ABSTRACT
Production, purification and characterisation of a black pigment from Termitomyces albuminosus as melanin is reported, for the first time, from shaken submerged culture condition using scanning electron microscopy (SEM), elemental analysis, ultraviolet–visible (UV-VIS), and Fourier transformed infrared spectroscopy (FTIR), electron paramagnetic resonance (EPR) and 13C (CP/MAS) NMR spectra. SEM results on T. albuminosus revealed nanogranular nature of melanin nanoparticles within size range of 400–100 nm with fractal dimension D = 1.195–1.73. Elemental analysis of melanin indicated 54.6% C, 3.5% H, 2.4% N, 26.9% O, and 12% S. UV-VIS and FTIR spectra confirmed to the characteristic of melanin and were identical to the reference commercial sepia melanin. Further validation of the identity of pigment as melanin was achieved by EPR analysis. Termitomyces albuminosus melanin is postulated to be DOPA-type melanin confirmed by 13C (CP/MAS) NMR spectral analysis showing chemical shift at 200–170 ppm carbonyl, 160–110 ppm aromatic region, and with high 40–30 ppm open chain aliphatic region. Chemical modification through oxidation and cysteinylation (Pheomelanin) is implied as indicated by relatively high sulphur content (12%).
KEYWORDS: Termitomyces, melanin, submerged fermentation, DOPA, purification
Introduction
Melanin biosynthesis is a common feature in kingdom fungi. The pigment not essential for hyphal growth appears as secondary metabolite. Melanins are most stable, amorphous polymers of phenolic compounds and can be classified into the following three types: eumelanins, pheomelanins and allomelanins. Melanin production helps in protection from extreme environmental conditions such as UV light, ionising radiation, resistance to heat or cold, phagocytosis, heavy metals, and oxidants and provides cell wall rigidity (Money et al. 1998; Plonka and Grabacka 2006; Pal et al. 2013; Casadevall et al. 2017). Despite its importance and ubiquity, many fundamental questions remain unanswered like details of its chemical structure and insolubility (Eisenman and Casadevall 2012). Some fungi undergo melanogenesis in response to certain environmental stress conditions such as extreme temperatures, dessiccation, hyperosmotic conditions, limited nutrients, pH changes, metal toxcicity, UV or ionisation stress, action of antagonistic microbes. Melanisation in fungi mostly seen in hyaline hyphae, sclerotia, appressoria, reproductive structures or conidia (Cordero and Casadevall 2017). Hyphal melanin is often found to be deposited as the outermost layer or internal layer in cell wall only with age or other stress (Bell and Wheeler 1986; Henson et al. 1999; Butler et al. 2001). Melanogenesis in pathogenic fungi plays a key role in pathogenesis in species such as Cryptococcus neoformans (Polacheck and Kwon-Chung 1988), Gaeumannomyces graminis var. tritici, Magnaporthe grisea, Alternaria alternata, Colletotrichum lagenarium, Cochliobolus heterostrophus (Henson et al. 1999), Paecilomyces variotti (Babitskaya et al. 2000a), Rhizoctonia solani (Chen et al. 2015) and Aspergillus spp. (Babitskaya et al. 2000a; Schmaler-Ripcke et al. 2009; Gonçalves et al. 2012; Pal et al. 2013). Melanins are reported from mushrooms such as Agaricus bisporus (Mendoza et al. 1979), Inonotus obliquus (Babitskaia et al. 2000b; Babitskaya et al. 2002), and Schizophyllum commune (Arun et al. 2015). Plant-associated symbiotic ectomycorrhizal fungus, Cenococcum geophilum, produces melanin under dehydrated conditions (Fernandez and Koide 2013). Fungi synthesise melanin by one of the two synthetic pathways: 1,8-dihydroxynaphthalene (DHN) intermediate and l-3,4-dihydroxyphenylanine (L-DOPA). Melanin synthesis involves copper containing metalloenzymes such as laccase and tyrosinase and in fungi also shows involvement of chitin cross-links to other cell wall polysaccharides and proteins (Eisenman and Casadevall 2012). Studies on melanins in mushrooms are limited to edible mushrooms such as Pleurotus cystidious var. formosensis, P. australis, and P. purpureoolivaceus from which darkly pigmented arthroconidia forming black pigment on mycelium or basidiomata has been characterised (Selvakumar et al. 2008). According to Mendoza et al. (1979), the spore wall of Agaricus bisporous and Agaricus campestris contain 26–28% and 24–26% crude (dry weright cell wall) melanin. Mushroom fruitbody decolourisation is very common due to oxidation of phenolic substrates into quinones leading to the formation of brown-coloured melanin in species such as A. bisporous, thus decreasing its commercial value (Weijn et al. 2013). Exo- and endomelanin complex of Inonotus obliquus and Phellinus robustus in submerged conditions demonstrate high-antioxidant and genoprotective properties (Bisko et al. 2002, 2007). Melanin in Auricularia auricula has been studied extensively (Zou et al. 2010; Bin et al. 2012; Zhang et al. 2015; Sun et al. 2016a). Melanin is found useful in the field of material science as coating material in electronic/bioelectronics, drug delivery and cosmetics as sunscreens, emphasising the importance of finding good, non-toxic melanin sources (Blumenberg 2017).
Symbiotic fungal species in Termitomyces Heim are found in Asian and African continents as exosymbionts cultivated by fungus growing termites belonging to subfamily – Macrotermitinae in their nest as food (Wood and Sands 1978). During tropical monsoon, fruitbodies from subterranean fungus combs emerge by forcing their way through very hard layer of inert matter using a hard, melanised perforatorium (Heim 1977; Kendrick 2001). Traditionally, these species are known to be most popular and highly prized edible mushrooms in Asia and Africa. Taxonomists have reported dark pigmentation in fruitbodies especially in organs like hypogeal pseudorrhiza and epigeal smooth or pointed umbo exhibiting brownish to greyish-black colouration, without commenting on chemical nature and role of such dark pigmentation, thus leaving the issue of its chemical identification and characterisation open (Otieno 1968; Pegler and Rayner 1969, 1969; Natarajan 1979; Van Der Westhuizen and Eicker 1990; Pegler and Vanhaecke 1994; Abdullah and Rusea 2009; De Kesel 2011; Srivastava et al. 2011; Tibuhwa 2012; Karun and Sridhar 2013; Aryal et al. 2016).
In spite of extraction of melanin from several edible mushroom species, there is no knowledge regarding edible melanin obtained from a symbiotic mushroom which can provide better source of mushroom melanin as this Termitomyces species is well consumed in entire Asian and African continent for its delicacy. The present study thus aimed to produce the dark melanin-like pigment from pure culture under controlled conditions, purify it and verify its chemical identity as melanin and characterise it structurally.
Materials and methods
Source and growth conditions of melanic culture
Fresh, healthy Termitomyces albuminosus fruitbodies were collected from Mardol, Goa during monsoon season and taxonomically identified using standard published Termitomyces keys (Heim 1942, 1977). Several pure cultures were obtained from sterile context tissue explants of pileus on 2% Malt Extract Agar (MEA) medium (Malt extract refined bacteriological grade 2% and Agar bacteriological grade 2%) with 0.01 mg/mL concentration of nalidixic acid and neomycin (HiMedia Chemicals Ltd., Mumbai, India). Growth, morphology, and pigmentation in colonies were monitored and a promising strain showing dark melanin like pigmentation was selected and microscopically checked for purity. The melanic strain was deposited in Goa University Fungus Culture Collection (WFCC Reg. no. 946) bearing GUFCC No. 20002 and maintained on Czapek Dox Agar (CDA) medium (0.5% sucrose, 0.2% sodium nitrate, 0.1% dipotassium phosphate, 0.05% magnesium sulphate heptahydrate, 0.05% potassium chloride, 0.001% ferrous sulphate heptahydrate, and 2% agar bacteriological grade), pH 5.5 and was incubated in incubator (Modern Industrial Corporation, Mumbai, India) at 28 ± 1 °C in dark.
Production of melanin in shaken submerged culture condition
Ten identical culture plugs were inoculated into 250 mL Erlenmeyer flasks containing 100 mL of Czapek Dox Solution (CDS) and were incubated on rotary shaker (Scigenics Biotech, Orbitek model LETT-A, Tamil Nadu, India) at 28 ± 1 °C, pH 5.5 for 1 week in dark with shaking at 150 rpm. Mycelial suspensions were obtained from pellets (Kalisz et al. 1986). Inoculum (10% v/v) was transferred into 2000 mL Erlenmeyer flasks containing 1000 mL of CDS having 5 g/L sucrose, pH 5.5 and incubated at 28 ± 1 °C for 20 days on rotary shaker at 150 rpm. Flasks were incubated at room temperature for 20 days. Insoluble melanin bound to mycelial biomass was extracted after 20 days.
Melanin extraction and purification
Termitomyces albuminosus pellet biomass was harvested using sterile stainless steel sieve of 100 µm mesh size, washed with sterile double distilled water three times, and oven dried at 70 °C overnight to a constant weight for estimation of mycelial dry weight. Melanin was extracted from the dry powdered fungal biomass using modification in previously described method (Sun et al. 2016b). Dry biomass powdered using mortar and pestle was subjected for melanin extraction in 100 mL 2 mol/L NaOH, in autoclave at 120 °C for 20 min. Extracts obtained were centrifuged at 5000 rpm for 5 min., supernatant was adjusted to pH 1.5 with 7 mol/L HCl, then kept at room temperature (RT) for 2 h and centrifuged at 8000 rpm for 20 min to collect the precipitate. The precipitate was washed three times with milliQ water, and dried and redisloved in 2 mol/L NaOH and surpernatent was collected after centrifugation at 8000 for 20 min. The supernatent pH was readjusted to pH 1.5 with 7 mol/L HCl and then kept at RT for 2 h. The precipitate was collected by centrifugation at 8000 rpm for 20 min. The precipitates obtained of crude mealnin were hydrolysed with 7 mol/L HCl at 100 °C for 2 h in order to remove bound carbohydrates and proteins. Then contents were cooled at RT and precipitate was collected by centrifugation at 8000 rpm for 20 min. The precipitate was washed three times with milliQ water to remove chloridion followed by drying at RT. The dried melanin was sequentially washed with chloroform, ethyl acetate and absolute ethanol in order to remove bound lipids, dried at RT and was transferred to a desiccator. Subsequently, the dried melanin was redissolved in 2.0 mol/L NaOH, followed by centrifugation at 8000 rpm for 20 min. The supernatant was adjusted to pH 1.5 and centrifuged at 8000 rpm for 20 min. The pure melanin was obtained after repeated washing of the precipitate with milliQ water and then drying to a constant weight in an oven at 60 °C. Purified melanin was stored in an air tight, moisture free amber bottle at −20 °C.
Morphology of melanin particles
Bright field microscopy
Culture from dark pigmented colonies of T. albuminosus and smaller melanised pellets were mounted in plain lactophenol. Pure melanin particles obtained by purification process were mounted in DPX on slides and examined using Nikon Eclipse E200 microscope with Nikon DS-fi2 camera and NIS element microscope imaging software.
Scanning electro microscopy (SEM)
Pure dried powdered melanin particles were fixed on carbon tape on aluminium stub and sputter coated with Palladium for 10 s (Quorum SC7620 Sputter Coater, UK) and examined by SEM at 5 kV (Vega 3 SB, TeScan, Advanced Scientific Equipment Pvt. Ltd., Bangalore, India).
Fractal analysis
SEM images of 10000× magnification were subjected to 11 different mathematical methods to compute fractal dimension using CMEIAS JFrad version 1.0 software freely available at http://cme.msu.edu/cmeias/ (Ji et al. 2015). The output data of melanin fractal dimensions were saved as *csv files and analysed statistically using the SYSTAT 13.
Elemental composition of melanin
The elemental composition CHN (O) of pure T. albuminosus melanin was determined with approximately 5 mg solid samples using elemental analyser (Thermo Finnigan, Italy model FLASH EA 1112 series, SAIF–IIT Bombay analytical laboratory, India) dispersed in water. The sulphur content was computed after addition of C, H, N, O percentages and qualitatively detected using Lassaigne’s test (Harki et al. 1997).
Ultraviolet–visible (UV-VIS) and Fourier transform infrared spectroscopy (FTIR)
UV-VIS spectrum was obtained in the range 190–750 nm using UV–VIS spectrophotometer (Shimadzu UV-2400) 0.1 mol/L NaOH as reference (Suryanarayanan et al. 2004; Selvakumar et al. 2008). A standard melanin spectrum was also obtained using Sepia officinalis melanin (Sigma, Aldrich Chemicals, India). For FTIR spectral analysis, the purified T. albuminosus melanin sample was mixed with KBr (1:10) and pressed into a 1 mm thin pellets. FTIR spectra were recorded between 4000 and 500 cm−1 in transmission/absorbance mode on FTIR spectrometer (Shimadzu IR Prestige 21, Japan) averaging of 40 scans. Spectral resolution was 4 cm−1, encoding interval 1 cm−1, Happ–Genzel apodisation and scanning speed 2.8 mm s−1 (Mbonyiryivuze et al. 2015).
Electron paramagnetic resonance (EPR) spectroscopy
EPR spectra were recorded using 25 mg samples at 77 K using ESR–JEOL, Japan model JES–FA200 ESR spectrometer for x band (SAIF–IIT Bombay analytical laboratory, India). Parameters used to acquire the spectra were as follows: modulation amplitude, 0.16 mT; modulation frequency 100 KHz; centre field, 325 mT; sweep width, 25 mT; sweep time, 2 min; microwave frequency, 9.1 GHz; microwave power, 0.1 mW; and temperature 77 K (Enochs et al. 1993).
NMR studies
Solid-state 13C (CP/MAS) NMR spectra were acquired on a Bruker Avance II 500 MHz spectrometer at Central Salt and Marine Chemicals Research Institute (CSMCRI) analytical laboratory, India.
Results
Cultural growth and melanin production
Termitomyces albuminosus colonies on CDA after 8 days showed 7.9 ± 0.17 cm diameter, initially cottony white but after 7–8 days of incubation, exhibited brownish to black pigmentation from central and older region. Termitomyces albuminosus hyphal growth characters were as per standard pure Termitomyces cultural descriptions (Botha and Eicker 1991). The pigmentation radiated towards the margin (Figure 1(a,b)). Repeated subcultures of melanogenic strain produced same results. In shaken submerged condition, T. albuminosus culture consistently produced spiky brown to black pellets (Figure 1(c,d)). Melanin yield from T. albuminosus in present study was found to be 0.0142 ± 0.005 g/L from pelletized biomass.
Figure 1.
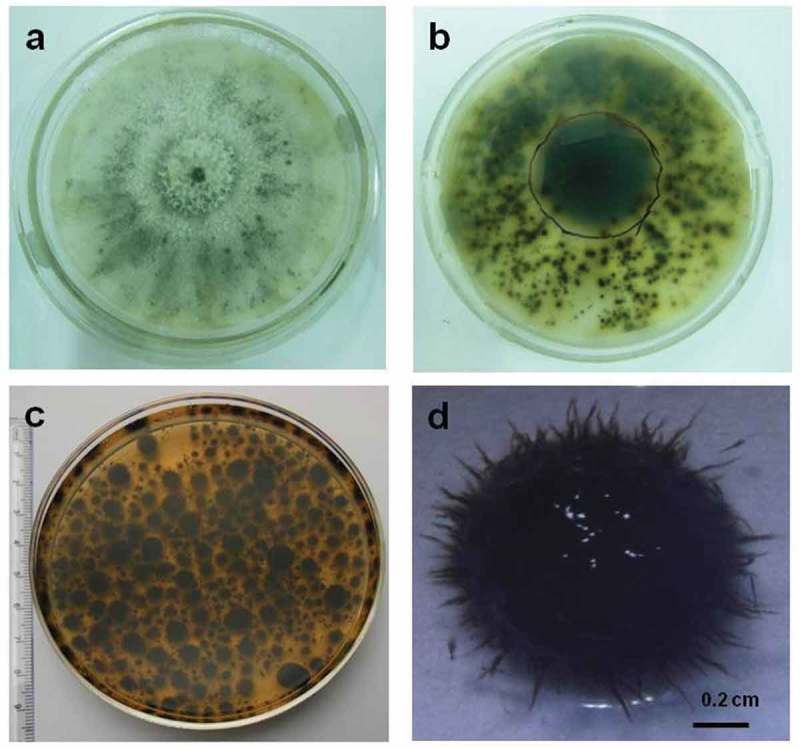
Melanin production in Termitomyces albuminosus Culture. (a) T. albuminosus colony surface view. (b) T. albuminosus colony reverse view. (c) T. albuminosus pellets production in submerged shaken condition. (d) Single-pellet morphology.
Melanin deposition sites and morphology of melanin granules
Micromorphologically T. albuminosus culture mat showed uniform deposition of brown–black pigment in hyphal cell wall and septa consistent with present knowledge (Figure 2(a)). Pellets showed central zone as dense black with brown peripheral spiky appendages (Figure 2(b)). Direct mount of purified melanin granules under bright field showed their polymorphic nature forming very thin, opaque amorphous black plates (Figure 2(c)). SEM images of purified sample showed the ultrafine structure of these thin amorphous plates comprising large clusters of almost spherical, compacted nanogranules. The plates show interesting but complex microtopography of nanogranules having 400–100 nm size (Figure 2(d,e,f)). Table 1 indicates the fractal analysis of pure melanin with fractal dimension D = 1.195–1.733.
Figure 2.

Microscopic analysis of Termitomyces melanin. (a) Cultural melanin with melanised hyphae showing cell wall bound and septal bound melanin under bright field view. (b) Pellet with spiky appendages cross section showing dark brown to black central core. (c) Pure dry melanin powder under bright field view. (d–f) Pure melanin granules at different magnifications under SEM view.
Table 1.
Fractal analysis of Melanin.
| Fractal dimensions methods | Mean ± SD |
|---|---|
| Dilation | 1.357 ± 0.050 |
| Euclidean distance map | 1.315 ± 0.048 |
| Box counting | 1.350 ± 0.081 |
| Fast | 1.155 ± 0.027 |
| Fast (hybrid) | 1.195 ± 0.034 |
| Parallel lines | 1.224 ± 0.032 |
| Cumulative intersection | 1.733 ± 0.084 |
| Mass radius (long) | 1.230 ± 0.051 |
| Mass radius (short) | 1.232 ± 0.050 |
| Corner (count) | 1.610 ± 0.078 |
| Corner (perimeter) | 1.616 ± 0.050 |
Note. Values are mean of (n = 3), ± SD (standard deviation).
Elemental composition
Elemental analysis of Termitomyces melanin mainly indicated C:H:N:O:S composition percentage as 54.679%, 3.544%, 2.492%, 26.924%, and 12.361% as listed in Table 2. The sulphur content was not directly estimated due to lack of S detection probe but derived stoichiometrically which is an alternative method and presence of S was confirmed by the positive Lassaigne’s test.
Table 2.
Elemental composition of melanin.
| Sample | Content % |
||||
|---|---|---|---|---|---|
| C | H | N | O | S | |
| Pure Termitomyces albuminosus melanin | 54.679 | 3.544 | 2.492 | 26.924 | 12.361 |
Note. The sulphur content was calculated from the equation (Harki et al. 1997).
S%= (100)–(∑ C %+ H % + N %+ O %).
UV -VIS and FTIR studies
UV-VIS spectrum showed absorption profile identical to standard sepia melanin. The absorption spectra of T. albuminosus melanin showed characteristic peak in the ultraviolet region at 233 nm and not in visible region (Figure 3(a)). Melanin from T. albuminosus culture also produced a linear form with a negative slope of −0.0026.
Figure 3.
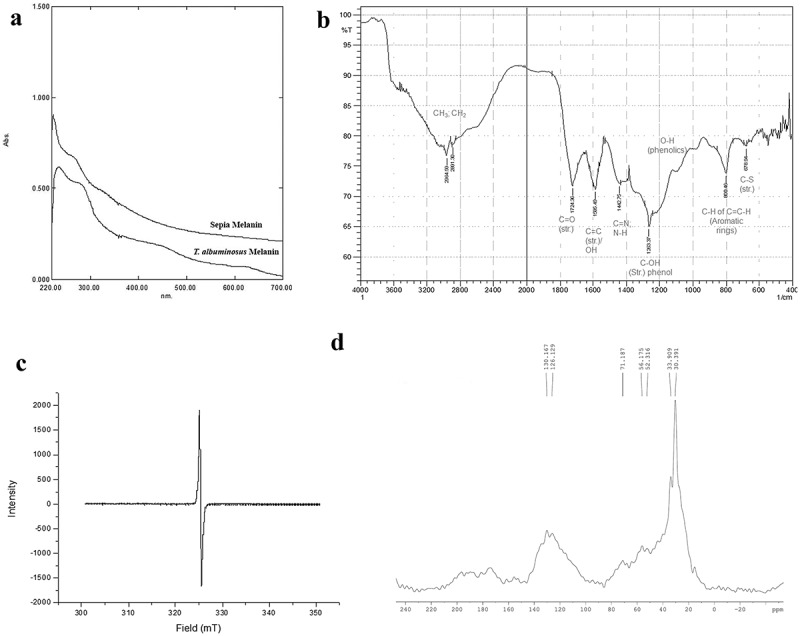
Spectral analysis of Termitomyces albuminosus melanin. (a) UV–VIS spectra of melanin. (b) FTIR spectrum of T. albuminosus melanin. (c) EPR of melanin. (d) 13C (CP/MAS) NMR spectra of melanin.
The infrared spectrum of melanin exhibited absorption band at 2964 cm−1 and 2891 cm−1, indicating the presence of CH3, CH2 aliphatic group. The 1724 cm−1, 1585 cm−1 and 1442 cm−1 bands indicate C = O, C = C and C = N / N–H group, whereas 1263 cm−1 indicates phenolic C–O–H band (Figure 3(b)). Table 3 provides a comparative view of FTIR spectral band analysis of T. albuminosus melanin with other fungal melanins. Termitomyces albuminosus melanin showed characteristic bands for aromatic rings and sulphur at 800 cm−1 and 678 cm−1.
Table 3.
FTIR spectroscopic characteristics of melanin.
| Fungus | Bands (cm−1) | Assignments | References |
|---|---|---|---|
| Phyllosticta capitalensis | 3352.5 1639.8 |
–OH, N–H bonds Conjugated carbonyl bonds |
Suryanarayanan et al. (2004) |
| Auricularia auricula | 1627.76 3422 2923.99 2853.83 |
Aromatic C=C & COO−group O–H stretching & NH2 groups Aliphathic group CH3 & CH2 |
Zhang et al. (2015) |
| Pleurotus cystidiosus | 3445.05 | OH group | Selvakumar et al. (2008) |
| Auricularia auricula | 3287.6 2925.8 2851.2 1702.3 1619.4 1378.8 |
OH & NH group CH3 group CH2 group C=O & COO– group |
Bin et al. (2012) |
| Termitomyces albuminosus | 2964 2891 1724 1585, 1442 1263 800 730, 710, 678 |
CH3 Aliphathic group CH2 group C=O stretching Overlapping O–H (def.) of C=C ring stretching C–O stretching due to phenol C–H (def.) of C=C–H (o.o.p.) from aromatic rings Weak absorption indicating C-S stretching |
Present study |
EPR spectroscopy
In the present study, EPR spectrum showed the peak at 2.00968 (G-value) for T. albuminosus melanin (Figure 3(c)).
NMR spectroscopy
13C (CP/MAS) NMR spectra are shown in Figure 3(d). Its spectral band assignments along with other reported melanins are summarised in Table 4. Characteristic chemical shift at 70–30 ppm representing =C–S and C–H carbon of open-chain aliphatic carbons present in cysteine/DOPA was observed in 13C NMR spectrum of Termitomyces.
Table 4.
13C NMR spectroscopic characteristics of melanin.
| Source and type of melanin | Chemical shift range (ppm) | Possible assignments | References |
|---|---|---|---|
| Oidiodendron tenuissimum, Trichoderma harzianum, Ulocladium atrum, Hendersonula toruloidea, Eurotium echinulatum | 220–160 | Carboxyl/carbonyl groups | Knicker et al. (1995) |
| 160–140 | Aromatic COR or CNR groups | ||
| 140–110 | Aromatic C–H carbons, guaiacyl C-2/C-6 Olefinic carbons | ||
| 110–90 | Anomeric carbon of carbohydrates, C-2/C-6 of Syringyl | ||
| 90–60 | Carbohydrate- derived structures (C-2 to C-5) in hexoses, C-2 of some amino acids & higher alcohols | ||
| 60–45 | Methoxyl groups, C-6 of carbohydrates, C-2 of most amino acids | ||
| 45–0 | Methylene groups in aliphatic rings & chains, methyl groups bound to carbon | ||
| Dopa melanin | 172 | Carbonyl carbon | Duff et al. (1988) |
| 143, 118 | Aromatic carbons | ||
| 55, 35 | Aliphatic carbons | ||
| Melanoma melanin | 173 | Carbonyl carbon | |
| 125 | Aromatic carbons | ||
| 53,33 | Aliphatic carbons | ||
| Sepia melanin | 173 | Carbonyl carbon | |
| 140–110 | Aromatic carbons | ||
| 70–30 | Aliphatic carbons | ||
| Sepia melanin | 200–160 | Carbonyl carbon | Adhyaru et al. (2003) |
| 160–135 | Aromatic & Indolic Cq (non-protonated) | ||
| 135–90 | Aromatic & Indolic CH (protonated) | ||
| 95–10 | Aliphatic carbons | ||
| Sepia melanin Free acid (MFA) | 200–160 | Carbonyl carbon | |
| 165–135 | Aromatic & Indolic Cq (non-protonated) | ||
| 135–100 | Aromatic & Indolic CH (protonated) | ||
| 95–10, 50–0 | Aliphatic carbons | ||
| Sepia melanin | 200–187, 167, 164 | Carbonyl carbon | Hervé et al. (1994) |
| 147–110 | Aromatic & ethylenic Cq (non-protonated) | ||
| 131–127, 119–95 | Aromatic & ethylenic CH (protonated) | ||
| 75–15 | Aliphatic carbons | ||
| T. albuminosus melanin | 200–170 | Carbonyl carbon | Present study |
| 160–110 | Aromatic carbons | ||
| 45–40 | =C–S | ||
| 71, 56, 52, 33, 30 | Aliphatic carbons in cysteine/DOPA |
Discussion
This is first report on formation of a dark melanin like pigment in Termitomyces colonies, a phenomenon noticed in natural fruitbodies and confirmation of the pigment as melanin. Despite taxonomic knowledge about universal occurrence of dark pigmentation in Termitomyces fruitbodies, no attention has been paid to establish its chemical identity as melanin. In addition, no reports have been found on melanogenesis in pure cultures of Termitomyces species. This may be due to availability of very few pure cultures available in world culture collections for scientific community to work. In spite of 90 total taxa recorded pending systematic revision and found listed in Index Fungorum mycological database (www.indexfungorum.org) indicating high diversity of Termitomyces species in Asia and Africa, the catalogues in World Federation for culture collection have only 11 Termitomyces strains listed globally. This may be due to relative lack of interest in high-frequency culturing of wild-edible Termitomyces species or failure to get healthy fruitbodies and viable spores for isolating mycelial cultures. The present study overcame the problem by obtaining several mycelial cultures from different Termitomyces species and zeroing down on a stable melanogenic strain of T. albuminosus able to show excellent growth on solid medium as well as under submerged culture conditions. Previously (Siddiquee et al. 2012, 2015) reported dark grey to black colouration in T. heimii and T. aurantiacus culture grown on Potato Dextrose Agar medium after 7 days but failed to identify the melanogenesis process. Zhang et al. (2015) reported melanin from culture free filtrate of Auricularia auricula in submerged culture conditions yielding 0.124–0.558 g/L. However, Sun et al. (2016b) reported yield of 2.22 g/L melanin in culture filtrate of A. auricula in complete medium containing lactose, yeast extract, tyrosine, calcium chloride and sodium chloride, but not estimated melanin bound to cultural biomass. In the present study, the final product of melanin accounted for about 0.012% (w/w) of dry biomass. Relatively T. albuminosus strain used in the present study yielded less melanin probably due to choice of the medium, being a symbiotic mushroom or many other physiological parameters which need to tested in future.
In melanised fungi, pigment is known to be localised in the cell wall, in the outermost layer or embedded within the wall as granules, layered in fibrils, or bound to cell wall chitin (Butler and Day 1998). In this study, Termitomyces melanin was microscopically detected to be present in cell wall or septa. Nanoparticle nature of melanin has been studied (Beltrán-García et al. 2014) and our results are consistent with the same. Consistent with the latest development in understanding the properties of such complex surfaces in topological quantum chemistry it would be interesting to see whether melanin nanogranules could also be subjected to topochemical studies (Bradlyn et al. 2017; Fiete 2017) which might explain some interesting properties. Melanins fractal dimensions results clearly implying that assembly of melanin nanogranules may occur in fractal pattern (Bridelli 1998; Eom et al. 2017). It has been known that melanin purification steps lead to dehydration thus making the polymer more aggregated and it results in loss of capacity for physiological interactions (Nicolaus 1968; Prota 1992). The aggregated structure of melanin is postulated to prevent reactive oxygen species formation because photoactive residues are less exposed (Beltrán-García et al. 2014) however the function of T. albuminosus melanin may be more complex as it is a mutualistic species with hypogeal anamorph and epigeal teleomorph (Piearce 1987) .
Melanin produced by DHN pathway contains carbon and oxygen only, while the L-DOPA pathway melanins also contain nitrogen. Melanin synthesised via the L-DOPA pathway is referred to as eumelanin. DOPA melanins in presence of oxygen and tyrosinase are also known to undergo cysteinylation (incorporation of cysteine in the polymer). These melanins, red or yellow-coloured pigments are termed as pheomelanins initially synthesised just like eumelanins and contain sulphur (El-Naggar and El-Ewasy 2017). Termitomyces melanin could be a form of sulphur-rich pheomelanin as this group mainly consists of sulphur-containing benzothiazine and benzathiozol derivatives. Generally, pheomelanins or DOPA melanin chemically modified by amino acids such as cys–DOPA melanins are known to have approx. 9–16% sulphur content. These findings are in accordance with those reported by Harki et al. (1997; Costa et al. 2015; Sun et al. 2016b). According to Ye et al. (2014), about 14.83% sulphur content was determined by elemental analysis from Lachnum YM404 strain. Also the effect of medium composition on melanin composition is known. According to Bull (1970), in Aspergillus nidulans melanin pigment varied in composition with response to growth medium and the most significant finding was the widely varying nitrogen content of the melanin in response to the growth medium. Bull (1970) reported percentage composition of melanin in Czapek Dox Medium as C = 56.40%, H = 6.55%, and N = 3.92–1.78% (on addition of DOPA & Catechol), indicating that melanin composition can vary from medium to medium. High sulphur content of melanin in Termitomyces is possible due to availability of sulphur-containing amino acids and sulphite reductase enzymes. Previously, Alofe (1991; Botha and Eicker 1992; Ijeh et al. 2016; Sun et al. 2017) reported sulphur-containing (methionine, cysteine) amino acids from Termitomyces umkowaani, T. sagittaeformis T. reticulatus, T. robustus, and T. microcarpus fruitbodies. These amino-acid compositions vary from one geographic region to another. Laccase enzyme which is known to play a key role in biosynthesis of melanin has been also reported from Termitomyces (Bose et al. 2007; Gangwar et al. 2016). Rahmad et al. (2014) identified sulphite reductase enzyme from T. heimii which plays a key role in sulphur assimilation. Our results indicate that Termitomyces species may have efficient sulphur metabolism involving an unidentified pathway linked to O-acetylserine to form cysteine (Leustek et al. 2000; Kopriva and Koprivova 2003). According to Plonka and Grabacka (2006), the possible melanin synthesis pathway in Termitomyces using laccase enzyme and source of sulphur pool as amino acids can be written as
DOPA→DOPAquinone→CysteinylDOPA→1,4-Benzothiazinylalanine→pheomalanin.
which is required to be tested in future as the present study only aimed at the characterisation of melanin pigment from genus Termitomyces.
The linear decrease in the absorption with increasing wavelength was observed for Termitomyces melanin similar to that reported by (Zhang et al. 2015). Absorption peaks in UV regions occur due to the presence of many conjugated structures in melanin molecule (Ou-Yang et al. 2004). The log of optical density of a melanin solution when plotted against wavelength produces a linear curve with negative slopes. Such characteristic straight lines with negative slopes have been obtained from some melanogenic fungi such as Phyllosticta capitalensis and Auricularia auricula with slope ranging −0.0015 to −0.0030 (Ellis and Griffiths 1974; Suryanarayanan et al. 2004; Bin et al. 2012; Zhang et al. 2015). The slopes of linear plots are often used to identify melanins and matching spectral features in the present work confirms the identity of T. albuminosus melanin.
TFTIR studies carried out by Sava et al. (2001) reported that absorption is reduced at 3450 cm−1 and 1650 cm−1, after acid hydrolysis treatment undertaken during purification step due to formation of reactions between phenolic and carboxylic groups to form lactones. Also treatment with chloroform and ethyl acetate could have reduced absorption at 2900–2850 cm−1 in spectra.
Melanin polymers are known to have paramagnetic character and o-semiquinone free radical with spin (S = 1/2). These unpaired electrons of free radicals obey EPR effect (Pilawa et al. 2017). Enochs et al. (1993) described a standardised and effective test for the identification of melanin pigment by identifying the presence of stable population of organic free radical signal. The G-value of fungal melanin is reported to be 2.0012 (Selvakumar et al. 2008). Termitomyces albuminosus melanin G-value is found to be somewhat higher which could be due to O-semiquinone free radicals. Bin et al. (2012) also showed higher G-value of 2.0042 for Auricularia auricula melanin. It has been known that sulphur-containing radicals show high G-value (Bolman et al. 1970); therefore, incorporation of a sulphur-rich scaffold in melanin of T. albuminosus may result in a high G-value.
TAliphatic amine structural elements are proposed to arise in 13C NMR spectrum from coupling of dopamine/quinone structural units which are unique to dopamine melanins (Della Vecchia et al. 2013; Chatterjee et al. 2014). Tian et al. (2003) reported that carbon-near sulphur shows chemical shift at 45–40 ppm and CH2 carbon–CH2–CH(NH2)–COOH of tyrosine/DOPA can also be seen around 40–35 ppm in 13C NMR spectrum which is consistent with our sulphur-containing melanin claim.
Conclusions
The present study successfully established the chemical identity of the dark pigment as a unique form of fungal melanin with high sulphur content. The exact structure of melanin polymers is difficult to elucidate and the benefit of incorporation of a sulphur scaffold in Termitomyces melanin needs further exploration as it may play functionally important roles at crucial and critical stages in the natural life cycle of Termitomyces holomorph in protecting the species from injury and damage.
Acknowledgments
TThe authors thank Goa University Fungus Culture Collection and Research Unit (GUFCCRU) for culture, Department of Botany, Goa University for providing Scanning Electron Microscope facility and University Science Instrumentation Centre (USIC), Goa University for sputter coating facility. The authors also thank SAIF IIT, Bombay for providing elemental analysis and CSMCRI for 13C NMR facility. A word of thanks also goes to Department of Chemistry, Goa University for providing FTIR facility. Special thanks to Senior sales Azhar Ullah Khan for providing Systat 13 software. RA De Souza acknowledges DST-Inspire Fellowship (SRF), Govt. of India.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdullah F, Rusea G.. 2009. Documentation of inherited knowledge on wild edible fungi from Malaysia. Blumea- Biodiversity, Evol Biogeo Plants. 54:35–38. [Google Scholar]
- Index Fungorum mycological database. Available from : www.indexfungorum.org. AccessedMay31, 2018.
- Adhyaru BB, Akhmedov NG, Katritzky AR, Bowers CR. 2003. Solid–state cross–polarization magic angle spinning 13C and 15N NMR characterization of sepia melanin, sepia melanin free acid and human hair melanin in comparison with several model compounds. Magn Reson Chem. 41(6):466–474. [Google Scholar]
- Alofe FV. 1991. Amino acids and trace minerals of three edible wild mushrooms from Nigeria. J Food Comp Anal. 4(2):167–174. [Google Scholar]
- Arun G, Eyini M, Gunasekaran P. 2015. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J Exp Biol. 53:380–387. [PubMed] [Google Scholar]
- Aryal HP, Ghimire SK, Budhathoki U. 2016. Termitomyces: New to the Science. J Plant Sci Res. 3(1):148–150. [Google Scholar]
- Babitskaia VG, Shcherba VV, Ikonnikova NV. 2000b. Melanin complex of the fungus Inonotus obliquus. Appl Biochem Microbiol. 36:377–381. [PubMed] [Google Scholar]
- Babitskaya VG, Scherba VV, Ikonnikova NV, Bisko NA, Mitropolskaya NY. 2002. Melanin complex from medicinal mushroom Inonotus obliquus (Pers.: Fr.) Pilat (Chaga) (Aphyllophoromycetidae). Int J Med Mushrooms. 4:139–145. [Google Scholar]
- Babitskaya VG, Shcherba VV, Filimonova TV, Grigorchuk EA. 2000a. Melanin pigments from the fungi Paecilomyces variotii and Aspergillus carbonarius. Appl Biochem Microbiol. 36(2):128–133. [PubMed] [Google Scholar]
- Bell AA, Wheeler MH. 1986. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 24(1):411–451. [Google Scholar]
- Beltrán-García MJ, Prado FM, Oliveira MS, Ortiz-Mendoza D, Scalfo AC, Jr A P, Medeiros MH, White JF, Di Mascio P. 2014. Singlet molecular oxygen generation by light-activated DHN-melanin of the fungal pathogen Mycosphaerella fijiensis in black Sigatoka disease of bananas. PloS one. 9:e91616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin L, Wei L, Xiaohong C, Mei J, Mingsheng D. 2012. In vitro antibiofilm activity of the melanin from Auricularia auricula, an edible jelly mushroom. Ann Microbiol. 62(4):1523–1530. [Google Scholar]
- Bisko NA, Mitropolskaya NY, Ikonnikova NV. 2002. Melanin complex from medicinal mushroom Inonotus obliquus (Pers.: Fr.) Pilat (Chaga) (Aphyllophoromycetideae). Int J Med Mushrooms. 4(2):139–145. [Google Scholar]
- Bisko NA, Shcherba VV, Mitropolskaya NY. 2007. Study of melanin complex from medicinal mushroom Phellinus robustus (P. Karst.) Bourd. et Galz. (Aphyllophoromycetideae). Int J Med Mushrooms. 9(2):177–184. [Google Scholar]
- Blumenberg M. 2017. Introductory Chapter: Melanin, a Versatile Guardian. In Melanin. InTechOpen, Rijeka. [Google Scholar]
- Bolman PS, Safarik I, Stiles DA, Tyerman WJ, Strausz OP. 1970. Electron paramagnetic resonance spectra of some sulfur-containing radicals. Can J Chem. 48(24):3872–3876. [Google Scholar]
- Bose S, Mazumder S, Mukherjee M. 2007. Laccase production by the white rot fungus Termitomyces clypeatus. J Basic Microbiol. 47(2):127–131. [DOI] [PubMed] [Google Scholar]
- Botha WJ, Eicker A. 1991. Cultural studies on the genus Termitomyces in South Africa. I. Macro-and microscopic characters of basidiome context cultures. Mycol Res. 95(4):435–443. [Google Scholar]
- Botha WJ, Eicker A. 1992. Nutritional value of Termitomyces mycelial protein and growth of mycelium on natural substrates. Mycol Res. 96(5):350–354. [Google Scholar]
- Bradlyn B, Elcoro L, Cano J, Vergniory MG, Wang Z, Felser C, Aroyo MI, Bernevig BA. 2017. Topological quantum chemistry. Nature. 547:298–305. [DOI] [PubMed] [Google Scholar]
- Bridelli MG. 1998. Self-assembly of melanin studied by laser light scattering. Biophys chem. 73(3):227–239. [DOI] [PubMed] [Google Scholar]
- Bull AT. 1970. Chemical composition of wild-type and mutant Aspergillus nidulans cell walls. Nat polysaccharide melanin constituents Microbiol 63:75–94. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Day AW. 1998. Fungal melanins: a review. Can J Microbiol. 44(12):1115–1136. [Google Scholar]
- Butler MJ, Day AW, Henson JM, Money NP. 2001. Pathogenic properties of fungal melanins. Mycologia. 93(1):1–8. [Google Scholar]
- Casadevall A, Cordero RJ, Bryan R, Nosanchuk J, Dadachova E. 2017. Melanin, radiation, and energy transduction in fungi. Microbiol Spectr. 5(2):FUNK-0037-2016. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Prados-Rosales R, Tan S, Itin B, Casadevall A, Stark RE. 2014. Demonstration of a common indole-based aromatic core in natural and synthetic eumelanins by solid-state NMR. Org Biomol Chem. 12(34):6730–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang C, Shu C, Zhu M, Zhou E. 2015. Isolation and characterization of a melanin from Rhizoctonia solani, the causal agent of rice sheath blight. Eur J Plant Pathol. 142(2):281–290. [Google Scholar]
- CMEIAS JFrad version 1.0 software Available from: http://cme.msu.edu/cmeias/AccessedNovember23, 2017.
- Cordero RJ, Casadevall A. 2017. Functions of fungal melanin beyond virulence. Fungal Biol Rev. 31(2):99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa TG, Feldhaus MJ, Vilhena FS, Heller M, Micke GA, Oliveira AS, Brighente I, Monteiro FB, Creczynski-Pasa TB, Szpoganicz B. 2015. Preparation, characterization, cytotoxicity and antioxidant activity of DOPA melanin modified by amino acids: melanin-like oligomeric aggregates. J Braz Chem Soc. 26(2):273–281. [Google Scholar]
- De Kesel A. 2011. Provisional macroscopic key to the edible mushrooms of tropical Africa: 100+ taxa from the Zambezian and Sudanian Region. MycoAfrica. 4(1):1–8. [Google Scholar]
- Della Vecchia NF, Avolio R, Alfè M, Me E, Napolitano A, d’Ischia M. 2013. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv Funct Mater. 23(10):1331–1340. [Google Scholar]
- Duff GA, Roberts JE, Foster N. 1988. Analysis of the structure of synthetic and natural melanins by solid-phase NMR. Biochemistry. 27(18):7112–7116. [DOI] [PubMed] [Google Scholar]
- Eisenman HC, Casadevall A. 2012. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol. 93(3):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DH, Griffiths DA. 1974. The location and analysis of melanins in the cell walls of some soil fungi. Can J Microbiol. 20(10):1379–1386. [Google Scholar]
- El-Naggar NE, El-Ewasy SM. 2017. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep. 7:42129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enochs WS, Nilges MJ, Swartz HM. 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic resonance (EPR) spectroscopy. Pigment Cell Melanoma Res. 6(2):91–99. [DOI] [PubMed] [Google Scholar]
- Eom T, Woo K, Cho W, Heo J, Jang D, Shin JI, Martin DC, Wie JJ, Shim BS. 2017. Nanoarchitecturing of natural melanin nanospheres by layer-by-layer assembly: macroscale anti-inflammatory conductive coatings with optoelectronic tunability. Biomacromolecules. 18(6):1908–1917. [DOI] [PubMed] [Google Scholar]
- Fernandez CW, Koide RT. 2013. The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol. 6(6):479–486. [Google Scholar]
- Fiete GA. 2017. Materials science: chemistry and physics happily wed. Nature. 547:287–288. [DOI] [PubMed] [Google Scholar]
- Mbonyiryivuze A, Mwakikunga B, Dhlamini SM, Maaza M. 2015. Fourier transform infrared spectroscopy for sepia melanin. Phys Mater Chem. 3(2):25 - 29. [Google Scholar]
- Gangwar R, Rasool S, Mishra S. 2016. Evaluation of cellobiose dehydrogenase and laccase containing culture fluids of Termitomyces sp. OE147 for degradation of Reactive blue 21. Biotechnol Rep. 12:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves RC, Lisboa HC, Pombeiro-Sponchiado SR. 2012. Characterization of melanin pigment produced by Aspergillus nidulans. World J Microbiol Biotechnol. 28(4):1467–1474. [DOI] [PubMed] [Google Scholar]
- Harki E, Talou T, Dargent R. 1997. Purification, characterisation and analysis of melanin extracted from Tuber melanosporum Vitt. Food Chem. 58(1–2):69–73. [Google Scholar]
- Heim R. 1942. Nouvelles etudes descriptives sur les agarics termitophiles d’Afrique tropicale. Arch Mus Natl Hist Nat ser. 6(18):107–166. [Google Scholar]
- Heim R. 1977. Termites et champignons. Les champignons termitophiles d’Afrique noire et d’Asie meridionale. Soc. Nouv. Edit. Paris: Boubee. [Google Scholar]
- Henson JM, Butler MJ, Day AW. 1999. The dark side of the mycelium: melanins of phytopathogenic fungi. Annu Rev Phytopathol. 37(1):447–471. [DOI] [PubMed] [Google Scholar]
- Hervé M, Hirschinger J, Granger P, Gilard P, Deflandre A, Goetz N. 1994. A 13C solid-state NMR study of the structure and auto-oxidation process of natural and synthetic melanins. Biochim Biophys Acta Protein Struct Mol Enzymol. 1204(1):19–27. [DOI] [PubMed] [Google Scholar]
- Ijeh II, Eke IN, Ugwu CC, Ejike EC. 2016. Myco-nourishment from the wild: chemical analyses of the nutritional and amino acid profile of Termitomyces robustus harvested from Uzuakoli, Nigeria. Nat Prod Chem Res. 4:225. [Google Scholar]
- Ji Z, Card KJ, Dazzo FB. 2015. CMEIAS JFrad: a digital computing tool to discriminate the fractal geometry of landscape architectures and spatial patterns of individual cells in microbial biofilms. Microb Ecol. 69(3):710–720. [DOI] [PubMed] [Google Scholar]
- Kalisz HM, Wood DA, Moore D. 1986. Regulation of extracellular laccase production of Agaricus bisporus by nitrogen sources in the medium. FEMS microbiol lett. 34(1):65–68. [Google Scholar]
- Karun NC, Sridhar KR. 2013. Occurrence and distribution of Termitomyces (Basidiomycota, Agaricales) in the Western Ghats and on the west coast of India. Czech Mycol. 65(2):233–254. [Google Scholar]
- Kendrick B. 2001. The fifth kingdom, 3rd:Sidney, BC, Canada; Mycologue Publisher [Google Scholar]
- Knicker H, Almendros G, Gonzalez-Vila FJ, Lüdemann HD, Martin F. 1995. 13C and 15N NMR analysis of some fungal melanins in comparison with soil organic matter. Org Geochem. 23:1023–1028. [Google Scholar]
- Kopriva S, Koprivova A. 2003. Sulphate assimilation: a pathway which likes to surprise In: Abrol YP, Ahmad A, eds. Sulphur in Plants. Dordrecht: Springer; p. 87–112. [Google Scholar]
- Leustek T, Martin MN, Bick JA, Davies JP. 2000. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Biol. 51:141–165. [DOI] [PubMed] [Google Scholar]
- Mendoza CG, Leal JA, Novaes-Ledieu M. 1979. Studies of the spore walls of Agaricus bisporus and Agaricus campestris. Can J Microbiol. 25(1):32–39. [DOI] [PubMed] [Google Scholar]
- Money NP, Caesar-TonThat TC, Frederick B, Henson JM. 1998. Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in Gaeumannomyces graminis var. graminis. Fungal Genet Biol. 24(1–2):240–251. [DOI] [PubMed] [Google Scholar]
- Natarajan K. 1979. South Indian Agaricales V: Termitomyces heimii. Mycologia. 71(4):853–855. [Google Scholar]
- Nicolaus RA. 1968. Melanins. Paris: Hermann. [Google Scholar]
- Otieno NC. 1968. Further contributions to a knowledge of termite fungi in East Africa: the genus Termitomyces Heim. Sydowia. 22:160–165. [Google Scholar]
- Ou-Yang H, Stamatas G, Kollias N. 2004. Spectral responses of melanin to ultraviolet A irradiation. J Investig Dermatol. 122(2):492–496. [DOI] [PubMed] [Google Scholar]
- Pal AK, Gajjar DU, Vasavada AR. 2013. DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med Mycol. 52(1):10–18. [DOI] [PubMed] [Google Scholar]
- Pegler DN, Rayner RW. 1969. A contribution to the Agaric flora of Kenya. Kew Bull. 23(3):347–412. [Google Scholar]
- Pegler DN, Vanhaecke M. 1994. Termitomyces of Southeast Asia. Kew Bull. 49(4):717–736. [Google Scholar]
- Piearce GD. 1987. The genus Termitomyces in Zambia. Mycologist. 1(3):111–116. [Google Scholar]
- Pilawa B, Zdybel M, Chodurek E. 2017. Application of electron paramagnetic resonance spectroscopy to examine free radicals in melanin polymers and the human melanoma malignum cells. In Melanin. InTechOpen, Rijeka. [Google Scholar]
- Plonka PM, Grabacka M. 2006. Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim Pol. 53:429–443. [PubMed] [Google Scholar]
- Polacheck I, Kwon-Chung KJ. 1988. Melanogenesis in Cryptococcus neoformans. Microbiology. 134(4):1037–1041. [DOI] [PubMed] [Google Scholar]
- Prota G. 1992. Melanins and melanogenesis. San Diego, CA: Academic Press. [Google Scholar]
- Rahmad N, Al-Obaidi JR, Rashid NM, Zean NB, Yusoff MH, Shaharuddin NS, Jamil NA, Saleh NM. 2014. Comparative proteomic analysis of different developmental stages of the edible mushroom Termitomyces heimii. Biol Res. 47:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava VM, Galkin BN, Hong MY, Yang PC, Huang GS. 2001. A novel melanin-like pigment derived from black tea leaves with immuno-stimulating activity. Food Res Int. 34(4):337–343. [Google Scholar]
- Schmaler-Ripcke J, Sugareva V, Gebhardt P, Winkler R, Kniemeyer O, Heinekamp T, Brakhage AA. 2009. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl Environ Microbiol. 75(2):493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar P, Rajasekar S, Periasamy K, Raaman N. 2008. Isolation and characterization of melanin pigment from Pleurotus cystidiosus (telomorph of Antromycopsis macrocarpa). World J Microbiol Biotechnol. 24(10):2125–2131. [Google Scholar]
- Siddiquee S, Rovina K, Naher L, Rodrigues KF, Uzzaman MA. 2015. Phylogenetic relationships of Termitomyces aurantiacus inferred from internal transcribed spacers DNA sequences. Adv Biosci Biotechnol. 6:358–367. [Google Scholar]
- Siddiquee S, Yee WY, Taslima K, Fatihah NH, Kumar SV, Hasan MM. 2012. Sequence analysis of the ribosomal DNA internal transcribed spacer regions in Termitomyces heimii species. Ann Microbiol. 62(2):797–803. [Google Scholar]
- Srivastava B, Dwivedi AK, Pandey VN. 2011. Morphological characterization and yield potential of Termitomyces spp. mushroom in Gorakhpur forest division. Bull Env Pharmacol Life Sci. 1(1):54–56. [Google Scholar]
- Sun L, Liu Q, Bao C, Fan J. 2017. Comparison of free total amino acid compositions and their functional classifications in 13 wild edible mushrooms. Molecules. 22(3):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang X, Chen W, Zhang L, Zhu H. 2016a. Production of natural edible melanin by Auricularia auricula and its physicochemical properties. Food Chem. 196:486–492. [DOI] [PubMed] [Google Scholar]
- Sun S, Zhang X, Sun S, Zhang L, Shan S, Zhu H. 2016b. Production of natural melanin by Auricularia auricula and study on its molecular structure. Food Chem. 190:801–807. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan TS, Ravishankar JP, Venkatesan G, Murali TS. 2004. Characterization of the melanin pigment of a cosmopolitan fungal endophyte. Mycol Res. 108(8):974–978. [DOI] [PubMed] [Google Scholar]
- Tian S, Garcia-Rivera J, Yan B, Casadevall A, Stark RE. 2003. Unlocking the molecular structure of fungal melanin using 13C biosynthetic labeling and solid-state NMR. Biochem. 42(27):8105–8109. [DOI] [PubMed] [Google Scholar]
- Tibuhwa DD. 2012. Termitomyces species from Tanzania, their cultural properties and unequalled basidiospores. J Biol Life Sci. 3:1. [Google Scholar]
- Van Der Westhuizen GC, Eicker A. 1990. Species of Termitomyces occurring in South Africa. Mycol Res. 94(7):923–937. [Google Scholar]
- Weijn A, Bastiaan-Net S, Wichers HJ, Mes JJ. 2013. Melanin biosynthesis pathway in Agaricus bisporus mushrooms. Fungal Genet Biol. 55:42–53. [DOI] [PubMed] [Google Scholar]
- Wood TG, Sands WA. 1978. The role of termites in ecosystems In: Brian MV, Ed. Production ecology of ants and termites. Cambridge, UK: Cambridge University Press; p. 245–292. [Google Scholar]
- Ye M, Guo GY, Lu Y, Song S, Wang HY, Yang L. 2014. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int J Biol Macromol. 63:170–176. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xiao G, Thring RW, Chen W, Zhou H, Yang H. 2015. Production and characterization of melanin by submerged culture of culinary and medicinal fungi Auricularia auricula. Appl Biochem Biotechnol. 176(1):253–266. [DOI] [PubMed] [Google Scholar]
- Zou Y, Xie C, Fan G, Gu Z, Han Y. 2010. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov Food Sci Emerg Technol. 11(4):611–615. [Google Scholar]


