Abstract
Trends towards dimensional approaches in understanding psychiatric disorders may also be applied to addictive disorders. Advances in our understanding of the neurobiology of addiction can inform these efforts. Furthermore, dimensional approaches to addiction, such as the proposed Addictions Neuroclinical Assessment (ANA), may be used in identifying novel addiction biomarkers, and refining ones that currently exist. These biomarkers, derived from both an understanding of the neurobiology of addiction and behavioral phenotypes, represent a departure from traditional markers of alcohol-relevant biomarkers, such as tests of liver function (LFTs). We posit that a potential addictionrelevant biomarker is reinforcer pathology, found to be relevant across addictions to different substances, and which may offer a target for modification through the use of episodic future thinking.
Dimensional Approaches to Addiction: Why Now?
In 2010, Thomas Insel, then director of the National Institute of Mental Health (NIMH), proposed a new framework for understanding psychiatric disease, the Research Domain Criteria (RDoC) initiative [1]. This dimensional approach echoed many previous efforts to augment and fundamentally modify psychiatric assessment and nosology via measures of brain function, but was proposed at a time of accelerating knowledge of genomics and neuroscience. Conceived primarily as a research framework and also as a conceptual alternative to the categorical, symptom-based Diagnostic and Statistical Manual of Mental Disorders (DSM), RDoC incorporates five domains, organized from cells to systems, for grounding psychiatric disease in neuroscience research. In 2016, George Koob, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), endorsed a similar framework for addictive diseases, the ANA [2]. While taking a different approach than RDoC, ANA is similar in four ways: first, it targets measures towards domains that are mechanistically important; second, it identifies three neuroscience domains that are not only grounded in the neurobiology of addiction [3], but also overlap with three of the five RDoC domains; third, it integrates measures for an understanding of the overall functioning of a domain; and fourth, it is a research approach for assessment of addiction, not capable, or intended to diagnose DSM-based alcohol/substance use disorders. The ANA domains, which encompass only a small fraction of the biomarker space for addiction, are incentive salience (see Glossary), negative emotionality, and executive function [2].
While these initiatives to deepen and sharpen assessment move forward in the research community, the USA faces an epidemic of opioid addiction and related deaths. In 2014, over 28 000 people in the USA died of opioid overdoses, translating to 76 deaths from opioids, or three deaths per hour; globally, opioid dependence contributed 9.2 million disability adjusted life years in 2010 [4,5]. Furthermore, alcohol use is found in nearly a quarter of opioid-related deaths and emergency department (ED) visits [6]. Given this crisis, leveraging movement towards dimensional approaches to understanding addiction specifically, and psychiatric pathology in general, is critical for preventing and reducing deaths from opioids, alcohol, and other drugs of abuse. A dimensional approach addresses a critical challenge in treating addiction: the significant heterogeneity of the disease within addiction to a specific agent, and similarity of disease processes across addiction to different agents. Moreover, these advances in the neurobiology of addiction may be used to identify biomarkers suitable for treatment intervention and assessment. The confluence between the public health crisis of addiction, recent advances in our understanding of the neurobiology of addiction, and the rise of dimensional approaches represents an opportunity for the development of new biomarkers that may be used in clinical care. Herein, we discuss the emergence of clinical measures that access dimensions of vulnerability and progression of addiction, viewing addiction as a process occurring in the brain and not merely as a collection, or quantitation of observed use and consequences of use. We discuss differences between biomarkers and intermediate phenotypes, the relevance of relying on the ANA to identify biomarkers, and offer several examples of potential biomarkers that may be used under this approach. We hypothesize that dimensional approaches to addiction offer a variety of heretofore-unexplored targets for use as biomarkers of addictive disorders.
Addiction Biomarkers
It is clear that the identification of additional putative addiction biomarkers will be critical in helping to refine possible treatments and prevention of substance abuse. An addiction biomarker could be any indicator of vulnerability or presence of a DSM addictive disorder. However, within the current DSM framework, this definition is not true. DSM addictive disorders are ‘use’ disorders. Therefore, it is not surprising that clinically relevant biomarkers for addictive disorders index use, history of use, and effects of use [7]. As shown in Table 1, across the progression of the development of addiction, from predisposition to consequences, different assays may be used, such as changes in LFTs. Consequences of use are measured in many other ways, such as impaired liver or cardiac function and, depending on the addictive agent, measures of consequence can be mechanistically informative, accessing prognostically useful pathophysiologic indicators, as well as overt dysfunction. For example, measures of consequence may be used to assess the functional impairment and prognosis of the brain or liver, as well as to better understand the mutagenic, teratogenic, and developmental effects of nicotine, alcohol, and other drugs [8]. However, none of these biomarkers are necessarily informative for the process of addiction; indeed, these represent particular changes and predispositions that lead to vulnerability to a use disorder and that maintain the particular use disorder. They may not be informative because they are viewed as endpoints of disease and because we lack data to understand, for instance, how differences in vulnerability for liver damage resulting from alcohol addiction can inform the maintenance of this addiction.
Table 1.
Measurements and Assays to Monitor the Progression of Substance Addiction
| Timeframe and/or metric | Assay for measurement of biomarker |
|---|---|
| Predisposition | Genes, teratogens, early life stress |
| Substance exposure | Drug levels (in urine, breath, and blood); drug metabolites in hair; cotinine; CO2; LFTs |
| Process | ANA domains, MRI, PET, pharmacodynamic responses to substances |
| Consequence | ANA domains, MRI, epigenetic modifications, LFTs |
Intermediate Phenotypes and Endophenotypes
The use of intermediate phenotypes, including heritable, disease-associated ‘endophenotypes’ [9,10], is an old idea in addiction medicine, and in psychiatry in general. However, this is not to say that psychiatrists have been nearly as successful as their counterparts in other areas of medicine. In psychiatry, a variety of potentially useful measures, including dexamethasone suppression tests, sleep EEGs, and event-related EEG potentials, including the P50 and P300 potential, have been known for decades but are scarcely used and, indeed, they are not integrated within the DSM framework. Notwithstanding, the present-day diagnostic framework for mental disorders might, without drastic revision, be improved with the aid of such measures. For addictive disorders, the story is much the same and, for example, ADH1B genotypes (alcohol), while associated with DSM criteria, are not integrated within this framework [11]. Furthermore, downregulation of striatal dopamine D2 receptor protein expression, observable by raclopride PET [12] and disturbances of the stress axis [13,14], have long been recognized to occur among patients who are addicts, but have not been incorporated into clinical practice, or used in clinical research in any systematic way. In sum, while addictive disorders are marked by multiple physiologic changes and associated with genetic variables, these markers have not yet been translated into clinical care.
Distinction between Biomarkers and Intermediate Phenotypes
The purpose of distinguishing between intermediate phenotypes and biomarkers that are not intermediate phenotypes is not to denigrate the utility of biomarkers. While sometimes used interchangeably, the distinction is important: a biomarker may be indicative of active disease, while an intermediate phenotype comprises a stepping-stone in the progress to disease [15]. Nevertheless, a biomarker that measures a consequence of a disease rather than an etiologic process can be useful clinically; for example, it can be used to measure current or recent exposure to an addictive agent. For biomarkers of any type to be most useful, they should be identified using big data initiatives, which may be used to explain the heterogeneity within disease types and to understand commonalities and distinctions with other diseases that may be similar, either at the level of etiology or clinical outcome. Relevant for ANA, it would be useful to consider, in particular, biomarkers based on different profiles of performance across tasks designed to measure ANA domains. An overly simplistic example of these patterns might discriminate: first, individuals with particularly strong salience for drug-related versus natural cues, but with less negative affective bias and less compromised executive functioning; second, individuals with a strong bias for negative affective stimuli and salience for drug-related cues and intact executive functioning; or third, individuals with strong salience for drug-related cues and compromised executive functioning, but with relatively little bias towards negative affective stimuli. Again, while an oversimplified description, these sorts of biomarkers might make use of big data, such that the biomarkers could comprise functional patterns across different neuroscience domains relevant for addiction, rather than focusing on an isolated specific function; however, this remains theoretical. To better identify biomarker patterns, we propose incorporating findings of epigenetic changes linked to substance abuse, as well as alterations to resting-state functional connectivity in relevant brain regions, with other sources of big data that may be integrated with the ANA domains. In addition, at least in the case of alcohol, integrating laboratory tests indicative of compromised liver function would also be informative, What is critical to remember is that biomarkers must indicate active disease, whereas intermediate phenotypes do not.
Biomarkers and Disease and/or Addiction
Historically, addiction is a disease defined by social outcomes but the major change in DSM-5, in addition to some minor changes in criteria, is that addictive disorders are now labeled as ‘use disorders’ replacing ‘dependence’ and ‘abuse’. As a result of this change, the name for these disorders has been more closely aligned with how they are diagnosed. Clearly, addiction is not merely dependence. Drug-tolerant individuals administered the drug in medical settings can frequently, or usually, be withdrawn from the agent without further consequences [16]. One way in which addiction-related biomarkers may be particularly useful is by diagnosing addiction without the traditional social outcomes (e.g., family discord, drug use in unsafe conditions, etc.), as in DSM-5, and also independently of whether patients have experienced tolerance or withdrawal to an addictive agent; indeed, some individuals may experience alterations in other domains of neuroscience function regardless of physical tolerance and withdrawal [43]. This concept is consistent with the belief that many addicted individuals consider themselves addicted to a given agent regardless of whether they are currently active users [17]. Furthermore, a nonaddicted user of an addicted agent may, by chance or circumstance, suffer an outcome qualifying them for diagnosis of a use disorder. For example, participants in Alcoholics Anonymous meetings usually consider themselves alcoholics while maintaining many years of sobriety. Potential prototype biomarkers that may be used for diagnosis include epigenetic changes and, as discussed below, reinforcer pathology.
ANA, Intermediate Phenotypes, and Biomarkers
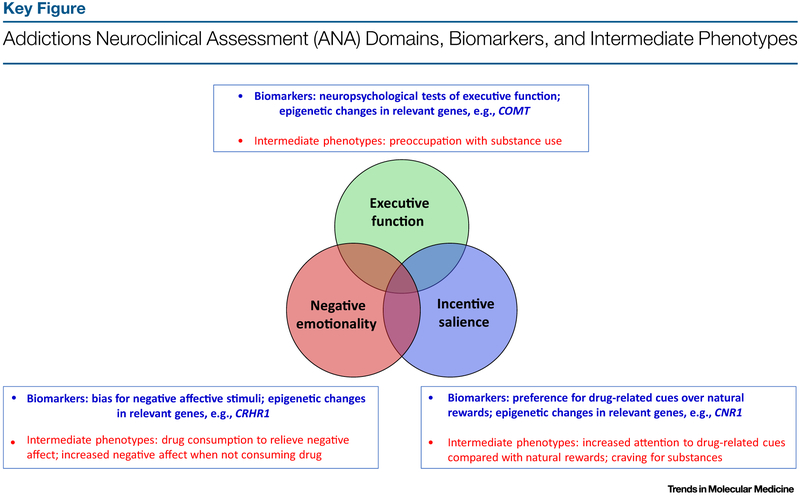
The ANA domains offer a useful rubric for organizing addiction-related biomarkers, while not encompassing all domains relevant to addiction. Beginning with incentive salience, or the concept that cues related to a given substance become themselves incentivized, or rewarding, as an addiction develops, there are various potential biomarkers. For example, preference for substance-related cues to the exclusion of natural rewards may be one of the most defining features of addiction specifically relevant to other psychiatric diseases [18]. Some individuals find natural rewards less pleasurable than do others even before the development of physical dependence and/or DSM-based use disorder on a given substance, while, in others, this shift towards substance-related rewards does not occur until later during the addictive process [19]. Concerning negative affect, a relevant biomarker may be a bias for negative affective stimuli compared with positive affective stimuli, for example as described in the probabilistic reward task, a measure of anhedonia [20]. Different aspects of compromised executive function may be found in any one of myriad behavioral tasks assessing various aspects of executive function, such as working memory as assessed by the N-back or digit span, or set-shifting as measured by the Wisconsin card sorting task [21]. In Figure 1 (Key Figure), we organize potential biomarkers against intermediate phenotypes representative of ANA domains.
Figure 1.
In this model, the ANA domains overlap with proposed biomarkers and intermediate phenotypes. Abbreviations: COMT, catechol-O-methyltransferase gene; CNR1, cannabinoid receptor 1; CRHR1, corticotropin-releasing hormone receptor 1 gene.
Reinforcer Pathology as a Prototype ‘Biomarker’
The neuroscience-based domains identified in RDoC and ANA may be used to generate biomarkers of addiction. Here, we exemplify reinforcer pathology as a new biomarker of a critical aspect of addiction because of the considerable amount of empirical data becoming available. The concept of reinforcer pathology is a recent development in the field of the behavioral economics of addiction specifically focused on how the window of time over which reinforcers are integrated can determine the relative valuation of alcohol and drugs versus other prosocial reinforcers, such as relationships and employment [22–24]. Indeed, critical to this concept is the interaction of the temporal window of integration [25,26]. Consider that alcohol and drug reinforcers are intense, reliable, and brief, while prosocial reinforcers (e.g., employment, relationships) are less intense, variable (e.g., a good, bad, or okay day of work), and may accrue value over a longer timeframe. Short temporal windows of integration may increase the value of intense, reliable, and brief reinforcers, such as drugs, and lead to a decline in the value of reinforcers that are less intense, variable, and accrue value over longer timeframes, such as prosocial reinforcers. Conversely, long temporal windows of integration should reverse the valuation of drug and prosocial reinforcers [25,26].
Reinforcer pathology can provide an understanding of the anhedonia that often occurs during the development of addiction [27,28] and suggests a novel approach to intervention development; namely, to increase the length of the temporal integration window so that individuals may value prosocial, longer term reinforcers over immediate ones, such as drugs [26]. However, to change that window requires that it can be measured. As such, the discounting of delayed reinforcers measures the temporal window of integration. Delay discounting (DD) refers to the decline in value of a reinforcer as a function of delay and is a process closely related to the phenotype of addiction and other disorders [29].
Given that reinforcer pathology, which stipulates enhanced valuation of a drug, and addiction-related loss of normal reward are inversely related and controlled by the temporal window of integration, then interventions that alter this temporal window should alter the valuation of drug and extended prosocial reinforcers (e.g., employment or relationships) [26]. Using a variety of methods, DD has been modified by eliciting either a positive future frame or a ‘scarcity’ frame [26]. Episodic future thinking (EFT) refers to simulating prospective, positive episodes that might occur in one’s personal future and is derived from the science of prospection, which also has the potential to modify delay discounting [26]. Prospection is important for understanding cognitive motivational processing and involves the prefrontal brain regions; individuals with alcohol use disorders demonstrate deficits in prospection, constricting their temporal window [30]. Engagement in EFT induces activation in regions previously associated with DD (i. e., lateral prefrontal cortices, anterior cingulate, and parietal cortex) and also increases coupling in regions of the amygdala and hippocampus not usually associated with DD, as determined from fMRI activity [31]. EFT has previously been shown to decrease DD in individuals with alcholol use disorder [32], smokers [33], as well as normal weight, overweight, and obese populations [34,35]. Consistent with the reinforcer pathology concept, EFT may reduce the demand for hypothetical drinks [32], self-administration of cigarettes [33], and self-administration and valuation of highly palatable snacks in overweight and/or obese adults and children [35,36]. Conversely, when smokers or obese individuals were exposed to a scarcity scenario (drastic income reduction), they discounted more (valued the future less) and drug craving and valuation of preferred foods increased [36]. One important future area of research would be to extend this model to address the ‘dark side’ of addiction, such as assessing the consequence of withdrawal. Indeed, opioid withdrawal, for example, dramatically shortens the temporal window [37]. As such, withdrawal data suggest that the temporal window would increase drug valuation further and decrease prosocial reinforcers [37]. Overall, these studies are consistent with, and provide support for, the hypothesis of reinforcer pathology as a prototype ‘biomarker’ for addictive disorders. We suggest that DD is a target for intervention development, with salutary effects of drug valuation and, perhaps, treatment outcomes.
Another possible biomarker relevant for the dimensional approach to addiction is the functional disruption of the hypothalamus-pituitary axis (HPA) among individuals addicted to alcohol. This outcome, manifested as attenuated HPA function during alcohol withdrawal [38], can predict relapse to alcohol drinking in men [39]. Of interest, it may provide a better understanding of the heterogeneity in HPA responses that has been observed for individuals presenting with alcholol use disorder, given that decreased cortisol responses to stress and at baseline have been associated with other psychiatric disorders frequently comorbid with alcholol use disorder, such as post-traumatic stress disorder (PTSD) [40]. Accordingly, women who are alcoholics do not appear to exhibit the same alterations in HPA activity consequent to heavy alcohol use [41]. Using HPA function as a putative biomarker for addiction might also address the critical need to emphasize negative emotionality and its role in perpetuating the addiction cycle.
Concluding Remarks
The utility of any biomarker is determined by both its predictive and explanatory power. The integration of addiction biomarkers into specific functional patterns across neuroscience domains (i.e., defining a specific profile of relative deficits and strengths, such as exaggerated negative affect, yet, preserved executive function) suggests that the measurement of weaknesses and strengths in these domains constitutes an important first step in the assessment of addictions. By contrast, clinical measures, and for example, a history of dysphoria or impulsive behavior, may represent weaker surrogates. Neuroscience-based biomarkers are influenced by genetic variability and, as reflected in the heritability of addiction [42], and individual vulnerability and resilience, in patients who are addicts, induced weaknesses and/or deficits and residual strengths and/or assets may be more paramount clinically compared with innate characteristics (see Outstanding Questions and Box 1). To understand the influence of genetic factors in a disease such as addiction, where secondary changes are profound, phenotype must be measured. When integrated with genetic background, the identification of different profiles may be one way that neuroscience-based biomarkers may help explain the heterogeneity present in an addictive disease, aiming to target weaknesses and strengths in any given individual, and moving towards precision medicine. Using dimensional approaches to addiction to identify new biomarkers may afford improved precision in treatment, thus alleviating the substantial public health burden of addictive disorders.
Outstanding Questions.
Which neuroscience domains should be incorporated into a functional profile that may serve as a biomarker of addictive disorders?
How can the field integrate genomics and neurobiology in to help develop and refine addiction-related biomarkers?
How can we test the utility of addiction-related biomarkers for clinical use?
Box.1. Clinician’s Corner.
The NIAAA has endorsed a novel framework for addictive disorders, the ANA, as a research-based, dimensional approach for addiction assessment. This approach is similar to other dimensional perspectives on psychiatric disorders, such as the RDoC initiative at the NIMH.
Central to this new perspective is avoiding the traditional approach of viewing addiction from the measurement of drug consumption and observable symptoms resulting from that use. Instead, by recognizing the heterogeneity of addiction across individuals, the commonalities across different types of addiction, and the centrality of neurobiology in addiction, the ANA seeks to identify and or refine biomarkers of the addictive process.
One such recently developed biomarker, reinforcer pathology, has shown that those with addiction have a short temporal view that renders the user most susceptible to brief intense reliable reinforcers and that using methods that increase that temporal view results in concomitant declines in substance valuation.
Development, use, and adoption of this NIAAA approach may begin to identify subtypes of addiction, show commonalities of this subtypes across substances, and provide novel targets for intervention development.
In the future, this effort may lead to a new understanding of addictive diseases and promote novel intervention directed by a dimensional understanding of addiction-related processes.
Supplementary Material
Highlights.
To better understand addiction, support is growing to undertake dimensional approaches that are based on neuroscience.
Neuroscience-based dimensional approaches may be used to identify and refine candidate biomarkers of addictive disorders.
Empirical evidence suggests that reinforcer pathology can be used as a biomarker of addictive disorders.
Acknowledgments
W.K.B. acknowledges support through NIH grants R01 DA039456, R01 DA034755 and R01 AA021529. D.G. and L.E.K. thank the Division of Intramural Clinical and Biological Research, NIAAA, and Roshni Janakiraman for her assistance in preparation of this manuscript.
Glossary
- Anhedonia:
lack of pleasure in things, particularly things that used to provide pleasure.
- Cotinine:
metabolite of nicotine.
- Delay discounting (DD):
a measure of the decline in value of a reinforcer as a function of the delay to its receipt.
- Dexamethasone suppression tests:
a test used to assess adrenal function by administering dexamethasone and measuring subsequent cortisol responses.
- Dimensional approaches:
frameworks for understanding disease that rely on continua of symptoms and/or underlying biological alterations, as contrasted with categorical approaches to understanding disease.
- Endophenotypes:
biologically derived markers of a given disease.
- Episodic future thinking:
the projection of one’s self into the future to pre-experience an event.
- Event-related EEG potentials:
a brain response resulting from a specific motor, sensory, or cognitive event and measured by electroencephalography (EEG).
- Executive function:
a set of abilities mediated by the prefrontal cortex of the brain, including functions such as planning, attention, inhibiting responses, and others.
- Incentive salience:
when stimuli associated with use of an addictive agent (e.g., alcohol) become themselves rewarding to an individual (i.e., the individual derives pleasure from being exposed to these cues for a given agent, even in absence of the agent itself).
- Intermediate phenotypes:
observable markers related to a given disease, which may be present even when the disease itself is not fully diagnosable (i.e., an individual does not meet all criteria for diagnosis, but may have one or more intermediate phenotypes and/or markers for this disease).
- N-back/Digit span:
two widely used measures to assess working memory, both of which require individuals to remember multiple items presented to them in sequence.
- Negative affective bias:
a preference for stimuli associated with unpleasant feelings.
- Negative emotionality:
a comprehensive set of emotional states related to unpleasant feelings or lack of feelings entirely (e.g., sadness, anxiety, malaise, or anhedonia).
- Positive future frame:
a narrative describing future positive outcomes.
- Probabilistic reward task:
a widely used task to assess an individual’s ability to learn by receiving rewards for making certain decisions and making subsequent decisions to maximize the receipt of rewards.
- Prospection:
construction and evaluation of possible future outcomes.
- Reinforcer pathology:
specifies discounting functions as a temporal window that integrates reinforcers over time. Longer windows of integration support valuation of lower intensity, and often variable, prosocial reinforcers, while shorter windows of integration support valuation of brief, intense, reliable reinforcers, such as drugs and food and may, in turn, lead to a decline in the value of prosocial reinforcers.
- Scarcity frame:
a narrative describing abrupt and extensive economic loss.
- Set-shifting:
the ability to change perspective or rules about a given task, mental flexibility.
- Wisconsin card sorting task:
a widely used task designed to assess set-shifting.
- Working memory:
memory dedicated to holding items in conscious awareness and for processing.
References
- 1.Insel T et al. (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 [DOI] [PubMed] [Google Scholar]
- 2.Kwako LE et al. (2016) Addictions neuroclinical assessment: a neuroscience-based frameworkfor addictive disorders. Biol. Psychiatry 80, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF and Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd RA et al. (2016) Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 65, 1445–1452 [DOI] [PubMed] [Google Scholar]
- 5.Degenhardt L et al. (2014) The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109, 1320–1333 [DOI] [PubMed] [Google Scholar]
- 6.Jones CM et al. (2014) Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 63, 881–885 [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann T and Spies C (2003) Use of biomarkers for alcohol use disorders in clinical practice. Addiction 98, 81–91 [DOI] [PubMed] [Google Scholar]
- 8.Zakhari S and Li TK (2007) Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology 46, 2032–2039 [DOI] [PubMed] [Google Scholar]
- 9.Cloninger CR et al. (1993) A psychobiological model of temperament and character. Arch. Gen. Psychiatry 50, 975–990 [DOI] [PubMed] [Google Scholar]
- 10.Gottesman II and Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645 [DOI] [PubMed] [Google Scholar]
- 11.Hart AB et al. (2016) Which alcohol use disorder criteria contribute to the association of ADH1B with alcohol dependence? Addict. Biol. 21, 924–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow ND et al. (1996) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 20, 1594–1598 [DOI] [PubMed] [Google Scholar]
- 13.Adinoff B et al. (2010)Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol-dependent women. Alcohol. Clin. Exp. Res. 34, 915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wand G (2005) The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism. J. Clin. Invest. 115, 2697–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenzenweger MF (2013) Endophenotype, intermediatepheno-type, biomarker: definitions, concept comparisons, clarifications. Depress. Anxiety 30, 185–189 [DOI] [PubMed] [Google Scholar]
- 16.Kosten TR and O’Connor PG (2003) Management of drug and alcohol withdrawal. N. Engl. J. Med. 348, 1786–1795 [DOI] [PubMed] [Google Scholar]
- 17.Morgenstern J et al. (1997) Affiliation with Alcoholics Anonymous after treatment: a study of its therapeutic effects and mechanisms of action. J. Consult. Clin. Psychol. 65, 768–777 [DOI] [PubMed] [Google Scholar]
- 18.Koob GF and Volkow ND (2009) Neurocircuitry of addiction. Neuropsychopharmacology 35, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litten RZ et al. (2015) Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol. Clin. Exp. Res. 39, 579–584 [DOI] [PubMed] [Google Scholar]
- 20.Pizzagalli DA et al. (2008) Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 43, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heaton RK (1993) Wisconsin Card Sorting Test: Computer Version 2, Psychological Assessment Resources [Google Scholar]
- 22.Bickel WK et al. (2011) The behavioral economics and neuroeconomics of reinforcer pathologies: implications for etiology and treatment of addiction. Curr. Psychiatry Rep. 13, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickel WK et al. (2014)The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu. Rev. Clin. Psychol. 10, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickel WK et al. (2017) Reinforcer pathology: the hohavioral economicsofabuse liability testing. Clin. Pharmacol. Ther. 101, 185–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bickel WK et al. (2011) The behavioral economics and neuroeconomics of reinforcer pathologies: implications for etiology and treatment of addiction. Curr. Psychiatry Rep. 13, 406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickel WK et al. (2017) Toward narrative theory: interventions for reinforcer pathology in health behavior In Impulsivity: How Time and Risk Influence Decision Making (Stevens JR, ed.), pp. 227–267, Springer International Publishing; [PubMed] [Google Scholar]
- 27.Koob GF (2006) The neurobiology ofaddiction: aneuroadaptational view relevant for diagnosis. Addiction 101, 23–30 [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND et al. (2003) The addicted human brain: insights from imaging studies. J. Clln. Invest. 111, 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickel WK et al. (2014) The behavioral-and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology 76, 518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths A et al. (2012) Prospective memory and future event simulation in individuals with alcohol dependence. Addiction 107, 1809–1816 [DOI] [PubMed] [Google Scholar]
- 31.Peters J and Buchel C (2010) Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron 66, 138–148 [DOI] [PubMed] [Google Scholar]
- 32.Snider SE et al. (2016) Episodic future thinking: expansion of the temporal window in individuals with alcohol dependence. Alcohol. Clin. Exp. Res. 40, 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein JS et al. (2016) Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology (Berl.J 233, 3771–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel TO et al. (2013) The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol. Sci. 24, 2339–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel TO et al. (2013) The future is now: comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appettte 71, 120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sze YY et al. (2017) Bleak present, bright future: online episodic future thinking, scarcity, delay discounting, and food demand. Clin. Psychol. Scl. 5, 683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giordano LA et al. (2002) Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Bert.) 163, 174–182 [DOI] [PubMed] [Google Scholar]
- 38.Adinoff B etai. (2005) Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, Part 1: Adrenocortical and pituitary glucocorticoid responsive¬ness. Alcohol. Clin. Exp. Res. 29, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junghanns K et al. (2003) Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 38, 189–193 [DOI] [PubMed] [Google Scholar]
- 40.Brady KT et al. (2006) The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J. Stud. Alcohol 67, 700–706 [DOI] [PubMed] [Google Scholar]
- 41.Adinoff B etai. (2010) Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol-dependent women. Alcohol. Clin. Exp. Res. 34, 915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman D et al. (2005) The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 6, 521–532 [DOI] [PubMed] [Google Scholar]
- 43.Ritz L et al. (2016) Clinical and biological risk factors for neuropsychological impairment in alcohol use disorder. PLoS One 11, e0159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.