Abstract
Background
Americans have a shorter life expectancy compared to almost all other high-income countries. We aim to estimate the impact of lifestyle factors on premature mortality and life expectancy in the US population.
Methods
Based on the Nurses’ Health Study (1980–2014, n=78,865) and the Health Professionals Follow-up Study (1986–2014, n=44,354), we defined five low-risk lifestyle factors as fulfilling either: never smoking, body mass index (BMI) 18.5–24.9 kg/m2, 30+ minutes/day moderate to vigorous physical activity, moderate alcohol intake, and a high diet quality score (upper 40%) and estimated hazard ratios (HRs) for the association of total lifestyle score (0–5 scale) with mortality. We used data from the NHANES (2013–2014) to estimate the distribution of the lifestyle score, and the US CDC WONDER database to derive the age-specific death rates of Americans. We applied life table method to estimate life expectancy by levels of the lifestyle score.
Results
During up to 34 years of follow-up, we documented 42,167 deaths. The multivariable-adjusted HRs for mortality in adults with five compared with zero low-risk factors were 0.26 (95% confidence interval [CI]: 0.22–0.31) for all-cause mortality, 0.35 (95% CI: 0.27–0.45) for cancer mortality and 0.18 (95% CI: 0.12–0.26) for CVD mortality. The population-attributable-risk of non-adherence to five low-risk factors was 60.7% (95% CI: 53.6%–66.7%) for all-cause mortality, 51.7% (95% CI: 37.1%–62.9%) for cancer mortality and 71.7% (58.1%–81.0%) for CVD mortality. We estimated that the life expectancy at age 50 was 29.0 years (95% CI: 28.3–29.8) for females and 25.5 years (95% CI: 24.7–26.2) for males who adopted zero low-risk lifestyle factors. In contrast, for those who adopted all five low-risk factors, we projected a life expectancy at age 50 of 43.1 years (95% CI: 41.3–44.9) for females and 37.6 years (95% CI: 35.8–39.4) for males. The projected life expectancy at age 50 was on average 14.0 (95% CI: 11.8–16.2) years longer among female Americans with five low-risk factors as compared to those with zero low-risk factors; for males, the difference was 12.2 (95% CI: 10.1–14.2) years.
Conclusion
Adopting a healthy lifestyle could substantially reduce premature mortality and prolong life expectancy in US adults.
Keywords: healthy lifestyle, life expectancy, premature death
INTRODUCTION
The United States (US) is one of the wealthiest nations worldwide, but Americans have a shorter life expectancy compared to almost all other high-income countries,1,2 ranking 53rd in the world for life expectancy at birth in 2015.3 In 2014, with a total health expenditure per capita of $9,402,4 the US was ranked first in the world for health expenditure as a percent of GDP (17.1%).4 However, the US health care system has primarily focused on drug discoveries and disease treatment, rather than prevention. Chronic diseases, such as cardiovascular disease (CVD) and cancer, are the most common and costly of all health problems, but are largely preventable.5 It has been widely acknowledged that unhealthy lifestyles are major risk factors for various chronic diseases and premature death.6
More than two decades ago, McGinnis et al. suggested that the nation’s major health policies should move to emphasize reducing unhealthy lifestyles.7,8 A meta-analysis9 of 15 studies including 531,804 participants from 17 countries with a mean follow-up of 13.24 years, suggested that approximately 60 percent of premature deaths could be attributed to unhealthy lifestyle factors, including smoking, excessive alcohol consumption, physical inactivity, poor diet, and obesity. A healthy lifestyle was associated with an estimated increase of 7.4–17.9 years in life expectancy in Japan,10 the UK,11 Canada,12 Denmark,13 Norway13 and Germany.13,14 However, a comprehensive analysis of the impact of adopting low-risk lifestyle factors on life expectancy in the US population is lacking. Therefore, we aimed to evaluate the potential impact of individual and combined lifestyle factors on premature death and life expectancy in the US population.
METHODS
The data, analytic methods, and study materials will be made available to other researchers from the corresponding author upon reasonable request for purposes of reproducing the results or replicating the procedure.
Overall Design
We first quantified the association between lifestyle-related low-risk factors and mortality based on cohort data from the Nurses’ Health Study (NHS)15,16 and the Health Professionals Follow-up Study (HPFS).17 Then, we used data from the NHANES (2013–2014) to estimate the distribution of the lifestyle-related factors among the US population.18 Furthermore, we derived the death rates of Americans from the CDC WONDER database.19 Finally, we combined the results from those three sources to estimate the extended life expectancy associated with different categories of each individual lifestyle factor and a combination of low-risk lifestyle factors.
Study Population
The NHS began in 1976, when 121,700 female nurses aged 30 to 55 years responded to a questionnaire gathering medical, lifestyle, and other health-related information. In 1980, 92,468 nurses also responded to a validated food frequency questionnaire (FFQ)15,16. The HPFS17 was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40–75 years completed a mailed questionnaire about their medical history and lifestyle, including a FFQ. We excluded participants with implausible energy intakes (female: <500 or >3500 kcal/day; male: <800 or >4200 kcal/day), a Body Mass Index (BMI) <18.5 kg/m2 at baseline, or with a missing value for BMI, physical activity, alcohol or smoking. After these exclusions, 78,865 female and 44,354 male participants remained in the analysis at baseline. The NHS and HPFS were approved by the institutional review board of Brigham and Women’s Hospital in Boston; completion of the self-administered questionnaire was considered to imply informed consent.
We used the NHANES (2013–2014)18 to estimate the population distribution of lifestyle related factors among American adults. The analytic population consisted of 2,128 adults aged 50 to 80 years with complete information on diet, BMI, physical activity, alcohol use and smoking status. We also excluded participants with BMIs of less than 18.5 kg/m2. The NHANES18 included a nationally representative sample of the US population. It was approved by the National Center for Health Statistics research ethics review board. Signed consents were obtained from all participants.
Data Collection
Diet in the NHS and HPFS was assessed every 4 years using a validated FFQ asking the frequency, on average, a participant had consumed a particular amount of a specific type of food during the previous year.15,16 Physical activity levels were investigated using a validated questionnaire and updated every 2 years.20 Body weight and smoking habits were self-reported and updated every two years. Alcohol consumption was also collected by the FFQ. Biennial questionnaires were used to collect information on potential confounders, such as age, ethnicity, multi-vitamin use, regular aspirin use, postmenopausal hormone use (NHS only), and the presence or absence of a family history of diabetes, cancer, or myocardial infarction.
Dietary data in the NHANES18 were collected by an interviewer-administered, computer-assisted, 24-hour dietary recall, which was an in-depth interview conducted by a trained interviewer who solicited detailed information about everything that the participant ate and drank in the prior twenty-four hours. Body weight and height were measured in a mobile examination center using standardized techniques and equipment. Smoking status was self-reported and included questions about numbers of cigarettes, pipes, or cigars smoked per day, and whether the participant had smoked at least 100 cigarettes in his or her lifetime. Participants also reported duration of moderate and vigorous physical activity during leisure time and at work. Usual alcohol intakes were recorded by two 24-hour dietary recalls.18
Low-risk Lifestyle Score
We included five lifestyle-related factors—diet, smoking, physical activity, alcohol consumption, and BMI. Because this study was focused on modifiable lifestyle factors, we did not include clinical risk factors such as hypertension, hypercholesterolemia, or medication use in the score.
Diet quality in the NHS, HPFS and NHANES was assessed using the Alternate Healthy Eating Index (AHEI) score (eMethod) that is strongly associated with the onset of cardiometabolic disease in the general population.21–23 We defined a healthy diet as a diet score in the top 40% of each cohort distribution. For smoking, we defined low-risk as never smoking. For physical activity, we classified low-risk as more than 30 minutes a day of moderate or vigorous activities (including brisk walking) that require the expenditure of at least 3 metabolic equivalents (METs) or more per hour. We defined low-risk alcohol consumption as moderate alcohol consumption, e.g. 5–15 g/day for females and 5–30 g/day for males. BMI was calculated as self-reported weight (kg)/height (m)2. Low-risk body weight was defined as BMI in the range of 18.5–24.9 kg/m2.
For each low-risk factor, the participant received a score of 1 if he or she met the criterion for low-risk. If the participant did not meet the criterion, he or she was classified as high-risk for that factor and received a score of 0. The sum of these five scores provided a total number of low-risk factors of 0, 1, 2, 3, 4 or 5 with higher scores indicating a healthier lifestyle.
Ascertainment of Deaths
In the NHS and HPFS, deaths were identified from state vital statistics records, the National Death Index, reports by the families, and the postal system.24 The follow-up for death in both cohorts was at least 98% complete. A physician reviewed death certificates or medical records to classify the cause of death according to ICD-8 in the NHS (ICD-9 in the HPFS).
We also derived the population all-cause, cardiovascular (I00-I99) and cancer mortality (C00-D48) rates for 2014 by gender and single-year ages ranging from 50 to 84 years from the CDC WONDER database of the US population.19 Because the database only provides mortality rates up to age 84 years, we estimated the all-cause and cause-specific mortality rates in single years of age from 85 to 105 years by extrapolation based on a Poisson regression model with both linear and quadratic terms for the midpoints of single-year age groups minus age 50.5 years (Supplemental Method, Supplemental Figure 1).
Statistical Analysis
Participants contributed person time from the return of the baseline questionnaire (NHS, 1980; HPFS, 1986) until the date of death, or the end of the follow-up period (30 June 2014 for NHS and 30 January 2014 for HPFS), whichever came first. We used Cox proportional hazard models to calculate the adjusted hazard ratios (HRs) of all-cause, cancer and cardiovascular mortality with their 95% confidence intervals (CIs) across categories of each individual factor and joint classification of number of low-risk factors (0, 1, 2, 3, 4 or 5).
Because lifestyle factors may affect mortality risk over an extended period of time, to best represent long-term effects, we calculated cumulative average levels of lifestyle factors using the latest two repeated measurements for our primary analysis of diet, physical activity and alcohol consumption. For example, in the NHS, mortality cases that occurred between 1980 and 1982 were examined in relation to physical activity based on data collected on the 1980 questionnaire; the average of 1980 and 1982 physical activity measurements was used to assess risk of mortality in the 1982–1984 follow-up period, the average of 1982 and 1984 physical activity measurements was used to assess risk of mortality in the 1984–1986 follow-up period, and so forth. For dietary AHEI score and alcohol, the average was calculated based on four-year repeated measurements. Smoking status was estimated based on both smoking history and most recent status updated every other year and classified into five categories: never, past, current smoking of 1–14, 15–24 and ≥25 cigarettes/day. To minimize the reverse causality bias resulting from weight loss due to preexisting illness, we applied the lifelong maximum BMI25. For example, we applied the maximum value of BMI at age 18 and BMI in 1980 to predict mortality between 1980 and 1982, and the maximum value of BMI at age 18, BMI in 1980 and BMI in 1982 to predict mortality between 1982 and 1984, and so forth. The same analytic strategy was applied to the HPFS. If data on low-risk factors were missing at a given time point, the last observation was carried forward. The following covariates were included in the multivariable model: age, ethnicity, current multivitamin use, current aspirin use, menopausal status and hormone use (females only), and family history of diabetes, myocardial infarction, or cancer. We applied competing risk regression model for cause-specific mortality by including lifestyle factors as exposure and other risk factors as unconstrained covariates, allowing the effects of the covariates vary across cause-specific mortality.26
We calculated the hypothetical population-attributable-risk (PAR), an estimation of the percentage of premature mortality in the study population that theoretically would not have occurred if all people had been in the low-risk category, assuming that the observed associations represent causal effects. For these analyses, we used a single binary categorical variable (with all 5 low-risk factors) and compared participants in the low-risk category with the rest of the population (without all 5 low-risk factors or with any high-risk factor) to calculate the HR. We combined these HRs with the prevalence of the low-risk category among American adults based on NHANES data to estimate the PAR.27
To calculate the life expectancy of participants following different levels of healthy lifestyles, we used life tables. We built the life table starting at age 50 years and ending at 105 years with the following three estimates to calculate the cumulative survival from 50 years onward: (1) Sex- and age- specific HRs of mortality associated with numbers of low-risk lifestyles, derived from the NHS and HPFS; (2) sex- and age-specific population mortality rate of all causes, cardiovascular mortality (I00-I99) and cancer mortality (C00-D48) from the US CDC WONDER database;19 (3) age- and sex-specific population prevalence of the number of low-risk lifestyles, derived from the NHANES.18 We fitted multivariable-adjusted Cox regression models for each gender separately to calculate the age specific hazard ratios for mortality by the number of low-risk factors as compared with zero low-risk factors. The model specification included linear and quadratic terms for the age variable (every 5-years, up to 85 years), and the interactions between the number of low-risk factors with linear and quadratic terms of age variable. The age specific hazard ratios for mortality were obtained as linear combinations of the relevant estimated coefficients, with age fixed at values corresponding to midpoints of 5-year age-group from age 50 onwards to age 85. The HR of age above 85 was assumed to be the same as that in the 85 years age group. Then we applied the age- and sex-specific HRs to estimate the life expectancy at different ages by the number of low-risk lifestyle factors (eMethod).
In the sensitivity analysis, we applied the sex-specific HRs (adjusted for age only) for all-cause and cause-specific mortality to test the robustness of our findings. To address the potential aging effect on the association between lifestyle and mortality, we conducted a sensitivity analysis limited to NHS and HPFS participants prior to age 75 years. We conducted three stratified analyses, one stratified by smoking status, another stratified by BMI status, to estimate the joint effect of other four lifestyle factors; the third was stratified by baseline disease status (with or without elevated cholesterol, hypertension or diabetes). To address the concern about the potential adverse effects of moderate alcohol intake, we created a healthy lifestyle score based on the other four low-risk factors without alcohol.
Since the binary variables could not account for the gradient in mortality risk with more extreme levels of these lifestyle factors, we conducted a third sensitivity analysis, in which we calculated an expanded low-risk score based on the associations between each lifestyle factor and mortality in the cohorts. We assigned scores of 1 (least healthy) to 5 (most healthy) to the categories of the lifestyle factors and summed the points across all 5 factors (score range, 5–25 points). For this analysis, the healthiest group was defined as never smoking, BMI between 18.5 and 22.9, moderate alcohol intake (5–14.9 g/day), moderate or vigorous activity duration of 6 hours/week or longer, and the highest quintile of the AHEI diet score.
We used SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) to analyze the data. Statistical significance was set at a two-tailed P value <0.05. We used Monte Carlo simulation (parametric bootstrapping) with 10,000 runs to calculate the confidence intervals of the life expectancy estimation with @RISK 7.5 (Palisade Corporation, Ithaca, NY, USA).
RESULTS
At baseline, participants with a higher number of low-risk lifestyle factors were slightly younger, more likely to use aspirin, and less likely to use multivitamin supplements (Table 1). During a median of 33.9 years follow-up of females and 27.2 years follow-up of males, 42,167 deaths were recorded (13,953 deaths from cancer and 10,689 deaths from CVD).
Table 1.
Participant characteristics* at baseline according the number of low-risk lifestyle factors
| The number of low-risk lifestyle factors† | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Nurses’ Health Study (1980) | ||||||
| N (%) | 5216(7.1) | 19200(26.3) | 26790(36.7) | 19563(26.8) | 7179(9.8) | 917(1.3) |
| Age, years | 47.2(6.9) | 46.7(7.1) | 46.1(7.2) | 45.8(7.3) | 45.7(7.3) | 45.7(7.3) |
| BMI, kg/m2 | 29.8(4.5) | 26.6(5.0) | 24.5(4.1) | 23.1(3.0) | 22.3(1.9) | 22.1(1.6) |
| Alternate Healthy Eating Index | 26.7(3.4) | 28.5(5.0) | 30.6(6.0) | 33.3(6.2) | 35.9(5.5) | 37.5(4.3) |
| Physical activity, hours/week | 1.7(1.2) | 2.4(2.1) | 3.6(2.8) | 5.1(2.9) | 6.5(2.1) | 7.1(1.2) |
| Alcohol consumption, gram/day | 5.6(12.6) | 6.2(12.4) | 6.3(10.8) | 6.5(9.1) | 7.1(6.8) | 9.5(2.8) |
| Past smoking, % | 48.5 | 33.1 | 27.7 | 22.9 | 15.7 | 0.0 |
| Current smoking, % | 51.5 | 41.9 | 28.8 | 18.2 | 9.8 | 0.0 |
| White, % | 97.9 | 97.7 | 97.6 | 97.4 | 97.4 | 97.8 |
| Multivitamin use, % | 73.2 | 69.8 | 66.3 | 62.0 | 60.3 | 57.8 |
| Regular aspirin use, % | 49.4 | 51.9 | 53.2 | 53.5 | 55.6 | 52.5 |
| Family history of diabetes, % | 34.3 | 30.8 | 28.3 | 26.2 | 25.0 | 25.1 |
| Family history of cancer, % | 13.0 | 13.3 | 14.1 | 14.1 | 14.7 | 14.1 |
| Family history of Myocardial Infarction, % | 27.3 | 25.6 | 24.6 | 24.1 | 24.0 | 23.5 |
| Health Professionals’ Follow-up Study (1986) | ||||||
| N (%) | 4388(11.4) | 12133(31.6) | 14151(36.9) | 9337(24.4) | 3680(9.6) | 665(1.7) |
| Age, years | 55.0(9.6) | 54.1(9.6) | 53.6(9.8) | 53.7(9.8) | 53.2(9.9) | 53.0(9.4) |
| BMI, kg/m2 | 28.2(3.2) | 27.1(3.4) | 25.8(3.3) | 24.7(2.8) | 23.8(2.0) | 23.2(1.2) |
| Alternate Healthy Eating Index | 39.5(6.7) | 42.9(9.5) | 47.2(10.7) | 51.6(10.4) | 55.8(8.9) | 58.6(6.8) |
| Physical activity, hours/week | 0.7(0.9) | 1.4(2.5) | 2.5(3.6) | 4.3(5.4) | 6.2(5.4) | 7.9(5.5) |
| Alcohol consumption, gram/day | 16.3(23.7) | 11.6(17.7) | 10.3(13.7) | 10.5(11.2) | 10.7(8.7) | 12.6(5.7) |
| Past smoking, % | 76.6 | 54.2 | 41.9 | 30.2 | 18.1 | 0.0 |
| Current smoking, % | 23.4 | 14.9 | 7.8 | 3.3 | 1.5 | 0.0 |
| White, % | 94.5 | 94.2 | 93.8 | 94.0 | 94.5 | 97.0 |
| Multivitamin use, % | 43.0 | 41.3 | 38.8 | 35.9 | 31.6 | 33.1 |
| Regular aspirin use, % | 68.3 | 68.3 | 70.4 | 70.0 | 72.3 | 73.3 |
| Family history of diabetes, % | 22.1 | 22.9 | 20.9 | 19.9 | 19.9 | 21.8 |
| Family history of cancer, % | 32.5 | 33.1 | 34.4 | 35.1 | 35.2 | 37.1 |
| Family history of Myocardial Infarction, % | 34.4 | 33.7 | 33.3 | 34.0 | 32.6 | 33.6 |
Values are means (SD) or percentages and are standardized to age distribution of the study population except age itself;
Low-risk lifestyle factors included: cigarette smoking (never smoking), physically active (≥3.5 hours/week moderate to vigorous intensity activity), high diet quality (upper 40% of alternative healthy eating index (AHEI), moderate alcohol intake of 5–15 g/day (female) or 5–30 g/day (male), and normal weight (body mass index 18.5–24.9 kg/m2).
Each individual component of a healthy lifestyle showed a significant association with risk of total mortality, cancer mortality and CVD mortality (Table 2). A combination of five low-risk lifestyle factors was associated with a HR (95% CI) of 0.26 (0.22–0.31) for all-cause mortality, 0.35 (0.27–0.465) for cancer mortality and 0.18 (0.12–0.26) for CVD mortality as compared with participants with zero low-risk factors. The PAR (95% CI) of non-adherence to 5 low-risk lifestyle factors was 60.7% (53.6%–66.7%) for all-cause mortality, 51.7% (37.1%–62.9%) for cancer mortality, and 71.7% (58.1%–81.0%) for cardiovascular mortality. We observed a similar association between the low-risk lifestyle factors and mortality prior to 75 years (Supplemental Table 1). The low-risk lifestyle factors were associated with lower risk of cause-specific mortality in females and males similarly (Supplemental Figure 2).
Table 2.
Hazard ratios (95% CIs) of total and cause-specific mortality according to individual lifestyle risk factors*
| Person | Deaths from any cause | Cancer deaths | Cardiovascular deaths | ||||
|---|---|---|---|---|---|---|---|
| Years | Cases | RR (95% CI) | Cases | RR (95% CI) | Cases | RR (95% CI) | |
| Body mass index (kg/m2) | |||||||
| 18.5–22.9 | 624140 | 5337 | 1.06 (1.02–1.09) | 1868 | 0.96 (0.91–1.02) | 1077 | 1.02 (0.94–1.10) |
| 23–24.9 | 677848 | 7289 | 1.0(ref.) | 2588 | 1.0(ref.) | 1716 | 1.0(ref.) |
| 25–29.9 | 1381081 | 17903 | 1.05 (1.02–1.08) | 5935 | 1.01 (0.96–1.06) | 4738 | 1.16 (1.10–1.23) |
| 30–34.9 | 518621 | 7427 | 1.25 (1.21–1.29) | 2371 | 1.12 (1.05–1.18) | 2006 | 1.66 (1.56–1.78) |
| ≥35 | 250013 | 4211 | 1.67 (1.61–1.74) | 1191 | 1.24 (1.16–1.33) | 1152 | 2.58 (2.39–2.79) |
| Cigarette smoking | |||||||
| Never | 1508401 | 13694 | 1.0(ref.) | 4324 | 1.0(ref.) | 3390 | 1.0(ref.) |
| Past | 1505488 | 23155 | 1.41 (1.38–1.44) | 7526 | 1.50 (1.44–1.56) | 6045 | 1.38 (1.32–1.44) |
| Current 1–14/day | 174422 | 2458 | 2.02 (1.93–2.10) | 873 | 2.00 (1.86–2.15) | 596 | 2.08 (1.91–2.27) |
| Current 15–24/day | 163678 | 1756 | 2.33 (2.21–2.45) | 729 | 2.28 (2.11–2.48) | 428 | 2.62 (2.37–2.91) |
| Current ≥25/day | 99716 | 1104 | 2.87 (2.70–3.06) | 501 | 2.97 (2.70–3.27) | 230 | 2.78 (2.43–3.19) |
| Alcohol consumption (g/day) | |||||||
| 0 | 1037840 | 16611 | 1.27 (1.24–1.30) | 4671 | 1.03 (0.98–1.08) | 4263 | 1.49 (1.41–1.57) |
| 1–4.9 | 1087210 | 10454 | 1.03 (1.00–1.06) | 3841 | 0.98 (0.93–1.03) | 2632 | 1.13 (1.07–1.20) |
| 5–14.9 | 773186 | 8041 | 1.0(ref.) | 2953 | 1.0(ref.) | 2007 | 1.0(ref.) |
| 15–29.9 | 345034 | 4009 | 0.99 (0.96–1.03) | 1417 | 0.99 (0.93–1.06) | 1017 | 0.97 (0.90–1.05) |
| ≥30 | 208434 | 3052 | 1.25 (1.19–1.30) | 1071 | 1.21 (1.13–1.30) | 770 | 1.17 (1.08–1.27) |
| Physical activity (hours/week) | |||||||
| 0 | 1089120 | 24254 | 1.0(ref.) | 6997 | 1.0(ref.) | 6177 | 1.0(ref.) |
| 0.1–0.9 | 921192 | 8239 | 0.65 (0.63–0.66) | 3044 | 0.71 (0.68–0.75) | 2159 | 0.69 (0.66–0.73) |
| 1.0–3.4 | 515731 | 3751 | 0.56 (0.54–0.58) | 1491 | 0.66 (0.62–0.70) | 930 | 0.54 (0.50–0.57) |
| 3.5–5.9 | 369688 | 2524 | 0.50 (0.48–0.52) | 1023 | 0.60 (0.56–0.64) | 590 | 0.44 (0.40–0.48) |
| ≥6 | 555972 | 3399 | 0.44 (0.43–0.46) | 1398 | 0.55 (0.52–0.58) | 833 | 0.39 (0.37–0.43) |
| Alternative healthy eating index | |||||||
| Fifth 1 | 736051 | 11125 | 1.0(ref.) | 3438 | 1.0(ref.) | 2588 | 1.0(ref.) |
| Fifth 2 | 701947 | 9228 | 0.86 (0.83–0.88) | 2983 | 0.89 (0.85–0.93) | 2306 | 0.89 (0.84–0.94) |
| Fifth 3 | 689795 | 8082 | 0.77 (0.75–0.79) | 2677 | 0.81 (0.77–0.85) | 2073 | 0.81 (0.76–0.86) |
| Fifth 4 | 672973 | 7250 | 0.70 (0.68–0.72) | 2511 | 0.76 (0.72–0.80) | 1954 | 0.75 (0.71–0.80) |
| Fifth 5 | 650937 | 6482 | 0.63 (0.61–0.65) | 2344 | 0.70 (0.67–0.74) | 1768 | 0.67 (0.63–0.71) |
| Number of 5 low-risk factors† | |||||||
| Zero | 458169 | 9286 | 1.0(ref.) | 2785 | 1.0(ref.) | 2430 | 1.0(ref.) |
| One | 1101853 | 16329 | 0.79 (0.77–0.81) | 5227 | 0.83 (0.79–0.87) | 4143 | 0.75 (0.71–0.79) |
| Two | 1053250 | 10908 | 0.61 (0.59–0.62) | 3821 | 0.68 (0.65–0.71) | 2719 | 0.54 (0.51–0.57) |
| Three | 596784 | 4408 | 0.47 (0.45–0.49) | 1607 | 0.53 (0.50–0.57) | 1101 | 0.40 (0.38–0.43) |
| Four | 208683 | 1113 | 0.35 (0.33–0.37) | 458 | 0.44 (0.40–0.49) | 270 | 0.28 (0.25–0.32) |
| Five | 32964 | 123 | 0.26 (0.22–0.31) | 55 | 0.35 (0.27–0.45) | 26 | 0.18 (0.12–0.26) |
| For not having five low-risk factors vs. all others (95% CI) | HRs | 0.39 (0.33–0.46) | 0.48 (0.37–0.63) | 0.28 (0.19–0.42) | |||
| PAR‡ (%) | 60.7 (53.6–66.7) | 51.7 (37.1–62.9) | 71.7 (58.1–81.0) | ||||
HR: Hazard ratio; PAR: Population-Attributable-Risk
Multivariable adjusted hazard ratio adjusted for age, sex, ethnicity, current multivitamin use, current aspirin use, family history of diabetes mellitus, myocardial infarction, or cancer, and, menopausal status and hormone use (females only).
Low-risk lifestyle factors included: cigarette smoking (never smoking), physically active (≥3.5 hours/week moderate to vigorous intensity activity), high diet quality (upper 40% of alternative healthy eating index (AHEI), moderate alcohol intake of 5–15 g/day (female) or 5–30 g/day (male), and normal weight (body mass index 18.5–24.9 kg/m2).
Estimation of PAR was based on the prevalence of not having five low-risk factors among American adults from NHANES data.
We observed a modest difference in HRs across age groups (Figure 1A). Using these age- and sex-specific HRs, we estimated that the life expectancy at age 50 was 29.0 years (95% CI: 28.3–29.8) for females and 25.5 years (95% CI: 24.7–26.2) for males who adopted zero low-risk lifestyle factors. In contrast, for those who adopted all five low-risk factors, we projected a life expectancy at age 50 of 43.1 years (95% CI: 41.3–44.9) for females and 37.6 years (95% CI: 35.8–39.4) for males (Figure 1B). Equivalently, females with five low-risk lifestyle factors could gain 14.0 (95% CI: 11.8–16.8) years of life expectancy on average, and males could gain 12.2 (95% CI: 10.1–14.2) years of life expectancy compared to those with zero low-risk lifestyle factors (Figure 1C). The preceding inferences were similar in sensitivity analyses using sex- specific HRs adjusted for age only (Supplemental Figure3A and 3B). Among females, on average, about 30.8% of the gained life expectancy at age 50 from adopting five versus zero low-risk lifestyle factors was attributable to reduced CVD death, and the remainder to lower cancer (21.2%) or other causes (48.0%) of mortality, respectively. For males, the corresponding percentage was 34.1%, 22.8% and 43.1%, respectively (Supplemental Figure 3C). We observed a consistent dose-response relationship between the increasing number of low-risk factors and gained life expectancy among both smokers and non-smokers (Supplemental Figure 4), among both normal weight and overweight adults (Supplemental Figure 5) and among individuals with and without chronic conditions at baseline (Supplemental Figure 6).
Figure 1. Life expectancy estimated based on overall mortality rate of Americans (CDC report), the prevalence of lifestyle factors using NHANES data 2013–2014 and age- and sex-specific hazard ratios (A: hazard ratio; B: life expectancy at age 50; C: life expectancy by age)*, †.
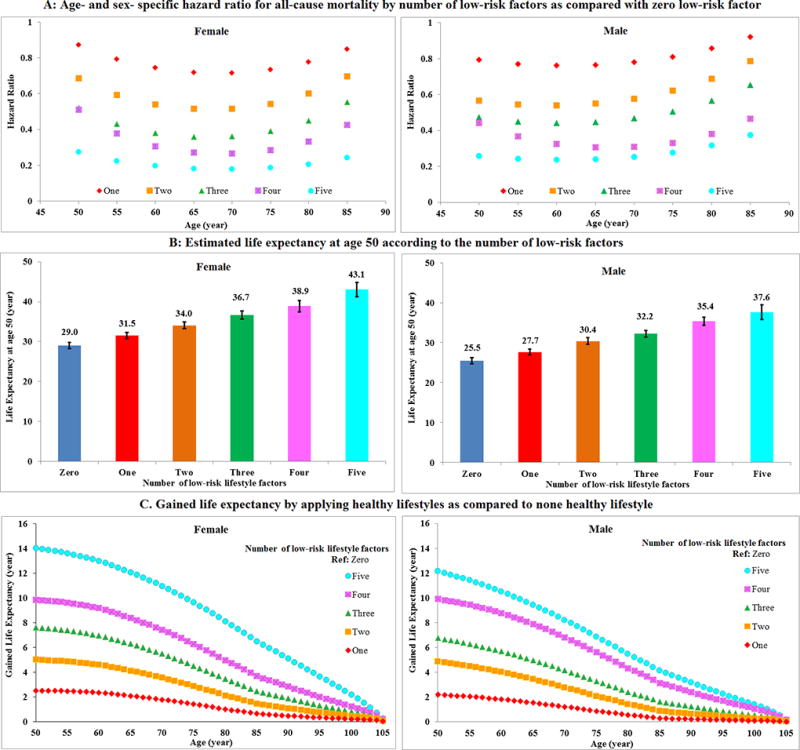
*Low-risk lifestyle factors included: cigarette smoking (never smoking), physically active (≥3.5 hours/week moderate to vigorous intensity activity), high diet quality (upper 40% of alternative healthy eating index (AHEI), moderate alcohol intake of 5–15 g/day (female) or 5–30 g/day (male), and normal weight (body mass index <25 kg/m2).
†The estimates of cumulative survival from 50 years of age onward among the 5 lifestyle risk factor groups were calculated by applying: (1) all-cause and cause-specific mortality rates were obtained from the US CDC WONDER database; (2) distribution of different numbers of low-risk lifestyles was based on the US NHANES 2013–2014; (3) multivariate-adjusted hazard ratios (sex-and age-specific) for all-cause mortality associated with the 5 low-risk lifestyles as compared to those without any low-risk lifestyle factors, adjusted for ethnicity, current multivitamin use, current aspirin use, family history of diabetes mellitus, myocardial infarction, or cancer, and menopausal status and hormone use (females only), were based on data from the NHS and HPFS.
In a sensitivity analysis using a low-risk score without moderate alcohol intake, the projected life expectancy at age 50 was on average 11.4 (95% CI: 9.5–13.3) years longer among female Americans with four low-risk factors as compared to those with zero low-risk factors; for males, the difference was 10.0 (95% CI: 9.2–10.9) years (Supplemental Figure 7).
We also estimated the gained life expectancy related to each of the lifestyle factors. As expected, increased exercise, not smoking or a reduced amount of smoking if a smoker, a healthy dietary pattern, moderate alcohol intake, and optimal body weight were all associated with longer life expectancy (Figure 2). The estimate based on the expanded low-risk score indicated a maximum of 20.5 years difference in life expectancy at age 50 in females (19.6 years among males) who adhered to the highest expanded lifestyle score compared to the lowest expanded score (Supplemental Figure 8).
Figure 2. Projected gained or lost life expectancy according to individual low-risk lifestyle factors (A: physical activity; B: smoking; C: diet; D: alcohol; E: body mass index)*.
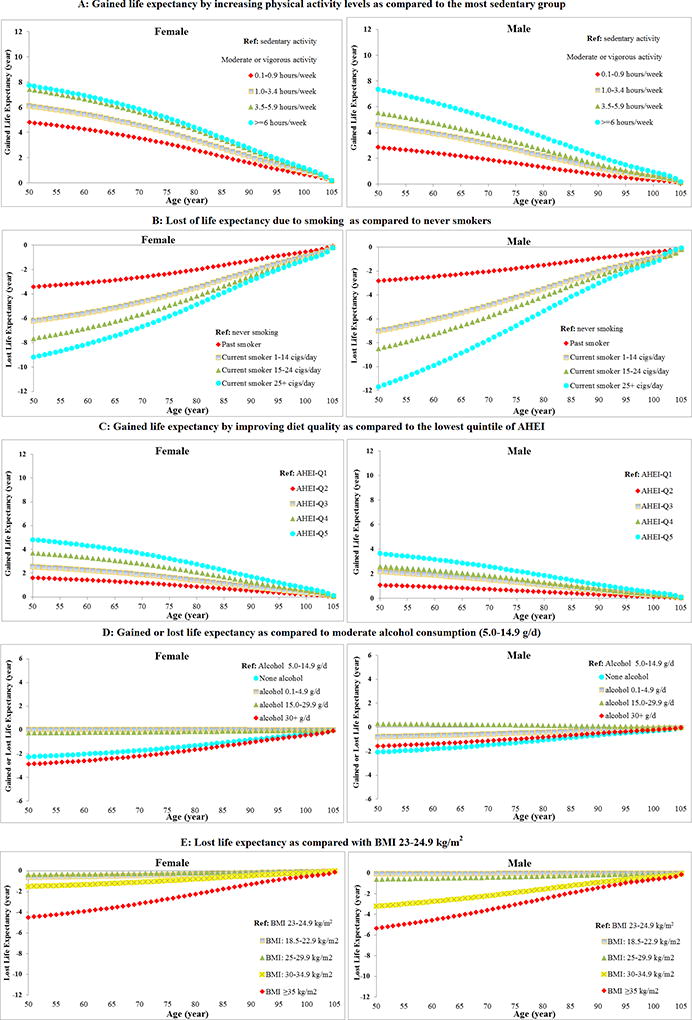
*The estimates of cumulative survival from 50 years of age onward among different levels of each lifestyle factor were calculated by applying: (1) all cause and cause-specific mortality rate were obtained from the US CDC WONDER database; (2) distributions of different groups of each lifestyle factor were based on the US NHANES 2013–2014; (3) multivariate-adjusted hazard ratios (gender-specific) for all-cause and cause-specific mortality associated with each lifestyle factor adjusted for ethnicity, current multivitamin use, current aspirin use, family history of diabetes mellitus, myocardial infarction, or cancer, menopausal status and hormone use (females only), were based on data from the NHS and HPFS.
DISCUSSION
We estimated that adherence to five low-risk lifestyle-related factors could prolong life expectancy at age 50 by 14.0 and 12.2 years for female and male US adults, compared to individuals who adopted zero low-risk lifestyle factor. These estimates suggest that Americans could narrow the life-expectancy gap between the US and other industrialized countries by adopting a healthier lifestyle. In 2014, the life expectancy for American adults at age 50 was 33.3 years for females and 29.8 years for males.28 We estimated that the life expectancies were 29.0 years for females and 25.5 years for males if they had zero low-risk factors, but could be extended to 43.1 years for females and 37.6 years for males if they adopted all five low-risk factors. However, in US adults, adherence to a low-risk lifestyle pattern has decreased during the last three decades, from 15% in 1988–1992 to 8% in 2001–2006,29 primarily driven by the increasing prevalence of obesity.
The life expectancy of Americans increased from 62.9 years in 1940 to 76.8 in 2000 and 78.8 in 2014.28 This increase could be due to a number of factors, such as improvements in living standards, improved medical treatment, substantial reduction in smoking30 and a modest improvement in diet quality.23 However, some unhealthy lifestyle factors may have counterbalanced the gain in life expectancy, particularly the increasing obesity epidemic30,31 and decreasing physical activity levels.32 In our study, three fourths of premature CVD deaths and half of premature cancer deaths in the U. S. could be attributed to lack of adherence to a low-risk lifestyle. There is still much potential for improvement in health and life expectancy, which depends not only on an individual’s efforts, but also the food, physical, and policy environments.33,34 A recent study found that low-income residents in relatively wealthy areas, such as New York and San Francisco, had significantly longer life expectancies than those in poorer regions, such as Gary, Indiana, and Detroit, Michigan.35 This phenomenon suggests that the living environment contributes to life expectancy beyond socio-economic status. For instance, the residents in affluent cities have more access to public health services and less exposure to smoking due to the more restricted policies regarding smoking in public.35 Studies36 have linked healthy eating and excise habits with built, social, and socioeconomic environment assets (access to parks, social ties, affluence), and unhealthy behaviors with built environment inhibitors (access to fast food outlets), suggesting that supporting environments for health lifestyle should be one part of the promotion of longevity for U.S. population. Prevention should be a top priority for national health policy and preventive care should be an indispensable part of the health care system.
Our estimation of gained life expectancy by adopting a low-risk lifestyle was broadly consistent with previous studies. A healthy lifestyle was associated with an estimated greater life expectancy of 8.3 (females) and 10.3 (males) years in Japan,10 17.9 years in Canada,12 13.9 years (females) and 17.0 years (males) in Germany,14 and 14 years difference in chronological age in the UK.11 Data from three European cohorts from Denmark, Germany and Norway13 suggested that males and females aged 50 years who had a favorable lifestyle would live 7.4 –15.7 years longer than those with an unfavorable lifestyle. These estimates were somewhat different, because of different definitions of a low-risk lifestyle and study population characteristics.10,12–14
We observed that adherence to a healthy diet pattern, moderate alcohol consumption, nonsmoking status, maintaining a normal weight and regular physical activity was each associated with a low risk of premature mortality. Smoking is a strong independent risk factor of cancer, diabetes, cardiovascular diseases and mortality potentially through inducing oxidative stress and chronic inflammation; and smoking cessation has been associated with a reduction of these excess risks.37–39 A healthy dietary pattern and its major food components have been associated with lower risk of morbidities and mortality of diabetes, cardiovascular disease, cancer and neurodegenerative disease;40 and its potential health benefits have been replicated in clinical trials.41 Physical activity and weight control significantly reduced the risk of diabetes, cardiovascular risk factors and breast cancer.42–44 Although no long-term trial of alcohol consumption on chronic disease risk has been conducted, cardiovascular benefits of moderate alcohol consumption have been consistently observed in large cohort studies.45 Results of our sensitivity analysis further indicated that combinations of the healthy lifestyle factors were particularly powerful; the larger the number of low-risk lifestyle factors, the longer was the potential prolonged life expectancy, regardless of the combined factors.46
A major strength of this study is the long follow-up of two large cohorts with detailed and repeated measurements of diet and lifestyle and low rates of loss to follow-up. Another important strength is the combination of the cohort estimates with a nationally representative study, the NHANES, which improved the generalizability of our findings. Although the hazard ratios between lifestyle factors and mortality were estimated based only on our cohort data, they were similar to those published in other populations9–14. As our cohorts included mostly Caucasian health professionals, we could not specifically examine the overall impact of lifestyle adherence among different ethnic subgroups; further studies are warranted to examine the impact of lifestyle factors in other ethnic and racial groups.
The current study has several limitations. First, diet and lifestyle factors were self-reported and thus measurement errors are inevitable. However, the use of repeated measures of these variables could reduce measurement errors and also represent long-term diet and lifestyle. Second, we counted the number of lifestyle factors based on the dichotomized value of each lifestyle factor, although the lifestyle factors were differentially associated with mortality. However, our analysis based on an expanded score considered different levels of each risk factor, and yielded similar results. Third, we did not fully consider the baseline comorbid conditions and background medical therapies. Although our stratification analysis by baseline chronic conditions of diabetes, hypertension and elevated cholesterol provided some support for the hypothesis that adopting a healthy lifestyle is important for both healthy individuals and those with existing chronic conditions, further studies among individuals with diagnosed cancer and cardiovascular diseases are warranted.
In conclusion, we estimate that adherence to a low-risk lifestyle could prolong life expectancy at age 50 by 14.0 and 12.2 years in female and male US adults compared to individuals without any of the low-risk lifestyle factors. Our findings suggest that the gap in life expectancy between the US and other developed countries could be narrowed by improving lifestyle factors.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
A comprehensive analysis of the impact of adopting low-risk lifestyle factors on life expectancy in the US population is lacking.
Adherence to five low-risk lifestyle-related factors (never smoking, a healthy weight, regular physical activity, a healthy diet, and moderate alcohol consumption) could prolong life expectancy at age 50 by 14.0 and 12.2 years for female and male US adults, compared to individuals who adopted zero low-risk lifestyle factor.
What is the clinical implication?
Americans could narrow the life-expectancy gap between the US and other industrialized countries by adopting a healthier lifestyle.
Prevention should be a top priority for national health policy and preventive care should be an indispensable part of the US health care system.
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Study that contributed data for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Sources of Funding
The cohorts were supported by grants of UM1 CA186107, R01 HL034594, R01 HL60712, R01 HL088521, P01 CA87969, UM1 CA167552, and R01 HL35464 from the National Institutes of Health. SK and EDA acknowledge grant support from the British Heart Foundation (SP/09/002) and UK Medical Research Council (G0800270).
Footnotes
Conflict of Interest
The authors have no competing interests.
References
- 1.Woolf SH, Aron L, editors. US Health in International Perspective: Shorter Lives, Poorer Health. Washington (DC): 2013. [PubMed] [Google Scholar]
- 2.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization W. Global Health Observatory Data Repository: Life expectancy - Data by country. Geneva, Switzerland: 2015. [Accessed February 14, 2018]. at http://apps.who.int/gho/data/view.main.WOMENLEXv. [Google Scholar]
- 4.World Bank. [Accessed July 12, 2017];World Development Indicators: Health systems. at http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS?year_high_desc=true.
- 5.Behrens G, Fischer B, Kohler S, Park Y, Hollenbeck AR, Leitzmann MF. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of U.S. women and men. Eur J Epidemiol. 2013;28:361–372. doi: 10.1007/s10654-013-9796-9. [DOI] [PubMed] [Google Scholar]
- 6.DHHS, Public Health Service. Ten Leading Causes of Death in the United States. Atlanta: Bureau of State Services; 1980. [Google Scholar]
- 7.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- 8.McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood) 2002;21:78–93. doi: 10.1377/hlthaff.21.2.78. [DOI] [PubMed] [Google Scholar]
- 9.Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163–170. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Tamakoshi A, Tamakoshi K, Lin Y, Yagyu K, Kikuchi S. Healthy lifestyle and preventable death: findings from the Japan Collaborative Cohort (JACC) Study. Prev Med. 2009;48:486–492. doi: 10.1016/j.ypmed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5:e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuel DG, Perez R, Sanmartin C, Taljaard M, Hennessy D, Wilson K, Tanuseputro P, Manson H, Bennett C, Tuna M, Fisher S, Rosella LC. Measuring Burden of Unhealthy Behaviours Using a Multivariable Predictive Approach: Life Expectancy Lost in Canada Attributable to Smoking, Alcohol, Physical Inactivity, and Diet. PLoS Med. 2016;13:e1002082. doi: 10.1371/journal.pmed.1002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Doherty MG, Cairns K, O'Neill V, Lamrock F, Jørgensen T, Brenner H, Schöttker B, Wilsgaard T, Siganos G, Kuulasmaa K, Boffetta P, Trichopoulou A, Kee F. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES) Eur J Epidemiol. 2016;31:455–468. doi: 10.1007/s10654-015-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K, Husing A, Kaaks R. Lifestyle risk factors and residual life expectancy at age 40: a German cohort study. BMC Med. 2014;12:59. doi: 10.1186/1741-7015-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiol. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 18.The Center for Disease Control and Prevention (CDC) [Accessed July 26, 2016];National Health and Nutrition Examination Surveys. at http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 19.The Center for Disease Control and Prevention (CDC) WONDER online database. [Accessed July 26, 2016];Underlying Cause of Death, 1999–2014. at http://wonder.cdc.gov/controller/datarequest/D76.
- 20.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 21.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WW. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements In US Diet Helped Reduce Disease Burden And Lower Premature Deaths, 1999–2012; Overall Diet Remains Poor. Health Aff (Millwood) 2015;34:1916–1922. doi: 10.1377/hlthaff.2015.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 25.Yu E, Ley SH, Manson JE, Willett W, Satija A, Hu FB, Stokes A. Weight History and All-Cause and Cause-Specific Mortality in Three Prospective Cohort Studies. Annal Intern Med. 2017;166:613–620. doi: 10.7326/M16-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, Poole EM, Tamimi R, Tworoger SS, Giovannucci E, Rosner B, Ogino S. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 28.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1–122. [PubMed] [Google Scholar]
- 29.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988–2006. Am J Med. 2009;122:528–534. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 34.Braveman P, Egerter S. Overcoming obstacles to health: report from the Robert Wood Johnson Foundation to the commission to built a healthier America. Washington (DC): 2008. Feb, 2008. [Google Scholar]
- 35.Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA. 2016;315:1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong MS, Chan KS, Jones-Smith JC, Colantuoni E, Thorpe RJ, Jr, Bleich SN. The neighborhood environment and obesity: Understanding variation by race/ethnicity. Prev Med. 2017 Nov 29; doi: 10.1016/j.ypmed.2017.11.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 38.Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O'Doherty MG, Buenode-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H CHANCES Consortium. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958–967. doi: 10.1016/S2213-8587(15)00316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 42.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59:252745. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valencia WM1, Stoutenberg M, Florez H. Weight loss and physical activity for disease prevention in obese older adults: an important role for lifestyle management. Curr Diab Rep. 2014;14:539. doi: 10.1007/s11892-014-0539-4. [DOI] [PubMed] [Google Scholar]
- 44.Hardefeldt PJ, Penninkilampi R, Edirimanne S, Eslick GD. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2017 Oct 17; doi: 10.1016/j.clbc.2017.10.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrens G, Fischer B, Kohler S, Park Y, Hollenbeck AR, Leitzmann MF. Healthy lifestyle behaviors and decreased risk of mortality in a large prospective study of U.S. women and men. Eur J Epidemiol. 2013;28:361–372. doi: 10.1007/s10654-013-9796-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


