Abstract
Adrenal chromaffin cells comprise the neuroendocrine arm of the sympathetic nervous system and secrete catecholamines to coordinate the appropriate stress response. Deletion of the serotonin (5-HT) transporter (SERT) gene in mice (SERT−/− mice) or pharmacological block of SERT function in rodents and humans augments this sympathoadrenal stress response (epinephrine secretion). The prevailing assumption is that loss of CNS SERT alters central drive to the peripheral sympathetic nervous system. Adrenal chromaffin cells also prominently express SERT where it might coordinate accumulation of 5-HT for reuse in the autocrine control of stress-evoked catecholamine secretion. To help test this hypothesis, we have generated a novel mouse model with selective excision of SERT in the peripheral sympathetic nervous system (SERTΔTH), generated by crossing floxed SERT mice with tyrosine hydroxylase Cre driver mice. SERT expression, assessed by western blot, was abolished in the adrenal gland but not perturbed in the CNS of SERTΔTH mice. SERT-mediated [3H] 5-HT uptake was unaltered in midbrain, hindbrain, and spinal cord synaptosomes, confirming transporter function was intact in the CNS. Endogenous midbrain and whole blood 5-HT homeostasis was unperturbed in SERTΔTH mice, contrasting with the depleted 5-HT content in SERT−/− mice. Selective SERT excision reduced adrenal gland 5-HT content by ≈ 50 % in SERTΔTH mice but had no effect on adrenal catecholamine content. This novel model confirms that SERT expressed in adrenal chromaffin cells is essential for maintaining wild-type levels of 5-HT and provides a powerful tool to help dissect the role of SERT in the sympathetic stress response.
Keywords: Adrenal chromaffin cell, Adrenal gland, Catecholamines, Serotonin, Serotonin transporter, Sympathetic nervous system
Graphical abstract
1. Introduction
The serotonin (5-hydroxytryptamine; 5-HT) transporter (SERT; Slc6a4) is critical for clearing extracellular 5-HT thereby limiting the availability of the monoamine for signaling via 5-HT receptors and maintaining its homeostasis. Although different tissues throughout the body express SERT, much work has focused on its role in the central nervous system (CNS). In the CNS, SERT is predominantly expressed on the presynaptic terminals of serotonergic neurons originating in the brainstem raphe nuclei. These neurons project extensively throughout the brain and spinal cord to regulate diverse behaviors and physiological processes including mood and autonomic function. Genetic variations that alter SERT expression or function disrupt 5-HT homeostasis and underlie a range of neurobehavioral disorders such as depression, anxiety, and autism (for recent reviews see [1, 2]). Beyond the brain, SERT has important functional roles in the gastrointestinal system, blood vessels, platelets, heart, lung, and pancreas [3–8]. SERT is also highly expressed in chromaffin cells of the adrenal medulla that comprise the neuroendocrine arm of the sympathetic nervous system [9]. In conjunction with postganglionic sympathetic neurons, adrenal chromaffin cells coordinate the physiological response to environmental, metabolic, and emotional / psychological stressors via the release of catecholamines and neuropeptides into the bloodstream. There is increasing evidence linking autonomic dysfunction and aberrant sympathoadrenal responses with depression, anxiety, and other diseases related to serotonergic signaling (for review see [10]). It has also been reported that selective 5-HT reuptake inhibitors (SSRIs), which antagonize SERT, enhance the sympathoadrenal counter-regulatory response to hypoglycemic stress in rodents [11] and humans [12, 13]. Moreover, compared to wild-type controls, SERT−/− mice with constitutive (global) knockout of the transporter display anxiety-related phenotypes and exaggerated adrenal catecholamine secretion in response to restraint stress [14]. However, mechanistic understanding of these effects remains unclear, including which tissues / cell types SERT acts upon to control the sympathetic stress response.
1.1 SERT and the sympathetic nervous system
Both chromaffin cells and postganglionic sympathetic neurons are excited by cholinergic preganglionic sympathetic neurons originating in the thoracic spinal cord. The prevailing assumption is that 5-HT controls sympathetic output by modulating CNS drive to these spinal cord neurons. Some central serotonergic raphe neurons directly innervate the sympathetic preganglionic neurons while others modulate the activity of sympathetic premotor neurons in the rostral ventrolateral medulla, which provide much of the central drive to the spinal preganglionic sympathetic neurons (see recent review [10]).
Recently, we proposed that chromaffin cells in the adrenal medulla might be a previously unrecognized hub at which peripheral 5-HT can control the sympathoadrenal stress response [10, 15]. Mammalian adrenal chromaffin cells contain small amounts of 5-HT (≈ 750 fold lower than epinephrine) but do not appear to synthesize it [4, 16, 17]. It is postulated that this adrenal 5-HT is accumulated by SERT-mediated uptake and is then transported into secretory vesicles by vesicular monoamine transporters where it is protected from metabolism by monoamine oxidase in the cytoplasm [10]. This raises the possibility that 5-HT might be released from chromaffin cells and act locally within the adrenal gland to control catecholamine secretion. Consistent with this, we demonstrated that SERT regulates the ability of 5-HT to inhibit catecholamine secretion via 5-HT1A receptors [15]. The inhibition does not involve altered membrane excitability, regulation of voltage-gated Ca2+ channels, or intracellular calcium handling, which are the typical mechanisms for autocrine / paracrine control of transmitter release [18, 19]. We have proposed that the distinct mechanism of 5-HT1A receptors, coupled with the modulatory influence of SERT, might fine-tune serotonergic signaling to control catecholamine secretion during periods of intense stimulation (i.e. stress) [10].
SERT might have additional effects on catecholamine secretion in the adrenal gland, for example control of the quantal size of unitary exocytotic events by a 5-HT receptor independent mechanism [15]. There is also evidence that SERT might modulate transcriptional regulation within the adrenal medulla. Acute stressors increase the expression / function of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis, to help replenish catecholamine stores during periods of increased demand [20, 21]. However, SERT−/− mice lack this response (tyrosine hydroxylase expression is not increased) and consequently acute restraint stress depletes adrenal catecholamine content in SERT−/− mice but not in wild-type controls that are able to maintain the stress-induced balance of increased catecholamine secretion and synthesis [22]. The relationship between SERT and upregulation of tyrosine hydroxylase is intriguing, but the underlying mechanism remains unknown.
1.2 A novel transgenic mouse model with selective excision of SERT in the peripheral sympathoadrenal system
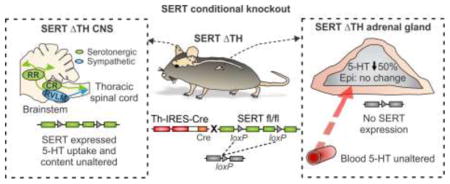
The SERT−/− mouse continues to be a valuable model for investigating serotonergic signaling and the mechanisms of antidepressants and other drugs that target SERT. However, the global nature of the knockout makes it difficult to determine where SERT might be acting to control the sympathetic stress response. Similarly, systemic SSRIs will block SERT function throughout the entire body. As noted above (section 1.1), altered SERT function in the brain and / or adrenal chromaffin cells could have an impact on the sympathoadrenal stress response. Added to this, lack of SERT in platelets disrupts delivery of gut-derived 5-HT to the adrenal gland (and other tissues) via the bloodstream [23]. With the recent generation of floxed SERT mice [24], spatial / temporal manipulation of SERT expression can help to address its cell / tissue specific role in various physiological functions. The first conditional knockout mice targeted SERT expression in CNS serotonergic neurons and subpopulations of SERT expressing neurons in the thalamus and cortex [24, 25]. In this paper, we describe the generation of a novel mouse model with selective excision of SERT from the sympathoadrenal system (SERTΔTH mice). SERT expression and function remains intact in the CNS of SERTΔTH mice and the endogenous 5-HT content of midbrain and whole blood was unaltered. In contrast, SERT expression in the adrenal gland was abolished and adrenal 5-HT content was dramatically reduced with no change in catecholamines. This novel model confirms that SERT expressed in adrenal chromaffin cells is essential for maintaining wild-type levels of 5-HT which might then be reused in an autocrine manner to modulate catecholamine secretion. It also provides a useful tool to help dissect the role of SERT in the sympathetic nervous system and to directly test the hypothesis that adrenal chromaffin cells form a peripheral hub for serotonergic control of the sympathoadrenal stress response.
2. Methods
2.1 Ethical approval / animals
All procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were performed as approved by the Institutional Animal Care and Use Committees of Vanderbilt University Medical Center and Rowan University. Mice were bred onsite at Vanderbilt University Medical Center and were maintained on a 12:12 hour light / dark cycle with food and water available ad libitum. Mice were weaned at 21 days of age and group housed 2–5 per cage with male and female littermates housed separately. Unless indicated, both male and female mice were used in this study and data was pooled for analysis.
2.2 Generation of SERTΔTH mice and maintenance of experimental mouse colonies
All mouse strains were maintained on a C57BL/6 background and had been backcrossed for at least 10 generations prior to this study. Floxed SERT (SERTfl/fl) mice were previously generated by the insertion of loxP sites into introns 2 and 4 of the SERT gene Slc6a4 [24]. Cre-mediated recombination of the loxP sites excises exons 3 and 4 of the floxed Slc6a4 allele and produces a reading frame shift that eliminates SERT function (Figure 1). To conditionally excise the floxed SERT allele in the sympathoadrenal system, female SERTfl/fl mice were crossed with heterozygous male Th-IRES-Cre mice (ThCre/wt; Figure 1), which were provided by Dr. Danny Winder at Vanderbilt University Medical Center. The Th-IRES-Cre mice (RRID:IMSR_EM:00254) are a knock-in mouse line in which the Cre transgene was inserted into the 3′-untranslated region (UTR) of the tyrosine hydroxylase gene, Th, via an internal ribosome entry sequence (IRES) [26]. Male offspring heterozygous for the floxed SERT allele and expressing Cre (SERTfl/wt;ThCre/wt) were bred to female SERTfl/fl mice to obtain the desired genotype (SERTfl/fl;ThCre/wt), which we term SERTΔTH. Male SERTΔTH progeny were subsequently crossed to female SERTfl/fl mice to maintain the colony and provide experimental SERTΔTH mice and Cre negative SERTfl/fl littermate controls (SERTfl/fl;Thwt/wt). SERTΔTH mice and SERTfl/fl littermate controls were born at the expected Mendelian frequency (25.4 ± 2.2 % SERTfl/fl male, 25.6 ± 2.4 % SERTfl/fl female, 24.4 ± 2.4 % SERTΔTH male, 24.8 ± 2.2 % SERTΔTH female, n = 63 litters) and litter size was typical for mice on a C57BL/6 background (6.8 ± 0.3 pups per litter, n = 63 litters). Average body weight of adult male SERTΔTH mice was similar to control littermates (24.8 ± 0.6 g SERTfl/fl Vs. 25.2 ± 0.6 g SERTΔTH, P = 0.57 Student’s t-test). No other overt phenotypes were noted and SERTΔTH mice were not readily distinguishable from littermates.
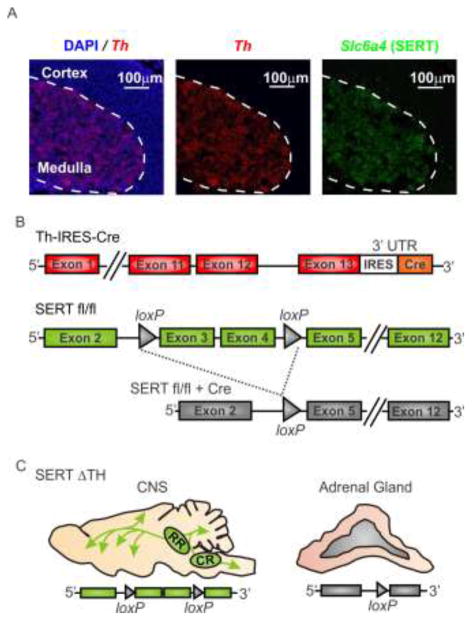
Figure 1. Strategy to generate conditional knockout of SERT in the sympathoadrenal system.
A) A multicolor in situ hybridization approach (RNAscope) was used to confirm co-expression of Slc6a4 (SERT) and tyrosine hydroxylase (Th), the rate-limiting enzyme for catecholamine biosynthesis, in the adrenal medulla. The merged image (left) shows Th (red) mRNA expression and DAPI-labeled nuclei (blue) in an adrenal gland section from a wild-type mouse. Th is expressed in chromaffin cells of the adrenal medulla but is absent from the adrenal cortex. The dashed line in all images indicates the demarcation of the adrenal medulla and cortex as defined by Th expression. In the same section, the Slc6a4 mRNA (SERT; green, right image) expression pattern is similar to Th and is restricted to the adrenal medullary chromaffin cells. B) Schematic representation of the strategy to conditionally excise the floxed SERT gene (SERTfl/fl) by Cre-mediated recombination. Th-IRES-Cre is a knock-in mouse with Cre inserted into the 3′-untranslated region (UTR) of tyrosine hydroxylase (Th) (upper panel). In the floxed SERT mouse (SERTfl/fl), loxP sites flank exons 3 and 4 of the Slc6a4 gene (middle panel). Cre-mediated recombination results in functional knockout of Slc6a4 (SERT) by excision of exons 3 and 4 (lower panel). C) SERTΔTH conditional knockout mice were generated by crossing SERTfl/fl with Th-IRES-Cre mice (for more detail see methods section 2.2). The illustration shows the predicted tissue specific excision of SERT from the adrenal gland but not the CNS of the SERTΔTH mice.
Th-IRES-Cre control mice lacking the floxed SERT allele were generated by maintaining a colony of ThCre/wt male x Thwt/wt female breedings. Constitutive (global) SERT knockout mice (SERT−/−; RRID:IMSR_JAX:008355) have been described previously [27] and were maintained onsite with homozygous breeding pairs.
2.3 Determination of mouse genotype
Genomic DNA was extracted from tail samples using the REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich, St Louis, MO) according to manufacturer’s instructions. PCR-based genotyping of the extracted DNA was performed as detailed below and genotypes determined following separation of the PCR products by 2 % agarose gel electrophoresis.
Cre genotyping
PCR thermocycler conditions were – initial step of 94 °C for 3 minutes; 35 cycles of 94 °C for 30 s, 59 °C for 1 minute and 72 °C for 1 minute; final extension step of 72 °C for 2 minutes and hold at 4 °C. Two pairs of oligonucleotide primers were used in the Cre PCR reaction. The first pair oIMR1084 5′-GCGGTCTGGCAGTAAAAACTATC-3′ and oIMR1085 5′-GTGAAACAGCATTGCTGTCACTT-3′ produced a 100 bp PCR product only if the Cre transgene was expressed. The second pair of primers, oIMR7338 5′-CTAGGCCACAGAATTGAAAGATCT-3′ and oiMR7339 5′-GTAGGTGGAAATTCTAGCATCATCC-3′, produced a 324 bp internal control band in all samples.
Floxed Slc6a4 allele genotyping
PCR thermocycler conditions were – initial step of 95 °C for 5 minutes; 35 cycles of 94 °C for 45 s, 56 °C for 1 minute and 72 °C for 1 minute; final extension of 72 °C for 10 minutes and hold at 4 °C. Oligonucleotide primer pair RB4780 5′-GGGTCTGGAGTCATAGGTAAT-3′ and RB4781 5′-GTGGGATGTCCTACTTTATGC-3′ produces 507 bp and 580 bp PCR products for the wild-type and floxed Slc6a4 alleles respectively.
Global SERT knockout mouse (SERT−/−) genotyping
PCR thermocycler conditions were – initial step of 95 °C for 5 minutes; 40 cycles of 95 °C for 30 s, 62 °C for 1 minute in cycle 1 decreasing by 0.3 °C for each subsequent cycle, 72 °C for 30 s; final extension of 72 °C for 7 minutes and hold at 10 °C. Oligonucleotide primers used in the PCR reaction were RB4796 5′-GCCAGAGGCCACTTGTGTAG-3′, RB4797 5′-AATGGTGAGGAGTGGTGGAG-3′ and RB4798 5′-CCTAGATACCAGGCCCACAA-3′ producing PCR products of 318 bp for the wild-type Slc6a4 allele and 210 bp for the knockout.
2.4 Preparation of tissue samples and whole blood collection
Mice were euthanized using carbon dioxide inhalation followed by cervical dislocation or rapid decapitation. Tissue samples were dissected on ice in Locke’s solution (154 mM NaCl, 5.6 mM KCl, 2.1 mM Na2HPO4, 10 mM glucose and 10 mM HEPES, pH 7.4) and, as indicated for each experiment, either used fresh or immediately frozen on dry ice and stored at −80 °C for later use. Each biological replicate was derived from tissue harvested from one mouse with the exception of hindbrain and spinal cord [3H] 5-HT uptake experiments in which tissue from two mice was combined. Hindbrain and midbrain dissections were based on anatomical landmarks and underlying raphe nuclei of the mouse brain [28]. Hindbrain: A coronal section was first made at the posterior end of the cerebellum (~ Bregma −8.24 mm) to discard spinal cord tissue and the cerebellum was then removed. The underlying brainstem tissue was cut in the coronal plane at the posterior end of the inferior colliculus (~ Bregma −5.4 mm) to dissect the hindbrain. Midbrain: Tissue was bound by the superior (~ Bregma −3.4 mm) and inferior (~ Bregma −5.4 mm) colliculi. After removal of the hindbrain tissue, the cortex was peeled back and a coronal section made at the anterior end of the superior colliculus. Spinal cord: The ribcage was used as a landmark to harvest primarily thoracic spinal cord tissue from which the preganglionic sympathetic innervation arises [29]. Transverse sections were made through the vertebrae and spinal cord, ~5 mm rostral to the first rib and ~ 5 mm caudal to the last rib, before the vertebrae were removed to isolate the spinal cord. Adrenal gland: Harvested adrenal glands were carefully trimmed of fat and immediately frozen on dry ice. For western blot experiments, the left and right adrenal glands from a single mouse were combined for each sample. Only the right adrenal gland was frozen for high performance liquid chromatography (HPLC) analysis. Whole blood: Whole blood samples were collected from euthanized (CO2 inhalation followed by cervical dislocation) male mice (8 – 17 weeks old) by cardiac puncture with a 26-gauge needle / 1 mL syringe coated with 5 mM EDTA solution (pH 8; Life Technologies, Grand Island, New York). Samples were immediately transferred to pre-chilled Eppendorf tubes containing EDTA solution (5 mM final concentration), frozen on dry ice and stored at −80 °C for later analysis by HPLC.
2.5 Multiplex fluorescent in situ hybridization (RNAscope)
RNAscope in situ hybridization was performed by the Molecular Neuroanatomy core of the Vanderbilt Silvio O. Conte Center for Neuroscience Research housed at the Saban Research Institute, Children’s Hospital of Los Angeles. Adrenal glands harvested from C57BL/6J mice (RRID:IMSR_JAX:000664; The Jackson Laboratory, Bar Harbor, ME) were immediately frozen in ice-cold isopentane and stored at −80 °C. Fresh-frozen glands were cryosectioned at 16 μM and post-fixed in 4 % paraformaldehyde (15 minutes at 4 °C). Fixed sections were dehydrated in ethanol (four 5-minute, room temperature washes at 50, 70, 100 and 100 % concentration respectively) and stored at −20 °C. Tyrosine hydroxylase (Th) and SERT (Slc6a4) mRNA transcripts were detected with commercially available RNAscope multiplex fluorescent in situ hybridization kits, equipment and RNA probes according to manufacturer’s instructions (Advanced Cell Diagnostics, Hayward, CA). Briefly, sections were permeabilized with protease pretreat 4 solution and then incubated for 2 hours at 40 °C with RNA target probes. RNA probes used were Mm-Slc6a4-C2 (probe region 452 – 1378 bp of accession number NM_010484.2) and Mm-Th-C1 (probe region 483 – 1603 bp of accession number NM_009377.1). The probes contain a tag that enables the target transcript to be fluorescently labeled during subsequent amplification steps and then visualized in a specific color channel. Control adrenal sections were incubated with positive or negative control probes as references for the signal intensity and background level in each color channel. The positive control probes targeted two housekeeping genes (C1 Polr2a and C2 Ppib) and the negative control probe targeted the bacterial gene DapB (in both color channels). Coverslips were mounted with ProLong Gold anti-fade reagent containing DAPI (Life Technology, Carlsbad, CA). Images were acquired, under 20X magnification, with a Zeiss Axio Observer inverted microscope fitted with a LSM700 confocal scanner and controlled by Zen software (Carl Zeiss Microscopy, Thornwood, NY). Excitation / emission spectra for each color channel were: C1 (Th and controls) 555 nm / 585 nm, C2 (Slc6a4 and controls) 639 nm / 669 nm, and DAPI 405 nm / 435 nm. Pseudo-colored images were exported in TIFF format and imported into CorelDRAW Graphics Suite 2017 (Corel Corporation, Ottawa, ON, Canada) where they were resized and globally adjusted for brightness and contrast for preparation of final figures.
2.6 Determination of SERT and TH protein expression by western blotting
Frozen tissue samples were homogenized with a pellet pestle in ice-cold RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 % Igepal CA-630 (NP-40), 0.5 % sodium deoxycholate and 0.1 % sodium dodecyl sulfate (SDS); Sigma-Aldrich, St Louis, MO) containing protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Homogenates were constantly rotated for 2 hours at 4 °C and the resulting protein lysates spun in a microcentrifuge for 20 minutes at 15000 xg and 4 °C. The supernatant was collected and the protein concentration determined by the bicinchoninic acid (BCA) assay method according to manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). 50 μg of each protein sample, mixed with Laemmli loading buffer (Bio-Rad Laboratories, Hercules, CA), was heated to 37 °C for 12 minutes and then separated by 10 % SDS-PAGE (Novex 10 % Tris-Glycine gels; Thermo Fisher Scientific, Waltham, MA) before overnight transfer at 10 V and 4 °C to polyvinylidene difluoride (PVDF) membranes (0.45 μm pore size; Immobilon-P, EMD Millipore, Burlington, MA). Membranes were blocked in 5 % non-fat milk in Tris-buffered saline, 0.1 % Tween 20 (TBST) for 1 hour at room temperature followed by overnight incubation at 4 °C in SERT primary antibody (1/1000 dilution, Guinea pig anti-5HTT, Cat # HTT-GP-Af1400, RRID:AB2571777 Frontier Institute, Japan). Membranes were washed 3 times, for 10 minutes each, in TBST and incubated in secondary antibody for 1 hour at room temperature (1/10000 dilution, Donkey or Goat anti-Guinea Pig HRP, Cat # 706-035-148, RRID:AB2340447 or Cat # 106-035-003, RRID:AB_2337402 respectively, Jackson ImmunoResearch, West Grove, PA). Following 3 further TBST washes, immunoreactive bands were detected by chemiluminesence (Clarity western ECL substrate; Bio-Rad Laboratories, Hercules, CA). Western blot images were acquired at multiple exposure times, to ensure linearity of data acquisition, with a ChemiDoc MP digital imaging system controlled by Image Lab 5.0 or 6.0 software (Bio-Rad Laboratories, Hercules, CA). Western blot membranes were stripped (25 mM glycine-HCl, 2 % sodium dodecyl sulfate, pH 2.0) and samples were re-probed for β-actin expression during a 1-hour room temperature incubation with a mouse monoclonal anti-β-actin peroxidase conjugated antibody (1/10000 dilution, Cat # A3854, RRID:AB_262011, Sigma-Aldrich, St Louis, MO). Adrenal gland tyrosine hydroxylase expression was detected following overnight incubation of membranes at 4 °C with rabbit anti-tyrosine hydroxylase primary antibody (1/2000 dilution, Cat # 2025-THRAB, RRID:AB2492276, PhosphoSolutions, Aurora, CO) and a 1-hour room temperature incubation with donkey anti-rabbit HRP secondary antibody (1/10000 dilution, Cat # 711-035-152, RRID:AB10015282 Jackson ImmunoResearch, West Grove, PA). Western blot images are representative of 4 – 10 biological replicates of each genotype (each from an individual mouse; age range 8 – 36 weeks) run in 3 – 6 independent experiments. Band densitometry analysis was performed using the volume tool of Image Lab 6.0 software with the volume of a defined membrane background region subtracted. SERT and tyrosine hydroxylase expression levels were normalized to the β-actin loading control of the same sample.
2.7 Synaptosomal [3H] 5-HT uptake
Freshly dissected midbrain, hindbrain and spinal cord tissue (age range of mice 17–35 weeks) was homogenized with a Teflon glass homogenizer (Wheaton Instruments, Millville, NJ) in a sucrose (0.32 μM)-HEPES (4.2 mM) solution (pH 7.4). Synaptosomal pellets were isolated by a two-step centrifugation process in which the homogenates were spun for 10 minutes at 1000 xg and 4 °C and the resulting supernatant spun for 13 minutes at 10000 xg and 4 °C. After the second centrifugation, the pellet was re-suspended in uptake assay buffer (5 mL for midbrain and 3.8 mL for hindbrain and spinal cord) of Krebs-Ringer HEPES solution (KRH; 120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 10 mM HEPES, pH 7.4) with glucose (10 mM), ascorbic acid (100 μM; Sigma-Aldrich, St Louis, MO) and pargyline (100 μM; Sigma-Aldrich, St Louis, MO). Uptake saturation assays were conducted by incubating synaptosomes with serial dilutions (6.25 – 200 nM final) of [3H] 5-HT (23.9 Ci/mmol; PerkinElmer Life Sciences, Waltham, MA). Non-specific uptake was determined by incubating parallel samples with 1 μM escitalopram (Sigma-Aldrich, St Louis, MO) in addition to [3H] 5-HT. 150 μl of the synaptosome suspension was pre-incubated with or without escitalopram for 10 minutes on ice and then equilibrated at 37 °C for 5 minutes in a shaking incubator before the addition of varying concentrations of [3H] 5-HT. After a 10-minute incubation, [3H] 5-HT uptake was terminated by filtering the samples through polyethyleneimine coated GF/B Whatman filters using a Brandel cell harvester (Brandel, Gaithersberg, MD) and rinsing the filters 3 times in ice-cold KRH solution. Dried filters were immersed in Econo-Safe scintillation fluid (Research Products International, Mount Prospect, IL) for 6 hours and accumulated radioactivity was quantified using a TriCarb 2900TR liquid scintillation counter controlled by QuantaSmart 1.31 software (Packard BioScience, PerkinElmer, Waltham, MA). For each independent experiment (biological replicate), samples with [3H] 5-HT alone were run in triplicate and samples with escitalopram also present (non-specific uptake) were run in duplicate. Technical replicate counts for each sample were averaged and non-specific uptake subtracted from total counts to determine SERT specific uptake at each concentration of [3H] 5-HT. Specific counts were normalized to total protein concentration of the sample, as quantified by a BCA assay. Data were fit with the Michaelis-Menten equation (using Prism 5 software; GraphPad Software Inc., La Jolla, CA) to derive saturation kinetic analysis parameters (Vmax and Km). Synaptosomes from SERT−/− mice were used as a negative control and, as expected, displayed no SERT-specific uptake. These data are included on graphs in the results section but were not subjected to curve fitting. Data presented are the average of 3 – 6 independent experiments.
2.8 Determination of tissue and whole blood monoamine content by HPLC
Midbrain and adrenal tissue was obtained from 11 – 16 week old mice; blood was collected from 9 – 17 week old mice. Tissue and whole blood biogenic amines and metabolites were extracted and determined using HPLC by the Vanderbilt Brain Institute Neurochemistry Core at Vanderbilt University. Frozen adrenal and midbrain tissue samples were homogenized with a tissue dismembrator in 100 – 750 μl of 0.1 M trichloroacetic acid (TCA), 10 mM sodium acetate, 0.1 mM EDTA, and 10.5 % methanol (pH 3.8). Isoproterenol (5 ng/mL) was included in the homogenization buffer for use as an internal standard. An aliquot of the homogenate (10 μl) was used in a BCA assay to measure tissue sample protein concentration, as detailed by the manufacturer (Thermo Fisher Scientific, Waltham, MA). The remaining homogenate was centrifuged at 10,000 xg for 20 minutes and the supernatant removed for analysis of monoamine content. Supernatant or whole blood samples (20 μl of each) were injected onto a Phenomenex Kinetex C18 HPLC column (100 x 4.60mm, 2.6u) using a Water 2707 autosampler and biogenic amine concentrations were determined using an Antec Decade II electrochemical detector (oxidation 0.65, 33 °C operating temperature; Antec Scientific, Zoeterwoude, The Netherlands). Biogenic amines were eluted with a mobile phase consisting of 89.5 % 0.1 M TCA, 10 mM sodium acetate, 0.1 mM EDTA and 10.5 % methanol (pH 3.8) delivered at a flow rate of 0.6 mL/min via a Waters 515 HPLC pump. HPLC control and data acquisition were managed by Empower software.
2.9 Statistical analysis
Graphing, curve fits, and statistical analyses were performed in OriginPro 2016 (OriginLab Corporation, Northampton, MA) and Prism 5 software (GraphPad Software Inc., La Jolla, CA). For each experimental dataset, ‘n’ denotes the number of biological replicates. The specific statistical tests used for each dataset are noted in the results section and / or figure legend with differences considered to be statistically significant if P < 0.05. Student’s t-test was used when comparing two groups and one-way ANOVA with Bonferroni post-hoc test was used for three or more groups. Staff performing HPLC analyses of monoamine content were blinded to sample genotype.
3. Results
3.1 SERT protein expression was abolished from the adrenal gland but remained intact in the CNS of SERTΔTH mice
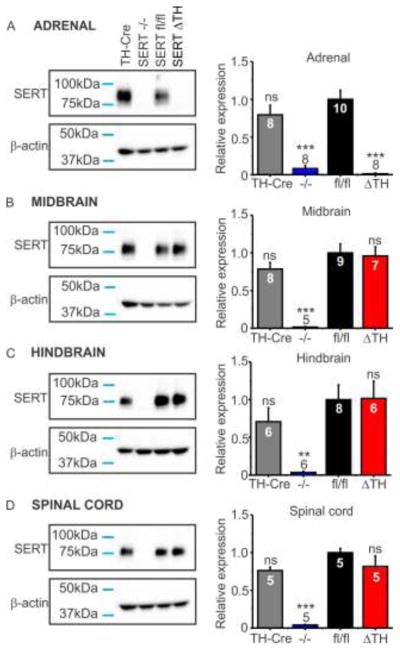
SERT is robustly expressed in chromaffin cells of the adrenal medulla but not in the adrenal cortex [9] (Figure 1a). Our conditional targeting strategy, using Th-IRES-Cre mediated excision of the floxed SERT allele, is therefore predicted to abolish SERT expression from the adrenal gland (Figure 1). This was confirmed using western blot experiments (Figure 2). As shown in the representative blot in Figure 2a, SERT protein expression was detected as a single band (~80 kDa) in the adrenal glands of the Th-IRES-Cre founder line (positive control) and was absent in SERT−/− mice (negative control). Robust SERT expression was also evident in the adrenal glands of SERTfl/fl mice but was not detected in SERTΔTH littermates (Figure 2a). This was confirmed in multiple samples from different mice and quantified following normalization of SERT expression to the β-actin loading control as shown in the bar graph in Figure 2a.
Figure 2. SERT protein expression was abolished from the adrenal gland but remained intact in the CNS of SERTΔTH mice.
SERT protein expression was determined in western blot analyses of tissue isolated from mice with the indicated genotypes: TH-Cre is the parent Th-IRES-Cre driver line (positive control); SERT−/− is the constitutive (global) SERT knockout line (negative control); SERTΔTH and SERTfl/fl are the conditional knockout and floxed control littermates respectively. Representative blots of SERT and β-actin expression (sample loading control) are shown from: A) the adrenal glands, B) midbrain, C) hindbrain, D) thoracic spinal cord. For each sample, SERT expression was normalized to the β–actin loading control and the mean data are expressed as a fraction of the SERTfl/fl control mean (bar graphs). Numbers (n values) denote the number of biological replicates, each from an individual mouse, and in the bar graphs are indicated within the bar of the corresponding genotype. Statistical significance was determined by one-way ANOVA followed by Bonferroni post-test for multiple pairwise comparisons relative to SERTfl/fl. A) In the adrenal gland, SERT expression was detected as a single band (~80 kDa) in TH-Cre and SERTfl/fl mice but was absent from both SERT−/− and SERTΔTH. Quantitative western blot analyses (bar graph) confirmed SERT was absent from the adrenal gland of SERTΔTH mice (F = 28.22, P <0.0001; ns, not significantly different; ***, p < 0.001 compared to SERTfl/fl). B–D) In the CNS tissues tested, SERT expression in SERTΔTH and TH-Cre mice was not significantly different from SERTfl/fl littermate controls but was absent in SERT−/− mice (Midbrain F = 14.62, P < 0.0001; Hindbrain F = 6.05, P = 0.004; Spinal cord F = 28.97, P < 0.0001; ns denotes not significantly different from SERTfl/fl; **, p < 0.01; ***, p < 0.001).
In contrast to the adrenal gland, SERT expression (assessed by western blot) was not altered in the CNS of SERTΔTH mice (Figure 2b–d). SERT is predominantly expressed on the presynaptic terminals of midbrain and hindbrain serotonergic raphe neurons that project widely throughout the CNS. This includes descending projections that innervate brainstem sympathetic premotor neurons and thoracic spinal cord preganglionic sympathetic neurons [10]. Western blot analyses (representative blots and quantification in bar graphs) confirmed that total SERT protein expression in the midbrain (Figure 2b), hindbrain (Figure 2c), and spinal cord (Figure 2d) of SERTΔTH mice was not significantly different from SERTfl/fl littermates. As expected, SERT−/− mice, which served as a negative control, lacked SERT expression in these tissues (Figure 2b–d). Additional experiments found no loss of SERT expression in the prefrontal cortex or hippocampus of SERTΔTH mice (supplemental data Figure S1).
3.2 SERT transport function and endogenous 5-HT homeostasis were unperturbed in the CNS of SERTΔTH mice
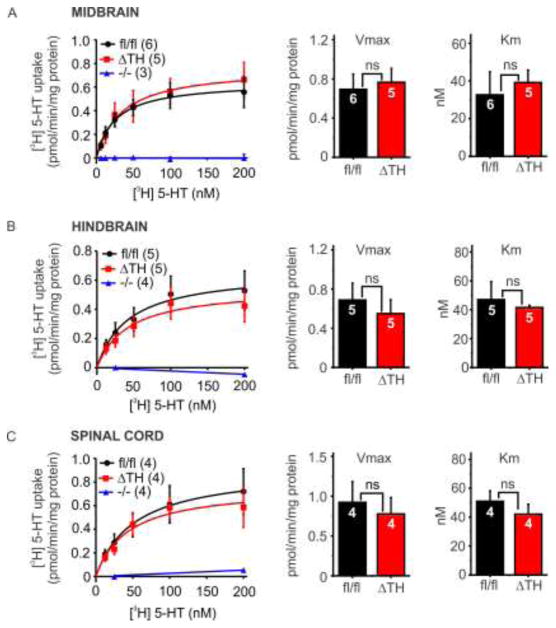
As reported above (section 3.1), SERT protein expression was abolished in the adrenal gland but remained intact in the CNS of SERTΔTH mice (Figure 2). To corroborate that CNS SERT was not targeted in the SERTΔTH mice we assessed transport function using [3H] 5-HT uptake assays. Synaptosomes were prepared from midbrain, hindbrain, or thoracic spinal cord and incubated for 10 minutes with increasing concentrations of tritiated 5-HT ([3H] 5-HT). Non-specific uptake of [3H] 5-HT was determined in the presence of escitalopram, a SSRI. As expected, synaptosomes prepared from SERT−/− mice did not display any specific uptake (total minus non-specific [3H] 5-HT uptake). In contrast, robust SERT-mediated uptake of [3H] 5-HT was observed in synaptosomes prepared from both SERTfl/fl and SERTΔTH mice (Figure 3). This uptake followed typical Michaelis-Menten kinetics and there was no significant difference in SERT transport capacity (Vmax) or affinity for 5-HT (Km) between SERTfl/fl and SERTΔTH mice (Figure 3).
Figure 3. SERT transport function (uptake of [3H] 5-HT) was not altered in the CNS of SERTΔTH mice.
Synaptosomes were prepared from A) midbrain, B) hindbrain, or C) thoracic spinal cord of SERTΔTH mice, SERTfl/fl littermates and SERT−/− mice, which served as a negative control. Synaptosomal preparations were incubated for 10 minutes with the indicated concentrations [3H] 5-HT. Non-specific uptake in the presence of escitalopram was subtracted from the data to obtain specific SERT-mediated uptake. Saturation curve data points show mean specific uptake at the indicated [3H] 5-HT concentration and the curved lines show the fit of the mean data with the Michaelis-Menten equation. SERT-mediated uptake was very similar in SERTΔTH and SERTfl/fl littermates and was absent from SERT−/− synaptosomes (negative control). SERT−/− data was fit with a straight line. The bar graphs show mean transport kinetics parameters obtained by fitting the data from individual experiments with the Michaelis-Menten equation. There was no significant difference in Vmax or Km of SERT-mediated uptake in any of the tissues tested (ns, not significant using Student’s t-test; midbrain Vmax P = 0.75, Km P = 0.67; hindbrain Vmax P = 0.55, Km P = 0.67; spinal cord Vmax P = 0.67, Km P = 0.40). Numbers (“n” values), indicated within the bars of each graph, denote biological replicates, each of which was from a fresh synaptosomal preparation.
To complement the [3H] 5-HT uptake experiments, we used HPLC to determine whether SERTΔTH mice exhibit alterations in endogenous 5-HT homeostasis as is seen in global SERT knockout mice [27, 30]. We measured endogenous 5-HT and 5-hydroxyindolacetic acid (5-HIAA), the major metabolite of 5-HT, in midbrain tissue taken from SERTΔTH mice, floxed littermate controls (SERTfl/fl), and global SERT knockout (SERT−/−) mice (Figure 4). There were no significant differences between SERTΔTH mice and SERTfl/fl littermates when comparing 5-HT (Figure 4a), 5-HIAA (Figure 4b), or the 5-HIAA: 5-HT ratio, a measure of 5-HT turnover in the tissue (Figure 4c). Consistent with previous reports [27, 30], loss of midbrain SERT expression in SERT−/− mice resulted in a ≈ 70 % reduction of 5-HT content and increased 5-HIAA: 5-HT ratio. Taken together, these data show that SERT expression and function remained intact in the CNS (brainstem and spinal cord) of SERTΔTH mice.
Figure 4. Endogenous midbrain 5-HT homeostasis was maintained in SERTΔTH mice.
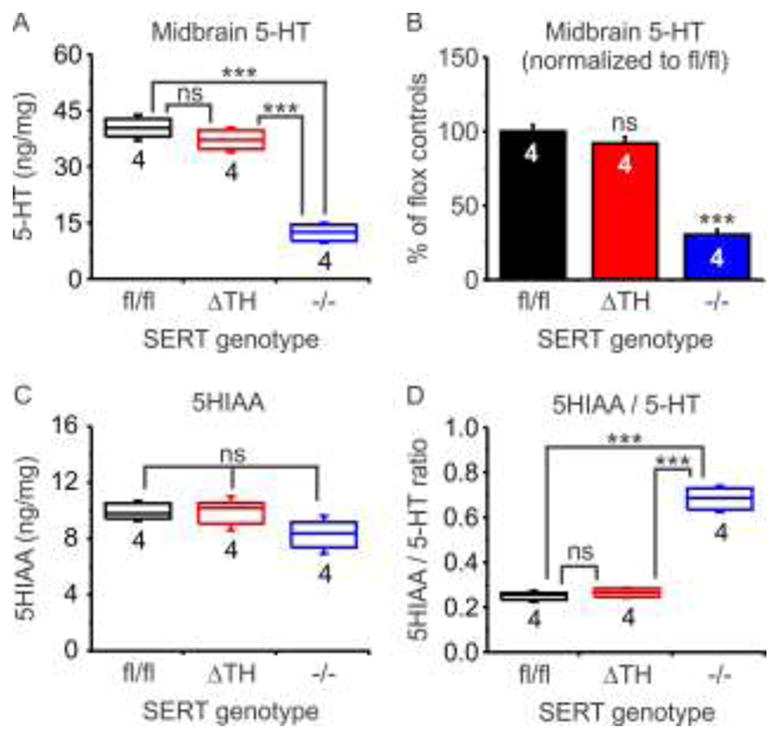
The amounts of endogenous 5-HT and its metabolite 5-HIAA were determined in midbrain tissue from SERTΔTH mice (n = 4), SERTfl/fl littermates (n = 4), and SERT−/− mice (n = 4) using HPLC and normalized to total protein content of the samples (ng / mg protein). Also shown is the 5-HIAA / 5-HT ratio that can be used as an indicator of 5-HT turnover. Box plots indicate the median with interquartile range and whiskers denote standard deviation. The number of biological replicates (n values) is indicated below the boxes. Statistical comparison was performed using one-way ANOVA followed by Bonferroni post-test for multiple pairwise comparisons. A) 5-HT content was not different in SERTΔTH and SERTfl/fl but was significantly reduced in SERT−/− (F = 94, P < 0.0001; ns, not significantly different, P = 0.55; *** P < 0.0001). B) Same data as in panel A is expressed as a percentage of the mean value detected in SERTfl/fl mice (F = 94, P < 0.0001; ns, not significantly different, P = 0.55; *** P < 0.001). C) 5-HIAA content was not significantly different across genotypes (F = 2.75, P = 0.12). D) Turnover of 5-HT assessed as the ratio of 5-HIAA / 5-HT was not significantly different in SERTΔTH and SERTfl/fl but was enhanced in SERT−/− (F = 162, P < 0.0001; ns, not significantly different, P = 1; *** P < 0.0001).
3.3 Whole blood 5-HT content was not altered in SERTΔTH mice
Whole blood contains a significant amount of 5-HT, most of which is synthesized and released from enterochromaffin cells in the gut and is then sequestered into platelets by SERT-mediated uptake [31]. This 5-HT plays an important role in platelet function and is also transported throughout the circulation. When plasma 5-HT content is elevated in rats by infusion of exogenous 5-HT, the adrenal gland accumulates one of the highest amounts of circulating 5-HT in the body [4]. Although peripheral 5-HT synthesis is unaltered in SERT−/− mice, whole blood 5-HT is dramatically reduced because it is not transported into platelets and therefore is rapidly metabolized. This is a potentially confounding factor when considering the role of SERT in other tissues including the adrenal gland. In the global SERT knockout (SERT−/−) mouse, both SERT function in adrenal chromaffin cells is lost and delivery of 5-HT via the circulation is impacted. In contrast, 5-HT is predicted to remain intact in whole blood from the conditional knockout mice (SERTΔTH). We confirmed this using HPLC analysis, which revealed no significant difference in whole blood 5-HT content between SERTΔTH and SERTfl/fl mice (Figure 5). As expected, 5-HT was virtually abolished in blood from SERT−/− mice and was below the limit of detection of the current assay (< 5pg/μl).
Figure 5. Whole blood 5-HT content was not altered in SERTΔTH mice.
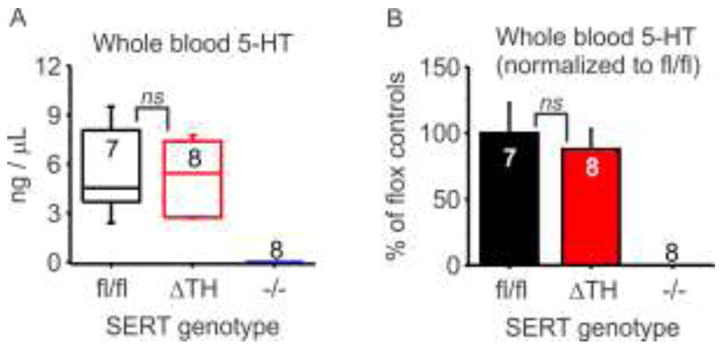
The 5-HT content of whole blood collected from SERTΔTH mice (n = 7), SERTfl/fl littermate controls (n = 8), and SERT−/− mice (n = 8) was determined using HPLC. A) Box plot indicates the median with interquartile range and whiskers denote standard deviation. The number of biological replicates (n values) is indicated below the boxes. 5-HT content was not significantly different in SERTΔTH and SERTfl/fl (ns, not significantly different, P = 0.65 Student’s t-test). Note: The 5-HT content of blood from SERT−/− mice was below the threshold of detection (< 5 pg / μL). B) Same data as panel in A expressed as a percentage of mean value of SERTfl/fl mice (ns, not significantly different, P = 0.65 Student’s t-test).
3.4 Adrenal gland 5-HT content was significantly reduced, but catecholamine content was unaltered in SERTΔTH mice
Adrenal gland 5-HT content is substantially reduced in SERT−/− rodents, consistent with the hypothesis that it is primarily derived from uptake rather than synthesis [4, 14, 15]. However, chromaffin cells in SERT−/− mice not only lack the transporter but are also exposed to dramatically reduced 5-HT in the circulation (section 3.3, Figure 5). Our results confirmed that SERTΔTH mice selectively lack expression of SERT in the adrenal gland but whole blood 5-HT levels remained intact (Figure 5). Therefore, we wanted to determine how this selective loss of SERT affected adrenal gland 5-HT content. We also wanted to ensure that the catecholamine content of the adrenal gland was not perturbed. Loss of adrenal SERT is unlikely to change catecholamine content given that no difference was observed in global SERT−/− mice [14, 15]. However, the targeting strategy used to generate the SERTΔTH mice utilizes a knock-in mouse line with Cre expressed in the 3′-UTR region of the tyrosine hydroxylase gene, the rate-limiting enzyme for catecholamine biosynthesis. Although previous reports showed that tyrosine hydroxylase expression is not disrupted in the Th-IRES-Cre mouse, we wanted to confirm this [26].
The glands isolated from SERTΔTH and SERTfl/fl littermates were of similar size as reflected in total protein content of adrenal gland homogenates (SERTfl/fl 340.6 ± 21.4 μg vs. SERTΔTH 340.3 ± 21.4 μg; P = 0.99 Students t-test). Using western blot analysis, there was no significant difference in the expression of tyrosine hydroxylase in SERTΔTH normalized to the mean of SERTfl/fl littermates (SERTΔTH = 0.90 ± 0.34, n = 4; SERTfl/fl = 1.00 ± 0.19, n = 4; P = 0.80, Student’s t test). Consistent with this, the catecholamine content of SERTΔTH adrenal glands was not significantly different from SERTfl/fl littermates (Figure 6). In contrast, there was a substantial (≈50 %), statistically significant reduction in the 5-HT content of SERTΔTH adrenal glands compared to SERTfl/fl littermates (Figure 6). Note that a similar reduction in 5-HT content was seen in both male and female mice (46 ± 3 %, n = 4 and 53 ± 6 %, n = 4; P = 0.33 Student’s t-test) so data from both sexes were combined in Figure 6. Together, these data demonstrate that SERT expressed in adrenal chromaffin cells plays a major role in determining adrenal gland 5-HT content and homeostasis in the context of wild-type 5-HT levels in the blood. Moreover, the SERTΔTH mice provide a novel conditional knockout model to dissect the role of peripheral 5-HT / SERT in the sympathoadrenal stress response.
Figure 6. Loss of SERT in the adrenal gland of SERTΔTH mice resulted in dramatically reduced 5-HT content but no change in catecholamines.
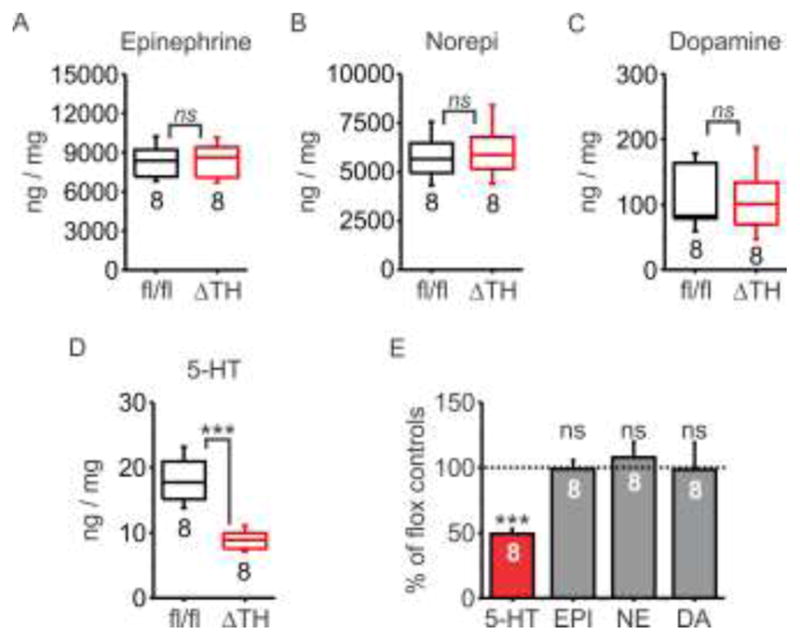
The monoamine content of whole adrenal glands from SERTΔTH (ΔTH; n = 8 mice, 4 male and 4 female) or SERTfl/fl littermates (fl/fl; n = 8, 4 male and 4 female) was determined using HPLC and normalized to total protein content of the samples (ng / mg protein). A–D) Box plots indicate the median with interquartile range and whiskers denote standard deviation. The number of biological replicates (n values) is indicated below the boxes. Statistical comparison was performed using an unpaired Student’s t-test. There was no significant difference in the amount of catecholamines detected in SERTΔTH mice compared to SERTfl/fl littermate controls (ns denotes not statistically significant: epinephrine, P = 0.91; norepinephrine P = 0.62; dopamine P = 0.96). In contrast, there was significantly less 5-HT in the adrenal glands of SERTΔTH mice compared to SERTfl/fl littermates (*** denotes P = 0.0001). E) Same data as panels A–D with the monoamine content of SERTΔTH expressed as a percentage of the mean value for SERTfl/fl mice (ns denotes not significantly different from SERTfl/fl, P = 0.6 – 0.9, *** P = 0.0001, unpaired Student’s t-test).
4. Discussion
In this paper, we report the development of a novel mouse model in which SERT was selectively excised from the peripheral sympathoadrenal system. The goal was to provide a new tool to test the significance of SERT expression in the adrenal medulla in the context of wild-type 5-HT levels in the circulation and SERT expression in the CNS. Multiple lines of evidence demonstrate that altered SERT function impacts the sympathetic stress response (for recent review see [10]). However, mechanistic understanding is lacking, including which tissues SERT acts upon to control peripheral catecholamine secretion. In the brain and spinal cord, serotonergic signaling can modulate central drive to the peripheral sympathetic nervous system. Additionally, we recently reported that the adrenal medulla might comprise a peripheral hub for serotonergic control of the sympathetic stress response [10, 15]. SERT is robustly expressed in adrenal chromaffin cells, specifically in epinephrine containing cells (~80 % of cells) [9], where it is proposed to mediate accumulation of 5-HT. In isolated chromaffin cells, SERT also controls a 5-HT1A receptor-mediated inhibition of catecholamine secretion [15]. This is consistent with a role in local, autocrine / paracrine control of catecholamine secretion and thereby the sympathoadrenal stress response [10]. Indeed, stress-evoked epinephrine secretion is enhanced in SERT−/− mice, and by SSRI treatment in both rodents and humans [11, 13, 14]. However, in these previous studies, genetic knockout and pharmacological block resulted in loss of SERT function throughout the entire body making it difficult to dissect the specific tissues in which SERT might be acting.
4.1 Selective excision of SERT in the adrenal gland
To selectively excise SERT from the peripheral sympathetic nervous system (i.e. adrenal chromaffin cells) we crossed floxed SERT mice [24] with a knock-in driver mouse line that harbors Cre in the 3′-UTR of tyrosine hydroxylase, the rate limiting enzyme for catecholamine biosynthesis (Th-IRES-Cre mouse) [26]. SERT and tyrosine hydroxylase are co-expressed in adrenal chromaffin cells [9] (Figure 1), but to the best of our knowledge they are not co-expressed in the same neurons within the CNS. Consistent with this, our data confirmed that SERT expression was abolished in the adrenal gland of the SERTΔTH mice but remained intact in the brain and spinal cord (Figure 2). To corroborate this expression data, we measured SERT-mediated [3H] 5-HT uptake in midbrain, hindbrain or spinal cord of SERTΔTH mice and found no difference from SERTfl/fl littermate controls (Figure 3). We also found no difference in the endogenous 5-HT content of midbrain tissue from SERTΔTH mice compared to SERTfl/fl littermates, which contrasted starkly with the disruption to 5-HT homeostasis seen in the CNS of SERT−/− mice (Figure 4) [27, 30]. Similarly, whole blood 5-HT content was not altered in SERTΔTH mice, which again contrasted starkly with SERT−/− mice in which blood 5-HT was virtually undetectable (Figure 5) [31].
There are some potential confounding factors with the use of Cre driver lines including disruption of endogenous gene expression and the possibility of transient expression during development leading to “off target” Cre expression. The Th-IRES-Cre driver line was generated using a knock-in approach that does not typically disrupt endogenous gene expression. Initial characterization of the founder Th-IRES-Cre mouse line confirmed that endogenous adrenal gland tyrosine hydroxylase expression was not altered [26] and we detected no difference in adrenal gland tyrosine hydroxylase expression in western blot experiments. Our HPLC analyses also confirmed there were no significant changes in the adrenal gland catecholamine content of SERTΔTH mice (figure 6). This was expected because no difference was seen in the catecholamine content of SERT−/− mice [14, 15], but it was important to confirm given the use of Th-IRES-Cre driver line.
Transient expression of tyrosine hydroxylase (and thus Cre) in non-catecholaminergic cells is known to occur during development of the Th-IRES-Cre driver mouse line [26]. Within the limits of the methodology used in this study, our data clearly demonstrate that the expression and function of SERT remained intact in the CNS and platelets of SERTΔTH mice, indicating that tyrosine hydroxylase / Cre and SERT are not co-expressed in the CNS or platelets of SERTΔTH mice. In addition, blood 5-HT content primarily reflects synthesis in the gut suggesting that this also remains intact in the SERTΔTH mouse, although a more detailed investigation is required to confirm this. It remains possible that there are discrete / scattered populations of neurons or other cell types in the SERTΔTH mice that have disrupted SERT expression and this would require further detailed investigation at the cellular level, for example using immunostaining approaches, in the specific regions of interest. Whilst CNS SERT expression did not appear to be disrupted, it is worth noting that SERT might be expressed in a subpopulation (≈30 %) of peripheral sympathetic neurons in the superior cervical ganglion [32]. We did not confirm this, but if present SERT will be excised from these peripheral noradrenergic neurons in SERTΔTH mice. Plasma epinephrine (>95%) is almost exclusively due to secretion from adrenal chromaffin cells whereas plasma norepinephrine is mostly due to overflow from sympathetic postganglionic nerve terminals (70 – 90 %) [33, 34]. Some stressors (e.g. hypoglycemia) preferentially recruit the sympathoadrenal response and others (e.g. cold stress) preferentially recruit the sympathoneural response [35–37]. Loss of SERT function via SSRI treatment selectively enhanced the increase in plasma epinephrine (i.e. sympathoadrenal) but not norepinephrine (i.e. sympathoneural) evoked by hypoglycemia [11]. Similarly, acute restraint stress selectively enhanced plasma epinephrine but not norepinephrine in SERT−/− mice [14]. The SERTΔTH model could therefore provide an opportunity to investigate not only the role of SERT in adrenal chromaffin cells but also its role in the superior cervical ganglion neurons, which remains unknown.
4.2 Adrenal SERT is a key determinant of gland 5-HT homeostasis
It has been proposed that the 5-HT content of adrenal chromaffin cells is due to SERT mediated uptake rather than synthesis [4, 9, 10]. Consistent with this, the adrenal gland 5-HT content was reduced by ≈ 65 – 80 % in SERT−/− mice or rats [4, 14, 15]. However, a caveat to using SERT−/− rodents is the significant disruption to circulating 5-HT due to loss of the transporter in platelets. Thus, the reduced 5-HT content of adrenal chromaffin cells could be due to loss of SERT-mediated uptake and / or due to reduced 5-HT delivery via the circulation. Our data using the SERTΔTH mice clarify this and confirm that SERT expressed in adrenal chromaffin cells is an important determinant of adrenal 5-HT content / homeostasis. Adrenal 5-HT was reduced by ≈ 50 % in SERTΔTH mice compared to SERTfl/fl littermates (Figure 6) even though blood 5-HT was unaltered (Figure 5). The ≈ 50 % reduction observed in SERTΔTH mice is lower than was previously reported for SERT−/− mice (≈80 %) [14, 15]. This indicates that there may be additional sources from which the SERTΔTH adrenal gland 5-HT content was derived but these remain unclear. The adrenal glands are highly vascularized and so this might reflect, at least in part, residual blood 5-HT content in the dissected adrenal glands. Whilst chromaffin cells do not appear to synthesize 5-HT, there is some evidence for local 5-HT synthesis in mast cells of the adrenal cortex [38]. Additionally, previous work from the Watts lab has found evidence for SERT-independent uptake of 5-HT [4]. One candidate for SERT independent 5-HT uptake is an organic cation transporter (OCT) family member. Studies suggest that OCT family members are expressed in rodent and human adrenal gland tissue, with some expression in the adrenal cortex [39–41]. It is unclear whether OCTs are also expressed in the adrenal medulla and their physiological role in the adrenal gland is not known. Of note, OCTs are inhibited by glucocorticoids produced in the adrenal cortex in response to stress [42].
4.3 Future directions
Genetic variants of SERT are thought to influence sensitivity to emotional / psychological stressors and subsequent development of depression and anxiety disorders [43]. Mounting evidence also links depression, anxiety and other disorders of serotonergic signaling to autonomic dysfunction / aberrant sympathoadrenal stress responses [44–47]. Appropriate recruitment of the sympathetic nervous system, including release of catecholamines from adrenal chromaffin cells, helps coordinate the physiological response to emotional / psychological, physical, or metabolic stressors. In situations where catecholamine secretion is insufficient or excessive, the response to stress can be maladaptive and lead to the development or exacerbation of disease. Depending on the nature of the stressor, aberrant adrenal catecholamine secretion can contribute to hypertension, heart failure, and loss of counterregulatory responses to hypoglycemia [48–51]. Failure of this hypoglycemic counterregulation can be a serious clinical problem in type-1 and some type-2 diabetics where glucagon is lacking and the response is predominantly mediated by adrenal epinephrine secretion [49]. Of note, pharmacological block of SERT augments the hypoglycemic counterregulatory response (increased adrenal epinephrine secretion) in both diabetic and non-diabetic human subjects [12, 13]. There is therefore considerable interest in the underlying mechanisms; however, it has been difficult to tease apart the role of SERT and to determine the tissues in which it is acting to mediate these effects. This promises to change with the recent development of floxed mice enabling the spatial / temporal manipulation of SERT expression.
Previously, SERTfl/fl mice were crossed with the ePet-Cre driver line to selectively excise SERT in CNS serotonergic neurons (SERTRapheΔ) [24]. As ePet (and thus Cre) is not expressed in the adrenal gland [52], SERT is predicted to remain intact in the adrenal chromaffin cells in this conditional knockout. In this paper, we introduce the SERTΔTH mouse with the inverse pattern of SERT excision (i.e. SERT is deleted from the adrenal gland but remains intact in the CNS). These complementary models will provide powerful tools to dissect the roles of SERT / 5-HT signaling in the CNS and peripheral sympathetic nervous system. Precise in vivo experimental approaches have been developed to enable glucose clamp studies in mice with remote blood sampling / drug administration to overcome the confounding effects of handling mice, which in of itself elicits a robust elevation of plasma catecholamines [53, 54]. Other acute stress paradigms include restraint or predation stress (emotional / psychological stressors), which are distinct from the metabolic stress of hypoglycemia. The sympathoadrenal response to restraint stress is augmented in SERT−/− mice [14], but more precise mechanistic understanding will be provided by exploiting the tissue selective knockouts provided by the SERT TH and SERTRapheΔ mice. It will also be important to assess the impact of sex and aging on the control of sympathoadrenal stress by SERT. In this paper, we found no significant sex difference in the effects of SERT on adrenal gland monoamine content, but a more detailed analysis is certainly warranted. There are reported changes in expression of adrenal gland catecholamine synthetic enzymes, epinephrine content, and stress-induced secretion of epinephrine in older rodents (> 12 months of age) [55, 56]. Reductions in human adrenal gland epinephrine secretion with older age have also been reported, but it has yet to be determined what influence this might have on the response to various stressors or the development of disease [57]. Finally, it is also important to note that adrenal chromaffin cells are a well-characterized neurosecretory model that enable detailed mechanistic investigation of stimulus-secretion coupling. Combining in vivo studies of the response to different stressors (e.g. restraint stress or hypoglycemia) with in vitro mechanistic understanding will enable a robust test of the hypothesis that the adrenal medulla comprises a peripheral serotonergic hub for control of the sympathetic stress response.
Supplementary Material
Acknowledgments
We thank Ginger Milne and Benlian Gao of the Vanderbilt Brain Institute Neurochemistry core laboratory in which HPLC analyses were performed. We thank Pat Levitt and Hsiao-Huei Wu for multiplex fluorescent in situ hybridization assays performed in the Molecular Neuroanatomy core of the Vanderbilt Silvio O. Conte Center for Neuroscience and housed at the Saban Research Institute, Children’s Hospital of Los Angeles. We also acknowledge Chris Svitek for initial help with the mouse colony. This work was supported by the American Heart Association [Grant 17GRNT33661156] and the National Institutes of Health [Grant P50 MH096972]. Generation of floxed SERT mice was supported by the National Institutes of Health [Grant MH105839 (to JYS)]. The sponsors had no role in the study design, collection / analysis / interpretation of the data, or in the writing of the manuscript for publication. The authors have no conflicts of interest to declare.
Abbreviations
- 3′-UTR
3′ untranslated region
- 5-HT
serotonin, 5-hydroxytryptamine
- 5-HIAA
5-hydroxyindolacetic acid
- HPLC
high performance liquid chromatography
- IRES
internal ribosome entry sequence
- OCT
organic cation transporter
- SERT
serotonin transporter
- SERT−/−
global SERT knockout mice
- SERTΔTH
sympathoadrenal system conditional SERT knockout
- SERTfl/fl
floxed SERT mice
- SSRI
selective serotonin reuptake inhibitor
- Th
tyrosine hydroxylase
- wt
wild-type
Footnotes
Conflicts of interest disclosure
This statements serves as a conflicts of interest disclosure. There are no conflicts to disclose.
Chemical compounds studied in this article: Escitalopram oxalate (PubChem CID: 146571)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 2011;131(1):61–79. doi: 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver KH, Duvernay MT, Hamm HE, Carneiro AM. Loss of Serotonin Transporter Function Alters ADP-mediated Glycoprotein alphaIIbbeta3 Activation through Dysregulation of the 5-HT2A Receptor. The Journal of biological chemistry. 2016;291(38):20210–9. doi: 10.1074/jbc.M116.736983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linder AE, Beggs KM, Burnett RJ, Watts SW. Body distribution of infused serotonin in rats. Clinical and experimental pharmacology & physiology. 2009;36(5–6):599–601. doi: 10.1111/j.1440-1681.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, Keating DJ. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology. 2017;158(5):1049–1063. doi: 10.1210/en.2016-1839. [DOI] [PubMed] [Google Scholar]
- 6.Penumatsa KC, Fanburg BL. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2014;306(4):L309–15. doi: 10.1152/ajplung.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in endocrinology, diabetes and obesity. 2013;20(1):14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacological reviews. 2012;64(2):359–88. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeter S, Levey AI, Blakely RD. Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol Cell Neurosci. 1997;9(3):170–84. doi: 10.1006/mcne.1997.0619. [DOI] [PubMed] [Google Scholar]
- 10.Brindley RL, Bauer MB, Blakely RD, Currie KPM. Serotonin and Serotonin Transporters in the Adrenal Medulla: A Potential Hub for Modulation of the Sympathetic Stress Response. ACS chemical neuroscience. 2017;8(5):943–954. doi: 10.1021/acschemneuro.7b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders NM, Wilkinson CW, Taborsky GJ, Jr, Al-Noori S, Daumen W, Zavosh A, Figlewicz DP. The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. American journal of physiology Endocrinology and metabolism. 2008;294(5):E853–60. doi: 10.1152/ajpendo.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briscoe VJ, Ertl AC, Tate DB, Davis SN. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes. 2008;57(12):3315–22. doi: 10.2337/db08-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briscoe VJ, Ertl AC, Tate DB, Dawling S, Davis SN. Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes. 2008;57(9):2453–60. doi: 10.2337/db08-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143(12):4520–6. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- 15.Brindley RL, Bauer MB, Blakely RD, Currie KP. An interplay between the serotonin transporter (SERT) and 5-HT receptors controls stimulus-secretion coupling in sympathoadrenal chromaffin cells. Neuropharmacology. 2016;110(Pt A):438–48. doi: 10.1016/j.neuropharm.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzwarth MA, Sawetawan C, Brownfield MS. Serotonin-immunoreactivity in the adrenal medulla: distribution and response to pharmacological manipulation. Brain Res Bull. 1984;13(2):299–308. doi: 10.1016/0361-9230(84)90131-x. [DOI] [PubMed] [Google Scholar]
- 17.Verhofstad AA, Jonsson G. Immunohistochemical and neurochemical evidence for the presence of serotonin in the adrenal medulla of the rat. Neuroscience. 1983;10(4):1443–53. doi: 10.1016/0306-4522(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 18.Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell Mol Neurobiol. 2010;30(8):1201–8. doi: 10.1007/s10571-010-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewell ML, Currie KPM. Control of CaV2 calcium channels and neurosecretion by heterotrimeric G protein coupled receptors. In: Stephens GJ, Mochida S, editors. Modulation of presynaptic calcium channels. Springer Publishing; 2013. pp. 101–130. [Google Scholar]
- 20.Sabban EL, Nankova BB, Serova LI, Hiremagalur B, Rusnak M, Saez E, Spiegelman B, Kvetňanský R. Regulation of Gene Expression of Catecholamine Biosynthetic Enzymes by Stress. In: David GE, Goldstein S, Richard M, editors. Advances in Pharmacology. Academic Press; 1997. pp. 564–567. [DOI] [PubMed] [Google Scholar]
- 21.Kvetnansky R, Lu X, Ziegler MG. Stress-triggered changes in peripheral catecholaminergic systems. Advances in pharmacology (San Diego, Calif) 2013;68:359–97. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
- 22.Armando I, Tjurmina OA, Li Q, Murphy DL, Saavedra JM. The serotonin transporter is required for stress-evoked increases in adrenal catecholamine synthesis and angiotensin II AT(2) receptor expression. Neuroendocrinology. 2003;78(4):217–25. doi: 10.1159/000073705. [DOI] [PubMed] [Google Scholar]
- 23.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat Rev Gastroenterol Hepatol. 2017;14(7):412–420. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY. Disruption of Transient Serotonin Accumulation by Non-Serotonin-Producing Neurons Impairs Cortical Map Development. Cell reports. 2015;10(3):346–358. doi: 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Petit EI, Dobrenis K, Sze JY. Spatiotemporal SERT expression in cortical map development. Neurochemistry international. 2016;98:129–37. doi: 10.1016/j.neuint.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis (New York, NY : 2000) 2004;40(2):67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 27.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Molecular pharmacology. 1998;53(4):649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Academic Press; San Diego, CA: 2004. [Google Scholar]
- 29.Harrison M, O’Brien A, Adams L, Cowin G, Ruitenberg MJ, Sengul G, Watson C. Vertebral landmarks for the identification of spinal cord segments in the mouse. Neuroimage. 2013;68:22–29. doi: 10.1016/j.neuroimage.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49(6):798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21(16):6348–61. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura M, Sato K, Shimada S, Tohyama M. Expression of norepinephrine and serotonin transporter mRNAs in the rat superior cervical ganglion. Brain Res Mol Brain Res. 1999;67(1):82–6. doi: 10.1016/s0169-328x(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5(4):552–9. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- 34.McCarty R. Learning about stress: neural, endocrine and behavioral adaptations. Stress. 2016;19(5):449–75. doi: 10.1080/10253890.2016.1192120. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein DS. Differential responses of components of the autonomic nervous system. Handb Clin Neurol. 2013;117:13–22. doi: 10.1016/B978-0-444-53491-0.00002-X. [DOI] [PubMed] [Google Scholar]
- 36.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol. 1998;275(4 Pt 2):R1247–55. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 37.Morrison SF, Reis DJ. Responses of sympathetic preganglionic neurons to rostral ventrolateral medullary stimulation. Am J Physiol. 1991;261(5 Pt 2):R1247–56. doi: 10.1152/ajpregu.1991.261.5.R1247. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre H, Compagnon P, Contesse V, Delarue C, Thuillez C, Vaudry H, Kuhn JM. Production and metabolism of serotonin (5-HT) by the human adrenal cortex: paracrine stimulation of aldosterone secretion by 5-HT. J Clin Endocrinol Metab. 2001;86(10):5001–7. doi: 10.1210/jcem.86.10.7917. [DOI] [PubMed] [Google Scholar]
- 39.Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335(3):743–53. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courousse T, Bacq A, Belzung C, Guiard B, Balasse L, Louis F, Le Guisquet AM, Gardier AM, Schinkel AH, Giros B, Gautron S. Brain organic cation transporter 2 controls response and vulnerability to stress and GSK3beta signaling. Mol Psychiatry. 2015;20(7):889–900. doi: 10.1038/mp.2014.86. [DOI] [PubMed] [Google Scholar]
- 41.Beery E, Middel P, Bahn A, Willenberg HS, Hagos Y, Koepsell H, Bornstein SR, Muller GA, Burckhardt G, Steffgen J. Molecular evidence of organic ion transporters in the rat adrenal cortex with adrenocorticotropin-regulated zonal expression. Endocrinology. 2003;144(10):4519–26. doi: 10.1210/en.2002-221001. [DOI] [PubMed] [Google Scholar]
- 42.Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121(1):89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 44.Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, Esler M, Alesci S, Masood A, Licinio J, Geracioti TD, Jr, Perini G, DeBellis MD, Holmes C, Vgontzas AN, Charney DS, Chrousos GP, McCann SM, Kling MA. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A. 2005;102(23):8303–8. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM, Sherwood A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom Med. 2015;77(2):136–44. doi: 10.1097/PSY.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010;84(2):228–34. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99(6):642–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, Carlezon WA, Jr, Kvetnansky R. Epinephrine: a short- and long-term regulator of stress and development of illness : a potential new role for epinephrine in stress. Cell Mol Neurobiol. 2012;32(5):737–48. doi: 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprague JE, Arbelaez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9(1):463–73. quiz 474–5. [PMC free article] [PubMed] [Google Scholar]
- 50.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13(3):315–23. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler MG, Elayan H, Milic M, Sun P, Gharaibeh M. Epinephrine and the metabolic syndrome. Curr Hypertens Rep. 2012;14(1):1–7. doi: 10.1007/s11906-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 52.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102(45):16472–7. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson L, Ansari T, McGuinness OP. Counterregulatory deficits occur within 24 h of a single hypoglycemic episode in conscious, unrestrained, chronically cannulated mice. American journal of physiology Endocrinology and metabolism. 2006;290(4):E678–84. doi: 10.1152/ajpendo.00383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tweedell A, Mulligan KX, Martel JE, Chueh FY, Santomango T, McGuinness OP. Metabolic response to endotoxin in vivo in the conscious mouse: role of interleukin-6. Metabolism. 2011;60(1):92–8. doi: 10.1016/j.metabol.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amano A, Tsunoda M, Aigaki T, Maruyama N, Ishigami A. Age-related changes of dopamine noradrenaline and adrenaline in adrenal glands of mice. Geriatr Gerontol Int. 2013;13(2):490–6. doi: 10.1111/j.1447-0594.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 56.McCarty R. Aged rats: diminished sympathetic-adrenal medullary responses to acute stress. Behav Neural Biol. 1981;33(2):204–12. doi: 10.1016/s0163-1047(81)91638-1. [DOI] [PubMed] [Google Scholar]
- 57.Esler M, Kaye D, Thompson J, Jennings G, Cox H, Turner A, Lambert G, Seals D. Effects of aging on epinephrine secretion and regional release of epinephrine from the human heart. J Clin Endocrinol Metab. 1995;80(2):435–42. doi: 10.1210/jcem.80.2.7852502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.