Abstract
The dendritic protrusions known as spines represent the primary postsynaptic location for excitatory synapses. Dendritic spines are critical for synaptic function, and their formation, modification, and turnover are thought to be important for mechanisms of learning and memory. At many synapses, dendritic spines form during the early postnatal period, and while many spines are likely being formed and removed throughout life, the net number are often gradually “pruned” during adolescence to reach a stable level in the adult. In neurodevelopmental disorders, spine pruning is disrupted, emphasizing the importance of understanding the processes governing spine pruning. Autophagy, a process through which cytosolic components and organelles are degraded, has recently been shown to control spine pruning in the mouse cortex, but, the mechanisms through which autophagy acts remain obscure. Here, we draw on three well-studied prototypical synaptic pruning events to focus on two governing principles of spine pruning: 1) activity-dependent synaptic competition and 2) non-neuronal contributions. We briefly review what is known about autophagy in the central nervous system and its regulation by metabolic kinases. We then propose a model in which autophagy in both neurons and non-neuronal cells contributes to spine pruning, and how other processes that regulate spine pruning could intersect with autophagy. We further outline future research directions to address outstanding questions on the role of autophagy in synaptic pruning.
I. Introduction
Neuronal networks are composed of balanced connections between excitatory, inhibitory and modulatory neurons. These balances are disrupted in a range of neurodevelopmental and neurodegenerative diseases, including autism spectrum disorders (ASD), schizophrenia, drug abuse, Down syndrome and Alzheimer’s disease. Understanding the basis of these “synaptopathies” promises deeper insight into the basis of the diseases and improved therapies (Dölen and Bear, 2009).
Within the brain, excitatory glutamatergic neuronal connections often occur between presynaptic release sites located en passant along axons and a postsynaptic site on a dendritic shaft or spine. Mature dendritic spines are typically 0.5–5 μm long with a narrow neck and wider head, and feature a range of shapes due to variations in the size of the head and neck length (Peters and Kaiserman-Abramof, 1970). The spine shape is correlated with the synaptic strength (Matsuzaki et al., 2001) stability (Trachtenberg et al., 2002) and function, as the spine neck segregates biochemical and electrical events between the spine head, where the postsynaptic density and neurotransmitter receptors are located, and the dendritic shaft (Koch and Zador, 1993; Yuste and Denk, 1995; Yuste, 2013).
Each spine is a dynamic structure, and in vivo, spine shape and the presence of the spine itself can change over short (minutes) and long (days to weeks) time scales (Alvarez and Sabatini, 2007; Trachtenberg et al., 2002; Yang et al., 2009). Immature spines, known as filopodia, lack a head and are predominantly observed during synaptogenesis, and presumably develop, into mature shapes in an activity dependent manner (Vaughn, 1989; Yuste and Bonhoeffer, 2004; Ziv and Smith, 1996). This modulation of morphology has led neuroscientists over the past century to postulate that such synapses are critical to memory storage, and that changes in spine structure and synaptic strength underlie forms of learning (Dickstein et al., 2013). Thus, understanding the processes through which dendritic spines are removed and restructured should provide important insight into the formation and maintenance of memories.
Neuronal connections begin to form prenatally and are continually refined throughout life (Huttenlocher and Dabholkar, 1997; LaMantia and Rakic, 1990; Rakic et al., 1986). From mid-embryonic development through adolescence, the number of synaptic contacts throughout the brain increases dramatically. During puberty and into adulthood, however, there is a net loss of synapses in many brain regions. This process, known as synaptic pruning, is critical for the establishment and function of mature neuronal networks. Synaptic pruning appears to occur through mutliple mechanisms as discussed below, but generally involves the removal of both pre- and postsynaptic elements (Purves and Lichtman, 1980). The removal of the presynaptic component, however, is more challenging to measure. Here, we will refer to synaptic pruning as the process in which both the pre-and postsynaptic elements are removed, and refer to spine pruning when only the postsynaptic structure has been measured experimentally.
Postnatal synaptic pruning was initially identified as “resorption” of neurites in Purkinje and granule cells by Ramon y Cajal (Yuste, 2015) and was reemphasized in studies of synapses and axons of the cortex almost thirty years ago (Huttenlocher, 1990; LaMantia and Rakic, 1990; Rakic et al., 1986). Significant insights have been made into mechanisms of synaptic pruning in the peripheral nervous system (neuromuscular junction; NMJ) (Purves and Lichtman, 1980; Sanes and Lichtman, 1999) and in the central nervous system (retinogeniculate synapses (Huberman, 2007) and cerebellum (Hashimoto and Kano, 2013), and these provide a foundation for the future characterization of pruning in the cerebral cortex. Here we review the literature on synaptic pruning events in these systems and propose research to address the relationship between synaptic autophagy and pruning.
II. Developmental synaptic pruning
Neuromuscular Junction
The study of the development and maturation of the neuromuscular junction (NMJ) provided many of the discoveries of mechanisms that regulate synaptic pruning. At birth, muscles are innervated by multiple motor axon terminals (Figure 1A), while the mature NMJ is composed of a single presynaptic input from a motor neuron that synapses on a postsynaptic specialization on the muscle surface. In rodents, “superfluous” axon terminals are removed over the first two postnatal weeks, illustrating fundamental principles of synaptic pruning including activity-dependent synaptic competition and non-neuronal contributions.
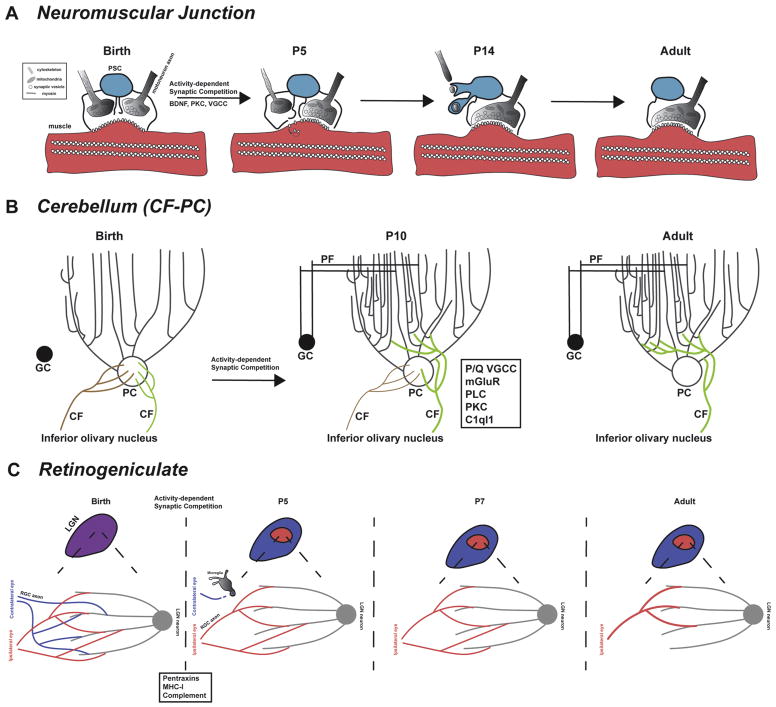
Figure 1. Schematic representations of NMJ, cerebellar, and retinogeniculate synaptic pruning events.
(A) At birth, individual muscles are innervated by multiple motor neuron axons. The NMJ is surrounded by a perisynaptic Schwann cell (PSC). By P5, selective strengthening of a single axon has occurred and pre- and post-synaptic elements are rearranged, including the beginning of axonal retraction and removal of nAChR from the muscle membrane. At P14, the “loser” axon retracts, leaving behind axosomes that are engulfed by PSCs and degraded. The “winner” axon expands its synaptic territory. In adulthood, all muscles are monoinnervated with a PSC delimiting the synaptic area. (B) At birth, cerebellar Purkinje cells (PCs) are innervated by multiple climbing fibers from the inferior olivary nucleus. At this time, CF axons synapse onto the PC soma. Over the first postnatal week, parallel fibers from cerebellar granule cells (GCs) synapse onto the distal dendrites of PCs. In addition, selective strengthening of a single CF input (green) occurs. The synapses of the stronger CF input begins to translocate onto the proximal dendrites of the PC. The weaker CF inputs (brown) are then withdrawn by adulthood. (C) At birth, retinal ganglion cell axons from both eyes innervate the lateral geniculate nucleus of the thalamus without any spatial segregation. During the first week postnatally, the RGC inputs segregate so that the ipsilateral RGC inputs innervate a smaller central area. Complement proteins coat synapses to be pruned from the contralateral eye (not shown). Microglia then phagocytose and degrade these synapses. Later stages of refinement include strengthening and further pruning of inputs from the ipsilateral eye.
Clusters of postsynaptic acetylcholine receptor (AChR) form in response to the arrival of motor neuron axons (Frank and Fischbach, 1979; Ko et al., 1977). Motor neuron axon terminals mature when they reach the muscle by synthesis and positioning of the presynaptic machinery required for neurotransmitter release (Chow and Poo, 1985; Evers et al., 1989; Kidokoro and Yeh, 1982; Xie and Poo, 1986). The postsynaptic stabilization occurs in regions of the muscle directly opposite to axon terminals, while AChR distal from axon terminals are removed (Balice-Gordon and Lichtman, 1993; Lin et al., 2005; Misgeld et al., 2005). Numerous proteins that play a role in this process have been identified, with contributions from neuronal, muscle and glial cells required for the formation of the synapse (See (Sanes and Lichtman, 1999) for review). The synaptic area is limited by perisynaptic Schwann cells (PSC), the glial cell of the NMJ, which at this stage ensure that AChR clusters are selectively present at the site of contact with the axon (Yang et al., 2001).
Pruning of superfluous motor neuron axons during the first two postnatal weeks is dependent on neuronal activity (Thompson et al., 1979), and the axons with relatively “weaker” synapses are removed (Buffelli et al., 2003) (Figure 1A). Differences in presynaptic strength related to altered presynaptic release probability are critical to the specification of the weaker inputs (Colman et al., 1997; Kopp et al., 2000). Alterations in the composition of the presynaptic terminal, including calcium channels (Urbano et al., 2002) and the organization of synaptic vesicles and active zone proteins (Chen et al., 2011; Fox et al., 2007; Umemori and Sanes, 2008) enable these changes in presynaptic strength. Intriguingly, the difference in synaptic strength alone is insufficient to drive synapse elimination, and asynchronous activity arising from disparate synaptic strengths between synaptic inputs is required (Buffelli et al., 2002; Favero et al., 2012; Thompson, 1983).
In addition to neuronal activity, survival signals such as brain-derived and glial-derived neurotrophic factor (BDNF and GDNF) are released from muscle and glia and contribute to the strengthening of ACh release (Henderson et al., 1994; Je et al., 2013, 2012). As the uptake of these factors by the axon is activity-dependent and they act to further enhance synaptic strength, they can initiate a feedforward cascade to strengthen a single axonal input (Snider and Lichtman, 1996). This provides an example of how non-neuronal cells contribute to selecting the “winning” axonal input via activity-dependent uptake of non-neuronally derived factors.
Regulatory signaling cascades contributing to presynaptic and postsynaptic maturation during this period also depend on intracellular calcium (Adams and Goldman, 1998; Dai and Peng, 1993) and protein kinase C (PKC) (Huang et al., 1992; Lanuza et al., 2002) (Figure 1A).
Together, these mechanisms lead to a synaptic competition that specifies the “winning” synaptic input that will remain and the “losers” to be eliminated. During this process, postsynaptic sites undergo morphological changes from a continuous plaque of membrane inserted AChR to a “pretzel-shaped area” with segments that lack postsynaptic AChR (Balice-Gordon and Lichtman, 1993; Marques et al., 2000), and the axon terminals withdraw from areas lacking the receptors (Balice-Gordon and Lichtman, 1993). The withdrawal of one innervating axon leads to the expansion of remaining axon terminals at the same postsynaptic site (Walsh and Lichtman, 2003). This process remains dynamic, as the withdrawing axon terminal can reinnervate the synapse and “win” the competition if the original “winner” axon terminal is selectively ablated (Turney and Lichtman, 2012).
Perisynaptic Schwann cells (PSCs) contribute to axon withdrawal by actively separating axon terminals from the postsynaptic membrane (Smith et al., 2013). Whether Schwann cells are able to selectively separate the losing axon terminals from the muscle based on their weaker synaptic strength or act to separate axon terminals indiscriminately remains controversial (Darabid et al., 2013; Jahromi et al., 1992; Robitaille, 1998; Rochon et al., 2001; Smith et al., 2013; Todd et al., 2010). The withdrawing axon terminal then forms a retraction bulb ensheathed by Schwann cells (Smith et al., 2013), leaving behind “axosomes,” which are filled with material from the presynaptic compartment including synaptic vesicle clusters and mitochondria (Bishop et al., 2004; Walsh and Lichtman, 2003) (Figure 1A). These axonal components are then degraded within the Schwann cell (Song et al., 2008). Finally, these events yield singly innervated, mature NMJs capable of triggering mature muscle activity (Figure 1A).
Cerebellum
Synaptic pruning has also been well characterized in synapses of the cerebellum. The mature cerebellar circuit consists of a Purkinje cell (PC) that receives strong inputs from a single climbing fiber (CF) arising from the inferior olivary nucleus onto its proximal dendrites, and weak inputs from many parallel fibers (PF) that arise from cerebellar granule cells onto its distal dendrites (Figure 1B). The PC is initially innervated by multiple weak CF inputs in the first postnatal week (Chedotal and Sotelo, 1993). During this period, CF inputs synapse onto the PC soma in a “pericellular nest” (Ito, 1984; Sugihara et al., 1999). By the end of this week, a single CF input is strengthened while others have become progressively weaker (Hashimoto and Kano, 2003). The principles of activity-dependent synaptic competition and non-neuronal contributions that regulate synaptic pruning at the NMJ are also illustrated at this synapse (Figure 1B).
The selective synaptic strengthening is thought to arise from enhanced presynaptic release due to increased probability of fusion of multiple synaptic vesicles and an increased number of active release sites, with no potentiation of quantal size (Hashimoto and Kano, 2003). This synaptic selection can be mimicked by a spike-timing dependent plasticity protocol in which the PC and a single CF input are coincidently activated. Interestingly, stimulating the stronger CF input elicits long-term potentiation, while activation of weaker CF inputs produces long-term depression (Bosman et al., 2008; Ohtsuki and Hirano, 2008).
A molecular mechanism for the selective strengthening of strong inputs and weakening of weaker inputs has recently been proposed. C1ql1, a protein expressed at the CF axon terminal, interacts with Bai3, the C1ql1 receptor expressed by PCs. Bidirectionally modulating this interaction disrupted the selective strengthening and selection of a winner CF during postnatal cerebellum development (Kakegawa et al., 2015). The downstream mechanisms underlying the role of C1ql1-Bai3 in CF-PC synapse maturation remain unexplored.
Following the differentiation of CF inputs, the strongest CF input translocates its synapses onto the proximal dendritic tree (Hashimoto and Kano, 2003) (Figure 1B). This event is critical to determining winner and loser CFs, as the CF that translocated generally continues to innervate the PC, while the remaining CF inputs onto the PC soma are pruned (Carrillo et al., 2013) (Figure 1B). An important contribution to the elimination of weak CF inputs, but not to the differentiation in strength between CF inputs, is the cerebellar granule cell parallel fiber (PF) to PC synapse. PF to PC synaptogenesis occurs during the second postnatal week (after CF input differentiation) (Altman, 1972). In mouse mutants with reduced PF to PC synapses or rodents whose cerebellum is irradiated in the first postnatal week to eliminate cerebellar granule cells, CF elimination is incomplete (Crepel et al., 1981; Hashimoto et al., 2001). In mice with reduced PF inputs and impaired CF elimination, CF inputs extend onto the distal PC dendrites and invade the space predominantly occupied by PF inputs in the “normal” cerebellum (Ichikawa et al., 2002). This demonstrates that heterosynaptic interactions, and perhaps competition, are responsible for the elimination of weak CF inputs to PCs.
Although CF input strengthening during the early phase of maturation in the first postnatal week occurs presynaptically (Hashimoto and Kano, 2003), several postsynaptic components in the PC have been implicated in controlling the second phase of CF input elimination during the second and third postnatal week (Hashimoto and Kano, 2013; Kano and Hashimoto, 2009) (Figure 1B). CF to PC synapses in mice deficient in the type 1 metabotropic glutamate receptor (mGluR1) undergo reduced elimination in the second and third postnatal week (Kano et al., 1997). It is hypothesized that PC-expressed mGluR1, which is activated by functional PF to PC synapses, is critical for CF elimination, providing a mechanism for heterosynaptic involvement in the late phase of CF elimination (K Hashimoto et al., 2009). Furthermore, elements of the signaling cascade downstream of mGluR1, such as PKC gamma (Kano et al., 1995), phospholipase cβ4 (Kano et al., 1998), and Gαq (Offermanns et al., 1997), are critical for CF synapse elimination. Postsynaptic PC P/Q type voltage-gated calcium channels (VGCCs) are also critical for CF input elimination (Hashimoto et al., 2011), possibly by providing the intracellular calcium influx required for synaptic plasticity and selective synaptic strengthening during the early phases of CF elimination (Kano and Hashimoto, 2009).
The downstream effectors of these signaling cascades have recently begun to be elucidated. Arc/Arg3.1, a protein locally synthesized at the dendritic spine, has been shown to act downstream of P/Q type VGCCs to mediate CF elimination and weakening CF to PC synaptic strength (Mikuni et al., 2013). While the role of postsynaptic Arc is not clear, these findings may indicate a molecular basis for postnatal CF elimination.
Retinogeniculate pathway
The neural circuits underlying vision also undergo significant postnatal refinement. At this synaptic pruning event, the contribution of microglia has been well investigated and illustrates the principle of non-neuronal contributions to synaptic pruning.
Retinal ganglion cells (RGCs) project to the dorsal lateral geniculate nucleus (dLGN) of the thalamus, where information is relayed to the primary visual cortex. During the first postnatal week, RGC axons from both eyes are intermixed in the dLGN with terminals from both eyes synapsing onto single dLGN neurons (Figure 1C). By the end of the first week, RGC axons segregate to ensure that each dLGN cell is innervated only by axons from one eye. Further refinement occurs over the next weeks as 2–3 RGC inputs per cell are selectively strengthened and the remainder are pruned (Huberman, 2007) (Figure 1C).
Similar to the CF to PC synapse in the cerebellum, RGC axon segregation is activity-dependent. Intriguingly, because eye opening has not occurred, the patterns of activity that induce synapse elimination differ from the CF to PC synapse. Nevertheless, blockade of spontaneous retinal activity in the first postnatal week with tetrodotoxin to block action potentials is sufficient to disrupt RGC axon segregation and synaptic maturation (Hooks and Chen, 2006; Shatz and Stryker, 1988). Experience-dependent activity, modified by dark rearing, does not affect this early stage of maturation but disrupts subsequent synaptic refinement (Hooks and Chen, 2006). Extensive research has focused on the characteristics of RGC spontaneous activity required for synaptic maturation in the early postnatal period, and is reviewed elsewhere (Butts et al., 2007; Penn et al., 1998; Stellwagen and Shatz, 2002; Torborg and Feller, 2005; Torborg et al., 2005). As discussed above for CF to PC synaptic elimination, this activity-dependence manifests through selective strengthening of individual inputs (Datwani et al., 2009; Ziburkus et al., 2009).
Efforts to address the mechanism of synaptic refinement that follows selective synaptic strengthening at the RGC to dLGN synapse have, surprisingly, identified proteins and processes associated with the immune system (Huberman, 2007). Early studies from Carla Shatz’s laboratory identified neuronally expressed Class I major histocompatibility complex (MHC-I) as a mediator of dLGN refinement and eye-specific segregation (Huh et al., 2000) (Figure 1C). Interestingly, MHCI appears to regulate synaptic plasticity, as MHC-I deficient mice showed enhanced long-term potentiation and disrupted LTD (Huh et al., 2000; Lee et al., 2014).
How might neuronal MHC-I molecules control synaptic plasticity? Upregulation of calcium-permeable AMPA receptors in the selective knockout mice biases synaptic plasticity toward long-term potentiation, possibly driving structural plasticity away from synapse elimination (Lee et al., 2014). The signaling pathways downstream of MHC-I that lead to alterations in AMPA receptor trafficking remain unknown. Another study identified the neuronal pentraxins (NPs) as mediators of dLGN maturation (Bjartmar et al., 2006) (Figure 1C). Pentraxins are synaptic homologs of a class of immune proteins that include acute phase proteins (Dodds et al., 1997). NPs were shown to be required for the segregation of eye-specific inputs to the dLGN in the early phase (spontaneous activity-dependent) of dLGN refinement (Bjartmar et al., 2006). The role of NPs in synaptic pruning appears to be independent of alterations in synaptic plasticity or neuronal firing patterns, but the precise mechanism remains unknown.
Recent studies by several groups further emphasize the role of the immune system in dLGN maturation. These studies demonstrate that synapse elimination is dependent on phagocytosis of synapses by microglia and astrocytes, suggesting that non-neuronal cells in the CNS may play an important role in developmental synaptic pruning and maturation. Pioneering work by Beth Stevens and Ben Barres demonstrated that mice lacking the critical complement cascade proteins C1 and C3 had reduced dLGN input segregation (Stevens et al., 2007) (Figure 1C). The complement cascade is best known for its role in the clearance of pathogens and debris in the periphery (Gasque, 2004). An initiating protein, C1q, opsonizes a target leading to a proteolytic cascade (including the protein C3) and either phagocytosis by macrophages or microglia, or cell lysis via the membrane attack complex (Gasque, 2004). Barres and Stevens noted developmentally-regulated expression of C1q from RGCs from P5 to P30 (Stevens et al., 2007). Immunofluorescence analysis demonstrated coating of synapses in the dLGN by C1q and C3 and engulfment of presynaptic components by microglia and astrocytes (Schafer et al., 2012; Stevens et al., 2007). The receptor for C3 (CR3) is only expressed by microglia and astrocytes in the CNS, suggesting that synaptic coating by these proteins signals these cells (Gasque et al., 1998). C1q and C3 coating, and microglial engulfment, were activity dependent and appeared to occur on the relatively smaller synapses present in the dLGN (Schafer et al., 2012).
A role for microglia in developmental synaptic pruning has been expanded to regions of the hippocampus (Paolicelli et al., 2011) and cortex (Chu et al., 2010), and recently to neurodegenerative and neuropsychiatric disease (Hong et al., 2016; Sekar et al., 2016; Stephan et al., 2012; Vasek et al., 2016), expanding on original observations by Peter and Edith McGeer (McGeer et al., 1989).
III. Neuronal Autophagy
We have recently reported that macroautophagy may play fundamental roles in synaptic pruning in the cortex (Tang et al., 2014). Here we review evidence suggesting roles for macroautophagy in synaptic pruning, speculate about the mechanism underlying this phenomenon, propose specific directions to answer major outstanding questions on the role of macroautophagy in synaptic pruning, and propose a “unified model” for the roles of neuronal and nonneuronal mechanisms in synaptic pruning.
Since the original description (and naming) of the lysosome, autophagy, and phagocytosis by Christian De Duve (Ohsumi, 2014; Sabatini and Adesnik, 2013), significant work has been conducted to understand the mechanisms underlying these processes. Macroautophagy (hereafter autophagy) is a multistep catabolic cellular process through which cytosolic proteins and organelles are degraded. Autophagy begins when substrates are sequestered into double membrane bound vesicles, known as autophagosomes. Autophagosomes then undergo retrograde trafficking toward the lysosome. En route, autophagosomes can fuse with endosomes to form amphisomes. Amphisomes or autophagosomes then fuse with the lysosome to form autolysosomes, in which the autophagosome cargo is degraded by lysosomal proteases (Figure 2).
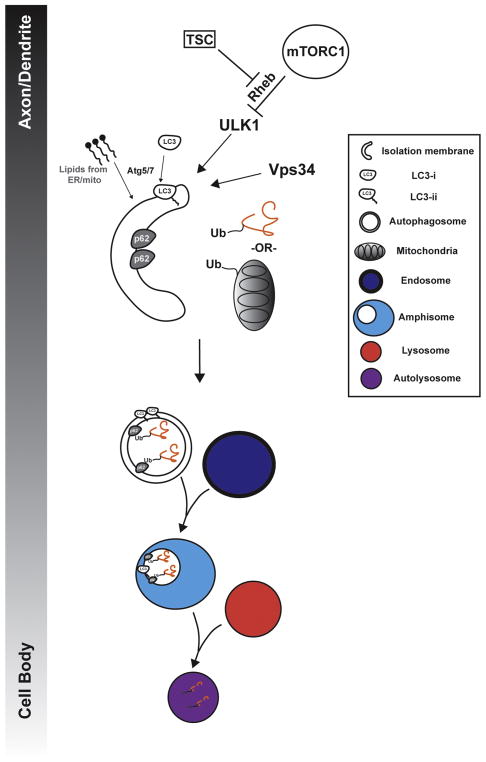
Figure 2. Control of neuronal autophagy by TSC and mTORC1.
mTORC1 and TSC control autophagy via regulation of ULK1, which controls formation of the nascent autophagosome (isolation membrane). The isolation membrane expands following addition of lipids from the endoplasmic reticulum or mitochondria. Autophagosome formation is dependent on many Atg genes including Atg5 and Atg7 and requires lipid conjugation of the Atg8 homolog LC3. Ubiquitinated cytosolic proteins or organelles bind to adapters on the inner membrane of the growing autophagosome, such as p62. In neurons, autophagosome formation typically occurs in axons or dendrites but can occur in the cell body. Subsequently, autophagosomes traffic retrogradely towards the cell body where they fuse with endosomes to form amphisomes. Amphisomes and autophagosomes then fuse with lysosomes, where autophagic cargo is degraded by luminal proteases.
Autophagy is dependent on many genes, most of which were originally described in brewer’s yeast. These genes are important for autophagosome membrane formation, cargo recognition and autophagic flux. Mouse models have been developed to provide for the conditional deletion of two genes encoding rate-limiting components of the autophagy machinery, Atg5 and Atg7 (Hara et al., 2006; Komatsu et al., 2006). Measuring the relative levels of the lipid-conjugated form (LC3-II) of the Atg8 homolog, LC3-I, and the cellular distribution of fluorophore tagged LC3 is often used to indicate the relative state of autophagic flux in neuronal systems (Klionsky et al., 2016). These tools have enabled the analysis of autophagy in the mammalian nervous system (Figure 2).
Given the wide interest neuronal autophagy at present and its control of a wide range of neuronal function and dysfunction, it will seem surprising that for many years, autophagy was widely considered to not occur in neurons at all, despite some very early morphological reports in Huntington’s disease autopsy (Roizin et al., 1979). The presence of autophagosomes in axons was essentially rediscovered by Peter Hollenbeck (Hollenbeck, 1993) and was gradually acknowledged in the field (Larsen and Sulzer, 2002) following research from multiple groups.
Although autophagy has been implicated in several cell biological processes in neurons, we will focus on the role for autophagy in synaptic pruning and neuronal development in this review. For background on other aspects of neuronal autophagy, please see other reviews in this edition.
In neurons, autophagosome formation has primarily been studied in the distal axon and soma (Hollenbeck, 1993; Maday and Holzbaur, 2016, 2014; Maday et al., 2012). Some reports have demonstrated autophagosome formation in dendrites, especially in response to particular stimuli (Hernandez et al., 2012; Shehata et al., 2012). Neurons also form autophagosomes in the cell body that can be exceedingly long lived, apparently through the lifetime of the organism, as seen by the accumulation of the neuronal aging pigments, lipofuscin and neuromelanin within autophagic organelles (Sulzer et al., 2008).
In axons, autophagosomes are trafficked retrogradely to the soma via dynein motors (Fu et al., 2014; Maday et al., 2014, 2012). During retrograde trafficking, autophagosome maturation occurs, as observed by increased acidification. Some argue that fusion with lysosomes occurs in neurites as opposed to solely in the cell body: this interpretation is largely dependent on the definition of the lysosome, which in the original sense, DeDuve and Alex Novikoff defined by the presence of acid hydrolases (Novikoff et al., 1956; Sabatini and Adesnik, 2013). Finally, in the soma, autophagosomes can fuse with conventional lysosomes where their contents are degraded (Figure 2).
In dividing cells, autophagy is a critical response to starvation and nutrient deprivation. Numerous signaling cascades convergently control autophagy by coordinately regulating different aspects of autophagosome formation, maturation and lysosomal fusion (Dunlop and Tee, 2014; Jung et al., 2010). Autophagy initiation is controlled by the activity of the serine/threonine kinase ULK1 (homolog of the yeast Atg1) (Ganley et al., 2009; Hosokawa et al., 2009; Jung et al., 2009; Russell et al., 2013) and the lipid kinase, Vps34 (Backer, 2008). ULK1 activity is negatively regulated by mammalian target of rapamycin (mTOR)-mediated activation of Rheb and AMBRA1 (Jung et al., 2009; Russell et al., 2013). ULK1 activity is also positively regulated by AMPK signaling to promote autophagy (Kim et al., 2011). Together these signaling cascades ensure that autophagy activity is tightly controlled (Figure 2). Nearly all labs that have studied these processes find that nutrient deprivation or serum starvation are relatively weak promoters of autophagy in neurons, and the molecular induction steps are likely to be somewhat different.
IV. Control of neuronal autophagy by mTOR
mTOR participates in two protein complexes, mTORC1 and mTORC2, that are integral to cellular signaling and can be differentiated by their sensitivity to the mTOR inhibitor, rapamycin. Although mTORC2 is critical for nutrient sensing in the periphery (Saxton and Sabatini, 2017) and is involved in plasticity in multiple brain circuits (Bockaert and Marin, 2015; Dadalko et al., 2015), mTORC1 has been most directly linked to the regulation of autophagy (Dunlop and Tee, 2014).
The role for mTOR regulation of neuronal autophagy has remained somewhat controversial, largely due to a variance in findings using rapamycin and its derivatives. In ventral midbrain dopamine neurons, mTOR activity appears to negatively regulate autophagy, as rapamycin induces LC3 puncta formation and conjugation of LC3-I to the lipidated LC3-II (Hernandez et al., 2012). Furthermore, genetic hyperactivation of mTOR following deletion of the mTORC1 inhibitors TSC1 and TSC2 (Tee et al., 2003, 2002; Zhang et al., 2003) in excitatory neurons in the cortex reduces LC3-II levels and increases p62, and decreases the number of LC3 puncta in the soma of primary cultured cortical neurons. This reduction in autophagy is rescued following pharmacological inhibition of mTOR with rapamycin both in vivo with an 8 day treatment regimen, and in culture, in an Atg7-dependent manner (Tang et al., 2014). Rapamycin treatment of wild-type mice in vivo and of wild-type primary cortical neurons was sufficient to elicit a small but significant increase in LC3-II or GFP-LC3 puncta, respectively (Tang et al., 2014) (Figure 2). Autophagy activity in TSC1/2 null cells, however, may be complicated by a compensatory increase in AMPK signaling (Di Nardo et al., 2014), suggesting a more complex relationship between autophagy and this protein complex.
Other reports have not observed a basal inhibition of autophagy by mTOR in the CNS (Fox et al., 2010; Maday and Holzbaur, 2016; Tsvetkov et al., 2010). Inhibition of mTOR with everolimus, a rapamycin-like compound, following a 6–8 week treatment paradigm failed to elicit an increased in LC3-II conjugation in vivo, but did engage other downstream signaling pathways of mTOR such as protein synthesis (Fox et al., 2010). mTOR inhibition with torin-1 failed to elicit autophagy activation in hippocampal cultures (Maday and Holzbaur, 2016). These results are intriguing given that, while the effect of rapamycin induced autophagy activation have been questioned, torin-1 mediated inhibition is reported to exert profound effects on autophagy (Thoreen et al., 2009). Differences in treatment paradigm, including the type, timing and concentration of mTORC1 inhibitor, and age may underlie these divergent conclusions about regulation of autophagy by mTORC1 in the CNS. Furthermore, differences in the regulation of autophagy between peripheral or dividing cells and neurons in the CNS has been clearly demonstrated in vivo. Starvation in the rodent induces autophagy in organs such as the kidney and liver but fails to do so in the brain (Mizushima et al., 2004). It is, however, possible that starvation-induced autophagy may not be mediated by LC3, and that measurements of GFP-LC3 are not representative of starvation-induced autophagy in the brain. Nevertheless, the possible regulation of autophagy upstream kinases, such as mTOR, suggest that autophagic dysfunction may contribute to neurodevelopmental disorders in which these kinases are hyperactivated, such as ASD.
V. Contribution of neuronal mTOR-dependent autophagy to dendritic spine pruning
Alterations in dendritic spine density have been proposed to play a role in the pathophysiology of ASDs. In ASD, human postmortem tissue demonstrates elevated spine densities in the temporal cortex (Hutsler and Zhang, 2010; Tang et al., 2014). Furthermore, in other neurodevelopmental disorders, such as Down, Angelman’s, Rett, schizophrenia, and Fragile X syndrome, changes in spine density or morphology are observed in postmortem studies and in animals models (Phillips and Pozzo-Miller, 2015). To address the underlying mechanism for increased spine density in human ASD cases, Tang et al. measured the spine density in postmortem samples of temporal cortex from cases at different ages and found significantly reduced spine pruning in ASD brains compared to controls. Interestingly, this was inversely correlated with levels of the autophagy marker LC3-II, suggesting that impaired autophagy, downstream of mTOR hyperactivation seen in ASD (Auerbach et al., 2011; Tang et al., 2014), may be responsible for the reduced synaptic pruning.
This hypothesis was addressed in a mouse model of tuberous sclerosis complex (TSC), a developmental disorder characterized by intellectual disability, epilepsy, and the presence of cortical tubers in addition to other peripheral dysfunction (Crino et al., 2006). TSC arises from loss-of-function mutations in the genes TSC1 (tuberin) and TSC2 (hamartin) (European Chromosome 16 Tuberous Sclerosis Consortium, 1993; Kandt et al., 1992; van Slegtenhorst et al., 1997). TSC1 and TSC2 inhibit mTOR signaling (Tee et al., 2002; Zhang et al., 2003) via direct inhibition of the Ras homolog enriched in brain, Rheb (Tee et al., 2003). Multiple studies have attempted to identify changes in dendritic spine density in mice carrying loss of function alleles in TSC1 and 2 with mixed results. Sabatini and colleagues reported deficits in both excitatory and inhibitory synaptic transmission in TSC-null mice but did not measure differences in spine density in vivo (Bateup et al., 2013, 2011). They however reported increased spine length, decreased spine density and disruption in soma size in primary neuronal culture (Tavazoie et al., 2005). Examination of hippocampal granule cells identified no effect of TSC1on spine density (Goorden et al., 2007). Interestingly, an increased spine density was observed in cerebellar Purkinje cells upon loss of TSC1 in 4 week old mice, following the classical period of synaptic reorganization seen postnatally (Tsai et al., 2012). Future work may elucidate the mechanism through which spine density is elevated in these TSC mutants. For example, excess spines may result from multiply innervated Purkinje cells (Kouichi Hashimoto et al., 2009) or spines may be present ectopically, for example on the soma where no spines are present in adulthood (Hashimoto and Kano, 2013). Finally, Meikle et al demonstrate a reduction in spine density on the apical dendrite of cortical neurons in TSC1 knockout mice (Meikle et al., 2008).
In contrast, Tang et al showed an increased spine density in cortical neurons from an excitatory neuronal specific TSC1 conditional knockout and TSC2 heterozygous mice by P30. Interestingly, spine density was unchanged at P20, suggesting that spinogenesis was intact but spine pruning was specifically deficient. Furthermore, this deficit in spine pruning was normalized by rapamycin (Tang et al., 2014). Differences in brain region, genetic background and mouse age may underlie the disparate conclusions on the role for TSC1/2 and mTOR signaling in controlling spine density.
What is the downstream effector for TSC1/2 that controls spine density? As described above, TSC1/2 loss of function leads to mTOR hyperactivation (Tee et al., 2003, 2002; Zhang et al., 2003). Downstream from mTOR, protein synthesis is disrupted in TSC null cells via mTOR-dependent regulation of the S6 kinase and 4E-BP. However, mTORC1 hyperactivity also inhibits autophagy in TSC null neurons (Tang et al., 2014). To address whether the loss of autophagy was the cause of spine pruning deficits in TSC1/2 null mice, Tang et al took two strategies. First, they showed that conditional deletion of Atg7, a required component of the autophagosome biogenesis machinery, in forebrain excitatory neurons was sufficient to phenocopy the spine pruning deficits observed in TSC1 null mice. Furthermore, both conditional knockouts for Atg7 and TSC1 null mice demonstrated social interaction deficits, thus providing face validity to these models for ASD. Tang et al then tested whether autophagy was necessary for the rescue of TSC spine pruning deficits by rapamycin. They generated mice harboring heterozygous TSC2 alleles and conditional deletion of Atg7 in forebrain excitatory neurons. Rapamycin treatment failed to normalize spine densities at P30 in these mice. These results indicate both necessity and sufficiency of neuronal autophagy for mediating spine pruning in cortical pyramidal neurons. Future studies will focus on the role of other genes involved in autophagy in controlling dendritic morphology and function.
Intriguingly, peripheral inflammatory insults, which have been implicated in development of diseases such as autism (Malkova et al., 2012; Osokine and Erlebacher, 2017) and schizophrenia (Depino, 2017; Miller and Goldsmith, 2017), also inhibits autophagic function in the brain (François et al., 2014). Furthermore, neonatal exposure to drugs of abuse can lead to neuropsychiatric phenotypes and have previously been shown to regulate autophagy (Cubells et al., 1994; Larsen and Sulzer, 2002; Larsen et al., 2002; Plessinger, 1998). This suggests that autophagic dysfunction, due to either inflammation or genetic insults, may underlie dysfunction in synaptogenesis in neurodevelopmental disorders.
VI. Suggested mechanisms for neuronal autophagy in autism and synaptic pruning
Mechanism of autophagic dysfunction in autism
Autophagic dysfunction in neurodevelopmental disorders can occur at different steps of the autophagy pathway (Figure 2). In many neurodevelopmental disorders where autophagy has been suggested to be deficient, mTOR is hyperactive. These include disorders with mutations in TSC1, TSC2, PTEN, and NF1. In these syndromes, hyperactive mTOR could lead to disrupted autophagosome biogenesis due to decreased ULK1 activity (Ganley et al., 2009; Jung et al., 2009; Tang et al., 2014). Alternatively, a recent report suggests a deficit in autophagosome-lysosome fusion during the process of mitophagy (Ebrahimi-Fakhari et al., 2016). Future studies will be required to specifically identify the steps in autophagy that are disrupted in these disorders and lead to synaptic dysfunction.
Genetic lesions associated with two other syndromes, Vici and Beta-propeller protein-associated neurodegeneration, provide insight into the steps in autophagy that can be disrupted in neuropsychiatric disease. In Vici syndrome, EPG5, a Rab7 effector which is required for autophagosome-lysosome fusion, is mutated (Wang et al., 2016). In Beta-propeller protein-associated neurodegeneration, the Wdr45 gene is disrupted, yielding inefficient autophagosome membrane elongation and the buildup of early autophagic structures (Saitsu et al., 2013). These syndromes are both associated with neurodevelopmental delay amongst other sequelae, although associated changes in spine density or synaptic function have yet to be described. Thus, autophagy can go awry at several different steps to yield developmental delay or intellectual disability.
Synaptic plasticity
During prototypical synaptic pruning events, synaptic plasticity establishes differential synaptic strengthening to specify the synapse that remain (Piochon et al., 2016). For example, changes in synaptic strength precede synaptic pruning and refinement in the NMJ (Buffelli et al., 2003; Colman et al., 1997; Kopp et al., 2000), the CF to PC synapse (Bosman et al., 2008; Ohtsuki and Hirano, 2008) and the RGC to LGN synapse (Datwani et al., 2009; Ziburkus et al., 2009). Furthermore, induction of LTD is sufficient to remove specific spines (Becker et al., 2008; Zhou et al., 2004).
Autophagy may be required for changes in synaptic strength during synaptic pruning events. Recently, autophagic function has been implicated in controlling synaptic strength and plasticity. Autophagy is important for GABA and glutamate receptor turnover (Rowland et al., 2006; Shehata et al., 2012), a process critical to changing synaptic strength during synaptic plasticity (Anggono and Huganir, 2012). Furthermore, autophagic activity is required for brain-derived neurotrophic factor (BDNF) mediated synaptic plasticity in the hippocampus (Nikoletopoulou et al., 2017). TSC1/2 deficiency, which reduces neuronal autophagy (Ebrahimi-Fakhari et al., 2016; Tang et al., 2014), leads to disruptions in synaptic plasticity in the hippocampus (Auerbach et al., 2011; Bateup et al., 2011; Chévere-Torres et al., 2012). Finally, presynaptic plasticity is controlled by autophagy in dopaminergic axons (Hernandez et al., 2012). Thus, autophagic activity, both pre-and postsynaptically, may contribute to synaptic plasticity, which is in turn required for selective synaptic strengthening.
A role for autophagy in the synaptic plasticity is further suggested because signaling cascades that control synaptic plasticity at these synapses also regulate autophagy. PKC signaling is required for CF to PC synaptic pruning (Kano et al., 1995) and NMJ maturation (Lanuza et al., 2002) and PKC inhibits autophagy (Jiang et al., 2010; Wei et al., 2016). Bai3 (Kakegawa et al., 2015), another protein required for maturation of the CF to PC synapse, is an inhibitor of autophagy (Lipinski et al., 2010). In the absence of Bai3, the selective strengthening and weakening of CF inputs is lost, suggesting a role in the pruning of the CF to PC synapse (Bosman et al., 2008; Ohtsuki and Hirano, 2008).
The semaphorins are a family of secreted and transmembrane proteins critical for axon guidance and other developmental processes (Yazdani and Terman, 2006). In the cerebellum, Semaphorin 3A and its receptor, PlexinA4, is required for strengthening of CF inputs during the early phase of CF to PC synaptic development (Uesaka et al., 2014). The late phase of pruning at this synapse is dependent on Semaphorin 7A/PlexinC1 (Uesaka et al., 2014). This interaction is required downstream of mGluR1 signaling required for CF pruning (see above). Furthermore, Semaphorin 3F and its receptors, Plexin A3/A4 and Nrp2, are required for the selective removal of corticospinal tract axons projecting from the visual cortex during postnatal development (Low et al., 2008). Semaphorin 3F and Nrp2 inhibit mTOR signaling and activate autophagy (Stanton et al., 2013), suggesting that activation of autophagy may be required for selective axon pruning in the visual system. Semaphorin 3A may also signal through Nrp2 (Cariboni et al., 2011), suggesting that Semaphorin/Nrp2 activation of autophagy may be important in the cerebellum. Finally, P/Q type calcium channels are required for CF to PC pruning and may locally increase neuronal calcium levels (Hashimoto et al., 2011). Increased intracellular calcium can activate autophagy (Høyer-Hansen et al., 2007), providing an additional route for dynamic regulation of autophagy during postnatal cerebellar development.
Of note, cerebellar pathology is especially prominent in mice lacking neuronal autophagy (Hara et al., 2006; Komatsu et al., 2006). Autophagy may thus play a critical role in synaptic refinement downstream of these molecular pathways during postnatal development.
The fact that autophagy regulates synaptic activity, via receptor trafficking and synaptic vesicle homeostasis, and that many cellular pathways implicated in controlling synaptic strength during synaptic pruning events suggests that autophagy may play a role in the first principle of developmental synaptic pruning that we describe above: activity-dependent synaptic competition.
Mitochondrial function
Autophagy may also control synaptic pruning via selective degradation of mitochondria. In addition to the role of mitochondria in ongoing synaptic function and plasticity (Cameron et al., 1991; Kang et al., 2008; Levy et al., 2003; Sun et al., 2013; Tang and Zucker, 1997; Weeber et al., 2002), proper mitochondrial function is critical to the formation (Courchet et al., 2013; Kimura and Murakami, 2014; Lee and Peng, 2008) and maintenance of synaptic contacts and strength (Li et al., 2004). In both the axon and the dendrite, mitochondrial localization is precisely regulated and localized to active synaptic contacts (Chada and Hollenbeck, 2004). Furthermore, mitochondrial function and content is coupled to synaptic activity (Bindokas et al., 1998; Hevner and Wong-Riley, 1993; Wong-Riley and Welt, 1980). These data suggest that the presence of functional mitochondria may strengthen a synaptic contact and preclude its pruning. In support of this hypothesis, mitochondrial content is sensitive to neuronal activity during critical periods in synaptic development (Tieman, 1984; Wong-Riley and Welt, 1980), and mitochondrial motility is more dynamic during synaptic maturation than during adulthood (Lewis et al., 2016; Smit-Rigter et al., 2016).
Autophagy plays a critical role in the clearance of damaged or dysfunctional mitochondria through mitophagy (Youle and Narendra, 2011). Mitophagy is a process through which damaged mitochondria are tagged and selectively degraded. In neurons, this can occur to mitochondria in axons (Ashrafi et al., 2014; Berthet et al., 2014) and in the somatodendritic region (Cai et al., 2012; Joselin et al., 2012). As mitophagy occurs in response to the loss of mitochondrial membrane potential, and mitochondrial membrane potential is sensitive to neuronal activity, it is possible that mitophagy locally controls mitochondrial content in response to neuronal activity (see above). Furthermore, local mitochondrial content can contribute to dendritic spine pruning and synaptic maintenance, suggesting the possibility that autophagy and mitophagy contribute to synaptic pruning via targeted degradation of mitochondria. In support of this, in TSC1-deficient neurons (Ebrahimi-Fakhari et al., 2016), which demonstrate deficient synaptic pruning (Tang et al., 2014), and in ASD brain (Tang et al., 2013), mitochondrial abnormalities are present.
Thus, the regulation of mitochondrial quality by autophagy may provide a cellular mechanism for the control of synaptic activity and, in turn, synaptic competition during developmental spine pruning by autophagy.
VII. Non-neuronal autophagy contributes to spine pruning
Does autophagy play a role in the second principle of developmental synaptic pruning, non-neuronal contributions? Recently, work from the Yoon group has addressed the role of non-neuronal autophagy in developmental spine pruning (Kim et al., 2017). Kim et al. argue that the reduction in autophagy inferred from postmortem tissue of ASD cases (Tang et al., 2014) may arise from non-neuronal cells such as microglia or astrocytes. Given the role of microglia and astrocytes in normal synaptic pruning (see above), the authors tested whether microglial autophagy was required for synaptic pruning. The authors used the same conditional allele of Atg7 (Komatsu et al., 2006) that Tang et al used., but Kim et al. used the Lyz2-Cre line to generate Atg7-deficient microglia. This Cre line is expressed throughout the myeloid lineage, giving rise to Atg7-deficient macrophages and other peripheral cells in these mice (Clausen et al., 1999). A caveat to these findings is this peripheral loss of autophagy, as recent evidence has emphasized the connection between peripheral immune cell function, the microbiome, and neurodevelopment (Vuong and Hsiao, 2017), and that this interaction could be governed by host cell autophagy (Chu et al., 2016). Nevertheless, Kim et al. found that in these conditional knockout mice, cortical pyramidal cells in the somatosensory cortex (the same areas that Tang et al examined) show increased spine density. In contrast to Tang et al., Kim et al. observe this difference at P15, prior to the classical window of spine pruning observed in the cortex and at a time in which Tang et al. observed no requirement for neuronal autophagy. It is it possible that non-neuronal versus neuronal autophagy contribute differentially at different stages of synaptic development. Alternatively, the results of Kim et al may reflect increased spinogenesis rather than deficient synaptic pruning. This interpretation is further supported by an increase in dendritic filopodia, the precursors of mature spines (Vaughn, 1989; Yuste and Bonhoeffer, 2004; Ziv and Smith, 1996), in neurons cocultured with microglia deficient in autophagy (Kim et al., 2017).
Why might microglial autophagy be required for proper spine density? Using a culture system, Kim et al. show that Atg7-deficient microglia are less efficient at degrading exogenous, purified synaptosomes. Interestingly, synaptosomal phagocytosis continues to be effective, but the presence of synaptosomal material in the degradative compartment is reduced. These findings suggest a role for microglia autophagy, seemingly downstream from phagocytosis, in regulating spine density. Why is spine density elevated if microglial phagocytosis is intact? It may be that impaired degradation of phagocytosed material leads backs up the phagocytic system, even if phagocytosis per se is intact.
Lysosomal degradation, possibly mediated by autophagy, in the Schwann cell is also critical in the transition from multi- to mono-innervation at the NMJ (Sanes and Lichtman, 1999; Song et al., 2008). By labeling cells with LysoTracker, Song et al. showed selectively and transiently enlarged lysosomal compartments proximal to and within motor axons receding from the NMJ, as well as in Bergmann glia surrounding receding cerebellar climbing fibers (Song et al., 2008). Using a mouse expressing GFP-tagged LC3, they further show that autophagosomal structures form in receding motor neuron axons at the NMJ. Both GFP-LC3 puncta and LysoTracker-positive organelles then form in Schwann cells to digest axonal material. Finally, disruption of autophagic flux and lysosomal storage by mutation of the membrane trafficking protein CLN3 resulted in the persistence of more retreating axons as well as slower rates of recession. In sum, the integrity of autophagic-lysosomal flux in motor axons and their surrounding glial populations appears crucial for the proper timing and degree of synaptic pruning in multiple brain structures.
VIII. Possible mechanisms for microglial autophagy in synaptic pruning
The importance of microglial autophagy to cortical synaptic refinement emphasizes that autophagy may contribute to both principles of synaptic pruning, including the contribution of non-neuronal cells (Figure 2). How may microglial autophagy control spine pruning? Microglial-dependent removal of synapses requires (1) active microglial migration, (2) phagocytosis of “tagged” material, (3) intracellular degradation of the phagocytosed components. The culture system used by Kim et al. suggests that engulfment of synaptic material is intact in microglia lacking autophagy (Kim et al., 2017). Indeed, the levels of engulfed synaptic markers (PSD-95 and synaptophysin) are elevated in microglia deficient for autophagy, as expected. These data suggest that microglial phagocytosis and migration to regions containing synapses that need to be pruned may remain intact without autophagy. Additional research is required to confirm the intact migration in the absence of microglial autophagy, as some signaling molecules involved in the control of microglial chemotaxis have been proposed to interact with autophagy machinery (Coly et al., 2017).
It may be that microglial autophagy’s primary role during synaptic pruning may be to degrade phagocytosed components. In support of this hypothesis, Song et al. demonstrate that the lysosomal compartment, and presumably its degradative capacity, increases during synaptic pruning at the NMJ in Schwann cells (Song et al., 2008). This suggests that autophagy and lysosomal activity can be recruited on demand during synaptic pruning events to degrade phagocytosed material.
How may microglial autophagy be activated during synaptic pruning? Autophagy can be upregulated via signaling downstream of Toll-like receptors/CR3 signaling (Sanjuan et al., 2007), the receptors required for microglial phagocytosis of synaptic material (Stephan et al., 2012). The role of autophagy proteins downstream of TLR/CR3 activation is complex, as both canonical autophagy and non- canonical LC3-associated phagocytosis (LAP) are regulated by these receptors (Sanjuan et al., 2007). LAP is a process in which canonical autophagy proteins such as LC3, Atg7 and Beclin-1 contribute to phagocytosis and promote efficient degradation of phagocytosed material independently of de novo membrane formation and sequestration of cytosolic components. Indeed, the deficit observed by Kim et al. is strikingly similar to that observed when LAP is disrupted. For example, in LAP, LC3, Atg7, and Beclin-1 are required for proper degradation of phagocytosed material: while LAP is required for full degradation, phagocytosis and internalization is not dependent on LAP function (Sanjuan et al., 2007). Thus, in concordance with Kim et al., the requirement for Atg7 in microglia-mediated synaptic pruning may be via LAP, in contrast to conventional autophagy. Further, both CR3-dependent phagocytosis, which is required for synaptic pruning by microglia, and LAP are independent of MyD88 signaling, suggesting a connection between these two processes (Hajishengallis et al., 2009; Sanjuan et al., 2007). Additional research is required to distinguish between LAP and canonical autophagy by addressing the role for specific autophagy-associated proteins, such as Rubicon, that contribute exclusively to either autophagy or LAP in microglia-mediated synaptic pruning (Martinez et al., 2015).
The temporal and functional connection between neuronal and microglial autophagy in spine pruning is an important future focus (Figure 3). In the synaptic pruning principles described above (Figure 1), the contribution of non-neuronal cells occurs after activity-dependent synaptic competition. It is tempting to hypothesize that neuronal autophagy and other mechanisms of spine pruning mark synapses for opsonization by complement, followed by subsequent phagocytosis and digestion of the labelled synaptic material. Indeed, there is a temporally specific transition in the localization of lysosomal upregulation from the axon to the apposed microglia during developmental pruning at the NMJ (Song et al., 2008).
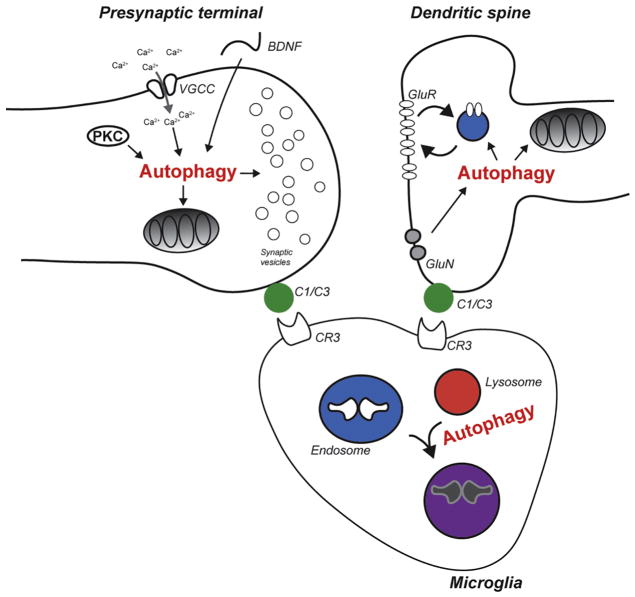
Figure 3. Neuronal and microglial autophagy contribute to synaptic pruning.
Presynaptic autophagy may be activated by BDNF, cytosolic calcium or PKC to modulate mitochondrial homeostasis and synaptic vesicle release. Postsynaptic autophagy is activated by NMDA receptor (GluN) signaling and controls both mitochondrial homeostasis and AMPA receptor (GluR1) trafficking. Weaker synaptic inputs are opsonized by C1/C3 during developmental synaptic pruning events. Opsonized synaptic contacts are phagocytosed and degraded in an autophagy-dependent fashion by microglia.
Alternatively, these processes may act independently. Microglia may select synapses to be pruned based on firing patterns and synaptic strength, as described at the NMJ and retinogeniculate synapse (Schafer et al., 2012; Smith et al., 2013; Tremblay et al., 2010) and require autophagy for the degradation of this material, whereas neuronal autophagy may be required for intrinsic neuronal mechanisms of structural plasticity (Cingolani and Goda, 2008; Piochon et al., 2016). Future research addressing the function of microglia in mice deficient in neuronal autophagy will be useful to define this.
IX. Future directions and conclusions
We have described evidence supporting roles for neuronal and non-neuronal autophagy in synaptic pruning, and their regulation by activity-dependent synaptic competition and the actions of non-neuronal cells. Here, we suggest experimental approaches to define how autophagy contributes to synaptic pruning.
Changes in presynaptic neuronal activity underlie synaptic pruning at the NMJ (Colman et al., 1997; Kopp et al., 2000), CF to PC synapse (Hashimoto and Kano, 2003) and the retinogeniculate synapse (Hooks and Chen, 2006). Could autophagy act presynaptically to mediate synaptic pruning? One possibility is that autophagy directly controls presynaptic strength. This is supported by the observation that loss of autophagy increases presynaptic dopamine release in adult mice (Hernandez et al., 2012). Furthermore, de novo autophagosome formation has been best described in the distal axon and contributes to mitochondrial homeostasis in this neuronal compartment (Ashrafi et al., 2014; Hollenbeck, 1993; Maday and Holzbaur, 2016). The importance of mitochondria for presynaptic plasticity (Sun et al., 2013) supports the possibility that autophagy regulates changes in presynaptic strength. Future experiments should focus on the direct observation of on-demand synthesis of autophagosomes, or changes in autophagosome content, in maturing axons undergoing changes in synaptic strength (Figure 3).
Alternatively, changes in presynaptic strength by other mechanisms could activate autophagy to remodel axonal terminals. Direct modulation of presynaptic autophagy by selectively changing synaptic strength, for example by tetrodotoxin (Shatz and Stryker, 1988), has not been demonstrated but would test this. Clearly, determining whether ontogenetic changes in presynaptic changes occur at prototypical synaptic pruning events in mice lacking autophagy specifically in the presynaptic compartment would address whether autophagy acts upstream or downstream of changes in presynaptic strength that might trigger synaptic pruning.
Autophagy may also act by mediating synaptic competition. The role of autophagy in controlling membrane trafficking has been clearly demonstrated in dividing and migrating cells (Coly et al., 2017; Sharifi et al., 2016). Thus, autophagy may contribute to changes in synaptic morphology such as axon retraction at the NMJ (Sanes and Lichtman, 1999) or translocation of CF inputs from the PC soma to the proximal dendrites (Hashimoto and Kano, 2013) that contribute to the competition between synapses during pruning events. Experiments demonstrating an absence of changes in axonal morphology at these events in the absence of autophagy, or the induction of neuronal autophagy during CF translocation, would provide evidence for this hypothesis.
Finally, microglial autophagy can contribute to the non-neuronal component of synaptic pruning (Kim et al., 2017). Defining the temporal relationship between contributions of neuronal and non-neuronal autophagy may be important for understanding respective roles in synaptic pruning. We suggest the use of a paradigm in which neuronal autophagy alters selective synaptic strengthening or synaptic competition leading to tagging of synapses by complement proteins and phagocytosis by microglia, which would require autophagy for proper degradation of synaptic material (Figure 3). This temporal pattern could be demonstrated by testing whether synaptic strengthening fails to occur while superfluous synapses remain in mice lacking microglial autophagy or complement proteins. An absence of complement receptor coating of synapses in mice lacking neuronal autophagy would support this hypothesis.
In conclusion, the elucidation of a role of autophagy in synaptic pruning is at an early stage, and there may be a multiplicity of roles that differ between systems, but the decades long study on features of synaptic pruning can be harnessed to focus these investigations.
Acknowledgments
OJL is supported by NIMH F30MH114390. Work by the Sulzer lab in this field is supported by the Simons, JPB, and Parkinson’s Foundations and NIDA R01 DA007418.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Adams L, Goldman D. Role for calcium from the sarcoplasmic reticulum in coupling muscle activity to nicotinic acetylcholine receptor gene expression in rat. J Neurobiol. 1998;35:245–257. doi: 10.1002/(SICI)1097-4695(19980605)35:3<245::AID-NEU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Takasaki KT, Saulnier JL, Denefrio CL, Sabatini BL. Loss of Tsc1 in vivo impairs hippocampal mGluR-LTD and increases excitatory synaptic function. J Neurosci. 2011;31:8862–8869. doi: 10.1523/JNEUROSCI.1617-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nägerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Berthet A, Margolis EB, Zhang Jue, Hsieh I, Zhang Jiasheng, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, Nakamura K. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci. 2014;34:14304–14317. doi: 10.1523/JNEUROSCI.0930-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindokas VP, Lee CC, Colmers WF, Miller RJ. Changes in mitochondrial function resulting from synaptic activity in the rat hippocampal slice. J Neurosci. 1998;18:4570–4587. doi: 10.1523/JNEUROSCI.18-12-04570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Marin P. mTOR in Brain Physiology and Pathologies. Physiol Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- Bosman LWJ, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the “winner” climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Busetto G, Cangiano L, Cangiano A. Perinatal switch from synchronous to asynchronous activity of motoneurons: link with synapse elimination. Proc Natl Acad Sci USA. 2002;99:13200–13205. doi: 10.1073/pnas.202471199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Kaliszewski CK, Greer CA. Organization of mitochondria in olfactory bulb granule cell dendritic spines. Synapse. 1991;8:107–118. doi: 10.1002/syn.890080205. [DOI] [PubMed] [Google Scholar]
- Cariboni A, Davidson K, Rakic S, Maggi R, Parnavelas JG, Ruhrberg C. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Hum Mol Genet. 2011;20:336–344. doi: 10.1093/hmg/ddq468. [DOI] [PubMed] [Google Scholar]
- Carrillo J, Nishiyama N, Nishiyama H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J Neurosci. 2013;33:7641–7653. doi: 10.1523/JNEUROSCI.4561-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Chedotal A, Sotelo C. The “creeper stage” in cerebellar climbing fiber synaptogenesis precedes the “pericellular nest”--ultrastructural evidence with parvalbumin immunocytochemistry. Brain Res Dev Brain Res. 1993;76:207–220. doi: 10.1016/0165-3806(93)90209-s. [DOI] [PubMed] [Google Scholar]
- Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31:512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chévere-Torres I, Kaphzan H, Bhattacharya A, Kang A, Maki JM, Gambello MJ, Arbiser JL, Santini E, Klann E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex. Neurobiol Dis. 2012;45:1101–1110. doi: 10.1016/j.nbd.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I, Poo MM. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci. 1985;5:1076–1082. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Khosravi A, Kusumawardhani IP, Kwon AHK, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, Targan SR, Xavier RJ, Ernst PB, Green DR, McGovern DPB, Virgin HW, Mazmanian SK. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science (80-) 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Colman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science (80-) 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- Coly PM, Gandolfo P, Castel H, Morin F. The autophagy machinery: A new player in chemotactic cell migration. Front Neurosci. 2017;11:78. doi: 10.3389/fnins.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Dupont JL. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadalko OI, Siuta M, Poe A, Erreger K, Matthies HJG, Niswender K, Galli A. mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci. 2015;35:8843–8854. doi: 10.1523/JNEUROSCI.0887-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Peng HB. Elevation in presynaptic Ca2+ level accompanying initial nerve-muscle contact in tissue culture. Neuron. 1993;10:827–837. doi: 10.1016/0896-6273(93)90199-2. [DOI] [PubMed] [Google Scholar]
- Darabid H, Arbour D, Robitaille R. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J Neurosci. 2013;33:1297–1313. doi: 10.1523/JNEUROSCI.2935-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino AM. Perinatal inflammation and adult psychopathology: From preclinical models to humans. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Wertz MH, Kwiatkowski E, Tsai PT, Leech JD, Greene-Colozzi E, Goto J, Dilsiz P, Talos DM, Clish CB, Kwiatkowski DJ, Sahin M. Neuronal Tsc1/2 complex controls autophagy through AMPK-dependent regulation of ULK1. Hum Mol Genet. 2014;23:3865–3874. doi: 10.1093/hmg/ddu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32. doi: 10.1016/j.neuroscience.2012.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS. Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J Biol Chem. 1997;272:21488–21494. doi: 10.1074/jbc.272.34.21488. [DOI] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Fragile x syndrome and autism: from disease model to therapeutic targets. J Neurodev Disord. 2009;1:133–140. doi: 10.1007/s11689-009-9015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop EA, Tee AR. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 2014;36:121–129. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Saffari A, Wahlster L, Di Nardo A, Turner D, Lewis TL, Conrad C, Rothberg JM, Lipton JO, Kölker S, Hoffmann GF, Han MJ, Polleux F, Sahin M. Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 2016;17:1053–1070. doi: 10.1016/j.celrep.2016.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-Z. [DOI] [PubMed] [Google Scholar]
- Evers J, Laser M, Sun YA, Xie ZP, Poo MM. Studies of nerve-muscle interactions in Xenopus cell culture: analysis of early synaptic currents. J Neurosci. 1989;9:1523–1539. doi: 10.1523/JNEUROSCI.09-05-01523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M, Busetto G, Cangiano A. Spike timing plays a key role in synapse elimination at the neuromuscular junction. Proc Natl Acad Sci USA. 2012;109:E1667–75. doi: 10.1073/pnas.1201147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Connor T, Chopra V, Dorsey K, Kama JA, Bleckmann D, Betschart C, Hoyer D, Frentzel S, Difiglia M, Paganetti P, Hersch SM. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington’s disease. Mol Neurodegener. 2010;5:26. doi: 10.1186/1750-1326-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fässler R, Hudson BG, John SWM, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- François A, Terro F, Quellard N, Fernandez B, Chassaing D, Janet T, Rioux Bilan A, Paccalin M, Page G. Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol Brain. 2014;7:56. doi: 10.1186/s13041-014-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Fischbach GD. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979;83:143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Nirschl JJ, Holzbaur ELF. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell. 2014;29:577–590. doi: 10.1016/j.devcel.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- Goorden SMI, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S. Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J Immunol. 2009;182:6690–6696. doi: 10.4049/jimmunol.0900524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hashimoto Kouichi, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63:106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, Mishina M, Hashikawa T, Konnerth A, Watanabe M, Kano M. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/S0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cell Mol Life Sci. 2013;70:4667–4680. doi: 10.1007/s00018-013-1405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin HS, Watanabe M, Sakimura K, Kano M. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci USA. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simmons L, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science (80-) 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Torres CA, Setlik W, Cebrián C, Mosharov EV, Tang G, Cheng HC, Kholodilov N, Yarygina O, Burke RE, Gershon M, Sulzer D. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Wong-Riley MT. Mitochondrial and nuclear gene expression for cytochrome oxidase subunits are disproportionately regulated by functional activity in neurons. J Neurosci. 1993;13:1805–1819. doi: 10.1523/JNEUROSCI.13-05-01805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science (80-) 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Huang CF, Tong J, Schmidt J. Protein kinase C couples membrane excitation to acetylcholine receptor gene inactivation in chick skeletal muscle. Neuron. 1992;9:671–678. doi: 10.1016/0896-6273(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science (80-) 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-I. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J Neurosci. 2002;22:8487–8503. doi: 10.1523/JNEUROSCI.22-19-08487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellum and Neural Control. Raven Pr; 1984. [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, Lu B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc Natl Acad Sci USA. 2012;109:15924–15929. doi: 10.1073/pnas.1207767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je HS, Yang F, Ji Y, Potluri S, Fu XQ, Luo ZG, Nagappan G, Chan JP, Hempstead B, Son YJ, Lu B. ProBDNF and mature BDNF as punishment and reward signals for synapse elimination at mouse neuromuscular junctions. J Neurosci. 2013;33:9957–9962. doi: 10.1523/JNEUROSCI.0163-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Cheng D, Liu W, Peng J, Feng J. Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem Biophys Res Commun. 2010;395:471–476. doi: 10.1016/j.bbrc.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW, Shen J, Slack RS, Park DS. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Hum Mol Genet. 2012;21:4888–4903. doi: 10.1093/hmg/dds325. [DOI] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Mitakidis N, Miura E, Abe M, Matsuda K, Takeo YH, Kohda K, Motohashi J, Takahashi A, Nagao S, Muramatsu S, Watanabe M, Sakimura K, Aricescu AR, Yuzaki M. Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron. 2015;85:316–329. doi: 10.1016/j.neuron.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Kandt RS, Haines JL, Smith M, Northrup H, Gardner RJ, Short MP, Dumars K, Roach ES, Steingold S, Wall S. Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease. Nat Genet. 1992;2:37–41. doi: 10.1038/ng0992-37. [DOI] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Watanabe M, Kurihara H, Offermanns S, Jiang H, Wu Y, Jun K, Shin HS, Inoue Y, Simon MI, Wu D. Phospholipase cbeta4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci USA. 1998;95:15724–15729. doi: 10.1073/pnas.95.26.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y, Yeh E. Initial synaptic transmission at the growth cone in Xenopus nerve-muscle cultures. Proc Natl Acad Sci USA. 1982;79:6727–6731. doi: 10.1073/pnas.79.21.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, Yoon SY. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry. 2017;22:1576–1584. doi: 10.1038/mp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Murakami F. Evidence that dendritic mitochondria negatively regulate dendritic branching in pyramidal neurons in the neocortex. J Neurosci. 2014;34:6938–6951. doi: 10.1523/JNEUROSCI.5095-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko PK, Anderson MJ, Cohen MW. Denervated skeletal muscle fibers develop discrete patches of high acetylcholine receptor density. Science (80-) 1977;196:540–542. doi: 10.1126/science.850796. [DOI] [PubMed] [Google Scholar]
- Koch C, Zador A. The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. J Neurosci. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kopp DM, Perkel DJ, Balice-Gordon RJ. Disparity in neurotransmitter release probability among competing inputs during neuromuscular synapse elimination. J Neurosci. 2000;20:8771–8779. doi: 10.1523/JNEUROSCI.20-23-08771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza MA, Garcia N, Santafé M, González CM, Alonso I, Nelson PG, Tomàs J. Pre- and postsynaptic maturation of the neuromuscular junction during neonatal synapse elimination depends on protein kinase C. J Neurosci Res. 2002;67:607–617. doi: 10.1002/jnr.10122. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell. 2008;19:150–158. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Brott BK, Kirkby LA, Adelson JD, Cheng S, Feller MB, Datwani A, Shatz CJ. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Turi GF, Kwon SK, Losonczy A, Polleux F. Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol. 2016;26:2602–2608. doi: 10.1016/j.cub.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto KI, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, Liu X, Eisenberg J, Liu J, Blenis J, Xavier RJ, Yuan J. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev Cell. 2010;18:1041–1052. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci USA. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Holzbaur ELF. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30:71–85. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Holzbaur ELF. Compartment-Specific Regulation of Autophagy in Primary Neurons. J Neurosci. 2016;36:5933–5945. doi: 10.1523/JNEUROSCI.4401-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur ELF. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur ELF. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, Lichtman JW. From plaque to pretzel: fold formation and acetylcholine receptor loss at the developing neuromuscular junction. J Neurosci. 2000;20:3663–3675. doi: 10.1523/JNEUROSCI.20-10-03663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, Kanneganti TD, Virgin HW, Green DR. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni T, Uesaka N, Okuno H, Hirai H, Deisseroth K, Bito H, Kano M. Arc/Arg3.1 is a postsynaptic mediator of activity-dependent synapse elimination in the developing cerebellum. Neuron. 2013;78:1024–1035. doi: 10.1016/j.neuron.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Goldsmith DR. Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology. 2017;42:299–317. doi: 10.1038/npp.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V, Sidiropoulou K, Kallergi E, Dalezios Y, Tavernarakis N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 2017;26:230–242. e5. doi: 10.1016/j.cmet.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Beaufay H, De Duve C. Electron microscopy of lysosomerich fractions from rat liver. J Biophys Biochem Cytol. 1956;2:179–184. [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson RF, Inoue Y, Kano M, Simon MI. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc Natl Acad Sci USA. 1997;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki G, Hirano T. Bidirectional plasticity at developing climbing fiber-Purkinje neuron synapses. Eur J Neurosci. 2008;28:2393–2400. doi: 10.1111/j.1460-9568.2008.06539.x. [DOI] [PubMed] [Google Scholar]
- Osokine I, Erlebacher A. Inflammation and autism: from maternal gut to fetal brain. Trends Mol Med. 2017;23:1070–1071. doi: 10.1016/j.molmed.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science (80-) 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science (80-) 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Phillips M, Pozzo-Miller L. Dendritic spine dysgenesis in autism related disorders. Neurosci Lett. 2015;601:30–40. doi: 10.1016/j.neulet.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Kano M, Hansel C. LTD-like molecular pathways in developmental synaptic pruning. Nat Neurosci. 2016;19:1299–1310. doi: 10.1038/nn.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstet Gynecol Clin North Am. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science (80-) 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science (80-) 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- Rochon D, Rousse I, Robitaille R. Synapse-glia interactions at the mammalian neuromuscular junction. J Neurosci. 2001;21:3819–3829. doi: 10.1523/JNEUROSCI.21-11-03819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizin L, Stellar S, Liu LC. Neuronal nuclear-cytoplasmic changes in Huntington’s chorea: electron microscope investigations. Adv Neurol. 1979;23:93–122. [Google Scholar]
- Rowland AM, Richmond JE, Olsen JG, Hall DH, Bamber BA. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci. 2006;26:1711–1720. doi: 10.1523/JNEUROSCI.2279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Adesnik M. Christian de Duve: Explorer of the cell who discovered new organelles by using a centrifuge. Proc Natl Acad Sci USA. 2013;110:13234–13235. doi: 10.1073/pnas.1312084110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, Kasai-Yoshida E, Sawaura N, Nishida H, Hoshino A, Ryujin F, Yoshioka S, Nishiyama K, Kondo Y, Tsurusaki Y, Nakashima M, Miyake N, Arakawa H, Kato M, Mizushima N, Matsumoto N. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet. 2013;45:445–9. 449e1. doi: 10.1038/ng.2562. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA Schizophrenia Working Group of the Psychiatric Genomics Consortium. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi MN, Mowers EE, Drake LE, Collier C, Chen H, Zamora M, Mui S, Macleod KF. Autophagy Promotes Focal Adhesion Disassembly and Cell Motility of Metastatic Tumor Cells through the Direct Interaction of Paxillin with LC3. Cell Rep. 2016;15:1660–1672. doi: 10.1016/j.celrep.2016.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science (80-) 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci. 2012;32:10413–10422. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-Rigter L, Rajendran R, Silva CAP, Spierenburg L, Groeneweg F, Ruimschotel EM, van Versendaal D, van der Togt C, Eysel UT, Heimel JA, Lohmann C, Levelt CN. Mitochondrial dynamics in visual cortex are limited in vivo and not affected by axonal structural plasticity. Curr Biol. 2016;26:2609–2616. doi: 10.1016/j.cub.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Smith IW, Mikesh M, Lee Yil, Thompson WJ. Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J Neurosci. 2013;33:17724–17736. doi: 10.1523/JNEUROSCI.3339-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, Lichtman JW. Are neurotrophins synaptotrophins? Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- Song JW, Misgeld T, Kang H, Knecht S, Lu J, Cao Y, Cotman SL, Bishop DL, Lichtman JW. Lysosomal activity associated with developmental axon pruning. J Neurosci. 2008;28:8993–9001. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton MJ, Dutta S, Zhang H, Polavaram NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH, Datta K. Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Res. 2013;73:160–171. doi: 10.1158/0008-5472.CAN-11-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SWM, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu H, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–148. doi: 10.1002/(SICI)1096-9861(19991115)414:2<131::AID-CNE1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem. 2008;106:24–36. doi: 10.1111/j.1471-4159.2008.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gutierrez Rios P, Kuo SH, Akman HO, Rosoklija G, Tanji K, Dwork A, Schon EA, Dimauro S, Goldman J, Sulzer D. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–361. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/S0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983;302:614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- Thompson W, Kuffler DP, Jansen JK. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4:271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman SB. Effects of monocular deprivation on geniculocortical synapses in the cat. J Comp Neurol. 1984;222:166–176. doi: 10.1002/cne.902220203. [DOI] [PubMed] [Google Scholar]
- Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci. 2010;30:11870–11882. doi: 10.1523/JNEUROSCI.3165-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Hansen KA, Feller MB. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat Neurosci. 2005;8:72–78. doi: 10.1038/nn1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tremblay M-È, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov AS, Miller J, Arrasate M, Wong JS, Pleiss MA, Finkbeiner S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc Natl Acad Sci USA. 2010;107:16982–16987. doi: 10.1073/pnas.1004498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turney SG, Lichtman JW. Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: evidence for synaptic competition and its mechanism. PLoS Biol. 2012;10:e1001352. doi: 10.1371/journal.pbio.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, Aiba A, Watanabe M, Kano M. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science (80-) 2014;344:1020–1023. doi: 10.1126/science.1252514. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sanes JR. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem. 2008;283:34053–34061. doi: 10.1074/jbc.M805729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Rosato-Siri MD, Uchitel OD. Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions (Review) Mol Membr Biol. 2002;19:293–300. doi: 10.1080/0968768021000035087. [DOI] [PubMed] [Google Scholar]
- Van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (80-) 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, Funk K, DeMasters BK, Jiang X, Bowen JR, Mennerick S, Robinson JK, Garbow JR, Tyler KL, Suthar MS, Schmidt RE, Stevens B, Klein RS. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry. 2017;81:411–423. doi: 10.1016/j.biopsych.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/S0896-6273(02)01142-X. [DOI] [PubMed] [Google Scholar]
- Wang Zheng, Miao G, Xue X, Guo X, Yuan C, Wang Zhaoyu, Zhang G, Chen Y, Feng D, Hu J, Zhang H. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol Cell. 2016;63:781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, Sweatt JD, Craigen WJ. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J Biol Chem. 2002;277:18891–18897. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- Wei H, Li Y, Han S, Liu S, Zhang N, Zhao L, Li S, Li J. cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl Stroke Res. 2016;7:497–511. doi: 10.1007/s12975-016-0484-4. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZP, Poo MM. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci USA. 1986;83:7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Cao G, Koirala S, Reddy LV, Ko CP. Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. J Neurosci. 2001;21:9572–9584. doi: 10.1523/JNEUROSCI.21-24-09572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Electrical compartmentalization in dendritic spines. Annu Rev Neurosci. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- Yuste R. The discovery of dendritic spines by Cajal. Front Neuroanat. 2015;9:18. doi: 10.3389/fnana.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Ziburkus J, Dilger EK, Lo FS, Guido W. LTD and LTP at the developing retinogeniculate synapse. J Neurophysiol. 2009;102:3082–3090. doi: 10.1152/jn.90618.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/S0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]