Abstract
The soil saprophyte and Tier I select agent Burkholderia pseudomallei can cause rapidly fatal infections in humans and animals. The capability of switching to an intracellular life cycle during infection appears to be a decisive trait of B. pseudomallei for causing disease. B. pseudomallei harbors multiple type VI secretion systems (T6SSs) orthologs of which are present in the surrogate organism Burkholderia thailandensis. Upon host cell entry and vacuolar escape into the cytoplasm, B. pseudomallei and B. thailandensis manipulate host cells by utilizing the T6SS-5 (also termed T6SS1) to form multinucleated giant cells for intercellular spread. Disruption of the T6SS-5 in B. thailandensis causes a drastic attenuation of virulence in wildtype but not in mice lacking the central innate immune adapter protein MyD88. This result suggests that the T6SS-5 is deployed by the bacteria to overcome innate immune responses. However, important questions in this field remain unsolved including the mechanism underlying T6SS-5 activity and its physiological role during infection. In this review, we summarize the current knowledge on the components and regulation of the T6SS-5 as well as its role in virulence in mammalian hosts.
Keywords: B. pseudomallei, type VI secretion system, intracellular life cycle, multinucleated giant cell formation, virulence
Introduction
Burkholderia pseudomallei is a soil dwelling Gram-negative bacterium that causes the potentially fatal disease melioidosis in humans and animals. Infection with B. pseudomallei may affect virtually any organ and may encompass a wide array of non-specific clinical manifestations ranging from acute pneumonia and sepsis to localized abscess formation, making diagnosis difficult (Currie, 2015; Wiersinga et al., 2018). The mortality rate can reach 40% despite appropriate antibiotic therapy. Southeast Asia and Northern Australia are hyperendemic regions (Cheng and Currie, 2005). However, reports of environmental B. pseudomallei isolates or melioidosis cases from Central and South America, Africa and South Asia indicate that the bacteria are found in the tropics worldwide (Mukhopadhyay et al., 2018; Rolim et al., 2018; Steinmetz et al., 2018; Torres et al., 2018). Furthermore, a recent comprehensive modeling study suggests vast underreporting of melioidosis cases and highlights the need to assess the true global burden and epidemiology of the disease (Limmathurotsakul et al., 2016).
Burkholderia thailandensis is used as a surrogate model for the Tier I Select Agent B. pseudomallei. Reports of human infections with B. thailandensis are exceedingly rare and the LD50 of B. thailandensis in mammalian animal models is at least 100 fold higher than that of B. pseudomallei (Smith et al., 1997; Brett et al., 1998; Lertpatanasuwan et al., 1999; Glass et al., 2006; West et al., 2008; Chang et al., 2017; Gee et al., 2018). Yet, at higher inocula via the respiratory tract, B. thailandensis causes rapidly fatal infections in mice and the manifestations such as neutrophil influx to the lungs, pulmonary inflammatory cytokine response, multifocal pneumonia and extra-pulmonary dissemination are similar to B. pseudomallei infections (West et al., 2008, 2012; Wiersinga et al., 2008a). Furthermore, both bacteria are facultative intracellular parasites and important regulatory systems and virulence factors of B. pseudomallei such as quorum sensing, type III and type VI secretion systems are conserved in B. thailandensis (Haraga et al., 2008; Majerczyk et al., 2014; Toesca et al., 2014). B. pseudomallei encodes six type VI secretion systems (T6SSs) and orthologs of five of them are present in B. thailandensis (Schell et al., 2007; Shalom et al., 2007). The analysis, so far, of three of the Burkholderia T6SSs revealed a high functional diversity: while the T6SS-1 and T6SS-4 are involved in interbacterial competition and metal ion acquisition, respectively, the T6SS-5 plays a central role in the intracellular life cycle of the bacteria (Schwarz et al., 2010; French et al., 2011; Russell et al., 2012; Si et al., 2017).
The Intracellular Life Cycle of Burkholderia Pseudomallei and Burkholderia Thailandensis
Since B. pseudomallei is only sporadically transmitted between humans and B. thailandensis infections of humans are extremely rare, the capacity of the bacteria for survival and virulence in mammals likely has its origin in the exposure of the bacteria to soil dwelling predators such as protozoa (Abbink et al., 2001; Ralph et al., 2004; Fang et al., 2016). B. pseudomallei is able to survive phagocytosis by protozoa, which has been suggested as pre-adaptation to avoid killing by mammalian phagocytes (Gao et al., 1997; Inglis et al., 2000; Strassmann and Shu, 2017). Indeed, B. pseudomallei and B. thailandensis are able to survive inside a range of mammalian phagocytic and non-phagocytic host cells (Jones et al., 1996; Sim et al., 2009; Bast et al., 2011; Lu et al., 2012; Whiteley et al., 2017).
A detailed discussion on the intracellular life cycle is beyond the scope of this review and we refer the reader to several comprehensive overviews on this topic (Allwood et al., 2011; Stone et al., 2014; Willcocks et al., 2016). In brief, upon passive or active entry into the host cell the bacteria are located in a membrane-bound vacuole (Jones et al., 1996; Figure 1A). Before lysosomal fusion B. pseudomallei and B. thailandensis escape the endocytic vacuole, a process that is significantly impaired in T3SS-3 mutants (Stevens et al., 2002; Vander Broek and Stevens, 2017). Once in the cytosol of the host cell the bacteria replicate and employ BimA to facilitate actin tail formation or the flagella fla2 system for intracellular motility (Stevens et al., 2005; French et al., 2011; Sitthidet et al., 2011). Intercellular spread of B. pseudomallei and B. thailandensis can occur directly without exposure of the bacteria to the extracellular milieu by the formation of multinucleated giant cells (MNGCs). MNGCs are the result of plasma membrane fusion and subsequent cytoplasmic mixing of the infected and neighboring host cell (Kespichayawattana et al., 2004; Boddey et al., 2007; French et al., 2011). These cell-cell fusions have been detected in lung tissue samples of melioidosis patients and mice infected with a low dose of B. pseudomallei (Wong et al., 1995; Conejero et al., 2011). Essential to MNGC formation is the Burkholderia T6SS-5 (also named cluster 1 T6SS) whose mechanism of action is still unknown (Pilatz et al., 2006; Burtnick et al., 2011; French et al., 2011; Suparak et al., 2011; Schwarz et al., 2014). Furthermore, findings on the T6SS-5 of Burkholderia mallei, which is closely related to B. pseudomallei, are discussed in the review (Schell et al., 2007; Losada et al., 2010).
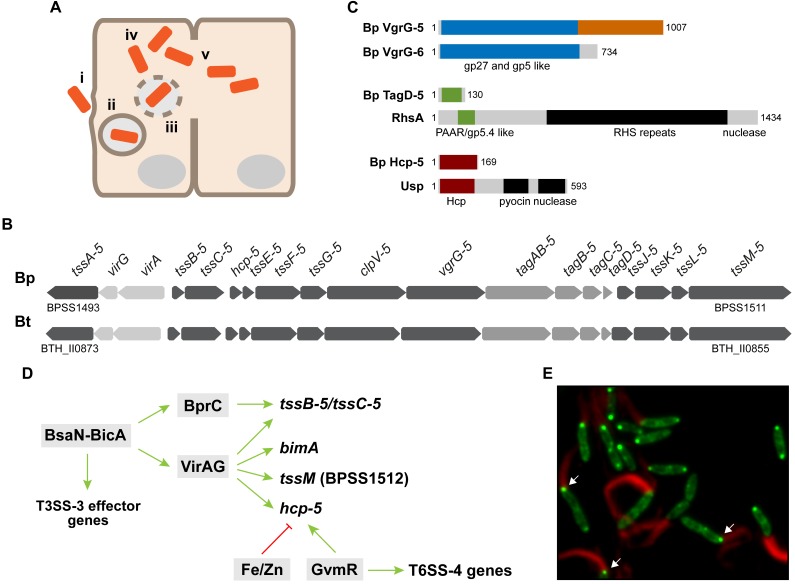
FIGURE 1.
(A) Schematic representation of the intracellular life cycle of Burkholderia pseudomallei and Burkholderia thailandensis: (i) entry into phagocytic or non-phagocytic cell, (ii) vacuole formation, (iii) vacuolar escape before lysosomal fusion, (iv) replication and intracellular motility in cytoplasm, and (v) T6SS-5-dependent induction of plasma membrane fusion of neighboring host cell leading to MNGCs. (B) Genetic organization of the T6SS-5 gene cluster in B. pseudomallei (Bp) and B. thailandensis (Bt). tss, tag and regulatory genes are highlighted in dark, mid and light gray, respectively. (C) Schematic representation of the B. pseudomallei (Bp) VgrG-5, TagD-5 and Hcp-5 domain organization in comparison with Bp VgrG-6 (UniProt: Q63II0), RhsA (PAAR domain containing protein of Dickeya dadantii; UniProt: E0SAK8) (Koskiniemi et al., 2013) and Usp (Hcp domain containing protein of E. coli; UniProt: Q1RG83), respectively. Blue, green, and red indicate domains conserved in VgrG, TagD, and Hcp proteins, respectively. The VgrG-5 CTD is indicated in orange and accessory (effector) domains are highlighted in black. (D) Summary of T6SS-5 regulators discussed in the text that affect expression of T6SS-5 and other genes involved in host-pathogen interaction [modified from (Chen et al., 2011)]. (E) Epifluorescence microscopy of B. thailandensis expressing a chromosomal clpV-5-sfgfp fusion during infection of Hela cells. ClpV-5-sfGFP shows foci formation at the bacterial cell pole and a diffuse localization in the cytoplasm. Actin was stained with Texas Red-Phalloidin. White arrows indicate the occurrence of actin tail formation and T6SS-5 expression and assembly in the same bacterial cell.
Components of the T6SS-5 Apparatus
The T6SS is a complex contractile injection system (CIS) exhibiting close structural and functional resemblance with other CIS such as myophage tails and R type pyocins (Veesler and Cambillau, 2011; Leiman and Shneider, 2012; Ge et al., 2015). The T6S apparatus is composed of 14 core components termed Tss (type VI secretion system) and PAAR, and variably present Tag (type VI secretion system associated) proteins serving regulatory, structural or effector functions (Shalom et al., 2007; Hsu et al., 2009; Aschtgen et al., 2010; Shneider et al., 2013; Cianfanelli et al., 2016a). Tss proteins assemble into three T6SS subcomplexes: a tubular system located in the cytoplasm consisting of the contractile sheath proteins TssB and TssC surrounding an inner tube formed by the Hcp (TssD) protein sharpened at one end by the TssI (VgrG) and PAARproteins, an envelope spanning membrane complex (TssM, TssL, and TssJ) and a base plate (TssE, TssF, TssG, and TssK) anchoring tube and sheath to the membrane complex (Cascales and Cambillau, 2012; Zoued et al., 2014; Nguyen et al., 2018). TssA was shown to initiate and coordinate sheath and tube polymerization during T6SS biogenesis (Zoued et al., 2016, 2017; Dix et al., 2018). The force-generating contraction of TssB and TssC acts as a molecular spring that pushes the inner Hcp tube tipped with the VgrG and PAAR spike proteins across the cell envelope into the target cell (Basler et al., 2012; Basler, 2015). Following translocation, the contracted sheath proteins are recycled by the ATPase ClpV (TssH) (Bonemann et al., 2009; Pietrosiuk et al., 2011; Kube et al., 2014; Kudryashev et al., 2015).
The vast majority of characterized T6SSs are employed by bacteria to inject toxic effector proteins into other prokaryotes (Russell et al., 2014a; Cianfanelli et al., 2016b; Hood et al., 2017; Sana et al., 2017). In addition, T6SSs specialized for effector protein delivery into eukaryotic cells including fungi and for the acquisition of metal ions have been described (Wang et al., 2015; Si et al., 2017; Trunk et al., 2018). This functional diversity extends to the mechanisms facilitating effector loading onto the T6SS. For instance, VgrG and PAAR (TagD) proteins can contain domains with effector function or act as carriers by binding to effector proteins while the Hcp tube can serve to translocate small (<25 kDa) effector proteins (Pukatzki et al., 2007; Silverman et al., 2013; Durand et al., 2014; Unterweger et al., 2015; Bondage et al., 2016; Ma et al., 2017; Quentin et al., 2018).
In addition to the canonical T6SSi subtype described above, which is predominantly found in Proteobacteria, other pathways (T6SSii-iv) have been identified that differ in composition and taxonomic distribution (Boyer et al., 2009; Broms et al., 2010; Russell et al., 2014b; Eshraghi et al., 2016; Bock et al., 2017). The Burkholderia T6SSs belong to the T6SSi pathway. Two different nomenclatures exist for naming the T6SS gene clusters and components: cluster 1–6 T6SS (Schell et al., 2007) and T6SS-1–T6SS-6 (Shalom et al., 2007). We adopted the nomenclature T6SS-1–T6SS-6 by Shalom et al., which is also universally used to name individual T6SS proteins. The T6SS-5 [cluster 1 T6SS according to the nomenclature by Schell et al. (2007)] consists of the 13 Tss core components that are encoded by the same gene cluster (Table 1 and Figure 1B). Furthermore, four tag genes, tagA/B-5, tagB-5, tagC-5 and tagD-5, are present in the T6SS-5 gene cluster whose role in T6SS-5 function is currently unknown. Primary sequence analysis indicates that TagA/B-5 and TagB-5 belong to the family of pentapeptide repeat proteins (PRP) (Shalom et al., 2007). Examples of characterized PRPs are the cytoplasmic quinolone resistance protein Qnr in E. coli and PipB2, a kinesin-recruiting T3SS effector protein in S. enterica sv. Typhimurium (Tran et al., 2005; Henry et al., 2006). TagA/B-5 is essential for MNGC formation, full virulence in mice and Hcp-5 secretion indicating a critical role in T6SS-5 activity but not as an effector protein (Hopf et al., 2014). TagD-5 is a PAAR-like protein comprising 130 amino acids that appears to lack effector domains and TagC-5 is a hypothetical protein of unknown function (DUF3540) (Figure 1C). Lastly, two regulatory genes are located within the T6SS-5 cluster encoding the two component regulator VirAG, which is required for transcriptional activation of T6SS-5 genes during infection (Chen et al., 2011).
Table 1.
Components of the T6SS-5 gene cluster in B. pseudomallei and B. thailandensis.
| Gene ID Bpa | Gene ID Btb | Tss/Tag nomenclature | Alternative name | T6SSi description/subcomplex |
|---|---|---|---|---|
| BPSS1493 | BTH_II0873 | tssA-5 | Sheath/tube assembly coordinationc | |
| BPSS1494 | BTH_II0872 | virG | Two component regulator VirAG; response regulator | |
| BPSS1495 | BTH_II0871 | virA | Two component regulator VirAG; sensor kinase | |
| BPSS1496 | BTH_II0870 | tssB-5 | Contractile sheath | |
| BPSS1497 | BTH_II0869 | tssC-5 | Contractile sheath | |
| BPSS1498 | BTH_II0868 | tssD-5 | hcp-5 | Tail tube/needle |
| BPSS1499 | BTH_II0867 | tssE-5 | Base plate | |
| BPSS1500 | BTH_II0866 | tssF-5 | Base plate | |
| BPSS1501 | BTH_II0865 | tssG-5 | Base plate | |
| BPSS1502 | BTH_II0864 | tssH-5 | clpV-5 | Sheath recycling AAA+ ATPase |
| BPSS1503 | BTH_II0863 | tssI-5 | vgrG-5 | Spike protein |
| BPSS1504 | BTH_II0862 | tagA/B-5 | Pentapeptide repeat protein | |
| BPSS1505 | BTH_II0861 | tagB-5 | Pentapeptide repeat protein | |
| BPSS1506 | BTH_II0860 | tagC-5 | Hypothetical | |
| BPSS1507 | BTH_II0859 | tagD-5 | PAAR like protein/spike tip | |
| BPSS1508 | BTH_II0858 | tssJ-5 | Membrane complex | |
| BPSS1509 | BTH_II0857 | tssK-5 | Base plate | |
| BPSS1510 | BTH_II0856 | tssL-5 | Membrane complex | |
| BPSS1511 | BTH_II0855 | tssM-5 | Membrane complex | |
aB. pseudomallei isolate K96243; bB. thailandensis isolate E264; cDix et al. (2018).
Regulation of T6SS-5 Gene Expression
The first evidence of the induction of T6SS-5 gene expression by a host cell derived signal has been provided by an in vivo expression technology (IVET) study (Shalom et al., 2007). The subsequent finding that the capability of vacuolar escape into the cytoplasm is a prerequisite for the activation of T6SS-5 genes suggested a cytoplasmic localization of the signal (Wong et al., 2015). Indeed, glutathione (GSH) and other low molecular weight (LMW) thiols such as cysteine have been identified to induce T6SS-5 gene expression (Wong et al., 2015). GSH is an antioxidant present at millimolar concentrations in the host cell cytoplasm. It contains one thiol group that acts as a reducing agent. Exposure of B. pseudomallei outside of host cells to reduced but not oxidized glutathione stimulated hcp-5 expression by approximately 1000 fold (Wong et al., 2015). However, it is important to note that so far LMW thiols have been shown to induce transcription of T6SS-5 genes but not secretory activity of the T6SS-5. At present, the signal(s) necessary to elicit T6SS-5 contraction and secretion are not known.
Low molecular weight thiols are sensed by the sensor histidine kinase VirA of the two component system VirAG, which forms a dimer that is reduced by thiols to the active monomeric form (Wong et al., 2015). During infection of host cells VirAG positively regulates expression of bimA and T6SS-5 genes (Chen et al., 2011). In trans overexpression of virAG in B. pseudomallei and B. thailandensis activates the T6SS-5 and leads to Hcp-5 secretion in culture media (Schell et al., 2007; Burtnick et al., 2011; Schwarz et al., 2014; Toesca et al., 2014). Furthermore, the transcription of T6SS-5 and T3SS-3 genes is co-regulated by BsaN encoded in the T3SS-3 gene cluster (Figure 1D). BsaN activates T3SS-3 effector and translocon genes, virAG and the regulatory gene bprC. BprC in turn induces expression of tssB-5 and tssC-5 (Chen et al., 2011, 2014). Expression of T6SS-5 genes was shown to be co-regulated with that of T6SS-4 and secondary metabolite genes as well as a gene located next to the T6SS-5 gene cluster encoding a deubiquitinase that is secreted by the type II secretion system (Shanks et al., 2009; Burtnick et al., 2014; Duong et al., 2018). Lastly, transcription of T6SS-5 genes is inhibited in the presence of iron and zinc (Burtnick and Brett, 2013).
Proteins Secreted by the T6SS-5
Taking advantage of the fact that virAG overexpression induces secretion of T6SS-5 in bacteria grown in culture medium, comparative mass spectrometric analysis of culture supernatants was performed to identify T6SS-5 effector proteins in B. thailandensis. Two proteins have been identified that were absent or of significantly lower abundance in the supernatant of a ΔtssK-5 mutant relative to the wildtype: Hcp-5, the inner tube forming protein of T6SSs and VgrG-5, the needle spike protein (Schwarz et al., 2014). Hcp-5 does not appear to carry effector domains (Figure 1C). VgrG-5 contains an N- terminal and middle domain related to gp5 and gp27 bacteriophage spike forming proteins that are conserved in all T6SSi VgrG proteins (Leiman et al., 2009). However, VgrG-5 possesses an additional domain, located at the C-terminus (VgrG-5 CTD), that is unique to the Burkholderia genus (Figure 1C; Burtnick et al., 2011; Schwarz et al., 2014; Toesca et al., 2014). Deletion of the VgrG-5 CTD abrogated cell-cell fusions and virulence in mice but did not affect secretion of Hcp-5 (Burtnick et al., 2011; Schwarz et al., 2014; Toesca et al., 2014). This result suggests that VgrG-5 is a specialized VgrG protein and that its CTD has essential effector function (Pukatzki et al., 2007; Durand et al., 2014; Schwarz et al., 2014). At present, VgrG-5 is the only T6SS-5 secreted protein identified with putative effector activity. Many other VgrG proteins containing additional domains at the C-terminus that display enzymatic activity, such as cross-linking of monomeric actin, have been described (Pukatzki et al., 2007; Ma et al., 2009; Suarez et al., 2010; Brooks et al., 2013). However, the VgrG-5 CTD lacks significant sequence similarity to proteins of known function and further studies will be required to determine whether the protein exhibits membrane fusion activity. Furthermore, it cannot be excluded that the protein acts as a carrier for as yet unidentified T6SS-5 effectors.
Role of the T6SS-5 in the Intracellular Life Cycle and in Virulence In Vivo
Several studies established a principal role of the B. pseudomallei and B. thailandensis T6SS-5 in inducing MNGC formation (Pilatz et al., 2006; Burtnick et al., 2011; French et al., 2011; Schwarz et al., 2014; Toesca et al., 2014). The function of host cell fusion in the pathogenesis of melioidosis, however, has yet to be determined. In vitro, B. pseudomallei and B. thailandensis are capable of stimulating MNGC formation in a range of primary and immortalized cells (Kespichayawattana et al., 2004; Welkos et al., 2015; Whiteley et al., 2017). Obvious potential benefits of this host cell manipulation are access to nutrients provided by uninfected host cells, and localized spread and replication without exposure to extracellular immune defense mechanisms.
Like T6SS-5 mutants, T3SS-3 mutants of B. pseudomallei display a host cell fusion defect (Suparak et al., 2005; Muangsombut et al., 2008; Gong et al., 2011). Since T3SS-3 mutants are impaired in vacuolar escape into the cytoplasm – a requirement for the induction of T6SS-5 gene expression– the role of T3SS-3 in MNGC formation could be indirect. To clarify the function of the T3SS-3 in MNGC formation, French et al. utilized a photothermal nanoblade to place a B. thailandensis T3SS-3 mutant from the extracellular milieu directly into the cytoplasm of the host cell thereby bypassing endocytic vesicle enclosure and escape (French et al., 2011). The finding that the mutant was able to induce host cell fusion following nanoblade delivery conclusively demonstrated that the T3SS-3 is not involved in this process.
In addition to T6SS-5 genes and bimA being co-regulated by VirAG, it has been shown that the deletion of structural components of the T6SS-5 reduced actin tail formation in B. pseudomallei and B. mallei (Burtnick et al., 2010;Chen et al., 2011). The underlying basis of this effect is currently unclear. Interestingly, however, the ability of the bacteria to move in the host cell cytoplasm is a prerequisite for the stimulation of cell-cell fusion. Disruption of intracellular motility of B. pseudomallei and B. thailandensis almost entirely abolished MNGC formation (French et al., 2011). This observation suggests a site-specific induction of T6SS-5 secretion inside the host cell that leads to cell-cell fusion. Alternatively, intracellular motility was proposed to be required to bring the plasma membrane of neighboring host cells into close proximity before they are punctured by the T6SS-5 to create a hemifusion zone leading to cell-cell fusion (Toesca et al., 2014). In support of these notions, fluorescence microscopy of B. thailandensis expressing clpV-5-sfgfp during infection showed actin tail formation and T6SS-5 expression in the same bacterial cell (Figure 1E; Schwarz et al., 2014).
The deletion of essential T6SS-5 genes drastically decreased virulence of B. pseudomallei and B. thailandensis in mammalian models of acute infection (Pilatz et al., 2006; Schwarz et al., 2010, 2014; Burtnick et al., 2011; Hopf et al., 2014). Intranasal inoculation of mice with B. pseudomallei wildtype and tssK-5 and tagA/B-5 mutants showed a significant attenuation of virulence upon T6SS-5 disruption (Pilatz et al., 2006; Hopf et al., 2014). CFU measurements of lung, liver, and spleen revealed that T6SS-5 mutants were able to disseminate to distant sites although the bacterial load in the organs was significantly lower compared with wildtype challenged mice. Likewise, the LD50 of a B. pseudomallei hcp-5 mutant in hamsters after intraperitoneal challenge was 1000 fold higher than that of the wildtype (Burtnick et al., 2011). Furthermore, after high dose pulmonary infection with B. thailandensis wildtype all mice succumbed whereas a tssK-5 mutant failed to cause lethal infections and to proliferate in the lung, liver and spleen (Schwarz et al., 2010). However, the tssK-5 mutant caused rapidly fatal infections in mice lacking the innate immune adapter molecule MyD88, which contributes to neutrophil recruitment and activation in mice infected with B. pseudomallei (Wiersinga et al., 2008b). The finding that the B. thailandensis tssK-5 mutant is highly virulent in MyD88-/- mice indicates that the T6SS-5 is required to overcome MyD88-dependent immune responses to establish an infection (Schwarz et al., 2010). In vitro, T6SS-5 mutants are able to multiply in the host cell cytoplasm (Shalom et al., 2007). Thus, the mere ability to replicate in the intracellular compartment appears to be a necessary but not sufficient trait of B. pseudomallei to cause disease. Lastly, virulence of T6SS-5 mutants of B. pseudomallei, B. thailandensis and B. mallei was attenuated in a cockroach model of infection (Fisher et al., 2012).
Conclusion and Future Perspective
Many fundamental questions remain unanswered since the discovery of the vital role of the T6SS-5 in B. pseudomallei-host cell interaction over 10 years ago. Critically, deciphering the mode of action of the T6SS-5 poses a challenge for the field as it still remains elusive although an essential candidate effector has been identified. In particular, important unsolved questions are: What is the exact subcellular localization of translocated VgrG-5 and does it function as membrane fusion protein? Is the VgrG-5 CTD sufficient for mediating cell-cell fusion or are other (T6SS-5) proteins involved in the process? Does the T6SS-5 employ host cellular factors to exert its function? In addition, investigating the molecular and cellular details of the MyD88-dependent immune response that facilitates control of T6SS-5 mutant bacteria will improve our understanding of T6SS-5 function. To answer these questions B. thailandensis will be an ideal model organism as work with this bacterium is less laborious and less restricted with respect to for example high throughput and in vivo imaging techniques compared with B. pseudomallei. Advancing knowledge on the molecular basis of the T6SS-5 – a key virulence determinant of B. pseudomallei – will benefit the development of strategies to disable the capacity of the pathogen to survive and proliferate in humans.
Author Contributions
JL and TW wrote the manuscript. SS conceived and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to those whose work was not mentioned due to space limitations.
Footnotes
Funding. JL was funded by the German Research Foundation (SFB766/Project B16). Work by SS was supported by the German Research Foundation (DFG SCHW 1517/1-1 and SCHW 1517/3-1) and the German Excellence Initiative.
References
- Abbink F. C., Orendi J. M., de Beaufort A. J. (2001). Mother-to-child transmission of Burkholderia pseudomallei. N. Engl. J. Med. 344 1171–1172. 10.1056/NEJM200104123441516 [DOI] [PubMed] [Google Scholar]
- Allwood E. M., Devenish R. J., Prescott M., Adler B., Boyce J. D. (2011). Strategies for intracellular survival of Burkholderia pseudomallei. Front. Microbiol. 2:170. 10.3389/fmicb.2011.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschtgen M. S., Thomas M. S., Cascales E. (2010). Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP. what else? Virulence 1 535–540. 10.4161/viru.1.6.13732 [DOI] [PubMed] [Google Scholar]
- Basler M., Pilhofer M., Henderson G. P., Jensen G. J., Mekalanos J. J. (2012). Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483 182–186. 10.1038/nature10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M. (2015). Type VI secretion system: secretion by a contractile nanomachine. Philos. Trans. R. Soc. Lon. Ser. B Biol. Sci. 370:20150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast A., Schmidt I. H., Brauner P., Brix B., Breitbach K., Steinmetz I. (2011). Defense mechanisms of hepatocytes against Burkholderia pseudomallei. Front. Microbiol. 2:277. 10.3389/fmicb.2011.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock D., Medeiros J. M., Tsao H. F., Penz T., Weiss G. L., Aistleitner K., et al. (2017). In situ architecture, function, and evolution of a contractile injection system. Science 357 713–717. 10.1126/science.aan7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey J. A., Day C. J., Flegg C. P., Ulrich R. L., Stephens S. R., Beacham I. R., et al. (2007). The bacterial gene lfpA influences the potent induction of calcitonin receptor and osteoclast-related genes in Burkholderia pseudomallei-induced TRAP-positive multinucleated giant cells. Cell Microbiol. 9 514–531. 10.1111/j.1462-5822.2006.00807.x [DOI] [PubMed] [Google Scholar]
- Bondage D. D., Lin J. S., Ma L. S., Kuo C. H., Lai E. M. (2016). VgrG C terminus confers the type VI effector transport specificity and is required for binding with PAAR and adaptor-effector complex. Proc. Natl. Acad. Sci. U.S.A. 113 E3931–E3940. 10.1073/pnas.1600428113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonemann G., Pietrosiuk A., Diemand A., Zentgraf H., Mogk A. (2009). Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 28 315–325. 10.1038/emboj.2008.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer F., Fichant G., Berthod J., Vandenbrouck Y., Attree I. (2009). Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104. 10.1186/1471-2164-10-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett P. J., DeShazer D., Woods D. E. (1998). Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48(Pt 1), 317–320. 10.1099/00207713-48-1-317 [DOI] [PubMed] [Google Scholar]
- Broms J. E., Sjostedt A., Lavander M. (2010). The role of the Francisella tularensis pathogenicity island in Type VI secretion, intracellular survival, and modulation of host cell signaling. Front. Microbiol. 1:136. 10.3389/fmicb.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks T. M., Unterweger D., Bachmann V., Kostiuk B., Pukatzki S. (2013). Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J. Biol. Chem. 288 7618–7625. 10.1074/jbc.M112.436725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick M. N., Brett P. J. (2013). Burkholderia mallei and Burkholderia pseudomallei cluster 1 type VI secretion system gene expression is negatively regulated by iron and zinc. PLoS One 8:e76767. 10.1371/journal.pone.0076767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick M. N., Brett P. J., DeShazer D. (2014). Proteomic analysis of the Burkholderia pseudomallei type II secretome reveals hydrolytic enzymes, novel proteins, and the deubiquitinase TssM. Infect. Immun. 82 3214–3226. 10.1128/IAI.01739-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick M. N., Brett P. J., Harding S. V., Ngugi S. A., Ribot W. J., Chantratita N., et al. (2011). The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 79 1512–1525. 10.1128/IAI.01218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick M. N., DeShazer D., Nair V., Gherardini F. C., Brett P. J. (2010). Burkholderia mallei cluster 1 type VI secretion mutants exhibit growth and actin polymerization defects in RAW 264.7 murine macrophages. Infect. Immun. 78 88–99. 10.1128/IAI.00985-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E., Cambillau C. (2012). Structural biology of type VI secretion systems. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 367 1102–1111. 10.1098/rstb.2011.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Luo J., Xu H., Li M., Zhang F., Li J., et al. (2017). Human infection with Burkholderia thailandensis, China, 2013. Emerg. Infect. Dis. 23 1416–1418. 10.3201/eid2308.170048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Schroder I., French C. T., Jaroszewicz A., Yee X. J., Teh B. E., et al. (2014). Characterization and analysis of the Burkholderia pseudomallei BsaN virulence regulon. BMC Microbiol. 14:206. 10.1186/s12866-014-0206-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wong J., Sun G. W., Liu Y., Tan G. Y., Gan Y. H. (2011). Regulation of type VI secretion system during Burkholderia pseudomallei infection. Infect. Immun. 79 3064–3073. 10.1128/IAI.05148-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A. C., Currie B. J. (2005). Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18 383–416. 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli F. R., Alcoforado Diniz J., Guo M., De Cesare V., Trost M., Coulthurst S. J. (2016a). VgrG and PAAR proteins define distinct versions of a functional type VI secretion system. PLoS Pathog. 12:e1005735. 10.1371/journal.ppat.1005735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli F. R., Monlezun L., Coulthurst S. J. (2016b). Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 24 51–62. 10.1016/j.tim.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Conejero L., Patel N., de Reynal M., Oberdorf S., Prior J., Felgner P. L., et al. (2011). Low-dose exposure of C57BL/6 mice to Burkholderia pseudomallei mimics chronic human melioidosis. Am. J. Pathol. 179 270–280. 10.1016/j.ajpath.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie B. J. (2015). Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respirat. Crit. Care Med. 36 111–125. 10.1055/s-0034-1398389 [DOI] [PubMed] [Google Scholar]
- Dix S. R., Owen H. J., Sun R., Ahmad A., Shastri S., Spiewak H. L., et al. (2018). Structural insights into the function of type VI secretion system TssA subunits. Nat. Commun. 9:4765. 10.1038/s41467-018-07247-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L. T., Schwarz S., Gross H., Breitbach K., Hochgrafe F., Mostertz J., et al. (2018). GvmR – A Novel LysR-Type transcriptional regulator involved in virulence and primary and secondary metabolism of Burkholderia pseudomallei. Front. Microbiol. 9:935. 10.3389/fmicb.2018.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E., Cambillau C., Cascales E., Journet L. (2014). VgrG, Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors. Trends Microbiol. 22 498–507. 10.1016/j.tim.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Eshraghi A., Kim J., Walls A. C., Ledvina H. E., Miller C. N., Ramsey K. M., et al. (2016). Secreted effectors encoded within and outside of the Francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe 20 573–583. 10.1016/j.chom.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Chen H., Zhu X., Mao X. (2016). Fatal melioidosis in a newborn from Hainan, China. Am. J. Trop. Med. Hygiene 95 444–446. 10.4269/ajtmh.15-0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher N. A., Ribot W. J., Applefeld W., DeShazer D. (2012). The Madagascar hissing cockroach as a novel surrogate host for Burkholderia pseudomallei, B. mallei B. thailandensis. BMC Microbiol. 12:117. 10.1186/1471-2180-12-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French C. T., Toesca I. J., Wu T. H., Teslaa T., Beaty S. M., Wong W., et al. (2011). Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc. Natl. Acad. Sci. U.S.A. 108 12095–12100. 10.1073/pnas.1107183108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L. Y., Harb O. S., Abu Kwaik Y. (1997). Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect. Immun. 65 4738–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P., Scholl D., Leiman P. G., Yu X., Miller J. F., Zhou Z. H. (2015). Atomic structures of a bactericidal contractile nanotube in its pre- and postcontraction states. Nat. Struct. Mol. Biol. 22 377–382. 10.1038/nsmb.2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. E., Elrod M. G., Gulvik C. A., Haselow D. T., Waters C., Liu L., et al. (2018). Burkholderia thailandensis isolated from infected wound, Arkansas, USA. Emerg. Infect. Dis. 24 2091–2094. 10.3201/eid2411.180821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M. B., Gee J. E., Steigerwalt A. G., Cavuoti D., Barton T., Hardy R. D., et al. (2006). Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J. Clin. Microbiol. 44 4601–4604. 10.1128/JCM.01585-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Cullinane M., Treerat P., Ramm G., Prescott M., Adler B., et al. (2011). The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One 6:e17852. 10.1371/journal.pone.0017852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraga A., West T. E., Brittnacher M. J., Skerrett S. J., Miller S. I. (2008). Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect. Immun. 76 5402–5411. 10.1128/IAI.00626-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T., Couillault C., Rockenfeller P., Boucrot E., Dumont A., Schroeder N., et al. (2006). The Salmonella effector protein PipB2 is a linker for kinesin-Proc. Natl. Acad. Sci. U.S.A. 103 13497–13502. 10.1073/pnas.0605443103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood R. D., Peterson S. B., Mougous J. D. (2017). From striking out to striking gold: discovering that type VI secretion targets bacteria. Cell Host Microbe 21 286–289. 10.1016/j.chom.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf V., Gohler A., Eske-Pogodda K., Bast A., Steinmetz I., Breitbach K. (2014). BPSS1504, a cluster 1 type VI secretion gene, is involved in intracellular survival and virulence of Burkholderia pseudomallei. Infect. Immun. 82 2006–2015. 10.1128/IAI.01544-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F., Schwarz S., Mougous J. D. (2009). TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol. Microbiol. 72 1111–1125. 10.1111/j.1365-2958.2009.06701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis T. J., Rigby P., Robertson T. A., Dutton N. S., Henderson M., Chang B. J. (2000). Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68 1681–1686. 10.1128/IAI.68.3.1681-1686.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. L., Beveridge T. J., Woods D. E. (1996). Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kespichayawattana W., Intachote P., Utaisincharoen P., Sirisinha S. (2004). Virulent Burkholderia pseudomallei is more efficient than avirulent Burkholderia thailandensis in invasion of and adherence to cultured human epithelial cells. Microb. Pathog. 36 287–292. 10.1016/j.micpath.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Koskiniemi S., Lamoureux J. G., Nikolakakis K. C., t’Kint de Roodenbeke C., Kaplan M. D., Low D. A., et al. (2013). Rhs proteins from diverse bacteria mediate intercellular competition. Proc. Natl. Acad. Sci. U.S.A. 110 7032–7037. 10.1073/pnas.1300627110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube S., Kapitein N., Zimniak T., Herzog F., Mogk A., Wendler P. (2014). Structure of the VipA/B type VI secretion complex suggests a contraction-state-specific recycling mechanism. Cell Rep. 8 20–30. 10.1016/j.celrep.2014.05.034 [DOI] [PubMed] [Google Scholar]
- Kudryashev M., Wang R. Y., Brackmann M., Scherer S., Maier T., Baker D., et al. (2015). Structure of the type VI secretion system contractile sheath. Cell 160 952–962. 10.1016/j.cell.2015.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman P. G., Basler M., Ramagopal U. A., Bonanno J. B., Sauder J. M., Pukatzki S., et al. (2009). Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U.S.A. 106 4154–4159. 10.1073/pnas.0813360106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman P. G., Shneider M. M. (2012). Contractile tail machines of bacteriophages. Adv. Exp. Med. Biol. 726 93–114. 10.1007/978-1-4614-0980-9_5 [DOI] [PubMed] [Google Scholar]
- Lertpatanasuwan N., Sermsri K., Petkaseam A., Trakulsomboon S., Thamlikitkul V., Suputtamongkol Y. (1999). Arabinose-positive Burkholderia pseudomallei infection in humans: case report. Clin. Infect. Dis. 28 927–928. 10.1086/517253 [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D., Golding N., Dance D. A., Messina J. P., Pigott D. M, Moyes C. L., et al. (2016). Predicted global distribution of and burden of melioidosis. Nat. Microbiol. 1:15008. 10.1038/nmicrobiol.2015.8 [DOI] [PubMed] [Google Scholar]
- Losada L., Ronning C. M., DeShazer D., Woods D., Fedorova N., Kim H. S., et al. (2010). Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol. Evol. 2 102–116. 10.1093/gbe/evq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Popov V., Patel J., Eaves-Pyles T. (2012). Burkholderia mallei and Burkholderia pseudomallei stimulate differential inflammatory responses from human alveolar type II cells (ATII) and macrophages. Front. Cell. Infect. Microbiol. 2:165. 10.3389/fcimb.2012.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A. T., McAuley S., Pukatzki S., Mekalanos J. J. (2009). Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5 234–243. 10.1016/j.chom.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Sun M., Dong W., Pan Z., Lu C., Yao H. (2017). PAAR-Rhs proteins harbor various C-terminal toxins to diversify the antibacterial pathways of type VI secretion systems. Environ. Microbiol. 19 345–360. 10.1111/1462-2920.13621 [DOI] [PubMed] [Google Scholar]
- Majerczyk C. D., Brittnacher M. J., Jacobs M. A., Armour C. D., Radey M. C., Bunt R., et al. (2014). Cross-species comparison of the Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei quorum-sensing regulons. J. Bacteriol. 196 3862–3871. 10.1128/JB.01974-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muangsombut V., Suparak S., Pumirat P., Damnin S., Vattanaviboon P., Thongboonkerd V., et al. (2008). Inactivation of Burkholderia pseudomallei bsaQ results in decreased invasion efficiency and delayed escape of bacteria from endocytic vesicles. Arch. Microbiol. 190 623–631. 10.1007/s00203-008-0413-3 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay C., Shaw T., Varghese G. M., Dance D. A. B. (2018). Melioidosis in South Asia (India, Nepal, Pakistan, Bhutan and Afghanistan). Trop. Med. Infect. Dis. 3:51. 10.3390/tropicalmed3020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. S., Douzi B., Durand E., Roussel A., Cascales E., Cambillau C. (2018). Towards a complete structural deciphering of Type VI secretion system. Curr. Opin. Struct. Biol. 49 77–84. 10.1016/j.sbi.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Pietrosiuk A., Lenherr, Falk S., Bonemann G., Kopp J., Zentgraf H., et al. (2011). Molecular basis for the unique role of the AAA+ chaperone ClpV in type VI protein secretion. J. Biol. Chem. 286 30010–30021. 10.1074/jbc.M111.253377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilatz S., Breitbach K., Hein N., Fehlhaber B., Schulze J., Brenneke B., et al. (2006). Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect. Immun. 74 3576–3586. 10.1128/IAI.01262-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S., Ma A. T., Revel A. T., Sturtevant D., Mekalanos J. J. (2007). Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U.S.A. 104 15508–15513. 10.1073/pnas.0706532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin D., Ahmad S., Shanthamoorthy P., Mougous J. D., Whitney J. C., Raunser S. (2018). Mechanism of loading and translocation of type VI secretion system effector TseNature microbiology. Nat. Microbiol. 3 1142–1152. 10.1038/s41564-018-0238-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph A., McBride J., Currie B. J. (2004). Transmission of Burkholderia pseudomallei via breast milk in northern Australia. Pediatric Infect. Dis. J. 23 1169–1171. [PubMed] [Google Scholar]
- Rolim D. B., Lima R. X. R., Ribeiro A. K. C., Colares R. M., Lima L. D. Q., Rodriguez-Morales A. J., et al. (2018). Melioidosis in South America. Trop. Med. Infect. Dis. 3:60. 10.3390/tropicalmed3020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., Peterson S. B., Mougous J. D. (2014a). Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 12 137–148. 10.1038/nrmicro3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., Wexler A. G., Harding B. N., Whitney J. C., Bohn A. J., Goo Y. A., et al. (2014b). A type VI secretion-related pathway in bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16 227–236. 10.1016/j.chom.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. B., Singh P., Brittnacher M., Bui N. K., Hood R. D., Carl M. A., et al. (2012). A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe 11 538–549. 10.1016/j.chom.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana T. G., Lugo K. A., Monack D. M. (2017). T6SS: the bacterial “fight club” in the host gut. PLoS Pathog. 13:e1006325. 10.1371/journal.ppat.1006325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A., Ulrich R. L., Ribot W. J., Brueggemann E. E., Hines H. B., Chen D., et al. (2007). Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64 1466–1485. 10.1111/j.1365-2958.2007.05734.x [DOI] [PubMed] [Google Scholar]
- Schwarz S., Singh P., Robertson J. D., LeRoux M., Skerrett S. J., Goodlett D. R., et al. (2014). VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect. Immun. 82 1445–1452. 10.1128/IAI.01368-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., West T. E., Boyer F., Chiang W. C., Carl M. A., Hood R. D., et al. (2010). Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068. 10.1371/journal.ppat.1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom G., Shaw J. G., Thomas M. S. (2007). In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology 153(Pt 8), 2689–2699. 10.1099/mic.0.2007/006585-0 [DOI] [PubMed] [Google Scholar]
- Shanks J., Burtnick M. N., Brett P. J., Waag D. M., Spurgers K. B., Ribot W. J., et al. (2009). Burkholderia mallei tssM encodes a putative deubiquitinase that is secreted and expressed inside infected RAW 264.7 murine macrophages. Infect. Immun. 77 1636–1648. 10.1128/IAI.01339-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider M. M., Buth S. A., Ho B. T., Basler M., Mekalanos J. J., Leiman P. G. (2013). PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500 350–353. 10.1038/nature12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si M., Zhao C., Burkinshaw B., Zhang B., Wei D., Wang Y., et al. (2017). Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. U.S.A. 114 E2233–E2242. 10.1073/pnas.1614902114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. M., Agnello D. M., Zheng H., Andrews B. T., Li M., Catalano C. E., et al. (2013). Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 51 584–593. 10.1016/j.molcel.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S. H., Liu Y., Wang D., Novem V., Sivalingam S. P., Thong T. W., et al. (2009). Innate immune responses of pulmonary epithelial cells to Burkholderia pseudomallei infection. PLoS One 4:e7308. 10.1371/journal.pone.0007308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitthidet C., Korbsrisate S., Layton A. N., Field T. R., Stevens M. P., Stevens J. M. (2011). Identification of motifs of Burkholderia pseudomallei BimA required for intracellular motility, actin binding, and actin polymerization. J. Bacteriol. 193 1901–1910. 10.1128/JB.01455-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Angus B. J., Wuthiekanun V., White N. J. (1997). Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 65 4319–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz I., Wagner G. E., Kanyala E., Sawadogo M., Soumeya H., Teferi M., et al. (2018). Melioidosis in Africa: time to uncover the true disease load. Trop. Med. Infect. Dis. 3:E62. 10.3390/tropicalmed3020062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. P., Stevens J. M., Jeng R. L., Taylor L. A., Wood M. W., Hawes P., et al. (2005). Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 56 40–53. 10.1111/j.1365-2958.2004.04528.x [DOI] [PubMed] [Google Scholar]
- Stevens M. P., Wood M. W., Taylor L. A., Monaghan P., Hawes P., Jones P. W., et al. (2002). An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol. Microbiol. 46 649–659. 10.1046/j.1365-2958.2002.03190.x [DOI] [PubMed] [Google Scholar]
- Stone J. K., DeShazer D., Brett P. J., Burtnick M. N. (2014). Melioidosis: molecular aspects of pathogenesis. Exp. Rev. Anti-Infect. Ther. 12 1487–1499. 10.1586/14787210.2014.970634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann J. E., Shu L. (2017). Ancient bacteria-amoeba relationships and pathogenic animal bacteria. PLoS Biol. 15:e2002460. 10.1371/journal.pbio.2002460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez G., Sierra J. C., Erova T. E., Sha J., Horneman A. J., Chopra A. K. (2010). A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 192 155–168. 10.1128/JB.01260-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suparak S., Kespichayawattana W., Haque A., Easton A., Damnin S., Lertmemongkolchai G., et al. (2005). Multinucleated giant cell formation and apoptosis in infected host cells is mediated by Burkholderia pseudomallei type III secretion protein BipB. J. Bacteriol. 187 6556–6560. 10.1128/JB.187.18.6556-6560.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suparak S., Muangsombut V., Riyapa D., Stevens J. M., Stevens M. P., Lertmemongkolchai G., et al. (2011). Burkholderia pseudomallei-induced cell fusion in U937 macrophages can be inhibited by monoclonal antibodies against host cell surface molecules. Microbes Infect. 13 1006–1011. 10.1016/j.micinf.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Toesca I. J., French C. T., Miller J. F. (2014). The Type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect. Immun. 82 1436–1444. 10.1128/IAI.01367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A. G., Montufar F. E., Gee J. E., Hoffmaster A. R., Elrod M. G., Duarte-Valderrama C., et al. (2018). Melioidosis is in the Americas: a call to action for diagnosing and treating the disease. Am. J. Trop. Med. Hygiene 99 563–564. 10.4269/ajtmh.18-0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J. H., Jacoby G. A., Hooper D. C. (2005). Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49 118–125. 10.1128/AAC.49.1.118-125.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunk K., Peltier J., Liu Y. C., Dill B. D., Walker L., Gow N. A. R., et al. (2018). The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 3 920–931. 10.1038/s41564-018-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterweger D., Kostiuk B., Otjengerdes R., Wilton A., Diaz-Satizabal L., Pukatzki S. (2015). Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae. EMBO J. 34 2198–2210. 10.15252/embj.201591163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Broek C. W., Stevens J. M. (2017). Type III secretion in the melioidosis pathogen Burkholderia pseudomallei. Front. Cell. Infect. Microbiol. 7:255. 10.3389/fcimb.2017.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veesler D., Cambillau C. (2011). A common evolutionary origin for tailed-bacteriophage functional modules and bacterial machineries. Microbiol. Mol. Biol. Rev. 75 423–433. 10.1128/MMBR.00014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Si M., Song Y., Zhu W., Gao F., Wang Y., et al. (2015). Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 11:e1005020. 10.1371/journal.ppat.1005020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Klimko C. P., Kern S. J., Bearss J. J., Bozue J. A., Bernhards R. C., et al. (2015). Characterization of Burkholderia pseudomallei strains using a murine intraperitoneal infection model and in vitro macrophage assays. PLoS One 10:e0124667. 10.1371/journal.pone.0124667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T. E., Frevert C. W., Liggitt H. D., Skerrett S. J. (2008). Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl. 1), S119–S126. 10.1016/S0035-9203(08)70028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T. E., Myers N. D., Liggitt H. D., Skerrett S. J. (2012). Murine pulmonary infection and inflammation induced by inhalation of Burkholderia pseudomallei. Int. J. Exp. Pathol. 93 421–428. 10.1111/j.1365-2613.2012.00842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley L., Meffert T., Haug M., Weidenmaier C., Hopf V., Bitschar K., et al. (2017). Entry, intracellular survival, and multinucleated-giant-cell-forming activity of burkholderia pseudomallei in human primary phagocytic and nonphagocytic cells. Infect. Immun. 85:e00468-17. 10.1128/IAI.00468-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W. J., de Vos A. F., de Beer R., Wieland C. W., Roelofs J. J., Woods D. E., et al. (2008a). Inflammation patterns induced by different Burkholderia species in mice. Cell Microbiol. 10 81–87. [DOI] [PubMed] [Google Scholar]
- Wiersinga W. J., Wieland C. W., Roelofs J. J., van der Poll T. (2008b). MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS One 3:e3494. 10.1371/journal.pone.0003494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W. J., Virk H. S., Torres A. G., Currie B. J., Peacock S. J., Dance D. A. B., et al. (2018). Melioidosis. Nat. Rev. Dis. Prim. 4:17107. 10.1038/nrdp.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks S. J., Denman C. C., Atkins H. S., Wren B. W. (2016). Intracellular replication of the well-armed pathogen Burkholderia pseudomallei. Curr. Opin. Microbiol. 29 94–103. 10.1016/j.mib.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Wong J., Chen Y., Gan Y. H. (2015). Host cytosolic glutathione sensing by a membrane histidine kinase activates the Type VI secretion system in an intracellular bacterium. Cell Host Microbe 18 38–48. 10.1016/j.chom.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Wong K. T., Puthucheary S. D., Vadivelu J. (1995). The histopathology of human melioidosis. Histopathology 26 51–55. 10.1111/j.1365-2559.1995.tb00620.x [DOI] [PubMed] [Google Scholar]
- Zoued A., Brunet Y. R., Durand E., Aschtgen M. S., Logger L., Douzi B., et al. (2014). Architecture and assembly of the Type VI secretion system. Biochim. Biophys. Acta 1843 1664–1673. 10.1016/j.bbamcr.2014.03.018 [DOI] [PubMed] [Google Scholar]
- Zoued A., Durand E., Brunet Y. R., Spinelli S., Douzi B., Guzzo M., et al. (2016). Priming and polymerization of a bacterial contractile tail structure. Nature 531 59–63. 10.1038/nature17182 [DOI] [PubMed] [Google Scholar]
- Zoued A., Durand E., Santin Y. G., Journet L., Roussel A., Cambillau C., et al. (2017). TssA: the cap protein of the type VI secretion system tail. Bioessays 39:1600262. 10.1002/bies.201600262 [DOI] [PubMed] [Google Scholar]