Abstract
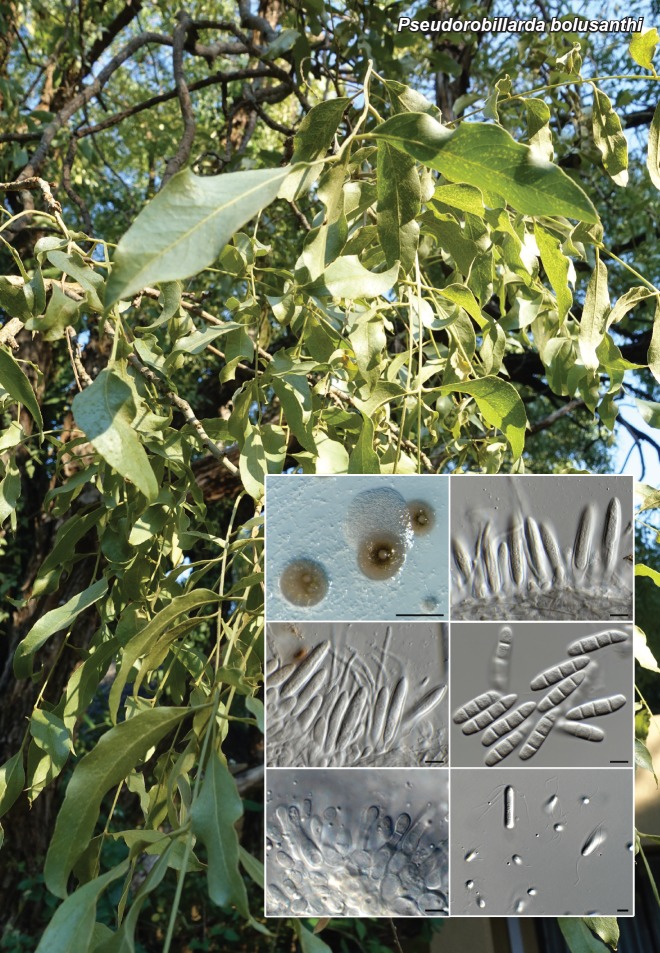
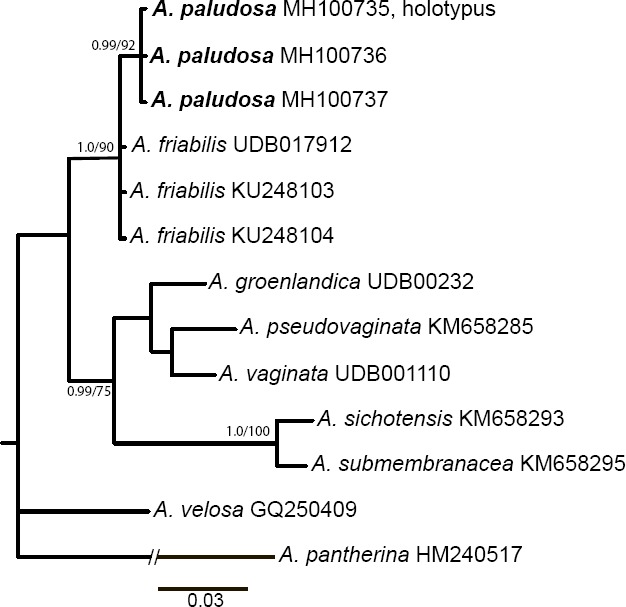
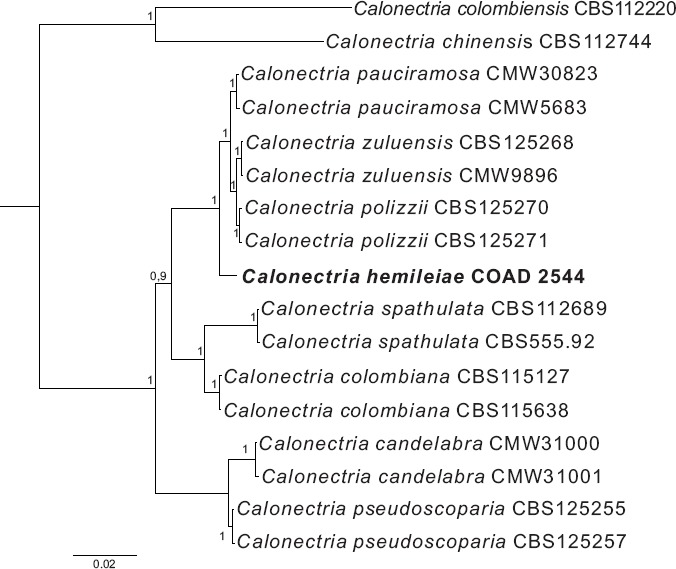
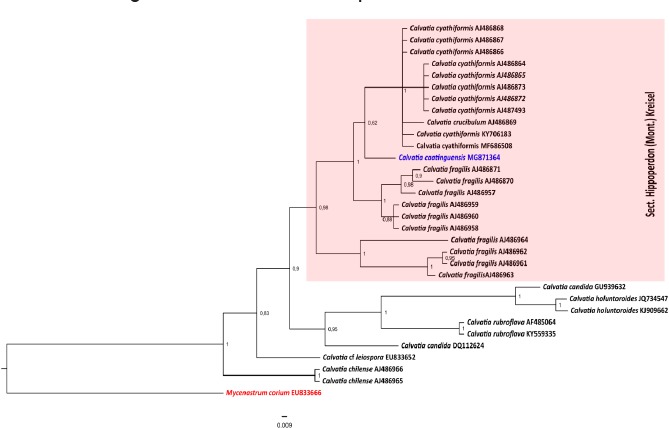
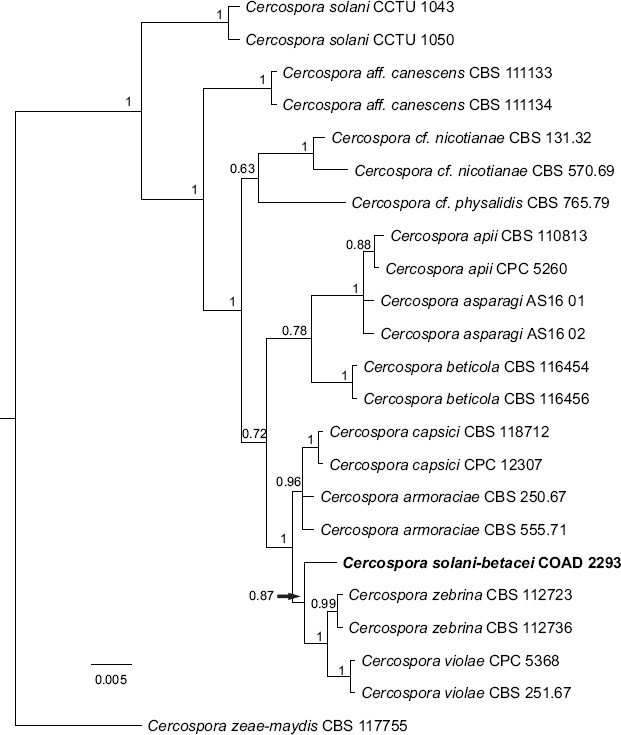
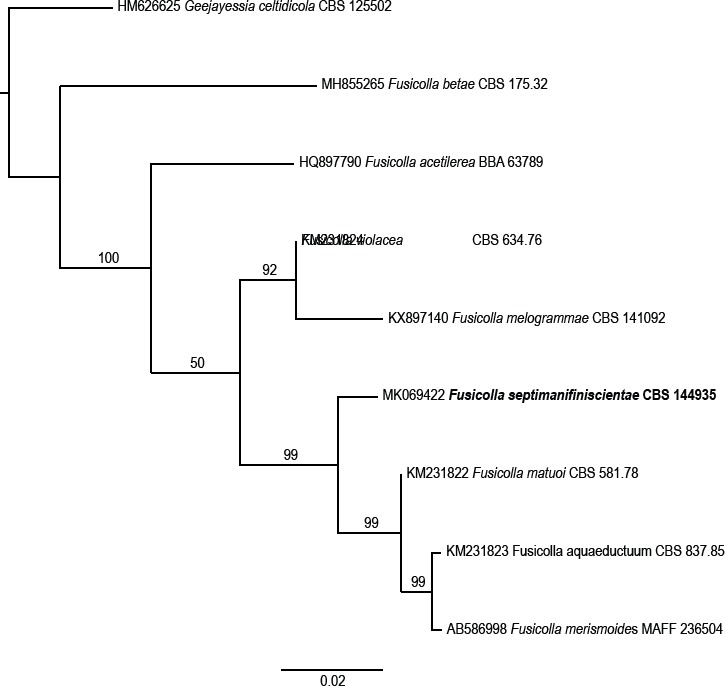
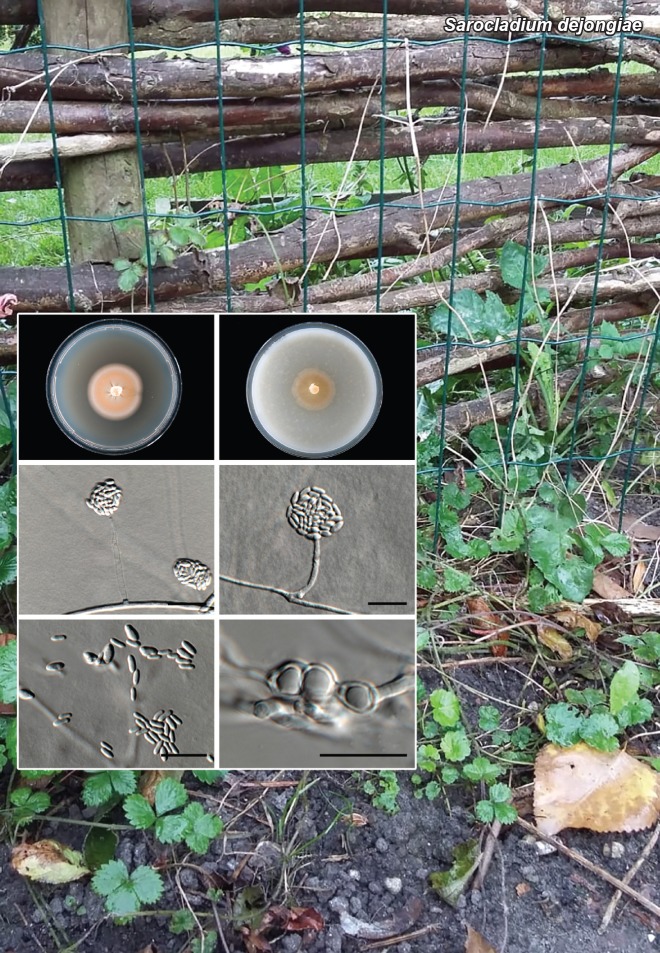
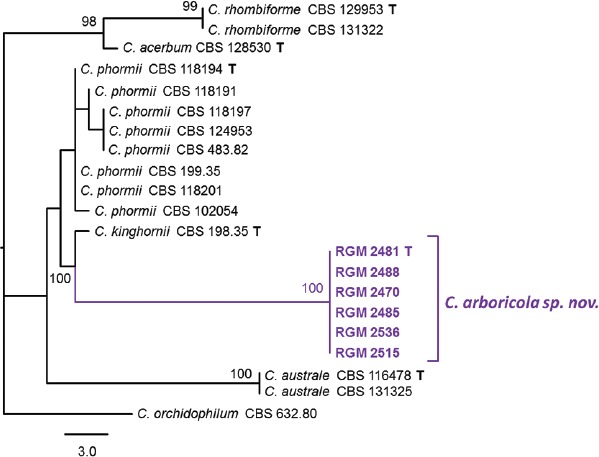
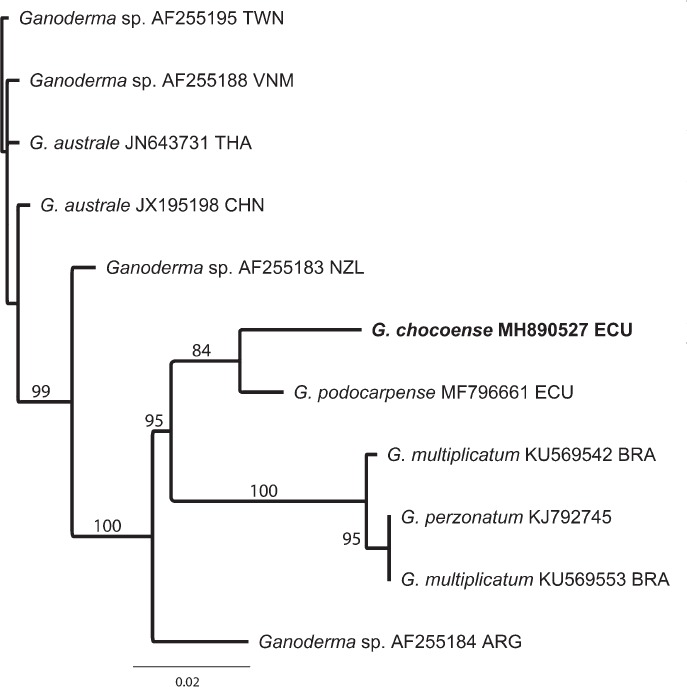
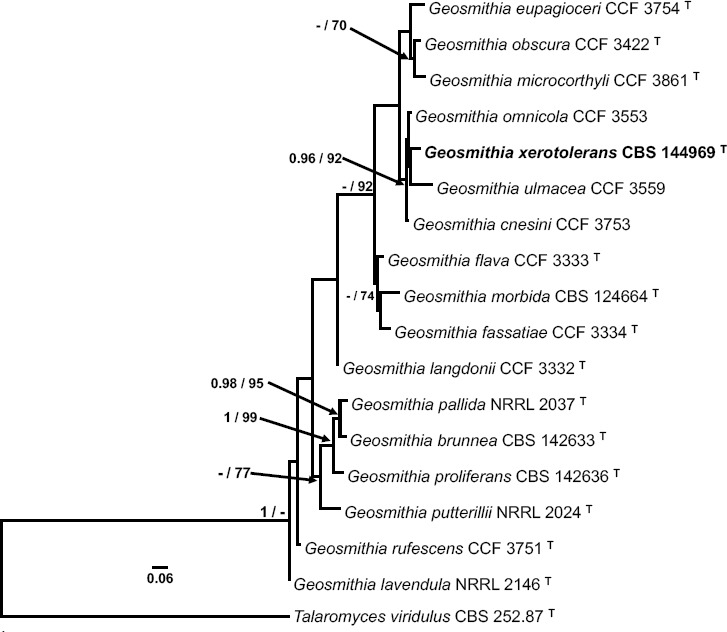
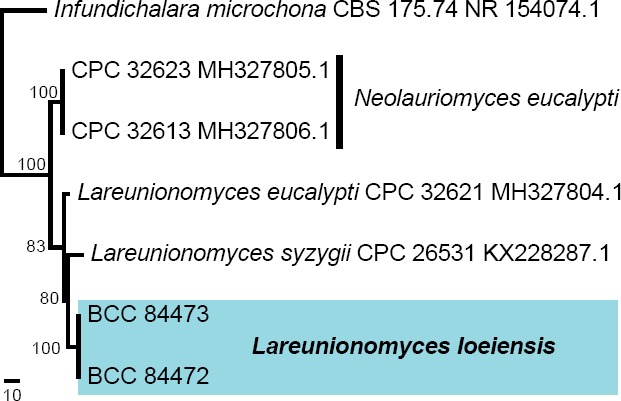
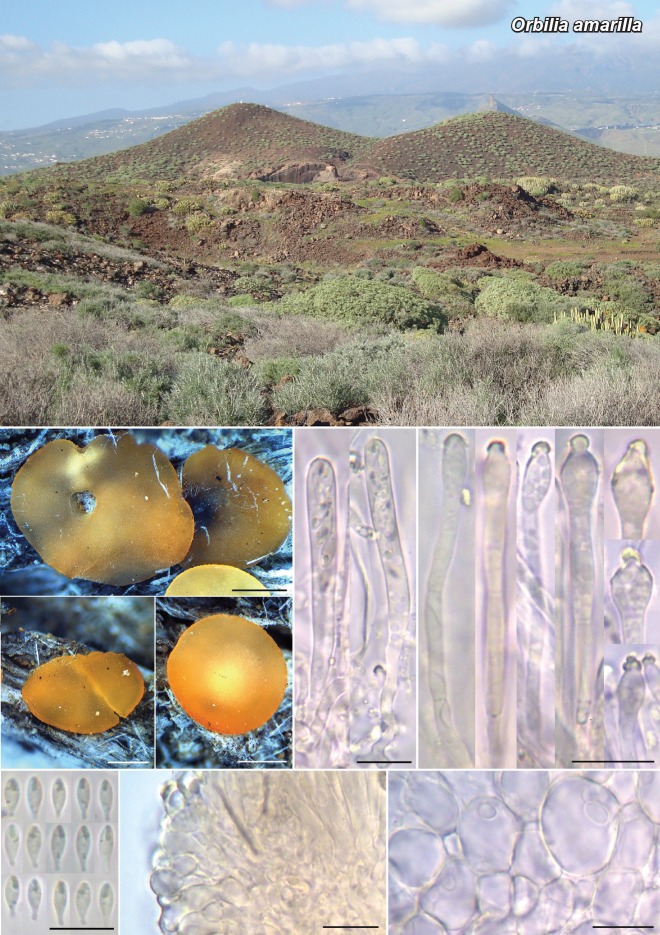
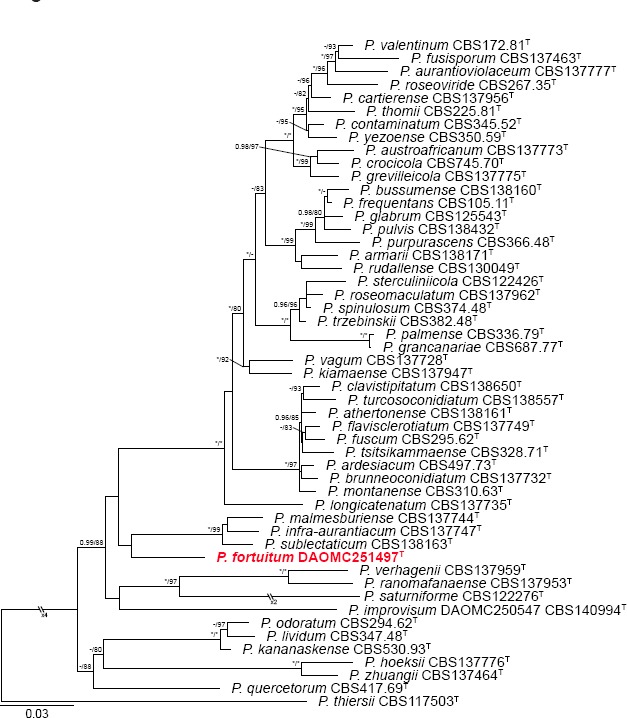
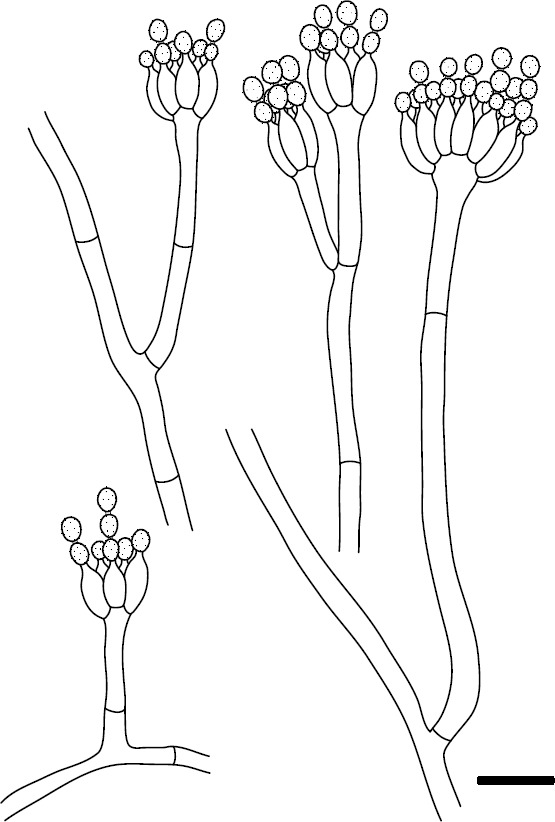
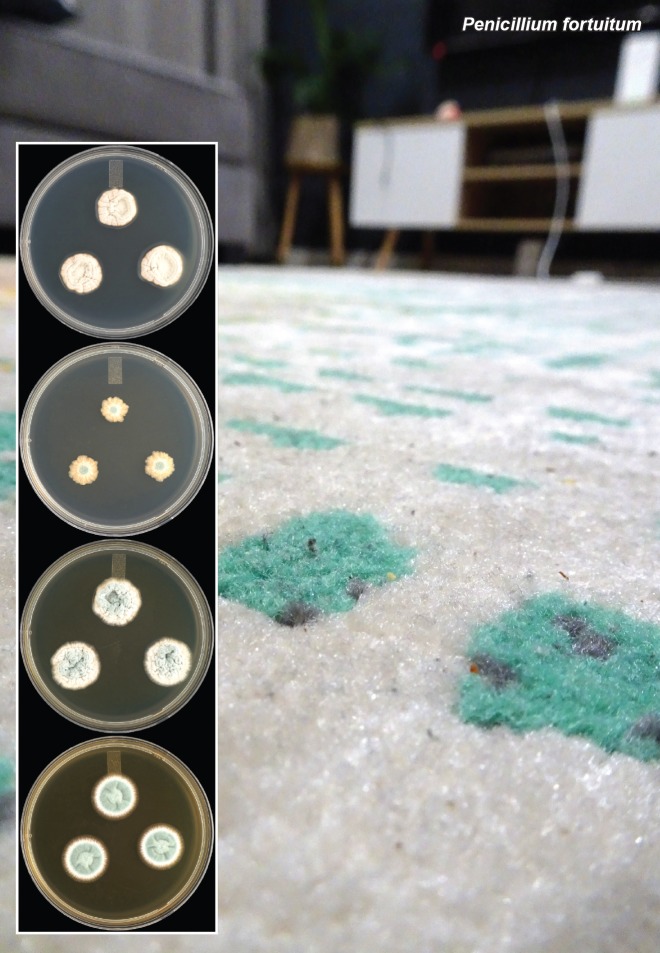
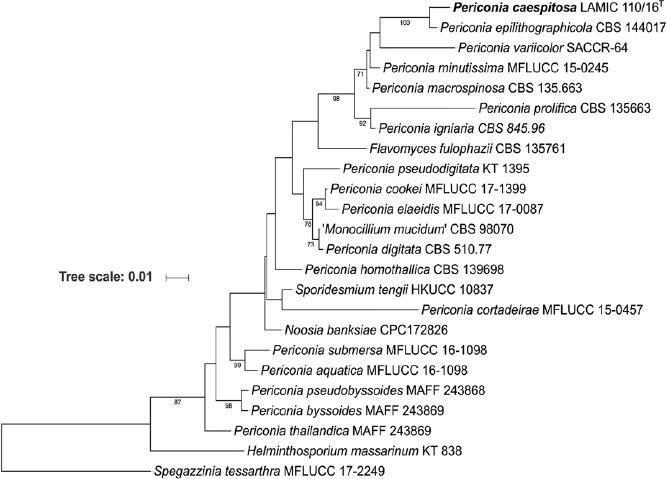
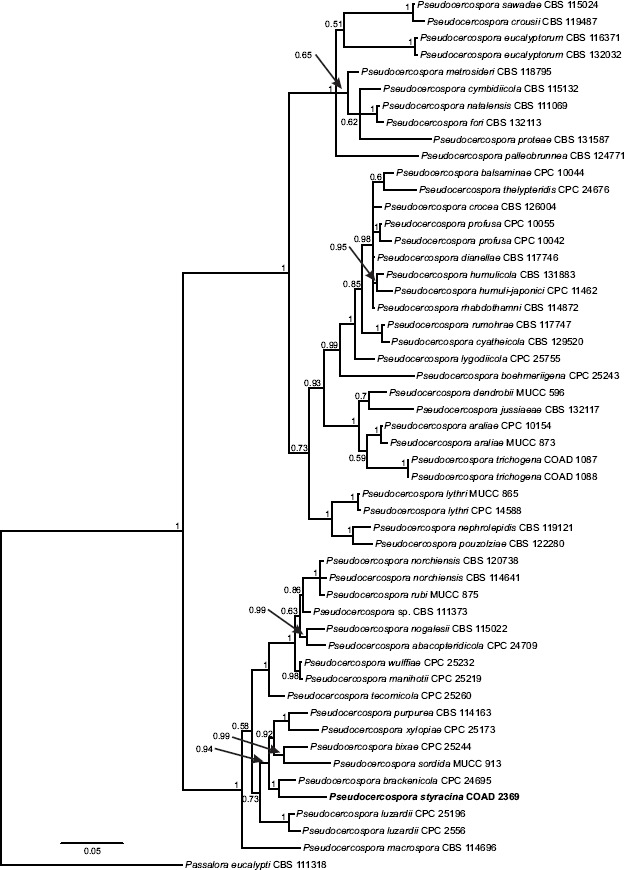
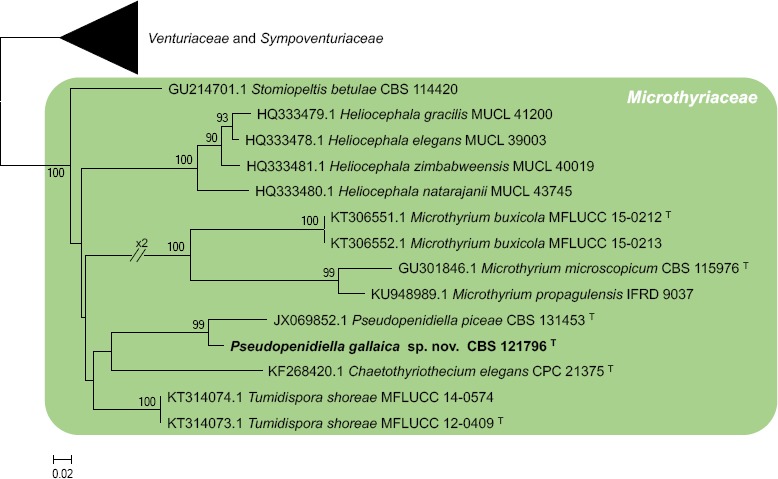
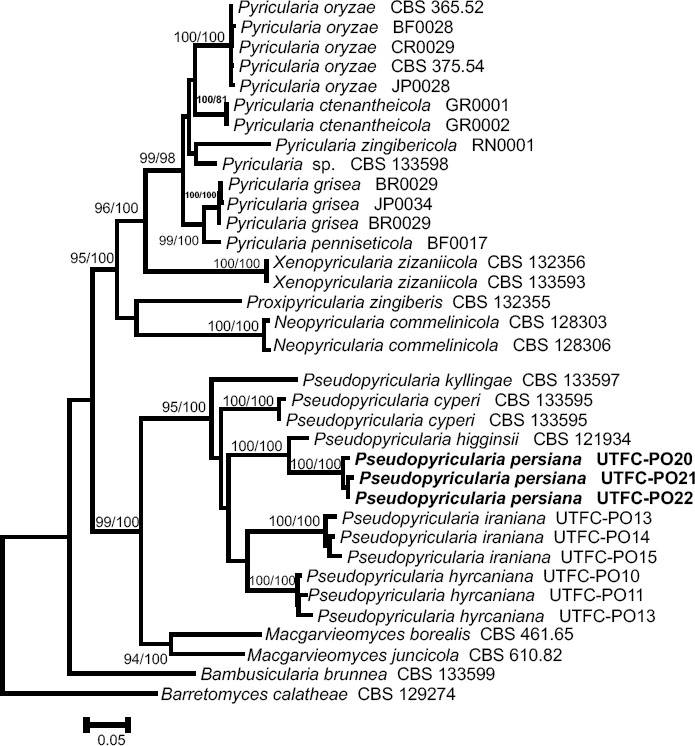
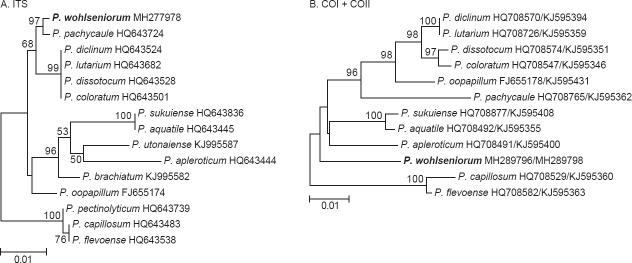
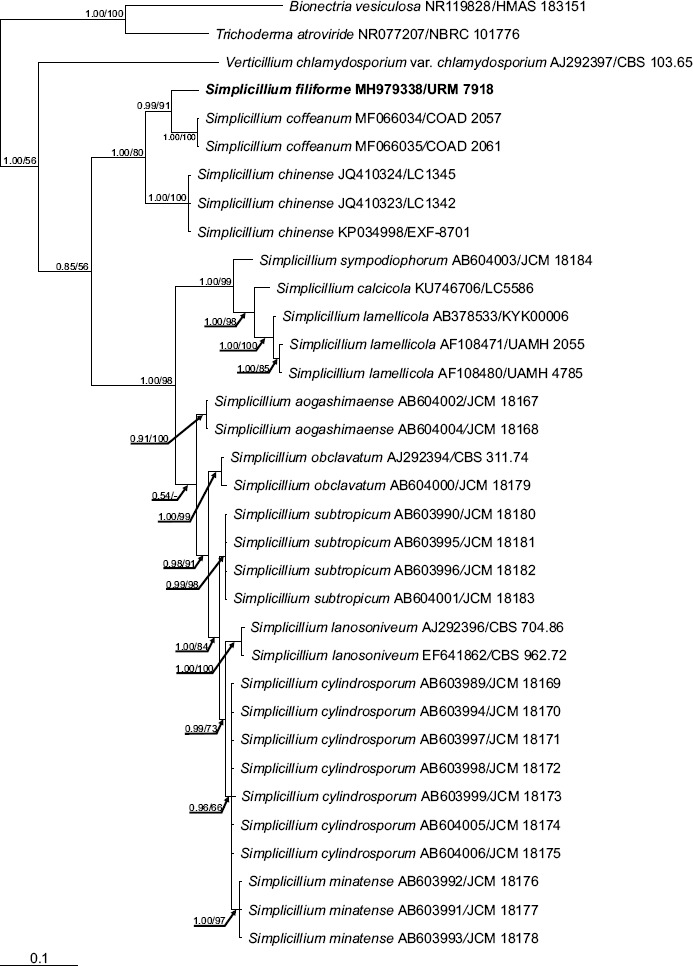
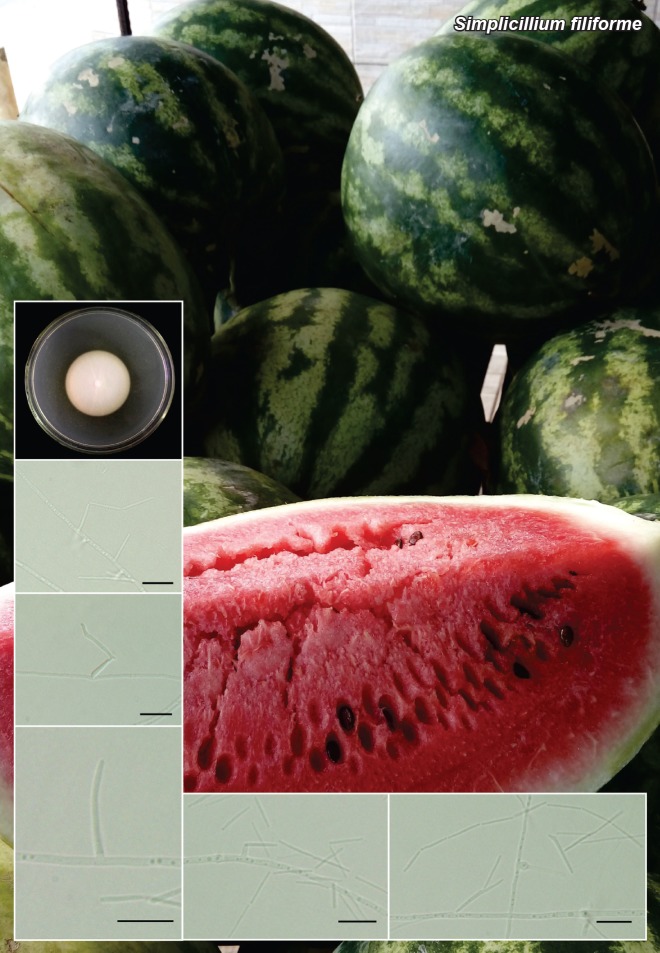
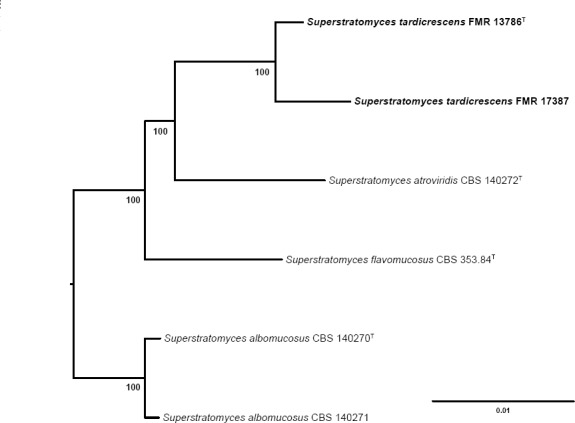
Novel species of fungi described in this study include those from various countries as follows: Angola, Gnomoniopsis angolensis and Pseudopithomyces angolensis on unknown host plants. Australia, Dothiora corymbiae on Corymbia citriodora, Neoeucasphaeria eucalypti (incl. Neoeucasphaeria gen. nov.) on Eucalyptus sp., Fumagopsis stellae on Eucalyptus sp., Fusculina eucalyptorum (incl. Fusculinaceae fam. nov.) on Eucalyptus socialis, Harknessia corymbiicola on Corymbia maculata, Neocelosporium eucalypti (incl. Neocelosporium gen. nov., Neocelosporiaceae fam. nov. and Neocelosporiales ord. nov.) on Eucalyptus cyanophylla, Neophaeomoniella corymbiae on Corymbia citriodora, Neophaeomoniella eucalyptigena on Eucalyptus pilularis, Pseudoplagiostoma corymbiicola on Corymbia citriodora, Teratosphaeria gracilis on Eucalyptus gracilis, Zasmidium corymbiae on Corymbia citriodora. Brazil, Calonectria hemileiae on pustules of Hemileia vastatrix formed on leaves of Coffea arabica, Calvatia caatinguensis on soil, Cercospora solani-betacei on Solanum betaceum, Clathrus natalensis on soil, Diaporthe poincianellae on Poincianella pyramidalis, Geastrum piquiriunense on soil, Geosmithia carolliae on wing of Carollia perspicillata, Henningsia resupinata on wood, Penicillium guaibinense from soil, Periconia caespitosa from leaf litter, Pseudocercospora styracina on Styrax sp., Simplicillium filiforme as endophyte from Citrullus lanatus, Thozetella pindobacuensis on leaf litter, Xenosonderhenia coussapoae on Coussapoa floccosa. Canary Islands (Spain), Orbilia amarilla on Euphorbia canariensis. Cape Verde Islands, Xylodon jacobaeus on Eucalyptus camaldulensis. Chile, Colletotrichum arboricola on Fuchsia magellanica. Costa Rica, Lasiosphaeria miniovina on tree branch. Ecuador, Ganoderma chocoense on tree trunk. France, Neofitzroyomyces nerii (incl. Neofitzroyomyces gen. nov.) on Nerium oleander. Ghana, Castanediella tereticornis on Eucalyptus tereticornis, Falcocladium africanum on Eucalyptus brassiana, Rachicladosporium corymbiae on Corymbia citriodora. Hungary, Entoloma silvae-frondosae in Carpinus betulus-Pinus sylvestris mixed forest. Iran, Pseudopyricularia persiana on Cyperus sp. Italy, Inocybe roseascens on soil in mixed forest. Laos, Ophiocordyceps houaynhangensis on Coleoptera larva. Malaysia, Monilochaetes melastomae on Melastoma sp. Mexico, Absidia terrestris from soil. Netherlands, Acaulium pannemaniae, Conioscypha boutwelliae, Fusicolla septimanifiniscientiae, Gibellulopsis simonii, Lasionectria hilhorstii, Lectera nordwiniana, Leptodiscella rintelii, Parasarocladium debruynii and Sarocladium dejongiae (incl. Sarocladiaceae fam. nov.) from soil. New Zealand, Gnomoniopsis rosae on Rosa sp. and Neodevriesia metrosideri on Metrosideros sp. Puerto Rico, Neodevriesia coccolobae on Coccoloba uvifera, Neodevriesia tabebuiae and Alfaria tabebuiae on Tabebuia chrysantha. Russia, Amanita paludosa on bogged soil in mixed deciduous forest, Entoloma tiliae in forest of Tilia × europaea, Kwoniella endophytica on Pyrus communis. South Africa, Coniella diospyri on Diospyros mespiliformis, Neomelanconiella combreti (incl. Neomelanconiellaceae fam. nov. and Neomelanconiella gen. nov.) on Combretum sp., Polyphialoseptoria natalensis on unidentified plant host, Pseudorobillarda bolusanthi on Bolusanthus speciosus, Thelonectria pelargonii on Pelargonium sp. Spain, Vermiculariopsiella lauracearum and Anungitopsis lauri on Laurus novocanariensis, Geosmithia xerotolerans from a darkened wall of a house, Pseudopenidiella gallaica on leaf litter. Thailand, Corynespora thailandica on wood, Lareunionomyces loeiensis on leaf litter, Neocochlearomyces chromolaenae (incl. Neocochlearomyces gen. nov.) on Chromolaena odorata, Neomyrmecridium septatum (incl. Neomyrmecridium gen. nov.), Pararamichloridium caricicola on Carex sp., Xenodactylaria thailandica (incl. Xenodactylariaceae fam. nov. and Xenodactylaria gen. nov.), Neomyrmecridium asiaticum and Cymostachys thailandica from unidentified vine. USA, Carolinigaster bonitoi (incl. Carolinigaster gen. nov.) from soil, Penicillium fortuitum from house dust, Phaeotheca shathenatiana (incl. Phaeothecaceae fam. nov.) from twig and cone litter, Pythium wohlseniorum from stream water, Superstratomyces tardicrescens from human eye, Talaromyces iowaense from office air. Vietnam, Fistulinella olivaceoalba on soil. Morphological and culture characteristics along with DNA barcodes are provided.
Keywords: ITS nrDNA barcodes, LSU, new taxa, systematics
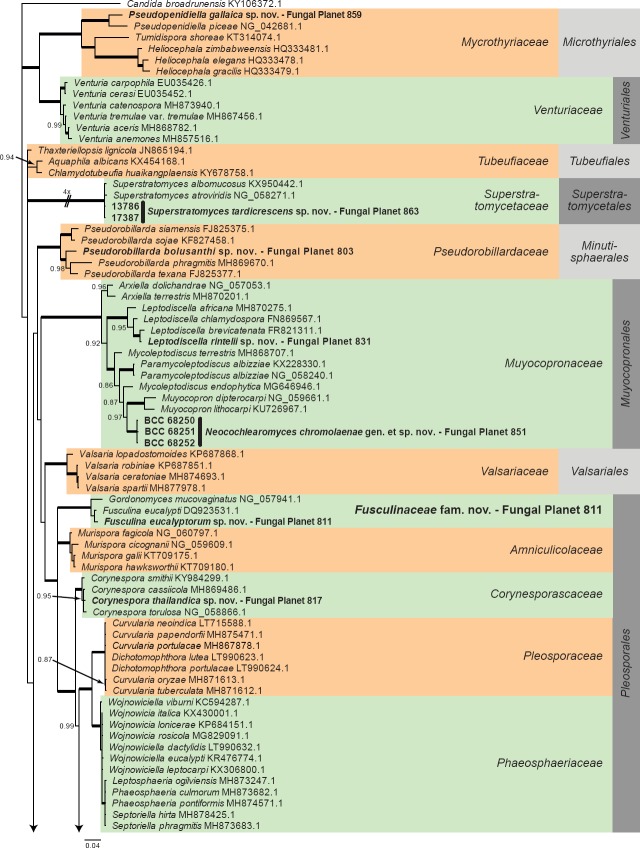
Overview Dothideomycetes phylogeny – part 1.
Consensus phylogram (50 % majority rule) of 2 478 trees resulting from a Bayesian analysis of the LSU sequence alignment (206 taxa including outgroup; 801 aligned positions; 464 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
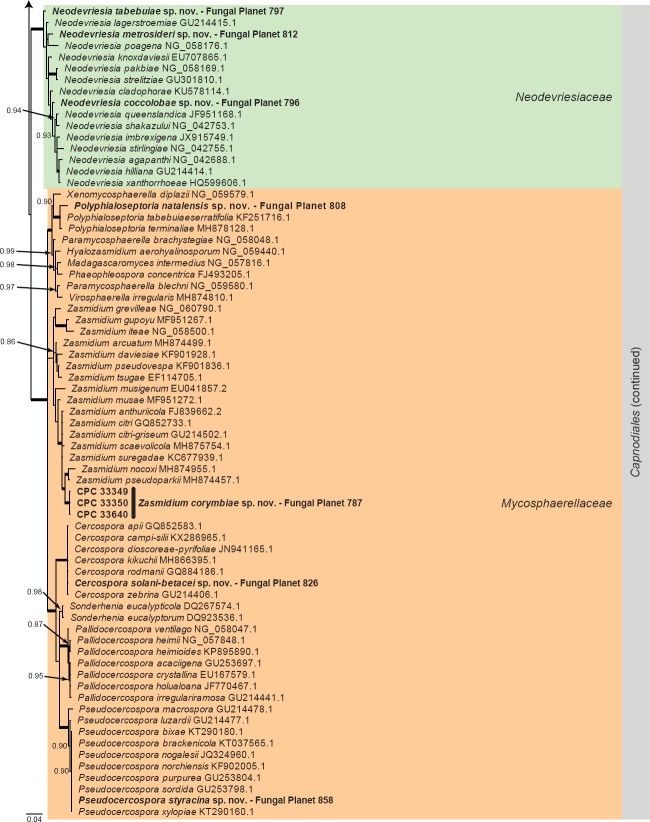
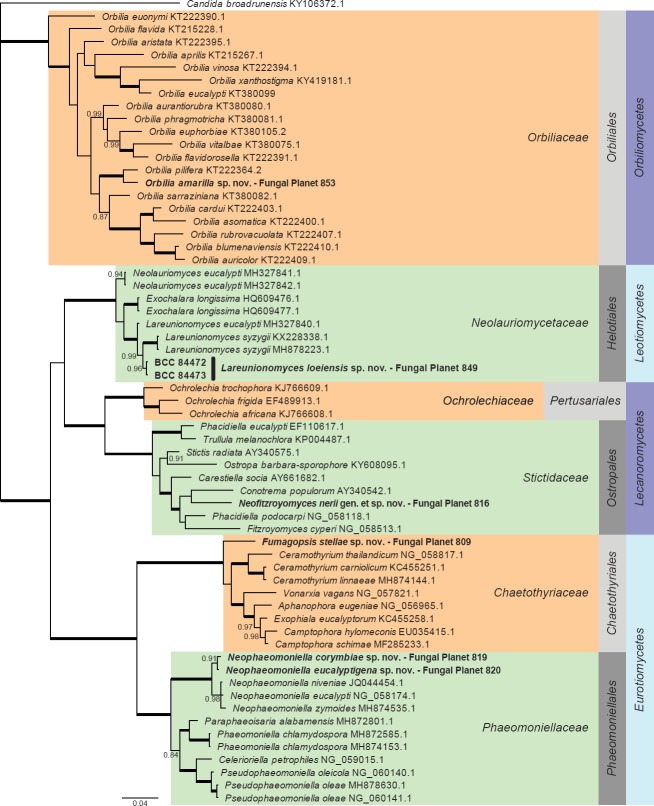
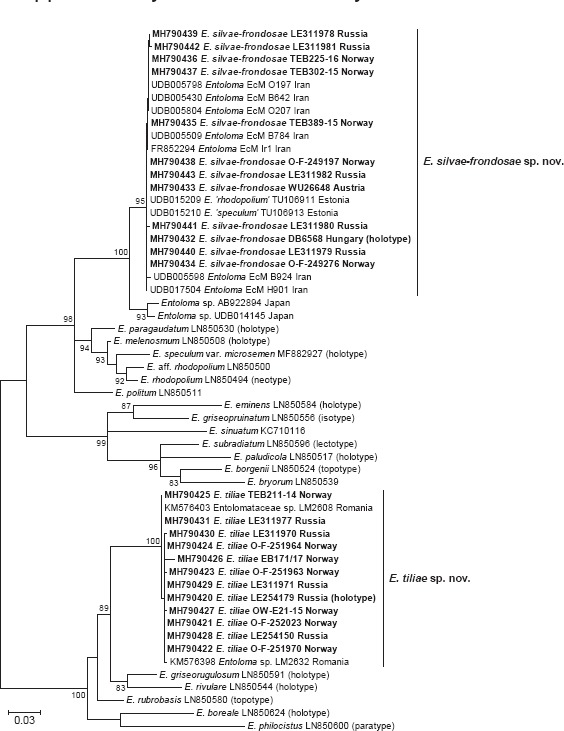
Overview Orbiliomycetes, Leotiomycetes, Lecanoromycetes and Eurotiomycetes phylogeny.
Consensus phylogram (50 % majority rule) of 12 452 trees resulting from a Bayesian analysis of the LSU sequence alignment (78 taxa including outgroup; 829 aligned positions; 360 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
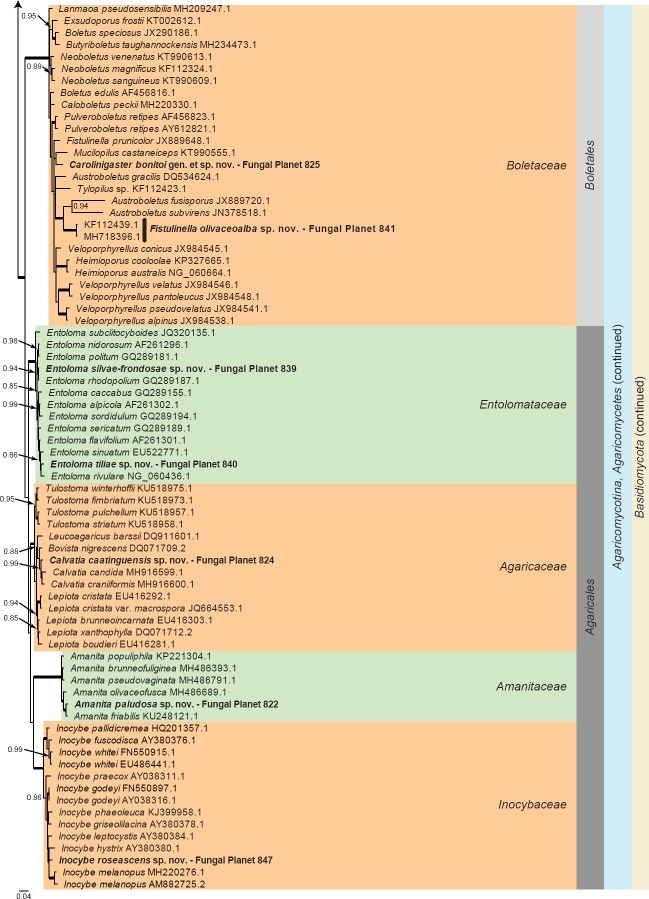
Overview Stramenopiles, Mucoromycota and Basidiomycota phylogeny – part 1.
Consensus phylogram (50 % majority rule) of 113 852 trees resulting from a Bayesian analysis of the LSU sequence alignment (141 taxa including outgroup; 980 aligned positions; 654 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders, classes, subdivisions and phyla are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to the Stramenopiles clade and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
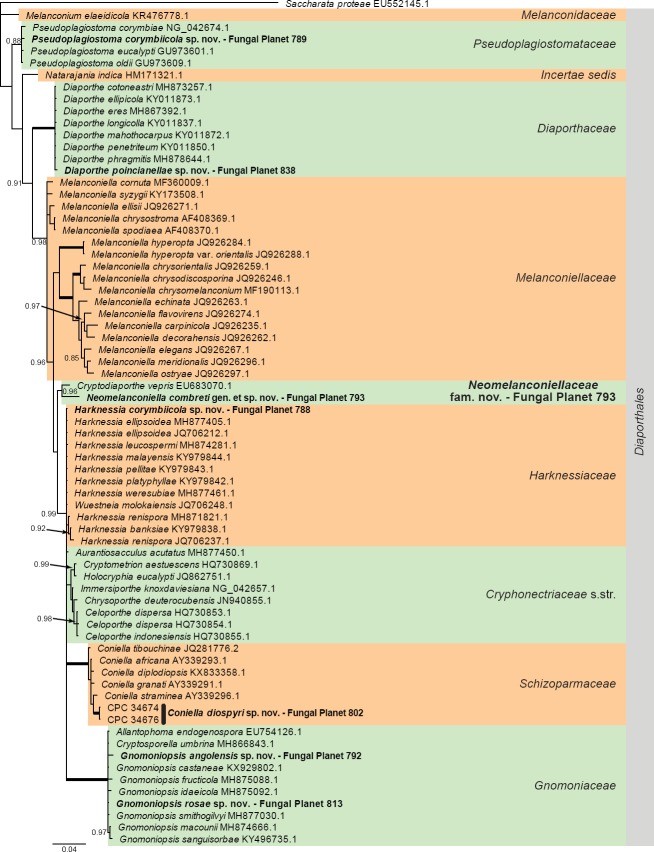
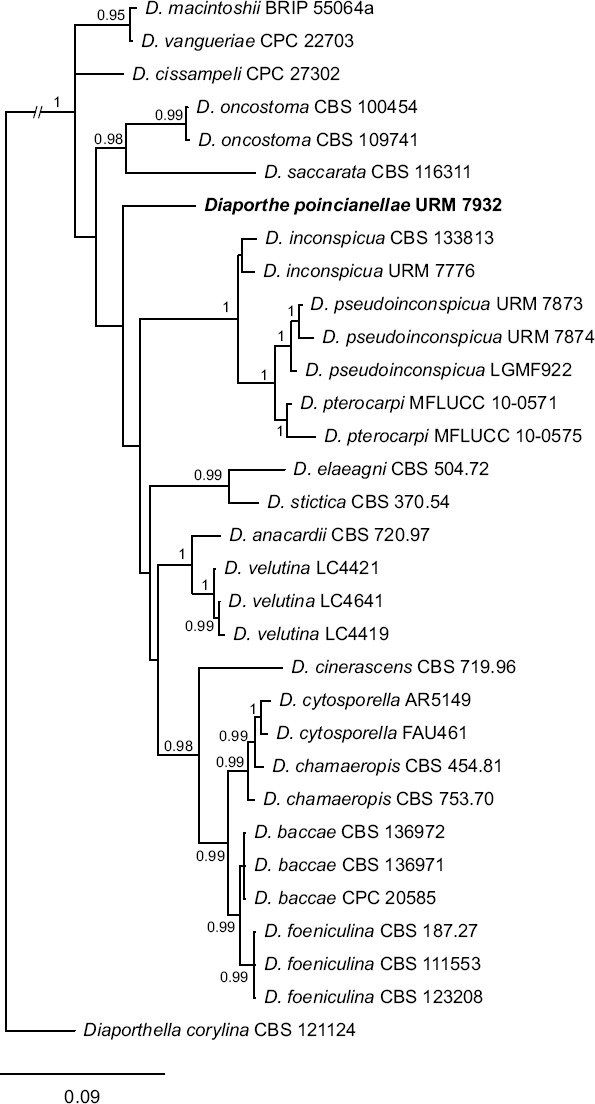
Overview Diaporthales (Sordariomycetes) phylogeny.
Consensus phylogram (50 % majority rule) of 1 052 trees resulting from a Bayesian analysis of the LSU sequence alignment (71 taxa including outgroup; 768 aligned positions; 176 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
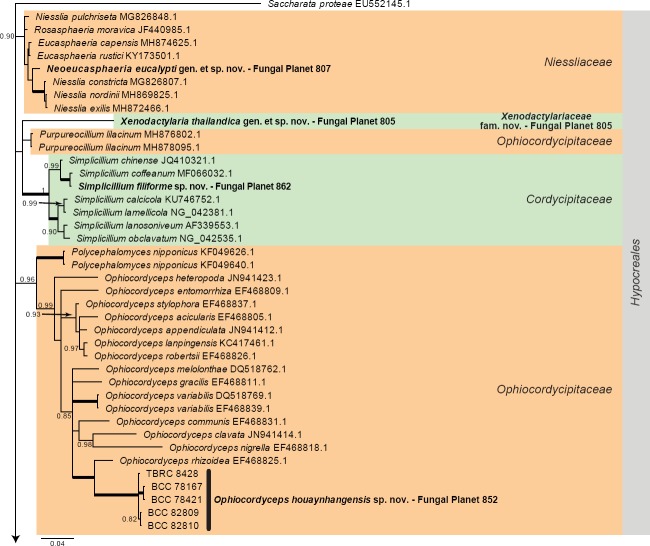
Overview Hypocreales (Sordariomycetes) phylogeny – part 1.
Consensus phylogram (50 % majority rule) of 3 078 trees resulting from a Bayesian analysis of the LSU sequence alignment (110 taxa including outgroup; 820 aligned positions; 339 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
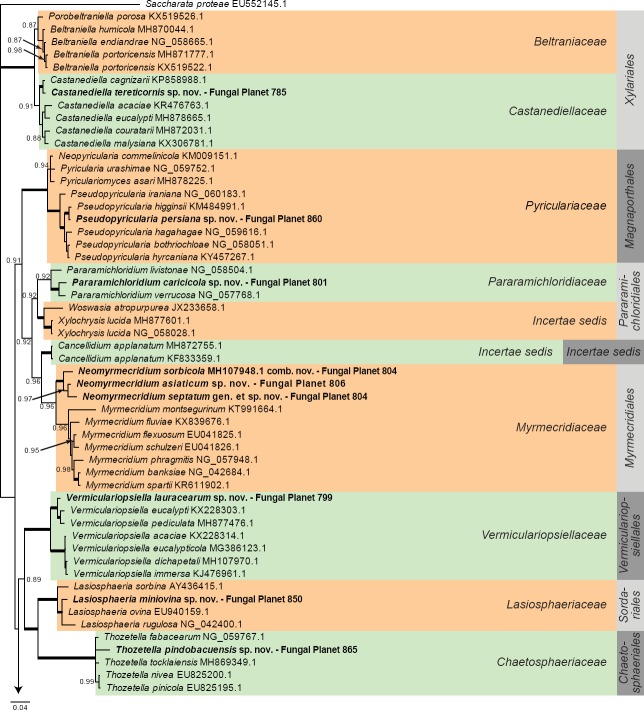
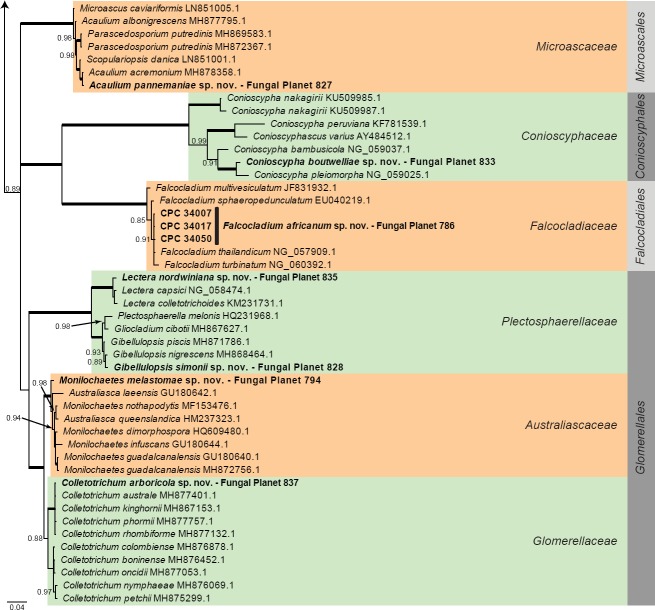
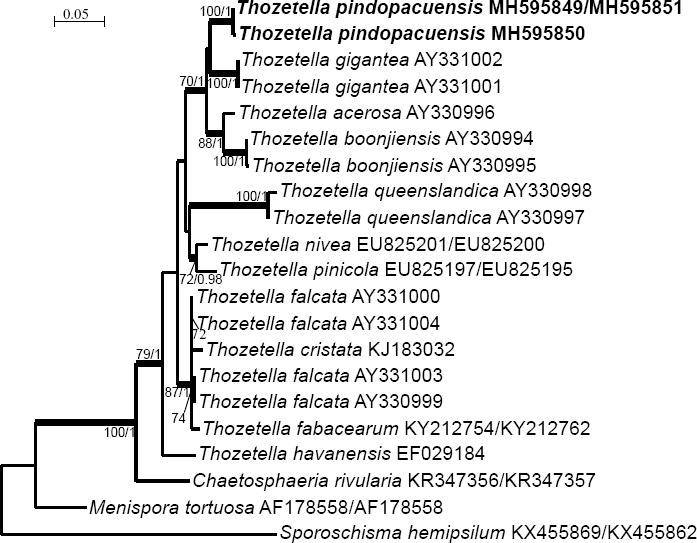
Overview other orders (Sordariomycetes) phylogeny – part 1.
Consensus phylogram (50 % majority rule) of 452 trees resulting from a Bayesian analysis of the LSU sequence alignment (102 taxa including outgroup; 782 aligned positions; 396 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S23436).
Acknowledgments
Tatiana M. Bulyonkova and colleagues are grateful to Dr Rodham Tulloss for his patient guidance and help, and to Dr Torbjørn Borgen Lindhardt for his invaluable advice. Thays G.L. Oliveira, Maria T.C. Felipe, Jadson D.P. Bezerra and Oliane M. C. Magalhães acknowledge financial support and/or scholarships from the CAPES (Finance Code 001), CNPq and FACEPE. Aline O.B. da Cunha, Alexandre R. Machado, Eder Barbier, Enrico Bernard and Cristina M. Souza-Motta acknowledge financial support and/or scholarships from the CAPES (Finance Code 001), CNPq, FACEPE, CECAV and ICMBio from Brazil. Rejane M.F. da Silva and colleagues express their gratitude to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a scholarship to Rejane M.F. da Silva and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a research fellowships and/or financial support to Gladstone A. da Silva, Cristina M. Souza-Motta, José L. Bezerra and Rafael J.V. de Oliveira (Processes 458622/2014-1 and 312186/2016-9). Olinto L. Pereira, Vanessa P. Abreu, Jackeline P. Andrade and colleagues would like to thank the CNPq, CAPES and FAPEMIG for financial support. The study of Olga V. Morozova was carried out within the framework of a research project of the Komarov Botanical Institute RAS ‘Herbarium funds of the BIN RAS’ (AAAA-A18-118022090078-2) with the support of the molecular work by the Russian Foundation for the Basic Research (project no. 15-29-02622). Anna M. Glushakova and Aleksey V. Kachalkin were supported by the Russian Foundation for Basic Research (RFBR), project no. 16-04-00624a. Janet Jennifer Luangsa-ard and colleagues were supported by ‘The Promotion Project on Science, Technology and Innovation Collaboration with ASEAN Member Countries under the Office of International Cooperation, MOST-Thailand’. They would also like to thank Ms Duangkaew Chongkachornphong, Ms Papawee Nupason (International Cooperation Section, BIOTEC) and Ms Bakeo Souvannalath (Director of Biotechnology Division, Biotechnology and Ecology Institute, BEI) for their kind cooperation. Javier Fernández-López and colleagues are grateful to Marian Glenn for checking the text, and were supported by DGICT projects CGL2012-35559 and CGL2015-67459-P. Javier Fernández-López was also supported by Predoctoral Grants (BES-2013-066429) from the Ministerio de Economía y Competitividad (Spain). Maria E. Ordoñez and colleagues acknowledge Pontificia Universidad Católica del Ecuador for financial support for project M13415. Taimy Cantillo is thankful to PEC-PG/CAPES for the PhD grant (proc. 12636134/2014) (Finance Code 001) and to the International Association for Plant Taxonomy (IAPT) for the Research Grant. Luis F.P. Gusmão is grateful to CNPq for Grant support (Proc. 303062/2014-2). Hugo Madrid was partially funded by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, project no. 11140562. Tor Erik Brandrud, Bálint Dima, Machiel E. Noordeloos and Egil Bendiksen thank the financial support of the Norwegian Taxonomy Initiative, with funding from the Norwegian Biodiversity Information Centre (NBIC); the majority of the Oslofjord material was sequenced through NorBOL (collections labelled NOBAS, CAFUN), and we thank Gunnhild Marthinsen and Katriina Bendiksen, NHM, University of Oslo as well as Rakel Blaalid, NINA, for performing the major work with the barcoding; the Kits van Waveren Foundation (Rijksherbariumfonds Dr E. Kits van Waveren, Leiden, Netherlands) contributed substantially to the costs of sequencing types. The Austrian Entoloma material (by Irmgard Krisai-Greilhuber) was sequenced within ABOL, subproject HRSFM University of Vienna, supported by the Austrian Federal Ministry of Education, Science and Research. Adriene M. Soares and colleagues would like to thank the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and the Instituto Brasileiro de Meio Ambiente (IBAMA) for support during field trips and R.L.M. Alvarenga for the figures. They also acknowledge CAPES for the Ph.D. scholarship of Adriene M. Soares, and CNPq (307601/2015-3), CAPES (CAPES-SIU 008/13), and FACEPE (APQ-0375-2.03/15) for financial support. Angus J. Carnegie acknowledges support from the Forestry Corporation of NSW, and David Sargeant for assistance with site photos. Adel Pordel and colleagues thank the University of Tehran for financial support. Luis Quijada acknowledges support from ‘Fundación Ramón Areces’. Robert W. Barreto and colleagues thank the World Coffee Research/Texas Agrilife for financial support, as well as the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Sara Salcedo-Sarmiento was supported by the ‘Programa de Estudante-Convênio de Pós-Graduação’ (PEC-PG) from CAPES. The research of Cobus M. Visagie and Keith A. Seifert was supported by grants from the Alfred P. Sloan Foundation Program on the Microbiology of the Built Environment. Blaise A. Darvaux acknowledges Keith A. Seifert for help with identification, Nicholas Mauriello for validating the Latin name, Mauricia Lawrence and Meagan Tillotson for help with material preparation. We are grateful to Gavin Phillips, Seed Bank Officer, Australian Botanic Garden, Mt Annan for field assistance and identification of plant species collected in New South Wales, Australia. Collection of specimens from Mungo National Park was supported by the ABRS Bush Blitz program, a partnership between the Australian Government, BHP and Earthwatch Australia. The National Geographic Okavango Wilderness Project is acknowledged for assistance and funding to J. Roux for material collected in Angola.
REFERENCES
- Abarca GH, Castañeda-Ruiz RF, Arias-Mota RA, et al. 2011. A new species of Heliocephala from México with an assessment of the systematic positions of the anamorph genera Heliocephala and Holubovaniella. Mycologia 103:631–640. [DOI] [PubMed] [Google Scholar]
- Alessio CL, Rebaudengo E. 1980. Inocybe, Iconographia Mycologica 29, Suppl. 3, Bd. 1 (Generalia et Descriptiones), Bd. 2 (Tabulae), Trento. [Google Scholar]
- Alfenas RF, Lombard L, Pereira OL, et al. 2015. Diversity and potential impact of Calonectria species in eucalyptus plantations in Brazil. Studies in Mycology 80:89–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfredo DS, Sousa JO, Souza EJ, et al. 2016. Novelties of gasteroid fungi, earthstars and puffballs, from the Brazilian Atlantic rainforest. In: de Madrid Anales del Jardín Botánico, Vol. 73 (2). Consejo Superior de Investigaciones Científicas. [Google Scholar]
- Alpago Novello L. 2006. Funghi rari e poco conosciuti della sinistra Piave in Valbelluna. Tipografia Milani, Verona. [Google Scholar]
- Alvarez LV, Groenewald JZ, Crous PW.2016. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Studies in Mycology 85:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, et al. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58:57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A, et al. 2014. Multi-gene analyses of Pseudocercospora spp. from Iran. Phytotaxa 184:245–264. [Google Scholar]
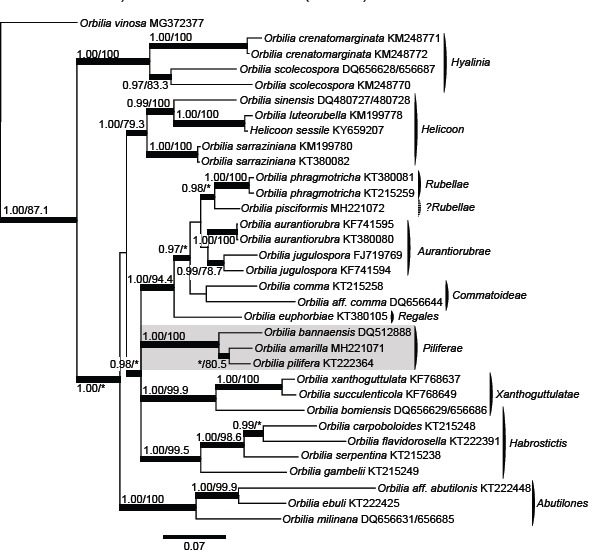
- Baral HO, Weber E, Gams W, et al. 2017. Generic names in the Orbiliaceae (Orbiliomycetes) and recommendations on which names should be protected or suppressed. Mycological Progress 17:5–31. [Google Scholar]
- Baseia IG, Calonge FD.2006. Geastrum hirsutum: a new earthstar fungus with a hairy exoperidium. Mycotaxon 95:301–304. [Google Scholar]
- Batista AC, Maia H da Silva. 1959. Uma nova doença fúngica de peixe ornamental. Anais da Sociedade de Biologia de Pernambuco 16: 153–159. [Google Scholar]
- Bizio E.2012. Considerazioni su alcune raccolte del genere Inocybe eseguite durante i lavori del 27° Comitato Scientifico A.G.M.T. 2010 di Piombino. Annali Micologici A.G.M.T. 5:47–57. [Google Scholar]
- Bon M.1997. Clé monographique du genre Inocybe (Fr.) Fr. -2ème partie: sous-genre Inocybe = Inocybium (Earle) Sing. Documents Mycologique 27: 1–77. [Google Scholar]
- Bosc L.1811. Mémoire sur quelques espèces de Champignons des parties méridionales de l’Amérique septentrionale. Magazin der Gesellschaft Naturforschenden Freunde Berlin 583 [Google Scholar]
- Bose T, Reynolds DR, Berbee ML.2014. Common, unsightly and until now undescribed: Fumiglobus pieridicola sp. nov., a sooty mold infesting Pieris japonica from western North America. Mycologia 106:746–756. [DOI] [PubMed] [Google Scholar]
- Bottomley AM.1948. Gasteromycetes of South Africa. Bothalia 4:473–810. [Google Scholar]
- Boursier J, Kuühner R.1928. Notes sur le genre Inocybe. Bulletin de la Société Mycologique de France 44:170–189. [Google Scholar]
- Brandrud TE, Bendiksen E, Jordal JB, et al. 2018. Entoloma species of the rhodopolioid clade (subgenus Entoloma; Tricholomatinae, Basidiomycota) in Norway. Agarica 38:21–46. [Google Scholar]
- Braun U, Crous PW, Nakashima C.2016. Cercosporoid fungi (Mycosphaerellaceae) 5. Species on dicots (Anacardiaceae to Annonaceae). IMA Fungus 7:161–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Nakashima C, Crous PW.2013. Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus 4:265–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon P, Buddie AG, Bridge PD, et al. 2012. Lectera, a new genus of the Plectosphaerellaceae for the legume pathogen Volutella colletotrichoides. MycoKeys 3:23–36. [Google Scholar]
- Cantrell SA, Hanlin RT, Emiliano A.2007. Periconia variicolor sp. nov., a new species from Puerto Rico. Mycologia 99:482–487. [DOI] [PubMed] [Google Scholar]
- Chachuła P, Vončina G, Kozik J.2011. Ophiocordyceps stylophora (Ascomycota, Hypocreales), new species for Poland. Polish Botanical Journal 56:321–326. [Google Scholar]
- Chaverri P, Salgado C, Hirooka Y, et al. 2011. Delimitation of Nectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology 68:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Verkley GJM, et al. 2010. Re-evaluation of Cryptosporiopsis eucalypti and Cryptosporiopsis-like species occurring on Eucalyptus. Fungal Diversity 44:89–105. [Google Scholar]
- Chen CC, Wu SH, Chen CY.2017. Three new species of Hyphodontia s.l. (Basidiomycota) with poroid or raduloid hymenophore. Mycological Progress 16:553–564. [Google Scholar]
- Chuaseeharonnachai C, Somrithipol S, Suetrong S, et al. 2017. Conioscypha nakagirii, a new species from naturally submerged wood in Thailand based on morphological and molecular data. Mycoscience 58:424–431. [Google Scholar]
- Chupp C. 1954. A monograph of the fungus genus Cercospora. Published by the author, Ithaca, New York, USA. [Google Scholar]
- Coker WC, Couch NC. 1928. The gasteromycetes of the eastern United States and Canada. Dover Publications, Inc; New York. [Google Scholar]
- Coronado-Ruiz C, Avendaño R, Escudero-Leyva E, et al. 2018. Two new cellulolytic fungal species isolated from a 19th-century art collection. Scientific Reports 87492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, St. Paul, Minnesota, USA. [Google Scholar]
- Crous PW, Braun U.2003. Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1:1–571.CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. 2013. Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75:37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, et al. 2007a. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58:33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, et al. 2009. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22:139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W.2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39:112–118. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50:19–22. [Google Scholar]
- Crous PW, Groenewald JZ.2016. They seldom occur alone. Fungal Biology 120:1392–1415. [DOI] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ.2017. The genera of fungi – G 4: Camarosporium and Dothiora. IMA Fungus 8:131–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Himaman W. 2007b. Falcocladium thailandicum. Fungal Planet No. 18. CBS-KNAW, Utrecht, Netherlands. [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG.2010. Phaeothecoidea melaleuca Crous & R.G. Sivas, sp. nov. Fungal Planet 61. Persoonia 25:142–143. [Google Scholar]
- Crous PW, Kendrick WB, Alfenas AC.1997. New species of hyphomycetes associated with Eucalyptus. South African Journal of Botany 63:286–290. [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2015a. Fungal Systematics and Evolution: FUSE 1. Sydowia 67:81–118. [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2018a. New and interesting fungi. 1. Fungal Systematics and Evolution 1:169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014a. Fungal Planet description sheets: 214–280. Persoonia 32:184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012a. A re-appraisal of Harknessia (Diaporthales), and the introduction of Harknessiaceae fam. nov. Persoonia 28:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012b. Fungal Planet description sheets: 107–127. Persoonia 28:138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Alfenas AC, et al. 1994. Cylindrocladium naviculatum sp. nov., and two new vesiculate Hyphomycete genera, Falcocladium and Vesicladiella. Mycotaxon 50:441–458. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2016a. Fungal Planet description sheets: 469–557. Persoonia 37:218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017a. Fungal Planet description sheets: 558–624. Persoonia 38:240–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017b. Fungal Planet description sheets: 625–715. Persoonia 39:270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2018b. Fungal Planet description sheets: 716–784. Persoonia 40:240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015b. Fungal Planet description sheets: 320–370. Persoonia 34:167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016b. Fungal Planet description sheets: 400–468. Persoonia 36:316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Schumacher RK, et al. 2014b. Fungal Planet description sheets: 281–319. Persoonia 33:212–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73:37–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R. et al. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS.1985. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology 26:1–60. [Google Scholar]
- De Hoog GS, Beguin H, Batenburg-Van de Vegte WH.1997. Phaeotheca triangularis, a new meristematic black yeast from a humidifier. Antonie van Leeuwenhoek 71:289–295. [DOI] [PubMed] [Google Scholar]
- DesRochers P, Ouellette GB.1994. Phaeotheca dimorphospora sp. nov.: description et caractéristiques culturales. Canadian Journal of Botany 72:808–817. [Google Scholar]
- Dissing H, Lange M.1962. Gasteromycetes of Congo. Bulletin du Jardin Botanique de l’État a Bruxelles 32:325–416. [Google Scholar]
- Domsch KH, Gams W, Anderson TH. 2007. Compendium of soil fungi, 2nd edn IHW Verlag, Germany. [Google Scholar]
- Douanla-Meli C, Langer E, Calonge FD.2005. Geastrum pleosporus sp. nov., a new species of Geastraceae identified by morphological and molecular phylogenetic data. Mycological Progress 4:239–250. [Google Scholar]
- Dring DM.1980. Contributions towards a rational arrangement of the Clathraceae. Kew Bulletin 35:1–96. [Google Scholar]
- Ellis MB. 1971. Dematiaceus Hyphomycetes. Commonwealth Mycological Institute, Kew, England. [Google Scholar]
- Ellis MB. 1976. More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, England. [Google Scholar]
- Evans HC, Araújo JPM, Halfeld VR, et al. 2018. Epitypification and re-description of the zombie-ant fungus, Ophiocordyceps unilateralis (Ophiocordycipitaceae). Fungal Systematics and Evolution 1:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr DF, Rossman A.Y 2018. Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved January 20, 2018, from http://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Fazolino EP, Trierveiler-Pereira L, Calonge FD, et al. 2010. First records of Clathrus (Phallaceae, Agaricomycetes) from the northeast region of Brazil. Mycotaxon 113:195–202. [Google Scholar]
- Fulgenzi TD, Halling RE, Henkel TW.2010. Fistulinella cinereoalba sp. nov. and new distribution records for Austroboletus from Guyana. Mycologia 102:224–232. [DOI] [PubMed] [Google Scholar]
- Gams W. 1971. Cephalosporium-artige Schimmelpilze (Hyphomycetes). Fischer Verlag, Stuttgart, Germany. [Google Scholar]
- Gao Y, Liu F, Duan W, et al. 2017. Diaporthe is paraphyletic. IMA Fungus 8:153–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W, Nirenberg H.1982. The genus Fusarium – a pictorial atlas. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 209:1–406. [Google Scholar]
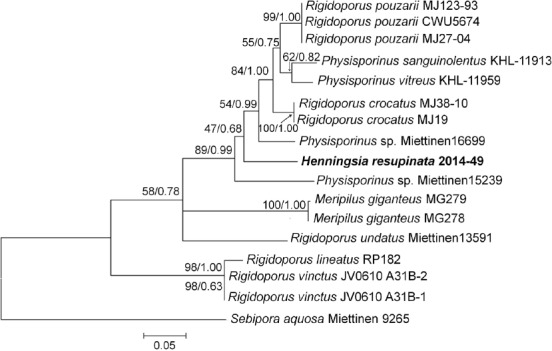
- Gibertoni TB, Ryvarden L.2014. Studies in Neotropical polypores 36. A note on the genus Henningsia. Synopsis Fungorum 32:55–57. [Google Scholar]
- Giraldo A, Crous PW.2019. Inside Plectosphaerellaceae. Studies in Mycology 92:227–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo A, Gené J, Sutton DA, et al. 2015. Phylogeny of Sarocladium (Hypocreales). Persoonia 34:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AAM, Pinho DB, Cardeal ZL, et al. 2018. Simplicillium coffeanum, a new endophytic species from Brazilian coffee plants, emitting antimicrobial volatiles. Phytotaxa 333:188–198. [Google Scholar]
- Gottlieb A, Wright J.1999. Taxonomy of Ganoderma from southern South America: subgenus Elfvingia. Mycological Research 103:1289–1298. [Google Scholar]
- Gouy M, Guindon S, Gascuel O.2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27:221–224. [DOI] [PubMed] [Google Scholar]
- Gräfenhan T, Schroers H-J, Nirenberg HI, et al. 2011. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68:79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, et al. 2013. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75:115–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia V, Sandoval-Denis M, Aiello D, et al. 2018. Neocosmospora perseae sp. nov., causing trunk cankers on avocado in Italy. Fungal Systematics and Evolution 1:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatimosim E, Schwartsburd PB, Barreto RW, et al. 2016. Novel fungi from an ancient niche: cercosporoid and related sexual morphs on ferns. Persoonia 37:106–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O.2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52:696–704. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, et al. 2010. PHYML Online – a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Research 33 (Web Server issue): W557–W559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen F, Mostert L, Crous PW.2007. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australasian Plant Pathology 36:277–285. [Google Scholar]
- Hennings P.1901. Beiträge zur Flora von Afrika. XXI. Fungi. camerunenses novi. III. Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 30:39–57. [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, et al. 2017. Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology 86:53–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Lombard L, et al. 2016. Fungal Systematics and Evolution: FUSE 2. Sydowia 68:193–230. [Google Scholar]
- Hirooka Y, Kawaradani M, Sato T.2014. Description of Gibellulopsis chrysanthemi sp. nov. from leaves of garland chrysanthemum. Mycological Progress 13:13–19. [Google Scholar]
- Hoffmann K, Discher S, Voigt K.2007. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycological Research 111:1169–1183. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Tubaki K.2004. Fusarium matuoi sp. nov. and its teleomorph Cosmospora matuoi sp. nov. Mycoscience 45:261–270. [Google Scholar]
- Houbraken J, Visagie CM, Meijer M, et al. 2014. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Studies in Mycology 78:373–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F.2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. [DOI] [PubMed] [Google Scholar]
- Hunter GC, Crous PW, Carnegie AJ, et al. 2011. Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Diversity 50:145–166. [Google Scholar]
- Hywel-Jones NL.1995. Cordyceps brunneapunctata sp. nov. infecting beetle larvae in Thailand. Mycological Research 99:1195–1198. [Google Scholar]
- Jeewon R, Yeung SYQ, Hyde KD.2009. A novel phylogenetic group within Thozetella (Chaetosphaeriaceae): a new taxon based on morphology and DNA sequence analyses. Canadian Journal of Microbiology 55:680–687. [DOI] [PubMed] [Google Scholar]
- Kadowaki K, Sato H, Yamamoto S, et al. 2014. Detection of the horizontal spatial structure of soil fungal communities in a natural forest. Population Ecology 56:301–310. [Google Scholar]
- Kasuya T, Hosaka K, Uno K, et al. 2012. Phylogenetic placement of Geastrum melanocephalum and polyphyly of Geastrum triplex. Mycoscience 53:411–426. [Google Scholar]
- Katoh K, Standley DM.2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KL, Judd DB. 1976. COLOR. Universal language and dictionary of names. National Bureau of Standards, Special Publication 440. [Google Scholar]
- Kirk PM.1983. New or interesting microfungi. Transactions of the British Mycological Society 80:449–467. [Google Scholar]
- Kirk PM.2014. Nomenclatural novelties. Index Fungorum 120:1–1. [Google Scholar]
- Klaubauf S, Tharreau D, Fournier E, et al. 2014. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79:85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen K.2015. A survey of boreal Entoloma with emphasis on the subgenus Rhodopolia. Mycological Progress 14:1–116. [Google Scholar]
- Kolařík M, Kirkendall LR.2010. Evidence for a new lineage of primary ambrosia fungi in Geosmithia Pitt (Ascomycota: Hypocreales). Fungal Biology 114:676–689. [DOI] [PubMed] [Google Scholar]
- Kolařík M, Kostovčík M, PaŽoutová S.2007. Host range and diversity of the genus Geosmithia (Ascomycota: Hypocreales) living in association with bark beetles in the Mediterranean area. Mycological Research 111:1298–1310. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour. 3rd ed Eyre Methuen, London. [Google Scholar]
- Kreisel H.1992. An emendation and preliminary survey of the genus Calvatia (Gasteromycetidae). Persoonia 14:431–439. [Google Scholar]
- Kreisel H.1994. Studies in the Calvatia complex (Basidiomycetes) 2. Feddes Repertorium 105:369–376. [Google Scholar]
- Kühner R, Romagnesi H.1954. Compléments à la “Flore analytique” I. Espèces Nouvelles ou critiques de Rhodophylles. Revue Mycologique 19:3–46. [Google Scholar]
- Kumar S, Stecher G, Tamura K.2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers H. 2002. Atlas de los colores. 1 st ed Barcelona, Blume. [Google Scholar]
- Kuyper TW.1986. A revision of the genus Inocybe in Europe. I. subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia, Supplement 3:1–247. [Google Scholar]
- Leite AG, Assis HK, Silva BDB, et al. 2011. Geastrum species from the Amazon Forest, Brazil. Mycotaxon 118:383–392. [Google Scholar]
- Leite AG, Baseia IG.2007. Novos registros de Geastraceae Corda para o Nordeste Brasileiro. Sitientibus Série Ciencias Biológicas 7:178–183. [Google Scholar]
- Levesque CA, De Cock AWAM.2004. Molecular phylogeny and taxonomy of the genus Pythium. Mycological Research 108:1363–1383. [DOI] [PubMed] [Google Scholar]
- Liu B.1984. The Gasteromycetes of China. Beihefte zur Nova Hedwigia 76:1–235. [Google Scholar]
- Liu F, Cai L.2012. Morphological and molecular characterization of a novel species of Simplicillium from China. Cryptogamie, Mycologie 33:137–144. [Google Scholar]
- Liu N, Hongsanan S, Yang J, et al. 2017. Periconia thailandica (Periconiaceae), a new species from Thailand. Phytotaxa 323:253–263. [Google Scholar]
- Liu X-Z, Wang Q-M, Göker M, et al. 2015. Towards an integrated phylogenetic classification of the Tremellomycetes. Studies in Mycology 81:85–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Chen SF, Mou X, et al. 2015a. New species, hyper-diversity and potential importance of Calonectria spp. from Eucalyptus in South China. Studies in Mycology 80:151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, et al. 2010. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology 66:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Houbraken J, Decock C, et al. 2016. Generic hyper-diversity in Stachybotriaceae. Persoonia 36:156–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2015b. Generic concepts in Nectriaceae. Studies in Mycology 80:189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes UP, Alfenas RF, Zambolim L, et al. 2018. A new species of Calonectria causing rot on ripe strawberry fruit in Brazil. Australasian Plant Pathology 47:1–11. [Google Scholar]
- Luangsa-ard JJ, Tasanathai K, Mongkolsamrit S, et al. 2008. Atlas of invertebrate-pathogenic fungi of Thailand, vol. 2. NSTDA publication. Darnsutha Press Co., Ltd. [Google Scholar]
- Ludwig E. 2007. Pilzkompendium. Band 2. Die größeren Gattungen der Agaricales mit farbigem Sporenpulver (ausgenommen Cortinariaceae). Beschreibungen + Abbildungen. Fungicon Verlag; Berlin. [Google Scholar]
- Luttrell ES.1951. Taxonomy of Pyrenomycetes. University of Missouri Studies 24:1–120. [Google Scholar]
- Madrid H, Gené J, Cano J, et al. 2012. A new species of Leptodiscella from Spanish soil. Mycological Progress 11:535–541. [Google Scholar]
- Mains EB.1958. North American entomogenous species of Cordyceps. Mycologia 50:169–222. [Google Scholar]
- Marin-Felix Y, Groenewald JZ, Cai L, et al. 2017. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology 86:99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, et al. 2019. Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology 92:47–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovskaja S, Kačergius A.2014. Morphological and molecular characterisation of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycological Progress 13:291–302. [Google Scholar]
- Matsushima T. 1975. Icones microfungorum a Matsushima lectorum. Matsushima, Kobe, Japan. [Google Scholar]
- Matsushima T. 1993. Matsushima mycological memoirs No. 7. Matsushima, Kobe, Japan. [Google Scholar]
- Matsushima T. 1996. Matsushima mycological memoirs No. 9. Matsushima, Kobe, Japan. [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, et al. 2011. A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Studies in Mycology 68:211–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
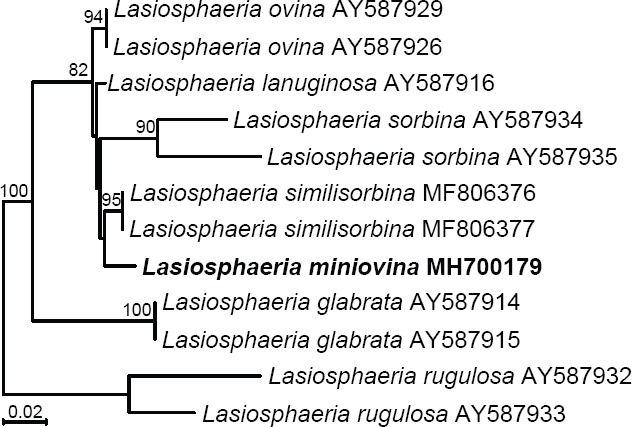
- Miller AN, Huhndorf SM.2004a. A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycological Research 108:26–34. [DOI] [PubMed] [Google Scholar]
- Miller AN, Huhndorf SM.2004b. Using phylogenetic species recognition to delimit species boundaries within Lasiosphaeria. Mycologia 96:1106–1127. [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE)M, 14 Nov. 2010, New Orleans, LA: 1–8. [Google Scholar]
- Milne I, Wright F, Rowe G, et al. 2004. TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20:1806–1807. [DOI] [PubMed] [Google Scholar]
- Möller A.1895. Brasilische Pilzblumen. Botanische Mitteilungen aus den Tropen 7:1–152. [Google Scholar]
- Morgan AP.1890. North American fungi. The Gasteromycetes. III. The Journal of the Cincinnati Society of Natural History 12:163–172. [Google Scholar]
- Morozova OV, Kovalenko AE, Rebriev Yu A, et al. 2014. Agaricoid and gasteroid fungi in the park of the Botanical Garden of the Komarov Botanical Institute – Botany: history, theory, practice (on the 300th anniversary of the founding of the VL Komarov Botanical Institute of the Russian Academy of Sciences): Proceedings of the International Scientific Conference: 142–149. [Google Scholar]
- Moyersoen B, Demoulin V.1996. Les Gastéromycètes de Corse: Taxonomie, Écologie, Chorologie. Lejeunia 152:1–130. [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario, Canada. [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka K, Kaifuchib S, Ômura S, et al. 2013. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience 54:42–53. [Google Scholar]
- Papendorf MC.1967. Leptodiscus africanus, sp. nov. Transaction British Mycological Society 50:687–690. [Google Scholar]
- Paulus B, Gadek P, Hyde K.2004. Phylogenetic and morphological assessment of five new species of Thozetella from an Australian rainforest. Mycologia 96:1074–1087. [PubMed] [Google Scholar]
- Peregrine WTH, Siddiqi MA.1972. A revised and annotated list of plant diseases in Malawi. Phytopathological Papers 16:1–51. [Google Scholar]
- Perera RH, Maharachchikumbura SSN, Bhat JD, et al. 2016. New species of Thozetella and Chaetosphaeria and new records of Chaetosphaeria and Tainosphaeria from Thailand. Mycosphere 7:1301–1321. [Google Scholar]
- Pitt JI.1979. Geosmithia gen. nov. for Penicillium lavendulum and related species. Canadian Journal of Botany 57:2021–2030. [Google Scholar]
- Pordel A, Khodaparast SA, McKenzie EHC, et al. 2017. Two new species of Pseudopyricularia from Iran. Mycological Progress 16:729–736. [Google Scholar]
- Poumarat S. 2003. Clé des Gastéromycètes épigés d’Europe. Phallales: Geastracea, Hysterangiaceae, Phallaceae; Agaricales: Lycoperdaceae, Mycenastraceae, Nidulariaceae, Phelloriniaceae, Tulostomataceae; Boletales: Sclerodermataceae (genres sécotioïdes exclus). Monographies Mycologiques de la FAMM, n° 2, 2ème édit. revue et augmentée, Edit. FAMM, Nice. [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, et al. 2013. Sizing up Septoria. Studies in Mycology 75:307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada L, Baral HO, Jaen-Molina R, et al. 2014. Phylogenetic and morphological circumscription of the Orbilia aurantiorubra group. Phytotaxa 175:001–018. [Google Scholar]
- Ramaley AW.1996. Comminutispora gen. nov. and its Hyphospora gen. nov. anamorph. Mycologia 88:132–136. [Google Scholar]
- Rao PR, Rao D.1964. The genus Periconia from India. Mycopathologia et Mycologia Applicata 22:285–310. [DOI] [PubMed] [Google Scholar]
- Raudabaugh DB, Iturriaga T, Carver A, et al. 2017. Coniella lustricola, a new species from submerged detritus. Mycological Progress 17:191–203. [Google Scholar]
- Réblová M, Gams W, Seifert KA.2011. Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68:163–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway R. 1912. Color standards and color nomenclature. Ridgway, Washington, DC. [Google Scholar]
- Rocha FB, Barreto RW, Bezerra JL, et al. 2010. Foliar mycobiota of Coussapoa floccosa, a highly threatened tree of the Brazilian Atlantic forest. Mycologia 102:1240–1252. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP.2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden L.2004. Neotropical polypores. Part 1. Introduction, Ganodermataceae & Hymenochaetaceae. Oslo: Fungiflora. Synopsis Fungorum 19:1–227. [Google Scholar]
- Ryvarden L, Gilbertson RL.1994. European Polypores. Part 2. Synopsis Fungorum 7:394–743. [Google Scholar]
- Sandoval-Denis M, Gené J, Sutton DA, et al. 2016. Redefining Microascus, Scopulariopsis and allied genera. Persoonia 36:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, et al. 1999. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91:286–298. [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9:1–997. CBS-KNAW Fungal Biodiversity Centre Utrecht, The Netherlands. [Google Scholar]
- Shearer CA.1973. Fungi of the Chesapeake Bay and its tributaries II. The genus Conioscypha. Mycologia 65:128–136. [Google Scholar]
- Sigler L, Tsuneda A, Carmichael JW.1981. Phaeotheca and Phaeosclera two new genera of dematiaceous hyphomycetes and a redescription of Sarcinomyces Lindner. Mycotaxon 12:449–467. [Google Scholar]
- Silva BDB, Sousa JO, Rodrigues ACM, et al. 2015. Geastrum hirsutum or G. trichiferum (Basidiomycota, Geastraceae): which name do use?. Mycosphere 6:459–462. [Google Scholar]
- Silva M, Barreto RW, Pereira OL, et al. 2016. Exploring fungal mega-diversity: Pseudocercospora from Brazil. Persoonia 37:142–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P, Grandi RAP.2013. Taxonomic studies of Thozetella Kuntze (anamorphic Chaetosphaeriaceae, Ascomycota). Nova Hedwigia 97:361–399. [Google Scholar]
- Silveira VD.1943. O gênero Calvatia no Brasil. Rodriguésia 16:63–80. [Google Scholar]
- Singtripop C, Hongsanan S, Li J, et al. 2016. Chaetothyrina mangiferae sp. nov., a new species of Chaetothyrina. Phytotaxa 255:21–33. [Google Scholar]
- Smith ME, Amses KR, Elliott TD, et al. 2015. Sequestrate fungi from Guyana I. Jimtrappea guyanensis gen. et sp. nov., Castellanea pakaraimophila gen. et sp. nov., and Costatisporia azurescens gen. et sp. nov. (Boletaceae, Boletales). IMA Fungus 6:297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogonov MV, Castlebury LA, Rossman AY, et al. 2008. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Studies in Mycology 62:1–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa JO, Silva BDB, Alfredo DS, et al. 2014. New records of Geastraceae (Basidiomycota: Phallomycetidae) from Atlantic rainforest remnants and relicts of northeastern Brazil. Darwiniana, Nueva serie 2:207–221. [Google Scholar]
- Stamatakis A.2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J.2008. A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75:758–771. [DOI] [PubMed] [Google Scholar]
- Stangl J.1989. Die gattung Inocybe in Bayern. Hoppea 46:1–394. [Google Scholar]
- Stuntz DE.1954. Studies on the genus Inocybe II. New and noteworthy species from Michigan. Papers of the Michigan Academy of Sciences, Arts & Letters 39:53–84. [Google Scholar]
- Subramanian CV.1955. Some species of Periconia from India. Journal of Indian Botanical Society 34:339–361. [Google Scholar]
- Summerbell RC, Gueidan C, Guarro J, et al. 2018. The Protean Acremonium. A. sclerotigenum/egyptiacum: Revision, food contaminant, and human disease. Microorganisms 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, et al. 2006. Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23:323–350. [Google Scholar]
- Sung GH, Hywel-Jones NL, Sung JM, et al. 2007. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57:5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunhede S.1989. Geastraceae (Basidiomycotina): Morphology, ecology, and systematics with a special emphasis of the North European species. Synopsis Fungorum 1:1–534. [Google Scholar]
- Sutton BC.1973. Hyphomycetes from Manitoba and Saskatchewan, Canada. Mycological Papers 132:1–143. [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. CMI, Kew, England. [Google Scholar]
- Sutton BC, Sarbhoy AK.1976. Revision of Chaetomella, and comments upon Vermiculariopsis and Thyriochaetum. Transactions of the British Mycological Society 66:297–303. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (*and their methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Takahashi H.1988. A new species of Boletus sect. Luridi and a new combination in Mucilopilus. Transactions of the Mycological Society of Japan 29:115–123. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82:75–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar GM.1972. Acremonium gamsii nov. sp. (Hyphomycetes). Acta Botanica Neerlandica 21:197–199. [Google Scholar]
- Trappe JM, Castellano MA, Halling RE, et al. 2013. Australasian sequestrate fungi 18: Solioccasus polychromus gen. & sp. nov., a richly colored, tropical to subtropical, hypogeous fungus. Mycologia 105:888–895. [DOI] [PubMed] [Google Scholar]
- Tsuneda A, Davey ML, Tsuneda I, et al. 2010. Two new dothideomycetous endoconidial genera from declining larch. Botany 88:471–487. [Google Scholar]
- Tulloss RE.2018. Amanita friabilis: description tabs. Available at: http://www.amanitaceae.org/?Amanita+friabilis (accessed: 2018.03.20). [Google Scholar]
- Udagawa S, Toyazaki N.1985. A new species of Leptodiscella. Mycotaxon 22:407–413. [Google Scholar]
- Van der Aa HA, Van Oorschot CAN.1985. A redescription of some genera with staurospores. Persoonia 12:415–425. [Google Scholar]
- Van Nieuwenhuijzen EJ, Miadlikowska JM, Houbraken JAMP, et al. 2016. Wood staining fungi revealed taxonomic novelties in Pezizomycotina: New order Superstratomycetales and new species Cyanodermella oleoligni. Studies in Mycology 85:107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. 2017. Mycosphaerellaceae – Chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viégas AP.1945. Alguns fungos do Brasil, X: Gasteromicetos. Bragantia 5:583–595. [Google Scholar]
- Visagie CM, Hirooka Y, Tanney JB, et al. 2014a. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology 78:63–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. 2014b. Identification and nomenclature of the genus Penicillium. Studies in Mycology 78:343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Seifert KA, et al. 2015. Four new Penicillium species isolated from the fynbos biome in South Africa, including a multigene phylogeny of section Lanata-Divaricata. Mycological Progress 1496 [Google Scholar]
- Voglmayr H, Jaklitsch WM.2017. Corynespora, Exosporium and Helminthosporium revisited – new species and generic reclassification. Studies in Mycology 87:43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57:1–44. [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom Fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92:135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic V, St-Arnaud M.2003. A new species of Pseudorobillarda, an endophyte from Thuja occidentalis in Canada, and a key to the species. Mycologia 95:955–958. [PubMed] [Google Scholar]
- Walker DM, Castlebury LA, Rossman AY, et al. 2010. Systematics of genus Gnomoniopsis (Gnomoniaceae, Diaporthales) based on a three gene phylogeny, host associations and morphology. Mycologia 102:1479–1496. [DOI] [PubMed] [Google Scholar]
- Wartchow F, Silva SM.2007. Primeira ocorrência de Calvatia cyathiformis (Basidiomycota) em Caatinga. Estado de Pernambuco, Brasil. Sitientibus Série Ciências Biológicas 7:176–177. [Google Scholar]
- Watling R. 1969. Colour Identification Chart. Edinburgh, Her Majesty's Stationery Office. [Google Scholar]
- Whiteside JO.1966. A revised list of plant diseases in Rhodesia. Kirkia 5:87–196. [Google Scholar]
- Woudenberg JHC, Sandoval-Denis M, Houbraken J, et al. 2017. Cephalotrichum and related synnematous fungi with notes on species from the built environment. Studies in Mycology 88:137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Feng B, Xu J, et al. 2014. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69:93–115. [Google Scholar]
- Wu G, Li YC, Zhu XT, et al. 2016. One hundred noteworthy boletes from China. Fungal Diversity 81:25–188. [Google Scholar]
- Wu SH.1990. The Corticiaceae (Basidiomycetes) subfamilies Phlebioideae, Phanerochaetoideae and Hyphodermoideae in Taiwan. Acta Botanica Fennica 142:1–123. [Google Scholar]
- Wu WP, Sutton BC.1995. Fumagopsis complexa sp. nov., a species with complicated conidial morphology. Mycological Research 99:1450–1452. [Google Scholar]
- Wu YM, Xu JJ, Kong JH, et al. 2015. New species of Graphium and Periconia from China. Mycotaxon 129:397–401. [Google Scholar]
- Zare R, Gams W.2001. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 71:1–50. [Google Scholar]
- Zare R, Gams W, Starink-Willemse M, et al. 2007. Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwigia 85:463–489. [Google Scholar]
- Zeller SM, Smith AH. 1964. The genus Calvatia in North America. Lloydia 27:148–186. [Google Scholar]
- Zelski SE, Raja HA, Miller AN, et al. 2015. Conioscypha peruensis sp. nov., its phylogenetic placement based on 28S rRNA gene, and a report of Conioscypha gracilis comb. nov. from Peru. Mycoscience 56:319–325. [Google Scholar]
- Zhao CL, Cui BK, Dai YC.2014. Morphological and molecular identification of two new species of Hyphodontia (Schizoporaceae, Hymenochaetales) from southern China. Cryptogamie, Mycologie 35:87–97. [Google Scholar]
- Zhukova EA, Morozova OV, Volobuev SV, et al. 2017. Basidial macromycetes and their impact on the state of green plantations of the State Russian Museum Gardens (Saint Petersburg). Mikologiya i Fitopatologiya 51:328–339. [Google Scholar]