Abstract
Glutamate is the neurotransmitter used at most excitatory synapses in the mammalian brain, including those in the olfactory bulb (OB). There, ionotropic glutamate receptors including N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) play a role in processes such as reciprocal inhibition and glomerular synchronization. Kainate receptors (KARs) represent another type of ionotropic glutamate receptor, which are composed of five (GluK1-GluK5) subunits. Whereas KARs appear to be heterogeneously expressed in the OB, evidence as to whether these KARs are functional, found at synapses, or modify synaptic transmission is limited. In the present study, coapplication of KAR agonists (kainate, SYM 2081) and AMPAR antagonists (GYKI 52466, SYM 2206) demonstrated that functional KARs are expressed by OB neurons, with a subset of receptors located at synapses. Application of kainate and the GluK1-selective agonist ATPA had modulatory effects on excitatory postsynaptic currents (EPSCs) evoked by stimulation of the olfactory nerve layer. Application of kainate and ATPA also had modulatory effects on reciprocal inhibitory postsynaptic currents (IPSCs) evoked using a protocol that evokes dendrodendritic inhibition. The latter finding suggests that KARs, with relatively slow kinetics, may play a role in circuits in which the relatively brief duration of AMPAR-mediated currents limits the role of AMPARs in synaptic transmission (e.g., reciprocal inhibition at dendrodendritic synapses). Collectively, our findings suggest that KARs, including those containing the GluK1 subunit, modulate excitatory and inhibitory transmission in the OB. These data further suggest that KARs participate in the regulation of synaptic circuits that encode odor information.
Keywords: glutamate receptors, olfaction, glutamate, GABA, ATPA, SYM 2081
INTRODUCTION
Glutamate is the neurotransmitter used at most excitatory synapses in the mammalian brain, including those in the olfactory bulb (OB). Both ionotropic and metabotropic glutamate receptors play a role in synaptic transmission and neuromodulation (Zhuo, 2017). Ionotropic glutamate receptors comprise three families, which are named based on their selective synthetic agonist: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate (Dingledine et al., 1999; Lodge, 2009; Alexander et al., 2017). In the central nervous system (CNS), rapid synaptic excitation is largely mediated by postsynaptic AMPA receptors (AMPARs) and NMDA receptors (NMDARs) (Koles et al., 2016), while kainate receptors (KARs) act principally to modulate neuronal excitability and synaptic transmission at both presynaptic and postsynaptic sites (Contractor et al., 2011; Lerma and Marques, 2013; Sihra and Rodriguez-Moreno, 2013).
In the OB, both AMPARs and NMDARs play a role in a number of processes including correlated spiking, reciprocal inhibition, and glomerular synchronization (Schoppa et al., 1998; Isaacson and Strowbridge, 1998; Schoppa and Westbrook, 2002; Halabisky and Strowbridge, 2003; Schoppa, 2006a). However, the potential role of KARs in such processes remains unclear. Studies that used a variety of techniques, including in situ hybridization (Gall C. et al., 1990), autoradiography (Nadi et al., 1980; Bailey et al., 2001), activity-dependent labeling (Edwards and Michel, 2003), and immunohistochemistry (Petralia et al., 1994; Montague and Greer, 1999; Davila et al., 2007), suggest that KARs are heterogeneously expressed in the OB. However, evidence as to whether KARs in the OB are functional, found at synapses, or modify synaptic transmission is limited.
KARs are tetrameric receptors comprised of the glutamate receptor subunits originally named GluR5–7, KA1, and KA2. New nomenclature for ligand-gated ion channels was introduced in 2009 (Collingridge et al., 2009), which re-named GluR5, GluR6, GluR7, KA1, and KA2 as GluK1-GluK5. GluK1–GluK3 form functional homomeric receptors when expressed in heterologous systems (Egebjerg et al., 1991; Sommer et al., 1992; Schiffer et al., 1997; Pinheiro P. and Mulle, 2006), although whether native KARs can exist as homomers remains unclear (Carta et al., 2014). GluK4 and GluK5 only form functional receptors when combined with one of the GluK1–GluK3 subunits (Lerma, 2006; Pinheiro P. and Mulle, 2006; Lerma and Marques, 2013; Carta et al., 2014), which generates KARs with varying kinetics and agonist affinities (Perrais et al., 2010; Carta et al., 2014).
KARs are widely dispersed in the CNS. Functional presynaptic KARs are found in brain regions including the hippocampus (Chittajallu et al., 1996; Rodriguez-Moreno et al., 1997; Clarke et al., 1997; Vignes et al., 1998; Negrete-Diaz et al., 2006; Andrade-Talavera et al., 2012), thalamus (Kidd et al., 2002; Andrade-Talavera et al., 2013), hypothalamus (Liu et al., 1999), cortex (Perkinton and Sihra, 1999; Kidd et al., 2002; Rodriguez-Moreno and Sihra, 2013), amygdala (Negrete-Diaz et al., 2012), and cerebellum (Falcon-Moya et al., 2018). Functional postsynaptic KARs are found in areas including the hippocampus (Castillo et al., 1997; Vignes and Collingridge, 1997; Cossart et al., 1998; Frerking et al., 1998), retina (DeVries and Schwartz, 1999), amygdala (Li H. and Rogawski, 1998), cortex (Wu et al., 2005; Campbell et al., 2007), auditory brainstem (Vitten et al., 2004), cerebellum (Bureau et al., 2000), and spinal cord (Li P. et al., 1999). Immunocytochemical (ICC) data, including our own, suggest that KARs in the OB are found on mitral/tufted (M/T) cells, the bulb’s principal output neurons, as well as interneurons including periglomerular (PG) cells and granule cells (Petralia et al., 1994; Montague and Greer, 1999; Davila et al., 2007). Our previous ICC data further suggest that GluK1-containing KARs are more prone to be located at or near synapses than GluK2/3-containing KARs (Davila et al., 2007). One goal of the present study was to examine the characteristics and distribution of functional KARs on M/T cells and interneurons in the OB, including the presence of KARs at synapses.
Only a few studies have provided evidence of functional KARs in the OB. A 2003 study that used 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzoquinoxaline-7-sulfonamide (NBQX), an AMPAR/KAR antagonist, as well as 4-(4-Aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine (SYM 2206), a noncompetitive AMPAR antagonist, examined the role of glutamate receptors in the OB (Lowe, 2003). In that study, mitral cell somatodendritic excitation was attributed to fast AMPAR- and KAR-mediated currents, as well as slow high-affinity NMDAR-mediated currents. However, this study was limited to mitral cells. Furthermore, these methods (flash photolysis of caged glutamate) do not distinguish between pre- and post-synaptic receptors, nor between synaptic and extrasynaptic receptors. In 2006, Schoppa reported a potential role for KARs in mediating synaptic events in granule cell s evoked by patterned olfactory nerve (“dynamic”) stimulation (Schoppa, 2006a). In our 2007 study, we found that KAR activation increases excitatory spontaneous activity but attenuates evoked glutamatergic transmission between OB neurons, likely via a presynaptic depolarizing mechanism (Davila et al., 2007). Another goal of the present study was to explore the potential roles of both presynaptic and postsynaptic KARs in mediating synaptic transmission in the OB.
Studies in various brain regions suggest that KARs modulate synaptic transmission via a variety of mechanisms (Contractor et al., 2011; Rodrigues and Lerma, 2012; Lerma and Marques, 2013; Negrete-Diaz et al., 2018). These include postsynaptic depolarization and mediation of a small component of the synaptic current at some excitatory synapses (e.g., mossy fiber-CA3 pyramidal cell synapse) (Castillo et al., 1997; Vignes and Collingridge, 1997; Lerma and Marques, 2013) and presynaptic modulation of the release of neurotransmitters such as glutamate (Chittajallu et al., 1996; Schmitz et al., 2000; Frerking et al., 2001; Rodriguez-Moreno and Sihra, 2004; Andrade-Talavera et al., 2012; Negrete-Diaz et al., 2012; Sihra and Rodriguez-Moreno, 2013; Andrade-Talavera et al., 2013; Rodriguez-Moreno and Sihra, 2013; Falcon-Moya et al., 2018) and GABA (Rodriguez-Moreno et al., 1997; Rodriguez-Moreno and Lerma, 1998; Liu et al., 1999; Cossart et al., 2001; Mathew et al., 2008). In contrast to AMPARs and NMDARs, some KAR-mediated modulation of synaptic transmission involves metabotropic (G protein-mediated)/non-canonical signaling in addition to traditional ionotropic receptor activity (Rodriguez-Moreno and Sihra, 2007; Rodrigues and Lerma, 2012; Lerma and Marques, 2013; Negrete-Diaz et al., 2018). The ionotropic pathway is responsible for membrane depolarization and the synaptic current as well as the facilitation of transmitter release at some synapses (Schmitz et al., 2001b; Cossart et al., 2001; Lerma and Marques, 2013). The metabotropic/non-canonical pathway is responsible for presynaptic facilitation (some) and inhibition of transmitter release (Rodriguez-Moreno and Lerma, 1998; Frerking et al., 2001; Negrete-Diaz et al., 2006; Jin et al., 2006; Bonfardin et al., 2010; Rodrigues and Lerma, 2012; Negrete-Diaz et al., 2012; Andrade-Talavera et al., 2012; Andrade-Talavera et al., 2013; Rodriguez-Moreno and Sihra, 2013; Lerma and Marques, 2013; Falcon-Moya et al., 2018). In the present study, we used prolonged agonist application in a subset of cells to determine if some of the observed effects may reflect metabotropic activity of KARs.
Different KAR subunit combinations bestow distinct ligand affinities and kinetic properties (Lerma and Marques, 2013). However, until recently, the lack of appropriate pharmacological tools to isolate KAR roles has limited the study of these receptors (Sihra et al., 2014). Some studies relied on potent competitive AMPAR/KAR antagonists such as the quinoxaline-2,3-dione derivatives (e.g., 6-cyano-7-nitroquinoxaline-2,3-dione disodium [CNQX] and NBQX) (Larsen and Bunch, 2011). Other studies have used the 2,3 benzodiazepines, 1-(4-Aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI 52466) (Donevan and Rogawski, 1993; Wilding and Huettner, 1995; Paternain et al., 1995) and 1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro- 7,8- methylenedioxy- 5H-2,3-benzodiazepine hydrochloride (GYKI 53655) (Wilding and Huettner, 1995; Paternain et al., 1995), which are selective and potent noncompetitive AMPAR antagonists with minimal activity at KARs. In the present study, we used GYKI 52466 (GYKI) and the noncompetitive selective AMPAR antagonist SYM 2206 (Pelletier et al., 1996; Li P. et al., 1999) to help pharmacologically isolate KAR-mediated currents.
The development of selective KAR agonists has further clarified the role of KARs in neuromodulation and synaptic transmission. KAR selective agonists include (RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA) and (2S,4R) 4-methylglutamic acid (SYM 2081). ATPA, a substituted analogue of AMPA, is a potent, relatively selective GluK1 agonist (Clarke et al., 1997; Hoo et al., 1999; Alt et al., 2004), with only weak activity at AMPARs (Clarke et al., 1997). SYM 2081, a gamma substituted glutamate analogue, is a potent and selective KAR agonist (Donevan et al., 1998; Small et al., 1998), with 500- to 2000- fold greater selectivity for homomeric GluK1 and GluK2 KARs over AMPARs (Donevan et al., 1998). In the present study, we used ATPA and SYM 2081 to determine whether KARs with different subunit combinations subserve different functions.
KAR-mediated excitatory postsynaptic currents (EPSCs) are present at some synapses in the hippocampus and are characteristically slower and smaller than AMPARmediated EPSCs (Castillo et al., 1997; Frerking et al., 1998; Cossart et al., 1998). Although KAR subunit composition influences EPSC kinetics (Contractor et al., 2003; Barberis et al., 2008; Fernandes et al., 2009), the findings that KAR co-assembly with Neto proteins alters agonist affinity and slows deactivation kinetics may help explain these findings (Copits et al., 2011; Straub et al., 2011a; Straub et al., 2011b; Fisher J. L. and Mott, 2013; Lerma and Marques, 2013). In the OB, KARs could be especially important in circuits in which the relatively brief duration of AMPAR-mediated currents limits the role of AMPARs in synaptic transmission (e.g., reciprocal inhibition at dendrodendritic synapses) (Schoppa et al., 1998; Isaacson and Strowbridge, 1998; Schoppa, 2006a). In the present study, we investigated a potential role for KARs in modulating dendrodendritic inhibition (DDI).
Our findings suggest that functional KARs are expressed by a variety of OB neurons, with a subset of receptors located at synapses. Our findings also suggest that KARs, including those composed of the GluK1 subunit, modulate both excitatory and inhibitory transmission in the OB. Collectively, these data support the notion that KARs participate in the regulation of OB synaptic circuits that encode odor information.
EXPERIMENTAL PROCEDURES
Animals
The protocols for all procedures that we performed were approved by the Florida State University Institutional Animal Care and Use Committee. This includes carrying out all experiments in accordance with the current (8th edition) of the National Institute of Health Guide for the Care and Use of Laboratory Animals. The total number of animals (Sprague-Dawley P1–P5 rat pups) used for culture experiments on 10 recording days was 60 (6 rat pups per set of OB cultures). The total number of animals (14- to 28-dayold Sprague-Dawley rats) used for OB slice experiments on 28 recording days was 56 (2 rats per day).
Tissue Culture
The preparation of primary cultures of OB neurons has previously been described (Trombley and Blakemore, 1999; Blakemore et al., 2006). Briefly, OBs harvested from male and female postnatal days 1–5 (P1–P5) Sprague-Dawley rat pups (Charles River, Wilmington, MA, USA) were cut into 1-mm cubes and then enzymatically treated for 1 h at 37°C in a Ca2-buffered papain solution (Worthington Biochemical, Lakewood, NJ). A fire-polished pipette was then used to triturate the tissue to achieve a single-cell suspension. The cells were plated onto 35-mm-diameter culture dishes on a confluent monolayer of OB astrocytes at varying cell densities (250,000–350,000 cells per dish).
Immunocytochemistry
Procedures were modified from our previous protocols (Trombley and Westbrook, 1990; Berkowicz et al., 1994; Blakemore et al., 2006). Briefly, OB cultures were incubated in blocking serum (5% goat serum) for 45 min, washed with PBS, and then incubated overnight at 4ºC in a 1:1,000 dilution with the primary antibody (glutamic acid decarboxylase [GAD] or vesicular glutamate transporter 1 [VGLUT1])(Chemicon International, Temecular, CA). After being washed in PBS, cultures were incubated in a Cy3-labeled secondary antibody (1:800 dilution) for 45 minutes and washed again in PBS. Primary neuronal cultures were examined for immunoreactivity 7–14 days after treatment with antibodies using fluorescent light microscopy.
Neuronal Identity
Several subtypes of projection neurons, mitral cells and tufted cells, exist in the OB (see Table 2, Nagayama et al., 2014)(Nagayama et al., 2014). These projection neurons form synapses with various types of interneurons (See Figure 1 in Nagayama et al., 2014 for a diagram illustrating the neuronal populations, layers, and synaptic connections in the OB). OB interneurons primarily include external tufted (ET) cells, periglomerular (PG) cells, short axon (SA) cells, and granule cells, which can be divided into various subtypes based on factors including bulb layer, projection patterns, morphology, and molecular properties (see Tables 1 & 3 in Nagayama et al., 2014).
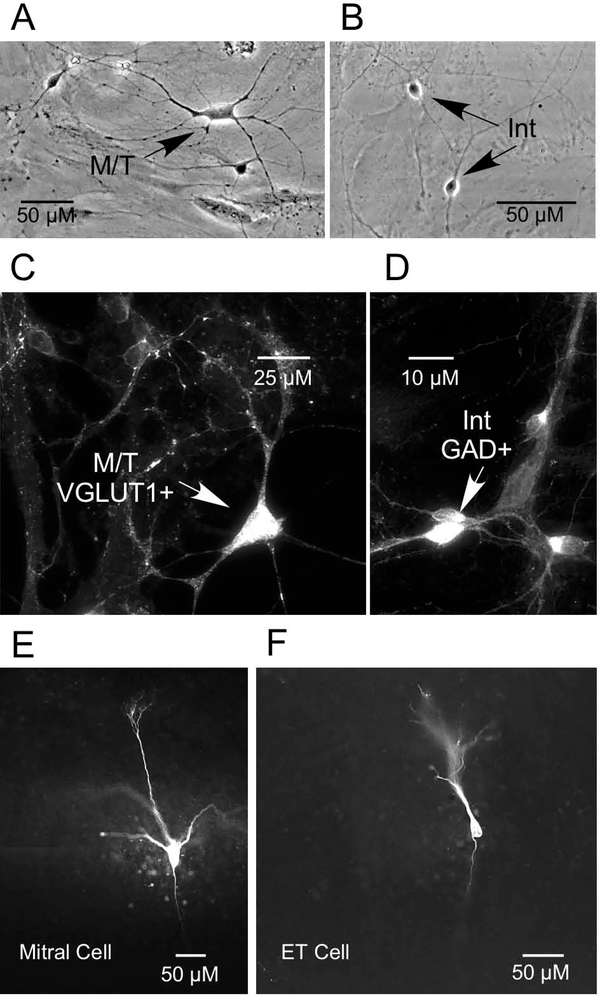
In the present study, our previous morphological criteria (Trombley and Westbrook, 1990) were used to identify presumptive mitral/tufted (M/T) cells and interneurons in OB cultures. M/T cells (Fig. 1A) were identified based on shape (pyramidal, multipolar) and soma size (20 to 40 μm), while the larger population of small-diameter interneurons (Fig. 1B) was identified based on soma size (5–10 μm). As multiple types of tufted cells exist, the term “M/T cells” is used to refer to mitral cells and the largest (internal) tufted cells (>20 μm) (Nagayama et al., 2014). Given the smaller interneurons have similarly sized cell bodies, these neurons are referred to collectively as “interneurons.”
1.
Neuronal identity. A-B) Photomicrographs of presumptive mitral/tufted cell (M/T) and interneuron (Int) in primary OB cultures. C-D) Immunolabeling of cultured OB neurons with glutamic acid decarboxylase (GAD), the GABA-synthesizing enzyme, and vesicular glutamate transporter 1 (VGLUT1) revealed that mitral/tufted cells (M/T) were VGLUT1 positive (+), while interneurons (Int) were GAD +). E-F) Photomicrographs of biocytinfilled mitral cell and external tufted (ET) cell in OB slice.
Expression of markers has also been used to subclassify neurons in the OB. For example, glutamic acid decarboxylase (GAD), the GABA-synthesizing enzyme, is a marker for GABAergic interneurons (PG cells, granule cells, superficial and deep SA cells)(Mugnaini et al., 1984; Gall C. M. et al., 1987; Trombley and Westbrook, 1990). The three subtypes of vesicular glutamate transporter (VGLUT) are markers of glutamatergic neurons at OB synapses (Gabellec et al., 2007). VGLUT1 is present at dendrodendritic synapses between mitral and tufted cells and interneurons in the glomerular layer and external plexiform layer, as well as in axonal synapses of the granule cell layer (Gabellec et al., 2007).
In the present study, immunocytochemistry was performed to further identify and correlate the size and morphology of OB neuronal subtypes with their physiological properties. Immunolabeling of cultured OB neurons with GAD and VGLUT1 revealed that presumptive M/T cells were VGLUT1 positive (+)(Fig. 1C), while presumptive interneurons were GAD + (Fig. 1D).
The three populations of OB interneurons that surround the glomeruli- PG cells, SA cells, and ET cells- are known collectively as juxtaglomerular (JG) cells (Pinching and Powell, 1971a; Shepherd G. M., 1972; Nagayama et al., 2014). These interneurons also consist of various subtypes (see Table 1, Nagayama et al., 2014). In the present study, mitral cells and JG cells in OB slices were identified and differentiated based on their morphology and location within the slice (Shepherd G.M. et al., 2004). Some of the cells from which we recorded were labeled with biocytin, as previously described (Corthell et al., 2014). Figures 1E and 1F show examples of a biocytin-filled mitral cell and ET cell in OB slices, respectively.
Preparation of OB Slices for Electrophysiology
OB slices were prepared for electrophysiology as previously described (Blakemore et al., 2006). OB slices were prepared from male and female 14- to 28-day-old SpragueDawley rats (Charles River, Wilmington, MA, USA) that had been killed by decapitation following halothane anesthesia. OBs were quickly removed and deposited in ice-cold oxygenated (95% O2-5% CO2) saline solution. A vibratory microtome (Vibratome, St. Louis, MO) was used to cut horizontal slices (400 μm). The slices were then incubated in a holding chamber for 30 min at 35°C before being stored at 20–24°C until use. For electrophysiology experiments, slices were positioned in a recording chamber and viewed with a Leica microscope (Leica Microsystems, Wetzlar, Germany) equipped with infrared differential interference contrast optics.
Electrophysiology
Primary culture.
Previously described methods were used to obtain whole-cell patchclamp recordings in cultured OB neurons (Blakemore et al., 2006). OBs were prepared for primary cultures as described above (Trombley and Blakemore, 1999). Whole cell voltage clamp recording in OB neurons took place at room temperature after the OB neurons had been cultured for 7–21 days. The culture dish (35-mm) was used as the recording chamber, and it was perfused at a rate of 0.5–2.0 ml/min with a bath solution containing (in mM) 162.5 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose. NaOH was used to achieve a pH of 7.3, and the final osmolarity was 325 mosmol/l. Patch-clamp electrodes were pulled from borosilicate glass to a final electrode resistance of 4–6 MΩ. These electrodes were filled with a solution containing (in mM) 145 CsCl or KMeSO4, 1 MgCl2, 10 HEPES, 4 Mg2+-ATP, 0.5 Mg2+-GTP, and 1.1 EGTA (pH 7.2; 310 mosmol/l).
A gravity-fed flow pipe perfusion system, comprised of 600-μm diameter square glass barrels, was used to apply drugs that had been diluted in recording solution. An electronic manipulator (Warner Instrument, Hamden, CT) was used to locate the flow pipes near the cell, and drug flow was controlled with pinch clamps. The rapid speed of the solution changes permitted the occurrence of peak drug responses within 100 ms. Neurons were perfused continuously with bath (control) solution except during application of drugs. The applied drugs were kainate (Tocris, Minneapolis, MN), GYKI 52466 (GYKI)(Sigma-Aldrich, St, Louis, MO), SYM 2206 (Tocris), SYM 2081 (Tocris), and tetrodotoxin (TTX)(Tocris).
OB slices.
Previously described methods were used to obtain whole-cell patch-clamp recordings from mitral cells and JG cells in OB slices (Berkowicz et al., 1994; Schoppa et al., 1998; Blakemore et al., 2006). For all experiments, the extracellular solution was oxygenated (95% O2-5% CO2) and contained (in mM) 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 2.5 KCl, 1.0 MgCl2, and 2 CaCl2, pH 7.3. Patch pipettes were pulled to a resistance of 1–3 MΩ in mitral cell recordings and 3–10 MΩ in JG cell recordings. Extracellular drugs were delivered by flow pipe or bath perfusion. Applied drugs included kainate (Tocris), GYKI 52466 (GYKI)(Sigma), ATPA (Tocris), SYM 2081 (Tocris), and lidocaine N-ethyl bromide (QX314)(Sigma). In some experiments, a 1.0% solution of biocytin was added to the pipette solution to aid neuron identification (Corthell et al., 2014).
To examine synaptic KARs, electrical stimulation of axons of olfactory sensory neurons (OSNs) was conducted using a bipolar tungsten electrode (125-μm tip separation; Frederick Haer, Brunswick, ME) or an extracellular patch pipette filled with extracellular solution to stimulate the olfactory nerve layer (ONL) and evoke EPSCs (voltage clamp) or excitatory postsynaptic potentials (EPSPs)(current clamp). Stimulatory pulses were generated via a computer, which triggered a stimulus isolation unit (stimulation ranged from 200 to 500 μA). To determine if KARs play a role in dendrodendritic inhibition (DDI), inhibitory postsynaptic currents (IPSCs) were induced by self-stimulation (using a brief 10 ms, 70 mV depolarizing voltage pulse) of mitral cells and ET cells to examine reciprocal inhibition from granule cells and PG cells, respectively.
EPSC (or EPSP) measurements were performed using a pipette solution containing (in mM) 125 KMeSO4, 2 MgCl2, 0.025 CaCl2, 1 EGTA, 2 Na+-ATP, 0.5 Na+-GTP, and 10 HEPES, pH 7.3. In the former experiments, 10 mM QX314 was added to the electrode solution to prevent action currents. IPSC measurements were performed using a pipette solution containing (in mM) 125 CsCl or KMeSO4, 2 MgCl2, 0.025 CaCl2, 1 EGTA, 2 Na+-ATP, 0.5 Na+-GTP, and 10 HEPES, pH 7.3. QX314 was also added to the electrode solution for these experiments.
Procedures
To examine membrane currents evoked by various combinations of agonists (kainate, SYM 2081, ATPA) and antagonists (SYM 2206, GYKI), whole-cell recordings were obtained from the cell bodies of randomly selected M/T cells and interneurons using AxoClamp and MultiClamp amplifiers (Axon Instruments, Sunnyvale, CA) in discontinuous (switch frequency of 10–15 kHz) or continuous voltage-clamp mode. Membrane currents were filtered at 1–3 kHz, digitized at 5–10 kHz, and analyzed using AxoGraph software (John Clements).
KAR-mediated currents in cultured M/T cells and interneurons were evaluated by measuring the kainate-evoked current in the presence of SYM 2206 or GYKI to block AMPAR-mediated currents; the percentage block was calculated using the following formula: [amplitude of current evoked by kainate alone - amplitude of current evoked by kainate plus SYM 2206 (or GYKI) / amplitude of current evoked by kainate alone] × 100. The residual (KAR-mediated) portion of the current was also expressed as a percentage of the control current and was estimated using the following formula: [amplitude of current evoked by kainate plus SYM 2206 (or GYKI) / amplitude of current evoked by kainate alone] × 100.
For slice experiments involving olfactory nerve layer (ONL) stimulation, individual traces (N=3–6) were averaged for each measurement recorded in a cell. The mean amplitude of this average was quantified using the “mouse measure” feature of Axograph, which allows you to determine the difference in amplitude between two points (i.e., baseline and current [EPSC] or voltage [EPSP] peak). A similar quantification method was used for experiments involving self-stimulation with a KMeSO4 electrode to measure IPSCs. For experiments involving self-stimulation with a CsCl electrode that caused a flurry of IPSCs, charge transfer during the first second after stimulation was determined for each trace using the area under the curve (AUC) measurement tool in AxoGraph. The average AUC for 3 traces was determined for each cell.
To investigate the presence of synaptic KARs, AP5 (to block NMDARs) and GYKI (to block AMPARs) were applied during synaptic events (EPSCs) evoked in mitral cells and JG cells in OB slices using ONL stimulation. The residual portion of the current, corresponding to that mediated by KARs, was expressed as a percentage of the control current and was estimated using the following formula: [amplitude of AP5 plus GYKI EPSC /amplitude of control EPSC] × 100.
The effects of kainate and ATPA on synaptic events (EPSCs) were investigated by comparing the control EPSC to the EPSC recorded in the presence of kainate or ATPA. The resulting degree of current potentiation or inhibition was expressed as a percentage of the control current and was estimated using the following formula: [amplitude of kainate (or ATPA) EPSC /amplitude of control EPSC] × 100. S imilar formulas were used to determine the effects of kainate and/or ATPA on other types of evoked synaptic events (EPSPs, IPSCs).
The effects of kainate and ATPA on the flurry of IPSCs evoked using the self-stimulation protocol with a CsCl electrode were measured by determining the charge transfer, as described above.
Data Analysis
GraphPad Prism (version 7; GraphPad Software Inc. La Jolla, CA, USA) was used for all statistical analyses. The Kolmogorov-Smirnov (KS) normality test and Shapiro-Wilk normality test were used to test for normality. The F test was used to assess for homogeneity of variance. As the sampled distributions had normal distributions and equal variances, the paired or unpaired t-test was used for all statistical analyses. Values are expressed as the mean ± SEM. Differences between means are reported as t(df)=x.xx, P ≤ 0.0x. Differences were considered statistically significant if the P value was ≤ 0.05, and P values are reported as P ≤ 0.05 or P ≤ 0.01.
RESULTS
What are the characteristics and distribution of KARs in the OB?
Although evidence from several studies suggests that functional KARs are expressed in the OB (Lowe, 2003; Schoppa, 2006a; Davila et al., 2007), the characteristics and distribution of KARs among various OB neuron types is unclear. In the present study, we applied a variety of AMPAR and KAR agonists and antagonists during whole-cell recording from OB neurons to isolate and examine KAR-mediated currents. As the speed of the onset and offset of drug application is superior in culture to slice, the majority of the experiments in this portion of our study were performed in OB cultures.
Kainic acid (kainate) is approximately 100-fold more selective for KARs over AMPARs (Huettner, 1990; Patneau et al., 1994; Paternain et al., 1995; Zhou et al., 1997). Currents activated by kainate but mediated by AMPARs do not desensitize, whereas currents activated by kainate and mediated by KARs strongly desensitize (Patneau and Mayer, 1991; Lerma et al., 1993). We used this differential response as one means of identifying functional KARs in cultured OB neurons. During whole-cell recording, application of 100 μM kainate activated currents in all M/T cells (N=21) and interneurons (N=8) examined, with mean peak current amplitudes of 143.27±22.61 pA (range: 43.44 to 442.5 pA) and 24.79±6.48 pA (range: 7.6 to 60.21 pA), respectively. In some cells (Fig. 2A), 100 μM kainate activated a large nondesensitizing (square) current, consistent with a significant AMPAR contribution to the current (Patneau and Mayer, 1991). In other cells (Fig. 2B), kainate activated a strongly desensitizing current, consistent with a significant KAR contribution to the current.
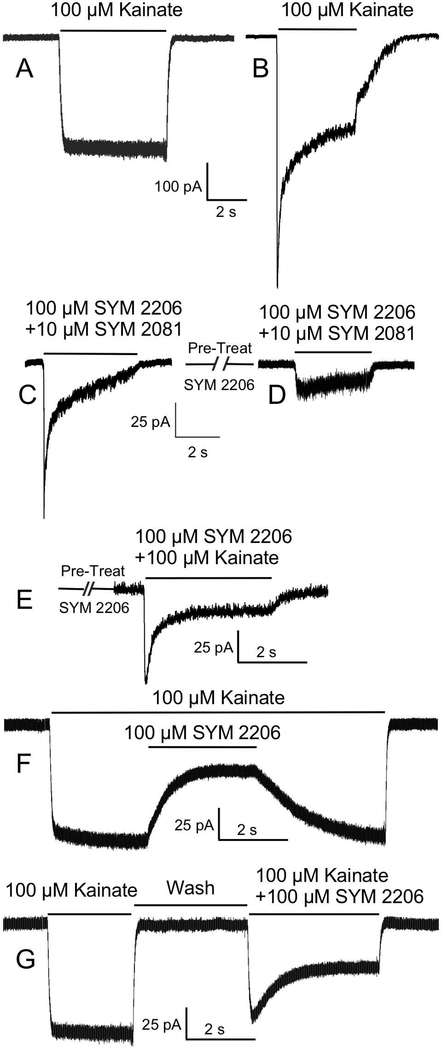
2.
Kainate receptor (KAR)-mediated currents in cultured olfactory bulb (OB) neurons isolated with KAR agonists (kainate or SYM 2081) and an AMPA receptor (AMPAR) antagonist (SYM 2206). A) Kainate activated a large nondesensitizing (square) current in some OB cells, consistent with a significant AMPAR contribution to the current. B) However, a strongly desensitizing current was seen in other cells, consistent with a significant KAR contribution to the current. C) Coapplication of the selective KAR agonist, SYM 2081, and SYM 2206 produced a prominent current in these cells. D) Same as “C” but with pretreatment with SYM 2206, reflecting the timing for the onset of the effects of SYM 2206 in blocking AMPARs. E) Coapplication of kainate and SYM 2206 after pretreatment with SYM 2206. F) Application of SYM 2206 during a current evoked by application of kainate. SYM 2206 blocked the AMPAR-mediated portion of this current, revealing the relatively large KAR-mediated component of the current. G) Currents evoked by sequential application of kainate and kainate +SYM 2206.
The selective KAR agonist SYM 2081 has 500- to 2000- fold greater selectivity for KARs over AMPARs and is a potent agonist at homomeric GluK1 and GluK2 KARs (Donevan et al., 1998). To ensure currents evoked by 10 μM SYM 2081 would not have actions at AMPARs, we combined administration of SYM 2081 with an AMPAR antagonist (SYM 2206) (Pelletier et al., 1996; Li P. et al., 1999). Coapplication of 10 μM SYM 2081 with 100 μM SYM 2206 produced currents in all cells examined (Fig 2C)(N=15). To further ensure AMPARs were blocked, we pretreated cells with 100 μM SYM 2206. Subsequent coapplication of 100 μM SYM 2206 with 3 μM SYM 2081 (N=19) or 10 μM SYM 2081 (N=41)(Fig. 2D) evoked qualitatively similar currents, but there was no visible current in 5 of the 19 OB neurons treated with the lower concentration of SYM 2081. The mean peak current amplitude evoked by 10 μM SYM 2081 + 100 μM SYM 2206 was 14.93±1.74 pA in M/T cells (N=36) and 7.27±2.68 pA in interneurons (N=5). As 100 μM SYM 2206 pretreatment completely blocked currents evoked by application of 50 μM AMPA (i.e., AMPARs)(N=5; data not shown), the currents evoked by coapplication of SYM 2081 and SYM 2206 were likely entirely mediated by KARs.
We next coapplied 100 μM SYM 2206 and 100 μM kainate following pretreatment with SYM 2206 to ensure AMPARs were blocked (Fig 2E). We observed KAR-mediated currents in most OB neurons examined (N=8/11), with a mean peak amplitude of 13.16±4.35 pA. We also coapplied 100 μM SYM 2206 during currents evoked by application of 100 μM kainate (Fig. 2F). In M/T cells (N=25) and interneurons (N=3), SYM 2206 blocked 91.72±2.13% (range: 49.83 to 100%) and 81.82±9.32% (range: 65 to 97.2%) of the kainate-evoked current, respectively; the residual KAR-mediated components of the current were 8.83±2.13% and 18.12±9.32% of the control current in M/T cells and interneurons, respectively. The example shown here represents an OB neuron with a relatively large KAR-mediated current (Fig 2F). Figure 2G shows sequential application of the drugs and no SYM 2206 pretreatment (N=9).
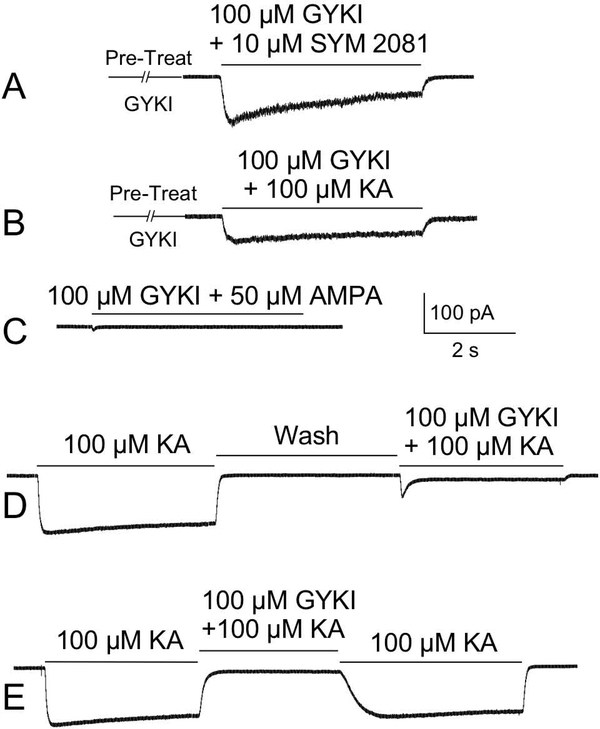
In other experiments, we used another AMPAR antagonist, GYKI 52466 (GYKI) (Donevan and Rogawski, 1993; Wilding and Huettner, 1995; Paternain et al., 1995), plus a KAR agonist (kainate or SYM 2081) to isolate KAR-mediated currents in both culture (Fig. 3) and OB slices (Fig. 4). Coapplication of 100 μM GYKI with 50 μM AMPA revealed that GYKI effectively blocked AMPARs in both M/T cells (N=7) and interneurons (N=3)(Fig. 3C). Following pretreatment with GYKI, coapplication of 100 μM GYKI and 10 μM SYM 2081 (Fig. 3A) evoked currents in all M/T cells (N=22) and interneurons (N=6) examined, with mean peak current amplitudes of 38.77±6.26 pA and 11.55±2.47 pA, respectively. Following pretreatment with GYKI, coapplication of 100 μM GYKI and 100 μM kainate (Fig. 3B) evoked currents in all M/T cells (N=27) and most interneurons (N=4/6) examined, with mean peak current amplitudes of 23.6±4.13 pA and 5.01±0.49 pA, respectively. Figure 3D shows sequential application of the drugs and no GYKI pretreatment (N=19). We also coapplied 100 μM GYKI during a current evoked by 100 μM kainate (Fig. 3E). In M/T cells (N=30) and interneurons (N=6), GYKI blocked 92.50±1.01% (range: 80.39 to 100%) and 89.32±2.20% (range: 78.63 to 93.41%) of the kainate-evoked current, respectively; the residual KAR-mediated components of the currents were 7.5±1.01% and 10.68±2.2% of the control current in M/T cells and interneurons, respectively. In the example shown in Figure 3E, most of the current in this cell is mediated by AMPARs (i.e., small KAR-mediated current).
3.
KAR-mediated currents in cultured OB neurons isolated with KAR agonists (SYM 2081 or kainate) and an AMPAR antagonist (GYKI). A) Coapplication of the selective KAR agonist, SYM 2081, and GYKI following pretreatment with GYKI. As GYKI blocked AMPARs, this current was virtually entirely KAR mediated. B) A similar current is evoked by coapplication of kainate (KA) and GYKI after pretreatment with GYKI. C) GYKI completely blocks AMPAR-mediated currents activated by application of AMPA. D) Sequential application of kainate and kainate + GYKI. E) Application of GYKI during a current evoked by application of kainate. GYKI blocked the AMPAR-mediated portion of this current, revealing the relatively small KAR-mediated component of the current.
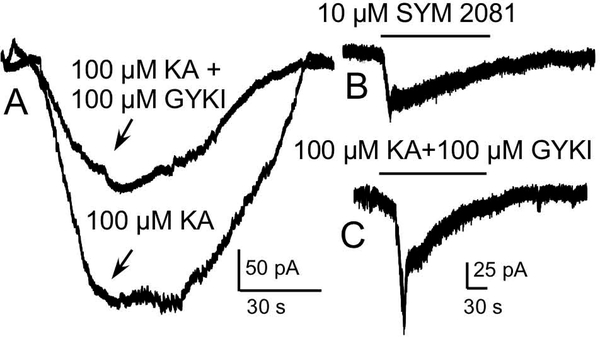
4.
KAR-mediated currents in neurons in OB slices. A) Coapplication of the AMPAR antagonist, GYKI, and kainate (KA) evoked a KAR-mediated current in this mitral cell. The kainate alone trace shows activation of both KARs and AMPARs for comparison. B) Current in a mitral cell evoked by application of the selective KAR agonist SYM 2081. C) Coapplication of GYKI and kainate evoked a current in this mitral cell.
We next examined KAR-mediated currents in neurons in OB slices. Application of 100 μM GYKI and 100 μM kainate evoked currents in all OB neurons (N=7) examined (Figs 4A, 4C), with a mean peak current amplitude of 86.56±19.44. For comparison, Figure 4A also shows the corresponding current in this mitral cell evoked by 100 μM kainate alone, which activated both AMPARs and KARs. Figure 4B shows the current in a mitral cell evoked by application of 10 μM SYM 2081.
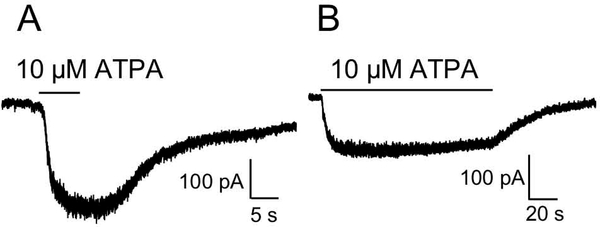
ATPA is a potent, relatively selective GluK1 agonist (Clarke et al., 1997; Hoo et al., 1999; Alt et al., 2004), with only weak activity at AMPARs (Clarke et al., 1997). Consistent with our finding that GluK1 is highly expressed in the OB (Davila et al., 2007), application of 10 μM APTA evoked currents in neurons in OB slices (mitral cells and JG cells)(Fig. 5). The mean amplitude of the current evoked by ATPA in these cells was 243.77±9.37 pA (N=5).
5.
Evidence for functional KARs containing the GluK1 subunit. Application of the selective GluK1 agonist, ATPA, evoked currents in neurons in OB slices (A: mitral cell; B: juxtaglomerular [JG] cell).
Are functional KARs located at synapses in the OB?
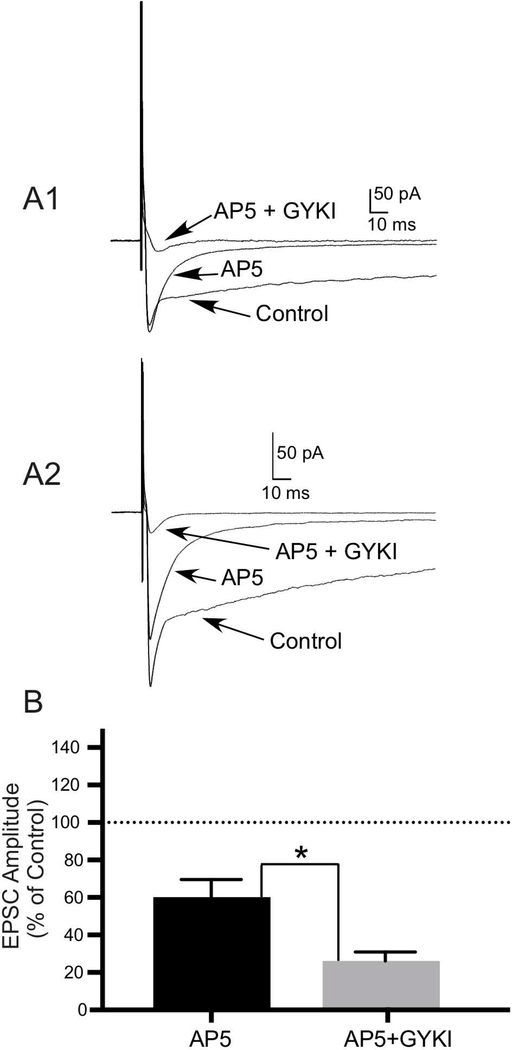
EPSCs mediated by KARs have been found at only a few central synapses (Lerma and Marques, 2013). To determine whether KARs in the OB are located at synapses, we evoked EPSCs in mitral cells (N=4) and JG cells (N=8) in OB slices by stimulation of the olfactory nerve layer (ONL). We then applied 100 μM AP5 (to block NMDARs) and 100 μM GYKI (to block AMPARs) to isolate KAR-mediated responses mediated by postsynaptic KARs. Application of AP5+GYKI reduced the mean amplitude of EPSCs in mitral cells (Fig. 6A1) and JG cells (Fig. 6A2) to 23.94±4.45% (N=4; t(3)=3.488, P ≤ 0.05) and 26.85±4.22% (N=8; t(7)=2.966, P ≤ 0.05) of the control current, respectively. These portions of the EPSCs in these cells were mediated by postsynaptic KARs. Comparison of the EPSCs recorded in the presence of AP5 alone (to isolate AMPA/KA receptors by blocking NMDARs) or the presence of AP5 +GYKI (to isolate KARs by blocking NMDARs + AMPARs) in a subset of cells revealed a significant difference in EPSC amplitudes (N=5; t(4)=2.952, P ≤ 0.05)(Fig. 6B).
6.
A subset of KARs in the OB is located at synapses. A1, A2) To determine whether KARs in the OB are located at synapses, excitatory postsynaptic currents (EPSCs) in neurons in OB slices were evoked by stimulation of the olfactory nerve layer (ONL). AP5 (to block NMDARs) and GYKI (to block AMPARs) were then coapplied to isolate EPSCs mediated by postsynaptic KARs. Compared to the control currents, application of 100 μM AP5 + 100 μM GYKI significantly reduced the mean amplitudes of EPSCs in mitral cells (A1)(N=4; P ≤ 0.05) and JG cells (A2)(N=8; P≤ 0.05), leaving visible the KAR-mediated portions of the responses. B) Histogram of data from a subset of cells showing a significant difference in the amplitudes of EPSCs recorded in the presence of 100 μM AP5 alone (to isolate AMPA/KA receptors by blocking NMDARs) or the presence of 100 μM AP5 + 100 μM GYKI (to isolate KARs by blocking NMDARs + AMPARs) expressed as a percentage of the control EPSC amplitude (N=5; P* ≤ 0.05). Values are the mean ± SEM.
Do KARs modulate glutamate-mediated (excitatory) transmission in the OB?
In the hippocampus, Chittajallu and colleagues were the first to demonstrate that kainate decreased glutamate release at Schaffer collateral-CA1 pyramidal cell synapses in a manner indicative of activation of presynaptic KARs (Chittajallu et al., 1996). Subsequent studies showed that presynaptic KARs modulate excitatory transmission but in a bidirectional manner. That is, low concentrations of kainate (25– 100 nM) can facilitate transmission (Lauri et al., 2001a; Schmitz et al., 2001b; Schmitz et al., 2001a; Andrade-Talavera et al., 2012), whereas higher kainate concentrations (200–500 nM) can inhibit transmission (Kamiya and Ozawa, 2000; Schmitz et al., 2000; Lauri et al., 2001a; Schmitz et al., 2001b; Schmitz et al., 2001a; Negrete-Diaz et al., 2006).
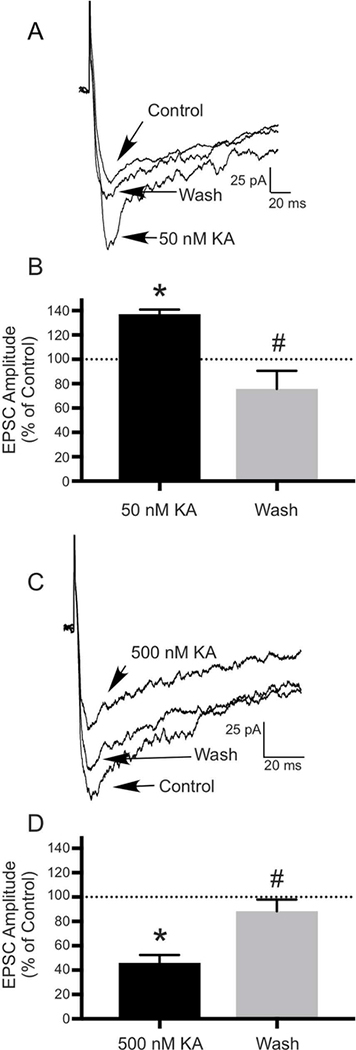
To determine whether similar modulatory mechanisms exist in the OB, we applied several concentrations of kainate during ONL stimulation and looked at its effects on EPSCs recorded from OB neurons. Consistent with previous studies, 50 nM kainate increased the mean amplitude of the glutamate-mediated EPSC to 137.21±3.62% of control in 4 (JG) cells (N=4; t(3)=9.208, P ≤ 0.01)(Figs. 7A, B). Following a wash, the EPSC recovered to 75.7±14.97% of control (N=4; t(3)=5.497, P ≤ 0.05)(Figs. 7A, 7B). Application of 500 nM kainate decreased the mean amplitude of the EPSC to 45.88±6.48% of control in 4 JG cells and 2 mitral cells (N=6; t(5)=5.143, P ≤ 0.01)(Figs. 7C, 7D). Following a wash, the EPSC recovered to 88.34±9.51% of control (N=6; t(5)=3.162, P ≤ 0.05)(Figs. 7C, 7D).
7.
Kainate has biphasic, concentration-dependent effects on EPSCs recorded in neurons in OB slices, suggesting a role for KARs in modulating excitatory transmission in the OB. EPSCs were evoked in neurons in OB slices by ONL stimulation. A) Compared to the control current, application of 50 nM kainate (KA) significantly increased the mean amplitude of the EPSC, with significant recovery of the current following a wash. B) Histogram showing the effect of 50 nM KA and recovery following a wash expressed as a percentage of the control EPSC amplitude (N=4; * P≤ 0.01 compared to control; # P ≤ 0.05 compared to 50 nM KA). C) Application of 500 nM kainate (KA) significantly decreased the mean amplitude of the EPSC, with significant recover of the current following a wash. D) Histogram showing the effect of 500 nM KA and recovery following a wash expressed as a percentage of the control EPSC amplitude (N=6; *P ≤ 0.01 compared to control; #P ≤ 0.05 compared to 500 nM KA). Values are the mean ± SEM.
Do GluK1-containing KARs mediate excitatory transmission in the OB?
The KAR subunit composition underlying the depression of excitatory transmission has been investigated in other brain regions. The selective GluK1 agonist ATPA (2 μM) mimicked kainate’s presynaptic depressant action in the CA1 region of the hippocampus, suppressing EPSCs evoked by mossy fiber (MF) stimulation ( Vignes et al., 1998). ATPA (1 to 2 μM) also decreased NMDAR-mediated EPSCs (Schmitz et al., 2000), consistent with a presynaptic effect on transmitter release. These results suggest that presynaptic KARs on excitatory synaptic terminals may contain GluK1. Given our prior ICC data suggesting the expression of GluK1 subunits at synapses in glomeruli (Davila et al., 2007), we investigated whether similar actions of ATPA occur in the OB by applying a range of concentrations of ATPA during ONL stimulation.
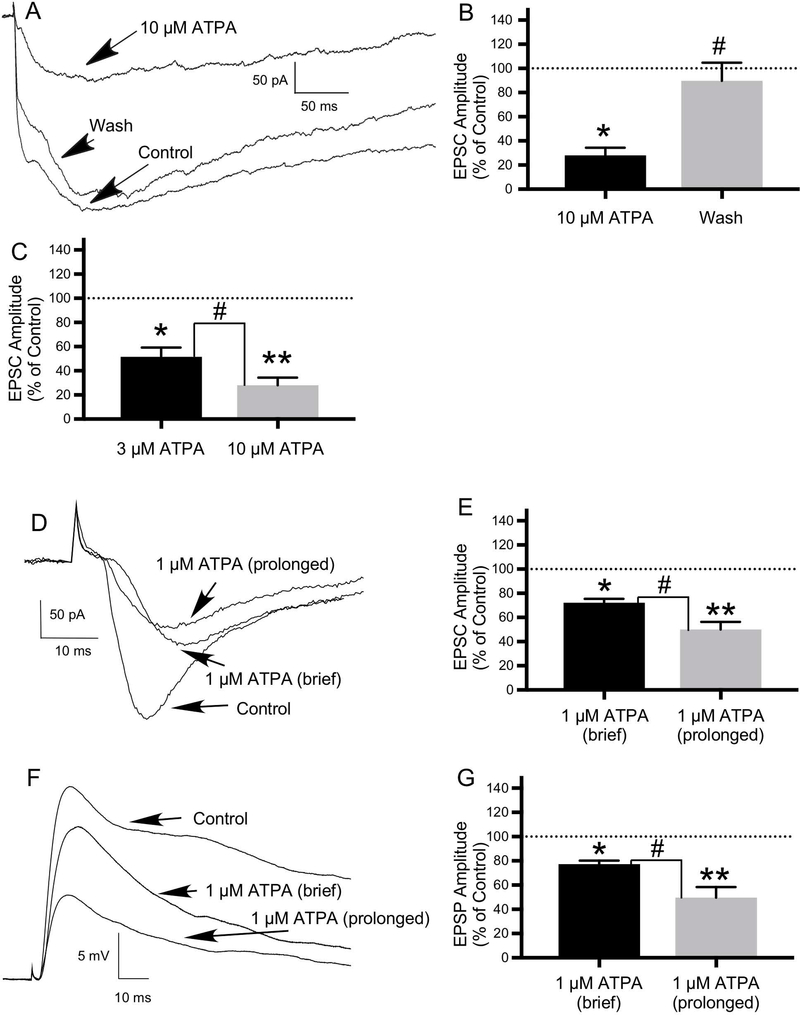
Application of a range of ATPA concentrations (1 μM, 3 μM and 10 μM) had qualitatively similar effects--decreasing the amplitude of the ONL-evoked EPSCs recorded in voltage clamp mode. Application of 3 μM and 10 μM (Figs. 8A, 8B) ATPA decreased the mean amplitude of the EPSC to 51.54±7.75% of control (N=7: 5 JG cells, 2 mitral cells; t(6)=2.562, P ≤ 0.05) and 28.09±6.18% of control (N=5: 4 JG cells, one mitral cell; t(4)=4.744, P ≤ 0.01), respectively. Following a wash, the EPSC amplitude recovered to 80.41±8.0% and 89.77±14.92% of control in cells treated with 3 μM ATPA (N=6; t(5)=2.715, P ≤ 0.05) and 10 μM ATPA (N=5; t(4)=4.076, P ≤ 0.05)(Figs. 8A, 8B), respectively. The higher dose of ATPA decreased the EPSC to a greater degree (t(9)=2.365, P ≤ 0.05)(Fig. 8C). In addition, 10 μM ATPA increased the frequency of synaptic failures.
8.
The GluK1-selective agonist, ATPA, inhibits EPSCs recorded in neurons in OB slices, suggesting a role for GluK1-containing KARs in modulating excitatory transmission in the OB. EPSCs were evoked in neurons in OB slices by ONL stimulation. A) Compared to the control current, application of 10 μM ATPA significantly decreased EPSC amplitude, with significant recovery of the current following a wash. B) Histogram showing the effect of 10 μM ATPA and recovery following a wash expressed as a percentage of the control EPSC amplitude (N=5; *P ≤ 0.01 compared to control; #P ≤ 0.05 compared to 10 μM ATPA). C) Histogram showing significant difference in the effects of 3 μM ATPA (N=7) and 10 μM ATPA (N=5) on EPSC amplitude expressed as a percentage of the control EPSC amplitude (#P ≤ 0.05; *P ≤ 0.05 compared to control; **P ≤ 0.01 compared to control). D) Compared with control, both brief (30 second) and prolonged (5–10 minute) application of 1 μM ATPA also significantly decreased EPSC amplitude, with greater effects with prolonged duration of ATPA application. E) Histogram showing significant difference in the effects of brief versus prolonged duration of 1 μM ATPA application on EPSC amplitude expressed as a percentage of the control EPSC amplitude (N=7; #P ≤ 0.05; *P ≤ 0.01 compared to control; **P ≤ 0.01 compared to control). F) Effects of 1 μM ATPA on ONL-evoked synaptic response recorded in current clamp mode (EPSP) in same cell as in “D” recorded in voltage clamp mode (EPSC). Compared with control, both brief and prolonged application of 1 μM ATPA significantly decreased excitatory postsynaptic potential (EPSP) amplitude, with greater effects with prolonged ATPA application. G) Histogram showing significant difference in the effects of brief versus prolonged duration of 1 μM ATPA application on EPSP amplitude expressed as a percentage of the control EPSP amplitude (N=5; #P ≤ 0.05; *P ≤ 0.05 compared to control; **P ≤ 0.05 compared to control). Values are the mean ± SEM.
Does prolonged KAR agonist (ATPA) application have greater effects?
The effects of prolonged application of a KAR agonist on excitatory transmission have been investigated in other brain regions. After a 4-minute perfusion of kainate (1 μM) to neurons in hippocampal slices, Negrete-Díaz and colleagues observed a long-lasting inhibition of CA3-evoked EPSCs (eEPSCs) (Negrete-Diaz et al., 2006). This kainatemediated depression of eEPSCs was 63.2 ±3.7% in the peak depression at 6 min after kainate application; 41 ± 2%, at 25 min; and 17.3 ± 2.3%, at 45 min and did not recover completely until 60 min after kainate application (see Fig 1C in Negrete-Díaz et al., 2006). To investigate potential differential effects of the duration of KAR agonist application in the OB, we applied ATPA for brief (e.g., 30 seconds) and prolonged (5–10 minutes) intervals and observed the effects on EPSCs (voltage clamp) and EPSPs (current clamp).
Application of 1 μM ATPA decreased EPSC amplitude, with increasing effects with longer duration of ATPA application (Figs. 8D, 8E). Brief (e.g., 30 seconds) application of 1 μM ATPA decreased the mean amplitude of the ONL-evoked EPSC to 72.15±3.25% of control (N=7: 5 JG cells, 2 mitral cells; t(6)=3.597, P ≤ 0.01). Prolonged (5–10 minute) application of 1 μM ATPA decreased the mean amplitude of the EPSC to 50.0±6.36% of control (N=7: 3 JG cells, 4 mitral cells; t(6)=4.0, P ≤ 0.01). This difference in the effects of brief versus prolonged application of ATPA on the EPSC was statistically significant (N=7; t(9)=3.102, P ≤ 0.05)(Fig. 8E).
In a subset of cells, the effects of 1 μM ATPA were also examined on ONL-evoked synaptic responses recorded in current clamp mode (Figs. 8F, 8G). Brief application of 1 μM ATPA decreased the mean amplitude of the ONL-evoked EPSP to 77.24±2.9% of control (N=5: 2 mitral cells, 3 JG cells; t(4)=7.143, P ≤ 0.05). Prolonged application of 1 μM ATPA decreased the mean amplitude of the EPSP to 49.63±8.84% of control (N=5: 3 mitral cells, 2 JG cells; t(4)=3.675, P ≤ 0.05). This difference in the effects of brief versus prolonged application of ATPA on the EPSP was statistically significant (N=5; t(5)=2.969, P ≤ 0.05)(Fig. 8G). ATPA had no effect on the EPSP recorded in one (mitral) cell and increased the amplitude of the EPSP in another (mitral) cell. Figures 8D and 8F show the effects of 1 μM ATPA on ONL-evoked synaptic responses in voltage clamp (EPSC) and current clamp (EPSP) recorded in the same cell.
Do KARs modulate GABA-mediated (inhibitory) transmission in the OB?
The OB is unusual in that mitral and tufted cells make reciprocal connections with some types of interneurons (PG cells, granule cells) at dendrodendritic synapses. There, glutamate-mediated activation of interneurons leads to feedback inhibition via GABA release from interneurons onto mitral and tufted cells at the same synaptic specialization (Ennis et al., 2007; Ennis et al., 2015). Although early studies indicated that reciprocal inhibition at dendrodendritic synapses between mitral cells and granule cells was driven by NMDARs (Schoppa et al., 1998; Isaacson and Strowbridge, 1998), data from a later study suggest a potential role for KARs on granule cells (Schoppa, 2006a).
In other brain regions, presynaptic KARs modulate inhibitory (GABAergic) transmission in a complex bidirectional manner, involving both depression and facilitation of GABA release (Lerma and Marques, 2013). As with glutamatergic synapses, a biphasic concentration-dependent response of presynaptic KARs to application of kainate has been found at GABAergic synapses. For example, in the hippocampus, application of a low dose of kainate (300 nM) potentiated some GABAergic synapses, whereas a higher dose of kainate (5 μM) inhibited some GABAergic synapses (Jiang et al., 2001).
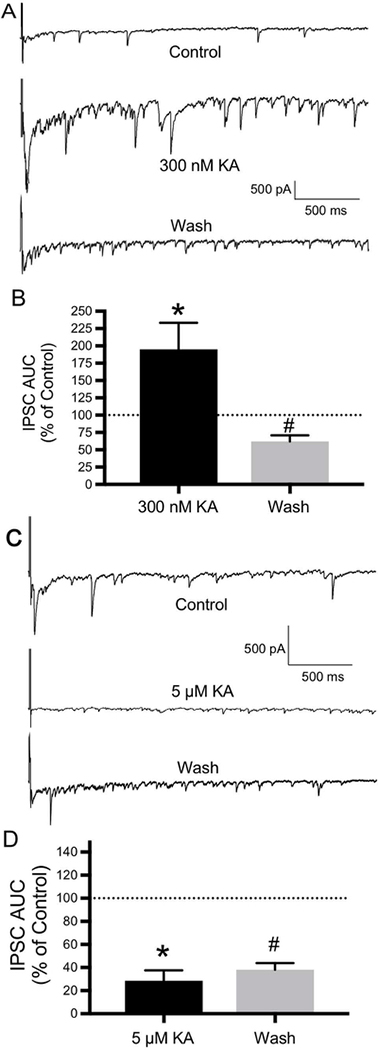
To test the hypothesis that KARs play a similar role in the OB, we investigated the effects of kainate on reciprocal inhibition. Reciprocal inhibition was induced by selfstimulation (using a brief depolarizing voltage pulse) of mitral cells in OB slices to examine reciprocal inhibition from granule cells. With use of a CsCl electrode, this selfstimulation protocol evoked a flurry of IPSCs. These data were quantified by determining the area under the curve (AUC) of the flurry of IPSCs, which represents the charge transfer during the first second after stimulation. Kainate had biphasic concentration-dependent effects. A low concentration of kainate (300 nM) had a potentiating effect on the flurry of IPSCs, increasing the AUC of IPSCs to 194.89±38.6% of control (N=5 mitral cells; t(4)=6.753, P ≤ 0.01)(Figs. 9A, 9B). Following a wash, the AUC of IPSCs recovered to 62±8.9% of control (N=5; t(4)=5.121, P ≤ 0.01)(Figs. 9A, 9B). A higher kainate concentration (5 μM) had an inhibitory effect on the flurry of IPSCs, decreasing the AUC of IPSCs to 28.59±8.95% of control (N=5 mitral cells; t(4)=4.183, P ≤ 0.05)(Figs. 9C, 9D). Following a wash, the AUC of IPSCs recovered towards the control value (to 38.07±5.75% of control), but the difference between drug and wash did not reach statistical significance (N=5; t(4)=0.205, P=≥ 0.05)(Figs. 9C, 9D). Consistent with its inhibitory effect, application of 5 μM also increased the frequency of synaptic failures.
9.
KARs modulate inhibitory transmission in the OB. Reciprocal inhibition was induced by self-stimulation (using a brief depolarizing voltage pulse) of mitral cells in OB slices to examine reciprocal inhibition from granule cells. With use of a CsCl electrode, this self-stimulation protocol evoked a flurry of inhibitory postsynaptic currents (IPSCs). These data were quantified by determining the area under the curve (AUC) of the flurry of IPSCs, which represents the charge transfer during the first second after stimulation. Kainate (KA) had biphasic concentration-dependent effects. A) Compared with control, a low concentration of kainate (300 nM) had a potentiating effect on reciprocal IPSCs, with significant recovery of the IPSCs following a wash. B) Histogram showing the effect of 300 nM KA and recovery following a wash expressed as a percentage of the control IPSC AUC (N=5; * P≤ 0.01 compared to control; #P ≤ 0.01 compared to 300 nM KA). C) A higher kainate concentration (5 μM) had an inhibitory effect on reciprocal IPSCs, with only limited recovery of the IPSCs following a wash. D) Histogram showing the effect of 5 μM KA and limited recovery following a wash expressed as a percentage of the control IPSC AUC (N=5; *P ≤ 0.05 compared to control; #P ≥ 0.05 compared to 5 μM KA). Values are the mean ± SEM.
Do GluK1-containing KARs mediate inhibitory transmission in the OB?
The subunit composition of the KARs mediating inhibitory transmission has also been investigated in other brain regions. As with excitatory transmission, ATPA influences inhibitory transmission in a bidirectional manner. For example, in the basolateral amygdala, application of a low dose of ATPA (300 nM) facilitated GABAergic transmission, whereas a high dose of ATPA (10 μM) inhibited GABAergic transmission (Braga et al., 2003). To see if similar mechanisms occur in the OB, we examined the effects of a two concentrations of ATPA (1 μM and 10 μM) on reciprocal inhibition.
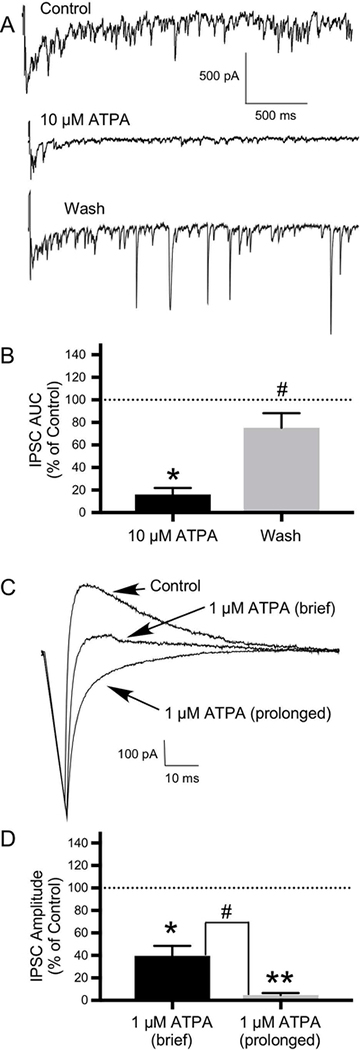
Reciprocal inhibition was induced by self-stimulation (using a brief depolarizing voltage pulse) of mitral cells and ET cells in OB slices to examine reciprocal inhibition from granule cells and PG cells, respectively. With use of a CsCl electrode, this self-stimulation protocol evoked a flurry of IPSCs recorded from OB neurons, which were quantified by determining the AUC. Application of 10 μM ATPA had an inhibitory effect on the flurry of reciprocal IPSCs, decreasing the AUC of IPSCs to 16.03±5.94% of control (N=5: 2 ET cells and 3 mitral cells; t(4)=3.35, P ≤ 0.05)(Figs. 10A, 10B). Following a wash, the AUC of IPSCs recovered to 75.18±13.08% of control (N=5; t(4)=2.826, P ≤ 0.05)(Figs. 10A, 10B).
10.
GluK1-containing KARs modulate inhibitory transmission in the OB. Reciprocal inhibition was induced by self-stimulation (using a brief depolarizing voltage pulse) of mitral cells and external tufted (ET) cells in OB slices to examine reciprocal inhibition from granule cells and periglomerular (PG) cells, respectively. A) With use of a CsCl electrode, this self-stimulation protocol evoked a flurry of IPSCs, which was quantified by determining the AUC. Compared with control, application of 10 μM ATPA significantly inhibited these reciprocal IPSCs, with significant recovery of IPSCs following a wash. B) Histogram showing the effect of 10 μM ATPA and recovery following a wash expressed as a percentage of the control IPSC AUC (N=5; *P ≤ 0.05 compared to control; # P≤ 0.05 compared to 10 μM ATPA). C) The effects of 1 μM ATPA on reciprocal inhibition in neurons in OB slices were also examined. With use of a KMeSO4 electrode, the self-stimulation protocol evoked a single IPSC. Both brief (30 seconds) and prolonged (5–10 minutes) application of ATPA significantly reduced IPSC amplitude, with greater effects with prolonged ATPA application. D) Histogram showing significant difference in the effects of brief (N=9) versus prolonged (N=7) duration of 1 μM ATPA application on IPSC amplitude expressed as a percentage of the control IPSC amplitude (#P ≤ 0.01; *P ≤ 0.01 compared to control; **P ≤ 0.01 compared to control). Values are the mean ± SEM.
We next examined the effects of both brief and prolonged application of 1 μM ATPA on reciprocal inhibition. With use of a KMeSO4 electrode, the self- stimulation protocol evoked a single IPSC. Brief (30 seconds) application of ATPA (1 μM) reduced the mean amplitude of the IPSC to 39.62±8.96% of control in 9 cells (N=9: 6 mitral cells, 3 ET cells; t(8)=4.207, P ≤ 0.01)(Figs. 10C, 10D) and had no effect in one ET cell. Prolonged (5–10 minutes) application of ATPA reduced the mean amplitude of the IPSC to 4.75±1.85% of control (N=7: 4 mitral cells, 3 ET cells; t(6)=4.257, P ≤ 0.01)(Figs. 10C, 10D). This difference in the effects of brief versus prolonged application of ATPA on the IPSC was statistically significant (t(9)=3.812, P ≤ 0.01)(Fig. 10D).
DISCUSSION
We and others have previously shown distinct laminar and cellular distributions of KAR subunits in the OB. In the present study, we used agonists and antagonists with actions at a variety of AMPAR and KAR subunits to investigate KAR-mediated currents in the OB. Our findings suggest that functional KARs composed of a variety of KAR subunits are widely expressed in the OB, on both principal (M/T) cells and interneurons, with a subset of receptors located at synapses. Presynaptic KARs appear to be important modulators of both excitatory and inhibitory transmission, potentially via both ionotropic and metabotropic activity. Along with previous results, our data also suggest that postsynaptic KARs may mediate synaptic responses and influence neuronal excitability. Lastly, our findings, combined with the previously described kinetic characteristics of KARs, suggest that KARs may play a role in DDI in the OB.
Selective AMPA and KA receptor agonists and antagonists used in this study
We and others have used a variety of AMPAR and KAR agonists and antagonists to isolate currents and synaptic events mediated by KARs. This section reviews the KAR subunit specificity and actions of these drugs.
GYKI 52466
GYKI 52466 is a highly selective noncompetitive inhibitor of AMPARs, with limited actions at KARs (Donevan and Rogawski, 1993; Wilding and Huettner, 1995; Paternain et al., 1995). In hippocampal neurons, GYKI 52466 strongly inhibited both the responses to AMPA and kainate at AMPARs (Paternain et al., 1995). GYKI 52466 also more weakly inhibited kainate-induced currents in cells lacking AMPARs (Paternain et al., 1995). The estimated half-maximal inhibition (IC50) was approximately 450 μM, suggesting that GYKI 52466 is approximately 45 times less potent at KARs than AMPARs (Paternain et al., 1995).
In cerebral cortical neurons and dorsal root ganglion (DRG) neurons, GYKI 52466 produced noncompetitive inhibition of AMPA-preferring and kainate-preferring receptors, respectively (Wilding and Huettner, 1995). However, antagonist potency was much greater against the former (IC50 for GYKI 52466 against AMPARs ~ 18 μM versus > 200 μM for KARs). Thus, whereas the concentration of GYKI used in the present study (100 μM) may have had some inhibitory actions on KARs, the effects were likely to be minimal.
SYM 2206
SYM 2206 is a relatively selective AMPAR antagonist (Pelletier et al., 1996; Li P. et al., 1999). Li and colleagues used SYM 2206 (100 μM) and AP5 (100 μM) to isolate the KAR-mediated components of EPSCs in the spinal cord (Li P. et al., 1999). This concentration of SYM 2206 was thought to produce maximal inhibition of AMPARs but less than 20–30% inhibition of KARs (Pelletier et al., 1996; Li P. et al., 1999). Thus, it is possible that the concentration of SYM 2206 used in the present study (100 μM) also had some inhibitory actions on KARs.
SYM 2081
SYM 2081 is a powerful and selective KAR agonist, with 500- to 2000- fold greater selectivity for homomeric GluK1 and GluK2 KARs over AMPARs (Donevan et al., 1998; Small et al., 1998). In Xenopus oocytes, SYM 2081 was a potent agonist at homomeric GluK1 and GluK2 KARs, with EC50s of 0.12 ±0.02 and 0.23±0.01 μM, respectively (Donevan et al., 1998). SYM 2081 was highly selective for KARs over AMPARs, with EC50s for GluA1 and GluA3 AMPARs of 132±44 μM and 453 ± 57 μM, respectively. Thus, at the concentration of SYM 2081 used in the present study (10 μM), SYM 2081 likely only had effects on KARs.
ATPA
ATPA is a potent, relatively selective GluK1 agonist (Clarke et al., 1997; Hoo et al., 1999; Alt et al., 2004), with only weak activity at AMPARs (Clarke et al., 1997). Radioligand binding studies have shown that ATPA is greater than 2000-fold more selective for GluK1 KARs compared with AMPARs (Clarke et al., 1997; Fritsch et al., 2014). In whole-cell recordings from GluK1-expressing HEK293 cells, ATPA evoked inward currents with an EC50 of 2.1±0.1 μM (Clarke et al., 1997). However, ATPA caused only weak activation of GluA1–4 (AMPA) receptors (EC50s for GluA1 and GluA4 of 386± 57 μM and 662±165 μM, respectively) (Clarke et al., 1997). Thus, effects of ATPA concentrations used in the present study (1, 3, 10 μM) were likely mediated by KARs.
ATPA also has effects at other KAR subunits. It acts as a partial agonist at the GluK2/GluK5 KAR (Alt et al., 2004), which is the brain’s most common type of heteromeric KAR (Perrais et al., 2010). This is potentially relevant given our previous situ hybridization data suggest that interneurons express mostly GluK2 and GluK5 (Davila et al., 2007). At high concentrations (IC50~ 2 mM), ATPA acts as an antagonist at homomeric GluK2 KARs (Alt et al., 2004). In binding studies, binding of ATPA to GluK3 was superior to that to GluK5 (Mollerud et al., 2016). Thus, some of the effects of ATPA in the present study may have been mediated by KAR subunits in addition to GluK1.
KARs are widely distributed in the OB
Evidence suggests that all KAR subunits are expressed in the OB, but with distinct cellular and laminar distributions (Gall C. et al., 1990; Petralia et al., 1994; Montague and Greer, 1999; Davila et al., 2007). One ICC study revealed light to moderate staining for GluK2/3 and GluK5 antibodies in the OB (Petralia et al., 1994). A subsequent study demonstrated labeling with a combined GluK1/2/3 antibody in the ONL, glomerular layer (GL), and external plexiform layer (EPL) of the OB, with localization to granule cells, mitral cells, and M/T cell dendrites (Montague and Greer, 1999). Similarly, our previous ICC experiments revealed GluK1/2/3 labeling in almost every bulb layer, particularly the GL and EPL, with localization to mitral cells and granule cells (Davila et al., 2007).
Results from our previous semiquantitative reverse transcription polymerase chain reaction (RT-PCR) experiments further support the notion that all KAR subunits are expressed in the OB but in varying amounts (GluK1 ≈ GluK2 ≈ GluK5 > GluK4 ≫ GluK3) (Davila et al., 2007). In situ hybridization revealed distinct laminar patterns of expression of KAR subunit mRNA: M/T cells expressed mostly GluK1 and GluK5, whereas interneurons express mostly GluK2 and GluK5 (Davila et al., 2007). Another study demonstrated high levels of expression of KAR mRNA in the glomerular, mitral cell, and granule cell layers of the OB, with denser labeling of PG and mitral cells than granule cells (Gall C. et al., 1990). In the zebrafish OB, use of an activity-dependent labeling method revealed KAR-mediated labeling of about 60–70% of JG cells, mitral cells, tyrosine hydroxylase-positive (dopaminergic) cells, and granule cells (Edwards and Michel, 2003).
Thus, evidence from previous experiments suggests that KAR receptors are widely distributed in the OB. However, only a few studies have provided evidence that KARs in the OB are functional (Lowe, 2003; Schoppa, 2006a; Davila et al., 2007). In the present study, we used flow-pipe application of a variety of AMPA and KA receptor agonists and antagonists during whole-cell recording to isolate and characterize KAR-mediated currents recorded from OB neurons.
Currents are evoked by a variety of agonists and antagonists
Application of kainate to OB neurons in primary culture evoked currents in most cells, suggesting that KARs are located both on principal cells (M/T cells) and interneurons. To isolate KARs, we co-applied various concentrations of KAR agonists (kainate, SYM 2081) and AMPA and/or KA receptor antagonists (SYM 2206 and GYKI), which also evoked currents in both M/T cells and interneurons.
At the concentrations used, both GYKI and SYM 2206 could have has some inhibitory actions on KARs as well as AMPARs (see Selective AMPA and KA receptor agonists and antagonists used in this study). Thus, the amplitudes of KAR-mediated currents evoked by the combinations of SYM 2206 plus KAR agonists (kainate, SYM 2081) as well as GYKI plus KAR agonists (kainate, SYM 2081) may have been underestimated.
Some currents may reflect activation of GluK1-containing KARs
GluK1-containing receptors mediate kainate-activated currents in DRG neurons (Partin et al., 1993; Clarke et al., 1997). In acutely isolated DRG neurons, the GluK1 selective agonist ATPA evoked inward currents (EC50=0.6±0.1 μM) more potently than kainate (EC50=12±2 μM) (Clarke et al., 1997). As our previous ICC studies showed labeling for GluK1/GluK2/GluK3 in almost every bulb layer (Davila et al., 2007), we used ATPA to determine if a subset of functional OB KARs contain GluK1.
Application of 10 μM ATPA evoked currents in neurons (mitral cells and JG cells) in OB slices with a mean peak amplitude of 243.77±9.37 pA. Others have reported somewhat smaller currents. For example, ATPA induced a small inward current (20–50 pA) in neurons in rat motor cortex slices (Ali et al., 2001). ATPA (10 μM) evoked currents in a subset (6 of 8) of lateral superior olive cells in rat auditory brainstem slices, with a mean peak amplitude of 51±20 pA (Vitten et al., 2004). As ATPA has effects at other KARs composed of other subunits, the ATPA-mediated currents observed in the present study may have been partially mediated by other KAR subunits in addition to GluK1.
Some currents reflect activation of KARs at synapses
Throughout the brain, AMPARs and NMDARs are found on the postsynaptic density of most glutamatergic synapses; however, EPSCs mediated by postsynaptic KARs have been found at only a few central synapses (Lerma and Marques, 2013)(see Potential roles of postsynaptic KARs in the OB). Our previous ICC double-labeling experiments with the synapse-specific protein synapsin (GluK1/2/3 or GluK1 + synapsin) support the expression of both synaptic and extrasynaptic KARs in the OB (Davila et al., 2007). To determine whether functional KARs in the OB are located at synapses, we evoked EPSCs in neurons in OB slices by stimulation of the ONL. To isolate KAR-mediated currents, we applied AP5 (to block NMDARs) and GYKI (to block AMPARs). We identified EPSCs mediated by postsynaptic KARs in a subset of mitral cells and JG cells examined.
Collectively, these results suggest that functional KARs are widely distributed among principal cells and interneurons of the OB, including a subset at synapses. This heterogeneous distribution has important implications to bulb function. As glutamate is released by OSNs (Berkowicz et al., 1994; Ennis et al., 1996), mitral cells (Trombley and Westbrook, 1990), and M/T cell primary dendrites (Schoppa and Westbrook, 2002; Urban and Sakmann, 2002), KARs on M/T cell primary dendrites may mediate both presynaptic and postsynaptic effects (Davila et al., 2007). KARs on secondary dendrites of M/T cells in the EPL may act as autoreceptors and presynaptically modulate glutamate release (Davila et al., 2007). KARs on granule cells and PG cells may play a role in the postsynaptic transduction of glutamatergic signals and/or regulate GABA release during DDI via presynaptic effects. The subsequent sections of this Discussion explore these potential actions of KARs in detail.
Presynaptic KARs and excitatory transmission
Presynaptic KARs modulate excitatory transmission in a variety of brain regions. Activation of KARs modulates transmitter release in a bidirectional manner, involving both depression and facilitation of glutamate release (Lerma and Marques, 2013; Sihra and Rodriguez-Moreno, 2013). In the present experiment, we found that kainate had biphasic effects on EPSCs evoked by ONL stimulation, consistent with a presynaptic site of action at KARs.
Kainate has biphasic effects on excitatory transmission in the OB
Chittajallu and colleagues (1996) were the first to demonstrate that kainate application caused a dose-dependent decrease in glutamate release in the hippocampus suggestive of the activation of presynaptic kainate autoreceptors (Chittajallu et al., 1996). Subsequent studies have shown that low concentrations of kainate (25– 100 nM) can facilitate transmission (Lauri et al., 2001a; Schmitz et al., 2001a; Schmitz et al., 2001b; Rodriguez-Moreno and Sihra, 2004; Andrade-Talavera et al., 2012), whereas higher concentrations of kainate (200–500 nM) can inhibit transmission (Kamiya and Ozawa, 2000; Schmitz et al., 2000; Lauri et al., 2001a; Schmitz et al., 2001a; Schmitz et al., 2001b; Negrete-Diaz et al., 2006).
At hippocampal MF synapses, a low concentration of kainate (50 nM) enhanced pharmacologically isolated AMPAR- and NMDAR-mediated EPSCs (Schmitz et al., 2001a; Schmitz et al., 2001b), which was attributed, in part, to an increase in transmitter release (Schmitz et al., 2001a). CNQX blocked this enhancement, suggesting a role for KARs (Schmitz et al., 2001a). Also at MF synapses, low kainate concentrations (20–50 nM) increased evoked EPSCs (Rodriguez-Moreno and Sihra, 2004). This effect was also blocked by CNQX, further suggesting a role for presynaptic KARs in facilitating glutamate release. Consistent with these prior studies, we found that a low concentration of kainate (50 nM) increased the amplitude of ONL-evoked EPSCs in OB neurons.
In regard to underlying mechanisms, kainate’s enhancement of excitatory synaptic transmission at MF synapses may be due to depolarization of presynaptic terminals by an ionotropic action of KARs (Schmitz et al., 2001b). Short-term synaptic plasticity at the MF-CA3 synapse is mediated by KARs whose activation results in prominent afterdepolarizations of MF axons and thus enhancing calcium influx into the presynaptic terminals (Kamiya et al., 2002). Consistent with these findings, two recent reviews (Rodrigues and Lerma, 2012; Lerma and Marques, 2013) concluded that most facilitation of transmitter release by KARs occurs via their ionotropic actions. We previously found that kainate (1 μM) increased spontaneous excitatory activity (EPSPs) recorded from interneurons (Davila et al., 2007), potentially reflecting kainate-induced depolarization of the presynaptic M/T cell.
A recent review of metabotropic/non-canonical mechanisms of presynaptic KARs regulating glutamate release sheds further light on underlying mechanisms (Negrete-Diaz et al., 2018). These authors define “direct metabotropic” actions as those involving G-proteins and “non-canonical” actions as those involving activation of intracellular cascades, but independent of any G-protein involvement. This review discusses studies in which relatively low concentrations of kainate facilitated glutamate release at hippocampal MF-CA3 synapses (Rodriguez-Moreno and Sihra, 2004; Andrade-Talavera et al., 2012) and at thalamo-cortical synapses (Andrade-Talavera et al., 2013) via noncanonical actions, which involved the G-protein-independent activation of the adenylyl cyclase/cAMP/protein kinase A signaling cascade. A (2018) study also demonstrated that kainate (3 μM) facilitates glutamate release in the cerebellum via a similar mechanism (Falcon-Moya et al., 2018).
At MF synapses, kainate (100–200 nM) inhibited the NMDAR-mediated EPSC, which was attributed to a decrease in glutamate release mediated by presynaptic KARs (Schmitz et al., 2000). Even higher concentrations of kainate (e.g., 1– 10 μM) also have been shown to depress synaptic transmission at MF synapses (Negrete-Diaz et al., 2006), at Schaffer collateral - CA1 pyramidal cells synapses (Chittajallu et al., 1996; Kamiya and Ozawa, 1998; Vignes et al., 1998; Frerking et al., 2001), and at medial geniculate nucleus-lateral amygdala synapses (Negrete-Diaz et al., 2012). Consistent with these prior studies, we found that a higher concentration of kainate (500 nM) decreased the amplitude of ONL-evoked EPSCs in OB neurons.
The mechanism(s) responsible for the KAR-mediated inhibition of excitatory transmission include presynaptic depolarization via traditional ionotropic actions of KARs (Chittajallu et al., 1996; Kamiya and Ozawa, 2000; Schmitz et al., 2000). However, evidence suggests that some of this inhibition in the hippocampus involves metabotropic signaling, since it was sensitive to G protein blockers (Frerking et al., 2001; Negrete-Diaz et al., 2006; Lerma and Marques, 2013). In the globus pallidus (Jin et al., 2006) and spinal cord (Rozas et al., 2003), activation of presynaptic KARs also decreased glutamate release via G-protein-coupled, metabotropic mechanisms.
Within glomeruli in the OB, OSNs release glutamate at synapses with M/T cells (Berkowicz et al., 1994; Ennis et al., 1996) and some JG cells including ET cells and a subset of PG cells (Nagayama et al., 2014; Ennis et al., 2015; Kosaka and Kosaka, 2016). Our finding that kainate had biphasic effects on EPSCs evoked by ONL stimulation is consistent with presynaptic modulation of excitatory transmission by KARs on OSN terminals and may provide a mechanism for gain control.
GluK1-containing KARs appear to regulate excitatory transmission in the OB: effects of ATPA
The GluK1 subunit contributes to presynaptic populations of KARs in the hippocampus (Vignes et al., 1998; Rodriguez-Moreno et al., 2000; Lauri et al., 2001b) and neocortex (Campbell et al., 2007). For example, GluK1-containing KARs on hippocampal mossy fibers reportedly function as autoreceptors to facilitate glutamate release at synapses (Lauri et al., 2001b). Our previous ICC experiments suggest that GluK1-containing KARs in the OB are located at or near synapses (Davila et al., 2007). Therefore, we investigated the actions of the GluK1-selective agonist ATPA (1 μM, 3 μM and 10 μM) on synaptic responses in OB neurons evoked by ONL stimulation.
Others have used ATPA to explore the subunit composition of KARs involved in the modulation of excitatory transmission. At the MF-CA3 synapse, ATPA (2 μM) depressed EPSCs evoked by MF stimulation in a manner consistent with a presynaptic effect (Vignes et al., 1998). Similar depression of MF-evoked EPSCs was observed by others with 1–2 μM ATPA (Schmitz et al., 2000). In the rat neocortex, a low dose of ATPA (1 μM) increased amplitudes of EPSCs evoked by intracortical stimulation, whereas a higher dose of ATPA (10 μM) decreased EPSC amplitudes (Campbell et al., 2007). Thus, our finding that all concentrations of ATPA had qualitatively similar effects-decreasing the amplitude of the ONL-evoked EPSC—is consistent with most previous findings. These findings collectively support the notion that GluK1-containing KARs modulate excitatory transmission, including that in the OB.
Prolonged duration of ATPA application had greater effects on synaptic events
Our finding that prolonged (5–10 minutes) application of 1 μM ATPA caused greater inhibition of ONL-evoked EPSCs and EPSPs than brief (30 seconds) application is potentially suggestive of metabotropic activity. Results from several studies suggest metabotropic activity of GluK1-containing KARs. In dorsal horn neurons, ATPA (10 μM) reduced AMPA-mediated EPSCs, which was inhibited by application of N-ethylmaleimide (Rozas et al., 2003). The authors concluded that modulation of glutamate release by GluK1-containing KARs in the spinal cord depends on the activation of a G protein. In the hippocampus, ATPA (1 μM) decreased fEPSP amplitude and reduced calcium influx at excitatory synaptic terminals (Salmen et al., 2012), which was abolished by inactivation of G proteins with pertussis toxin.
Our investigation and findings of the effects of prolonged application of a KAR agonist are relevant in the context of previous studies in other brain regions. For example, Negrete-Díaz and colleagues perfused neurons in hippocampal slices with kainate (1 μM) for 4 minutes and observed a long-lasting inhibition of CA3-evoked EPSCs (eEPSCs) that suggested that kainate’s effects lasted for 1 hour (Negrete-Diaz et al., 2006). These long-lasting effects outlasted the change in holding current (which lasted only 6 minutes) induced by the activation of ionotropic KARs. Additional experiments suggested that presynaptic KARs at MF synapses mediate these inhibitory effects of kainate on glutamate release via a metabotropic mechanism (Negrete-Diaz et al., 2006).
The greater inhibition of ONL-evoked EPSCs and EPSPs we observed with prolonged application of ATPA (1 μM) may reflect the time course of the metabotropic activity of presynaptic KARs on OSN terminals. However, evidence from the hippocampus suggests the possibility of metabotropic activity of other types of receptors. Based on their finding that ATPA depolarizes CA3 interneurons and increases the frequency of spontaneous IPSCs recorded in CA3 pyramidal neurons, Schmitz and colleagues hypothesized that the inhibitory effects of ATPA on synaptic transmission might actually be an indirect effect of GABA release onto metabotropic GABAB receptors on MF terminals rather than a direct effect on excitatory transmission (Schmitz et al., 2000). Consistent with this notion, blockade of metabotropic GABAB receptors with the GABAB antagonist, SCH50911, significantly reduced ATPA’s inhibitory effects.
In the OB, PG cells have a non-conventional relationship with OSNs due to the lack of an anatomically defined synapse (Ennis et al., 2015). PG cells may release GABA that binds to presynaptic GABAB receptors on OSNs and inhibits glutamate release via effects of GABAergic spillover rather than via a traditional inhibitory synapse (Pinching and Powell, 1971b; Aroniadou-Anderjaska et al., 2000; Wachowiak et al., 2005; Ennis et al., 2015; Lizbinski and Dacks, 2017). Thus, the further decrease in EPSC/EPSP amplitudes observed with prolonged ATPA application may reflect activation of GluK1-containing KARs on PG cells, which then release GABA that acts via spillover to activate metabotropic GABAB receptors on OSN terminals.
Presynaptic KARs and inhibitory transmission
Presynaptic KARs in other brain regions also influence inhibitory transmission. Activation of KARs modulates transmitter release in a complex bidirectional manner, involving both depression and facilitation of GABA release (Lerma and Marques, 2013). In the present experiment, we found that kainate had biphasic effects on reciprocal IPSCs evoked by a self-stimulation protocol, consistent with presynaptic and/or postsynaptic sites of action of KARs.
Kainate has biphasic effects on inhibitory transmission in the OB
A biphasic concentration-dependent response of presynaptic KARs to application of kainate has been found at some GABAergic synapses. For example, in the hippocampus, application of a low dose of KA (300 nM) potentiated some GABAergic synapses, whereas a high dose of KA (5 μM) inhibited some GABAergic synapses (Jiang et al., 2001).
In the hippocampus, activation of presynaptic KARs appears to be, at least in part, responsible for kainate-induced inhibition of GABAergic synaptic transmission (Clarke et al., 1997; Rodriguez-Moreno et al., 1997; Rodriguez-Moreno and Lerma, 1998). A range of concentrations of kainate (0.3–300 μM) decreased the amplitude of evoked IPSCs in the hippocampus (Rodriguez-Moreno et al., 1997). Kainate also increased synaptic failures and decreased miniature IPSC (mIPSC) frequency, suggesting a presynaptic site of action. Consistent with these findings, we found that a relatively high kainate concentration (5 μM) inhibited reciprocal IPSCs recorded in OB neurons and also increased the frequency of synaptic failures.
The inhibitory activity of KARs on transmitter release has largely been attributed to metabotropic effects (Rodrigues and Lerma, 2012). In a seminal study, kainate’s inhibitory action on hippocampal IPSCs was sensitive to pertussis toxin as well as inhibitors of phospholipase C and protein kinase C (Rodriguez-Moreno and Lerma, 1998). Inhibition of GABAergic transmission via a presynaptic metabotropic action of KARs has also been observed in the globus pallidus (Jin and Smith, 2007) and hypothalamus (Bonfardin et al., 2010).
Evidence that presynaptic KARs can facilitate GABA release has been observed in brain regions including the hippocampus (Jiang et al., 2001; Cossart et al., 2001), hypothalamus (Liu et al., 1999; Bonfardin et al., 2010), and prefrontal cortex (Mathew et al., 2008). Kainate (250 nM) increased the amplitude of IPSCs recorded from layer II/III pyramidal neurons in the prefrontal cortex (Mathew et al., 2008). Kainate (250 nM) also increased mIPSC frequency but not amplitude in hippocampal CA1 interneurons (Cossart et al., 2001) and layer II/III pyramidal neurons (Mathew et al., 2008), indicative of a presynaptic effect on transmitter release. Consistent with these findings, we found that a low concentration of kainate (300 nM) potentiated reciprocal IPSCs recorded from OB neurons.
In contrast to the inhibitory effects, the facilitation of GABA release by presynaptic KARs likely reflects conventional ionotropic activity (Rodrigues and Lerma, 2012; Lerma and Marques, 2013). The facilitation of GABAergic transmission is thought to reflect activation of ionotropic KARs located on GABAergic axons (Cossart et al., 2001) and/or depolarization of interneurons via axonal KARs (Semyanov and Kullmann, 2001).
Consistent with these prior studies, our findings suggest that KARs on OB neurons modulate GABA release in a bidirectional manner that may involve several types of circuits. In the OB, M/T cells provide excitatory (glutamatergic) input to PGs and granule cells, which provide feedback, feedforward, and/or lateral inhibition of M/T cells at reciprocal GABAergic dendrodendritic synapses (Shepherd G.M. et al., 2004; Ennis et al., 2007; Ennis et al., 2015). Thus, kainate’s effects on inhibitory transmission in the OB may reflect the activation of presynaptic KARs on PG cells and granule cells, resulting in a reduction or increase in the release of GABA. These effects could also be due to activation of presynaptic KARs on mitral cells and ET cells, resulting in a reduction or increase in glutamate release that then indirectly influences GABA release.
Several studies support the existence of kainate autoreceptors on M/T cells, which could modulate glutamate release by a presynaptic mechanism. Glutamate released from mitral cell primary and secondary dendrites activated both NMDA and non-NMDA (AMPA/KA) autoreceptors (Salin et al., 2001). Results from this study further suggest that such AMPA/KA autoreceptors may play a role in mitral cell self-excitation. Consistent with these findings, Lowe (2003) reported that self-excitation of the mitral cell somatodendritic membrane relies on the activation of NMDA, AMPA, and kainate autoreceptors (Lowe, 2003).
ET cells also play a key role in intraglomerular inhibition (Ennis et al., 2007; Ennis et al., 2015). OSNs release glutamate at synapses with some PG cell subclasses (Ennis et al., 2007; Nagayama et al., 2014; Kosaka and Kosaka, 2016). However, most (67% of) PG cells receive their strongest drive from an ON-->ET-->PG circuit with only weak or no monosynaptic OSN input (Shao et al., 2009). After receiving excitatory (glutamatergic) input from ET cells, GABAergic PG cells provide intraglomerular feedback inhibition to ET cells and feed forward inhibition to M/T cells (Ennis et al., 2015). An activitydependent labeling method revealed KAR-mediated labeling of JG cells in the zebrafish OB (Edwards and Michel, 2003). Thus, KARs on JG cells including PG cells and ET cells could modulate inhibitory transmission at these synapses via presynaptic and/or postsynaptic mechanisms.
Short axon (SA) cells are a type of JG cell that express GAD67 and tyrosine hydroxylase (Kosaka and Kosaka, 2008; Kiyokage et al., 2010) and co-release GABA and dopamine (Ennis et al., 2015). Although a subset of SA cells receive ON input, the majority (≈70%) receive excitatory (glutamatergic) input from ET cells (Ennis et al., 2015). SA cells primarily target mitral cells and ET cells, although they also may synapse with other SA cells and PG cells (Ennis et al., 2015). The activity-dependent labeling method also revealed KAR-mediated labeling of tyrosine hydroxylase-positive cells in the zebrafish OB (Edwards and Michel, 2003). Thus, KARs on SA cells may modulate the complex inhibitory/excitatory circuit effects mediated by these cells.
GluK1-containing KARs appear to regulate inhibitory transmission in the OB: effects of ATPA
The selective GluK1 agonist ATPA has been used to explore the subunit composition of KARs mediating inhibitory transmission in various brain regions. In the amygdala, ATPA bidirectionally modulates the strength of synaptic transmission from GABAergic interneurons to pyramidal cells (Braga et al., 2003). Low concentrations of ATPA (0.3 μM) reduced synaptic failures and increased miniature IPSC (mIPSC) frequency. In contrast, higher concentrations of ATPA (10 μM) increased synaptic failures and decreased mIPSC frequency. As our prior ICC data suggest the expression of GluK1 subunits at OB synapses (Davila et al., 2007), we also investigated the actions of several concentrations of ATPA (1 and 10 μM) on reciprocal inhibition.
Findings in other brain regions have shown mixed (potentiating, inhibiting, no) effects of ATPA on inhibitory transmission. In the neocortex, ATPA (1 μM) decreased the amplitude of fast-spiking interneuron to pyramidal cell unitary IPSCs in a manner consistent with a presynaptic site of action (Ali et al., 2001). In the hypothalamus, ATPA (3 μM) increased mIPSC frequency but not amplitude, also suggestive of a presynaptic site of action (Bonfardin et al., 2010). Application of (S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxybenzyl) pyrimidine-2,4-dione (UBP302) (10 μM), a selective antagonist of GluK1-containing KARs (More et al., 2004), abolished ATPA’s effect on mIPSC frequency (Bonfardin et al., 2010), further suggesting that GABAergic terminals express functional presynaptic GluK1-containing KARs. In hippocampal neurons, ATPA (10 μM) depressed monosynaptically activated inhibitory postsynaptic potentials (IPSPs) similar to kainate (5 μM) in a manner consistent with a presynaptic effect (Clarke et al., 1997).
Our findings that ATPA (1 and 10 μM) inhibited reciprocal IPSCs is consistent with (most) previous findings and implies a role for GluK1-containing KARs in modulating inhibitory transmission in the OB. ATPA’s effects may reflect the activation of presynaptic GluK1-containing KARs on PG cells and granule cells, resulting in a reduction in the release of GABA. ATPA also may activate GluK1-containing KARs on mitral cells and ET cells, resulting in a reduction in glutamate release that then leads to a decrease in GABA release. As ATPA also has actions at other KAR subunits, some of the observed effects may have been mediated by additional KAR subunits. Our finding that prolonged (5 to 10 minute) versus brief (30 second) application of 1 μM ATPA had greater effects on reciprocal IPSCs further suggests that some of the observed effects of ATPA on inhibitory transmission may reflect metabotropic actions of GluK1containing KARs.
Potential roles of postsynaptic KARs in the OB
In addition to presynaptic KARs, some cells also express postsynaptic KARs that play a role in synaptic transmission. Postsynaptic KARs that mediate synaptic responses were initially demonstrated in the hippocampus, at MF-CA3 pyramidal cell synapses (Castillo et al., 1997; Vignes and Collingridge, 1997) and Schaffer collateral-CA1 interneuron synapses (Cossart et al., 1998; Frerking et al., 1998). Synaptic responses mediated by postsynaptic KARs have also been found at synapses between parallel fibers and Golgi cells in the cerebellum (Bureau et al., 2000), at thalamocortical connections ( Kidd and Isaac, 2001), at synapses between afferent sensory fibers and dorsal horn neurons in the spinal cord (Li P. et al., 1999), as well as in the basolateral amygdala (Li H. and Rogawski, 1998), retina (DeVries and Schwartz, 1999), anterior cingulate cortex (Wu et al., 2005), and auditory brainstem (Vitten et al., 2004). In the present study, we used similar methods (electrical stimulation of a pathway during application of AP5 and an AMPAR antagonist) to isolate KAR-mediated synaptic responses (EPSCs) in neurons in the OB that were consistent with the presence of postsynaptic KARs.
Postsynaptic KARs may modulate transmission in the OB in several ways. As OSNs (which synapse with M/T cell primary dendrites, ET cells, and some PG cells), mitral cells, ET cells, and M/T cell primary dendrites all release glutamate (Trombley and Westbrook, 1990; Berkowicz et al., 1994; Ennis et al., 1996; Schoppa and Westbrook, 2002; Urban and Sakmann, 2002), OB KARs may mediate both presynaptic and postsynaptic effects (Davila et al., 2007). In addition to presynaptic modulation of GABA release, KARs on granule cells and PG cells may take part in the postsynaptic transduction of glutamatergic signals during DDI (see KAR kinetics and dendrodendritic inhibition in the OB).
Kainate receptor subunit composition and kinetics
In the hippocampus, the glutamate-mediated activation of postsynaptic KARs yields small amplitude EPSCs, with slower activation and deactivation kinetics than seen with AMPAR-mediated EPSCs (Castillo et al., 1997; Frerking et al., 1998; Cossart et al., 1998; Lerma and Marques, 2013). KAR-mediated EPSCs with slower kinetics than AMPAR-mediated EPSCs have also been observed at cortical (Wu et al., 2005), thalamocortical (Kidd and Isaac, 2001), and cerebellar (Bureau et al., 2000) synapses. One determinant of KAR-mediated EPSC kinetics is receptor subunit composition (Contractor et al., 2003; Barberis et al., 2008; Fernandes et al., 2009; Lerma and Marques, 2013).
In heterologous expression systems, even sub-maximal glutamate levels cause rapid and complete desensitization of homomeric GluK1–3 receptors (Sommer et al., 1992; Heckmann et al., 1996; Schiffer et al., 1997; Paternain et al., 1998; Fisher M. T. and Fisher, 2014). However, inclusion of a GluK4 or GluK5 subunit in recombinant receptors slows deactivation and changes the agonist concentration-dependence of desensitization (Barberis et al., 2008; Mott et al., 2010; Fisher J. L. and Mott, 2011; Fisher M. T. and Fisher, 2014). Most native postsynaptic KARs are heteromeric receptors comprised of GluK1 or GluK2 plus GluK4 or GluK5 (Petralia et al., 1994; Darstein et al., 2003; Fernandes et al., 2009; Fisher M. T. and Fisher, 2014). Thus, the slow kinetics of EPSCs mediated by native KARs compared with the fast kinetics of EPSCs mediated by recombinant homomeric KARs partially reflect the biophysical properties of heteromeric KARs (Barberis et al., 2008; Carta et al., 2014).
Two integral membrane proteins, Neuropilin Tolloid-like 1 and Neuropilin Tolloid-like 2 (Neto1 and Neto2) (Zhang et al., 2009; Straub et al., 2011a; Tang et al., 2011; Straub et al., 2011b) are auxiliary subunit proteins of KARs. The coexpression of Neto1 and Neto2 with other traditional KAR subunits influences KAR trafficking, pharmacology, and channel kinetics (Straub et al., 2011a; Straub et al., 2011b; Copits et al., 2011; Fisher J. L. and Mott, 2012; Fisher J. L. and Mott, 2013; Palacios-Filardo et al., 2016). In fact, Lerma & Marques concluded that the higher agonist affinity and slowed deactivation kinetics conferred by inclusion of Neto proteins in KARs helps to explain why KARmediated EPSCs are slower than AMPAR-mediated EPSCs (Lerma and Marques, 2013). Further support for this conclusion comes from the finding that cultured hippocampal neurons transfected with Neto proteins had KAR-mediated EPSCs with slower onset and decay rates than neurons transfected with GluK4 or GluK5 (Palacios-Filardo et al., 2016).
These findings may have implications for the kinetics of KARs in the OB. Our previous in situ hybridization studies suggest that M/T cells express mostly GluK1 and GluK5, whereas interneurons express mostly GluK2 and GluK5 (Davila et al., 2007). Thus, the kinetics of OB neurons may be similar to those of native heteromeric KARs in other brain regions composed of GluK1 or GluK2 plus GluK4 or GluK5. Other in situ hybridization studies have revealed widespread expression of RNA for Neto1 (Ng et al., 2009) and Neto2 (Magdaleno et al., 2006) ([dataset] URL: http://www.informatics.jax.org/assay/MGI:4944601 accessed 5–28-18) in the mouse OB. Thus, KAR-mediated EPSCs in the OB also may have slow kinetics due to the heteromeric composition of KARs and/or expression of Neto proteins, which could have important implications to circuit function (see next section).
KAR kinetics and dendrodendritic inhibition in the OB
The functional significance of the slower kinetics of KARs includes a role for a prolonged current in synaptic integration (Frerking and Ohliger-Frerking, 2002; Goldin et al., 2007; Sachidhanandam et al., 2009; Pinheiro P. S. et al., 2013; Lerma and Marques, 2013). For example, modeling of hippocampal EPSCs indicates that KAR-mediated synaptic currents cause tonic depolarizations over a large range of firing frequencies. In contrast, the AMPAR-mediated portion of the current is restricted to phasic or transient excitation (Frerking and Ohliger-Frerking, 2002). In the OB, KARs could be especially important in circuits in which the relatively brief duration of AMPAR-mediated currents limits the role of AMPARs in synaptic transmission (i.e., reciprocal inhibition at dendrodendritic synapses) (Schoppa et al., 1998; Isaacson and Strowbridge, 1998; Schoppa, 2006a).
Dendrodendritic inhibition (DDI) occurs at reciprocal synapses between M/T cells and PG cells (Shepherd G.M. et al., 2004; Ennis et al., 2007; Ennis et al., 2015) and between M/T cells and granule cells (Price and Powell, 1970; Woolf et al., 1991; Ennis et al., 2015). At the latter synapses, M/T cells release glutamate that results in a dualcomponent response in granule cells, comprised of a fast AMPAR-mediated response and a slower NMDAR-mediated response (Trombley and Westbrook, 1990; Schoppa et al., 1998; Isaacson and Strowbridge, 1998; Aroniadou-Anderjaska et al., 1999; Chen et al., 2000; Isaacson, 2001). Granule cell activation results in inhibition of M/T cells mediated via GABAA receptors (Chen et al., 2000; Isaacson and Vitten, 2003; Dietz and Murthy, 2005).
In 1998, Schoppa and colleagues demonstrated that NMDAR activation is required for DDI between mitral cells and granule cells, with the primary purpose of AMPAR-mediated depolarization being to help relieve the voltage-dependent magnesium block of NMDARs (Schoppa et al., 1998). Isaacson & Strowbridge (1998) similarly declared that while both NMDARs and non-NMDARs play a role in DDI, the apparent role of nonNMDAR depolarization is to unblock NMDARs (Isaacson and Strowbridge, 1998). Voltage recordings revealed that effective spike stimulation in granule cells during DDI relied on the slow kinetics of NMDARs (Schoppa et al., 1998). Thus, the slower kinetics of KARs compared with AMPARs may suggest a role for KARs in mediating DDI.
Data from a subsequent study more clearly suggest a role for KARs in DDI. Schoppa (2006a) found that fast spiking in granule cells observed during “dynamic” stimulus conditions (i.e., patterned stimulation of the ON) contrasts with the delayed spiking dependent on slow NMDARs observed during the “static” stimulus recording conditions (i.e., single stimulation of one or a few cells) of DDI (Schoppa et al., 1998), with the former relying on activation of AMPA/KA receptors on granule cells (Schoppa, 2006a). Schoppa used cyclothiazide (CTZ), a drug that can distinguish between AMPAR and KARs, to determine that a subset of the synaptic events (glutamatergic EPSCs) evoked in granule cells by dynamic stimulation were mediated either by CTZ-insensitive KARs or AMPARs comprised of CTZ-insensitive subunits (Schoppa, 2006a).
Consistent with these findings, we found that kainate and ATPA had effects on reciprocal IPSCs evoked using a protocol that evokes DDI. Kainate (300 nM) potentiated reciprocal IPSCs, whereas kainate (5 μM) inhibited reciprocal IPSCs and increased the frequency of synaptic failures. ATPA (1 μM, 10 μM) also decreased the amplitude of reciprocal IPSCs, with greater effects seen with prolonged application. These inhibitory effects of these agonists could be explained by activation of KARs on mitral and tufted cells, resulting in a reduction in the release of glutamate and/or the activation of KARs on PG cells and granule cells, resulting in a reduction in the release of GABA. The finding that ATPA decreased the amplitude of reciprocal IPSCs suggests a role for GluK1-containing KARs at these synapses. The greater effect with prolonged application of ATPA further suggests the possibility of metabotropic actions of KARs.
A role for KARs in odor information processing
Synchronization of activity is a key characteristic of neuronal networks in many CNS regions. In the OB, OSN excitation of OB neurons synchronizes the activity of M/T cells projecting to the same glomerulus (Carlson et al., 2000; Schoppa and Westbrook, 2001; Schoppa and Westbrook, 2002; Christie and Westbrook, 2006; Schoppa, 2006b). This results in spatial patterns (“odor maps”) (Rubin and Katz, 1999; Belluscio and Katz, 2001; Wachowiak and Cohen, 2001; Chen and Shepherd, 2002) and temporal patterns (Schoppa and Westbrook, 2002; Christie and Westbrook, 2006; Schoppa, 2006b) of odor-evoked oscillations among OB neurons that underlie the OB’s role in encoding olfactory information.
This network behavior in the OB is also strongly influenced by GABAergic inhibitory circuits (Schoppa et al., 1998; Isaacson and Strowbridge, 1998; Christie et al., 2001; Nusser et al., 2001; Urban and Sakmann, 2002; Schoppa, 2006b). The activity of these inhibitory circuits is controlled by excitatory synaptic transmission, mediated, at least in part, by AMPA/KA receptors (Schoppa, 2006a). Studies in other brain regions suggest roles for excitatory and inhibitory circuits in generating electrical oscillations (Fisahn, 2005; Buzsaki and Wang, 2012). In the hippocampus, results from knockout mice suggest that GluK1- and GluK2-containing KARs play distinct roles in kainate-induced gamma oscillations that depend on intact inhibitory neurotransmission (Fisahn et al., 2004; Fisahn, 2005).
Activation of the ionotropic receptors for glutamate (AMPA, kainate, and NMDA receptors) is critical to both the synchronization of glomerular excitation and to the timing of reciprocal inhibition, hence, control of both the temporal and spatial patterns of OB activity that underlie olfactory coding. Given this, the broad goal of this study was to explore the mechanisms by which activation of KARs contributes to the activity of circuits that mediate olfactory encoding. This involved determining the distribution of functional KARs, their role in modulating synaptic transmission, and their contribution to network activity. In the present study, flow-pipe application of KAR agonists (kainate, SYM 2081, ATPA) and AMPAR antagonists (SYM 2206 and GYKI) evoked currents in M/T cells and interneurons in cultures and OB slices. Application of AP5 and GYKI to neurons in OB slices during ONL stimulation isolated EPSCs mediated by postsynaptic KARs. Application of kainate and ATPA had modulatory effects on evoked EPSCs and IPSCs, with greater effects with prolonged application of ATPA.
Together, these findings suggest that functional KARs composed of a variety of KAR subunits are expressed on both principal (M/T) cells and interneurons, with a subset of receptors located at synapses. These findings also suggest that presynaptic and/or postsynaptic KARs are important modulators of excitatory and inhibitory transmission in the OB, potentially via both ionotropic and metabotropic activity. Lastly, given the slow kinetics of KARs in other brain regions, our findings that kainate and ATPA modulate reciprocal IPSCs suggest that KARs may play a role in reciprocal inhibition at dendrodendritic synapses. Collectively, these data further suggest that KARs participate in the regulation of synaptic circuits that encode odor information.
HIGHLIGHTS.
Functional kainate receptors are expressed by olfactory bulb neurons, with a subset of receptors located at synapses.
Kainate receptor agonists (kainate and ATPA) modulate excitatory and inhibitory transmission in the olfactory bulb.
Kainate receptors appear to participate in the regulation of synaptic circuits that encode odor information.
Acknowledgments
FUNDING:
This work was supported in part by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health [grant number: DC-04320] and in part by the NIDCD Chemosensory Training Program [grant: T32 DC000044].
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- AP5
2-amino-5-phosphonopentanoic acid
- ATPA
(RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid
- AUC
area under the curve
- CNS
central nervous system
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione disodium
- DDI
dendrodendritic inhibition
- CTZ
cyclothiazide
- DRG
dorsal root ganglion
- EPSC
excitatory postsynaptic current
- EPSP
excitatory postsynaptic potential
- EPL
external plexiform layer
- ET cells
external tufted cells
- GABA
gamma-Aminobutyric acid
- GAD
glutamic acid decarboxylase
- GL
glomerular layer
- GYKI 52466
1-(4-Aminophenyl)-4methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride
- GYKI 52466
GYK
- GYKI 53655
1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-3,4-dihydro −7,8- methylenedioxy- −5H-2,3-benzodiazepine hydrochloride
- ICC
immunocytochemical
- IPSC
inhibitory postsynaptic current
- IPSP
inhibitory postsynaptic potential
- JG cells
juxtaglomerular cells
- KAR
kainate receptor
- mIPSC
miniature IPSC
- MF
mossy fiber
- M/T cell
mitral/tufted cell
- NBQX
2,3-dioxo-6- nitro-1,2,3,4-tetrahydrobenzoquinoxaline7-sulfonamide
- Neto1
Neuropilin tolloid-like 1
- Neto2
Neuropilin tolloid-like 2
- NMDA
N-methyl-D-aspartate receptor
- NMDAR
NMDA receptor
- OB
olfactory bulb
- ON
olfactory nerve
- ONL
olfactory nerve layer
- OSN
olfactory sensory neuron
- PG cell
periglomerular cell
- QX314
lidocaine N-ethyl bromide
- RT-PCR
reverse transcription polymerase chain reaction
- SYM 2081
(2S,4R) 4-methylglutamic acid
- SYM 2206
4-(4Aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine
- UBP302
(S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxybenzyl) pyrimidine-2,4-dione
- TTX
tetrodotoxin
- VGLUT1
vesicular glutamate transporter 1
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Magdaleno S, et al. , Neto2 RNA in situ Gene Expression Assay, Mouse Genome Informatics (MGI), 4/11/2011, Accession ID: MGI 4944601; URL: http://www.informatics.jax.org/assay/MGI:4944601. Accessed May 28, 2018 [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD, Pawson AJ, Sharman JL, et al. (2017) THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand-gated ion channels. Br J Pharmacol 174 Suppl 1:S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Rossier J, Staiger JF, Audinat E (2001) Kainate receptors regulate unitary IPSCs elicited in pyramidal cells by fast-spiking interneurons in the neocortex. J Neurosci 21:2992–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D (2004) Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 46:793–806. [DOI] [PubMed] [Google Scholar]
- Andrade-Talavera Y, Duque-Feria P, Negrete-Diaz JV, Sihra TS, Flores G, RodriguezMoreno A (2012) Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+ -calmodulin at mossy fiber-CA3 synapses. J Neurochem 122:891–899. [DOI] [PubMed] [Google Scholar]
- Andrade-Talavera Y, Duque-Feria P, Sihra TS, Rodriguez-Moreno A (2013) Presynaptic kainate receptor-mediated facilitation of glutamate release involves PKA and Ca(2+) -calmodulin at thalamocortical synapses. J Neurochem 126:565–578. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Ennis M, Shipley MT (1999) Dendrodendritic recurrent excitation in mitral cells of the rat olfactory bulb. J Neurophysiol 82:489–494. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT (2000) Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol 84:1194–1203. [DOI] [PubMed] [Google Scholar]
- Bailey A, Kelland EE, Thomas A, Biggs J, Crawford D, Kitchen I, Toms NJ (2001) Regional mapping of low-affinity kainate receptors in mouse brain using [(3)H](2S,4R)4-methylglutamate autoradiography. Eur J Pharmacol 431:305–310. [DOI] [PubMed] [Google Scholar]
- Barberis A, Sachidhanandam S, Mulle C (2008) GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci 28:6402–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Katz LC (2001) Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci 21:2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowicz DA, Trombley PQ, Shepherd GM (1994) Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol 71:2557–2561. [DOI] [PubMed] [Google Scholar]
- Blakemore LJ, Resasco M, Mercado MA, Trombley PQ (2006) Evidence for Ca(2+)permeable AMPA receptors in the olfactory bulb. Am J Physiol Cell Physiol 290:C925935. [DOI] [PubMed] [Google Scholar]
- Bonfardin VD, Fossat P, Theodosis DT, Oliet SH (2010) Glia-dependent switch of kainate receptor presynaptic action. J Neurosci 30:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H (2003) Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci 23:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Dieudonne S, Coussen F, Mulle C (2000) Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A 97:6838–6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Mathew SS, Hablitz JJ (2007) Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology 53:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A (2000) Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20:2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Fievre S, Gorlewicz A, Mulle C (2014) Kainate receptors in the hippocampus. Eur J Neurosci 39:1835–1844. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA (1997) Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388:182–186. [DOI] [PubMed] [Google Scholar]
- Chen WR, Shepherd GM (2002) Putting odor maps in sync. Nat Neurosci 5:505–506. [DOI] [PubMed] [Google Scholar]
- Chen WR, Xiong W, Shepherd GM (2000) Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron 25:625–633. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM (1996) Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379:78–81. [DOI] [PubMed] [Google Scholar]
- Christie JM, Schoppa NE, Westbrook GL (2001) Tufted cell dendrodendritic inhibition in the olfactory bulb is dependent on NMDA receptor activity. J Neurophysiol 85:169–173. [DOI] [PubMed] [Google Scholar]
- Christie JM, Westbrook GL (2006) Lateral excitation within the olfactory bulb. J Neurosci 26:2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, et al. (1997) A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389:599–603. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M (2009) A nomenclature for ligandgated ion channels. Neuropharmacology 56:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT (2011) Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci 34:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF (2003) Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci 23:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Robbins JS, Frausto S, Swanson GT (2011) Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloidlike (NETO) proteins. J Neurosci 31:7334–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthell JT, Olcese J, Trombley PQ (2014) Melatonin in the mammalian olfactory bulb. Neuroscience 261:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y (1998) GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1:470–478. [DOI] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C (2001) Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29:497–508. [DOI] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF (2003) Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci 23:80138019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila NG, Houpt TA, Trombley PQ (2007) Expression and function of kainate receptors in the rat olfactory bulb. Synapse 61:320–334. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA (1999) Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature 397:157–160. [DOI] [PubMed] [Google Scholar]
- Dietz SB, Murthy VN (2005) Contrasting short-term plasticity at two sides of the mitralgranule reciprocal synapse in the mammalian olfactory bulb. J Physiol 569:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61. [PubMed] [Google Scholar]
- Donevan SD, Beg A, Gunther JM, Twyman RE (1998) The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther 285:539–545. [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA (1993) GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron 10:51–59. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Michel WC (2003) Pharmacological characterization of ionotropic glutamate receptors in the zebrafish olfactory bulb. Neuroscience 122:1037–1047. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S (1991) Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature 351:745748. [DOI] [PubMed] [Google Scholar]
- Ennis M, Hamilton KA, Hayar A (2007) Neurochemistry of the main olfactory system. In: Handbook of Neurochemistry and Molecular Neurobiology: Sensory Neurochemistry, vol. 20 (Johnson DA, ed), pp. 137–204. Heidelberg, Germany: Springer. [Google Scholar]
- Ennis M, Puche AC, Holy T, Shipley MT (2015) The Olfactory System In: The Rat Nervous System: Fourth Edition, vol. (Paxinos G, ed), pp. 761–803. London, U.K.: Elsevier Inc. . [Google Scholar]
- Ennis M, Zimmer LA, Shipley MT (1996) Olfactory nerve stimulation activates rat mitral cells via NMDA and non-NMDA receptors in vitro. Neuroreport 7:989–992. [DOI] [PubMed] [Google Scholar]
- Falcon-Moya R, Losada-Ruiz P, Sihra TS, Rodriguez-Moreno A (2018) Cerebellar Kainate Receptor-Mediated Facilitation of Glutamate Release Requires Ca(2+)Calmodulin and PKA. Front Mol Neurosci 11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A (2009) High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron 63:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A (2005) Kainate receptors and rhythmic activity in neuronal networks: hippocampal gamma oscillations as a tool. J Physiol 562:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ (2004) Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci 24:9658–9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD (2011) Distinct functional roles of subunits within the heteromeric kainate receptor. J Neurosci 31:17113–17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD (2012) The auxiliary subunits Neto1 and Neto2 reduce voltagedependent inhibition of recombinant kainate receptors. J Neurosci 32:12928–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Mott DD (2013) Modulation of homomeric and heteromeric kainate receptors by the auxiliary subunit Neto1. J Physiol 591:4711–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MT, Fisher JL (2014) Contributions of different kainate receptor subunits to the properties of recombinant homomeric and heteromeric receptors. Neuroscience 278:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA (1998) Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1:479–486. [DOI] [PubMed] [Google Scholar]
- Frerking M, Ohliger-Frerking P (2002) AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci 22:7434–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA (2001) Kainate receptors depress excitatory synaptic transmission at CA3-->CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci 21:2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Gasior M, Kaminski RM, Rogawski MA (2014) Role of GluK1 kainate receptors in seizures, epileptic discharges, and epileptogenesis. J Neurosci 34:5765–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellec MM, Panzanelli P, Sassoe-Pognetto M, Lledo PM (2007) Synapse-specific localization of vesicular glutamate transporters in the rat olfactory bulb. Eur J Neurosci 25:1373–1383. [DOI] [PubMed] [Google Scholar]
- Gall C, Sumikawa K, Lynch G (1990) Levels of mRNA for a putative kainate receptor are affected by seizures. Proc Natl Acad Sci U S A 87:7643–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW (1987) Evidence for coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol 266:307–318. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crepel V, Cossart R (2007) Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci 27:9560–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Strowbridge BW (2003) Gamma-frequency excitatory input to granule cells facilitates dendrodendritic inhibition in the rat olfactory Bulb. J Neurophysiol 90:644–654. [DOI] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J (1996) Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J 71:1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo K, Legutko B, Rizkalla G, Deverill M, Hawes CR, Ellis GJ, Stensbol TB, Krogsgaard-Larsen P, et al. (1999) [3H]ATPA: a high affinity ligand for GluR5 kainate receptors. Neuropharmacology 38:1811–1817. [DOI] [PubMed] [Google Scholar]
- Huettner JE (1990) Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5:255–266. [DOI] [PubMed] [Google Scholar]
- Isaacson JS (2001) Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A 98:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW (1998) Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron 20:749–761. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Vitten H (2003) GABA(B) receptors inhibit dendrodendritic transmission in the rat olfactory bulb. J Neurosci 23:2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Xu J, Nedergaard M, Kang J (2001) A kainate receptor increases the efficacy of GABAergic synapses. Neuron 30:503–513. [DOI] [PubMed] [Google Scholar]
- Jin XT, Pare JF, Raju DV, Smith Y (2006) Localization and function of pre- and postsynaptic kainate receptors in the rat globus pallidus. Eur J Neurosci 23:374–386. [DOI] [PubMed] [Google Scholar]
- Jin XT, Smith Y (2007) Activation of presynaptic kainate receptors suppresses GABAergic synaptic transmission in the rat globus pallidus. Neuroscience 149:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S (1998) Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol 509 ( Pt 3):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S (2000) Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol 523 Pt 3:653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S, Manabe T (2002) Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J Neurosci 22:9237–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Coumis U, Collingridge GL, Crabtree JW, Isaac JT (2002) A presynaptic kainate receptor is involved in regulating the dynamic properties of thalamocortical synapses during development. Neuron 34:635–646. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT (2001) Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J Neurophysiol 86:1139–1148. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, et al. (2010) Molecular identity of periglomerular and short axon cells. J Neurosci 30:1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles L, Kato E, Hanuska A, Zadori ZS, Al-Khrasani M, Zelles T, Rubini P, Illes P (2016) Modulation of excitatory neurotransmission by neuronal/glial signalling molecules: interplay between purinergic and glutamatergic systems. Purinergic Signal 12:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K (2008) Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci Res 60:349–354. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K (2016) Neuronal organization of the main olfactory bulb revisited. Anat Sci Int 91:115–127. [DOI] [PubMed] [Google Scholar]
- Larsen AM, Bunch L (2011) Medicinal chemistry of competitive kainate receptor antagonists. ACS Chem Neurosci 2:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL (2001a) A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron 32:697–709. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Delany C, VR JC, Bortolotto ZA, Ornstein PL, J TRI, Collingridge GL (2001b) Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptormediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology 41:907–915. [DOI] [PubMed] [Google Scholar]
- Lerma J (2006) Kainate receptor physiology. Curr Opin Pharmacol 6:89–97. [DOI] [PubMed] [Google Scholar]
- Lerma J, Marques JM (2013) Kainate receptors in health and disease. Neuron 80:292–311. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellstrom B (1993) Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A 90:11688–11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Rogawski MA (1998) GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology 37:1279–1286. [DOI] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M (1999) Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397:161–164. [DOI] [PubMed] [Google Scholar]
- Liu QS, Patrylo PR, Gao XB, van den Pol AN (1999) Kainate acts at presynaptic receptors to increase GABA release from hypothalamic neurons. J Neurophysiol 82:1059–1062. [DOI] [PubMed] [Google Scholar]
- Lizbinski KM, Dacks AM (2017) Intrinsic and Extrinsic Neuromodulation of Olfactory Processing. Front Cell Neurosci 11:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D (2009) The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology 56:6–21. [DOI] [PubMed] [Google Scholar]
- Lowe G (2003) Flash photolysis reveals a diversity of ionotropic glutamate receptors on the mitral cell somatodendritic membrane. J Neurophysiol 90:1737–1746. [DOI] [PubMed] [Google Scholar]
- Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A, Cheung T, Cornelius T, et al. (2006) BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol 4:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SS, Pozzo-Miller L, Hablitz JJ (2008) Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci 28:725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollerud S, Kastrup JS, Pickering DS (2016) A pharmacological profile of the highaffinity GluK5 kainate receptor. Eur J Pharmacol 788:315–320. [DOI] [PubMed] [Google Scholar]
- Montague AA, Greer CA (1999) Differential distribution of ionotropic glutamate receptor subunits in the rat olfactory bulb. J Comp Neurol 405:233–246. [DOI] [PubMed] [Google Scholar]
- More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, et al. (2004) Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology 47:46–64. [DOI] [PubMed] [Google Scholar]
- Mott DD, Rojas A, Fisher JL, Dingledine RJ, Benveniste M (2010) Subunit-specific desensitization of heteromeric kainate receptors. J Physiol 588:683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH, Wouterlood FF (1984) Immunocytochemical localization of GABA neurons and dopamine neurons in the rat main and accessory olfactory bulbs. Neurosci Lett 47:221–226. [DOI] [PubMed] [Google Scholar]
- Nadi NS, Hirsch JD, Margolis FL (1980) Laminar distribution of putative neurotransmitter amino acids and ligand binding sites in the dog olfactory bulb. J Neurochem 34:138–146. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F (2014) Neuronal organization of olfactory bulb circuits. Front Neural Circuits 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Diaz JV, Duque-Feria P, Andrade-Talavera Y, Carrion M, Flores G, RodriguezMoreno A (2012) Kainate receptor-mediated depression of glutamatergic transmission involving protein kinase A in the lateral amygdala. J Neurochem 121:36–43. [DOI] [PubMed] [Google Scholar]
- Negrete-Diaz JV, Sihra TS, Delgado-Garcia JM, Rodriguez-Moreno A (2006) Kainate receptor-mediated inhibition of glutamate release involves protein kinase A in the mouse hippocampus. J Neurophysiol 96:1829–1837. [DOI] [PubMed] [Google Scholar]
- Negrete-Diaz JV, Sihra TS, Flores G, Rodriguez-Moreno A (2018) Non-canonical Mechanisms of Presynaptic Kainate Receptors Controlling Glutamate Release. Front Mol Neurosci 11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, et al. (2009) Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol 7:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I (2001) Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol 86:2823–2833. [DOI] [PubMed] [Google Scholar]
- Palacios-Filardo J, Aller MI, Lerma J (2016) Synaptic Targeting of Kainate Receptors. Cereb Cortex 26:1464–1472. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A (1993) Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11:1069–1082. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J (1995) Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14:185–189. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J (1998) Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology 37:1249–1259. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML (1991) Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron 6:785–798. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V (1994) Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron 12:357–371. [DOI] [PubMed] [Google Scholar]
- Pelletier JC, Hesson DP, Jones KA, Costa AM (1996) Substituted 1,2dihydrophthalazines: potent, selective, and noncompetitive inhibitors of the AMPA receptor. J Med Chem 39:343–346. [DOI] [PubMed] [Google Scholar]
- Perkinton MS, Sihra TS (1999) A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes). Neuroscience 90:1281–1292. [DOI] [PubMed] [Google Scholar]
- Perrais D, Veran J, Mulle C (2010) Gating and permeation of kainate receptors: differences unveiled. Trends Pharmacol Sci 31:516–522. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ (1994) Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol 349:85–110. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP (1971a) The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci 9:305–345. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP (1971b) The neuropil of the periglomerular region of the olfactory bulb. J Cell Sci 9:379–409. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C (2006) Kainate receptors. Cell Tissue Res 326:457–482. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crepel V, Perrais D, Mulle C (2013) Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb Cortex 23:323–331. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP (1970) The synaptology of the granule cells of the olfactory bulb. J Cell Sci 7:125–155. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Lerma J (2012) Metabotropic signaling by kainate receptors. WIREs Membr Transp Signal 1:399–410. [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J (1997) Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19:893–901. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lerma J (1998) Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20:1211–1218. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lopez-Garcia JC, Lerma J (2000) Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc Natl Acad Sci U S A 97:1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Sihra TS (2004) Presynaptic kainate receptor facilitation of glutamate release involves protein kinase A in the rat hippocampus. J Physiol 557:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Sihra TS (2007) Kainate receptors with a metabotropic modus operandi. Trends Neurosci 30:630–637. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Sihra TS (2013) Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+-calmodulin and PKA in cerebrocortical synaptosomes. FEBS Lett 587:788–792. [DOI] [PubMed] [Google Scholar]
- Rozas JL, Paternain AV, Lerma J (2003) Noncanonical signaling by ionotropic kainate receptors. Neuron 39:543–553. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC (1999) Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron 23:499–511. [DOI] [PubMed] [Google Scholar]
- Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C (2009) Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J Neurosci 29:5000–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Lledo PM, Vincent JD, Charpak S (2001) Dendritic glutamate autoreceptors modulate signal processing in rat mitral cells. J Neurophysiol 85:1275–1282. [DOI] [PubMed] [Google Scholar]
- Salmen B, Beed PS, Ozdogan T, Maier N, Johenning FW, Winterer J, Breustedt J, Schmitz D (2012) GluK1 inhibits calcium dependent and independent transmitter release at associational/commissural synapses in area CA3 of the hippocampus. Hippocampus 22:57–68. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF (1997) Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron 19:1141–1146. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA (2000) Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron 27:327–338. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA (2001a) Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A 98:11003–11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA (2001b) Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science 291:1972–1976. [DOI] [PubMed] [Google Scholar]
- Schoppa NE (2006a) AMPA/kainate receptors drive rapid output and precise synchrony in olfactory bulb granule cells. J Neurosci 26:12996–13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE (2006b) Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron 49:271–283. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL (1998) Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci 18:6790–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL (2001) Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron 31:639–651. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL (2002) AMPA autoreceptors drive correlated spiking in olfactory bulb glomeruli. Nat Neurosci 5:1194–1202. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM (2001) Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nat Neurosci 4:718–723. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT (2009) Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101:1988–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM (1972) Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52:864–917. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA (2004) Olfactory bulb In: The synaptic organization of the brain, vol. (Shepherd GM, ed), pp. 165–216. New York City, U.S.: Oxford University Press. [Google Scholar]
- Sihra TS, Flores G, Rodriguez-Moreno A (2014) Kainate receptors: multiple roles in neuronal plasticity. Neuroscientist 20:29–43. [DOI] [PubMed] [Google Scholar]
- Sihra TS, Rodriguez-Moreno A (2013) Presynaptic kainate receptor-mediated bidirectional modulatory actions: mechanisms. Neurochem Int 62:982–987. [DOI] [PubMed] [Google Scholar]
- Small B, Thomas J, Kemp M, Hoo K, Ballyk B, Deverill M, Ogden AM, Rubio A, et al. (1998) LY339434, a GluR5 kainate receptor agonist. Neuropharmacology 37:1261–1267. [DOI] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinanen K, Sakmann B, Seeburg PH (1992) A glutamate receptor channel with high affinity for domoate and kainate. EMBO J 11:1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Hunt DL, Yamasaki M, Kim KS, Watanabe M, Castillo PE, Tomita S (2011a) Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat Neurosci 14:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Zhang W, Howe JR (2011b) Neto2 modulation of kainate receptors with different subunit compositions. J Neurosci 31:8078–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, McInnes RR (2011) Neto1 is an auxiliary subunit of native synaptic kainate receptors. J Neurosci 31:10009–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Blakemore LJ (1999) Mammalian olfactory bulb neurons. In: The Neuron in Tissue Culture, vol. (Haynes LW, ed), pp. 585–592. [Google Scholar]
- Trombley PQ, Westbrook GL (1990) Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol 64:598–606. [DOI] [PubMed] [Google Scholar]
- Chichester UK: Wiley. Trombley PQ, Westbrook GL (1990) Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol 64:598–606. [DOI] [PubMed] [Google Scholar]
- Urban NN, Sakmann B (2002) Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 542:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Clarke VR, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL (1998) The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology 37:1269–1277. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL (1997) The synaptic activation of kainate receptors. Nature 388:179–182. [DOI] [PubMed] [Google Scholar]
- Vitten H, Reusch M, Friauf E, Lohrke S (2004) Expression of functional kainate and AMPA receptors in developing lateral superior olive neurons of the rat. J Neurobiol 59:272–288. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB (2001) Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32:723–735. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT (2005) Inhibition [corrected] of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol 94:2700–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE (1995) Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol Pharmacol 47:582–587. [PubMed] [Google Scholar]
- Woolf TB, Shepherd GM, Greer CA (1991) Serial reconstructions of granule cell spines in the mammalian olfactory bulb. Synapse 7:181–192. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Zhao MG, Toyoda H, Ko SW, Zhuo M (2005) Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol 94:18051813. [DOI] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, et al. (2009) A transmembrane accessory subunit that modulates kainatetype glutamate receptors. Neuron 61:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LM, Gu ZQ, Costa AM, Yamada KA, Mansson PE, Giordano T, Skolnick P, Jones KA (1997) (2S,4R)-4-methylglutamic acid (SYM 2081): a selective, high-affinity ligand for kainate receptors. J Pharmacol Exp Ther 280:422–427. [PubMed] [Google Scholar]
- Zhuo M (2017) Cortical kainate receptors and behavioral anxiety. Mol Brain 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]