Abstract
Background
microRNAs have been reported to play critical roles in cancer and to have potential as diagnostic biomarkers. During miRNA biogenesis, one strand of the miRNA hairpin precursor is preferentially selected as a functionally mature miRNA, while the other strand is typically degraded. Arm switching occurs when the strand preference is changed. This preference can be different and can change dynamically depending upon the species, tissue types, or development stages. Due to recent advances in next-generation sequencing methods, arm switching has been observed in a variety of cancers.
Methods
A tumour miRNA-Seq dataset was collected from The Cancer Genome Atlas (TCGA). The support vector machine (SVM) method combined with 5-fold cross validation was applied to select the best combination of arm-switched miRNA tumour markers. Survival analysis was also applied to identify patient survival associated miRNA markers.
Findings
We observed 51 arm-switched miRNAs and of these, 7 were associated with patient survival. Twenty-three 1-combination arm switching miRNAs with excellent diagnostic value were identified. Interestingly, ovarian cancer showed a significant difference in arm switching pattern compared with 32 other cancers.
Interpretation
These results suggest that arm switching miRNAs could be used as potential biomarkers for various cancers.
Fund
This work was partially supported by the National Natural Science Foundation of China (no. 61472158, 61572227), and University of Macau Faculty of Health Sciences (MYRG2016-00101-FHS).
Keywords: microRNA, Arm switching, Pan-cancer, Biomarker, SVM, Survival analysis
Research in context.
Evidence before this study
microRNA is a ~20 nucleotide RNA derived from a hairpin-like precursor miRNA. miRNA acts as an important regulator of mRNA expression levels. miRNAs have been reported to play critical roles in tumours and to have potential as diagnostic biomarkers. Arm switching denotes a biochemical phenomenon where the mature miRNA switches between one of the two arms of precursor miRNA and has been observed to occur in different species, tissue types, or development stages. Arm switching is also found in different tumours and their adjacent normal tissues. With advances in next-generation sequencing in tumours, particularly The Cancer Genome Atlas (TCGA) dataset, it has now become possible to mine and identify dynamic changes in RNA transcripts, and to associate these with patient survival information. The TCGA miRNA-Seq dataset makes searching arm switching events in pan-cancers now possible.
Added value of this study
miRNA expression levels can be utilized as potential diagnostic biomarkers for many cancers, while the application of arm switching miRNA in this context remains lacking. We identified and then compared arm switching events between tumour tissues and tumour-adjacent tissues and also applied artificial intelligence to exploit these results in order to obtain a panel of biomarkers. Hence, although the mechanism of arm switching remains unclear, we identified arm switching miRNAs with potential use as biomarkers for various cancers with excellent diagnostic and prognostic value.
Implications of all the available evidence
Our results suggest that miRNA arm switching occurs in specific cancers in a specific pattern. Such knowledge would be useful not only in enlightening basic molecular biology mechanisms, but also in gaining knowledge in pathophysiology of cancer.
Alt-text: Unlabelled Box
1. Introduction
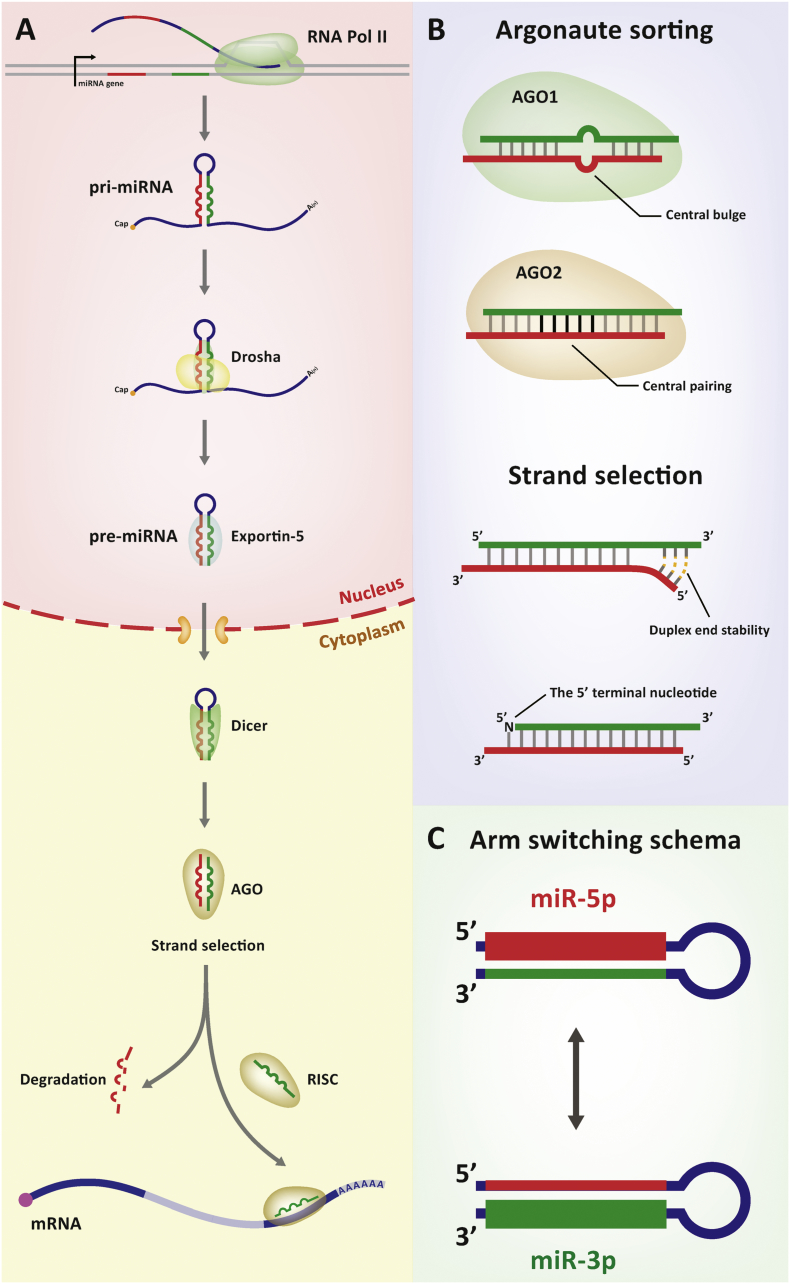
miRNAs are single-stranded endogenous small non-coding RNAs that comprise 1–3% of the human genome [1]. miRNAs have been found to play a crucial role in maintaining and dynamically altering the levels of mRNAs. One of the well-known functions of miRNA is the post-transcriptional regulation of gene expression [2]. miRNAs are initially transcribed as primary transcripts (pri-miRNAs) by RNA polymerase II (Pol II) and are subsequently cleaved to 65–70 nucleotide precursors (pre-miRNAs) by RNA endonuclease Drosha [3,4]. pre-miRNAs occur as thermodynamically stable hairpin structures that are exported to the cytoplasm via Exportin-5 and processed to a short double-stranded RNA duplex by RNA endonuclease Dicer. miRNA duplexes are then loaded into particular types of AGO proteins. Following AGO loading, one strand is degraded [2,3]. The other strand preferentially remains and forms a mature RNA-induced silencing complex (RISC) that binds to the complementary mRNA and cleaves the transcript products. This action prevents the translation of mRNAs into proteins [5,6]. Canonical miRNA biogenesis is shown in Fig. 1A.
Fig. 1.
Canonical miRNA biogenesis and arm switching schema. (A) Biogenesis of miRNA is shown from transcription of the primary miRNA to targeting of mRNA via the RISC complex. (B) Argonaute (AGO) sorting and selection of the two strands of miRNA duplex are shown. The two arms are labelled with red and green, respectively. (C) Arm switching schema is shown. The blue line represents the pre-miRNA also known as the hairpin. The red and green lines represent the locations of the miR-5p and miR-3p arms, respectively. The thickness of the red or green line indicates the expression level.
In the short double-stranded RNA duplex, the strand with presumable biological activity is named the guide strand, while the discarded strand is termed the passenger strand (or miRNA star) [7]. Fig. 1B shows the models of AGO sorting and strand selection. The central mismatching and bulge on the miRNA duplex can promote sorting of the duplex to the appropriate type of AGO protein in Drosophila [7]. In contrast, the four human AGO proteins (AGO1–4) are associated with miRNA duplexes without any clear preference [4]. Strand selection is not random, and some selection factors have been revealed. Duplex end stability is one determinant factor, where the strand whose 5′ end has less structure is favoured to be chosen as the guide strand [8,9]. The 5′ terminal nucleotide effects the choice of guide strand and different AGO proteins prefer different nucleotides. For example, AGO1 tends to select the strand with U at the 5′ end as a guide strand [[9], [10], [11]]; while the pocket within AGO2 prefers a pA or pU 5′ terminus [12].
The asymmetry of miRNA strand selection does not necessarily imply that the passenger strand is non-functional. Many studies have demonstrated that the passenger strand can also constitute a mature RISC and interact with target genes [[13], [14], [15]]. The 5′ and 3′ side of the pre-miRNA hairpin structure define the two arms of miRNA. The miR-5p and miR-3p represent 5′ mature miRNA and 3′ mature miRNA, respectively, as shown in Fig. 1C. In different tissues, developmental stages and species, the biological system may select different arms as the dominant expressed one [[16], [17], [18]]. The phenomenon of alternative strand selection has been termed ‘arm switching’ [19]. The molecular mechanisms that underlie miRNA strand selection and that lead to arm switching are not entirely clear. Previous studies have suggested that Dicer cleavage governs arm switching [[20], [21], [22]]. Alternative Drosha processing which changes the thermodynamic stability of the miRNA duplex ends can also explain an arm switching event [23]. Both arms could be co-accumulated as miRNA pairs in some tissues while being subjected to arm selection in other tissues [18]. Arm switching can frequently occur (~11%) in insects between orthologous miRNAs [24]. Other studies have shown that two arms from the same precursor tend to have different targeting properties [25]. In brief, arm switching provides a basic mechanism to evolve the function of miRNA and may be a fundamental mechanism of miRNA diversification [14,19,[26], [27], [28]].
The importance of miRNAs in cancer has been demonstrated via direct interaction with oncogenes or tumour suppressor genes [29]. Differentially expressed miRNAs have been proposed as biomarkers for cancers [30,31]. Circulating miRNA could also be a potential biomarker for cancer diagnosis and prognosis [32]. Moreover, some cancer-related studies have integrated miR-5p and miR-3p together [[33], [34], [35]]. For example, miR-193a-5p and miR-193a-3p were found significantly increased in breast cancer and revealed that strand expression preference may be a means of modulating miRNA function [36]. miR-5p and miR-3p differential expression between tumour tissue and tumour-adjacent tissue has been reported in gastric cancer [37]. miR-5p and miR-3p have been found that co-express and cross-target in colon cancer cells [38]. Moreover, dysregulation of miR-5p/miR-3p specific miRNAs were found in gastric cancer in a TCGA data set [39]. Furthermore, a study of specific differences in the function of an arm-switched miRNA in a lung cancer has been performed [40]. Recently, a database for small RNA sequencing in human cancer research can visualize expression of arm switching miRNAs has been published [41]. Although the mechanism of arm switching is not fully understood, some studies have linked arm switching to cancers. Nonetheless, there are still no miRNA biomarkers based on arm switching to our knowledge. In this study, we investigated the arm switching pattern in different tumours and demonstrated that some pre-miRNAs tend to select different arms in tumour and tumour-adjacent tissues. We also correlated arm switching events in pan-cancers with patient survival. Our results support the notion that miRNA arm switching could be a novel biomarker for different cancers.
2. Materials and methods
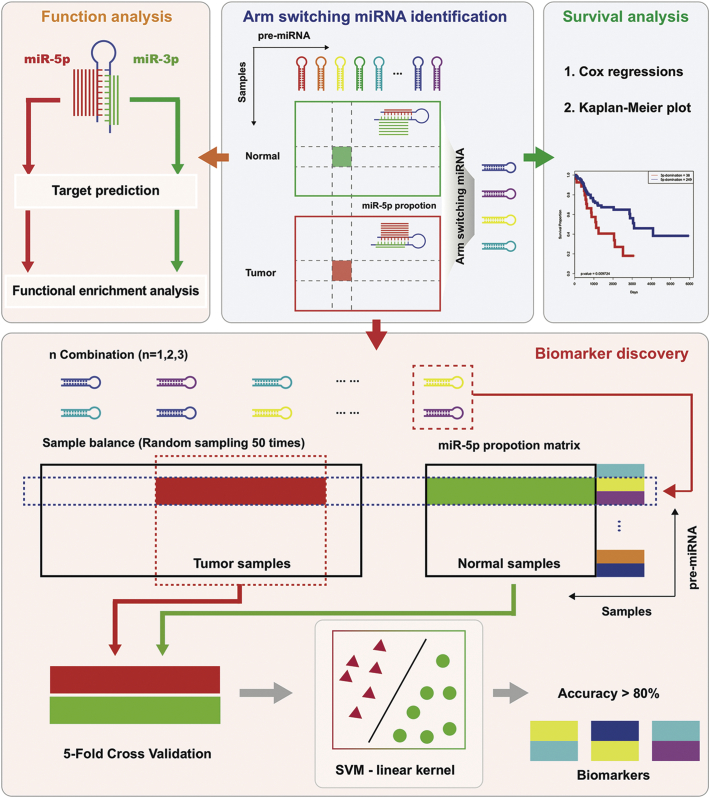
In order to discover novel tumour biomarkers based on miRNA arm switching, we implemented a workflow as shown in Fig. 2. The overall workflow contains four parts: arm switching miRNA identification, enrichment analysis, survival analysis, and biomarker discovery. The arm switching miRNA identification module marks strand selection preference between tumour and tumour-adjacent samples for each pre-miRNA initially and then identifies arm-switched miRNAs. The function analysis module annotates different arms of pre-miRNA and reveals the function for each arm for each biomarker candidate. The survival analysis module is used to identify arm-switched miRNAs which are associated with patient survival. The biomarker discovery module applies SVM to find combination biomarkers of arm-switched miRNAs.
Fig. 2.
Workflow of this study. The study is divided into four parts: arm switching miRNA identification, functional enrichment analysis, survival analysis, and biomarker discovery. Data sources and tools used in each part are provided in the methods.
2.1. Data sources
Thirty three cancer types including 11158 datasets were collected from the public database TCGA (http://cancergenome.nih.gov/). A summary table of the data sets that includes the cancer types are shown in Supplementary Table 1. The sample types we collected were tumour tissue and tumour-adjacent tissue (Normal tissue). Processed miRNA-Seq data were directly extracted from tab-delimited (.txt) data files in level 3. The survival information was obtained from the clinical files and incomplete samples were excluded from the survival analysis. Basic information of miRNAs was extracted from miRBase (Release 21, http://www.mirbase.org/) [42]. The annotation of TCGA miRNA-Seq level 3 data is based on miRBase v16, so there are only 1046 pre-miRNAs of which 318 pre-miRNAs are annotated with two arms. The experiment validated targets were extracted from miRTarBase 7.0 [43] and TarBase 8.0 [44].
2.2. Arm switching miRNA identification
To evaluate the arm switching patterns, we calculated a miR-5p ratio for each pre-miRNA in each sample originally developed by Hu et al. [45]. We defined an average miR-5p ratio arm switch criteria for multi-samples in this study as:
miR-5p (or miR-3p) represents the expression RPM value of miR-5p (or miR-3p). N is the number of samples in each tumour. We determine the dominant arm by averaging the miR-5p ratio. The ratio was adopted as a measure of the arm selection, where 1 ≥ ratio > 0.5 indicates miR-5p dominant expression, and 0.5 > ratio ≥0 indicates miR-3p dominant expression.
In normal or tumour samples, we labelled each pre-miRNA by the above calculation. We then defined an arm switching event between normal and tumour samples by finding a transformation between miR-5p and miR-3p dominant expression.
2.3. Enrichment analysis
The experimentally validated targets were extracted from miRTarBase 7.0 [43] and TarBase 8.0 [44]. Bioconductor R package clusterProfiler [46] and GOSemSim [47] were applied to perform enrichment analysis and to measure the similarity of GO terms, respectively. We chose the Wang method to measure the semantic similarity of GO terms [48].
2.4. Survival analysis
Multivariate Cox regressions were performed with the coxph function from the R survival library to test statistical significance [49].
2.5. Biomarker discovery
miRNA expression profiles can be used for classifying tumours [50], and up- or down- regulated miRNAs could be a biomarker for diagnosis [51]. Briefly, in most studies, miRNA signatures are the expression of miRNA. In this study, we applied arm switching events in place of expression levels to discover the differences in arm switching signatures between tumour and normal tissues.
The workflow of biomarker discovery is shown in Fig. 2. In the TCGA dataset, the number of tumour samples was always more than normal samples and it was necessary to balance the sample number before training. We sampled the same number of tumour samples as normal samples for each cancer. We randomly sampled 50 times to make the negative and positive training sets to have equal size, and the average classification accuracy is based on each time. For each k-combination miRNA, we applied SVM with linear kernel and combined a 5-fold cross validation method to select the best combination of tumour markers [52]. The average classification accuracy of each k-combination miRNA should be above 80% to meet the conditions of selection.
3. Results
3.1. Arm switching events identified between tumour and tumour-adjacent
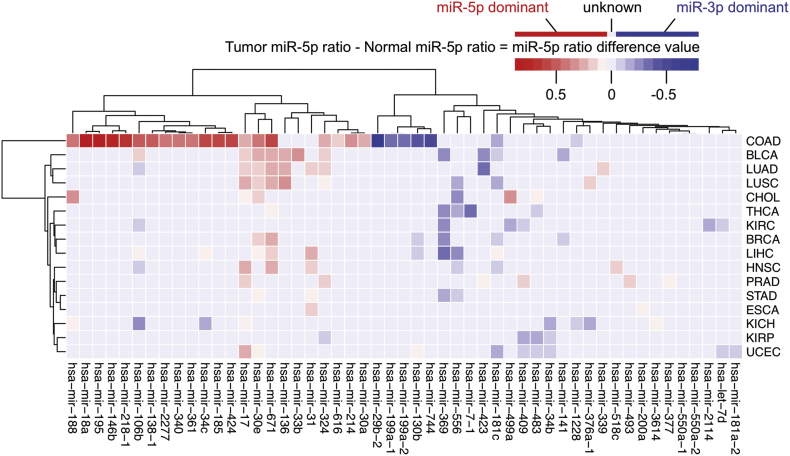
Based on the workflow described in methods (Fig. 2), we searched for arm switching miRNAs between tumour and tumour-adjacent tissues. We identified 51 miRNAs that displayed arm switching in 16 different tumours: Colon adenocarcinoma (COAD), Kidney renal papillary cell carcinoma (KIRP), Uterine corpus endometrial carcinoma (UCEC), Kidney chromophobe (KICH), Kidney renal clear cell carcinoma (KIRC), Esophageal carcinoma (ESCA), Prostate adenocarcinoma (PRAD), Bladder urothelial carcinoma (BLCA), Head and neck squamous cell carcinoma (HNSC), Lung adenocarcinoma (LUAD), Lung squamous cell carcinoma (LUSC), Cholangiocarcinoma (CHOL), Liver hepatocellular carcinoma (LIHC), Breast invasive carcinoma (BRCA), Thyroid carcinoma (THCA), and Stomach adenocarcinoma (STAD) with a criteria that the number of the tumour-adjacent samples is >8. Fig. 3 shows 51 arm switching miRNAs detected from 16 tumours vs. tumour-adjacent tissues (normal tissues). Results of basic statistical analysis of these arm switching miRNAs are in Supplementary Table 2.
Fig. 3.
Heat map of arm switching miRNA in different tumours. The value in the heat map was defined as the difference between tumour miR-5p ratio and tumour-adjacent (normal) miR-5p ratio. Red indicates the miR-5p dominantly expressed in a certain tumour compared to its adjacent tissue. Blue indicates the miR-3p dominantly expressed and light grey indicates unknown (not expressed or not switched). Rows indicate cancer types and columns indicate arm-switched miRNAs.
We found some miRNAs that could frequently arm switch. They were hsa-mir-30e (in 10 tumours), hsa-mir-17 (in 9 tumours), hsa-mir-671 (in 8 tumours), hsa-mir-106b (in 7 tumours), hsa-mir-181c (in 7 tumours), hsa-mir-556 (in 7 tumours), hsa-mir-324 (in 6 tumours), and hsa-mir-1228 (in 5 tumours). While most arm-switching events were consistent in arm preference (miR-5p to miR-3p or vice versa) in tumour tissues, hsa-mir-106b and hsa-mir-130b showed different arm preference depending upon the tumour (miR-5p dominant in some, miR-3p in others). COAD showed the most number of arm-switched miRNAs.
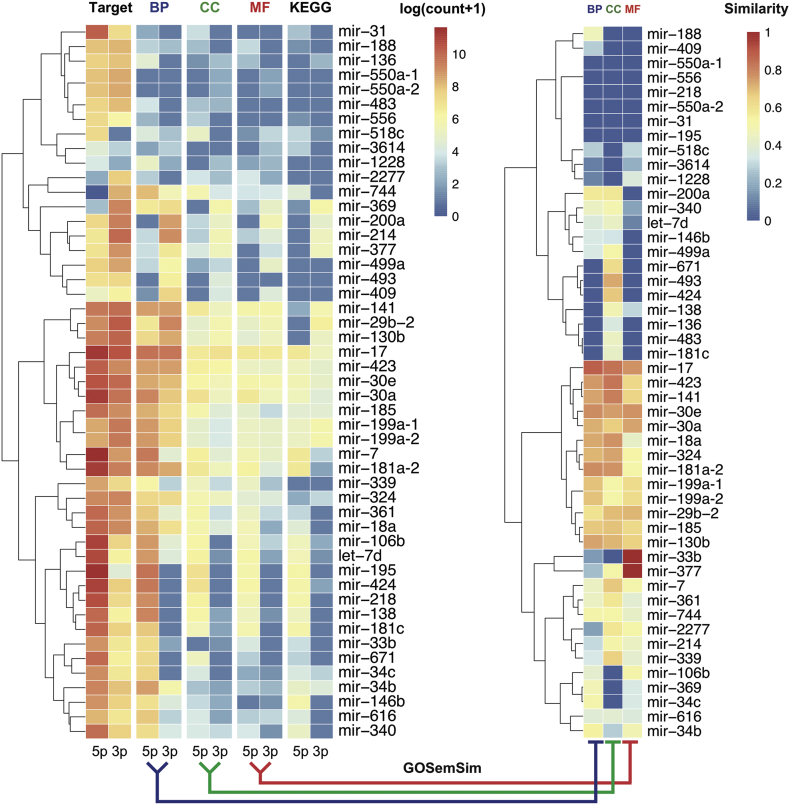
3.2. Functional enrichment analysis of paired miR-3p and miR-5p
In order to explore the arm switching effect of the 51 pre-miRNAs, we performed GO and KEGG enrichment analysis for miR-3p and miR-5p arms, respectively. For this analysis, we used the experimentally validated targets of 51 paired miRNAs extracted from miRTarBase 7.0 [43] and TarBase 8.0 [44]. The results of these 51 arm switching miRNAs in heat map format are shown in Fig. 4. The full enrichment analysis results in text format are shown in Supplementary Table 3. We found that for most pre-miRNAs, the respective targets of the two individual arms are associated with different terms, and similarity of terms shows different functions. While the arm switching event of some miRNAs do not affect the function, such as mir-30e and mir-17.
Fig. 4.
Heat map of functional comparison of arm-switched miRNAs. The left heat map shows the target gene count, GO term count (BP, biological process; CC, cellular compartment; MF, molecular function), and KEGG pathway count. The value in the heat map is log (count +1). Rows are pre-miRNAs and columns are miR-5p and miR-3p arms, respectively. The right heat map shows the semantic similarity between enriched terms of the two arms. The detailed results of enrichment analysis of arm switching miRNAs are provided in Supplementary Table 3.
Heat map of functional comparison of arm-switched miRNAs. The left heat map shows the target gene count, GO term count (BP, biological process; CC, cellular compartment; MF, molecular function), and KEGG pathway count. The value in the heat map is log (count +1). Rows are pre-miRNAs and columns are miR-5p and miR-3p arms, respectively. The right heat map shows the semantic similarity between enriched terms of the two arms. The detailed results of enrichment analysis of arm switching miRNAs are provided in Supplementary Table 3.
3.3. Novel tumour biomarkers based on miRNA arm switching
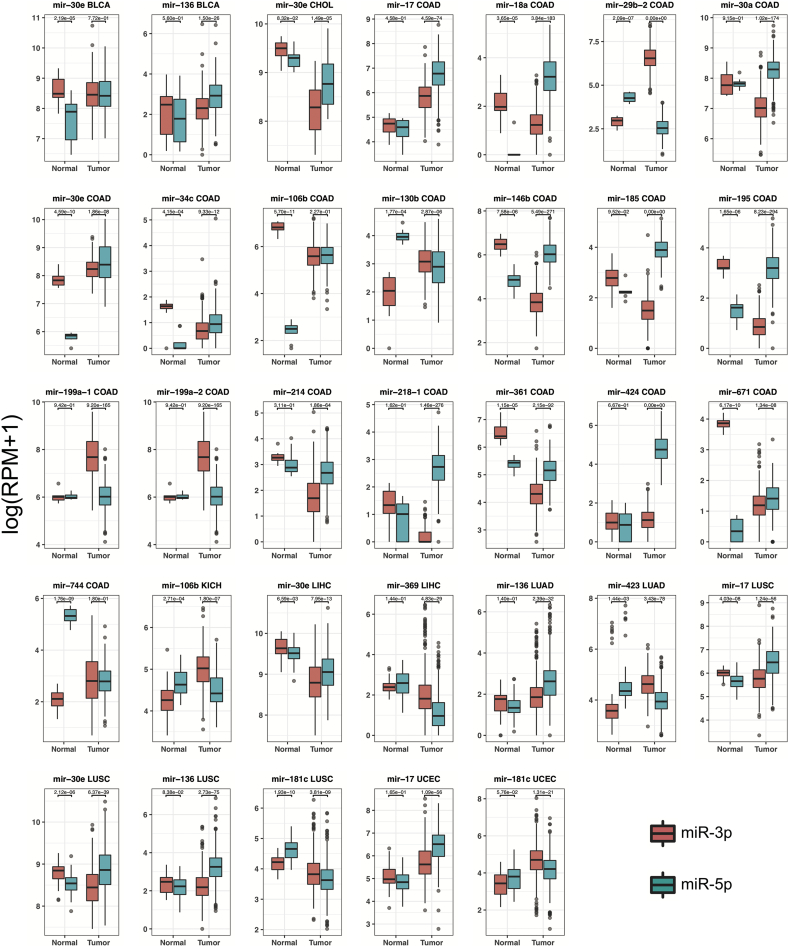
We applied SVM with 5-fold cross validation method to exhaustively search n (n = 1, 2, 3) combination of 51 arm switching miRNAs for 16 cancers (tumour-adjacent samples number ≥ 8). One-, 2- and 3-combination biomarkers with average accuracy are listed in Supplementary Table 4 whose average classification power accuracy is >80%. From Supplementary Table 4, we found 23 unique arm switching miRNAs from 8 cancers (BLCA, CHOL, COAD, KICH, LIHC, LUAD, LUSC, and UCEC). Boxplots of expression from the 2 arms of the 23 potential biomarkers that reached statistical significance are shown in Fig. 5.
Fig. 5.
Boxplot of expression of two arms in arm switching miRNA biomarkers. The boxplot illustrates the expression distribution of two arms from the pre-miRNA in different sample types (tumour or tumour-adjacent), using the log of RPM plus 1. The products from different arms are distinguished by colour (red, miR-3p and blue, miR-5p). The significance level value is shown above the box plots (t-test, p-value). The tissue types are marked below the box plots (Normal (tumour-adjacent) and tumour). The y-axis represents the expression level of reads log (RPM + 1).
Among the best biomarkers, hsa-mir-29b-2 selects miR-29-3p arm as dominantly expressed in tumour and the miR-5p ratio of hsa-mir-29b-2 can classify COAD tumour and normal with 100% accuracy. As a tumour promoter, miR-29-3p mediates epithelial-mesenchymal transition (EMT) and promotes metastasis in breast cancer and colon cancer [53]. The miR-5p ratio of hsa-mir-136 could classify tumour and normal in LUAD (accuracy: 0.83), LUSC (accuracy: 0.92), and BLCA (accuracy: 0.81). The miR-5p ratio of hsa-mir-30e could classify tumour and normal in BLCA (accuracy: 0.83), CHOL (accuracy: 0.93), COAD (accuracy: 0.96), LIHC (accuracy: 0.81), and LUSC (accuracy: 0.82). miR-136-5p and miR-30e-5p are dominantly expressed in tumour tissue (see Fig. 3).
3.4. Arm switching events identified in pan-cancer
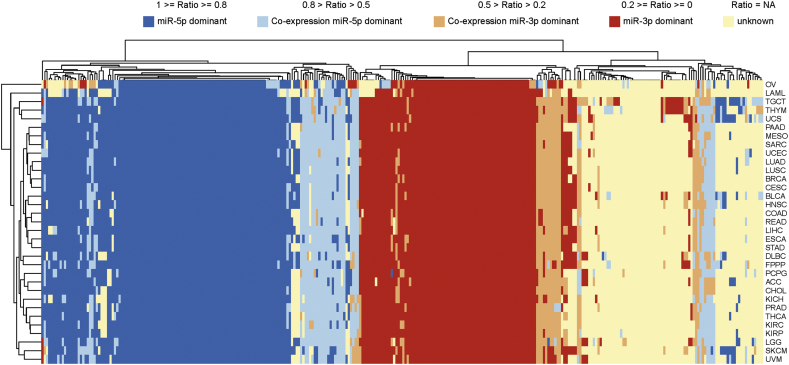
We also investigated arm switching between 33 different tumour types. Fig. 6 shows a landscape of arm switching events in pan-cancer. In Fig. 6, Ovarian serous cystadenocarcinoma (OV) is significantly different from other cancers in arm switching patterns. In most miRNAs, we could observe three patterns: miR-5p preference, miR-3p preference, and not expressed in tumours compared to others. When pre-miRNA arm preference changes, it usually does not change in many different cancers, which suggests arm switching may be a specific event for each cancer and not a general cancer mechanism.
Fig. 6.
Heat map of 318 pre-miRNA arm selection preference events in pan-cancer. Blue represents miR-5p is the dominant arm and red indicates miR-3p is dominant. The ratio was adopted as a measure of the arm selection, where 1 ≥ ratio ≥ 0.8 indicates miR-5p dominant expression, 0.8 > ratio > 0.5 indicates co-expression miR-5p dominant expression, 0.5 > ratio > 0.2 indicates co-expression miR-3p dominant expression, and 0.2 ≥ ratio ≥ 0 indicates miR-3p dominant expression. Arm switching degree under the detection threshold or when data is unavailable are filled by light orange.
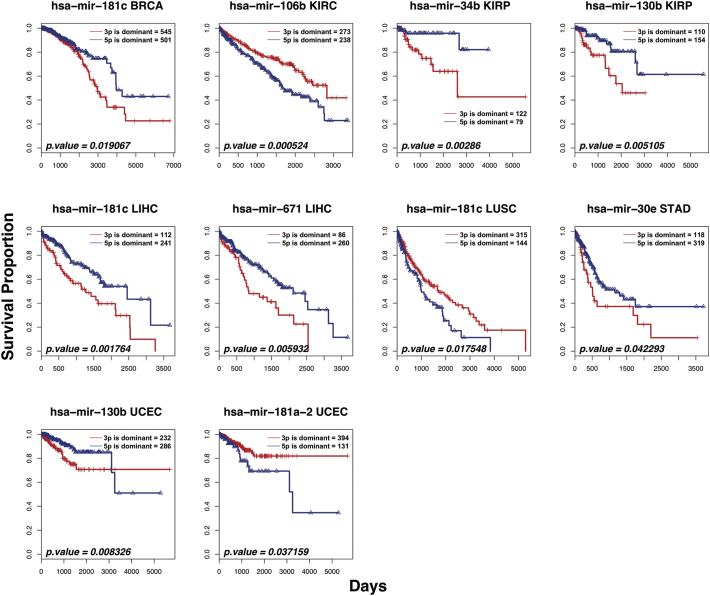
We tested the association of the identified 51 miRNAs based on miR-5p expression ratio of pre-miRNA with patient survival. We found 7 miRNAs that were highly associated with patient survival. The Kaplan-Meier plots of survival association with the 7 biomarkers are shown in Fig. 7. Ten associations were identified where arm-switched miRNAs were associated with increased survival. Some of the miRNAs were associated with increased survival in more than one cancer. For example, hsa-mir-181c was associated with BRCA, LIHC, and LUSC. Conversely, 3 cancers (KIRP, LIHC, and UCEC) had more than one arm-switched miRNA associated with it. The strand dominance did not favour miR-5p or miR-3p as some miRNAs were represented by both strands. While those miRNAs identified were significant (p < .05, Cox regression), some of the differences in survival were dramatic such as hsa-mir-34b (KIRP) and hsa-mir-181a-2 (UCEC).
Fig. 7.
Kaplan-Meier plot of survival associated biomarkers. Seven different pre-miRNAs biomarkers in 7 tumours are shown. The survival proportion and survival in days are indicated. Blue and red represent dominant expression of miR-5p or miR-3p, respectively. Number of samples and statistical significance from Cox proportional hazards regression are indicated inside the plots. Abbreviations for the tumour types are: BRCA, Breast invasive carcinoma; KIRC, Kidney renal clear cell carcinoma; KIRP, Kidney renal papillary cell carcinoma; LIHC, Liver hepatocellular carcinoma; LUSC, Lung squamous cell carcinoma; STAD, Stomach adenocarcinoma; UCEC, Uterine corpus endometrial carcinoma.
4. Discussion
Cancer is a complex disease that involves multiple factors that play a role in its development and progression. miRNA is one of these key factors, and its dysregulated expression has been altered in different cancer types and during several stages of cancer. In the present study, we applied and validated miRNA arm switching expression as a basis for biomarker discovery. We identified 51 pre-miRNAs that displayed arm switching in different cancer types. All arms from 51 pre-miRNAs have been associated with cancer from previous studies. A summary of these studies are shown in Supplementary Table 5. We observed that the miR-5p and miR-3p were treated separately in those studies and were found dysregulated in different tumours. Some were reported as arm switching miRNAs or analysed as miR-3p and miR-5p together by previous studies. For example, in 2015, Pundhir et al. found mir-30e and mir-30a could arm switch in 9 different tissue cell lines based on ENCODE datasets [54]. Earlier studies have found that two arms of mir-17 could participate in the same biological process. In 2013, Yang et al. found that miR-17-5p and miR-17-3p could target TIMP3 at the same time to promote the growth and invasion of prostate cancer [55]. Shan et al. found that miR-17-5p and miR-17-3p induced liver cancer by targeting PTEN and GalNT7 [56]. Impressively, some arm switching miRNAs from the 51 pre-miRNAs could be circulating miRNA biomarkers for cancers. They were serum miRNAs: miR-369-5p, miR-30e-5p, and miR-423-3p and plasma miRNAs: miR-671-3p, miR-483-3p, and miR-744-5p.
While the TCGA database has proven to be a rich resource for biomarker studies, our results would be strengthened if we could validate some of our findings in independent data sets. Taking a sampling of datasets from the GEO database, we were able to confirm arm switching of mir-324, mir-181c, mir-556, mir-195, mir-424, mir-185, and mir-483 in the same direction (miR-5p dominant to miR-3p dominant or vice versa) and in same or similar tumour types. The GEO dataset, platform, tumour type and number of samples and t-test values are provided in Supplementary Table 6. GO and KEGG analysis of their experimentally verified targets are provided in Supplementary Fig. 1. As we did not exhaustively search the GEO database and we confirmed in different microarray and sequencing platforms, there may be more arm-switched miRNAs that could be validated.
We also identified 7 arm switching miRNAs associated with patient survival and 23 high accuracy 1-combination arm switching miRNAs that classify tumour and normal and many 2- and 3-combination biomarkers. Based on the enrichment analysis, we were not able to determine whether the two arms of miRNA work cooperatively or not. The arm switching pattern generated from 33 tumours denote that arm switching does not frequently occur, and most miRNAs tend to conservatively choose one arm as the dominantly expressed one.
Some studies analysed miR-3p and miR-5p forms together. Dual strands of hsa-mir-150 and hsa-mir-145 act as tumour-suppressors in prostate cancer and lung cancer respectively [57,58]. In previous studies, miR-195-5p tends to suppress the development of the tumour. In colon cancer, miR-195-5p promotes cell apoptosis and suppresses tumour cell invasion and metastasis by downregulating the Bcl-2 gene [59]. In liver cancer, miR-195-5p targets PHF19 and CBX4 to suppress the development of the tumour [60,61]. Based on Supplementary Table 3, we found that miR-195-5p has a well-defined biological function, while miR-195-3p had no enriched pathways and non-significant GO terms. miR-17-5p and miR-17-3p can enrich the same KEGG pathways related to cancer, such as Transcriptional mis-regulation in cancer (hsa05202), Colorectal cancer (hsa05210), Glioma (hsa05214), Bladder cancer (hsa05219), Chronic myeloid leukemia (hsa05220), Hepatocellular carcinoma (hsa05225), and Gastric cancer (hsa05226).
The expression levels of miRNAs may be small, and this limits sensitivity. Nonetheless, our study demonstrates the concept of using miRNA arm-switching in cancer tissues to observe dynamic changes in miRNA at the molecular level. As sequencing technologies advance, and costs for sequence determination decrease rapidly, the feasibility of sequencing tissues for biomarkers is becoming greater. Our studies indicate here specific miRNA arms that should be interrogated as markers when tissue is available. Finally, while the function of RNA arm switching events remains to be elucidated, our results provide additional motivation for its investigation. Such knowledge would be useful not only in enlightening basic molecular biology mechanisms, but also in gaining knowledge in pathophysiology of cancer.
The following are the supplementary data related to this article.
GO and KEGG analysis results of verified targets of two arms.
TCGA dataset used in this study.
Results of basic statistical analysis of arm switching miRNAs.
Enrichment analysis results.
Combination biomarker list.
Information of 51 arm switching miRNAs.
External dataset validation of arm-switched miRNAs from TCGA database.
Acknowledgments
Acknowledgements
The authors would like to thank The Cancer Genome Atlas (TCGA) for making the dataset available publicly. We also thank all members of the Wong Laboratory who provided us with critical comments and feedback. This work was performed in part at the high performance computing cluster (HPCC) which is supported by the Information and Communication Technology Office (ICTO) of the University of Macau.
Funding sources
This work was partially supported by the National Natural Science Foundation of China (no. 61472158, 61572227), and University of Macau Faculty of Health Sciences (MYRG2016-00101-FHS).
Declarations of interests
The authors have no conflicts of interest.
Author contributions
Garry Wong and Liang Chen designed research and wrote the manuscript; Liang Chen and Huiyan Sun performed the analysis; Changliang Wang, Yang Yang, and Menglei Zhang performed data collection and pre-processing of data. All authors approved the manuscript.
References
- 1.Zhao Y., Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32(4):189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 5.Pillai R.S. MicroRNA function: multiple mechanisms for a tiny RNA? RNA (New York, NY) 2005;11(12):1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 7.Okamura K., Liu N., Lai E.C. Distinct mechanisms for microRNA strand selection by Drosophila Argonautes. Mol Cell. 2009;36(3):431–444. doi: 10.1016/j.molcel.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khvorova A., Reynolds A., Jayasena S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115(2):209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 9.Noland C.L., Doudna J.A. Multiple sensors ensure guide strand selection in human RNAi pathways. RNA (New York, NY) 2013;19(5):639–648. doi: 10.1261/rna.037424.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H.Y., Yan Z., Xu Y. Sequence features associated with microRNA strand selection in humans and flies. BMC Genomics. 2009;10:413. doi: 10.1186/1471-2164-10-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghildiyal M., Xu J., Seitz H., Weng Z., Zamore P.D. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA (New York, NY) 2010;16(1):43–56. doi: 10.1261/rna.1972910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H.I., Katsura A., Yasuda T. Small-RNA asymmetry is directly driven by mammalian Argonautes. Nat Struct Mol Biol. 2015;22(7):512–521. doi: 10.1038/nsmb.3050. [DOI] [PubMed] [Google Scholar]
- 13.Ruby J.G., Stark A., Johnston W.K., Kellis M., Bartel D.P., Lai E.C. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007;17(12):1850–1864. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura K., Phillips M.D., Tyler D.M., Duan H., Chou Y.T., Lai E.C. The regulatory activity of microRNA* species has substantial influence on microRNA and 3' UTR evolution. Nat Struct Mol Biol. 2008;15(4):354–363. doi: 10.1038/nsmb.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo L., Lu Z. The fate of miRNA* strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu N., Okamura K., Tyler D.M., Phillips M.D., Chung W.J., Lai E.C. The evolution and functional diversification of animal microRNA genes. Cell Res. 2008;18(10):985–996. doi: 10.1038/cr.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang H.R., Schoenfeld L.W., Ruby J.G. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev. 2010;24(10):992–1009. doi: 10.1101/gad.1884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ro S., Park C., Young D., Sanders K.M., Yan W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 2007;35(17):5944–5953. doi: 10.1093/nar/gkm641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S., Hui J.H., Marco A., Ronshaugen M. MicroRNA evolution by arm switching. EMBO Rep. 2011;12(2):172–177. doi: 10.1038/embor.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudoit S., Fridlyand J. A prediction-based resampling method for estimating the number of clusters in a dataset. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0036. RESEARCH0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz D.S., Hutvagner G., Du T., Xu Z., Aronin N., Zamore P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115(2):199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 22.Wilson R.C., Tambe A., Kidwell M.A., Noland C.L., Schneider C.P., Doudna J.A. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol Cell. 2015;57(3):397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H., Ye C., Ramirez D., Manjunath N. Alternative processing of primary microRNA transcripts by Drosha generates 5′ end variation of mature microRNA. PLoS One. 2009;4(10) doi: 10.1371/journal.pone.0007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marco A., Hui J.H., Ronshaugen M., Griffiths-Jones S. Functional shifts in insect microRNA evolution. Genome Biol Evol. 2010;2:686–696. doi: 10.1093/gbe/evq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marco A., Macpherson J.I., Ronshaugen M., Griffiths-Jones S. MicroRNAs from the same precursor have different targeting properties. Silence. 2012;3(1):8. doi: 10.1186/1758-907X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L., Zhang H., Zhao Y., Yang S., Chen F. Selected isomiR expression profiles via arm switching? Gene. 2014;533(1):149–155. doi: 10.1016/j.gene.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 27.de Wit E., Linsen S.E., Cuppen E., Berezikov E. Repertoire and evolution of miRNA genes in four divergent nematode species. Genome Res. 2009;19(11):2064–2074. doi: 10.1101/gr.093781.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12(12):846. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 29.Hayes J., Peruzzi P.P., Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Liu R., Chen X., Du Y. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58(3):610–618. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 31.Lee E.J., Gusev Y., Jiang J. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell P.S., Parkin R.K., Kroh E.M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X., Zeng R., Wu S., Zhong J., Yang L., Xu J. Comprehensive expression analysis of miRNA in breast cancer at the miRNA and isomiR levels. Gene. 2015;557(2):195–200. doi: 10.1016/j.gene.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H., Yang S., Guo L., Zhao Y., Shao F., Chen F. Comparisons of isomiR patterns and classification performance using the rank-based MANOVA and 10-fold cross-validation. Gene. 2015;569(1):21–26. doi: 10.1016/j.gene.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Mitra R., Lin C.C., Eischen C.M., Bandyopadhyay S., Zhao Z. Concordant dysregulation of miR-5p and miR-3p arms of the same precursor microRNA may be a mechanism in inducing cell proliferation and tumorigenesis: a lung cancer study. RNA (New York, NY) 2015;21(6):1055–1065. doi: 10.1261/rna.048132.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai K.W., Leung C.M., Lo Y.H. Arm selection preference of MicroRNA-193a varies in breast cancer. Sci Rep. 2016;6:28176. doi: 10.1038/srep28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S.C., Liao Y.L., Ho M.R., Tsai K.W., Lai C.H., Lin W.C. miRNA arm selection and isomiR distribution in gastric cancer. BMC Genomics. 2012;(13 Suppl 1):S13. doi: 10.1186/1471-2164-13-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choo K.B., Soon Y.L., Nguyen P.N., Hiew M.S., Huang C.J. MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells. J Biomed Sci. 2014;21:95. doi: 10.1186/s12929-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo W.T., Su M.W., Lee Y.L. Bioinformatic interrogation of 5p-arm and 3p-arm specific miRNA expression using TCGA datasets. J Clin Med. 2015;4(9):1798–1814. doi: 10.3390/jcm4091798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin M.H., Chen Y.Z., Lee M.Y. Comprehensive identification of microRNA arm selection preference in lung cancer: miR-324-5p and -3p serve oncogenic functions in lung cancer. Oncol Lett. 2018;15(6):9818–9826. doi: 10.3892/ol.2018.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung I.F., Chang S.J., Chen C.Y. YM500v3: a database for small RNA sequencing in human cancer research. Nucleic Acids Res. 2017;45(D1) doi: 10.1093/nar/gkw1084. D925-D31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou C.H., Shrestha S., Yang C.D. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karagkouni D., Paraskevopoulou M.D., Chatzopoulos S. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018;46(D1) doi: 10.1093/nar/gkx1141. D239-D45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu W., Wang T., Yue E., Zheng S., Xu J.H. Flexible microRNA arm selection in rice. Biochem Biophys Res Commun. 2014;447(3):526–530. doi: 10.1016/j.bbrc.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu G., Li F., Qin Y., Bo X., Wu Y., Wang S. GOSemSim: an R package for measuring semantic similarity among GO terms and gene products. Bioinformatics. 2010;26(7):976–978. doi: 10.1093/bioinformatics/btq064. [DOI] [PubMed] [Google Scholar]
- 48.Wang J.Z., Du Z., Payattakool R., Yu P.S., Chen C.F. A new method to measure the semantic similarity of GO terms. Bioinformatics. 2007;23(10):1274–1281. doi: 10.1093/bioinformatics/btm087. [DOI] [PubMed] [Google Scholar]
- 49.Therneau T.M., Grambsch P.M. Springer Science & Business Media; 2013. Modeling survival data: extending the Cox model. [Google Scholar]
- 50.Lu J., Getz G., Miska E.A. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 51.Andorfer C.A., Necela B.M., Thompson E.A., Perez E.A. MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol Med. 2011;17(6):313–319. doi: 10.1016/j.molmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Kohavi R. Vol. 1995. Ijcai; Montreal, Canada: 1995. A study of cross-validation and bootstrap for accuracy estimation and model selection; pp. 1137–1145. [Google Scholar]
- 53.Jiang H., Zhang G., Wu J.H., Jiang C.P. Diverse roles of miR-29 in cancer (review) Oncol Rep. 2014;31(4):1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 54.Pundhir S., Gorodkin J. Differential and coherent processing patterns from small RNAs. Sci Rep. 2015;5:12062. doi: 10.1038/srep12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X., Du W.W., Li H. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013;41(21):9688–9704. doi: 10.1093/nar/gkt680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shan S.W., Fang L., Shatseva T. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J Cell Sci. 2013;126(Pt 6):1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 57.Okato A., Arai T., Kojima S. Dual strands of pre-miR150 (miR1505p and miR1503p) act as antitumor miRNAs targeting SPOCK1 in naive and castration-resistant prostate cancer. Int J Oncol. 2017;51(1):245–256. doi: 10.3892/ijo.2017.4008. [DOI] [PubMed] [Google Scholar]
- 58.Mataki H., Seki N., Mizuno K. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7(44):72084–72098. doi: 10.18632/oncotarget.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B., Tan Z., Song Y. Study on the molecular regulatory mechanism of MicroRNA-195 in the invasion and metastasis of colorectal carcinoma. Int J Clin Exp Med. 2015;8(3):3793–3800. [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng C., Li J., Wang Q. MicroRNA-195 functions as a tumor suppressor by inhibiting CBX4 in hepatocellular carcinoma. Oncol Rep. 2015;33(3):1115–1122. doi: 10.3892/or.2015.3734. [DOI] [PubMed] [Google Scholar]
- 61.Xu H., Hu Y.W., Zhao J.Y. MicroRNA-195-5p acts as an anti-oncogene by targeting PHF19 in hepatocellular carcinoma. Oncol Rep. 2015;34(1):175–182. doi: 10.3892/or.2015.3957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GO and KEGG analysis results of verified targets of two arms.
TCGA dataset used in this study.
Results of basic statistical analysis of arm switching miRNAs.
Enrichment analysis results.
Combination biomarker list.
Information of 51 arm switching miRNAs.
External dataset validation of arm-switched miRNAs from TCGA database.