Abstract
Background
Social anxiety disorder (SAD) is a disabling psychiatric condition with a genetic background. Brain alterations in gray matter (GM) related to SAD have been previously reported, but it remains to be elucidated whether GM measures are candidate endophenotypes of SAD. Endophenotypes are measurable characteristics on the causal pathway from genotype to phenotype, providing insight in genetically-based disease mechanisms. Based on a review of existing evidence, we examined whether GM characteristics meet two endophenotype criteria, using data from a unique sample of SAD-patients and their family-members of two generations. First, we investigated whether GM characteristics co-segregate with social anxiety within families genetically enriched for SAD. Secondly, heritability of the GM characteristics was estimated.
Methods
Families with a genetic predisposition for SAD participated in the Leiden Family Lab study on SAD; T1-weighted MRI brain scans were acquired (n = 110, 8 families). Subcortical volumes, cortical thickness and cortical surface area were determined for a-priori determined regions of interest (ROIs). Next, associations with social anxiety and heritabilities were estimated.
Findings
Several subcortical and cortical GM characteristics, derived from frontal, parietal and temporal ROIs, co-segregated with social anxiety within families (uncorrected p-level) and showed moderate to high heritability.
Interpretation
These findings provide preliminary evidence that GM characteristics of multiple ROIs, which are distributed over the brain, are candidate endophenotypes of SAD. Thereby, they shed light on the genetic vulnerability for SAD. Future research is needed to confirm these results and to link them to functional brain alterations and to genetic variations underlying these GM changes.
Fund
Leiden University Research Profile ‘Health, Prevention and the Human Life Cycle’.
Keywords: Social anxiety disorder, Family study, Endophenotypes, Structural MRI
Highlights
-
•
Gray matter (GM) characteristics were examined exploring their potential as endophenotypes of social anxiety disorder (SAD).
-
•
Several GM characteristics co-segregated with social anxiety within families genetically enriched for SAD and were heritable.
-
•
These findings suggest that GM characteristics are candidate endophenotypes of SAD.
Research in context.
Evidence before this study
Social anxiety disorder (SAD) is a prevalent psychiatric condition characterized by intense fear of negative evaluation in social situations. SAD typically develops during late childhood or adolescence and has a strong negative impact on patients' lives. Previous studies showed that SAD has a familial background. However, it's unknown which heritable characteristics make children and adolescents vulnerable for developing SAD. The endophenotype approach could be helpful to shed more light on the genetic susceptibility to SAD. Endophenotypes are measurable characteristics which are associated with the disorder, heritable, and co-segregate with the disorder within families of patients. Alterations in brain structure are candidate endophenotypes of SAD, as gray matter (GM) characteristics have been shown to be highly heritable. Furthermore, several studies have shown abnormalities of brain structure in SAD.
Added value of this study
To investigate whether specific GM characteristics could serve as endophenotypes for SAD, family studies are needed. The Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) is a unique neuroimaging study, in which patients with SAD as well as their family-members of two generations were investigated. Selected families were genetically enriched for SAD and due to the family-design of the LFLSAD, we were able to investigate two endophenotype criteria. First, we examined whether GM characteristics co-segregated with social anxiety within the families. Second, we estimated the heritability of the GM characteristics. Our results show that several GM characteristics meet both endophenotype criteria, making them promising candidate endophenotypes of social anxiety.
Implications of all available evidence
The findings provide preliminary evidence that several GM characteristics are genetically linked to social anxiety. Thereby, the results of this study shed light on the genetic vulnerability for SAD.
Alt-text: Unlabelled Box
1. Introduction
Patients who suffer from social anxiety disorder (SAD) are characterized by an intense fear of negative evaluation by others in social situations [1,2]. As a result, SAD-patients try to avoid social situations as much as possible, which lead to disability and serious impairments in important areas of life such as education, work, and social activities [[3], [4], [5], [6], [7], [8], [9], [10]]. The disorder has a high prevalence [11,12], is often chronic [13,14], and has a typical onset during late childhood and early adolescence [[15], [16], [17], [18], [19], [20]]. Furthermore, SAD is associated with high psychiatric comorbidity [[21], [22], [23]], adding to its burden on patients. Insight in the development of and vulnerability for SAD is therefore of great importance, as this might aid in developing preventive interventions and effective treatments.
Previous studies indicate that the pathogenesis of SAD is complex: environmental, biological, temperamental, and genetic factors are shown to play a interacting role [[24], [25], [26]]. With respect to the latter, the heritability of SAD is estimated to be between 39 and 56% [[27], [28], [29], [30]]. However, despite the promising results of a handful of studies investigating the genetic background of SAD [[30], [31], [32], [33], [34], [35], [36]], the genetic variants underlying the vulnerability for SAD are at present still largely unidentified. Detecting such ‘SAD genes’ is difficult due to several factors. First of all, SAD is a polygenic disorder, and it is widely assumed that various genetic variants, influenced by environmental factors, are involved in its development [[37], [38], [39]]. Furthermore, SAD is a heterogeneous disorder, and the diagnosis is based on clinical interviews and not on biologically-based parameters [40,41]. Thus, investigating endophenotypes might facilitate in unravelling the genetic vulnerability for complex psychiatric disorders like SAD [42].
Endophenotypes are measurable traits located on the causal pathway from genotype to phenotype [43,44], and include, for example, neurobiological changes in brain structure and function. Criteria for endophenotypes are the following [[45], [46], [47]]: (1) they are associated with the disorder; (2) they are state-independent traits, already present in a preclinical state; (3) they are heritable; (4) they co-segregate with the disorder within families of probands, with non-affected family members showing altered levels of the endophenotype in comparison to the general population. As reviewed in our earlier work [48], endophenotypes have the potential to shed more light on the mechanisms involved in the etiology of SAD.
In the present work, we provide a comprehensive overview of existing evidence and investigate whether gray matter (GM) structural brain characteristics, as measured with magnetic resonance imaging (MRI), are candidate endophenotypes of SAD. Based on previous findings, and as summarized in Bas-Hoogendam et al. (2016) [48], there are two important reasons to do so. To start, differences in GM between SAD-patients and healthy controls have been reported for a number of subcortical, frontal, temporal and parietal regions [49,50,59,60,[51], [52], [53], [54], [55], [56], [57], [58]] – see Table 1 for an overview of MRI-studies on GM in SAD. Furthermore, changes in brain structure were shown to be associated with clinical characteristics [49,50,[54], [55], [56], [57], [58],60,61], while treatment-related changes in brain structure in SAD patients have also been described [[62], [63], [64]]. Although it should be noted that the findings reported in these studies are heterogeneous (see Table 1 and review by Brühl and colleagues [65]), and have small effect sizes [60], a machine learning study was able to discriminate SAD-patients from healthy controls based on GM changes over the whole brain [66]. Furthermore, higher levels of social anxiety in healthy women were related to increased volumes of the amygdala, nucleus accumbens, and striatal regions like the putamen and caudate nucleus [67], while structural brain alterations have also been reported in anxious children and adolescents [[68], [69], [70], [71], [72]]. In addition, changes in brain structure have been reported in participants who were classified as being ‘behaviorally inhibited’ [[73], [74], [75], [76], [77], [78], [79]], which refers to the innate, temperamental trait associated with an increased vulnerability for developing SAD [80]. Together, these results suggest that structural brain alterations in GM might be related to SAD.
Table 1.
Overview results of studies on GM in SAD.
| Publication | Method | Group | Subcortical areas |
||||
|---|---|---|---|---|---|---|---|
| Amy | HiC | Thal | Putamen | Caudate | |||
| Potts et al., 1994 [193] | Manual segmentation caudate, thalamus, putamen | 22 SAD vs 22 HC | n.a. | n.a. | = | = | = |
| Cassimjee et al., 2010 [64] | Whole brain VBM (SPM) | 11 SAD - treatment effect | = | = | = | = | = |
| Irle et al., 2010 [61] | Manual segmentation amygdala & hippocampus | 24 SAD vs 24 HC | − | − | n.a. | n.a. | n.a. |
| Liao et al., 2011 [50] | Whole brain VBM (SPM) | 18 SAD vs 18 HC | = | – | = | = | = |
| Syal et al., 2012 [55] | Whole brain CT FreeSurfer; volumes amygdala & hippocampus | 13 SAD vs 13 HC | = | = | n.a. | n.a. | n.a. |
| Frick et al., 2013 [51] | Whole brain CT using FACE | 14 male SAD vs 12 HC | = | = | = | = | = |
| Meng et al., 2013 [53] | Whole brain VBM (SPM) | 20 SAD vs 19 HC | - And negative correlation with disease duration | = | - And positive correlation with age of onset | = | = |
| Talati et al., 2013 – sample 1 [49] | Whole brain VBM (SPM) | 16 SAD vs 20 HC (16 PD) | = | + | = | = | = |
| Talati et al., 2013 – sample 2 [49] | Whole brain VBM (SPM) | 17 SAD vs 17 HC | = | = | = | = | = |
| Brühl et al., 2014 [54] | Whole brain & ROIs CT FreeSurfer; volumes subcortical ROIs | 46 SAD vs 46 HC | = | = | = | = | = |
| Frick et al., 2014 [66] | Whole brain VBM (SPM) + ROI approach; SVM study | 14 SAD vs 12 HC | = | = | = | = | = |
| Frick et al., 2014 [58] | Whole brain VBM (SPM) | 48 SAD vs 29 HC | = | = | = | = | = |
| Irle et al., 2014 [57] | Whole brain VBM (SPM); manual segmentation parietal ROIs | 67 SAD vs 64 HC | = | = | = | = | = |
| Machado-de-Sousa et al., 2014 [194] | Manual segmentation amygdala & hippocampus | 12 SAD, 12 SA, 14 HC | + | + | n.a. | n.a. | n.a. |
| Talati et al., 2015 [62] | Whole brain VBM (SPM) | 14 SAD - treatment effect | = | = | - After treatment | - After treatment | - After treatment |
| Tükel et al., 2015 [56] | Whole brain VBM (SPM) | 27 SAD vs 27 HC | = | = | = | = | = |
| Månnson et al., 2016, 2017 [195,196] | ROIs (amygdala, ACC, insula, hippocampus) as well as whole brain VBM (SPM) | 13 SAD - treatment effect | - After treatment | = | = | = | = |
| Steiger et al., 2016 [63] | Whole brain cortical volume & CT using Freesurfer | 33 SAD -treatment effect | = | = | = | = | = |
| Bas-Hoogendam et al., 2017 [60] | Whole brain VBM (FSL) | 178 SAD vs 213 HC | = | = | = | + | = |
| Zhao et al., 2017 [59] | Whole brain VBM (SPM) & whole brain CT using Freesurfer | 24 SAD vs 41 HC (and 37 MDD) | = | = | − | − | = |
| Publication | Frontal regions |
Parietal regions |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MPFC | DLPFC | VLPFC | OFC | PMC | ACC | PCC | Par | PC | |
| Potts et al., 1994 [193] | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a | n.a. | n.a. |
| Cassimjee et al., 2010 [64] | = | = | = | = | = | = | = | = | = |
| Irle et al., 2010 [61] | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Liao et al., 2011 [50] | + | = | = | = | = | = | = | = | = |
| Syal et al., 2012 [55] | = | − | = | − | − | = | − | − | − |
| Frick et al., 2013 [51] | = | = | = | = | = | Pos. relation symptoms | = | = | = |
| Meng et al., 2013 [53] | = | = | = | = | = | = | = | = | − |
| Talati et al., 2013 – sample 1 [49] | − | = | = | = | − | − | − | − | − |
| Talati et al., 2013 – sample 2 [49] | = | − | = | − | + | = | = | + | = |
| Brühl et al., 2014 [54] | = | + | = | = | = | + ROI approach | = | + | + |
| Frick et al., 2014 [66] | = | = | = | = | = | = | = | = | = |
| Frick et al., 2014 [58] | = | = | = | = | = | = | Pos. relation symptoms | = | = |
| Irle et al., 2014 [57] | = | = | = | = | + | = | = | Both + and – (neg. relation LSAS avoidance) | Both + and – (neg. relation LSAS avoidance) |
| Machado-de-Sousa et al., 2014 [194] | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Talati et al., 2015 [62] | = | = | = | = | = | = | = | = | = |
| Tükel et al., 2015 [56] | = | = | = | = | = | = | = | + | + |
| Månnson et al., 2016, 2017 [195,196] | - after treatment | = | = | = | = | = | = | = | - after treatment |
| Steiger et al., 2016 [63] | = | Relation with treatment success | = | = | = | = | = | - After treatment | = |
| Bas-Hoogendam et al., 2017 [60] | = | = | = | = | = | = | = | = | = |
| Zhao et al., 2017 [59] | − | = | = | − | − | + | = | + | = |
| Publication | Temporal regions |
Occipital regions |
Cerebellum | ||
|---|---|---|---|---|---|
| Ins | TC | OCC | FFG | ||
| Potts et al., 1994 [193] | n.a. | n.a. | n.a. | n.a. | n.a. |
| Cassimjee et al., 2010 [64] | = | - After treatment | = | = | - After treatment |
| Irle et al., 2010 [61] | n.a. | n.a. | n.a. | n.a. | n.a. |
| Liao et al., 2011 [50] | = | − | = | = | n.a. |
| Syal et al., 2012 [55] | − | − | = | − | n.a. |
| Frick et al., 2013 [51] | = | + | = | + | n.a. |
| Meng et al., 2013 [53] | = | = | = | = | n.a. |
| Talati et al., 2013 – sample 1 [49] | = | + | + | + | n.a. |
| Talati et al., 2013 – sample 2 [49] | = | both – and + | = | = | n.a. |
| Brühl et al., 2014 [54] | + (ROI approach, uncorrected) | + (ROI approach, uncorrected) | = | = | n.a. |
| Frick et al., 2014 [66] | = | = | = | = | n.a. |
| Frick et al., 2014 [58] | = | = | + | + | n.a. |
| Irle et al., 2014 [57] | = | = | = | = | n.a. |
| Machado-de-Sousa et al., 2014 [194] | n.a. | n.a. | n.a. | n.a. | n.a. |
| Talati et al., 2015 [62] | = | = | = | = | + after treatment |
| Tükel et al., 2015 [56] | = | + | = | + | n.a. |
| Månnson et al., 2016, 2017 [195,196] | = | = | = | = | n.a. |
| Steiger et al., 2016 [63] | = | = | - After treatment | = | n.a. |
| Bas-Hoogendam et al., 2017 [60] | = | = | = | = | n.a. |
| Zhao et al., 2017 [59] | + | + | = | = | n.a. |
=: no difference; +: increase; −: decrease; n.a.: not data available.
ACC: anterior cingulate cortex; Amy: amygdala; CT: cortical thickness; DLPFC: dorsolateral prefrontal cortex; FFG: fusiform gyrus; GM: gray matter; HC: healthy control participants; HiC: hippocampus; Ins: insula; MDD: patients with major depressive disorder; MPFC: medial prefrontal cortex; Occ: occipital cortex; OFC: orbitofrontal cortex; Par: parietal cortex; PC: (pre)cuneus; PCC: posterior cingulate cortex; PD: patients with panic disorder; PMC: premotor cortex; ROI: region of interest; SA: social anxiety; SAD: patients with social anxiety disorder; SVM: support vector machine; TC: temporal cortex; Thal: thalamus; VBM: voxel-based morphometry; VLPFC: ventrolateral prefrontal cortex.
A second reason to consider GM brain characteristics as candidate endophenotypes is the fact that numerous studies, both in healthy controls as well as in several patient groups, have indicated that brain structure is to a great extent determined by genetic influences. For example, studies revealed that genetic variants affect the thickness and surface area of cortical GM [[81], [82], [83], [84], [85], [86]], as well as intracranial volume (ICV) [87] and subcortical brain volumes [[88], [89], [90], [91], [92]]; the findings with respect to subcortical volumetric measures have recently been replicated and extended in a genome-wide association analysis in over 40,000 individuals [93]. In addition, the neuroanatomical shape of subcortical structures has been shown to be significantly heritable [94,95]. Furthermore, the results of studies in various patient populations, for example in twins (dis)concordant for bipolar disorder [96] and in families with multiple cases of schizophrenia [97] corroborate with these findings, showing that both the volume as well as the shape of subcortical structures are heritable. A meta-analysis of twin studies confirmed that global brain volumes, volumes of subcortical brain areas, as well as measures of cortical thickness, are all highly or moderately-to-highly heritable [98]; see also the review by Peper and colleagues [99].
The present work used MRI data from the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) [100] to explore whether GM brain characteristics (volumes of subcortical structures; estimations of cortical thickness (CT), and measures of cortical surface area (CSA)) are endophenotypes of SAD. The LFLSAD is a multiplex (i.e., families were selected based on a minimum of two (sub)clinical SAD cases within one nuclear family), multigenerational (i.e., multiple nuclear families encompassing two generations from the same family took part) family study on SAD, in which nine families who were genetically enriched for SAD were included (total n = 132). Such a family design is particularly powerful to investigate genetic and environmental influences on SAD-related characteristics [101].
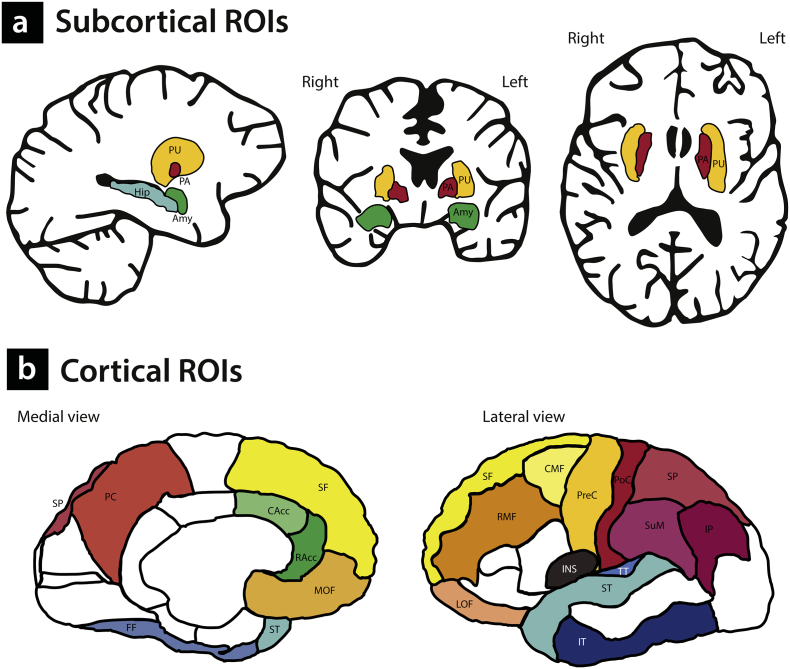
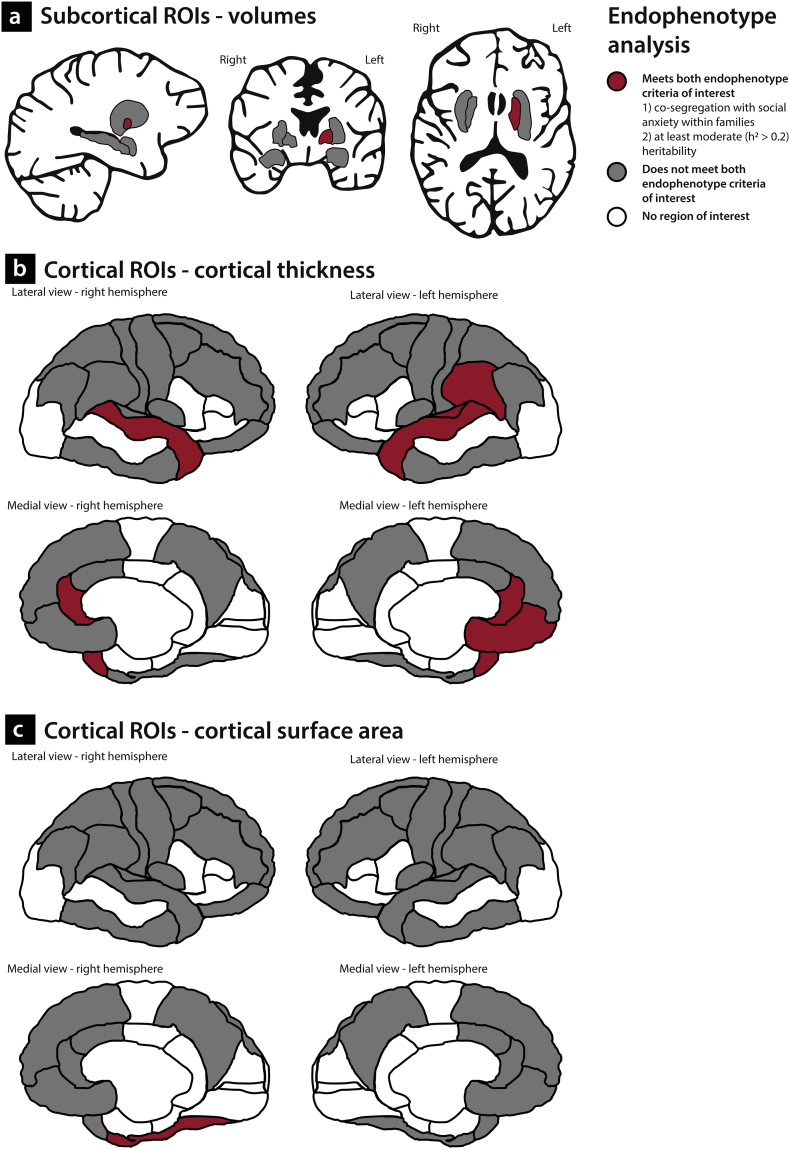
We examined two endophenotype criteria. First, we investigated whether alterations in GM brain characteristics co-segregate with social anxiety within the families (first element of endophenotype criterion 4); second, we estimated the heritability of these measures (endophenotype criterion 3). The structural brain phenotypes were established using the FreeSurfer software package (version 5.3) and we employed a hypothesis-driven region-of-interest (ROI) approach based on the results of previous studies. With respect to the subcortical volumes, we focused on the putamen and pallidum, based on the findings of a recent mega-analysis on SAD reporting increased GM related to SAD in these regions [60], which were recently replicated [67]. In addition, we investigated the association between social anxiety and volumes of the amygdala and hippocampus, given the fact that volumetric changes in these areas in SAD have been reported [52,53,61], although it should be noted that other studies were not able to replicate these effects (see for example [54,60] and Table 1). These subcortical ROIs are displayed in Fig. 1a.
Fig. 1.
Subcortical and cortical Regions of Interest (ROIs). Subcortical ROIs (1a) Amy: amygdala; Hip: hippocampus; PA: pallidum; PU: putamen. Cortical ROIs (1b)Frontal regions (yellow) CMF: caudal middle frontal; LOF: lateral orbitofrontal; MOF: medial orbitofrontal. PreC: precentral; RMF: rostral middle frontal. SF: superior frontal. Anterior cingulate (green) CAcc: caudal anterior cingulate. RAcc: rostral anterior cingulate. Insula (purple) INS: insula. Parietal regions (red) IP: inferior parietal; PC: precuneus; PoC: postcentral; SuML supramarginal; SP: superior parietal. Temporal regions (blue) FF: fusiform gyrus; IT: inferior temporal; ST: superior temporal; TT: transverse temporal.
With respect to the estimates of CT, it should be noted that only a handful of studies have investigated SAD-related alterations in CT, with mixed results (Table 1). To determine cortical ROIs for the present study, we used the findings from previous work, starting with the work by Brühl and colleagues [54], who investigated CT in a sample of 46 SAD-patients and 46 matched healthy controls; they reported SAD-related increases in CT in the anterior cingulate cortex (ACC), the insula, the dorsolateral prefrontal cortex (DLPFC) including the middle frontal gyrus and the superior frontal lobule, the temporal pole and the parietal cortex [54]. Most of these findings were recently replicated by Zhao and colleagues [59], who described significant cortical thickening in the ACC, the insula, the superior frontal cortex, as well as in the temporal pole and parietal areas in SAD; in addition, this study mentioned cortical thinning in the orbitofrontal cortex, precentral cortex and the rostral medial frontal cortex. Other work, by Syal and colleagues [55], reported on cortical thinning in 13 SAD-patients, in several temporal, frontal and parietal regions, as well as in the insula and cingulate areas. The selected ROIs based on the results of these three studies are illustrated in Fig. 1b (cortical parcellations as defined in the Desikan-Killiany atlas [102]).
As there are, to the best of our knowledge, no studies on measures of CSA in SAD, the same cortical ROIs were used to investigate alterations in CSA related to SAD. It is of importance to investigate the measures of CT and CSA separately, as it has been shown that these neuroimaging phenotypes reflect different features of cerebral cortical structure. That is, neurons in the cortex are organized in columns running perpendicular to the surface of the brain; CT represents the number of cells within these columns, whereas the size of the CSA is determined by the number of columns in a certain area [103,104]. Previous research indicated that brain size is primarily determined by the size of CSA (and not by CT) [105]; in addition, CT and CSA are genetically independent and follow different developmental trajectories [[106], [107], [108], [109], [110], [111], [112], [113]]. Furthermore, CT and CSA have different predictive values with respect to the development of psychopathology [114,115].
Other, non ROI (sub)cortical areas were investigated on an exploratory basis only; results are reported in the Supplementary Material and only briefly mentioned in the Results section. Analyses were corrected for multiple comparisons at a false discovery rate (FDR) of 5% [116], but given the divergent findings of previous studies (Table 1), the innovative nature of the present study (to the best of our knowledge, this is the first comprehensive family study on social anxiety) and the fact that brain regions are likely biologically not independent but constitute structural and functional networks (cf. the work of Brühl et al. [54]), uncorrected p-values are reported and discussed as well.
2. Materials and methods
2.1. Participants
Participants included families genetically enriched for SAD, who were part of the LFLSAD (total sample: n = 132, from nine families). The background, objectives and methods of this multiplex multigenerational family study, as well as the clinical characteristics of the LFLSAD sample and an a priori power analysis are extensively described elsewhere [100]; in addition, a preregistration of the study is available online at https://osf.io/e368h/ [117]. In brief, the LFLSAD sample consists of families who were selected based on the presence of a primary diagnosis of SAD in a parent (aged 25–55 years old; the so-called “proband”) with a child, living at home and aged 8–21 years of age (“proband's SA-child”) who met criteria for clinical or subclinical SAD. The age-criterion was based on the fact that adolescence appears to be a critical period for the development of clinical levels of SAD [17,18], while we used the ‘living at home’ criterion to minimize the impact of environmental influences, other than the family environment, on the child's phenotype and on the gene-environment interaction, in order to optimize the ability to detect the genotype-endophenotype-phenotype connection.
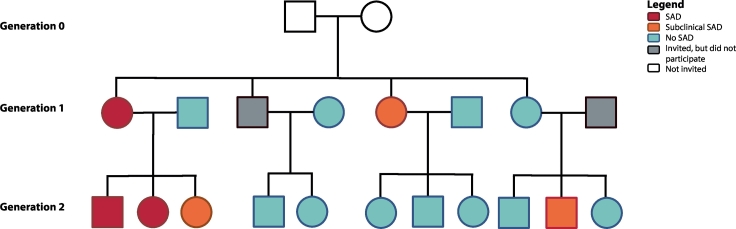
In addition to the proband and proband's SA-child, the proband's partner and other children from this nuclear family (aged 8 years or older), as well as the proband's sibling(s), with their partners and children (aged 8 years or older) were invited to participate. This way, the sample consisted of family members of two generations (generation 1: generation proband; generation 2: generation proband's SA-child), as depicted in Fig. 2.
Fig. 2.
Example of a family within the LFLSAD.
Families were included based on the combination of a parent with SAD (‘proband’; depicted in red) and a proband's child with SAD (red) or (sub)clinical SA (orange). In addition, family members of two generations were invited, independent from the presence of SAD within these family members (no SAD: light blue; did not participate: gray). Grandparents (generation 0; white) were not invited for participation. This family is slightly modified to guarantee anonymity; however, the number of family members and the frequency of (sub)clinical SAD are depicted truthfully. Squares and circles represent men and women, respectively. This figure is a reprint of Fig. 1 of Bas-Hoogendam et al., 2018, International Journal of Methods in Psychiatric Research [100].
Exclusion criteria for the LFLSAD were comorbidity other than internalizing disorders in the proband or proband's SA-child, especially developmental disorders like autism; other family members were included independent from the presence of psychopathology. Furthermore, general MRI contraindications, like metal implants, pregnancy or dental braces, were exclusion criteria for the MRI experiment.
Although we collected MRI data from nine families (n = 113) [100], data from one family were excluded from the present analysis, as the proband from this family was not able to participate in the MRI experiment due to an MRI contraindication, which limited the analyses on the data of this proband's family members (n = 3). Therefore, the remaining sample consisted of 110 family members (56 males) from eight families (mean number of participating family members per family: 13·8; range 5–28). These family members were, according to the design, divided over two generations (generation 1: n = 51, 24 males; age (mean ± SD, range) 46·5 ± 6·7 years, 34·3–61.5 years; generation 2: n = 59, 32 males, age 18·1 ± 6·0 years, 9·0–32·2 years) who differed significantly in age (β = −30·3, p < 0·001), but not in male/female ratio (χ2(1) = 0·56, p = 0·57).
2.2. Ethics
The LFLSAD study was approved by the Medical Ethical Committee of the Leiden University Medical Center (P12.061). Prior to entering the study, interested family members received verbal and written information on the objectives and procedure of the study; information letters were age-adjusted, to make them understandable for participants of all ages. All participants provided informed consent according to the Declaration of Helsinki; both parents signed the informed consent form for their children, while children between 12 and 18 years of age signed the form themselves as well. Every participant received €75 for participation in the LFLSAD (duration whole test procedure, including breaks: 8 h) and travel expenses were reimbursed. Furthermore, participants were provided with lunch/dinner, snacks and drinks during their visit to the lab. Confidentiality of the research data was maintained by the use of a unique research ID number for each participant.
2.3. Data collection LFLSAD: extensive phenotyping
All included family members participated in a range of measurements, as described in Bas-Hoogendam et al. [100]. The presence of DSM-IV diagnoses, with special attention to (sub)clinical SAD, was determined using the Mini-International Neuropsychiatric Interview (M.I.N.I.)-Plus (version 5.0.0) [118,119] or the M.I.N.I.-Kid interview (version 6.0) [120,121]; these interviews were conducted by experienced clinicians and were recorded. The diagnosis of clinical SAD was established using the DSM-IV-TR criteria for the generalized subtype of SAD, but the clinician verified whether the DSM-5 criteria for SAD were also met. A diagnosis of subclinical SAD was established when participants met the criteria for SAD as described in the DSM-5, but did not show impairing limitations in important areas of functioning (criterion G) [1].
Furthermore, participants completed age-appropriate questionnaires on several anxiety-related constructs, including, among others, the level of self-reported social anxiety symptoms (Liebowitz Social Anxiety Scale (LSAS-SR) [122,123] or the Social Anxiety Scale for adolescents (SAS-A) [124]), the intensity of fear of negative evaluation (revised Brief Fear of Negative Evaluation (BFNE) – II scale) [125,126] and the level of trait anxiety (State-Trait Anxiety Inventory (STAI) [127]. The severity of self-reported depressive symptoms was evaluated using the Beck Depression Inventory (BDI) – II) [128,129] or the Children's Depression Inventory (CDI) [130,131]. In order to enable analysing the scores of the age-specific questionnaires, z-scores were computed as described previously [100]. In addition, an estimate of cognitive functioning was obtained using two subtests of the Wechsler Adult Intelligence Scale IV (WAIS-IV) [132] or Wechsler Intelligence Scale for Children III (WISC) [133] consisting of the similarities (verbal comprehension) and block design (perceptual reasoning) subtests.
2.4. MRI procedure and data acquisition
Prior to the MRI scan, all participants were informed about the MRI safety procedures and they were told that they could refrain from continuing the experiment at any time. Children and adolescents were familiarized with the MRI scanner using a mock scanner [134]. State anxiety was assessed before and after the MRI scan by a Dutch-translation of the STAI [127]. Scanning was performed using a 3.0 T Philips Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands), equipped with a 32-channel Sensitivity Encoding (SENSE) head coil.
The MRI session (total duration of the MRI protocol: 54 min 47 s) consisted of several structural and functional scans, as described in the design paper on this project [100]. Of interest for the present work is a high-resolution T1-weighted scan, with the following characteristics: 140 slices, resolution 0·875 mm × 0·875 mm × 1·2 mm, FOV = 224 mm × 168 mm × 177·333 mm, TR = 9·8 ms, TE = 4·59 ms, flip angle = 8°. All structural MRI scans were inspected by a neuroradiologist; no clinically relevant abnormalities were reported in any of the participants.
2.5. MRI processing
Reconstruction of cortical surface, cortical parcellation and CT estimation, as well as segmentation of subcortical brain structures, was performed using standard procedures in the FreeSurfer software (version 5.3). This software is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/) and the technical details of these procedures are described elsewhere [[135], [136], [137], [138], [139], [140], [141], [142]]. These procedures resulted in the extraction of volumes for seven bilateral subcortical GM regions (amygdala, caudate, hippocampus, nucleus accumbens, pallidum, putamen and thalamus) and the lateral ventricles, as well as in the segmentation of the cortex into 68 (34 left and 34 right) GM regions based on the Desikan-Killiany atlas [102]. For these regions, mean CT, defined as the closest distance from the gray/white boundary to the gray/cerebral spinal fluid boundary at each location of each participant's reconstructed cortical surface, as well as mean CSA, was determined. The method for the measurement of CT have been validated against both histological analysis [143] and manual measurements [144,145], and FreeSurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths [146,147]. Subcortical ROIs in the current study were the amygdala, hippocampus, pallidum and putamen; cortical ROIs were the superior frontal gyrus, the caudal middle frontal gyrus, the rostral middle frontal gyrus, the lateral orbitofrontal gyrus, the medial orbitofrontal gyrus, the precentral gyrus, the caudal anterior cingulate, the rostral anterior cingulate, the insula, the superior parietal gyrus, the inferior parietal cortex, the precuneus, the supramarginal gyrus, the postcentral gyrus, the temporal pole, the inferior temporal gyrus, the superior temporal gyrus, the fusiform gyrus and the transverse temporal gyrus.
Both the subcortical segmentations as well as the segmentations of the cortical GM regions were visually inspected for accuracy and statistically evaluated for outliers according to standardized protocols designed to facilitate harmonized image analysis across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols/). This quality control resulted in the exclusion of, on average, 2·0% (SD: 4·0%) of the segmentations per participant for the subcortical measures (absolute number: 0·3 segmentations, range: 0–3; SD: 0·6) and 3·4% (SD: 3·2%) of the segmentations per participant for the cortical measures (absolute number: 2·3 segmentations, range: 0–8; SD: 2·2). In addition, data of one participant (age 9·0 y, generation 2) had to be excluded completely from the analyses because FreeSurfer was not able to reliably reconstruct the brain from the T1-weighted scan. This was due to excessive movement during data acquisition, which was present during both the structural as well as the functional MRI scans of this participant (relative motion parameters exceeded 2.5 mm) [148].
Data of the FreeSurfer segmentations are available at https://osf.io/m8q2z [149].
2.6. Statistical analysis
Incidental missing values on the self-report questionnaires were replaced by the mean value of the completed items. We investigated differences between participants with and without (sub)clinical SAD by fitting regression models in R [150], with (sub)clinical SAD as the independent variable and the outcomes of the self-report questionnaires (self-reported social anxiety (z-score), fear of negative evaluation, level of trait anxiety and level of state anxiety before and after the MRI scan) as dependent variables of interest. Gender and age were included as covariates, and genetic correlations between family members were modeled by including random effects. P-values were corrected for multiple comparisons (seven tests, Bonferroni corrected p-value = 0·007). In addition, we compared the presence of (comorbid) psychopathology between participants in the (sub)clinical SAD and no SAD group by performing chi-square tests using IBM SPSS Statistics for Windows (Version 23.0. Armonk, NY: IBM Corp.), while applying a Bonferroni-corrected p-value (p = .005 [10 tests]).
Next, we investigated whether GM brain characteristics are candidate endophenotypes of SAD by focusing on two endophenotype criteria. First, the ‘co-segregation of the candidate endophenotype with the disorder within families’ (first element of endophenotype criterion 4) was examined, by performing multiple regression using a linear mixed model in R [150]. (Sub)clinical SAD was used as the independent variable, as we considered the clinical and subclinical SAD cases to reflect the same phenotype; the GM brain characteristics (subcortical volumes; CT; CSA) were dependent variables. Again, correlations between family members were modeled by including random effects; age (centered) and gender were included as covariates of no interest. In addition, total ICV (centered), mean global cortical thickness (GCT) (centered) or total global cortical surface area (GCSA) (centered) were added as covariates for the analyses on subcortical volumes, CT and cortical surface area, respectively. Furthermore, in order to obtain a reliable estimate of the main effect of (sub)clinical SAD, a (sub)clinical SAD-by-age interaction term as well as an analysis-dependent interaction term ((sub)clinical SAD-by-total ICV; (sub)clinical SAD-by-mean GCT; (sub)clinical SAD-by-total GCSA) were included in the model. As data on the presence of subclinical SAD were, due to technical reasons, lost for eight family members, data from these participants could not be used for this analysis (remaining sample: n = 101). For reasons of completeness, we also investigated the relationship between GM brain characteristics and two continuous measures of social anxiety: self-reported levels of social anxiety (z-scores, based on the LSAS and SAS-A) and levels of fear of negative evaluation (FNE) (sample: n = 109). Because of the non-normal distribution of most of the dependent variables, we confirmed the robustness of the used linear mixed model by checking the distribution of the residuals of the phenotypes showing significant results using Shapiro-Wilk normality tests in R; results showed that these residuals followed a normal distribution. Analyses were corrected for multiple comparisons at a false discovery rate (FDR) of 5% [116]. In addition to these analyses of interest, we performed two sensitivity analyses to examine whether the results of the association analyses were driven by (comorbid) psychopathology other than SAD or by the severity of depressive symptoms as measured by the BDI-II or the CDI. Therefore, we excluded all participants with past and/or present (comorbid) psychopathology other than SAD (sensitivity analysis 1; note however, that the results may be biased, as the majority of the probands, on which the selection of the families was based, were excluded as well) or added the z-score of the level of depressive symptoms as a covariate in the analyses (sensitivity analysis 2).
Second, the heritability of the GM brain characteristics (h2) was estimated (endophenotype criterion 3) by jointly modelling the GM brain characteristics and SAD on which the selection of the families was based. Random effects were included to model the familial relationships [151]. Age (centered and standardized), gender and total ICV (centered and standardized; analyses on subcortical volume), mean GCT (centered and standardized; analyses on CT) or total GCSA (centered and standardized; analyses on surface area) were included as covariates. This approach takes the ascertainment process into account. We tested whether the genetic variance was significantly different from zero (cf. [152]) by using likelihood ratio tests. Significance levels are reported for heritability estimates >0·10. Again, a FDR of 5% was applied.
3. Results
3.1. Sample characteristics
Characteristics of the sample are summarized in Table 2. Seventeen participants were diagnosed with clinical SAD, while an additional 22 were classified as having subclinical SAD (total group (sub)clinical SAD n = 39); the validity of these diagnoses was substantiated by the scores on the self-report questionnaires as described previously [100]. The family members with (sub)clinical SAD did not differ from family members without SAD (n = 62) with respect to male/female ratio, age and estimated IQ. However, family members in the (sub)clinical SAD group were more often diagnosed with depression (past) and dysthymia (present), although these differences were not significant at a Bonferroni-corrected p-value. In addition, the prevalence of depressive episodes within the sample as a whole was in the range of the general population [153,154], as reported in the design paper on the LFLSAD [100]. Furthermore, participants with (sub)clinical SAD self-reported significantly higher levels of social anxiety, FNE, trait anxiety, and increased levels of depressive symptoms. Groups did not differ with respect to state anxiety related to the MRI scan. None of the participants with SAD received treatment for the disorder before entering the study [100].
Table 2.
Characteristics of participants with and without (sub)clinical SAD.
| (Sub)clinical SAD (n = 39) | No SAD (n = 62) | Statistical analysis | |
|---|---|---|---|
| Demographics | |||
| Male / Female (n) | 20 / 19 | 31 /31 | χ2(1) = 0.02, p = 1.00 |
| Generation 1 / Generation 2 (n) | 19 / 20 | 27 / 35 | χ2(1) = 0.26, p = .68 |
| Age in years (mean ± SD); range | 30.3 ± 15.5; 9.2–59.6 | 31.3 ± 15.2; 9.4–61.5 | β (± SE) = −1.0 ± 3.1, p = .76 |
| Estimated IQ (mean ± SD) | 104.3 ± 12.2 | 105.6 ± 10.5 | β (± SE) = −2.1 ± 2.2, p = .33 |
| Diagnostic information(n) | |||
| Clinical SAD | 17 | 0 | χ2(1) = 32.5, p < .001 ⁎⁎ |
| Depressive episode - present | 1 | 1 | χ2(1) = 0.15, p = 1.00 |
| Depressive episode - past | 12 | 9 | χ2(1) = 4.8, p = .04 ⁎ |
| Dysthymia - present | 3 | 0 | χ2(1) = 5.3, p = .05 ⁎ |
| Dysthymia - past | 1 | 1 | χ2(1) = 0.2, p = 1.00 |
| Panic disorder lifetime | 5 | 2 | χ2(1) = 3.9, p = .10 |
| Agoraphobia - present | 3 | 2 | χ2(1) = 1.2, p = .35 |
| Agoraphobia - past | 0 | 2 | χ2(1) = 1.2, p = .53 |
| Separation anxiety | 0 | 1 | χ2(1) = 0.8, p = 1.00 |
| Specific phobia | 2 | 3 | χ2(1) = 0.02, p = 1.00 |
| Generalized anxiety disorder - present | 1 | 0 | χ2(1) = 1.7, p = .37 |
| Self-report measures (mean ± SD) | |||
| Social anxiety symptoms (z-score) | 3.0 ± 3.3 | 0.6 ± 1.5 | β (± SE) = 2.6 ± 0.5, p < .001 ⁎⁎ |
| FNE | 23.3 ± 12.3 | 12.8 ± 8.0 | β (± SE) = 10.6 ± 1.9, p < .001 ⁎⁎ |
| Depressive symptoms (z-score) | 0.0 ± 0.9 | −0.5 ± 0.7 | β (± SE) = 0.5 ± 0.2, p < .001⁎⁎ |
| STAI - trait | 38.8 ± 9.4 | 33.1 ± 8.5 | β (± SE) = 5.5 ± 1.8, p = .002 ⁎⁎ |
| STAI - state pre scan | 35.2 ± 7.5 | 32.2 ± 8.8 | β (± SE) = 2.8 ± 1.6, p = .08 |
| STAI - state post scan | 30.8 ± 6.4 | 28.5 ± 6.4 | β (± SE) = 2.2 ± 1.3, p = .09 |
FNE: fear of negative evaluation; SAD: social anxiety disorder; SD: standard deviation; SE: standard error; STAI: state-trait anxiety inventory;
Significant at uncorrected p-value of 0.05. ** Significant at Bonferroni-corrected p-value.
3.2. General imaging phenotypes
Values of general imaging phenotypes are presented in Table 3. Participants with and without (sub)clinical SAD did not differ with respect to total ICV, mean GCT and total GCSA, but there were effects of age and gender on these phenotypes, in line with previous findings [155,156].
Table 3.
General imaging characteristics participants with and without (sub)clinical SAD.
| (Sub)clinical SADa | No SADa | Effect of (sub)clinical SADb |
Effect of social anxiety (z-score)b |
Effect of FNEb |
Effect of ageb, c |
Effect of genderb, c |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | β | SE | p | |||
| Total ICV | 1,599,832.3 ± 161,567.6 | 1,628,908.4 ± 163,820.3 | −0.06 | 0.07 | 0.41 | 0.05 | 0.07 | 0.49 | −0.07 | 0.07 | 0.27 | −0.13 | 0.06 | 0.04 ⁎ | −0.70 | 0.07 | <0.001 ⁎⁎ |
| Mean GCT | 2.55 ± 0.13 | 2.54 ± 0.14 | 0.05 | 0.06 | 0.45 | 0.01 | 0.06 | 0.88 | −0.03 | 0.06 | 0.66 | −0.69 | 0.05 | <0.001 ⁎⁎ | 0.07 | 0.06 | 0.28 |
| Total GCSA | 174,163.3 ± 16,561.2 | 176,417.4 ± 17,792.7 | 0.00 | 0.07 | 0.99 | 0.05 | 0.06 | 0.38 | 0.02 | 0.06 | 0.71 | −0.38 | 0.05 | <0.001 ⁎⁎ | −0.59 | 0.07 | <0.001 ⁎⁎ |
FNE: fear of negative evaluation; GCSA: global cortical surface area (mm2); GCT: global cortical thickness (mm); ICV: intracranial volume (mm3); SAD: social anxiety disorder; SE: standard error.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender and family structure. Reported p-values are uncorrected for multiple comparisons.
Uncorrected mean ± standard deviation.
Coefficients represent standardized values.
Effects of age and gender are reported for the models including (sub)clinical SAD, but are comparable to the effects of these covariates in the models including social anxiety (z-score) and FNE. Values of the covariates are reported in Supplementary Table 2.
Significant at uncorrected p-value of 0.05. ** Significant at Bonferroni-corrected p-value.
3.3. Volumes of subcortical brain structures
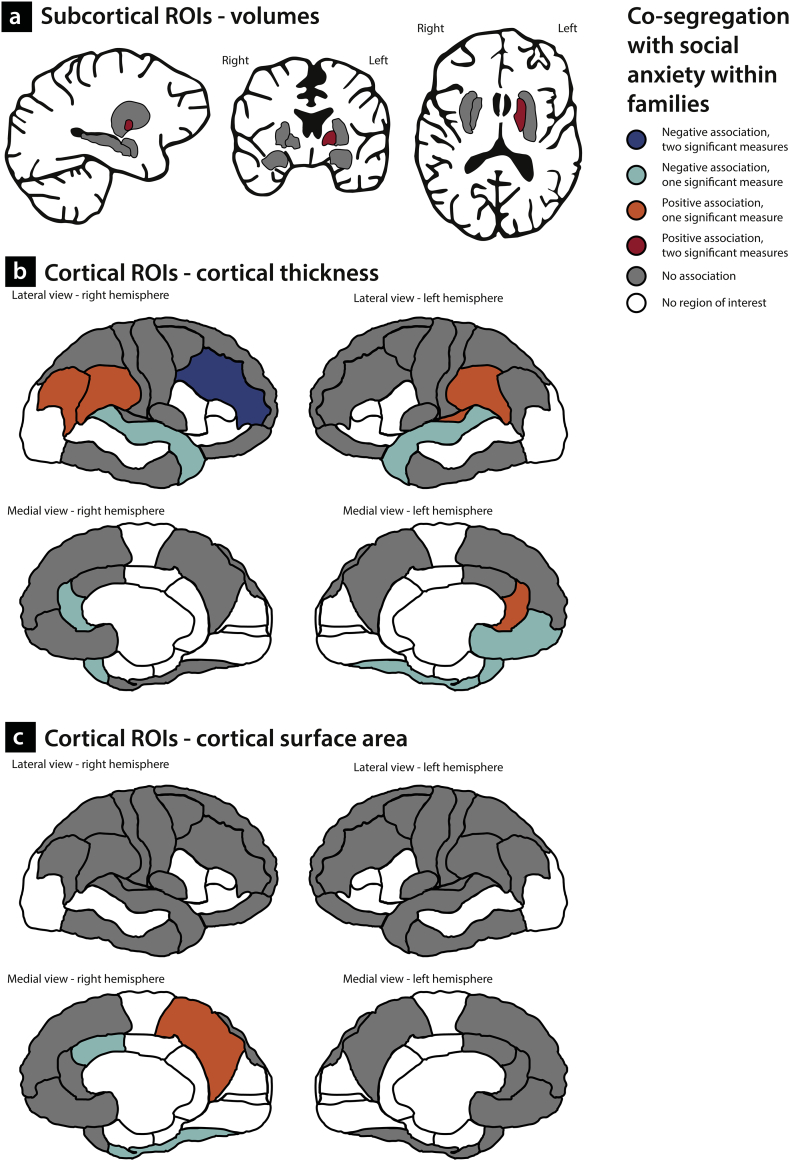
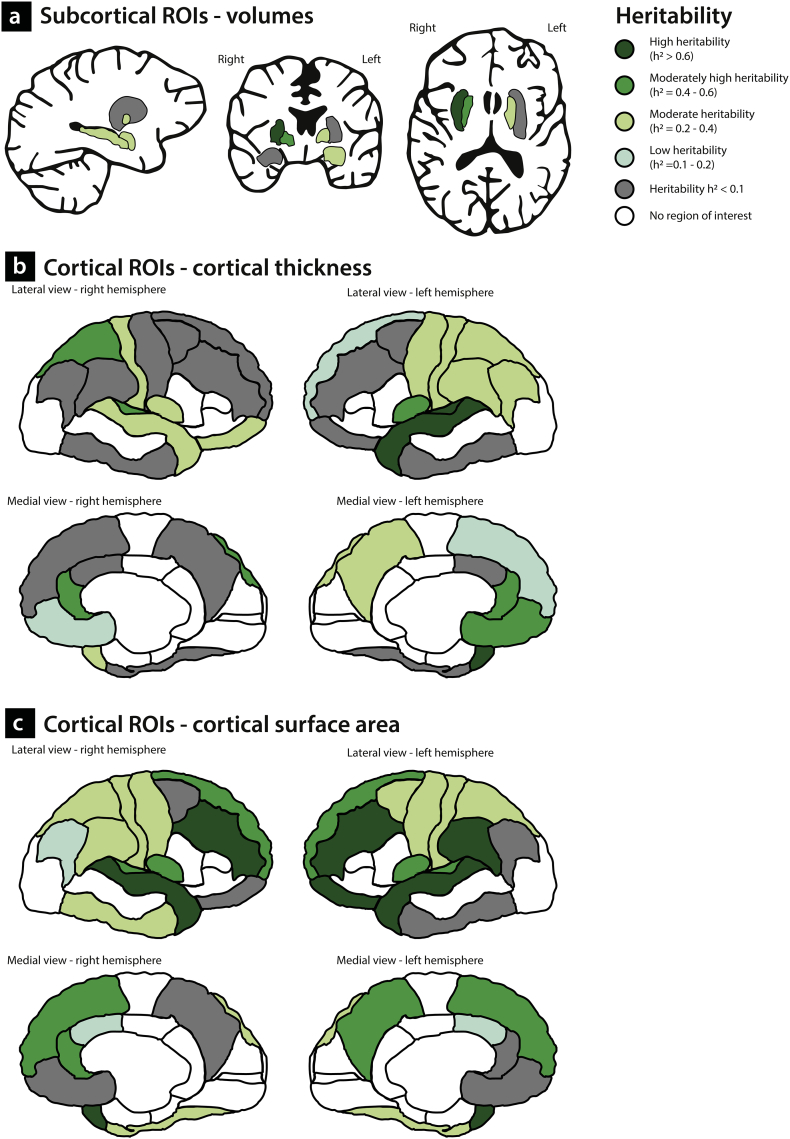
Using three different models, we investigated whether indices of social anxiety ((sub)clinical SAD, z-score of SA, or FNE) were associated with volumes of the subcortical ROIs. Results of the analyses are displayed in Table 4 and Supplementary Table 1. There were no significant associations between the indices of social anxiety and subcortical volumes at the FDR-corrected significance level, but there were positive relationships between the level of self-reported social anxiety and FNE on the one hand and volume of the left pallidum at the other at an uncorrected significance level of p < 0·05 (Fig. 3a). Furthermore, volume of the left pallidum was moderately heritable (h2 = 0·28). Heritability estimates of the volumes of other subcortical ROIs are depicted in Fig. 4a and listed in Table 4.
Table 4.
Effects of social anxiety on volumes of subcortical ROIs; heritability estimates.
| (Sub)clinical SADa | No SADa | Effect of (sub)clinical SADb |
Effect of social anxiety (z-score)b |
Effect of FNEb |
Heritability estimate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | h2 | p | ||||
| Amygdala | L | 1511.0 ± 150.4 | 1552.9 ± 190.7 | −0.11 | 0.08 | 0.19 | 0.04 | 0.08 | 0.58 | −0.01 | 0.08 | 0.94 | 0.34 | 0.009 ⁎⁎ |
| R | 1515.1 ± 192.3 | 1541.4 ± 196.8 | −0.04 | 0.08 | 0.62 | 0.07 | 0.08 | 0.40 | 0.05 | 0.08 | 0.55 | < 0.10 | n.a. | |
| Hippocampus | L | 5009.3 ± 611.3 | 5150.4 ± 544.7 | −0.09 | 0.08 | 0.26 | 0.01 | 0.08 | 0.89 | 0.07 | 0.08 | 0.39 | 0.37 | 0.001 ⁎⁎ |
| R | 4782.6 ± 547.2 | 4782.3 ± 494.5 | 0.04 | 0.08 | 0.65 | 0.00 | 0.08 | 0.98 | 0.08 | 0.08 | 0.33 | 0.29 | 1.1x10- 5⁎⁎ | |
| Pallidum | L | 1777.5 ± 283.8 | 1711.3 ± 256.1 | 0.08 | 0.08 | 0.35 | 0.21 | 0.08 | 0.01 ⁎ | 0.21 | 0.08 | 0.01 ⁎ | 0.28 | 0.038 ⁎ |
| R | 1516.4 ± 220.0 | 1497.8 ± 203.5 | 0.00 | 0.08 | 0.96 | 0.08 | 0.08 | 0.33 | 0.12 | 0.08 | 0.13 | 0.45 | 1.7x10−5⁎⁎ | |
| Putamen | L | 6741.4 ± 1028.2 | 6480.6 ± 931.6 | 0.15 | 0.09 | 0.09 | 0.07 | 0.08 | 0.40 | 0.08 | 0.09 | 0.35 | < 0.10 | n.a. |
| R | 5103.5 ± 688.4 | 5153.7 ± 568.2 | −0.04 | 0.07 | 0.57 | 0.07 | 15.9 | 0.54 | 0.06 | 0.06 | 0.39 | 0.61 | 5.5x10−6⁎⁎ | |
FNE: fear of negative evaluation; L: left; n.a.: not applicable; R: right; SAD: social anxiety disorder; SE: standard error.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, total intracranial volume (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD* age (centered) and (sub)clinical SAD*total intracranial volume (centered). Values of the covariates are reported in Supplementary Table 1. Reported p-values are uncorrected for multiple comparisons.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, total intracranial volume (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD*age (centered) and (sub)clinical SAD*total intracranial volume (centered). Values of the covariates are reported in Supplementary Table 1. Reported p-values are uncorrected for multiple comparisons.
Uncorrected mean ± standard deviation.
Coefficients represent standardized values.
Significant at uncorrected p-value of 0.05. ** Significant at FDR-corrected p-value.
Fig. 3.
Relationship between indices of social anxiety and gray matter characteristics.
Fig. 4.
Heritability estimates of gray matter characteristics.
3.4. Cortical thickness of ROIs
Results of the analyses with respect to the thickness of cortical ROIs are displayed in Table 5 and Supplementary Table 1. Again, we used three different models to test for associations between cortical thickness and, respectively, (sub)clinical SAD, self-reported levels of SA (z-score), and FNE. None of the associations was significant at the FDR-corrected significance level; at the uncorrected level (p < 0·05), indices of social anxiety were negatively correlated with CT of the right rostral middle frontal gyrus (effect of (sub)clinical SAD and effect of self-reported social anxiety), the left medial orbitofrontal cortex (effect of self-reported social anxiety), the right rostral ACC (effect of (sub)clinical SAD), the left and right superior temporal gyrus (effect of (sub)clinical SAD and effect of FNE, respectively) and the left fusiform gyrus (effect of self-reported social anxiety). Furthermore, there were positive relationships between social anxiety and CT of the left rostral ACC (effect of FNE), the right inferior parietal cortex (effect of (sub)clinical SAD), the left and right supramarginal gyrus (effect of (sub)clinical SAD and effect of FNE, respectively), the left temporal pole (effect of (sub)clinical SAD) and the left transverse temporal gyrus (effect of (sub)clinical SAD) (Fig. 3b). It should be noted that there were significant interactions between (sub)clinical SAD and age with respect to the thickness of the right rostral middle frontal gyrus and the left supramarginal gyrus. These interactions are illustrated in Supplementary Fig. 1.
Table 5.
Effects of social anxiety on thickness of cortical ROIs; heritability estimates.
| Effect of (sub)clinical SADb |
Effect of social anxiety (z-score)b |
Effect of FNEb |
Heritability estimate |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Sub)clinical SADa | No SADa | β | SE | p | β | SE | p | β | SE | p | h2 | p | ||
| Superior frontal | L | 2.93 ± 0.17 | 2.93 ± 0.20 | −0.07 | 0.04 | 0.07 | −0.07 | 0.04 | 0.10 | 0.01 | 0.04 | 0.85 | 0.11 | n.s. |
| R | 2.92 ± 0.17 | 2.93 ± 0.20 | −0.06 | 0.04 | 0.15 | 0.00 | 0.04 | 1.00 | 0.02 | 0.04 | 0.63 | < 0.10 | n.a. | |
| Caudal middle frontal | L | 2.68 ± 0.16 | 2.65 ± 0.17 | 0.03 | 0.06 | 0.56 | 0.04 | 0.06 | 0.53 | 0.07 | 0.06 | 0.25 | < 0.10 | n.a. |
| R | 2.66 ± 0.16 | 2.63 ± 0.17 | 0.05 | 0.06 | 0.44 | 0.03 | 0.06 | 0.60 | −0.02 | 0.06 | 0.73 | < 0.10 | n.a. | |
| Rostral middle frontal | L | 2.51 ± 0.17 | 2.51 ± 0.18 | 0.00 | 0.05 | 0.93 | −0.04 | 0.05 | 0.41 | 0.06 | 0.05 | 0.26 | < 0.10 | n.a. |
| R | 2.44 ± 0.17 | 2.48 ± 0.18 | −0.13 | 0.05 | 0.02 ⁎ | −0.12 | 0.05 | 0.03 ⁎ | −0.08 | 0.05 | 0.15 | < 0.10 | n.a. | |
| Lateral orbitofrontal | L | 2.80 ± 0.25 | 2.81 ± 0.20 | −0.08 | 0.06 | 0.18 | −0.06 | 0.05 | 0.28 | −0.05 | 0.06 | 0.34 | < 0.10 | n.a. |
| R | 2.71 ± 0.23 | 2.72 ± 0.22 | −0.04 | 0.06 | 0.51 | −0.02 | 0.05 | 0.71 | −0.01 | 0.05 | 0.86 | 0.20 | n.s. | |
| Medial orbitofrontal | L | 2.60 ± 0.20 | 2.62 ± 0.21 | −0.08 | 0.06 | 0.18 | −0.12 | 0.06 | 0.04 ⁎ | 0.06 | 0.06 | 0.32 | 0.48 | 0.035 ⁎ |
| R | 2.71 ± 0.28 | 2.67 ± 0.21 | 0.05 | 0.06 | 0.41 | 0.03 | 0.06 | 0.60 | 0.09 | 0.06 | 0.13 | 0.19 | n.s. | |
| Precentral | L | 2.61 ± 0.17 | 2.59 ± 0.15 | −0.01 | 0.06 | 0.90 | 0.02 | 0.06 | 0.75 | 0.03 | 0.06 | 0.62 | 0.22 | n.s. |
| R | 2.59 ± 0.15 | 2.58 ± 0.16 | −0.01 | 0.06 | 0.83 | 0.02 | 0.06 | 0.76 | 0.04 | 0.06 | 0.55 | < 0.10 | n.a. | |
| Caudal anterior cingulate | L | 2.96 ± 0.25 | 2.91 ± 0.26 | 0.05 | 0.09 | 0.58 | 0.12 | 0.09 | 0.16 | 0.02 | 0.09 | 0.80 | < 0.10 | n.a. |
| R | 2.72 ± 0.21 | 2.76 ± 0.25 | −0.08 | 0.08 | 0.29 | −0.15 | 0.08 | 0.06 | −0.08 | 0.08 | 0.33 | < 0.10 | n.a. | |
| Rostral anterior cingulate | L | 3.13 ± 0.26 | 3.08 ± 0.25 | 0.05 | 0.07 | 0.51 | −0.04 | 0.07 | 0.60 | 0.14 | 0.07 | 0.05 ⁎ | 0.48 | 0.024 ⁎ |
| R | 3.05 ± 0.25 | 3.15 ± 0.23 | −0.18 | 0.08 | 0.02 ⁎ | −0.07 | 0.08 | 0.36 | 0.09 | 0.08 | 0.27 | 0.48 | 0.016 ⁎⁎ | |
| Insula | L | 3.16 ± 0.20 | 3.17 ± 0.20 | −0.05 | 0.06 | 0.44 | −0.07 | 0.06 | 0.23 | 0.02 | 0.06 | 0.77 | 0.43 | 0.001 ⁎⁎ |
| R | 3.16 ± 0.21 | 3.15 ± 0.20 | −0.01 | 0.07 | 0.93 | −0.04 | 0.07 | 0.53 | −0.06 | 0.07 | 0.38 | 0.29 | 0.046 ⁎ | |
| Superior parietal | L | 2.20 ± 0.14 | 2.19 ± 0.14 | 0.02 | 0.05 | 0.76 | 0.08 | 0.05 | 0.14 | 0.01 | 0.05 | 0.81 | 0.35 | n.s. |
| R | 2.17 ± 0.16 | 2.15 ± 0.15 | 0.07 | 0.05 | 0.19 | 0.02 | 0.05 | 0.68 | −0.02 | 0.05 | 0.63 | 0.53 | 0.002 ⁎⁎ | |
| Inferior parietal | L | 2.53 ± 0.16 | 2.50 ± 0.16 | 0.08 | 0.05 | 0.11 | −0.03 | 0.05 | 0.55 | −0.03 | 0.05 | 0.52 | 0.35 | n.s. |
| R | 2.56 ± 0.14 | 2.52 ± 0.15 | 0.11 | 0.05 | 0.03 ⁎ | 0.07 | 0.05 | 0.12 | 0.01 | 0.05 | 0.86 | < 0.10 | n.a. | |
| Precuneus | L | 2.46 ± 0.21 | 2.44 ± 0.19 | 0.01 | 0.05 | 0.86 | 0.09 | 0.05 | 0.06 | 0.03 | 0.05 | 0.45 | 0.30 | 0.045 ⁎ |
| R | 2.45 ± 0.19 | 2.44 ± 0.20 | −0.01 | 0.06 | 0.84 | 0.06 | 0.05 | 0.29 | −0.06 | 0.05 | 0.28 | < 0.10 | n.a. | |
| Supramarginal | L | 2.64 ± 0.17 | 2.58 ± 0.16 | 0.13 | 0.06 | 0.03 ⁎ | 0.04 | 0.06 | 0.53 | 0.02 | 0.06 | 0.73 | 0.23 | n.s. |
| R | 2.62 ± 0.17 | 2.58 ± 0.17 | 0.06 | 0.06 | 0.32 | 0.08 | 0.06 | 0.14 | 0.12 | 0.06 | 0.04 ⁎ | < 0.10 | n.a. | |
| Postcentral | L | 2.11 ± 0.18 | 2.09 ± 0.12 | 0.02 | 0.07 | 0.77 | 0.08 | 0.07 | 0.26 | 0.02 | 0.07 | 0.78 | 0.19 | n.s. |
| R | 2.03 ± 0.16 | 2.06 ± 0.13 | −0.13 | 0.07 | 0.07 | −0.03 | 0.07 | 0.66 | −0.05 | 0.07 | 0.50 | 0.34 | n.s. | |
| Temporal pole | L | 3.63 ± 0.27 | 3.49 ± 0.28 | 0.25 | 0.09 | 0.01 ⁎ | 0.07 | 0.09 | 0.48 | 0.14 | 0.10 | 0.15 | 0.11 | n.s. |
| R | 3.53 ± 0.36 | 3.50 ± 0.38 | 0.05 | 0.10 | 0.59 | −0.02 | 0.09 | 0.85 | −0.06 | 0.10 | 0.51 | < 0.10 | n.a. | |
| Inferior temporal | L | 2.80 ± 0.14 | 2.77 ± 0.19 | 0.06 | 0.07 | 0.38 | 0.02 | 0.06 | 0.74 | −0.06 | 0.07 | 0.33 | < 0.10 | n.a. |
| R | 2.77 ± 0.15 | 2.78 ± 0.17 | −0.05 | 0.07 | 0.47 | 0.01 | 0.07 | 0.89 | −0.06 | 0.07 | 0.41 | < 0.10 | n.a. | |
| Superior temporal | L | 2.84 ± 0.17 | 2.87 ± 0.19 | −0.21 | 0.07 | 0.002 ⁎ | −0.09 | 0.07 | 0.15 | −0.08 | 0.06 | 0.21 | 0.74 | 7.5x10−5⁎⁎ |
| R | 2.89 ± 0.15 | 2.89 ± 0.17 | −0.07 | 0.06 | 0.28 | −0.05 | 0.06 | 0.39 | −0.17 | 0.06 | 0.01 ⁎ | 0.23 | n.s. | |
| Fusiform | L | 2.70 ± 0.16 | 2.71 ± 0.16 | −0.06 | 0.06 | 0.34 | −0.14 | 0.06 | 0.02 ⁎ | −0.09 | 0.06 | 0.15 | < 0.10 | n.a. |
| R | 2.69 ± 0.14 | 2.69 ± 0.17 | 0.00 | 0.06 | 0.97 | 0.02 | 0.06 | 0.69 | 0.07 | 0.06 | 0.26 | < 0.10 | n.a. | |
| Transverse temporal | L | 2.46 ± 0.30 | 2.34 ± 0.27 | 0.15 | 0.07 | 0.04 ⁎ | 0.09 | 0.07 | 0.22 | 0.03 | 0.07 | 0.66 | 0.64 | 1.7x10−6⁎⁎ |
| R | 2.49 ± 0.28 | 2.45 ± 0.33 | 0.02 | 0.08 | 0.83 | 0.01 | 0.08 | 0.89 | 0.03 | 0.08 | 0.72 | 0.47 | 0.001 ⁎⁎ | |
FNE: fear of negative evaluation; L: left; n.a.: not applicable; n.s.: not significant; R: right; SAD: social anxiety disorder; SE: standard error.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, mean global cortical thickness (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD* age (centered) and (sub)clinical SAD* mean global cortical thickness (centered). Values of the covariates are reported in Supplementary Table 1. Reported p-values are uncorrected for multiple comparisons.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, mean global cortical thickness (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD*age (centered) and (sub)clinical SAD*mean global cortical thickness (centered). Values of the covariates are reported in Supplementary Table 1. Reported p-values are uncorrected for multiple comparisons.
Uncorrected mean ± standard deviation.
Coefficients represent standardized values.
Significant at uncorrected p-value of 0.05. ** Significant at FDR-corrected p-value.
Considering the regions showing an association between CT and social anxiety in the first place, heritability analyses revealed that CT of the left medial orbitofrontal cortex, the bilateral rostral ACC, the left superior temporal gyrus and the left transverse temporal gyrus displayed moderately high (h2 = 0·4–0.6) or even high (h2 > 0·6) heritability. Furthermore, CT of the left supramarginal gyrus and the right superior temporal gyrus had moderate heritability (h2 between 0·2 and 0·4). These heritability estimates, as well as the estimates for ROIs in which there was no association with social anxiety, are illustrated in Fig. 4b and summarized in Table 5.
3.5. Cortical surface area of ROIs
Results of the analyses with respect to the average CSA of the cortical ROIs are displayed in Table 6 and Supplementary Table 1. There were no significant relationships between the measures of social anxiety at the corrected significance level, but self-reported social anxiety was negatively related to the CSA of the right fusiform gyrus at the uncorrected level. In addition, the level of FNE was negatively related to the CSA of the right caudal ACC and positively associated with CSA of the right precuneus (Fig. 3c). Analyses on the heritability of CSA of these ROIs indicated that CSA of the right fusiform gyrus was moderately high (h2 = 0·33). Heritability estimates of other ROIs are depicted in Fig. 4c and listed in Table 6.
Table 6.
Effects of social anxiety on surface area of cortical ROIs; heritability estimates.
| (Sub)clinical SADa | No SADa | Effect of (sub)clinical SADb |
Effect of social anxiety (z-score)b |
Effect of FNEb |
Heritability estimate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | h2 | p | ||||
| Superior frontal | L | 7412.1 ± 884.9 | 7448.6 ± 809.4 | 0.09 | 0.05 | 0.07 | 0.01 | 0.05 | 0.91 | −0.01 | 0.05 | 0.89 | 0.59 | 0.003 ⁎⁎ |
| R | 7112.2 ± 809.1 | 7268.8 ± 845.4 | −0.04 | 0.05 | 0.44 | 0.00 | 0.05 | 0.94 | 0.07 | 0.05 | 0.15 | 0.48 | 0.001 ⁎⁎ | |
| Caudal middle frontal | L | 2416.5 ± 360.0 | 2398.2 ± 400.2 | 0.05 | 0.07 | 0.46 | 0.08 | 0.07 | 0.24 | 0.10 | 0.07 | 0.13 | 0.31 | 0.036 ⁎ |
| R | 2241.5 ± 371.9 | 2206.5 ± 355.9 | 0.07 | 0.07 | 0.36 | 0.02 | 0.07 | 0.83 | 0.01 | 0.07 | 0.90 | 0.54 | 0.057 | |
| Rostral middle frontal | L | 5947.1 ± 805.4 | 6080.3 ± 862.2 | 0.00 | 0.05 | 0.97 | −0.02 | 0.05 | 0.59 | 0.00 | 0.04 | 0.97 | 0.66 | 2.7x10−4⁎⁎ |
| R | 6277.7 ± 924.3 | 6347.6 ± 934.5 | 0.04 | 0.04 | 0.33 | 0.00 | 0.04 | 0.92 | −0.01 | 0.04 | 0.83 | 0.79 | 1.6x10−5⁎⁎ | |
| Lateral orbitofrontal | L | 2566.5 ± 215.2 | 2644.7 ± 287.5 | 0.03 | 0.06 | 0.56 | 0.08 | 0.06 | 0.16 | −0.02 | 0.05 | 0.75 | 0.76 | 9.3x10−6⁎⁎ |
| R | 2569.0 ± 260.1 | 2596.0 ± 282.4 | 0.01 | 0.08 | 0.94 | 0.01 | 0.07 | 0.90 | −0.12 | 0.07 | 0.09 | < 0.10 | n.a. | |
| Medial orbitofrontal | L | 1913.4 ± 239.6 | 1942.0 ± 253.0 | 0.00 | 0.07 | 0.97 | 0.06 | 0.07 | 0.41 | 0.03 | 0.07 | 0.73 | < 0.10 | n.a. |
| R | 1872.9 ± 205.4 | 1872.6 ± 195.2 | 0.04 | 0.08 | 0.61 | 0.06 | 0.07 | 0.45 | 0.06 | 0.07 | 0.41 | < 0.10 | n.a. | |
| Precentral | L | 4821.7 ± 450.3 | 4887.3 ± 491.4 | −0.04 | 0.06 | 0.54 | 0.02 | 0.06 | 0.77 | 0.05 | 0.06 | 0.45 | 0.26 | 0.035 ⁎ |
| R | 4924.3 ± 502.2 | 4925.0 ± 461.3 | 0.04 | 0.06 | 0.48 | 0.10 | 0.06 | 0.09 | 0.03 | 0.06 | 0.57 | 0.24 | 0.049 ⁎ | |
| Caudal anterior cingulate | L | 641.3 ± 120.6 | 678.0 ± 172.2 | −0.05 | 0.08 | 0.54 | 0.06 | 0.08 | 0.43 | −0.01 | 0.08 | 0.89 | 0.17 | n.s. |
| R | 819.9 ± 178.4 | 834.2 ± 150.3 | −0.02 | 0.09 | 0.79 | −0.05 | 0.08 | 0.51 | −0.16 | 0.08 | 0.05⁎ | 0.16 | n.s. | |
| Rostral anterior cingulate | L | 828.2 ± 161.3 | 850.7 ± 155.6 | −0.01 | 0.07 | 0.93 | 0.05 | 0.07 | 0.50 | −0.01 | 0.07 | 0.94 | < 0.10 | n.a. |
| R | 673.7 ± 158.3 | 711.7 ± 117.4 | −0.07 | 0.08 | 0.36 | 0.02 | 0.08 | 0.79 | −0.06 | 0.08 | 0.42 | 0.44 | 0.002 ⁎⁎ | |
| Insula | L | 2277.4 ± 188.0 | 2275.6 ± 225.0 | 0.04 | 0.06 | 0.56 | 0.00 | 0.06 | 0.98 | −0.04 | 0.06 | 0.47 | 0.49 | 0.004 ⁎⁎ |
| R | 2272.2 ± 271.2 | 2327.7 ± 262.0 | −0.03 | 0.08 | 0.69 | −0.08 | 0.07 | 0.30 | 0.06 | 0.07 | 0.39 | 0.45 | 0.002 ⁎⁎ | |
| Superior parietal | L | 5611.9 ± 584.8 | 5609.0 ± 693.8 | 0.00 | 0.06 | 0.99 | 0.04 | 0.06 | 0.54 | 0.00 | 0.06 | 0.99 | 0.39 | 0.029 ⁎ |
| R | 5620.2 ± 734.3 | 5631.8 ± 664.2 | 0.00 | 0.07 | 0.98 | 0.06 | 0.06 | 0.35 | 0.09 | 0.07 | 0.19 | 0.35 | n.s. | |
| Inferior parietal | L | 4751.4 ± 749.4 | 4880.4 ± 663.4 | −0.02 | 0.06 | 0.78 | −0.02 | 0.06 | 0.67 | −0.01 | 0.05 | 0.78 | < 0.10 | n.a. |
| R | 5618.7 ± 811.9 | 5843.9 ± 918.8 | −0.10 | 0.06 | 0.06 | −0.09 | 0.05 | 0.10 | −0.04 | 0.06 | 0.43 | 0.17 | n.s. | |
| Precuneus | L | 3853.2 ± 504.3 | 3922.2 ± 504.2 | 0.00 | 0.05 | 0.94 | 0.02 | 0.05 | 0.73 | 0.02 | 0.05 | 0.66 | 0.47 | 2.4x10−5⁎⁎ |
| R | 4063.1 ± 527.7 | 4095.5 ± 534.8 | 0.04 | 0.06 | 0.48 | 0.03 | 0.06 | 0.65 | 0.13 | 0.06 | 0.02⁎ | < 0.10 | n.a. | |
| Supramarginal | L | 3966.7 ± 608.7 | 4110.0 ± 664.4 | −0.09 | 0.06 | 0.16 | −0.01 | 0.06 | 0.85 | −0.02 | 0.06 | 0.67 | 0.75 | 1.1x10−6⁎⁎ |
| R | 3895.4 ± 616.5 | 3922.6 ± 652.1 | 0.06 | 0.06 | 0.30 | −0.03 | 0.06 | 0.62 | −0.05 | 0.06 | 0.36 | 0.32 | 0.005 ⁎⁎ | |
| Postcentral | L | 4249.8 ± 634.9 | 4323.8 ± 511.6 | −0.02 | 0.06 | 0.70 | −0.03 | 0.06 | 0.60 | −0.02 | 0.06 | 0.79 | 0.29 | 0.034 ⁎ |
| R | 4086.7 ± 597.0 | 4147.1 ± 536.8 | −0.01 | 0.06 | 0.89 | 0.03 | 0.06 | 0.63 | −0.06 | 0.06 | 0.35 | 0.22 | 0.049 ⁎ | |
| Temporal pole | L | 474.7 ± 59.4 | 484.3 ± 64.4 | −0.05 | 0.09 | 0.56 | 0.03 | 0.08 | 0.74 | −0.02 | 0.09 | 0.82 | 0.15 | 0.028 ⁎ |
| R | 403.2 ± 64.7 | 407.1 ± 52.8 | 0.01 | 0.09 | 0.93 | 0.02 | 0.09 | 0.80 | 0.02 | 0.09 | 0.82 | 0.34 | 0.005 ⁎⁎ | |
| Inferior temporal | L | 3544.0 ± 452.0 | 3583.9 ± 591.1 | 0.02 | 0.06 | 0.70 | −0.02 | 0.06 | 0.75 | −0.04 | 0.06 | 0.51 | < 0.10 | n.a. |
| R | 3314.4 ± 508.7 | 3367.0 ± 490.0 | 0.01 | 0.05 | 0.91 | −0.02 | 0.06 | 0.69 | −0.02 | 0.05 | 0.70 | 0.38 | 0.004 ⁎⁎ | |
| Superior temporal | L | 3947.8 ± 457.5 | 3921.2 ± 476.2 | 0.06 | 0.06 | 0.30 | 0.04 | 0.06 | 0.49 | 0.07 | 0.05 | 0.20 | 0.92 | 5.8x10−6⁎⁎ |
| R | 3773.6 ± 407.2 | 3675.4 ± 315.8 | 0.08 | 0.05 | 0.15 | 0.00 | 0.05 | 0.96 | 0.06 | 0.05 | 0.26 | 0.75 | 3.8x10−4⁎⁎ | |
| Fusiform | L | 3405.1 ± 446.8 | 3371.7 ± 474.6 | 0.09 | 0.07 | 0.18 | −0.08 | 0.07 | 0.22 | 0.01 | 0.06 | 0.92 | 0.34 | 0.004 ⁎⁎ |
| R | 3162.8 ± 408.3 | 3274.9 ± 451.4 | −0.09 | 0.06 | 0.13 | −0.12 | 0.05 | 0.02⁎ | −0.04 | 0.05 | 0.47 | 0.33 | 3.6x10−6⁎⁎ | |
| Transverse temporal | L | 482.6 ± 75.0 | 495.4 ± 77.4 | −0.03 | 0.08 | 0.71 | −0.01 | 0.08 | 0.92 | 0.01 | 0.08 | 0.93 | 0.55 | 0.004 ⁎⁎ |
| R | 361.1 ± 57.5 | 368.8 ± 67.7 | −0.02 | 0.08 | 0.79 | 0.02 | 0.08 | 0.84 | 0.03 | 0.08 | 0.72 | 0.52 | 0.002 ⁎⁎ | |
FNE: fear of negative evaluation; L: left; n.a.: not applicable; n.s.: not significant; R: right; SAD: social anxiety disorder; SE: standard error.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, total global cortical surface area (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD* age (centered) and (sub)clinical SAD* total global cortical surface area (centered). Values of the covariates are reported in Supplementary Table 1.
Main effects of (sub)clinical SAD, social anxiety (z-score) and FNE are corrected for age (centered), gender, total global cortical surface area (centered) and family-structure. Furthermore, the models including (sub)clinical SAD contained the interaction terms (sub)clinical SAD*age (centered) and (sub)clinical SAD*total global cortical surface area (centered). Values of the covariates are reported in Supplementary Table 1.
Reported p-values are uncorrected for multiple comparisons.
Uncorrected mean ± standard deviation.
Coefficients represent standardized values.
Significant at uncorrected p-value of 0.05. ** Significant at FDR-corrected p-value.
3.6. Sensitivity analyses
Results of the sensitivity analyses showed comparable associations between the indices of social anxiety and the GM characteristics as the main analyses of interest. That is, in both sensitivity analyses (sensitivity analysis 1: participants with past and/or present (comorbid) psychopathology other than SAD were excluded; remaining n = 70; sensitivity analysis 2: the level of depressive symptoms was added as a covariate), we found a positive association with volume of the left pallidum, changes in cortical thickness in frontal, parietal and temporal areas, as well as alterations in cortical surface area of the precuneus and fusiform gyrus (all at p < .05, uncorrected). These findings are illustrated in Supplementary Fig. 2, Supplementary Fig. 3; detailed statistics are available in Supplementary Table 2, Supplementary Table 3.
3.7. Other subcortical and cortical brain regions (non-ROIs)
For reasons of completeness, results of the association analyses on subcortical and cortical regions that were not a priori selected (non-ROIs) are reported in Supplementary Table 4. In brief, none of the subcortical non-ROIs showed an association with any of the indices of social anxiety. With respect to the cortical measurements: cortical thickness was positively related to indices of social anxiety in some regions (right banks of the superior temporal sulcus, bilateral lingual gyrus, right lateral occipital gyrus and left pars triangularis), while indices of social anxiety were related to cortical surface area of the left parahippocampal gyrus, the right pars opercularis and the right banks of the superior temporal sulcus. However, none of these results survived multiple comparisons correction.
4. Discussion
Aim of the present study was to investigate whether structural gray matter (GM) brain characteristics could serve as candidate endophenotypes of social anxiety disorder (SAD) [48]. Data from the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) were used, as the multiplex, multigenerational family design of this study enables investigating two important endophenotype criteria [100]. First of all, we investigated whether the candidate endophenotypes co-segregated with social anxiety within the families, by studying the association between GM characteristics and three indices of social anxiety in families genetically enriched for SAD: the diagnosis of (sub)clinical SAD, self-reported levels of social anxiety, and self-reported levels of fear of negative evaluation (FNE). Secondly, we examined the heritability of the GM phenotypes.
We investigated subcortical brain volumes, cortical thickness (CT) measures and estimates of cortical surface area (CSA) and used a hypothesis-driven region-of-interest (ROI) approach, focussing on regions in which SAD-related alterations have been reported previously (Fig. 1), although it should be noted that the results of these studies, as summarized in Table 1, often lack consistency. Findings of these analyses will be considered in the following. We start with reviewing GM characteristics meeting both criteria for being a candidate endophenotype of social anxiety, as they (1) co-segregated with social anxiety within families, and (2) were at least moderately heritable. Next, we discuss the results of the association and heritability analyses in more detail, and consider them in the light of previous work.
4.1. Candidate endophenotypes of SAD
When combining the results of the association analyses with those of the heritability analyses, several GM characteristics turn out to be promising candidate endophenotypes of social anxiety, although it should be noted that the results of the association analyses did not survive correction for multiple comparisons. We summarized these findings in Fig. 5. This figure illustrates that the structural changes in GM which are genetically related to SAD are widespread over the brain, as they involve subcortical (pallidum) as well as cortical areas, including frontal, parietal and temporal regions. Interestingly, several of these cortical areas, namely the medial orbitofrontal cortex, the ACC, the supramarginal gyrus and the fusiform gyrus, are part of the extended neurobiological model of SAD as proposed by Brühl and colleagues [65]. This model of SAD, which is mainly based on data from functional MRI and the results of resting-state and functional connectivity studies, describes a hyperactive fear and anxiety circuit [157,158], consisting of the amygdala, insula, ACC, and prefrontal cortex, as well as hyperactive but less connected parieto-occipital regions. Furthermore, recent studies on connectivity showed widespread changes in functional networks in SAD [[159], [160], [161]]. Together, these findings converge with the results of the present study, as they indicate that the neurobiological brain changes related to SAD are not limited to the regions traditionally implicated in fear and anxiety, but are distributed in larger networks in the brain (cf. the recent commentary by Frick [162]). Although it is difficult to relate the structural alterations described here to functional brain changes, the results of functional MRI studies on SAD offer an interesting starting point. Most fMRI studies on SAD employ paradigms involving faces, as these are anxiety-provoking stimuli, and the results often point to increased brain responses in several brain areas, including the candidate endophenotype regions of the present study (Fig. 5). The rostral ACC, for example, a region involved in emotional processing, resolving emotional conflicts, and guiding socially-driven human interactions [[163], [164], [165]], showed increased activation in patients with SAD in response to angry [166], disgust [167] and sad faces [168]. Furthermore, several studies reported increased responsiveness of the superior temporal gyrus and the fusiform gyrus related to facial emotion processing in SAD [[169], [170], [171], [172]], while increased activation of the medial orbitofrontal cortex was found when patients with SAD looked at harsh faces [173]. However, as these results provide only indirect indications of the psychological alterations which might be related to the structural GM changes, future studies, for example using advanced MR sequences, are needed to gain more insight in the cellular bases of structural brain alterations [174] and to link them more directly to functional brain changes related to SAD. In addition, animal studies, in particular in non-human primates, enabling a translational approach, should further advance our understanding of the molecular and genetic underpinnings of anxiety-related brain changes (cf. [26,175]).
Fig. 5.
Overview of gray matter candidate endophenotypes of social anxiety.
4.2. Co-segregation of GM characteristics with social anxiety within families
When we consider the results of the association analyses, no significant associations between social anxiety and the GM characteristics were present at an FDR-corrected significance level (Table 4, Table 5, Table 6). At an uncorrected significance level (p < 0·05), several interesting patterns with respect to the association with social anxiety emerged (Fig. 3), which deserve to be discussed in detail.
To start with the subcortical ROIs, we found a positive association between both the level of self-reported social anxiety as well as with the level of self-reported FNE on the one hand and the volume of the left pallidum on the other (Fig. 3, Table 4). This result is in line with findings of a mega-analysis on 174 patients with SAD and 213 healthy control participants, showing larger GM volume in the dorsal striatum, including the pallidum and the putamen; in this study, the increase in GM was positively related to the level of self-reported social anxiety [60]. Recently, the positive relationship between social anxiety and volume of the dorsal striatum was replicated in a sample of healthy young women with a broad range of social anxiety levels [67], while a study on the structural correlates of ‘intolerance of uncertainty’, a psychological construct that is related to anxiety, indicated a positive relationship between intolerance of uncertainty and bilateral striatal volume, in particular the putamen and pallidum [176]. Interestingly, these findings and the increase in pallidum volume reported in the present work fit within the recent focus on the striatum as being an important structure in the anxiety circuitry of the brain [177] and are potentially reflective of the role of the pallidum and putamen in processing emotions and reward [178], as both processes have been shown to be associated with altered brain activation levels in these regions in patients with SAD [170,[179], [180], [181]].
Next, we investigated cortical GM characteristics. We examined estimates of CT as well as of CSA separately, as these measures show different developmental courses, are genetically independent, and have distinct associations with the risk of developing psychopathology [[106], [107], [108], [109], [110], [111], [112],114,115]. Our results converge with these findings, as there were no cortical ROIs in which both the estimates of CT and CSA were associated with social anxiety (cf. Fig. 3b and c).
The analyses on CT (Table 5) revealed that social anxiety was related to cortical thickening of the left rostral ACC, the right inferior parietal cortex, the left and right supramarginal gyrus, the left temporal pole, and the left transverse temporal gyrus; furthermore, there were associations between social anxiety and cortical thinning of the right rostral middle frontal gyrus, the left medial orbitofrontal gyrus, the right rostral ACC, the bilateral superior temporal gyrus, and the left fusiform gyrus (Fig. 3b). To facilitate the discussion, we summarized these findings together with the results of previous studies on the association between social anxiety and CT [54,55,59] in Table 7. This summary shows the divergence of the results with respect to the relation between social anxiety and CT. That is, our results showing decreases in CT in frontal ROIs coincide with those of Syal et al. (2012) [55] and Zhao et al. (2017) [59], while Brühl and colleagues (2014) [54] reported increased CT in frontal areas. The increases in CT in the left rostral ACC and several parietal regions found in the present study are in line with the results described by Brühl et al. (2014) [54] and Zhao et al. (2017) [59], but it should be noted that Syal et al. (2012) [55] outlined decreased CT in parietal regions; furthermore, the cortical thinning of the right rostral ACC of the present work has not been described previously. In addition, we found both increases as well as decreases in CT in the temporal ROIs; the increase in CT of the temporal pole corresponded to the results of Brühl et al. (2014) [54] and Zhao et al. (2017) [59] (but note that Syal et al. [55] reported a decrease in CT in this area), while the decreases in CT (superior temporal gyrus and fusiform gyrus) were in line with the data of Syal and colleagues (2012) [55] and with the results of a voxel-based morphometry study involving 68 anxiety patients without comorbidity [182]. Furthermore, it should be mentioned that we could not replicate previous findings on SAD-related changes in CT in frontal areas like the superior frontal gyrus, the caudal middle frontal cortex, the lateral orbitofrontal gyrus, and the precentral gyrus, nor did we found changes in CT in the caudal ACC, the insula, the superior parietal gyrus, the precuneus, the postcentral gyrus, and the inferior temporal gyrus. Taken together, the inconsistency of the results, as well as the small effect sizes [54] and the fact that p-values often don't survive comparison for multiple comparisons (this study and [54,55]) indicate that studies with large sample sizes and meta-analyses such as those performed by the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) Consortium [[183], [184], [185]] are needed to increase the reproducibility and validity of results of studies on the relation between social anxiety and cortical thickness [186]. The results of the present study could serve as a starting point for such future studies.
Table 7.
Summary of results with respect to the association between social anxiety disorder and cortical thickness.
| Present work |
Previous work |
||||
|---|---|---|---|---|---|
| LFLSAD [100] 39 (sub)clinical SAD-participantswith their family members (n = 62) |
Syal et al. (2012) [55] 13 SAD-patients vs 13 HC |
Brühl et al. (2014) [54] 46 SAD-patients vs 46 HC |
Zhao et al. (2017) [59] 24 SAD-patients vs 41 HC |
||
| Frontal | Superior frontal | n.s. | − | + | + |
| Caudal middle frontal | n.s. | − | + | + | |
| Rostral middle frontal | − | − | + | − | |
| Lateral orbitofrontal | n.s. | − | n.s. | − | |
| Medial orbitofrontal | − | − | n.s. | n.s. | |
| Precentral | n.s. | − | n.s. | − | |
| ACC | Caudal anterior cingulate | n.s. | n.s. | + | + |
| Rostral anterior cingulate | + (left) and - (right) | n.s. | + | + | |
| Insula | Insula | n.s. | − | + | + |
| Parietal | Superior parietal | n.s. | − | + | + |
| Inferior parietal | + | n.s. | + | n.s. | |
| Precuneus | n.s. | − | + | n.s. | |
| Supramarginal | + | − | n.s. | + | |
| Postcentral | n.s. | − | n.s. | n.s. | |
| Temporal | Temporal pole | + | − | + | + |
| Inferior temporal | n.s. | − | n.s. | + | |
| Superior temporal | − | − | n.s. | n.s. | |
| Fusiform gyrus | − | − | n.s. | n.s. | |
| Transverse temporal | + | − | n.s. | n.s. | |
HC: healthy control; n.s.: not significant; SAD: social anxiety disorder; +: increase; −: decrease.
To the best of our knowledge, this study was the first to explore the relationship between social anxiety and CSA, although Steiger and colleagues investigated changes in cortical volume, which is the product of CT and CSA, in a treatment study on SAD-patients [63]. Results showed decreases in CSA in the right caudal ACC and right fusiform gyrus, as well as an increase in CSA in the right precuneus (Table 6 and Fig. 3c).
4.3. Heritability of GM characteristics
We used a newly developed model to estimate the heritability of the GM brain characteristics, which is, to the best of our knowledge, the only available analysis model taking the specific ascertainment process of the present study and the familial relationships between the participants into account [151]. As expected based on the results of previous studies [81,[84], [85], [86],88,91,98], the majority of the GM measures of interest were (at least) moderately heritable (Fig. 4; Table 4, Table 5, Table 6). It should be noted that we could not replicate the significant heritability estimates of some of the GM measures as reported in other work, but estimates of heritability are often highly variable across studies [98] and across brain regions [86]; we refer to the recent work of Patel and colleagues reporting on the effects of different estimation approaches on heritability estimates [187]. Furthermore, these divergent results could also be due to the relatively small sample size and specific data structure of the present study, in which a limited number of families (n = 8) was included, with a broad range in the size of the families (range in number of participating family members per family 5–28).
4.4. Limitations and suggestions for future studies
The present study is unique as it is the first neuroimaging family-study on SAD involving two generations, enabling the investigation of the potential of structural GM characteristics as candidate endophenotypes of SAD. Several limitations of the present study should be mentioned. First of all, the sample size of the MRI sample of the LFLSAD was relatively small, which was partly caused by the loss of data points due to technical reasons and as a result of thorough quality control. Secondly, as this was a cross-sectional study, the trait stability of the GM characteristics (endophenotype criterion 2) could not be investigated. Third, we should mention the issue of psychiatric comorbidity, which was present in the LFLSAD sample as could be expected based on the comorbidity associated with SAD [[21], [22], [23],41]. We performed two sensitivity analyses to address this issue; in the first analysis, we excluded participants with past and/or present (comorbid) psychopathology other than SAD, in the second we added the level of depressive symptoms as a covariate. The results of these sensitivity analyses were in line with those of the main analyses, but these analyses were limited by a small sample size (sensitivity analysis 1) and the fact that we only controlled for the level of depressive symptoms (sensitivity analysis 2), and not for other comorbidity. Furthermore, as the regression models tested were already complex and computationally demanding due to the family structure of the sample, we could not investigate the potentially moderating or mediating effects of factors like trait anxiety, education level, IQ, and socioeconomic status [188,189], nor did we examine the non-linear effects of age on the GM characteristics [107]. As technical advances are constantly being made, future studies will most likely be able to perform more advanced analyses taking these factors into account. In addition, as the LFLSAD did not include control families from the general population, we were not able to assess the second part of endophenotype criterion 4, namely, whether the levels of the candidate endophenotypes differed between nonaffected family members and participants from the general population. Furthermore, as most of the results presented here did not survive corrections for multiple comparisons, future studies, preferably with a longitudinal design and larger sample sizes, are needed to confirm these findings. In addition, as we have not yet analysed the genetic data that was acquired in the LFLSAD [100], we could not link the GM changes to genetic variations. Moreover, future studies should investigate to which extent the GM alterations are specific to social anxiety (cf [59]). Finally, we employed a ROI approach in this study, as this enabled implementing the complex family structure of the sample in the analyses. However, as vertex-based and voxel based morphometry studies have the potential to detect more subtle alterations in brain structure [174,190,191], we recommend these techniques for future studies when they become available for family studies with complex (family) designs.
To conclude, the results of this study suggest that several structural GM alterations are heritable and co-segregate with social anxiety within families genetically enriched for SAD. Thereby, these GM characteristics are promising candidate endophenotypes of SAD and have the potential to offer novel insights in the genetic neurobiological vulnerability for this disabling psychiatric condition. Future replication studies are important to confirm these preliminary findings.
The following are the supplementary data related to this article
Illustration of interaction (sub)clinical SAD x age
Relationship between indices of social anxiety and gray matter characteristics in selection of LFLSAD sample: participants with (comorbid) psychopathology other than (sub)clinical SAD were excluded (sensitivity analysis 1)
Relationship between indices of social anxiety and gray matter characteristics, corrected for level of depressive symptoms (sensitivity analysis 2)
Detailed statistics of effects of social anxiety on, and heritability estimates of parameters of interest: general imaging phenotypes (tab 1); subcortical volumes (tab 2); cortical thickness (tab 3); cortical surface area (tab 4).
Detailed statistics of effects of social anxiety on parameters of interest in sample without comorbidity (sensitivity analysis 1): subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).
Detailed statistics of effects of social anxiety on parameters of interest, corrected for level of depressive symptoms (sensitivity analysis 2): subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).
Detailed statistics of effects of social anxiety on, and heritability estimates of non-ROIs: subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).
Funding sources
The Leiden Family Lab study on Social Anxiety Disorder and Janna Marie Bas-Hoogendam are funded by Leiden University Research Profile ‘Health, Prevention and the Human Life Cycle’ and the Institute of Psychology of Leiden University. These funding sources had no involvement in writing this paper nor in the decision to submit this work for publication.
Janna Marie Bas-Hoogendam has full access to all the data in the study and has final responsibility for the decision to submit this work for publication.
Declaration of interests
Janna Marie Bas-Hoogendam received a travel grant to present preliminary results of this study at the WASAD-SFB-TRR58 2017 meeting, organized by the World Association for Stress Related and Anxiety Disorders (from 14 to 16 September 2017, Würzburg, Germany) [192]. Nic J.A. van der Wee has consulted for Lilly, Wyeth, Servier, Pfizer and GlaxoSmithKline.
The authors report no further conflicts of interest.
Author contributions
Design of the LFLSAD: Janna Marie Bas-Hoogendam, Henk van Steenbergen, Jeanine J. Houwing-Duistermaat, Nic J.A. van der Wee, P. Michiel Westenberg.
Data acquisition: Janna Marie Bas-Hoogendam.
Data analysis: Janna Marie Bas-Hoogendam.
Statistical support: Renaud L.M. Tissier, Jeanine J. Houwing-Duistermaat, Henk van Steenbergen.
Literature search: Janna Marie Bas-Hoogendam.
Writing the present paper: Janna Marie Bas-Hoogendam.
Critically reviewing the present paper: Henk van Steenbergen, Renaud L.M. Tissier, Jeanine J. Houwing-Duistermaat, Nic J.A. van der Wee, P. Michiel Westenberg.
Acknowledgements
We thank Anita Harrewijn, Melle van der Molen and Irene M. van Vliet for their contribution to the design of the LFLSAD and their role in the recruitment of the families. Furthermore, we are grateful to several master students (Marjolein Barendse, Tanja Kreuk, Saskia van Leuverden, Farah Mesbahi, Eefje Poppelaars) and the support team of the Leiden Institute for Brain and Cognition (LIBC) who assisted in acquiring the MRI data. We would also like to acknowledge Lara Wierenga who provided support for creating Fig. 3, Fig. 4, Fig. 5.
In addition, we thank the Lorentz Center (Leiden, the Netherlands) for their financial and practical support in organizing the workshop ‘Endophenotypes of Social Anxiety Disorder: Can we detect them and are they useful in clinical practice?’, which took place 14 to 18 December 2015 (https://www.lorentzcenter.nl/lc/web/2015/754/info.php3?wsid=754).
Contributor Information
Janna Marie Bas-Hoogendam, Email: j.m.hoogendam@fsw.leidenuniv.nl.
Henk van Steenbergen, Email: HvanSteenbergen@FSW.leidenuniv.nl.
Renaud L.M. Tissier, Email: r.l.m.tissier@fsw.leidenuniv.nl.
Jeanine J. Houwing-Duistermaat, Email: J.Duistermaat@leeds.ac.uk.
P.Michiel Westenberg, Email: westenberg@fsw.leidenuniv.nl.
Nic J.A. van der Wee, Email: N.J.A.van_der_Wee@lumc.nl.
References
- 1.American Psychiatric Association . 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [Google Scholar]
- 2.Stein M.B., Stein D.J. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks S.M., Spijker J., Licht C.M.M. Disability in anxiety disorders. J Affect Disord. 2014;166:227–233. doi: 10.1016/j.jad.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Fehm L., Pelissolo A., Furmark T., Wittchen H.-U. Size and burden of social phobia in Europe. Eur Neuropsychopharmacol. 2005;15:453–462. doi: 10.1016/j.euroneuro.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Acarturk C., de Graaf R., van Straten A., Ten Have M., Cuijpers P. Social phobia and number of social fears, and their association with comorbidity, health-related quality of life and help seeking: a population-based study. Soc Psychiatry Psychiatr Epidemiol. 2008;43:273–279. doi: 10.1007/s00127-008-0309-1. [DOI] [PubMed] [Google Scholar]
- 6.Stein M.B., Kean Y.M. Disability and quality of life in social phobia: epidemiologic findings. Am J Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- 7.Aderka I.M., Hofmann S.G., Nickerson A., Hermesh H., Gilboa-Schechtman E., Marom S. Functional impairment in social anxiety disorder. J Anxiety Disord. 2012;26:393–400. doi: 10.1016/j.janxdis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Wittchen H.U., Jacobi F., Rehm J. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Vos T., Allen C., Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craske M.G., Stein M.B., Eley T.C. Anxiety disorders. Nat Rev Dis Prim. 2017;3:17024. doi: 10.1038/nrdp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graaf R., ten Have M., van Gool C., van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012;47:203–213. doi: 10.1007/s00127-010-0334-8. [DOI] [PubMed] [Google Scholar]
- 12.Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco C., Xu Y., Schneier F.R., Okuda M., Liu S.-M., Heimberg R.G. Predictors of persistence of social anxiety disorder: a national study. J Psychiatr Res. 2011;45:1557–1563. doi: 10.1016/j.jpsychires.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Wittchen H.-U., Fehm L. Epidemiology and natural course of social fears and social phobia. Acta Psychiatr Scand. 2003;108:4–18. doi: 10.1034/j.1600-0447.108.s417.1.x. [DOI] [PubMed] [Google Scholar]
- 15.Beesdo-Baum K., Knappe S., Asselmann E. The ‘Early Developmental Stages of Psychopathology (EDSP) study’: a 20-year review of methods and findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:851–866. doi: 10.1007/s00127-015-1062-x. [DOI] [PubMed] [Google Scholar]
- 16.Miers A.C., Blöte A.W., de Rooij M., Bokhorst C.L., Westenberg P.M. Trajectories of social anxiety during adolescence and relations with cognition, social competence, and temperament. J Abnorm Child Psychol. 2013;41:97–110. doi: 10.1007/s10802-012-9651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miers A.C., Blöte A.W., Heyne D.A., Westenberg P.M. Developmental pathways of social avoidance across adolescence: The role of social anxiety and negative cognition. J Anxiety Disord. 2014;28:787–794. doi: 10.1016/j.janxdis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Haller S.P.W., Cohen Kadosh K., Scerif G., Lau J.Y.F. Social anxiety disorder in adolescence: how developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Dev Cogn Neurosci. 2015;13:11–20. doi: 10.1016/j.dcn.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merikangas K.R., He J.-P., Burstein M. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leigh E., Clark D.M. Understanding social anxiety disorder in adolescents and improving treatment outcomes: applying the cognitive model of Clark and Wells (1995) Clin Child Fam Psychol Rev. 2018 doi: 10.1007/s10567-018-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruscio A.M., Brown T.A., Chiu W.T., Sareen J., Stein M.B., Kessler R.C. Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med. 2008;38:15–28. doi: 10.1017/S0033291707001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erwin B.A., Heimberg R.G., Juster H., Mindlin M. Comorbid anxiety and mood disorders among persons with social anxiety disorder. Behav Res Ther. 2002;40:19–35. doi: 10.1016/s0005-7967(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 23.Meier S.M., Petersen L., Mattheisen M., Mors O., Mortensen P.B., Laursen T.M. Secondary depression in severe anxiety disorders: a population-based cohort study in Denmark. Lancet Psychiatry. 2015;2:515–523. doi: 10.1016/S2215-0366(15)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong Q.J.J., Rapee R.M. The aetiology and maintenance of social anxiety disorder: a synthesis of complimentary theoretical models and formulation of a new integrated model. J Affect Disord. 2016;203:84–100. doi: 10.1016/j.jad.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 25.Hirshfeld-Becker D.R. Familial and temperamental risk factors for social anxiety disorder. New Dir Child Adolesc Dev. 2010;2010:51–65. doi: 10.1002/cd.262. [DOI] [PubMed] [Google Scholar]
- 26.Fox A.S., Kalin N.H. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandelow B., Baldwin D., Abelli M. Biological markers for anxiety disorders, OCD and PTSD – a consensus statement. Part I: neuroimaging and genetics. World J Biol Psychiatry. 2016;17:321–365. doi: 10.1080/15622975.2016.1181783. [DOI] [PubMed] [Google Scholar]
- 28.Isomura K., Boman M., Rück C. Population-based, multi-generational family clustering study of social anxiety disorder and avoidant personality disorder. Psychol Med. 2015;45:1581–1589. doi: 10.1017/S0033291714002116. [DOI] [PubMed] [Google Scholar]
- 29.Smoller J.W. The genetics of stress-related disorders: PTSD, depression and anxiety disorders. Neuropsychopharmacology. 2015;41:297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scaini S., Belotti R., Ogliari A. Genetic and environmental contributions to social anxiety across different ages: a meta-analytic approach to twin data. J Anxiety Disord. 2014;28:650–656. doi: 10.1016/j.janxdis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Stein M.B., Chen C.-Y., Jain S. Genetic risk variants for social anxiety. Am J Med Genet Part B Neuropsychiatr Genet. 2017;174:120–131. doi: 10.1002/ajmg.b.32520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelernter J., Page G.P., Stein M.B., Woods S.W. Genome-wide linkage scan for loci predisposing to social phobia: evidence for a chromosome 16 risk locus. Am J Psychiatry. 2004;161:59–66. doi: 10.1176/appi.ajp.161.1.59. [DOI] [PubMed] [Google Scholar]
- 33.Fyer A.J., Costa R., Haghighi F. Linkage analysis of alternative anxiety phenotypes in multiply affected panic disorder families. Psychiatr Genet. 2012;22:123–129. doi: 10.1097/YPG.0b013e328353956a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otowa T., Hek K., Lee M. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016:1–9. doi: 10.1038/mp.2016.11. [DOI] [PubMed] [Google Scholar]
- 35.Stein M.B., Jang K.L., Livesley W.J. Heritability of social anxiety-related concerns and personality characteristics: a twin study. J Nerv Ment Dis. 2002;190:219–224. doi: 10.1097/00005053-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Stein M.B., Chartier M.J., Lizak M.V., Jang K.L. Familial aggregation of anxiety-related quantitative traits in generalized social phobia: clues to understanding ‘disorder’ heritability? Am J Med Genet. 2001;105:79–83. [PubMed] [Google Scholar]
- 37.Gottschalk M.G., Domschke K. Novel developments in genetic and epigenetic mechanisms of anxiety. Curr Opin Psychiatry. 2016;29(1):32–38. doi: 10.1097/YCO.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 38.Binder E.B. The genetic basis of mood and anxiety disorders - changing paradigms. Biol Mood Anxiety Disord. 2012;2:1–3. doi: 10.1186/2045-5380-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munafò M.R., Flint J. Common or rare variants for complex traits? Biol Psychiatry. 2014;75:752–753. doi: 10.1016/j.biopsych.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Bearden C.E., Reus V.I., Freimer N.B. Why genetic investigation of psychiatric disorders is so difficult. Curr Opin Genet Dev. 2004;14:280–286. doi: 10.1016/j.gde.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Hyett M.P., McEvoy P.M. Social anxiety disorder: looking back and moving forward. Psychol Med. 2018:1–8. doi: 10.1017/S0033291717003816. [DOI] [PubMed] [Google Scholar]
- 42.Iacono W.G. Endophenotypes in psychiatric disease: prospects and challenges. Genome Med. 2018;10:1–3. doi: 10.1186/s13073-018-0526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenzenweger M.F. Thinking clearly about the endophenotype-intermediate phenotype-biomarker distinctions in developmental psychopathology research. Dev Psychopathol. 2013;25:1347–1357. doi: 10.1017/S0954579413000655. [DOI] [PubMed] [Google Scholar]
- 44.Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 45.Glahn D.C., Thompson P.M., Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenzenweger M.F. Endophenotype, intermediate phenotype, biomarker: definitions, concept comparisons, clarifications. Depress Anxiety. 2013;30:185–189. doi: 10.1002/da.22042. [DOI] [PubMed] [Google Scholar]
- 47.Puls I., Gallinat J. The concept of endophenotypes in psychiatric diseases meeting the expectations? Pharmacopsychiatry. 2008;41(Suppl. 1):S37–S43. doi: 10.1055/s-2008-1081462. [DOI] [PubMed] [Google Scholar]
- 48.Bas-Hoogendam J.M., Blackford J.U., Brühl A.B., Blair K.S., van der Wee N.J.A., Westenberg P.M. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci Biobehav Rev. 2016;71:362–378. doi: 10.1016/j.neubiorev.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 49.Talati A., Pantazatos S.P., Schneier F.R., Weissman M.M., Hirsch J. Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol Psychiatry. 2013;73:75–84. doi: 10.1016/j.biopsych.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao W., Xu Q., Mantini D. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–177. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Frick A., Howner K., Fischer H., Eskildsen S.F., Kristiansson M., Furmark T. Cortical thickness alterations in social anxiety disorder. Neurosci Lett. 2013;536:52–55. doi: 10.1016/j.neulet.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 52.Machado-de-Sousa J.P., de Osório F.L., Jackowski A.P. Increased amygdalar and hippocampal volumes in young adults with social anxiety. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng Y., Lui S., Qiu C. Neuroanatomical deficits in drug-naïve adult patients with generalized social anxiety disorder: a voxel-based morphometry study. Psychiatry Res. 2013;214:9–15. doi: 10.1016/j.pscychresns.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Brühl A.B., Hänggi J., Baur V. Increased cortical thickness in a frontoparietal network in social anxiety disorder. Hum Brain Mapp. 2014;35:2966–2977. doi: 10.1002/hbm.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syal S., Hattingh C.J., Fouché J.-P. Grey matter abnormalities in social anxiety disorder: a pilot study. Metab Brain Dis. 2012;27:299–309. doi: 10.1007/s11011-012-9299-5. [DOI] [PubMed] [Google Scholar]
- 56.Tükel R., Aydın K., Yüksel Ç., Ertekin E., Koyuncu A., Taş C. Gray matter abnormalities in patients with social anxiety disorder: a voxel-based morphometry study. Psychiatry Res. 2015;234:106–112. doi: 10.1016/j.pscychresns.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Irle E., Barke A., Lange C., Ruhleder M. Parietal abnormalities are related to avoidance in social anxiety disorder: a study using voxel-based morphometry and manual volumetry. Psychiatry Res. 2014;224:175–183. doi: 10.1016/j.pscychresns.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Frick A., Engman J., Alaie I. Enlargement of visual processing regions in social anxiety disorder is related to symptom severity. Neurosci Lett. 2014;583:114–119. doi: 10.1016/j.neulet.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y., Chen L., Zhang W. Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine. 2017 doi: 10.1016/j.ebiom.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bas-Hoogendam J.M., van Steenbergen H., Pannekoek J.N. Voxel-based morphometry multi-center mega-analysis of brain structure in social anxiety disorder. NeuroImage Clin. 2017;16:678–688. doi: 10.1016/j.nicl.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irle E., Ruhleder M., Lange C. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J Psychiatry Neurosci. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talati A., Pantazatos S.P., Hirsch J., Schneier F. A pilot study of gray matter volume changes associated with paroxetine treatment and response in social anxiety disorder. Psychiatry Res. 2015;231:279–285. doi: 10.1016/j.pscychresns.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steiger V.R., Brühl A.B., Weidt S. Pattern of structural brain changes in social anxiety disorder after cognitive behavioral group therapy: a longitudinal multimodal MRI study. Mol Psychiatry. 2016;00:1–8. doi: 10.1038/mp.2016.217. [DOI] [PubMed] [Google Scholar]
- 64.Cassimjee N., Fouche J.-P., Burnett M. Changes in regional brain volumes in social anxiety disorder following 12 weeks of treatment with escitalopram. Metab Brain Dis. 2010;25:369–374. doi: 10.1007/s11011-010-9218-6. [DOI] [PubMed] [Google Scholar]
- 65.Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in Social Anxiety Disorder–a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Frick A., Gingnell M., Marquand A.F. Classifying social anxiety disorder using multivoxel pattern analyses of brain function and structure. Behav Brain Res. 2014;259:330–335. doi: 10.1016/j.bbr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Günther V., Ihme K., Kersting A., Hoffmann K.-T., Lobsien D., Suslow T. Volumetric associations between amygdala, nucleus accumbens, and socially anxious tendencies in healthy women. Neuroscience. 2018;374:25–32. doi: 10.1016/j.neuroscience.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 68.Gold A.L., Steuber E.R., White L.K. 2017. Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology. published online May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller S.C., Aouidad A., Gorodetsky E., Goldman D., Pine D.S., Ernst M. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val66Met polymorphism? J Am Acad Child Adolesc Psychiatry. 2013;52:184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gold A.L., Brotman M.A., Adleman N.E. Comparing brain morphometry across multiple childhood psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2016;55:1027–1037. doi: 10.1016/j.jaac.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strawn J.R., Hamm L., Fitzgerald D.A., Fitzgerald K.D., Monk C.S., Phan K.L. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81–88. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milham M.P., Nugenta A.C., Drevets W.C. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 73.Sylvester C.M., Barch D.M., Harms M.P. Early childhood behavioral inhibition predicts cortical thickness in adulthood. J Am Acad Child Adolesc Psychiatry. 2015 doi: 10.1016/j.jaac.2015.11.007. published online Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz C.E., Kunwar P.S., Greve D.N. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Arch Gen Psychiatry. 2010;67:78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clauss J.A., Seay A.L., Vanderklok R.M. Structural and functional bases of inhibited temperament. Soc Cogn Affect Neurosci. 2014;9:2049–2058. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherbuin N., Windsor T.D., Anstey K.J., Maller J.J., Meslin C., Sachdev P.S. Hippocampal volume is positively associated with behavioural inhibition (BIS) in a large community-based sample of mid-life adults: the PATH through life study. Soc Cogn Affect Neurosci. 2008;3:262–269. doi: 10.1093/scan/nsn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrós-Loscertales A., Meseguer V., Sanjuán A. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. Neuroimage. 2006;33:1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 78.Levita L., Bois C., Healey A. The Behavioural Inhibition System, anxiety and hippocampal volume in a non-clinical population. Biol Mood Anxiety Disord. 2014;4:4. doi: 10.1186/2045-5380-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuentes P., Barrós-Loscertales A., Bustamante J.C., Rosell P., Costumero V., Ávila C. Individual differences in the Behavioral Inhibition System are associated with orbitofrontal cortex and precuneus gray matter volume. Cogn Affect Behav Neurosci. 2012;12:491–498. doi: 10.3758/s13415-012-0099-5. [DOI] [PubMed] [Google Scholar]
- 80.Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson P.M., Cannon T.D., Narr K.L. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 82.Wen W., Thalamuthu A., Mather K.A. Distinct genetic influences on cortical and subcortical brain structures. Sci Rep. 2016;6:32760. doi: 10.1038/srep32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joshi A.A., Leporé N., Joshi S.H. The contribution of genes to cortical thickness and volume. Neuroreport. 2011;22:101–105. doi: 10.1097/WNR.0b013e3283424c84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen C.-H., Peng Q., Schork A.J. Large-scale genomics unveil polygenic architecture of human cortical surface area. Nat Commun. 2015;6:7549. doi: 10.1038/ncomms8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eyler L.T., Prom-Wormley E., Panizzon M.S. Genetic and environmental contributions to regional cortical surface area in humans: a magnetic resonance imaging twin study. Cereb Cortex. 2011;21:2313–2321. doi: 10.1093/cercor/bhr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strike L.T., Hansell N.K., Couvy-Duchesne B. Genetic complexity of cortical structure: differences in genetic and environmental factors influencing cortical surface area and thickness. Cereb Cortex. 2018 doi: 10.1093/cercor/bhy002. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adams H.H.H., Hibar D.P., Chouraki V. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci. 2016;19:1569. doi: 10.1038/nn.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hibar D.P., Stein J.L., Renteria M.E. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stein J.L., Medland S.E., Vasquez A.A. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whelan C.D., Hibar D.P., van Velzen L.S. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage. 2015;128:125–137. doi: 10.1016/j.neuroimage.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.den Braber A., Bohlken M.M., Brouwer R.M. Heritability of subcortical brain measures: a perspective for future genome-wide association studies. Neuroimage. 2013;83C:98–102. doi: 10.1016/j.neuroimage.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 92.Rentería M.E., Hansell N.K., Strike L.T. Genetic architecture of subcortical brain regions: common and region-specific genetic contributions. Genes Brain Behav. 2014;13:821–830. doi: 10.1111/gbb.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Satizabal C.L., HHH Adams, Hibar D.P. Genetic architecture of subcortical brain structures in over 40,000 individuals worldwide. bioRxiv. 2017 [preprint] [Google Scholar]
- 94.Roshchupkin G.V., Gutman B.A., Vernooij M.W. Heritability of the shape of subcortical brain structures in the general population. Nat Commun. 2016;7:13738. doi: 10.1038/ncomms13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ge T., Reuter M., Winkler A.M. Multidimensional heritability analysis of neuroanatomical shape. Nat Commun. 2016;7:13291. doi: 10.1038/ncomms13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bootsman F., Brouwer R.M., Kemner S.M. Contribution of genes and unique environment to cross-sectional and longitudinal measures of subcortical volumes in bipolar disorder. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.09.023. published online Oct 8. [DOI] [PubMed] [Google Scholar]
- 97.Roalf D.R., Vandekar S.N., Almasy L. Heritability of subcortical and limbic brain volume and shape in multiplex-multigenerational families with schizophrenia. Biol Psychiatry. 2015;77:137–146. doi: 10.1016/j.biopsych.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blokland G.A.M., de Zubicaray G.I., McMahon K.L., Wright M.J. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15:351–371. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peper J.S., Brouwer R.M., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bas-Hoogendam J.M., Harrewijn A., Tissier R.L.M. The Leiden Family Lab study on Social Anxiety Disorder: a multiplex, multigenerational family study on neurocognitive endophenotypes. Int J Methods Psychiatr Res. 2018;27 doi: 10.1002/mpr.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams J.T., Blangero J. Power of variance component linkage analysis to detect quantitative trait loci. Ann Hum Genet. 1999;63:545–563. doi: 10.1017/S0003480099007848. [DOI] [PubMed] [Google Scholar]
- 102.Desikan R.S., Ségonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 103.Rakic P. Specification of cerebral cortical areas. Science (80-) 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 104.Geschwind D.H., Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Im K., Lee J.-M., Lyttelton O., Kim S.H., Evans A.C., Kim S.I. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- 106.Winkler A.M., Kochunov P., Blangero J. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 108.Tamnes C.K., Herting M.M., Goddings A.-L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37:3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Panizzon M.S., Fennema-Notestine C., Eyler L.T. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hogstrom L.J., Westlye L.T., Walhovd K.B., Fjell A.M. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- 111.Chen C.-H., Fiecas M., Gutiérrez E.D. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 2013;110:17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winkler A.M., Greve D.N., Bjuland K.J. Joint analysis of cortical area and thickness as a replacement for the analysis of the volume of the cerebral cortex. Cereb Cortex. 2018;28:738–749. doi: 10.1093/cercor/bhx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prasad K.M., Goradia D., Eack S. Cortical surface characteristics among offspring of schizophrenia subjects. Schizophr Res. 2010;116:143–151. doi: 10.1016/j.schres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bois C., Ronan L., Levita L. Cortical surface area differentiates familial high risk individuals who go on to develop schizophrenia. Biol Psychiatry. 2015;78:413–420. doi: 10.1016/j.biopsych.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 116.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 117.Bas-Hoogendam J.M., Harrewijn A., van der Molen M.J.W. 2014. Profiling Endophenotypes in Social Anxiety Disorder: A Neurocognitive Approach. General Background and Key Question of Project. [Google Scholar]
- 118.Sheehan D.V., Lecrubier Y., Sheehan K.H. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 2):22–33. [PubMed] [Google Scholar]
- 119.van Vliet IM, de Beurs E. [The MINI-International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders]. Tijdschr Psychiatr 2007; 49: 393–7. [PubMed]
- 120.Bauhuis O., Jonker K., Verdellen C., Reynders J., Verbraak M. De introductie van een Nederlandstalig instrument om DSM-IV-Tr-diagnoses bij kinderen te stellen. Kind Adolesc Prakt. 2013;12:20–26. [Google Scholar]
- 121.Sheehan D.V., Sheehan K.H., Shytle R.D. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 122.Fresco D.M., Coles M.E., Heimberg R.G. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 123.Mennin D.S., Fresco D.M., Heimberg R.G., Schneier F.R., Davies S.O., Liebowitz M.R. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J Anxiety Disord. 2002;16:661–673. doi: 10.1016/s0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- 124.La Greca A.M., Lopez N. Social anxiety among adolescents: linkages with peer relations and friendships. J Abnorm Child Psychol. 1998;26:83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- 125.Carleton R.N., McCreary D.R., Norton P.J., Asmundson G.J.G. Brief fear of negative evaluation scale-revised. Depress Anxiety. 2006;23:297–303. doi: 10.1002/da.20142. [DOI] [PubMed] [Google Scholar]
- 126.Leary M.R. A brief version of the fear of negative evaluation scale. Pers Soc Psychol Bull. 1983;9:371–375. [Google Scholar]
- 127.Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, CA: 1970. STAI manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 128.Beck A., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 129.Van der Does A. Harcourt; Amsterdam: 2002. Handleiding bij de Nederlandse versie van Beck Depression Inventory—second edition (BDI—II—NL)[Manual for the Dutch version of the Beck Depression Inventory—second edition (BDI—II—NL).] [Google Scholar]
- 130.Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 131.Timbremont B., Braet C. Lisse: Swets & Zeitlinger. 2002. Children's Depression Inventory: Nederlandstalige versie [Children's Depression Inventory: Dutch Version] [Google Scholar]
- 132.Wechsler D., Coalson D., Raiford S. Pearson; San Antonio, TX: 2008. WAIS-IV Technical and Interpretive Manual. [Google Scholar]
- 133.Wechsler D. The Psychological Corporation; San Antonio, TX: 1991. Manual for the Wechsler Intelligence Scale for Children - Third Edition (WISC-III) [Google Scholar]
- 134.Galván A. Neural plasticity of development and learning. Hum Brain Mapp. 2010;31:879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 136.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 138.Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 140.Fischl B., Salat D.H., Busa E. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 141.Fischl B., van der Kouwe A., Destrieux C. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 142.Fischl B., Salat D.H., van der Kouwe A.J.W. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 143.Rosas H.D., Liu A.K., Hersch S. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 144.Kuperberg G.R., Broome M.R., McGuire P.K. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 145.Salat D.H., Buckner R.L., Snyder A.Z. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 146.Han X., Jovicich J., Salat D. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 147.Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Savalia N.K., Agres P.F., Chan M.Y., Feczko E.J., Kennedy K.M., Wig G.S. Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum Brain Mapp. 2016;38:472–492. doi: 10.1002/hbm.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bas-Hoogendam J.M., van Steenbergen H., Tissier R.L.M., Houwing-Duistermaat J.J., Westenberg P.M., van der Wee N.J.A. Gray matter characteristics as endophenotypes of Social Anxiety Disorder. Database Open Sci Framew. 2018 [dataset] [Google Scholar]
- 150.Core Team R. 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 151.Tissier R., Tsonaka R., Mooijaart S.P., Slagboom E., Houwing-Duistermaat J.J. Secondary phenotype analysis in ascertained family designs: application to the Leiden longevity study. Stat Med. 2017;36:2288–2301. doi: 10.1002/sim.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ganjgahi H., Winkler A.M., Glahn D.C., Blangero J., Kochunov P., Nichols T.E. Fast and powerful heritability inference for family-based neuroimaging studies. Neuroimage. 2015;115:256–268. doi: 10.1016/j.neuroimage.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jacobi F., Wittchen H.-U., Hölting C. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS) Psychol Med. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- 154.Vandeleur C.L., Fassassi S., Castelao E. Prevalence and correlates of DSM-5 major depressive and related disorders in the community. Psychiatry Res. 2017;250:50–58. doi: 10.1016/j.psychres.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 155.Gennatas E.D., Avants B.B., Wolf D.H. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci. 2017;37 doi: 10.1523/JNEUROSCI.3550-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mutlu A.K., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 157.Etkin A. Neurobiology of anxiety: from neural circuits to novel solutions? Depress Anxiety. 2012;29:355–358. doi: 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- 158.Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cui Q., Vanman E.J., Long Z. Social anxiety disorder exhibit impaired networks involved in self and theory of mind processing. Soc Cogn Affect Neurosci. 2017;12:1284–1295. doi: 10.1093/scan/nsx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yuan C., Zhu H., Ren Z. Precuneus-related regional and network functional deficits in social anxiety disorder: a resting-state functional MRI study. Compr Psychiatry. 2018;82:22–29. doi: 10.1016/j.comppsych.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 161.Yang X., Liu J., Meng Y. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.12.011. published online Dec. [DOI] [PubMed] [Google Scholar]
- 162.Frick A. Common and distinct gray matter alterations in social anxiety disorder and major depressive disorder. EBioMedicine. 2017;21:53–54. doi: 10.1016/j.ebiom.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 165.Lavin C., Melis C., Mikulan E., Gelormini C., Huepe D., Ibañez A. The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Front Neurosci. 2013;7:64. doi: 10.3389/fnins.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blair K.S., Geraci M., Korelitz K. The pathology of social phobia is independent of developmental changes in face processing. Am J Psychiatry. 2011;168:1202–1209. doi: 10.1176/appi.ajp.2011.10121740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amir N., Klumpp H., Elias J., Bedwell J.S., Yanasak N., Miller L.S. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 168.Labuschagne I., Phan K.L., Wood A. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol. 2011;15:1–14. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- 169.Ziv M., Goldin P.R., Jazaieri H., Hahn K.S., Gross J.J. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3:5. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Binelli C., Subirà S., Batalla A. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64C:205–217. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 171.Evans K.C., Wright C.I., Wedig M.M., Gold A.L., Pollack M.H., Rauch S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 172.Straube T., Mentzel H.-J., Miltner W.H.R. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 173.Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lerch J.P., van der Kouwe A.J.W., Raznahan A. Studying neuroanatomy using MRI. Nat Neurosci. 2017;20:314–326. doi: 10.1038/nn.4501. [DOI] [PubMed] [Google Scholar]
- 175.McGregor N.W., Dimatelis J.J., Van Zyl P.J. A translational approach to the genetics of anxiety disorders. Behav Brain Res. 2018;341:91–97. doi: 10.1016/j.bbr.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 176.Kim M.J., Shin J., Taylor J.M., Mattek A.M., Chavez S.J., Whalen P.J. Intolerance of Uncertainty Predicts Increased Striatal Volume. Emotion. 2017;17:895–899. doi: 10.1037/emo0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lago T., Davis A., Grillon C., Ernst M. Striatum on the anxiety map: Small detours into adolescence. Brain Res. 2017;1654:177–184. doi: 10.1016/j.brainres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Arsalidou M., Duerden E.G., Taylor M.J. The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2012;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cremers H.R., Veer I.M., Spinhoven P., Rombouts S.A.R.B., Roelofs K. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front Behav Neurosci. 2015;8 doi: 10.3389/fnbeh.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shah S.G., Klumpp H., Angstadt M., Nathan P., Phan K.L. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 181.Heitmann C.Y., Feldker K., Neumeister P. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum Brain Mapp. 2016;37:1559–1572. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van Tol M.-J., van der Wee N.J.A., van den Heuvel O.A. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 183.Bearden C.E., Thompson P.M. Emerging global initiatives in neurogenetics: the Enhancing Neuroimaging Genetics through Meta-analysis (ENIGMA) consortium. Neuron. 2017;94:232–236. doi: 10.1016/j.neuron.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thompson P.M., Stein J.L., Medland S.E. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Groenewold N., Bas-Hoogendam J.M., Amod A.R. F27. subcortical volumes in social anxiety disorder: preliminary results from enigma-anxiety. Biol Psychiatry. 2018;83:S247–S248. [Google Scholar]
- 186.Blackford J.U. Leveraging statistical methods to improve validity and reproducibility of research findings. JAMA Psychiat. 2017;74:119–120. doi: 10.1001/jamapsychiatry.2016.3730. [DOI] [PubMed] [Google Scholar]
- 187.Patel S., Patel R., Park M.T.M., Masellis M., Knight J., Chakravarty M.M. Heritability estimates of cortical anatomy: the influence and reliability of different estimation strategies. Neuroimage. 2018;178:78–91. doi: 10.1016/j.neuroimage.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 188.Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front Neurosci. 2014;8 doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Noble K.G., Houston S.M., Brito N.H. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ashburner J., Friston K.J. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 191.Clarkson M.J., Cardoso M.J., Ridgway G.R. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage. 2011;57:856–865. doi: 10.1016/j.neuroimage.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 192.Bas-Hoogendam J.M., van Steenbergen H., van der Wee N.J.A., Westenberg P.M. Subcortical brain volumes as endophenotypes of social anxiety disorder— preliminary findings from the Leiden Family Study on Social Anxiety Disorder; part of ‘Abstracts of the WASAD Conference 2017, 14–16 September, Würzburg, Germany’. J Neural Transm. 2017;124:1277–1328. [Google Scholar]
- 193.Potts N.L., Davidson J.R., Krishnan K.R., Doraiswamy P.M. Magnetic resonance imaging in social phobia. Psychiatry Res. 1994;52:35–42. doi: 10.1016/0165-1781(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 194.Machado-De-Sousa J.P., Osório F De L., Jackowski A.P. Increased amygdalar and hippocampal volumes in young adults with social anxiety. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Månsson K.N.T., Salami A., Frick A. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2016;6:e727. doi: 10.1038/tp.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Månsson K.N.T., Salami A., Carlbring P., Boraxbekk C.-J., Andersson G., Furmark T. Structural but not functional neuroplasticity one year after effective cognitive behaviour therapy for social anxiety disorder. Behav Brain Res. 2017;318:45–51. doi: 10.1016/j.bbr.2016.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Illustration of interaction (sub)clinical SAD x age
Relationship between indices of social anxiety and gray matter characteristics in selection of LFLSAD sample: participants with (comorbid) psychopathology other than (sub)clinical SAD were excluded (sensitivity analysis 1)
Relationship between indices of social anxiety and gray matter characteristics, corrected for level of depressive symptoms (sensitivity analysis 2)
Detailed statistics of effects of social anxiety on, and heritability estimates of parameters of interest: general imaging phenotypes (tab 1); subcortical volumes (tab 2); cortical thickness (tab 3); cortical surface area (tab 4).
Detailed statistics of effects of social anxiety on parameters of interest in sample without comorbidity (sensitivity analysis 1): subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).
Detailed statistics of effects of social anxiety on parameters of interest, corrected for level of depressive symptoms (sensitivity analysis 2): subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).
Detailed statistics of effects of social anxiety on, and heritability estimates of non-ROIs: subcortical volumes (tab 1); cortical thickness (tab 2); cortical surface area (tab 3).