Abstract
Fish consumption is an important route of exposure to persistent organic pollutants (POPs) in dolphins as well as humans. In order to assess the potential risks associated with these contaminants, 39 whole fish and 37 fillets from fish representing species consumed by dolphins and humans captured from Charleston Harbor and tributaries, South Carolina, USA, were measured for a suite of POPs. Polychlorinated biphenyls (PCBs) were the predominant contaminant with concentrations ranging from 5.02 to 232.20 ng/g in whole fish and5.42–131.95 ng/g in fillets (weight weight ww) followed by total organochlorine pesticides (OCPs) and polybrominated diphenyl ethers (PBDEs). Total POPs levels varied by location and species with general trends indicating significantly higher levels in fish from the Cooper (93.4 ng/g ww) and Ashley Rivers (56.2 ng/g ww) compared to Charleston Harbor (31.6 ng/g ww). Mullet and spot were found to have significantly higher PCBs, OCPs and total POPs, 2–3 times higher than red drum; mullet were also significantly higher in OCPs compared to seatrout. PCB concentrations in whole fish and fillets exceeded EPA human screening values for cancer risk in all fish sampled. For PCBs in fillets, all samples had values of maximum allowable meals per month that were less than the EPA, FDA guidelines for recommended fish meals per month, suggesting lower (more stringent) allowable fish meals per month. All fish exceeded PBDE wildlife values and all fish except two exceeded the level where 95% of the dolphin population would have tissue levels below the health effect threshold. Considering that POP concentrations in fish potentially consumed by humans exceed human health effect thresholds levels, consumption advisories should be considered as a prudent public health measure.
Keywords: Persistent organic pollutants (POPs), Charleston Harbor, Marine fish, Human risk assessment, Dolphin
1. Introduction
Fish consumption is an important route of exposure for Persistent Organic Pollutants (POPs) to species high on the food chain such as dolphins and humans. Due to their persistence, bioaccumulation potential and toxicity, many POPs remain a concern for coastal environmental and human health (Loganathan and Kannan, 1994). These toxic chemicals are relatively resistant to natural degradation in the environment and fish and other aquatic organisms accumulate these chemicals through the food chain. As a result, international regulation and mitigation actions have been put in place to regulate their usage (Stockholm Convention, 2014). While global POP levels are declining slowly in marine fish populations, variation in pollutant concentrations remains high, indicating ongoing consumer risk of exposure to POPs via contaminated fish exceeding U.S. Environmental Protection Agency (USEPA) screening values (Bonito et al., 2016). As such, monitoring fish tissue contamination allows detection of levels that may be harmful to consumers, thus informing actions and policies for protecting public health and the environment.
Many POPs are still present in the environment and consumption of contaminated food is the primary route of human exposure (Patandin et al., 1999). Of all food sources, fish can accumulate some of the highest POP levels (Kannan et al., 1995; Haug et al., 2010; Schecter et al., 2010a, 2010b). Fish constitute a major source of protein and it has been suggested that a large segment of the world’s population is exposed to POPs through seafood (Asplund et al., 1994; Schecter et al., 2010a, 2010b). Fish are an increasingly important part of the human diet and fish consumption has increased by about 30% in the United States (U.S.) over the last several decades (Loke et al., 2012). Seafood consumption in the U.S. had the largest increase in two decades, by nearly 1 pound per person in 2015, to an average of 15.5 pounds per year, or just over 4.75 ounces per week (http://www.st.nmfs.noaa.gov/commercial-fisheries/fus/fus15/index). Recreational fishermen sometimes eat large quantities of fish from a few local sources. Even where state consumption advisories are in place, anglers are often unaware of these advisories or choose to ignore them (Beehler et al., 2001; Niederdeppe et al., 2015). These anglers may be at greater risk from specific polluted areas and species of fish (Beehler et al., 2001; Toppe, 2016). Fish contaminant data by species for specific rivers and tributaries are necessary to provide risk analysis for local populations of recreational anglers.
Epidemiological studies have shown high blood levels of several organic contaminants in human populations such as those in Greenland that depend on local traditional diets of fish, and sea mammals (Deutch et al., 2006) and in the Great Lakes region of the US. The New York State Angler Cohort study of fish consumption and blood levels of POPs support the premise that long-term dietary consumption of contaminated fish by recreational anglers, even at comparatively low concentrations in the fish contribute significantly to body burden of POPs (Bloom et al., 2005). Human health effects are of concern with respect to the legacy POPs, such as PCBs and pesticides, and more contemporary chemicals like PBDEs (de Wit, 2002). The health benefits of eating fish have been well documented especially with omega-3 fatty acids (Daviglus et al., 2002; Mozaffarian and Rimm, 2006) including a lower risk of cancer among a cohort of Lake Ontario fish-eaters (Callahan et al., 2016). However, relatively fatty fish high in the food web also bioaccumulate contaminants and may pose a potential risk to human health. Thus, contaminant data on fish is an increasingly important element to inform public health decisions and potential health risks for local human and wildlife populations. One of the important factors that influence pollutant levels in fish is the variable distribution of pollutants in the environment as well as the biological and ecological characteristics of the fish. High variation in POP levels have been observed when examining fish depending upon where they were harvested. One such recent study found wide variation among yellowfin tuna on a global scale depending upon where they were captured (Nicklisch et al., 2017).
Health assessment studies of bottlenose dolphins in Charleston, South Carolina, have documented POP concentrations above established thresholds for concern, thus warranting additional investigation in this area (Fair et al., 2010). Some of the highest perfluorinated alkyl acids (PFAAs) and PBDEs found globally in marine mammals have been observed in dolphins from Charleston (Houde et al., 2005; Fair et al., 2010). As a long-lived apex predator which often exhibits a high degree of site fidelity, the bottlenose dolphin is a useful sentinel species for monitoring the health of the environment and signaling emerging public health issues (Bossart, 2011). Based on the high contaminant levels reported in Charleston dolphins, investigations have focused on their foodweb (Houde et al., 2006). Evidence suggests that Gullah/Geechee African American fishers, other recreational anglers and bottlenose dolphins in the Charleston Harbor area share similarities in the most common fish species consumed (Pate and McFee, 2012; Ellis, 2013; Perkinson et al., 2016). Since the Gullah/Geechee African American population participate in local subsistence fishing they may be a potentially vulnerable and susceptible group to pollutant exposure from consumption of seafood. Therefore, it is important to monitor the levels of POPs in fish species from this region.
In order to assess the potential health risks associated with these contaminants several species of fish were collected from the estuarine waters of Charleston and analyzed for PCBs, OCPs and PBDEs. Here, we measured POP levels in fish species commonly consumed by bottlenose dolphins in the Charleston Harbor estuarine area and the Gullah/Geechee anglers, focusing on fish species consumed by both. Our study objectives were to 1) investigate the levels and profiles of a suite of contaminants (PCBs, pesticides, PBDEs) in fish from the Charleston area; 2) evaluate fish size and geographic location related changes in contaminant levels and 3) assess the potential health-related risks of human and dolphin exposure through fish consumption.
2. Methods
2.1. Sample collection and preparation
Fish were collected at three sites in the Charleston Harbor (CH), Ashley River (AR) and Cooper River (CR) by the South Carolina Department of Natural Resources as part of their trammel net Inshore Fisheries monitoring program of estuarine fish (Arnott et al., 2010) or directed sampling. A total of 39 fish from the 3 sites (CH = 15; AR = 13; CR = 11) were collected for whole fish contaminants analysis and 37 fish (CH = 10; AR = 10; CR = 17) collected for fillet contaminant analysis during 2014 (Table 1). The following five known dolphin prey fish species (Pate and McFee, 2012) were collected: Atlantic croaker (Micropogonias undulatus); red drum (Sciaenops ocellatus); spot (Leiostomus xanthurus), spotted seatrout (Cynoscion nebulosus) and striped mullet (Mugil cephalus). These same 5 fish are also commonly caught and consumed by the Gullah-Geechee African American community and other recreational anglers. We also included one additional fish species, southern flounder (Paralichthys lethostigma), for sampling based on fish consumption questionnaire data from the Gullah-Geechee community (Ellis, 2013). Additional information collected included the total length and total weight of each fish and geographic coordinates. Each specimen was wrapped individually in heavy aluminum foil, placed in a polyethylene bag, sealed, and kept on ice for 3–6 h until frozen at −20 °C for sample preparation and analysis. Analyses of chemical contaminants were performed on tissue homogenate prepared from individual whole fish representing fish consumed by dolphins. For assessment of human consumption, individual skin-on fillets were analyzed. A fillet included the flesh tissue and skin from head to tail beginning at the mid-dorsal line including the belly flap. Filleting was conducted on cutting boards covered with heavy duty aluminum foil and changed between samples. Whole fish and fillet samples were ground using a Hobart Grinder (Elk Grove Village, IL). Before each specimen was processed, all equipment was thoroughly cleaned with detergent, rinsed in isopropanol and washed with distilled water.
Table 1.
Fish demographics across all sample and by whole fish or fillet. Categorical variables are reported as n (%) and continuous variables are reported as mean (SD).
| All Sample (n = 76) | Whole Fish (n = 39) | Fillets (n = 37) | |
|---|---|---|---|
| Species | |||
| Croaker | 7 (9.22) | 5 (12.8) | 2 (5.41) |
| Flounder | 9 (11.8) | 0 (0.00) | 9 (24.3) |
| Mullet | 18 (23.7) | 9 (23.1) | 9 (24.3) |
| Red Drum | 14 (18.4) | 9 (23.1) | 5 (13.5) |
| pot | 13 (17.1) | 7 (18.0) | 6 (16.2) |
| Seatrout | 15 (19.7) | 9 (23.1) | 6 (16.2) |
| Location | |||
| Charleston Harbor | 25 (30.9) | 15 (38.5) | 10 (27.0) |
| Ashley River | 23 (28.4) | 13 (33.3) | 10 (27.0) |
| Cooper River | 28 (36.8) | 11 (28.2) | 17 (46.0) |
| Length (mm) | |||
| Croaker | 207.6 (55.7) | 206.2 (39.0) | 211.0 (5.66) |
| Flounder | 392.6 (47.3) | 392.6 (47.3) | |
| Mullet | 259.7 (35.2) | 235.4 (31.7) | 283.0 (47.5) |
| Red Drum | 441.1 (94.6) | 464.3 (74.6) | 399.2 (120.6) |
| Spot | 231.7 (93.5) | 177.9 (39.8) | 294.5 (101.4) |
| Seatrout | 390.3 (62.4) | 412.0 (66.8) | 357.7 (40.5) |
| Weight (g) | |||
| Croaker | 129.4 (32.0) | 136.2 (30.1) | 112.5 (0.71) |
| Flounder | 757.6 (304.7) | - | 757.6 (304.7) |
| Mullet | 246.3 (48.1) | 267.1 (41.1) | 225.6 (47.5) |
| Red Drum | 1092.4 (735.6) | 1217.3 (212.0) | 867.4 (795.0) |
| Spot | 242.2 (252.8) | 122.1 (58.7) | 382.2 (325.0) |
| Seatrout | 527.3 (153.3) | 577.1 (134.7) | 452.5 (159.8) |
2.2. Analysis of PCBs, PBDEs and pesticides in fish samples
Samples from whole fish and fillet were analyzed for PCBs, PBDEs and OCPs as listed in Table 1. Total numbers of congeners were 121 for PCBs, 8 for PBDEs and 16 for OCPs (Table S1). Samples from individual fish (6–20 g ea) were used for the analysis of PCBs, PBDEs and OCPs. Methodological analysis details are provided in Supplemental Material 1. Analysis of PCBs, PBDEs and OCPs in fish samples was performed by GC-MS (GC7890 and MS5975 Agilent Technologies, Atlanta, GA) under electron ionization (EI) mode (Software: ChemStation).
2.3. Statistical analysis
Descriptive statistics were calculated for whole fish and fillet samples for biological variables (weight and length, Table S2) and for total PCBs, PBDEs, OCPs and total POP levels. The associations between contaminant load with fish length and percent lw of homogenized fish tissue for whole and fillet fish were evaluated using Pearson’s or Spearman’s rank correlation where appropriate.
2.3.1. Total contaminant load, capture location, and species
The associations between total PCB, PBDE, OCP, TIC, and Total POPs levels by fish wet weight (ww) or lipid weight (lw), by whole fish or fillet within geographic location (AR, CR, or CH), and by species were determined using a series of linear regression models. Comparisons of contaminant levels between geographic locations and between fish species were conducted using linear contrasts from the models. Contaminant levels were natural log transformed to meet model assumptions. Given the limited sample size, pairwise comparisons were considered for any model with an overall p < 0.20. Additionally, for pairwise comparisons we considered both the un-adjusted p-value and the Tukey’s HSD (honest significant difference) adjusted p-value. Due to the small sample size for each species within a geographic location, we did not consider models with additional covariates (e.g. fish length) or a location by species interaction. Thus, we did not conduct formal hypothesis testing to compare differences in contaminant loading for species within location or location by species. However, difference between species within location and differences between the same species across locations were examined graphically. Fish species examined in the current study were also grouped into feeding guilds based on feeding strategy and prey items to examine this factor in the accumulation of POPs.
2.3.2. Patterns in contaminant levels of all contaminants
We also developed heat maps of contaminant loading across the observed organic pollutants by ww or lw, and by whole fish or fillet, to identify possible patterns of contaminant loads by fish species and location. For all heat maps, contaminants were natural log transformed and then centered and scaled so that their relative magnitude would be similar. We used complete linkage clustering to generate the sample clusters for the heat maps.
2.3.3. Risk-based consumption advisory estimation
We used USEPA’s modified approach for estimating maximum allowable meal per month of fish for each fish species by capture location based on the carcinogenic effects of total PCBs (Hites et. al, 2004; USEPA, 2000a). This approach to estimate maximum allowable meals per month (CRmm) is based on the maximum acceptable individual risk level (ARL), consumer body weight in kg (BW), the cancer slope factor (CSF), the average days per month (Tap), meal size in kg (MS), and the concentration of the contaminant in the given fish sample (Cm). The EPA expression for estimating CRmm is:
For each fish sample we estimated the CRmm based on the observed levels of total PCBs in mg/kg ww. ARL was set at 10−5 mg/kg ww, consumer BW at 70 kg, Tap at 30.44 d/mo, and MS at 0.227 kg (equivalent to 8 oz). The CSF for total PCBs was set at 2.0 based on the conservative upper-bound (mg/kg ww)/day described in (USEPA, 2000a). The estimated CRmm for each sample was also qualitatively compared to the nutritional recommendation thresholds set by the Food and Drug Administration (FDA, 2018) and the USEPA joint fish consumption advice (USEPA, 2000a) which suggests consuming 340 g cooked fish per week and the American Heart Association recommendation to consume two 99 g servings of fish per week (AHA, 2016). A conversion factor of 1.33 was used to convert the suggested mass of cooked fish per week to the corresponding mass of uncooked fish (Minnesota Department of Health, 2016) and monthly recommended intake was estimated assuming an average of 4.33 weeks per month.
An important step toward understanding the persistence of POPs and their impacts on organisms is defining their interactions with the xenobiotic elimination systems. Congeners of organochlorine pesticides, PCBs and PBDEs were identified by Nicklisch et al. (2016) to inhibit P-glycoprotein (P-gp), which play a major role in the disposition of xenobiotics and cellular defense. As another indicator of risk, we also considered levels of Transporter Inhibiting Compounds (TICs), which include a subset of the POPs shown to interact P-gp which potentially reduces the ability to eliminate the contaminant from the body. Nicklisch et al. (2016) defined a set of 10 POPs that were inhibitory: DDT, DDD, DDE, BDE-47, BDE-100, PCB-146, PCB-170, PCB-187, dieldrin and endrin. Our study measured levels of 8 of these 10 compounds (dieldrin and endrin were not measured) thus, our TIC measure is the sum of the levels of the 8 observed compounds that we used to determine their environmental levels in our fish samples from Charleston.
3. Results
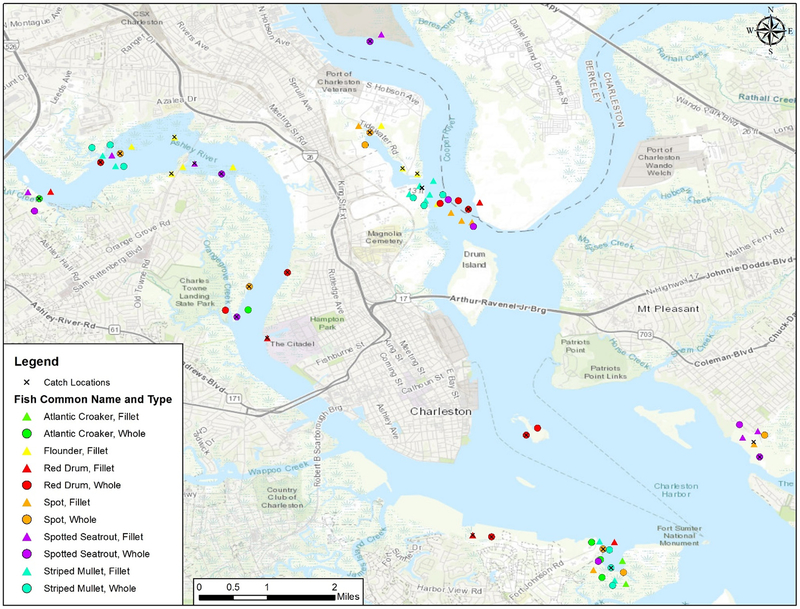
Data were collected from 39 whole fish and 37 fillets. Among whole fish, the number of fish from each capture location was similar with 15, 13 and 11 (38.5%, 33.3%, and 28.2%) of the samples coming from the Charleston Harbor, Ashley River, and Cooper River, respectively. Among fillets, 10 (27%) of the fish were captured in Charleston Harbor, 10 in the Ashley River, and 17 (46%) in the Cooper River. Both sample types were collected from croaker, mullet, red drum, spot and seatrout, whereas only fillets were collected from flounder. Fig. 1 shows the physical location of where each fish was captured by type (whole or fillet) and by species. Biological data of fish collected, including length and weight, are provided in Table S2.
Fig. 1.
Capture location for all study samples by type (whole or fillet) and by species (Atlantic croaker, red drum, spot, spotted seatrout, striped mullet, southern flounder). The study included 17 individual capture site (6 Charleston Harbor, 7 Ashley River, and 4 Cooper River sites) and these sites included multiple fish captures. The capture location for fish caught at the same site was perturbed to allow all fish to be visible on the map and the actual geographic location for each capture site is marked by a black X.
3.1. Association between total contaminant levels, % lipids and length
Initially, we examined the associations between fish tissue contaminant levels (total PCBs, total PDBEs, total OCPs, and total POPs) and percent fish weight as lipids and fish length. Also, Fig. S1 is a plot of % lipid and length by ng/g ww and lw of total POPs in whole fish.
3.1.1. Whole fish, contaminants per ww
When fish length was examined for associations with contaminant levels (total PCBs, PBDEs, OCPs, and total POPs) only one significant association was found. Total OCPs (ng/g ww) were negatively correlated with fish length (r = −0.42, p = 0.008). Length is negatively correlated with % lipids (rho = −0.49, p = 0.002). Total PCBs, OCPs, and total POPs (ng/g ww) were positively correlated with fish lipid content (% lipids), with correlation coefficients ranging from 0.39 to0.67 (p = 0.015, < 0.001, and 0.001 respectively). The strongest correlation with percent lipids was with total OCPs.
3.1.2. Whole fish, contaminants per lw
In whole fish samples, total PBDEs and total OCPs in ng/g lw were positively correlated with fish length (r = 0.33, p = 0.038; r = 0.35, p = 0.029, respectively).
3.1.3. Fillets, contaminants per ww
In fillets, percent lipids were also positively correlated with total PCBs, PBDEs, OCPs, and Total POPs in ng/g ww with correlations ranging between 0.42 and 0.54 (p = 0.002, 0.011, < 0.001, and <0.001 respectively). The strongest correlation was between percent lipids and total POPs though the correlation between percent lipids and total OCPs was similar. Fillet contaminant concentrations were not compared to length as the concentrations represent only the muscle tissue and not as relevant biologically as whole fish contaminant loads.
3.1.4. Fillets, contaminants per lw
In fillets, percent lipids were also negatively correlated with total PCBs, PBDEs, OCPs, and total POPs in ng/g ww with correlations ranging between −0.39 and −0.45 (p = 0.018, 0.009, 0.005, and 0.010 respectively). The strongest correlation was between percent lipids and total OCPs though all of the estimated correlations between the other contaminants and percent lipids were similar.
3.2. Associations between total contaminant levels, capture location and species
3.2.1. Whole fish, contaminants per ww
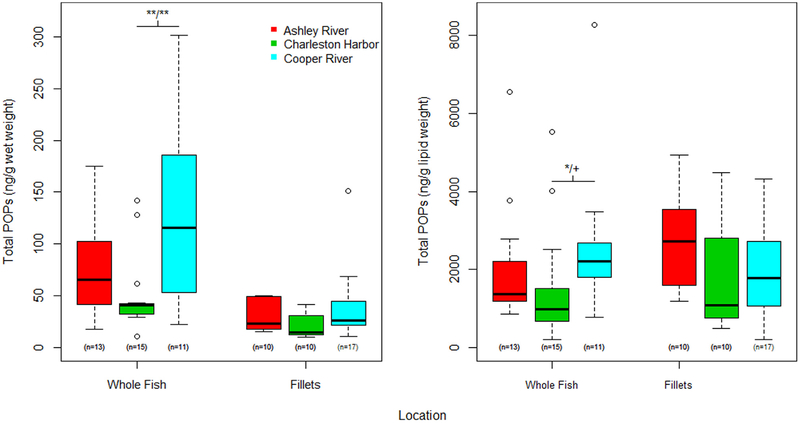
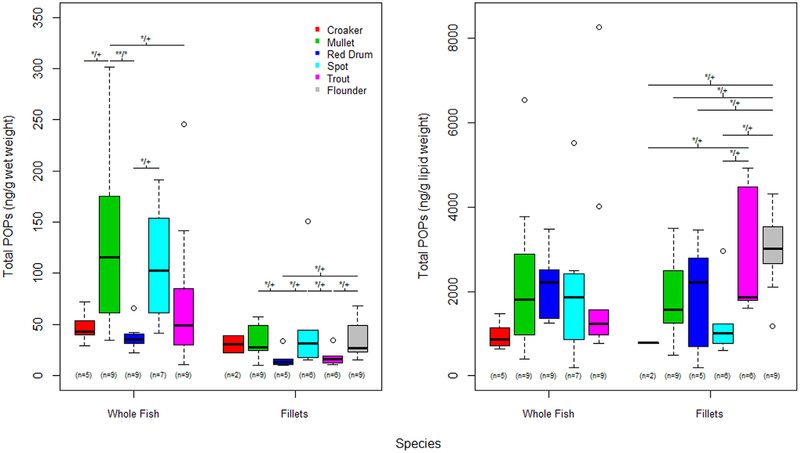
The overall summary of POPs is listed in Table 2; by location and sample type in Fig. 2 and by species and sample type in Fig. 3. There were differences in total PCBs, OCPs, total POPs, and TICs in ng/g ww by capture location in whole fish (ANOVA p = 0.019, 0.055, 0.014, and0.006 respectively). In pair-wise comparisons, fish captured in the Cooper River locations had significantly higher levels of each of these contaminants and these differences remained significant even after adjusting for multiple comparisons. There were also significant differences in total PCBs, OCPs, total POPs, and TICs in ng/g ww by species in whole fish samples (ANOVA p = 0.049, < 0.001, 0.010, and 0.034 respectively). Mullet and spot had significantly higher levels of PCBs, OCPs, total POPs, and TICs relative to red drum. These differences in pollutant class levels remained significant between mullet and red drum for all comparisons and for OCPs and total POPs between spot and red drum after adjusting for multiple comparisons. Additionally, mullet and spot had higher levels of total OCPs relative to seatrout, even after adjusting for multiple comparisons.
Table 2.
Mean (SD) contaminant levels in ng/g wet weight by capture location and by species. P-values at the top of each column are for one-way ANOVA of either location or species. Given the limited sample size, pairwise comparisons of all groups were evaluated if the p-value for the ANOVA model was p < 0.2.
| ng/g Wet Weight | |||||||
|---|---|---|---|---|---|---|---|
| Type | Group | n | PCB | PDBE | OCP | Total POPs | TIC |
| Location | p = 0.019 | p = 0.647 | p = 0.055 | p = 0.014 | p = 0.006 | ||
| Charleston Harbor | 15 | 31.6 (30.2) | 1.82 (1.34) | 16.5 (8.43) | 49.9 (36.1) | 36.8 (32.5) | |
| Cooper River | 11 | 93.4 (72.9)a,A | 1.92 (1.12) | 37.2 (35.3)a,A | 132.5 (92.9)a,A | 119.7 (89.9)a,A | |
| Ashley River | 13 | 56.2 (41.3) | 1.99 (0.94) | 17.4 (6.46) | 75.6 (47.0)a | 64.1 (43.6) | |
| Whole | Species | p = 0.049 | p = 0.356 | p < 0.001 | p = 0.010 | p = 0.034 | |
| Mullet | 9 | 87.1 (57.9) | 2.35 (1.13) | 39.7 (36.1) | 129.1 (84.1) | 112.8 (82.0) | |
| Spot | 7 | 77.1 (50.0) | 2.25 (1.07) | 30.0 (15.1) | 109.3 (60.6) | 93.4 (61.1) | |
| Croaker | 5 | 28.3 (11.0) | 1.49 (0.38) | 17.7 (6.30)b | 47.5 (16.3)b | 35.8 (14.6) | |
| Red Drum | 9 | 23.9 (8.93)b,c,B | 1.41 (0.41) | 12.3 (3.26)b,c,B,C | 37.6 (12.1)b,c,B,C | 28.6 (10.7)b,c,B | |
| Seatrout | 9 | 61.3 (72.9) | 1.92 (1.72) | 13.1 (6.11)b,c,B,C | 76.3 (75.0) | 66.4 (73.2) | |
| Location | p = 0.066 | p = 0.122 | p = 0.590 | p = 0.114 | p = 0.058 | ||
| Charleston Harbor | 10 | 14.4 (10.5) | 0.53 (0.47) | 5.94 (1.91) | 20.9 (12.0) | 15.1 (10.7) | |
| Cooper River | 17 | 29.6 (29.4)a,A | 0.77 (0.55) | 7.65 (3.98) | 38.0 (33.3)a,A | 32.1 (31.2)a,A | |
| Ashley River | 10 | 21.5 (12.5) | 0.93 (0.72)a | 7.48 (3.66) | 29.9 (15.1) | 22.5 (13.5) | |
| Fillet | Species | p = 0.163 | p = 0.053 | p = 0.009 | p = 0.082 | p = 0.131 | |
| Mullet | 9 | 23.8 (13.2) | 0.84 (0.66) | 7.93 (3.08) | 32.6 (16.1) | 26.7 (15.1) | |
| Spot | 6 | 39.7 (46.4) | 0.99 (0.87) | 8.09 (4.65) | 48.8 (51.7) | 41.5 (48.4) | |
| Croaker | 2 | 22.6 (12.3) | 0.85 (0.68) | 7.07 (1.15) | 30.5 (11.8) | 23.1 (12.2) | |
| Red Drum | 5 | 12.7 (9.50)c | 0.26 (0.10)b,c,d,B,D | 3.76 (0.49)b,c,d,B,C,D | 16.7 (9.71)b,c,d | 12.9 (9.53) | |
| Seatrout | 6 | 12.5 (7.06)c | 0.50 (0.29) | 5.18 (2.08)b,d | 18.2 (8.61)c,d | 13.0 (6.92)c | |
| Flounder | 9 | 25.0 (15.5) | 0.92 (0.48) | 8.91 (3.52) | 34.8 (17.8) | 27.1 (18.1) | |
Unadjusted p < 0.05 compared to Charleston Harbor;
Adjusted p < 0.10 compared to Charleston Harbor.
Unadjusted p < 0.05 compared to Mullet;
Adjusted p < 0.10 compared to Mullet.
Unadjusted p < 0.05 compared to Spot;
Adjusted p < 0.10 compared to Spot.
Unadjusted p < 0.05 compared to Flounder;
Adjusted p < 0.10 compared to Flounder.
Fig. 2.
Distribution of Total POPs in ng/g wet weight and ng/g lipid weight by location and sample type (whole fish vs. fillet). Boxes represent the 25th 50th and 75th percentiles for the observed distribution, the whiskers represent 1.5 × inner-quartile range (IQR), and points outside the whiskers represent any observed value that is less than or greater than 1.5 × IQR. The number of samples per group are shown below each box. Note, lines above the boxes indicate significant relationships; with **/** above the line represent the observed range for the unadjusted p-value/Tukey HSD adjusted p-value where ** is p < 0.01, * is p < 0.05, + is p not significant (only used for adjusted p-values).
Fig. 3.
Distribution of Total POPs in ng/g wet weight and ng/g lipid weight by species and sample type (whole fish vs. fillet). Boxes represent the 25th 50th and 75th percentiles for the observed distribution, the whiskers represent 1.5 × inner-quartile range (IQR), and points outside the whiskers represent any observed value that is less than or greater than 1.5 × IQR. The number of samples per group are shown below each box. Note, lines above the boxes indicate significant relationships; with **/** above the line represent the observed range for the unadjusted p-value/Tukey HSD adjusted p-value where ** is p < 0.01, * is p < 0.05, + is p not significant (only used for adjusted p-values).
3.2.2. Fillets, contaminants per ww
Mean levels of all contaminant classes in fillets (ng/g ww), capture location, and species are provided in Table 2. Total PCBs, PBDEs, total POPs, and TICs in ng/g ww differed by capture location in fillets (ANOVA p = 0.066, 0.122, 0.114, and 0.058 respectively) as shown in Table 2 and Fig. 2. Similar to the findings in whole fish, fish captured in the Cooper River locations had significantly higher levels of PCBs, total POPs and TICs and these differences remained significant even after adjusting for multiple comparisons. Fish collected in the Ashley River had higher levels of PBDEs relative to fish captured in the harbor, though this difference was not significant after adjusting for multiple comparisons. There were also differences in total PCBs, PBDEs, OCPs, total POPs, and TICs in ng/g ww by species in fillet samples (ANOVA p = 163, 0.053, 0.009, 0.082, and 0.131 respectively). Mullet, spot, and flounder, all had significantly higher levels of PBDEs, OCPs, and total POPs relative to red drum. These differences remained significant between mullet or flounder and red drum for PBDEs and OCPs and for OCPs between spot and red drum after adjusting for multiple comparisons. Mullet and spot also had higher levels of total OCPs relative to seatrout, though this difference was not significant after adjusting for multiple comparisons. Spot also had significantly higher levels of OCPs relative to red drum and seatrout and higher levels of TICs relative to red drum, though these differences were not significant after adjusting for multiple comparisons.
3.2.3. Whole fish, contaminants per lw
Total PCBs and total POPs in ng/g lipid differed by capture location in whole fish (ANOVA p = 0.028 and 0.049 respectively; Table 3). Specifically, whole fish samples from the Charleston Harbor had significantly lower levels of PCBs and total POPs relative to samples captured in the Cooper River and significantly lower PCBs relative to fish from the Ashley River. There was also notable difference in total PBDEs and OCPs by species (ANOVA p = 0.069 and 0.159 respectively). Red drum had significantly higher levels of PBDEs than croaker, mullet and spot, though this difference remained statistically significant only for levels in red drum relative to mullet after adjusting for multiple comparisons. Additionally, red drum had significantly higher levels of OCPs relative to croaker and seatrout, though this difference was not significant after adjusting for multiple comparisons.
Table 3.
Mean (SD) contaminant levels in ng/g lipid weight by capture location and by species. P-values at the top of each column are for one-way ANOVA of either location or species. Given the limited sample size, pairwise comparisons of all groups were evaluated if the p-value for the ANOVA model was p < 0.2.
| ng/g Lipid Weight | ||||||
|---|---|---|---|---|---|---|
| Type | Group | n | PCB | PDBE | OCP | Total POPs |
| Location | p = 0.028 | p = 0.736 | p = 0.316 | p = 0.049 | ||
| Charleston Harbor | 15 | 1059.4 (1101.9) | 49.2 (49.7) | 409.6 (385.3) | 1518.3 (1464.7) | |
| Cooper River | 11 | 2031.0 (2009.4)a,A | 42.9 (40.8) | 539.1 (306.9) | 2613.3 (17.2)a,A | |
| Ashley River | 13 | 1680.4 (1474.1)a,A | 44.6 (27.7) | 374.5 (174.5) | 2088.5 (1566.7) | |
| Whole | Species | p = 0.497 | p = 0.069 | p = 0.159 | p = 0.454 | |
| Mullet | 9 | 1870.1 (1796.4) | 31.0 (25.0) | 413.3 (251.9) | 2314.5 (1907.7) | |
| Spot | 7 | 1472.7 (1202.5) | 44.0 (62.4) | 503.8 (556.2) | 2020.5 (1773.1) | |
| Croaker | 5 | 628.9 (188.1) | 27.2 (17.4) | 306.2 (169.5) | 962.3 (340.1) | |
| Red Drum | 9 | 1453.4 (458.9) | 66.8 (24.7)b,c,e,B | 597.3 (256.1)e,f | 2117.4 (746.9) | |
| Seatrout | 9 | 1857.0 (2428.0) | 51.7 (57.8) | 310.0 (86.4) | 2218.8 (2476.3) | |
| Location | p = 0.089 | p = 0.036 | p = 0.264 | p = 0.136 | ||
| Charleston Harbor | 10 | 1064.3 (796.6) | 37.2 (30.5) | 645.7 (654.7) | 1747.2 (1384.2) | |
| Cooper River | 17 | 1428.6 (834.9) | 45.6 (34.0) | 462.5 (326.5) | 1936.7 (1158.3) | |
| Ashley River | 10 | 1873.6 (870.2)a,A | 73.5 (30.3)a,h,A,H | 743.7 (445.0) | 2689.8 (1155.8)a,A | |
| Fillet | Species | p = 0.073 | p = 0.006 | p = 0.031 | p = 0.031 | |
| Mullet | 9 | 1269.6 (774.8)d | 41.6 (25.8) | 439.2 (243.0) | 1750.4 (1000.6) | |
| Spot | 6 | 950.8 (660.4)d | 26.9 (25.4) | 283.1 (206.8) | 1260.7 (861.9) | |
| Croaker | 2 | 563.5 (106.6)d | 19.5 (10.1) | 195.2 (99.2) | 778.2 (17.5) | |
| Red Drum | 5 | 1316.9 (971.2)d | 37.4 (36.9) | 517.9 (425.5) | 1872.3 (1387.2) | |
| Seatrout | 6 | 1785.4 (964.3) | 69.6 (33.8)c,e,g | 898.8 (746.8)c,e | 2753.8 (1522.3)c,e | |
| Flounder | 9 | 2012.2 (757.0) | 78.0 (29.5)b,c,e,g,C,G | 858.1 (410.7)c,e,g,C | 2948.3 (920.7)b,c,e,g,C | |
Unadjusted p < 0.05 compared to Charleston Harbor;
Adjusted p < 0.10 compared to Charleston Harbor.
Unadjusted p < 0.05 compared to Mullet;
Adjusted p < 0.10 compared to Mullet.
Unadjusted p < 0.05 compared to Spot;
Adjusted p < 0.10 compared to Spot.
Unadjusted p < 0.05 compared to Flounder; D: Adjusted p < 0.10 compared to Flounder.
Unadjusted p < 0.05 compared to Croaker; E: Adjusted p < 0.10 compared to Croaker.
Unadjusted p < 0.05 compared to Trout; F: Adjusted p < 0.10 compared to Trout.
Unadjusted p < 0.05 compared to Red Drum;
Adjusted p < 0.10 compared to Red Drum.
Unadjusted p < 0.05 compared to Cooper River;
Adjusted p < 0.10 compared to Cooper River.
3.2.4. Fillets, contaminants per lw
Total PCBs, PBDEs, and total POPs in ng/g ww differed by capture location in fillets (ANOVA p = 0.089, 0.036, and 0.136 respectively; Table 3). Specifically, samples from the Charleston Harbor had significantly lower levels of PCBs, PBDEs, and total POPs relative to samples from the Ashley River. Samples from the Cooper River also had lower levels of PBDEs relative to samples from the Ashley River. Levels of total PCBs, PBDEs, OCPs, and total POPs also differed by species (ANOVA p = 0.073, 0.006, 0.031, and 0.031 respectively). Flounder had significantly higher levels of PCBs, PBDEs, OCPs, and total POPs relative to croaker, mullet, spot, and red drum. Differences between flounder and spot in PBDEs, OCPs, and total POPs and differences between flounder and red drum in PBDEs remained significant after adjusting for multiple comparisons. Seatrout also had higher levels of PBDEs, OCPs, and total POPs relative to spot and croaker, though this difference was not significant after adjusting for multiple comparisons. Mean levels of all contaminant classes in ng/g lw by fish type (whole or fillet), capture location, and species are provided in Table 2.
3.3. Transport inhibiting compounds (TICs), ecological variables
Generally mean levels of TICs were higher in whole fish compared to fillets, however, fillets had greater ranges (6.37–277.6 ng/g) than whole fish (5.74–131.9 ng/g). There was a significant association between contaminant load in both whole fish and fillet and TICs for location (Table 2). TIC levels in whole fish were significantly higher for mullet and spot. TIC levels in fillets were highest for spot, mullet and flounder. Cooper River fish had significantly higher mean TIC values than the other two locations, Ashley River and Charleston Harbor. Whole fish red drum had significantly lower levels of TICs when compared to all other species even after adjusted for multiple comparisons.
Fish species examined in the current study were grouped into feeding guilds based on feeding strategy and prey items (Table 4). Species were grouped into herbivore, low predator and mid-predator, as well as habitat use. After adjusting for multiple comparisons, mullet(9.18% lipid) and spot (9.80% lipid) whole fish had significantly higher lipid concentrations than red drum (2.44% lipid, p = 0.007 and 0.005 respectively). Mullet and spot were found to have significantly higher PCBs, OCPs and total POPs than red drum and additionally, mullet were significantly higher in OCPs compared to seatrout.
Table 4.
Mean contaminant levels (ng/g wet weight) and percent lipids in whole fish for species grouped into feeding guilds based on feeding strategy and prey items.
| Feeding Guild | Fish | PCBs | PBDEs | OCPs | Total POPs | Lipid % |
|---|---|---|---|---|---|---|
| Mid-predator | Red Drum1 | 42.6a | 1.67 | 12.7a,A | 56.9a,A | 3.83a,A |
| Nekton (crustaceans, fish) | Seatrout1 | |||||
| Low-predator | Croaker2 | 56.8 | 1.94 | 24.8a,A | 83.6 | 8.46a,A |
| Benthic infauna/epifauna | Spot2 | |||||
| Herbivore | Mullet3 | 87.1 | 2.35 | 39.7 | 129.1 | 9.18 |
| Detritivores Planktivores Herbivores |
p < 0.05 compared to herbivores unadjusted.
p < 0.05 compared to herbivores adjusted.
Mississippi Sound and the Gulf of Mexico. Gulf Research Reports, 6: 145–152; https://drive.google.com/open?id=1lA4fRQ19UtDSzhcsLFw-h7SJVxUlvvxO.
Smith et al. (1984).
3.4. Individual contaminants and congeners
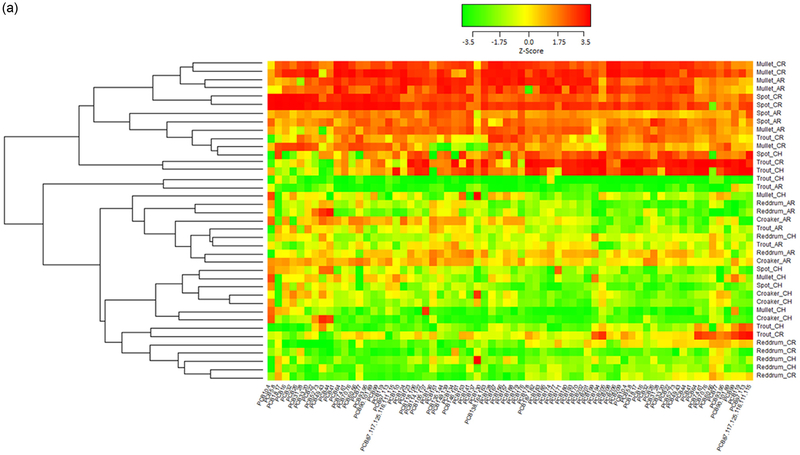
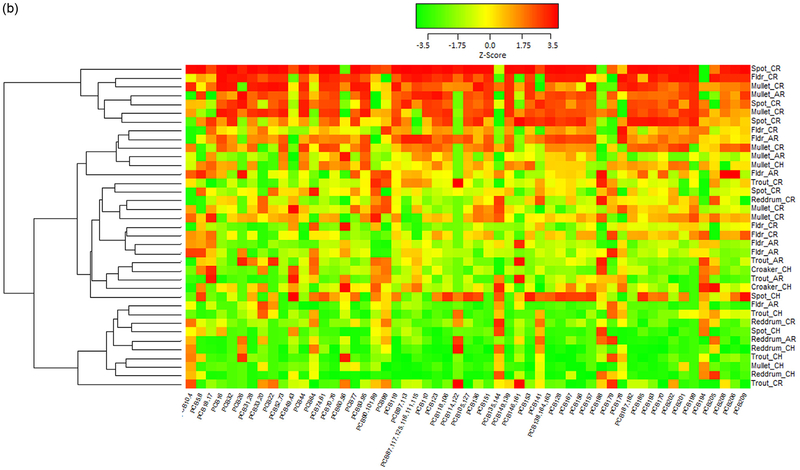
Contaminant levels described above are based on a sum of the observed levels of different contaminants within each contaminant class. In order to identify possible patterns of contaminant loadings in the different samples, we used complete linkage clustering to cluster samples according to the levels of the individual contaminants for all total POPs, for PCBs only, for PBDEs only, and for OCPs only in each fish by ww and lw in fillets or whole fish. Only contaminants with detectable levels in at least 75% of samples were considered for clustering. Although, we considered all 4 contaminant groups, only the clustering of PCBs suggested patterns in the distribution of individual contaminants by sample location and species.
The heat map of PCBs in ng/g ww in whole fish (Fig. 4A) indicate that mullet and spot generally have the highest PCB load and red drum have the lowest across all PCBs. Additionally, fish captured in the Cooper and Ashley Rivers have elevated levels of all PCBs relative to a majority of the other fish. However, red drum and more generally, most fish captured in the Charleston harbor had lower levels of all PCBs compared to the average. Fig S2A heat map show that mullet and spot in the Ashley and Cooper river sites generally have the highest PBDE for all but BDE183 and BDE209. Those fish with high levels of BDE183 (denoted by red color) are predominantly in the harbor and many of them are red drum. The heat map depicting PCBs by ww in fillets (Fig. 4B) shows a similar pattern to what was observed in whole fish with mullet having higher than average levels and fish caught in the Cooper River tending to have higher levels. However, the pattern is not as distinct as it is in the whole fish samples. The PBDE pattern in fillets (ng/g ww, Fig. S2B) is similar to whole fish with mullet and spot generally have the highest PBDE load.
Fig. 4.
A. Heat map of PCBs wet weight ng/g in whole fish by fish species (Atlantic croaker, red drum, spot, spotted sea trout, striped mullet) and location (CH=Charleston Harbor, CR=Cooper River, AR=Ashley River). All PCB values were normalized to ensure all PCBs were on the same scale. Red indicates levels higher than the average across all fish, yellow indicates levels near the mean, and green indicates levels that are lower than average. The dendogram on the left side of the heat map show the clusters found using complete linkage clustering and Euclidean distance between points as the distance metric. Note, only contaminants with detectable levels in at least 75% of samples were considered for clustering. B. Heat map of PCBs wet weight ng/g in fillets by fish species (Atlantic croaker, red drum, spot, spotted sea trout, striped mullet, flounder) and location (CH=Charleston Harbor, CR=Cooper River, AR=Ashley River). All PCB values were normalized to ensure all PCBs were on the same scale. Red indicates levels higher than the average across all fish, yellow indicates levels near the mean, and green indicates levels that are lower than average. The dendogram on the left side of the heat map show the clusters found using complete linkage clustering and Euclidean distance between points as the distance metric. Note, only contaminants with detectable levels in at least 75% of samples were considered for clustering.
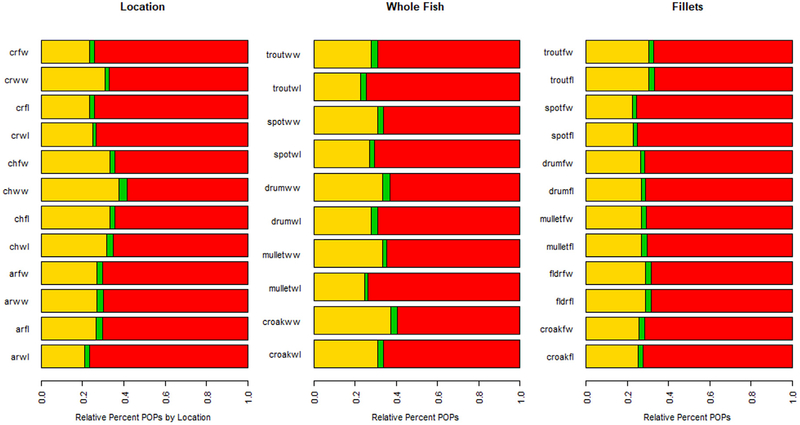
The relative percentage of individual POPs for fish species by location and ww and lw are illustrated in Fig. 5. PCB was the dominant contaminant in all species with concentrations ranging from 5.02 to 232.20 ng/g in whole fish and 5.42–131.95 ng/g in fillets on a ww basis. Generally, lw data had higher relative percentages of PCBs. The relative percent of PCB concentrations in whole fish (ww) ranged from 60% in croaker to 80% in seatrout. The second highest contaminant class, OCPs, comprised from 17% in seatrout to 37% in croaker; PBDE was the least dominant with less variation ranging from 2% to 3% for all species. (Table 2). In comparing individual contaminants between whole fish to fillet ww, whole fish consistently had higher total POP levels than fillets. Comparisons of total PCB levels between whole and fillet fish ranged from 1.3 times higher in whole croaker to 4.9 in whole seatrout; OCP levels ranged from 2.5 time higher in whole croaker to 5 times higher in whole mullet; PBDE levels ranged from 1.8 times higher in whole croaker to 5.4 times higher in whole red drum compared to fillets for these species. General trends show that fish collected from Charleston Harbor have lower percentages of PCBs compared to fish from the Cooper and Ashley Rivers.
Fig. 5.
Relative % of POPs (yellow = OCP, green = PBDE, red = PCB) by wet weight and lipid weight and by fillet or whole fish for location and by fish species. The first panel is by location (CR = Cooper River, CH = Charleston Harbor, AR= Ashley River), wet or lipid weight (wet = wet weight, lip = lipid weight), and by whole fish or fillt (whl = whole fish, fil = fillet). The second and third panels are by Species (Croak = croaker, Mull = mullet, Drum = reddrum, Spot = spot, and Trout = sea trout) and wet or lipid weight.
Among 121 PCB congeners analyzed in this study, the major contributors in whole fish (ww) are: 8% for 90/101/89, 6% for 153% and 5% for 187/182, 118/106%, 180% and 4.5% for 99%, and 4.3% for 138/164/163. The top pesticides in whole fish were total DDTs (55%); followed by BHCs at 22%, CHLs at 20%, mirex at 2% and HCB at 1% (Table 5). This pattern was different in fillets with 55% BHCs, 23% DDTs, 11% for CHL and also for mirex and 1% for HCB. In whole fish, mean percentage of total DDE was 72.8% while in fillets, mean percentage was 32.0%.
Table 5.
Mean (SD) (ranges) of pesticide concentrations in ng/g wet weight measured in whole fish and fillet.
| DDTsa | BHCsb | CHLsc | HCBd | Mirex | |
|---|---|---|---|---|---|
| Whole Fish | 12.1 (19.0) (0.65–107.6) | 4.86 (3.10) (1.05–15.98) | 4.53 (3.40) (0.47–15.0) | 0.20 (0.17) (0.03–1.01) | 0.35 (0.45) (0.01–1.81) |
| Fillet | 1.64 (2.31) (0.05–10.3) | 3.84 (1.53) (1.82–8.38) | 0.78 (0.86) (0.05–3.56) | 0.08 (0.08) (0.01–0.37) | 0.73 (0.51) (0.01–2.25) |
Total DDT = Sum of o.p-DDE, p.p-DDE, o.p-DDD, o.p-DDT/p.p-DDD, and p.p-DDT.
Total BHC (Benzenehexachloride) = Sum of α, β, γ, δ BHC.
Total CHL (Chlordanes) - Sum of a-, c-chlordane, cis-nonachlor, trans-nonachlor, oxychordane.
HCB = Hexachlorobenzene.
Mean PBDE concentration in whole fish was 1.15 ng/g ww (range0.13–3.74) and in fillets 0.41 ng/g ww (range 0.02–1.17). Of the 8 measured PBDEs congeners in whole fish, BDE 47 accounted for 60% of the total PBDEs. Mean concentrations of other PBDE congeners in whole fish were as follows: BDE 100 > 99–209 > 154 > 153. The mean BDE 47 level was 4.2 times higher than next highest mean BDE level summarized in all species and locations. In fillets, BDE 47 comprised over 55% of the total PBDEs followed by BDE 100 > 99 > 153 > 154 > 209 > 28 > 154. The mean BDE 47 level was 4.6 times higher than next highest mean BDE level summarized across all species and locations. Total PBDE concentrations (ww) were not different for fish from any of the locations averaging 1.91 ng/g in whole fish and 0.74 in fillets (Table 2). When whole fish were compared on a lw basis, significantly higher total PBDE levels were found in flounder compared to mullet and red drum. For fillets, red drum had significantly higher PBDE levels than mullet on a lipid basis. For whole fish on a ww basis, seatrout and red drum has significantly lower total PBDEs than mullet and spot while in fillets only red drum were significantly lower than mullet and spot. There were also several differences in PBDE congener profiles among fish species and location. Examining locations, BDE 183 and 209 were higher in fish from Charleston Harbor and Ashley River, respectively. In whole fish, higher levels of BDE 28 occurred in mullet/spot than red drum and BDE 99 were higher in spot than red drum and seatrout. In fillets (ww) BDE 100 and BDE 153 were both higher in the Cooper River than Charleston Harbor. BDE 28 was higher in fillets from flounder and spot than red drum; BDE 47 was higher in spot than in red drum and seatrout; BDE 100 was higher in flounder and spot compared to red drum and mullet was higher than red drum.
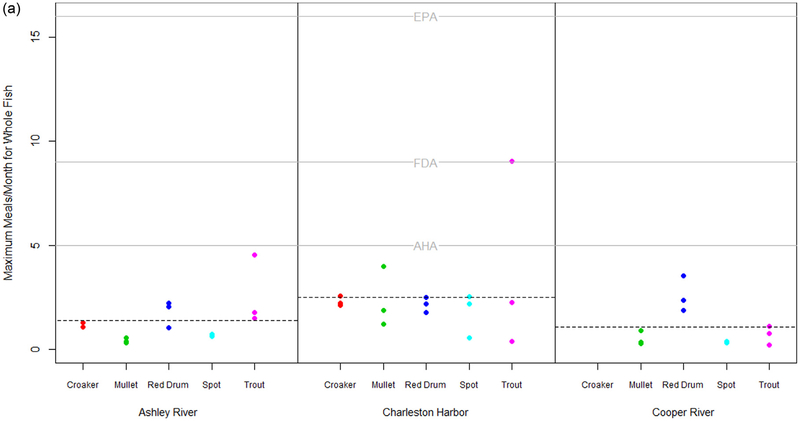
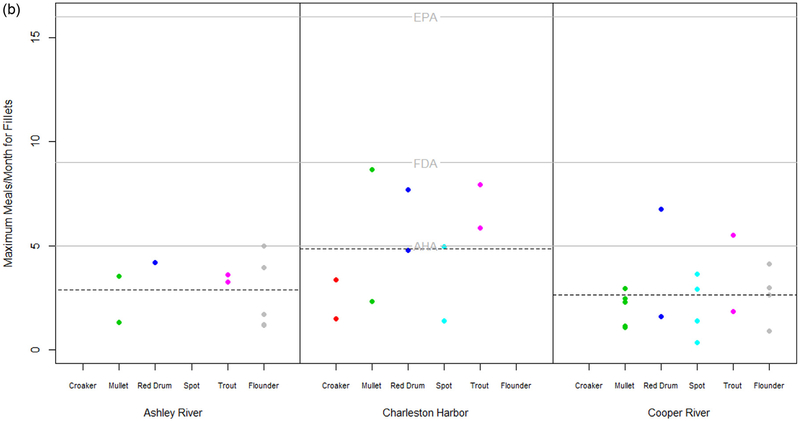
3.5. Evaluation of risk based consumption for humans and marine mammals
In order to better understand the implications of contaminant levels in fish captured in the estuaries of Charleston, SC, we estimated the maximum allowable meals per month based on PCB levels in whole fish and fillets using the EPA method and then compared these estimated values to the guidelines for the recommended minimum monthly fish consumption levels provided by the AHA (2016) ≥ 5 meals/mo) and the FDA (2018) (9 meals/mo and to the USEPA threshold for unrestricted fish consumption (> 16 meals/mo). The estimated maximum allowable meals per month based on PCB levels in whole fish for all fish species and capture locations ranged between 0.2 and 9 meals per month (Fig. 6A). The average number of allowable meals per month across fish species was higher for the Charleston Harbor relative to both the Ashley and Cooper Rivers. The estimated maximum allowable meals per month based on PCB levels in fillets for all fish species and capture locations ranged between 0.4 and 8.6 meals per month (Fig. 6B). Similar to what was observed in whole fish, the average number of allowable meals per month across fish species was higher for the Charleston Harbor relative to both the Ashley and Cooper Rivers. All estimated values of maximum meals per month for fillets exceeded the USEPA (2000a) and FDA (2018) guidelines for recommended fish meals per month, suggesting the consumption of fish meals per month should be decreased for fish from the Ashley and Cooper Rivers. Only 6 fillets out of 37 samples were above the AHA’s (2016) recommended guideline of 5 meals per month. These 6 samples included 2 croakers, 1 red drum, 1 mullet from Charleston Harbor and 1 croaker and 1 red drum from Cooper River.
Fig. 6.
a. Estimated maximum allowable meals per month based on PCB in ng/g wet weight in whole fish using the EPAs method (cite EPA document). Grey solid lines represent the recommended minimum monthly fish consumption levels provided by the American Heart Association (AHA) and the Food and Administration (FDA) and the US EPAs threshold for unrestricted fish consumption. Dashed lines represent the mean estimated maximum allowable meals across fish captures within a location. b. Estimated maximum allowable meals per month based on PCB in ng/g wet weight in fillets using the EPAs method (cite EPA document). Grey solid lines represent the recommended minimum monthly fish consumption levels provided by the American Heart Association (AHA) and the Food and Administration (FDA) and the US EPAs threshold for unrestricted fish consumption. Dashed lines represent the mean estimated maximum allowable meals across fish captures within a location.
We also compared our data to EPA screening values for recreational fishers based on concentrations for non-cancer health endpoints and assumption that consumption over a lifetime of four 8-oz meals per month would not generate a chronic, systemic health risk (USEPA, 2000b). All fish were below the EPA screening values for DDT(0.117 ppm), mirex (0.8 ppm), lindane (0.0307 ppm), HCB(0.025 ppm), although 3 out of 39 whole fish exceeded the CHL(0.114 ppm) value. When comparison to the PBDE fish tissue advisory level based on a non-cancer risk of 210 μg/kg ww (California EPA, 2011), none of the fish, either as fillets or whole, in our study exceed the California human health value. In contrast, all fish exceeded the European Union (EU) Environmental Quality Standards (EQS) PBDE level of 0.0085 μg/kg (European Union, 2013). Due to the lack of PBDE screening guidelines for marine mammals, we compared our data to wildlife screening values for mink at 21 μg/kg ww (California EPA, 2011) and kestrel 8.7 μg/kg (Environment Canada, 2013). Using these values as a proxy for dolphins, whole fish PBDE concentrations in 95% of our samples exceed the wildlife value for kestrels and 80% of the samples exceed the value for mink.
4. Discussion
Our study is the first study to report POP levels in fish from Charleston, SC and risks for humans and marine mammals from fish consumption. Even within a small geographic area, pollutants in fish varied significantly by location of capture and species. General trends in total POPs concentrations indicated significantly higher levels in fish from the Cooper (93.4 ng/g ww) and Ashley Rivers (56.2 ng/g ww) compared to fish from the Charleston Harbor (31.6 ng/g ww). In particular, fish from the Cooper River had significantly higher levels of all contaminants other than PBDEs which were highest in the Ashley River. This was observed in both whole fish and fillets. Intuitively, one might assume that the levels of pollutants in fish from a small geographic area would have less variation than studies with larger global scales and thus, few differences might occur. POP levels in yellowfin tuna sampled globally varied significantly among sites by more than 36-fold, highlighting that geographic difference is important to address with implications for assessment of health risks (Nicklisch et al., 2017). However, a review of studies measuring POPs in a wide variety of marine fish consumed by humans found that while some contaminants varied spatially regional trends were less distinct and confounded by finer scale factors such as trophic level and habitat type (Bonito et al., 2016). Thus, the ability to detect spatial differences in contaminant levels decreased as spatial scale increased. Geographic variation is an important factor when assessing health risks connected to fish consumption given the patchy distribution of contaminants in the aquatic environment. Studies investigating pollutant levels in fish have suggested that seafood captured from industrialized regions have higher levels (Ueno et al., 2003; Lu et al., 2017; Batt et al., 2017). In the Charleston metropolitan area, there are numerous industrial sites along the Cooper and Ashley Rivers including two EPA Superfund sites for coal tar and heavy metals, (EPA. National Priorities List. United States Environmental Protection Agency https://www.epa.gov/superfund/superfund-national-priorities-list-npl accessed 2/18/18). Many of the legacy contaminants such as PCBs and DDTs were banned decades ago in the US and are now widely distributed in the environment. Emerging chemicals such as PBDEs are not regulated thus, it is difficult to determine sources. Higher POP concentrations are known to occur in urban areas which could be from industrial and chemical use and manufacturing, but also from waste water treatment plant discharges, a potential point source of PCB and PBDE contamination (Samara et al., 2006). Highest concentrations of PBDEs in fish have been found in areas draining urbanized watersheds (Johnson and Olson, 2001). It is not surprising that higher concentrations of contaminants were found in the urbanized Cooper and Ashley Rivers. Also, PBDE levels were higher in Charleston dolphins that frequented the more urbanized/industrialized Cooper Subbasin compared to those in an adjacent estuary (Adams et al., 2014). Limited published POPs data exist for fish species in the Charleston estuarine area and no risk assessment analysis has been performed. In modeling dolphin PCB accumulation, Hickie et al. (2013) found higher PCB concentrations in fish from the inner Charleston Harbor, Ashley River, and Cooper River compared with other estuaries to the north and south of Charleston. Generally, the concentrations of POPs found in fish in our study were comparable to values reported in other studies, with higher levels of PCBs in fish from the tributaries of Charleston Harbor, although values were not as high as levels found in some other regions of the world (Fairey et al., 1997; Kannan et al., 1995).
4.1. Fish species — biological and ecological characteristics
Contaminant concentration varied by species as well. In whole fish, mullet and spot were found to have significantly higher levels of all contaminant classes (PCBs, OCPs and total POPs) than red drum and mullet were higher in OCPs than seatrout. In fillets on a ww basis, mullet, spot and flounder were significantly higher than red drum in PBDE, OCP, total POPs. When fillets were compared as lw, flounder had significantly higher PBDE, OCP, total POPs than spot. For PBDEs, higher levels were generally found in species with higher fat content such as mullet and spot. When whole fish were examined on a ww basis, this trend was present. However, lw comparisons indicated higher levels in red drum for whole fish and in seatrout in fillets. It should also be noted that due to the small sample size for each species within a geographic location, our data and therefore analysis/interpretation has some limitations as to whether the differences found are conclusive specifically to location or species or the combination of the two.
Fish length were positively associated with PBDE and OCP concentrations but not PCBs (lw). Previous studies have shown positive correlations between fish length and contaminant concentration (Fairey et al., 1997; Ikemoto et al., 2008; Blocksom et al., 2010) although these relationships are not consistent in all studies and for all POPs. No correlation was found between PCBs and length in five Baltic fish species, however, DDT was related to length in cod (Szlinder-Richert, 2009) while and in another study both PBDE and PCBs were positively correlated with fish length (Manchester-Neesvig et al., 2001). The environment in which a fish spends the majority of its time can potentially affect POP accumulation as well as its trophic level. Often fish consumption advisories use species characteristics to characterize pollutant concentrations with expectations to find higher levels in fish based on trophic position and habitat type. Bioaccumulation, increasing pollutant levels with increasing trophic levels are determined not only on the food web but also the chemical and species examined and in marine fishes these patterns may not be as clear as terrestrial food webs (Bonito et al., 2016). Feeding habitat also plays a role in the intake and accumulation of POPs. Since POPs are hydrophobic they absorb to sediments and thus, would be more available to benthic species than pelagic species. Mullet and spot were found to have significantly higher PCBs, OCPs and total POPs than red drum and additionally, mullet were significantly higher in OCPs compared to seatrout. Striped mullet was found to have the highest level of all contaminants measured. Striped mullet are detritivores and primarily feed on soft mud and other detritus (Cardona et al., 2015) and are also known to be significant forging fish for commercial fisheries as they are an important prey for upper-level piscivores (Wenner et al., 1990). As such, they are in close proximity to sediments and accumulate greater amounts of POPs. Species with higher lipid amounts also tend to have higher contaminants as observed in the present study with mullet and spot having the highest mean lipid percentage of all species, 9.1% and 9.8%, respectively. Since PCBs are lipophilic they tend to accumulate in fatty tissues and organs (USEPA, 2009). A more mid-level species, red drum, with only 2.4% lipid had significantly lower levels of these contaminants compared to mullet. Similar to our study, Blocksom et al. (2010) found that OCPs and PBDE concentrations were also positively related to fish with higher lipid composition. When fish were grouped into feeding guilds based on feeding strategy, the herbivore fish, mullet, had significantly higher levels of OCPs compared to both mid-level (red drum, sea trout) and low-level predators (croaker, spot) (Table 4). PCBs were detected in all the fish samples collected from the 500 sampling sites in one of the largest fish contaminant monitoring efforts in the U.S., The National Lake Fish Tissue Study (USEPA, 2009). Concentrations in bottom dwellers were generally higher in PCB and other POPs than levels detected in predators similar to findings in our study, although they measured only fillets for predators and whole bodies for bottom dwellers. Many biological and ecological variables can influence POP concentrations and bioaccumulation (Borga et al., 2004) and fish movement patterns and residence time in contaminated areas are important determinants of tissue contaminant levels. Tagging and acoustic tracking studies have shown some differences in movement patterns between the species we studied (Wenner et al., 1990; Craig et al., 2015; SCDNR Inshore Fisheries Section, unpublished data). Spotted seatrout and sexually immature red drum (TL < ~800 mm) typically remain within localized estuarine areas (< 1–2 km) year-round (Wenner et al., 1990). By contrast, striped mullet and southern flounder can migrate hundreds of kms. However, in the case of southern flounder, migrations are generally associated with winter spawning activity in large, sexually mature individuals (for females > ~400 mm; Midway and Scharf, 2012; Craig et al., 2015). Immature fish are thought to reside in the same estuary until they mature, and during summer months southern flounder of all sizes often stay within very restricted areas (several kms), exposing them at the same time to localized contaminants. Spot and Atlantic croaker are seasonal inhabitants of SC estuaries, moving to into offshore waters during winter months.
4.2. Risk assessment – human and wildlife health
Humans represent one of the top receptors for aquatic contaminants and public health concerns relate to consumption of fish with high levels of contaminants. In our study the toxicity hazard is dominated by PCBs. PCBs, chemicals manufactured and utilized extensively by industry, were banned in the U.S. in 1979 because of evidence that they accumulate in the environment and can pose human health risks (ATSDR, 2000). While PCB concentrations were lower in fillets than whole fish, more favorable for human consumption, but overall fillets had high levels of POPs. More so, the higher PCB levels in whole fish is disturbing for wildlife, such as dolphins, since they are piscivorous species and these fish represent their primary food. An individually-based (IB) model to predict PCB concentration in dolphins was developed by Hickie et al. (2013) for the Charleston dolphin population. The bioaccumulation model estimated that dietary PCB concentrations not exceed 5 ng/g to reach a condition where 95% of the population would have tissue levels below the health effect threshold. In the current study, all whole fish except for two, a mullet and seatrout from Charleston Harbor, exceeded the 5 ng/g threshold PCB levels. The highest PCB levels in whole fish were 33 times higher than the 5.1 ng/g recommended level. Therefore, the current concentrations of POPs in fish in the Charleston area continue to put the wild dolphin population at risk.
The risks of exposure to POP pollutants are a combination of the toxicity of the contaminant, the concentration in fish tissues and consumption rates. We estimated the maximum allowable meals per month for each sample based on the USEPA approach and compared these estimated maximum allowable meals to the USEPA threshold for unrestricted fish consumption, the AHA (2016) and FDA (2018) guidelines for minimum monthly fish consumption. Although the estimated number of allowable meals per month was higher in Charleston Harbor compared to the two rivers, the maximum allowable meals per month based on PCB levels in fillets for all fish species and capture locations ranged between 0.4 and 8.6 meals per month. Most importantly, all estimated values of maximum allowable meals per month for fillets were less than the EPA and FDA (2018) guidelines for recommended fish meals per month. This suggests that recommendations should be made for advisories for lower (more stringent) allowable fish meals per month. Only 6 fillets out of 37 samples were above the AHA (2016) recommended guideline of 5 meals per month. All estimated values of maximum meals per month for whole fish were less than the EPA guidelines and all but one sample were below all three thresholds, suggesting that more stringent fish consumption guidelines (lower number of maximum meals per month) should be recommended.
The EPA methods used were developed to control health risks by providing risk-based consumption advice regarding contaminated fish and these were based on estimates of potential cancer risks. Other methods may provide consumption advice based on non-cancer endpoints such as immune/endocrine and neurobehavior. Recommendations based on these can occur at lower levels than those used in cancer effects, however, quantitative risk and threshold levels are not yet established (Carpenter et al., 2002). The above recommendations for fish in our study was based on PCBs which was the predominant contaminant, however, many other contaminants can be present in these fish, some which we measured with available screening values. It should also be noted that other contaminants with potential harmful effects such as mercury were not analyzed in this study. Besides PCBs, screening values for several other contaminants for recreational fishers based on concentrations for non-cancer health endpoints (USEPA, 2000a). Applying these to our data, all fish were below the EPA screening values for DDT, mirex, lindane and HCB, although 3 out of 39 whole fish exceeded the CHL value. The EPA values are more conservative than the FDA (2018) action levels since they consider health risks of local consumers and vulnerable populations (i.e., recreational, tribal, subsistence) that may consume larger quantities of fish than the general population. Fish consumption advisories recommending that at-risk groups limit consumption of fish have been issued by U.S. federal and state agencies that are concerned about exposure to toxicants in fish, such as mercury and PCBs (USEPA, 2009).
The fish PCB concentrations reported in this study are comparable to levels found in fish from other regions in the US such as the coast of Georgia (Sajwan et al., 2008) and mid-continental rivers (Blocksom et al., 2010). Typically, the most abundant PCB congeners are hexa-138 and 153 and penta-PCBs 101 and 118 which is in accordance with findings in our study (Szlinder-Richert et al., 2009). PCB 153 is highly persistent and is not metabolized, as such it bioaccumulates in animal and human tissue (Eisler and Belisle, 1996). Also, two congeners CB138 and CB153 (coeluting isomers) accounted for 44% of total blubber PCBs in Charleston dolphins (Fair et al., 2010).
Generally, the levels of PBDE in fish from Charleston SC are among the reported low average range of values (Staskal et al., 2008; Quinete et al., 2011) compared to the more highly exposed fish like salmonids from Lake Michigan (Manchester-Neesvig et al., 2001). Total PBDE concentrations in whole fish and fillets did not differ between location of fish or species. Further, the lack of differences in species specific differences in PBDE congener profiles detected in fish in our study reflect an average contamination profiles which nonpoint sources are likely to be the main contributors. It is common to find non-specific sources related to PBDE levels (Dodder et al., 2002) although specific sources may be evident in some areas (Mariussen et al., 2008). As found in other studies, BDE 47, the tetrabromo diphenyl ether, had the highest concentrations in whole fish and fillets with levels 4-folds higher than the next highest congener. Since BDE-47 biomagnifies more readily than the more halogenated congeners, it is generally found at high concentrations in fish tissues as confirmed by other studies (Hale et al., 2001; Covaci et al., 2005). PBDE 47 was the dominant congener followed by 99 and 100, similar to findings reported for other U.S. systems (Brown et al., 2006; Batt et al., 2017). PBDEs are used as flame retardants in plastics, textile coatings, and polyurethane foams. Like PCBs, they are hydrophobic and lipophilic, tend to bioaccumulate in tissue, and biomagnify in the food web (Darnerud et al., 2001). PBDEs represent a potential environmental threat since they are not regulated in the U.S. and adverse health effects have reported developmental, neurological and endocrine effects (Darnerud et al., 2001; de Wit, 2002).
While several guidelines are available to assess the hazardous risk to human health from many contaminants, less data exists for PBDEs and limited reference doses (RfDs) are available for PBDE congeners. California EPA published a fish tissue advisory level based on non-cancer risk of 210 μg/kg ww (California EPA, 2011). None of the fish, either as fillets or whole, in our study exceed the California human health value. A more conservative standard for PBDEs developed by the European Union (EU) Environmental Quality Standards (EQS) is0.0085 μg/kg (European Union, 2013). Using the EU EQS, all of our fish exceed this value. Similarly, in a study by Lu et al. (2017) fish samples (mean value of 9.35 μg/kg; range 2.30–24.47 μg/kg) from the River Thames, UK, were several orders of magnitude higher than the EU EQS. When Eljarrata and Barceló (2017) compared published data on PBDEs in fish around the world, PBDE levels clearly exceeded the established EQS, with 25% of fish samples exceeding up to ten thousand times. They concluded that while levels of pollution by PBDEs will decrease over the next years due to the ban in their use, however, is not expected that this decrease will reach the EQS values by the end of 2021 and new strategies are needed to minimize impacts. While there are no PBDE guidelines for marine mammals, available wildlife screening values exist for mink at 21 μg/kg ww (California EPA, 2011) and kestrel8.7 μg/kg (Environment Canada, 2013). Using these values as a proxy for dolphins, whole fish PBDE concentrations in 95% of our samples exceed the wildlife value for kestrels and 80% of the samples exceed the value for mink.
As another indicator of risk, results from the TIC values, calculated using specific POPs shown to be inhibitors of transported proteins, reinforce the important role of geographic location in the overall pollutant levels in fish. The close relationship between TICs and location demonstrated the Cooper River as the area with the highest TIC values, twice that of the Ashley River and three times that of Charleston Harbor. TIC levels in whole fish were significantly higher for mullet and spot. TIC levels in fillets were highest for spot, mullet and flounder. These species are frequently consumed by recreational anglers, especially the Gullah-Geechee fishers. Chronic POP exposures could potentially affect the cell protective mechanisms by altering P-gp drug transporter molecules, an important cellular defense in humans (Gottesman et al., 2002). Results from Nicklisch et al. (2016) reported that P-gp binds some POPs but are relatively ineffective against their bioaccumulation. In our study the environmental levels of TICs in fish from different sites provided insight as to how these levels varied both spatially and within species. Similar to Nicklisch et al. (2017) the most contaminated fish in our study also had higher TIC levels. TICs is one method that reflects real world pollutant mixtures since some POP congeners could inhibit important cell defense proteins, thus, enhancing the accumulation of chemicals that would otherwise be eliminated. Therefore, high levels of TICs might enhance the accumulation of these chemicals.
Balancing the health benefits and risks of eating fish rely on information regarding the specific fish and shellfish species consumed and the frequency and amount of consumption (Domingo, 2016). The health benefits of eating fish have been well described having high-quality protein, low in saturated fats with other nutrients such as vitamin D, selenium, and iodine and particularly the primary dietary source of n-3 fatty acids (Harper et al., 2001; Oken et al., 2012). These beneficial effects have supported recommendations from organizations as the AHA to eat fish twice weekly. However, studies over the last several years have shown that the environmental contaminants in fish and shellfish can also indicate a risk for the health of some consumers. The high variability in contaminant concentration even within the same species makes it difficult to predict contaminant levels in a species. Oken et al. (2012) emphasizes that there is a relative lack of information toward integrating the health, ecological, and economic impacts of different fish choices and the challenges of providing clear and simple guidance. Certainly, in order to balance the health benefits and risks of regular fish consumption information on pollutants in local fish is paramount.
Some measures can be taken to lower exposures. The fillet samples in this study included skin and removal of skin and trimming fat is recommended to decrease levels and to broil, bake or poach rather than fry (SCDHEC, 2018). Generally, high concentrations of POPs occur near urban areas in near coastal environments, and fish in less urbanized more open ocean should have lower POP levels. The species of fish should be considered and selecting fish with lower amounts of lipid generally will have lower POP levels. Currently, there is no South Carolina fish consumption advisories issued for any of the fish in our study in estuarine or marine waters for either PCB or Hg (SCDHEC, 2018). Our study suggests that specific advisories may be warranted for women and children and restrictions for species in specific bodies of water. To provide for a more accurate assessment of risks, it is necessary to establish specific fish consumption for the local population and effective risk management. The residents of the Charleston Harbor area and Sea Islands include populations that depend extensively on fishing for their livelihood as well as their primary protein source. Epidemiology and seafood contamination studies are urgently needed to be completed to assess the health impacts of exposure to POPs along this coast. In particular, an assessment of exposure to Chemicals of Emerging Concern (CECs) should be performed in regard to establishing associated trends and potential health risks. Diet and seafood in particular, are major sources of PFASs and PBDEs, two emerging CECs that act as endocrine disruptors and immune-modulators (Falandysz et al., 2006; Haug et al., 2010; Holzer et al., 2011). Subsistence fisherman and those who consume seafood have higher body burdens of these contaminants (Haug et al., 2010; Holzer et al., 2011; Bloom et al., 2009; Kamen et al., 2012). The African American Gullah population of Coastal Carolina have elevated levels of PFASs and PBDEs, which has been associated with markers of autoimmunity (Miller et al., 2012). Similar to other African American communities, Gullah women are disproportionately affected by systemic lupus erythematosus (Lim et al., 2014). With local seafood consumption being a dietary staple, potential pollutant contamination of the seafood is of high concern to the health of the Gullah community (Spruill et al., 2013; Kamen et al., 2012). In parallel, common bottlenose dolphins (Tursiops truncatus) resident to coastal estuarine areas of Charleston with high site fidelity are among marine mammals with the highest levels of PBDEs and PFASs and associated immune and other detrimental health effects, highlighting the hazardous nature of these chemicals (Fair et al., 2007, 2013; Houde et al., 2005). Although we know dolphins’ resident to Charleston accumulate extremely high levels of these chemicals we know little about exposures in humans living adjacent to these areas, and particularly those consuming local seafood, and in the context of alterations being brought about increased POPs bioavailability. We suspect that elevated contaminant levels in coastal seafood will give rise to increased contaminant burdens in humans who consume coastal seafood. Studies are needed to examine body burden of these POPs in humans and the fish species consumed (exposure science), integrating biomarkers of exposure and health effects (effects), and modeling exposure routes via fish consumption (risk assessment).
5. Conclusion
Our study found POP concentrations in fish potentially consumed by humans and wildlife (dolphins) exceed human health and wildlife values. While the risk/benefit assessment is complicated, consumption of several species of fish including in the Charleston Harbor and its tributaries may pose risks as several contaminants were identified as potential chemicals of concern. The PCB concentrations in whole fish and fillets exceeded human screening values for cancer risk in all fish sampled. Thus, there is a need to conduct more studies on fish in areas that are fished by recreational and subsistence consumers, screening-level risk assessments with further studies on contaminant sources and mitigation measures for a cleaner environment. In the meantime, consumption advisories should be considered as a prudent public health measure.
Supplementary Material
Acknowledgements
We would like to thank Bill Roumillat and the SCDNR Inshore Fisheries Section for sample collection, Philip Wolf and Mikala Randich for spatial mapping, Jamelle Ellis, and research support funding from the Medical University of South Carolina (Dr. Vena), South Carolina Clinical & Translational Research Institute, Medical University of South Carolina’s CTSA, NIH/NCRR Grant Number UL1RR029882 (Dr. Wolf) and SCDNR Funding: National Marine Fisheries Service RecFIN grant; South Carolina Saltwater Recreational Fishing License funds.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has as its purpose an interest to cause the advertised product to be used or purchased because of this publication.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2018.08.001.
References
- Adams J, Speakman T, Zolman E, Wirth E, Mitchum G, Bossart G, Fair P, 2014. The relationship between land use and emerging and legacy contaminants in an apex predator, the bottlenose dolphin (Tursiops truncatus), from two adjacent estuarine watersheds. Environ. Res 135, 346. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2000. Toxicological Profile for Polychlorinated Biphenyls. U.S. Department of Health and Human Services, Public Health Service, Atlanta, Georgia. 〈http://www.atsdr.cdc.gov/toxprofiles/tp17.pdf〉. [PubMed] [Google Scholar]
- AHA (American Heart Association), 2016. Fish and Omega-3 fatty acids. 〈http://www.heart.org/HEARTORG/HealthyEating/HealthyDietGoals/Fish-and-Omega-3-FattyAcids_UCM_303248〉 (Accessed 23 March 2018).
- Arnott SA, Roumillat WA, Archambault JA, Wenner CA, Gerhard JI, Darden TL, Denson MR, 2010. Spatial synchrony and temporal dynamics of juvenile red drum (Sciaenops ocellatus) populations in South Carolina, USA. Mar. Ecol. Prog. Ser 415, 221–236. [Google Scholar]
- Asplund L, Svensson BG, Nilsson A, Eriksson U, Jansson B, Jensen S, Wideqvist U, Skerfving S, 1994. Polychlorinated –biphenyls, 1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane (p,p’-DDT) and 1,1,-dichloro-2,2,-bis(chlorophenyl)-ethylene (p,p’-DDE) in human plasma related to fish consumption. Arch. Environ. Health 49, 477–486. [DOI] [PubMed] [Google Scholar]
- Batt AL, Wathen JB, Lazorcha JM, Olsen AR, Kincaid TM, 2017. Statistical survey of persistent organic pollutants, risk estimations to humans and wildlife through consumption of fish from U.S. rivers. Environ. Sci. Technol 51, 3021–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehler Gregory P., McGuinness Bridget M., Vena JE, 2001. Polluted fish, sources of knowledge, and the perception of risk, contextualizing African American anglers’ sport fishing practices. Hum. Organ 60.3, 288–297. [Google Scholar]
- Blocksom KA, Walters DM, Jicha TM, Lazorchak JM, Angradi TR, Bolgrien DW, 2010. Persistent organic pollutants in fish tissue in the mid-continental great rivers of the United States. Sci. Total Environ 408, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Bloom M, Vena JE, Moysich K, Swanson M, Olson J, 2005. Profiles of orthopolychlorinated biphenyl congeners, dichlorodiphenyldichloroethylene, hexachlorobenzene, and mirex among male Lake Ontario sportfish consumers: the New York State Angler cohort study. Environ. Res 97, 178–194. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Vena JE, Olson JR, Kostyniak PJ, 2009. Assessment of polychlorinated biphenyl congeners, thyroid stimulating hormone, and free thyroxine among New York state anglers. Int. J. Hyg. Environ. Health 212, 599–611. [DOI] [PubMed] [Google Scholar]
- Bonito LT, Hamdoun A, Sandin SA, 2016. Evaluation of the global impacts of mitigation on persistent, bioaccumulative and toxic pollutants in marine fish. Peer J. 4, e1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borga K, Fisk AT, Hoekstra PF, Muir DCG, 2004. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs. Environ. Toxicol. Chem 23, 2367–2385. [DOI] [PubMed] [Google Scholar]
- Bossart GD, 2011. Marine mammals as sentinel species for oceans and human health.Vet. Pathol 48, 676–690. [DOI] [PubMed] [Google Scholar]
- Brown FK, Winkler J, Visita P, Dhaliwaj J, Petreas M, 2006. Levels of PBDEs, PCDDs, PCDFs and coplanar PCBs in edible fish from California coastal waters. Chemosphere 70, 276–286. [DOI] [PubMed] [Google Scholar]
- California EPA, 2011. Development of Fish Contaminant Goals and Advisory Tissue Levels for Common Contaminants in California Sport Fish: Polybrominated Diphenyl Ethers (PBDEs). Office of Environmental Health Hazard Assessment, Oakland, CA: https://oehha.ca.gov/fish/report/development-fish-contaminant-goals-and-advisory-tissue-levels-common-contaminants. [Google Scholar]
- Callahan CL, Vena JE, Green J, Swanson M, Mu L, Bonner MR, 2016.Consumption of Lake Ontario sport fish and the incidence of colorectal cancer in the New York state Angler cohort study (NYSACS). Environ. Res 154, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona L, 2015. Food and feeding of Mugilidae. In: Crosetti D, Blaber S (Eds.), Biology, Ecology and Culture of Grey Mullet (Mugilidae). CRC Press, London. [Google Scholar]
- Carpenter DO, Acaro K, Spink DC, 2002. Understanding the human health effects of chemical mixtures. Environ. Health Perspect 110 (Suppl. 1), 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Bervoets L, Hoff P, Voorspoels S, Voets J, Van Campenhout K, Blust R, Schepens P, 2005. Polybrominatediphenyl ethers (PBDE) in freshwater mussels and fish from Flanders, Belgium. J. Environ. Monit 7, 132–136. [DOI] [PubMed] [Google Scholar]
- Craig JK, Smith WE, Scharf FS, Monaghan JP, 2015. Estuarine residency and migration of southern flounder inferred from conventional tag returns at multiple spatial scales. Mar. Coast. Fish.: Dyn. Manag. Ecosyst. Sci 7, 450–463. [Google Scholar]
- Darnerud PO, Gunnar S, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M, 2001. Polybrominated diphenyl Ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect 109 (Suppl. 1), 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus M, Sheeeshka J, Murkin E, 2002. Health benefits from eating fish. Comments Toxicol 8, 345–374. [Google Scholar]
- Deutch B, Dyerberg J, Pedersen HS, Asmund G, Møller P, Hansen JC, 2006. Dietary composition and contaminants in north Greenland, in the 1970s and 2004. Sci. Total Environ 370, 372–381. [DOI] [PubMed] [Google Scholar]
- Dodder NG, Strandberg B, Hites RA, 2002. Concentrations and spatial variations of polybrominated diphenyl ethers and several organochlorine compounds in fishes from the northeastern United States. Environ. Sci. Technol 36, 146–151. [DOI] [PubMed] [Google Scholar]
- Domingo JL, 2016. Nutrients and chemical pollutants in fish and shellfish. balancing health benefits and risks of regular fish consumption. Crit. Rev. Food Sci. Nutr 56, 979–988. [DOI] [PubMed] [Google Scholar]
- Eisler R, Belisle AA, 1996. Planar PCB Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review. Contaminant Hazard Reviews Report No. 31. U.S. Department of the Interior, National Biological Service, Washington, D.C. [Google Scholar]
- Eljarrata E, Barceló D, 2017. How do measured PBDE and HCBD levels in river fish compare to the European environmental quality Standards? Environ. Res 160, 203–211. [DOI] [PubMed] [Google Scholar]
- Ellis JH, 2013. Fishing and Consumption Patterns in the Gullah/Geechee Sea Island Population (Ph.D. dissertation). Environmental Health Sciences, Norman J. Arnold School of Public Health, University of South Carolina. [Google Scholar]
- Environment Canada. Canadian Environmental Protection Act, 1999 Federal Environmental Quality Guidelines Polybrominated Diphenyl Ethers (PBDEs), 2013. 〈http://www.ec.gc.ca/ese-ees/05DF7A37-60FF-403F-BB37-0CC697DBD9A3/FEQG_PBDE_EN.pdf〉.
- European Union 2013. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. OJ L226. 〈http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?Uri=OJ:L:2013:226:0001:0017:EN〉 PDF (Accessed 07/2018).
- Fair PA, Mitchum G, Hulsey TC, Adams J, Zolman E, McFee W, Wirth E, Bossart GD, 2007. Polybrominated diphenyl ethers (PBDEs) in blubber of free-ranging bottlenose dolphins (Tursiops truncatus) from two southeast Atlantic estuarine areas. Arch. Environ. Contam. Toxicol 53, 483–494. [DOI] [PubMed] [Google Scholar]
- Fair PA, Adams J, Mitchum G, Hulsey TC, Reif J, Houde M, Muir D, Wirth E, Wetzel D, Zolman ES, McFee W, Bossart GD, 2010. Contaminant blubber burdens in Atlantic bottlenose dolphins (Tursiops truncatus) from two southeast U.S. estuarine areas: concentrations and patterns of PCBs, Pesticides, PBDEs, PFCs, and PAHs. Sci. Total. Environ 408, 1577–1597. [DOI] [PubMed] [Google Scholar]
- Fair PA, Romano TA, Reif JS, Schaefer AM, Hulsey TC, Bossart GB, Adams J, Houde M, Muir DC, Rice C, Peden-Adams M 2013. Association between plasma concentrations of perfluorochemicals (PFCs) and immune and clinical chemistry parameters in bottlenose dolphins (Tursiops truncatus). Environ. Toxicol. Chem, 32, pp. 736–746. [DOI] [PubMed] [Google Scholar]
- Fairey R, Taberski K, Lamerdin S, Johnson E, Clark J, Downing JW, Newman J, Petreas M, 1997. Organochlorines and other environmental contaminants in muscle tissues of sportfish collected from San Francisco Bay. Mar. Poll. Bull 34, 1058–1071. [Google Scholar]
- Falandysz J, Albanis T, Bachmann J, Bettinetti R, Bochentin I, Boti V, Bristeau S, Daehne B, Dagnac T, Galassi S, Jeannot R, Oehlmann J, Orlikowska A, Sakkas V, Szczerski R, Valsamaki V, Schulte-Oehlmann U, 2006. Some chemical contaminant of surface sediments at the Baltic Sea coastal region with special emphasis on androgenic and anti-androgenic compounds. J. Environ. Sci. Health A Toxicol. Hazard. Subst. Environ. Eng 41, 2127–2162. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration), 2018. What pregnant women and parents should know. 〈http://www.fda.gov/downloads/Food/FoodborneillnessContaminants/Metals/UCM4000358.pdf〉 (Accessed 23 March 2018).
- Gottesman MM, Fojo T, Bates SE, 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey MJ, Mainor EP, Duff TM, Gaylor WHMO, 2001. Polybrominated diphenyl either flame retardants in Virginia freshwater fishes (USA). Environ. Sci. Technol 35, 4585–4591. [DOI] [PubMed] [Google Scholar]
- Harper CR, Jacobson TA, 2001. The fats of life: the role of omega-3 fatty acids in the prevention of coronary heart disease. Arch. Intern. Med 161, 2185–2192. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G,Alexander J, Meltzer HM, Knutsen HK, 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int 36, 772–778. [DOI] [PubMed] [Google Scholar]
- Hickie BE, Cadieux MA, Riehl KN, Bossart GD, Alava JJ, Fair PA 2013.Modeling PCB-bioaccumulation in the Bottlenose Dolphin (Tursiops truncatus): estimating a dietary threshold concentration, Environ. Sci. Technol, 47, pp. 12314–12324. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ, 2004. Global assessment of organic contaminants in farmed salmon. Science 303, 226–229. [DOI] [PubMed] [Google Scholar]
- Holzer J, Goen T, Just P, Reupert R, Rauchfuss K, Kraft M, Muller J, Wilhelm M,2011. Perfluorinated compounds in fish and blood of anglers at Lake Mohne, Sauerland area, Germany. Environ. Sci. Technol 45, 8046–8052. [DOI] [PubMed] [Google Scholar]
- Houde M, Wells RS, Fair PA, Bossart GD, Hohn AA, Rowles TK, Sweeney JC, Solomon KR, Muir DC, 2005. Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ. Sci. Technol 39, 6591–6598. [DOI] [PubMed] [Google Scholar]
- Houde M, Bujas TAD, Small J, Wells R, Fair PA, Bossart G, Solomon KR, Muir DCG, 2006. Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ. Sci. Technol, 40, pp. 4138–4144. [DOI] [PubMed] [Google Scholar]
- Ikemoto T, Tu NPC, Watanabe MX, Okuda N, Omori K, Tanabe S, 2008. Analysis of biomagnification of persistent organic pollutants in the aquatic food web of the Mekong Delta, South Vietnam using stable carbon and nitrogen isotopes. Chemistry 72, 104–114. [DOI] [PubMed] [Google Scholar]
- Johnson A, Olson N, 2001. Analysis and occurrence of polybrominated diphenyl ethers in Washington state freshwater fish. Arch. Environ. Contam. Toxicol 41, 339–344. [DOI] [PubMed] [Google Scholar]
- Kamen DL, Peden-Adams MM, Vena JE, Gilkeson GS, Hulsey TC, Moultrie L, Stevens BE, 2012. Seafood consumption and persistent organic pollutants as triggers of autoimmunity among Gullah African Americans. Arthr. Res. Ther 14 (Suppl.3) (A19-A). [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R, 1995. Geographical distribution and accumulation features of organochlorine residues in fish tropical Asia and Oceania. Environ. Sci. Technol 29, 2673–2683. [DOI] [PubMed] [Google Scholar]
- Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C, 2014. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthr. Rheum 66, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan BG, Kannan K, 1994. Global organochlorine contamination trends: an overview. Ambio 23, 187–191. [Google Scholar]
- Loke M, Geslani C, Takenaka B, Leung PS, 2012. An overview of seafood consumption and supply sources: Hawai’i versus U.S Honolulu (HI): University of Hawaii. 9 p. (Economic Issues; EI–22). Available at: 〈http://www.fpir.noaa.gov/SFD/pdfs/seafood/EI-22.pdf〉. [Google Scholar]
- Lu Q, Monika D, Jürgens MD, Johnson AC, Graf C, Sweetman A, Crosse J, Whitehead P, 2017. Persistent Organic Pollutants in sediment and fish in the River Thames Catchment (UK). Sci. Total Environ 576, 78–84. [DOI] [PubMed] [Google Scholar]
- Manchester-Neesvig JB, Valters K, Sonzogni WC, 2001. Comparison of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in Lake Michigan salmonids. Environ. Sci. Technol 35, 1072–1077. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fjeld E, Breivik K, Steinnes E, Borgena A, Kjellberg G, Schlabacha M, 2008. Elevated levels of polybrominated diphenylethers (PBDEs) in fish from Lake Mjøsa, Norway. Sci. Total Environ 390, 132–141. [DOI] [PubMed] [Google Scholar]
- Midway SR, Scharf FS, 2012. Histological analysis reveals larger size at maturity for southern flounder with implications for biological reference points. Mar. Coast. Fish.: Dyn. Manag. Ecosyst. Sci 4, 628–638. [Google Scholar]
- Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ, 2012. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J. Autoimmun 39, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnesota Department of Health, 2016. How much is a serving of fish? 〈http://www.health.state.ms.us/divs/eh/fish/eating/serving.html〉 (Accessed 23 March 2018).
- Mozaffarian D, Rimm EB, 2006. Fish intake, contaminants, and human health: evaluating the risks and the benefits. Clinical review. JAMA, 296, pp. 1885–1899. [DOI] [PubMed] [Google Scholar]
- Nicklisch SCT, Rees SD, McGrath AP, Gokirmak T, Bonito LT, Vermeer LM, 2016. Global marine pollutants inhibit P-glycoprotein; environmental levels, inhibitory effects, and cocrystal structure. Sci. Adv 15, e160000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklisch SCT, Bonito LT, Sandin S, Hamdoun A, 2017. Geographic differences in persistent organic pollutant levels of yellowfin tuna (PMID: ). Environ. Health Perspect 125, 067014. 10.1289/EHP518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederdeppe J, Connelly N, Labuer T, Knuth B, 2015. Using theory to identify beliefs associated with intentions to follow fish consumption advisories among anglers living in the Great Lakes Region. Risk Anal. 35, 1996–2008. [DOI] [PubMed] [Google Scholar]
- Oken E, Choi AL, Karagas MR, Mariën K, Rheinberger CM, Schoeny R,Sunderland E, Korrick S, 2012. Which fish should i eat? Perspectives influencing fish consumption choices. Environ. Health Perspect 120, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, Dagnelie PC, Mulder PG, Op de Coul E, van der Veen JE, Weisglas-Kuperus N, Sauer PJ, 1999. Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: a comparison between breast-feeding, toddler, and long-term exposure. Environ. Health Perspect 107, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate SM, McFee WE, 2012. Prey species of bottlenose dolphins (Tursiops truncatus) from South Carolina waters. Southeast. Naturalist 11 (1), 1–22. [Google Scholar]
- Perkinson MT, Faith TD, Vahey GM, Vena JE, Williams EM, 2016. Quantifying the seafood consumption patterns of recreational anglers in Charleston and Berkeley Counties, South Carolina. Environ. Health Insights 10, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinete N, Lavandier R, Dias P, Taniguchi S, Montone R, Moreira I, 2011. Specific profiles of polybrominated diphenylethers (PBDEs) and polychlorinated biphenyls (PCBs) in fish and tucuxi dolphins from estuary of Paraiba do Sul River, Southeastern Brazil. Mar. Poll. Bull 62, 440–446. [DOI] [PubMed] [Google Scholar]
- Sajwan KS, Kumar KS, Nune S, Fowler A, Richardson JP, Loganathan BG, 2008. Persistent organochlorine pesticides, polychlorinated biphenyls, polybrominated diphenyl ethers in fish from coastal waters off Savannah, GA, USA. Toxicol. Environ. Chem 90, 81–96. [Google Scholar]
- Samara F, Tsai CW, Aga DS, 2006. Determination of potential sources of PCBs and PBDEs in sediments of the Niagara River. Environ. Pollut 139, 594–606. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, Birnbaum L, 2010a. Perfluorinated compounds, polychlorinated biphenyls and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Health Perspect 118, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, Birnbaum L, 2010b. Polybrominated diphenyl ethers (PBDEs) and hexabromocylodecane (HBCD) in composite US food samples. Environ. Health Perspect 118, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Carolina Department of Health and Environmental Control (SCDHEC). 2018. South Carolina Fish Consumption Advisories. Fish Advisory Website 〈www.scdhec.gov/fish〉.
- Spruill IJ, Leite RS, Fernandes JK, Kamen DL, Ford ME, Jenkins C, Hunt KJ, Andrews JO, 2013. Successes, challenges and lessons learned: community-engaged research with South Carolina’s Gullah population. Intern. J. Comm. Res. Engag 2013, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholm Convention. The Stockholm Convention on Persistent Organic Pollutants (POPs), 2014. (Accessed March 2018) at 〈http://chm.pops.int〉.
- Szlinder-Richert J, Barska I, Mazerski J, Usydus Z, 2009. PCBs in fish from the southern Baltic Sea: levels, bioaccumulation features, and temporal trends during the period from 1997 to 2006. Mar. Poll. Bull 58, 85–92. [DOI] [PubMed] [Google Scholar]
- Toppe J, 2016. Which fish to eat: enjoying the benefits while minimizing the risks. Procedia Food Science 6.International Conference of Sabaragamuwa University of Sri Lanka (ICSUSL 2015), pp. 47–50. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA), 2000a. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, 3rd edition. 2 Risk Assessment and Fish Consumption Limits, Washington, DC: (U.S. EPA. EPA-823-B-00–008). 〈http://www.epa.gov/waterscience/fish/advice/volume2/index.html〉. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA), 2000b. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Volume I: Fish Sampling and Analysis, Third edition. Office of Science and Technology. Office of Water. U.S. Environmental Protection Agency, Washington, D.C. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA), 2009. The National Study of Chemical Residues in Lake Fish Tissue. EPA-823-R-09–006. U.S. Environmental Protection Agency, Office of Water, Washington, DC. [Google Scholar]
- Ueno D, Takahashi S, Tanaka H, Subramanian AN, Fillmann G, Nakata H, Lam PKS, Zheng J, Muchtar M, Prudente M, Chung KH, Tanabe S, 2003. Global Pollution monitoring of PCBs and organochlorine pesticides using skipjack tuna as a bioindicator. Arch. Environ. Contam. Toxicol 45, 378–389. [DOI] [PubMed] [Google Scholar]
- Wenner CA, Roumillat WA, Moran JE Jr, Maddox MB, Daniel LB III, Smith JW, 1990. Investigations on the life history and population dynamics of marine recreational fishes in South Carolina: Part 1. Report to Fish Restoration Act under Project F-37. South Carolina Department of Natural Resources, Marine Resources Division, Charleston, South Carolina. [Google Scholar]
- de Wit CA, 2002. An overview of brominated flame retardants in the environment. Chemosphere 46, 583–624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.