Abstract
Paediatric shock is still a common emergency of public health importance with an estimated 400,000–500,000 reported cases annually. Mortality due to paediatric shock has varied over the years. Data in 1980s show that mortality rates due to septic shock in children were over 50%; but by the end of the year 2000 data indicated that though a marked decline in mortality rates had been achieved, it had stagnated at about 20%. Descriptions of paediatric shock reveal the lack of a common definition and there are important gaps in evidence-based management in different settings. In well-resourced healthcare systems with well-functioning intensive care facilities, the widespread implementation of shock management guidelines based on the Paediatric Advanced Life Support and European Paediatric Advanced Life Support courses have reduced mortality. In resource limited settings with diverse infectious causative agents, the Emergency Triage Assessment and Treatment (ETAT) approach is more pragmatic, but its impact remains circumscribed to centres where ETAT has been implemented and sustained. Advocacy for common management pathways irrespective of underlying cause have been suggested. However, in sub Saharan Africa, the diversity of underlying causative organisms and patient phenotypes may limit a single approach to shock management.
Data from a large fluid trial (the FEAST trial) in East Africa have provided vital insight to shock management. In this trial febrile children with clinical features of impaired perfusion were studied. Rapid infusion of fluid boluses, irrespective of whether the fluid was colloid or crystalloid, when compared to maintenance fluids alone had an increased risk of mortality at 48 h. All study participants were promptly managed for underlying conditions and comorbidity such as malaria, bacteraemia, severe anaemia, meningitis, pneumonia, convulsions, hypoglycaemia and others. The overall low mortality in the trial suggests the potential contribution of ETAT, the improved standard of care and supportive treatment across the subgroups in the trial. Strengthening systems that enable rapid identification of shock, prompt treatment of children with correct antimicrobials and supportive care such as oxygen administration and blood transfusion may contribute to better survival outcomes in resources limited settings.
Keywords: Shock, Paediatric, Aetiology, Pathophysiology and management
African relevance
-
•
Shock in African children remain poorly defined.
-
•
Paediatric shock is a leading cause for morbidity and mortality in Africa.
-
•
There are no uniform guidelines for the management of shock in children.
Introduction
Paediatric shock is a common emergency that is responsible for high morbidity and mortality [1], [2]. Globally, an estimated 400,000–500,000 reported cases of paediatric septic shock occur annually [3]. In the last three decades, there have been improvements in mortality outcomes in paediatric shock from rates of about 50% to 20% by the year 2000 [3]. However, 20% mortality from a single syndrome cause remains unacceptably high.
Shock has been defined in various ways (Table 1), but terms such as compensated shock, decompensated shock, impaired circulation and severely impaired circulation are used and sometimes interchanged. Table 2 describes these terms. The cardinal clinical features and laboratory markers for shock are well documented, but its clinical recognition may be challenging especially in resources-limited settings. Moreover, delay in identifying and promptly treating shock often results in progression to metabolic deregulation especially metabolic acidosis [1], [2]. Compensated shock may advance into clinically unredeemable decompensated states with fatal organ dysfunction [4]. While disability especially among neonatal shock survivors is not infrequent [5], it is very rare to have established shock resolve spontaneously without appropriate treatment [6]. In an attempt to improve recognition, treatment and outcomes of paediatric shock in resources-limited settings, training courses such as ETAT (Emergency Triage Assessment and Treatment) [7]; PALS (Paediatric Advanced Life Support) [8]; EPLS (European Paediatric Life Support Course) (2006) [9]; have been developed to equip clinicians with skills in the rapid assessment and treatment modalities of children with shock [10]. The overall impact on child survival where these initiatives have been successfully implemented is tangible [10]. Nevertheless clinicians, especially in resource-limited settings, still find it challenging to diagnose shock and take initial management decisions for a number of reasons. Firstly, while clinical shock is common, there are a confusing number of different definitions (Table 1). Secondly, there are varied practices with a striking lack of consensus on definitive policies and guidelines on the management of paediatric shock. Thirdly, there are conflicting schools of thought about fluids; on one hand, rapid fluid administration, as supported by a systematic review in 2009, is widely taught and practiced [11]; on the other hand conservative fluid resuscitation in early stages of shock has been recommended [12]. Fourthly, contentious debate surrounds data that have attempted to answer theoretically simple but clinically complex questions on what type of fluid to give, how much fluid to give, how rapidly to infuse fluids and who should receive fluids? [13], [14].
Table 1.
Defining shock.
| Shock criterion | Definition of shock |
|---|---|
| FEAST inclusion criteria [24] | History of fever or temperature ≥ 37.5 °C or < 36.0 °C Plus Impaired consciousness (prostration or coma) and/or respiratory distress (deep breathing or increased work of breathing) Plus ≥ 1 of: CRT > 2 s; lower limb temperature gradient; weak pulse; HR > 180 (<12 months), >160 (12 months–5 years), >140 (>5 years) |
| ACCCM clinical practice parameters for hemodynamic support of pediatric & neonatal septic shock (2007 update) [11] | Hypothermia or hyperthermia Plus Clinical signs of inadequate tissue perfusion including any of the following: decreased or altered mental status; CRT > 2 s, diminished pulses, mottled cool extremities (cold shock); flash capillary refill, bounding peripheral pulses, wide pulse pressure (warm shock); urine output <1 ml/kg/h Hypotension not necessary for clinical diagnosis of septic shock, but its presence in a child with clinical suspicion of infection is confirmatory |
| Paediatric Advanced Life Support (PALS): 2010 American Heart Association guidelines for cardiopulmonary resuscitation [8] | No single sign confirms the diagnosis Typical signs of compensated shock include: tachycardia; cool and pale distal extremities; CRT > 2 s despite warm ambient temperature; weak peripheral pulses compared with central pulses; normal systolic blood pressure Decompensated shock characterised by signs & symptoms consistent with inadequate delivery of oxygen to tissues (pallor, peripheral cyanosis, tachypnoea, mottling of skin, decreased urine output, metabolic acidosis, depressed mental status); also weak or absent peripheral pulses, weak central pulses, hypotension (systolic BP < 70 mmHg 1–12 months; <70 mmHg + (2 × age in years) 1–10 yrs; <90 mmHg ≥ 10 years) |
| 2016 Surviving Sepsis 3 definitions [72] |
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be identified as an acute change in total SOFA (Sequential [Sepsis-related] Organ Failure Assessment) score ≥ 2 points consequent to the infection. The baseline SOFA score can be assumed to be zero in patients not known to have preexisting organ dysfunction. A SOFA score ≥ 2 reflects an overall mortality risk of approximately10%in a general hospital population with suspected infection. Even patients presenting with modest dysfunction can deteriorate further, emphasizing the seriousness of this condition and the need for prompt and appropriate intervention, if not already being instituted Septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality. Patients with septic shock can be identified with a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP(mean arterial pressure) ≥65 mmHg and having a serum lactate level > 2 mmol/L (18 mg/dL) despite adequate volume resuscitation. With these criteria, hospital mortality is in excess of 40% |
| European Paediatric Life Support Course (2006) [9] | Compensated circulatory failure: although the blood pressure is normal, poor skin perfusion (CRT > 2 s, mottled, cool peripheries, peripheral cyanosis), weak peripheral pulse, tachycardia: HR > 180 (3 months–2 yrs); >140 (2–10 yrs); >100 (>10 yrs), tachypnoea (RR > 40 <1 yr; >34 (1–2 yrs); >30 (2–5 yrs); >24 (5–12 yrs) and oliguria are observed Decompensated circulatory failure: hypotension (SBP < 70 mmHg (1–12 months); <70 + (2 × age in yrs) mmHg (1–10 yrs); <90 mmHg (>10 yrs); decreased mental status |
| WHO/ETAT [73], [74] | The presence of cold hands or feet with both capillary refill time > 3 s and weak and fast pulse |
Table 2.
Describing shock.
| Classification of shock | Description |
|---|---|
| Compensated shock or impaired circulation | The early phase of shock in which the body’s compensatory mechanisms (such as increased heart rate, vasoconstriction, increased respiratory rate) are able to maintain adequate perfusion to the brain and vital organs. Typically, the patient is normotensive in compensated shock [75] |
| Decompensated shock or severely impaired circulation | The late phase of shock in which the body’s compensatory mechanisms (such as increased heart rate, vasoconstriction, increased respiratory rate) are unable to maintain adequate perfusion to the brain and vital organs. Typically, the patient is hypotensive in decompensated shock [75] |
Our article reviews identification, common aetiology, pathophysiology and management of non-trauma paediatric shock with a view of highlighting pragmatic approaches for good prognosis and outcomes in African children in resources limited settings.
Aetiology and pathophysiology
Shock is a multi-aetiological syndrome, with various clinical categories based on the underlying pathophysiology [4]. Essentially, in shock, delivery and utilization of oxygen and nutrients at cellular and/or tissue levels is grossly compromised. These result from various underlying mechanisms.
Hypovolemic shock
Hypovolemic shock is the most common type of shock and involves a large deficiency of intravascular fluid volume [4]. Even though it is globally recognised as one of the commonest causes of mortality, the underlying causes are not homogenous across diverse geographical locales. Gastroenteritis is a leading infectious cause of hypovolaemia in children. It may be very aggressive and can result in massive losses in circulating volumes within a few hours [12]. Gastroenteritis is caused by a number of pathogens including, but not limited to Salmonella, Shigella, Campylobacter species (spp), Escherichia coli, and Vibrio cholerae as well as viruses such as rotaviruses, adenoviruses, and enteroviruses which may lead to fulminant, fluid loss and shock [15]. Non-infectious conditions such as trauma and burns can cause massive blood or fluid losses [16]. Additionally, hypovolaemia due to third spacing, though rare in children can present a diagnostic dilemma. It results from leaky capillary syndrome where patients appear oedematous yet have substantial intravascular fluid deficiency [17].
Distributive shock
Abnormal peripheral vasodilation results in distributive shock, which physiologically involves very slow and poor distribution of oxygen to the cells and tissues [18]. The pathophysiology is due to sudden sustained incongruous relaxation of peripheral vascular tone [18]. The resultant vasodilation expands venous blood pooling, resulting in circulatory fluid deficit without an actual fluid loss. This category of shock is common in anaphylaxis [19], but may also occur in sepsis.
Cardiogenic shock
Cardiogenic shock results mainly from pathological insufficiency in cardiac contractility. This leads to a reduced stroke volume [20] and cardiac output (CO) [4]. Consequently, there is a decrease in both delivery of oxygen to the body cells and tissues and removal of cellular metabolic wastes.
Obstructive shock
In other instances, there is a localized cause of a diminished or absent systemic or pulmonary blood flow. This is obstructive shock [21]. The impairment to blood flow may result in CO insufficiency and hence, shock. Common conditions are congenital cardiac defects including coarctation of the aorta, interrupted aortic arch or severe aortic valvular stenosis [21]. Conversely, acquired blockade may be caused by acute cardiac tamponade or pulmonary conditions like tension pneumothorax or pulmonary embolism.
Management of paediatric shock
Management in a well resourced versus resource-limited settings: more studies may be needed
Management of a condition with such diverse underlying pathophysiology is often challenging. In developed countries, there has been advocacy to adopt one common approach for delivering care to critically sick children in a standardised manner targeting key aspects of deregulated physiology, regardless of the underlying aetiology [22]. For instance, in the management of paediatric shock, the application of PALS [8], EPLS [9] and other similar training packages to ensure early accurate recognition and rapid resuscitation has, in settings with intensive care facilities, been coupled with a marked improvement in prognosis [10].
The situation is distinctly dissimilar in poorly resourced hospitals in sub-Saharan Africa (SSA), where essential point-of-care tests, intensive care monitoring and high-quality clinical care are frequently lacking. Most in-patient deaths in African hospitals occur within 24 h of admission [23], and hypotensive shock is a leading cause. Adoption and implementation of simple treatment algorithms for the common approaches for treatment and prevention of shock could be of great benefit. The FEAST study used an all-inclusive or ‘soft’ definition of shock (Table 1) and evaluated the effectiveness and safety of bolus fluids (20 ml/kg in 1 h) versus conservative management (maintenance fluids only) in non-hypovolaemic ‘septic” shock, irrespective of aetiology [24]. This study found that in African children, rapid infusion of fluid boluses irrespective of fluid type compared to maintenance fluids alone (3141 participants) increased the mortality at 48hours (RR 1.45, CI 1.13–1.86), P = .003 and at 28 days follow up (RR 1.39, CI 1.11–1.74), P = .004. In addition, further sub-analyses of syndromes in the recruited study participants, including malaria, coma, anaemia, and impaired metabolism all gave the same results [24]. In a nutshell, fluid boluses in febrile children with impaired perfusion are ineffective and carry a higher risk compared to maintenance fluids alone [24]. These findings should be considered when developing protocols for the management of shock in different patient phenotypes and settings. Surprisingly, over 5 years since the main publication of this study [24], little enthusiasm to adopt the recommendations in the treatment guidelines remains a hindrance to advancing management of paediatric shock in Africa. Instead, these findings have continued to generate intense debate. In some debates the need for re-evaluation of management of shock in well-resourced settings have been proposed [13], [14]. In Africa, evaluation of management of shock in patients that were excluded in the FEAST study is needed. These categories include gastroenteritis, neonates and severe acute malnutrition (SAM). Some pilot studies are underway. The FEAST study recommendations have not been formally adopted by WHO as yet, though some caution is expressed for giving fluids in impaired circulation without the WHO definition of shock [25]. This has meant that many national guidelines on shock management in Sub Saharan Africa remain either unchanged, or in some settings clinicians have adopted recommendations from published data. This scenario indicates that more robust guidelines are urgently needed.
Childhood sepsis: does this require a syndromic treatment approach?
In all infectious emergencies in children a multifaceted pathophysiology underlies the common clinical picture [26], [27]. The pathophysiology may be due to a wide range of causes such as viral, parasitic, fungal, and non-infectious disorders [28], [29], [30]; yet the clinical presentation is similar irrespective of the underlying trigger. This has generated a school of thought in which a generic approach to care should be considered [31]. However, the challenge to this is that these etiologic agents may require specific guidelines over and above a common approach to management. Where the causative agents are few and have similar pathophysiology, it is plausible to have a common treatment approach.
Evaluation of the management of shock in severe malaria and severe sepsis syndrome
There is a better understanding of the clinical spectrum, pathophysiology and outcomes of severe falciparum malaria in African children since 1995 [32]. These include a composite range of physiological derangements, multi-organ dysfunction and biochemical abnormalities. These features are also characteristic of severe sepsis syndromes [33]. Bacterial co-infections have also been demonstrated to occur in in children with severe malaria [34]. The clinical dilemma in practice in resource limited settings is that simple bedside and laboratory assessments fail to distinguish between severe malaria and microbiologically proven sepsis [35].
Respiratory distress, a common feature of severe malaria in children, and originally thought to be caused by congestive cardiac failure, is now recognised as an indicator of metabolic acidosis, and is one of the predictors of poor outcome [36]. A series of studies from Kenya, which pre-date the FEAST study and were each of small sample sizes, appeared to established hypovolaemia as a feature of severe malaria in children [37]. Low central venous pressure, severe tachycardia and delayed capillary refill time (CRT), all clinical signs of impaired perfusion, were commonly observed at the time of admission [37]. In a trial from Ghana, delayed CRT, a simple test of impaired perfusion, was an independent predictor of mortality in severe malaria [38]. Studies from Gabon have documented moderate dehydration in children with severe malaria, and a clear correlation between dehydration and depth of coma [39]. Currently, the vast majority of children with severe malaria in SSA hospitals receive no specific fluid management apart from blood transfusion, which is recommended for the management of severe anaemia [40]. Intravenous 10% glucose 80 ml/kg/day is given as maintenance fluid requirements in some centres [41]. With the FEAST study indicating that rapid and early restoration of intravascular volume results in poor outcomes [24], there is urgent need for direction in the guidelines in SSA.
The WHO currently recommends rapid intravenous administration of 20 ml/kg Ringers Lactate solution or 0.9% saline only for the treatment of advanced hypotensive shock (defined as cold hands or feet and weak rapid pulse and capillary refill time >3 s) in children [42], [43]. Evaluation in 3837 Brazilian children attending the emergency department, using these criteria identified shock in only 4 children (0.1%), all of whom died [44]. The guidelines state that boluses of fluid may be repeated twice, to a maximum total volume of 60 ml/kg. Extreme caution is advised and recommends that fluid restoration is withheld or stopped in the presence of an enlarging liver or signs indicating raised jugular venous pressure. Hepatomegaly is common in malaria endemic areas and none of these signs is very sensitive. Although this approach is broadly recommended for all conditions except severe malnutrition and anaphylactic shock, recent WHO guidelines for the management of severe malaria, gastroenteritis and severe malnutrition differ with respect to both clinical assessment and the type and volume of fluid recommended for resuscitation [42]. Development of an evidence-based approach to fluid management of the critically sick child in resource-poor settings is urgently needed.
Encephalopathy and meningitis
Doubt had been raised over the safety of volume expansion in children with severe meningitis or encephalopathy complicated by septic shock. In these encephalopathies, cerebral autoregulation fails and cerebral perfusion is dependent on adequate cardiac output, but some studies have shown that modest fluid restriction may be detrimental to brain perfusion and outcome in severe meningitis [45]. International guidelines now advocate correction of fluid deficits in children with meningitis [46], [47].
Severe malarial anaemia
Among children with severe malarial anaemia [48], highest mortality is observed in those with concomitant respiratory distress (symptomatic SMA) [49]. Case fatality rates of children with symptomatic SMA range from 20–30%, rising to >35% when associated with impaired consciousness [49]. The majority of deaths occur in children whilst awaiting transfusion [49]. There is now evidence to suggest that the haemodynamic characteristics of symptomatic SMA are more characteristic of hypovolaemia than of cardiac failure [50]. As a result, it has been reasoned that moderate volume expansion with an intravenous bolus of fluid might restore circulation, and thus stabilize the condition of children awaiting transfusion for symptomatic SMA. Preliminary evidence provided by a small randomised controlled trial suggested that pre-transfusion management with an intravenous bolus of 0.9% saline 20 ml/kg was safe, and improved clinical and biochemical markers of hypovolaemia and improved outcome, compared to a control group [51]. These findings need to be read with caution as they pre-date the very large FEAST trial in which bolus fluid was detrimental in SMA.
Severe malnutrition and HIV
There have been few detailed studies examining the pathophysiology and outcome of severe bacterial or malaria infections in HIV-infected children in Africa [52], [53], [54]. Current WHO guidelines for the treatment of shock in children with severe malnutrition (severe wasting or kwashiorkor) differ markedly from recommendations for children with normal nutritional status, with extreme caution being expressed in relation to the use of isotonic volume expansion [12].
Neonates
Shock is an independent predictor of neonatal mortality and carries an increased risk of neurological impairment in those who survive [55]. Hypovolaemia is rarely the primary cause of shock in neonates, however, myocardial dysfunction is common in extremely preterm neonates and term neonates with perinatal asphyxia. The most common cause of shock in neonates is sepsis, nevertheless even in developed countries the management of neonatal septic shock is challenging. There are many differences in the physiological response of a neonate compared to a child [56]. Firstly, the structure and function of cardiomyocytes is different with impaired ability to increase stroke volume and contractility. The presence of a patent ductus arteriousus (PDA) or persistent pulmonary hypertension (PPHN) can also complicate management. Furthermore the presentation of shock in neonates is much more variable than in older children and can even present with a normal blood pressure.
Various treatment guidelines for neonates have been written [11] but data on the survival and neurological outcomes following different management approaches of neonatal shock is limited. The development and testing of, and advocacy for neonatal shock guidelines are needed to aid assessment, diagnosis and management. Future research should focus on these.
Possible risks of volume expansion in shock management
To support a generic approach to volume expansion in sick children, the benefits should substantially outweigh risks. The major risks of volume expansion are volume overload, manifest as pulmonary oedema, brain swelling or raised intracranial pressure, and acute allergic reactions to infusion fluids. Most hospitals in resource-poor settings lack the clinical personnel and basic equipment that are essential for meticulous patient monitoring, and this represents a major barrier to the widespread endorsement of volume expansion. Data from Kenya suggest, however, that pulmonary or cerebral oedema rarely complicate volume expansion in children with severe malaria, while no adverse reactions were observed in 135 children who received HAS [37], [51], [57], [58]. Globally, serious adverse events and allergic reactions to HAS are extremely rare [59].
Whilst rapid and early restoration of volume depletion using fluid boluses of 20–40 ml/kg may be regarded as safe in shocked, previously healthy, well-nourished children with ready access to intensive care facilities, this may not be true of children with poor nutritional status and/or anaemia, who are usually managed on general paediatric hospital wards in SSA, and who have no access to intensive care. In such settings slow volume restoration may be more relevant. Particular concern has been raised in relation to children with septic shock complicating encephalopathy or meningitis, severely anaemic children, and those with severe malnutrition and HIV.
Volume expansion with Human albumin solution – risks and benefits
An early meta-analysis suggested that HAS may cause harm when administered predominantly to adults with a wide range of underlying conditions [60]. This conclusion has not been endorsed by further meta-analyses [61]. Several resuscitation fluids are available for the treatment of severe dehydration or shock in children. Simple electrolyte solutions are of proven benefit in most situations where excess water and electrolytes depletion has resulted from gastroenteritis or burns. In conditions where hypotensive shock results from a sepsis-like syndrome, the choices include isotonic crystalloids or colloids [62]. Isotonic crystalloids, such as Ringers Lactate and 0.9% (normal) saline, equilibrate freely across the intra- and extra-vascular compartments of the extracellular space, so are favoured for restoration of circulating volume. A meta-analysis of the combined data for isotonic and colloid fluids has also been undertaken [58]. Up to 40 ml/kg of 0.9% saline or HAS was found to be safe and corrected haemodynamic indices of hypovolaemia [37]. In some parts of SSA, 19–50% of all paediatric admissions receive a blood transfusion[49], [63]. In many hospitals, transfusion services are poorly developed and cannot provide adequate supplies of blood for emergency transfusions [64], [65]. Although blood could be used for volume expansion, WHO guidelines suggest that in well nourished children with Hb > 5 g/dl the benefit is questionable [66], [67], [68]. Any short-term benefit needs to be balanced by the risk of exposure to unsafe blood. In areas where the seroprevalence of HIV, hepatitis B and C and syphilis is high, 57% of donated blood may be unscreened for some of these infections [69]. Blood transfusions remain a high risk for preventable HIV infection in SSA [70]. The cost of transfusion is substantial, estimated at between US$30 and US$50 in most localities across Africa, a cost that is often recouped from families [71].
Effective treatment protocols for paediatric shock in resources limited settings
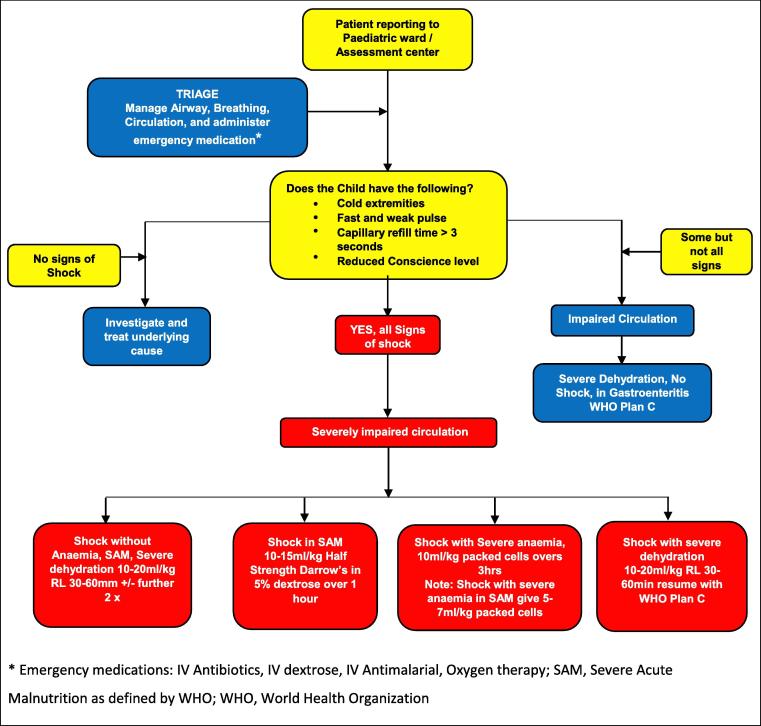
Given the challenges associated with definitions and treatment protocols for shock in children, it is important to guide all health workers, but especially those at the district level health facilities. Updated treatment guidelines based on current evidence are urgently needed in SSA. Prompt management for underlying conditions and comorbidity such as malaria, bacteraemia, severe anaemia, meningitis, pneumonia, convulsions, hypoglycaemia and others using national guidelines are important. In addition, supportive care such as oxygen administration is recommended. Fig. 1 provides a practical example of how to incorporate different resuscitation strategies depending on presentation. Appendix A (data supplement) provides two further algorithms, one from Malawi and one from Myanmar, that addresses different resuscitation strategies depending on presentation.
Fig. 1.
Assessment and management of Impaired Circulation in Children.
Conclusions
In our review, despite many definitions of shock and contentious debate surrounding whether or not to use bolus fluids, there are key undisputable aspects that need strengthening in resources limited settings. Training of health workers in recognition and prioritization of treatment of patients with shock remains the most essential initial step. Early diagnosis and appropriate treatment of underlying cause are essential. Finally, the objective knowledge translation of the currently available data and systematic reviews into guidelines should be considered.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
We would like to acknowledge the paediatric patients of Mbale Regional Referral Hospital and Busitema University where the lead author works for contributing to what is known about shock in African children
Author contribution
P-OO conceived the idea, drafted the manuscript, proofread and approved the final version of the manuscript. JN, GO, KB, JSB and CT edited, proofread and approved the final version of the manuscript.
Footnotes
Peer review under responsibility of African Federation for Emergency Medicine.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.afjem.2017.10.002.
Appendix A. Supplementary data
References
- 1.Mbevi G., Ayieko P., Irimu G. Prevalence, aetiology, treatment and outcomes of shock in children admitted to Kenyan hospitals. BMC Med. 2016;14(1):184. doi: 10.1186/s12916-016-0728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silveira Rde C., Giacomini C., Procianoy R.S. Neonatal sepsis and septic shock: concepts update and review. Rev Bras Ter Intensiva. 2010;22(3):280–290. [PubMed] [Google Scholar]
- 3.Kutko M.C., Calarco M.P., Flaherty M.B. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4(3):333–337. doi: 10.1097/01.PCC.0000074266.10576.9B. [DOI] [PubMed] [Google Scholar]
- 4.Thomovsky E., Johnson P.A. Shock pathophysiology. Compend Contin Educ Vet. 2013;35(8):E2. quiz E2. [PubMed] [Google Scholar]
- 5.Bolton C.F., Young G.B., Zochodne D.W. The neurological complications of sepsis. Ann Neurol. 1993;33(1):94–100. doi: 10.1002/ana.410330115. [DOI] [PubMed] [Google Scholar]
- 6.León A.L., Hoyos N.A., Barrera L.I. Clinical course of sepsis, severe sepsis, and septic shock in a cohort of infected patients from ten Colombian hospitals. BMC Infect Dis. 2013;13:345. doi: 10.1186/1471-2334-13-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization; Geneva: 2005. Emergency triage assessment and treatment (ETAT) 2 v. [Google Scholar]
- 8.Kleinman M.E., Chameides L., Schexnayder S.M. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S876–S908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 9.RCUK . 2nd ed. Resuscitation Council (UK); London: 2006. European paediatric life support course. [Google Scholar]
- 10.Han Y.Y., Carcillo J.A., Dragotta M.A. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112(4):793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 11.Brierley J., Carcillo J.A., Choong K. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Pocket book of hospital care for children. 2nd ed. Guidelines for the management of common illnesses with limited resources. Geneva; 2013. [PubMed]
- 13.Duke T., Mason E. WHO guidelines on fluid resuscitation in children with shock. Lancet. 2014;383(9915):411–412. doi: 10.1016/S0140-6736(14)60053-2. [DOI] [PubMed] [Google Scholar]
- 14.Kiguli S., Akech S.O., Mtove G. WHO guidelines on fluid resuscitation in children: missing the FEAST data. BMJ. 2014;348:f7003. doi: 10.1136/bmj.f7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roshdy A. Infection management in patients with sepsis and septic shock in resource-limited settings: focus on appropriate antimicrobial. Intensive Care Med. 2016;42(12):2115–2116. doi: 10.1007/s00134-016-4473-6. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez G., Reines H.D., Wulf-Gutierrez M.E. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouillette J.N. Third spacing is bad for health care. J Fla Med Assoc. 1995;82(3):211–212. [PubMed] [Google Scholar]
- 18.Chawla L.S., Busse L.W., Brasha-Mitchell E. The use of angiotensin II in distributive shock. Crit Care. 2016;20(1):137. doi: 10.1186/s13054-016-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel J., Hofbeck M., Spiller G. Safety and efficacy of terlipressin in pediatric distributive shock: a retrospective analysis in 20 children. Paediatr Drugs. 2017;19(1):35–41. doi: 10.1007/s40272-016-0199-8. [DOI] [PubMed] [Google Scholar]
- 20.Parm Ü., Metsvaht T., Sepp E. Mucosal surveillance cultures in predicting Gram-negative late-onset sepsis in neonatal intensive care units. J Hosp Infect. 2011;78(4):327–332. doi: 10.1016/j.jhin.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Morita S., Sakurai K., Watanabe Y. Obstructive shock caused by a giant hiatus hernia. Intern Med. 2014;53(23):2755. doi: 10.2169/internalmedicine.53.3280. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein B., Maitland K. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux E.M., Maitland K. Intravenous fluids–getting the balance right. N Engl J Med. 2005;353(9):941–944. doi: 10.1056/NEJMe058135. [DOI] [PubMed] [Google Scholar]
- 24.Maitland K., Kiguli S., Opoka R.O. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Pocket book of hospital care for children: Second edition. Guidelines for the management of common childhood illnesses; 2013 [cited 2017: 29.09.2017]; Available from: http://www.who.int/maternal_child_adolescent/documents/child_hospital_care/en/. [PubMed]
- 26.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40(12):845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R., Janeway C., Jr. Innate immunity. N Engl J Med. 2000;343(5):338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 28.Sáez-Llorens X., Vargas S., Guerra F. Application of new sepsis definitions to evaluate outcome of pediatric patients with severe systemic infections. Pediatr Infect Dis J. 1995;14(7):557–561. doi: 10.1097/00006454-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 30.Saez-Llorens X., McCracken G.H., Jr. Sepsis syndrome and septic shock in pediatrics: current concepts of terminology, pathophysiology, and management. J Pediatr. 1993;123(4):497–508. doi: 10.1016/s0022-3476(05)80942-4. [DOI] [PubMed] [Google Scholar]
- 31.Brilli R.J., Goldstein B. Pediatric sepsis definitions: past, present, and future. Pediatr Crit Care Med. 2005;6(3 Suppl):S6–S8. doi: 10.1097/01.PCC.0000161585.48182.69. [DOI] [PubMed] [Google Scholar]
- 32.Marsh K1., Forster D., Waruiru C. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332(21):1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 33.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 34.Olupot-Olupot P., Urban B.C., Jemutai J. Endotoxaemia is common in children with Plasmodium falciparum malaria. BMC Infect Dis. 2013;13:117. doi: 10.1186/1471-2334-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkley J., Mwarumba S., Bramham K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93(3):283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 36.English M., Waruiru C., Amukoye E. Deep breathing in children with severe malaria: indicator of metabolic acidosis and poor outcome. Am J Trop Med Hyg. 1996;55(5):521–524. doi: 10.4269/ajtmh.1996.55.521. [DOI] [PubMed] [Google Scholar]
- 37.Maitland K., Pamba A., Newton C.R. Response to volume resuscitation in children with severe malaria. Pediatr Crit Care Med. 2003;4(4):426–431. doi: 10.1097/01.PCC.0000090293.32810.4E. [DOI] [PubMed] [Google Scholar]
- 38.Evans J.A., May J., Ansong D. Capillary refill time as an independent prognostic indicator in severe and complicated malaria. J Pediatr. 2006;149(5):676–681. doi: 10.1016/j.jpeds.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 39.Planche T., Onanga M., Schwenk A. Assessment of volume depletion in children with malaria. PLoS Med. 2004;1(1):e18. doi: 10.1371/journal.pmed.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English M. Life-threatening severe malarial anaemia. Trans R Soc Trop Med Hyg. 2000;94(6):585–588. doi: 10.1016/s0035-9203(00)90197-4. [DOI] [PubMed] [Google Scholar]
- 41.Taylor T.E., Borgstein A., Molyneux M.E. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86(2):99–109. [PubMed] [Google Scholar]
- 42.World Health Organization; Geneva: 2000. Management of the child with a serious infection or severe malnutrition. [Google Scholar]
- 43.World Health Organization; Geneva, Switzerland: 2005. Hospital Care for Children: guidelines for the management of common illnesses with limited resources. [Google Scholar]
- 44.Tamburlini G., Di M., Maggi R.S. Evaluation of guidelines for emergency triage assessment and treatment in developing countries. Arch Dis Child. 1999;81(6):478–482. doi: 10.1136/adc.81.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell K.R., Sugarman L.I., Eskenazi A.E. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J Pediatr. 1990;117(4):515–522. doi: 10.1016/s0022-3476(05)80682-1. [DOI] [PubMed] [Google Scholar]
- 46.Carcillo J.A., Fields A.I. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30(6):1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 47.Mackway-Jones K., editor. Advanced paediatric life support: the practical approach. 3rd ed. BR MED J Publishing Group; London: 2004. [Google Scholar]
- 48.Wang H., Liddell C.A., Coates M.M. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):957–979. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lackritz E.M., Campbell C.C., Ruebush T.K., 2nd Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340(8818):524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 50.English M.C., Waruiru C., Marsh K. Transfusion for respiratory distress in life-threatening childhood malaria. Am J Trop Med Hyg. 1996;55(5):525–530. doi: 10.4269/ajtmh.1996.55.525. [DOI] [PubMed] [Google Scholar]
- 51.Maitland K., Pamba A., English M. Pre-transfusion management of children with severe malarial anaemia: a randomised controlled trial of intravascular volume expansion. Br J Haematol. 2005;128(3):393–400. doi: 10.1111/j.1365-2141.2004.05312.x. [DOI] [PubMed] [Google Scholar]
- 52.Newton C.R. Interaction between Plasmodium falciparum and human immunodeficiency virus type 1 on the central nervous system of African children. J Neurovirol. 2005;11(Suppl 3):45–51. doi: 10.1080/13550280500511881. [DOI] [PubMed] [Google Scholar]
- 53.Kublin J.G., Patnaik P., Jere C.S. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365(9455):233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 54.Whitworth J.A., Hewitt K.A. Effect of malaria on HIV-1 progression and transmission. Lancet. 2005;365(9455):196–197. doi: 10.1016/S0140-6736(05)17752-6. [DOI] [PubMed] [Google Scholar]
- 55.Adams-Chapman I., Stoll B.J. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19(3):290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 56.McKiernan C.A., Lieberman S.A. Circulatory shock in children: an overview. Pediatr Rev. 2005;26(12):451–460. doi: 10.1542/pir.26-12-451. [DOI] [PubMed] [Google Scholar]
- 57.Maitland K., Pamba A., English M. Randomized trial of volume expansion with albumin or saline in children with severe malaria: preliminary evidence of albumin benefit. Clin Infect Dis. 2005;40:538–545. doi: 10.1086/427505. [DOI] [PubMed] [Google Scholar]
- 58.Akech S., Gwer S., Idro R. Volume expansion with albumin compared to gelofusine in children with severe malaria: results of a controlled trial. PLoS Clin Trials. 2006;1(5):e21. doi: 10.1371/journal.pctr.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubois M.J., Vincent J.L. Use of albumin in the intensive care unit. Curr Opin Crit Care. 2002;8(4):299–301. doi: 10.1097/00075198-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Cochrane Injuries Group Albumin Reviewers Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317(7153):235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi P.T., Yip G., Quinonez L.G. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27(1):200–210. doi: 10.1097/00003246-199901000-00053. [DOI] [PubMed] [Google Scholar]
- 62.Falk J.L., Rackow E.C., Weil M.H. Colloid and crystalloid fluid resuscitation. Acute Care. 1984;10(2):59–94. [PubMed] [Google Scholar]
- 63.Snow R., Newton C.R.J.C., Weil M.H. Fogarty International Center, National Institutes of Health; Bethesda, Maryland: 2003. The public health burden of Plasmodium falciparum malaria in Africa: Deriving the numbers; pp. 1–75. Working Paper No. 11. [Google Scholar]
- 64.World Health Organization; Geneva: 2001. The prevention and management of severe anaemia in children in malaria-endemic regions of Africa. [Google Scholar]
- 65.World Health Organization/Blood Transfusion Service; Geneva: 2001. Clinical use of blood in medicine, obstetrics, paediatrics, surgery & anaesthesia, trauma & burns (the) pp. 1–337. [Google Scholar]
- 66.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- 67.Holzer B.R., Egger M., Teuscher T. Childhood anemia in Africa: to transfuse or not transfuse? Acta Trop. 1993;55(1–2):47–51. doi: 10.1016/0001-706x(93)90047-f. [DOI] [PubMed] [Google Scholar]
- 68.Obonyo C.O., Steyerberg E.W., Oloo A.J. Blood transfusions for severe malaria-related anemia in Africa: a decision analysis. Am J Trop Med Hyg. 1998;59(5):808–812. doi: 10.4269/ajtmh.1998.59.808. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization, Blood Transfusion Safety, Department of blood transfusion safety and clinical technology; Geneva: 2000. Global database of blood safety. Summary report 1998–1999. [Google Scholar]
- 70.Colebunders R., Greenberg A.E., Francis H. Acute HIV illness following blood transfusion in three African children. Aids. 1988;2(2):125–127. doi: 10.1097/00002030-198804000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Hensher M., Jefferys E. Financing blood transfusion services in sub-Saharan Africa: a role for user fees? Health Policy Plan. 2000;15(3):287–295. doi: 10.1093/heapol/15.3.287. [DOI] [PubMed] [Google Scholar]
- 72.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO . World Health Organization; Geneva: 2005. Emergency Triage Assessment and Treatment (ETAT). Manual for participants. [Google Scholar]
- 74.WHO . World Health Organization; Geneva: 2005. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resource. [PubMed] [Google Scholar]
- 75.The Free Dictionery. [cited 2017: 29.09.2017]; Available from: http://www.thefreedictionary.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.