Abstract
Background
Diffuse intrinsic pontine glioma (DIPG) is a uniformly fatal CNS tumor diagnosed in 300 American children per year. Radiation is the only effective treatment and extends overall survival to a median of 11 months. Due to its location in the brainstem, DIPG cannot be surgically resected. Immunotherapy has the ability to target tumor cells specifically; however, little is known about the tumor microenvironment in DIPGs. We sought to characterize infiltrating immune cells and immunosuppressive factor expression in pediatric low- and high-grade gliomas and DIPG.
Methods
Tumor microarrays were stained for infiltrating immune cells. RNA was isolated from snap-frozen tumor tissue and Nanostring analysis performed. DIPG and glioblastoma cells were co-cultured with healthy donor macrophages, T cells, or natural killer (NK) cells, and flow cytometry and cytotoxicity assays performed to characterize the phenotype and function, respectively, of the immune cells.
Results
DIPG tumors do not have increased macrophage or T-cell infiltration relative to nontumor control, nor do they overexpress immunosuppressive factors such as programmed death ligand 1 and/or transforming growth factor β1. H3.3-K27M DIPG cells do not repolarize macrophages, but are not effectively targeted by activated allogeneic T cells. NK cells lysed all DIPG cultures.
Conclusions
DIPG tumors have neither a highly immunosuppressive nor inflammatory microenvironment. Therefore, major considerations for the development of immunotherapy will be the recruitment, activation, and retention of tumor-specific effector immune cells.
Keywords: DIPG, tumor microenvironment, immunotherapy, tumor-associated macrophage, NK cell
Key Points
1. DIPG tumors contain few immune cells suggesting limited immune surveillance
2. The few immune cells that are in the tumor are less suppressive than those in adult brain tumors
3. Immunosuppression in DIPG may be less of an obstacle than in adult tumors
Importance of the Study
Over a hundred clinical trials have resulted in no improvements to the overall survival of DIPG patients. Immunotherapy holds the potential to eliminate transformed cells while leaving the critical structures within the brainstem intact. However, little is known about the immune microenvironment in pediatric CNS tumors in general and DIPG in particular. We sought to provide insights about the opportunities and challenges that may be encountered in the development of DIPG-specific immunotherapies. We show that in contrast to therapies in development for adult GBM, DIPG-specific immunotherapies should not focus on overcoming the tumor microenvironment, but rather on methods to recruit and activate cytotoxic cells in an environment with few antigen-presenting cells and a low mutational burden. Our work provides critical background to investigators currently developing immunotherapies for DIPG.
In recent years, CNS tumors have overtaken leukemia as the leading cause of pediatric cancer-related death.1 This is largely due to improvements in treatment of pediatric leukemia, particularly the advent of molecularly targeted therapeutics and immunotherapy for relapsed disease. In contrast, treatment for most pediatric CNS tumors has changed little in several decades, relying primarily on surgical tumor removal followed by chemotherapy and/or radiation to target remaining cancer. While outcomes by subtype vary,2 patients with aggressive tumors are in desperate need of new therapeutic options.
Approximately 300 children per year in the United States are diagnosed with diffuse intrinsic pontine glioma (DIPG), a uniformly fatal tumor that arises from an oligodendroglial precursor cell in the ventral pons at a median of ~6–7 years old.3 Surgical resection of the tumor is impossible due to its location, and dozens of clinical trials have yet to reveal a chemotherapy or molecularly targeted agent with efficacy. The standard therapy for DIPG is radiation, which mitigates symptoms temporarily and extends progression-free and overall survival by several months. However, death of patients with DIPG is currently inevitable, with a median survival of only 11 months from diagnosis.4 Because of its ability to specifically target transformed cells while leaving healthy cells intact, immunotherapy is a particularly appealing strategy to treat DIPG.
DIPGs are characterized by a point mutation in the genes encoding histone 3.3 (H3.3; ~70% of DIPG) or 3.1 (H3.1; 15%), causing a K27M alteration to the histone and epigenetic changes throughout the genome. Although these mutations appear to drive unique oncogenic programs, with H3.3-K27M mutations associated with TP53 loss and PDGFRA amplification, and H3.1-K27M with an ACVR1 mutation,5,6 DIPGs are similar to other pediatric and adult CNS tumors in their low mutational burden. This results in a paucity of neo-epitopes that can be targeted by T cells, coupled with few infiltrating lymphocytes, resulting in an immunologically “cold” tumor that responds poorly to immune checkpoint inhibition.7 This leaves cellular immunotherapies, including chimeric antigen receptor (CAR) T cells or ex vivo expanded natural killer (NK) cells, promising candidates for the treatment of DIPG. T cell–based approaches targeting the mutant K27M allele8,9 or the ganglioside GD210 have shown promise in preclinical studies.
To date, there has been little investigation of the tumor microenvironment (TME) of pediatric CNS tumors, a critical first step in the rational development of effective immunotherapy strategies. However, in contrast to adult high-grade gliomas, which have extensive immune cell infiltration,11 high-grade pediatric tumors often have a notable absence of a myeloid or lymphoid infiltrate12–14 that fails to correlate with survival.14 Because DIPG tumors are rarely surgically resected, tumor tissue from these patients is infrequently available; therefore most prior studies have not evaluated immune cell infiltration in DIPG patient tumors. A recent study showed that the DIPG microenvironment has fewer inflammatory cells and is immunologically inert relative to adult glioblastoma (GBM).15 Herein, we demonstrate that DIPG tumors have few infiltrating immune cells and are not enriched for the expression of several genes associated with immune dysfunction. To predict responses of adoptively transferred immunotherapy cells, we also employed a reductionist approach to examine how patient-derived DIPG cell cultures interact with immune cells in vitro. In contrast to macrophages cultured with adult GBM cells, macrophages cultured with DIPG cells are not repolarized to an immunosuppressive phenotype, and NK cells are capable of killing DIPG cells. Together, our data suggest that in contrast to immunotherapy approaches for adult GBM, which must overcome the immunosuppressive TME, the primary considerations in development of immune therapies for DIPG will be the recruitment of effector cells to the tumor and local support for their persistence and activation.
Materials and Methods
Ethics
Tumor microarrays (TMAs) were constructed and immune cells isolated from donor blood collected under Seattle Children’s Research Institute IRB #14412. Frozen tumor samples were collected under Seattle Children’s Hospital IRB #14449. Pediatric patients were defined as <18 years of age.
CD163 Kaplan–Meier Analysis
Kaplan–Meier plots were generated using the R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl). Datasets used included “Paugh” pediatric glioma (GBM only)16 and The Cancer Genome Atlas adult GBM.17
TMA Construction, Immunohistochemistry Staining, and Image Analysis
TMAs were constructed as previously described13 and included triplicate cores from pediatric low-grade glioma (pLGG) (19 pilocytic astrocytoma, 4 ganglioglioma), pediatric high-grade cortical glioma (pHGG) (11 anaplastic astrocytoma, 16 GBM), DIPG (n = 9), and control (12 nontumor pediatric brain). Details can be found in the Supplementary Methods.
RNA Isolation and Nanostring
RNA was isolated from flash-frozen tissue using the RNeasy Mini Kit (Qiagen), then concentrated with the RNeasy Minelute Kit (Qiagen) according to the manufacturer’s protocols. RNA was incubated with the Nanostring PanCancer Immune Profiling Codeset and run on a Nanostring nCounter SPRINT instrument according to the manufacturer’s protocols. Data analysis information is included in Supplementary Methods.
Cell Cultures
Validated SU-DIPG-IV, SU-DIPG-XIII, and SU-DIPG-XVII were from the Monje Lab. U87 cells were purchased from American Type Culture Collection. All cultures were routinely mycoplasma tested and cultured in NeuroCult NSC (neural stem cell) Proliferation media (Stemcell) supplemented with epidermal growth factor and basic fibroblast growth factor (Stemcell).18 U87 cells were maintained in NeuroCult media for at least 10 passages prior to use in experiments. Cell culture information can be found in Supplementary Table 1.
Macrophage-Tumor Co-Culture
Cluster of differentiation (CD)14+ monocytes were isolated from healthy donor peripheral blood mononuclear cells (PBMCs) using positive selection (Stemcell) and differentiated to macrophages with 6 days of culture in Roswell Park Memorial Institute 1640 media (Gibco) supplemented with 10% fetal bovine serum (Hyclone) (RP10) and 10 ng/mL granulocyte-macrophage colony-stimulating factor (R&D Systems). Macrophages were reseeded at 5e5 cells/well in a 6-well plate. Cells were washed extensively the following day with NeuroCult media. Following macrophage equilibration in NeuroCult media for 24 hours, 5e5 tumor cells were added in an additional 2 mL of NeuroCult media for 72 hours prior to collection.
Macrophage Flow Cytometry
Following co-culture, adherent cells were detached with Versene (Gibco), incubated with Fc Block (BD Biosciences), stained according to standard protocols, and run on a BD Fortessa instrument. Antibodies used are included in Supplementary Table 1. Data were analyzed with FlowJo (Treestar).
T-Cell Chromium Release Assay and Intracellular Cytokine Staining
Allogeneic, major histocompatibility complex (MHC)–unmatched total T cells were isolated from PBMCs using negative selection (Miltenyi), activated using Dynabeads (ThermoFisher), and cultured in RP10 supplemented with 30 U/mL interleukin (IL)-2 (R&D) for 5 days. Dynabeads were removed and T cells (effectors) rested overnight in fresh media containing IL-2, then mixed with 51Cr-labeled tumor cells (target) in RP10 for 6 hours, and percentage of lysis determined as previously described.19 Concomitantly, activated T cells were incubated with unlabeled tumor cells at a 1:1 ratio in RP10 for 6 hours in the presence of Brefeldin A (Sigma), then stained for intracellular interferon-gamma (IFNγ) followed by flow cytometry. Antibodies are included in Supplementary Table 1.
NK Cell Chromium Release Assay and Intracellular Cytokine Staining
NK cells were isolated from PBMCs using negative selection (Miltenyi) and incubated overnight in RP10 containing 1000 U/mL IL-2. Effector NK cells were then mixed with 51Cr-labeled tumor cells (target) in RP10 for 4 hours, and percentage of lysis determined as previously described.19 Concomitantly, NK cells were incubated with unlabeled tumor cells at a 1:1 ratio in RP10 for 4 hours in the presence of Brefeldin A (Sigma), then stained for intracellular IFNγ and tumor necrosis factor alpha (TNFα) followed by flow cytometry. Antibodies are included in Supplementary Table 1.
Reproducibility and Statistics
One-way ANOVA with Tukey’s multiple comparisons test was performed unless noted otherwise. For in vitro experiments, at least 3 biological replicates were used and performed in triplicate. Statistical analysis was performed in GraphPad Prism, and *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Results
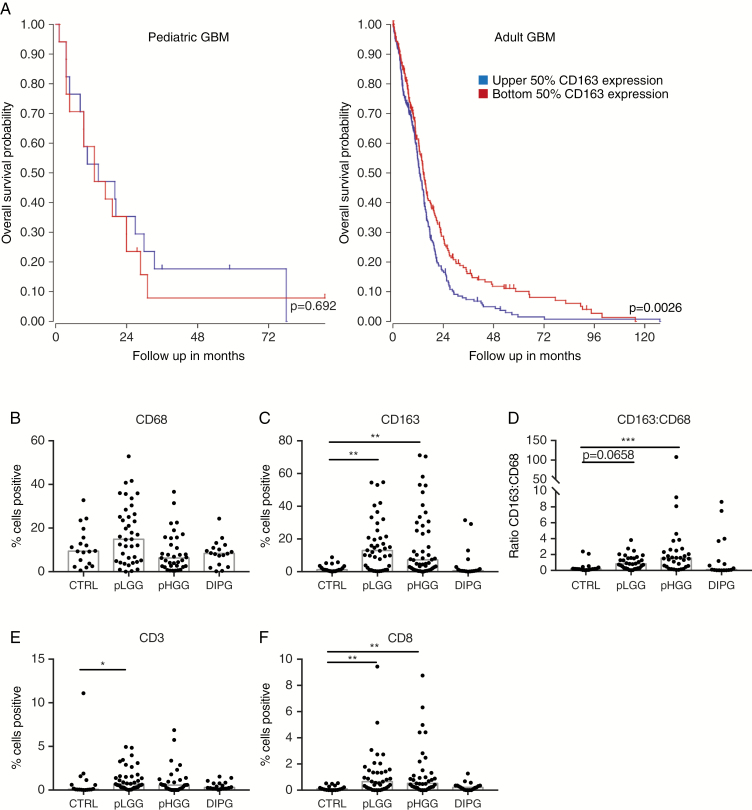
Previous reports have demonstrated significant variation in immune infiltrate between subtypes of adult20 and pediatric10,12,14 CNS tumors. Furthermore, the role of particular immune cells is not necessarily the same even in adult and pediatric tumors with the same histopathological classification. For example, the extent of infiltration by immunosuppressive CD163+ tumor-associated macrophages (TAMs) is not related to survival in pediatric GBM but is in adult GBM (Fig. 1A); TAM infiltration is associated with a poor prognosis.21 Previous studies have demonstrated that immune infiltrate in pediatric CNS tumors has no bearing on survival.14 Furthermore, pediatric CNS tumors may not establish an immunosuppressive microenvironment as is seen in adult CNS tumors,13 and instead reflect a failure of immune surveillance. It is not clear, however, whether impaired immune surveillance is the result of poor lymphocyte trafficking to the tumor site or minimal expression of ligands for activating immune receptors expressed by lymphocytes. Therefore, we sought to characterize the immune microenvironment in DIPG as a first step toward the rational development of immunotherapy tailored specifically to these tumors.
Fig. 1.
Immune infiltrate in pediatric CNS tumors. (A) R2 software was used to examine survival by CD163 expression in pediatric (n = 34) and adult GBM (n = 504). (B, C) Percentage of cells positive for CD68 or CD163. (D) Ratio of CD163 to CD68 positive cells. (E, F) Percentage of cells positive for CD3 or CD8. Bars represent median. Significance evaluated by one-way ANOVA.
We used immunohistochemistry (IHC) to compare immune cell infiltration in pediatric tumor or normal adjacent tissue from surgical resection (pLGG, pHGG) or autopsy (DIPG) using TMAs (representative images in Supplementary Fig. 1). To confirm no confounding effects, we also examined pHGG samples from both surgery and autopsy and found no significant differences in immune infiltration (Supplementary Fig. 2). Infiltrating myeloid cells, including microglia, can express the general macrophage marker CD68 as well as CD163, a marker for alternatively activated macrophages, which contribute to immunosuppression. The median number of total CD68+ macrophages/microglia is similar among all samples, representing approximately 10% of cells (Fig. 1B). However, whereas pLGG and pHGG samples have a 10.4- and 5.9-fold increase over control, respectively, in the number of CD163+ macrophages, DIPG samples do not have increased CD163 positivity (Fig. 1C). Furthermore, the ratio of CD163+ to CD68+ macrophages, a measure of immunosuppression by TAMs,22 is elevated 8.0-fold over control in pHGG, but was not different from control in DIPG (Fig. 1D). We also evaluated infiltrating T cells: Because of the low mutational burden of these tumors, low numbers of T cells were expected. Nevertheless, although pLGG and pHGG had increased infiltration of T cells, in particular cytotoxic CD8+ T cells (6.5- and 5.1-fold, respectively), DIPG tumors had no significant increase in any subset of T cells over control (Fig. 1E, F). Furthermore, effects of cell density in the tumor samples were examined (Supplementary Fig. 3), with no differences in interpretation of results from percent area positive. Together, this suggests that DIPG cells neither recruit TAMs nor repolarize macrophages to an immunosuppressive phenotype. However, perhaps in part due to the absence of myeloid/antigen-presenting cells to initiate an antitumor immune response, DIPGs also do not recruit the effector lymphocytes that may be capable of lysing transformed cells. Notably, our IHC data showing that DIPG samples do not have as extensive myeloid infiltration as seen in low- and high-grade pediatric gliomas and adult GBMs are consistent with transcript-level data maintained in a public database (Supplementary Fig. 4) and in our own samples (Supplementary Fig. 5). Data for CD3 expression is also consistent between our TMA analysis and a public database; however, increased CD8 positivity in pLGG and pHGG was not seen in the public database, a discrepancy possibly due to regulation of CD8 expression at the translational level.23 Neither CD3 nor CD8 transcripts were above the noise threshold in our analysis. Importantly, the level of immune cell infiltration did not correlate with patient survival (Supplementary Fig. 6).
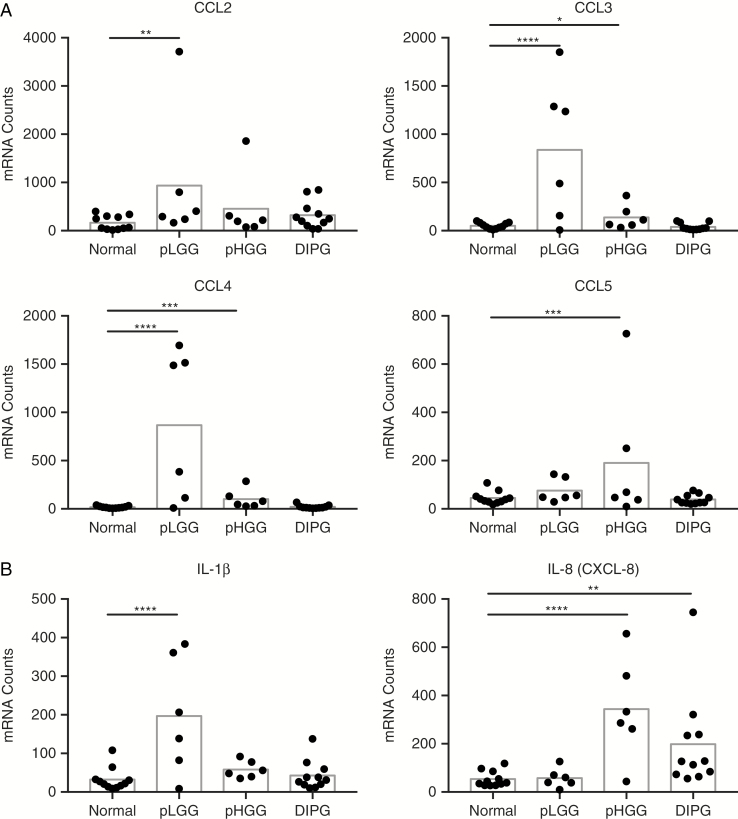
To explain the differences in infiltrating leukocytes, we used Nanostring transcript analysis of immune cell–specific chemokine genes in pLGG, pHGG, DIPG, and nontumor controls. Although most chemokines were not above background, the chemokines most important for monocyte and lymphocyte chemotaxis, chemokine C-C ligands 2 to 5 (CCL2–5), were all increased in the pLGG samples, with CCL2 (5.6-fold), CCL3 (16-fold), and CCL4 (48-fold) significant over control (Fig. 2A). This is consistent with the increased infiltration of pLGG by both macrophages and T cells (Fig. 1). Furthermore, the presence of detectable proinflammatory mediators in pLGG (IL-1B) and pHGG (IL-8) (Fig. 2B) suggests that immune cells in those tumors detect transformed cells and respond by recruiting additional leukocytes, a phenomenon not seen in DIPG. Therefore, there are 2 possibilities: Either DIPGs form an extremely immunosuppressive microenvironment that precludes any immune cell activation, or DIPG cells are poorly visible to the immune system.
Fig. 2.
Expression of chemokines and inflammatory cytokines by pediatric CNS tumors. (A) Nanostring analysis of RNA isolated from snap-frozen tissue for the expression of CCL2–5. (B) Nanostring analysis of inflammatory cytokines. Bars represent mean. Significance evaluated by one-way ANOVA.
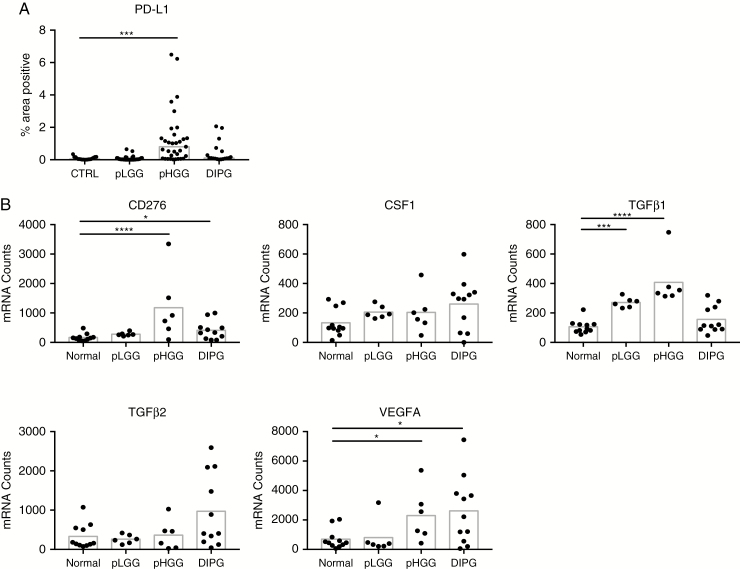
To examine the former possibility, we turned to our analysis of TMAs by IHC and transcripts by Nanostring. We found that cells within the pHGG microenvironment express higher levels of immunosuppressive factors including programmed death ligand 1 (PD-L1) (20-fold; Fig. 3A), B7-H3 (7-fold), and transforming growth factor beta 1 (TGFβ1) (3.9-fold; Fig. 3B) than either pLGG or DIPG, consistent with the increased ratio of CD163 to CD68 (Fig. 1D) and the well-documented immunosuppression that occurs in adult GBM.24 In DIPG, only B7-H3 (2.4-fold) and vascular endothelial growth factor (VEGF)A (3.8-fold) are increased relative to control. Therefore, we conclude that DIPG tumors express factors most associated with immunosuppression in CNS tumors at lower levels than HGGs; therefore, the absence of immune cells in DIPG likely reflects a failure of immunosurveillance. Additional transcript data are presented in the hyperlinked Supplementary file.
Fig. 3.
Expression of factors associated with immunosuppression by pediatric CNS tumors. (A) Percentage of total area of spots staining positive for PD-L1. Bars represent median. Significance evaluated by one-way ANOVA. (B) Nanostring analysis of immunosuppressive factors found in pediatric brain tumors. Bars represent mean. Significance evaluated by one-way ANOVA.
Because there are so few leukocytes present in DIPGs, we next sought to directly evaluate the impact of DIPG tumor cells on immune cell polarization and functions should they be either recruited more effectively or introduced locally. To answer this question, we performed a series of co-culture experiments to examine the effects of patient-derived DIPG cell cultures on isolated immune cell populations from healthy donors. In addition to 3 DIPG cultures (Supplementary Table 4), we included the adult GBM U87 cell line as a known positive control for immunosuppression. Their effect on immune cells has been extensively characterized and recapitulates features of pro-tumor immune modulation found in GBM tumors.25
Macrophages are exquisitely sensitive to their microenvironment, and readily modulate phenotype and functions in response to local conditions.26 We and others27,28 have investigated them as a possible immunotherapy for solid tumors such as DIPG that are deficient in myeloid cells necessary to promote an immune response. To determine the soluble factors produced by tumor cells that may play a role in macrophage polarization, we collected conditioned media and performed a Luminex-based assay on a panel of cytokines and growth factors (Supplementary Fig. 7). Both SU-DIPG-IV and U87 cells produced VEGF and IL-6 at high levels, along with a small amount of IL-10, all of which have been implicated in immunosuppressive polarization of macrophages.29,30 This was in contrast to SU-DIPG-XIII and SU-DIPG-XVII, which produced less VEGF (6.5% and 19%, respectively, of the VEGF produced by U87), while IL-6 and IL-10 were not detected. Notably, SU-DIPG-IV and U87 produced chemokines that attract circulating monocytes (Supplementary Fig. 8) and higher levels of macrophage colony-stimulating factor (Supplementary Fig. 7), while SU-DIPG-XIII and -XVII did not, suggesting that monocytes may migrate toward tumors already predisposed to repolarize and support macrophages. We also collected media from the primary pediatric GBM line SU-pcGBM-II and the long-term GBM cultures LN229, U138, and T98G (Supplementary Figs. 7–8). Although there was considerable variation in the amount of each cytokine produced by the various cells, it does not appear that U87 produces these factors at levels that exceed other GBM cultures. Furthermore, differences in soluble factor production could not be explained by differences in cell growth rate (Supplementary Fig. 9).
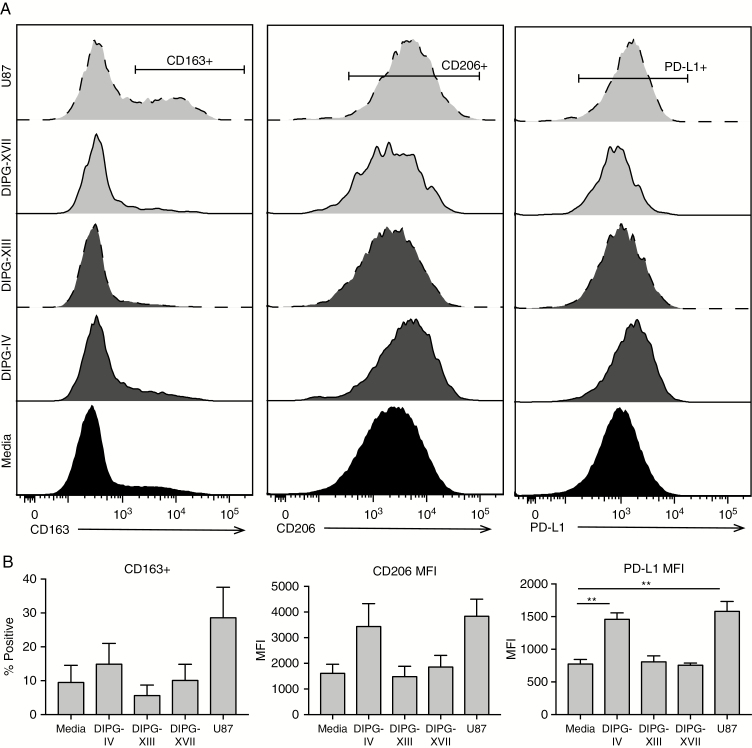
Because they did not produce the necessary soluble factors, we hypothesized that neither SU-DIPG-XIII nor -XVII would repolarize macrophages to an immunosuppressive phenotype. We differentiated healthy donor monocytes to a neutral “M0” phenotype, then added an equal number of brain tumor cells for 72 hours and evaluated canonical markers of polarization on the macrophages by flow cytometry (Fig. 4). As expected, following culture with immunosuppressive U87 GBM cells, macrophages upregulated expression of the receptors CD163 by 3.3-fold and CD206, a scavenger receptor that is increased on anti-inflammatory macrophages, by 2.4-fold, as well as PD-L1, which contributes to T-cell exhaustion, by 2-fold. SU-DIPG-IV, the lone H3.1-K27M culture, followed trends similar to U87, albeit to a lesser magnitude. The H3.3-K27M DIPG cultures had little effect on macrophage phenotype, which is supported by analysis of macrophage-specific immune regulatory transcripts following co-culture with cancer cells (Supplementary Fig. 10).
Fig. 4.
DIPG cells do not repolarize macrophages. (A) CD163, CD206, and PD-L1 on macrophages following co-culture with tumor cells, by flow cytometry. (B) Proportion (CD163+) or mean fluorescence intensity (CD206, PD-L1) of macrophages following co-culture with tumor cells. N = 3, error bars represent SEM. Significance evaluated by one-way ANOVA.
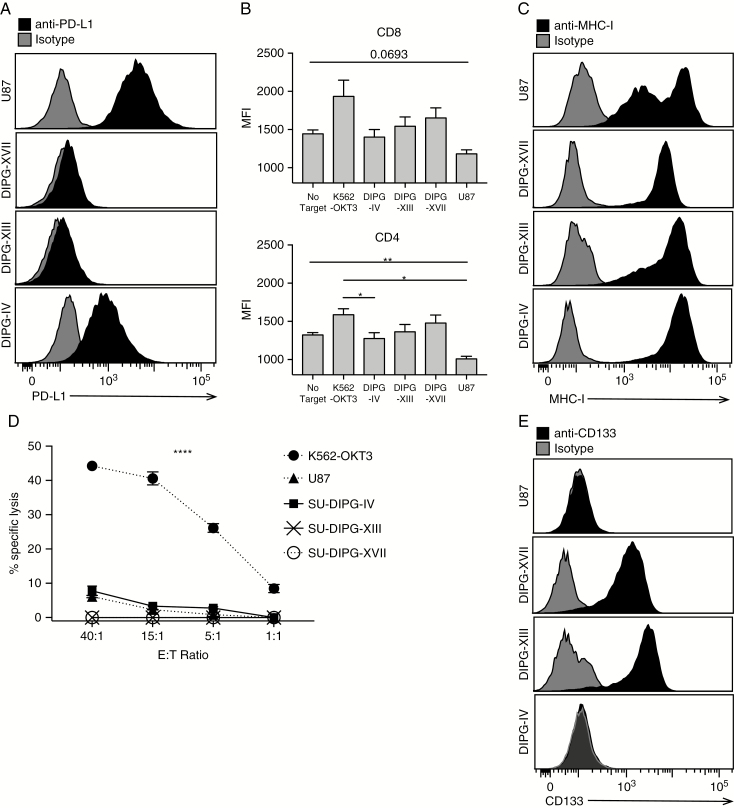
In support of the observation that PD-L1 and other immunosuppressive factors are present at only low levels in the DIPG TME (Fig. 3), we found that immunosuppressive U87 cells express higher levels of PD-L1 (Fig. 5A) than DIPG cells, although DIPG tumor cell cultures secrete similar levels of TGFβ as do U87 cells (Supplementary Fig. 7). Additional GBM cells also have higher surface levels of PD-L1 than H3.3-K27M DIPG cells, albeit not as high as U87 (Supplementary Fig. 11). Consistent with this, upon exposure to U87 cells, IFNγ production is decreased by 24% and 18%, respectively, for CD3/28-activated allogeneic CD4+ and CD8+ T cells relative to the No Target control; DIPG cells do not suppress IFNγ production (Fig. 5B). However, despite expression of MHC class I (Fig. 5C), which should induce killing by activated T cells specific for foreign MHC, T cells fail to efficiently lyse DIPG cell cultures relative to immunosuppressive U87 (Fig. 5D). This is consistent with previous observations that cancer stem cells are also not efficiently targeted for immune destruction by T cells31; SU-DIPG-XIII and -XVII are 100% positive for the stem cell marker CD133 (Fig. 5E). Collectively, these data suggest that both the cell of origin and low mutational burden contribute to poor T-cell activation in response to DIPG tumor cells. Therefore, we hypothesize that successful adoptive T-cell therapies for DIPG patients will include the provision of appropriate costimulation mediated by antigen presenting cells in the TME.
Fig. 5.
T cells do not effectively kill DIPG cells. (A) PD-L1 expression on DIPG cells by flow cytometry. (B) Mean fluorescence intensity of IFNγ in activated CD8 and CD4 T cells following incubation with tumor cells. N = 3, error bars represent SEM. Significance evaluated by one-way ANOVA. (C) MHC-I expression on DIPG cells, by flow cytometry. (D) Lysis of cell cultures following 6-hour incubation with activated T cells at the noted effector:target ratios. N = 3. Error bars represent SEM. Significance evaluated by 2-way ANOVA. (E) CD133 expression on DIPG cells, by flow cytometry.
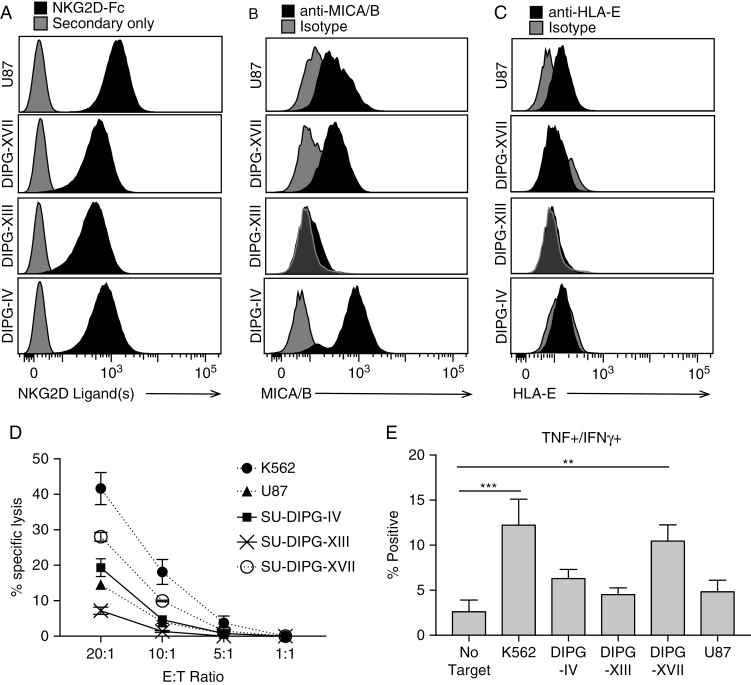
Finally, we examined the ability of NK cells to kill DIPG cells. NK cells target transformed or virally infected cells that either downregulate surface expression of MHC-I (“missing self”) or upregulate expression of NK cell activating ligands. The presence of MHC-I on DIPG and U87 (Fig. 5C) suggests they will not be targeted for elimination based on a lack of expression of MHC alone. Using flow cytometry, we found that all DIPG cultures and U87 express at least one of the 8 known stress ligands (including MHC class I polypeptide-related sequence A/B [MICA/B] and UL16 binding proteins 1–6) for the activating receptor natural killer group 2, member D (NKG2D) (Fig. 6A). More specifically, SU-DIPG-IV, SU-DIPG-XVII, and U87 cells express MICA/MICB, while SU-DIPG-XIII does not (Fig. 6B). NKG2D ligands can also be detected ubiquitously in tumor samples (Supplementary Fig. 12). Furthermore, no DIPG cells expressed detectable levels of human leukocyte antigen E (HLA-E) (Fig. 6C), which is a regulated component of an inhibitory ligand that binds to NKG2C. Together, expression of these surface ligands suggests that NK cells should have at least some activity against DIPG cells, in spite of their expression of MHC-I. Indeed, in a cytotoxicity assay, all DIPG cell cultures were lysed to at least some degree (Fig. 6D), ranging from 28% lysis for SU-DIPG-XVII to 7% for SU-DIPG-XIII at an effector-to-target ratio of 20:1. Although many factors contribute to susceptibility of NK cell–mediated killing, it is noteworthy that NK cells are 4 times more effective against SU-DIPG-XVII than -XIII, in spite of their similar effects on macrophage phenotypes (Fig. 4) and T-cell functions (Fig. 5). This may be due to the absence of MICA/B expression on SU-DIPG-XIII, and/or to its secretion of large amounts of platelet derived growth factor with 2 B-chains (PDGF-BB) (Supplementary Fig. 7), which inhibits NK cell cytotoxicity.32 Finally, although all cell cultures induced some expression of TNFα and IFNγ production by CD56hi NK cells, only SU-DIPG-XVII induced enough NK cells to produce both cytokines to be significant relative to the No Target control (10.5% dual positive vs 2.6%; Fig. 6E).
Fig. 6.
NK cells can kill DIPG cells dependent on NKG2D ligand expression. (A) NKG2D ligand expression, (B) MICA/B expression, and (C) HLA-E expression on DIPG cells. (D) Lysis of cell cultures following 4-hour incubation with NK cells at the noted effector:target ratios. N = 3. Error bars represent SEM. Significance evaluated by 2-way ANOVA. (E) Percentage of CD56hi NK cells expressing both TNF and IFNγ following a 4-hour incubation with the indicated cell cultures. N = 3, error bars represent SEM. Significance evaluated by one-way ANOVA.
Discussion
Despite the success in treating certain cancers with immunotherapy, such as acute lymphoblastic leukemia using CD19-directed CAR T cells and metastatic melanoma using checkpoint blockade, most malignancies, including CNS tumors, remain untreatable with this modality, although recent successes suggest a breakthrough may be on the horizon.8–10,33 Most research performed to date has been on adult GBM. However, examination of the correlation between tumor immune infiltrate and patient survival14 (Fig. 1) suggests that the role of the immune system in the development and progression of pediatric brain tumors may be strikingly different from that of adult tumors. Furthermore, like DIPG, many pediatric CNS tumors are entities unique to childhood and often arise in distinct cell types and locations; hence, in order to design effective therapies that harness the power of the immune system, extrapolation of results from adult GBM to pediatric brain tumors will need to be validated in appropriate models of pediatric disease.
The uniqueness of pediatric brain tumors is supported by our evaluation of the immune infiltrate and immunomodulatory landscape in 3 types of pediatric brain tumor: pLGG, typified by World Health Organization grade I pilocytic astrocytoma, pHGG, including both anaplastic astrocytoma and GBM, and DIPG. We found that although both pLGG and pHGG have a considerable accumulation of CD163+ macrophages and CD8+ T cells, DIPG tumors do not have an increase in any immune cells over nontumor control tissue, nor do they express chemokines necessary to recruit these cells. This finding is consistent with a recent study showing low inflammation in the DIPG microenvironment.15 The absence of inflammatory mediators is an important consideration in the development of immunotherapy: In a tumor such as DIPG, which expresses neither the chemokines nor the inflammatory cytokines required to recruit immune cells, cellular products may best be delivered intratumorally, which may be possible thanks to the neurosurgical advances that have made stereotactic biopsy routine.
Several studies have demonstrated that the TME of adult GBM is highly immunosuppressive, effectively shielding the tumor from the body’s defense mechanisms. However, a recent study of pediatric brain tumors14 shows little expression of programmed cell death protein 1 or PD-L1 in tumor infiltrating cells or the presence of soluble NKG2D ligands in serum, suggesting that pediatric brain tumors do not rely on these mechanisms of immune evasion. We found that DIPGs had only a marginal increase in B7-H3, as well as VEGFA, although an important caveat to our findings is that DIPG samples evaluated in TMA analysis were collected during autopsy, and levels of VEGFA may be altered during postmortem ischemia. Recent rigorous analysis of gene transcript expression as a function of postmortem interval has demonstrated that none of the transcripts we evaluated are altered in the brain upon delayed collection,34 suggesting that our DIPG patient tissue collection had minimal impact on our analyses.
Because of their potential to secrete immunomodulatory factors as well as support a polyclonal antitumor immune response through the production of cytokines, and presentation of immunogenic peptides to infiltrating T cells in the context of activating costimulatory molecules, our group and others are interested in developing engineered macrophages as a cellular immunotherapy.27,28 In our studies, only macrophages cultured with U87s or the H3.1-K27M mutant culture increased the expression of markers associated with immune suppression, suggesting that at least some DIPG patient tumors will not subvert the effects of immune-stimulatory adoptively transferred macrophages. The importance of macrophages/microglia in facilitating effective T-cell interactions with DIPG tumor cells is supported by our observation that none of the tumor cell cultures tested were effectively lysed by allogeneic T cells. Although this is consistent with previous observations that allogeneic T cells do not effectively kill cells of astrocytic or oligodendroglial origin,35 the lack of activity against SU-DIPG-XIII and -XVII was striking. This suggests that some DIPG tumor cells may have as yet undefined mechanisms of evading T-cell recognition, possibly by the lack of any distinct antigenic peptides sufficient to induce T-cell activity, even in healthy adult donors, in which an expansive memory cell population persists. Furthermore, as additional immune checkpoints such as the axes of TIM3 (T-cell immunoglobulin and mucin-domain containing–3), LAG3 (lymphocyte-activation gene 3), and TIGIT (T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domains) are elucidated, future studies may reveal the role they play in DIPG immune surveillance.
In contrast to T cells, NK cells were more effective when directly in contact with DIPG cell cultures. The mechanism for effective NK cell activation is likely the expression of one or more activating ligands for NKG2D, which is sufficient to induce cytokine production and targeted cytotoxic granule release. However, in adult GBM, expression of NKG2D ligands suppresses NK cell lysis to some degree as a result of TGFβ secretion, which inhibits NKG2D transcription and reduces its surface expression.36 The observation of poor NK-mediated lysis of SU-DIPG-XIII, which did not express the activating NKG2D ligand MICA/B but did secrete high levels of the NK-suppressive factor PDGF-BB, suggests that some amount of immunosuppression may play a role in local antitumor immunity. Although determining precise strategies for the development of NK cell–based immunotherapies is beyond the scope of the current work, we and others19,37,38 have demonstrated the utility of ex vivo expansion of NK cells for immunotherapy. If delivered locally or effectively recruited to the DIPG TME, NK cells may therefore represent a promising avenue for targeting DIPG tumors that express activating ligands, including, but not limited to, those for NKG2D.
Although our current study is limited by the existence of only a single DIPG cell culture with the H3.1 mutation, it is tempting to speculate that differences in tumor cell effect on immune cell function may be due in part to underlying differences in epigenetic modifications between H3.1- versus H3.3-K27M cell cultures. A previous study has demonstrated differences in gene expression patterns between H3.1-K27M and H3.3-K27M DIPG tumors, with the former associated with an expression signature similar to GBMs of the mesenchymal subtype.5 This subtype of GBM has greater immune cell infiltration and activation of macrophages; indeed, H3.1 DIPG tumors have increased accumulation of macrophages based on a 2-fold higher expression of CD145. Although further studies and larger cohorts are necessary, accumulated data suggest that effective immunotherapies for DIPG may be dependent on the underlying changes to the DIPG epigenome.
In conclusion, DIPG tumors are poorly infiltrated by the immune cells responsible for tumor elimination, nor do they express significant amounts of a number of immunosuppressive factors. Most DIPG cell cultures do not repolarize macrophages, but can be lysed by NK cells, suggesting that myeloid cell–based immunotherapies or interventions that improve recruitment of myeloid cells and cytotoxic lymphocytes such as NK cells may be an effective strategy to eliminate DIPG tumors, especially if therapies may be locally administered during biopsy.
Funding
This work was supported by the Sarah Hughes Foundation and the Pediatric Brain Tumor Research Fund.
Supplementary Material
Acknowledgments
We are grateful to Dr Michelle Monje for the initial culture and verification of all DIPG patient-derived cell cultures. We also thank Matthew Biery for suggestions on cell culture techniques, and Xumei Deng for data abstraction.
Conflict of interest statement. The authors declare no conflicts of interest.
Authorship statement. NAPL designed and performed all experiments and data analysis with help from KD and HMK. VH performed microscopy. AD and JS procured all patient samples and performed data abstraction. CW constructed TMAs and performed IHC staining. GD selected appropriate cases for TMAs. SNF analyzed Nanostring data. NAV and SESL provided clinically appropriate interpretations of data and edited the manuscript. CAC designed experiments, interpreted data, and edited the manuscript. NAPL and CAC wrote the manuscript.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl_5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lieberman NAP, Vitanza NA, Crane CA. Immunotherapy for brain tumors: understanding early successes and limitations. Expert Rev Neurother. 2018;18(3):251–259. [DOI] [PubMed] [Google Scholar]
- 3. Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooney T, Lane A, Bartels U, et al. Contemporary survival endpoints: an International Diffuse Intrinsic Pontine Glioma Registry study. Neuro Oncol. 2017;19(9):1279–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chheda ZS, Kohanbash G, Okada K, et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018;215(1):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ochs K, Ott M, Bunse T, et al. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology. 2017;6(7):e1328340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med. 2018;24(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glass R, Synowitz M. CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol. 2014;128(3):347–362. [DOI] [PubMed] [Google Scholar]
- 12. Griesinger AM, Birks DK, Donson AM, et al. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol. 2013;191(9):4880–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haberthur K, Brennan K, Hoglund V, et al. NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther. 2016;17(12):1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plant AS, Koyama S, Sinai C, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol. 2018;137(2):269–278. [DOI] [PubMed] [Google Scholar]
- 15. Lin GL, Nagaraja S, Filbin MG, Suvà ML, Vogel H, Monje M. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lieberman NAP, DeGolier K, Haberthur K, et al. An uncoupling of canonical phenotypic markers and functional potency of ex vivo-expanded natural killer cells. Front Immunol. 2018;9:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bieńkowski M, Preusser M. Prognostic role of tumour-infiltrating inflammatory cells in brain tumours: literature review. Curr Opin Neurol. 2015;28(6):647–658. [DOI] [PubMed] [Google Scholar]
- 21. Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15(8):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber M, Moebius P, Büttner-Herold M, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas—an immunohistochemical study. Br J Cancer. 2015;113(3):510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Araki K, Morita M, Bederman AG, et al. Translation is actively regulated during the differentiation of CD8+ effector T cells. Nat Immunol. 2017;18(9):1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(Suppl 7:vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 27. Andreesen R, Hennemann B, Krause SW. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol. 1998;64(4):419–426. [DOI] [PubMed] [Google Scholar]
- 28. Moyes KW, Lieberman NA, Kreuser SA, et al. Genetically engineered macrophages: a potential platform for cancer immunotherapy. Hum Gene Ther. 2017;28(2):200–215. [DOI] [PubMed] [Google Scholar]
- 29. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wheeler KC, Jena MK, Pradhan BS, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS One. 2018;13(1):e0191040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maccalli C, Volontè A, Cimminiello C, Parmiani G. Immunology of cancer stem cells in solid tumours. A review. Eur J Cancer. 2014;50(3):649–655. [DOI] [PubMed] [Google Scholar]
- 32. Gersuk GM, Westermark B, Mohabeer AJ, Challita PM, Pattamakom S, Pattengale PK. Inhibition of human natural killer cell activity by platelet-derived growth factor (PDGF). III. Membrane binding studies and differential biological effect of recombinant PDGF isoforms. Scand J Immunol. 1991;33(5):521–532. [DOI] [PubMed] [Google Scholar]
- 33. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu Y, Wang L, Yin Y, Yang E. Systematic analysis of gene expression patterns associated with postmortem interval in human tissues. Sci Rep. 2017;7(1):5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171(1):368–379. [DOI] [PubMed] [Google Scholar]
- 36. Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.