Summary
Imagination is an internal simulation of real-life events and a common treatment tool for anxiety disorders, however, the neural processes by which imagination exerts behavioral control are unclear. This investigation tests if and how imagined exposures to a threatening stimulus, conditioned in the real world, influence neural and physiological manifestations of threat. We found that imagined and real extinction are equally effective in the reduction of threat-related neural patterns and physiological responses elicited upon re-exposure to real-world threatening cues. Network connectivity during the extinction phase showed that imagined, like real extinction, engaged the ventromedial prefrontal cortex (vmPFC) as a central hub. vmPFC, primary auditory cortex, and amygdala activation during imagined and real extinction predictive individual differences in extinction success. The nucleus accumbens, however, predicted extinction success in the imagined extinction group alone. We conclude that deliberate imagination can attenuate reactions to threat through perceptual and associative learning mechanisms.
Keywords: imagery, extinction, fear conditioning, prefrontal cortex, auditory, MVPA
eTOC Blurb
Reddan et al. demonstrate that threat responses can be extinguished through imagined simulations of the conditioned stimuli. Like real extinction, imagined extinction engages the ventromedial prefrontal cortex, amygdala, and related perceptual cortices. Nucleus accumbens activity predicts an individual’s ability to successfully extinguish via imagination.
Threat likelihoods are rapidly acquired across diverse and dynamic environments. The diminution of learned threat, however, is a slower and more mutable process (Ledoux, 2000). Fear, therefore, often persists into times of safety (Vervliet et al., 2013). Maladaptive defensive reactions negatively impact quality of life, and underlie emotional disorders, such as post-traumatic stress disorder (PTSD), phobias, and anxiety. Extinction learning, the repeated presentation of a conditioned stimulus without its aversive consequence, is one of the most effective ways of reducing learned threat in the laboratory (Graham and Milad, 2011). Extinction is theoretically a core process of exposure therapy, the most prescribed treatment for fear-related disorders (Taylor et al., 2003). Exposure therapy is impractical in some cases because the recreation of cues associated with a traumatic event may be difficult or unethical to reconstruct (e.g., a war zone), or because the intensity of re-exposure is overwhelming for the patient (Storch and McKay, 2013). Imagination creates a unique opportunity to simulate re-exposure in a controlled way so that a patient may immerse themselves at their own pace. Simulation, in theory, provides a method by which persistent and maladaptive threat memories can be targeted for treatment, independent of external stimuli (Arbuthnott et al., 2001). This investigation demonstrates the utility of imagination as a threat-extinction tool, and proposes a neural mechanism for imagined extinction that includes a network of brain regions known to support the real extinction learning. These findings expand our understanding of how the human brain modifies threat representations and, in turn, our ability to treat threat-related disorders using mental action.
Imagination, also called mental imagery or mental practice, is a conscious simulation of a stimulus or an event that can impact perception, cognition, and emotion (Holmes and Mathews, 2005; Kosslyn et al, 2006). There is growing evidence that imagination induces neural plasticity (Pascual-Leone et al., 2005), activates perceptual and motor cortices (Kosslyn et al., 2001; Zatorre and Halpern, 2005), enhances real-world performance (Shepard and Metzler, 1971) and supports the prediction of future events (Schacter et al., 2007; Mendelsohn et al., 2014), but its role in emotion regulation remains unclear (Holmes and Matthews, 2010). When effective, imagination holds a unique advantage as an emotion regulation tool because it is changeable, internally directed, and dissociated from immediate sensory input (Taylor et al., 1998).
The clinical advantages of imagination are harnessed by imaginal exposure therapy, where patients imagine triggering events until threat sensations subside (van Minnen and Foa, 2006). Despite imagination’s longstanding recognition in the clinic, it has received little attention from the neuroscientific learning community (Mendelsohn et al., 2014; Agren et al., 2017; Soeter and Kindt, 2012; Dadds et al., 1997). This is due in part, to the clinic’s reliance on behavior and self-report in non-neurotypical populations. When considering behavioral and self-report evidence alone, it is difficult to separate the unique effects of imagination from expectancy or other complex cognitive phenomena that shape emotion and learning. It is also related to the field’s focus on translational research: Animal models of imagination are difficult to construct, though they have been attempted (Johnson et al., 2009; Lucantonio et al., 2015; Redish, 2016; Takahashi et al., 2013; Holland, 1990). The current study bridges the gap between the clinic and the laboratory, and in doing so, demonstrates that a scientific interrogation of imagination’s role in fear regulation may provide novel insight into how threat memories are represented, accessed, and modified by mental action.
Extinction is a distributed neural process involving the ventromedial prefrontal cortex (vmPFC), hippocampus, and amygdala (Quirk and Mueller, 2008). More recent evidence extends this network to include the nucleus accumbens (NAc) and related perceptual circuitry (Apergis-Schoute et al., 2014; Levita et al., 2012). Learning and plasticity in these regions underlie the creation and sustainment of extinction memories. Imagination may influence emotion processing either by directly modifying affective substrate downstream of sensory cortices, such as the amygdala, or by activating and altering the representation of existing emotional memories via interactions among the PFC, hippocampus, and NAc (Holmes and Mathews, 2010). Behavioral evidence suggests an interaction; vividness of imagination is positively correlated with both memory strength and physiological arousal (Acosta and Vila, 1990; Miller et al., 2008; Mullally and Maguire, 2013). Furthermore, the vmPFC and hippocampus are part of an imagination network that supports mental time travel (Buckner and Carroll, 2007, Schacter et al., 2007), and the orbitofrontal cortex drives learning related to imagined outcomes (Takahashi et al., 2013). In cases of deliberation, the NAc is recruited to simulate the outcomes of all possible options (Stott and Redish, 2014), and its size is related to individual differences in imaginal ability (Jung et al., 2016).
Drawing across this wide range of evidence, we propose the following mechanism: Imagination of the conditioned threat stimulus will activate stimulus-specific perceptual representations that will, in turn, engage the neurocircuitry which underlie threat acquisition: the vmPFC, hippocampus, amygdala, and NAc. In the absence of any danger, repeated imaginings will have the same effect as actual exposures— Neural and physiological responses to the conditioned threat stimulus will diminish.
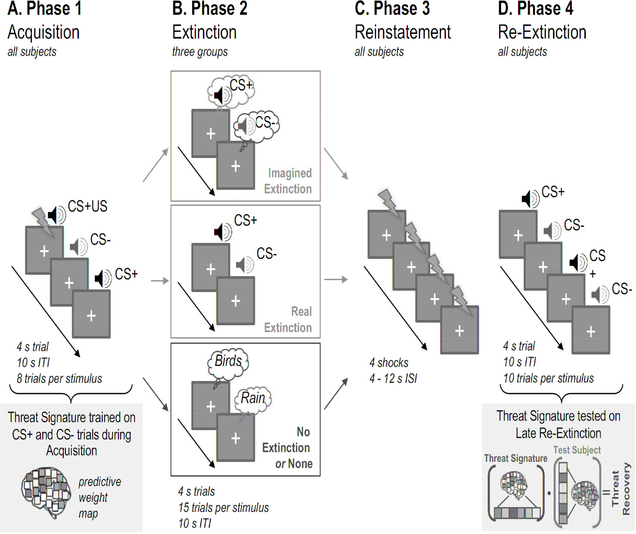
To test the effectiveness of imagined extinction and its neural mechanism, our participants underwent auditory threat conditioning, and then were randomized into three groups (Figure 1). The first group performed imagined extinction, that is, they were directed to “play” the conditioned tones “in their head” to the best of their ability. The second underwent real extinction, which consisted of actual exposures to the conditioned auditory stimuli. The third underwent no extinction and instead was directed to imagine two neutral sounds from nature (“birds singing” and “rain falling”), as a control for the general effects of imagination on arousal. The threat memory was then reinstated in all participants through four unsignaled shocks, after which all participants were re-exposed to the conditioned auditory stimuli. Functional MRI images were collected for each phase, and skin conductance responses (SCR) were recorded continuously. We hypothesized that both real and imagined extinction would attenuate the neural and bodily expression of threat memory following reinstatement. We expected that defensive responding would perseverate through re-exposure only in the third group, which had no extinction at all.
Figure 1. Schematic of the Protocol and Methods.
This experiment was divided into four phases. (1) Acquisition. All participants (N=68) underwent an auditory threat-conditioning paradigm. The CS+ tone was paired with an electric shock 33% of the time. The CS− tone was never paired with shock. A whole brain multivariate threat-predictive pattern was trained on these trials using support vector machines. (2) Extinction. Participants were randomly divided into one of three ‘Extinction’ Sessions: Imagined Extinction (N=20), Real Extinction (N=22), and No Extinction (or None, N=24). Trial structure was conserved across groups, and imagined extinction trial order was yoked to real extinction stimulus presentation. (3) Reinstatement. All participants underwent reinstatement, where they received four unsignaled shocks presented 4 – 12 s apart. (4) Re-Extinction. Participants were then re-exposed to the conditioned stimuli to assess the effectiveness of the extinction sessions. The threat-predictive pattern was applied to participant-specific brain maps during the threat recovery test period, which was comprised of the last 5 trials of reextinction. The threat-predictive pattern was applied by taking the dot product between the unthresholded pattern and participant-specific brain maps. The dot product reflects the magnitude of similarity between two vectors.
Results
Imagined extinction reduces whole-brain threat expression to conditioned stimuli
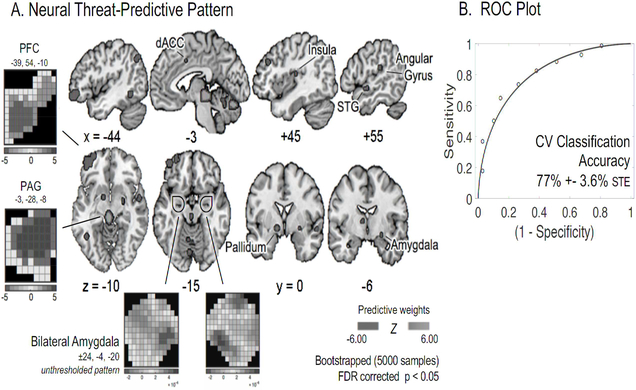
We developed a novel whole-brain fMRI predictive pattern, sensitive and specific to threat, in order to test how effectively imagined extinction reduces threat responses in the brain (Figure 2a). This threat-predictive pattern is a multivariate pattern classifier that can be applied to novel activation maps to assess threat expression in subsequent neural activity. It was developed using a linear support vector machine (SVM) trained on subject-wise (n = 68) univariate brain activation to the unreinforced CS+ (threatening) and CS− (non-threatening) stimuli during threat acquisition. It yielded a classification accuracy of 77% (+− 3.6 STE, P < 0.0001) in a modified leave-three-subjects-out cross-validation (CV) procedure (Figure 2b), where one subject from each of the three groups was left out of each fold to reduce potential bias from group assignment. The threat-predictive pattern’s sensitivity and specificity to threat were 71% (CI: 61% - 79%) and 84% (CI: 76% - 91%), respectively. It had a positive predictive value (PPV) of 81% (CI: 72% - 90%), and an effect size of 0.82, estimated by nonparametric area under the curve. When thresholded (bootstrapped 5,000 samples) and corrected for multiple comparisons (FDR corrected, P < 0.05), the neural threat-predictive pattern revealed a distributed network of threat representation including the PFC, periaqueductal gray (PAG), insula, globus pallidus, and dorsal anterior cingulate cortex (dACC; see Table S1 for complete network). These regions are consistent with those reported in the existing threat conditioning literature, and with the univariate map contrasting the CS+ and CS− stimuli in this study (see Figure S1 and Table S2; Critchley and Garfinkel, 2014, Fullana et al., 2016).
Figure 2. Multivariate Neural Threat-Predictive Pattern Trained on Acquisition.
A. Neural Threat-Predictive Pattern. A distributed pattern of threat was developed using a linear support vector machine trained on subject-wise (N=68) univariate brain activation to the unreinforced CS+ (threatening) and CS− (non-threatening) stimuli during threat acquisition. When thresholded (bootstrapped 5,000 samples) and corrected for multiple comparisons (FDR p < 0.05), the ‘threat-predictive pattern’ revealed a distributed network of threat representation including the PFC, periaqueductal gray (PAG), insula, subregions of the basal ganglia, and dorsal anterior cingulate (dACC). B. ROC Plot. The neural threat-predictive pattern yielded a classification accuracy of 77% (+− 3.6 STE, p < .0001, AUC: 0.82) in a modified leave-three-subjects-out cross-validation (CV) procedure. One subject from each of the three groups was left out of each fold to reduce potential bias from group assignment.
Next we used the neural threat-predictive pattern to evaluate the effectiveness of imagined extinction relative to the control conditions. The pattern was applied to participant-specific brain maps during the re-extinction phase. Reinstatement is often followed by the immediate recovery of defensive reactions, even if extinction learning was successful (Vervliet et al., 2013). However, reinstatement effects are transient in neurotypical humans, lasting only a few trials. (Haaker et al., 2014). We therefore examined whether threat representation was preserved during the late phase of re-extinction (last 5 trials). If extinction learning were less effective or absent, defensive reactions should be maintained during this later phase (Craske et al., 2008). The pattern was applied by taking the dot product between the unthresholded threat-predictive pattern (all voxels) and participant-specific brain maps. This amounts to taking a weighted average over the test data, where the pattern defines the weights, yielding a single value for a given data image. The resulting metric is known as “pattern expression” (or “pattern response”) and is used to assess the magnitude of the activation in a pre-defined pattern (i.e., here, defined to differentiate CS+ vs. CS−, and thus serve as a measure of neural threat response). The pattern expression is influenced by both the spatial similarity between the threat-predictive pattern and test data, as well as the magnitude of activation (Woo et al., 2017).
A positive pattern expression value indicates that the tested brain activity is classified as ‘threatening,’ while a negative value indicates that the brain activity is classified as ‘non-threatening.’ Group differences in the magnitude of the neural threat-predictive pattern expression were analyzed and the effectiveness of the three experimental conditions was compared. Data from two participants were lost during this phase due to a technical error during data collection; therefore, only 66 participants were included in this and the following fMRI analyses.
In this case, as is standard in the field, we trained and tested the threat-predictive pattern in a cross-validated manner (leave-3-participants-out), so that the pattern responses estimated for each individual were always obtained from patterns trained on other participants’ data, avoiding circularity when testing CS+ vs. CS− effects on pattern expression (see Methods section for more detail; Varoquaux et al., 2017). Group (intervention) effects were always estimated on independent contrasts from the CS+ vs. CS− contrast used to train the pattern, and tests of intervention effects are also non-circular, with unbiased estimates of effect sizes.
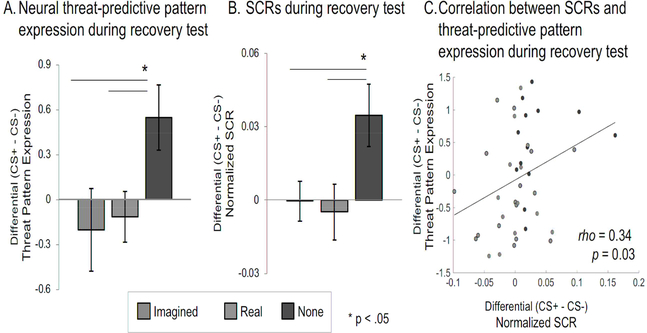
There was a main effect of group as indicated by a one-way ANOVA (F(2,63) = 3.54, P = 0.03, η2= 0.10; Figure 3a; see Figure S2a for data distribution). As hypothesized, only the no extinction group exhibited a distributed neural threat response during the late re-exposure (recovery test) period. The threat-predictive pattern expression was positive on average in the no extinction group ( = 0.55, n = 24), and significantly greater than both imagined extinction x = −0.20, CI = [−1.46, −0.04], n = 20, t(37.9) = 2.14, P = 0.04, Hedges g = 0.64) and real extinction (x = −0.11, CI = [−1.22,−0.11], n = 22, t(42.2) = 2.40, P = 0.02, Hedges g = −0.69) groups as indicated by two-sample t-tests with unequal variance. There was no evidence for a difference between the real and imagined extinction group (t(31.8) = 0.27, P = 0.79; Bayes factor in favor of the null = 7.62), indicating no detectable differences in the reduction of neural threat expression between these groups and that the null hypothesis is favored 7.62 times more than the alternative.
Figure 3. Imagined Extinction Reduces Neural and Physiological Threat Expression.
A. Neural Threat-Predictive Pattern Expression during the Recovery Test. The threat-predictive pattern was applied to brain activity during the late re-extinction recovery test. Only the no extinction group (‘none’, N=24) demonstrated threat recovery during late re-extinction, indicated by a comparison of average threat-predictive pattern expression values between groups. This may indicate that both imagined (N=20) and real (N=22) extinction successfully generated an extinction memory. Data are represented as mean ± SEM. B. SCRs during the Recovery Test. Physiological findings, indicated by SCR, complimented the neural findings. Relative to no extinction (N=13), imagined (N=12) and real extinction (N=17) showed a reduction in threat-related physiological arousal during the recovery test. Data are represented as mean ± SEM. Participants (N=24) who did not demonstrate a discriminatory SCR during acquisition, defined as greater SCR to the CS+ relative to the CS− during either the first or last half of threat-acquisition on average, were excluded from SCR analysis because we were unable to determine if their SCR were representative of threat-related arousal. C. Correlation between SCRs and Threat-Predictive Pattern Expression during the Recovery Test. SCRs were positively correlated with expression of the threat-predictive pattern (rho = 0.34, p = 0.03), indicating a link between the neural and physiological expressions of threat.
Because the amygdala is a region of interest, we performed this analysis again only in this region, bilaterally. The neural threat-predictive pattern was masked and then applied to the same test phase, late re-extinction. Similar to the results of the whole brain threat expression, there was a non-significant trend where only the no extinction group exhibited positive ‘threat’ pattern expression (see Figure S2d for details).
Together, these findings show that only the no extinction group expressed the threat pattern following the (no extinction) general imagery session. The results suggest that imagined and real extinction were both effective in diminishing neural pattern reinstatement of threat.
For consistency with prior research, a univariate analysis of the threat recovery period was also performed. Similar, but weaker, results were found in the amygdala and hippocampus, whereas both regions activated more to the CS+ relative to the CS− in the no extinction group than in the other two groups. Indeed, the univariate whole brain maps contrasting CS+ > CS− revealed threat-related responses in the amygdala, insula, and hippocampus of only the no extinction group (see Figure S3 and Table S3 for details).
Imagined extinction reduces bodily threat expression to conditioned stimuli
The physiological SCR results, in the participants who showed reliable physiological threat acquisition (n = 42), complemented the neural findings: relative to no extinction (x = 0.03, n = 13), imagined extinction ( = −0.0004, n = 12, t(20.1) = 2.31, P = 0.03, CI = [−0.07 −0.003], Hedges g = 0.88) and real extinction ( = −0.005, n = 17, t(26.2) = 2.31, P = 0.03, CI = [−0.07,−0.004], Hedges g = - 0.82) showed a reduction in threat-related physiological arousal (F(2,39) = 3.61, P = 0.036, =0.16, Figure 3b; see Figure S2b for data distribution) during the late re-extinction phase. There was no difference between the real and imagined extinction groups during this period (t(26.5) = 0.32, P = 0.75, Bayes factor in favor of the null = 2.75). Furthermore, participants’ SCR positively correlated with expression of the neural threat-predictive pattern (Spearman’s rho = 0.34, P = 0.03, Figure 3c), signifying a link between the neural and physiological expressions of threat. Participants (n = 24) who did not demonstrate a discriminatory SCR during acquisition, defined as greater SCR to the CS+ relative to the CS− during either the first or last half of threat-acquisition on average, were excluded from this analysis because we were unable to determine if their SCR were representative of threat-related arousal (see Figure S4 for SCR analysis without this criteria). Importantly, this failure to produce discriminatory SCRs was not related to the order by which stimuli were presented or the counterbalancing of two different Hz tones (see Table S4 for an analysis of order effects in SCR).
To demonstrate that the inclusion of the neural data from SCR non-responders was valid, we retested the recovery of the neural threat pattern expression in the participants whose SCR was not excluded (N = 42). The findings yield the same conclusions as this analysis with the complete sample (F(2,39) = 4.45, P = 0.02, η2 = 0.10). The no extinction group (x = 0.47, n = 13), was significantly larger than both imagined (x = −0.31, n = 12, t(21.4) = −2.62, P = 0.02, CI = [−1.40, −0.16], Hedges g = −1.02) and real extinction ( = −0.21, n = 17, t(26.94) = −2.68, P = 0.01, CI = [−1.20, −0.16], Hedges g = −0.94) groups as indicated by two-sample t-tests with unequal variance. Real extinction did not differ from imagined (t(22.1) = −0.33, P = 0.74, CI = [−0.70 0.51], Hedges g = −0.12).
Neural Network Supporting Imagined Extinction
To examine whether brain regions involved in real extinction are also engaged during imagined extinction, we performed a network connectivity analysis among known extinction circuitry (Herry et al., 2010) including, vmPFC, hippocampus (CA1), laterobasal amygdala (Amg-LB), central nucleus of the amygdala (Amyg-CM), NAc, PAG, and the primary auditory cortex. Anatomical masks of these regions were taken from the SPM anatomy toolbox, except for the vmPFC, NAC, and PAG, which were derived from Neurosynth (Yarkoni et al., 2011). Average signal from the specified regions of interest (ROIs) was extracted across the entire extinction BOLD timecourse without contrast. A contrast was not used because it would not be meaningful to contrast the imagined stimuli in the no extinction group (imagined sounds of birds singing and rain falling), and contrasting imagined stimuli may mask important activity related to imaginal processes.
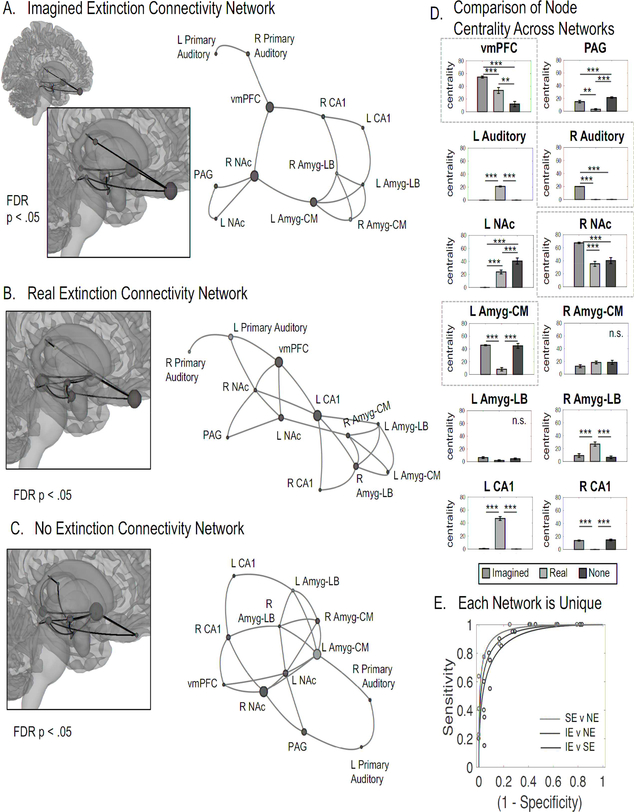
This analysis was performed on each group. While each group yielded discriminable networks, which can be accurately classified as either imagined extinction (Figure 4a), real extinction (Figure 4b), or no extinction (Figure 4c) using SVM (Figure 4e), imagined and real extinction have a strikingly similar construction where the vmPFC is a central hub, indicated by a comparison of the vmPFC’s betweenness centrality, calculated using the number of shortest paths that pass through the node, across groups (Figure 4d), connected to the primary auditory cortex, CA1, and NAc. The nodes with the highest betweenness centrality in the imagined extinction group, which were also significantly higher than either real or no extinction, were the right NAc, vmPFC, and right primary auditory cortex, indicating that imagined extinction may engage perceptual and learning circuitry to update the value of the conditioned stimuli. (see Table S5 for the complete statistical report on the betweenness centrality comparisons).
Figure 4. Neural Networks Supporting Extinction Learning.
Functional connectivity between a priori regions of interest (vmPFC, hippocampus (CA1), laterobasal amygdala (Amg-LB), central nucleus of the amygdala (Amyg-CM), NAc, PAG, and the primary auditory cortex) across the entire extinction session was investigated without a contrast. Links are plotted if the partial correlation test between two nodes was significant after FDR correction within each group. The link length between each pair of nodes is related to the absolute value of the t-statistic associated with the partial correlation between each pair, however, link length is a graphical representation of this value, adjusted to exist in a 2D space. Here, shorter links represent greater functional connectivity. The size of each node represents betweenness centrality, which is the number of shortest paths that pass through the node, rescaled for plotting purposes with a sigmoid function. Node colors indicate clusters determined by ward linkage. A. Imagined Extinction Connectivity Network. The most central nodes (i.e., nodes most connected with other nodes) of the imagined extinction network included the right Nac, vmPFC, left Amg-CM, and right Primary Auditory Cortex. B. Real Extinction Connectivity Network. The most central nodes of real extinction included the left CA1, vmPFC, and right Nac. C. No Extinction Connectivity Network. The most central nodes during the no extinction, unrelated imagination included left NAc, left Amyg-CM, and right NAc. D. Comparison of Nodal Centrality Across Networks. 10 out of 12 nodes yielded group significant differences in betweenness centrality in a one-way ANOVA test, FDR corrected for multiple comparisons (p < 0.05). Data are represented as mean ± SEM Asterisks represent pairwise significant difference via a post hoc t-test. The most central nodes to imagined extinction are indicated by a dashed box. E. Each Network is Unique. In order to test the separability of each matrix, and thereby assess their relative similarity, we trained three different binary linear support vector machine classifiers on participant connectivity data (the partial correlations between each pair of nodes). Leave-one-out cross-validated accuracy scores revealed that each group was linearly separable from one another with high accuracy (Real v None: 91% +− 4.2% STE; Imagined v None: 89% +− 4.8% STE; Imagined v Real: 88% +− 5.0% STE).
Imagined and Real Extinction Recruit vmPFC
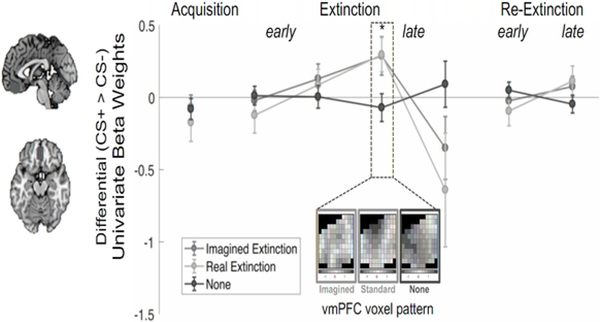
Given the centrality of vmPFC as a hub of network connectivity during both imagined and real extinction, we further examined the univariate timecourse activation of this region. We applied the vmPFC mask to the univariate beta weights, and, using repeated-measures ANOVA, compared differential activation in the vmPFC (CS+ minus CS−) across groups and time (in bins). All acquisition trials were averaged into one bin. The 15 extinction trials were divided into four bins, two early and two late, by averaging four trials in the first three bins and three trials in the last bin. This was decided post hoc in order to better analyze the finer timecourse without sacrificing power. Early re-extinction was comprised of the first five trials, and late re-extinction was the last five trials. There was a significant main effect of time (F(6,378) = 3.72, P = 0.001) and a significant interaction between group and time (F(12,378) = 1.83, P = 0.04; Figure 5).
Figure 5. Imagined and real extinction recruit the vmPFC.
A. vmPFC activation across experimental phases. Left. Depiction of vmPFC anatomical mask used in this analysis. Due to the role of the vmPFC during extinction, the vmPFC was an a priori region of interest. This bilateral mask was created with Neurosynth. Right. Average differential (CS+ > CS−) BOLD signal in the vmPFC rose over the course of extinction, peaked in the third bin, and then decreased in the imagined and real extinction groups. The no extinction group exhibited little to no change in activation across binned trials. The spatial voxel patterns during the third extinction time point are displayed, revealing (nonsignificant) similarity in the activity patterns between the imagined and real groups. During late re-extinction, vmPFC activity increased in the imagined and real groups, but not in the no extinction group, which demonstrated threat responses during this phase. Data are represented as mean ± SEM.
Descriptively, vmPFC activation during imagined and real extinction was nearly synchronous: it rose slowly and peaked towards the end of extinction training. Pairwise t-tests of unequal variance at peak activation (in the third bin) indicated no difference between imagined and real extinction, (t(39.01) = −0.04, P = 0.96, CI=[−0.37, 0.35], Hedges g = −0.01), but vmPFC activation during no extinction was significantly lower than both imagined (t(35.93) = 2.18, P = 0.035, CI = [0.03,0.69], Hedges g = 0.66) and real extinction (t(41.26) = 2.39, P = 0.02, CI = [0.06, 0.67], Hedges g = 0.70).
Additionally, correlations of voxel-wise activity patterns at peak activation suggest that activations in the vmPFC are spatially analogous in imagined and real extinction (R = 0.51, P = 0.25; Figure 5), but more data is required to assess significance (see also Figure S5). These results are consistent with previous reports indicating vmPFC activity increases during extinction learning (Schiller and Delgado, 2010; Schiller et al., 2013) and further indicate the ability of imagined extinction to recruit vmPFC in a manner similar to real extinction.
Brain Regions that Predict the Success of Imagined and Real Extinction
Finally, to better understand individual differences in the activation of extinction networks related to extinction success (as defined by the expression of the neural threat-predictive pattern during the recovery test period), we ran a post hoc univariate ordinary least squares regression, controlling for nuisance signal in the ventricles and white matter, on whole brain maps of differential activation (CS+ minus CS−) during extinction at peak activation (third time bin) with subject threat-predictive pattern expression scores as the predictor within each group (Figure 6a-b). When the analysis was thresholded and corrected for multiple comparisons (FDR corrected, P < 0.05, k = 5), no significant correlates of extinction success were found in the no extinction group. Therefore, the no extinction group is not displayed in Figure 6 nor discussed here.
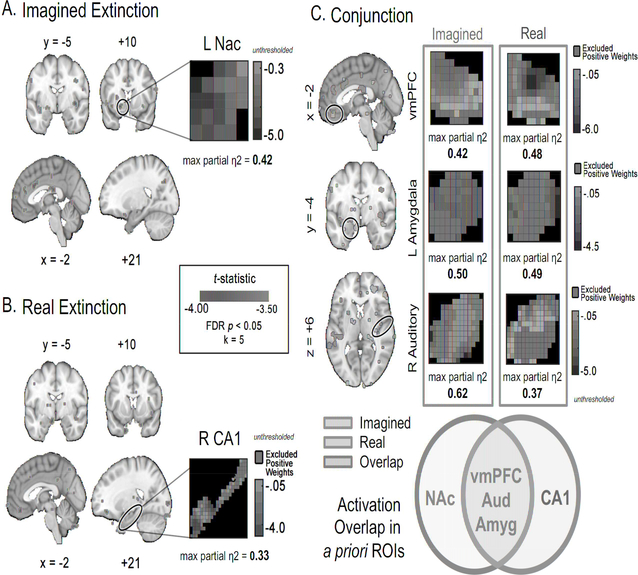
Figure 6. Brain regions that predict the success of imagined and real extinction.
A linear regression was performed on whole brain maps of differential activation (CS+ > CS−) during extinction (time point 3) with participants’ threat-predictive pattern expression scores during the recovery test as the predictor. Because we were most interested in brain regions that predicted extinction success, we focused on the negatively correlated results (see Table S6 for a complete table of activation). When thresholded and corrected for multiple comparisons (FDR corrected, P < 0.05), both imagined (A) and real extinction (B) revealed activity in the vmPFC, primary auditory cortex, and amygdala were related to extinction success. No significant correlates of extinction success were found in the no extinction group. C. Conjunction. Signal in a priori ROIs that survived correction in the whole brain analysis was assessed. The NAc predicted extinction success uniquely in the imagined extinction group, while CA1 predicted extinction success uniquely in the real extinction group. Effect sizes in the ROIs were estimated via max partial η2 which ranges from 0 to
To compare the imagined and real extinction FDR corrected thresholded maps, we plotted the conjunction of the FDR thresholded maps (Figure 6c). We report significant activity anywhere within the bilateral anatomical masks of the a priori ROIs used in the connectivity analysis. Here, we included the entire amygdala, instead of subnuclei, to provide a more general overview. In this descriptive analysis, individual voxels need not overlap between the two maps in order to conclude that there is activity in the same ROI. Instead, significant activity in any voxel within the ROI was used to construct the Venn diagram descriptive summary of the two maps (Figure 6c). Because we were most interested in brain regions that predicted extinction success, we focused on the negatively correlated results alone, as a negative correlation meant that the threat-predictive pattern expression had decreased. The max effect size (partial η2) was estimated within each ROI to compare the importance of the effect between groups. Activity in vmPFC (Imagined: partial η2 = 0.42; Real: partial η2 = 0.48), primary auditory cortex (Imagined: partial η2 = 0.62; Real: partial η2 = 0.37) and amygdala (Imagined: partial η2 = 0.50; Real: partial η2 = 0.49) were negatively correlated with threat recovery in both the imagined and real extinction groups (refer to Figure S6 for the distribution of the data supporting these effects). The NAc (partial η2 = 0.42) predicted extinction success uniquely in the imagined extinction group, while CA1 (partial η2 = 0.33) predicted extinction success uniquely in the real extinction group. It is possible that imagined extinction does not influence learning in the hippocampus the same way as real extinction does, but instead changes representations of stimulus value via the NAc.
Discussion
Imagination is an important cognitive and emotion regulation tool that allows simulation of the real world. This investigation demonstrates that imagined extinction can reduce learned responses to threat. Using a novel neural threat-predictive pattern, we found that imagined extinction reduces threat-related brain activity, and using SCR, we found that imagined extinction reduces threat-related physiology upon re-exposure. Furthermore, we demonstrate that the neural mechanisms underlying imagined and real extinction share a ‘common core’ network of brain regions, wherein the vmPFC acts as a central hub of connectivity among the hippocampus, NAc, and auditory cortex. By contrast, non-specific imagination (no extinction) depends upon a different network of brain activity and is not effective against the recovery of threat-related neural activation and arousal.
People differ in both their imaginal ability and threat response magnitudes (Miller et al., 2008). We therefore expected individual differences in the efficacy of imagined extinction. When exploring these individual differences, we found that activation strength in the vmPFC, auditory cortex, and amygdala were related to extinction efficacy in both the imagined and real extinction groups. Activity in the NAc, however, predicted extinction efficacy in the imagined extinction group alone, while activity in the hippocampus was uniquely related to the efficacy of real extinction. Together with the connectivity network analysis, this evidence suggests that imagined extinction alters learned threat responses by updating value representations associated with the conditioned stimuli, through interactions among the auditory cortex, vmPFC, amygdala, and NAc. How does imagined extinction change the value of a threat representation learned through experience with the real world? There are several possible ways by which imagination may induce neural processes that change one’s responses to learned threat. Here we discuss two possible learning mechanisms.
Imagined Extinction as New Learning.
Our findings provide strong evidence that imagined extinction engages the perceptual circuitry active during the encoding of real-world threat contingencies. One interpretation of this finding is that imagined extinction is a ‘true’ simulation of real extinction learning. Simulation of real exposures may activate the extinction circuitry to form a new stimulus-outcome association that competes with the original learned threat association for expression. Like real extinction, vmPFC activity during imagined extinction may reflect integration of information distributed across the amygdala, NAc, and hippocampus (Nieuwenhuis and Takashima, 2011; Hartley and Phelps, 2010). As this information is integrated in a safe context, the vmPFC may suppress representations in the limbic system, which are no longer accurate or relevant. This interpretation views imagined extinction as real extinction without the external stimulus. It follows then, that imagined extinction, like real extinction, would be marked by a gradual reduction in physiological responses to the conditioned threat stimulus (Vervliet et al., 2013). We did not find evidence for this; as real extinction SCR responses decreased through time, imagined extinction responses, which were initially lower, slightly rose (Figure S4). However, the efficacy of exposure therapy is not well predicted by threat expression during or at the end of exposure (Craske et al., 2008), and SCR results in this investigation are possibly underpowered to fully examine this hypothesis. We also found differences in the degree to which the two processes involved the hippocampus, a region that is central to formation and recall of an extinction memory. Therefore, we maintain the position that though imagined and real extinction share ‘core’ processes and accomplish comparable reductions in threat responding upon re-exposure, as a whole, they are functionally distinct.
Another type of new learning that imagination may influence is belief formation. This type of learning is more similar to cognitive approaches to emotion regulation (McRae, 2016). For example, instead of learning something new about reinforcement contingencies in their environment, a person engaging in imagined extinction training may learn something new about their agency within it. Clinical reports indicate that it is not just the vividness of one’s imagination that predicts therapeutic outcomes, but the interaction between vividness and one’s sense of control over the imagery (Richardson, 1969; Singer and Pope, 1978). Hence, imagination can be used to increase one’s internal locus of control, which may alter - not existing memories - but the way one approaches future threats. Thus, imagination may provide a stepping stone toward real exposure therapy. Alternatively, imagined exposures can yield adverse effects. Anxiety-driven imagination that strengthens the belief that one is unsafe is unlikely to have positive therapeutic outcomes (Jones and Davey, 1990). Indeed, emotional imagery is a hallmark of PTSD reexperiencing (Holmes and Mathews, 2010). In this way, imagination can exasperate a fear and promote avoidance behaviors. Drawing across this disparate evidence, we conclude that if imagination is well directed, so that the person feels safe and in control, one can reconceptualize learned threat contingencies in a wider context and change the value of the threatening stimulus via expectancy violation, which is a key component of extinction learning (Craske et al., 2014; Rescorla and Wagner, 1972).
Imagined Extinction as ‘Unlearning.’
Instead of engendering new learning, imagined extinction may alter the existing threat association itself, whereby one ‘unlearns’ the threat association. Repeated imagination of actions or events that never actually happened can lead to their being falsely remembered as having occurred in reality (Thomas et al., 2007), especially if the imagined events are consistent with existing knowledge. Intrusions of imagination on existing memories are thought to occur through a reconsolidation process (Schiller and Phelps, 2011; Schacter et al., 2011). Reconsolidation occurs after a consolidated memory re-enters a labile state following reactivation. The memory may be ‘updated’ with new information and then reconsolidated. During imagined extinction, the perceptual simulation of real exposures may recreate the original learning context and reactivate the original threat memory in the amygdala, thereby assuming the state of initial learning. Similar to the theory behind gradual extinction learning (Gershman et al., 2015), which posits that a gradual reduction in reinforcement reduces the magnitude of prediction errors allowing extinction to become an extended state of acquisition learning, imagined extinction may, while recreating the original learning state, diminish or even omit prediction errors due to the lack of external input. Despite the lack of input, percepts continue to be experienced without external reinforcement. This may cause the memory trace to weaken its associations with threat-related physiology. Reconsolidation, of course, can only take place after the memory has first been consolidated. This experiment occurred in a single visit, so we are unable to assess ‘reconsolidation.’ However, in this timeframe, imagined extinction may become an extension of the acquisition learning, so that only one stimulus-outcome memory is formed.
One way to disentangle these two learning hypotheses would be to test the durability of imagined extinction. Real extinction suppresses the expression of a fear memory. It does not modify nor erase the original memory; this is why extinguished fear responses spontaneously recover over time (Quirk and Mueller, 2008). Reconsolidation modifies the original threat memory, and therefore prevents the return of fear both spontaneously over time and after reinstatement (Schacter et al., 2011). Evidence from the clinic suggests that the effects of imaginal processes endure through time (Zoellner et al., 2017); however, a controlled investigation of imagined extinction assessing more than one visit would be necessary in order to determine whether imagined extinction is vulnerable to spontaneous recovery.
When interpreting the finding that imagined extinction engages perceptual circuitry active during acquisition, it is important to note that this experiment used a unimodal auditory conditioning paradigm. There is growing evidence that auditory processing has a direct impact on the learning structures supporting emotional behaviors. For example, amygdala-dependent threat-learning induces long lasting changes in the sensitivity of the auditory cortex, in both the mouse and the human, and these changes persist after extinction (Bakin and Weinberger, 1990; Weinberger, 1998; Wigestrand et al., 2016). Indeed, Apergis-Schoute et al (2014) found auditory association cortex BOLD signal is resistant to extinction. This suggests that sensory-cortical processing of threatening stimuli can be long lasting, even when immediate threat and corresponding amygdala activation have subsided. It is possible that this grounding of the CS in the sensory region it was learned may allow quicker responding to the threatening stimulus in the future. It is also possible that auditory sensation itself can gate negative arousal: Music has been shown to alleviate pain during surgery recovery, even when the music is played while a person is under anesthesia (Hole et al., 2015; Kahloul et al., 2017). This evidence also suggests that the choice of sounds imagined by the no extinction control group could have affected their performance. In this case, if the sounds of birds or rain were affectively calming, for example, this might produce extinction-like effects, which would work against the group differences (more powerful effects of both real and imagined extinction) that we observed here. To better understand the role of perceptual processes in imagined extinction, future studies could test for such unconditioned effects of affective qualities of multimodal imagined stimuli.
Nothing in the real world can be perfectly represented by the brain, but a “good enough” approximation allows one to predict and navigate their environment. Imagination is a process by which information about one’s environment can be simulated and reorganized in order to improve predictions and learn under reduced risk. Imagination, when reduced to this basic functionality, is not unique to humans. Animals demonstrate deliberation when making choices, and this deliberation is thought to be analogous to imagined simulations of possible outcomes (Redish, 2016). Moser and Moser (2011) demonstrate that the resting mouse will both ‘replay’ past actions and ‘preplay’ future ones in a maze task. Furthermore, Takahashi et al (2013) show that rats imagine outcomes when inferring paths through a mechanism that requires the integration of reinforcement histories of environmental cues by the orbitofrontal cortex. The human brain, however, may use imagination to draw upon richer experiences and cognitive frameworks in order to influence memory, learning, emotion, decision making, expectations, and beliefs. In this way, imagination is a process that can inform the study of cognition and behavior in both human and non-human animals.
This investigation has strong implications for the treatment of anxiety and threat-related disorders. While the integration of imagination with exposure therapy is not new, our approach to threat simulation is. Clinical applications of imagination are not pure exposure tools: Patients have expectations of recovery; they sometimes are trained to control their breath; and imagination may be combined with cognitive restructuring technique, or other talk-based therapies (Craske et al., 2014). In this experiment, the only difference between the real and imagined extinction procedure is the existence of the external stimulus. This allowed us to directly test if stimulus re-exposure is critical to extinction learning, or if can be internally simulated. We conclude that an internal simulation of a real-world experience can alter the way one responds to that situation in the future. Indeed, imagined exposures to threatening stimuli are effective in the reduction of learned threat responses, and evoke a network of brain activation similar to real extinction. These novel findings bridge a long-standing gap between clinical practice and cognitive neuroscience. Once a topic reserved only for poets and philosophers, imagination is now being regarded by psychologists as an important cognitive tool for both decision-making and emotion regulation.
STAR Methods
CONTACT FOR RESOURCE SHARING
Further information and requests for resources and code should be directed to and will be fulfilled by the Lead Contact, Daniela Schiller (daniela.schiller@mssm.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Sixty-eight (45 females, average age=29.64 STD 15.89, 45 participants were white, 5 African American, 9 Hispanic/Latino, 8 Asian, and 1 Native American) adult healthy members of the New York community, who had not participated in a shock-related experiment in at least six months prior to recruitment participated in this experiment. The gender identity of the participants is not known. Participants were only asked to provide their biological sex as male or female.
Participants were recruited through internal and surrounding communities near Icahn School of Medicine at Mount Sinai and New York University. All participants gave informed consent and were paid for their participation. Participants were not aware that there were multiple experimental conditions. This study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board.
METHOD DETAILS
Experimental Procedures
Eligibility Criteria.
Participants were required to be between the ages of 18 to 65. Participants who met criteria for disorders under the DSM-IV were excluded from this study. Eligibility was determined by the SCID (structured clinical interview for DSM-IV), which was conducted by the experimenter prior to the scheduling of the MRI experiment.
Randomization.
Participants were randomly assigned to an experimental condition (imagined, real, or no extinction) and order (A or B) by the experimenter upon arrival. A power analysis was not calculated for this study. Sample sizes were determined based on previously published studies of threat conditioning and extinction, At least 20 participants were decided to be necessary in each of the three groups.
Shock Work-Up Procedure.
Mild electric shocks were delivered through a bar electrode attached to the right wrist. A Grass Instruments stimulator was used with cable leads that were magnetically shielded and grounded through an RF filter. Shock strength was determined by a work-up procedure. In this procedure, the subject was first given a mild shock (200 ms duration, 50 pulses/s) which was gradually increased to a level the subject indicated as maximally “uncomfortable, but not painful” (the maximum shock that could given was 50 V). The average shock strength of participants in this experiment was 24.28 V (STD 10.19).
Stimuli.
Several auditory stimuli were used in this experiment: (1) a 800 Hz pure “high” tone, (2) a 170 Hz pure “low” tone, (3) a sound clip of birds singing, and (4) a sound clip of rain falling. The tones were used because they had been previously used in threat conditioning experiments (Apergis-Schoute, Schiller, LeDoux, and Phelps, 2014). The nature sounds were selected for the no extinction control session because the sounds of birds singing and rain falling are quite commonly associated with relaxation, and therefore, they control for the aspect of imagination which we intended to control for: its relaxing, internally-directed properties.
Practice Session.
All 68 participants completed a “practice session” before the experiment in which they were asked to listen to and imagine four types of auditory stimuli. This practice allowed participants to familiarize themselves with the cued auditory imagination procedure. The following auditory stimuli were presented for 4 seconds two times each: (1) a 800 Hz pure “high” tone, (2) a 170 Hz pure “low” tone, (3) a sound clip of birds singing, and (4) a sound clip of rain falling. Participants were then told the cue words which will indicate to them which sound to imagine and when. The cue words were: high, low, birds, and rain, respectively. Participants were then cued to imagine each sound twice to practice the cued-imagination process. Participants were given the cue word ‘stop,’ to indicate when they should stop imagining the sound after each 4 s cued-imagination trial.
Phase 1: Threat Acquisition.
All 68 participants underwent an auditory threat-conditioning paradigm inside of the fMRI environment. The CSs were two easily distinguishable pure tones (800 and 170 Hz). They were delivered through MR-designed headphones, and were outside of the range of scanner noise. The US was a mild electric shock to the right wrist. All CSs were presented for 4 s, with a 10 s fixed inter-trial-interval (ITI). One of the tones was designated as the CS+ and was paired with shock on 33% of the trials. The other tone was the CS− and was never paired with shock. Assignment of the high and low tones to the CS+ and CS− conditions was counterbalanced across participants. Participants were not informed of the reinforcement contingency. They were told that they might receive shocks during the experiment, but that no further information could be given. Acquisition consisted of 8 randomized repetitions of CS+ tones paired with shock, 8 randomized repetitions of unreinforced CS+ tones, and 8 randomized repetitions of CS− tones.
Phase 2: Extinction Training.
Participants then underwent one of three “extinction training sessions.” The first control group underwent real extinction training, which consisted of 15 pseudorandomized unreinforced presentations of each CS+ and CS− tone. The second control group was cued to imagine two neutral sounds from nature, which they heard previously during the practice session: birds singing and rain falling. There were 15 pseudorandomized cues to imagine each the birds and the rain. The third group, which was of main theoretical interest, was cued to imagine the CS+ and CS−, 15 times each, in a pseudorandomized order yoked to the presentation pattern of the real extinction-training group. No shocks were delivered during any of the extinction training sessions.
Phase 3: Reinstatement.
All 68 participants underwent reinstatement of the threat memory: Four unsignaled shocks were delivered in temporally random pattern, whereby shocks were delivered between 4 to 12 seconds apart.
Phase 4: Re-Extinction.
All 68 participants were exposed again to the CS+ and CS− tones. They received 10 presentations of each, in a randomized order.
DATA COLLECTION
Skin Conductance Signal Acquisition.
Skin conductance was assessed with shielded Ag–AgCl electrodes attached to the middle phalanges of the second and third fingers of the left hand using BIOPAC systems EDA module. The electrode cables were grounded through an RF filter panel.
Neuroimaging Acquisition.
The study was conducted at the NYU Center for Brain Imaging using a 3T Siemens Allegra scanner and a 32 channel Siemens head coil. Functional scans used a gradient echo sequence, TR = 2 s, TE = 20, flip ANGLE = 90, 183 FOV = 192, 3 mm slice thickness. A total of 39 axial slices were sampled for whole brain coverage. The in-plane resolution was 3 mm x 3 mm. Functional image acquisition was divided into four runs, one for each session, however the run for phase 3, reinstatement was not analyzed due to it short duration (approximately 1 minute). Between runs there was a break of approximately 15–30 s where the experimenter checked with the participant to make sure they were comfortable and alert. The scanning session ended with MPRAGE anatomical scans to obtain a 3D volume for slice selection.
QUANTIFICATION AND STATISTICAL ANALYSIS
SCR Preprocessing and Signal Decomposition.
Skin conductance analysis was performed using the Matlab package, Ledalab (Benedek and Kaernbach, 2010). Raw traces were downsampled to 100 hz, then preprocessed with the software’s adaptive smoothing function, which employs a gaussian low-pass filter to the data. Finally, the signal was deconvolved using a continuous decomposition analysis (CDA). This analysis separates the traces into continuous signals of tonic and phasic activity. The ‘phasic’ driving component of the signal can then be analyzed while controlling for slow, tonic changes. Event-related responses to stimuli were assessed by extracting the average phasic activity of SCRs occurring within a half a second after stimulus onset until a half a second after stimulus offset for each trial. The SCR analysis included only trials that did not coterminate with a presentation of the US.
SCR Data Normalization.
Event-related phasic SCRs were next range normalized and log transformed within subjects, across all trials. This procedure was done so that individual variability in the signal would not bias further analyses.
Outlier Removal.
Participants who did not demonstrate greater SCRs to the CS+ relative to the CS− on average across all acquisition trials (N=24) were removed from analysis because it would be meaningless to investigate threat recovery in the signal of participants who did not demonstrate initial learning. It is important to note that individual differences in SCR are known to exist and that individuals vary in the reliability of their responding due to the equipment, properties of the skin, or psychological traits; Ben-Shakhar, 1985; Naveteur and Baque, 1987). A lack of a conditioned SCR does not mean the person did not learn cognitively or autonomically, only that the learning is not evident in their SCR signal.
Imaging Preprocessing.
Imaging data were preprocessed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and custom Matlab (MATLAB, The MathWorks, Inc., Natick, MA) code available from the authors’ website (http://canlab.colorado.edu). Images were first unwarped and realigned to the first image of the series using a six-parameter, rigid-body transformation. The realigned images were then coregistered to each participant’s T2-weighted structural image and normalized to the 152 MNI template using a 12-parameter nonlinear and affine transformation. The images were resliced during preprocessing from 3×3×3 voxels to 2×2×2 which is standard in SPM. Spatially normalized images were smoothed with a 6 mm full-width-at-half-maximum (FWHM) Gaussian kernel and filtered with a high pass filter (180 sec cutoff).
Univariate Analysis.
A univariate general linear model (GLM) was used to create images for the prediction analysis (Figure 2). For Phase 1: Acquisition, the model included three boxcar regressors, one for each stimulus presentation type: reinforced CS+, unreinforced CS+, and CS−. For the remaining phases, a single trial model, where each trial is modeled by one regressor was applied (Rissman et al., 2004; Mumford et al., 2012; Atlas et al., 2010). All regressors were convolved with a double gamma HRF function, and an additional 24 covariate regressors modeled movement effects (6 realignment parameters demeaned, their 1st derivatives, and the squares of these 12 regressors). Univariate analyses are reported in Figures 5 and 6 as well as Supplementary Figures 1, 3, and 6.
Classification analysis.
Using a cross-validated pattern classification procedure, we developed a whole brain neural pattern predictive of threat. This combination of multivariate pattern analysis and machine learning allows for the creation of a single integrated model of threat experiencing that accounts for activity distributed across networks. This approach has greater power and less bias than mass univariate approaches (Reddan et al., 2017). First, a support vector machine classifier was trained on single subject first-level GLM contrast images to distinguish between responses to the CS+ > baseline and responses to the CS− > baseline during Phase 1: Acquisition. In order to validate the neural threat-predictive pattern and reduce the chance of overfitting, we used a modified leave-one-subject out cross validation procedure which splits the data into “training” and “testing” data. Typically, a leave-one-subject-out procedure uses N-1 subjects for training and the remaining subject for testing. Because our subjects were divided into three groups for the extinction phase, we used a stricter N-3 procedure, where one subject from each group was held out. This efficient procedure allows every data point to serve as both training and test data. The resulting cross-validated neural threat-predictive pattern consists of the weights of each voxel in predicting the unreinforced CS+ or CS− stimulus presentation plus the intercept. However, to determine the voxels that made the most reliable contributions to the classification, we performed a bootstrap test. This involved taking 5,000 samples with replacement from the training dataset and repeating the prediction process with each bootstrap sample. This distribution was then converted into a z-value at each voxel and thresholded based on a voxel’s corresponding p-value, FDR corrected p < 0.05.
All pattern expression analyses using the test data were performed with the full set of nonthresholded weights, which included all nonzero voxels, unless otherwise specified (i.e., partial pattern expression looking at just the amygdala). We use the thresholded map to elucidate which voxels were most important for the classification. The non-thresholded weight map was applied to test data in an independent, leave-k-subjects-out fashion so that the participant whose data were being tested was not included in the neural-threat-predictive pattern (Varoquaux et al., 2017). That is, the weight map from the cross-validation fold that held out the participant to be tested was used to assess threat pattern expression in that subject. In this way, the pattern expression estimates for each individual were obtained from patterns trained on other participants’ data, avoiding circularity. Pattern expression is calculated by taking the dot product between the neural threat pattern (classifier map) and a ‘test’ activation map, in this case, activation to the CS+ and CS− during the ‘recovery test period.’ A positive pattern expression is produced when positive data values are found in regions that are positive (CS+ > CS−) in the classifier map and negative data values are found in regions negative (CS− > CS+) in the classifier map.
Network Analysis Preprocessing.
Preprocessing for the extinction network connectivity analysis (Figure 4) was completed using in-house MATLAB software (canlab_connectivity_preproc, www.github.com/canlab). Time course data were bandpass filtered (.008 – .25 window) and linear trend was removed. Nuisance regressors included the 24 motion parameters, spikes, and the top five principle components of signal from the white matter and ventricles.
Additional details about the specific analyses supporting the figures in this paper can be found in both the Results sections of the main paper and in the figure legends.
DATA AND SOFTWARE AVAILABILITY
Data and analysis scripts for this paper can be found at:
Mendeley: http://dx.doi.org/10.17632/46npy3y2zg.1
Open Science Framework: https://osf.io/68ywz/
https://github.com/canlab/Attenuating_neural_threat_expression_with_imagination_2018/
Supplementary Material
Supplementary Table 6. Significant regions of activation during extinction that predict the neural threat-predictive pattern expression during recovery test for imagined and real extinction groups (FDR q < 0.05, k =5). Related to Figure 6.
Highlights.
Imagined extinction reduces neural and physiological conditioned threat responses
Ventromedial prefrontal cortex is central to both real and imagined extinction
Nucleus accumbens uniquely predicts the success of imagined extinction
Acknowledgements
The authors would like to thank Dino Levy for discussions on the conceptualization of this study. Funding was provided by NIDA R03 DA035305 and Klingenstein-Simons Fellowship Award in the Neurosciences to D.S; and NIDA R01 DA035484 to T.D.W
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta A, and Vila J (1990). Emotional Imagery: Effect of Autonomic Response Information on Physiological Arousal. Cogn. Emot 4, 15–160. [Google Scholar]
- Agren T, Björkstrand J, and Fredrikson M (2017). Disruption of human fear reconsolidation using imaginal and in vivo extinction. Behav. Brain Res 319, 9–15. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Schiller D, LeDoux JE, and Phelps EA (2014). Extinction resistant changes in the human auditory association cortex following threat learning. Neurobiol. Learn. Mem 113, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott KD, Arbuthnott DW, and Rossiter L (2001). Guided imagery and memory: Implications for psychotherapists. J. Couns. Psychol 48, 123–132. [Google Scholar]
- Atlas LY, Bolger B, Lindquist MA, and Wager TD (2010). Brain mediators of predictive cue effects on perceived pain. J. Neurosci 30, 12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, and Weinberger NM (1990). Classical conditioning induces CS−specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 536, 271–286. [DOI] [PubMed] [Google Scholar]
- Benedek M, and Kaernbach C (2010). A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 190, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shakhar G (1985). Standardization Within Individuals: A Simple Method to Neutralize Individual Differences in Skin Conductance. Psychophysiology 22, 292–299. [DOI] [PubMed] [Google Scholar]
- Buckner RL, and Carroll DC (2007). Self-projection and the brain. Trends Cogn. Sci. 11, 49–57. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zalikowsky M, Mystkowski J, Chowdhury N, Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther 46, 5–27. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway C, Zbozinek T, and Vervliet B (2014). Maximizing Exposure Therapy: An Inhibitory Learning Approach. Behav. Res. Ther 58, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H, and Garfinkel S (2014). Neural correlates of fear: insights from neuroimaging. Neurosci. Neuroecon 3, 111–125. [Google Scholar]
- Dadds MR, Bovbjerg DH, Redd WH, and Cutmore TRH (1997). Imagery in human classical conditioning. Psychol. Bull 122, 89–103. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, and Radua J (2016). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol. Psychiatry, 21, 500–508. [DOI] [PubMed] [Google Scholar]
- Graham BM, and Milad MR (2011) The study of fear extinction: Implications for anxiety disorders. Am. J. Psychi 168, 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Norman KA and Niv Y (2015). Discovering latent causes in reinforcement learning, Curr. Opin. Behav. Sci 5, 43–50. [Google Scholar]
- Haaker J, Golkar A, Hermans D, and Lonsdorf TB (2014). A review on human reinstatement studies: an overview and methodological challenges Learn. Mem. 21, 424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA and Phelps EA (2010). Changing Fear: The Neurocircuitry of Emotion Regulation. Neuropsychopharmacology 35, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, and Lüthi A (2010). Neuronal circuits of fear extinction. Eur. J. Neurosci 31, 599–612. [DOI] [PubMed] [Google Scholar]
- Hole J, Hirsch M, Ball E, and Meads C (2015). Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet, 386, 1659–1671. [DOI] [PubMed] [Google Scholar]
- Holland PC (1990). Event representation in Pavlovian conditioning: Image and action. Cognition 37, 105–131. [DOI] [PubMed] [Google Scholar]
- Holmes EA, and Mathews A (2005). Mental Imagery and Emotion: A Special Relationship? Emotion 5, 489–497. [DOI] [PubMed] [Google Scholar]
- Holmes EA, and Mathews A (2010). Mental imagery in emotion and emotional disorders. Clin. Psychol. Rev 30, 349–362. [DOI] [PubMed] [Google Scholar]
- Johnson A, Fenton A, Kentros C, and Redish D (2009). Looking for cognition in the structure within the noise. Trends Cogn. Sci 13, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, and Davey GC (1990). The effects of cued UCS rehearsal on the retention of differential “fear” conditioning: An experimental analogue of the “worry” process. Behav. Res. Ther 28, 159–164. [DOI] [PubMed] [Google Scholar]
- Jung RE Flores RA, and Hunter D (2016). A. new measure of imagination ability: Anatomical brain imaging correlates. Front. Psychol 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahloul M, Mhamdi S, Nakhli MS, Nadhir A, Azzaza M, Chaouch A, and Naija W (2017). Effects of music therapy under general anesthesia in patients undergoing abdominal surgery. Libyan J Med 12, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, and Thompson WL (2001). Neural Foundations of Imagery. Nat. Rev. Neurosci 2, 635–642. [DOI] [PubMed] [Google Scholar]
- Kosslyn S, Thompson W, and Ganis G (2006). The Case for Mental Imagery (New York: Oxford University Press; ). [Google Scholar]
- Ledoux JE (2000). Emotion Circuits in the Brain. Ann. Rev. Neurosci 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Levita L, Hoskin R, and Champi S (2012). Avoidance of harm and anxiety : A role for the nucleus accumbens. NeuroImage 62, 189–198. [DOI] [PubMed] [Google Scholar]
- Lucantonio F, Gardner MPH, Mirenzi A, Newman LE, Takahashi YK, and Schoenbaum G (2015). Neural Estimates of Imagined Outcomes in Basolateral Amygdala Depend on Orbitofrontal Cortex. J Neurosci. 35, 16521–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K (2016). Cognitive emotion regulation: a review of theory and scientific findings. Curr. Opin. Behav. Sci 10, 119–124. [Google Scholar]
- Mendelsohn A, Pine A, and Schiller D (2014). Between thoughts and actions: motivationally salient cues invigorate mental action in the human brain. Neuron 81, 207–17. [DOI] [PubMed] [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EW, McLean A, and Lang PJ (2008). Individual differences in imagery and the psychophysiology of emotion. Cogn. Emot 1, 367–390. [Google Scholar]
- Moser EI, and Moser MB (2011). Seeing into the future. Nature 469, 303–304. [DOI] [PubMed] [Google Scholar]
- Mullally SL, and Maguire EA (2013). Memory, Imagination, and Predicting the Future: A Common Brain Mechanism? Neuroscientist 20, 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, and Poldrack RA (2012). Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage 59, 2636–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveteur J, and Baque EF (1987). Individual differences in electrodermal activity as a function of subjects’ anxiety. Pers. Individ. Dif 8, 615–626. [Google Scholar]
- Nieuwenhuis IL, and Takashima A (2011). The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res 218, 325–34. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, and Merabet LB (2005). The Plastic Human Brain Cortex. Ann. Rev. Neurosci 28, 377–401. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, and Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan MC, Lindquist MA, and Wager TD (2017). Effect Size Estimation in Neuroimaging. JAMA Psychiatry 74, 207–208. [DOI] [PubMed] [Google Scholar]
- Redish AD (2016). Vicarious trial and error. Nature Reviews Neuroscience 17, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, and Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement In Classical Conditioning II: Current Research and Theory, Prokasy AH, ed. (New York: Appleton-Century-Croft; ), pp. 64–99. [Google Scholar]
- Richardson A (1969). Mental Imagery. (New York: Springer; ). [Google Scholar]
- Rissman J, Gazzaley A, and D’Espositio M (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage 23, 752–63. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, and Buckner RL (2007). Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci 8, 657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, and St. Jacques PL (2011). Memory distortion: an adaptive perspective. Trends Cogn. Sci 15, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, and Delgado M (2010). Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci 14, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils M, and Phelps EA (2013). Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. PNAS 110, 20040–20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, and Phelps EA (2011). Does Reconsolidation Occur in Humans? Front. Behav. Neurosci 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, and Metzler J (1971) Mental Rotation of Three-Dimensional Objects. Science 171, 701–703. [DOI] [PubMed] [Google Scholar]
- Singer JL, and Pope KS (1978). The Power of Human Imagination: New Methods in Psychotherapy. (New York: Plenum Press; ). [Google Scholar]
- Soeter M, and Kindt M (2012) Erasing fear for an imagined threat event. Psychoneuroendocrinology, 37, 1769–1779. [DOI] [PubMed] [Google Scholar]
- Storch E, and McKay D (2013). Therapist Barriers to the Dissemination of Exposure Therapy In Handbook of Treating Variants and Complications in Anxiety Disorders, Brand J, Reid JM and Mckay D, eds. (New York: Springer; ), pp 363–373. [Google Scholar]
- Stott JJ, and Redish AD (2014). A functional difference in information processing between orbitofrontal cortex and ventral striatum during decision-making behaviour. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Chang CY, Lucantonio F, Haney RZ, Berg BA, and Yau H (2013). Neural Estimates of Imagined Outcomes in the Orbitofrontal Cortex Drive Behavior and Learning. Neuron 80, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Pham LB, Rivkin ID, and Armor DA (1998). Harnessing the imagination: Mental simulation, self-regulation, and coping. Am. Psychol 53, 429–439. [DOI] [PubMed] [Google Scholar]
- Taylor S, Thordarson DS, Maxfield L, Fedoroff IC, Lovell K, and Ogrodniczuk J (2003). Comparative efficacy, speed, and adverse effects of three PTSD treatments: Exposure therapy, EMDR, and relaxation training. J. Cons. Clin. Psychol 71, 330–338. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Hannula DE, and Loftus EF (2007). How Self-Relevant Imagination Affects Memory for Behaviour. Appl. Cogn. Psychol 21, 69–86. [Google Scholar]
- van Minnen A, and Foa E (2006). The Effect of Imaginal Exposure Length on Outcome of Treatment for PTSD. J. Trauma Stress 19, 427–438. [DOI] [PubMed] [Google Scholar]
- Varoquaux G, Raamana PR, Engemann DA, Hoyos-Idrobo A, Schwartz Y, and Thirion B (2017). Assessing and tuning brain decoders: Cross-validation, caveats, and guidelines. NeuroImage, 145B, 166–179. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, and Hermans D (2013). Fear Extinction and Relapse: State of the Art. Ann. Rev. Clin. Psychol 9, 215–248. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack R, and Nichols T (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM (1998). Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol. Learn. Mem 70, 226–251. [DOI] [PubMed] [Google Scholar]
- Wigestrand MB, Schiff HC, Fyhn M, Ledoux JE, and Sears RM (2016). Primary auditory cortex regulates threat memory specificity. Learn. Mem 24, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Chang LJ, Lindquist MA, and Wager TD (2017). Building better biomarkers: Brain models in translational neuroimaging. Nat. Neurosci. Reviews 20, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, and Halpern AR (2005). Mental concerts: Musical imagery and auditory cortex. Neuron 47, 9–12. [DOI] [PubMed] [Google Scholar]
- Zoellner LA, Telch M, Foa EB, Farach FJ, McLean CP, Gallop R, and Gonzalez-Lima F (2017). Enhancing Extinction Learning in Posttraumatic Stress Disorder With Brief Daily Imaginal Exposure and Methylene Blue: A Randomized Controlled Trial. J. Clin. Psychiatry 78, 782–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 6. Significant regions of activation during extinction that predict the neural threat-predictive pattern expression during recovery test for imagined and real extinction groups (FDR q < 0.05, k =5). Related to Figure 6.