Abstract Abstract
The order Melanosporales comprises a large group of ascomycetes, most of them mycoparasites, characterized by the production of usually ostiolate, translucent ascomata, unitunicate asci, and unicellular, pigmented ascospores with germ pores or germ slits. The most studied taxa are Melanospora and Sphaerodes, but the boundaries with other morphologically closely related genera are not well resolved. In this study, the taxonomy of Melanospora and related taxa have been re-evaluated based on the analysis of nuclear rDNA, actin and elongation factor genes sequences of fresh isolates and numerous type and reference strains. The genus Melanospora has been restricted to species with ostiolate ascoma whose neck is composed of intermixed hyphae, and with a phialidic asexual morph. Microthecium has been re-established for species of Melanospora and Sphaerodes without a typical ascomatal neck or, if present, being short and composed of angular cells similar to those of the ascomatal wall, and usually producing bulbils. Three new genera have been proposed: Dactylidispora, possessing ascospores with a raised rim surrounding both terminal germ pores; Echinusitheca, with densely setose, dark ascomata; and Pseudomicrothecium, characterized by ascospores with indistinct germ pores. Dichotomous keys to identify the accepted genera of the Melanosporales, and keys to discriminate among the species of Melanospora and Microthecium, as well as a brief description of the accepted species of both genera, are also provided.
Keywords: Ceratostomataceae , Dactylidispora , Echinusitheca , Melanosporales , Microthecium , Pseudomicrothecium , soil, Sphaerodes , 4 new taxa
Introduction
The family Ceratostomataceae (Winter 1887) includes nearly 100 species, often mycoparasitic and characterized by ostiolate and rostrate, or less frequently non-ostiolate, translucent ascomata, unitunicate and evanescent asci, brown or exceptionally hyaline, unicellular ascospores with a germ pore at each end, or less frequently with only one germ pore or a germ slit, and phialidic asexual morphs or bulbils. Currently, that family is included in the Melanosporales (Chaudhary et al. 2006, Zhang et al. 2006, Hibbett et al. 2007, Li et al. 2016, Schultes et al. 2017), although historically it had been placed in Aspergillales (Gaümann 1964), Hypocreales (Alexopoulos 1962, Spatafora and Blackwell 1994a, Rehner and Samuels 1995, Jones and Blackwell 1998, Zhang and Blackwell 2002) and Sphaeriales (Bessey 1950, Dennis 1968). This family comprises 11 sexually reproducing genera, i.e. Arxiomyces, Melanospora, Persiciospora, Pteridiosperma, Pustulipora, Rhytidospora, Scopinella, Setiferotheca, Sphaerodes, Syspastospora and Vittatispora. Melanospora, the largest genus of this family (more than 50 species), was established by Corda (1837) to accommodate Ceratostomachionea and two new species, Melanosporazamiae and Melanosporaleucotricha, with the former chosen later as the type species (Kowalski 1965). Melanospora is characterized by usually ostiolate ascomata with a long neck and a translucent, pale yellow to reddish brown ascomatal wall, and mostly smooth-walled, brown, ellipsoidal to citriform, rarely discoid or fusoid ascospores, with a depressed germ pore at each end, occasionally surrounded by a raised rim (Guarro et al. 2012). Related genera are Microthecium and Sphaerodes. The former was erected by Corda (1842) to distinguish Mi.zobelii from Melanospora spp. by the presence of non-ostiolate, usually immersed ascomata; and Sphaerodes was introduced by Clements (1909) to separate Melanosporaepisphaeria from Melanospora spp. by its reticulate ascospores. However, the generic boundaries between Melanospora and its relatives remained obscure. Doguet (1955) carried out a revision of Melanospora, synonymizing several species and transferring additional species from other genera, mostly from Sphaeroderma, which had been proposed by Fuckel (1877) and distinguished from Melanospora by the absence of an ascomatal neck. Doguet (1955) considered the production of a neck as a non-stable taxonomic character influenced by the nature of the substrate where the fungus grows, and segregated the genus in several sections on the basis of the morphology of the ascomata (presence or absence of neck, and its size when present) and ascospores (shape and ornamentation). The most comprehensive revision of Melanospora and related genera was carried out by Cannon and Hawksworth (1982), based mainly on the structure of the ascospore wall under SEM, resulting in the transfer of species of Microthecium to Melanospora and to Sphaerodes. However, recent molecular studies demonstrated that these two latter genera are polyphyletic (Zhang and Blackwell 2002, Fan et al. 2012, Li et al. 2016, Schultes et al. 2017). Other genera included in the family are: Arxiomyces, which produces ovoid to ellipsoidal ascospores with a rounded apex and a truncate base with a large sunken germ pore (Cannon and Hawksworth 1982, 1983); Persiciospora, characterized by ascospores with a pitted wall and a faint reticulation (Cannon and Hawksworth 1982); Pteridiosperma, with ascospores ornamented with longitudinal wing-like appendages (Krug and Jeng 1979); Pustulipora, distinguished by its ascospores with a germ pore at each end surrounded by a blistered, rarely cushion-like structure showing an irregular pustulate appearance (Cannon 1982); Rhytidospora, characterized by non-ostiolate ascomata with a cephalothecoid ascomatal wall (Krug and Jeng 1979); Scopinella, producing brown, cuboid-ellipsoidal ascospores with two prominent longitudinal germ slits (Cannon and Hawksworth 1982); Setiferotheca, which produces ascospores similar to those of Arxiomyces and ascomata with a crown of dark brown setae surrounding the ostiole (Matsushima 1995); Syspastospora, possessing ascomata with a long neck composed of parallel arranged hyphae and cylindrical ascospores with a large terminal slightly sunken germ pore at each end (Cannon and Hawksworth 1982); and Vittatispora, which produces ascomata similar to those of Syspastospora and citriform ascospores with a longitudinal, thick, hyaline ridge (Chaudhary et al. 2006). Practically all taxonomic studies on these fungi have been based exclusively on the morphological characterization of the reproductive structures of preserved fungarium specimens, since unfortunately due to their mycoparasitism, many of these fungi do not grow in pure culture or do not produce ascomata in absence of their hosts. On the other hand, obtaining reliable nucleotide sequences from members of the Melanosporales is also difficult because of the usually large amount of DNA of their hosts. Based on the study of several freshly-isolated soil-borne fungi and of reference and type strains obtained from various culture collections, we have re-examined the phylogenetic relationships of the most relevant genera of the Ceratostomataceae. Consequently, the genus Melanospora has been redefined, Microthecium has been re-established, and three new genera have been proposed.
Materials and methods
Fungal isolates
The strains included in this study are listed in Table 1. Fresh isolates were obtained from samples following previously described procedures for the activation of dormant ascospores in soil using acetic acid and phenol solutions (Stchigel et al. 2001, García et al. 2003). Ascomata were transferred to 55 mm diam. Petri dishes containing oatmeal agar (OA; oatmeal flakes, 30 g; agar-agar, 20 g; distilled water, 1 L) using a sterile needle, which were then incubated at 15, 25 and 35 °C.
Table 1.
Isolates and reference strains of members of Melanosporales included in the combined phylogenetic study.
| Taxa | Strain | Source | GenBank accession number | |||
|---|---|---|---|---|---|---|
| LSU | ITS | act | tef1 | |||
| Dactylidispora ellipsospora | NBRC 31376T | Forest soil, Papua New Guinea, Buin, Bougainville Island | KP981451 | 03137601* | KP981545 | KP981579 |
| Dactylidispora singaporensis | NBRC 30865T | Soil, Singapore | KP981452 | 03086502* | KP981546 | KP981580 |
| Echinusitheca citrispora |
CBS 137837T = FMR 12767T |
Forest soil, USA, North Carolina, Great Smoky Mountains National Park, Cataloochee Creek Campground | KP981453 | KP981477 | KP981547 | KP981581 |
| Nectria cinnabarina | CBS 127383 | Austria, Niederösterreich, Litschau | HM534894 | HM534894 | – | HM534873 |
| Melanospora damnosa | CBS 113681 | Soil, France, Pont d’Espagne | KP981454 | KP981478 | KP981543 | KP981582 |
| Melanospora kurssanoviana | NBRC 8098 | Unknown | KP981455 | KP981479 | KP981548 | KP981583 |
| Melanospora verrucispora | NBRC 31375T | Forest soil, Papua New Guinea, Kebil, Chimb Dist. | KP981456 | KP981480 | KP981549 | KP981584 |
| Melanospora zamiae | NBRC 7902 | Unknown | KP981457 | 00790201* | KP981544 | KP981585 |
| Microthecium ciliatum | NBRC 9829 | Soil, unknown | KP981458 | KP981481 | KP981524 | KP981586 |
| Microthecium compressum | NBRC 8627 | Unkown | KP981459 | 00862701* | KP981525 | KP981587 |
| Microthecium fayodii | FMR 12363 | Soil, Tennessee, Great Smoky Mountains National Park, Cosby Creek trail | KP981460 | KP981482 | KP981526 | KP981588 |
| Microthecium fimbriatum | NBRC 8523 | Unknown | KP981461 | KP981483 | KP981527 | KP981589 |
| Microthecium fimicola | NBRC 8354 | Unknown | KP981462 | KP981484 | KP981528 | KP981590 |
| FMR 5483 | Soil, Australia, Moara | KP981463 | KP981485 | KP981529 | KP981591 | |
| FMR 12370 | Soil, Spain, Gran Canaria | KP981464 | KP981486 | KP981530 | KP981592 | |
| FMR 13418 | Soil, Spain, Aragon, Los Valles Occidentales | KP981465 | KP981487 | KP981531 | KP981593 | |
| Microthecium fusisporum | NBRC 8806 | Unknown | KP981466 | 00880601* | KP981532 | KP981594 |
| Microthecium japonicum | FMR 12371 | Soil, Spain, Gran Canaria, Pico de Osorio | KP981467 | KP981488 | KP981533 | KP981595 |
| Microthecium levitum |
FMR 6218 = CBS 966.97 |
Soil, Nepal, Bhadgaon | KP981468 | KP981489 | KP981534 | KP981596 |
| FMR 10098 | Soil, Nigeria, Enugu, Nsukka | KP981469 | KP981490 | KP981535 | KP981597 | |
| FMR 13884 | Soil, Spain, Catalonia, Vall Fosca | KP981470 | KP981491 | KP981536 | KP981598 | |
| Microthecium quadrangulatum | CBS 112763T | Soil, Spain, Asturias, Muniellos Biological Absolute Reserve | KP981471 | KP981492 | KP981537 | KP981599 |
| Microthecium retisporum | NBRC 8366 | Soil, Japan | KP981472 | 00836601* | KP981538 | KP981600 |
| Microthecium sepedonioides | FMR 11933 | Forest soil, Spain, Aragón, valle de Ordesa | KP981473 | KP981493 | KP981539 | KP981601 |
| Microthecium sp. |
FMR 6725 = CBS 102190 |
Desert soil, Egypt, Sinai | KP981474 | KP981494 | KP981540 | KP981602 |
| Microthecium sp. |
FMR 7183 = CBS 108937 |
Forest soil, New South Wales, Sydney, Blue Mountains | KP981475 | KP981495 | KP981541 | KP981603 |
| Microthecium tenuissimum | CBS 112764T | Soil, Spain, Murcia, Sierra de Espuña, Umbria de Peña Apartada | KY628706 | KY628705 | – | – |
| Microthecium zobelii | NBRC 9442 | Decaying carpophore of Coriolusflabelliformis | KP981476 | 00944201* | KP981542 | KP981604 |
| Pseudallescheria fusoidea | CBS 106.53T | Soil, Panama, Guipo | EF151316 | AY878941 | – | – |
| Pseudomicrothecium subterraneum | BJTC FAN1001T | From Tuberindicum, China, Yunnan | JN247804 | – | – | – |
| Vittatispora coorgii | BICC 7817T | Soil, India, Western Ghats, Coorg District, Kakkabe | DQ017375 | – | – | – |
BICC: Biocon culture collection, Bangalore, India; BJTC: Capital Normal University, Beijing, China; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; FMR: Facultat de Medicina, Reus, Spain; NBRC: Biological Resource Center, Chiba, Japan. T zindicates type strains. * sequences retrieved from NBRC database.
Morphological study
For cultural characterization, isolates were grown for up to 30 d on OA, potato carrot agar (PCA; grated potatoes, 20 g; grated carrot, 20 g; agar-agar, 20 g; L-chloramphenicol, 100 mg; distilled water, 1 L), and potato dextrose agar (PDA; Pronadisa, Madrid, Spain) at 5, 10, 15, 20, 25, 30, 35 and 40 °C. Color notations in parentheses are from Kornerup and Wanscher (1984). Vegetative and reproductive structures were examined under an Olympus BH-1 brightfield microscope by direct mounting in lactic acid and water of the ascomata and/or microcultures grown on OA and PDA. Pictures were obtained with a Zeiss Axio Imager M1 brightfield microscope. The samples for scanning electron microscopy (SEM) were processed according to Figueras and Guarro (1988), and SEM micrographs were taken at 15 keV with a Jeol JSM 840 microscope.
Molecular study
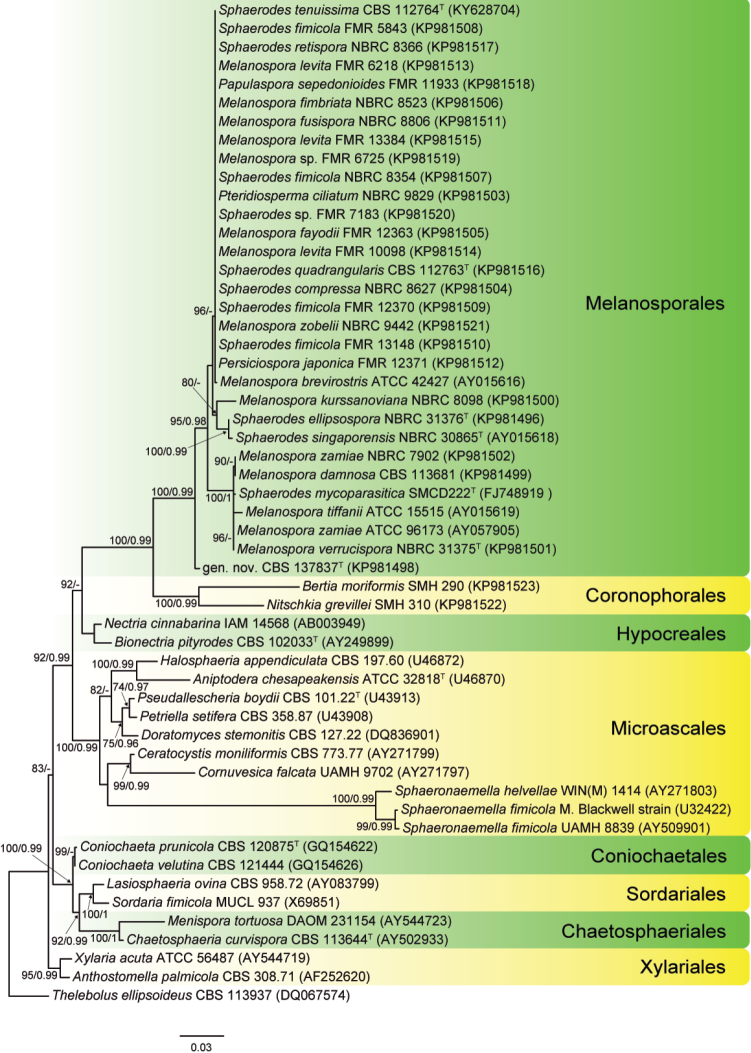
The DNA of the fungal isolates (Table 1) was extracted and purified directly from the colonies according to the Fast DNA Kit protocol (MP Biomedicals, Solon, Ohio). The amplification of the small subunit (SSU), the D1−D3 domains of the large subunit (LSU) and the internal transcribed spacer region (ITS) of the nuclear rDNA, and the fragments of actin (act) and translation elongation factor 1-α (tef1) genes were performed according to White et al. (1990) (SSU), Vilgalys and Hester (1990) (LSU), Cano et al. (2004) (ITS), Voigt and Wöstermeyer (2000) (act) and Houbraken et al. (2007) (tef1). A BigDye Terminator 3.1 cycle sequencing kit (Applied Biosystems Inc., Foster City, California) was used to sequence both strands with a combination of the same primers used in the amplification. PCR products were purified and sequenced at Macrogen Europe (Amsterdam, The Netherlands) with a 3730XL DNA analyzer (Applied Biosystems), and the consensus sequences were obtained using SeqMan (version 7.0.0; DNASTAR, Madison, WI, USA). A phylogenetic study based on the analysis of SSU sequences of the isolates and type and reference strains of the Melanosporales and of some members of the Chaetosphaeriales, Coniochaetales, Coronophorales, Hypocreales, Microascales, Sordariales and Xylariales, using Thelebolusellipsoideus (Thelebolales) as outgroup, was performed to confirm the taxonomic placement of our isolates. A subsequent study, carried out to infer the phylogenetic relationships among members of the Melanosporales, was based on the analysis of a combined data set including the ITS, LSU, act and tef1 sequences of our isolates and of type and reference strains of a large number of the Melanosporales, including Nectriacinnabarina and Pseudallescheriafusoidea as outgroups. The Maximum-Likelihood (ML) and Bayesian Inference (BI) methods were used in phylogenetic analyses as described by Hernández-Restrepo et al. (2016). Bootstrap support (BS) ≥70 and posterior probability values (PP) ≥0.95 were considered significant. The sequences generated in this study were deposited in GenBank (Table 1 and Fig. 1) and the alignments used in the phylogenetic analyses were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S17079). Sequences retrieved from GenBank and NBRC included in the SSU and combined analyses are shown in Fig. 1 and Table 1, respectively.
Figure 1.
RAxML phylogram obtained from SSU sequences of isolates and type and reference strains included in the Melanosporales, and strains belonging to the orders Chaetosphaeriales, Coniochaetales, Coronophorales, Hypocreales, Microascales, Sordariales and Xylariales. Thelebolusellipsoideus was used as outgroup. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. Type strains of the different species are indicated with T.
Results
The SSU phylogenetic study was based on an alignment of 1023 bp and produced a single ML tree (Fig. 1) inferred from a RAxML analysis. The members of the Melanosporales including our isolates were placed in a highly supported main clade (100 % BS / 0.99 PP), and the isolate CBS 137837, whose morphological features did not match any previously described taxon, occurred as a basal branch clearly separated from the other Melanosporales, which grouped together with a high support (95 % BS / 0.98 PP) and separated into three subclades. The first one (96 % BS / - PP), contained most of the isolates morphologically identified as Melanospora, Persiciospora and Sphaerodes, including the type and reference strains of Melanosporabrevirostris, M.fimbriata, M.fusispora, M.levita, M.zobelii, Papulasporasepedonioides, Pteridiospermaciliatum, Sphaerodescompressa, S.fimicola, S.retispora, S.quadrangularis and S.tenuissima, without significant genetic variation among them. The second subclade (80 % BS / - PP) comprised the type strains of Sphaerodesellipsospora and Sphaerodessingaporensis and a reference strain of Melanosporakurssanoviana, which resulted clearly separated from the other two, which grouped with high support (100 % BS / 0.98 PP). In the third subclade (100 % BS / 1 PP) were nested the type species of Melanospora (M.zamiae), the type strains of Melanosporaverrucispora and Sphaerodesmycoparasitica, and reference strains of Melanosporadamnosa and Melanosporatiffanii.
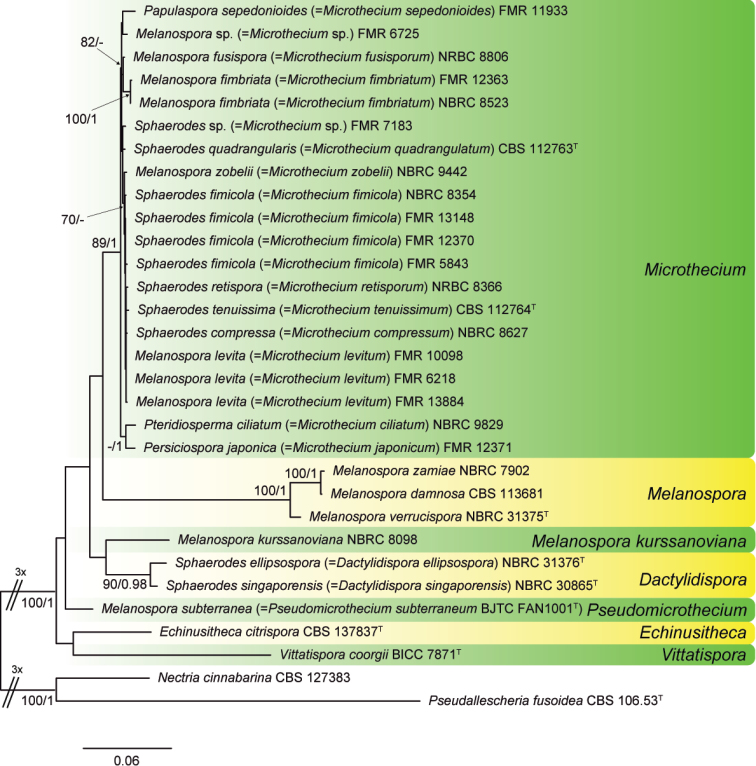
The lengths of the individual alignments used in the combined data set were 802 bp (LSU), 535 bp (ITS), 727 bp (act) and 846 bp (tef1), respectively, and the final total alignment was 2910 bp. In the ML tree derived from the RAxML analysis of the combined data set (Fig. 2), the Melanosporales were highly supported (100 % BS / 1 PP) and subdivided into seven lineages. The first clade (89 % BS / 1 PP; Clade Microthecium) grouped all our isolates, with the exception of CBS 137837, and type or reference strains of Melanosporafimbriata, M.fusispora, M.levita, M.zobelii, Papulasporasepedonioides, Pteridiospermaciliatum, Sphaerodescompressa, S.fimicola, S.retispora, S.quadrangularis and S.tenuissima. All the fungi belonging to this clade have non-ostiolate ascomata, or when a neck is present, it is short and composed of angular cells similar to those of the ascomatal wall. Also, bulbils (microsclerotial-like asexual propagules) are present in most of these species. In spite of the high morphological variability shown by members of this clade, the loci used in the phylogenetic analysis were not able to separate the species from each other. The second clade (100% BS / 1 PP; Clade Melanospora) comprised the type species of Melanospora, M.zamiae, the type strain of M.verrucispora and a reference strain of M.damnosa. The members of this clade produce ostiolate ascomata with a long neck composed of hyphae irregularly arranged and ending in a crown of setae. In addition, an asexual morph is commonly present, which is characterized by solitary, sessile, flask-shaped phialides producing from rounded to ellipsoidal conidia. The third lineage comprised only a reference strain of Melanosporakurssanoviana, which failed to sporulate in pure culture. The fourth clade (90 % BS / 0.98 PP; Clade Dactylidispora) was composed of the type strains of Sphaerodesellipsospora and S.singaporensis, both characterized by ascospores with a raised rim surrounding the germ pores. Finally, the isolate CBS 137837 and the type strains of Melanosporasubterranea and Vittatisporacoorgii formed three independent branches. The isolate CBS 137837 produces globose, non-ostiolate, densely setose, dark ascomata and smooth-walled ascospores with a depressed germ pore at each end, while the other two species of this clade also possess morphological features unique in the Melanosporales, e.g. ascospores with indistinct germ pores in M.subterranea and with a longitudinal, thick, hyaline ridge in V.coorgii.
Figure 2.
RAxML phylogram obtained from the combined ITS, LSU, act and tef1 sequences of our isolates and type and reference strains of the order Melanosporales. Nectriacinnabarina and Pseudallescheriafusoidea were used as outgroup. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers are indicated in Table 1. Type strains of the different species are indicated with T.
Taxonomy
Key to the accepted genera of the Melanosporales producing sexual morphs (adapted from Cannon and Hawksworth 1982)
| 1 | Ascospores with two longitudinal germ slits | Scopinella |
| – | Ascospores with germ pores | 2 |
| 2 | Ascospores with a broad germ pore and a small basal appendage | 3 |
| – | Ascospores with a germ pore at each end | 4 |
| 3 | Ascomata with a crown of dark brown setae surrounding the ostiole | Setiferotheca |
| – | Ascomata without setae | Arxiomyces |
| 4 | Ascospores oblong or cylindric-fusiform, and germ pores crateriform | Syspastospora |
| – | Ascospores and germ pores otherwise | 5 |
| 5 | Ascomata ostiolate; neck long, composed of hyphae | 6 |
| – | Ascomata non-ostiolate or ostiolate; neck absent or short, conical, composed of angular cells similar to those of the ascomatal wall | 7 |
| 6 | Neck composed of irregularly arranged hyphae | Melanospora |
| – | Neck composed of parallel arranged hyphae | Vittatispora |
| 7 | Ascospores with indistinct germ pores | Pseudomicrothecium |
| – | Ascospores with conspicuous germ pores | 8 |
| 8 | Germ pores surrounded by hyaline structures | 9 |
| – | Germ pores without such structures | 10 |
| 9 | Germ pores with a raised rim | Dactylidispora |
| – | Germ pores with a blistered, rarely cushion-like structure | Pustulipora |
| 10 | Ascomatal wall cephalothecoid | Rhytidospora |
| – | Ascomatal wall not cephalothecoid | 11 |
| 11 | Ascomata dark, densely setose | Echinusitheca |
| – | Ascomata translucent, glabrous or surrounded by hyphae-like hairs | Microthecium |
Dactylidispora
Y. Marín, Stchigel, Guarro & Cano gen. nov.
812079
Type species.
Dactylidisporaellipsospora (Takada) Y. Marín, Stchigel, Guarro & Cano. Holotype and ex-type strain: NBRC 31376.
Description.
Ascomata superficial, globose to pyriform, ostiolate or not, yellowish-brown, appearing dark brown when the ascospores are mature, glabrous or setose; necks cellular, short, conical, with a crown of setae surrounding the ostiole; ascomatal wall membranaceous, of textura angularis. Paraphyses absent. Asci 8-spored, broadly clavate, short-stipitate, without apical structures, evanescent. Ascospores one-celled, at first hyaline, becoming brown to dark brown when mature, fusiform or citriform, umbonate and truncate at the ends, smooth-walled, with one germ pore at each end; germ pores depressed, surrounded by a raised rim. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, solitary, flask-shaped. Conidia hyaline, subglobose to ovoid, smooth-walled.
Etymology.
From Greek δακτυλίδης–, ring, and from Latin –spora, spore, due to the raised rim that surrounds the germ pores of the ascospores.
Notes.
The most distinctive characteristic of Dactylidispora is the production of smooth-walled ascospores with a germ pore at each end surrounded by a raised rim. Vittatispora, proposed as a new genus by Chaudhary et al. (2006), also produces a raised rim surrounding the germ pores. However, both genera can be easily distinguished by the nature of the ascomatal neck, which is composed of angular cells in Dactylidispora and of parallel arranged hyphae in Vittatispora; and by the presence of a hyaline ridge running the entire vertical length of the ascospore between the germ pores in Vittatispora. Moreover, in our phylogenetic study (Fig. 2), Vittatispora also constituted a lineage independent from the other members of the Melanosporales. Pustulipora is also morphologically similar to Dactylidispora being characterized by blistered, rarely cushion-like structures surrounding the germ pore (Cannon 1982). However, unfortunately, Pustulipora could not be included into this phylogenetic study since living cultures were not available.
The presence of a raised rim was also described in Melanosporacollipora (Stchigel et al. 1997), which is here transfered to Dactylidispora even though it was not possible to include this species in the phylogenetic study.
Dactylidispora collipora
(Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812080
Melanospora collipora Stchigel & Guarro, in Stchigel, Guarro & Figueras, Mycol. Res. 101: 446. 1997. [Basionym]
Notes.
This species produces ascomata with a crown of setae around the ostiole, ellipsoidal ascospores, and bulbils.
Dactylidispora ellipsospora
(Takada) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812081
Microthecium ellipsosporum Takada, in Kobayasi et al., Bull. natn. Sci. Mus., Tokyo 16: 527. 1973. [Basionym]
≡ Sphaerodesellipsospora (Takada) D. García, Stchigel & Guarro, Stud. Mycol. 50: 67. 2004.
Notes.
Dactylidisporaellipsospora is characterized by non-ostiolate ascomata, fusiform ascospores and absence of asexual morph.
Dactylidispora singaporensis
(Morinaga, Minoura & Udagawa) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812082
Melanospora singaporensis Morinaga, Minoura & Udagawa, Trans. Mycol. Soc. Japan 19: 142. 1978. [Basionym]
≡ Sphaerodessingaporensis (Morinaga, Minoura & Udagawa) D. García, Stchigel & Guarro, Stud. Mycol. 50: 67. 2004.
Notes.
Dactylidisporasingaporensis is distinguished by its ostiolate ascomata, citriform ascospores, and phialidic asexual morph.
Echinusitheca
Y. Marín, Stchigel, Dania García, Guarro, A.N. Mill. & Cano gen. nov.
812084
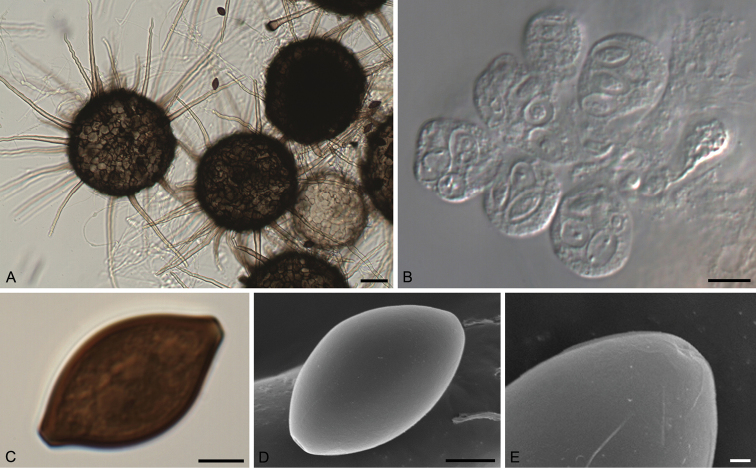
Figure 3.
Echinusithecacitrispora (CBS 137837T). A Ascomata B Asci C, D Ascospores E Depressed germ pore. Scale bars: 50 μm (A); 10 μm (B); 5 μm (C, D); 1 μm (E).
Type species.
Echinusithecacitrispora Y. Marín, Stchigel, Dania García, Guarro, A.N. Mill. & Cano. Holotype and ex-type strain, respectively: CBS H-21596, CBS 137837 = FMR 12767.
Description.
Ascomata superficial or immersed, solitary to gregarious, globose, non-ostiolate, strongly setose, semi-translucent, pale brown to brown, appearing black when ascospores are mature; setae straight, becoming sinuous toward apex, pale brown to brown, non-septate, rarely 1-septate, thick-walled, verrucose to tuberculate, sometimes branched; ascomatal wall membranaceous, of textura angularis to textura globulosa. Asci 8-spored, globose to subglobose, non-stipitate, without apical structures. Ascospores at first hyaline, becoming brown to dark brown when mature, ellipsoidal, one-celled, smooth-walled, with a depressed germ pore at each end.
Etymology.
From Latin echinus–, sea urchin, and from Greek –τείχος, wall, because of the ascomata resemblance to a sea urchin, due to the abundance of setae.
Notes.
This genus is characterized by dark, strongly setose, non-ostiolate ascomata. Apart from Echinusitheca, the other genera of the Melanosporales characterized by the production of dark semi-translucent ascomata are Arxiomyces and Scopinella, but both genera differ from Echinusitheca by the production of long ascomatal necks. Moreover, Scopinella can be easily distinguished from Echinusitheca by its cuboid-ellipsoidal ascospores with two prominent longitudinal germ slits, and Arxiomyces by its ellipsoidal ascospores that are rounded at the apex and truncated at the base, and with a broad germ pore that bears a mucilaginous and collapsing appendage.
Echinusitheca citrispora
Y. Marín, Stchigel, Dania García, Guarro, A.N. Mill. & Cano sp. nov.
812085
Type.
USA, North Carolina, Great Smoky Mountains National Park, Cataloochee Creek Campground (35.1375; -83.4915), forest soil, 15 July 2008, A.N. Miller, M. Calduch and A.M. Stchigel, holotype CBS H-21596, cultures ex-type CBS 137837 = FMR 12767.
Description.
Colonies on PDA attaining a diam. of 70–75 mm after 14 d at 35 °C, cottony and granulose due to the presence of a large number of ascomata, white with grey to black dots, depressed at the centre and margins fringed; reverse yellowish-white to pale yellow (4A2 to 4A3) and with olive brown (4F2) dots. Colonies on OA attaining a diam. of 50–60 mm in 14 d at 35 °C, cottony and granulose due to the presence of numerous ascomata, margins arachnoid, white to orange white (5A2) with brownish grey dots (5F2); reverse yellowish-white to golden grey (4A2 to 4C2). Minimum, maximum, and optimum temperature of growth are 20, 40 and 35 °C, respectively. Mycelium composed of hyaline to pale yellow, septate, branched, smooth-walled hyphae, 1–3 µm diam. Ascomata non-ostiolate, immersed into the mycelium, solitary or gregarious, globose, 130–280 µm diam., setose, semi-translucent, pale brown to brown, appearing black when ascospores are mature; setae straight, becoming sinuous toward apex, 20–200 µm long, 5–20 µm wide at base, tapering gradually to a rounded tip of 2–5 µm diam., pale brown to brown, non-septate or rarely 1-septate, thick-walled, verrucose to tuberculate, sometimes branched at apex; ascomatal wall membranaceous, 30–40 µm thick, composed of 5–6 layers of flattened cells of 5–30 µm diam. of textura angularis to textura globulosa. Asci 8-spored, globose to subglobose, 20–25 × 15–20 µm, soon evanescent, non-stipitate, without apical structures, irregularly disposed at the centrum. Ascospores irregularly arranged in the asci, one-celled, at first hyaline, becoming brown to dark brown when mature, smooth- and thick-walled, ellipsoidal, 20–27 × 10–15 µm, with one germ pore at each end; germ pores 0.75–2 µm diam., depressed. Asexual morph absent.
Etymology.
From Latin citrum-, lemon, and -spora, spore, referring to the lemon-shaped ascospores.
Melanospora
Corda, Icon. fung. (Prague) 1: 24. 1837, emend.
Figure 4.
Morphological features of the genus Melanospora. Melanosporadamnosa (CBS 113681). A Ascoma B Ascomatal neck D Detail of hyphal neck F Ascospores H Ascospore germinating. Melanosporazamiae (NBRC 7902) C Ascomatal neck E Detail of ascomatal wall. Melanosporaverrucispora (NBRC 31375T) G Ascospores I Phialidic asexual morph. Scale bars: 50 μm (A); 10 μm (B–E, I); 5 μm (F–H).
Type species.
Melanosporazamiae Corda, Icon. fung. (Prague) 1: 24. 1837. Representative strain: NBRC 7902.
Description.
Ascomata superficial to immersed, globose to subglobose, ostiolate, yellowish-orange or reddish, tomentose or glabrous, usually with a long neck composed of intermixed hypha, with a crown of rigid, hyaline, septate, smooth- and thick-walled setae; ascomatal wall membranaceous, translucent, of textura angularis. Periphyses present. Paraphyses absent. Asci 8-spored, clavate, rounded at apex, without apical structures, thin-walled, evanescent. Ascospores one-celled, at first hyaline, becoming brown to dark brown when mature, fusiform, ellipsoidal or citriform, smooth-walled, reticulate or verrucose, with a terminal apiculate or depressed germ pore at each end. Asexual morph phialidic, hyaline. Bulbils uncommon.
Notes.
This genus is distinguished by translucent ascomata with a neck composed of intermixed hyphae and with an apical crown of setae, smooth or ornamented ascospores with an apiculate germ pore at each end, and a phialidic asexual morph. The neck of Melanospora spp. is morphologically similar to those of Syspastospora and Vittatispora, which are also composed of hyphae. Syspastospora was introduced in 1982 by Cannon and Hawksworth to accommodate Melanosporaparasitica, with three additional species described later (S.boninensis, S.cladoniae and S.tropicalis). This genus differs from Melanospora in the production of cylindrical to barrel-shaped ascospores with a large, slightly sunken germ pore at both ends (ellipsoidal, citriform or fusiform, having much smaller, apiculate or depressed germ pores in Melanospora). Vittatispora can be distinguished from Melanospora by the production of ascospores with a thick, hyaline, longitudinal ridge and a raised rim surrounding the germ pores. Moreover, Syspastospora and Vittatispora differs from Melanospora in the structure of the ascomatal neck, which is composed of hyphae in a parallel arrangement in both genera (interwoven hyphae in Melanospora).
Melanospora is now restricted to species with ascoma bearing a neck composed of interwoven hyphae and mostly ending in a crown of setae. This kind of neck differentiates this genus from Microthecium, which has a neck composed of angular cells similar to those of the ascomatal wall and possessing a crown of setae surrounding the ostiole rather than disposed at apex of the neck. The only exception is Melanosporamycoparasitica that does not have this sort of neck, being short, cellular and without the crown of setae at the top of this, although this could be due to the fact that it was described and illustrated at an early stage of ascomal development. In a study on the development and cytology of Melanosporatiffanii, Kowalski (1965) illustrated early stages of development with the neck appearing similar to that of M.mycoparasitica.
Long hyphal necks are produced in Melanosporaarenaria, Melanosporacaprina, Melanosporachionea, Melanosporalangenaria, Melanosporalongisetosa and Melanosporawashingtonensis; therefore, these have been kept in the emended genus Melanospora, although they were not included in the phylogenetic study.
Key to the species of Melanospora
| 1 | Ascospores with the surface ornamented | 2 |
| – | Ascospores smooth-walled | 4 |
| 2 | Ascospores irregularly verrucose | M. verrucispora |
| – | Ascospores reticulate | 3 |
| 3 | Ascospores coarsely reticulate | M. mycoparasitica |
| – | Ascospores slightly reticulate | M. tiffanii |
| 4 | Ascospores discoid-ellipsoidal | 5 |
| – | Ascospores otherwise | 7 |
| 5 | Asci 4-spored; ascospores 14–19 × 12–14 × 8–9 μm | M. longisetosa |
| – | Asci 8-spored; ascospores smaller | 6 |
| 6 | Neck 250–400 μm long; ascospores 7.5–16 × 6–12 × 4–7 μm | M. chionea |
| – | Neck 150–200(–260) μm long; ascospores 10.5–12(–13.5) × 9–10.5(–12) × 7–9 μm | M. washingtonensis |
| 7 | Ascomata usually narrower than 100 μm; ascospores citriform to rhomboidal | M. damnosa |
| – | Ascomata usually broader than 100 μm; ascospores ellipsoidal to citriform | 8 |
| 8 | Ascomata strongly tomentose; neck 1500–2000 μm long | M. caprina |
| – | Ascomata weakly or not tomentose; neck shorter than 1500 μm | 9 |
| 9 | Neck shorter than 250 μm long | M. zamiae |
| – | Neck longer than 800 μm long | 10 |
| 10 | Setae longer than 100 μm | M. arenaria |
| – | Setae up to 50 μm long | M. lagenaria |
Melanospora arenaria
L. Fisch. & Mont., in Montagne, Annls. Sci. Nat., Bot., sér. 4 5: 337. 1856.
Notes.
Melanosporaarenaria is characterized by ascomata with a long neck and ellipsoidal to citriform, smooth-walled ascospores. It is similar to Melanosporacaprina, but differs in having less tomentose ascomata with a shorter neck. Also, it is similar to M.lagenaria, differing only by the size of the setae at the top of the ascomatal neck. Molecular data is necessary to confirm that both species correspond to different species since the size of the setae could be influenced by the culture media on where these grew.
Melanospora caprina
(Fr.) Sacc., Syll. fung. (Abellini) 2: 462. 1883.
Sphaeria caprina Fr., Fl. Danic. 11: tab. 1859, fig. 2. 1825. [Basionym]
≡ Ceratostomacaprinum (Fr.) Fr., Summa veg. Scand., Section Post. (Stockholm): 396. 1849.
≡ Cerastomacaprinum (Fr.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 522. 1875.
= Sphaeriavervecina Desm., Annls Sci. Nat., Bot., sér. 2 17: 13. 1842.
≡ Melanosporavervecina (Desm.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 126. 1870.
= Melanosporavervecina f. arundinis Sacc., Syll. fung. (Abellini) 2: 461. 1883.
Notes.
Melanosporacaprina is distinguished from the other species of the genus by its larger, white, densely tomentose ascomata with a very long neck, and ellipsoidal to citriform, smooth-walled ascospores with slightly apiculate germ pores.
Melanospora chionea
(Fr.) Corda, Icon. fung. (Prague) 1: 24. 1837.
Ceratostoma chioneum Fr., Observ. mycol. (Havniae) 2: 340. 1818. [Basionym]
≡ Sphaeriachionea (Fr.) Fr., Syst. mycol. (Lundae) 2: 446. 1823.
≡ Melanosporachioneavar.chionea (Fr.) Corda, Icon. fung. (Prague) 1: 24, tab. 7, fig. 297. 1837.
= Sphaeriabiformisvar.brachystoma Pers., Syn. meth. fung. (Göttingen) 1: 60. 1801.
≡ Melanosporachioneavar.brachystoma (Pers.) Sacc., Syll. fung. (Abellini) 2: 461. 1883.
= Sphaerialeucophaea Fr., Elench. fung. (Greifswald) 2: 92. 1828.
≡ Ceratostomaleucophaeum (Fr.) Fr., Summa veg. Scand., Section Post. (Stockholm): 396. 1849.
≡ Melanosporachioneavar.leucophea (Fr.) Sacc., Syll. fung. (Abellini) 2: 461. 1883.
= Melanosporaantarctica Speg., Boln Acad. nac. Cienc. Córdoba 11: 233. 1888.
Notes.
This species is characterized by white, tomentose ascomata and discoid, smooth-walled ascospores with depressed germ pores.
Melanospora damnosa
(Sacc.) Lindau, in Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 353. 1897.
Sphaeroderma damnosum Sacc., Riv. Patol. veg. 4: 64. 1895. [Basionym]
Notes.
Melanosporadamnosa is distinguised by the production of ascomata with a short neck and citriform to rhomboidal, smooth-walled ascospores with a slightly apiculate germ pore at each end.
Melanospora lagenaria
(Pers.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 126. 1870.
Sphaeria lagenaria Pers., Syn. meth. fung. (Göttingen) 1: 58. 1801. [Basionym]
≡ Ceratostomalagenaria (Pers.) Fr. [as ‘lagenarium’], Syst. veg., Edn 16: 392. 1827.
≡ Auerswaldialagenaria (Pers.) Rabenh., Hedwigia 1: 116. 1857.
≡ Cerastomalagenaria (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 522. 1875.
≡ Phaeostomalagenaria (Pers.) Munk [as ‘lagenarium’], Dansk bot. Ark. 17: 82. 1957.
= Melanosporalagenariavar.tetraspora Rehm, Hedwigia 30: 259. 1891.
Notes.
Melanosporalagenaria is similar to M.caprina, but the former has less tomentose ascomata with shorter necks ending in a poorly developed crown of setae. This species is also similar to M.arenaria. For morphological comparison see Notes of the latter species.
Melanospora longisetosa
P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 130. 1982.
Notes.
This species is characterized by the formation of 4-spored asci and discoid, smooth-walled ascospores.
Melanospora mycoparasitica
(Vujan.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812086
Sphaerodes mycoparasitica Vujan., Mycol. Res. 113: 1173. 2009. [Basionym]
Notes.
Melanosporamycoparasitica is distinguished by its fusiform, coarsely reticulate ascospores.
Melanospora tiffanii
Kowalski, Mycologia 57: 279. 1965.
Notes.
This species is distinguished by its fusiform, slightly reticulate ascospores.
Melanospora verrucispora
Takada, in Kobayasi et al., Bull. natn. Sci. Mus., Tokyo 16: 525. 1973.
Notes.
This species is characterized by irregularly verrucose ascospores.
Melanospora washingtonensis
Nitzan, J.D. Rogers & D.A. Johnson, Sydowia 56: 282. 2004.
Notes.
This species is similar to M.chionea, but they differ in the length of the neck [150–200(–266) μm in M.washingtonensis vs. 250–400 μm in M.chionea] and in the size of the ascospores [10.5–12(–13.5) × 9–10.5(–12) × 7–9 μm in M.washingtonensis vs. 7.5–16 × 6–12 × 4–7 μm in M.chionea], as well as in the presence of a phialidic asexual morph in M.washingtonensis.
Melanospora zamiae
Corda., Icon. fung. (Prague) 1: 24. 1837.
= Melanosporaleucotricha Corda, Icon. fung. (Prague) 1: 25. 1837.
= Melanosporacoemansii Westend., Bull. Acad. R. Sci. Belg., Cl. Sci., sér. 2 2: 579. 1857.
= Melanosporacirrhata Berk. in Cooke, Grevillea 16: 102. 1888.
= Melanosporaglobosa Berl., Malpighia 5: 409. 1891.
= Melanosporapampeana Speg., Anal. Mus. nac. Hist. nat. B. Aires 6: 287. 1898.
= Melanosporatownei Griffiths, Bull. Torrey bot. Club 26: 434. 1899.
= Melanosporarhizophila Peglion & Sacc., Annls mycol. 11: 16. 1913.
= Melanosporamattiroloana Mirande [as ‘mattiroliana’], Bull. Soc. mycol. Fr. 32: 72. 1916.
= Melanosporaschmidtii Sacc., Syll. fung. (Abellini) 24: 650. 1926.
= Melanosporaasclepiadis Zerova, J. Inst. Bot. Acad. Sci. Ukraine 12: 155. 1937.
Notes.
Melanosporazamiae is characterized by the production of ellipsoidal to citriform, smooth-walled ascospores with a depressed germ pore at each end. Doguet (1955) described the presence of bulbils; however, later studies did not mention the presence of such sort of propagules (Calviello 1973, Cannon and Hawksworth 1982), which rarely occur in the genus.
Doubtful species
Melanospora aculeata
E.C. Hansen, Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn 59: 15. 1877.
Notes.
Cultures of this species are not available, but it was originally described as producing small asci (18–21 × 7–8 μm) and ascospores (4–6 × 3–4 μm). This species produced ostiolate ascomata without a neck, typical of Microthecium; however, such small ascospores have never been seen in Microthecium.
Melanospora endobiotica
Woron., Notul. syst. Inst. cryptog. Horti bot. petropol. 3: 31. 1924.
Notes.
Cultures are not available, and no illustrations were included in the protologue. It was reported as morphologically similar to Melanosporarhizophila [now considered a synonym of Melanosporazamiae (Doguet 1955)] when it was first described (Woronichin 1924).
Excluded species
Melanospora arachnophila
Fuckel, Jb. nassau. Ver. Naturk. 23–24: 127. 1870.
Notes.
This species possesses cylindrical asci and hyaline ascospores, features never seen in Melanospora. It was previously excluded from Melanospora by Doguet (1955).
Melanospora argadis
Czerepan., Nov. sist. Niz. Rast. 3: 177. 1966.
Notes.
This species shows morphological features never observed in Melanospora, e.g. small asci (10–14 × 5–6.5 μm) and olivaceous ascospores (5–5.5 × 3–3.5 μm). The original description is not detailed enough to ascertain its possible taxonomical placement.
Melanospora exsola
Bat. & H.P. Upadhyay, Atas Inst. Micol. Univ. Recife 2: 331. 1965.
Notes.
This species is excluded from Melanospora due to its dark brown, non-translucent, setose ascomata and its small ascospores (4.5–12 × 4–7 μm), which seem to indicate a closer relationship with Chaetomium.
Melanospora gigantea
(Massee & Crossl.) Massee & Crossl., Fungus Flora of Yorkshire (Leeds): 215. 1905.
Notes.
Descriptions of this species and of its basionym, Sphaerodermagigantea, were not found.
Melanospora lucifuga
(Jungh.) Sacc., Syll. fung. (Abellini) 2: 464. 1883.
Notes.
Cultures are not available, and the original description does not mention asci and ascospores. Therefore, we agree with Doguet (1955) in the exclusion of this fungus from Melanospora.
Melanospora kurssanoviana
(Beliakova) Czerepan., Notul. syst. Sect. cryptog. Inst. bot. Acad. Sci. U.S.S.R. 15: 84. 1962.
Notes.
In our phylogenetic study, M.kurssanoviana was placed in an independent lineage far from Melanospora. Unfortunately, the only living culture available is sterile. We did not find any distinctive morphological feature to differentiate this species from other members of the Melanosporales in the original description and in the drawing to introduce it as a new genus.
Melanospora macrospora
P. Karst., Hedwigia 30: 299. 1891.
Notes.
Doguet (1955) excluded this species due to the production of very large cylindrical asci (480–500 × 33–36 μm) and ascospores (42–52 × 28–35 μm), morphological features not observed in any other member of the Melanosporales.
Melanospora octahedrica
Pat., Cat. Rais. Pl. Cellul. Tunisie (Paris): 109. 1897.
Notes.
This species is transferred to Scopinella due to the morphology of its ascospores, i.e. octahedral ascospores with two prominent longitudinal germ slits.
Scopinella octahedrica
(Pat.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812087
Basionym.
Melanosporaoctahedrica Pat., Cat. Rais. Pl. Cellul. Tunisie (Paris): 109. 1897.
Melanospora pascuensis
Stchigel & Guarro, Mycol. Res. 103: 1305. 1999.
Notes.
This species is excluded from Melanospora since its neck is cellular or absent, instead it is characterized by a dark ring-like structure around the germ pores of the ascospores (Stchigel et al. 1999). This fungus could represent a new genus since such structure is unique in the Melanosporales, and these kind of structures resulted in being phylogenetically informative, as in the case of Dactylidispora, which is distinguished by its ascospores with a raised rim around the germ pores. The type strain of this specimen was contaminated with another fungus and it could not be included in the molecular study.
Melanospora setchellii
(Harkn.) Sacc. & P. Syd., Syll. fung. (Abellini) 16: 564. 1902.
Notes.
This species is excluded from Melanospora since it produces cylindrical asci with the ascospores uniseriately disposed, a feature never observed in this genus.
Melanospora vitrea
(Corda) Sacc., Syll. fung. (Abellini) 2: 463. 1883.
Sphaeronaema vitreum Corda, Icon. fung. (Prague) 1: 25. 1837. [Basionym]
Notes.
Doguet (1955) excluded this species due to its oblong, pale yellow ascospores.
Microthecium
Corda, Icon. fung. (Prague) 5: 30, 74. 1842, emend.
Figure 5.
Morphological features of the genus Microthecium. Microtheciumlevitum (FMR 10098). A Non-ostiolate ascoma E Asci G Ascospores K Ascospore (SEM). Microtheciumfayodii (FMR 12363). B Ostiolate ascomata F Ascospores O Variable shaped bulbils. Microtheciumfimicola (FMR 5483). C Detail of cellular neck M Ascospores (SEM) P Bulbil. Microtheciumquadrangulatum (CBS 112763T). D Crown of setae around the ostiole L Ascospore SEM. Microtheciumretisporum (NBRC 8366). H Ascospores N Asexual morph. Microtheciumjaponicum (FMR 12371) I Ascospores J Ascospore SEM. Microtheciumsepedonioides (FMR 11933) Q Bulbil. Scale bars: 50 μm (A, B, O); 20 μm (C, D); 10 μm (E–I, P, Q); 5 μm (J, L–N); 2.5 μm (K).
= Sphaerodes Clem., Gen. fung. (Minneapolis): 44, 173. 1909.
= Pteridiosperma J.C. Krug & Jeng, Mycotaxon 10: 44. 1979.
= Persiciospora P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 133. 1982.
Type species.
Microtheciumzobelii Corda, Icon. fung. (Prague) 5: 74. 1842. Representative strain: NBRC 9442.
Description.
Ascomata ostiolate or not, superficial or immersed, globose to subglobose or pyriform, yellowish-orange, orange-brown or reddish, tomentose or glabrous; necks short or absent, conical, composed of angular cells similar to those of the ascomatal wall, usually with a crown of hyaline, septate, smooth- and thick-walled setae around the ostiole; ascomatal wall membranaceous, translucent, of textura angularis. Periphyses present. Paraphyses absent. Asci 8-spored, clavate, rounded at apex, without apical structures, thin-walled, evanescent. Ascospores one-celled, at first hyaline, becoming brown to dark brown when mature, ellipsoidal, fusiform, navicular, citriform, plataniform or spindle-shaped, smooth, reticulate, pitted or wrinkled, with a terminal apiculate or depressed germ pore at each end. Asexual morph phialidic, hyaline. Bulbils usually produced, pale orange to reddish-orange.
Notes.
Microthecium has translucent ascomata of textura angularis, cellular necks short or absent, ascospores smooth-walled or ornamented with a depressed or apiculate germ pore at each end, often producing bulbils and a phialidic asexual morph. Dactylidispora, Pustulipora and Pseudomicrothecium produce ascomata similar to Microthecium. However, the two first genera can be distinguished by the presence of a raised rim and blistered structure surrounding the germ pores of the ascospores, respectively, while Pseudomicrothecium differs in the production of 2-spored asci and ascospores with indistinct germ pores.
The species Mi.africanum, Mi.beatonii, Mi.brevirostratum, Mi.episphaerium, Mi.foveolatum, Mi.geoporae, Mi.hypomyces, Mi.internum, Mi.lenticulare, Mi.marchicum, Mi.masonii, Mi.micropertusum, Mi.moureai, Mi.nectrioides, Mi.pegleri and Mi.perplexum were not included in the phylogenetic study because we could not locate any specimens since the holotypes or living cultures of most of them are not available. However, these species were transferred to Microthecium based on their complete and well-illustrated descriptions.
Key to the species of Microthecium
| 1 | Sexual morph absent, only producing bulbils | Mi. sepedonioides |
| – | Sexual morph present | 2 |
| 2 | Ascomata non-ostiolate | 3 |
| – | Ascomata ostiolate | 13 |
| 3 | Ascospores with an ornamented surface | 4 |
| – | Ascospores smooth-walled or nearly so | 8 |
| 4 | Ascospores pitted and with wing-like ridges | Mi. foveolatum |
| – | Ascospores coarsely reticulate | 5 |
| 5 | Asci 4-spored | 6 |
| – | Asci 8-spored | 7 |
| 6 | Ascospores (25–)28–34(–40) × 14–18(–20) µm | Mi. beatonii |
| – | Ascospores 22–28 × 12–15 × 9–11 µm | Mi. perplexum |
| 7 | Ascospores 25–34 × 12–18 µm | Mi. episphaerium |
| – | Ascospores 17–20 × 10–12 × 7–9 µm | Mi. retisporum |
| 8 | Ascomata smaller than 120 µm | Mi. tenuissimum |
| – | Ascomata longer than 120 µm | 9 |
| 9 | Ascospores shorter than 20 µm | 10 |
| – | Ascospores longer than 20 µm | 11 |
| 10 | Ascospores 15–19 × 11–13 × 8–9 µm, with the narrow faces coarsely reticulate and the others smooth | Mi. compressum |
| – | Ascospores 10–17 × 8–12 × 9–10 µm, entirely smooth-walled | Mi. levitum |
| 11 | Ascospores fusiform | Mi. hypomyces |
| – | Ascospores citriform | 12 |
| 12 | Ascospores 28–30 × 12–13(–15) µm | Mi. geoporae |
| – | Ascospores 18–25 × 8.5–12 × 6–9 µm | Mi. zobelii |
| 13 | Ascospores with wing-like appendages | 14 |
| – | Ascospores otherwise | 15 |
| 14 | Ascospores wrinkled, (12–)13–18 × (7–)8–10 µm | Mi. ciliatum |
| – | Ascospores pitted, (17–)20–22(–24) × 12–14 × 10–12 µm | Mi. lenticulare |
| 15 | Ascospores ornamentated | 16 |
| – | Ascospores smooth-walled | 23 |
| 16 | Ascospores punctate or punctate-reticulate | 17 |
| – | Ascospores reticulate or striate-reticulate | 19 |
| 17 | Ascospores punctate, ellipsoidal | Mi. africanum |
| – | Ascospores punctate-reticulate, ellipsoidal-fusiform | 18 |
| 18 | Ascospores delicately punctate, asexual morph and bulbils present | Mi. japonicum |
| – | Ascospores coarsely punctate, asexual morph and bulbils absent | Mi. moreaui |
| 19 | Ascospores striate-reticulate | 20 |
| – | Ascospores reticulate | 21 |
| 20 | Ascospores with inconspicuous ridges forming a very coarse reticulum, 18–22(–28) × 9.5–11(–13) × 8–9 µm | Mi. micropertusum |
| – | Ascospores without ridges or reticulum, 26–36 × 13–17 μm | Mi. masonii |
| 21 | Ascospores with 4–6 prominent longitudinal ribs | Mi. quadrangulatum |
| – | Ascospores without longitudinal ribs | 22 |
| 22 | Ascospores spindle-shaped, 19.5–22 × 8.5–11 µm | Mi. internum |
| – | Ascospores citriform to fusiform, 14–20 × 10–17 µm | Mi. fimicola |
| 23 | Crown of setae absent | Mi. nectrioides |
| – | Crown of setae present around the ostiole | 24 |
| 24 | Ascospores citriform | Mi. marchicum |
| – | Ascospores otherwise | 25 |
| 25 | Ascospores ellipsoid to citriform, often somewhat plataniform | 26 |
| – | Ascospores otherwise | 28 |
| 26 | Bulbils present | Mi. fallax |
| – | Bulbils absent | 27 |
| 27 | Ascospores 21–34 × 11–17 µm | Mi. brevirostrum |
| – | Ascospores 18–22 × 9–11 µm | Mi. fimbriatum |
| 28 | Ascospores ellipsoid to fusiform | Mi. fusisporum |
| – | Ascospores ellipsoid to navicular | 29 |
| 29 | Ascospores (9.5–)11–12(–13) × 4–4.5 µm | Mi. pegleri |
| – | Ascospores longer than 15 µm | 30 |
| 30 | Ascospores 16–24 × 8–12 µm | Mi. fayodii |
| – | Ascospores 25–30 × 11–15 µm | Mi. brevirostratum |
Microthecium africanum
(J.C. Krug) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812088
Persiciospora africana J.C. Krug, Mycologia 80: 416. 1988. [Basionym]
Notes.
Microtheciumafricanum is characterized by ostiolate ascomata and punctate, ellipsoidal ascospores. Two asexual morphs with different conidia have been reported: (i), 1–4(–5)-celled, globose and smooth-walled at first but becoming cylindrical and coarsely verrucose later; (ii), 1–2-celled, large, usually cylindrical and smooth-walled (Krug 1988). However, the type strain was probably not a pure culture because the SSU and LSU sequences match with different species of Fusarium and the pictures of the conidia type (i) resemble the chlamydospores produced by several species of this genus.
Microthecium beatonii
D. Hawksw., Trans. Mycol. Soc. Japan 18: 145. 1977.
≡ Sphaerodesbeatonii (D. Hawksw.) P.F. Cannon & D. Hawksw., Bot. J. Linn. Soc. 84: 145. 1982.
Notes.
This species is characterized by non-ostiolate ascomata, 4-spored asci and very coarsely reticulate, citriform ascospores. These morphological features are also observed in Microtheciumperplexum, but this species produces ascospores with only a third of the surface coarsely reticulate while the rest remains smooth-walled. Microtheciumepisphaerium and Mi.retisporum differ from Mi.beatonii in the production of 8-spored asci. Moreover, Mi.retisporum produces a phialidic asexual morph and bulbils, which are absent in the other mentioned species, and smaller ascospores (17–20 × 10–12 × 7–9 µm) than in Mi.beatonii [28–34(–40) × 14–18(–20) µm], in Mi.episphaerium (25–34 × 12–18 µm) and in Mi.perplexum (22–28 × 12–15 × 9–11 µm).
Microthecium brevirostratum
(Moreau) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812089
Melanospora brevirostrata Moreau, Bull. Trimest. Soc. mycol. Fr. 61: 59. 1945. [Basionym]
Notes.
Microtheciumbrevirostratum together with Mi.fayodii and Mi.pegleri produces ostiolate ascomata, smooth-walled, ellipsoidal to navicular or citriform ascospores and bulbils. Microtheciumbrevirostratum is easily distinguished by ascospores with apiculate germ pores and the presence of a phialidic asexual morph (ascospores show depressed germ pores and lack an asexual morph in other species). Microtheciumfayodii and Mi.pegleri differ in the size of the ascospores, Mi.pegleri having the smallest ascospores in Microthecium [(9.5–)11–12(–13) × 4–4.5 µm].
Microthecium brevirostrum
(Fuckel) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812090
Ceratostoma brevirostre Fuckel, Bot. Ztg. 19: 250. 1861. [Basionym]
≡ Melanosporabrevirostris (Fuckel) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 94. 1914.
= Ceratostomahelvellae Cooke, Grevillea 1: 175. 1873.
≡ Melanosporahelvellae (Cooke) Sacc., Syll. fung. (Abellini) 2: 462. 1883.
= Melanosporasphaerodermoides Grove, J. Bot., Lond. 23: 132. 1885.
= Melanosporasphaerodermoidesvar.sphaerodermoides Grove, J. Bot., Lond. 23: 132. 1885.
= Thielaviasoppittii Crossl., Naturalist, London: 7. 1901.
= Roselliniaaurea McAlpine, Fungus Diseases of stone-fruit trees in Australia: 102. 1902.
≡ Sphaerodermaaureum (McAlpine) Sacc. & D. Sacc., Syll. fung. (Abellini) 17: 781. 1905.
≡ Melanosporaaurea (McAlpine) Doguet, Botaniste 39: 124. 1955.
= Melanosporasphaerodermoidesvar.rubella Pidopl., Mikrobiol. Zh. 9: 61. 1948.
= Melanosporacamelina Faurel & Schotter, Revue Mycol., Paris 30: 144. 1965.
= Melanosporatulasnei Udagawa & Cain, Can. J. Bot. 47: 1932. 1970.
Notes.
Microtheciumbrevirostrum, Mi.fallax and Mi.fimbriatum produce ostiolate ascomata and ellipsoidal to citriform, often plataniform, smooth-walled ascospores with an apiculate germ pore at each end. Microtheciumfimbriatum is easily distinguished by its smaller (100–110 µm diam.), reddish ascomata, while Mi.fallax differs in the production of bulbils.
Microthecium ciliatum
Udagawa & Takada, Trans. Mycol. Soc. Japan 15: 23. 1974.
≡ Pteridiospermaciliatum (Udagawa & Y. Takada) J.C. Krug & Jeng, Mycotaxon 10: 45. 1979.
Notes.
This species is characterized by non-ostiolate ascomata and ellipsoidal to fusiform ascospores ornamented with wing-like appendages and wrinkles, and the production of a phialidic asexual morph and bulbils. Microtheciumlenticulare and Mi.foveolatum also present ascospores with wing-like appendages, but these are pitted and not wrinkled (as in Mi.ciliatum), and neither species produces bulbils. Microtheciumfoveolatum and Mi.ciliatum are characterized by non-ostiolate ascomata and the production of a phialidic asexual morph, whereas Mi.lenticulare has ostiolate ascomata and lacks an asexual morph.
Microthecium compressum
Udagawa & Cain, Can. J. Bot. 47: 1921. 1970.
≡ Sphaerodescompressa (Udagawa & Cain) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 145. 1982.
Notes.
This species is distinguished by the production of non-ostiolate ascomata and citriform, bilaterally flattened ascospores, with the narrow faces coarsely reticulate and the widest faces smooth or nearly so, along with the production of a phialidic asexual morph.
Microthecium episphaerium
(W. Phillips & Plowr.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 98. 1914.
Melanospora episphaeria W. Phillips & Plowr., Grevillea 10: 71. 1881. [Basionym]
≡ Sphaerodermaepisphaerium (W. Phillips & Plowr.) Sacc., Syll. fung. (Abellini) 2: 460. 1883.
≡ Sphaerodesepisphaerium (W. Phillips & Plowr.) Clem. [as ‘episphaericum’], Gen. fung. (Minneapolis): 1‒227. 1909.
≡ Vittadinulaepisphaeria (W. Phillips & Plowr.) Clem. & Shear, Gen. fung., Edn 2 (Minneapolis): 281. 1931.
= Sphaerodermaepimyces Höhn., Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Math.-naturw. Klasse Abt. I 116: 103. 1907.
≡ Melanosporaepimyces (Höhn.) Doguet, Botaniste 39: 125. 1955.
Notes.
Microtheciumepisphaerium shows non-ostiolate ascomata and very coarsely reticulate, citriform ascospores. For morphological comparison see Notes of Mi.beatonii.
Microthecium fallax
(Zukal) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812772
Melanospora fallax Zukal, Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 98: 547. 1889. [Basionym]
= Melanosporaanomala Hotson, Proc. Amer. Acad. Arts & Sci 48.: 257. 1912.
= Melanospora cervicula Hotson, Proc. Amer. Acad. Arts & Sci. 48: 254. 1912.
= Melanosporapapillata Hotson, Proc. Amer. Acad. Arts & Sci 48.: 251. 1912.
= Melanosporaphaseoli Roll-Hansen, Blyttia 6: 73. 1948.
Notes.
This species is characterized by ostiolate ascomata, ellipsoidal to citriform, often plataniform, smooth-walled ascospores, and production of bulbils. For morphological comparison see Notes of Mi.brevirostrum.
Microthecium fayodii
(Vuill.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812091
Melanospora fayodii Vuill. [as ‘fayodi’], Bull. Séanc. Soc. Sci. Nancy, Sér. 2 8: 33. 1887. [Basionym]
Notes.
This species is characterized by ostiolate ascomata, ellipsoidal to navicular or citriform, smooth-walled ascospores, and production of bulbils. For morphological comparison see Notes of Mi.brevirostratum.
Microthecium fimbriatum
(Rostr.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812092
Sphaeroderma fimbriatum Rostr., Oest. Grönl. Svampe: 25. 1894. [Basionym]
≡ Melanosporafimbriata (Rostr.) Petch, Trans. Br. mycol. Soc. 21: 253. 1938.
Notes.
Microtheciumfimbriatum produces ostiolate ascomata, and citriform to plataniform, smooth-walled ascospores with a strongly apiculate and tuberculate germ pore at each end. Although the ascomata was described as small and reddish in the protologue, the strain included in this study (NBRC 8523) shows larger (250–380 µm diam.), orange-brown ascomata. Moreover, our isolate produces bulbils. For morphological comparison see Notes of Mi.brevirostrum.
Microthecium fimicola
(E.C. Hansen) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812093
Melanospora fimicola E.C. Hansen, Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn 59: 15. 1876. [Basionym]
≡ Sphaerodermafimicola (E.C. Hansen) Sacc., Syll. fung. (Abellini) 2: 460. 1883.
≡ Sphaerodesfimicola (E.C. Hansen) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 146. 1982.
= Melanosporaornata Zukal, Verh. zool.-bot. Ges. Wien 35: 340. 1886.
≡ Sphaerodesornata (Zukal) Arx, Gen. Fungi Sporul. Cult., Edn 3 (Vaduz): 156. 1981.
= Sphaerodermahulseboschii Oudem., Contrib. Flora Mycol. d. Pays-Bas 11: 23. 1886.
≡ Melanosporahulseboschii (Oudem.) Doguet, Botaniste 39: 121. 1955.
= Melanosporaaffine Sacc. & Flageolet, Bull. Soc. Mycol. Fr. 12: 67. 1896.
= Melanosporamanginii Vincens [as ‘mangini’], Bull. Soc. Mycol. Fr. 33: 69. 1917.
≡ Sphaerodesmanginii (Vincens) Arx, Gen. Fungi Sporul. Cult., Edn 3 (Vaduz): 156. 1981.
Notes.
Microtheciumfimicola is characterized by ostiolate ascomata and coarsely reticulate ascospores with strongly apiculate germ pores at both ends. The other species with ostiolate ascomata and reticulate ascospores are Mi.internum and Mi.quadrangulatum. The main differences among them are the shape and size of the ascospores, being citriform in Mi.fimicola, spindle-shaped in Mi.internum and fusiform in Mi.quadrangulatum. The production of bulbils has only been observed in our fresh isolates of Mi.fimicola, although this was not previously reported.
Microthecium foveolatum
Udagawa & Y. Horie, in Hawksworth & Udagawa, Trans. Mycol. Soc. Japan 18: 149. 1977.
≡ Pteridiospermafoveolatum (Udagawa & Y. Horie) J.C. Krug & Jeng, Mycotaxon 10: 45. 1979.
Notes.
This species is easily distinguished by its non-ostiolate ascomata, ellipsoidal to fusiform ascospores ornamented with small pores and thick wing-like ridges usually longitudinal but often oblique, and production of phialidic asexual morph. For morphological comparison see Notes of Mi.ciliatum.
Microthecium fusisporum
(Petch) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812094
Sphaeroderma fusisporum Petch, Naturalist, London: 58. 1936. [Basionym]
≡ Melanosporafusispora (Petch) Doguet, Botaniste 39: 215. 1955.
= Melanosporafusisporavar.fusispora (Petch) Doguet, Botaniste 39: 215. 1955.
= Melanosporafusisporavar.parvispora Matsush., Matsush. Mycol. Mem. 8: 24. 1995.
Notes.
Microtheciumfusisporum is related to Mi.nectrioides, both possessing ostiolate ascomata and smooth-walled ascospores. However, Mi.nectrioides can be distinguished by the absence of the crown of setae around the ostiole and its citriform ascospores, being fusiform in Mi.fusisporum.
Microthecium geoporae
(W. Oberm.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 98. 1914.
Guttularia geoporae W. Oberm., Mykol. Zentbl. 3: 9. 1913. [Basionym]
Notes.
This species produces non-ostiolate ascomata and citriform, smooth-walled ascospores. Other species previously placed in Melanospora characterized by the production of non-ostiolate ascomata and smooth-walled ascospores are Mi.hypomyces, Mi.levitum and Mi.zobelii. Microtheciumhypomyces is distinguished by its fusiform ascospores (citriform in the other species), and Mi.levitum by the presence of bulbils and a phialidic asexual morph. Microtheciumgeoporae and Mi.zobelii are distinguished by the size of their ascospores [28–30 × 12–13(–15) µm in Mi.geoporae and 18–25 × 8.5–12 × 6–9 µm in Mi.zobelii]. Microtheciumtenuissimum shows similar morphological features to these species but its ascospores are finely reticulate under SEM and its ascomata are smaller (less than 120 µm) than in the other species.
Microthecium hypomyces
(Höhn.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 123: 50. 1914.
Sphaeroderma hypomyces Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 116: 102. 1907. [Basionym]
≡ Melanosporahypomyces (Höhn.) Doguet, Botaniste 39: 215. 1955.
Notes.
This species is characterized by non-ostiolate ascomata and fusiform, smooth-walled ascospores. For morphological comparison see Notes of Mi.geoporae.
Microthecium internum
(Tehon & G.L. Stout) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812095
Melanospora interna Tehon & G.L. Stout, Mycologia 21: 181. 1929. [Basionym]
Notes.
This species produces ostiolate ascomata and spindle-shaped ascospores with a coarse and irregular reticulum. For morphological comparison see Notes of Mi.fimicola.
Microthecium japonicum
(Y. Horie, Udagawa & P.F. Cannon) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812096
Persiciospora japonica Y. Horie, Udagawa & P.F. Cannon, Mycotaxon 25: 233. 1986. [Basionym]
Notes.
Microtheciumjaponicum is characterized by ostiolate ascomata and ellipsoidal to fusiform, punctate-reticulate ascospores, similar to Mi.moureai. However, Mi.japonicum produces a phialidic asexual morph and bulbils (absent in Mi.moureai) and delicately reticulate ascospores (coarsely reticulate in Mi.moureai).
Microthecium lenticulare
(Udagawa & T. Muroi) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812097
Pteridiosperma lenticulare Udagawa & T. Muroi [as ‘lenticularis’], Trans. Mycol. Soc. Japan 22: 20. 1981. [Basionym]
Notes.
Microtheciumlenticulare produces ostiolate ascomata and pitted-walled ascospores with wing-like appendages. For morphological comparison see Notes of Mi.ciliatum.
Microthecium levitum
Udagawa & Cain, Can. J. Bot. 47: 1917. 1970.
≡ Sphaerodeslevita (Udagawa & Cain) D. García, Stchigel & Guarro, Stud. Mycol. 50: 67. 2004.
Notes.
This species is characterized by non-ostiolate ascomata, citrifrom and smooth-walled ascospores with umbonate and tuberculate germ pores, presence of bulbils and phialidic asexual morph. For morphological comparison see Notes of Mi.geoporae.
Microthecium marchicum
(Lindau) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812099
Chaetomium marchicum Lindau, Hedwigia 35: 56. 1896. [Basionym]
≡ Sphaerodermamarchicum (Lindau) Sacc. & P. Syd., Syll. fung. (Abellini) 14: 627. 1899.
Notes.
Microtheciummarchicum is characterized by its ostiolate ascomata and citrifrom, smooth-walled ascospores. Its ascospores are similar to those of Mi.geoporae, Mi.hypomyces, Mi.levitum and Mi.zobelii, but all of them produce non-ostiolate ascomata.
Microthecium masonii
(Kirschst.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812100
Ceratostoma masonii Kirschst., Trans. Br. mycol. Soc. 18: 306. 1934. [Basionym]
≡ Persiciosporamasonii (Kirschst.) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 135. 1982.
Notes.
Microtheciummasonii is characterized by ostiolate ascomata and ellipsoidal to fusiform, faintly striate-reticulate ascospores. The same type of ascospore ornamentation is also observed in Mi.micropertusum, but this latter species is easily distinguished by the presence of inconspicuous ridges forming a very coarse reticulum and a phialidic asexual morph.
Microthecium micropertusum
(Y. Horie, Udagawa & P.F. Cannon) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812101
Sphaerodes micropertusa Y. Horie, Udagawa & P.F. Cannon, Mycotaxon 25: 236. 1986. [Basionym]
Notes.
Microtheciummicropertusum is distinguished by its ostiolate ascomata, fusiform to citriform or nearly rhombic in outline ascospores with inconspicuous ridges forming a coarse reticulum, and presence of phialidic asexual morph. For morphological comparison see Notes of Mi.masonii.
Microthecium moreaui
(P.F. Cannon & D. Hawksw.) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812102
Persiciospora moreaui P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 134. 1982. [Basionym]
Notes.
Microtheciummoreaui is characterized by its ostiolate ascomata, ellipsoidal and pitted-walled ascospores, and production of bulbils. For morphological comparison see Notes of Mi.japonicum.
Microthecium nectrioides
(Marchal) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812103
Sphaeroderma nectrioides Marchal, Bull. Soc. R. Bot. Belg. 23: 25. 1884. [Basionym]
≡ Melanosporanectrioides (Marchal) Doguet, Botaniste 39: 121. 1955.
= Melanosporaasparagi G. Arnaud, Ann. Serv. Epiph. 2: 273. 1915.
Notes.
This species produces ostiolate ascomata and citriform, smooth-walled ascospores. For morphological comparison see Notes of Mi.fusisporum.
Microthecium pegleri
(D. Hawksw. & A. Henrici) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812104
Melanospora pegleri D. Hawksw. & A. Henrici, Kew Bull. 54: 795. 1999. [Basionym]
Notes.
Microtheciumpegleri is characterized by ostiolate ascomata, ellipsoidal to plano-convex, smooth-walled ascospores and presence of bulbils. For morphological comparison see Notes of Mi.brevirostratum.
Microthecium perplexum
D. Hawksw., Trans. Mycol. Soc. Japan 18: 151. 1977.
≡ Sphaerodesperplexa (D. Hawksw.) P.F. Cannon & D. Hawksw., Bot. J. Linn. Soc. 84: 148. 1982.
Notes.
This species produces non-ostiolate ascomata, 4-spored asci and citrifrom ascospores usually with smooth walls, but one third of these are coarsely reticulated. For morphological comparison see Notes of Mi.beatonii.
Microthecium quadrangulatum
(D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812105
Sphaerodes quadrangularis D. García, Stchigel & Guarro, Stud. Mycol. 50: 64. 2004. [Basionym]
Notes.
Microtheciumquadrangulatum is characterized by ostiolate ascomata and fusiform, reticulate ascospores with strongly apiculate germ pores. For morphological comparison see Notes of Mi.fimicola.
Microthecium retisporum
Udagawa & Cain, Can. J. Bot. 47: 1926. 1970.
≡ Sphaerodesretispora (Udagawa & Cain) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 149. 1982.
= Microtheciumretisporumvar.inferius Udagawa & Cain [as ‘inferior’], Can. J. Bot. 47: 1928. 1970.
≡ Sphaerodesretisporavar.inferior (Udagawa & Cain) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 149. 1982.
≡ Sphaerodesinferior (Udagawa & Cain) D.W. Li & N.P. Schultes, in Schultes, Murtishi & Li, Fungal Biology 121: 901. 2017.
= Microtheciumretisporumvar.retisporum Udagawa & Cain, Can. J. Bot. 47: 1926. 1970.
≡ Sphaerodesretisporavar.retispora (Udagawa & Cain) P.F. Cannon & D. Hawksw., J. Linn. Soc., Bot. 84: 149. 1982.
Notes.
This species is characterized by non-ostiolate ascomata, reticulate citriform ascospores with apiculate germ pores, a phialidic asexual morph and presence of bulbils. For morphological comparison see Notes of Mi.beatonii.
Microthecium sepedonioides
(Preuss) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812106
Papulaspora sepedonioides Preuss, Linnaea 24: 112. 1851. [Basionym]
Notes.
Microtheciumsepedonioides only produces bulbils and the sexual morph has never been observed.
Microthecium tenuissimum
(D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812107
Sphaerodes tenuissima D. García, Stchigel & Guarro, Stud. Mycol. 50: 65. 2004. [Basionym]
Notes.
This species is characterized by non-ostiolate ascomata and citriform, ellipsoidal in lateral view, finely reticulate ascospores with strongly apiculate germ pores. For morphological comparison see Notes of Mi.geoporae.
Microthecium zobelii
Corda, Icon. fung. (Prague) 5: 74. 1842.
≡ Sphaeriazobelii (Corda) Tul. & C. Tul., Fungi hypog.: 186. 1851.
≡ Ceratostomazobelii (Corda) Berk., Journal of the Royal Horticultural Society 4: 402. 1860.
≡ Melanosporazobelii (Corda) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 127. 1870.
= Melanosporazobeliivar.zobelii (Corda) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 127. 1870.
= Melanosporacoprophila Zukal, Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 98: 544. 1889.
= Melanosporamarchicum Lindau, Hedwigia 35: 56. 1896.
= Melanosporazobeliivar.minor Pidopl., Mikrobiol. Zh. 9(2-3): 60. 1948.
Notes.
Microtheciumzobelii produces non-ostiolate ascomata, and citriform, smooth-walled ascospores with slightly apiculate germ pores. For morphological comparison see Notes of Mi.geoporae.
Doubtful species
Microthecium ryvardenianum
Aramb. & Gamundí, Agarica 6: 124. 1985.
Notes.
This species is considered as doubtful because it presents morphological features atypical of Microthecium (e.g. allantoid ascospores when immature becoming striate when mature).
Pseudomicrothecium
Y. Marín, Stchigel, Guarro, Cano, gen. nov .
812108
Type species.
Pseudomicrotheciumsubterraneum (L. Fan, C.L. Hou, P.F. Cannon & Yong Li) Y. Marín, Stchigel, Guarro & Cano. Holotype and ex-isotype strain: BJTC FAN1001, K[M] 172128.
Description.
Ascomata non-ostiolate, globose, translucent, pale brown to brown, appearing dark brown when the ascospores are mature, glabrous or setose; ascomatal wall membranaceous, of textura angularis. Asci 2-spored, clavate, short-stipitate, without apical structures, evanescent. Ascospores one-celled, at first hyaline, becoming dark brown to blackish when mature, ellipsoidal to citriform, umbonate and truncate at both ends, with a terminal indistinct germ pore at each end. Asexual morph absent.
Etymology.
The name refers to the morphological resemblance to Microthecium.
Notes.
The new genus Pseudomicrothecium is proposed here to accommodate Melanosporasubterranea because it constitutes a separate lineage in our phylogenetic study. This genus is characterized by its non-ostiolate ascomata, similar to those of Microthecium, 2-spored asci and smooth-walled ascospores with an indistinct germ pore at each end. Asci containing two ascospores have only been observed in some species of Scopinella (i.e. Scopinellagallicola and S.sphaerophila). However, Scopinella can be easily distinguished from Pseudomicrothecium by the production of ostiolate ascomata with long necks and cuboid-ellipsoidal ascospores with two prominent longitudinal germ slits.
Pseudomicrothecium subterraneum
(L. Fan, C.L. Hou, P.F. Cannon & Yong Li) Y. Marín, Stchigel, Guarro & Cano comb. nov.
812109
Basionym.
Melanosporasubterranea L. Fan, C.L. Hou, P.F. Cannon & Yong Li, Mycologia 104: 1434. 2012.
Discussion
We have revised the taxonomy of relevant members of the family Ceratostomataceae based on the analyses of the SSU, LSU, ITS, act and tef1 nucleotide sequences. This study strongly supported the order Melanosporales proposed by Zhang and Blackwell in 2007 (Hibbett et al. 2007). The phylogenetic inference showed seven lineages corresponding to the genera Dactylidispora, Echinusitheca, Melanospora, Microthecium, Pseudomicrothecium and Vittatispora, and to Melanosporakurssanoviana. Our results agree with previous studies (Zhang and Blackwell 2002, Fan et al. 2012) which already suggested and demonstrated that the ornamentation of the ascospores under SEM, a feature traditionally used to delimit most of the genera in the Melanosporales, is not useful for estimating phylogenetic relationships among these fungal taxa. Similarly, the morphology of the ascospores is of weak taxonomic value and a poor predictor for the generic delimitation of members of the family Sordariaceae, resulting in the synonymy of two relevant genera, i.e. Gelasinospora and Neurospora (Dettman et al. 2001, García et al. 2004, Nygren et al. 2011). In our study, two of the largest genera of the Melanosporales, Melanospora and Microthecium, grouped species with both smooth and ornamented ascospore walls. By contrast, a phylogenetic study of the Lasiosphaeriaceae (Miller and Huhndorf 2005) revealed that the morphology of the ascomatal wall was more phylogenetically informative than that of the ascospores, with several new genera proposed (i.e. Immersiella) or emended (i.e. Lasiosphaeria, Lasiosphaeris and Schizothecium) (Miller and Huhndorf 2004, Cai et al. 2005). Here, the erection of the new genus Echinusitheca is a clear example of the relevance of the ascomatal morphology in the taxonomy of these fungi, and in fact this taxon together with Arxiomyces and Scopinella are the only genera in the Melanosporales that show dark semi-transluscent ascomata. In this context, although Echinusitheca has ascospores similar to those of Melanospora and Microthecium, this genus constitutes one of the lineages phylogenetically most distant within this order.
Another lineage considerably distant from the other members of the Melanosporales is constituted by the clade represented only by the species Melanosporakurssanoviana, suggesting that this fungus could represent a new genus. However, this new taxon is at this moment not proposed because its colonies, in spite of attempts to induce sporulation, remain sterile and a detailed morphological study was not possible. The infertility of the cultures is probably due to the fact that an important part of the members of this fungal group show a peculiar habitat developing a certain degree of mycoparasitism and requiring the presence of the host to complete the biologic cycle and develope reproductive structures. The mycoparasitism of Melanospora, Syspastospora and the species previously placed in Persiciospora and Sphaerodes has already been demonstrated by numerous authors (Doguet 1955, Calviello 1973, Jordan and Barnett 1978, Harveson and Kimbrough 2000, 2001), and this ability has been exploited in the biocontrol of phytopathogenic fungi (Vujanovic and Goh 2009, Goh and Vujanovic 2010, Kim and Vujanovic 2016, 2017).
The genus Sphaeronaemella, which is characterized by pale and translucent ascomata, was thought to be related to Melanospora (Cannon and Hawksworth 1982). However, we do not agree with this relationship because it differs from the Melanosporales in the production of hyaline ascospores, as opposed to the pigmented ones in the members of that order. By contrast, our results correlate with those of other authors that demonstrated a closer phylogenetic relationship of this genus with the members of the order Microascales (Spatafora and Blackwell 1994b, Hausner and Reid 2004). In fact, our SSU tree seems to indicate that Sphaeronaemella could represent a new family of the Microascales; however, further studies including more taxa and additional genes are needed to more accurately confirm its taxonomic status.
The placement of our isolate of Persiciosporajaponicum in the Microthecium clade once more demonstrated that the ornamentation of the ascospores, which is pitted in Persiciospora spp., is of poor taxonomic value, and consequently all the species of Persiciospora should be transferred to Microthecium. As it was above mentioned, the species of this latter genus show a typical cellular ascomatal neck which is also present in Persiciospora and constitutes a common feature in both genera. Surprisingly, in some previous phylogenetic studies, the species of Persiciospora were placed in the Hypocreales, closely related to Nectria (Zhang and Blackwell 2002, Maharachchikumbura et al. 2015, Schultes et al. 2017). However, this could be probably explained by a possible contamination of the cultures of Persiciospora spp. with an hypocrealean host (Fan et al. 2012). The same situation may have occurred with the cultures of Scopinella and Syspastospora, which led to a possible erroneous classification of both taxa in the Hypocreales (Zhang and Blackwell 2002, Chaudhary et al. 2006, Fan et al. 2012, Maharachchikumbura et al. 2015, Schultes et al. 2017).
Pteridiospermaciliatum, a member of the Melanosporales with ascospores ornamented with longitudinal wing-like ridges that anastomose each other to form a well defined reticulum (a distinctive feature of Pteridiosperma), was also found in the Microthecium clade, proving once again that the ascospore ornamentation is not phylogenetically informative. Consequently, we have synonymyzed the genus Pteridiosperma with Microthecium since Pteridiosperma spp. show non-ostiolate ascomata, or if ostiolate, they show a short neck composed of angular cells, which are typical morphological characteristics of Microthecium.
Another genus that our results demonstrated should be synonymized and included in Microthecium is Sphaerodes because its type species, S.episphaerium, shows morphological features (non-ostiolate ascomata) that fit with the current circumscription of that emended genus. Most of the species of Sphaerodes, with the exception of S.ellipsospora and S.singaporensis, which are now located in the new genus Dactylidispora, and S.mycoparasitica, which is now placed in Melanospora, are also transferred to Microthecium since these produce non-ostiolate or ostiolate ascomata without a neck, or less frequently with a short neck composed of angular cells similar to the ascomatal ones. Another relevant feature of the genus Microthecium is the production of bulbils. These propagules are typical of Papulaspora, an anamorphic genus that encompasses more than 40 species. Although it was initially accepted as a genus without a sexual morph (Hotson 1912), its link with species of Melanospora and Chaetomium has been reported (Roll-Hansen 1948, Zhang et al. 2004). In our phylogenetic study Papulasporasepedonioides, the type species of the genus, was nested in the Microthecium clade, and therefore transferred to this genus. The relationship of this species with the Melanosporales had already previously been demonstrated by Davey et al. (2008) and Li et al. (2016). However, it has been demonstrated that Papulaspora is a polyphyletic genus, and other species of the genus have been reported as belonging to the classes Leotiomycetes and Sordariomycetes (Ascomycota). Therefore, the other species of Papulaspora not linked to the species of Microthecium should be transferred to other taxonomic groups. The relationship of some species of Papulaspora with the Melanosporales is also suggested by the production of similar phialidic synanamorphs (Van Beyma 1931, Hotson 1942).
The most recent new combination performed in Sphaerodes, S.inferior, was done to accommodate S.retisporavar.inferior since it was not clustering with S.retisporavar.retispora (Schultes et al. 2017). However, we suspected that the sequences of S.retisporavar.retispora deposited in GenBank were contaminated with the hypocrealean host. In order to corroborate it, we studied such sequenced strain demonstrating that it was effectively contaminated. Therefore, S.inferior is here considered a synonym of Mi.retisporum since the morphological differences are insufficient to recognize this variety as a different species.
There are important morphological differences among the strains of Microthecium that suggest the presence of several additional cryptic species in the genus; however, our phylogenetic study, in spite of having used five loci, was not able to resolve the boundaries among them.
Supplementary Material
Acknowledgments
This work was supported by the Spanish “Ministerio de Economía y Competitividad”, grant CGL2017-88094-P. The new genus Echinusitheca was recovered from samples collected during fieldwork supported by a National Science Foundation grant (DEB-0515558) to ANM.
Citation
Marin-Felix Y, Guarro J, Cano-Lira JF, García D, Miller AN, Stchigel AM (2018) Melanospora (Sordariomycetes, Ascomycota) and its relatives. MycoKeys 44: 81–122. https://doi.org/10.3897/mycokeys.44.29742
References
- Alexopoulos CJ. (1962) Introductory mycology. 2nd edn. John Wiley, New York, 613 pp. [Google Scholar]
- Bessey EA. (1950) Morphology and Taxonomy of Fungi. Blakiston, Philadelphia, Toronto, 791 pp 10.5962/bhl.title.5663 [DOI] [Google Scholar]
- Cai L, Jeewon R, Hyde KD. (2005) Phylogenetic evaluation and taxonomic revision of Schizothecium based on ribosomal DNA and protein coding genes. Fungal Diversity 19: 1–21. [Google Scholar]
- Calviello BO. (1973) Contribución al estudio de ascomycetes argentinos. I. Comunicaciones del Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’. Botánica 2: 31–39. [Google Scholar]
- Cannon PF. (1982) Pustulipora, a new genus of the Melanosporaceae. Mycotaxon 15: 523–528. [Google Scholar]
- Cannon PF, Hawksworth DL. (1982) A re-evaluation of Melanospora Corda and similar pyrenomycetes, with a revision of the British species. The Journal of the Linnean Society, Botany 84: 115–160. 10.1111/j.1095-8339.1982.tb00363.x [DOI] [Google Scholar]
- Cannon PF, Hawksworth DL. (1983) Arxiomyces, a new name for Phaeostoma von Arx & E. Müller. Transactions of the British Mycological Society 8(3): 644–645. 10.1016/S0007-1536(83)80143-0 [DOI] [Google Scholar]
- Cano J, Guarro J, Gené J. (2004) Molecular and morphological identification of Colletotrichum species of clinical interest. Journal of Clinical Microbiology 42(6): 2450–2454. 10.1128/JCM.42.6.2450-2454.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P, Campbell J, Hawksworth DL, Sastry KN. (2006) Vittatispora, a new melanosporaceous genus from Indian soil. Mycologia 98(3): 460–467. 10.1080/15572536.2006.11832681 [DOI] [PubMed] [Google Scholar]
- Clements FE. (1909) Genera of Fungi. H. W. Wilson, Minneapolis, 227 pp. [Google Scholar]
- Corda ACJ. (1837) Icones fungorum hucusque cognitorum. Volume 1. Calve, Prague, 32 pp. [Google Scholar]
- Corda ACJ. (1842) Icones fungorum hucusque cognitorum. Volume 5. Calve, Prague, 92 pp. [Google Scholar]
- Davey ML, Tsuneda A, Currah RS. (2008) Evidence that the gemmae of Papulasporasepedonioides are neotenous perithecia in the Melanosporales. Mycologia 100(4): 626–635. 10.3852/08-001R [DOI] [PubMed] [Google Scholar]
- Dennis RWG. (1968) British Ascomycetes. Cramer, Lehre, Germany, 455 pp. [Google Scholar]
- Dettman JR, Harbinski FM, Taylor JW. (2001) Ascospore morphology is a poor predictor of the phylogenetic relationships of Neurospora and Gelasinospora. Fungal Genetics and Biology 34(1): 49–61. 10.1006/fgbi.2001.1289 [DOI] [PubMed] [Google Scholar]
- Doguet G. (1955) Le genre Melanospora biologie, morphologie, développement, systématique. Botaniste 39: 1–313. [Google Scholar]
- Fan L, Hou C, Cannon PF, Li Y. (2012) A new species of Melanospora on truffles from China. Mycologia 104(6): 1433–1442. 10.3852/11-338 [DOI] [PubMed] [Google Scholar]
- Figueras MJ, Guarro J. (1988) A scanning electron microscopic study of ascoma development in Chaetomiummalaysiense. Mycologia 80(3): 298–306. 10.2307/3807625 [DOI] [Google Scholar]
- Fuckel L. (1877) Symbolae mycologicae. Beiträge zur Kenntniss der rheinischen Pilze. Dritter Nachtrag. Jahrbücher des Nassauischen Vereins für Naturkunde 29-30: 1–39.
- García D, Stchigel AM, Guarro J. (2003) A new species of Poroconiochaeta from Russian soils. Mycologia 95(3): 525–529. 10.1080/15572536.2004.11833099 [DOI] [PubMed] [Google Scholar]
- García D, Stchigel AM, Cano J, Guarro J, Hawksworth DL. (2004) A synopsis and re-circumscription of Neurospora (syn. Gelasinospora) based on ultrastructural and 28S rDNA sequence data. Mycological Research 108(10): 1119–1142. 10.1017/S0953756204000218 [DOI] [PubMed] [Google Scholar]
- Gaümann E. (1964) Die Pilze. Birkhäuser Verlag, Basel, Switzerland, 541 pp 10.1007/978-3-0348-6860-0 [DOI] [Google Scholar]
- Goh YK, Vujanovic V. (2010) Sphaerodesquadrangularis biotrophic mycoparasitism on Fusariumavenaceum. Mycologia 102(4): 757–762. 10.3852/09-171 [DOI] [PubMed] [Google Scholar]
- Guarro J, Gene J, Stchigel AM, Figueras MJ. (2012) Atlas of Soil Ascomycetes. CBS Biodiversity Series no. 10. CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands, 486 pp. [Google Scholar]
- Harveson RM, Kimbrough JW. (2000) First report of Persiciosporamoreaui, a parasite of Fusariumoxysporum, in the western hemisphere. Mycotaxon 76: 361–365. [Google Scholar]
- Harveson RM, Kimbrough JW. (2001) Parasitism and measurement of damage to Fusariumoxysporum by species of Melanospora, Sphaerodes, and Persiciospora. Mycologia 93(2): 249–257. 10.2307/3761645 [DOI] [Google Scholar]
- Hausner G, Reid J. (2004) The nuclear small subunit ribosomal genes of Sphaeronaemellahelvellae, Sphaeronaemellafimicola, Gabarnaudiabetae, and Cornuvesicafalcata: phylogenetic implications. Canadian Journal of Botany 82(6): 752–762. 10.1139/b04-046 [DOI] [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Elliott ML, Canning G, McMillan VE, Crous PW. (2016) Take-all or nothing. Studies in Mycology 83: 19–48. 10.1016/j.simyco.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüssler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. (2007) A higher-level phylogenetic classification of the Fungi. Mycological Research 111(5): 509–547. 10.1016/j.mycres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Hotson JW. (1912) Cultural studies of fungi producing bulbils and similar propagative bodies. Proceedings of the American Academy of Arts and Sciences 48(8): 227−306. 10.2307/20022828 [DOI]
- Hotson HH. (1942) Some species of Papulaspora associated with rots of gladiolus bulbs. Mycologia 34(4): 391−399. 10.2307/3754980 [DOI]
- Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. (2007) Polyphasic taxonomy of AspergillussectionUsti Studies in Mycology 59: 107−128. 10.3114/sim.2007.59.12 [DOI] [PMC free article] [PubMed]
- Jones KG, Blackwell M. (1998) Phylogenetic analysis of ambrosial species in the genus Raffaelea based on 18S rDNA sequences. Mycological Research 102(6): 661–665. 10.1017/S0953756296003437 [DOI] [Google Scholar]
- Jordan EG, Barnett HL. (1978) Nutrition and parasitism of Melanosporazamiae. Mycologia 70(2): 300–312. 10.2307/3759028 [DOI] [Google Scholar]
- Kim SH, Vujanovic V. (2016) Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp. and mycotoxins production. Applied Microbiology and Biotechnology 100(12): 5257–5272. 10.1007/s00253-016-7539-z [DOI] [PubMed] [Google Scholar]
- Kim SH, Vujanovic V. (2017) Biodegradation and biodetoxification of Fusarium mycotoxins by Sphaerodesmycoparasitica AMB Express 7: 145. 10.1186/s13568-017-0446-6 [DOI] [PMC free article] [PubMed]
- Kornerup A, Wanscher JH. (1984) Methuen handbook of colour. 3rd edn. Eyre Methuen, London, England, 252 pp. [Google Scholar]
- Kowalski DT. (1965) The development and cytology of Melanosporatiffanii. Mycologia 57(2): 279–290. 10.2307/3756829 [DOI] [Google Scholar]
- Krug JC. (1988) A new species of Persiciospora from African soil. Mycologia 80(3): 414–417. 10.2307/3807643 [DOI] [Google Scholar]
- Krug JC, Jeng RS. (1979) Rhytidospora and Pteridiosperma, gen. nov. (Melanosporaceae). Mycotaxon 10(1): 41–45. [Google Scholar]
- Li D-W, Schultes NP, Vossbrinck C. (2016) Olpitrichumsphaerosporum: a new USA record and phylogenetic placement. Mycotaxon 131(1): 123–133. 10.5248/131.123 [DOI] [Google Scholar]
- Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu J-K, Liu Z-Y, Norphanphoun C, Pang K-L, Perera RH, Senanayake IC, Shang Q, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J. (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Diversity 72(1): 199–301. 10.1007/s13225-015-0331-z [DOI] [Google Scholar]
- Matsushima T. (1995) Matsushima Mycological Memoirs No. 8. Matsushima Mycological Memoirs 8: 1–44. [Google Scholar]
- Miller AN, Huhndorf S. (2004) Using phylogenetic species recognition to delimit species boundaries within Lasiosphaeria. Mycologia 96(5): 1106–1127. 10.1080/15572536.2005.11832909 [DOI] [PubMed] [Google Scholar]
- Miller AN, Huhndorf S. (2005) Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi). Molecular Phylogenetics and Evolution 35(1): 60–75. 10.1016/j.ympev.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Nygren K, Strandberg R, Wallberg A, Nabholz B, Gustafsson T, García D, Cano J, Guarro J, Johannesson H. (2011) A comprehensive phylogeny of Neurospora reveals a link between reproductive mode and molecular evolution in fungi. Molecular Phylogenetics and Evolution 59(3): 649–63. 10.1016/j.ympev.2011.03.023 [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1995) Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany 73(S1): 816–823. 10.1139/b95-327 [DOI]
- Roll-Hansen F. (1948) Melanosporaphaseolii n.sp. Blyttia 6: 73–76. [Google Scholar]
- Schultes NP, Murtishi B, Li DW. (2017) Phylogenetic relationships of Chlamydomyces, Harzia, Olpitrichum, and their sexual allies, Melanospora and Sphaerodes. Fungal Biology 121(10): 890–904. 10.1016/j.funbio.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Spatafora JW, Blackwell M. (1994a) Cladistic analysis of partial ssrDNA sequences among unitunicate perithecial ascomycetes and its implications on the evolution of centrum development. In: Hawksworth DL. (Ed.) Ascomycete Systematics: problems and perspectives in the nineties.Plenum Press, New York, 233–241.
- Spatafora JW, Blackwell M. (1994b) The polyphyletic origins of ophiostomatoid fungi. Mycological Research 98(1): 1–9. 10.1016/S0953-7562(09)80327-4 [DOI] [Google Scholar]
- Stchigel AM, Cano J, Guarro J. (1999) A new species of Melanospora from Easter Island. Mycological Research 103(10): 1305–1308. 10.1017/S095375629900845X [DOI] [Google Scholar]
- Stchigel AM, Cano J, Mac Cormack WP, Guarro J. (2001) Antarctomycespsychrotrophicus gen. et sp. nov., a new ascomycete from Antarctica. Mycological Research 105(3): 377–382. 10.1017/S0953756201003379 [DOI] [Google Scholar]
- Stchigel AM, Guarro J, Figueras MJ. (1997) A new species of Melanospora from India. Mycological Research 101(4): 446–448. 10.1017/S0953756296002948 [DOI] [Google Scholar]
- Van Beyma JFH. (1931) Untersuchungen über Russtaupilze. Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde 29(2): 1–40. [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococcus Journal of Bacteriology 172(8): 4238−4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed]
- Voigt K, Wöstemeyer J. (2000) Reliable amplification of actin genes facilitates deep-level phylogeny. Microbiological Research 155(3): 179–195. 10.1016/S0944-5013(00)80031-2 [DOI] [PubMed] [Google Scholar]
- Vujanovic V, Goh YK. (2009) Sphaerodesmycoparasitica sp. nov., a new biotrophic mycopaparasite on Fusariumavenaceum, F. graminearum and F.oxysporum. Mycological Research 113(10): 1172–1180. 10.1016/j.mycres.2009.07.018 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JS, White TJ. (Eds) PCR protocol: a guide to methods and applications.Academic Press, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Winter G. (1887) Dr. L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. E. Kummer, Leipzig, Germany, 928 pp. [Google Scholar]
- Woronichin NN. (1924) Fungi nonnulli novi e Caucaso. III. Notulae Systematicae ex Instituto Cryptogamico Horti Botanici Petropolitani 3(2): 31–32. [Google Scholar]
- Zhang N, Blackwell M. (2002) Molecular phylogeny of Melanospora and similar pyrenomycetous fungi. Mycological Research 106(2): 148–155. 10.1017/S0953756201005354 [DOI] [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH. (2006) An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98(6): 1076–1087. 10.1080/15572536.2006.11832635 [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang R-L, Hu H. (2004) Bulbils exist in root of Cypripediumflavum. Acta Botanica Yunnanica 26(5): 495–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.