Abstract
Background
Encapsulation devices have the potential to enable cell-based insulin replacement therapies (such as human islet or stem cell–derived β cell transplantation) without immunosuppression. However, reasonably sized encapsulation devices promote ischemia due to high β cell densities creating prohibitively large diffusional distances for nutrients. It is hypothesized that even acute ischemic exposure will compromise the therapeutic potential of cell-based insulin replacement. In this study, the acute effects of high-density ischemia were investigated in human islets to develop a detailed profile of early ischemia induced changes and targets for intervention.
Methods
Human islets were exposed in a pairwise model simulating high-density encapsulation to normoxic or ischemic culture for 12 hours, after which viability and function were measured. RNA sequencing was conducted to assess transcriptome-wide changes in gene expression.
Results
Islet viability after acute ischemic exposure was reduced compared to normoxic culture conditions (P < 0.01). Insulin secretion was also diminished, with ischemic β cells losing their insulin secretory response to stimulatory glucose levels (P < 0.01). RNA sequencing revealed 657 differentially expressed genes following ischemia, with many that are associated with increased inflammatory and hypoxia-response signaling and decreased nutrient transport and metabolism.
Conclusions
In order for cell-based insulin replacement to be applied as a treatment for type 1 diabetes, oxygen and nutrient delivery to β cells will need to be maintained. We demonstrate that even brief ischemic exposure such as would be experienced in encapsulation devices damages islet viability and β cell function and leads to increased inflammatory signaling.
Human pancreatic islets under high density and hypoxic conditions similar to the ones in encapsulation devices show lower viability and insulin secretion. RNAseq analysis shows increase expression of for genes related to inflammation and hypoxia and decrease in expression of genes associated to nutrient transport and metabolism.
Islet transplantation (ITx), a form of cell-based insulin replacement therapy, is an attractive approach for the treatment of uncontrolled or “brittle” type 1 diabetes. However, large scale application of ITx is limited by the need for lifelong immunosuppression and a shortage of human cadaveric pancreas donors, which is exacerbated by the need for islets from multiple pancreata per patient to achieve insulin independence.1,2 Encapsulation (immunoisolation) devices have the potential to address these critical limitations. They have demonstrated efficacy in protecting transplanted islets even in the absence of immunosuppression, and may allow for alternative cell-based insulin replacement therapies for type 1 diabetes, for example, by enabling the use of stem cell-derived β cells (SC-βs) which can be sourced on a virtually unlimited scale.3-10
However, encapsulating therapeutic β cell doses into reasonably sized (postage stamp) immunoisolation devices creates a high-density environment in which the availability of nutrients (especially glucose and oxygen) is limited, leading to ischemia.7 This has ultimately hindered the clinical application of macroencapsulation in β cell replacement therapies. Insufficient oxygen is a particular concern for the insulin secreting β cells within islets. β cells lack sufficient levels of the enzyme lactate dehydrogenase α to generate ATP using anaerobic glycolysis, and overexpression of lactate dehydrogenase α in β cells diminishes their glucose responsiveness.11,12 Moreover, once separated from their native vasculature, islets depend solely on diffusion for the delivery of oxygen and energetic substrates. Deprivation of these critical nutrients and the accumulation of toxic metabolites lead to impaired insulin secretion and eventual cell death.13-19 These effects are even more pronounced in islets with large diameters, and clinical ITx outcomes are improved for patients receiving preparations composed of smaller average diameter islets.20-22
Considering the recent surge of interest in immunoisolation devices and the benefits that their application can offer, it will be of critical importance to understand how these devices impact β cell physiology to facilitate successful clinical outcomes. Therefore, we investigated the viability, insulin secretion, and transcriptional adaptations of a highly purified human islets with an emphasis on β cell function, stress, and inflammation after acute ischemic exposure in high-density “pellet” model. This model has been characterized previously in the context of islet shipping, and is used here to simulate and study the effects of the high density environments created by delivery of therapeutic β cell doses in reasonably sized immunoisolation devices.23,24
MATERIALS AND METHODS
Islet Source and Maintenance
Human islets (n = 11 independent preparations) were obtained from the Integrated Islet Distribution Program, the University of Minnesota, the University of Arizona, the McGill University Health Centre, and the University of California-San Francisco. Islets were cultured in oxygen permeable silicone rubber membrane GRex vessels (Wilson Wolf, St Paul, MN). Islet culture media consisted of CMRL 1066 (Mediatech, Inc., Manassas, VA) supplemented with 0.5% human serum albumin ( BioChemed Services, Winchester, VA), 1% heparin (SAGENT, Schaumburg, IL), 1% l-glutamine (Mediatech, Inc.), and 1% penicillin/streptomycin (GE Healthcare Life Sciences, Logan, UT). Before experiments, islets were maintained at 25°C and ambient pO2 supplemented with 5% CO2.
Induction of Pelletized Culture
After standard culture, islets from each donor were divided into normoxic and ischemic conditions. Ischemia was modeled through high-density “pelletized” culture in which 10000 islet equivalents (IE) as quantified by DNA were allowed to settle in the bottom of a 1.5-mL centrifuge tube.25 This condition was selected because it mimics both traditional islet shipping conditions as well as the high-density environments that can result from encapsulation. For the normoxic condition, an equal number of islets were cultured in a 10-cm2 GRex vessel (Wilson Wolf). Normoxic and ischemic islets were cultured for 12 hours at 37°C, ambient O2, and 5% CO2.
Islet Encapsulation
To test the effects of high density encapsulation on human islets and verify comparable effects to pelletized culture, 8000 IE as quantified by DNA were loaded into 4.5 μL (0.34 cm2) TheraCyte (TheraCyte, Laguna Hills, CA) devices using a Hamilton syringe. Devices were then placed into oxygen permeable, 10 cm2 GRex vessels (Wilson Wolf) filled with culture media and maintained for 12 hours at 37°C, ambient O2, and 5% CO2. Encapsulated islets were compared with matched pellets and controls prepared as described above.
Measurement of DNA
Islets were suspended in 1 mL of AT Buffer (1 M solution of ammonium hydroxide in nanopure water, supplemented with 0.2% Triton X-100) and sonicated using a Sonic Dismembrator Model 500 (Fisher Scientific, Waltham, MA) for 30 seconds at 11% amplitude. DNA was assessed using a Quant-iT PicoGreen dsDNA kit (Life Technologies, Carlsbad, CA) according to manufacturer instructions. 96 well plates were read using a SpectraMax M5 plate reader and SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
Measurement of Oxygen Consumption Rate Normalized to DNA
After 12 hours, islets and media were mixed to ensure homogenous sampling. Oxygen consumption rate (OCR) was conducted as previously described.26 Briefly, islets were resuspended in Media 199 (Mediatech, Inc.) warmed to 37°C and divided evenly between 3 OCR chambers of known volumes, or conducted on whole devices as in the case of encapsulated islets (Instech Laboratories, Inc., Plymouth Meeting, PA). Measurements of pO2 in each chamber over time were recorded using fiber optic sensors and NeoFox viewer software (Ocean Optics, Inc., Dunedin, FL). The oxygen consumption rate in nanomoles of O2 per minute was then estimated from the slope of the decline in pO2 over time. This value was normalized to the DNA content of each chamber (as described above) to give a final measurement of OCR/DNA (nmol O2/min*mg DNA).
Measurement of % Viability by Fluorescein Diacetate/Propidium Iodide Staining
From each condition, 100-μL samples of islets were combined with 377.6 μL of dithizone (Sigma-Aldrich, St. Louis, MO). To this, 1.39 μL of fluorescein diacetate (Sigma-Aldrich) and 21.05 μL of propidium iodide (Sigma-Aldrich) were added for final concentrations of 0.067 and 4.0 μM, respectively. Islets were incubated in the dark for 20 minutes, after which they were allowed to settle and 100 μL of islets were placed on a slide for imaging. To determine the proportion of live versus dead cells, islets were imaged on a Zeiss Observer.Z1 (Zeiss, Oberkochen, Germany) using the 10× objective and an AxioCam MRm camera with ZEN 2012 (blue addition) software (Zeiss).
β cell Function
The Biorep Technologies Peri-4.2 Perifusion System (Biorep Techonologies, Inc., Miami Lakes, FL) was used to measure dynamic glucose-stimulated insulin secretion (GSIS). Triplicate measurements using 100 IE each underwent baseline stimulation with 2.8 mM glucose in oxygen-saturated (95% O2) Krebs-Ringer Bicarbonate buffer for 20 minutes before and after a 40-minute stimulation period with 16.7-mM glucose. Perfusate was collected at a rate of 100 μL/min in a 96-well plate. Perfusate was tested for insulin content using an insulin enzyme linked immunosorbent assay (ELISA, Mercodia, Winston-Salem, NC). ELISA plates were read using Softmax Pro software and a SpectraMax M5 plate reader (Molecular Devices). Insulin content was normalized to DNA as described above.
RNA Library Preparation
Paired normoxic and ischemic islets from 4 donors were selected at random for high throughput RNA sequencing (RNAseq). For RNA samples, islets were collected in microcentrifuge tubes and washed twice 1× Dulbecco's phosphate buffered saline to remove serum. RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA quality was assessed by Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and a minimum RIN of 7.0 was required for inclusion in the study. Libraries were prepared using the Illumina TruSeq Stranded mRNA Sample Preparation Kit (Illumina, San Diego, CA). mRNA was sequenced using Illumina HiSeq 2000 sequencer and quantified into transcripts using EA- Quintiles mRNAv8 pipeline.
RNAseq Analysis
Briefly, 50 × 50 base, paired-end reads were checked for quality by comparison to intergenic and ribosomal sequences. Sequencing reads were aligned to the human University of California Santa Cruz known gene transcriptome using RNA-Seq by Expectation Maximization v1.2.0 and transcript abundance was quantified. An average of 97.4 ± 0.4% of total reads mapped to reference genomes. The aligned reads for each gene were summarized as described in Li and Dewey.27 All genes and isoforms have been assigned a normalized coverage rate in fragments per kilobase per million mapped reads (FPKM). Differential gene expression was performed using the edgeR R package scripts (bioconductor.org) using paired sample statistical procedures.28,29 A paired-sample (generalized paired t test) analysis was conducted to investigate the effects of ischemia while adjusting for baseline differences between patients. Genes with a P value less than 0.05 following Benjamini-Hochberg correction were considered to be statistically significant.
Functional Analysis of Differentially Expressed Genes
Significantly upregulated or downregulated genes were submitted to KOBAS 2.0 to determine which canonical pathways were enriched by ischemia, as well as to determine associated gene ontology (GO) terms.30 Significant enrichments were defined as a corrected P value less than 0.05 following a Fisher exact test with Benjamini Hochberg correction in KOBAS 2.0. To summarize GO enrichment and reduce redundant terms, ReviGO was used to cluster similar GO terms using small (0.5) SimRel similarity and the whole Uniprot as the database for categorical GO term sizes.31 Significant pathways were manually curated to remove pathways containing redundant or nonspecific gene findings. Preferential association between the lists of upregulated and downregulated genes was also evaluated.
RT qPCR Array
Results from RNAseq were compared with quantitative reverse transcriptase polymerase chain reaction results for a single islet preparation. Purified islet total RNA was run on RT2 Profiler PCR Array Human Hypoxia Signaling Pathway plates (Qiagen) using iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Irvine, CA). Fold changes were determined according to manufactures instructions. Gene expression was normalized to housekeeping genes RPLP0, B2M, and HPRT1.
Statistics
Statistical analysis of physiological parameters was conducted in SAS 9.4 (SAS Institute Inc., Cary, NC). OCR/DNA data were analyzed using Wilcoxon Signed Rank Test. Matched fluorescein diacetate/propidium iodide and OCR/DNA data were analyzed using a Student t test. Perifusion data were analyzed using a generalized linear mixed model for repeated measurements. Fold changes from the RT qPCR array were correlated to fold changes from RNAseq using linear regression analysis in GraphPad Prism version 6.07 (GraphPad Software, Inc., La Jolla, CA). Unless otherwise indicated, data represent mean ± standard deviation.
RESULTS
Donor Metrics
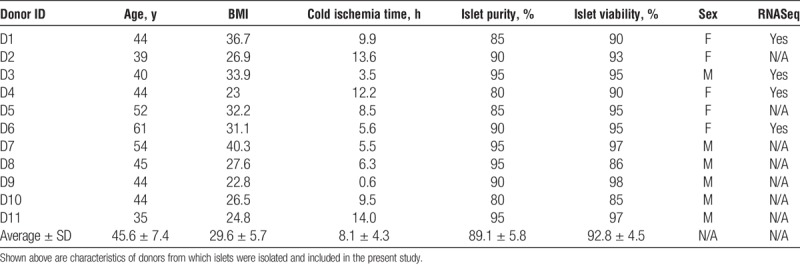
Individual donor characteristics and islet quality parameters (% viability measured by membrane integrity staining, purity, and cold ischemia time) as reported by the distributing isolation center are presented in Table 1.
TABLE 1.
Donor metrics
Pellet Model Characterization and Comparison to Encapsulation
The viability of islets from n = 2 human islet preparations was compared under matched normoxic, pelletized, and encapsulated conditions to confirm that the ischemic damage caused by the pellet condition is similar to what is caused in devices loaded at high densities.7 Pelletized islets and encapsulated islets showed similar % reductions in OCR/DNA values versus control (57.7 ± 19.5% and 80.3 ± 21.1%, respectively).
Islet Viability
Islet viability was determined in n = 8 independent islet preparations. Average OCR/DNA values for normoxic islets align closely with clinical averages observed for both islet auto and allotransplantation.32,33 Islet OCR/DNA values are reduced after warm ischemic exposure (P < 0.01, Figure 1), which correlates to lower, although not entirely diminished, viability. In a subcohort of n = 4 independent islet preparations, OCR/DNA results were compared with fluorescein diacetate/propidium iodide staining. Staining revealed a significant loss of viability for control versus pelletized islets (% 87.3 ± 1.0% vs 8.8 ± 5.5%, respectively, P < 0.01). OCR/DNA measurements for matched samples were similarly reduced for control vs. pelletized islets (122.9 ± 53.3 and 53.4 ± 12.0 nmol O2/min*mg DNA, respectively, P < 0.05).
FIGURE 1.
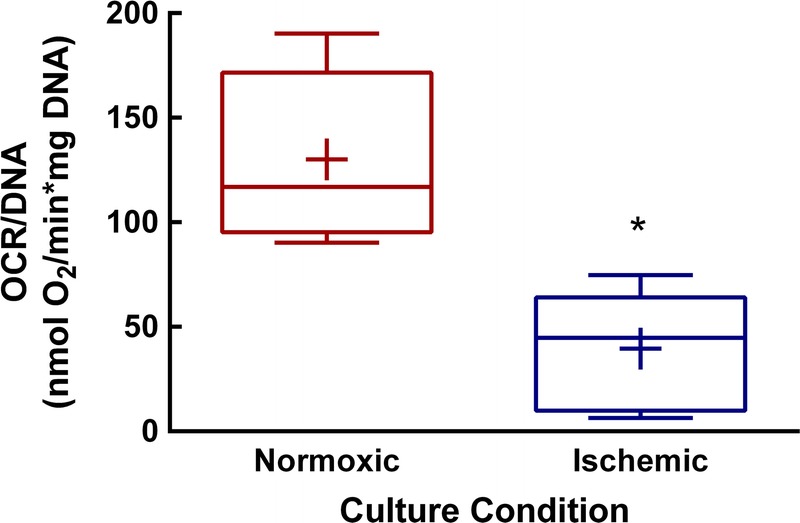
Human islet viability is reduced after acute ischemia. After 12 hours of control (normoxic) or ischemic exposure, islet viability was determined by OCR/DNA. Shown above are values for n = 8 paired experiments. *P = 0.01. Data mean are indicated by +, whiskers indicate minimum and maximum values. Box bounds indicate upper and lower quartiles, and the median value is indicated by the line within the box.
B Cell Function
GSIS was measured in a subset of control and ischemic islet pairs (n = 4 independent preparations, Figure 2). GSIS of ischemic islets is virtually absent compared with control islets. Basal secretion was not different between control and ischemic islets (Figure 3A). However, under stimulatory glucose concentrations (Figure 3B), ischemic islets secrete less insulin than control islets (P < 0.01). Normoxic islets show elevated (P < 0.01) insulin secretion in response to stimulatory glucose concentrations, whereas ischemic islets are unresponsive.
FIGURE 2.
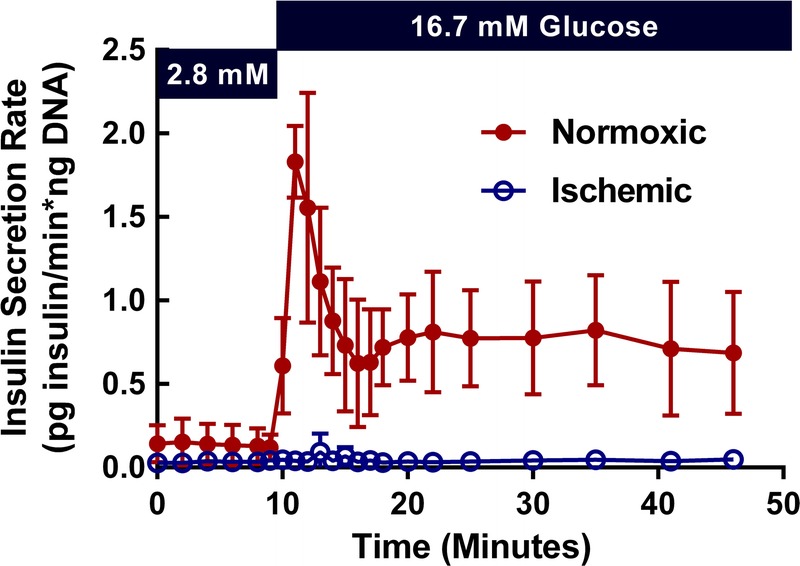
Human islet function is absent after acute ischemic exposure. To determine β cell function, GSIS was measured on a perifusion system. Shown above are the insulin secretion profiles for control and ischemic islets. The figure indicates mean ± SD values for n = 4 pairs of islets.
FIGURE 3.
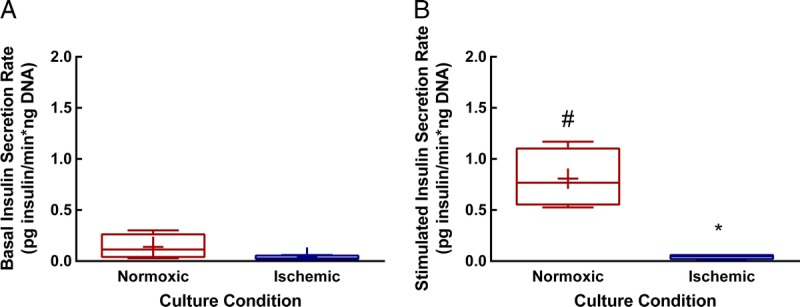
Basal and stimulated insulin secretion in normoxic versus ischemic human islets. Area under the curve was calculated and divided by the corresponding time interval to give the average insulin secretion rate for basal and stimulated portions of the curves shown in Figure 2. A, there is no significant difference in the rate of insulin secretion under basal conditions. B, ischemic islets show significantly lower insulin secretion than do normoxic islets under stimulated conditions. *p = 0.006 versus stimulated normoxic condition. Similarly, ischemic islets do not have a significant increase in insulin secretion under stimulatory glucose conditions, whereas normoxic islets do show a significant increase. #P = 0.009 versus basal rate. Means for n = 4 samples are indicated by +, whiskers indicate minimum and maximum values. Box bounds indicate upper and lower quartiles, and the median value is indicated by the line within the box.
Global Gene Expression Changes After Ischemia
An average of 19 932 ± 138 genes were detected with RNAseq. Of these, 657 genes were differentially expressed in ischemic versus control islets (P < 0.05) (Figure 4A). Genes most upregulated include immune molecules interleukins 6 and 8 (IL6, IL8), chemokine 3 (CCL3), and chemokine 3 like (CXCL3). Genes most downregulated include the inward rectifier K+ channel Kir 3.1 (KCNJ3) and Na+-coupled neutral amino acid transporter 4 (SLC38A4) (Figure 4B).
FIGURE 4.
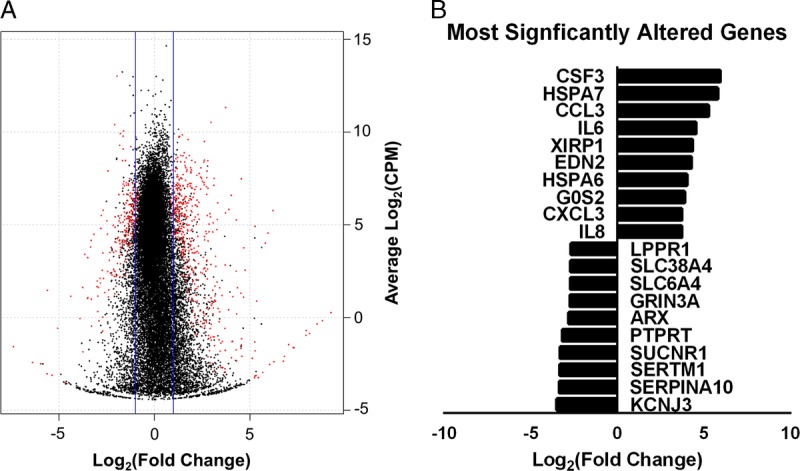
RNAseq summary. Shown above is a summary of differential expression for RNAseq data. A, A volcano plot indicating the distribution of genes detected by log2 (fold change) and log2 (CPM). Differentially expressed genes are shown in red, and nondifferentially expressed genes are indicated in black. B, The most upregulated and downregulated genes in ischemic islets as determined by fold change are shown. Genes had a minimum CPM cutoff value of 1. CPM, counts per million.
Fold changes calculated from RNAseq correlated (P < 0.0001, r2 = 0.75) to fold changes calculated by RT qPCR array (Figure S1, SDC, http://links.lww.com/TP/B421). The high correlation between independent measures of gene expression corroborates findings from RNAseq model.
Functional Analysis of Differentially Expressed Genes
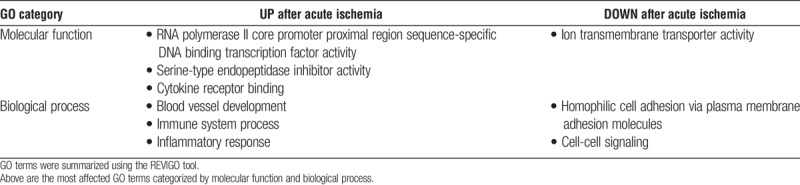
Canonical pathways significantly associated with differentially expressed genes were identified (Table 2). Specific differentially expressed genes associated with inflammation and cytokine signaling, and also associated with pathways for the HIF-1α response, AP-1 (FOS) signaling, and ion transport are detailed in Figure 5. Enriched GO terms were in agreement with pathway findings, showing enhancement of cytokine signaling and inflammation as well as downregulation of cellular ion transport (Table 3). Genes associated with hypoxia and nutrient deprivation (including SLC2A1, HK1, HMOX1, ARG1, and UPP1) were also upregulated (P < 0.05).
TABLE 2.
Signaling pathways enriched following acute ischemia in human islets
FIGURE 5.

Heatmaps for significantly enriched pathways. Genes in enriched pathways as well as related DE genes of interest were plotted as heatmaps. A, A heatmap detailing changes in inflammatory genes. B, Changes in genes involved in the hypoxia response, cell death, ion transport, and cell damage. FPKM values were converted to FPKM + 1 before log transformation. Values in heat maps represent log2 (fold change).
TABLE 3.
GO terms associated with significantly upregulated or downregulated genes after acute ischemia in human islets
DISCUSSION
Ischemia remains a pervasive issue that directly influences the outcomes of cell-based insulin replacement therapies, such as ITx. In this study, we implement an ischemic model that has previously been used to study islet shipping and captures the conditions seen in high density islet encapsulation, including a significant reduction of islet viability which is associated with oxygen and nutrient deprivation, and consistent with results from the transcriptome analysis.19,23,24 Furthermore, we demonstrate that the viable population of islets following acute ischemia exhibits significant β cell dysfunction corresponding to global alterations in gene expression. Inflammatory responses were evident in the gene signatures of islets after ischemia, with concurrent downregulation of genes involved in nutrient and ion transport. The persistence of β cell dysfunction due to ischemic stress has been previously demonstrated, whether due to cellular reprogramming or ischemia-reperfusion injury, and the endurance of a concurrent proinflammatory signature is likely, given the present work.19,34,35 Interestingly, kidney capsule islet transplants represent a commonly used in vivo correlate to our high-density in vitro model in which islets are transplanted as a “pellet” below the renal capsule.36 Although this method induces less ischemic stress than what might be experienced in an immunoisolation device, when these islets are exposed to nerve growth factor (which has an anti-apoptotic effect), graft function is improved.37-39 This supports our finding that even acute ischemia is detrimental to islet viability and function and indicates that early mitigation of these factors is beneficial to transplant outcomes.
The differential gene expression profile caused by acute ischemia was predominantly increased inflammatory and stress response signaling. More specifically, there was increased expression of key inflammatory genes (including TNF and IL6), which are known to contribute to islet death in the peritransplant period by the instant blood mediated inflammatory reaction.40 The link between hypoxia and this proinflammatory profile has been demonstrated previously in rat islets.41 This increased inflammatory signaling may be important not only from an immunological standpoint, but also in the context of β cell function. TNFα has previously been shown to inhibit GSIS in β cell lines as well as downstream insulin signaling in adipocytes.42-44 The potential damage to insulin signaling is compelling, as autocrine insulin signaling in β cells has been demonstrated to influence insulin expression.45 Key genes involved in the coupling of glucose sensing to insulin secretion (for example KCNJ11, which encodes the major subunit of the ATP-sensitive K+ channel) are also significantly downregulated. Together with the effects of inflammatory factors, these changes may underlie the significant loss of β cell function. By mitigating these damaging adaptations, loss of islets in the peritransplant period may be minimized and ultimately help to facilitate lower curative doses of islets for diabetes reversal.46
Lifelong immunosuppression remains a critical barrier which limits the application of β cell replacement therapies. Implantable macroencapsulation devices are a possible solution and have been demonstrated to be alloprotective in animal models as well as in humans.3,47 However, packing therapeutic β cell doses into practically sized devices results in a high-density environment in which diffusion of oxygen and nutrients is severely limited. This study demonstrates that such high-density environments are damaging to β cell function and may ultimately affect transplant outcomes.5,48 Addressing the effects if ischemia by ensuring adequate nutrient delivery will be critical to the success of cell-based insulin replacement therapies going forward. With respect to maintaining oxygenation, numerous advances have been made with regard to supplying oxygen to the pancreas during the preservation period as well as to isolated islets during culture.49,50 Attempts have also been made to supply islets in encapsulation devices with oxygen, and this remains an active area of research although a permanent solution has yet to be achieved.3
ACKNOWLEDGMENTS
The authors would like to acknowledge the members of the Papas laboratory and collaborators, including William Purivs, Nicholas Price, Ivan Georgiev, Anna Maria Burachek, Alexandra Hoeger, Diana Molano, Jana Jandova, Kristina Rivera, Aikaterina Assimacopoulos, and Breonna Smith for their support. The authors would like to thank our collaborators at the University of California-San Francisco, McGill University Health Centre, and the University of Minnesota as well as the Integrated Islet Distribution Program for supplying islets used in these studies, as well as Dr. Linda Tempelman and Dr. Jan Burt for their advisement and mentorship. The authors would additionally like the ARCS Foundation Phoenix Chapter for their generous support.
Footnotes
Support for this study was provided by the Sanofi-Aventis Group, Icagen, Inc., the Heart, Lung, and Blood Training Grant (T32HL007249), NIH/NIDDK Phase 1 SBIR Grant (Giner, Inc.) R43 DK100999, NIH/NIDDK DP3 Grant (K.K. Papas) 1DP3 DK106933-01, and the Juvenile Diabetes Research Foundation (JDRF Grant JDRF: 5-2013-141 and 3-SRA-2015-40-Q-R).
K.K.P. declares a financial interest in Giner, Inc. This interest has been properly disclosed to the University of Arizona Institutional Review Committee and is managed by a Financial Conflict of Interest Management Plan in accordance with its conflict of interest policies.
K.E.S. coordinated and contributed to physiological assessments and analyses, contributed directly to manuscript preparation and content, figures, and review. A.C.K. coordinated and conducted RNAseq analysis and directly contributed to article preparation and content, figures, and review. C.M. contributed to physiological assessments, analysis, and article review. C.S.W. contributed to physiological assessments, analysis, and article review. F.M.MC. contributed to RNAseq analysis and article review. L.V.S. contributed to physiological assessments and article review. V.B. contributed to RNAseq analysis and article review. P.S. contributed to study design, article review, and funding. A.C.G. conducted statistical analysis and article review. J.B.S. performed fluorescence microscopy and quantification and contributed to article review. J.P.K. consulted on physiological assessments and article review. R.M.L. contributed to study design, consulted on physiological assessments, and article review. S.W.L. contributed to study design, article preparation, content, statistics, and review. K.K.P. is the principal investigator, contributed directly to study funding, conception, design, data analysis and presentation, article preparation, and review.
REFERENCES
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakuma Y, Ricordi C, Miki A, et al. Factors that affect human islet isolation. Transplant Proc. 2008;40:343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig B, Reichel A, Steffen A, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A. 2013;110:19054–19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasikala M, Rao GV, Vijayalakshmi V, et al. Long-term functions of encapsulated islets grafted in nonhuman primates without immunosuppression. Transplantation. 2013;96:624–632. [DOI] [PubMed] [Google Scholar]
- 5.Sörenby AK, Kumagai-Braesch M, Sharma A, et al. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model. Transplantation. 2008;86:364–366. [DOI] [PubMed] [Google Scholar]
- 6.Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014;67–68:93–110. [DOI] [PubMed] [Google Scholar]
- 7.Papas KK, Avgoustiniatos ES, Suszynski TM. Effect of oxygen supply on the size of implantable islet-containing encapsulation devices. Panminerva Med. 2016;58:72–77. [PubMed] [Google Scholar]
- 8.Domínguez-Bendala J, Inverardi L, Ricordi C. Stem cell-derived islet cells for transplantation. Curr Opin Organ Transplant. 2011;16:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk K, Hao E, Lahmy R, et al. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12:807–814. [DOI] [PubMed] [Google Scholar]
- 10.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcazar O, Tiedge M, Lenzen S. Importance of lactate dehydrogenase for the regulation of glycolytic flux and insulin secretion in insulin-producing cells. Biochem J. 2000;352(Pt 2):373–380. [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 13.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of langerhans. Diabetes. 1993;42:12–21. [DOI] [PubMed] [Google Scholar]
- 14.Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis. 2015;7:1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ip M, Mokhlesi B. Sleep and glucose intolerance/diabetes mellitus. Sleep Med Clin. 2007;2:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, Zhang Q, Tan J, et al. Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and protective effects of antioxidant intervention. Nutr Diabetes. 2014;4:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SJ, Huang HH, Kover K, et al. Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis. 2010;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giuliani M, Moritz W, Bodmer E, et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14:67–76. [DOI] [PubMed] [Google Scholar]
- 19.Lablanche S, Cottet-Rousselle C, Argaud L, et al. Respective effects of oxygen and energy substrate deprivation on beta cell viability. Biochim Biophys Acta. 2015;1847:629–639. [DOI] [PubMed] [Google Scholar]
- 20.Tannock IF. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972;45:515–524. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann R, Zuellig RA, Kugelmeier P, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. [DOI] [PubMed] [Google Scholar]
- 22.Suszynski TM, Wilhelm JJ, Radosevich DM, et al. Islet size index as a predictor of outcomes in clinical islet autotransplantation. Transplantation. 2014;97:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichii H, Sakuma Y, Pileggi A, et al. Shipment of human islets for transplantation. Am J Transplant. 2007;7:1010–1020. [DOI] [PubMed] [Google Scholar]
- 24.Papas KK, Colton CK, Gounarides JS, et al. NMR spectroscopy in beta cell engineering and islet transplantation. Ann N Y Acad Sci. 2001;944:96–119. [DOI] [PubMed] [Google Scholar]
- 25.Colton CK, Papas KK, Pisania A, et al. Characterization of islet preparations. In: Halberstadt C, Emerich D, eds. Cellular transplantation: From laboratory to clinic. Oxford, UK: Elsevier, Inc; 2007:85–133. [Google Scholar]
- 26.Papas KK, Pisania A, Wu H, et al. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98:1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie C, Mao X, Huang J, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39(Web Server issue):W316–W322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supek F, Bošnjak M, Škunca N, et al. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzmann JP, Pepper AR, Gala-Lopez B, et al. Human islet viability and function is maintained during high-density shipment in silicone rubber membrane vessels. Transplant Proc. 2014;46:1989–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papas KK, Bellin MD, Sutherland DE, et al. Islet oxygen consumption rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS One. 2015;10:e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantley J, Walters SN, Jung MH, et al. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant. 2013;22:2147–2159. [DOI] [PubMed] [Google Scholar]
- 35.Emamaullee J, Liston P, Korneluk RG, et al. XIAP overexpression in islet beta-cells enhances engraftment and minimizes hypoxia-reperfusion injury. Am J Transplant. 2005;5:1297–1305. [DOI] [PubMed] [Google Scholar]
- 36.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp. 2007:404 doi(9):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hata T, Sakata N, Yoshimatsu G, et al. Nerve growth factor improves survival and function of transplanted islets via TrkA-mediated β cell proliferation and revascularization. Transplantation. 2015;99:1132–1143. [DOI] [PubMed] [Google Scholar]
- 38.Pierucci D, Cicconi S, Bonini P, et al. NGF-withdrawal induces apoptosis in pancreatic beta cells in vitro. Diabetologia. 2001;44:1281–1295. [DOI] [PubMed] [Google Scholar]
- 39.Shimoke K, Sasaya H, Ikeuchi T. Analysis of the role of nerve growth factor in promoting cell survival during endoplasmic reticulum stress in PC12 cells. Methods Enzymol. 2011;490:53–70. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16:620–626. [DOI] [PubMed] [Google Scholar]
- 41.de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12:867–875. [PubMed] [Google Scholar]
- 42.Peraldi P, Hotamisligil GS, Buurman WA, et al. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS, Murray DL, Choy LN, et al. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HE, Choi SE, Lee SJ, et al. Tumour necrosis factor-alpha-induced glucose-stimulated insulin secretion inhibition in INS-1 cells is ascribed to a reduction of the glucose-stimulated Ca2+ influx. J Endocrinol. 2008;198:549–560. [DOI] [PubMed] [Google Scholar]
- 45.Xu GG, Rothenberg PL. Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine beta-cell regulation. Diabetes. 1998;47:1243–1252. [DOI] [PubMed] [Google Scholar]
- 46.Bennet W, Groth CG, Larsson R, et al. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125–133. [DOI] [PubMed] [Google Scholar]
- 47.Sweet IR, Yanay O, Waldron L, et al. Treatment of diabetic rats with encapsulated islets. J Cell Mol Med. 2008;12(6B):2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki K, Bonner-Weir S, Trivedi N, et al. Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation. 1998;66:21–28. [DOI] [PubMed] [Google Scholar]
- 49.Papas KK, Avgoustiniatos ES, Tempelman LA, et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412–3414. [DOI] [PubMed] [Google Scholar]
- 50.Scott WE, 3rd, Weegman BP, Ferrer-Fabrega J, et al. Pancreas oxygen persufflation increases ATP levels as shown by nuclear magnetic resonance. Transplant Proc. 2010;42:2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


