Abstract
In this study a series of diacylhydrazine and acylhydrazone derivatives were designed and synthesized according to the method of active group combination and the principles of aromatic group bioisosterism. The structures of the novel derivatives were determined on the basis on 1H-NMR, IR and ESI-MS spectral data. All of the compounds were evaluated for their in vivo insecticidal activity against the third instar larvae of Spodoptera exigua Hiibner, Helicoverpa armigera Hubner, Plutella xyllostella Linnaeus and Pieris rapae Linne, respectively, at a concentration of 10 mg/L. The results showed that all of the derivatives displayed high insecticidal activity. Most of the compounds presented higher insecticidal activity against S. exigua than the reference compounds tebufenozide, metaflumizone and tolfenpyrad, and approximately identical insecticidal activity against H. armigera, P. xyllostella and P. rapae as the references metaflumizone and tolfenpyrad.
Keywords: diacylhydrazine, acylhydrazone, aromatic diamide, insecticidal activity, synthesis
1. Introduction
Synthetic pesticides have performed major functions in feed, food and fiber production for many years. To a considerable extent, these pesticides may be expected to be used well into the future, although the type of pesticides used may shift toward materials with novel modes of action and lower risk to humans and other non-target organisms [1,2].
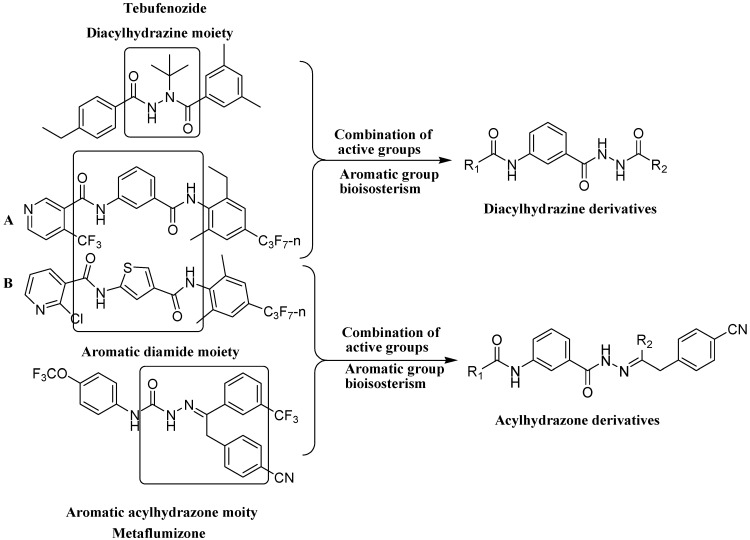
The phthalic diamide flubendiamide and the anthranilic diamides chlorantraniliprole and cyantraniliprole were successively designed and synthesized by Japanese pesticide companies in 1998 and DuPont in 2001; these novel insecticides act on the ryanodine receptor (RyR) [3,4,5,6,7]. Bayer and Syngenta have also developed the meta-amino benzamides A and B (Figure 1), both of which are considered RyR pesticides [8,9,10]. Compounds A and B present excellent insecticidal activities against pests of different orders, such as Lepidoptera, Diptera, Coleoptera, Hemiptera, and Isoptera, and feature high selectivity, low mammalian toxicity, and environmental friendliness. As determined through electrophysiological and Ca2+-release studies these insecticides act by activating insect RyR, a non-voltage-gated calcium channel, to affect calcium release from intracellular stores by locking channels in a partially opened state [3,11,12]. All of the RyR insecticides described above contain two amide structures that are important to their insecticidal activity.
Figure 1.
Design of the skeleton of diacylhydrazine and acylhydrazone derivatives.
Diacylhydrazines have been identified as one of the most important types of insect regulators since the discovery of the N-tert-butyl-N,N'-diacylhydrazines in the mid-1980s by Rohm and Haas [13,14,15]. Several commercial compounds, such as tebufenozide, methoxyfenozide, chromafenozide, and halofenozide, are all classified as diacylhydrazines, and all of these insecticides affect the ecdysone receptor complex, leading to precocious lethal molting, especially in caterpillars [16,17]. Diacylhydrazines have attracted significant attention because of their high insecticidal selectivity, simple structure, and low toxicity to vertebrates [15].
Metaflumizone, a semicarbazone compound with a structure containing an acylhydrazone moiety, is a novel sodium channel blocker insecticide recently introduced to the Chinese market in 2010 by BASF. It provides excellent control of most economically important pests belonging to the orders Lepidoptera, Coleoptera, Hymenoptera, Hemiptera, Isoptera, Diptera, and Siphonaptera. Metaflumizone presents low risk to pollinators and beneficial insects, as well as humans and the environment. Insect strains that are resistant to organophosphates, carbamates, and imidacloprid do not display cross-resistance to metaflumizone. Thus, this insecticide demonstrates great potential use in Integrated Pest Management (IPM) and resistance management strategies [2,18,19]. Moreover many other acylhydrazone compounds also show strong insecticidal activity [20,21].
Plant pests, such as the beet armyworm (Spodoptera exigua Hiibner), diamondback moth (Helicoverpa armigera Hubner), cotton bollworm (Plutella xyllostella Linnaeus), and cabbage caterpillar (Pieris rapae Linne), are harmful to crops all over the world. Unfortunately, pest control of these species has become increasingly difficult because of development of resistance to traditional insecticides. Poor pest control leads to enormous losses of crop production because of long-term use of conventional pesticides [3,22].
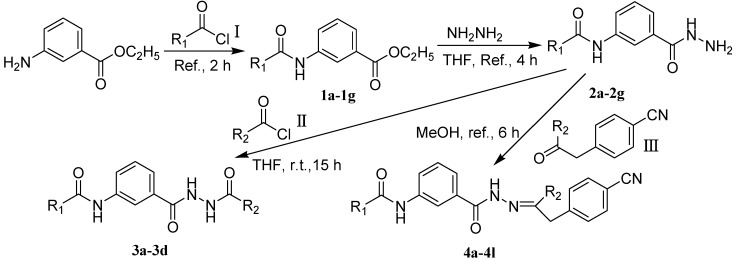
In the present work, we sought to incorporate an aromatic diamide unit into an aromatic diacylhydrazine or acylhydrazone moiety according to the method of active group combination and the principle of aromatic group bioisosterism. A total of four diacylhydrazine derivatives and 12 acylhydrazone derivatives were designed; the structures of these compounds combined a meta-amino benzamide with diacylhydrazine or acylhydrazone active groups (Scheme 1) and they were identified by IR, ESI-MS, and 1H-NMR spectroscopy. The insecticidal activities of the resulting compounds against the third instarlarvae of beet armyworm, diamondback moth, cotton bollworm, and cabbage caterpillar were screened. Combination of critical components is expected to improve the biological activities of these pesticides.
Scheme 1.
Synthesis of diacylhydrazine and acylhydrazone derivatives.
2. Results and Discussion
2.1. Chemistry
Scheme 1 shows the route used successfully for the preparation of the four diacylhydrazine derivatives 3a–3d and 12 acylhydrazone derivatives 4a–4l. The raw materials 3-aminoethyl benzoate and acyl chlorides R1COCl (I) were dissolved in chloroform and heated to reflux to prepare the 3-acylamino ethyl benzoates 1a–1g. Using alcohol as a solvent, 3-acylaminobenzoyl hydrazines 2a–2g were obtained by reaction of compounds 1a–1g with hydrazine hydrate. Then, compounds 2a–2g were reacted with acyl chlorides R2COCl (II) in tetrahydrofuran at room temperature to prepare the diacylhydrazine derivatives 3a–3d. Again using alcohol as a solvent and trifluoroacetic acid as a catalyst, compounds 2a–2g were reacted with 2-(4-nitrilo)benzyl-1-substituedphenyl ketones II to form acylhydrazone derivatives 4a–4l. The structures of all of the diacylhydrazine and acylhydrazone derivatives were effectively determined through 1H-NMR, ESI-MS, and IR spectroscopy.
2.2. Insecticidal Activities
Table 1 shows that all of the diacylhydrazine and acylhydrazone derivatives 3a–3d, 4a–4l display strong insecticidal activity against the third instar larvae of beet armyworm (S. exigua) at a concentration of 10 mg/L. Most of the synthesized compounds indicated higher insecticidal activity than the reference compounds tebufenozide, metaflumizone, and tolfenpyrad. Among the synthesized compounds, the mortality caused by compounds 3b, 4b, 4c, 4d, 4f and 4l exceeded 95% at the 72 h time point. Moreover the third instar larvae of beet armyworm showed 100% mortality within 72 h when treated with compounds 4b, 4d, and 4l. Table 1 further demonstrates that insect mortality presents a positive relationship with administration time.
Table 1.
Insecticidal activities of diacylhydrazine and acylhydrazone derivatives (concentration, 10 mg/L) against the third instar larvae of beet armyworm.
| Compound | R1 | R2 | Mortality (%) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| 3a | 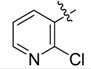 |
 |
12.50 | 29.17 | 70.83 |
| 3b | 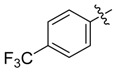 |
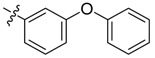 |
45.83 | 66.67 | 95.83 |
| 3c | 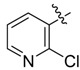 |
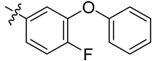 |
16.67 | 37.50 | 79.17 |
| 3d | 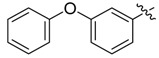 |
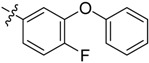 |
41.67 | 66.67 | 91.67 |
| 4a | 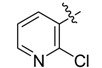 |
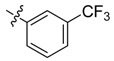 |
45.83 | 66.67 | 87.50 |
| 4b | 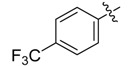 |
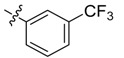 |
50.00 | 75.00 | 100.00 |
| 4c | 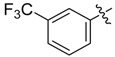 |
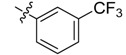 |
50.00 | 79.17 | 95.83 |
| 4d | 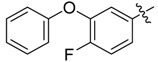 |
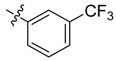 |
54.17 | 79.17 | 100.00 |
| 4e | 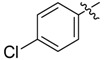 |
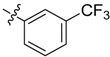 |
45.83 | 66.67 | 91.67 |
| 4f | 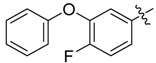 |
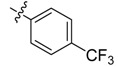 |
54.17 | 70.83 | 95.83 |
| 4g | 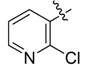 |
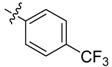 |
41.67 | 70.83 | 91.67 |
| 4h | 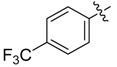 |
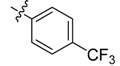 |
37.50 | 75.00 | 91.67 |
| 4i | 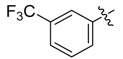 |
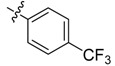 |
45.83 | 70.83 | 91.67 |
| 4j | 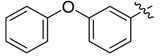 |
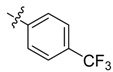 |
37.50 | 66.67 | 87.50 |
| 4k | 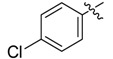 |
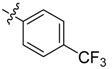 |
41.67 | 70.83 | 87.50 |
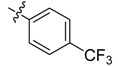
| 4l | 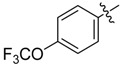 |
 |
58.33 | 83.33 | 100.00 |
| Reference Compounds | |||||
| Tebufenozide | 12.50 | 37.50 | 66.67 | ||
| Metaflumizone | 37.50 | 45.83 | 66.67 | ||
| Tolfenpyrad | 45.83 | 66.67 | 79.17 | ||
Table 2 presents the mortality data of cotton bollworm (H. armigera), diamondback moth (P. xyllostella), and cabbage worm (P. rapae) exposed to acylhydrazone derivatives 4a–4l at a concentration of 10 mg/L for 72 h. Compounds 4a–4l revealed strong insecticidal activity against the third instar larvae of these species. Insect mortalities from exposure to these compounds were approximately identical to those observed from exposure to the reference compounds metaflumizone and tolfenpyrad. Among the synthesized acylhydrazone derivatives, 4b, 4c, 4d, 4f and 4l showed 100% mortalities against the third instar larvae of P. rapae. Compound 4f in particular displayed broad spectrum insecticidal activity.
Table 2.
Insecticidal activities of acylhydrazone derivatives (concentration, 10 mg/L; treatment time, 72 h).
| Compound | Mortality(%) | ||
|---|---|---|---|
| H. armigera | P. xyllostella | P. rapae | |
| 4a | 87.50 | 79.17 | 91.67 |
| 4b | 91.67 | 95.83 | 100.0 |
| 4c | 91.67 | 87.50 | 100.0 |
| 4d | 95.83 | 87.50 | 100.0 |
| 4e | 79.17 | 83.33 | 91.67 |
| 4f | 95.83 | 100.0 | 100.0 |
| 4g | 87.50 | 87.50 | 95.83 |
| 4h | 83.33 | 87.50 | 95.83 |
| 4i | 87.50 | 75.50 | 91.67 |
| 4j | 83.33 | 83.33 | 91.67 |
| 4k | 91.67 | 70.83 | 91.67 |
| 4l | 79.17 | 95.83 | 100.0 |
| Metaflumizone | 87.50 | 83.33 | 87.50 |
| Tolfenpyrad | 91.67 | 91.67 | 95.83 |
According to the data in Table 1 and Table 2, it could be found that the presence of fluorine was important for the insecticidal activity of the synthesized compounds. Comparing the differences between the substituent groups and the position of the substituents on the benzene ring, it could be presumed that relatively weak electron-withdrawing effects could strengthen the insecticidal activities of the acylhydrazone derivatives, however electron-donating effects (such as seen in 4j) and strong electron-withdrawing effects (such as in 4h and 4i) could not do so.
3. Experimental Section
3.1. General Procedures
All reagents were chemically pure and solvents were dried according to standard methods. 1H-NMR spectra were obtained on an AM-500 spectrometer (Bruker, Karlsruhe, Germany) with DMSO-d6 as the solvent. IR spectra were recorded on an IR-200 spectrophotometer (Nicolet, Thermo Electron, Madison, WI, USA) using KBr disks. Mass spectra were recorded under ESI conditions on a Q-TOF spectrometer (Micromass, Waters Corp., Manchester, UK). Melting points were measured on a WRS-1A-type melting point apparatus (Shanghai, China) and are reported uncorrected. Analytical TLC was carried out on pre-coated silica gel plates, and spots were visualized through UV illumination (254 nm).
3.2. General Procedure for the Preparation of 1a–1g
3-Aminoethyl benzoate (30 mmol) was dissolved in chloroform (25 mL) in an ice-water bath. Then, an acyl chloride R1COCl (I, 30 mmol) was dissolved in chloroform (25 mL) and added dropwise to this solution. The mixture was reacted at 25 °C in a water bath for 5 h and then refluxed for 2 h. The reaction mixture was subsequently cooled to room temperature and filtered under vacuum to obtain the 3-acylaminoethyl benzoates 1a–1g with yields of 85%–95%.
3.3. General Procedure for the Preparation of 2a–2g
A mixture of 3-acylaminoethyl benzoates 1a–1g (20 mmol), 80% hydrazine hydrate (100 mmol), and ethanol (100 mL) was stirred and heated under reflux for 4 h. The reaction mixture was cooled to room temperature and filtered under vacuum. The solid obtained was washed with water (50 mL) to provide the 3-acylaminobenzoyl hydrazines 2a–2g in yields of 80%–95%.
3.4. General Procedure for Preparation of 3a–3d and 4a–4l
3-Acylaminobenzoyl hydrazines 2a–2g (5 mmol) and NaOH (5.7 mmol) were dissolved in dry tetrahydrofuran (50 mL). Acyl chlorides R2COCl (II, 5.7 mmol) dissolved in dry tetrahydrofuran (30 mL) were then dropped slowly into the above solution at 0 °C in an ice-water bath. The solution was reacted at room temperature for 15 h. After completion of the reaction, the reaction mixture was concentrated in vacuo and mixed with saturated NaHCO3 solution (25 mL). The mixture was stirred for 30 min and then filtered under vacuum. The filtrate was recrystallized with DMF and water (volume ratio, 2:1) to afford the target compounds 3a–3d.
3-Acylaminobenzoyl hydrazines 2a–2g (2 mmol), 2-(4-nitrilo)benzyl-1-substituted phenyl ketones (III, 2 mmol), and two or three drops of trifluoroacetic acid were dissolved in methanol (15 mL) and n-hexane (2 mL). This solution was stirred and heated under reflux for 6 h, after which the reaction mixture was concentrated in vacuo and filtered under vacuum. The filtrate was recrystallized with methanol to yield the target compounds 4a–4l. All 16 compounds are novel compounds and their physical and spectral data are listed below.
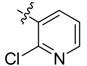
N-(2-Chloropyridyl-3-formyl)-3-(2-chloropyridyl-3-formylamino)benzoyl hydrazine (3a). White needle-like crystals, yield 75.2%, m.p. 253.0–253.2 °C. HR-ESI-MS m/z: 430.0471 [M+H]+ (calcd for C19H14Cl2N5O3, 430.0468). 1H-NMR (DMSO-d6) δ: 10.84, 10.73, 10.61 (each s, 1H, NH), 8.54 (d, J = 2.4Hz, 2H,), 8.26 (s, 1H), 8.10 (dd, J = 7.5, 1.7 Hz, 1H), 7.98 (dd, J = 7.5, 1.7 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.60–7.48 (m, overlapped, 3H). IR (KBr): ν 3246, 2360, 1701, 1656, 1580, 1492, 1400, 1283, 1268, 912, 750, 653 cm−1.
N-(3-Phenoxy)benzoyl-3-(3-phenoxylbenzoylamino)benzoyl hydrazine (3b). White powder, yield 76.6%, m.p. 239.6–240.8 °C. HR-ESI-MS m/z: 520.1474 [M+H]+ (calcd for C28H21F3N3O4, 520.1479). 1H-NMR (DMSO-d6) δ: 10.67, 10.57, 10.52 (each s, 1H, NH), 8.30 (s, 1H), 8.18 (d, J = 8.1 Hz, 2H), 8.01 (d, J = 8.1 Hz, 1H), 7.93 (d, J = 8.2 Hz, 2H), 7.71 (d, J = 7.7 Hz, 1H), 7.66 (d, J = 7.7 Hz, 1H), 7.50–7.54 (m, overlapped, 3H), 7.43 (t, J = 7.8 Hz, 2H), 7.25 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.4 Hz, 1H), 7.08 (d, J = 8.1 Hz, 2H).
N-(4-Fluoro-3-phenoxy)benzoyl-3-(2-chloropyridyl-3-formylamino)benzoyl hydrazine (3c). White powder, yield 78.3%, m.p. 185.6–186.4 °C. 1H-NMR (DMSO-d6) δ: 10.89, 10.60, 10.46 (each s, 1H, NH), 8.55 (dd, J = 4.0, 1.6 Hz, 1H), 8.28 (s, 1H), 7.99 (dd, J = 6.2, 1.6 Hz, 1H), 7.95 (dd, J = 6.7, 1.0 Hz, 1H), 7.92 (m, 1H), 7.82 (dd, J = 6.5, 1.8 Hz, 1H), 7.66 (d, J = 6.6 Hz, 1H), 7.56–7.61 (m, overlapped, 2H), 7.49 (t, J = 6.6 Hz, 1H), 7.42 (dd, J = 7.1, 6.6 Hz, 2H),7.17 (t, J = 6.1 Hz, 1H), 7.06 (d, J = 6.5 Hz, 2H). IR (KBr): ν 3478, 3411, 1704, 1615, 1577, 1392, 1388, 1164, 902, 714 cm−1.
N-(4-Fluoro-3-phenoxy)benzoyl-3-(3-phenoxybenzoylamino)benzoyl hydrazine (3d). White powder, yield 72.6%, m.p. 221.8–222.1 °C. HR-ESI-MS m/z: 562.1777 [M+H]+ (calcd for C33H25FN3O5, 562.1773). 1H-NMR (DMSO-d6) δ: 10.60, 10.51, 10.47 (each s, 1H, NH), 8.55 (s, 1H), 8.26 (s, 1H), 7.99 (d, J = 7.6 Hz, 1H), 7.84–7.76 (m, overlapped, 2H), 7.67 (d, J = 7.9 Hz, 1H), 7.59 (m, 4H), 7.40–7.50 (m, overlapped, 5H), 7.24 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.4 Hz, 2H), 7.08 (d, J = 8.1 Hz, 3H). IR (KBr): ν 3296, 3222, 3072, 1648, 1612, 1580, 1545, 1477, 1274, 900, 685 cm−1.
N-[3-(2-Chloropyridyl-3-formylamino)]benzoyl-1-(3-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4a). White needle-like crystals, yield 63.4%, m.p. 253.0–253.2 °C. HR-ESI-MS m/z: 562.1256 [M+H]+ (calcd for C29H20ClF3N5O2, 562.1252). 1H-NMR (DMSO-d6) δ: 11.39, 10.86 (each s, 1H, NH), 8.55 (dd, J = 4.8,1.7 Hz, 1H), 8.16 (d, J = 6.2 Hz, 1H), 8.10 (dd, J = 7.5, 1.6 Hz, 1H), 8.03 (s, 1H), 7.92 (d, J = 8.2 Hz, 1H), 7.88 (d, J = 6.0 Hz, 1H), 7.72–7.82 (overlapped, 4H), 7.58(dd, J = 7.5, 4.9 Hz, 1H), 7.52 (s, 1H), 7.51 (d, J = 7.1 Hz, 1H), 7.40 (d, J = 8.1 Hz, 2H), 4.58 (s, 2H, -CH2-). IR (KBr): ν 3275, 2230,1671, 1550, 1400, 1309,1103, 1068, 841, 750 cm−1.
N-[3-(4-Trifluoromethyl)benzoylamino]benzoyl-1-(3-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4b). Off-white powder, yield 68.6%, m.p. 217.9–218.5 °C. 1H-NMR (DMSO-d6) δ: 11.35, 10.65 (each s, 1H, NH), 8.16 (d, J = 7.8 Hz, 3H), 7.99 (s,1H), 7.93 (s, 1H), 7.92 (d, J =8.8 Hz, 3H), 7.76 (d, J = 8.0 Hz, 2H), 7.60 (s, 1H), 7.50 (s, 1H), 7.49 (d, J = 7.4 Hz, 1H), 7.39 (d, J = 8.0 Hz, 2H), 4.58 (s, 2H, -CH2-). IR (KBr): ν 3222, 2360, 2227, 1651, 1550, 1321, 1162, 862, 685 cm−1.
N-[3-(3-Trifluoromethyl)benzoylamino]benzoyl-1-(3-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4c). Off-white powder, yield 77.9%, m.p. 184.0–184.5 °C. HR-ESI-MS m/z: 595.1560 [M+H]+ (calcd for C31H21F6N4O2, 595.1563). 1H-NMR (DMSO-d6) δ: 11.36, 10.67 (each s, 1H, NH), 8.33 (s, 1H), 8.29 (d, J = 7.9 Hz, 1H), 8.23 (s, 1H), 8.03 (overlapped, 2H), 7.99 (d, J = 7.9 Hz, 1H), 7.55–7.82 (m, overlapped, 5H), 7.52 (s, 1H), 7.51 (d, J = 6.7 Hz, 1H),7.39 (d, J = 8.2 Hz, 2H), 4.59 (s, 2H, -CH2-). IR (KBr): ν 3225, 2357, 2227, 1680, 1651, 1550, 1336,1108, 853, 697 cm−1.
N-(4-Fluoro-3-phenoxylbenzoylamino)benzoyl-1-(3-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4d). Off-white powder, yield 74.3%, m.p.196.3–196.8 °C. HR-ESI-MS m/z: 637.1858 [M+H]+ (calcd for C36H25F4N4O3, 637.1857). 1H-NMR (DMSO-d6) δ: 11.34, 10.46 (each s, 1H, NH), 8.18 (s, 1H), 8.08 (s, 1H), 7.88–7.98 (m, 3H), 7.65–7.85 (m, 4H), 7.58–7.70 (m, 2H), 7.48 (s, 1H), 7.36–7.47 (m, 3H), 7.18 (m, 1H), 7.06 (d, J = 7.3 Hz, 2H), 4.59 (s, 2H, -CH2-). IR (KBr): ν 2360, 2227, 1671, 1648, 1559, 1333, 1277, 1212, 1135, 876, 750 cm−1.
N-(4-Chlorobenzoylamino)]benzoyl-1-(3-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4e). White powder, yield 70.3%, m.p. 197.0–197.3 °C. HR-ESI-MS m/z: 561.1298 [M+H]+ (calcd for C30H21ClF3N4O2, 561.1300). 1H-NMR (DMSO-d6) δ: 11.35, 10.52 (each s, 1H, NH), 8.23 (s, 1H), 8.17 (s, 1H), 8.01 (d, J = 8.4 Hz, 3H), 7.94 (s, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.63 (d, J = 8.5 Hz, 3H), 7.49 (s, 1H), 7.48 (d, J = 6.7 Hz,1H), 7.40 (d, J = 8.2 Hz, 2H), 4.60 (s, 2H, -CH2-). IR (KBr): ν 3255, 2227, 1659, 1542, 1341, 1109, 1074, 826, 750 cm−1.
N-(4-Fluoro-3-phenoxylbenzoylamino)benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4f). Off-white powder, yield 74.3%, m.p. 187.1–187.4 °C. HR-ESI-MS m/z: 637.1859 [M+H]+ (calcd for C36H25F4N4O3, 637.1858). 1H-NMR (DMSO-d6) δ: 11.33, 10.45 (each s,1H, NH), 8.18 (s, 1H), 7.90–8.10 (m, 4H), 7.70–7.82 (m, 5H), 7.60 (m,1H), 7.35–7.50 (m, 6H), 7.18 (t, J = 7.3 Hz, 1H), 7.06 (d, J = 8.1 Hz, 2H), 4.57 (s, 2H, -CH2-). IR (KBr): ν 3187, 2369, 2225, 1677, 1648, 1556, 1503, 1324, 1271.3, 1212, 1132, 753, 691 cm−1.
N-[3-(2-Chloropyridyl-3-formylamino)]benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4g). White powder, yield 65.7%, m.p. 244.4–245.2 °C. HR-ESI-MS m/z: 562.1254 [M+H]+ (calcd for C29H20ClF3N5O2, 562.1252). 1H-NMR (DMSO-d6) δ: 11.39, 10.85 (each s, 1H, NH), 8.55 (dd, J = 4.4 Hz, 1H), 8.16 (s, 2H), 8.10 (m, 2H), 7.90 (d, J = 5.8 Hz, 1H), 7.77 (d, J = 8.0 Hz, 2H), 7.72 (d, J = 8.1 Hz, 1H), 7.62 (s, 1H), 7.58 (dd, J = 7.4, 4.9 Hz, 1H), 7.46–7.54 (m, 2H), 7.40 (d, J = 8.0 Hz, 2H), 4.59 (s, 2H, -CH2-). IR (KBr): ν 3275, 3249, 2227, 1671, 1527, 1397, 1350, 1168, 1103, 1068, 747, 547 cm−1.
N-(4-Trifluoromethylbenzoylamino)benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4h). Off-white powder, yield 78.3%, m.p. 228.7–229.0 °C. HR-ESI-MS m/z: 595.1568 [M+H]+ (calcd for C31H21F6N4O2, 595.1563). 1H-NMR (DMSO-d6) δ: 11.36, 10.67 (each s, 1H, NH), 8.24 (s, 1H), 8.18 (d, J = 8.0 Hz, 2H), 8.00 (s, 2H), 7.94 (d, J = 8.3 Hz, 2H), 7.70–7.82 (m, 5H), 7.49–7.55 (m, 2H), 7.40 (d, J = 8.3 Hz, 2H), 4.59 (s, 2H, -CH2-). IR (KBr): ν 3228, 2233, 1683, 1656, 1559, 1324, 1130, 1065, 853 cm−1.
N-(3-Trifluoromethylbenzoylamino)benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4i). White powder, yield 72.5%, m.p. 190.5–190.8 °C. HR-ESI-MS m/z: 595.1565 [M+H]+ (calcd for C31H21F6N4O2, 595.1563). 1H-NMR (DMSO-d6) δ: 11.35, 10.66 (each s, 1H, NH), 8.32 (s, 1H), 8.29 (d, J = 7.2 Hz, 2H), 8.23 (s, 1H), 8.18 (s, 1H), 7.96–8.05 (overlapped, 4H), 7.75–7.84 (m, overlapped, 4H), 7.63 (s, 1H), 7.52 (s, 1H), 7.51 (d, J = 7.3 Hz, 1H), 7.41 (d, J = 8.2 Hz, 2H), 4.60 (s, 2H, -CH2-). IR (KBr): ν 3267, 2230, 1659, 1512, 1336, 1142, 1074, 823, 553 cm−1.
N-(3-Phenoxylbenzoylamino)benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4j). Off-white powder, yield 70.3%, m.p. 189.4–189.6 °C. HR-ESI-MS m/z: 619.1949 [M+H]+ (calcd for C36H26F3N4O3, 619.1952). 1H-NMR (DMSO-d6) δ: 11.34, 10.47 (each s, 1H, NH), 8.22 (s, 1H), 7.95–8.10 (m, 3H), 7.71–7.82 (overlapped, 5H), 7.61 (s, 1H), 7.56 (t, J = 7.9 Hz, 1H), 7.48 (s, 1H), 7.47 (d, J = 6.5 Hz, 1H), 7.44 (t, J = 7.9 Hz, 2H), 7.39 (d, J = 8.1 Hz, 2H), 7.25 (dd, J = 8.0, 1.9 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.08 (d, J = 8.0 Hz, 2H), 4.58 (s, 2H,-CH2-). IR (KBr): ν 3187, 2225, 1677, 1648, 1539, 1480, 1327, 1271, 1235, 1124, 868, 750 cm−1.
N-(4-Chlorobenzoylamino)]benzoyl-1-(4-trifluoromethyl)phenyl-2-(4-cyano)phenyl ethanone hydrazone (4k). White powder, yield 70.3%, m.p. 208.5–208.7 °C. HR-ESI-MS m/z: 561.1304 [M+H]+ (calcd for C30H21ClF3N4O2, 561.1300). 1H-NMR (DMSO-d6) δ: 11.36, 10.52 (each s, 1H, NH), 8.23 (s, 1H), 7.95–8.10 (overlapped, 5H), 7.70–7.82 (overlapped, 4H), 7.63 (d, J = 8.5 Hz, 2H), 7.50 (s, 1H), 7.49 (d, J = 6.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 2H), 4.58 (s, 2H, -CH2-). IR (KBr): ν3225, 2233, 1680, 1645, 1559, 1486, 1327, 1127, 1065, 850, 753, 600 cm−1.
N-(4-Trifluoromethoxy benzoylamino)]benzoyl-1-(4-trifluoromethyl) phenyl-2-(4-cyano) phenyl ethanone hydrazone (4l). White powder, yield 70.3%, m.p. 249.5–249.8 °C. HR-ESI-MS m/z: 611.1518 [M+H]+ (calcd for C31H21F6N4O3, 611.1512). 1H-NMR (DMSO-d6) δ: 11.45, 10.58 (each s, 1H, NH), 8.35 (s, 1H), 8.19 (s, 1H), 8.16 (d, J = 7.9 Hz, 2H), 8.08 (s, 1H), 7.98 (d, J = 7.2 Hz, 1H), 7.91 (d, J = 9.0 Hz, 2H), 7.77 (d, J = 7.8 Hz, 2H), 7.68 (t, J = 7.7 Hz, 1H), 7.64 (s, 1H), 7.35–7.45 (m, overlapped, 4H), 4.61 (s, 2H, -CH2-). IR (KBr): ν 3222, 2230, 1683, 1645, 1536, 1509, 1256, 1171, 853, 697 cm−1.
3.5. Insecticidal Activity Bioassays
Wheat leaf discs measuring 0.5 cm × 0.5 cm were treated with 5 μL of 1.0 mg of test samples dissolved in 100 mL of acetone. Acetone was used as a negative control, whereas tebufenozide, metaflumizone, and tolfenpyrad were used as positive controls. The third instar larvae of S. exigua were allowed to feed on the discs. Cohorts of 24 beet armyworms were treated each time and bioassays were replicated three times. After 24, 48, and 72 h, the numbers of knocked-down larvae (indications: the larvae were narcotized, their bodies were very soft and immobile, and responses to stimuli disappeared completely) were recorded [23,24]. Insecticidal activity results are listed in Table 1.
Bioassays for insecticidal activity against cotton bollworm, diamondback moth, and cabbage worm was performed according to the method described above, except that the third instar larvae of S. exigua were replaced by the third instar larvae of H. armigera, P. xyllostella, and P. rapae. Metaflumizone and tolfenpyrad were used as positive controls, and the numbers of knocked-down larvae were recorded 72 h after exposure. Bioassay results are listed in Table 2.
4. Conclusions
In conclusion, four novel diacylhydrazine 3a–3d and 12 acylhydrazone derivatives 4a–4l were designed and synthesized according to the method of active group combination and the principle of aromatic group bioisosterism. The resultant analogs were evaluated (concentration, 10 mg/L) for their insecticidal activity against the third instar larvae of S. exigua, H. armigera, P. xyllostella, and P. rapae in vivo. Bioassays of these analogs showed high insecticidal activity. Most of the synthesized compounds presented higher insecticidal activity against S. exigua than the reference compounds tebufenozide, metaflumizone, and tolfenpyrad. Insecticidal activities against H. armigera, P. xyllostella, and P. rapae similar to that of the reference compounds metaflumizone and tolfenpyrad were also obtained. The results above motivate us to further explore novel diacylhydrazine and acylhydrazone derivatives as insecticidal agents; new findings will be reported in future work.
Acknowledgments
This work was supported by the Wheat Innovation Team of the Modern Agricultural Industry Technology System of Shandong Province and High Level Talents Fund of Qingdao Agricultural University (No. 6631110).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/04/5625/s1.
Author Contributions
Jialong Sun designed the research; Jialong Sun and Yuanming Zhou performed the research and analyzed the data; Yuanming Zhou wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 3a–3d and 4a–4l are available from the authors.
References
- 1.Seiber J.N., Kleinschmidt L.A. Contribution of pesticide residue chemistry to improving food and environmental safety: past and present accomplishments and future challenges. J. Agric. Food Chem. 2011;59:7536–7543. doi: 10.1021/jf103902t. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee N.S., Gupta S., Varghese E. Degradation of metaflumizone in soil: Impact of varying moisture, light, temperature, atmospheric CO2 level, soil type and soil sterilization. Chemosphere. 2013;90:729–736. doi: 10.1016/j.chemosphere.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Luo M., Chen Q.C., Wang J., Hu C.Y., Lu J., Luo X.M., Sun D.Q. Novel chlorantraniliprole derivatives as potential insecticides and probe to chlorantraniliprole binding site on ryanodine receptor. Bioorg. Med. Chem. Lett. 2014;24:1987–1992. doi: 10.1016/j.bmcl.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 4.Tohnishi M., Nakao H., Kohno E., Nishida T., Furuya T., Shimizu T., Seo A., Sakata K., Fujioka S. Phthalic Acid Diamide Derivatives, Fluorine-Containing Aniline Compounds as Starting Material, Agricultural and Horticultural Insecticides, and A Method for Application of the Insecticides. 6,559,341. U.S. Patent. 2003 Mar 20;
- 5.Masali T., Yasokawa N., Tohnishi M., Nishimatsu T., Tsubata K., Inoue K., Motoba K., Hirooka T. Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol. Pharmacol. 2006;69:1733–1739. doi: 10.1124/mol.105.020339. [DOI] [PubMed] [Google Scholar]
- 6.Lahm G.P., Cordova D., Barry J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009;17:4127–4133. doi: 10.1016/j.bmc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Hughes K.A., Lahm G.P., Selby T.P., Stevenson T.M. Cyano Anthranilamide Insecticides. WO 2004/067528. 2004 Aug 12;
- 8.Akihiko Y., Yukiyishi W., Katsuaki W., Tetsuya M., Katsuhiko S., Eiichi S., Akira E. Insecticidal 3-Acylaminobenzanilides. WO 2007/017075. 2007 Feb 15;
- 9.Pierre J., Patricia D., William L., Peter M., Thomas P., Peter R., Werner Z. Insecticidal Compounds. WO 2007/128410. 2007 Nov 15;
- 10.Zhang Y.B. The insecticedes of ryanodine receptor inhibitors and varieties structural character and mechanism. Agrochemicals. 2012;51:157–162. [Google Scholar]
- 11.Li Y.X., Mao M.Z., Li Y.M., Xiong L.X., Li Z.M., Xu J.Y. Modulations of high-voltage activated Ca2+channels in the central neurones of Spodoptera exigua by chlorantraniliprole. Physiol. Entomol. 2011;36:230–234. doi: 10.1111/j.1365-3032.2011.00788.x. [DOI] [Google Scholar]
- 12.Cordova D., Benner E.A., Sacher M.D., Rauh J.J., Sopa J.S., Lahm G.P., Selby T.P., Stevenson T.M., Flexner L., Gutteridge S., et al. Anthranilicdiamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006;84:196–214. [Google Scholar]
- 13.Wing K.D. RH-5849, a nonsteroidal ecdysone agonist: effects on a drosophila cell line. Science. 1988;241:467–469. doi: 10.1126/science.3393913. [DOI] [PubMed] [Google Scholar]
- 14.Sun R.F., Zhang Y.L., Chen L., Li Y.Q., Li Q.S., Song H.B., Huang R.Q., Bi F.C., Wang Q.M. Design, synthesis, and insecticidal activities of new N-benzoyl-N'-phenyl-N'-sulfenylureas. J. Agric. Food Chem. 2009;57:3661–3668. doi: 10.1021/jf900324a. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Yang Z.K., Fan Z.J., Wu Q.J., Zhang Y.J., Mi N., Wang S.X., Zhang Z.C., Song H.B., Liu F. Synthesis and insecticidal activity of N-tert-Butyl-N,N'-diacylhydrazines containing 1,2,3-Thiadiazoles. J. Agric. Food Chem. 2011;59:628–634. doi: 10.1021/jf104004q. [DOI] [PubMed] [Google Scholar]
- 16.Yanagi M., Tsukamoto Y., Watanabe T., Kawagishi A. Development of a novel lepidopteran insect control agent chromafenozide. J. Pestic. Sci. 2006;31:163–164. doi: 10.1584/jpestics.31.163. [DOI] [Google Scholar]
- 17.Aguirre O.U., Martínez A.M., Campos-García J., Hernández L., Figueroa J.I., Lobit P., Viñuela E., Chavarrieta J.M., Smagghe G., Pineda1 S. Foliar persistence and residual activity of methoxyfenozide against beet armyworm (Lepidoptera: Noctuidae) Insect Sci. 2013;20:734–742. doi: 10.1111/j.1744-7917.2012.01576.x. [DOI] [PubMed] [Google Scholar]
- 18.Salgado V.L., Hayashi J.H. Metaflumizone is a novel sodium channel blocker insecticide. Vet. Parasitol. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Hempel K., Hess F.G., Bogi C., Fabian E., Hellwig J., Fegert I. Toxicological properties of metaflumizone. Vet. Parasitol. 2007;150:190–195. doi: 10.1016/j.vetpar.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Che Z.P., Zhang S.Y., Shao Y.H., Fan L.L., Xu H., Yu X., Zhi X.Y., Yao X.J., Zhang R. Synthesis and quantitative structure-activity relationship (QSAR) study of novel N-arylsulfonyl-3-acylindole arylcarbonyl hydrazone derivatives as nematicidal agents. J. Agric. Food Chem. 2013;61:5696–5705. doi: 10.1021/jf400536q. [DOI] [PubMed] [Google Scholar]
- 21.Qu H., Yu Y., Zhi X.Y., Lv M., Xu H. Natural-product-based insecticidal agents 14.Semisynthesis and insecticidal activity of new piperine-based hydrazone derivatives against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett. 2013;23:5552–5557. doi: 10.1016/j.bmcl.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Cao G., Han Z. Tebufenozide resistance selected in Plutella xylostella and its cross-resistance and fitness cost. Pest. Manag. Sci. 2006;62:746–751. doi: 10.1002/ps.1234. [DOI] [PubMed] [Google Scholar]
- 23.Mao C.H., Wang Q.M., Huang R.Q., Bi F.C., Chen L., Liu Y.X., Shang J. Synthesis and insecticidal evaluation of novel N-oxalyl derivatives of tebufenozide. J. Agric. Food Chem. 2004;52:6737–6741. doi: 10.1021/jf048834e. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J.W., Hu Z., Li S.K., Wu W.J. Synthesis and insecticidal activities of new ester-derivatives of Celangulin-V. Int. J. Mol. Sci. 2011;12:9596–9604. doi: 10.3390/ijms12129596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.