Abstract
Background & Aims
Most patients with esophageal adenocarcinoma (EAC) present de novo. Although this may be due to inadequate screening strategies, the precise reason for this observation is not clear. We compared survival of patients with prevalent EAC with and without synchronous BE/intestinal metaplasia of the esophagus (IM) at the time of EAC diagnosis.
Methods
Clinical data were studied using Cox Proportional Hazards regression to evaluate the effect of synchronous BE/IM on EAC survival independent of age, sex, TNM stage and tumor location. Two cohorts from the Mayo Clinic and a U.K.
multicenter prospective cohort were included.
Results
The Mayo cohort had 411 EAC patients with 49.3% with BE/IM demonstrating a survival benefit as compared to those without (hazard ratio (HR), 0.44; 95% CI: 0.34 – 0.57, P<0.001). In a multivariable analysis BE/IM was associated with better survival independent of age, sex, stage and tumor location and length (adjusted HR: 0.66, 95% CI: 0.5–0.88, P=0.005). The UK cohort contained 1417 patients, 45% with BE/IM demonstrating a survival benefit as compared with non-BE/IM patients (HR 0.59, 95% CI: 0.5-0.69, P<0.001) with continued significance in multivariable analysis that included age, sex, stage, and tumor location (adjusted HR 0.77, 95% CI: 0.64-0.93, P=0.006).
Conclusion
Two types of esophageal adenocarcinoma can be characterized based on the presence or absence of Barrett’s epithelium. These findings have implications for understanding the etiology of EAC and determining prognosis as well as for development of optimal clinical strategies to identify patients at risk.
Keywords: Esophageal adenocarcinoma, Barrett’s esophagus, Survival, Esophagus
Background
Esophageal adenocarcinoma (EAC) is a major public health concern due to rapidly increasing incidence rates and with fewer than 20% patients surviving beyond five years. One of the most well defined risk factors is the presence of the precursor lesion Barrett’s Esophagus (BE) which may occur on the background of heartburn symptoms or remain clinically silent. There has therefore been a concerted effort over several decades to identify and monitor patients with BE characterized by intestinal metaplasia1. However, the overwhelming majority of patients who develop EAC present de novo2 and therefore do not benefit from endoscopic surveillance programs3–5. Research efforts have therefore focused on finding easily identifiable factors that might select an at risk group for more systematic screening 6–10. Similarly, researchers have been developing less expensive and easier to use screening devices and biomarker assays applied to biopsies, cytology specimens and blood samples 11, 12, 13.
It has been assumed that the reflux-induced, inflammation to cancer sequence is similar across all patients who develop EAC such that identification of BE would give us years of surveillance to treat incident dysplasia or cancer before the development of incurable adenocarcinoma. However, in some patients who develop EAC it has been observed that no Barrett’s is present at the time of surgical resection 14. In these patients, it is assumed that the cancer grows over and/or replaces the previously extant metaplasia.
However, another quite distinct explanation could be possible. If a more rapidly evolving and/or aggressive form of EAC developed from a small and easily missed area of esophageal metaplastic epithelium, or if the stage of intestinalization between inflammation and cancer was more ephemeral, less prominent or absent14,15, 25 attenuating identification with our current screening strategies, then a large proportion of prevalent EAC could be explained. In keeping with this there is some molecular evidence that catastrophic genomic events such as chromothripsis could conceivably lead to more rapid progression to cancer15, 16.
We thus hypothesized that there might be a group of patients with EAC without co-existing Barrett’s Esophagus/IM (non- BE/IM) at the time of cancer diagnosis who have a more aggressive form of EAC which might lead to a poorer prognosis when compared to those with prevalent Barrett’s Esophagus/IM (BE/IM). To address this question we compared the clinico-pathological characteristics and survival in two types of patients: those who present with EAC in the context of histologic and/or endoscopically identifiable IM of the esophagus to those who present with adenocarcinoma without identifiable intestinal metaplasia (non-BE/IM). This question was evaluated in two distinct but contemporaneous cohorts for whom we had high quality pathological and outcome data available.
Methods
Study population
Two cohorts were independently collected and analyzed. At the Mayo Clinic, Rochester all patients with a diagnosis of EAC treated in the years 2011-2012 were included regardless of the stage and treatment modality and a retrospective analysis was conducted. These years were selected to allow us to estimate 5 years mortality. The study was approved by the Mayo Clinic Institutional Review Board (IRB: 17-003675 on 5/15/2017). A prospective multicenter cohort was studied from the UK Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS). The UK based consortium was set-up in 2010 in order to prospectively collect clinical and molecular data to inform patient management strategies and as the vehicle for whole-genome sequencing data as part of the International Cancer Genome Consortium. The study was registered (UKCRNID 8880) and approved by the relevant institutional ethics committees (REC 07/H0305/52 and 10/H0305/1) and all subjects provided individual informed consent. This analysis included all OCCAMS patients diagnosed with EAC from 2002-2017 from 25 sites across the UK, all stages of disease, all treatment modalities, and all locations as reported by Siewert classifications 17, and follow-up for all patients is up to 5 years.
Although these two cohorts were studied independently, the following common methodology was employed. Patients were excluded for the following reasons: esophageal squamous cell carcinoma (Mayo n=75, OCCAMS n=122), non-primary esophageal cancer (Mayo n=17, OCCAMS n=37), and absence of pathology specimens (Mayo n=12). For this study we are interested in all EAC cases presenting outside of Barrett’s surveillance programs, because surveillance detected cases are likely to be biased by being earlier stage. Patients with EAC detected in a surveillance program more than one year after identification of Barrett’s esophagus were excluded from the Mayo cohort (n=97). The OCCAMS cohort includes 135 patients who self-reported being in surveillance for Barrett’s as there was no information regarding the timeline for Barrett’s diagnosis and subsequent surveillance. These are evaluated for their effect on survival in the results section. All patients had BE/IM at the time of their cancer diagnosis.
Pathology Review
A strict expert pathology review was performed by one specialized gastrointestinal pathologist with over 30 years of experience in the Mayo clinic Cohort (T.C.S). In the OCCAMS cohort pathology was reviewed by two or more pathologists for all cases: the upper-gastrointestinal pathologist at the referring hospital followed by review performed by the OCCAMS central study upper-GI pathologist with over 20 years of experience. The pathology reviews were abstracted from prior interpretation.
The T, N, M cancer stage were assigned in both cohorts according to the American Joint Committee on Cancer (AJCC) 7th edition18 using the available information in the medical record including clinical notes, endoscopic ultrasound (EUS), positron emission tomography (PET), endoscopic mucosal resection (EMR) and histopathological evaluation following surgical resection. In patients who underwent neoadjuvant therapy prior to surgical resection, the most advanced stage identified either prior to treatment or at the time of surgical resection was used.
Barrett’s esophagus was defined by visual changes identified endoscopically in the pre-staging evaluation together with pathology demonstrating IM at time of surgical resection when reviewed by expert GI pathologists in the recruiting hospitals. IM was also identified in cases without macroscopic evidence of Barrett’s upon expert review of the pathology specimen. In a small group of patients who were treated with chemo-radiation therapy without endoscopic or surgical resection, BE/IM was ascertained using visual endoscopic appearance and endoscopic biopsies from the tumor. The reviewing pathologists followed a specific synoptic report proposed by the College of American Pathologists (www.cap.org/cancerprotocols) and the OCCAMS Consortium protocol in which both require the proximal and distal resection margins and tumor to be thoroughly evaluated for BE. In addition, extensive sampling is performed for all tumor borders of the resected esophagus/tumor and the tumor bed making sampling error less likely. The number of biopsies varied based on the tumor size.
Statistical Analysis
Baseline characteristics were compared between the two groups using chi-square χ 2 for categorical variables and student t-test for continuous ones. The primary outcome was overall survival. Survival in the Mayo cohort was ascertained from the date of diagnosis to death or the date of data collection (administrative censoring), using online death records from U.S. Social Security Death Index. Survival time in the OCCAMS Consortium was evaluated from the date of diagnosis to the date the patient was last seen in clinic, or the date of death. Survival was plotted using a Kaplan-Meier curve with statistical comparison between the two groups using the log-rank test. A Cox proportional hazard regression model was used to examine the impact of BE/IM on overall survival. Unadjusted and adjusted hazard ratios were calculated adjusting for possible predefined confounders. In both cohorts age and sex were included.
Predefined subgroup analyses were performed to examine if the survival effect of BE/IM was instead driven by the differential survival seen by stage, treatment, chemo-sensitivity, or due to the location of the tumor (i.e. IM being more likely to be involved due to cardia involvement). Each cohort was independently evaluated for these subgroups based on the specific data available. Two sided P<0.05 was considered statistically significant. A final multivariable model is evaluated in both cohorts that includes all significant subgroups, age, and sex.
Missing value imputation was performed on the UK cohort to evaluate how these missing data may be influencing the survival effects. The missing values for variables (e.g. TNM stages, Siewert class, age at diagnosis, sex) were imputed using a multivariable chained equations method that applied random forest to categorical variables, and used the distribution means for continuous variables19. Imputation was performed for 20 iterations with the Cox multivariable regression model evaluated on each iteration. We report on the mean values of the Cox regression model iterations. The imputed data was not used when reporting the primary or final Cox regression analyses. No imputation was performed on the Mayo Cohort.
All analysis on the Mayo cohort was performed using STATA 14.0 (College Station, Texas, USA). All analysis on the OCCAMS cohort was performed using R v3.4.3, with the packages ‘survival’ v2.41-3, ‘coxme’ v2.2-7, ‘mice’ v.3.0.0, and ‘survminer’ v0.4.
Results
Patient Characteristics
Retrospective cohort Mayo Clinic
There were 411 patients with prevalent EAC treated in the Mayo Clinic during the years 2011 and 2012 who met the inclusion criteria. 204 patients (49.3%) had evidence of associated IM with or without a macroscopically visible BE segment, leaving 207 with no associated IM. The mean age was 64.0 ± 10.7 years (interquartile range IQR: 57 -72 years) without a meaningful clinical difference between the two groups (P=0.06). The cohort was male predominant 85.2% as expected for this disease. The BE/IM and non-BE/IM group were similar in terms of BMI, family history of esophageal cancer and smoking (P>0.05) (Table 1).
Table 1.
Baseline characteristics of included patients, comparing patients with identified BE/IM to those without BE/IM in the Mayo Clinic (A) and OCCAMS cohort (B)
| (A) Mayo | (B) OCCAMS | |||||||
|---|---|---|---|---|---|---|---|---|
| BE/IM, n=204 | Non-BE/IM, n=207 | P value | Total, n=411 | BE/IM n=634 | Non-BE/IM, n=783 | P value | Total n=1417 | |
| Age at diagnosis, mean years (SD) | 65 (10.4) | 63 (11) | 0.02 | 64 (10.7) | 67 (9) | 66 (9.8) | 0.06 | |
| Male, n (%) | 172 (84) | 178 (86) | 0.6 | 350 (85.2) | 539 (85) | 640 (81.7) | 0.1 | 1179 (83.2) |
| BMI, mean (SD, IQR) | 29.4 (5, 26-32) | 29 (5.4, 25-33) | 0.6 | 29 (5.3, 25-32) | 28 (5, 25-31) | 27 (4.8, 24-29) | < 0.001 | |
| History or current Smoking, n (%) | 128 (63) | 128 (62) | 0.2 | 256 (62.3) | 362 (57.1) | 456 (58.2) | 0.07 | 818 (57.7) |
| Family history of EAC, n (%) | 9 (4.4) | 11 (5.3) | 0.6 | 20 (5) | 39 (6.2) | 39 (5) | 0.662 | 78 (5.5) |
| TNM Stage, n (%) | <0.001 | < 0.001 | ||||||
| I | 62 (30.4) | 10 (4.8) | 72 (17.5) | 53 (8.4) | 17 (2.2) | 70 (4.9) | ||
| II | 55 (27) | 37 (17.9) | 92 (22.4) | 323 (50.9) | 339 (43.3) | 662 (46.7) | ||
| III | 61 (29.9) | 93 (44.9) | 154 (37.5) | 195 (30.8) | 285 (36.4) | 480 (33.9) | ||
| IV | 26 (12.7) | 67 (32.4) | 93 (22.6) | 8 (1.3) | 46 (5.9) | 54 (3.8) | ||
| Missing | 0 | 0 | 0 | 55 (8.7) | 96 (12.3) | 151 (10.7) | ||
| T stage, n (%) | <0.001 | <0.001 | ||||||
| T0 | 0 | 0 | 0 | 10 (1.6) | 3 (0.4) | 13 (0.9) | ||
| T1 | 66 (32.4) | 11 (5.3) | 77 (18.7) | 70 (11) | 21 (2.7) | 91 (6.4) | ||
| T2 | 41 (20.1) | 23 (11.1) | 64 (15.6) | 86 (13.6) | 60 (7.7) | 146 (10.3) | ||
| T3 | 46 (37.3) | 107 (51.7) | 183 (44.5) | 380 (59.9) | 511 (65.3) | 891 (62.9) | ||
| T4 | 1 (0.5) | 4 (1.9) | 5 (1.2) | 34 (5.4) | 95 (12.1) | 129 (9.1) | ||
| Missing | 20 (9.8) | 62 (30) | 82 (20) | 52 (8.2) | 93 (11.9) | 145 (10.2) | ||
| N stage, n (%) | <0.001 | < 0.001 | ||||||
| N0 | 89 (43.6) | 32 (15.5) | 121 (29.4) | 155 (24.4) | 146 (18.6) | 301 (21.2) | ||
| N1 | 77 (37.8) | 84 (40.6) | 161 (39.2) | 205 (32.3) | 230 (29.4) | 435 (30.7) | ||
| N2 | 12 (5.9) | 20 (9.7) | 32 (7.8) | 139 (21.9) | 190 (24.3) | 329 (23.2) | ||
| N3 | 5 (2.5) | 8 (3.9) | 13 (3.2) | 76 (12) | 139 (17.8) | 215 (15.2) | ||
| Missing | 21 (10.3) | 63 (30.4) | 84 (20.4) | 53 (8.4) | 78 (10) | 131 (9.2) | ||
| Location (Siewert class), n (%) | 0.8 | < 0.001 | ||||||
| 1 (Distal 1-5cm above EGJ) | 109 (53.4) | 117 (56.5) | 226 (55) | 222 (35) | 173 (22.1) | 395 (27.9) | ||
| 2 (1cm above- 2cm below EGJ) | 86 (42.2) | 80 (38.6) | 166 (40.4) | 213 (33.6) | 288 (36.8) | 501 (35.4) | ||
| 3 (2-5 cm below EGJ) | -- | -- | -- | 50 (7.9) | 125 (16) | 175 (12.4) | ||
| Missing | 4 (2) | 6 (2.9) | 10 (2.4) | 149 (23.5) | 197 (25.2) | 346 (24.4) | ||
| Tubular esophagus above 5 cm | 5 (2.4) | 4 (2) | 9 (2.2) | -- | -- | -- | ||
| Treatment, n (%) | <0.001 | < 0.001 | ||||||
| Endoscopic therapy | 37 (18.1) | 2 (1) | 39 (9.5) | -- | -- | -- | ||
| Esophagectomy alone | 31 (15.2) | 10 (4.8) | 41 (9.9) | 137 (21.6) | 114 (14.6) | 251 (17.7) | ||
| Neoadjuvant chemotherapy & esophagectomy | 93 (45.6) | 91 (44) | 184 (44.8) | 416 (65.6) | 518 (66.2) | 934 (65.9) | ||
| Esophagectomy & adjuvant chemo-radiation | 5 (2.4) | 4 (1.9) | 9 (2.2) | -- | -- | -- | ||
| Chemo and/or radiation therapy alone | 29 (14.2) | 79 (38.2) | 108 (26.3) | 25 (3.9) | 76 (9.7) | 101 (7.1) | ||
| Palliative | 4 (2) | 17 (8.2) | 21 (5.1) | 13 (2.1) | 76 (9.7) | 89 (6.3) | ||
| Missing | 5 (2.4) | 4 (1.9) | 9 (2.2) | -- | -- | -- | ||
Prospective Multicenter Cohort OCCAMS Consortium
In the UK multi-center cohort, 1417 patients who had recorded information regarding BE status, stage, chemotherapy, and survival were included. 634 (45 %) had Barret’s adjacent to the tumor, while 783 (55%) did not. The mean age was 66 ± 9.5 years (IQR: 60-73) without a meaningful clinical difference between the groups (P>0.05). The two groups were similar in male predominance (83%), history of smoking, and family history of EAC (P>0.05), though BE/IM patients had a slightly increased BMI (P<0.001) (Table 1).
Lesion Features
Mayo Clinic
Most tumors (n=226, 55%) occurred at the distal esophagus (Siewert Type I) comprising 109 (54.3%) in the BE/IM and 117 (56.5%) in the non-BE/IM group. 166 patients had Siewert Type II tumors spanning the EGJ with 86 patients (42.2%) in the BE/IM group and 80 patients (38.6%) in the non-BE/IM. 2% of all the tumors were in the middle of the esophagus with the distal end of the tumor above 5 cm from EGJ.
Cancers were divided between stages with stage I (n=72, 17.5%), stage II (n= 92, 22.4%), stage III (n=154, 37.5%) and stage IV (n=93, 22.6%). Patients in the prevalent BE/IM group presented at earlier stages with 30.4% stage I, 27% stage II, 29.9% stage III and 12.7% stage IV as compared to more advanced stages in the non-BE/IM group 4.8%, 17.9%, 44.9% and 32.4% respectively (P<0.001) (Table 1). Tumor length was overall similar for both groups when matched for stages I-III (Supplementary Table 1). Nevertheless, at stages I and IV and tumor lengths 3-6 cm and > 6 cm, respectively, non-BE/IIM trended toward association with longer tumor length. Both groups were treated similarly with neoadjuvant chemoradiation therapy followed by esophagectomy (45.6% in the BE/IM and 44% in non-BE/IM). Patients in the non-BE/IM group were more likely to undergo radiation, and/or chemotherapy alone 79 (38.2%) compared to 29 patients (14.2%) in the BE/IM group where there was a higher prevalence of stage IV disease. Endoscopic therapy was more common among the BE/IM group.
OCCAMS Consortium
28% of tumors (n=395) were in the distal esophagus (Siewert Type I), with 35% of these occurring in the BE/IM group. 35% (n=501) of tumors were classified as spanning the esophagogastric (Siewert Type II), with 33% (n=213) in the BE/IM group compared with 36% (n=288) in the non-BE/IM group. The majority of patients received neoadjuvant chemotherapy and esophagectomy (66%, n=934) with no difference between the two groups. The BE/IM group was more likely to receive esophagectomy only (22%, n=137) while the non-BE/IM patients were more likely to receive chemo and/or radiotherapy as the only treatment (9.7%, n=76). Most patients in the cohort were TNM stages II (47%, n=662) or III (34%, n=480). BE/IM was likely to be found in stage II patients (51%, n=323), while stage III patients were more often in the non-BE/IM group (36%, n=285). Patients with BE/IM tended to be more commonly associated with early stages (TNM I, 8.4%), however these accounted for only 5% (n=70) of all cases (Table 1).
Survival
Mayo Clinic
The median overall survival for the entire cohort was 4 years (IQR: 1.3-6.5). Among BE/IM patients, the median survival was 5.8 years (IQR: 2.5 – 7.2), compared to 2.3 years (IQR: 0.9 – 5.6) for the non-BE/IM group (P<0.001) (Figure 1). The 5-year mortality was 219/411 (53.3%) in the entire cohort. This was significantly lower in the BE/IM group 75/203 (36.8%) compared to the non-BE/IM 144/207 (69.6%) in non-BE/IM, P<0.001.
Figure 1.
Overall survival time in years comparing esophageal adenocarcinoma with and without BE/IM, (A): Mayo Clinic (P<0.001), (B): OCCAMS (P<0.001)
Comparing overall survival, the unadjusted model demonstrated a significant survival benefit in the BE/IM group (hazard ratio (HR), 0.44; 95% confidence interval (CI), 0.34 – 0.57, P<0.001) (Figure 1). A multivariable Cox regression analysis including age at diagnosis, sex, tumor location and length and TNM stage resulted in an adjusted hazard ratio (aHR) of 0.66 (95% CI: 0.5–0.88, P=0.005) indicating better survival associated with BE/IM phenotype independent of the above factors.
OCCAMS Consortium
The median overall survival for the OCCAMS cohort was 1.6 years (IQR: 0.9-2.6). Among BE/IM patients the median survival was 3.4 years (IQR 2.9-4.4), compared to 2.0 years (IQR 1.9-2.3) for the non-BE/IM group (P<0.001). In the 864 patients that were diagnosed prior to 2014, 92% received curative treatment and the 5-year mortality was 444/864 (51%), with 42% (164/386) in the BE/IM patients and 58% (280/478) in non-BE/IM patients (P<0.001). When comparing overall survival, the unadjusted model demonstrated a significant difference between the two groups with a survival benefit in the BE/IM group (HR 0.58, 95% CI: 0.49-0.68, P<0.001) (Figure 1). A multivariable analysis including age at diagnosis, sex, tumor location, and TNM stage resulted in an aHR of 0.77 (95% CI: 0.64-0.93, P=0.006).
Subgroup analysis
Mayo Clinic
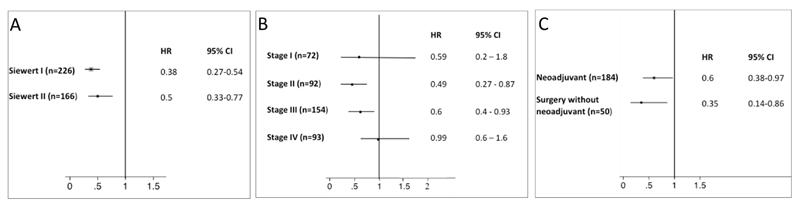
In a multivariable Cox proportional hazards regression that included age at diagnosis and sex for all the models, we performed predefined subgroup analyses to determine if the survival difference was influenced by location (Siewert classification), TNM stage or receiving neoadjuvant therapy. In patients with tumors classed as either Siewert I (n=226, HR=0.38, 95% CI: 0.27-0.54, P<0.001) or Siewert II (n=166, HR=0.5, 95% CI: 0.33-0.77, P=0.002) the BE/IM group showed better survival indicating that the effect of BE/IM is independent of esophageal location (Figure 2A).
Figure 2.
Forest plots for subgroup analysis HR in Mayo Clinic cohort for BE/IM vs non-BE/IM adjusting for age and sex including: (A) location based on Siewert classification, (B) TNM stage, (C) Surgery with/without neoadjuvant therapy.
In a subgroup analysis for each TNM stage, there was a benefit for BE/IM patients in TNM stages II (n=92, HR=0.49, 95% CI: 0.27-0.87, P=0.01) and III (n=154, HR=0.6, 95% CI: 0.4-0.93, P=0.02) (Figure 2B). There was no difference in survival between BE/IM and non-BE/IM in stage I (HR: 0.59, 95%CI: 0.2 – 1.8, P=0.35) and IV (HR: 0.99, 95% CI: 0.6 – 1.6, P=0.97).
In patients who underwent esophagectomy after neoadjuvant chemoradiation (n=184, HR=0.6, 95% CI: 0.38-0.97) a better survival for BE/IM patients persisted (Figure 2C). The subgroup of patients receiving surgery without neoadjuvant therapy (n=50), the HR was consistent with other subgroups and suggests that the benefit of BE/IM persist (HR: 0.35, 95% CI: 0.14 – 0.86, P=0.02). Finally, we performed a subgroup analysis excluding patients who did not undergo surgical or endoscopic resection (i.e. chemo-radiation therapy alone or palliative therapy) to minimize the risk of tissue sampling error. BE/IM was associated with superior survival compared to non-BE/IM HR: 0.65 (95%: 0.44 – 0.96, P=0.03) adjusting for age, sex, tumor location and length and TNM stage.
OCCAMS Consortium
In a multivariable Cox proportional hazards regression that included age at diagnosis and sex we performed subgroup analyses to determine if other factors could be driving the survival difference observed these patients. Patients undergoing surveillance for Barrett’s, tumor location (i.e. Siewert classification), TNM stage, tumor differentiation (i.e. poor, moderate, well), surgery with/without neoadjuvant chemotherapy, and response to chemotherapy (i.e. change in tumor stage pre- and post-surgery), were all tested and had no effect (see Supplementary Figures 1-5).
We additionally assessed whether the effect of BE/IM depends on stage, and how strong the dependence is. We added a random term for the BE/IM-TNM stage interaction to the model. The estimated variance for this term was 1.52x10-5, so the impact of TNM stage on the hazard ratio can be considered negligible.
Finally, we evaluated the impact of the missing data in each category by imputing the missing data points based on observed distributions, and assessing the multivariable Cox regression from the full dataset. We performed 20 iterations of the imputation and a consistent improvement to the aHR of BE/IM resulted (mean aHR=0.64 ± 0.008, P<0.001) indicating that the survival difference is robust to missing data.
Discussion
In this study we have demonstrated the association between the presence of gross Barrett’s esophagus and/or histologic IM on EAC survival in two independent cohorts. In a retrospective, single center cohort from the USA we found that EAC on the background of BE/IM presented at earlier stages and had better survival even after adjusting for disease stage, tumor location and length, and neoadjuvant treatment. These findings were similar in the OCCAMS cohort, a larger cohort of patients with EAC collected from multiple hospitals in the UK.
While both cohorts demonstrated an improved survival when there was associated Barrett’s, there was a difference in median overall survival which was 1.3 years (IQR: 0.6-2.3) in the UK OCCAMS cohort versus the 4 years (IQR: 1.3-6.5) survival seen in the Mayo cohort. It is noteworthy that the UK data reflects the typical 5-year survival for EAC of 12-20%20, 21. The Mayo clinic is a tertiary referral center and there was a higher proportion of early stage tumors (6% in OCCAMS vs 17% in Mayo) and the UK population was slightly older (66 ± 9.4 years for OCCAMS, 64.0 ± 10.7 for Mayo). Additionally, in both cohorts the survival benefit persisted with exclusion of neo-adjuvant therapy exposure to cancers with pre-existing BE/IM. On the other hand, more aggressive use of neo-adjuvant therapy at Mayo may explain some of the overall improved survival differences as well 22.
A key question is what it means from an etiological perspective when Barrett’s and/or IM is not identified at the time of esophageal adenocarcinoma resection or diagnosis. Several possibilities could be considered. First, that IM was present but then eradicated by tumor growth and this scenario has been proposed previously 23, 24, however in the Mayo cohort a finding of BE/IM was overall similar for both groups within cancer stage I-III, independent of the length of the tumor making overgrowth of gross intestinal metaplasia by a larger tumor less likely an explanation in the non-BE group. On the other hand, in specific subgroups of stage and tumor length, there appeared to be differences supporting the possibility of tumor overgrowth in some patients. Second, is that there is acquisition of sudden genomic instability that allows extant intestinal metaplasia to progress rapidly to cancer that is no longer visible in the tumor25. Investigations into molecular mechanisms including mutational signatures, copy-number differences, or recurrent gene mutation analyses that could explain these difference have so far been inconclusive. This is likely due to the complexity of the molecular profile of EAC26. Work is ongoing to examine the molecular characteristics in the subset of patients in the OCCAMS cohort with whole genome sequencing data. Third, the cancer derives through a molecular sequence not involving IM27.
While the initial study at the Mayo clinic was limited due to the use of specimens from a single center, the demographics of these patients are representative of the typical patient who develops EAC - middle to older age white man with increased BMI. This limitation has been addressed by the independent identification of similar findings in the OCCAMS cohort, which includes patients prospectively recruited from 25 different hospitals across the UK. A second concern may be the assuredness with which we propose the existence of these two types of cancer based only on finding BE/IM at diagnosis and/or resection, with the possibility of missing a small focus of IM. Systematic pathology review for the presence of IM could help mitigate this limitation, and the prospective OCCAMS cohort addressed this since the presence of BE/IM is systematically assessed in all patient samples as part of the study protocol. Furthermore, in a small subset of the OCCAMS patients an independent review of their pathological reports was undertaken, and the survival advantage remained for the BE/IM group.
One important finding that also argues for a different phenotype of esophageal cancer is that cancers without IM accounted for almost half the EACs from the years studied and were mostly distal EACs. This stands in contrast to data demonstrating that the majority of esophageal adenocarcinomas develop in a segment of Barrett’s mucosa28. As a result, this data further suggests the existence of a different phenotype of esophageal adenocarcinoma rather than misclassifying the IM type that would have more likely arisen in the presence of long segment Barrett’s mucosa. Another limitation might be in determining whether the poorer prognosis of EAC without IM results from poor response to therapy since most patients with stage II and III EAC receive chemotherapy, and in the USA radiation therapy. Given the inaccuracy of endoscopic ultrasound and PET/CT for assessing lymph node involvement and T stage before therapy, it would be difficult to compare response before and after treatment. However, analysis of patients with stage II cancer not treated with neo-adjuvant therapy showed persistent survival benefit of Barrett’s/IM cancers when compared to non-BE/IM cancers, as did an analysis of response based on the differences between pre- and post-resection tumor staging. Furthermore, the advanced presentation of non-BE/IM adenocarcinoma before therapy suggests a more aggressive cancer. Finally, another concern is whether we are confusing cancers of the esophago-gastric junction with similarly true esophageal adenocarcinoma which may carry a different molecular signature and prognosis. However, in both cohorts the survival advantage of BE/IM was present regardless of the location of the tumor. In our study, the patients without BE and distal adenocarcinoma extending to EGJ but not Siewert II adenocarcinoma had similar demographics to those with Barrett’s patients as all tumors extended > 1 cm above the gastroesophageal junction17. Furthermore, when we excluded all patients with extension of tumor into the cardia, there was a persistent decrease in overall survival when compared to Barrett’s/IM related tumors.
In conclusion, this study suggests that there are phenotypically two types of esophageal adenocarcinoma – one with grossly visible and/or histologically identifiable intestinal metaplasia in the esophagus and one without. Furthermore, the presence or absence of these findings may influence the ability for early detection in this disease through screening for BE. It may also have ramifications for tumor behavior and/or response to therapy and therefore prognosis. Longitudinal and detailed molecular characterization studies are required to shed further light on the natural history of EAC that presents de novo, in order to develop evidenced based screening and prevention strategies for this highly lethal malignancy on a population basis. Finally, sequencing will be needed to determine ultimately if this new phenotype is an intestinal metaplasia independent pathway.
Supplementary Material
Table 2. Predictors for survival in EAC patients presented as hazard ratios from multivariate cox regression model including Barrett’s phenotype, sex, age at diagnosis, Siewert classification and TNM stage in both cohorts and the tumor length in the Mayo cohort.
| Mayo | OCCAMS | |||||
|---|---|---|---|---|---|---|
| Total, N= 411 | Adjusted HR | 95% CI | Total, N= 1417 | Adjusted HR | 95% CI | |
| Barrett's Esophagus | ||||||
| Non-BE/IM | 207 | Reference | — | 783 | Reference | |
| BE/IM | 204 | 0.66 | 0.5 - 0.88 | 634 | 0.77 | 0.64-0.93 |
| Sex | ||||||
| Male | 350 | Reference | 1179 | 1.1 | 0.84-1.43 | |
| Female | 61 | 1.02 | 0.72-1.44 | 238 | Reference | |
| Age at diagnosis (years) | 411 | 1.02 | 1.01-1.04 | 1417 | 1.01 | 1.0-1.02 |
| Siewert Classification | ||||||
| 1 | 226 | Reference | — | 395 | Reference | |
| 2 | 166 | 0.75 | 0.56 - 1 | 501 | 0.85 | 0.7-10.5 |
| 3 | 0 | — | — | 175 | 0.92 | 0.7-1.2 |
| Tubular | 9 | 1.24 | 0.56 - 2.74 | — | — | — |
| Missing | 10 | 1.88 | 0.9 – 3.95 | |||
| TNM stage | ||||||
| I | 72 | Reference | — | 70 | Reference | |
| II | 92 | 2 | 1.22 - 3.44 | 662 | 3.25 | 1.44-7.33 |
| III | 154 | 3 | 1.8 – 5.1 | 480 | 6.25 | 2.77-14.13 |
| IV | 93 | 6.9 | 4 - 11.9 | 54 | 10.02 | 4.14-24.23 |
| Missing | 0 | — | — | |||
| Tumor length | ||||||
| 1-3 cm | 161 | Reference | ||||
| 3 cm<Length ? 6 cm | 129 | 0.82 | 0.57 - 1.18 | |||
| >6 cm | 69 | 1.1 | 0.7 - 1.63 | |||
| Missing | 52 | 1.1 | 0.68 - 1.7 | |||
| Log-Rank P<0.001 | Log-Rank P<0.001 | |||||
Funding
None
Abbreviations
- BE
Barrett’s esophagus
- BMI
Body mass index
- EAC
Esophageal adenocarcinoma
- EGJ
esophageal gastric junction
- EMR
endoscopic mucosal resection
- IM
Intestinal metaplasia
Footnotes
Disclosure:
DAK: pharmaceutical trial with Shire
JBK: Listed as an inventor under an intellectual property development agreement between Mayo Clinic and Exact Sciences (Madison WI) under which royalties may be paid.
DAA: Listed as an inventor under an intellectual property development agreement between Mayo Clinic and Exact Sciences (Madison WI) under which royalties may be paid.
KKW: Wang receives research support from CSA Medical and C2 Therapeutics
TS, PGI, TCS, YQ, SK, MG, RJC and AKR: None to declare
RCF: Listed as an inventor on patents pertaining to Cytosponge and associated assays which have been licensed by the Medical Research Council to Medtronic
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg D, Ness-Jensen E, Mattsson F, et al. Risk of oesophageal adenocarcinoma in individuals with Barrett's oesophagus. Eur J Cancer. 2017;75:41–46. doi: 10.1016/j.ejca.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Visrodia K, Singh S, Krishnamoorthi R, et al. Systematic review with meta-analysis: prevalent vs. incident oesophageal adenocarcinoma and high-grade dysplasia in Barrett's oesophagus. Aliment Pharmacol Ther. 2016;44:775–84. doi: 10.1111/apt.13783. [DOI] [PubMed] [Google Scholar]
- 4.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett's oesophagus. Gut. 2016;65:1252–1260. doi: 10.1136/gutjnl-2014-308865. [DOI] [PubMed] [Google Scholar]
- 6.Rubenstein JH, Thrift AP. Risk factors and populations at risk: selection of patients for screening for Barrett's oesophagus. Best Pract Res Clin Gastroenterol. 2015;29:41–50. doi: 10.1016/j.bpg.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Almers LM, Graham JE, Havel PJ, et al. Adiponectin May Modify the Risk of Barrett's Esophagus in Patients With Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2015;13(13):2256–2264.e3. doi: 10.1016/j.cgh.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett's esophagus: a case-control study. Clin Gastroenterol Hepatol. 2014;12:229–238 e3. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein JH, Morgenstern H, McConell D, et al. Associations of diabetes mellitus, insulin, leptin, and ghrelin with gastroesophageal reflux and Barrett's esophagus. Gastroenterology. 2013;145:1237–44 e1-5. doi: 10.1053/j.gastro.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett's esophagus among men. Am J Gastroenterol. 2013;108:353–62. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offman J, Fitzgerald RC. Alternatives to Traditional Per-Oral Endoscopy for Screening. Gastrointest Endosc Clin N Am. 2017;27:379–396. doi: 10.1016/j.giec.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med. 2015;12:e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallick R, Patnaik SK, Wani S, et al. A Systematic Review of Esophageal MicroRNA Markers for Diagnosis and Monitoring of Barrett's Esophagus. Dig Dis Sci. 2016;61:1039–50. doi: 10.1007/s10620-015-3959-3. [DOI] [PubMed] [Google Scholar]
- 14.Clark GW, Smyrk TC, Burdiles P, et al. Is Barrett's metaplasia the source of adenocarcinomas of the cardia? Arch Surg. 1994;129:609–14. doi: 10.1001/archsurg.1994.01420300051007. [DOI] [PubMed] [Google Scholar]
- 15.Sottoriva A, Kang H, Ma Z, et al. A Big Bang model of human colorectal tumor growth. Nat Genet. 2015;47:209–16. doi: 10.1038/ng.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nones K, Waddell N, Wayte N, et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat Commun. 2014;5:5224. doi: 10.1038/ncomms6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–9. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 18.Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–317. doi: 10.3322/caac.21399. [DOI] [PubMed] [Google Scholar]
- 19.Stef van Buuren KG-O. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- 20.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 21.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–6. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiesi G, Park W, Gunder M, et al. Long-term survival based on pathologic response to neoadjuvant therapy in esophageal cancer. J Surg Res. 2017;216:65–72. doi: 10.1016/j.jss.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasoma P, Wickramasinghe K, Ma Y, et al. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus. 2007;20:36–41. doi: 10.1111/j.1442-2050.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 24.Cameron AJ, Lomboy CT, Pera M, et al. Adenocarcinoma of the esophagogastric junction and Barrett's esophagus. Gastroenterology. 1995;109:1541–6. doi: 10.1016/0016-5085(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 25.Contino G, Vaughan TL, Whiteman D, et al. The Evolving Genomic Landscape of Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterology. 2017;153:657–673 e1. doi: 10.1053/j.gastro.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secrier M, Li X, de Silva N, et al. Corrigendum: Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2017;49:317. doi: 10.1038/ng0217-317a. [DOI] [PubMed] [Google Scholar]
- 27.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut. 2012;61:970–6. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 28.Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at barrett's esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol. 2014;109:527–34. doi: 10.1038/ajg.2014.10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.