Abstract
Mitochondrial dysfunction characterized by impaired bioenergetics, oxidative stress and aldehydic load is a hallmark of heart failure. Recently, different research groups have provided evidence that selective activation of mitochondrial detoxifying systems that counteract excessive accumulation of ROS, RNS and reactive aldehydes is sufficient to stop cardiac degeneration upon chronic stress, such as heart failure. Therefore, pharmacological and non-pharmacological approaches targeting mitochondria detoxification may play a critical role in the prevention or treatment of heart failure. In this review we discuss the most recent findings on the central role of mitochondrial dysfunction, oxidative stress and aldehydic load in heart failure, highlighting the most recent preclinical and clinical studies using mitochondria-targeted molecules and exercise training as effective tools against heart failure.
Keywords: Mitochondria, Redox imbalance, Aldehydes, Cardiovascular diseases, Therapy, Exercise training
Introduction
Heart failure is a multifactorial degenerative disease, characterized by impaired capacity to fill the left ventricle and/or eject blood to match the demands. It has been considered the final common pathway of several acute and chronic diseases including myocardial infarction, myocarditis, valvular heart disease, hypertension, diabetes, obesity and chemotherapy [1–3]. Despite the advances in pharmacological and non-pharmacological interventions, heart failure continues to be a major public health problem, affecting over 25 million people worldwide [4] and costing about $100 billion/yr in the United States [5].
Over the last decades, heart failure treatment relied mainly on drugs that reduce cardiac workload by targeting neurohumoral over-activation with drugs such as beta-blockers, ACE inhibitors and AT1 antagonists [6,7]. Although these interventions promote symptom relief, they have a marginal impact on heart failure outcome such as mortality and rehospitalization rates [8]. Therefore, the identification of intrinsic mechanisms responsible for the continuous cardiomyocyte degeneration during the establishment and progression of heart failure is needed for the development of more effective therapies.
Current studies have focused on identifying intracellular targets that act as central nodes in the suppression or retardation of heart failure development and/or progression [9–13]. Many such intracellular candidates have been described in preclinical studies as critical players in cardiomyocyte degeneration during heart failure [14–18]. However, none of them has been translated into the clinic yet. Perhaps it is insufficient to treat heart failure by focusing on individual targets in a condition where a wide range of intracellular pathways is activated at different stages of the disease progression. Yet, identifying the optimal intracellular node(s) that should be inhibited to counteract chronic cardiomyocyte dysfunction and cardiac degeneration remains a major challenge in the heart failure treatment.
Mitochondrial (dys)function in heart failure
Among the myriad of intracellular components, mitochondria have been considered a strategic and dynamic node of influence, with unique biochemical, morphological and spatial features. Mitochondria are double membrane organelles containing over 1200 proteins, mainly encoded by the nucleus, translated in the cytosol and imported through mitochondrial outer and inner membrane (TOM-TIM) complexes. Only 13 proteins (all subunits of the electron transport chain) are encoded by the mitochondrial DNA. Mitochondria use redox oxidation to generate an electrochemical gradient required for ATP synthesis through oxidative phosphorylation. Mitochondrial energy metabolism is also the main source of oxidants; therefore playing a unique role in cardiac redox signaling and oxidative stress. In addition to their central role in ATP synthesis and redox homeostasis, mitochondria are involved in fatty acid and amino acid oxidation; maintenance of metabolites, co-factors and ions homeostasis; and heme and iron-sulfur cluster synthesis [19–21].
There are three different subpopulations of cardiac mitochondria: subsarcolemmal, perinuclear and intermyofibrillar. These mitochondrial subtypes, which account for ~35% of cardiomyocyte volume, differ in their morphology and function [22,23]. Intermyofibrillar mitochondria form a well-organized network of long and dense organelles oriented by the contractile myofilaments. This specific mitochondria architecture along the cardiomyocyte facilitates both physical and chemical interactions between mitochondria and other intracellular structures, such as the sarcoplasmic reticulum, which are critical for the heart physiology [22,23]. Disruption of these interactions is sufficient to reduce cardiomyocyte contractility properties and induce death [24].
Mitochondria have both direct and indirect impact on cardiomyocyte physiology by regulating bioenergetics [cardiomyocytes have high demand for ATP synthesis and oxygen consumption], redox signaling [physiological response], oxidative stress [pathological response], calcium handling, contractility properties, necrosis and apoptosis. Therefore, maintenance of cardiac mitochondrial function and integrity is critical for human health. Eukaryotic cells have developed several mechanisms of surveillance to protect mitochondrial integrity. The three main levels of surveillance include (1) a multilayer network of detoxifying systems that counteracts excessive accumulation of oxygen- and aldehyde-meditated mitochondrial toxicity; (2) a protein quality control machinery, mediated by chaperones and proteases that are responsible for the maintenance of mitochondrial proteostasis; and (3) an interconnected mitochondrial network that controls mitochondrial morphology and number through mitochondrial fusion and fission, and mitophagy [24]. Pre-clinical studies have demonstrated that activating these mitochondrial surveillance systems is sufficient to protect against heart failure [25].
Several mitochondrial abnormalities have been associated with decompensated heart failure in both humans and experimental animal models [24]. The different types of dysfunction include inefficient energy production, excessive mitochondrial accumulation of ions, ROS and RNS, aldehydic load, disrupted mitochondrial interaction with other organelles (i.e. endoplasmic reticulum) and changes in mitochondrial number, mass and morphology due to unbalanced mitochondrial dynamics and impaired mitophagy [24]. This review is focused solely on detoxifying systems that counteract ROS-, RNS- and aldehyde- meditated mitochondrial toxicity in heart failure. The role of other mitochondrial mechanisms of surveillance in heart failure has been recently reviewed [24,26–28].
Reactive oxygen and nitrogen species in heart failure
ROS and RNS are broad terms used to refer to all the reactive chemical species derived from oxygen and nitrogen, respectively [29], including free radicals (e.g. OH˙, O2˙-, NO˙) and non-radicals (e.g. H2O2). These molecules have different half-life, reactivity and distribution within the cell. Therefore, features such as time, location and concentration should be considered when studying the effect of ROS and RNS in cardiac physiology and disease. Moreover, the method used to detect these species and the secondary products should be specific, suitable and sensitive enough. The current approaches to measure these ROS and RNS directly and indirectly include electron paramagnetic resonance (EPR) spectroscopy, liquid chromatography–mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC) and redox-active probes [30,31].
The role of ROS in tissue degeneration has been studied and reviewed in the last decades [32–34]. But despite the extensive investigation in the field of oxidative stress, commonly defined as the imbalance between ROS generation and antioxidant defense, our current understanding of the so called “equilibrium state” is insufficient; there is a gap in the current knowledge about the physiological role of both oxidants and antioxidants in a time- and location-dependent manner [29]. This gap may have contributed to the difficulty in designing effective interventions and the failure of antioxidants in clinical trials [29]. Most studies have not considered the role of targeted reactive species as signaling molecules as well as the consequences of its scavenging in redox-mediated processes, such as ischemic preconditioning [35,36]; therefore, reinforcing the need for a better understanding of ROS biological functions.
ROS and RNS have many different intracellular sources, including ETC, xanthine oxidase, NADPH oxidase and nitric oxide synthase (for details see refs [37,38]). In cardiac myocytes, mitochondria have been considered the major source of such products [32]. Mitochondrial respiration generates superoxide (O2˙-) through electron leakage at the complexes I and III of the ETC, which are transferred to free O2. Superoxide is then dismutated to hydrogen peroxide (H2O2) by the mitochondrial superoxide dismutase (SOD) and, in the presence of free iron (Fe2+), H2O2 can be reduced via Fenton reaction to originate the hydroxyl radical (OH˙), a highly reactive free radical [39]. Additionally, in the presence of nitric oxide (NO˙), superoxide can generate peroxynitrite (ONOO˙). H2O2 is eliminated by antioxidant enzymes (e.g. catalase, peroxidase) that mostly require NADPH as an electron donor (Figure 1). ROS can also be produced by the metabolism of drugs and xenobiotics, such as the anti-cancer drug, doxorubicin [29], which has been linked to the development of cardiomyopathies [2]. In fact, cardiac accumulation of ROS and RNS has been reported in several cardiac diseases, including cardiomyopathies (i.e. ischemic, chagasic, dilated, hypertensive and idiopathic) and heart failure in both rodents and humans [40–42].
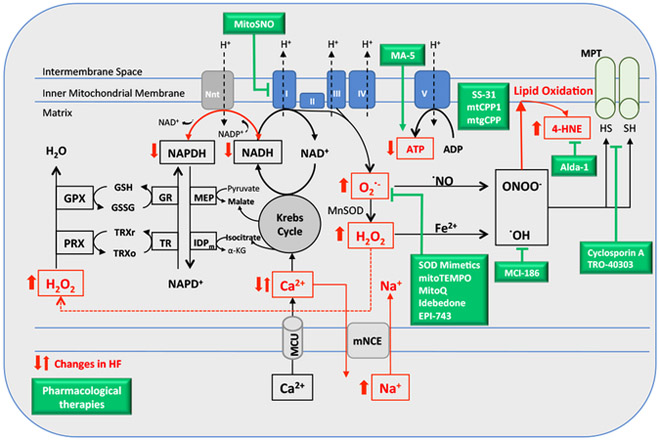
Figure 1.
Proposed model for the sources of reactive oxygen species (ROS) in heart failure based on previous studies [38,57,232], which focused in the interplay between defective Ca2+ handling and NAD(P)H/NAD(P)+ redox state in mitochondrial ROS generation. In green boxes, the pharmacological therapies listed in Table 1. IDPm, mitochondrial NADP+ dependent isocitrate dehydrogenase; MEP, malic enzyme; Nnt, nicotinamide nucleotide transhydrogenase; GR, glutathione reductase; TR, thioredoxin reductase; MCU, mitochondrial Ca2+ Uniporter; Mn-SOD, Mn2+ dependent superoxide dismutase; PRX, peroxiredoxin; GPX, glutathione peroxidase; TRXr/o, reduced/oxidized thioredoxin; GSH/GSSG, reduced/oxidized glutathione; α-KG, α-ketoglutarate; mNCE, Na+/Ca2+ exchanger; MPT, mitochondrial permeability transition.
Due to its important role in energy supply (ATP synthesis) and ROS production/metabolism, mitochondrial dysfunction has been strongly linked to the development of metabolic and oxidative stress related diseases, such as cardiovascular diseases [43]. In fact, increased levels of ROS have been reported in failing hearts [16,44]; however, the cellular mechanisms that trigger its production and accumulation as well as the downstream effects that lead to the disease progression are still elusive, and may differ in distinct cardiomyopathies [40,41]. For example, the accumulation of succinate (a Krebs intermediate) during cardiac ischemia is sufficient to cause massive ROS generation through induction of reverse electron transport in the ETC during reperfusion [45]. This conserved metabolic response has been proposed as a critical process responsible for ischemia-reperfusion injury and, therefore, a promising therapeutic target in ischemic heart diseases [45]. However, its long-term impact on ischemic cardiomyopathy or heart failure remains to be determined.
The source of ROS in heart failure with reduced ejection fraction (HFrEF) seems to be different from heart failure with preserved ejection fraction (HFpEF, also known as diastolic heart failure). While the cardiomyocytes have been described as the main source of ROS in HFrEF [38], the endothelial cells seem to play an important role in ROS generation in HFpEF [46]. In fact, reactive species generated in endothelial cells, as a consequence of coronary microvascular inflammation, have been proposed to propagate the damage to cardiomyocytes through paracrine mechanisms, contributing to the maladaptive cardiac remodeling [38,46]. Notably, the association between ROS production and inflammatory processes has been extensively reported in the literature and may play a role in cardiovascular diseases [47–50].
Mitochondrial calcium overload has also been linked to increased ROS release and reduced ATP production in heart failure [51]. The relationship between Ca2+ and ROS generation in mitochondria has been extensively studied and reviewed [38,52–54]. Mitochondrial calcium activates Krebs cycle dehydrogenases involved in the oxidative phosphorylation, maintaining a pool of reduced NADH and contributing to cellular energy homeostasis and maintenance of adequate cardiac work [55,56]. In heart failure, this process is disrupted, and disturbed mitochondrial Ca2+ uptake culminates in NADPH oxidation and ROS generation and accumulation. This effect is partially explained by a shift in the activity of the mitochondrial enzyme nicotinamide nucleotide transhydrogenase (Nnt) in the heart under pressure overload [57]. This enzyme converts NADH to NADPH, an essential substrate for antioxidant enzymes. However, under overload conditions, Nnt catalyzes the opposite reaction, then weakening the cellular antioxidant defense. Other studies have also demonstrated the importance of NADPH for the ROS scavenger enzymes, to provide reduced glutathione, glutaredoxin, and thioredoxin pools [58,59]. Deletion of key enzymes in the generation and/or recycling of NADPH directly impacts cell capacity to remove ROS [59], mainly the H2O2, an abundant product in failing cardiomyocytes, involved in hypertrophy signaling [60] and modulation of mitochondrial enzymes activity [61,62] (Figure 1).
Both defective Ca2+ and Na+ handling were proposed to initiate a vicious cycle leading to changes in NAD(P)H/NAD(P)+ redox state and oxidative stress, which potentially affects EC coupling, cardiac dysfunction and heart failure [39,54,57,63]. Accumulation of cytosolic Na+, which has been described in heart failure [39,64], has detrimental effects on mitochondrial Ca2+ uptake, once the Ca2+ efflux occurs through a Na+ /Ca2+ exchanger (mNCE). Consistent with this mechanism, inhibition of mNCE rescues mitochondrial Ca2+ uptake, prevents ROS accumulation and attenuates cardiac pathological remodeling in a guinea pig model of heart failure [65] (Figure 1).
The negative impact of ROS in heart failure includes the activation of a wide range of signaling pathways related to cell death [66], proliferation of cardiac fibroblasts, activation of matrix metalloproteinases, mtDNA damage [67], mitochondrial dysfunction [68], impaired calcium handling [69] and contractility [70], and cardiac hypertrophy [15], all of which ultimately leading to maladaptive myocardial remodeling and cardiac dysfunction. For example, the oxidation and activation of the kinase CaMKII (Ca2+-calmodulin–dependent protein kinase) mediated by H2O2 triggers mitochondrial permeability transition, oxidative stress-mediated myocardial damage, necrosis and heart failure [62,71]. CaMKII contributes to the regulation of cardiac excitation–contraction coupling, and its delta isoform has been implicated in apoptotic signaling and in mediating the transition to heart failure [72,73].
Furthermore, it has recently been proposed that ROS can directly modify microRNAs [74] and alter its expression [75]; therefore causing both proteome remodeling [76] and metabolic shift in heart failure [76–78]. miRNAs are a class of small non-coding RNA involved in posttranscriptional regulation of protein expression [79]. Therefore, modifications in miRNA expression can lead to expressive changes in protein levels and disrupt down-stream gene regulation in affected tissues. Several microRNAs have been reported to modulate mitochondrial function [80,81]. For example, miR-210 regulates mitochondrial respiration and metabolism during hypoxic stress by repressing the Iron-Sulfur Cluster Assembly Proteins ISCU1/2 [82]. ISCU1/2 facilitates the assembly of [4Fe-4S] and [2Fe-2S] iron-sulfur clusters required by enzymes such as aconitase (TCA cycle) and the mitochondrial respiratory complexes. Chen et al reported that treatment with miR-210 increases O2˙- and H2O2 production in cellular model [83] and several miRNAs were identified in the mitochondrial fraction (mitomiRs) and profiled in failing hearts following TAC (transverse aortic constriction) in mice [84]. However, their functional consequences in heart diseases are still to be elucidated.
In addition to their direct damage of biomolecules and related downstream signaling, increased levels of ROS can induce cellular responses through distinct mechanisms such as the production of other classes of reactive molecules (aldehydes) [85], ROS-induced ROS release [86–88] and the recently described ROS-mediated paracrine signaling [89]. Interestingly, the latter mechanism proposes that ROS indirectly mediates the communication between cardiomyocytes and pericardial cells in the heart. Using Drosophila, Lim et al (2014) postulated that cardiac ROS induce downstream signals in the pericardial cells that, in turn, act on the cardiomyocytes in a paracrine way to modulate their function, in a process that does not require diffusion of ROS between cells [89].
For the development of effective therapy for a multifactorial disease as heart failure, it is crucial not to limit our studies to local damage in cardiomyocyte, but also its crosstalk with non-myocytes cells and remote organs. To elucidate the role of ROS as signaling molecules in cardiomyopathies, ROS should be broadly investigated, considering each species own reactivity and stability, cellular concentration and distribution, as well as its sources and metabolism in specific cell types.
Lipid peroxidation and aldehyde metabolism in heart failure
Lipid peroxidation is an oxidative degradation process triggered by a chain reaction of excess ROS attacking cell membrane or subcellular organelle membrane phospholipid and polyunsaturated fatty acid (PUFA) [90]. Lipid peroxidation is characterized by the attack of free radicals (e.g. OH·- and O2·-) on the double bonds of unsaturated lipid to produce lipid peroxide. The result of this chemical reaction is the generation of highly reactive and damaging carbonyls, such as different aldehydes, ketones, alkanes [91]. Two examples of the highly reactive lipid peroxidation aldehydes are 4-hydroxy-nonenal (4HNE) and malondialdehyde (MDA), which are generated from linoleic acid or arachidonic acid [91]. Reactive aldehydes can form adducts with proteins and DNA thus leading to cellular damage. As compared to the average half-life of ROS, which is only micro- to milliseconds in vivo, reactive aldehydes are stable for 1–2 seconds, diffuse through cell membranes and amplify the effects of ROS [92]. The damage caused by reactive aldehydes has therefore been implicated in many acute and chronic human diseases such neurodegenerative diseases, alcoholic liver disease, diabetes, cancer and various cardiovascular diseases [27,93–97].
The coupling of oxidative phosphorylation from respiration with electron transport chain across the mitochondria inner membrane is a major site and source of abundant ROS, and consequent, aldehyde generation [98]. The presence of at least six aldehyde dehydrogenase (ALDH) isozymes, among a total of 19 human ALDH isozymes, within the mitochondria highlights the critical protective role of ALDHs for the removal and metabolism of exogenic and endogenic reactive aldehydes to preserve mitochondrial homeostasis and function [99]. One of the better-characterized ALDH isozyme is the mitochondrial ALDH2 (EC 1.2.1.3). ALDH2 is a tetrameric non-P450 detoxifying enzyme, most efficient in metabolizing short-chain aliphatic aldehydes like acetaldehyde and propionaldehyde. However, ALDH2 also participates in the catalysis of other reactive lipid peroxidation-derived aldehydes, such as 4HNE and MDA [100,101]. Importantly, a common single amino acid substitution variant of ALDH2 (E594K, or the ALDH2*2 allele) that renders the inactive enzyme, exists in about 540 million East Asians and their descendants [102].
Carriers of the ALDH2*2 variant allele typically exhibit the “alcohol flushing syndrome” due to their inability to metabolize acetaldehyde from alcohol drinking efficiently. Accumulation of toxic acetaldehyde not only causes the symptoms of facial flushing, palpitation, headache, vomiting, but it is also highly associated with upperaerodigestive track cancers and liver, colorectal and female breast cancers [102]. In 2007, the WHO International Agency for Research on Cancer (IARC) classified alcoholic beverage as a Group 1 carcinogen (carcinogenic to human), with an emphasis on acetaldehyde toxicity specially for ALDH2*2 enzyme-deficient human subjects [103].
The importance of mitochondrial ALDH2 enzyme for cardiovascular function and cardiovascular diseases is well documented. The subject has been reviewed extensively in recently years [104–113]. Chen et al. first reported a critical role of ALDH2 in εPKC-mediated cardioprotection against ischemia and reperfusion (I/R) injury [114]. A small molecule ALDH2-specific activator, Alda-1, was shown to be effective in reducing I/R injury in an animal model by increasing the ALDH2 enzyme activity directly [114]. X-ray crystal structures showed that Alda-1 binds in the catalytic tunnel site of the ALDH2, close to its substrate site. Alda-1 not only enhances the rate of productive encounter between the aldehyde substrate and the critical active site amino acid residues, but also corrects the conformational defect of the ALDH2*2 mutant enzyme and thus restoring ALDH2*2 activity allosterically [115]. These observations underlie the potential for the development of Alda-1-like compounds as therapeutics for the enhancement of ALDH2 activity and especially for human subjects carrying the defective ALDH2*2 variant [27,108].
More recent work indicated that ALDH2 and its polymorphism play a role in the etiology and pathology of heart failure. Two common features of heart failure are oxidative stress and mitochondrial dysfunction, both intricately related to ALDH2 function. In separate studies, downregulation of ALDH2 was associated with cardiac damage in both rat and mouse model of myocardial infarction and pressure overload [116,117], and in tissues obtained from end-stage heart failure patients receiving heart transplantation [116]. In two different genetic models, ALDH2 knockout and dominant-negative ALDH2 mutant overexpression, there was an exacerbated left ventricular dilation and cardiomyocyte death 4 weeks after myocardial infarction [116]. On the other hand, cardiac overexpression of a functional ALDH2 attenuated and protected heart function and remodeling against myocardial infarction [116] and chronic alcohol intake [118].
Therapies targeting mitochondria in heart failure
As previously discussed, mitochondrial dysfunction, oxidative stress and aldehydic load have been linked to the development and progression of heart failure. Therefore, mitochondria may serve as one of the most promising targets for heart failure treatment [25,119,120]. Several strategies targeting mitochondria, such as small molecules [25,115], and mitochondria targeting peptides and antioxidants [121] have been tested in preclinical and clinical studies in heart failure (Table 1). Coenzyme Q10 (CoQ10 or ubiquinone) supplementation has been considered a safe and effective therapeutic option to treat heart failure [122]. CoQ10 is a component of the ETC, mediating the electron transport from Complexes I and II to Complex III. Its reduced form, ubiquinol, acts as an antioxidant inside mitochondria, once it can react with peroxyl radicals and regenerate vitamin E [123], thus protecting the cell against lipid peroxidation.
Table 1.
Pharmacological therapies targeting mitochondria in cardiac diseases.
| Agent | Chemical Property | Action | Diseases | Clinical Status | References |
|---|---|---|---|---|---|
| EUK-8 | Synthetic salen-manganese complex | SOD mimetic | Dilated Cardiomyopathy; Pressure overload induced HF |
Pre-clinical Pre-clinical |
[205] [206] |
| M40403 | Mn2+ containing synthetic compound | SOD mimetic | I/R heart injury | Pre-clinical | [207,208] |
| Me2DO2A | Mn2+ containing synthetic compound | SOD mimetic | I/R heart injury | Pre-clinical | [209] |
| XJB-5-131 | Mitochondria-targeted nitroxide | SOD mimetic/ROS scavenger | I/R heart injury; Huntington’s Disease |
Pre-clinical Pre-clinical |
[210,211] [212] |
| mitoTEMPO | Mitochondria-targeted nitroxide | ROS scavenger | Diabetic cardiomyopathy; Hypertension |
Pre-clinical Pre-clinical |
[213] [214,215] |
| MCI-186 (Edaravone) | Small molecule | Free radical scavenger | I/R heart injury; Pressure overload induced HF; Acute Ischemic Stroke; Amyotrophic lateral sclerosis (ALS) |
Phase 4 Pre-clinical Phases 2-4 Phases 1-3 |
[140,216] [217] [218] [219,220] |
| MitoQ | Ubiquinone derivative | Free radical scavenger | I/R heart injury; Pressure overload; Peripheral Artery Disease (PAD); Cardiovascular Function |
Pre-clinical Pre-clinical Clinical Trial Clinical Trial |
[123,129] [130] NCT03506633 NCT03586414 |
| EPI-743 | Para-benzoquinone analog | Free radical scavenger | Mitochondrial Respiratory Chain Diseases | Phase 2 | [128,221] |
| Idebenone | Short chain quinone | Free radical scavenger | Friedreich's ataxia; Mitochondrial cardiomyopathy |
Phase 3 Pre-clinical |
[222] [223] |
| SS-31 (Elamipretide, MPT-131) | Szeto-Schiller tetrapeptide | Cardiolipin protection | Friedreich's ataxia; I/R heart injury; Pressure overload induced HF; HFrEF HFpEF Congestive Heart Failure |
Pre-clinical Phase 2 Pre-clinical Phase 2 Phase 2 Phase 1 |
[153] [157,224,225] [154,155] [9,14] NCT02814097 NCT02914665 |
| mtCPP-1 | Peptide | Cardiolipin protection | No studies | Pre-clinical | [158] |
| mtgCPP | Peptide | Cardiolipin protection | No studies | Pre-clinical | [159] |
| Cyclosporine A | Small molecule | MPT inhibition | I/R heart injury; Post-MI heart failure | Phase 3 | [141,142,226] |
| TRO-40303 | Small molecule | MPT inhibition | I/R heart injury; Post-MI heart failure | Phase 2 | [143,227–229] |
| Alda-1 | Small molecule | Increase ALDH2 activity | I/R heart injury; Post-MI heart failure | Phase 1 | [114,162,163] |
| MA-5 | Synthetic compound | Increase ATP synthesis | Mitochondrial Diseases | Pre-clinical | [149,172,230] |
| Metformin | Biguanide | ETC inhibition | I/R heart injury; Post-MI heart failure; HFpEF |
Phase 2 Phase 2 |
[145,231] NCT03629340 |
| Vericiguat | Small molecule | sGC stimulator | HFrEF; HFpEF |
Phase 3 Phase 2 |
[136] [137,138] |
Preclinical studies have demonstrated a depletion of CoQ10 in different heart failure models [124,125], thus suggesting a role for CoQ10 in the pathophysiology of heart failure. And chronic supplementation with CoQ10 counteracts excessive oxidative stress and cardiomyocyte remodeling in diabetic cardiomyopathy in rodents [125]. Recently, CoQ10 has been tested in patients with chronic heart failure. The clinical trial named Q-SYNBIO was a small study conducted between the years 2003– 2010 in 420 chronic heart failure patients worldwide. Despite its limitations, the study reported promising results, such as reduction in major adverse cardiovascular events and lower rates of hospitalization and mortality among the patients treated with CoQ10 [126]. Another ongoing randomized, double-blind and placebo-controlled design clinical trial currently tests whether ubiquinol treatment (active form of Coenzyme Q10) improves the outcome of heart failure patients with preserved ejection fraction (HFpEF) (estimated enrollment of 250 participants) [127]. In the same direction, short-chain synthetic CoQ analogues have also been developed (e.g. EPI-743 and Idebenone) and tested in genetic mitochondrial disease [128]. An open-label trial demonstrated that sustained treatment with EPI-743 improved quality of life in individuals with genetically-confirmed mitochondrial disease (the study included 13 children and 1 adult) [128].
In 2001, Murphy’s group synthetized a mitochondria targeting antioxidant, named MitoQ, which comprises an exogenous ubiquinone attached to a triphenylphosphonium lipophilic cation [123]. Sustained MitoQ treatment protected hearts against ex vivo ischemia-reperfusion injury. This cardioprotection was associated with reduced mitochondrial dysfunction and cell death [129]. MitoQ also reduced mitochondrial ROS production and restored ATP synthesis in a pressure-overload induced heart failure model. However, the treatment was not sufficient to alter the left ventricle function and remodeling [130]. Chronic MitoQ supplementation improved vascular function in healthy older adults (55 subjects enrolled) [131], but not the outcome of Parkinson disease in patients (128 subjects enrolled) [132]. The impact of MitoQ supplementation in heart failure patients remains to be determined.
Another promising strategy for treatment of heart failure relies on targeting the nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate (NO-sGC-cGMP) pathway [133]. Under oxidative stress, this pathway is compromised, and cells present lower levels of cGMP [134]. The downregulation of this signal has been linked to the development of several heart failure symptoms, such as vascular stiffness and reduced coronary blood flow [133]. Among the new molecules being engineered to preserve the cGMP level, the most advanced therapy is the sGC stimulator Vericiguat [135]. The molecule was tested in two parallel phase II studies in both heart failure with reduced (NCT01951625; 456 subjects enrolled) [136] and preserved (NCT01951638; 477 subjects enrolled) [137,138] ejection fraction. The drug was well tolerated in HFrEF patients and, despite no effect on NT-pro-BNP levels, it has been currently tested in a Phase III Study (NCT02861534; ongoing; estimated enrollment: 4872 subjects). In patients with HFpEF, the drug was also ineffective in reducing the levels of the disease severity markers [137,138]. However, as it improved patient’s quality of life, the clinical investigation will continue in the recently posted Phase II Trial (NCT03547583; ongoing; estimated enrollment: 735 subjects).
There are other types of antioxidant with potential against cardiac diseases, such as radical scavengers (MCI186/Edaravone, XJB-5–131) and superoxide dismutase mimetics (EUK8/EUK134, M40403, Me2DO2A, MnTBPA) [25]. Notably, edaravone (Radicava) has just received the FDA approval for ALS (amyotrophic lateral sclerosis) treatment and is currently under clinical trial for acute ischemic stroke in Japan. Regarding its application for cardiac diseases, the efforts seem to be concentrated on ischemia-reperfusion injury. Eto et al reported myocardial protection against I/R injury in rabbit hearts treated with edaravone immediately after reperfusion. Using electron paramagnetic resonance (EPR), the authors demonstrated increased OH˙ production after reperfusion, associated with loss of cardiac function and I/R injury, which was mitigated by edaravone [139]. Furthermore, in a small pilot study carried out in 80 patients with acute myocardial infarction, administration of edaravone prior to reperfusion was associated with a better clinical outcome, such as decrease in reperfusion arrhythmias and myocardial stunning [140].
Other strategies targeting mitochondria disorders include blocking the mitochondrial permeability transition (cyclosporine A [141,142], TRO40303 [143]), transient inhibition of the ETC (mitoSNO) [144], AMPK activation (metformin, AICAR, resveratrol) [145–148], modulating mitochondrial enzymes activity (MA-5 [149]) and targeting the mitochondrial inner membrane (SS31, also known as MTP-131, Bendavia, Elamipretide) [9,14]. Among this list, one attractive compound is the SS-31, a peptide that associates with cardiolipin (CL), a phospholipid that localizes in the mitochondrial inner membrane [150]. Lately, CL has been considered a critical target for drug development as it has been shown to interact with several mitochondrial proteins and metabolite carriers, such as the ETC complexes, cytochrome c and ADP/ATP carrier; therefore affecting mitochondrial bioenergetics [151]. Szeto et al demonstrated that SS-31 improves mitochondrial bioenergetics, accelerating ATP recovery after ischemia and reducing ischemic kidney injury [152]. The authors proposed that SS-31 protects CL from peroxidation, which could explain the MTP inhibition. Similarly, in Friedreich ataxia disease, SS-31 treatment increased the enzymatic activity of iron-sulphur enzymes (aconitase, ETC complexes II and III), accompanied by an improvement in the mitochondrial membrane potential and ATP content [153].
Preclinical studies have demonstrated a protective effect of SS-31 in animal models of pressure overload-induced heart failure[154,155] and hypertensive cardiomyopathy. In the latter study, SS31 was shown to prevent ROS accumulation in the mitochondria and ameliorate angiotensin II-induced cardiac hypertrophy and diastolic dysfunction [156]. Additionally, Elamipretide (or Bendavia), a drug form of SS-31, has recently been tested in two clinical trials in patients with heart failure with reduced ejection fraction (HFrEF, 36 patients enrolled) (NCT02388464) and patients with ST-segment elevation myocardial infarction (STEMI, 300 patients enrolled) (NCT01572909 - EMBRACE) [14,157]. Both trials demonstrated acceptable safety and tolerability; however, the phase 2a trial EMBRACE did not show an improvement in the primary endpoint after treatment (myocardial infarct size). It is worth mentioning that Cerrato’s group has developed two modified versions of the peptide SS-31, called mtCPP-1 [158] and mtgCPP [159], in the last two years. In preliminary studies, both peptides have shown greater efficiency and antioxidant capacity (2–3 fold) than SS-31. Despite its potential to become new drug candidates, studies on cardiomyopathies have not yet been described.
Finally, small molecules targeting mitochondria have emerged as promising therapeutic strategies in the last decade. One example is the previously mentioned Alda-1 [115], an agonist of the mitochondrial enzyme aldehyde dehydrogenase 2 (ALDH2), which accumulate functions in the ethanol metabolism [97] and redox biology [160], removing intracellular toxic aldehydes (e.g. acetaldehyde, 4-HNE) [161]. Notably, in an animal model of myocardial infarction-induced heart failure, selective activation of ALDH2 by Alda-1 improves the clinical outcome of heart failure by decreasing the overload of reactive aldehydes and preserving mitochondrial bioenergetics [162].
In a rat model of post-myocardial infarction-induced cardiomyopathy, Gomes et al., also showed a protective role of ALDH2. Without an intervention, ALDH2 activity was reduced by 80% within the first two week of coronary artery ligation with a concomitant increase of aldehydic loads and loss in cardiac mitochondria function in the myocardium [163]. However, a sustained treatment with Alda-1 for 4 weeks starting 24 hours after the coronary artery ligation increased ALDH2 activity, reduced lipid peroxidation, 4HNE protein adducts, and prevented mitochondria damage [163]. Furthermore, sustained activation of ALDH2 by Alda-1, from week 4 to week 10, was effective in preventing myocardial hypertrophy, fibrosis and cardiac dysfunction [162]. The absence of 4HNE-protein adducts accumulation and preservation of mitochondria function was clearly associated with the improvement of heart failure outcome [162].
The ALDH2*2 (rs671) variant is an independent risk factor of coronary heart disease in many population-based studies [164–168]. In 248 patients with coronary heart disease, the variant ALDH2 genotypes (GG & GA) were associated with increased serum levels of 4-HNE, severity of coronary artery stenosis and atherosclerosis [169]. Oxidized low-density lipoprotein induces ER stress, apoptosis and 4-HNE protein adducts production in mouse smooth muscle cells. Using an in vitro model of atherosclerosis, Alda-1 was effective in reducing 4-HNE-protein adducts, ER stress and apoptosis in smooth muscle cells [169]. In addition, activation of ALDH2 by Alda-1 reduced ROS and reactive aldehydes levels, mitigated calcium overload and dramatically improved resuscitation survival in an in vivo model of post-myocardial arrest dysfunction [170]. These studies established a critical connection between ALDH2 activity, cardiac function and heart failure. These studies also identified ALDH2 as a potential target for intervention and suggest that ALDH2 activators, such as the small molecule Alda-1, as a therapeutic for heart failure [108,113,171] (Figure 2).
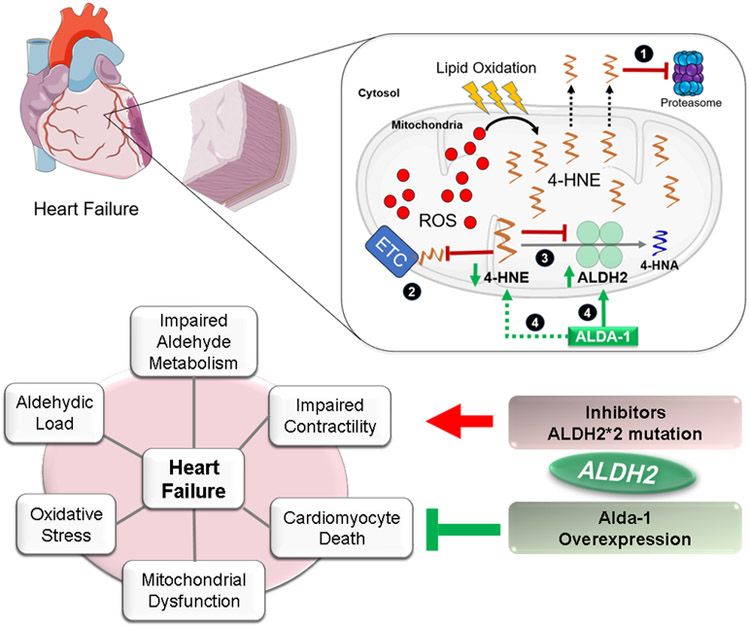
Figure 2.
Proposed model for the aldehydic overload in heart failure and for the role of aldehyde dehydrogenase 2 (ALDH2) in cardiac diseases. In the first scheme, during the progression of post-MI cardiomyopathy, excessive ROS production (free radicals) leads to lipid oxidation and 4-HNE generation and accumulation. The aldehyde inhibits (1) the proteasome [11,233], (2) the electron transport chain [162,234,235], (3) ALDH2, among other intracellular targets [236]. (4) Pharmacological activation of ALDH2 by Alda-1 improves cardiac outcome and 4-HNE removal [162,163,237]. The second scheme summarizes data from preclinical studies using either ALDH2 transgenic mice or pharmacological ALDH2 inhibitors or activators that elucidated the potential pathways regulated by ALDH2 and aldehydic load in cardiac diseases, including heart failure. Activation of ALDH2 or ALDH2 overexpression confer cardiac protection by counteracting aldehydic load, mitochondrial dysfunction, oxidative stress, impaired contractility and death in failing hearts, whereas inactivating mutation in ALDH2 or the use of selective inhibitors of the enzyme, exacerbate all the disease. This figure was produced using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Mitochonic Acid 5 (MA-5) is another recently described molecule [172], which consists in a synthetic derivative of the plant hormone, indole-3-acetic acid (IAA). MA-5 increases cellular ATP levels independently of the membrane potential and ETC complexes, possibly through the facilitation of ATP synthase oligomerization. The first studies with this compound reported promising results in fibroblasts from patients with mitochondrial diseases, and its protective effects on animal models of heart failure are to be evaluated [149,172].
To date, there are few studies describing the toxicity (pre-clinical studies) and safety (clinical trials) of the molecules depicted in Table 1. Sustained Alda-1 treatment (for 6 weeks) did not change hemodynamic parameters, circulating aspartate aminotransferase and alanine aminotransferase activities, as well as serum uric acid and creatinine levels in healthy animals [162]. Regarding its use in patients, Alda (FP-045) has successfully completed the Phase I Clinical Study this year, showing safety and tolerability in a dose escalation study in healthy humans (24 subjects). The trial is registered by Foresee Pharmaceuticals in the ANZCTR (Australian New Zealand Clinical Trials Registry) (Trial code: ACTRN12618000195257p). Despite the recent and ongoing clinical tests with MitoQ supplementation (NCT02597023, 55 subjects enrolled; NCT03586414, planned enrolment: 60 subjects), recent studies have raised some concerns regarding the drug toxicity, as it has been reported to inhibit mitochondrial respiratory chain complexes [173] and cause mitochondrial depolarization [174]. In the same direction, the free radical scavenger MCI-186 (Edavarone), which has been considered safe in clinical trials (FDA, NDA: 209176), can be toxic when administered by continuous intravenous infusion.
Remarkably, great efforts have been made not only to identify and address mitochondrial targets, but many studies have also focused on developing effective strategies to increase tissue and cell permeability [159] and deliver molecules inside mitochondria [175,176] as well as alternative ways of drug administration [177]. Combined strategies to approach and treat oxidative stress and mitochondrial dysfunction, based on the increasing understanding of the role of reactive species and cellular antioxidants, seem to be a promising way to design effective interventions and develop new therapies for a complex and multifactorial disease such as heart failure.
Exercise and mitochondrial dysfunction in heart failure
Exercise intolerance is a hallmark of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). Over the last decades, it has been proposed that exercise intolerance is not only a consequence of heart failure but it indeed has a major contribution to cardiac degeneration and heart failure progression [178–180]. Therefore, exercise has been used as a diagnostic, prognostic and therapeutic tool in heart failure [181,182]. More recently, exercise training has been included in the European Society of Cardiology (ESC) Guideline recommendations for treatment of heart failure as an important strategy in prevention and treatment of heart failure, as a non-pharmacological adjuvant therapy [178–181,183].
Overall, exercise training can improve the outcome of patients with heart failure by improving quality of life, reducing hospitalization and relieving symptoms [184–186]. The cardiovascular benefits of exercise training include reduction of neurohumoral activation (both SNS and RAS), decrease of inflammatory markers, better vascular function, physiological cardiac remodeling effect and improve of skeletal muscle mass and contractility properties [187–190]. Intrinsically, the positive effects of exercise training in cardiomyocytes are associated, but not limited, to increased levels of calcium-handling-related proteins [191], contractility properties [70,192], antioxidant capacity [188,193] and protein quality control [11,187,194]. However, these intracellular adjustments still need to be validated as causal mechanisms of exercise benefits in heart failure. Therefore, the causal molecular mechanism underlying the benefits of exercise training in failing hearts remains unknown.
Over the last decade, several studies have demonstrated that changes in mitochondrial function are critical to induce exercise benefits in heart failure [195]. Overall, exercise training improves the efficiency of mitochondrial oxidative phosphorylation [68], reduces mitochondrial permeability transition [68], increases the mitochondrial clearance of toxic molecules including ROS and aldehydes [196,197] and increases mitochondria number, size and clearance [68] in heart failure. In this context, exercise training would improve left ventricular function in heart failure animals by reestablishing mitochondrial functionality.
The mitochondrial benefits of exercise training have been demonstrated in both animal models and patients with heart failure. Aerobic exercise improves mitochondrial dysfunction in a rat model of heart failure induced by myocardial infarction [11,68]. This response is mainly characterized by the rescue of mitochondrial oxygen consumption, reduction of mitochondrial hydrogen peroxide release and calcium-induced mitochondrial permeability transition, and reestablishment of mitochondrial number and size in failing hearts [11,68]. Indeed, these exercise-induced mitochondrial changes positively affect cardiac protein quality control machinery in heart failure [11]. Short-term aerobic exercise training also prevents mitochondrial dysfunction and oxidative stress in an animal model of doxorubicin-induced heart failure [198]. Low-intensity aerobic intermittent training attenuates mitochondrial dysfunction with a positive impact on cardiac function and remodeling in animal model of myocardial infarction-induced heart failure [199] and pressure overload-induced heart failure [200].
Cardiac and skeletal muscle mitochondrial abnormalities lead to the metabolic dysfunction seen in heart failure patients; therefore contributing to disease progression [25]. Therefore, increasing energy expenditure during exercise should have a direct and positive impact on mitochondrial bioenergetic efficiency and lately increase the peak oxygen consumption (a predictor of mortality in heart failure patients [201,202]). In fact, even modest increase in peak oxygen consumption (an indicator of mitochondrial metabolism) induced by a 3 month aerobic exercise training is sufficient to lower the risk of cardiovascular mortality, hospitalization and all-cause mortality in patients with chronic systolic heart failure [203].
Exercise training is also effective in improving peak oxygen consumption with a positive impact on diastolic function and quality of life in patients with heart failure with preserved ejection fraction [182]. The latest Cochrane review, which included 33 trials with 4740 people with heart failure (predominantly with HFrEF and New York Heart Association classes II and III) provides evidence that exercise training reduces the risk of hospital admissions and confers improvements in quality of life of heart failure patients [204].
In summary, the ability of exercise training to improve mitochondria functionality with a clear impact on cardiomyocyte viability highlights an important intracellular node underlying the benefits of exercise training in heart failure. However, further integrative studies comparing different exercise training protocols and time as well as studies investigating the role of mitochondrial metabolism and its extension to cytosolic systems and whether this connectivity is required to improve cardiac fitness induced by exercise in heart failure are needed.
Conclusions
As discussed here, heart failure is a degenerative disease that affects millions of people worldwide. Although extensive efforts have been made over the last decades in the development of better drugs, the available therapies (mainly targeting neurohumoral hyperactivity) still have a marginal effect on heart failure outcome such as mortality. Therefore, identifying the optimal intracellular node(s) that slow down, stop or even reverse cardiomyocyte dysfunction and cardiac degeneration remains a major challenge for developing better therapies.
Recently, mitochondria have attracted considerable attention from both academia and industry, since mitochondrial dysfunction is a hallmark of many diseases, including heart failure. However, are mitochondrial dysfunction benign or do they actually contribute to heart failure? In this review, we examined the evidence from animal studies and from human clinical trials, and it seems that intrinsic mitochondrial changes in the affected cardiomyocyte are critical to heart failure pathophysiology. Overall, these studies support a decisive role for (but not restricted to) impaired cardiac mitochondrial electron transport chain activity, oxidative stress and aldehydic load in cardiomyocyte dysfunction and consequent heart failure establishment/progression.
But, why is there such a central role for mitochondria in heart failure? First, mitochondria are the powerhouse of the cell. Second, mitochondria account for ~35% of cardiomyocyte volume and form a long, dynamic and well-organized network, which facilitates both physical and chemical interactions between mitochondria and other intracellular structures. Third, exciting body of recent research focusing on the cellular and molecular mechanisms involved in heart failure pathophysiology indicates mitochondria as strategic and dynamic nodes of influence, virtually affecting every single biochemical process in cardiac cells. In that sense, dysfunctional mitochondria can quickly propagate damage within cardiomyocyte in failing hearts, where mitochondrial surveillance mechanisms are usually disrupted. Therefore, different emerging therapeutic strategies including small molecules and peptides individually targeting such mitochondrial abnormalities have the potential to improve the function of the failing heart. In fact, several preclinical studies have succeeded in improving heart failure outcome. Some of these molecules are now in clinical trials.
As last note, improving mitochondrial functionality by using mitochondria-targeted therapeutics might be an important strategy to enhance the effectiveness of nonpharmacological therapies, such as exercise. Exercise is a well-known and very effective intervention, improving cardiac mitochondrial metabolism in both health and disease. However, it should be performed at a moderate to high intensity to maximize its impact on cardiac physiology. Considering the exercise limitations observed in heart failure patients, its benefits are only marginal in this population. We believe that the optimal management of heart failure requires a combination of pharmacological and nonpharmacological therapies that synergistically potentiates mitochondrial functionality with a positive impact on cardiac performance and patient healthspan and lifespan.
Highlights.
Heart failure affects over 25 million people worldwide
Heart failure therapies target mainly neurohumoral over-activation
Mitochondrial dysfunction and oxidative stress are hallmarks of heart failure
Mitochondrial-derived aldehydes accumulate in failing hearts
Therapies targeting mitochondrial detoxification improves heart failure outcome
Acknowledgments
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [FAPESP 2012/05765–2, 2013/07937–8, 2015/20783–5, 2015/22814–5, 2017/16694–2 to JCBF]; Conselho Nacional de Pesquisa e Desenvolvimento – Brasil [CNPq 303281/2015–4, 470880/2012–0, 407306/2013–7 to JCBF]; and National Institutes of Health [NIAAA MERIT Award AA011147 to D-MR]. LA holds fellowship from FAPESP [2016/00900–0 and 2017/14426–0] and RAP holds fellowship from Capes. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Abbreviations
- 4-HNE
4-hydroxynonenal ISCU 1/2 iron-sulfur cluster assembly protein 1/2
- ACE
angiotensin converting enzyme
- ALDH2
aldehyde dehydrogenase 2
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- BCL-2
b-cell lymphoma 2
- CaMKII
Ca2+-calmodulin-dependent protein kinase
- CL
cardiolipin
- CoQ10
coenzyme q10
- EC
excitation contraction
- εPKC
protein kinase C epsilon
- EPR
electron paramagnetic resonance
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FDA
Food and Drug Administration
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HPLC
high-performance liquid chromatography
- HSP70
heat shock protein 70
- IARC
International Agency for Research on Cancer
- IR
ischemia reperfusion
- ISCU 1/2
iron-sulfur cluster assembly protein ½
- JNK
c-Jun N-terminal kinase
- LC-MS
liquid chromatography-mass spectrometry
- LV
left ventricle
- MDA
malonaldehyde
- MI
myocardial infarction
- miR-210
microRNA210
- miRNA
microRNA
- mNCE
mitochondrial Na+ Ca2+ exchanger
- MPT
mitochondrial permeability transition
- Nnt
nicotinamide nucleotide transhydrogenase
- NO-sGC-cGMP
nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate
- p53
cellular tumor antigen p53
- PUFA
polyunsaturated fatty acid
- RAS
renin-angiotensin system
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STEMI
st-fragment elevation myocardial infarction
- SNS
sympathetic nervous system
- TAC
transverse aortic constriction
- TIM
transporter of inner membrane
- TOM
transporter of outer membrane
- WHO
World Health Organization
Footnotes
Disclosure
DM-R and C-HC hold patents related to Alda and ALDH2, now licensed to Foresee Pharmaceuticals. However, they do not hold stocks of the company and none of this research was supported by the company. The other authors have no disclosure.
References
- [1].Jessup M, Brozena S, Heart failure, new Engl. J. Med. Rev. Artic. Med. 348 (2003) 2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- [2].Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL, Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies, J. Mol. Cell. Cardiol. 52 (2012) 1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- [3].Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO, Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure, JACC Hear. Fail. 1 (2013) 93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- [4].Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G, Heart failure: preventing disease and death worldwide, ESC Hear. Fail. 1 (2014) 4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- [5].Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C, A reevaluation of the costs of heart failure and its implications for allocation of health resources in the united states, Clin. Cardiol. 37 (2014) 312–321. doi: 10.1002/clc.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Senni M, Gavazzi A, Gheorghiade M, Butler J, Heart failure at the crossroads : moving beyond blaming stakeholders to targeting the heart, Eur. J. Heart Fail. 17 (2015) 760–763. doi: 10.1002/ejhf.315. [DOI] [PubMed] [Google Scholar]
- [7].Brum PC, Bacurau AVN, Medeiros A, Ferreira JCB, Vanzelli AS, Negrão CE, Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function, Brazilian J. Med. Biol. Res. 44 (2011) 827–835. doi: 10.1590/S0100-879X2011007500075. [DOI] [PubMed] [Google Scholar]
- [8].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines, J. Am. Coll. Cardiol. 62 (2013) e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- [9].Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K, Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure, Circ. Hear. Fail. 9 (2016). doi: 10.1161/CIRCHEARTFAILURE.115.002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Teerlink JR, Felker GM, McMurray JJV, Solomon SD, Adams KF, Cleland JGF, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial, Lancet. 388 (2016) 2895–2903. doi: 10.1016/S0140-6736(16)32049-9. [DOI] [PubMed] [Google Scholar]
- [11].Campos JC, Queliconi BB, Dourado PMM, Cunha TF, Zambelli VO, Bechara LRG, Kowaltowski AJ, Brum PC, Mochly-Rosen D, Ferreira JCB, Exercise Training Restores Cardiac Protein Quality Control in Heart Failure, PLoS One. 7 (2012) 1–12. doi: 10.1371/journal.pone.0052764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferreira JCB, Brum PC, Mochly-Rosen D, βIIPKC and εPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure, J. Mol. Cell. Cardiol. 51 (2012) 479–484. doi: 10.1016/j.yjmcc.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Palaniyandi SS, Ferreira JCB, Brum PC, Mochly-Rosen D, PKCβII inhibition attenuates myocardial infarction induced heart failure and is associated with a reduction of fibrosis and pro-inflammatory responses., J. Cell. Mol. Med. 15 (2011) 1769–77. doi: 10.1111/j.1582-4934.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Daubert MA, Yow E, Dunn G, Marchev S, Barnhart H, Douglas PS, O’Connor C, Goldstein S, Udelson JE, Sabbah HN, Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide, Circ. Hear. Fail. 10 (2017). doi: 10.1161/CIRCHEARTFAILURE.117.004389. [DOI] [PubMed] [Google Scholar]
- [15].Ferreira JCB, Koyanagi T, Palaniyandi SS, Fajardo G, Churchill EN, Budas G, Disatnik MH, Bernstein D, Brum PC, Mochly-Rosen D, Pharmacological inhibition of βIIPKC is cardioprotective in late-stage hypertrophy, J. Mol. Cell. Cardiol. 51 (2011) 980–987. doi: 10.1016/j.yjmcc.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferreira JCB, Boer BN, Grinberg M, Brum PC, Mochly-Rosen D, Protein quality control disruption by PKCβII in heart failure; rescue by the selective PKCβII inhibitor, βIIV5–3, PLoS One. 7 (2012) 1–11. doi: 10.1371/journal.pone.0033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Budas G, Costa HM, Ferreira JCB, Teixeira da Silva Ferreira A, Perales J, Krieger JE, Mochly-Rosen D, Schechtman D, Identification of εPKC targets during cardiac ischemic injury., Circ. J 76 (2012) 1476–85. doi: 10.1253/circj.CJ-11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D, Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion., Cardiovasc. Res. 85 (2010) 385–94. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nunnari J, Suomalainen A, Mitochondria: In sickness and in health, Cell. 148 (2012) 1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friedman JR, Nunnari J, Mitochondrial form and function, Nature. 505 (2014) 335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ, Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association, 2016. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Palmer JW, Tandler B, Hoppel C, Biochemical Properties of Subsarcolemmal and Interfibrillar Mitochondria Isolated from Rat Cardiac Muscle, J. Biol. Chem. Chem. 252 (1977) 8731–8739. [PubMed] [Google Scholar]
- [23].Hollander JM, Thapa D, Shepherd DL, Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: influence of cardiac pathologies, AJP Hear. Circ. Physiol. 307 (2014) H1–H14. doi: 10.1152/ajpheart.00747.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Palaniyandi SS, Qi X, Yogalingam G, Ferreira JCB, Mochly-Rosen D, Regulation of mitochondrial processes: A target for heart failure, Drug Discov. Today Dis. Mech. 7 (2010) 1–14. doi: 10.1016/j.ddmec.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JGF, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M, Expert consensus document: Mitochondrial function as a therapeutic target in heart failure, Nat. Rev. Cardiol. 14 (2017) 238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Disatnik M-H, Hwang S, Ferreira JCB, Mochly-Rosen D, New therapeutics to modulate mitochondrial dynamics and mitophagy in cardiac diseases, J. Mol. Med. 93 (2015) 279–287. doi: 10.1007/s00109-015-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gross ER, Zambelli VO, Small BA, Ferreira JCB, Chen C-H, Mochly-Rosen D, A Personalized Medicine Approach for Asian Americans with the Aldehyde Dehydrogenase 2*2 Variant, Annu. Rev. Pharmacol. Toxicol. 55 (2015) 107–127. doi: 10.1146/annurev-pharmtox-010814-124915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Campos JC, Bozi LHM, Bechara LRG, Lima VM, Ferreira JCB, Mitochondrial Quality Control in Cardiac Diseases, Front. Physiol. 7 (2016) 1–9. doi: 10.3389/fphys.2016.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winterbourn CC, Reconciling the chemistry and biology of reactive oxygen species, Nat. Chem. Biol. 4 (2008) 278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- [30].Davies MJ, Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods, Methods. 109 (2016) 21–30. doi: 10.1016/j.ymeth.2016.05.013. [DOI] [PubMed] [Google Scholar]
- [31].Cheng G, Zielonka M, Dranka B, Kumar SN, Myers CR, Bennett B, Garces AM, Dias Duarte Machado LG, Thiebaut D, Ouari O, Hardy M, Zielonka J, Kalyanaraman B, Detection of mitochondria-generated reactive oxygen species in cells using multiple probes and methods: Potentials, pitfalls, and the future., J. Biol. Chem. (2018). doi: 10.1074/jbc.RA118.003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JCB, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, Vercesi AE, Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health, Antioxid. Redox Signal. 18 (2013) 2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- [33].Wenzel P, Kossmann S, Münzel T, Daiber A, Redox regulation of cardiovascular inflammation – Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species, Free Radic. Biol. Med. 109 (2017) 48–60. doi: 10.1016/j.freeradbiomed.2017.01.027. [DOI] [PubMed] [Google Scholar]
- [34].Yan Y, Finkel T, Autophagy as a regulator of cardiovascular redox homeostasis, Free Radic. Biol. Med. 109 (2017) 108–113. doi: 10.1016/j.freeradbiomed.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertrán E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Förstermann U, Giniatullin R, Giricz Z, Görbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustín P, Hillion M, Huang J, Ilikay S, Jansen-Dürr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz L-O, Korac B, Korkmaz KS, Koziel R, Kračun D, Krause K-H, Křen V, Krieg T, Laranjinha J, Lazou A, Li H, Martínez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milković L, Miranda-Vizuete A, Mojović M, Monsalve M, Mouthuy P-A, Mulvey J, Münzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavićević A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Antón N, Rodríguez-Mañas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schröder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanić V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentová K, Van Breusegem F, Varisli L, Veal EA, Yalçın AS, Yelisyeyeva O, Žarković N, Zatloukalová M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt HHHW, Di Lisa F, Daiber A, European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS)., Redox Biol. 13 (2017) 94–162. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schmidt HHHW, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, Cuadrado A, Antioxidants in Translational Medicine., Antioxid. Redox Signal. 23 (2015) 1130–43. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Burgoyne JR, Mongue-Din H, Eaton P, Shah AM, Redox signaling in cardiac physiology and pathology, Circ. Res. 111 (2012) 1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- [38].Münzel T, Gori T, Keaney JF, Maack C, Daiber A, Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications, Eur. Heart J. 36 (2015) 2555–2564. doi: 10.1093/eurheartj/ehv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nickel A, Löffler J, Maack C, Myocardial energetics in heart failure, Basic Res. Cardiol. 108 (2013). doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- [40].Seddon M, Looi YH, Shah AM, Oxidative stress and redox signalling in cardiac hypertrophy and heart failure, Heart. 93 (2007) 903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bayeva M, Ardehali H, Mitochondrial dysfunction and oxidative damage to sarcomeric proteins, Curr. Hypertens. Rep. 12 (2010) 426–432. doi: 10.1007/s11906-010-0149-8. [DOI] [PubMed] [Google Scholar]
- [42].Gupta S, Wen J-J, Garg NJ, Oxidative Stress in Chagas Disease, Interdiscip. Perspect. Infect. Dis. 2009 (2009) 1–8. doi: 10.1155/2009/190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xiao M, Zhong H, Xia L, Tao Y, Yin H, Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria, Free Radic. Biol. Med. 111 (2017) 316–327. doi: 10.1016/j.freeradbiomed.2017.04.363. [DOI] [PubMed] [Google Scholar]
- [44].Disatnik M-H, Ferreira JCB, Campos JC, Gomes KS, Dourado PMM, Qi X, Mochly-Rosen D, Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction., J. Am. Heart Assoc. 2 (2013) e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP, Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS, Nature. 515 (2014) 431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paulus WJ, Tschöpe C, A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation., J. Am. Coll. Cardiol. 62 (2013) 263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- [47].Ortona E, Maselli A, Delunardo F, Colasanti T, Giovannetti A, Pierdominici M, Relationship between redox status and cell fate in immunity and autoimmunity., Antioxid. Redox Signal. 21 (2014) 103–22. doi: 10.1089/ars.2013.5752. [DOI] [PubMed] [Google Scholar]
- [48].Zhou R, Yazdi AS, Menu P, Tschopp J, A role for mitochondria in NLRP3 inflammasome activation., Nature. 469 (2011) 221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- [49].Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K-Y, Sack MN, Kastner DL, Siegel RM, Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS)., J. Exp. Med. 208 (2011) 519–33. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S, TLR signalling augments macrophage bactericidal activity through mitochondrial ROS., Nature. 472 (2011) 476–80. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Santulli G, Xie W, Reiken SR, Marks AR, Mitochondrial calcium overload is a key determinant in heart failure, Proc. Natl. Acad. Sci. 112 (2015) 11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gorski PA, Ceholski DK, Hajjar RJ, Altered myocardial calcium cycling and energetics in heart failure - A rational approach for disease treatment, Cell Metab. 21 (2015) 183–194. doi: 10.1016/j.cmet.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Limbu S, Hoang-Trong TM, Prosser BL, Lederer WJ, Jafri MS, Modeling Local X-ROS and Calcium Signaling in the Heart, Biophys. J. 109 (2015) 2037–2050. doi: 10.1016/j.bpj.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bertero E, Maack C, Calcium Signaling and Reactive Oxygen Species in Mitochondria., Circ. Res. 122 (2018) 1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- [55].Cox DADA, Matlib MAMA, A role for the mitochondrial Na(+)-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria., J. Biol. Chem. 268 (1993) 938–947. [PubMed] [Google Scholar]
- [56].Glancy B, Willis WT, Chess DJ, Balaban RS, Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria, Biochemistry. 52 (2013) 2793–2809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nickel AG, Von Hardenberg A, Hohl M, Löffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CRD, Blacker TS, Hall AR, Duchen MR, Kästner L, Lipp P, Zeller T, Müller C, Knopp A, Laufs U, Böhm M, Hoth M, Maack C, Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure, Cell Metab. 22 (2015) 472–484. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- [58].Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huhe TL, Control of Mitochondrial Redox Balance and Cellular Defense against Oxidative Damage by Mitochondrial NADP+-dependent Isocitrate Dehydrogenase, J. Biol. Chem. 276 (2001) 16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- [59].Dey S, Sidor A, O’Rourke B, Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes, J. Biol. Chem. 291 (2016) 11185–11197. doi: 10.1074/jbc.M116.726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L, Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction., J Pharmacol Exp Ther. 318 (2006) 214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- [61].Nulton-Persson AC, Szweda LI, Modulation of Mitochondrial Function by Hydrogen Peroxide, J. Biol. Chem. 276 (2001) 23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- [62].Erickson JR, ling A. Joiner M, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME, A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation, Cell. 133 (2008) 462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O’Rourke B, Maack C, Elevated cytosolic Na+increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes, Circulation. 121 (2010) 1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O’Rourke B, Maack C, Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes., Circulation. 121 (2010) 1606–13. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B, Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a guinea pig model of heart failure, Circ. Res. 115 (2014) 44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Luczak ED, Anderson ME, CaMKII oxidative activation and the pathogenesis of cardiac disease, J. Mol. Cell. Cardiol. 73 (2014) 112–116. doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tsutsui H, Kinugawa S, Matsushima S, Oxidative stress and heart failure., Am. J. Physiol. Heart Circ. Physiol. 301 (2011) H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- [68].Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, Jannig PR, Gomes KMS, Zambelli VO, Rocha-Resende C, Guatimosim S, Brum PC, Mochly-Rosen D, Gottlieb RA, Kowaltowski AJ, Ferreira JCB, Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure., Autophagy. 13 (2017) 1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ferreira JCB, Moreira JBN, Campos JC, Pereira MG, Mattos KC, Coelho MA, Brum PC, Angiotensin receptor blockade improves the net balance of cardiac Ca(2+) handling-related proteins in sympathetic hyperactivity-induced heart failure., Life Sci. 88 (2011) 578–85. doi: 10.1016/j.lfs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [70].Paulino EC, Ferreira JCB, Bechara LR, Tsutsui JM, Mathias W, Lima FB, Casarini DE, Cicogna AC, Brum PC, Negrão CE, Exercise training and caloric restriction prevent reduction in cardiac Ca2+-handling protein profile in obese rats., Hypertens. (Dallas, Tex. 1979). 56 (2010) 629–35. doi: 10.1161/HYPERTENSIONAHA.110.156141. [DOI] [PubMed] [Google Scholar]
- [71].Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP, CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis, Nat. Med. 22 (2016) 175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- [72].Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, Brown JH, The δcisoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure, Circ. Res. 92 (2003) 912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- [73].Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A, CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury, Cardiovasc. Res. 73 (2007) 689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [74].Wang J-X, Gao J, Ding S-L, Wang K, Jiao J-Q, Wang Y, Sun T, Zhou L-Y, Long B, Zhang X-J, Li Q, Liu J-P, Feng C, Liu J, Gong Y, Zhou Z, Li P-F, Oxidative Modification of miR-184 Enables It to Target Bcl-xL and Bcl-w., Mol. Cell. 59 (2015) 50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- [75].Magenta A, Greco S, Gaetano C, Martelli F, Oxidative stress and microRNAs in vascular diseases., Int. J. Mol. Sci. 14 (2013) 17319–46. doi: 10.3390/ijms140917319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Huang Z-P, Neppl RL, Wang D-Z, MicroRNAs in cardiac remodeling and disease., J. Cardiovasc. Transl. Res. 3 (2010) 212–8. doi: 10.1007/s12265-010-9165-y. [DOI] [PubMed] [Google Scholar]
- [77].Pinti MV, Hathaway QA, Hollander JM, Role of microRNA in metabolic shift during heart failure, Am. J. Physiol. - Hear. Circ. Physiol. 312 (2017) H33–H45. doi: 10.1152/ajpheart.00341.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen J-F, Callis TE, Wang D-Z, microRNAs and muscle disorders., J. Cell Sci. 122 (2009) 13–20. doi: 10.1242/jcs.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bartel DP, MicroRNAs: genomics, biogenesis, mechanism, and function., Cell. 116 (2004) 281–97. [DOI] [PubMed] [Google Scholar]
- [80].Tomasetti M, Neuzil J, Dong L, MicroRNAs as regulators of mitochondrial function: Role in cancer suppression, Biochim. Biophys. Acta - Gen. Subj. 1840 (2014) 1441–1453. doi: 10.1016/j.bbagen.2013.09.002. [DOI] [PubMed] [Google Scholar]
- [81].Wang J-X, Jiao J-Q, Li Q, Long B, Wang K, Liu J-P, Li Y-R, Li P-F, miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1., Nat. Med. 17 (2011) 71–8. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- [82].Chan SY, Zhang Y-Y, Hemann C, Mahoney CE, Zweier JL, Loscalzo J, MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2., Cell Metab. 10 (2009) 273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chen Z, Li Y, Zhang H, Huang P, Luthra R, Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression., Oncogene. 29 (2010) 4362–8. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- [84].Wang X, Song C, Zhou X, Han X, Li J, Wang Z, Shang H, Liu Y, Cao H, Mitochondria Associated MicroRNA Expression Profiling of Heart Failure., Biomed Res. Int. 2017 (2017). doi: 10.1155/2017/4042509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Csala M, Kardon T, Legeza B, Lizák B, Mandl J, Margittai É, Puskás F, Száraz P, Szelényi P, Bánhegyi G, On the role of 4-hydroxynonenal in health and disease, Biochim. Biophys. Acta - Mol. Basis Dis. 1852 (2015) 826–838. doi: 10.1016/j.bbadis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- [86].Zandalinas SI, Mittler R, ROS-induced ROS release in plant and animal cells, Free Radic. Biol. Med. (2017). doi: 10.1016/j.freeradbiomed.2017.11.028. [DOI] [PubMed] [Google Scholar]
- [87].Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ, Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes., J. Exp. Med. 192 (2000) 1001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zorov DB, Juhaszova M, Sollott SJ, Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release., Physiol. Rev. 94 (2014) 909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lim HY, Wang W, Chen J, Ocorr K, Bodmer R, ROS regulate cardiac function via a distinct paracrine mechanism, Cell Rep. 7 (2014) 35–44. doi: 10.1016/j.celrep.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kato Y, Osawa T, Detection of lipid-lysine amide-type adduct as a marker of PUFA oxidation and its applications., Arch. Biochem. Biophys. 501 (2010) 182–7. doi: 10.1016/j.abb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [91].Esterbauer H, Schaur RJ, Zollner H, Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes, Free Radic. Biol. Med. 11 (1991) 81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [92].Voulgaridou G-P, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A, DNA damage induced by endogenous aldehydes: current state of knowledge., Mutat. Res. 711 (2011) 13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [93].Fritz KS, Petersen DR, An overview of the chemistry and biology of reactive aldehydes., Free Radic. Biol. Med. 59 (2013) 85–91. doi: 10.1016/j.freeradbiomed.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Galligan JJ, Smathers RL, Fritz KS, Epperson LE, Hunter LE, Petersen DR, Protein carbonylation in a murine model for early alcoholic liver disease., Chem. Res. Toxicol. 25 (2012) 1012–21. doi: 10.1021/tx300002q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ferreira JCB, Mochly-Rosen D, Nitroglycerin use in myocardial infarction patients., Circ. J. 76 (2012) 15–21. doi: 10.1253/circj.CJ-11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Josan S, Xu T, Yen Y-F, Hurd R, Ferreira J, Chen C-H, Mochly-Rosen D, Pfefferbaum A, Mayer D, Spielman D, In vivo measurement of aldehyde dehydrogenase-2 activity in rat liver ethanol model using dynamic MRSI of hyperpolarized [1-(13) C]pyruvate., NMR Biomed. 26 (2013) 607–12. doi: 10.1002/nbm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ueta CB, Campos JC, Albuquerque RPE, Lima VM, Disatnik M-H, Sanchez AB, Chen C-H, de Medeiros MHG, Yang W, Mochly-Rosen D, Ferreira JCB, Cardioprotection induced by a brief exposure to acetaldehyde: role of aldehyde dehydrogenase 2., Cardiovasc. Res. 114 (2018) 1006–1015. doi: 10.1093/cvr/cvy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Raha S, Robinson BH, Mitochondria, oxygen free radicals, disease and ageing., Trends Biochem. Sci. 25 (2000) 502–8. doi: 10.1016/S0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- [99].Marchitti SA, Brocker C, Stagos D, Vasiliou V, Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily., Expert Opin. Drug Metab. Toxicol. 4 (2008) 697–720. doi: 10.1517/17425255.4.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Breitzig M, Bhimineni C, Lockey R, Kolliputi N, 4-Hydroxy-2-nonenal: a critical target in oxidative stress?, Am. J. Physiol. Cell Physiol. 311 (2016) C537–C543. doi: 10.1152/ajpcell.00101.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yoval-Sánchez B, Rodríguez-Zavala JS, Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts., Chem. Res. Toxicol. 25 (2012) 722–9. doi: 10.1021/tx2005184. [DOI] [PubMed] [Google Scholar]
- [102].Brooks PJ, Enoch M-A, Goldman D, Li T-K, Yokoyama A, The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption., PLoS Med. 6 (2009) e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group, Carcinogenicity of alcoholic beverages., Lancet. Oncol. 8 (2007) 292–3. doi: 10.1016/S1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- [104].Budas GR, Disatnik M-H, Mochly-Rosen D, Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target?, Trends Cardiovasc. Med. (2009). doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chen C-H, Sun L, Mochly-Rosen D, Mitochondrial aldehyde dehydrogenase and cardiac diseases., Cardiovasc. Res. 88 (2010) 51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mochly-Rosen D, Zakhari S, Focus on: The cardiovascular system: what did we learn from the French (Paradox)?, Alcohol Res. Health. 33 (2010) 76–86. [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang Y, Ren J, ALDH2 in alcoholic heart diseases: Molecular mechanism and clinical implications, Pharmacol. Ther. (2011). doi: 10.1016/j.pharmthera.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chen C-H, Ferreira JCB, Gross ER, Mochly-Rosen D, Targeting aldehyde dehydrogenase 2: new therapeutic opportunities., Physiol. Rev. 94 (2014) 1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Xu F, Sun Y, Shang R, Li M, Cui L, Cui Z, Chen Y, The Glu504Lys polymorphism of aldehyde dehydrogenase 2 contributes to development of coronary artery disease., Tohoku J. Exp. Med. 234 (2014) 143–50. doi: 10.1620/tjem.234.143. [DOI] [PubMed] [Google Scholar]
- [110].Hu N, Zhang Y, Nair S, Culver BW, Ren J, Contribution of ALDH2 polymorphism to alcoholism-associated hypertension., Recent Pat. Endocr. Metab. Immune Drug Discov. 8 (2014) 180–5. doi: 10.2174/1872214808666141020162000. [DOI] [PubMed] [Google Scholar]
- [111].Pang J-J, Barton LA, Chen Y-G, Ren J, Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: from bench to bedside., Sheng Li Xue Bao. 67 (2015) 535–44. [PubMed] [Google Scholar]
- [112].Pang J, Wang J, Zhang Y, Xu F, Chen Y, Targeting acetaldehyde dehydrogenase 2 (ALDH2) in heart failure-Recent insights and perspectives., Biochim. Biophys. Acta. 1863 (2017) 1933–1941. doi: 10.1016/j.bbadis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- [113].Münzel T, Daiber A, The potential of aldehyde dehydrogenase 2 as a therapeutic target in cardiovascular disease., Expert Opin. Ther. Targets. 22 (2018) 217–231. doi: 10.1080/14728222.2018.1439922. [DOI] [PubMed] [Google Scholar]
- [114].Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, Mochly-Rosen D, Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart., Science. 321 (2008) 1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD, Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant, Nat. Struct. Mol. Biol. 17 (2010) 159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J, Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice., J. Am. Heart Assoc. 3 (2014) e000779. doi: 10.1161/JAHA.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shen C, Wang C, Fan F, Yang Z, Cao Q, Liu X, Sun X, Zhao X, Wang P, Ma X, Zhu H, Dong Z, Zou Y, Hu K, Sun A, Ge J, Acetaldehyde dehydrogenase 2 (ALDH2) deficiency exacerbates pressure overload-induced cardiac dysfunction by inhibiting Beclin-1 dependent autophagy pathway., Biochim. Biophys. Acta. 1852 (2015) 310–8. doi: 10.1016/j.bbadis.2014.07.014. [DOI] [PubMed] [Google Scholar]
- [118].Doser TA, Turdi S, Thomas DP, Epstein PN, Li S-Y, Ren J, Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction., Circulation. 119 (2009) 1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [119].Smith RAJ, Hartley RC, Cochemé HM, Murphy MP, Mitochondrial pharmacology, Trends Pharmacol. Sci. 33 (2012) 341–352. doi: 10.1016/j.tips.2012.03.010. [DOI] [PubMed] [Google Scholar]
- [120].Kloner RA, Brown DA, Csete M, Dai W, Downey JM, Gottlieb RA, Hale SL, Shi J, New and revisited approaches to preserving the reperfused myocardium, Nat. Rev. Cardiol. 14 (2017) 679–693. doi: 10.1038/nrcardio.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Enns GM, Treatment of mitochondrial disorders: Antioxidants and beyond, J. Child Neurol. 29 (2014) 1235–1240. doi: 10.1177/0883073814538509. [DOI] [PubMed] [Google Scholar]
- [122].Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM, Coenzyme Q10 and Heart Failure: A State-of-the-Art Review., Circ. Heart Fail. 9 (2016) e002639. doi: 10.1161/CIRCHEARTFAILURE.115.002639. [DOI] [PubMed] [Google Scholar]
- [123].Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RAJ, Murphy MP, Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties, J. Biol. Chem. 276 (2001) 4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- [124].Garjani A, Andalib S, Biabani S, Soraya H, Doustar Y, Garjani A, Maleki-Dizaji N, Combined atorvastatin and coenzyme Q10 improve the left ventricular function in isoproterenol-induced heart failure in rat., Eur. J. Pharmacol. 666 (2011) 135–41. doi: 10.1016/j.ejphar.2011.04.061. [DOI] [PubMed] [Google Scholar]
- [125].De Blasio MJ, Huynh K, Qin C, Rosli S, Kiriazis H, Ayer A, Cemerlang N, Stocker R, Du X-J, McMullen JR, Ritchie RH, Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110α) signaling., Free Radic. Biol. Med. 87 (2015) 137–47. doi: 10.1016/j.freeradbiomed.2015.04.028. [DOI] [PubMed] [Google Scholar]
- [126].Okonko DO, Shah AM, Heart failure: Mitochondrial dysfunction and oxidative stress in CHF, Nat. Rev. Cardiol. 12 (2015) 6–8. doi: 10.1038/nrcardio.2014.189. [DOI] [PubMed] [Google Scholar]
- [127].Pierce JD, Mahoney DE, Hiebert JB, Thimmesch AR, Diaz FJ, Smith C, Shen Q, Mudaranthakam DP, Clancy RL, Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose., BMC Cardiovasc. Disord. 18 (2018) 57. doi: 10.1186/s12872-018-0796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Enns GM, Kinsman SL, Perlman SL, Spicer KM, Abdenur JE, Cohen BH, Amagata A, Barnes A, Kheifets V, Shrader WD, Thoolen M, Blankenberg F, Miller G, Initial experience in the treatment of inherited mitochondrial disease with EPI-743, Mol. Genet. Metab. 105 (2012) 91–102. doi: 10.1016/j.ymgme.2011.10.009. [DOI] [PubMed] [Google Scholar]
- [129].Adlam VJ, Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury, FASEB J. 19 (2005) 1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- [130].Ribeiro Junior RF, Dabkowski ER, Shekar KC, O´Connell KA, Hecker PA, Murphy MP, MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload, Free Radic. Biol. Med. 117 (2018) 18–29. doi: 10.1016/j.freeradbiomed.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR, Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults., Hypertens. (Dallas, Tex. 1979). 71 (2018) 1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RAJ, Murphy MP, Taylor KM, Protect Study Group A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease., Mov. Disord. 25 (2010) 1670–4. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- [133].Breitenstein S, Roessig L, Sandner P, Lewis KS, Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure., Handb. Exp. Pharmacol. 243 (2017) 225–247. doi: 10.1007/164_2016_100. [DOI] [PubMed] [Google Scholar]
- [134].Pacher P, Beckman JS, Liaudet L, Nitric oxide and peroxynitrite in health and disease., Physiol. Rev. 87 (2007) 315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Follmann M, Ackerstaff J, Redlich G, Wunder F, Lang D, Kern A, Fey P, Griebenow N, Kroh W, Becker-Pelster E-M, Kretschmer A, Geiss V, Li V, Straub A, Mittendorf J, Jautelat R, Schirok H, Schlemmer K-H, Lustig K, Gerisch M, Knorr A, Tinel H, Mondritzki T, Trübel H, Sandner P, Stasch J-P, Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure., J. Med. Chem. 60 (2017) 5146–5161. doi: 10.1021/acs.jmedchem.7b00449. [DOI] [PubMed] [Google Scholar]
- [136].Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CSP, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B, SOCRATES-REDUCED Investigators and Coordinators, Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial., JAMA. 314 (2015) 2251–62. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- [137].Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise A-V, Mueller K, Roessig L, Gheorghiade M, Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study., Eur. Heart J. 38 (2017) 1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Filippatos G, Maggioni AP, Lam CSP, Pieske-Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise A-V, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B, Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study., Eur. J. Heart Fail. 19 (2017) 782–791. doi: 10.1002/ejhf.800. [DOI] [PubMed] [Google Scholar]
- [139].Eto M, Kajihara N, Morita S, Tominaga R, A novel electron paramagnetic resonance spin-probe technique demonstrates the relation between the production of hydroxyl radicals and ischemia-reperfusion injury, Eur. J. Cardio-thoracic Surg. 39 (2011) 465–470. doi: 10.1016/j.ejcts.2010.08.011. [DOI] [PubMed] [Google Scholar]
- [140].Tsujita K, Shimomura H, Kawano H, Hokamaki J, Fukuda M, Yamashita T, Hida S, Nakamura Y, Nagayoshi Y, Sakamoto T, Yoshimura M, Arai H, Ogawa H, Effects of edaravone on reperfusion injury in patients with acute myocardial infarction., Am. J. Cardiol. 94 (2004) 481–484. doi: 10.1016/j.amjcard.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [141].Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier J-P, Derumeaux G, Ovize M, Effect of cyclosporine on reperfusion injury in acute myocardial infarction., N. Engl. J. Med. 359 (2008) 473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- [142].Ottani F, Latini R, Staszewsky L, La Vecchia L, Locuratolo N, Sicuro M, Masson S, Barlera S, Milani V, Lombardi M, Costalunga A, Mollichelli N, Santarelli A, De Cesare N, Sganzerla P, Boi A, Pietro Maggioni A, Limbruno U, Cyclosporine A in Reperfused Myocardial Infarction the Multicenter, Controlled, Open-Label CYCLE Trial, J. Am. Coll. Cardiol. 67 (2016) 365–374. doi: 10.1016/j.jacc.2015.10.081. [DOI] [PubMed] [Google Scholar]
- [143].Atar D, Arheden H, Berdeaux A, Bonnet J-L, Carlsson M, Clemmensen P, Cuvier V, Danchin N, Dubois-Rande J-L, Engblom H, Erlinge D, Firat H, Halvorsen S, Hansen HS, Hauke W, Heiberg E, Koul S, Larsen A-I, Le Corvoisier P, Nordrehaug JE, Paganelli F, Pruss RM, Rousseau H, Schaller S, Sonou G, Tuseth V, Veys J, Vicaut E, Jensen SE, Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results, Eur. Heart J. 36 (2015) 112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- [144].Methner C, Chouchani ET, Buonincontri G, Pell VR, Sawiak SJ, Murphy MP, Krieg T, Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts., Eur. J. Heart Fail. 16 (2014) 712–7. doi: 10.1002/ejhf.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yin M, van der Horst ICC, van Melle JP, Qian C, van Gilst WH, Sillje HHW, de Boer RA, Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure, AJP Hear. Circ. Physiol. 301 (2011) H459–H468. doi: 10.1152/ajpheart.00054.2011. [DOI] [PubMed] [Google Scholar]
- [146].Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M, Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase., Circulation. 119 (2009) 2568–77. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- [147].Beauloye C, Bertrand L, Horman S, Hue L, AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure., Cardiovasc. Res. 90 (2011) 224–33. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- [148].Chan AYM, Dolinsky VW, Soltys C-LM, Viollet B, Baksh S, Light PE, Dyck JRB, Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt., J. Biol. Chem. 283 (2008) 24194–201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Matsuhashi T, Sato T, ichiro Kanno S, Suzuki T, Matsuo A, Oba Y, Kikusato M, Ogasawara E, Kudo T, Suzuki K, Ohara O, Shimbo H, Nanto F, Yamaguchi H, Saigusa D, Mukaiyama Y, Watabe A, Kikuchi K, Shima H, Mishima E, Akiyama Y, Oikawa Y, Hsin-Jung HO, Akiyama Y, Suzuki C, Uematsu M, Ogata M, Kumagai N, Toyomizu M, Hozawa A, Mano N, Owada Y, Aiba S, Yanagisawa T, Tomioka Y, Kure S, Ito S, Nakada K, ichiro Hayashi K, Osaka H, Abe T, Mitochonic Acid 5 (MA-5) Facilitates ATP Synthase Oligomerization and Cell Survival in Various Mitochondrial Diseases, EBioMedicine. 20 (2017) 27–38. doi: 10.1016/j.ebiom.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Szeto HH, First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics, Br. J. Pharmacol. 171 (2014) 2029–2050. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G, Functional role of cardiolipin in mitochondrial bioenergetics, Biochim. Biophys. Acta - Bioenerg. 1837 (2014) 408–417. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- [152].Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng F-Y, Zhao Z, Ganger M, Tow CY, Seshan SV, Mitochondria-Targeted Peptide Accelerates ATP Recovery and Reduces Ischemic Kidney Injury, J. Am. Soc. Nephrol. 22 (2011) 1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zhao H, Li H, Hao S, Chen J, Wu J, Song C, Zhang M, Qiao T, Li K, Peptide SS-31 upregulates frataxin expression and improves the quality of mitochondria: Implications in the treatment of Friedreich ataxia, Sci. Rep. 7 (2017) 1–11. doi: 10.1038/s41598-017-10320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, Rabinovitch PS, Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides, Circ. Hear. Fail. 6 (2013) 1067–1076. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lu HI, Lee FY, Wallace CG, Sung PH, Chen KH, Sheu JJ, Chua S, Tong MS, Huang TH, Chen YL, Shao PL, Yip HK, SS31 therapy effectively protects the heart against transverse aortic constriction-induced hypertrophic cardiomyopathy damage, Am. J. Transl. Res. 9 (2017) 5220–5237. [PMC free article] [PubMed] [Google Scholar]
- [156].Dai D-F, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS, Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy., J. Am. Coll. Cardiol. 58 (2011) 73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Jánosi A, Merkely B, Godlewski J, Halaby R, Korjian S, Daaboul Y, Chakrabarti AK, Spielman K, Neal BJ, Weaver WD, EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention., Eur. Heart J. 37 (2016) 1296–1303. doi: 10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]
- [158].Cerrato CP, Pirisinu M, Vlachos EN, Langel Ü, Novel cell-penetrating peptide targeting mitochondria, FASEB J. 29 (2015) 4589–4599. doi: 10.1096/fj.14-269225. [DOI] [PubMed] [Google Scholar]
- [159].Cerrato CP, Langel Ü, Effect of a Fusion Peptide by Covalent Conjugation of a Mitochondrial Cell-Penetrating Peptide and a Glutathione Analog Peptide, Mol. Ther. - Methods Clin. Dev. 5 (2017) 221–231. doi: 10.1016/j.omtm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sun L, Ferreira JCB, Mochly-Rosen D, ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance., Sci. Transl. Med. 3 (2011) 107ra111. doi: 10.1126/scitranslmed.3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zambelli VO, Gross ER, Chen C-H, Gutierrez VP, Cury Y, Mochly-Rosen D, Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain., Sci. Transl. Med. 6 (2014) 251ra118. doi: 10.1126/scitranslmed.3009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gomes KMS, Campos JC, Bechara LRG, Queliconi B, Lima VM, Disatnik M-H, Magno P, Chen C-H, Brum PC, Kowaltowski AJ, Mochly-Rosen D, Ferreira JCB, Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling., Cardiovasc. Res. 103 (2014) 498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gomes KMS, Bechara LRG, Lima VM, Ribeiro MAC, Campos JC, Dourado PM, Kowaltowski AJ, Mochly-Rosen D, Ferreira JCB, Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post-myocardial infarction cardiomyopathy: benefits of Alda-1., Int. J. Cardiol. 179 (2015) 129–138. doi: 10.1016/j.ijcard.2014.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Xu F, Chen YG, Xue L, Li RJ, Zhang H, Bian Y, Zhang C, Lv RJ, Feng JB, Zhang Y, Role of aldehyde dehydrogenase 2 Glu504lys polymorphism in acute coronary syndrome., J. Cell. Mol. Med. 15 (2011) 1955–62. doi: 10.1111/j.1582-4934.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Han H, Wang H, Yin Z, Jiang H, Fang M, Han J, Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: a meta-analysis., Gene. 526 (2013) 134–141. doi: 10.1016/j.gene.2013.05.002. [DOI] [PubMed] [Google Scholar]
- [166].Mizuno Y, Hokimoto S, Harada E, Kinoshita K, Nakagawa K, Yoshimura M, Ogawa H, Yasue H, Variant Aldehyde Dehydrogenase 2 (ALDH2*2) Is a Risk Factor for Coronary Spasm and ST-Segment Elevation Myocardial Infarction., J. Am. Heart Assoc. 5 (2016). doi: 10.1161/JAHA.116.003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mizuno Y, Harada E, Morita S, Kinoshita K, Hayashida M, Shono M, Morikawa Y, Murohara T, Nakayama M, Yoshimura M, Yasue H, East asian variant of aldehyde dehydrogenase 2 is associated with coronary spastic angina: possible roles of reactive aldehydes and implications of alcohol flushing syndrome., Circulation. 131 (2015) 1665–1673. doi: 10.1161/CIRCULATIONAHA.114.013120. [DOI] [PubMed] [Google Scholar]
- [168].Li Y-Y, Wang H, Wu J-J, Kim HJ, Yang X-X, Geng H-Y, Gong G, ALDH2 gene G487A polymorphism and coronary artery disease: a meta-analysis including 5644 participants., J. Cell. Mol. Med. 22 (2018) 1666–1674. doi: 10.1111/jcmm.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yang M-Y, Wang Y-B, Han B, Yang B, Qiang Y-W, Zhang Y, Wang Z, Huang X, Liu J, Chen Y-D, Ren J, Cao F, Xu Y, Activation of aldehyde dehydrogenase 2 slows down the progression of atherosclerosis via attenuation of ER stress and apoptosis in smooth muscle cells., Acta Pharmacol. Sin. 39 (2018) 48–58. doi: 10.1038/aps.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Woods C, Shang C, Taghavi F, Downey P, Zalewski A, Rubio GR, Liu J, Homburger JR, Grunwald Z, Qi W, Bollensdorff C, Thanaporn P, Ali A, Riemer K, Kohl P, Mochly-Rosen D, Gerstenfeld E, Large S, Ali Z, Ashley E, In Vivo Post-Cardiac Arrest Myocardial Dysfunction Is Supported by Ca2+/Calmodulin-Dependent Protein Kinase II-Mediated Calcium Long-Term Potentiation and Mitigated by Alda-1, an Agonist of Aldehyde Dehydrogenase Type 2., Circulation. 134 (2016) 961–977. doi: 10.1161/CIRCULATIONAHA.116.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Yavari A, Ashrafian H, Potentiating mitochondrial aldehyde dehydrogenase 2 to treat post-infarction heart failure., Cardiovasc. Res. 103 (2014) 429–431. doi: 10.1093/cvr/cvu175. [DOI] [PubMed] [Google Scholar]
- [172].Suzuki T, Yamaguchi H, Kikusato M, Matsuhashi T, Matsuo A, Sato T, Oba Y, Watanabe S, Minaki D, Saigusa D, Shimbo H, Mori N, Mishima E, Shima H, Akiyama Y, Takeuchi Y, Yuri A, Kikuchi K, Toyohara T, Suzuki C, Kohzuki M, Anzai J, Mano N, Kure S, Yanagisawa T, Tomioka Y, Toyomizu M, Ito S, Osaka H, Hayashi K, Abe T, Mitochonic Acid 5 (MA-5), a Derivative of the Plant Hormone Indole-3-Acetic Acid, Improves Survival of Fibroblasts from Patients with Mitochondrial Diseases, Tohoku J. Exp. Med. 236 (2015) 225–232. doi: 10.1620/tjem.236.225. [DOI] [PubMed] [Google Scholar]
- [173].Sun C, Liu X, Di C, Wang Z, Mi X, Liu Y, Zhao Q, Mao A, Chen W, Gan L, Zhang H, MitoQ regulates autophagy by inducing a pseudo-mitochondrial membrane potential., Autophagy. 13 (2017) 730–738. doi: 10.1080/15548627.2017.1280219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gottwald EM, Duss M, Bugarski M, Haenni D, Schuh CD, Landau EM, Hall AM, The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue., Physiol. Rep. 6 (2018) e13667. doi: 10.14814/phy2.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Jameson VJA, Cochemé HM, Logan A, Hanton LR, Smith RAJ, Murphy MP, Synthesis of triphenylphosphonium Vitamin E derivatives as mitochondria-targeted antioxidants, Tetrahedron. 71 (2015) 8444–8453. doi: 10.1016/j.tet.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Apostolova N, Victor VM, Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications, Antioxid. Redox Signal. 22 (2015) 686–729. doi: 10.1089/ars.2014.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Degli Esposti L, Rossi F, Ravanetti F, Pinelli S, Alinovi R, Erreni M, Rossi S, Condorelli G, Post H, Tampieri A, Catalucci D, Inhalation of peptide-loaded nanoparticles improves heart failure, Sci. Transl. Med. 10 (2018) 1–12. doi: 10.1126/scitranslmed.aan6205. [DOI] [PubMed] [Google Scholar]
- [178].Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J, Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure., Circulation. 121 (2010) 237–44. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- [179].Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, Kannel WB, Vasan RS, Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study., Eur. J. Heart Fail. 15 (2013) 742–6. doi: 10.1093/eurjhf/hft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, V Georgiopoulou V, Butler J, Laukkanen JA, Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study., Eur. J. Heart Fail. 16 (2014) 180–8. doi: 10.1111/ejhf.37. [DOI] [PubMed] [Google Scholar]
- [181].Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C, Exercise and heart failure: an update., ESC Hear. Fail. 5 (2018) 222–232. doi: 10.1002/ehf2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Edelmann F, Gelbrich G, Düngen H-D, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B, Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study., J. Am. Coll. Cardiol. 58 (2011) 1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- [183].Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution, Eur. J. Heart Fail. 18 (2016) 891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- [184].Belardinelli R, Georgiou D, Cianci G, Purcaro A, Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome., Circulation. 99 (1999) 1173–82. doi: 10.1161/01.CIR.99.9.1173. [DOI] [PubMed] [Google Scholar]
- [185].Tabet J-Y, Meurin P, Ben Driss A, Weber H, Renaud N, Grosdemouge A, Beauvais F, Cohen-Solal A, Benefits of exercise training in chronic heart failure., Arch. Cardiovasc. Dis. 102 (2009) 721–30. doi: 10.1016/j.acvd.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [186].Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G, Effect of exercise on coronary endothelial function in patients with coronary artery disease., N. Engl. J. Med. 342 (2000) 454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- [187].Cunha TF, Bacurau AVN, Moreira JBN, Paixão NA, Campos JC, Ferreira JCB, Leal ML, Negrão CE, Moriscot AS, Wisløff U, Brum PC, Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure., PLoS One. 7 (2012) e41701. doi: 10.1371/journal.pone.0041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Cunha TF, Bechara LRG, Bacurau AVN, Jannig PR, Voltarelli VA, Dourado PM, Vasconcelos AR, Scavone C, Ferreira JCB, Brum PC, Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats., J. Appl. Physiol. 122 (2017) 817–827. doi: 10.1152/japplphysiol.00182.2016. [DOI] [PubMed] [Google Scholar]
- [189].Bacurau AVN, Jardim MA, Ferreira JCB, Bechara LRG, Bueno CR, Alba-Loureiro TC, Negrao CE, Casarini DE, Curi R, Ramires PR, Moriscot AS, Brum PC, Sympathetic hyperactivity differentially affects skeletal muscle mass in developing heart failure: role of exercise training., J. Appl. Physiol. 106 (2009) 1631–40. doi: 10.1152/japplphysiol.91067.2008. [DOI] [PubMed] [Google Scholar]
- [190].Pereira MG, Ferreira JCB, Bueno CR, Mattos KC, Rosa KT, Irigoyen MC, Oliveira EM, Krieger JE, Brum PC, Exercise training reduces cardiac angiotensin II levels and prevents cardiac dysfunction in a genetic model of sympathetic hyperactivity-induced heart failure in mice., Eur. J. Appl. Physiol. 105 (2009) 843–50. doi: 10.1007/s00421-008-0967-4. [DOI] [PubMed] [Google Scholar]
- [191].Bueno CR, Ferreira JCB, Pereira MG, Bacurau AVN, Brum PC, Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure., J. Appl. Physiol. 109 (2010) 702–9. doi: 10.1152/japplphysiol.00281.2010. [DOI] [PubMed] [Google Scholar]
- [192].Oliveira RSF, Ferreira JCB, Gomes ERM, Paixão NA, Rolim NPL, Medeiros A, Guatimosim S, Brum PC, Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice., J. Physiol. 587 (2009) 3899–910. doi: 10.1113/jphysiol.2009.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ferreira JCB, V Bacurau A, Bueno CR, Cunha TC, Tanaka LY, Jardim MA, Ramires PR, Brum PC, Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice., Exp. Biol. Med. (Maywood). 235 (2010) 497–505. doi: 10.1258/ebm.2009.009165. [DOI] [PubMed] [Google Scholar]
- [194].Cunha TF, Moreira JBN, Paixão NA, Campos JC, Monteiro AWA, Bacurau AVN, Bueno CR, Ferreira JCB, Brum PC, Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice., J. Appl. Physiol. 112 (2012) 1839–46. doi: 10.1152/japplphysiol.00346.2011. [DOI] [PubMed] [Google Scholar]
- [195].Campos JC, Gomes KMS, Ferreira JCB, Impact of exercise training on redox signaling in cardiovascular diseases., Food Chem. Toxicol. 62 (2013) 107–19. doi: 10.1016/j.fct.2013.08.035. [DOI] [PubMed] [Google Scholar]
- [196].Campos JC, Fernandes T, Bechara LRG, da Paixão NA, Brum PC, de Oliveira EM, Ferreira JCB, Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training., Oxid. Med. Cell. Longev. 2015 (2015). doi: 10.1155/2015/464195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ueta CB, Gomes KS, Ribeiro MA, Mochly-Rosen D, Ferreira JCB, Disruption of mitochondrial quality control in peripheral artery disease: New therapeutic opportunities., Pharmacol. Res. 115 (2017) 96–106. doi: 10.1016/j.phrs.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Kavazis AN, Smuder AJ, Min K, Tümer N, Powers SK, Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72., Am. J. Physiol. Heart Circ. Physiol. 299 (2010) H1515–24. doi: 10.1152/ajpheart.00585.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Kraljevic J, Marinovic J, Pravdic D, Zubin P, Dujic Z, Wisloff U, Ljubkovic M, Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart., Cardiovasc. Res. 99 (2013) 55–64. doi: 10.1093/cvr/cvt080. [DOI] [PubMed] [Google Scholar]
- [200].Emter CA, Baines CP, Low-intensity aerobic interval training attenuates pathological left ventricular remodeling and mitochondrial dysfunction in aortic-banded miniature swine., Am. J. Physiol. Heart Circ. Physiol. 299 (2010) H1348–56. doi: 10.1152/ajpheart.00578.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].O’Neill JO, Young JB, Pothier CE, Lauer MS, Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers., Circulation. 111 (2005) 2313–8. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- [202].Malhotra R, Bakken K, D’Elia E, Lewis GD, Cardiopulmonary Exercise Testing in Heart Failure., JACC. Heart Fail. 4 (2016) 607–16. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- [203].Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou M-C, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE, HF-ACTION Investigators, Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training., Circ. Heart Fail. 5 (2012) 579–85. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh S, Exercise-based rehabilitation for heart failure., Cochrane database Syst. Rev. (2014) CD003331. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Kawakami S, Matsuda A, Sunagawa T, Noda Y, Kaneko T, Tahara S, Hiraumi Y, Adachi S, Matsui H, Ando K, Fujita T, Maruyama N, Shirasawa T, Shimizu T, Antioxidant EUK -8, prevents murine dilated cardiomyopathy., … J. Off. J. …. 73 (2009) 2125–2134. doi: 10.1253/circj.CJ-09-0204. [DOI] [PubMed] [Google Scholar]
- [206].van Empel VPM, Bertrand AT, van Oort RJ, van der Nagel R, Engelen M, van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ, EUK-8, a Superoxide Dismutase and Catalase Mimetic, Reduces Cardiac Oxidative Stress and Ameliorates Pressure Overload-Induced Heart Failure in the Harlequin Mouse Mutant, J. Am. Coll. Cardiol. 48 (2006) 824–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- [207].Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP, A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats., Science. 286 (1999) 304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- [208].Masini E, Cuzzocrea S, Mazzon E, Marzocca C, Mannaioni PF, Salvemini D, Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo, Br. J. Pharmacol. 136 (2002) 905–917. doi: 10.1038/sj.bjp.0704774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Nistri S, Boccalini G, Bencini A, Becatti M, Valtancoli B, Conti L, Lucarini L, Bani D, A new low molecular weight, MnII-containing scavenger of superoxide anion protects cardiac muscle cells from hypoxia/reoxygenation injury., Free Radic. Res. 49 (2015) 67–77. doi: 10.3109/10715762.2014.979168. [DOI] [PubMed] [Google Scholar]
- [210].Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P, Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants., Crit. Care Med. 35 (2007) S461–7. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- [211].Escobales N, Nuñez RE, Jang S, Parodi-Rullan R, Ayala-Peña S, Sacher JR, Skoda EM, Wipf P, Frontera W, Javadov S, Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats, J. Mol. Cell. Cardiol. 77 (2014) 136–146. doi: 10.1016/j.yjmcc.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Xun Z, Rivera-Sánchez S, Ayala-Peña S, Lim J, Budworth H, Skoda EM, Robbins PD, Niedernhofer LJ, Wipf P, McMurray CT, Targeting of XJB-5–131 to Mitochondria Suppresses Oxidative DNA Damage and Motor Decline in a Mouse Model of Huntington’s Disease, Cell Rep. 2 (2012) 1137–1142. doi: 10.1016/j.celrep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T, Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy, Free Radic. Biol. Med. 90 (2016) 12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI, Therapeutic targeting of mitochondrial superoxide in hypertension, Circ. Res. 107 (2010) 106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Dikalova AE, Kirilyuk IA, Dikalov SI, Antihypertensive effect of mitochondria-targeted proxyl nitroxides, Redox Biol. 4 (2015) 355–362. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, Tanaka E, The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease, Int. J. Mol. Sci. 14 (2013) 13909–13930. doi: 10.3390/ijms140713909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zhang W-W, Bai F, Wang J, Zheng R-H, Yang L-W, James EA, Zhao Z-Q, Edaravone inhibits pressure overload-induced cardiac fibrosis and dysfunction by reducing expression of angiotensin II AT1 receptor., Drug Des. Devel. Ther. 11 (2017) 3019–3033. doi: 10.2147/DDDT.S144807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Edaravone Acute Infarction Study Group, Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters., Cerebrovasc. Dis. 15 (2003) 222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- [219].Abe K, Aoki M, Tsuji S, Itoyama Y, Sobue G, Togo M, Hamada C, Tanaka M, Akimoto M, Nakamura K, Takahashi F, Kondo K, Yoshino H, Abe K, Aoki M, Tsuji S, Itoyama Y, Sobue G, Togo M, Hamada C, Sasaki H, Yabe I, Doi S, Warita H, Imai T, Ito H, Fukuchi M, Osumi E, Wada M, Nakano I, Morita M, Ogata K, Maruki Y, Ito K, Kano O, Yamazaki M, Takahashi Y, Ishiura H, Ogino M, Koike R, Ishida C, Uchiyama T, Mizoguchi K, Obi T, Watanabe H, Atsuta N, Aiba I, Taniguchi A, Sawada H, Hazama T, Fujimura H, Kusaka H, Kunieda T, Kikuchi H, Matsuo H, Ueyama H, Uekawa K, Tanaka M, Akimoto M, Ueda M, Murakami A, Sumii R, Kudou T, Nakamura K, Morimoto K, Yoneoka T, Hirai M, Sasaki K, Terai H, Natori T, Matsui H, Kotani K, Yoshida K, Iwasaki T, Takahashi F, Kondo K, Yoshino H, Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial, Lancet Neurol. 16 (2017) 505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- [220].Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, Hamada C, Kondo K, Yoneoka T, Akimoto M, Yoshino H, Edaravone ALS Study Group, Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients., Amyotroph. Lateral Scler. Frontotemporal Degener. 15 (2014) 610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Martinelli D, Catteruccia M, Piemonte F, Pastore A, Tozzi G, Dionisi-Vici C, Pontrelli G, Corsetti T, Livadiotti S, Kheifets V, Hinman A, Shrader WD, Thoolen M, Klein MB, Bertini E, Miller G, EPI-743 reverses the progression of the pediatric mitochondrial disease-Genetically defined Leigh Syndrome, Mol. Genet. Metab. 107 (2012) 383–388. doi: 10.1016/j.ymgme.2012.09.007. [DOI] [PubMed] [Google Scholar]
- [222].Hausse AO, Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia, Heart. 87 (2002) 346–349. doi: 10.1136/heart.87.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Lerman-Sagie T, Rustin P, Lev D, Yanoov M, Leshinsky-Silver E, Sagie A, Ben-Gal T, Munnich A, Dramatic improvement in mitochondrial cardiomyopathy following treatment with idebenone, J. Inherit. Metab. Dis. 24 (2001) 28–34. doi: 10.1023/A:1005642302316. [DOI] [PubMed] [Google Scholar]
- [224].Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH, The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin, J. Am. Soc. Nephrol. 24 (2013) 1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Wu D, Soong Y, Zhao G-MM, Szeto HH, A highly potent peptide analgesic that protects against ischemia-reperfusion-induced myocardial stunning., Am. J. Physiol. Heart Circ. Physiol. 283 (2002) H783–H791. doi: 10.1152/ajpheart.00193.2002. [DOI] [PubMed] [Google Scholar]
- [226].Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, André-Fouët X, Derumeaux G, Piot C, Vernhet H, Revel D, Ovize M, Effect of Cyclosporine on Left Ventricular Remodeling After Reperfused Myocardial Infarction, J. Am. Coll. Cardiol. 55 (2010) 1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- [227].Schaller S, Paradis S, Ngoh GA, Assaly R, Buisson B, Drouot C, Ostuni MA, Lacapere J-J, Bassissi F, Bordet T, Berdeaux A, Jones SP, Morin D, Pruss RM, TRO40303, a New Cardioprotective Compound, Inhibits Mitochondrial Permeability Transition, J. Pharmacol. Exp. Ther. 333 (2010) 696–706. doi: 10.1124/jpet.110.167486. [DOI] [PubMed] [Google Scholar]
- [228].Le Lamer S, Paradis S, Rahmouni H, Chaimbault C, Michaud M, Culcasi M, Afxantidis J, Latreille M, Berna P, Berdeaux A, Pietri S, Morin D, Donazzolo Y, Abitbol JL, Pruss RM, Schaller S, Translation of TRO40303 from myocardial infarction models to demonstration of safety and tolerance in a randomized Phase I trial, J. Transl. Med. 12 (2014) 1–15. doi: 10.1186/1479-5876-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Butt N, Bache-Mathiesen LK, Nordrehaug JE, Tuseth V, Munk PS, Bonarjee V, Hall TS, Jensen SE, Halvorsen S, Firat H, Atar D, Larsen AI, Administration of the Mitochondrial Permeability Transition Pore Inhibitor, TRO40303, prior to Primary Percutaneous Coronary Intervention, Does Not Affect the Levels of Pro-Inflammatory Cytokines or Acute-Phase Proteins., Cardiology. 138 (2017) 122–132. doi: 10.1159/000475460. [DOI] [PubMed] [Google Scholar]
- [230].Suzuki T, Yamaguchi H, Kikusato M, Hashizume O, Nagatoishi S, Matsuo A, Sato T, Kudo T, Matsuhashi T, Murayama K, Ohba Y, Watanabe S, Kanno S. -i., Minaki D, Saigusa D, Shinbo H, Mori N, Yuri A, Yokoro M, Mishima E, Shima H, Akiyama Y, Takeuchi Y, Kikuchi K, Toyohara T, Suzuki C, Ichimura T, Anzai J. -i., Kohzuki M, Mano N, Kure S, Yanagisawa T, Tomioka Y, Toyomizu M, Tsumoto K, Nakada K, Bonventre JV, Ito S, Osaka H, Hayashi K. -i., Abe T, Mitochonic Acid 5 Binds Mitochondria and Ameliorates Renal Tubular and Cardiac Myocyte Damage, J. Am. Soc. Nephrol. 27 (2016) 1925–1932. doi: 10.1681/ASN.2015060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Sun D, Yang F, Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism., Biochem. Biophys. Res. Commun. 486 (2017) 329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- [232].Maack C, Bhm M, Targeting mitochondrial oxidative stress in heart failure: Throttling the afterburner, J. Am. Coll. Cardiol. 58 (2011) 83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- [233].Grune T, Davies KJA, The proteasomal system and HNE-modified proteins., Mol. Aspects Med. 24 ([s.d.]) 195–204. [DOI] [PubMed] [Google Scholar]
- [234].Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ, 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites., J. Neurochem. 72 (1999) 1617–24. [DOI] [PubMed] [Google Scholar]
- [235].Wu J, Luo X, Yan L-J, Two dimensional blue native/SDS-PAGE to identify mitochondrial complex I subunits modified by 4-hydroxynonenal (HNE)., Front. Physiol. 6 (2015) 98. doi: 10.3389/fphys.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Doorn JA, Hurley TD, Petersen DR, Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal, Chem. Res. Toxicol. 19 (2006) 102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- [237].Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, Mochly-Rosen D, Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart., Science. 321 (2008) 1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]