Abstract
It is becoming well established that the gut microbiome has a profound impact on human health and disease. In this review, we explore how steroids can influence the gut microbiota and, in turn, how the gut microbiota can influence hormone levels. Within the context of the gut microbiome-brain axis, we discuss how perturbations in the gut microbiota can alter the stress axis and behaviour. In addition, human studies on the possible role of gut microbiota in depression and anxiety are examined. Finally, we present some of the challenges and important questions that need to be addressed by future research in this exciting new area at the intersection of steroids, stress, gut-brain axis and human health.
Keywords: androgen, microbiota, oestrogen, steroid hormones, stress
1 |. INTRODUCTION
Within each human gastrointestinal tract, there is an exclusive combination of different communities of organisms, including bacteria, viruses, archaea, protozoa and fungi, which are collectively referred to as the gut microbiota and outnumber the total amount of human cells in the human body.1 The collection of these microorganisms, their genomes and the factors that they produce are all part of the gut microbiome.2 Increasing evidence suggests that these microorganisms actively participate in shaping and maintaining our physiology almost as an extra organ.3
The gut microbiota composition is associated with many health benefits, including the maintenance of intestinal homoeostasis, protection against pathogens and appropriate immune responses.4,5 Conversely, many gastrointestinal disorders, such as irritable bowel syndrome and inflammatory bowel disease,6 are associated with an imbalance in this microbial population. Furthermore, differences in diversity and composition of the microbiota have been linked to extra-intestinal diseases, ranging from obesity7 and asthma8 to a variety of brain disorders.3,9–14 The gut microbiota helps break down food and, in doing so, produces metabolites that can directly influence the physiology of host cells, including brain cells. Moreover, immune responses to pathogenic bacteria produce cytokines and lymphokines that can affect brain physiology.15 Because the nervous system is a master regulator of host function, this allows microbes to influence a broad range of complex physiological processes. An improved mechanistic understanding of how bacterial molecules act on the nervous system could yield improved therapeutics for treating behavioural and neurological disorders.16
The human gut microbiota is usually stable and resilient to transient perturbations.17 However, microbial composition or activity of the gut can be modified by a variety of factors, including internal factors such as hormonal changes, or external factors such as diet, antibiotics and stress.3,18 In this review, the influence of steroids and stress on the gut microbiome-brain axis is discussed, as well as the challenges that face future research in this area.
2 |. STEROID HORMONES INFLUENCE THE GUT MICROBIOTA
There is mounting evidence that steroid hormones can affect the gut microbiota. In support of steroids influencing the gut bacterial communities, sex differences have been noted in the composition of the gut microbiota, with specific phyla, family and genera variances occurring with clear effects of gonadectomy and hormone replacement on gut bacteria in rodents.19–21 In mice, the sex differences in the gut microbiota observed between males and females are decreased after castration, indicating a role for gonadal steroids in modulating the gut microbiota.22 Administering testosterone propionate (1250 μg) to female rats on the day of birth decreased the diversity of the gut microbiota in adulthood by increasing the Firmicutes-to-Bacteroidetes ratio.23 In adult rats, ovariectomy caused shifts in the relative abundances of two major phyla, Bacteroidetes and Firmicutes.23 In their study, Moreno-Indias et al23 found that hormonal changes (either early androgenisation or ovariectomy) were more impactful on the gut microbiota than the nutritional changes of switching to a high fat diet. Another study using female oestrogen receptor (ER)β knockout mice found that ERβ influences the gut microbiota in a diet-specific manner.24 When mice were switched from a diet containing oestrogenic isoflavones to one lacking isoflavones, the major phyla including Proteobacteria, Bacteroidetes and Firmicutes differed dependent on ERβ status.24 However, it is important to note that ERβ knockout mice have reduced ovarian function,25,26 which could have contributed to some of the alterations in the gut microbiota that were detected. Finally, in rats bred based on their running capacity, ovariectomy alters gut microbes compared to sham controls more in rats with low running capacity than in those with high running capacity, which was suggested to be linked to a potential role for gut microbiota in protecting individuals with increased aerobic capacity to the cardiometabolic risks associated with menopause.27
Sex differences in the gut microbiota have been reported in humans as well. For example, men have higher levels of Bacteroidetes and Prevotella than women,28,29 suggesting a role for sex factors such as differential sex chromosomal gene expression or differences in gonadal hormone levels in the modulation of the gut microbiota. However, it should be noted that more recent metagenomic studies have reported mixed results with respect to sex differences in micro-biota in humans. Some studies note modest to no effects of sex on the human gut microbiota,30,31 whereas other findings reveal substantial sex differences.32 Other studies suggest that steroids influence the gut microbial communities. For example, the gut microbiota undergoes profound changes during pregnancy in women.33 Koren et al33 found a large shift in the gut microbiota from the first to third trimester, with an increase in overall diversity and a proliferation of Proteobacteria and Actinobacteria, resulting in changes in metabolism. Although the dramatic shift in steroid hormones (eg, oestrogens and progestins) could very well contribute directly to these changes in the gut microbiota, it was also suggested that changes in the immune system at the mucosal surfaces could alter the microbiota.33
Although gonadal steroids can alter the gut microbiota, it appears that, in turn, the gut microbiome can influence hormone levels.34 In postmenopausal women, gut microbiota diversity was positively associated with the ratio of oestrogen metabolites in urine.35,36 In mice, transferring the gut microbiota of adult males to immature females caused an elevation in testosterone levels and metabolomic changes in recipient females.37 In addition, the transfer of gut microbiota from male non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D) to the female NOD T1D mice led to increased testosterone, metabolic changes and protection against type 1 diabetes, which usually shows a strong female-to-male sex bias.37 These studies highlight the reciprocal connections between sex hormones and gut microbiota. Taken together, these studies provide strong evidence that the gut microbiota is influenced by gonadal steroid hormones and steroid levels can be altered by the gut microbiome.
In addition to gonadal steroid hormones influencing the gut microbiota, there is evidence that some endocrine disrupting chemicals (EDCs), such as bisphenol A (BPA) or ethinyl-oestradiol (EE), may alter the gut bacterial composition. BPA, which binds to oestrogen receptors, is classified both as an EDC and as a metabolism-disrupting compound (MDC) that can cause obesity.38 EE, which is present in birth control pills, is secreted in urine and can accumulate in regenerated drinkable water. EDCs and MDCs exert their effects on peripheral organs and the central nervous system (CNS) throughout life, although they can be particularly dangerous during the critical period when they can have permanent deleterious effects that may drive a variety of diseases in adulthood.38 In a recent study,39 California mice, which are biparental, were fed diets containing BPA, EE or a control diet around conception through weening aiming to investigate their effects on the gut micro-biota in parents and their offspring. The gut microbiota differed in both males and females of the parental generation exposed to BPA or EE compared to non-exposed controls. Similar effects were observed in the offspring that were exposed indirectly to EDCs through the placental exchanges and milk of their mothers.39 Presumably, EDCs may affect the gut microbiota partly by disrupting the endocrine equilibrium of animals and perhaps also by directly acting on the gut microbiota.
3 |. THE GUT MICROBIOME-BRAIN AXIS
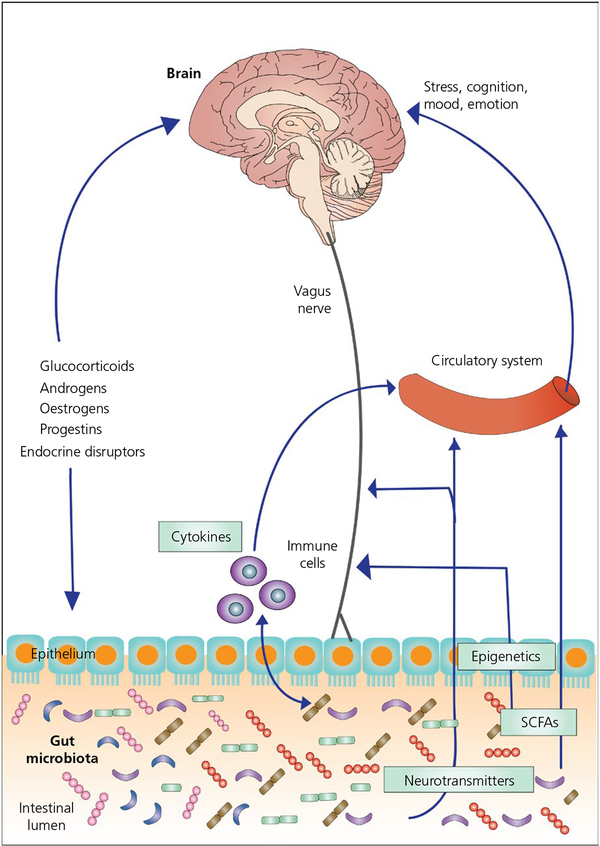
The communication between the gut microbiome and the CNS occurs in a bidirectional fashion and is referred to as the gut microbiome-brain axis (Figure 1).9,11,40,41 This axis includes immune, neural, endocrine and metabolic pathways,42–45 which work together to permit each end organ to communicate and influence the functioning of the other. The enteric nervous system, as well as the sympathetic and parasympathetic divisions of the autonomic nervous system, play major roles in the communication within this axis.44,46,47 Changes to the microbial environment, as a result of different stressors, are associated with changes to gut barrier, motility and immune system activation. Conversely, perturbation of this axis results in alterations in the stress-response and behaviour, which have been proposed to be involved in several CNS diseases, such as anxiety,48,49 depression,50,51 autism,52,53 Parkinson’s disease,54,55 Alzheimer’s disease56 and stroke/brain injury.57
FIGURE 1.
The gut microbiome-brain axis. The gut microbiome (which consists of the microbiota, their genomes and their products) can influence brain function through a variety of mechanisms, including the production of neurotransmitters and short chain fatty acids (SCFAs), the modulation of the release of cytokines by immune cells and the vagus nerve. Conversely, the brain can influence the gut microbiota via regulation of endocrine systems (eg, hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes)
In the case of autism, there are several independent lines of evidence indicating that the gut microbiota is a factor. Compared with neurotypical controls, children with autism show an increase in Clostridium, Bacteroidetes and Lactobacillus species, whereas Firmicutes species are higher in neurotypical controls.58–60 Moreover, treatment with the antibiotic vancomycin, which is poorly absorbed and therefore preferentially affects the gut microbiota, improves symptoms of autism.61 Autism has been associated with reduced levels of short chain fatty acids, including acetate, propionate and valerate, which indicates a poorer fermentation of carbohydrates, presumably because of a relative absence of beneficial bacteria.60 In addition, there is a correlation between the severity of autism and gastrointestinal symptoms.62 Studies using a variety of mouse models of autism support the idea that the gut microbiota is involved in autism.63,64 However, in the clinical studies discussed above, it should be noted that causality is difficult to demonstrate given that children with autism often have eating habits different from those of neurotypical children.
As reported in clinical studies, Parkinson’s disease (PD) is accompanied by dysbiosis of the gut microbiome, resulting in altered levels of microbes (eg, Bifidobacteriaceae, Christensenellaceae, Lachnospiraceae, Lactobacillaceae, Pasteurellaceae and Verrucomicrobiaceae families)65 and concentrations of short chain fatty acids.66 Animal models of PD support these findings and have enabled the functional manipulation of the gut microbiota. For example, mice that overexpress α-synuclein reveal that bacteria within the gut regulate motor deficits and neuroinflammation.67 Indeed, antibiotic treatment ameliorates, whereas microbial colonisation with microbiota from PD patients promotes, the neurological phenotype in these mice.67 These clinical and basic studies suggest that the gut microbiota has an important role in the physical impairments associated with PD.
With regard to Alzheimer’s disease (AD), a clinical study investigating the role of gut microbiota in AD pathogenesis reveals that cognitively impaired patients with brain amyloidosis, compared to controls, have lower levels of the anti-inflammatory Eubacterium rectale and an increase in the pro-inflammatory Escherichia/Shigella.68 In support of these clinical findings, altered gut microbiota have been observed in a variety of mouse models of AD.69–71 Furthermore, in an APPSWE/PS1ΔE9 mouse model of AD, changes in the gut microbiota by long-term antibiotic treatment decreases amyloid-β plaque formation.69 Germ-free APP transgenic mice have decreased amyloid-β pathology compared to control mice.70 Moreover, colonisation of these germ-free APP mice with the microbiota of conventionally-raised APP mice increased amyloid-β levels.70 These findings suggest that dysbiosis of the gut microbiota can contribute to AD pathology and manipulation of the gut microbial community may offer therapeutic opportunities for AD treatment.
There are several mechanisms by which the gut microbiome influences brain function and these work in concert with reciprocal signalling from the brain.72 For example, gut bacteria may influence central neurotransmitters by altering systemic levels of precursors of neurotransmitters.73 Moreover, neurotransmitters such as serotonin, which is involved in pain transmission and affect, can be produced by Candida, Streptococcus and Enterococcus spp.74–77 Lactobacillus and Bifidobacterium species have been shown to synthesise and release GABA, whereas Bacillus and Saccharomyces species can produce norepinephrine.54 These microbially synthesised neurotransmitters can act locally and also cross the mucosal layer of the gut to influence systems in the periphery and potentially the CNS. It has been proposed that serotonergic enterochromaffin cells in the gut epithelium act as chemosensors and transduce chemosensory information to the nervous system.78 In future studies, it will be important to determine whether these serotonergic cells function in disorders, such as depression, within the gut microbiome-brain axis.
The gut microbiota also produce active mediators including short chain fatty acids,79,80 which can inhibit histone deacetylases and lead to epigenetic changes.81,82 These substances also use the vagal nerve to signal the brain and influence behaviour and other centrally-mediated functions, such as the stress response.83 Indeed, studies show that modulation of the gut microbiome can impact the development of the stress system,84 as well as reduce stress levels.85
Similar conditions that alter the composition and diversity of the gut microbiota can result in changes in metabolites that influence signaling systems. The chemical signals generated by these bacteria may also alter the accessibility of these signals to the periphery and the brain (eg, by altering the lining of the gut). In close association with the gut microbiota is the intestinal epithelium, which acts as a physical barrier that protects against luminal contents, as well as being involved with the development and maintenance of the mucosal immune system.86 Dysregulation within the epithelial layer can increase its permeability, thereby disrupting intestinal immune homeostasis.87 A disruption of the gut barrier, sometimes referred to as “leaky gut”, allows microbial products and viable bacteria to translocate from the intestinal lumen.88 A compromised gut barrier is associated with consequences beyond the gastrointestinal tract including an altered blood brain barrier89 and alcohol dependence.90
4 |. THE GUT MICROBIOTA AND DEPRESSION AND ANXIETY
Disorders of the CNS, such as depression and anxiety, are highly debilitating and can profoundly impact quality of life.91 Over-activity of the main stress axis, the hypothalamic-pituitary-adrenal (HPA) axis, is characterised by elevated levels of cortisol and disruption of negative-feedback and is commonly associated with depression.92 Interestingly, activity of the HPA axis can be impacted by the gut microbiota. Germ-free raised BALB/c mice show an exaggerated HPA axis stress response compared to mice raised under specific pathogen-free (SPF) conditions, which have a microbiota that lacks specific pathogenic microbes. The HPA axis stress response can be normalised in adulthood by colonising the gut with Bifidobacterium infantis,78 whereas reconstitution with the enteropathogenic Escherichia coli heightened the stress response even further.84
In addition to these acute effects of the gut microbiota on brain function, microbiota also appear to have programming effects. If BALB/c mice are exposed to faeces from SPF mice early in life (6 weeks of age), the previously germ-free mice show a normal stress response, whereas those reconstituted as adults (≥8 weeks) do not.84 Germ-free mice of the NMRI strain also show reduced anxiety-related behaviour, and conventionalising them (ie, exposing them to feces of conventionally colonised or SPF mice) at birth, but not in adulthood, normalises this behaviour.79 These findings suggest that microbiota influence brain development as well. Indeed, a recent study found that Germ-free Swiss Webster mice were less sociable and did not show the typical preference for investigating novel mice found in conventionally colonised mice.3 Conventionally colonising these mice at day 21 only partially normalised their behaviour,3 suggesting that gut microbiota must be present even earlier for normal social behaviour.
Reduced anxiety and depressive-like behaviours, as well as changes in the GABAergic system, were seen following treatment of mice with Lactobacillus rhamnosus (JB-1).83 Interestingly, these behavioural and neurochemical changes do not occur following vagotomy, indicating that the vagus nerve is a key route of communication between probiotic bacteria and the brain.83 A recent study showed that faecal microbiota transplantation from depressed patients (characterised by decreased microbial richness and diversity) to antibiotics-treated recipient animals induces a depressive-like phenotype in the recipient animals compared to the control recipient group.51
The maternally separated rat model is a model of depression that has been used to demonstrate the role of the gut microbiota in stress-related disorders.93 Maternally separated rats present with behaviours indicative of depression and anxiety had increased systemic immune response and dysbiosis of the faecal microbiota compared to nonsepa-rated controls.93 Furthermore, maternal separation reduced swimming and increased time spent immobile in the forced swim test, induced changes in central neurotransmitters and enhanced peripheral inter-leukin-6 release.73 However, treatment with the probiotic B. infantis reverses these behavioural changes and restores the immune system response and the basal noradrenaline concentrations in the brainstem of rats undergoing maternal separation.73 Lactobacillus plantarum treatment of mice exposed to early-life stress ameliorated anxiety-and depression-like behaviours, balanced the immune response, and modulated neurotransmitters related to affective disorders.94 Altered HPA axis activity and gut physiology are retained in germ-free mice that are subjected to maternal separation, whereas both anxiety-and depression-like behaviours are not.95 Interestingly, the behavioural phenotype is re-established when germ-free mice are colonised in adulthood with the gut microbiota from non-maternally separated control mice but not the gut microbiota of maternally separated animals. These findings suggest a convergence of microbial and host factors.95
Bilateral olfactory bulbectomy in mice induces depression-and anxiety-like behaviours in mice, which are associated with increased immediate early gene expression, serotonin levels, gut motility and alterations in intestinal microbiota profiles.96 Given that similar changes in behaviours, motility and microbiota composition were also induced via central corticotrophin-releasing hormone administration, it was suggest that the altered HPA axis activity functions in this model.96 Both manipulations show the impact of the brain on microbiota composition and indicate that the gut microbiome-brain axis is a two-way street.
Although much of the evidence on the role of gut microbiota in stress-related disorders originates from animal models and other basic research studies, findings from recent human studies are coming to the forefront. The analysis of faecal samples from patients with depression reveals differences in α diversity and levels of specific gut bacterial taxa compared to controls.97 In particular, levels of Bacteroidetes, Proteobacteria and Actinobacteria were increased, whereas Firmicutes were reduced, in samples from depressed patients compared to controls.97 Another study indicates that depression is associated with increased levels of Enterobacteriaceae and Alistipes but a reduction of Faecalibacterium. By contrast, Naseribafrouei et al98 reported no differences in species richness or diversity between depressed patients and controls. However, the same study did find a general under-representation of Bacteroidetes in depressed patients and a significant interaction between the genus Alistipes and Oscillibacter with depression.98
Steenbergen et al99 showed that a multispecies probiotic reduced negative thoughts associated with sad mood, which was suggested to support the concept of probiotics intervention as a preventive approach for depression.99 Patients affected by chronic fatigue syndrome often suffer from anxiety and depression.100 Rao et al101 found that treating these patients with Lactobacillus casei strain Shirota caused an increase in Lactobacillus and Bifidobacteria spp. and a decrease in anxiety symptoms. It was proposed that the improvement seen in the stress-related symptoms is a result of improved gut physiology by the Lactobacillus casei strain Shirota.101
5 |. FUTURE DIRECTIONS
Given the mounting evidence of the effects of the gut microbiome on brain function, future research may be targeted to finding efficient ways of promoting a healthy microbiota (eg, by identifying pre-and probiotics that produce relatively stable changes in the microbiota). Conversely, identifying dietary habits and food additives that negatively impact the brain will be just as important. Diet has profound effects on the structure and activity of gut microorganisms.102–106 For example, a diet rich in fats promotes the growth of the proinflammatory Gram-negative microorganisms, which in turn can alter immune homeostasis.107,108 In addition, the gut microbiota of obese individuals has a higher percentage of energy-efficient bacteria than that of lean individuals.109–112 Because changes in diet affect the gut microbiota, these changes may also influence brain and behaviour. For example, a recent study focused on the negative effects of emulsifiers (common additives in foods such as peanut butter, mayonnaise, salad dressings, bread, ice cream and many other processed foods) on gut microbiota. The addition of emulsifiers to the diet of mice induced systemic inflammation, metabolic syndrome and obesity.113 These emulsifier-induced effects appear to occur by compromising the mucus lining of the gut, allowing gut microbiota to encroach on the epithelium, and altering the species composition of the gut microbiota, thereby increasing its pro-inflammatory potential. Physiological effects were absent in germ-free mice fed emulsifiers, suggesting that the microbiota probably mediates these effects.113 Preliminary results suggest that the same emulsifiers also increase anxiety-related behaviours and brain neuropeptide systems involved in these behaviours (M. K. Holder, B. Chassaing, N. V. Peters, J. Whylings, A. Gewirtz and G. J. de Vries, unpublished observations.).
Adding factors to the diet that promote a healthy microbiota, and avoiding factors that do the opposite, appears to be the most direct way of promoting brain health. However, as indicated in the present review, the gut microbiota is influenced by a variety of factors, including hormones and the activity of the gut microbiome-brain axis. Identifying the links between the gut microbiome and brain is clinically important and may provide insight into the aetiology of behavioural disorders discussed above that have an important social component.62
Understanding the role of the gut microbiome in human health presents a number of challenges. Although germ-free mice have been very effective in studying the gut microbiome, they have their limitations and caveats as discussed above, including alterations in blood-brain barrier, neuroanatomy, stress response and behaviour.79,84,95 Good animal models are essential for studying causal links and identify potential targets for therapeutic interventions. However, it is critical to move beyond strictly correlational studies in animal studies that reveal changes in the gut microbiota under different conditions. In the future, more functional studies that manipulate the gut microbiota in specific and directed ways will serve to move the field forward. To achieve this more specific manipulation of the gut microbiota, the use of bacteriophage therapy has been proposed.114 Bioengineered phages may be able to provide targeted infection and lysis of bacteria in the gut, and thus offer therapeutic options and a much more complete understanding of the gut microbiome-brain axis. In addition, the isolation and growing in culture of specific anaerobic microbes will enable essential add-back experiments in animal models and provide important tools for treating human disease in the future. Given the profound effects of the endocrine system on human health, it will be critical to gain a better understanding of how hormones influence the gut microbiome-brain axis. For example, it will be essential to determine how age-related changes in oestrogen and androgen levels affect the gut microbiota and the impact on vulnerability to disease. In addition, because of the interesting sexually dimorphic relationship between the gut microbiome and steroid hormones coming from peripheral glands (eg, testis, ovary and adrenal glands), it will be important in the future to study the role of neuro-active steroids (ie, steroids directly synthesised and/or metabolised in the nervous system) in the gut-brain axis. Indeed, in this context, it is important to highlight that neuroactive steroids represent important physiological regulators of the nervous system and that their levels are differentially affected in the two sexes by nervous pathologies, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, traumatic brain injury, spinal cord injury, stroke, diabetic encephalopathy, autism, anxiety and depression.115,116 Moreover, neuro-active steroids play an important role in neuroinflammatory processes in the brain, which in turn modulate behavioural states.117–119 Because these neuroinflammatory processes are responsive to peripheral immune activation, eg, by gut dysbiosis, these molecules may play an important integrative role in the gut microbiome-brain axis.
ACKNOWLEDGEMENTS
We thank Kiran Sandhu for her expertise with respect to the preparation of Figure 1 and the Fondazione Cavalieri-Ottolenghi (Torino), Department of Neuroscience (Torino), and University of Torino, for their support for the organisation of the International Meeting on Steroids and Nervous System, which hosted the roundtable that was the impetus for this review.
REFERENCES
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Wang P, Hu X, Chen F. The gut microbiota: a treasure for human health. Biotechnol Adv. 2016;34:1210–1224. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi J, Shanahan F. The normal intestinal microbiota. Curr Opin Infect Dis. 2007;20:508–513. [DOI] [PubMed] [Google Scholar]
- 6.Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495–507. [DOI] [PubMed] [Google Scholar]
- 7.Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. [DOI] [PubMed] [Google Scholar]
- 8.Adami AJ, Bracken SJ. Breathing better through bugs: asthma and the microbiome. Yale J Biol Med. 2016;89:309–324. [PMC free article] [PubMed] [Google Scholar]
- 9.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–413. [DOI] [PubMed] [Google Scholar]
- 10.Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol. 2015;91:1–62. [DOI] [PubMed] [Google Scholar]
- 11.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. [DOI] [PubMed] [Google Scholar]
- 12.Gulden E, Wong FS, Wen L. The gut microbiota and type 1 diabetes. Clin Immunol. 2015;159:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Cepero AA, Palacios C. Association of the intestinal microbiota and obesity. P R Health Sci J. 2015;34:60–64. [PubMed] [Google Scholar]
- 14.Melli LC, do C-RM, Araujo-Filho HB, Sole D, de Morais MB. Intestinal microbiota and allergic diseases: a systematic review. Allergol Immunopathol (Madr). 2015;44:177–188. [DOI] [PubMed] [Google Scholar]
- 15.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 16.Yang NJ, Chiu IM. Bacterial signaling to the nervous system through toxins and metabolites. J Mol Biol. 2017;429:587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondot S, de Wouters T, Dore J, Lepage P. The human gut microbiome and its dysfunctions. Dig Dis. 2013;31:278–285. [DOI] [PubMed] [Google Scholar]
- 18.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373–403. [DOI] [PubMed] [Google Scholar]
- 19.Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasarevic E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields CT, Chassaing B, Paul MJ, Gewirtz AT, de Vries GJ. Vasopressin deletion is associated with sex-specific shifts in the gut microbiome. Gut Microbes. 2017;July 31:1–13. 10.1080/19490976.2017.1356557. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Indias I, Sanchez-Alcoholado L, Sanchez-Garrido MA, et al. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology. 2016;157:4888–4898. [DOI] [PubMed] [Google Scholar]
- 24.Menon R, Watson SE, Thomas LN, et al. Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 2013;79:5763–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor-beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tetel MJ, Pfaff DW. Contributions of estrogen receptor-alpha and estrogen receptor-beta to the regulation of behavior. Biochim Biophys Acta. 2010;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox-York KA, Sheflin AM, Foster MT, et al. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol Rep. 2015;3:pii: e12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller S, Saunier K, Hanisch C, et al. Differences in fecal micro-biota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominianni C, Sinha R, Goedert JJ, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE. 2015;10:e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. [DOI] [PubMed] [Google Scholar]
- 32.Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS ONE. 2016;11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuhrman BJ, Feigelson HS, Flores R, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99:4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goedert JJ, Jones G, Hua X, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107:pii: djv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 38.Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javurek AB, Spollen WG, Johnson SA, et al. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol. 2014;592:2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1–9. [DOI] [PubMed] [Google Scholar]
- 42.El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther. 2015;37:954–967. [DOI] [PubMed] [Google Scholar]
- 43.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 44.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. [DOI] [PubMed] [Google Scholar]
- 46.Cryan JF, Dinan TG. More than a gut feeling: the microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology. 2015;40:241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis DJ, Doerr HM, Grzelak AK, et al. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci Rep. 2016;6:33726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res. 2016;36:889–898. [DOI] [PubMed] [Google Scholar]
- 50.Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. 2015;28:1–6. [DOI] [PubMed] [Google Scholar]
- 51.Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. [DOI] [PubMed] [Google Scholar]
- 52.Inoue R, Sakaue Y, Sawai C, et al. A preliminary investigation on the relationship between gut microbiota and gene expressions in peripheral mononuclear cells of infants with autism spectrum disorders. Biosci Biotechnol Biochem. 2016;80:2450–2458. [DOI] [PubMed] [Google Scholar]
- 53.O’Mahony SM, Stilling RM, Dinan TG, Cryan JF. The microbiome and childhood diseases: focus on brain-gut axis. Birth Defects Res C Embryo Today. 2015;105:296–313. [DOI] [PubMed] [Google Scholar]
- 54.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheperjans F Gut microbiota, 1013 new pieces in the Parkinson’s disease puzzle. Curr Opin Neurol. 2016;29:773–780. [DOI] [PubMed] [Google Scholar]
- 56.Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016;74:624–634. [DOI] [PubMed] [Google Scholar]
- 57.Winek K, Dirnagl U, Meisel A. The gut microbiome as therapeutic target in central nervous system diseases: implications for stroke. Neurotherapeutics. 2016;13:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parracho H, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. [DOI] [PubMed] [Google Scholar]
- 59.Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. [DOI] [PubMed] [Google Scholar]
- 60.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finegold SM, Molitoris D, Song YL, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16. [DOI] [PubMed] [Google Scholar]
- 62.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep. 2013;15:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelop-mental disorders. Cell. 2013;155:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim JS, Lim MY, Choi Y, Ko G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol Brain. 2017;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. [DOI] [PubMed] [Google Scholar]
- 67.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. [DOI] [PubMed] [Google Scholar]
- 69.Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harach T, Marungruang N, Duthilleul N, et al. Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandscheid C, Schuck F, Reinhardt S, et al. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimers Dis. 2017;56:775–788. [DOI] [PubMed] [Google Scholar]
- 72.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. [DOI] [PubMed] [Google Scholar]
- 74.Lyte M Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9:e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyte M Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014;5:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;81:7221–7239. [DOI] [PubMed] [Google Scholar]
- 77.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 81.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. [DOI] [PubMed] [Google Scholar]
- 82.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics. 2012;39:375–384. [DOI] [PubMed] [Google Scholar]
- 83.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26:1615–1627. [DOI] [PubMed] [Google Scholar]
- 86.Huang XZ, Zhu LB, Li ZR, Lin J. Bacterial colonization and intestinal mucosal barrier development. World J Clin Pediatr. 2013;2:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coskun M Intestinal epithelium in inflammatory bowel disease. Front Med (Lausanne). 2014;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hyland NP, Quigley EM, Brint E. Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol. 2014;20:8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111:E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. [DOI] [PubMed] [Google Scholar]
- 92.Dinan TG, Scott LV. Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J Anat. 2005;207:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y-W, Liu W-H, Wu C-C, et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016;1631:1–12. [DOI] [PubMed] [Google Scholar]
- 95.De PG, Blennerhassett P, Lu J, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6:7735. [DOI] [PubMed] [Google Scholar]
- 96.Park AJ, Collins J, Blennerhassett PA, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. [DOI] [PubMed] [Google Scholar]
- 98.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. [DOI] [PubMed] [Google Scholar]
- 99.Steenbergen L, Sellaro R, van HS, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. [DOI] [PubMed] [Google Scholar]
- 100.Wessely S, Chalder T, Hirsch S, Wallace P, Wright D. Psychological symptoms, somatic symptoms, and psychiatric disorder in chronic fatigue and chronic fatigue syndrome: a prospective study in the primary care setting. Am J Psychiatry. 1996;153:1050–1059. [DOI] [PubMed] [Google Scholar]
- 101.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rezzi S, Ramadan Z, Martin FP, et al. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res. 2007;6:4469–4477. [DOI] [PubMed] [Google Scholar]
- 104.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. [DOI] [PubMed] [Google Scholar]
- 106.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/−mice. Nature. 2012;487:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris). 2008;56:305–309. [DOI] [PubMed] [Google Scholar]
- 109.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. [DOI] [PubMed] [Google Scholar]
- 111.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 113.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–U192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giatti S, Romano S, Pesaresi M, et al. Neuroactive steroids and the peripheral nervous system: an update. Steroids. 2015;103:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melcangi RC, Giatti S, Garcia-Segura LM. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: sex-specific features. Neurosci Biobehav Rev. 2016;67:25–40. [DOI] [PubMed] [Google Scholar]
- 117.Slowik A, Lammerding L, Hoffmann S, Beyer C. Brain inflammasomes in stroke and depressive disorders: regulation by estrogen. J Neuroendocrinol. 2017;May 6 10.1111/jne.12482. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 118.Vasconcelos AR, Cabral-Costa JV, Mazucanti CH, Scavone C, Kawamoto EM. The role of steroid hormones in the modulation of neuroinflammation by dietary interventions. Front Endocrinol (Lausanne). 2016;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giatti S, Boraso M, Melcangi RC, Viviani B. Neuroactive steroids, their metabolites, and neuroinflammation. J Mol Endocrinol. 2012;49:R125–R134. [DOI] [PubMed] [Google Scholar]