Abstract
Zika virus (ZIKV) outbreaks pose a massive public health threat in several countries. We have developed an in vivo model to investigate the host-ZIKV interaction in Drosophila. We have found that a strain of ZIKV replicates in wild-type flies without reducing their survival ability. We have shown that ZIKV infection triggers RNA interference, and that mutating Dicer-2, results in enhanced ZIKV load and increased susceptibility to ZIKV infection. Using a flavivirus-specific antibody, we have found that ZIKV is localized in the gut and fat body cells of the infected wild-type flies and results in their perturbed homeostasis. In addition, Dicer-2 mutants display severely reduced insulin activity, which could contribute towards the increased mortality of these flies. Our work establishes the suitability of Drosophila as the model system to study host-ZIKV dynamics, which is expected to greatly advance our understanding of the molecular and physiological processes that determine the outcome of this disease.
Introduction
Zika virus (ZIKV) is a hitherto understudied member of the Flaviviridae, a viral family that also includes yellow fever, West Nile virus (WNV) and Dengue virus (DENV) (1). In recent years, due to the wide geographical distribution of the mosquito vector, ZIKV suddenly expanded its range dramatically and severe outbreaks appeared in the Americas and other parts of the world (2). Being a vector-borne virus, ZIKV-induced outbreaks are difficult to control. As vector control is the only viable alternative for alleviating the disease, a thorough understanding of host-ZIKV interaction is critical. Thus, there is an urgent need to develop an in vivo model for identifying and characterizing the number and types of molecular components that directly or indirectly participate in the host immune response against ZIKV. Since host innate immune responses are evolutionary conserved across many phyla (3), investigating the effect of ZIKV infection on the immune signaling and function of animal models will be particularly insightful because it could potentially lead to the identification of anti-ZIKV immune mechanisms in humans.
The use of the fruit fly Drosophila melanogaster has led to significant advances in the characterization of the molecular events leading to the activation of immune responses against infectious microorganisms, including viral pathogens (4, 5). Apart from those viruses that naturally infect Drosophila (6), previous work indicates that the fly is also a suitable model for dissecting host interactions with human pathogenic viruses including ZIKV (7, 8). Drosophila for instance was instrumental in deciphering antiviral immune mechanisms against Sindbis virus (SINV), Vesicular stomatitis virus (VSV) and WNV and in particular the importance of RNA interference (RNAi) against these viruses (9–11). Apart from unraveling the cellular and molecular basis of antiviral immunity, Drosophila is also a suitable model for understanding host-virus interaction and the associated pathology (12, 13). Few compelling evidences further indicate that Drosophila can be a reliable model to analyze virus tropism (14). The insect-specific viruses Drosophila C Virus (DCV) and Flock house virus (FHV) for example, have been shown to infect the fat body, digestive tract, trachea and egg chamber, which results in infection-induced pathologies (12, 15, 16). These findings are of paramount importance to elucidate the physiological mechanisms that regulate the complex interactions between insects and viral pathogens. Comparative genomics studies have addressed the conservation between Drosophila and mosquitoes and shown that Drosophila developmental genes are largely conserved in three vector mosquito species (17). Deciphering the complete genome sequences of the mosquito vectors Anopheles gambiae and A. aegypti has enabled the identification and comparison of antiviral immune genes like Dicer-2 and Ago-2 (18–20). In the context of host pathology, forward genetic screens in Drosophila have identified genes regulating Plasmodium growth in A. gambiae (21). Therefore, molecular and functional characterization of innate immune factors acting against ZIKV and the consequent ZIKV-induced pathogenesis will potentially lead to a comprehensive understanding of the host-ZIKV interactions, which in turn will potentially lead to novel strategies for blocking ZIKV transmission.
In the absence of a classical adaptive immune system, Drosophila relies on innate defenses for immunity against viral infections. For instance, the Toll, Immune deficiency (Imd) and Janus kinase/signal transduction and activators of transcription (JAK/STAT) signaling pathways in Drosophila participate in antiviral responses; however, each of those pathways confers antiviral effects against certain viruses (22–24). The central antiviral immune response in the fly involves the RNAi mechanism, a conserved sequence-specific nucleic-acid-based immune defense that is induced by double stranded RNA (dsRNA). In Drosophila, RNAi involves the ribonuclease Dicer-2 that recognizes and cleaves dsRNA to generate viral small interfering RNAs (siRNAs) (25, 26). These siRNAs are loaded onto Argonaute-2 (Ago-2) and guide the RNA-induced silencing complex (RISC) to complementary RNAs in the cell, leading to their sequence-specific degradation (27, 28). RNAi mediates a strong antiviral response against a range of viruses in Drosophila (5, 7, 23, 29–32). Upon infection with RNA viruses, Drosophila Dicer-2 and Ago-2 null mutants display a substantial increase in viral replication and rapid decrease in survival (11, 30–33). The major importance of RNAi in host antiviral defense is further reinforced by the identification of viral proteins that act in vivo as suppressors of this mechanism (31, 33–35).
We have developed an in vivo model for studying the molecular basis of the host immune response to ZIKV infection and the occurring pathophysiological defects. We show that Drosophila flies are able to support ZIKV replication, which leads to the activation of stress-induced genes Turandot and Diedel and induction of the RNAi pathway. We find that the two central mediators in RNAi, Ago-2 and Dicer-2 have differential function in the context of ZIKV infection. While Ago-2 is dispensable, Dicer-2 regulates ZIKV replication and renders resistance to infection. In addition, we find that ZIKV exhibits tissue tropism by infecting the fat body, crop and gut of the adult fly. The tissue-specific infiltration of ZIKV results in local pathologies marked by perturbed homeostasis of the gut and the fat body lipid droplets. Furthermore, we find that the ZIKV mediated perturbed homeostasis is aggravated in Dicer-2 mutants along with severely reduced insulin signaling resulting in significantly increased sensitivity to the infection. These are important findings because they demonstrate that using the Drosophila-ZIKV model enables the identification of host factors with anti-ZIKV immune activity and allows the characterization of tissue-specific effects that occur in the host during the infection process.
Materials and methods
Fly stocks
The following fly lines were used: w1118 (wild-type and background control), Ago-2414, the null allele Ago-2321 in trans to Df[BSC558](36) , Dicer-2R461X (37), Dicer-2L811fsX (37) compared to rescue with a Dicer-2 genomic transgene (BL33053), esg-Gal4 (NP5130, Drosophila Genomics Resource Center). Flies were reared on standard medium at 25°C. All fly lines were tested for Wolbachia infection and cured whenever necessary (38).
Zika virus stock preparation
Vero cells (ATCC, Manassas, VA, USA) were grown in EMEM (ATCC) supplemented with 10% fetal bovine serum (Gemini Bio-Products), penicillin/streptomycin (VWR), gentamicin (Sigma Aldrich), and amphotericin B (Quality Biological). ZIKV strain MR766 was added to Vero cells at MOI of 0.1 and incubated for 4–6 days. The supernatants were centrifuged at 1,500 rpm for 5 min and filtered (0.45 μm) before being concentrated via SnakeSkin dialysis tubing 3.5K MWCO (Thermo Scientific) in polyethylene glycol 8000 powder (Alfa Aesar) until all liquid was drawn out. The tubing was then placed in PBS overnight at 4°C. The reconstituted ZIKV was then aliquoted and stored at −80°C. ZIKV titers were determined using plaque assays on Vero cells as previously described (39). Briefly, ZIKV stocks were serially diluted and adsorbed to confluent monolayers of Vero cells. After 3 hours, the inoculum was removed, and cells were overlaid with semisolid medium containing 1% carboxymethyl cellulose (Sigma Aldrich). Cells were further incubated for 5 days, fixed with 4% paraformaldehyde (Electron Microscopy Sciences), and stained with 0.5% aqueous crystal violet solution (Sigma Aldrich) for plaque visualization. Titers were expressed as plaque forming units (PFU) per milliliter.
Fly infection
To avoid heterogeneity in ZIKV load, only adult female flies were used in these experiments. Injections were performed by anesthetizing the flies with CO2. For each experiment, two to five-day old adult female flies were injected with ZIKV suspensions in PBS (pH 7.5) using a nanoinjector (Nanoject III, Drummond Scientific). ZIKV stocks were prepared in PBS, pH 7.5. Heat-inactivated ZIKV stocks were generated by exposing the virus inoculum to 56oC for 1 hour in a water bath. Heat-inactivated or live ZIKV solution (11,000 PFU/fly) (100 nl) was injected into the thorax of flies and injection of the same volume of PBS acted as negative control. Injected flies were then maintained at 25°C and transferred to fresh vials every third day throughout the experiment. They were collected at the indicated time points and directly processed for RNA analysis. Flies that died right after injection were not considered for further analysis. Statistical analyses of the differences in ZIKV titers between fly strains and experimental conditions were conducted with data from three independent experiments.
RNA analysis
Total RNA was extracted from 10 adult female flies, using Trizol according to manufacturer’s protocol. Total RNA (500 ng - 1 μg) was used to synthesize cDNA using the High Capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative RT-PCR (qRT-PCR) experiments were performed with technical triplicates and gene-specific primers (Table I) using iQ SYBR Green Supermix (Bio-Rad) and a CFX96 Real-Time PCR detection system (Bio-Rad). Quantification was performed from three biological replicates for both test and control treatments. ZIKV copy numbers were estimated by using previously described primers (ZIKV F9027; ZIKV R9197c) (40). Fold changes were calculated with the delta delta Ct method using RpL32 as a housekeeping gene. Absolute copy numbers of ZIKV were extrapolated by a standard curve constructed out of 6-point dilution series of viral cDNA.
Table I.
List of the gene-specific primers used in the study
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| Rpl32 | gatgaccatccgcccagca | cggaccgacagctgcttggc |
| NS5 | ccttggattcttgaacgagga | agagcttcattctccagatcaa |
| Drosomycin | gacttgttcgccctcttcg | cttgcacacacgacgacag |
| Diptericin | gctgcgcaatcgcttctact | tggtggagttgggcttcatg |
| Cecropin A1 | tcttcgttttcgtcgctctc | cttgttgagcgattcccagt |
| Metchnikowin | tcttggagcgatttttctgg | aataaattggacccggtcttg |
| Tep1 | agtcccataaaggccgactga | cacctgcatcaaagccatattg |
| Vago | tgcaactctgggaggatagc | aattgccctgcgtcagttt |
| Diedel | gtgcgtgcaatcgaaaacta | cgtactgctggttcctcctc |
| Turandot A | gaagatcgtgaggctgacaac | gtcctgggcgtttttgataa |
| Turandot M | gctgggaaaggtaaatgctg | aggcgctgtttttctgtgac |
| Argonaute-2 | ccggaagtgactgtgacagatcg | cctccacgcactgcattgctcg |
| Dicer-2 | gtatggcgatagtgtgactgcgac | gcagcttgttccgcagcaatatagc |
| Listericin | gagttagggccgctcgtc | cctcctccacagcaatatcct |
| Vir-1 | gatcccaattttcccatcaa | gattacagctgggtgcacaa |
| 4E-BP | tcctggaggcaccaaacttatc | ggagccacggagattcttca |
| Impl2 | aagagccgtggacctggta | ttggtgaacttgagccagtcg |
| Upd-3 | gccgcgatataaagatacaaagata | actttgcttttgtaacgctgttagt |
| Lsd-1 | tgagccggcgacagcaacagt | cgtaggcggccgaaatggtg |
| Lsd-2 | agtgtactagccgatacg | tctgactcccggatct |
| Lipin | gggcatgaatgaaatcgag | tcaccaccttgtcgttgtg |
| Mdy | cgttctccaatatggacgtg | aaaagcagagccagcaaag |
| Spitz | cgcccaagaatgaaagagag | aggtatgctgctggtggaac |
| Vein | tcacacatttagtggtggaag | ttgtgatgcttgaattggtaa |
| Keren | cgtgtttggcaacaacaagt | tgtggcaatgcagtttaagg |
Fly survival
For each fly strain, three groups of 20 female flies were injected with ZIKV and one group was injected with PBS for control. Following injection, flies were maintained at a constant temperature of 25°C with a 12 h light/dark cycle and mortality was recorded daily. Fly deaths occurring within one day of injection were attributed to injury and they were not included in the results. Log-rank (Mantel-Cox) was used to analyze the survival curves.
Nile Red staining of neutral lipids
Fat body tissues from w1118 untreated, PBS or ZIKV injected adult female flies were dissected and fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Fixed tissues were then rinsed twice in PBS, incubated for 30 min in 1:1000 dilution of 0.05% Nile Red prepared in 1 mg/ml of methanol, and finally mounted with Vectashield (Vector labs, H1200). Images were taken using Zeiss LSM 510 confocal microscope. To quantify LD size, the area of the three largest LDs per cell from 25 fat body cells was measured using ImageJ. This experiment was repeated three times with three samples for each experiment.
Immunostaining and antibodies
Anti-Prospero antibody (1:30), Anti-PH3 (1:500) and Anti-4G2 (1:100) antibody were purchased from DSHB and Abcam, respectively. Secondary antibodies included AlexaFluor 488, 555 and 633 (Invitrogen). DAPI was used for nuclear marking (Invitrogen). Phalloidin-FITC and Phalloidin TRITC were used for actin staining (Sigma). Standard procedures were followed for immunostaining. Briefly, fly tissues were dissected and fixed in PBS containing 4% formaldehyde for 30 min. Following double rinsing in PBS containing 0.1% Triton X-100, the samples were incubated overnight at 4°C with the primary antibody. The samples were then blocked with 1% BSA for 2 hours followed by 2-hour incubation with secondary antibody at room temperature. Finally, the samples were mounted with Vectashield medium (Vector Laboratories). Images were acquired with Zeiss LSM 510 confocal microscope and processed using Adobe Photoshop CS6. Fluorescence intensity plots were generated from a single slice of 40X confocal images using ImageJ software. A line segment was drawn across the two points of expression and the plot profile function was used to generate a fluorescence intensity plot for the desired channel. The raw data file generated by these plot profiles were analyzed in Excel with each plot value corresponding to the peak value creating intensity plots.
Fly climbing ability
Climbing assays were carried out as previously described (41, 42). Groups of 10 adult female flies were transferred into empty vials and incubated for 1 hour at room temperature for acclimatization. The flies were gently tapped down to the bottom of the vials and then the number of flies reaching an 8 cm mark was counted after 18 sec of climbing. The experiment was repeated three times.
Statistical analysis
An unpaired two-tailed Student’s t-test was used for statistical analysis of data using GraphPad Prism (GraphPad Software). Log-rank (Mantel-Cox) within GraphPad Prism program was used to analyze the survival curves. The p values < 0.05 were considered statistically significant.
Results
Drosophila flies support ZIKV replication without succumbing to the infection
We first investigated whether Drosophila flies can support replication of ZIKV. For this, we injected 11,000 PFU/fly of the strain MR766 into the thorax of w1118 adult flies and estimated viral copy numbers using ZIKV gene-specific primers. ZIKV belongs to the Flaviviridae family of viruses with an 11 kb single-stranded positive-stranded RNA genome (43, 44). The positive- stranded RNA serves as the mRNA for translation of a large polyprotein, which in turn codes for three structural and seven non-structural (NS) proteins (44). Among the latter, NS5 is the largest and most crucial NS protein in the viral replication complex because it performs both methyltransferase and polymerase functions, and therefore it forms an important therapeutic target for interfering with viral RNA production (45, 46). We estimated ZIKV copy numbers in the infected flies compared to PBS control treated flies at three time-points post infection using primer sequences against NS5, as previously described (40). We found strongly elevated levels of ZIKV copies at 8 days post infection (dpi) (200-fold increase compared to 4 dpi), which declined subsequently at 12 dpi (Fig. 1A) and did not change at 20 dpi (Fig. 1A). To test whether infection of Drosophila with ZIKV affects the survival of wild-type flies, we injected w1118 flies with MR766 and estimated survival rates over time. We found that infection with this ZIKV strain failed to reduce fly survival, which was similar to the survival of PBS injected controls (Fig. 1B). These results indicate that although ZIKV does not kill wild-type Drosophila through direct delivery into the hemolymph, it can replicate efficiently in the infected flies.
Figure 1. ZIKV replicates in Drosophila adult flies and triggers RNAi and the expression of Turandot genes.
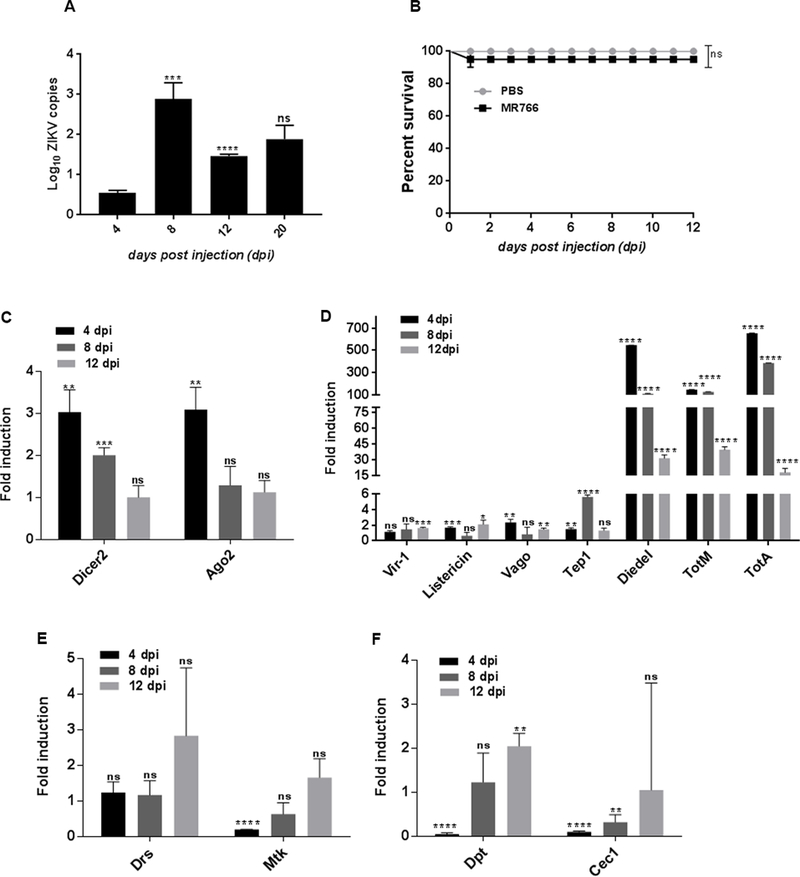
(A) Drosophila female w1118 adult flies were injected with ZIKV (African strain MR766; 110 million PFUs/ml) and ZIKV load was estimated at several days post injection (dpi). Absolute ZIKV copy numbers were quantified via qRT-PCR. (B) Survival of w1118 adult flies after intrathoracic injection with ZIKV was monitored at 24-hour intervals for 12 days. Injections with PBS served as negative controls. Grey and black lines depicting the survival of PBS and ZIKV-injected flies are superimposed, but for clarity, they are shown in parallel. Data represent the mean ± SD of three biological replicates of at least 20 female flies. Log-rank (Mantel-Cox) was used for statistical analysis; ns denotes no significant differences between experimental treatments. (C-F) ZIKV-infected flies were processed for RNA analysis and gene expression levels were determined by quantitative RT-PCR at 4, 8 and 12dpi. (C) Transcript levels of RNAi machinery, Ago-2 and Dicer-2. (D) Transcript levels of JAK/STAT gene targets including the Turandot (Tot) genes TotA and TotM, the antiviral cytokine Diedel, the thioester-containing protein Tep1, and the antiviral STAT regulated target genes Vago, Vir-1 and Listericin. (E) Transcript levels of Toll signaling gene readouts Drosomycin (Drs) and Metchnikowin (Mtk) throughout the stages of infection. (F) Transcript levels of Imd signaling gene readouts Diptericin (Dpt) and Cecropin (Cec) in flies responding to ZIKV infection. All data were normalized to the housekeeping gene RpL32 shown relative to wild-type flies injected with PBS (sterile control). Three independent experiments were carried out with 10 flies per sample in triplicates (*p=0.0215, **p=0.002, ***p=0.0006, ****p<0.0001, 0.0002). Bars represent the mean ± SD and ns denotes no significant differences between experimental treatments. Statistical analysis was performed using Student’s t-test.
Adult flies injected with ZIKV activate RNAi and stress-induced Turandot genes
Drosophila lacks an adaptive immune system and solely relies on innate immune mechanisms (4). At the molecular level, sensing of viral infection results in induction of signaling pathways and the production of antiviral effectors. To examine whether ZIKV infection stimulates innate immunity in Drosophila, we injected w1118 adult flies with the MR766 strain and estimated the time-course expression of genes regulated by immune signaling pathways in the fly. We analyzed the activation of the RNAi pathway that forms a potent antiviral defense in Drosophila (5, 31, 32), the JAK/STAT pathway that is required but not sufficient for the inducible antiviral response in the fly (47), and the Toll and Imd pathways that regulate the activation of the nuclear factor κB (NF-κB) transcription factors DIF/Dorsal and Relish, respectively (24, 48, 49). We found that Dicer-2, encoding the sole siRNA-producing Dicer-2 protein that acts as a pattern recognition receptor and as a component of the RISC in Drosophila (29), was significantly upregulated at 4 and 8 dpi with ZIKV, whereas Ago-2, encoding the Argonaute protein in the RISC (31), was significantly induced at 4 dpi only compared to control injections with PBS (Fig. 1C). JAK/STAT is a mammalian antiviral signaling pathway and is a crucial component of the interferon response (50). While insects do not possess interferon activity, JAK/STAT has been instrumental in providing immunity against several viruses in Drosophila and mosquitoes (22, 51, 52). In corroboration with previous findings, we found a strong (up to 500-fold) upregulation of the antiviral cytokine Diedel and the JAK/STAT regulated genes Turandot (Tot), TotA and TotM in ZIKV injected flies (53, 54). We also found a moderate upregulation of Thioester-containing protein 1 (Tep1) at 8 dpi (24), and slight upregulation of the STAT regulated antiviral genes Vago, Vir-1 and Listericin mainly at 12 dpi (22, 55, 56) (Fig. 1D). In case of bacterial challenge, Tot genes are regulated through JAK/STAT signaling (57). To examine whether the ZIKV-induced Tot genes are also regulated by JAK/STAT signaling, we estimated TotA and TotM transcript levels in hop mutants infected with ZIKV. Unlike bacterial infection, mutating hop did not affect TotA and TotM induction upon ZIKV infection (Fig. S1A). We also found no significant upregulation of Upd-3 (Fig. S1B), the ligand necessary for JAK/STAT mediated TotA activation (57), further emphasizing on JAK/STAT independent regulation of Tot genes in case of ZIKV infection. Also, there was no significant change in the mRNA levels of the Toll regulated antimicrobial peptide (AMP) gene Drosomycin and a slight decrease of Metchnikowin at 4 dpi (Fig. 1E); however, we noticed reduced transcript levels of the Imd regulated AMP genes Diptericin at 4 dpi and Cecropin-A1 at 4 and 8 dpi, and increase of Diptericin at 12 dpi (4) (Fig. 1F). We then asked whether injection with inactivated ZIKV alters immune signaling regulation in Drosophila. Inactivated viruses are incapable of replication, but are still capable of binding cells and performing endocytosis and entry to the cells (35, 58). Similar to the effect of live ZIKV, we found robust induction of RNAi and upregulation of TotM in flies injected with the heat-inactivated ZIKV (Fig. S1C and D). These results demonstrate that Drosophila can sense the presence of different ZIKV components and respond by activating certain antiviral immune mechanisms controlled by RNAi and the stress induced Tot genes. Interestingly, the latter are regulated independently of JAK/STAT signaling.
Dicer-2 controls ZIKV replication and resistance to the infection
RNAi plays a major role in the insect immune response to certain viral infections (5). To test whether activation of RNAi confers resistance to ZIKV infection in Drosophila, we estimated the ZIKV load and survival ability of flies carrying loss-of-function mutations in Ago-2 or Dicer-2. Ago-2 plays a pivotal immune role against viral infections in the fly (11, 31, 33). Time-course infection revealed significant increase in ZIKV load at the early time-point (4 dpi) in Ago-2 mutant flies (Ago-2414) (Fig. 2A). However, ZIKV load in Ago-2 mutants for the later time-points remained unaffected compared to the w1118 background controls (Fig. 2A). In line with the ZIKV load results, Ago-2 mutant flies were able to survive infection with ZIKV at similar levels compared to their background controls (Fig. 2B). We further confirmed the survival ability of ZIKV infected Ago-2414 in another trans-heterozygous null allelic combination Ago-2321/Df (Fig. S2A). In contrast, Dicer-2 mutants (Dicer-2L811fsX) contained higher ZIKV load at both 4 and 8 dpi. ZIKV load increased up to 5 and 11 times, respectively (Fig. 2C). Dicer-2 mutant flies infected with ZIKV succumbed at a much faster rate with 50% of the infected mutants dying after 6.5 days post injection compared to 100% survival of their background controls (Fig. 2D). The survival defect of ZIKV infected Dicer-2L811fsX null mutant flies was also validated in another null mutant of Dicer-2 (Dicer-2R416X) (Fig. S2B). Similar to Dicer-2L811fsX mutants, ZIKV infected Dicer-2R416X flies succumbed at a faster rate as compared to the wild-type (w1118) flies (Fig. S2B). We further observed that the survival defect of ZIKV infected Dicer-2 (Dicer-2L811fsX) mutant flies was rescued by the introduction of a genomic copy of Dicer-2 (Fig. S2C). Of note, WNV, an arbovirus of the Flaviviridae family that can readily infect Drosophila, shows an opposite replication pattern in RNAi mutants, as it amplifies at higher titers in Ago-2 (but not in Dicer-2) deficient flies than in controls (11). It has also been shown that Dicer-2 can regulate Toll signaling and the expression of Vago, a secreted protein with antiviral activity (55). However, unlike previous studies, here we found increased expression of Vago in Dicer-2 mutants infected with ZIKV. Vago mRNA levels were 1.5-fold higher in ZIKV infected Dicer-2 mutant flies compared to the background controls (Fig. S2D). These findings indicate that Dicer-2 is crucial in regulating ZIKV replication, while Ago-2 regulates the replication at a modest level. In particular, Ago-2 confers a tolerant phenotype during the early stages of ZIKV infection, while Dicer-2 is important for conferring resistance to ZIKV.
Figure 2. Inactivating Dicer-2 results in enhanced ZIKV load and compromised fly survival.
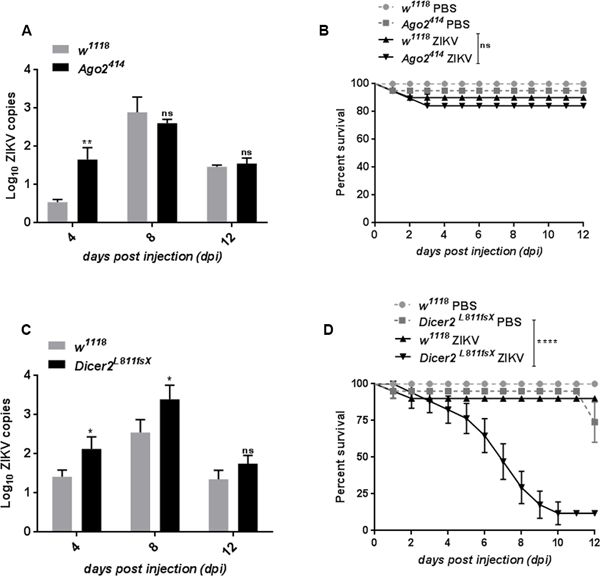
ZIKV load and survival in Ago2414 and Dicer2L811fsX loss-of-function mutant flies and w1118 background controls. (A) Quantitative RT-PCR analysis depicting ZIKV load in Ago-2 mutant flies compared to the w1118 controls. (B) Survival analysis of w1118 and Ago-2 mutant flies injected with PBS and ZIKV. (C) ZIKV load in Dicer-2 mutant compared to the wild-type control flies. (D) Survival analysis of w1118 and Dicer-2 mutant flies injected with PBS and ZIKV. All ZIKV load data represent the mean and standard deviation of three independent experiments in triplicates (*p=0.05, **p=0.04). For survival experiments, three groups of 20 female flies from each fly strain were injected with ZIKV and fly survival was monitored at 24-hour intervals and up to 12 dpi. Log-rank (Mantel-Cox) was used for survival data analysis (****p<0.0001; ns denotes no significant differences between experimental treatments).
Characterization of ZIKV tropism reveals replication in the midgut, fat body and crop of the infected flies
Once inside the host, viruses need to propagate for their own survival, and thereby they interact with certain cell types or host tissues, also known as viral tropism, which determines the outcome of infection (59). In Drosophila, there have been compelling evidences depicting the tissue tropism of DCV, Nora virus, FHV, VSV and Bluetongue virus (BTV) in the infected flies. (14). In order to examine ZIKV tissue tropism, we used the flavivirus-specific antibody 4G2, which detects the viral envelope protein (60–62). We noticed strong 4G2 positive signal in the midgut of the ZIKV-infected flies (Fig. 3A), while the midgut of the uninfected flies was devoid of 4G2 expression (Fig. 3A). We also examined 4G2 expression in the midgut of both uninfected controls and ZIKV-infected wild-type flies. We generated fluorescence intensity plots across two linear points of 4G2 expression (yellow line in Fig. 3A) to precisely analyze 4G2 expression. Intensity plot showed two peaks of 4G2 fluorescence corresponding to the yellow line in ZIKV-infected gut tissue (magenta line) (Fig. 3B), while negligible 4G2 intensity was observed for the expression in uninfected gut tissue (green line) (Fig. 3B). Similar to midgut, the crop (digestive organ) of the ZIKV-infected flies also showed numerous 4G2 positive ZIKV particles (Fig. 3C) as compared to the uninfected flies, in which no expression of 4G2 was detected (Fig. 3C). However, unlike DCV infection (12), we were not able to detect any morphological defect in the crop of ZIKV infected flies as compared to the uninfected controls (Fig. 3C). Similar to the midgut, the intensity plot for 4G2 expression in the crop revealed sharp peak for ZIKV-infected crop tissue while diminished 4G2 intensity was found for the uninfected crop (Fig. 3D). Furthermore, closer examination revealed 4G2 positive signal in the primary metabolic/immune organ, the fat body of the infected flies, and no signal was observed in the fat body of uninfected flies (Fig. 3E). Measurement of intensity plots showed two strong peaks corresponding to 4G2 expression in ZIKV-infected fat body while a flat-line expression was observed for 4G2 expression in uninfected fat body tissue (Fig. 3F). We also examined 4G2 expression in the reproductive organ and noticed that similar to the uninfected flies, there was no expression of 4G2 in the egg chambers of the infected flies (Fig. S3). To further validate the expression of 4G2 in these different tissues of the infected wild-type flies, we next estimated ZIKV load in these tissues. Analysis through qRT-PCR revealed elevated level of ZIKV copies in fat body, gut and crop of the infected flies (Fig. 3G). These findings suggest that ZIKV depict a broad tissue tropism in Drosophila by infecting the midgut, crop and fat body of the infected flies.
Figure 3. Midgut, crop and fat body of ZIKV infected flies shows flavivirus-specific 4G2 expression.
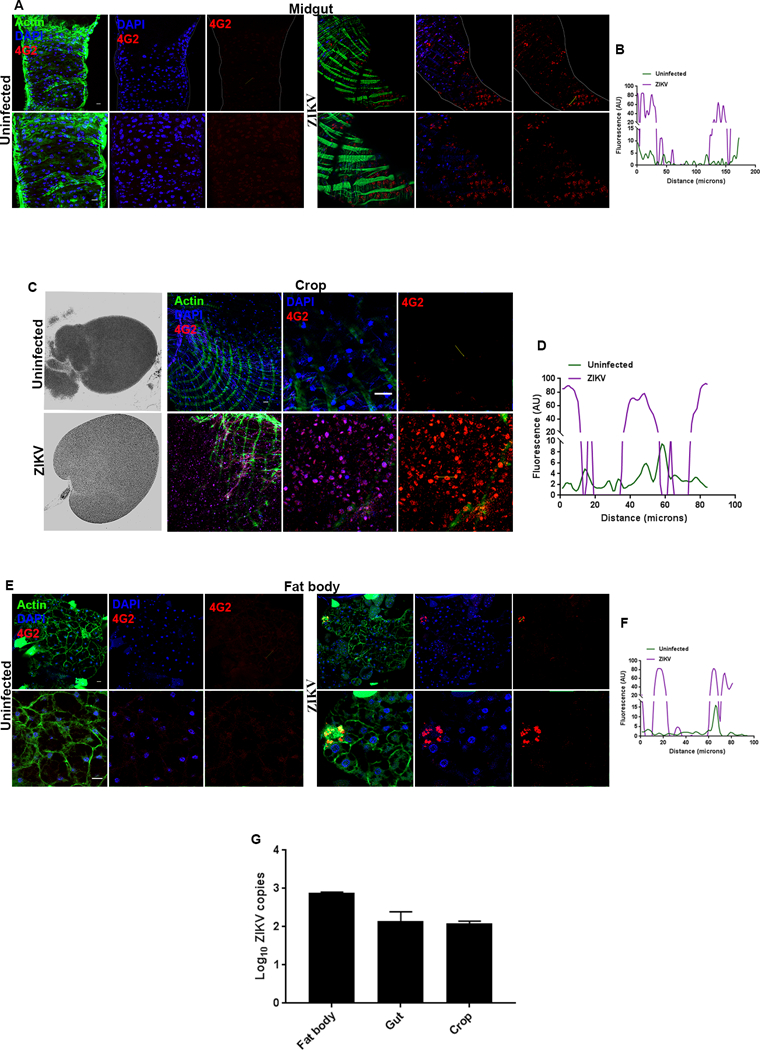
Representative midgut, crop and fat body images from uninfected w1118 adult flies and flies infected with ZIKV at 8 days post injection (8 dpi). The expression of ZIKV was detected through anti-flavivirus antibody, 4G2. (A) The first panel shows dissected midgut from uninfected w1118 flies along with the enlarged images in the lower panel, while the second panel shows dissected midgut from ZIKV infected adult flies. (B) Quantification of the 4G2 fluorescence intensity in midgut in uninfected and ZIKV-infected flies. The intensity plot corresponds to a line connecting two points (marked by yellow line in the images). Fluorescence intensity is represented in pseudocolors where magenta marks the intensity for ZIKV infection and green for uninfected control (AU, arbitrary unit). (C) Expression of 4G2 in the crop (organ in the digestive tract) in uninfected controls and ZIKV-infected adult flies. (D) Quantification of the 4G2 fluorescence intensity in crop from uninfected and ZIKV-infected flies. The intensity plot corresponds to a line connecting two points (marked by yellow line in the images; AU, arbitrary unit). (E) Expression of 4G2 in the fat body tissue from uninfected controls and ZIKV-injected adult flies. (F) Measurement of fluorescence intensity of 4G2 expression in the fat body from uninfected controls and ZIKV-injected flies (AU, arbitrary unit). (G) ZIKV load in the fat body, gut and crop tissue from ZIKV-injected flies (8 dpi). In all images, 4G2 was marked in red, Actin in green and nuclei were marked in blue. Scale bars: 100 microns.
Midgut from ZIKV-infected wild-type and Dicer-2 mutant flies differ in their ability to induce intestinal stem cell proliferation
Characterizing the physiological events occurring during the course of a microbial infection is essential for a better understanding of host-microbe dynamics. Infection with certain microbial pathogens induces pathophysiological responses in Drosophila (63). For instance, adult flies infected with Salmonella typhimurium, Listeria monocytogenes and Mycobacterium marinum demonstrate various infection-induced pathologies (64, 65). However, virus-induced pathologies in Drosophila are still poorly understood (12, 13, 66). In correlation with tissue tropism of ZIKV, we next aimed at investigating whether viral infection of the midgut affects the overall gut homeostasis. Drosophila gut homeostasis is primarily maintained by epithelial renewal, where the stress or infection-induced damage results in enterocyte (EC, the main gut cell type) loss that is compensated by over-proliferation of intestinal stem cells (ISCs)(67). Interestingly, flies compromised in the ability to renew the gut epithelium succumb to infection, demonstrating the importance of ISC-induced epithelium renewal in anti-bacterial immunity (68, 69).
In order to investigate the gut-related pathologies upon ZIKV infection, ZIKV was injected in flies carrying an ISC-specific reporter construct (esgGal4UAS-GFP)(70), where Escargot (Esg) is a transcription factor that maintains stemness in ISCs (71). In the absence of infection, the expression of Escargot was restricted to a few dispersed subsets of ISCs (Fig. 4A). In contrast to the midgut of the uninfected flies, the midgut from ZIKV-infected individuals displayed a strong increase in ISC proliferation marked by enhanced expression of Escargot (Fig. 4A). The increased number of ISCs was further validated by quantification showing that up to 60% of the total midgut cells were comprised of Escargot-positive ISCs (Fig. 4B). In agreement with the enhanced expression of esg-GFP, we noticed significant enrichment of escargot mRNA in the midgut of ZIKV-infected wild-type flies as compared to the uninfected controls (Fig. 4C). In order to understand the causative mechanism for the increased susceptibility, we next examined the midgut-related defects in ZIKV-infected Dicer-2 (Dicer-2L811fsX) mutant flies. We found that unlike wild-type flies, ZIKV infected Dicer-2 mutants showed a significant reduction in the mRNA expression of escargot (Fig. 4C).
Figure 4. ZIKV-infected Dicer-2 mutant flies fail to trigger infection-induced intestinal stem cell proliferation.
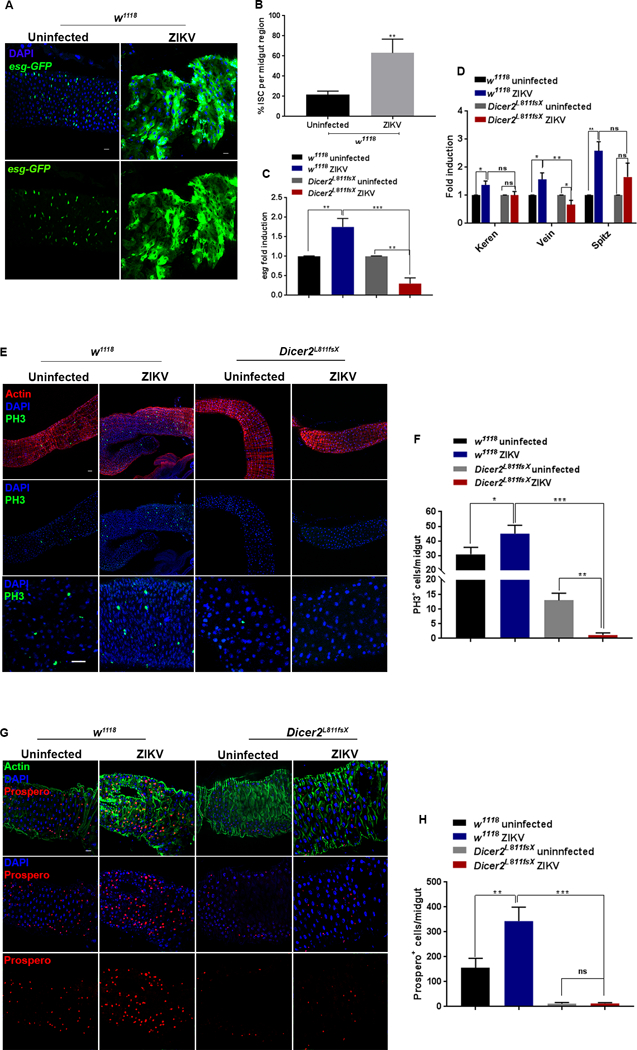
(A) Representative images of gut in ZIKV infected wild-type (w1118) flies at 8 days post injection (8 dpi) and in uninfected controls. Intestinal stem cells (ISC) were marked by the expression of an esgGal4UAS-GFP reporter. (B) Percentage of esg positive cells or ISC per midgut region. (C) Quantitative RT-PCR analysis showing the mRNA level of escargot in the midgut of uninfected and ZIKV-infected wild-type and Dicer-2 mutant flies. (D) Quantitative RT-PCR analysis of genes encoding EGFR signaling ligands, Keren, Vein and Spitz in midgut of the uninfected controls and ZIKV-infected wild-type and Dicer-2 mutant flies. (E) Representative images of midgut in uninfected and ZIKV-infected wild-type (w1118) and Dicer-2 mutant flies. Midgut tissues were stained with anti-PH3 (green) to mark the mitotically active cells. Cytoarchitecture was marked with Actin (red) and nuclei were stained with DAPI (blue). (F) Quantification of PH3-positive cells per midgut tissue in uninfected and ZIKV-infected wild-type and Dicer-2 mutant flies. (G) Proportion of enteroendocrine (EE) cells in the midgut of uninfected and ZIKV-infected wild-type and Dicer-2 mutant flies. EE cells were stained with anti-Prospero antibody (red). Actin was marked in green while nuclei were stained with DAPI (blue). (H) Quantification of Prospero positive cells per midgut tissue. Levels of mRNA were normalized against RpL32 and three independent experiments were performed. In all graphs, bars represent mean ± SD. Statistical analysis was performed using Student’s t-test (*p<0.05, **p < 0.05, ***p<0.05); ns denotes no significant differences between experimental treatments. Scale bars: 100 microns.
We then investigated the signaling pathways regulating ZIKV-induced ISC proliferation. Infection-induced ISC proliferation is regulated by several signaling pathways (67). However, EGFR signaling is the core regulatory mechanism for ISC proliferation. Activation of EGFR signaling is sufficient to induce ISC proliferation while its inactivation in ISCs renders them unable to proliferate upon infection (67). We next analyzed the transcriptional activation of EGFR signaling components in wild-type and Dicer-2 infected flies. qRT-PCR analysis showed that mRNA level of EGFR ligands, Spitz, Keren and Vein were significantly upregulated in the ZIKV-infected wild-type flies as compared to the uninfected individuals (Fig. 4D). However, in case of Dicer-2, although there was a significant reduction in level of Vein, there was no change in expression of Keren and Spitz (Fig. 4D).
For further characterization of ISCs, we next examined the overall rate of proliferation in the midgut cells using antibodies against mitosis marker, phospho-histone 3 (PH3). We found that even in uninfected condition, the midgut of Dicer-2 mutant flies showed a markedly reduced rate of proliferation as compared to the uninfected wild-type flies (Fig. 4E). Quantification analysis further showed that the number of mitotically active PH3-marked cells was reduced 10 times in the midgut of Dicer-2 mutant flies as compared to the uninfected wild-type flies (Fig. 4F). Upon ZIKV infection, the midgut of wild-type flies showed a significantly increased number of mitotic cells, but the midgut of Dicer-2 mutants failed to trigger proliferation and was devoid of any mitotically active PH3-positive cells (Fig. 4E and F).
Mitotically active ISCs divide to generate the enteroendocrine cells (EE) in the Drosophila midgut. Primarily involved in regulating intestinal physiology, their immune role has been recently characterized (71–73). Similar to the rate of proliferation, we noticed a strikingly reduced number of Prosper-marked EE cells in the midgut of uninfected Dicer-2 mutant flies as compared to the uninfected wild-type flies (Fig. 4G and H). Upon ZIKV infection, although there was no difference in the proportion of EE cells between infected and uninfected Dicer-2 mutants, there was significant increase in the number of Prospero-marked EE cells in the midgut of the wild-type flies (Fig. 4G). Quantification analysis revealed that in case of ZIKV-infected wild-type flies, the number of midgut-specific Prospero positive EE cells increased 3 times as compared to the uninfected wild-type controls (Fig. 4H). These findings indicate that the midgut of resistant wild-type flies is naturally more proliferative than the midgut of susceptible Dicer-2 mutant flies. Upon ZIKV infection, the midgut of wild-type cells responds promptly triggering enhanced proliferation while the midgut of Dicer-2 mutant flies refrains from undergoing any proliferation
Fat body from ZIKV-infected flies shows perturbed lipid metabolism with Dicer-2 mutants suffering from lipodystrophy and severely reduced insulin activity
To further characterize the ZIKV induced-pathology in Drosophila, we next examined the effect of ZIKV infection on fat body homeostasis. Being an adipose tissue, fat body acts as the reservoir for lipids, which in turn are stored in specialized organelles, called lipid droplets (LDs). Emerging evidence indicates that except for lipid metabolism, LDs might participate in host immune activities against microbial infections by acting as a source of antibacterial and antiviral proteins (74). Here we found that the fat body from ZIKV-infected w1118 flies consistently displayed accumulation of enlarged lipid droplets (Fig. 5A). Upon ZIKV infection, the size of Nile Red marked fat body LDs increased 3–4-fold as compared to those in uninfected flies (Fig. 5B). Because lipid forms the primary source of energy reserve, we asked whether perturbed lipid homeostasis could contribute to the susceptibility of ZIKV-infected Dicer-2 mutant flies. In contrast to the increased size of LDs in ZIKV-infected w1118 flies, ZIKV-infected Dicer-2 mutants displayed a dispersed set of LDs with a strikingly diminished size (Fig. 5A). Reduced size of LDs is a sign of lipodystrophy, which in turn could lead to faster death and has also been shown to be a morphological characteristic of the fat body in FHV-infected flies (29, 75). Quantification analysis showed that the size of fat body LDs in ZIKV-infected Dicer-2 mutants was reduced to one-third compared to those in ZIKV-infected w1118 flies (Fig. 5B).
Figure 5. The increased susceptibility of Dicer-2 mutant flies to ZIKV infection is linked tolipodystrophy and Impl2-induced wasting.
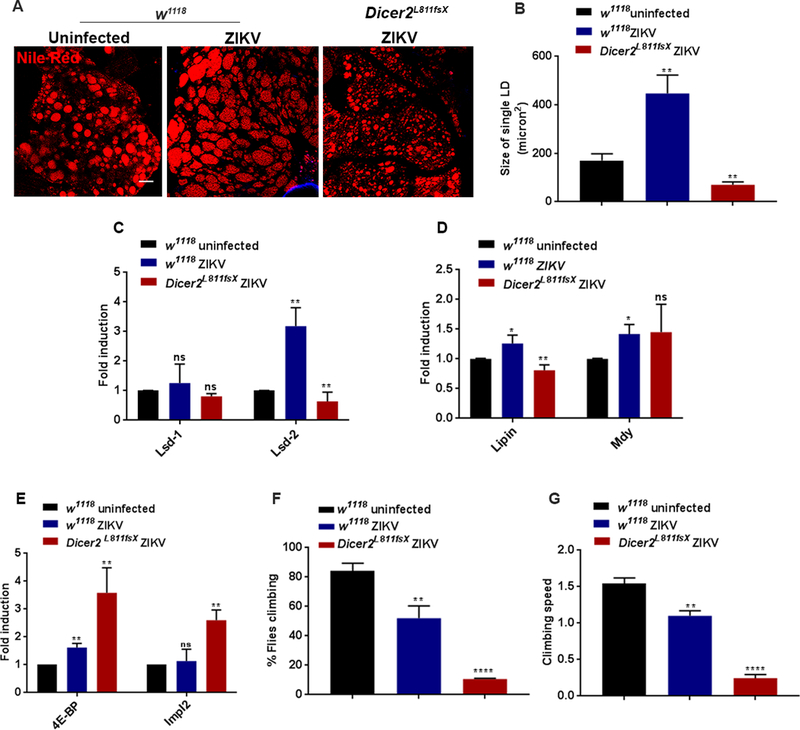
(A) Representative images of fat body lipid droplets in ZIKV-infected wild-type (w1118) flies, Dicer-2 mutants and uninfected background controls at 8 days post injection (8 dpi). The neutral lipids were marked with Nile Red. (B) Quantification of lipid droplet size in ZIKV infected wild-type and Dicer-2 mutant flies as well as in uninfected controls. (C-D) Expression analysis of lipid-metabolism related genes in ZIKV-infected w1118 flies and Dicer-2 mutants, and in uninfected w1118 individuals. (C) Lsd-1 and Lsd-2 were used as read-outs for lipolysis, while (D) Lipin and mdy were used as read-outs for lipogenesis. (E) Quantitative RT-PCR analysis of the Foxo target gene 4E-BP and insulin antagonist Impl2 in ZIKV-infected wild-type (w1118) flies, Dicer-2 mutants and uninfected background controls. (F, G) Climbing ability and speed of climbing in uninfected wild-type controls and ZIKV-infected wild-type and Dicer-2 mutant flies. Levels of mRNA were normalized against RpL32 and three independent experiments were performed. In all graphs, bars represent mean ± SD. Statistical analysis was performed using Student’s t-test (*p<0.05, **p < 0.05, **** p<0.0001); ns denotes no significant differences between experimental treatments. Scale bars: 100 microns.
We next assessed the expression status of lipid metabolism genes in ZIKV-infected w1118 and Dicer-2 mutant flies. In Drosophila, Perilipin-like domain containing proteins (76), DmPLIN1 (Lsd-1) and DmPLIN2 (Lsd-2) modulate the rate of lipolysis (77–79). Lsd-1 is broadly expressed in LDs of fat body cells and promotes lipolysis (78, 80). Lsd-2 functions in opposite fashion to Lsd-1 and protects triacylgycerides stores in a dose-dependent manner (80). We therefore analyzed the transcript levels of Lsd-1 and Lsd-2 in ZIKV-infected w1118 and Dicer-2 mutant flies. We found that mRNA levels of Lsd-2 were significantly upregulated in ZIKV-infected w1118 flies while ZIKV-infected Dicer-2 mutant flies showed reduced expression of Lsd-2 (Fig. 5C). However, mRNA levels of Lsd-1 were not altered in any of the infected strains (Fig. 5C).
We further examined the expression of genes regulating lipogenesis in the ZIKV-infected flies. Lipin and mdy are major regulators of lipid biogenesis in Drosophila; knockdown of lipin and mdy in the fly results in reduced lipid storage and increased lethality (75, 81). While ZIKV-infected w1118 flies showed upregulation of lipin and mdy, ZIKV-infected Dicer-2 mutants showed reduced expression of lipin only (Fig 5D).
One of the pathways regulating lipid metabolism is the insulin signaling pathway (82, 83), which is indispensable for the growth of an organism and forms an important regulator of survival in Drosophila responding to microbial infection (65). Here we found that expression of 4E-BP, a negative regulator of insulin signaling (84), significantly increased in ZIKV-infected w1118 flies (Fig. 5E), suggesting reduced insulin activity and further emphasizing the possibility that these flies suffered from metabolic defects. However, ZIKV-infected w1118 flies showed normal level of Impl2 (Fig. 5E), which is a known antagonist of insulin signaling that has been implicated recently in wasting phenotypes (84–86). Interestingly, ZIKV-infected Dicer-2 mutants exhibited significant reduction in insulin signaling, as indicated by 3.5-fold increase in 4E-BP mRNA levels and markedly increased mRNA levels of Impl2 (Fig. 5E), a condition that leads to wasting syndrome and inevitably death of the infected flies (65, 84, 85). In addition, ZIKV-infected w1118 flies were less active and showed mild defect in climbing ability (Fig. 5F and G); however, the climbing ability and speed were severely affected in the Dicer-2 mutants with only 10% of these flies being able to climb compared to 50% of the w1118 individuals (Fig. 5F and G).
We next wanted to determine whether the observed pathologies in ZIKV-infected Dicer-2 mutants are linked to the Dicer-2 mutation per se or they are resulting from the enhanced ZIKV load. To address this issue, we investigated the occurrence of these pathologies in uninfected Dicer-2 mutant flies. The fat body LDs in uninfected Dicer-2 mutant flies were similar in size to those in background control uninfected flies (Fig. S4A and B). Although uninfected Dicer-2 mutant flies showed a mild defect in climbing (Fig. S4C and D), unlike the ZIKV-infected Dicer-2 mutants, there was no change in the activation of insulin signaling as shown by the normal levels of 4E-BP and Impl2 (Fig. S4E). In order to further ascertain whether the LD phenotype is indeed a genuine phenotype owing to Dicer-2 mutation induced ZIKV enrichment, we next used a fly strain carrying Dicer-2 genomic construct in the background of Dicer-2 mutation. We found that the LDs of ZIKV-infected Dicer-2 rescue carrying flies were similar in size to those of the wild-type controls (Fig. S4F and G). Further, we examined the level of insulin signaling and found no change in mRNA level of 4E-BP as compared to the ZIKV-infected wild-type flies (Fig. S4H). Collectively these findings indicate that Drosophila infection with ZIKV induces perturbed lipid metabolism followed by reduced insulin activity. These pathologies deteriorate severely in Dicer-2 mutants, which sustain enhanced ZIKV burden, defects that result in increased mortality upon ZIKV infection.
Discussion
Our results suggest that Drosophila is a suitable model for dissecting the molecular and pathophysiological basis of host-ZIKV dynamics. Our findings indicate that Drosophila flies subjected to intrathoracic injection of the ZIKV African strain MR766 are capable of sustaining replication of this virus. Using gene-specific primers designed against ZIKV NS5, a crucial molecule for ZIKV replication, we show that ZIKV copy number increases steadily up to 8 dpi before reaching a peak, and subsequently declines at 12 dpi and remains constant thereafter. These findings indicate that NS5 is a reliable indicator for estimating in vivo ZIKV gene expression and replication. Interestingly, enhanced amplification of ZIKV does not compromise fly survival, signifying that Drosophila wild-type flies are resistant to ZIKV infection.
One of the primary innate immune defense mechanisms in Drosophila is the induction of antimicrobial peptides (AMP) regulated by the Toll and Imd signaling pathways, which have been shown to protect against certain viruses, although the mechanistic details are still poorly understood. Drosophila Toll is induced by and regulates Drosophila X virus (DXV) and VSV infection, while in Aedes mosquitoes it is critical against DENV infection (6, 24, 57, 87, 88). Previous studies also indicate that Imd signaling participates in the control of SINV and Cricket paralysis virus (CrPV) infection in Drosophila, but the level of its involvement in the overall antiviral response is currently unclear (48, 89). Here we have found that ZIKV infected flies fail to trigger significant activation of Toll and Imd regulated AMP encoding genes. This finding denotes that the antiviral activities of these pathways are probably virus-specific and they are not directed against ZIKV infection. Unlike Toll and Imd, JAK/STAT is a canonical mammalian antiviral pathway and is a crucial component of the interferon response (50). Though its antiviral role is increasingly perceived in Drosophila and mosquitoes, accumulating evidence indicates that its contribution might also be virus-specific. While flies mutant for JAK are more sensitive to DCV and CrPV, these mutant flies display a rather weak phenotype to SINV, VSV and DXV (22, 23). While activation of JAK/STAT restricts DENV infection, it has no effect on resistance of Aedes aegypti to ZIKV or Chikungunya virus (52, 90). A recent transcriptomic analysis further showed that components of JAK/STAT signaling are not induced in ZIKV-infected mosquitoes (91). In line with these results, our data show that the viral regulated JAK/STAT targets Vago, Vir-1 and Listericin, which are known to be induced by DCV, show only moderate upregulation upon ZIKV infection. Also, the Tot genes, which are activated by bacterial infection and regulated by JAK/STAT signaling, are markedly upregulated in wild-type flies upon ZIKV infection. Primarily induced upon stress (54, 92), the role of Tot genes in the fly antiviral immune response is still unclear. Here, we provide evidence that Tot gene regulation through JAK/STAT signaling in Drosophila might be restricted to bacterial infections only and is probably not a general feature of viral infections. Tot gene induction upon ZIKV infection indicates a virus-specific mechanism of activation for certain genes in the fly, which could imply a general stress response rather than an antiviral immune mechanism. Predominantly upregulated upon septic injury, Diedel is a negative regulator of JAK/STAT signaling although its exact molecular function is still unknown (53, 93). ZIKV injection in Drosophila results in significant upregulation of Diedel throughout the course of infection. This finding suggests that ZIKV infection results in negative regulation of JAK/STAT signaling, which could form a potential strategy of ZIKV to evade the host immune response. The NS5 protein of flaviviruses, which is critically involved in replication, is also able to mediate antagonism of JAK/STAT signaling. DENV infection has been associated with loss of STAT2 expression (94), while WNV infection results in failed JAK activation (95). In line with these findings, our data further stress that the interaction of flaviviruses with JAK/STAT signaling is particularly complex and requires future thorough examination.
In Drosophila, the main antiviral RNAi pathway relies on siRNAs and involves Ago-2 and Dicer-2 as the central operating genes (5, 29, 31, 32). Our findings show that Ago-2 and Dicer-2 are significantly upregulated in Drosophila flies infected with ZIKV implying their anti-ZIKV role. Our finding implicating that Dicer-2 regulate ZIKV infection and provide resistance, is in contrast to a recent work reporting that Dicer-2 is dispensable in regulating ZIKV replication in Drosophila (8). This discrepancy could be attributed to the genetic nature of the strains used in the recent study. Here we further show that while Dicer-2 is instrumental in regulating ZIKV replication, the effect of Ago-2 is only marginal. This distinction in the level of surveillance between the two RNAi components is surprising but not unusual. The distinct functions of Ago-2 and Dicer-2 in the context of viral infection have been documented in Drosophila in case of WNV, where Ago-2 substantially regulates viral replication while Dicer-2 controls viral load at a modest level (11). Several studies in mosquitoes have implicated the siRNA, and in particular, Dicer-2 based immunity in case of ZIKV infection (96, 97). In addition, there have been instances where knockdown of Ago-2 in mosquito cells has only a modest/marginal effect on replication of Dengue and Orthobunyaviruses (98, 99). In a recent report, knockdown of Ago-2 did not lead to increased ZIKV replication, while knockdown of Dicer-2 increased ZIKV replication in mosquito cells (97). It is likely that direct dicing of RNA is more relevant in these cases than RISC-mediated antiviral response. Previous evidence also suggests that independent of RNAi, Dicer-2 can regulate Toll signaling and expression of antiviral gene, Vago. (55, 100). In the context of ZIKV infection, Dicer-2 loss results in enhanced expression of Vago suggesting that the induction of Dicer-2 by Vago might be DCV-specific and not a general antiviral response. How exactly RNAi effectors regulate viral replication is thus an intriguing question and our findings further highlight the specificity of certain RNAi signaling components against different viral pathogens. Identification of putative ZIKV dsRNA targets recognized by Dicer-2 may provide mechanistic insight into how it regulates ZIKV replication in Drosophila.
Our study indicates the importance of siRNAs in anti-ZIKV immunity. It is only recently that other forms of small RNAs, miRNAs and piRNAs, have been implicated in mosquito antiviral immunity. Deep sequencing has revealed accumulation of piRNAs during infection of mosquitoes with Dengue and Chikungunya virus (101, 102). Infection of A. aegypti with a Mexican strain of ZIKV results in modulation of miRNAs and dramatic increase of siRNAs and piRNAs (103). The role of piRNAs and miRNAs in Drosophila antiviral immunity has also been demonstrated recently. Although infection with DXV, DCV or SINV failed to trigger any viral-derived piRNAs and mutation in piRNAs including Ago3, Aub and Piwi had no effect on Drosophila survival (104), flies and cells infected with DCV showed reduced expression of miR-8–3p, a known antagonist of viral replication in Bombyx mori (105). Further experiments would provide an insight into the contribution of these RNAi pathways in the regulation of ZIKV infection.
For successful infection, a virus should target certain cell types or tissues in the host. The ability to colonize and replicate in a specific host tissue also determines the nature and extent of viral pathogenesis (12, 15, 16, 106). Interestingly, the available information of tissue tropism is restricted to insect-specific viruses and the tissue tropism in the case of human pathogenic viruses has not been investigated in detail. Unlike the insect-specific viruses, infection with flaviviruses is asymptomatic and no defect in survival has been reported. This could be one of the reasons for the lack of tissue tropism related studies in the case of human pathogenic viruses. Our findings indicate that firstly, 4G2, a flavivirus specific antigen is a reliable marker for in vivo detection of ZIKV. Using expression of 4G2 as indicator of ZIKV infection, our study provides the first demonstration of a human pathogenic virus infecting the immune/metabolic tissues of the fly. Infection of these organs further implies the relevance of the tissue tropism in dictating the level of viral pathogenesis that can be local, and not always demonstrated at the organismal level (defect in survival).
Although significant advances on the cellular and genetic basis of antiviral immunity in Drosophila have been made, the effects of viral infection on fly physiology have not been widely explored (13, 66). For instance, DCV infection triggers intestinal obstruction marked by crop enlargement due to the disrupted movement of the ingested food in the fly midgut (12). In Drosophila, cytotoxic agents such as bleomycin or paraquat or pathogenic bacterial infection can trigger gut remodeling and in the process increase ISC proliferation (73, 107, 108). The importance of cell renewal in regulating viral infection has been recently demonstrated in mosquitoes and has been proposed to be one of the primary determinants for the susceptibility to Dengue infection. (109). Our finding that ZIKV-infected Dicer-2 mutant flies fail to trigger midgut proliferation as compared to the ZIKV-infected wild-type flies which display considerable increase in ISC proliferation, suggests that in manner similar to Dengue infection, ISC proliferation might also be acting as a determinant for fly susceptibility to ZIKV. Apart from gut-related perturbations (shown in this study), mutation in Dicer-2 has been linked with a large number of perturbations including stress, perturbed metabolism (110), and thus could be considered as the host factor dictating the sensitivity or predisposition to infection. Ago-2 in contrast, has not been shown to be involved in any tissue-specific perturbations, which might in turn explain why Ago-2 mutant flies are refractory to ZIKV infection as compared to Dicer-2 mutant flies. Future investigations will explore the extent to which the restricted proliferation of the midgut contributes to the mortality of these susceptible flies. In particular, future efforts could focus on examining the contribution of other pathways (such as Wingless, Hippo or Notch) to ZIKV induced ISC proliferation, and whether or how the latter could interfere with ZIKV replication.
Emerging evidence indicates that viral infection can modulate lipid metabolism. Hepatitis C virus (HCV) and DENV infection in the hepatoma and kidney cell lines has been linked to enhanced lipogenesis and sharp increase in LD numbers (111, 112). In addition, liver steatosis or fatty liver (the accumulation LDs) is a commonly observed pathology in HCV infected patients (113, 114). Although the mechanism of lipid accumulation is not known, it has been proposed that certain viruses might reside in LDs to promote their own assembly and replication (115). A recent finding has implicated the role of mosquito gut LDs in regulating replication of DENV (116). The perturbed lipid metabolism has also been observed in Drosophila S2 cells where infection with FHV results in increased transcription of genes required for lipid biogenesis (117). Although further studies are required, these previous results suggest that modulation in lipid metabolism could be a general response upon viral infection. Our findings reveal that ZIKV infection in Drosophila flies results in multiple pathologies, most of which are closely associated with lipid metabolic processes. To our knowledge, this is the first in vivo demonstration of perturbed lipid metabolism upon viral infection in Drosophila. While a moderate ZIKV load results in enlarged LDs in wild-type flies, an enhanced load in Dicer-2 mutants results in reduced accumulation of LDs. Reduced size of LDs is a sign of lipodystrophy, which in turn could lead to accelerated death (75, 118).
The reduced energy storage could further be attributed to strikingly decreased insulin signaling in ZIKV-infected Dicer-2 mutant flies. Insulin signaling is one of the main metabolic signaling pathways and previous evidence indicates that reduction in insulin signaling results in depletion of energy reserves, which can lead to infection-induced wasting (65). Impl2, a secreted antagonist of insulin signaling (86), has been implicated recently in robust wasting phenotypes (84, 85). Transcriptional activation of Impl2 in ZIKV-infected Dicer-2 mutant flies further confirms that enhanced ZIKV load could induce host wasting, which points out a potential interplay between host metabolism and viral infection. A fascinating future question pertains to the mechanism by which ZIKV regulates lipid metabolism of the host and how this regulation affects the propagation of the virus. It would also be interesting to investigate the participation of Dicer-2 in ZIKV-induced perturbed lipid metabolism.
Infection-induced behavioral changes are not uncommon. These can be attributed to the pathogen infecting certain tissues and organs in the host or they can be caused by the host per se in an attempt to store limited resources (119). Of note, locomotion of an organism is strongly correlated with its state of metabolism (120, 121). Mosquitoes infected with DENV or Wolbachia (wMelPop) have been proved more active than uninfected individuals (122, 123). Virus infection-induced behavioral changes are also evident in our study showing that ZIKV-infected Dicer-2 mutant flies (which have been metabolically impaired) suffer from lethargy and display impaired locomotion. Whether this behavioral defect is the result of muscular or nervous dysfunction remains unknown and it will be further explored to yield more insights into the mechanistic processes governing host-ZIKV interactions.
Taken altogether, our results illustrate the efficacy of Drosophila as model for understanding host-ZIKV interactions. Our findings reveal that ZIKV can replicate in Drosophila and the resulting infection can cause severe pathophysiological defects. The key insights into identifying the genetic basis of the interplay between Drosophila and ZIKV will undoubtedly assist in unraveling conserved anti-ZIKV immune mechanisms in mammals, perhaps even humans, and will contribute towards developing efficient strategies for blocking the transmission of ZIKV and other flaviviruses by mosquito vectors.
Supplementary Material
Acknowledgments
We thank Prof. Jean-Luc Imler (University of Strasbourg, France) for providing the Ago-2 mutant, Prof. Eric C. Lai (Sloan-Kettering Institute, New York) for trans-combination of Ago-2 and deficiency line, Prof. Richard Carthew (Northwestern University, Illinois) for sharing the Dicer-2 mutants, and Dr. Alex Jeremic’s lab (George Washington University, Department of Biological Sciences) for providing assistance with confocal microscopy. We also thank members of the Department of Biological Sciences at GWU for critical reading of the manuscript.
References
- 1.Musso D, and Gubler DJ. 2016. Zika Virus. Clin. Microbiol. Rev. 29: 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wikan N, and Smith DR. 2016. Zika virus: history of a newly emerging arbovirus. Lancet. Infect. Dis. 16: e119–e126. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R 2007. Recognition of microorganisms and activation of the immune response. Nature 449: 819–826. [DOI] [PubMed] [Google Scholar]
- 4.Buchon N, Silverman N, and Cherry S. 2014. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 14: 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, and Ding SW. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312: 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, and Cherry S. 2012. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 36: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes TT, Allen AL, Bardin JE, Christian MN, Daimon K, Dozier KD, Hansen CL, Holcomb LM, and Ahlander J. 2012. Drosophila as a genetic model for studying pathogenic human viruses. Virology 423: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Gordesky-Gold B, Leney-Greene M, Weinbren NL, Tudor M, and Cherry S. 2018. Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 24: 57–68 e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, and Andino R. 2009. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature 458: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau CK, Thompson CB, and Cherry S. 2009. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 138: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chotkowski HL, Ciota AT, Jia Y, Puig-Basagoiti F, Kramer LD, Shi PY, and Glaser RL. 2008. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology 377: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chtarbanova S, Lamiable O, Lee KZ, Galiana D, Troxler L, Meignin C, Hetru C, Hoffmann JA, Daeffler L, and Imler JL. 2014. Drosophila C virus systemic infection leads to intestinal obstruction. J. Virol 88: 14057–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eleftherianos I, Won S, Chtarbanova S, Squiban B, Ocorr K, Bodmer R, Beutler B, Hoffmann JA, and Imler JL. 2011. ATP-sensitive potassium channel (K(ATP))-dependent regulation of cardiotropic viral infections. Proc. Natl. Acad. Sci. U S A. 108: 12024–12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, and Cherry S. 2014. Viruses and antiviral immunity in Drosophila. Dev. Comp. Immunol. 42: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson TC, Schneemann A, and Johnson J. 2012. Oocyte destruction is activated during viral infection. Genesis 50: 453–465. [DOI] [PubMed] [Google Scholar]
- 16.Lautie-Harivel N, and Thomas-Orillard M. 1990. Location of Drosophila C virus target organs in Drosophila host population by an immunofluorescence technique. Biol. Cell. 69: 35–39. [DOI] [PubMed] [Google Scholar]
- 17.Behura SK, Haugen M, Flannery E, Sarro J, Tessier CR, Severson DW, and Duman-Scheel M. 2011. Comparative genomic analysis of Drosophila melanogaster and vector mosquito developmental genes. PloS One 6: e21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair CD 2011. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future. Microbiol. 6: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell CL, Black W. C. t., Hess AM, and Foy BD 2008. Comparative genomics of small RNA regulatory pathway components in vector mosquitoes. BMC. Genomics. 9: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, and Christophides GK. 2007. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316: 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt SM, Jaramillo-Gutierrez G, Kumar S, Barillas-Mury C, and Schneider DS. 2008. Use of a Drosophila model to identify genes regulating Plasmodium growth in the mosquito. Genetics 180: 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, and Imler JL. 2005. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 6: 946–953. [DOI] [PubMed] [Google Scholar]
- 23.Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, Pfeffer S, Hoffmann JA, and Imler JL. 2013. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 190: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambon RA, Nandakumar M, Vakharia VN, and Wu LP. 2005. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. U S A. 102: 7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein E, Caudy AA, Hammond SM, and Hannon GJ. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366. [DOI] [PubMed] [Google Scholar]
- 26.Elbashir SM, Lendeckel W, and Tuschl T. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes. Dev. 15: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura K, Ishizuka A, Siomi H, and Siomi MC. 2004. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes. Dev. 18: 1655–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rand TA, Ginalski K, Grishin NV, and Wang X. 2004. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl. Acad. Sci. U S A. 101: 14385–14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, and Imler JL. 2006. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat. Immunol. 7: 590–597. [DOI] [PubMed] [Google Scholar]
- 30.Mueller S, Gausson V, Vodovar N, Deddouche S, Troxler L, Perot J, Pfeffer S, Hoffmann JA, Saleh MC, and Imler JL. 2010. RNAi-mediated immunity provides strong protection against the negative-strand RNA vesicular stomatitis virus in Drosophila. Proc. Natl. Acad. Sci. U S A. 107: 19390–19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, and Andino R. 2006. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes. Dev. 20: 2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zambon RA, Vakharia VN, and Wu LP. 2006. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell. Microbiol. 8: 880–889. [DOI] [PubMed] [Google Scholar]
- 33.Nayak A, Berry B, Tassetto M, Kunitomi M, Acevedo A, Deng C, Krutchinsky A, Gross J, Antoniewski C, and Andino R. 2010. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat. Struct. Mol. Biol. 17: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Li WX, and Ding SW. 2002. Induction and suppression of RNA silencing by an animal virus. Science 296: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 35.Shelly S, Lukinova N, Bambina S, Berman A, and Cherry S. 2009. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen J, Duan H, Bejarano F, Okamura K, Fabian L, Brill JA, Bortolamiol-Becet D, Martin R, Ruby JG, and Lai EC. 2015. Adaptive regulation of testis gene expression and control of male fertility by the Drosophila hairpin RNA pathway. [Corrected]. Mol. Cell. 57: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, and Carthew RW. 2004. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117: 69–81. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira L, Ferreira A, and Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delvecchio R, Higa LM, Pezzuto P, Valadao AL, Garcez PP, Monteiro FL, Loiola EC, Dias AA, Silva FJ, Aliota MT, Caine EA, Osorio JE, Bellio M, O’Connor DH, Rehen S, de Aguiar RS, Savarino A, Campanati L, and Tanuri A. 2016. Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, and Tang JW. 2012. A diagnostic polymerase chain reaction assay for Zika virus. J. Med. Virol. 84: 1501–1505. [DOI] [PubMed] [Google Scholar]
- 41.Feany MB, and Bender WW. 2000. A Drosophila model of Parkinson’s disease. Nature 404: 394–398. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, and Chung J. 2006. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 43.Petersen LR, Jamieson DJ, Powers AM, and Honein MA. 2016. Zika Virus. N. Engl. J. Med. 374: 1552–1563. [DOI] [PubMed] [Google Scholar]
- 44.Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, Yang Y, Tesh RB, Bourne N, Barrett AD, Vasilakis N, Weaver SC, and Shi PY. 2016. An Infectious cDNA Clone of Zika Virus to Study Viral Virulence, Mosquito Transmission, and Antiviral Inhibitors. Cell Host Microbe 19: 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim SP, Noble CG, and Shi PY. 2015. The dengue virus NS5 protein as a target for drug discovery. Antiviral. Res. 119: 57–67. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Tan XF, Thurmond S, Zhang ZM, Lin A, Hai R, and Song J. 2017. The structure of Zika virus NS5 reveals a conserved domain conformation. Nat. Commun. 8: 14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myllymaki H, and Ramet M. 2014. JAK/STAT pathway in Drosophila immunity. Scand. J. Immunol. 79: 377–385. [DOI] [PubMed] [Google Scholar]
- 48.Costa A, Jan E, Sarnow P, and Schneider D. 2009. The Imd pathway is involved in antiviral immune responses in Drosophila. PloS One 4: e7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hetru C, and Hoffmann JA. 2009. NF-kappaB in the immune response of Drosophila. Cold. Spring. Harb. Perspect. Biol. 1: a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen GC 2001. Viruses and interferons. Ann. Rev. Microbiol. 55: 255–281. [DOI] [PubMed] [Google Scholar]
- 51.Paradkar PN, Trinidad L, Voysey R, Duchemin JB, and Walker PJ. 2012. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc. Natl. Acad. Sci. U S A. 109: 18915–18920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza-Neto JA, Sim S, and Dimopoulos G. 2009. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. U S A. 106: 17841–17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coste F, Kemp C, Bobezeau V, Hetru C, Kellenberger C, Imler JL, and Roussel A. 2012. Crystal structure of Diedel, a marker of the immune response of Drosophila melanogaster. PloS One 7: e33416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekengren S, and Hultmark D. 2001. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 284: 998–1003. [DOI] [PubMed] [Google Scholar]
- 55.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, and Imler JL. 2008. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat. Immunol. 9: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 56.Goto A, Yano T, Terashima J, Iwashita S, Oshima Y, and Kurata S. 2010. Cooperative regulation of the induction of the novel antibacterial Listericin by peptidoglycan recognition protein LE and the JAK-STAT pathway. J. Biol. Chem. 285: 15731–15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, and Perrimon N. 2003. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 5: 441–450. [DOI] [PubMed] [Google Scholar]
- 58.Lamiable O, Kellenberger C, Kemp C, Troxler L, Pelte N, Boutros M, Marques JT, Daeffler L, Hoffmann JA, Roussel A, and Imler JL. 2016. Cytokine Diedel and a viral homologue suppress the IMD pathway in Drosophila. Proc. Natl. Acad. Sci. U S A. 113: 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFadden G, Mohamed MR, Rahman MM, and Bartee E. 2009. Cytokine determinants of viral tropism. Nat. Rev. Immunol. 9: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, and Wrammert J. 2016. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. U S A. 113: 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metz SW, Gallichotte EN, Brackbill A, Premkumar L, Miley MJ, Baric R, and de Silva AM. 2017. In Vitro Assembly and Stabilization of Dengue and Zika Virus Envelope Protein Homo-Dimers. Sci. Rep. 7: 4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiasny K, Kiermayr S, Holzmann H, and Heinz FX. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J. Virol. 80: 9557–9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider DS, and Ayres JS. 2008. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambers MC, Song KH, and Schneider DS. 2012. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster. PloS One 7: e50679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dionne MS, Pham LN, Shirasu-Hiza M, and Schneider DS. 2006. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 16: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 66.Arnold PA, Johnson KN, and White CR. 2013. Physiological and metabolic consequences of viral infection in Drosophila melanogaster. J. Exp. Biol. 216: 3350–3357. [DOI] [PubMed] [Google Scholar]
- 67.Bonfini A, Liu X, and Buchon N. 2016. From pathogens to microbiota: How Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 64: 22–38. [DOI] [PubMed] [Google Scholar]
- 68.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, and Edgar BA. 2009. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, and Penninger JM. 2009. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta D, Xiang J, and Edgar BA. 2013. RNA expression profiling from FACS-isolated cells of the Drosophila intestine. Curr. Protoc. Stem. Cell. Biol. 27: Unit 2F 2. [DOI] [PubMed] [Google Scholar]
- 71.Dutta D, Buchon N, Xiang J, and Edgar BA. 2015. Regional Cell Specific RNA Expression Profiling of FACS Isolated Drosophila Intestinal Cell Populations. Curr. Protoc. Stem. Cell. Biol. 34: 2F 2 1–14. [DOI] [PubMed] [Google Scholar]
- 72.Beebe K, Park D, Taghert PH, and Micchelli CA. 2015. The Drosophila Prosecretory Transcription Factor dimmed Is Dynamically Regulated in Adult Enteroendocrine Cells and Protects Against Gram-Negative Infection. G3 5: 1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatterjee M, and Ip YT. 2009. Pathogenic stimulation of intestinal stem cell response in Drosophila. J. Cell. Physiol. 220: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roingeard P, and Melo RC. 2017. Lipid droplet hijacking by intracellular pathogens. Cell. Microbiol. 19. [DOI] [PubMed] [Google Scholar]
- 75.Ugrankar R, Liu Y, Provaznik J, Schmitt S, and Lehmann M. 2011. Lipin is a central regulator of adipose tissue development and function in Drosophila melanogaster. Mol. Cell. Biol. 31: 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, and Kimmel AR. 2001. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm. Genome. 12: 741–749. [DOI] [PubMed] [Google Scholar]
- 77.Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, and Kuhnlein RP. 2003. Control of fat storage by a Drosophila PAT domain protein. Curr. Biol. 13: 603–606. [DOI] [PubMed] [Google Scholar]
- 78.Beller M, Bulankina AV, Hsiao HH, Urlaub H, Jackle H, and Kuhnlein RP. 2010. PERILIPIN-dependent control of lipid droplet structure and fat storage in Drosophila. Cell. Metab. 12: 521–532. [DOI] [PubMed] [Google Scholar]
- 79.Teixeira L, Rabouille C, Rorth P, Ephrussi A, and Vanzo NF. 2003. Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech. Dev. 120: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 80.Bi J, Xiang Y, Chen H, Liu Z, Gronke S, Kuhnlein RP, and Huang X. 2012. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell. Sci. 125: 3568–3577. [DOI] [PubMed] [Google Scholar]
- 81.Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, and Oliver B. 2008. COPI complex is a regulator of lipid homeostasis. PLoS Biol 6: e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmitt S, Ugrankar R, Greene SE, Prajapati M, and Lehmann M. 2015. Drosophila Lipin interacts with insulin and TOR signaling pathways in the control of growth and lipid metabolism. J. Cell. Sci. 128: 4395–4406. [DOI] [PubMed] [Google Scholar]
- 83.DiAngelo JR, and Birnbaum MJ. 2009. Regulation of fat cell mass by insulin in Drosophila melanogaster. Mol. Cell. Biol. 29: 6341–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, and Perrimon N. 2015. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev. Cell. 33: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Figueroa-Clarevega A, and Bilder D. 2015. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell. 33: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, and Stocker H. 2008. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramirez JL, and Dimopoulos G. 2010. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev. Comp. Immunol. 34: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xi Z, Ramirez JL, and Dimopoulos G. 2008. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4: e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, and Hardy RW. 2009. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog 5: e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jupatanakul N, Sim S, Anglero-Rodriguez YI, Souza-Neto J, Das S, Poti KE, Rossi SL, Bergren N, Vasilakis N, and Dimopoulos G. 2017. Engineered Aedes aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus. PLoS Negl Trop Dis 11: e0005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Etebari K, Hegde S, Saldana MA, Widen SG, Wood TG, Asgari S, and Hughes GL. 2017. Global Transcriptome Analysis of Aedes aegypti Mosquitoes in Response to Zika Virus Infection. mSphere 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Marshall KE, Westwood JT, Clark MS, and Sinclair BJ. 2011. Divergent transcriptomic responses to repeated and single cold exposures in Drosophila melanogaster. J. Exp. Biol. 214: 4021–4029. [DOI] [PubMed] [Google Scholar]
- 93.Muller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, and Boutros M. 2005. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436: 871–875. [DOI] [PubMed] [Google Scholar]
- 94.Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, and Jacobs M. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79: 5414–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo JT, Hayashi J, and Seeger C. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 79: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anglero-Rodriguez YI, MacLeod HJ, Kang S, Carlson JS, Jupatanakul N, and Dimopoulos G. 2017. Aedes aegypti Molecular Responses to Zika Virus: Modulation of Infection by the Toll and Jak/Stat Immune Pathways and Virus Host Factors. Front. Microbiol. 8: 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varjak M, Donald CL, Mottram TJ, Sreenu VB, Merits A, Maringer K, Schnettler E, and Kohl A. 2017. Characterization of the Zika virus induced small RNA response in Aedes aegypti cells. PLoS Negl Trop Dis 11: e0006010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dietrich I, Shi X, McFarlane M, Watson M, Blomstrom AL, Skelton JK, Kohl A, Elliott RM, and Schnettler E. 2017. The Antiviral RNAi Response in Vector and Non-vector Cells against Orthobunyaviruses. PLoS Negl Trop Dis 11: e0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miesen P, Ivens A, Buck AH, and van Rij RP. 2016. Small RNA Profiling in Dengue Virus 2-Infected Aedes Mosquito Cells Reveals Viral piRNAs and Novel Host miRNAs. PLoS Negl Trop Dis 10: e0004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Z, Wu D, Liu Y, Xia X, Gong W, Qiu Y, Yang J, Zheng Y, Li J, Wang YF, Xiang Y, Hu Y, and Zhou X. 2015. Drosophila Dicer-2 has an RNA interference-independent function that modulates Toll immune signaling. Sci. Adv. 1: e1500228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, and Myles KM. 2012. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog 8: e1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hess AM, Prasad AN, Ptitsyn A, Ebel GD, Olson KE, Barbacioru C, Monighetti C, and Campbell CL. 2011. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC. Microbiol. 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saldana MA, Etebari K, Hart CE, Widen SG, Wood TG, Thangamani S, Asgari S, and Hughes GL. 2017. Zika virus alters the microRNA expression profile and elicits an RNAi response in Aedes aegypti mosquitoes. PLoS Negl Trop Dis 11: e0005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petit M, Mongelli V, Frangeul L, Blanc H, Jiggins F, and Saleh MC. 2016. piRNA pathway is not required for antiviral defense in Drosophila melanogaster. Proc. Natl. Acad. Sci. U S A. 113: E4218–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monsanto-Hearne V, Asad S, Asgari S, and Johnson KN. 2017. Drosophila microRNA modulates viral replication by targeting a homologue of mammalian cJun. J. Gen. Virol. 98: 1904–1912. [DOI] [PubMed] [Google Scholar]
- 106.Cherry S, and Perrimon N. 2004. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat. Immunol. 5: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buchon N, Broderick NA, Chakrabarti S, and Lemaitre B. 2009. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes. Dev. 23: 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amcheslavsky A, Jiang J, and Ip YT. 2009. Tissue damage-induced intestinal stem cell division in Drosophila. Cell. Stem. Cell. 4: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taracena ML, Bottino-Rojas V, Talyuli OAC, Walter-Nuno AB, Oliveira JHM, Anglero-Rodriguez YI, Wells MB, Dimopoulos G, Oliveira PL, and Paiva-Silva GO. 2018. Regulation of midgut cell proliferation impacts Aedes aegypti susceptibility to dengue virus. PLoS Negl Trop Dis 12: e0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim DH, Oh CT, Lee L, Hong JS, Noh SH, Hwang S, Kim S, Han SJ, and Lee YS. 2011. The endogenous siRNA pathway in Drosophila impacts stress resistance and lifespan by regulating metabolic homeostasis. FEBS. Lett. 585: 3079–3085. [DOI] [PubMed] [Google Scholar]
- 111.Herker E, and Ott M. 2012. Emerging role of lipid droplets in host/pathogen interactions. J. Biol. Chem. 287: 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, and Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5: e1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Negro F 2004. Hepatitis C virus and liver steatosis: when fat is not beautiful. J. Hepatol. 40: 533–535. [DOI] [PubMed] [Google Scholar]
- 114.Negro F, and Sanyal AJ. 2009. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver. Int. 29 Suppl 2: 26–37. [DOI] [PubMed] [Google Scholar]
- 115.Welte MA 2015. Expanding roles for lipid droplets. Curr. Biol. 25: R470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chotiwan N, Andre BG, Sanchez-Vargas I, Islam MN, Grabowski JM, Hopf-Jannasch A, Gough E, Nakayasu E, Blair CD, Belisle JT, Hill CA, Kuhn RJ, and Perera R. 2018. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog 14: e1006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castorena KM, Stapleford KA, and Miller DJ. 2010. Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication. BMC. Genomics. 11: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krahmer N, Farese RV Jr., and Walther TC. 2013. Balancing the fat: lipid droplets and human disease. EMBO. Mol. Med. 5: 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hart BL 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12: 123–137. [DOI] [PubMed] [Google Scholar]
- 120.Berrigan D, and Partridge L. 1997. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp. Biochem. Physiol. A. Physiol. 118: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 121.Glazier DS 2005. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. Camb. Philos. Soc. 80: 611–662. [DOI] [PubMed] [Google Scholar]
- 122.Evans O, Caragata EP, McMeniman CJ, Woolfit M, Green DC, Williams CR, Franklin CE, O’Neill SL, and McGraw EA. 2009. Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J. Exp. Biol. 212: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lima-Camara TN, Bruno RV, Luz PM, Castro MG, Lourenco-de-Oliveira R, Sorgine MH, and Peixoto AA. 2011. Dengue infection increases the locomotor activity of Aedes aegypti females. PloS One 6: e17690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


