Abstract
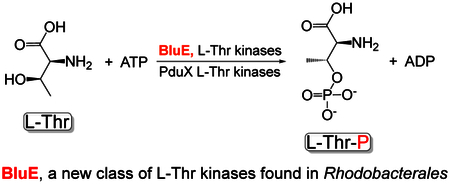
Several of the enzymes involved in the conversion of adenosylcobyric acid (AdoCby) to adenosylcobamide (AdoCba) are yet to be identified and characterized in some cobamide (Cba)-producing prokaryotes. Using a bioinformatics approach, we identified the bluE gene (locus tag RSP_0788) of Rhodobacter sphaeroides 2.4.1 as a putative functional homologue of the L-threonine kinase enzyme (PduX, EC 2.7.1.177) of S. enterica. In AdoCba, (R)-1-aminopropan-2-ol O-phosphate (AP-P) links the nucleotide loop to the corrin ring; most known AdoCba producers derive AP-P from L-Thr-O-3-phosphate (L-Thr-P). Here, we show that RsBluE has L-Thr independent ATPase activity in vivo and in vitro. We used 31P-NMR spectroscopy to show that RsBluE generates L-Thr-P at the expense of ATP and is unable to use L-Ser as substrate. BluE from R. sphaeroides or Rhodobacter capsulatus restored AdoCba biosynthesis in S. enterica ΔpduX and R. sphaeroides ΔbluE mutant strains. R. sphaeroides ΔbluE strains exhibited a decreased pigment phenotype that was restored by complementation with BluE. Finally, phylogenetic analyses revealed that bluE was restricted to the genomes of a few Rhodobacterales that appear to have a preference for a specific form of Cba, namely Coα-(α−5,6-dimethylbenzimidazolyl-Coβ-adenosylcobamide (a.k.a. adenosylcobalamin, AdoCbl; coenzyme B12, CoB12).
Abbreviated summary
Aminopropanol-phosphate (AP-P) links the nucleotide loop to the ring in vitamin B12. The PduX enzyme is the kinase that is commonly responsible converting L-Thr to L-Thr-P, which is in turn decarboxylated to make AP-P. Rhodobacterales lack pduX. Instead they have the non-orthologous bluE gene that encodes an enzyme that, unlike PduX, is specific for L-Thr and cannot use L-Ser. Phylogenetic analysis show that bluE is restricted to the Rhodobacterales.
Introduction
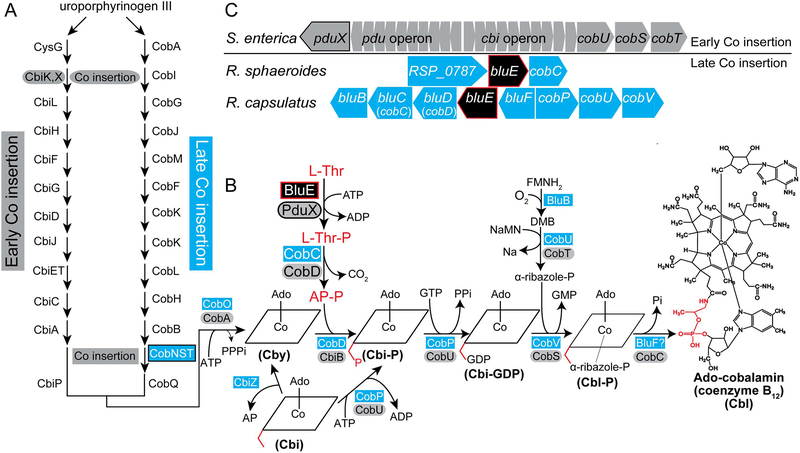
Bacteria and archaea that synthesize cobamides (Cbas) use one of two pathways, the O2-dependent or O2-independent pathway (Blanche et al., 1993, Roessner et al., 2001) [reviewed in (Escalante-Semerena & Warren, 2008)]. There are several differences between these pathways, but the one that is most frequently used to distinguish one from the other is the timing of insertion of the cobalt ion into the tetrapyrrole ring. In the O2-dependent pathway, cobalt insertion occurs late in the branch of the pathway that assembles the corrin ring. In contrast, in the O2-independent pathway cobalt insertion occurs at the second committed step of the corrin ring biosynthetic pathway (Fig. 1). For this reason, the pathways are also referred to as the late- and early-cobalt insertion pathways. The early-Co-insertion pathway has been extensively studied in Salmonella enterica sv. Typhimurium LT2 [reviewed in (Escalante-Semerena, 2007, Escalante-Semerena & Warren, 2008)] and Bacillus megaterium (Biedendieck et al., 2010, Collins et al., 2013, Beck et al., 1997, Leech et al., 2002, Raux et al., 1998). In this pathway, de novo synthesis of the corrin ring occurs only in the absence of oxygen due to the oxygen lability of precorrin 5B (Moore et al., 2013), the product of the cobalt-precorrin 5A acylhydrolase (CbiG) enzyme (Schroeder et al., 2009). The late-Co-insertion pathway is functional under normoxic and anoxic conditions, and has been primarily studied in Pseudomonas denitrificans (Blanche et al., 1991, Cameron et al., 1991b, Cameron et al., 1991a, Crouzet et al., 1990, Debussche et al., 1991, Thibaut et al., 1990, Zumft, 1997) and Rhodobacter capsulatus (Biel, 1992, Heldt et al., 2005, McGoldrick et al., 2005, Pollich & Klug, 1995, Vlcek et al., 1997). Both pathways share several homologous enzymes, however, while most of the enzymes within the early-Co-insertion pathway of S. enterica have been identified, the functional equivalents of some enzymes have not been identified in the late-Co-insertion pathway. For example, it is not known which phosphatase dephosphorylates adenosylcobalamin-phosphate (AdoCbl-P) to produce the final product, adenosylcobalamin (AdoCbl). Also unknown is the kinase that generates L-Thr-P, which is converted to (R)-1-aminopropan-2-ol O-phosphate (a.k.a (R)-1-amino-2-propanol-O-2-phosphate, AP-P), which tethers the nucleotide loop to the corrin ring (Fig. 1, highlighted red). In S. enterica, PduX (locus tag STM2058, hereafter SePduX) phosphorylates L-Thr (Fan & Bobik, 2008, Fan et al., 2009). Notably, the functional equivalent of SePduX has not been identified in any organism that uses the late-Co-insertion pathway.
Figure 1. AdoCbl biosynthetic pathways in bacteria and archaea.
A. Schematic depicting the early- and late-cobalt insertion pathways of AdoCbl biosynthesis. B. Late steps of the AdoCbl biosynthetic pathway, with proteins in the early-cobalt-insertion (a.k.a. O2-independent, blue rectangles) and late-cobalt-insertion (a.k.a. O2-dependent, gray ovals) pathways. The BluE enzyme is shown in a black box with red trim. The (R)-1-aminopropan-2-ol O-phosphate (AP-P) moiety is highlighted in red in the structure scheme. Exogenous Cbi (a.k.a (CN)2Cbi) enters the pathway as indicated. Exogenous Cby (a.k.a (CN)2Cby) enters the pathway at the location it appears on in the figure. Exogenous corrinoids, including complete Cbas such as Cbl are adenylated by CobA/CobO upon transport into the cell and prior to entering the pathway. C. Genetic layout of the L-threonine kinase-encoding genes bluE in the late-cobalt-insertion, AdoCbl-synthesizing bacteria R. sphaeroides and R. capsulatus, and pduX in S. enterica, an early-cobalt-insertion, AdoCbl-synthesizing bacterium. Figure key: Ado, Adenosyl; Cby, cobyric acid; Cbi-P, cobinamide phosphate; Cbi-GDP, cobinamide-GDP; Cbl-P, cobalamin phosphate; Cbl, cobalamin; AP-P, (R)-1-aminopropan-2-ol O-phosphate; AP, (R)-1-aminopropan-2-ol; L-Thr-P, L-threonine-O-3-phosphate; L-Thr, L-threonine; α-ribazole-P, α-ribazole phosphate; DMB, 5,6-dimethylbenzimidazole; NaMN, nicotinic acid mononucleotide; Nm, Nicotinic Acid; PPi, pyrophosphate; Pi, orthophosphate; CbiK/CbiX, anaerobic cobaltochelatase; CobN/CobS/CobT, aerobic hydrogenobyrinic acid a,c-diamide cobaltochelatase; CobA/CobO, ATP:Co(I)rrinoid Adenosyltransferase; CbiB/CobD, AdoCbi-P synthase; CobS/CobV, AdoCba-5′P synthase; CobD/CobC, L-Thr-P decarboxylase; CobT/CobU, NaMN:DMB phosphoribosyltransferase; CobU/CobP, AdoCbi kinase / AdoCbi-P guanylyltransferase; PduX/BluE, L-Thr kinase; CobC/BluF, AdoCba-5′-P phosphatase; CbiZ, cobinamide amidohydrolase; pdu, genes of the Cba-dependent 1,2-propanediol degradation operon; cbi, genes of the early steps of the early-Co-insertion cobalamin biosynthesis operon.
The bluE gene was identified in R. capsulatus, BB1 as the second gene in an operon named bluFEDCB (Fig. 1) because disruption of the operon resulted in a reduction of red pigmentation or a “blush” phenotype (Pollich & Klug, 1995). In R. capsulatus precursor molecules of the initial steps for AdoCbl synthesis enters the bacteriochlorophyll (Bch) synthesis pathway and are incorporated into bacteriochlorophyll (Willows & Kriegel, 2009). In addition, AdoCbl is a required cofactor for enzymes that synthesize bacteriochlorophyll such as BchE/ChlE and is needed for transcriptional activation of genes that lead to carotenoid biosynthesis (Yin & Bauer, 2013, Gough et al., 2000). Therefore, cells with incomplete AdoCbl production have a pigmentation phenotype (Yin & Bauer, 2013). Disruption of the bluE gene by a Tn5 element blocked AdoCbl biosynthesis resulting in an auxotrophy and blush phenotype that was corrected by cobalamin (Cbl) but not by cobinamide (Cbi), a Cbl precursor (Fig. 1) (Pollich & Klug, 1995). BluD is homologous to the AdoCbi-P synthase (CbiB, EC 6.3.1.10) of S. enterica, (CobD in the late-Co-insertion pathway), BluC is homologous to the L-Thr-P decarboxylase (CobD, EC 4.1.1.81) of S. enterica, (CobC in the late-Co-insertion pathway), and BluB was later identified in Sinorhizobium meliloti and Rhodospirillum rubrum as a 5,6-dimethylbenzimidazole (DMB) synthase (Gray & Escalante-Semerena, 2007, Campbell et al., 2006, Taga et al., 2007). BluB activity has not been identified in S. enterica. To date, the putative functions of the proteins encoded by the bluF and bluE genes have not been identified. BluF is likely the last missing enzyme in the late-Co-insertion pathway (Pollich et al., 1996), the AdoCbl-P phosphatase (CobC) of S. enterica (Zayas & Escalante-Semerena, 2007) (unpublished data). Here we report the identification and initial characterization of BluE (locus tag RSP_0788). Our data support the conclusion that BluE is the L-threonine (L-Thr) kinase of Rhodobacter sphaeroides 2.4.1.
We found that RsBluE failed to use L-Ser as substrate to produce L-Ser-P. L-Ser-P is used by some Cba producing organisms such as Sulfurospirillum multivorans to make ethanolamine phosphate (EA-P) (Keller et al., 2016), which serves as the nucleotide loop linker in norCbas (e.g. Coα−5,6-dimethylbenzimidazolyl-176-cobamide, norCbl, norB12).(Kräutler et al., 2003). NorCbas have a nucleotide loop linker that lacks a methyl group at position 176. The S. multivorans L-Ser-P/L-Thr-P decarboxylase, CobD (locus tag SMUL_1544) preferentially uses L-Ser-P over L-Thr-P (Keller et al., 2016) to produce EA-P. S. multivorans naturally produces norpseudo-Cbl (Coα-adeninyl-176-norcobamide, norpseudo-B12) because the Cba-dependent PCE reductive dehalogenase (PceA) enzymes in S. multivorans has a preference for norCbas (Keller et al., 2018, Keller et al., 2013) and norpseudo-Cbas in particular (Keller et al., 2018, Keller et al., 2014, Keller et al., 2013, Kräutler et al., 2003). Pseudo-Cbas are cobamides with adenine as the lower axial ligand, as opposed to DMB in Cbl. The enzyme that generates L-Ser-P has not been identified in S. enterica or S. multivorans and it appears R. sphaeroides cannot incorporate L-Ser-P into Cba synthesis via BluE.
Lastly, the RsBluE enzyme was found to be restricted to a subclass of Rhodobacterales with genetic attributes that indicate they have a strong preference for AdoCbl. That is to say, these Rhodobacter species prefer and synthesize only a Cba with DMB as the lower ligand base and AP-P as the nucleotide linker; Coα-(α−5,6-dimethylbenzimidazolyl-Coβ-adenosylcobamide (a.k.a. adenosylcobalamin, AdoCbl; coenzyme B12, CoB12).
Results
BluE is the L-Thr kinase involved in AdoCbl biosynthesis in Rhodobacterales.
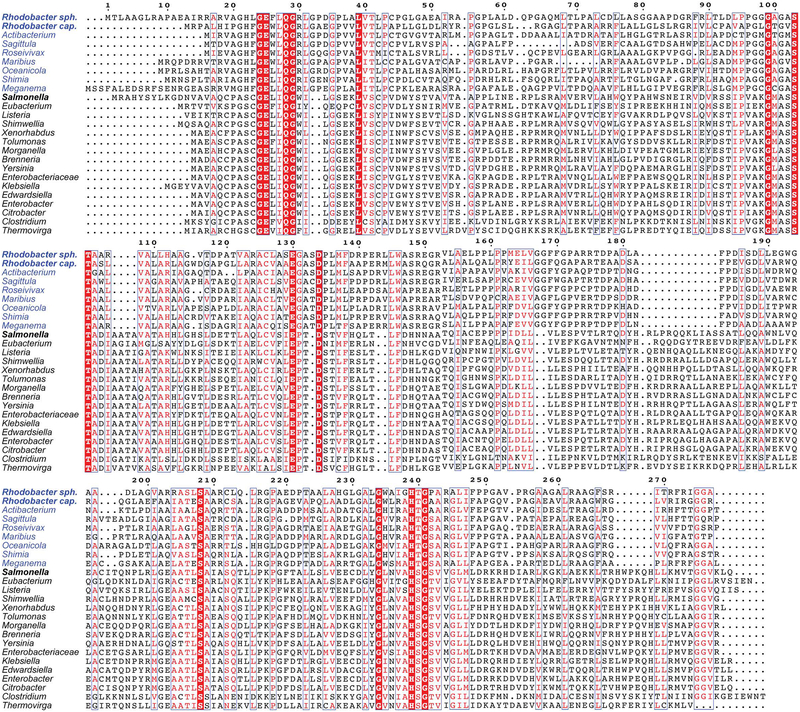
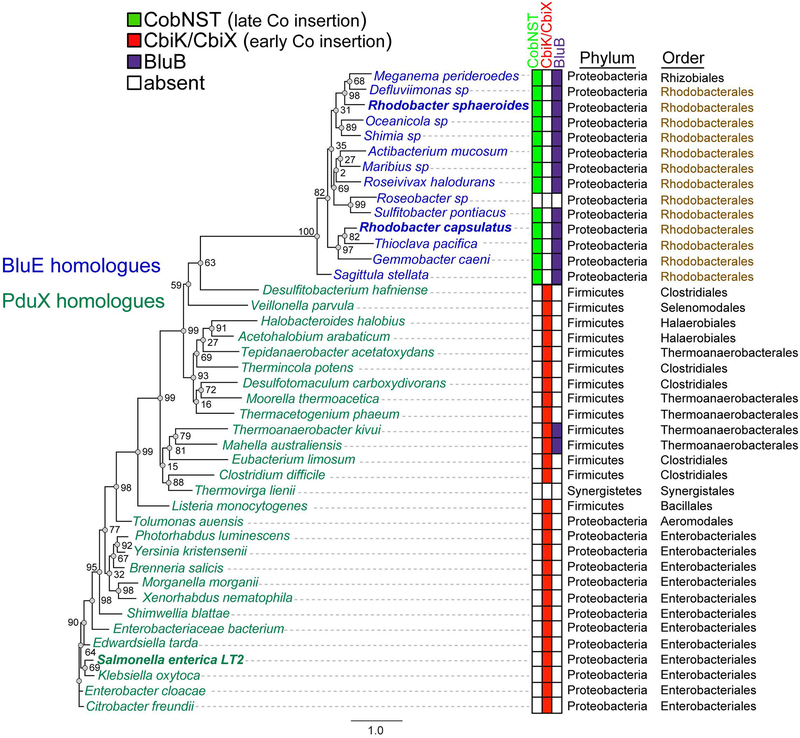
Previous bioinformatics analysis of the genomes of AdoCba producers (Rodionov et al., 2003) did not identify homologues of the L-Thr kinase (PduX in S. enterica, SePduX) in organisms that use the late-cobalt-insertion pathway for AdoCbl biosynthesis (Fig. 1A). Because of significant protein sequence differences, BLASTP (Altschul et al., 1997) searches using SePduX failed to retrieve BluE sequences and vice versa. The bluE (locus RCAP_rcc02055, accession number Z46611) gene from R. capsulatus was first identified as part of an AdoCbl biosynthetic gene cluster but its function was not elucidated (Pollich & Klug, 1995). All bluE homologues identified thus far have been found clustered with other AdoCbl biosynthetic genes. BluE proteins were on average about 23–35 residues (5–6 kDa) shorter than SePduX (Fig. 2). The BluE proteins from R. capsulatus SB1003 (RcBluE; 27 kDa, 265 amino acids) and R. sphaeroides (RsBluE, locus RSP_0788; 28 kDa, 277 amino acids) displayed 47–52% end-to-end identity among the Rhodobacterales analyzed (Fig. 2), but only a 19% identity to SePduX (33 kDa, 300 amino acids). This generally falls below the threshold of most default BLAST queries, and it is likely the reason why it was overlooked in previous bioinformatics analysis of AdoCbl genes (Rodionov et al., 2003). Despite the low sequence identity, and notable gaps in the sequence of BluE relative to SePduX (residues 105–108, 180–190, and 283–288), the sequence alignment revealed multiple conserved residues. Several of these residues were previously identified as important for SePduX function (Fan & Bobik, 2008, Fan et al., 2009) (Fig. 2). A phylogenetic tree was constructed from SePduX and BluE from R. sphaeroides and R. capsulatus protein sequences (Fig. 3). We identified two distinct phylogenetic clusters. At the bottom of this tree were closely related homologues of SePduX (green lettering). This cluster contained organisms that synthesized homologues of the cobaltochelatase SeCbiK or CbiX (EC 4.99.1.3, red squares) and therefore use the early-cobalt-insertion pathway for AdoCba biosynthesis (Fig. 1A). At the top of the tree is a cluster containing organisms that synthesize BluE homologues (blue lettering). This BluE cluster contained members only from the order Rhodobacterales, with one exception, a representative from the Rhizobiales (i.e., Maganema perideroedes). This cluster was comprised only of organisms that contained all three subunits of the cobaltochelatase CobNST (EC 6.6.1.2, green squares), which is a marker for the late-cobalt-insertion pathway. Rhodobacterales are known to use the late-cobalt-insertion pathway [reviewed in (Mattes et al., 2017)]. The one exception in the cluster is a Roseobacter species, which oddly possessed genes that encoded two of the three subunits of the late-Co-insertion pathway cobaltochelatase CobST but not CobN and lacked all the genes of the early de novo biosynthetic pathway. It had all the genes for the late steps of the pathway (cobCDOPUV, bluEF) and must therefore salvage precursors such as cobyric acid (Cby) or cobinamide (Cbi) (Fig. 1B) in order to assemble the nucleotide loop and produce AdoCbl. The Rhodobacterales that encoded BluE also encoded the 5,6-dimethylbenzimidazole (DMB) synthase, BluB (EC 1.14.99.40, purple squares) (Gray & Escalante-Semerena, 2007, Taga et al., 2007). Possible implications of this genetic affiliation are discussed below.
Figure 2. Sequence alignment of representative PduX and BluE proteins.
Genera names of organisms with BluE homologues are colored blue and PduX homologues are colored black. Residues that are 100% conserved are highlighted in red with white lettering. Areas with a high degree of conservation greater than 75% but less than 100% and or residues with similar properties are boxed in blue with red letter.
Figure 3. Phylogenetic analysis of the distribution of PduX and BluE proteins.
Maximum likelihood phylogenetic tree of homologous proteins based on the amino acid sequence of SePduX (dark green) and RsBluE or RcBluE (blue). Order Rhodobacterales is highlighted in brown. Color-coded table of the presence or absence of the cobaltochelatase CbiK or CbiX (red squares), which is indicative of the early-cobalt-insertion pathway, the presence of all three subunits of the cobaltochelatase CobNST (lime green squares) in organisms that use the late-cobalt-insertion pathway and BluB (purple squares) the O2-dependent DMB synthase, which is indicative of a physiological reliance on Cbas with DMB as the lower ligand base. Sequences were aligned using the MUSCLE (Edgar, 2004) plugin within Geneious R8.1.7 software (Biomatters Ltd.) with 100 iterations and default settings. Maximum likelihood phylogenic tree was generated with the online PhyML (Guindon et al., 2010) tool on the ATCG Montpellier Bioinformatics Platform available at http://www.atgc-montpellier.fr/phyml/, using Jones-Taylor-Thornton substitution model (Jones et al., 1992) with 500 bootstrap replicates. Bootstrap support for each node is shown as a percent value. The scale bar is provided as a reference for branch lengths. Gene locus tags are available in Table S1.
R. capsulatus and R. sphaeroides BluE restore AdoCbl-dependent growth of a S. enterica ΔpduX strain.
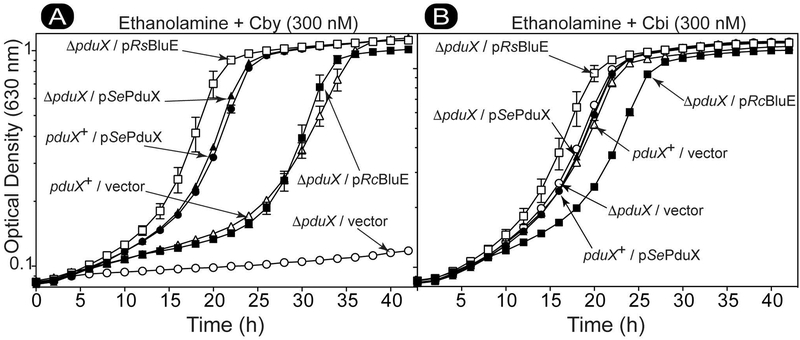
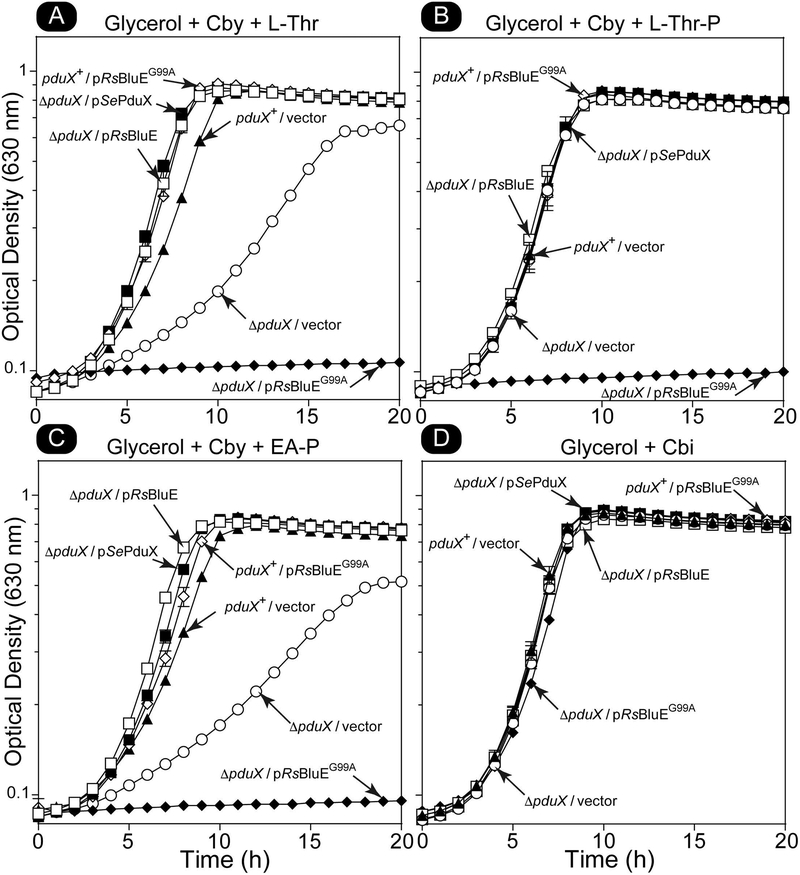
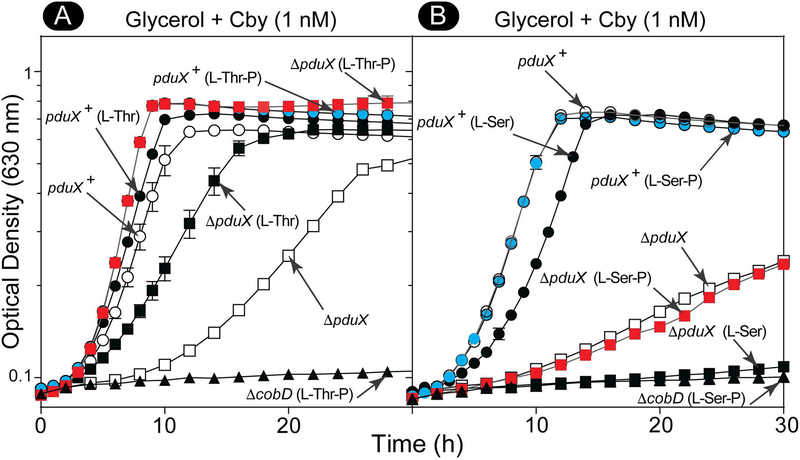
S. enterica encodes two methionine synthases, the Cba-dependent (MetH, EC 2.1.1.13) (Drennan et al., 1994, Bandarian et al., 2002) and the Cba-independent methionine synthase (MetE, 2.1.1.14) (Peariso et al., 2001, Gonzalez et al., 1992, Hondorp & Matthews, 2004), both of which synthesize methionine from homocysteine and methyl-tetrahydrofolate. The Cba-dependent MetH enzyme has a 57-fold greater enzymatic efficiency than MetE (Taylor & Weissbach, 1973, Jakubowski, 1990), so picomolar concentrations of Cba is sufficient to meet the methionine synthesis needs of S. enterica under laboratory conditions (Bradbeer, 1982). We used a S. enterica ΔmetE ΔpduX mutant strain to show in vivo that BluE had L-Thr kinase function. It has been observed by us and others (Fan & Bobik, 2008) that when a S. enterica ΔmetE ΔpduX strain is grown on minimal glycerol medium with relatively high levels of exogenous Cby (i.e., >5 nM), the strain does not display a robust growth phenotype relative to the wild-type strain. One reason for this was the very low levels of Cba required by the Cba-dependent methionine synthase (MetH) (Andersson & Roth, 1989). The results also suggested the presence of at least one other enzyme with L-Thr kinase activity within S. enterica. This hypothetical, redundant kinase activity only restored AdoCba synthesis in a ΔpduX mutant strain when the intracellular levels of Cby were high. For this reason, growth analysis was performed with a low Cby concentration (1 nM) to clearly distinguish the ΔpduX growth phenotype when assaying MetH Cba-dependent growth. Likewise, when cells were grown under conditions that demanded a much higher level of AdoCba production, such as growth with ethanolamine as the sole carbon and energy source (Roof & Roth, 1988, Roof & Roth, 1989), a S. enterica ΔpduX mutant strain did not grow (Fig. 4A, open circles) (Fan & Bobik, 2008).
Figure 4. RsBluE and RcBluE restore AdoCbl synthesis in a S. enterica ΔpduX strain.
Growth analysis of S. enterica pduX+ and ΔpduX strains harboring plasmids expressing bluE+ from R. sphaeroides (R.s. bluE+), R. capsulatus (R.c. bluE+), PduX from S. enterica (S.e. pduX+), or containing the empty vector pBAD24 (vector). Cells grown aerobically at 37°C in NCE minimal medium with ethanolamine (90 mM) as the sole carbon and energy source, supplemented with DMB (0.15 mM), arabinose (0.5 mM), ampicillin (0.1 mg mL−1), MgSO4 (1 mM), Fe(III)-citrate (0.05 mM), and A. Cby (300 nM) or B. Cbi (300 nM). A representative graph of three independent growth experiments performed in technical triplicate. Error bar represent the standard error of the mean. Figure key: ΔpduX/pRsBluE (□), ΔpduX/pSePduX (▲), ΔpduX/pRcBluE (■), ΔpduX/vector (○), pduX+/pSePduX (●), pduX+/vector (△).
Figure 4 shows the growth behavior of S. enterica ΔpduX mutant strains expressing genes from plasmids encoding R. sphaeroides locus RSP_0788 (pRsBluE, open squares), R. capsulatus locus RCAP_rcc02055 (pRcBluE, closed squares), S. enterica pduX+ (pSePduX, closed triangles), and the corresponding vector-only controls. The culture medium contained ethanolamine (90 mM) as the carbon and energy source supplemented with cobalamin precursors Cby (300 nM) or Cbi (300 nM) and DMB (0.15 mM). Aerobic growth on ethanolamine requires a high concentration of Cba or corrinoid Cba precursors (e.g. Cby or Cbi) (Roof & Roth, 1988, Roof & Roth, 1989) and base (e.g. DMB or adenine) to keep up with the AdoCba demand necessary to utilize this carbon and energy source (Anderson et al., 2008). The use of Cby allowed us to probe the AP-P synthesis branch directly, while using Cbi functions as a positive control by bypassing the AP-P linker synthesis and attachment branch (Fig. 1). RcBluE restored AdoCba biosynthesis in the S. enterica ΔpduX strain allowing the culture to reach the same cell density as that of the cultures of wildtype S. enterica pduX+. Expression of S. enterica pduX+ (pSePduX, closed triangles) improved growth over that of the wild-type pduX+ strain (open triangles) whether or not the chromosomal pduX+ gene was deleted (closed triangles vs closed circles); the strains grew with similar doubling times (i.e., 3.7 h) (Fig. 4A, closed circles and triangles). However, cultures of strains producing RsBluE or SePduX displayed a much shorter lag time (12 and 16 h, respectively) than cultures of the wild-type strain carrying the empty cloning vector, or the strain producing RcBluE (i.e., 26 h) (Fig. 4A, closed squares). RsBluE restored AdoCba synthesis in a ΔpduX strain with roughly the same doubling time (i.e., 2.7 h) as SePduX (Fig. 4A, open squares vs closed triangles). Figure 4B shows the effect of adding the Cba precursor Cbi, which enters the pathway downstream of the enzymes that synthesize and attach the AP-P linker moiety (Fig. 1B). As expected all the strains grew like wildtype, except for a slightly longer lag time for the ΔpduX/pRcBluE strain. These results showed that BluE from R. sphaeroides or R. capsulatus efficiently substituted for PduX in S. enterica. Using sequence alignments of SePduX and BluE homologues (Fig. 2), we targeted conserved residues for substitution. Expression of a R. sphaeroides bluE allele encoding variant RsBluEG99A, failed to restore AdoCba biosynthesis in a S. enterica ΔpduX strain under conditions that demanded either low (Fig. S1A) or high (Fig. S1B) AdoCba levels for growth. Figure 5 shows the effect of the RsBluEG99A variant on Cba-dependent growth of S. enterica when precursors for each enzyme in the AP-P synthesis and attachment branch of the Cba biosynthesis pathway was provided exogenously. As expected, when L-Thr was provided to a ΔpduX strain producing the RsBluEG99A variant it failed to grow (Fig. 5A, closed diamonds), but grew well in a wild-type pduX+ strain background (open diamonds), presumably because the presence of the native wild-type SePduX enzyme produced from the chromosomal copy of the gene compensated for the presence of the defective RsBluEG99A enzyme produced from a plasmid. Surprisingly, when the product of SePduX/RsBluE and substrate of SeCobD (L-Thr decarboxylase), L-Thr-P, was provided to bypass the L-Thr kinase function, the ΔpduX/pRsBluEG99A strain was still unable to grow (Fig. 5B). Likewise, when ethanolamine phosphate (EA-P), the product of SeCobD and substrate of SeCbiB (AdoCbi-P synthase) was provided the ΔpduX/pRsBluEG99A strain was still unable to grow. SeCbiB was previously shown to use EA-P as a Cba linker in S. enterica to generate nor-Cbas (Zayas et al., 2007). Only when the entire linker synthesis and attachment branch is bypassed (Fig. 1) by the addition of Cbi (Fig. 5D) was the ΔpduX/pRsBluEG99A strain able to grow. The implication of these results are discussed below.
Figure 5. RsBluEG99A variant disrupts all the enzymes in the entire AP-P synthesis and attachment branch in S. enterica in vivo.
Representative graphs of growth analyses of S. enterica cells grown aerobically at 37°C in NCE minimal medium with glycerol (22 mM) ampicillin (0.1 mg mL−1), and MgSO4 (1 mM) Cby (1 nM) and supplemented with A. L-Thr (1 mM), B. L-Thr-P (1 mM), or C. ethanolamine phosphate (EA-P, 1 mM). Cbi (1 mM) was used as the corrinoid in place of Cby in panel D. Experiments were replicated in two independent experiments, each performed in triplicate. Error bar represent the standard error of the mean. Figure key: ΔpduX/pRsBluE (□), ΔpduX/pSePduX (■), ΔpduX/pRsBluEG99A (◆), pduX+/pRsBluEG99A (◇), pduX+/vector (▲), ΔpduX/vector (○).
Inactivation of bluE in R. sphaeroides causes AdoCbl-dependent growth phenotypes.
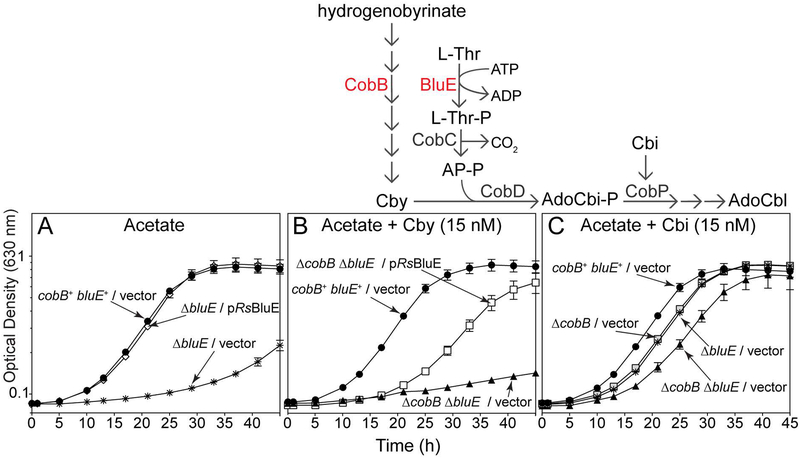
Since the presence of RsBluE restored growth of a S. enterica ΔpduX strain under AdoCbl dependent growth conditions (Fig. 4, 5, S1), the function of this gene was assessed in R. sphaeroides. R. sphaeroides cells grown aerobically with acetate (30 mM) as the sole source of carbon and energy. R. sphaeroides uses a Cbl-dependent ethylmalonyl-CoA mutase (Erb et al., 2008) and methylmalonyl-CoA (Banerjee, 2003) mutase to catabolize short chain fatty acids such as acetate (Gray & Escalante-Semerena, 2009b, Banerjee, 2003). When no cobamide precursors were added to the medium, ΔbluE strains failed to grow compared to bluE+ controls (Fig. 6A, asterisks vs circles). We also observed a “blush” phenotype for the ΔbluE strain and noted that the amount of light-harvesting complex 1 (LH1 B875) reaction center pigments of this strain were reduced relative to those in cultures of the ΔbluE strain growing in the presence of AdoCbl (Fig. S2A,B) after measurements were normalized for the cell density of each culture. The blush phenotype was also corrected by the addition of CNCbl to the medium (Fig. S2C)
Figure 6. Growth analysis of R. sphaeroides ΔbluE and ΔbluE ΔcobB strains.
Growth analysis of R. sphaeroides bluE+, ΔbluE, and ΔcobB ΔbluE strains harboring a plasmid expressing R. sphaeroides bluE+ or carrying the empty pBBR1MCS-2 plasmid (vector). Cells were grown overnight in Sistrom’s medium and cultures were prepared as described in Materials and Methods. Cell growth was monitored normoxically at 30°C in Sistrom’s medium with acetate (30 mM), kanamycin (0.01 mg mL−1), and when noted, Cby (15 nM) or Cbi (15 nM). Growth experiments were performed in triplicate in three independent experiments. Error bars represent the standard error of the mean. Pathway represents a simplified schematic of the roles of CobB (hydrogenobyrinate a,c-diamide synthase) and BluE (L-Thr kinase) in the synthesis of cobalamin in R. sphaeroides. CobD, AdoCbi-P synthase; CobC, L-Thr-P decarboxylase; CobP, AdoCbi kinase / AdoCbi-P guanylyltransferase; BluE, L-Thr kinase; Cby, cobyric acid; Cbi, cobinamide, AP-P, (R)-1-aminopropan-2-ol O-phosphate. Figure key: Panel A: cobB+ bluE+/vector (●), ΔbluE/pRsBluE (○), ΔbluE/vector (✲), Panel B: ΔcobB ΔbluE/pRsBluE (□), ΔcobB ΔbluE/vector (▲), cobB+ bluE+/pRsBluE (●), Panel C: cobB+ bluE+/vector (●), ΔcobB ΔbluE/vector (▲), ΔcobB/vector (□), ΔbluE/vector (✲).
To further validate BluE as a L-Thr kinase, we used a R. sphaeroides ΔcobB strain, which could not synthesize adenosylcobyric acid (AdoCby) de novo under the aerobic conditions tested because of the disruption of the early steps O2-depenent (late-Co-insertion) pathway (Fig. 1A). AdoCba biosynthesis in the ΔcobB strain was restored by feeding Cby, the substrate for the adenosylcobinamide-phosphate (AdoCbi-P) synthase (CobD; CbiB in the early-Co-insertion pathway) enzyme (Fig. 6, pathway scheme). As expected, R. sphaeroides ΔcobB ΔbluE mutants did not grow in medium containing Cby, unless bluE+ was provided on a plasmid (Fig. 6B, triangles). Although growth of the ΔbluE/pRsBluE strain was delayed, the doubling time (6 h) and final density (OD630 = 0.6) of the culture was similar to that of the cobB+ bluE+ strain (4 h, OD630 = 0.6; Fig. 6B, circles vs squares). Even when the AP-P synthesis branch of the pathway is bypassed by the addition of the Cbl precursor Cbi (Fig. 6, pathway scheme) to the medium, all mutant strains displayed a slight delay in the onset of exponential growth relative to the cobB+ bluE+ (Fig. 6C). What this might indicate about the physiology of Cba biosynthesis in this organism is discussed below. Collectively, results shown in figure 6 validated BluE as a bona fide L-Thr kinase in R. sphaeroides.
Enrichment of RsBluE and SePduX.
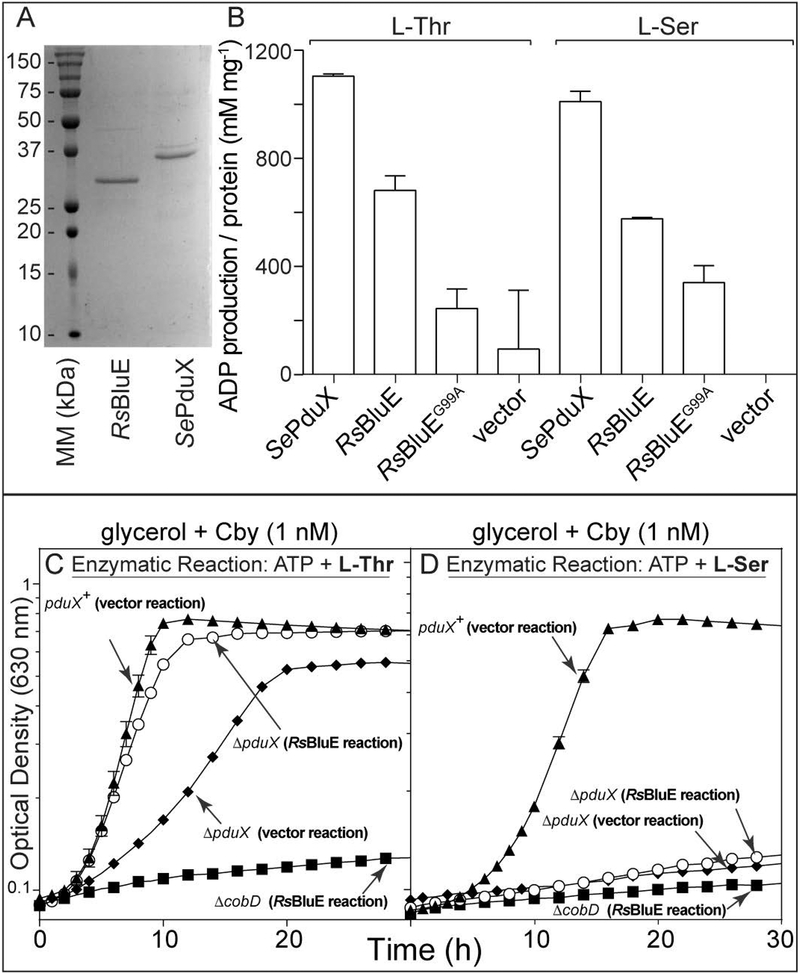
Overproduction of RsBluE and SePduX resulted, in both cases, in the production of substantial quantities (~12 mg g−1 cells) of insoluble protein. Several strains of E. coli overexpression strains (BL21, BL21/RIL, Rosetta 2/plysS, BL21/plysSRARE2, C41, C43, MDS 42, and Lemo21), and S. enterica at various temperatures (15, 25, 37°C), with different lysis methods (chemical lysis, French press, and sonication), buffers (HEPES, Tris-HCl), pH (7.0, 7.5, 7.9, 8.0, and 8.5), under normoxic and anoxic conditions were tried with little success at improving the quantity of soluble protein isolated. Tags used to increase protein solubility such as maltose binding protein (MBP) resulted in an inability to detect protein production either due to poor expression or proteolysis prior to cell harvesting. Several different detergents were screened in an attempt to solubilize proteins. Detergents used included 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), n-dodecyl-β-D-maltopyranoside (DDM), Fos-choline-16 (FOS16), Triton-X, sarkosyl, and sodium dodecyl sulfate (SDS). The proteins were only soluble in SDS or sarkosyl. Attempts to completely remove the detergent after solubilization resulted in protein precipitation. A concentration of ~1% (w/v) sarkosyl was maintained to keep the proteins in solution. N-terminally His6-tagged protein was overproduced, solubilized, and purified to 70% purity with the detergent sarkosyl as described in the Experimental procedures section. The sarkosyl concentration was reduced to ~1% (w/v) by dialysis. Figure 7A shows a representative SDS-PAGE gel of purified RsBluE and SePduX. Both enzymes were active in the presence of ~1% (w/v) sarkosyl. Proteins isolated in this manner were used for all in vitro assessment of ATPase activity using the ADP-Glo™ ATPase Assay kit described under Materials & Methods.
Figure 7. In vitro ATPase activity assay for RsBluE.
A. SDS-PAGE-gel of purified, sarkosyl-solubilized RsBluE and SePduX. B. ATPase activity assay performed with ADP-Glo™ ATPase Assay kit (Promega). Reaction mixtures contained HEPES buffer (50 mM, pH 7.0 at 25°C), MgCl2 (1 mM), ATP (0.1 mM), L-Thr or L-Ser (10 mM), enrichment samples of SePduX, RsBluE, and RsBluEG99A sarkosyl-solubilized protein (12 μM) incubated at 25°C for 1 h. Negative control reaction mixtures contained extracts of sarkosyl-solubilized protein obtained from cells expressing the empty overexpression vector pTEV5 (vector). ATP to ADP conversion was quantified from the luminescence (relative light units; RLU) after subtracting the background from the no-enzyme control and comparing the value to a standard curve (Fig. S3). Graph titles indicate the growth medium and the substrate constituents of the reaction mixtures used to supplement the growth medium. The source of the protein extracts used in the reactions are in parentheses in bold typeface next to the strain genotype (RsBluE reaction or vector resection). Representative graphs of two independent experiments performed in triplicate. Error bars represent the standard deviation from the mean. C and D. Growth analysis of S. enterica cells grown normoxically at 37°C in NCE minimal medium with glycerol (22 mM), MgSO4 (1 mM), Cby (1 nM), and filter sterilized RsBluE reaction mixtures (6% v/v) described above (reactions from panel B) containing either ATP (0.08 mM) and L-Thr (0.8 mM, panel C) or L-Ser (0.8 mM, panel D). The final concentrations of substrates after dilution of the filtered reaction mixtures into the medium are in parentheses. Graphs are representative of two independent experiments performed in triplicate. Error bars represent the standard error of the mean. Figure key: Panel C, D: (▲) pduX+ strain supplemented with filter sterilized negative control enzymatic reaction containing ATP, and L-Thr (panel C) or L-Ser (panel D) and sarkosyl-solubilized protein extracts from cells carrying the empty vector; (◆) ΔpduX strain supplemented with filter sterilized negative control enzymatic reaction containing ATP, and L-Thr (panel C) or L-Ser (panel D) and sarkosyl-solubilized protein extracts from cells carrying the empty vector; (○) ΔpduX strain supplemented with filtered enzymatic reaction containing ATP, and L-Thr (panel C) or L-Ser (panel D) and purified sarkosyl-solubilized RsBluE protein; (■) ΔcobD strain supplemented with filtered enzymatic reaction containing ATP and L-Thr (panel C) or L-Ser (panel D) and purified sarkosyl-solubilized RsBluE protein.
RsBluE has ATPase and L-Thr kinase activities in vitro.
Figure 7B shows representative results of an ATP activity assay using the ADP-Glo™ Assay kit. Sarkosyl-solubilized RsBluE and SePduX (12 μM) exhibited ATPase activity when provided with ATP (0.1 mM) and L-Thr (10 mM). ATP to ADP conversion was quantified by comparison to a standard curve (Fig. S3). SePduX converted 99% of the ATP to ADP, RsBluE converted 61%, and the RsBluEG99A variant converted 21% after 1 h incubation at 25°C. There was residual 8% ATPase activity detected in the reaction with extracts from the vector control. With L-Ser as the substrate, SePduX converted 90% of the ATP to ADP, RsBluE converted 51%, and the RsBluEG99A variant converted 30%. There was no activity detected in the reactions with extracts from the vector control. These data implied that sarkosyl-solubilized SePduX was a more efficient enzyme than RsBluE in vitro under the conditions tested. These data also suggested that SePduX and RsBluE might be able to use L-Ser as substrate to generate O-phospho-L-serine (L-Ser-P).
We employed a biological assay to verify the generation of L-Thr-P and L-Ser-P by sarkosyl-solubilized RsBluE. RsBluE reactions were filter sterilized and added to minimal glycerol medium supplemented with Cby used to grow S. enterica strains (Fig. 7C, D). RsBluE reactions containing L-Thr and ATP restored near wild-type growth (Fig. 7C, open circles vs triangles) of a ΔpduX strain when compared to the same strain grown in medium containing the reactions of extracts from the vector control (Fig. 7C, open circles vs diamonds). These results showed that RsBluE had L-Thr kinase activity in vitro. A ΔcobD strain was included as a negative control since only AP-P, not L-Thr-P, would restore growth of the ΔcobD strain (Fig. 7D, diamonds). Figure 7D shows the growth of strains in medium supplemented with reactions containing L-Ser and ATP. The presence of L-Ser from the vector only or RsBluE reactions strongly inhibited growth of ΔpduX strains (Fig. 7D, diamonds and circles). The wild-type strain also exhibited an increased lag from 2 to 6 hours but eventually reached full density (Fig. 7D, triangles). These results suggested that L-Ser had a slight inhibitory effect on the growth of S. enterica, an effect that was greatly exacerbated in the ΔpduX strain. This idea was further explored (see below).
Optimal conditions for the RsBluE in vitro activity assay.
The reaction conditions for the L-Thr kinase reaction were optimized (Fig. S8). The optimal pH was 8 in HEPES buffer (50 mM) (Fig. S8A). Sarkosyl-solubilized RsBluE was 57% and 67% more active at low protein concentrations (2 μM) than at 12 and 24 μM protein, respectively (Fig. S8B). However, the total percent conversion after a 1-h incubation period at 25°C was greater at higher concentrations of protein; 81% at 24 μM, 54% at 12 μM, and 20% at 2 μM (data not shown). Salts were not required for activity, and CaCl2 (100 mM) decreased the activity of the enzyme by 75% (Fig. S8C). However, a divalent metal ion (1 mM) was required for RsBluE ATPase activity, with optimal activity observed with MnCl2. RsBluE used MgCl2, ZnCl2, NiCl2, and CaCl2 equally well. CoCl2 did not support the ATPase activity of RsBluE as well as the other metals but did perform better than the no metal control (Fig. S8D). The concentration of L-Thr did not affect the ATPase activity for RsBluE. There was a slight drop in activity at 50 and 100 mM L-Thr concentration (Fig. S8E) and increasing the concentration of L-Ser inhibited RsBluE ATPase activity (Fig. S8F). RsBluE hydrolyzed ATP in the absence of L-Thr, and in the presence of L-Ser, D-Ser, D-Thr, L-Tyr, or L-Val (10 mM), without only slightly higher activity levels for L-Ser, D-Ser, and D-Thr relative to the ATP only control and L-Tyr and L-Val had similar activity levels to that of the ATP only control (Fig. S8G). RsBluE ATPase activity is independent of potential amino acid substrate. Figure S8H shows the activity of RsBluE as a function of ATP concentration. RsBluE activity ATP activity increased with increasing ATP concentration (1–20 mM) as was observed in figure S7A. RsBluE was assayed for inhibition by known ATPase inhibitors (Fig. S8I). ADP was the most effective inhibitor followed by pyrophosphate (PPi), with a 52% and 12% decrease in activity, respectively. Surprisingly, ortho-phosphate (Pi) and adenosine 5′-[γ-thio]triphosphate (ADP-γ-S) increased the activity of RsBluE by 8% and 22%, respectively. It may be possible that RsBluE can hydrolyze ADP-γ-S. There are precedents of enzymes using ADP-γ-S. as substrate (Smith et al., 2011). Further testing of RsBluE with ADP-γ-S will be needed. The inhibition of RsBluE by one of its products, ADP, could have implications for how this enzyme is regulated.
Detergent-free RsBluE and SePduX can be isolated in low concentrations.
While the sarkosyl-solubilized proteins displayed robust ATPase activity in vitro, and kinase activity using a sensitive biological assay (Fig. 7C), we did not detect L-Thr kinase activity using 31P-NMR spectroscopy. The implication being that sthe sarkosyl-solubilized protein had kinase activity that was present but reduced or impaired. The mechanism of sarkosyl solubilization of proteins is generally achieved by the partial unfolding of proteins. While this did not appear to affect the ATPase activity of RsBluE, it did have an effect on the transfer of the phosphoryl moiety to L-Thr. For this reason, we attempted to purify RsBluE and SePduX without the aid of detergents. While most of produced protein was insoluble, small quantities of His6-tagged protein bound to a Ni-NTA affinity chromatography resin and were eluted with imidazole (200 mM). Notably, these proteins were only soluble for < 60 min before precipitating. Neither temperature (4, 25°C), dialysis to remove imidazole, or the addition of glycerol improved protein stability. RsBluE and SePduX were purified to a purity of 87% and 89% respectively (see Experimental procedures). This window of protein stability afforded the opportunity to assess protein function without detergent. Extracts from cells carrying the empty vector pTEV5 were isolated in an identical manner and fractions eluted at the same imidazole concentration (200 mM) were collected and used as negative controls (Fig. S4). Proteins isolated in this manner were used to assay the generation of L-Thr-P by 31P-NMR spectroscopy. Concerns about possible interference by the N-terminal His6-tag lead us to test the solubility and purification of previously published non-cleavable C- and N-terminally tagged SePduX proteins (Fan et al., 2009, Fan & Bobik, 2008). SePduX exhibited the same poor solubility regardless of the placement or cleavability of the His6- or His8-tag. The majority of the protein was found in the insoluble fraction and only small amounts of limited stability protein was isolated (Fig. S5).
RsBluE generates L-Thr-P in vitro.
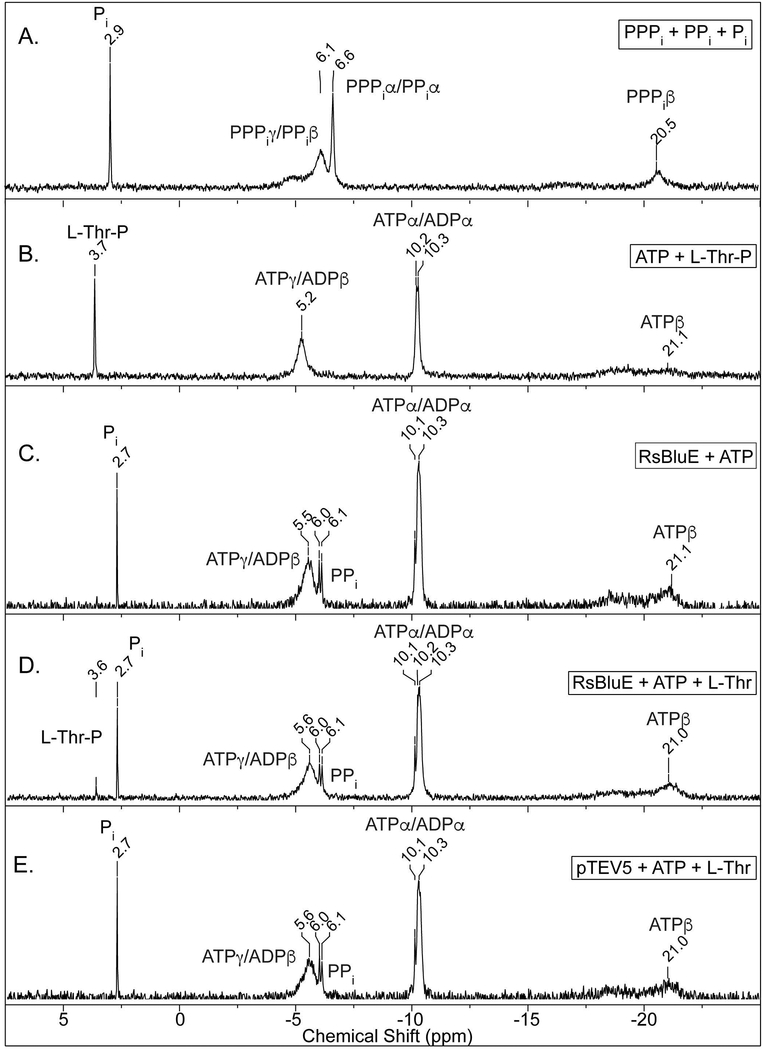
We used 31P-NMR spectroscopy to confirm that RsBluE transferred a phosphate from ATP to L-Thr to generate L-Thr-P; figure 8 shows representative 31P-NMR spectra. Panel A shows the spectrum for standards of polytriphosphate (PPPi), pyrophosphate (PPi), and ortho-phosphate. Panel B shows the spectrum of the reaction mixture without enzyme but containing ATP and L-Thr-P. Enzymatic reactions containing ATP and RsBluE (panel C), ATP, L-Thr, and RsBluE (panel D), and ATP, L-Thr, and extracts from cells carrying the pTEV5 empty vector (panel E). For those experiments we used detergent-free protein extracts obtained as described above and in Experimental prodecures. As a result, some conversion of ATP to ADP and free phosphate (Pi) and pyrophosphate (PPi) was observed, likely due to contaminating proteins with ATP/ADP hydrolysis activities. This was confirmed by the presence of signals with chemical shifts corresponding to PPi (6.1 ppm) and Pi (2.9 ppm) in the reactions containing extracts from cells expressing the empty vector pTEV5, which was included as a negative control (Fig. 8E). When RsBluE was incubated with ATP and L-Thr a signal with a chemical shift of 3.6 ppm corresponding to authentic L-Thr-P was detected (Fig. 8D). This signal was absent in the spectrum of reaction mixtures containing only RsBluE and ATP (Fig. 8C), or cell-free extracts of cells carrying the empty pTEV5 vector (Fig. 8E). These results supported the idea that RsBluE was a bona fide L-Thr kinase. GTP was also tested as a phosphor donor and failed to generate GDP or L-Thr-P (data not shown), indicating RsBluE could not use GTP as a phosphoryl donor. SePduX was previously shown to have very low levels of activity with GTP, CTP, or UTP and a strong preference for ATP (Fan & Bobik, 2008).
Figure 8. 31P-NMR spectra of RsBluE reaction products.
Representative 31P-NMR spectra of duplicate independent experiments. Reaction mixtures containing MgCl2 (1 mM), ATP (3 mM), L-Thr (6 mM), and 10 μL of detergent-free RsBluE protein (11 μM) were incubated at 25°C for 1 h. A. No enzyme reactions with AMP, sodium ortho-phosphate (Pi), sodium pyrophosphate (PPi), and sodium polytriphosphate (PPPi) standards. B. No enzyme reactions with L-Thr-P and ATP standards. C. Reaction containing ATP and RsBluE. D. Reaction containing ATP, L-Thr, and RsBluE. E. Reaction containing ATP, L-Thr, and extracts from cells carrying pTEV5 empty vector. Conditions used for the acquisition of the spectra are described in the Materials and Methods section.
L-Ser inhibits a S. enterica ΔpduX strain in vivo.
To address the L-Ser inhibition shown in figure 7D (diamonds and circles), we grew S. enterica strains in minimal glycerol + Cby medium supplemented with either L-Thr, L-Thr-P, L-Ser, or L-Ser-P (Fig. 9). The addition of L-Thr-P improved the growth rates and cell yields of the pduX+ and ΔpduX strains (red squares, cyan circles; overlapping) over those of the pduX+ strain with no supplements (open circles). L-Thr also improved the growth behavior of wildtype (pduX+, black circles) and ΔpduX strains (black squares) to a slightly lesser extent than L-Thr-P. These results suggested that exogenous L-Thr was used by PduX. Notably, the ΔpduX strain grew well in the absence of L-Thr or L-Thr-P (Fig. 9, open squares). Possible explanations for the growth behavior of the ΔpduX strain are discussed below.
Figure 9. Growth analysis of S. enterica strains in the presence of L-Thr, L-Thr-P, L-Ser, and L-Ser-P.
Growth analysis of S. enterica cells grown normoxically at 37°C in NCE minimal medium with glycerol (22 mM), MgSO4 (1 mM), and A. Cby (5 nM) supplemented with L-Thr or L-Thr-P (1 mM), or B. Cby (1 nM) supplemented with L-Ser or L-Ser-P (1 mM). The amino acid or phospho-amino acid supplement is indicated in parentheses next to the strain genotype. Representative graphs of two independent experiments performed in triplicate. Error bars represent the standard error of the mean. Figure key: Panel A: ΔpduX (□), ΔpduX (L-Thr) (■), ΔpduX (L-Thr-P) (■), pduX+ (○), pduX+ (L-Thr) (●), pduX+ (L-Thr-P) (●), ΔcobD (L-Thr-P) (▲), Panel B: ΔpduX (□), ΔpduX (L-Ser) (■), ΔpduX (L-Ser-P) (■), pduX+ (○), pduX+ (L-Ser) (●), pduX+ (L-Ser-P) (●), ΔcobD (L-Ser-P) (▲).
As shown in figure 7D, the addition of L-Ser caused a slight increase in the lag time of the wild-type strain compared to the addition of L-Thr (Fig. 7C), and strongly inhibited growth of a ΔpduX strain (Fig. 7D, diamonds), even when expressing pduX+ from a plasmid (Fig. 7D, circles). The addition of L-Ser-P did not affect the growth of the S. enterica pduX+ strain (Fig. 9B, blue circles vs open circles), or ΔpduX strain (Fig. 9B, red squares vs open squares). As expected, the addition of L-Thr-P or L-Ser-P did not affect the growth behavior of the ΔcobD strain (Fig. 9A, 9B solid triangles), which requires AP-P for growth.
To further validate the above in vivo results, we performed 31P-NMR analyses of the RsBluE reaction mixtures containing ATP and L-Ser (Fig. S6). RsBluE did not synthesize L-Ser-P from ATP and L-Ser (Fig. S6C).
SePduX and RsBluE use L-Thr more efficiently than L-Ser, and ATP is the limiting substrate.
We measured the specific activity of sarkosyl-solubilized SePduX and RsBluE using a pyruvate kinase/lactate dehydrogenase coupled assay that indirectly measured the generation of ADP via the consumption of NADH (see Experimental procedures) (Bergmeyer et al., 1985) (Fig. S7). Shown in figure S7 are the specific activities of RsBluE (Fig. S7A) and SePduX (Fig. S7B) as a function of ATP, L-Thr (Figs. S7C, D), and L-Ser (Figs. S7E, F). The concentration of L-Thr was held constant at 50 mM as the ATP concentration was increased, and vice versa, the ATP concentration was held at 50 mM while L-Thr or L-Ser concentrations were increased. SePduX and RsBluE had comparable specific activities for ATP, that is, 1.0 and 0.93 μmol ATP per minute per mg of protein (Table 1). However, complete substrate saturation was not reached. The rate of ATP hydrolysis did not change as a function of the concentration of L-Thr (Fig. S7C, S7D). Even when no L-Thr was present in the reaction mixture, both enzymes hydrolyzed ATP to ADP. This is also illustrated in figure S8G. SePduX specific activity declined by 9% when L-Thr was the substrate and 35% when L-Ser was used, resulting in a 29% difference in activity between L-Thr and L-Ser as substrates. The effect was more severe for RsBluE, with a 17% decline in activity when L-Thr was present and 55% with L-Ser, for a 46% difference in specific activity between L-Thr and L-Ser as substrates. This decrease in RsBluE activity in the presence of increasing L-Ser concentration is illustrated graphically by the gradual downward slope of the curve in figures S7E and S8F in contrast to the relatively flat line for the RsBluE reactions with L-Thr (Figs. S7C and S8E), or the SePduX reaction with L-Ser (Fig. S7F). This result suggested an inhibitory effect by L-Ser on RsBluE.
Table 1. Comparison of RsBluE and SePduX activities as a function of substrates.
Specific activity values for purified and sarkosyl solubilized RsBluE and SePduX enzymes were assayed for ATPase activity in the presence and absence of L-Thr or L-Ser. Values are reported as mean ± standard deviation of three measurements of activity at 100 mM ATP and 50 mM L-Thr or L-Ser. Activity was measured with a NADH consumption assay (see Materials and Methods).
| ATP (μmol ATP min−1 mg−1) |
L-Thr (μmol ATP min−1 mg−1) |
L-Ser (μmol ATP min−1 mg−1) |
|
|---|---|---|---|
| RsBluE | 0.93 ± 0.02 | 0.77 ± 0.02 | 0.42 ± 0.01 |
| SePduX | 1.00 ± 0.03 | 0.91 ± 0.03 | 0.65 ± 0.02 |
Discussion
The bluE genes of R. capsulatus and R. sphaeroides encode L-Thr kinases.
In vivo (Fig. 4, 6) and in vitro (Fig. 8) data provide strong support to our assignment of function to BluE proteins from R. capsulatus (RcBluE) and R. sphaeroides (RsBluE) as L-Thr kinases that synthesize L-Thr-O-3-phosphate (L-Thr-P). Although some differences between RcBluE and RsBluE were observed during their in vivo function analysis (Fig. 4), those differences may be due to several factors. We can only speculate about the possibilities, such as structural differences in RcBluE that prevent optimal interaction with native S. enterica proteins in the pathway. This might explain why the ΔpduX / pRcBluE strain had a slightly longer lag time when grown on media supplement with Cbi (Fig. 5B), a condition which bypasses the need for BluE or PduX (Fig. 1B). RcBluE may be simply a less catalytically efficient enzyme. At the moment insufficient information is available to compare catalytic effciencies.
Rhodobacterales may have evolved RsBluE to avoid synthesizing nor-Cbl.
Our data (Figs. S5, S6) support the idea that Rhodobacter sphaeroides and probably other Rhodobacterales that possess the BluE enzyme may have a preference or requirement for Cbl, that is, the Cba that has AP-P, not EA-P, as the nucleotide linker. The restriction against the synthesis of norCbas with EA-P as the linker derived from L-Ser-P, was made clear by the results of in vitro experiments (Fig. 7B, S6, S5, S7F). Based on those data we suggest that the L-Thr-P decarboxylase (CobC; CobD in the early-Co-insertion pathway) (Fig.1) in these bacteria likely also have a strong preference for L-Thr-P, given that growth was poorly supported by L-Ser-P relative to the response to L-Thr-P (Fig. 9). The catalytic activities of Cba-dependent enzymes from these bacteria must be analyzed to gain insights into the specificity of the enzymes for Cbl. While the data presented here suggests that Rhodobacterales prefer Cbl, other than the inability of R. sphaeroides to use pseudo-Cbl (Gray & Escalante-Semerena, 2009a), the ability of all other Rhodobacterales that synthesize BluE to use other form of Cbas has not been tested. At this time the Cba preference of these Rhodobacterales remains speculative.
Phylogenetic analyses suggest that BluE is not the L-Thr kinase of the late-cobalt-insertion pathway.
At first glance, it appears that BluE is found exclusively in organisms that synthesize the CobNST cobaltochelatase of the late-cobalt-insertion pathway (Fig. 3). BLASTP searches using SePduX retrieved homologues only from organisms that use the early-cobalt-insertion pathway. The only exception being T. lienii, which lacks all the genes of the early corrinoid biosynthesis pathway. BluE appears to be restricted only to the order Rhodobacterales and is not found in any other group that uses the late- or early-cobalt-insertion pathway.
The BluE protein is the L-Thr kinase of a subgroup of Rhodobacterales with a preference for AdoCbas with AP-P as the nucleotide linker and DMB as the ribotide base.
While BluE is present in R. sphaeroides and R. capsulatus it is not found in other AdoCba producing purple photosynthetic bacteria such as Rhodopseudomonas palustris (Order Rhizobiales) or Rhodospirillum rubrum (Order Rhodospirillales). In addition, BluE is not found in Rhodobacterales such as Silicibacter pomeroyi, Dinoroseobacter shibae, Ruegeria pomeroyi, and Jannashia. Homologues of PduX or BluE have not been identified in these other purple photosynthetic bacteria or in archaea, leaving open the question of how these organisms produce L-Thr-P. Recent work identifying a bifunctional version of CobD in M. Mazei that has both L-Thr decarboxylase and L-Thr kinase activities might offer a clue (Tavares et al., 2018). It is not clear what evolutionary or metabolic distinction may be responsible for the restriction of BluE to this small group of Rhodobacterales.
Importantly, Rhodobacterales that possess BluE also encode the 5,6-dimethylbenzimidazole (DMB) synthase, BluB (EC:1.13.11.79) (Gray & Escalante-Semerena, 2007, Taga et al., 2007) (Fig. 3). Organisms that encode BluB appear to have a strong preference for DMB as the lower ligand base and do not synthesize or use Cbas with other bases (Taga et al., 2007, Gray & Escalante-Semerena, 2007, Campbell et al., 2006). Several of these Rhodobacterales also encode CbiZ, an amidohydrolase (EC 3.5.1.90) known to cleave AdoCbas between the secondary amine of the (R)-1-aminopropan-2-ol (AP) moiety that links the nucleotide to an ester on the corrinoid ring to form the nucleotide loop (Gray & Escalante-Semerena, 2009a). CbiZ from R. sphaeroides has been shown to cleave nucleotide loops that do not have DMB as the base, such as Adopseudo-Cbl, which has adenine as the base, or to a lesser extent, AdoCbi, which only has the AP moiety without a nucleotide attached (Gray & Escalante-Semerena, 2009a). The activity of CbiZ allows cobyric acid (Cby, the product of the CbiZ reaction) (Fig. 1) to re-enter the biosynthetic pathway thereby allowing R. sphaeroides to remodel salvaged Cbas by incorporating the preferred DMB base into the final product (Woodson & Escalante-Semerena, 2004, Gray & Escalante-Semerena, 2009b, Gray & Escalante-Semerena, 2009a). The presence of both BluE and BluB in these Rhodobacterales could imply a preference for the Cba with DMB as the base and AP-P as the nucleotide linker, known as adenosylcobalamin (AdoCbl, Coα-(α−5,6-dimethylbenzimidazolyl-Coβ-adenosylcobamide).
The genetic makeup of these Rhodobacterales led us to speculate about the evolutionary and or environmental context for the presence of these particular sets of genes. The Cbl-dependent ethylmalonyl-CoA mutase and methylmalonyl-CoA mutase enzymes in these organisms may require Cbl for optimal functionally and may not be as enzymatically active with other Cbas such as norCbas (EA-P as the linker rather than AP-P) or Cbas with lower ligands bases other than DMB, such as pseudo-Cbl (adenine as the base). To our knowledge the enzymatic activity of these enzymes with different Cba cofactors has not been reported.
In the environmental context, pseudo-Cbl is the dominant corrinoid found in marine surface water. While pseudo-Cbl is produced by other prokaryotes, cyanobacteria are thought to be the primary producer of pseudo-Cbl in marine environments. Thaumarchaeota (early-Co-insertion pathway) and Rhodobacterales (late-Co-insertion pathway) are thought to be the primary producers of Cbl (Cbas with DMB as the base) in marine environments (Heal et al., 2017, Doxey et al., 2015). Norpsuedo-Cbl has not been directly measured in marine waters but organohalide respiring bacteria that possess genes for norpseudo-Cbl dependent reductive dehalogenase (Kräutler et al., 2003) are known to inhabit marine subsurface sediments (Futagami et al., 2009) and have association with marine sponges (Liu et al., 2017). Eukaryotes cannot synthesize Cbas and must rely on dietary sources or symbiotic relationships with bacteria to acquire it. Eukaryotes can only take up and use Cbl and do use other Cbas such as pseudo-Cbl or norpseudo-Cbl. Some Rhodobacterales have symbiotic associations with marine phytoplankton (Amin et al., 2012, Buchan et al., 2014), that appear to obtain AdoCbl from their bacterial symbionts (Croft et al., 2005, Croft et al., 2006). The presence of cbiZ in the genomes of many Rhodobacterales confers an advantage to these microorganisms by allowing them to salvage pseudo-Cbl, which is the dominant marine species of Cbas, and potentially norpseudo-Cbl, and remodel them into Cbl, their preferred Cba. CbiZ activity has not been tested against norCbas, so its ability to cleave norpseudo-Cbl is unknown. The enzymatic preference towards producing only AdoCbl may be a result of evolutionary pressure on some Rhodobacterales in symbiotic relationships to produce only AdoCbl to accommodate their eukaryotic host’s specific metabolic requirements.
In vivo BluE phenotypes revel potential protein-protein interactions indicative of Cba biosynthesis enzyme complex.
It was surprising that the absence of an enzyme like CobB, which is involved in the early steps of the corrinoid ring biosynthesis pathway had impaired growth (Fig. 6C) when that portion of the pathway was bypassed by the addition of Cbi (Fig. 1B, Fig. 6, pathway scheme). One explanation for this result is CobB and possibly other proteins in the early steps of the pathway may interact with proteins in the late steps of the pathway such as CobD (CbiB in the early-Co-insertion pathway), in a Cba biosynthetic protein complex. Removal of either CobB (Fig. 6C, open squares) or BluE (asterisks) disrupts the protein complex and is further exacerbated by the removal of both (closed triangles). This would explain why the ΔcobB ΔbluE/pRsBluE strain had delayed growth (Fig. 6B, open squares vs Fig. 6A open diamonds), because while the enzymatic activity of CobB is not required, the presence of the protein itself may be required to stabilize a protein complex. These results are similar to the growth behavior of the RsBluEG99A variant in S. enterica (Fig. 5). Cumulatively, the results in figure 5 showed in S. enterica that only when the entire AP-P linker synthesis and attachment branch of the Cba synthesis pathway was bypassed by the addition of Cbi (Fig. 5D) was the ΔpduX/pRsBluEG99A strain able to grow. These results obtained with S. enterica suggest that in R. sphaeroides the RsBluE protein not only interacts with protein of the early steps like CobB but also with the L-Thr-P decarboxylase (CobD/CobC) and the AdoCby-P synthase (CbiB/CobD) enzymes. We speculate that this may occur through the formation of a multiprotein biosynthetic complex anchored to the membrane by the transmembrane AdoCby-P synthase (CbiB/CobD) protein (Zayas et al., 2007). Evidence supporting the idea of a Cba biosynthetic protein complex has been previously presented by our lab and others (Raux et al., 1996, O’Toole et al., 1993).
Conclusions
SePduX was the first enzyme reported to phosphorylate free L-Thr (Fan & Bobik, 2008), and it is annotated as a member of the GHMP kinase family (Bork et al., 1993). Here we add BluE from the Rhodobacterales as a new member of that family. Further investigation of BluE may reveal mechanistic differences with SePduX. There are several gaps in the late-cobalt-insertion AdoCba biosynthesis pathway, and within AdoCbl producers like R. sphaeroides, which appear to have a strong preference for the Cbas they synthesize and use. We have identified BluE as the L-Thr kinase for some of these Rhodobacterales. How other organisms that do not possess bluE or pduX generate L-Thr-P or L-Ser-P remains an open question.
Experimental procedures
Phylogenetic analysis and tree construction.
Sequences were obtained using Basic Local Alignment Search Tool for Protein (BLASTP) (Altschul et al., 1997). The protein sequences of the following proteins were used to search for homologues in the Integrated Microbial Genome (IMG) database (Markowitz et al., 2006). RsBluE (RSP_0788), RcBluE (RCAP_rcc02055), SePduX (STM2058), SeCbiK (STM2025), RsBluB (RSP_3218), RsCobN (RSP_2827), RsCobS (RSP_1977), RsCobT (RSP_1976) DhCbiX (DeshaDRAFT_4176) from Desulfitobacterium hafniense Y51, and BmCbiX (BMQ_2618) from Bacillus megaterium QM B1551. Only finished or permanent draft genomes with bit scores > 50 or e values > 1.0e−7 and pduX homologues adjacent to Cba biosynthetic or utilization genes on the chromosome were used in the analysis. The bluE homologues identified were always associates with Cba genes on the chromosomes. Locus tags and phylogenetic information for PduX and BluE homologues used to construct the phylogenic tree are listed in Table S3. Sequence header files were simplified with the find/replace and grep functions of TextWrangler (Bare Bones Software). Outliers and sequences with extreme divergence were not included nor were alleles not associated with AdoCba biosynthetic gene clusters on the chromosome. FASTA formatted sequences were aligned using the MUSCLE (Edgar, 2004) plugin within Geneious R8.1.7 software (Biomatters Ltd.) with 100 iterations and default settings. Phylogenetic trees were constructed with maximum likelihood using the online PhyML (Guindon et al., 2010) tool on the ATCG Montpellier Bioinformatics Platform (http://www.atgc-montpellier.fr/phyml/) using Jones-Taylor-Thornton substitution model (Jones et al., 1992) with 500 bootstrap replicates. The resulting tree was edited using Figtree (Rambaut, 2007) and Illustrator CS6 (Adobe). We used the packages ape (Paradis et al., 2004), geiger (Harmon et al., 2008), and diversitree (FitzJohn, 2012) of the R program (R Core Development Team, 2015) to represent in the phylogeny the presence or absence of CbiK, CbiX, CobNST, or BluB within the genome of each species. We plotted the presence/absence data in the phylogeny using the function trait.plot of the diversitree (FitzJohn, 2012) package. The presence of CbiK or CbiX homologues are represented as blue boxes on Fig. 3 All three subunits of CobNST had to be present on the chromosome for an organism to receive a green box on Fig. 3, denoting the presence of the complete cobaltochelatase of the late-Co-insertion pathway. ESPript 3.0 (Gouet et al., 1999) was used to generate an image of the alignment. Locus tags of bluE and pduX homologues used in the phylogenetic analysis are available in the Supplemental Information section (Table S1).
Bacterial strains and growth conditions.
The genotypes of strains used in this work are described in Table S2. All S. enterica strains carried a deletion of the chromosomal copy of the metE gene that encodes the AdoCbl-independent methionine synthase (MetE) enzyme (Peariso et al., 2001). The absence of MetE demands that the cell uses the Cba-dependent methionine synthase (MetH) enzyme to methylate homocysteine (Drennan et al., 1994, Hall et al., 2001, Taylor & Weissbach, 1973). All S. enterica strains used in this work also carried a mutation in the arabinose operon (allele ara-9) that prevented the utilization of L-(+)-arabinose as a carbon and energy source. Deletions of the ΔmetE and ΔpduX genes in S. enterica were constructed using the phage lambda Red recombinase system as described elsewhere (Datsenko & Wanner, 2000).
All Rhodobacter strains were derivatives of Rhodobacter sphaeroides 2.4.1. R. sphaeroides was grown at 30 °C in Sistrom’s medium A (Sistrom, 1960) lacking aspartate and glutamate and supplemented with CoCl2 (1 mg L−1). Succinate (10 mM) or acetate (30 mM) were used as sole carbon sources.
Rhodobacter strains construction.
R. sphaeroides genes were inactivated using published protocols (Gray & Escalante-Semerena, 2009b). Primers (Table S3) were used to amplify ~1,500-bp fragments upstream and downstream of the gene of interest. Fragments were fused using overlap extension PCR and cloned into EcoRI and XbaI restriction sites of pK18mobsacB as described below under Plasmid construction to generate plasmid pRsBLUE9. The pRsBLUE9 deletion plasmid was transformed into E. coli S17-λ cells, which contained conjugal transfer functions for bi-parental mating. R. sphaeroides cells were inoculated into 5 mL of Sistrom’s medium A containing succinate (10 mM) for 48 h. Cells of E. coli strains carrying deletion constructs were inoculated into lysogeny broth (LB) (Bertani, 1951, Bertani, 2004) with kanamycin (0.05 mg mL−1) overnight and sub-cultured (20 % v/v) into LB with no antibiotic the next morning. After 2.5 h of sub-cultured E. coli growth, recipient R. sphaeroides cells (1.5 mL) were harvested by centrifugation at 6,000 x g for 5 min, after which 0.5 mL of a culture of E. coli donor strain was added to the tube containing R. sphaeroides cells and centrifuged again at 6,000 x g for 5 min. Cells were washed with Sistrom’s medium (1 mL), pelleted (6,000 x g, for 5 min) and re-suspended in 0.1 mL of Sistrom’s medium. Cells were plated as a single droplet onto a LB agar plate and incubated for 24 h. Conjugation mixtures were streaked onto a Sistrom’s medium agar plate containing kanamycin (0.01 mg mL−1) and succinate (10 mM). Plates were incubated in an Advanced™Anoxomat® III Anaerobic jar and placed 50 cm away from the light source in a light box chamber equipped with 8 incandescent white light bulbs (60 W, 120V, 840 lumens each). Single colonies were inoculated into 50 mL of Sistrom’s medium supplemented with succinate (10 mM) and kanamycin (0.01 mg mL−1) and grown photosynthetically in sealed bottles with N2 gas in the headspace. For counter selection, cells were inoculated (10% v/v) into 500 mL of Sistrom’s medium containing succinate (10 mM) and sucrose (0.3 M, added after autoclaving). After cultures reached full density (2–3 days), cells were harvested by centrifugation at 6,000 x g. Pellets were re-suspended in 2–3 mL of Sistrom’s medium, diluted in 10-fold increments, and a 0.1-mL sample was plated at dilutions 10−7 and 10−8. Plates were incubated in an Advanced™Anoxomat® III Anaerobic jar and cells were grown photosynthetically as described above. Isolated colonies were screened using PCR for in-frame bluE deletions with primers listed in Table S3. Positive deletion strains were checked for kanamycin sensitivity to ensure loss of chromosomally inserted plasmid. For conjugations of complementation vector (pRsBluE10), the same protocol was followed up to incubation on Sistrom’s agar plate with kanamycin.
Plasmid construction.
R. sphaeroides strain 2.4.1 and R. capsulatus strain SB1003 were gifts from Timothy Donohue (University of Wisconsin, Madison WI). Genomic DNA was isolated using Wizard® SV Genomic DNA Purification kit (Promega). Oligonucleotide primers were purchased from Integrated DNA Technologies Inc. Primers for cloning were designed using the Saccharomyces Genome Database web-based primer design tool http://www.yeastgenome.org/cgi-bin/web-primer. Genes were PCR amplified from the appropriate genomic DNA template with PCR Extender Polymerase (5 Prime) and the primer pairs listed in Table S3. PCR products and vectors were treated with FastDigest™ restriction endonucleases (Fermentas) indicated in the primer name in Table S3 and purified with the Wizard® SV Gel and PCR Clean-Up kit (Promega). Cloning vectors were treated with Fast AP alkaline phosphatase (Fermentas). PCR fragments and cloning vectors were ligated together using T4 Ligase (New England BioLabs) and introduced into E. coli DH5α (Raleigh et al., 1989, Woodcock et al., 1989) via electroporation (Calvin & Hanawalt, 1988). Plasmid DNA was purified using the Wizard® Plus SV Miniprep kit (Promega). Plasmid sequence was confirmed by sequencing using BigDye® (ABI PRISM) protocols, and sequencing reactions were resolved at the University of Wisconsin-Madison Biotechnology Center. The list of resulting plasmids can be found in Table S2. Primers for site directed mutagenesis were designed with the QuikChange Primer Design tool found at http://www.genomics.agilent.com/primerDesignProgram.jsp. DNA for site-directed mutagenesis was amplified using PfuUltra II Fusion DNA polymerase (Stratagene), and site-directed mutagenesis was performed using the QuikChange protocol from Stratagene. Genes used in this study were cloned into cloning vectors pBBR1MCS-2 (Kovach et al., 1995) for complementation studies in R. sphaeroides, or pBAD24 and pBAD30 (Guzman et al., 1995) for arabinose-dependent gene expression used in complementation studies in S. enterica. Plasmid pTEV5 (Rocco et al., 2008) was used to overexpress the gene of interest to isolate protein needed for biochemical studies.
S. enterica growth media and conditions.
No-carbon essential (NCE) (Berkowitz et al., 1968) was used as minimal growth medium with either glycerol (22 mM) or ethanolamine hydrochloride (90 mM) as the carbon and energy source. When added to the medium, the following supplements were at the indicated concentrations: trace minerals, (10 mL L−1) (Balch & Wolfe, 1976); MgSO4 (1 mM), 5,6-dimethylbenzimidazole (DMB, 0.15 mM), ampicillin (0.1 mg mL−1), arabinose (0.5 mM). All corrinoids (dicyanocobyric acid [(CN)2Cby], dicyanocobinamide [(CN)2Cbi], and cyanocobalamin (CNCbl)) were added at 1 nM or 5 nM final concentration when glycerol was used or 300 nM when ethanolamine was used as the carbon and energy source. Fe(III)-citrate (0.05 mM) was also added to the medium when ethanolamine was used. (CN)2Cby was a gift from Paul Renz (Universität-Hohenheim, Stuttgart, Germany). When indicated L-Thr, L-Ser, L-Thr-P, and L-Ser were present in the medium at 1 mM. For growth analysis involving RsBluE reactions, the reactions were filtered with sterile 0.2-μm Spin-X centrifuge filters (Corning) and added to the growth medium at 8% (v/v) concentration. All chemicals were purchased from Sigma-Aldrich. S. enterica strains were cultured in nutrient broth (NB, Difco Laboratories) (0.8% w/v) containing NaCl (85 mM). Lysogeny broth was used as rich medium to culture E. coli strains unless otherwise indicated. For complementation studies strains were grown in minimal medium in triplicate in sterile 96-well tissue culture plates (Falcon). A 1% (v/v) inoculum of an overnight cell culture grown in NB was used when glycerol was the carbon and energy source, and a 5% inoculum (v/v) was used for ethanolamine minimal medium in 0.2 mL total volume. Growth was monitored using Gen5 software (BioTek Instruments) at 37˚C with continuous shaking using the slow setting of an EL808 Ultra or PowerWave XS Microplate Reader (BioTek Instruments). Cell density measurements at 630 nm were acquired every 30 min for 24 or 72 h. Data were analyzed using the Prism v6 software package (GraphPad Software). Growth analyses were performed in triplicate in three independent experiments. Representative growth curves are shown with the error bars representing the standard error of the mean.
Rhodobacter growth medium, conditions, and analysis.
Relevant R. sphaeroides strains were grown for three days at 30°C in Sistrom’s medium with succinate (10 mM) and kanamycin (0.01 mg mL−1). Cells were washed twice with Sistrom’s medium after centrifugation at 6,000 x g for 5 min. Cells were sub-cultured (1:50) into 0.2 mL Sistrom’s medium with acetate (30 mM) and kanamycin and grown in a 96-well microtiter plate. Plates were incubated at 30°C inside a temperature-controlled chamber of a PowerWave XS Microplate Reader (BioTek Instruments). Plates were continuously shaken using the medium setting of the instrument and cell density was monitored at 630 nm. Data were analyzed using Prism v6 software package (GraphPad Software). Growth analyses were performed in triplicate in three independent experiments. Representative growth curves are shown with the error bars representing the standard error of the mean. Pigment analysis was performed with culture samples (100 μL) pipetted into a 96-well plate and scanned (A600–1000) using a Spectramax (Molecular Devices) UV-vis spectrophotometer.
Solubilization and enrichment of RsBluE and SePduX proteins.
E. coli C43 (λDE3) (JE6664) (Miroux & Walker, 1996) cells carrying plasmids pPDU23 or pRsBLUE4 encoding N-terminally His6 tagged S. enterica pduX+ or R. sphaeroides bluE+ genes cloned into pTEV5 (Rocco et al., 2008), respectively, were grown in 4 L of LB. E. coli does not encode PduX or homologues in its genome and is not known to synthesize or use L-Thr-P. Cells were centrifuged at 6,000 x g for 10 min. Cell pellets were placed at −20°C until used. Cell pellets were thawed in 30 mL of bind buffer [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES-NaOH, 50 mM, pH 7.9 at 4°C), NaCl (500 mM), imidazole (5 mM)], and lysed (1.9 × 108 kPa) using a TS Series (1.1 kW) Bench top cell disrupter (Constant Systems Ltd.), equipped with a cooling jacket on the disruptor head to maintain a 6°C temperature using a Neslab ThermoFlex 900 recirculating chiller (Thermo Scientific). Cell particulates were removed by centrifugation at 39,000 x g for 20 min. The supernatant containing soluble proteins was discarded. The pellet containing insoluble RsBluE or SePduX was re-suspended in 30 mL of bind buffer along with 12% (w/v) sarkosyl, followed by incubation at room temp for 30 min with shaking until the pellet was solubilized. Remaining debris was removed by centrifugation at 20,000 x g for 10 min. Sarkosyl was removed stepwise by dialysis into bind buffer containing 2% (w/v), then 1% (w/v) sarkosyl, followed by three rounds of dialysis into bind buffer without sarkosyl. Each dialysis step was performed in 2 L of buffer at 4ºC for 3 h. After the final dialysis the protein solution had approximately 1–2% sarkosyl present. The presence of solubilized protein was confirmed by SDS-PAGE (Laemmli, 1970) followed by staining with Coomassie Blue R (Sasse, 1991). Precipitated protein was removed by centrifugation at 20,000 x g for 10 min. Ni(NTA)-affinity chromatography was used with a 1.5-mL bed volume of HisPur Ni-NTA resin (Thermo Scientific). The column was equilibrated with bind buffer [HEPES (50 mM pH 7.9 at 4°C), NaCl (500 mM), imidazole (5 mM)] before supernatant was applied to the column. Due to the viscosity of the solution caused by sarkosyl, movement of the supernatant across the column was facilitated by using a P1 peristaltic pump (GE Healthcare) with a flow rate of 1 mL min−1. After binding to the column, the column was washed with four column volumes of bind buffer, before proteins were eluted by stepwise increases in the imidazole concentration from 20 to 100 mM with 20-mM steps; 5-mL fractions were collected for each step. The His6 tagged proteins did not bind to the column due to interference by sarkosyl and were found in the flow through. His6 tagged proteins were treated with rTEV protease (1:100, rTEV:His6-protein ratio) for 3 h at 25°C in bind buffer containing 1,4-dithiothreitol (DTT, 1 mM). It is unclear if the tag was removed, as sarkosyl-solubilized protein did not bind a Ni column. The protein was dialyzed into bind buffer containing ethylenediaminetetraacetic acid (EDTA, 1 mM) at room temperature for 1 h, then dialyzed twice more against the same buffer devoid of EDTA. Final dialysis was performed until the protein began to slowly precipitate to obtain the minimal concentration of sarkosyl required to maintain the enriched RsBluE and SePduX proteins in solution. Precipitated protein was removed by centrifugation at 6,000 x g for 5 min. Proteins were concentrated using Amicon Ultracel 10,000 MWCO centrifugal filters (Millipore), flash frozen drop-wise into liquid N2, and stored at −80ºC until used. Further attempts to completely remove sarkosyl by treatment with BioBeads™ SM-2 Resin (BioRad) or Pierce™ Detergent Removal Spin (ThermoFisher) resulted in complete protein precipitation. Cells harboring the empty cloning vector, pTEV5, were subjected to the same purification process and sarkosyl solubilized extracts were used as a control for enzyme assays described below. Protein concentrations were determined using a NanoDrop 1000 spectrophotometer (Thermo Scientific), using the theoretical molecular weights and A280 molar extinction coefficients for each protein, which were obtained from the ExPASy Protparam database (Gasteiger et al., 2003). Protein concentrations was verified using Bradford Assay Bio-Rad kit (Bradford, 1976). Proteins were resolved by SDS-PAGE. Protein purity was estimated using band densitometry with a Fotodyne imaging system and Foto/Analyst v.5.00 software (Fotodyne Inc.) for image acquisition and TotalLab v.2005 software for analysis (Nonlinear Dynamics). RsBluE and SePduX were purified to 70% purity.
Purification of detergent-free RsBluE and SePduX proteins.
Due to the partial potential effects of unfolding and concern of potential interference of sarkosyl on the enzymatic activity, we attempted to purify RsBluE and SePduX without sarkosyl or other detergents. Protein overexpression and cell lysis proceeded as described above with the exception that cell pellets were not frozen but used immediately after harvesting. Sarkosyl was not present in buffers, and after cell lysis and centrifugation to remove cellular debris, the soluble fraction was retained. The discarded insoluble fraction contained most of the overproduced proteins. The soluble fraction was applied to a HisPur Ni-NTA resin (Thermo Scientific) as described above. A small quantity of soluble His6-tagged protein was bound to the resin and was eluted with imidazole (200 mM). This protein remained in solution for ~30–60 min before precipitating. Fractions containing soluble protein were immediately assayed for activity after elution from the column as described below. Figure S4 shows representative SDS-PAGE gels of whole cell extracts and fractions containing RsBluE, SePduX, and extracts from cells harboring the empty vector pTEV5, which was used as control. Protein purity was estimated using band densitometry with a Fotodyne imaging system and Foto/Analyst v.5.00 software (Fotodyne Inc.) for image acquisition and TotalLab v.2005 software for analysis (Nonlinear Dynamics). His6-tagged RsBluE and SePduX were purified to 87% and 89% purity, respectively (Fig. 7A) Protein identity was verified by in-gel trypsin digestion, followed by MALDI mass spectrometry, peptide mass fingerprinting and protein identification via Mascot (Matrix Science) protein identification software and peptide sequence databases performed by the Proteomics and Mass Spectrometry Core Facility at the University of Georgia (Athens, GA). Previously published non-cleavable C- and N-terminally His tagged SePduX proteins (Fan et al., 2009, Fan & Bobik, 2008) were purified using published methods (Fan et al., 2009) and those outlined above. Plasmids encoding non-cleavable C- and N-terminally His tagged SePduX proteins were a gift from Thomas Bobik (Iowa State University).
ATPase activity assay.
ATPase activity was assessed using the ADP-Glo™ ATPase Assay kit (Promega) (Zegzouti et al., 2009). Per the following manufacturer’s instructions, this two-step endpoint assay was used in the following manner. Reaction mixtures containing ATP, L-Thr, and RsBluE or SePduX, were incubates for 1 hr and stopped with a proprietary reagent that depleted any remaining unused ATP. Then a secondary reagent was added, which converted the RsBluE- or SePduX-generated ADP back to ATP. ATP was then measured using an ATP-dependent luciferase reaction. The resulting luminescence was measured at 560 nm with a SpectraMax Plus Gemini EM microplate spectrophotometer (Molecular Devices) equipped with SoftMax Pro v4 software. Samples (25 μL each) taken from reaction mixtures containing HEPES buffer (50 mM, pH 7.9 at 25°C), MgCl2 (1 mM), ATP (0.1 mM or 0.01 mM), L-Thr (0.01 mM or 10 mM), and sarkosyl-solubilized protein (0.14 μM or 12 μM) were incubated at 25°C for 1 h. A 5-μL sample of the reaction mixture was used in the ADP-Glo™ assay. Nunc 96-well round bottom black polypropylene microtiter plates (Thermo Fisher) were used to minimize background. ATP to ADP conversion was quantified from the luminescence (relative light units; RLU) after subtracting the background from the no-enzyme control and comparing the value to a standard curve of luminescence vs % ATP to ADP conversion (Fig. S3) and converted into units of ADP produced (mM) per mg of protein. Reactions were performed in triplicate in two independent experiments. Error bars represent the standard deviation. For ATPase inhibition assays the following inhibitors were used adenosine diphosphate (ADP, 20 mM), sodium pyrophosphate (PPi, 10 mM), sodium phosphate monobasic (Pi, 10 mM), adenosine 5′-[γ-thio]triphosphate (ADP-γ-S, 0.2 mM), α,β-methyleneadenosine 5′-triphosphate (AMP-CPP, 10 mM), P1,P3-di(adenosine-5′) triphosphate ammonium salt (Ap3A, 10 mM).
Phosphorous nuclear magnetic resonance (31P-NMR) analysis of the L-threonine kinase reaction products.
Proton-decoupled 31P-NMR spectra were obtained using a Varian Unity Inova 500 MHz spectrometer (Chemical Sciences Magnetic Resonance Facility, University of Georgia, Athens, GA) with the following parameters: pulse angle 45°, relaxation delay 1 s, excitation pulse 3.88 μs, spectral width 12.11 kHz, acquisition time 0.81 s. Chemical shifts were referenced to a H3PO4 (85%) standard set to 0.0 ppm. Reaction mixtures (0.5 mL) consisted of HEPES buffer (50 mM, pH 7.5 at 25°C), MgCl2 (1 mM), ATP (3 mM), L-Thr or L-Ser (6 mM). Immediately after elution from the Ni-NTA column, 10-μL samples of detergent-free protein (11 μM) were added to the reaction mixtures, which were incubated at 25°C for 1 h. Reaction mixtures used as negative controls contained L-Thr, L-Thr-P, L-Ser-P, Pi, and AMP (3 mM each), or ATP, sodium pyrophosphate (PPi), and sodium tripolyphosphate (PPPi) (6 mM each). Protein was removed from reaction mixtures by filtration using Amicon Ultracel filters (Millipore) with 10-kDa molecular mass size exclusion cut offs. Reaction mixtures were brought up to a final volume of 0.6 mL in D2O (17% v/v). 31P-NMR data were processed with MestReNova software version v7.0.0–8333 (Mestrelab Research). Representative spectra are shown from one of two independent reactions with detergent-free enzyme acquired from two independent protein purifications.
Spectrophotometric measurement of RsBluE and SePduX ATPase activities as a function of substrate.
ATPase activity were measured using an NADH-consuming assay (Bergmeyer et al., 1985, Horswill & Escalante-Semerena, 2002, Wilson, 1962, Havemann & Bobik, 2003). All substrate stocks were made fresh. Reaction mixtures (0.1 mL) contained HEPES buffer (50 mM, pH 7.0 at 25°C), MgCl2 (5 mM), phosphoenolpyruvate (PEP, 3 mM), NADH (0.1 mM), pyruvate kinase (1 U), lactate dehydrogenase (1.5 U); mixtures were incubated at 25°C with measurements taken every 11 s over a 20-min period. For the ATPase specific activity L-Thr concentration was held at 50 mM while the ATP concentration was varied (0–100 mM). To measure the effect of L-Thr on the ATPase activity, ATP concentration was held at 50 mM while the concentration of L-Thr was varied (0–100 mM). Reactions were started by the addition of RsBluE or SePduX (3 μM). The absorbance at 340 nm was monitored in a 96-well plate using the SpectraMax Plus UV-visible spectrophotometer (Molecular Devices) equipped with SoftMax Pro v6.2 software. Enzyme activities were calculated as described elsewhere (Wilson, 1962). Specific activity data are presented with standard deviations from two independent experiments performed in technical triplicate, with error bars representing the standard deviation.
Supplementary Material
Acknowledgments
The authors declare no conflict of interest. This work was supported by NIH grant R37 GM40313 to J.C.E.-S. N.K.T. was supported in part by NIH grant F31 GM095230 and Advanced Opportunity Fellowship awarded by the Graduate School of the University of Wisconsin, Madison. We thank Paul Renz (Universität Stuttgart) for his gift of (CN)2Cby, Tim Donohue (University of Wisconsin-Madison) for providing R. sphaeroides strain 2.4.1 and R. capsulatus strain SB1003 (University of Wisconsin-Madison), and Paul Merchant for technical assistance. Thanks also to Dongtao Cui at the Chemical Sciences Magnetic Resonance facility (University of Georgia) for training and assistance with NMR experiments. We are grateful to Paula Pappalardo (University of Georgia) for assistance with R and phylogenetic analysis. Thank you to Thomas Bobik for providing plasmids encoding pduX.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Miller W, and Lipmann DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin SA, Green DH, Gardes A, Romano A, Trimble L, and Carrano CJ (2012) Siderophore-mediated iron uptake in two clades of Marinobacter spp. associated with phytoplankton: the role of light. Biometals 25: 181–192. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Lango J, Carkeet C, Britten A, Kräutler B, Hammock BD, and Roth JR (2008) One pathway can incorporate either adenine or dimethylbenzimidazole as an alpha-axial ligand of B12 cofactors in Salmonella enterica. J. Bacteriol 190: 1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DI, and Roth JR (1989) Mutations affecting regulation of cobinamide biosynthesis in Salmonella typhimurium. J. Bacteriol 171: 6726–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, and Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol 32: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, and Ludwig ML (2002) Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat. Struct. Biol 9: 53–56. [DOI] [PubMed] [Google Scholar]
- Banerjee R (2003) Radical carbon skeleton rearrangements: catalysis by coenzyme B12-dependent mutases. Chem. Rev 103: 2083–2094. [DOI] [PubMed] [Google Scholar]
- Beck R, Raux E, Thermes C, Rambach A, and Warren M (1997) CbiX: a novel metal-binding protein involved in sirohaem biosynthesis in Bacillus megaterium. Biochem. Soc.Trans 25: 77S. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bergmeyer J, Grassl M, and Berger R (1985) Methods of enzymatic analysis. vol. iv “enzymes 2: Esterases, glycosidases, lyases, ligases”. 3rd Edition Weinheim; Deerfield Beach, Florida; Basel: Verlag Chemie, 1984. 426 S., 258 DM. Acta Biotechnologica 5: 114–114. [Google Scholar]
- Berkowitz D, Hushon JM, Whitfield HJ Jr., Roth J, and Ames BN (1968) Procedure for identifying nonsense mutations. J. Bacteriol 96: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G (2004) Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol 186: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedendieck R, Malten M, Barg H, Bunk B, Martens JH, Deery E, Leech H, Warren MJ, and Jahn D (2010) Metabolic engineering of cobalamin (vitamin B12) production in Bacillus megaterium. Microb. Biotechnol 3: 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel AJ (1992) Oxygen-regulated steps in Rhodobacter capsulatus tetrapyrrole biosynthetic pathway. J. Bacteriol 174: 5272–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F, Couder M, Debussche L, Thibaut D, Cameron B, and Crouzet J (1991) Biosynthesis of vitamin B12: stepwise amidation of carboxyl groups b, d, e, and g of cobyrinic acid a,c-diamide is catalyzed by one enzyme in Pseudomonas denitrificans. J. Bacteriol 173: 6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F, Thibaut D, Debussche L, Hertle R, Zipfel F, and Muller G (1993) Parallels and decisive differences in vitamin-B12 biosyntheses. Angew. Chem. Int. Ed. Engl 32: 1651–1653. [Google Scholar]
- Bork P, Sander C, and Valencia A (1993) Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci 2: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C, (1982) Cobalamin transport in microorganisms In: B12. Dolphin D (ed). New York: John Wiley & Sons, pp. 31–56. [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Buchan A, LeCleir GR, Gulvik CA, and Gonzalez JM (2014) Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol 12: 686–698. [DOI] [PubMed] [Google Scholar]
- Calvin NM, and Hanawalt PC (1988) High-efficiency transformation of bacterial cells by electroporation. J. Bacteriol 170: 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Blanche F, Rouyez MC, Bisch D, Famechon A, Couder M, Cauchois L, Thibaut D, Debussche L, and Crouzet J (1991a) Genetic analysis, nucleotide sequence, and products of two Pseudomonas denitrificans cob genes encoding nicotinate-nucleotide: dimethylbenzimidazole phosphoribosyltransferase and cobalamin (5’-phosphate) synthase. J. Bacteriol 173: 6066–6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Guilhot C, Blanche F, Cauchois L, Rouyez MC, Rigault S, Levy-Schil S, and Crouzet J (1991b) Genetic and sequence analyses of a Pseudomonas denitrificans DNA fragment containing two cob genes. J. Bacteriol 173: 6058–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Taga ME, Mistry K, Lloret J, Anderson PJ, Roth JR, and Walker GC (2006) Sinorhizobium meliloti bluB is necessary for production of 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc. Natl. Acad. Sci. U S A 103: 4634–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins HF, Biedendieck R, Leech HK, Gray M, Escalante-Semerena JC, McLean KJ, Munro AW, Rigby SE, Warren MJ, and Lawrence AD (2013) Bacillus megaterium has both a functional BluB protein required for DMB synthesis and a related flavoprotein that forms a stable radical species. PLoS One 8: e55708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, and Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93. [DOI] [PubMed] [Google Scholar]
- Croft MT, Warren MJ, and Smith AG (2006) Algae need their vitamins. Eukaryot. Cell 5: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J, Cameron B, Cauchois L, Rigault S, Rouyez MC, Blanche F, Thibaut D, and Debussche L (1990) Genetic and sequence analysis of an 8.7-kilobase Pseudomonas denitrificans fragment carrying eight genes involved in transformation of precorrin-2 to cobyrinic acid. J. Bacteriol 172: 5980–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, and Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L, Couder M, Thibaut D, Cameron B, Crouzet J, and Blanche F (1991) Purification and partial characterization of cob(I)alamin adenosyltransferase from Pseudomonas denitrificans. J. Bacteriol 173: 6300–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey AC, Kurtz DA, Lynch MD, Sauder LA, and Neufeld JD (2015) Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J 9: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan CL, Huang S, Drummond JT, Matthews RG, and Ludwig ML (1994) How a protein binds B12: A 3.0Å X-ray structure of B12-binding domains of methionine synthase. Science 266: 1669–1674. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb TJ, Retey J, Fuchs G, and Alber BE (2008) Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclade of coenzyme B12-dependent acyl-CoA mutases. J. Biol. Chem 283: 32283–32293. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena JC (2007) Conversion of cobinamide into adenosylcobamide in bacteria and archaea. J. Bacteriol 189: 4555–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC, and Warren MJ, (2008) Biosynthesis and Use of Cobalamin (B12) In: EcoSal - Escherichia coli and Salmonella: cellular and molecular biology. Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM & Squires CL (eds). Washington, D. C.: ASM Press, pp. [Google Scholar]
- Fan C, and Bobik TA (2008) The PDUX enzyme of Salmonella enterica is an L-threonine kinase used for coenzyme B12 synthesis. J. Biol. Chem 283: 11322–11329. [DOI] [PubMed] [Google Scholar]
- Fan C, Fromm HJ, and Bobik TA (2009) Kinetic and functional analysis of L-threonine kinase, the PduX enzyme of Salmonella enterica. J. Biol. Chem 284: 20240–20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzJohn RG (2012) Diversitree: comparative phylogenetic analyses of diversification in R. Meth. Ecol. Evol 3: 1084–1092. [Google Scholar]
- Futagami T, Morono Y, Terada T, Kaksonen AH, and Inagaki F (2009) Dehalogenation Activities and Distribution of Reductive Dehalogenase Homologous Genes in Marine Subsurface Sediments. Appl. Environ. Microbiol 75: 6905–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, and Bairoch A (2003) ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31: 3784–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JC, Banerjee RV, Huang S, Sumner JS, and Matthews RG (1992) Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: two solutions to the same chemical problem. Biochemistry 31: 6045–6056. [DOI] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, and Metoz F (1999) ESPript: multiple sequence alignments in PostScript. Bioinformatics 15: 305–308. [DOI] [PubMed] [Google Scholar]
- Gough SP, Petersen BO, and Duus JO (2000) Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor. Proc. Natl. Acad. Sci. U S A 97: 6908–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, and Escalante-Semerena JC (2007) Single-enzyme conversion of FMNH2 to 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc. Natl. Acad. Sci. U S A 104: 2921–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, and Escalante-Semerena JC (2009a) The cobinamide amidohydrolase (cobyric acid-forming) CbiZ enzyme: a critical activity of the cobamide remodelling system of Rhodobacter sphaeroides. Mol. Microbiol 74: 1198–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, and Escalante-Semerena JC (2009b) In vivo analysis of cobinamide salvaging in Rhodobacter sphaeroides strain 2.4.1. J. Bacteriol 191: 3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, and Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, and Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol 177: 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Vander Kooi CW, Stasik CN, Stevens SY, Zuiderweg ER, and Matthews RG (2001) Mapping the interactions between flavodoxin and its physiological partners flavodoxin reductase and cobalamin-dependent methionine synthase. Proc. Natl. Acad. Sci. USA 98: 9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, and Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24: 129–131. [DOI] [PubMed] [Google Scholar]
- Havemann GD, and Bobik TA (2003) Protein content of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol 185: 5086–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal KR, Qin W, Ribalet F, Bertagnolli AD, Coyote-Maestas W, Hmelo LR, Moffett JW, Devol AH, Armbrust EV, Stahl DA, and Ingalls AE (2017) Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc. Natl. Acad. Sci. U S A 114: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt D, Lawrence AD, Lindenmeyer M, Deery E, Heathcote P, Rigby SE, and Warren MJ (2005) Aerobic synthesis of vitamin B12: ring contraction and cobalt chelation. Biochem. Soc. Trans 33: 815–819. [DOI] [PubMed] [Google Scholar]
- Hondorp ER, and Matthews RG (2004) Oxidative stress inactivates cobalamin-independent methionine synthase (MetE) in Escherichia coli. PLoS Biol 2: e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horswill AR, and Escalante-Semerena JC (2002) Characterization of the propionyl-CoA synthetase (PrpE) enzyme of Salmonella enterica: Residue Lys592 is required for propionyl-AMP synthesis. Biochemistry 41: 2379–2387. [DOI] [PubMed] [Google Scholar]
- Jakubowski H (1990) Proofreading in vivo: editing of homocysteine by methionyl-tRNA synthetase in Escherichia coli. Proc. Natl. Acad. Sci 87: 4504–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, and Thornton JM (1992) The Rapid Generation of Mutation Data Matrices From Protein Sequences. Comput. Appl. Biosci 8: 275–282. [DOI] [PubMed] [Google Scholar]
- Keller S, Kunze C, Bommer M, Paetz C, Menezes RC, Svatos A, Dobbek H, and Schubert T (2018) Selective Utilization of Benzimidazolyl-Norcobamides as Cofactors by the Tetrachloroethene Reductive Dehalogenase of Sulfurospirillum multivorans. J Bacteriol 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Ruetz M, Kunze C, Krautler B, Diekert G, and Schubert T (2013) Exogenous 5,6-dimethylbenzimidazole caused production of a non-functional tetrachloroethene reductive dehalogenase in Sulfurospirillum multivorans. Environ. Microbiol 16: 3361–3369. [DOI] [PubMed] [Google Scholar]
- Keller S, Ruetz M, Kunze C, Krautler B, Diekert G, and Schubert T (2014) Exogenous 5,6-dimethylbenzimidazole caused production of a non-functional tetrachloroethene reductive dehalogenase in Sulfurospirillum multivorans. Environ. Microbiol 16: 3361–3369. [DOI] [PubMed] [Google Scholar]
- Keller S, Treder A, von Reuss SH, Escalante-Semerena JC, and Schubert T (2016) The SMUL_1544 Gene Product Governs Norcobamide Biosynthesis in the Tetrachloroethene-Respiring Bacterium Sulfurospirillum multivorans. J Bacteriol 198: 2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, and Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166: 175–176. [DOI] [PubMed] [Google Scholar]
- Kräutler B, Fieber W, Osterman S, Fasching M, Ongania K-H, Gruber K, Kratky C, Mikl C, Siebert A, and Diekert G (2003) The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is Norpseudo-B12, a new type of natural corrinoid. Helv. Chim. Acta 86: 3698–3716. [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Leech HK, Raux-Deery E, Heathcote P, and Warren MJ (2002) Production of cobalamin and sirohaem in Bacillus megaterium: an investigation into the role of the branchpoint chelatases sirohydrochlorin ferrochelatase (SirB) and sirohydrochlorin cobalt chelatase (CbiX). Biochem. Soc. Trans 30: 610–613. [DOI] [PubMed] [Google Scholar]
- Liu J, Lopez N, Ahn Y, Goldberg T, Bromberg Y, Kerkhof LJ, and Haggblom MM (2017) Novel Reductive Dehalogenases From The Marine Sponge Associated Bacterium Desulfoluna spongiiphila. Environ. Microbiol. Rep 9: 537–549. [DOI] [PubMed] [Google Scholar]
- Markowitz VM, Korzeniewski F, Palaniappan K, Szeto E, Werner G, Padki A, Zhao X, Dubchak I, Hugenholtz P, Anderson I, Lykidis A, Mavromatis K, Ivanova N, and Kyrpides NC (2006) The integrated microbial genomes (IMG) system. Nucleic Acids Res 34: D344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes TA, Deery E, Warren MJ, and Escalante-Semerena JC, (2017) Cobalamin Biosynthesis and Insertion In: Encyclopedia of Inorganic and Bioinorganic Chemistry. Scott RA (ed). Chichester, UK: John Wiley & Sons, Ltd, pp. 1–24. [Google Scholar]
- McGoldrick HM, Roessner CA, Raux E, Lawrence AD, McLean KJ, Munro AW, Santabarbara S, Rigby SE, Heathcote P, Scott AI, and Warren MJ (2005) Identification and characterization of a novel vitamin B12 (cobalamin) biosynthetic enzyme (CobZ) from Rhodobacter capsulatus, containing flavin, heme, and Fe-S cofactors. J. Biol. Chem 280: 1086–1094. [DOI] [PubMed] [Google Scholar]
- Miroux B, and Walker JE (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol 260: 289–298. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, Howard MJ, Rigby SE, and Warren MJ (2013) Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proc. Natl. Acad. Sci. U S A 110: 14906–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Rondon MR, and Escalante-Semerena JC (1993) Analysis of mutants of defective in the synthesis of the nucleotide loop of cobalamin. J. Bacteriol 175: 3317–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Claude J, and Strimmer K (2004) APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Peariso K, Zhou ZS, Smith AE, Matthews RG, and Penner-Hahn JE (2001) Characterization of the zinc sites in cobalamin-independent and cobalamin-dependent methionine synthase using zinc and selenium X-ray absorption spectroscopy. Biochemistry 40: 987–993. [DOI] [PubMed] [Google Scholar]
- Pollich M, and Klug G (1995) Identification and sequence analysis of genes involved in late steps of cobalamin (vitamin B12) synthesis in Rhodobacter capsulatus. J. Bacteriol 177: 4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollich M, Wersig C, and Klug G (1996) The bluF gene of Rhodobacter capsulatus is involved in conversion of cobinamide to cobalamin (vitamin B12). J. Bacteriol 178: 7308–7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Development Team, (2015) R: A language and environment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing, pp. [Google Scholar]
- Raleigh EA, Lech K, and Brent R, (1989) Selected topics from classical bacterial genetics In: Curr. Protoc. Mol. Biol Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA & Struhl K (eds). New York: Wiley Interscience, pp. 1.4. [Google Scholar]
- Rambaut A, (2007) FigTree In. University of Edinburgh, Edinburgh UK: Institute of Evolutionary Biology, University of Edinburgh, Ashworth Laboratories; pp. FigTree is designed as a graphical viewer of phylogenetic trees and as a program for producing publication-ready figures.. [Google Scholar]
- Raux E, Lanois A, Levillayer F, Warren MJ, Brody E, Rambach A, and Thermes C (1996) Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol 178: 753–t767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux E, Lanois A, Warren MJ, Rambach A, and Thermes C (1998) Cobalamin (vitamin B12) biosynthesis: identification and characterization of a Bacillus megaterium cobI operon. Biochem. J 335: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco CJ, Dennison KL, Klenchin VA, Rayment I, and Escalante-Semerena JC (2008) Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid 59: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, and Gelfand MS (2003) Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem 278: 41148–41159. [DOI] [PubMed] [Google Scholar]
- Roessner CA, Santander PJ, and Scott AI (2001) Multiple biosynthetic pathways for vitamin B12: variations on a central theme. Vitam. Horm 61: 267–297. [DOI] [PubMed] [Google Scholar]
- Roof DM, and Roth JR (1988) Ethanolamine utilization in Salmonella typhimurium. J. Bacteriol 170: 3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof DM, and Roth JR (1989) Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium. J. Bacteriol 171: 3316–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, (1991) Detection of proteins In: Curr. Protoc. Mol. Biol Ausubel FA, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA & Struhl K (eds). New York: Wiley Interscience, pp. 10.16.11–10.16.18. [Google Scholar]
- Schroeder S, Lawrence AD, Biedendieck R, Rose RS, Deery E, Graham RM, McLean KJ, Munro AW, Rigby SE, and Warren MJ (2009) Demonstration that CobG, the monooxygenase associated with the ring contraction process of the aerobic cobalamin (vitamin B12) biosynthetic pathway, contains a Fe-S center and a mononuclear non-heme iron center. J. Biol. Chem 284: 4796–4805. [DOI] [PubMed] [Google Scholar]
- Sistrom WR (1960) A requirement for sodium in the growth of Rhodopseudomonas spheroides. J. Gen. Microbiol 22: 778–785. [DOI] [PubMed] [Google Scholar]
- Smith DM, Fraga H, Reis C, Kafri G, and Goldberg AL (2011) ATP binds to proteasomal ATPases in pairs with distinct functional effects, implying an ordered reaction cycle. Cell 144: 526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, and Walker GC (2007) BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature 446: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares NK, Zayas CL, and Escalante-Semerena JC (2018) The Methanosarcina mazei MM2060 gene encodes a bifunctional kinase/decarboxylase enzyme involved in cobamide biosynthesis. Biochemistry, doi: 10.1021/acs.biochem.8b00546; published online on 6.27/2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, and Weissbach H, (1973) N5-methylenetetrahydrofolate-homocysteine methyltransferases In: The Enzymes. Boyer PD (ed). New York: Academic Press, Inc., pp. 121–165. [Google Scholar]
- Thibaut D, Couder M, Crouzet J, Debussche L, Cameron B, and Blanche F (1990) Assay and purification of S-adenosyl-L-methionine:precorrin-2 methyltransferase from Pseudomonas denitrificans. J. Bacteriol 172: 6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek C, Paces V, Maltsev N, Paces J, Haselkorn R, and Fonstein M (1997) Sequence of a 189-kb segment of the chromosome of Rhodobacter capsulatus SB1003. Proc. Natl. Acad. Sci. U. S. A 94: 9384–9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willows RD, and Kriegel AM, (2009) Biosynthesis of bacteriochlorophylls in purple bacteria. In: The Purple Phototrophic Bacteria, Advances in Photosynthesis and Respiration Hunter N, Daldal F, Thurnauer MC & Beatty JT (eds). Berlin: Springer Science+Business Media BV, pp. 57–79. [Google Scholar]
- Wilson AC P. a.A.B. (1962) Regulation of flavin synthesis by Escherichia coli. J. Gen. Microbiol 28: 283–303. [DOI] [PubMed] [Google Scholar]
- Woodcock DM, Crowther PJ, Doherty J, Jefferson S, De Cruz E, Noyer-Weidner M, Smith SS, Michael MZ, and Graham MW (1989) Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucl. Acids Res 17: 3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JD, and Escalante-Semerena JC (2004) CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea. Proc. Natl. Acad. Sci. USA 101: 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JD, Zayas CL, and Escalante-Semerena JC (2003) A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity. J. Bacteriol 185: 7193–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, and Bauer CE (2013) Controlling the delicate balance of tetrapyrrole biosynthesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci 368: 20120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas CL, Claas K, and Escalante-Semerena JC (2007) The CbiB protein of Salmonella enterica is an integral membrane protein involved in the last step of the de novo corrin ring biosynthetic pathway. J. Bacteriol 189: 7697–7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas CL, and Escalante-Semerena JC (2007) Reassessment of the late steps of coenzyme B12 synthesis in Salmonella enterica: Evidence that dephosphorylation of adenosylcobalamin-5’-phosphate by the CobC phosphatase is the last step of the pathway. J. Bacteriol 189: 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H, Zdanovskaia M, Hsiao K, and Goueli SA (2009) ADP-Glo: A Bioluminescent and homogeneous ADP monitoring assay for kinases. Assay Drug Dev. Technol 7: 560–572. [DOI] [PubMed] [Google Scholar]
- Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev 61: 533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.