Abstract
With increased numbers of older people a higher burden of neurological disorders worldwide is predicted. Stroke and other cerebrovascular diseases do not necessarily present with different phenotypes in Africa but their incidence is rising in tandem with the demographic change in the population. Age remains the strongest irreversible risk factor for stroke and cognitive impairment. Modifiable factors relating to vascular disease risk, diet, lifestyle, physical activity and psychosocial status play a key role in shaping the current spate of stroke related diseases in Africa. Hypertension is the strongest modifiable risk factor for stroke but is also likely associated with co-inheritance of genetic traits among Africans. Somewhat different from high-income countries, strokes attributed to cerebral small vessel disease (SVD) are higher >30% among sub-Saharan Africans. Raised blood pressure may explain most of the incidence of SVD-related strokes but there are likely other contributing factors including dyslipidaemia and diabetes in some sectors of Africa. However, atherosclerotic and cardioembolic diseases combined also appear to be common subtypes as causes of strokes. Significant proportions of cerebrovascular diseases are ascribed to various forms of infectious disease including complications of human immunodeficiency virus. Cerebral SVD leads to several clinical manifestations including gait disturbance, autonomic dysfunction and depression. Pathological processes are characterized by arteriolosclerosis, lacunar infarcts, perivascular spaces, microinfarcts and diffuse white matter changes, which can now all be detected on neuroimaging. Except for isolated cases of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy or CADASIL, hereditary arteriopathies have so far not been reported in Africa. Prevalence estimates of vascular dementia (2–3%), delayed dementia after stroke (10–20%) and vascular cognitive impairment (30–40%) do not appear to be vastly different from those in other parts of the world. However, given the current demographic transition in both urban and rural settings these figures will likely rise. Wider application of neuroimaging modalities and implementation of stroke care in Africa will enable better estimates of SVD and other subtypes of stroke. Stroke survivors with SVD type pathology are likely to have low mortality and therefore portend increased incidence of dementia.
Keywords: Africa, Alzheimer’s disease, cerebrovascular disease, small vessel disease, stroke, vascular cognitive impairment
Introduction
Worldwide neurological disorders are an important cause of disability and mortality. The global burden of neurological disorders has increased substantially over the past 25 years because of increases in the general population as well as in proportions of older people [36, 40]. Life expectancy at age 65 years has increased in almost all countries from 1970 to 2016. The increases occurred across successive decades suggesting implications for lower mortality and improved health care. Despite these positives, however, among all the common neurological disorders worldwide, cerebrovascular disease (CVD) and in particular stroke account for the largest proportions (47–67%) of total disability-adjusted life-years and deaths [54]. Low-and-middle income countries (LMICs) bear a high global burden of not only neurological but also cardiovascular disorders [89, 137], which until recently used to include stroke. With the recently revised ICD-11 classification [134], stroke is now finally considered a brain disease or a neurological disorder. While there are considerable geographical variations, the prevalence of stroke in a large number of sub-Saharan African countries is reported to be the highest with an estimated overall Africa rate of 981 per 100,000 [54, 106]. These accord with previously measured yearly stroke incidence rates age-standardised to the WHO world population of 109/100 000 in rural (Hai District) and 316/100,000 in urban (Dar-es-Salaam) Tanzania [149] and estimated rates of 258/100,000 in rural South Africa [83]. Current prevalence and incidence figures may actually be underestimates given there has been an incremental increase of stroke cases of about 10% every 4 years in the last decade [3]. Africa has also experienced a steady increase in stroke admissions to hospital and mortality over the past three decades. In a recent Ghanaian study, comprising over 12,000 stroke admissions with equal gender distribution, the rate of stroke admissions had increased from 5/1000 in 1983 to 14/1000 in 2010 corresponding to a 260% rise over the period. Stroke mortality rates increased from 3/1000 to 7/1000 deaths over the same period [132]. In rural Tanzania, both mild cognitive impairment (MCI) and dementia particularly vascular dementia (VaD) were associated with excess mortality relative to those without cognitive impairment [110].
Vascular risk factors (VRFs) impact on brain health to produce clinically silent disease or overt stroke, all of which lead to cognitive dysfunction,[9, 53] depression [141, 142] and frailty [63, 124]. It is estimated by 2025, three in four persons will be living with hypertension with increasing clustering of other risks including diabetes, obesity and metabolic syndrome [102]. These constitute a heightened but less recognized threat to brain health, mental capital and socioeconomic progress in Africa. Hypertensive small-vessel disease (SVD) is thought to be the main risk factor for these infarcts, which may be associated with subtle deficits in physical and cognitive function that often go unnoticed, particularly in older age. In recent years, SVD has taken precedence as a radiological concept [151] and refers to an intracranial disorder which involves pathological changes within and at the surfaces of brain microvessels including perforating arteries and arterioles, capillaries and venules. SVD comprises tissue injury in both the cortical and subcortical grey and white matter. However, SVD may often coexist with atherosclerosis involving large extracranial vessels and cardioembolic (CE) disease [78].
Apart from depressive illness, an important consequence of CVD in the survivors is cognitive impairment. In recent years, vascular cognitive impairment (VCI) has been implemented as an umbrella term to encompass all conditions and causes of CVD including hereditary forms that lead to early and severe forms of dementia syndromes [56]. Degrees of impairment with the VCI concept remains challenging to define in the clinic. Thus, developments within the guises of DSM V defined neurocognitive disorder, mild and severe forms of VCI have been proposed as minor and major neurocognitive disorders. The latter being consistent with VaD, which is widely regarded as the second most common cause of dementia. VaD may culminate from global or focal effects of vascular disease. It incorporates behavioural symptoms and locomotor abnormalities e.g. Parkinsonian-like gait disorder, dysarthria and autonomic dysfunction. Within the spectrum of CVD and VaD, the most common vascular contributor to dementia appears to be cerebral SVD [151]. As people worldwide survive longer [39, 127] including in Africa and stroke management improves, the incidence of SVD type of VaD is likely to rise.
In this review, we focus on stroke and cerebrovascular diseases in Africa. We also review current trends in vascular risk factors associated with stroke subtypes. The recognition of subtypes of strokes was an important step forward towards current pathological classification based on vascular aetiology. We further appraise the vascular causes of cognitive impairment and their contribution to neurodegenerative processes. The incidence of hypertension is increasing in Africa suggesting that the incidence of cognitive impairment associated with subcortical ischemic lesions or Binswanger’s disease involving subcortical structures and the WM resulting from changes in intracranial small vessels will rise.
Search Methods
We searched PubMed, Science Direct and Web of Science databases between 1970 and 2017 to identify reports of CVD and stroke published from Africa over the last 45 years. For this systematic review, we searched for studies on stroke prevalence, incidence, morbidity, mortality, TOAST, risk factors, cerebrovascular accident in all Africa. Both hospital and non-hospital cases as well as population-based studies were included. We searched for cognitive impairment, mild cognitive impairment, VCI, dementia and VaD. All of the countries in Africa were systematically used as search items in segments. Thus, each segment included countries in North, West, Central, East and Southern African countries. We also specifically looked at all abstracts from sub-Saharan countries. In total, we initially found 2,350 titles or abstracts. These were pruned to 523 and finally we selected 120 abstracts to pursue further. Only abstracts in English were included. Case or study details were not available for all and in the final analysis, 65 key papers were included. The INTERSTROKE, SIREN and Tanzanian Stroke Incidence Study (TSIP) or Hai District projects outputs were identified to be the mainstay of this systematic review.
Age, Gender and Ethnicity
In high-income countries (HICs), old age remains as one of the strongest risk factors for ischaemic strokes and other types of CVD. It involves arteriosclerotic changes in the cerebral vasculature and cardiovascular system and often both, that may begin in middle age considerably prior to the manifestation of an overt or clinically evident event. Early or subtle changes may not necessarily be recognised clinically but are evident radiologically as white matter (WM) changes or silent lesions, most often in the form of subcortical ischaemic changes. For example, in developed countries typically 20–22% of healthy elderly people will exhibit magnetic resonance imaging (MRI)-defined silent brain infarcts and up to 50% of these are detected in selected patient cohorts [148]. We do not have similar statistics for Africa.
As in HICs, increasing age is the strongest non-modifiable risk factor for stroke in Africa as demonstrated in different cohort and population studies including the TSIP [149], INTERSTROKE [96] and SIREN [109]. Background and ethnicity also contribute to differences in the age at which strokes occur [38]. For first ever strokes, black patients were younger by a decade (mean age 51) than white patients. While stroke severity was similar; more black compared to white patients exhibited cerebral haemorrhage (27% vs 15%). This was also evident in the SIREN case-control study, which recorded baseline odds ratios for age > 50 years was 5.5 vs 3.9 for haemorrhagic and ischemic strokes respectively, [109]. Population-attributable risk for ischemic stroke was 61% second to hypertension at 87%.
As above, lacunar strokes (28% vs 22%) and total anterior circulation infarcts (28% vs 22%) tend to be more common in black Africans. Interestingly, large vessel atherosclerosis and ischemic heart disease were very uncommon (1%) as a cause in black patients with stroke. This is consistent with the lack of carotid artery stenosis as a significant cause of stroke in an incident cohort in Tanzania [68] but may not be universal in Africa (see below). Hypertension (70% vs 68%) and diabetes (14 vs 15%) were as common in black as in white stroke patients, but mean cholesterol levels were lower (4.6 vs 5.3 mmol/L) and cigarette smoking less frequent in black patients (23 vs 54%) [109].
Stroke Subtypes in Africa
Compared to the HICs, the overall proportions of haemorrhagic strokes in sub-Saharan Africa (SSA) are phenomenally high [75]. Whereas this figure is ~12% in HICs, in SSA it ranged from 15% in Kenya [67], 41% in Ethiopia [47] and an incredible 50% recorded to have intracerebral haemorrhages with 16% sub-arachnoid haemorrhages [132] in hospital-based samples. Patients with ischemic stroke were significantly older compared to those with intracerebral haemorrhages. Among these 16% had sub-arachnoid haemorrhage [132]. In an earlier study from Ethiopia [156], haemorrhagic strokes were reported to be even higher at 57% of all patients and 59% among those who had a CT scan during the reported period from 2000 to 2001. Hypertension (>140/90 mmHg) and lack of adherence to medication were cited as the main risk factors for the haemorrhagic strokes, which also included subarachnoid haemorrhage. However, the proportions of haemorrhagic strokes in the community may actually be closer to that in developed countries at 17% indicated by a Tanzania study involving urban and rural populations [150].
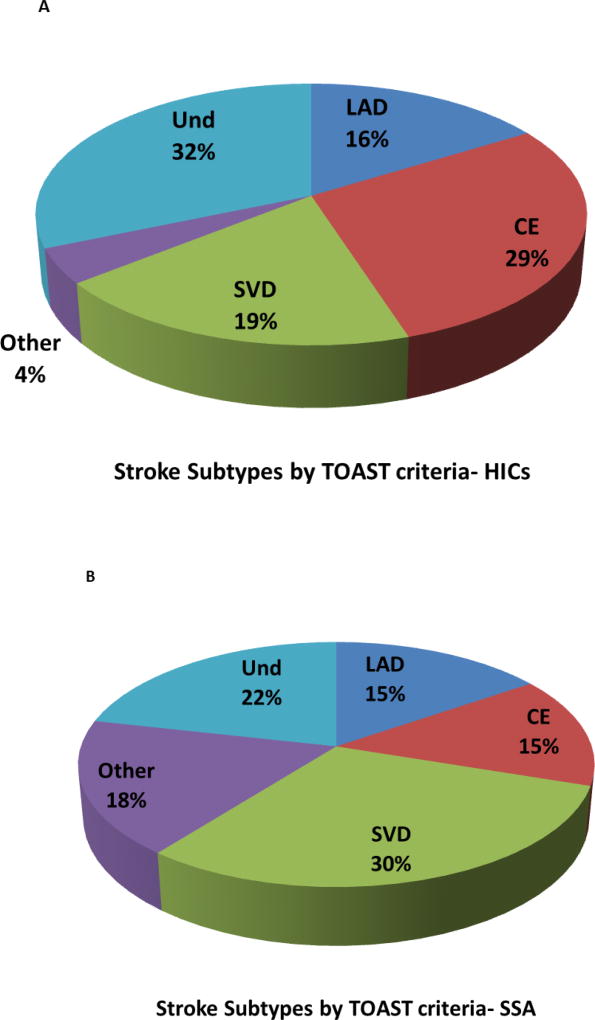
In recent years, the classification systems for stroke have enabled better understanding of the pathophysiology of CVD, particularly with a view to identifying precise substrates of motor and cognitive function outcomes and designing trials to reduce recurrent stroke injury. These systems have provided knowledge on the frequencies of ischaemic strokes in hospital and community based settings. While none of the subtype classification systems is flawless [15, 86], the Trial of Org 10172 in Acute Stroke Treatment (TOAST) has been recently widely utilised for evaluating the pathophysiology of clinical stroke. With regard to the developed countries, the TOAST classification [1] suggests atherosclerotic or large artery disease (LAD) and cerebral embolism (CE) are the main causes of infarctions associated with major arterial territories, which may be admixed in cortical and subcortical regions (Figure 1). Thromboembolic events are responsible for up to 50% of all ischaemic strokes whereas intracranial SVD causes 20–25% of the infarcts (Figure 1). Strokes of undetermined causes are identified most frequently but this category may contain several cases admixed with small artery occlusion [27, 84]. This analysis also suggested that SVD rather than LAD is more common in non-white populations [55, 144].
Figure 1. Stroke subtypes by the TOAST criteria in Africa.
1A and 1B, Pathophysiology of stroke subtypes according to TOAST [1]. Figures show proportions (mean percent) of strokes resulting from large artery disease (LAD), CE, cardioembolic (CE), lacunar infarction or small vessel disease (SVD), other (Other) and undermined (Und) causes. 1A, In HICs, CE is the most common cause of strokes with SVD a little less frequent. Patients with CE having small infarcts commonly develop VaD. The frequency of ICH in these cohorts was a mean of 15% (range 9.6–14%). Chart was constructed from 13 different studies involving 12,931 patients from both hospital and non-hospital or community based cohorts in Western Europe and USA [14, 22, 27, 55, 57, 64, 74, 84, 113, 116, 130, 133, 144]. 1B, In SSA, SVD is the most frequent cause of stroke. All types of primary insults causing stroke or cerebrovascular disease can lead to cognitive impairment. Chart shows proportions of stroke pathophysiology collated from 5 different studies involving 2,843 patients from both hospital and community based cohorts in Ghana, Kenya, Nigeria, Mozambique, South Africa, Sudan, Uganda [59, 67, 79, 96, 109] The risk factors associated with particularly SVD include hypertension, diabetes mellitus, hyperlipidaemia, hyperhomocysteinaemia, chronic kidney disease, infection and obstructive sleep aponea. Lifestyle factors such as smoking, obesity, alcohol abuse and regular meat consumption are other factors. Abbreviations: CE, cardioembolic; HIC, high-income countries; LVD, large vessel disease; SSA, sub-Saharan Africa; SVD, small vessel disease; TOAST, Trial of Org 10172 in Acute Stroke Treatment; Und, undetermined. Chart 1A was adapted from [70].
The meagre available data on TOAST classification in Africa over the last 20 years from 2000–2017 indicates that in the hospital based populations frequency of LAD is more or less similar to that in HICs at 15% [60, 79, 97, 109]. Whereas CE strokes were a less common cause of strokes in Africa, small vessel disease (SVD) at nearly 30% was much higher than in white populations of HICs (Figure 1). Strokes from other causes also tend to be higher in Africa. The higher SVD frequencies among Africans was also noted in the South London Ethnicity and Stroke Study which reported SVD at 29% per TOAST classification in black people of African origin [55]. It is plausible that certain genetic factors predispose to more SVD among Africans [2]. In this respect, we have recently reported an association between a risk locus on the apolipoprotein L1 (APOL1) gene, SNP rs73885319, and SVD ischaemic stroke in a cohort of indigenous West African stroke patients [5].
The lacunar subtype of ischaemic strokes was common followed by large-artery atherosclerosis and CE strokes. Eighty seven percent of haemorrhagic strokes were attributed to hypertension on the basis of the SMASH-U classification [109] providing further impetus to address this risk factor at the population level to mitigate the occurrence of this fatal stroke type among Africans. Indeed, although hypertension and current alcohol use were significantly associated with haemorrhagic stroke compared with ischaemic stroke, dyslipidaemia, diabetes, and cardiac disease were conversely more associated with ischaemic than haemorrhagic strokes. Small vessel alterations are very common and mostly involve arteriolosclerosis. They are associated with lacunar infarcts predominantly occurring in the WM, basal ganglia and thalamus. WM disease or subcortical leukoencephalopathy with incomplete infarction is a common pathological change associated with dementia [41]. Others features include borderzone (watershed) infarctions, laminar necrosis and cerebral amyloid angiopathy (CAA).
While it is possible that CAA is contributory as a cause of haemorrhagic strokes mainly cortical in many Africans [109], there appear no established reports of familial CAA. There are now more than 10 different hereditary CAAs caused by mutations in different genes [123, 155] but none have been yet described in Africa. CAA most often occurs in Alzheimer’s disease [99] but it can be found in CVD in the absence of Alzheimer pathology [35]. This association may be attributable to the apolipoprotein E (APOE) ε4 allele, as Africans tend to have a high frequency of the allele.
The undetermined and other causes of stroke reported in Africa may include whole spectrum of vasculopathies and angiopathies, which either lead to stenosis or alter the haemodynamics of flow (Table 1). Many of them related to prevalent infectious diseases and several reported in younger and paediatric patients [60, 100]. However, these include cerebral amyloid angiopathy, aneurysms, fibromuscular dysplasia, arterial dissections and collagen vascular disease [59]. Furthermore, CVD involving haematological and metabolic disorders have also been reported that include sickle cell anaemia, systemic lupus erythematosus, prothrombotic states, antiphospholipid syndrome, hyper-homocysteinemia and mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes have also been reported. A recently described large vessel vasculopathy involving the aorta and femoral and carotid arteries, resulting in either multiple aneurysm formation or occlusive disease was reported in younger subjects. An infective agent was not identified but aetiologically these lesions might be the result of a leucocytoclastic vasculitis of the vasa vasorum or periadventitial vessels [34]. Hospital-based retrospective studies have reported strokes due to direct cardiac or systemic large vessel abnormalities include mitral stenosis, cardiac-arrhythmias cardio-myopathy, left ventricular hypertrophy, and infective endocarditis, and atrial septal aneurysm, carotid plaques with or without stenosis.
Table 1.
Common and rare causes of stroke pathophysiology in Africa
| Primary or Secondary Vascular Disorder(s)* |
Common conditions | Predominant Tissue changes |
Frequencies in Africa (rare/low+, medium ++, high+++) |
|---|---|---|---|
| Atherosclerotic disease | Cardiac and carotid atherosclerosis | Cortical and territorial infarcts; WML | ++ |
| Infarcts, laminar necrosis, rarefaction | ++ | ||
| Embolic disease | Cardioembolism | Large and small infarcts | +++ |
| Arteriolosclerosis | Cerebral small vessel disease | Cortical infarcts, lacunar infarcts/lacunes, microinfarcts, WML | +++ |
| Hypertensive vasculopathy | +++ | ||
| Non-atherosclerotic non-inflammatory vasculopathies | Arterial dissections (carotid, vertebral and intracranial), fibromuscular dysplasia, dolichoectatic basilar artery, large artery kinking and coiling, radiation induced angiopathy, moyamoya disease | No pattern of brain infarctions: haemodynamic, thromboembolic, or due to occlusion of a perforating artery. Subarachnoid haemorrhage; lacunar infarcts, PVS | + |
| Aneurysms- sacular, berry, fusifom, cerebral | Haemorrhagic infarcts, herniation | ++ | |
| Vascular malformations: cavernous hemiangioma, arteriovenous, capillary | Rarefaction, WML | ||
| Cerebral venous thrombosis | Subcortical infarcts(thalamus), lobar haemorrhages | ||
| Amyloid angiopathies | Hereditary CAAs (Amyloid β, prion protein, cystatin C, transthyretin, gelsolin) | Cortical microinfarcts, lacunar infarcts, WML | + |
| Monogenic stroke disorders | CADASIL, CARASIL, retinal vasculopathy with cerebral leukodystrophies (RVCLs), Moyamoya disease, Hereditary angiopathy, nephropathy, aneurysm and muscle cramps(HANAC), COL4 disorders | Lacunar infarcts/lacunes, microinfarcts, WML | + |
| Monogenic disorders involving stroke | Fabry disease, familial hemiplegic migraine, hereditary haemorrhagic telangiectasia, Vascular Ehlers-Danlos syndrome, Marfan syndrome, Psuedoxanthoma elasticum, Arterial tortuosity syndrome, Loeys-Dietz syndrome, polycystic kidney disease; Neurofibromatosis type 1 (von Ricklinghausen disease), Carney syndrome (Faciallentiginosis and myxoma) | Cortical and subcortical infarcts, haemorraghic infarcts | + |
| Metabolic disorders | Mitochondrial disorders (MELAS, MERRF, Leigh’s disease, MIRAS), Fibromuscular dysplasia, Menkes disease, Homocystinuria, Tangier’s disease | Cortical and subcortical stroke-like lesions, microcystic cavitation, cortical petechial haemorrahges, gliosis, WML | ++ |
| Haematological disorders | Paraproteinaemia, coagulopathies (antiphospholipid antibodies, SLE, nephrotic syndrome, Sneddon syndrome, deficiencies in clotting cascade factors e.g. protein S, C, Z, antithrombin III, plasminogen) | Cortical and subcortical infarcts, ICH and subarachnoid haemorrhages | ++ |
| Vasospastic disorders | Subarachnoid haemorrahge, Migraine related strokes, paroxysmal hypertension, drug induced vasconstriction | Cortical and subcortical small infarcts | + |
Data summarised and updated from several source references. Africa also has proportionally large numbers of young strokes and cerebrovascular disorders [60, 100]. Countries reporting were: included: Benin, Botswana, Democratic Republic of Congo, Egypt, Ethiopia, Ghana, Kenya, Malawi, Mozambique, Nigeria, Senegal, Sudan, Tanzania, Tunisia, Uganda, South Africa, Zambia, Zimbabwe. Several disorders may also occur with other co-morbidities such as coronary artery disease, congestive heart failure, hypertension, diabetes, hyperlipidaemia, hypercoagulability, renal disease, atrial fibrillation and valvular heart disease.
Other miscellaneous causes of stroke including mechanical, invention induced or rare genetic syndromes such as trauma, iatrogenic, decompression sickness, air or fat embolism, transplantation and Werner’s syndrome can lead to cognitive impairment.
Abbreviations: CAA, cerebral amyloid angiopathy; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CARASIL; cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy; ICH, intracerebral haemorrhage; MCA, middle cerebral artery; MELAS, Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis and Stroke-like Episodes; MERRF, Myoclonic epilepsy with ragged red fibres; MIRAS; PCA, posterior cerebral artery; PVS, perivascular spaces; SLE, systemic lupus erythematosus; WML, white matter lesion.
Among the secondary causes of stroke or cerebral ischaemia, many sectors of Africa are afflicted with various vasculitides associated with infections. Both primary CNS vasculitis and systemic vasculitis with CNS manifestations occur but their frequencies are not known. Primary angiitis of the CNS or cerebral vasculitis per se appear to be rare. However, the most important rapidly emerging cause of ischaemic strokes is related to HIV infection [23, 24]. Several mechanisms may be involved including opportunistic infections, vasculopathy, CE and coagulopathy. While the occurrence of stroke and HIV infection might often be co-incidental, HIV-associated vasculopathy describes various cerebrovascular changes. These include stenosis or aneurysms, vasculitis, and accelerated atherosclerosis caused directly or indirectly by HIV infection. A range of vasculitides with CNS involvement particularly in relation to HIV has been reported [25, 34, 61]. These non-specific vasculitides do not fit into any of the characteristic patterns of systemic vasculitis. As part of the immunocompromise caused by HIV, a granulomatous inflammation may involve the leptomeninges, small arteries and veins as a primary angiitis of the CNS associated with high mortality. Cerebral vasculitis could also occur in later stages of the Schistosomiasis mansoni infection in endemic areas that causes severe vascular damage in cerebral vessels to produce stroke-like episodes [32].
Systemic vasculitides with CNS manifestations occur with almost the same frequency as in developed countries [49]. Systemic large vessel vasculitides include Takayasu arteritis (age <50 years) and giant cell arteritis (GCA) or cranial arteritis (age >50 years). In GCA, the involvement of CNS arteries is very rare (<2%). In one retrospective study, 20% of the patients identified with Takayasu disease had a primarily cerebrovascular presentation [61]. Neuroimaging revealed increased velocities in the anterior circulation with turbulence and multiple areas of hypoperfusion in the 7 cases investigated. Other vasculitides included Bechet’s disease with 13% of the patients having CNS involvement [4]. Medium-sized vessels are affected in classical polyarteritis nodosa. In classical polyarteritis nodosa, CNS involvement with headaches and encephalopathy is known in up to 20%. Small vessel vasculitides are separated into immune complex-mediated e.g. cryoglobulinaemic, immunoglobulin (Ig)A-associated, hypocomplementaemic anti-C1q and ANCA-associated variants including microscopic polyangiitis, granulomatosis with polyangiitis Wegener and eosinophilic granulomatosis with polyangiitis Churg–Strauss. In these small vessel vasculitides, CNS involvement is rare recorded to be ~10% in granulomatosis with polyangiitis Wegener and ~15% in eosinophilic granulomatosis with polyangiitis Churg–Strauss. Hypersensitivity vasculitis resulting in several patterns of vasculitis and angiocentric immunoproliferative vasculitis are also recognised in Africa [34].
Cerebral small vessel diseases in Africa
Several familial stroke disorders also appear to cause cognitive impairment or dementia. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common form of hereditary SVDs [33]. CADASIL is caused by over 250 distinct mutations in the NOTCH3 gene. Recent extensive exome analysis suggested that the frequency of distinctive epidermal growth factor region cysteine altering NOTCH3 mutations appear to be 100-fold greater than simply based on CADASIL prevalence [126]. The frequencies of NOTCH3 mutations are not known among North or sub-Saharan Africans but these frequencies among African Americans are estimated to be 0.7/1000 compared to 1.6/1000 in the European population and a phenomenal 11.4/1000 in the South Asians. However, genotyping confirmed mutations in NOTH3 or appropriate morphological evidence inn skin biopsies have been reported in peoples of Tunisia, Sudan, Tanzania and South Africa [28, 122]. In most of these cases, the relevant clinical and laboratory features of CADASIL are indicated by the characteristic radiological WM disease and family history, ischaemic events, migraine or cognitive impairment. All of the skin biopsies were positive, and the onset of migraine in our patients was considerably earlier. In two of the South African cases, a new finding was the normality of visual, somatosensory and auditory evoked potentials. Other familial forms of cerebral arteriopathies such as cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy or CARASIL [58] or the cerebroretinal vasculopathies [155] have not been reported. In the latter, retinal microvessels undergo severe distortions and become tortuous predictive of the SVD type of pathology with multilaminated vascular basement membranes in the brain [65]. There is also not doubt that some rarer and less characterised hereditary SVDs including the recently characterised collagen IV (COL4) disorders [136, 147] also exist in Africa.
Vascular and Other Risk Factors for Stroke
Key vascular disease risk factors for stroke include hypertension, diabetes, dyslipidaemia and cardiac disease (Table 2). There is a high burden of these cardiometabolic disorders in SSA that may also be modified by different genetic traits [2]. Lifestyle factors including lack of a healthy diet e.g. regular meat but low consumption of leafy vegetables, physical inactivity as well as psychosocial factors e.g. exposure to stressful situations are listed has high risk in both men and women as well as for both stroke types. Both urban and rural Africa are also not spared from emerging risk factors of air pollution (indoor household air pollution from solid fuels and outdoor ambient particulate matter pollution) and stress, which likely contribute to the current trends in stroke incidence [48].
Table 2.
Unmodifiable and Modifiable Risk Factors for All Strokes in Africa
| Factor | Parameter definition |
INTERSTROKE† OR and PAR |
SIREN† OR and PAR |
|---|---|---|---|
| Age | >50 years | - | 4.5 |
| Education | Some vs none | - | 1.4 |
| Family history of CVD | Self- reported CVD or stroke | - | 1.3 |
| Hypertension | >140/90 mm Hg | 4.0 | 19.4 |
| Diabetes | Glucose >7 mmol/L; HbA1c 6.5% | 1.5 | 2.6 |
| Dyslipidemia | Total cholesterol >5.2 mmol/L | 2.5 | 1.9 |
| Smoking (tobacco) | Current or former smoker | 1.6 | 4.4 |
| Cardiac disease†† | Any cardiac problem‡ | 3.6 | 1.7 |
| Waist-to-Hip ratio | Measured in cm | 1.6 | 1.5 |
| Low Physical Activity | Moderate activity, >4hr per week | 0.95 | 2.1¶ |
| Psychosocial Factors | Stress, life events, depression | N/A | 1.9 |
| Alcohol intake | >14–21 drinks per week | 5.9 | - |
| Diet | Healthy diet | 0.78‖ | - |
| Meat intake | Daily or weekly | - | 1.6 |
| Salt intake | Added salt | - | 2.1 |
| Sugar intake | Daily or weekly | - | 1.2 |
| Low Vegetable intake | Not daily or weekly | - | 2.4 |
Data summarised from two major studies assessing risk factors in Africa [96, 109]. The countries represented in Africa were Ghana, Mozambique, Nigeria, Tanzania, South Africa, Sudan and Uganda.
Sample sizes for the stroke cases were 973 and 2118, respectively.
For atrial fibrillation or flutter associated with ischaemic strokes in INTERSTROKE study OR value was 4.4.
cardiomyopathy, heart failure, ishaemic heart disease and valvular heart diseases.
OR for regular physical inactivity.
OR for healthy cardiovascular diet intake.
Abbreviations: INTERSTROKE, International case-control study in stroke ; N/A, not available; OR, odds ratio; PAR, population attributable risk; SIREN, Stroke Investigative Research and Educational Network.
Hypertension
Africans have a higher burden of hypertension with resultant cardiovascular mortality and end-organ damage than Caucasians [83, 88]. As a matter of fact, individuals of African ancestry have been shown to be more susceptible to elevations in blood pressure compared to Caucasians, with the increase in stroke risk being 3-times greater in individuals of African ancestry compared to Caucasians [62]. Individuals of African ancestry have greater burden of vascular risk factors (e.g., early age of onset, greater severity, worse control of BP) and may also have more brain injury from high BP and other vascular risk factors [30, 82]. In the INTERSTROKE study the adjusted OR for hypertension among Africans was reported to be of 4.0 (99% CI 2.6–6.2). This was the highest within different regions included in the survey. However, in the recent SIREN study, an even higher estimate of 19 (95% CI 12–31) for stroke overall was reported with a higher effect size for haemorrhagic strokes compared to ischaemic types [109]. Hypertension [91] in LMICs is exacerbated by a lack of or ineffective population campaigns (i.e. salt reduction), obesity [37, 94, 120] or weak health care systems [42, 72].
The high prevalence of hypertension among Africans may be attributed to decreased production and increased degradation of nitric oxide (NO) resulting in endothelial dysfunction [88] Effective interventions are needed to minimize the projected cardiovascular disease trends. Inorganic nitrate supplementation was shown to reduce systolic blood pressure, improve endothelial function and cerebral blood flow in young and older healthy participants [17, 29, 76, 87, 118, 152]. Inorganic nitrate supplementation may be associated with improved executive function and motor skills [51, 69, 152]. The mechanisms linking dietary nitrate to improved cardiovascular health appear to be mediated by an increased NO generation via the non-enzymatic (nitrate) NO pathway [81].
Diabetes
It is projected that by 2030, SSA will have 18.7 million people with type 2 diabetes representing 1.6 times greater numbers since the year 2000 [153]. As elsewhere, diabetes in Africa is attributable to both genetic and lifestyle factors. Self-reported diabetes or measured values of more than 6.5% HbA1c was considered a relatively strong factor for stroke [96] and among the top 11 modifiable factors associated with stroke [109]. Diabetes was more associated with ischaemic strokes than haemorrhagic strokes. In the SIREN study, there was nearly three times greater frequency of diabetes among stroke patients than controls and stronger risk for stroke in those under 50 years compared to those older than 50 years (OR 5.8 vs 2.4). While it is not clear whether this affects cerebral SVD, it is plausible that high numbers of diabetics will eventually contribute to the burden of cognitive impairment.
Dyslipidaemia
Dyslipidaemia is mainly characterized by increases in triglycerides, apolioprotein B and low-density lipoprotein and decreased high-density lipoprotein. In general, the proportions of these lipids are similarly between HICs and Africa. In the INTERSTROKE study, the prevalence of apoliopoprotein B/A1 in stroke controls was nearly 2 fold higher in Africans compared to Western Europeans, North Americans and Australians with ORs for stroke as 2.5 vs 1.8, and PARs 45 vs 24, respectively [96]. As with diabetes, dyslipidaemia was among the top 11 modifiable risk factors associated with stroke [109]. Moreover, younger patients (<50 years) are at greater at risk for stroke than those above 50 years of age (ORs 2.8 vs 1.7). In addition to lifestyle habits, genetic factors likely play a significant role in determining the variability in blood lipid concentrations among Africans [2].
Carotid Artery Disease
The TOAST classification suggests there the frequency of LAD strokes is similar between SSA and HICs (Figure 1). While LAD may incorporate other systemic vessels including the aorta and coronary arteries, carotid artery stenosis or disease (CAD) is a key substrate of strokes. The true prevalence of carotid artery stenosis or disease (CAD) in Africa is unknown. The prevalence and severity of CAD was significantly higher in a post-mortem sample of Nigerians compared to similar examination undertaken four decades ago.[46, 154]. Our previous Tanzanian Stroke Incidence study indicated there was no significant CAD in the Hai District [68]. However, recent studies suggest carotid artery stenosis or disease (CAD) by assessing carotid intima-medial thickness (CIMT) in a hospital-based cohort of hypertensive Nigerian Africans appears a sensitive indicator for the stratification of cardiovascular phenotype and risk [105]. It also better predicts stroke than other cardiovascular risk calculators of Western origin [108]. Similarly, in a multiracial North American study, racial disparities showed that blacks had more severe arteriolar sclerosis and atherosclerosis [20] whereas significant racial disparities were reported in stroke among blacks showing more severe disease, [125] worse cognitive outcomes,[43] and higher mortality.[106]
Smoking
Consistent data from the INTERSTROKE and SIREN studies suggest former or current smokers are at high risk of any stroke. In a South African study of young patients (mean 44, range 15–49 years) hypertension (31%) and smoking (19%) were the most commonly encountered risk factors (Hoffmann M, 1998). Similarly, in the SIREN cohort tobacco smoking was the second strongest factor after hypertension as a risk for all strokes. ORs were 76> 7.5 >5.5 in order of hypertension > smoking > age for haemorrhagic strokes with similar order for ischaemic strokes. However, the population attributable risk was much less at 1.5% below the order hypertension (87%) > age (61%) > dyslipidaemia (38%) > raised waist hip ratio (30%) > > regular meat consumption (28%) > diabetes (26%) [109]..
Neuroimaging of stroke in Africa
Neuroimaging is an important tool to document in vivo the impact of vascular damage to the brain. Neuroimaging also complements histopathology to decipher the pathophysiology of VCI, and is a crucial technique for the identification of such damage in patients with cognitive decline. While large city hospitals and private practice-based institutions may have computed tomography (CT) and magnetic resonance imaging (MRI), the overall availability of neuroimaging services in Africa is insufficient. In a recent Ghanaian survey of hospital services for acute stroke care, 64% of 11 surveyed hospitals had a functional CT scan but only 18 % offered 24 hours services. Functional MRI and carotid Doppler ultrasonography services were available in 27% and 21% respectively of the surveyed hospitals [19]. In places where imaging services are available their affordability and accessibility when maintained and functioning are also of concern. Thus, to provide evidence-based stroke services and improve stroke care in resource poor settings, public health policy campaigns are necessary to not only reduce stroke-related morbidity and mortality [19] but also enhance stroke diagnosis. In one study, clinical accuracy for stroke diagnosis was ~66% while diagnostic errors were apparent in 35%, particularly in those patients at older age [103]. This underscores significant misdiagnosis despite use of current diagnostic criteria and scoring systems and the potential for providing inadequate care of patients. However, research studies have reported that WMHs were consistently more severe in patients with stroke than in patients without [98]. This finding was independent of age and gender [7]. Similarly, global and medial temporal lobe atrophy determined by MRI were associated with cognitive decline after stroke [8]. Prospective studies in large cohorts are necessary to determine the patterns of WM changes and brain atrophy in predicting stroke and its recurrence as well as other CVDs independent of other known risk factors. Wider use of neuroimaging would also allow detection of subclinical vascular brain disease and SVD associated with hypertension, other vascular disease risk factors or frailty.
Vascular Cognitive Impairment, Vascular Cognitive Disorders and VaD in Africa
Increasing evidence shows that higher cumulative vascular risk factor load worsens cognition [13]. The mechanisms by which individuals of African ancestry within studies including African-Americans [31] are more prone to VCI and vascular cognitive disorders (VCD) [43, 115] are not fully understood but hypertension is likely to be a significant one. Cognitive impairment resulting from brain vascular changes or injury is likely to be the most prevalent worldwide. There are sparse data on VCI and VCD in Africa. In sub-Saharan Africa, cognitive impairment from all causes was estimated to range from 6.3% to 25% in adults over 50 years of age [85]. Given these estimates, the true prevalence of VCI is likely high in Africa.
The concept of VCI [53, 56, 95] has been very useful but it continually challenges the correlation between the pathophysiology and degree of impaired cognition in the continuum of VCI. Recently, the VCI diagnostic concept was refined in a Delphi type of analysis [139, 140]. The consensus adopted use of ‘Mild’ and ‘Major’ forms of VCI. The use of ‘Mild’ and ‘Major’ subdivisions for the severity of impairment accords with the revised terminology in Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) and with the introduction of categories of mild and major VCD [18]. The major neurocognitive disorder classification, meant to describe frank dementia as a substitute for VaD appears to fit better with patients, and more adapted to neurodegenerative cognitive disorders for which memory impairment is not predominant but comprises prominent frontal lobe change [128]. However, the Major form of VCI is proposed to have four main subtypes including subcortical ischaemic vascular dementia or SIVaD, multi-infarct dementia, post-stroke dementia (PSD) and mixed dementias, which could be determined by significant co-existing neurodegenerative pathology.
Cognitive impairment or dementia following stroke is relatively common [77, 93, 115]. Incident dementia after stroke or PSD may develop within three months or after a stabilisation period of one year or more after the stroke [13, 21, 117]. However, PSD may have a complex aetiology with varying combinations of LAD as well as VaD and neurodegenerative pathology. Stroke injury could unmask other pre-existing disease processes such as Alzheimer’s disease. Majority of PSD in the absence of significant neurodegenerative pathology appears to be VaD [13, 92] fulfilling relevant clinical guidelines for VCI [56]. Furthermore, the presence of any age-related hippocampal Alzheimer type of lesions did not differentiate demented from non-demented post-stroke subjects [6].
There are few prospective studies on VCI or VaD in Africa (Table 3). Consistent with the previous studies on VCI and PSD, our Nigerian study in older stroke survivors [7] found that ~40% had VCI while 8% developed dementia three months after the event. Multiple regression analysis revealed that older age, low education, pre-stroke cognitive decline and medial temporal lobe atrophy were independently associated with cognitive dysfunction whereas pre-stroke daily intake of fish was protective. In a recent Ghanaian study [131], 34% of stroke survivors developed VCI whereas 14% had VaD. Increasing age, lack of formal education and worse functional disability on the modified Rankin scale were again identified as strong risk factors. Survivors diagnosed with VaD had the poorest health-related quality of life. These reports suggest that nearly 50% of older West African stroke survivors develop cognitive impairment or dementia. More than 30% of stroke survivors may develop delirium within days after the stroke majority 66% of which is hypoactive [101]. Those with severe stroke injury and developing were more prone to succumb to dementia or early death. These observations are further consistent with a study on mild cognitive impairment [121], where the presence of vascular risk factors ranging from hypertension (64%) to stroke (14%) were contributed to MCI (27%) and dementia (8%) in South Africans. In accord with previous conclusions on modifiable risk factors, all these studies collectively emphasize the vital role of education and healthy nutrition in building reserves to improve or sustain cognitive function after stroke or CVD.
Table 3.
Proportions of VaD estimated from Dementia prevalence estimates in various countries in Africa
| Country, Location; Type of sample |
Sample size, Age (yrs)* |
Dementia Prevalence (%) |
Alzheimer’s Disease (%) |
VaD (%) | Reference |
|---|---|---|---|---|---|
| Africa, 10 countries; Community | 2.4% | 57% | 27% | [50] | |
| SSA, 5 countries (Benin, Botswana, CAR, Congo, Nigeria); Community/clinic¶ | 10,413; >65 yrs | 0–10% | 54–83% | 8–31% | [85] |
| Nigeria, Abeokuta/Ibadan; Hospital/ Clinic | 240,294; | 0.05% | 57% | 17% | [16] |
| Egypt, Al Kharga District; Community | 8173, >50 yrs | 2.3% | 51% | 29% | [45] |
| South Africa, Durban; Hospital | 140; >60 yrs‡ | 8% | - | 40% | [121] |
| Egypt, Al-Quseir city, | 2,222; >60 yrs† | 3.8% | 48% | 37% (1.4%)† | [44] |
| Tanzania, Hai District; Community | 1198; >60 yrs | 6.4% | 48% | 41% (2.6%)†† | [112],[111] |
| Egypt, Quena/Aswan; Community | 691; >60 yrs† | 5.1% | - | - | [73] |
| Congo, Brazzaville, Bangui, CAR; Community | 910; | 6.1% | 69,%, 83% | 31%, 18% | [129] |
Data summarised from several references as shown. VaD cases in prospective stroke survivors studies not included [7, 131].
Age in years (yrs) shows youngest age from which sample was screened.
Range shown from studies in 4 SSA countries included in the analysis [85].
Dementia was 2.0% in >50 year olds, 3.8% >60 years and 13.5%–100% in individuals over age 80 years [44].
Crude prevalence rate [111].
Vascular risk factors were strongly associated with 80% of dementia cases.
Abbreviations: CAR, Central African Republic, SSA, sub-Saharan Africa.
Previous systematic analyses suggested the prevalence of dementia in LMICs is relatively lower than that in HICs [71, 119]. A more recent analysis involving 10 studies estimated an overall prevalence of ~2.4% in adults over 50 years. This converts to nearly a million people with dementia in Africa [50]. Dementia prevalence was higher in women with little variation between regions. Only few African studies have determined rates of dementia subtypes. In general, the prevalence of Alzheimer’s disease is greater than VaD. VaD varies between 17 and 41% for all causes of dementia depending on the type of study sample (Table 3). This is in accord with the previous systematic analysis, which reported Alzheimer disease was 57% whereas VaD was 27%. The main risk factors for dementia even in Africa were older age, female gender and presence of cardiovascular disease. In the Egyptian study [44], the prevalence of VaD in over 60 year olds was estimated as 1.4%. In our Tanzanian study [111], while there was a greater proportion of VaD, the overall prevalence was estimated to be 2.6%. A previous diagnosis of diabetes mellitus was independently associated with greater odds of having VaD than Alzheimer’s disease in the Hai District.
As with all other dementias, confirmation of VaD diagnosis in all these studies is only definitive after post-mortem examination. Autopsies are rarely performed in Africa but much needed to reflect true prevalence and incidence rates of VaD that may also be influenced by inconsistencies in diagnostic criteria, sampling methods and variations in subject or country demographics, morbidity and mortality trends.
Treatment and Interventions
While it is important to uncover new knowledge, particularly from genetically different African populations with wide allelic diversity and low linkage disequilibrium [10, 12, 104, 143], it is imperative to design effective lifestyle, dietary and pharmacological interventional and translational strategies for the best health impact [26]. Model stroke services in Africa are varied and currently sparse in respect of focused systems of care delivery including pre-hospital, hyperacute, acute, subacute and rehabilitative phases of care [114]. There is paucity of data on the organization of prehospital stroke services while only three African countries (South Africa, Egypt and Morocco) have reported experiences on thrombolysis. The uptake of dedicated stroke units also appears limited but slowly growing across the continent.
Thrombolytic therapy (thrombolysis) using recombinant tissue plasminogen activator (rtPA) for acute ischemic stroke care was not available in any of recently surveyed hospitals. Aspirin therapy is often administered in study hospitals. Although only a fraction of stroke patients receive rtPA even in the developed countries (e.g. <10% in the US) despite efforts to increase use of such reperfusion therapies [66], 25–30% of those who undergo the reperfusion treatment experience successful arterial recanalization. In Africa, the most frequent subtype of ischaemic stroke was cerebral SVD, which may further benefit from rtPA because of the low risk of haemorrhagic transformation [135] in this TOAST subtype.
In addition, endovascular thrombectomy has become available in the developed countries, which can achieve considerably higher recanalization rates approaching 90% in acute ischemic stroke patients [52]. Availability of several new devices such as a stent retriever has opened a new era in management of large vessel occlusion caused by large artery disease and CE stroke. In Africa, the sparse number of stroke units as well as the high cost of such reperfusion treatments, limited resources, pre-hospital delay, and insufficient post-hospital infrastructures may deter against their widespread use. However, implementation of rtPA and endovascular thrombectomy hold promise in acute stroke management. There are several economic and regulatory hurdles to overcome this issue but health care professionals and policy makers should cooperate to improve stroke care delivery with the aid of scientific organizations. The World Stroke Organization (WSO) has produced the global stroke services guidelines and action plan as a template [80], which may be adapted to guide development of protocols for developing stroke care services in all regions of the world, but particularly LMICs such as those of Africa where stroke care services are currently poorly organised. Task shifting and task sharing approaches to effective stroke care are therefore very important in Africa given the low availability of competent stroke clinicians [11, 90].
Prevention
Risk factor control is important to prevent stroke worldwide [48]. For instance, Japan had the highest stroke mortality in the world in 1965 [145]. However, it rapidly declined by ~80% during the period from 1965 to 1990. In parallel, the prevalence of hypertension declined substantially from 1965 to 1990 [145]. Salt consumption in the northern part of Japan was more than 25 g/day in the 1950s but now it is decreased to ~13 g/day partially due to population and community campaigns, although this figure is still higher than that of the WHO recommended intake of 5g/day to prevent cardiovascular disease [146]. (http://apps.who.int/iris/bitstream/handle/10665/43685/9789241547178_eng.pdf;jsessionid=E128B9F03597B7D6B189EA6AE2C532B1?sequence=1). In Africa, primitive, primary and secondary prevention models exist mostly within the context of clinical research with promising results [107, 138]. However, salt reduction by population or regional campaigns need to be vigorously undertaken in Africa to reduce the burden and cognitive sequelae of stroke incidence. The prevalence of obesity is also increasing, which will inevitably increase the prevalence of diabetes mellitus and metabolic disorders. The time for management of these modifiable risk factors is ripe for prevention of stroke in African countries.
Summary
Stroke and other CVDs are increasing in Africa. Hypertension is the strongest modifiable risk factor for stroke in Africa. Other modifiable factors include diabetes, dyslipidaemia, physical inactivity and unhealthy diet. In contrast to HICs, strokes attributed to cerebral SVD appear high among sub-Saharan Africans. Raised blood pressure may explain most of the incidence of SVD-related strokes but there are likely other contributing vascular risk factors including dietary constituents which influence vascular disease. High burdens of disease will contribute higher incidence of cognitive impairment and dementia. Despite incomplete data, it is apparent that controlling risk factors and prevention strategies will be beneficial for reducing the morbidity and mortality as well as lessen the burden of dementia.
Highlights.
Hypertension is the strongest modifiable risk factor for stroke in Africa.
Cerebral SVD appears high among sub-Saharan Africans.
Prevalence estimates of VaD is 2–3% and delayed dementia after stroke is 10–20%.
Demographic transition in both urban and rural settings will likely increase strokes.
It timely control vascular risk to reduce stroke and cognitive impairment in Africa.
Acknowledgments
We are thankful to Arthur Oakley and Janet Slade as loyal members of the Neurovascular Research Group for providing continued technical support.
Funding Sources
RNK’s research on elderly stroke survivors has been supported by the Medical Research Council, RCUK Newcastle Centre for Brain Ageing and Vitality, Medical Research Council (UK), Alzheimer’s Research UK, the Dunhill Medical Trust, UK and the Newcastle National Institute for Health Research Biomedical Research Centre in Ageing and Age Related Diseases, Newcastle upon Tyne Hospitals National Health Service Foundation Trust. MOO and ROA are supported by the National Institutes of Health (NIH) U54 HG007479 and Grant CTR16A012 from the College of Medicine, University of Ibadan, Nigeria. ROA is also supported by the International Brain Research Organization (IBRO) Return Home Fellowship. MI is supported by JSPS KAKENHI Grant Number 17H04670.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
We declare no competing interests.
References
- 1.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Adebamowo SN, Tekola-Ayele F, Adeyemo AA, Rotimi CN. Genomics of Cardiometabolic Disorders in Sub-Saharan Africa. Public Health Genomics. 2017;20:9–26. doi: 10.1159/000468535. [DOI] [PubMed] [Google Scholar]
- 3.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS One. 2014;9:e100724. doi: 10.1371/journal.pone.0100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajose FO, Adelowo O, Oderinlo O. Clinical presentations of Behcet's disease among Nigerians: a 4-year prospective study. Int J Dermatol. 2015;54:889–897. doi: 10.1111/ijd.12554. [DOI] [PubMed] [Google Scholar]
- 5.Akinyemi R, Tiwari HK, Arnett DK, Ovbiagele B, Irvin MR, Wahab K, Sarfo F, Srinivasasainagendra V, Adeoye A, Perry RT, Akpalu A, Jenkins C, Arulogun O, Gebregziabher M, Owolabi L, Obiako R, Sanya E, Komolafe M, Fawale M, Adebayo P, Osaigbovo G, Sunmonu T, Olowoyo P, Chukwuonye I, Obiabo Y, Onoja A, Akinyemi J, Ogbole G, Melikam S, Saulson R, Owolabi M S. Investigators. APOL1, CDKN2A/CDKN2B, and HDAC9 polymorphisms and small vessel ischemic stroke. Acta Neurol Scand. 2018;137:133–141. doi: 10.1111/ane.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinyemi RO, Allan L, Oakley A, Kalaria R. Hippocampal Neurodegenerative Pathology in Post-stroke Dementia Compared to Other Dementias and Ageing Controls. Front Neurosci. 2017;11:717. doi: 10.3389/fnins.2017.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinyemi RO, Allan L, Owolabi MO, Akinyemi JO, Ogbole G, Ajani A, Firbank M, Ogunniyi A, Kalaria RN. Profile and determinants of vascular cognitive impairment in African stroke survivors: the CogFAST Nigeria Study. J Neurol Sci. 2014;346:241–249. doi: 10.1016/j.jns.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Akinyemi RO, Firbank M, Ogbole GI, Allan LM, Owolabi MO, Akinyemi JO, Yusuf BP, Ogunseyinde O, Ogunniyi A, Kalaria RN. Medial temporal lobe atrophy, white matter hyperintensities and cognitive impairment among Nigerian African stroke survivors. BMC Res Notes. 2015;8:625. doi: 10.1186/s13104-015-1552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer's disease and vascular dementia. Curr Alzheimer Res. 2013;10:642–653. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 10.Akinyemi RO, Ovbiagele B, Akpalu A, Jenkins C, Sagoe K, Owolabi L, Sarfo F, Obiako R, Gebreziabher M, Melikam E, Warth S, Arulogun O, Lackland D, Ogunniyi A, Tiwari H, Kalaria RN, Arnett D, Owolabi MO S.I.a.M.o.t.H.A. Consortium. Stroke genomics in people of African ancestry: charting new paths. Cardiovasc J Afr. 2015;26:S39–49. doi: 10.5830/CVJA-2015-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinyemi RO, Owolabi MO, Adebayo PB, Akinyemi JO, Otubogun FM, Uvere E, Adeniji O, Adeleye O, Aridegbe O, Taiwo FT, Ogun SA, Ogunniyi A. Task-shifting training improves stroke knowledge among Nigerian non-neurologist health workers. J Neurol Sci. 2015;359:112–116. doi: 10.1016/j.jns.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemi RO, Owolabi MO, Oyeniyi T, Ovbiagele B, Arnett DK, Tiwari HK, Walker R, Ogunniyi A, Kalaria RN S.g.o.H.A. Consortium. Neurogenomics in Africa: Perspectives, progress, possibilities and priorities. J Neurol Sci. 2016;366:213–223. doi: 10.1016/j.jns.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, O'Brien JT, Kalaria RN. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzamora MT, Sorribes M, Heras A, Vila N, Vicheto M, Fores R, Sanchez-Ojanguren J, Sancho A, Group IS, Pera G. Ischemic stroke incidence in Santa Coloma de Gramenet (ISISCOG), Spain. A community-based study. BMC Neurol. 2008;8:5. doi: 10.1186/1471-2377-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27:493–501. doi: 10.1159/000210432. [DOI] [PubMed] [Google Scholar]
- 16.Amoo G, Akinyemi RO, Onofa LU, Akinyemi JO, Baiyewu O, Ogunlesi AO, Ogunniyi A. Profile of clinically-diagnosed dementias in a neuropsychiatric practice in Abeokuta, South-Western Nigeria. African Journal of Psychiatry. 2011;14:377–382. doi: 10.4314/ajpsy.v14i5.5. [DOI] [PubMed] [Google Scholar]
- 17.Ashor AW, Lara J, Siervo M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: a systematic review and meta-analysis. J Hypertens. 2017;35:1353–1359. doi: 10.1097/HJH.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 18.A.P. Association. Diagnostic and Statistical Manual of Mental Disorders APA. United States; 2013. [Google Scholar]
- 19.Baatiema L, Otim M, Mnatzaganian G, Aikins AD, Coombes J, Somerset S. Towards best practice in acute stroke care in Ghana: a survey of hospital services. Bmc Health Services Research. 2017;17 doi: 10.1186/s12913-017-2061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528–534. doi: 10.1212/WNL.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bejot Y, Aboa-Eboule C, Durier J, Rouaud O, Jacquin A, Ponavoy E, Richard D, Moreau T, Giroud M. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke. 2011;42:607–612. doi: 10.1161/STROKEAHA.110.595553. [DOI] [PubMed] [Google Scholar]
- 22.Bejot Y, Caillier M, Ben Salem D, Couvreur G, Rouaud O, Osseby GV, Durier J, Marie C, Moreau T, Giroud M. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry. 2008;79:1344–1348. doi: 10.1136/jnnp.2008.150318. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin LA, Allain TJ, Mzinganjira H, Connor MD, Smith C, Lucas S, Joekes E, Kampondeni S, Chetcuti K, Turnbull I, Hopkins M, Kamiza S, Corbett EL, Heyderman RS, Solomon T. The Role of Human Immunodeficiency Virus-Associated Vasculopathy in the Etiology of Stroke. J Infect Dis. 2017;216:545–553. doi: 10.1093/infdis/jix340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11:878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin LA, Bryer A, Lucas S, Stanley A, Allain TJ, Joekes E, Emsley H, Turnbull I, Downey C, Toh CH, Brown K, Brown D, Ison C, Smith C, Corbett EL, Nath A, Heyderman RS, Connor MD, Solomon T. Arterial ischemic stroke in HIV: Defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm. 2016;3:e254. doi: 10.1212/NXI.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birbeck GL, Meyer AC, Ogunniyi A. Nervous system disorders across the life course in resource-limited settings. Nature. 2015;527:S167–171. doi: 10.1038/nature16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogiatzi C, Wannarong T, McLeod AI, Heisel M, Hackam D, Spence JD. SPARKLE (Subtypes of Ischaemic Stroke Classification System), incorporating measurement of carotid plaque burden: a new validated tool for the classification of ischemic stroke subtypes. Neuroepidemiology. 2014;42:243–251. doi: 10.1159/000362417. [DOI] [PubMed] [Google Scholar]
- 28.Bohlega S, Al Shubili A, Edris A, Alreshaid A, Alkhairallah T, AlSous MW, Farah S, Abu-Amero KK. CADASIL in Arabs: clinical and genetic findings. BMC Med Genet. 2007;8:67. doi: 10.1186/1471-2350-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bond V, Jr, Curry BH, Adams RG, Asadi MS, Millis RM, Haddad GE. Effects of dietary nitrates on systemic and cerebrovascular hemodynamics. Cardiol Res Pract. 2013;2013:435629. doi: 10.1155/2013/435629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, Dagher AP, Cooper L. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR Am J Neuroradiol. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 32.Camuset G, Wolff V, Marescaux C, Abou-Bacar A, Candolfi E, Lefebvre N, Christmann D, Hansmann Y. Cerebral vasculitis associated with Schistosoma mansoni infection. BMC Infect Dis. 2012;12:220. doi: 10.1186/1471-2334-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 34.Chetty R. Vasculitides associated with HIV infection. J Clin Pathol. 2001;54:275–278. doi: 10.1136/jcp.54.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen DL, Hedera P, Premkumar DR, Friedland RP, Kalaria RN. Amyloid-beta protein angiopathies masquerading as Alzheimer's disease? Ann N Y Acad Sci. 1997;826:390–395. doi: 10.1111/j.1749-6632.1997.tb48490.x. [DOI] [PubMed] [Google Scholar]
- 36.G.B.D.M. Collaborators. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.G.B.D.O. Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connor MD, Modi G, Warlow CP. Differences in the nature of stroke in a multiethnic urban South African population: the Johannesburg hospital stroke register. Stroke. 2009;40:355–362. doi: 10.1161/STROKEAHA.108.521609. [DOI] [PubMed] [Google Scholar]
- 39.Corraini P, Henderson VW, Ording AG, Pedersen L, Horvath-Puho E, Sorensen HT. Long-Term Risk of Dementia Among Survivors of Ischemic or Hemorrhagic Stroke. Stroke. 2017;48:180–186. doi: 10.1161/STROKEAHA.116.015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.G.B.D. DALYs, H. Collaborators. Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Ackerman IN, Ademi Z, Adou AK, Adsuar JC, Afshin A, Agardh EE, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Almazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Ameh EA, Amini H, Ammar W, Anderson HR, Anderson BO, Antonio CA, Anwari P, Arnlov J, Arsic Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Avila MA, Awuah B, Bachman VF, Badawi A, Bahit MC, Balakrishnan K, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu A, Basu S, Basulaiman MO, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Bertozzi-Villa A, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Bienhoff K, Bikbov B, Biryukov S, Blore JD, Blosser CD, Blyth FM, Bohensky MA, Bolliger IW, Bora Basara B, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brayne CE, Brazinova A, Breitborde NJ, Brenner H, Briggs AD, Brooks PM, Brown JC, Brugha TS, Buchbinder R, Buckle GC, Budke CM, Bulchis A, Bulloch AG, Campos-Nonato IR, Carabin H, Carapetis JR, Cardenas R, Carpenter DO, Caso V, Castaneda-Orjuela CA, Castro RE, Catala-Lopez F, Cavalleri F, Cavlin A, Chadha VK, Chang JC, Charlson FJ, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Cirillo M, Coates MM, Coffeng LE, Coggeshall MS, Colistro V, Colquhoun SM, Cooke GS, Cooper C, Cooper LT, Coppola LM, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Danawi H, Dandona L, Dandona R, Dansereau E, Dargan PI, Davey G, Davis A, Davitoiu DV, Dayama A, De Leo D, Degenhardt L, Del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, Dharmaratne SD, Dherani MK, Diaz-Torne C, Dicker D, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duan L, Duber HC, Ebel BE, Edmond KM, Elshrek YM, Endres M, Ermakov SP, Erskine HE, Eshrati B, Esteghamati A, Estep K, Faraon EJ, Farzadfar F, Fay DF, Feigin VL, Felson DT, Fereshtehnejad SM, Fernandes JG, Ferrari AJ, Fitzmaurice C, Flaxman AD, Fleming TD, Foigt N, Forouzanfar MH, Fowkes FG, Paleo UF, Franklin RC, Furst T, Gabbe B, Gaffikin L, Gankpe FG, Geleijnse JM, Gessner BD, Gething P, Gibney KB, Giroud M, Giussani G, Gomez Dantes H, Gona P, Gonzalez-Medina D, Gosselin RA, Gotay CC, Goto A, Gouda HN, Graetz N, Gugnani HC, Gupta R, Gupta R, Gutierrez RA, Haagsma J, Hafezi-Nejad N, Hagan H, Halasa YA, Hamadeh RR, Hamavid H, Hammami M, Hancock J, Hankey GJ, Hansen GM, Hao Y, Harb HL, Haro JM, Havmoeller R, Hay SI, Hay RJ, Heredia-Pi IB, Heuton KR, Heydarpour P, Higashi H, Hijar M, Hoek HW, Hoffman HJ, Hosgood HD, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu G, Huang C, Huang JJ, Husseini A, Huynh C, Iannarone ML, Iburg KM, Innos K, Inoue M, Islami F, Jacobsen KH, Jarvis DL, Jassal SK, Jee SH, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Juel K, Kan H, Karch A, Karema CK, Karimkhani C, Karthikeyan G, Kassebaum NJ, Kaul A, Kawakami N, Kazanjan K, Kemp AH, Kengne AP, Keren A, Khader YS, Khalifa SE, Khan EA, Khan G, Khang YH, Kieling C, Kim D, Kim S, Kim Y, Kinfu Y, Kinge JM, Kivipelto M, Knibbs LD, Knudsen AK, Kokubo Y, Kosen S, Krishnaswami S, Kuate Defo B, Kucuk Bicer B, Kuipers EJ, Kulkarni C, Kulkarni VS, Kumar GA, Kyu HH, Lai T, Lalloo R, Lallukka T, Lam H, Lan Q, Lansingh VC, Larsson A, Lawrynowicz AE, Leasher JL, Leigh J, Leung R, Levitz CE, Li B, Li Y, Li Y, Lim SS, Lind M, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Lofgren KT, Logroscino G, Looker KJ, Lortet-Tieulent J, Lotufo PA, Lozano R, Lucas RM, Lunevicius R, Lyons RA, Ma S, Macintyre MF, Mackay MT, Majdan M, Malekzadeh R, Marcenes W, Margolis DJ, Margono C, Marzan MB, Masci JR, Mashal MT, Matzopoulos R, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, Meaney PA, Medina C, Mehndiratta MM, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah GA, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Mohamed Ibrahim N, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montanez Hernandez JC, Montico M, Montine TJ, Mooney MD, Moore AR, Moradi-Lakeh M, Moran AE, Mori R, Moschandreas J, Moturi WN, Moyer ML, Mozaffarian D, Msemburi WT, Mueller UO, Mukaigawara M, Mullany EC, Murdoch ME, Murray J, Murthy KS, Naghavi M, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KM, Nejjari C, Neupane SP, Newton CR, Ng M, Ngalesoni FN, Nguyen G, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Ohno SL, Olusanya BO, Opio JN, Ortblad K, Ortiz A, Pain AW, Pandian JD, Panelo CI, Papachristou C, Park EK, Park JH, Patten SB, Patton GC, Paul VK, Pavlin BI, Pearce N, Pereira DM, Perez-Padilla R, Perez-Ruiz F, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips BK, Phillips DE, Piel FB, Plass D, Poenaru D, Polinder S, Pope D, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Pullan RL, Qato DM, Quistberg DA, Rafay A, Rahimi K, Rahman SU, Raju M, Rana SM, Razavi H, Reddy KS, Refaat A, Remuzzi G, Resnikoff S, Ribeiro AL, Richardson L, Richardus JH, Roberts DA, Rojas-Rueda D, Ronfani L, Roth GA, Rothenbacher D, Rothstein DH, Rowley JT, Roy N, Ruhago GM, Saeedi MY, Saha S, Sahraian MA, Sampson UK, Sanabria JR, Sandar L, Santos IS, Satpathy M, Sawhney M, Scarborough P, Schneider IJ, Schottker B, Schumacher AE, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serina PT, Servan-Mori EE, Shackelford KA, Shaheen A, Shahraz S, Shamah Levy T, Shangguan S, She J, Sheikhbahaei S, Shi P, Shibuya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh A, Singh JA, Singh L, Skirbekk V, Slepak EL, Sliwa K, Soneji S, Soreide K, Soshnikov S, Sposato LA, Sreeramareddy CT, Stanaway JD, Stathopoulou V, Stein DJ, Stein MB, Steiner C, Steiner TJ, Stevens A, Stewart A, Stovner LJ, Stroumpoulis K, Sunguya BF, Swaminathan S, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, Te Ao BJ, Tediosi F, Temesgen AM, Templin T, Ten Have M, Tenkorang EY, Terkawi AS, Thomson B, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tonelli M, Topouzis F, Toyoshima H, Traebert J, Tran BX, Trillini M, Truelsen T, Tsilimbaris M, Tuzcu EM, Uchendu US, Ukwaja KN, Undurraga EA, Uzun SB, Van Brakel WH, Van De Vijver S, van Gool CH, Van Os J, Vasankari TJ, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Wagner GR, Wagner J, Waller SG, Wan X, Wang H, Wang J, Wang L, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Wenzhi W, Werdecker A, Westerman R, Whiteford HA, Wilkinson JD, Williams TN, Wolfe CD, Wolock TM, Woolf AD, Wulf S, Wurtz B, Xu G, Yan LL, Yano Y, Ye P, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Zaki ME, Zhao Y, Zheng Y, Zonies D, Zou X, Salomon JA, Lopez AD, Vos T. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, Kalaria RN. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewhurst MJ, Walker RW. Hypertension in Sub-Saharan Africa; prevalence, prescriptions, pitfalls and paradigms. J Hum Hypertens. 2016;30:221–222. doi: 10.1038/jhh.2015.93. [DOI] [PubMed] [Google Scholar]
- 43.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke. 2013;44:138–145. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- 44.El Tallawy HN, Farghly WM, Badry R, Rageh TA, Shehata GA, Hakeem MN, Abd El Hamed M, Sayd MA, Hamed Y, Kandil MR. Prevalence of dementia in Al-Quseir city, Red Sea Governorate, Egypt. Clin Interv Aging. 2014;9:9–14. doi: 10.2147/CIA.S48325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Tallawy HN, Farghly WM, Shehata GA, Rageh TA, Hakeem NA, Abo-Elfetoh N, Hegazy AM, Rayan I, El-Moselhy EA. Prevalence of dementia in Al Kharga District, New Valley Governorate, Egypt. Neuroepidemiology. 2012;38:130–137. doi: 10.1159/000335655. [DOI] [PubMed] [Google Scholar]
- 46.Erete EI, Ogun OG, Oladapo OO, Akang EE. Prevalence and severity of atherosclerosis in extra cranial carotid arteries in Nigeria: an autopsy study. BMC Cardiovasc Disord. 2012;12:106. doi: 10.1186/1471-2261-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkabu SG, Agedie Y, Mihretu DD, Semere A, Alemu YM. Ischemic and Hemorrhagic Stroke in Bahir Dar, Ethiopia: A Retrospective Hospital-Based Study. J Stroke Cerebrovasc Dis. 2018 doi: 10.1016/j.jstrokecerebrovasdis.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 48.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJ, Forouzanfar MH I. Global Burden of Diseases, S. Risk Factors, G. Stroke Experts Writing, Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 49.Genga E, Oyoo O, Adebajo A. Vasculitis in Africa. Curr Rheumatol Rep. 2018;20:4. doi: 10.1007/s11926-018-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George-Carey R, Adeloye D, Chan KY, Paul A, Kolcic I, Campbell H, Rudan I. An estimate of the prevalence of dementia in Africa: A systematic analysis. J Glob Health. 2012;2:020401. doi: 10.7189/jogh.02.020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Gomis M, Davalos A. Recanalization and Reperfusion Therapies of Acute Ischemic Stroke: What have We Learned, What are the Major Research Questions, and Where are We Headed? Front Neurol. 2014;5:226. doi: 10.3389/fneur.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S C.o.E. American Heart Association Stroke Council, C.o. C.N.C.o.C.R. Prevention, Intervention, S. Council on Cardiovascular, Anesthesia, Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.G.B.D.N.D.C. Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulli G, Rutten-Jacobs LC, Kalra L, Rudd AG, Wolfe CD, Markus HS. Differences in the distribution of stroke subtypes in a UK black stroke population - final results from the South London Ethnicity and Stroke Study. BMC Med. 2016;14:77. doi: 10.1186/s12916-016-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 57.Hajat C, Heuschmann PU, Coshall C, Padayachee S, Chambers J, Rudd AG, Wolfe CD. Incidence of aetiological subtypes of stroke in a multi-ethnic population based study: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2011;82:527–533. doi: 10.1136/jnnp.2010.222919. [DOI] [PubMed] [Google Scholar]
- 58.Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, Kawata H, Koyama A, Arima K, Takahashi T, Ikeda M, Shiota H, Tamura M, Shimoe Y, Hirayama M, Arisato T, Yanagawa S, Tanaka A, Nakano I, Ikeda S, Yoshida Y, Yamamoto T, Ikeuchi T, Kuwano R, Nishizawa M, Tsuji S, Onodera O. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann M. Stroke in the young: The multiethnic prospective durban stroke data bank results. J Stroke Cerebrovasc Dis. 1998;7:404–413. doi: 10.1016/s1052-3057(98)80124-9. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann M. Stroke in the young in South Africa--an analysis of 320 patients. S Afr Med J. 2000;90:1226–1237. [PubMed] [Google Scholar]
- 61.Hoffmann M, Corr P, Robbs J. Cerebrovascular findings in Takayasu disease. J Neuroimaging. 2000;10:84–90. doi: 10.1111/jon200010284. [DOI] [PubMed] [Google Scholar]
- 62.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, Howard VJ R.E.f. Geographic, I. Racial Differences in Stroke. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–3375. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howlett SE, Rockwood K. Ageing: Develop models of frailty. Nature. 2014;512:253. doi: 10.1038/512253d. [DOI] [PubMed] [Google Scholar]
- 64.Ihle-Hansen H, Thommessen B, Wyller TB, Engedal K, Fure B. Risk factors for and incidence of subtypes of ischemic stroke. Funct Neurol. 2012;27:35–40. [PMC free article] [PubMed] [Google Scholar]
- 65.Jen J, Cohen AH, Yue Q, Stout JT, Vinters HV, Nelson S, Baloh RW. Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS) Neurology. 1997;49:1322–1330. doi: 10.1212/wnl.49.5.1322. [DOI] [PubMed] [Google Scholar]
- 66.Joo H, Wang G, George MG. Use of intravenous tissue plasminogen activator and hospital costs for patients with acute ischaemic stroke aged 18–64 years in the USA. Stroke Vasc Neurol. 2016;1:8–15. doi: 10.1136/svn-2015-000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jowi JO, Mativo PM. Pathological sub-types, risk factors and outcome of stroke at the Nairobi Hospital, Kenya. East Afr Med J. 2008;85:572–581. doi: 10.4314/eamj.v85i12.43535. [DOI] [PubMed] [Google Scholar]
- 68.Jusabani A, Gray WK, Swai M, Walker R. Post-stroke carotid ultrasound findings from an incident Tanzanian population. Neuroepidemiology. 2011;37:245–248. doi: 10.1159/000334610. [DOI] [PubMed] [Google Scholar]
- 69.Justice JN, Johnson LC, DeVan AE, Cruickshank-Quinn C, Reisdorph N, Bassett CJ, Evans TD, Brooks FA, Bryan NS, Chonchol MB, Giordano T, McQueen MB, Seals DR. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: a pilot trial in middle-aged and older adults. Aging (Albany NY) 2015;7:1004–1021. doi: 10.18632/aging.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 71.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P G. World Federation of Neurology Dementia Research. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kayima J, Wanyenze RK, Katamba A, Leontsini E, Nuwaha F. Hypertension awareness, treatment and control in Africa: a systematic review. BMC Cardiovasc Disord. 2013;13:54. doi: 10.1186/1471-2261-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khedr E, Fawi G, Abbas MA, Mohammed TA, El-Fetoh NA, Al Attar G, Noaman M, Zaki AF. Prevalence of mild cognitive impairment and dementia among the elderly population of Qena Governorate, Upper Egypt: a community-based study. J Alzheimers Dis. 2015;45:117–126. doi: 10.3233/JAD-142655. [DOI] [PubMed] [Google Scholar]
- 74.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 75.Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, Barker-Collo S, Connor M, Roth GA, Sacco R, Ezzati M, Naghavi M, Murray CJ, Feigin VL I. Global Burden of Diseases, G. Risk Factors Study Stroke Expert, The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart. 2014;9:101–106. doi: 10.1016/j.gheart.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr. 2016;55:451–459. doi: 10.1007/s00394-015-0872-7. [DOI] [PubMed] [Google Scholar]
- 77.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM. S. Oxford Vascular, Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Limbole EB, Magne J, Lacroix P. Stroke characterization in Sun Saharan Africa: Congolese population. Int J Cardiol. 2017;240:392–397. doi: 10.1016/j.ijcard.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 80.Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke. 2014;9(Suppl A100):4–13. doi: 10.1111/ijs.12371. [DOI] [PubMed] [Google Scholar]
- 81.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 82.Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, Decarli C, Wright CB. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke. 2011;42:2639–2641. doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015;15:54. doi: 10.1186/s12883-015-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, Harris D, Williams EB, Horgan G, Kyne L, McCormack PM, Duggan J, Moore A, Crispino-O'Connell G, Kelly PJ. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke. 2010;41:1579–1586. doi: 10.1161/STROKEAHA.109.575373. [DOI] [PubMed] [Google Scholar]
- 85.Mavrodaris A, Powell J, Thorogood M. Prevalences of dementia and cognitive impairment among older people in sub-Saharan Africa: a systematic review. Bull World Health Organ. 2013;91:773–783. doi: 10.2471/BLT.13.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McArdle PF, Kittner SJ, Ay H, Brown RD, Jr, Meschia JF, Rundek T, Wassertheil-Smoller S, Woo D, Andsberg G, Biffi A, Brenner DA, Cole JW, Corriveau R, de Bakker PI, Delavaran H, Dichgans M, Grewal RP, Gwinn K, Huq M, Jern C, Jimenez-Conde J, Jood K, Kaplan RC, Katschnig P, Katsnelson M, Labovitz DL, Lemmens R, Li L, Lindgren A, Markus HS, Peddareddygari LR, Pedersen A, Pera J, Redfors P, Roquer J, Rosand J, Rost NS, Rothwell PM, Sacco RL, Sharma P, Slowik A, Sudlow C, Thijs V, Tiedt S, Valenti R, Worrall BB, Study NS. Agreement between TOAST and CCS ischemic stroke classification: the NINDS SiGN study. Neurology. 2014;83:1653–1660. doi: 10.1212/WNL.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McRae MP. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. J Chiropr Med. 2009;8:15–24. doi: 10.1016/j.jcm.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 89.Mensah GA, Roth GA, Sampson UK, Moran AE, Feigin VL, Forouzanfar MH, Naghavi M, Murray CJ, Mortality GBD. C. Causes of Death, Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Afr. 2015;26:S6–10. doi: 10.5830/CVJA-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meretoja A, Acciarresi M, Akinyemi RO, Campbell B, Dowlatshahi D, English C, Henninger N, Poppe A, Putaala J, Saini M, Sato S, Wu B, Brainin M, Norrving B, Davis S. Stroke doctors: Who are we? A World Stroke Organization survey. Int J Stroke. 2017;12:858–868. doi: 10.1177/1747493017701150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mok V, Leung EY, Chu W, Chen S, Wong A, Xiong Y, Lam W, Ho CL, Wong KS. Pittsburgh compound B binding in poststroke dementia. J Neurol Sci. 2010;290:135–137. doi: 10.1016/j.jns.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 93.Mok VC, Lam BY, Wong A, Ko H, Markus HS, Wong LK. Early-onset and delayed-onset poststroke dementia - revisiting the mechanisms. Nat Rev Neurol. 2017;13:148–159. doi: 10.1038/nrneurol.2017.16. [DOI] [PubMed] [Google Scholar]
- 94.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 96.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S I. investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 97.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S I. investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 98.Ogbole GI, Owolabi MO, Yusuf BP. White Matter Changes on Magnetic Resonance Imaging: A Risk Factor for Stroke in an African Population? Journal of Stroke & Cerebrovascular Diseases. 2013;22:E227–E233. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Ogeng'o JA, Cohen DL, Sayi JG, Matuja WB, Chande HM, Kitinya JN, Kimani JK, Friedland RP, Mori H, Kalaria RN. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly east Africans. Brain Pathol. 1996;6:101–107. doi: 10.1111/j.1750-3639.1996.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 100.Ogeng'o JA, Olabu BO, Mburu AN, Sinkeet SR. Pediatric stroke in an African country. J Pediatr Neurosci. 2010;5:22–24. doi: 10.4103/1817-1745.66676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ojagbemi A, Owolabi M, Bello T, Baiyewu O. Stroke severity predicts poststroke delirium and its association with dementia: Longitudinal observation from a low income setting. Journal of the Neurological Sciences. 2017;375:376–381. doi: 10.1016/j.jns.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oluyombo R, Akinwusi PO, Olamoyegun MA, Ayodele OE, Fawale MB, Okunola OO, Olanrewaju TO, Akinsola A. Clustering of cardiovascular risk factors in semiurban communities in south-western Nigeria. Cardiovasc J Afr. 2016;27:1–6. doi: 10.5830/CVJA-2016-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Onwuekwe IO, Ezeala-Adikaibe BA, Ohaegbulam SC, Chikani MC, Amuta J, Uloh HN. Stroke mimics - A study of CT images in Nigerian African stroke patients. Journal of Neurological Sciences-Turkish. 2008;25:143–149. [Google Scholar]
- 104.Owolabi M, Peprah E, Xu H, Akinyemi R, Tiwari HK, Irvin MR, Wahab KW, Arnett DK, Ovbiagele B. Advancing stroke genomic research in the age of Trans-Omics big data science: Emerging priorities and opportunities. J Neurol Sci. 2017;382:18–28. doi: 10.1016/j.jns.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Owolabi MO, Agunloye AM, Umeh EO, Akpa OM. Can common carotid intima media thickness serve as an indicator of both cardiovascular phenotype and risk among black Africans? Eur J Prev Cardiol. 2015;22:1442–1451. doi: 10.1177/2047487314547656. [DOI] [PubMed] [Google Scholar]
- 106.Owolabi MO, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, Tiwari H, Arulogun O, Akpalu A, Sarfo FS, Obiako R, Owolabi L, Sagoe K, Melikam S, Adeoye AM, Lackland D, Ovbiagele B. H.A.C. Members of the, The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26:S27–38. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owolabi MO, Akinyemi RO, Hurst S, Arulogun O, Olaniyan O, Gebregziabher M, Salako BL, Ovbiagele B. Tailored Hospital-based Risk Reduction to Impede Vascular Events After Stroke (THRIVES) study: qualitative phase protocol. Crit Pathw Cardiol. 2014;13:29–35. doi: 10.1097/HPC.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Owolabi MO, Akpa OM, Agunloye AM. Carotid IMT is more associated with stroke than risk calculators. Acta Neurol Scand. 2016;133:442–450. doi: 10.1111/ane.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Obiako R, Owolabi L, Ovbiagele B S. Team. H.A.C. as part of, Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health. 2018 doi: 10.1016/S2214-109X(18)30002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paddick SM, Kisoli A, Dotchin CL, Gray WK, Chaote P, Longdon A, Walker RW. Mortality rates in community-dwelling Tanzanians with dementia and mild cognitive impairment: a 4-year follow-up study. Age Ageing. 2015;44:636–641. doi: 10.1093/ageing/afv048. [DOI] [PubMed] [Google Scholar]
- 111.Paddick SM, Longdon A, Kisoli A, Gray WK, Dotchin CL, Jusabani A, Iqbal A, Hughes J, Teodorczuk A, Chaote P, Walker RW. The prevalence of dementia subtypes in rural Tanzania. Am J Geriatr Psychiatry. 2014;22:1613–1622. doi: 10.1016/j.jagp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Paddick SM, Longdon AR, Kisoli A, Dotchin C, Gray WK, Dewhurst F, Chaote P, Kalaria R, Jusabani AM, Walker R. Dementia prevalence estimates in sub-Saharan Africa: comparison of two diagnostic criteria. Glob Health Action. 2013;6:19646. doi: 10.3402/gha.v6i0.19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palm F, Urbanek C, Wolf J, Buggle F, Kleemann T, Hennerici MG, Inselmann G, Hagar M, Safer A, Becher H, Grau AJ. Etiology, risk factors and sex differences in ischemic stroke in the Ludwigshafen Stroke Study, a population-based stroke registry. Cerebrovasc Dis. 2012;33:69–75. doi: 10.1159/000333417. [DOI] [PubMed] [Google Scholar]
- 114.Pandian JD, William AG, Kate MP, Norrving B, Mensah GA, Davis S, Roth GA, Thrift AG, Kengne AP, Kissela BM, Yu C, Kim D, Rojas-Rueda D, Tirschwell DL, Abd-Allah F, Gankpe F, deVeber G, Hankey GJ, Jonas JB, Sheth KN, Dokova K, Mehndiratta MM, Geleijnse JM, Giroud M, Bejot Y, Sacco R, Sahathevan R, Hamadeh RR, Gillum R, Westerman R, Akinyemi RO, Barker-Collo S, Truelsen T, Caso V, Rajagopalan V, Venketasubramanian N, Vlassovi VV, Feigin VL. Strategies to Improve Stroke Care Services in Low- and Middle-Income Countries: A Systematic Review. Neuroepidemiology. 2017;49:45–61. doi: 10.1159/000479518. [DOI] [PubMed] [Google Scholar]
- 115.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with prestroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 116.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 117.Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997;28:785–792. doi: 10.1161/01.str.28.4.785. [DOI] [PubMed] [Google Scholar]
- 118.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75 e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 120.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 121.Ramlall S, Chipps J, Pillay BJ, Bhigjee AL. Mild cognitive impairment and dementia in a heterogeneous elderly population: prevalence and risk profile. Afr J Psychiatry (Johannesbg) 2013;16 doi: 10.4314/ajpsy.v16i6.58. [DOI] [PubMed] [Google Scholar]
- 122.Retief C, Schutte CM, Baker MK. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) S Afr Med J. 2009;99:461–465. [PubMed] [Google Scholar]
- 123.Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing. 2015;44:545–547. doi: 10.1093/ageing/afv043. [DOI] [PubMed] [Google Scholar]
- 125.Ruland S, Gorelick PB. Stroke in Black Americans. Curr Cardiol Rep. 2005;7:29–33. doi: 10.1007/s11886-005-0007-5. [DOI] [PubMed] [Google Scholar]
- 126.Rutten JW, Dauwerse HG, Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, Bernal-Quiros M, Lesnik Oberstein SA. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016;3:844–853. doi: 10.1002/acn3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sacco RL, Dong C. Declining stroke incidence and improving survival in US communities: evidence for success and future challenges. JAMA. 2014;312:237–238. doi: 10.1001/jama.2014.7693. [DOI] [PubMed] [Google Scholar]
- 128.Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens P B. Internationlal Society for Vascular, D. Cognitive. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samba H, Guerchet M, Ndamba-Bandzouzi B, Mbelesso P, Lacroix P, Dartigues JF, Preux PM. Dementia-associated mortality and its predictors among older adults in sub-Saharan Africa: results from a 2-year follow-up in Congo (the EPIDEMCA-FU study) Age Ageing. 2016;45:681–687. doi: 10.1093/ageing/afw097. [DOI] [PubMed] [Google Scholar]
- 130.Saposnik G, Caplan LR, Gonzalez LA, Baird A, Dashe J, Luraschi A, Llinas R, Lepera S, Linfante I, Chaves C, Kanis K, Sica RE, Rey RC. Differences in stroke subtypes among natives and caucasians in Boston and Buenos Aires. Stroke. 2000;31:2385–2389. doi: 10.1161/01.str.31.10.2385. [DOI] [PubMed] [Google Scholar]
- 131.Sarfo FS, Akassi J, Adamu S, Obese V, Ovbiagele B. Burden and Predictors of Poststroke Cognitive Impairment in a Sample of Ghanaian Stroke Survivors. Journal of Stroke & Cerebrovascular Diseases. 2017;26:2553–2562. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarfo FS, Akassi J, Awuah D, Adamu S, Nkyi C, Owolabi M, Ovbiagele B. Trends in stroke admission and mortality rates from 1983 to 2013 in central Ghana. Journal of the Neurological Sciences. 2015;357:240–245. doi: 10.1016/j.jns.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 133.Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke. 2003;34:2050–2059. doi: 10.1161/01.STR.0000079818.08343.8C. [DOI] [PubMed] [Google Scholar]
- 134.Shakir R, Davis S, Norrving B, Grisold W, Carroll WM, Feigin V, Hachinski V. Revising the ICD: stroke is a brain disease. Lancet. 2016;388:2475–2476. doi: 10.1016/S0140-6736(16)31850-5. [DOI] [PubMed] [Google Scholar]
- 135.Shobha N, Fang J, Hill MD. Do lacunar strokes benefit from thrombolysis? Evidence from the Registry of the Canadian Stroke Network. Int J Stroke. 2013;8(Suppl A100):45–49. doi: 10.1111/j.1747-4949.2012.00932.x. [DOI] [PubMed] [Google Scholar]
- 136.Siitonen M, Borjesson-Hanson A, Poyhonen M, Ora A, Pasanen P, Bras J, Kern S, Kern J, Andersen O, Stanescu H, Kleta R, Baumann M, Kalaria R, Kalimo H, Singleton A, Hardy J, Viitanen M, Myllykangas L, Guerreiro R. Multi-infarct dementia of Swedish type is caused by a 3'UTR mutation of COL4A1. Brain. 2017 doi: 10.1093/brain/awx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Silberberg D, Anand NP, Michels K, Kalaria RN. Brain and other nervous system disorders across the lifespan - global challenges and opportunities. Nature. 2015;527:S151–154. doi: 10.1038/nature16028. [DOI] [PubMed] [Google Scholar]
- 138.Singh A, Jenkins C, Calys-Tagoe B, Arulogun OS, Sarfo S, Ovbiagele B, Akpalu A, Melikam S, Uvere E, Owolabi MO. Stroke Investigative Research and Education Network: Public Outreach and Engagement. J Community Med Health Educ. 2017;7 doi: 10.4172/2161-0711.1000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, O'Brien J, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I, group V, Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 140.Skrobot OA, O'Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I, group V, Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017;13:624–633. doi: 10.1016/j.jalz.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 141.Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: an update. Am J Geriatr Psychiatry. 2011;19:99–103. doi: 10.1097/jgp.0b013e318202fc8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 143.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, Kotze MJ, Lema G, Moore JH, Mortensen H, Nyambo TB, Omar SA, Powell K, Pretorius GS, Smith MW, Thera MA, Wambebe C, Weber JL, Williams SM. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ueshima H. Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb. 2007;14:278–286. doi: 10.5551/jat.e529. [DOI] [PubMed] [Google Scholar]
- 146.Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. doi: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Verdura E, Herve D, Bergametti F, Jacquet C, Morvan T, Prieto-Morin C, Mackowiak A, Manchon E, Hosseini H, Cordonnier C, Girard-Buttaz I, Rosenstingl S, Hagel C, Kuhlenbaumer G, Leca-Radu E, Goux D, Fleming L, Van Agtmael T, Chabriat H, Chapon F, Tournier-Lasserve E. Disruption of a miR-29 binding site leading to COL4A1 upregulation causes pontine autosomal dominant microangiopathy with leukoencephalopathy. Ann Neurol. 2016;80:741–753. doi: 10.1002/ana.24782. [DOI] [PubMed] [Google Scholar]
- 148.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 149.Walker R, Whiting D, Unwin N, Mugusi F, Swai M, Aris E, Jusabani A, Kabadi G, Gray WK, Lewanga M, Alberti G. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. 2010;9:786–792. doi: 10.1016/S1474-4422(10)70144-7. [DOI] [PubMed] [Google Scholar]
- 150.Walker RW, Jusabani A, Aris E, Gray WK, Mitra D, Swai M. A prospective study of stroke sub-type from within an incident population in Tanzania. S Afr Med J. 2011;101:338–344. doi: 10.7196/samj.4511. [DOI] [PubMed] [Google Scholar]
- 151.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wightman EL, Haskell-Ramsay CF, Thompson KG, Blackwell JR, Winyard PG, Forster J, Jones AM, Kennedy DO. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–158. doi: 10.1016/j.physbeh.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 153.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 154.Williams AO, Loewenson RB, Lippert DM, Resch JA. Cerebral atherosclerosis and its relationship to selected diseases in Nigerians: a pathological study. Stroke. 1975;6:395–401. doi: 10.1161/01.str.6.4.395. [DOI] [PubMed] [Google Scholar]
- 155.Yamamoto Y, Craggs L, Baumann M, Kalimo H, Kalaria RN. Molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol Appl Neurobiol. 2011;37:94–113. doi: 10.1111/j.1365-2990.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 156.Zenebe G, Alemayehu M, Asmera J. Characteristics and outcomes of stroke at Tikur Anbessa Teaching Hospital, Ethiopia. Ethiop Med J. 2005;43:251–259. [PubMed] [Google Scholar]