Abstract
Prior research has indicated that brain regions and networks that support semantic memory, top-down and bottom-up attention, and cognitive control are all involved in divergent creative thinking. Kernels of evidence suggest that neural processes supporting episodic memory—the retrieval of particular elements of prior experiences—may also be involved in divergent thinking, but such processes have typically been characterized as not very relevant for, or even a hindrance to, creative output. In the present study, we combine functional magnetic resonance imaging with an experimental manipulation to test formally, for the first time, episodic memory’s involvement in divergent thinking. Following a manipulation that facilitates detailed episodic retrieval, we observed greater neural activity in the hippocampus and stronger connectivity between a core brain network linked to episodic processing and a frontoparietal brain network linked to cognitive control during divergent thinking relative to an object association control task that requires little divergent thinking. Stronger coupling following the retrieval manipulation extended to a subsequent resting-state scan. Neural effects of the episodic manipulation were consistent with behavioral effects of enhanced idea production on divergent thinking but not object association. The results indicate that conceptual frameworks should accommodate the idea that episodic retrieval can function as a component process of creative idea generation, and highlight how the brain flexibly utilizes the retrieval of episodic details for tasks beyond simple remembering.
Keywords: core network, divergent thinking, episodic memory, fMRI, frontoparietal control network
Divergent thinking is the ability to generate creative ideas by combining diverse types of information in novel ways (Guilford 1967), and is one facet of creative thinking that has been linked to real-world creative achievement (Torrance 1981). The dominant contemporary view in the cognitive neuroscience of creativity is that semantic memory is a key source of divergent creative thinking, in line with longstanding cognitive science accounts of creative cognition (Welch 1946; Mednick 1962; Torrance 1962; Mehndelsohn 1976; Finke et al. 1992; Smith et al. 1995). The dominant contemporary view also points to interactions between frontoparietal cognitive control and dorsal and ventral attention brain networks in the service of divergent thinking (Fink et al. 2015; Sun et al. 2016), as well as brain regions implicated in semantic cognition (Noonan et al. 2013; Davey et al. 2015a, 2015b, 2016). To this end, a recent meta-analysis of functional neuroimaging studies (Wu et al. 2015) indicates that activity in lateral prefrontal, anterior cingulate, and posterior parietal and temporal cortices underlies component processes of divergent thinking, which are typically thought to include semantic retrieval and expansion (Howard-Jones et al. 2005; Abraham et al. 2012), inhibition and cognitive control (Fink et al. 2009; Chrysikou and Thompson-Schill 2011; Mayseless et al. 2015), top-down and bottom-up attention (Fink et al. 2010, 2012), and cognitive flexibility (Roberts et al. 2017).
While much research has been justifiably devoted to these behavioral and neural underpinnings, a small but growing number of studies also suggest a meaningful role for episodic memory in divergent thinking that may point to the need to reconsider the dominant contemporary view. Episodic memory is a neurocognitive system that is classically characterized as supporting the recollection of past experiences specific to a time and place (Tulving 1983, 2002). Strikingly, during the past decade, episodic memory, and the neural processes that support it, have been found to be engaged during tasks beyond simple remembering that may also involve the retrieval and reconstruction of episodic details for completion, such as future thinking or simulation, decision making, and problem solving (for review, see Schacter et al. 2012; Moscovitch et al. 2016; Schacter et al. 2017; Roberts and Addis 2017). Studies on this topic have indicated that episodic memory may be recruited for additional tasks where particular details of past experiences are leveraged to generate, or assemble and maintain, mental content that incorporates specific elements of prior events.
Recently, this more expansive view of the role of episodic memory has begun to extend to the domain of creative cognition: kernels of empirical evidence suggest that episodic retrieval may likewise contribute to divergent thinking. Behaviorally, 3 kinds of evidence have emerged: (1) episodic memories are sometimes drawn upon during divergent thinking (Gilhooly et al. 2007; Runco and Acar 2010; Storm and Patel 2014); (2) a cognitive manipulation that facilitates episodic retrieval enhances the generation of ideas on divergent thinking tasks (Madore et al. 2015, 2016a); and (3) the specificity (Ononye et al. 1993) and episodic detail (Addis et al. 2016) of imagined future events correlate with divergent thinking. Neurally, a core network of brain regions (Schacter et al. 2007; Benoit and Schacter 2015) that overlaps substantially with the default network (Buckner et al. 2008; Andrews-Hanna et al. 2010, 2014; Yeo et al. 2011) and is recruited for remembering past events and simulating future events, also exhibits consistent activity during tasks that measure divergent thinking (for review, see Jung et al. 2013). These tasks include creative writing (Shah et al. 2013), poetry generation (Liu et al. 2015), book illustrations (Ellamil et al. 2012), and a classic index of divergent thinking (Guilford et al. 1960), the production of creative and alternate uses for everyday objects (Fink et al. 2012; Benedek et al. 2014). In particular, nodes of the medial temporal lobe subsystem of the core network implicated in detailed event and scene processing (Andrews-Hanna et al. 2010), such as the hippocampus, parahippocampal gyrus, and inferior parietal lobule, have been found to exhibit greater activity during these tasks compared with various controls (for additional evidence with hippocampal amnesic patients, see Duff et al. 2013). Studies of between-network functional connectivity (for review, see Beaty et al. 2016) using multivariate independent components analysis (ICA) and univariate region of interest (ROI) approaches have also suggested that components of the core network and frontoparietal cognitive control and dorsal and ventral attention networks may couple and decouple during various stages of divergent thinking (Ellamil et al. 2012; Wei et al. 2014; Beaty et al. 2015, 2017; Mayseless et al. 2015), and during rest in high versus low creative individuals (Takeuchi et al. 2012; Beaty et al. 2014).
While such evidence collectively suggests a link between episodic memory and divergent thinking, there are several caveats to this claim. First, neuroimaging work that implicates the core network and medial temporal lobe subsystem in divergent thinking has not been linked specifically to behavioral indices of episodic retrieval. Moreover, medial temporal lobe structures have been associated with both semantic and episodic memory (Eichenbaum and Cohen 2001). Second, behavioral work has shown that particular episodic memories are drawn on infrequently during divergent thinking (Gilhooly et al. 2007) and that episodic details from past (vs. future) events do not correlate strongly with divergent thinking (Addis et al. 2016). Third, researchers have tended to downplay the role of episodic memory in divergent thinking and some have suggested that episodic retrieval is not very relevant for, and may even be a hindrance to, creative output (Gilhooly et al. 2007; Runco and Acar 2010; Fink et al. 2015).
To examine this fundamental and as yet unresolved issue in the cognitive neuroscience of creativity, we designed and carried out a whole-brain functional magnetic resonance imaging (fMRI) study to formally test, for the first time, both behavioral and neural aspects of episodic memory’s involvement in divergent thinking using an experimental manipulation that impacts episodic retrieval. To accomplish this objective, we adopted a manipulation that we have referred to in previous work as an episodic specificity induction (ESI)—brief training in recollecting the details of a past experience (for review, see Schacter and Madore 2016). The logic of this approach is straightforward: cognitive tasks that involve episodic memory should be affected by an ESI when given prior to task completion, whereas cognitive tasks that do not involve episodic memory should not be affected by an ESI prior to task completion. To this end, previous behavioral work has indicated that ESI, compared with various control inductions that do not target episodic retrieval, improves subsequent performance on tasks that involve the retrieval and reconstruction of episodic details, such as episodic remembering and imagining (Madore et al. 2014), means-end problem solving (Madore and Schacter 2014) and divergent thinking (Madore et al. 2015, 2016a). Critically, induction effects have not been observed on tasks that do not require episodic retrieval, such as describing pictures (Madore et al. 2014) and defining objects (Madore and Schacter 2016). Moreover, on episodic memory and simulation tasks the effects of ESI are limited to the production of episodic—but not semantic—details (Madore et al. 2014). With respect to divergent thinking, ESI increases the production of both old ideas from memory (e.g., novel uses of an object based on remembering a past experience involving that use) and new ideas from imagination generated for the first time during study (e.g., novel uses of an object that are not based on remembering any one prior experience involving that use), without affecting performance on nonepisodic tasks like typical semantic associates (Madore et al. 2015, 2016a) and convergent creative riddles (Madore et al. 2015). The ESI effect has likewise been exhibited for typical generative divergent thinking measures (Guilford et al. 1960) that include total responses that are deemed feasible or appropriate in everyday life and that fit under distinct and flexible categories (Madore et al. 2015, 2016a).
Importantly, the selective behavioral effects of ESI have recently been linked to the medial temporal lobe subsystem of the core network. An fMRI study (Madore et al. 2016b) found that following ESI relative to a control induction, participants exhibited greater activity in the hippocampus and inferior parietal lobule when imagining future events relative to comparing and defining objects semantically. Resting-state functional connectivity analyses with hippocampal and inferior parietal lobule seed regions and the rest of the brain also revealed stronger core network coupling following ESI relative to a control induction, suggesting short-term, functional reorganization of brain networks as a function of induction. Imagined events contained more episodic—but not semantic—details following ESI and were not rated as more similar to past experiences or thoughts than imagined events following a control induction.
Based on this body of work, we have suggested that the ESI impacts episodic retrieval orientation—a flexible, goal-directed strategy for retrieving an episode when presented with a cue (Morcom and Rugg 2012). Receiving an induction focused on episodic details should affect performance on subsequent tasks, like divergent thinking, that may also require filling in an event with specific details as done during the ESI (for review, see Schacter and Madore 2016). The biasing of a specific retrieval orientation via ESI should show differential effects on subsequent tasks where an episodic retrieval orientation is invoked relative to tasks where it is not. An extension of this logic is that retrieval orientation effects via ESI could be observed not just during formal tasks that recruit episodic mechanisms but during subsequent rest where default or core network activity is also often exhibited (Andrews-Hanna et al. 2010).
Because ESI is a reliable tool for probing episodic memory’s involvement in tasks that are not typically considered to be “episodic memory tasks,” we think that it can help to shed light on the theoretically fundamental but currently unsettled and formally untested question of whether brain regions and networks that support episodic retrieval also play a meaningful role in divergent creative thinking, rather than their being irrelevant to, or a hindrance to, creative output. Specifically, we hypothesize that during divergent thinking relative to an object association control task, following ESI compared with a control induction, (1) greater blood oxygen level-dependent (BOLD) activity in key medial temporal lobe subsystem structures of the core network and (2) greater core network and frontoparietal control network coupling should be observed; that (3) the effects of ESI should show short-term, functional reorganization into a resting period; and that (4) behavioral performance in the scanner and during a postscan interview on a divergent thinking task (generating alternate uses of objects)—but not an object association control task (generating typical associates of objects) that involves primarily semantic imagery and little divergent thinking—should be enhanced by ESI on generative measures such as old and new idea production as well as total and appropriate responses that fit under distinct categories.
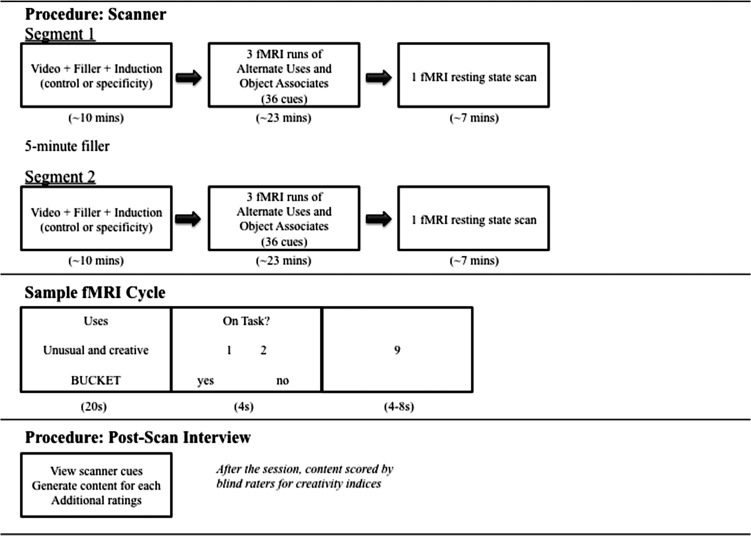
To test these predictions, 32 participants completed a within-subjects fMRI experiment (see Fig. 1 for a design schematic). The experiment consisted of 2 segments in the scanner and one postscan interview. In each segment in the scanner, participants (1) watched 1 of 2 short videos, completed a brief filler task, and then received the ESI or a control induction; (2) completed 3 runs of task-based fMRI during which they viewed 36 object cues for 20 s each and generated creative uses for the cue (i.e., divergent thinking: the Alternate Uses Task [AUT]) or typical associates of the cue (i.e., object control: Object Association Task [OAT]), pressing a button whenever they thought of a response in the trial window (as in previous related work; see Materials and Methods); and (3) underwent a resting-state fMRI scan. The number of responses/button presses, reaction time of each response, and an engagement rating were collected for each trial during task runs. Different stimuli were used in each segment and counterbalanced across participants. After scanning was completed, verbal reports and additional ratings for each cue were collected in the postscan interview to further characterize performance. For the critical fMRI analyses, we examined activity and connectivity during the main tasks following the inductions via (1) whole-brain activation patterns with univariate regression models and (2) between-network functional connectivity with multivariate ICA. We then assessed functional connectivity in the resting-state scans via a univariate seed-to-voxel approach.
Figure 1.
Design schematic. Participants completed a within-subjects fMRI experiment in a single session. (Top) Scanning procedure. In the first segment of scanning, participants watched a short video, completed a brief filler task, and then were asked questions about the video in the form of an episodic specificity induction (ESI) or impressions control induction. Immediately following the induction manipulation, participants completed 3 fMRI runs during which they viewed 36 object cues (12 per run) and either generated unusual and creative uses for the cue (i.e., divergent thinking: Alternate Uses Task, or AUT) or typical object associates (i.e., object control: Object Association Task, or OAT). After completing these 3 runs, participants underwent a resting-state fMRI run followed by a brief filler task. They then completed the second segment where they received new stimuli in the same sequence (i.e., video, induction, and task cues). (Middle) Sample trial cycle during the fMRI main task runs. For each trial, participants viewed a screen for 20 s with the top line providing the task (i.e., uses or associates), the second line providing a reminder of instructions (i.e., Unusual and creative or typical objects), and the third line providing an object cue in capital letters (e.g., BUCKET). Participants pressed a button each time they generated a use or object during the trial window. After each trial, participants completed a task engagement rating for 4 s (i.e., on or off task), followed by a jittered odd/even baseline task for 4, 6, or 8 s. (Bottom) Postscan procedure. Participants underwent a postscan interview after completing the 2 main segments in the scanner. They viewed the 72 cues from the scanner in the same order, and trial-by-trial verbally stated what they had thought about for each cue and provided additional phenomenological ratings. Participants’ verbal output was transcribed and audio-recorded, and they labeled each divergent thinking response as an old idea from memory or new idea from imagination. Individual difference assessments for creativity, personality, imagery, memory, and language were also collected. Raters blind to hypothesis and induction later scored participants’ output to further characterize performance.
Materials and Methods
Participants
Thirty-two young adults (rangeage = 18–29 years, M = 20.97, standard deviation [SD] = 3.11; 23 females, 9 males) participated in the study, and were recruited via advertisements posted at universities in Boston, MA. Participants were all right-handed, native speakers of English and monolingual, had normal or corrected-to-normal vision, and no history of neurological or psychological impairment. None of the participants had completed previous ESI experiments. Participants gave written informed consent and were treated in a manner approved by Harvard University’s ethics committee. Five additional participants completed the study but were excluded from analyses due to excessive movement in the scanner (n = 4) or task noncompliance (n = 1).
Design Overview
Participants completed one within-subjects fMRI session at the imaging center that lasted approximately 4.5 h (Fig. 1). Before entering the scanner, participants received materials explaining the study and completed 3 practice trials of each main task (approximately 0.5 h). During the scan itself, participants completed 2 main segments in the scanner (approximately 2 h). In each segment in the scanner, participants (1) viewed 1 of 2 short videos, completed a short filler task, and then received either the ESI or impressions control induction; (2) viewed 36 object cues after receiving an induction, and for each cue generated alternate uses of the object (i.e., divergent thinking: AUT) or typical associates of the object (i.e., object control: OAT), pressing a button each time they thought of a response during the trial; and (3) completed a resting-state scan. Different but similar stimuli were used in each segment (e.g., video, induction, and cues) and counterbalanced across participants. Following the 2 segments in the scanner, participants completed a postscan interview where they viewed each cue they had seen in the scanner (in the same order) and verbally generated what they had thought about for each one (approximately 2 h). This overall design was derived from Madore et al. (2016b).
Induction Materials and Procedure
To begin each segment in the scanner, participants received 1 of the 2 induction manipulations in the scanner (but were not scanned). They first watched a 2-min video of a man and woman performing activities in a kitchen, followed by a 2-min number judgment filler task. Participants viewed the computer screen showing the video and filler task via a mirror attached to the scanner head coil, and wore scanner-compatible headphones. They then received either the ESI or an impressions control induction about the video they had watched over a loudspeaker and responded out-loud. Participants confirmed that they could hear the questions and responded appropriately. Videos and induction sequences were counterbalanced across participants. The ESI and control inductions were also audio-recorded, lasted approximately 5 min in length, and did not differ in length or overall word count significantly (see Madore et al. 2014 for full induction interview scripts). As a manipulation check, we transcribed and scored participants’ responses about the respective video they had watched prior to each induction using the Autobiographical Interview (AI) scoring protocol (Levine et al. 2002). We found that significantly (P < 0.001) more internal/episodic details were generated following ESI (M = 84.06, SD = 17.86) relative to control induction (M = 7.44, SD = 6.07), indicating that ESI facilitates detailed retrieval of induction content.
The ESI was comprised of a set of questions derived from the Cognitive Interview (CI), a forensic protocol that has been found to reliably boost accurate details of eyewitness memory (Fisher and Geiselman 1992). In our previous work, the ESI has produced consistent increases in the episodic detail of remembered and imagined events as measured by the AI (Levine et al. 2002), as well as key generative measures of divergent creative thinking. For the ESI, participants were told that they were the chief expert about the video they had watched, and were then asked to recall as many details about the setting, people, and actions of the video respectively using 3 mental imagery probes and open-ended follow-up questions focused on content they had generated.
To match the structure of the ESI, the impressions control also involved reflecting back on and verbally reporting content associated with the video in response to a question set. Participants were first asked for their global impressions of the video, and then generated additional opinions about the setting, people, and actions of the video (e.g., adjectives used to describe each category of information, when they thought the video was made, what equipment they thought was used to make it). This induction did not facilitate detailed episodic retrieval and did not include mental imagery probes. In previous work we have sometimes included a math control induction in addition to or instead of the impressions control. The math control involves completing basic math problems after the video and filler task without any verbal questions or reports (Madore et al. 2016b). In the present study, we used the impressions control alone because it is a more stringent baseline than the math control and because the ESI has been found to have indistinguishable effects on key behavioral and fMRI indices compared with either control induction.
fMRI Materials and Procedure
Following each induction, participants completed 4 fMRI runs. For the first 3 runs participants viewed a total of 36 object cues and completed the main tasks (AUT and OAT), and for the last run participants completed a resting-state scan.
Main Tasks
Each main task run was 7 m in duration (beginning and ending with 16 s of fixation), and included random presentation of 6 AUT and 6 OAT trials for 20 s each. Thus, 18 divergent thinking and 18 object control trials were included per segment. Participants pressed a button each time they generated an alternate use or object associate during the respective trial; the number of button presses and the response time of each button press during scanning were collected for subsequent analyses. After each main task trial, participants completed one engagement rating to verify task compliance (as in Beaty et al. 2015) for 4 s followed by a basic odd/even number judgment, which served as a rest period (M = 6 s; jittered at 4, 6, or 8 s). Participants responded during scans using an MR-compatible 5-button response box in their right hand.
To ensure a methodologically sound paradigm, we derived our parameters for main task trial number, length, and idea response mode from AUT fMRI studies (e.g., Fink et al. 2015 for trial number; Sun et al. 2016 for trial length; Abraham et al. 2012 for response mode) and from our previous induction fMRI study (Madore et al. 2016b). Because less personally original ideas on the AUT are sometimes generated before more personally original ones (Gilhooly et al. 2007), we also selected 20 s as a trial length to allow enough time for multiple AUT responses judged as creative to be generated (Sun et al. 2016). A behavioral pilot with a separate batch of 12 young adult participants found induction differences on the AUT using the parameters finalized for the present study, though it should be noted that there is considerable variation in trial number (e.g., 8–23), length (e.g., 12–60 s), and idea response mode (e.g., button presses, verbal report after each trial, no report) within the AUT fMRI literature (see Fink et al. 2009; Benedek et al. 2014; Beaty et al. 2015; Mayseless et al. 2015 for relevant examples).
The 72 cues (i.e., 36 per segment) were common, everyday household objects as drawn from previous fMRI studies on divergent thinking (Beaty et al. 2015). The cues were divided into 4 lists of 18, and did not differ significantly with respect to number of syllables (M = 1.93, SD = 0.83) or words (M = 1.26, SD = 0.44) per cue. As in previous work (Madore et al. 2016b), the order of cue list was counterbalanced across induction sequence and task and randomly assigned to participants. None of the cues were related to the content of the videos.
For the main task trials, the computer screen showed 3 lines of text for 20 s, with the top line stating the task (i.e., uses or objects), the middle line stating a reminder of instructions (i.e., unusual and creative or typical associates), and the bottom line stating a cue in capital letters (i.e., BUCKET). For the AUT (Guilford et al. 1960; Guilford 1967), participants were instructed to generate silently to themselves as many unusual and creative uses for the object cue as they could during the trial’s duration, pressing a button whenever they thought of a new use. We stressed the importance of being both creative and generating as many uses as possible because past research has suggested that type of instruction can impact AUT performance (Nusbaum et al. 2014). For the OAT (Madore et al. 2015, 2016a), participants were instructed to generate silently to themselves as many typical associates of the object cue as they could during the trial’s duration, pressing a button whenever they thought of a related object. Control tasks resembling the OAT have been used in previous fMRI research as a comparison to AUT (Abraham et al. 2012) and episodic simulation performance (Gerlach et al. 2011). Both the AUT and OAT involve assembling, integrating, and generating information in response to an object cue, but the AUT involves divergent thinking and episodic imagery (Madore et al. 2015, 2016a; Addis et al. 2016). After each 20 s-duration, the screen changed and participants were asked to judge in 4 s whether they were able to stay engaged during the preceding trial (i.e., 1 indicating yes and 2 indicating no).
Resting State
After the 3 main task runs in each segment, participants completed the resting-state scan for 7 m, 08 s. The screen was turned off during this scan and participants were instructed to keep their eyes open.
Postscan Interview
Five minutes after the scanning session, participants completed a postscan interview in an adjacent testing room to verify task compliance in the scanner and obtain additional measures of task performance (as in Madore et al. 2016b). Participants viewed each cue they had seen in the scanner trial-by-trial and were asked to report verbally whatever they had thought about in response to each cue in a self-paced manner without probing or input from the experimenter. Participants were also instructed to not include any additional information that they had not thought about in the scanner. Cues appeared in the same order as in the scanner to reduce cognitive load. Previous AUT fMRI research (Abraham et al. 2012; Sun et al. 2016) has suggested that behavioral performance in the scanner correlates positively with output collection in a postscan interview (e.g., number of ideas generated in the scanner correlates positively with number of ideas collected in a postscan interview regarding the scanner cues). Behavioral pilot testing on a separate batch of participants (n = 12) where button presses were provided before later verbal responses about the button presses replicated this pattern.
After reporting the content of their thoughts for each cue, participants completed additional ratings related to their responses. During the postscan interview itself, participants’ responses were audio-recorded and transcribed, which allowed us to later present participants with their ideas for these additional ratings after their verbal reports. There were 3 ratings for each trial that used a 1–5 Likert scale (i.e., 1 indicating not at all up to 5 indicating extremely). For each main task trial, participants rated how difficult it was to generate responses. For each AUT trial, participants additionally rated how similar the generated uses were to previous experiences or thoughts, and how creative (original and novel) the uses were to them. For each OAT trial, participants additionally rated how familiar and how typical (semantically and thematically related to the cue) the generated associates were to them. At the end of the session, participants also viewed and labeled each alternate use that they had generated as an old or new idea, an old idea being a previous memory or thought before the study and a new idea being a new thought that came to mind for the first time during the study (Gilhooly et al. 2007; Benedek et al. 2014; Silvia et al. 2015). Questionnaires regarding individual differences in creativity, personality, imagery, memory, and language were also completed at the end of the session (an analysis of individual differences is not presented here because it goes beyond the aims of the present study and would be severely underpowered in a study designed for group-level analyses).
In addition to obtaining measures from participants in the scanner and the postscan interview, we had 2 independent raters blind to induction and hypothesis later score postscan responses to further characterize performance. Before scoring of the experimental trials commenced, the 2 raters individually completed practice scoring on 20 responses from an independent dataset and obtained high inter-rater reliability (Cronbach’s αs > 0.91 on all measures). For the AUT, various dimensions related to use generation and quality were scored trial-by-trial as in previous work (Guilford et al. 1960; Guilford 1967; Benedek et al. 2014; Madore et al. 2015, 2016a; Addis et al. 2016). Total uses is simply the number of uses generated, excluding repetitions. Appropriate uses is the number of uses generated that are feasible and useful in everyday life (e.g., using a safety pin as a bracelet charm is appropriate but using a safety pin as a laser is not). Over 98% of uses across the sample were deemed appropriate by the raters. Categories of uses is the number of distinct categories that total uses can be classified under (e.g., using a safety pin as a bracelet charm and as an earring would be classified under one category of jewelry). Categories of appropriate uses is the number of distinct categories that appropriate uses can be classified under (e.g., using a safety pin as a laser and as a mini-gun would fall under one category of weaponry but the uses are inappropriate and thus would not be scored). Elaboration is a rating of the level of detail of each use, ranging from 0 to 2. Creativity is a rating of the level of perceived originality and appropriateness of each use, ranging from 1 to 4, with scores of 3 and 4 reserved for only a few uses across the sample. Sometimes an originality index is used in place of a creativity rating, in which points are awarded for each use that is statistically infrequent in the sample (see Guilford 1967). We did not include an originality index in the present study because it is sample-specific and biases could be introduced with this method (e.g., more uses generated following ESI would become more frequent in the sample, and would subsequently not be awarded points, masking an effect of the manipulation). For each OAT trial, the total number of objects associated with the cue was scored trial-by-trial (based on Abraham et al. 2012; Madore et al. 2015, 2016a; e.g., associating a safety pin with a diaper and a shirt would be scored as objects, but sewing would not). Over 98% of responses across the sample were considered objects by the raters. For each of these variables, we averaged the scores across trials to create a standardized index of performance.
fMRI Acquisition, Preprocessing, and Analysis Parameters
Neuroimaging data were acquired on a 3-T Siemens Magnetom Prisma MRI scanner with a 32-channel head coil. Anatomical images were acquired with a T1-weighted magnetization-prepared rapid gradient multiecho sequence (176 sagittal slices; repetition time [TR] = 2530 ms; echo time [TE] = 1.64 ms; flip angle = 7°; 1-mm3 voxels; field of view [FoV] = 256 mm). All BOLD data were acquired with a T2*-weighted multiband echo planar imaging (EPI) sequence that incorporated multiband radiofrequency pulses and simultaneous multislice (SMS) acquisition (Feinberg et al. 2010; Moeller et al. 2010; Xu et al. 2013). For the 6 (3 per segment) BOLD main task runs, the EPI parameters included 84 interleaved axial-oblique slices, TR = 2000 ms, TE = 30 ms, flip angle = 80°, 1.5-mm3 nominal voxels, 6/8 partial Fourier, FoV = 204 mm, SMS = 3. For the 2 (1 per segment) BOLD resting-state runs, the EPI parameters included 64 interleaved axial-oblique slices, TR = 650 ms, TE = 34.80 ms, flip angle = 52°, 2.3-mm3 nominal voxels, 6/8 partial Fourier, FoV = 207 mm, SMS = 8. The acquisition parameters were different for the task and resting-state runs to maximize scanner capabilities.
fMRI Main Task Analyses: Univariate Regression Models
For task runs, neuroimaging data were preprocessed with SPM12 (Wellcome Department of Imaging Neuroscience, London, UK) to minimize scanner noise and artifacts. The first 4 functional images were removed to minimize T1-saturation effects. Preprocessing steps included slice-time correction, realignment, spatial normalization to the Montreal Neurological Institute (MNI) template (without resampling), and spatial smoothing (with a 3-mm full-width half maximum [FWHM] Gaussian kernel). A modest smoothing kernel of 3-mm was applied to preserve the spatial resolution of the functional data.
Univariate analyses of the preprocessed data were conducted with general linear models (GLMs). On an individual participant basis, 2 first-level models were created (i.e., one for each induction, with each induction consisting of 3 fMRI runs). Each model contained 2 events of interest (AUT and OAT). The 20 s duration for each AUT and OAT trial was modeled with a boxcar function convolved with canonical hemodynamic response function yielding regressors. The entire 20 s duration of each trial was modeled because previous creativity fMRI studies have used this approach consistently with similar trial lengths (cf. Fink et al. 2009, 2010, 2012, 2015; Sun et al. 2016). We also modeled the rating phase for each trial with a similar boxcar function (i.e., the 4 s duration for the alternate use rating and object control rating). Six regressors representing movement related variance (3 for rotation and 3 for rigid-body translation) and regressors modeling each fMRI run were also entered into each model. It should be noted that we did not examine BOLD activity associated with each particular button press within each trial because differences emerged for this variable on the alternate uses task as a function of induction, which could skew results in terms of the number of trials modeled per induction. In our previous induction fMRI work (Madore et al. 2016b), we also focused on analyzing separate construct and elaborate phase regressors for each episodic simulation and semantic control trial of 20 s; the task structure of the present study (i.e., generating multiple responses during a 20 s trial rather than just one) was not well suited for a construct-elaborate approach.
To assess across-participant whole-brain differences in activation associated with the AUT, we first computed contrast images for AUT > OAT for each participant using their first-level models (i.e., 2 contrast images [one per induction] for each participant). These first-level contrast images were then entered into random-effects one-sample t tests at the second level. We examined AUT > OAT differences for each induction separately to confirm typical patterns of BOLD activity on divergent thinking after each induction (Wu et al. 2015). For the critical induction analysis, we entered the first-level contrast images from all participants into a random-effects paired t test at the second level (where each pair of images included the respective contrast image following the control induction and contrast image following ESI for each participant separately). This analysis procedure was derived from our previous induction fMRI study (Madore et al. 2016b) and allowed us to test whether ESI differentially affected BOLD activity during divergent thinking.
We also utilized parametric modulation to rule out a key alternative explanation of potential induction-related findings. Because the strength of BOLD activity during fMRI, particularly in the hippocampus and lateral prefrontal cortex (for review, see Simons and Spiers 2003; e.g., see Martin et al. 2011), can be modulated by the amount of information that is generated and potentially encoded during a task, rather than a shift in retrieval orientation, and because the ESI increased the number of responses generated and potentially encoded during the AUT selectively, it was important to rule out this account of the data. To test the possibility, at the first level we modeled the number of button presses exhibited on each alternate use and object control trial separately by including the number of button presses as a trial-by-trial parametric modulator representing the amount of information generated, as well as potentially encoded, per trial. The parametric modulation regressor was modeled orthogonally and linearly, and mean-centered according to SPM algorithms. We then recomputed the contrast of AUT > OAT following the control induction and the contrast of AUT > OAT following ESI at the first level for each participant, which represents task-related activity independent of the variance associated with the number of button presses.
At the second level we re-entered the first-level contrast images for AUT > OAT following each induction into a random-effects paired t test (i.e., the set of contrast images across participants for AUT > OAT following ESI compared with the set of contrast images across participants for AUT > OAT following control). This parametric modulation analysis allowed us to identify greater BOLD activity derived from the AUT > OAT contrast following ESI > control induction after accounting for variance related to the button presses (i.e., assumed to reflect generation, and potential encoding, rather than a shift in retrieval orientation).
fMRI Main Task Analyses: Multivariate Between-Network ICA
Along with measuring mean BOLD activations, we assessed between-network functional connectivity during the main tasks following the inductions using multivariate ICA (Calhoun et al. 2001a, 2001b) as implemented in the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012). That is, we extracted functional brain networks related to AUT and OAT performance following ESI and the control induction by identifying independent spatiotemporal voxel clusters (i.e., components comprising a timecourse and a spatial map) and correlational patterns (i.e., connectivity) between networks (for a related example, see Beaty et al. 2017; for a related proof of principle with autobiographical memory, see Tailby et al. 2017). For preprocessing in the CONN toolbox, we entered our initially preprocessed data from the univariate analyses including parameters associated with grey matter, white matter, cerebrospinal fluid, and movement. We then used separate computational algorithms in the GIFT program (Calhoun et al. 2001a, 2001b) to determine the number of independent components that should be extracted for our main task BOLD data, which has been recommended for ICA with task-based fMRI (Calhoun et al. 2001a, 2001b). We entered the appropriate values from GIFT into the CONN toolbox for the number of components to be extracted (in this case, 26) and dimensionality reduction (64). Two raters blind to induction visually inspected the spatial patterns and timecourses of the 26 extracted components independently (see Kelly et al. 2010) and determined that 24 of the 26 components were nonartifactual (inter-rater agreement was 100% on which components were nonartifact and artifact). We then quantified between-network connectivity by treating each group-level spatial map from the 24 nonartifactual components as separate ROIs, and computing ROI-to-ROI correlations of timecourses within participants. Induction-related differences in connectivity among components were assessed by performing an interaction test on Fisher’s z-transformed correlations.
fMRI Resting-State Analyses: Univariate Seed-to-Voxel Connectivity
The raw data from the resting-state scans were preprocessed using SPM12 as in the task analyses. The first 4 time points of each resting-state run were removed to maximize stability of the BOLD signal. Realignment, spatial normalization to the MNI template, and resampling at 2-mm3 were also performed. Functional-connectivity specific preprocessing steps for resting data were then implemented with FSL 4.1.7 (FMRIB) and SPM12 (Van Dijk et al. 2010). Data from each run were concatenated and spatially smoothed with a 4-mm FWHM kernel and temporally filtered (low-pass) to retain frequencies below 0.08 Hz. Partial regression was used to create a series of nuisance regressors reflecting spurious noise and systematic variance of non-neural sources. The regressors included 6 motion parameters and their derivatives, the averaged signal within cerebrospinal fluid, an ROI within deep white matter, and an ROI comprising the whole brain (i.e., global signal regression; see Murphy and Fox 2017). We also included each regressor’s first temporal derivative to correct for potential temporal shifts in BOLD signal.
A seed (i.e., ROI) to voxel analysis (Van Dijk et al. 2010) was then carried out to test for induction-related effects during the resting-state scans. A 6-mm sphere in the left anterior hippocampus centered on the peak voxel from our task analysis (xyz −32, −14, −20) was selected as the seed, given the results from our main task analyses and a priori hypotheses. Whole-brain correlation images (one per participant) were created by using the averaged time series across all voxels comprising the seed and the time series corresponding to each voxel across the brain with Pearson’s product moment correlation. Statistical analyses of the correlation data were conducted on approximately normally distributed Fisher z-transformations. SPM12 was used to compare the strength of seed-to-voxel connectivity following ESI and following the control induction using a random-effects paired t test at the second level (i.e., ESI > control Induction and vice versa). This analysis procedure was derived from our previous induction fMRI study (Madore et al. 2016b).
Significance Testing
For the univariate main task and resting-state analyses, we used cluster-extent thresholding. We ran Monte Carlo simulations with 10 000 iterations with estimated spatial autocorrelation values of 7.06–7.47 to yield parameters with a significance threshold of P < 0.05 corrected for multiple voxel-wise comparisons (Slotnick et al. 2003; see Thakral et al. 2017 for a recent example of this approach). For the whole-brain main task analyses, the parameters of P < 0.001, uncorrected with an extent threshold of 17 contiguously activated voxels (1.5-mm3) were applied. For the seed-to-voxel resting-state analysis, the parameters of P < 0.001, uncorrected with an extent threshold of 16 contiguously activated voxels (2-mm3) were applied. To minimize false positives that can occur with cluster-extent thresholding in fMRI analyses (see Eklund et al. 2016; but also see Cox et al. 2017), we adopted a computational method that does not employ a random-field theory approach, derived our spatial autocorrelation values from the respective group residual mean-square images, and used a conservative cluster defining threshold (i.e., P < 0.001 vs. P < 0.01).
For the assessment of between-network coupling, we used FDR thresholding as implemented in the CONN toolbox. Multiple comparisons across internetwork correlations were accounted for with the significance threshold of FDR-corrected P < 0.05.
Visualizations and localizations across fMRI analyses were completed via MRIcron (Rorden et al. 2007) and xjview (www.alivelearn.net/xjview, date last accessed 2 June 2017). All materials and data from the present study are available upon request.
Results
Behavioral Analyses: Scanner Metrics
To verify task compliance for the AUT and OAT during scanning and examine induction effects, we first ran a series of repeated-measures analyses of variance (ANOVAs) on the in-scanner behavioral data as a function of task and induction (see Table 1 for descriptive statistics). Participants rated themselves as engaged on task for at least 17 out of 18 trials per task per induction during scanning (Ps > 0.12). Participants also generated their first response early in the 20 s trial window (e.g., 3–4 s) and their last response late in the trial window (e.g., 15–16 s); first and last reaction times did not vary significantly as a function of induction (Ps > 0.21). The first OAT response was generated significantly earlier than the first AUT response and the last OAT response was generated significantly later than the last AUT response (Ps < 0.009) by a magnitude of 1 s or less per trial. Participants also produced multiple responses per task per induction; a significantly greater number of such responses was produced for the OAT relative to the AUT (P < 0.001), as in previous work using related control tasks (Fink et al. 2009; Abraham et al. 2012; Madore et al. 2015, 2016a). These results confirm that participants were performing the tasks adequately in the scanner.
Table 1.
Descriptive statistics for scanning behavioral data. Numeric values are presented as mean per trial (with standard deviation in parentheses)
| Scanner metrics | Control induction | Specificity induction |
|---|---|---|
| AUT responses/button presses | 4.18 (1.44) | 4.44 (1.60) |
| OAT responses/button presses | 7.44 (2.54) | 7.31 (2.45) |
| AUT total trials (out of 18) | 15.16 (2.82) | 15.03 (3.17) |
| OAT total trials (out of 18) | 15.22 (3.16) | 15.13 (2.52) |
| AUT first reaction time | 4.22 s (1.16) | 4.18 s (1.23) |
| OAT first reaction time | 3.21 s (0.77) | 3.07 s (0.76) |
| AUT last reaction time | 15.67 s (1.96) | 15.85 s (1.96) |
| OAT last reaction time | 16.47 s (1.59) | 16.22 s (1.92) |
Critically, as hypothesized, participants generated significantly more responses on the AUT following ESI relative to the control induction in the scanner, and did not differ in the number of responses generated on the OAT as a function of induction (induction × task interaction: F[1, 31] = 5.24, P = 0.029, ηp2 = 0.15), as confirmed by pairwise comparisons (induction effect on AUT: P = 0.028, ηp2 = 0.15; induction effect on OAT: P = 0.37, ηp2 = 0.03). This pattern of induction-related findings replicates and extends behavioral studies of divergent thinking (Madore et al. 2015, 2016a), and indicates for the first time that the ESI manipulation selectively affected divergent thinking performance in an fMRI setting but did not affect performance on an object control task.
Behavioral Analyses: Postscan Metrics
To confirm task compliance and further characterize ESI effects on task responses generated during scanning, we ran another series of repeated-measures ANOVAs on the behavioral postscan data as a function of task and induction (see Table 2 for descriptive statistics). Phenomenological ratings confirmed that participants completed the tasks in the scanner as expected, and these ratings did not vary as a function of induction (Ps > 0.15). Specifically, participants found generating AUT responses in the scanner to be somewhat difficult and OAT responses a little difficult; as in previous related work (Abraham et al. 2012), the AUT was rated as significantly more difficult than a related control task (P < 0.001). Because there were differences in task difficulty on the AUT and OAT, we covaried out this rating (with difference score, average rating, and trial-wise rating indices where appropriate) in all induction-related main task behavioral and neural analyses and obtained the same results. These supplementary analyses rule out differences in task difficulty as a plausible explanation for induction-related findings. Average ratings also indicated that participants judged their generated uses as dissimilar to previous experiences and thoughts, and creative. In addition, they judged their generated objects from the OAT to be very familiar, and very typical.
Table 2.
Descriptive statistics for postscan behavioral data. Numeric values are presented as mean per trial (with standard deviation in parentheses)
| Postscan ratings (1–5, higher = more) | Control induction | Specificity induction |
|---|---|---|
| AUT difficulty | 3.43 (0.60) | 3.30 (0.68) |
| OAT difficulty | 1.70 (0.52) | 1.76 (0.45) |
| AUT similarity | 2.46 (0.81) | 2.45 (0.73) |
| AUT creativity | 2.89 (0.67) | 2.98 (0.72) |
| OAT familiarity | 4.35 (0.51) | 4.30 (0.55) |
| OAT typicality | 4.28 (0.49) | 4.28 (0.48) |
| Postscan generative metrics | Control induction | Specificity induction |
| AUT total uses | 2.25 (0.84) | 2.54 (0.94) |
| OAT total objects | 4.48 (1.17) | 4.31 (1.29) |
| AUT old ideas | 1.16 (0.70) | 1.23 (0.63) |
| AUT new ideas | 1.09 (0.54) | 1.31 (0.74) |
| AUT appropriate uses | 2.21 (0.74) | 2.54 (0.94) |
| AUT categories of uses | 2.09 (0.77) | 2.25 (0.70) |
| AUT categories of appropriate uses | 2.06 (0.66) | 2.24 (0.70) |
| AUT elaboration (0–2; higher = more detailed) | 0.60 (0.36) | 0.60 (0.34) |
| AUT creativity (1–4, higher = more original and appropriate) | 2.37 (0.19) | 2.36 (0.13) |
Focusing on the postscan verbal reports, participants labeled their total uses (excluding repetitions) on the AUT as consisting of both old and new ideas (e.g., approximately 50% of each idea type). Raters blind to induction and hypothesis (Cronbach’s αs > 0.91 on all measures) scored total uses generated by participants on the AUT for various dimensions (see Guilford et al. 1960 and Addis et al. 2016, for related examples; also see Materials and Methods). Total uses were scored as “brief” to “somewhat detailed” and “somewhat creative”; these average elaboration and creativity ratings for total uses did not vary significantly as a function of induction (Ps > 0.76), and were similar when other use dimensions were analyzed, consistent with prior work (Madore et al. 2015, 2016a). Correlations between total output in the postscan interview for the AUT and OAT and total button presses in the scanner were also significantly positive (r = 0.67, P < 0.001 for AUT and r = 0.63, P < 0.001 for OAT). These correlations did not vary significantly as a function of induction (Fisher r-to-z transformation tests, Ps > 0.26). Relatedly, there was significantly less output in the postscan interview for the AUT and OAT than button presses in the scanner (P < 0.001), as in previous related fMRI work (Abraham et al. 2012), and this did not vary significantly as a function of induction (P = 0.68). These aspects of the data suggest that participants performed the main tasks in the scanner adequately and reported relevant information in the postscan interview about their task performance in the scanner.
Critically, with respect to induction effects and as hypothesized, the output generated by participants in the postscan interview exhibited a similar significant pattern to that of button presses in the scanner following ESI compared with the control. In response to the cues they had seen in the scanner previously, participants generated significantly more total uses that were also deemed appropriate and as fitting into distinct categories for those AUT cues that had come after ESI compared with the control but did not show significant induction-related differences in total objects generated for OAT cues (induction × task interactions: Fs[1, 31] > 5.91, Ps < 0.021, ηp2s > 0.16), as confirmed by pairwise comparisons (induction effect on AUT: Ps < 0.015, ηp2s > 0.18; induction effect on OAT: P = 0.19, ηp2 = 0.06). We also found that both old and new idea production, as indicated by participant labels of their total uses on divergent thinking, were boosted significantly following ESI relative to the control (P < 0.001, ηp2 = 0.32). This pattern of findings replicates and extends previous behavioral evidence (Madore et al. 2015, 2016a) where ESI effects on these measures of alternate use—but not object association—performance were exhibited in terms of total and appropriate uses that come from distinct categories, and from old and new ideas, to an fMRI setting.
fMRI Analyses: Overview
All reported results are derived from statistical parameters that survive a significance threshold of P < 0.05 corrected for multiple voxel-wise comparisons or multiple component comparisons, respectively. See Materials and Methods for full details on analyses conducted and significance testing. The results are collapsed across the variable of induction order (i.e., specificity followed by control versus control followed by specificity) because it did not affect behavioral or neural outcomes significantly.
fMRI Main Task Analyses: Univariate Regression Models
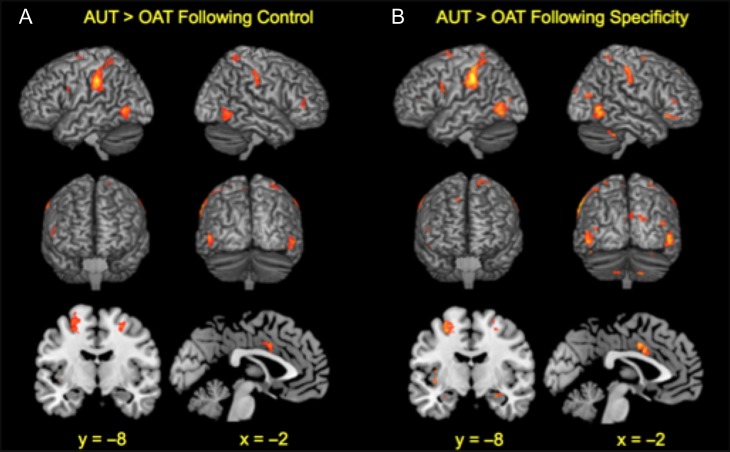
Fifteen trials per task per induction were analyzed for the task fMRI runs, after excluding trials where participants indicated they were off-task in the scanner or could not generate their scanner responses in the postscan interview. To initially characterize main task performance across the whole brain, the AUT > OAT contrast following each induction was analyzed separately. Participants exhibited distributed and expected patterns of significantly greater BOLD activity for the AUT > OAT contrast following each induction in posterior parietal cortex (e.g., inferior parietal lobule and precuneus), lateral prefrontal cortex (e.g., superior, middle, and inferior frontal gyri), temporal cortex (e.g., middle temporal and fusiform gyri), dorsal anterior cingulate cortex, precentral and postcentral gyri, and insula, among other regions (see Fig. 2 and Table 3). In addition, significantly greater BOLD activity was observed in medial temporal lobe (i.e., hippocampus and amygdala) for the AUT > OAT contrast following ESI (and the control induction, subthreshold). These activity patterns are in line with those reported by Wu et al. (2015) in their neural meta-analysis of divergent thinking, and show overlap with the core network evident during episodic memory and simulation (Benoit and Schacter 2015).
Figure 2.
fMRI main task results following each induction. Mean BOLD activation from univariate regressions exhibited for the Alternate Uses Task (AUT) > Object Association Task (OAT) following (A) the control induction and (B) the episodic specificity induction with statistical parameters that survive a significance threshold of P < 0.05 corrected for multiple voxel-wise comparisons. Results are projected onto surface and slice templates from MRIcron (Rorden et al. 2007).
Table 3.
MNI coordinates of peak activation in fMRI main task analyses following each induction. Provided at a statistical threshold of P < 0.05 after correcting for multiple voxel-wise comparisons.
| Brain region | x | y | z | Z-score |
|---|---|---|---|---|
| Alternate uses > object association following control induction | ||||
| L inferior parietal lobule | −48 | −31 | 36 | 5.79 |
| L posterior middle temporal gyrus | −51 | −62 | −2 | 5.20 |
| R cerebellum | 22 | −68 | −24 | 4.81 |
| R posterior middle temporal gyrus | 58 | −58 | −6 | 4.71 |
| L insula | −38 | −12 | −2 | 4.60 |
| L dorsal anterior cingulate cortex | 0 | 5 | 36 | 4.56 |
| R postcentral gyrus | 60 | −24 | 42 | 4.50 |
| L superior parietal lobule | −26 | −44 | 64 | 4.46 |
| R superior parietal lobule | 30 | −54 | 70 | 4.36 |
| R precentral gyrus | 27 | −7 | 56 | 4.29 |
| L middle frontal gyrus | −50 | 5 | 24 | 4.29 |
| L superior frontal gyrus | −26 | −10 | 62 | 4.27 |
| R inferior parietal lobule | 32 | −38 | 44 | 4.26 |
| L superior frontal gyrus | −20 | −1 | 70 | 4.25 |
| L inferior parietal lobule | −44 | −38 | 53 | 4.18 |
| L inferior frontal gyrus | −26 | 32 | −16 | 4.17 |
| L precuneus | −6 | −56 | 62 | 4.11 |
| R inferior frontal gyrus | 57 | 38 | 4 | 3.98 |
| L precuneus | −8 | −49 | 62 | 3.90 |
| R cerebellum | 22 | −67 | −46 | 3.85 |
| L superior parietal lobule | −34 | −48 | 64 | 3.83 |
| R superior parietal lobule | 24 | −48 | 68 | 3.79 |
| L fusiform gyrus | −46 | −40 | −18 | 3.64 |
| Alternate uses > object association following specificity induction | ||||
| L inferior parietal lobule | −58 | −31 | 42 | 5.96 |
| L dorsal anterior cingulate cortex | 0 | 2 | 38 | 5.46 |
| R posterior middle temporal gyrus | 56 | −66 | −1 | 5.25 |
| R cerebellum | 30 | −74 | −24 | 5.20 |
| L middle occipital gyrus | −52 | −67 | −1 | 5.08 |
| L superior frontal gyrus | −22 | −2 | 68 | 5.00 |
| R postcentral gyrus | 46 | −26 | 42 | 4.98 |
| R inferior frontal gyrus | 46 | 26 | −10 | 4.66 |
| R postcentral gyrus | 33 | −34 | 41 | 4.66 |
| L insula | −39 | −12 | 0 | 4.63 |
| R cerebellum | 46 | −43 | −36 | 4.56 |
| R middle occipital gyrus | 46 | −79 | 17 | 4.48 |
| L insula | −39 | −7 | 10 | 4.47 |
| R superior parietal lobule | 32 | −49 | 64 | 4.44 |
| R superior frontal gyrus | 26 | −6 | 66 | 4.43 |
| L cerebellum | −18 | −73 | −22 | 4.40 |
| R cerebellum | 15 | −74 | −49 | 4.40 |
| L inferior frontal gyrus | −33 | 38 | −13 | 4.38 |
| L precentral gyrus | −44 | 2 | 22 | 4.34 |
| R calcarine | 9 | −78 | 2 | 4.33 |
| L fusiform gyrus | −48 | −49 | −20 | 4.32 |
| L amygdala | −26 | −2 | −25 | 4.31 |
| R fusiform gyrus | 48 | −37 | −20 | 4.30 |
| R superior frontal gyrus | 14 | 42 | 47 | 4.20 |
| R anterior hippocampus | 32 | −12 | −14 | 4.20 |
| L cerebellum | −12 | −78 | −48 | 4.20 |
| R cuneus | 18 | −88 | 23 | 4.15 |
| L postcentral gyrus | −28 | −44 | 70 | 4.05 |
| L superior frontal gyrus | −15 | 6 | 68 | 4.02 |
| L inferior frontal gyrus | −46 | 34 | 11 | 3.96 |
| L middle occipital gyrus | −44 | −80 | 10 | 3.94 |
| R lingual gyrus | 12 | −74 | −4 | 3.92 |
| R amygdala | 33 | −2 | −18 | 3.88 |
| L precuneus | −9 | −55 | 65 | 3.81 |
| R cuneus | 6 | −84 | 30 | 3.73 |
| R inferior frontal gyrus | 52 | 38 | 6 | 3.50 |
L, left and R, right.
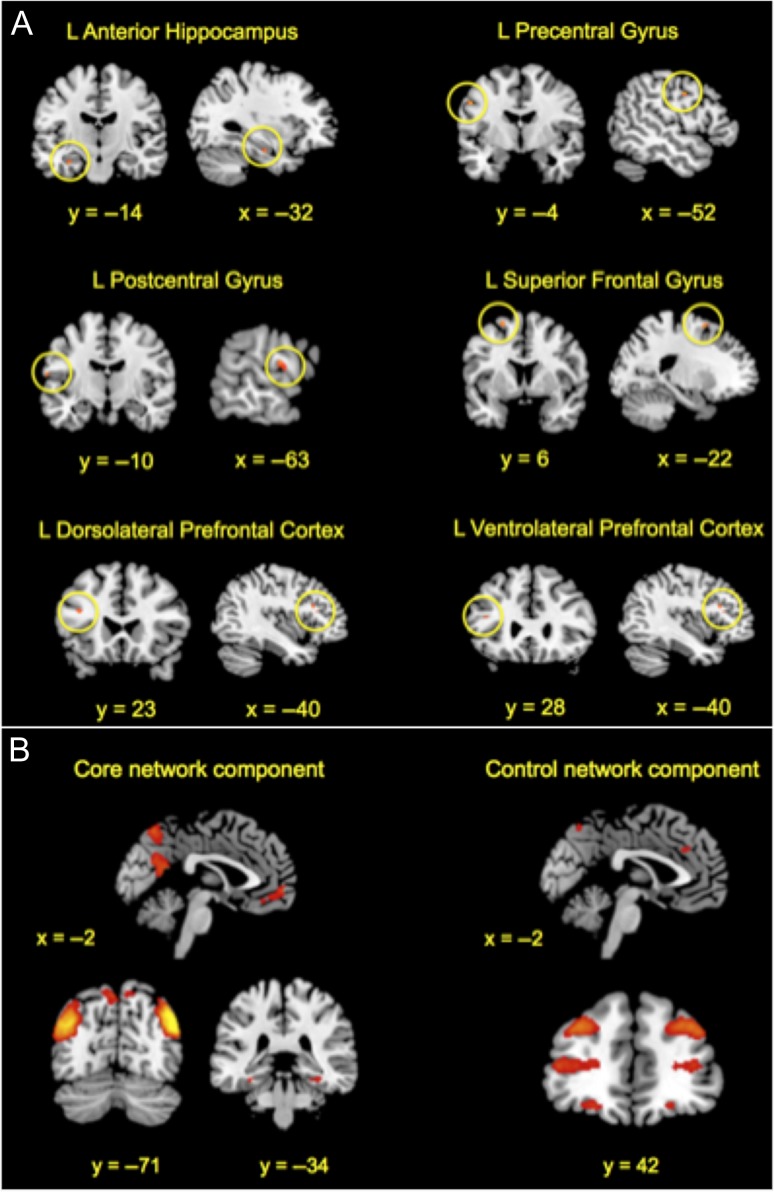
Critically, to formally test for ESI-related effects on divergent thinking performance across the whole brain for the first time, the AUT > OAT contrast for the ESI > control induction was analyzed (i.e., the interaction contrast). Task effects differed significantly as a function of induction, in that participants exhibited significantly greater BOLD activity in left anterior hippocampus, left inferior (i.e., ventrolateral), middle (i.e., dorsolateral), and superior frontal gyri, and left precentral and postcentral gyri for the AUT > OAT contrast following ESI relative to the control induction (see Fig. 3 and Table 4). No patterns of significant activation emerged for the other induction-related contrasts. The left anterior hippocampal finding replicates and extends our previous ESI-related fMRI work on episodic simulation (xyz −34, −16, −12 in Madore et al. 2016b) to a second task that involves episodic processes, divergent thinking (xyz −32, −14, −20).
Figure 3.
fMRI main task results as a function of induction. BOLD activation exhibited for the Alternate Uses Task (AUT) > Object Association Task (OAT) for the Specificity Induction > Control Induction in terms of (A) mean activation from univariate regressions and (B) between-network functional connectivity from multivariate ICA with statistical parameters that survive a significance threshold of P < 0.05 corrected for multiple voxel-wise comparisons or multiple component comparisons, respectively. Results are projected onto slice templates from MRIcron (Rorden et al. 2007). L, left and R, right.
Table 4.
MNI coordinates of peak activation in fMRI main task analyses as a function of induction. Provided at a statistical threshold of P < 0.05 after correcting for multiple voxel-wise comparisons L, left and R, right; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex.
| Brain region | x | y | z | Z-score |
|---|---|---|---|---|
| Alternate uses > object association for specificity induction > control induction | ||||
| L precentral gyrus | −52 | −4 | 38 | 4.64 |
| L superior frontal gyrus | −22 | 6 | 64 | 4.46 |
| L middle frontal gyrus (DLPFC) | −40 | 23 | 23 | 4.19 |
| L anterior hippocampus | −32 | −14 | −20 | 4.19 |
| L postcentral gyrus | −63 | −10 | 16 | 4.08 |
| L inferior frontal gyrus (VLPFC) | −40 | 28 | 17 | 3.49 |
| Alternate uses > object association for specificity induction > control induction in parametric modulation analysis (to account indirectly for generation/encoding) | ||||
| R fusiform gyrus | 39 | −44 | −19 | 4.70 |
| R inferior occipital gyrus | 42 | −70 | −8 | 4.55 |
| L precentral gyrus | −52 | −4 | 38 | 4.36 |
| L superior frontal gyrus | −22 | 6 | 64 | 4.35 |
| L middle frontal gyrus (DLPFC) | −40 | 23 | 23 | 4.30 |
| L anterior hippocampus | −32 | −13 | −20 | 4.15 |
| R middle frontal gyrus (DLPFC) | 51 | 18 | 32 | 3.90 |
| R superior frontal gyrus | 38 | 4 | 60 | 3.85 |
| L inferior frontal gyrus (VLPFC) | −40 | 28 | 17 | 3.79 |
While the induction-related results indicate a role for episodic retrieval in divergent thinking that is consistent with our claim noted earlier that ESI impacts performance by influencing participants’ retrieval orientation (Schacter and Madore 2016), we also re-examined the AUT > OAT contrast for the ESI > control induction to assess indirectly an alternative explanation of these neural effects. Because more button presses were exhibited during divergent thinking following ESI relative to the control, it was important to address the possibility that ESI-related fMRI effects were attributable to the amount of information that was generated and potentially encoded during scanning, rather than to a change in retrieval orientation (for review, see Simons and Spiers 2003; e.g., see Martin et al. 2011). In brief, we conducted the second-level interaction analysis described above but included a parametric modulation regressor in the first-level models: the number of button presses, which served as an indirect measure of the amount of information that was generated and thus potentially encoded during each task block. In doing so, the task interaction was independent of the effect of button presses. When we applied this procedure, we again found patterns of significantly greater BOLD activity in the same clusters in left anterior hippocampus, left inferior, middle, and superior frontal gyri, and left precentral gyrus following ESI relative to the control during divergent thinking compared with the object control. Additional patterns of significantly greater BOLD activation were also observed in clusters in right fusiform gyrus, right inferior occipital gyrus, and right middle and superior frontal gyrus for the AUT > OAT contrast for the ESI > control induction (Table 4). No patterns of significant activation emerged for the other induction-related contrasts when the parametric modulation analysis was applied. These results, though indirect, indicate that the main ESI-related BOLD findings cannot be fully explained by increased generation and encoding of alternate uses following ESI versus the control induction.
fMRI Main Task Analyses: Multivariate ICA and Between-Network Coupling
Having established effects of ESI on particular activation patterns across the whole brain, we next used multivariate spatiotemporal ICA to determine functional connectivity during the main tasks as a function of induction (i.e., via an interaction contrast with 24 nonartifactual extracted components) and coupling between these networks for the first time. An independent component where the spatiotemporal map represented the core network (including bilateral parahippocampal gyrus, angular gyrus, medial prefrontal cortex, precuneus, and retrosplenial cortex/posterior cingulate; Benoit and Schacter 2015), and an independent component where the spatiotemporal map represented the frontoparietal control network (including bilateral lateral prefrontal cortex, anterior cingulate, and precuneus; Vincent et al. 2008), exhibited significantly stronger between-network coupling during the AUT > OAT following ESI > control induction (Fig. 3). None of the other 22 nonartifactual components exhibited differential coupling as a function of induction. These results fit with recent accounts of functional network interactions during divergent thinking (Beaty et al. 2016; Roberts and Addis 2017) and related tasks that involve core network coupling (Schacter et al. 2012, 2017; Moscovitch et al. 2016; Roberts et al. 2017).
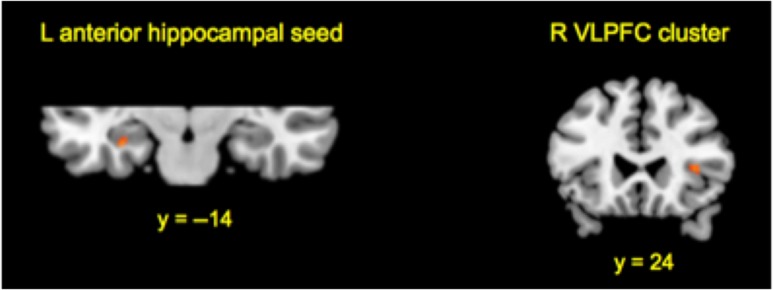
fMRI Resting-State Analyses: Univariate Seed-to-Voxel Connectivity
To examine short-term, functional reorganization following ESI that persisted into rest, we examined functional connectivity during the resting-state scans. We examined seed-to-voxel functional connectivity (i.e., ROI-to-whole brain) by using the left anterior hippocampal region that emerged from the main task univariate analysis as the seed (a 6-mm sphere around the peak coordinate xyz −32, −14, −20) and measuring its connectivity strength with the rest of the brain (as in Madore et al. 2016b). Significantly stronger functional connectivity was exhibited between the left anterior hippocampal seed and right inferior frontal gyrus (i.e., ventrolateral prefrontal cortex bordering on anterior insula; xyz 36, 24, 6) following ESI relative to the control induction (Fig. 4). No other significant patterns of connectivity emerged.
Figure 4.
fMRI resting-state results as a function of induction. Neural connectivity profiles exhibited for the specificity induction > control induction using a univariate seed-to-voxel approach with statistical parameters that survive a significance threshold of P < 0.05 corrected for multiple voxel-wise comparisons. Results are projected onto slice templates from MRICron (Rorden et al. 2007). L, left and R, right; VLPFC, ventrolateral prefrontal cortex.
Discussion
The contribution of episodic memory to divergent thinking is an important but poorly understood issue in the cognitive neuroscience of creativity. Using fMRI together with an experimental manipulation of episodic specificity, the results of the present study provide the first formal evidence that episodic retrieval can serve as a component process of divergent thinking, both neurally and behaviorally. Following both ESI and control inductions, participants exhibited significantly greater BOLD activity in frontoparietal cognitive control and dorsal and ventral attention brain networks consistently implicated in divergent thinking relative to various control conditions (Wu et al. 2015), as well as brain regions consistently implicated in semantic cognition (Noonan et al. 2013; Davey et al. 2015a, 2015b, 2016), including lateral prefrontal, anterior cingulate, and posterior parietal and temporal cortices. Critically, and as hypothesized, following ESI relative to the control induction, participants exhibited significantly greater BOLD activity in the hippocampus during divergent thinking but not an object association control task that involves semantic imagery and little divergent thinking, and significantly stronger between-network functional connectivity of components extracted from ICA that corresponded to the core network and frontoparietal control network respectively. The neural effects of ESI extended into rest, where significantly greater coupling was observed between the hippocampus and lateral prefrontal cortex using univariate seed-to-voxel connectivity. Similar induction effects were evident in measures of behavioral performance. Participants made more responses during divergent thinking following ESI relative to the control induction in the scanner, with no differences during the OAT as a function of induction. The postscan interview revealed that ESI increased the number of uses on divergent thinking that were appropriate, fit under distinct categories, and were labeled by participants as old ideas from memory and new ideas from imagination. That is, an episodic retrieval manipulation increased not only the retrieval of old ideas but the production of new ideas that were not associated with any one particular past experience, as in our previous induction-related behavioral work (Madore et al. 2015, 2016a). We ruled out differences in task difficulty as an alternative explanation for induction-related effects.
These behavioral and neural results together present novel evidence that episodic memory is involved in divergent thinking because they show selective and simultaneous behavioral and neural effects of an episodic retrieval manipulation on a subsequent task that involves divergent thinking, but not on a subsequent task that involves little divergent thinking or episodic imagery. The results of the present study thus highlight the need for conceptual frameworks in the cognitive neuroscience of creativity to accommodate the idea that episodic retrieval can serve as a component process of creative output during divergent thinking, along with more frequently studied elements such as semantic memory, top-down and bottom-up attention, and cognitive control (see Dietrich and Kanso 2010 for review). Our findings derived from the combination of a behavioral manipulation and neuroimaging with univariate and multivariate analytic techniques indicate, in a formal test for the first time, that episodic memory contributes to novel idea generation during divergent thinking, rather than being irrelevant to, or a hindrance to, creative output.
Episodic Retrieval Supports Divergent Thinking in the Brain
How can episodic memory, a neurocognitive system classically characterized as supporting the retrieval of past experiences, likewise support divergent thinking? Recent conceptual work has suggested that engagement of the hippocampus (and core network more broadly) is exhibited during generative tasks that nominally involve the retrieval and reconstruction of episodic details for completion, such as simulation, decision making, and problem solving (for review, see Schacter et al. 2012; Moscovitch et al. 2016; Schacter et al. 2017; Roberts and Addis 2017). Relatedly, we have previously hypothesized (Schacter and Madore 2016) that an experimental manipulation that facilitates detailed episodic retrieval and biases a specific retrieval orientation (Morcom and Rugg 2012) should likewise facilitate performance on subsequent tasks that nominally involve this sort of episodic processing for completion, in this case divergent thinking. From this perspective, idea generation during divergent thinking is attributable, at least in part, to the retrieval and reconstruction of specific episodic details supported by activity in the hippocampus (and core network more broadly). The fMRI results of the present study fit with this retrieval orientation interpretation. Greater BOLD activity was observed in the left anterior hippocampus during divergent thinking selectively following ESI relative to control induction. This finding replicates and extends our previous fMRI results on ESI and future event simulation (Madore et al. 2016b) to divergent thinking, and lends support to current conceptual frameworks and data patterns focused on the role of the hippocampus in flexible cognition (Rubin et al. 2014), associative and relational processing (Roberts et al. 2017), spontaneous and internally directed thought (Smallwood, et al. 2012; Andrews-Hanna et al. 2014), event construction (Romero and Moscovitch 2012) and event models (Radvansky and Zacks 2014; Richmond and Zacks 2017).
We have recently suggested (Schacter and Madore 2016; Madore et al. 2016b) that contributions of episodic memory to a variety of tasks beyond simple remembering, such as imagining the future, solving means-end problems, and thinking creatively, may be explained by an event construction account in particular, and we think that the patterns of data from the current study add novel support for this interpretation by combining simultaneous behavioral and neural effects of ESI in a single formal study of divergent thinking. Event construction, the assembly of an event bound in space and time with details related to settings, people, and actions, may be facilitated by a retrieval orientation that focuses on assembling an event with these kinds of specific details. This point also fits under the theoretical framework of an event model (Radvansky and Zacks 2014; Richmond and Zacks 2017), which is constructed at least in part of elements of previous episodic memories that are situated in a specific place and time related to physical entities and action sequences. Ideas may be generated on divergent thinking by invoking the construction of a small-scale event or a series of small-scale events retrieved and reconstructed from elements of prior episodic experiences. The ESI manipulation, by instructing participants to generate specific elements of a prior episode or construct an event, may support subsequent idea generation at the point where the retrieval of prior episodic elements is invoked to construct an event and complete a task with this sort of nominal requirement.
Amount of Generated/Encoded Information Cannot Fully Explain Key Neural Effects of Divergent Thinking
Following ESI, we also observed selectively increased engagement of lateral prefrontal regions typically implicated in cognitive control (Vincent et al. 2008), including dorsolateral and ventrolateral cortices (Race et al. 2009), during divergent thinking. Because ESI increased the number of responses during divergent thinking in the scanner and because lateral prefrontal and hippocampal engagement during fMRI can be modulated by the amount of information that is generated and potentially encoded during a task (for review, see Simons and Spiers 2003), it is possible that increases in the amount of generated/encoded information, rather than a shift in retrieval orientation, accounts for the observed effects of ESI. To address this issue, we ran a parametric modulation analysis and tentatively excluded this alternative explanation of the data. This is an important point, because previous neuroimaging studies of divergent thinking have not verified or ruled out the possibility that lateral prefrontal and hippocampal activation are attributable at least in part to successful encoding during task performance, along with generation as hypothesized. The induction-related patterns of hippocampal and lateral prefrontal coactivation, then, point more to the influence of episodic retrieval and executive control processes during divergent thinking.
Core and Control Brain Networks Flexibly Couple During Divergent Thinking
Using multivariate ICA, we also found evidence of stronger between-network functional coupling of a component comprising core network nodes (Benoit and Schacter 2015), including bilateral parahippocampal gyrus, angular gyrus, medial prefrontal cortex, precuneus, and retrosplenial/posterior cingulate cortex, and a component comprising frontoparietal control network nodes (Vincent et al. 2008), including bilateral lateral prefrontal cortex, anterior cingulate cortex, and precuneus, during divergent thinking selectively following ESI relative to a control induction. The core network component included medial temporal lobe subsystem regions typically implicated in detailed event and scene processing, such as the parahippocampal gyrus, angular gyrus, and retrosplenial cortex, as well as medial prefrontal subsystem regions and a midline core thought to be involved in crosstalk between the 2 subsystems (Andrews-Hanna et al. 2010). It should be noted that the core network component excluded the hippocampus, which may have resulted from this region exhibiting a different temporal profile of task-related activation from other core network regions (for related evidence, see Roberts et al. 2017). Nevertheless, this overarching data pattern fits with emerging frameworks that emphasize the role of the core network and its flexible coupling with other networks in the service of goal-directed tasks that comprise multiple component processes, including divergent thinking (Beaty et al. 2016; Roberts and Addis 2017; for related discussion of core and frontoparietal coupling, see Ellamil et al. 2012; Beaty et al. 2015, 2017; Liu et al. 2015; Mayseless et al. 2015). We also found evidence that functional coupling extended into a rest period (with a hippocampal seed on the one hand and a cluster of voxels in lateral prefrontal cortex on the other), which suggests that there is short-term, functional reorganization of core and control networks following ESI.
Limitations and Future Directions
There are a few caveats and future directions of the present study that should be noted, some of which are specific to the design of our study and some of which are more broadly related to investigations involving the cognitive neuroscience of creativity and related forms of cognition. In terms of study-specific points, we are cautious in interpreting too heavily the resting-state results because it is unclear whether it is the ESI itself or the after effects of the ESI manipulation during the main tasks that drives the later resting-state findings. Our findings speak to a retrieval orientation account of the data (i.e., a specific retrieval orientation is invoked via ESI and persists through the main tasks and resting-state scan), but clearer results would be possible if resting-state scans were acquired directly before and directly after ESI. Second, because ESI is currently presented as a verbal interview between research assistant and participant, it was not possible to scan participants during the induction phase of the experiment (due to reduced sound quality and increased head motion impacting data collection and analyses). While our behavioral manipulation check indicated that episodic retrieval is targeted by ESI (see Materials and Methods), in the sense that more internal/episodic details (as scored using the AI protocol, Levine et al. 2002) were generated following ESI relative to the control, future work could pinpoint mechanisms underlying the induction manipulation’s effect with more precision by scanning the induction phase and examining neural effects. Adopting a self-administered version of the CI (Gabbert et al. 2009), which ESI is based on, may assist in this effort. Third, while the present study concerns divergent thinking, it will be valuable for future work to assess whether core network involvement extends to convergent thinking, the ability to come up with one correct solution to a given problem (for related evidence with hippocampal amnesic patients, see Warren et al. 2016), as well as whether seemingly left-lateralized, induction-related effects in our study, as also observed to some extent in the Wu et al. (2015) meta-analysis, can further our understanding of divergent thinking. Fourth, it should be noted that recent evidence suggests that core or default network involvement from group-level analyses differs when scanning data acquired from many sessions are analyzed on an individual-by-individual basis (Braga and Buckner 2017), so this sort of approach should be carried out in further work focused on tasks that are thought to engage the core network.
The findings from our study also raise several broader points related to investigations involving the cognitive neuroscience of creativity. First, divergent thinking is typically measured via quantity (i.e., number of ideas) and quality (e.g., novelty or originality) facets. An important question raised from the current findings is what measure or measures best capture divergent thinking. In the present study, the ESI manipulation affected the quantity of responses generated during divergent thinking but did not affect the average level of quality ratings of creativity (i.e., on average, ideas generated following ESI and the control induction were both rated as “a little” to “somewhat creative” by blind raters and as “creative” by participants themselves). Nonetheless, the number of old ideas from memory and the number of new (i.e., personally novel) ideas from imagination generated for the first time during the experiment were boosted following ESI. We think that this pattern of findings highlights that episodic retrieval can serve as a component process of divergent thinking because it indicates that episodic memory—rather than being irrelevant to, or a hindrance to, creative output—actually enhances creative idea production: ESI affected creative idea production whether measured via quantity of old/novel ideas or quantity of ideas matched in terms of a creativity rating (e.g., 4 responses rated as “creative” or “new ideas” following ESI and 2 responses rated as “creative” or “new ideas” following the control induction means twice as many “creative” or “new idea” responses were generated following ESI relative to control). A significant challenge for future work will be to pinpoint whether particular cognitive and neural contributions to divergent thinking and creative cognition more broadly are best predicted by different facets or stages of creativity (for related examples, see Jung et al. 2015; Kleinmintz et al. 2017), as well as whether “quality” is best characterized as the generation of personally new ideas, a creativity rating from blind research assistants or experts, a creativity rating from participants, an index based on sample-specific or normative originality, or some combination of these measures.
Relatedly, contemporary work including the present study has mainly concentrated on uncovering similarities in the contributions of episodic memory to tasks beyond simple remembering, such as imagining the future, solving social problems, and thinking creatively. While episodic retrieval and event construction may similarly affect certain forms of simulation, problem solving, and creative thinking, an important avenue for future work will be to determine when and how other cognitive processes recruited for these seemingly overlapping tasks may work in distinct ways. The adoption of particular behavioral manipulations and neuroimaging techniques should shed light on similarities as well as differences in episodic memory and related tasks. For example, cognitive inductions that target nonepisodic processes may uncover differences among such tasks as future simulation, problem solving, and creative thinking. Neuroimaging techniques, such as transcranial magnetic stimulation of particular regions/networks subserving particular processes, or multivariate pattern classifiers that can or cannot decode from BOLD signal in a particular ROI any task distinctions between episodic memory, episodic future simulation, and divergent thinking based on old or new ideas, may also inform this issue.
Conclusions
While these limitations should be addressed systematically in future work, the collective results reported here converge on the idea that the boundary conditions and neural expressions of a constructive episodic memory system lie beyond simple remembering (cf. Schacter and Addis 2007; Moscovitch et al. 2016). Additional studies should examine how episodic memory interacts with other component processes during divergent thinking and related tasks, and which brain networks underlie such capabilities, using experimental tools such as ESI that can isolate component processes. This approach should shed light on how the brain flexibly utilizes the retrieval of episodic details in the service of a variety of cognitive functions.
Notes
We thank Jyotika Bindra, Kristina Tummino, and Stephanie McMains for assistance with various aspects of the experiment, and the University of Minnesota Center for Magnetic Resonance Research for neuroimaging sequences. The authors declare no competing financial interests. Conflict of Interest: None declared.
Funding
National Institute of Mental Health (Grant MH060941) to D.L.S. Imagination Institute funded by the John Templeton Foundation (Grant RFP-15-12) to R.E.B. Rutherford Discovery Fellowship (Grant RDF-10-UOA-024) and Marsden Fund Grant (Grant 12-UOA-254) to D.R.A. This research was carried out at the Harvard Center for Brain Science, and involved the use of instrumentation supported by the National Institutes of Health Shared Instrumentation Grant Program (Grant S10OD020039).
References
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kroger S, Schweckendiek J, Stark R, Windmann S, Hermann C. 2012. Creativity and the brain: uncovering the neural signature of conceptual expansion. Neuropsychologia. 50:1906–1917. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Musicaro R, Schacter DL. 2016. Divergent thinking and constructing episodic simulations. Memory. 24:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain’s default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, Yarkoni T. 2014. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting state connectivity, and fMRI meta-analyses. Neuroimage. 91:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. 2014. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Kaufman SB, Silvia PJ. 2015. Default and executive network coupling supports creative idea production. Sci Rep. 5:10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, Schacter DL. 2016. Creative cognition and brain network dynamics. Trends Cogn Sci. 20:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Wilkins RW, Jauk E, Fink A, Silvia PJ, Hodges DA, Koschutnig K, Neubauer AC. 2014. Creativity and the default network: a functional connectivity analysis of the creative brain at rest. Neuropsychologia. 64:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Christensen AP, Benedek M, Silvia PJ, Schacter DL. 2017. Creative constraints: brain activity and network dynamics underlying semantic interference during idea production. Neuroimage. 148:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Fink A, Koschutnig K, Reishofer G, Ebner F, Neubauer AC. 2014. To create or to recall? neural mechanisms underlying the generation of creative new ideas. Neuroimage. 88:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. 2015. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 75:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. 2017. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 95:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. 2001. a. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. 2001. b. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 13:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Thompson-Schill SL. 2011. Dissociable brain states linked to common and creative object use. Hum Brain Mapp. 32:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. 2017. fMRI clustering and false-positive rates. Proc Natl Acad Sci USA. 114:3370–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, Jefferies E. 2015. a. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J Neurosci. 35:15230–15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Rueschemeyer SA, Costigan A, Murphy N, Krieger-Redwood K, Hallam G, Jefferies E. 2015. b. Shared neural processes support semantic control and action understanding. Brain Lang. 142:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, Krieger-Redwood K, Bernhardt BC, Smallwood J, Jefferies E. 2016. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 137:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. 2010. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull. 136:822–848. [DOI] [PubMed] [Google Scholar]
- Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D. 2013. Hippocampal amnesic disrupts creative thinking. Hippocampus. 23:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. 2001. From conditioning to conscious recollection: memory systems of the brain. Oxford (England): Oxford University Press. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. 2012. Evaluative and generative modes of thought during the creative process. Neuroimage. 59:1783–1794. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil L, Yacoub E. 2010. Multiplexed echo planar imaging for sub-second whole brain fMRI and fast diffusion imaging. PLoS One. 5:e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Benedek M, Koschutnig K, Pirker E, Berger E, Sabrina M, Neubauer AC, Papousek I, Weiss EM. 2015. Training of verbal creativity modulates brain activity in regions associated with language- and memory-related demands. Hum Brain Mapp. 36:4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Resihofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC. 2009. The creative brain: investigation of brain activity during creative problem solving by means of EEG and fMRI. Hum Brain Mapp. 30:734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Gebauer D, Resihofer G, Koschutnig K, Ebner F. 2010. Enhancing creativity by means of cognitive simulation: evidence from an fMRI study. Neuroimage. 52:1687–1695. [DOI] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Benedek M, Reishofer G, Ischebeck A, Weiss EM, Ebner F. 2012. Stimulating creativity via the exposure to other people’s ideas. Hum Brain Mapp. 33:2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke RA, Ward TB, Smith SM. 1992. Creative cognition: theory, research, and applications. Cambridge (MA): MIT Press. [Google Scholar]
- Fisher RP, Geiselman RE. 1992. Memory-enhancing techniques for investigative interviewing: the cognitive interview. Springfield (IL): Charles C. Thomas Books. [Google Scholar]
- Gabbert F, Hope L, Fisher RP. 2009. Protecting eyewitness evidence: examining the efficacy of a self-administered interview tool. Law Hum Behav. 33:298–307. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. 2011. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage. 55:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooly KJ, Fioratou E, Anthony SH, Wynn V. 2007. Divergent thinking: strategies and executive involvement in generating novel uses for familiar objects. Br J Psychol. 98:611–625. [DOI] [PubMed] [Google Scholar]
- Guilford JP. 1967. The nature of human intelligence. New York (NY): McGraw Hill. [Google Scholar]
- Guilford JP, Christensen PR, Merrifield PR, Wilson RC. 1960. Alternate uses manual. Menlo Park (CA): Mind Garden. [Google Scholar]
- Howard-Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G. 2005. Semantic divergence and creative story generation: an fMRI investigation. Cogn Brain Res. 25:240–250. [DOI] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA. 2013. The structure of creative cognition in the human brain. Front Hum Neurosci. 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Wertz CJ, Meadows CA, Ryman SG, Vakhtin AA, Flores RA. 2015. Quantity yields quality when it comes to creativity: a brain and behavioral test of the equal-odds rule. Front Psychol. 6:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulous D, Jia Z, Lim KO, Hoptman MJ. 2010. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinmintz OM, Abecasis D, Tauber A, Geva A, Chistyakov AV, Kreinin I, Klein E, Shamay-Tsoory S. 2017. Participation of the left inferior frontal gyrus in human originality. Brain Struct Funct. doi:10.1007/s00429-017-1500-5. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. 2002. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 17:677–689. [PubMed] [Google Scholar]
- Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, Braun AR. 2015. Brain activity and connectivity during poetry composition: toward a multidimensional model of the creative process. Hum Brain Mapp. 36:3351–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Addis DR, Schacter DL. 2015. Creativity and memory: effects of an episodic specificity induction on divergent thinking. Psychol Sci. 26:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Gaesser B, Schacter DL. 2014. Constructive episodic simulation: dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. J Exp Psychol Learn Mem Cogn. 40:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Jing HG, Schacter DL. 2016. a. Divergent creative thinking in young and older adults: extending the effects of an episodic specificity induction. Mem Cognit. 44:974–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. 2014. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 29:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. 2016. Remembering the past and imagining the future: selective effects of an episodic specificity induction on detail generation. Q J Exp Psychol (Hove). 69:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Szpunar KK, Addis DR, Schacter DL. 2016. b. Episodic specificity induction impacts activity in a core brain network during construction of imagined future experiences. Proc Natl Acad Sci USA. 113:10696–10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. 2011. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci USA. 108:13858–13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayseless N, Eran A, Shamay-Tsoory SG. 2015. Generating original ideas: the neural underpinning of originality. Neuroimage. 116:232–239. [DOI] [PubMed] [Google Scholar]
- Mednick SA. 1962. The associative basis of the creative process. Psychol Rev. 69:220–232. [DOI] [PubMed] [Google Scholar]
- Mehndelsohn GA. 1976. Associative and attentional processes in creative performance. J Pers. 44:341–369. [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 63:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Rugg MD. 2012. Retrieval orientation and the control of recollection: an fMRI study. J Cogn Neurosci. 24:2372–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. 2016. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu Rev Psychol. 67:105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Fox MD. 2017. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambdon Ralph MA. 2013. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of the dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 25:1825–1850. [DOI] [PubMed] [Google Scholar]
- Nusbaum EC, Silvia PJ, Beaty RE. 2014. Ready, set, create: what instructing people to “be creative” reveals about the meaning and mechanisms of divergent thinking. Psychol Aesthet Creat Arts. 8:423–432. [Google Scholar]
- Ononye GC, Blinn-Pike LM, Smith DE. 1993. Creativity and future time perspective: exploring fantasy and realistic measures. Creat Res J. 6:449–456. [Google Scholar]
- Race EA, Kuhl BA, Badre D, Wagner AD. 2009. The dynamic interplay between cognitive control and memory In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge (MA): MIT Press; p. 705–723. [Google Scholar]
- Radvansky GA, Zacks JM. 2014. Event cognition. New York (NY): Oxford University Press. [Google Scholar]
- Richmond LL, Zacks JM. 2017. Constructing experience: event models from perception to action. Trends Cogn Sci. doi:10.1016/j.tics.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RP, Addis DR forthcoming 2017. A common mode of processing governing divergent thinking and future imagination In: Jung RE, Vartanian O, editors. The Cambridge Handbook of the neuroscience of creativity. New York (NY): Cambridge University Press. [Google Scholar]
- Roberts RP, Schacter DL, Addis DR. 2017. a. Scene construction and relational processing: separable constructs? Cereb Cortex. 10.1093/cercor/bhx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RP, Wiebels K, Sumner RL, van Mulukom V, Grady CL, Schacter DL, Addis DR. 2017. An fMRI investigation of the relationship between future imagination and cognitive flexibility. Neuropsychologia. 95:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero K, Moscovitch M. 2012. Episodic memory and event construction in aging and amnesia. J Mem Lang. 67:270–284. [Google Scholar]
- Rorden C, Karnath H, Bonilha L. 2007. Improving lesion-symptom mapping. J Cogn Neurosci. 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, Cohen NJ. 2014. The role of the hippocampus in flexible cognition. Front Hum Neurosci. 8:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runco MA, Acar SA. 2010. Do tests of divergent thinking have an experiential bias? Psychology of Aesthetics. Creat Arts. 4:144–148. [Google Scholar]
- Schacter DL, Addis DR. 2007. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B. 362:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. 2007. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 8:657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. 2012. The future of memory: remembering, imagining, and the brain. Neuron. 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Szpunar KK. 2017. Escaping the past: contributions of the hippocampus to future thinking and imagination In: Hannula DE, Duff MC, editors. The hippocampus from cells to systems: structure, connectivity, and functional contributions to memory and flexible cognition. New York (NY): Springer; p. 439–465. [Google Scholar]
- Schacter DL, Benoit RG, Szpunar KK. 2017. Episodic future thinking: mechanisms and functions. Curr Opin Behav Sci. 17:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Madore KP. 2016. Remembering the past and imagining the future: identifying and enhancing the contribution of episodic memory. Mem Stud. 9:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah C, Erhard K, Ortheil H, Kaza E, Kessler C, Lotze M. 2013. Neural correlates of creative writing: an fMRI study. Hum Brain Mapp. 34:1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Nusbaum EC, Beaty RE. 2015. Old or new? Evaluating the old/new scoring method for divergent thinking tasks. J Creat Behav. 10.1002/jocb.101. [DOI] [Google Scholar]
- Simons JS, Spiers HJ. 2003. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 4:637–648. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. 2003. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res. 17:75–82. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. 2012. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 5:60–70. [DOI] [PubMed] [Google Scholar]
- Smith SM, Ward TB, Finke RA. 1995. The creative cognition approach. New York (NY): Oxford University Press. [Google Scholar]
- Storm BC, Patel TN. 2014. Forgetting as a consequence and enabler of creative thinking. J Exp Psychol Learn Mem Cogn. 40:1594–1609. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen Q, Zhang Q, Li Y, Li H, Wei D, Yang W, Qiu J. 2016. Training your brain to be more creative: brain functional and structural changes induced by divergent thinking training. Hum Brain Mapp. 37:3375–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Rayner G, Wilson S, Jackson G. 2017. The spatiotemporal substrates of autobiographical recollection: using event-related ICA to study cognitive networks in action. Neuroimage. 152:237–248. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki H, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. 2012. The association between resting functional connectivity and creativity. Cereb Cortex. 22:2921–2929. [DOI] [PubMed] [Google Scholar]
- Thakral PP, Benoit RG, Schacter DL. 2017. Imagining the future: the core episodic simulation network dissociates as a function of time course and the amount of simulated information. Cortex. 90:12–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance PE. 1962. Guiding creative talent. Englewood Cliffs (NJ): Prentice-Hall. [Google Scholar]
- Torrance PE. 1981. Empirical validation of criterion-referenced indicators of creative ability through a longitudinal study. Creat Child Adult Q. 6:136–140. [Google Scholar]
- Tulving E. 1983. Elements of episodic memory. Oxford (England): Clarendon Press. [Google Scholar]
- Tulving E. 2002. Episodic memory: from mind to brain. Annu Rev Psychol. 53:1–25. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurosci. 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Kurczek J, Duff MC. 2016. What relates newspaper, definite, and clothing? an article describing deficits in convergent problem solving and creativity following hippocampal damage. Hippocampus. 6:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J. 2014. Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex. 51:92–102. [DOI] [PubMed] [Google Scholar]
- Welch L. 1946. Recombination of ideas in creative thinking. J Appl Psychol. 30:638–643. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Zhang Q, Zhang M, Qiu J. 2015. A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Hum Brain Mapp. 36:2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Ugurbil K. 2013. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 83:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. . 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]