Abstract
What is the role of a single dose of oral corticosteroids for those with acute sore throat? Using the GRADE framework according to the BMJ Rapid Recommendation process, an expert panel make a weak recommendation in favour of corticosteroid use. The panel produced these recommendations based on a linked systematic review triggered by a large randomised trial published in April 2017. This trial reported that corticosteroids increased the proportion of patients with complete resolution of pain at 48 hours. Box 1 shows all of the articles and evidence linked in this Rapid Recommendation package. The infographic provides the recommendation together with an overview of the absolute benefits and harms of corticosteroids in the standard GRADE format. Table 2 below shows any evidence that has emerged since the publication of this article. Clinicians and their patients can find consultation decision aids to facilitate shared decision making in MAGICapp (www.magicapp.org/goto/guideline/JjXYAL/section/j79pvn).
What you need to know:
Sore throat is one of the most common reasons for primary care appointments, and international guidance varies about whether to use corticosteroids to treat it, but a trial published in April 2017 suggested that costicosteroids might be effective
We make a weak recommendation to use a single dose of oral corticosteroids, in those presenting with acute sore throat, after performing a systematic review of the new evidence in this rapid recommendation publication package
The recommendation is weak and shared decision making is needed because corticosteroids did not help all patient reported outcomes and patients’ preferences varied substantially
Steroids somewhat reduced the severity and duration of pain by one day, but time off school or work was unchanged. Harm seems unlikely with one steroid dose.
The treatment is inexpensive and likely to be offered in the context of a consultation that would have taken place anyway
Box 1: Linked articles in this BMJ Rapid Recommendations cluster
-
Aertgeerts B, Agoritsas T, Siemieniuk RAC, et al. Corticosteroids for sore throat: a clinical practice guideline. BMJ 2017;358:j4090. doi:10.1136/bmj.j4090
Summary of the results from the Rapid Recommendation process
-
Sadeghirad B, Siemieniuk RA, Brignardello-Petersen R, et al. Corticosteroids for treatment of sore throat: a systematic review and meta-analysis of randomised trials. BMJ 2017;358:j3887. doi:10.1136/bmj.j3887
Review of all available randomised trials that assessed corticosteroids as adjunct treatment versus standard care for sore throat.
-
MAGICapp (www.magicapp.org/goto/guideline/JjXYAL/section/j79pvn)
Expanded version of the results with multilayered recommendations, evidence summaries, and decision aids for use on all devices
Acute sore throat is defined as pain in the throat for less than 14 days. Acute sore throat could be caused by pharyngitis, nasopharyngitis, tonsillitis, peritonsillar abscess, or retropharyngeal abscess. Some patients with sore throat also experience headache, fever, muscle stiffness, cough, and general malaise.
Acute sore throat is common, but only a minority of patients will visit their general practitioner.1 A survey reported that the main reasons are to establish the cause of the symptoms, obtain pain relief, and to gain information on the course of the disease.2 Data from Dutch and Flemish primary care databases show that, for every 1000 consecutive patients consulting a general practitioner, 50 present with an acute sore throat.3 4 In the US, more than 92 million visits by adults to primary care practices and emergency departments between 1997 and 2010 were recorded.5 Sore throat presenting as acute tonsillitis is also the commonest cause for emergency admission to otorhinolaryngology services in the US.6
Acute sore throat is a self limiting disease and typically resolves after 7-10 days in adults and 2-7 days in children.7 Most infections are of viral origin; only a few are caused by a bacterial infection, of which group A β-haemolytic streptococcus, Haemophilus influenzae, and Moraxella catarrhalis are the most common pathogens. Evidence suggests that the time to resolution is not associated with the type of pathogen.7 About 2% of patients initially presenting with sore throat will have a mononucleosis infection caused by an Epstein-Barr virus, which could prolong the duration of symptoms.8
Some patients experience unacceptable morbidity and inconvenience, and miss school or work due to recurrent sore throat.9 Pain is a common reason for work or school absence. Complications of sore throat are rare: about 0.2% of patients with tonsillitis will develop a peritonsillar abscess.10
The diagnosis of an acute sore throat is based on signs and symptoms. The Centor clinical prediction rules can be used to help predict whether the sore throat is caused by a bacterial pathogen, and thus guide the decision whether to prescribe an antibiotic.11 12
Most guidelines recommend paracetamol or ibuprofen as the first choice treatment.13 The use of corticosteroids is mentioned in few, and is generally discouraged (table 1). Antibiotics are probably not helpful for pain relief in an episode of acute sore throat caused by viruses, but may help those with a bacterial infection.14 15 Recommended management of sore throat varies widely, and table 1 summarises current guidelines.
Table 1.
Current guidance for treatment of patients with sore throat
| Ibuprofen | Paracetamol | Antibiotics | Corticosteroids | ||
|---|---|---|---|---|---|
| For adults | For children | ||||
| EBM guidelines11 | Supportive | Supportive | Conditionally | Supportive | Not applicable |
| SIGN6 | Supportive | Supportive | Conditionally | Not supportive | No comment |
| NHG12 | Supportive | Supportive | Conditionally | Not recommended | No comment |
| BC guidelines13 | No comment | No comment | Against | No comment | No comment |
| UpToDate14 | Against | No comment | No comment | Supportive | No comment |
How the recommendation was created
A large randomised controlled trial published in April 201721 found that corticosteroids increased the proportion of patients with complete resolution of symptoms at 48 hours. However, corticosteroids did not seem to decrease the duration of moderately bad symptoms, pain severity, healthcare attendance, days missed from school or work, or the consumption of delayed antibiotics. This study adds to the body of evidence that suggests that, although corticosteroids probably have benefits in patients with sore throat, these benefits may be modest.22 23 24 25 The Rapid Recommendations team felt that the study, when considered in context of the full body of evidence, might change practice.26
Our international panel—including general practitioners, general internists, paediatricians, an otorhinolaryngologist, epidemiologists, methodologists, statisticians, and people with lived experience of sore throat—decided what was the scope of the recommendation and the outcomes that are most important to patients. After a parallel team conducted a systematic review on the benefits and harms of corticosteroids,16 and a systematic search for evidence about patients’ values and preferences (appendix 1 on bmj.com), the panel met to discuss the evidence and formulate a recommendation. No person had financial conflicts of interest; intellectual and professional conflicts were minimised and managed (appendix 2 on bmj.com).
The panel followed the BMJ Rapid Recommendations procedures for creating a trustworthy recommendation,26 27 including using the GRADE approach to critically appraise the evidence and create recommendations (appendix 3 on bmj.com).28 The panel considered the balance of benefits, harms, and burdens of the drug, the quality of the evidence for each outcome, typical and expected variations in patient values and preferences, and acceptability.29 Recommendations can be strong or weak, for or against a course of action.
The evidence
The linked systematic review reports the effects of corticosteroids when added to standard care in patients with acute sore throat.16
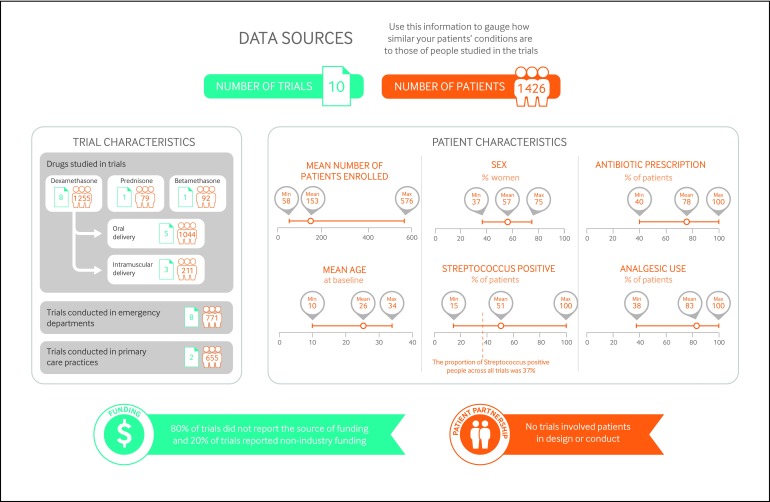
Figure 1 gives an overview of the number and types of patients included, the study funding, and patient involvement, as well as a summary of the benefits and harms of corticosteroids for treating acute sore throat.
Fig 1 Characteristics of patients and trials included in systematic review of effects of corticosteroids on acute sore throat
The panel identified eight patient-important outcomes needed to inform the recommendation: complete resolution of pain, time to onset of pain relief, pain severity, need for antibiotics, days missed from school or work, recurrence of symptoms, duration of bad or non-tolerable symptoms, and adverse effects. The included studies reported on all patient-important outcomes, except for duration of bad or non-tolerable symptoms. Regarding pain, the panel appraised the likelihood of complete resolution of pain at 24 hours and 48 hours, as well as the mean time to complete resolution of pain and the mean time to onset of pain relief.
Although most of the studies (80%) were conducted in emergency departments, they accounted for 54% of all patients enrolled across studies. The remaining 46% were enrolled in the studies conducted in primary care settings, and the panel was therefore confident that the evidence was applicable to them as well. Most of the studies focused in adults only (60%). The studies that focused only on children (three studies, 2% of all the patients enrolled in the studies) did not include children younger than 5 years old, and thus the recommendation does not apply to younger ages.
Since the randomised controlled trials focused on patients who did not have recurrent episodes of sore throat, the panel was less confident of the applicability of the evidence to such patients, and the recommendation therefore does not apply to them. Similarly, the panel did not consider patients with sore throat after surgery or intubation, nor immunocompromised patients.
Understanding the recommendation
The recommendation for using corticosteroids made by the panel was weak because of the modest reduction of symptoms and the large variability in patient preferences.
The panel is confident that the recommendation applies to almost all patients with acute sore throat: children 5 years and older and adults, severe and not severe sore throat, patients who receive immediate antibiotics and those who receive deferred antibiotics, patients with a viral or bacterial sore throat, and patients who seek care in the emergency department as well as those who attend primary care. The systematic review contained adequate representation from such groups and settings, and results were consistent (that is, absence of credible subgroup effects), for example, between trials of children and adults, and those seen in emergency departments and in primary care offices.16
Absolute benefits and harms
Although the evidence indicates that the treatment works on average, it did not reduce the severity of pain dramatically and failed to improve several other patient-important outcomes.
The infographic explains the recommendation and provides an overview (GRADE summary of findings) of the absolute benefits and harms of corticosteroids. Estimates of baseline risk for effects come from the control arms of the trials.16 The infographic also leads to point-of-care formats in the MAGICapp, including consultation decision aids designed to support shared decision making with patients.17
Considering the evidence and its certainty, the panel was confident that:
Corticosteroids increase the chance of complete resolution of pain at 24 and 48 hours, reduce the severity of pain, and shorten the time to onset of pain relief (GRADE high to moderate quality evidence)
Corticosteroids are unlikely to reduce recurrence or relapse of symptoms or days missed from school or work (GRADE moderate quality evidence)
-
A single dose of corticosteroids is unlikely to cause serious adverse events
The randomised trials did not report any major event attributable to single dose corticosteroids (GRADE moderate quality evidence)
The panel also considered evidence from observational studies that used higher doses of steroids. A large retrospective US cohort study of private insurance claims assessed adverse events in 327 452 adults who received an outpatient prescription of corticosteroids.18 There was a small absolute increase in the rate of sepsis, venous thromboembolism, and fracture in the first 30 days (GRADE low quality evidence, due to suboptimal verification of diagnosis in large databases and confounding by indication19). The panel agreed that such events seemed unlikely with single dose steroids
Similarly, among paediatric populations, indirect evidence from a meta-analysis of 44 randomised trials did not report any major adverse events in patients with conditions requiring a short course of corticosteroids (such as asthma, bronchiolitis, croup, wheeze, and pharyngitis or tonsillitis)20
There are no differences in the relative effects of corticosteroids (when compared with usual care) between primary care settings and emergency departments
It is unlikely that new information will change interpretation for outcomes that are high to moderate quality of evidence.
The panel was less confident about whether:
Corticosteroids reduced antibiotic use, due to a lack of improvement or worsening of symptoms in patients not prescribed antibiotics immediately when consulting the physician (GRADE low quality evidence)
Corticosteroids reduced the average time to complete resolution of pain (GRADE low quality evidence).
Values and preferences
The weak recommendation for corticosteroids reflects a high value on a modest reduction of symptom severity and the time that it takes to achieve such improvement, and a substantial and important increase in the chance of complete resolution of pain at 48 hours.
The panel, including the patient representatives, felt that the values and preferences are likely to vary greatly across patients, which justifies a weak recommendation. For example, achieving complete pain resolution 12 hours earlier may be of little importance for patients who feel less busy in their daily life, have higher tolerance to pain, or whose symptoms are not so severe; whereas it may be very important to patients whose ability to go to school or to perform at work are compromised, care givers wishing to reduce their children’s pain, or patients experiencing their pain as severe.
The panel believes that there is great variability in how much reduction in pain severity or time to complete pain resolution each patient would consider important. However, the greater the reduction in hours to achieve complete resolution of pain, the more likely it is that typical patients would place high value on those outcomes. Patients who place a high value in reducing the symptoms by any amount (such as patients with lower tolerance to pain or with severe symptoms) are more likely to accept receiving corticosteroids.
The weak recommendation for corticosteroids also reflects the concerns that the panel had with acceptability. Specifically, how acceptable is it to treat a condition that is usually not severe and is self limiting with a drug that many patients, practitioners, and other stakeholders know is almost always used for more severe diseases.
The systematic search for empirical data on patients’ values and preferences related to sore throat identified 4149 references that were screened at the title and abstract level. From these, we screened 99 full text articles, from which only two provided relevant information on patients’ values and preferences (see appendix 1 on bmj.com). Neither of the studies provided additional data that had not been raised by the panel members: the panel had identified appropriate patient-important outcomes and considered the variability in patient values and preferences regarding sore throat management.
Practical issues and other considerations
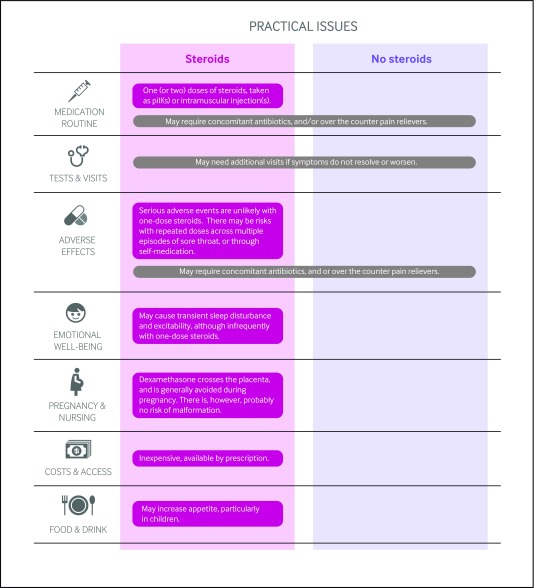
Figure 2 outlines the key practical issues for patients and clinicians discussing adjunct steroids for sore throat, which are also accessible along with the evidence as decision aids to support shared decision-making in MAGICapp. Steroids are typically given as 10 mg dexamethasone (or adapted to weight for children: 0.6 mg/kg, up to a maximum dose of 10 mg), typically taken as pill or intramuscular injection.
Fig2 Practical issues about use of corticosteroids to treat acute sore throat
The risks may outweigh the benefits when larger cumulative doses of corticosteroids are given to patients who experience multiple episodes of sore throat, either through multiple visits or for patients who self medicate if prescribed more than one pill for their previous episode. To mitigate this issue, clinicians should administer the medication in office if possible or prescribe only one dose per visit.
Costs and resources
The panel focused on the patient perspective rather than that of society when formulating the recommendation. Given the low cost of corticosteroids for treating sore throat, implementation of this recommendation is unlikely to have an important impact on the costs for health funders. The treatment is inexpensive and likely to be offered in the context of a consultation that would have taken place anyway. Nevertheless, it remains uncertain whether it may increase the proportion of patients visiting a doctor to get a prescription of corticosteroids.
Uncertainties for future research
Key research questions to inform decision makers and future guidelines include:
Are there any severe adverse effects of using one-dose of steroids for treating sore throat?
What are the effects of corticosteroids, in addition to standard care, in patients with recurrent episodes of acute sore throat?
Updates to this article
Table 2 shows evidence which has emerged since the publication of this article. As new evidence is published, a group will assess the new evidence and make a judgment on to what extent it is expected to alter the recommendation.
Table 2.
New evidence which has emerged after initial publication
| Date | New evidence | Citation | Findings | Implications for recommendation(s) |
|---|---|---|---|---|
| There are currently no updates to the article | ||||
Education in practice
How do you currently approach giving advice for those with acute sore throat? Do you consider offering corticosteroids?
The recommendation for corticosteroid use is weak, and patient’s preferences are likely to vary. What information could you share with your patient to help reach a decision together?
Have you learnt one thing from this article that might alter how you consult with patients with sore throat? How might you share this information with colleagues to learn together?
To what extent do you practice shared decision making for such preference-sensitive decisions?
How patients were involved in the creation of this article
Five people with lived experience of sore throat were full panel members. These panel members identified important outcomes, and led the discussion on values and preferences. These patient representatives agreed that while small reductions in pain severity and time to complete pain resolution (for example 12 compared to 24 hours) were important to them, these values may not be shared by all patients; they expected moderate to great variability in how much importance other patients would place in small reductions in pain. These panel members participated in the teleconferences and email discussions and met all authorship criteria.
Web Extra.
Extra material supplied by the author
Infographic:
Appendix 1: Results of the search for evidence about patients’ values and preferences
Appendix 2: Full list of authors’ declarations of interests
Appendix 3: Methodology for development of BMJ Rapid Recommendations
Appendix 4: The full information available on the MAGICapp
This BMJ Rapid Recommendation article is one of a series that provides clinicians with trustworthy recommendations for potentially practice changing evidence. BMJ Rapid Recommendations represent a collaborative effort between the MAGIC group (www.magicproject.org) and The BMJ. A summary is offered here and the full version including decision aids is on the MAGICapp (www.magicapp.org), for all devices in multilayered formats. Those reading and using these recommendations should consider individual patient circumstances, and their values and preferences and may want to use consultation decision aids in MAGICapp to facilitate shared decision making with patients. We encourage adaptation and contextualization of our recommendations to local or other contexts. Those considering use or adaptation of content may go to MAGICapp to link or extract its content or contact The BMJ for permission to reuse content in this article.
Competing interests: All authors have completed the BMJ Rapid Recommendations interests disclosure form and a detailed, contextualised description of all disclosures is reported in appendix 2 on bmj.com. As with all BMJ Rapid Recommendations, the executive team and The BMJ judged that no panel member had any financial conflict of interest. Professional and academic interests are minimised as much as possible, while maintaining necessary expertise on the panel to make fully informed decisions.
Funding: This guideline was not funded.
Transparency: B Aertgeerts affirms that the manuscript is an honest, accurate, and transparent account of the recommendation being reported; that no important aspects of the recommendation have been omitted; and that any discrepancies from the recommendation as planned (and, if relevant, registered) have been explained.
References
- 1.Evans CE, McFarlane AH, Norman GR, Neale KA, Streiner DL. Sore throats in adults: who sees a doctor?Can Fam Physician 1982;28:453-8.pmid:21286075. [PMC free article] [PubMed] [Google Scholar]
- 2.van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief?Ann Fam Med 2006;4:494-9. 10.1370/afm.609 pmid:17148626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIVEL Primary care database: Netherlands Institute for Health Services Research; 2012. www.nivel.nl/en/dossier/nivel-primary-care-database.
- 4.Truyers C, Goderis G, Dewitte H, Akker Mv, Buntinx F. The Intego database: background, methods and basic results of a Flemish general practice-based continuous morbidity registration project. BMC Med Inform Decis Mak 2014;14:48 10.1186/1472-6947-14-48 pmid:24906941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med 2014;174:138-40. 10.1001/jamainternmed.2013.11673. pmid:24091806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird JH, Biggs TC, King EV. Controversies in the management of acute tonsillitis: an evidence-based review. Clin Otolaryngol 2014;39:368-74. 10.1111/coa.12299. pmid:25418818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD. TARGET Programme Team. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013;347:f7027 10.1136/bmj.f7027. pmid:24335668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rea TD, Russo JE, Katon W, Ashley RL, Buchwald DS. Prospective study of the natural history of infectious mononucleosis caused by Epstein-Barr virus. J Am Board Fam Pract 2001;14:234-42.pmid:11458965. [PubMed] [Google Scholar]
- 9.Scottish Intercollegiate Guidelines Network. Management of sore throat and indications for tonsillectomy (SIGN 117). 2010. www.sign.ac.uk/sign-117-management-of-sore-throat-and-indications-for-tonsillectomy.html.
- 10.Little P, Stuart B, Hobbs FD, et al. DESCARTE investigators. Predictors of suppurative complications for acute sore throat in primary care: prospective clinical cohort study. BMJ 2013;347:f6867 10.1136/bmj.f6867 pmid:24277339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atula T. Corticosteroids for sore throat. Evidence-Based Medicine Guidelines 2014. www.ebm-guidelines.com/ebmga/ltk.naytaartikkeli?p_artikkeli=evd07095#refs.
- 12.Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat?JAMA 2000;284:2912-8. 10.1001/jama.284.22.2912 pmid:11147989. [DOI] [PubMed] [Google Scholar]
- 13.Chiappini E, Regoli M, Bonsignori F, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther 2011;33:48-58. 10.1016/j.clinthera.2011.02.001. pmid:21397773. [DOI] [PubMed] [Google Scholar]
- 14.Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev 2006;(4):CD000023 10.1002/14651858.CD000023.pub3. pmid:17054126. [DOI] [PubMed] [Google Scholar]
- 15.van Driel ML, De Sutter AI, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev 2016;9:CD004406 10.1002/14651858.CD004406.pub4. pmid:27614728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadhegirad B, Siemieniuk R, Brignardello-Petersen R, et al. Corticosteroids for treatment of sore thoat: a systematic review and meta-analysis of randomized trials. BMJ 2017;358:j3887 10.1136/bmj.j3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ 2015;350:g7624 10.1136/bmj.g7624. pmid:25670178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415 10.1136/bmj.j1415. pmid:28404617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agoritsas T, Merglen A, Shah ND, O’Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA 2017;317:748-59. 10.1001/jama.2016.20029. pmid:28241362. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes RM, Oleszczuk M, Woods CR, Rowe BH, Cates CJ, Hartling L. The Cochrane Library and safety of systemic corticosteroids for acute respiratory conditions in children: an overview of reviews. Evid Based Child Health 2014;9:733-47. 10.1002/ebch.1980 pmid:25236311. [DOI] [PubMed] [Google Scholar]
- 21.Hayward GN, Hay AD, Moore MV, et al. Effect of oral dexamethasone without immediate antibiotics vs placebo on acute sore throat in adults: a randomized clinical trial. JAMA 2017;317:1535-43. 10.1001/jama.2017.3417 pmid:28418482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward G, Thompson M, Heneghan C, Perera R, Del Mar C, Glasziou P. Corticosteroids for pain relief in sore throat: systematic review and meta-analysis. BMJ 2009;339:b2976 10.1136/bmj.b2976. pmid:19661138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward G, Thompson MJ, Perera R, Glasziou PP, Del Mar CB, Heneghan CJ. Corticosteroids as standalone or add-on treatment for sore throat. Cochrane Database Syst Rev 2012;10:CD008268 10.1002/14651858.CD008268.pub2. pmid:23076943. [DOI] [PubMed] [Google Scholar]
- 24.Korb K, Scherer M, Chenot JF. Steroids as adjuvant therapy for acute pharyngitis in ambulatory patients: a systematic review. Ann Fam Med 2010;8:58-63. 10.1370/afm.1038. pmid:20065280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing A, Villa-Roel C, Yeh B, Eskin B, Buckingham J, Rowe BH. Effectiveness of corticosteroid treatment in acute pharyngitis: a systematic review of the literature. Acad Emerg Med 2010;17:476-83. 10.1111/j.1553-2712.2010.00723.x pmid:20536799. [DOI] [PubMed] [Google Scholar]
- 26.Siemieniuk RA, Agoritsas T, Macdonald H, Guyatt GH, Brandt L, Vandvik PO. Introduction to BMJ Rapid Recommendations. BMJ 2016;354:i5191 10.1136/bmj.i5191. pmid:27680768. [DOI] [PubMed] [Google Scholar]
- 27.Vandvik PO, Otto CM, Siemieniuk RA, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 2016;354:i5085 10.1136/bmj.i5085. pmid:27680583. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD. pmid:18436948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013;66:726-35. 10.1016/j.jclinepi.2013.02.003. pmid:23570745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infographic:
Appendix 1: Results of the search for evidence about patients’ values and preferences
Appendix 2: Full list of authors’ declarations of interests
Appendix 3: Methodology for development of BMJ Rapid Recommendations
Appendix 4: The full information available on the MAGICapp