. (2002) Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Alimentary pharmacology & therapeutics 16: 1333–1339 [DOI] [PubMed] [Google Scholar]
- [37].Eichholzer M, Tonz O, Zimmermann R (2006) Folic acid: a public-health challenge. Lancet 367: 1352–1361 [DOI] [PubMed] [Google Scholar]
- [38].Volpe SL (2013) Magnesium in disease prevention and overall health. Adv Nutr 4: 378S–383S [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schwingshackl L, Hoffmann G (2015) Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 115: 780–800 [DOI] [PubMed] [Google Scholar]
- [40].Cheng J, Brar PS, Lee AR, Green PH (2010) Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol 44: 267–271 [DOI] [PubMed] [Google Scholar]
- [41].Dickey W, Kearney N (2006) Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol 101: 2356–2359 [DOI] [PubMed] [Google Scholar]
- [42].Valletta E, Fornaro M, Cipolli M, Conte S, Bissolo F, Danchielli C (2010) Celiac disease and obesity: need for nutritional follow-up after diagnosis. Eur J Clin Nutr 64: 1371–1372 [DOI] [PubMed] [Google Scholar]
- [43].Kabbani TA, Goldberg A, Kelly CP, et al. (2012) Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Alimentary pharmacology & therapeutics 35: 723–729 [DOI] [PubMed] [Google Scholar]
- [44].Wu JH, Neal B, Trevena H, et al. (2015) Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br J Nutr 114: 448–454 [DOI] [PubMed] [Google Scholar]
- [45].Miranda J, Lasa A, Bustamante MA, Churruca I, Simon E (2014) Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum Nutr 69: 182–187 [DOI] [PubMed] [Google Scholar]
- [46].Kobayashi M, Hashimoto Y, Taniuchi S, Tanabe S (2004) Degradation of wheat allergen in Japanese soy sauce. Int J Mol Med 13: 821–827 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed for the current study are not publicly accessible, but are available from the corresponding author on reasonable request.