Abstract
Maternal nutrition is critically important for fetal development. Recent human studies demonstrate a strong connection between diet during pregnancy and offspring risk for neuropsychiatric disorders including depression, anxiety, and attention-deficit/hyperactivity disorder. Animal models have emerged as a crucial tool for understanding maternal nutrition’s contribution to prenatal programming and the later development of neuropsychiatric disorders. This review highlights preclinical studies examining how maternal consumption of the three macronutrients (protein, fats, and carbohydrates) influence offspring negative valence behaviors relevant to neuropsychiatric disorders. We highlight the translational aspects of animal models, and so examine exposure periods that mirror the neurodevelopmental stages of human gestation. Due to our emphasis on programmed changes in neurobehavioral development, studies that continue diet exposure until assessment in adulthood are not discussed. The presented research provides a strong foundation of preclinical evidence of nutritional programming of neurobehavioral impairments. Alterations in risk assessment and response were observed alongside neurodevelopmental impairments related to neurogenesis, synaptogenesis, and synaptic plasticity. To date, the large majority of studies utilized rodent models and the field could benefit from additional study of large animal models. Additional future directions are discussed, including the need for additional studies examining how sex as a biological variable affects the contribution of maternal nutrition to prenatal programming.
Keywords: Animal model, Behavior, Maternal Diet, Neuropsychiatric disorders, Nutrition, Prenatal programming
Introduction
Neuropsychiatric disorders are a major global health concern. They are highly prevalent and lack effective prevention and treatment strategies, consequently imposing enormous societal costs (1-5). The origins and mechanisms of neuropsychiatric disorders are of great consequence to the public and scientific community, with findings from epidemiological and animal studies indicating that early life conditions greatly contribute to risk of mental disorders (6, 7). The fetal programming hypothesis posits that prenatal environmental factors influence long-term neuropsychiatric outcomes by altering epigenetic control of neural processes or disrupting neural function during critical periods of development (7, 8). The prenatal environment is significantly influenced by nutrient availability, and nutritional programming specifically investigates the residual impact of fetal nutrient imbalance (9). The mother supplies offspring nutrition during gestation and lactation, with an excess or deficiency in most nutrients impacting fetal neurodevelopment (10). The placenta facilitates maternofetal nutrient exchange in utero, buffering glucose fluctuations, storing lipids, and producing a few essential amino acids (11) (Figure 1). In addition to diet, maternal metabolic conditions (e.g. maternal obesity, diabetes) alter nutrient balance and placental function (10, 12); clinical and animal models implicate these conditions in increased offspring risk of neuropsychiatric disorders (13-15). Maternal nutrition and metabolic state are highly inter-related and associated with diet, making their contributions to offspring neuropsychiatric impairments difficult to differentiate.
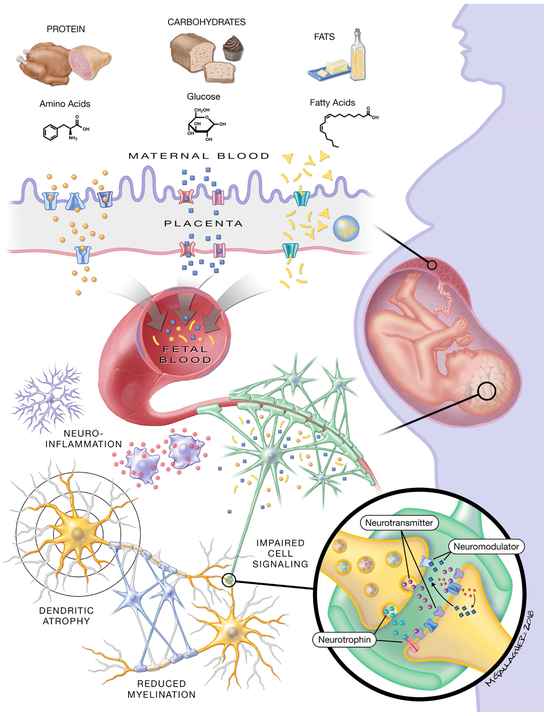
Figure 1. Maternal Diet and Fetal Macronutrient Availability.
Food sources rich in protein, carbohydrates, and fats increase the presence of amino acids, glucose, and fatty acids in maternal circulation. These nutrients enter fetal circulation via different methods of placental transport. Amino acids provided by dietary protein require active transport across the placenta, and the placenta can produce select amino acids like glutamate into the fetal circulatory system. The transport of glucose (derived from sugars and grains) across the placenta is facilitated by glucose transport proteins, causing changes in fetal blood glucose concentrations that closely mirror maternal levels. Fatty acids derived from triglycerides present in dietary fats like butter and oil can freely diffuse across the placental boundary and fatty acid transport proteins provide additional energy-dependent transfer. While many fatty acids directly enter fetal circulation, the placenta can convert the fatty acids back to triglycerides for storage in lipid droplets. After entering fetal circulation, macronutrients traverse the blood-brain-barrier via similar mechanisms to placental transport. As described throughout this review, nutritional programming results in altered neural function and development (depicted in gray). Discussed mechanisms include elevated neuroinflammation, dendritic atrophy and instability, and delayed glial maturation resulting in reduced myelination. Many of the reported studies investigated altered expression, production, and function of cell signaling molecules and receptors. Inadequate fetal nutrition is associated with changes in neurotrophin (e.g. brain-derived neurotrophic factor signaling), neurotransmitter (e.g. dopamine), and neuromodulator systems (e.g. endocannabinoid signaling).
Epidemiologic studies exploring nutritional programming are complicated by substantial variation in nutrition and limitations in modifying the diet of pregnant mothers and newborns. Animal models allow precise control of diet content and the ability to limit manipulation to critical periods of development. Notably, developmental ontogeny varies depending on the model utilized; neurodevelopmental processes that occur during late gestation in humans take place postnatally in rodents (16, 17) (Figure 2). It is therefore necessary that translational efforts consider differences in nutrient transfer and demand between the intrauterine and extrauterine environments. Adopting an endophenotype approach to neuropsychiatric research, this review focuses on evidence from animal models demonstrating nutritional programming of offspring negative valence behaviors (18, 19). In humans, the negative valence domain encompasses fear and sadness and, while adaptive in many contexts, negative valence behaviors are dysregulated in multiple psychopathologies (20, 21). Although not equivalent to the complex spectrum of behavioral endophenotypes in humans, animal models can reliably investigate aspects of the negative valence system via behavioral assays. Just as excessive fear and anxiety can impair a person’s ability to evaluate and respond to a stressful or threatening situation, an animal’s behavioral response can reveal altered appraisal and avoidance of a threatening stimulus, giving insight to potentially dysregulated defensive response (22-24) (Figure S1). In rodents, changes in thigmotaxis (the tendency to avoid open, exposed areas) and passive coping behaviors can reliably indicate altered threat response, similar to anxiety in humans (25-29). Importantly, major components of negative valence networks are well-defined and conserved among mammals. Investigation of the corticolimbic system, involved in risk assessment and response, gives insight to neurobiological aspects of neuropsychiatric disorders in humans (30).
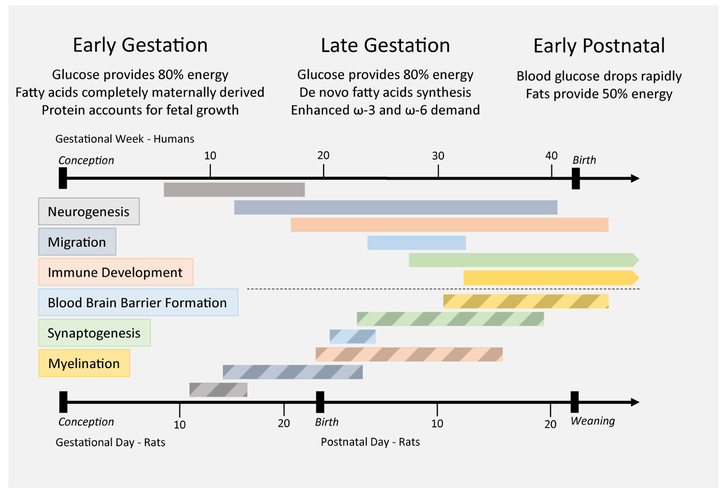
Figure 2. Neurodevelopmental Ontogeny and Nutrient Requirements.
Nutritional requirements for developing offspring change in accordance with physiological demand. During in utero development, glucose provided by circulating maternal glucose is the main source of energy for the developing fetus. Availability of energy and amino acids determine the rate of protein synthesis, and protein accretion is critical to sustaining fetal growth in early and late gestation. During advanced gestation demands for omega-3 (ω-3) and omega-6 (ω-6) essential fatty acids increase, and the fetus is capable of some de novo fatty acid synthesis to assist in lipid accumulation. Immediately following birth, blood glucose drops rapidly as the continual source of maternal glucose via the placenta is replaced with nutrition provided by nursing. Lipids account for a substantial source of energy in the early postnatal stage as fat accumulation continues. The importance of these nutritional changes across development is conserved across animal models, however the timing varies according to developmental rate. Presented are paired neurodevelopmental timelines for humans (above, in gestational weeks post-conception; solid bars) and rats (below, in gestational days post-conception and postnatal days after birth; striped bars) (similar in mice). Different brain regions have asynchronous development; therefore, the general stages of brain development are presented in this image. Discordant schedules of neurodevelopment are evident between the two species. In particular, processes that occur during late gestation in humans take place postnatally in rats. Weaning in rodents is triggered naturally by sexual maturity around three weeks of age, marking the beginning of adolescence/adulthood. It is important to note that different rat and mouse species have innately varied timelines, and for some of the animal models discussed in this review weaning occurred at the natural point for that species, which may be a number of days before or after postnatal day 21.
We focus on negative valences behaviors and neural outcomes from animal models relevant to global dietary patterns and seek to investigate programming influence of maternal diet independent from metabolic disorders. This review highlights the translational aspects of animal models, and so examines exposure periods that mirror the neurodevelopmental stages of human gestation (16, 17). Studies are included that examine diet manipulation during gestation (conception to birth), lactation (birth to weaning), or both combined, defined here as perinatal exposure. Due to our emphasis on programmed changes in neurobehavioral development, all studies provided diet intervention (switched to control diet) by weaning. The correction of nutrient balance prior to neurobehavioral assessment in adulthood allows for the examination of long-term changes in offspring behavior. Tables are provided to supply key details for the presented behavioral studies. We concentrate on neurobehavioral differences resulting from maternal dietary manipulation of the three macronutrients: protein, fats, and carbohydrates.
Protein
Proteins are macronutrients comprised of amino acid subunits that are required for growth and the production of cellular receptors, transporters, and signaling molecules like neurotransmitters (31). Amino acids require active transport across the placenta and blood brain barrier. While many are produced endogenously, nine essential amino acids must be obtained from dietary sources (11). The placenta prioritizes resources to sustain fetal growth; however, animal models demonstrate that placental amino acid transport is reduced at the detriment of fetal amino acids when protein restriction limits maternal amino acid availability (32-37). Protein restriction is highly relevant as millions of people worldwide consume dietary staples that are poor sources of amino acids, both in terms of quality and quantity (38). While the effects of gestational protein restriction on overall growth are well-documented, limited animal studies investigated the effect of maternal protein restriction on offspring negative valence behaviors (Table 1). The few animal models examining this relationship have focused on casein. Unlike the sources typically available in protein deficient human diets, casein is a high-quality protein that provides all essential amino acids. As a rat model demonstrated that protein quality alone significantly affects offspring development (39), this discrepancy is important to note when translating findings to the human condition. Nevertheless, preclinical research provides important insights regarding the effect of inadequate dietary protein during development on offspring behavioral regulation.
Table 1.
Protein
| Source | Model | Strain/ Species |
Subject Sex |
Diet Manipulation | Diet Exposure Period |
Behavioral Assays | Testing Age |
Significant Outcomes |
|---|---|---|---|---|---|---|---|---|
| Reyes-Castro 2012 (44) | Rat | Wistar | Male | 10% protein (control 20%) | Gestation, Lactation, Perinatal | Open field test, Elevated plus maze | 10 weeks | Gestational, lactational, and perinatal restriction impaired risk assessment (EPM). |
| Reyes-Castro 2012 (43) | Rat | Wistar | Female | 10% protein (control 20%) | Gestation, Lactation, Perinatal | Open field test, Elevated plus maze, Operant conditioning & Progressive ratio | 12-22 weeks | Perinatal and gestational restriction increased thigmotaxis (EPM, OFT), gestational restriction decreased motivation (PR). |
| Crossland 2017 (41) | Mouse | C57BL6/J | Male | 8% protein (control 20%) | Pre-conception perinatal | Open field test, Elevated plus maze, Light/dark transition, Forced swim test, Tail suspension test, Fear conditioning | 8-12 weeks | Increased thigmotaxis (EPM) |
| Belluscio 2014 (40) | Mouse | CF-1 | Male and female | 9% protein (control 20%) | Perinatal | Open field test, Elevated plus maze, Tail suspension test, Cage escape | 3-9 weeks | Decreased motivation (CE), increased thigmotaxis and decreased rearing and head dipping (OFT, EPM). Increased behavioral despair in females only (TST). |
| Pillay 2016 (42) | Mouse | African Striped Mouse | Male and female | 10% protein (control 19%) | Pre-conception perinatal | Open field test | 10-11 weeks | Increased thigmotaxis (OFT) |
Diet manipulation values presented as percent of g/kg. Abbreviations: EPM=Elevated Plus Maze, OFT=Open Field Test, PR=Progressive Ratio, CE=Cage Escape, TST=Tail Suspension Test
Perinatal protein restriction beginning at conception consistently increased threat aversion and reduced passive coping behaviors in adult mouse offspring; females further exhibited increased behavioral despair (40). When perinatal protein restriction began prior to conception, mouse offspring displayed less profound behavioral alterations, elevating only thigmotaxic response (41, 42). These findings suggest that initiating protein restriction weeks prior to conception may allow maternal acclimation to the diet, potentially lessening the impact on the fetus. Additional studies in rats found that perinatal and gestational restriction similarly enhanced stress sensitivity, with females demonstrating increased risk aversion and frustrative non-reward (43). In contrast, males exhibited decreased threat avoidance with any exposure to maternal protein deficiency (44). Overall, perinatal protein restriction increased stress sensitivity and fear response in males and females, and a single study in rats suggests sex-specificity. These studies further noted increased behavioral despair in females only, consistent with higher rates of clinical depression in women (45). However, few studies examined depression-like responses in both male and female offspring, and the field would benefit from additional research to validate this finding.
Preclinical behavioral outcomes are supported by evidence that maternal protein restriction is detrimental to offspring neural functions related to the negative valence system. Two models investigated the effects of perinatal protein restriction on neurotransmitter systems important in behavioral regulation, finding that mouse offspring displayed a hyperactive dopaminergic system attributed to hypomethylation (46) and rat offspring displayed desensitization of serotonergic receptors (47). While global alterations were observed in rat offspring, including decreased brain weight and protein levels, hippocampal neurogenesis appears to be particularly disrupted by gestational or perinatal protein deficiency (48, 49). These disturbances are highly relevant to negative valence behaviors; the hippocampus is a crucial part of the limbic system and fear circuitry, and impaired hippocampal neurogenesis is a potential contributor to neuropsychiatric impairment in humans (50-52). Perinatal and gestational protein restricted rat offspring displayed evidence of impaired hippocampal development, exhibiting reduced brain-derived neurotrophic factor (BDNF) levels, brain volume, and neuron population (48, 49). Neurotrophic growth factors like BDNF and insulin-like growth factor (IGF) are important for healthy neurodevelopment (53, 54) and are decreased in mice and rats exposed to maternal protein deficiency (34, 35, 48). Preclinical evidence suggests that decreased hippocampal BDNF could reflect elevated stress hormones due to protein restriction (43, 44, 55, 56), as altered BDNF in this region is specifically associated with prenatal stress-induced methylation changes (57, 58).
Demonstrating the complexity of nutritional programming, nutrient changes due to protein restriction are not limited to amino acids. Gestational protein restriction decreased maternal lipid availability in rats, lowering fetal brain fatty acid levels and potentially contributing to the long-lasting reductions in myelin produced by early lactation protein restriction (59, 60). Clinical studies show that myelin deficits are associated with neuropsychiatric disorders, and both clinical and preclinical evidence supports the importance of sufficient brain fatty acids for neurobehavioral health (60, 61). Clearly, altering maternal protein content triggers multiple compensatory changes as the body attempts to optimize both maternal and fetal health. In addition to the aforementioned associations with offspring neural health, maternal fatty acids, steroid hormones, and growth factors regulate placental amino acid transport, alluding to the complex interrelation of mechanistic components (32). Future research expanding on the presented findings should consider these potential mechanisms and additionally investigate the impact of protein quality on offspring neurobehavioral health. Importantly, casein is the standard protein utilized in laboratory animal chow, and so is consistent with other models of dietary manipulation, such as Western-style diets.
Western-Style Diets
Considerable attention has been given to the neurobehavioral impacts of developmental exposure to highly-palatable dietary patterns. These diets are calorically dense, provide increased calories from fat, and incorporate sugar as a noteworthy source of carbohydrates. When consumed consistently, this dietary pattern, referred to as Western-style diet (WSD), produces metabolic impairments including obesity, disrupted glucose and insulin homeostasis, and altered metabolic hormones. The WSD and resulting metabolic disorders have considerable global prevalence and reviews of clinical and preclinical research demonstrate that each contribute to increased risk of offspring neuropsychiatric disorders (13-15, 62-64). Small animal models of WSD-induced obesity report alterations in maternal oocyte quality and placental function that independently influence fetal nutrient availability and neurodevelopment (65, 66). However, findings from chronic maternal WSD models are rarely able to distinguish between the effects of diet and metabolic state. A recent study in nonhuman primates showed these unique influences as perinatal WSD exposure and maternal obesity, but not maternal insulin resistance, differentially impaired offspring behavioral regulation (67). While maternal WSD models are fairly common, there is limited literature investigating the long-term effects on neurobehavioral development in the absence of maternal obesity.
To examine diet-induced changes without metabolic impairments, we focus on acute models of maternal WSD consumption: those that begin diet exposure a maximum of two weeks pre-conception and do not produce differences in maternal body weight before conception (Table 2). A rat model concluded that WSD during lactation decreased risk aversion and elevated exploratory activity at weaning, consistent with disinhibition (68). In adulthood, rat offspring exposed to lactation WSD likewise demonstrated impaired risk assessment in males, but conversely increased inhibition in males and females (69). A similar disconnect between avoidance and inhibition resulted from a perinatal WSD model in Oldfield mice, with females exhibiting increased freezing behaviors (70). Unlike typical laboratory strains, both control and WSD offspring exhibited an atypical preference for exposed areas, complicating the interpretation. Another rat model investigating sustained threat response found that WSD during lactation shortened threat evasion but did not increase immobility, suggesting altered risk aversion but not conclusively behavioral despair (71). While mouse offspring with gestational WSD exposure exhibited similarly reduced risk aversion (72), investigations of gestational and perinatal WSD exposure did not alter fear response in rats (69, 73).
Table 2.
Western-style diet.
| Source | Model | Strain/ Species |
Subject Sex |
Diet Manipulation | Diet Exposure Period |
Behavioral Assays | Testing Age |
Significant Outcomes |
|---|---|---|---|---|---|---|---|---|
| Wright 2011(69) | Rat | Wistar | Male and female | Chow and cafeteria diet: DAC 9.53g fat (2.70g control), 5.71g sucrose (control DAC 2.70g fat, 1.75g sucrose) | Gestation, Lactation, Perinatal | Open field test, Elevated plus maze | 10 weeks | Lactation WSD decreased risk aversion in males (EPM, OFT). Lactation WSD decreased activity and passive coping behaviors in males and females (OFT, EPM). |
| Janthakhin 2017 (73) | Rat | Wistar | Male | Chow: 45% energy lard fat, 17.5% energy sucrose (control 0% lard, 0% sucrose) | Perinatal | Open field test | 3-5 months | No differences. |
| Speight 2017 (68) | Rat | Wistar | Male and female | Chow and cafeteria diet: DAC 11.63g fat, 5.95g sucrose (control DAC 3.325g fat, 1.97g sucrose) | Lactation | Open field test, Elevated plus maze, Home-cage activity | 3 weeks | Decreased risk aversion and increased activity and rearing (OFT, EPM). |
| Giriko 2013 (71) | Rat | Wistar | Male | Chow: 18% ration lard fat, 2% ration sucrose (control 0% lard, 0% sucrose) | Lactation | Forced swim test, Foot-shock, Open field test (activity) | 8-14 weeks | Decreased climbing and swimming (FST) and increased aggressive response (foot-shock). |
| Johnson 2017 (70) |
Mouse | Oldfield | Male and female | Chow: 15% ration lard fat, 20% ration sugar (control 0% lard, 10% sugar) | Pre-conception perinatal |
Elevated plus maze, Voluntary wheel running, Home-cage activity | 12 weeks | All animals had increased number of entries into open arms compared to closed arms. Increased immobility in females (EPM). Decreased head dipping but increased rearing in males (EPM). Decreased activity (home-cage). |
| Ribeiro 2018 (72) |
Mouse | Swiss | Male and Female | Chow and cafeteria diet | Gestation | Light-dark transition test, Open field test (activity) | 4 weeks | Decreased risk aversion in males and females, exaggerated in males (LDT). |
Cafeteria diets are provided in addition to nutritionally complete chow and vary depending on the model, but typically are an assortment of candy and chips. Nutritional or energy intake not available from Wright 2011 so reported averages were taken from a different publication from the same group (135). Ribeiro 2018 (72) did not report nutritional values comparable to other cafeteria diet models but additional information regarding component products’ energy and nutritional content is available. Open field tests that did not consider zone differences were used to assess activity only, not threat response. DAC=daily average consumption, calculated experimentally, OFT=Open field test, EPM=Elevated plus maze, FST=Forced swim test, LDT= Light-dark transition
These results indicate that early developmental exposure to WSD, particularly during lactation in rat models, impairs risk assessment and modulates later-life stress sensitivity. The importance of this exposure window could be due to the changing nutrition requirements of neonates. Mother’s milk is extremely lipid dense, mostly in saturated fats like those elevated in WSD, suggesting that nursing offspring are more susceptible to maternal WSD effects (74). Additionally, during the early postnatal period rodents undergo important neurodevelopmental processes that, if disrupted, could be responsible for the observed perturbations in behavior (75). Rat and mouse offspring provided evidence that perinatal or lactation WSD exposure resulted in dendritic atrophy and spine instability in the amydgala, hippocampus, and prefrontal cortex (73, 76, 77), with abnormal dendritic environments implicated in various psychopathologies (78). A porcine model also demonstrated hippocampal disturbances, as perinatal WSD reduced hippocampal volume and altered neurogenic mechanisms (79, 80). The dopamine system contributes to attentional and impulse control, and several studies of perinatal WSD in rats demonstrate persistent impairments in dopamine transmission (81-83). The impairments in fear and anxiety circuits observed in animal models provide strong evidence that maternal WSD exposure disrupts neurobehavioral development in a manner highly translatable to human neuropsychiatric disorders.
There are a number of physiological pathways by which maternal WSD alters the course of offspring neurobehavioral development, including inducing neuroinflammatory response and altering the microbiotic environment (77, 79). Major confounding factors of the current WSD literature are the limited models investigating programmed WSD effects independent from maternal metabolic state and the considerable disparity in diet formulation. While this is true with any investigation of diet-derived outcomes, it is particularly pronounced in WSD models, as the experimental manipulation is designed to emulate a multi-faceted dietary pattern and not a single targeted factor. In fact, the three main aspects of the WSD (caloric density, increased fat, and increased sugar) have each been individually associated with altered offspring neurobehavioral outcomes. The following sections discuss how specific alterations in maternal carbohydrate and fat sources each independently alter offspring neurobehavioral development.
Carbohydrates
The WSD is associated with increased consumption of simple carbohydrates and sugars, typically in the form of sucrose or fructose-derived sweeteners. Sucrose is a dimer of glucose and fructose, both becoming freely available in the blood following a meal. Glucose has enhanced importance during gestation; the fetus derives 80% of its energy from glucose, with fetal blood glucose mirroring maternal fluctuations (11). Unlike glucose, fructose is not regulated by insulin and produces unique metabolic consequences as it is slowly converted to glucose by the liver. Despite the body of metabolic programming research investigating the impact of gestational diabetes on offspring risk of neuropsychiatric disorder (84), there is limited mechanistic insight regarding how glucose/insulin homeostasis or fructose influences offspring neurobehavioral development, particularly in the absence of metabolic disorders. Maintaining our focus on maternal nutrition, we identified models of moderate sugar intake without gestational diabetes. Importantly, these studies did not alter maternal weight gain or induce diabetes in offspring. To date, only one preclinical source investigated the influence of maternal sugar intake on offspring behavioral programming (Table S1).
Choi et al. (85) examined the effect of added sugar during gestation on behavior of male mice. Gestational sucrose exposure impaired risk assessment, induced hyperactivity, and decreased spontaneous alternation behavior (suggesting inattention or behavioral inflexibility). Aberrant attentive control and impulsivity were associated with altered striatal dopamine transport and receptor expression, despite normal dopaminergic neuron density. Changes in striatal dopamine function are believed to be key to ADHD pathology; although striatal dopamine transporter activity in humans is associated with trait impulsivity (86), but not conclusively with ADHD (87). Nonetheless, the outcomes from Choi et al. suggest maternal sugar consumption may contribute to the pathophysiology of dopamine-related neurobehavioral abnormalities.
Despite limited behavioral research, the few neural studies evaluating maternal sugar consumption indicate several mechanisms of impaired hippocampal neurogenesis. One group found that gestational exposure to sucrose-sweetened beverages accelerated neurodegeneration, with decreased central IGF levels in sucrose-exposed rat offspring suggesting impaired neuroprotection contributed to hippocampal atrophy (88, 89). Increased maternal plasma glucose could contribute to the uninhibited apoptosis, as preclinical models of gestational diabetes demonstrated that maternal glucose and insulin levels directly influence fetal plasma IGF and neural IGF expression in neonates (90, 91). The involvement of neurotrophic factors is supported by a rat model of elevated maternal fructose consumption, with increased histone modification suppressing BDNF production (92). Evidence of fructose-induced epigenetic modification is supported by a study in adult mice demonstrating that fructose consumption alters transcript abundance and other epigenetic controls, including DNA methylation (93). Yet another group found that perinatal fructose exposure modulated expression of several hippocampal neurosteroidgenic enzymes in rat offspring, consistent with preclinical evidence that glucocorticoids contribute to hippocampal atrophy and associated neurobehavioral impairments (94-96).
These perinatal models of increased sugar exposure indicate the importance of further fetal programming research. Despite the limited studies and differences in sugar type, disturbances in a variety of mechanistic pathways disrupted cell signaling and neurogenesis. Further study is needed concerning potential differences between maternal sucrose or fructose consumption, as each results in unique patterns of maternal glucose and insulin response, impacting placental function (97). Additionally, it is unclear how glucose and fructose differentially affect the brain, or even if fructose can cross the blood-brain-barrier. Recent clinical evidence suggests that peripheral levels of glucose determine central fructose concentrations (98), and that fructose could influence neural function by altering cerebral blood flow (99). Other aspects of carbohydrate intake, such as carbohydrate complexity and glycemic index, should be addressed in follow-up studies. Although current research is limited, preliminary evidence clearly suggests that maternal carbohydrate intake impacts offspring neurodevelopment.
Fatty Acids
The WSD is characterized by a high percentage of saturated fats (prevalent in most animal products) rather than unsaturated fats common in plants and fish. Dietary fat is a triglyceride: a macronutrient composed of three fatty acid chains that cannot traverse the placental boundary unless broken down into component fatty acids (11). Maternal fatty acid consumption determines fetal availability, and animal research indicates that diets low in protein or high in sugar alter fetal fatty acid levels (59, 97). These changes directly influence offspring brain fatty acid profiles, impacting neurodevelopment; fatty acids are utilized in the brain for myelin synthesis, membrane components, cellular signaling, and energy (100, 101). Altered fatty acid profiles are implicated in neuropsychiatric disorders (102), with specific poly-unsaturated fatty acids (PUFA) particularly significant to fetal neurodevelopment (103). Termed essential fatty acids because they must be derived from the diet, omega-3 (ω-3) and omega-6 (ω-6) PUFAs compete for access to the enzymatic pathway that produces long-chain products utilized throughout the brain (Figure 3) (104). While long-chain ω -3 and ω -6 molecules are both crucial to neural function, the ω-3 end-product has an enhanced role in neurodevelopment (104, 105). The ratio of ω-6/ω-3 is critical for brain development, and minor dietary changes in essential fatty acids can dramatically affect cerebral lipid profiles and neural function (106, 107). In excess, maternal PUFAs are associated with similar neurobehavioral phenotypes to WSD: mouse and rat offspring demonstrated impaired risk assessment and decreased hippocampal neurogenesis and synaptic transmission (108-110). Considering that current dietary practices reflect a ω-6/ω-3 ratio of 15/1 (significantly skewed in comparison to the 1/1 ratio maintained in a hunter-gatherer diet) (111), the influence of maternal fatty acids has important ramifications for offspring neurobehavioral development.
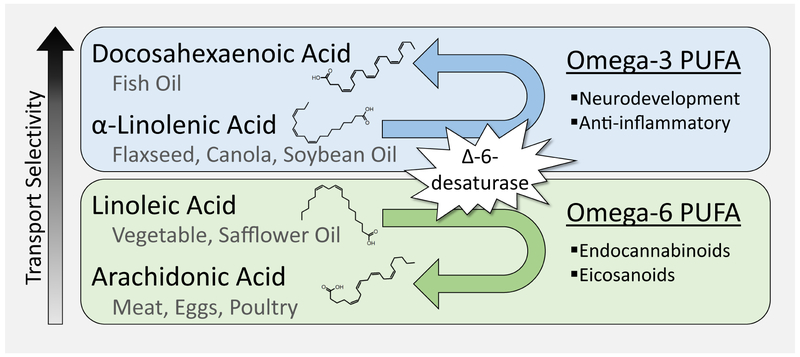
Figure 3. Essential Fatty Acid Balance.
Essential fatty acids are poly-unsaturated fatty acids (PUFA) that can only be obtained from dietary sources. These PUFA have one of the double bonds in their hydrocarbon chain located on the third or sixth carbon from the end, omega-3 (ω-3) or omega-6 (ω-6) PUFA respectively. The most basic essential fatty acids are linoleic acid (18:2 (18 carbon chain, two double bonds); ω-6) and α-linolenic acid (18:3; ω-3). Once consumed, commonly from plant-based oils, these molecules can be endogenously modified by a step-wise pathway of desaturase enzymes which convert them into long-chain molecules: arachidonic acid (20:4; ω-6) and docosahexaenoic acid (22:6; ω-3). Linoleic and α-linolenic acids compete for access to these enzymes, meaning excess of either contributes to a relative deficiency of the opposing long-chain product. Arachidonic and docosahexaenoic acids have unique neurodevelopmental functions and can also be obtained directly from diet. PUFAs can cross both the placenta and the blood-brain-barrier via gradient-dependent diffusion and active transport. The relative abundance of these nutrients in fetal blood and in cerebrospinal fluid suggest that essential fatty acids can be preferentially transported in a selective order: docosahexaenoic acid> α-linolenic acid>linoleic acid>arachidonic acid. The exact controls and implications of this transport selectivity are uncertain; however, it is clear that dietary maternal fatty acid imbalance has important ramifications for fetal essential fatty acid availability and neural function.
In an effort to simulate how essential fatty acids are altered in human diets, animal models swap fat sources with moderate levels of ω-3 PUFAs (like canola or flaxseed oils) for low ω-3, high ω-6 fat sources (like safflower or sunflower oils), producing experimental diets that are equally calorie and lipid dense (Table 3). Animal studies commonly examine a high ω-6/ω-3 ratio that results in a relative shortage of ω-3 PUFA, reflecting the trend of ω-3 deficient foods in current dietary patterns. Adult mice with perinatal exposure to high maternal ω-6/ω-3 consistently exhibited increased thigmotaxic, risk aversion behaviors (112-114). Rat offspring also displayed elevated anxiety-like behaviors, and further showed that high maternal ω-6/ω-3 exaggerated physiological stress response and increased behavioral despair (115). Although these studies demonstrated increased brain ω-6/ω-3 ratio during fetal and early postnatal development, the observed behavioral phenotypes in adulthood were not accompanied by long-term changes in brain essential fatty acid availability (113-115).
Table 3.
Fatty Acids.
| Source | Model | Strain/ Species |
Subject Sex |
Diet Manipulation | Diet Exposure Period |
Behavioral Assays | Testing Age |
Significant Outcomes |
|---|---|---|---|---|---|---|---|---|
| High ω-6/ω-3 Models | ||||||||
| Jones 2013 (112) | Mouse | C57BL/6J | Male and Female | Safflower oil, 51.3/1 (control: soybean oil, 6.9:1) | Pre-conception perinatal |
Elevated plus maze, Open field test (activity) |
8-9 weeks | Increased risk aversion (EPM). |
| Sakayori 2016a (113) |
Mouse | C57BL/6N | Male and Female | Safflower oil, 74.4/0.3 (control: canola oil, 2.2/1) | Pre-conception perinatal |
Open field test, Elevated plus maze |
13-15 weeks |
Increased thigmotaxis and risk aversion (OFT, EPM). |
| Sakayori 2016b (114) |
Mouse | C57BL/6N | Male and Female | Safflower oil, 74.4/0.3 (control: canola oil, 2.2/1) | Pre-conception perinatal |
Open field test, Elevated plus maze, Forced swim test | 13-15 weeks |
Increased thigmotaxis (OFT, EPM). Further increased risk aversion in males (OFT). Increased activity in females (EPM). |
| Chen 2013 (115) | Rat | Sprague- Dawley |
Male | Sunflower oil, 61/0 (control: sunflower plus fish oil, 2.6/1) | Perinatal | Elevated plus maze, Forced swim test | 10 weeks | Increased thigmotaxis (EPM) and behavioral despair (FST). |
| Supplement Models | ||||||||
| Roversi 2016 (134) |
Rat | Wistar | Male and female | 3 g/kg daily gavage: Hydrogenated vegetable fat, water | Pre-conception perinatal |
Elevated plus maze | 6-7 weeks | Decreased open arm time and head dipping (EPM). |
| Pase 2017 (133) | Rat | Wistar | Male | 3 g/kg daily gavage: Hydrogenated vegetable fat, soybean/fish oil mix (control) | Gestation, Lactation |
Novel object recognition, Y-maze | 12 weeks | Any HVF reduced novelty preference, with lactation period HVF showed long-term novelty aversion (NOR). Any HVF decreased spontaneous alternations (Y-maze). |
| Ferraz 2008 (130) |
Rat | Wistar | Male | 3 g/kg daily gavage: Coconut fat, fish oil, no supplement (control) | Pre-conception perinatal |
Open field test, Elevated plus maze, Forced swim test | 15 weeks | Fish oil decreased immobility time (FST). |
High ω-6/ω-3 models elevate ω-6 at the expense of ω-3, producing relative ω-3 deficiency. Diet manipulations indicate the source of fat used to generate the experimental ω-6/ω-3 ratios listed. These sources used different oils to modify the ratio except for Chen 2013, which supplemented the deficient diet with fish oil to alter essential fatty acid ratio. Supplement models provided animals with nutritionally complete chow, and thus did not examine ω-3 deficiency. Supplements were isocaloric and normolipidic except for water controls, which led for Pase 2017 to use a combination soybean/fish oil supplement as a control. Abbreviations: HVF=Hydrogenated vegetable fat, OFT= Open field test; EPM= Elevated plus maze, FST= Forced swim test.
Despite normal brain lipid profiles, perinatal fatty acid exposure impacts long-term neural function. Studies investigating the effect of essential fatty acid rehabilitation during different developmental stages found that altered maternal ω-6/ω-3 ratio through lactation impaired dopamine and serotonin release, reduced myelin yield, and delayed brain growth in adult mouse and rat offspring (116-119). Evidence from the perinatal period demonstrates that these neural processes, as well as hippocampal development and microglia activation, are already disrupted before weaning (120-125). Pre-weaning examinations additionally show ω-6 PUFA alterations impair hippocampal neuroplasticity via altered endocannabinoid signaling and glucocorticoid inhibition (126, 127). This literature suggests that the timing of altered brain lipids during critical periods of development could contribute to neurobehavioral impairments later in life. However, a second interpretation is possible: the observed neural impairments in adulthood are not due to programmed changes in brain development, but rather the length of time between essential fatty acid rehabilitation and neurobehavioral assessment. Brain ω-3 PUFA levels take about eight weeks to normalize after diet rehabilitation (128), and the lack of sustained neural impairment in early interventions is potentially due to the extended recuperation period (116, 129). Although there is a wealth of potential mechanisms for fatty acid programming, few behavioral or neural outcomes have been examined more than eight weeks after diet intervention, highlighting an important future direction for fatty acid and nutritional programming research.
Supportive evidence of PUFA programming is provided by models of fatty acid supplementation to nutritionally complete chow. Perinatal exposure to fish oil supplement decreased behavioral despair in adult rat offspring (130, 131), reflecting clinical interest in associations between depression and ω-3 PUFAs (132). Non-essential fatty acids also present strong evidence of lasting neurobehavioral impairment. Maternal supplement with hydrogenated vegetable fats, like those found in margarine, induced stress sensitivity and behavioral inflexibility, decreased hippocampal plasticity factors, and increased neuroinflammation at the detriment of cellular function in adult rat offspring (133, 134). Strikingly, rats exposed to hydrogenated fat during gestation or lactation exhibited decreased hippocampal ω-3 PUFA nine weeks after diet intervention (133). The long-term depletion of ω-3 PUFA with maternal hydrogenated fat exposure could be due to interference caused by the presence of trans fats in offspring neural lipid profile. Trans fats are essentially nonexistent in natural food sources, and it is possible that developing brains have an impaired ability to accommodate this unusual lipid form. By extension, it follows that the influence of fatty acid availability on offspring behavioral programming is moderated by the capacity of the developing brain to efficiently optimize neural lipid content. The observed effects of maternal ω-3 deficiency on offspring negative valence behaviors could be compounded when altered fatty acid availability is accompanied by protein deficiency, calorie density, and increased sugar.
Conclusion
Current animal literature supports the programming effect of maternal nutrition on offspring negative valence behaviors. Maternal protein deficiency and fatty acid manipulation exaggerated fear response, with exposure to sustained threat inducing behavioral despair in a potentially sex-dependent manner. Alternatively, WSD exposure during the lactation period impaired risk assessment and response. Furthermore, a model of elevated gestational sugar impaired attention and impulse control. These behavioral alterations are supported by long-term disruptions in neural processes associated with neuropsychiatric disorders. Perinatal nutritional programming resulted in persistent impairments in synaptic plasticity and neurotransmitter systems as well as neurogenic, apoptotic, and brain growth anomalies. Reported outcomes were observed after macronutrient supply was normalized, suggesting that nutrition during critical periods of perinatal development contributes to programming of offspring neurobehavioral impairment.
The strong foundation of literature supports continued investigation of nutritional programming mechanisms. To date, the manner by which maternal macronutrients trigger neurobehavioral abnormalities is under-studied, though evidence from each macronutrient model implicates altered placental function. Importantly, the placenta is regulated by many factors modified by diet, including nutrient availability, maternal stress response, inflammation, and offspring growth factors. Initiating diet manipulation immediately prior to conception or cross-fostering offspring are strengths of animal models and useful in limiting potentially confounding metabolic effects. Current limitations of animal models, including the inconsistency of diet formulations and use of physiologically irrelevant nutritional values, can be improved to enhance translatability. To date, a significant portion of animal studies have exclusively investigated the hippocampus. Examining other brain regions will help generate a more holistic understanding of observed neurobehavioral impairments and increase relevance to human psychopathology. These translational efforts can be further enhanced by increased use of animal models with more complex behavioral phenotypes and similar developmental ontogeny and neuroanatomy to humans. While the presented literature included a near-balanced mix of male and female offspring, sex was often not considered in statistical analysis and future studies should investigate the extensive contributions of sex to neurobehavioral outcomes. Altogether, the presented literature has paved the way for focused, future research to identify the contribution of maternal nutrition to offspring neuropsychiatric risk.
Supplementary Material
Acknowledgements
This publication was supported by grant number R01 MH107508R01 (ES) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to acknowledge Michael Gallagher of subQstudio for his work in creating Figure 1.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, et al. (2007): School readiness and later achievement. Developmental psychology. 43:1428–1446. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 3.Pelham WE, Foster EM, Robb JA (2007): The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 7:121–131. [DOI] [PubMed] [Google Scholar]
- 4.Breslau J, Miller E, Breslau N, Bohnert K, Lucia V, Schweitzer J (2009): The impact of early behavior disturbances on academic achievement in high school. Pediatrics. 123:1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jester JM, Nigg JT, Buu A, Puttler LI, Glass JM, Heitzeg MM, et al. (2008): Trajectories of childhood aggression and inattention/hyperactivity: differential effects on substance abuse in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 47:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB (2007): Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 20:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. (2010): Early Life Programming and Neurodevelopmental Disorders. Biological Psychiatry. 68:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon EJ, Kim YJ (2017): What is fetal programming?: a lifetime health is under the control of in utero health. Obstetrics & Gynecology Science. 60:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgieff MK (2007): Nutrition and the developing brain: nutrient priorities and measurement. The American Journal of Clinical Nutrition. 85:614S–620S. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JL, Regnault TRH (2016): Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients. 8:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao PNS, Shashidhar A, Ashok C (2013): In utero fuel homeostasis: Lessons for a clinician. Indian Journal of Endocrinology and Metabolism. 17:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA (2011): Obesity and the placenta: A consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta. 32:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. (2012): Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics. 129:e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera HM, Christiansen KJ, Sullivan EL (2015): The role of maternal obesity in the risk of neuropsychiatric disorders. Frontiers in Neuroscience. 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Lieshout RJ, Voruganti LP (2008): Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. Journal of Psychiatry & Neuroscience : JPN. 33:395–404. [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy B, Finlay BL, Darlington RB, Anand KJS (2007): Extrapolating brain development from experimental species to humans. NeuroToxicology. 28:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice D, Barone S (2000): Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives. 108:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderzhanova E, Kirmeier T, Wotjak CT (2017): Animal models in psychiatric research: The RDoC system as a new framework for endophenotype-oriented translational neuroscience. Neurobiology of Stress. 7:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalueff AV, Ren-Patterson RF, LaPorte JL, Murphy DL (2008): Domain interplay concept in animal models of neuropsychiatric disorders: A new strategy for high-throughput neurophenotyping research. Behavioural Brain Research. 188:243–249. [DOI] [PubMed] [Google Scholar]
- 20.Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, et al. (2006): Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 60:991–997. [DOI] [PubMed] [Google Scholar]
- 21.Shaw P, Stringaris A, Nigg J, Leibenluft E (2014): Emotion dysregulation in attention deficit hyperactivity disorder. The American Journal of Psychiatry. 171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler EJ, Hyman SE (2010): Animal models of neuropsychiatric disorders. Nature Neuroscience. 13:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homberg JR, Kyzar EJ, Nguyen M, Norton WH, Pittman J, Poudel MK, et al. (2016): Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neuroscience & Biobehavioral Reviews. 65:292–312. [DOI] [PubMed] [Google Scholar]
- 24.LeDoux JE, Pine DS (2016): Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. American Journal of Psychiatry. 173:1083–1093. [DOI] [PubMed] [Google Scholar]
- 25.Simon P, Dupuis R, Costentin J (1994): Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behavioural Brain Research. 61:59–64. [DOI] [PubMed] [Google Scholar]
- 26.Walf AA, Frye CA (2007): The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filgueiras GB, Carvalho-Netto EF, Estanislau C (2014): Aversion in the elevated plus-maze: role of visual and tactile cues. Behav Processes. 107:106–111. [DOI] [PubMed] [Google Scholar]
- 28.Sorregotti T, Cipriano AC, Cruz FC, Mascarenhas DC, Rodgers RJ, Nunes-de-Souza RL (2018): Amygdaloid involvement in the defensive behavior of mice exposed to the open elevated plus-maze. Behavioural Brain Research. 338:159–165. [DOI] [PubMed] [Google Scholar]
- 29.Sorregotti T, Mendes-Gomes J, Rico JL, Rodgers RJ, Nunes-de-Souza RL (2013): Ethopharmacological analysis of the open elevated plus-maze in mice. Behavioural Brain Research. 246:76–85. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann SG, Ellard KK, Siegle GJ (2012): Neurobiological Correlates of Cognitions in Fear and Anxiety: A Cognitive-Neurobiological Information Processing Model. Cognition & emotion. 26:282–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman HR (1999): Amino Acid and Protein Requirements: Cognitive Performance, Stress, and Brain Function. In: Research IoMUCoMN, editor The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. Washington DC: National Academies Press (US). [PubMed] [Google Scholar]
- 32.Vaughan OR, Rosario FJ, Powell TL, Jansson T (2017): Chapter Eight - Regulation of Placental Amino Acid Transport and Fetal Growth In: Huckle WR, editor. Progress in Molecular Biology and Translational Science: Academic Press, pp 217–251. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez PN, Gasperowicz M, Barbeito-Andrés J, Klenin N, Cross JC, Hallgrímsson B (2016): Chronic Protein Restriction in Mice Impacts Placental Function and Maternal Body Weight before Fetal Growth. PLoS ONE. 11:e0152227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao H, Sathishkumar KR, Yallampalli U, Balakrishnan M, Li X, Wu G, et al. (2012): Maternal protein restriction regulates IGF2 system in placental labyrinth. Frontiers in bioscience (Elite edition). 4:1434–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiaratti MR, Malik S, Diot A, Rapa E, Macleod L, Morten K, et al. (2015): Is Placental Mitochondrial Function a Regulator that Matches Fetal and Placental Growth to Maternal Nutrient Intake in the Mouse? PLoS ONE. 10:e0130631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Pond WG, Ott T, Bazer FW (1998): Maternal Dietary Protein Deficiency Decreases Amino Acid Concentrations in Fetal Plasma and Allantoic Fluid of Pigs. The Journal of Nutrition. 128:894–902. [DOI] [PubMed] [Google Scholar]
- 37.Rees WD, Hay SM, Buchan V, Antipatis C, Palmer RM (1999): The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. British Journal of Nutrition. 81:243–250. [PubMed] [Google Scholar]
- 38.Semba RD (2016): The rise and fall of protein malnutrition in global health. Annals of nutrition & metabolism. 69:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akyol A, Cetin AK, Gulec A, Dasgin H, Ayaz A, Onbasilar I (2018): Maternal low-quality protein diet exerts sex-specific effects on plasma amino acid profile and alters hepatic expression of methyltransferases in adult rat offspring. Journal of Developmental Origins of Health and Disease. 1–8. [DOI] [PubMed] [Google Scholar]
- 40.Belluscio LM, Berardino BG, Ferroni NM, Ceruti JM, Cánepa ET (2014): Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiology & Behavior. 129:237–254. [DOI] [PubMed] [Google Scholar]
- 41.Crossland RF, Balasa A, Ramakrishnan R, Mahadevan SK, Fiorotto ML, Van den Veyver IB (2017): Chronic Maternal Low Protein Diet in Mice Affects Anxiety, Night-Time Energy Expenditure and Sleep Patterns, but Not Circadian Rhythm in Male Offspring. PLOS ONE. 12:e0170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillay N, Rimbach R, Rymer T (2016): Pre- and postnatal dietary protein deficiency influences anxiety, memory and social behaviour in the African striped mouse Rhabdomys dilectus chakae. Physiology & Behavior. 161:38–46. [DOI] [PubMed] [Google Scholar]
- 43.Reyes-Castro LA, Rodriguez JS, Charco R, Bautista CJ, Larrea F, Nathanielsz PW, et al. (2012): Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. International Journal of Developmental Neuroscience. 30:39–45. [DOI] [PubMed] [Google Scholar]
- 44.Reyes-Castro LA, Rodriguez JS, Rodriguez-González GL, Chavira R, Bautista CJ, McDonald TJ, et al. (2012): Pre- and/or postnatal protein restriction developmentally programs affect and risk assessment behaviors in adult male rats. Behavioural Brain Research. 227:324–329. [DOI] [PubMed] [Google Scholar]
- 45.van Loo HM, Aggen SH, Gardner CO, Kendler KS (2017): Sex similarities and differences in risk factors for recurrence of major depression. Psychological Medicine.1–9. [DOI] [PubMed] [Google Scholar]
- 46.Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, et al. (2010): Early life protein restriction alters dopamine circuitry. Neuroscience. 168:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes de Souza S, Orozco-Solis R, Grit I, Manhaes de Castro R, Bolaños-Jiménez F (2008): Perinatal protein restriction reduces the inhibitory action of serotonin on food intake. European Journal of Neuroscience. 27:1400–1408. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Xu RJ (2007): The effects of perinatal protein malnutrition on spatial learning and memory behaviour and brain-derived neurotrophic factor concentration in the brain tissue in young rats. Asia Pacific journal of clinical nutrition. 16 Suppl 1:467–472. [PubMed] [Google Scholar]
- 49.Lister James P, Blatt Gene J, DeBassio William A, Kemper Thomas L, Tonkiss J, Galler Janina R, et al. (2005): Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 15:393–403. [DOI] [PubMed] [Google Scholar]
- 50.Tovote P, Fadok JP, Lüthi A (2015): Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 16:317. [DOI] [PubMed] [Google Scholar]
- 51.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ (2012): Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 76:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeCarolis NA, Eisch AJ (2010): Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology. 58:884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anlar B, Sullivan KA, Feldman EL (1999): Insulin-Like Growth Factor-I and Central Nervous System Development. Horm Metab Res. 31:120–125. [DOI] [PubMed] [Google Scholar]
- 54.Castrén E (2014): Neurotrophins and Psychiatric Disorders In: Lewin GR, Carter BD, editors. Neurotrophic Factors. Berlin, Heidelberg: Springer Berlin Heidelberg, pp 461–479. [Google Scholar]
- 55.Guzmán C, Cabrera R, Cárdenas M, Larrea F, Nathanielsz PW, Zambrano E (2006): Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. The Journal of Physiology. 572:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CRW, Jackson AA, et al. (1996): Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 17:169–172. [DOI] [PubMed] [Google Scholar]
- 57.Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Zare Mehrjerdi F, Karimi M (2017): Effect of Maternal Stress Prior to Conception on Hippocampal BDNF Signaling in Rat Offspring. Molecular Neurobiology. 54:6436–6445. [DOI] [PubMed] [Google Scholar]
- 58.Boersma GJ, Lee RS, Cordner ZA, Ewald ER, Purcell RH, Moghadam AA, et al. (2014): Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics. 9:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torres N, Bautista CJ, Tovar AR, Ordáz G, Rodríguez-Cruz M, Ortiz V, et al. (2010): Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. American Journal of Physiology - Endocrinology and Metabolism. 298:E270–E277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montanha-Rojas EA, Ferreira AA, Tenório F, Barradas PC (2005): Myelin basic protein accumulation is impaired in a model of protein deficiency during development. Nutritional Neuroscience. 8:49–56. [DOI] [PubMed] [Google Scholar]
- 61.Fields RD (2008): White matter in learning, cognition and psychiatric disorders. Trends in neurosciences. 31:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker KD, Loughman A, Spencer SJ, Reichelt AC (2017): The impact of obesity and hypercaloric diet consumption on anxiety and emotional behavior across the lifespan. Neuroscience & Biobehavioral Reviews. 83:173–182. [DOI] [PubMed] [Google Scholar]
- 63.Contu L, Hawkes CA (2017): A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. International Journal of Molecular Sciences. 18:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan EL, Nousen EK, Chamlou KA (2014): Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiology & Behavior. 123:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. (2012): High Fat Diet Induced Developmental Defects in the Mouse: Oocyte Meiotic Aneuploidy and Fetal Growth Retardation/Brain Defects. PLOS ONE. 7:e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T (2008): High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. The FASEB Journal. 23:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson JR, Gustafsson HC, DeCapo M, Takahashi DL, Bagley JL, Dean TA, et al. (2018): Maternal Diet, Metabolic State, and Inflammatory Response Exert Unique and Long-Lasting Influences on Offspring Behavior in Non-Human Primates. Frontiers in Endocrinology. 9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Speight A, Davey WG, McKenna E, Voigt J-PW (2017): Exposure to a maternal cafeteria diet changes open-field behaviour in the developing offspring. International Journal of Developmental Neuroscience. 57:34–40. [DOI] [PubMed] [Google Scholar]
- 69.Wright T, Langley-Evans SC, Voigt J-P (2011): The impact of maternal cafeteria diet on anxiety-related behaviour and exploration in the offspring. Physiology & Behavior. 103:164–172. [DOI] [PubMed] [Google Scholar]
- 70.Johnson SA, Javurek AB, Painter MS, Murphy CR, Conard CM, Gant KL, et al. (2017): Effects of a maternal high-fat diet on offspring behavioral and metabolic parameters in a rodent model. Journal of Developmental Origins of Health and Disease. 8:75–88. [DOI] [PubMed] [Google Scholar]
- 71.Giriko CÁ, Andreoli CA, Mennitti LV, Hosoume LF, Souto TdS, Silva AVd, et al. (2013): Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. International Journal of Developmental Neuroscience. 31:731–739. [DOI] [PubMed] [Google Scholar]
- 72.Ribeiro ACAF, Batista TH, Veronesi VB, Giusti-Paiva A, Vilela FC (2018): Cafeteria diet during the gestation period programs developmental and behavioral courses in the offspring. International Journal of Developmental Neuroscience. 68:45–52. [DOI] [PubMed] [Google Scholar]
- 73.Janthakhin Y, Rincel M, Costa A-M, Darnaudéry M, Ferreira G (2017): Maternal high-fat diet leads to hippocampal and amygdala dendritic remodeling in adult male offspring. Psychoneuroendocrinology. 83:49–57. [DOI] [PubMed] [Google Scholar]
- 74.Uauy R, Castillo C (2003): Lipid Requirements of Infants: Implications for Nutrient Composition of Fortified Complementary Foods. The Journal of Nutrition. 133:2962S–2972S. [DOI] [PubMed] [Google Scholar]
- 75.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013): Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 0:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rincel M, Lépinay AL, Janthakhin Y, Soudain G, Yvon S, Da Silva S, et al. (2018): Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Structure and Function. 223:883–895. [DOI] [PubMed] [Google Scholar]
- 77.Hatanaka Y, Wada K, Kabuta T (2016): Maternal high-fat diet leads to persistent synaptic instability in mouse offspring via oxidative stress during lactation. Neurochemistry International. 97:99–108. [DOI] [PubMed] [Google Scholar]
- 78.Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM (2011): Dendritic spine pathology in neuropsychiatric disorders. Nature Neuroscience. 14:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Val-Laillet D, Besson M, Guérin S, Coquery N, Randuineau G, Kanzari A, et al. (2017): A maternal Western diet during gestation and lactation modifies offspring’s microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. The FASEB Journal. 31:2037–2049. [DOI] [PubMed] [Google Scholar]
- 80.Clouard C, Gerrits WJJ, Kemp B, Val-Laillet D, Bolhuis JE (2016): Perinatal Exposure to a Diet High in Saturated Fat, Refined Sugar and Cholesterol Affects Behaviour, Growth, and Feed Intake in Weaned Piglets. PLoS ONE. 11:e0154698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romaní-Pérez M, Lépinay AL, Alonso L, Rincel M, Xia L, Fanet H, et al. (2016): Impact of perinatal exposure to high-fat diet and stress on responses to nutritional challenges, food-motivated behaviour and mesolimbic dopamine function. International Journal Of Obesity. 41:502. [DOI] [PubMed] [Google Scholar]
- 82.Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker C-D (2008): Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology. 197:83–94. [DOI] [PubMed] [Google Scholar]
- 83.Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD (2011): Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 176:225–236. [DOI] [PubMed] [Google Scholar]
- 84.Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, et al. (2016): Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. American Journal of Obstetrics and Gynecology. 215:380.e381–380.e387. [DOI] [PubMed] [Google Scholar]
- 85.Choi CS, Kim P, Park JH, Gonzales ELT, Kim KC, Cho KS, et al. (2015): High sucrose consumption during pregnancy induced ADHD-like behavioral phenotypes in mice offspring. The Journal of Nutritional Biochemistry. 26:1520–1526. [DOI] [PubMed] [Google Scholar]
- 86.Costa A, la Fougère C, Pogarell O, Möller H-J, Riedel M, Ettinger U (2013): Impulsivity is related to striatal dopamine transporter availability in healthy males. Psychiatry Research: Neuroimaging. 211:251–256. [DOI] [PubMed] [Google Scholar]
- 87.Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U (2012): Striatal Dopamine Transporter Alterations in ADHD: Pathophysiology or Adaptation to Psychostimulants? A Meta-Analysis. American Journal of Psychiatry. 169:264–272. [DOI] [PubMed] [Google Scholar]
- 88.Kuang H, Sun M, Lv J, Li J, Wu C, Chen N, et al. (2014): Hippocampal apoptosis involved in learning deficits in the offspring exposed to maternal high sucrose diets. The Journal of Nutritional Biochemistry. 25:985–990. [DOI] [PubMed] [Google Scholar]
- 89.He A, Zhang Y, Yang Y, Li L, Feng X, Wei B, et al. (2017): Prenatal high sucrose intake affected learning and memory of aged rat offspring with abnormal oxidative stress and NMDARs/Wnt signaling in the hippocampus. Brain Research. 1669:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oliver MH, Harding JE, Breier BH, Gluckman PD (1996): Fetal insulin-like growth factor (IGF)-I and IGF-II are regulated differently by glucose or insulin in the sheep fetus. Reproduction, Fertility and Development. 8:167–172. [DOI] [PubMed] [Google Scholar]
- 91.Haghir H, Rezaee A-A-R, Sankian M, Kheradmand H, Hami J (2013): The effects of induced type-I diabetes on developmental regulation of insulin & insulin like growth factor-1 (IGF-1) receptors in the cerebellum of rat neonates. Metabolic Brain Disease. 28:397–410. [DOI] [PubMed] [Google Scholar]
- 92.Wu KLH, Wu C-W, Tain Y-L, Huang L-T, Chao Y-M, Hung C-Y, et al. (2016): Environmental stimulation rescues maternal high fructose intake-impaired learning and memory in female offspring: Its correlation with redistribution of histone deacetylase 4. Neurobiology of Learning and Memory. 130:105–117. [DOI] [PubMed] [Google Scholar]
- 93.Meng Q, Ying Z, Noble E, Zhao Y, Agrawal R, Mikhail A, et al. (2016): Systems Nutrigenomics Reveals Brain Gene Networks Linking Metabolic and Brain Disorders. EBioMedicine. 7:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohashi K, Ando Y, Munetsuna E, Yamada H, Yamazaki M, Nagura A, et al. (2015): Maternal fructose consumption alters messenger RNA expression of hippocampal StAR, PBR, P450(1β), 11β-HSD, and 17β-HSD in rat offspring. Nutrition Research. 35:259–264. [DOI] [PubMed] [Google Scholar]
- 95.Mizuno G, Munetsuna E, Yamada H, Ando Y, Yamazaki M, Murase Y, et al. (2017): Fructose intake during gestation and lactation differentially affects the expression of hippocampal neurosteroidogenic enzymes in rat offspring. Endocrine Research. 42:71–77. [DOI] [PubMed] [Google Scholar]
- 96.Sapolsky RM (2000): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 57:925–935. [DOI] [PubMed] [Google Scholar]
- 97.Asghar ZA, Thompson A, Chi M, Cusumano A, Scheaffer S, Al-Hammadi N, et al. (2016): Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Scientific Reports. 6:25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hwang JJ, Jiang L, Hamza M, Dai F, Belfort-DeAguiar R, Cline G, et al. (2017): The human brain produces fructose from glucose. JCI Insight. 2:e90508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Page KA, Chan O, Arora J, Belfort-DeAguiar R, Dzuira J, Roehmholdt B, et al. (2013): Effects of Fructose vs Glucose on Regional Cerebral Blood Flow in Brain Regions Involved With Appetite and Reward Pathways. JAMA : the journal of the American Medical Association. 309:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sastry PS (1985): Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 24:69–176. [DOI] [PubMed] [Google Scholar]
- 101.Romano A, Koczwara JB, Gallelli CA, Vergara D, Micioni Di Bonaventura MV, Gaetani S, et al. (2017): Fats for thoughts: An update on brain fatty acid metabolism. The International Journal of Biochemistry & Cell Biology. 84:40–45. [DOI] [PubMed] [Google Scholar]
- 102.Genevieve Y, Julie C (2005): Omega-3 fatty acids and neuropsychiatric disorders. Reprod Nutr Dev. 45:1–28. [DOI] [PubMed] [Google Scholar]
- 103.Brenna JT (2011): Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life. Maternal & Child Nutrition. 7:59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME (2015): Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Research. 1597:220–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bernardi JR, Escobar RdS, Ferreira CF, Silveira PP (2012): Fetal and Neonatal Levels of Omega-3: Effects on Neurodevelopment, Nutrition, and Growth. The Scientific World Journal. 2012:202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simopoulos A (2002): The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine and Pharmacotherapy. 56:365–379. [DOI] [PubMed] [Google Scholar]
- 107.Clandinin MT, Jumpsen J (1997): Fatty Acid Metabolism in Brain in Relation to Development, Membrane Structure, and Signaling In: Yehuda S, Mostofsky DI, editors. Handbook of Essential Fatty Acid Biology: Biochemistry, Physiology, and Behavioral Neurobiology. Totowa, NJ: Humana Press, pp 15–65. [Google Scholar]
- 108.Raygada M, Cho E, Hilakivi-Clarke L (1998): High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings' aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. Journal of Nutrition. 128:2505–2511. [DOI] [PubMed] [Google Scholar]
- 109.Sussman D, Germann J, Henkelman M (2014): Gestational ketogenic diet programs brain structure and susceptibility to depression & anxiety in the adult mouse offspring. Brain and Behavior. 5:e00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rincel M, Lépinay AL, Delage P, Fioramonti J, Théodorou VS, Layé S, et al. (2016): Maternal high-fat diet prevents developmental programming by early-life stress. Translational Psychiatry. 6:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simopoulos AP, Faergeman O, Bourne PG (2011): Action plan for a healthy agriculture, healthy nutrition, healthy people. World Rev Nutr Diet. 102:1–5. [DOI] [PubMed] [Google Scholar]
- 112.Jones KL, Will MJ, Hecht PM, Parker CL, Beversdorf DQ (2013): Maternal diet rich in omega-6 polyunsaturated fatty acids during gestation and lactation produces autistic-like sociability deficits in adult offspring. Behav Brain Res. 238:193–199. [DOI] [PubMed] [Google Scholar]
- 113.Sakayori N, Kikkawa T, Tokuda H, Kiryu E, Yoshizaki K, Kawashima H, et al. (2016): Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells. 34:470–482. [DOI] [PubMed] [Google Scholar]
- 114.Sakayori N, Tokuda H, Yoshizaki K, Kawashima H, Innis SM, Shibata H, et al. (2016): Maternal Nutritional Imbalance between Linoleic Acid and Alpha-Linolenic Acid Increases Offspring's Anxious Behavior with a Sex-Dependent Manner in Mice. Tohoku J Exp Med. 240:31–37. [DOI] [PubMed] [Google Scholar]
- 115.Chen HF, Su HM (2013): Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J Nutr Biochem. 24:70–80. [DOI] [PubMed] [Google Scholar]
- 116.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S (2002): Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. Journal of Lipid Research. 43:1209–1219. [PubMed] [Google Scholar]
- 117.Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, et al. (2004): Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. Journal of Neurochemistry. 89:695–702. [DOI] [PubMed] [Google Scholar]
- 118.Berkow SE, Campagnoni AT (1983): Essential Fatty Acid Deficiency: Effects of Cross-Fostering Mice at Birth on Myelin Levels and Composition. The Journal of Nutrition. 113:582–591. [DOI] [PubMed] [Google Scholar]
- 119.Berkow SE, Campagnoni AT (1981): Essential Fatty Acid Deficiency: Effects of Cross-Fostering Mice at Birth on Brain Growth and Myelination. The Journal of Nutrition. 111:886–894. [DOI] [PubMed] [Google Scholar]
- 120.Coti Bertrand P, O'Kusky JR, Innis SM (2006): Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 136:1570–1575. [DOI] [PubMed] [Google Scholar]
- 121.McKenna MC, Campagnoni AT (1979): Effect of Pre- and Postnatal Essential Fatty Acid Deficiency on Brain Development and Myelination. The Journal of Nutrition. 109:1195–1204. [DOI] [PubMed] [Google Scholar]
- 122.Innis SM, de la Presa Owens S (2001): Dietary Fatty Acid Composition in Pregnancy Alters Neurite Membrane Fatty Acids and Dopamine in Newborn Rat Brain. The Journal of Nutrition. 131:118–122. [DOI] [PubMed] [Google Scholar]
- 123.Kuperstein F, Yakubov E, Dinerman P, Gil S, Eylam R, Salem N, et al. (2005): Overexpression of dopamine receptor genes and their products in the postnatal rat brain following maternal n-3 fatty acid dietary deficiency. Journal of Neurochemistry. 95:1550–1562. [DOI] [PubMed] [Google Scholar]
- 124.Kuperstein F, Eilam R, Yavin E (2008): Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. Journal of Neurochemistry. 106:662–671. [DOI] [PubMed] [Google Scholar]
- 125.Fan C, Sun W, Fu H, Dong H, Xia L, Lu Y, et al. (2015): Dietary ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy affect hippocampal neurogenesis and apoptosis in mouse offspring. Nutr Hosp. 32:1170–1179. [DOI] [PubMed] [Google Scholar]
- 126.Thomazeau A, Bosch-Bouju C, Manzoni O, Layé S (2017): Nutritional n-3 PUFA Deficiency Abolishes Endocannabinoid Gating of Hippocampal Long-Term Potentiation. Cerebral Cortex. 27:2571–2579. [DOI] [PubMed] [Google Scholar]
- 127.Dinel AL, Rey C, Bonhomme C, Le Ruyet P, Joffre C, Layé S (2016): Dairy fat blend improves brain DHA and neuroplasticity and regulates corticosterone in mice. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). 109:29–38. [DOI] [PubMed] [Google Scholar]
- 128.Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N (2001): Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. Journal of Lipid Research. 42:419–427. [PubMed] [Google Scholar]
- 129.Lozada LE, Desai A, Kevala K, Lee JW, Kim HY (2017): Perinatal Brain Docosahexaenoic Acid Concentration Has a Lasting Impact on Cognition in Mice. J Nutr. 147:1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferraz AC, Kiss A, Araújo RL, Salles HM, Naliwaiko K, Pamplona J, et al. (2008): The antidepressant role of dietary long-chain polyunsaturated n-3 fatty acids in two phases in the developing brain. Prostaglandins Leukot Essent Fatty Acids. 78:183–188. [DOI] [PubMed] [Google Scholar]
- 131.McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford KE, et al. (2007): Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Postmortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biological Psychiatry. 62:17–24. [DOI] [PubMed] [Google Scholar]
- 132.Wani AL, Bhat SA, Ara A (2015): Omega-3 fatty acids and the treatment of depression: a review of scientific evidence. Integrative Medicine Research. 4:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pase CS, Roversi K, Roversi K, Vey LT, Dias VT, Veit JC, et al. (2017): Maternal trans fat intake during pregnancy or lactation impairs memory and alters BDNF and TrkB levels in the hippocampus of adult offspring exposed to chronic mild stress. Physiology & Behavior. 169:114–123. [DOI] [PubMed] [Google Scholar]
- 134.Roversi K, Pase CS, Roversi K, Vey LT, Dias VT, Metz VG, et al. (2016): Trans fat intake across gestation and lactation increases morphine preference in females but not in male rats: Behavioral and biochemical parameters. European Journal of Pharmacology. 788:210–217. [DOI] [PubMed] [Google Scholar]
- 135.Wright TM, King MV, Davey WG, Langley-Evans SC, Voigt J-PW (2014): Impact of cafeteria feeding during lactation in the rat on novel object discrimination in the offspring. British Journal of Nutrition. 112:1933–1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.