Abstract
The idea that astrocytes provide support for neurons has a long history, but whether neurons play an instructive role in these processes is poorly understood. To address this question, we co-culture astrocytes with genetically labeled neurons, permitting their separation by flow cytometry, and test whether the presence of neurons influences the astrocyte transcriptome. We find that numerous pathways are regulated in the co-cultured astrocytes, in a time-dependent matter coincident with synaptic maturation. In particular, the induction of glutathione metabolic genes is prominent, resulting in increased glutathione production. We show that the induction of the glutathione pathway is mediated by astrocytic metabotropic glutamate receptors. Using a candidate approach, we identify direct binding of the nuclear factor E2-related factor, NRF2, to several of the induced genes. Blocking nuclear accumulation of astrocytic NRF2 abolishes neuron-induced glutathione gene induction and glutathione production. Our results suggest that astrocyte transcriptional and metabolic profiles are tightly coupled to the activity of neurons, consistent with the model that astrocytes dynamically support healthy brain function.
Keywords: astrocyte, neuron, co-culture, RNA-seq, glutathione, NRF2
Table of Contents
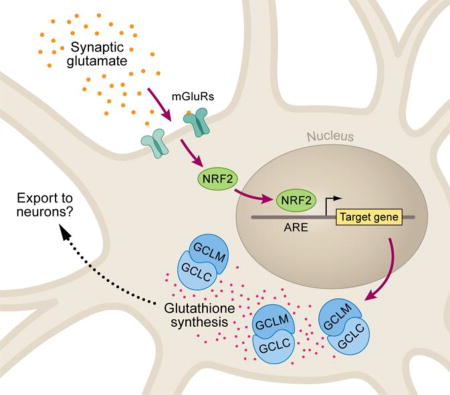
In astrocyte-neuronal co-cultures, neuronal synaptic activity induces astrocytic expression of glutathione pathway genes encoding metabolic enzymes. The transcriptional inductions and downstream glutathione production are dependent on astrocytic mGluR signaling and involve NRF2 transcription factor binding.
Introduction
Our understanding of the contribution of astrocytes to neuronal development has advanced rapidly, including the impact of astrocytes on dendritic morphology, synapse formation, and activity-dependent spine pruning (Chung, Allen, & Eroglu, 2015; Molofsky et al., 2012; Ullian, Christopherson, & Barres, 2004). Several astrocyte-derived molecules have been identified as permissive and/or instructive to synaptogenesis, including Hevin (Sparcl1), Thrombospondins, and Sparc (Christopherson et al., 2005; Kucukdereli et al., 2011). However, whether these molecules are transcriptionally regulated in response to neuronal signals remains an open question.
There is precedent for the idea that specific signaling, identity, and structural pathways are indeed induced in astrocytes by neurons. For example, medium spiny neurons in the striatum selectively activated astrocyte calcium signaling depending on their dopamine receptor subtype (Martin, Bajo-Graneras, Moratalla, Perea, & Araque, 2015). Further, certain glutamate transporter (Swanson et al., 1997) and connexin (Koulakoff, Ezan, & Giaume, 2008) genes were induced in astrocytes in neuronal co-cultures. It is not clear whether these particular neuronal gene inductions in astrocytes were isolated events or represented more global transcriptional changes in astrocytes, but the broad effects of neuronal sonic hedgehog signaling on Bergmann glia function in the cerebellum suggested the latter might be the case (Farmer et al., 2016), and a recent culture study provided more support for such broad transcriptional changes (Hasel et al, 2017).
We sought to identify neuron-induced transcriptional responses in astrocytes during a window of synapse maturation. To better control the cellular environment in which the astrocytes were studied, we performed the experiments in vitro, isolating the astrocyte-neuron interaction from other cell types such as oligodendrocytes, microglia, endothelia, and neuronal progenitors. Additionally, the in vitro co-culture system was amenable to molecular analyses at different time points during neuronal maturation and in response to pharmacological manipulation. An efficient separation of co-cultured astrocytes and neurons was required for downstream analyses. We addressed this issue by culturing wild-type astrocytes with neurons from TdTomato-expressing mice followed by flow cytometry. Our co-culture system resulted in a highly enriched astrocyte population suitable for transcriptomic analyses. These analyses highlighted a metabolic pathway induced in astrocytes, in part by the transcription factor NRF2, only in the presence of mature, synaptically active neurons.
Materials and methods
Astrocyte and neuronal cultures
Care and handling of mice was in accordance with institutional IACUC guidelines. Cortical astrocytes and hippocampal neurons were each cultured according to the Banker protocol (Kaech & Banker, 2006), except tissue was dissociated with papain according to (Brewer & Torricelli, 2007) and neurons were plated directly on to astrocytes rather than on to glass coverslips. Astrocytes were first plated in astrocyte growth media (AGM), consisting of DMEM (ThermoFisher) with 10% fetal bovine serum and 2mM glutamax (ThermoFisher) on 10cm or 6-well plates (ThermoFisher) or glass chamber slides (Lab Tek). For imaging, glass slides were treated with poly-l-lysine for >2h at 37°C before plating cells. When confluent, cells were switched to N2 media (1% N2 supplement (ThermoFisher) in MEM (ThermoFisher) with 1mM sodium pyruvate and 1uM Ara-C (Sigma) for drug incubations and/or neuronal co-culture. The percent of astrocytes and neurons in cultures was determined by GFAP and NeuN staining, respectively. Neuronal contamination with this procedure was negligible (<0.1% in all cultures). Mice constitutively expressing TdTomato were generated by crossing Hprttm1(CAG-cre)Mnn mice (Jackson Laboratory, RRID:IMSR_JAX:004302) to Gt(ROSA)26Sortm9(CAG-tdTomato)Hze reporter mice (Jackson Laboratory, RRID:IMSR_JAX:007905) to remove the stop cassette. Offspring were then backcrossed to C57Bl6 (Jackson Laboratory, RRID:JAX:000664) for >6 generations, and hippocampal neurons were isolated from heterozygous P0 pups. Single-cell suspensions were prepared as above. Dissociated neurons were plated onto astrocytes at defined density (Very Low Density, 125/mm2 or 1×10^6 onto a 10cm plate). Replacement N2 media with AraC was added to the co-cultures after 24h to inhibit proliferation of non-neuronal cells. For collection of neuronal conditioned media (NCM), hippocampal neurons were plated on poly-l-lysine-treated plates at high density (1000/mm2 or 2.1×10^6 onto a 6cm plate) and grown in Neuronal Growth Media (2% B27 Supplement (ThermoFisher), 2mM Glutamax in Neurobasal-A, 1uM Ara-C). Cultures were stained for GFAP and NeuN and were >90% neuronal. Media was exchanged every other day, and media used for NCM was from DIV 7–10, and filtered through .22uM filter (Millex). Control media was NGM from same stock.
Fluorescence Activated Cell Sorting
Naïve astrocytes (not cultured with neurons) and neuronal-astrocyte co-cultures were each trypsinized and centrifuged five minutes at 500 × g. After removing supernatant, cells were resuspended in DPBS without Calcium or Magnesium (ThermoFisher). RFP− FACS selection windows were set to capture all of the naïve astrocytes (Supplementary Figure 1), while RFP+ windows were set to have at least 5× higher TdTomato expression than the highest naïve astrocytes. Cells were sorted into DPBS and transferred immediately to TRIzol (Invitrogen) for RNA extraction.
RNA-seq library construction and analysis
Total RNA was extracted from cells with Trizol. Ribosomal RNA was removed using the Ribogone kit (Clontech). The SMARTer Universal Low Input RNA Kit for Sequencing and the SMARTer Low Input Library Prep Kit (Clontech) were used to synthesize cDNA and construct indexed Illumina libraries. The libraries were sequenced on a HiSeq 2000 machine at Oregon State University. The reads were split into individual samples based on the Illumina index. Reads were then aligned to the mm9 mouse genome and transcript-level and gene-level expression tallied in R using Subread with default output parameters, including normalizing counts in each sample by the library size. (Liao, Smyth, & Shi, 2013)(RRID:SCR_009803). Differential expression was analyzed using EdgeR (D. J. McCarthy, Chen, & Smyth, 2012)(RRID:SCR_012802). Significant differential expression was defined as genes with greater than 1.6-fold expression differences between co-culture and naïve astrocytes, with adjusted p-values less than .05 by both tagwise and common dispersion estimates. Gene Ontology analysis was performed with AMIGO (Carbon et al., 2009)(RRID:SCR_002143) and DAVID (D. W. Huang et al., 2007)(RRID:SCR_003033). Gene set enrichment analysis (Wang & Cairns, 2014)(RRID:SCR_003199) was conducted in R using the Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations (Kanehisa & Goto, 2000)(RRID:SCR_001120). Raw read files and gene-level expression data may be found in the Gene Expression Omnibus under GSE69731.
qRT-PCR
Total RNA after TRIzol purification was converted to cDNA with the Superscript III First Strand Kit (Invitrogen). Real time qPCR reactions were analyzed using SYBR Select master mix (Applied Biosystems) on a QuantStudio6 machine (Applied Biosystems). Three technical replicates were analyzed for each sample and primer set, and all experiments contained at least three biological replicates. Expression for every primer set was normalized to the geometric mean of four housekeeping gene primers (Aip, Cxxc1, Hprt, Srp14). One-way t-test was used to evaluate fold expression in co-cultured astrocytes relative to naïve astrocytes. Comparisons between co-cultured astrocytes treated with different drugs were analyzed by student’s t-test or Kruskal-Wallis one way analysis of variance (ANOVA), depending on the number of treatments, and corrected for multiple comparisons by an adjusted Bonferroni correction. The Bonferroni correction was adjusted for the correlation between the tested genes using gene expression counts between naïve astrocytes and co-cultured astrocytes from RNAseq data, which gives an r2 value of 0.99. Therefore we used the corrected p < 0.048 threshold for significance throughout the manuscript. Primers used for all experiments are listed in Supplementary Table 2.
Fluorescence staining and imaging
For Thioltracker staining, cells were incubated according to manufacturer’s instructions (20uM for 30m in N2 media), washed with DPBS, and then fixed with 4% PFA in PBS for seven minutes. This fixation protocol was also used for immunofluorescence. Cells were permeablized with 0.1% TritonX-100 in PBS and blocked for 30m with 10% Normal Goat Serum (ThermoFisher) in PBS. Primary antibodies were incubated overnight at 4°C. Primary antibodies used were rabbit anti-GFAP (Z0334, Dako, RRID:AB_10013382), rabbit anti-GCLM (ab81445, Abcam, RRID:AB_1860504), rabbit anti-NRF2 (D1Z9C, Cell Signaling, RRID:AB_2715528), Alexa Fluor 488-conjugated NeuN (Millipore, MAB377X, RRID:AB_2149209), mouse anti-GFAP (ab4648, Abcam, RRID:AB_449329), rabbit anti-Synaptophysin (ab32594, Abcam, RRID:AB_778204), and guinea pig anti-Homer (#160004, Synaptic Systems, RRID:AB_10549720). Secondary antibodies were incubated for 1h at room temperature. Secondary antibodies used were Alexa Fluor 633 goat anti-rabbit (A21070, Thermo Fisher, RRID:AB_2535731), Alexa Fluor 488 goat anti-rabbit (A11034, Thermo Fisher, RRID:AB_2576217), Alexa Fluor donkey anti-mouse (A21202, Thermo Fisher, RRID:AB_141607), and Alex Fluor goat anti-guinea pig 647 (A21450, Thermo Fisher, RRID:AB_141882). Confocal images were acquired with a LD Plan NEOFLUAR 20× objective (Zeiss, 421350-9970) on a LSM710 (Zeiss) using identical conditions for all samples within a single experiment. Image analysis was done with CellProfiler (Carpenter et al., 2006)(RRID:SCR_007358), scripts provided by request. For determining NRF2 nuclear:cytoplasm ratios, because of the low constitutive expression levels of NRF2, we elected to use a method independent of choosing regions of interest, but which still controlled for differences in cell size. In our unbiased analysis, nuclear domains were identified with the nuclear marker Draq5 (ThermoFisher 62251). Cell boundaries were determined based on a threshold of GFAP immunofluorescence using the Global Otsu propagation algorithm from the Cell Profiler ‘Identify Secondary Objects’ module. Cytoplasm was defined as the total cell area minus the nuclear domain. All threshold parameters were kept constant across conditions. The mean pixel intensities (integrated pixel intensity divided by area) in nucleus and cytoplasm were calculated on a per-cell basis, and these pixel ratios were used for statistical analysis with one-way ANOVA in Prism5 (Graphpad Software, Inc., RRID:SCR_002798). For Thioltracker and GCLM analysis, each cell’s mean pixel intensity in a particular culture was normalized to the population mean for the parallel untreated naïve astrocyte culture. The normalized intensities were used for statistical analysis with student’s t-test or one-way ANOVA, depending on the number of comparisons, in Prism5.
Chromatin Immunoprecipitation
Chromatin preparation and immunoprecipitation was performed as described (Ballas et al., 2001) with modifications as follows: After formaldehyde-fixed cells were collected, they were resuspended in HB buffer (0.25M Sucrose, 25mM KCl, 5mM MgCl2, 20mM Tricine-KOH, pH 7.8) and homogenized ten times with a Dounce homogenizer. IGEPAL CA-630 was added to 0.3% and homogenized another ten times. Following centrifugation at 4500 × g for 5m, nuclei were resuspended in L3 buffer (10mM Tris-HCl pH 8.0, 1mM EDTA, 1mM EGTA, 0.15% SDS) and sheared with a Covaris S220 focused ultrasonicator (200 cycles/burst, 5% duty cycle, power level 4, 12m). NRF2 immunoprecipitation was performed overnight at 4°C with anti-NRF2 antibody (D1Z9C, Cell Signaling) and attached to protein-G Dynabeads (ThermoFisher) at 4°C for 2h. Bead washes and DNA purification was performed as described in Ballas et al., 2001. qPCRs were run as above. A one-way t-test was used to evaluate NRF2 binding in co-cultured astrocytes relative to naïve astrocytes, while comparison between co-cultured astrocytes and trigonelline-treated astrocytes was analyzed by student’s t-test.
Western Blot
Western blot for GCLM (rabbit anti-GCLM ab81445, Abcam, RRID:AB_1860504) and alpha-tubulin (DSHB # AA4.3, RRID:AB_579793) was performed using conditions recommended by the manufacturer. Protein lysates were collected in RIPA buffer with protease inhibitors (Roche #11873580001) and separated on 4–12% NuPage Bis-Tris gels (NP0335) in MOPS buffer according to manufacturer’s directions and transferred to nitrocellulose. Membranes were blocked 1h with 2% milk in PBS + 0.1% Tween-20 (Ptween), and incubated with primary antibodies overnight at 4°C. Membranes were washed 3× 5m with Ptween and secondary antibodies (goat anti-rabbit IgG-IR800 and goat anti-mouse IgG-IR680, Thermofisher 35571 and 35518, RRID:AB_614947, RRID:AB_614942) incubated at room temperature for 1h. Blots were washed again and visualized and quantified with Odyssey CLx imager (LI-COR, RRID:SCR_014579).
Drug incubations
Drugs were added to media after 24h of co-culture unless otherwise noted. TTX (Alamone T-550) was used at a final concentration of 1uM, CNQX (Tocris 0190) at 5uM, LY341495 (Tocris 1209) at 1uM, MPEP (Tocris 1212) at 20uM, (S)-3,5-DHPG (Tocris 0805) at 10mM, Glutamate (Fluka 49621) at 10mM, and Trigonelline (Sigma T5509) at 75uM. Gene expression and ChIP comparisons in drug-treated co-culture samples were always compared to drug-treated naïve astrocytes.
Results
Astrocytes co-cultured with TdTomato-labeled neurons can be purified for transcriptome analysis by fluorescence-activated cell sorting (FACS)
To capture both contact-mediated and secreted events in our experimental design, we modified an indirect contact co-culture system of astrocytes and neurons published previously (Kaech & Banker, 2006). To prepare the astrocytes, dissociated cortices of wild-type mice (P0-P1) were cultured in serum-containing media for three passages, which resulted in cultures highly enriched for astrocytes (>95%, data not shown) and (Ballas, Lioy, Grunseich, & Mandel, 2009) and with a near-complete absence of neurons (<0.1%, according to (K. D. McCarthy & de Vellis, 1980), hereafter called naïve astrocytes. Separately, dissociated neurons were isolated from hippocampi of P0 mice constitutively expressing the TdTomato red fluorescent protein (RFP). For the co-cultures, the hippocampal neurons were plated directly onto astrocytes, rather than onto glass coverslips. Both naïve astrocytes and astrocyte-neuron co-cultures were then maintained in minimal media for up to seven days in culture. During this time, we observed increasing length and complexity of dendritic arborization for the neurons in co-culture (Fig. 1A).
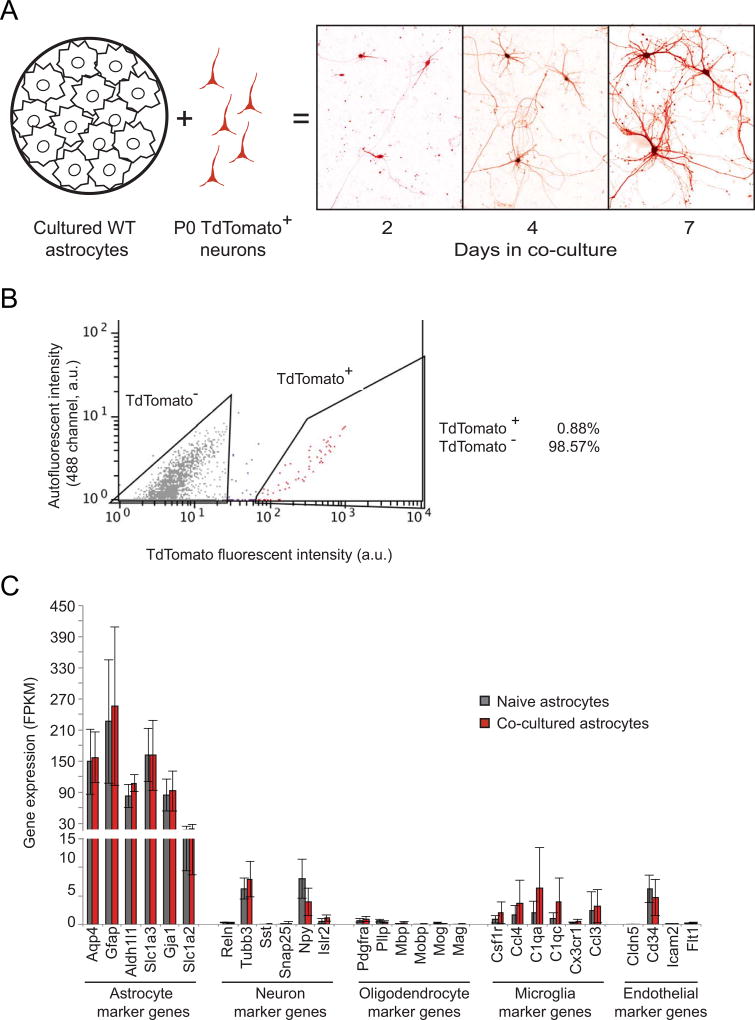
Figure 1. Fluorescence-activated cell sorting (FACS) of astrocytes co-cultured with TdTomato-labeled neurons enriches for astrocyte-specific transcripts.
A) Schematic of co-culture preparation and neuronal morphology changes in culture. Neurons isolated from hippocampi of P0 pups constitutively expressing a TdTomato transgene were cultured for up to seven days after plating on top of P0-isolated cortical wild-type astrocytes cultured for three passages. B) Representative FACS results of a astrocyte-neuron co-culture. Intensity of TdTomato fluorescence (561nm) is on the x-axis, autofluorescence (488nm) is on the y axis. Cells were sorted into TdTomato− (grey) and TdTomato+ (red) bins based on gates shown. C) Gene expression levels (FPKM) for genes enriched in the brain cell types indicated. Averages include all time points (n=9), error bars represent the 95% confidence intervals. No statistically significant differences are observed in these genes between naïve astrocytes (grey) and co-cultured astrocytes (red).
FACS of wild-type naïve cultures resulted in high levels of expression of astrocyte-specific marker genes in the RFP-negative pool, as expected (Fig. 1B, gray bars, and Supplementary Fig. 1). Combined with the relatively low expression levels of neuronal, oligodendrocyte, microglia, and endothelial identifier genes (as identified by (Zhang et al., 2014)), our results are in alignment with previously published astrocyte transcriptomes (Cahoy et al., 2008; Zhang et al., 2014), Supplementary Fig. 2). We therefore refer to the isolated RFP− population hereafter as astrocytes. We utilized the same FACS gating criteria for the astrocyte-neuron co-cultures as those for naïve astrocytes (Supplementary Fig. 1). Importantly, despite the addition of neurons into the co-culture to ~10% of the total cell number, we observed no statistically significant enrichment in neuronal marker RNA in these samples relative to naïve astrocytes (Fig. 1B and Supplementary Fig. 2). Taken together, these data indicate the high specificity of astrocyte isolation achieved by our co-culture experimental design and FACS sorting.
RNA-seq results reveal astrocytic genes and pathways that are differentially regulated during neuronal maturation in co-culture
To identify genes differentially expressed between naïve astrocytes and astrocytes in neuronal co-culture during neuronal maturation, we performed RNA-seq analysis at three developmental time points (two, four, and seven days of co-culture, n=3 replicates for each time point). We identified a total of 1122 genes that were differentially expressed relative to naïve astrocytes, in at least one of the three time points (Fig. 2A). Of the differentially expressed genes, only 88 and 11 were down- and up-regulated, respectively, at all three time points. In addition, among differentially expressed genes, there was a slight bias towards down-regulation at two days and four days of development (57.6% and 54.9% of genes down-regulated compared to naïve astrocytes, respectively). In contrast, we found a stronger bias at seven days in co-culture (69.9% of genes down-regulated compared to naïve astrocytes).
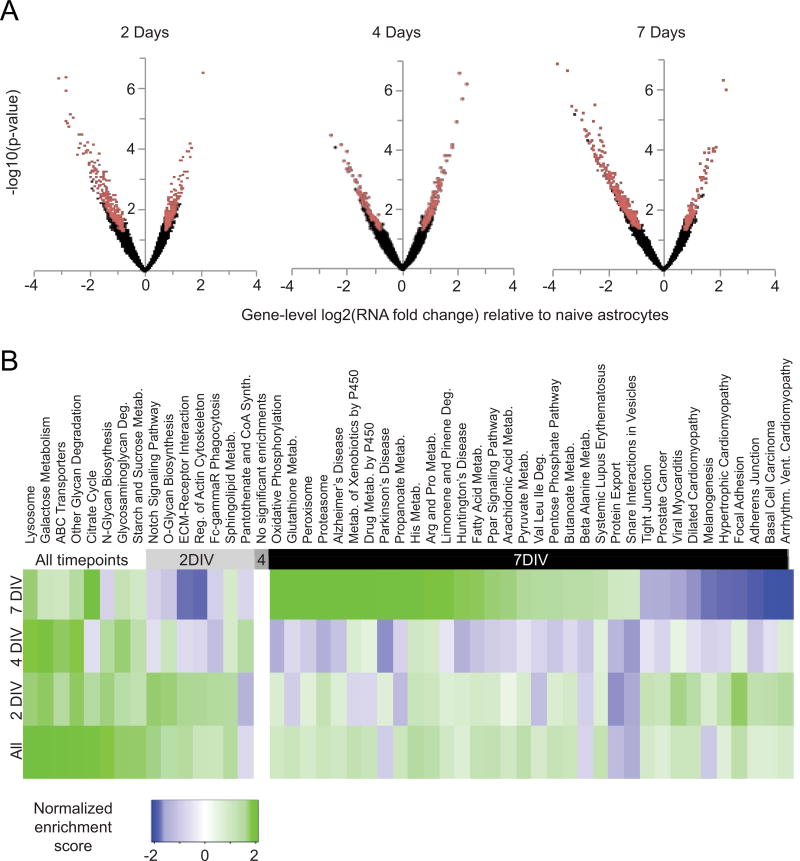
Figure 2. Co-culture of astrocytes with neurons results in transcriptome changes in astrocytes during neuronal maturation.
A) Volcano plots of RNA-seq analysis showing all genes expressed in astrocytes with increasing days in co-culture. The x-axis shows the magnitude of change (astrocytes co-cultured with neurons / naïve astrocytes). The y-axis shows significance values; a false discovery rate (FDR)-adjusted P value of .001 is 3 on this scale. Red dots are genes differentially expressed between co-cultured and naïve astrocytes, defined as those with greater than 1.6-fold expression differences and adjusted p-values less than .05 by both tagwise and common dispersion estimates. Black dots are genes not classified as differentially expressed. B) Gene Set Enrichment Analysis (GSEA) results showing biological pathways whose genes are coordinately up- or down-regulated. Pathways are defined according to the annotated KEGG database (Kanehisa & Goto, 2000). The normalized enrichment score is higher when pathway genes are more differentially expressed between naïve astrocytes and astrocytes in neuronal co-culture. Therefore, green boxes represent pathways up-regulated in astrocytes in neuronal co-culture, while blue boxes represent pathways down-regulated in neuronal co-culture. Only significant results (FDR< .01) are shown.
Although individual gene expression differences between naïve and co-cultured astrocytes were significant, standard Gene Ontology (GO) enrichment analysis did not reveal any clusters of genes significantly enriched in particular molecular functions or biological processes. Therefore, to broaden our analysis to include even subtle gene expression changes, we further analyzed the entire expression dataset using Gene Set Enrichment Analysis (GSEA) with regards to the KEGG molecular pathways annotation (Fig. 2B). In this analysis, all expressed genes were first ranked with respect to their expression change between naïve and co-cultured astrocytes. The members of a particular pathway defined by the KEGG annotation were then identified and, according to their rank, an enrichment score was calculated (top of the list = high enrichment score). The sum of the enrichment scores for a given pathway was then compared to a random distribution, and after correcting for multiple comparisons, a normalized enrichment score for each pathway was computed. Using this analysis, we found, for example, that co-cultured astrocytes up-regulated lysosomal genes, as well as those for glycan degradation and biosynthesis (represented by green-shaded boxes in the All time points category, Fig. 2B). When we separated the data as a function of time in culture, we identified pathways that were differentially regulated only early in development (e.g. Notch signaling is up-regulated after two days co-culture). At the four-day time point, no pathways reached significance, for reasons we have not yet determined. However, at seven days of co-culture, many pathways were both significantly up- and down-regulated. Nearly half (11/24) of these enriched pathways were metabolic, and we chose to focus on this group in particular (Fig. 2B).
Glutathione production is increased in astrocytes exposed to neurons
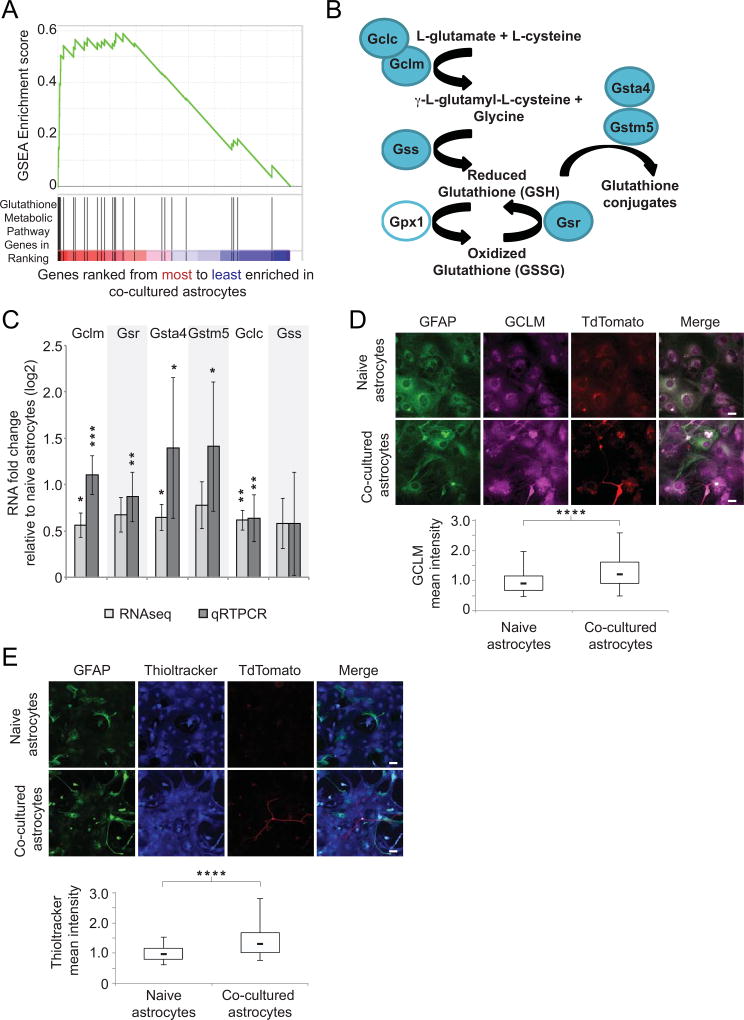
Among the metabolic pathways most highly enriched for induction in astrocytes after seven days of neuronal co-culture was the pathway related to glutathione metabolism. Glutathione is an important antioxidant that protects cells against toxins and oxidative stress. The majority of the genes in this pathway clustered at the very top of the list of genes differentially expressed between co-cultured astrocytes and naïve astrocytes (black vertical lines in the red shading in Fig. 3A), resulting in a highly significant enrichment score according to GSEA (green line, Fig. 3A). The first dedicated members in the pathway, glutamate-cysteine ligase catalytic subunit (Gclc) and glutamate-cysteine ligase modifier subunit (Gclm), form a complex to produce the intermediate dipeptide gamma-L-glutamyl-L-cysteine (Fig. 3B). It is the expression levels and activity of the regulatory subunit Gclm that determine the amount of glutathione synthesized by the downstream enzyme glutathione synthetase (Gss, Fig. 3B, (C. S. Huang, Anderson, & Meister, 1993). When ranking all ~9000 astrocyte-expressed genes by their induction after seven days co-culture, Gclm ranked 60th and Gclc ranked 77th (Figure 3A). These two critical pathway genes were up-regulated 2.1- and 1.6-fold, respectively, in independent samples of astrocytes in neuronal co-culture relative to naïve astrocytes (Fig. 3C, n=3, p < 0.01). In addition to these biosynthetic genes, several other glutathione pathway genes (shown in Fig. 3B) showed similar up-regulation in co-cultured astrocytes, including glutathione reductase (Gsr), which recovers oxidized glutathione, and the glutathione s-transferases alpha-4 (Gsta4) and mu-5 (Gstm5), which conjugate glutathione to potentially toxic electrophilic compounds. Subsequent qRT-PCR verified the up-regulation of these genes in independent samples (Fig. 3C), as well as changes in expression of other genes identified by our RNA-seq data (Supplementary Fig. 3, Supplementary Table 1). Notably, none of the transcriptional changes resulted in changes in the morphology of astrocytes in co-culture compared with naïve astrocytes, as assessed by GFAP staining (Fig. 3D, 3E, and data not shown). Following the observation of transcriptional induction of glutathione production enzymes, we measured whether the protein level of the master regulator of glutathione metabolism, GCLM, was also up-regulated by neuronal co-culture. After seven days of co-culture, astrocytes showed a 34% increase in levels of cellular GCLM relative to naïve astrocytes by immunofluorescence comparing population medians (Fig. 3D, n=5, p < 0.0001). In addition, we analyzed cultures by western blotting and observed a 57% increase in GCLM levels in astrocyte-neuron co-cultures relative to naïve astrocytes (when normalized to alpha-tubulin loading control, Supplementary Fig. 4, n=3, p < 0.05).
Figure 3. Glutathione metabolic genes and glutathione production in astrocytes are up-regulated in neuronal co-culture.
A) Gene Set Enrichment Analysis (GSEA) showing KEGG-defined glutathione metabolic genes in co-cultured astrocytes relative to naïve astrocytes after seven days in culture. The heat map shows the ranking of genes according to their expression difference between astrocytes in co-culture with neurons relative to naïve astrocytes. Black vertical lines indicate rank location of glutathione metabolism genes annotated by KEGG. Green line designates the running sum of the calculation for the total enrichment score. Positive enrichment scores indicate enrichment at the top of the ranked list. B) Schematic of proteins in the glutathione synthesis pathway. Enzymes with blue fill were identified as up-regulated in astrocytes in response to neuronal co-culture. C) qRT-PCR results for glutathione pathway genes from three independent biological replicates after neuronal co-culture confirm induction observed in RNA-seq data. Alternative white and light gray shaded regions align gene names with each result. This designation applies to all following figures. * p < 0.05, ** p < 0.01, *** p < 0.001 relative to naïve astrocytes using one-way t-test. Error bars show 95% confidence intervals. D) Immunofluorescence results for GCLM protein in naïve astrocytes and astrocytes co-cultured with neurons. Top, representative images, with indicated agents, scale bar, 20 µm. Bottom, box plots for cells measuring mean-normalized GCLM pixel intensities. Results shown are averages of three replicates. Upper bar is 95th percentile, and lower bar is 5th percentile. The top of each box is the 75th percentile, while the bottom is the 25th percentile. The bar within each box represents the median. **** p < 0.0001 relative to naïve astrocytes using student’s t-test. E) Immunofluorescence results using Thioltracker as a measure of glutathione levels in naïve astrocytes and astrocytes co-cultured with neurons. At top are representative images, with indicated agents, scale bar, 40 µm. Bottom, box plots as in D for cells measuring mean-normalized Thioltracker pixel intensities, n=5. **** p < 0.0001 relative to naïve astrocytes using student’s t-test.
To determine whether the increases in astrocyte glutathione production pathway mRNA and protein after neuronal co-culture were reflected in increased glutathione, we utilized the glutathione-specific fluorescent dye Thioltracker (Mandavilli & Janes, 2010). After seven days, astrocytes in co-culture showed a 35% increase, relative to naïve astrocytes, in levels of cellular glutathione (when comparing population medians, Fig. 3E, n=5, p < 0.0001), indicating that the gene expression changes we identified affected the metabolic output of the astrocytes.
The up-regulation of glutathione metabolic pathway genes requires neuronal activity
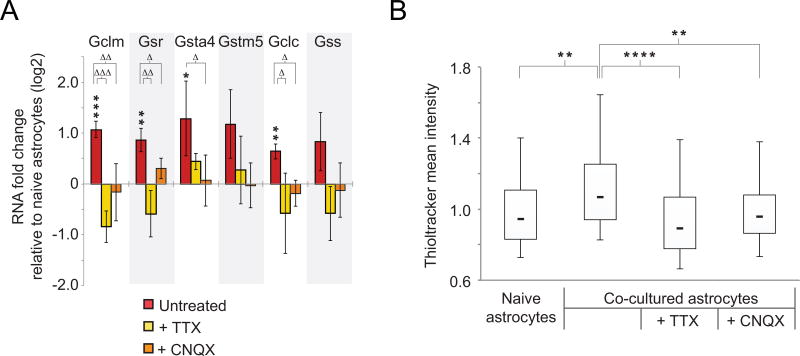
We next tested whether neuronal activity was required for the increase in gene expression of the glutathione pathway genes and glutathione synthesis. Suggestive of this possibility was the finding that none of these genes were up-regulated by RNAseq after four days of neuronal co-culture, results that were recapitulated by qRT-PCR, (Supplementary Table 1 and data not shown). We therefore concentrated on the seven-day co-cultures. We first confirmed that untreated co-cultured astrocytes showed the expected increased RNA levels compared to naïve astrocytes (Fig. 4A, asterisks). This comparison also served as an internal control for the subsequent experiments. To test for a requirement for neuronal activity, we exposed co-cultured astrocytes to the voltage-gated sodium channel blocker tetrodotoxin (TTX), or the ionotropic glutamate receptor blocker CNQX (Honore et al., 1988; Kao, 1964), and determined the fold change in expression level compared to treated naïve astrocytes. Treatment with TTX, which blocks neuronal action potentials, also blocked the up-regulation of the Gclc, Gsr, and Gclm genes due to neuronal co-culture (Fig. 4A, triangles, n=3, p < 0.048). Treatment with CNQX, which prevents activation of post-synaptic AMPA/kainite receptors, also blunted the up-regulation of these genes in neuronal co-culture, as well as that of Gsta4 (Fig. 4A, triangles, n=3, p < 0.048). Therefore, blocking with TTX and CNQX restored gene expression in co-cultured astrocytes to levels that were not significantly different from treated naïve astrocytes (Fig. 4A, n=3, minimum p > 0.11 for all genes after TTX and CNQX treatments). We further reasoned that the observed increase in glutathione production (Fig. 3E) would also be blunted under conditions of reduced neuronal activity. Indeed, we found by Thioltracker staining that TTX and CNQX both reduced the increase in cellular glutathione after neuronal co-culture from 35% to −6% and to 1% at the population medians (Fig. 4B, n=3, p < 0.0001 and p < 0.01, respectively).
Figure 4. Astrocyte glutathione metabolic up-regulation is dependent on neuronal activity.
A) qRT-PCR results for glutathione metabolic genes in astrocytes co-cultured with neurons relative to naïve astrocytes. Cultures were untreated or treated with the indicated pharmacological blockers of neuronal synaptic activity (n=3). * p < 0.048, ** p < 0.01, *** p < 0.001 relative to naïve astrocytes using one-way t-test; Δ p < 0.048, ΔΔ p < 0.01, ΔΔΔ p < 0.001 relative to untreated co-cultured astrocytes using Kruskal-Wallis one-way analysis of variance. Error bars show 95% confidence intervals. B) Box plots of Thioltracker (as in Fig. 3E) comparing naïve astrocytes and astrocytes co-cultured with neurons in the presence or absence of inhibitors of neuronal activity TTX and CNQX (n=3 replicates). ** p < 0.01, **** p < 0.0001 between indicated conditions using one-way analysis of variance.
The neuronal signal for up-regulation of a set of astrocyte glutathione pathway genes is a secreted molecule that depends on astrocytic metabotropic glutamate receptors (mGluRs)
To determine whether induction of the glutathione pathway by neurons was through a soluble factor or required cell-cell contact, we exposed naïve astrocytes to neuronal conditioned media (NCM) collected from hippocampal neurons that had been in culture for ten days, and compared gene expression changes to astrocytes in media that had never been exposed to cells (Control media). As in the co-cultures, NCM increased the expression of the glutathione pathway genes Gclm, Gsr, Gsta4 and Gstm5 by 74%, 45%, 23%, and 22%, respectively, compared to control medium (Fig. 5A, asterisks, n=3, p < 0.048). While significant, these expression changes did not increase to the same levels as those we observed with neuronal co-culture, perhaps suggesting that full induction requires factors in addition to those present in the conditioned media. Further, NCM treatment did not significantly increase expression of Gclc or Gss (35%, p=0.11, 49%, p = .10, respectively) again consistent with lower inducing activity in the NCM. Taken together, the data indicate that a neuronally-secreted factor is sufficient to induce significant glutathione gene expression changes in astrocytes.
Figure 5. A set of astrocyte glutathione genes is up-regulated in response to secreted glutamate from neuronal cultures.
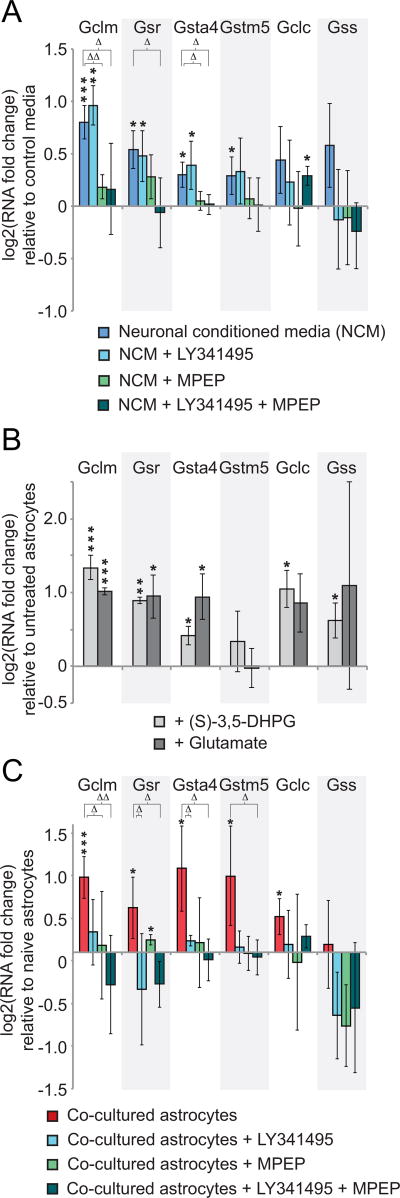
A) qRT-PCR results for glutathione metabolic genes in naïve astrocytes exposed to conditioned media (NCM) from neurons grown in isolation for 10DIV, with or without inhibitors of metabotropic glutamate receptors (mGluRs) LY341495 and MPEP (n=4). * p < 0.048, ** p < 0.01, *** p < 0.001 relative to control media not incubated with neurons using one-way t-test; Δ p < 0.048, ΔΔ p < 0.01 relative to untreated astrocytes in NCM using Kruskal-Wallis one-way analysis of variance. B) qRT-PCR results for glutathione metabolic genes in naïve astrocytes exposed to glutamate or the mGluR agonist (S)-3,5-DHPG (n=3). * p < 0.048, ** p < 0.01, *** p< 0.001 relative to untreated astrocytes using one-way t-test. C) qRT-PCR results for glutathione metabolic genes in astrocytes co-cultured with neurons relative to naïve astrocytes. Cultures were treated with mGluR antagonists LY341495 and MPEP singly or in combination (n=3). * p < 0.048, ** p < 0.01, *** p < 0.001 relative to naïve astrocytes using one-way t-test; Δ p < 0.048, ΔΔ p < 0.01 relative to untreated co-cultured astrocytes using Kruskal-Wallis one-way analysis of variance. Error bars show 95% confidence intervals in all panels.
Given the predominantly glutamatergic nature of the hippocampal neurons used in our experimental preparation (~90%, data not shown and (Benson, Watkins, Steward, & Banker, 1994)), an obvious candidate for a secreted signal for the induced genes was glutamate itself. Many glutamate receptors have been identified in astrocytes, so we used a pharmacological approach to determine which receptors might be involved in the astrocytic response. The metabotropic glutamate receptor antagonist MPEP, which is highly selective for group I mGluRs (Gasparini et al., 1999), when included in the NCM, effectively blocked astrocyte Gclm and Gsta4 gene up-regulation to only 13% and 3% induction, respectively (Fig. 5A, triangles, n=3, p < 0.048). Conversely, activation of mGluRs in naïve astrocytes by treatment with either the mGluR group I agonist (S)-3,5-DHPG (Schoepp, Goldsworthy, Johnson, Salhoff, & Baker, 1994), or glutamate, was sufficient to induce these genes 252% and 33%, respectively, for the agonist and 201% and 92% with glutamate (Fig. 5B, n=3). In contrast to the Gclm and Gsta4 genes, the inclusion of the group II mGluR antagonist LY341495 (Kingston et al., 1998) was required together with MPEP in the NCM to fully block the up-regulation of the Gsr gene to −4% of control conditioned media levels (Fig. 5A, n=3, triangles, p < 0.048). mGluR agonist or glutamate was again sufficient to induce Gsr transcription in the naïve astrocytes 86% and 92% (Fig. 5B, asterisks, n=3, p < 0.048). In contrast to the above genes, induction of the Gstm5 gene was not blocked by NCM containing the mGluR antagonists (Fig. 5A), and no significant up-regulation of Gstm5 was observed in response to agonist or glutamate (Fig. 5B). The lack of response for Gstm5 may reflect the much lower induction of Gstm5 by NCM compared to neuronal co-culture. Taken together our results suggest that mGluR activation is a link between neuronal activity and increased expression for some critical components of the glutathione pathway.
We also tested the role of astrocyte mGluRs in the astrocyte neuronal co-cultures. For this purpose, we included the mGluR antagonists MPEP and LY341495 in the culture medium, individually and together. In co-culture, MPEP treatment alone blocked only Gclm induction to 6% (Fig. 5C, triangles, n=3, p < 0.048). Treatment with both drugs, however, blocked the up-regulation of the Gclm, Gsr, and Gsta4 genes, to −23%, −23%, and −6%, respectively relative to naïve astrocytes (Fig. 5C, triangles, n=3, p < 0.048). In contrast to the NCM results, which did not show a significant effect, Gstm5 gene induction after co-culture was blocked by the combined mGluR antagonism to −4% of naïve astrocyte values (Fig. 5C, triangles, n=3, p < 0.048). The block of Gstm5 induction is surprising in this context, as it was also not induced by glutamate (Fig. 5B). Combined, these results suggest that in the neuron-astrocyte co-culture, where induction is stronger than after incubation with conditioned media, astrocytic group II mGluRs play a more significant role in the induction of these glutathione pathway genes. Despite these differences, the lack of induction by co-culture in the presence of mGluR antagonists supports a critical role of glutamate release and activation of mGluRs in the astrocyte metabolic response to neuronal activity.
Astrocyte neuronal co-culture induces Nuclear factor erythroid2-related factor (NRF2) translocation and binding in the astrocytes
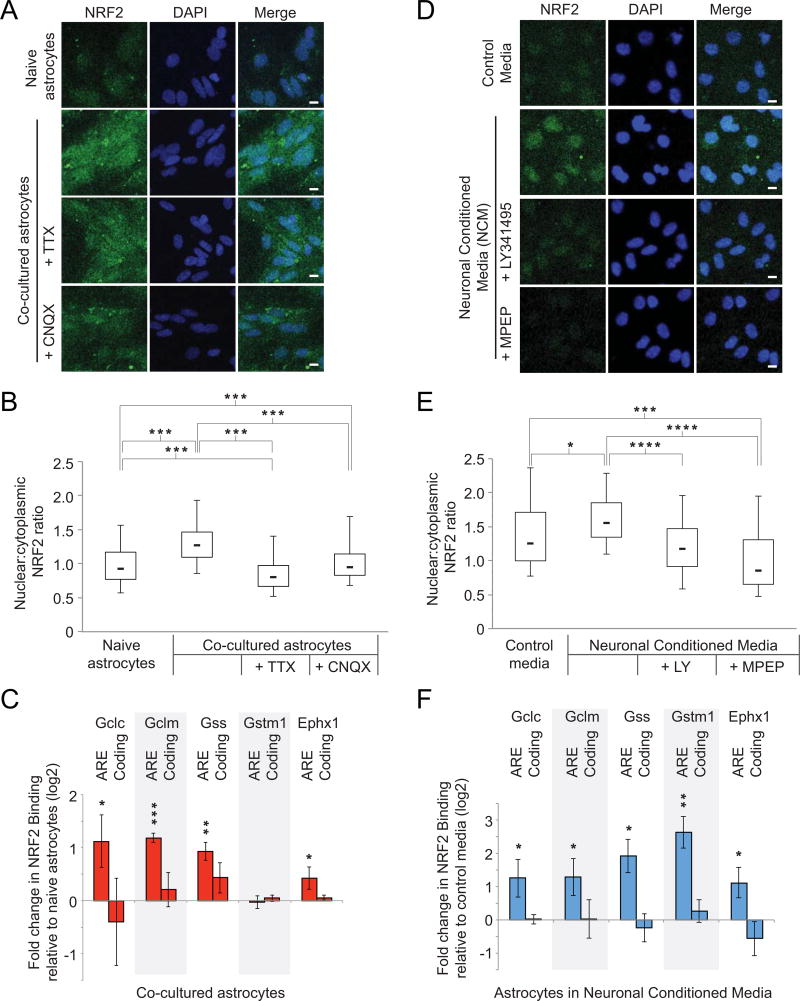
To determine the mechanism of up-regulation of glutathione genes in response to neuronal glutamate signaling, we focused on the transcription factor, NRF2. NRF2 regulates gene expression in the glutathione metabolic pathway in response to oxidative or toxic stress (Venugopal & Jaiswal, 1998), both of which cause NRF2 to translocate to the nucleus instead of being constitutively degraded in the cytoplasm. In the nucleus, NRF2 binds to specific DNA sequences termed Antioxidant Response Elements (AREs) in the upstream regulatory regions of its target genes, where it serves as a transcriptional activator (Itoh et al., 1999). While we observed no change in levels of Nrf2 mRNA either by RNA-seq or qRT-PCR (Supplementary Fig. 5), we measured a significant 38% increase in the ratio of nuclear NRF2 protein immunofluorescence relative to cytoplasmic NRF2 in astrocytes co-cultured with neurons for seven days relative to naïve astrocytes (Fig. 6A and 6B, n=5, p < 0.001). Incubation with either TTX or CNQX significantly blunted this increase to −13% and 3% relative to naïve astrocytes (Fig. 6A and 6B, n=3, p < 0.001). This inhibitory effect by TTX and CNQX suggested that astrocyte NRF2 nuclear translocation was induced by neuronal synaptic activity in the co-culture.
Figure 6. Astrocyte-neuron co-culture results in increased NRF2 nuclear localization and chromatin binding at glutathione metabolic gene promoters relative to naïve astrocytes.
A) Representative immunocytochemistry for NRF2 and DAPI for naïve astrocytes and astrocytes in co-culture with neurons, with and without inhibitors of neuronal activity TTX and CNQX. Scale bar is 10 µm. B) Box plots as in Fig. 3D showing the nuclear:cytoplasmic ratio of NRF2 immunofluorescence in astrocytes alone or in co-culture with neurons (n=3). *** p < 0.001 between indicated conditions using one-way analysis of variance. C) Chromatin immunoprecipitation results for NRF2 at reported antioxidant response element (ARE) binding sites for glutathione metabolic genes and other antioxidant-response genes (Gstm1, Ephx1). Control sites are within coding sequences (n=3). * p < 0.048, ** p < 0.01, *** p < 0.001 relative to naïve astrocytes using one-way t-test. Error bars show 95% confidence intervals. D) Immunocytochemistry as in A) for astrocytes exposed to control media or neuronal conditioned media, with and without inhibitors of mGluR activity MPEP and LY341495. Scale bar is 10 µm. E) Box plots as in B in astrocytes exposed to neuronal conditioned media (NCM), or NCM in combination with mGluR inhibitors LY341495 and/or MPEP (n=3). * p < 0.05, *** p < 0.001, **** p < 0.0001 between indicated conditions using one-way analysis of variance. F) Chromatin immunoprecipitation at AREs and control regions as in C) comparing astrocytes exposed to NCM relative to those incubated with control media (n=3). * p < 0.048, ** p < 0.01 relative to naïve astrocytes using one-way t-test. Error bars show 95% confidence intervals.
To test whether NRF2 translocation manifested in increased binding to the glutathione genes that were up-regulated in neuronal co-culture, we performed chromatin immunoprecipitation (ChIP) at reported AREs within promoters of the Gclc, Gclm, and Gss genes (Malhotra et al., 2010). Gstm5 lacks an annotated ARE. Results showed that NRF2 binding increased at AREs for Gclc, Gclm, and Gss after co-culture with neurons by 2.1-, 2.3-, and 1.8-fold, respectively (Fig. 6C, asterisks, n=3, p < 0.048). The increased binding was specific to AREs, as no statistically significant differences were observed at coding regions of the same genes (Fig. 6C). NRF2 binding was not increased at the ARE for Gstm1, a glutathione pathway gene which has an identified ARE but where we observed no induction in astrocytes after neuronal co-culture (Supplementary Table 1). The antioxidant response gene Ephx1, which is not in the glutathione pathway but is a known NRF2 target (Gorrini, Harris, & Mak, 2013) also exhibited increased ARE binding by NRF2 after neuronal co-culture (1.3-fold, Fig. 6C, asterisks, n=3, p < 0.048).
We next treated astrocytes with Neuronal Conditioned Media (NCM) and measured the nuclear:cytoplasm ratio of NRF2. Incubation with NCM led to a modest but significant increase in the ratio of nuclear to cytoplasmic NRF2 relative to incubation in control medium (24% increase at the population median, Fig. 6D and 6E, n=3, p < 0.05,), and this effect was inhibited by the mGluR antagonists LY341495 and MPEP to −6% and −32% of levels at the population median, respectively, relative to NCM (Fig. 6E, n=3, p < .0001).Further, NRF2 in astrocytes exposed to NCM also showed increased binding at AREs adjacent to the glutathione pathway genes Gclc, Gclm, and Gss by 2.2-, 2.2-, and 3.7-fold, respectively, relative to unconditioned media (Fig. 6F, n=3, p<.048), suggesting that astrocyte NRF2 activity is increased by a soluble signal in NCM. Thus, NRF2 nuclear localization induced by neuronal signaling is dependent on mGluR activation and manifests in increased binding to specific glutathione metabolic genes.
Neuronal-induced cellular glutathione induction requires NRF2
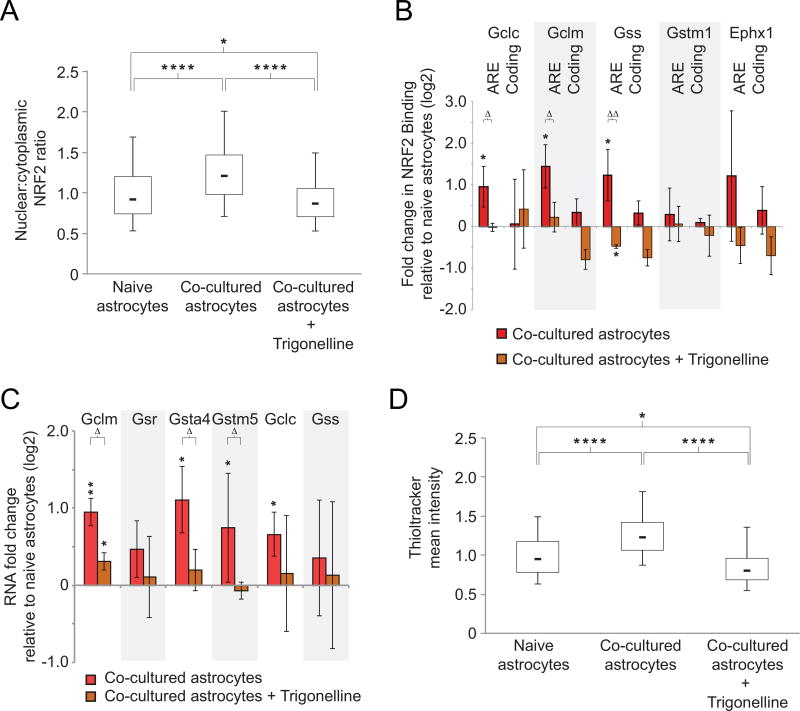
Trigonelline is an alkaloid component of coffee and fenugreek that blocks nuclear transport of NRF2 and NRF2-dependent effects of oxidative stress (Boettler et al., 2011). We treated our co-culture system with trigonelline to determine whether translocation of NRF2 was necessary for the changes in gene expression levels and glutathione production we observed in neuronal co-culture. Trigonelline treatment inhibited the neuron-induced increase in NRF2 nuclear:cytoplasm ratio to −5% of untreated naïve astrocyte levels at the population median (Fig. 7A, n= 3, p < 0.0001). Then we again confirmed the binding of NRF2 to Gclc, Gclm, and Gss in astrocytes in untreated co-culture compared to naïve astrocytes for glutathione pathway members (Fig. 7B, asterisks, n=3, p < 0.048). As predicted, trigonelline treatment prevented enrichment of NRF2 at the respective AREs in astrocytes in co-culture, to −2%, 10%, and −21%, respectively, relative to the untreated astrocytes in co-culture (Fig. 7B, triangles, n=3, p < 0.048). There was again no effect of Trigonelline after co-culture on the binding of NRF2 at the Gstm1 gene. For gene expression studies, we first confirmed that the astrocytes in co-cultures showed increased glutathione gene expression changes compared to naïve astrocytes (Fig. 7C, asterisks, n=3, p < 0.048). Trigonelline treatment significantly inhibited the gene expression increases relative to untreated co-cultures (Fig. 7C, triangles, n=3, p < 0.048). The Gsr gene was not affected, which may reflect the presence of additional regulatory elements that influence its expression. Importantly, trigonelline inhibited cellular glutathione induction after neuronal co-culture to −15% of naïve astrocyte levels at the population median (Fig. 7D, n=3, p < 0.0001).
Figure 7. Blocking NRF2 activity with trigonelline eliminates neuron-induced astrocyte glutathione pathway activation.
A) Box plot as in Fig. 6B showing immunofluorescence staining for NRF2 in the nucleus relative to the cytoplasm of astrocytes when co-cultured with neurons, with and without the NRF2 inhibitor, trigonelline. * p < 0.05, **** p < 0.0001 to indicated conditions using one-way analysis of variance. B) Chromatin immunoprecipitation results for NRF2 at AREs and control coding regions following trigonelline treatment (brown bars) or untreated (red bars) (n=3). * p < 0.048, ** p < 0.01 relative to naïve astrocytes using one-way t-test; Δ p < 0.048, ΔΔ p < 0.01, ΔΔΔ p < 0.001 relative to untreated co-cultured astrocytes using student’s t-test. Error bars show 95% confidence intervals. C) qRT-PCR for glutathione metabolic genes in astrocytes co-cultured with neurons in the presence or absence of trigonelline. Values on Y-axis are fold change of RNA for astrocytes in co-culture with neurons relative to naïve astrocytes (n=3). * p < 0.048, ** p < 0.01 relative to naïve astrocytes using one-way t-test; Δ p < 0.048 relative to co-cultured astrocytes using student’s t-test. Error bars show 95% confidence intervals. D) Box plot as in Fig. 3E showing Thioltracker immunofluorescence of naïve astrocytes or astrocytes co-cultured with neurons in the presence or absence of trigonelline (n=3). * p < 0.05, **** p < 0.0001 between indicated conditions using one-way analysis of variance.
Discussion
In this work, we have demonstrated that genes encoding enzymes in the glutathione pathway are induced in astrocytes by neuronal co-culture. Gclm is the primary regulator of glutathione metabolism (C. S. Huang et al., 1993), and exemplifies the signaling cascade we have identified: strong astrocyte induction of Gclm gene expression requires neuronal activity (Fig. 4A), mGluR function (Fig. 5C), and NRF2 binding (Figs. 7B and C). Other glutathione pathway genes respond similarly (Gsta4), while some responses differed (e.g., Gclc induction did not respond to mGluR inhibition, Fig. 5C). The outliers suggest that the signaling cascade we identify here is not the only input to the regulation of glutathione genes within astrocytes, and that other regulatory contributions will need to be explored.
Our results link together neuronal activity and the astrocyte production of glutathione through the nuclear localization of NRF2. Other groups have demonstrated that stimulated neuronal activity is capable of inducing NRF2 in astrocytes in culture, with high potassium or AP5/gabazine treatments (Habas, Hahn, Wang, & Margeta, 2013). We show here, by comparison with naïve astrocytes, that basal neuronal activity is sufficient to induce astrocytic NRF2 nuclear localization, DNA binding, and transcriptional activation of select genes in the glutathione metabolic pathway. In our experiments, these effects require the activation of astrocyte mGluRs, as either type I (mGlu5) or type II (mGlu3) antagonists were able to block nuclear accumulation of NRF2 (Fig. 6E) and neuronal induction of glutathione pathway genes Gclm, Gsr, Gsta4, and Gstm5 (Fig. 5C). However, the signaling pathway between mGluR activation and NRF2 nuclear localization remains unclear. Several phosphorylation pathways have been proposed to lie downstream of mGluRs, including PKC and PI3-kinase (Butt, Vanzulli, Papanikolaou, De La Rocha, & Hawkins, 2017; Codazzi et al., 2006; Hou & Klann, 2004; Zou et al., 2013), and Cdk5 and GSK3 have been shown to regulate NRF2 stability (X. Chen et al., 2016; Jimenez-Blasco, Santofimia-Castano, Gonzalez, Almeida, & Bolanos, 2015; Rojo, Sagarra, & Cuadrado, 2008), but the full pathway has yet to be elucidated.
The biological role for activity-dependent astrocyte glutathione induction remains to be determined. Enzymes that use glutathione in its reduced form (GSH) are capable of scavenging reactive oxygen (ROS) and nitrogen species to maintain cellular redox balance and protect protein sulfhydryl groups from damaging oxidation (Lu, 2009). Glutathione’s central role in this critical cellular antioxidant system is especially important when cells are undergoing oxidative stress caused by increased mitochondrial energy production or external perturbations (i.e., peroxide) (Ribas, Garcia-Ruiz, & Fernandez-Checa, 2014). Neurons undergoing active synaptic transmission have increased production of ROS (Baxter & Hardingham, 2016), yet a weak antioxidant capacity relative to astrocytes (Bell et al., 2015; Dringen, Pawlowski, & Hirrlinger, 2005; Eftekharpour, Holmgren, & Juurlink, 2000). Therefore, astrocytes in co-culture can provide glutathione extracellularly, where it is used by neurons to protect against oxidative stress induced by enhanced neuronal activity, 6-hydroxydopamine, or peroxide (Baxter & Hardingham, 2016; Dringen et al., 2005; Iwata-Ichikawa, Kondo, Miyazaki, Asanuma, & Ogawa, 1999). Conversely, pre-treatment of astrocytes in co-culture with acivicin, an inhibitor of the glutathione transporter gamma-glutamyltransferase, prevents astrocyte glutathione release and causes a reduction in neuronal glutathione levels (Dringen, Pfeiffer, & Hamprecht, 1999) and prevents astrocyte-mediated survival of neurons undergoing oxidative stress (Jimenez-Blasco et al., 2015). Similarly, treatment of astrocytes with buthionine sulfoxamine (BSO), an inhibitor of GSS, also results in lowered glutathione levels and a subsequent lack of neuroprotection after oxidative stress (Chatterjee, Noack, Possel, Keilhoff, & Wolf, 1999; Y. Chen et al., 2001; Gegg, Clark, & Heales, 2005). These independent studies are consistent with the interpretation that neurons depend on astrocytes for the provision of glutathione for antioxidant purposes, and support the hypothesis that the up-regulation of astrocyte glutathione we observed in co-culture may be used, at least in part, to meet the demands for export, and not exclusively for in situ redox regulation.
While the neuronal dependence on astrocyte glutathione is well documented in vitro, the role of astrocyte glutathione production in vivo is less well understood. Studies of Nrf2 knockout mice have demonstrated that NRF2 is dispensable for mouse development (Chan, Lu, Chang, & Kan, 1996), although there may be subtle behavioral effects (Muramatsu et al., 2013) and numerous organs become more susceptible to environmental toxins (Chan & Kan, 1999; Loboda et al., 2017; Shanmugam et al., 2017). Similarly, Gclm knockout mice are viable and fertile despite having a small fraction (9-35%) of wild-type levels of glutathione, yet they too exhibit a variety of subtle behavioral phenotypes (Y. Chen et al., 2012; Kulak, Cuenod, & Do, 2012). While not absolutely required for normal brain development, glutathione homeostasis may still be important for maintenance of normal brain function after environmental or disease-related insult. In support of this idea, glutathione synthetase (Gss) expression in astrocytes, one of the genes identified in our analysis, is down-regulated in epileptic neurodegeneration in proportion to the degree of neuronal loss (Papageorgiou et al., 2018), and Nrf2 knockout mice appear to be more sensitive to neural damage induced by valproic acid (Furnari, Saw, Kong, & Wagner, 2014). Further, overexpression or activation of NRF2 results in neuronal survival in models of neurodegeneration (Buendia et al., 2016; Xiong, MacColl Garfinkel, Li, Benowitz, & Cepko, 2015). Our results suggest this could be due in part to the induction of glutathione downstream of NRF2. Further, Due to the increased levels of reactive oxygen species in many neurological diseases (Lin & Beal, 2006) and the persistence of astrocyte antioxidant capacity during ageing (Liddell, Robinson, Dringen, & Bishop, 2010), the control of glutathione production by astrocytes may be a useful therapeutic.
In addition to glutathione metabolism, we observed several other pathways related to bioenergetics that were up-regulated in astrocytes in co-culture (Mamczur et al., 2015), including genes encoding the production of pyruvate, the end product of glycolysis. These genes include the complement of lactate dehydrogenase genes (Ldha, Ldhb, Ldhc, and Ldhd), critical enzymes for converting pyruvate to lactate. Interestingly, a recent study by Hasel et al. (Hasel et al., 2017), published during the course of our work, also analyzed astrocyte transcriptomes in neuronal co-culture. Their approach relied on RNA profiling followed by in silico species separation between mouse astrocytes and rat neurons. In their system, stimulating neuronal activity with bicuculine induced genes expressed in the lactate biosynthetic pathway. The impact of astrocyte lactate on neurons, through an astrocyte-neuron lactate shuttle, has been controversial (Erlichman et al., 2008; Machler et al., 2016; Pellerin et al., 2007). However, a recent elegant study showed that neurons do not rely on lactate from astrocytes (Diaz-Garcia et al., 2017). Nonetheless, the shared induction of genes in astrocytes involved in pyruvate and lactate synthesis, in both our study and Hasel et al., lends further mechanistic support for how neurons influence the metabolic status in astrocytes through transcriptional changes.
The Hasel et al. study found significant up-regulation of glutamate transporter genes when astrocytes were co-cultured with neurons, in the absence of any treatment to stimulate neuronal activity, while our study did not detect any up-regulation of these genes. The differences may be technical; our study was developmentally focused, while theirs centered on a later time point, when neurons may be even more synaptically active and require astrocyte contributions to glutamate homeostasis. In addition, Hasel, et al. used a higher proportion of neurons to astrocytes than used in our study, so higher levels of neuronal activity may skew their results more towards other pathways. Finally, the media used for their rat/mouse co-culture contained glutathione, which obviated the need for astrocyte production of this critical (and potentially rate-limiting) antioxidant, and may explain why they observed no increases in glutathione metabolic gene expression such as we report here. In any case, the next important step is to confirm the role of the glutathione pathway, newly identified in our study, in mice.
Although we observed numerous genes up-regulated at the three developmental time points we examined, this analysis did not reveal any of the factors established previously to influence neuronal synaptic formation or physiology, including the thrombospondin and protocadherin gamma families, glypicans 4 and 6, as well as Sparc and Sparcl1 (Hevin). This omission suggests that these factors aren’t transcriptionally regulated by neuronal interaction. However, we cannot exclude the possibility that the particular astrocyte-neuron pair influences the astrocyte transcriptome, since many of the previous studies used retinal ganglion cells in contrast to hippocampal neurons. Furthermore, these factors were identified as secreted by co-culturing in separate dimensions using an insert (Geissler & Faissner, 2012; Kaech & Banker, 2006; Pyka, Busse, Seidenbecher, Gundelfinger, & Faissner, 2011). The insert system, however, limits analyses to astrocyte-neuron interactions mediated entirely by extracellular signaling or metabolite exchange, and may not identify the full range of neuron-astrocyte communication, especially contact-mediated events. Conversely, our list of astrocyte genes differentially expressed during neuronal maturation includes genes whose regulation is contact-dependent (i.e. neuronal conditioned media does not induce them), such as members of the heparan sulfate proteoglycan (HSPG) degradation pathway Naglu and Gusb. As some HSPGs are known to promote axon outgrowth and synaptic connectivity (Farhy Tselnicker, Boisvert, & Allen, 2014; Johnson-Green, Dow, & Riopelle, 1992), their potentially selective degradation by astrocytes may be a mechanism for regulating neuronal maturation. Thus, beyond the glutathione metabolic pathway, our results provide additional candidates for non-cell autonomous factors that influence synaptic formation and physiology.
Supplementary Material
Supplementary Fig. 4. GCLM western blot. Representative western blot for GCLM protein in extracts prepared from naïve astrocytes and astrocyte-neuron co-cultures. Quantification shows level of GCLM in co-cultured astrocytes relative to naïve astrocytes for three experiments, normalized to alpha tubulin.
Supplementary Fig. 5. Nrf2 transcription is unchanged in co-cultured astrocytes relative to naïve astrocytes. Independent methods (RNAseq n=3, qRT-PCR n=3) indicate no significant changes in Nrf2 RNA levels after astrocyte-neuron co-culture by one-way t-test.
Supplementary Fig. 1. Representative FACS plots for naïve astrocytes and co-cultures. Representative flow cytometry profiles of wild type naïve astrocytes (top), TdTomato+ neurons (middle), and astrocyte-neuron co-culture (bottom, same as in Fig 1B). X-axis is fluorescence in the TdTomato channel (561nm). Y-axis is fluorescence in the GFP channel (488nm). Cells sorted into the RFP-negative pool are marked in grey; cells sorted into the RFP-positive pool are marked in red.
Supplementary Fig. 2. Cell type-specific gene counts compared with results in (Cahoy et al., 2008). The expression levels of genes highly enriched in non-astrocyte cell types in the brain are shown for our study (red bars, normalized counts) and Cahoy et al. (cultured astrocytes, blue bars, normalized using geometric mean of astrocyte marker genes from Fig. 1C. B) Expression levels of genes highly enriched in non-astrocyte cell types in the brain are shown for the co-cultured astrocytes from our study (red bars) and Zhang et al (2014) (green bars) in FPKM.
Supplementary Fig. 3. qRT-PCR verification of RNA-seq results. qRT-PCR for genes differentially expressed in at least one time point, performed on samples independently isolated from RNA-seq samples. Genes analyzed can be found in Supplementary Table 1.
Acknowledgments
The authors thank members of the Mandel lab for advice and suggestions throughout this work. We also thank Caitlin Miller for her technical expertise, the OHSU Flow Cytometry Core, and Dr. Shannon McWeeney for bioinformatics instruction. The work was supported by grants from the Rett Syndrome Research Trust and the NIH (HD050563 to GM and F32 NS078886 to JM).
Footnotes
The authors have no conflict of interest regarding the research contained herein.
References
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31(3):353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12(3):311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Hardingham GE. Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes. Free Radic Biol Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Hardingham GE. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23(5):279–295. doi: 10.1007/BF01188497. [DOI] [PubMed] [Google Scholar]
- Boettler U, Sommerfeld K, Volz N, Pahlke G, Teller N, Somoza V, Marko D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J Nutr Biochem. 2011;22(5):426–440. doi: 10.1016/j.jnutbio.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2(6):1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Butt AM, Vanzulli I, Papanikolaou M, De La Rocha IC, Hawkins VE. Metabotropic Glutamate Receptors Protect Oligodendrocytes from Acute Ischemia in the Mouse Optic Nerve. Neurochem Res. 2017 doi: 10.1007/s11064-017-2220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S Web Presence Working, G. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25(2):288–289. doi: 10.1093/bioinformatics/btn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96(22):12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93(24):13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Noack H, Possel H, Keilhoff G, Wolf G. Glutathione levels in primary glial cultures: monochlorobimane provides evidence of cell type-specific distribution. Glia. 1999;27(2):152–161. [PubMed] [Google Scholar]
- Chen X, Liu Y, Zhu J, Lei S, Dong Y, Li L, Zhao Y. GSK-3beta downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci Rep. 2016;6:20196. doi: 10.1038/srep20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Curran CP, Nebert DW, Patel KV, Williams MT, Vorhees CV. Effect of chronic glutathione deficiency on the behavioral phenotype of Gclm−/− knockout mice. Neurotoxicol Teratol. 2012;34(4):450–457. doi: 10.1016/j.ntt.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77(6):1601–1610. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015;7(9):a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, Grohovaz F. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26(13):3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Garcia CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 2017;26(2):361–374. e364. doi: 10.1016/j.cmet.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79(1–2):157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19(2):562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharpour E, Holmgren A, Juurlink BH. Thioredoxin reductase and glutathione synthesis is upregulated by t-butylhydroquinone in cortical astrocytes but not in cortical neurons. Glia. 2000;31(3):241–248. doi: 10.1002/1098-1136(200009)31:3<241::aid-glia50>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28(19):4888–4896. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy Tselnicker I, Boisvert MM, Allen NJ. The role of neuronal versus astrocyte-derived heparan sulfate proteoglycans in brain development and injury. Biochem Soc Trans. 2014;42(5):1263–1269. doi: 10.1042/BST20140166. [DOI] [PubMed] [Google Scholar]
- Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Murai KK. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016;351(6275):849–854. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- Furnari MA, Saw CL, Kong AN, Wagner GC. Altered behavioral development in Nrf2 knockout mice following early postnatal exposure to valproic acid. Brain Res Bull. 2014;109:132–142. doi: 10.1016/j.brainresbull.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38(10):1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Clark JB, Heales SJ. Co-culture of neurones with glutathione deficient astrocytes leads to increased neuronal susceptibility to nitric oxide and increased glutamate-cysteine ligase activity. Brain Res. 2005;1036(1–2):1–6. doi: 10.1016/j.brainres.2004.11.064. [DOI] [PubMed] [Google Scholar]
- Geissler M, Faissner A. A new indirect co-culture set up of mouse hippocampal neurons and cortical astrocytes on microelectrode arrays. J Neurosci Methods. 2012;204(2):262–272. doi: 10.1016/j.jneumeth.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- Habas A, Hahn J, Wang X, Margeta M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci U S A. 2013;110(45):18291–18296. doi: 10.1073/pnas.1208764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Hardingham GE. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun. 2017;8:15132. doi: 10.1038/ncomms15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24(28):6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Anderson ME, Meister A. Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268(27):20578–20583. [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72(6):2334–2344. doi: 10.1046/j.1471-4159.1999.0722334.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Blasco D, Santofimia-Castano P, Gonzalez A, Almeida A, Bolanos JP. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015;22(11):1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Green PC, Dow KE, Riopelle RJ. Neurite growth modulation associated with astrocyte proteoglycans: influence of activators of inflammation. Glia. 1992;5(1):33–42. doi: 10.1002/glia.440050106. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1(5):2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY. Tetrodotoxin: Mechanism of Action. Science. 1964;144(3616):319. doi: 10.1126/science.144.3616.319-a. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia. 2008;56(12):1299–1311. doi: 10.1002/glia.20698. [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108(32):E440–449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav Brain Res. 2012;226(2):563–570. doi: 10.1016/j.bbr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41(10):e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR, Robinson SR, Dringen R, Bishop GM. Astrocytes retain their antioxidant capacity into advanced old age. Glia. 2010;58(12):1500–1509. doi: 10.1002/glia.21024. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Loboda A, Stachurska A, Sobczak M, Podkalicka P, Mucha O, Jozkowicz A, Dulak J. Nrf2 deficiency exacerbates ochratoxin A-induced toxicity in vitro and in vivo. Toxicology. 2017;389:42–52. doi: 10.1016/j.tox.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, Weber B. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016;23(1):94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38(17):5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamczur P, Borsuk B, Paszko J, Sas Z, Mozrzymas J, Wisniewski JR, Rakus D. Astrocyte-neuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes. Glia. 2015;63(2):328–340. doi: 10.1002/glia.22753. [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Janes MS. Detection of intracellular glutathione using ThiolTracker violet stain and fluorescence microscopy. Curr Protoc Cytom. 2010;Chapter 9(Unit 9):35. doi: 10.1002/0471142956.cy0935s53. [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349(6249):730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu H, Katsuoka F, Toide K, Shimizu Y, Furusako S, Yamamoto M. Nrf2 deficiency leads to behavioral, neurochemical and transcriptional changes in mice. Genes Cells. 2013;18(10):899–908. doi: 10.1111/gtc.12083. [DOI] [PubMed] [Google Scholar]
- Papageorgiou IE, Valous NA, Lahrmann B, Janova H, Klaft ZJ, Koch A, Kann O. Astrocytic glutamine synthetase is expressed in the neuronal somatic layers and down-regulated proportionally to neuronal loss in the human epileptic hippocampus. Glia. 2018;66(5):920–933. doi: 10.1002/glia.23292. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pyka M, Busse C, Seidenbecher C, Gundelfinger ED, Faissner A. Astrocytes are crucial for survival and maturation of embryonic hippocampal neurons in a neuron-glia cell-insert coculture assay. Synapse. 2011;65(1):41–53. doi: 10.1002/syn.20816. [DOI] [PubMed] [Google Scholar]
- Ribas V, Garcia-Ruiz C, Fernandez-Checa JC. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105(1):192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63(2):769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Shanmugam G, Narasimhan M, Conley RL, Sairam T, Kumar A, Mason RP, Rajasekaran NS. Chronic Endurance Exercise Impairs Cardiac Structure and Function in Middle-Aged Mice with Impaired Nrf2 Signaling. Front Physiol. 2017;8:268. doi: 10.3389/fphys.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17(3):932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47(3):209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17(24):3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- Wang X, Cairns MJ. SeqGSEA: a Bioconductor package for gene set enrichment analysis of RNA-Seq data integrating differential expression and splicing. Bioinformatics. 2014;30(12):1777–1779. doi: 10.1093/bioinformatics/btu090. [DOI] [PubMed] [Google Scholar]
- Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Chen C, Zhong Y, An J, Zhang X, Yu Y, Fu J. PI3K/Akt pathway mediates Nrf2/ARE activation in human L02 hepatocytes exposed to low-concentration HBCDs. Environ Sci Technol. 2013;47(21):12434–12440. doi: 10.1021/es401791s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 4. GCLM western blot. Representative western blot for GCLM protein in extracts prepared from naïve astrocytes and astrocyte-neuron co-cultures. Quantification shows level of GCLM in co-cultured astrocytes relative to naïve astrocytes for three experiments, normalized to alpha tubulin.
Supplementary Fig. 5. Nrf2 transcription is unchanged in co-cultured astrocytes relative to naïve astrocytes. Independent methods (RNAseq n=3, qRT-PCR n=3) indicate no significant changes in Nrf2 RNA levels after astrocyte-neuron co-culture by one-way t-test.
Supplementary Fig. 1. Representative FACS plots for naïve astrocytes and co-cultures. Representative flow cytometry profiles of wild type naïve astrocytes (top), TdTomato+ neurons (middle), and astrocyte-neuron co-culture (bottom, same as in Fig 1B). X-axis is fluorescence in the TdTomato channel (561nm). Y-axis is fluorescence in the GFP channel (488nm). Cells sorted into the RFP-negative pool are marked in grey; cells sorted into the RFP-positive pool are marked in red.
Supplementary Fig. 2. Cell type-specific gene counts compared with results in (Cahoy et al., 2008). The expression levels of genes highly enriched in non-astrocyte cell types in the brain are shown for our study (red bars, normalized counts) and Cahoy et al. (cultured astrocytes, blue bars, normalized using geometric mean of astrocyte marker genes from Fig. 1C. B) Expression levels of genes highly enriched in non-astrocyte cell types in the brain are shown for the co-cultured astrocytes from our study (red bars) and Zhang et al (2014) (green bars) in FPKM.
Supplementary Fig. 3. qRT-PCR verification of RNA-seq results. qRT-PCR for genes differentially expressed in at least one time point, performed on samples independently isolated from RNA-seq samples. Genes analyzed can be found in Supplementary Table 1.