Abstract
Drug addiction is a behavioral disorder characterized by dysregulated learning about drugs and associated cues that result in compulsive drug seeking and relapse. Learning about drug rewards and predictive cues is a complex process controlled by a computational network of neural connections interacting with transcriptional and molecular mechanisms within each cell to precisely guide behavior. The interplay between rapid, temporally specific neuronal activation, and longer-term changes in transcription is of critical importance in the expression of appropriate, or in the case of drug addiction, inappropriate behaviors. Thus, these factors and their interactions must be considered together, especially in the context of treatment. Understanding the complex interplay between epigenetic gene regulation and circuit connectivity will allow us to formulate novel therapies to normalize maladaptive reward behaviors, with a goal of modulating addictive behaviors, while leaving natural reward-associated behavior unaffected.
Keywords: Dopamine, Addiction, Reinforcement, Epigenetics, Circuits
1 INTRODUCTION
Drug addiction is a chronic relapsing disorder characterized by maladaptive learning about drugs and predictive cues that leads to compulsive drug seeking (Leshner, 1997). The major goal of addiction treatment is to correct these maladaptive behaviors and minimize relapse events without disrupting normal reward-seeking behaviors (e.g., seeking food or water). Because drugs hijack endogenous reward circuitry to elicit their rewarding and reinforcing effects, this has proved difficult (Dackis and O’Brien, 2005; Leshner, 1997). For example, simply pharmacologically inhibiting receptors or circuits involved in these pathways often has side effects that suppress the rewarding value of natural stimuli, making them undesirable and ineffective as long-term treatments (Aberman and Salamone, 1999; Leshner, 1997; Salamone et al., 2002). Thus, it is critical to understand the different neural codes that encode information about rewarding stimuli and drug stimuli, and the neural basis of how these types of behaviors become dysregulated for long-periods of time to drive drug addiction.
While a large body of work has focused on neural circuit dysfunction as well as genetic factors contributing to addiction (Koob and Volkow, 2010; Kreek et al., 2005; Nestler and Aghajanian, 1997; Volkow et al., 2013), less work has taken a broad scale multidisciplinary approach to understand how genes, circuits, and complex behaviors act together to control acute and chronic responses to drugs. Thus, to develop new targets we propose work aimed at understanding the complex interplay between genetic and circuit factors, as modulating each in isolation will likely lead to ineffective treatment strategies.
Here, we will outline how epigenetic factors converge with changes in neural circuit activity and connectivity to control complex behaviors and drug addiction.
2 DRUG ADDICTION IS A BEHAVIORAL LEARNING DISORDER
2.1 LEARNING ABOUT RESPONSE CONTINGENCIES
DRUG SELF-ADMINISTRATION AS AN ANIMAL MODEL OF ADDICTION.
Currently, a large amount of what we know about drug-induced plasticity comes from work using noncontingent acute drug administration. For example, the acute effects of many drugs are commonly modeled with intraperitoneal injections, subcutaneous pellets, and other forms of passive drug delivery. However, the acute effects of drugs are not what characterize human drug addiction. Additionally, animal models that rely on passive drug delivery do not reflect other equally crucial aspects of the addiction phenomenon. Similarly, while different paradigms are often used for studying the rewarding properties of addictive substances, reward is in fact a subjective measure difficult to interpret in many animal paradigms frequently used in the field. Importantly, other, equally crucial aspects of addiction such as social context of drug use are not assessed by animal models that still mostly rely on passive drug delivery (Heilig et al., 2016).
To model these aspects of drug addiction in animals, we use drug self-administration where animals are given access to a lever that when pressed results in the delivery of an intravenous injection of drug. The delivery of drug can be paired with discrete and contextual cues that allow for studies that assess how drug consumption changes over time, how predictive cues enhance motivation, and how cues can precipitate release.
Unlike genetic disorders, drug addiction is, primarily, a behavioral disorder that only develops after repeated exposure to a drug of abuse (Dackis and O’Brien, 2005; Koob and Volkow, 2010; Leshner, 1997). It is characterized by an inability to inhibit inappropriate behavioral responses to drugs and associated stimuli (Goldstein and Volkow, 2002). Both the consumption of natural rewards and drug rewards relies on reinforcement learning, where a stimulus (e.g., a reinforcer such as food) acts to maintain a particular behavior (such as eating) (Skinner, 1958). In the case of drug reinforcers, drug taking is driven initially by positive reinforcement, where an individual takes drug for the positive euphoric effects (i.e., the “high”) (Everitt and Robbins, 2005). This positive experience reinforces the previous behavior (smoking, consuming, or injecting the drug), driving the individual to learn to seek out and take the drug again to get the “high.” This same process occurs with natural rewards, and the ability to learn effective strategies for seeking reinforcers is critical to survival. Accordingly, many of these behavioral processes and the neural circuits controlling them are evolutionarily conserved across many species ranging from honey bees to humans (Balleine and O’Doherty, 2010; Gil, 2010; Tobler et al., 2006).
Because reinforcement learning relies on learning contingencies between actions and outcomes, it is a plastic process that requires quick adaptations changes in the environment, internal state, and need (Kandel and Schwartz, 1982; Kauer and Malenka, 2007; Shimizu et al., 2000). This behavioral process requires the ability to rapidly recall information, process information in real time, and reconsolidate this information. This is encoded by precise neural connections between brain regions and relies on both circuit connectivity and molecular and transcriptional factors to rapidly update information, and then maintain this information for when it is needed subsequently (Maren and Baudry, 1995; Sweatt, 2004). Evidence has shown that repeated drug exposure acts on some circuits to prevent plasticity and impair the ability of individuals to update these types of information (O’Brien et al., 1998). For example, with natural rewards, if the reward is no longer presented, or is very difficult to obtain, individuals will reduce the behavior because the effort or cost required to obtain the reward is too great (Bouton and Todd, 2014; Todd et al., 2014a, b). This adaptive response allows animals to learn new contingencies and allocate their behavior toward other scenarios and contexts that have a greater likelihood of resulting in rewards being obtained. This results in synaptic remodeling in the form of altered excitability and changes in cellular morphology in reward-related brain regions such as the nucleus accumbens (NAc) and prefrontal cortex (PFC) (Robinson, 1999; Robinson and Kolb, 2004). However, in the case of drug reinforcers these processes are often dysregulated, where drug seeking and taking can become compulsive and do not adapt to changing contingencies at both the behavioral and brain level (Everitt, 2014). Accordingly, individuals put enormous amounts of disproportionate effort into working to obtain drug at the expense of other adaptive behaviors. Thus, the inability to update information and extinguish mal-adaptive behavioral responses forms the basis of pathological drug seeking that often underlies relapse.
2.2 LEARNING ABOUT DRUG-ASSOCIATED CUES
In addition to learning about instrumental responses and outcomes, individuals also incorporate information about cues and contexts that inform about drug availability and guide environmentally appropriate behavioral responses (Schultz, 2006). In healthy individuals, associations between salient experiences and the environments within which they occur are the basis of decision-making and guide behavior toward advantageous outcomes. These learning processes play a critical role in survival and are essential for animals to successfully navigate their environment (Schultz, 2006). In drug addiction, discrete cues, such as drug paraphernalia, are associated with consummatory processes and can begin to acquire value on their own (Everitt, 2014; O’Brien et al., 1998). In human imaging studies, the presentation of these drug-associated cues has been shown to activate reward pathways (Young et al., 2014), and they can act to increase craving and drug seeking/motivation for consuming the drug (Goldman et al., 2013).
An organism’s ability to learn about these associations relies on a complex learning processes where information is encountered, consolidated within the brain, and upon exposure to the stimulus again the same neural circuits need to be reactivated. The number of times a memory is reconsolidated makes the strength of that memory stronger and makes the ability to reorganize the synaptic architecture that encodes that information less plastic. In the case of drug addiction, the presentation of the drug cues happens many times each day, making these associations particularly strong (Everitt, 2014; Lee et al., 2006b). These strong associations contribute not only to enhanced neural responses to these stimuli, but also create an ability to replace them with new information as it becomes available.
An inability to extinguish these associations facilitates reinstatement of drug seeking and taking when the stimulus is encountered even after a period of abstinence and it is thought to be a predominant process precipitating relapse (Everitt, 2014; Goldman et al., 2013; O’Brien et al., 1990; Weiss, 2010; Young et al., 2014). Thus, identifying the specific neuronal ensembles/subpopulations that drive these behaviors, and determining strategies to manipulate their activity to promote extinction of drug associations, is critical to understand the mechanisms of addiction.
3 DRUG-INDUCED PLASTICITY: HOW IS DRUG-ASSOCIATED INFORMATION STORED FOR LONG PERIODS OF TIME IN THE BRAIN?
A major focus of neuroscience research is understanding how information is stored in the brain and how we access that information later to guide behavior. Behavior in general is a complex process controlled by neural circuits that integrate information across a wide range of sensory modalities and balance that information with ever changing internal states. Precise networks of neural circuits that are sensory, homeostatic, attribute value to stimuli, and coordinate motor outputs all synchronously activate to guide the precise behavior that is necessary in that very moment (Anderson, 2016; Betley et al., 2013; Delgado et al., 2004; Fu et al., 2014; Schultz, 2006). Predictive cues and new information can change the neural code and behavioral response on a subsecond timescale, which requires tight and temporally specific signaling from all of the neurons involved.
This is controlled by a complex computational network of neural connections, the temporally specific activity of which guides behaviors toward appropriate outcomes. However, in psychiatric disease, especially drug addiction, these systems are dysregulated in an activity-dependent manner. Enhanced activation of specific reward and reinforcement pathways, such as the dopamine system, can lead to remodeling of these circuits as well as changes in synaptic connectivity that increase specific connections while decreasing others (Creed et al., 2016; MacAskill et al., 2014; Pascoli et al., 2014). The question at hand is how drug-induced changes in circuit dynamics, cell identity, and responsive gene expression interact with each other to produce these effects. Because addiction results in long-term changes in responses to drug-associated cues, that last long beyond the lifetime of any single protein or transcript in a given cell the question remains: how are these maintained long-term and how can we reverse them?
Tightly regulated control of DNA conformation and modification can increase or decrease the expression level of a particular gene over long periods (Jaenisch and Bird, 2003). This process termed epigenetic remodeling (discussed in Section 5) allows for potent and precise activity-dependent control over gene expression and it is via this mechanism that long-lasting, stable effects on gene expression can outlive an initial transient signal. This is important because protein turnover and posttranslational modifications, while relatively stable, only last a matter of weeks (Pratt et al., 2002). This allows for protein production that can change the activity of a cell, and its cellular response to a stimulus over the course of years. Conversely, repeated activation of a particular cell type can also change the epigenetic landscape of the cell to increase or decrease the probability of subsequent activation (Sweatt, 2009). It is through these complementary processes that organisms learn information and are able to update it quickly, efficiently, and for long periods of time.
The interplay between quick temporally specific neuronal activation and longer-term changes in transcription is of critical importance in the expression of appropriate, or in the case of drug addiction, inappropriate behaviors (Fig. 1). Thus, both of these factors and their interaction must be considered together, especially in the context of treatment. Understanding the complex interplay between these two factors will allow us to formulate novel epigenetic therapies that are likely to normalize mal-adaptive reward behaviors, with a goal of modulating addictive, but not natural reward behaviors.
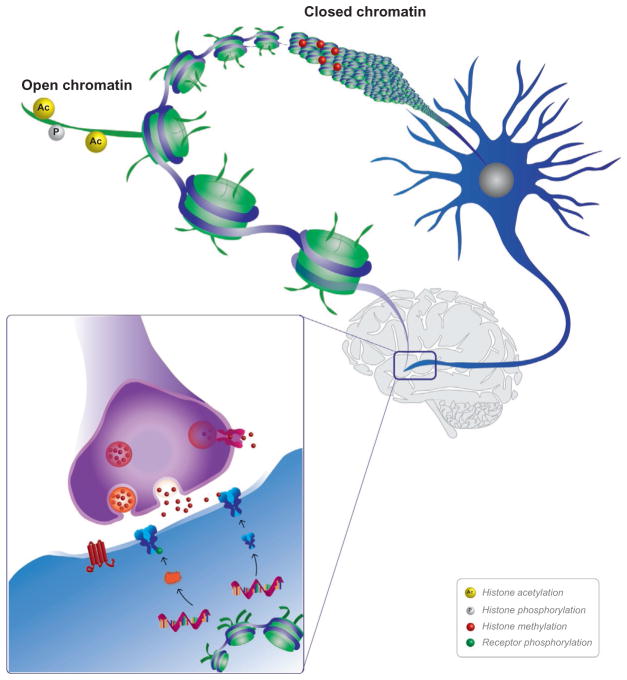
FIG. 1.
Bidirectional cross-talk between neuronal activity and the epigenome. The brain is a complex computational machine that must respond to information on a subsecond timescale and then store this information for long periods of time. This occurs via a complex interplay between neuronal activity and DNA modifications. Upon the presentation of a salient environmental stimulus, neurons are activated in various regions of the brain. In the case of drugs of abuse, these regions are often the reward pathway containing the ventral tegmental area and its projections to mid- and forebrain structures. This activation triggers activity-dependent molecular signaling cascades that can act to modify the DNA (blue) and the histone proteins that it is wrapped around (green). These modifications make DNA more accessible and can alter the expression of genes involved in synaptic function (inset). These epigenetic changes to chromatin (comprised of DNA and associated histone proteins) can change basal gene expression which can alter the composition of membrane receptors or increase regulatory proteins that can phosphorylate other effectors downstream. Epigenetic mechanisms allow for the initial activity-dependent changes to be solidified and outlast the life of any individual protein.
Below, we will review three main points: (1) What neural circuits control learning about drugs and associated stimuli, (2) How changes in these pathways drive epigenetic reorganization and (3) How chromatin changes drive long-lasting changes in neural circuit function to dysregulate reward-related behaviors indefinitely.
4 THE NEURAL CIRCUITS CONTROLLING MOTIVATED BEHAVIORS AND THEIR DYSREGULATION IN DRUG ADDICTION
4.1 THE SHIFT FROM ACUTE PHARMACOLOGY TO REMODELED NEURAL CIRCUITS
The initial component of drug addiction is the “high” induced by the drug. These euphoric effects are what initially reinforces drug taking and drives individuals to seek out the drug again. While drugs of abuse have a variety of mechanisms of action, nearly all of them increase dopamine transmission in reward-related brain regions (Di Chiara and Imperato, 1988). Stimulants, such as cocaine and amphetamine, inhibit dopamine uptake and drive active dopamine release from nerve terminals, respectively (Seiden et al., 1993). Other drugs of abuse, such as nicotine or opioids, act indirectly to enhance VTA firing rate. (Laviolette and van der Kooy, 2004; Nader and van der Kooy, 1997; Pierce and Kumaresan, 2006).
This ability to increase dopamine signaling has been shown to be critically involved in reinforcement processes as dopamine depletion or pharmacological blockade of dopamine receptors reduces motivation for food and drug (Aberman and Salamone, 1999; Aberman et al., 1998; Salamone et al., 2002; Woolverton and Virus, 1989). Most stimulant drugs of abuse like cocaine increase dopamine levels by binding to and inhibiting the dopamine transporter. The ability of cocaine to bind to the dopamine transporter is directly linked to the rewarding and motivational value of the drug (Chen et al., 2006; Siciliano and Jones, 2017), the consummatory process of drug taking (Roberts et al., 1977), and the ability to form associations between predictive cues and the rewarding effects of the drug (Calipari et al., 2017). Indeed, inhibiting the ability of drugs to increase dopamine levels, either by depleting dopamine, or altering the ability of cocaine to bind to the dopamine transporter, can completely block the ability of animals to associate cocaine with predictive cues. Therefore, the ability to increase dopamine levels initially is critical to the reinforcement process that begins the cycle of drug taking that ultimately results in addiction.
Drug-induced increases in dopamine signaling are typically long lasting. For example, DAT inhibition and the resulting “high” from a single cocaine injection can last 30min or more (Volkow et al., 1997a); however, neural signals in the brain that encode information are temporally specific and time-locked to behavioral events (Hart et al., 2014, 2015; Hollon et al., 2014; Howe et al., 2013). In the case of dopamine, it acts to predict reward availability and value (Lak et al., 2014, 2016; Medic et al., 2014; Sackett et al., 2017; Saddoris et al., 2015, 2017). When an individual encounters something that will likely be rewarding, dopamine is released and acts to coordinate downstream circuits to initiate action generation to obtain the rewarding stimulus (Ko and Wanat, 2016). Therefore, drugs enhance reinforcement learning by increasing dopamine and increasing the probability that animals will continue to execute behaviors that result in the drug being delivered. However, in addition to encoding information about rewards and their value, dopamine acts to update information and refine future behavior. The way this is achieved is by error signal (Schultz and Dickinson, 2000; Schultz et al., 1997). When a cue predicting a reward of a particular value is presented, dopamine is increased to reflect that value. However, if the value of the reward was not similar to that previously predicted, there will be a subsequent dopamine signal to the reward itself (Schultz et al., 1997). If the reward was smaller than expected, dopamine will decrease and if it was larger than expected, dopamine will increase. This acts as feedback to change the neural response to the cue and therefore refine expectation to allow for adaptive behaviors. The administration of drugs of abuse, including ethanol, cocaine, nicotine, and amphetamine, has all been shown to potentiate the amplitude of transient dopamine signals in the NAc (Cheer et al., 2007; Covey et al., 2016; Howard et al., 2013; Robinson et al., 2009). Thus, in the presence of a drug that increases dopamine signaling, the error signal will always be enhanced, making the neural representation of the reward greater than the value of the reward itself. The next time the cue is presented, the prediction will be that the reward is even bigger than the subsequent encounter. Thus, one major issue with drugs is not the initial high induced, but rather the ability of these drugs to hijack the reward prediction system to change reward circuits so that they respond maximally to drug predictive cues.
This dopamine reward signal is not only critical in predicting future rewards, but also in driving an organism to choose one reward over another (Lak et al., 2014, 2016; Medic et al., 2014). The predictive dopamine signal allows for the rank ordering of available rewards and allows organisms to make decisions between them. This has been shown in monkeys where electrophysiological recordings were conducted during the presentation of three separate juice rewards. The VTA dopamine signal to each cue was largest for each monkey’s preferred juice and guided the animal to choose that reward over the others available (Lak et al., 2014). In drug addiction, because the dopamine prediction signal is augmented, it guides animals to bias choices toward the drug over alternative reinforcers, and even in the face of negative consequences. Indeed, this has been seen in humans as well as in animal studies. For example, monkeys with a history of drug self-administration will choose heroin over a food reward (Negus and Rice, 2009) and will even administer drug to the point of death (Downs et al., 1979). Similar effects can be seen in rodent models, where a subset of rats will choose cocaine over sucrose rewards (Lenoir et al., 2013). Recent work has aimed at trying to find treatment interventions that shift choice away from maladaptive, and toward more adaptive rewards (Vandaele et al., 2016).
The ability of drug-associated stimuli to enhance motivated behaviors and drive drug seeking is a critical aspect of drug addiction (O’Brien et al., 1990, 1998). In addition to guiding decision making and choice, following long-term exposure, these cues can act as reinforcers in their own right, highlighting the powerful control they exert over motivation (Everitt and Robbins, 2005). These cue responses can be elicited even after long-term abstinence of many years and have been implicated in precipitating relapse. Cue-elicited neural responses highlight that the key factor in the development of addiction is not simply the pharmacological action of the drug. The drug binding to its site of action is critical to the initial reinforcement (Thomsen et al., 2009); however, this sets in motion a complicated circuit of learning that recruits not only the system the drug acts on, but other systems across the brain (Pascoli et al., 2015). Eventually, the ability of drugs to enhance reward circuits becomes an auxiliary component of motivation. Thus, to understand drug addiction we must focus on pinpointing the precise neural circuits in the brain that control this type of learning, understanding how they are initiated, and how they interact with the pharmacological effects of drugs to become pathological.
4.2 CHANGES IN DOPAMINERGIC ENCODING OF INFORMATION IN ADDICTION
While dopamine signaling is critical for self-administration and controls the motivation to self-administer drugs, long-term cocaine intake leads to increased motivation paired with paradoxical reductions in dopamine function. In the striatum, human imaging studies have shown reduced responses to dopaminergic challenges (Park et al., 2013; Tomasi et al., 2010; Volkow et al., 1997b). Analogous neurochemical alterations have been particularly well documented in the dopamine system of rodents with effects that include hypodopaminergia, tolerance to cocaine, and enhanced responsivity to cues (Calipari et al., 2013a,b, 2014; Ferris et al., 2012, 2013a,b, 2015). Thus, leading to the hypothesis that dopamine signaling is initially critical to the development of addiction; however, the maintenance of craving and seeking is driven by information being shifted to other neural circuits. Indeed, even after long-term abstinence from cocaine, animals still show tolerance to the effects of cocaine (Siciliano et al., 2016). This hypofunctional dopamine system is observed across many striatal regions as well as at varying periods of withdrawal (Park et al., 2013; Tomasi et al., 2010; Volkow et al., 1997a,b, 2007, 2013). In addition to presynaptic release being altered in the VTA to NAc projections, other studies have also seen decreases in D2 receptor expression levels which could contribute to further reductions in the ability of dopamine to update information about drug contingencies, thus impairing dopamine-dependent learning processes such as extinction (Ferris et al., 2012; Park et al., 2013; Tomasi et al., 2010; Volkow et al., 1997a,b, 2007, 2013).
In addition to reductions in the ability of the dopamine system to respond to salient stimuli and update new information about drug rewards, the primary locus of control shifts from ventral regions of the striatum to more dorsal regions. Initially, reward and reinforcement is controlled by the posterior dorsomedial striatum and NAc, but shifts to the anterior dorsolateral striatum (Murray et al., 2012), a structure that is considered a critical mediator of habit control. This anatomical shift is thought to underlie the transition from goal-directed to habitual drug seeking (Belin and Everitt, 2008; Dackis and Gold, 1985). Therefore, not only are there changes in dopaminergic responsivity, but wide-scale reorganization of the circuits controlling motivated behaviors. This is a critical consideration as these types of changes prevent further learning-induced plasticity and can drive compulsive behaviors. However, this is even more important in the context of other glutamatergic circuits across the brain, where changes in striatal circuits lead to more widespread alterations in other brain regions (Baler and Volkow, 2006; Decot et al., 2017; Kalivas and Volkow, 2005; Koob and Volkow, 2016; Moeller et al., 2010).
4.3 DOPAMINE-INDUCED CHANGES IN THE NEURAL CIRCUIT REMODELING OF DOWNSTREAM INPUTS
While dopamine signaling plays a critical role in reward learning, its function in the brain is largely neuromodulatory. Dopamine acts on D1-type and D2-type receptor families, which are G protein-coupled receptors (Surmeier et al., 2007). Because these are metabotropic receptors the signaling occurring through them does not trigger action potentials on their own, but rather modulates the probability of converging glutamatergic inputs to drive action potential firing as well as altering many other aspects of cellular metabolism and function via complex signaling cascades. Dopamine acts as a high pass filter increasing the probability that high frequency inputs will generate action potentials and decreasing background low frequency firing (Surmeier et al., 2007). This serves one of two functions: (1) To increase the activity of downstream neurons in driving behavior and (2) Increasing the strength of these specific inputs via changes in plasticity. This can occur in all of the projection regions of the VTA, has been seen in the PFC, NAc, and amygdala, and gives further support that these initial dopaminergic changes can induce widespread circuit dysfunction in a range of circuits across the brain to drive maladaptive behaviors (Decot et al., 2017).
4.3.1 Enduring changes in synaptic connectivity
The dopamine signaling pathway involved in reward learning is illustrated in Fig. 2. These regions have been shown to be critical in learning about aversive and rewarding stimuli, and are dysregulated in drug addiction. The NAc is a region of particular interest in drug addiction as it integrates information from VTA dopamine projections with glutamatergic inputs from the hippocampus, basolateral amygdala (BLA), PFC, and a number of inputs from sensory and motor regions onto medium spiny neurons (MSNs) and neighboring interneurons. Thus, the NAc integrates information about internal state, reward, aversion, and motivation to guide appropriate decision making. In the NAc, dopaminergic signals are integrated by two largely nonoverlapping populations of GABAergic projection neurons (MSNs) that project to downstream regions such as the ventral pallidum (VP) and back to the VTA (Surmeier et al., 2007).
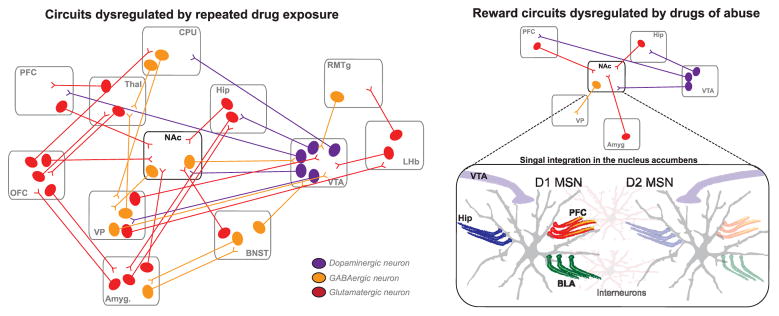
FIG. 2.
Neural circuits implicated in drug addiction. A complex network of brain regions participates in the encoding of information associated with drug addiction. (left) Network diagram of interconnected brain regions involved in drug addiction. Orange, GABAergic populations; red, glutamatergic projections; purple, dopaminergic projections. (right) The integration of signaling in the nucleus accumbens (NAc). The NAc is comprised of two major populations of projection neurons: D1 and D2 receptor containing medium spiny neurons. In basic reward processing, these neurons integrate information from glutamatergic inputs from the prefrontal cortex (PFC), hippocampus (Hip), and amygdala (Amyg.) with dopaminergic projections originating in the ventral tegmental area (VTA). While in dorsal regions of the striatum D1 and D2 MSNs signal through direct and indirect motor pathways, in ventral regions of the striatum populations project to the ventral pallidum (VP). Amyg., amygdala; BNST, bed nucleus of the stria terminalis; CPU, caudate putamen; Hip, hippocampus, LHb, lateral habenula; OFC, orbitofrontal cortex; RMTg, rostromedial tegmental nucleus; Thal, thalamus.
The GABAergic projections neurons in the NAc are most often defined by their expression of D1 and D2 dopamine receptors. D1 dopamine receptors are Gq coupled and activation of these receptors increases the probability of action potential generation by excitatory inputs in this population, while D2 receptors are Gi coupled and are inhibitory (Surmeier et al., 2007). Indeed, work with in vivo calcium imaging has shown that increasing dopamine levels via cocaine injection leads to a suppression of the population activity in D2 MSNs, while increasing the activity of D1 MSNs (Calipari et al., 2016; Luo et al., 2011). D1 and D2 MSNs have been proposed to control discrete aspects of behavior as optogenetically stimulating D1 MSNs can result in a place preference and reinforcement while stimulating D2 MSNs results in aversion (Kravitz et al., 2012; Lobo et al., 2010). Thus, increases in dopamine activate D1 MSNs to drive animals toward rewarding stimuli. However, it is likely more complicated than just D1 or D2 signaling controlling reward and aversion, and rather is more likely controlled by a complex regulatory process that finely tunes the activity of these two populations (Soares-Cunha et al., 2016). This is highlighted by the overlapping inputs of sensory and cortical systems in both populations of neurons (Guo et al., 2015, see Fig. 3). In addition, D1 and D2 MSN populations were recently shown to have lateral inhibitory effects on each other (Dobbs et al., 2016), which allows activation in one pathway to regulate the output of the other, further refining signaling. As a result of this tightly controlled neuronal regulation, there is an interest in how repeated drug exposure alters the synaptic connections and excitability of these two pathways in isolation and how they contribute to behaviors associated with drug addiction.
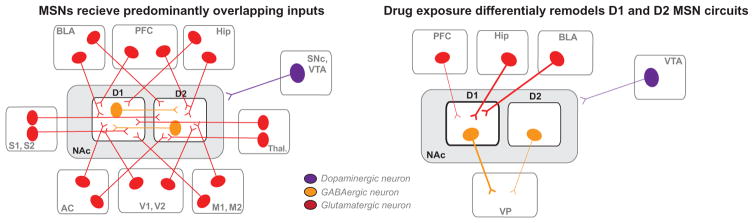
FIG. 3.
D1 and D2 medium spiny neurons in the NAc. (left) D1 and D2 medium spiny neurons in the NAc receive overlapping projections from cortical and sensory areas. These overlapping inputs allow these populations to balance information about internal state with other environmental factors to execute goal-oriented behaviors. These populations have been shown to be activated during self-administration for food and drug rewards. (right) Cocaine exposure changes the balance of inputs onto each of these populations differentially. Work from a number of groups has outlined the projection and cell type-specific effects of cocaine in the NAc (discussed in Section 4.3.1). Cocaine exposure leads to increased activity in D1 MSNs. This is likely driven by an enhancement of activity from hippocampal (Hip) and basolateral amygdala (BLA) projections. Further dopaminergic inputs from the ventral tegmental area (VTA) and glutamatergic inputs from the prefrontal cortex (PFC) are weakened. This reorganization of connectivity leads to greater signaling of D1 MSNs relative to D2 via downstream regions such as the ventral pallidum (VP) to drive enhanced behavioral responses to cocaine and associated stimuli. AC—anterior cingulate; M1, M2—motor cortices; S1, S2—sensory cortices; SNc—substantia nigra pars compacta; Thal—thalamus; V1, V2—visual cortices.
The role of D1 and D2 MSNs in reward and aversion sheds light onto how rewarding and aversive stimuli alters the balance of signaling through these two neuronal populations to guide behavior (Calipari et al., 2016; Creed et al., 2016; Kravitz et al., 2012; Lüscher, 2016; MacAskill et al., 2014; Pascoli et al., 2014); however, even more important is the nature of signaling that occurs in response to drug-associated stimuli and how this is changed following repeated drug exposure (Bock et al., 2013; Creed et al., 2016; Lüscher, 2016; MacAskill et al., 2014; Pascoli et al., 2014). Temporally specific signaling originating from D1 MSNs in the NAc was identified to be critical in the encoding of information about drug associations. The extent to which this D1 signal was increased to predictive cues was positively correlated with drug seeking. Chronic cocaine exposure enhanced drug-cue evoked D1 signals to both prevent extinction and facilitate reinstatement (Calipari et al., 2016). Further, these increased responses to drug-associated contextual cues by D1 MSNs were still present after a 2-week withdrawal period, suggesting that the changes in the population activity were stable and long-lasting (Calipari et al., 2016). This is particularly interesting because recent work with food self-administration has shown that both D1 and D2 MSN activity is stimulated, suggesting that something is fundamentally different about the encoding of food and drug rewards (Natsubori et al., 2017).
The cell type-specific neuroadaptations that increase responses to drugs and associated cues are of interest not only in the NAc, but across the brain. In the case of D1 and D2 MSNs not only have increases in activity been observed, but increases in the synaptic connectivity of specific glutamatergic inputs onto each population of neurons. Initially, a large body of work found that changes in glutamate signaling and extrasynaptic glutamate levels within the NAc were associated with relapse and withdrawal from cocaine self-administration (Baker et al., 2003; Kalivas et al., 2003; Kau et al., 2008; Pierce et al., 1996; Reid and Berger, 1996). These spine changes are consistent with increases in synaptic strength observed during the same period, where α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/N-methyl-D-aspartate (AMPA/NMDA) ratio is increased in these neurons following withdrawal (Conrad et al., 2008; Kourrich et al., 2007). The increase in AMPA receptor levels after withdrawal has been shown to be mediated by the insertion of AMPA receptors that lack the GluR2 subunit, thus rendering the receptors calcium permeable (Conrad et al., 2008). Indeed, calcium entry into these dendritic spines is potentiated during withdrawal from cocaine self-administration (Ferrario et al., 2012). Further, blocking the insertion of these calcium-permeable AMPA receptors in a nonspecific or projection-specific fashion is capable of blocking drug seeking (Lee et al., 2013). These changes highlight the long-term reorganization of receptor composition that could act to guide signaling via specific signaling cascades at specific times during withdrawal. This shift to allow more calcium-mediated signaling cascades could change the transcriptional landscape to promote different gene networks and could have potent effects on drug craving.
This work was further refined to show that specific projections from the PFC were critical in controlling drug seeking and relapse. Activity in the infralimbic cortex suppresses cocaine seeking and inactivation drives cocaine seeking (Lalumiere et al., 2012). Further, cocaine self-administration results in depressed prelimbic cortex excitability, which is associated with cocaine seeking. Rescuing this hypoactivity was capable of ablating this compulsive seeking, highlighting the causal role of reduced PFC activity in the process (Chen et al., 2013). However, following these studies, it was unclear as to which cells in the NAc these cortical projections were synapsing on. Recent work has demonstrated that drug seeking is associated with reduced AMPA/NMDA ratio (signifying reduced synaptic strength) at medial pre-frontal cortex (mPFC) to D1 MSN synapses. Conversely, increased AMPA/NMDA ratio was observed in ventral hippocampal inputs to this D1 population (Pascoli et al., 2014). Other studies have also shown specific potentiation of glutamatergic inputs onto D1 MSN. For example, cocaine exposure selectively enhances amygdala inputs to D1, but not D2 MSNs (but see: Pascoli et al., 2014), further highlighting cell type and pathway-specific adaptations that underlie cocaine-associated behaviors (MacAskill et al., 2014). These data demonstrate that remodeling of synaptic inputs is a complicated process that relies on converging factors in the environment to activate specific input circuits at specific times to induce long-term potentiation (LTP) in these circuits, but not others.
In addition to the inputs onto D1 and D2 MSNs, the outputs are also differentially altered by cocaine and show functionally distinct effects. Both D1 and D2 MSNs have been shown to project to the VP (Kupchik et al., 2015) rather than through direct and indirect pathways like in more dorsal regions of the striatum. Chronic cocaine exposure was shown to enhance D1 outputs to the VP while weakening outputs from D2 neurons (Creed et al., 2016), which is consistent with previous imaging work showing enhanced signaling through D1 pathways relative to D2 (Calipari et al., 2016). Interestingly, this work also showed that D1 and D2 projections to the VP had distinct roles in controlling behavioral outputs of cocaine, with the D1 pathway being more involved in locomotor sensitization and D2 in motivational deficits associated with cocaine exposure. It is likely that these two projection populations are responsible for balancing motivation and external information with changing internal states to help animals to make decisions; however, this is dysregulated in addiction. Further, work with reinforcement tasks has shown that reducing plasticity in the D2 pathway, or increasing its activity acts to alter cue-driven seeking behaviors (Bock et al., 2013; Heinsbroek et al., 2017). Importantly, the dysfunction in both of these pathways results from dysregulated plasticity, suggesting that increasing the ability of these cells to adapt to changing environments would have advantageous effects on addictive behaviors.
4.3.2 Enduring changes in morphology maintain new synaptic connections
Changes in afferents and efferents on specific populations of neurons are maintained for long periods of time through stable changes in neuronal morphology. Dendritic spines are protrusions of the dendritic membrane upon which a majority of excitatory synapses are formed in the brain. Spine shape and size is often indicative of LTP or long-term depression (LTD) in glutamatergic projection populations (Okamoto et al., 2004). Spine head size is reflective of synaptic strength such that spines with large heads typically have a larger content of functional surface AMPA receptors than spines with small heads (Heinsbroek et al., 2017). Thus, LTD is often associated with shrinkage and atrophy of spines, while LTP is associated with both the formation of new spines and the enlargement of existing spines.
Spine changes are induced by activity-dependent signaling within each neuron which starts a molecular cascade that rapidly recruits the polymerization of actin and cytoskeletal-associated proteins to change spine size and alter stability (Blanpied and Ehlers, 2004; Lisman, 2003; Matus, 2005; Rao and Craig, 2000). The process by which spines can increase and decrease size and synaptic function is particularly important in drug addiction, as it provides a mechanism by which stable changes in circuit dynamics can be maintained so that activation of the pathway is more or less sensitive to environmental stimuli and subsequent drug exposure. Indeed, changes in spine morphology have been directly associated with the propensity to seek out drugs in animal models (Cahill et al., 2016; Dietz et al., 2012; Gipson et al., 2013; Li et al., 2004; Robinson et al., 2001; Russo et al., 2010). Furthermore, changes in the ability to remodel synaptic connections in reward-related brain regions are critical to responding to and recognizing drug-associated stimuli (Shen et al., 2009; Dumitriu et al., 2012; Gipson et al., 2013). These spine changes have been observed with concomitant changes in excitatory glutamatergic inputs (Conrad et al., 2008; Kourrich et al., 2007), and, as mentioned previously, with associated changes in excitability (Lee et al., 2013), extrasynaptic glutamate levels (Baker et al., 2003; Kalivas et al., 2003; Kau et al., 2008; Pierce et al., 1996; Reid and Berger, 1996), and enhanced connectivity of specific glutamatergic inputs from cortical areas (MacAskill et al., 2014; Pascoli et al., 2014).
Acute withdrawal from cocaine exposure results in increases in thin dendritic spines; however, following a withdrawal period there is a persistent and long-lasting change in spine size and dendritic branching in both the NAc and more dorsal regions of the striatum (Jedynak et al., 2007; Shen et al., 2009). It is likely that this maturation of spines acts to solidify the synaptic connections that contain information about cocaine and associated stimuli (Bourne and Harris, 2007). Indeed, work on projections from the amygdala to the NAc has shown that over withdrawal, immature synapses were strengthened to form functional synaptic connections. Preventing this process from occurring was capable of preventing drug seeking (Lee et al., 2013).
While the stability and size of these spines is interesting, even more interesting is the rapid plasticity that they undergo upon presentation of a drug- or reward-cue stimulus. Both contingent and noncontingent cue presentation produces rapid changes in spine head diameter in the NAc (Gipson et al., 2013; Stankeviciute et al., 2014). Cue presentation results in rapid increases in spine head diameter within 15min, which is directly correlated with cocaine seeking. These spine head changes are associated with enhanced AMPA/NMDA ratios, suggesting that these proteins were “primed” to be inserted into the cellular membrane upon cue presentation. Further supporting the hypothesis of priming, an acute injection of cocaine following withdrawal from self-administered cocaine rapidly induces the surface expression of calcium permeable AMPA receptors (Anderson et al., 2008). These data together suggest that the ability of synapses to remodel quickly and efficiently is critical to learning-related processes and is altered following cocaine exposure in a way that potentiates the ability to rapidly upregulate proteins involved in synaptic connectivity.
While the ability of synapses and circuits to rapidly upregulate activity is enhanced following drug exposure, potentially more interesting is a concomitant decrease in the ability to induce LTD within these same brain regions (Martin et al., 2006). Therefore, cocaine experience seems to “prime” some responses while “desensitizing” others. Together, these changes converge to control an enhanced ability to respond to cues and drugs, and a reduced ability to inhibit these responses with changing environments. This could explain the deficits in drug-addicted individuals in appropriately extinguishing drug-associated behaviors. The question currently remains whether these changes are occurring within the same circuit or in a cell type or projection-specific manner. As mentioned previously, the maturation of synapses in the amygdala to NAc projection was shown to be critically involved in drug seeking. Further, changes over withdrawal from cocaine exposure have been shown to occur initially in both D1 and D2 MSN populations (Lee et al., 2006a), however, after a long withdrawal period only the D1 MSNs show an increase in the number of spines.
An important thing to note is that the changes in dendritic spines seem to be different to some extent across drug classes. Cocaine has been shown to increase the number and density of spines in the NAc, VTA, PFC, and OFC (Robinson and Kolb, 2004; Robinson et al., 2001). Conversely, opiates have been shown to decrease the number of spines in the NAc, hippocampus, and PFC (Russo et al., 2007; Sklair-Tavron et al., 1996). These findings are paradoxical as the behavioral phenotypes are similar. The changes in spines are interesting in this context, but are not surprising given the diverse roles of different cell types and projections in controlling these behaviors. For example, if the output of the NAc is driven by the balance between D1 and D2 MSNs, then both an increase in D1 and a decrease in D2 activity could potentially drive the same behavior. Therefore, moving forward it will be important to determine the source of the plasticity in order to make conclusions about how to effectively target these systems.
Together, effects of some drug classes may differ from others in some ways, however, these studies have a fundamental degree of overlap: there is a lack of dynamic plasticity across the brain that prevents adaptive learning processes. Thus, in order to make progress, we must understand the molecular basis for this activity-dependent morphological and circuit reorganization that underlies reward learning.
5 HOW ARE CHANGES IN NEURAL MORPHOLOGY AND FUNCTION MAINTAINED?
The behavioral, circuit, and morphology data point to one important adaptation in drug-addicted individuals. Synapses are strengthened for long periods of time that last far beyond the life span of any individual protein involved in the process (McPherson, 2015). These long-lasting changes in synaptic strength are critical mediators of drug seeking and relapse, thus understanding how they are maintained is of critical importance, both on a basic neuroscience and drug abuse treatment level.
At the heart of experience-dependent plasticity lies the capacity of neural circuits to undergo activity-induced structural and functional changes. The maintenance of such permanent change requires efficient posttranslational and transcriptional regulation (Sweatt, 2013). The ability to turn genes on and off is controlled by epigenetic regulation, where the structure of DNA is modified to increase or decrease the probability of gene expression. This process is emerging as the primary molecular mechanism underlying plasticity in postmitotic neurons. Thus, understanding how this process is dysregulated in disease will shed light onto the basic process, its importance in behavioral control, and provide potential targets for treatment of these complex disorders.
5.1 THE EPIGENOME AS THE MOLECULAR HUB OF INFORMATION ENCODING IN ADDICTION
EPIGENETICS.
The term epigenetics was coined by Conrad Waddington to describe a conceptual solution to a fundamental consideration—and conundrum—in developmental biology. All the different types of cells that make up our body, bar exceptions in our reproductive and immune systems, have exactly the same genome. Yet, how can the identical DNA template produce vastly different gene products and yield distinct cell types like myocytes or neurons? Waddington reasoned there must be a mechanism above the level of DNA that controls the readout of genes encoded in its nucleotide sequence—coining the term epigenetics. These epigenetic mechanisms specify certain sets of genes that are turned into functional products in neurons, for instance, yet not in myocytes. Diverse epigenetic marks are set up during early cell fate decisions, in due course forming a memory system that perpetuates cellular phenotypes over the lifespan of our bodies. In view of that, classic epigenetics is the study of a change in gene expression or cellular phenotype that is stably inherited by a cell and that is not associated with changes in DNA sequence. Today, many epigenetic modifications are known to be highly dynamic with critical functions in neuronal plasticity that are implicated in neurodegenerative and psychiatric disorders.
Although many cellular phenomena may be considered epigenetic, the primary focus of the field has been to illuminate environment–genome interactions at the level of chromatin. Chromatin describes the DNA–protein packaging complex that determines the accessibility of DNA in eukaryotic cells, making it the focal point of transcriptional gene regulation. The basic repeating unit of the chromatin structure is the nucleosome: ~146 base pairs of genomic DNA wrapped around a protein octamer, assembled from two molecules each of histone H2A, H2B, H3, and H4. In essence, the nucleosome constitutes a platform for complex chemical modifications—i.e., epigenetic marks—that dynamically regulate chromatin architecture and gene transcription (Rivera and Ren, 2013). The entirety of these epigenetic features has been denoted the epigenome—it defines neuronal identity and expresses the regulatory channels that operate at the interface of genome and environment (Day and Sweatt, 2011).
Posttranslational modifications of histones elicit structural and functional changes within chromatin and regulate various epigenetic processes. To date, numerous histone modifications have been identified and include acetylation, methylation, phosphorylation, ubiquitylation, sumoylation, and ADP-ribosylation (Berger, 2007; Kouzarides, 2007). Acetylation, for instance, along with methylation, is the most extensively studied histone modification, and has broad effects on chromatin function and nuclear signaling pathways (Berndsen and Denu, 2008; Shahbazian and Grunstein, 2007). Histone acetylation is regulated by the opposing actions of histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs acetylate-specific lysine residues of histone proteins, which neutralizes their positive charge, and can thus help to decondense chromatin leading to active gene transcription (Berndsen and Denu, 2008). Additionally, histone acetylation marks can be bound by small protein modules called bromodomains, often referred to as “readers.” These domains are conserved within many chromatin-associated proteins—including HATs themselves—that regulate transcription-mediated biological processes, and whose aberrant activities are correlated with several human diseases (Bannister and Kouzarides, 2011; Burdge and Lillycrop, 2010; Filippakopoulos and Knapp, 2014).
Indeed, drug addiction is one of these syndromes. Epigenetic remodeling has emerged as a potent regulator of drug-induced plasticity and has been implicated in addiction to stimulants, opiates, ethanol, and nicotine (Walker et al., 2015). For example, hypermethylation of the dopamine transporter gene has been observed in human alcoholics and was shown to be predictive of addiction severity (Ponomarev, 2013). In human heroin addicts, impairments in both glutamatergic neurotransmission and chromatin remodeling were observed, including increased enrichment of lysine-27 acetylated histone H3 (H3K27) that affected GluR2, the subunit of the AMPA receptor that confers calcium permeability (Egervari et al., 2017). Similar changes were seen in rodents that underwent heroin self-administration and these changes were directly linked to self-administration behavior. Other work with cocaine has shown reduced levels of H3K9 dimethylation in the nucleus NAc, which was found to be mediated through the repression of G9a, a methyltransferase (Maze et al., 2010). These reductions led to increases in immediate early gene expression, and thus, could act to prime these genes to respond quickly and to a greater extent upon cellular activation. Together, changes in the ability of transcription to dynamically respond to the changing cellular microenvironment is important as it dysregulates not only the protein composition of cells, but their ability to quickly respond in a temporally specific fashion to information.
6 EPIGENETIC REGULATION IS THE KEY TO A CENTRAL PROPERTY OF NEURAL NETWORKS: PLASTICITY
The exact mechanisms by which circuit activity can directly manipulate chromatin structure in its participating neurons remain opaque. Neurons continually adapt to a changing environment, and thus require an acutely responsive system that adjusts chromatin structure and gene transcription. Epigenetic changes can be transient, such as histone acetylation or phosphorylation, or long-lived, such as specific histone methylation and DNA methylation, and both of these processes are likely involved in addiction (Fass et al., 2013; Peña et al., 2014; Robison and Nestler, 2011; Rudenko and Tsai, 2014). Importantly, modification of the epigenetic landscape provides a mechanism by which the transcriptional response to stimuli can be permanently altered, thus providing a molecular route to lasting modifications of neuronal and circuit functions.
Several types of epigenetic modifications have been associated with cognitive functions, including DNA methylation, and the posttranslational modification of histone proteins by acetylation, methylation, and phosphorylation (Alarcón et al., 2004; Dulac, 2010; Gräff and Tsai, 2013a; Korzus et al., 2004; Levenson et al., 2004; Nelson and Monteggia, 2011; Wood et al., 2006). Yet histone acetylation in particular has spurred considerable interest, and is most robustly associated with promoting associative learning and memory formation, which, as discussed previously, is one of the critical learning processes dysregulated in drug addiction.
As early as 1979, it was found that the acetylation state of histones is altered when rats undergo memory consolidation (Schmitt and Matthies, 1979). More recent evidence confirmed these findings, showing that specific forms of associative learning about cues and predicted outcomes correlate with increased histone acetylation (Levenson et al., 2004). For example, following contextual fear conditioning, acetylation of H3K14 was significantly increased in the hippocampus, whereas acetylation of H4 was unchanged (Levenson et al., 2004). Conversely, H4 acetylation was selectively increased after latent inhibition training (Schmitt and Matthies, 1979). Latent inhibition involves a process where a familiar stimulus takes longer to acquire a new meaning because of extended experience with the stimulus (Lubow and Moore, 1959). Thus, with latent inhibition the ability to update information about that stimulus is particularly difficult. Interestingly, H4 hyperacetylation is observed within 30min of a single cocaine injection, highlight that related processes are recruited with paradigms of drug exposure (Kumar et al., 2005). Thus, this specific modification could underlie some of the difficulty with replacing cocaine-associated memories with new and updated information. These early results not only highlight that histone acetylation accompanies memory consolidation and learning about drug rewards, but show that different learning paradigms elicit distinct epigenetic signatures in the brain. Given what we know about the processes involved in these different memory types (i.e., extinction vs latent inhibition, vs reinforcement learning) interventions could be targeted at specific epigenetic marks that help to enhance specific types of learning.
Many follow-up studies have corroborated the implied link between histone hyperacetylation and memory formation for different memory types and phases, such as reconsolidation and extinction learning, which as discussed previously, are basic learning processes that are critical mediators of volitional drug consumption (Bannerman et al., 2014; Gräff et al., 2012; Oliveira et al., 2011; Rogge and Wood, 2013; Schneider et al., 2013; White and Wood, 2013; Wood et al., 2006). Newer studies using chromatin immunoprecipitation have revealed that memory-induced histone acetylation is specific to certain genes (Alarcón et al., 2004; Fischer, 2014; Korzus et al., 2004). These include genes that are important for neuronal plasticity, such as the immediate-early genes Erg1, Creb, and Bdnf, which showed an increase in expression following contextual fear learning, concomitant with the increase in histone acetylation (Bannerman et al., 2014; Korzus et al., 2004; Oliveira et al., 2011). Indeed, all of these molecules have been shown to be critically involved in different stages of the addiction process and the expression of maladaptive reward behaviors (Go et al., 2016; Hoffmann et al., 2017; Li et al., 2016; Rovaris et al., 2017; Sun et al., 2015; Zhang et al., 2016). Relevantly, during this time window, Ca2+-induced cAMP signaling that engages the ERK and PKA pathways in the hippocampus and the amygdala directly phosphorylate and thus activate the transcription factor cAMP-response element-binding protein (CREB), a process critical to long-term memory (Dash et al., 1990; Freytag et al., 2017; Serita et al., 2017; Stevens, 1994). In fact, windows of CREB serine 133 phosphorylation coincide with sensitive periods during which inhibition of transcription impairs memory storage, highlighting the importance of regulated gene expression in learning and memory (Bourtchouladze et al., 1998). Aptly, acute administration of cocaine phosphorylates and thus activates CREB to induce transcription-dependent neuroplasticity in the reward pathway, most prominently in the NAc. Work has shown that overexpressing CREB enhances cocaine reinforcement and increasing CREB following withdrawal potentiates cocaine-induced seeking behavior, showing a potentiation of both drug effects and seeking (Larson et al., 2011).
It is tempting to attempt to identify a single gene that controls the addictive phenotype, yet the complex nature of a disorder involving learning mechanisms make it more likely that a complex network of interconnected genes regulates the connectivity of neurons across the brain. Accordingly, recent evidence showed that CREB phosphorylation alone is not sufficient to drive such gene expression. Instead, it is the interaction between phosphorylated CREB and the histone acetyltransferase CREB-binding protein (CBP) that is critical for memory formation (Fischer et al., 2010; Gräff and Tsai, 2013b; Haettig et al., 2011; Levenson et al., 2004; Stefanko et al., 2009). This finding highlights the importance of dynamic chromatin regulation in neuroplasticity in response to drugs of abuse: CBP catalyzes the acetylation of histone H3 lysines through its HAT domain, resulting in chromatin remodeling and relaxation of chromatin structure that enables the transcription of CREB target genes (Wang et al., 2013). Learning-induced transcription occurs rapidly and is restricted to precisely timed windows of dynamic histone acetylation and is dependent on a number of signaling cascades converging simultaneously (Dulac, 2010; Fischer, 2014; Gräff and Tsai, 2013a; Hsieh and Eisch, 2010; Sharma, 2010; Tsankova et al., 2007). Accordingly, the sudden increase in acetylation was found to be critically dependent on production of the cofactor acetyl-CoA by ACSS2, which “fuels” CBP-mediated acetylation and effective induction of genes functioning in experience-driven synaptic change, including Fos, Arc, and Egr2 (Mews et al., 2017). Thus, CREB acts in concert with a wider range of other factors to induce learning-related plasticity.
Similarly, following cocaine self-administration, rapid increases in CREB levels are linked to dynamic increases in promoter-proximal H3K9 and H3K14 acetylation at the Cdk5, Bdfn, and Fosb genes, which have key functions in neural plasticity and become upregulated by virtually all drugs of abuse (Bibb et al., 2001; Graham et al., 2007; Hope et al., 1994). As mentioned earlier (see Section 4.3.2) repeated exposure to cocaine leads to persistent alterations in spine morphology and synaptic strength that involve increases in calcium permeable AMPA-receptor levels. Because CREB is activated through calcium-dependent signaling within the cell, this provides a potential mechanism by which epigenetic modifications could control plasticity specifically in the neurons recruited into the drug-reward circuit, putatively constituting a molecular feedforward loop that could work to potentiate responses to drug-associated stimuli.
7 THE INTERFACE BETWEEN NEURONAL ACTIVATION AND EPIGENETIC REMODELING
Together, a large body of work has shown that both changes in neural circuit dynamics and epigenetic regulation can alter reward-related behaviors (Dudai and Morris, 2013; Russo and Nestler, 2013). A major question in neuroscience is how these activity-dependent changes at the level of the circuit interface with DNA changes to guide behaviors. The first step is understanding how information is transmitted from a synaptic signal, in the form of an action potential or receptor activation, to the nucleus to trigger the necessary molecular machinery for epigenetic remodeling on a fast time scale. In this view, epigenetic regulation is a gating device that arbitrates acute and transient gene expression in response to upstream neural activity. Circuit activity triggers intracellular signaling cascades such as the PKA or MAPK/ERK pathways that are activated by G protein-coupled receptors and calcium, and therefore, transmit circuit activity information to the nucleus (Ménard et al., 2015). In the nucleus, epigenetic signatures demarcate and regulate genes with functions in synaptic remodeling for memory circuit formation (Sweatt, 2013). Remarkably, but not surprisingly, the plasticity mechanisms linked to drug addiction correspond to well-described neuronal and circuit plasticity in learning and memory, and involve most of the same brain regions (outlined in Fig. 2).
7.1 DRUG-INDUCED TRANSIENT CHANGES IN CHROMATIN STRUCTURE
Epigenetic modifications serve two major functions in differentiated neurons. First, they act to determine which genes are upregulated on a transient timescale upon cellular activation. Second, they act to control stable gene expression on a timescale that extends beyond the initial transient signal. The interplay between these two types of epigenetic modifications is relatively unstudied. Thus, better insight into how drug-induced transient changes in chromatin structure lead to stable and long-lasting epi-genetic regulation of gene expression is needed.
The focus of recent studies has been on the initial chromatin-localized events that occur on a timescale of minutes to regulate neuronal gene expression. Neuronal activity signals can be transmitted to the nucleus through the engagement of G protein-coupled receptors that stimulates cAMP signaling to set off a signaling cascade via the PKA pathway and members of the mitogen-activated protein kinases (MAPKs), which can directly phosphorylate histones to prompt further changes in chromatin structure, including transcriptionally activating histone acetylation (Gräff and Tsai, 2013a; Nestler, 2016). Additionally, signals of neural activity are transmitted to chromatin via the calcium/calmodulin-dependent kinase II (CaMKII), which becomes activated upon cellular depolarization and influx of calcium. CamKII stimulates transcription of BDNF, a well-known neurotrophin involved in neuroplasticity, by phosphorylating and thus releasing the DNA methylation “reader” methyl CpG-binding protein 2 (MeCP2), a highly abundant chromosomal protein within the brain, from its promoter (Im et al., 2010; Nott et al., 2016; Zhou et al., 2006).
This process is important in both drug addiction and reinforcement learning, and interestingly these changes have been liked to both chromatin modifications and changes in cellular morphology. MeCP2 in the dorsal striatum was shown to control escalated cocaine consumption, a process thought to mimic the uncontrolled use seen in human addicts. A critical aspect of this study is that MeCP2 only regulated escalated cocaine consumption, not consumption on restricted access schedules, suggesting that it plays a role in drug-induced plasticity, rather than the rewarding effects of cocaine in general. Further, the actions of MeCP2 in this specific context were found to be via alterations in BDNF (Im et al., 2010). Similarly, striatal BDNF transmission is known to increase the motivation to self-administer cocaine (Graham et al., 2007, 2009; Grimm et al., 2003; Hall et al., 2003; Horger et al., 1999; Im et al., 2010; Lu et al., 2004; Schoenbaum et al., 2007), and increases have been linked to the increased spine changes that are characteristic of cocaine exposure (Zhou et al., 2006). BDNF activates the enzyme nitric oxide synthase, leading to nitrosylation and dismissal of chromatin-bound HDAC2, thus ultimately increasing histone acetylation at genes involved in neural plasticity for LTP and learning (Nott et al., 2008). Notably, whereas inhibition of HDAC2 and HDAC3 enhances histone acetylation and synaptic plasticity, deficit of other HDACs, namely HDAC4, has the opposite effect (Gräff and Tsai, 2013a). These data indicate that histone acetylation is a highly specific epigenetic mark that is controlled by different regulatory processes in a time-sensitive window with gene-specific transcriptional outcomes.
Whereas activity-induced gene expression and protein synthesis is transient, the circuit rewiring linked to associative learning and memory storage is long-lasting (Tonegawa et al., 2015). Notably, histone acetylation is known as a highly dynamic modification that rapidly turns over. Equally, even the extended half-life of channel proteins such as AMPA and NMDA receptors—whose expression is manipulated by drugs of abuse—is transitory when compared to timescales of pathological states of addiction, as drug relapse can occur even after years of abstinence and clinical intervention. Therefore, persistent changes in transcriptional regulation caused by drugs of abuse are likely maintained by the complex interplay of short lived epigenetic marks—e.g., transient histone acetylation with dramatic effects on gene expression—that regulate synaptic and circuit strengths and permanent epigenetic aberrations that preserve transcriptional dysregulation in concert with alterations at the synapse and cell signaling.
7.2 TRANSIENT CHANGES AS A SCAFFOLD FOR LONG-TERM EPIGENETIC CHANGES
All of the aforementioned mechanisms rely on acute changes that are transient in nature and are likely involved in quick and adaptive responses of cellular circuits to environmental information. But the question is how these precisely timed processes ultimately lead to permanently altered epigenetic landscapes that underlie dysregulated transcription in addiction.
Results support an emerging view that rapid changes in DNA methylation—traditionally viewed as a permanent and immutable mark in postmitotic cells—are involved in activity-dependent regulation of neuronal gene transcription. The maintenance DNA methyltransferase, DNMT1, is highly expressed across the brain, and transient increases in DNMT1 expression are not only seen with Pavlovian learning, but have been reported after administration of drugs of abuse, specifically methamphetamine (Goto et al., 1994; Numachi et al., 2007). In fact, following chronic exposure to drug, increases in DNA methylation in the striatum are persistent and evident even after extended periods of withdrawal (Mychasiuk et al., 2013). Notably, in the case of Pavlovian learning, DNMT inhibition in the mPFC blocked memory recall even when applied acutely 1 month after training (Miller and Sweatt, 2007). Furthermore, upon synaptic activity, DNA demethylation is initiated by Tet family proteins that oxidize 5-methylcytosine and activate the base-excision repair pathway, dysregulation of which has been found to prevent homeostatic synaptic plasticity (Yu et al., 2015). These findings highlight the dynamic nature of the neuronal DNA methylome and suggests an important role for DNA methylation in the stabilization of epigenetic change that is instigated by drugs of abuse (Feng et al., 2015). In fact, both acute and chronic cocaine has been shown to cause hypomethylation of the FosB promoter in the striatum, linked to decreased binding of MeCP2 and upregulation of FosB expression (Anier et al., 2010). Intriguingly, repeated drug exposure causes accumulation of its splicing variant ΔFosB in D1 MSNs due to its increased stability (Nestler et al., 2001). This provides a partial window into how neuronal activity patterns can be integrated over time to license permanent changes in chromatin structure and gene regulation, and endure even after ΔFosB protein expression is reduced following prolonged withdrawal.
As outlined earlier, the aforementioned changes in chromatin structure produce plasticity at the synaptic and circuit level, including alterations of the AMPA and NMDA receptor levels and their subunit composition. Just like with dendritic spines where the thin spines serve as a scaffold to create more mature spines, transient epigenetic marks can set a series of events in place that help to consolidate information permanently only if the stimulus is incredibly salient or encountered repeatedly over long periods of time. This would provide gaiting so that the long-term changes would only happen after repeated exposure.
7.3 PERMANENT EPIGENETIC CHANGES AS MEDIATORS OF GENE PRIMING
Evidence from rodent addiction models supports the view that permanent epigenetic adaptations underlie persistent anomalies in transcription regulation induced by drugs of abuse across the brain. While these permanent marks could change basal levels of gene expression, it is important to understand how they would alter stimulus-induced gene expression. Specifically, repeated cocaine exposure alters the inducibility of key genes, referred to as gene priming and desensitization. Such latent transcriptional dysregulation has been linked to epigenetic priming in the context of immunology, but is unexplored in neuropsychiatric disorders. Notably, most of these changes are not manifested in altered steady-state levels of mRNA, but rather, are latent and reflected in dysregulated expression of genes upon challenge with drug at a later time point. A similar phenomenon is notably seen with AMPA receptor trafficking where the insertion into the membrane is faster after withdrawal from cocaine exposure (Lee et al., 2013; MacAskill et al., 2014), and it is possible that this is driven by epigenetic changes at the gene level.
Mounting evidence suggests that aberrations in the epigenetic landscape are responsible for these persistent changes in gene expression. In this view, alterations in chromatin architecture “scar” gene regulatory regions to permanently prime specific gene sets for rapid induction or repression in response to future stimuli. Accordingly, drug-induced changes in the epigenetic landscape persist and distinctly prime neuronal populations that participate in the reward circuit for aberrant transcriptional responses in the future. The extant literature supports this assertion but has been insufficiently mechanistic. DNA methylation, which, as discussed earlier, is permanently altered by the epigenetic processes set off by drugs of abuse, and could underlie this process. The methylation state of genes acts to control both transcription and splicing events in plasticity and surface receptor genes. Thus, deepening our knowledge of the causal roles epigenetic mechanisms play in long-lasting transcription dysregulation will spur development of novel treatments to manipulate chromatin pathways in neuropsychiatric disorders.
8 BIDIRECTIONAL CROSS-TALK BETWEEN THE EPIGENOME AND CELLULAR ACTIVITY
Together, a large body of work has shown the critical role of epigenetic modifications in drug addiction (Walker et al., 2015). Similarly, many studies have defined the specific neural adaptations at the circuit level that underlie theses same maladaptive behaviors (Dumitriu et al., 2012; Robinson, 1999; Robinson et al., 2001). Thus, understanding the basic process by which activity-dependent changes in the epigenome can feed back and alter cellular excitability in specific populations will lead to a better understanding of how certain neurons are recruited to respond to specific stimuli in health and disease. The ability to strengthen some synapses while weakening others is a critical mediator of learning and helps to guide appropriate behaviors. This process relies on precise temporal activity in both the pre- and postsynaptic cell, which through activity-dependent calcium signaling, leads to the maturation and stability of that particular synapse.
Changes in the resting membrane potential, gene priming, and stable receptor expression levels can all alter the probability that a specific cell will fire, and thus can increase the incorporation of these neurons into memory ensembles and strengthen synaptic connections. Work has shown that increasing CREB levels within neurons are sufficient to increase their incorporation into ensembles that encode specific memories, highlighting a role of activity-dependent molecular signaling in the process (Hsiang et al., 2014). Maintenance of these tonic levels of neurotransmitter, changes in transporter function, and postsynaptic receptor content have been shown to be regulated by epigenetic modifications at the chromatin level. Specific methyl and acetyl marks can act to change stable expression levels of proteins involved in this process, such as AMPA and NMDA receptors, which can change the speed and efficiency with which new synapses can be formed and destabilized. This can also change the response magnitude of these cells and circuits to salient stimuli in the environment, thus driving maladaptive behaviors. These specific processes have been shown to be dysregulated in both human addicts and rodent models of drug-addiction (Breiter et al., 1997; Calipari et al., 2016; Dackis and O’Brien, 2005; Volkow et al., 2005). Thus, basal epigenetic regulation of membrane-associated proteins can alter the excitability of neurons and concomitant behavioral processes associated with addiction.
Conversely, repeated stimulation of strengthened synapses can result in activity-dependent epigenetic remodeling via calcium-dependent signaling via effectors like CREB (Nestler, 2013). This increase in the activity level of neurons can lead to the activation of immediate early genes and concomitant wide scale changes in the accessibility of DNA and transcriptional processes. In addition, these changes can lead to a feedforward loop in which activity-dependent epigenetic changes lead to enhanced sensitivity to subsequent inputs. If these inputs are in pathways driving reinforcement learning this can act to increase self-administration and drug seeking.
Thus, it is the communication between the nuclear changes in DNA conformation/ transcription and the precise changes in membrane excitability that allow for the refinement of information at the level of each individual neuron. A lack of plasticity in this process is what underlies drug addiction in a way that results in the strong and stable storage and expression of drug-associated memories over all others (Fig. 4).
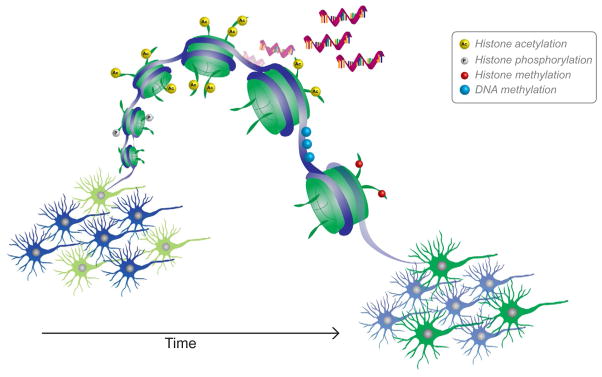
FIG. 4.
Epigenetic modifications to solidify neuronal ensembles. Drug-induced neuronal activation causes rapid changes in chromatin structure. Epigenetic signatures in neurons of the reward circuit gate intracellular signaling to regulate the temporally sensitive transcription of genes involved in synaptic plasticity. Upon initial activation, transient changes in chromatin modifications, like phosphorylation and acetylation, alter transcription in the short-term. Over time, epigenetic changes become more permanent, such as DNA methylation that can alterthe ability of genes to be induced by neuronal activation. Such gene priming or desensitization can modify the ability of neurons to rapidly and efficiently respond to drug-associated cues. Thus, repeated exposure to drugs causes permanent changes in the epigenetic landscape that alter the inducibility of key genes, which solidifies which neurons will be activated and act to drive drug seeking.
9 FOCUSING TO THE FUTURE
9.1 GOALS OF TREATMENT
The goal of drug addiction treatment has historically focused on two main problems: (1) Helping individuals stop consuming drugs once they have started and (2) Preventing relapse in abstinent individuals. Both of these processes involve learning and updating information on a rapid timescale. Consumption relies on learning about response-contingency outcomes and drug seeking and relapse on learning about predictive cues. Together, the goal of all of these treatment strategies is to increase the probability of individuals learn new contingencies, allocate behavior away from drug seeking and taking, and instead toward more adaptive responses. Thus, in the case of both consummatory and appetitive processes increasing the metaplasticity of neural circuits so that they can weaken associations between drugs and associated cues and replace them with more adaptive information about natural rewards will be important.
Applying pharmacological approaches to correcting behavioral disorders has been largely ineffective, likely because addiction is characterized by dysregulated learning, which is controlled by complex interconnected networks, not just the reward circuit, of neural signaling in a specific context. The neural circuit changes induced by drug exposure trigger a molecular cascade that can act to alter subsequent transcription through epigenetic remodeling. This remodeling can feed forward then act to change the connectivity and excitability of the circuit. Thus, to come up with effective therapies we need to understand the interplay between the molecular targets and the circuit disruption that controls discrete aspects of behavior. Altering either in isolation will likely not be sufficient to reverse the problem.
9.2 COMPLEX INTERPLAY BETWEEN CIRCUITS AND TRANSCRIPTION
While it is interesting to speculate which factor is more important in regulating addiction, and thus which to target for treatment, both epigenetic and circuit factors play critical roles, possibly in different temporal aspects of the behavior. For example, transcription happens on the time scale on minutes, however, the high perceived by drugs of abuse occur within seconds. Within 5s, and intravenous cocaine injection has sufficiently to elevated dopamine levels in reward-related brain regions in rodents (Yorgason et al., 2011). In fact, it is the speed at which the drug does this that predicts the euphoric effects of the drug in humans, and the reinforcing effects of the drug in animal models (Balster and Schuster, 1973; Volkow et al., 1995). Therefore, responding and updating information in real-time requires neural connections across the brain.
Further, drug-induced learning processes can be manipulated by altering circuit excitability. Optogenetic studies have shown that maladaptive drug seeking can be blocked by reversing activity deficits in PFC projections into the NAc (Chen et al., 2013). Further, the expression of cocaine-associated memories can be inhibited by blocking hippocampal or cortical projections to the NAc (Pascoli et al., 2014). Inhibiting the BLA to NAc projection is capable of inhibiting cocaine-induced locomotor sensitization (MacAskill et al., 2014). Finally, associative learning about drug rewards and predictive cues can be abolished by inhibiting D1 MSN activity during cue-cocaine pairing (Calipari et al., 2016). These are selected examples of many studies defining circuit activity as a critical regulator of these behavioral learning processes.
Similarly, epigenetic factors are also involved in learning about cocaine and associated stimuli. For example, blocking protein synthesis following a learning task can prevent memory consolidation, thus the ability to acutely upregulate specific proteins is critical (Bourtchouladze et al., 1998). Similar results have been seen with small molecules that inhibit epigenetic writers and erasers. HDACs have been shown to bidirectionally control associative learning for cocaine and predictive cues (Kumar et al., 2005). HDAC inhibitors enhanced associative learning for cocaine and predictive cues. Conversely, reducing histone acetylation in the NAc by virally expressed HDACs decreases the ability of animals to make associations between cocaine and predictive cues (Renthal et al., 2007). Thus, epigenetic modifications are critical in the stabilization and expression of these learned behaviors.
Therefore, circuit activity is necessary for the expression of behavior and the ability to update information in real-time, while epigenetic information is necessary to maintain this information for the long-term. As a result, treatments that target the circuit dynamic changes, without normalizing the molecular basis for this change will likely only be transiently effective. However, targeting epigenetic writers and erasers, without activating the circuits in the appropriate context to allow for plasticity, will likely not lead to appropriate circuit remodeling to reduce drug craving.
9.3 WHAT TO TARGET AND HOW
On an epigenetic level, insight into the kinetic mechanisms of writers, readers, and erasers of chromatin modifications is anticipated to provide novel clinically relevant diagnostic, prognostic, and therapeutic avenues in the treatment of psychiatric disorders. Discussed earlier, different learning paradigms can result in different types of epigenetic modifications, that can feed back and influence neural circuit stability, making understanding the discrete aspects of learning and memory that underlie these changes critical moving forward. For example, targeting epigenetic marks that are responsible for stabilizing synaptic connections in specific pathways or cell types could be effective as treatments. As new tools develop to tackle these complex problems, we will get a more comprehensive view of how different marks are altered by drug exposure across the brain.
Epigenetic avenues of treatment are particularly appealing as epigenetic mechanisms are not only implicated in the molecular pathophysiology of drug addiction, but function as the principle regulators that are common to different neuropsychiatric disorders. Highly informative to current efforts in developing novel therapeutic approaches to drug addiction are recent insights from epigenetic treatments in posttraumatic stress disorder (PTSD). In PTSD, histone acetylation facilitates memory formation and long-term consolidation of fear. The learning process and mechanisms governing this process are similar in the case of drug abuse, where long-term associations between predictive cues and drugs can precipitate relapse (O’Brien et al., 1998).
Dynamic histone acetylation is further involved in the reconsolidation of memories following retrieval, which allows for memory updating and new associative learning linked to disease progression (Torregrossa and Taylor, 2013). Pharmacological agents that target these processes to manipulate traumatic memories are anticipated to be extremely useful in prevention and treatment of PTSD and other psychiatric disorders, including drug addiction (Kwapis and Wood, 2014). However, in order to use this as a treatment, we need to first understand what makes these memories accessible and renders the affected circuits plastic. Because drug addiction activates a brain wide network of circuits, it is important to understand how, when, and which neurons undergo plasticity in order to target them effectively.
Because cue learning is the factor that plays the largest role in relapse, treatment could potentially be aimed at delivering epigenetic drugs during cue exposure to facilitate extinction learning. Once these neural ensembles are activated, they can be targeted with epigenetic modifying drugs such as HDAC inhibitors (HDACi). In the case of PTSD, HDACi are being used in clinical trials to enhance extinction learning in combination with cognitive behavioral therapy. Whereas the original trauma memory is not erased, such epigenetic pharmacotherapy enhances activity-induced histone acetylation and new learning that has shown preliminary success. Similarly, drug addiction may be treated by pairing cognitive behavioral therapy with epigenetic interventions, for instance, HDACi to facilitate extinction learning. The power of this approach is that epigenetic pathways that function in an activity-dependent manner, are targeted across different brain regions.
Thus, we propose a novel two-pronged approach to treat pathological states of addiction: combining cognitive behavioral therapy with pharmacological targeting of epigenetic plasticity mechanisms in a clinical setting to reverse permanent alterations in neural and circuit function caused by drugs of abuse.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci. 2016;17:692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JHJ, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2010;35:2450–2461. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, Seeburg PH. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:1–15. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr Opin Struct Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. doi: 10.1101/lm.5.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP. A fundamental role for context in instrumental learning and extinction. Behav Processes. 2014;104:13–19. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Bagot RC, Gancarz AM, Walker DM, Sun H, Wang ZJ, Heller EA, Feng J, Kennedy PJ, Koo JW, Cates HM, Neve RL, Shen L, Dietz DM, Nestler EJ. Bidirectional synaptic structural plasticity after chronic cocaine administration occurs through Rap1 small GTPase signaling. Neuron. 2016;89:566–582. doi: 10.1016/j.neuron.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJR, Jones SR, Porrino LJ. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci. 2013a;38:3749–3757. doi: 10.1111/ejn.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013b;38:2385–2392. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PEM, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA. Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci. 2016;43:1661–1673. doi: 10.1111/ejn.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of reinforcing and Anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decot HK, Namboodiri VMK, Gao W, McHenry JA, Jennings JH, Lee SH, Kantak PA, Jill Kao YC, Das M, Witten IB, Deisseroth K, Shih YYI, Stuber GD. Coordination of brain-wide activity dynamics by dopaminergic neurons. Neuropsychopharmacology. 2017;42:615–627. doi: 10.1038/npp.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, Damez-Werno D, Dietz KC, Scobie KN, Ferguson D, Christoffel D, Ohnishi Y, Hodes GE, Zheng Y, Neve RL, Hahn KM, Russo SJ, Nestler EJ. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–1113. doi: 10.1016/j.neuron.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs DA, et al. Continuous stimulant self-administration in rhesus monkeys. Res Comm Psychol Psychiat Behav. 1979;4:39–49. [Google Scholar]
- Dudai Y, Morris RGM. Memorable trends. Neuron. 2013;80:742–750. doi: 10.1016/j.neuron.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, LaPlant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus Accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egervari G, Landry J, Callens J, Fullard JF, Roussos P, Keller E, Hurd YL. Striatal H3K27 acetylation linked to glutamatergic gene dysregulation in human heroin abusers holds promise as therapeutic target. Biol Psychiatry. 2017;81:585–594. doi: 10.1016/j.biopsych.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fass DM, Reis SA, Ghosh B, Hennig KM, Joseph NF, Zhao WN, Nieland TJF, Guan JS, Kuhnle CEG, Tang W, Barker DD, Mazitschek R, Schreiber SL, Tsai LH, Haggarty SJ. Crebinostat: a novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology. 2013;64:81–96. doi: 10.1016/j.neuropharm.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Shao N, Szulwach KE, Vialou V, Huynh J, Zhong C, Le T, Ferguson D, Cahill ME, Li Y, Koo JW, Ribeiro E, Labonte B, Laitman BM, Estey D, Stockman V, Kennedy P, Couroussé T, Mensah I, Turecki G, Faull KF, Ming G, Song H, Fan G, Casaccia P, Shen L, Jin P, Nestler EJ. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat Neurosci. 2015;18:536–544. doi: 10.1038/nn.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Goussakov I, Stutzmann GE, Wolf ME. Withdrawal from cocaine self-administration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines. PLoS One. 2012:7. doi: 10.1371/journal.pone.0040898. [DOI] [PMC free article] [PubMed]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology. 2012;37:1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Melchior JR, Roberts DCS, España RA, Jones SR. Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci. 2013a;38:2628–2636. doi: 10.1111/ejn.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Nerosci. 2013b;4:693–703. doi: 10.1021/cn400026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. A single amphetamine infusion reverses deficits in dopamine nerve-terminal function caused by a history of cocaine self-administration. Neuropsychopharmacology. 2015;40:1826–1836. doi: 10.1038/npp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13:337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- Fischer A. Epigenetic memory: the Lamarckian brain. EMBO J. 2014;33:945–967. doi: 10.1002/embj.201387637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Freytag V, Probst S, Hadziselimovic N, Boglari C, Hauser Y, Peter F, Gabor Fenyves B, Milnik A, Demougin P, Vukojevic V, de Quervain DJF, Papassotiropoulos A, Stetak A. Genome-wide temporal expression profiling in Caenorhabditis elegans identifies a Core gene set related to long-term memory. J Neurosci. 2017;37:6661–6672. doi: 10.1523/JNEUROSCI.3298-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M. Reward expectations in honeybees. Commun Integr Biol. 2010;3:95–100. doi: 10.4161/cib.3.2.10621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go BS, Barry SM, McGinty JF. Glutamatergic neurotransmission in the prefrontal cortex mediates the suppressive effect of intraprelimbic cortical infusion of BDNF on cocaine-seeking. Eur Neuropsychopharmacol. 2016;26:1989–1999. doi: 10.1016/j.euroneuro.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, Ehrman RN, Wang Z, Li Y, Suh JJ, Kampman K, O’Brien CP, Childress AR, Franklin TR. Reward-related brain response and craving correlates of marijuana cue exposure: a preliminary study in treatment-seeking marijuana-dependent subjects. J Addict Med. 2013;7:8–16. doi: 10.1097/ADM.0b013e318273863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013a;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013b;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- Gräff J, Woldemichael BT, Berchtold D, Dewarrat G, Mansuy IM. Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat Commun. 2012;3:991. doi: 10.1038/ncomms1997. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LF, Nestler EJ, Self DW. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L, Luo M. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One. 2015;10:e0123381. doi: 10.1371/journal.pone.0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW, Phillips PEM. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci. 2014;34:698–704. doi: 10.1523/JNEUROSCI.2489-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AS, Clark JJ, Phillips PEM. Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiol Learn Mem. 2015;117:84–92. doi: 10.1016/j.nlm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016;17:592–599. doi: 10.1038/nrn.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW. Loss of plasticity in the D2-Accumbens Pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Crouzin N, Moreno E, Raivio N, Fuentes S, McCormick PJ, Ortiz J, Vignes M. Long-lasting impairment of mGluR 5-activated intracellular pathways in the striatum after withdrawal of cocaine self-administration. Int J Neuropsychopharmacol. 2017;20:72–82. doi: 10.1093/ijnp/pyw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon NG, Arnold MM, Gan JO, Walton ME, Phillips PEM. Dopamine-associated cached values are not sufficient as the basis for action selection. Proc Natl Acad Sci U S A. 2014;111:18357–18362. doi: 10.1073/pnas.1419770111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CD, Daberkow DP, Ramsson ES, Keefe KA, Garris PA. Methamphetamine-induced neurotoxicity disrupts naturally occurring phasic dopamine signaling. Eur J Neurosci. 2013;38:2078–2088. doi: 10.1111/ejn.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang HL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, Ye L, Niibori Y, Deisseroth K, Frankland PW, Josselyn SA. Manipulating a “cocaine engram” in mice. J Neurosci. 2014;34:14115–14127. doi: 10.1523/JNEUROSCI.3327-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiol Dis. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz J. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine–glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Ko D, Wanat MJ. Phasic dopamine transmission reflects initiation vigor and exerted effort in an action- and region-specific manner. J Neurosci. 2016;36:2202–2211. doi: 10.1523/JNEUROSCI.1279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus Accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, Laforge S, Laforge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DEH, Truong HT, Russo SJ, LaPlant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Wood MA. Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends Neurosci. 2014;37:706–720. doi: 10.1016/j.tins.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lak A, Stauffer WR, Schultz W. Dopamine prediction error responses integrate subjective value from different reward dimensions. Proc Natl Acad Sci. 2014;111:2343–2348. doi: 10.1073/pnas.1321596111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lak A, Stauffer WR, Schultz W. Dopamine neurons learn relative chosen value from probabilistic rewards. Elife. 2016:5. doi: 10.7554/eLife.18044. [DOI] [PMC free article] [PubMed]
- Lalumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUR-OSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006a;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006b;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Augier E, Vouillac C, Ahmed SH. Current Protocols in Neuroscience. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2013. A choice-based screening method for compulsive drug users in rats. Unit 9.44. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, Wu H, Wang X, Wang X. NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience. 2016;315:45–69. doi: 10.1016/j.neuroscience.2015.11.063. [DOI] [PubMed] [Google Scholar]
- Lisman J. Actin’s actions in LTP-induced synapse growth. Neuron. 2003;38:361–362. doi: 10.1016/s0896-6273(03)00257-5. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Luo Z, Volkow ND, Heintz N, Pan Y, Du C. Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: in vivo optical microprobe [Ca2+]i imaging. J Neurosci. 2011;31:13180–13190. doi: 10.1523/JNEUROSCI.2369-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C. The emergence of a circuit model for addiction. Annu Rev Neurosci. 2016;39:257–276. doi: 10.1146/annurev-neuro-070815-013920. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Baudry M. Properties and mechanisms of long-term synaptic plasticity in the mammalian brain: relationships to learning and memory. Neurobiol Learn Mem. 1995;63:1–18. doi: 10.1006/nlme.1995.1001. [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005;15:67–72. doi: 10.1016/j.conb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS. Eating locally: microautophagy and protein turnover at the synapse. Neuron. 2015;88:619–621. doi: 10.1016/j.neuron.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Medic N, Ziauddeen H, Vestergaard MD, Henning E, Schultz W, Farooqi IS, Fletcher PC. Dopamine modulates the neural representation of subjective value of food in hungry subjects. J Neurosci. 2014;34:16856–16864. doi: 10.1523/JNEUROSCI.2051-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Gaudreau P, Quirion R. Signaling pathways relevant to cognition-enhancing drug targets. Handb Exp Pharmacol. 2015;228:59–98. doi: 10.1007/978-3-319-16522-6_3. [DOI] [PubMed] [Google Scholar]
- Mews P, Donahue G, Drake AM, Luczak V, Abel T, Berger SL. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. 2017;546:381–386. doi: 10.1038/nature22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Ilnytskyy S, Kolb B. Persistent gene expression changes in NAc, mPFC, and OFC associated with previous nicotine or amphetamine exposure. Behav Brain Res. 2013;256:655–661. doi: 10.1016/j.bbr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, Watanabe M, de Kerchove d’Exaerde A, Mimura M, Takata N, Tanaka KF. Ventrolateral striatal medium spiny neurons positively regulate food-incentive, goal-directed behavior independently of D1 and D2 selectivity. J Neurosci. 2017;37:2723–2733. doi: 10.1523/JNEUROSCI.3377-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Monteggia LM. Epigenetics in the mature mammalian brain: effects on behavior and synaptic transmission. Neurobiol Learn Mem. 2011;96:53–60. doi: 10.1016/j.nlm.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431–443. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Reflections on: “a general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function”. Brain Res. 2016;1645:71–74. doi: 10.1016/j.brainres.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- Nott A, Cheng J, Gao F, Lin YT, Gjoneska E, Ko T, Minhas P, Zamudio AV, Meng J, Zhang F, Jin P, Tsai LH. Histone deacetylase 3 associates with MeCP2 to regulate FOXO and social behavior. Nat Neurosci. 2016;19:1497–1505. doi: 10.1038/nn.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numachi Y, Shen H, Yoshida S, Fujiyama K, Toda S, Matsuoka H, Sora I, Sato M. Methamphetamine alters expression of DNA methyltransferase 1 mRNA in rat brain. Neurosci Lett. 2007;414:213–217. doi: 10.1016/j.neulet.2006.12.052. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Okamoto KI, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Oliveira AMM, Estévez MA, Hawk JD, Grimes S, Brindle PK, Abel T. Subregion-specific p300 conditional knock-out mice exhibit long-term memory impairments. Learn Mem. 2011;18:161–169. doi: 10.1101/lm.1939811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Volkow ND, Pan Y, Du C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci. 2013;33:15827–15836. doi: 10.1523/JNEUROSCI.1935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Lüscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Peña CJ, Bagot RC, Labonté B, Nestler EJ. Epigenetic signaling in psychiatric disorders. J Mol Biol. 2014;426:3389–3412. doi: 10.1016/j.jmb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I. Epigenetic control of gene expression in the alcoholic brain. Alcohol Res. 2013;35:69–76. doi: 10.1164/rccm.201010-1579PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JM, Petty J, Riba-Garcia I, Robertson DHL, Gaskell SJ, Oliver SG, Beynon RJ. Dynamics of protein turnover, a missing dimension in proteomics. Mol Cell Proteomics. 2002;1:579–591. doi: 10.1074/MCP.M200046-MCP200. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Signaling between the actin cytoskeleton and the postsynaptic density of dendritic spines. Hippocampus. 2000;10:527–541. doi: 10.1002/1098-1063(2000)10:5<527::AID-HIPO3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Rivera CM, Ren B. Mapping human epigenomes. Cell. 2013;155:39–55. doi: 10.1016/j.cell.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38:94–110. doi: 10.1038/npp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris DL, Schuch JB, Grassi-Oliveira R, Sanvicente-Vieira B, da Silva BS, Walss-Bass C, Müller D, Stolf AR, von Diemen L, Cereser KMM, Pianca TG, Szobot CM, Kessler FHP, Roman T, Bau CHD. Effects of crack cocaine addiction and stress-related genes on peripheral BDNF levels. J Psychiatr Res. 2017;90:78–85. doi: 10.1016/j.jpsychires.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Rudenko A, Tsai LH. Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology. 2014;80:70–82. doi: 10.1016/j.neuropharm.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Russo S, Nestler E. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DA, Saddoris MP, Carelli RM. Nucleus accumbens shell dopamine preferentially tracks information related to outcome value of reward. eNeuro. 2017:4. doi: 10.1523/ENEURO.0058-17.2017.ENEURO.0058-17.2017. [DOI] [PMC free article] [PubMed]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77:903–911. doi: 10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Carelli RM. Prior cocaine experience impairs normal phasic dopamine signals of reward value in accumbens shell. Neuropsychopharmacology. 2017;42:766–773. doi: 10.1038/npp.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Matthies H. Biochemical studies on histones of the central nervous system. III Incorporation of [14C]-acetate into the histones of different rat brain regions during a learning experiment. Acta Biol Med Ger. 1979;38:683–689. [PubMed] [Google Scholar]
- Schneider A, Chatterjee S, Bousiges O, Selvi BR, Swaminathan A, Cassel R, Blanc F, Kundu TK, Boutillier AL. Acetyltransferases (HATs) as targets for neurological therapeutics. Neurotherapeutics. 2013;10:568–588. doi: 10.1007/s13311-013-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Stalnaker TA, Shaham Y. A role for BDNF in cocaine reward and relapse. Nat Neurosci. 2007;10:935–936. doi: 10.1038/nn0807-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–676. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Serita T, Fukushima H, Kida S. Constitutive activation of CREB in mice enhances temporal association learning and increases hippocampal CA1 neuronal spine density and complexity. Sci Rep. 2017;7:42528. doi: 10.1038/srep42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Sharma SK. Protein acetylation in synaptic plasticity and memory. Neurosci Biobehav Rev. 2010;34:1234–1240. doi: 10.1016/j.neubiorev.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Shen H-w, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Jones SR. Cocaine potency at the dopamine transporter tracks discrete motivational states during cocaine self-administration. Neuropsychopharmacology. 2017;42:1893–1904. doi: 10.1038/npp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Fordahl SC, Jones SR. Cocaine self-administration produces long-lasting alterations in dopamine transporter responses to cocaine. J Neurosci. 2016;36:7807–7816. doi: 10.1523/JNEUROSCI.4652-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. Reinforcement today. Am Psychol. 1958;13:94–99. doi: 10.1037/h0049039. [DOI] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ. Reappraising striatal D1-and D2-neurons in reward and aversion. Neurosci Biobehav Rev. 2016;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addict Biol. 2014;19:972–974. doi: 10.1111/adb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. CREB and memory consolidation. Neuron. 1994;13:769–770. doi: 10.1016/0896-6273(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Sun W-L, Eisenstein SA, Zelek-Molik A, McGinty JF. A single brain-derived neurotrophic factor infusion into the dorsomedial prefrontal cortex attenuates cocaine self-administration-induced phosphorylation of Synapsin in the nucleus accumbens during early withdrawal. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu049. . pii: pyu049. [DOI] [PMC free article] [PubMed]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The emerging field of neuroepigenetics. Neuron. 2013;80:624–632. doi: 10.1016/j.neuron.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’doherty JP, Dolan RJ, Schultz W. Human neural learning depends on reward prediction errors in the blocking paradigm. J Neurophysiol. 2006;95:301–310. doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Mechanisms of renewal after the extinction of discriminated operant behavior. J Exp Psychol Anim Learn Cogn. 2014a;40:355–368. doi: 10.1037/xan0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiol Learn Mem. 2014b;108:52–64. doi: 10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010:5. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed]
- Tonegawa S, Liu X, Ramirez S, Redondo R. Perspective memory engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Vandaele Y, Cantin L, Serre F, Vouillac-Mendoza C, Ahmed SH. Choosing under the influence: a drug-specific mechanism by which the setting controls drug choices in rats. Neuropsychopharmacology. 2016;41(2):646–657. doi: 10.1038/npp.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997a;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997b;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Heller EA, Nestler EJ. Regulation of chromatin states by drugs of abuse. Curr Opin Neurobiol. 2015;30:112–121. doi: 10.1016/j.conb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Marshall CB, Ikura M. Transcriptional/epigenetic regulator CBP/p300 in tumorigenesis: structural and functional versatility in target recognition. Cell Mol Life Sci. 2013;70:3989–4008. doi: 10.1007/s00018-012-1254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Advances in animal models of relapse for addiction research. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. CRC Press; Boca Raton, FL: 2010. pp. 1–25. [PubMed] [Google Scholar]
- White AO, Wood MA. Does stress remove the HDAC brakes for the formation and persistence of long-term memory? Neurobiol Learn Mem. 2013;112:61–67. doi: 10.1016/j.nlm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Hawk JD, Abel T. Combinatorial chromatin modifications and memory storage: a code for memory? Learn Mem. 2006;13:241–244. doi: 10.1101/lm.278206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Jones SR, España RA. Low and high affinity dopamine transporter inhibitors block dopamine uptake within 5 sec of intravenous injection. Neuroscience. 2011;182:125–132. doi: 10.1016/j.neuroscience.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Franklin TR, Roberts DCS, Jagannathan K, Suh JJ, Wetherill RR, Wang Z, Kampman KM, O’Brien CP, Childress AR. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci. 2014;34:5038–5043. doi: 10.1523/JNEUROSCI.4977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Su Y, Song H. Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Dev Psychopathol. 2015;27:1251–1265. doi: 10.1017/S0954579414000868.Child-evoked. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang L, Lu K, Liu Y, Tu G, Zhu M, Ying L, Zhao J, Liu N, Guo F, Zhang L, Zhang L. Cocaine-induced synaptic structural modification is differentially regulated by dopamine D1 and D3 receptors-mediated signaling pathways. Addict Biol. 2016;112:61–67. doi: 10.1111/adb.12462. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao W, Ho HH, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JAJ, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]