Abstract
Preeclampsia (PE) is a common cause of maternal morbidity, characterized by impaired trophoblast invasion and spiral artery transformation resulting in progressive uteroplacental hypoxia. Given the primary role of LIN28A and LIN28B in modulating cell metabolism, differentiation, and invasion, we hypothesized that LIN28A and/or LIN28B regulates trophoblast differentiation and invasion, and that its dysregulation may contribute to PE. Here we show that LIN28B is expressed ∼1300-fold higher than LIN28A in human term placenta and is the predominant paralog expressed in primary human trophoblast cultures. The expression of LIN28B mRNA and protein levels are significantly reduced in gestational age–matched preeclamptic vs. normal placentas, whereas LIN28A expression is not different. First trimester human placental sections displayed stronger LIN28B immunoreactivity in extravillous (invasive) cytotrophoblasts and syncytial sprouts vs. villous trophoblasts. LIN28B overexpression increased HTR8 cell proliferation, migration, and invasion, whereas LIN28B knockdown in JEG3 cells reduced cell proliferation. Moreover, LIN28B knockdown in JEG3 cells suppressed syncytin 1 (SYN-1), apelin receptor early endogenous ligand (ELABELA), and the chromosome 19 microRNA cluster, and increased mRNA expression of ITGβ4 and TNF-α. Incubation of BeWo and JEG3 cells under hypoxia significantly decreased expression of LIN28B and LIN28A, SYN-1, and ELABELA, whereas TNF-α is increased. These results provide the first evidence that LIN28B is the predominant paralog in human placenta and that decreased LIN28B may play a role in PE by reducing trophoblast invasion and syncytialization, and by promoting inflammation.—Canfield, J., Arlier, S., Mong, E. F., Lockhart, J., VanWye, J., Guzeloglu-Kayisli, O., Schatz, F., Magness, R. R., Lockwood, C. J., Tsibris, J. C. M., Kayisli, U. A., Totary-Jain, H. Decreased LIN28B in preeclampsia impairs human trophoblast differentiation and migration.
Keywords: C19MC, inflammation, cell invasion, DOHaD
Preeclampsia (PE) is a major pregnancy complication characterized by hypertension and proteinuria (1) and is the second most common cause of maternal morbidity (2–4). Epidemiologic studies indicate that a history of PE correlates with the later development of cardiovascular disease (5). Although both maternal and placental factors contribute to its pathogenesis, the etiology of PE is incompletely understood. Maternally driven PE is attributed to a maternal milieu that is predisposed or susceptible to hypertension (6). Alternatively, placenta-driven PE appears to develop in response to placental exposure to pathologic hypoxia (7).
During the first trimester of human pregnancy, maternal spiral arteries undergo extensive remodeling mediated primarily by invasive extravillous trophoblasts (EVTs), leading to loss of myogenic tone and adequate placental perfusion (8). Impaired EVT invasion accompanied by poor spiral vascular remodeling elicits insufficient placental perfusion (7, 9). The resulting pathologic placental hypoxia can induce fetal growth restriction (10) and/or stimulate placental secretion of soluble factors into the maternal circulation, such as the VEGF receptor splice variant soluble fms-like tyrosine kinase 1, leading to elevated maternal blood pressure (11, 12). That placental hypoxia drives development of PE is supported by hypoxia-induced down-regulation of syncytialization mediator syncytin 1 (SYN-1), causing impaired syncytiotrophoblast (ST) formation found in PE placentas (13–16).
Alterations in microRNA (miRNA) expression are also associated with PE; specifically, the largest human miRNA cluster on chromosome 19 (C19MC) is highly expressed in normal human placentas. However, in PE, placental C19MC miRNAs are often down-regulated (17), but up-regulated in maternal serum (18).
The RNA binding protein LIN28 is documented to both regulate and be regulated by the let-7 family of miRNAs (19, 20). The highly conserved LIN28/let-7 switch governs developmental timing, stem cell self-renewal, cell differentiation, invasion, glucose metabolism, and embryonic growth (21–23). In humans, two LIN28 paralogs, LIN28A and LIN28B, share 65% sequence identity. The role of LIN28 in cell metabolism, differentiation, growth, and invasion suggests that LIN28 plays key roles in EVT differentiation and invasion, and that alterations in LIN28 expression contribute to the pathogenesis of PE.
MATERIALS AND METHODS
Ethical approval
Collection of 15 human placentas from normotensive pregnancies and 13 human placentas from PE-affected pregnancies was approved by the institutional review board of the University of South Florida (Protocol 00015578) after obtaining written informed patient consent. Additional total RNA from 6 normotensive and 6 PE pregnancies was provided by A. Karumanchi (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA). For all samples used in this study, PE was diagnosed according to criteria established by National High Blood Pressure Education Working Group. According to these guidelines, PE is defined by blood pressure that is >140 mmHg systolic or >90 mmHg diastolic, accompanied by proteinuria, in a woman who was normotensive throughout the first 20 wk of gestation. Placentas from the first trimester (7 and 8 wk gestation, n = 2) and early pregnancies (20 wk gestation, n = 2) were obtained from voluntary terminations of uncomplicated pregnancies.
Placental tissue collection
For RNA and protein analysis, tissue samples were excised from the fetal compartment of the placenta, placed in conical tubes, frozen on dry ice, and stored at −80°C. For immunohistochemistry, tissue samples were excised, fixed in formalin, and embedded in paraffin. The clinical characteristics of the patients are listed in Table 1.
TABLE 1.
Clinical characteristics of patients used in this study
| Characteristic | Normal (n = 15) | PE (n = 13) |
|---|---|---|
| Age [(yr) median ± sem] | 27.5 ± 1.32 | 26 ± 1.27 |
| Gravida (range) | 2 (1–12) | 2 (1–4) |
| Gestational age [(wk) median ± sem] | 38.6 ± 0.24 | 37.6 ± 0.45 |
| Body mass index in labor and delivery [(kg/m²) median ± sem] | 36.3 ± 3.09 | 40.7 ± 4.44 |
| Race (%) | ||
| White | 3 (20) | 6 (46.2) |
| Black | 5 (33.3) | 3 (23.1) |
| Asian | 1 (6.7) | 0 |
| Hispanic | 4 (26.7) | 4 (30.8) |
| Unknown | 2 (13.3) | 0 |
| Cigarette use (%) | 2 (13.3) | 2 (15.4) |
| Chronic hypertension (%) | 1 (6.7) | 2 (15.4) |
| DM [(gestational DM) (%)] | 4 (2) (26.7) | 2 (1) (13.3) |
| Mg2+ | 0 | 11 (84.6)* |
| Aspirin (%) | 0 | 2 (15.4) |
| Anti-inflammatory (%) | 1 (6.7) | 2 (15.4) |
| Mode of delivery (%) | ||
| Vaginal delivery | 5 (33.33) | 4 (30.7) |
| Cesarean section | 10 (66.67) | 9 (69.2) |
| Indication for cesarean section (%) | ||
| Failure of labor to progress | 0 | 4 (30.8)* |
| Other | 0 | 1 (7.7) |
| Repeat | 9 (60) | 2 (15.4) |
| Fetal sex (%) | ||
| Male | 4 (26.7) | 8 (61.5) |
| Female | 11 (73.3) | 5 (38.5) |
| Fetal weight [(g) mean ± sem] | 3444 ± 141.5 | 3160 ± 168.8 |
DM, diabetes mellitus.
P < 0.05 vs. normal cohort.
Isolation and culturing of primary decidual cells, cytotrophoblasts, and STs
Term decidual cells (DCs) and cytotrophoblasts (CTs) were isolated as previously described (24, 25) from placentas from uncomplicated pregnancies undergoing term cesarean delivery under University of South Florida institutional review board approval. STs were obtained by spontaneous differentiation of CTs after 72 h in culture as previously described (26).
Immunohistochemistry
Immunohistochemistry was performed on placental sections as previously described (27) using the primary antibody (rabbit anti-LIN28B, 40 μg/ml, ab71415, RRID:AB_2135050; Abcam, Cambridge, United Kingdom) and detected using a biotinylated goat anti-rabbit secondary antibody (BA-1000, RRID:AB _2313606; Vector Laboratories, Burlingame, CA, USA) together with the avidin–biotin–peroxidase complex (Vectastain ABC Kit, pk6200, RRID:AB_2336826; Vector Laboratories) followed by 3,3′-diaminobezidine (sk-4100, RRID:AB_2336382; Vector Laboratories) incubation as a substrate and counterstained with hematoxylin (H3404, RRID:AB_2336843; Vector Laboratories).
Quantification of immunohistochemistry
LIN28B immunoreactivity was semiquantitatively evaluated using the histology score (H score) method as previously described (28). Briefly, 3 different high-power fields for each section were imaged and scored by 3 separate individuals unaware of the sections’ status using the following scale: 0+ (no staining or weak staining), 100+ (moderate and detectable staining), and 200+ (distinct staining). The average score for each section was considered the H score.
Cell culture
BeWo cells [CCL-98; American Type Culture Collection (ATCC), Manassas, VA, USA, Manassas, VA, USA] were cultured in F-12K medium supplemented with 10% fetal bovine serum (FBS; MilliporeSigma, Burlington, MA, USA). JEG3 cells (ATCC HTB-36) were grown in MEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS. HTR8/SVneo (CRL-3271; ATCC) cells were cultured in Rosewell Park Memorial Institute (RPMI) 1640 supplemented with 5% FBS. Cell lines were maintained at 37°C and 5% CO2. The JEG3, BeWo, and HTR8/SVneo cells used in this study were authenticated by STR analysis through ATCC.
Transfections
JEG3 cells were transfected with scrambled short hairpin RNA (shRNA) or an shRNA targeting LIN28B (TG311156; OriGene Technologies, Rockville, MD, USA) using Lipofectamine 2000 according to the manufacturer’s instructions (Thermo Fisher Scientific). HTR8/SVneo cells were transfected with green fluorescent protein (GFP) or pFRT/FLAG/HA-DEST LIN28B plasmid, a gift from T. Tuschl (The Rockefeller University, New York, NY, USA) (43798; Addgene, Cambridge, MA, USA) (29) using 25 kDa linear polyethylenimine (23966; Polysciences, Philadelphia, PA, USA). Briefly, 105 cells per well were seeded in triplicate in a 12-well plate. Plasmids were diluted in sterile water followed by the addition of NaCl (final concentration of 150 mM) and polyethylenimine (final concentration of 0.05 µg/μl). The mixture was vortexed and incubated at room temperature for 15 min; then 500 µl of RPMI 1640 containing 5% FBS medium was added, and the transfection mixture was pipetted onto cells in each well.
Hypoxia
BeWo or JEG3 cells were seeded in 12-well plates and allowed to attach overnight while being housed under normal culture conditions (21% oxygen, 5% CO2) at 37°C in standard CO2 water-jacketed cell culture incubators. The following day, the medium was replaced with fresh medium, and the normal culture condition (21% oxygen) cells were placed back in the same incubator. The cells that were to be subjected to hypoxic conditions (1% oxygen) were placed in a polycarbonate, airtight hypoxia incubator chamber (27310; StemCell Technologies, Vancouver, BC, Canada) and flushed with a gas mixture of 1% oxygen, 5% CO2, and 94% nitrogen for 10 min at a flow rate of 20 L/min. The sealed chamber was then placed back in the same 37°C water-jacketed incubator. The chamber was flushed at a flow rate of 20 L/min for 10 min every 24 h for the duration of the experiment. Oxygen levels were monitored using a portable oxygen meter (803; Shenzhen Tomtop Technology, Shenzhen, China) and averaged 1.13 ± 0.14% oxygen throughout the duration of the experiments.
RNA and protein extractions
Placental samples were homogenized in Qiazol and used to isolate RNA. Primary cells and cultured cells were lysed using Qiazol. Total RNA was extracted using the miRNeasy Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. For protein isolation, placental specimens or tissue cultures were homogenized in lysis buffer as previously reported (30).
Immunoblotting
Total protein lysates were fractionated by PAGE as previously described (30, 31), transferred to a nitrocellulose membrane, and blocked for 1 h at room temperature in Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA). The membranes were then incubated overnight with LIN28B (2 μg/ml; ab71415, RRID:AB_2135050; Abcam) and α-tubulin (0.35 μg/ml, 2144, SRRID:AB_2210548; Cell Signaling Technology, Danvers, MA, USA) primary antibodies followed by probing with the secondary antibody IRDye 680 goat anti-rabbit (0.2 μg/ml, 926-68073, RRID:AB_10954442; Li-Cor). The resulting immunoblots were scanned and quantitatively assessed using the Odyssey infrared imaging system (Li-Cor).
Real-time quantitative PCR
cDNA was synthesized using M-MuLV reverse transcriptase according to the manufacturer’s instructions (M0253L; New England Biolabs, Ipswich, MA, USA) and relative mRNA expression levels were determined using TaqMan Fast Advanced Mastermix (4366072; Thermo Fisher Scientific) and TaqMan real-time quantitative PCR (qPCR) primers/probes [(LIN28A: HS_00702808_s1, LIN28B: HS_01013729_m1, TNF-α: HS_01113624_g1, SYN-1: HS_01926764_u1, ITGβ4: HS_00236216_m1, apelin receptor early endogenous ligand (ELABELA): HS_04274421_m1; Thermo Fisher Scientific)] with the Quantstudio 3 qPCR machine (Thermo Fisher Scientific). For miRNA expression analysis, TaqMan miRNA Reverse Transcription Kit (4366597; Applied Biosystems; Thermo Fisher Scientific) and miRNA-specific TaqMan primers/probe (miR-516a: 002416, miR-516b: 001150, miR-517a: 002402, miR-518c: 002401, miR-519d: 002403, let-7g: 478580, U18: 001204; Thermo Fisher Scientific) were used as previously described (32). Relative expression was determined using the δ-δCt method.
Scratch wound assays
HTR8/SVneo cells were seeded in a 24-well dish and transfected the following day with a plasmid encoding either GFP or LIN28B. Seventy-two hours later, a single scratch wound was established in each well, and the cells were thoroughly washed with sterile PBS and photographed. After 24 h, the cells were stained with crystal violet and rephotographed for analysis of wound closure as previously described (31).
Transwell migration assays
Transwell migration assays were performed as previously described (33, 34). Briefly, HTR8/SVneo cells were seeded in a 12-well dish and transfected the following day with a plasmid encoding GFP (control) or LIN28B. After 72 h, 2 × 104 transfected cells were seeded in serum-free RPMI 1640 in the upper chamber of a Matrigel-coated Transwell insert. RPMI 1640 containing 5% FBS was placed in the lower chamber. Twenty-four hours later, nonmigrated cells were removed from the upper chamber with a cotton-tipped swab. The remaining migrated cells were then stained with crystal violet and imaged. The number of migrated cells per field was counted to determine the migration.
Statistical analyses
Results are presented as means ± sem. All data were tested for normality and equivalence of variance to determine the appropriate statistical tests. A 1-way ANOVA with Tukey’s post hoc test was performed for multiple group analyses. Multiple 2-way Student’s t tests with Holm-Sidak correction for multiple comparisons were performed to determine changes in mean gene expression. For analysis of demographic data, Student’s t test, χ2, or Fisher’s exact test were used to determine significant differences in the cohorts. All statistical analyses were performed by Prism 7 software (GraphPad Software, La Jolla, CA, USA), and results were considered significant when adjusted P < 0.05.
RESULTS
LIN28B is predominant paralog expressed in human placentas
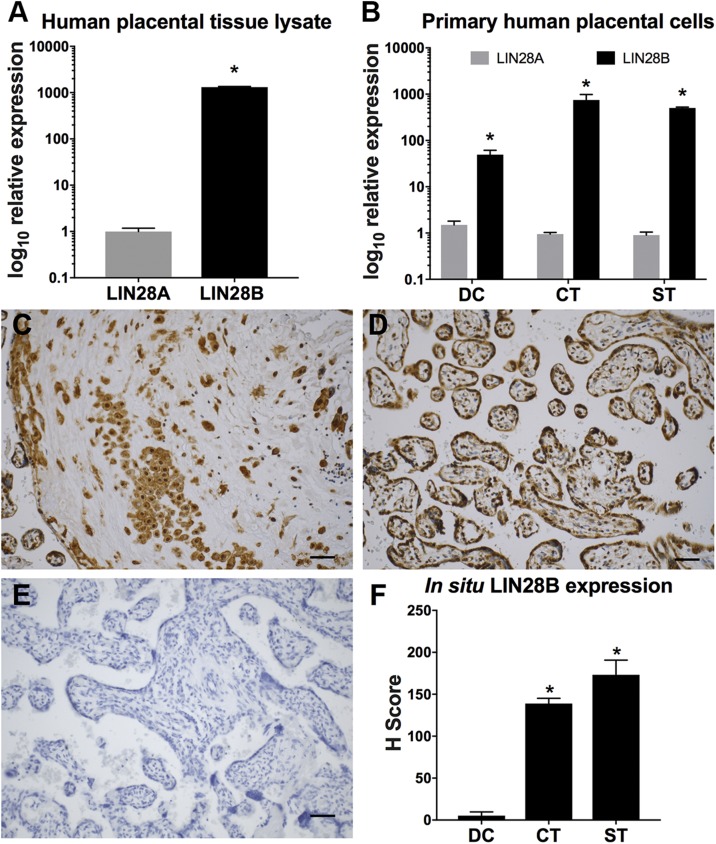
To identify the specific LIN28 paralog expressed in human placenta, qPCR analysis for LIN28A and LIN28B was performed on whole placenta tissue lysate; we found that LIN28B is expressed ∼1300-fold higher than LIN28A (Fig. 1A). Furthermore, primary cultures of DCs, CTs, and STs isolated from normal term placentas revealed ∼50-, 750-, and 500-fold higher LIN28B expression than LIN28A, respectively (Fig. 1B). Immunohistochemical analysis of normal human-term placental specimens also detected higher levels of LIN28B in CTs and STs compared to DCs (Fig. 1C–F). These observations identified LIN28B as the predominant paralog expressed in human placenta, leading to a primary focus on LIN28B expression and function for the remainder of this study.
Figure 1 .
LIN28B is predominant LIN28 paralog expressed in human placenta. A, B) qPCR analysis of LIN28A and LIN28B mRNA expression in term human placenta (A) and isolated primary DCs, CTs, and STs (B) normalized to GAPDH. C–E) Representative LIN28B immunohistochemistry images in term human placenta of invasive trophoblasts (C), villous trophoblasts (D), and nonimmune rabbit IgG–only control (E). F) In situ protein LIN28B expression in DCs, CTs, and STs of placentas from normotensive pregnancies. Scale bars, 35 μm; n = 10 placental sections (F). Results represent means ± sem. *P < 0.05 vs. LIN28A (A, B) vs. DC (F).
LIN28B is decreased in placentas from PE pregnancies
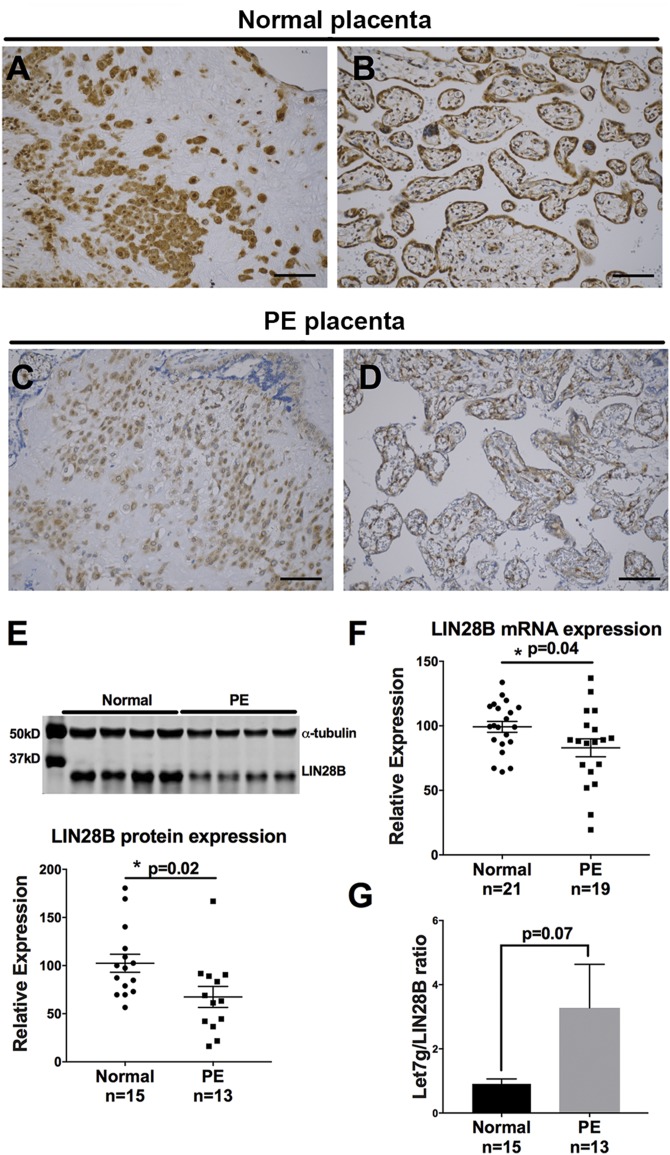
To determine whether LIN28B is dysregulated in placentas of PE-affected pregnancies, immunohistochemical analysis of LIN28B was performed. Compared to normotensive pregnancies, placentas from PE-affected pregnancies exhibited decreased LIN28B expression in both invasive trophoblasts and chorionic villi (Fig. 2A–D). Furthermore, LIN28B protein and mRNA levels were significantly decreased in placentas from PE-affected pregnancies compared to placentas from normotensive pregnancies (Fig. 2E, F), whereas there was no difference in LIN28A mRNA or protein levels (Supplemental Fig. S1). As indicated in Fig. 2G, qPCR measurements for let-7g, a member of the let-7 family that is highly sensitive to LIN28B expression (29), revealed higher let-7g/LIN28B ratio in PE vs. normotensive pregnancy placentas; however, it did not reach significance (P = 0.07).
Figure 2 .
LIN28B is decreased in placenta of PE pregnancies. A–D) Representative images of LIN28B immunostaining of invasive trophoblasts (A, C) and villous trophoblasts (B, D) in placentas from normotensive pregnancies (A, B) and in preeclamptic (PE) placental tissues (C, D). Scale bars, 35 μm. E) Representative immunoblot for LIN28B and α-tubulin in term placenta lysates from 4 normotensive pregnancies vs. 4 PE pregnancies (top) and densitometric analysis of immunoblotting results for LIN28B normalized to α-tubulin from tissue lysates from normotensive pregnancies (n = 15) and PE pregnancies (n = 13) (bottom). F) qPCR analysis for LIN28B mRNA expression in placental lysates from normotensive pregnancies (n = 21) and from preeclamptic pregnancies (n = 19). G) Let-7g/LIN28B protein ratio determined by qPCR analysis for let-7g normalized to U18 and LIN28B immunoblot normalized to α-tubulin in placental lysates from normotensive pregnancies and PE pregnancies. Results represent means ± sem. *P < 0.05 vs. normal.
LIN28B is highly expressed in invasive EVTs in first trimester and early pregnancy human placentas in situ and increased trophoblast invasion and migration in vitro
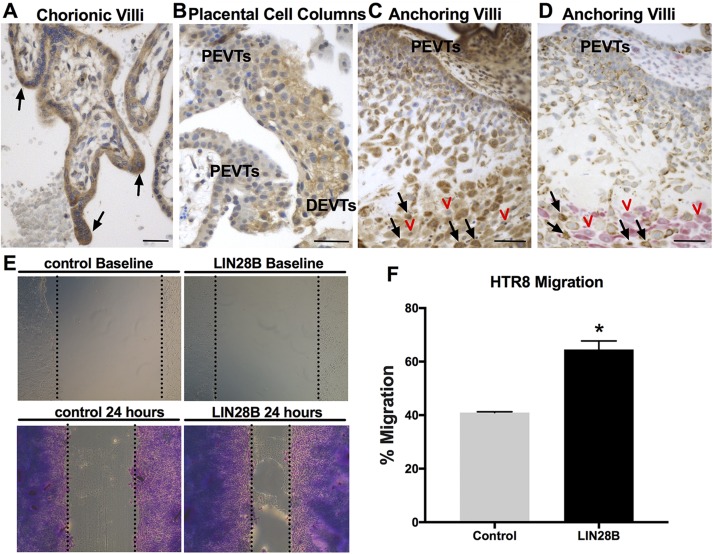
Human trophoblasts can differentiate to STs or EVTs, with the latter including proximal EVTs (proliferative), distal EVTs (invasive), interstitial CTs, and/or endovascular CTs, all of which can be detected in first trimester and early pregnancy placental sections. To determine the precise in situ LIN28B expression pattern among different trophoblast cell types, first trimester and early pregnancy human placental sections were immunostained for LIN28B. Compared to villous CTs, syncytial sprouts and EVTs in trophoblastic cell columns in the anchoring villi displayed stronger LIN28B expression (Fig. 3A, B). Moreover, a gradual increase in LIN28B immunoreactivity is evident from proximal EVTs to distal invasive EVTs in the cell columns. Interstitial CTs (cytokeratin positive) invading the decidua (vimentin positive) exhibited the strongest LIN28B expression (Fig. 3C, D). These observations provide supporting in situ evidence for the involvement of LIN28B in trophoblast differentiation toward an invasive phenotype and/or syncytialization. To support these findings with functional in vitro studies, overexpression of LIN28B in a first trimester human trophoblast (HTR8/SVneo) cell line resulted in a 1.6-fold increase (P < 0.05) in migration compared to control (Fig. 3E, F). Furthermore, overexpression of LIN28B resulted in an increase in HTR8/SVneo cell invasion; however, it did not reach significance (P = 0.053) (Supplemental Fig. S2).
Figure 3 .
LIN28B is highly expressed in syncytial sprouts and invasive trophoblasts in first trimester placentas and increases trophoblast migration in vitro. A–C) Representative images of immunohistochemical staining for LIN28B in human first trimester placenta. LIN28B is highly expressed in syncytial sprouts of chorionic villi (black arrows, A). LIN28B expression increases from proximal EVTs (PEVTs) to distal invasive EVTs (DEVTs) in placental cell columns (B). LIN28B expression is lowest in PEVTs and increased in interstitial trophoblasts (black arrows) invading maternal decidua (red arrowheads) in anchoring villi (C). D) Representative serial section of tissue shown in C double immunostained for DC marker vimentin (pink) and trophoblast marker cytokeratin-7 (brown). Red arrowheads and black arrows in D correspond to red arrowheads and black arrows in C. E) Representative results of scratch wound assay at time of scratch application (top) and after 24 h (bottom) from HTR8/SVneo cells transfected with either GFP (control) or LIN28B. F) Quantification of migration after 24 h. Scale bars, 35 mm. Results represent means ± sem. *P < 0.05 vs. control.
LIN28B expression regulates trophoblast proliferation, differentiation, and inflammation
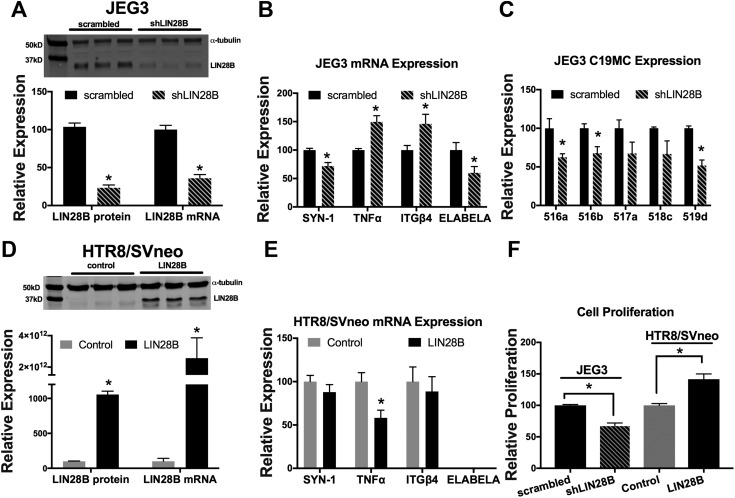
To determine whether the reduction in LIN28B expression observed in placentas of PE-complicated pregnancies also affects trophoblast differentiation, inflammation, and C19MC miRNAs, LIN28B was transiently knocked down in JEG3 cells (Fig. 4A). In these experiments, JEG3 cells were used because of their high expression of LIN28B. LIN28B knockdown elicited a 29% reduction in mRNA levels of the trophoblast differentiation marker SYN-1 and a ∼50% increase in mRNA expression of both the proinflammatory cytokine TNF-α and the integrin subunit enriched on noninvasive trophoblasts, ITGβ4 (Fig. 4B). Furthermore, knockdown of LIN28B resulted in a 41% reduction in mRNA expression of ELABELA (Fig. 4B), a gene whose deficiency was shown to result in development of PE-like symptoms (35). Knockdown of LIN28B also resulted in a 33% to 49% decrease in 5 randomly selected miRNAs of the C19MC cluster, of which 3 (miR-516a, miR-516b, and miR-519d) were significantly decreased (P < 0.05, Fig. 4C). Conversely, overexpression of LIN28B in HTR8/SVneo cells (Fig. 4D), in which LIN28B is undetectable, resulted in a 42% decrease in TNF-α mRNA levels, whereas SYN-1 and ITGβ4 levels were unchanged (Fig. 4E). HTR8/SVneo cells do not normally express ELABELA, and overexpression of LIN28B did not induce ELABELA expression. Furthermore, knockdown of LIN28B in JEG3 cells significantly reduced cell proliferation, whereas overexpression of LIN28B in HTR8/SVneo cells significantly increased cell proliferation (Fig. 4F).
Figure 4 .
LIN28B regulates trophoblast proliferation, differentiation, inflammation, and C19MC miRNA expression. A) Representative immunoblot, densitometric quantification, and qPCR analysis of LIN28B levels 72 h after control or LIN28B shRNA-mediated knockdown in JEG3 cells. B, C) qPCR analysis of indicated gene (B) and miRNA (C), normalized to GAPDH and U18, respectively, after transient transfection of JEG3 cells with control or LIN28B shRNA. D) Representative immunoblot, densitometric quantification, and qPCR analysis of LIN28B levels 72 h after transient overexpression of GFP (control) or LIN28B encoding plasmids in HTR8/SVneo cells. E) qPCR analysis of indicated genes 72 h after transient overexpression of GFP or LIN28B in HTR8/SVneo cells. F) Cell proliferation analysis 72 h after transient knockdown of LIN28B in JEG3 cells or transient overexpression of LIN28B in HTR8/SVneo cells. Results represent means ± sem. *P < 0.05 shLIN28B vs. scrambled (A–C) or LIN28B vs. control (D, E).
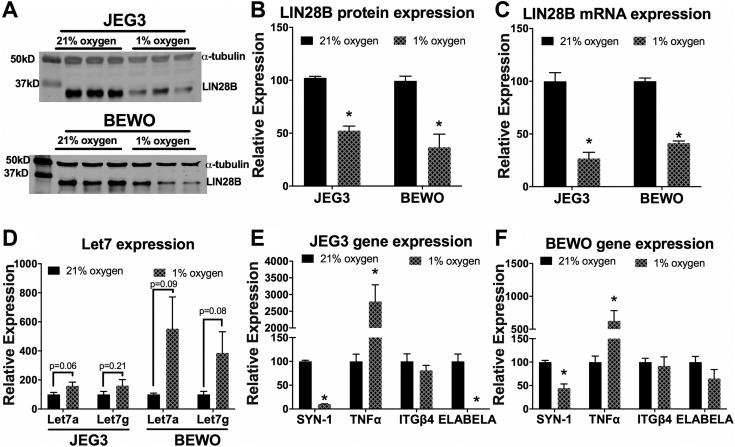
Hypoxia decreased LIN28B expression in BeWo and JEG3 cells
Shallow implantation is associated with impaired trophoblast invasion and remodeling of the maternal spiral arteries, resulting in insufficient placental perfusion (36, 37). The current results demonstrate that trophoblasts are the main cell type expressing LIN28B, which prompted assessment of the effect of hypoxia on LIN28B expression. Accordingly, BeWo cells, which also express high levels of LIN28B, and JEG3 cells cultured for 72 h in hypoxia (1% O2) exhibited a >50% reduction in LIN28B mRNA and protein levels compared to standard culture conditions (21% O2) (Fig. 5A–C). Moreover, LIN28A mRNA and protein levels were also decreased under hypoxic vs. normoxic conditions (Supplemental Fig. S3). Furthermore, exposure of JEG3 and BeWo cells to 1% oxygen resulted in an increase in the expression of let-7a and let-7g. However, this increase did not reach statistical significance (Fig. 5D). In addition, hypoxia significantly reduced SYN-1, whereas TNF-α was significantly increased in JEG3 and BeWo cells (Fig. 5E, F). The expression of ITGβ4 was unchanged by hypoxia; however, ELABELA mRNA was reduced to undetectable levels in JEG3 cells after exposure to hypoxic conditions and trended toward a significant decrease in BeWo cells (Fig. 5E, F).
Figure 5 .
Hypoxia reduces expression of LIN28B in JEG3 and BeWo cells. A) Representative immunoblot for LIN28B in JEG3 and BeWo cells cultured in standard culture conditions (21% oxygen) or hypoxia (1% oxygen) for 72 h. B, C) Densitometric quantification of LIN28B protein expression normalized to α-tubulin (B) and qPCR analysis for LIN28B mRNA expression normalized to GAPDH (C) after 72 h of normal culture conditions or hypoxia. D) qPCR analysis for let-7a and let-7g in JEG3 and BeWo cells after culture in standard culture conditions or hypoxia for 72 h. E, F) qPCR analysis for indicated genes expressed by JEG3 cells (E) and BeWo cells (F) after culture in standard culture conditions or hypoxia for 72 h. Results represent means ± sem. *P < 0.05 for 1 vs. 21% oxygen standard culture conditions.
DISCUSSION
The current study revealed that LIN28B is the predominant paralog in human placentas and is significantly down-regulated in placentas of PE affected pregnancies; that increased LIN28B expression is positively correlated with EVT invasion, migration, and proliferation; that knockdown of LIN28B in JEG3 cells significantly decreased levels of SYN-1, ELABELA, and C19MC miRNA, and increased expression of ITGβ4, an integrin subunit enriched on noninvasive trophoblasts, as well as the proinflammatory cytokine TNF-α, whereas overexpression of LIN28B in HTR8/SVneo cells decreased TNF-α expression; and that incubation under hypoxia significantly decreased LIN28B and SYN-1 expression in BeWo and JEG3 cells, while it increased TNF-α expression.
Results presented in this study reveal LIN28B as the predominant paralog in human placentas. Although LIN28A and LIN28B share 65% sequence identity including the N-terminal cold shock domain and the C-terminal zinc-knuckle domain and share similar functions, such as regulation of let-7 maturation and thousands of mRNAs, LIN28B is composed of a distinct set of 50 aa at the C-terminal domain that includes a nuclear localization signal (38). These findings suggest a unique role for LIN28B in human pregnancy.
Previous studies have shown that LIN28B selectively binds to a common GGAG motif in the loop region of the pri– and pre–let-7 family, resulting in inhibition of DROSHA or DICER1 RNase III processing (39–43). The let-7 family consists of 12 members in humans, with suppression of let-7g by LIN28B well documented (44). The LIN28B/let-7 axis contributes to control of cellular metabolism by regulating genes in the insulin-PI3K-mTOR pathway (23). Embryonic knockout of LIN28B in mice leads to postnatal dwarfism and glucose intolerance, whereas mice overexpressing LIN28B grow larger and exhibit increased insulin sensitivity and higher glucose metabolism compared to wild-type mice (22, 23). Importantly, fetal muscle–specific LIN28B knockout resulted in long-term adverse effects on growth, insulin resistance, and impaired glucose uptake compared to wild-type controls (45), suggesting a crucial role for LIN28B in both prenatal and postnatal development. Furthermore, genome-wide association studies have linked LIN28B with human height, age at puberty and menarche, and type 2 diabetes (46–49). Therefore, the reduction in LIN28B in the placentas of PE-complicated pregnancies reported here may help explain why children from PE-complicated pregnancies are often born small for gestational age and are at increased risk of developing metabolic- and cardiovascular-related diseases later in life (50, 51).
Sufficient early EVT invasion into the decidua accompanied by proper remodeling of the spiral arteries is required for normal pregnancy. Such EVT invasion bears striking similarities to that of cancer metastasis (52, 53). In this regard, LIN28B is reported to increase cancer cell proliferation and invasion (54, 55). Accordingly, the current study demonstrates that invasive interstitial EVTs in first trimester placental sections express higher levels of LIN28B compared to noninvasive proximal EVTs, and that elevated LIN28B expression increases proliferation, migration, and invasion of HTR8/SVneo cells in vitro. Additionally, LIN28B knockdown in JEG3 cells results in increased ITGβ4, which is an integrin subunit that is enriched on noninvasive trophoblasts. Collectively, these findings suggest that high LIN28B expression is required for proper trophoblast migration and invasion.
Development of PE is also associated with aberrant expression of several proinflammatory cytokines. Specifically, TNF-α levels are elevated in the amniotic fluid and plasma of pregnancies affected by PE (56, 57). This study shows that LIN28B knockdown results in elevated levels of TNF-α, indicating a potential link between decreased placental LIN28B expression and the proinflammatory milieu of PE. Moreover, LIN28B knockdown in JEG3 cells significantly reduced mRNA levels of both SYN-1 and ELABELA, a placenta-derived hormone whose depletion results in the onset of PE-like symptoms in mice (35). Considered together, these findings strongly suggest that LIN28B plays a role in PE-associated trophoblast differentiation, inflammation, and endocrine signaling. Acting as an RNA-binding protein, LIN28B may directly and indirectly regulate the expression of any or all of these genes, thus justifying the need for further investigation.
Although LIN28B is documented to inhibit miRNA maturation, the present study showed that LIN28B knockdown reduced the expression of several C19MC miRNAs. These unexpected results may be explained by a recent study demonstrating that LIN28 binds directly to a consensus DNA sequence at promoter regions and recruits the CpG demethylase TET1 to activate transcription (58). Moreover, analysis of published PAR-CLIP results indicate that LIN28B can bind to CpG rich-Alu repeats dispersed throughout C19MC (29) that can function as independent promoters for RNA polymerase III and transcriptionally activate C19MC (59–61). Furthermore, our group recently showed that overexpression of LIN28B induces the expression of some miRNAs of the C19MC cluster (62). Additional studies are required to elucidate the signal transduction pathway or pathways responsible for LIN28B regulation of C19MC.
Insufficient perfusion elicits pathologic uteroplacental hypoxia (37). The current study demonstrates that exposure of BeWo and JEG3 cells to hypoxia reduced LIN28B mRNA and protein expression. Moreover, as previously reported, hypoxia reduced SYN-1 expression. Recently LIN28 was shown to be phosphorylated and stabilized by MAPK/ERK (63), and that MAPK/ERK is down-regulated by hypoxia (64). Furthermore, in endothelial cells and cancer cell lines, hypoxia can induce the expression of let-7 (65). These changes in gene expression induced by hypoxia could be responsible for the reduction in LIN28B found in the current study. Furthermore, human TargetScan analysis reveals that the genes studied in the current report that are shown to affected by hypoxia are not predicted targets of let-7. Therefore, it is likely that these genes are directly or indirectly regulated by LIN28B because it is an RNA binding protein that can target many mRNA transcripts. Both hypoxia- and LIN28B-dependent reduction in SYN-1 expression likely results in placental insufficiency as a result of reduced numbers of STs, which play a role in placental gas and nutrient transport (66, 67). Unlike a previous study, which showed that knockdown of LIN28A in a first trimester human trophoblast-like ACH-3P cell line induced spontaneous syncytialization, the present study found that knockdown of LIN28B reduced SYN-1. Further studies are required to clearly elucidate the role or roles that LIN28B may play in trophoblast differentiation.
Analysis of the demographic data associated with the two cohorts used in this study indicate significant differences between normal and PE pregnancies related to the indication for cesarean section. Of the 13 PE samples currently studied, 4 of the 9 subjects who underwent cesarean section were due to failure of labor to progress, while 9 of the 15 normal subjects underwent repeat cesarean sections. Here we identify a global decrease in the expression of LIN28B, which is indicative of changes in protein stability and/or protein degradation, as well as transcriptional changes. Therefore, these changes are not likely due to the delay in delivery caused by the failure of labor to progress. However, further studies are warranted to determine the effect of cesarean section and mode of delivery on the placental expression of LIN28B.
It is possible that the reduction in LIN28B expression observed in placentas of PE-affected pregnancies arises early in pregnancy. Specifically, on the basis of the known roles of LIN28B and the data shown here, decreased LIN28B expression during blastocyst development could potentially cause impaired placentation and insufficient trophoblast invasion of the spiral arteries. The resulting poor spiral artery remodeling could result in pathologically prolonged hypoxia, further decreasing LIN28B expression, thereby triggering a potential feed-forward loop. However, it is also possible that the down-regulation of LIN28B is initially triggered by placental hypoxia, of which the origin is not yet known. Additional studies are required to further investigate the cause of the reduction in LIN28B.
The developmental origin of health and disease states that environmental factors that interfere with normal fetal development, such as poor nutrition of the mother or fetus, are likely contributors to lifelong changes in the metabolism of the child (68). However, the precise cellular mechanisms responsible are largely unknown. The strong correlation that exists between fetal LIN28B expression and both development and long-term changes in growth and metabolism (22, 23, 45) indicates the relevance of investigating whether the decrease in LIN28B expression observed in placentas of PE-complicated pregnancies contributes to adverse outcomes in adult health. Future studies directed at testing this hypothesis may confirm LIN28B as a contributing factor to the underlying mechanism of the developmental origin of postnatal diseases and may set the stage for further molecular and epidemiologic studies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Ananth Karumanchi (Beth Israel Deaconess Medical Center, Harvard Medical School) for providing total placental RNA from normal and preeclampsia affected pregnancies. J.C. was supported by the American Heart Association (Grant 15PRE25850019), and H.T.J. was supported by the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (Grant R01HL128411). The authors declare no conflicts of interest.
Glossary
- C19MC
chromosome 19 microRNA cluster
- CT
cytotrophoblast
- DC
decidual cell
- ELABELA
apelin receptor early endogenous ligand
- EVT
extravillous trophoblast
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- miRNA
microRNA
- PE
preeclampsia
- qPCR
quantitative PCR
- RPMI
Rosewell Park Memorial Institute
- shRNA
short hairpin RNA
- ST
syncytiotrophoblast
- SYN-1
syncytin 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. Canfield and H. Totary-Jain designed research; J. Canfield, S. Arlier, E. F. Mong, J. Lockhart, and J. VanWye performed the experiments; O. Guzeloglu-Kayisli, F. Schatz, R. R. Magness, C. J. Lockwood, J. C. M. Tsibris, and U. A. Kayisili contributed reagents; J. Canfield and H. Totary-Jain analyzed the data; and J. Canfield and H. Totary-Jain wrote the manuscript.
REFERENCES
- 1.Smyth, A., Ronco, C., Garovic, V. D. (2017) Preeclampsia: a cardiorenal syndrome in pregnancy. Curr. Hypertens. Rep. 19, 15 [DOI] [PubMed] [Google Scholar]
- 2.Henderson, J. T., Thompson, J. H., Burda, B. U., Cantor, A. (2017) Preeclampsia screening: evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA 317, 1668–1683 [DOI] [PubMed] [Google Scholar]
- 3.Ghulmiyyah, L., Sibai, B. (2012) Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 36, 56–59 [DOI] [PubMed] [Google Scholar]
- 4.Shih, T., Peneva, D., Xu, X., Sutton, A., Triche, E., Ehrenkranz, R. A., Paidas, M., Stevens, W. (2016) The rising burden of preeclampsia in the United States impacts both maternal and child health. Am. J. Perinatol. 33, 329–338 [DOI] [PubMed] [Google Scholar]
- 5.Paauw, N. D., Luijken, K., Franx, A., Verhaar, M. C., Lely, A. T. (2016) Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin. Sci. (Lond.) 130, 239–246 [DOI] [PubMed] [Google Scholar]
- 6.Ness, R. B., Roberts, J. M. (1996) Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am. J. Obstet. Gynecol. 175, 1365–1370 [DOI] [PubMed] [Google Scholar]
- 7.Redman, C. W., Sargent, I. L. (2005) Latest advances in understanding preeclampsia. Science 308, 1592–1594 [DOI] [PubMed] [Google Scholar]
- 8.Degner, K., Magness, R. R., Shah, D. M. (2017) Establishment of the human uteroplacental circulation: a historical perspective. Reprod. Sci. 24, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karumanchi, S. A., Bdolah, Y. (2004) Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 145, 4835–4837 [DOI] [PubMed] [Google Scholar]
- 10.Krishna, U., Bhalerao, S. (2011) Placental insufficiency and fetal growth restriction. J. Obstet. Gynaecol. India 61, 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts, J. M., Edep, M. E., Goldfien, A., Taylor, R. N. (1992) Sera from preeclamptic women specifically activate human umbilical vein endothelial cells in vitro: morphological and biochemical evidence. Am. J. Reprod. Immunol. 27, 101–108 [DOI] [PubMed] [Google Scholar]
- 12.Nagamatsu, T., Fujii, T., Kusumi, M., Zou, L., Yamashita, T., Osuga, Y., Momoeda, M., Kozuma, S., Taketani, Y. (2004) Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145, 4838–4845 [DOI] [PubMed] [Google Scholar]
- 13.Kudo, Y., Boyd, C. A., Sargent, I. L., Redman, C. W. (2003) Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim. Biophys. Acta 1638, 63–71 [DOI] [PubMed] [Google Scholar]
- 14.Chen, C. P., Wang, K. G., Chen, C. Y., Yu, C., Chuang, H. C., Chen, H. (2006) Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG 113, 152–158 [DOI] [PubMed] [Google Scholar]
- 15.Lee, X., Keith, J. C., Jr., Stumm, N., Moutsatsos, I., McCoy, J. M., Crum, C. P., Genest, D., Chin, D., Ehrenfels, C., Pijnenborg, R., van Assche, F. A., Mi, S. (2001) Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta 22, 808–812 [DOI] [PubMed] [Google Scholar]
- 16.Knerr, I., Beinder, E., Rascher, W. (2002) Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 186, 210–213 [DOI] [PubMed] [Google Scholar]
- 17.Hromadnikova, I., Kotlabova, K., Ondrackova, M., Pirkova, P., Kestlerova, A., Novotna, V., Hympanova, L., Krofta, L. (2015) Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 34, 437–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hromadnikova, I., Kotlabova, K., Ivankova, K., Krofta, L. (2017) First trimester screening of circulating C19MC microRNAs and the evaluation of their potential to predict the onset of preeclampsia and IUGR. PLoS One 12, e0171756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piskounova, E., Polytarchou, C., Thornton, J. E., LaPierre, R. J., Pothoulakis, C., Hagan, J. P., Iliopoulos, D., Gregory, R. I. (2011) Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147, 1066–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan, S. R., Daley, G. Q. (2010) Lin28: a microRNA regulator with a macro role. Cell 140, 445–449 [DOI] [PubMed] [Google Scholar]
- 21.Tsialikas, J., Romer-Seibert, J. (2015) LIN28: roles and regulation in development and beyond. Development 142, 2397–2404 [DOI] [PubMed] [Google Scholar]
- 22.Zhu, H., Shah, S., Shyh-Chang, N., Shinoda, G., Einhorn, W. S., Viswanathan, S. R., Takeuchi, A., Grasemann, C., Rinn, J. L., Lopez, M. F., Hirschhorn, J. N., Palmert, M. R., Daley, G. Q. (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 42, 626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu, H., Shyh-Chang, N., Segrè, A. V., Shinoda, G., Shah, S. P., Einhorn, W. S., Takeuchi, A., Engreitz, J. M., Hagan, J. P., Kharas, M. G., Urbach, A., Thornton, J. E., Triboulet, R., Gregory, R. I., Altshuler, D., Daley, G. Q.; DIAGRAM Consortium ; MAGIC Investigators . (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockwood, C. J., Arcuri, F., Toti, P., Felice, C. D., Krikun, G., Guller, S., Buchwalder, L. F., Schatz, F. (2006) Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am. J. Pathol. 169, 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang, Z., Tadesse, S., Norwitz, E., Mor, G., Abrahams, V. M., Guller, S. (2011) Isolation of Hofbauer cells from human term placentas with high yield and purity. Am. J. Reprod. Immunol. 66, 336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, M. J., Wang, Z., Yee, H., Ma, Y., Swenson, N., Yang, L., Kadner, S. S., Baergen, R. N., Logan, S. K., Garabedian, M. J., Guller, S. (2005) Expression and regulation of glucocorticoid receptor in human placental villous fibroblasts. Endocrinology 146, 4619–4626 [DOI] [PubMed] [Google Scholar]
- 27.Yen, C. F., Liao, S. K., Huang, S. J., Tabak, S., Arcuri, F., Lee, C. L., Arici, A., Petraglia, F., Wang, H. S., Kayisli, U. A. (2017) Decreased endometrial expression of leukemia inhibitory factor receptor disrupts the STAT3 signaling in adenomyosis during the implantation window. Reprod. Sci. 24, 1176–1186 [DOI] [PubMed] [Google Scholar]
- 28.Lockwood, C. J., Murk, W., Kayisli, U. A., Buchwalder, L. F., Huang, S. T., Funai, E. F., Krikun, G., Schatz, F. (2009) Progestin and thrombin regulate tissue factor expression in human term decidual cells. J. Clin. Endocrinol. Metab. 94, 2164–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafner, M., Max, K. E., Bandaru, P., Morozov, P., Gerstberger, S., Brown, M., Molina, H., Tuschl, T. (2013) Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA 19, 613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totary-Jain, H., Sanoudou, D., Dautriche, C. N., Schneller, H., Zambrana, L., Marks, A. R. (2012) Rapamycin resistance is linked to defective regulation of Skp2. Cancer Res. 72, 1836–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santulli, G., Wronska, A., Uryu, K., Diacovo, T. G., Gao, M., Marx, S. O., Kitajewski, J., Chilton, J. M., Akat, K. M., Tuschl, T., Marks, A. R., Totary-Jain, H. (2014) A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J. Clin. Invest. 124, 4102–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Totary-Jain, H., Sanoudou, D., Ben-Dov, I. Z., Dautriche, C. N., Guarnieri, P., Marx, S. O., Tuschl, T., Marks, A. R. (2013) Reprogramming of the microRNA transcriptome mediates resistance to rapamycin. J. Biol. Chem. 288, 6034–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, L., Song, W. Y., Xie, Y., Hu, L. L., Hou, X. M., Wang, R., Gao, Y., Zhang, J. N., Zhang, L., Li, W. W., Zhu, C., Gao, Z. Y., Sun, Y. P. (2018) miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 9, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu, J. J., Lin, P., Lv, X. Y., Yan, X. J., Wang, H. X., Zhu, C., Tsang, B. K., Yu, X. G., Wang, H. (2009) Low molecular mass polypeptide-2 in human trophoblast: over-expression in hydatidiform moles and possible role in trophoblast cell invasion. Placenta 30, 305–312 [DOI] [PubMed] [Google Scholar]
- 35.Ho, L., van Dijk, M., Chye, S. T. J., Messerschmidt, D. M., Chng, S. C., Ong, S., Yi, L. K., Boussata, S., Goh, G. H., Afink, G. B., Lim, C. Y., Dunn, N. R., Solter, D., Knowles, B. B., Reversade, B. (2017) ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 357, 707–713: [DOI] [PubMed] [Google Scholar]
- 36.Fisher, S. J. (2015) Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 213 (Suppl 4), S115–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutter, D., Kingdom, J., Jaeggi, E. (2010) Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int. J. Pediatr. 2010, 401323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayr, F., Heinemann, U. (2013) Mechanisms of Lin28-mediated miRNA and mRNA regulation—a structural and functional perspective. Int. J. Mol. Sci. 14, 16532–16553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loughlin, F. E., Gebert, L. F., Towbin, H., Brunschweiger, A., Hall, J., Allain, F. H. (2011) Structural basis of pre–let-7 miRNA recognition by the zinc knuckles of pluripotency factor Lin28. Nat. Struct. Mol. Biol. 19, 84–89 [DOI] [PubMed] [Google Scholar]
- 40.Newman, M. A., Thomson, J. M., Hammond, S. M. (2008) Lin-28 interaction with the let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagan, J. P., Piskounova, E., Gregory, R. I. (2009) Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat. Struct. Mol. Biol. 16, 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heo, I., Joo, C., Kim, Y. K., Ha, M., Yoon, M. J., Cho, J., Yeom, K. H., Han, J., Kim, V. N. (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 [DOI] [PubMed] [Google Scholar]
- 43.Lehrbach, N. J., Armisen, J., Lightfoot, H. L., Murfitt, K. J., Bugaut, A., Balasubramanian, S., Miska, E. A. (2009) LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 16, 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali, P. S., Ghoshdastider, U., Hoffmann, J., Brutschy, B., Filipek, S. (2012) Recognition of the let-7g miRNA precursor by human Lin28B. FEBS Lett. 586, 3986–3990 [DOI] [PubMed] [Google Scholar]
- 45.Shinoda, G., Shyh-Chang, N., Soysa, T. Y., Zhu, H., Seligson, M. T., Shah, S. P., Abo-Sido, N., Yabuuchi, A., Hagan, J. P., Gregory, R. I., Asara, J. M., Cantley, L. C., Moss, E. G., Daley, G. Q. (2013) Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 31, 1563–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, S. W., Lee, S. T., Sohn, Y. B., Cho, S. Y., Kim, S. H., Kim, S. J., Kim, C. H., Ko, A. R., Paik, K. H., Kim, J. W., Jin, D. K. (2012) LIN28B polymorphisms are associated with central precocious puberty and early puberty in girls. Korean J. Pediatr. 55, 388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widén, E., Ripatti, S., Cousminer, D. L., Surakka, I., Lappalainen, T., Järvelin, M. R., Eriksson, J. G., Raitakari, O., Salomaa, V., Sovio, U., Hartikainen, A. L., Pouta, A., McCarthy, M. I., Osmond, C., Kajantie, E., Lehtimäki, T., Viikari, J., Kähönen, M., Tyler-Smith, C., Freimer, N., Hirschhorn, J. N., Peltonen, L., Palotie, A. (2010) Distinct variants at LIN28B influence growth in height from birth to adulthood. Am. J. Hum. Genet. 86, 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voight, B. F., Scott, L. J., Steinthorsdottir, V., Morris, A. P., Dina, C., Welch, R. P., Zeggini, E., Huth, C., Aulchenko, Y. S., Thorleifsson, G., McCulloch, L. J., Ferreira, T., Grallert, H., Amin, N., Wu, G., Willer, C. J., Raychaudhuri, S., McCarroll, S. A., Langenberg, C., Hofmann, O. M., Dupuis, J., Qi, L., Segrè, A. V., van Hoek, M., Navarro, P., Ardlie, K., Balkau, B., Benediktsson, R., Bennett, A. J., Blagieva, R., Boerwinkle, E., Bonnycastle, L. L., Bengtsson Boström, K., Bravenboer, B., Bumpstead, S., Burtt, N. P., Charpentier, G., Chines, P. S., Cornelis, M., Couper, D. J., Crawford, G., Doney, A. S., Elliott, K. S., Elliott, A. L., Erdos, M. R., Fox, C. S., Franklin, C. S., Ganser, M., Gieger, C., Grarup, N., Green, T., Griffin, S., Groves, C. J., Guiducci, C., Hadjadj, S., Hassanali, N., Herder, C., Isomaa, B., Jackson, A. U., Johnson, P. R., Jørgensen, T., Kao, W. H., Klopp, N., Kong, A., Kraft, P., Kuusisto, J., Lauritzen, T., Li, M., Lieverse, A., Lindgren, C. M., Lyssenko, V., Marre, M., Meitinger, T., Midthjell, K., Morken, M. A., Narisu, N., Nilsson, P., Owen, K. R., Payne, F., Perry, J. R., Petersen, A. K., Platou, C., Proença, C., Prokopenko, I., Rathmann, W., Rayner, N. W., Robertson, N. R., Rocheleau, G., Roden, M., Sampson, M. J., Saxena, R., Shields, B. M., Shrader, P., Sigurdsson, G., Sparsø, T., Strassburger, K., Stringham, H. M., Sun, Q., Swift, A. J., Thorand, B., Tichet, J., Tuomi, T., van Dam, R. M., van Haeften, T. W., van Herpt, T., van Vliet-Ostaptchouk, J. V., Walters, G. B., Weedon, M. N., Wijmenga, C., Witteman, J., Bergman, R. N., Cauchi, S., Collins, F. S., Gloyn, A. L., Gyllensten, U., Hansen, T., Hide, W. A., Hitman, G. A., Hofman, A., Hunter, D. J., Hveem, K., Laakso, M., Mohlke, K. L., Morris, A. D., Palmer, C. N., Pramstaller, P. P., Rudan, I., Sijbrands, E., Stein, L. D., Tuomilehto, J., Uitterlinden, A., Walker, M., Wareham, N. J., Watanabe, R. M., Abecasis, G. R., Boehm, B. O., Campbell, H., Daly, M. J., Hattersley, A. T., Hu, F. B., Meigs, J. B., Pankow, J. S., Pedersen, O., Wichmann, H. E., Barroso, I., Florez, J. C., Frayling, T. M., Groop, L., Sladek, R., Thorsteinsdottir, U., Wilson, J. F., Illig, T., Froguel, P., van Duijn, C. M., Stefansson, K., Altshuler, D., Boehnke, M., McCarthy, M. I.; MAGIC investigators ; GIANT Consortium . (2010) Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42, 579–589; erratum: 43, 388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, J., Zhang, L., Fan, R., Guo, N., Xiong, C., Wang, L., Jin, S., Li, W., Lu, J. (2013) The polymorphism in the let-7 targeted region of the Lin28 gene is associated with increased risk of type 2 diabetes mellitus. Mol. Cell. Endocrinol. 375, 53–57 [DOI] [PubMed] [Google Scholar]
- 50.Hakim, J., Senterman, M. K., Hakim, A. M. (2013) Preeclampsia is a biomarker for vascular disease in both mother and child: the need for a medical alert system. Int. J. Pediatr. 2013, 953150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu, C. S., Nohr, E. A., Bech, B. H., Vestergaard, M., Catov, J. M., Olsen, J. (2009) Health of children born to mothers who had preeclampsia: a population-based cohort study. Am. J. Obstet. Gynecol. 201, 269.e1–269.e10 [DOI] [PubMed] [Google Scholar]
- 52.Murray, M. J., Lessey, B. A. (1999) Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. 17, 275–290 [DOI] [PubMed] [Google Scholar]
- 53.Holtan, S. G., Creedon, D. J., Haluska, P., Markovic, S. N. (2009) Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin. Proc. 84, 985–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo, Y., Chen, Y., Ito, H., Watanabe, A., Ge, X., Kodama, T., Aburatani, H. (2006) Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384, 51–61 [DOI] [PubMed] [Google Scholar]
- 55.Balzeau, J., Menezes, M. R., Cao, S., Hagan, J. P. (2017) The LIN28/let-7 pathway in cancer. Front. Genet. 8, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serin, I. S., Ozçelik, B., Basbug, M., Kiliç, H., Okur, D., Erez, R. (2002) Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 100, 143–145; erratum: 104, 86 [DOI] [PubMed] [Google Scholar]
- 57.Kupferminc, M. J., Peaceman, A. M., Wigton, T. R., Rehnberg, K. A., Socol, M. L. (1994) Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am. J. Obstet. Gynecol. 170, 1752–1757; discussion 1757–1759 [PubMed] [Google Scholar]
- 58.Zeng, Y., Yao, B., Shin, J., Lin, L., Kim, N., Song, Q., Liu, S., Su, Y., Guo, J. U., Huang, L., Wan, J., Wu, H., Qian, J., Cheng, X., Zhu, H., Ming, G. L., Jin, P., Song, H. (2016) Lin28A binds active promoters and recruits Tet1 to regulate gene expression. Mol. Cell 61, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen, P. N., Huang, C. J., Sugii, S., Cheong, S. K., Choo, K. B. (2017) Selective activation of miRNAs of the primate-specific chromosome 19 miRNA cluster (C19MC) in cancer and stem cells and possible contribution to regulation of apoptosis. J. Biomed. Sci. 24, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borchert, G. M., Lanier, W., Davidson, B. L. (2006) RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 13, 1097–1101 [DOI] [PubMed] [Google Scholar]
- 61.Saito, Y., Suzuki, H., Tsugawa, H., Nakagawa, I., Matsuzaki, J., Kanai, Y., Hibi, T. (2009) Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene 28, 2738–2744 [DOI] [PubMed] [Google Scholar]
- 62.Mong, E. F., Akat, K. M., Canfield, J., Lockhart, J., VanWye, J., Matar, A., Tsibris, J. C. M., Wu, J. K., Tuschl, T., Totary-Jain, H. (2018) Modulation of LIN28B/Let-7 signaling by propranolol contributes to infantile hemangioma involution. Arterioscler. Thromb. Vasc. Biol. 38, 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsanov, K. M., Pearson, D. S., Wu, Z., Han, A., Triboulet, R., Seligson, M. T., Powers, J. T., Osborne, J. K., Kane, S., Gygi, S. P., Gregory, R. I., Daley, G. Q. (2017) LIN28 phosphorylation by MAPK/ERK couples signalling to the post-transcriptional control of pluripotency. Nat. Cell Biol. 19, 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kučera, J., Netušilová, J., Sladeček, S., Lánová, M., Vašíček, O., Štefková, K., Navrátilová, J., Kubala, L., Pacherník, J. (2017) Hypoxia downregulates MAPK/ERK but not STAT3 signaling in ROS-dependent and HIF-1–independent manners in mouse embryonic stem cells. Oxid. Med. Cell. Longev. 2017, 4386947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choudhry, H., Mole, D. R. (2016) Hypoxic regulation of the noncoding genome and NEAT1. Brief. Funct. Genomics 15, 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nwabuobi, C., Arlier, S., Schatz, F., Guzeloglu-Kayisli, O., Lockwood, C. J., Kayisli, U. A. (In press) hCG: biological functions and clinical applications. Int. J. Mol. Sci. 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni, X. T., Duan, T., Yang, Z., Guo, C. M., Li, J. N., Sun, K. (2009) Role of human chorionic gonadotropin in maintaining 11beta-hydroxysteroid dehydrogenase type 2 expression in human placental syncytiotrophoblasts. Placenta 30, 1023–1028 [DOI] [PubMed] [Google Scholar]
- 68.Jun-Hao, E. T., Gupta, R. R., Shyh-Chang, N. (2016) Lin28 and let-7 in the metabolic physiology of aging. Trends Endocrinol. Metab. 27, 132–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.