Abstract
Maternal obesity negatively impacts the placenta, being associated with increased inflammation, decreased mitochondrial respiration, decreased expression of brain-derived neurotrophic factor (BDNF), and its receptor, tropomyosin receptor kinase B (TRKB). TRKB induction by 7,8-dihydroxyflavone (7,8-DHF) improves energy expenditure in an obesity animal model. We hypothesized that TRKB activation would improve mitochondrial respiration in trophoblasts from placentas of obese women. Placentas were collected from lean (pre-pregnancy BMI < 25) and obese (pre-pregnancy BMI > 30) women at term following cesarean section delivery without labor. Cytotrophoblasts were isolated and plated, permitting syncytialization. At 72 hours, syncytiotrophoblasts (STs) were treated for 1 hour with 7,8-DHF (10 nM–10 M), TRKB antagonists (ANA-12 (10 nM–1 M), Cyclotraxin B (1 nM–1M)), or vehicle. Mitochondrial respiration was measured using the XF24 Extracellular Flux Analyzer. TRKB, MAPK, and PGC1α were measured using Western blotting. Maternal obesity was associated with decreased mitochondrial respiration in STs; however, 7,8-DHF increased basal, ATP-coupled, maximal, spare capacity, and nonmitochondrial respiration. A 10 μM dose of 7,8-DHF reduced spare capacity in STs from lean women, with no effect on other respiration parameters. 7,8-DHF had no effect on TRKB phosphorylation; however, there was a concentration-dependent decrease of p38 MAPK phosphorylation and increase of PGC1α in STs from obese, but not in lean women. TRKB antagonism attenuated ATP-coupled respiration, maximal respiration, and spare capacity in STs from lean and obese women. 7,8-DHF improves mitochondrial respiration in STs from obese women, suggesting that the obese phenotype in the placenta can be rescued by TRKB activation.
Keywords: mitochondrial respiration, neurotrophin, obesity, placenta
Introduction
The prevalence of obesity in women older than 20 years in the United States is greater than 40%.1 Obesity during pregnancy increases the risk of pregnancy-induced hypertension,2 gestational diabetes,3 and preeclampsia.4 Fetal and neonatal consequences from maternal obesity include increased risk of stillbirth,5 preterm delivery,6 and congenital abnormalities.7 The placenta is a fetal organ that functions as an immunological barrier between the mother and the developing fetus, transports nutrients and waste, and synthesizes hormones that support pregnancy8,9; however, maternal obesity adversely affects placental function. Maternal obesity increases hypoxia,10 inflammation,11,12 oxidative stress,12 and mitochondrial dysfunction12,13 in the placenta. It is hypothesized that an adverse intrauterine environment, including the placenta, is involved in programming the fetus for disease later in life.14–16 The offspring of obese (OB) women have increased prevalence of cardiovascular disease, hypertension, diabetes, and dysregulation of the hypothalamic–pituitary–adrenal axis.17–19 There has also been growing interest in understanding the role of maternal obesity in regulating brain development, offspring behavior, and onset of neuropsychiatric disease in adulthood20; however, the mechanisms underlying fetal programming of neuropsychiatric and other diseases is unknown.
The placenta secretes a variety of neuropeptides, neurohormones, and neurotransmitters,21–27 including brain-derived neurotrophic factor (BDNF). BDNF binds to tropomyosin receptor kinase B (TRKB), a membrane-bound receptor tyrosine kinase, and induces phosphorylation at several tyrosine residues resulting in induction of several cell signaling cascades, including MEK1 (mitogen-activated protein (MAP) or extracellular signal-regulated (Erk) kinase) and MAPK, that are associated with maintaining energy homeostasis. We recently identified decreased BDNF, decreased total TRKB, increased phosphorylation at tyrosine 817, and increased phosphorylation of p38 MAPK in placentas from OB women.28 These alterations in signaling are similar to those seen in neurodegenerative diseases,29 affective disorders,30 and obesity.31,32 Brain-derived neurotrophic factor has the potential to ameliorate the effects of obesity in the placenta, as it increases mitochondrial function33,34 and protects from oxidative stress35 in neurons and human choriocarcinoma cells; however, peripherally administered BDNF has low bioavailability.36 7,8-Dihydroxyflavone (7,8-DHF) is a flavonoid derivative that induces TRKB and TRKB-dependent signaling.37,38 Although the temporal phosphorylation patterns of TRKB seen with 7,8-DHF differ from that of BDNF,39 TRKB phosphorylation is induced,39 mitochondrial respiration is increased,40 levels of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC1α), a master regulator of mitochondrial biogenesis and antioxidant response is increased,40 and markers of obesity are decreased in a diet-induced obesity animal model.41 Based on these findings, we hypothesized that 7,8-DHF would improve mitochondrial respiration in placentas of OB women.
Materials and Methods
Placenta Collection and Cytotrophoblast Isolation
The research protocol was approved by the institutional review board of the University of Texas Health Science Center at San Antonio. Rather than studying women with a range of body mass index (BMI) including lean (LN), overweight, and OB, we chose to compare 2 well-separated phenotypes, LN and OB, to demonstrate the effects of obesity. We have previously shown inflammation and oxidative stress increase with maternal BMI12,13; however, the increase in inflammatory markers and indicators of oxidative stress was more pronounced in the cases of maternal obesity. Placentas from uncomplicated, term pregnancies of LN (pre/early pregnancy BMI: 18.5-24.9 kg/m2, n = 6; Table 1) and OB (pre/early pregnancy BMI >30 kg/m2, n = 12; Table 1) women were immediately collected following elective cesarean delivery at term in the absence of labor with informed consent from patients in the Labor and Delivery Unit of University Hospital San Antonio. Exclusion criteria for the study were abnormal oral glucose tolerance test, concurrent diseases (diabetes, preeclampsia, hypertension, and infections), tobacco or drug/medication use, excessive weight gain/loss prior to pregnancy, and labor with regular contractions. Primary cytotrophoblasts (CTs) were isolated in the manner previously described.42
Table 1.
Maternal and Fetal Characteristics.
| Lean (n = 6) | Obese (n = 12) | |
|---|---|---|
| Pre/early pregnancy BMI, kg/m2 | 21.2 ± 1.1 | 34.0 ± 1.0 a |
| Gestational age, weeks | 39.3 ± 0.5 | 39.2 ± 0.2 |
| Maternal weight gain, (lbs) | 23.8 ± 3.9 | 15.8 ± 3.0 |
| Birth weight, g | 3262 ± 127 | 3418 ± 91 |
| Parity | 1.5 ± 0.3 | 1.4 ± 0.3 |
| Maternal age at delivery, years | 30.8 ± 1.5 | 26.6 ± 2.3 |
| Ethnicity (Hispanic/non-Hispanic) | 5/1 | 9/3 |
Abbreviations: BMI, body mass index; LN, lean.
a P < .0001 versus LN.
Primary CT Cell Culture and Treatment
Cytotrophoblasts were isolated from each placenta and plated at a density of 8.5 × 105 cells and allowed to syncytialize to syncytiotrophoblasts (STs) over 72 hours in Seahorse XF24 plates (Seahorse Biosciences, Santa Clara, CA) and 24-well, flat bottom culture plates for subsequent protein studies. Cytotrophoblasts were cultured in a 5% carbon dioxide (CO2) humidified atmosphere at 37°C in complete media (Dulbecco’s modified Eagle medium, Ham F-12 nutrient mixture, l-glutamine, penicillin, streptomycin, and gentamicin) that was changed daily. At 72 hours, the STs were treated for 1 hour with or without increasing concentrations of the BDNF agonist 7,8-DHF (10 nmol/L-10 μmol/L final, cells treated in quadruplicate per dose) or the BDNF antagonists ANA-12 (10 nmol/L-1 μmol/L, cells treated in triplicate per dose), or cyclotraxin B (1 nmol/L-1 μmol/L, cells treated in triplicate per dose; Tocris Bioscience, Bristol, UK). Following treatment, STs in Seahorse plates were cultured in Seahorse media supplemented with glucose and pyruvate, incubated in CO2-free incubator at 37°C for 1 hour, and oxygen consumption was measured.43 Corresponding STs (treated in duplicate per dose) in 24-well, flat-bottomed plates were harvested for protein analysis.
Measurement of Oxygen Consumption Rates
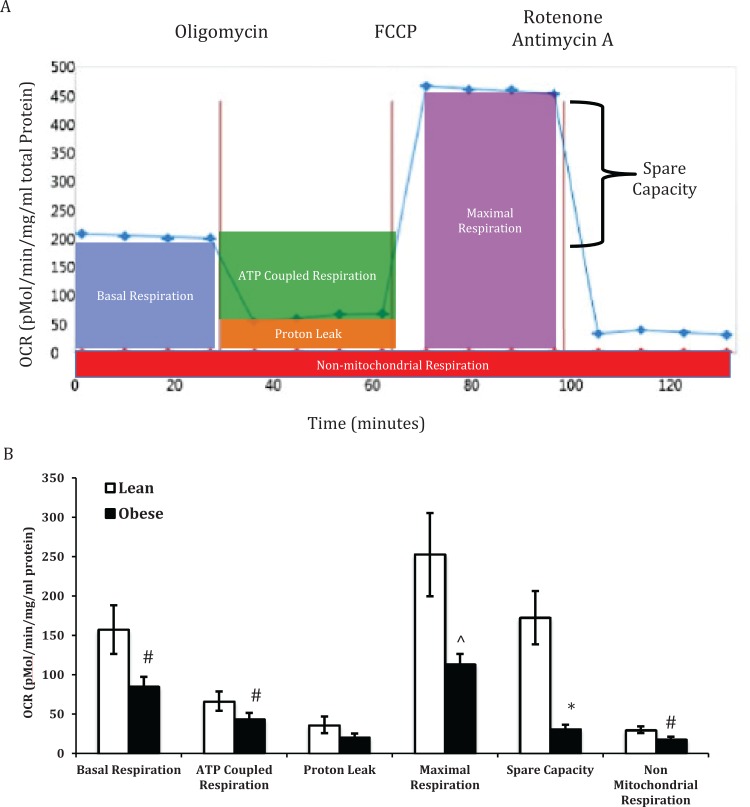
The Seahorse XF24 analyzer (Seahorse Biosciences) was used to measure oxygen consumption rates (OCRs) upon challenge with various reagents in STs as described previously.44,45 Basal respiration is measured to establish baseline rates. At 25 minutes, oligomycin is injected, which blocks the proton pore (F0 subunit) and inhibits adenosine triphosphate (ATP) synthesis. At 65 minutes, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone was injected, uncoupling ATP synthesis from the electron transport chain. This resulted in a rapid increase in OCR without the generation of ATP. Injection of rotenone and antimycin A at 100 minutes inhibits complexes I and III, resulting in complete depletion of mitochondrial-dependent respiration (Figure 1A). Nonmitochondrial respiration was measured following the inhibition of complexes I and III. Spare capacity was calculated by subtracting basal from maximal respiration and is an index of the cells ability to respond to stress. Proton leak was calculated by subtracting nonmitochondrial respiration from ATP-coupled respiration.
Figure 1.
Oxygen consumption rates (OCRs) in placentas from lean (LN) and obese (OB) women. Representative OCR versus time trace in syncytiotrophoblasts from the placenta of a LN woman (A) following injection of oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), rotenone, and antimycin A. Basal respiration, adenosine triphosphate (ATP)-coupled respiration, proton leak, maximal respiration, spare capacity, and nonmitochondrial respiration in placentas from LN and OB women (B). Oxygen consumption rate was normalized to total cellular protein. Values are mean ± standard error of the mean (SEM); #: P < .05 versus LN; ⁁: P < .01 versus LN; *: P < .001 versus LN; n = 6 LN and 12 OB.
Western Blot
Twenty micrograms of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described.13 Tropomyosin receptor kinase B (1:500 rabbit anti-TrkB; Santa Cruz, CA), phosphorylated TRKB at Y817 (1:1000 rabbit anti-TrkB phospho Y817; Abcam, Cambridge, MA), p38 MAPK (1:1000 rabbit anti-p38 antibody; Cell Signaling), phosphorylated p38 MAPK (1:1000 rabbit anti-phosphorylated p38 antibody; Cell Signaling), PGC1α (1:1000 rabbit anti- PGC1α antibody; Cell Signaling, Beverly, MA), and β actin (loading control; 1:1000 mouse anti-β-actin antibody; Sigma, St. Louis, MO) were measured by Western blotting. The membranes were then washed, incubated with the appropriate peroxidase-conjugated secondary antibody, and visualized.
Statistical Analysis
Data are reported as mean ± standard error of the mean. Parametric statistical analysis was performed using Student t test, 1- or 2-way analysis of variance. The post hoc analysis was completed using Fisher’s Least Significant Difference (LSD). P values <.05 were considered statistically significant. Analyses were performed using StatPlus: mac Pro Software (AnalystSoft Inc, Walnut, California) and GraphPad Prism version 7 (GraphPad Software).
Results
Demographic and Clinical Characteristics
Maternal and fetal characteristics are presented in Table 1. By design, prepregnancy/first-trimester BMI was significantly greater in the OB group compared to the LN group (P < .0001). There were no differences across groups in gestational age, maternal age at delivery, parity, or birth weight (P > .05). Each group contained equal numbers of male and female fetuses. The majority of the participants were Hispanic.
Characterization of ST Mitochondrial Phenotype in Placentas From LN and OB Women
Basal respiration, ATP-coupled respiration, proton leak, maximal respiration, and nonmitochondrial respiration from STs isolated from the placenta of LN women are shown in Figure 1A. Maternal obesity was associated with decreased basal respiration, ATP-coupled respiration, maximal respiration, spare capacity, and nonmitochondrial respiration by 30% or more (Figure 1B).
Cellular Response to TRKB Agonist, 7,8-DHF
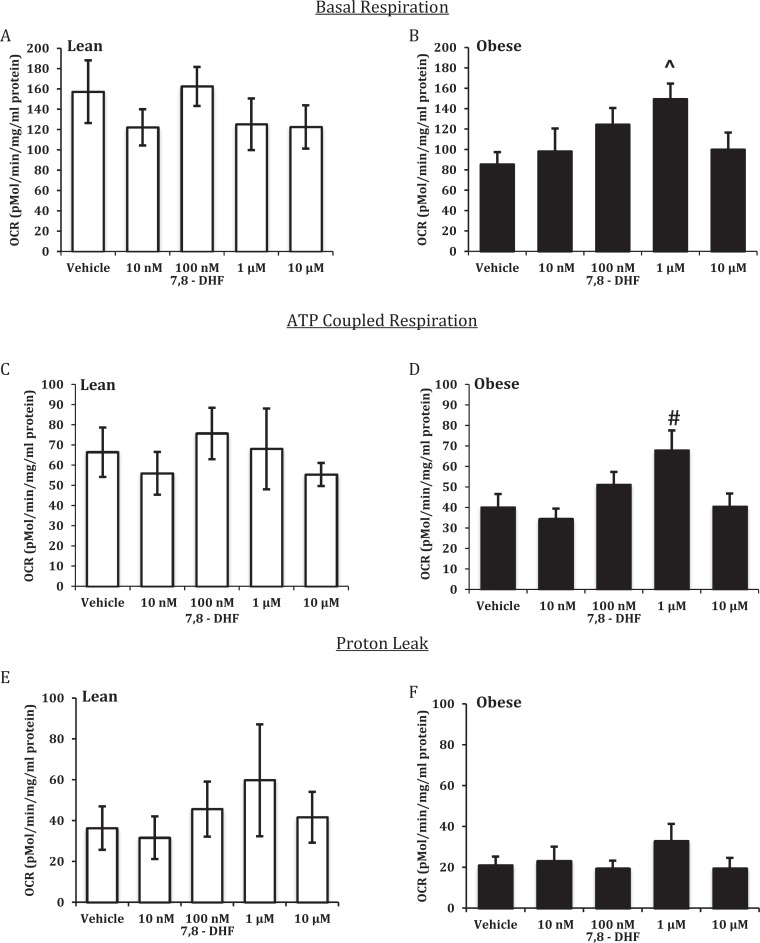
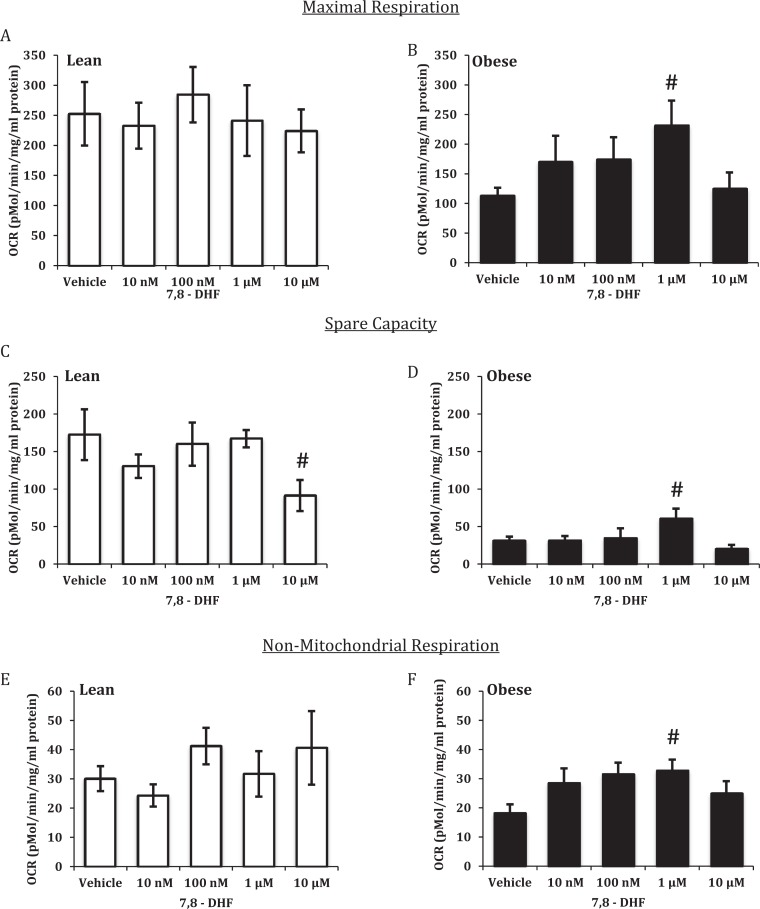
The addition of increasing concentrations of 7,8-DHF to ST from LN women resulted in no effect on basal respiration (Figure 2A), ATP-coupled respiration (Figure 2C), proton leak (Figure 2E), maximal respiration (Figure 3A), or nonmitochondrial respiration (Figure 3E). Acute treatment with 10 μmol/L 7,8-DHF gave a significant reduction in spare capacity (Figure 3C). In STs from OB women, 7,8-DHF had no effect on proton leak (Figure 2F); conversely, 1 μmol/L 7,8-DHF significantly increased basal respiration (Figure 2B), ATP-coupled respiration (Figure 2D), maximal respiration (Figure 3B), spare capacity (Figure 3D), and nonmitochondrial respiration (Figure 3F).
Figure 2.
Effect of 7,8-dihydroxyflavone on basal and adenosine triphosphate (ATP)-coupled respiration and proton leak in syncytiotrophoblasts from lean (LN) and obese (OB) women. Basal respiration (A and B), ATP-coupled respiration (C and D), and proton leak (E and F). Oxygen consumption rates (OCR)was normalized to total cellular protein. Values are mean ± standard error of the mean (SEM); #: P < .05 versus vehicle (OB); ⁁: P < .01 versus vehicle (OB); n = 6 LN and 12 OB.
Figure 3.
Effect of 7,8-dihydroxyflavone on maximal and nonmitochondrial respiration and spare capacity in syncytiotrophoblasts from lean (LN) and obese (OB) women. Maximal respiration (A and B), spare capacity (C and D), and nonmitochondrial respiration (E and F). Oxygen consumption rates (OCR) was normalized to total cellular protein. Values are mean ± standard error of the mean (SEM); #: P < .05 versus vehicle (OB); n = 6 LN and 12 OB.
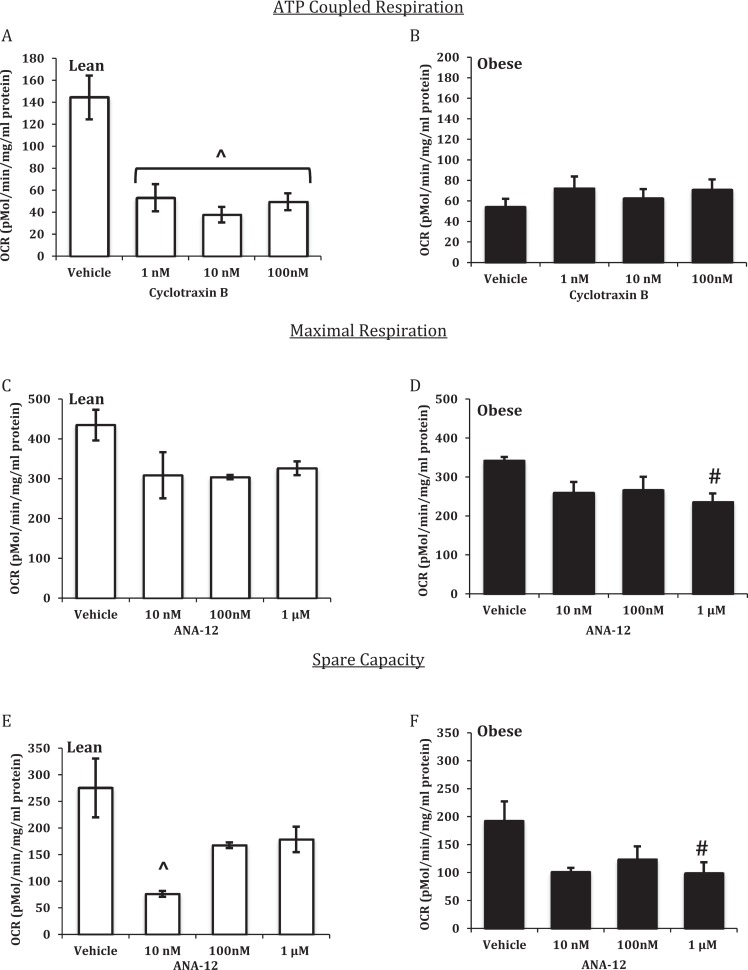
Cellular Response to TRKB Antagonists ANA-12 and Cyclotraxin B
Increasing concentrations of cyclotraxin B significantly decreased ATP-coupled respiration in STs from placentas of LN women (Figure 4A) but had no effect on STs from placentas of OB women (Figure 4B). Acute treatment with ANA-12 had no effect on maximal respiration in STs isolated from placentas from LN women (Figure 4C); however, 1 μmol/L ANA-12 significantly decreased maximal respiration in STs isolated from placentas of OB women (Figure 4D). ANA-12 of 10 nmol/L significantly decreased spare capacity in STs from placentas of LN women (Figure 4E), while 1 μmol/L of ANA-12 significantly decreased spare capacity in STs isolated from placentas of OB women (Figure 4F).
Figure 4.
Concentration-dependent mitochondrial respiration responses to cyclotraxin B and ANA-12. Adenosine triphosphate (ATP)-coupled respiration (A and B), maximal respiration (C and D), and spare capacity (E and F) in syncytiotrophoblasts from lean (LN) and obese (OB) women. Oxygen consumption rates (OCR) were normalized to total cellular protein. Values are mean ± standard error of the mean (SEM); ⁁: P < .01 versus vehicle (LN); #: P < .05 versus vehicle (OB); n = 3 LN and 5 OB.
Regulation of Cell Signaling Cascades Associated With TRKB Agonism
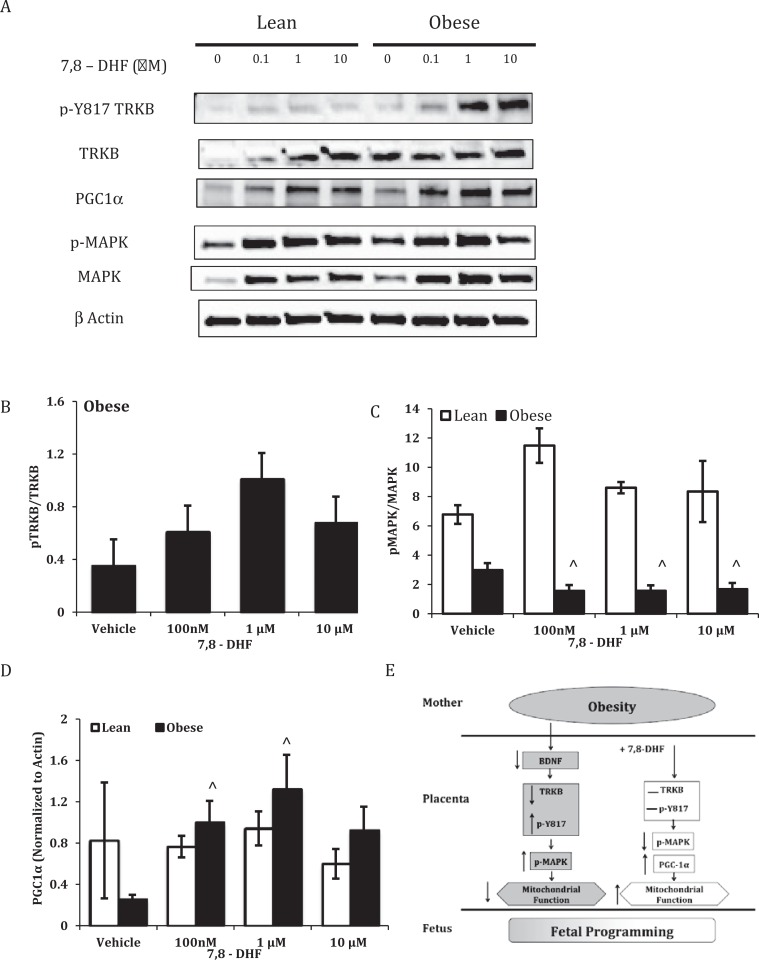
Increasing concentrations of 7,8-DHF had no effect on phosphorylation at Y817 of TRKB (Figure 5A and B) in trophoblast of OB women; however, there was a dose-dependent decrease in phosphorylation of p38 MAPK by 7,8-DHF in STs from OB but not LN women (Figure 5A and C).
Figure 5.
Tropomyosin receptor kinase B (TRKB) phosphorylation at tyrosine 817, p38 mitogen-activated protein kinase (MAPK) phosphorylation at threonine 180/tyrosine 182, proliferator-activated receptor-gamma (PPARγ) coactivator 1α (PGC-1α)levels, and proposed model for effect of 7,8-dihydroxyflavone (DHF) in placentas from lean (LN) and obese (OB) women. Representative Western blots and quantification of pY817 (A and B), pp38 MAPK (A and C), and PGC-1α (A and D) in cell lysates from LN and OB women. Samples were normalized to total TRKB, p38, and β actin, respectively. Values are mean ± standard error of the mean (SEM); ⁁: P < .01 versus vehicle (OB); n = 3 LN and 11 OB.
Regulation of PGC1α
Increasing concentrations of 7,8-DHF significantly increased PGC1α in STs from placentas of OB women but had no effect in trophoblast from LN women (Figure 5D).
Discussion
Maternal obesity is associated with placental dysfunction, which increases the risk of pregnancy complications, poor perinatal outcome, and fetal programming. We recently identified dysregulation of BDNF/TRKB signaling in placentas from OB women,28 which corresponds with decreased circulating BDNF in the plasma32 and decreased BDNF messenger RNA (mRNA) expression in the ventromedial hypothalamus of OB individuals.31 Tropomyosin receptor kinase B, the BDNF cognate receptor, mediates the transduction of several cell signaling cascades that are necessary for preventing oxidative stress,46 improving mitochondrial respiration,47 and increasing OCRs.34 This study builds upon our understanding of neuropeptide signaling in the placenta and uses a compound that potentially ameliorates maternal obesity-associated placental mitochondrial dysregulation by inducing TRKB signaling. Strengths of the study are the well-defined patient groups, the predominance of 1 ethnic group (Hispanic), and collection of tissue at term in the absence of labor to avoid additional oxidative stress. Limitations would include the generalizability of the data to other ethnicities and the small sample size. The study design does not allow us to distinguish if the causative factor is obesity prior to pregnancy or the continuing dietary habits of OB individuals. However, these data contribute to our understanding of the role of TRKB modulation on mitochondrial respiration in the context of obesity.
In this study, we find that maternal obesity decreased mitochondrial and nonmitochondrial respiration in STs, recapitulating our published data.12 Although BDNF was found to improve respiration in brain mitochondrial preparations,33,34 it has poor therapeutic potential when administered peripherally. From a screen of over 60 compounds, 7,8-DHF was found to induce TRKB dimerization, autophosphorylation, and cross the blood–brain barrier.38 In a diet-induced obesity animal model, 7,8-DHF decreased bodyweight, circulating Tumor necrosis factor alpha (TNF-α) concentrations, hepatic and muscular free fatty acids, and increased energy expenditure,41 whereas it did not affect body weight in mice receiving standard chow.41 We hypothesized that treating STs with 7,8-DHF would improve cellular respiration in the context of maternal obesity. Syncytiotrophoblasts from placentas of LN and OB women underwent acute treatment with the TRKB agonist, 7,8-DHF from 10 nmol/L to 10 µmol/L. Cellular respiration parameters in cells isolated from placentas of LN women were not affected by this acute treatment with 7,8-DHF, other than a significant decrease in spare capacity seen at 10 μmol/L 7,8-DHF, which suggests a diminished ability to respond to cellular stress and possible cytotoxicity. Although not statistically significant, both basal and maximal respiration started to decrease at 10 μmol/L 7,8-DHF in trophoblast from both LN and OB women. Interestingly, Chen et al had shown that DHF concentrations of 100 µmol/L and upward had no effect on viability of immortalized mouse hippocampal cells.48 In contrast to trophoblast isolated from LN women, induction of placental TRKB by 1 μmol/L 7,8-DHF in trophoblast isolated from OB women improved both mitochondrial (basal, ATP-coupled, maximal, and spare capacity) and nonmitochondrial respiration. Indeed basal, ATP-coupled, maximal, and nonmitochondrial respiration were returned to levels that were no different from those measured in trophoblast from LN women. This suggests that the effective dose of 7,8-DHF controls cellular respiration in a metabolic status-specific manner similar to other descriptions in the literature.41,49 The relationship between TRKB activation, subsequent induction of MEK and MAPK, and mitochondrial respiration has been shown in previous reports.33,34,40 Conversely, inhibition of MEK/ERK kinases and MEK1/2 in neuronal mitochondrial preparations coincubated with increasing concentrations of BDNF resulted in diminished respiratory control index, a measure of mitochondrial integrity, ATP synthesis, and oxygen consumption.33,34
We next examined cellular respiration following treatment with TRKB-specific antagonists ANA-12 and cyclotraxin B which have differing modes of action. ANA-12 is a nonpeptide derived, noncompetitive receptor antagonist that binds to the high- and low-affinity sites on the extracellular domain of TRKB.50 Incubation with increasing concentrations of ANA-12 decreased neurite outgrowth in a TRKB-specific cell model of neuronal differentiation (nnr5-PC12-TRKB) but not in nnr5-PC12-TRKA and nnr5-PC12-TRKC lines.50 Additionally, systemic administration of ANA-12 resulted in decreased anxious and depressive behavior in rodent models without affecting neuron survival.50 Cyclotraxin B is a BDNF-derived, noncompetitive inhibitor that allosterically alters TRKB confirmation, thus modulating its activation capacity in a BDNF-dependent and -independent manner.51 The cellular and physiological responses to cyclotraxin B are comparable to that of ANA-12. These studies provide evidence that ANA-12 and cyclotraxin B are selective and specific for TRKB. In this study, ANA-12 and cyclotraxin B significantly decreased ATP-coupled respiration and spare capacity in trophoblast isolated from LN women, bringing levels to those seen in untreated trophoblast from OB women or lower. In trophoblast from placentas of OB women, ANA-12 further significantly decreased maximal respiration and spare capacity while cyclotraxin B had no effect on ATP-coupled respiration, suggesting a possible floor effect that is mitochondrial, electron transport chain (ETC) complex specific. However, the effect of TRKB antagonists in inhibiting respiration in trophoblast from both LN and OB women provides evidence of a central role of TRKB in ST respiration.
Tropomyosin receptor kinase B activation is associated with induction of PI3K, Protein Kinase B (AKT), MAPK, and phospholipase C (PLCγ) signaling.52 We have shown that maternal obesity is associated with increased phosphorylation in the placenta of TRKB at tyrosine 817,28 a residue associated with induction of PLCγ, Protein kinase C (PKC), and MAPK.53–55 However, in our study, addition of 7,8-DHF in vitro did not induce a further significant increase in phosphorylation of TRKB at Y817. Phosphorylation of p38 MAPK was significantly decreased in cells isolated from placentas of OB women and treated with 7,8-DHF, whereas there was no effect in trophoblast from a LN woman, which supports data that suggest 7,8-DHF decreases the inflammatory profile associated with obesity.41 A possible mechanism for recovery of respiration is modulation of PGC-1α, a key regulator of metabolism (Figure 5E). PGC-1α is a transcriptional coactivator of PPARγ, a transcription factor that is required for placenta development.56 PGC-1α mRNA expression is decreased in white adipose tissue of morbidly OB patients57 and animal models of obesity.58 p38 MAPK removes the negative regulatory unit from PGC-1α permitting mitochondrial respiration and regulation of the antioxidant response59–62; however, sustained p38 MAPK phosphorylation may serve as a negative PGC-1α transcriptional regulator.58 Collectively, these studies suggest that PGC-1α expression would be decreased in placentas from OB women, contributing to mitochondrial dysregulation. Increasing concentrations of 7,8-DHF significantly increased PGC-1α in trophoblast from OB women, suggesting a possible mechanism through which mitochondrial respiration can recover from the adverse effects of maternal obesity. In addition to the effect via TRKB, we are cognizant that 7,8-DHF is reported to have general antioxidant activity, which was not examined here and which may have contributed to the amelioration of impaired mitochondrial respiration. Clinically, it would also be necessary to determine whether 7,8-DHF could cross the placenta and assess the effects on the developing fetus.
Various factors can influence placental function and theoretically could contribute to pregnancy outcome and fetal programming for disease onset in adult life. Maternal obesity influences placenta size, respiration, metabolism and function, fetal oxygen supply, and fetal growth and development. Improvement in trophoblast respiration via 7,8-DHF induction of TRKB signaling may ameliorate the effect of obesity on the placenta and on pregnancy outcomes.
Acknowledgments
The authors would like to thank Drs L.C. Evans and S. Muralimanoharan for their technical support and Drs K. Ireland and E. Rodriguez for screening and consenting patients for the placenta study. The authors would also like to thank the participants in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the Eunice Kennedy National Institute of Child Health and Human Development (HD076259; AM and LM).
References
- 1. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2):234–239. [DOI] [PubMed] [Google Scholar]
- 3. Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30(8):2070–2076. [DOI] [PubMed] [Google Scholar]
- 4. O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374. [DOI] [PubMed] [Google Scholar]
- 5. Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197(3):223–228. [DOI] [PubMed] [Google Scholar]
- 6. Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F378–F382. [DOI] [PubMed] [Google Scholar]
- 7. Reece EA. Obesity, diabetes, and links to congenital defects: a review of the evidence and recommendations for intervention. J Matern Fetal Neonatal Med. 2008;21(3):173–180. [DOI] [PubMed] [Google Scholar]
- 8. Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinolo Metab. 2005;90(7):4299–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aye IL, Lager S, Ramirez VI, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes.(Load). 2015;39(8):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barker DJ. The effect of nutrition of the fetus and neonate on cardiovascular disease in adult life. Proc Nutr Soc. 1992;51(2):135–144. [DOI] [PubMed] [Google Scholar]
- 15. Barker DJ. Intrauterine programming of coronary heart disease and stroke. Acta Paediatr Suppl. 1997;423:178–182; discussion 183. [DOI] [PubMed] [Google Scholar]
- 16. Barker DJ, Gluckman PD, Robinson JS. Conference report: fetal origins of adult disease—report of the First International Study Group, Sydney, 29-30 October 1994. Placenta. 1995;16(3):317–320. [DOI] [PubMed] [Google Scholar]
- 17. Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185(1-2):135–144. [DOI] [PubMed] [Google Scholar]
- 18. Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006;572(pt 1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol. 2013;34(1):27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84(6):1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Odagiri E, Sherrell BJ, Mount CD, Nicholson WE, Orth DN. Human placental immunoreactive corticotropin, lipotropin, and beta-endorphin: evidence for a common precursor. Proc Nati Acad Sci U S A. 1979;76(4):2027–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramamoorthy S, Leibach FH, Mahesh VB, Ganapathy V. Active transport of dopamine in human placental brush-border membrane vesicles. Am J Physio. 1992;262(5 pt 1):C1189–C1196. [DOI] [PubMed] [Google Scholar]
- 24. Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X, Xie C, Zhang Y, Fan Z, Yin Y, Blachier F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids. 2015;47(1):45–53. [DOI] [PubMed] [Google Scholar]
- 27. Garces MF, Sanchez E, Torres-Sierra AL, et al. Brain-derived neurotrophic factor is expressed in rat and human placenta and its serum levels are similarly regulated throughout pregnancy in both species. Clin Endocrinol (Oxf). 2014;81(1):141–151. [DOI] [PubMed] [Google Scholar]
- 28. Prince CS, Maloyan A, Myatt L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta. 2017;49:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7(5):695–702. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida T, Ishikawa M, Niitsu T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7(8):e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mou Z, Hyde TM, Lipska BK, et al. Human obesity associated with an intronic SNP in the brain-derived neurotrophic factor locus. Cell Rep. 2015;13(6):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–123. [DOI] [PubMed] [Google Scholar]
- 33. Markham A, Cameron I, Bains R, et al. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. EurJ Neurosci. 2012;35(3):366–374. [DOI] [PubMed] [Google Scholar]
- 34. Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20(5):1189–1196. [DOI] [PubMed] [Google Scholar]
- 35. Fujita K, Tatsumi K, Kondoh E, et al. Differential expression and the anti-apoptotic effect of human placental neurotrophins and their receptors. Placenta. 2011;32(10):737–744. [DOI] [PubMed] [Google Scholar]
- 36. Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401–416. [DOI] [PubMed] [Google Scholar]
- 37. Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TRKB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22(3):399–408. [DOI] [PubMed] [Google Scholar]
- 38. Jang SW, Liu X, Yepes M, et al. A selective TRKB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Obianyo O, Chan CB, et al. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TRKB receptor. J Biol Chem. 2014;289(40):27571–27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agrawal R, Tyagi E, Vergnes L, Reue K, Gomez-Pinilla F. Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim Biophys Acta. 2014;1842(4):535–546. [DOI] [PubMed] [Google Scholar]
- 41. Chan CB, Tse MC, Liu X, et al. Activation of muscular TRKB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol. 2015;22(3):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bax CM, Ryder TA, Mobberley MA, Tyms AS, Taylor DL, Bloxam DL. Ultrastructural changes and immunocytochemical analysis of human placental trophoblast during short-term culture. Placenta. 1989;10(2):179–194. [DOI] [PubMed] [Google Scholar]
- 43. Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010;(46):e2511 DOI:10.3791/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maloyan A, Mele J, Muralimanohara B, Myatt L. Measurement of mitochondrial respiration in trophoblast culture. Placenta. 2012;33(5):456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han BH, D’Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7(1):38–53. [DOI] [PubMed] [Google Scholar]
- 47. Nakagawa T, Tsuchida A, Itakura Y, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49(3):436–444. [DOI] [PubMed] [Google Scholar]
- 48. Chen J, Chua KW, Chua CC, et al. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011;499(3):181–185. [DOI] [PubMed] [Google Scholar]
- 49. Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19(6):1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TRKB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121(5):1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cazorla M, Jouvenceau A, Rose C, et al. Cyclotraxin-B, the first highly potent and selective TRKB inhibitor, has anxiolytic properties in mice. PloS One. 2010;5(3):e9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. [DOI] [PubMed] [Google Scholar]
- 53. Chen LW, Lin MW, Hsu CM. Different pathways leading to activation of extracellular signal-regulated kinase and p38 MAP kinase by formyl-methionyl-leucyl-phenylalanine or platelet activating factor in human neutrophils. J Biomed Sci. 2005;12(2):311–319. [DOI] [PubMed] [Google Scholar]
- 54. Yang JM, Vassil AD, Hait WN. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol. 2001;60(4):674–680. [PubMed] [Google Scholar]
- 55. McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Molr Cell. 1999;4(4):585–595. [DOI] [PubMed] [Google Scholar]
- 57. Semple RK, Crowley VC, Sewter CP, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord. 2004;28(1):176–179. [DOI] [PubMed] [Google Scholar]
- 58. Crunkhorn S, Dearie F, Mantzoros C, et al. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282(21):15439–15450. [DOI] [PubMed] [Google Scholar]
- 59. Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci U S A. 2001;98(17):9713–9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fan M, Rhee J, St-Pierre J, et al. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18(3):278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66(3):562–573. [DOI] [PubMed] [Google Scholar]