Abstract
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder that results from the death of dopamine (DA) neurons. Over recent years, differentiated or undifferentiated neural stem cells (NSCs) transplantation has been widely used as a means of cell replacement therapy. However, compelling evidence has brought attention to the array of bioactive molecules produced by stem cells, defined as secretome. As described in the literature, other cell populations have a high‐neurotrophic activity, but little is known about NSCs. Moreover, the exploration of the stem cell secretome is only in its initial stages, particularly as applied to neurodegenerative diseases. Thus, we have characterized the secretome of human neural progenitor cells (hNPCs) through proteomic analysis and investigated its effects in a 6‐hydroxidopamine (6‐OHDA) rat model of PD in comparison with undifferentiated hNPCs transplantation. Results revealed that the injection of hNPCs secretome potentiated the histological recovery of DA neurons when compared to the untreated group 6‐OHDA and those transplanted with cells (hNPCs), thereby supporting the functional motor amelioration of 6‐OHDA PD animals. Additionally, hNPCs secretome proteomic characterization has revealed that these cells have the capacity to secrete a wide range of important molecules with neuroregulatory actions, which are most likely support the effects observed. Overall, we have concluded that the use of hNPCs secretome partially modulate DA neurons cell survival and ameliorate PD animals’ motor deficits, disclosing improved results when compared to cell transplantation approaches, indicating that the secretome itself could represent a route for new therapeutic options for PD regenerative medicine. stem cells translational medicine 2018;7:829–838
Keywords: Parkinson's disease, Dopaminergic neurons, Human neural progenitor cells, Secretome
Introduction
Parkinson's Disease (PD) is the second most common chronic neurodegenerative disorder in the elderly, and it is estimated to affect about 1% of population over 60 years of age 1, 2. Clinically, PD is mainly characterized as a motor disease. The diagnosis currently available depends on the identification of cardinal features, such as bradykinesia, muscular rigidity, postural instability, and tremor that show a nonlinear progression during the development of the disease 3, 4. These motor deficits are the result of the progressive loss of dopamine (DA) neurons in the nigrostriatal pathway, particularly in the substantia nigra pars compacta (SNpc), leading to the reduction of DA levels in the striatum 3, 5, 6. Despite pharmacological advances, the current treatments do not address the etiology of the disease and the degenerative process is not avoided 7, 8, 9, 10. Therefore, new strategies based on the use of stem cells, such as neural stem cells (NSCs), have emerged as an alternative therapy for PD 11, 12. NSCs are multipotent stem cells isolated from fetal and adult nervous system tissues with the ability to self‐renew and differentiate into specialized functional neurons and glial cells, making them an interesting source of cells for neuronal repair after injury or disease 12, 13, 14. In fact, different reports have already shown that the transplantation of NSCs display therapeutic effects, such as the capacity to protect and regenerate damaged DA neurons, as well as the potential to improve function in animal models of PD. For instance, Harrower and colleagues 15 showed a reliable long‐term survival and integration of transplanted NSCs in the striatum of rats lesioned with 6‐hydroxydopamine (6‐OHDA). According to the authors, an increase in dopaminergic (DAergic) fiber densities and synapse formation was observed. Similarly, another study demonstrated that the transplantation of adult NSCs (expanded from the subependymal zone) in the striatum of 6‐OHDA‐lesioned rats led to a functional recovery in these animals, a fact that was positively correlated with DA transporter immunoreactivity re‐establishment in the host tissue 16. Even though, growing evidence suggests that the effects mediated by stem cell transplants are not associated with the generation of new neurons or glial cells 17, 18. In fact, stem cells can secrete a wide panel of bioactive agents (e.g., growth factors, cytokines, and [micro]vesicles), which is defined as secretome. The latter is believed to be important in the modulation of several biological processes, such as cell survival, proliferation and differentiation, immunomodulation, antiapoptosis, and stimulation of tissue adjacent cells 18, 19, 20. Currently, there are no studies regarding the application of NSCs secretome per se in animal models of PD, nor its effect on DAergic neuronal cell populations. Nevertheless, some reports have suggested them as neurotrophic‐factor secreting cells 21. Indeed, different studies showed that important neurotrophic factors, such as glial cell line‐derived neurotrophic factor (GDNF), brain‐derived neurotrophic factor (BDNF), stem cell factor (SCF), and insulin growth factor (IGF), were found to be increased after NSCs transplantation, thereby supporting NSCs mediated effects 21, 22, 23, 24. Having this in mind, it would be interesting to further explore the impact of the human progenitor cells (hNPCs) secretome on the reversion of PD, when compared to more conventional approaches like cell transplantation. Following this, we analyzed the effects of hNPCs secretome on DA neurons survival and motor function of a 6‐OHDA‐rat model of PD, comparing it with the outputs obtained from animals’ transplanted undifferentiated hNPCs, and highlighted possible new neuroregulatory molecules that mediate these actions.
Materials and Methods
Primary Culture of hNPCs and Collection of the Secretome
hNPCs cell culture was performed as previously described by our group 25, 26, and according to the protocols and strict ethical guidelines previously established and approved by the Conjoint Health Research Ethics Board (CHREB, University of Calgary, AB, ID: E‐18786) 27, 28, 29. Regarding conditioned media (CM) collection, at passage 5 (P5), hNPCs were enzymatically dissociated and plated into new tissue culture flasks at a density of 5,000 cells/cm2 for experiments, and 12,000 cells/cm2 for proteomic procedures. After 14–20 days of growth in culture, the cells were centrifuged and the supernatant was discarded. Then, Neurobasal A medium (TermoFisher Scientific, 10888‐022, Waltham, MA, https://www.thermofisher.com/pt/en/home.html) supplemented with 1% kanamycin (TermoFisher Scientific, 15160‐047) was added to the cells. After 24 h, this medium, containing the factors secreted by hNPCs was collected, centrifuged at 250 g for 10 min to remove any cell debris, and then stored at −80°C until it was required for further experiments.
6‐OHDA Lesions
All the experiments were done after the consent from the Portuguese national authority for animal research, Direção Geral de Alimentação e Veterinária (ID: DGAV28421, Lisbon, Portugal) and Ethical Subcommittee in Life and Health Sciences (SECVS; ID: SECVS‐008/2013, University of Minho, Braga, Portugal), and conducted in accordance with the local regulations on animal care and experimentation (European Union Directive 2010/63/EU). Eight‐weeks‐old Wistar‐Han male rats (Charles River, Barcelona, Spain, http://www.criver.com/) were housed in pairs, in appropriate cages, under standard controlled conditions (12‐hour light/12‐hour dark cycles; room temperature (RT) at 22–24°C and 55% humidity; food and water ad libitum). For surgical procedures, animals were anesthetized intraperitoneally (i.p.) with ketamine (75 mg/kg) plus medetomidine (0.5 mg/kg), placed on a stereotaxic frame (Stoelting, Wood Dale, IL, https://www.stoeltingco.com/), and unilaterally injected using a 30‐gauge needle Hamilton syringe (Hamilton, Bonaduz, Switzerland, https://www.hamiltoncompany.com/), with either vehicle (0.2 mg/ml of ascorbic acid in 0.9% NaCl; sham group, n = 9) or 6‐OHDA hydrochloride (Sigma, H4381, St. Louis, MO, http://www.sigmaaldrich.com/portugal.html; 6‐OHDA group, n = 15) directly into the medial forebrain bundle (MFB; coordinates related to Bregma: AP = −4.4 mm, ML = − 1.0 mm, DV = −7.8 mm 30) at a rate of 0.5 μl/min. Sham animals received 2 μl of 0.2 mg/ml of ascorbic acid (Sigma, A1968) in 0.9% NaCl and the 6‐OHDA animals were injected with 2 μl of 6‐OHDA hydrochloride (4 μg/μL) with 0.2 mg/ml of ascorbic acid in 0.9% NaCl. The needle was left in place for 4 min after each injection to avoid any backflow. The PD model with 6‐OHDA injections into the MFB was chosen due to their relevance to study anti‐PD properties of novel therapies, such as cell replacement strategies or the application of new drugs or cell‐free therapeutic tools (e.g., hNPCs secretome) 31, 32, 33. Animals presenting more than 100 rotations in the apomorphine‐induced turning behavior (rotameter test) were consider having a complete lesion, as previously described by our group 34.
Surgical Treatment: Injection of hNPCs and hNPCs Secretome
Five weeks after 6‐OHDA injections, the animals received hNPCs transplants or hNPCs secretome. After anesthesia administration, animals were unilaterally injected, as described in previous section, with either vehicle (Neurobasal A medium; 6‐OHDA control group, n = 5), sterile saline (sham group, n = 9), hNPCs (n = 5), or hNPCs CM (n = 5) directly in the SNpc (coordinates related to Bregma: AP = − 5.3 mm, ML = −1.8 mm, DV = −7.4 mm) and into four striatum coordinates (coordinates related to Bregma: AP = −1.3 mm, ML = 4.7 mm, DV = −4.5 mm, and − 4.0 mm; AP = −0.4 mm, ML = 4.3 mm, DV = −4.5 mm, and 4.0 mm; AP = 0.4 mm, ML = 3.1 mm, DV = −4.5 mm, and − 4.0 mm; AP = 1.3 mm, ML = 2.7 mm; DV = −4.5 mm, and − 4.0 mm 30). 6‐OHDA‐control group received 4 μl of Neurobasal A medium in the SNpc and 2 μl in each coordinate of striatum at a rate of 0.5 μl/min. Cell transplanted groups received 200,000 cells in SNpc and 50,000 cells in each coordinate of striatum. CM‐injected animals received 4 μl in the SNpc and 2 μl in each coordinate of striatum at a rate of 0.5 μl/min. The needle was left in place for 4 min after each injection to avoid any backflow.
Behavioral Assessment
Behavioral analysis was performed 3 weeks after 6‐OHDA injections for the PD model characterization and after treatments at 1, 4, and 7 weeks following surgeries (Fig. 1). Motor coordination and balance of the animals was evaluated using the Rotarod test. The skilled paw reaching test (staircase test) was used to assess the independent forelimb extension and grasping skills. Finally, the extension of DA depletion was evaluated using the apomorphine‐turning behavior test (rotameter test). Understanding that apomorphine is a strong DA agonist, the continuous overstimulation of the DAergic system could lead to an inadequate interpretation of the treatments impact on the functional outcomes. Therefore, this test was only used to select the animals that were truly injured upon 6‐OHDA lesions 35, 36, 37. All behavioral tests were performed as previously described 30, 34.
Figure 1.
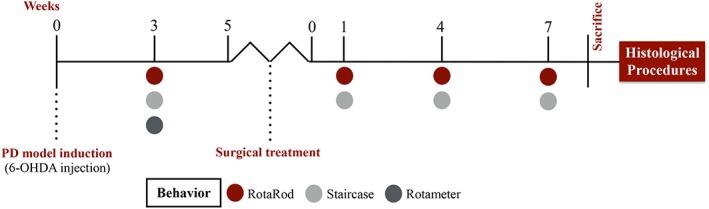
Experimental design. The PD model was induced by a 6‐OHDA unilateral injection. Three weeks later, the animals were submitted to a first behavioral analysis (using the RotaRod, Staircase, and Rotameter tests) to characterize the model. After this, the animals were treated with hNPCs or their secretome, which were injected in the SNpc and striatum. Then, behavior analysis was performed 1, 4, and 7 weeks after treatments, using the RotaRod and Staircase tests. Finally, the animals were sacrificed to proceed with the histological analysis. Abbreviations: 6‐OHDA, 6‐hydroxidopamine; hNPCs, human neural progenitor cells; SNpc, substantia nigra pars compacta.
TH Immunohistochemistry
After 13 weeks (including the development of the lesion and consequent treatment) animals were sacrificed with sodium pentobarbital (Eutasil, 60 mg/kg, i.p.; Ceva Saúde Animal, Algés, Portugal, http://www.ceva.pt/) and transcardially perfused using 4% paraformaldehyde (Merck, Lisbon, Portugal, http://www.emdgroup.com/emd/index.html) in 0.01 M phosphate‐buffered saline (PBS). Four series of striatal and mesencephalon coronal sections, 50 μm thick, were obtained using a vibratome (Leica, VT1000S, Wetzlar, Germany, http://www.leicabiosystems.com/) and processed as free‐floating sections. Sections were immersed for 20 min in 0.01 M PBS with 3% H2O2 for the inhibition of endogenous peroxidase activity, and then permeabilized for 10 min with 0.1% PBS‐Triton (PBS‐T). After this, the sections were blocked for 2 h with 5% new born calf serum (NBCS; ThermoFisher Scientific, 16010159, Waltham, MA) in 0.01 M PBS, and incubated overnight at 4°C with rabbit tyrosine hydroxylase (TH) primary antibody (1:2,000; Merck Millipore, AB152, Billerica, MA, http://www.merckmillipore.com/PT/en?bd=1) diluted in 0.01 M PBS with 2% of NBCS (1:2,000). Afterward, sections were sequential incubated during 30 min at RT with a biotinylated secondary antibody (ThermoFisher Scientific, TP‐125‐HL) and then with the streptavidine‐peroxidase solution (ThermoFisher Scientific, TP‐125‐HL). The antigen visualization was performed using 25 mg of 3,3′‐diaminobenzidine tetrahydrocloride (DAB; Sigma, D5905) in 50 ml of Tris‐HCl 0.05 M (pH = 7.6) with 12.5 μl of H2O2. Sections were then mounted on superfrost slides and allowed to dry in the dark. After 24 h, thionin counter‐coloration was performed and the sections were mounted using entellan (Merck Millipore, 1079610500). To ensure a representative sampling among the animals, four identical TH‐labeled slices covering the entire mesencephalon were chosen, including all the portions of the SNpc. Using a brightfield microscope (Olympus, BX51, Tokyo, Japan, https://www.olympus-global.com/) equipped with a digital camera (CANIMPEX Enterprises, PixeLINK PL‐A622, Halifax, NS, Canada), and with the help of Visiopharm software (Visiopharm, V2.12.3.0, Hørsholm, Denmark, https://www.visiopharm.com/), the boundaries of SNpc area were drawn. The delineation of this region was performed through identification of anatomic standard reference points and with the help of the rat brain atlas 38. Counting of total TH‐positive cells in the SNpc area was performed on both hemispheres, and the data presented as the percentage (%) of remaining TH‐positive cells in the lesioned side compared to the control side. All the analysis was performed under blind conditions.
Striatal Fiber Density Measurement
TH‐immunostained striatal sections (four sections per animal) representing the coordinates of injection sites within the striatum were photographed (×1 objective) using brightfield illumination (Olympus, SZX16). All image analysis was completed using the ImageJ software (National Institute of Health, v1.48, Bethesda, MD, https://imagej.nih.gov/ij/). The optical density of TH‐positive fibers was measured by densitometry, as previously described 30, 39. The data are presented as percentages of the contralateral striatum (intact side).
Immunostaining
Striatal and mesencephalon coronal sections (including SNpc) were obtained and processed as described above. As first approach, sections were permeabilized with 0.3% PBS‐T for 10 min. Then, the sections were blocked with a solution of 10% NCBS in 0.01 M PBS for 30 min. After that, the samples were incubated overnight at 4°C with the primary antibodies namely, rabbit TH for DA neurons detection (1:1,000) and with the mouse Human Nuclear Antigen (HNA, 1:200; Merck Millipore, MAB1281) for hNPCs detection. Sections were afterward incubated with the secondary antibodies: Alexa Fluor 594 goat anti‐mouse (1:1,000; ThermoFisher Scientific, A11005) and Alexa Fluor 488 goat anti rabbit (1:1,000; ThermoFisher Scientific, A11008) during 2 h at RT. All sections were incubated with the nuclear counterstain 4′,6‐diamidino‐2‐phenylindole‐dihydrochloride (DAPI, 1:1,000; Sigma). Finally, the slides were mounted in Immu‐Mount (Thermo Scientific) and observed at a confocal point‐scanning microscope (Olympus, FV1000).
Untargeted Mass Spectrometry Proteomic Analysis: IDA and SWATH Acquisitions
Three biological replicates of hNPCs CM were first concentrated (×100) using a 5 kDA cut‐off concentrator (Vivaspin, GE Healthcare, Little Chalfont, U.K., http://www3.gehealthcare.com/en/global_gateway) by ultracentrifugation at 3,000 g during 45 min, as previously described 40. Afterwards, the secreted proteins, were precipitated with Trichloroacetic acid (TCA)—Acetone method 40 and the washed pellets were resuspended in 40 μL ×2 Laemmli buffer (BioRad, Hercules, CA, http://www.bio-rad.com/), aided by ultrasonication and denaturation at 95°C. Prior to protein digestion, 10 μL of each replicate were used to create a pooled sample for protein identification. After denaturation, samples were alkylated with acrylamide and subjected in gel digestion by using the short‐GeLC approach 41, and the formed peptides were desalted using OMIX tips with C18 stationary phase (Agilent Technologies, Glostrup, Denmark, http://www.agilent.com) before liquid chromatography‐tandem mass spectrometry (LC–MS/MS). Samples were then analyzed on a Triple TOF 5600 System (Sciex, Framingham, MA, https://sciex.com) in two phases: information‐dependent acquisition (IDA) of the pooled samples for library generation and, Sequential Window Acquisition of All Theoretical Mass Spectra (SWATH) acquisition of each individual sample to performed protein identification from the SWATH analysis (detailed in Supporting Information Table S1). A specific library of precursor masses and fragment ions was created by combining all files from the IDA experiments, and used for subsequent SWATH processing. Libraries were obtained using ProteinPilot software (Sciex, v5.1) searching against a database composed by Homo sapiens from SwissProt (release at April 2016) and the sequence of the recombinant protein malE‐GFP. SWATH data processing was performed using SWATH processing plug‐in for PeakView (Sciex, v2.0.01). Briefly peptides were selected automatically from the library and up to 15 peptides with up to 5 fragment ions were chosen per protein identified below 5% local false discovery rate (FDR) from ProteinPilot search. The chromatographic profiles of the target fragment ions of those peptides were extracted using an extracted‐ion chromatogram (XIC) window of 4 minutes with 100 ppm XIC width. Proteins with at least 1 peptide with a FDR below 1% were considered for identification, and the ones that were just identified in a single biological replicate were not considered for analysis. The final list of proteins was used to functional characterize the hNPCs CM by Gene Ontology and Pathways analysis using the Protein Analysis Through Evolutionary Relationships (PANTHER) classification system (http://pantherdb.org) 30.
Statistical Analysis
Data evaluation for animal behavior tests after 6‐OHDA injections was performed using an independent Student's t test (RotaRod and Rotameter tests), and repeated measures ANOVA if an evaluation along time was desired (Staircase test). After treatments, the behavior and histological data were analyzed using a one‐way ANOVA to compare the mean values for the four groups. If an evaluation along time was required (RotaRod and Staircase tests), a mixed design factorial ANOVA was performed. Multiple comparisons between groups were accomplished through the Bonferroni statistical test. The significance value was set as p ≤ .05 and all the results are presented as mean ±SEM (standard error of the mean).
Results
Phenotypic Characterization of 6‐OHDA Lesions
To evaluate the functional integrity of the DAergic system, after 6‐OHDA injections (Fig. 2A), and therefore select the animals that were truly injured, the rotameter test was performed at the end of behavior assessment (RotaRod and staircase tests). Three weeks after the 6‐OHDA injections, statistical analysis revealed differences in the number of apomorphine‐induced turning rotations in the 6‐OHDA‐injected animals when compared to the sham group (t (12) = 9.398, p < .0001; Fig. 2B). In addition, also the motor performance of the animals was affected by the 6‐OHDA injections. Regarding motor coordination and balance, measured by the rotarod test, it was found to be impaired in animals injected with 6‐OHDA (t (19) = 4.034, p < .001; Fig. 2C). In the staircase test, performed to assess the forelimb use and skilled motor function, we also observed that the 6‐OHDA‐injected animals were remarkably affected when compared to sham animals (F1,22) = 48.727, p < .0001, η2 partial = .689; Fig. 2D).
Figure 2.
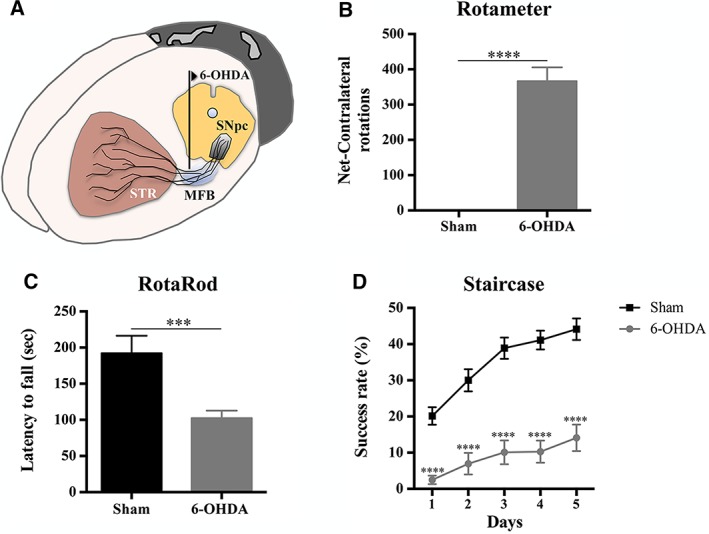
Behavioral characterization of 6‐OHDA lesions. (A): The animals received unilateral injections of 6‐OHDA directly in the MFB. (B): Rotameter revealed that the animals injected with 6‐OHDA exhibited an intense turning behavior when compared to sham group. 6‐OHDA‐injected animals also presented significant impairment in motor coordination and balance (C) and in the paw‐reaching test performance (D). For the Rotameter test, n = 9 for sham group and n = 13 for 6‐OHDA group. For the RotaRod test, n = 7 for sham group and n = 14 for 6‐OHDA group. For the Staircase test, n = 9 for sham group and n = 15 for 6‐OHDA group. Data are presented as mean ±SEM. ***p ≤ .001; ****p ≤ .0001. Abbreviations: 6‐OHDA, 6‐hydroxidopamine; MFB, medial forebrain bundle; sec, seconds; SNpc, substantia nigra pars compacta; STR, striatum.
Injection of hNPCs CM Reduces the Motor Deficits of 6‐OHDA‐Lesioned Animals
To address the effects of hNPCs transplantation and its CM (i.e., secretome) in 6‐OHDA‐injected animals, the motor performance was evaluated at 1, 4, and 7 weeks after treatment by using the RotaRod and staircase tests, as previously described 30. Regarding motor coordination and balance (assessed by the RotaRod test), the results showed a significant effect for the factor treatment (F(3,18) = 20.034, p ≤ .0001, η2 partial = .770) and for the factor time (weeks; F(3,54) = 2.824, p ≤ .05, η2 partial = 0.136), but no interaction between the factors (F(9,54) = .751, p = .661). When we compared CM‐injected animals with cells‐transplanted animals, we observed a significant improvement of motor coordination performance promoted by the hNPCs CM when compared to hNPCs‐injected group (p ≤ .05, Fig. 3A). Similar results were also observed in the staircase test, used to assess the forelimb use and the fine motor coordination of the animals. Indeed, after CM injection, statistical analysis revealed a significant effect for the treatment (F(3,20) = 21.740, p ≤ .0001, η2 partial = .765) and for the factor time (F(1.634,32.681) = 37.954, p ≤ .0001, η2 partial = .665), but no interaction between these two factors (F(4.902,32.681) = 0.672, p = .645). Comparing the animals injected with hNPCs CM with the untreated group (6‐OHDA), a post hoc analysis revealed that the injection of hNPCs CM led to a significant improvement on the success rate of eaten pellets (p ≤ .05, Fig. 3B). In addition, we were also able to observe that hNPCs CM‐injected animals presented a better motor performance than those transplanted with hNPCs (p ≤ .05, Fig. 3B). Finally, the hNPCs treatment did not lead to any motor improvements, in both tests, when compared with the untreated group.
Figure 3.
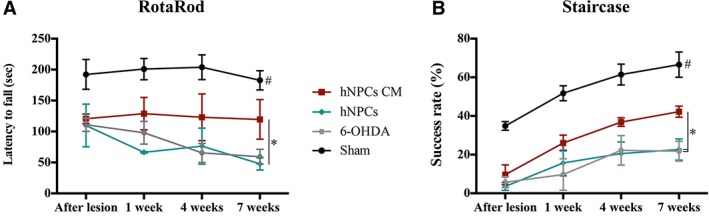
Behavioral characterization 1, 4, and 7 weeks after hNPCs transplantation and hNPCs CM (i.e., secretome) injection in the SNpc and striatum. (A): Latency to fall was measured in the accelerating RotaRod test, demonstrating that the hNPCs CM‐injected animals had a statistical significant improvement in their motor coordination when compared to the hNPCs‐transplanted group (at 7 weeks after injection). (B): The Staircase test also demonstrated a statistical significant improvement of the forelimb coordination of the hNPCs CM‐injected animals when compared to both the untreated group 6‐OHDA and the hNPCs‐transplanted group (at 7 weeks after injection). For the RotaRod test, n = 7 for sham group, n = 5 for 6‐OHDA group, n = 5 for hNPCs transplanted group and n = 5 for NCPs CM‐injected animals. For the Staircase test, n = 9 for sham group, n = 5 for 6‐OHDA group, n = 5 for hNPCs transplanted group and n = 5 for hNCPs CM‐injected animals. Data are presented as mean ±SEM. *p ≤ .05; Sham animals statistically different from all the other groups: # p ≤ .0001. Abbreviations: 6‐OHDA, 6‐hydroxidopamine; hNPCs, human neural progenitor cells; hNPCs CM, human neural progenitor cells‐conditioned media; SNpc, substantia nigra pars compacta.
Injection of hNPCs CM Restores TH Deficits
To analyze the effects of the 6‐OHDA injections, as well as the resulting treatments with hNPCs CM or hNPCs cell transplantation, histological analyses for TH were performed. From the results, we observed that there was a significant decrease of DA neurons after the injection of 6‐OHDA into the MFB (Fig. 4A–4D). After treatment, statistical analyses (F(3,23) = 109.868, p ≤ .0001, η2 = .943) demonstrated that the injection of the hNPCs CM most likely play a role in the DA neurons survival, leading to a significant higher number of TH‐positive cells in the SNpc when compared with the untreated group 6‐OHDA (p < .05, Fig. 4E). The same observations were also found in the striatum, by assessing TH‐positive fibers through densitometry analysis (Fig. 4F–4I). Statistical analysis (F(3,20) = 91.258, p ≤ .0001, η2 = .942) revealed that hNPCs CM was able to increase the TH expression levels when compared to the untreated group 6‐OHDA (p ≤ .05, Fig. 4J). To emphasize that, it was observed that the treatment with hNPCs led to a small increase of TH‐positive cells in the SNpc, even though nonsubstantial, and no differences were found in the striatum when compared with the untreated group. Moreover, an immunostaining against HNA was performed to identify the transplanted hNPCs in both SNpc and striatum, as it is a specific marker for human cells. Results showed that hNPCs were only found in one animal in the SNpc (Supporting Information Fig. S1) 8 weeks post transplantation.
Figure 4.
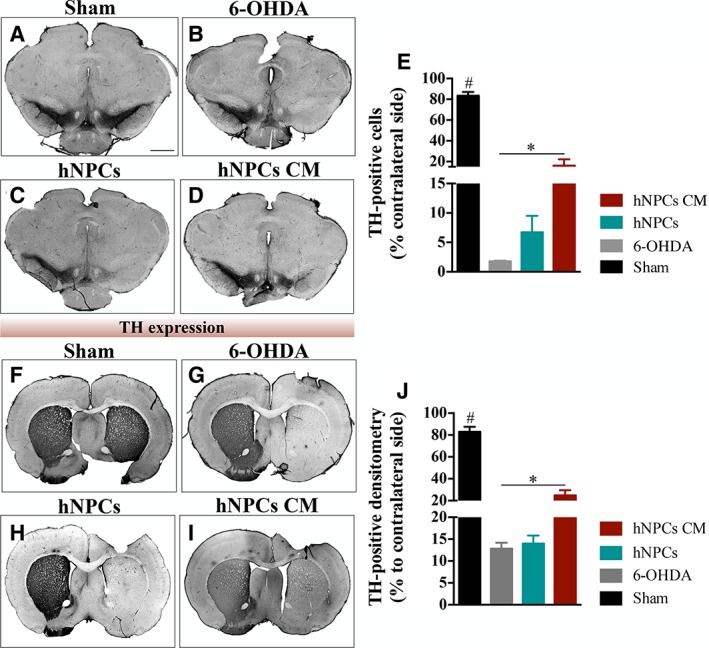
Representative micrographs of brain slices stained for TH. Compared to the sham group (A, F), all the animals that were submitted to 6‐OHDA injection presented a reduction of TH expression levels. However, animals injected with (D, I) hNPCs CM (i.e., secretome) had significantly more TH‐positive staining when compared to the (B, G) 6‐OHDA untreated group, both in (E) SNpc and (J) striatum. Data are presented as mean ±SEM. *p ≤ .05; Sham animals statistically different from all the other groups: #, p ≤ .0001. (Scale bar: 2000 μm). Abbreviations: 6‐OHDA, 6‐hydroxidopamine; hNPCs, human neural progenitor cells; hNPCs‐CM, human neural progenitor cells‐conditioned media; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase.
hNPCs Secretome as a Source of Proteins with Neuroregulatory Potential
To identify potential molecules in the hNPCs secretome that could be involved in the prosperous responses of the secretome‐injected animals, we characterized the latter through a nontargeted proteomic analysis based on a combined mass spectrometry (MS) approach. From these results, it was possible to identify 595 proteins (according to the UniProtKB/Swiss‐Prot classification; Supporting Information Table S2) in the hNPCs secretome, from which 401 proteins were common to the 3 replicates (Fig. 5A). From these, we focused on specific proteins with actions in CNS, finding important molecules in the context of PD. (Fig. 5B‐5C; highlighted in Table 1).
Figure 5.
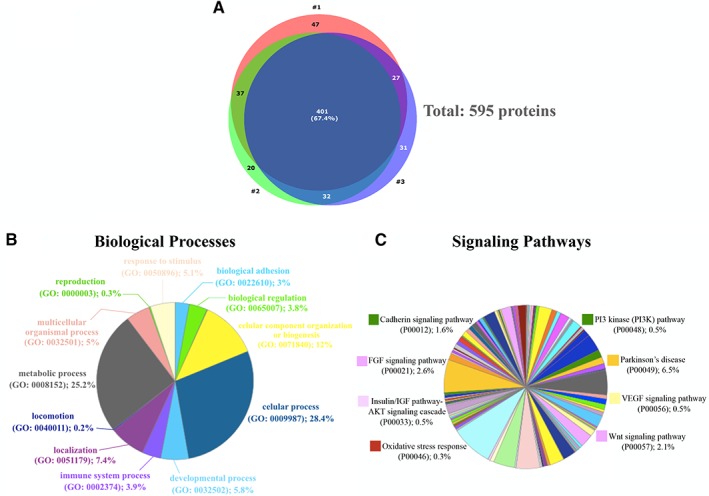
hNPCs secretome as a source of neuroregulatory molecules for PD. (A): Through combined MS analysis (IDA and STWAH acquisitions.) it was possible to identified 595 proteins in the hNPCs CM, from which 401 proteins were common to the 3 replicates as shown in the Venn Diagram. In accordance with Gene Ontology analysis of biological processes (B) and Protein Analysis Through Evolutionary Relationships (PANTHER) pathways (C), these proteins have neuroregulatory properties, n = 3. Abbreviations: hNPCs, human neural progenitor cells; hNPCs‐CM, human neural progenitor cells‐conditioned media; IDA, information‐dependent; MS, mass spectrometry; PD, Parkinson's disease; SWATH, Sequential Window Acquisition of All Theoretical Mass Spectra.
Table 1.
Combined list of reproducible proteins with neuroregulatory potential
| Accession number | Accession name | Protein Name_UNIPROT recommended |
|---|---|---|
| P19022 | CADH2_HUMAN | Cadherin_2 |
| P10909 | CLUS_HUMAN | Clusterin |
| P01034 | CYTC_HUMAN | Cystatin‐C |
| Q9UBP4 | DKK3_HUMAN | Dickkopf‐related protein 3 |
| P02751 | FINC_HUMAN | Fibronectin |
| P09382 | LEG1_HUMAN | Galectin‐1 |
| P07093 | GDN_HUMAN | Glia‐derived nexin (GDN) |
| P36955 | PEDF_HUMAN | Pigment epithelium‐derived factor |
| Q06830 | PRDX1_HUMAN | Peroxiredoxin‐1 |
| Q99497 | PARK7_HUMAN | Protein deglycase DJ‐1 |
| O75326 | SEM7A_HUMAN | Semaphorin‐7A (SEM7A) |
| P00441 | SODC_HUMAN | Superoxide dismutase [Cu‐Zn] |
| P04179 | SODM_HUMAN | Superoxide dismutase [Mn], mitochondrial |
| Q16881 | TRXR1_HUMAN | Thioredoxin reductase 1, cytoplasmic |
| P62258 | 1433E_HUMAN | 14‐3‐3 protein epsilon |
| P27348 | 1433T_HUMAN | 14‐3‐3 protein theta |
| P63104 | 1433Z_HUMAN | 14‐3‐3 protein zeta/delta |
Discussion
In this study we used a well‐defined rat model of PD, induced by a unilateral injection of 6‐OHDA into the MFB 34. This model leads to the DA neurons degeneration mimicking the appearance of the main motor deficits associated with the PD 34, 42. As shown in the rotameter test (Fig. 2B), 6‐OHDA‐injected animals displayed an intense turning behavior when compared with the sham group, indicating a clear decline in the functional integrity of the DAergic system. Moreover, we verified that the motor function of these animals was also affected, which is in agreement with previous reports 30, 43, 44. In fact, the animals presented impairments in motor coordination and balance, as assessed by the rotarod test (Fig. 2C), and in the skilled motor function addressed by the staircase test (Fig. 2D). Regarding the effects of hNPCs CM and hNPCs on motor coordination and balance (assessed by the rotarod test), we observed that the CM injection led to a significant improvement of the motor performance of the injected animals when compared with the hNPCs‐transplanted group (Fig. 3A). Similar outcomes were also seen in the staircase test, in which we verified that the injection of hNPCs secretome improved the success rate of eaten pellets in the CM‐injected animals when compared with the untreated group 6‐OHDA and to those transplanted with hNPCs (Fig. 3B). In addition to this, we also observed that the administration of the hNPCs secretome significantly increase SNpc and striatal TH‐positive neurons and fibers, respectively (Fig. 4), when compared with the untreated group 6‐OHDA. This improvement is most likely mediating the positive motor functional gains that were observed. Such evidences were not observed in hNPCs‐transplanted animals, probably due to the low rate survival of cells upon transplantation, as we observed the presence of them in just one animal (Supporting Information Fig. S1). In fact, hNPCs have been described as a potential stem cell source for the treatment of neurological disorders, including PD. Thus, to further understand which molecules present in the hNPCs secretome could be involved in the observed results, a nontargeted proteomic approach by MS was performed. In addition to the well‐known neurotrophic factors such as GDNF, BDNF, SCF, among others, our proteomic analysis revealed the secretion of important neuroregulatory candidates, such as 14‐3‐3 proteins, PEDF, Galectin‐1, Cystatin C, Clusterin, GDN, SEM7A, and Cadherin‐2 (Table 1), with important roles on the migration, differentiation, and neuroprotection mechanisms both in vitro and in vivo 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55. Concerning (for instance) GDN, although its role in PD remains undiscovered, studies have described this molecule as a modulator of neuronal survival and neurite outgrowth 52. From these, PEDF was found to have important actions in the context of PD 47, 56. As stated by Falk and colleagues 57, PEDF is not only neurotrophic but also neuroprotective in both 6‐OHDA and rotenone primary midbrain culture models of PD. Evidence of neuroprotective action of this neuroregulatory molecule has been linked to their capacity for incite the activation of nuclear factor NF‐κB signaling cascade, allowing NF‐κB to act as a transcription factor that induces the expression of genes that are crucial to neuronal protection and survival, such as BDNF and GDNF 56. Such evidence reinforces the importance of BDNF in PD, as it has been suggested that the downregulation of BDNF expression in the SNpc can be one of the initial steps at PD onset, which leads to an increased sensitization of DA neurons 30, 58. Still, Zheng and colleagues 59 have also suggested that PEDF can decrease mitochondria‐derived reactive oxygen species generation, and subsequently down‐regulate VEGF‐A expression, possibly through the inhibition of the janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) activation. In addition, Yasuda and colleagues 60 have recognized that PEDF is a promoter of protective effects on various neuronal populations, including DA neurons, indicating that an acute damage induces a rise in PEDF levels in the CNS, thereby supporting the hypothesis that PEDF acts as a natural PD neuroprotective factor that has been correlated with motor performance ameliorations. DJ‐1, also identified in hNPCs secretome, it is a multifunctional protein deeply linked to PD, and the loss of its function is thought to result in the onset of PD 61, 62. DJ‐1 is a stress sensor and its expression is increased upon various stresses, including oxidative stress 61, 63, 64, modulating signaling pathways critical to cell survival 65, 66. Moreover, studies showed that the administration of DJ‐1 protein prevented DAergic cell death and restored locomotion in a 6‐OHDA‐rat model of PD, suggesting DJ‐1 as a possible pharmaceutical target for PD 67, 68. In addition to DJ‐1, we also found TrxR1, Prdx1, and SOD enzymes, which have been reported as important antioxidant agents and modulators of oxidative stress 69, 70, 71. For instance, Arodin and colleagues 72, using a Caenorhabditis elegans (C. elegans) model, concluded that in the absence of TrxR1, DA neurons were significantly more sensitive to 6‐OHDA, suggesting that this molecule is important for neuronal survival in DA‐induced cell death. Regarding Prdx1, its overexpression in a DAergic neuronal cell line has shown to revert the effects of 6‐OHDA, acting as ROS scavenger that led to DA neurons cell survival and protection 73. Similarly, SOD enzymes are often regarded as the first line of defense against ROS 70, 74. Fibronectin for instance, has also been described to exert neuroinflammatory and neuroprotective roles 75. Evidence showed that this glycoprotein could bind integrin and growth factor receptors (such as IGF‐1 receptor) to transactivate intracellular signaling events, such as the PI3K/AKT pathway, leading to the increase of growth factor‐like neuroprotective actions 75. Indeed, accumulating evidence suggests that the normal functioning of the PI3K/AKT pathway ensures the neuroprotective defense in degenerating DA neurons, by limiting apoptosis, preventing microglia activation and neuroinflammation, as well as preventing ROS accumulation, thereby keeping oxidative stress levels under control 76. Interestingly, the results of a study about the impact of Cadherin on PI3K/AKT activation suggested that Cadherin‐2 is indeed involved in the activation of this signaling cascade in DA neurons, being triggered by the glial cell line‐derived neurotrophic factor (GDNF; of pivotal importance for DA neuronal maintenance and development) 54. Finally, Dickkopf 3, was another molecule identified in the secretome of hNPCs, which has been described as an important modulator of DAergic neuronal differentiation through the Wnt/β‐catenin signaling pathway 77. Still, as previously stated by Teixeira and colleagues 25, all the mentioned proteins have already been identified in different cell secretomes by distinct proteomic‐based techniques, supporting the results stated by our proteomic analysis. Hereupon, our findings suggest that the modulation effect in DA neurons triggered by hNPCs secretome could be related with the presence/expression of several secreted factors, as those discussed above. This is in line with previous secretome studies from our lab, which demonstrated similar effects in the same PD model, as well as in the proteomic profile, by using a different stem cell population like human mesenchymal stem cells 30. Nevertheless, further research is required to carefully define which molecules are imperative for the stem cells secretome‐mediated neuroprotective and regenerative properties. Elucidation of the implicated molecular pathways is also a crucial step toward improving our understanding of the secreted factor profile and its clinical utility.
Conclusion
The findings of this study demonstrated that the injection of hNPCs secretome enhanced the densities and fibers of TH‐positive cells, a fact that probably explains the improved behavioral performance of secretome‐injected animals when compared to the untreated group 6‐OHDA and those transplanted with cells. The presence of important neuroregulatory molecules within the secretome such as PEDF, DJ‐1, Cadherin‐3, anti‐oxidant proteins, among others, might play a role in the observed outcomes. In fact, it has been described that they are involved in different therapeutic mechanisms, including DA neurons cell survival and protection, opening in this way, new therapeutical opportunities for PD. Overall, our results strongly suggest that the use of the secretome per se may be considered as a possible tool for the treatment of PD, as the secretome was able to better modulate DAergic neuronal survival and animal behavior performance when compared to cell transplantation.
Authors Contributions
M.P.B.: designed and performed most of the experiments, collected and analyzed the data, and drafted the manuscript; F.G.T.: performed the stereotaxic surgeries, helped with the data interpretation and with manuscript writing; S.I.A. and B.M.: performed and collected the data regarding the proteomic analysis. L.A.B.: provided study material, and helped with the manuscript writing. A.J.S.: conceived and financially supported the study, participated in its design and coordination, and helped with the manuscript writing. All authors read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Figure S1
Supporting Information Table S2
Supporting Information
Acknowledgments
This work was supported by Portuguese Foundation for Science and Technology: Ciência 2007 Program and IF Development Grant (IF/00111/2013) to A.J.S., Ph.D. scholarships to S.I.A. (SFRH/BD/81495/ 2011); Canada Research Chair in Biomedical Engineering (LAB). This article has been developed under the scope of the project NORTE‐01‐0145‐FEDER‐000013 and NORTE‐01‐0145‐FEDER‐000023, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). This work has been funded by FEDER funds, through the Competitiveness Factors Operational Programme (COMPETE), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of the projects POCI‐01‐0145‐FEDER‐007038, PTDC/NEU‐NMC/0205/2012, UID/NEU/04539/2013, and POCI‐01‐0145‐FEDER‐007440 cofunded by the Programa Operacional Factores de Competitividade, QREN, the European Union (FEDER), and by the National Mass Spectrometry Network under the contract REDE/1506/REM/2005.
References
- 1. Dexter DT, Jenner P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic Biol Med 2013;62:132–144. [DOI] [PubMed] [Google Scholar]
- 2. Pringsheim T, Jette N, Frolkis A et al. The prevalence of Parkinson's disease: A systematic review and meta‐analysis. Mov Disord 2014;29:1583–1590. [DOI] [PubMed] [Google Scholar]
- 3. Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet 2009;373:2055–2066. [DOI] [PubMed] [Google Scholar]
- 4. Jankovic J. Parkinson's disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368–376. [DOI] [PubMed] [Google Scholar]
- 5. Langston JW. The Parkinson's complex: Parkinsonism is just the tip of the iceberg. Ann Neurol 2006;59:591–596. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Kim HJ, Lee JY et al. Phenotype analysis in patients with early onset Parkinson's disease with and without parkin mutations. J Neurol 2011;258:2260–2267. [DOI] [PubMed] [Google Scholar]
- 7. Weiner WJ. Advances in the diagnosis, treatment, and understanding of Parkinson's disease and parkinsonism. Rev Neurol Dis 2006;3:191–194. [PubMed] [Google Scholar]
- 8. Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat 2008;4:743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jimenez‐Shahed J. A review of current and novel levodopa formulations for the treatment of Parkinson's disease. Ther Deliv 2016;7:179–191. [DOI] [PubMed] [Google Scholar]
- 10. Anisimov SV. Cell‐based therapeutic approaches for Parkinson's disease: Progress and perspectives. Rev Neurosci 2009;20:347–381. [DOI] [PubMed] [Google Scholar]
- 11. Han F, Baremberg D, Gao J et al. Development of stem cell‐based therapy for Parkinson's disease. Transl Neurodegener 2015;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci 2012;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buzhor E, Leshansky L, Blumenthal J et al. Cell‐based therapy approaches: The hope for incurable diseases. Regen Med 2014;9:649–672. [DOI] [PubMed] [Google Scholar]
- 14. Fu MH, Li CL, Lin HL et al. Stem cell transplantation therapy in Parkinson's disease. Springerplus 2015;4:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrower TP, Tyers P, Hooks Y et al. Long‐term survival and integration of porcine expanded neural precursor cell grafts in a rat model of Parkinson's disease. Exp Neurol 2006;197:56–69. [DOI] [PubMed] [Google Scholar]
- 16. Richardson RM, Broaddus WC, Holloway KL et al. Grafts of adult subependymal zone neuronal progenitor cells rescue hemiparkinsonian behavioral decline. Brain Res 2005;1032:11–22. [DOI] [PubMed] [Google Scholar]
- 17. Pluchino S, Zanotti L, Rossi B et al. Neurosphere‐derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005;436:266–271. [DOI] [PubMed] [Google Scholar]
- 18. Pluchino S, Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia 2013;61:1379–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skalnikova H, Motlik J, Gadher SJ et al. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics 2011;11:691–708. [DOI] [PubMed] [Google Scholar]
- 20. Cossetti C, Alfaro‐Cervello C, Donega M et al. New perspectives of tissue remodelling with neural stem and progenitor cell‐based therapies. Cell Tissue Res 2012;349:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drago D, Cossetti C, Iraci N et al. The stem cell secretome and its role in brain repair. Biochimie 2013;95:2271–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ourednik J, Ourednik V, Lynch WP et al. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol 2002;20:1103–1110. [DOI] [PubMed] [Google Scholar]
- 23. Yasuhara T, Matsukawa N, Hara K et al. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci 2006;26:12497–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebert AD, Beres AJ, Barber AE et al. Human neural progenitor cells over‐expressing IGF‐1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp Neurol 2008;209:213–223. [DOI] [PubMed] [Google Scholar]
- 25. Teixeira FG, Panchalingam KM, Assuncao‐Silva R et al. Modulation of the mesenchymal stem cell secretome using computer‐controlled bioreactors: Impact on neuronal cell proliferation, survival and differentiation. Sci Rep 2016;6:27791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teixeira FG, Carvalho MM, Neves‐Carvalho A et al. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev 2015;11:288–297. [DOI] [PubMed] [Google Scholar]
- 27. Mendez I, Sanchez‐Pernaute R, Cooper O et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 2005;128:1498–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendez I, Dagher A, Hong M et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: A pilot study (Report of three cases.). J Neurosurg 2002;96:589–596. [DOI] [PubMed] [Google Scholar]
- 29. Baghbaderani BA, Mukhida K, Sen A et al. Bioreactor expansion of human neural precursor cells in serum‐free media retains neurogenic potential. Biotechnol Bioeng 2010;105:823–833. [DOI] [PubMed] [Google Scholar]
- 30. Teixeira FG, Carvalho MM, Panchalingam KM et al. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson's disease. Stem Cells Translational Medicine 2017;6:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson LH, Grealish S, Kirik D et al. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci 2009;30:625–638. [DOI] [PubMed] [Google Scholar]
- 32. Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson's disease. Prog Brain Res 2009;175:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boix J, Padel T, Paul G. A partial lesion model of Parkinson's disease in mice–Characterization of a 6‐OHDA‐induced medial forebrain bundle lesion. Behav Brain Res 2015;284:196–206. [DOI] [PubMed] [Google Scholar]
- 34. Carvalho MM, Campos FL, Coimbra B et al. Behavioral characterization of the 6‐hydroxidopamine model of Parkinson's disease and pharmacological rescuing of non‐motor deficits. Mol Neurodegener 2013;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bibbiani F, Costantini LC, Patel R et al. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol 2005;192:73–78. [DOI] [PubMed] [Google Scholar]
- 36. Poewe W, Wenning GK. Apomorphine: An underutilized therapy for Parkinson's disease. Mov Disord 2000;15:789–794. [DOI] [PubMed] [Google Scholar]
- 37. Trenkwalder C, Chaudhuri KR, Garcia Ruiz PJ et al. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson's disease–Clinical practice recommendations. Parkinsonism Relat Disord 2015;21:1023–1030. [DOI] [PubMed] [Google Scholar]
- 38. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, Academic Press, 2009. [Google Scholar]
- 39. Febbraro F, Andersen KJ, Sanchez‐Guajardo V et al. Chronic intranasal deferoxamine ameliorates motor defects and pathology in the alpha‐synuclein rAAV Parkinson's model. Exp Neurol 2013;247:45–58. [DOI] [PubMed] [Google Scholar]
- 40. Anjo SI, Lourenco AS, Melo MN et al. Unraveling mesenchymal stem cells' dynamic secretome through nontargeted proteomics profiling. Methods Mol Biol 2016;1416:521–549. [DOI] [PubMed] [Google Scholar]
- 41. Anjo SI, Santa C, Manadas B. Short GeLC‐SWATH: A fast and reliable quantitative approach for proteomic screenings. Proteomics 2015;15:757–762. [DOI] [PubMed] [Google Scholar]
- 42. Simola N, Morelli M, Carta AR. The 6‐hydroxydopamine model of Parkinson's disease. Neurotox Res 2007;11:151–167. [DOI] [PubMed] [Google Scholar]
- 43. Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6‐OHDA model. J Neurosci Methods 2006;158:219–223. [DOI] [PubMed] [Google Scholar]
- 44. Truong L, Allbutt H, Kassiou M et al. Developing a preclinical model of Parkinson's disease: A study of behaviour in rats with graded 6‐OHDA lesions. Behav Brain Res 2006;169:1–9. [DOI] [PubMed] [Google Scholar]
- 45. Fraga JS, Silva NA, Lourenco AS et al. Unveiling the effects of the secretome of mesenchymal progenitors from the umbilical cord in different neuronal cell populations. Biochimie 2013;95:2297–2303. [DOI] [PubMed] [Google Scholar]
- 46. Chen J, Lee CT, Errico SL et al. Increases in expression of 14‐3‐3 eta and 14‐3‐3 zeta transcripts during neuroprotection induced by delta9‐tetrahydrocannabinol in AF5 cells. J Neurosci Res 2007;85:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yabe T, Sanagi T, Yamada H. The neuroprotective role of PEDF: Implication for the therapy of neurological disorders. Curr Mol Med 2010;10:259–266. [DOI] [PubMed] [Google Scholar]
- 48. Sakaguchi M, Okano H. Neural stem cells, adult neurogenesis, and galectin‐1: From bench to bedside. Dev Neurobiol 2012;72:1059–1067. [DOI] [PubMed] [Google Scholar]
- 49. Kajitani K, Nomaru H, Ifuku M et al. Galectin‐1 promotes basal and kainate‐induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ 2009;16:417–427. [DOI] [PubMed] [Google Scholar]
- 50. Gauthier S, Kaur G, Mi W et al. Protective mechanisms by cystatin C in neurodegenerative diseases. Front Biosci (Schol Ed) 2011;3:541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wicher G, Fex‐Svenningsen A, Velsecchi I et al. Extracellular clusterin promotes neuronal network complexity in vitro. Neuroreport 2008;19:1487–1491. [DOI] [PubMed] [Google Scholar]
- 52. Farmer L, Sommer J, Monard D. Glia‐derived nexin potentiates neurite extension in hippocampal pyramidal cells in vitro. Dev Neurosci 1990;12:73–80. [DOI] [PubMed] [Google Scholar]
- 53. Pasterkamp RJ, Peschon JJ, Spriggs MK et al. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003;424:398–405. [DOI] [PubMed] [Google Scholar]
- 54. Zuo T, Qin JY, Chen J et al. Involvement of N‐cadherin in the protective effect of glial cell line‐derived neurotrophic factor on dopaminergic neuron damage. Int J Mol Med 2013;31:561–568. [DOI] [PubMed] [Google Scholar]
- 55. Gao X, Bian W, Yang J et al. A role of N‐cadherin in neuronal differentiation of embryonic carcinoma P19 cells. Biochem Biophys Res Commun 2001;284:1098–1103. [DOI] [PubMed] [Google Scholar]
- 56. Falk T, Gonzalez RT, Sherman SJ. The yin and yang of VEGF and PEDF: Multifaceted neurotrophic factors and their potential in the treatment of Parkinson's disease. Int J Mol Sci 2010;11:2875–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Falk T, Zhang S, Sherman SJ. Pigment epithelium derived factor (PEDF) is neuroprotective in two in vitro models of Parkinson's disease. Neurosci Lett 2009;458:49–52. [DOI] [PubMed] [Google Scholar]
- 58. Baquet ZC, Bickford PC, Jones KR. Brain‐derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci 2005;25:6251–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng Z, Chen H, Zhao H et al. Inhibition of JAK2/STAT3‐mediated VEGF upregulation under high glucose conditions by PEDF through a mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci 2010;51:64–71. [DOI] [PubMed] [Google Scholar]
- 60. Yasuda T, Fukuda‐Tani M, Nihira T et al. Correlation between levels of pigment epithelium‐derived factor and vascular endothelial growth factor in the striatum of patients with Parkinson's disease. Exp Neurol 2007;206:308–317. [DOI] [PubMed] [Google Scholar]
- 61. Ariga H, Takahashi‐Niki K, Kato I et al. Neuroprotective function of DJ‐1 in Parkinson's disease. Oxid Med Cell Longev 2013;2013:683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miyazaki S, Yanagida T, Nunome K et al. DJ‐1‐binding compounds prevent oxidative stress‐induced cell death and movement defect in Parkinson's disease model rats. J Neurochem 2008;105:2418–2434. [DOI] [PubMed] [Google Scholar]
- 63. Martinat C, Shendelman S, Jonason A et al. Sensitivity to oxidative stress in DJ‐1‐deficient dopamine neurons: An ES‐derived cell model of primary Parkinsonism. PLoS Biol 2004;2:e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yokota T, Sugawara K, Ito K et al. Down regulation of DJ‐1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun 2003;312:1342–1348. [DOI] [PubMed] [Google Scholar]
- 65. Aleyasin H, Rousseaux MW, Marcogliese PC et al. DJ‐1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A 2010;107:3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim RH, Peters M, Jang Y et al. DJ‐1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 2005;7:263–273. [DOI] [PubMed] [Google Scholar]
- 67. Inden M, Taira T, Kitamura Y et al. PARK7 DJ‐1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis 2006;24:144–158. [DOI] [PubMed] [Google Scholar]
- 68. Paterna JC, Leng A, Weber E et al. DJ‐1 and Parkin modulate dopamine‐dependent behavior and inhibit MPTP‐induced nigral dopamine neuron loss in mice. Mol Ther 2007;15:698–704. [DOI] [PubMed] [Google Scholar]
- 69. Vlamis‐Gardikas A, Holmgren A. Thioredoxin and glutaredoxin isoforms. Methods Enzymol 2002;347:286–296. [DOI] [PubMed] [Google Scholar]
- 70. Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson's disease: A mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci 2008;1147:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu H, Santo A, Li Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 2012;237:143–149. [DOI] [PubMed] [Google Scholar]
- 72. Arodin L, Miranda‐Vizuete A, Swoboda P et al. Protective effects of the thioredoxin and glutaredoxin systems in dopamine‐induced cell death. Free Radic Biol Med 2014;73:328–336. [DOI] [PubMed] [Google Scholar]
- 73. Lee YM, Park SH, Shin DI et al. Oxidative modification of peroxiredoxin is associated with drug‐induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem 2008;283:9986–9998. [DOI] [PubMed] [Google Scholar]
- 74. Filograna R, Godena VK, Sanchez‐Martinez A et al. Superoxide dismutase (SOD)‐mimetic M40403 is protective in cell and fly models of paraquat toxicity: Implications for Parkinson disease. J Biol Chem 2016;291:9257–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Yin L, Chen Z. Neuroprotective role of fibronectin in neuron‐glial extrasynaptic transmission. Neural Regen Res 2013;8:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jha SK, Jha NK, Kar R et al. p38 MAPK and PI3K/AKT signalling cascades in Parkinson's disease. Int J Mol Cell Med 2015;4:67–86. [PMC free article] [PubMed] [Google Scholar]
- 77. Fukusumi Y, Meier F, Gotz S et al. Dickkopf 3 promotes the differentiation of a rostrolateral midbrain dopaminergic neuronal subset in vivo and from pluripotent stem cells in vitro in the mouse. J Neurosci 2015;35:13385–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1
Supporting Information Table S2
Supporting Information


