Abstract
Recent advances in materials development and tissue engineering has resulted in a substantial number of bioinspired materials that recapitulate cardinal features of bone extracellular matrix (ECM) such as dynamic inorganic and organic environment(s), hierarchical organization, and topographical features. Bone mimicking materials, as defined by its self-explanatory term, are developed based on the current understandings of the natural bone ECM during development, remodeling, and fracture repair. Compared to conventional plastic cultures, biomaterials that resemble some aspects of the native environment could elicit a more natural molecular and cellular response relevant to the bone tissue. Although current bioinspired materials are mainly developed to assist tissue repair or engineer bone tissues, such materials could nevertheless be applied to model various skeletal diseases in vitro. This review summarizes the use of bioinspired materials for bone tissue engineering, and their potential to model diseases of bone development and remodeling ex vivo. We largely focus on biomaterials, designed to re-create different aspects of the chemical and physical cues of native bone ECM. Employing these bone-inspired materials and tissue engineered bone surrogates to study bone diseases has tremendous potential and will provide a closer portrayal of disease progression and maintenance, both at the cellular and tissue level. We also briefly touch upon the application of patient-derived stem cells and introduce emerging technologies such as organ-on-chip in disease modeling. Faithful recapitulation of disease pathologies will not only offer novel insights into diseases, but also lead to enabling technologies for drug discovery and new approaches for cell-based therapies.
1. Introduction
Animal models have greatly advanced our understanding of the origin and progression of many human diseases and tissue repair. The most widely used animal models are in mice (i.e. transgenic mice) that re-create many aspects of disease pathologies and have been a vital tool for basic understandings and discovery of new therapeutic targets and approaches. However, only a few of these models replicate all the underlying features mostly due to species differences in genetic background, and often fall short in predicting the pathophysiology of many human diseases [1]. On the other hand, employing human cells along with the necessary microenvironment (i.e., niche) could be an alternative approach; however the commonly used two-dimensional (2-D) cultures often do not replicate the complex three-dimensional (3-D) environment as well as the dynamic physicochemical cues of the microenvironment [2]. Improved ex vivo systems that facilitate modeling of human disease by providing key attributes of the native environment may overcome the drawbacks of both the animal studies and in vitro 2D cultures [3, 4]. To this end, efforts have been made to develop ex vivo cultures that are 3-D, with tissue-specific physicochemical cues including, but not limited to mechanical properties, chemical components, growth factor signaling, oxygen tension, and fluid flow [5–9].
“Bioinspired” materials are materials engineered to mimic the structure, properties, and function of naturally occurring extracellular matrices (ECM). Natural biological materials such as bone ECMs are hierarchically organized displaying nanoscale to macroscopic features and combine chemical and physical properties that integrate and perform specific functions at individual size scales. The multifaceted effects of these distinct properties of the ECM are manifested at the cellular, tissue, and organ level. This is important for skeletal tissues that are known to be functionally hierarchical, highly organized, and undergo coordinated spatiotemporal events during development and repair [10]. Over the years, a substantial number of biomaterials—decellularized bone tissues, ECM proteins and polysaccharides, and synthetic materials—have been developed as bone grafts or scaffolds for engineering bone tissues, which elicit the necessary cellular response that contribute to bone regeneration [11–14]. While decellularized bone ECM provides various physicochemical cues relevant to bone tissues, synthetic or hybrid biomaterials are modular, which means individual elements of the biomaterial can be manipulated independently to enable defined experimental conditions, relatively easy to manufacture, and reproducible.
Particular attention has been given to designing biomaterials that mimic different physicochemical attributes of bone ECM during bone developmental processes or homeostasis [15]. These bioinspired materials are fabricated by incorporating key factors that are important for bone formation and function. They can be categorized into their functionalized components based on inorganic substances in the form of minerals (e.g. calcium phosphate; CaP) or organic elements (i.e. collagenous and non-collagenous) [15], or matrix metalloproteinases (MMP)-sensitive materials [16]. While there are a substantial number of biomaterials used to engineer bone tissues to study bone metastasis [17] and bone repair [18], less attention has been given to their potential to study bone disorders. When combined with appropriate cells and/or under the right culture conditions (e.g. biochemical factors), these same materials could potentially be applied to develop ex vivo models recapitulating various attributes of bone diseases. Different cell populations such as bone marrow-derived MSCs (BM-MSCs) and pluripotent stem cells (embryonic stem cells, ESCs and induced pluripotent stem cells, iPSCs) have been used to engineer bone tissues ex vivo due to their ability to differentiate into osteoblasts [19–21]. Given the importance of BM-MSCs in bone repair and iPSCs in disease modeling, respectively, we will focus on studies involving these two cell populations. Based on our current understandings of bone ECM and bone biology in development and disease, we will outline various potential strategies available for disease modeling involving bioinspired materials, diseased cells, and induction protocols. In vitro platforms with increasing complexity, such as bone-on-a-chip and bioengineered systems, which integrate mechanical stimuli will also be discussed.
2. ECM and growth factors during bone development and remodeling
During development, bone is formed by either intramembranous (IM) or endochondral (EC) ossification [10]. Flat bones are formed by IM ossification in which neural crest-derived mesenchymal cells proliferate and condense into compact nodules and differentiate into osteoblasts. The osteoblasts secrete a collagen-proteoglycan matrix (osteoid), which mineralizes to form calcified tissue. On the other hand, long bones are formed by EC ossification. Longitudinal growth of skeletal bones is characterized by EC ossification with ordered zones of proliferating and differentiating chondrocytes in the growth plate. These processes are characterized by distinct ECM composition and properties. The ECM in the growth plate is composed of type II, IX, X, and XI collagen, large proteoglycans (aggrecan) that contain glycosaminoglycans (GAGs; chondroitin sulfate), hyaluronic acid (HyA), and other molecular components such as matrilins, cartilage oligomeric matrix protein, and MMPs [22, 23]. The proteoglycans interacts noncovalently via hyaluronan-binding motifs with HyA, an important nonproteoglycan polysaccharide [24]. The most abundant collagen in the growth plate cartilage is type II collagen [22], a homotrimer composed of three identical α1(II) collagen chains encoded by the COL2A1 gene [25]. Hypertrophic chondrocytes in the growth plate secrete collagen type X and also induces expression of the collagenase matrix metalloproteinase-13 (MMP13), which is a prerequisite for invasion of blood vessels, osteoclasts, and osteogenic cells to form ossification and maturation of bone [23].
Bone is a highly dynamic tissue constantly undergoing remodeling throughout the lifetime of an individual [26]. The function and homeostasis of bone tissues are maintained by highly coordinated activities of cells residing within the bone organ, which include osteoblasts, osteocytes, and osteoclasts; 90% of cells in mature bone tissue are osteocytes [27–29]. Once bone tissue is formed, osteoblasts get trapped within the mineralized ECM and transform into osteocytes. In addition to osteoblasts and osteocytes, bone tissue also contains multinucleated osteoclasts, which are formed by the fusion of mononuclear progenitors of macrophages. The osteoclasts degrade the bone matrix by creating an acidic microenvironment that liberates Ca2+ and PO43- ions during bone remodeling [30]. Bone remodeling is a highly coordinated process involving communication between multiple cell types present in the bone tissue. Osteoblasts can affect osteoclast formation, differentiation, and apoptosis through OPG/RANKL/RANK, RANKL/LGR4/RANK, Ephrin2/ephB4, and Fas/FasL pathways. Conversely, osteoclasts influence bone formation by osteoblasts via the d2 isoform of the vacuolar (H+) ATPase (v-ATPase) V0 domain (Atp6v0d2), complement component 3a, semaphorin 4D, microRNAs, and growth factors, released from the resorbed bone matrix, such as TGF-β and IGF-1 [31]. Each of the processes involves a complex spatiotemporal progression at the molecular, cellular and tissue level where the cells, soluble factors, and ECM have defined roles. In addition to the bone tissue specific cell types, other cells that are involved in bone development and remodeling, include endothelial cells of the vasculature, peripheral neurons, and immune cells of the hematopoietic system [32–36].
Collagenous and non-collagenous proteins make up the organic components in mature bone while minerals make up the inorganic components. Type I collagen, a triple-helical molecule containing two identical αl(I) chains and a α2(I) chain, is the major collagenous protein in bone [37]. Collagen α chains are modified by several post-translational modifications including hydroxylation of lysyl or prolyl residues, glycosylation of hydroxylysine with galactose and glucose residues, and formation of intra- and intermolecular covalent crosslinks [38]. Collagen molecules are ~1.5 nm in width that co-assemble laterally to form a microfibril. Multiple microfibrils further assemble to form a fibril of ~50-300 nm in width. [39, 40]. In the periodic 67 nm cross-striated pattern of the collagen fibril, the less dense 40 nm-long gap zone has been implicated as the place where apatite crystals nucleate to form an amorphous phase, and subsequently grow and spread through the fibrils, leading to arrays of HA nanocrystals embedded within and oriented along the collagen fibrils, forming intrafibrillar mineralization [41–45]. Following intrafibrillar mineralization, HA crystals also grow on the surface of the collagen fibrils, leading to extrafibrillar mineralization [46].
In addition to type I collagen, trace amounts of type III, V, and VI may be present during certain stages of bone formation and may regulate collagen fibril diameter [47–49]. On the other hand, non-collagenous proteins comprise 10-15% of the total protein content in bone. These include various families of proteins encompassing serum-derived proteins (e.g. albumin), matricellular proteins (e.g. thrombospondins), small integrin-binding ligand-linked glycoprotein (SIBLING; e.g. bone sialoprotein), carboxylated proteins (e.g. osteocalcin), small leucine rich proteoglycans (SLRP; e.g. biglycan), and MMPs [50, 51]. Among the MMPs, MMP-2, MMP-9, MMP-13, and MMP-14 have a major role in modulating bone remodeling [52]. A series of growth factors are also involved in bone development and fracture repair[53, 54], including Bone Morphogenic Protein (BMP) [55], Vascular Endothelial Growth Factor (VEGF) [56], Notch [57, 58], Wnt [59], Sonic hedgehog [60], Indian hedgehog [61], Transforming Growth Factor β (TGFβ), Fibroblast Growth Factor (FGF) [62], Platelet-derived Growth Factor (PDGF) [63], and Insulin-like Growth Factor (IGF) [64]. Amongst the many growth factors, BMPs are the most important growth factors for bone formation during embryogenesis and in postnatal tissues.
Bone is a unique tissue in which one of the main ECM component is inorganic mineral and hence structurally bone ECM can be characterized as a composite. The apatite-like mineral phase of the bone ECM is around 60-70% dry weight depending upon the site and stage of development [37]. In addition to calcium and phosphate, the mineral phase of the apatite contains various substituents such as carbonate, magnesium, and acid phosphate [51]. There is a substantial heterogeneity with respect to crystallinity and the mineral content of bone tissue. The initially nucleated crystals are amorphous CaP (ACP) [65–67] that matures to crystalline HA minerals [41, 43, 68]. Thus, with time, bone mineral develops from a poorly crystalline apatite with high HPO42- content to a mineral with more organized structure of higher crystallinity, more carbonate substitutions, and lower acid phosphate content [69–71].
3. Cells sources to engineer bone tissues ex vivo
Various stem cell populations such as pluripotent and multipotent stem cells have been extensively used to engineer bone tissues due to their ability to differentiate into bone forming cells, osteoblasts [19, 20, 72]. Amongst them multipotent BM-MSCs have been extensively studied towards engineering bone tissues. BM-MSCs from patients with skeletal pathologies have also been used to determine the cellular changes associated with various bone diseases [73, 74]. The ability to derive induced pluripotent stem cells (iPSCs) from somatic cells has opened up immense opportunities to model developmental and hereditary genetic disorders in vitro [75]. Stem cells are suitable for disease modeling because they 1) allow relatively high cell number for experimental manipulation, 2) recapitulates disease progression during commitment, differentiation, and maturation, and 3) can be isogenic and allows differentiation into multiple lineages to study the contribution of cell-matrix and cell-cell interactions. Patient-derived cells from bone diseases could model to illuminate the dynamic epigenetic, transcriptional/post-transcriptional, and translational/post-translational processes during different stages of disease progression. Disease modeling of monogenic diseases can be readily achieved by using iPSCs [76, 77] because they are cell autonomous and usually have defined readouts reflecting the pathophysiology of the affected cells. In contrast, developing in vitro models of most diseases [78, 79], require complex systems, such as multiple cell populations that can organize into rudimentary organ subunits and tissue specific extracellular cues. Recent efforts in the area of organoids and organ-on-chip systems have allowed the modeling of various human diseases with relevant pathophysiology.
3.1. iPSCs derived from patients with skeletal disorders
A number of iPSC lines are established from patients with rare genetic disorders. Osteogenesis imperfecta (OI) is caused by mutations in the COL1A1 or COL1A2 genes. To understand the cardinal features of the diseases ex vivo, iPSCs have been derived from patients with OI and the corresponding gene-corrected cells [80]. In OI, the folding process of collagen into a triple helical structure is disrupted resulting in an abnormal collagen protein structures. The most common type of mutations observed in OI are single base changes that lead to the replacement of one Gly in the (Gly-Xaa-Yaa)n repeating sequence of the triple helix by another amino acid residue. Such changes are postulated to affect α2β1-integrin binding [81], which could have significant impact in cell-matrix interactions. Another disease with bone abnormalities is craniometaphyseal dysplasia (CMD), which is a rare disorder characterized by progressive hyperostosis of craniofacial bones and widened metaphyses of long bones. Patients with CMD suffer from deafness, blindness, facial paralysis and severe headache due to hyperostosis and compression of the brain. Mutations of the autosomal dominant form have been identified in the progressive ankylosis gene (ANKH), a pyrophosphate (PPi) transporter that channels intracellular PPi into extracellular matrix, and in Connexin 43 (Cx43) for a recessive form [82, 83]. Extracellular PPi acts as a potent inhibitor of mineralization, thus influencing the ECM properties. Furthermore, Cx43 is a main component of gap junctions in osteoblasts, osteocytes, osteoclasts. Cx43 are required for osteoblast and osteoclast differentiation and function [84], and the compromised Cx43 could significantly limit the cell-cell communication needed for osteoclastogenesis and bone remodeling. Studies showed that hiPSCs with ANKH mutations formed fewer osteoclasts, expressed lower levels of osteoclast specific genes, and resorbed less bone [85]. Another disease with iPSCs generated from patients is Marfan syndrome (MFS), a life-threatening, autosomal dominant disease with mutations identified in Fibrillin-1 (FBN1) microfibrils and elastic fibers [86]. FBN1 sequesters BMP and TGF-β in the ECM and its aberrant changes during MFS disease affects their bioavailability, leading to premature depletion of MSCs and osteoprogenitor cells and enhanced bone resorption [87–89]. Skeletal abnormalities include long limbs and digits, deformities of vertebrae and anterior chest, and increased height. hiPSCs generated from MFS patients were found to faithfully represent pathologic skeletogenesis associated with MFS and impaired osteogenic differentiation due to activation of TGF-β signaling and its crosstalk with BMP signaling [90, 91].
Similarly, several iPSCs from patients with developmental disorders related to EC ossification have been derived. Individuals with a lethal form of metatropic dysplasia have aberrant EC ossification and cortical bone morphology, caused by a dominant mutation in the calcium channel gene TRPV4. TRPV4 act as a transducer of mechanical loading to regulate cartilage ECM biosynthesis [92]. Another pathology related to compromised EC ossification is Thanatophoric dysplasia type 1 and achondroplasia caused by gain-of-function mutations in the FGFR3 gene. Accumulation of FGFR3 protein suppresses the differentiation and proliferation of chondrocytes [93], and patients manifest symptoms such as femoral bowing, short limb stature, and skeletal dysplasia. Expression profiling in FGFR3-related chondrodysplasias revealed about 8% of the modulated genes are related to extracellular matrix (ECM) structure and dynamics, including those coding the basement membrane or ECM structural components, aggrecan turnover, glycosaminoglycan (GAG) and proteoglycan biosynthesis, diversification and sulfation. Genes involved in cell-cell interaction or adhesion, including CD44, NCAM1, integrins, cadherins and protocadherins are also affected [94]. Fibrodysplasia ossificans progressive (FOP) is another rare, debilitating genetic disease caused by constitutive activation of the ACVR1 gene [95]. Mutations in the ACVR1 gene lead to overgrowth of bone and cartilage and fusion of joints. Concurrent with disease manifestation, FOP-derived iPSCs exhibited enhanced mineralization and chondrogenesis in vitro [96].
iPSCs have also been generated from patients with rheumatoid arthritis [97] and osteoarthritis [97, 98]. Although bone loss is a secondary symptom as a consequence of immune-related dysfunction, integration of hiPSC-derived organoids with diseased immune cells and an arthritic environment (biochemical and biomechanical) could lead to disease models in vitro. Bioreactors involving mechanical loading and organ-on-chip platforms could be used to establish multifaceted complex environments pertaining to the diseases to study secondary conditions and the influence of immune environment on disease maintenance and progression.
3.2. MSCs isolated from bone diseases
Given the inherent potential of MSCs to contribute to adipose, cartilage and bone tissues [99–103], they have been extensively studied towards their application to regenerate skeletal tissues and their function during skeletal diseases [74, 99, 104–108]. Cell studies comparing the differentiation potential of MSCs derived from osteoporotic patients with that of normal MSCs have shown dysregulated osteogenic and adipogenic processes, involving increased adipocyte formation, along with reduced differentiation potential into osteogenic cells [73]. Osteoporotic MSCs demonstrate decreased TGF-β production, as well as decreased capacity to synthesize and maintain a type I collagen-rich ECM. Furthermore, these cells had diminished alkaline phosphatase activity and less calcium deposition, and reduced ability to form mature bone cells [104]. Dysregulated cell-cell interactions between osteoblasts, osteocytes and osteoclasts regulating the RANK/RANKL, osteoprotegerin, Wnt/sclerostin pathways contribute to disease progression [109]. Autoimmune diseases such as rheumatoid arthritis (RA) and its management using glucocorticoids, increase the risk for bone loss [106]. MSCs isolated from patients with RA display impaired inhibition of Th17 cells. Th17 cells secrete IL-17, an important cytokine that induces osteoclast differentiation and bone resorption [74, 107]. These studies demonstrate the potential of MSCs derived from patients to study diseases of bone loss by re-creating tissue specific environments ex vivo.
4. Bioinspired materials for bone disease modeling
Substantial knowledge of skeletal development, fracture healing, and diseases have identified various key ECM molecules/properties and growth factors that contribute to bone formation, as well as maintenance of tissue homeostasis [10]. These understandings have enabled design of biomaterials with chemical and physical cues as well as growth factor immobilization to direct cellular functions relevant to bone tissue formation. To our knowledge, there are no studies directly employing bioinspired materials to study bone disorders in development and remodeling. Most bioinspired materials developed so far are used as grafts for bone repair or as scaffolds (i.e., artificial ECM) to engineer bone tissue surrogates [17, 18, 110–113]. However, these approaches can be easily extended to create ex vivo models of bone disorders in development and remodeling. Bioinspired materials can contribute to bone disease modeling either as an (i) active component, or (ii) passive component. In the case of an active component, the bioinspired materials can be used to model and study the role of ECM regulation in bone tissue functions and how perturbations in these interactions could contribute to bone diseases. In the latter case, the bioinspired materials can be used as a scaffold to create functional bone tissues from diseased cells. In this section we discuss the role of bioinspired materials in bone tissue engineering and their potential contributions to disease modeling.
4.1 Bioinspired materials to mimic biochemical cues
4.1.1 CaP and collagen-based materials
CaP-based biomaterials are the most commonly used scaffolds for bone tissue engineering. CaP-based materials are either osteoinductive, osteoconductive, or a combination of both. A comprehensive list of CaP-based biomaterials for bone tissue repair is summarized elsewhere [18]. CaP-biomaterials such as ceramics have been used for bone and dental restorations [114]. Composite materials containing both organic phase and inorganic CaP minerals have been developed by physical dispersion of CaP particles [115–121] or through biomineralization [122, 123]. Biomineralization is a naturally occurring process that supports mineralization of living systems. Biomineralization requires functional groups of the organic matrices to nucleate CaP minerals and support their growth. A number of factors such as structure, hydrophobicity and functional groups of the organic template has been shown to play a key role in promoting biomineralization [41, 124–128]. Similarly, the crystalline nature of CaP minerals can be controlled through different experimental parameters such as the pH, temperature, and reactants [114].
Another widely used material for bone tissue formation is collagen, individually or as hybrids with calcium phosphate minerals. Several physical forms of collagens were developed over the years, including injectable hydrogels, membranes, or sponges with a number of materials as commercialized products. Synthetic materials that combine type I collagen with HA or other forms of CaP, have also been developed [129–131]. Some examples include, injectable collagen hydrogels in the form of composites such as collagen/HA/chitosan or collagen/HA/alginate [132, 133]. In addition to this review, we suggest literature summarizing collagen materials for bone tissue engineering elsewhere [134]. Besides collagen, collagen-derived peptides are also heavily used in bone tissue engineering. For example, collagen-derived peptides Ala-Gly-Glu-Ala (DGEA) that engage with α2β1 integrin was used to improve the biofunctionality of alginate hydrogels. MSCs cultured with the help of these hydrogels exhibited increased levels of osteocalcin expression and mineral deposition [135]. In fact, many of the collagen-based products are commercialized for bone tissue repair [136–138]. These include collagen sponges such as Collagraft™ that combines HA/TCP with bovine collagen. INFUSE® Bone Graft is a recombinant human bone morphogenetic protein-2 (rhBMP-2) applied to an absorbable collagen sponge carrier for lumbar spine fusion, tibial fracture repair, and maxillofacial and dental bone grafting. OssiMend™ Bone Graft Matrix combines porous mineral with collagen for use in orthopaedic and spinal surgery.
4.1.2. CaP/Collagen-based biomaterials for disease modeling
Osteoinductive biomaterials such as CaP-based materials can induce osteogenic differentiation of stem cells in vitro and also support neo-bone tissue formation through recruitment of endogenous cells [20, 101, 139, 140] without the addition of exogenous osteoinductive factors. Thus, these materials offers a tool to engineer ex vivo bone tissue surrogates either through the use of cells derived from patients or healthy individuals. For example, patient-derived diseased stem cells can be in vitro cultured in 3-D osteoinductive materials (e.g. TCP, BCP) to undergo material-induced osteogenic commitment and mineralization to study IM ossification (Figure 1A). Addition of biochemical factors and immune cells could further be used to replicate diseases that arise from systemic biochemical or cellular factors such as hyperparathyroidism, treatment-induced bone loss, or autoimmune diseases [141–143]. Moving forward, the cell-laden constructs could be implanted in vivo in immune-compromised animals to undergo vascularization and host cell infiltration (e.g. hematopoietic progenitors, osteoprogenitors) [144–146] and establishment of bone remodeling. In addition, disease models can also be established with osteoconductive materials (e.g. HA, collagen) with diseased cells, where the latter often requires addition of exogenous osteoinductive molecules such as chemicals or BMPs, for osteogenesis and mineralization (Figure 1B). Such tissue-engineered platforms could be used to decouple the contribution of ECM, cells, and microenvironmental cues on disease progression and maintenance.
Figure 1.
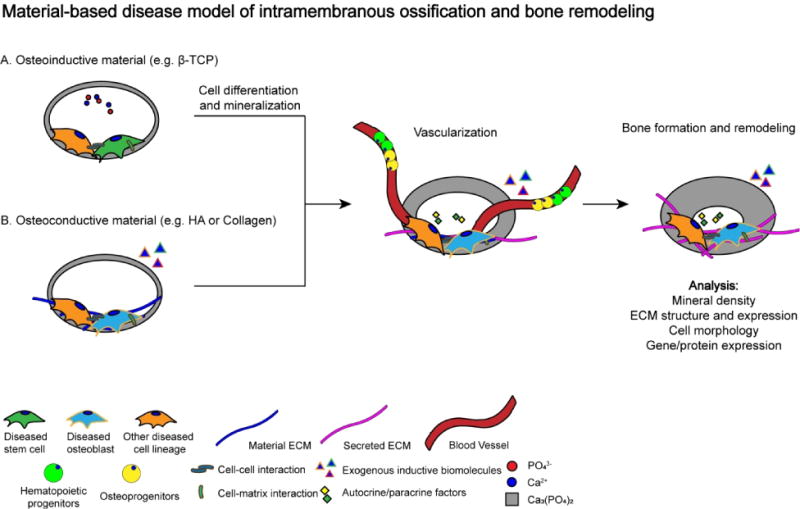
Proposed disease model of material-based intramembranous ossification and bone remodeling. (A) Diseased bone formation using osteoinductive material (i.e. β-TCP, BCP). (B) Diseased bone formation using osteoconductive material (i.e. HA, collagen). Diseased stem cell: iPSC, ESC, BMMSC. Other diseased cell lineage: pre-osteoclast, immune cells, endothelial cell etc.
4.1.3 Bioinspired materials to study disorders involving endochondral ossification
During skeletal development and repair, EC ossification remains a key biological process with the involvement of cartilage formation. Development through EC ossification and fracture healing processes are spatiotemporally dynamic and do not completely resemble the homeostatic native bone ECM [147, 148]. This lack of resemblance suggests that materials design aimed at mimicking the composition of mature bone ECM (mainly CaP minerals and collagens) may be insufficient to faithfully recreate the process of EC ossification. Biomaterial designs that leverage the temporal ECM changes during the developmental process have the potential to recapitulate EC ossification. Specifically, materials that facilitate the initial inflammatory or cartilaginous tissue formation in the early reparative stage of fracture healing may be used. In order to achieve EC ossification, the materials should support the underlying biological features including hypertrophic chondrocyte growth and eventual bone tissue formation. Most of the traditional tissue engineering steps involving stem cells include: 1) priming with chondrogenic supplements for a cartilage template, 2) hypertrophic conditions with thyroxine, β-glycerophosphate, dexamethasone, ascorbic acid, and IL-1β to generate hypertrophic cartilage, and finally 3) implanting subcutaneously into immune-deficient mice for calcification. This strategy, referred to as “developmental engineering”, is envisioned to be more adequate for bone regeneration by following the highly regulated spatiotemporal stages of EC ossification. Although a number of different biomaterials are used for chondrogenic differentiation [149], we refer only to those used specifically for studies related to EC ossification. Some materials have been found to better facilitate the process over others. For example, increased EC ossification foci were observed in human demineralized bone (DBM) implants suspended in a HyA carrier compared with DBM implants suspended in a glycerol solution [150]. In vitro priming of MSCs within collagen/HyA scaffolds supported significantly more cartilage tissue formation and VEGF production in vitro, and led to more bone formation within critical-sized bone defects compared to collagen/hydroxyapatite materials [151]. HyA is known to be a major component of cartilage ECM [152] and, therefore, could play a regulatory role in both chondrogenesis and EC ossification [23]. Alginate, chitosan, and fibrin hydrogels were used for chondrogenesis and hypertrophy of MSCs in vitro and EC ossification in vivo. Both alginate and fibrin constructs facilitated vascularization, endochondral bone formation, as well as the development of a bone marrow in vivo. Alginate constructs accumulated significantly more mineral and supported greater bone formation especially in the central regions of the constructs [153]. To shorten the in vitro chondrogenic priming period, MSC micropellets were mixed with fibrin that demonstrated comparable bone formation to standard pellets implanted in vivo [154]. It is interesting to note that although fibrin participates during early stages of bone repair, it was determined as nonessential for fracture repair [155]. Other studies have recapitulated EC ossification by mixing MSCs with ECM components such as collagen-GAG [156, 157], type I collagen mesh/IL-1β [158, 159], hyaluronan-gelatin [160], gelatin methacrylate embedded with cartilage-derived matrix particles [161], collagen/HyA acid [151], collagen/HA [151], and HA/TCP composites [162]. Using an alternative strategy, endochondral bone formation by scaffold-free constructs was developed that comprised of human BM-MSCs embedded with bioactive microparticles for controlled delivery of TGF-β1 and BMP-2. The microparticles were formulated to release TGF-β1 to induce cartilage formation followed by BMP-2 in a sustained manner to promote remodeling into bone. Microparticle-incorporated constructs with both TGF-β1 and BMP-2 promoted the most bone formation and bone bridging compared to those with no growth factor or single growth factor [163].
In order to model skeletal diseases due to aberrant endochondral ossification, biochemical cues are required, while materials serve as scaffolds that provide the necessary 3-D environment and ECM-based physicochemical cues to support chondrogenesis, followed by hypertrophy and bone tissue formation (Figure 2). Upon culture, the embedded cells secrete their own ECM as a function of time. The intermediate tissue-engineered hypertrophic cartilage can also be implanted into immune-deficient animals to promote vascularization and eventual bone formation (Figure 2). While in vivo implantation forms ossification, however, to avoid xenogenic effects, more efforts should be emphasized to establish methods that enable abundant in vitro calcification similar to the levels achieved in vivo. Such an in vitro approach could limit the artifacts, if any, involved with in vivo transplantation of human cells into mice recipients. A recent study demonstrated that large 3-D MSC ball-like constructs cultured under a hypoxia condition formed region-specific chondrogenic differentiation and mineralization within the cartilage tissue in vitro [164], suggesting that all stages of EC ossification could be recapitulated in vitro.
Figure 2.
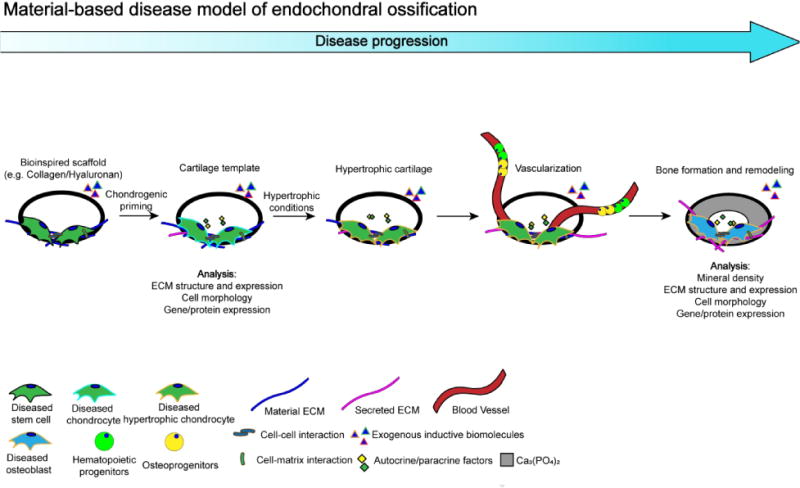
Proposed disease model of material-based endochondral ossification. Diseased bone formation through endochondral ossification using bioinspired scaffold (i.e. collagen, hyaluronan, fibrin). Diseased stem cell: iPSC, ESC, BMMSC. Other diseased cell lineage: pre-osteoclast, immune cells, endothelial cell etc. Diseased cell: osteoblast.
4.1.4 CaP materials to study bone mineral environment
The mineral environment of the bone tissue has been touted to pay a key role in maintaining tissue homeostasis and repair. Indeed, bone mineral density (BMD) serves as a marker for bone health. The mineral environment of native tissue is very dynamic due to tissue remodeling. Such a dynamic environment can be mimicked ex vivo, in the absence of osteocytes and osteoclasts, through the crystalline nature of the CaP minerals. The dissolution of CaP minerals to Ca2+ and PO43- ions often results in their supersaturation, leading to subsequent precipitation of Ca2+ and PO43- ions onto CaP minerals [114, 165]. Such processes, termed as dynamic dissolution-precipitation, govern the local levels of extracellular Ca2+ and PO43- ions and has been shown to regulate osteogenic commitment of stem cells [101, 102, 166]. A study by Germaini et al. compared the functions of carbonated HA (CHA) with that of HA and results suggest that carbonated HA has distinctive effects on osteoblasts and pre-osteoclasts compared to HA materials [167]. Studies have demonstrated TCP undergoes higher dissolution rates compared to HA [165, 168], which correlates with their ability to promote osteogenic differentiation of MSCs [169] and bone regeneration [168]. However, excessive dissolution of CaP moieties from ACP surfaces adversely affect osteogenic differentiation of MSCs compared to crystalline CaP surfaces [170, 171]. This is further supported by a recent study which reported the osteostimulatory effect of exogenous Ca2+ and PO43- supplements on MSCs observed within a stringent concentration range [172]. Together the findings suggest that only those mineralized materials that maintain a tightly regulated and optimal extracellular concentration of extracellular Ca2+ and PO43- can support osteogenic differentiation of stem cells.
Several pathways have been postulated regarding the contribution of CaP-materials assisted osteogenic differentiation and bone regeneration through extracellular Ca2+ and PO43 ions [102, 169, 173, 174]. Ca2+ ions have been shown to promote osteogenic differentiation of MSCs through L-type voltage-gated calcium channels (L-VGCC) [173, 174]. BMP2 signaling is regulated by calcium phosphate ions. For example, BMP2 expression increases when cells are cultured in presence of TCP, calcium phosphate crystals, and Ca2+ [175–177]. The dissolution- precipitation of CaP minerals could facilitate various biological signaling through the adsorption/sequestration of growth factors and their subsequent release, including BMP2 [178–180]. Our studies using biomineralized materials and BM-MSCs have shown that phosphate ions released from the materials, due to dissociation of CaP minerals, promote osteogenic differentiation of progenitor cells via SLC20a1-ATP-adenosine signaling pathway (Figure 3) [102, 181]. In addition to promoting the osteogenic differentiation, cells on these materials undergo limited adipogenic differentiation [181]. This CaP-material mediated inhibition of adipogenic differentiation could be reversed through adenosine A2B receptor signaling intervention [181]. Emerging studies implicate the importance of purinergic P1 and P2 family of adenosine and adenosine triphosphate (ATP) receptors in bone and cartilage functions [182–184]. Reflecting the findings from biomaterial studies, mouse models with knockout of adenosine A2B receptor showed low bone mass, delayed fracture repair with MSCs displaying diminished osteogenic differentiation [185] and bone formation [186]. That the in vivo findings mirror the in vitro findings using biomineralized materials suggest the potential of bioinspired materials as an active component to study bone disorders and the underlying mechanisms ex vivo. Findings from materials study could be used to investigate whether alterations to SLC20a1-ATP-adenosine signaling occurs in diseased patient cells. Furthermore, disorders of mineral metabolism including hyperphosphatemia/hypophosphatemia and abnormal calcium regulation can potentially be studied using such materials. Osteoinductive CaP-based biomaterials could thus be used as an in vitro tool to explore and unravel the molecular mechanism through which the mineral environment of bone ECM maintains bone tissue regeneration and homeostasis (Figure 4A). CaP-based materials could also be prime tools to study diseases of bone loss or excess bone formation associated with abnormal bone remodeling. In native bone tissue, in situ bone resorption by osteoclasts creates local increases in the extracellular calcium [187] (and phosphate), which could be important for osteoblast formation during remodeling.
Figure 3.
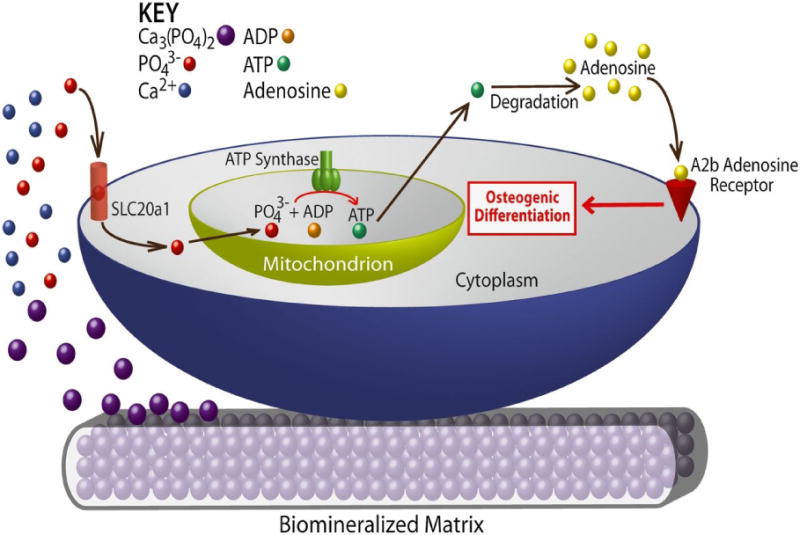
Schematic model of mineralized matrix-induced osteogenic differentiation through inorganic phosphate (adapted from Ref. [102]).
Figure 4.
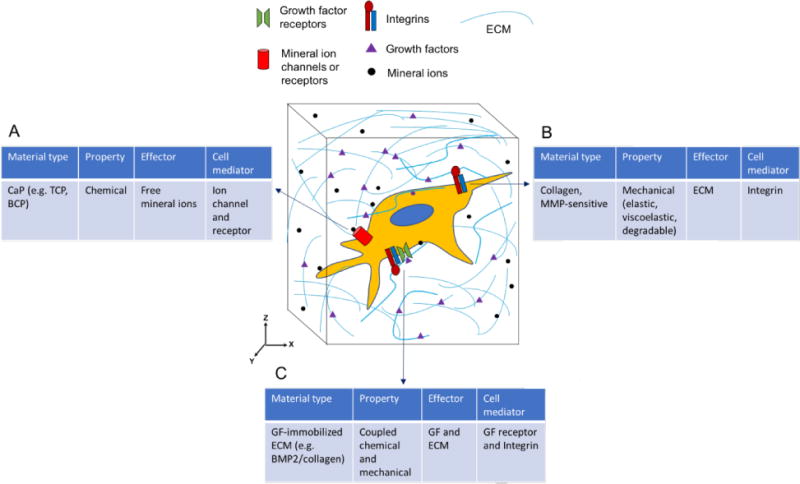
Types of materials to explore molecular targets involved during osteogenesis. (A) Osteoinductive calcium phosphate materials release mineral ions to bind ion channels or receptors. (B) Mechanical properties of ECM modulate integrin and mechanotransduction. (C) Immobilized growth factor (GF) on ECM couples chemical and mechanical signaling through direct interaction between GF receptors and integrins.
4.2 Bioinspired materials to mimic biophysical cues
Besides biochemical cues, collagen-based biomaterials also offer an ECM-like nanofibrous topography and facilitate ECM degradation by endogenous enzymes that promote tissue remodeling. The mechanical property of the biomaterials determines cell behaviors in spreading, polarization and differentiation by modulating mechanotransduction. The influence of mechanical properties of the biomaterial on osteogenic differentiation of stem cells and progenitor cells have been extensively studied [188–194]. Cell behaviors such as spreading, polarization, proliferation, and differentiation are determined by integrins sensing the mechanical cues of ECM to modulate mechanotransduction [195–199]. Integrin associated complexes connect ECM proteins through integrin transmembrane receptors with the intracellular actin cytoskeleton. Hydrogels are attractive tools for studying mechanobiology given that their mechanical properties can be easily tuned to achieve the targeted values and properties such as linear elasticity. Most hydrogels are non-adhesive and to improve the cell-matrix interaction they are often coated with ECM proteins or functionalized with peptide units such as integrin-binding ligands [195–198]. The ability of MSCs to differentiate into osteoblasts is increased on relatively more rigid materials [200]. hMSC differentiation is also impacted by stress stiffening in nonlinear elastic materials [201]. This phenomenon was found to affect through a previously unknown stress-stiffening-mediated mechanotransduction pathway involving microtubule-associated protein DCAMKL1. Unlike most hydrogels that are used ex vivo, natural ECMs such as collagens are viscoelastic. Using viscoelastic hydrogels with dynamic mechanical cues, recent studies have shown materials with relatively faster stress-relaxation increased cell spreading, proliferation, osteogenic differentiation of MSCs, and bone formation [202, 203]. In diseases such as OI, absence of α2(I) chains in the collagen fibrils correlate with reduced stiffness, as well as a reduced failure strength of collagen fibrils [204]. Materials mimicking or allowing control of the dynamic physical properties of ECM proteins [205] can thus be applied to unravel unknown mechanotransducers that are potentially relevant to in vivo disease settings (Figure 4B).
4.3 Cell-responsive materials to mimic ECM degradation
A number of synthetic materials has been employed towards bone tissue engineering [21, 170]. While such synthetic materials offer control over many material properties, they lack degradation which is important in bone tissue formation and remodeling. MMPs are highly involved in bone development, remodeling, and disease [52, 206]. By leveraging this native biological process, linear oligopeptide substrates of MMPs were crosslinked with poly(ethylene glycol) (PEG) macromers functionalized with integrin-binding domains to create 3-D scaffolds for bone tissue regeneration [16]. When physically bound with BMP-2, the MMP-degradable hydrogel promoted cell infiltration and enhanced bone regeneration in a critical rat cranium defect. Furthermore, MMP-sensitive hyaluronic acid (HyA)-based hydrogel was used for BMP-2 delivery where maleimide-modified HyA macromers were crosslinked with difunctional MMP-sensitive peptides to permit protease-mediated hydrogel degradation and BMP-2 release [207]. Degradation of MMP-responsive materials can be used to study the effect of MMP on dynamic self-regulation of cells. For example, secretion of MMP by cells degrade the surrounding ECM, which could affect cell-matrix interaction by regulating signaling related to cell spreading, de novo ECM secretion, growth factor release; all of which influence osteogenesis and bone formation. Diseased cells involved in bone development can be cultured in MMP-responsive materials to explore MMP function and related targets, in diseases previously unknown to involve MMPs. Similarly, cell-responsive disulfide-modified PEG hydrogels that undergo tunable degradation in response to extracellular molecules with thiol groups [208] can be applied to study the effect of matrix degradation on ECM dynamics (Figure 4B).
4.4 Bioinspired materials with immobilized growth factors to explore coupling of biochemical cues and mechanotransduction
In native tissue, growth factors are bound to the ECM, as both proteins and GAGs have growth factor binding sites [209]. Growth factors can also be sequestered by the ECM through electrostatic interactions [210]. Towards this, many growth factors or their derivatives have been covalently tethered or sequestered through physical interactions to collagen or CaP based materials [211–216]. BMPs are the most frequently used growth factor for bone tissue repair owing to its osteoinductive properties. To anchor BMP molecules onto collagen-based scaffolds, The CBDs have been incorporated into recombinant BMP2 [217, 218], BMP3 [219], and BMP4 [220, 221]. When coupled with collagen carriers, these CBD-BMP fusion proteins were found to stimulate greater bone formation compared to materials soaked with recombinant BMPs. In contrast to collagen-based scaffolds, CaPs offer minimal functional groups for covalent bonding. Thus, efforts have been focused on engineering BMP-mimetic peptides with a CaP-binding domain to improve their anchoring to CaP carriers. In one such instance, a modular peptide containing a BMP2 mimetic sequence was fused with an HA-binding domain of a γ-carboxyglutamate-rich motif of osteocalcin [222]. HA biomaterials functionalized with this modular peptide promoted osteogenic differentiation of MSCs in vitro, and enhanced bone repair in vivo [223, 224]. To circumvent the undesirable off-target effects of BMP2 in the surrounding tissue, peptide mimics of BMP2 were covalently bound to alginate hydrogels to enable local presentation and to control stem cell differentiation [225].
Angiogenesis is key to bone formation and is influenced by angiogenic factor VEGF [226]. New blood vessels bring oxygen and nutrients, as well as inflammatory and bone precursor cells to reach the regenerating tissue. Hence, several materials encoded with VEGF were used for bone tissue formation and repair [227–229]. To improve the coupling of recombinant VEGF to CaP carriers, a co-precipitation method for VEGF deposition onto BCP ceramics was developed [230]. Compared with superficially adsorbed VEGF, co-precipitated BCP/VEGF exhibited reduced VEGF burst release, and showed enhanced vascularization and osteointegration when implanted into mouse cranial defects [230]. Apart from these activities, a peptide termed “QK” that mimics the region in the VEGF binding interface to VEGF receptor, was synthesized with an osteocalcin-derived HA-binding domain. This peptide-coated HA microparticle was found to stimulate endothelial cell proliferation and migration [231]. When implanted intramuscularly into sheep, the HA-binding QK peptide that was coated onto β-TCP disks, through immersion, stimulated significantly higher blood vessel ingrowth [232]. A synthetic bone graft involving β-TCP matrix combined recombinant PDGF has been approved by the FDA as a dental product (GEM21S®). Similar to BMPs, PDGF molecules has been engineered with CBD domains to enhance anchoring to collagen-based scaffolds [215]. In another study, heparin was first crosslinked onto collagen-rich demineralized bone matrix (DBM), and recombinant PDGF was attached to the heparin-DBM via the heparin binding domains of PDGF [233]. Furthermore, by modeling natural growth factor-ECM interactions, collagen has been covalently affixed with heparin, followed by the noncovalent binding of growth factors that naturally bear heparin-binding domains [234, 235].
When combined with substrates of varying mechanical property, the direct interaction between GF receptors and integrins on mechanotranduction and cell behavior, and possibly disease, can be studied. It has been shown that integrin α1β1 and bone morphogenetic protein receptor (BMPR) IA formed a complex and co-localized in several cell types [236] and the coupling of physical and biochemical signaling was investigated on how growth factors were presented to cells [237]. For example, murine C2C12 cells on stiff surfaces responded to both matrix bound BMP‐2 and soluble BMP‐2 in the culture media while that on softer matrices responded only to matrix bound BMP‐2. Matrix bound BMP-2 was sufficient to induce β3 integrin–dependent C2C12 cell spreading by overriding the low mechanical rigidity of the biomaterial (i.e., soft matrices). This phenomenon is due to convergence of BMP receptor and β3 integrin signaling that control both focal adhesion dynamics and Smad signaling to cell migration and fate commitment [238]. However, the growth factor induced osteogenic differentiation of the C2C12 cells required a minimal threshold stiffness (3.5 kPa) below which the presence of bound BMP‐2 mimetic‐peptide had no effect [239]. These results imply that compared to physical properties of biomaterials, chemical attributes such as those presented by growth factors are more robust on affecting cell function. Similarly, studies have shown increased osteogenic differentiation of stem cells with higher adhesion peptide density [240], and osteogenic differentiation of stem cells on soft matrices when cultured in osteogenic medium [199]. Furthermore, studies investigating matrix rigidity often employ primed cells (cells exposed to pro-differentiating medium) [241]. Together these findings suggest that chemical cues are active regulators of cell processes whereas physical cues support the effects of chemical cues in a secondary role. Furthermore, a distinct mechanism of matrix-bound GF presentation that is different to soluble cues occurs, possibly coupled through the direct interaction between integrins and growth factor receptors that feel the substrate stiffness. During aging and disease, the mechanical property of bone ECM is drastically affected [242, 243], which could impact GF binding and affect the coupled integrin-GF receptor signaling. Such materials could be used to identify unexplored signaling targets for intervention (Figure 4C).
5. Bioinspired materials in complex ex vivo systems to study bone function
Biomechanical cues are important to the function of native bone tissue. Employing systems that incorporate the relevant biomechanical cues could be applied to materials to study disease ex vivo. Various forms of mechanical stimuli such as shear stress and compressive loading are present in bone [244, 245], all of which are possible to be applied in vitro to 3-D cultures [246, 247]. In the latest advancement, organ-on-chip platforms that attempt to mimic tissue-specific structure and function are promising tools to improve in vitro disease modeling and enable validation of drug efficacy [9]. To induce pathophysiology, various forms of mechanical or biochemical stress could be provided using inflammatory cytokines and cells, bacterial or viral challenge, and mechanical stretch and strain. Integrating mechanical loading with organ-on-chips could lead to better mimics of bone modeling. This section introduces some systems that can be integrated to disease models for higher complexity with potential to further improve recapitulation of disease in the body.
5.1 Ex vivo systems providing mechanical stimuli
Bone is constantly influenced by mechanical loading. The strain magnitude during walking was estimated to be approximately -430 με (compression strain), but up to 850 με (tensile strain) when running [248]. During daily functional activities, large strains (>1000 με) occur only a few times a day, while small strains (<10 με) occur thousands of times a day [245]. Under experimental settings, increased osteogenic differentiation was observed when tensile forces were applied to MSCs seeded in collagen-GAG scaffolds cyclically (1 Hz) at 5% strain magnitude [246], and collagen coated silicone scaffolds at 2.5% at 0.17 Hz [249]. A 3-D in vitro co-culture system was also adopted to investigate the effect of loading on osteocyte-osteoblast interactions [5]. Osteocyte MLO-Y4 cells were embedded in type I collagen gels with MC3T3-E1 or MG63 cells layered on top. Cyclic compression of 10 Hz at 2.5 N to induce 4000-4500 με within gels increased prostaglandin E2 (PGE2) in MLO-Y4 cells, and increased type I pro-collagen synthesis in the co-culture. This co-culture model facilitates the elucidation of osteocyte-osteoblast communication during bone formation as a result of mechanical loading. In addition to mechanical loading, fluid shear stress had been postulated to occur in the lacunar-canalicular pores [244] and bone marrow [250]. The effect of fluid flow-induced shear stress on osteoprogenitor cells in 3-D scaffolds has been extensively studied. In general, unidirectional flow promoted early proliferation and osteogenic differentiation [247, 251], and intermittent pulsatile and oscillatory flow also increased cell response compared to continuous flow [252, 253].
5.2 Organ-on-a-chip systems
The development of technological platforms that generate three-dimensional (3D) organoids recapitulating the structural and biological features of native tissues has led to promising in vitro systems for disease modeling. Current research in this area has focused on the development of organ- and tissue-on-chip platforms ranging from traditional monolayer cultures to multicellular three-dimensional organoids. Organ-on-a-chip, which contain engineered microtissues that capture the physiological complexity of the native tissues within a continuous perfusion device, can be developed in a reproducible and cost-effective manner. These microphysiological systems also have the potential to supplement preclinical animal studies during drug discovery and development in order to improve the translatability of the drugs to the clinic. In fact, functional organs-on-chips simulating the lungs, gut, bone, heart, skeletal muscle, tumor, motor neurons, blood-brain barrier, and immune function have been developed [9, 254–260]. During bone development and remodeling, the vasculature, peripheral neurons, and hematopoietic system are highly involved in its physiological and pathological states. It would be ideal if a combination of some or all of the relevant cell types can be integrated into an ex vivo platform to model various etiologies of bone disorders. To date, only the vasculature has been shown to be integrated with bone [261–263]. A number of microphysiological systems consisting of the vasculature have been developed that are applied to studies in cancer metastasis, tumor angiogenesis, endothelial dysfunction, and organ regeneration using reconstituted natural ECM materials such as type I collagen [264–272], a mixture of thymosinbeta4/chitosan/collagen [273], and fibrin [262]. Below we discuss the advancements in the area of bone-on-chip systems. Though the focus of these studies were not bone disease modeling, when used appropriate cells and culture conditions these systems can be easily extended to study bone diseases.
5.2.1 Bone-on-a-chip platform
A triculture bone perivascular (BoPV) niche-on-a-chip system was developed to investigate the progression and drug resistance of breast cancer cells colonizing the bone. Human endothelial and bone marrow-derived mesenchymal stem cells (MSCs) were cultured in a native decellularized bone matrix measuring 6 mm height × 3 mm length × 1 mm thickness and exposed to interstitial flow and oxygen gradients. Breast cancer cells were perfused into the engineered bone and the cells were found to colonize within the tissue. Furthermore, disseminated breast cancer cells exposed to flow exhibited a slow proliferative state and increased drug resistance in the BoPV niche-on-a-chip model [261]. In another bone-on-a-chip model, multiple cell populations comprising of primary hMSCs, hMSC-derived osteoblasts, and HUVECs were encapsulated in fibrin gels to create a bone-mimicking environment of osteoblasts with perfused microvasculature and mural cells. Employing this system, the authors have validated the extravasation of cancer cells, which showed that the human breast cancer cells were able to be perfused into the microvasculature and extravasate. These studies demonstrate the efficacy of such systems as a drug screening platform and a potential tool to investigate specific molecular pathways involved in cancer progression [262].
5.2.2 Bone marrow-on-a-chip platform
To establish the cellular diversity and complex functions of living bone marrow in vitro, bone marrow-on-a-chip was developed with a functional hematopoietic niche [263, 274] (Figure 5). This was achieved by filling type I collagen gel containing bone-inducing (i.e., osteoinductive) demineralized bone powder and BMP2 or BMP4 in a microfabricated poly(dimethylsiloxane) (PDMS) device with a central cylindrical cavity measuring 1 mm in height and 4 mm in diameter, and implanting subcutaneously in the back of mouse. The implanted device was excised once the bone marrow was formed, and inserted into a microfluidic system for in vitro culture. The engineered bone marrow (eBM) retained hematopoietic stem and progenitor cells with in vivo-like ratios for at least 1 week ex vivo. In addition, the eBM modeled organ-level marrow toxicity responses, and protective effects of radiation countermeasure drugs that were not possible with conventional bone marrow culture methods.
Figure 5.
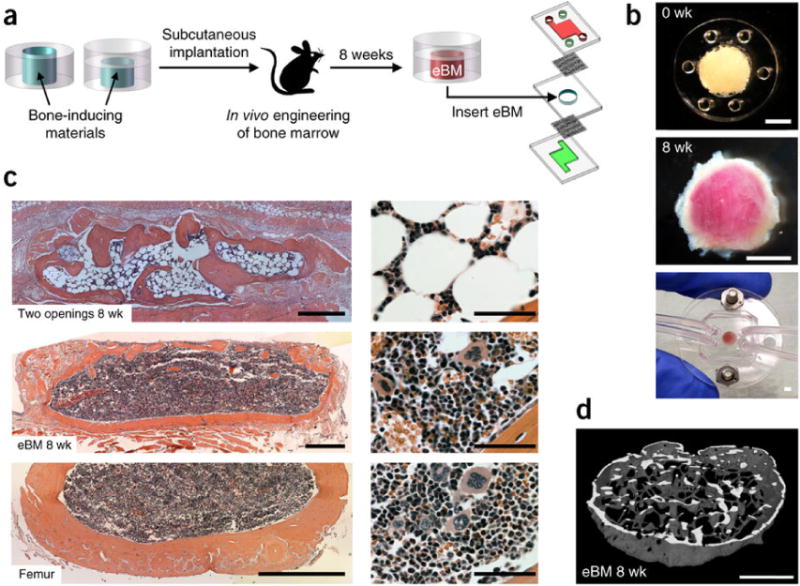
Bone marrow-on-a-chip. (A) Workflow to generate a bone marrow-on-a-chip system. (B) Top, PDMS device containing bone-inducing materials before implantation. Center, white cylindrical bone with pink marrow within eBM 8 weeks after implantation. Bottom, in vitro bone marrow (BM) chip microdevice. (C) H&E-stained sections of the eBM formed in the PDMS device with two openings (top), or one lower opening (center) at 8 weeks following implantation, bone BM in a normal adult mouse femur (bottom). (D) 3-D reconstruction of micro-CT data from eBM 8 weeks after implantation (from Ref. [263] with permission).
6. Summary and future outlook
Tremendous advances in bone biology, materials science, stem cells, and tissue engineering have led to in vitro tools to improve the potential of translating discoveries from the bench to bedside. In contrast to the frequent use of bioinspired materials as bone grafts for bone tissue repair and as scaffolds for bone tissue engineering, such materials are less commonly used as tools to study congenital disease of development and disorders of bone remodeling. These material-based tools and tissue engineered bone surrogates are valuable for acquiring novel insights to disease progression that are not able to be studied on 2-D tissue culture surfaces, and enables validation of drugs for therapeutic targets. Needless to say, the integration of bioinspired material, cells, and soluble factors are minimal elements required to model disease in vitro and in vivo. The value of bioinspired materials is their ability to test and dissect the role of individual factors through controllable manufacturing. However, technical limitations to biomaterials-based approaches described in this review exist and can be improved. For example, in addition to inducing cell differentiation, a material that facilitates cells to undergo tissue morphogenesis that forms macroscopic lamellar structures of native bone would be desirable. When stem cell-laden biomaterials are used to study developmental process such as EC ossification or remodeling, the final bone formation steps often require implantation into animals. Although this aids bone formation, the participation of host xenogenic, immune-compromised environment could introduce deviations and may not recapitulate the development process entirely. In addition to establishing and perfecting the components necessary for generating models of tissues, analyses of these tissue surrogates to determine how adequate they mimic the disease initiation, progression, and maintenance comparable to actual human disease are also crucial. Advancements in biomaterial synthesis, microfabrication, and imaging can lead to in vitro platforms with increased complexity by providing controlled microenvironmental stimuli, such as geometry, mechanical force, pH, temperature, and oxygen tension. Yet, it is important to be aware that increasing complexity does not necessarily emulate the physiological state, and it is necessary to ensure that the stimuli applied to cells ex vivo, are representative of what they are “felt” in the body. Rapid developments in organ-on-chip systems affirm that bone-on-a-chip can soon be integrated with other tissues/organ-chips as a multi-organ-on-chip system to study the systemic effects of bone on other organs, and vice versa, during drug treatments and disease. This will tremendously improve our ability to acquire accurate new insights into disease progression, and validation of therapeutic treatments for humans that are not possible in current cultures or animal models.
Acknowledgments
We thank Zhao Ma for drawing the proposed illustrations of material-based disease modeling. The authors acknowledge the financial support from National Institutes of Health (NIH, R01 AR063184 and R01 AR071552).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.M.G.S. Consortium. Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O’Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 2.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 3.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting Organ-Level Lung Functions on a Chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D. A 3d Human Neural Cell Culture System for Modeling Alzheimer’s Disease. Tissue Engineering Part A. 2015;21:S78–S78. doi: 10.1038/nprot.2015.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez M, Evans BAJ, Riccardi D, Evans SL, Ralphs JR, Dillingham CM, Mason DJ. A new method to investigate how mechanical loading of osteocytes controls osteoblasts. Front Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song AS, Najjar AM, Diller KR. Thermally induced apoptosis, necrosis, and heat shock protein expression in 3D culture. J Biomech Eng. 2014;136(7) doi: 10.1115/1.4027272. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Xu X, Lu Y, Xie X. Physiological oxygen tension reduces hepatocyte dedifferentiation in in vitro culture. Sci Rep. 2017;7(1):5923. doi: 10.1038/s41598-017-06433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 10.Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zujur D, Kanke K, Lichtler AC, Hojo H, Chung UI, Ohba S. Three-dimensional system enabling the maintenance and directed differentiation of pluripotent stem cells under defined conditions. Sci Adv. 2017;3(5):e1602875. doi: 10.1126/sciadv.1602875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian XF, Heng BC, Ge Z, Lu K, Rufaihah AJ, Fan VT, Yeo JF, Cao T. Comparison of osteogenesis of human embryonic stem cells within 2D and 3D culture systems. Scand J Clin Lab Invest. 2008;68(1):58–67. doi: 10.1080/00365510701466416. [DOI] [PubMed] [Google Scholar]
- 13.Shekaran A, Sim E, Tan KY, Chan JK, Choolani M, Reuveny S, Oh S. Enhanced in vitro osteogenic differentiation of human fetal MSCs attached to 3D microcarriers versus harvested from 2D monolayers. BMC Biotechnol. 2015;15:102. doi: 10.1186/s12896-015-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour A, Mezour MA, Badran Z, Tamimi F. (*) Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Eng Part A. 2017;23(23–24):1436–1451. doi: 10.1089/ten.TEA.2017.0026. [DOI] [PubMed] [Google Scholar]
- 15.Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biology. 2016;52–54:397–412. doi: 10.1016/j.matbio.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. P Natl Acad Sci USA. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitarski HFAM, Falank C, Reagan MR. 3D Tissue Engineered in Vitro Models of Cancer in Bone. ACS Biomater Sci & Eng. 2017 doi: 10.1021/acsbiomaterials.7b00097. Ahead of Press Published Online: June 9, 2017 http://pubs.acs.org/doi/abs/10.1021/acsbiomaterials.7b00097. [DOI] [PMC free article] [PubMed]
- 18.Renth AN, Detamore MS. Leveraging “Raw Materials” as Building Blocks and Bioactive Signals in Regenerative Medicine. Tissue Eng Part B-Re. 2012;18(5):341–362. doi: 10.1089/ten.teb.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Wen C, Kang H, Shih YR, Hwang Y, Varghese S. In vivo comparison of biomineralized scaffold-directed osteogenic differentiation of human embryonic and mesenchymal stem cells. Drug Deliv Transl Res. 2016;6(2):121–31. doi: 10.1007/s13346-015-0242-2. [DOI] [PubMed] [Google Scholar]
- 21.Kang H, Shih YR, Nakasaki M, Kabra H, Varghese S. Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci Adv. 2016;2(8):e1600691. doi: 10.1126/sciadv.1600691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myllyharju J. Extracellular matrix and developing growth plate. Curr Osteoporos Rep. 2014;12(4):439–45. doi: 10.1007/s11914-014-0232-1. [DOI] [PubMed] [Google Scholar]
- 23.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int J Biochem Cell B. 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Myllyharju J. Extracellular Matrix and Developing Growth Plate. Current Osteoporosis Reports. 2014;12(4):439–445. doi: 10.1007/s11914-014-0232-1. [DOI] [PubMed] [Google Scholar]
- 25.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–8. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Critical Reviews in Oral Biology & Medicine. 1992;3(3):269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- 28.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289(5484):1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 29.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016;143(15):2706–15. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 33.Lafage-Proust MH, Roche B, Langer M, Cleret D, Vanden Bossche A, Olivier T, Vico L. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep. 2015;4:662. doi: 10.1038/bonekey.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gkiatas I, Papadopoulos D, Pakos EE, Kostas-Agnantis I, Gelalis I, Vekris M, Korompilias A. The Multifactorial Role of Peripheral Nervous System in Bone Growth. Front Phys. 2017;5 [Google Scholar]
- 35.Grassel S. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Research & Therapy. 2014;16(6) doi: 10.1186/s13075-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raggatt LJ, Partridge NC. Cellular and Molecular Mechanisms of Bone Remodeling. Journal of Biological Chemistry. 2010;285(33):25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013;2:447. doi: 10.1038/bonekey.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–33. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piez KA, Miller A. The structure of collagen fibrils. J Supramol Struct. 1974;2(2–4):121–37. doi: 10.1002/jss.400020207. [DOI] [PubMed] [Google Scholar]
- 40.Bancelin S, Aime C, Gusachenko I, Kowalczuk L, Latour G, Coradin T, Schanne-Klein MC. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat Commun. 2014;5:4920. doi: 10.1038/ncomms5920. [DOI] [PubMed] [Google Scholar]
- 41.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk NAJM. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature Materials. 2010;9(12):1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein, 1. Nature Materials. 2003;2(8):552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 43.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li CH, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. P Natl Acad Sci USA. 2010;107(14):6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu LN, Jee SE, Jiao K, Tonggu L, Li M, Wang L, Yang YD, Bian JH, Breschi L, Jang SS, Chen JH, Pashley DH, Tay FR. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nature Materials. 2017;16(3):370–378. doi: 10.1038/nmat4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, Stevens MM. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. P Natl Acad Sci USA. 2012;109(35):14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XM, Cui FZ, Ge J, Wang Y. Hierarchical structural comparisons of bones from wild-type and liliput(dtc232) gene-mutated Zebrafish. Journal of Structural Biology. 2004;145(3):236–245. doi: 10.1016/j.jsb.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Keene DR, Sakai LY, Bachinger HP, Burgeson RE. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J Cell Biol. 1987;105(5):2393–402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalickova K, Susic M, Willing MC, Wenstrup RJ, Cole WG. Mutations of the alpha2(V) chain of type V collagen impair matrix assembly and produce ehlers-danlos syndrome type I. Hum Mol Genet. 1998;7(2):249–55. doi: 10.1093/hmg/7.2.249. [DOI] [PubMed] [Google Scholar]
- 49.Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–31. doi: 10.1242/jcs.169748. [DOI] [PubMed] [Google Scholar]
- 50.Alford AI, Kozloff KM, Hankenson KD. Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol. 2015;65:20–31. doi: 10.1016/j.biocel.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 51.GR P, Boskey AL. The Composition of Bone. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. John Wiley & Sons; Ames USA: 2013. pp. 49–56. [Google Scholar]
- 52.Paiva KBS, Granjeiro JM. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog Mol Biol Transl. 2017;148:203–303. doi: 10.1016/bs.pmbts.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14(11):1805–15. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 54.Baylink DJ, Finkelman RD, Mohan S. Growth-Factors to Stimulate Bone-Formation. Journal of Bone and Mineral Research. 1993;8:S565–S572. doi: 10.1002/jbmr.5650081326. [DOI] [PubMed] [Google Scholar]
- 55.Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–8. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilton MJ, Tu XL, Wu XM, Bai ST, Zhao HB, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long FX. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nature Medicine. 2008;14(3):306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashley JW, Ahn J, Hankenson KD. Notch Signaling Promotes Osteoclast Maturation and Resorptive Activity. Journal of Cellular Biochemistry. 2015;116(11):2598–2609. doi: 10.1002/jcb.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YP, Li YP, Paulson C, Shao JZ, Zhang XL, Wu MR, Chen W. Wnt and the Wnt signaling pathway in bone development and disease. Front Biosci-Landmrk. 2014;19:379–407. doi: 10.2741/4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinto N, Iwamoto M, Enomoto-Iwamoto M, Noji S, Ohuchi H, Yoshioka H, Kataoka H, Wada Y, Yuhao G, Takahashi HE, Yoshiki S, Yamaguchi A. Fibroblasts expressing Sonic hedgehog induce osteoblast differentiation and ectopic bone formation. Febs Lett. 1997;404(2–3):319–23. doi: 10.1016/s0014-5793(97)00014-8. [DOI] [PubMed] [Google Scholar]
- 61.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Gene Dev. 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su N, Jin M, Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2 doi: 10.1038/boneres.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caplan AI, Correa D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. J Orthopaed Res. 2011;29(12):1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 64.Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:437. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Termine JD, Posner AS. Infrared analysis of rat bone: age dependency of amorphous and crystalline mineral fractions. Science. 1966;153(3743):1523–5. doi: 10.1126/science.153.3743.1523. [DOI] [PubMed] [Google Scholar]
- 66.Termine JD, Posner AS. Infra-red determinaion of the percentage of crystallinity in apatitic calcium phosphates. Nature. 1966;211(5046):268–70. doi: 10.1038/211268a0. [DOI] [PubMed] [Google Scholar]
- 67.Termine JD, Wuthier RE, Posner AS. Amorphous-crystalline mineral changes during endochondral and periosteal bone formation. Proc Soc Exp Biol Med. 1967;125(1):4–9. doi: 10.3181/00379727-125-31999. [DOI] [PubMed] [Google Scholar]
- 68.Dey A, Bomans PHH, Muller FA, Will J, Frederik PM, de With G, Sommerdijk NAJM. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nature Materials. 2010;9(12):1010–1014. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 69.Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues (review) J Biomed Opt. 2000;5(3):259–68. doi: 10.1117/1.429994. [DOI] [PubMed] [Google Scholar]
- 70.Boskey AL. Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging. Curr Osteoporos Rep. 2006;4(2):71–5. doi: 10.1007/s11914-006-0005-6. [DOI] [PubMed] [Google Scholar]
- 71.Verdelis K, Lukashova L, Wright JT, Mendelsohn R, Peterson MG, Doty S, Boskey AL. Maturational changes in dentin mineral properties. Bone. 2007;40(5):1399–407. doi: 10.1016/j.bone.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang H, Shih YV, Varghese S. Direct Conversion of Human Pluripotent Stem Cells to Osteoblasts With a Small Molecule. Curr Protoc Stem Cell Biol. 2018;44:1F 21 1–1F 21 6. doi: 10.1002/cpsc.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 74.Sun Y, Deng W, Geng L, Zhang L, Liu R, Chen W, Yao G, Zhang H, Feng X, Gao X, Sun L. Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J Immunol Res. 2015;2015:284215. doi: 10.1155/2015/284215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol. 2012;13(11):713–26. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 77.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12(6):656–68. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu H, Lensch MW, Cahan P, Daley GQ. Investigating monogenic and complex diseases with pluripotent stem cells. Nat Rev Genet. 2011;12(4):266–75. doi: 10.1038/nrg2951. [DOI] [PubMed] [Google Scholar]
- 80.Deyle DR, Khan IF, Ren G, Wang PR, Kho J, Schwarze U, Russell DW. Normal collagen and bone production by gene-targeted human osteogenesis imperfecta iPSCs. Mol Ther. 2012;20(1):204–13. doi: 10.1038/mt.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yigit S, Yu H, An B, Hamaia S, Farndale RW, Kaplan DL, Lin YS, Brodsky B. Mapping the Effect of Gly Mutations in Collagen on alpha2beta1 Integrin Binding. J Biol Chem. 2016;291(36):19196–207. doi: 10.1074/jbc.M116.726182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nature Genetics. 2001;28(1):37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- 83.Hu Y, Chen IP, de Almeida S, Tiziani V, Do Amaral CMR, Gowrishankar K, Passos-Bueno MR, Reichenberger EJ. A Novel Autosomal Recessive GJA1 Missense Mutation Linked to Craniometaphyseal Dysplasia. Plos One. 2013;8(8) doi: 10.1371/journal.pone.0073576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52(1):157–66. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen IP, Luxmi R, Kanaujiya J, Hao ZF, Reichenberger EJ. Craniometaphyseal Dysplasia Mutations in ANKH Negatively Affect Human Induced Pluripotent Stem Cell Differentiation into Osteoclasts. Stem Cell Rep. 2017;9(5):1369–1376. doi: 10.1016/j.stemcr.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereira L, Dalessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic Organization of the Sequence Coding for Fibrillin, the Defective Gene-Product in Marfan-Syndrome (Vol 2, Pg 961, 1993) Human Molecular Genetics. 1993;2(10):1762–1762. doi: 10.1093/hmg/2.10.1762. [DOI] [PubMed] [Google Scholar]
- 87.Wohl AP, Troilo H, Collins RF, Baldock C, Sengle G. Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1. J Biol Chem. 2016;291(24):12732–46. doi: 10.1074/jbc.M115.704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smaldone S, Clayton NP, del Solar M, Pascual G, Cheng SH, Wentworth BM, Schaffler MB, Ramirez F. Fibrillin-1 Regulates Skeletal Stem Cell Differentiation by Modulating TGFbeta Activity Within the Marrow Niche. J Bone Miner Res. 2016;31(1):86–97. doi: 10.1002/jbmr.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, Sakai LY, Karsenty G, Ramirez F. Fibrillin-1 and -2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J Cell Biol. 2010;190(6):1107–21. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quarto N, Leonard B, Li SL, Marchand M, Anderson E, Behr B, Francke U, Reijo-Pera R, Chiao E, Longaker MT. Skeletogenic phenotype of human Marfan embryonic stem cells faithfully phenocopied by patient-specific induced-pluripotent stem cells. P Natl Acad Sci USA. 2012;109(1):215–220. doi: 10.1073/pnas.1113442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quarto N, Li S, Renda A, Longaker MT. Exogenous activation of BMP-2 signaling overcomes TGFbeta-mediated inhibition of osteogenesis in Marfan embryonic stem cells and Marfan patient-specific induced pluripotent stem cells. Stem Cells. 2012;30(12):2709–19. doi: 10.1002/stem.1250. [DOI] [PubMed] [Google Scholar]
- 92.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316–21. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henderson JE, Naski MC, Aarts MM, Wang D, Cheng L, Goltzman D, Ornitz DM. Expression of FGFR3 with the G380R achondroplasia mutation inhibits proliferation and maturation of CFK2 chondrocytic cells. J Bone Miner Res. 2000;15(1):155–65. doi: 10.1359/jbmr.2000.15.1.155. [DOI] [PubMed] [Google Scholar]
- 94.Schibler L, Gibbs L, Benoist-Lasselin C, Decraene C, Martinovic J, Loget P, Delezoide AL, Gonzales M, Munnich A, Jais JP, Legeai-Mallet L. New Insight on FGFR3-Related Chondrodysplasias Molecular Physiopathology Revealed by Human Chondrocyte Gene Expression Profiling. Plos One. 2009;4(10) doi: 10.1371/journal.pone.0007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY, Ryoo HM. Molecular Consequences of the ACVR1(R206H) Mutation of Fibrodysplasia Ossificans Progressiva. Journal of Biological Chemistry. 2010;285(29):22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsumoto Y, Hayashi Y, Schlieve CR, Ikeya M, Kim H, Nguyen TD, Sami S, Baba S, Barruet E, Nasu A, Asaka I, Otsuka T, Yamanaka S, Conklin BR, Toguchida J, Hsiao EC. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J Rare Dis. 2013;8 doi: 10.1186/1750-1172-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung SM, Kang JY, Min HK, Koh JH, Suh YS, Lee JH, Lee J, Lee JY, Kim JM, Kwok SK, Park KS, Park SH, Kim HY, Ju JH. Generation of Disease-Specific Induced Pluripotent Stem Cells from Patients with Rheumatoid Arthritis and Osteoarthritis. Annals of the Rheumatic Diseases. 2014;73:851–851. [Google Scholar]
- 98.Wei YY, Zeng W, Wan R, Wang J, Zhou Q, Qiu SJ, Singh SR. Chondrogenic Differentiation of Induced Pluripotent Stem Cells from Osteoarthritic Chondrocytes in Alginate Matrix. Eur Cells Mater. 2012;23:1–12. doi: 10.22203/ecm.v023a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang NS, Zhang C, Hwang YS, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1(1):97–106. doi: 10.1002/wsbm.26. [DOI] [PubMed] [Google Scholar]
- 100.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63(1):148–158. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phadke A, Shih YR, Varghese S. Mineralized synthetic matrices as an instructive microenvironment for osteogenic differentiation of human mesenchymal stem cells. Macromol Biosci. 2012;12(8):1022–32. doi: 10.1002/mabi.201100289. [DOI] [PubMed] [Google Scholar]
- 102.Shih YRV, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proceedings of the National Academy of Sciences. 2014;111(3):990–995. doi: 10.1073/pnas.1321717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phadke A, Hwang Y, Kim SH, Kim SH, Yamaguchi T, Masuda K, Varghese S. Effect of scaffold microarchitecture on osteogenic differentiation of human mesenchymal stem cells. Eur Cell Mater. 2013;25:114–129. doi: 10.22203/ecm.v025a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 105.Kaneto CM, Pereira Lima PS, Prata KL, Dos Santos JL, de Pina Neto JM, Panepucci RA, Noushmehr H, Covas DT, de Paula FJA, Silva WA., Jr Gene expression profiling of bone marrow mesenchymal stem cells from Osteogenesis Imperfecta patients during osteoblast differentiation. Eur J Med Genet. 2017;60(6):326–334. doi: 10.1016/j.ejmg.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Una CR, Adachi JD, Lems WF, Cooper C, Lane NE, I.O.F.C. Inflammation, G. Bone Structure Working Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28(2):429–446. doi: 10.1007/s00198-016-3769-2. [DOI] [PubMed] [Google Scholar]
- 107.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Undale AH, Westendorf JJ, Yaszemski MJ, Khosla S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84(10):893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- 110.Salamanna F, Contartese D, Maglio M, Fini M. A systematic review on in vitro 3D bone metastases models: A new horizon to recapitulate the native clinical scenario? Oncotarget. 2016;7(28):44803–44820. doi: 10.18632/oncotarget.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future? Mater Today. 2016;19(2):69–87. [Google Scholar]
- 112.Thibault RA, Mikos AG, Kasper FK. Scaffold/Extracellular matrix hybrid constructs for bone-tissue engineering. Adv Healthc Mater. 2013;2(1):13–24. doi: 10.1002/adhm.201200209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biol. 2016;52–54:397–412. doi: 10.1016/j.matbio.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chemical reviews. 2008;108(11):4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 115.Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, Schuetz MA. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Science Translational Medicine. 2012;4(141):141ra93–141ra93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 116.Hwang NS, Varghese S, Lee HJ, Zhang Z, Elisseeff J. Biomaterials Directed In Vivo Osteogenic Differentiation of Mesenchymal Cells Derived from Human Embryonic Stem Cells. Tissue Engineering Part A. 2013;19:1723–1732. doi: 10.1089/ten.tea.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yaylaoǧlu M, Korkusuz P, Örs Ü, Korkusuz F, Hasirci V. Development of a calcium phosphate–gelatin composite as a bone substitute and its use in drug release. Biomaterials. 1999;20(8):711–719. doi: 10.1016/s0142-9612(98)00199-9. [DOI] [PubMed] [Google Scholar]
- 118.Song J, Xu J, Filion T, Saiz E, Tomsia AP, Lian JB, Stein GS, Ayers DC, Bertozzi CR. Elastomeric high‐mineral content hydrogel‐hydroxyapatite composites for orthopedic applications. Journal of Biomedical Materials Research Part A. 2009;89(4):1098–1107. doi: 10.1002/jbm.a.32110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhumiratana S, Grayson WL, Castaneda A, Rockwood DN, Gil ES, Kaplan DL, Vunjak-Novakovic G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials. 2011;32(11):2812–2820. doi: 10.1016/j.biomaterials.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28(16):2622–2630. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rezwan K, Chen Q, Blaker J, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 122.Gkioni K, Leeuwenburgh SC, Douglas TE, Mikos AG, Jansen JA. Mineralization of hydrogels for bone regeneration. Tissue Engineering Part B: Reviews. 2010;16(6):577–585. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 123.Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue engineering. 2007;13(5):927–938. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 124.Song J, Malathong V, Bertozzi CR. Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J Am Chem Soc. 2005;127(10):3366–72. doi: 10.1021/ja043776z. [DOI] [PubMed] [Google Scholar]
- 125.Phadke A, Zhang C, Hwang Y, Vecchio K, Varghese S. Templated mineralization of synthetic hydrogels for bone-like composite materials: role of matrix hydrophobicity. Biomacromolecules. 2010;11(8):2060–8. doi: 10.1021/bm100425p. [DOI] [PubMed] [Google Scholar]
- 126.Nudelman F, Lausch AJ, Sommerdijk NAJM, Sone ED. In vitro models of collagen biomineralization. Journal of Structural Biology. 2013;183(2):258–269. doi: 10.1016/j.jsb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 127.Gower LB. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chemical Reviews. 2008;108(11):4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Varghese S, Lele AK, Srinivas D, Mashelkar RA. Role of hydrophobicity on structure of polymer-metal complexes. Journal of Physical Chemistry B. 2001;105(23):5368–5373. [Google Scholar]
- 129.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cells Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 130.Kane RJ, Ma PX. Biomimetic Nanofibrous Scaffolds for Bone Tissue Engineering Applications. Biomimetics: Advancing Nanobiomaterials and Tissue Engineering. 2013:69–89. [Google Scholar]
- 131.Venugopal J, Prabhakaran MP, Zhang YZ, Low S, Choon AT, Ramakrishna S. Biomimetic hydroxyapatite-containing composite nanofibrous substrates for bone tissue engineering. Philos T R Soc A. 2010;368(1917):2065–2081. doi: 10.1098/rsta.2010.0012. [DOI] [PubMed] [Google Scholar]
- 132.Huang Z, Feng QL, Yu B, Li SJ. Biomimetic properties of an injectable chitosan/nano-hydroxyapatite/collagen composite. Mat Sci Eng C-Mater. 2011;31(3):683–687. [Google Scholar]
- 133.Sotome S, Uemura T, Kikuchi M, Chen J, Itoh S, Tanaka J, Tateishi T, Shinomiya K. Synthesis and in vivo evaluation of a novel hydroxyapatite/collagen-alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mat Sci Eng C-Bio S. 2004;24(3):341–347. [Google Scholar]
- 134.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191–200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 135.Mehta M, Madl CM, Lee S, Duda GN, Mooney DJ. The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. Journal of Biomedical Materials Research Part A. 2015;103(11):3516–3525. doi: 10.1002/jbm.a.35497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, Nomura S, Maeda T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials. 2005;26(31):6158–6166. doi: 10.1016/j.biomaterials.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 137.Geistlich Bio-Gide. 2018 < https://www.geistlich-na.com/en-us/professionals/membranes/bio-gide/user-benefits/>.
- 138.Orthopaedic Synthetic Bone Grafts. < http://collagenmatrix.com/products/orthopaedic/orthopaedic-synthetic-bone-grafts/-scc-composite-strips-pads>.
- 139.Kang HM, Shih YRV, Hwang Y, Wen C, Rao V, Seo T, Varghese S. Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta Biomaterialia. 2014;10(12):4961–4970. doi: 10.1016/j.actbio.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kang HM, Wen C, Hwang YS, Shih YRV, Kar M, Seo SW, Varghese S. Biomineralized matrix-assisted osteogenic differentiation of human embryonic stem cells. Journal of Materials Chemistry B. 2014;2(34):5676–5688. doi: 10.1039/C4TB00714J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu B, Zhao XL, Yang CZ, Crane J, Xian LL, Lu W, Wan M, Cao X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. Journal of Bone and Mineral Research. 2012;27(9):2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Canalis E. Mechanisms of glucocorticoid-induced osteoporosis. Curr Opin Rheumatol. 2003;15(4):454–457. doi: 10.1097/00002281-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 143.Nozaki T, Takahashi K, Ishii O, Endo S, Hioki K, Mori T, Kikukawa T, Boumpas DT, Ozaki S, Yamada H. Development of an ex vivo cellular model of rheumatoid arthritis - Critical role of CD14-positive monocyte/macrophages in the development of pannus tissue. Arthritis Rheum-Us. 2007;56(9):2875–2885. doi: 10.1002/art.22849. [DOI] [PubMed] [Google Scholar]
- 144.Tasso R, Fais F, Reverberi D, Tortelli F, Cancedda R. The recruitment of two consecutive and different waves of host stem/progenitor cells during the development of tissue-engineered bone in a murine model. Biomaterials. 2010;31(8):2121–9. doi: 10.1016/j.biomaterials.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 145.Tortelli F, Tasso R, Loiacono F, Cancedda R. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials. 2010;31(2):242–9. doi: 10.1016/j.biomaterials.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 146.Shih YR, Kang H, Rao V, Chiu YJ, Kwon SK, Varghese S. In vivo engineering of bone tissues with hematopoietic functions and mixed chimerism. Proc Natl Acad Sci U S A. 2017;114(21):5419–5424. doi: 10.1073/pnas.1702576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Studer D, Millan C, Ozturk E, Maniura-Weber K, Zenobi-Wong M. Molecular and Biophysical Mechanisms Regulating Hypertrophic Differentiation in Chondrocytes and Mesenchymal Stem Cells. Eur Cells Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. [DOI] [PubMed] [Google Scholar]
- 148.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury-International Journal of the Care of the Injured. 2005;36(12):1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 149.Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, Weiss P, Guicheux J, Noel D. Cartilage Tissue Engineering: Towards a Biomaterial-Assisted Mesenchymal Stem Cell Therapy. Curr Stem Cell Res T. 2009;4(4):318–329. doi: 10.2174/157488809789649205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oakes DA, Lee CC, Lieberman JR. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop Relat R. 2003;(413):281–290. doi: 10.1097/01.blo.0000073347.50837.16. [DOI] [PubMed] [Google Scholar]
- 151.Thompson EM, Matsiko A, Kelly DJ, Gleeson JP, O’Brien FJ. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Engineering Part A. 2016;22(5–6):556–567. doi: 10.1089/ten.TEA.2015.0457. [DOI] [PubMed] [Google Scholar]
- 152.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 153.Sheehy EJ, Mesallati T, Vinardell T, Kelly DJ. Engineering cartilage or endochondral bone: a comparison of different naturally derived hydrogels. Acta Biomater. 2015;13:245–53. doi: 10.1016/j.actbio.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 154.Knuth CA, Witte-Bouma J, Ridwan Y, Wolvius EB, Farrell E. Mesenchymal stem cell-mediated endochondral ossification utilising micropellets and brief chondrogenic priming. Eur Cell Mater. 2017;34:142–161. doi: 10.22203/eCM.v034a10. [DOI] [PubMed] [Google Scholar]
- 155.Yuasa M, Mignemi NA, Nyman JS, Duvall CL, Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao CG, Bible JE, Obremskey WT, Flick MJ, Degen JL, Barnett JV, Cates JMM, Schoenecker JG. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification (vol 125, pg 3117, 2015) Journal of Clinical Investigation. 2015;125(9):3723–3723. doi: 10.1172/JCI84059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Farrell E, van der Jagt OP, Koevoet W, Kops N, van Manen CJ, Hellingman CA, Jahr H, O’Brien FJ, Verhaar JA, Weinans H, van Osch GJ. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15(2):285–95. doi: 10.1089/ten.tec.2008.0297. [DOI] [PubMed] [Google Scholar]
- 157.Farrell E, Both SK, Odorfer KI, Koevoet W, Kops N, O’Brien FJ, Baatenburg de Jong RJ, Verhaar JA, Cuijpers V, Jansen J, Erben RG, van Osch GJ. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mumme M, Scotti C, Papadimitropoulos A, Todorov A, Hoffmann W, Bocelli-Tyndall C, Jakob M, Wendt D, Martin I, Barbero A. Interleukin-1beta modulates endochondral ossification by human adult bone marrow stromal cells. Eur Cell Mater. 2012;24:224–36. doi: 10.22203/ecm.v024a16. [DOI] [PubMed] [Google Scholar]
- 159.Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, Barbero A, Manz MG, Martin I. Engineering of a functional bone organ through endochondral ossification. P Natl Acad Sci USA. 2013;110(10):3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang JI, Durbhakula MM, Angele P, Johnstone B, Yoo JU. Lunate arthroplasty with autologous mesenchymal stem cells in a rabbit model. Journal of Bone and Joint Surgery-American. 2006;88a(4):744–752. doi: 10.2106/JBJS.E.00669. [DOI] [PubMed] [Google Scholar]
- 161.Visser J, Gawlitta D, Benders KEM, Toma SMH, Pouran B, van Weeren PR, Dhert WJA, Malda J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–182. doi: 10.1016/j.biomaterials.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 162.Yang WX, Both SK, van Osch GJVM, Wang YN, Jansen JA, Yang F. Effects of in vitro chondrogenic priming time of bone-marrow-derived mesenchymal stromal cells on in vivo endochondral bone formation. Acta Biomaterialia. 2015;13:254–265. doi: 10.1016/j.actbio.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 163.Dang PN, Herberg S, Varghai D, Riazi H, Varghai D, McMillan A, Awadallah A, Phillips LM, Jeon O, Nguyen MK, Dwivedi N, Yu XH, Murphy WL, Alsberg E. Endochondral Ossification in Critical-Sized Bone Defects via Readily Implantable Scaffold-Free Stem Cell Constructs. Stem Cell Transl Med. 2017;6(7):1644–1659. doi: 10.1002/sctm.16-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sasaki JI, Matsumoto T, Egusa H, Matsusaki M, Nishiguchi A, Nakano T, Akashi M, Imazato S, Yatani H. In vitro reproduction of endochondral ossification using a 3D mesenchymal stem cell construct. Integr Biol-Uk. 2012;4(10):1207–1214. doi: 10.1039/c2ib20027a. [DOI] [PubMed] [Google Scholar]
- 165.Radin S, Ducheyne P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. Journal of biomedical materials research. 1993;27(1):35–45. doi: 10.1002/jbm.820270106. [DOI] [PubMed] [Google Scholar]
- 166.Chai YC, Roberts SJ, Desmet E, Kerckhofs G, van Gastel N, Geris L, Carmeliet G, Schrooten J, Luyten FP. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials. 2012;33(11):3127–3142. doi: 10.1016/j.biomaterials.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 167.Germaini MM, Detsch R, Grunewald A, Magnaudeix A, Lalloue F, Boccaccini AR, Champion E. Osteoblast and osteoclast responses to A/B type carbonate-substituted hydroxyapatite ceramics for bone regeneration. Biomedical Materials. 2017;12(3) doi: 10.1088/1748-605X/aa69c3. [DOI] [PubMed] [Google Scholar]
- 168.Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AM, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proceedings of the National Academy of Sciences. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barradas AM, Monticone V, Hulsman M, Danoux C, Fernandes H, Tahmasebi Birgani Z, Barrere-de Groot F, Yuan H, Reinders M, Habibovic P, van Blitterswijk C, de Boer J. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integrative biology: quantitative biosciences from nano to macro. 2013;5(7):920–31. doi: 10.1039/c3ib40027a. [DOI] [PubMed] [Google Scholar]
- 170.Ter Brugge P, Wolke J, Jansen J. Effect of calcium phosphate coating crystallinity and implant surface roughness on differentiation of rat bone marrow cells. Journal of biomedical materials research. 2002;60(1):70–78. doi: 10.1002/jbm.10031. [DOI] [PubMed] [Google Scholar]
- 171.Hu Q, Tan Z, Liu Y, Tao J, Cai Y, Zhang M, Pan H, Xu X, Tang R. Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. Journal of Materials Chemistry. 2007;17(44):4690–4698. [Google Scholar]
- 172.Liu YK, Lu QZ, Pei R, Ji HJ, Zhou GS, Zhao XL, Tang RK, Zhang M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: implication for bone tissue engineering. Biomedical Materials. 2009;4(2):025004. doi: 10.1088/1748-6041/4/2/025004. [DOI] [PubMed] [Google Scholar]
- 173.Wen L, Wang Y, Wang H, Kong L, Zhang L, Chen X, Ding Y. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2012;424:439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- 174.Barradas A, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen JP, van Blitterswijk CA, de Boer J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33(11):3205–3215. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 175.Chen Z, Wu C, Gu W, Klein T, Crawford R, Xiao Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35(5):1507–18. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 176.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79(4):414–22. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nakade O, Takahashi K, Takuma T, Aoki T, Kaku T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J Bone Miner Metab. 2001;19(1):13–9. doi: 10.1007/s007740170055. [DOI] [PubMed] [Google Scholar]
- 178.Suárez-González D, Lee JS, Lan Levengood SK, Vanderby R, Jr, Murphy WL. Mineral coatings modulate β-TCP stability and enable growth factor binding and release. Acta biomaterialia. 2012;8(3):1117–1124. doi: 10.1016/j.actbio.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suarez-Gonzalez D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials. 2012;33(2):713–21. doi: 10.1016/j.biomaterials.2011.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu YL, Hunziker EB, Layrolle P, De Bruijn JD, De Groot K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Engineering. 2004;10(1–2):101–108. doi: 10.1089/107632704322791745. [DOI] [PubMed] [Google Scholar]
- 181.Kang H, Shih YR, Varghese S. Biomineralized matrices dominate soluble cues to direct osteogenic differentiation of human mesenchymal stem cells through adenosine signaling. Biomacromolecules. 2015;16(3):1050–61. doi: 10.1021/acs.biomac.5b00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends Endocrinol Metab. 2013;24(6):290–300. doi: 10.1016/j.tem.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rumney RM, Wang N, Agrawal A, Gartland A. Purinergic signalling in bone. Front Endocrinol (Lausanne) 2012;3:116. doi: 10.3389/fendo.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wesselius A, Bours MJ, Agrawal A, Gartland A, Dagnelie PC, Schwarz P, Jorgensen NR. Role of purinergic receptor polymorphisms in human bone. Front Biosci (Landmark Ed) 2011;16:2572–85. doi: 10.2741/3873. [DOI] [PubMed] [Google Scholar]
- 185.Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K. A2B Adenosine Receptor Promotes Mesenchymal Stem Cell Differentiation to Osteoblasts and Bone Formation in Vivo. Journal of Biological Chemistry. 2012;287(19):15718–15727. doi: 10.1074/jbc.M112.344994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Corciulo C, Wilder T, Cronstein BN. Adenosine A2B receptors play an important role in bone homeostasis. Purinergic Signal. 2016;12(3):537–47. doi: 10.1007/s11302-016-9519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Silver IA, Murrills RJ, Etherington DJ. Microelectrode Studies on the Acid Microenvironment beneath Adherent Macrophages and Osteoclasts. Experimental Cell Research. 1988;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 188.Yuan HP, Fernandes H, Habibovic P, de Boer J, Barradas AMC, de Ruiter A, Walsh WR, van Blitterswijk CA, de Bruijn JD. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. P Natl Acad Sci USA. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang H, Zeng HJ, Hao LJ, Zhao NR, Du C, Liao H, Wang YJ. Effects of hydroxyapatite microparticle morphology on bone mesenchymal stem cell behavior. Journal of Materials Chemistry B. 2014;2(29):4703–4710. doi: 10.1039/c4tb00424h. [DOI] [PubMed] [Google Scholar]
- 190.Choi S, Murphy WL. A screening approach reveals the influence of mineral coating morphology on human mesenchymal stem cell differentiation. Biotechnology Journal. 2013;8(4):496–+. doi: 10.1002/biot.201200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malard O, Bouler JM, Guicheux J, Heymann D, Pilet P, Coquard C, Daculsi G. Influence of biphasic calcium phosphate granulometry on bone ingrowth, ceramic resorption, and inflammatory reactions: Preliminary in vitro and in vivo study. Journal of Biomedical Materials Research. 1999;46(1):103–111. doi: 10.1002/(sici)1097-4636(199907)46:1<103::aid-jbm12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 192.Wang L, Barbieri D, Zhou H, de Bruijn JD, Bao C, Yuan H. Effect of particle size on osteoinductive potential of microstructured biphasic calcium phosphate ceramic. J Biomed Mater Res A. 2015;103(6):1919–29. doi: 10.1002/jbm.a.35325. [DOI] [PubMed] [Google Scholar]
- 193.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13(10):979–87. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27(28):4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 197.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen SC, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature Materials. 2014;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jha AK, Jackson WM, Healy KE. Controlling osteogenic stem cell differentiation via soft bioinspired hydrogels. PLoS One. 2014;9(6):e98640. doi: 10.1371/journal.pone.0098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011;26(4):730–8. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 201.Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nature Materials. 2016;15(3):318–+. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 202.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials. 2016;15(3):326–+. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, Duda G, Mooney D. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Advanced Healthcare Materials. 2017;6(1) doi: 10.1002/adhm.201601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ. Molecular and Mesoscale Mechanisms of Osteogenesis Imperfecta Disease in Collagen Fibrils. Biophys J. 2009;97(3):857–865. doi: 10.1016/j.bpj.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Licup AJ, Munster S, Sharma A, Sheinman M, Jawerth LM, Fabry B, Weitz DA, MacKintosh FC. Stress controls the mechanics of collagen networks. P Natl Acad Sci USA. 2015;112(31):9573–9578. doi: 10.1073/pnas.1504258112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Paiva KBS, Granjeiro JM. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Archives of Biochemistry and Biophysics. 2014;561:74–87. doi: 10.1016/j.abb.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 207.Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kar M, Shih YRV, Velez DO, Cabrales P, Varghese S. Poly(ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery. Biomaterials. 2016;77:186–197. doi: 10.1016/j.biomaterials.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 211.Chen B, Lin H, Wang JH, Zhao YN, Wang B, Zhao WX, Sun WJ, Dai JW. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28(6):1027–1035. doi: 10.1016/j.biomaterials.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 212.Bentz H, Schroeder JA, Estridge TD. Improved local delivery of TGF-beta 2 by binding to injectable fibrillar collagen via difunctional polyethylene glycol. Journal of Biomedical Materials Research. 1998;39(4):539–548. doi: 10.1002/(sici)1097-4636(19980315)39:4<539::aid-jbm6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 213.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31(2):226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 214.Saito W, Uchida K, Ueno M, Matsushita O, Inoue G, Nishi N, Ogura T, Hattori S, Fujimaki H, Tanaka K, Takaso M. Acceleration of bone formation during fracture healing by injectable collagen powder and human basic fibroblast growth factor containing a collagen-binding domain from Clostridium histolyticum collagenase. Journal of Biomedical Materials Research Part A. 2014;102(9):3049–3055. doi: 10.1002/jbm.a.34974. [DOI] [PubMed] [Google Scholar]
- 215.Lin H, Chen B, Sun WJ, Zhao WX, Zhao YN, Dai JW. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials. 2006;27(33):5708–5714. doi: 10.1016/j.biomaterials.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 216.Shu XL, Feng J, Feng J, Huang XM, Li LQ, Shi QS. Combined delivery of bone morphogenetic protein-2 and insulin-like growth factor-1 from nano-poly (gamma-glutamic acid)/beta-tricalcium phosphate-based calcium phosphate cement and its effect on bone regeneration in vitro. J Biomater Appl. 2017;32(5):547–560. doi: 10.1177/0885328217737654. [DOI] [PubMed] [Google Scholar]
- 217.Han XL, Zhang W, Gu J, Zhao H, Ni L, Han JJ, Zhou Y, Gu YN, Zhu XS, Sun J, Hou XL, Yang HL, Dai JW, Shi Q. Accelerated Postero-Lateral Spinal Fusion by Collagen Scaffolds Modified with Engineered Collagen-Binding Human Bone Morphogenetic Protein-2 in Rats. Plos One. 2014;9(5) doi: 10.1371/journal.pone.0098480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen B, Lin H, Zhao YN, Wang B, Zhao YH, Liu YP, Liu ZH, Dai JW. Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. Journal of Biomedical Materials Research Part A. 2007;80a(2):428–434. doi: 10.1002/jbm.a.30900. [DOI] [PubMed] [Google Scholar]
- 219.Han B, Perelman N, Tang BW, Hall F, Shors EC, Nimni ME. Collagen-targeted BMP3 fusion proteins arrayed on collagen matrices or porous ceramics impregnated with Type I collagen enhance osteogenesis in a rat cranial defect model. J Orthopaed Res. 2002;20(4):747–755. doi: 10.1016/S0736-0266(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 220.Lu HX, Kawazoe N, Kitajima T, Myoken Y, Tomita M, Umezawa A, Chen GP, Ito Y. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials. 2012;33(26):6140–6146. doi: 10.1016/j.biomaterials.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 221.Shiozaki Y, Kitajima T, Mazaki T, Yoshida A, Tanaka M, Umezawa A, Nakamura M, Yoshida Y, Ito Y, Ozaki T, Matsukawa A. Enhanced in vivo osteogenesis by nanocarrier-fused bone morphogenetic protein-4. Int J Nanomed. 2013;8:1349–1360. doi: 10.2147/IJN.S44124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomaterialia. 2010;6(1):21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lu Y, Lee JS, Nemke B, Graf BK, Royalty K, Illgen R, Vanderby R, Markel MD, Murphy WL. Coating with a Modular Bone Morphogenetic Peptide Promotes Healing of a Bone-Implant Gap in an Ovine Model. Plos One. 2012;7(11) doi: 10.1371/journal.pone.0050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, Murphy WL. Influence of Hydroxyapatite-Coated and Growth Factor-Releasing Interference Screws on Tendon-Bone Healing in an Ovine Model. Arthroscopy. 2009;25(12):1427–U92. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Madl CM, Mehta M, Duda GN, Heilshorn SC, Mooney DJ. Presentation of BMP-2 Mimicking Peptides in 3D Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells. Biomacromolecules. 2014;15(2):445–455. doi: 10.1021/bm401726u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42(6):556–61. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Quinlan E, Lopez-Noriega A, Thompson EM, Hibbitts A, Cryan SA, O’Brien FJ. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J Tissue Eng Regen M. 2017;11(4):1097–1109. doi: 10.1002/term.2013. [DOI] [PubMed] [Google Scholar]
- 228.Knaack S, Lode A, Hoyer B, Rosen-Wolff A, Gabrielyan A, Roeder I, Gelinsky M. Heparin modification of a biomimetic bone matrix for controlled release of VEGF. Journal of Biomedical Materials Research Part A. 2014;102(10):3500–3511. doi: 10.1002/jbm.a.35020. [DOI] [PubMed] [Google Scholar]
- 229.Jin K, Li B, Lou LX, Xu YF, Ye X, Yao K, Ye J, Gao CY. In vivo vascularization of MSC-loaded porous hydroxyapatite constructs coated with VEGF-functionalized collagen/heparin multilayers. Sci Rep-Uk. 2016;6 doi: 10.1038/srep19871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, Hofstetter W, Klenke FM. Vegf Incorporated into Calcium Phosphate Ceramics Promotes Vascularisation and Bone Formation in Vivo. Eur Cells Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 231.Lee JS, Johnson AJW, Murphy WL. A Modular, Hydroxyapatite-Binding Version of Vascular Endothelial Growth Factor. Advanced Materials. 2010;22(48):5494–5498. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 232.Suarez-Gonzalez D, Lee JS, Diggs A, Lu Y, Nemke B, Markel M, Hollister SJ, Murphy WL. Controlled Multiple Growth Factor Delivery from Bone Tissue Engineering Scaffolds via Designed Affinity. Tissue Engineering Part A. 2014;20(15–16):2077–2087. doi: 10.1089/ten.tea.2013.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sun B, Chen B, Zhao YN, Sun WJ, Chen KS, Zhang J, Wei ZL, Xiao ZF, Dai JW. Crosslinking Heparin to Collagen Scaffolds for the Delivery of Human Platelet-Derived Growth Factor. J Biomed Mater Res B. 2009;91b(1):366–372. doi: 10.1002/jbm.b.31411. [DOI] [PubMed] [Google Scholar]
- 234.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64(1–3):103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 235.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJA, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. Journal of Biomedical Materials Research. 2002;62(2):185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 236.Zu Y, Liang X, Du J, Zhou S, Yang C. Binding of integrin alpha1 to bone morphogenetic protein receptor IA suggests a novel role of integrin alpha1beta1 in bone morphogenetic protein 2 signalling. J Biomech. 2015;48(14):3950–4. doi: 10.1016/j.jbiomech.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 237.Crouzier T, Fourel L, Boudou T, Albiges-Rizo C, Picart C. Presentation of BMP-2 from a soft biopolymeric film unveils its activity on cell adhesion and migration. Adv Mater. 2011;23(12):H111–8. doi: 10.1002/adma.201004637. [DOI] [PubMed] [Google Scholar]
- 238.Fourel L, Valat A, Faurobert E, Guillot R, Bourrin-Reynard I, Ren K, Lafanechere L, Planus E, Picart C, Albiges-Rizo C. beta3 integrin-mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J Cell Biol. 2016;212(6):693–706. doi: 10.1083/jcb.201508018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zouani OF, Kalisky J, Ibarboure E, Durrieu MC. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials. 2013;34(9):2157–66. doi: 10.1016/j.biomaterials.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 240.Frith JE, Mills RJ, Cooper-White JJ. Lateral spacing of adhesion peptides influences human mesenchymal stem cell behaviour. J Cell Sci. 2012;125(Pt 2):317–27. doi: 10.1242/jcs.087916. [DOI] [PubMed] [Google Scholar]
- 241.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 242.Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333–48. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ. Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils. Biophys J. 2009;97(3):857–65. doi: 10.1016/j.bpj.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33(3):317–25. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 246.Byrne EM, Farrell E, McMahon LA, Haugh MG, O’Brien FJ, Campbell VA, Prendergast PJ, O’Connell BC. Gene expression by marrow stromal cells in a porous collagen-glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J Mater Sci-Mater M. 2008;19(11):3455–3463. doi: 10.1007/s10856-008-3506-2. [DOI] [PubMed] [Google Scholar]
- 247.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99(20):12600–5. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lanyon LE, Hampson WG, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand. 1975;46(2):256–68. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 249.Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile Strain as a Regulator of Mesenchymal Stem Cell Osteogenesis. Annals of Biomedical Engineering. 2010;38(5):1767–1779. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- 250.Gurkan UA, Akkus O. The Mechanical Environment of Bone Marrow: A Review. Annals of Biomedical Engineering. 2008;36(12):1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 251.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Jansen JA, Mikos AG. Flow perfusion culture of marrow stromal osteoblasts in titanium fiber mesh. J Biomed Mater Res A. 2003;64(2):235–41. doi: 10.1002/jbm.a.10365. [DOI] [PubMed] [Google Scholar]
- 252.Jaasma MJ, O’Brien FJ. Mechanical stimulation of osteoblasts using steady and dynamic fluid flow. Tissue Engineering Part A. 2008;14(7):1213–1223. doi: 10.1089/ten.tea.2007.0321. [DOI] [PubMed] [Google Scholar]
- 253.Du DJ, Furukawa KS, Ushida T. 3D Culture of Osteoblast-Like Cells by Unidirectional or Oscillatory Flow for Bone Tissue Engineering. Biotechnol Bioeng. 2009;102(6):1670–1678. doi: 10.1002/bit.22214. [DOI] [PubMed] [Google Scholar]
- 254.Ahn J, Sei YJ, Jeon NL, Kim Y. Tumor Microenvironment on a Chip: The Progress and Future Perspective. Bioengineering (Basel) 2017;4(3) doi: 10.3390/bioengineering4030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Aung A, Bhullar IS, Theprungsirikul J, Davey SK, Lim HL, Chiu YJ, Ma X, Dewan S, Lo YH, McCulloch A, Varghese S. 3D cardiac mutissues within a microfluidic device with real-time contractile stress readout. Lab Chip. 2016;16(1):153–62. doi: 10.1039/c5lc00820d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Agrawal G, Aung A, Varghese S. Skeletal muscle-on-a-chip: an in vitro model to evaluate tissue formation and injury. Lab Chip. 2017;17(20):3447–3461. doi: 10.1039/c7lc00512a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Aung A, Theprungsirikul J, Lim HL, Varghese S. Chemotaxis-driven assembly of endothelial barrier in a tumor-on-a-chip platform. Lab Chip. 2016;16(10):1886–98. doi: 10.1039/c6lc00184j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Uzel SG, Platt RJ, Subramanian V, Pearl TM, Rowlands CJ, Chan V, Boyer LA, So PT, Kamm RD. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci Adv. 2016;2(8):e1501429. doi: 10.1126/sciadv.1501429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim D, Haynes CL. On-Chip Evaluation of Neutrophil Activation and Neutrophil-Endothelial Cell Interaction during Neutrophil Chemotaxis. Anal Chem. 2013;85(22):10787–10796. doi: 10.1021/ac4020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Biselli E, Agliari E, Barra A, Bertani FR, Gerardino A, De Ninno A, Mencattini A, Di Giuseppe D, Mattei F, Schiavoni G, Lucarini V, Vacchelli E, Kroemer G, Di Natale C, Martinelli E, Businaro L. Organs on chip approach: a tool to evaluate cancer -immune cells interactions. Sci Rep-Uk. 2017;7 doi: 10.1038/s41598-017-13070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Marturano-Kruik A, Nava MM, Yeager K, Chramiec A, Hao L, Robinson S, Guo E, Raimondi MT, Vunjak-Novakovic G. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1714282115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. P Natl Acad Sci USA. 2015;112(1):214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11(6):663–+. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 264.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. In vitro model of tumor cell extravasation. PLoS One. 2013;8(2):e56910. doi: 10.1371/journal.pone.0056910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cross VL, Zheng Y, Choi NW, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Buchanan CF, Verbridge SS, Vlachos PP, Rylander MN. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhes Migr. 2014;8(5):517–524. doi: 10.4161/19336918.2014.970001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13(8):1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 269.Chen MB, Whisler JA, Froese J, Yu C, Shin YJ, Kamm RD. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat Protoc. 2017;12(5):865–880. doi: 10.1038/nprot.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tan A, Fujisawa K, Yukawa Y, Matsunaga YT. Bottom-up fabrication of artery-mimicking tubular co-cultures in collagen-based microchannel scaffolds. Biomater Sci-Uk. 2016;4(10):1503–1514. doi: 10.1039/c6bm00340k. [DOI] [PubMed] [Google Scholar]
- 271.Kim JA, Kim HN, Im SK, Chung S, Kang JY, Choi N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics. 2015;9(2) doi: 10.1063/1.4917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cho HS, Seo JH, Wong KHK, Terasaki Y, Park J, Bong K, Arai K, Lo EH, Irimia D. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep-Uk. 2015;5 doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chiu LLY, Montgomery M, Liang Y, Liu HJ, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. P Natl Acad Sci USA. 2012;109(50):E3414–E3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Torisawa YS, Mammoto T, Jiang E, Jiang A, Mammoto A, Watters AL, Bahinski A, Ingber DE. Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng Part C-Me. 2016;22(5):509–515. doi: 10.1089/ten.TEC.2015.0507. [DOI] [PubMed] [Google Scholar]


