Abstract
The prevalence of obesity in combination with sarcopenia (the age-related loss of muscle mass and strength or physical function) is increasing in adults aged 65 years and older. A major subset of adults over the age of 65 is now classified as having sarcopenic obesity, a high-risk geriatric syndrome predominantly observed in an ageing population that is at risk of synergistic complications from both sarcopenia and obesity. This Review discusses pathways and mechanisms leading to muscle impairment in older adults with obesity. We explore sex-specific hormonal changes, inflammatory pathways and myocellular mechanisms leading to the development of sarcopenic obesity. We discuss the evolution, controversies and challenges in defining sarcopenic obesity and present current body composition modalities used to assess this condition. Epidemiological surveys form the basis of defining its prevalence and consequences beyond comorbidity and mortality. Current treatment strategies, and the evidence supporting them, are outlined, with a focus on calorie restriction, protein supplementation and aerobic and resistance exercises. We also describe weight loss-induced complications in patients with sarcopenic obesity that are relevant to clinical management. Finally, we review novel and potential future therapies including testosterone, selective androgen receptor modulators, myostatin inhibitors, ghrelin analogues, vitamin K and mesenchymal stem cell therapy.
Adults over the age of 65 constitute 13% of the global population and are the fastest growing demographic subgroup; this group is expected to reach 2.1 billion people in 2050 (REF1). Within this population, obesity has steadily increased2,3, and in the United States, 38.5% of men and 43.1% of women are currently classified as having obesity4. Worldwide, these rising rates presumably offset gains in life expectancy5, with age-adjusted life expectancy dropping by roughly 0.17 years from 2014 to 2015 (REFS6–10).
Sarcopenia, which is the loss of muscle mass and strength or physical function, naturally occurs in ageing. Sarcopenia synergistically worsens the adverse effects of obesity in older adults, resulting in sarcopenic obesity. Sarcopenic obesity is appropriately characterized as a confluence of two epidemics — an ageing population and rising obesity rates11. As elevated BMI, functional impairment, increased mortality and reduction in quality of life10 are observationally associated, addressing sarcopenic obesity is important for preventing longterm disability in the older adults at high risk12. In this Review, we describe the aetiology and pathogenesis of sarcopenic obesity, as well as the associated adverse outcomes for aged individuals beyond reduced function and mortality, and highlight evidence-based and novel therapies targeting this high-risk population.
Biological pathways to sarcopenic obesity
Age-related changes in body composition.
Multiple factors are responsible for changes to body composition with ageing. Body fat increases until the seventh decade of life and thereafter decreases13,14. Vertebral compression results in a reduction in height15, which affects anthropometric measures such as BMI. Muscle mass declines after peaking in the fourth decade16, such that weight is mostly gained as fat rather than lean mass. This age-related reduction in lean mass17,18 accounts, in part, for reduced resting metabolic rates19. Other aetiological factors that cause a decline in resting metabolic rates include reduced physical activity20, reduced mitochondrial volume and reduced oxidative capacity21,22. Age-related decreases in the components of total energy expenditure (such as, resting metabolic rates, thermic effect of food and physical activity) contribute largely to the gradual increase in body fat.
The age-related decline in resting metabolic rates can also result from factors independent of changes to body composition, such as adaptive thermogenesis23,24, which is considered a defence mechanism against weight loss25. The reduction in energy expenditure as we age is not proportionally associated with a reduced drive to eat, which furthers fat build-up and leads to small yearly positive changes in energy balance that might lead to weight gain25–27. Considerable inter-individual variability to weight loss suggests that adaptive thermogenesis plays a part in energy balance28 in sarcopenic obesity. Muscle mass loss with ageing29 correlates with decreased resting metabolic rates and metabolic adaptation, which perpetuates the development of obesity30–32. As most individuals with sarcopenic obesity are sedentary, small changes in their muscle mass can markedly alter daily energy expenditure, which in turn affects adaptive thermogenesis and exacerbates a vicious cycle in their metabolic development33–36.
Sex-specific hormonal changes: oestrogen and testosterone.
Sex-specific changes in muscle and fat composition are partly due to age-related changes in oestrogen and testosterone. In women, menopause increases body weight and fat mass, specifically in visceral areas37, but decreases fat-free mass38. This shift in fat deposition to the centre of the body (which accounts for 15–20% of total fat stores) expands waist circumference and reduces muscle mass38–40. Oestrogen can attenuate these changes41 by modulating inflammation in skeletal muscle through satellite cell activation42,43.
In males, testosterone promotes muscle regeneration through satellite cell activation44,45. Testosterone levels decline by approximately 1% per year, which can negatively affect muscle mass and fat distribution in ageing46. Testosterone levels in the highest quartile (496–1,340 nmol/l) are associated with reduced lean muscle loss47 and reduced visceral fat redistribution48 in older men aged ≥65 years and in individuals with obesity49. Testosterone increases muscle protein synthesis by increasing amino acids utilization in skeletal muscle and increases androgen receptor expression44,45,50. Current data on supplementation for muscle strengthening are conflicting51,52. A 2016 study reported that treatment with testosterone for 1 year did not improve physical function in men >65 years of age with age- reduced levels of testosterone (serum testosterone concentration <275 ng/dl)53. Levels of dehydroepiandrosterone sulfate, the biological precursor of testosterone, also decrease with age in both men and women54,55 (for a comprehensive review on the effects of testosterone on body composition see REF56).
Inflammatory pathways.
A number of inflammatory pathways are common to muscle and visceral fat. Obesity activates macrophages, mast cells and T lymphocytes, promoting a low-level inflammation that results in the secretion of tumour necrosis factor (TNF), leptin and growth hormone (GH)57–59. All such secretory changes lead to insulin resistance, which is increased by muscle catabolism60, promoting gain in fat mass and a loss of muscle mass57. Leptin upregulates the pro-inflammatory cytokines IL-6 and TNF, which results in a reduction in the anabolic actions of insulinlike growth factor 1 (IGF1)61. This reduction in IGF1, along with the age-related reductions in testosterone, increases the likelihood of incident frailty62. Elevated cytokine levels observed in hypogonadal states are associated with truncal obesity, which exacerbates the development of sarcopenia44,45. Adiponectin is negatively correlated with age and obesity and counters the effects of leptin. Elevated TNF directly inhibits adiponectin63, arresting muscle protein synthesis and mitochondrial processes64. Obesity also induces leptin resistance, promoting reduced muscle fatty oxidation and ectopic fat deposition65,66.
Myocellular mechanisms.
A number of mechanisms might explain the reduction of muscle mass and strength in sarcopenic obesity, including type II muscle fibre atrophy, reduction in motor neurons, collagen deposition and fibre necrosis67–70. Older adults (those ≥65 years) are at risk of developing anabolic resistance owing to reduced post-prandial amino acid availability, reduced muscle perfusion and a reduced digestive capacity resulting from splanchnic sequestration of amino acids71.
Ageing stimulates the infiltration of fat into muscle72,73, which might negatively affect sarcopenia74, as described below, and obesity promotes the deposition of fat in the liver, heart, pancreas and skeletal muscle (FIG. 1). The deposition of intramyocellular lipids promotes lipotoxicity and inflammation and induces dedifferentiation of mesenchymal adipocyte-like progenitor cells that express fatty tissue genes75. The regeneration potential of muscle is impaired, which might promote fibrosis, thereby promoting insulin resistance76–79, partially owing to impaired mitochondrial fatty acid oxidation and increased lipolysis76,80. A reduction in the number of mitochondria and elevated production of reactive oxygen species occur in muscle following the deposition of intramyocellular lipids. This process can impair muscle function and might reduce the oxidative capacity of muscle81. Potential mechanisms explaining these changes include age-related reductions in protea some activity, deficiencies in ubiquitylation and autophagy and impairments in removing degraded proteins and end products82–84.
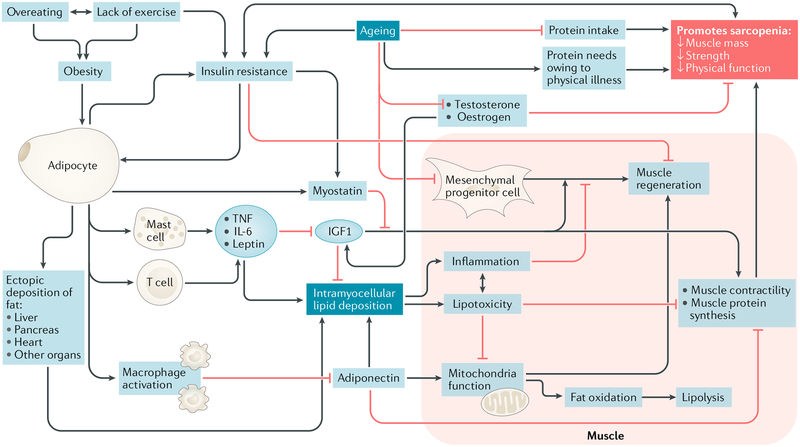
Fig. 1 |. A proposed model of mechanisms leading to sarcopenic obesity.
The proposed interplay between adipose and muscle tissue, which is believed to contribute to the development of sarcopenic obesity, is shown. The black lines are stimulatory, while red lines with flat ends indicate inhibition. IGF1, insulin-like growth factor 1; TNF, tumour necrosis factor.
Pro-inflammatory lipids also secrete paracrine hormones and cytokines that promote a feedforward cycle by producing intramyocellular lipids. This lipotoxicity impairs muscle fibre contractility and interferes with muscle protein synthesis, exacerbating sarcopenia82–84. Lipid deposition can also occur in spaces previously occupied by muscle, impairing new muscle tissue growth. One study reported an increase in intramyo cellular lipid deposition after young, healthy men and women aged 19–28 years were exposed to 30 days of leg disuse, which resulted in lower extremity muscle mass loss85. This finding could be due to skeletal muscle preferentially depositing fat for a source of energy as opposed to glucose86,87. While muscle cells can regenerate through satellite mesenchymal progenitor cells, their numbers decline with age, which contributes to reduced muscle function88,89. Myostatin can be upregulated in skeletal muscle, inhibiting muscle genesis90. In sum, individuals with obesity are at risk of inflammation, which can lead to the preferential mobilization of muscle instead of fat91.
The role of exercise.
Exercise can affect hormonal balance, reduce oxidative stress, induce mitochondrial synthesis, alter immunological and motor function and improve muscle oxidative capacity92–95. Increased muscle protein synthesis with exercise sensitizes muscle insulin action and promotes anabolism96–100. Sarcopenia is associated with reduced muscle protein synthesis, partly owing to decreased anabolic stimulation (which can result from a lack of regular exercise). Aerobic exercise101, resistance training102–104 and their combination105 increase muscle protein synthesis in older adults despite age-related decreases in anabolic signalling106–109. Muscle satellite cells located between myofibres and their surrounding basal lamina are recruited into existing muscle fibres by physical activity110,111. Muscle injury activates satellite cells to regenerate muscle by releasing IGF1, fibroblast growth factor and mechano growth factor, all of which stimulate the differentiation and proliferation of muscle satellite cells112,113. Circulating inflammatory biomarkers, including IL-6, C-reactive protein and TNF, are downregulated by aerobic exercise and strength training, although the relationship is less clear with combined aerobic and resistance activities114–118. Elevated levels of IL-6 and TNF and low levels of IGF1 are associated with reduced muscle mass, reduced muscle strength, reduced muscle mobility and reduced muscle function, suggesting a marked role of exercise in attenuating these muscular changes with ageing117,119,120.
Aerobic activity can improve the oxidative capacity of muscle by counteracting the negative effect of intra- myocellular lipids and accelerating lipolysis, which results in an increase in capillary density121. The synthesis of mitochondria in myocytes is upregulated to meet the demands associated with an increase in capillary density, which in turn leads to increased oxygen extraction and metabolism122 through the induction of calcium and metabolic signalling pathways such as those involving 5’-AMP-activated serine/threonine-protein kinase (AMPK) and sirtuins123. These mediators stimulate mitochondrial production, which promotes improved fatty acid metabolism124.
Myocyte apoptosis can be abrogated by physical activity125,126, while mechanisms of cellular quality control, including autophagy, mitophagy and mitochondriogenesis127, contribute to the development of sarcopenic obesity and could be potential targets for therapy. Reduced cytokine production can lead to improved glucose metabolism, insulin sensitivity and muscle protein synthesis, which might dampen the progression of sarcopenic obesity.
Ageing leads to reduced cardiopulmonary status owing to inefficient oxygen extraction and a concomitant reduction in metabolically active muscle mass128. Peak oxygen consumption is potentially inversely related to frailty129,130, suggesting that improvements in VO2 max following aerobic training counteract frailty131,132. Following a 12-week diet–exercise intervention in male and female frail adults with obesity aged 69 ± 1 years, investigators reported reduced skeletal muscle levels of mRNA for TLR4, IL6 and TNF, increased mechano growth factor mRNA and increased fat-free mass in the exercise group, and these results were independent of weight loss133. Separately, resistance exercises resulted in increased TNF mRNA and protein from skeletal muscle biopsy samples in frail adults134. Expression of skeletal muscle TNF, IL-1β and nitric oxide synthase, inducible in patients with heart failure was reduced following aerobic training, suggesting that aerobic exercise has anti-inflammatory effects135. Furthermore, a 12-week aerobic and resistance programme increased serum levels of ghrelin and adiponectin by 47% and 55%, respectively, and reduced circulating levels of CD14+ and CD16+ inflammatory monocytes, adding additional evidence to the anti-inflammatory effects of exercise136.
Resistance exercise increases the number and size of fast twitching muscle fibres (IIA and IIX), which improve glucose metabolism in muscle and muscle protein synthesis102,103,137–139. Muscle protein synthesis is also improved by nutrient-stimulated vasodilation and nutrient transport to local muscle myofibrils112,124. Muscle fascicle length and muscle tendon stiffness reportedly increased after strength training (leg press and extension) over 14 weeks in a cohort of men and women aged over 65 years140. In a study of eight young adults (aged 18–29 years) and seven older adults (aged 67–81 years), isometric knee extension at varying degrees of maximal voluntary contraction followed by a 6-week resistance programme demonstrated early increases of isometric knee extensor maximal force (which is a marker of voluntary muscle contraction) and increases in motor unit discharge rates (which is a magnitude ofthe speed of neural activation)141. Resistance training has also been shown to reduce levels of cytokines, such as resistin, leptin and IL-6 (REF142).
Leptin and adiponectin stimulate and inhibit, respectively, the deposition of intramuscular lipids (Fig. 1); however, defining their precise roles in physical activity continues to be challenging. For instance, the concentration of leptin in the systemic circulation is suppressed following resistance exercise143 but also in individuals with overweight or obesity following a physical training intervention143–145. Resistance training seems to be more efficient in reducing leptin levels than aerobic training alone146, though conflicting evidence exists147. A study into the effects of aerobic activity in patients who had recovered from breast cancer reported that individuals who were randomized to the aerobic exercise group demonstrated reductions in insulin and leptin and increases in the adiponectin:leptin ratio but no significant changes in adiponectin compared with participants in the usual activity group148. These results parallel those from studies in inactive men aged 65–82 years who were overweight. Investigators assigned participants to partake in varying intensities of resistance exercises. The investigators reported no alterations in concentrations of leptin, but participants had intensity-dependent changes in adiponectin139,145 — high-intensity resistance training led to an increase in the concentrations of adiponectin for 24 hours after exercise in inactive adults who were overweight139.
Summary of mechanisms.
The core biological factors that underlie sarcopenic obesity are age-related changes in metabolism and body composition and the presence of concurrent environmental obesogenic factors and physical illnesses that develop with the ageing process. Incremental metabolic changes over time promote fat deposition with a pro-inflammatory cascade of events. In tandem, crosstalk with biologically active muscle tissue leads to a negative feedback cycle that promotes progressive gain in fat mass and loss of lean mass and muscle strength. In a pre-frail and frail population, a strategy combining physical training and nutritional intervention was more likely to result in stable or reduced IL-6 levels in individuals who demonstrated improved physical performance than in those with lower physical performance149. Calorie restriction and physical activity might impede and halt these processes. While we have a better understanding of the role of physical activity in reversing sarcopenic obesity, the effect of a lifetime of inactivity on the development of sarcopenic obesity is still unclear.
Assessing body composition
Gold standard methods to assess body composition, including CT and MRI, allow clinicians to accurately analyse adipose tissue and muscle mass150 (FIG. 2). Steven Heymsfield and colleagues have argued the importance of using measures beyond muscle mass when diagnosing sarcopenic obesity151. The strengths and limitations of each method to assess body composition to diagnose sarcopenia and sarcopenic obesity have been reviewed elsewhere152–155.
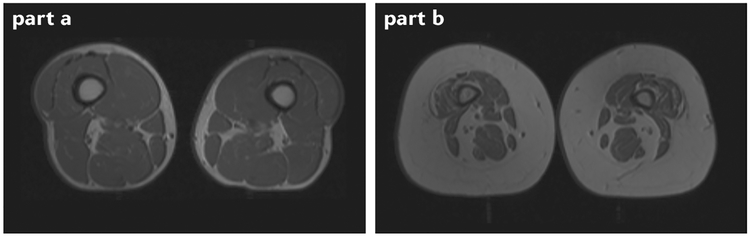
Fig. 2 |. MRi of individuals with and without obesity.
Cross-sectional MRI of the quadriceps area of an individual without obesity with normal muscle characteristics (part a) and an individual with obesity with small muscles and infiltration by adipose tissue (part b) is shown. More muscle tissue is visible in part a than in part b, and the higher intensity signals seen in part b indicate fat infiltration of the muscle. Images courtesy of Edward Weiss, St Louis University School of Medicine, St Louis, MO, USA.
Dual-energy X-ray absorptiometry (DXA) is recommended for the assessment of appendicular lean mass in the diagnosis of sarcopenia156 owing to its affordability, availability and diagnostic accuracy157. DXA correlates well with gold standard measures of body composition, such as MRI, and with bioelectrical impedance, which can also measure fat and segmental muscle mass158–160. Assessments of lean mass are highly reproducible and can be used for clinical monitoring, while a detailed assessment of visceral fat is not as accurate150,161–163. In a 2013 report by the International Society for Clinical Densitometry, the recommendation to perform DXA for assessing total body composition and for a regional analysis of fat and muscle in patients with muscle weakness or poor physical function was categorized as fair157.
Bioelectrical impedance is a simple, non-invasive, inexpensive, rapid and portable diagnostic tool. Reductions in muscle mass result in an increase in connective tissue164–166 that can interfere with the assessment of muscle mass. Variable hydration status also impacts its accuracy167. To use bioelectrical impedance, tissue hydration must be constant and the body must be cylindrical168,169; both assumptions are challenged in sarcopenia and obesity. Thus, an overestimation of the total volume of water and extracellular fluid in the body leads to aberrant values. Further, whether the bioelectrical impedance prediction equations are valid when applied to different ethnic groups is unclear170, despite specific adaptations and adjustments171–173. Biological differences between different ethnic populations might influence the relationship between skeletal mass and resistance174. Other notable limitations include large standard errors and population specificity175. Cut-off points might not capture such determinants, particularly when levels of fat mass are high, which questions the utility of bioelectrical impedance for the assessment of body composition by professional societies169 who recommend adjustment to population-specific, age-appropriate equations169,176. Further validation of bioelectrical impedance results is needed in individuals aged ≥80 years, as they are at increased risk of sarcopenic obesity177,178. Of note, current bioelectrical impedance systems permit an improved protocol that involves segmental analyses in clinical settings, as reviewed elsewhere179.
An evolving definition
The current definitions of sarcopenic obesity are based on the individual definitions of sarcopenia and obesity (TABLE 1), but presently there is no consensus that defines the cut-off points for either of these diseases, which makes arriving at an accurate diagnosis of sarcopenic obesity challenging. The term sarcopenia is defined differently throughout the literature (TABLE 1), leading to confusion in the medical community and preventing any inter-study comparisons. Without a consistent definition of sarcopenia, investigators are limited in their ability to identify participants for interventional research.
Table 1 |.
Selected definitions of sarcopenia with or without obesity
| Author, year and study name (when applicable) | Sarcopenia component | Measurement modality (cut-off points) | Obesity component (cut-off points) | Validated population |
|---|---|---|---|---|
| Newman, 2003 (ref.469) | ALM divided by height squared | DXA (men <7.23kg/m2; women <5.67kg/m2) | BMI (≥30kg/m2) | New Mexico Elder Health Survey |
| ALM divided by height and fat mass | DXA (lowest twentieth percentile of residuals (sex-specific)) | BMI (≥30kg/m2) | Health ABC study | |
| Baumgartner, 2000 (ref.470) | ALM divided by height squared | DXA (men <7.26kg/m2; women <5.45kg/m2) | Body fat (men >27%; women >38%) | New Mexico Aging Process Study |
| Baumgartner, 2004 (ref.191) | ALM divided by height squared | DXA (men <7.26kg/m2; women <5.45kg/m2) | Body fat (men ≥28%; women ≥40%) | New Mexico Elder Health Survey |
| Villareal, 2005, ASN–TOS186 | ALM divided by height squared | ALM (<5.45kg/m2, sex is not specified) | BMI (≥30kg/m2) | Young healthy population |
| Bouchard, 2009 (ref.188) | ALM divided by height squared | DXA (men <8.51kg/m2; women <6.29kg/m2) | Body fat (men ≥28%; women ≥35%) | Nutrition as a Determinant of Successful Aging study |
| Fielding, 2011, IWGSP180 | Physical function | Gait speed (<1m/s) | NA | NA |
| Lean mass | DXA (less than the twentieth percentile healthy adults, ALM divided by height squared: men ≤7.23kg/m2; women ≤5.67kg/m2) | NA | Health ABC | |
| Cruz-Jentoft, 2010, EWGSOP156 | ALM divided by height squared | DXA (men ≤7.26kg/m2; women ≤5.50kg/m2) | NA | Rosetta study |
| DXA (men ≤7.25kg/m2; women ≤5.67kg/m2) | NA | Health ABC study | ||
| DXA (men ≤7.23kg/m2; women ≤5.67kg/m2) | NA | Health ABC study | ||
| Residuals | DXA (ALM (fat mass divided by height), men: −2.29; women: −1.73) | NA | Health ABC study | |
| SMI divided by height squared | BIA (men ≤8.87kg/m2; women ≤6.42kg/m2) | NA | Taiwanese population | |
| ALM divided by height squared | (men: severe ≤8.50kg/m2, moderate 8.51–10.75kg/m2. Women: severe ≤5.75kg/m2; moderate 5.76–6.75kg/m2) | NA | NHANES III study | |
| Muscle strength | Handgrip strength (men <30kg; women <20kg) | NA | InCHIANTI study | |
| Muscle strength based on BMI category | Handgrip strength (males: BMI <24 kg/m2: <29.0kg; BMI 24.1–26.0 kg/m2: <30.0kg; BMI 26.1–28.0 kg/m2: <30.0kg; and BMI >28 kg/m2: <32.0kg; females: BMI <23 kg/m2: <17.0kg; BMI 23.1–26.0 kg/m2: <17.3kg; BMI 26.1–29.0 kg/m2: <18.0kg; and BMI >29 kg/m2: <21.0kg) | NA | Cardiovascular Health Study | |
| Physical performance | SPPB (≤8) | NA | EPESE study | |
| Gait speed over 6m (<1m/s) | NA | Health ABC study | ||
| Gait speed over 6m (<1.175m/s) | NA | Health ABC study | ||
| Gait speed over 15 ft (men ≤1.73m: <0.65m/s; and >1.73m: <0.76m/s; women: ≤1.59m: <0.65m/s; and >1.59m: <0.76m/s) | NA | Cardiovascular Health Study | ||
| Gait speed over 4m (<0.8m/s) | NA | InCHIANTI study | ||
| Studenski, 2014, FNIH182 | Weakness | Handgrip strength (men <26kg; women <16kg) | NA | Multiple study cohorts |
| Handgrip strength:BMI (men <1.0; women <0.56) | NA | Multiple study cohorts | ||
| ALM | Men <19.75kg; women <15.02kg | NA | Multiple study cohorts | |
| ALM:BMI | Men <0.789; women <0.512 | NA | Multiple study cohorts | |
| Asian Working Group for Sarcopenia, 2014 (ref.185) |
ALM divided by height squared | DXA (men <7.0kg/m2; women <5.4kg/m2) | NA | NA |
| BIA (men <7.0kg/m2; women <5.7kg/m2) | NA | NA | ||
| Strength | Handgrip strength (men <26kg; women <18kg) | NA | NA | |
| Performance | Gait speed over 6m (<0.8m/s) | NA | NA |
ABC, Ageing, Body and Body Composition; ALM, appendicular lean mass; ASN, American Society of Nutrition; BIA, bioelectrical impedance; DXA, dual-energy X-ray absorptiometry; EPESE, Established Populations for the Epidemiologic Study of the Elderly; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; IWGSP, International Working Group on Sarcopenia; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; SMI, skeletal muscle index; SPPB, short performance physical battery; TOS, The Obesity Society.
Current definitions of sarcopenia incorporate variations of muscle mass, strength and anthropometric measures including mid-arm and calf circumference. The International Working Group for the Study of Sarcopenia (IWGS) provided a consensus definition for sarcopenia180 as the combination of low whole-body or appendicular lean mass and poor physical functioning (gait speed ≤1 m/s). The European Working Group for the Study of Sarcopenia (EWGSOP)156 identified sarcopenia cut-off points and tools for its measurement. They recognized the lack of diagnostic criteria for sarcopenia but integrated low muscle mass and function (strength or performance) in their terminology, believing that the relationship between these two measures is not linear nor bidirectional73,156,181. This algorithm was meant for clinical application using gait speed (<0.8 m/s) before muscle mass or strength measurement. EWGSOP recommended that muscle mass is assessed by DXA or bioelectrical impedance, using mathematical thresholds and formulas presented in their consensus document. Hand grip strength cut-off points are dependent on an individual’s BMI.
The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project182 suggested a causal, indirect relationship between muscle mass and function in their definition of sarcopenia. The FNIH suggested testing for low lean mass using DXA (defined using appendicular lean mass) and reduced muscle function using handgrip strength. FNIH stated that sex-specific cut-off points could be adjusted for BMI. The separate criteria for muscle mass and strength implied the need to target interventions for individuals with low mass or low strength. FNIH deliberately avoided the term sarcopenia to differentiate between qualitative (strength) and quantitative (mass) components.
These definitions provide excellent negative percent agreements on the absence of sarcopenia; however, there is poor overlap in identifying individuals with sarcopenia183. Ethnic-specific differences result in inaccurate prevalence estimates184. The Asian Working Group for Sarcopenia185 provided guidance for individuals of Asian descent. They suggested using handgrip strength and gait speed for initial testing and/or screening followed by the EWGSOP approach for muscle mass measurement, strength and physical performance, with different, lower, cut-off points (TABLE 1).
Obesity is defined as an unhealthy excess body fat that increases the risk of medical illness and mortality186. As with sarcopenia, no consensus defines obesity cutoff points. Instead, cutoff points are premised on sex-specific, whole-body DXA. The American Association of Clinical Endocrinology187 recommends the use of the WHO body fat thresholds for the diagnosis of obesity — (men >25% body fat and women >35% body fat). The WHO thresholds also used BMI for obesity (≥30 kg/m2) or waist circumference (men ≥102 cm and women ≥88 cm) as a visceral fat surrogate. The International Society of Clinical Densitometry, the American Heart Association and The Obesity Society all recognize the lack of specific thresholds, while the American College of Sports Medicine suggests cut-off points of 28% and 35% for men and women, respectively188,189. Others applied mathematical body fat thresholds of reference populations to provide sufficient power that might not be based on distal outcomes190,191. Body fat has better predictive validity on the development of the metabolic syndrome192 and cardiovascular disease risk193 than BMI.
Body composition modalities have advantages and disadvantages in assessing changes in fat or muscle distribution. We suggest DXA for research purposes, as it is more readily available to provide the necessary information. If DXA is unavailable, a stand-alone or portable bioelectrical impedance system can be used. We advocate caution when using bioelectrical impedance equations; they must account for age, sex, levels of physical activity, body fat and ethnicity. Feasible anthropometric indices as surrogates for adiposity, including BMI and waist circumference, have poor sensitivity. In one study, BMI correctly classified 41.0% of men and 45.1% of women as being obese and waist circumference correctly classified 64.2% of men and 81.0% ofwomen as being obese194. We believe that anthropometric measures should be used with great caution when assessing body composition and only if other imaging is unavailable.
With ageing, fat preferentially accumulates both viscerally and ectopically rather than as abdominal subcutaneous fat. Rapid accumulation of intra-abdominal fat is exacerbated by physical inactivity, hormonal changes, reduced responsiveness to thyroid hormone and leptin resistance186. As central fat accumulation predominates, and loss of muscle occurs peripherally, the prototype of sarcopenic obesity is easily recognized (‘fat frail’). This prototype is not inconsistent with intramuscular fat accumulation, which contributes to inflammation, mitochondrial dysfunction and insulin resistance within muscle and reduces muscle protein synthesis74,195.
Prevalence of sarcopenic obesity
A shortcoming in ascertaining accurate prevalence rates for sarcopenic obesity is the lack of a consistent definition for either sarcopenia or obesity. A review of eight definitions for sarcopenic obesity noted a 19-fold to 26-fold variation in sex-specific rates178. The analysis showed that definitions for sarcopenia were highly dependent on mathematical thresholds, reference populations and muscle mass definitions. A comparison of the rates of sarcopenic obesity using bioelectrical impedance to define sarcopenia and percentage of body fat to define obesity showed increasing rates with age196. In another study, the authors identified individuals with a BMI ≥35 kg/m2 and evaluated the prevalence of sarcopenic obesity using DXA-defined body fat in 120 predominantly female adults (46.9 ± 11.0 years). The investigators reported rates that ranged from 0–84.5% in women to 0–100% in males depending upon the definition applied197. In a population-based cohort using National Health and Nutrition Examination Survey (NHANES) data that applied the aforementioned FNIH criteria for appendicular lean mass, rates of sarcopenic obesity were 12.6% in men and 33.5% in women. The rates of sarcopenic obesity increased with age, reaching 48.0% and 27.5% in females and males, respectively, in those aged over 80 years198. In a cohort of individuals from South Korea’s Korean Sarcopenic Obesity Study, an ongoing epidemiological, prospective cohort of healthy volunteers aged 20–80 years, prevalence of sarcopenic obesity ranged from 1.3–15.4% in men to 0.8–22.3% in women199.
The prevalence of low muscle strength with obesity is less clear. Data from the InCHIANTI study noted rates of 3.2–8.7% using the low knee extensor strength with either high BMI or waist circumferrence200. Investigators from the Cardiovascular Health Study used low grip strength and high waist circumference to define low muscle strength and obesity. Rates approached 11.1%201, while data from FNIH classified 4.1% of men and 14.0% of women as having sarcopenic obesity using high BMI and low grip strength as measures of obesity and low muscle strength, respectively202. Overlap using different diagnostic criteria of sarcopenic obesity is limited183 but ranges from 2.1% to 4.1%. These findings are also observed when evaluating the overlap of sarcopenia-only definitions, which is less than 50%183.
Consequences of sarcopenic obesity
Cross-sectional and longitudinal studies are subject to the same definitional challenges as prevalence studies. Despite the crucial need for a consensus definition, here we describe the clinical importance of sarcopenic obesity.
Disability or impairments.
Richard Baumgartner and colleagues were the first to characterize the association between sarcopenia (as defined by appendicular lean mass) and percent body fat on incident disability191. In their analysis, when compared with a healthy body composition, sarcopenic obesity was associated with a relative risk of incident disability over 8 years that was 2.63 (95% CI 1.19–5.85). In addition, when compared with a healthy body composition, a combination of obesity, as defined by percentage of body fat, with low muscle mass represented odds ratios of difficulty ascending and descending stairs that were 2.60 and 2.35 higher, respectively203. The Concord Health and Aging project204 used the FNIH criteria for sarcopenia with elevated body fat to evaluate frailty and reported that sarcopenic obesity resulted in an increased risk of frailty (OR 2.00, 95% CI 1.42–2.82), activity of daily living disability (OR 1.58, 95% CI 1.12–2.24) and instrumental activity of daily living disability (OR 1.36, 95% CI 1.05–1.76). The above results contrast with an earlier cross-sectional study that defined sarcopenic obesity by low muscle mass and elevated body fat and did not demonstrate differences in disability compared with controls205. Another group used DXA to assess body mass and its relationship with physical capacity and found mixed results188. Data from the Quebec Longitudinal Study applied definitions of sarcopenic obesity comprising Baumgarter’s definitions of sarcopenia and obesity as defined by body fat191 and found global physical capacity scores were no different between obese groups (sarcopenia versus non-sarcopenia (P = 0.14 in men and P = 0.19 in women)), but lower scores were observed than with the non-sarcopenic non-obese group (P < 0.05)188. Women with sarcopenia alone had higher scores than people with obesity without sarcopenia and than individuals with obesity and sarcopenia (P < 0.01).
Muscle strength is a stronger predictor of long-term functional decline than muscle mass206. Data from the Osteoarthritis Initiative showed that a combination of low knee extensor strength with high BMI was associated with reduced gait speed and reduced Late-Life Function and Disability Index and Short Form-12 scores207, which indicate a lower degree of physical function208 and decreased self-reported health status209.
Low handgrip strength and elevated BMI were strongly associated with an increased risk of functional decline210. In addition, data from the UK Biobank study found an association between high BMI, low grip strength and reduced long-term physical activity211. Data from the InCHIANTI study showed that mobility disability trajectories and gait speed over 6 years were steepest in individuals with obesity as defined by BMI and low muscle strength200. An increase in mobility disability and risk of hospitalization (OR 2.10, 95% CI 1.14–3.88) was associated with low muscle strength and abdominal obesity in an 11-year follow-up study (OR 1.36, 95% CI 1.04–1.78). High BMI and low muscle strength were related to limitation in mobility at 2-year follow-up (OR 3.88, 95% CI 1.08–13.91)212. In another study, abdominal visceral fat and quadriceps muscle area served as markers for central obesity and sarcopenia, respectively, and were associated with postural instability213.
Metabolic impairments.
A study from the South Korean NHANES conducted an evaluation of sarcopenia (as defined by muscle mass) with obesity (as defined by a BMI ≥25 kg/m2). The authors reported that individuals with sarcopenic obesity were at an increased risk of dyslipidaemia (OR 2.82, 95% CI 1.76–4.51)214 and had significant positive associations with insulin resistance as defined by HOMA scores and triglycerides215. In another study, low handgrip strength and high waist circumference and/or BMI were significantly associated with elevated levels of IL-6, C-reactive protein and IL-1 (REF57) but conflicted with results using the FNIH criteria to define low lean mass216. By contrast, low muscle strength (as defined by the FNIH criteria) with an elevated BMI was not associated with differences in metabolic components among groups of postmenopausal women aged 55–75 years217.
Comorbidities.
Individuals with sarcopenic obesity have a higher risk (OR 3.51, 95% CI 2.15–5.75) of radiographic knee osteoarthritis218 than individuals in the non-sarcopenic obesity group. One study reported that risk of falling was highest in individuals with low muscle mass and/or strength with obesity as defined by percentage of body fat219, but spine and total BMD were lower in individuals who were sarcopenic obese and dynapenic obese than in individuals with obesity alone220. A study that evaluated participants over 6 years reported that the combination of obesity as defined by BMI and low handgrip strength suggested an increased risk of type 2 diabetes mellitus (OR 3.57, 95% CI 2.04–6.24), an association that was not observed with cardiovascular disease221. The rate of depression has been reported as being highest in patients with sarcopenic obesity (defined as low handgrip strength and obesity defined by BMI) (OR 1.79, 95% CI 1.10–2.89) over 4 years222 compared with non-obese individuals in the highest tertile of grip strength. These data were confirmed in another study that defined sarcopenic obesity as low muscle mass or muscle strength, with obesity defined by percentage of body fat223. Individuals with low muscle mass and high waist circumference had worse psychological health and higher stress than individuals with normal muscle mass and normal waist circumference. Finally, an area of interest for researchers now is the role of sarcopenic obesity in cancer224, which further demonstrates its relationship with adverse health events.
Mortality.
Epidemiological studies investigating the relationship between sarcopenic obesity and mortality have reported conflicting results198,204,221,225–228. A longitudinal study from 2017 demonstrated small differences in all-cause mortality between obesity as defined by both BMI and low muscle strength and low muscle strength alone229. Others showed that mortality was significantly elevated in people with sarcopenic obesity, which was defined using mid-arm circumference (HR 1.46, 95% CI 1.23–1.73) and muscle strength with waist circumference (HR 1.23, 95% CI 1.09–1.38)230. Sarcopenic obesity (defined by muscle mass assessed by bioelectrical impedance and percentage of body fat) was associated with an increased mortality (HR 1.29, 95% CI 1.03–1.60)230. Muscle strength also affects mortality independent of muscle mass. Investigators from the Health, Aging and Body Composition study reported that low quadriceps strength was associated with increased mortality231. Similar results were reported in another study that showed that reduced leg isometric strength and increased waist circumference were associated with increased mortality232 (HR 2.46, 95% CI 1.34–4.52). These results were further corroborated in the MiniFinland Health Examination Study, which also showed that reduced muscle strength is associated with increased mortality (HR 1.30, 95% CI 1.09–1.54)228. Cutoffs specific to individuals from South Korea predicted higher mortality risk than the FNIH cut-off233. Finally, a recent meta-analysis found that mortality was highest in patients with sarcopenic obesity (HR 1.24, 95% CI 1.12–1.37) compared with healthy individuals, but the authors acknowledged that they had used multiple definitions of sarcopenic obesity in their study230.
Quality of life.
Few studies have evaluated the effect of sarcopenic obesity on quality of life. Sarcopenic obesity (as defined by low appendicular lean mass normalized for height2 and increased BMI) was associated with unfavourable scores on the Medical Outcomes Survey234. Another study reported no differences in Short Form-36 scores, which provide a measure of quality of life235, between individuals with obesity and low handgrip strength and individuals with normal indices236. The EuroQOL score was dependent on cardiovascular fitness rather than sarcopenic obesity237. Future studies need to focus on health-related quality of life and patient-reported outcomes in sarcopenic obesity before we are able to draw firm conclusions.
Institutionalization and health-care utilization.
Few studies, and no known longitudinal studies, have evaluated the relationship between sarcopenic obesity and institutionalization. Peggy Cawthon and colleagues238 reported that neither sarcopenia nor the components that define weakness increased the risk of hospitalization or short-term nursing facility stay. A population-based cohort study that defined sarcopenia using the EWGSOP criteria found an increased incidence of long-term care certification in patients with sarcopenia239. Low muscle mass or strength is causally associated with long-term care placement. The relationship with obesity is clearer, whereby an elevated BMI is associated with admission to a nursing home240. Midlife obesity also increases the risk of long-term care placement241, an association that persists in older adults with obesity242.
Treatments for sarcopenic obesity
Lifestyle interventions, including calorie restriction and physical activity, are hallmarks of treating sarcopenic obesity (TABLE 2). Few clinical trials specifically focus on sarcopenic obesity243; however, intentional weight loss in older adults improves morbidity and physical function186. Following a meta-analysis of randomized trials of adults with obesity aged ≥55 years, which had follow-up times of ≥4 years, investigators reported a 16% reduction in mortality (95% CI 0.71–0.99)243. In the United States, while Medicare covers weight loss therapy244, no major societies outline targeted therapies for sarcopenic obesity187,245.
Table 2 |.
Potential approved therapies in sarcopenic obesity
| component | goal | Suggested approach |
|---|---|---|
| Calorie restriction | Lose body fat and improve physical function | 500–1,000kcal per day |
| ~0.5kg per week aiming for 8–10% weight loss at 6 months followed by weight loss maintenance | ||
| No specific diets are proven in this population | ||
| Aerobic exercises | Improve cardiorespiratory fitness | 150 min per week of moderate to vigorous aerobic exercise |
| Resistance exercises | Improve muscle strength and mass; attenuate loss of muscle and bone during weight loss efforts | 60–75 min of resistance training 3 times weekly, separated by one day focusing on strength, balance and flexibility |
| Protein supplementation | Mitigate loss of muscle mass and strength | 1.0–1.2g/kg per day of protein in divided doses (25–30g daily) |
| 2.5–2.8g leucine daily | ||
| Calcium supplementation | Prevent potential disturbances in bone metabolism | 1,200 mg per day of supplemental calcium, preferably through dietary measures |
| Vitamin D supplementation | Prevent potential disturbances in bone metabolism | 1,000 IU vitamin D per day, ideally maintaining blood levels ≥30 ng/ml |
IU, international units.
Dennis Villareal’s work best corresponds to participants with sarcopenic obesity as defined by obesity with evidence of physical frailty246,247. In this cohort of patients, weight loss alone or exercise alone improved physical function; however, a combination of weight loss and regular exercise improved physical function and ameliorated frailty more than either intervention alone246. Moreover, another study reported that weight loss plus combined aerobic and resistance exercise was the most effective method for improving functional status of adults aged 65 years and older with obesity247 (FIG. 3). Hung-Ting Chen and colleagues248 evaluated four groups of individuals with sarcopenic obesity according to different exercise interventions (aerobic, resistance, combined aerobic and resistance), and controls who were prohibited from engaging in exercise, and demonstrated that individuals in the resistance training group had the greatest improvements in strength.
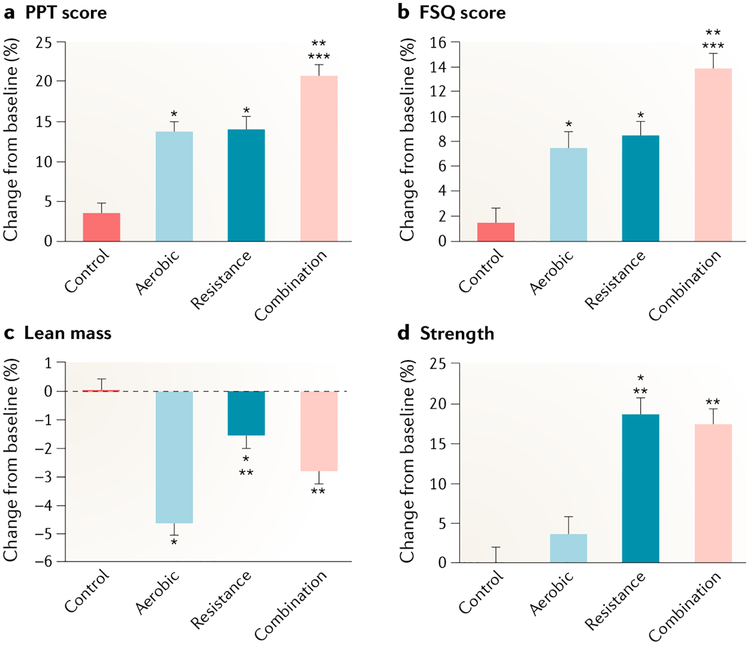
Fig. 3 |. Mean percentage changes in physical function and lean mass during the weight loss interventions.
Measures used included a physical performance test (PPT) (scores range from 0 to 36, with higher scores indicating better functional status) (part a); the Functional Status Questionnaire (FSQ) (scores range from 0 to 36, with higher scores indicating better functional status) (part b); lean mass (part c); and strength (measured as total one repetition maximum (that is, the total of the maximum weight a participant can lift, in one attempt, in the bicep curl, bench press, seated row, knee extension, knee flexion and leg press)) (part d). Scores on the PPT were used as an objective measure of frailty (primary outcome), and scores on the FSQ were used as a subjective measure of frailty. Percentage changes are presented as least-squares-adjusted means; T bars indicate standard errors. *P < 0.05 for the comparison with the control group. **P < 0.05 for the comparison with the aerobic group. ***P < 0.05 for the comparison with the resistance group. Figure adapted with permission from REF247, New England Journal of Medicine, Villareal, D. T. et al. Aerobic or resistance exercise, or both in dieting obese older adults, 376, 1943–1955 Copyright © (2017) Massachusetts Medical Society. Reprinted with permission.
Dietary strategies: calorie restriction and protein supplementation.
Dieticians are multidisciplinary team members integral to developing lifestyle interventions whose delivery is often grounded in behavioural theories and motivational interviewing249. Weight loss trials tend to restrict calories by 500–1,000 kcal per day250. Initial weight loss goals of ~0.5 kg per week can lead to an 8–10% loss in 6 months, with most patients sustaining an 8–10 kg loss in weight during this period of time. We are unaware of specifically tested diets in sarcopenic obesity. As in other populations, diets in patients with sarcopenic obesity lead to weight loss251, with adherence to a diet predicting weight loss success252.
Strategies that optimize protein anabolism during weight loss, such as consumption before exercise or spreading out of protein during the day, can prevent weight loss-induced sarcopenia247,250. Energy deficits created by acute calorie restriction could downregulate muscle protein synthesis and increase proteolysis, which contributes to reduced muscle mass247,253,254; however, chronic calorie restriction does not seem to reduce muscle protein synthesis, but it might increase it105,255. Increased dietary protein stimulates muscle protein synthesis71,256. The source of protein, timing of intake257 and specific amino acid constituents can also be factors in increasing muscle mass and strength. High protein intake (1.2 g of protein per kg per day) during weight loss might eliminate the beneficial effect of weight loss on insulin sensitivity in skeletal muscle258. Distributing protein intake throughout the day259 or pulse feeding at main meals260 could be beneficial for the stimulation of muscle protein synthesis in patients with sarcopenic obesity.
The PROT-Age group recommends 1.0–1.1 g/kg protein per day in divided doses, acknowledging that a ‘one size fits all protein recommendation’ fails to account for the complex physiological changes of ageing71. Generally, 25.0–30.0 g of protein containing 2.5–2.8 g of leucine can slow frailty261,262. Early pilot studies demonstrate that meals enhanced with protein and coupled with a weight loss intervention improve physical function263. For example, a high- protein diet in conjunction with resistance training preserved appendicular lean mass during weight loss264. In a pilot study, participants with sarcopenic obesity undergoing a weight loss programme augmented by a high-protein diet showed improvements in muscle strength and Short Form-36 scores (REF265). We need further evidence to support the effect of supplemental protein on functional outcomes in patients with sarcopenic obesity266–268. High-protein diets consisting of 1.0–1.2 g per kg per day should be prescribed with caution to prevent renal dysfunction269 as evidenced by observational data270–272, as higher doses have recently demonstrated no changes in lean mass273. We recommend the importance of ensuring adequate protein intake in countering weight loss-induced sarcopenia in individuals with sarcopenic obesity participating in programmes. Careful medical monitoring and dietary planning are required when optimizing protein intake while limiting calorie restriction, and this often needs to be administered under the auspices of a registered dietician with expertise in this population. The challenges in limiting calories are recognized, and hence we believe that alternative approaches are crucially needed to augment muscle mass and strength.
Resistance training and aerobic exercise.
Several professional societies3,187,245,274 recommend that all older adults engage in at least 150 min per week of moderate to vigorous aerobic exercise, with two sessions of resistance exercises consisting of strength training, flexibility and balance. Aerobic exercise and resistance training are safe, even in patients who are at a high risk of falling275. Aerobic exercise improves cardiorespiratory fitness and has beneficial effects on mortality276–278. Even minimal resistance exercise improves muscle strength and mass279,280, and progressive resistance exercises counter sarcopenia by increasing strength. As with any exercise programme, clinical consultation and medical clearance is advised.
A Cochrane review reporting physical outcomes of progressive resistance exercises for older people identified 33 trials that significantly improved physical abilities (standardized mean difference 0.14, 95% CI 0.05–0.22) in 2,172 participants, with improvements in muscle strength (standardized mean difference 0.84, 95% CI 0.67–1.00) in 73 trials281 (3,059 participants). The LIFE study282, a structured, moderate-intensity physical activity programme, demonstrated reduced persistent mobility disability (HR 0.72, 95% CI 0.57–0.91) compared with a health education programme. Evaluation of four groups of men and women aged 60–75 years with sarcopenia demonstrated that 2 days of high-resistance concentric exercise with one bout of low-resistance exercise increased muscle expression of pro-inflammatory cytokine receptors, maximized muscle mass and total lean mass and improved knee extension283. A secondary subset analysis ofthe LIFE pilot study found that the short physical performance battery — an objective assessment tool for the evaluation of lower extremity function (higher score equals better function)284 — of patients with sarcopenia improved from 7.4 to 8.7 when compared with the successful ageing group285. Although the LIFE study is considered a standard for physical activity in older adults282, we acknowledge its lack of evidence in sarcopenic obesity and the lack of power in this pilot trial.
High-intensity resistance training combined with short resting intervals improves body composition, muscle and functional performance in men aged 68 ± 4.1 years286. High-speed resistance training over 12 weeks induced greater improvements in muscle power and functional capacity than low-speed training287. In this study of 60 women of Hispanic descent aged over 60 years, high-speed training consisted of individuals performing exercises as fast as possible (1 second or less) and was compared with low-speed resistance training (3 seconds). The authors of this study also demonstrated that two versus three training sessions per week for 12 weeks of high-speed resistance training were equally effective for improving physical performance and quality of life288. High-velocity knee extension training at 240° of movement per second increases the expression of MYH6 and MYH9 mRNA and improves muscle enhancement138.
The effect of power training (moving resistance at higher speed) on function requires further investigation289,290. A pilot study of patients with sarcopenic obesity (defined using EWGSOP criteria for sarcopenia and BMI for obesity) were randomized to a strength and hypertrophy group or a high-speed circuit group for 15 weeks289. High-speed circuit training was associated with nonsignificant improvements between groups in short performance physical battery (mean difference 1.1, 95% CI–0.1 to 2.4; P = 0.08) and power (mean difference 158 W, 95% CI 2–315; P = 0.01). We note that while these trials enrolled patients with sarcopenic obesity in each arm, they are small, short-term studies.
Other exercise therapies, including tai chi or yoga, could potentially be beneficial; however, to our knowledge, no studies have evaluated these modalities in sarcopenic obesity. Tai chi and The Otago Exercise Programme (a home-based balance and strength fall prevention programme) have been shown to be effective at preventing falls and improving physical function, mobility and functional measures of lower extremity strength in older adults291. A meta-analysis of 18 trials (n = 3,824), including study participants greater than 65 years who participated in tai chi for a minimum of 4 weeks (range 1–12 months) 1–3 times per week, demonstrated a reduction in falls of 20% (relative risk (RR) 0.8, 95% CI 0.72–0.88)292. In addition, yoga has been shown to improve mobility in participants 60 years of age and older, with no restriction on their characteristics, whose follow-up ranged from 8 to 24 weeks (total duration 8–36 hours of yoga)293. A meta-analysis of 28 studies demonstrated a positive effect of aquatic exercises on physical functioning (RR 0.70, 95% CI 0.48–0.92) compared with no training (control group)294. Furthermore, the data suggested that aquatic exercises are as effective as land-based exercises (standardized mean difference 0.39, 95% CI 0.12–0.66). Finally, while training until failure might be an approach for muscle strengthening and endurance295, we generally recommend exercising until fatigue rather than failure, as exercising until failure can increase the risk of musculoskeletal injury.
We advocate individualized exercise treatment for patients with sarcopenic obesity because of the associated medical comorbidity and disability. As previously described, the exercise programme296 should begin at a fairly low-to-moderate intensity, duration and frequency to minimize injury and maximize adherence; this approach progressively induces exercise adaptations246,247. Aerobic activity should target ~65% of the peak heart rate, aiming to reach 70-–85% of peak heart rate over the duration of the exercise regimen. Resistance activities, on the other hand, should focus on 1–2 sets of 8–12 repetitions at ~65% of one repetition maximum, which is defined as the maximal amount of force a person generates in a single repetition, with the aim of advancing to a goal of 2–3 sets of 75% of one repetition maximum over time. These activities are recommended even for frail, older adults246,247.
Calorie restriction and physical activity.
A trial of older adults with obesity247 consisted of a hypocaloric diet with an energy deficit of 500–750 kcal per day on average, 1 g high-quality protein, plus either 60 min of progressive aerobic exercise and resistance training or 75–90 min of both aerobic exercise and resistance training, three times a week. The findings demonstrated increases in physical performance test scores (higher score equals higher level of function), more so in the combined aerobic and resistance exercise group (27.9 to 33.4 points (21% increase)) than in the aerobic (29.3 to 33.2 points (14% increase)) or resistance group (28.8 to 32.7 points (14% increase)) alone. Other activities for weight loss therapy in older adults reflect similar components and outcomes and have produced similar findings250,297. There are few multicomponent studies in patients with sarcopenic obesity. A meta-analysis showed that aerobic exercise and resistance were useful tools to preserve fat-free mass in adults aged ≥50 years who were engaged in a moderate energy restriction-induced weight loss programme298. Increased muscle mass and reduced total and visceral fat over an 8-week intervention were observed in predominantly female individuals, whose mean age was 69 years, with sarcopenic obesity engaged in resistance training248. A resistance programme of participants fulfilling the EWGSOP criteria for sarcopenia and obesity as defined by percentage body fat demonstrated reduced rates of sarcopenia and improved physical function following three training sessions weekly over a 12-week period compared with a control group receiving no intervention297. A combined treatment of diet and exercise improved physical function in frail older men with obesity aged ≥65 years for 1 year, despite resulting in a reduction in oestradiol levels and only a modest increase in testosterone levels299.
Combining both diet and exercise can positively improve adipose markers of adiponectin and significantly reduce leptin levels. In response to a 6-month randomized diet and exercise intervention, levels of C-reactive protein and IL-6 decreased in older adults (age ≥65 years) with obesity (BMI ≥30 kg/m2) compared with controls (−2.5 versus 0.8 mg/l (P < 0.05) and −2.4 versus 1.6 pg/ml (P < 0.05), respectively)300. Yet, the positive effects on circulating cytokines, adiponectin and TNF were due to diet and not exercise301, which is consistent with the direct effect of exercise on or within muscle not being reflected in the circulation133,134. A study that investigated the effect of diet or diet and exercise interventions in individuals aged 50–79 years with overweight or obesity reported that levels of adiponectin increased in individuals with overweight or obesity compared with controls (diet resulted in 9.5% increase in adiponectin (P < 0.001), and diet and exercise resulted in a 6.6% increase in adiponectin (P < 0.001)). Furthermore, levels of leptin in individuals with obesity or overweight decreased by 27.1% in the diet group and 40.1% in the diet and exercise group302.
Investigators in the LIFE pilot study reported that individuals in the physical activity group had reductions in IL-8 but no differences in other inflammatory markers303. A 12-week aerobic exercise regime in combination with a low glycaemic index diet or high glycaemic index diet resulted in reductions in leptin levels in two groups of participants who had elevated levels of adiponectin, suggesting that the reductions in leptin were a result of exercise training and independent of dietary glycaemic index304. Another study reported that in postmenopausal women with overweight or obesity, adding aerobic activity to calorie restriction increased serum concentrations of adiponectin (6.9 μg/ml for individuals in the group without aerobic activity versus 8.5 μg/ml for individuals in the group with aerobic activity (P < 0.001))305. Levels of adiponectin were also elevated following a multicomponent, randomized lifestyle intervention study that investigated the mRNA expression of adiponectin and its receptor in skeletal muscle in adults with impaired glucose tolerance who were aged ≥60 years and had a BMI of 30–40 kg/m2 (REF306). These data suggest that improved insulin sensitivity is due, in part, to the distribution of adiponectin across various tissues and an upregulation in the expression of its receptor. Other conflicting data suggest that in patients with knee osteoarthritis, weight training combined with walking three times a week for 1 hour does not have any significant effect on levels of TNF, IL-6 or C-reactive protein307. The addition of weight loss of 0.3 kg per week for 6 months to physical activity in older community-dwelling adults with obesity or overweight results in a greater reduction in serum levels of leptin and IL-6 than either physical activity alone or a successful ageing health education intervention308.
Risks of weight loss in older adults
Energy restriction with a hypocaloric diet with or without exercise results in the loss of approximately one-quarter of lean mass per unit weight, which could worsen sarcopenia and osteopenia154. A total of 33 intervention studies lasting 8–24 weeks reported that unopposed calorie restriction without resistance training leads to the loss of muscle mass and loss of handgrip strength of up to 4.6% and 1.7 kg, respectively309. Unopposed diet therapy without exercise in older frail adults ≥65 years with obesity (BMI ≥30 kg/m2) led to a marked loss of lean mass at 6 months and 1 year (−3.5 kg and −3.2 kg, respectively) compared with the diet and exercise group, where the loss of lean mass was partially mitigated (−1.7 kg and −1.8 kg, respectively)246. In the Look AHEAD trial, total skeletal mass decreased in both of the intensive lifestyle groups and in the diabetes support and education group (−1.4 kg; P < 0.001). The researchers reported that patients in the intervention group regained appendicular lean mass during the second year and that weight loss was 5.2 kg less in the intervention group than in participants in the control group, whose weight did not markedly change after the second year310. A review of 52 studies reported that loss of fat-free mass as a proportion of overall weight was attenuated after combining exercise with calorie restriction298.
Weight loss in younger adults (age 45–65 years) led to loss of lean mass after calorie restriction (4% reduction in lean mass; P < 0.0001), which was partially lessened by augmentation with aerobic activity (2% reduction in lean mass in participants who had augmented weight loss with aerobic activity; P = 0.05)311. One study evaluated the effectiveness of low-fat diets versus carbohydrate restricted diets with or without progressive resistance exercise on fat-free mass in 42 men with the metabolic syndrome whose age was 59 ± 7 years. Percent weight loss from appendicular lean mass dropped markedly more in the low-fat and no exercise group than in the other groups, suggesting that this intervention has a detrimental effect on appendicular lean mass312.
Obesity is inversely related to BMD and fractures313 but might increase the fracture risk through bone quality314,315 or frailty132,316 independent of BMD. Adipose tissue has been shown to be inversely associated with bone material strength and positively associated with cortical porosity, indicating an adverse effect of adipose tissue on bone microstructure317. Calorie restriction alters bone metabolism, resulting in the loss of BMD in the hip without effects on lumbar spine318, even after a 4-month restriction319. Increases in bone markers such as osteocalcin and of carboxy-terminal telopeptide (C-telopeptide) and N-terminal telopeptide (N-telopeptide) oftype I collagen were observed. Levels of osteocalcin were increased in the diet-only group (36 ± 11.6%), yet its levels were no different than baseline in individuals on diet coupled with exercise; increased differences were reported in the disposition index (an index of insulin secretion after correction for insulin resistance) in the diet–exercise group (92.4 ± 11.4%) compared with the diet-only group (61.9 ± 15.3%) at 12 months320. Loss of BMD in older adults with obesity seems to continue during long-term lifestyle change in the opposite direction to the weight changes321. These findings suggest that BMD and markers of bone turnover following long-term calorie restriction show larger changes in patients than in healthy control participants advised to continue their current diet322.
In one study, the authors reported that trabecular bone microarchitecture was no different in calorie- restricted participants (~35% less calories than controls) than in middle-aged individuals eating a Western diet323. Furthermore, trabecular geometry, cortical geometry and strength were no different in individuals undergoing intentional weight loss through calorie restriction or weight maintenance for 6 months324, which suggests that calorie restriction has protective effects on bone quality. However, 2017 data from the Look AHEAD trial showed that long-term intentional weight loss was associated with a 39% increased risk of fragility fractures325. Very-low energy or protein-sparing diets to induce rapid weight loss are not recommended owing to potential loss of muscle mass, strength and bone and risks of dramatic fluid, electrolyte and water shifts owing to protein shifts; however, a preliminary, short-term study in a population of individuals ≥65 years of age suggests potential benefits326. Studies emphasize exercise training during calorie restriction to prevent an increase in bone turn-over327 and an increase in serum levels of sclerostin328, thus minimizing bone loss. Whether weight loss and exercise lower overall risk of falls and fractures despite the decline in BMD is unknown, suggesting the need for formal evaluation in future studies.
Supplementation with calcium and vitamin D.
Conventional strategies to minimize the effect of weight loss on bone metabolism, including up to 1,200 mg supplemental calcium per day and 800–1,000 international units (IU) per day of vitamin D3, are needed to minimize the risk of weight loss-induced BMD reduction329. Oral calcium should be coupled with vitamin D to mitigate the potential risks of unopposed supplementation330. Supplementing vitamin D in patients with sarcopenic obesity can potentially influence and improve muscle function331 and proximal muscle weakness332 through the actions ofvitamin D metabolites333. Vitamin D deficiency is associated with an increased risk of falls and fractures, and reduced muscle mass and strength334–338, independent of obesity. We agree with the American Geriatrics Society recommendation of 1,000 IU of vitamin D3 per day with calcium among non-institutionalized adults aged ≥65 years334, to maintain serum levels of vitamin D at ≥30 ng/ml.
Future directions and emerging therapies
We anticipate that a deeper understanding of sarcopenic obesity will emerge over the next decade, which will ultimately bridge the divide between clinical practice and research. Here, we outline the major gaps of knowledge and advancements needed to further the field (BOX 1).
Box 1 |. Emerging therapies in sarcopenic obesity.
Anamorelin
A ghrelin analogue used in cancer cachexia that could promote appetite and enhance lean mass with anti-inflammatory and anabolic properties.
Bariatric surgery
The safety and efficacy of different procedures (Roux-en-Y, gastric band and gastric sleeve) are currently unknown but can be considered in carefully selected older adults aged 65 years and older.
Mesenchymal stem cells
Shared precursors of muscle, bone and cartilage that hold promise in the regeneration of muscle tissue. Barriers exist but these cells may play a promising role in the future management of sarcopenia.
Myostatin inhibitors
A treatment type with biological plausibility for improving physical function by enhancing skeletal muscle growth development. This class of therapy can directly inhibit muscle loss, with data suggesting improvements in physical function in patients with cancer.
Neuromuscular activation
Whole-body vibration therapy (using electrical stimuli) or tai chi can enhance muscle contraction efficiency and function.
Periodization strategies
Systematic variation in training specificity, intensity and volume used in sports programmes to achieve peak physical performance. May be feasibly prescribed in sedentary, frail, older adults to improve function but it is premature to endorse these strategies.
Testosterone and selective androgen receptor modulators
Important regulators of body composition that increase muscle and bone mass by increasing insulin-like growth factor 1 (IGF1) and decreasing inflammatory markers. Data on their impact on muscle strength and function are conflicting. Selective androgen receptor modulators (that is, enobosarm) preferentially target androgen receptors on muscle and bone, sparing the androgenic impact elsewhere in the body. Early efficacy studies demonstrate improved lean mass and function in patients with cancer.
Weight loss therapies
Anti-obesity medications (liraglutide, lorcaserin, phentermine, topiramate, bupropion and orlistat) are approved for use in non-geriatric populations with weight loss as an indication. Their use is restricted to off-label use for weight loss, and few data exist on their safety and efficacy in this population.
Vitamin K
Inhibits bone resorption and osteoclast formation and may be helpful in mitigating bone loss following intentional weight loss. Supplementation may increase bone resorption markers, although conflictive data exist on its effect on BMD and fractures.
Harmonizing a definition.
The most notable barrier to advancing the science in targeting this condition is the lack of a consistent definition for sarcopenic obesity. While the criteria for identifying and classifying subcutaneous or visceral adiposity are somewhat consistent, major progress is needed regarding the definition of sarcopenia. Advancing our understanding of the relative contributions of strength and muscle mass — as well as their differences — might help. The introduction in 2016 of an International Classification of Diseases 10 code for sarcopenia (M62.84) will permit clinical recognition and promote its diagnosis, classification and drug development339,340. Different populations, ethnicities and sexes require specific diagnostic thresholds; therefore, integrating highly accurate body composition measures into clinical settings will encourage clinical identification of sarcopenic obesity. The disparate classification has impeded progress in this field.
Integrating methods for analysing body composition into clinical practice.
To promote the translation of methods for assessing body composition, including CT, MRI and DXA, into routine care, we acknowledge the need to remove regulatory and operational obstacles, particularly in the United States. For instance, DXA is routinely performed for screening and assessment of osteoporosis and is generally covered by insurance for this indication341. Older adults often receive gold standard imaging, which can accurately ascertain muscle and fat content, for indications other than sarcopenic obesity, such as abdominal pain or back pain342,343. Assessing muscle strength (using handgrip dynamometry) and muscle mass (using DXA, bioelectrical impedance or other modalities) can fill a clinical gap in identifying sarcopenic obesity. Widespread availability of DXA even in low-resource areas344 permits this evaluation. Future studies should focus on dissemination and implementation strategies of using such diagnostics.
Epidemiology and clinical outcomes.
Further work is required to elucidate the descriptive epidemiology of sarcopenic obesity regarding important outcomes beyond weight loss, comorbidity and mortality. Though experts currently debate a unifying definition, one will ultimately become accepted, standardized and implemented. Until then, useful and cost-effective measures, including grip strength, gait speed, the short performance physical battery and/or bioelectrical impedance or DXA, should continue to be used in clinical and research arenas345–347. Focusing on patient-centred outcomes, including physical function and quality of life, is important. Additional trials in sarcopenic obesity can clarify the mechanisms underlying interactions between fat, muscle and bone that explain alterations in short-term and long-term outcomes. Improved characterization of biological signalling will permit full comprehension of the differences between sarcopenia and sarcopenic obesity. The association of resource and cost data in health systems and third-party payers (insurers) will escalate the importance of sarcopenic obesity.
Dietary composition and restriction.
No specific interventions have tested diets for the treatment of sarcopenic obesity. While diets should be individualized, the composition of carbohydrates, fats and protein have differed in clinical trials. Adjusting these components might differentially affect muscle mass, strength and weight. Research should distinguish appropriate diets, the type of protein to administer (such as whey or casein) and potentially the timing of intake in relation to exercise, as well as whether recommendations should be based on ideal or total body weight. The specific composition of essential amino acids (for example, leucine or creatine) and vitamin D supplementation requires structured interventions to ascertain dosing and monitoring. For instance, leucine-rich protein can activate metabolic pathways involved in testosterone and IGF1 homeostasis348–351. Such elements will allow tailored dietary interventions.
Exercise and combined interventions.
While aerobic and resistance exercises are core components in the treatment of sarcopenic obesity, the specific frequency, intensity, time and types (aerobic, resistance or both) should be considered. The relationship of resistance exercises with respect to dietary composition requires evaluation. Longitudinal studies should verify whether weight loss plus combined aerobic and resistance training prolongs physical independence in sarcopenic obesity. Such studies might translate to older adults who have access to health membership benefits in community-based exercise centres352. Assessing aquatic therapies353–355 or tai chi356, in isolation or in tandem with other types of physical activities, might prove useful for treating patients with sarcopenic obesity. The addition of pharmacotherapy, such as testosterone supplementation, to progressive resistance training augmented the improvements in body composition, including reduced fat mass and improved lean mass357. However, whether or not physical activity should be combined with novel and promising treatments requires systematic and further investigation.
Periodization strategies.
Periodization, which is a systematic variation in physical training specificity, intensity and volume within periods, has emerged as a potential strategy to improve muscle performance358. Periodization is typically used in sports programmes aiming to achieve peak physical performance while minimizing overtraining risk. Linear periodization reduces training volume while increasing training intensity or load between cycles359. Periodized resistance training in older adults demonstrated equal efficacy in physical function and physiological outcomes when compared with non-periodized resistance training360. In patients with sarcopenic obesity (defined using handgrip strength and BMI), no differences were observed in strength, power or short performance physical battery following a 10-week periodization strategy of strength and endurance training with concentric and eccentric movements290. Preliminary studies indicate that periodization results in increases in serum levels of irisin and decreases in IL-1β361. Leptin might also be reduced further with periodized resistance training362. While periodization could feasibly be prescribed in sedentary or frail older adults to improve physical function, it is premature to endorse this training as superior to non-periodized training358. Longer-term investigations in older populations with sarcopenic obesity are needed.
Whole-body vibration therapy.
Whole-body vibration therapy is a novel therapy that could increase muscle contraction efficiency and function with similar efficacy to resistance training, though data on its efficacy are mixed. This safe and convenient technique is associated with a low risk of injury363,364. Whole-body vibration therapy uses the transmission of mechanical stimuli through the person’s body365,366 to activate the primary ends of muscle spindles, which leads to neuromuscular activation367–369. The participant stands on a vibrating platform where electrical signals are delivered through the body, and thus primary endings of muscle spindles are activated.
Hengting Chen and colleagues370 identified 10 randomized trials of whole-body vibration therapy showing its usefulness in younger adults (difference (d) = 0.35 (95% CI 0.05–0.64; P = 0.02)), but this usefulness was not seen in older adults (d = −0.04 (95% CI −0.28 to 0.21; P = 0.78)). The review included heterogeneous studies using different methodologies, training and vibration characteristics. Separately, Ricky Lau and colleagues371 reviewed 13 trials of older adults and found significant treatment effects on knee extension dynamic strength (d = 0.63; P = 0.006), isometric strength (d = 0.57; P = 0.003) and functional measures such as sit-to-stand (d = 0.72; P < 0.001). Whole-body vibration therapy was as efficient as a fitness programme at increasing knee extension and lower leg muscle mass in non-institutionalized men aged 60–80 years old372 and improved quality of life and functional measures373.
Summative effects of the combination of whole body vibration therapy and resistance exercises374–377 or of whole-body vibration therapy and vitamin D367 are mixed. Others hypothesize that pathways contributing to weight loss as a result of whole-body vibration therapy could inhibit adipogenesis, increase energy expenditure and reduce muscle mass378. Augmenting existing squatting exercises with whole-body vibration therapy failed to improve muscle mass in younger men aged 18–30 years379. Future research should focus on type, frequency and duration of treatment380.
Weight loss medications.
None of the six FDA-approved medications for weight loss are approved for use in older adults aged over 65 years, and few have been evaluated in terms of changes in body composition. In nine older adults prescribed liraglutide, a weight decrease of 2.0 kg (−1.5 kg fat mass and −0.9% android fat) was observed, with a marginal improvement of 0.03 kg/m2 in skeletal muscle index (absolute muscle mass normalized by height squared)381. Lorcaserin leads to more fat loss than placebo in patients with diabetes mellitus (−12.1% versus −5.9%; P = 0.008) and more trunk obesity (3.65% versus −0.36%). When compared with controls, patients treated with lorcaserin had greater fat mass loss than lean mass loss382. Topiramate negatively affects BMD383 but might not affect lean mass384,385. Phentermine minimally alters lean mass386. Bupropion can blunt olanzapine-associated weight gain without affecting bone metabolism387 and in combination with naltrexone can lead to a reduction in fat mass without altering lean mass388. Orlistat promotes the weight loss via fat and visceral adipose tissue loss but minimally changes lean mass389–391. In carefully selected individuals, industry-sponsored trials should evaluate these agents in both older adults with obesity and patients with sarcopenic obesity.
Bariatric surgery.
Bariatric surgery improves weight and metabolic outcomes and reduces mortality. In carefully selected patients, this could be considered a treatment for sarcopenic obesity in older adults ≥65 years392. Its safety and efficacy in sarcopenic obesity is unknown other than one study that evaluated the influence of sarcopenic obesity on gastric bypass and sleeve gastrectomy results393. The population of participants had a mean age of 44 years, and no documented differences were observed in weight loss results or comorbidity resolution. Bariatric surgery leads to loss of fat mass394, alters gut hormones395 and can exacerbate weight loss-induced sarcopenia396–398 and osteoporosis399–402. Carefully designed studies are needed before promoting this intervention.
Testosterone.
Obesity negatively affects serum levels of testosterone and disrupts the actions of testosterone by increasing its aromatization to oestrogen403 and down regulating follicle-stimulating hormone and luteinizing hormone404, thus exacerbating hypogonadotropic hypogonadism. Testosterone is an important regulator of body composition with ageing, as it increases muscle and bone mass, increases IGF1 levels, decreases inflammatory markers405 and alters biomarkers of bone turnover in adults with hypogonadism. Testosterone deficiency can impair muscle adaptation to exercise owing to reduced expression of IGF1 and increased inflammatory cytokines. However, reductions in TNF and IL-6 observed in older men with hypogonadism can be reversed following testosterone treatment406.
Supplementation with testosterone promotes IGF1 mRNA and protein expression, leading to increased lean mass through increased muscle protein synthesis52. Increases in IGF1 following testosterone administration might improve muscle mass and strength enhanced by exercise52,56,406, and in men over 60 years with low testosterone, gains in lean mass following testosterone supplementation ranged from 1.6 kg to 6.20 kg (REF407). Androgen therapy also reduces fat mass (−1.78%). Therapy with testosterone and GH in older men aged 65–80 years with normal testosterone levels resulted in greater improvements in lean mass with both treatments than with either alone56,408. In select older men over the age of 60 years with testosterone deficiency and frailty, body composition and quality of life improved following supplementation with testosterone409–411. Three years of testosterone administration in patients with low levels of testosterone resulted in an increase in lean mass (0.9 kg, 95% CI 0.5–1.4; P < 0.001)412. Testosterone deficiency and treatment in older men have been reviewed elsewere413.
There are conflicting data on the effect of testosterone supplementation on muscle strength and function50,51,357,414. In the ‘Testosterone Trials’, treatment with testosterone improved participants’ results in the 6 min walk test compared with placebo (20.5% versus 12.6%; P = 0.003)53. Elsewhere, testosterone-associated increases in lean mass were accompanied by improvements in handgrip strength, knee extension and leg press and chest press exercises415,416. A meta-analysis of testosterone supplementation found effect sizes of 0.47 (95% CI 0.12–0.84) for upper and 0.63 (95% CI 0.03–1.28) for lower extremity strength417, without sustained improvements in body composition418. Healthy men with reduced levels of testosterone had no improvements in muscle strength or mobility after 6 months of supplementation350. Another trial of testosterone treatment found improved stair-climbing power and strength351.
Improvements in lean mass do not directly result in improved function after testosterone therapy409. With ageing, muscle strength often drops before muscle mass73. The nonlinear relationship between mass and function suggests that hypertrophy rather than muscle fibre hyperplasia materializes without neuronal plasticity. A reason for the observed significance in some studies might be a patient’s intrinsic threshold for functional impairment. Frail older adults with testosterone deficiency could require minimal incremental gains in mass to realize benefit. Therefore, we suggest an individualized approach. Comorbidity and health status, small sample sizes, minimal changes in testosterone levels following treatment and the lack of practice sessions before initiating strength testing could have contributed to the negative findings. Areas of future research should identify responders to androgen supplementation in those with low lean mass or strength.
Testosterone potentially augments diet-induced loss of fat mass in individuals with BMI ≥30 kg/m2 and low testosterone levels. Over 56 weeks, testosterone-treated participants (mean age 53 years) had greater reductions in fat mass (mean between-group difference 2.9 kg; P = 0.04) and visceral fat (−2,678 mm2; P = 0.04) than controls. Testosterone-treated participants also had greater lean mass regain during weight maintenance (mean between-group difference 3.4 kg; P = 0.002) following the very-low-energy diet, suggesting that weight loss was exclusively fat mass419. As multicomponent interventions can attenuate lean mass losses, studies should evaluate whether testosterone replacement helps preserve muscle and bone mass during weight loss in patients with sarcopenic obesity.
Adverse events associated with testosterone supplementation include polycythemia420, possible cardiovascular events421, venous thromboembolism422 and prostatism423. Those who favour supplementation cite a lack of credible evidence related to cardiovascular risk424. Future cost–benefit analyses should compare the relative benefits regarding body and bone composition with disability risk. To date, the American Association of Clinical Endocrinologists187, the Endocrine Society425 and the Obesity Society186 have not recommended testosterone supplementation as a treatment for sarcopenia or obesity.
Selective androgen receptor modulators.
Selective androgen receptor modulators (SARMs) target androgen receptors on muscle and bone but do not activate androgenic effects elsewhere. SARMs indirectly affect nonmuscle androgen receptor pathways mediated by muscle fibroblasts426. Enobosarm, a non-steroidal SARM, successfully increased muscle mass and physical function in patients without cancer and in those with advanced lung, colorectal or breast cancer or lymphoma427–429. Other studies showed increases in lean mass without improvements in strength or physical performance in patients with sarcopenia428,430. Early trials demonstrated a greater total lean mass of 1.3 kg (P < 0.001), better physical function as measured by reduced stair climb time (P = 0.013), a trend towards lower blood levels of glucose of 6.9 ± 2.5 mg/dl (P = 0.052) and lower insulin sensitivity (−27.5% versus 2.6%; P = 0.013) with 3 mg per day of GTx-024 (an orally bioavailable nonsteroidal SARM) than with placebo429. Enobosarm significantly increased lean mass compared with baseline (1.0 kg, 95% CI −4.8 to 11.5; P = 0.046) versus controls (0.02 kg, 95% CI −5.8 to 6.7; P = 0.88). SARM treatment in older women with low lean mass, a self-reported mobility disability and a short physical performance battery score between 4 and 9 provided improvements in muscle mass without benefits of improved strength430. SARMs that have been developed in the past 5 years have not demonstrated adverse effects431 and have restored cortical and trabecular bone in orchidectomized mice432. Transdermal SARMs could emerge in the future433. A review of SARMs has been conducted elsewhere434, but they could be of benefit to patients with sarcopenic obesity who require muscle mass improvements rather than strength. However, conclusive evidence is still needed.
Anamorelin.
Anamorelin, an oral ghrelin analogue, is effective for improving appetite in patients with cancer cachexia and might hold promise in patients with sarcopenic obesity. Its anti-inflammatory and anabolic properties might counter the negative nitrogen balance observed in sarcopenia. Anamorelin is safe, well tolerated and stimulates appetite by promoting expression of GH, IGF1 and IGF-binding protein 3 (REFS435,436), which reverses muscle atrophy in mice437. A meta-analysis of 1,641 patients with cancer demonstrated improved total body weight, lean mass and quality of life (1.78, 95% CI 1.28–2.28, P < 0.001; 1.10, 95% CI 0.35–1.85, P = 0.004; 0.19, 95% CI 0.08–0.30, P = 0.0006, respectively)438. A review of four studies demonstrated high heterogeneity with improved symptom scores, and in three studies, improved lean mass was shown439. Anamorelin improved lean mass in patients with cancer cachexia compared with placebo (2.09 kg, 95% CI 0.94–3.25; P = 0.0006)440 but failed to improve muscle power or handgrip strength in patients with inoperable nonsmall-cell lung cancer441. Lack of functional improvements were also observed in patients with unresectable non-small-cell lung cancer442. Adverse effects are no different than with placebo. It is unclear whether the improved lean mass observed in patients treated with anamorelin has differential effects on intramuscular fat in patients with sarcopenic obesity. As no studies have established improved function or strength, further evaluation is needed, particularly in the subset of patients with sarcopenic obesity who have low lean mass with intact muscle strength.
Myostatin inhibitors.
Individuals with sarcopenia have elevated levels of myostatin443, a negative regulator of skeletal muscle growth development444. Myostatin is also expressed in adipose tissue and is inversely related to insulin resistance445. In vitro trials demonstrate that myostatin inhibitors increase muscle mass and strength446, suppress irisin, downregulate inflammatory cytokines and improve insulin resistance447. Its expression drops following weight loss because it directly influences adipocyte metabolism448, and myostatin inhibitors can directly inhibit muscle loss. Myostatin inhibitors reduce expression of myostatin in both aerobic and resistance exercise449 and might be beneficial in treating sarcopenic obesity. Early data suggest improved physical function in patients with non-small-cell lung cancer427. Interventions should directly measure changes in levels of myostatin and corresponding changes in muscle mass, strength, function and insulin sensitivity.
Vitamin K.
Vitamin K might have a role in mitigating bone loss following intentional weight loss by inhibiting bone resorption450 and osteoclast formation451. Its deficiency has been associated with increased risk of fragility fractures452–455, particularly in patients who are malnourished456,457. Vitamin K supplementation can restore serum levels of the vitamin458,459 and might increase bone resorption markers460. Conflicting data exist; in some studies, vitamin K antagonists demonstrate no differences in BMD or fracture rates461, while other data suggest lessening of steroid-induced BMD462 and sex-specific improvements in insulin sensitivity463. One study464 reported that over 3 years vitamin K supplementation was not implicated in the age-related changes in skeletal muscle or adipose tissue mass in older community-dwelling adults. Our understanding of the complex relationship between vitamin K and weight loss-induced effects on bone, muscle and fat in sarcopenic obesity is currently in its infancy465.
Mesenchymal stem cells.
Muscle, bone and cartilage derived from mesenchymal cells share common precursor mesenchymal stem cells. In mice, transplantation of satellite cells into damaged muscle leads to self-renewal and muscle regeneration466,467. An early human study suggests a role for mesenchymal stem cells in managing frailty468. The cost, regulatory constraints and potential ethical barriers of applying such technology into clinical settings need to be addressed further.
Conclusions
The growing challenges associated with sarcopenic obesity will probably worsen with the changing demographic distribution of our ageing population. Effective evidence based therapies can be helpful for improving physical function in older adults. We encourage further agreement on defining sarcopenic obesity within both research and clinical settings. In our opinion, a lack of a consensus definition is one of the greatest limitations to advancing the science. Without being able to accurately identify populations of patients, there will be continued difficulties in targeting further obesity subtypes. Clarifying the mechanisms that contribute to sarcopenic obesity might elucidate novel therapies to improve function, quality of life and prevent institutionalization. A number of novel therapies independently hold promise or could be considered adjunctively for those who have struggled with a lifetime of reduced motivation. These potential strategies should be key research questions in future work.
Key points.
Body composition changes that occur with the ageing process can lead to sarcopenic obesity, an increasingly prevalent disorder owing to the increased prevalence of obesity in an ageing population.
Hormonal, inflammatory and myocellular mechanisms impact underlying biological processes that promote fat deposition and loss of lean mass and strength.
Definitions of sarcopenia and obesity can vary considerably, prompting difficulties in the diagnosis and epidemiological understanding of sarcopenic obesity as well as the development of treatment strategies for this disease.
Lifestyle interventions including calorie restriction and physical activity consisting of aerobic and resistance exercises are the cornerstones of therapy.
Clinicians and researchers need to be aware of weight loss-induced sarcopenia and osteopenia.
Novel, promising therapies, including weight loss medications, bariatric surgery, whole-body vibration therapy, periodization (a systematic variation in physical training specificity, intensity and volume within periods), testosterone, selective androgen receptor modulators, anamorelin, myostatin inhibitors, vitamin K and mesenchymal stem cells, require further investigation.
Acknowledgements
The authors would like to thank R. Masutani and R. Dokko for their help with preparing the manuscript and E. Weiss for contributing to the images in figure 2. J.A.B.’s research reported in this publication was supported in part by the US National Institute on Aging of the US National Institutes of Health under award number K2 3AG051681. Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by cooperative agreement number U48DP005018 from the Centers for Disease Control and Prevention and the Dartmouth Clinical and Translational Science Institute under award number UL1TR001086 from the US National Center for Advancing Translational Sciences. D.T.V.’s research was supported in part by DK109950 from the US National Institute of Diabetes, Digestive and Kidney Diseases, AG031176 from the US National Institutes on Aging, CX000906 from the Veterans Affairs Office of Research and Development, 1–14-LLY-38 from the American Diabetes Association and the Alkek Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or of the Department of Veterans Affairs, nor represents the official position of the Centers for Disease Control and Prevention.
Glossary
- Sarcopenia
The loss of muscle mass, strength or physical function with age.
- Oxidative capacity
The maximal ability of muscle to utilize oxygen per g of muscle per hour.
- Thermic effect of food
The amount of energy expended owing to the body’s processing and storage of food.
- Fat-free mass
A term interchangeably used with muscle mass and lean mass; it refers to the mass of all visceral organs, muscles (smooth and skeletal), bones, ligaments and tendons but does not include fat that is present in the marrow of bones or internal organs.
- Waist circumference
An anthropometric measure of central obesity (subcutaneous and visceral) measured at the level of the iliac crest.
- Visceral fat
A measurement of the adiposity located among organs within the abdominal cavity; it is associated with inflammation and increased cardiometabolic risk.
- Intramyocellular lipids
Fat depositions within the muscle structure.
- Myostatin
A transforming growth factor-related protein that is synthesized and secreted in skeletal muscle and negatively regulates muscle mass and function.
- VO2 max
The maximal amount of oxygen used per kg of body weight during maximal exercise.
- Lean mass
A term that refers to the mass of all visceral organs, muscles (smooth and skeletal), bones, ligaments and tendons but excludes fat from bone.
- Appendicular lean mass
Muscle mass consisting of the sum of the upper and lower limbs.
- Grip strength
A measurement used in the ascertainment of upper extremity strength; it is assessed using the dominant hand with a hand-held dynamometer.
- Knee extensor strength
A measure of lower extremity strength. The test is performed using a dynamometer with the participant sitting with hips and knees flexed at 90°; the participant extends his or her knee and pushes against a resistance pad — the results are measured in kilograms or pounds.
- Quadriceps muscle area
Cross-sectional 2D area at the level of the quadriceps muscle of the lower limb.
- Skeletal muscle index
Absolute muscle mass (in kg) normalized for height (muscle mass in kg divided by height (in m)).
- Absolute muscle mass
Muscle mass consisting of all limbs and muscle from visceral organs.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Endocrinology thanks W. Kemmler and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Nations United, Department of Economic Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248 (United Nations, 2017). [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD & Ogden CL Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315, 2284–2291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson NB, Hayes LD, Brown K, Hoo EC & Ethier KA CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. MMWR Suppl 63, 3–27 (2014). [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD & Ogden CL Prevalence of obesity among adults and youth: United States, 2015–2016. Centers for Disease Control and Prevention https://www.cdc.gov/nchs/data/databriefs/db288.pdf (2017).These are the most updated prevalence rates of the obesity epidemic in the United States.
- 5.Organisation for Economic Co-operation and Development. Obesity Update 2017. OECD www.oecd.org/health/obesity-update.htm (2017).
- 6.Acciai F & Firebaugh G Why did life expectancy decline in the United States in 2015? A gender specific analysis. Soc. Sci. Med 190, 174–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig DS Lifespan weighed down by diet. JAMA 315, 2269–2270 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Olshansky SJ et al. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med 352, 1138–1145 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Preston SH, Stokes A, Mehta NK & Cao B Projecting the effect of changes in smoking and obesity on future life expectancy in the United States. Demography 51, 27–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart ST, Cutler DM & Rosen AB Forecasting the effects of obesity and smoking on U.S. life expectancy. N. Engl. J. Med 361, 2252–2260 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roubenoff R Sarcopenic obesity: the confluence of two epidemics. Obes. Res 12, 887–888 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Patterson RE, Frank LL, Kristal AR & White E A comprehensive examination of health conditions associated with obesity in older adults. Am. J. Prev. Med 27, 385–390 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM et al. Comparisons of percentage body fat, body mass index, waist circumference, and waiststature ratio in adults. Am. J. Clin. Nutr 89, 500–508 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heo M, Faith MS, Pietrobelli A & Heymsfield SB Percentage of body fat cutoffs by sex, age, and race- ethnicity in the US adult population from NHANES 1999–2004. Am. J. Clin. Nutr 95, 594–602 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Xu W et al. Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone 48, 307–311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayer AA et al. The developmental origins of sarcopenia. J. Nutr. Health Aging 12, 427–432 (2008).This is a review that evaluates the development of and life-course influences on muscle mass and strength.
- 17.Abizanda P et al. Energetics of aging and frailty: the FRADEA Study. J. Gerontol. A Biol. Sci. Med. Sci 71, 787–796 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinsier RL, Schutz Y & Bracco D Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am. J. Clin. Nutr 55, 790–794 (1992). [DOI] [PubMed] [Google Scholar]
- 19.Gallagher D et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am. J. Physiol 275, E249–E258 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Wilson MM & Morley JE Invited review: aging and energy balance. J. Appl. Physiol. (1985) 95, 1728–1736 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Conley KE et al. Ageing, muscle properties and maximal O(2) uptake rate in humans. J. Physiol 526, 211–217 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conley KE, Jubrias SA & Esselman PC Oxidative capacity and ageing in human muscle. J. Physiol 526, 203–210 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannon B & Nedergaard J Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol 214, 242–253 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Dulloo AG, Seydoux J & Jacquet J Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol. Behav 83, 587–602 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Doucet E et al. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int. J. Obes. Relat. Metab. Disord 24, 906–914 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Doucet E et al. Changes in energy expenditure and substrate oxidation resulting from weight loss in obese men and women: is there an important contribution of leptin? J. Clin. Endocrinol. Metab 85, 1550–1556 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Bray GA Effect of caloric restriction on energy expenditure in obese patients. Lancet 2, 397–398 (1969).This is an early article that highlights the metabolic changes with energy restriction in patients with obesity.
- 28.Major GC, Doucet E, Trayhurn P, Astrup A & Tremblay A Clinical significance of adaptive thermogenesis. Int. J. Obes. (Lond.) 31, 204–212 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Cohn SH et al. Changes in body chemical composition with age measured by total-body neutron activation. Metabolism 25, 85–95 (1976). [DOI] [PubMed] [Google Scholar]
- 30.Tremblay A, Royer MM, Chaput JP & Doucet E Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int. J. Obes. (Lond.) 37, 759–764 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Astrup A et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am. J. Clin. Nutr 69, 1117–1122 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum M, Hirsch J, Gallagher DA & Leibel RL Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr 88, 906–912 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Napier JR, Thomas MF, Sharma M, Hodgkinson SC & Bass JJ Insulin-like growth factor-I protects myoblasts from apoptosis but requires other factors to stimulate proliferation. J. Endocrinol 163, 63–68 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Roth SM et al. Ultrastructural muscle damage in young versus older men after high-volume, heavy- resistance strength training. J. Appl. Physiol. (1985) 86, 1833–1840 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Klem ML, Wing RR, Lang W, McGuire MT & Hill JO Does weight loss maintenance become easier over time? Obes. Res 8, 438–444 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Wing RR & Hill JO Successful weight loss maintenance. Annu. Rev. Nutr 21, 323–341 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Tremollieres FA, Pouilles JM & Ribot CA Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am. J. Obstet. Gynecol 175, 1594–1600 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Sowers M et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J. Clin. Endocrinol. Metab 92, 895–901 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milewicz A, Tworowska U & Demissie M Menopausal obesity — myth or fact? Climacteric 4, 273–283 (2001). [PubMed] [Google Scholar]
- 40.Gambacciani M et al. Climacteric modifications in body weight and fat tissue distribution. Climacteric 2, 37–44 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Guo SS, Zeller C, Chumlea WC & Siervogel RM Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am. J. Clin. Nutr 70, 405–411 (1999).This study presents the natural patterns of change in body composition in older adults and the influence of physical activity.
- 42.Enns DL & Tiidus PM Estrogen influences satellite cell activation and proliferation following downhill running in rats. J. Appl. Physiol. (1985) 104, 347–353 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Gambacciani M et al. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas 39, 125–132 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Kadi F Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharmacol 154, 522–528 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bondanelli M et al. Activation of the somatotropic axis by testosterone in adult men: evidence for a role of hypothalamic growth hormone-releasing hormone. Neuroendocrinology 77, 380–387 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Yeap BB Are declining testosterone levels a major risk factor for ill-health in aging men? Int. J. Impot. Res 21, 24–36 (2009). [DOI] [PubMed] [Google Scholar]
- 47.LeBlanc ES et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J. Clin. Endocrinol. Metab 96, 3855–3863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller KK Androgen deficiency: effects on body composition. Pituitary 12, 116–124 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Kaplan SA, Lee JY, O’Neill EA, Meehan AG & Kusek JW Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 16, 169–172 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Urban RJ et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am. J. Physiol 269, E820–E826 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Snyder PJ et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J. Clin. Endocrinol. Metab 84, 2647–2653 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Ferrando AA et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab 282, E601–E607 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Snyder PJ et al. Effects of testosterone treatment in older men. N. Engl. J. Med 374, 611–624 (2016).This is a randomized trial of testosterone therapy in symptomatic older men that demonstrates no benefit in vitality or walking distance following supplementation.
- 54.Orentreich N, Brind JL, Rizer RL & Vogelman JH Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab 59, 551–555 (1984). [DOI] [PubMed] [Google Scholar]
- 55.Nafziger AN, Bowlin SJ, Jenkins PL & Pearson TA Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J. Lab. Clin. Med 131, 316–323 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Giannoulis MG, Martin FC, Nair KS, Umpleby AM & Sonksen P Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr. Rev 33, 314–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrager MA et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. (1985) 102, 919–925 (2007).This is an epidemiological study that shows that obesity directly affects inflammation and negatively affects muscle strength.
- 58.Forsythe LK, Wallace JM & Livingstone MB Obesity and inflammation: the effects of weight loss. Nutr. Res. Rev 21, 117–133 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Park HS, Park JY & Yu R Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract 69, 29–35 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Zamboni M, Mazzali G, Fantin F, Rossi A & Di Francesco V Sarcopenic obesity: a new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis 18, 388–395 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Hamrick MW Role of the cytokine-like hormone leptin in muscle-bone crosstalk with aging. J. Bone Metab 24, 1–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeap BB et al. Associations of insulin-like growth factor-I and its binding proteins and testosterone with frailty in older men. Clin. Endocrinol. (Oxf.) 78, 752–759 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Wang Y et al. Adiponectin inhibits tumor necrosis factor-alpha-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res 114, 792–805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang CH, Frost RA, Nairn AC, MacLean DA & Vary TC TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am. J. Physiol. Endocrinol. Metab 282, E336–E347 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Cartwright MJ, Tchkonia T & Kirkland JL Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp. Gerontol 42, 463–471 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shulman GI Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med 371, 1131–1141 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Thomas DR Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr 26, 389–399 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Nilwik R et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol 48, 492–498 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Delbono O Neural control of aging skeletal muscle. Aging Cell 2, 21–29 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Stenholm S et al. Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 11, 693–700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauer J et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc 14, 542–559 (2013).This is a position paper from the PROT-Age study group that recommends an average daily intake of at least 1.0–1.2 g protein per kg body weight per day.
- 72.Gallagher D et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am. J. Clin. Nutr 72, 694–701 (2000).This study includes ethnically heterogeneous study populations, which allow the development of prediction formulas for healthy percentage body fat.
- 73.Goodpaster BH et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci 61, 1059–1064 (2006).This study shows that loss of strength with ageing is much more rapid than the concomitant loss of muscle mass, suggesting a decline in muscle quality.
- 74.Visser M et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci 60, 324–333 (2005).This study shows that in sarcopenic obesity reflected by low cross-sectional thigh muscle area, greater fat infiltration into muscle is associated with mobility loss.
- 75.Sepe A, Tchkonia T, Thomou T, Zamboni M & Kirkland JL Aging and regional differences in fat cell progenitors — a mini-review. Gerontology 57, 66–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalinkovich A & Livshits G Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev 35, 200–221 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Kob R et al. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology 16, 15–29 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Stinkens R, Goossens GH, Jocken JW & Blaak EE Targeting fatty acid metabolism to improve glucose metabolism. Obes. Rev 16, 715–757 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Aon MA, Bhatt N & Cortassa SC Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol 5, 282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruce CR et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J. Clin. Endocrinol. Metab 88, 5444–5451 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Kohara K Sarcopenic obesity in aging population: current status and future directions for research. Endocrine 45, 15–25 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Carnio S et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8, 1509–1521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcell TJ Sarcopenia: causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci 58, M911–M916 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA & Leeuwenburgh C Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp. Gerontol 45, 138–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manini TM et al. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutr 85, 377–384 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Kusminski CM, Shetty S, Orci L, Unger RH & Scherer PE Diabetes and apoptosis: lipotoxicity. Apoptosis 14, 1484–1495 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Nilsson MI et al. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J 27, 3905–3916 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Shefer G, Rauner G, Stuelsatz P, Benayahu D & Yablonka-Reuveni Z Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J 280, 4063–4073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zwetsloot KA, Childs TE, Gilpin LT & Booth FW Non-passaged muscle precursor cells from 32-month old rat skeletal muscle have delayed proliferation and differentiation. CellProlif 46, 45–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakuma K et al. The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol. (Oxf.) 197, 151–159 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Srikanthan P, Hevener AL & Karlamangla AS Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE 5, e10805 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joseph AM, Adhihetty PJ & Leeuwenburgh C Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol 594, 5105–5123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schubert MM, Sabapathy S, Leveritt M & Desbrow B Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med 44, 387–403 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Roque FR et al. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br. J. Pharmacol 168, 686–703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumaraguru U, Nandakumar S, Chi D, Stone M & Krishnaswamy G Resistance training and aerobic exercise alters immune function (87.25). J. Immunol 184, 87.25 (2010). [Google Scholar]
- 96.Carraro F, Stuart CA, Hartl WH, Rosenblatt J & Wolfe RR Effect of exercise and recovery on muscle protein synthesis in human subjects. Am. J. Physiol 259, E470–E476 (1990). [DOI] [PubMed] [Google Scholar]
- 97.Drummond MJ et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J. Physiol 587, 1535–1546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujita S et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56, 1615–1622 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levenhagen DK et al. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am. J. Physiol. Endocrinol. Metab 280, E982–E993 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Sheffield-Moore M et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am. J. Physiol. Endocrinol. Metab 287, E513–E522 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Short KR, Vittone JL, Bigelow ML, Proctor DN & Nair KS Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab 286, E92–E101 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Yarasheski KE, Zachwieja JJ & Bier DM Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol 265, E210–E214 (1993). [DOI] [PubMed] [Google Scholar]
- 103.Yarasheski KE et al. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am. J. Physiol 277, E118–E125 (1999). [DOI] [PubMed] [Google Scholar]
- 104.Hasten DL, Pak-Loduca J, Obert KA & Yarasheski KE Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am. J. Physiol. Endocrinol. Metab 278, E620–E626 (2000). [DOI] [PubMed] [Google Scholar]
- 105.Villareal DT, Smith GI, Sinacore DR, Shah K & Mittendorfer B Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 19, 312–318 (2011).This study demonstrates that a multicomponent exercise programme consisting of strength, endurance, flexibility and balance increases the basal rate of muscle protein synthesis without affecting the magnitude of the muscle protein anabolic response to feeding.
- 106.Cuthbertson D et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19, 422–424 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Volpi E, Mittendorfer B, Rasmussen BB & Wolfe RR The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab 85, 4481–4490 (2000).This study highlights the anabolic response to feeding in elderly individuals that underscores sarcopenia of ageing.
- 108.Volpi E, Sheffield-Moore M, Rasmussen BB & Wolfe RR Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286, 1206–1212 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peterson CM, Johannsen DL & Ravussin E Skeletal muscle mitochondria and aging: a review. J. Aging Res 2012, 194821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET & Wiswell RA Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33, 242–253 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Petrella JK, Kim JS, Mayhew DL, Cross JM & Bamman MM Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J. Appl. Physiol. (1985) 104, 1736–1742 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Kang JS & Krauss RS Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care 13, 243–248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thornell LE Sarcopenic obesity: satellite cells in the aging muscle. Curr. Opin. Clin. Nutr. Metab. Care 14, 22–27 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Bruunsgaard H, Bjerregaard E, Schroll M & Pedersen BK Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J. Am. Geriatr. Soc 52, 237–241 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Kohut ML et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun 20, 201–209 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Forti LN et al. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age (Dordr.) 36, 9704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beyer I, Mets T & Bautmans I Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 15, 12–22 (2012). [DOI] [PubMed] [Google Scholar]
- 118.Woods JA, Wilund KR, Martin SA & Kistler BM Exercise, inflammation and aging. Aging Dis 3, 130–140 (2012). [PMC free article] [PubMed] [Google Scholar]
- 119.Visser M et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci 57, M326–M332 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Cappola AR et al. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab 88, 2019–2025 (2003). [DOI] [PubMed] [Google Scholar]
- 121.Suetta C et al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J. Appl. Physiol. (1985) 97, 1954–1961 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Marzetti E et al. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. FreeRadic. Biol. Med 44, 160–168 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Canto C et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marzetti E et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res 29, 35–42 (2017).This study provides an overview of the interplay between physical activity, exercise and sarcopenia.
- 125.Hood DA Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr Metab 34, 465–472 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Mooren FC & Kruger K Exercise, autophagy, and apoptosis. Prog. Mol. Biol. Trans! Sci 135, 407–422 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Wohlgemuth SE et al. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res 14, 315–324 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lambert CP & Evans WJ Adaptations to aerobic and resistance exercise in the elderly. Rev. Endocr. Metab. Disord 6, 137–143 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Moayedi Y et al. The prognostic significance of frailty compared to peak oxygen consumption and B-type natriuretic peptide in patients with advanced heart failure. Clin. Transplant https://doi.org/10.1111/ctr.13158 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Jones S et al. Assessment of exercise capacity and oxygen consumption using a 6 min stepper test in older adults. Front. Physiol 8, 408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Binder EF et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J. Am. Geriatr. Soc 50, 1921–1928 (2002).This is a randomized controlled trial demonstrating that intensive exercise training can improve measures of physical function.
- 132.Villareal DT, Banks M, Siener C, Sinacore DR & Klein S Physical frailty and body composition in obese elderly men and women. Obes. Res 12, 913–920 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Lambert CP, Wright NR, Finck BN & Villareal DT Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. (1985) 105, 473–478 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE & Semenkovich CF Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 15, 475–482 (2001). [DOI] [PubMed] [Google Scholar]
- 135.Gielen S et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J. Am. Coll. Cardiol 42, 861–868 (2003). [DOI] [PubMed] [Google Scholar]
- 136.Markofski MM et al. Exercise training modifies ghrelin and adiponectin concentrations and is related to inflammation in older adults. J. Gerontol. A Biol. Sci. Med. Sci 69, 675–681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Andersen LL et al. Changes in the human muscle force-velocity relationship in response to resistance training and subsequent detraining. J. Appl. Physiol. (1985) 99, 87–94 (2005). [DOI] [PubMed] [Google Scholar]
- 138.Englund DA, Sharp RL, Selsby JT, Ganesan SS & Franke WD Resistance training performed at distinct angular velocities elicits velocity-specific alterations in muscle strength and mobility status in older adults. Exp. Gerontol 91, 51–56 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Fatouros IG et al. Intensity of resistance exercise determines adipokine and resting energy expenditure responses in overweight elderly individuals. Diabetes Care 32, 2161–2167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reeves ND, Maganaris CN & Narici MV Effect of strength training on human patella tendon mechanical properties of older individuals. J. Physiol 548, 971–981 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamen G & Knight CA Training-related adaptations in motor unit discharge rate in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci 59, 1334–1338 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Prestes J et al. Effects of resistance training on resistin, leptin, cytokines, and muscle force in elderly post-menopausal women. J. Sports Sci 27, 1607–1615 (2009). [DOI] [PubMed] [Google Scholar]
- 143.Nindl BC et al. Leptin concentrations experience a delayed reduction after resistance exercise in men. Med. Sci. Sports Exerc 34, 608–613 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Bouassida A et al. Leptin, its implication in physical exercise and training: a short review. J. Sports Sci. Med 5, 172–181 (2006). [PMC free article] [PubMed] [Google Scholar]
- 145.Fatouros IG et al. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J. Clin. Endocrinol. Metab 90, 5970–5977 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Rostas I et al. In middle-aged and old obese patients, training intervention reduces leptin level: a metaanalysis. PLoS ONE 12, e0182801 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nikseresht M, Sadeghifard N, Agha-Alinejad H & Ebrahim K Inflammatory markers and adipocytokine responses to exercise training and detraining in men who are obese. J. Strength Cond. Res 28, 3399–3410 (2014). [DOI] [PubMed] [Google Scholar]
- 148.Friedenreich CM et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr. Relat. Cancer 18, 357–369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Haider S et al. Change in inflammatory parameters in prefrail and frail persons obtaining physical training and nutritional support provided by lay volunteers: a randomized controlled trial. PLoS ONE 12, e0185879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Woodrow G Body composition analysis techniques in the aged adult: indications and limitations. Curr. Opin. Clin. Nutr Metab. Care 12, 8–14 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Heymsfield SB, Gonzalez MC, Lu J, Jia G & Zheng J Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc 74, 355–366 (2015).This is a crucial review that elicits important questions related to loss of lean tissues with dieting.
- 152.Baracos V et al. Advances in the science and application of body composition measurement. JPEN J. Parenter. Enteral Nutr 36, 96–107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Earthman CP Body composition tools for assessment of adult malnutrition at the bedside: a tutorial on research considerations and clinical applications. JPEN J. Parenter. Enteral Nutr 39, 787–822 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Heymsfield SB, Gonzalez MC, Shen W, Redman L & Thomas D Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes. Rev 15, 310–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Teigen LM, Kuchnia AJ, Mourtzakis M & Earthman CP The use of technology for estimating body composition strengths and weaknesses of common modalities in a clinical setting [formula: see text]. Nutr. Clin. Pract 32, 20–29 (2017). [DOI] [PubMed] [Google Scholar]
- 156.Cruz-Jentoft AJ et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kendler DL et al. The Official Positions of the International Society for Clinical Densitometry: indications of use and reporting of DXA for body composition. J. Clin. Densitom 16, 496–507 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Fakhrawi DH et al. Comparison of body composition by bioelectrical impedance and dual-energy x-ray absorptiometry in overweight/obese postmenopausal women. J. Clin. Densitom 12, 238–244 (2009). [DOI] [PubMed] [Google Scholar]
- 159.Bridge P et al. Validation of longitudinal DXA changes in body composition from pre- to mid-adolescence using MRI as reference. J. Clin. Densitom 14, 340–347 (2011). [DOI] [PubMed] [Google Scholar]
- 160.Xu L et al. Comparisons of body-composition prediction accuracy: a study of 2 bioelectric impedance consumer devices in healthy chinese persons using DXA and MRI as criteria methods. J. Clin. Densitom 14, 458–464 (2011). [DOI] [PubMed] [Google Scholar]
- 161.Park YW, Heymsfield SB & Gallagher D Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int. J. Obes. Relat. Metab. Disord 26, 978–983 (2002). [DOI] [PubMed] [Google Scholar]
- 162.Kaul S et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 20, 1313–1318 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE & Kelly TL Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 20, 1109–1114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Das SK et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab 284, E1080–E1088 (2003). [DOI] [PubMed] [Google Scholar]
- 165.Schautz B, Later W, Heller M, Muller MJ & Bosy-Westphal A Total and regional relationship between lean and fat mass with increasing adiposity — impact for the diagnosis of sarcopenic obesity. Eur. J. Clin. Nutr 66, 1356–1361 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Bosy-Westphal A & Muller MJ Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int. J. Obes. (Lond.) 39, 379–386 (2015).This review argues for the importance of standardized definitions in skeletal muscle mass.
- 167.Bosy-Westphal A et al. Quantification of whole-body and segmental skeletal muscle mass using phase- sensitive 8-electrode medical bioelectrical impedance devices. Eur. J. Clin. Nutr 71, 1061–1067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Houtkooper LB, Lohman TG, Going SB & Howell WH Why bioelectrical impedance analysis should be used for estimating adiposity. Am. J. Clin. Nutr 64, 436S–448S (1996). [DOI] [PubMed] [Google Scholar]
- 169.Kyle UG et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin. Nutr 23, 1430–1453 (2004).This study demonstrates the application of bioelectrical impedance equations in clinical practice, as advocated by ESPEN.
- 170.Chumlea WC et al. Body composition estimates from NHANES III bioelectrical impedance data. Int. J. Obes. Relat. Metab. Disord 26, 1596–1609 (2002).This study presents normative bioelectrical impedance analysis equations validated using a population-based cohort of American adults.
- 171.Janssen I, Heymsfield SB, Baumgartner RN & Ross R Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. (1985) 89, 465–471 (2000). [DOI] [PubMed] [Google Scholar]
- 172.Mally K, Trentmann J, Heller M & Dittmar M Reliability and accuracy of segmental bioelectrical impedance analysis for assessing muscle and fat mass in older Europeans: a comparison with dual-energy X-ray absorptiometry. Eur. J. Appl. Physiol. (1985) 111, 1879–1887 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Yu SC, Powell A, Khow KS & Visvanathan R The performance of five bioelectrical impedance analysis prediction equations against dual x-ray absorptiometry in estimating appendicular skeletal muscle mass in an adult Australian population. Nutrients 8, 189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chien MY, Huang TY & Wu YT Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J. Am. Geriatr. Soc 56, 1710–1715 (2008). [DOI] [PubMed] [Google Scholar]
- 175.Roubenoff R Applications of bioelectrical impedance analysis for body composition to epidemiologic studies. Am J. Clin. Nutr 64, 459S–462S (1996). [DOI] [PubMed] [Google Scholar]
- 176.Kyle UG et al. Bioelectrical impedance analysis — part I: review of principles and methods. Clin. Nutr 23, 1226–1243 (2004). [DOI] [PubMed] [Google Scholar]
- 177.Batsis JA, Mackenzie TA, Lopez-Jimenez F & Bartels SJ Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr. Res 35, 1031–1039 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Batsis JA et al. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J. Am. Geriatr. Soc 61, 974–980 (2013).This study applies multiple definitions of sarcopenic obesity on a cohort of American adults and emphasizes the varied prevalences of this disorder.
- 179.Dehghan M & Merchant AT Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J 7, 26 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fielding RA et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc 12, 249–256 (2011).This is a consensus statement from the International Working Group on Sarcopenia that presents evidence and a definition for the identification of sarcopenia.
- 181.Janssen I, Baumgartner RN, Ross R, Rosenberg IH & Roubenoff R Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol 159, 413–421 (2004). [DOI] [PubMed] [Google Scholar]
- 182.Studenski SA et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci 69, 547–558 (2014).This report details recommendations from the Foundation of the National Institutes on Health Sarcopenia Project, which aggregated multiple cohorts of older adults and created cut-off points to permit identification of low muscle mass and low muscle strength.
- 183.Kemmler W et al. Prevalence of sarcopenia and sarcopenic obesity in older German men using recognized definitions: high accordance but low overlap! Osteoporos. Int 28, 1881–1891 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Cruz-Jentoft AJ et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43, 748–759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen LK et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc 15, 95–101 (2014). [DOI] [PubMed] [Google Scholar]
- 186.Villareal DT et al. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr 82, 923–934 (2005).This study highlights current guidelines for the management of older adults with obesity.
- 187.Garvey WT et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract 22 (Suppl. 3), 1–203 (2016).Guidelines for the management of obesity by the AACE/ACE.
- 188.Bouchard DR, Dionne IJ & Brochu M Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring) 17, 2082–2088 (2009). [DOI] [PubMed] [Google Scholar]
- 189.Durstine J in ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities 4th edn (eds Moore GE, Durstine JL & Painter PL) (Human Kinetics, 2016). [Google Scholar]
- 190.Baumgartner RN et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol 147, 755–763 (1998).This is a landmark study that demonstrates the prevalence of sarcopenia in a cohort of older adults in the New Mexico Elder Health Survey.
- 191.Baumgartner RN et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res 12, 1995–2004 (2004).This study highlights the synergistic problems of sarcopenia and obesity that lead to impairments in instrumental activities of daily living.
- 192.Fox CS et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116, 39–48 (2007). [DOI] [PubMed] [Google Scholar]
- 193.Despres JP Body fat distribution and risk of cardiovascular disease: an update. Circulation 126, 1301–1313 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Batsis JA et al. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int. J. Obes. (Lond.) 40, 761–767 (2016).This study emphasizes the challenges of using BMI as a marker for obesity. BMI has a poor sensitivity in ascertaining body fat-defined obesity that worsens with age.
- 195.Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P & Kooi ME Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 14, 357–367 (2006). [DOI] [PubMed] [Google Scholar]
- 196.Kemmler W, von Stengel S, Engelke K, Sieber C & Freiberger E Prevalence of sarcopenic obesity in Germany using established definitions: baseline data of the FORMOsA study. Osteoporos. Int 27, 275–281 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Johnson Stoklossa CA et al. Prevalence of sarcopenic obesity in adults with class II/III obesity using different diagnostic criteria. J. Nutr. Metab 2017, 7307618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F & Bartels SJ Low lean mass with and without obesity, and mortality: results from the 1999–2004 National Health and Nutrition Examination Survey. J. Gerontol. A Biol. Sci. Med. Sci 72, 1445–1451 (2017).This report details the definition of low lean mass determined by the Foundation of the National Institutes of Health, as well as the adverse association between low lean mass and mortality.
- 199.Kim TN et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int. J. Obes. (Lond.) 33, 885–892 (2009). [DOI] [PubMed] [Google Scholar]
- 200.Stenholm S et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int. J. Obes. (Lond.) 33, 635–644 (2009).This study details data from the InCHIANTI study that prove that obesity combined with low muscle strength increases the risk of mobility disability with age.
- 201.Stephen WC & Janssen I Sarcopenic-obesity and cardiovascular disease risk in the elderly. J. Nutr. Health Aging 13, 460–466 (2009). [DOI] [PubMed] [Google Scholar]
- 202.Alley DE et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A Biol. Sci. Med. Sci 69, 559–566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rolland Y et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic- obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am. J. Clin. Nutr 89, 1895–1900 (2009). [DOI] [PubMed] [Google Scholar]
- 204.Hirani V et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 46, 413–420 (2017). [DOI] [PubMed] [Google Scholar]
- 205.Zoico E et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int. J. Obes. Relat. Metab. Disord 28, 234–241 (2004). [DOI] [PubMed] [Google Scholar]
- 206.Schaap LA, Koster A & Visser M Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev 35, 51–65 (2013).This is a meta-analysis that proves the longitudinal impact of BMI on incident loss of muscle mass and muscle strength.
- 207.Batsis JA, Zbehlik AJ, Pidgeon D & Bartels SJ Dynapenic obesity and the effect on long-term physical function and quality of life: data from the osteoarthritis initiative. BMC Geriatr 15, 118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sayers SP et al. Validation of the late-life function and disability instrument. J. Am. Geriatr. Soc 52, 1554–1559 (2004). [DOI] [PubMed] [Google Scholar]
- 209.Ware J Jr, Kosinski M & Keller SDA 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care 34, 220–233 (1996). [DOI] [PubMed] [Google Scholar]
- 210.Yang M, Jiang J, Hao Q, Luo L & Dong B Dynapenic obesity and lower extremity function in elderly adults. J. Am. Med. Dir. Assoc 16, 31–36 (2015). [DOI] [PubMed] [Google Scholar]
- 211.Kim Y et al. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. Int. J. Obes. (Lond.) 41, 1361–1368 (2017).This study analyses data from the UK Biobank study and shows that advanced obesity and poor strength at baseline independently predict lower activities at follow-up.
- 212.Jung S et al. Obesity and muscle weakness as risk factors for mobility limitation in community-dwelling older Japanese women: a two-year follow-up investigation. J. Nutr. Health Aging 20, 28–34 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Ochi M et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr. Gerontol. Int 10, 233–243 (2010). [DOI] [PubMed] [Google Scholar]
- 214.Baek SJ et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Invest 37, 247–260 (2014). [DOI] [PubMed] [Google Scholar]
- 215.Chung JY, Kang HT, Lee DC, Lee HR & Lee YJ Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch. Gerontol. Geriatr 56, 270–278 (2013). [DOI] [PubMed] [Google Scholar]
- 216.Batsis JA, Mackenzie TA, Jones JD, Lopez-Jimenez F & Bartels SJ Sarcopenia, sarcopenic obesity and inflammation: results from the 1999–2004 National Health and Nutrition Examination Survey. Clin. Nutr 35, 1472–1483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Aubertin-Leheudre M, Lord C, Goulet ED, Khalil A & Dionne IJ Effect of sarcopenia on cardiovascular disease risk factors in obese postmenopausal women. Obesity (Silver Spring) 14, 2277–2283 (2006). [DOI] [PubMed] [Google Scholar]
- 218.Lee S, Kim TN & Kim SH Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum 64, 3947–3954 (2012). [DOI] [PubMed] [Google Scholar]
- 219.Scott D et al. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle- aged and older adults. Obesity (Silver Spring) 22, 1568–1574 (2014). [DOI] [PubMed] [Google Scholar]
- 220.Scott D et al. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community- dwelling older adults. Calcif. Tissue Int 99, 30–42 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Atkins JL et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population- based cohort study of older men. J. Am. Geriatr. Soc 62, 253–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hamer M, Batty GD & Kivimaki M Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. Int. J. Obes. (Lond.) 39, 1717–1720 (2015).This study includes longitudinal data that suggest an association between sarcopenic obesity and depression.
- 223.Ishii S et al. The association between sarcopenic obesity and depressive symptoms in older Japanese adults. PLoS ONE 11, e0162898 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mei KL, Batsis JA, Mills JB & Holubar SD Sarcopenia and sarcopenic obesity: do they predict inferior oncologic outcomes after gastrointestinal cancer surgery? Perioper. Med. (Lond.) 5, 30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Androga L, Sharma D, Amodu A & Abramowitz MK Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int. Rep 2, 201–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Malhotra R et al. Sarcopenic obesity definitions by body composition and mortality in the hemodialysis patients. J. Ren. Nutr 27, 84–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rossi AP et al. Dynapenic abdominal obesity as a predictor of worsening disability, hospitalization, and mortality in older adults: results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci 72, 1098–1104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stenholm S et al. Obesity and muscle strength as long-term determinants of all-cause mortality — a 33-year follow-up of the Mini-Finland Health Examination Survey. Int. J. Obes. (Lond.) 38, 1126–1132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hamer M & O’Donovan G Sarcopenic obesity, weight loss, and mortality: the English Longitudinal Study of Ageing. Am. J. Clin. Nutr 106, 125–129 (2017). [DOI] [PubMed] [Google Scholar]
- 230.Tian S & Xu Y Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int 16, 155–166 (2016). [DOI] [PubMed] [Google Scholar]
- 231.Newman AB et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci 61, 72–77 (2006).This study demonstrates the importance of muscle strength as opposed to muscle mass as a key determinant of mortality.
- 232.Rossi AP et al. Dynapenic abdominal obesity as predictor of mortality and disability worsening in older adults: a 10-year prospective study. Clin. Nutr 35, 199–204 (2016). [DOI] [PubMed] [Google Scholar]
- 233.Moon JH et al. Predictive values of the new sarcopenia index by the Foundation for the National Institutes of Health sarcopenia project for mortality among older Korean adults. PLoS ONE 11, e0166344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Messier V et al. Metabolic profile and quality of life in class I sarcopenic overweight and obese postmenopausal women: a MONET study. Appl. Physiol. Nutr. Metab 34, 18–24 (2009). [DOI] [PubMed] [Google Scholar]
- 235.Ware J et al. User’s Manual for the SF-36v2 Health Survey (QualityMetric Incorporated, 2007). [Google Scholar]
- 236.Silva Neto LS, Karnikowiski MG, Tavares AB & Lima RM Association between sarcopenia, sarcopenic obesity, muscle strength and quality of life variables in elderly women. Rev. Bras. Fisioter 16, 360–367 (2012). [PubMed] [Google Scholar]
- 237.Pedrero-Chamizo R et al. Higher levels of physical fitness are associated with a reduced risk of suffering sarcopenic obesity and better perceived health among the elderly: the EXERNET multi-center study. J. Nutr. Health Aging 19, 211–217 (2015). [DOI] [PubMed] [Google Scholar]
- 238.Cawthon PM et al. Sarcopenia and health care utilization in older women. J. Gerontol. A Biol. Sci. Med. Sci 72, 95–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Akune T et al. Incidence of certified need of care in the long-term care insurance system and its risk factors in the elderly of Japanese population-based cohorts: the ROAD study. Geriatr. Gerontol. Int 14, 695–701 (2014). [DOI] [PubMed] [Google Scholar]
- 240.Zizza CA, Herring A, Stevens J & Popkin BM Obesity affects nursing-care facility admission among whites but not blacks. Obes. Res 10, 816–823 (2002). [DOI] [PubMed] [Google Scholar]
- 241.Elkins JS et al. Midlife obesity and long-term risk of nursing home admission. Obesity (Silver Spring) 14, 1472–1478 (2006). [DOI] [PubMed] [Google Scholar]
- 242.Valiyeva E, Russell LB, Miller JE & Safford MM Lifestyle-related risk factors and risk of future nursing home admission. Arch. Intern. Med 166, 985–990 (2006). [DOI] [PubMed] [Google Scholar]
- 243.Kritchevsky SB et al. Intentional weight loss and allcause mortality: a meta-analysis of randomized clinical trials. PLoS ONE 10, e0121993 (2015).This is an important meta-analysis that evaluates randomized controlled trials of weight loss interventions in older adults and their impact on mortality.
- 244.Batsis JA, Huyck KL & Bartels SJ Challenges with the Medicare obesity benefit: practical concerns & proposed solutions. J. Gen. Intern. Med 30, 118–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jensen MD et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation 129, S102–S138 (2014).This report provides clinical obesity guidelines endorsed by multiple national societies.
- 246.Villareal DT et al. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med 364, 1218–1229 (2011).This is a randomized trial of older adults with frailty and obesity that compares diet, exercise type and controls and demonstrates that physical function improves and muscle and bone loss is mitigated with concomitant exercise therapy.
- 247.Villareal DT et al. Aerobic or resistance exercise, or both in dieting obese older adults. N. Engl. J. Med 376, 1943–1955 (2017).This is a randomized trial of older adults with obesity and frailty demonstrating that weight loss coupled with both aerobic and resistance activities is associated with improved physical function.
- 248.Chen HT, Chung YC, Chen YJ, Ho SY & Wu HJ Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J. Am. Geriatr. Soc 65, 827–832 (2017). [DOI] [PubMed] [Google Scholar]
- 249.McTigue KM et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med 139, 933–949 (2003). [DOI] [PubMed] [Google Scholar]
- 250.Batsis JA et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J. Am. Geriatr. Soc 65, 257–268 (2017).This is a comprehensive systematic review of randomized trials in older adults with obesity highlighting the importance of the improvement in physical function through diet and exercise.
- 251.Johnston BC et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 312, 923–933 (2014). [DOI] [PubMed] [Google Scholar]
- 252.Dansinger ML, Gleason JA, Griffith JL, Selker HP & Schaefer EJ Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293, 43–53 (2005). [DOI] [PubMed] [Google Scholar]
- 253.Areta JL et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab 306, E989–E997 (2014). [DOI] [PubMed] [Google Scholar]
- 254.Pasiakos SM et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J 27, 3837–3847 (2013). [DOI] [PubMed] [Google Scholar]
- 255.Campbell WW et al. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring) 17, 1332–1339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wolfe RR, Miller SL & Miller KB Optimal protein intake in the elderly. Clin. Nutr 27, 675–684 (2008). [DOI] [PubMed] [Google Scholar]
- 257.Schoenfeld BJ, Aragon AA & Krieger JW The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J. Int. Soc. Sports Nutr 10, 53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Smith GI et al. High-protein intake during weight loss therapy eliminates the weight-loss-induced improvement in insulin action in obese postmenopausal women. Cell Rep 17, 849–861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Deutz NE et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin. Nutr 33, 929–936 (2014).These are the European Society for Clinical Nutrition and Metabolism workshop recommendations on protein requirements in elderly individuals.
- 260.Bouillanne O et al. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: a randomized controlled trial. Clin. Nutr 32, 186–192 (2013). [DOI] [PubMed] [Google Scholar]
- 261.Beasley JM et al. Protein intake and incident frailty in the Women’s Health Initiative observational study. J. Am. Geriatr. Soc 58, 1063–1071 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tieland M et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc 13, 720–726 (2012). [DOI] [PubMed] [Google Scholar]
- 263.Porter Starr KN et al. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J. Gerontol. A Biol. Sci. Med. Sci 71, 1369–1375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Verreijen AM et al. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr. J 16, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sammarco R et al. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: a pilot study. Obes. Facts 10, 160–167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Avenell A & Handoll HH Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst. Rev 1, CD001880 (2010). [DOI] [PubMed] [Google Scholar]
- 267.Cawood AL, Elia M & Stratton RJ Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev 11, 278–296 (2012). [DOI] [PubMed] [Google Scholar]
- 268.Milne AC, Potter J, Vivanti A & Avenell A Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst. Rev 2, CD003288 (2009).This is a systematic Cochrane review of randomized and quasi-randomized controlled trials of oral protein and energy supplementation in older people, which shows a small but consistent weight gain in older people.
- 269.Brenner BM, Meyer TW & Hostetter TH Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med 307, 652–659 (1982). [DOI] [PubMed] [Google Scholar]
- 270.Klahr S The modification of diet in renal disease study. N. Engl. J. Med 320, 864–866 (1989). [DOI] [PubMed] [Google Scholar]
- 271.Lentine K & Wrone EM New insights into protein intake and progression of renal disease. Curr. Opin. Nephrol. Hypertens 13, 333–336 (2004). [DOI] [PubMed] [Google Scholar]
- 272.Pedrini MT, Levey AS, Lau J, Chalmers TC & Wang PH The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann. Intern. Med 124, 627–632 (1996). [DOI] [PubMed] [Google Scholar]
- 273.Bhasin S et al. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern. Med 178, 530–541 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Garber CE et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc 43, 1334–1359 (2011). [DOI] [PubMed] [Google Scholar]
- 275.Gillespie LD et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev 9, CD007146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lin X et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc 4, e002014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hwang CL, Wu YT & Chou CH Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J. Cardiopulm. Rehabil. Prev 31, 378–385 (2011). [DOI] [PubMed] [Google Scholar]
- 278.Sui XM et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 298, 2507–2516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Taaffe DR, Duret C, Wheeler S & Marcus R Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J. Am. Geriatr. Soc 47, 1208–1214 (1999). [DOI] [PubMed] [Google Scholar]
- 280.Brown AB, McCartney N & Sale DG Positive adaptations to weight-lifting training in the elderly. J. Appl. Physiol. (1985) 69, 1725–1733 (1990). [DOI] [PubMed] [Google Scholar]
- 281.Liu CJ & Latham NK Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev 3, CD002759 (2009).This is a systematic Cochrane review of randomized controlled trials reporting physical outcomes of progressive resistance therapy that demonstrates improved strength and performance.
- 282.Pahor M et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311, 2387–2396 (2014).This study reports on a gold standard, structured, long-term physical activity intervention, consisting of 3–4 times of aerobic, resistance and flexibility training per week, that demonstrates improvements in mobility disability over a 2.6-year follow-up.
- 283.Stec MJ et al. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp. Gerontol 99, 98–109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME & Wallace RB Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med 332, 556–561 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu CK et al. The impact of sarcopenia on a physical activity intervention: the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P). J. Nutr. Health Aging 18, 59–64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Villanueva MG, Lane CJ & Schroeder ET Short rest interval lengths between sets optimally enhance body composition and performance with 8 weeks of strength resistance training in older men. Eur. J. Appl. Physiol 115, 295–308 (2015). [DOI] [PubMed] [Google Scholar]
- 287.Ramirez-Campillo R et al. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp. Gerontol 58, 51–57 (2014). [DOI] [PubMed] [Google Scholar]
- 288.Ramirez-Campillo R et al. Effects of different doses of high-speed resistance training on physical performance and quality of life in older women: a randomized controlled trial. Clin. Interv. Aging 11, 1797–1804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Balachandran A, Krawczyk SN, Potiaumpai M & Signorile JF High-speed circuit training versus hypertrophy training to improve physical function in sarcopenic obese adults: a randomized controlled trial. Exp. Gerontol 60, 64–71 (2014). [DOI] [PubMed] [Google Scholar]
- 290.Vasconcelos KS et al. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: a randomized controlled trial. Braz. J. Phys. Ther 20, 432–440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Son NK, Ryu YU, Jeong HW, Jang YH & Kim HD Comparison of 2 different exercise approaches: Tai Chi versus Otago, in community-dwelling older women. J. Geriatr. Phys. Ther 39, 51–57 (2016). [DOI] [PubMed] [Google Scholar]
- 292.Huang ZG, Feng YH, Li YH & Lv CS Systematic review and meta-analysis: Tai Chi for preventing falls in older adults. BMJ Open 7, e013661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Youkhana S, Dean CM, Wolff M, Sherrington C & Tiedemann A Yoga-based exercise improves balance and mobility in people aged 60 and over: a systematic review and meta-analysis. Age Ageing 45, 21–29 (2016). [DOI] [PubMed] [Google Scholar]
- 294.Waller B et al. The effect of aquatic exercise on physical functioning in the older adult: a systematic review with meta-analysis. Age Ageing 45, 593–601 (2016). [DOI] [PubMed] [Google Scholar]
- 295.Nobrega SR & Libardi CA Is resistance training to muscular failure necessary? Front. Physiol 7, 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Aguirre LE & Villareal DT Physical exercise as therapy for frailty. Nestle Nutr. Inst. Workshop Ser 83, 83–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liao CD et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: a CONSORT- compliant prospective randomized controlled trial. Medicine (Baltimore) 96, e7115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Weinheimer EM, Sands LP & Campbell WW A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr. Rev 68, 375–388 (2010).This is a systematic review assessing the effects of energy restriction and exercise on fat-free mass and suggests that exercise is an effective tool in combating sarcopenia-induced weight loss.
- 299.Armamento-Villareal R, Aguirre LE, Qualls C & Villareal DT Effect of lifestyle intervention on the hormonal profile of frail, obese older men. J. Nutr. Health Aging 20, 334–340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Villareal DT et al. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am. J. Clin. Nutr 84, 1317–1323 (2006). [DOI] [PubMed] [Google Scholar]
- 301.Bouchonville M et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int. J. Obes. (Lond.) 38, 423–431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Abbenhardt C et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J. Intern. Med 274, 163–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Beavers KM et al. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. SportsExerc 42, 2189–2196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kelly KR et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med. Sci. Sports Exerc 46, 920–926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wang X, You T, Murphy K, Lyles MF & Nicklas BJ Addition of exercise increases plasma adiponectin and release from adipose tissue. Med. Sci. Sports Exerc 47, 2450–2455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.O’Leary VB et al. Enhanced adiponectin multimer ratio and skeletal muscle adiponectin receptor expression following exercise training and diet in older insulin-resistant adults. Am. J. Physiol. Endocrinol. Metab 293, E421–E427 (2007). [DOI] [PubMed] [Google Scholar]
- 307.Nicklas BJ et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am. J. Clin. Nutr 79, 544–551 (2004).This is an early trial suggesting that resistance training is important for improving body composition and muscle strength in elderly individuals who are obese, an effect which is improved with caloric restriction.
- 308.Beavers KM, Ambrosius WT, Nicklas BJ & Rejeski WJ Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J. Am. Geriatr. Soc 61, 1089–1094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zibellini J et al. Effect of diet-induced weight loss on muscle strength in adults with overweight or obesity — a systematic review and meta-analysis of clinical trials. Obes. Rev 17, 647–663 (2016). [DOI] [PubMed] [Google Scholar]
- 310.Gallagher D et al. Changes in skeletal muscle and organ size after a weight-loss intervention in overweight and obese type 2 diabetic patients. Am. J. Clin. Nutr 105, 78–84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Weiss EP, Jordan RC, Frese EM, Albert SG & Villareal DT Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med. Sci. Sports Exerc 49, 206–217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wood RJ et al. Preservation of fat-free mass after two distinct weight loss diets with and without progressive resistance exercise. Metab. Syndr. Relat. Disord 10, 167–174 (2012). [DOI] [PubMed] [Google Scholar]
- 313.Tang X et al. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PLoS ONE 8, e55077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nielson CM et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS). J. Bone Miner. Res 26, 496–502 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Compston JE et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med 124, 1043–1050 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ensrud KE et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J. Am. Geriatr. Soc 57, 492–498 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sundh D et al. A high amount of local adipose tissue is associated with high cortical porosity and low bone material strength in older women. J. Bone Miner. Res 31, 749–757 (2016). [DOI] [PubMed] [Google Scholar]
- 318.Zibellini J et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J. Bone Miner. Res 30, 2168–2178 (2015). [DOI] [PubMed] [Google Scholar]
- 319.Soltani S, Hunter GR, Kazemi A & Shab-Bidar S The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int 27, 2655–2671 (2016). [DOI] [PubMed] [Google Scholar]
- 320.Colleluori G, Napoli N, Phadnis U, Armamento- Villareal R & Villareal DT Effect of weight loss, exercise, or both on undercarboxylated osteocalcin and insulin secretion in frail, obese older adults. Oxid. Med. Cell. Longev 2017, 4807046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Waters DL et al. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J. Nutr. Health Aging 17, 3–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Villareal DT et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Miner. Res 31, 40–51 (2016).This is a 2-year randomized trial of caloric restriction, which had been previously shown to lead to bone loss at important sites of osteoporotic fractures as represented by reductions in BMD.
- 323.Villareal DT et al. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell 10, 96–102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Pop LC et al. Moderate weight loss in obese and overweight men preserves bone quality. Am. J. Clin. Nutr 101, 659–667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Johnson KC et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the Look AHEAD randomized clinical trial. J. Bone Miner. Res 32, 2278–2287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Haywood CJ et al. Very low calorie diets for weight loss in obese older adults—a randomized trial. J. Gerontol. A Biol. Sci. Med. Sci 73, 59–65 (2017). [DOI] [PubMed] [Google Scholar]
- 327.Shah K et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J. Bone Miner. Res 26, 2851–2859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Armamento-Villareal R et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J. Bone Miner. Res 27, 1215–1221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Moyer VA Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 158, 691–696 (2013). [DOI] [PubMed] [Google Scholar]
- 330.Anderson JJ et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J. Am. Heart Assoc 5, e003815 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hassan-Smith ZK et al. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 12, e0170665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Beaudart C et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab 99, 4336–4345 (2014). [DOI] [PubMed] [Google Scholar]
- 333.Malafarina V, Uriz-Otano F, Malafarina C, Martinez JA & Zulet MA Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 101, 42–50 (2017). [DOI] [PubMed] [Google Scholar]
- 334.American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J. Am. Geriatr. Soc 62, 147–152 (2014). [DOI] [PubMed] [Google Scholar]
- 335.Bischoff-Ferrari HA et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower- extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr 80, 752–758 (2004). [DOI] [PubMed] [Google Scholar]
- 336.Scott D et al. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. (Oxf.) 73, 581–587 (2010). [DOI] [PubMed] [Google Scholar]
- 337.Wicherts IS et al. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab 92, 2058–2065 (2007). [DOI] [PubMed] [Google Scholar]
- 338.Zittermann A et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am. J. Clin. Nutr 95, 91–100 (2012). [DOI] [PubMed] [Google Scholar]
- 339.Anker SD, Morley JE & von Haehling S Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 7, 512–514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Cao B Future healthy life expectancy among older adults in the US: a forecast based on cohort smoking and obesity history. Popul. Health Metr 14, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Preventive US Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann. Intern. Med 154, 356–364 (2011). [DOI] [PubMed] [Google Scholar]
- 342.Maddalo M et al. Validation of a free software for unsupervised assessment of abdominal fat in MRI. Phys. Med 37, 24–31 (2017). [DOI] [PubMed] [Google Scholar]
- 343.van Vugt JLA et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J. Cachexia Sarcopenia Muscle 8, 285–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Curtis JR et al. The geographic availability and associated utilization of dual-energy X-ray absorptiometry (DXA) testing among older persons in the United States. Osteoporos. Int 20, 1553–1561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Maggio M et al. Instrumental and non-instrumental evaluation of 4-meter walking speed in older individuals. PLoS ONE 11, e0153583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nascimento LR et al. Different instructions during the ten-meter walking test determined significant increases in maximum gait speed in individuals with chronic hemiparesis. Rev. Bras. Fisioter 16, 122–127 (2012). [DOI] [PubMed] [Google Scholar]
- 347.Roberts HC et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40, 423–429 (2011). [DOI] [PubMed] [Google Scholar]
- 348.Witard OC, McGlory C, Hamilton DL & Phillips SM Growing older with health and vitality: a nexus of physical activity, exercise and nutrition. Biogerontology 17, 529–546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Garatachea N et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res 18, 57–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Emmelot-Vonk MH et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299, 39–52 (2008). [DOI] [PubMed] [Google Scholar]
- 351.Travison TG et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J. Gerontol. A Biol. Sci. Med. Sci 66, 1090–1099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nguyen HQ et al. Health care use and costs associated with use of a health club membership benefit in older adults with diabetes. Diabetes Care 31, 1562–1567 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bergamin M et al. Water-versus land-based exercise in elderly subjects: effects on physical performance and body composition. Clin. Interv. Aging 8, 1109–1117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.de Souza Vasconcelos KS et al. Land-based versus aquatic resistance therapeutic exercises for older women with sarcopenic obesity: study protocol for a randomised controlled trial. Trials 14, 296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Simmons V & Hansen PD Effectiveness of water exercise on postural mobility in the well elderly: an experimental study on balance enhancement. J. Gerontol. A Biol. Sci. Med. Sci 51, M233–M238 (1996). [DOI] [PubMed] [Google Scholar]
- 356.Li F et al. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized contolled trial. Ann. Behav. Med 23, 139–146 (2001). [DOI] [PubMed] [Google Scholar]
- 357.Hildreth KL et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J. Clin. Endocrinol. Metab 98, 1891–1900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Strohacker K, Fazzino D, Breslin WL & Xu X The use of periodization in exercise prescriptions for inactive adults: a systematic review. Prev. Med. Rep 2, 385–396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Steele J Intensity; in-ten-si-ty; noun. 1. Often used ambiguously within resistance training. 2. Is it time to drop the term altogether? Br. J. Sports Med 48, 1586–1588 (2014). [DOI] [PubMed] [Google Scholar]
- 360.Conlon JA et al. Periodization strategies in older adults: impact on physical function and health. Med. Sci. Sports Exerc 48, 2426–2436 (2016). [DOI] [PubMed] [Google Scholar]
- 361.Prestes J et al. Understanding the individual responsiveness to resistance training periodization. Age(Dordr.) 37, 9793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Botero JP et al. Effects of long-term periodized resistance training on body composition, leptin, resistin and muscle strength in elderly postmenopausal women. J. Sports Med. Phys. Fitness 53, 289–294 (2013). [PubMed] [Google Scholar]
- 363.Ebersbach G, Edler D, Kaufhold O & Wissel J Whole body vibration versus conventional physiotherapy to improve balance and gait in Parkinson’s disease. Arch. Phys. Med. Rehabil 89, 399–403 (2008). [DOI] [PubMed] [Google Scholar]
- 364.Zhang L et al. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: a pilot randomized controlled trial. Clin. Rehabil 28, 59–68 (2014). [DOI] [PubMed] [Google Scholar]
- 365.Rogan S, Hilfiker R, Herren K, Radlinger L & de Bruin ED Effects of whole-body vibration on postural control in elderly: a systematic review and meta-analysis. BMC Geriatr 11, 72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Rogan S et al. Feasibility and effects of applying stochastic resonance whole-body vibration on untrained elderly: a randomized crossover pilot study. BMC Geriatr 15, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Verschueren SM et al. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: a 6-month randomized, controlled trial. J. Bone Miner Res 26, 42–49 (2011). [DOI] [PubMed] [Google Scholar]
- 368.Cardim AB et al. Does whole-body vibration improve the functional exercise capacity of subjects with COPD? A meta-analysis. Respir. Care 61, 1552–1559 (2016). [DOI] [PubMed] [Google Scholar]
- 369.Burke D & Schiller HH Discharge pattern of single motor units in the tonic vibration reflex of human triceps surae. J. Neurol. Neurosurg. Psychiatry 39, 729–741 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Chen H, Ma J, Lu B & Ma XL The effect of whole-body vibration training on lean mass: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 96, e8390 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lau RW et al. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin. Rehabil 25, 975–988 (2011). [DOI] [PubMed] [Google Scholar]
- 372.Bogaerts A et al. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci 62, 630–635 (2007). [DOI] [PubMed] [Google Scholar]
- 373.Chang SF, Lin PC, Yang RS & Yang RJ The preliminary effect of whole-body vibration intervention on improving the skeletal muscle mass index, physical fitness, and quality of life among older people with sarcopenia. BMC Geriatr 18, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Bemben DA, Palmer IJ, Bemben MG & Knehans AW Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 47, 650–656 (2010). [DOI] [PubMed] [Google Scholar]
- 375.Fjeldstad C, Palmer IJ, Bemben MG & Bemben DA Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas 63, 79–83 (2009). [DOI] [PubMed] [Google Scholar]
- 376.Machado A, Garcia-Lopez D, Gonzalez-Gallego J & Garatachea N Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand. J. Med. Sci. Sports 20, 200–207 (2010). [DOI] [PubMed] [Google Scholar]
- 377.von Stengel S, Kemmler W, Engelke K & Kalender WA Effect of whole-body vibration on neuromuscular performance and body composition for females 65 years and older: a randomized-controlled trial. Scand. J. Med. Sci. Sports 22, 119–127 (2012). [DOI] [PubMed] [Google Scholar]
- 378.Cristi-Montero C, Cuevas MJ & Collado PS Whole-body vibration training as complement to programs aimed at weight loss. Nutr. Hosp 28, 1365–1371 (2013). [DOI] [PubMed] [Google Scholar]
- 379.Lamont HS et al. Effects of a 6-week periodized squat training with or without whole-body vibration upon short-term adaptations in squat strength and body composition. J. Strength Cond. Res 25, 1839–1848 (2011). [DOI] [PubMed] [Google Scholar]
- 380.Wei N, Pang MY, Ng SS & Ng GY Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): a randomized controlled trial. Geriatr. Gerontol. Int 17, 1412–1420 (2017). [DOI] [PubMed] [Google Scholar]
- 381.Perna S et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin. Exp. Res 28, 1251–1257 (2016). [DOI] [PubMed] [Google Scholar]
- 382.Apovian C, Palmer K, Fain R, Perdomo C & Rubino D Effects of lorcaserin on fat and lean mass loss in obese and overweight patients without and with type 2 diabetes mellitus: the BLOSSOM and BLOOM-DM studies. Diabetes Obes. Metab 18, 945–948 (2016). [DOI] [PubMed] [Google Scholar]
- 383.Simko J et al. The effect of topiramate and lamotrigine on rat bone mass, structure and metabolism. J. Neurol. Sci 340, 80–85 (2014). [DOI] [PubMed] [Google Scholar]
- 384.Eliasson B et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int. J. Obes. (Lond.) 31, 1140–1147 (2007). [DOI] [PubMed] [Google Scholar]
- 385.Yaman M et al. Effects of topiramate use on body composition and resting metabolic rate in migraine patients. Neurol. Sci 34, 225–229 (2013). [DOI] [PubMed] [Google Scholar]
- 386.Cordero-Maclntyre ZR et al. Effect of a weight-reduction program on total and regional body composition in obese postmenopausal women. Ann. NY Acad. Sci 904, 526–535 (2000). [DOI] [PubMed] [Google Scholar]
- 387.Gadde KM, Zhang W & Foust MS Bupropion treatment of olanzapine-associated weight gain: an open-label, prospective trial. J. Clin. Psychopharmacol 26, 409–413 (2006). [DOI] [PubMed] [Google Scholar]
- 388.Smith SR et al. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes Obes. Metab 15, 863–866 (2013). [DOI] [PubMed] [Google Scholar]
- 389.Kujawska-Luczak M et al. The effect of orlistat versus metformin on body composition and insulin resistance in obese premenopausal women: 3-month randomized prospective open-label study. Arch. Med. Sci 13, 725–731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Smith SR et al. Orlistat 60 mg reduces visceral adipose tissue: a 24-week randomized, placebo- controlled, multicenter trial. Obesity (Silver Spring) 19, 1796–1803 (2011). [DOI] [PubMed] [Google Scholar]
- 391.Smith TJ et al. Efficacy of orlistat 60 mg on weight loss and body fat mass in US Army soldiers. J. Acad. Nutr. Diet 112, 533–540 (2012). [DOI] [PubMed] [Google Scholar]
- 392.Batsis JA & Dolkart KM Evaluation of older adults with obesity for bariatric surgery: geriatricians’ perspective. J. Clin. Gerontol. Geriatr 6, 45–53 (2015). [Google Scholar]
- 393.Mastino D et al. Bariatric surgery outcomes in sarcopenic obesity. Obes. Surg 26, 2355–2362 (2016). [DOI] [PubMed] [Google Scholar]
- 394.Cole AJ et al. Long-term body composition changes in women following roux-en-Y gastric bypass surgery. JPEN J. Parenter. Enteral Nutr 41, 583–591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Anderwald CH et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care 35, 2580–2587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lyytinen T, Liikavainio T, Paakkonen M, Gylling H & Arokoski JP Physical function and properties of quadriceps femoris muscle after bariatric surgery and subsequent weight loss. J. Musculoskelet. Neuronal Interaa 13, 329–338 (2013). [PubMed] [Google Scholar]
- 397.Thibault R, Huber O, Azagury DE & Pichard C Twelve key nutritional issues in bariatric surgery. Clin. Nutr 35, 12–17 (2016). [DOI] [PubMed] [Google Scholar]
- 398.Vaurs C et al. Determinants of changes in muscle mass after bariatric surgery. Diabetes Metab 41, 416–421 (2015). [DOI] [PubMed] [Google Scholar]
- 399.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR & Greenspan SL Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J. Clin. Endocrinol. Metab 89, 1061–1065 (2004). [DOI] [PubMed] [Google Scholar]
- 400.Ko BJ et al. Relationship between bariatric surgery and bone mineral density: a meta-analysis. Obes. Surg 26, 1414–1421 (2016). [DOI] [PubMed] [Google Scholar]
- 401.Shanbhogue VV et al. Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study. Eur. J. Endocrinol 176, 685–693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Yu EW, Lee MP, Landon JE, Lindeman KG & Kim SC Fracture risk after bariatric surgery: roux-en-Y gastric bypass versus adjustable gastric banding. J. Bone Miner. Res 32, 1229–1236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Cohen PG Obesity in men: the hypogonadal estrogen receptor relationship and its effect on glucose homeostasis. Med. Hypotheses 70, 358–360 (2008). [DOI] [PubMed] [Google Scholar]
- 404.Freeman EW, Sammel MD, Lin H & Gracia CR Obesity and reproductive hormone levels in the transition to menopause. Menopause 17, 718–726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Nettleship JE, Pugh PJ, Channer KS, Jones T & Jones RD Inverse relationship between serum levels of interleukin-1beta and testosterone in men with stable coronary artery disease. Horm. Metab. Res 39, 366–371 (2007). [DOI] [PubMed] [Google Scholar]
- 406.Corrales JJ et al. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J. Endocrinol 189, 595–604 (2006). [DOI] [PubMed] [Google Scholar]
- 407.Neto WK et al. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age (Dordr.) 37, 9742 (2015).This is a meta-analysis demonstrating that testosterone supplementation in elderly men leads to increases in lean mass and potentially reductions in fat mass.
- 408.Giannoulis MG et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J. Clin. Endocrinol. Metab 91, 477–484 (2006). [DOI] [PubMed] [Google Scholar]
- 409.Schroeder ET et al. Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur. J. Appl. Physiol 112, 1123–1131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Hyde Z et al. Low free testosterone predicts frailty in older men: the health in men study. J. Clin. Endocrinol. Metab 95, 3165–3172 (2010). [DOI] [PubMed] [Google Scholar]
- 411.Krasnoff JB et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J. Clin. Endocrinol. Metab 95, 2790–2799 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Storer TW et al. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J. Clin. Endocrinol. Metab 102, 583–593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Saad F, Rohrig G, von Haehling S & Traish A Testosterone deficiency and testosterone treatment in older men. Gerontology 63, 144–156 (2017). [DOI] [PubMed] [Google Scholar]
- 414.Bhasin S et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J. Clin. Endocrinol. Metab 90, 678–688 (2005).This is a randomized trial of dose-dependent testosterone supplementation that demonstrates that older men are as responsive as young men to the anabolic effects of testosterone; however, older men have lower testosterone clearance rates, higher increments in haemoglobin and a higher frequency of adverse effects.
- 415.Srinivas-Shankar U et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab 95, 639–650 (2010). [DOI] [PubMed] [Google Scholar]
- 416.Page ST et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab 90, 1502–1510 (2005). [DOI] [PubMed] [Google Scholar]
- 417.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA & Ostir GV Androgen treatment and muscle strength in elderly men: a meta-analysis. J. Am. Geriatr. Soc 54, 1666–1673 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.O’Connell MD et al. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J. Clin. Endocrinol. Metab 96, 454–458 (2011). [DOI] [PubMed] [Google Scholar]
- 419.Ng Tang Fui M et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med 14, 153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Ohlander SJ, Varghese B & Pastuszak AW Erythrocytosis following testosterone therapy. Sex. Med. Rev 6, 77–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Albert SG & Morley JE Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin. Endocrinol. (Oxf.) 85, 436–443 (2016). [DOI] [PubMed] [Google Scholar]
- 422.Martinez C et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ 355, i5968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Basaria S et al. Adverse events associated with testosterone administration. N. Engl. J. Med 363, 109–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Morgentaler A et al. Fundamental concepts regarding testosterone deficiency and treatment: international expert consensus resolutions. Mayo Clin. Proc 91, 881–896 (2016). [DOI] [PubMed] [Google Scholar]
- 425.Bhasin S et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab 95, 2536–2559 (2010). [DOI] [PubMed] [Google Scholar]
- 426.Dubois V, Laurent M, Boonen S, Vanderschueren D & Claessens F Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci 69, 1651–1667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Crawford J et al. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr. Oncol. Rep 18, 37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Dobs AS et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol 14, 335–345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Dalton JT et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2, 153–161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Papanicolaou DA et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J. Nutr. Health Aging 17, 533–543 (2013). [DOI] [PubMed] [Google Scholar]
- 431.Bhattacharya I et al. Safety, pharmacokinetic, and pharmacodynamic evaluation after single and multiple ascending doses of a novel selective androgen receptor modulator in healthy subjects. Clin. Ther 38, 1401–1416 (2016). [DOI] [PubMed] [Google Scholar]
- 432.Watanabe K et al. BA321, a novel carborane analog that binds to androgen and estrogen receptors, acts as a new selective androgen receptor modulator of bone in male mice. Biochem. Biophys. Res. Commun 478, 279–285 (2016). [DOI] [PubMed] [Google Scholar]
- 433.Saeed A et al. 2-Chloro-4-[[(1R,2R)-2-hydroxy-2-methyl-cyclopentyl]amino]-3-methyl-benzonitrile: a transdermal selective androgen receptor modulator (SARM) for muscle atrophy. J. Med. Chem 59, 750–755 (2016). [DOI] [PubMed] [Google Scholar]
- 434.von Haehling S & Anker SD Treatment of cachexia: an overview of recent developments. Int. J. Cardiol 184, 736–742 (2015). [DOI] [PubMed] [Google Scholar]
- 435.Garcia JM & Polvino WJ Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm. IGF Res 19, 267–273 (2009). [DOI] [PubMed] [Google Scholar]
- 436.Garcia JM & Polvino WJ Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple- dose study in healthy volunteers. Oncologist 12, 594–600 (2007). [DOI] [PubMed] [Google Scholar]
- 437.Koshinaka K et al. Therapeutic potential of ghrelin treatment for unloading-induced muscle atrophy in mice. Biochem. Biophys. Res. Commun 41 2, 296–301 (2011). [DOI] [PubMed] [Google Scholar]
- 438.Nishie K, Yamamoto S, Nagata C, Koizumi T & Hanaoka M Anamorelin for advanced non-small-cell lung cancer with cachexia: systematic review and meta-analysis. Lung Cancer 112, 25–34 (2017). [DOI] [PubMed] [Google Scholar]
- 439.Bai Y et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support. Care Cancer 25, 1651–1659 (2017).This is a meta-analysis of four randomized trials demonstrating that lean body mass improved with anamorelin, a novel ghrelin receptor agonist.
- 440.Garcia JM et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol 16, 108–116 (2015). [DOI] [PubMed] [Google Scholar]
- 441.Temel JS et al. Anamorelin in patients with non-small- cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17, 519–531 (2016). [DOI] [PubMed] [Google Scholar]
- 442.Katakami N et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (0N0-7643-04). Cancer 124, 606–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Bergen HR III et al. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet. Muscle 5, 21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.McPherron AC, Lawler AM & Lee SJ Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90 (1997). [DOI] [PubMed] [Google Scholar]
- 445.White TA & LeBrasseur NK Myostatin and sarcopenia: opportunities and challenges — a mini-review. Gerontology 60, 289–293 (2014). [DOI] [PubMed] [Google Scholar]
- 446.Camporez JP et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl Acad. Sci. USA 113, 2212–2217 (2016).This study evaluates a treatment consisting of anti- myostatin antibody administration for 4 weeks, showing improved muscle mass and strength in young and old mice.
- 447.Dong J et al. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. (Lond.) 40, 434–442 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Buehring B & Binkley N Myostatin — the holy grail for muscle, bone, and fat? Curr Osteoporos. Rep 11, 407–414 (2013). [DOI] [PubMed] [Google Scholar]
- 449.Santos AR et al. Different resistance-training regimens evoked a similar increase in myostatin inhibitors expression. Int. J. Sports Med 36, 761–768 (2015). [DOI] [PubMed] [Google Scholar]
- 450.Sato Y, Honda Y, Kuno H & Oizumi K Menatetrenone ameliorates osteopenia in disuse- affected limbs of vitamin D- and K-deficient stroke patients. Bone 23, 291–296 (1998). [DOI] [PubMed] [Google Scholar]
- 451.Schurgers LJ & Vermeer C Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 30, 298–307 (2000). [DOI] [PubMed] [Google Scholar]
- 452.Hart JP et al. Electrochemical detection of depressed circulating levels of vitamin K1 in osteoporosis. J. Clin. Endocrinol. Metab 60, 1268–1269 (1985). [DOI] [PubMed] [Google Scholar]
- 453.Bitensky L et al. Circulating vitamin K levels in patients with fractures. J. Bone Joint Surg. Br 70, 663–664 (1988). [DOI] [PubMed] [Google Scholar]
- 454.Hodges SJ, Akesson K, Vergnaud P, Obrant K & Delmas PD Circulating levels of vitamins K1 and K2 decreased in elderly women with hip fracture. J. Bone Miner. Res 8, 1241–1245 (1993). [DOI] [PubMed] [Google Scholar]
- 455.Hodges SJ et al. Depressed levels of circulating menaquinones in patients with osteoporotic fractures of the spine and femoral neck. Bone 12, 387–389 (1991). [DOI] [PubMed] [Google Scholar]
- 456.Binkley N et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J. Bone Miner. Res 24, 983–991 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Binkley NC & Suttie JW Vitamin K nutrition and osteoporosis. J. Nutr 125, 1812–1821 (1995). [DOI] [PubMed] [Google Scholar]
- 458.Caillot-Augusseau A et al. Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: observations in two cosmonauts. Clin. Chem 46, 1136–1143 (2000). [PubMed] [Google Scholar]
- 459.Douglas AS et al. Carboxylation of osteocalcin in post-menopausal osteoporotic women following vitamin K and D supplementation. Bone 17, 15–20 (1995). [DOI] [PubMed] [Google Scholar]
- 460.Macdonald HM et al. Vitamin K1 intake is associated with higher bone mineral density and reduced bone resorption in early postmenopausal Scottish women: no evidence of gene-nutrient interaction with apolipoprotein E polymorphisms. Am. J. Clin. Nutr 87, 1513–1520 (2008). [DOI] [PubMed] [Google Scholar]
- 461.Jamal SA, Browner WS, Bauer DC & Cummings SR Warfarin use and risk for osteoporosis in elderly women. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med 128, 829–832 (1998). [DOI] [PubMed] [Google Scholar]
- 462.Yonemura K, Kimura M, Miyaji T & Hishida A Short-term effect of vitamin K administration on prednisolone-induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcif. Tissue Int 66, 123–128 (2000). [DOI] [PubMed] [Google Scholar]
- 463.Yoshida M et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 31, 2092–2096 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Shea MK, Dawson-Hughes B, Gundberg CM & Booth SL Reducing undercarboxylated osteocalcin with vitamin k supplementation does not promote lean tissue loss or fat gain over 3 years in older women and men: a randomized controlled trial. J. Bone Miner. Res 32, 243–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Shah K, Gleason L & Villareal DT Vitamin K and bone health in older adults. J. Nutr. Gerontol. Geriatr 33, 10–22 (2014). [DOI] [PubMed] [Google Scholar]
- 466.Collins CA et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005). [DOI] [PubMed] [Google Scholar]
- 467.Sacco A, Doyonnas R, Kraft P, Vitorovic S & Blau HM Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Tompkins BA et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase ii randomized, double-blind, placebo-controlled clinical trial. J. Gerontol. A Biol. Sci. Med. Sci 72, 1513–1522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Newman AB et al. Sarcopenia: alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc 51, 1602–1609 (2003).This evaluates two sarcopenia definitions in the Health, Aging and Body Composition study and suggests differential prevalence rates by obesity status, highlighting the importance of fat mass in the evaluation of sarcopenia.
- 470.Baumgartner RN Body composition in healthy aging. Ann. NY Acad. Sci 904, 437–448 (2000). [DOI] [PubMed] [Google Scholar]