Abstract
Anandamide (AEA) is the N-acyl ethanolamide of arachidonic acid, an agonist of cannabinoid and non-cannabinoid receptors in the body. The kidneys are enriched in AEA and in enzymes that metabolize AEA, but the roles of AEA and its metabolites in the kidney remain poorly understood. This system likely is involved in the regulation of renal blood flow and hemodynamics and of tubular sodium and fluid reabsorption. It may act as a neuromodulator of the renal sympathetic nervous system. AEA and its cyclooxygenase-2 metabolites, the prostamides, in the renal medulla may represent a unique antihypertensive system involved in the long-term control of blood pressure. AEA and its metabolites are also implicated as modulators of inflammation and mediators of signaling in inflammation. AEA and its metabolites may be influential in chronic kidney disease states associated with inflammation and cardiovascular diseases associated with hyperhomocysteinemia. The current knowledge of the roles of AEA and its derivatives highlights the need for further research to define and potentially exploit the role of this endocannabinoid system in the kidney.
Keywords: Anandamide, Endocannabinoid, Prostamide, Kidney, Review
2. INTRODUCTION
The endocannabinoids comprise a large and heterogenous group of endogenous fatty acid-derived lipids that share in common the ability to bind and activate endogenous CB receptors. The first identified and most intensively studied endocannabinoid is AEA (AEA), the N-acyl ethanolamide derivative of the C20,4 fatty acid, arachidonic acid (1). Given the subsequent recognition of the ubiquitous distribution of AEA, AEA-metabolizing enzymes, and their receptors, it is not surprising that AEA has been linked with so many diverse physiologic and pathophysiologic actions and functions including modulatory effects on sensory and autonomic nerve signaling (2), regulation of energy consumption and balance (3), and initiation and control of inflammation (4). Although the kidneys are known to contain a rich supply of AEA and enzymes and transporters that control the disposition of AEA, the role(s) of AEA and its metabolites in this organ remain poorly understood. The kidneys play a crucial role in the regulation of electrolyte and water balance and the maintenance of the extracellular fluid volume, an essential determinant of blood pressure. In addition, they contribute to acid-base balance, glucose and calcium homeostasis, and they serve as an endocrine organ, producing renin, erythropoietin, kinins, 1,25-dihydroxycholecalciferol, and other hormones. This review focuses on the current evidence regarding the roles of AEA on kidney functions and kidney disease. Critical gaps in our understanding that need to be addressed for a better understanding of its roles in kidney health and disease are highlighted.
3. REGIONAL AND CELLULAR DISTRIBUTION OF AEA IN THE KIDNEY
The kidneys are among a small number of tissues characterized by their higher than average AEA content (5). The cellular localization that accounts for this high AEA level in the kidney remains unknown. In a limited analysis, AEA was detected in isolated cultured renal endothelial cells and mesangial cells (6), but other specific cell types have not been evaluated. In an assessment of the general regional distribution of AEA in kidney, AEA was found to be relatively concentrated in the renal medullary region compared to the renal cortex (7). In contrast, three other related fatty acids with acylated acidic groups, including the 2-glycerol ester of arachidonic acid (2-AG) and the non-CB N-ethanolamides of palmitate and oleate, showed comparable concentrations in the cortex and medulla. This finding suggested that AEA serves a unique functional role in the renal medulla. The identification of cells responsible for the differential enrichment of AEA in the renal medulla is currently hampered by the lack of specific and sensitive tools and methods, for example, the lack of an AEA-specific antibody for immunohistochemistry. The approach of using primary cultured cells derived from kidney as described above (6) is potentially meaningful but has the drawback of post-isolation changes as the cells adapt to their culture environment.
AEA is principally recognized as neuronal in origin, which is consistent with its proposed major neuromodulatory function. Although neuronal cells are a relatively minor constituent of the total cell number and mass of the kidney, the kidneys are innervated by the renal sympathetic nerve (8). Whether the AEA content of the kidney is attributable to renal nerve cells or other cells is not yet known. Non-neuronal cell types can synthesize and release AEA such as macrophages (9), thereby demonstrating cells need not be neuronal in origin to have this property. In the kidneys, an interesting non-neuronal cellular candidate for AEA enrichment is the renomedullary interstitial cell. These cells possess lipid-rich granules which degranulate in response to elevated blood pressure, releasing antihypertensive substances (10).
3.1. Biosynthesis of AEA
The biosynthetic pathway of AEA synthesis begins with the formation of membrane-bound N-arachidonyl phosphatidyl ethanolamine (NAPE) by transfer of the fatty acyl group at the sn-1 position of glycerophospholipids to the primary amino group of phosphatidylethanolamine. This reaction is catalyzed by N-acyltransferases that are either calcium-dependent (11) or independent (12). The system responsible for synthesis of NAPE in kidney remains unknown.
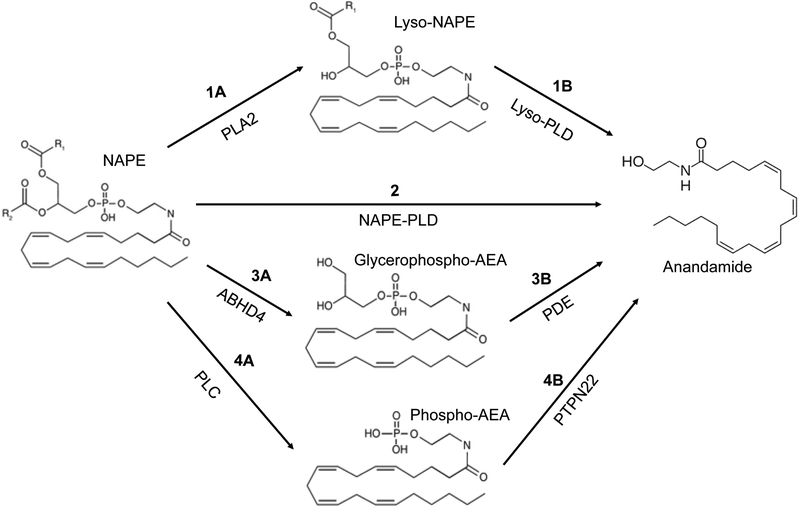
After the formation of NAPE, the next step, which appears rate limiting, is the conversion of NAPE to AEA. Four different mechanisms of synthesis of AEA from NAPE have been described as shown in Fig. 1. The first involves the conversion of NAPE by phospholipase A2 to form N-arachidonyl lysophosphatidylethanolamine followed by lysophospholipase D-catalyzed release of AEA (see steps 1A and 1B in Fig. 1) (15, 16). NAPE may also be directly converted to AEA by NAPE-specific phospholipase D (step 2) (14) . A third pathway consists of conversion of NAPE to glycerophospho-AEA by the enzyme ABHD4 (step 3A) followed by phosphodiesterase-catalyzed cleavage of the phosphoglycerol moiety to release AEA (step 3B). Last, NAPE may also be converted to AEA by phospholipase C-catalyzed formation of phospho-AEA (step 4A) followed by removal of the phosphate group by an enzyme in the phosphatase family such as PTPN22 (step 4B) (13). In regard to these four possibilities, NAPE-PLD has been identified and found to be expressed at high levels in kidneys. Mice carrying NAPE-PLD gene knockouts have been generated, but no data have been reported regarding the effect of this knockout on renal AEA. In regard to the third pathway, recently a member of the glycerophosphodiester phosphodiesterase family, GDE4, having lysophospholipase D activity with the ability to liberate AEA from N-arachidonyl lysophosphatidylethanolamine has been identified in the kidney (16, 17).
Figure 1.
Biosynthetic pathways of anandamide. Anandamide (AEA) can be generated from N-arachidonoyl-phosphatidylethanolamine (NAPE) by 1) release of Lyso-NAPE via phospholipase A2 (PLA2) followed by cleavage by Lyso-phospholipase D (Lyso-PLD), 2) by NAPE-PLD, 3) by ABHD4-catalyzed formation of glycerophospho-AEA followed by phosphodiesterase (PDE), or 4) by release of phospho-AEA by phospholipase C followed by dephosphorylation by PTPN22. The pathway required for synthesis of AEA in the kidney is unknown.
3.2. AEA- metabolizing enzymes in the kidney
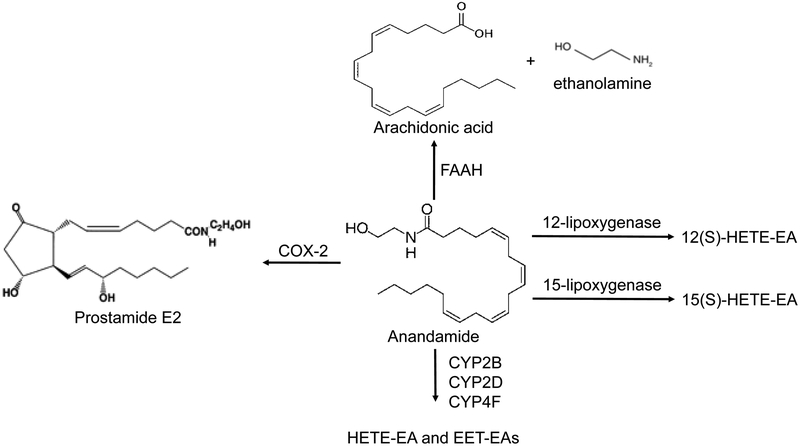
Given the high AEA content of kidneys, the kidneys not surprisingly are also enriched in several enzymes that can metabolize this lipid substrate (Fig. 2). Some of these enzymes may play roles in the termination of AEA action, whereas others may contribute unique roles in the generation of bioactive signaling mediators under physiologic or disease conditions.
Figure 2.
Metabolic pathways of anandamide. Anandamide (AEA) can be metabolized by 1) fatty acyl amide hydrolase (FAAH) to release free arachidonic acid and ethanolamine, 2) 12- or 15-lipoxygenases to form 12(S)-hydroxyeicosatetraenoic acid-ethanolamine (HETE)-EA and 15(S)-HETE-EA and other HETEs, 3) by cytochrome P450s to form HETE-EAs and EET-EAs, and 4) by cyclooxygenase-2 (COX-2) to form prostamide E2 and other prostamides.
3.2.1. Fatty acid amide hydrolase
The enzyme, fatty acid amide transferase (FAAH), is an integral membrane serine hydrolase that catalyzes the hydrolysis of AEA to form arachidonic acid and ethanolamine. Cloning of the enzyme activity indicated the existence of two distinct FAAH family members which are expressed in a species and tissue dependent manner (18). FAAH-1 is produced in both humans and rodents, and is the main form found in brain, liver, lung, testis, prostate, kidney, and small intestine. FAAH-2 on the other hand is found only in humans and is detected in the heart, lung, liver, kidney, prostate, and ovary (19).
The biological significance of FAAH-catalyzed AEA hydrolysis is that it inactivates the ability of AEA to stimulate either CB receptors or TRPV1 ion channels. However, FAAH should not be viewed strictly as an inactivating enzyme, in that arachidonic acid formed from its reaction is converted to a wide range of eicosanoid metabolites having diverse biologic activities and roles. An important quantitative contribution of FAAH to the kidney AEA level is suggested by the finding that mice homozygous for FAAH knockout show a 3–4-fold increase in AEA concentration (20). The relative importance of FAAH as a controlling factor is likely to depend on the specific tissue or cell type. Tissues such as brain exhibit a relative high turnover of endogenous AEA by FAAH, as suggested by the ~10-fold increase in AEA levels in mice homozygous for the FAAH mutation. Corresponding increases in kidney and brain AEA content also occur in animals treated with chemical inhibitors of FAAH.
The observation that mice with chemical inhibition or genetic knockout of FAAH show no detectable AEA hydrolytic activity in the kidney establishes FAAH as the principle AEA hydrolytic enzyme in the mouse kidney (5). Biochemical analysis of the regional distribution within the mouse kidney revealed a higher relative FAAH activity in renal cortex than medulla (7). Immunohistochemical analysis using a FAAH-specific antibody suggested two distinct staining patterns in the kidney. In the cortex, proximal and distal tubules showed diffuse but significant staining. Individual cells in the glomeruli and in renal vasculature also were positive for staining. Also in the cortex but particularly in the renal medulla, cortical and medullary collecting ducts, respectively, exhibited more intense staining indicative of the highest relative FAAH expression in the kidney. These findings are consistent with AEA having an important role in the proximal and distal tubular cells, glomeruli, and renal collecting ducts.
3.2.2. Cyclooxygenase-2
Cyclooxygenases are membrane bound oxygenases that catalyze the metabolism of arachidonic acid to prostaglandin H2, which is subsequently converted to specific prostaglandins by prostaglandin synthases. The kidney is unique among tissues in its high basal expression of COX-2 (21). COX-2 is differentially expressed in renal medullary cells and in the macula densa cells which exhibit upregulated COX-2 in response to low sodium concentration in the tubular fluid (22).
The identification of AEA as a substrate of recombinant COX-2 (23) raises questions regarding the biological activities of the COX-2 metabolites of AEA, the prostamides. In contrast, COX-1 appeared inactive (23). The basis for this differential activity of COX-2 versus COX-1 towards AEA is proposed to be due to a slightly enlarged side pocket of the arachidonic acid-binding site in COX-2, which is absent in the COX-1 structure. This side pocket permits the enzyme to accommodate the bulky ethanolamine side group of AEA (24).
In the kidney, FAAH and COX-2 exhibit opposing patterns of distribution as assessed by enzymatic activity assays, Western blotting and immunohistochemistry (7), suggesting a relationship between their functions. The higher AEA content of the renal medulla in concert with the higher medullary expression of COX-2 suggests a novel role of the prostamides in the renal medulla. Prostamide E2, the ethanolamide of prostaglandin E2, is the major product formed in reactions of renal medullary microsomes with AEA (7). Prostamides E2 and D2 have been identified in the kidneys of mice treated with AEA (25). The dependence of the rate of prostamide formation on the FAAH activity is suggested by the higher level of prostamides in kidneys of AEA-treated FAAH knockout mice compared to wild-type mice.
3.2.3. Lipooxygenases
Lipoxygenases are a family of iron-containing dioxygenases that catalyze the hydroperoxidation of polyunsaturated fatty acids containing a cis,cis-1,4-pentadiene structure. The products of lipoxygenase enzyme-mediated metabolism of arachidonic acid are hydroperoxyeicosatetraenoic acids (HpETEs) which then rapidly convert to hydroxyeicosatetraenoic acids (HETEs). Of the four specific types of lipoxygenase known, AEA was demonstrated to be a substrate of leukocyte 12-lipoxygenase and 15-lipoxygenase, but not 5-lipoxygenase or the platelet-type 12-lipoxygenase (26). The products of 12- and 15- lipoxygenation of AEA, 12(S)- and 15(S)-Hydroperoxyeicosatetraenoyl-EA (12- and 15-HpETE-EA), are subsequently reduced to form 12(S)- and 15(S)-HETE-EA. The roles of these metabolites in the kidney have not yet been investigated, but at least one of the lipoxygenases is known to be significantly expressed in kidneys under basal conditions. The proximal and distal tubules and mesangial cells of mouse kidneys express high levels of leukocyte 12-lipoxygenase and 15-lipoxygenase (27, 28).
3.2.4. Cytochrome P450s
The cytochrome P450s are a superfamily of microsomal heme-containing monooxygenases that participate in the oxidative metabolism of many endogenous and xenobiotic substrates. P450s in several CYP subfamilies have been shown to metabolize arachidonic acid to epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs). The EETs may also be further metabolized by microsomal epoxide hydrolase to form DHETEs.
Human kidney microsomal P450s can also catalyze the oxidation of AEA to produce the corresponding ethanolamides of 20-HETE and 5,6-, 8,9-, 11,12- and 14,15-EET (29). CYP4F2, a major P450 expressed in proximal and distal tubules, was shown to be active in the conversion of AEA to 20-HETE-EA. Two additional members of the P450 family found in the kidney, CYP2B6 and CYP2D6, were shown to form EET-EAs and HETE-EAs (30, 31). Whereas 20-HETE has been associated with increased blood pressure and several of the EET metabolites with decreased blood pressure, the biological activities of the corresponding metabolites of AEA and their physiological significance for renal function is not known.
3.3. Membrane transporters of AEA
After its release from cells, the actions of AEA, like other neurotransmitters, may be terminated by either of two possible mechanisms: metabolism to inactive products by metabolizing enzymes or by carrier-mediated reuptake. AEA, being a neutral lipid, may cross back into the cell by lipid diffusion. However, evidence supports the existence of membrane carriers that can translocate AEA across cell membranes, and thereby influence its biological activity. Following the injection of radiolabeled AEA as tracer, AEA was found to be rapidly cleared from the blood, mainly by movement into tissues (32). The extent and rate of this uptake into tissues occurred at different rates, with the adrenal glands, lungs, and kidneys being the most prolific in this regard. The basis of this rapid movement into tissues, whether mainly by diffusion or by carrier-mediated uptake remains to be determined.
Recently, a potential AEA carrier in neurons, FLAT, has been identified (33). FLAT exhibits a FAAH-1-like variant structure but lacks detectable hydrolytic activity. Heterologous expression of this protein in HEK293 cells conferred AEA-binding activity and the ability to accumulate AEA. Moreover, a series of chemical inhibitors previously demonstrated to inhibit AEA cellular transport were shown to inhibit AEA accumulation in FLAT-expressing cells. Expression of the FLAT gene was detected in a wide array of neural and non-neural cell types, including liver, lung and kidney. Further research is needed to ascertain the significance and roles of this carrier protein in the kidney and other tissues.
4. RECEPTORS FOR AEA AND PROSTAMIDE IN THE KIDNEY
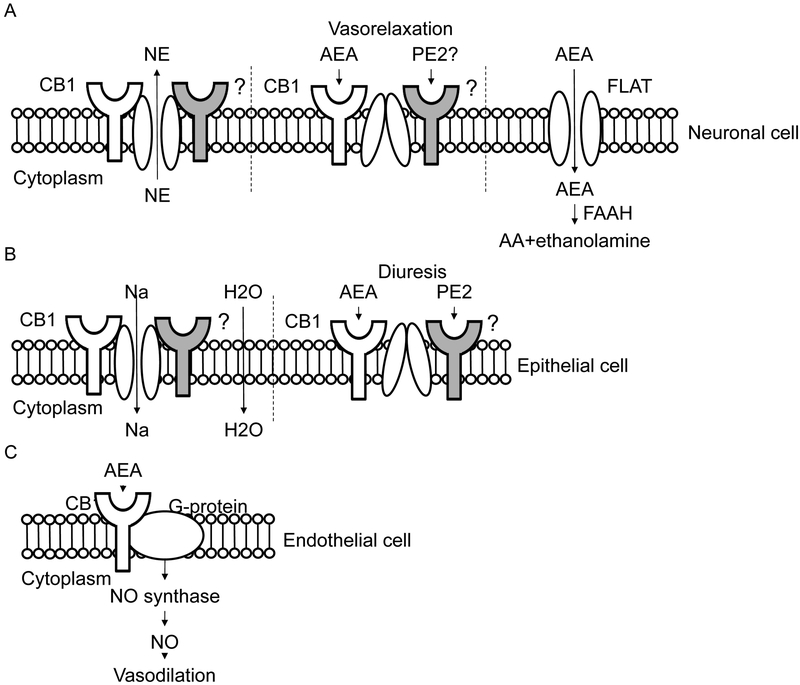
AEA and AEA metabolites can elicit their physiological and behavioral effects through a number of possible receptor systems (Fig. 3). The cellular localization patterns of these receptors in the kidney and their signaling pathways can provide important clues as to the functional roles and activities of AEA and its metabolites in the kidneys.
Figure 3.
Potential transporters and mechanisms by which anandamide and prostamide E2 can exert their effects on (A) sensorimotor neural cells resulting in inhibition of sympathetic tone , (B) renal tubular epithelial cells involved in sodium excretion and diuresis and (C) vascular endothelial cells resulting in nitric oxide-dependent vasorelaxation. The cytoplasmic side of the membrane is indicated.
4.1. Cannabinoid (CB) receptors
AEA may elicit its effects by activating either of two types of CB receptors: CB1 and CB2. The CB receptors are G-protein-coupled receptors, and their cellular distribution in kidney has been studied using both direct (RT-PCR, Western blot, and immunohistochemistry) and indirect approaches (based on manipulation of isolated cultured cell models using pharmacological or genetic tools). These data suggest that CB1 receptors are the predominant type of CB receptor expressed in rodent and human kidney and are localizable to the proximal and distal convoluted tubule and intercalated cells in the collecting ducts (34). This pattern of expression has a striking resemblance to the pattern of FAAH distribution, suggesting that AEA may act directly in renal cortex and medulla, respectively, on CB-1 receptors located on proximal and distal tubular cells and the collecting duct. Cultured cell model-based data, on the other hand, suggested that CB1 receptors are expressed and are functional in podocytes and mesangial cells of the glomerulus (35, 36), proximal tubular cells of the cortex (37, 38), and thick ascending limb cells of Henle’s loop (39).
4.2. Transient receptor potential vanilloid type I channels
The identification of AEA as a ligand activator of TRPV1 has led to its recognition as a “non-CB” receptor for AEA (40). (TRPV1) ion channel is a ligand-gated cation channel involved in mechano- and chemosensory function. The activation of TRPV1 channels in kidney by capsaicin produces diuresis and natriuresis mediated in part by an increased glomerular filtration rate and by activation of sensory nerves in the renal pelvis (41). TRPV1 activation provides a protective mechanism against salt-induced blood pressure elevation (42). However, assessment of the specific contribution of AEA-induced TRPV1 channel modulation is complicated by the multiple other sensory stimuli capable of activating TRPV1 channels. To our knowledge, the role of TRPV1 receptors in modulation of blood pressure has not been studied under conditions of elevated AEA such as in mice with FAAH gene knockout mutations or treated with FAAH inhibitors. AEA levels were reported to be elevated in TRPV1 knockout but not wild type mice with hypertension induced by uninephrectomy and deoxycorticosterone acetate-salt treatment (42).
4.3. Prostamide receptors
The finding that prostamides have no meaningful activity at prostanoid receptors suggests that prostamides exert their biological activities through unique receptors (43). Recent progress towards the identification of prostamide receptors was demonstrated by the identification of prototype prostamide F2α antagonists capable of blocking prostamide F2α- and bimatoprost-induced effects on calcium signaling in feline iris cells, an effect independent of any known prostaglandin receptors including the FP receptor (44). In mouse kidney, the major prostamide formed is prostamide E2, and it is unclear at present what receptors prostamide E2 may be acting through.
5. ACTIONS OF AEA AND ITS METABOLITES ON THE KIDNEY
The most critical function of the kidneys is the maintenance of electrolyte and fluid homeostasis, which is considered a crucial mechanism for the long-term control of blood pressure. There are three principal mechanisms by which the kidneys regulate salt and water balance: renal hemodynamics and microcirculation, renal excretory functions, and neuromodulation of the sympathetic nervous system input to the kidneys. Current evidence supports the capacity of AEA or AEA metabolites to regulate these three basic mechanisms.
5.1. Renal hemodynamics and microcirculation
In a normal individual, the kidneys receive ~20% or 1.2 L/min of the cardiac output delivered by the renal artery. After branching of the artery, the blood enters the renal microcirculation of the glomerular capillaries, entering via the afferent arterioles and leaving by the efferent arterioles. The hydrostatic pressure in the glomerular capillaries is highly autoregulated to maintain a constant glomerular filtration rate (GFR) over a range of mean arterial pressures. This autoregulation of GFR occurs by differential regulation of the resistances of the afferent and efferent arterioles to blood flow. Deutsch et al. reported that AEA induced a reversible vasodilation of preglomerular juxtamedullary afferent arterioles isolated and perfused in vitro (6). This effect was blocked by either a selective CB1 antagonist or an NO synthase inhibitor. These results are consistent with a model whereby CB1-receptors present on the endothelial cells of afferent arterioles stimulates nitric oxide synthase-mediated production of nitric oxide which diffuses to the adjacent smooth muscle to produce the observed vasodilation. The non-selective COX inhibitor, indomethacin, had no apparent effect on the AEA-induced vasodilation, suggesting that COX metabolites of AEA do not contribute. Vasodilation of the afferent arterioles would be predicted to increase the hydrostatic pressure in the glomerular capillaries, thereby increasing the GFR. Data from an in vivo inulin clearance study in rats receiving AEA intraarterially showed that AEA decreased GFR (45). p-Aminohippuric acid clearance was increased suggesting that AEA increased total renal blood flow, likely explained by a decrease in peripheral vascular resistance. Further experiments showed that AEA increased the arteriolar diameters of both the afferent and efferent arterioles, but the efferents were more sensitive. Predominant vasodilation of the efferent arterioles is consistent with decreased hydrostatic pressure in the glomerular capillaries and a lowering of GFR. It is noteworthy that the vasodilating effect of AEA on both the afferent and efferent arterioles was CB1 receptor-mediated, because the CB1 receptor antagonist, AM281, completely blocked the responses to AEA.
Blood leaving the efferent arterioles of juxtamedullary glomeruli enters the renal medullary microcirculation and travels down the descending and up the ascending branches of the vasa recta. The renal medullary circulation is considered to have an important influence on the control of sodium excretion and blood pressure (46). Renomedullary blood flow, in contrast to total renal blood flow and the glomerular filtration rate, is not well regulated and is proportional to renal perfusion pressure. The proportionality of medullary blood flow to perfusion pressure is considered essential for pressure-induced sodium excretion.
Although the influence of AEA on renal medullary blood flow has not been reported, Li et al. found that intramedullary infusion of the AEA analogue, methanandamide, had no effect on either renal cortical or medullary blood flow (47). Methanandamide is a metabolically stable analogue of AEA that retains CB-1 receptor-binding capacity. In contrast, intramedullary infusion of AEA resulted in decreased mean arterial pressure, an effect which was blocked by a selective CB1 receptor antagonist, AM251, but not a TRPV1 receptor-antagonist, capsazepine. The lack of effect of methAEA on renal medullary blood flow is in agreement with published data from our group showing that renal medullary blood flow is unaffected by intramedullary infusions of AEA (7).
5.2. Tubular absorption of sodium and water
The tubular epithelial cells of the kidney beginning with the proximal convoluted tubule and ending with the medullary collecting ducts control the reabsorption of sodium and water. It has been known for many years that CBs can alter this renal tubular excretory function. Δ9 tetrahydrocannabinol (THC), the main psychoactive constituent of marijuana, increased urine output along with sodium and potassium excretion after oral administration to rats (48). The CB-1 receptor agonist, AM2389, exhibited a stronger diuretic effect than the nonselective CB agonists, THC and AM4054 (49). The specific nephron segment mediating the effect of THC and CB receptor agonists and its mechanism remain unknown.
The intramedullary infusion of methanandamide, a metabolically stable analogue of AEA, increased the urine formation rate without changing either sodium excretion or the cortical or medullary blood flow in rats (47). This diuretic effect occurred independently of either the CB1 receptor or the TRPV1 vanillinoid receptor assessed using antagonists of these receptors. This infusion of methanandamide also decreased systemic pressure in a CB1 receptor-dependent manner. However, in a related study of AEA infusion into the mouse renal medulla, excretion of sodium and potassium and urine formation rate were all increased, and there was no effect on either total renal or medullary blood flow (7). The diuretic and natriuretic effects of AEA were only partially blocked by a CB-1 receptor antagonist, suggesting a non-CB receptor based mechanism. The finding that celecoxib blocked the effect of AEA suggested that the action of AEA was mediated indirectly by its COX-2 metabolite, prostamide-E2. Intramedullary infusion of prostamide E2 mimicked the effects of AEA in stimulating natriuresis and diuresis but not affecting intramedullary blood flow. These observations support a role of prostamide E2 as a mediator of AEA effects on renal excretory function in mice. In contrast, Silva et al demonstrated that AEA inhibited oxygen consumption in suspensions of rat medullary thick ascending limb cells, consistent with an inhibitory effect of AEA on sodium transport (39). However, this effect was not blocked by a cyclooxygenase inhibitor.
5.3. Neuromodulation of renal sympathetic nerve activity
A number of observations support a sympathoinhibitory role of AEA in the modulation of sympathetic neurotransmission. THC and AEA inhibited the exocytotic release of radiolabeled norepinephrine evoked by electrical stimulation of isolated rat atria and vas deferens pre-loaded with [3H[-norepinephrine (50). This effect occurred through CB-1 receptors located on presynaptic sympathetic nerve terminals, and it has been proposed to underly the prolonged depressor response to an intravenous bolus of AEA (51). The sympathoinhibitory effect was shown to depend on pre-existing sympathetic tone, as it occurred in either anesthetized or conscious spontaneously hypertensive rats or urethane-anesthetized normal rats (which exhibit an elevated sympathetic tone) but not in conscious normotensive rats (which lack significant basal sympathetic tone) (52). This is further supported by the observation that the vasodepressor effect of AEA is blocked by the alpha adrenergic antagonist, phentolamine (53). It is noteworthy that spontaneously hypertensive rats treated with a FAAH inhibitor, showed normalized blood pressure, a fact which supports a role of endogenous AEA in the sympathoinhibitory effect (54).
The kidney and its functions are under the control of the renal sympathetic nerve. Activation of the sympathetic nervouse system increases tubular sodium reabsorption, the release of renin and the renin-angiotensin-aldosterone system, and renal vascular resistance (8), all of which contribute to the elevation of blood pressure. The possibility that renal AEA contributes to regulation of the renal sympathetic nerve is supported by the observation that AEA could inhibit the potassium chloride-stimulated release of norepinephrine from renal sympathetic nerves in freshly dissected rat kidney arterial trees (6). Interestingly, this effect was only partially blocked by a CB-1 receptor antagonist, raising the question of the nature of the CB receptor-independent mechanism. Given the evidence already discussed for metabolism of AEA by the kidneys into prostamides and other metabolites, it will be interesting to examine the roles of metabolites in this neuromodulatory function.
6. POTENTIAL ROLES OF THE RENAL AEA SYSTEM IN DISEASE STATES
6.1. Antihypertensive system
Treatment of normotensive rats and mice with CB-1 antagonists alone has little effect on blood pressure, and mice with CB-1 receptor gene knockouts show no difference in baseline pressure compared to their wild-type littermates (53, 55). These results suggest that CB-1 receptors are not tonically active in tissues controlling blood pressure including the CNS and kidneys. In contrast, protective antihypertensive effects of the endocannabinoid system have been described in experimental models of hypertension including spontaneous hypertensive rats (SHRs) and Dahl salt-sensitive hypertensive rats (56). Administration of a CB-1 antagonist to SHRs increased blood pressure, whereas a chemical inhibitor of FAAH decreased it. Although the mechanisms of the protective effects were attributed to effects of endocannabinoids on cardiac contractility and vascular resistance, a role for AEA in the kidney in the mechanism of antihypertensive response cannot be excluded.
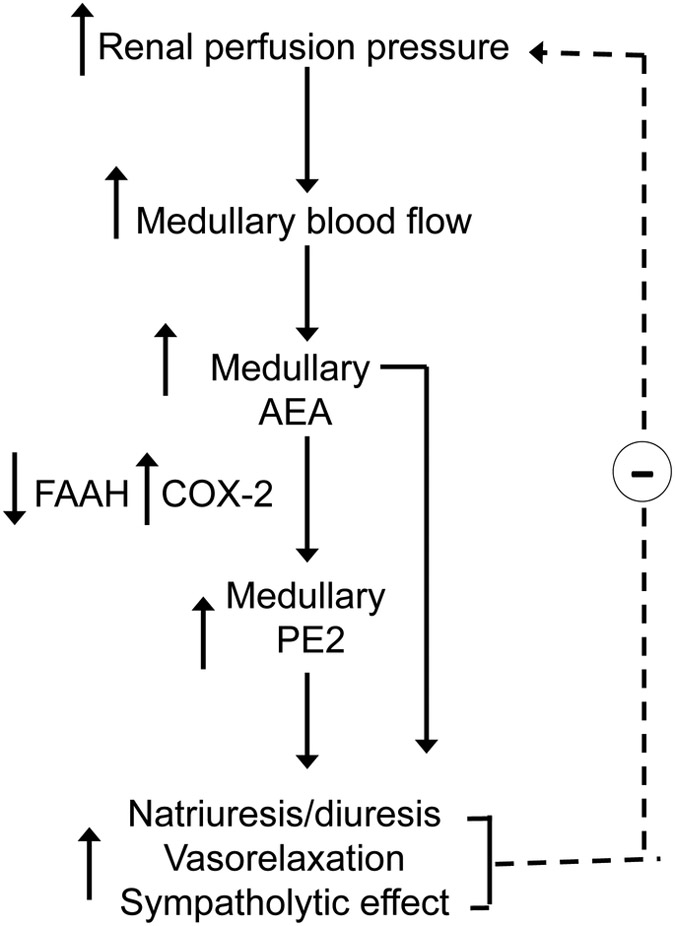
Our group has proposed that AEA and/or AEA metabolites may represent a novel renal antihypertensive system and may be released from kidney in response to hypertensive stimuli according to the model of Muirhead (7) (Fig. 4). This system is a proposed physiologic counterpart to the pro-hypertensive, sodium- and volume-conserving renin-angiotensin system of the kidney. According to Muirhead (57, 58), the renal medulla responds to increased medullary blood flow by secreting a vasodepressor neutral lipid termed medullipin. The antihypertensive effects of medullipin were proposed to be due to three of its intrinsic properties: vasodilator, inhibitor of sympathetic activation, and promoter of renal salt and water excretion. The identity of medullipin has remained elusive, but if elucidated, it has potential to lead to development of novel anti hypertension strategy. AEA and its COX-2 metabolite, prostamide E2, possess several of the properties proposed for medullipin. Both of these lipids are N-acylated on their acid groups and are therefore uncharged at physiologic pH consistent with the proposed neutral chemical property suggested for medullipin (59). After an i.v. bolus infusion, AEA elicits a ‘tri-phasic’ blood pressure response featuring an initial hypotensive response followed by a brief hypertensive action and then by a more prolonged hypotensive effect mediated by its vasodepressor actions (52). AEA also has actions consistent with sympathoinhibitory properties. AEA blocked exocytotic release of norepinephrine from electrically stimulated tissues (50) and inhibited KCl-stimulated norepinephrine release from isolated renal arteries (6). After acute elevation of blood pressure, the AEA content is elevated in the nucleus tractus solitarius, a mid-brain region involved in the involuntary control of blood pressure. Moreover, AEA microinjected into this region significantly prolonged the baroreflex-mediated inhibition of the renal sympathetic nerve (60). This potential mechanism is described in more detail above in the section on neural regulation by AEA. The response of endogenous AEA in the kidneys to renal sympathetic nerve stimulation or after acute or chronic hypertensive stimuli needs to be investigated. Lastly, AEA also exhibits the third property ascribed to medullipin by Muirhead, as it has the capacity to stimulate excretion of sodium and water following injection into the renal medulla of mice (7).
Figure 4.
Proposed role of anandamide in the renal medulla. When renal perfusion pressure increases, medullary blood flow increases, and synthesis and release of AEA in the renal medulla is stimulated. The low FAAH-high COX-2 expression of the renal medulla favors the local release of AEA and/or its metabolism by COX-2 to prostamide E2 (PE2). AEA and/or PE2 could exert local apocrine effects or longer range endocrine effects by entering the circulation. Local apocrine effects include the tubular reabsorption of sodium and water resulting in lowered extracellular fluid volume and blood pressure. Systemic actions include vasorelaxation and an inhibitory effect on central sympathetic outflow to the heart and vasculature. The net effect of the three actions is lowering of arterial blood pressure and deceased renal perfusion pressure, restoring the system to homeostatic balance.
6.2. Modulation of inflammation
FAAH knockout mice exhibit an anti-inflammatory phenotype in various models of inflammation (61), including the λ-carageenan–induced hind paw inflammation model (62), the 2,4-dinitrofluorobenzene –induced cutaneous allergic dermatitis model (63), and the nitrobenzene sulfonic acid or dextran sulfate sodium-induced colitis model (64). The finding that in most of these models the anti-inflammatory phenotype is diminished by co-treatment with either CB-1 or CB2 antagonists (65) has led to the view of AEA as an anti-inflammatory factor. However, not all of these models show a reversal of effect by the CB antagonists (62, 63). These findings suggest that lipid metabolites of AEA are responsible for the anti-inflammatory phenotype associated with the FAAH knockout genotype.
A number of chronic kidney diseases are associated with long-term inflammation including diabetic nephropathy, glomerular nephritis, and interstitial nephritis. Therefore, the question is raised as to the role of AEA and the endocannabinoid system in either the initiation or progression of such diseases. Few studies have addressed this question directly. In obese Zucker rats with proteinuria and increased plasma creatinine, a model for obesity-induced renal failure, treatment with a CB-1 receptor antagonist was associated with protection rather than exacerbation of disease markers (66). The mechanism of the CB-1 antagonist appeared however not to involve an effect on inflammation, but rather by improving lipid metabolism and the serum lipid profile of obese Zucker rats (67, 68). Barutta et al also described a protective effect of a CB-1 antagonist against albuminuria in mice with streptozotocin-induced diabetes, a model of diabetic nephropathy (69). Although treatment with the CB-1 antagonist ameliorated the albuminuria in the model, there were no visible signs of ultrastructural damage to the glomeruli or tubules of the kidney. Thus, the protective effect of the CB antagonist appeared to be on an early process in the development of the disease, prior to tissue damage and onset of inflammation.
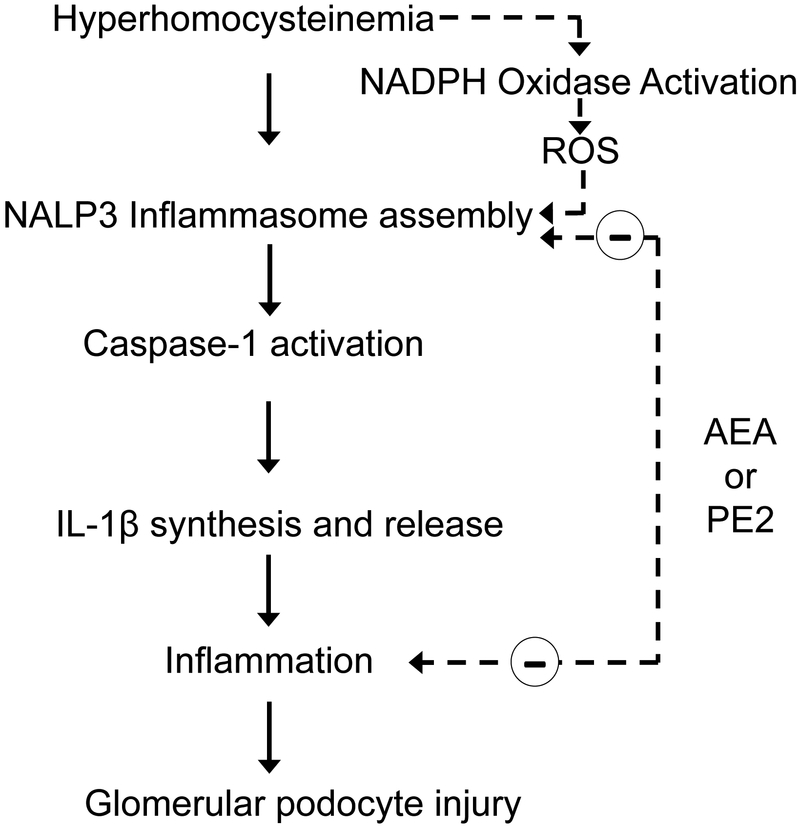
Another chronic kidney disease associated with chronic inflammation is glomerular nephritis associated with elevated homocysteine levels in the blood or hyperhomocysteinemia (hHcys) (Fig. 5). hHCys induces assembly and activation of NLRP3 inflammasomes in podocytes, as indicated by increased co-localization of three major inflammasome proteins: NALP3, ASC and caspase-1, and elevated caspase-1 activity and IL-1β secretion by podocytes (70–73). AEA was found to inhibit hHCys-induced inflammasome formation and activation but that its anti-inflammatory potency is markedly reduced by co-treatment with a COX-2 inhibitor (celecoxib) (74). The prostamide, PGE2-EA, showed a 10-fold higher potency than AEA in the inhibition of Hcys-induced caspase-1 activity and IL-1β production. In addition, PGE2-EA was effective in preventing a morphological change in F-actin fiber staining associated with Hcys-induced inflammation and it also prevented podocyte dysfunction by restoring Hcys-induced suppression of VEGF production and secretion and by inhibiting the Hcys-induced decrease in podocin and increase in desmin. Whether podocytes are capable of generating AEA or the source of AEA in glomeruli are the glomerular mesangial cells is unclear at present. Together, our results demonstrate that PGE2-EA has anti-inflammatory properties and is capable of protecting podocytes from Hcys-induced injury by inhibiting NLRP3 inflammasome activation.
Figure 5.
Proposed pathway of hyperhomocysteinemia-induced podocyte injury leading to glomerulopathy and role of anandamide and prostamide E2. High homocysteine levels either directly or indirectly through increased NADPH oxidase activity and secondary production of ROS increases NALP3 inflammasome assembly and activation. The resulting activation of caspase-1 and conversion of proIL-1β into IL-1β produces a pro-inflammatory state that injures podocytes and results in podocyte dysfunction. Both AEA and PE2 have inhibitory effects on hyperhomocysteinemia-induced NALP3 inflammasome activation and possess anti-inflammatory activity.
7. SUMMARY AND PERSPECTIVE
Over 60% of the world population will develop hypertension or chronic kidney diseases in their lifetime. AEA and its prostamide metabolites have the capacity to modulate key renal functions that impact these diseases. Future research into the pathogenesis of these diseases will need to focus on the sources of endogenous AEA and its metabolites and the mechanisms of their responses to pathophysiologic stimuli including elevated blood pressure and hHCys-induced renal pathologies. A better understanding of the pathophysiologic roles and mechanisms of AEA, prostamides, and other AEA metabolites will benefit efforts to develop better therapies and diagnostic markers for these diseases.
8. ACKNOWLEDGEMENTS
JKR is supported by grant R56DK102539 from the National Institutes of Health
Abbreviations:
- AEA
anandamide
- CB
cannabinoid
- COX
cyclooxygenase
- EET
epoxyeicosatrienoic acid
- FAAH
fatty acid amide hydrolase
- GFR
glomerular filtration rate
- HETE
hydroxyeicosatetraenoic acids
- HETE-EA
hydroxyeicosatetraenoic acid-ethanolamide
- HpETE
hydroperoxyeicosatetraenoic acids
- HpETE-EA
hydroperoxyeicosatetraenoic acid ethanolamide
- hHCys
hyperhomocysteinemia
- NAPE
N-arachidonyl phosphatidyl ethanolamine
- NAPE-PLD
NAPE-selective phospholipase D
- SHR
spontaneously hypertensive rats
- THC
Δ9 tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid 1
9. REFERENCES
- 1.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A and Mechoulam R: Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258(5090), 1946–9 (1992) [DOI] [PubMed] [Google Scholar]
- 2.Ralevic V and Kendall DA: Cannabinoid modulation of perivascular sympathetic and sensory neurotransmission. Curr Vasc Pharmacol, 7(1), 15–25 (2009) [DOI] [PubMed] [Google Scholar]
- 3.Bermudez-Silva FJ, Viveros MP, McPartland JM and Rodriguez de Fonseca F: The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav, 95(4), 375–82 (2010) doi:10.1016/j.pbb.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Turcotte C, Chouinard F, Lefebvre JS and Flamand N: Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol, 97(6), 1049–1070 (2015) doi:10.1189/jlb.3RU0115-021R [DOI] [PubMed] [Google Scholar]
- 5.Long JZ, LaCava M, Jin X and Cravatt BF: An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J Lipid Res, 52(2), 337–44 (2011) doi:10.1194/jlr.M012153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G and Moore LC: Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest, 100(6), 1538–46 (1997) doi:10.1172/jci119677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritter JK, Li C, Xia M, Poklis JL, Lichtman AH, Abdullah RA, Dewey WL and Li PL: Production and actions of the anandamide metabolite prostamide E2 in the renal medulla. J Pharmacol Exp Ther, 342(3), 770–9 (2012) doi:10.1124/jpet.112.196451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johns EJ, Kopp UC and DiBona GF: Neural control of renal function. Compr Physiol, 1(2), 731–67 (2011) doi:10.1002/cphy.c100043 [DOI] [PubMed] [Google Scholar]
- 9.Gaskari SA, Liu H, D’Mello C, Kunos G and Lee SS: Blunted cardiac response to hemorrhage in cirrhotic rats is mediated by local macrophage-released endocannabinoids. J Hepatol, 62(6), 1272–7 (2015) doi:10.1016/j.jhep.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maric C, Harris PJ and Alcorn D: Changes in mean arterial pressure predict degranulation of renomedullary interstitial cells. Clin Exp Pharmacol Physiol, 29(12), 1055–9 (2002) [DOI] [PubMed] [Google Scholar]
- 11.Cadas H, Gaillet S, Beltramo M, Venance L and Piomelli D: Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci, 16(12), 3934–42 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin XH, Okamoto Y, Morishita J, Tsuboi K, Tonai T and Ueda N: Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J Biol Chem, 282(6), 3614–23 (2007) doi:10.1074/jbc.M606369200 [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY and Kunos G: A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A, 103(36), 13345–50 (2006) doi:10.1073/pnas.0601832103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto Y, Tsuboi K and Ueda N: Enzymatic formation of anandamide. Vitam Horm, 81, 1–24 (2009) doi:10.1016/S0083-6729(09)81001-7 [DOI] [PubMed] [Google Scholar]
- 15.Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I and Ueda N: Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J, 380(Pt 3), 749–56 (2004) doi:10.1042/BJ20040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohshima N, Kudo T, Yamashita Y, Mariggiò S, Araki M, Honda A, Nagano T, Isaji C, Kato N, Corda D, Izumi T and Yanaka N: New members of the mammalian glycerophosphodiester phosphodiesterase family: GDE4 and GDE7 produce lysophosphatidic acid by lysophospholipase D activity. J Biol Chem, 290(7), 4260–71 (2015) doi:10.1074/jbc.M114.614537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi K, Okamoto Y, Rahman IA, Uyama T, Inoue T, Tokumura A and Ueda N: Glycerophosphodiesterase GDE4 as a novel lysophospholipase D: a possible involvement in bioactive N-acylethanolamine biosynthesis. Biochim Biophys Acta, 1851(5), 537–48 (2015) doi:10.1016/j.bbalip.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 18.Giang DK and Cravatt BF: Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A, 94(6), 2238–42 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei BQ, Mikkelsen TS, McKinney MK, Lander ES and Cravatt BF: A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem, 281(48), 36569–78 (2006) doi:10.1074/jbc.M606646200 [DOI] [PubMed] [Google Scholar]
- 20.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR and Lichtman AH: Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A, 98(16), 9371–6 (2001) doi:10.1073/pnas.161191698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breyer MD and Harris RC: Cyclooxygenase 2 and the kidney. Curr Opin Nephrol Hypertens, 10(1), 89–98 (2001) [DOI] [PubMed] [Google Scholar]
- 22.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN and Breyer MD: Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest, 94(6), 2504–10 (1994) doi:10.1172/jci117620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Ives D and Ramesha CS: Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem, 272(34), 21181–6 (1997) [DOI] [PubMed] [Google Scholar]
- 24.Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR and Marnett LJ: Amino acid determinants in cyclooxygenase-2 oxygenation of the endocannabinoid anandamide. Biochemistry, 42(30), 9041–9 (2003) doi:10.1021/bi034471k [DOI] [PubMed] [Google Scholar]
- 25.Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Burk R, Cravatt BF and Woodward D: Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res, 45(4), 757–63 (2004) doi:10.1194/jlr.M300475-JLR200 [DOI] [PubMed] [Google Scholar]
- 26.Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I and Inoue K: Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta, 1254(2), 127–34 (1995) [DOI] [PubMed] [Google Scholar]
- 27.González-Núñez D, Solé M, Natarajan R and Poch E: 12-Lipoxygenase metabolism in mouse distal convoluted tubule cells. Kidney Int, 67(1), 178–86 (2005) doi:10.1111/j.1523-1755.2005.00068.x [DOI] [PubMed] [Google Scholar]
- 28.Gohara A, Eltaki N, Sabry D, Murtagh D, Jankun J, Selman SH and Skrzypczak-Jankun E: Human 5-, 12- and 15-lipoxygenase-1 coexist in kidney but show opposite trends and their balance changes in cancer. Oncol Rep, 28(4), 1275–82 (2012) doi:10.3892/or.2012.1924 [DOI] [PubMed] [Google Scholar]
- 29.Snider NT, Kornilov AM, Kent UM and Hollenberg PF: Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther, 321(2), 590–7 (2007) doi:10.1124/jpet.107.119321 [DOI] [PubMed] [Google Scholar]
- 30.Sridar C, Snider NT and Hollenberg PF: Anandamide oxidation by wild-type and polymorphically expressed CYP2B6 and CYP2D6. Drug Metab Dispos, 39(5), 782–8 (2011) doi:10.1124/dmd.110.036707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snider NT, Sikora MJ, Sridar C, Feuerstein TJ, Rae JM and Hollenberg PF: The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther, 327(2), 538–45 (2008) doi:10.1124/jpet.108.141796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willoughby KA, Moore SF, Martin BR and Ellis EF: The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther, 282(1), 243–7 (1997) [PubMed] [Google Scholar]
- 33.Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A and Piomelli D: A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci, 15(1), 64–9 (2012) doi:10.1038/nn.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larrinaga G, Varona A, Pérez I, Sanz B, Ugalde A, Cándenas ML, Pinto FM, Gil J and López JI: Expression of cannabinoid receptors in human kidney. Histol Histopathol, 25(9), 1133–8 (2010) [DOI] [PubMed] [Google Scholar]
- 35.Lim JC, Lim SK, Park MJ, Kim GY, Han HJ and Park SH: Cannabinoid receptor 1 mediates high glucose-induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. Am J Physiol Renal Physiol, 301(1), F179–88 (2011) doi:10.1152/ajprenal.00032.2010 [DOI] [PubMed] [Google Scholar]
- 36.Lim SK and Park SH: The high glucose-induced stimulation of B1R and B2R expression via CB(1)R activation is involved in rat podocyte apoptosis. Life Sci, 91(19–20), 895–906 (2012) doi:10.1016/j.lfs.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 37.Lim JC, Lim SK, Han HJ and Park SH: Cannabinoid receptor 1 mediates palmitic acid-induced apoptosis via endoplasmic reticulum stress in human renal proximal tubular cells. J Cell Physiol, 225(3), 654–63 (2010) doi:10.1002/jcp.22255 [DOI] [PubMed] [Google Scholar]
- 38.Sampaio LS, Taveira da Silva R, Lima D, Sampaio CL, Iannotti FA, Mazzarella E, Di Marzo V, Vieyra A, Reis RA and Einicker-Lamas M: The Endocannabinoid System in Renal Cell: Regulation of Na+ Transport by CB1 Receptors Through Distinct Cell Signaling Pathways. Br J Pharmacol (2014) doi:10.1111/bph.13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva GB, Atchison DK, Juncos LI and García NH: Anandamide inhibits transport-related oxygen consumption in the loop of Henle by activating CB1 receptors. Am J Physiol Renal Physiol, 304(4), F376–81 (2013) doi:10.1152/ajprenal.00239.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D and Högestätt ED: Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature, 400(6743), 452–7 (1999) doi:10.1038/22761 [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y and Wang DH: Segmental regulation of sodium and water excretion by TRPV1 activation in the kidney. J Cardiovasc Pharmacol, 51(5), 437–42 (2008) doi:10.1097/FJC.0b013e318168d120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao F, Sui D, Garavito RM, Worden RM and Wang DH: Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension, 53(2), 228–35 (2009) doi:10.1161/HYPERTENSIONAHA.108.117499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodward DF, Liang Y and Krauss AH: Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol, 153(3), 410–9 (2008) doi:10.1038/sj.bjp.0707434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spada CS, Krauss AH, Woodward DF, Chen J, Protzman CE, Nieves AL, Wheeler LA, Scott DF and Sachs G: Bimatoprost and prostaglandin F(2 alpha) selectively stimulate intracellular calcium signaling in different cat iris sphincter cells. Exp Eye Res, 80(1), 135–45 (2005) doi:10.1016/j.exer.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 45.Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M and Saruta T: Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol, 15(6), 1488–94 (2004) [DOI] [PubMed] [Google Scholar]
- 46.Mattson DL: Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol, 284(1), R13–27 (2003) doi:10.1152/ajpregu.00321.2002 [DOI] [PubMed] [Google Scholar]
- 47.Li J and Wang DH: Differential mechanisms mediating depressor and diuretic effects of anandamide. J Hypertens, 24(11), 2271–6 (2006) doi:10.1097/01.hjh.0000249706.42230.a8 [DOI] [PubMed] [Google Scholar]
- 48.Sofia RD, Knobloch LC, Harakal JJ and Erikson DJ: Comparative diuretic activity of delta9-tetrahydrocannabinol, cannabidiol, cannabinol and hydrochlorothiazide in the rat. Arch Int Pharmacodyn Ther, 225(1), 77–87 (1977) [PubMed] [Google Scholar]
- 49.Paronis CA, Thakur GA, Bajaj S, Nikas SP, Vemuri VK, Makriyannis A and Bergman J: Diuretic effects of cannabinoids. J Pharmacol Exp Ther, 344(1), 8–14 (2013) doi:10.1124/jpet.112.199331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME and Kunos G: Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol, 118(8), 2023–8 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varga K, Lake KD, Huangfu D, Guyenet PG and Kunos G: Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension, 28(4), 682–6 (1996) [DOI] [PubMed] [Google Scholar]
- 52.Lake KD, Martin BR, Kunos G and Varga K: Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension, 29(5), 1204–10 (1997) [DOI] [PubMed] [Google Scholar]
- 53.Varga K, Lake K, Martin BR and Kunos G: Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol, 278(3), 279–83 (1995) [DOI] [PubMed] [Google Scholar]
- 54.Godlewski G, Alapafuja SO, Batkai S, Nikas SP, Cinar R, Offertaler L, Osei-Hyiaman D, Liu J, Mukhopadhyay B, Harvey-White J, Tam J, Pacak K, Blankman JL, Cravatt BF, Makriyannis A and Kunos G: Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol, 17(11), 1256–66 (2010) doi:10.1016/j.chembiol.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lake KD, Compton DR, Varga K, Martin BR and Kunos G: Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther, 281(3), 1030–7 (1997) [PubMed] [Google Scholar]
- 56.Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertaler L, Mackie K, Rudd MA, Bukoski RD and Kunos G: Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation, 110(14), 1996–2002 (2004) doi:10.1161/01.cir.0000143230.23252.d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muirhead EE: The medullipin system of blood pressure control. Am J Hypertens, 4(10 Pt 2), 556s–568s (1991) [DOI] [PubMed] [Google Scholar]
- 58.Muirhead EE: Renal vasodepressor mechanisms: the medullipin system. J Hypertens Suppl, 11(5), S53–8 (1993) [PubMed] [Google Scholar]
- 59.Bergstrom G, Gothberg G, Karlstrom G and Rudenstam J: Renal medullary blood flow and renal medullary antihypertensive mechanisms. Clin Exp Hypertens, 20(1), 1–26 (1998) [DOI] [PubMed] [Google Scholar]
- 60.Brozoski DT, Dean C, Hopp FA and Seagard JL: Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res, 1059(2), 197–202 (2005) doi:10.1016/j.brainres.2005.08.030 [DOI] [PubMed] [Google Scholar]
- 61.Schlosburg JE, Kinsey SG and Lichtman AH: Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J, 11(1), 39–44 (2009) doi:10.1208/s12248-008-9075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH and Lichtman AH: Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A, 101(29), 10821–6 (2004) doi:10.1073/pnas.0401292101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tüting T and Zimmer A: Attenuation of allergic contact dermatitis through the endocannabinoid system. Science, 316(5830), 1494–7 (2007) doi:10.1126/science.1142265 [DOI] [PubMed] [Google Scholar]
- 64.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M and Lutz B: The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest, 113(8), 1202–9 (2004) doi:10.1172/JCI19465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D and Vaughan CW: Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol, 147(3), 281–8 (2006) doi:10.1038/sj.bjp.0706510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, Barbosa I, Dedio J, Maffrand JP, Le Fur G, O’Connor S and Herbert JM: Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int, 72(11), 1345–57 (2007) doi:10.1038/sj.ki.5002540 [DOI] [PubMed] [Google Scholar]
- 67.Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O’Connor SE, Janiak P and Herbert JM: The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab, 7(1), 65–72 (2005) doi:10.1111/j.1463-1326.2004.00374.x [DOI] [PubMed] [Google Scholar]
- 68.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrié P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM and Bensaid M: Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology, 46(1), 122–9 (2007) doi:10.1002/hep.21641 [DOI] [PubMed] [Google Scholar]
- 69.Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, Rastaldi MP, Perin PC and Gruden G: Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes, 59(4), 1046–54 (2010) doi:10.2337/db09-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi F, Xia M, Li N, Zhang C, Tang L and Li PL: Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension, 53(1), 90–6 (2009) doi:10.1161/hypertensionaha.108.115675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi F, Zhang AY, Janscha JL, Li PL and Zou AP: Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int, 66(5), 1977–87 (2004) doi:10.1111/j.1523-1755.2004.00968.x [DOI] [PubMed] [Google Scholar]
- 72.Han H, Wang Y, Li X, Wang PA, Wei X, Liang W, Ding G, Yu X, Bao C, Zhang Y, Wang Z and Yi F: Novel role of NOD2 in mediating Ca2+ signaling: evidence from NOD2-regulated podocyte TRPC6 channels in hyperhomocysteinemia. Hypertension, 62(3), 506–11 (2013) doi:10.1161/HYPERTENSIONAHA.113.01638 [DOI] [PubMed] [Google Scholar]
- 73.Yi F, dos Santos EA, Xia M, Chen QZ, Li PL and Li N: Podocyte injury and glomerulosclerosis in hyperhomocysteinemic rats. Am J Nephrol, 27(3), 262–8 (2007) doi:10.1159/000101471 [DOI] [PubMed] [Google Scholar]
- 74.Xia M, Li G, Abais JM, Boini K, Li P-L and Ritter JK: Protective action of prostamide E2 on hyperhomocysteinemia-induced NLRP3 inflammasome activation and prodocyte injury Submitted (2015) [Google Scholar]