Abstract
Glutamate receptors are the most abundant excitatory neurotransmitter receptors in the brain, responsible for mediating the vast majority of excitatory transmission in neuronal networks. The AMPA- and NMDA-type ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels that mediate the fast synaptic responses, while metabotropic glutamate receptors (mGluRs) are coupled to downstream signaling cascades that act on much slower timescales. These functionally distinct receptor sub-types are co-expressed at individual synapses, allowing for the precise temporal modulation of postsynaptic excitability and plasticity. Intriguingly, these receptors are differentially distributed with respect to the presynaptic release site. While iGluRs are enriched in the core of the synapse directly opposing the release site, mGluRs reside preferentially at the border of the synapse. As such, to understand the differential contribution of these receptors to synaptic transmission, it is important to not only consider their signaling properties, but also the mechanisms that control the spatial segregation of these receptor types within synapses. In this review, we will focus on the mechanisms that control the organization of glutamate receptors at the postsynaptic membrane with respect to the release site, and discuss how this organization could regulate synapse physiology.
Keywords: AMPA receptors, Metabotropic glutamate receptors, Synaptic transmission, Synaptic plasticity, Super-resolution imaging, Electron microscopy
1. Introduction
Synapses are the fundamental elements of neuronal networks that enable the processing, encoding, and retrieval of information in the brain, and pathological disruptions in synapse structure are broadly held to underlie the development of neurological disorders such as autism and schizophrenia (Volk et al., 2015). To maintain and adjust the efficiency of synaptic signaling, synapses are built from a broad array of components that assemble into large macromolecular machineries. At the presynaptic terminal, action potentials trigger the fast release of synaptic vesicles. Synaptic vesicles are docked at the active zone and primed for exocytosis by protein complexes containing e.g. Rab3-interacting molecules (RIM) and soluble N-ethylmaleimide-sensitive factor activating protein receptors (SNARE) (Sudhof, 2012). The release of glutamate is closely aligned with the postsynaptic receptors that are stably anchored in the opposing postsynaptic density (PSD), a complex molecular machine containing a plethora of scaffolding proteins and signaling molecules (Okabe, 2007; Sheng and Hoogenraad, 2007). How are these molecular complexes organized and precisely positioned to sustain synaptic transmission? In this review we will focus particularly on the functional distribution of glutamate receptors at the postsynaptic membrane.
2. Functional organization of postsynaptic glutamate receptors
2.1. Impact of glutamate receptor distribution on probability of receptor activation
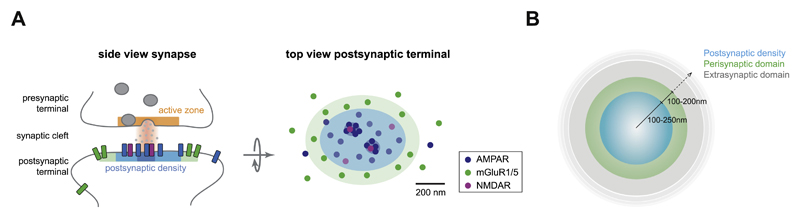
At excitatory synapses, the postsynaptic effects of glutamate are mediated by different types of glutamate receptors; the ionotropic glutamate receptors (iGluRs) comprising AMPA- and NMDA- and kainate-type receptors, and the metabotropic glutamate receptors (mGluRs). The principal iGluRs, the AMPA and NMDA-type receptors act on millisecond timescales to mediate the majority of fast, basal synaptic transmission. In contrast, the postsynaptic group I mGluRs, i.e. mGluR1 and mGluR5, respond much slower and have much longer-lasting physiological effects. Intriguingly, these functionally distinct receptor types are spatially segregated with respect to the presynaptic release site. While AMPA and NMDA receptors are highly enriched in the core of the PSD opposing the presynaptic release site, mGluRs are preferentially enriched in the perisynaptic domain, much further away from the vesicle release site, and seem to be largely excluded from the PSD (Baude et al., 1993; Lujan et al., 1996; Nusser et al., 1994) (Fig. 1A). We define the perisynaptic domain as an annular ring of 100–200 nm surrounding the PSD, whereas the extrasynaptic domain is everything beyond the perisynaptic domain, and thus starts 100–200 nm away from the edge of the PSD (Fig. 1B).
Fig. 1. Subsynaptic segregation of glutamate receptor types at the postsynaptic membrane.
(A) Side view of an excitatory synapse with an active zone (orange) at the presynaptic terminal and postsynaptic density (PSD) (blue), and perisynaptic domain (green) at the postsynaptic terminal (left). A single release event of glutamate is predicted to create a subsynaptic hotspot of maximally activated postsynaptic glutamate receptors (dark blue shaded area) aligned with the presynaptic vesicle release site. Top view of the lateral patterning of the postsynaptic membrane with a central PSD (blue) containing AMPA- (dark blue) and NMDA-type (pink) receptors, and a surrounding perisynaptic domain (green) enriched in mGluR1/5 (dark green) (right). Additionally, PSD components, most notably AMPARs, are organized in ~1–3 distinct nanodomains per synapse (dark blue shaded area). (B) Top view of an excitatory postsynapse to make a clear distinction between the PSD, on average 500–1000 nm in diameter, the perisynaptic domain, an annulus of 100–200 nm surrounding the PSD, and the extrasynaptic domain, everything beyond the perisynaptic domain.
The spatial segregation of receptor types has important functional implications as the distinct localization with respect to the presynaptic release site is predicted to greatly impact the activation kinetics of these receptors types. As has been extensively investigated by numerous computational models that incorporate realistic features of glutamate release and synapse geometry single release events produce a very steep peak in synaptic cleft glutamate concentration, restricted to a small area (< 100 nm) for only a brief period of time (~100 μs) (Boucher et al., 2010; Franks et al., 2003; Raghavachari and Lisman, 2004; Uteshev and Pennefather, 1996; Xie et al., 1997; Xu-Friedman and Regehr, 2004). Importantly, the affinity of AMPARs for glutamate is relatively low and the number of glutamate molecules bound to AMPAR subunits determines the open probability of the receptor (Rosenmund et al., 1998). Initially it was thought that receptor activation requires binding of at least two glutamate molecules, however recently it was proposed that in the presence of auxiliary subunits binding of a single glutamate molecule might be sufficient for receptor activation (Coombs et al., 2017; Greger et al., 2017). However, binding of a single glutamate molecule was also shown to be sufficient to desensitize AMPARs (Robert and Howe, 2003), and although the rate of AMPAR desensitization upon binding of a single glutamate molecule is similar when bound to two to four glutamate molecules, the rate of glutamate dissociation is predicted to be slower with at least two glutamate molecules bound (Robert and Howe, 2003). As a result of these biophysical properties, computational models predict that the probability of AMPAR opening is highest near vesicle release sites, producing local hotspots (< 0.03 μm2) of maximally activated AMPARs that cover only a fraction of the total PSD area (~25% of an average PSD in a CA1 synapse) (Franks et al., 2003; Raghavachari and Lisman, 2004). Importantly, this suggests that not the absolute number, but the density of AMPARs with respect to the presynaptic release site determines the size of the synaptic response. Similarly, although to a lesser extent, the activation probability of NMDARs is also location-dependent. Even though NMDARs have a higher affinity for glutamate and desensitize slower than AMPARs (Erreger et al., 2005), the slow binding rate puts a considerable limit on the opening probability of NMDARs during the short-lived glutamate peak. This is particularly significant for GluN2B-containing NMDARs that are three times more likely to become activated when directly opposing the release site than when displaced > 200 nm. In contrast, the activation probability of GluN2A-containing receptors falls below 50% only when displaced > 300 nm from the release site (Santucci and Raghavachari, 2008). Indeed, receptor non-saturation has been demonstrated experimentally at different types of synapses, where increasing presynaptic release or focal application of exogenous glutamate resulted in larger amplitude responses (Liu et al., 1999; McAllister and Stevens, 2000; Pankratov and Krishtal, 2003).
Even further displaced from the release site are the mGluRs, located at the perisynaptic domain surrounding the PSD, strongly constraining the activation probability of these receptors. The binding affinity of group I mGluRs for glutamate is comparable to AMPARs as measured in heterologous systems (Conn and Pin, 1997; Traynelis et al., 2010), and although one glutamate molecule is sufficient to activate mGluR5 dimers, occupation of both subunits is required for optimal activation (Kniazeff et al., 2004; Niswender and Conn, 2010). Thus, these bio-physical properties predict that the low concentration of glutamate at the periphery of the synapse during single release events limits mGluR activation. Moreover, glutamate transporters co-localizing with mGluRs at the perisynaptic domain (Dehnes et al., 1998; He et al., 2000) compete for the residual glutamate that diffuses out of the synaptic cleft, which further enhances the rapid uptake of glutamate, and thereby virtually eliminating the probability of mGluRs to sense glutamate during single release events (Brasnjo and Otis, 2001). Functionally this would imply that mGluRs only respond when cleft glutamate concentration builds up such that it “spills over” to the perisynaptic domain, for instance during sustained high-frequency synaptic stimulation. Consistently, the activation kinetics of group I mGluRs are very fast (< 10 ms), and the deactivation time is slow (~50 ms) (Marcaggi et al., 2009; Rondard and Pin, 2015). Thus, also the intrinsic kinetic profile of mGluRs predicts that these receptors function as integrators of activity and are sensitive to high-frequency (> 20 Hz) pulses of release (Greget et al., 2011; Marcaggi et al., 2009). Indeed, at cerebellar synapses, trains of stimuli with a minimal frequency of 20 Hz are required to elicit mGluR1-mediated excitatory postsynaptic currents (EPSCs) (Tempia et al., 1998).
Taken together, the nanoscale segregation of glutamate receptor subtypes differentially determines their activation probabilities, providing synapses with a powerful means to encode synaptic activity patterns. In the following, we will present an overview of the literature on the molecular organization of excitatory synapses, focusing in particular on the subsynaptic distribution of glutamate receptors at the postsynaptic membrane, and explore the potential physiological consequences of this organization and mechanisms that could control the entry and distribution of receptors in the synapse.
2.2. Subsynaptic segregation of glutamate receptor types
The activation of the distinct receptor subtypes and their contribution to synaptic transmission is controlled by their lateral distribution across the postsynaptic membrane. To better understand the functional organization of glutamate receptors at excitatory synapses, we will first discuss the distinct distribution patterns of the different postsynaptic glutamate receptors in relation to the presynaptic release site. Although glutamatergic synapses can vary tremendously in their structural, molecular and functional properties, we mainly focus on mature hippocampal excitatory synapses which have been most extensively studied in the context of the functional organization of glutamate receptors.
AMPARs are concentrated at the PSD opposing the presynaptic vesicle release site to ensure fast and efficient synaptic transmission (Fig. 1A). Measuring the subsynaptic distribution of receptors has been challenging owing to the limited resolution of conventional light microscopy. Electron microscopy (EM) immunogold labeling techniques provide the highest achievable resolution and have been instrumental in precisely determining receptor distribution at synapses. At neocortical synapses, AMPAR localization was found preferentially at the edge of the PSD (Bernard et al., 1997; Kharazia and Weinberg, 1997), but generally AMPARs can be found anywhere in the PSD (Chen et al., 2008; Dani et al., 2010; Masugi-Tokita et al., 2007; Somogyi et al., 1998; Tang et al., 2016; Tarusawa et al., 2009), varying greatly between synapses and synapse types (MacGillavry et al., 2011). Also, variations in distribution between different AMPAR subtypes have been suggested. AMPARs form hetero-tetrameric complexes composed of different combinations of four subunits, i.e. GluA1–4. In the adult hippocampus, the most prevalent combinations are GluA1/2 and GluA2/3 heteromers, as well as GluA1 homomers (Lu et al., 2009; Wenthold et al., 1996). At hippocampal synapses, GluA1 tends to localize more towards the edge of the PSD, whereas GluA3 localizes significantly more central (Jacob and Weinberg, 2015). Peripheral localization of GluA1 was most prominent at small synapses, whereas at larger synapses GluA1 was localized more central, similar to GluA3 (Jacob and Weinberg, 2015). Super-resolution studies on individual synapses have corroborated the notion of receptor hotspots, demonstrating that AMPARs form distinct subsynaptic regions of high molecular density, of around 70 to 80 nm in diameter, hereinafter referred to as nanodomains (MacGillavry et al., 2013; Nair et al., 2013; Tarusawa et al., 2009). Most synapses were shown to contain one to three distinct nanodomains, each consisting of around 20 receptors (MacGillavry et al., 2013; Nair et al., 2013; Tang et al., 2016). Importantly, this heterogeneous organization was found to extend to presynaptic sites where key proteins involved in vesicle docking and priming, such as RIM1/2, also form distinct nanodomains within the presynaptic active zone that marked sites of preferred vesicle release. Additionally, these presynaptic nanodomains are spatially aligned with postsynaptic AMPAR nanodomains, forming a trans-synaptic molecular ‘nano-column’ (Biederer et al., 2017; Tang et al., 2016). This striking level of subsynaptic molecular organization provides a simple, but powerful mechanism to efficiently modulate the efficiency of synaptic transmission.
Like AMPARs, NMDARs are preferentially enriched at the PSD, but tend to localize more towards the center of the PSD (Chen et al., 2008; Kharazia and Weinberg, 1997; Perez-Otano et al., 2006; Racca et al., 2000), potentially accompanied with reduced AMPAR densities (Chen et al., 2008). Functionally this is intriguing and might entail that the generally more central NMDARs allow the AMPARs to turn over quickly at the periphery of the PSD, accounting for dynamic modulation of synaptic transmission as suggested by mathematical models (Freche et al., 2011). However, this pattern is not universal among all synapses and synapse types. Clear clustered subsynaptic distributions of NMDARs, comparable to AMPAR nanodomains (Jezequel et al., 2017; MacGillavry et al., 2013), have been found at the center of the PSD (Chen et al., 2008), or either in central or peripheral regions of the PSD (Dani et al., 2010; Perez-Otano et al., 2006). Whether GluN2A- and GluN2B-containing receptors distribute differentially remains to be studied. In conclusion, both AMPARs and NMDARs are organized in higher density nanodomains within the PSD that may serve to optimize the open probability of these receptors by alignment with the presynaptic release site.
In stark contrast to AMPA and NMDA receptors, postsynaptic group I mGluRs (mGluR1/5) seem to be largely excluded from the PSD, but accumulate in the perisynaptic domain surrounding the PSD (Baude et al., 1993; Lujan et al., 1996; Nusser et al., 1994) (Fig. 1A). Quantifications of immunogold labeling clearly demonstrate that the levels of mGluR1/5 significantly peak at the edge of the PSD (within 60 nm), but decrease further away from the PSD, reaching a uniform labeling density at the extrasynaptic dendritic membrane (Lujan et al., 1996). A more recent super-resolution study confirmed the perisynaptic accumulation of mGluR5 co-localizing with Norbin (Westin et al., 2014), a neuron-specific protein that interacts with and regulates the signaling properties of mGluR5 (Wang et al., 2009). Also, single-molecule tracking studies corroborate that a large fraction of the total mGluR5 pool is extrasynaptic and highly mobile (Aloisi et al., 2017; Renner et al., 2010; Sergé et al., 2002), but a small fraction can become reversibly immobilized at synaptic Homer clusters (~8%) (Aloisi et al., 2017; Renner et al., 2010; Sergé et al., 2002).
It is unknown whether mGluRs are distributed homogeneously throughout the perisynaptic domain, or are mGluRs perhaps enriched in local, perisynaptic nanodomains? Although speculative, such a non-random organization of mGluRs in nanodomains would aid in facilitating downstream signaling processes. It is becoming increasingly clear that clustering of receptors and their effectors in signaling platforms, or signalosomes, contributes to the efficacy and fidelity of signal transduction (Kasai and Kusumi, 2014). Many G-protein coupled receptors (GPCRs), including group I mGluRs (Francesconi et al., 2009; Kumari et al., 2013), have been found to localize in insoluble membrane domains, or lipid rafts, using biochemical approaches (Insel et al., 2005; Pontier et al., 2008). Such a local accumulation of receptor complexes could provide a platform for highly efficient and localized signal transduction, even when receptors and effectors are present at low numbers (Kusumi et al., 2012). A recent single-molecule tracking study confirmed the presence of GPCR signalosomes at the plasma membrane of non-neuronal cells. Using the α2A-adrenergic receptor as a prototypical GPCR, this study found that receptors were confined at hotspots where they preferentially interacted with their cognate G-protein complexes (Sungkaworn et al., 2017). Thus, perhaps mGluRs are also organized in functional nanodomains together with its G-proteins and likely other signaling molecules. Indeed, immuno-labeling EM studies found that components of the mGluR signaling complex, including Gq-protein alpha subunits and phospholipase C beta (PLCb), are also localized in the perisynaptic domain (Nakamura et al., 2004; Tanaka et al., 2000). The perisynaptic domain also contains components of the endocytic apparatus forming a stable endocytic zone (EZ) that is tightly linked to the PSD via specific protein-protein interactions (Blanpied et al., 2002, 2003; Lu et al., 2007). The EZ functions to locally internalize synaptic receptors, and acts as a mechanism for local retention of receptors for fast exchange between the synaptic and extrasynaptic receptor pool (Lu et al., 2007; Petrini et al., 2009). The close co-localization of the EZ and the perisynaptic mGluRs might facilitate the fast desensitization and local turn-over of receptors after activation to rapidly and dynamically respond to high-frequency inputs.
3. Downstream effects of glutamate receptor positioning
3.1. Regulation of synaptic transmission by subsynaptic AMPA receptor organization
The enrichment of AMPARs in nanodomains aligned with the presynaptic release site suggests that this subsynaptic pool of receptors contributes the most to synaptic responses, while receptors outside of nanodomains contribute only little. Computationally, this can be addressed systematically (MacGillavry et al., 2013; Nair et al., 2013; Tang et al., 2016), but experimentally it is highly challenging to specifically measure the contribution of receptors enriched in nanodomains. Nevertheless, as we will discuss here, the subsynaptic organization of AMPARs in nanodomains is predicted to influence both basal and plasticity-regulated synaptic transmission.
Important in this respect is the recent demonstration that spontaneous release events are distributed over a much larger area of the active zone than evoked synaptic responses (Tang et al., 2016), consistent with earlier suggestions that spontaneous and evoked release are mechanistically distinct events (Kavalali, 2015). Additionally, in contrast to spontaneous events, evoked vesicle fusion events were found to preferentially take place at subsynaptic hotspots marked by RIM1/2, that were transsynaptically aligned with postsynaptic nanodomains (Tang et al., 2016). Thus, evoked release is more likely to activate the receptors that are enriched in nanodomains, while spontaneous quantal events are likely to probe a larger fraction of the postsynaptic pool of receptors, including areas that are less dense in receptors. One prediction from this organization is that the variance in the peak amplitude of evoked EPSCs is much smaller than of mEPSCs (MacGillavry et al., 2013), which has indeed been confirmed experimentally (Freche et al., 2011). Alignment of presynaptic release with postsynaptic nanodomains thus gives rise to more reliable synaptic transmission (Fig. 2).
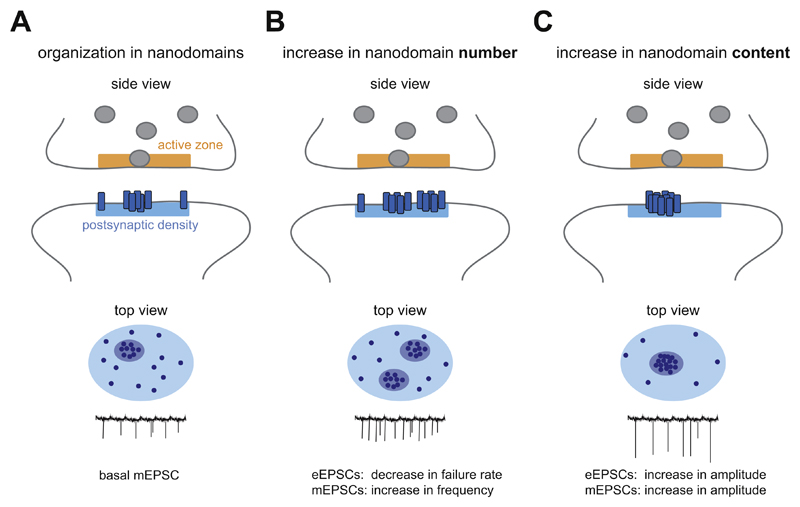
Fig. 2. Physiological implications of AMPAR organization.
(A) Overview of AMPAR organization in distinct nanodomains, aligned with the presynaptic release site in side view (top), top view (middle) and the hypothetical mEPSC trace (bottom), that is maintained throughout this figure. (B) An increase in the number of nanodomains per synapse is likely to increase the probability that a spontaneous event activates receptors, and could be measured as a decrease in the failure rate of evoked responses (eEPSCs), or an increase in mEPSC frequency. (C) An increase in the number of receptors in a nanodomain aligned with the presynaptic site of vesicle release is predicted to enhance synaptic transmission, which could be measured as an increase in the amplitude of eEPSCs or mEPSCs.
How could changes in postsynaptic nanodomain organization affect synaptic responses? Since evoked release events are spatially aligned with postsynaptic nanodomains, evoked EPSCs are likely more directly influenced by changes in receptor organization than spontaneous events. As such, an increase in the number of postsynaptic receptor nanodomains is predicted to decrease the failure rate of evoked responses (Fig. 2B), while an increase in nanodomain content (i.e. more receptors per nanodomain) would increase the amplitude of evoked responses (Fig. 2C).
Given that spontaneous release events seem to take place at random positions, not preferentially aligned with postsynaptic nanodomains, changes in nanodomain organization are probably less directly associated with changes in mEPSCs. Nevertheless, computational simulations suggest that the amplitude of mEPSCs is highly dependent on the location relative to the receptor nanodomain, such that release events on non-clustered regions of the synapse are predicted to produce very modest, and likely undetectable mEPSCs and that most of the recorded mEPSCs reflect “on-cluster” events (MacGillavry et al., 2013; Nair et al., 2013). Thus, similar as for evoked responses, changes in nanodomain content are predicted to alter mEPSC amplitude (Fig. 2C). More speculative, one could predict that because spontaneous events ‘probe’ a larger fraction of the postsynaptic membrane, a change in the number of nanodomains per synapse, increases the probability that a spontaneous event activates receptors, and could be measured as a change in mEPSC frequency (Fig. 2B). Thus, while a change in mEPSC frequency is generally interpreted as a change in presynaptic function or in the number of synaptic connections, perhaps it could also be interpreted as a change in the functional organization of postsynaptic receptors. For instance, the genetic removal of individual GluA2-containing AMPA receptors significantly reduced mEPSC frequency, but not amplitude, even though both the total number of synapses and measures of presynaptic function are not affected in (Lu et al., 2009). This might suggest that in the remaining fraction of functional synapses, GluA2-lacking AMPA receptors are still capable of populating subsynaptic nanodomains that can produce similar response amplitudes as normal synapses. It would be of interest to investigate how the subunit composition and levels of AMPARs at individual synapses is related to the molecular organization of synapses.
In a recent study, GluA1-containing AMPA receptors were selectively recruited to the PSD by an elegant optogenetic approach. Using this recruitment assay, it was shown that adding GluA1-containing AMPA receptors to existing synapses significantly increased synaptic responses as measured by evoked EPSCs, glutamate uncaging, and mEPSC frequency, but did not affect the amplitude of mEPSCs (Sinnen et al., 2017). These experiments thus indicate that the global addition of receptors to a synapse does not necessarily increase quantal amplitude, and confirm predictions that adding receptors specifically to nanodomains aligned with presynaptic sites of vesicle release is required to enhance synaptic transmission (Liu et al., 2017). Also, it could indicate that the number of receptor slots in a nanodomain is limited, and is in most cases saturated, such that it cannot be further increased. Thus, it will be important to dissect the mechanisms that specifically control the integration of receptors in nanodomains after these receptors entered the synaptic membrane.
While the role of GluA1/2 receptors in synaptic transmission has been vigorously investigated, the contribution of GluA3 to synaptic transmission has long been regarded as non-essential. The deletion of GluA3-subunits has minimal effects on synaptic currents (Lu et al., 2009), and does not prevent the induction of long-term potentiation (LTP) and depression (LTD) (Meng et al., 2003; Reinders et al., 2016). However, recent studies on slice preparations from cerebellum (Gutierrez-Castellanos et al., 2017), and hippocampus (Renner et al., 2017) found that synaptic potentiation by increasing cyclic-AMP (cAMP) levels was completely abolished in GluA3 knock-out (KO) mice. It was furthermore shown that cAMP-mediated potentiation increased the open-channel probability of GluA3-containing AMPA receptors, thereby enhancing their contribution to the synaptic response. Thus, GluA3-containing AMPA receptors might form a distinct subpopulation of ‘silent’ AMPARs that become activated by modulatory inputs that activate cAMP signaling pathways. Interestingly, potentiation by cAMP had a drastic effect on mEPSC frequency, but no alterations in presynaptic function were observed, suggesting that the number of functional postsynaptic nanodomains was increased, or that the increased contribution of GluA3-containing AMPA receptors in pre-existing nanodomains ‘unsilenced’ these domains. In this respect it is interesting to note that ectopic expression of GluA3 can depress synaptic responses (Shi et al., 2001). Perhaps that these ectopically expressed, silent GluA3-containing AMPA receptors, exchange with AMPA receptors present in nanodomains, effectively silencing these receptor domains.
The strength of synaptic transmission is highly regulated by changes in synaptic activity patterns, and the dynamic trafficking of AMPARs to and from synapses underlies the expression of LTP and LTD (Huganir and Nicoll, 2013). Following on the discussion above, it is attractive to speculate that specifically altering the number or composition of nanodomains, or transsynaptic “modules” (Liu et al., 2017), underlies the expression of long-term plasticity. Consistent with this idea it was found that activity-induced potentiation of synapses involves an increase in the spatial alignment with presynaptic domains (Tang et al., 2016). It will be of great interest to further investigate how exactly synaptic potentiation affects postsynaptic receptor organization, and to test whether this involves an increase in the number of nanodomains, the number of receptors within nanodomains, an increase in effective alignment with the presynaptic release machinery, or a combination of these processes.
3.2. Modulation of synaptic transmission and plasticity by perisynaptic mGluRs
At the postsynaptic membrane, group I mGluRs are spatially segregated from the synaptic iGluRs in the PSD, at considerable distance from the hotspot of glutamate release (Fig. 1A). Nevertheless, the activity of synaptic mGluRs has been shown to modulate synaptic transmission and several forms of plasticity, and disruption of mGluR function has been implicated in neurological diseases, most notably Fragile X syndrome, the most common form of inherited intellectual disability (Bear et al., 2004; Lüscher and Huber, 2010). Group I mGluRs are GPCRs and can trigger a wide variety of effector systems. Group I mGluRs are canonically linked to Gαq/11 -proteins which activate PLC. PLC in turn, hydrolyzes the phospholipid PIP2 (phosphatidylinositol 4,5-bisphosphate) to form diacylglycerol (DAG) and the soluble second-messenger IP3 (inositol tris-phosphate). IP3 activates IP3 receptors on the endoplasmic reticulum (ER) triggering the release of Ca2+ from internal stores, which, together with DAG, activates protein kinase C (PKC) (Niswender and Conn, 2010). As a result of these signaling events, mGluR activity generally increases postsynaptic excitability through the modulation of several ion channels such as calcium-dependent and independent cationic channels, HCN channels, small-conductance K+ (SK) channels, and the regulation of NMDAR currents (Anwyl, 1999; Fitzjohn et al., 1996; Heidinger et al., 2002).
On longer time scales, the activity of mGluRs has been implicated in several forms of plasticity. Numerous studies have confirmed that mGluR5 is involved in the induction and expression of LTP, as shown by pharmacological blockade in vitro and in vivo (Balschun and Wetzel, 2002; Bashir et al., 1993a; Bortolotto et al., 1994; Francesconi et al., 2004; Neyman and Manahan-Vaughan, 2008), and by genetic deletion (Jia et al., 1998; Lu et al., 1997). These effects seem to reflect a metaplastic effect in which prior activation of mGluRs primes the induction of subsequent LTP by increasing the excitability (Cohen et al., 1999; Cohen et al., 1998), and even prolongs the expression of LTP by inducing local protein synthesis (Raymond et al., 2000). On the other hand, selective activation of group I mGluRs, or low-frequency synaptic stimulation, is sufficient to induce a form of synaptic depression that is independent of NMDARs, termed mGluR-LTD (Bashir et al., 1993b; Lüscher and Huber, 2010; Palmer et al., 1997). Unlike NMDAR-dependent depression, this form of LTD involves the protein synthesis-dependent endocytosis of AMPARs (Huber et al., 2000; Snyder et al., 2001; Waung and Huber, 2009).
The contribution of mGluRs to synaptic signaling and plasticity is likely to be not equal at all synapses. Using two-photon imaging of calcium transients in response to glutamate uncaging it was observed that these responses had much larger amplitudes in spines that contained a prominent ER structure (Holbro et al., 2009). Intriguingly, low-frequency stimulation of these ER-containing spines induced a long-lasting, mGluR-dependent depression of synaptic responses, while ER-lacking spines did not respond to this stimulus. This would suggest that mGluR activity is most prominent in a subset of spines that contain an ER. Interesting in this regard is that in the hippocampal CA1 region, the reported values of spines containing an ER has been variable (20–70%) (Holbro et al., 2009; Ng et al., 2014; Spacek and Harris, 1997), but considerably lower than in Purkinje neurons in the cerebellum, where virtually all spines contain an ER (Harris and Stevens, 1988; Wagner et al., 2011) and mGluR1-mediated signaling has a prominent role in synaptic transmission and plasticity. Interestingly in this respect is that mGluR activation itself can regulate ER complexity in dendrites (Cui-Wang et al., 2012), indicating that the activity of mGluRs and the ER are tightly coupled. Also, mGluR-mediated EPSCs, and mGluR-LTD could not be induced in directly coupled CA3-CA1 synapses, but was only apparent after activation of several (> 7) inputs on a single CA1 neuron (Fan et al., 2010), additionally suggesting that mGluR-mediated responses can only be triggered in a subset of spines. Together, these results suggest that only in a subset of synapses, mGluRs are functionally coupled to the ER to effectively modulate synaptic transmission. This would imply that ER-containing synapses carry most of the excitatory drive, and it will be important to determine how mGluRs functionally interact with the receptors in the core of the PSD, but also with compartments in the spines, such as the ER and spine apparatus. In conclusion, mGluRs can be regarded as sensors of synaptic activity providing critical feedback control, effectively gating the excitatory flow in neuronal networks.
4. Mechanisms underlying the subsynaptic positioning of glutamate receptors
4.1. Regulation of synaptic entry and retention of glutamate receptors
The distribution of glutamate receptors at synapses is highly heterogeneous, with a refined level of organization that has direct consequences for synaptic physiology. What underlies the entry of receptors into the PSD before they become stably anchored? Also, what mechanisms retain and position receptors once they entered the PSD? The trafficking of receptors into and away from the synaptic membrane is a highly regulated and dynamic process, and underlies the activity-dependent modulation of synaptic strength (Huganir and Nicoll, 2013; Kessels and Malinow, 2009). The level of receptors expressed at the synaptic membrane is governed by a series of vesicular trafficking steps and exocytosis of receptors from intracellular compartments (Kennedy and Ehlers, 2011; van der Sluijs and Hoogenraad, 2011; Wu et al., 2017), but lateral diffusion of receptors is the key final step by which receptors are inserted in the synaptic membrane (Makino and Malinow, 2009; Penn et al., 2017) (Fig. 3A). Synaptic entry of receptors seems to be tightly regulated at the border of the PSD with specific receptors being selected by mechanisms that remain largely unknown.
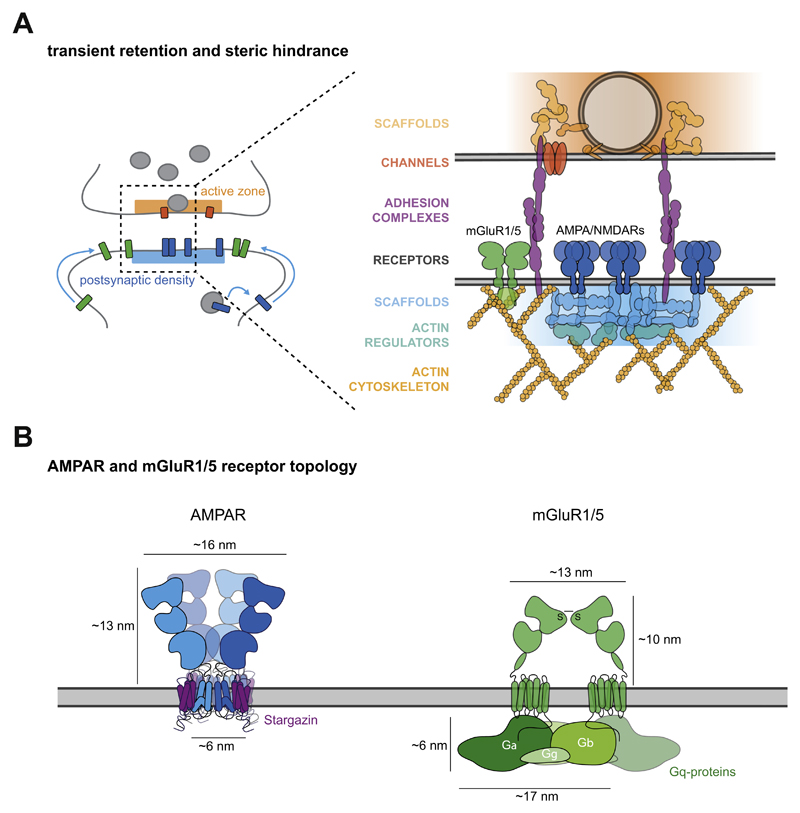
Fig. 3. Mechanisms underlying the dynamic positioning of glutamate receptors.
(A) Side view of a synapse showing the subsynaptic distribution of the AMPA-, NMDA- and mGluR1/5-type receptors established by mechanisms regulating the synaptic entry and retention of these glutamate receptor types. The zoom of the synapse in side view reveals possible mechanisms underlying the distinct subsynaptic positioning of the glutamate receptor types; transient retention of glutamate receptors via intracellular interactions with scaffolding proteins and extracellular interactions with synaptic cleft proteins, and steric hindrance due to molecular crowding of the different synaptic components and cytoskeletal hindrance at the border of the PSD. (B) Side view of the tetrameric AMPAR (blue) in complex with Stargazin (magenta) based on (Greger et al., 2017) (left), and dimeric mGluR1/5 coupled to its cognate Gq-proteins (green) based on (Nishimura et al., 2010) (right). This figure shows the Y-shaped GluA2 homomer (Greger et al., 2017) and the closed-closed resting conformation of an mGluR dimer (Muto et al., 2007). Models are approached to scale.
How does the border of the PSD discriminate between receptor types to establish the remarkable nanoscale segregation of AMPARs and mGluRs? Here we will discuss potential mechanisms that could contribute to the formation and maintenance of the dynamic distribution of glutamate receptors at the synapse. To control receptor entry the PSD might act as a ‘gate keeper’ selecting receptors for entry based on specific properties, perhaps in an activity-regulated manner. Thus, we will make the distinction between processes that control the entry of receptors into the synapse, and processes that control the anchoring and positioning of receptors at distinct locations within the postsynaptic membrane (i.e. within or outside of subsynaptic nanodomains). Making this distinction might be important to understand and delineate processes that specifically control the (activity-induced) changes in the number of synaptic components, and processes that structurally (re-) organize the synapse or partition it into distinct nanodomains. We will specifically discuss the relative contribution of interactions of receptors with intracellular and extracellular protein complexes, steric hindrance due to molecular crowding, and cytoskeletal hindrance to the entry and retention of receptors. Here we will focus on AMPARs and mGluRs to narrow the scope of this review, as the AMPARs are the predominant iGluRs and are intriguingly different in their subsynaptic positioning compared to mGluRs.
4.2. Intracellular interactions with scaffolding proteins
At the synapse receptors can engage in a multitude of interactions with the numerous scaffolding proteins that form the PSD just below the membrane (Fig. 3A). Indeed, decades of research have principally focused on identifying and characterizing protein-protein interactions with intracellular scaffolding proteins (Okabe, 2007; Sheng and Hoogenraad, 2007). AMPARs can interact with scaffolding proteins via their PDZ ligand in their C-terminal domain (CTD). The GluA1 subunits have a long CTD containing a type-I PDZ ligand, whereas the GluA2/3 subunits have a short CTD with a type-II PDZ ligand. GluA1 can directly interact with the PDZ type I containing protein SAP97, whereas GluA2/3 can directly interact with the PDZ type II containing proteins PICK1 and GRIP/ABP (Anggono and Huganir, 2012). The differences in CTD have been ascribed to underlie the subunit-specific trafficking of AMPARs (Malinow and Malenka, 2002). In particular, it is broadly held that synaptic activity promotes the entry of GluA1-containing AMPA receptors, while GluA2/3 receptors can traffic constitutively to the synapse (Hayashi et al., 2000; Shi et al., 2001). Recent genetic studies, however, challenged this idea. It was demonstrated that removal of the GluA1 CTD, or even complete depletion of the AMPAR GluA1–3 subunits, did not prevent the induction of LTP or LTD (Granger and Nicoll, 2014; Granger et al., 2013). Interestingly, a more recent study in which the GluA1 and GluA2 CTD were genetically exchanged demonstrated that these tails are necessary and sufficient for plasticity-mediated receptor trafficking and hippocampal learning paradigms (Zhou et al., 2018). Thus, the CTDs of AMPAR subunits can differentially control the distribution and trafficking of AMPARs in and to synapses.
SAP97, a member of the membrane-associated guanylate kinase (MAGUK) family (also including PSD-95, PSD-93 and SAP102), is a particularly interesting candidate to regulate the specific synaptic entry of GluA1 receptors, as SAP97 has been reported to concentrate at the edge of the PSD (DeGiorgis et al., 2006; Valtschanoff et al., 2000). Also, the two SAP97 isoforms, αSAP97 and βSAP97, were found to differentially regulate the targeting of GluA1 to the center or the periphery of the synaptic membrane respectively, as shown by dSTORM super-resolution imaging (Goodman et al., 2017). The palmitoylated αSAP97 isoform is stably anchored within the PSD and regulates the amount of synaptic binding sites for GluA1-containing AMPA receptors. On the other hand, the L27 domain-containing βSAP97 isoform is much more dynamic and regulates the cluster size and density of GluA1-containing AMPA receptors at the periphery of the PSD (Regalado et al., 2006; Waites et al., 2009). The α- and βSAP97 isoforms also differentially regulate synaptic transmission, with αSAP97 increasing and βSAP97 decreasing synaptic currents (Waites et al., 2009). Thus, the alternative splicing of the SAP97 N-terminus may be an important factor in the regulation of synaptic entry of AMPARs. Additionally, the S845 phosphorylation on the GluA1 subunit, which is a substrate of protein kinase A (PKA), has been shown to regulate the perisynaptic surface pool of GluA1 homomers, and dephosphorylation of S845 in turn removes the perisynaptic GluA1 homomers (He et al., 2009). Interestingly, these perisynaptic GluA1 homomers are implicated in the induction of PKA-dependent LTP, which might involve activity-induced recruitment of perisynaptic GluA1 homomers to synaptic sites (He et al., 2009; Park et al., 2016). On the other hand, genetic removal of the GluA1 PDZ ligand did not seem to affect synaptic targeting or CA1 hippocampal LTP (Kim et al., 2005), and the exact contribution of the GluA1 CTD and interaction with SAP97 isoforms to synaptic entry remains to be elucidated.
Once in the synapse, what scaffolding proteins could control the subsynaptic accumulation of AMPARs in nanodomains? By far the most prominent candidate is PSD-95, also part of the MAGUK family, that is highly enriched in the PSD. PSD-95 is stably anchored at the postsynaptic membrane via palmitoylation (Craven et al., 1999; El-Husseini et al., 2000), and interacts with AMPAR auxiliary proteins, including the transmembrane AMPAR regulatory protein (TARP) family, to retain AMPARs at synaptic sites (Bats et al., 2007; Schnell et al., 2002). PSD-95 distribution within individual PSDs is highly heterogeneous, forming distinct subsynaptic nanodomains (Broadhead et al., 2016; Fukata et al., 2013; MacGillavry et al., 2013; Tang et al., 2016) that are enriched with AMPARs as found by EM (Chen et al., 2008), and super-resolution microscopy (MacGillavry et al., 2013; Nair et al., 2013; Tang et al., 2016). Thus, PSD-95 is likely to play a critical role in the immobilization of AMPARs in nanodomains. Indeed, immobilization of AMPARs within the synapse is found to be highly heterogeneous (Li and Blanpied, 2016; Nair et al., 2013) with restricted zones of diffusion (Ehlers et al., 2007; Kerr and Blanpied, 2012), where they can be retained for long periods of time (Adesnik et al., 2005). Furthermore, AMPARs within nanodomains are largely immobilized, whereas the AMPARs outside nanodomains are much more mobile (Nair et al., 2013). The mechanisms involved in the clustering of MAGUKs underlying the formation of AMPAR nanodomains, however, remain largely unexplored. Palmitoylation of MAGUKs might be crucial as it is involved in maintaining PSD-95 nanodomains (Fukata et al., 2013), and is essential for receptor binding (Jeyifous et al., 2016). Moreover, along with MAGUKs there are many other scaffolding proteins present in the PSD, such as Shanks, SAPAPs, and Homers, that together create a laminated structure providing a highly linked platform likely involved in the retention and subsynaptic positioning of receptors (Burette et al., 2012; Harris and Weinberg, 2012; Valtschanoff and Weinberg, 2001) (Fig. 3A). As such, also these scaffolding molecules that reside in the deeper layers of the PSD and link to the cytoplasmic actin cytoskeleton, were found co-enriched in receptor nanodomains, suggesting a highly interlinked postsynaptic “super-complex” (Frank and Grant, 2017). To what extent each of these components simply constitute the nanodomain, or are instructive in the formation of the transsynaptic nano-column however, is as yet unknown.
Unlike the well-studied AMPAR-scaffold interactions, identification of scaffolding proteins that regulate the lateral diffusion of mGluRs is largely lacking. Interestingly, similar to GluA1, mGluRs also contain a typical C-terminal type-I PDZ ligand known to interact with Shank and Tamalin (Kitano et al., 2002; Tu et al., 1999). However, this domain seems primarily involved in surface trafficking, and might not be involved in the positioning of the receptor (Kitano et al., 2002). The most prominent candidate, however, is the scaffolding protein Homer, which can interact with the CTD of group I mGluRs (Enz, 2012; Tu et al., 1999), and is generally proposed as the protein regulating the sub-synaptic positioning of mGluRs. Indeed, Homer overexpression induces clustering of group I mGluRs in heterologous cell systems (Ciruela et al., 2000; Tadokoro et al., 1999), Homer and group I mGluRs co-localize at the light microscopy level (Tadokoro et al., 1999), move together in developing hippocampal neurons, and mGluR5 becomes more mobile when the binding with long Homer1b/c forms is disrupted by mutation or co-expression of Homer1a, a short Homer isoform that acts as dominant negative disrupting the interaction of mGluR5 with the longer Homer isoforms (Brakeman et al., 1997; Sergé et al., 2002; Xiao et al., 1998).
A recent study identified alterations in the mGluR5-Homer crosstalk in a new Fmr1 KO mouse, a model for Fragile X syndrome (Aloisi et al., 2017). In these Fmr1 KO mice, mGluR5 displayed increased mobility, specifically the small synaptic fraction, which was attributed to the disruption of the mGluR5-Homer1b/c interaction by overexpression of Homer1a (Aloisi et al., 2017). However, although these findings show that the small fraction of mGluR5 that was able to enter the PSD (8% of total mGluR5 pool) is dynamically regulated by Homer, evidence for a role of Homer in clustering mGluRs at its preferred location within the perisynaptic domain is lacking. Moreover, given that Homer is highly enriched in the PSD, distant from the mGluRs that are concentrated in the perisynaptic domain, Homer might not be the prominent scaffold for mGluRs at perisynaptic sites. Rather, Homer might function as an adaptor protein, mediating mGluR signaling by forming a link between mGluR5 and downstream signaling pathways (Shiraishi-Yamaguchi and Furuichi, 2007). Intriguingly, the same study found that the loss of interaction between mGluR5 and Homer1b/c resulted in a tighter association between mGluR5 and NMDARs, associated with abnormal NMDAR functioning and plasticity (Aloisi et al., 2017). Importantly, this effect was rescued by knockdown of Homer1a. This supports the idea that a correct balance between the binding of long and short Homer forms to mGluR in specific physiological conditions are essential for proper mGluR signaling.
Apart from Homer, a few other candidates could underlie the synaptic entry and retention of mGluRs. For instance, Norbin is an accessory protein of mGluRs shown to be important for mGluR surface expression and signaling (Wang et al., 2009), and was also found to accumulate in the perisynaptic domain (Westin et al., 2014). Thus, Norbin could potentially play an important role in regulating the availability and positioning of mGluRs modulating mGluR function in synapses. Additionally, other CTD interaction partners have been determined such as Calmodulin (Minakami et al., 1997), Filamin-A (Enz, 2002), Siah-1A (Ishikawa et al., 1999), Preso1 (Hu et al., 2012), Tamalin (Kitano et al., 2002), and Shank (Tu et al., 1999). However, whether these proteins play a role in mGluR positioning, rather than in regulating mGluR trafficking or signaling, remains to be established.
4.3. Extracellular interactions with synaptic cleft proteins
For AMPARs, the mechanisms underlying the targeting to and positioning at synapses have largely been ascribed to the intracellular tail of AMPARs (Shi et al., 2001; Anggono and Huganir, 2012; Shepherd and Huganir, 2007). Recent studies however suggest that the extracellular domain of AMPARs could also have an instructive role in subsynaptic targeting (Diaz-Alonso et al., 2017; Elegheert et al., 2016; Matsuda et al., 2016; Watson et al., 2017). AMPARs contain two extracellular domains, the ligand-binding domain (LBD) and the distant N-terminal domain (NTD) which encompass 50% of the receptor, extending 13 nm into the synaptic cleft. Removing the NTD from GluA1 was shown to prevent its synaptic targeting and impaired the maintenance of LTP (Diaz-Alonso et al., 2017; Watson et al., 2017). Moreover, the fusion of a GFP tag at the NTD (Diaz-Alonso et al., 2017; Granger et al., 2013; Greger et al., 2017), or coupling large quantum dots (S.H. Lee et al., 2017), seemed to hamper the entry of GluA1 receptors, possibly by interfering with endogenous NTD interactions, but also see (Nabavi et al., 2014). Interestingly, the NTD sequence of different AMPAR subunits is highly variable. Indeed, deleting the NTD from GluA2 did not prevent synaptic entry (Diaz-Alonso et al., 2017), and replacing the GluA1 NTD with the GluA2 NTD promoted the synaptic entry of GluA1 receptors (Watson et al., 2017), suggesting that subunit-specific trafficking of AMPARs can in part be mediated by extracellular interactions. An interesting model in this respect would be that while the CTDs of AMPARs determine the trafficking to and from synapses, the NTD of AMPARs mediate the anchoring and positioning, and perhaps instruct the transsynaptic alignment of the receptors within the PSD with the vesicle release site at the presynaptic active zone.
How does the NTD mediate receptor positioning? Several proteins have been found to interact with the extracellular domain of different AMPAR subunits (Fig. 3A), such as N-cadherin (Saglietti et al., 2007), neuroligin-1 (Budreck et al., 2013), pentraxins (Farhy-Tselnicker et al., 2017; S.J. Lee et al., 2017; O'Brien et al., 1999), LRRTMs (Schwenk et al., 2012), and the EphB2 receptor via ephrinBs (Dalva et al., 2000; Grunwald et al., 2001). However, their precise role in regulating synaptic entry or retention of AMPARs remains to be established (Biederer et al., 2017). Interestingly, the postsynaptic adhesion protein LRRTM2 was also found to form stable nanodomains (Chamma et al., 2016), and LRRTM2 knockdown resulted in decreased AMPAR-mediated synaptic currents (de Wit et al., 2009; Soler-Llavina et al., 2011), and might thus be an interesting candidate for the retention and subsynaptic positioning of AMPARs. Recent studies on GluD2 and kainate receptors found a similar requirement for NTD-dependent interactions in synaptic retention (Elegheert et al., 2016; Matsuda et al., 2016), suggesting that extracellular, and perhaps transsynaptic, interactions with glutamate receptors are a more general instructive mechanism to control synaptic entry and positioning.
4.4. Steric hindrance
In addition to direct biochemical interactions between receptors and other proteins, alternative, more indirect mechanisms are likely to contribute to the positioning of receptors. As the name implies, the PSD is an extremely densely packed structure with numerous synaptic proteins forming an intricate network just underneath the cell membrane (Burette et al., 2012; Sheng and Hoogenraad, 2007), and is thus likely to impose a physical barrier for receptors diffusing in the synaptic membrane (Fig. 3A). Most directly, PSD-95 is attached to the membrane via palmitoylation and forms an intricate lateral structure close to the cytoplasmic face of the postsynaptic membrane. Indeed, computational modeling predicts that simply by molecular crowding, the PSD can trap receptors for hours, even in the absence of interactions (Santamaria et al., 2010). Additionally, using the heterogeneous distribution of PSD-95 as a template, measured experimentally with single-molecule localization microscopy, it was computationally predicted that receptor size contributes considerably to the extent that receptors can diffuse through the synapse and exchange with the extrasynaptic membrane. Importantly, also experimentally it was shown that while a single-pass transmembrane protein with one PDZ motif is efficiently targeted to the synapse, to the same extent as AMPARs, the much larger AMPAR was far less mobile (Li et al., 2016). Moreover, the diffusion properties of a single-pass transmembrane probe lacking an intracellular PSD-95 binding site was highly heterogeneous within individual synapses, and correlated inversely with the local density in PSD-95, i.e. mobility of this probe was significantly restricted in high-density PSD-95 nanodomains (Li and Blanpied, 2016). Thus, molecular crowding, in concert with molecular binding, can trap and limit the exit of receptors from the PSD and might as such favor the subsynaptic positioning of receptors in high-density scaffold nanodomains. Conversely, the PSD may act as an exclusion matrix or sieve that filters on molecular size to regulate receptor entry. This implies that different receptor subtypes must have different structures and geometries to contribute, probably in concert with scaffold interactions, to the distinct subsynaptic patterns of receptors (Fig. 3B).
The transmembrane domain (TMD) of glutamate receptors contributes to steric hindrance in the molecular crowded PSD (Li et al., 2016). The AMPAR TMD sector forms a pore of approximately 5.5 nm in diameter (Sobolevsky et al., 2009), similar for different combinations of AMPAR subunits (Herguedas et al., 2016). On the other hand, mGluR forms dimers and each TMD consists of seven TM helices that each have a diameter of 3.5 nm (Muto et al., 2007). Thus, the TMD of an mGluR dimer is similar in size to an AMPAR tetramer. However, based on the crystal structures from group II mGluRs, highly similar in structure to group I mGluRs, several conformational states have been predicted that also affect the proximity of the two TMDs relative to each other (Muto et al., 2007). Interestingly, the distance between the TMDs of the mGluR dimer highly varies between the resting state (large) and the active state (small) of the receptor, varying the degree of steric hindrance in the synaptic membrane (Muto et al., 2007). This suggests that the regulation of synaptic entry and positioning of mGluRs is an activity-dependent process. Importantly, AMPARs additionally closely assemble with a variety of auxiliary subunits in the TMD that regulate AMPAR properties such as trafficking, expression, and functioning (Greger et al., 2017) (Fig. 3B). Recent cryo-EM studies reveal that homo-tetrameric GluA2, also suggested for hetero-tetrameric AMPAR complexes (Kim et al., 2010), can interact with one to four Stargazins, with a preferred stoichiometry of one to two depending on Stargazin expression (Twomey et al., 2016; Zhao et al., 2016). In conclusion, although AMPARs and mGluRs differ broadly in the assembly of their TMD, the overall size of the TMD in the plane of the synaptic membrane contributing to steric hindrance appears to be highly similar.
Together with the TMD, the cytoplasmic CTD may also contribute to aggravating steric hindrance of the receptor types. The CTD of the GluA1 and GluA2/3 subunits are only 81 and 50 amino acid residues long respectively, whereas mGluR5a and mGluR5b have a very large CTD of 350 and 382 residues respectively. Unfortunately, because the CTDs are largely unfolded structures, the intracellular structures of mGluRs and the AMPAR/TARP complex have yet to be crystallized. However, the most striking difference between the two receptor types in their CTDs, is that unlike iGluRs, mGluRs are coupled to Gq-proteins. Gq-proteins assemble close to the cytoplasmic face of the synaptic membrane by interacting in the pocket formed between the second and third intracellular TM loops of mGluR (De Blasi et al., 2001) (Fig. 3B). The Gq-proteins are composed of α-, β-, and ƴ-subunits with a total estimated size of 17.3 nm long, 17.3 nm wide and, 6.09 nm high based on the crystal structure (Nishimura et al., 2010). Even though the interaction with Stargazins also adds some bulk to the AMPAR CTD (Fig. 3B), this is considerably less than the Gq-proteins interacting with the mGluR CTD. The differences suggest that the molecular size of the CTD in full assembly with its other constituents might contribute to the segregation of AMPAR and mGluRs, where the molecular crowded PSD acts as a size exclusion matrix regulating receptor entry. Interestingly, the full complex of Gq-proteins binds to the resting conformation of mGluRs, and upon activation the β- and ƴ-subunits uncouple, perhaps alleviating steric hindrance due to molecular crowding. This notion is supported by a study showing increased mobility of mGluR5 upon activation with its specific agonist DHPG (Sergé et al., 2002). This, in addition to the conformational changes upon activation, furthermore supports that the regulation of synaptic entry of mGluRs is an activity-dependent process.
The significant accumulation of synaptic cleft molecules could also hinder receptor entry via steric hindrance with the extracellular domain of receptors. AMPARs consist of two globular extracellular structures, the NTD and LBD, whereas mGluRs consist of one extracellular LBD and a small cysteine-rich region (Fig. 3B). Interestingly, the density map of a tetrameric AMPAR can accommodate two dimeric crystal structures of the mGluR1 extracellular domain (Greger et al., 2017; Kunishima et al., 2000; Nakagawa et al., 2005). Although there are slight differences between the extracellular domains of AMPARs and mGluRs, the overall conformation results in a high similarity in the secondary structure of these receptors and it is thus not likely that steric hindrance contributes to the segregation of these receptors types. Rather, adhesion molecules are suggested to be key to the transsynaptic alignment of the presynaptic vesicle release site with postsynaptic AMPAR nanodomains (Biederer et al., 2017; Tang et al., 2016). Several active zone proteins, such as Liprins, LAR, RIM, but also other pre- and postsynaptic components, are likely to be part of the lateral oligomerization forming a transsynaptic nanocolumn (Biederer et al., 2017). Also, some adhesion proteins specifically concentrate at the postsynaptic edge, such as SynCAM1 or more towards the center, such as EphB2 (Perez de Arce et al., 2015), or the classical synaptic adhesion molecule N-cadherin that initially localizes throughout the synaptic cleft, but at later stages forms distinct clusters at the edge (Elste and Benson, 2006; Uchida et al., 1996) providing a heterogenous localization pattern possibly resembling the transsynaptic nanocolumns. Together, adhesion molecules can contribute to the retention and positioning of receptors by imposing diffusional barriers.
4.5. Cytoskeletal hindrance
The confinement of receptors within specific subsynaptic areas may also arise from structures that compartmentalize the synaptic membrane leading to steric hindrance counteracting free receptor diffusion and/or accumulations of receptor clusters (Kusumi et al., 2005). Modeling studies have sought to investigate the possibility that this can lead to the clustering of receptors by considering a boundary with small openings or a stochastic gate that allows receptor escape (Earnshaw and Bressloff, 2006; Holcman and Triller, 2006). Holcman and Triller (2006) modeled the PSD as two simplified compartments: a central region with both bound and unbound scaffolding molecules to receptors and a surrounding annulus that represents a fence formed by transmembrane proteins and submembraneous cytoskeleton. By adding a small opening to this fence to allow for few receptors to escape, this model could reproduce fluorescence recovery after photobleaching (FRAP) data measured for AMPARs. However, these models treat the interior of the PSD as a homogeneous compartment, and therefore cannot explain the subsynaptic distribution of AMPARs. Rather, these models might be useful to consider the boundary of the PSD as a gatekeeper to receptor entry by varying the size of the small opening to better understand entry of different receptors.
The perisynaptic actin cytoskeleton could impose a diffusional barrier to receptor entry into the synapse. At the perisynaptic membrane actin is in close proximity to the cytoplasmic surface forming an intricate mesh-like structure of sub-membranous filaments, whereas actin is largely absent from the PSD (Burette et al., 2012; Frost et al., 2010; Morone et al., 2006; Westin et al., 2014) (Fig. 3A). However, there is no clear evidence that the actin filamentous meshwork is enriched at the perisynaptic membrane. Thus, actin is likely not to be involved in the subsynaptic positioning of iGluRs, but might be involved in the exclusion of the larger mGluRs from the PSD and perhaps even in clustering mGluRs at perisynaptic sites. The actin-based membrane skeleton (MSK) may act as a gatekeeper or fence, but also transmembrane proteins attached to the MSK may behave as pickets that result in steric hindrance and nonspecific corralling of receptor diffusion at the perisynaptic domain, where only a few receptors ‘hop the fence’ of the PSD (Morone et al., 2006; Sako and Kusumi, 1994). Also, freely diffusing receptors may cluster when encountering these fences/pickets, a process called diffusion-limited aggregation (DLA). In support of this picket-fence model an EM study revealed that in non-neuronal cells the actin-based MSK can partition the cell membrane limiting receptor diffusion within these compartments (Morone et al., 2006). The size of these MSK meshes was determined to range from 50 to 200 nm (Morone et al., 2006), which would also allow receptors to accumulate in the perisynaptic domain. Additionally, the recent finding that G-proteins are co-clustered with GPCRs at the cell surface defined by the actin cytoskeleton further support this notion (Sungkaworn et al., 2017). Although to date there is no ultrastructural evidence for actin meshes at the perisynaptic membrane, it is possible that the actin MSK is an important player in the gatekeeper role of the PSD, perhaps by forming a fence that only allows some receptors to pass.
5. Conclusions and future prospects
The molecular organization of synapses is undoubtedly a critical determinant of the efficiency of synaptic transmission. The complexity of synapse organization has indeed been underlined by extensive genetic and biochemical approaches that over the past decades have resulted in a comprehensive “parts list” of synapses. Yet, how are these components properly assembled into the large macromolecular complexes that organize the glutamate receptors at the surface? Emerging evidence demonstrates that the structure and molecular organization of synapses is highly heterogeneous and organized in distinct subsynaptic nanodomains (Biederer et al., 2017), but we are only starting to understand how, within individual synapses, different proteins find their correct location. Undoubtedly, the overall assembly of synapses is directed by specific protein-protein interactions via well-defined protein interaction motifs (Kim and Sheng, 2004). These core biochemical processes give rise to the stable molecular complexes that effectively concentrate receptors at synaptic sites and couple these receptors to intracellular scaffolding, adaptor, and signaling proteins. At the same time, these mechanisms enable the dynamic modifications of synaptic structure in response to activity. However, while these mechanisms can explain the assembly and stoichiometry of specific components into molecular complexes, to date it is not fully understood how these mechanisms contribute to the spatial organization of molecules at the synapse, i.e. how proteins are positioned relative to each other within individual synapses. Moreover, apart from these classic biochemical operations, the contribution of biophysical processes such as steric hindrance, membrane composition (Tulodziecka et al., 2016), and phase transitions (Zeng et al., 2016) are only beginning to be explored in the context of synapse organization.
We have discussed potential mechanisms that could work globally to organize the synapse in functional domains and mechanisms that act on specific receptor subtypes, but many questions about the structural organization of synapses remain unanswered. What is the exact composition of a nanodomain? Is there a fixed number of proteins enriched in these domains and are there proteins that are exclusively found within the domain? Are AMPARs with different subunit compositions co-enriched in nanodomains? How do nanodomains develop? Are they present in early, newly formed PSDs, or do they form in response to specific activity patterns? Clearly, there is a strong need for experimental directions that can tag or disrupt specific aspects of synapse organization, without affecting overall synapse structure. Ongoing developments in super-resolution, single-molecule tracking, and EM tomography will be key in determining how synapses are built from their numerous components.
Alterations in glutamatergic synapse structure and function seem to represent a common hallmark of many cognitive disorders (Volk et al., 2015). Intriguingly, these disorders span a broad clinical spectrum, including intellectual disability, autism spectrum disorder, and schizophrenia, but all seem to stem from a common defect; synaptic dysfunction. Indeed, these disorders are frequently associated with loss of synapses, or changes in morphology of dendritic spines. Given that many disease-associated genes are components of the glutamate receptor-associated complexes or can regulate glutamate receptor function through the actin cytoskeleton, indicates that disruptions in the precise positioning of glutamate receptors can underlie the development of these diseases. Future directions aimed at understanding the spatial organization of glutamate receptors will therefore not only be indispensable for a deeper insight in the regulation of synaptic transmission and plasticity, but will also contribute to the identification of disease mechanisms.
Acknowledgements
We would like to thank Dr. Thomas Blanpied, Sai Sachin Divakaruni, Dr. Helmut Kessels, Feline Lindhout, Dieudonnée van de Willige, and all members of the MacGillavry lab for discussions and critical reading of the manuscript. This work was supported by NWO (ALW-VENI 863.13.020, ALW-VIDI 171.029 and the Graduate Program of Quantitative Biology and Computational Life Sciences), the European Research Council (ERC-StG 716011), and a NARSAD Young Investigator Award (24995).
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Aloisi E, Le Corf K, Dupuis J, Zhang P, Ginger M, Labrousse V, Spatuzza M, Georg Haberl M, Costa L, Shigemoto R, Tappe-Theodor A, et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01191-2. 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993a;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Jane DE, Sunter DC, Watkins JC, Collingridge GL. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur J Pharmacol. 1993b;239:265–266. doi: 10.1016/0014-2999(93)91009-c. [DOI] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Kaeser PS, Blanpied TA. Transcellular Nanoalignment of synaptic function. Neuron. 2017;96:680–696. doi: 10.1016/j.neuron.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Aging. 2003;24:1095–1104. doi: 10.1016/j.neurobiolaging.2003.04.004. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Bashir ZI, Davies CH, Collingridge GL. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Boucher J, Kroger H, Sik A. Realistic modelling of receptor activation in hippocampal excitatory synapses: analysis of multivesicular release, release location, temperature and synaptic cross-talk. Brain Struct Funct. 2010;215:49–65. doi: 10.1007/s00429-010-0273-x. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Broadhead MJ, Horrocks MH, Zhu F, Muresan L, Benavides-Piccione R, DeFelipe J, Fricker D, Kopanitsa MV, Duncan RR, Klenerman D, Komiyama NH, et al. PSD95 nanoclusters are postsynaptic building blocks in hippocampus circuits. Sci Rep. 2016;6 doi: 10.1038/srep24626. 24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, Scheiffele P, et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extra-cellular coupling. Proc Natl Acad Sci U S A. 2013;110:725–730. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burette AC, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman MH, Weinberg RJ. Electron tomographic analysis of synaptic ultrastructure. J Comp Neurol. 2012;520:2697–2711. doi: 10.1002/cne.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamma I, Letellier M, Butler C, Tessier B, Lim KH, Gauthereau I, Choquet D, Sibarita JB, Park S, Sainlos M, Thoumine O. Mapping the dynamics and nanoscale organization of synaptic adhesion proteins using monomeric streptavidin. Nat Commun. 2016;7 doi: 10.1038/ncomms10773. 10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith J, Leapman R, Reese T. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, Chan WY, McIlhinney RA. Homer-1c/Vesl-1L modulates the cell surface targeting of metabotropic glutamate receptor type 1alpha: evidence for an anchoring function. Mol Cell Neurosci. 2000;15:36–50. doi: 10.1006/mcne.1999.0808. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C. Hippocampus. 1998;8:160–170. doi: 10.1002/(SICI)1098-1063(1998)8:2<160::AID-HIPO8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. J Neurophysiol. 1999;82:3139–3148. doi: 10.1152/jn.1999.82.6.3139. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Coombs ID, MacLean DM, Jayaraman V, Farrant M, Cull-Candy SG. Dual effects of TARP gamma-2 on glutamate efficacy can account for AMPA receptor autoinactivation. Cell Rep. 2017;20:1123–1135. doi: 10.1016/j.celrep.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven S, El-Husseini A, Bredt D. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, Watson D, Harris KM, Ehlers MD. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:309–321. doi: 10.1016/j.cell.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin J, Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgis JA, Galbraith JA, Dosemeci A, Chen X, Reese TS. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biol. 2006;35:239–250. doi: 10.1007/s11068-007-9017-0. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Alonso J, Sun YJ, Granger AJ, Levy JM, Blankenship SM, Nicoll RA. Subunit-specific role for the amino-terminal domain of AMPA receptors in synaptic targeting. Proc Natl Acad Sci U S A. 2017;114:7136–7141. doi: 10.1073/pnas.1707472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw BA, Bressloff PC. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J Neurosci. 2006;26:12362–12373. doi: 10.1523/JNEUROSCI.3601-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M, Heine M, Groc L, Lee M, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, Kohda K, Miura E, Rossmann M, Mitakidis N, Motohashi J, et al. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science. 2016;353:295–299. doi: 10.1126/science.aae0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A, Craven S, Chetkovich D, Firestein B, Schnell E, Aoki C, Bredt D. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- Enz R. The actin-binding protein Filamin-a interacts with the metabotropic glutamate receptor type 7. FEBS Lett. 2002;514:184–188. doi: 10.1016/s0014-5793(02)02361-x. [DOI] [PubMed] [Google Scholar]
- Enz R. Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front Mol Neurosci. 2012;5:52. doi: 10.3389/fnmol.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ster J, Gerber U. Activation conditions for the induction of metabotropic glutamate receptor-dependent long-term depression in hippocampal CA1 pyramidal cells. J Neurosci. 2010;30:1471–1475. doi: 10.1523/JNEUROSCI.5619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, van Casteren ACM, Lee A, Chang VT, Aricescu AR, Allen NJ. Astrocyte-secreted glypican 4 regulates release of neuronal pentraxin 1 from axons to induce functional synapse formation. Neuron. 2017;96(428–445):e413. doi: 10.1016/j.neuron.2017.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, Collingridge GL. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci Lett. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Cammalleri M, Sanna PP. The metabotropic glutamate receptor 5 is necessary for late-phase long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1022:12–18. doi: 10.1016/j.brainres.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RA, Grant SG. Supramolecular organization of NMDA receptors and the postsynaptic density. Curr Opin Neurobiol. 2017;45:139–147. doi: 10.1016/j.conb.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freche D, Pannasch U, Rouach N, Holcman D. Synapse geometry and receptor dynamics modulate synaptic strength. PLoS One. 2011;6:e25122. doi: 10.1371/journal.pone.0025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J Cell Biol. 2013;202:145–161. doi: 10.1083/jcb.201302071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L, Baddeley D, Ambroziak W, Waites CL, Garner CC, Soeller C, Montgomery JM. N-terminal SAP97 isoforms differentially regulate synaptic structure and postsynaptic surface pools of AMPA receptors. Hippocampus. 2017;27:668–682. doi: 10.1002/hipo.22723. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Nicoll RA. LTD expression is independent of glutamate receptor subtype. Front Synaptic Neurosci. 2014;6:15. doi: 10.3389/fnsyn.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Watson JF, Cull-Candy SG. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron. 2017;94:713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Greget R, Pernot F, Bouteiller JM, Ghaderi V, Allam S, Keller AF, Ambert N, Legendre A, Sarmis M, Haeberle O, Faupel M, et al. Simulation of postsynaptic glutamate receptors reveals critical features of glutamatergic transmission. PLoS One. 2011;6:e28380. doi: 10.1371/journal.pone.0028380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP, Bonhoeffer T, Klein R. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Castellanos N, Da Silva-Matos CM, Zhou K, Canto CB, Renner MC, Koene LMC, Ozyildirim O, Sprengel R, Kessels HW, De Zeeuw CI. Motor learning requires Purkinje cell synaptic potentiation through activation of AMPA-receptor subunit GluA3. Neuron. 2017;93:409–424. doi: 10.1016/j.neuron.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1988;8:4455–4469. doi: 10.1523/JNEUROSCI.08-12-04455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Weinberg RJ. Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Rothstein JD, Morrison JH. Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol. 2000;418:255–269. [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci. 2002;22:5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herguedas B, Garcia-Nafria J, Cais O, Fernandez-Leiro R, Krieger J, Ho H, Greger IH. Structure and organization of heteromeric AMPA-type glutamate receptors. Science. 2016;352:aad3873. doi: 10.1126/science.aad3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci U S A. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, Park JM, et al. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci. 2012;15:836–844. doi: 10.1038/nn.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Jacob AL, Weinberg RJ. The organization of AMPA receptor subunits at the postsynaptic membrane. Hippocampus. 2015;25:798–812. doi: 10.1002/hipo.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyifous O, Lin EI, Chen X, Antinone SE, Mastro R, Drisdel R, Reese TS, Green WN. Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation. Proc Natl Acad Sci U S A. 2016;113:E8482–E8491. doi: 10.1073/pnas.1612963113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezequel J, Johansson EM, Dupuis JP, Rogemond V, Grea H, Kellermayer B, Hamdani N, Le Guen E, Rabu C, Lepleux M, Spatola M, et al. Dynamic disorganization of synaptic NMDA receptors triggered by autoantibodies from psychotic patients. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01700-3. 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Kasai RS, Kusumi A. Single-molecule imaging revealed dynamic GPCR dimerization. Curr Opin Cell Biol. 2014;27:78–86. doi: 10.1016/j.ceb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JM, Blanpied TA. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J Neurosci. 2012;32:658–673. doi: 10.1523/JNEUROSCI.2927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels H, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Tangential synaptic distribution of NMDA and AMPA receptors in rat neocortex. Neurosci Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kim CH, Takamiya K, Petralia RS, Sattler R, Yu S, Zhou W, Kalb R, Wenthold R, Huganir R. Persistent hippocampal CA1 LTP in mice lacking the C-terminal PDZ ligand of GluR1. Nat Neurosci. 2005;8:985–987. doi: 10.1038/nn1432. [DOI] [PubMed] [Google Scholar]
- Kim KS, Yan D, Tomita S. Assembly and stoichiometry of the AMPA receptor and transmembrane AMPA receptor regulatory protein complex. J Neurosci. 2010;30:1064–1072. doi: 10.1523/JNEUROSCI.3909-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Kumari R, Castillo C, Francesconi A. Agonist-dependent signaling by group I metabotropic glutamate receptors is regulated by association with lipid domains. J Biol Chem. 2013;288:32004–32019. doi: 10.1074/jbc.M113.475863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KG. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson's fluid-mosaic model. Annu Rev Cell Dev Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jin C, Cai E, Ge P, Ishitsuka Y, Teng KW, de Thomaz AA, Nall D, Baday M, Jeyifous O, Demonte D, et al. Super-resolution imaging of synaptic and extra-synaptic AMPA receptors with different-sized fluorescent probes. elife. 2017;6 doi: 10.7554/eLife.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Wei M, Zhang C, Maxeiner S, Pak C, Calado Botelho S, Trotter J, Sterky FH, Sudhof TC. Presynaptic neuronal Pentraxin receptor organizes excitatory and inhibitory synapses. J Neurosci. 2017;37:1062–1080. doi: 10.1523/JNEUROSCI.2768-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TP, Blanpied TA. Control of transmembrane protein diffusion within the postsynaptic density assessed by simultaneous single-molecule tracking and localization microscopy. Front Synaptic Neurosci. 2016;8:19. doi: 10.3389/fnsyn.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TP, Song Y, MacGillavry HD, Blanpied TA, Raghavachari S. Protein crowding within the postsynaptic density can impede the escape of membrane proteins. J Neurosci. 2016;36:4276–4295. doi: 10.1523/JNEUROSCI.3154-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Liu KK, Hagan MF, Lisman JE. Gradation (approx. 10 size states) of synaptic strength by quantal addition of structural modules. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Kerr JM, Blanpied TA. Lateral organization of the postsynaptic density. Mol Cell Neurosci. 2011;48:321–331. doi: 10.1016/j.mcn.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Mutoh H, Dimitrov D, Beato M, Knopfel T. Optical measurement of mGluR1 conformational changes reveals fast activation, slow deactivation, and sensitization. Proc Natl Acad Sci U S A. 2009;106:11388–11393. doi: 10.1073/pnas.0901290106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Budisantoso T, Mitakidis N, Sugaya Y, Miura E, Kakegawa W, Yamasaki M, Konno K, Uchigashima M, Abe M, Watanabe I, et al. Transsynaptic modulation of kainate receptor functions by C1q-like proteins. Neuron. 2016;90:752–767. doi: 10.1016/j.neuron.2016.04.001. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J Biol Chem. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, Yuasa S, Usukura J, Kusumi A. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174:851–862. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Alfonso S, Aow J, Malinow R. GluA1 trafficking and metabotropic NMDA: addressing results from other laboratories inconsistent with ours. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0145. 20130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33:13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AN, Doherty AJ, Lombroso PJ, Emptage NJ, Collingridge GL. Rapid regulation of endoplasmic reticulum dynamics in dendritic spines by NMDA receptor activation. Mol Brain. 2014;7:60. doi: 10.1186/s13041-014-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K, Hakoshima T, Itoh H. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci U S A. 2010;107:13666–13671. doi: 10.1073/pnas.1003553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Pankratov YV, Krishtal OA. Distinct quantal features of AMPA and NMDA synaptic currents in hippocampal neurons: implication of glutamate spillover and receptor saturation. Biophys J. 2003;85:3375–3387. doi: 10.1016/S0006-3495(03)74757-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Sanderson TM, Amici M, Choi SL, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL. Calcium-permeable AMPA receptors mediate the induction of the protein kinase A-dependent component of long-term potentiation in the Hippocampus. J Neurosci. 2016;36:622–631. doi: 10.1523/JNEUROSCI.3625-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Arce K, Schrod N, Metzbower SW, Allgeyer E, Kong GK, Tang AH, Krupp AJ, Stein V, Liu X, Bewersdorf J, Blanpied TA, et al. Topographic mapping of the synaptic cleft into adhesive nanodomains. Neuron. 2015;88:1165–1172. doi: 10.1016/j.neuron.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier SM, Percherancier Y, Galandrin S, Breit A, Gales C, Bouvier M. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J Biol Chem. 2008;283:24659–24672. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado MP, Terry-Lorenzo RT, Waites CL, Garner CC, Malenka RC. Transsynaptic signaling by postsynaptic synapse-associated protein 97. J Neurosci. 2006;26:2343–2357. doi: 10.1523/JNEUROSCI.5247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders NR, Pao Y, Renner MC, da Silva-Matos CM, Lodder TR, Malinow R, Kessels HW. Amyloid-beta effects on synapses and memory require AMPA receptor subunit GluA3. Proc Natl Acad Sci U S A. 2016;113:E6526–E6534. doi: 10.1073/pnas.1614249113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MC, Albers EH, Gutierrez-Castellanos N, Reinders NR, van Huijstee AN, Xiong H, Lodder TR, Kessels HW. Synaptic plasticity through activation of GluA3-containing AMPA-receptors. elife. 2017;6 doi: 10.7554/eLife.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondard P, Pin JP. Dynamics and modulation of metabotropic glutamate receptors. Curr Opin Pharmacol. 2015;20:95–101. doi: 10.1016/j.coph.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sako Y, Kusumi A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol. 1994;125:1251–1264. doi: 10.1083/jcb.125.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria F, Gonzalez J, Augustine GJ, Raghavachari S. Quantifying the effects of elastic collisions and non-covalent binding on glutamate receptor trafficking in the post-synaptic density. PLoS Comput Biol. 2010;6:e1000780. doi: 10.1371/journal.pcbi.1000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci DM, Raghavachari S. The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput Biol. 2008;4:e1000208. doi: 10.1371/journal.pcbi.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt D, Nicoll R. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A, Klocker N, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Sergé A, Fourgeaud L, Hemar A, Choquet D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22:3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnen BL, Bowen AB, Forte JS, Hiester BG, Crosby KC, Gibson ES, Dell'Acqua ML, Kennedy MJ. Optogenetic control of synaptic composition and function. Neuron. 2017;93(646–660):e645. doi: 10.1016/j.neuron.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Fuccillo MV, Ko J, Sudhof TC, Malenka RC. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc Natl Acad Sci U S A. 2011;108:16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkaworn T, Jobin ML, Burnecki K, Weron A, Lohse MJ, Calebiro D. Single-molecule imaging reveals receptor-G protein interactions at cell surface hot spots. Nature. 2017;550:543–547. doi: 10.1038/nature24264. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proc Natl Acad Sci U S A. 1999;96:13801–13806. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Nakagawa S, Kushiya E, Yamasaki M, Fukaya M, Iwanaga T, Simon MI, Sakimura K, Kano M, Watanabe M. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Tulodziecka K, Diaz-Rohrer BB, Farley MM, Chan RB, Di Paolo G, Levental KR, Waxham MN, Levental I. Remodeling of the postsynaptic plasma membrane during neural development. Mol Biol Cell. 2016;27:3480–3489. doi: 10.1091/mbc.E16-06-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI. Elucidation of AMPA receptor-stargazin complexes by cryo-electron microscopy. Science. 2016;353:83–86. doi: 10.1126/science.aaf8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, Pennefather PS. A mathematical description of miniature postsynaptic current generation at central nervous system synapses. Biophys J. 1996;71:1256–1266. doi: 10.1016/S0006-3495(96)79325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtschanoff JG, Burette A, Davare MA, Leonard AS, Hell JW, Weinberg RJ. SAP97 concentrates at the postsynaptic density in cerebral cortex. Eur J Neurosci. 2000;12:3605–3614. doi: 10.1046/j.1460-9568.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P, Hoogenraad CC. New insights in endosomal dynamics and AMPA receptor trafficking. Semin Cell Dev Biol. 2011;22:499–505. doi: 10.1016/j.semcdb.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Volk L, Chiu SL, Sharma K, Huganir RL. Glutamate synapses in human cognitive disorders. Annu Rev Neurosci. 2015;38:127–149. doi: 10.1146/annurev-neuro-071714-033821. [DOI] [PubMed] [Google Scholar]
- Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol. 2011;13:40–48. doi: 10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Specht CG, Hartel K, Leal-Ortiz S, Genoux D, Li D, Drisdel RC, Jeyifous O, Cheyne JE, Green WN, Montgomery JM, Garner CC. Synaptic SAP97 isoforms regulate AMPA receptor dynamics and access to presynaptic glutamate. J Neurosci. 2009;29:4332–4345. doi: 10.1523/JNEUROSCI.4431-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, Nestler E, Aperia A, Flajolet M, Greengard P. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326:1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JF, Ho H, Greger IH. Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. elife. 2017;6 doi: 10.7554/eLife.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin L, Reuss M, Lindskog M, Aperia A, Brismar H. Nanoscopic spine localization of Norbin, an mGluR5 accessory protein. BMC Neurosci. 2014;15:45. doi: 10.1186/1471-2202-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, Sudhof TC. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature. 2017;544:316–321. doi: 10.1038/nature21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xie X, Liaw JS, Baudry M, Berger TW. Novel expression mechanism for synaptic potentiation: alignment of presynaptic release site and postsynaptic receptor. Proc Natl Acad Sci U S A. 1997;94:6983–6988. doi: 10.1073/pnas.94.13.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Structural contributions to short-term synaptic plasticity. Physiol Rev. 2004;84:69–85. doi: 10.1152/physrev.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell. 2016;166(1163–1175):e1112. doi: 10.1016/j.cell.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen S, Yoshioka C, Baconguis I, Gouaux E. Architecture of fully occupied GluA2 AMPA receptor-TARP complex elucidated by cryo-EM. Nature. 2016;536:108–111. doi: 10.1038/nature18961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Liu A, Xia S, Leung C, Qi J, Meng Y, Xie W, Park P, Collingridge GL, Jia Z. The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat Neurosci. 2018;21:50–62. doi: 10.1038/s41593-017-0030-z. [DOI] [PubMed] [Google Scholar]