Abstract
Carbohydrate response element-binding protein (ChREBP) has an important role in the carbohydrate-mediated regulation of hepatic de novo lipogenesis, but the mechanism for how it regulates plasma triacylglycerol (TAG) levels has not been established. This study aimed to clarify the role of ChREBP in regulation of plasma TAG levels. We analyzed the metabolic changes in mice infected with an adenovirus expressing ChREBP Δ196 (Ad-ChREBP). Compared with adenovirus harboring green fluorescent protein infected mice, Ad-ChREBP-infected mice had higher plasma free fatty acid levels and paradoxically lower plasma 3-hydroxybutyrate levels through decreased fatty acid oxidation, rather than ketogenesis. Consistent with their hepatomegaly and increased lipogenic gene expression, the liver TAG contents were much higher. Regarding lipid composition, C16:0 was much lower and C18:1n-9 was much higher, compatible with increased stearoyl CoA desaturase-1 and ELOVL fatty acid elongase 6 expression. Furthermore, Ad-ChREBP-infected mice had decreased plasma TAG and very low density lipoprotein (VLDL)-TAG levels, consistent with decreased Angiopoietin-like protein 3 (Angptl3) and increased fibroblast growth factor (Fgf21) mRNA and protein levels. Finally, Ad-ChREBP infection increased white adipose tissue Ucp1 mRNA levels with increased plasma Fgf21 levels. Because Fgf21 and Angptl3 are known to activate and suppress lipolysis in adipose tissues and oxidative tissues, ChREBP appears to regulate plasma TAG levels by modulating Fgf21 and Angptl3 levels. Thus, ChREBP overexpression led to dissociation of hepatic steatosis from hyperlipidemia.
Keywords: Chrebp, carbohydrate response element-binding protein, fatty liver, Fgf21, fibroblast growth factor 21, Angptl3, angiopoietin-like 3, Angptl8, angiopoietin-like 8
1. Introduction
Excess dietary carbohydrate and fat intakes occasionally cause obesity, glucose intolerance, and dyslipidemia [1,2]. These metabolic disorders are associated with non-alcoholic fatty liver disease (NAFLD) [3,4,5]. NAFLD is characterized by excess triglyceride accumulation in the liver. The size of the hepatic triacylglycerol (TAG) pool is determined by several pathways [1]: (1) free fatty acid (FFA) supply from peripheral adipose tissues; (2) intestinal absorption from dietary fatty acids; (3) de novo lipogenesis; (4) fatty acid oxidation; and (5) very low density lipoprotein (VLDL) secretion.
De novo lipogenesis plays an important role in the regulation of hepatic lipid contents [5]. In a previous study, de novo lipogenesis was two-fold higher in subjects with NAFLD than in subjects without NAFLD [5]. De novo lipogenesis is a process that converts excess carbohydrate into fatty acids for storage [6]. Insulin and glucose induce expression of genes related to de novo lipogenesis [6]. Insulin stimulates sterol regulatory element-binding protein 1c (SREBP1c) and glucose stimulates carbohydrate response element-binding protein (ChREBP) [6]. Both of these transcription factors regulate the expression of common lipogenic genes, fatty acid synthase (Fasn) and acetyl CoA carboxylase 1 (Acc1), in the liver and consequently, de novo lipogenesis [6]. ChREBP is induced by a high-carbohydrate diet and activated ChREBP induces lipogenic gene expression [7,8,9,10]. Moreover, regulation of lipid composition plays an important role in glucose and lipid metabolism [11,12]. Elovl6 and Scd1 are also regulated by SREBP1c and ChREBP [13,14,15,16]. Thus, ChREBP has important roles in regulation of de novo lipogenesis and lipid composition.
Hyperlipidemia is controlled by several factors, including hepatic lipid synthesis, plasma lipid secretion, and plasma lipid clearance [17]. For example, hereditary hypertriglyceridemia is caused by decreased lipid clearance through defective lipoprotein lipase activity [18]. Although ChREBP has an important role in the regulation of liver de novo lipogenesis and lipid secretion, the relationship between ChREBP and plasma lipid clearance has not been established. Several secretory proteins are known to regulate plasma triglyceride levels. Fibroblast growth factor-21 (Fgf21) regulates plasma triglyceride levels through decreased VLDL secretion in the liver and increased TAG disposal in adipose tissues [19]. Angiopoietin-like protein 3 (Angptl3) and Angiopoietin-like protein 8 (Angptl8) can regulate plasma TAG levels through inhibition of lipoprotein lipase [20,21]. Because Fgf21 is a target gene for ChREBP [22], we hypothesized that ChREBP may regulate plasma TAG levels by modulating secretory protein levels.
In the present study, we examined the effect of ChREBP on lipid metabolism with special reference to plasma triglyceride metabolism. Clarification of the role of ChREBP in lipid metabolism will be useful for therapeutic strategies in the treatment of non-alcoholic fatty liver disease (NAFLD) and hyperlipidemia.
2. Materials and Methods
2.1. Establishment of Mice Infected with an Adenovirus Harboring with ChREBP Δ196
Animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978). All animal care was approved by the Animal Care Committee of the University of Gifu (No. 25-16, approval date: 2 May 2013; No. 22-26, approval date: 8 November 2010). Mice were housed at 23 °C on a 12-h/12-h light/dark cycle. At 8–9 weeks of age, male C57BL/6J mice (n = 6 per group) were intravenously infected with an adenovirus harboring ChREBP lacking the N-terminal 196 amino acids (ChREBP Δ196) or enhanced green fluorescent protein (GFP) as a control [23,24]. ChREBP Δ196 lacks two nuclear export signals and mimics ChREBP-β [25,26]. Mice had free access to water and an autoclaved CE-2 diet (25.5% protein, 4.6% fat, 48.9% carbohydrate; CLEA Japan, Tokyo, Japan). Mice were euthanized after 5 days by cervical dislocation. All tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C until further analysis for hepatic TAG and cholesterol contents or quantitative polymerase chain reaction (PCR).
2.2. Liver Metabolites and Plasma Profile Measurements
Liver glucose-6-phosphate (G6P) and glycogen contents were measured as described [16,23]. Liver lipids were extracted by the Bligh and Dyer method [27] and measured using triglyceride and cholesterol E-tests (Wako Pure Chemicals, Osaka, Japan). Blood plasma was collected from the retro-orbital venous plexus after an 18-h fast (for glucose, insulin, FFA, and 3-hydroxybutyrate (OHBA)) or 6-h fast (for TAG and cholesterol). Blood glucose and OHBA levels were measured using a Free Style Freedom monitoring system (Nipro, Osaka, Japan). Plasma insulin, FFA, Fgf21, Angptl3, Angptl8, triglyceride, and total cholesterol levels were determined using commercial assay kits: mouse insulin enzyme-linked immunosorbent assay (ELISA) (H type) (Shibayagi, Gunma, Japan), NEFA C-test (Wako Pure Chemicals), mouse/rat FGF21 ELISA (R&D Systems, Minneapolis, MN, USA), Angptl3 ELISA kit (Thermo Fisher Scientific, Middletown, VA, USA), Angptl8 ELISA kit (Cloud-Clone Corp., Wuhan, China), triglyceride E-test, and cholesterol E-test, respectively. The TAG contents and cholesterol levels in lipoprotein fractions and the VLDL particle numbers were analyzed by Skylight Biotech Inc. (Akita, Japan) using gel-permeation high-performance liquid chromatography as described [28]. For serum lipoprotein analysis, serum samples obtained from six mice after a 6-h fast were pooled and measured. Lipid composition assays using gas chromatography analysis methods after methanolysis were performed by Toray Research Center (Tokyo, Japan). For hepatic lipid composition analysis, liver samples obtained from six mice after a 6-h fast were pooled and measured.
2.3. RNA Isolation and Quantitative Real-Time PCR
Total RNA isolation, cDNA synthesis, and real-time PCR analysis were performed as described [22,23,29,30,31]. Equal amounts of RNA from six mice were pooled and cDNA was synthesized. Real-time PCR amplifications were performed in triplicate. The relative amounts of mRNA were calculated by the comparative Ct method. Pol2 expression was evaluated as an internal control.
2.4. Statistical Analysis
All values are presented as mean ± standard deviation. Data were analyzed by Student’s t-test. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Adenoviral Overexpression of ChREBP Causes Hepatomegaly without Obesity
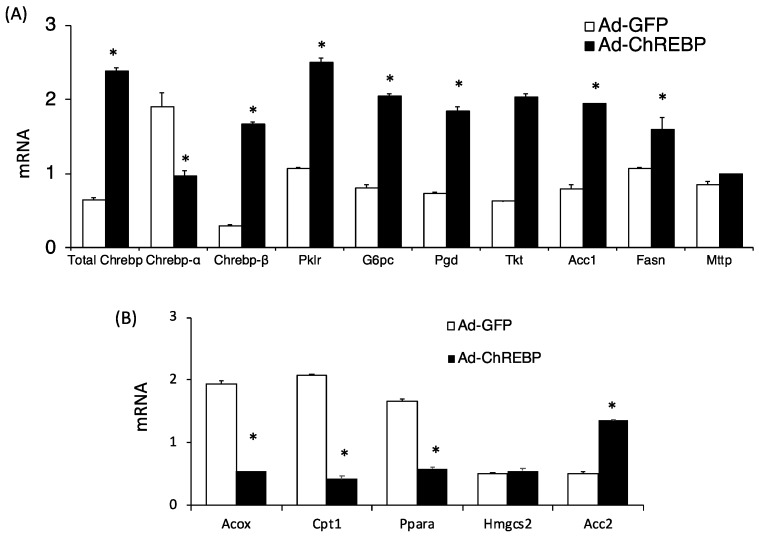
We examined the metabolic effects of ChREBP overexpression in mice fed a normal chow diet. Initially, total Chrebp mRNA levels in Ad-ChREBP-infected mice were increased by about four times compared with those in Ad-GFP-infected mice (Figure 1A). Endogenous mouse Chrebp-β mRNA was similarly increased. In contrast, modest decreases were observed for endogenous mouse Chrebp-α (Figure 1A). ChREBP target genes (Pklr, G6pc, Pgd, Tkt, Acc1, and Fasn) were increased by ChREBP overexpression. Mttp expression was only modestly increased. Thus, Ad-ChREBP successfully infected the liver of C57BL/6J mice (Figure 1A).
Figure 1.
Hepatic mRNA analysis in Ad-GFP-infected and Ad-ChREBP-infected mice. The phenotypes of Ad-ChREBP-infected mice were evaluated. Male C57BL/6J mice at 8 weeks of age were infected with an adenovirus expressing ChREBP Δ196 or GFP. The mice were examined and analyzed after 5 days. (A) ChREBP (total Chrebp, Chrebp-α, Chrebp-β) and ChREBP target genes (Pklr, G6pc, Pgd, Tkt, Acc1, Fasn, Mttp). (B) Genes related to fatty acid oxidation and ketogenesis (Acox, Cpt1, Ppara, Hmgcs2, Acc2). Equal amounts of RNA from 6 mice were pooled, and cDNA was synthesized. RT-PCR analyses were performed in triplicate. Pol2 expression was evaluated as an internal control. Data represent means ± SD (n = 3 per group). * p < 0.05.
Regarding phenotypes, Ad-ChREBP-infected mice showed severe hepatomegaly without body weight gain, adiposity, and increased food intake (Table 1). Plasma glucose levels were lower and plasma FFA levels were higher compared with those in Ad-GFP-infected mice. Plasma OHBA levels were much lower despite the decreased plasma glucose levels (Table 1). Plasma ketone levels are regulated by acetyl CoA supply from fatty acid oxidation, ketogenesis, and use of ketones in peripheral tissues [32]. Regarding ketogenic gene expression, Hmgcs2 levels were similar, while genes related to fatty acid oxidation were altered to promote decreased fatty acid oxidation that supplies acetyl CoA as a source of ketone bodies (Figure 1B).
Table 1.
Phenotypic comparisons between Ad-GFP-infected and Ad-ChREBP-infected mice.
| Ad-GFP | Ad-ChREBP | |
|---|---|---|
| BW (g) | 24.35 ± 1.47 | 22.46 ± 0.66 |
| Liver (%BW) | 6.01 ± 0.31 | 9.80 ± 1.69 |
| WAT (%BW) | 1.38 ± 0.16 | 1.09 ± 0.45 |
| BAT (%BW) | 0.56 ± 0.36 | 0.48 ± 0.10 |
| Food intake (g) | 4.03 ± 0.58 | 3.46 ± 0.56 |
| Plasma glucose (mg/dl) | 110.7 ± 25.5 | 46.7 ± 13.5 * |
| Plasma OHBA (mM) | 1.28 ± 0.15 | 0.61 ± 0.36 * |
| Plasma FFA (mM) | 0.56 ± 0.22 | 1.35 ± 0.65 * |
| Plasma Insulin (ng/mL) | 0.32 ± 0.16 | 0.43 ± 0.25 |
| Plasma total cholesterol (mg/dl) | 76.21 ± 9.23 | 59.36 ± 13.01 * |
| Plasma triglyceride (mg/dl) | 92.94 ± 28.54 | 65.91 ± 25.54 * |
| Liver glycogen (mg/g liver) | 112.6 ± 38.1 | 20.3 ± 3.3 * |
| Liver glucose-6-phosphate (μmoles/g liver) | 0.41 ± 0.06 | 0.19 ± 0.05 * |
| Liver total cholesterol (mg/g liver) | 1.21 ± 0.25 | 1.61 ± 0.45 |
| Liver triglyceride (mg/g liver) | 10.5 ± 3.5 | 73.8 ± 37.9 * |
Abbreviations: BW, body weight; WAT, white adipose tissue; BAT, brown adipose tissue; OHBA, 3-hydroxybutyrate; FFA, free fatty acid. Data represent means ± SD (n = 6 per group). * p < 0.05.
Liver G6P and glycogen contents in Ad-ChREBP-infected mice were much lower than those in Ad-GFP-infected mice (Table 1). Compatible with these changes, G6pc mRNA levels were higher in Ad-ChREBP-infected mice (Figure 1A). Plasma triglyceride levels and cholesterol levels were both significantly lower, while liver lipid contents differed. Liver cholesterol levels were similar in the two groups, while liver triglyceride contents were much higher because of increased Fasn and Acc expression (Table 1). Thus, Ad-ChREBP-infected mice showed altered lipid metabolism compared with Ad-GFP-infected mice.
3.2. Adenoviral ChREBP Overexpression Causes Higher Triglyceride Contents with Altered Lipid Composition
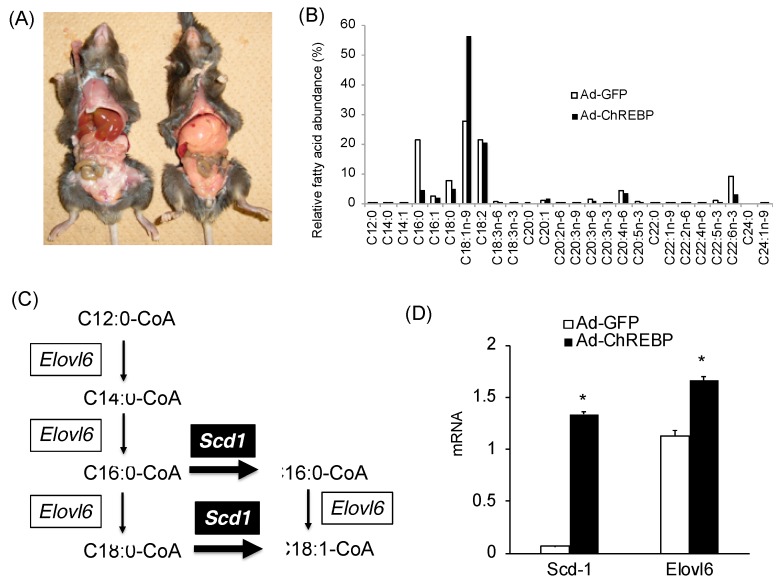
Histological analyses were not performed. However, the gross anatomical views revealed hepatomegaly with fatty liver (Figure 2A). Liver triglyceride contents in Ad-ChREBP-infected mice were about seven times higher, as shown in Table 1. Regarding fatty acid composition in Ad-ChREBP-infected mice, saturated fatty acids (C16:0 and C18:0) and a monounsaturated fatty acid (C16:1) were decreased, while C18:1n-9 (oleic acid) and C20:1 were increased (Figure 2B). C20:3n-6, C20:4n-6, and C22:6n-3 were decreased. C16 acyl CoA levels were lower in Ad-ChREBP-infected mice, while C18 acyl CoA levels were much higher (Figure 2B). Consistent with these findings, mRNA levels of ELOVL fatty acid elongase 6 (Elovl6), a fatty acid-elongating enzyme, were increased in Ad-ChREBP-infected mice (Figure 2C,D). Furthermore, consistent with the increased C18:1n-9 levels, mRNA levels of stearoyl CoA desaturase 1 (Scd1) were increased in Ad-ChREBP-infected mice (Figure 2C,D).
Figure 2.
Effects of ChREBP on the liver lipid composition. (A) Gross anatomical views of representative mice. Hepatomegaly with fatty liver was observed in Ad-ChREBP-infected mice. (B) Liver lipid composition. Data are representative of six pooled samples. (C) Scd1 and Elovl6 are involved in the fatty acid elongation and lipid unsaturation pathway, respectively. (D) Hepatic mRNA levels of stearoyl CoA desaturase-1 (Scd1) and ELOVL fatty acid elongase 6 (Elovl6). Data represent means ± SD (n = 3 per group). * p < 0.05 vs. Ad-GFP.
3.3. ChREBP Overexpression Lowers Plasma Triglyceride Levels by Modulating Angptl3 and Fgf21 Levels
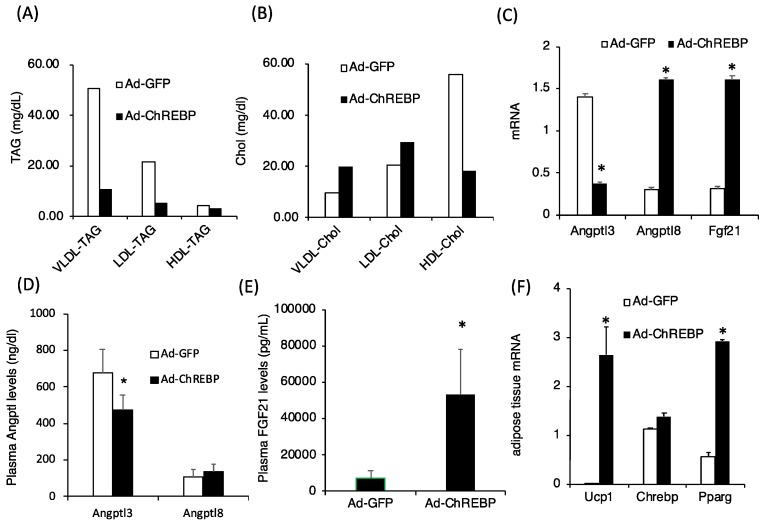
The lipid profiles, plasma triglyceride, and plasma cholesterol levels were significantly lower in Ad-ChREBP-infected mice (Table 1). Regarding the plasma lipoprotein profiles, plasma VLDL-TAG, LDL-TAG, and HDL-TAG levels were much lower in Ad-ChREBP-infected mice (Figure 3A). Plasma VLDL-Chol and LDL-Chol levels were relatively higher and HDL-Chol levels were relatively lower in Ad-ChREBP-infected mice (Figure 3B). Angptl3 and Angptl8 are known to regulate lipoprotein lipase activity. Angptl3 mRNA levels were decreased, while Angptl8 mRNA levels were increased (Figure 3C). Regarding protein levels, plasma Angptl3 levels were significantly decreased, and plasma Angptl8 levels were slightly increased (Figure 3D). Fgf21 is known to regulate plasma lipid levels. Fgf21 mRNA and protein levels in Ad-ChREBP infected mice were much higher than those in Ad-GFP infected mice (Figure 3E). Compatible with the increased plasma Fgf21 levels, mRNA levels of uncoupling protein 1 (Ucp1), specific to brown adipose tissue, and peroxisome proliferator-activating protein gamma (Pparg) were increased (Figure 3F). ChREBP mRNA levels in adipose tissues were not affected by Ad-ChREBP infection (Figure 3F).
Figure 3.
Effects of ChREBP on plasma lipid profiles and secretory proteins. (A) Plasma very low-density lipoprotein (VLDL)-triacylglycerol (TAG), low-density lipoprotein (LDL)-TAG, and high-density lipoprotein (HDL)-TAG levels. (B) Plasma VLDL-cholesterol (VLDL-Chol), LDL-cholesterol (LDL-Chol), and HDL-cholesterol (HDL-Chol) levels. Data are representative of pooled samples from six mice. (C) Hepatic mRNA levels of angiopoietin-like protein (Angptl) 3, Angptl8, and fibroblast growth factor-21 (Fgf21). Data represent means ± SD (n = three per group). * p < 0.05 vs. Ad-GFP. (D) Plasma Angptl3 and Angptl8 levels. Data represent means ± SD (n = six per group). * p < 0.05 vs. Ad-GFP. (E) Plasma Fgf21 levels. Data represent means ± SD (n = six per group). * p < 0.05 vs. Ad-GFP. (F) mRNA levels of uncoupling protein 1 (Ucp1) and peroxisome proliferator-activating protein gamma (Pparg). Data represent means ± SD (n = three per group). * p < 0.05 vs. Ad-GFP.
4. Discussion
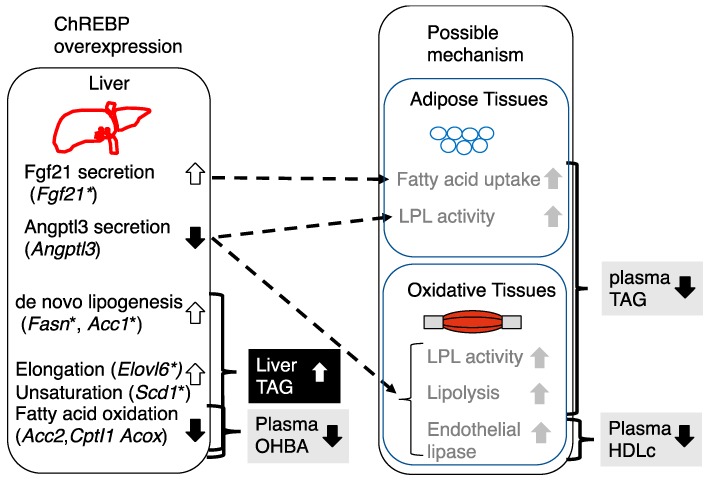
In this study, we have clarified the role of hepatic ChREBP in glucose and lipid metabolism. Consistent with a previous paper [33], we confirmed that ChREBP overexpression increased hepatic triglyceride contents, altered hepatic lipid composition, and lowered plasma glucose levels. Moreover, we observed the following effects: (1) ChREBP overexpression significantly lowered plasma ketone levels through decreased fatty acid oxidation gene expressions, and (2) ChREBP overexpression significantly lowered plasma TAG levels through altered plasma Angptl3 and Fgf21 levels, associated with increased WAT Ucp1 mRNA levels. These findings suggest that ChREBP may reciprocally regulate liver and plasma triglyceride levels (Figure 4). High carbohydrate diets such as high fructose and sucrose diets are known to promote hepatic ChREBP transcription activity [7,8,9,10]. Thus, ChREBP overexpression leads to dissociation of hepatic steatosis from hyperlipidemia.
Figure 4.
Schematic representation of the phenotypic changes in Ad-ChREBP-infected mice and possible mechanism of how ChREBP overexpression impacts liver and plasma lipid metabolism. A high-sucrose diet or high-fructose diet feeding is known to activate liver ChREBP transcription activity [7,8,9,10]. ChREBP promotes the following effects: (1) lowering of plasma 3-hydroxybutyrate (OHBA) through decreased fatty acid oxidation; (2) increase in liver triglyceride contents through increased de novo lipogenesis, fatty acid elongation and unsaturation, and decreased fatty acid oxidation; (3) lowering of plasma triglyceride; (4) lowering of plasma HDL cholesterol (HDLc). Fgf21 is known to lower plasma TAG levels by increasing TAG disposal. Angptl3 is known to inhibit lipoprotein lipase (LPL) and endothelial lipase and thereby inhibition of Angptl3 lowers plasma TAG and HDLc levels. * represented as ChREBP-target gene.
Plasma OHBA levels in Ad-ChREBP-infected mice were much lower than those in Ad-GFP-infected mice, while plasma FFA levels were much higher. Plasma ketone levels are regulated by acetyl CoA supply from fatty acid oxidation, ketogenesis, and use of ketones in peripheral tissues [32]. Regarding ketogenesis, the levels of Hmgcs2, encoding a rate-limiting enzyme in the ketogenic pathway [32], were similar. In contrast, for fatty acid oxidation, Acox and Cpt1 mRNA levels in the fatty acid oxidation pathway were decreased, compatible with a previous paper [33]. Moreover, levels of Acc2 mRNA, which suppress CPT1 activity through mitochondrial malonyl CoA production [34], were increased. Acetyl CoA is used for glycogenosis (or gluconeogenesis) and de novo lipogenesis. Although we did not measure the hepatic acetyl CoA contents, much of the acetyl CoA pool may be used to meet the increased demand for de novo lipogenesis upon ChREBP overexpression. Therefore, the lower plasma OHBA levels in Ad-ChREBP-infected mice may arise through decreased fatty acid oxidation, rather than ketogenesis. Interestingly, ChREBP−/− mice also showed lower plasma OHBA levels through decreased fatty acid oxidation, decreased supply of acetyl CoA because of lower plasma FFA levels, and lower cytosolic NAD-to-NADH ratios [31,35,36]. Although it appears contradictory that ChREBP overexpression and deletion lead to decreases in ketogenesis by decreasing β-oxidation, these findings are consistent with in vivo evidence that both Chrebp gene deletion and Chrebp gene activation fail to prevent the development of high-fat diet-induced fatty liver [31,33]. Therefore, ChREBP activity plays some roles in the regulation of ketogenesis.
In Ad-ChREBP-infected mice, both hepatic G6P and glycogen contents were decreased. G6P is one of the candidate mediators for glucose signals that activate ChREBP transcriptional activity [37]. Hepatic G6P levels are correlated with hepatic glycogen contents [38]. ChREBP knockout mice had higher hepatic G6P and glycogen contents than wild-type mice [11,36]. In the present study, hepatic G6P and glycogen contents were reduced through increased G6pc mRNA levels in Ad-ChREBP-infected mice. G6pc is a target gene for ChREBP [36,39]. Thus, ChREBP negatively regulates liver glycogen contents.
In Ad-ChREBP-infected mice, hepatomegaly caused by fatty liver was seen. These effects arise through increased gene expression related to lipogenesis. Moreover, pentose phosphate shunt pathway genes (Pgd, Tkt), which produce cytosolic NADPH for lipogenesis, were increased in Ad-ChREBP-infected mice. These findings were compatible with previous papers describing findings in ChREBP−/− mice [33,36].
Fatty acid elongation and unsaturation are important processes for TAG synthesis [11,12]. Regarding fatty acid composition, the amounts of C18:1n-9 (oleic acid) were much higher in Ad-ChREBP-infected mice compared with Ad-GFP-infected mice. In contrast, the amounts of palmitic acid (C16:0) were much lower in Ad ChREBP-infected mice. These findings were compatible with those in a previous paper [33]. Elovl6 is known to elongate the carboxyl chain of fatty acyl CoA [12,15] and is regulated by not only SREBP, but also ChREBP [13]. Elovl6 mRNA was increased in Ad-ChREBP-infected mice and thus the amounts of C18 were higher than the amounts of C16. Moreover, ChREBP regulates Scd1 mRNA expression [16]. Scd1 catalyzes the rate-limiting step in the formation of monounsaturated fatty acids, specifically oleate and palmitoleate, from stearoyl-CoA and palmitoyl-CoA [11]. Consistent with a previous paper [33], Scd1 mRNA was increased in Ad-ChREBP-infected mice. Unlike other unsaturated fatty acids, oleic acid cannot suppress ChREBP transcriptional activities [40]. This may also contribute to increased liver TAG storage. Consequently, the amounts of oleic acid in Ad-ChREBP-infected mice were much higher due to increased Elovl6 and Scd1 expression.
Regarding the physiological roles of oleic acid, oleic acid is more suitable for lipid storage than palmitic acid because of its lower melting point [41]. Moreover, palmitic acid is incorporated into DAG and consequently activates the proinflammatory PKCu–NF-κB pathway, while oleic acid is incorporated into TAG [42]. Therefore, palmitic acid is known to cause insulin resistance by attenuating insulin signaling, while oleic acid has a protective effect against insulin resistance and type 2 diabetes mellitus [42]. Compatible with these observations, our findings and previous results [33] showed that Ad-ChREBP-infected mice had lower plasma glucose levels or improved insulin resistance. Therefore, ChREBP promotes TAG storage in the liver without ameliorating plasma glucose by converting a palmitic acid into oleic acid.
In Ad-ChREBP-infected mice, plasma TAG, VLDL-TAG, LDL-TAG, and HDL-TAG levels were decreased compared with Ad-GFP-infected mice. Plasma TAG levels are regulated by de novo lipogenesis, plasma lipid secretion, and peripheral lipolysis [17]. As de novo lipogenesis and hepatic lipid secretion were rather increased due to increased gene expression (Fasn, Acc1, Mttp), we hypothesized that peripheral lipolysis may be increased by secretory factors that promote lipolysis. Angptl3 is known to increase lipolysis by inhibiting lipoprotein lipase [20] and Fgf21 is known to promote TAG disposal in adipose tissues [19]. In Ad-ChREBP-infected mice, hepatic Angptl3 mRNA and plasma Angptl3 protein levels were decreased, while hepatic Fgf21 mRNA and plasma Fgf21 protein levels were significantly increased. In support of these findings, Fgf21 is known to induce adipose tissue Ucp1 and Pparg mRNA expression [43,44]. Interestingly, Ucp1 and Pparg mRNA expression were induced in white adipose tissue. Taken together with the finding that Ad-ChREBP was not present in white adipose tissue, these observations suggest that increased plasma Fgf21 levels could modulate adipose tissue function in Ad-ChREBP-infected mice. The findings suggest that decreased Angptl3 and increased Fgf21 may promote peripheral TAG disposal. Angptl8 is known to increase plasma TAG levels by suppressing lipoprotein lipase [21] and Angptl8 is a target gene for ChREBP [45]. Although Angptl8 mRNA levels were increased, plasma Angptl8 protein levels were not significantly increased. In Ad-ChREBP-infected mice, only Angptl8 mRNA levels were significantly increased. Moreover, in Angptl3−/− mice, Angptl8 administration did not increase plasma triglyceride levels [21,46]. These observations suggest that Angptl3, rather than Angptl8, may contribute to the lower plasma TAG levels in Ad-ChREBP-infected mice.
The mechanism for how ChREBP regulates Angptl3 expression has not been established and further investigations are needed. Meanwhile, plasma cholesterol levels in Ad-ChREBP infected mice were lower. VLDL-Chol and LDL-Chol levels were higher and HDL-Chol levels were lower. Regarding plasma HDL-Chol levels, lower Angptl3 mRNA levels lead to lower plasma HDL-Chol levels [47]. In particular, Angptl3 inhibits the phospholipase activity of endothelial lipase, and lowering Angptl3 may thereby decrease plasma HDL-Chol levels [48]. These observations are compatible with the findings in our Ad-ChREBP-infected mice. Thus, ChREBP may regulate plasma lipid levels partly through FGF21 and Angptl3.
5. Conclusions
We clarified that (1) ChREBP negatively regulates plasma ketone levels, and (2) ChREBP differently regulates hepatic and plasma triglyceride levels. Under normal conditions, excess intake of carbohydrates such as fructose or sucrose is converted into triglyceride through glucose-activated ChREBP. ChREBP promotes hepatic de novo lipogenesis by induction of lipogenic gene expression. ChREBP lowers plasma ketone levels by inhibiting fatty acid oxidation. Furthermore, ChREBP lowers plasma TAG levels through TAG disposal in adipose tissue and oxidative tissues by decreasing plasma Angptl3 levels and increasing plasma Fgf21 levels. Therefore, the discrepancies between plasma and liver TAG levels in Ad-ChREBP mice revealed that ChREBP reciprocally regulates hepatic and plasma TAG levels in different manners. With the intake of a high-fructose or high-sucrose diet, Fgf21 and Angptl3, rather than Angptl8, may be better targets for the improvement of hypertriglyceridemia.
Acknowledgments
We thank Alison Sherwin, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author Contributions
K.I. conceived and designed the experiments; K.I., K.T., T.K., performed the experiments; K.T. analyzed the data; Y.H. and J.T. provided support in the literature review; K.I. wrote and revised the paper. All the authors approved the final version of the manuscript.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Iizuka K: No. 17K00850, 26500005; Takeda J: No. 17K19902; Horikawa Y: No. 18H02779), and research grants from Eli Lilly, MSD, and Novartis Pharma (Iizuka K and Takeda J).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Moore J.B., Gunn P.J., Fielding B.A. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6:5679–5703. doi: 10.3390/nu6125679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu S., Sievenpiper J.L., de Souza R.J., Cozma A.I., Mirrahimi A., Carleton A.J., Ha V., Di Buono M., Jenkins A.L., Leiter L.A., et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabbrini E., Magkos F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients. 2015;7:4995–5019. doi: 10.3390/nu7064995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning J.D., Horton J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004;114:147–152. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iizuka K. The transcription factor carbohydrate-response element-binding protein (ChREBP): A possible link between metabolic disease and cancer. Biochim. Biophys. Acta. 2017;1863:474–485. doi: 10.1016/j.bbadis.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Wahed A., Guilmeau S., Postic C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Hannou S.A., Haslam D.E., McKeown N.M., Herman M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018;128:545–555. doi: 10.1172/JCI96702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ntambi J.M., Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004;43:91–104. doi: 10.1016/S0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 12.Shimano H. Novel qualitative aspects of tissue fatty acids related to metabolic regulation: Lessons from Elovl6 knockout. Prog. Lipid Res. 2012;51:267–271. doi: 10.1016/j.plipres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Bae J.S., Oh A.R., Lee H.J., Ahn Y.H., Cha J.Y. Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochem. Biophys. Res. Commun. 2016;478:1060–1066. doi: 10.1016/j.bbrc.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki M., Dobrzyn A., Man W.C., Chu K., Sampath H., Kim H.J., Ntambi J.M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 15.Kumadaki S., Matsuzaka T., Kato T., Yahagi N., Yamamoto T., Okada S., Kobayashi K., Takahashi A., Yatoh S., Suzuki H., et al. Mouse Elovl-6 promoter is an SREBP target. Biochem. Biophys. Res. Commun. 2008;368:261–266. doi: 10.1016/j.bbrc.2008.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka K., Bruick R.K., Liang G., Horton J.D., Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy S.M. Pathogenesis of hyperlipoproteinemia. J. Lipid Res. 1984;25:1611–1618. [PubMed] [Google Scholar]
- 18.Wang H., Eckel R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 19.Schlein C., Talukdar S., Heine M., Fischer A.W., Krott L.M., Nilsson S.K., Brenner M.B., Heeren J., Scheja L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016;23:441–453. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T., Furukawa H. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002;277:33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 21.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E., Valenzuela D.M., Murphy A.J., Cohen J.C., Hobbs H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 23.Iizuka K., Takeda J., Horikawa Y. Hepatic overexpression of dominant negative Mlx improves metabolic profile in diabetes-prone C57BL/6J mice. Biochem. Biophys. Res. Commun. 2009;379:499–504. doi: 10.1016/j.bbrc.2008.12.100. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka K., Horikawa Y. Regulation of lipogenesis via BHLHB2/DEC1 and ChREBP feedback looping. Biochem. Biophys. Res. Commun. 2008;374:95–100. doi: 10.1016/j.bbrc.2008.06.101. [DOI] [PubMed] [Google Scholar]
- 25.Li M.V., Chang B., Imamura M., Poungvarin N., Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006;55:1179–1189. doi: 10.2337/db05-0822. [DOI] [PubMed] [Google Scholar]
- 26.Herman M.A., Peroni O.D., Villoria J., Schön M.R., Abumrad N.A., Blüher M., Klein S., Kahn B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki M., Yamashita S. Recent Advances in Analytical Methods on Lipoprotein Subclasses: Caluculation of Particle Numbers from Lipid Levels by Gel Permeation HPLC Using “Spherical Particle Model”. J. Oleo Sci. 2016;65:265–282. doi: 10.5650/jos.ess16020. [DOI] [PubMed] [Google Scholar]
- 29.Kato T., Iizuka K., Takao K., Horikawa Y., Kitamura T., Takeda J. ChREBP-KnockoutMice Show Sucrose Intolerance and Fructose Malabsorption. Nutrients. 2018;10:340. doi: 10.3390/nu10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niwa H., Iizuka K., Kato T., Wu W., Tsuchida H., Takao K., Horikawa Y., Takeda J. ChREBP Rather Than SHP Regulates Hepatic VLDL Secretion. Nutrients. 2018;10:321. doi: 10.3390/nu10030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W., Tsuchida H., Kato T., Niwa H., Horikawa Y., Takeda J., Iizuka K. Fat and carbohydrate in western diet contribute differently to hepatic lipid accumulation. Biochem. Biophys. Res. Commun. 2015;461:681–686. doi: 10.1016/j.bbrc.2015.04.092. [DOI] [PubMed] [Google Scholar]
- 32.Grabacka M., Pierzchalska M., Dean M., Reiss K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int. J. Mol. Sci. 2016;17:2093. doi: 10.3390/ijms17122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Investig. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Elheiga L., Matzuk M.M., Abo-Hashema K.A., Wakil S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 35.Burgess S.C., Iizuka K., Jeoung N.H., Harris R.A., Kashiwaya Y., Veech R.L., Kitazume T., Uyeda K. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J. Biol. Chem. 2008;283:1670–1678. doi: 10.1074/jbc.M706540200. [DOI] [PubMed] [Google Scholar]
- 36.Iizuka K., Miller B., Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 37.Li M.V., Chen W., Harmancey R.N., Nuotio-Antar A.M., Imamura M., Saha P., Taegtmeyer H., Chan L. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP) Biochem. Biophys. Res. Commun. 2010;395:395–400. doi: 10.1016/j.bbrc.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villar-Palasí C., Guinovart J.J. The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 1997;11:544–558. doi: 10.1096/fasebj.11.7.9212078. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen K.B., Zhang P., Doumen C., Charbonnet M., Lu D., Newgard C.B., Haycock J.W., Lange A.J., Scott D.K. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. Am. J. Physiol. Endocrinol. Metab. 2007;292:E788–E801. doi: 10.1152/ajpendo.00510.2006. [DOI] [PubMed] [Google Scholar]
- 40.Dentin R., Benhamed F., Pégorier J.P., Foufelle F., Viollet B., Vaulont S., Girard J., Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermudez B., Lopez S., Ortega A., Varela L.M., Pacheco Y.M., Abia R., Muriana F.J. Oleic acid in olive oil: from a metabolic framework toward a clinical perspective. Curr. Pharm. Des. 2011;17:831–843. doi: 10.2174/138161211795428957. [DOI] [PubMed] [Google Scholar]
- 42.Palomer X., Pizarro-Delgado J., Barroso E., Vázquez-Carrera M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018;29:178–190. doi: 10.1016/j.tem.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E., et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutchak P.A., Katafuchi T., Bookout A.L., Choi J.H., Yu R.T., Mangelsdorf D.J., Kliewer S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Z., Berhane F., Fite A., Seyoum B., Abou-Samra A.B., Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 2014;4:5013. doi: 10.1038/srep05013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haller J.F., Mintah I.J., Shihanian L.M., Stevis P., Buckler D., Alexa-Braun C.A., Kleiner S., Banfi S., Cohen J.C., Hobbs H.H., et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J. Lipid Res. 2017;58:1166–1173. doi: 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musunuru K., Pirruccello J.P., Do R., Peloso G.M., Guiducci C., Sougnez C., Garimella K.V., Fisher S., Abreu J., Barry A.J., et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]