Abstract
Male hypogonadism associated with obesity is a very prevalent condition and is increasing in parallel with the epidemic prevalence of obesity. Low testosterone levels promote higher fat mass with reduced lean mass. Male hypogonadism is related to an increase in associated cardiometabolic complications, such as hypertension, type 2 diabetes mellitus, the metabolic syndrome, and cardiovascular disease. Its influence as a comorbidity of obesity is becoming more evident and should be evaluated and treated in at-risk patients. Mechanisms involved in this relationship include body composition changes, the presence of adipokines, insulin resistance, and other factors, some of which are still unknown. Weight loss and treatment to replace testosterone levels improve the metabolic profile and quality of life in patients with obesity and hypogonadism; these beneficial effects depend on treatment modality and duration of therapy. The use of testosterone replacement therapy may be indicated, as it has not been shown to increase cardiovascular risk, and retrospective studies suggest a reduction in events in men with metabolic syndrome and type 2 diabetes.
Keywords: adipose tissue, male hypogonadism, obesity, testosterone
INTRODUCTION
In recent decades, the prevalence of obesity and overweight has increased substantially. Globally, the number of overweight and obese individuals increased from 857 million in 1980 to 2.1 billion in 2013.1 To explain this pronounced increase, the influence of factors such as increased caloric intake, dietary changes, decreased physical activity, and alterations in the intestinal microbiome must be considered.2 Obesity is directly related to a large number of comorbidities, such as type 2 diabetes (T2DM), nonalcoholic fatty liver disease, osteoarthrosis, cardiovascular disease, and cancer arising from the severe metabolic alterations caused by excess weight.3
Obesity is considered the most frequent cause of male hypogonadism.4,5 Male hypogonadism is the clinical syndrome resulting from the inability to produce physiological concentrations of testosterone, normal amounts of sperm, or both. It can adversely affect multiple organ functions and quality of life.6,7
The increase in life expectancy and prevalence of obesity in Western industrialized countries has given rise to an increasing number of cases of male hypogonadism. Hypogonadism is estimated to affect between 2.1% and 12.8% of adult men in the general population, and its prevalence will increase to 6.5 million by 2025 as a result of an aging population.8 Androgen deficiency leads to reduced fertility, sexual dysfunction, decreased muscle mass, alterations in bone mineralization, and lipid metabolism disorders in men.
Numerous epidemiological studies have shown a negative correlation between obesity and testosterone levels, and several meta-analyses have shown that weight loss produces a proportional increase in testosterone concentrations.9 Furthermore, an increase in adipose tissue has been observed in patients with hypogonadism. Patients in testosterone replacement therapy have shown a reduction in abdominal circumference and adipose tissue, together with an increase in muscle mass.10 The relationship between hypogonadism and obesity is, therefore, bidirectional.
There is also a greater prevalence of hypogonadism in patients with conditions directly related to obesity, such as T2DM and metabolic syndrome (MS).11,12 The pathophysiological links between all these disorders are complex, but adipose tissue plays an important role, especially when localized to the abdomen.10
In this review, we summarize current knowledge about the close relationship between obesity and male hypogonadism.
MECHANISMS INVOLVED IN THE RELATIONSHIP BETWEEN MALE HYPOGONADISM AND OBESITY
Obesity-associated hypogonadism is characterized by normal or low levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and decreased plasma testosterone.5
The gonadal axis (hypothalamus–pituitary–gonadal axis) is regulated by a set of hypothalamic neurons that respond to stimulation by the kisspeptin peptide hormones. These neurons produce gonadotropin-releasing hormone (GnRH), which is released in pulses every 60–90 min, stimulating the pulsatile secretion of the pituitary gonadotropins, LH and FSH, into the bloodstream. LH stimulates Leydig cells to produce testosterone, while FSH, in conjunction with intratesticular testosterone, acts on Sertoli cells and seminiferous tubules to stimulate spermatogenesis. In turn, the hormone inhibin is produced in Sertoli cells, which provides negative feedback on the production of FSH in the pituitary gland. Testosterone acts directly through androgen receptors and by its conversion into two active metabolites, dihydrotestosterone, by the enzyme 5-α reductase, and estradiol, by the enzyme aromatase. Testosterone and estradiol provide negative feedback to the hypothalamus and pituitary gland to suppress the secretion of gonadotropins.7
Obesity in men is associated with an increase in the aromatase enzyme activity of adipocytes, which results in a higher peripheral conversion of testosterone to estradiol and, traditionally, it has been thought that the consequent rise in serum estradiol exerts negative feedback on the secretion of LH from the pituitary gland, due to the presence of estrogen receptors in the hypothalamus–pituitary–gonadal axis, thereby suppressing the axis and leading to a reduction in testosterone levels. Reduced plasma testosterone worsens obesity since it promotes changes in body composition, stimulating an increase in adipose tissue, mainly in the abdomen. Obesity, in turn, may have a direct impact on testosterone levels, contributing to reduced testosterone and increased adipose tissue, creating a negative cycle called the hypogonadism-obesity cycle. The extension of this theory is based on the fact that adipose tissue is an endocrine organ that secretes various factors that influence the pathogenesis of obesity and affect testosterone production, contributing to the onset of hypogonadism. This is known as the hypogonadism-obesity-adipokine hypothesis.10
However, the role of estradiol is controversial in light of recent findings. Since in men, most of the circulating estradiol (80%) derive from peripheral aromatization of testosterone or conversion of estrone and only 10%–20% is directly secreted by the testes,13 lower testosterone levels should lead to lower estradiol levels. In a meta-analysis studying the relationship between body mass index (BMI) and reproductive hormones, MacDonald et al.14 found a direct relationship between BMI and estradiol in only 4 of 10 studies determining estradiol levels. In fact, large population-based studies using liquid chromatography-tandem mass spectrometry (LC-MS/MS) have reported lower estradiol levels in hypogonadal men compared with eugonadal men.15,16 Indeed, increased estradiol levels have only been reported in extremely obese men,17,18 suggesting that increased estradiol contributes to the development of hypogonadism only in extremely obese men.
TESTOSTERONE AND BODY COMPOSITION
Testosterone influences metabolism. One of the mechanisms is through changes in body composition. In fact, testosterone inhibits fat accumulation through its binding to the androgen receptor, which has a higher density in visceral adipose tissue than in subcutaneous adipose tissue.19 In addition, testosterone plays an inhibitory role in lipoprotein lipase activity by inhibiting lipid accumulation and favoring lipolysis in visceral adipocytes.
By contrast, decreased testosterone levels could be implicated in the lack of negative regulation of both the differentiation of preadipocytes into mature adipocytes and pluripotent mesenchymal stem cells into adipocytes, which leads to increased adipose tissue related to hypogonadism.20 Central or abdominal fat is more closely related to testosterone levels than other forms of obesity.21 Abate et al.22 showed that the accumulation of subcutaneous fat in the trunk was highly predictive of low levels of free testosterone (FT). Couillard et al.23 in the HERITAGE study, which included 217 men aged 17–64 years, found that abdominal fat was the most important parameter related to testosterone levels. Vermeulen et al.24 found that testosterone levels negatively correlated with body fat percentage and abdominal fat in a study of 57 men between 70 and 80 years old. Garaulet et al.25 carried out a study with 80 obese people (29 men), between 30 and 70 years old. They found an inverse relationship between testosterone levels and fat mass percentage and total abdominal fat. Finally, Dhindsa et al.26 conducted a study on 138 men with T2DM, with a mean age of 59 years and a mean BMI of 31.8 kg m−2, finding an inverse correlation between FT and total testosterone (TT) levels and total subcutaneous and trunk fat.
Visceral fat is the most metabolically active fraction of body fat mass. It constitutes a significant proportion of intra-abdominal fat. Testosterone in men has been shown to be a lipolytic hormone, with selective activity at the intra-abdominal level.27 In 1990, Seidell et al.28 found a negative correlation between FT and visceral fat determined by computed tomography in 23 healthy men between 25 and 50 years old. Subsequently, Tsai et al.29 showed an inverse relationship between TT levels and visceral fat accumulation (also determined by computed tomography), but not other fatty deposits, in a population of 110 males after 7.5 years of follow-up. In the previously mentioned study by Couillard et al.,23 in addition to increased abdominal adiposity, they also found greater accumulation of visceral fat in patients with low testosterone. In 2007, Nielsen et al.30 in a population of 685 young men, 70 of them obese, found that the degree of visceral fat measured by dual-energy X-ray absorptiometry and magnetic resonance imaging was inversely related to free and bioavailable TT. Furthermore, in a multiple linear regression analysis, visceral fat was independently and inversely related to FT and bioavailable testosterone.30 However, Abate et al.22 found that subcutaneous fat in the trunk, but not visceral fat, was highly predictive of plasma concentrations of testosterone. In fact, patients with prostate cancer who were on antiandrogen hormone treatment develop an increase in central adiposity and percentage of fat mass, with a decrease in lean mass. While Smith et al.31 observed that this change in body fat affected mainly subcutaneous and nonvisceral depots, Hamilton et al.32 reported a higher increase in visceral abdominal fat area than in subcutaneous abdominal fat area. With respect to testosterone replacement therapy, some studies show a clear decrease in visceral fat, while others find either no decrease in abdominal or visceral fat mass or a decline in subcutaneous fat in the limbs but not in the abdomen.9
Therefore, in conclusion, the evidence suggests the existence of a link between testosterone deficiency and the development and progression of obesity, but it remains unclear if this association is more apparent in visceral or in subcutaneous depots.
In addition to increased fat mass, decreased muscle mass is a key feature in obesity that contributes to several related metabolic disorders. Testosterone is an anabolic hormone and its effect on increase in muscle mass is well documented.12 The administration of testosterone stimulates the synthesis of muscle proteins, causes hypertrophy of muscle fiber, and increases the myonuclear content per fiber and satellite cells, which give the muscle strength. In addition, testosterone exerts inhibitory effects on the differentiation of multipotent mesenchymal cells into adipogenic cells and promotes their differentiation into cells of myogenic lineage.20 Multiple cross-sectional studies demonstrate the positive association between testosterone levels and muscle mass in men.28,29,30,33,34,35
ROLE OF ADIPOCYTOKINES IN MALE HYPOGONADISM ASSOCIATED WITH OBESITY
Adipose tissue, as an endocrine and metabolic organ, expresses and secretes active metabolites such as leptin, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). Leptin stimulates GnRH-producing cells in the hypothalamus to induce the release of LH and in the testicule stimulates the production of testosterone under normal conditions. This stimulation of hypothalamic GnRH-producing neurons is not directly done by leptin, whose receptors are expressed scarcely on them, but by kisspeptins. Hypothalamic cells produce kisspeptins and express the leptin receptor. Obesity is typically associated with higher levels of leptin derived from the high percentage of fat mass, which does not decrease after the exogenous administration of leptin, suggesting that obese individuals are resistant to it.36
Most obese individuals have high serum leptin concentrations mainly due to diet-induced expansion of adipocytes.37,38 Despite hyperleptinemia, these patients are considered to be resistant to the effects of leptin. This hyperleptinemia seen in obesity may play a role in hypogonadism and subfertility due to the development of leptin resistance, akin to insulin resistance. Based on in vitro and rodent studies, several mechanisms of leptin resistance have been proposed, including impaired transport across the blood–brain barrier, impaired leptin signaling by suppressor of cytokine signaling 3 (SOCS3), impaired leptin receptor trafficking, saturation of leptin signaling pathways, endoplasmic reticulum stress, and downmodulation of leptin's neural circuitry.39,40 A few of these mechanisms have been confirmed in human studies.41 There may be other factors, including genetic factors, that make certain individuals predisposed to the adverse reproductive effects of central leptin resistance. Leptin concentrations have been found to inversely correlate with testosterone concentrations, even after controlling for sex hormone-binding globulin (SHBG) and estradiol, and leptin concentrations were the best hormonal predictor of low androgen concentrations in obesity.42
It has been proposed that this resistance to leptin also exists at the central level, favoring hypogonadism.43 In addition, elevated leptin levels may directly inhibit the production of testosterone in Leydig cells, further decreasing it.43 Both TNF-α and IL-6 are also expressed in adipose tissue. An elevation in their levels creates a pro-inflammatory state involved in the pathophysiology of obesity and insulin resistance and also negatively influences the secretion of gonadotropins.44
Adiponectin is an adipocyte-specific secretory protein which exhibits antiatherogenic, anti-inflammatory, and antidiabetic properties. It is produced mainly by visceral adipose tissue. Interestingly and in contrast to other adipocytokines, for example, leptin and adiponectin levels are reduced among obese individuals in comparison with lean controls and increase concomitantly with weight loss. Compared to eugonadal patients, hypogonadal men have higher adiponectin levels which are reduced by testosterone replacement therapy. Overall, the evidence suggests that testosterone exerts a negative regulatory role on adiponectin secretion in humans.45 While this is expected to be metabolically unfavorable, testosterone treatment-induced reductions in adiponectin were not accompanied by reduced insulin sensitivity.
In conclusion, the interplay among testosterone, adipocytokines such as leptin and adiponectin, and glucose metabolism is complex, bidirectional, and linked with body composition in a way that makes it difficult to determine the precise role of these factors in the overall regulation of whole body metabolism.
TESTOSTERONE AND INSULIN RESISTANCE
Most studies suggest that the effects of testosterone on insulin resistance occur through changes in body composition. Many of these studies linking hypogonadism with insulin levels have been performed in men with prostate cancer in antiandrogen hormone therapy. These patients present an increase in visceral fat, which is related to an increase in insulin resistance, aggravated by a concomitant decrease in muscle mass.21 Increased abdominal fat causes higher concentrations of free fatty acids to be delivered to the liver. With more free fatty acids, there is a higher production of hepatic glucose and a decrease in insulin uptake. This results in hyperinsulinemia and increased insulin resistance in peripheral tissues, which also leads to further release of insulin by β-cells.46 Several studies of obese hypogonadal patients with T2DM on testosterone treatment versus placebo or diet and exercise found a decrease in insulin resistance (measured by Homeostasis Model Assessment for Insulin Resistance [HOMA-IR]) in the testosterone replacement therapy group.47,48,49 Nevertheless, Grossmann et al.50 in a meta-analysis reported that the effect of testosterone in insulin resistance was nonsignificant when measured using a more stringent computer-based equation, different from the simple linear equation used in previously reported studies. On the other side, some studies have found higher prevalence of insulin resistance among patients with hypogonadism compared to those without it51,52 and negative correlations between insulin resistance and testosterone levels.53,54,55,56,57
In summary, the current evidence is consistent with a bidirectional relationship between visceral fat and testosterone levels, creating a self-perpetuated cycle that promotes insulin resistance.
OTHER MECHANISMS LINKING OBESITY AND HYPOGONADISM
Another proposed mechanism for the influence of obesity on the development of hypogonadism is through its effects on sleep quality. Studies have established that obstructive sleep apnea (OSA) associated with obesity disrupts the gonadal axis, interfering with the secretion of LH at night and, therefore, with testosterone levels. It has been reported that OSA is independently associated with decreased LH pulse amplitude, decreased mean serum LH and testosterone levels, and disruption of serum testosterone levels associated with the onset of the first rapid eye movement (REM) sleep. Men with OSA present higher circulating leptin levels, regardless of age and BMI.5 In addition, the relationship between obesity and sleep seems to be bidirectional. Lack of sleep has recently been implicated as a risk factor for the development of obesity and its complications. Although the exact mechanism is unclear, it appears to be mediated by alterations in the concentrations of different neuroendocrine modulators, including leptin and cortisol.58 Nonetheless, other studies establish that OSA is not a factor favoring hypogonadism independent of obesity.59
Most circulating testosterone is bound to SHBG or albumin and only 1%–3% circulates as FT. Changes in SHBG greatly affect the interpretation of testosterone concentrations.60 Obesity is negatively related to SHBG levels, which in turn affect the serum levels of testosterone. Frequently, in moderate obese men, there are “falsely” low levels of TT with normal FT. The liver secretes SHBG into the blood, where it binds testosterone with high affinity, regulating its bioavailability. Traditionally, it has been postulated that the link between obesity and SHBG may be mediated by insulin resistance and compensatory hyperinsulinemia, which suppresses hepatic SHBG production. Nevertheless, more recently, it has been shown that liver fat, but not visceral fat or total body fat, influences SHBG levels,61 and fatty liver is considered the main risk for lower SHBG levels. The free hormone hypothesis, which states that only free steroids diffuse into cells, is still the best explanation for the clinical manifestations of steroid hormone deficiency, but it has been proposed that SHBG leaves the blood circulation in some tissues, interacting with proteins on the plasma membranes of specific cell types. This may contribute to the delivery of SHBG-bound sex steroids via endocytotic mechanisms or lead to cell membrane receptor-mediated signaling,62 so reduced SHBG could result in decreased testosterone action in this situation. On the other side, lower levels of SHBG allow greater availability of FT as a substrate for aromatization to estradiol in adipose tissue.
Due to the direct effect of obesity on reducing circulating SHBG, it is important to consider SHBG levels when diagnosing hypogonadism.61
PREVALENCE OF MALE HYPOGONADISM ASSOCIATED WITH OBESITY
There has been ongoing interest in the relationship between obesity and hypogonadism. As early as 1977, Glass et al.56 observed that testosterone levels were lower in obese patients than in normal-weight controls. Since then, numerous studies have confirmed this relationship and have established a high prevalence of hypogonadism in obese patients.
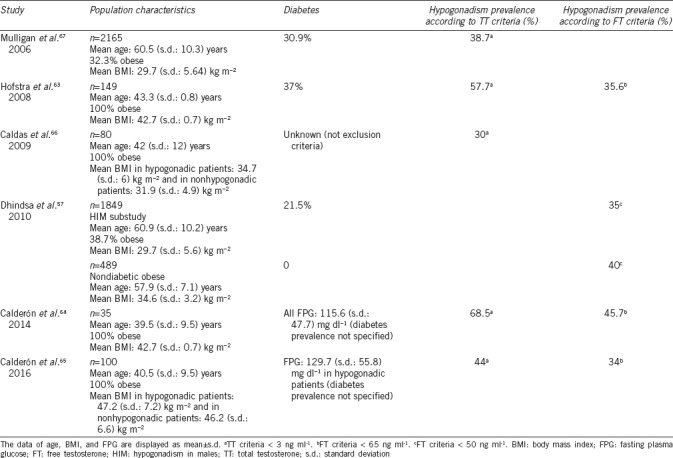
In 2008, Hofstra et al.63 reported a prevalence of hypogonadism of 57.7% according to TT criteria (<3 ng ml−1) and 35.6% according to FT criteria (<65 pg ml−1) in a population of 149 men between 18 and 66 years of age with a mean BMI of 42.7 (standard deviation [s.d.]: 0.7) kg m−2 and a prevalence of T2DM of 37%. Calderón et al.64 found a 68.5% prevalence of hypogonadism according to TT criteria (<3 ng ml−1) in a population of 35 men prior to bariatric surgery (mean BMI: 42.7 [s.d.: 0.7] kg m−2, mean age: 39.5 [s.d.: 9.5] years) and 45.7% according to FT criteria (<65 pg ml−1). Although the prevalence of T2DM was not specified, mean fasting glucose was >110 mg dl−1, indicating that a significant percentage of patients could be diabetic. A recent study by the same research group, conducted with 100 men (mean age: 40.5 [s.d.: 9.5] years, BMI ≥35 kg m−2), found a prevalence of hypogonadism according to TT and/or FT criteria (considering normal range for TT to be 2.88–8.94 ng ml−1 and for FT to be 65–183 pg ml−1) of 45%.65 When considering only TT, the prevalence was 44% and decreased to 34% when considering only FT. Again, the prevalence of T2DM is not specified, but mean fasting glucose in the group of patients with hypogonadism is 129.7 (s.d.: 55.8) mg dl−1, thus the number of diabetic patients could be significant. In 2009, Caldas et al.66 studied 80 men with MS (mean BMI >30 kg m−2, mean age: 42 [s.d.: 12] years) to determine the relationship between insulin and hypogonadism and found a prevalence of hypogonadism of 30%, according to TT criteria <3 ng ml−1. In this case, the prevalence of T2DM was not specified, but it was not an exclusion criterion. However, the largest study on hypogonadism is the Hypogonadism in Males study,67 which included 2165 men aged ≥45 years. Defining hypogonadism as TT <300 ng dl−1 (<3 ng ml−1), a prevalence of 38.7% was found. Of the patients with hypogonadism, 32.3% were obese and 30.9% had T2DM. In a substudy of this same study,57 data from 1849 men (1451 nondiabetic and 398 diabetic) were examined to determine the prevalence of hypogonadism in patients with obesity and T2DM. Of this sample, 489 were nondiabetic obese. A prevalence of hypogonadism, defined as FT <50 pg ml−1, of 34.6% (s.d.: 3.2%) was observed in this group, whereas in obese patients with T2DM, this prevalence increased to 36.4% (s.d.: 3.2%) (Table 1).
Table 1.
Studies reported hypogonadism prevalence in obese males
TREATMENT OF HYPOGONADISM ASSOCIATED WITH OBESITY
Weight loss: changes in lifestyle and bariatric surgery
Several studies have shown that in people at risk, intensive lifestyle interventions, including nutritional counseling and physical activity, can reduce body weight and insulin resistance and improve hypogonadism associated with obesity. Loss of body weight is associated with an increase in gonadotropins, TT, and FT, and in most studies with a decrease in estrogen levels.68
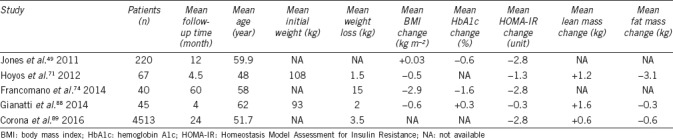
Few randomized clinical trials have specifically assessed the impact of diet and physical activity on testosterone levels in obese men, and those available have obtained contradictory results. Some show an increase in testosterone, while others find no change or even a decrease.68 In the studies analyzed in the meta-analysis by Corona et al.,68 the increase in testosterone induced by lifestyle interventions was modest (Table 2). This likely reflects the relatively limited results of diet and physical activity on body weight loss. Changes in lifestyle, however, should be the first measure proposed in patients with obesity-associated hypogonadism.
Table 2.
Modifications in anthropometric characteristics, insulin resistance, and testosterone levels after testosterone treatment in male patients with hypogonadism
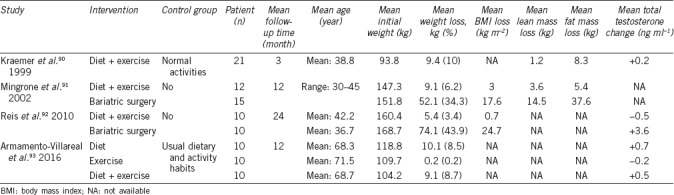
Nonetheless, most of the weight lost with diet and exercise is regained in the long term in the majority of patients. Consequently, bariatric surgery is another option for reducing body weight and treating hypogonadism associated with obesity.69 Our knowledge of the long-term effects of bariatric surgery is based only on observational, nonrandomized studies that are often of poor methodological quality. In recent years, several studies have evaluated the impact of bariatric surgery on testosterone levels in men, showing increased levels and even complete recovery of gonadal axis function in most cases.18 The beneficial effects of weight loss are more striking in younger patients and in nondiabetic controls with a higher degree of obesity presurgery, likely due to greater weight loss.18,70 At the same time, the normalization of testosterone levels contributes to the reduction in weight, waist circumference, and fat mass, which enhances the beneficial effects of bariatric surgery in terms of decreased development of T2DM, MS, cardiovascular disease, and reduced mortality (Table 2). In a study published in 2014, comparing the effect of bariatric surgery in hypogonadal obese patients versus obese men with normal gonadal status, a greater decrease in waist circumference was observed in the hypogonadal patients with the same weight loss, suggesting that bariatric surgery may have a specific effect on abdominal adiposity in hypogonadal patients.69 For these reasons, several articles propose hypogonadism associated with obesity as a potential new indication for bariatric surgery.
Testosterone treatment
The effect of testosterone treatment in men with hypogonadism is beneficial in most cases, although relatively long periods of time are often required to observe their metabolic effects. In addition, the temporal course of these effects differs depending on the dose or formulation chosen, among other variables. Many studies have demonstrated minimal or moderate effects on body weight and body composition, although the results have been evaluated in the short term. Hoyos et al.71 failed to demonstrate a beneficial effect of testosterone treatment on weight or MS and suggested the need for long-term studies. Systematic reviews of studies with testosterone therapy in men with hypogonadism reveal that changes in fat mass and lean mass occurring at 12–16 weeks of treatment stabilize at 6–12 months and that there is even a marginal improvement for years. In a recently published 56-week clinical trial,72 it was reported that in men receiving very low energy diet combined with testosterone undecanoate or matching placebo, patients assigned to testosterone mainly lost body fat, while those receiving placebo lost both fat and lean mass. Cohorts of hypogonadal patients who received long-acting testosterone undecanoate showed a decrease in body weight of almost 5% after 1 year of treatment and more than 13% at 5 years.73,74 Likewise, a significant reduction in waist circumference has also been reported.73,75 These effects are most notable in patients with higher BMI.76 Long-term treatment also produces sustained improvements in weight and waist circumference in a subgroup of obese men with hypogonadism and T2DM.77
As a conclusion, the authors suggest that long-term treatment with testosterone produces beneficial effects on weight loss and waist circumference independently of diet and exercise. These effects are superior to other drugs alone or in combination with behavioral and lifestyle modifications (Table 3). However, we have to take into account that most of these studies were not properly well-designed, double-blind, placebo-controlled trials.
Table 3.
Modifications in anthropometric characteristics and testosterone levels after lifestyle intervention or bariatric surgery in male patients with hypogonadism
Beyond the relative benefits of different methods of testosterone administration and the duration of treatment, response to treatment also depends on patient demographics. Serum levels of testosterone obtained after intramuscular administration of testosterone correlate with age and body composition. Younger and heavier men have been shown to achieve lower concentrations of total and bioavailable testosterone. Similar results have been described with transdermal testosterone formulations, demonstrating that hypogonadal men with obesity and T2DM have a lower probability of attaining normal gonadal status compared to nonobese, nondiabetic men.78 The mechanisms underlying this body composition and lower age-dependent response remain to be determined.
With regard to the safety of testosterone treatment in these patients, there has been reluctance due to the observation in some studies of a possible increased risk of cardiovascular events.79,80 In response, the United States Food and Drug Administration and the European Medicine Agency claim that there is no substantial evidence that testosterone treatment has adverse effects on cardiovascular health in men with hypogonadism.81,82,83 However, it should be emphasized that there are no long-term safety studies with testosterone. The studies that have raised these concerns are retrospective and with significant methodological limitations. It is also true that in many of these studies, testosterone doses are greater than those recommended in standard clinical practice. On the other hand, in a recent large meta-analysis of major cardiovascular events in placebo-controlled randomized trials, no increase in cardiovascular events was found. In fact, the results suggest a reduction in cardiovascular events in those with MS and T2DM.84 Another recent large retrospective study has also shown no increase in cardiovascular events.85
Treatment with aromatase inhibitors
One of the mechanisms involved in the pathophysiology of hypogonadism associated with obesity is the increased aromatase activity in adipose tissue. Aromatase converts testosterone to estradiol, which has an inhibitory effect on the production of LH in the pituitary gland. The use of letrozole, an aromatase inhibitor, has been investigated. De Boer et al.86 analyzed the effect of treatment with letrozole at doses of 7.5 mg and 17 mg per week for 6 weeks in 10 men (mean age of 48.2 [s.d.: 2.3] years and mean BMI of 42.1 [s.d.: 2.6] kg m−2), observing a significant decrease in estradiol levels and a significant increase in LH and TT levels, with stable levels of SHBG. Loves et al.87 reported similar results in a population of 12 men (mean age of 48.4 [s.d.: 3.3] years and mean BMI of 45.7 [s.d.: 3.0] kg m−2) after 6 months of treatment with letrozole at a dose of 2.5 mg weekly. In addition, Loves et al.87 observed that FT levels increased to supraphysiological levels in 50% of patients, suggesting FT levels as the best marker in the follow-up of patients taking letrozole and the need, in some cases, for dose reduction in long-term treatment.
Finally, it is important to note that aromatase inhibitors have been associated with increased osteoporosis risk, and therefore should be used with caution in patients at high risk for bone fractures.
CONCLUSION
Male hypogonadism associated with obesity is very prevalent and is increasing in parallel to the increasing prevalence of obesity. Hypogonadism perpetuates obesity, especially central obesity and, as a consequence, related cardiometabolic complications, such as T2DM and cardiovascular disease. The importance of hypogonadism as a comorbidity of obesity in men is gradually becoming clear.
Our review enabled us to conclude that screening for the presence of hypogonadism is advisable in obese men, especially in those with T2DM. In addition, despite changes in lifestyle to achieve significant weight loss should be the basis of treatment, in some cases, testosterone therapy may be indicated, as in those men with multiple signs and symptoms of hypogonadism and concomitant reduced levels of testosterone. In obese men with hypogonadism, this treatment has shown to improve body composition and to have beneficial effects on metabolic risk factors and the underlying pathophysiological mechanisms. Its use has not been shown to increase the risk of cardiovascular events in this population. The presence of hypogonadism associated with obesity should be taken into account when establishing the indication for bariatric surgery. Randomized controlled clinical trials are needed to reinforce the available evidence.
AUTHOR CONTRIBUTIONS
MMV, JCFG and AMG contributed to the manuscript conception, data search, drafting, reviewing, and critically revising the manuscript. MDF contributed to drafting and reviewing the manuscript. FJT contributed to critically revising the manuscript. All authors have read and approved the final manuscript and agree with the order of presentation of the authors.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We thank Maria Repice for English language editing. JCFG was supported by a research contract from Servicio Andaluz de Salud (SAS) (B-0033-2014); AMG was the recipient of a postdoctoral grant (Rio Hortega CM 14/00078) and is now the recipient of a postdoctoral grant (Juan Rodes CM 17/00023) from the Spanish Ministry of Economy and Competitiveness. This work was supported in part by a grant from Servicio Andaluz de Salud (PI-0173-2013). The funding organizations played no role in the present manuscript.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleich S, Cutler D, Murray C, Adams A. Why is the developed world obese? Annu Rev Public Health. 2008;29:273–95. doi: 10.1146/annurev.publhealth.29.020907.090954. [DOI] [PubMed] [Google Scholar]
- 3.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 5.Saboor Aftab SA, Kumar S, Barber TM. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clin Endocrinol (Oxf) 2013;78:330–7. doi: 10.1111/cen.12092. [DOI] [PubMed] [Google Scholar]
- 6.Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S, et al. Guidelines on Male Hypogonadism. European Association of Urology. [Last accessed on 2018 Apr 03]. Available from: http://www.uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Hypogonadism2015.pdf .
- 7.Basaria S. Male hypogonadism. Lancet. 2014;383:1250–63. doi: 10.1016/S0140-6736(13)61126-5. [DOI] [PubMed] [Google Scholar]
- 8.Zarotsky V, Huang MY, Carman W, Morgentaler A, Singhal PK, et al. Systematic literature review of the risk factors, comorbidities and consequences of hypogonadism in men. Andrology. 2014;2:819–34. doi: 10.1111/andr.274. [DOI] [PubMed] [Google Scholar]
- 9.Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16:581–606. doi: 10.1111/obr.12282. [DOI] [PubMed] [Google Scholar]
- 10.Lamm S, Chidakel A, Bansal R. Obesity and hypogonadism. Urol Clin North Am. 2016;43:239–45. doi: 10.1016/j.ucl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–8. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 12.Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism. 2013;62:457–78. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Baird DT, Horton R, Longcope C, Tait JF. Steroid dynamics under steady-state conditions. Recent Prog Horm Res. 1969;25:611–64. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 15.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European male ageing study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 16.Dhindsa S, Batra M, Kuhadiya N, Dandona P. Oestradiol concentrations are not elevated in obesity-associated hypogonadotrophic hypogonadism. Clin Endocrinol. 2014;80:464. doi: 10.1111/cen.12236. [DOI] [PubMed] [Google Scholar]
- 17.Aarts E, van Wageningen B, Loves S, Janssen I, Berends F, et al. Gonadal status and outcome of bariatric surgery in obese men. Clin Endocrinol. 2014;81:378–86. doi: 10.1111/cen.12366. [DOI] [PubMed] [Google Scholar]
- 18.Pellitero S, Olaizola I, Alastrue A, Martínez E, Granada ML, et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg. 2012;22:1835–42. doi: 10.1007/s11695-012-0734-9. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- 20.Tirabassi G, delli Muti N, Buldreghini E, Lenzi A, Balercia G. Central body fat changes in men affected by post-surgical hypogonadotropic hypogonadism undergoing testosterone replacement therapy are modulated by androgen receptor CAG polymorphism. Nutr Metab Cardiovasc Dis. 2014;24:908–13. doi: 10.1016/j.numecd.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Grossmann M, Zajac JD. Androgen deprivation therapy in men with prostate cancer: how should the side effects be monitored and treated? Clin Endocrinol (Oxf) 2011;74:289–93. doi: 10.1111/j.1365-2265.2010.03939.x. [DOI] [PubMed] [Google Scholar]
- 22.Abate N, Haffner SM, Garg A, Peshock RM, Grundy SM. Sex steroid hormones, upper body obesity and insulin resistance. J Clin Endocrinol Metab. 2002;87:4522–7. doi: 10.1210/jc.2002-020567. [DOI] [PubMed] [Google Scholar]
- 23.Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE family study. J Clin Endocrinol Metab. 2000;85:1026–31. doi: 10.1210/jcem.85.3.6427. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22(5 Suppl):110–6. [PubMed] [Google Scholar]
- 25.Garaulet M, Perex-Llamas F, Fuente T, Zamora S, Tebar FJ. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol. 2000;143:657–66. doi: 10.1530/eje.0.1430657. [DOI] [PubMed] [Google Scholar]
- 26.Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi GM, et al. The effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care. 2007;30:1860–1. doi: 10.2337/dc07-0337. [DOI] [PubMed] [Google Scholar]
- 27.Armellini F, Zamboni M, Bosello O. Hormones and body composition in humans: clinical studies. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S18–21. doi: 10.1038/sj.ijo.0801270. [DOI] [PubMed] [Google Scholar]
- 28.Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 29.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24:485–91. doi: 10.1038/sj.ijo.0801183. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab. 2007;92:2696–705. doi: 10.1210/jc.2006-1847. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Lee H, McGovern F, Fallon MA, Goode M, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–94. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–83. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 2010;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 34.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 35.Yuki A, Otsuka R, Kozakai R, Kitamura I, Okura T, et al. Relationship between low free testosterone levels and loss of muscle mass. Sci Rep. 2013;3:1818. doi: 10.1038/srep01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou SH, Mantzoros C. 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol. 2014;223:T49–62. doi: 10.1530/JOE-14-0245. [DOI] [PubMed] [Google Scholar]
- 37.Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF. The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations. Diabetes. 1996;45:992–4. doi: 10.2337/diab.45.7.992. [DOI] [PubMed] [Google Scholar]
- 38.Considine RV, Caro JF. Leptin in humans: current progress and future directions. Clin Chem. 1996;42(6 Pt 1):843–4. [PubMed] [Google Scholar]
- 39.Villanueva EC, Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. 2008;32(Suppl 7):S8–12. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, et al. Leptin's role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty GK, Matarese G, Magkos F, Moon HS, Liu X, et al. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol. 2011;165:249–54. doi: 10.1530/EJE-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–80. doi: 10.1210/jcem.84.10.6082. [DOI] [PubMed] [Google Scholar]
- 43.Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update. 2011;17:667–83. doi: 10.1093/humupd/dmr017. [DOI] [PubMed] [Google Scholar]
- 44.Ramírez S, Claret M. Hypothalamic ER stress: a bridge between leptin resistance and obesity. FEBS Lett. 2015;589:1678–87. doi: 10.1016/j.febslet.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–7. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 46.Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf) 2013;78:321–9. doi: 10.1111/cen.12086. [DOI] [PubMed] [Google Scholar]
- 47.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 48.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 49.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossmann M, Hoermann R, Wittert G, Yeap BB. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Clin Endocrinol (Oxf) 2015;83:344–51. doi: 10.1111/cen.12664. [DOI] [PubMed] [Google Scholar]
- 51.Guay A, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med. 2007;4(4 Pt 1):1046–55. doi: 10.1111/j.1743-6109.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 52.Amin R, Pathan F, Aktaruzzaman A, Kabir N. Insulin resistance and hypogonadism. Delta Med Col J. 2013;1:3–7. [Google Scholar]
- 53.Zheng R, Cao L, Cao W, Chu X, Hu Y, et al. Risk factors for hypogonadism in male patients with type 2 diabetes. J Diabetes Res 2016. 2016:5162167. doi: 10.1155/2016/5162167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebrahimi F, Christ-Crain M. Metabolic syndrome and hypogonadism-two peas in a pod. Swiss Med Wkly. 2016;146:w14283. doi: 10.4414/smw.2016.14283. [DOI] [PubMed] [Google Scholar]
- 55.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39:82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45:1211–9. doi: 10.1210/jcem-45-6-1211. [DOI] [PubMed] [Google Scholar]
- 57.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beccutia G, Pannaina S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–12. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittert G. The relationship between sleep disorders and testosterone. Curr Opin Endocrinol Diabetes Obes. 2014;21:239–43. doi: 10.1097/MED.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 60.Cooper LA, Page ST, Amory JK, Anawalt BD, Matsumoto AM. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing-implications for the interpretation of total testosterone measurements. Clin Endocrinol (Oxf) 2015;83:828–33. doi: 10.1111/cen.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26:376–83. doi: 10.1016/j.tem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230:R13–25. doi: 10.1530/JOE-16-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, et al. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66:103–9. [PubMed] [Google Scholar]
- 64.Calderón B, Galdón A, Calañas A, Peromingo R, Galindo J, et al. Effects of bariatric surgery on male obesity-associated secondary hypogonadism: comparison of laparoscopic gastric bypass with restrictive procedures. Obes Surg. 2014;24:1686–92. doi: 10.1007/s11695-014-1233-y. [DOI] [PubMed] [Google Scholar]
- 65.Calderón B, Gómez-Martín JM, Vega-Piñero B, Martín-Hidalgo A, Galindo J, et al. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2016;4:62–7. doi: 10.1111/andr.12135. [DOI] [PubMed] [Google Scholar]
- 66.Caldas AD, Porto AL, Motta LD, Casulari LA. Relationship between insulin and hypogonadism in men with metabolic syndrome. Arq Bras Endocrinol Metabol. 2009;53:1005–11. doi: 10.1590/s0004-27302009000800015. [DOI] [PubMed] [Google Scholar]
- 67.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 69.Samavat J, Facchiano E, Lucchese M, Forti G, Mannucci E, et al. Hypogonadism as an additional indication for bariatric surgery in male morbid obesity? Eur J Endocrinol. 2014;171:555–60. doi: 10.1530/EJE-14-0596. [DOI] [PubMed] [Google Scholar]
- 70.Luconi M, Samavat J, Seghieri G, Iannuzzi G, Lucchese M, et al. Determinants of testosterone recovery after bariatric surgery: is it only a matter of reduction of body mass index? Fertil Steril. 2013;99:1872–9. doi: 10.1016/j.fertnstert.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 71.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 72.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14:153. doi: 10.1186/s12916-016-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Francomano D, Ilacqua A, Bruzziches R, Lenzi A, Aversa A. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology. 2014;83:167–73. doi: 10.1016/j.urology.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 74.Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol 2014. 2014:527470. doi: 10.1155/2014/527470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3:73–83. doi: 10.1111/cob.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15:1903–26. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 77.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014;8:e339–49. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Winter A, Marte A, Kelly M, Funaro M, Schlegel P, et al. Predictors of poor response to transdermal testosterone therapy in men with metabolic syndrome. J Urol. 2014;191(Suppl 4):e528. [Google Scholar]
- 79.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vigen R, O’Donnell CI, Baron A, Grunwald GK, Maddox TM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 81.FDA Briefing Document. Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) 2016. [Last accessed on 2017 Dec 12]. Available from: https://www.fda. gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/reproductivehealthdrugsadvisorycommittee/ucm530330.pdf .
- 82.FDA. Advisory Committee Industry Briefing Document. Testosterone Therapy. Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. 2014. [Last accessed on 2017 Dec 16]. Available from: https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Pink%20Sheet/76/36/Testosterone%20replacement%20therapy%20FDA%20background%20documents%20for%20Sept%2017%20AC%20meeting.pdf .
- 83.European Medicines Agency. No Consistent Evidence of an Increased Risk of Heart Problems with Testosterone Medicines. 2014. [Last accessed on 2017 Dec 20]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/11/WC500177618.pdf .
- 84.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–51. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 85.Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacol. 2014;48:1138–44. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes, Diabetes Obes Metab. 2005;7:211–5. doi: 10.1111/j.1463-1326.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 87.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol. 2008;158:741–7. doi: 10.1530/EJE-07-0663. [DOI] [PubMed] [Google Scholar]
- 88.Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, et al. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37:2098–107. doi: 10.2337/dc13-2845. [DOI] [PubMed] [Google Scholar]
- 89.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 90.Kraemer WJ, Volek JS, Clark KL, Gordon SE, Puhl SM, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc. 1999;31:1320–9. doi: 10.1097/00005768-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 91.Mingrone G, Greco AV, Giancaterini A, Scarfone A, Castagneto M, et al. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis. 2002;161:455–62. doi: 10.1016/s0021-9150(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 92.Reis LO, Favaro WJ, Barreiro GC, de Oliveira LC, Chaim EA, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33:736–44. doi: 10.1111/j.1365-2605.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 93.Armamento-Villareal R, Aguirre LE, Qualls C, Villareal DT. Effect of lifestyle intervention on the hormonal profile of frail, obese older men. J Nutr Health Aging. 2016;20:334–40. doi: 10.1007/s12603-016-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


