Significance
Clotting of blood is not exclusive to host physiology; pathogens are also able to generate clots as part of their life cycle. Here, we show that coagulases, enzymes responsible for bacteria-mediated clotting, can act as public goods in clinical conditions. Coagulases, secreted by producers, generate protective layers of fibrin around the bacteria, shielding them from antimicrobials and host immune factors. Remarkably, we find that this protection is also conferred onto strains that do not produce coagulases but still benefit from those made by others. Although this is a social trait, overexploitation of coagulases does not occur due to spatial segregation and population viscosity. Our study provides a social evolution perspective on the critical role of coagulases.
Keywords: public goods, coagulases, biofilms, social evolution, Staphylococcus aureus
Abstract
Coagulation is an innate defense mechanism intended to limit blood loss and trap invading pathogens during infection. However, Staphylococcus aureus has the ability to hijack the coagulation cascade and generate clots via secretion of coagulases. Although many S. aureus have this characteristic, some do not. The population dynamics regarding this defining trait have yet to be explored. We report here that coagulases are public goods that confer protection against antimicrobials and immune factors within a local population or community, thus promoting growth and virulence. By utilizing variants of a methicillin-resistant S. aureus we infer that the secretion of coagulases is a cooperative trait, which is subject to exploitation by invading mutants that do not produce the public goods themselves. However, overexploitation, “tragedy of the commons,” does not occur at clinically relevant conditions. Our micrographs indicate this is due to spatial segregation and population viscosity. These findings emphasize the critical role of coagulases in a social evolution context and provide a possible explanation as to why the secretion of these public goods is maintained in mixed S. aureus communities.
Intravenous catheters are widely used in clinical practice for long-term venous access in patients requiring continuous perfusion (1–5). Intended for the administration of fluids, blood transfusions, medications, nutritional support, chemotherapy, and hemodynamic monitoring (1–3, 5), these devices have become a major source of healthcare-associated infections. The impact of endovascular infections is substantial, both in terms of morbidity and financial resources expended (6–11). Since i.v. catheters are in direct contact with the bloodstream, their surfaces become coated with plasma, platelets, red blood cells, and ECM proteins such as albumin, fibrinogen, fibronectin, and laminin (12), making the host vasculature highly susceptible to microbial colonization and biofilm formation (13). Typically, biofilms are regarded as communities embedded in a bacterially produced matrix where the extracellular polymeric substance (EPS) is composed of self-secreted polysaccharides, proteins, and nucleic acids. However, during infections, pathogens are also able to sense environmental cues and utilize the surrounding ECM components to form a host-derived matrix (HDM).
Staphylococcus aureus is one such versatile opportunistic pathogen that is frequently associated with endovascular infections. Its ability to interact with ECM components via secreted factors is a defining feature of S. aureus infections, where a vast array of its virulence genes coding for adhesins/invasins, toxins, and modulins contribute to its colonization, dissemination, and persistence in host tissue. However, certain staphylococcal factors are secreted out into the surrounding environment, where they specifically interact with the aforementioned ECM components found in blood. In terms of sociobiology, these secreted factors may act as “public goods” that benefit all individuals present within a local population or community (14, 15). Biofilm development and maintenance in vivo is often dependent on and can be influenced by public goods (16, 17). Granted that the expression of these public goods can prove to be costly to the producer, they provide a communal benefit and can, therefore, be potentially favored by all individuals within the population (18). However, this type of behavior poses an evolutionary conundrum because it is vulnerable to exploitation by cheats which are “free riders” that do not cooperate in producing the public goods but can still benefit at the expense of producers (14). This dilemma is well-known in the fields of economics and human morality, where it is termed the “tragedy of the commons” (19). The tragedy is that, as a group, individuals stand to benefit from cooperation, but cooperation is not stable because each individual can gain by selfishly pursuing its own short-term interests. Despite the merits of investigating these mechanistic and evolutionary theories in a host–pathogen context (20), little is known about public good virulence factors that interact with physiological components during infections and their influence on population dynamics. Therefore, we use a highly relevant in vitro clinical model to explore the theoretical underpinnings of the social dynamics occurring during staphylococcal bloodstream infections. We focus on the two known coagulases of S. aureus, staphylocoagulase (Coa) and von Willebrand factor-binding protein (vWbp).
Coa and vWbp are two hemostasis factors that allow S. aureus to usurp the physiological blood coagulation cascade. Both coagulases trigger a conformational change and induce a functionally active catalytic site in the host coagulation zymogen, prothrombin (ProT) (21). The enzymatically active staphylothrombin complexes (ProT•Coa and ProT•vWbp) then facilitate clotting of plasma by cleaving fibrinogen from its substrate form to insoluble polymerizing fibrin fibrils. This dense fibrous clot is further strengthened by the cross-linking activity of the transglutaminase factor XIIIa, a fibrin-stabilizing enzyme that is nonproteolytically activated by the ProT•vWbp•fXIII complex (22). Despite Coa- and vWbp-mediated clots being vital for S. aureus during infection, phenotypes of low expression levels, loss-of-function mutations, and/or complete deficiency of coagulases are observed among clinical isolates (23–30). In this sense, we propose that secretion of coagulases is a defining feature of S. aureus fitness during infection, but it is also a costly and potentially exploitable trait—a public good. We test this hypothesis using our model organisms: a community-acquired methicillin-resistant S. aureus, USA300 LAC, that produces Coa and vWbp; a Δcoa mutant that does not produce Coa; and a ΔcoaΔvwbp double mutant that does not produce Coa and vWbp. The mutants, Δcoa and ΔcoaΔvwbp, represent cheats that do not produce the potential public goods of interest, whereas LAC represents producers.
Previous work established a simple yet elegant in vitro model for studying pathogens found in clinical infections (31–33). Termed “wound-like” media (WLM), this model is formulated to represent physiological components encountered within blood and host vasculature. Previously, it has been used to study polymicrobial communities found in human wounds and the efficacy of various antimicrobials as potential treatments (31, 33–35). Here, we utilize the WLM to mimic biofilms and septic thrombi associated with endovascular infections. To supply an infection-like environment, we constitute the WLM with a chopped-meat-based medium, heparinized plasma, and hemolyzed blood. Heparin is a glycosaminoglycan that effectively inhibits the clotting of blood and is frequently used in clinical practice as a prophylaxis against thrombosis. The anticoagulant properties are mediated by heparin’s interaction with the enzyme inhibitor antithrombin that inactivates thrombin, factor Xa, and other proteases, thereby preventing the endogenous conversion of fibrinogen to fibrin clots (36). Despite therapeutic doses of heparin being administered alongside venous catheter installations, septic thrombi continue to be a major concern for patients where bacteria circumvent the proteolytic process of blood coagulation.
Results
Coagulases Mediate Clotting of WLM.
The first aim of our study was to utilize the WLM to identify potential public goods of S. aureus that facilitate clotting and form thrombi similar to those observed in vivo (SI Appendix, Fig. S1). We initially screened the clotting ability of select S. aureus gene knockout mutants. Inoculation of fresh WLM with S. aureus Newman wild type triggered clotting, whereas the ΔsaePQRS mutant was unable to generate clots within 24 h (SI Appendix, Fig. S2A). The Sae regulatory system (S. aureus exoprotein expression) controls the expression of several genes encoding proteins known to interact with host ECM components (37). Therefore, we screened various S. aureus factors that could potentially mediate clotting of the WLM: fibronectin binding proteins (FnbpA and FnbpB) that bind to fibrinogen/fibrin (38, 39); clumping factor (ClfA) that binds to fibrinogen/fibrin (40); ECM and plasma binding protein (Empbp/Emp) that interacts with fibrinogen/fibrin and vitronectin (41); extracellular adherence protein (Eap) that binds to fibrinogen, fibronectin, vitronectin, thrombospondin, and collagen (42, 43); and the prothrombin-activating proteins (Coa and vWbp) (44, 45). With the exception of clfA, most, if not all, of these genes are transcriptionally activated by the saeRS two-component system (37, 46, 47), of which coa was found to mediate the clotting of the WLM (SI Appendix, Fig. S2B). Disruption in any of the other genes did not affect the ability of S. aureus to clot the WLM (SI Appendix, Fig. S2).
These phenotypic data led us to construct our final working strains: USA300 LAC, Δcoa, and ΔcoaΔvwbp. Inoculation of fresh WLM with LAC triggered clotting, whereas the Δcoa and ΔcoaΔvwbp mutants were unable to generate clots within 24 h. We also constructed a Δvwbp mutant, but this strain was able to clot the WLM within 24 h and therefore was excluded from the study. This observation was most likely because of Coa compensating for the lack of vWbp. However, vWbp is known to form a functionally active complex with ProT, fibrinogen, and factorXIII (22) and is suggested to compensate for the lack of Coa during endovascular infections (48–50)—hence the inclusion of the ΔcoaΔvwbp double mutant within our study. Therefore, we consider coagulases Coa and vWbp as potential public goods that contribute to the ability of S. aureus to generate robust clots (Fig. 1).
Fig. 1.
Clotting of WLM is facilitated by S. aureus coagulases. WLM was inoculated with LAC, Δcoa, Δvwbp, or ΔcoaΔvwbp and incubated for 24 h at 37 °C. Cultures were poured into a Petri dish to assess coagulation. Images represent three independent determinations.
Nonproducers Coexist in Close Proximity to the Coagulase Producers Within Clots.
In vivo, pathogenic bacteria often grow as aggregates that are found interspersed throughout the infected host tissue (51–56). Typically, studies focus on visualizing bacterial glycocalyx; however, this is equivocal, considering in vivo biofilms are a combination of both the bacteria-derived EPS and HDM (57). This is the case with septic thrombi, where secreted coagulases allow S. aureus to utilize the ECM substrates to generate clots incorporating the HDM as part of the biofilm. To verify this phenomenon, we used a lectin dye, Con A, that does not discriminate between the EPS and HDM to provide an accurate spatial context of bacteria in our in vitro model. Due to its ability to selectively bind α-mannopyranosyl and α-glucopyranosyl residues, we have previously used Con A to stain matrix components in sections from infected wounds (58).
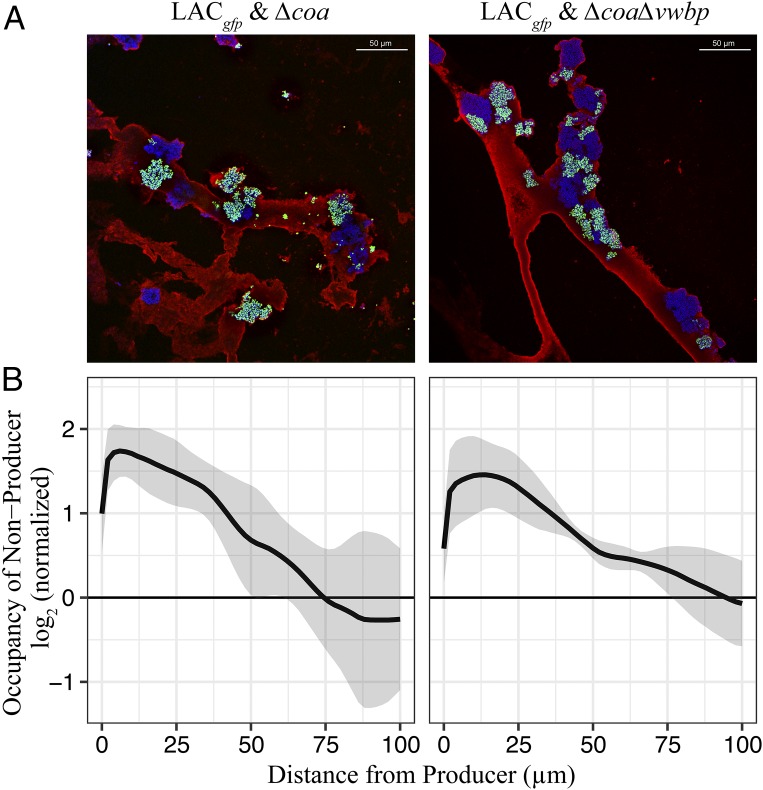
Thin sections of coagulated WLM that had been cocultured for 18 h with LAC and Δcoa or LAC and ΔcoaΔvwbp were stained and visualized using confocal laser scanning microscopy. Micrographs revealed clonal clumps of nonproducers and producers coaggregating, enmeshed in a web of fibrous matrix (Fig. 2A and SI Appendix, Figs. S3A and S4). The staphylococcal aggregates were interspersed throughout the fibrous HDM, all the while enclosed and segregated by fibrin strands. The HDM served as a scaffold to which the bacteria could adhere and reside within. Analyses of the spatial organization of the aggregates revealed that the nonproducers were situated in close proximity to the producers, more than what is expected to be random (Fig. 2B and SI Appendix, Fig. S3B). However, both nonproducers and producers were situated closer to themselves than they were to each other (SI Appendix, Figs. S5 and S6). This suggested that the majority of the clumps were clonal. Therefore, we believe that clonal clumps were able to coaggregate, but individual cells of the two strains rarely intermixed. It should be reiterated that monocultures of nonproducers are unable to coagulate the WLM; therefore, the observed fibrous architecture is orchestrated only in the presence of producers, where coagulases induce fibrinogen cleavage to polymerize protective layers of fibrin around the staphylococci.
Fig. 2.
Clumps of coagulase producers and nonproducers coaggregate within clots. WLM inoculated with LAC and Δcoa or ΔcoaΔvwbp visualized by confocal fluorescence microscopy. (A) Overlays of representative Z stacks from the cocultures visualized: LACgfp (green) & Δcoa (blue); LACgfp (green) & ΔcoaΔvwbp (blue). See SI Appendix, Figs. S3–S7 for reverse controls Δcoagfp (green) & LAC (blue). Images reveal clumps of both producers and nonproducers, interspersed throughout the fibrous host derived matrix (red). (Scale bars: 50 μm.) (B) Occupancy of nonproducers (Δcoa or ΔcoaΔvwbp) is plotted as a function of distance away from producers (LAC). Occupancy is defined as the fraction of nonproducers at a certain distance normalized to the fraction of nonproducers in the entire image, such that an image with randomly distributed pixels would have an occupancy of one at any distance (log2 = 0). Continuous lines represent means; shaded regions represent 95% confidence intervals. LACgfp & Δcoa (n = 6); LACgfp & ΔcoaΔvwbp (n = 6).
Taken together, these micrographs indicate that nonproducers are able to integrate themselves into the fibrin clots generated by LAC. If coagulases provide a fitness advantage for nonproducers we consider them public goods, even though they act on the surrounding host ECM in generating clots and not directly on the surrounding bacterial cells. Therefore, we decided to measure a classical parameter that is both dependent on community structure and biofilm formation: antimicrobial tolerance. We test this parameter because it could be a potential benefit of clot formation that may also be shared with the nonproducers, which would suggest coagulases are public goods (that can potentially be cheated on).
Access to Coagulases Confers Enhanced Antimicrobial Tolerance.
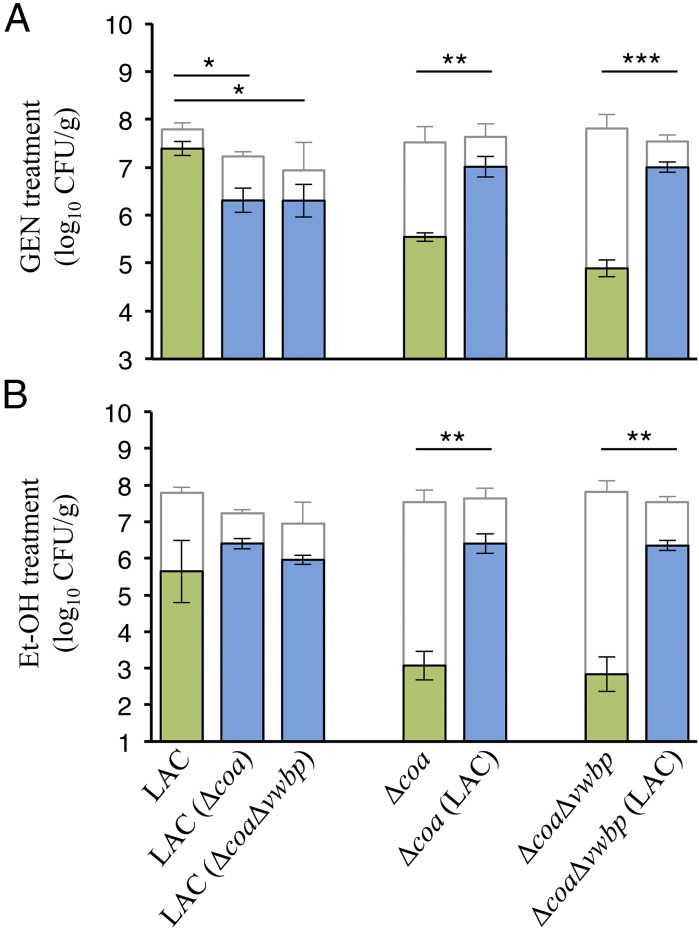
Biofilm-related antimicrobial tolerance has been observed in vivo and in vitro; therefore, having visualized the marked phenotypic differences in the clotting ability of our staphylococcal strains, we sought to determine if the expression of or having access to public goods affected their antimicrobial susceptibilities. Our experiments examined the consequences of variation for a single trait, whether or not they produce coagulases. In the monoculture groups WLM was inoculated with only the LAC, Δcoa, or ΔcoaΔvwbp, whereas in the coculture groups WLM was inoculated with a 1:1 mixture of both the LAC and Δcoa or LAC and ΔcoaΔvwbp. Monocultures and cocultures were grown overnight in WLM, and antimicrobial tolerance was determined as described in Materials and Methods. We assessed the tolerance of S. aureus strains to an aminoglycoside (gentamicin) and ethanol. As shown in Fig. 3, monocultures of producers (LAC) were more tolerant to both gentamicin and ethanol in comparison with the monocultures of nonproducers (Δcoa and ΔcoaΔvwbp). Notably, the nonproducers displayed an increase in tolerance against both antimicrobials when present in cocultures with producers in comparison with their monocultures. We also saw a decrease in the tolerance of LAC against gentamicin when cocultured with nonproducers in comparison with its monoculture.
Fig. 3.
Clots generated by coagulases contribute to enhanced antimicrobial tolerance. Monocultures of LAC, Δcoa, or ΔcoaΔvwbp; cocultures of LAC and Δcoa, or LAC and ΔcoaΔvwbp were grown in WLM for 18 h at 37 °C. (A) Gentamicin and (B) ethanol tolerances of samples from these cultures were then assessed by enumeration of CFUs on selective agar plates. Green and blue colored bars represent the number of cells viable of after gentamicin or ethanol treatment, whereas the white regions represent the control treatment (PBS) for each group. Green labels with only one strain listed are monocultures; the blue labels with two strains listed are cocultures. The tolerance profiles pertain to the strain listed outside the parentheses, whereas the strain listed inside the parentheses denotes what it was cocultured with. Data represent three independent trials (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with post hoc Tukey test; error bars denote ±SEM; n = 3).
The observed tolerance profiles are owed to the thrombus generated in the presence of coagulases; monocultures of nonproducers are unable to clot the WLM and thereby do not generate the HDM, leaving them susceptible to antimicrobials. However, when cocultured with producers, nonproducers are incorporated into the septic thrombi, whereby secreted coagulases result in fibrinogen cleavage and fibrin clots surrounding the staphylococcal communities (Fig. 2A and SI Appendix, Figs. S3A and S4). Therefore, we emphasize that here the benefits of coagulases accrue not only to the producers within the thrombus but also to the nonproducers residing therein. This distinction is important in terms of sociobiology because it leads to the fundamental question of what favors the cooperative production of coagulases.
Stressors Can Select for Cooperators and Defer a Tragedy of the Commons.
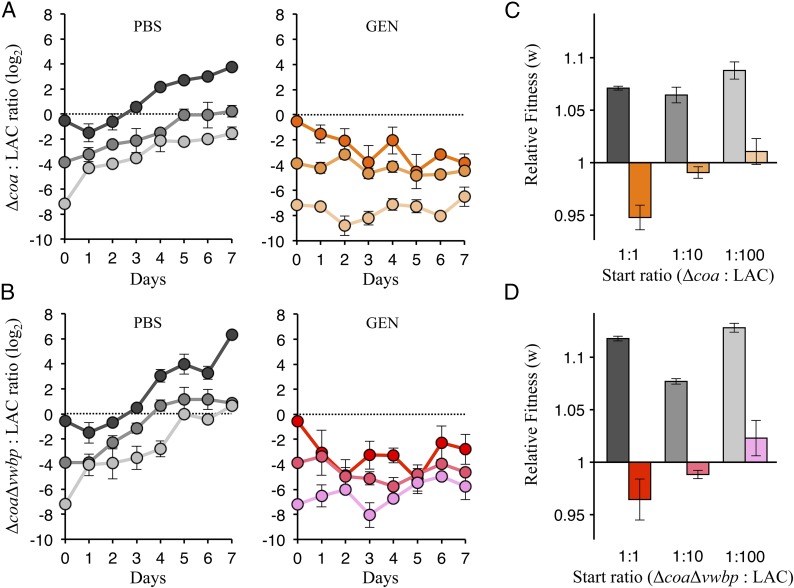
Despite administration of antibiotics and/or anticoagulants to prevent recurring clots, S. aureus is able to reconstitute the thrombus. Here we used the WLM to investigate how stressors influence the relative fitness of producers vs. nonproducers within the septic thrombi. In this assay, we follow the population dynamics over several days and generations as opposed to the assay used in the previous results. The relative fitness experiment consisted of a control, PBS, or an antibiotic, gentamicin. These experiments were carried out to assess if the secretion of coagulases, our public goods of interest, is cheatable.
We initiate the experiment by inoculating the WLM with a 1:1, 1:10, or 1:100 mixture of Δcoa:LAC or ΔcoaΔvwbp:LAC. After 18 h of growth, the WLM cultures were subjected to parallel but different treatments (PBS or gentamicin). Then, all subsequent rounds were initiated with the treated population from the preceding round. To calculate the relative frequency of producers vs. nonproducers, bacteria were enumerated after each round of treatment. As we propagated the bacterial population through the different selection rounds, we found that the nonproducers were favored under conditions without gentamicin (PBS treatments) (Fig. 4), where at day 7 the ratios of nonproducers to producers was significantly higher than what we initially started with at day 0 (P < 0.05, one-way ANOVA with post hoc Tukey test) (Fig. 4 A and B). In addition, the fitness of the nonproducers was higher than that of the producers (Fig. 4 C and D). This was true for all of the PBS-treated samples irrespective of whether we analyzed the cocultures harboring Δcoa or ΔcoaΔvwbp, indicating a fitness cost of producing coagulases and suggesting that our nonproducers are cheats. Intuitively this makes sense: As cheats are not taxed with the costs of producing coagulases, they can divert resources toward other metabolic activities and can thereby increase in frequency.
Fig. 4.
Penalty resulting from overexploitation defers a tragedy of the commons. The 1:1 (darkest), 1:10, or 1:100 (lightest) starting mixtures of Δcoa:LAC (A and C) or ΔcoaΔvwbp:LAC (B and D) were grown repeatedly in WLM for 18 h at 37 °C. The population from these cultures was then subjected to PBS (tones of black) or gentamicin [Δcoa:LAC (orange tones), and ΔcoaΔvwbp:LAC (red tones)] treatment and propagated through multiple days of culturing. Population ratios were assessed by enumeration of CFUs on selective agar plates. (A) Log2-transformed Δcoa:LAC ratios and (B) ΔcoaΔvwbp:LAC ratios are plotted for each day. (C) Relative fitness (w) of the mutant for the Δcoa:LAC mixtures and (D) the ΔcoaΔvwbp:LAC mixtures. Black horizontal line at 1.0 represents where the mutants and LAC are equally fit. The relative fitness of both Δcoa and ΔcoaΔvwbp was significantly higher at the lowest start ratio in comparison with the highest start ratio for the gentamicin-treated samples (P < 0.05, two-way ANOVA with an interaction term and post hoc Tukey test; error bars denote ±SEM; n = 3 for each start ratio).
In contrast, the producers were favored under conditions of gentamicin treatment (Fig. 4) and cooperation was maintained, thereby making the coagulase-dependent fibrous matrix sustainable. The ratios of cheats to producers at day 7 was not significantly different from what we initially started with at day 0 in the gentamicin-treated samples (P > 0.05, one-way ANOVA with post hoc Tukey test), except for the 1:1 Δcoa:LAC (Fig. 4 A and B). The relative fitness of the cheats for the gentamicin-treated samples was significantly less in comparison with their PBS counterparts (P < 0.001, two-way ANOVA with an interaction term and post hoc Tukey test) (Fig. 4 C and D). Furthermore, consistent with social evolution theory (14), we find the fitness of our cheats to be frequency-dependent for the gentamicin-treated samples; the relative fitness of both Δcoa and ΔcoaΔvwbp was significantly higher at the lowest start ratio in comparison with the highest start ratio (P < 0.05, two-way ANOVA with an interaction term and post hoc Tukey test) (Fig. 4 C and D). By fitting a linear model, we saw that the relative fitness of both Δcoa (P = 0.0033; R2 = 0.732) and ΔcoaΔvwbp (P = 0.0217; R2 = 0.554) increased significantly as their starting frequency decreased (SI Appendix, Fig. S9). Overall, the above suggests that the staphylococcal community stands to benefit when the structural integrity of the thrombus remains uncompromised, whereas a higher frequency of cheats delays clotting of the WLM, resulting in a more fluid biofilm, leaving the community more susceptible to gentamicin. In addition, since cheats do not clot the WLM, one can expect the fluid layers to harbor more cheats than producers. Therefore, cheats are kept in check by the stressor and a tragedy of the commons is deferred because overexploitation of producers results in immediate consequential penalties. Undoubtedly, access to public goods confers benefits within the WLM; however, as our in vitro model lacks the immunological factors encountered within blood, we sought to determine if the benefits of secreted public goods could be recapitulated in fresh human blood.
Coagulases Enhance Staphylococcal Survival in Human Blood.
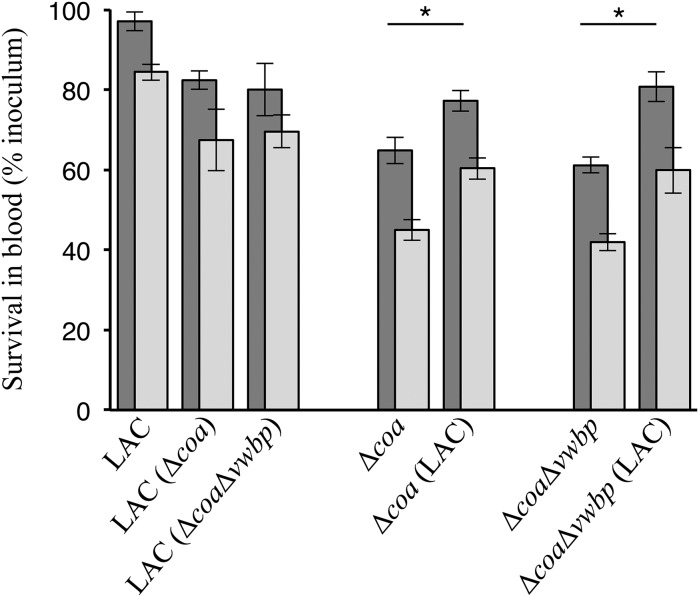
The abundant complement proteins in blood serum, antimicrobial peptides, and polymorphonuclear neutrophils provide a highly specific and rapid response against invading pathogens (59–61). For this reason, we analyzed the contribution of coagulases toward staphylococcal survival in fresh heparinized human blood. As before, monoculture groups were inoculated with only the LAC, Δcoa, or ΔcoaΔvwbp, whereas the coculture groups were inoculated with a 1:1 mixture of both the LAC and Δcoa, or LAC and ΔcoaΔvwbp. Colony-forming units (CFUs) were obtained after 45 and 90 min of incubation. Monoculture of LAC displayed a slight reduction in CFUs in human blood (Fig. 5). In contrast, the monocultures of the mutants, Δcoa and ΔcoaΔvwbp, suffered a large reduction in CFUs, exhibiting a defect in survival in human blood (Fig. 5). Interestingly, coculturing the cheats with producers significantly improved the survival of both Δcoa and ΔcoaΔvwbp in human blood (Fig. 5). This trend held for both time points analyzed in our experiment. These data indicate that coagulases function as public goods and enhance staphylococcal survival in human blood.
Fig. 5.
Access to coagulases enhances staphylococcal survival in blood. Monocultures of LAC, Δcoa, or ΔcoaΔvwbp; cocultures of LAC and Δcoa, or LAC and ΔcoaΔvwbp were incubated in heparinized human blood for 45 (dark gray) or 90 (light gray) min and bacterial survival assessed by bacteria enumeration of CFUs on selective agar plates. The labels with only one strain listed are monocultures; the labels with two strains listed are cocultures. The survival profiles pertain to the strain listed outside the parentheses, whereas the strain listed inside the parentheses denotes what it was cocultured with. Data generated from three independent trials (*P < 0.05, one-way ANOVA with post hoc Tukey test based on the 90-min data; error bars denote ±SEM; n = 3).
Discussion
A key aspect of this work was the utilization of relevant infection models in identifying coagulases as public goods that can be exploited. Many studies that focus on the sociobiology of biofilms are carried out in broth media that bear little or no clinical relevance (62). Therefore, the contributions of public goods toward the pathogenesis of staphylococcal infections in the presence of ECM components, but not in their absence, are overlooked. Typically, septic thrombi are not considered biofilms, where coagulation and clot formation are regarded as part of the host’s innate defense mechanism. However, these infections often involve aggregates of bacterial cells in a milieu of HDM, with the bacteria harnessing the surrounding ECM proteins to protect themselves. Therefore, with the growing number of biofilm-related infections and emerging knowledge, the term “biofilm” is constantly being redefined to incorporate new findings. S. aureus is one such pathogen that is able to co-opt the physiological coagulation cascade and utilize host proteins as part of its immune evasion and survival strategy during infections. In that sense, we propose that septic thrombi are a form of HDM biofilms that culminate in the presence of select staphylococcal virulence factors. During these events, interactions with the abundant host plasma glycoproteins fibrinogen, fibrin, and fibronectin are particularly important. Although FnbpA, FnbpB, ClfA, Empbp/Emp, and Eap are known to interact with these ECM components, their binding to fibrinogen does not precipitate fibrinogen cleavage and/or fibrin clot formation. The ability to catalytically convert fibrinogen to fibrin and generate clots is attributed to our primary public goods of interest, Coa and vWbp. However, vWbp displays lower binding affinity to ProT than Coa, where the subsequent ProT•vWbp complex displays different catalytic activity than ProT•Coa and generates fibrin monomers at a reduced rate (21). Perhaps the enzyme kinetics explain the disparity in the coagulation of our WLM, where the Δvwbp mutant was able to generate clots within 24 h, but Δcoa did not. Regardless, the fact that both staphylothrombin complexes interact with host fibrinogen and contribute toward clotting is an important attribute.
One of the major hallmarks associated with biofilm infections is their increased tolerance to antimicrobials. In the case of staphylococcal infections, one possible explanation for this common theme is the presence of fibrin deposits within the biofilm (63, 64). Using the WLM model, we observed this phenomenon in our antimicrobial tolerance assay (Fig. 3). Most notably, after being cocultured with producers in coagulated WLM, the protection against antimicrobials was also conferred onto the cheats. In addition to the producers’ being fortified, aggregates of cheats were also incorporated within the polymerized fibrin meshwork (Fig. 2A and SI Appendix, Figs. S3A and S4). Antimicrobials are able to rapidly bind planktonic bacteria, but binding biofilm-encased cells requires longer periods of time due to the reduced rate of penetration (65, 66). This allows biofilm-residing cells to physiologically respond to the antimicrobial and adopt a more antimicrobial-tolerant state before killing concentrations of the antibiotics can be achieved (65, 67, 68). The penetrating ability of gentamicin is dependent on its electrical charge and the basophilic HDM poses a challenge for the positively charged antibiotic. Studies have demonstrated that positively charged aminoglycosides such as tobramycin and gentamicin become sequestered in the negatively charged biofilm periphery due to ionic interactions (69, 70). Therefore, the poor efficacy of gentamicin and ethanol in our tolerance assay most likely owes, in part, to their inability to access the fibrin-encased staphylococcal aggregates.
We further demonstrate the benefit conferred upon access to coagulases in human blood as well, where S. aureus must overcome major hurdles posed by the host immune system (71, 72). Monocultures of producers displayed much higher survival rates in human blood compared with monocultures of cheats (Fig. 5). However, the survival of cheats improved dramatically upon being cocultured with producers that were able to generate clots (Fig. 5). Our observations align with other studies that have demonstrated that fibrin-encased bacteria do not activate immune cells and can thus escape phagocytosis (63, 73). Earlier work suggests that Coa is probably responsible for the formation of a fibrin shield directly around bacterial cells, while vWbp induces fibrin formation toward the periphery of the staphylococcal community (74, 75). We surmise that the fibrinogen and fibrin coat formed in the immediate vicinity prevents attachment of complement proteins and antimicrobial peptides, whereas those formed distal to the staphylococcal aggregates inhibit opsonin recognition by immune cells and act as a physical obstacle for incoming phagocytes. Most antimicrobial peptides circulating in blood have a positive charge that allows them to bind the negatively charged cell surfaces of bacteria. However, akin to the same way gentamicin becomes sequestered, the positively charged antimicrobials most likely become sequestered in the negatively charged biofilm as well. Together, these barriers generated by Coa and vWbp interfere with complement activation, binding of antimicrobials, formation of membrane attack complexes, and opsonophagocytosis of the fibrin-encased staphylococci.
Of course, the benefits of the ensuing coagulation product rely on the fact that the cheats situate themselves in close proximity to the producers (Fig. 2B and SI Appendix, Fig. S3B), where the concentration of public goods is likely to be high. This phenomenon is evidenced in our micrographs, where clumps of both cheats and producers were incorporated into the fibrin meshwork (Fig. 2A and SI Appendix, Figs. S3A and S4). Coaggregation allows cheats to be situated near the public goods and thereby maximize their benefit and exploitation of the secreted coagulases. Cooperative production of public goods is associated with fitness costs that divert resources away from primary metabolism. Hence, coaggregation can lead to competition for resources between producers and cheats. Monoinfection of LAC harbors only producers that cooperatively secrete coagulases; thus, the costs of producing public goods are shared, whereas in coinfections, which harbor both producers and cheats, Δcoa and ΔcoaΔvwbp benefit from the coagulases secreted by LAC without incurring the costs of producing the public goods themselves. This phenomenon is evident in our relative fitness assay (Fig. 4), where cheats increase in frequency, as they outcompete the cooperative producers (PBS). However, the repeated exposure to gentamicin defers a tragedy of the commons, where the producers retain the majority (Fig. 4). Still, when producers become more common, there is more opportunity for the cheats to exploit cooperators and benefit, as indicated by the increasing relative fitness of the cheats as their starting frequency decreases.
Our results suggest that the population dynamics are regulated by spatial organization and limited dispersal (population viscosity) (76), rather than active communication and coordination among constituent members. Even though coaggregation occurs, clumping is what ensures that relatives are kept close together in S. aureus; in doing so the indiscriminate acts of sharing public goods have a higher probability of benefitting relatives. This is evidenced in our micrographs, where the cheats and producers are situated closer to themselves than they are to each other (SI Appendix, Figs. S5 and S6). The immediate clumping neighbor will benefit more than an aggregate population situated further away simply because more coagulases and the subsequent fibrin product will be readily accessible to them. Perhaps this is a possible explanation as to how cheats are kept in check during infections where coagulases provide a benefit against host immune factors. Cheats can coreside and reap the benefits of being in a clot without incurring any of the costs of producing coagulases, but they do not outcompete the producers due to limited dispersal. In this sense, limited dispersal can be highly conducive to the secretion of coagulases where active kin recognition mechanisms may not be essential for maintaining cooperation (77). Thus, during infection where immune factors and antimicrobials are present, coagulases are social and cheatable public goods, but overexploitation does not occur as explained by Hamilton’s theory on limited dispersal (76).
Materials and Methods
Bacterial Strains and Growth Conditions.
USA300 LAC and mutants in this background, Δcoa and ΔcoaΔvwbp, were used for all experiments unless otherwise stated. Nonfluorescent strains were used in experiments unless otherwise stated. Strains were grown in Tryptic soy broth (TSB) with 100 μg/mL rifampicin or streptomycin before inoculation of WLM or human blood. Enumeration of CFUs was done on selective Tryptic soy agar (TSA) plates infused with 20 μg/mL rifampicin or streptomycin.
Construction of Strains.
Regions upstream and downstream of coa were amplified using primers HC375/HC376 and HC377/HC378, and the pJB38 (78) backbone was amplified using primers HC367/HC368. The resulting fragments were fused using the Gibson assembly master mix (New England Biolabs), generating plasmid pHC83. A plasmid for deleting vwbp, pHC85, was generated by the same method, using primers HC387/HC370 and HC371/HC372. Deletion plasmid were electroporated into S. aureus RN4220, selecting on TSA plates containing chloramphenicol (10 μg/mL) at 30 °C. The plasmids were then transduced into S. aureus strain LAC. Individual colonies were streaked on TSA Cam plates incubated at 42 °C to select for integration into the chromosome. Single colonies were grown in TSB at 30 °C and diluted 1:500 in fresh media for four successive days before diluting to 10−6 and plating on TSA containing 0.3 μg/mL anhydrotetracycline to select for loss of the plasmid. Colonies were screened for sensitivity to Cam, and CamS colonies were screened by PCR for deletion of coa or vwbp. Refer to SI Appendix, Table S1 for oligonucleotide sequences of primers used.
Fluorescent Tagging of Strains.
USA300 LAC wild type (AH1263) was tagged with green fluorescence by transferring plasmid pCM11 (79) carrying sGFP from strain AH1331, yielding strain JH992. Likewise, Δcoa (AH4035) was tagged by transferring pCM11, yielding strain JH994. The plasmids were transferred between strains using standard phage transduction protocols outlined by Olson (80).
Screening Phenotypes Using the WLM.
The WLM consisted of 45% Bolton broth, 50% bovine plasma (heparinized), and 5% laked horse blood. Glass 16- × 100-mm test tubes with caps were autoclaved, and 4 mL of WLM was aseptically dispensed into each tube. The overnight TSB cultures were centrifuged for 10 min at 6,300 × g and resuspended in the same volume of 1× PBS, repeated twice to rinse all antibiotics. The optical densities of all overnight cultures were normalized for all of the strains. The glass tubes were then inoculated with 10 μL of 104 to 105 CFU/mL of S. aureus. The tubes were then incubated at 37 °C for 24 h. S. aureus Newman and mutants in this background, Δeap and ΔsaePQRS, were acquired from University of Leicester and prepared as outlined in Guggenberger et al. (75). A plasmid-cured version of USA300 LAC (JE2) and transposon mutants in this background, fnbpA::Tn, fnbpB::Tn, clfA::Tn and empbp::Tn, and coa::Tn, were retrieved from the Nebraska Transposon Mutant Library (81).
Staining and Imaging.
WLM was prepared in the same manner as described above, with the exception of a 460-μL volume being placed in a 5-cm × 0.5-cm glass tube and inoculated with 7.5 μL of 104 to 105 CFU/mL of S. aureus. Imaging was performed on frozen sample sections. Briefly, coagulated WLM was removed from tubes after 18 h, placed in a Tissue-Tek vinyl specimen Cryomold (Sakura Finetek) containing a cryomatrix of OCT (optimum cutting temperature) compound (Thermo Fisher Scientific), and then immediately placed in a freezer at −80 °C to allow the OCT compound to solidify. Frozen sections were securely anchored using deep-waffled, large-face block holders (Electron Microscopy Sciences). Frozen OCT-embedded samples were sectioned using an OTF5000 cryostat (Bright Instrument Co., Ltd.) to a thickness of 6–8 μm and were then directly transferred to Superfrost Plus microscope slides (Thermo Fisher Scientific) and stored at −20 °C until ready for visualization. Frozen sample sections were prepared for staining by air drying at room temperature for 5 min, washed three times in 1× PBS (2 min each time), fixed in 4% paraformaldehyde at room temperature for 15 min, washed three times in 1× PBS (2 min each time), and allowed to air-dry at room temperature for 5 min before addition of the stain. Matrix components were visualized by staining sections with 100 μg/mL Texas Red-conjugated Con A (Invitrogen) in the dark for 5 min at room temperature, washing three times in 1× PBS (5 min each time), and then mounting with Prolong Gold Antifade reagent (Molecular Probes) supplemented with DAPI to stain DNA, sealed with a coverglass (Fisher-brand), and then analyzed by confocal laser scanning microscopy.
All samples were visualized using an A1 confocal (Nikon) on a Ti-E inverted microscope (Nikon) equipped with CFI Plan Fluor 40× oil objective/N.A. 1.3 (differential interface contrast N2) (Nikon). Images were acquired using an N-STORM superresolution iXon Ultra 897 electron multiplying CCD camera (Andor) controlled with NIS-Elements Ar software version 4.51.01 (Nikon). For each sample, multiple Z stacks were taken from distinct areas within the clots, and optical sections within each Z stack were collected using a step size of 0.3 μm. All instrument settings were uniformly consistent and maintained between each set of experimental conditions. Z-stack images were then adjusted for γ, brightness, and contrast (identically for compared image sets) using NIS-Elements Ar software version 4.50.00 (Nikon) before spatial distribution analysis.
Image Analyses.
All image analyses were performed with R 3.4.3 (82). Thresholds were set for each channel with Otsu’s method (83). A dilation algorithm was applied to correct for the GFP signal being weaker than the DAPI signal; blue pixels within a three-pixel distance from a green pixel were designated as green. See representative dilated images in SI Appendix, Fig. S8. Dilation was done using a seven-pixel-diameter disk-formed mathematical kernel implemented in the mmand package (84). Occupancy was determined using an algorithm described in Liu et al. (85), but in two dimensions. Briefly, 10,000 random pixels of the focal channel were picked. Pixels within 100 μm of the focal pixel were grouped in distance bins to the nearest even integer. For each distance bin, the number of target pixels and total pixels was determined. The counts for the 10,000 pixels were then aggregated. The occupancy was defined as the fraction of target pixels in each distance bin normalized to the fraction of target pixels in the entire image, such that an image with randomly distributed pixels would have an occupancy of 1 at any distance (log2 = 0). Analyses without the dilation algorithm resulted in similar trends but a higher degree of occupancy of nonproducers in close proximity to the producers (SI Appendix, Fig. S7).
Antimicrobial Tolerance Assay.
A 4-mL volume of WLM media was prepared as described above. Sections of coagulated WLM or 460 μL uncoagulated planktonic WLM cultures were suspended in 1 mL of 300 μg/mL gentamicin, 35% ethanol, or 1× PBS for 5 h. Samples were then centrifuged and resuspended in 1 mL of Dey–Engley broth and allowed to sit for 10 min, after which they were centrifuged and resuspended in 1 mL of 1× PBS, homogenized, vortexed, serially diluted, and plated on selective TSA for enumeration of CFUs. CFU per gram was calculated for each sample. Even though Dey–Engley broth is frequently used to neutralize antiseptics to avoid false-negative results due to drug carryover, to our knowledge its neutralizing properties do not extend to antibiotics. Therefore, in our assay, it was used as a rinsing agent.
Relative Fitness Assay.
A 4-mL volume of WLM media was prepared as described above. Sections of coagulated WLM were suspended in 1 mL of 1× PBS for 5 h, homogenized, vortexed, serially diluted, and plated on selective TSA for enumeration of CFUs. CFU per gram was calculated for each sample. The 10−2 dilution (∼104 – 105 CFU/mL) of the WLM homogenate was used to inoculate fresh WLM; this procedure was repeated every 18 h, while propagating the bacteria through multiple days of culturing.
A similar setup was used for the gentamicin-treated rounds, with the exception that the sections of coagulated WLM were suspended in 300 μg/mL gentamicin for 5 h. Samples were then centrifuged and resuspended in 1 mL of Dey–Engley broth and allowed to sit for 10 min, after which they were centrifuged and resuspended in 1 mL of 1× PBS, homogenized, vortexed, serially diluted, and plated on selective TSA for enumeration of CFUs. CFU per gram was calculated for each sample. The homogenate was used to inoculate fresh WLM for the following day; this procedure was repeated at every 18 h, while propagating the bacteria through multiple days of culturing. The relative fitness (w) was calculated as , where ; the dilution factor accounts for the homogenate being inoculated into fresh WLM each day.
Blood Survival Assay.
Overnight cultures were centrifuged for 10 min at 6,300 × g and resuspended in the same volume of 1× PBS, repeated twice to rinse all antibiotics. The optical densities of all cultures were normalized for all of the strains to generate a suspension of 1 × 107 CFU/mL. Fresh whole blood was collected from consenting human volunteers by venous puncture using a 0.6- × 19- × 305-mm and 23-gauge × 3/4-inch × 12-inch push-button blood collection set (Becton Dickinson); 4 mL of blood was collected into 13- × 75-mm vacutainers with 75 USP units of freeze-dried sodium heparin (Becton Dickinson). Then, 450 μL of blood was aliquoted into a 1-mL Eppendorf tube and inoculated with 50 μL of bacteria sample (1 × 105 CFU/mL). Samples were incubated at 37 °C with slow rotation. One hundred-microliter aliquots were removed at times 0, 45, and 90 min, mixed 1:1 with fresh 2% saponin/PBS, and incubated on ice for 30 min. Five 1:10 serial dilutions were prepared and 100-μL aliquots spread on selective TSA for enumeration of CFUs.
Ethics Statement.
The protocol for venous blood collection was approved by the Texas Tech University Health Sciences Center (TTUHSC) Institutional Review Board. Consent was obtained from healthy volunteers as mandated by the Clinical Research Institute at TTUHSC in compliance with ethical practices. No clinically admitted patients or children were involved in this study.
Supplementary Material
Acknowledgments
We thank Lauren Choate and Anette Løth for technical assistance. Confocal images were generated in the Image Analysis Core Facility supported in part by Texas Tech University Health Sciences Center. This work was supported by the Danish Council for Independent Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804850115/-/DCSupplemental.
References
- 1.O’Grady NP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR Recomm Rep. 2002;51:1–29. [PubMed] [Google Scholar]
- 2.Registered Nurses’ Association of Ontario 2004. Assessment and device selection for vascular access (Registered Nurses’ Association of Ontario, Toronto)
- 3.Registered Nurses’ Association of Ontario 2005. Care and maintencance to reduce vascular access complications (Registered Nurses’ Association of Ontario, Toronto)
- 4.Climo M, et al. Prevalence of the use of central venous access devices within and outside of the intensive care unit: Results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention. Infect Control Hosp Epidemiol. 2003;24:942–945. doi: 10.1086/502163. [DOI] [PubMed] [Google Scholar]
- 5.O’Grady NP, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimick JB, et al. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg. 2001;136:229–234. doi: 10.1001/archsurg.136.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Warren DK, et al. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006;34:2084–2089. doi: 10.1097/01.CCM.0000227648.15804.2D. [DOI] [PubMed] [Google Scholar]
- 8.Blot SI, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591–1598. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 9.Renaud B, Brun-Buisson C. ICU-Bacteremia Study Group Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584–1590. doi: 10.1164/ajrccm.163.7.9912080. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Vital signs: Central line-associated blood stream infections–United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–248. [PubMed] [Google Scholar]
- 11.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893–898. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- 13.Mehall JR, Saltzman DA, Jackson RJ, Smith SD. Fibrin sheath enhances central venous catheter infection. Crit Care Med. 2002;30:908–912. doi: 10.1097/00003246-200204000-00033. [DOI] [PubMed] [Google Scholar]
- 14.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 15.Rumbaugh KP, et al. Kin selection, quorum sensing and virulence in pathogenic bacteria. Proc R Soc B. 2012;279:3584–3588. doi: 10.1098/rspb.2012.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 17.Madsen JS, et al. Facultative control of matrix production optimizes competitive fitness in Pseudomonas aeruginosa PA14 biofilm models. Appl Environ Microbiol. 2015;81:8414–8426. doi: 10.1128/AEM.02628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckling A, et al. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol Ecol. 2007;62:135–141. doi: 10.1111/j.1574-6941.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 19.Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 20.Pollitt EJ, West SA, Crusz SA, Burton-Chellew MN, Diggle SP. Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infect Immun. 2014;82:1045–1051. doi: 10.1128/IAI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc Natl Acad Sci USA. 2009;106:7786–7791. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. J Biol Chem. 2013;288:28283–28292. doi: 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiśniewska K, Garbacz K, Piechowicz L. Genotypic screening of atypical Staphylococcus aureus strains isolated from clinical samples for presence of selected adhesin genes. Med Mal Infect. 2008;38:549–553. doi: 10.1016/j.medmal.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Młynarczyk G, et al. Coagulase-negative variants of methicillin-resistant Staphylococcus aureus subsp. aureus strains isolated from hospital specimens. Zentralbl Bakteriol. 1998;288:373–381. doi: 10.1016/s0934-8840(98)80010-8. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KR, Roberson J, Gillespie BE, Luther DA, Oliver SP. Identification and differentiation of coagulase-negative Staphylococcus aureus by polymerase chain reaction. J Food Prot. 1997;60:686–688. doi: 10.4315/0362-028X-60.6.686. [DOI] [PubMed] [Google Scholar]
- 26.Rotun SS, et al. Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg Infect Dis. 1999;5:147–149. doi: 10.3201/eid0501.990118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonsale N, et al. Specific identification of Staphylococcus aureus by Staphychrom II, a rapid chromogenic staphylocoagulase test. J Clin Microbiol. 2004;42:1962–1964. doi: 10.1128/JCM.42.5.1962-1964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malinowski E, Lassa H, Klossowska A, Smulski S, Kaczmarowski M. Atypical Staphylococcus aureus as an aetiological agent of mastitis in cows. Bull Vet Inst Pulawy. 2009;53:383–387. [Google Scholar]
- 29.Vandenesch F, et al. Coagulase deficiency in clinical isolates of Staphylococcus aureus involves both transcriptional and post-transcriptional defects. J Med Microbiol. 1994;40:344–349. doi: 10.1099/00222615-40-5-344. [DOI] [PubMed] [Google Scholar]
- 30.Fox LK, Besser TE, Jackson SM. Evaluation of a coagulase-negative variant of Staphylococcus aureus as a cause of intramammary infections in a herd of dairy cattle. J Am Vet Med Assoc. 1996;209:1143–1146. [PubMed] [Google Scholar]
- 31.Sun Y, Dowd SE, Smith E, Rhoads DD, Wolcott RD. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008;16:805–813. doi: 10.1111/j.1524-475X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi U, et al. A post-planktonic era of in vitro infectious models: Issues and changes addressed by a clinically relevant wound like media. Crit Rev Microbiol. 2016;43:453–465. doi: 10.1080/1040841X.2016.1252312. [DOI] [PubMed] [Google Scholar]
- 33.Dalton T, et al. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One. 2011;6:e27317. doi: 10.1371/journal.pone.0027317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Smith E, Wolcott R, Dowd SE. Propagation of anaerobic bacteria within an aerobic multi-species chronic wound biofilm model. J Wound Care. 2009;18:426–431. doi: 10.12968/jowc.2009.18.10.44604. [DOI] [PubMed] [Google Scholar]
- 35.Dowd SE, et al. Effects of biofilm treatments on the multi-species Lubbock chronic wound biofilm model. J Wound Care. 2009;18:508, 510–512. doi: 10.12968/jowc.2009.18.12.45608. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- 37.Mainiero M, et al. Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J Bacteriol. 2010;192:613–623. doi: 10.1128/JB.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fröman G, Switalski LM, Speziale P, Höök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987;262:6564–6571. [PubMed] [Google Scholar]
- 39.Jönsson K, Signäs C, Müller HP, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 40.Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 41.Hussain M, et al. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J Bacteriol. 2001;183:6778–6786. doi: 10.1128/JB.183.23.6778-6786.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jönsson K, McDevitt D, McGavin MH, Patti JM, Höök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 43.McGavin MH, Krajewska-Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedrich R, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 45.Bjerketorp J, Jacobsson K, Frykberg L. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234:309–314. doi: 10.1016/j.femsle.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 46.Harraghy N, et al. Sae is essential for expression of the staphylococcal adhesins Eap and Emp. Microbiology. 2005;151:1789–1800. doi: 10.1099/mic.0.27902-0. [DOI] [PubMed] [Google Scholar]
- 47.Goerke C, et al. Role of Staphylococcus aureus global regulators sae and sigmaB in virulence gene expression during device-related infection. Infect Immun. 2005;73:3415–3421. doi: 10.1128/IAI.73.6.3415-3421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crosby HA, Kwiecinski J, Horswill AR. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host-pathogen interactions. Adv Appl Microbiol. 2016;96:1–41. doi: 10.1016/bs.aambs.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baddour LM, Tayidi MM, Walker E, McDevitt D, Foster TJ. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. J Med Microbiol. 1994;41:259–263. doi: 10.1099/00222615-41-4-259. [DOI] [PubMed] [Google Scholar]
- 50.Claes J, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–1676. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjarnsholt T, et al. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Kirketerp-Møller K, et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol. 2008;46:2717–2722. doi: 10.1128/JCM.00501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marrie TJ, Nelligan J, Costerton JW. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982;66:1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- 54.Marrie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984;19:687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marrie TJ, Noble MA, Costerton JW. Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol. 1983;18:1388–1398. doi: 10.1128/jcm.18.6.1388-1398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoodley P, et al. Direct demonstration of Staphylococcus biofilm in an external ventricular drain in a patient with a history of recurrent ventriculoperitoneal shunt failure. Pediatr Neurosurg. 2010;46:127–132. doi: 10.1159/000319396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen LD, et al. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology. 2007;153:2312–2320. doi: 10.1099/mic.0.2007/006122-0. [DOI] [PubMed] [Google Scholar]
- 58.Watters C, et al. Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol (Berl) 2013;202:131–141. doi: 10.1007/s00430-012-0277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson BJ, Kim Y, Peterson PK, Quie PG, Michael AF. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978;20:388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verbrugh HA, Van Dijk WC, Peters R, Van Der Tol ME, Verhoef J. The role of Staphylococcus aureus cell-wall peptidoglycan, teichoic acid and protein A in the processes of complement activation and opsonization. Immunology. 1979;37:615–621. [PMC free article] [PubMed] [Google Scholar]
- 61.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 62.Roberts AEL, Kragh KN, Bjarnsholt T, Diggle SP. The limitations of in vitro experimentation in understanding biofilms and chronic infection. J Mol Biol. 2015;427:3646–3661. doi: 10.1016/j.jmb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Kwiecinski J, et al. Staphylokinase control of Staphylococcus aureus biofilm formation and detachment through host plasminogen activation. J Infect Dis. 2016;213:139–148. doi: 10.1093/infdis/jiv360. [DOI] [PubMed] [Google Scholar]
- 64.Vanassche T, et al. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J Infect Dis. 2013;208:92–100. doi: 10.1093/infdis/jit130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2005;49:2467–2473. doi: 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 67.Szomolay B, Klapper I, Dockery J, Stewart PS. Adaptive responses to antimicrobial agents in biofilms. Environ Microbiol. 2005;7:1186–1191. doi: 10.1111/j.1462-2920.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 68.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 69.Tseng BS, et al. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol. 2013;15:2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatch RA, Schiller NL. Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:974–977. doi: 10.1128/aac.42.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 72.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 73.Vanassche T, et al. Inhibition of staphylothrombin by dabigatran reduces Staphylococcus aureus virulence. J Thromb Haemost. 2011;9:2436–2446. doi: 10.1111/j.1538-7836.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 74.Thomer L, et al. Antibodies against a secreted product of Staphylococcus aureus trigger phagocytic killing. J Exp Med. 2016;213:293–301. doi: 10.1084/jem.20150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guggenberger C, Wolz C, Morrissey JA, Heesemann J. Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS Pathog. 2012;8:e1002434. doi: 10.1371/journal.ppat.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 77.Nadell CD, Foster KR, Xavier JB. Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol. 2010;6:e1000716. doi: 10.1371/journal.pcbi.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bose JL, Fey PD, Bayles KW. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol. 2013;79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28:55–61. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 80.Olson ME. Bacteriophage transduction in Staphylococcus aureus. In: Bose JL, editor. The Genetic Manipulation of Staphylococci: Methods and Protocols. Springer; New York: 2016. pp. 69–74. [Google Scholar]
- 81.Fey PD, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4:e00537-e12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.R Core Team 2017. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
- 83.Otsu N. Threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern Syst. 1979;9:62–66. [Google Scholar]
- 84.Clayden J. 2017. mmand: Mathematical Morphology in Any Number of Dimensions, R Package Version 1.5.3.
- 85.Liu W, Russel J, Burmølle M, Sørensen SJ, Madsen JS. Micro-scale intermixing: A requisite for stable and synergistic co-establishment in a four-species biofilm. ISME J. 2018;12:1940–1951. doi: 10.1038/s41396-018-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.