Abstract
Mesenchymal stem cells (MSCs) are broadly distributed cells that retain postnatal capacity for self-renewal and multilineage differentiation. MSCs evade immune detection, secrete an array of anti-inflammatory and anti-fibrotic mediators, and very importantly activate resident precursors. These properties form the basis for the strategy of clinical application of cell-based therapeutics for inflammatory and fibrotic conditions. In cardiovascular medicine, administration of autologous or allogeneic MSCs in patients with ischemic and nonischemic cardiomyopathy holds significant promise. Numerous preclinical studies of ischemic and nonischemic cardiomyopathy employing MSC-based therapy have demonstrated that the properties of reducing fibrosis, stimulating angiogenesis, and cardiomyogenesis have led to improvements in the structure and function of remodeled ventricles. Further attempts have been made to augment MSCs' effects through genetic modification and cell preconditioning. Progression of MSC therapy to early clinical trials has supported their role in improving cardiac structure and function, functional capacity, and patient quality of life. Emerging data have supported larger clinical trials that have been either completed or are currently underway. Mechanistically, MSC therapy is thought to benefit the heart by stimulating innate anti-fibrotic and regenerative responses. The mechanisms of action involve paracrine signaling, cell-cell interactions, and fusion with resident cells. Trans-differentiation of MSCs to bona fide cardiomyocytes and coronary vessels is also thought to occur, although at a nonphysiological level. Recently, MSC-based tissue engineering for cardiovascular disease has been examined with quite encouraging results. This review discusses MSCs from their basic biological characteristics to their role as a promising therapeutic strategy for clinical cardiovascular disease.
I. INTRODUCTION
Heart disease is the leading cause of death for both men and women in the United States and even worldwide (248). Ischemic heart disease (IHD), specifically coronary artery disease, is the most common type of heart disease and a major contributor to IHD-related morbidity and mortality (248). Following insults to the myocardium, left ventricular remodeling occurs with a subsequent decrease in myocardial function and efficiency (276). The fundamental driving force of cardiac remodeling is the formation of myocardial scar tissue that replaces the necrotic myocardium injured by an ischemic insult (139). Noncontractile fibrosis leads to infarct expansion and extension (386), processes that drive the formation of a spherical shape to the ventricle (86, 91). Such cardiomyopathies, either ischemic or nonischemic in nature, can lead to heart failure and cause a marked deterioration in patients' quality of life and functional capacity (276). Although advances in medicine and surgery have lowered cardiovascular disease mortality, they merely serve as transient “delayers” of an inevitably progressive disease process that carries significant morbidity (238).
The concept of stem cell use as a therapeutic strategy for cardiovascular disease initially emerged in animal studies over 2 decades ago (231) and in clinical trials 10 years later (53, 138). Due to the heart's limited self-regenerative capacity, investigators have attempted to identify an “optimal” cell-based therapy to assist in myocardial self-repair and restoration of cardiac function.
A number of cell-based strategies are being explored for cardiac regeneration. Generally, they are classified under two major categories: 1) those aiming at directly remuscularizing the myocardial scar or 2) those targeting endogenous mechanisms of repair (143).
The first category includes myocardial transplantation of cell types with unequivocal myogenic ability, such as uncommitted induced pluripotent stem cells (iPSC) or embryonic stem cells (ESC) (22), iPSC/ESC-derived human cardiomyocytes (61, 97, 304, 405), or ESC/iPSC-derived cardiomyocyte precursors (97, 240, 405). Gene therapy-based approaches aiming at converting scar tissue fibroblasts into cardiomyocyte-like cells (288, 340) or forcing massive dedifferentiation and proliferation of surviving cardiomyocytes (68, 95) are also under consideration, although still far from clinical application due to concerns over safety and technical feasibility (87). Importantly, technological advancements have made the ex vivo manufacturing of transplantable cardiac cell products with unequivocal capacity to form contractile human myocardium clinically feasible and has been successfully applied in preclinical animal models of cardiac remuscularization (61, 97, 304). However, further studies are needed for optimizing this strategy to enhance functional recovery, since some of the existing experiments regarding long-term engraftment of bona fide human myocardium have not definitively shown recovery of heart function (61, 97, 99, 143, 304). For example, ex vivo tissue engineering approaches, whereby human cardiomyogenic cells are combined with mesenchymal stem cells (MSCs), vasculogenic cells (42, 60, 405), and/or neurons (224, 389) may be required prior to engraftment, for meaningful cardiac regeneration.
The second category includes adult, undifferentiated progenitor cells such as bone marrow mononuclear cells (BMCs) (147, 260, 318, 396), MSCs (11, 119, 137), and resident adult cardiac progenitors (CPCs) (62, 228). Although most of these cell types entered the clinical arena based on the hypothesis that they possessed myogenic differentiation capacity (191), further mechanistic studies revealed critical contributions of their anti-inflammatory and antifibrotic properties, as well as stimulation of endogenous cardiovascular progenitor and cardiomyocyte proliferation cell programs (144, 219, 229, 291, 349). Nonetheless, genetic lineage-fate mapping studies show that, under the proper conditions, endogenous CPCs (145, 348, 368), and to a smaller extent BMCs (289, 396) and MSCs (60, 144, 290) produce new cardiomyocytes in the postnatal heart, albeit at a functionally insignificant level. Paradoxically, compared with ESC/iPSC-based strategies, engraftment of MSCs and CPCs is lower but leads to significant heart regeneration and recovery of heart function (62, 97, 119, 137, 143, 144, 147, 174, 175, 228, 229, 284, 290, 321, 322, 385). Ex vivo tissue engineering approaches, whereby MSCs are combined or fused with adult human CPCs (175, 291, 392), ESC/iPSC-derived CPCs (42), endothelial progenitors (417), and possibly neurons (224, 389) may improve long-term engraftment and/or differentiation of the adult cell grafts and therefore lead to a meaningful level of myocardial regeneration and functional recovery.
Compared with the cell sources discussed above, MSCs are particularly attractive for cardiac regenerative cell-based therapy as well as many other disease processes (44, 118, 200, 263, 284, 365), for a number of reasons. They are easy to isolate and amplify from multiple sources, including the bone marrow (277) and the heart itself (60); they hold unequivocal postnatal multilineage potential; and are immunotolerant permitting its use as an allogeneic “off-the-shelf” product, either alone or in combination with other cells (42, 175, 392).
In this review, we discuss the biology, mechanisms of action, reparative effects, preclinical and clinical data, and potential utilization of MSCs for cardiovascular disease.
II. UNDERSTANDING AND DEFINING THE MESENCHYMAL STEM CELL
In 1970, Dr. A. J. Friedenstein and colleagues (104) identified a rare population of plastic adherent bone marrow (BM) stromal cells. Further characterization indicated that this population comprises ∼0.01% of nucleated BM cells, which are now commonly referred to as MSCs. Friedenstein et al. demonstrated that these cells resembled fibroblasts and formed single-cell colonies, hence calling them colony-forming unit fibroblasts (CFU-Fs). Furthermore, they were easily expandable and were capable of differentiating into mesenchymal tissue types, highlighting their crucial role in regulating the hematopoietic niche (105). However, because methods of isolation, expansion, and characterization of these cells have varied among investigators over the last several decades, study outcome measurements have been rather complex to compare thus hampering progress in the field of cell therapy. In response to this need, the 2006 International Society for Cellular Therapy proposed that the minimal criteria for defining an MSC are (82) 1) plastic adherence under standard culture conditions; 2) expression of CD105, CD73, and CD90 but not CD45, CD34, CD14 or CD11b, CD79α or CD19, or HLA-DR surface markers; and 3) retain in vitro multilineage differentiation capacity into osteoblasts, chondroblasts, and adipocytes. Importantly however, these criteria may not all apply to MSCs derived from all species as the process of culturing and expanding can differ between them (269). Nevertheless, human MSCs are considered true stem cells due to their ability of self-replicate while maintaining their multipotent potential, as well as their multilineage differentiation capacity into mesoderm-derived tissues (277).
III. SOURCES AND TYPES
MSCs are ubiquitously found throughout the human body and can be derived from multiple organ systems including, but not limited to, bone marrow, heart, peripheral blood, adipose tissue, placenta, and umbilical cord (142). Of all tissue types, bone marrow and adipose tissue remain the most common studied sources of MSCs. MSCs can be easily harvested in large quantities from these tissue types, rendering them as rather appealing sources (173). Of note, much controversy still exists as to whether adipose tissue-derived cells are bona fide MSCs (59).
Neonatal and fetal tissue-derived MSCs have been used in preclinical models of heart failure and have demonstrated a lower immunogenic profile (27). Isolation of fetal MSCs from amniotic fluid has demonstrated that they have a similar phenotype and lineage capacity as postnatal MSCs (161). These amniotic fluid-derived MSCs (AFMSCs) have excellent in vitro expansion potential as well as multipotent differentiation capability (273). Compared with conventional MSCs, AFMSCs are thought to express the transcription factors Oct-4, Nanog, and stage-specific embryonic antigen-4 (SSEA-4) (312). Furthermore, AFMSCs express similar immunologic features, such as HLA-A, -B, and -C (MHC Class I), as MSCs from other organ tissues, including bone marrow (360). MSCs derived from umbilical cord blood and umbilical cord cells have also been used in the context of human cardiac disease (401) and cardiovascular tissue engineering (37), yet their isolation can be difficult (31, 179).
Peripheral blood has been shown to harbor MSCs as well as mesenchymal progenitor cells (MPC), a subpopulation which shares many of the same phenotypic features as MSCs (423). MPCs can also be isolated from bone marrow aspirate (336). Although MPCs share some common CD surface markers as MSCs, they additionally express a number of embryonic markers specific to an early level of differentiation including SSEA-4, TRA-1-81, and Oct-3/4 (274). MSC-like cells found in cardiac tissue have been used in cardiac regenerative medicine, although they do not completely meet all definition requirements of an MSC (151). Additionally, these cells have not been popularized in the clinical setting due to their limited tissue availability (21).
IV. CULTURE AND EXPANSION
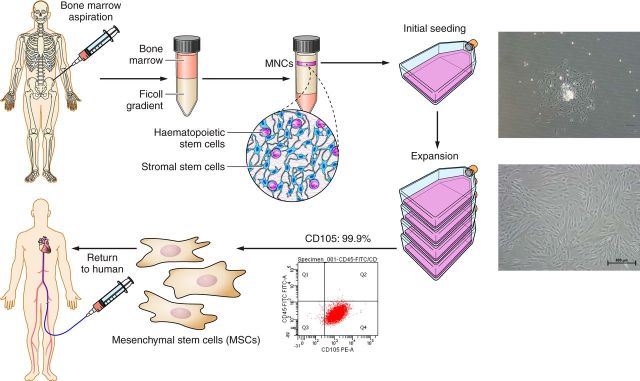
The ex vivo propagation of MSCs is a three-step process: 1) separation of nucleated cells from nonnucleated cells by Percoll or Ficoll density gradient centrifugation, 2) adherence of cells to plastic tissue culture flasks, and 3) passaging of adhered MSCs via trypsinization (201). Mononuclear cell (MNC) enrichment is first achieved by fractionating samples obtained from bone marrow, cord blood, or peripheral blood using density gradient centrifugation. Enriched MNCs are then resuspended in culture medium containing appropriate quantities of fetal bovine serume (FBS), or FBS substitutes, and allowed to adhere to plastic culture flasks for 2 days. Subsequently, culture medium is replaced with fresh medium to remove nonadherent cells. Remaining adherent cells are allowed to grow for the next 2-3 wk with periodic medium change. Initially, by 5–7 days after primary culture, fibroblast-like cells and small round-shaped cells (monocytes) are found in the heterogeneous adherent cell layer of the culture flasks and form colonies. After trypsinization, these cells are subcultured and begin to appear more uniformly spindle shaped. After two to three passages, MSCs are ready to be cryopreserved. To confirm their phenotypic characterization, monoclonal antibodies against specific cell-surface markers are used to perform fluorescence-activated cell sorting (FACS) analysis (Figure 1). Typically, a 10 ml bone marrow aspirate sample can generate ∼50–400 million MSCs (277).
FIGURE 1.
Mesenchymal stem cell (MSC) expansion from P-0 to P-1. First step of MSC manufacturing involves bone marrow (BM) harvesting from healthy donors. MSC manufacturing begins with mononuclear enrichment (MNC) using Ficoll gradient. Cells are cultured for 3–4 wk in tissue culture flasks and then cryopreserved until they are ready to be infused. Representative fluorescence-activated cell sorting (FACS) analysis of CD105+ expression of MSCs isolated from BM. CD105 expression >90%.
V. REGULATION OF MSC DIFFERENTIATION
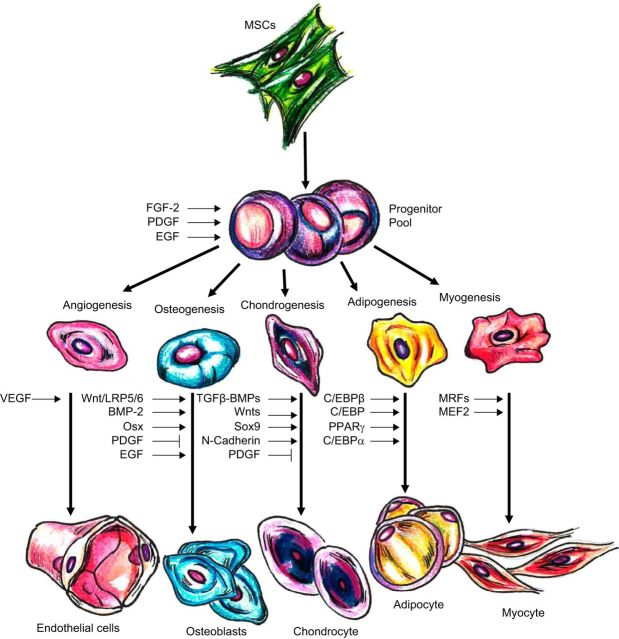
A. Osteogenesis, Chondrogenesis, and Adipogenesis
Human bone marrow-derived MSCs exposed to dexamethasone, ascorbic acid, and β-glycerol phosphate can be induced to differentiate into osteogenic cells as determined by morphology, expression of alkaline phosphatase, alteration of osteocalcin mRNA production, reactivity with anti-osteogenic cell surface antibodies, and formation of a mineralized extracellular matrix (Figure 2) (38, 165). Micro-RNA analysis suggests that modulation of specific micro-RNAs (miRNAs), which either inhibit or stimulate target mRNAs, govern the early osteogenic differentiation of human MSCs (319). MSCs can be stimulated to differentiate into a chondrogenic phenotype when cultured in the presence of dexamethasone and transforming growth factor-β3 (TGF-β3) (223). With dexamethasone being a common denominator in the MSC osteogenic-chondrogenic-adipogenic differentiation axis, combining it with insulin, indomethacin, and 1-methyl-3-isobutylxanthine can cause MSC adipogenesis and expression of peroxisome proliferator-activated receptor (PPAR)-γ (77). Furthermore, culture-expanded MPCs have been shown to serve as precursors for mesenchymal tissues including bone, cartilage, and lung parenchyma when infused intravenously (270).
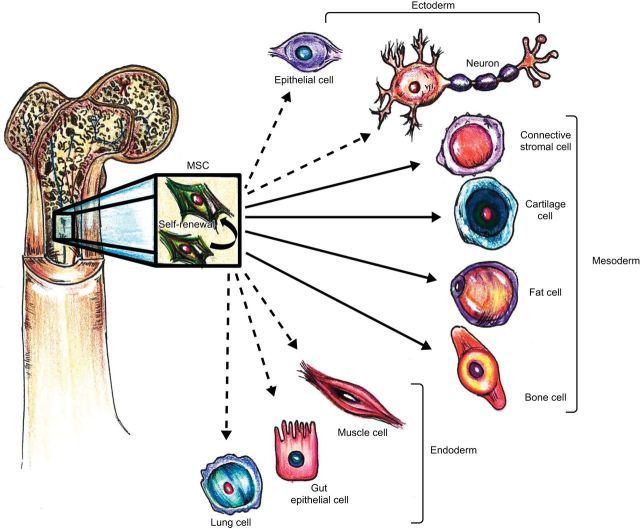
FIGURE 2.
Multipotential fate of mesenchymal stem cells. MSCs have the ability to self-renew within the bone marrow (curved arrow). They can also differentiate into cell types of the mesodermal lineage (solid arrows) as well as the ectodermal and endodermal lineages (dashed arrows), although their in vivo transdifferentiation capacity is debatable. [Adapted from Uccelli et al. (363), with permission from Nature Publishing Group.]
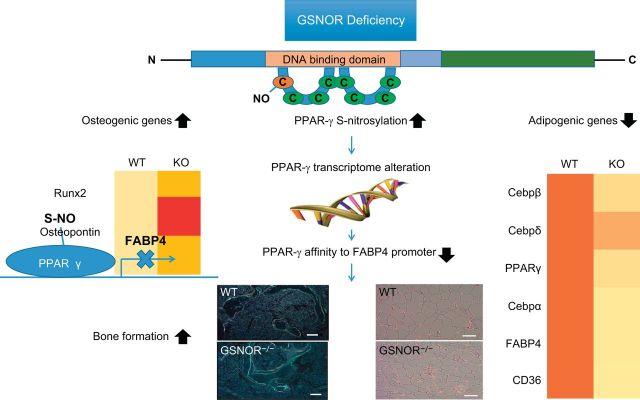
Various mediators participate in balancing MSC lineage differentiation. In this regard, vascular endothelial growth factor (VEGF) regulates the balance between MSC osteoblastogenesis and adipogenesis, with reduced expression of VEGF resulting in greater MSC differentiation into adipocytes (218). This regulation mechanism involves transcription factors RUNX2, PPAR-γ2, and nuclear envelope proteins lamin A/C. Similarly, Cao et al. (44) found PPAR-γ to be an essential “equilibrator” between osteogenesis and adipogenesis. The study showed that S-nitrosoglutathione reductase (GSNOR)-deficient mice had higher levels of S-nitrosylated PPAR-γ and hence reduced adipogenesis with increased osteoblastogenesis, compared with wild-type mice (44). Increased levels of S-nitrosylated PPAR-γ decrease its binding affinity to fatty acid-binding protein 4 (FABP4), its target promoter, and result in inhibition of PPAR-γ transcriptional activity through a negative-feedback mechanism (44) (Figure 3).
FIGURE 3.
Regulation of MSC osteogenesis and adipogenesis. Deficiency of S-nitrosoglutathione reductase (GSNOR) leads to increased levels of S-nitrosylated PPAR-γ with a decreased affinity for fatty acid-binding protein 4 (FABP4), its target promoter. Alterations in the PPAR-γ transcriptome modify expression of various genes responsible for regulating the balance between osteogenesis and adipogenesis. [Adapted from Cao et al. (44), with permission from American Society of Clinical Investigation.]
B. Cardiomyogenesis
Differentiation of MSCs into cardiomyocytes has been demonstrated both in vivo and in vitro. In vitro exposure of MSCs to the DNA methyltransferase 5-azacytidine (5-Aza) induces cardiomyogenic differentiation (60, 226), characterized by the expression of myocardial-specific proteins and formation of myotubes (358). Similarly, exposure of MSCs to growth factor cocktails containing bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-4 (FGF-4), or insulin, steroids (dexamethasone), and antioxidant supplements can also result in differentiation into cardiomyocyte-like cells (332, 408). Furthermore, coculture of bone marrow-derived MSCs with ventricular myocytes creates a conditioned medium that allows for MSC differentiation into cardiomyocytes with expression of sarcomeric α-actinin, desmin, cardiac troponins, SERCA2, and ryanodine receptor (208). Differentiated bone marrow-derived MSCs exhibit α-actinin positivity, express cardiac transcription factors, and form gap junctions with native myocytes when cocultured together; however, differentiation into cardiomyocytes does not commence when they are separated from ventricular myocytes by a semipermeable membrane (399).
Of note, an important limitation in these studies is that there is little information regarding the potential mechanisms through which they induce cardiomyocyte differentiation of MSCs. For example, it is unclear whether 5-Aza specifies MSCs to a cardiomyocytic lineage, or may be ectopically inducing cardiomyocyte differentiation due to random, hypomethylation-induced, transcription of cardiac specific genes (310).
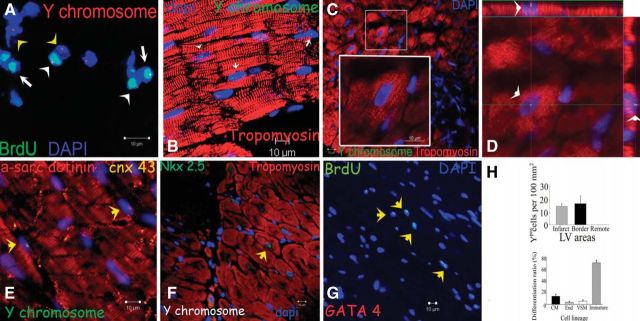
In vivo studies have demonstrated the ability of MSCs to differentiate into cardiac muscle-like when transplanted into damaged myocardial tissue (171, 378). When human MSCs were transplanted into fetal sheep early in gestation, MSCs remained engrafted up to 13 years and differentiated into cardiomyocyte-like cells, as well as site-specific myocytes, chondrocytes, and adipocytes (212). Sex-mismatched transplantation of male porcine MSCs in female swine with chronic (290) or acute (144) experimental myocardial infarction have unequivocally demonstrated the long-term engraftment and cardiomyocyte differentiation of MSCs, through the identification of Y-chromosome bearing cardiomyocytes within the female myocardium (Figure 4). In addition, Cre-LoxP-based genetic lineage fate-mapping studies in mice have identified a platelet-derived growth factor receptor, alpha polypeptide (Pdgfra)+ MSC lineage of mesodermal origin residing within the embryonic and adult heart, as a source of bona fide cardiomyocytes in response to myocardial damage (60). Intriguingly, a similar approach recently illustrated that the adult bone marrow likely contains more than one Pdgfra+ MSC lineages: a mesoderm-derived that participates in skeletogenesis, and a neural crest-derived lineage that establishes the hematopoietic stem cell niche (162). Further studies are needed to determine whether a lineal relationship exists between the Pdgfra+ resident cardiomyogenic MSCs and the bone marrow MSCs.
FIGURE 4.
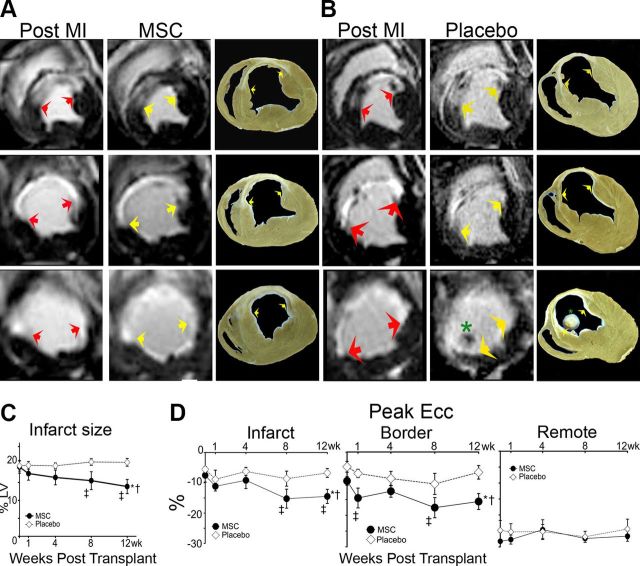
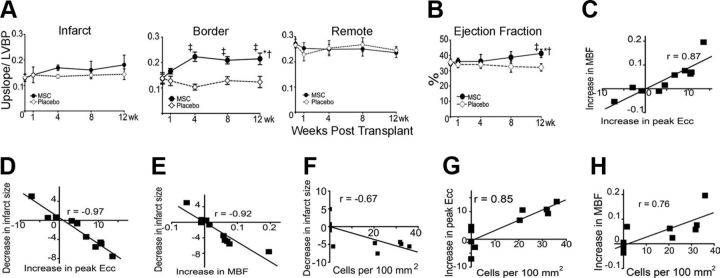
Cardiogenic potential of transplanted MSCs. A: cluster of Ypos/BrdUpos cells (white arrowheads) located in infarct and border zones of treated hearts 12 wk after MSC implantation. Some of the transplanted MSCs do not exhibit BrdUpos signal (green), but maintain Ypos signal (red, yellow arrowheads). Conversely, another group shows BrdUpos signal (white arrows) and negative Y chromosome signal due to technique sensitivity. B: cluster of Ypos cells (green, white arrows) in the border zone of MSC-treated animals colocalizing with tropomyosin (red). C: evidence of cardiac differentiation in a panoramic view of an infarct border zone of MSC-treated hearts. Inset depicts one Ypos (green) myocyte costained with tropomyosin. High magnification of the square is shown in the inset. D: confocal microscopy analysis of the same cell by orthogonal section of a z-stack (arrows point the cell analyzed in xy-plane). E: two transplanted Ypos cells (green, arrows) coupled with the resident cardiomyocytes by expressing connexin-43 (orange). F: evidence of cardiac commitment in the transplanted cell by the colocalization of Ypos signal with the cardiac transcription factor Nkx2.5 (green, arrow). Nuclei were counterstained with DAPI in all of the immunofluorescence assays. G: cluster of BrdUpos cells (green) in the border zone of MSC-treated animals exhibiting colocalization with transcription factor GATA-4 (red, arrows). H: quantitation of transplanted cells according to Y chromosome cell tracking. Ypos cells show no preference in distribution according to LV areas (top). Importantly, at 12 wk posttransplantation, implanted MSCs showed commitment to repopulate the three major cardiac cell lineages and maintain a reservoir of nondifferentiated cells (bottom). Cell quantification per unit area for the Y chromosome (n = 6 for MSC-treated hearts, n = 4 for placebo-treated hearts). At least four tissue sections for infarct, border, and remote zone per heart were evaluated. Total area evaluated is 2,673.34 mm2. CM, cardiomyocyte; End, endothelial cells; VSM, vascular smooth muscle. [From Quevedo et al. (290).]
Collectively, these findings indicate that, although MSCs are not a major cellular source for cardiomyocytes, they are capable of differentiating into cardiomyocytes under proper conditions.
C. Endothelial and Vascular Smooth Muscle Differentiation
Treating MSCs with VEGF and fetal calf serum supports their differentiation into endothelial cells measured by the expression of endothelial-specific markers, including kinase insert domain receptor (KDR), FMS-like tyrosine kinase (FLT)-1, and von Willebrand factor (261). Notably, these cells can form capillary-like structures in vitro, which may be an important indicator of angiogenic potential (261, 290). Ikhapoh et al. (160) furthered these findings by demonstrating that VEGF mediates MSC differentiation into endothelial cells by increasing the expression of VEGF receptor (VEGFR)-2, which stimulates Sox18 and upregulates endothelial cell-specific markers. Our group corroborated these findings in an in vivo porcine model, by injecting male MSCs into female swine, and demonstrated Y-chromosome colocalization of donor MSCs in endothelial, vascular smooth muscle, and cardiac cell lineages (290) (Figure 5). Vascular smooth muscle differentiation has been associated with TGF-β-induced activation of Notch ligand and signaling (190). Interestingly, subpopulations of MSCs that highly express CD146 are strongly associated with lineage commitment towards vascular smooth muscle cells (93). Using a murine model, investigators were able to regenerate all three layers of the vascular wall by induction of MSCs together with recombinant human-BMP-2 (rh-BMP-2) seeded on a vascular patch, which promoted tubelike formation 90 days following aortic implantation (25).
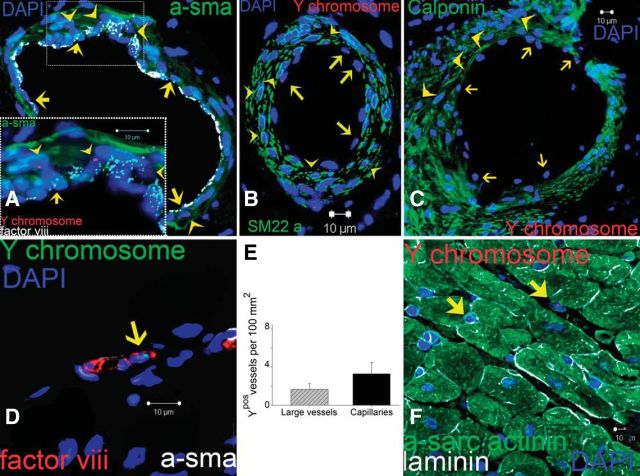
FIGURE 5.
Vascular differentiation of transplanted MSCs. A: representative image of a vessel containing numerous Ypos cells colocalized with smooth muscle actinin (a-sma in green, arrowheads) and endothelial cells (factor VIII-related antigen in white, arrows). High magnification of the inset to visualize the Ypos cells that colocalize with sma (arrowheads) and factor VIII-related antigen (white, arrows) demonstrating vascular smooth muscle and endothelial commitment, respectively. B and C: confirmation of Ypos cells commitment into vascular structures as depicted by colocalization with SM22-α (B) and calponin (C, arrowheads in both pictures). Ypos cells also commit to endothelial cell lineages (arrows). D: capillary formation with the incorporation of Ypos cell (arrow) costained with factor VIII-related antigen depicting the luminal surface of the vessel. E: assessment of vessel number per unit area according to their respectively size. F: Ypos cells also reside in the interstitial compartment (arrows) of border myocardium in a nondifferentiated stage (n = 6 for MSC-treated hearts, n = 4 for placebo). At least 4 tissue sections from infarct, border, and remote zone were evaluated per animal. [From Quevedo et al. (290).]
D. Nonmesenchymal Tissue Differentiation
MSCs are a heterogeneous population of multipotent cells, ontogenically related to both mesodermal and neuroectodermal lineages (36, 162, 251). As such, they have been documented to differentiate into a variety of tissues. Skeletal muscle MSC differentiation has been reported with the strongest potential observed among adipose-derived MSCs, compared with bone marrow-derived MSCs, using cultured cell media containing human satellite cell cultures mixed with MSC growth medium (347). Importantly, the Pax3 and Pax7 genes are required for myogenic commitment and expressed by MSCs during skeletal muscle differentiation (50). Bitto et al. (32) cocultured MSCs with myoblasts, added dexamethasone and basic fibroblast growth factor (bFGF), implanted motor nerves for stimulation, and observed in vivo myogenic differentiation (32). Areas of differentiation expressed myogenic markers such as α-sarcomeric actin. Induced mycoytes can also exhibit rhythmic calcium fluxes, potassium-induced calcium fluxes, and express β-myosin heavy chain and desmin (400).
Human and rat MSCs can be induced to differentiate into neurons through an incompletely understood approach involving their exposure to β-mercaptoethanol (BME), Dulbecco's modified Eagle's medium (DMEM), and dimethylsulfoxide (DMSO) (395). These cells exhibit neuronal properties and expression of neuron-specific enolase (NSE) and neurofilament-M (33, 395). MSCs injected into the lateral ventricle of neonatal mice were found to migrate throughout the forebrain and cerebellum 12 days after injection. Importantly, these cells were capable of differentiating into mature astrocytes, expressing glial fibrillary acidic protein, and neurons expressing neurofilament protein (185). Engraftment of fractionated human MSCs into rat livers demonstrated differentiation of human MSC xenografts into hepatocyte-like cells as evidenced by the presence of human-specific alpha-fetoprotein (AFP), albumin, asialoglycoprotein receptor (AGPR), and cytokeratins 18 and 19 (315). This differentiation potential has been suggestively attributed to MSC gene reprogramming (315) and can be accelerated by promoting the mesenchymal-to-epithelial (MET) transition through inhibition of Ras-related C3 botulinum toxin substrate 1 (Rac1) (353).
MSCs have also been shown to acquire an epithelial cell phenotype in various tissues such as intestine (98, 324), lung (241), and esophagus (172). These processes are mediated by protein substrate-MSC interactions (241) and even cell fusion with native epithelial cells (98). Infusion of human bone marrow-derived MSCs into mice with radiation-induced gastrointestinal tract failure leads to rapid recovery of small intestinal structure and function by prevention of apoptosis and enhancement of endogenous repair and proliferation (324). Similarly, MSC-treated rats with induced caustic esophageal injury demonstrated homing of MSCs to sites of injury with Dil-labeled epithelial cells originating from transplanted MSCs (172). More recently, evidence regarding MSC differentiation into corneal endothelial and epithelial cells has been shown, albeit inconclusive results (140). Figure 6 provides a schematic summary of the regulation of MSC differentiation.
FIGURE 6.
Transcription factors and signaling molecules participating in regulation of MSC differentiation. Various molecules and factors induce MSC differentiation into several cell lines. BMP-2, bone morphogenetic protein-2; EGF, epidermal growth factor; FGF-2, fibroblast growth factor-2; LRP5/6, low-density lipoprotein receptor-related protein-5/6; MEF2, myocyte enhancer factor-2; MRF, myogenic regulatory factors; Osx, Osterix; PDGF, platelet-derived growth factor; RUNX2, runt-related transcription factor-2; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor. [Adapted from Karantalis et al. (173).]
E. Wnt and TGF-β Pathways
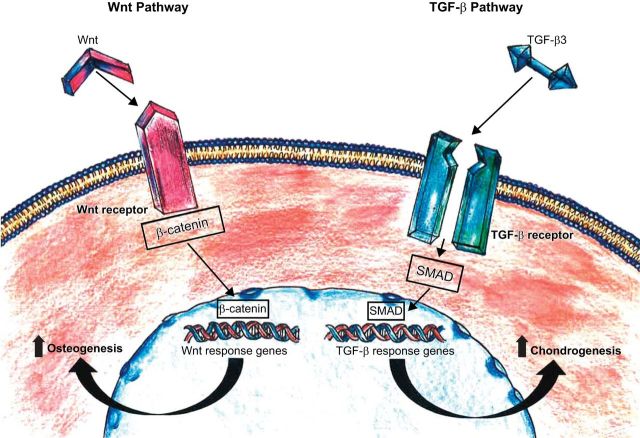
Although many signaling pathways have been implicated in MSC differentiation, the Wnt canonical pathway and the TGF-β superfamily pathway are the most widely studied (13) (Figure 7). The Wnt family comprises 19 genes that produce secreted lipid-modified glycoproteins involved in regulating osteogenesis in vivo by acting directly on MSCs (217). This signaling pathway, through a series of intracellular events, is involved in modulation of osteoblast proliferation and survival (388). The Wnt glycoproteins stabilize and increase levels of β-catenin, which is transported to the nucleus and binds to Lef1/Tcf transcription factors, leading to modified gene expression and promotion of osteoblast growth (23). Day et al. (71) demonstrated that signaling via the Wnt pathway results in greater ossification during skeletogenesis and that inhibition of β-catenin suppresses osteogenesis while promoting chondrogenesis. Multiple Wnt ligands (Wnt2, Wnt4, Wnt5a), receptors (FZD2, 3, 4, 5), coreceptors, and inhibitors are expressed by MSCs (94); the interactive balance between their up- and downregulation control MSC differentiation (388).
FIGURE 7.
Major molecular pathways of MSC differentiation. The Wnt and TGF-β signaling pathways are responsible for regulating the differentiation of MSCs into osteoblasts and chondrocytes while promoting their proliferation and survival through activation of intracellular cascades with subsequent modification of gene expression. [Adapted from Williams et al. (393), with permission from American Heart Association.]
The TGF-β superfamily includes various ligand growth factors, differentiation factors, anti-Müllerian hormone (AMH), Activin, Nodal, BMPs, and TGF-β, and participates in developmental skeletogenesis and differentiation of MSCs into chondrocytes (13). TGF-β3 induces chondrogenesis in MSCs by upregulating cartilage-specific gene expression, which is achieved by specific intracellular signaling cascades consisting of SMAD proteins, mitogen-activated protein (MAP) kinases, p38, extracellular-signal regulated kinase (ERK)-1, and c-Jun NH2-terminal kinase (JNK) (10, 221, 223) (Figure 6). Other pathways such as FGF (235), PDGF (125), and epidermal growth factor (EGF) (188) also participate in regulating MSC differentiation via interactions with the Wnt and TGF-β pathways (13).
VI. IMMUNOLOGY OF MSCs
MSCs exert both immunomodulatory and immunosuppressive activity, and this is demonstrated both in vitro and in vivo (4, 26, 65, 80, 101, 169, 196, 216, 222, 246, 247, 292, 294, 296–298, 323, 341, 343, 344, 361, 363, 411, 416). By interacting with cells of the adaptive and innate immune system, they suppress release of pro-inflammatory cytokines while supporting an anti-inflammatory state (363). They constitutively express major histocompatibility complex (MHC) class I, but not class II, and lack T-cell costimulatory molecules CD40, CD80, CD86, or B7 (79, 225). MSCs are not targeted by cytotoxic lymphocytes or natural killer cells and, additionally, when in coculture with allogeneic lymphocytes, do not induce lymphocyte proliferation (306). The latter is at least partially caused by secreted soluble factors including hepatocyte growth factor, TGF-β1, interferon (IFN)-γ, nitric oxide (NO), interleukin (IL)-2, indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and IL-10 (19, 80, 361).
A. Innate Immunity
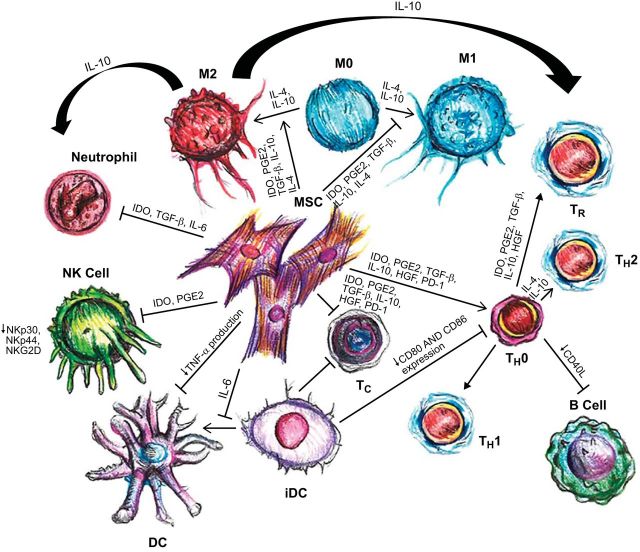
Neutrophils, the most abundant white blood cell type, are essential to the innate system as one of the initial responders to, particularly, bacterial infection and/or injury. They rapidly mobilize to sites of inflammation and are activated to degrade bacterial microorganisms through the respiratory burst, a quick release of reactive oxygen species (363). MSCs have the capability of attenuating the respiratory burst and protecting both resting and activated neutrophils from spontaneous apoptosis via an IL-6 pathway (292) (Figure 8).
FIGURE 8.
Immune profile of mesenchymal stem cells (MSCs). Graphic summary of the interactions between MSC and the immune system. MSCs can suppress proliferation of both T helper (TH) and cytotoxic T cells (Tc) through multiple pathways. Differentiation of MSCs to TH2 and regulatory T-cells (Treg) is triggered, resulting in an anti-inflammatory environment. Interleukin (IL)-6 blocks the maturation of dendritic cells (DC) by inhibiting upregulation of CD40, CD80, and CD86, which subsequently reduces T-cell activation. Monocytes are stimulated by MSCs to preferentially differentiate towards the M2 phenotype. IL-10, produced by M2 macrophages, can boost the formation of Treg, and simultaneously reduces neutrophil tissue migration. Neutrophils (polymorphonuclear granulocytes; PMN) have a longer life span; however, production of reactive oxygen species (ROS) is decreased. Natural killer (NK) cell proliferation and cytotoxic activity are both suppressed. B-cell proliferation is inhibited, and production of antibodies is reduced. HGF, hepatocyte growth factor; IDO, indoleamine-pyrrole-2-3-dioxygenase; PGE2, prostaglandin E2; and TGF-β, transforming growth factor-β. (Adapted from van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: iummunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm 2013: 181020, 2013.)
Dendritic cells are antigen-presenting cells that specialize in presenting naive T-cells with antigens after induction by proinflammatory cytokines. While the expression of MHC class I and II cell-surface markers (including CD11c and CD83), and other costimulatory molecules are increased upon dendritic cell maturation, they are downregulated in the presence of MSCs, thus weakening dendritic cell function (363). MSCs have also been found to prevent maturation of hematopoietic stem cells as well as immature monocytes into dendritic cells (169, 294). Furthermore, production of tumor necrosis factor (TNF)-α, a strong proinflammatory molecule, by dendritic cells is reduced by MSCs (4) (Figure 8).
Natural killer (NK) cells also play an important role in the innate immune system, specifically in antiviral and antitumor immune activity, through cell lysis and cytokine release. Cytolytic activity is based on activating and inhibitory surface receptors on the NK cells that recognize specific target-cell ligands (247). MSCs decrease expression of certain NK-cell surface activating receptors leading to a suppressed inflammatory state (Figure 8). The ability of resting NK cells to proliferate and produce IFN-γ after in vitro culture with IL-2 or IL-15 is diminished in the presence of MSCs (343). Alternatively, cytokine-activated NK cells target and destroy autologous and allogeneic MSCs, which can be partially rescued with the addition of IFN-γ (341, 344).
With respect to cardiac immunology, following an acute myocardial infarction (MI), two major subtypes of macrophages are found in the heart: 1) M1 macrophages, which are proinflammatory and release IL-1β, TNF- α, as well as IFN-γ; and 2) M2 macrophages, which are anti-inflammatory, promote angiogenesis, and help form scar tissue (196). MSCs have been found to augment the differentiation of M2 macrophages without decreasing their debris-clearance properties (101), thereby curbing the proinflammatory state post-MI.
B. Adaptive Immunity
T-cells, which require T-cell receptor (TCR) activation to exert effector functions, are central to the adaptive immune system (363). Both autologous and allogeneic MSCs can inhibit proliferation of T-cells that are stimulated by certain antigens or mitogens (80, 297, 361). Inhibition is mediated by T-cell arrest in G0/G1 phase of the cell cycle; administration of IL-2, however, can partly reverse this effect (26, 411). Furthermore, the antiproliferative effect of MSCs on T-cells causes a shift from a proinflammatory state, with decreased IFN-γ production, to an anti-inflammatory state with an increase in IL-4 production by T-helper (TH2) cells (4) (Figure 8).
Another main lymphocyte-type of the adaptive immune system is the B-cell, which is involved in antibody production and activated through T-cell-dependent and -independent pathways (363). MSCs can prevent B-cell maturation and differentiation, through arrest in the G0/G1 phase, and expression of chemokine receptors (65, 416). Although some reports (14, 65, 113) have shown that, in vitro, MSCs are capable of inhibiting B-cell survival, proliferation, and function, others (296, 359) have demonstrated conflicting results. Nevertheless, because of the strong immunosuppressive effect MSCs have on T-cells, which influence B-cell responses, MSCs may still exert immunomodulatory actions on B lymphocytes in vivo (Figure 8).
The activity of CD8+ cytotoxic T-lymphocytes (CTLs), a major type of T-cells that are responsible for MHC-restricted killing of target cells, is attenuated by MSCs (298). Although pretreatment of MSCs with IFN-γ was found to increase cell-surface expression of MHC class I molecules, they are not targets of CTLs (246). An important T-cell subtype is the regulatory T-cell, which is involved in tolerance to self-antigens by suppressing the immune system (363). Their production is increased by MSC-induced release of IL-10 from plasmacytoid DCs (pDCs) (222) (Figure 8). Moreover, MSCs enhance regulatory T-cell proliferation through their release of HLA-G5 (323). Thus the combined effect of MSCs inhibiting T-cell proliferation and cytotoxicity while supporting production of regulatory T-cells can help control an immune response. While the notion exists that excessive modulation may weaken the immune system, Toll-like receptors (TLRs) found on MSC cell-surfaces serve as a foolproof mechanism by impairing Notch signaling with subsequent reversal of MSC immunosuppression (216, 275, 357). Indeed, Abarbanell et al. (2) found TLR-2 to be a vital component of postinfarction, MSC-mediated myocardial recovery.
C. Mechanistic Insights Into Immunomodulatory Properties of MSCs
Two main mechanisms have been shown to underlie MSC-mediated immunosuppression: 1) cell-to-cell contact and 2) release of soluble factors as mentioned earlier (363). It has been proposed that the initial step enabling immunosuppression is cell contact between target cells and MSCs via adhesion molecules such as programmed cell death 1 (PD1) receptor and its corresponding ligands, respectively, resulting in decreased production and release of pro-inflammatory cytokines (14). Contact between MSCs and NK cells allows for downregulation of NK-cell activating receptors such as NKp30, NKp44, and NKG2D (343). MSC-mediated immunosuppression also relies on multiple soluble factors, which are not exclusively independent from one another. Molecules like NO (314) and IDO (239) are released by MSCs after release of IFN-γ by target cells (187). Other factors such as TGF-β1 (80), hepatocyte growth factor (HGF) (4), IL-6 (183), HLA-G5 (323), and PGE2 (343) are released autonomously by MSCs without requiring cross talk between cells. Soluble immunosuppressive factors can act alone or in combination to reduce T-cell proliferation and cytotoxicity as well as NK-cell cytotoxicity and to generate regulatory T-cells (363).
MSCs have been shown to attenuate inflammation in acute MI (83), by decreasing the production of TNF-α and IL-6 (proinflammatory) while increasing IL-10 (anti-inflammatory), as well as in cases of acute myocarditis (163) and cardiac dysfunction secondary to endotoxemia (384). Yet these effects depend on the degree of cardiac inflammation and are MSC-dose dependent (301). Cytokines released into the cardiac microenvironment during an inflammatory state can also affect MSC function through stimulation of MSC receptors. Activation of TNF-α receptor 1 and 2 leads to a reduction of gene expression of proinflammatory cytokines in MSCs, including IL-1β, IL-6, and TNF-α (17), and alters the expression of VEGF (232), an important paracrine mediator in post-MI recovery (233).
Although some in vivo studies have demonstrated MSCs' immunomodulatory properties, the effects may be less than or different from in vitro studies due to different factors including, but not limited to, variable MSC doses, culturing conditions, sources and techniques of isolation and/or expansion, and kinetics (199). Still, in vivo transplantation of MSCs have shown promising results in various studies of immune-dependent disease processes. Chapel et al. (49) found that systemically infused MSCs homed to injured tissues in a model of radiation-induced multiorgan failure. Intravenous MSC infusion has also been shown to prolong skin allograft survival (19) as well as offer treatment for steroid-resistant, acute graft-versus-host-disease (198) (GVHD).
It has been suggested that MSCs may lose their immunoprivileged state upon differentiation (391). This is partially supported by findings from a study on transplanted allogeneic MSCs using a post-MI rat model that demonstrated MSC elimination from the heart with no effect on cardiac function (158). Furthermore, this study found that, in vitro, MSC differentiation into myogenic, endothelial, or smooth muscle cells leads to a change in MHC-immune antigen profile with higher expression of MHC-Ia and II and lower expression of MHC Ib, thus transforming them from immunotolerant to immunogenic. Corroborating this evidence, allogeneic MSCs transplanted into post-MI swine mostly engrafted as undifferentiated cells with no induction of an immune response and the potential to stimulate endogenous cardiac repair (144, 290).
VII. MSCs AT THE CELLULAR LEVEL
A. Paracrine Effects
MSCs are known to produce and secrete a plethora of factors that contribute to their paracrine actions. These soluble molecules have been shown to contribute to cardiac functional recovery by aiding endogenous repair mechanisms (114–116, 182, 205, 243). Additionally, soluble factors released by MSCs not only stimulate cardiomyocyte regeneration and angiogenesis, but they further support antifibrosis and antiapoptotic activity as well as inhibit a proinflammatory state as previously mentioned (46, 146, 181). MSCs' paracrine action can also aid in myocardial recovery even from remote tissues as shown in preclinical (328) and clinical studies (174). This global trophic effect has been attributed to the activation of the Janus kinase (JAK)-signal transducer and activator of transcription-3 (STAT3) signaling pathway by MSC-derived IL-6-type factors and the subsequent increase in production of host-tissue derived factors (HGF and VEGF) that mediate activation of endogenous cardiac repair mechanisms (327).
Hypoxic MSCs that overexpress the prosurvival gene Akt (MSC-Akts) upregulate genes that encode for soluble factors, including VEGF, FGF-2, HGF, and insulin-like growth factor-I (IGF-I), which may be responsible for mediating cardioprotective effects. Conditioned medium from hypoxic MSC-Akts, in vitro, has been shown to significantly inhibit hypoxia-induced apoptosis and stimulate strong spontaneous contraction of adult rat cardiomyocytes, while in vivo, conditioned medium injected into post-MI rat myocardium has demonstrated attenuation of infarct size with improvement of cardiac function (115). These effects were observed rather early (<72 h post-infarction) and thus were suggested to be due to myocardial protection and enhancement of cardiac function rather than donor-cell engraftment and/or cardiomyogenesis (114, 115).
Secreted frizzled related protein 2 (Sfrp2), a paracrine factor released by MSC-Akts, also mediates myocardial survival and repair postischemic injury through the Wnt signaling pathway (243). Sfrp-2-treated cardiomyocytes have increased cellular levels of β-catenin, which leads to overexpression of antiapoptotic genes (243). Marrow-derived MSCs cocultured with neonatal rat ventricular myoctes (NRVMs) can enhance expression of the transmembrane potassium channel and increased the transient outward potassium current by secreting bFGF (27). Human MSCs in coculture with neonatal mouse ventricular cardiac myocytes (nMCMs), injured by incubation with endotoxin or IL-1β, protected cardiomyocyte calcium handling. Importantly, the presence of a semi-permeable membrane in this study suggested that paracrine signaling was operable (309).
The conditioned medium of murine MSCs contains bFGF, placental growth factor (PGF), monocyte chemoattractant protein-1 (MCP-1), and VEGF, all of which increase endothelial and smooth muscle cell proliferation in a dose-dependent manner (182). Following distal femoral artery ligation, mice injected with MSCs demonstrated an improvement in distal limb perfusion, function, and appearance with a concomitant reduction in muscle atrophy and fibrosis compared with controls. Local muscle levels of bFGF and VEGF proteins were elevated with colocalization of transplanted, labeled MSCs and VEGF. However, labeled MSCs continuously decreased weeks 1–4 and did not incorporate into blood vessels (182). Although the paracrine factors secreted by MSCs show beneficial effects in myocardial recovery, they have been shown to have more profound effects when released through administration of actual cells rather than conditioned medium alone (144). Furthermore, the secreted protein profile can be altered depending on the inflammatory setting, levels of oxygen within the cardiac microenvironment, and mechanical stress experienced by the MSCs and resident cells (153, 206, 354).
B. Exosomes and Extracellular Vesicles
More recently, MSCs have been shown to secrete microvesicles, macromolecular complexes, smaller phospholipid particles called exosomes, and extracellular vesicles (EVs) (244), all of which are involved in intercellular communication. Nucleic acids, in the form of mRNA, microRNA and non-protein encoding RNA, have been found in MSC-released exosomes (129), which interestingly differ from their parent cell RNA content (244, 422).
Some studies have demonstrated the ability of exosomal mRNA and microRNA, or exosomal shuttle RNA (esRNA), to be delivered to recipient cells resulting in translation of the proteins encoded from the transferred RNA within these new cells (366). In agreement, investigators have implicated the exchange of nucleic acids as a key exosomal function (373). Other exosome-mediated roles include transfer of proteins and lipids as well as modification of downstream genetic signaling with target recipient cells (373). On a larger scale, exosomes play important roles in immunological responses, as they are involved in antigen presentation, and are also involved in angiogenesis, coagulation, as well as programmed cell death (167, 355). EVs, which are similar in function and composition to exosomes, are also continuously released by cells and participate in genetic exchange (372). Endothelial progenitor cell (EPC)-derived EVs are involved in transfer of mRNAs and microRNAs to help induce endothelial-cell angiogenesis (78, 410). Furthermore, this transfer of genetic material exists as a bidirectional process between stem cells and differentiated endogenous cells (43). In addition to RNA molecules, EVs derived from embryonic stem cells contain proteins such at Oct-4 and Wnt-3 (148).
MSCs produce and secrete exosomes to a greater degree than muscle or human embryonic kidney stem cell lines (406). Common surface antigens of both MSCs and exosomes alone, including CD9, CD29, CD44, and CD89, have been found on exosomes derived from MSCs (193). Exosomes, and related extracellular membrane vesicles secreted by MSCs (i.e., EVs), are active components of intracardiac communication using horizontal transfer of information between cells (337). The first report of MSC-derived exosomes to be studied was by Lai et al. using a murine ischemia/reperfusion injury model (193). They reported that exogenously administered purified exosomes can reduce infarct size and concluded that MSCs exert their cardioprotective paracrine effect through exosomal release. When exosomes derived from MSCs overexpressing the transcription factor GATA-4 were transplanted into the border zones of post-MI rat hearts, cardiac contractile function was restored and infarct size was reduced. The observed cardioprotective effects were at least in part due to transfer of miR-19a in cardiomyocytes, resulting in reduced cardiomyocytic expression of the phosphatase and tensin homoglog (PTEN) and secondary activation of the Akt and ERK signaling pathways (409). MSCs can also release large quantities of EVs under hypoxic conditions (30). Infarcted hearts treated with intramyocardial injection of MSC-EVs exhibited significantly improved blood flow through angiogenesis-based mechanisms, as well as reduced infarct size while preserving systolic and diastolic function.
The use of exosomes and EVs has also been studied as a therapeutic strategy for renal and neurological diseases (300, 398). Renal injury was attenuated in rats receiving MSCs, MSC-conditioned medium (MSC-CM), or exosomes purified from the MSC-CM, by blunting the increase in creatinine, urea, and fractional excretion of sodium (FeNa) (300). Furthermore, addition of RNase to MSC-CM or MSC-CM-derived exosomes inhibited these effects, suggesting that exchange of RNA particles plays a major role. Similarly, injured renal proximal tubular tubular epithelial cells (PTECs) treated with MSC-EVs have been found to have enhanced incorporation of these vesicles with increased EV-mediated transporting miRNAs involved in renal recovery (215). Ischemic brain parenchymal cells have demonstrated an increase in neurite branch number and total neurite length after MSC treatment (398). These findings have been partially attributed to transfer of microRNAs 133b (miR-133b) and cel-miR-56 from MSC-derived exosomes to neural cells.
Exosome-mediated transfer of genetic information has been studied in the context of inflammatory states (259), including ischemic heart disease (121). Cardiac endothelial cell (CED)-derived exosomes have been reported to induce regulatory B-cells involved in immunosuppressive functions (339). However, while the tissue reparative potential of MSC-derived secretome may not depend on their cellular counterpart (15), this does not necessarily hold true in the immune response setting. Similarly, bone marrow MSCs and adipose tissue MSCs have been shown to exhibit immunosuppressive effects on lymphocyte proliferation via EV secretion, but isolated EVs have not (122). This corroborates the aforementioned discussion regarding the necessary cell-to-cell contact between MSCs and target cells for proper immunomodulation. Alternatively, treatments using MSC-derived EVs alone seem to impart different immunomodulatory effects than their cells of origin (74).
C. Mitochondrial Transfer
Another method of intercellular communication employed by MSCs is through mitochondrial transfer. Damaged cells that are devoid of functional mitochondria or mitochondrial DNA can benefit from mitochondrial transfer by MSCs (57), via a restoration of impaired oxidative phosphorylation and bioenergetics caused by dysfunctional mitochondria (214). The physiological importance of mitochondrial transfer and its role in rescuing mammalian-cell aerobic respiration in somatic cells with dysfunctional mitochondria was initially studied almost a decade ago by Spees et al. (345). While the exact underlying mechanisms still remain unclear, the presence of tunneling nanotubules and gap junctions containing connexin-43 have been linked to the process of mitochondrial transfer (266, 367). Inhibition of nanotubule formation has been associated with a decreased capacity for mitochondrial transfer (209). Miro1, a mitochondrial Rho-GTPase, is a key regulator in intercellular mitochondrial transport (197) and has been shown to enhance the mitochondrial donor capacity of MSCs (5). Treatment of injured epithelial cells in asthma models using MSCs expressing Miro1 results in increased rescue of epithelial injury as well as reversal of airway responsiveness and remodeling (5). Furthermore, MSCs express higher levels of Miro1, compared with lung epithelial cells or fibroblasts.
The interplay between MSCs and cardiomyocytes using mitochondrial transfer is also essential in providing therapeutic insights into heart disease (194). Coculture of human MSCs and rat cardiac myocytes resulted in transfer of mitochondria within nanotubes, suggesting a possible contribution to myocardial repair (279). Although the above-mentioned studies have demonstrated a unidirectional transfer of mitochondria by MSCs, mitochondrial transfer to MSCs can also occur by vascular smooth muscle cells (VSMCs), which results in an upregulation of MSC proliferation (367). Regardless, MSC-mediated mitochondrial transfer holds great promise in ischemia-reperfusion injury disease patterns, such as ischemic cardiomyopathy (342).
D. Reconstitution of the Cardiac Niche
MSCs have been shown to be a component of, contribute to, and support stem cell niches, or microenvironments, thereby maintaining tissue homeostasis (162, 178, 237). These cell niches are comprised of resident stem cells and supporting cells and exist in a variety of tissue structures and organs including hair follicles (265), epidermis (58), intestinal epithelium (407), bone marrow (413), the brain (330), and the heart (364). Stem cell niches, including cardiac niches, represent specialized dynamic entities, that regulate the quiescent and active states of resident stem cells (203) through cell-to-cell signaling triggered by cytokine/chemokine release, cell surface adhesion molecules, shear forces, innervation, and oxygen tension (346, 379). Remarkably, populations of epicardially derived MSC-like stem cells have been found to occupy perivascular cardiac niches (60).
The stromal derived factor-1 (SDF-1)/chemokine receptor type 4 (CXCR4) axis is a major cell-signaling axis involved in homing of MSCs to injured cardiac tissue (112). Accordingly, Src family protein kinases (SFK) are activated by the SDF-1/CXCR4 axis and may also be critical in ischemic cardiac recruitment of MSCs (56). While homing of MSCs to injured tissue remains a fundamental characteristic of their cardioreparative abilities, interactions between some protein factors, such as Fas and Fas ligand, within the cardiac milieu may activate stem cell apoptosis with loss of implanted MSCs (134). Importantly, MSCs play a critical role in cardiac niche homeostasis by enhancing endogenous repair mechanisms following myocardial injury (203). Expression of mitogens like periostin (189), neuregulin (29), and neuropeptide Y (381) purportedly promote cardiac regeneration by stimulating cell cycle reentry of adult differentiated cardiomyocytes, although others failed to record such an effect (302). An increase in cardiomyocyte mitosis and survival has also been demonstrated using FGF1/p38 MAP kinase inhibitor therapy (90). It was also found that, after acute MI, rat hearts treated with FGF1/p38 MAP kinase inhibitor resulted in reduced scarring, reduced wall thinning, and markedly improved cardiac function (90).
VIII. IN VIVO MECHANISM OF ACTION
A. Engraftment, Differentiation, and Fusion
Engraftment and differentiation rates of MSCs are relatively low compared with other cellular effects they render, including their paracrine actions (203). Still, numerous reports regarding these mechanisms of action for both autologous and allogeneic MSCs exist in the literature, albeit conflicting evidence (180, 227, 285, 329, 335, 356). Porcine hearts directly injected with autologous, Di-I-labeled MSCs into the infarct zone, 2 wk following left anterior descending (LAD) artery occlusion, were found to have marked engraftment in the host myocardium as well as markers for myocardial-specific proteins troponin T, tropomyosin, myosin heavy chain, and α-actinin (329). Similarly, allogeneic bromodeoxyuridine-labeled MSCs, delivered via direct intramyocardial injections 1 mo after MI induction, engrafted in the peri-infarct zone and differentiated into cardiomyocytes (227). In another porcine acute MI model, intravenous infusion of Di-I-labeled allogeneic MSCs demonstrated engraftment in the infarct and peri-infarct regions, and cells were also found to engraft in the lungs (285). Engraftment of MSCs following intramyocardial injection has also been demonstrated in a dog model of chronic ischemic cardiomyopathy (335). Interestingly however, the engrafted cells expressed markers of endothelial and smooth muscle cell lineage, but no evidence for cardiomyocyte differentiation was documented (335). Indeed, the capacity of MSCs to differentiate into endothelium and smooth muscle cells in addition to cardiomyocytes has previously been documented (290).
Others have found little to no engraftment or differentiation in myocardial injury models (180, 356). Human β-galactosidase+ MSCs transdiaphragmatically delivered to rat left ventricles engrafted in host myocardium at a rate of only 0.44% 4 days following injection (356). Significantly, most cells were found in the lungs, spleen, and liver. The engrafted cells however began to appear morphologically indistinguishable from the host cardiac myocytes and, starting as early as 2 wk post-injection, revealed de novo expression of desmin, β-myosin heavy chain, α-actinin, cardiac troponin T, and phospholamban with sarcomeric organization of contractile proteins (356). In another study, mouse hearts that were administered human placenta-derived amniotic MSCs (AMCs) following left anterior descending (LAD) artery ligation did not demonstrate any immunohistological evidence of engraftment, while those that were treated with c-kit+ AMCs showed engraftment yet no cardiac differentiation of cells (180). Similarly, by utilizing a combination of in vivo bioluminescence imaging and DNA-based quantitation of cell engraftment approaches, Wu and colleagues (369, 370) recorded a fast decline in the engraftment of intramyocardially injected mouse MSCs and no improvement in cardiac function, over a period of 4–6 wk following transplantation. Interestingly, compared with MSCs, engraftment of bone marrow mononuclear cells was robust and resulted in a modest functional benefit in the heart function of female FVB mice with experimental myocardial infarction (369, 370).
These inconsistent findings on MSC survival have led to the concept of cell transiency, suggesting that MSCs' engraftment persists only for a limited time (166). In a swine acute-MI model, Tao et al. (352) found that autologous bone marrow-derived MSCs engrafted within the ischemic myocardium at week 3 but not at week 10 post-injection (352). Analogously, immunohistochemistry studies against the Y-chromosome by Dixon et al. (81) showed that transplanted male allogeneic MPCs into post-MI female sheep hearts were readily apparent in host border zones 1 h post-injection but absent at 8 wk post-MI, indicating a failure of prolonged engraftment (81). Although the reasons behind this phenomenon are not exactly clear, they may possibly be related to the MSC source, mode of delivery (352), and/or disease model employed (i.e., acute vs. chronic). In fact, an intravenous approach to cellular delivery has been to shown to result in insufficient MSC engraftment (150), and intramyocardial administration may be more superior to the intravenous or intracoronary routes (103). Yet, others have proposed that the cardioreparative properties of MSCs do not depend on permanent engraftment (267). Notwithstanding this, efforts to enhance MSC survival, and thus their therapeutic efficacy, have been increasingly made (69, 307, 403, 418). Combined therapy with simvastatin has been shown to promote MSC survival and cardiovascular differentiation almost fourfold in infarcted Chinese miniswine (403). Transfected MSCs with miRNA-133a mimic can improve MSC survival both in vitro and in vivo with downregulation of pro-apoptotic genes apoptotic peptidase activating factor (Apaf)-1, caspase-9, and caspase-3 (69). Inhibition of inositol hexakisphosphate kinases (IP6Ks) has been shown to lead to enhanced Akt activation increasing MSC engraftment and preserving myocardial function following MI (418). More recently, biomaterial delivery vehicles have been developed to evaluate MSC tissue retention (307). Roche et al. (307) compared engraftment rates of MSCs administered via saline injection versus injectable hydrogel or epicardial patch biomaterial carriers. Human MSC delivered to infarct-border zones of postinfarct rats by any of these methods demonstrated that there was an 8- to 14-fold and 47- to 59-fold higher engraftment rate when injectable hydrogels and epicardial patches, respectively, were used compared with saline injection. Furthermore, all biomaterials retained 50–60% of MSCs that were present immediately prior to injection, with only 10% retention for the saline injection control group (307).
Another proposed mechanism that may contribute to MSC cellular action is the process of MSC fusion with different cell types including neurons, hepatocytes (8), and even cardiomyocytes (254, 256). At 2 days (and 1 wk) following human MSC delivery to murine hearts via a collagen patch, fusion of transplanted cells with recipient cardiac cells was detected using a Cre-LoxP-based reporter system (102). Fusion products were also found in distal organs such as the stomach, small intestines, and liver. Importantly, these fused cells were located perivascularly, suggesting a hematological migration route (102). Overexpression of Akt by MSCs can lead to higher engraftment of cells within infarcted myocardium with low levels of cellular fusion (254). However, similar to their engraftment properties, MSC fusion with cardiomyocytes seems to be an early and transient event (254). Furthermore, its occurrence is rare and, if present, infrequently takes place. As such, it is questionable whether or not this mechanism is considered a substantial contributor, if at all, to cardiac functional improvement.
IX. ANTIFIBROSIS, ANGIOGENESIS, AND CELLULAR REGENERATION
A. Antifibrosis and Prosurvival
Transplantation of MSCs confers cardioprotection by limiting the degree of apoptotic cell death in the heart. Apoptotic cardiac and neuronal cells secrete high levels of HGF, a potent chemoattractant for MSC migration towards injurious sites (375). Significantly, recruitment of MSCs toward damaged tissue is reduced upon blockage of HGF bioactivity. In a rat cardiac ischemia-reperfusion model, intracoronary infusion of wild-type MSCs induced the highest degree of myocardial functional recovery and antiapoptosis while infusion of STAT3 knockout MSCs exhibited similar recovery as control (282). STAT3 knockout rats also exhibited higher levels of inflammatory cytokines, HGF, and caspase-3 than wild type. These findings demonstrate the importance of the STAT3 signaling pathway in reducing proinflammatory and proapoptotic factors. MSC-derived growth factors alone have also been shown to reduce cardiomyocyte apoptosis when studied in in vivo acute-MI (114, 252) models, with a particular emphasis on MSCs' paracrine effects. Garikipati et al (108) employed serial pinhole gated SPECT-CT to evaluate cardiac function and cell tracking using PKH26 dye in ischemic myocardium treated with rat fetal heart-derived MSCs (fcMSCs). An abundance of PKH26-labeled fcMSCs were found in both infarct and peri-infarct regions. Importantly, fcMSC-treated hearts had fewer apoptotic cells; upregulated gene expression of VEGF, bFGF, and HGF-1; overexpressed the antiapoptotic protein Bcl2; and had a significantly lower expression of the proapoptotic protein Bax compared with control. Our group has highlighted the cytoprotective properties of autologous (196) and allogeneic (197) MSCs through their ability to reduce myocardial fibrosis in post-MI injected swine as assessed by cardiac MRI. Others (213) have found that treating ischemic swine with MSCs overexpressing Akt significantly enhances the antifibrotic effects of MSCs while protecting them from apoptosis. Enhancement of MSCs' antiapoptotic effects can be accomplished through genetic engineering with overexpression of certain transcription markers (115, 207), as described below. MSCs can therefore be a useful tool in inhibiting or diminishing the apoptotic stimuli that trigger myocyte loss following ischemic cardiac events.
Formation of scar not only initiates cardiac remodeling to a spherical shape but is also closely interrelated with endogenous myogenesis. Type I collagen is the most frequently found collagen in fibrotic tissues, and the presence of tissue fibrosis has been associated with dysregulation of myocyte regeneration and repair (6, 35). Indeed, exposure of MSCs to type I collagen leads to a downregulation of growth and inflammatory gene factors with a resultant decrease in MSC-induced myoblast proliferation potential (73). Matrix metalloproteinases (MMPs) are a group of essential molecules that maintain extracellular matrix (ECM) homeostasis and ECM remodeling plays a large role in regulating myocyte migration, differentiation, and regeneration (55). MSCs release a combination of various MMPs and tissue inhibitors that are involved in extracellular remodeling (245). Notably, the ratio of MMPs to tissue inhibitors can be modulated by overexpression of certain factors, which can reverse the process of cardiac remodeling (334, 351). Moreover, MSCs are capable of regulating the ECM degradative potential of cardiac fibroblasts, thereby supporting an indirect antifibrotic mechanism (242). Indeed, the reduction of fibrosis in scarred tissues, which involves ECM degradation, aids in improving the renegerative capacity of endogenous myocytes (325). Regardless of the method of cardiac neomyogenesis, the process seems to occur under significant manipulation in vitro while biological in vivo influences may not be adequate for driving cardiac regeneration (356).
B. Neovascularization and Angiogenesis
Postnatal blood vessel formation, or neovascularization, is conducted through angiogenesis or arteriogenesis (40) and is of particular importance in myocardial functional restoration. Following myocardial injury, changes in vessel fluid shear stress serve as a molding force for local recruitment of smooth muscle cells and differentiation of newly formed capillary vessels into arteries or veins (40, 402). MSCs can contribute to neovascularization by 1) releasing growth factors and/or cytokines, 2) differentiating into endothelial cells or 3) vascular smooth muscle cells, and 4) acting as perivascular cells.
Secretion of proangiogenic and proarteriogenic factors by MSCs is the main mechanism suggested to play a role in neovascularization (120). VEGF signaling is essential in the formation of new blood vessels, primarily through its binding interaction with PDGF receptor (PDGFR) (16), and is upregulated 30-fold by hypoxia-inducible transcription factors (303). Our group has shown that downregulation of PDGFR- α, crucial for VEGF-A action, in MSCs derived from GSNOR-deficient mice markedly decreases the capacity for MSC-mediated neovascularization (120). These results are in agreement with the significance of VEGF in stem cell-mediated cardioprotection (233). Of note, exogenously administered VEGF alone does not meet the full potential of vascular regeneration but rather MSCs and their secretomes containing VEGF are superior (253). bFGF is another well-studied proangiogenic and proarteriogenic factor that facilitates blood vessel morphogenesis (47). In a murine MI model, transplantation of MSCs overexpressing connexin-43 resulted in augmented bFGF and VEGF expression and an increase in vascular density, compared with connexin-43 knockout cells (377).
Other factors in the cardiac microenvironment, such as NO, can modulate the process of neovascularization. We have also shown that GSNOR-deficient mice exhibit a decreased propensity for vessel formation, highlighting the inhibitory properties of S-nitrosylation signaling in MSC-mediated vascularization (120). Furthermore, blockade of NO synthase, an enzyme responsible for NO production, resulted in normalization of vessel tube formation, whereas NO donors reduced tube formation (120), at least partially due to suppressed secretion of VEGF and HGF from MSCs (380). In addition to the indirect action of released soluble factors, MSCs can directly exchange exosomes and microvesicles containing factors crucial for neovascularization (51, 326). These extracellular vesicles are composed of VEGF, bFGF, angiogenin, MCP-1, and many other proangiogenic cytokines that are important in neovascularization-related cell signaling (51).
MSCs have also been shown to undergo endothelial cell differentiation (290) and form isolated cell aggregates expressing the endothelial cell-specific markers von Willebrand's antigen and CD31 (109). When cocultured with endothelial cells, MSCs promote the generation and maturation of new vessels in a time- and dose-dependent manner (84). Interestingly, the MSC's tissue of origin seems to play an important role in their vasculogenic capacity, since MSCs derived from Wharton's jelly have a higher endothelial differentiation potential compared with others (52). Differentiation of MSCs into vascular smooth muscle cells has also been suggested by the expression of α-smooth muscle actin, although expression of this marker is not coronary smooth muscle cell lineage-specific and could indicate differentiation into myofibroblasts (70). Smooth muscle cell transdifferentiation can be stimulated by soluble factors, such as TGF-β1 (7), and even through mechanical stimulation (404). Within the perivascular niche, MSCs and their tissue-specific progeny have been identified among pericytes, or perivascular mural cells (45). These progenitor cells express surface markers specific for MSCs, myogenic cells, and the endothelial cell phenotype (66). In addition to their ability of differentiating into endothelial and smooth muscle cells, they can act as pericytes by triggering the tubelike formation of endothelial cells and development of robust vascular networks (41). In vivo studies for ischemic heart disease have found MSC transplantation to significantly increase capillary bed density in viable myocardium versus scarred (280). These findings however are closely related to MSCs' paracrine role by upregulation of VEGF and bFGF rather than their engraftment and differentiation properties (210). Furthermore, various growth factors and proteins can amplify much of their angiogenic and arteriogenic potential (24, 85).
C. Cardiomyocyte Regeneration
As described above, cocktails of growth factors and cytokines can stimulate the cardiomyocyte differentiation potential of MSCs (123, 226, 332, 408). TGF-β receptor signaling negatively regulates cardiomyocyte formation and inhibition of the receptor using a 1,4-dihydropyridine inducer of type II TGF-β receptor degradation-1 (ITD-1) analog stimulates mesodermal stem cell differentiation into cardiomyocytes (390). BMP-4 can markedly enhance MSC cardiomyogenic differentiation, suppress skeletal myogenesis, and increase the presence of the longer action potential, characteristic of cardiomyocytes (123). Mechanical (110) and electric (111) stimuli can also influence the differentiation of MSCs into cardiomyocytes. However, while many investigators have been successful in promoting MSC cardiomyogenic differentiation (171, 332, 378, 408), others have not been able to reproduce such findings (184, 313). Alternatively, some authors propose that perivascular cells within the cardiac niche contribute to cardiomyogenesis (107). Our group validated the ability for MSCs to induce proliferation and differentiation of resident cardiac stem cells thus enhancing cardiomyocyte regeneration (144).
D. Stimulation of Endogenous Stem Cells
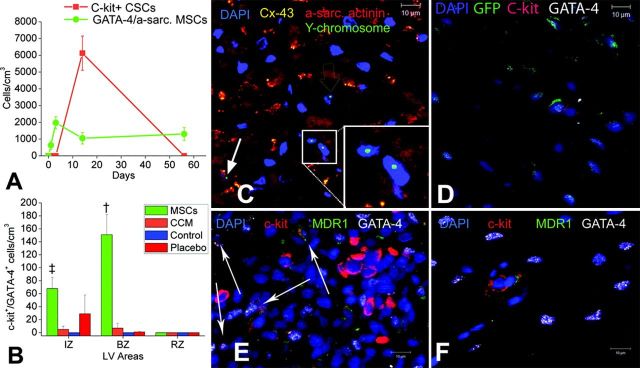
MSCs have also been reported to stimulate the proliferation of endogenous cardiac stem cells (CSCs). Hatzistergos et al. (144) demonstrated that in vivo transendocardial injections (TESI) of allogeneic bone marrow-derived MSCs into the infarct-border zones of post-MI swine led to a 20-fold increase in endogenous c-Kit+ CSCs, a 6-fold increase in GATA-4+ CSCs, and a 4-fold increase in mitotic myocytes (Figure 9). Histological examination of the infarct-border zones displayed specific niches containing chimeric clusters of exogenous MSCs and endogenous c-Kit+ CSCs. These cells formed connections with each other and neighboring cardiomyocytes through connexin-43 gap junctions and N-cadherin. Additional in vitro experiments revealed that coculture of c-Kit+ CSCs and MSCs increased c-Kit+ CSCs 10-fold compared with c-Kit+ CSCs cultures alone (144). Furthermore, c-Kit+ CSCs proliferated into adult cardioblasts expressing Nkx2-5, an important transcription factor implicated in cardiac structural remodeling (320). In agreement, Williams et al. (392) administered a combination of human MSCs and human c-Kit+ CSCs intramyocardially to swine in an acute ischemic cardiomyopathy model and found that combination therapy conferred an enhanced engraftment of stem cells, greater reduction in infarct size, substantial improvement in left ventricular volumes, and stronger contractility than either cell type alone. Together, these findings help illustrate the cooperative effect of MSCs and CSCs, by means of their complex biological interactions, in the cardiac niche. Nevertheless, the question whether MSCs, together or without CSC therapy, trigger the formation of new temporary niches or preserves preexisting and/or dysfunctional niches still remains to be answered (202).
FIGURE 9.
MSC cardiomyogenesis. A: graph depicting the contribution of cardiomyocyte precursors following exogenous administration of MSCs (green line) and endogenous CSCs (orange line), during cardiac repair after MI. MSC differentiation occurs rapidly after delivery. At 2 wk, MSCs activate endogenous expansion of c-kit+ CSCs (orange line). B: 2 wk following TEI, the number of c-kit+ cells coexpressing GATA-4 is greater in MSCs versus non-MSCs treated hearts. The cardiac precursors are preferentially located in the IZ and BZ of the MI, indicating an active process of endogenous regeneration (‡P = 0.019; †P < 0.0001) C and D: the 2-wk-old chimeric myocardium contains mature cardiomyocytes (open arrow), immature MSCs (arrowheads, inset), and cardiac precursors of MSCs origin (arrow), coupled to host myocardium by connexin-43 gap junctions. Interestingly, endogenous c-kit+ CSCs are found in close proximity to MSCs (D). E: cluster of c-kit+ CSCs in an MSC-treated heart; numerous CSCs are committed to cardiac lineage documented by GATA-4 and MDR-1 coexpression (arrows). F: few, isolated c-kit+ cells were found in non-MSC-treated animals. [From Hatzistergos et al. (144), with permission from American Heart Association.]
Recently, MSCs have been studied in the setting of endothelial dysfunction and vascular disease (284). Our group found that individuals with heart failure demonstrated increased endothelial progenitor cell (EPC) colony formation, enhanced flow-mediated vasodilation (FMD), and reduced levels of VEGF following transendocardial administration of allogeneic MSCs (284). Indeed, these functional parameters are known to strongly correlate with and contribute to the pathophysiology of cardiomyopathy (88, 234, 387). The therapeutic potential for MSCs to restore endogenous endothelial stem cells to near-normal levels is of tremendous value to understanding cardiovascular disease and further progressing treatment strategies.
X. AUGMENTATION OF MSC EFFECTS
The restorative capacity of MSCs has mainly been limited by its poor cell engraftment and survival. As such, attempts have been made to enhance the cellular and therapeutic effects of MSCs by preconditioning with growth factors and/or drugs and utilizing genetic modification.
A. Preconditioning
Pretreatment of MSCs with VEGF leads to an upregulation of prosurvival factors, Akt and Bcl-xL, and downregulation of cell cycle inhibitors, p16 and p21, thus increasing MSC proliferation while reducing cellular stress (281). In vitro treatment of MSCs with IGF-I with subsequent infusion into post-MI rat tail veins results in marked expression of cell surface CXCR4 after 48 h. Four weeks following transplantation, these pretreated MSCs demonstrate higher engraftment in the infarct-border zones, enhanced capillary density, and an attenuation of cardiac dysfunction, LV chamber enlargement, and scar formation (128). Trimetazidine, an anti-ischemic drug used for treating angina, can be used to protect against peroxide-induced membrane damage and loss of cellular viability when preconditioned with MSCs (394). Furthermore, hearts transplanted with trimetazidine-preconditioned MSCs showed an improvement in cardiac function post-LAD ligation with overexpression of Akt and Bcl-2. Supernatants of heme oxygenase (HO)-1 MSCs can suppress hypoxia-induced apoptosis and cause MSCs to produce protective cytokines such as TGF-β, IL-1β, and VEGF (412). The paracrine action of HO1-1 MSCs includes enhancement of LV function, increase in angiogenesis, and inhibition of cardiomyocyte apoptosis in a rat myocardial infarction model (412). Priming MSCs with a “cocktail” of growth factors, including FGF-2, IGF-I, and BMP-2, improved their cytoprotective effects on cardiomyocytes through an increase in gap junction formation (132). Similarly, in a rat MI model, transplanted growth factor-treated MSCs resulted in a reduction of infarct size with cardiac functional improvement.
B. Genetic Modification
With the use of a murine model, genetically modified MSCs overexpressing C-C chemokine receptor type 1 (CCR1) significantly increases chemokine-induced MSC migration and prevents apoptosis in vitro while enhancing MSC engraftment and myocardial function, thereby preventing cardiac remodeling, in vivo (155). Preischemic infusion of MSCs derived from tumor necrosis factor receptor (TNFR)-1 knockout mice, but not TNFR2 knockout mice, restores cardiac function after ischemic-reperfusion injury (177). This suggests that TNFR2 signaling is responsible for exerting beneficial effects of MSCs, whereas TNFR1 negatively modulates MSCs' paracrine action. Modification of survivin (SVV)-engineered MSCs under hypoxic conditions leads to increased secretion of VEGF (96). Transplantation of SVV-engineered MSCs after permanent LAD ligation can increase capillary density and reduce infarct size. Importantly, cell viability is enhanced and cardiac function is restored at 1 wk and as late as 28 days after intramyocardial tranplanation (96). SDF-1 is strongly chemotactic for lymphocytes, and its interaction with its receptor CXCR-4 can function as a target for myocardial perseveration (414). Autologous MSCs that overexpress SDF-1 can improve myocardial function though a direct increase of cardiomyocyte survival rather than triggering endogenous cardiac myocyte regeneration (414). Similarly, overexpression of antiapoptotic genes Akt (115) and Bcl-2 (207) enhances myocardial functional recovery by increasing cellular survival and inhibiting cell death, as mentioned above. Thus cell preconditioning and genetic modification serve as rather promising options in augmenting MSC-based therapy.
XI. PRECLINICAL STUDIES
A. Ischemic Cardiomyopathy in Murine Models
Many of the initial MSC studies for heart disease using murine models were performed over a decade ago and focused on the capability of these cells to transdifferentiate into cardiomyocytes (117, 295). Toma et al. (356) were among the first to investigate the use of MSCs in enhancing the functional capacity of diseased myocardium. They found that human MSCs (hMSCs) derived from adult bone marrow had the potential to undergo myogenic differentiation after transplantation into adult murine myocardium, underlying the basis for its use for cellular cardiomyoplasty (356). In a similar study by Gojo et al. (117), bone marrow-derived MSCs primed with 5-azacytidine were injected into mouse recipient myocardium and exhibited in vivo cardiovasculogenesis. Together, these findings have prompted the utilization of MSCs in murine ischemic models (70, 180, 230). Davani et al. (70) found that autologous MPCs directly injected into ischemic rat hearts improved left ventricular performance and increased vessel density 30 days following implantation. Furthermore, MPCs engrafted and differentiated into smooth muscle and endothelial cells (70). In a different study, infarcted rat myocardium treated with MSCs modified with Akt restored cardiac performance to near normal and prevented remodeling (230). Recently, Kim et al. (180) examined a subpopulation of MSCs, AMC-derived induced pluripotent stem cells (MiPSCs), in treating mice that underwent LAD ligation. MiPSC-treated mice had a greater, more sustained improvement in ventricular function and stem cell engraftment than unselected AMCs or c-kit+ AMCs (180). Still, many studies using murine models for ischemic cardiomyopathy employed cardiomyocyte transplantation rather than MSCs (249, 299, 415).
B. Ischemic Cardiomyopathy in Large Animal Models
Favorable data from small animal models have paved the way for MSC-based cardiomyoplasty to be studied in larger animal species such as swine (9, 141, 287, 290, 305, 321, 322, 329, 420), sheep (81, 135), and dogs (272, 335). Arrays of experiments have ranged from using autologous (287, 329, 420) and allogeneic (9, 141, 290, 321, 335, 371) MSCs in acute (141, 287, 329), subacute (9, 321, 371), and chronic ischemic cardiomyopathy (290, 322, 335, 420) models delivered via multiple routes of administration (9, 133, 272, 287, 305, 321, 322, 329, 335, 420) (Figure 10).
FIGURE 10.
Delivery methods of MSC therapy. A: intravenous (IV) infusion of mesenchymal stem cells (MSCs; peripheral IV not shown). B: administration of MSCs through transendocardial injection (TESI). C: direct epicardial injection of MSCs. D: delivery of MSCs via intracoronary infusion.
During the acute phase following an MI, the proinflammatory and proapoptotic cardiac milieu counter the proreparative effects of MSCs, but strong homing signals and alterations in ECM regulation can help harness MSCs' therapeutic actions. In an acute MI model, swine were injected with autologous MSCs directly into the myocardium 2 wk following a 60-min LAD occlusion (329). Transplanted MSCs engrafted and demonstrated expression of muscle-specific proteins as early as 2 wk post-injection with significant attenuation of left ventricular contractile dysfunction at 4 wk after injection (329). Qi et al. (287) delivered magnetically labeled autologous MSCs to postacute MI swine via intracoronary infusion and noted that cell treatment substantially improved ejection fraction (EF) and blunted MI size increases at 8 wk post-infusion. Although certain routes of delivery like intravenous (133) and intracoronary (287) infusions offer practical techniques for administration, there is potential for cell entrapment in other organs, such as the lungs, and thrombosis and/or coronary artery occlusion leading to ischemia, respectively (18, 331). Many investigators have thus employed catheter-based delivery systems, such as transendocardial stem cell injection, or TESI, to prevent such complications while maintaining cell therapeutic efficacy (9, 130, 141, 144, 290, 321). Perin et al. (272) found that transendocardial injections of MSCs were superior to intracoronary delivery in a canine model of acute MI, validated by greater cell retention, increased vascularity, and higher functional improvement. On the other hand, Rigol et al. (305) used a porcine model of acute MI to compare the same two delivery routes and found that intracoronary infusion increased neovascularization compared with TESI, but both modes of delivery had comparable engraftment rates.
MSC-based therapy has also been tested in chronic ischemic cardiomyopathy using large animal models and the previously mentioned delivery methods, although most chronic models have employed TESI or direct intramyocardial injections (322, 335, 420). In a chronic MI, the fibrotic process has stabilized and tissue disorganization makes the myocardium prone to further dysfunction. Zhou et al. (420) directly injected swine with chronic ischemic injury with ex vivo expanded autologous MSCs 4 wk following placement of an ameroid constrictor around the proximal circumflex artery (420). Six weeks post-injection, the authors found that the MSC-treated group displayed a 40% improvement in myocardial function, as assessed by regional wall thickening, compared with a 4% change in saline-treated animals. The MSC-treated group demonstrated an increase in EF by 20% and, compared with control, had a higher vascular density as calculated by capillary-to-myocyte ratio (420).
The first preclinical randomized study of bone marrow-derived allogeneic MSCs administered to pigs was conducted by Amado et al. (9). This study highlighted the safety and efficacy of allogeneic MSCs, by demonstrating no signs of rejection and profound infarct size reductions with restoration of cardiac function to near-normal levels, respectively (9). Hashemi et al. (141) corroborated the safe use of allogeneic MSCs and revealed that endomyocardial injections of allogeneic MSCs in an acute MI porcine model did not produce any toxic effects clinically or by histology (141). Using a swine model of chronic ischemic cardiomyopathy, we found that allogeneic MSCs, delivered transendocardially to ischemic myocardium, were able to differentiate into cardiomyocytes, vascular smooth muscle, and endothelial cells (290). These results were accompanied by a reduction in infarct size, an increase in EF, and improvements in regional contractility and myocardial blood flow which were at least partially attributable to their trilineage differentiation potential (290) (Figures 11 AND 12). In a similar study by Silva et al. (335), however using a canine chronic ischemic model, intramyocardial injections of MSCs increased EF in the cell-treated group, yet only a trend in reduced fibrosis and increased vascular density was observed (335). Furthermore, MSCs coexpressed markers of vascular smooth muscle and endothelial cell lineages, but not cardiomyocytes (335). Therefore, allogeneic MSC therapy has proved to be both safe and efficacious in cardiac regenerative medicine, supporting its role as an excellent “off-the-shelf” product.
FIGURE 11.
Infarct size assessment and regional myocardial function. A and B: sequential short axis heart sections from base (top) to apex (bottom) of delayed gadolinium enhancement MRI images depicting the infarct extension (white) before treatment and 12 wk following MSC therapy (A) compared with the placebo (B). Comparable gross pathology sections are shown adjacent to the MRI images. Arrows delineate the infarct extension, and the asterisk illustrates the presence of a thrombus near the apex in a placebo animal. C: reduction in infarct size following 8 and 12 wk post MSC transplantation versus placebo (*P < 0.001 within MSC group ANOVA, †P < 0.05 between groups ANOVA, ‡P < 0.001 vs. preinjection status by Student-Newman-Keuls test). D: myocardial strain analysis represented by peak Ecc decreased in response to MSC treatment in infarct (left) and border (middle) areas but remained constant in the remote uninfarcted zone (right) (*P < 0.05 for ANOVA within MSC group, †P < 0.05 ANOVA between groups, ‡P < 0.05 vs. preinjection status by Student-Newman-Keuls test). At least five MRI time points were analyzed for MSC-treated hearts (n = 6) and placebo-treated hearts (n = 4). For week 4 tagged MRI images and week 8 delayed contrast enhancement MRI analysis, MSCs (n = 4) and placebo (n = 4). [From Quevedo et al. (290).]
FIGURE 12.
Myocardial blood flow and segmental contractility showed improvement related to the cell engraftment. A: quantitative analysis of first-pass perfusion MRI in the infarct (left), border (middle), and remote (right) myocardium demonstrating the improvement in the MSC-treated group compared with placebo group (*P < 0.05 for ANOVA within MSC, †P < 0.001 ANOVA between groups, ‡P < 0.05 vs. preinjection status by Student-Newman-Keuls test). LVBP, left ventricular blood pool. B: LV ejection fraction improves in MSC group at 12 wk posttransplantation (*P < 0.05 for ANOVA within MSC, †P < 0.05 ANOVA between groups, ‡P < 0.05 vs. preinjection status by Student-Newman-Keuls). C–H: the functional outcomes in heart function (i.e., infarct size reduction, increase in contractility, and increase in tissue perfusion) showed related interaction between them (C–E) and with the magnitude of cells detected (F–H) at 12 wk after injection (P < 0.05 for all Pearson correlations). At least five time points for first-pass perfusion MRI image analysis. MSCs-treated hearts (n = 6) and placebo (n = 4) hearts for most of the time points except at 4 wk for ejection fraction (n = 8, 4 MSC and 4 placebo). [From Quevedo et al. (290).]
Despite the relatively few large animal studies of subacute ischemic cardiomyopathy, the beneficial effects of MSCs observed in these experiments have been sustained (9, 321, 371). Schuleri et al. (321) described a porcine subacute ischemic cardiomyopathy model in which swine were intramyocardially injected with allogeneic MSCs 3 days following LAD occlusion. Compared with the placebo and no intervention group, MSC-treated animals demonstrated a reduction in apoptosis, improvements in regional and global LV function, and mature vessels 8 wk post-injection. Importantly, resting myocardial blood flow was increased as early as 1 wk post-injection in the MSC-treated group, suggesting that improvements in tissue perfusion earlier than restoration of LV function as well as reduction of infarct size may serve as a valuable method for predicting successful myocardial regeneration. Vela et al. (371) examined the histopathological properties of canine hearts treated with MSCs 7 days post-MI and found that the cell-treated group exhibited less necrotic myocardium and more ECM deposition than the control group during the 2 wk post-treatment subacute phase.
While many preclinical studies utilizing MSC therapy for cardiac disease have presented auspicious findings, there still remain inconsistencies with regards to cardiac remodeling and functional data, and such discordant results may be related to cell dose and delivery method. Schuleri et al. (322) randomized post-LAD occlusion swine to receive 20 million autologous MSCs, 200 million autologous MSCs, or placebo. While both cell treatment groups improved regional contractility and myocardial blood flow, only the 200 million dose group exhibited a significant reduction in infarct size (322). Allogeneic STRO-3+ MPCs, a subset of MSCs (124), were isolated from male sheep and injected into female sheep in a dose-dependent manner with cardiac assessments at 4 and 8 wk post-MI showing improvements in EF at all cell doses but reductions in infarct expansion and left ventricular volumes only in the low-dose groups (135). In another dose-dependent study, allogeneic MPCs that were transplanted into post-MI sheep had the greatest influence on LV remodeling at lower MPC concentrations by altering collagen dynamics, MMPs, and tissue inhibitors within the border zone (81). These findings lend support to the notion that perhaps a “threshold” effect exists whereby the concentration of cells delivered, rather than total cell number, determines the therapeutic outcome.
C. Nonischemic Cardiomyopathy
In rat model of nonischemic, dilated cardiomyopathy, Nagaya et al. (250) reported that autologous MSCs injected 5 wk following immunization significantly increased capillary density and reduced myocardial collagen volume fraction, with consequent improvements in LV chamber volume and hemodynamics. Furthermore, a proportion of the engrafted MSCs expressed cardiomyocyte and vascular cell markers (250). The same group studied acute myocarditis by injecting rats with porcine myosin and treating them with autologous MSCs 7 days following induction of myocarditis (258). MSC transplantation reduced the proinflammatory cardiac microenvironment, by inhibiting increases in CD68-positive inflammatory cells and MCP-1 expression, increased capillary density, and improved cardiac function (258). Similar in vitro findings, within the same study, support MSCs' paracrine effects as playing the major role even in nonischemic cardiac repair (258). Xenografted adult human MSCs into canine LV anterior walls with complete heart block provided pacemaker function when >700,000 cells were delivered, and stabilized as early as day 10 with persistence to 6 wk post-injection (278). Importantly, these functional biological pacemakers were catecholamine-responsive and did not manifest any cellular or humoral rejection (278). Thus MSCs can be of great benefit for a wide range of cardiac pathologies.
D. Safety Profile
MSC cell-based therapy for cardiovascular disease (138), and many other disease processes (268, 283), has been shown to be safe in both preclinical (9, 144, 322) and clinical studies (137, 147, 308). Allogeneic MSCs offer similar safety properties to autologous cells (137), with the benefit of avoiding MSCs derived from aged and aberrant microenvironments, since these cells are obtained from young and healthy donors (293). However, long-term safety measures have not been studied as extensively as the more immediate and apparent outcomes.
A major concern regarding the long-term safety of MSC therapy has been the potential for arrhythmogenicity. In a murine post-MI model, Lai et al. (192) observed that direct injection of MSCs mitigated the reduction in conduction velocity and ion channel gene expression, which are decreased after an infarction, but did not promote arrhythmogenicity. In agreement, Wei et al. (382) demonstrated that while transplanted MSCs do not acquire the electrophysiological characteristics of mature cardiomyocytes, they are not proarrhythmic and can alleviate the electrical vulnerability associated with injured myocardium. In support of MSCs' paracrine effects, it was found that hypoxic, but not normoxic, MSC-conditioned medium was antiarrhythmic when injected into border zones of infarcted rat myocardium (159). Further resolution of proarrhythmic concerns was substantiated by data from clinical trials supporting MSCs' antiarrhythmic actions (138).
Another equally central issue vis-à-vis chronic MSC safety is tumorogenicity. Conflicting evidence exists with respect to MSCs' potential for tumor formation (28, 204, 311, 362). Rosland et al. (311) reported in vitro spontaneous malignant transformation of human bone marrow-derived MSCs with a high proliferation rate and altered morphology. Moreover, it is not clear whether MSCs themselves spontaneously transform into malignant cells or if tumorogenesis occurs through MSC-stromal cell communications. However, Bernardo et al. (28) cultured human bone marrow-derived MSCs until senescence or passage 25 and did not note any telomerase activity expression, human telomerase reverse transcriptase (hTERT), alternative lengthening of telomeres (ALTs), or chromosomal abnormalities, all indicative of carcinogenesis. While these favorable findings contend against MSC-induced tumorogenicity, many of these studies have been either performed in vitro or in vivo preclinical models (28, 204, 311, 362). Therefore, additional long-term clinical studies are required to provide more robust conclusions.
XII. CLINICAL TRIALS
There have been significant efforts to advance the application of cell-based therapeutics for heart disease, from the bench to bedside. To date, the majority of stem cell treatments under clinical trials are with adult bone marrow-derived cells (173), whereas there is cautious optimism for cardiac progenitors derived from within the adult heart (62, 228) or differentiated ESCs/iPSCs (240) that have just started coming down the pipeline. Overall, an increasing number of patients have been successfully receiving cell therapy for a period of time, with no health hazards reported. However, the conclusions regarding their efficacy as a potential treatment for heart disease are mixed. Whereas in some occasions meta-analyses of the reported effects following bone marrow-derived cell therapy identified significant clinical benefit (3, 63, 64, 75, 76, 168, 186), others report opposing effects (72, 100, 131, 255). To this end, the ACCRUE database (meta-Analysis of Cell-based CaRdiac study; NCT01098591) is sought to improve data analysis by collecting and providing access to individual patient data (IPD) from multinational, randomized, and cohort studies of cell-based therapy in patients with ischemic heart disease. For example, a recent IPD-based meta-analysis of 767 patients from 12 randomized controlled trials who received intracoronary administration of bone marrow-derived or cardiosphere-derived cells after acute MI illustrated no significant clinical benefit over the 485 controls (131). The inability of the ACCRUE meta-analysis to record the positive effects of intracoronary cell transplantation previously reported by others may reflect the impact of using more rigorous statistical approaches that are based on IPD rather than study-level data (186). However, it may also be due to important differences between the 12 different studies, such as the methods in cell preparation and delivery; differences in the administered cell types which included bone marrow mononuclear cells, cardiosphere-derived cells, and CD133+ and CD34+ bone marrow cells; as well as important differences in patient characteristics. Nonetheless, the ACCRUE study strongly suggests that intracoronary administration of cell-based therapeutics may not be the optimal therapeutic strategy in its present form.
A. Acute and Subacute Myocardial Infarction
MSCs' cardioreparative capacity shown in numerous preclinical studies (9, 70, 180, 290, 321, 322, 329, 335, 356) along with their excellent safety track record (195) have allowed for their use as a promising therapeutic agent in clinical trials. To date, multiple phase I and II clinical trials have been completed using intravenous (138, 308), intracoronary (54, 152, 227, 228), and intramyocardial (11, 137, 147, 271, 393) delivery routes. Hare et al. (138) investigated the safety and efficacy of intravenously delivered allogeneic human MSCs in acute MI patients. In this phase I randomized, double-blinded study, MSC-treated, but not placebo-treated, patients experienced a significantly improved global symptom score, increased EF, LV reverse remodeling, improved pulmonary function testing, and reduced ventricular arrhythmias 6 mo post-infusion. These encouraging data led to a phase II trial (ClinicalTrials.gov identifier: NCT00877903), using a similar study design, with preliminary results showing decreased LV remodeling and treatment-emergent serious adverse events (TE-SAEs). This study is still ongoing. Rodrigo et al. (308) performed a phase I study evaluating the safety and efficacy of autologous bone marrow-derived ex vivo expanded MSCs delivered by TESI in acute MI patients. They highlighted the feasibility and safety of this delivery system, noting a similar 5-yr event-free survival in MSC-treated and control groups (308). The APOLLO trial, a randomized, double-blinded, placebo-controlled, phase I/IIa study for ST-elevation MI (STEMI), assessed the safety and feasibility of adipose-derived MSCs administered intracoronarily acutely after an MI, and importantly, no TE-SAEs were noted. Cell-treated patients exhibited a 50% reduction in infarct size and a significant improvement in perfusion, albeit only a trend in cardiac functional improvement. The ongoing AMICI trial is designed to evaluate the safety and efficacy of allogeneic MPCs infused intracoronarily in patients with acute STEMI (ClinicalTrials.gov identifier: NCT01781390).
In a subacute MI model, Chen et al. (54) delivered autologous MSCs via intracoronary infusion almost 3 wk following percutaneous intervention (PCI) and observed improvements in LV chamber dimensions, ejection fraction, and perfusion defects in cell-treated patients versus placebo. The CADUCEUS trial was a phase I randomized, dose-escalation study examining the safety and efficacy of intracoronary delivery of autologous cardiosphere-derived stem cells (CDCs) (which include MSC-like cardiac cells) 2–4 wk post-MI (227). At 6 mo post-infusion, an increase in viable heart mass, regional contractility, regional systolic wall thickening, and a reduction in scar mass were found in the CDC group, but no changes in left-ventricular end-systolic volume (LVESV), end-diastolic volume (LVEDV), or EF were observed. Furthermore, no major adverse cardiac events (MACE) or tumorogenicity were found in treatment or control groups. Importantly, a follow-up to that trial showed that safety outcomes and therapeutic regeneration were maintained even until 1 yr post-treatment (228). Together, these data strongly suggest a role for MSCs in the context of acute and subacute MI.
B. Chronic Ischemic Cardiomyopathy
Translational data obtained from rigorous chronic ischemic models in large animal models has permitted implementation of MSC-based therapy in clinical trials for patients with chronic ischemic cardiomyopathy. MSCs' in vivo mechanisms of action, as described above, have been distinguished as ostensibly operative in human trials. Importantly, their unique immunological phenotype together with their effective ability to suppress cells of both the innate and adaptive immune systems (363) has allowed for their use as an allograft in clinical trials.
Our group performed an early-phase clinical trial, in which eight patients were administered either autologous bone marrow-derived MSCs or whole bone marrow by TESI, demonstrating reverse remodeling with restoration of regional cardiac function in the infarct zones treated with autologous MSCs (393). The two follow-up studies were the randomized, double-blinded TAC-HFT (Figure 13) and POSEIDON (Figure 14) trials comparing bone marrow MSCs versus mononuclear cells and allogeneic versus autologous MSC therapy, respectively, in chronic ischemic cardiomyopathy patients. These phase I/II trials found that at 1 yr, autologous MSCs, but not mononuclear cells, reduced infarct size, improved regional contractility, and increased functional capacity (147) and that allogeneic MSCs are equivalent to autologous MSCs in reducing scar size as well as improving end-diastolic chamber volumes and sphericity index (137). Importantly, low-dose allogeneic MSCs (20 million cells) produced the greatest reductions in LV chamber dimensions and increased EF (137). Interestingly, in a secondary analysis of these two prospective trials, older patients (≥60 yr) exhibited a similar improvement in functional capacity and quality of life measures (6-min walking distance and Minnesota Living With Heart Failure Questionnaire, respectively) (Figure 15), in addition to equivalent reductions in MI size (data not shown), compared with young patients (<60 yr) (119). In an effort to examine cell-based therapy in improving LV function for ischemic cardiomyopathy patients undergoing coronary artery bypass grafting (CABG), the PROMETHEUS trial found that intramyocardial injection of autologous MSCs into infarcted, nonrevascularized segments resulted in a concordant improvements in scar size, perfusion, and contraction compared with revascularized and nontreated segments (174). The impact on cardiac structure and function was less prominent in revascularized, but noninjected segments, emphasizing MSCs' largest effects as being local with a present, yet reduced, concordance of phenotypic effects in regions adjacent to injection sites (174).
FIGURE 13.
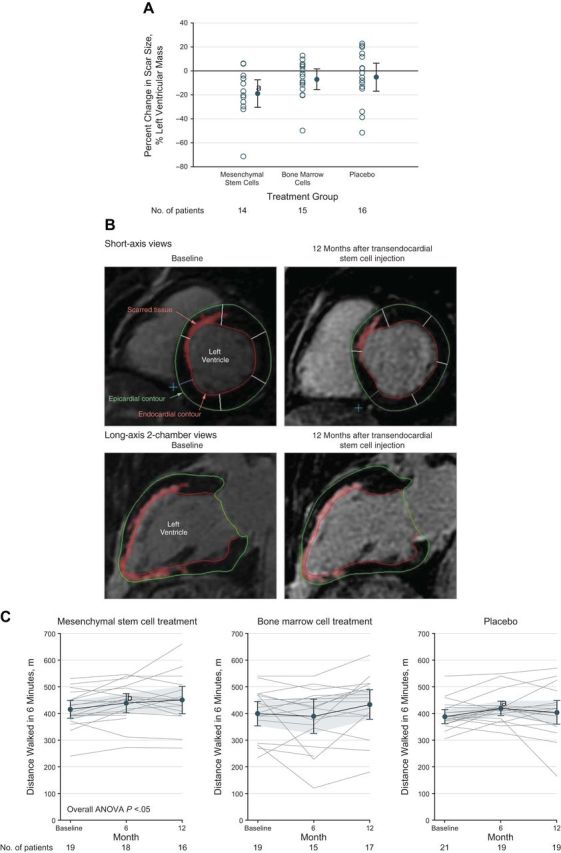
Impact of autologous MSC therapy versus whole bone marrow or placebo in patients with ischemic cardiomyopathy. Panels depict results from the TAC-HFT trial: percent change in scar size as a percentage of left ventricular mass and impact on functional capacity. A: the 14 patients treated with mesenchymal stem cells (MSCs) exhibited a significant reduction in scar size (P = .004) as a percentage of left ventricular mass with no differences for the 15 patients treated with bone marrow cells or the 16 patients in the placebo group. B: short-axis and long-axis views of the basal area of a patient's heart, with delayed tissue enhancement delineated at the septal wall (short-axis) and anterior and inferior walls (long-axis) as well as the entire apex (long-axis). Delayed tissue enhancement corresponds to scarred tissue and is depicted brighter than the nonscarred tissue. The red, green, and white lines demarcate the endocardial, epicardial contours, and borders of the segments, respectively. Twelve months after injection of mesenchymal stem cells, scar mass was reduced from 30.85 g at baseline to 21.17 g (short axis) and delayed tissue enhancement receded in the midinferior and basal anterior walls (long axis). C: patients in the mesenchymal stem cell group exhibited a significant increase in 6-min walk distance when 6-mo and 12-mo time points were compared with baseline in a repeated measures model (P = 0.03). No significant difference was observed for patients in the bone marrow cell group (P = 0.73) or in the placebo group (P = 0.25). [From Heldman et al. (147), with permission from American Medical Association.]
FIGURE 14.
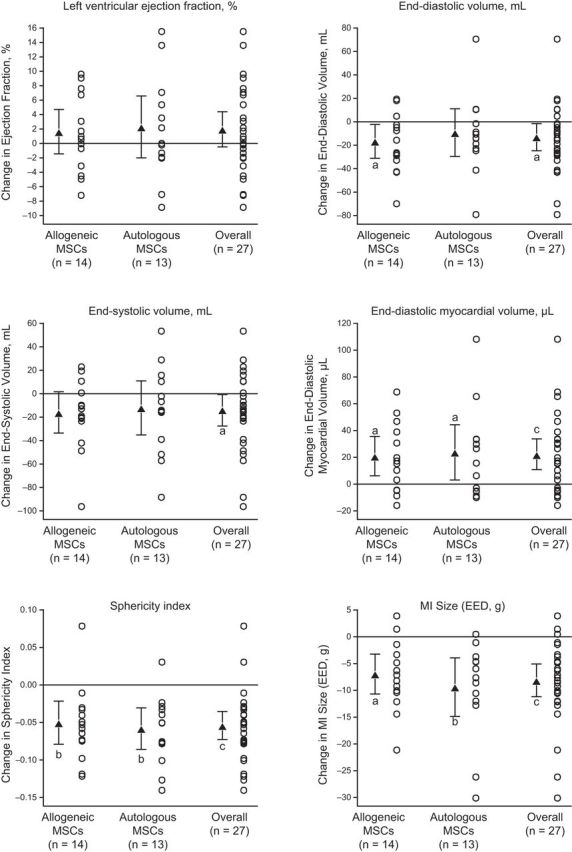
Impact of allogeneic versus autologous MSC therapy in patients with ischemic cardiomyopathy. Panels depict results from the POSEIDON trial: computer tomography (CT) parameters change from baseline. MSCs, mesenchymal stem cells; EED, early enhancement effect. Mean changes from baseline to 13 mo are noted by triangles and depict change in cardiac phenotype assessed by cardiac CT scan. Error bars indicate 95% confidence intervals (CIs). Individual patient changes from baseline are shown as circles. Shown are changes in cardiac structural and functional parameters from baseline to 13-mo follow-up in allogeneic, autologous, and combined patient groups. Within-group P values are noted as aP < 0.05, bP < 0.01, and cP < 0.001. [From Hare et al. (137).]
FIGURE 15.
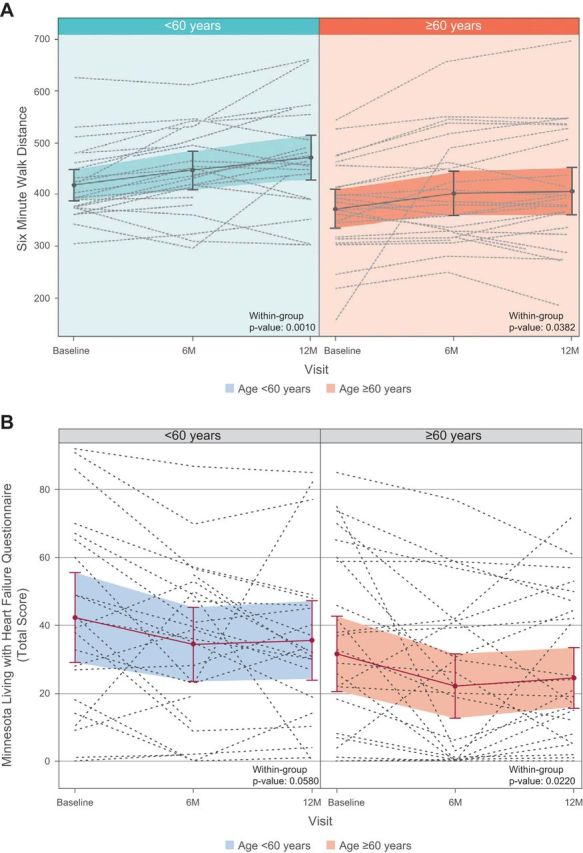
Patient functional capacity and quality of life following MSC therapy in old and young patients. A: graphic representation shows the estimated mean 6-min walk distance values and individual patient values in each age group at each time point, using a repeated-measures model. Both age groups increased similarly until 6 mo post-TESI. Although the older age group plateaus by 1 yr post-TESI, average distances between age groups do not differ significantly (P = 0.954 and P = 0.288 at 6 mo and 1 yr post-TESI, respectively; between-group comparison). B: in this graphic representation of the estimated mean Minnesota Living With Heart Failure Questionnaire, the total score values and individual patient values in each age group at each time point are depicted, using a repeated-measures model. The total scores of both age groups improved in a parallel fashion at 6 mo post-TESI. Both groups plateaued by 1 yr post-TESI with similar mean total scores on between-group comparison (P = 0.524 and P = 0.871 at 6 mo and 1 yr post-TESI, respectively; between-group comparison). TESI, transendocardial stem cell injection. [From Golpanian et al. (119), with permission from Elsevier.]
The phase II randomized, placebo-controlled, double-blinded PRECISE trial investigated TESI injections of adipose-derived MSCs isolated from liposuction aspirates into ischemic cardiomyopathy patients and demonstrated improved myocardial perfusion, exercise capacity, and cardiac function at 6 mo post-treatment (271). Importantly, perfusion was conserved for 12 subsequent months. In a smaller phase II study, 30 LVAD-recipient patients were randomized to either intramyocardial injection of allogeneic (STRO-3+) MPCs, during time of surgical implantation, or medium (11). Although 90 days following randomization successful weaning from LVAD support was observed in half of the MPC group and 20% of control patients, no significant difference existed. Nevertheless, this trial was preliminary, and allogeneic MPCs were safe and feasible with the prospect of possible efficacy in a future study with a larger sample size. Currently, an ongoing clinical trial using autologous VEGF-A165-stimulated adipose-derived MSCs, MyStromalCell, aims to establish the safety and efficacy of this cell therapy, by using functional capacity, quality of life, and cardiac imaging parameters, in patients with chronic myocardial ischemic and refractory angina (286).
C. Phase III, Ongoing, and Planned Clinical Trials
Based on findings from the APOLLO trial, the phase IIb/III ADVANCE trial (ClinicalTrials.gov identifier: NCT01216995) has been initiated and is enrolling up to 375 patients with an STEMI to evaluate the safety and efficacy of intracoronary infusion of two doses of adipose-derived MSCs. In the phase III C-CURE trial for ischemic heart failure, endomyocardial injection of autologous MSCs exposed to a cardiogenic cocktail resulted in a significant restoration of LVEF and LVESV as well as improvements in functional capacity and quality of life compared with control patients (20). A different, ongoing phase III trial is estimated to enroll 1,730 subjects and will evaluate the efficacy and safety of ex vivo-expanded allogeneic MPCs (CEP-41750) for treatment of chronic ischemic cardiomyopathy. Cells are to be delivered by TESI and efficacy outcomes will be assessed until 60 mo post-treatment (ClinicalTrials.gov identifier: NCT02032004). The TRIDENT trial (ClinicalTrials.gov identifier: NCT02013674) compares two different cell doses (20 million vs. 100 million allogeneic MSCs) administered via TESI for ischemic cardiomyopathy. The POSEIDON-DCM trial (ClinicalTrials.gov identifier: NCT01392625) aims to evaluate the role of autologous and allogeneic MSCs in patients with nonischemic, dilated cardiomyopathy. A multicenter phase II, randomized, placebo-controlled study, named the CONCERT-CHF trial under the National Heart, Lung, and Blood Institute's (NHBLI) Cardiovascular Cell Therapy Research Network (CCTRN), is investigating the safety and efficacy of combination cell therapy in subjects with chronic heart failure. The trial is designed to have patients receive transendocardial injections of either a combination of autologous MSCs and c-kit+ CSCs, autologous MSCs alone, autologous CSCs alone, or placebo. The estimated subject population will be 144 randomized patients who will be followed for safety and efficacy outcomes until 1 yr following treatment.
XIII. ENGINEERED HEART TISSUE
A. Enhancement of Cell Delivery and Retention
Because of MSCs' low retention rates, investigators have explored different options to optimize cell delivery. Transmyocardial transplantation of MSCs using microcapsules, which consist of agarose, dextran sulfate, ECM proteins, collagen, and fibrin, can be accomplished and supports long-term survival of MSCs (34). Of note, these injectable microcapsules degrade slowly and do not cause foreign body reactions (34). MSCs can undergo anoikis, a form of apoptosis induced by cell detachment from the surrounding ECM. As such attempts have been made to develop products such as grapheme oxide (GO) to protect implanted MSCs from this and improve their therapeutic efficacy (264). Huang et al. (156) were the first to use proteomics to identify proteins involved in the migration capacity and tissue-specific functions of MSCs derived from different tissues, implicating that such tools can be vital in future MSC-based tissue engineering studies. Other techniques, such as coating MSCs with synthetic and/or biological materials or combining them with fibrin glue prior to implantation, also have been employed to facilitate MSC recruitment to and engraftment within injured tissues (176, 397).
B. Implantation of Biomimetic Scaffolds and Matrices
In a murine acute MI model, in vivo implantation of three-dimensional polyelectrolyte complex (PEC)-based scaffolds loaded with MSCs demonstrate an increase in EF, improvements in neovascularization, as well as blunted scar size expansion and LV dilation (48). Elastic, biodegradable, nanofibrous cardiac patches seeded with MSCs can be transplanted onto the epicardium of infarct regions in post-LAD ligation rats and allow MSCs to migrate towards infarct sites, attenuate LV wall expansion, reduce fibrosis, and promote angiogenesis 4 wk post-transplantation (170). In another murine chronic MI model, MSC-seeded plasma-coated poly ε-caprolactone (PCL) grafts were used to stabilize cardiac function and dilation (126). MSC-patch animals only experienced a 6% relative decrease in EF 4 wk post-implantation, compared with a 13 and 20% decrease in sham-treated animals and acellular patch implantation, respectively (126).
Investigators have engineered PBHV/gelatin scaffolds resembling myocardial structural properties that, following stem cell culture, can guide initial MSC and nonmyocyte cell lineages towards cardiomyogenesis as shown by expression of sarcomeric proteins and cardiac transcription factors GATA-4, Nkx2.5, and TBX-5 (67). In an interesting study by Haneef et al. (136), electrostimulation of MSC-seeded collagen matrices significantly increased MSC proliferation and induced expression of cardiac markers troponin I, connexin 43, sarcomeric α-actinin, slow myosin, fast myosin and desmin, compared with nonstimulated control matrices. Alternatively, scaffold-free MSC assembly into three-dimensional microtissues (3D-MTs) has been studied. Successful in vitro generation of MSC 3D-MTs prior to transplantation has displayed sufficient viability and ECM formation, which offers a strategic delivery tool for cardiac therapy (89). Three-dimensional in vitro cardiac scar models have also been used to examine the paracrine effects of implanted MSCs in ischemic myocardium and resident myofibroblasts, thereby elucidating their role in attenuating the progression of cardiac fibrosis (106).
C. Decellularization and Recellularization
Decellularization is a process whereby cells are removed from tissues or organs using detergents, enzymes, and/or salts, with conservation of ECM composition, structure, mechanisms, and bioactivity. Combining this process with recellularization, or cell repopulation, with stem cells like MSCs can serve as an important bioengineering instrument for cardiac regenerative medicine (317). Vincentelli et al. (374) injected autologous MSCs and bone marrow mononuclear cells into decellularized porcine pulmonary valve scaffolds with subsequent implantation into the pulmonary artery in 14 lambs during cardiopulmonary bypass. Four months post-implantation, MSC-seeded valves had lower mean transvalvular and distal gradients compared with the mononuclear cell-seeded group. Furthermore, while the valves from the MSC group were found to have similar ECM organization to native pulmonary valves, mononuclear cell-seeded valves underwent significant valve thickening and inflammatory cell infiltration (374). Human myocardial ECM sheets derived from tissue decellularization have been analyzed and deemed to be suitable biologic scaffolds for cell seeding and cell-matrix interaction studies (257). Using a translational approach, Huang et al. (154) found that fabricated autologous bone marrow-derived MSC sheet fragments, which retained endogenous ECM, preserved cardiac function and attenuated fibrosis LV and remodeling compared with control when transplanted into infarcted porcine hearts. Decellularization of cardiac structures, and even whole hearts, provide organ-specific scaffolds that are involved in cell-ECM communication (127). As such, preservation of the ECM following decellularization may serve as a major influence in promoting MSC differentiation and tissue valve regeneration.
Decellularized tissue-engineered heart valves have also been used as models to demonstrate the superior recellularization potential of engineered leaflets seeded with human MSCs as well as similar tensile properties and collagen content compared with decellularized native valves (350). Likewise, Hoerstrup et al. (149) noted that expanded human MSCs seeded onto fabricated polymer-based trileaflet heart valves organized into viable tissue and ECM formation. Collagen types I and III, ASMA, and vimentin were identified in the engineered leaflets, and these valves demonstrated similar morphological and mechanical properties to native valves tissue (149). Utilizing various growth factors as well can accelerate the recellularization process (157, 338). Acellular porcine heart valves incubated with platelet gel, a storage vehicle for growth factors, markedly enhances sheep MSC repopulation through sustained release of bFGF and TGF-β1 rendering them as dynamic, bioactive matrices (338). The combination of HGF and fibronectin play a major role in sheep MSC adhesion, re-endothelialization, and reconstitution of myofibroblasts in porcine decellularized valve constructs (157).
Although most cardiac decellurization/recellularization reports are on heart valves, uses for other myocardial tissue types have also been explored (220, 262, 383). Decellularized porous bovine pericardia that were seeded with BrdU-labeled MSCs and successfully implanted to repair surgically created right ventricular defects in a murine model (383). Upon retrieval of the implanted patched 4 and 12 wk post-operatively, myocardial and nonmyocardial tissue regeneration was observed in the MSC-patch group but not the control-patch group. Importantly, no tissue adhesion, aneurysmal dilation, or thrombus formation was seen in the MSC-patch group, supporting its potential to preserve ventricular wall structures (383). While whole heart decellularization to construct bioartificial hearts has been described (12), few reports of recellularization using other types of stem cells (220, 262) have been made, and thus future studies utilizing MSCs are required.
D. Clinical Application of Engineered Heart Tissue
The goal of engineered heart tissue is the ex vivo manufacturing of fully functional, healthy heart tissue that may be used clinically for direct, damaged tissue replacement or drug screening applications (92, 164, 333, 376, 419). Compared with cell-based therapy, heart tissue engineering bears multiple layers of complexity, since it requires the integration of several factors that should eventually recapitulate the functional, cellular, extracellular, and molecular characteristics of the healthy human heart. Zimmermann et al. (421) observed partial reversal of functional deterioration in rats with experimental MI, following epicardial implantation of thick, multi-loop rings of contractile engineered heart tissue from neonatal rat heart cells. Okano and colleagues (236) and Bursac and colleagues (211) later demonstrated that the functional and structural properties of engineered mouse heart tissue could be significantly improved by combining stem cell-derived cardiomyocytes with noncardiomyocytes. Similarly, Ye et al. (405) showed in a porcine model of MI that transplantation of engineered contractile heart tissue composed of a mixture of human iPSC-derived cardiomyocytes, endothelial cells, and smooth muscle cells is therapeutically superior to using hiPSC cardiomyocytes alone. More recently, Burridge et al. (39) demonstrated that the contractility of engineered human heart tissue from iPSC-derived cardiomyocytes may be significantly improved in the presence of endothelial cells and MSCs. Importantly, transplantation of skeletal myoblast cell sheets received conditional approval in Japan as a clinical treatment for cardiovascular disease (1, 316), making it the first engineered tissue-based therapeutic modality to receive approval worldwide.
XIV. CONCLUSIONS AND FUTURE DIRECTIONS
Significant advancements have been made in MSC-based cardiac regenerative medicine and engineering. Although a large body of preclinical and clinical work supports their safety and restorative effects, data regarding their exact mechanisms of action remain incompletely understood. Strategies involving MSC-based genetic and tissue engineering are underway in an effort to improve delivery and retention of cell therapeutics. However, many of these results remain premature and await clinical testing. MSC paracrine effects are known to be a key part of their therapeutic potential, yet many of their secreted factors and cell-to-cell interactions are yet to be determined. Their distinctive immunological profile supports their clinical application, especially as an “off-the-shelf” product with the use of allogeneic MSCs. The evolution of MSC therapy and cardioregenerative medicine have demonstrated that MSCs may benefit from the addition of other cell types, and such cell “cocktails” can provide more marked improvements in cardiac repair. This principle is under active investigation in a multicenter study through the CCTRN. Data from robust preclinical studies have paved the way for clinical trials that have shown promising signs of MSC therapeutic benefits for patients with cardiac disease. Recently, MSC-based tissue engineering studies for heart disease have shown encouraging findings, but translation to the clinical setting is still required. Current phase III clinical trials and possible near-future tissue engineering trials implementing MSCs for cardiac repair will turn the page to the next chapter of heart disease treatments.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01 HL110737, R01 HL107110, R01 HL084275, and 5UM HL113460 (to J. M. Hare) and grants from the Starr Foundation and the Soffer Family Foundation.
DISCLOSURES
J. M. Hare reports equity interest and board membership in Vestion Inc and Longeveron LLC. K. E. Hatzistergos reports equity interest in Vestion Inc.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: J. M. Hare, Louis Lemberg Professor of Medicine, Professor of Biomedical Engineering and Cellular and Molecular Pharmacology, Director, Interdisciplinary Stem Cell Institute, Biomedical Research Building, 1501 N.W. 10th Ave., Rm. 910, PO Box 016960 (R125), Miami, FL 33101 (e-mail: jhare@med.miami.edu).
REFERENCES
- 1.Stem the tide. Nature : 163–164, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Abarbanell AM, Wang Y, Herrmann JL, Weil BR, Poynter JA, Manukyan MC, Meldrum DR. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol : H1529–H1536, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Int Med : 989–997, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood : 1815–1822, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport and enhances mesenchymal stem cell rescue efficacy. EMBO J : 994–1010, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol : C661–C669, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Alimperti S, You H, George T, Agarwal SK, Andreadis ST. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. J Cell Sci : 2627–2638, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature : 968–973, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA : 11474–11479, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFbeta-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun : 564–569, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, Klodell CT, Szady A, Parides MK, Jeffries NO, Skerrett D, Taylor DA, Rame JE, Milano C, Rogers JG, Lynch J, Dewey T, Eichhorn E, Sun B, Feldman D, Simari R, O'Gara PT, Taddei-Peters WC, Miller MA, Naka Y, Bagiella E, Rose EA, Woo YJ. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation : 2287–2296, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubin H, Kranz A, Hulsmann J, Lichtenberg A, Akhyari P. Decellularized whole heart for bioartificial heart. Methods Mol Biol : 163–178, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther : 1226–1238, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol : 1482–1490, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol : 359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol : 489–500, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao C, Guo J, Lin G, Hu M, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J : 56–62, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation : 863–868, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol : 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol : 2329–2338, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Bayes-Genis A, Soler-Botija C, Farre J, Sepulveda P, Raya A, Roura S, Prat-Vidal C, Galvez-Monton C, Montero JA, Buscher D, Izpisua Belmonte JC. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J Mol Cell Cardiol : 771–780, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, Puceat M, Niederlander N, Alekseev AE, Zingman LV, Terzic A. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med : 405–420, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature : 638–642, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WM. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arteriosclerosis Thrombosis Vasc Biol : 802–808, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belmokhtar K, Bourguignon T, Worou ME, Khamis G, Bonnet P, Domenech J, Eder V. Regeneration of three layers vascular wall by using BMP2-treated MSC involving HIF-1alpha and Id1 expressions through JAK/STAT pathways. Stem Cell Rev : 847–859, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells : 1753–1760, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Benzhi C, Limei Z, Ning W, Jiaqi L, Songling Z, Fanyu M, Hongyu Z, Yanjie L, Jing A, Baofeng Y. Bone marrow mesenchymal stem cells upregulate transient outward potassium currents in postnatal rat ventricular myocytes. J Mol Cell Cardiol : 41–48, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res : 9142–9149, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell : 257–270, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med : 387–397, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells : 625–634, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Bitto FF, Klumpp D, Lange C, Boos AM, Arkudas A, Bleiziffer O, Horch RE, Kneser U, Beier JP. Myogenic differentiation of mesenchymal stem cells in a newly developed neurotised AV-loop model. BioMed Res Int : 935046, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Molecules Dis : 632–636, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Blocki A, Beyer S, Dewavrin JY, Goralczyk A, Wang Y, Peh P, Ng M, Moonshi SS, Vuddagiri S, Raghunath M, Martinez EC, Bhakoo KK. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium. Biomaterials : 12–24, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science : 807–810, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science : 1775–1779, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev : 87–92, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem : 278–294, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Burridge PW, Metzler SA, Nakayama KH, Abilez OJ, Simmons CS, Bruce MA, Matsuura Y, Kim P, Wu JC, Butte M, Huang NF, Yang PC. Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am J Transl Res : 724–735, 2014. [PMC free article] [PubMed] [Google Scholar]
- 40.Buschmann I, Schaper W. Arteriogenesis versus angiogenesis: two mechanisms of vessel growth. News Physiol Sci : 121–125, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA, Zhang Z, Vacanti JP, Hauschka PV, Grottkau BE. Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev : 437–445, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Calderon D, Planat-Benard V, Bellamy V, Vanneaux V, Kuhn C, Peyrard S, Larghero J, Desnos M, Casteilla L, Puceat M, Menasche P, Chatenoud L. Immune response to human embryonic stem cell-derived cardiac progenitors and adipose-derived stromal cells. J Cell Mol Med : 1544–1552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res : 98–110, 2011. [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Y, Gomes SA, Rangel EB, Paulino EC, Fonseca TL, Li J, Teixeira MB, Gouveia CH, Bianco AC, Kapiloff MS, Balkan W, Hare JM. S-nitrosoglutathione reductase-dependent PPARgamma denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J Clin Invest : 1679–1691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caplan AI. All MSCs are pericytes? Cell Stem Cell : 229–230, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem : 1076–1084, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature : 298–307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceccaldi C, Bushkalova R, Alfarano C, Lairez O, Calise D, Bourin P, Frugier C, Rouzaud-Laborde C, Cussac D, Parini A, Sallerin B, Fullana SG. Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomaterialia : 901–911, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med : 1028–1038, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Charytonowicz E, Matushansky I, Castillo-Martin M, Hricik T, Cordon-Cardo C, Ziman M. Alternate PAX3 and PAX7 C-terminal isoforms in myogenic differentiation and sarcomagenesis. Clin Transl Oncol : 194–203, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, Guo ZK, Zhong J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PloS One : e115316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol : 629–640, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, Zhang JJ, Lin S, Liao LM, Zhao RC. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J : 1443–1448, 2004. [PubMed] [Google Scholar]
- 54.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol : 92–95, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhesion Migration : 337–341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng M, Huang K, Zhou J, Yan D, Tang YL, Zhao TC, Miller RJ, Kishore R, Losordo DW, Qin G. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol : 49–53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho YM, Kim JH, Kim M, Park SJ, Koh SH, Ahn HS, Kang GH, Lee JB, Park KS, Lee HK. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PloS One : e32778, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi HR, Byun SY, Kwon SH, Park KC. Niche interactions in epidermal stem cells. World J Stem Cells : 495–501, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi YH, Kurtz A, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther : 3–17, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, Biben C, Zoellner H, Colvin EK, Pimanda JE, Biankin AV, Zhou B, Pu WT, Prall OW, Harvey RP. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell : 527–540, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature : 273–277, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation : S54–S64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, Watt SM, Martin-Rendon E. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PloS One : e37373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev : CD006536, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood : 367–372, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell : 301–313, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Cristallini C, Cibrario Rocchietti E, Accomasso L, Folino A, Gallina C, Muratori L, Pagliaro P, Rastaldo R, Raimondo S, Saviozzi S, Sprio AE, Gagliardi M, Barbani N, Giachino C. The effect of bioartificial constructs that mimic myocardial structure and biomechanical properties on stem cell commitment towards cardiac lineage. Biomaterials : 92–104, 2014. [DOI] [PubMed] [Google Scholar]
- 68.D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nature Cell Biol : 627–638, 2015. [DOI] [PubMed] [Google Scholar]
- 69.Dakhlallah D, Zhang J, Yu L, Marsh CB, Angelos MG, Khan M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol : 241–251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation Suppl 1: II253–258, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell : 739–750, 2005. [DOI] [PubMed] [Google Scholar]
- 72.De Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv : 156–167, 2014. [DOI] [PubMed] [Google Scholar]
- 73.De Lisio M, Jensen T, Sukiennik RA, Huntsman HD, Boppart MD. Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res Ther : 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant : 2615–2627, 2015. [DOI] [PubMed] [Google Scholar]
- 75.Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart : 225–232, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Delewi R, Hirsch A, Tijssen JG, Schachinger V, Wojakowski W, Roncalli J, Aakhus S, Erbs S, Assmus B, Tendera M, Goekmen Turan R, Corti R, Henry T, Lemarchand P, Lunde K, Cao F, Huikuri HV, Surder D, Simari RD, Janssens S, Wollert KC, Plewka M, Grajek S, Traverse JH, Zijlstra F, Piek JJ. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J : 989–998, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res : 700–709, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood : 2440–2448, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol : 358–363, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood : 3838–3843, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH 3rd, Martens TP, Itescu S, Schuster MD, Plappert T, St John-Sutton MG, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation : S220–S229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. Interernational Society for Cellular Therapy position statement. Cytotherapy : 315–317, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, Liu B, Liu QM. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy : 469–478, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Duffy GP, Ahsan T, O'Brien T, Barry F, Nerem RM. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Engineering Part A : 2459–2470, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells : 2991–3001, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Eaton LW, Weiss JL, Bulkley BH, Garrison JB, Weisfeldt ML. Regional cardiac dilatation after acute myocardial infarction: recognition by two-dimensional echocardiography. N Engl J Med : 57–62, 1979. [DOI] [PubMed] [Google Scholar]
- 87.Ebert AD, Diecke S, Chen IY, Wu JC. Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: where do we stand? EMBO Mol Med : 1090–1103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eleuteri E, Di Stefano A, Tarro Genta F, Vicari C, Gnemmi I, Colombo M, Mezzani A, Giannuzzi P. Stepwise increase of angiopoietin-2 serum levels is related to haemodynamic and functional impairment in stable chronic heart failure. Eur J Cardiovasc Prevent Rehab : 607–614, 2011. [DOI] [PubMed] [Google Scholar]
- 89.Emmert MY, Wolint P, Wickboldt N, Gemayel G, Weber B, Brokopp CE, Boni A, Falk V, Bosman A, Jaconi ME, Hoerstrup SP. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials : 6339–6354, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA : 15546–15551, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erlebacher JA, Weiss JL, Weisfeldt ML, Bulkley BH. Early dilation of the infarcted segment in acute transmural myocardial infarction: role of infarct expansion in acute left ventricular enlargement. J Am Coll Cardiol : 201–208, 1984. [DOI] [PubMed] [Google Scholar]
- 92.Eschenhagen T, Didie M, Heubach J, Ravens U, Zimmermann WH. Cardiac tissue engineering. Transplant Immunol : 315–321, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Espagnolle N, Guilloton F, Deschaseaux F, Gadelorge M, Sensebe L, Bourin P. CD146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J Cell Mol Med : 104–114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells : 849–860, 2004. [DOI] [PubMed] [Google Scholar]
- 95.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature : 376–381, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, Fang J, Huang Z, Lin Y, Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail : 1023–1030, 2009. [DOI] [PubMed] [Google Scholar]
- 97.Fernandes S, Chong JJ, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Reports : 753–762, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrand J, Noel D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Menard A, Megraud F, Varon C. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PloS One : e19569, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science : 1247391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Francis DP, Mielewczik M, Zargaran D, Cole GD. Autologous bone marrow-derived stem cell therapy in heart disease: discrepancies and contradictions. Int J Cardiol : 3381–3403, 2013. [DOI] [PubMed] [Google Scholar]
- 101.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res : 159–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freeman BT, Kouris NA, Ogle BM. Tracking fusion of human mesenchymal stem cells after transplantation to the heart. Stem Cells Transl Med : 685–694, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J : 1114–1122, 2006. [DOI] [PubMed] [Google Scholar]
- 104.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinetics : 393–403, 1970. [DOI] [PubMed] [Google Scholar]
- 105.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues Cloning in vitro and retransplantation in vivo. Transplantation : 331–340, 1974. [DOI] [PubMed] [Google Scholar]
- 106.Galie PA, Stegemann JP. Injection of mesenchymal stromal cells into a mechanically stimulated in vitro model of cardiac fibrosis has paracrine effects on resident fibroblasts. Cytotherapy : 906–914, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galvez BG, Sampaolesi M, Barbuti A, Crespi A, Covarello D, Brunelli S, Dellavalle A, Crippa S, Balconi G, Cuccovillo I, Molla F, Staszewsky L, Latini R, Difrancesco D, Cossu G. Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle. Cell Death Differ : 1417–1428, 2008. [DOI] [PubMed] [Google Scholar]
- 108.Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PloS One : e100982, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garzoni LR, Rossi MI, de Barros AP, Guarani V, Keramidas M, Balottin LB, Adesse D, Takiya CM, Manso PP, Otazu IB, Meirelles Mde N, Borojevic R. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp Cell Res : 3406–3418, 2009. [DOI] [PubMed] [Google Scholar]
- 110.Ge D, Liu X, Li L, Wu J, Tu Q, Shi Y, Chen H. Chemical and physical stimuli induce cardiomyocyte differentiation from stem cells. Biochem Biophys Res Commun : 317–321, 2009. [DOI] [PubMed] [Google Scholar]
- 111.Genovese JA, Spadaccio C, Chachques E, Schussler O, Carpentier A, Chachques JC, Patel AN. Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Front Biosci : 2996–3002, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther : 97–108, 2011. [DOI] [PubMed] [Google Scholar]
- 113.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood : 2821–2827, 2005. [DOI] [PubMed] [Google Scholar]
- 114.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Med : 367–368, 2005. [DOI] [PubMed] [Google Scholar]
- 115.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J : 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 116.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res : 1204–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res : 51–59, 2003. [DOI] [PubMed] [Google Scholar]
- 118.Golpanian S, DiFede DL, Pujol MV, Lowery MH, Levis-Dusseau S, Goldstein BJ, Schulman IH, Longsomboon B, Wolf A, Khan A, Heldman AW, Goldschmidt-Clermont PJ, Hare JM. Rationale and Design of the AllogeneiC Human Mesenchymal Stem Cells (hMSC) in Patients With Aging fRAilTy via intravenoUS delivery (CRATUS) Study A phase I/II, Randomized, Blinded and Placebo Controlled Trial to Evaluate the Safety and Potential Efficacy of Allogeneic Human Mesenchymal Stem Cell Infusion in Patients with Aging Frailty. Oncotarget : 11899–11912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol : 125–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gomes SA, Rangel EB, Premer C, Dulce RA, Cao Y, Florea V, Balkan W, Rodrigues CO, Schally AV, Hare JM. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci USA : 2834–2839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gonzalez-Calero L, Martin-Lorenzo M, Alvarez-Llamas G. Exosomes: a potential key target in cardio-renal syndrome. Front Immunol : 465, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gouveia de Andrade AV, Bertolino G, Riewaldt J, Bieback K, Karbanova J, Odendahl M, Bornhauser M, Schmitz M, Corbeil D, Tonn T. Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev : 1374–1376, 2015. [DOI] [PubMed] [Google Scholar]
- 123.Grajales L, Garcia J, Geenen DL. Induction of cardiac myogenic lineage development differs between mesenchymal and satellite cells and is accelerated by bone morphogenetic protein-4. J Mol Cell Cardiol : 382–391, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino AC. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev : 953–963, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, Watzek G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets : 29–35, 2004. [DOI] [PubMed] [Google Scholar]
- 126.Guex AG, Frobert A, Valentin J, Fortunato G, Hegemann D, Cook S, Carrel TP, Tevaearai HT, Giraud MN. Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomaterial : 2996–3006, 2014. [DOI] [PubMed] [Google Scholar]
- 127.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell : 17–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo J, Lin G, Bao C, Hu Z, Chu H, Hu M. Insulin-like growth factor 1 improves the efficacy of mesenchymal stem cells transplantation in a rat model of myocardial infarction. J Biomed Sci : 89–97, 2008. [DOI] [PubMed] [Google Scholar]
- 129.Gusachenko ON, Zenkova MA, Vlassov VV. Nucleic acids in exosomes: disease markers and intercellular communication molecules. Biochem Biokhim : 1–7, 2013. [DOI] [PubMed] [Google Scholar]
- 130.Gyongyosi M, Blanco J, Marian T, Tron L, Petnehazy O, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, Kertesz I, Balkay L, Pavo N, Posa A, Emri M, Galuska L, Kraitchman DL, Wojta J, Huber K, Glogar D. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging : 94–103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye LA, Surder D, Corti R, Huikuri H, Miettinen J, Wohrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G, Investigators A . Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res : 1346–1360, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol : 933–943, 2008. [DOI] [PubMed] [Google Scholar]
- 133.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol : 525–536, 2008. [DOI] [PubMed] [Google Scholar]
- 134.Ham O, Lee SY, Song BW, Cha MJ, Lee CY, Park JH, Kim IK, Lee J, Seo HH, Seung MJ, Choi E, Jang Y, Hwang KC. Modulation of Fas-Fas ligand interaction rehabilitates hypoxia-induced apoptosis of mesenchymal stem cells in ischemic myocardium niche. Cell Transplant : 1329–1341, 2015. [DOI] [PubMed] [Google Scholar]
- 135.Hamamoto H, Gorman JH 3rd Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thoracic Surg : 794–801, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haneef K, Lila N, Benadda S, Legrand F, Carpentier A, Chachques JC. Development of bioartificial myocardium by electrostimulation of 3D collagen scaffolds seeded with stem cells. Heart Int : e14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA : 2369–2379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol : 2277–2286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hare JM, Walford GD, Hruban RH, Hutchins GM, Deckers JW, Baughman KL. Ischemic cardiomyopathy: endomyocardial biopsy and ventriculographic evaluation of patients with congestive heart failure, dilated cardiomyopathy and coronary artery disease. J Am Coll Cardiol : 1318–1325, 1992. [DOI] [PubMed] [Google Scholar]
- 140.Harkin DG, Foyn L, Bray LJ, Sutherland AJ, Li FJ, Cronin BG. Concise reviews: can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells : 785–791, 2015. [DOI] [PubMed] [Google Scholar]
- 141.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J : 251–259, 2008. [DOI] [PubMed] [Google Scholar]
- 142.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signaling : 12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hatzistergos KE, Hare JM. Cell therapy: targeting endogenous repair versus remuscularization. Circ Res : 659–661, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res : 913–922, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM. cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci USA : 13051–13056, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol : 585–592, 1996. [DOI] [PubMed] [Google Scholar]
- 147.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA : 62–73, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med : 1605–1618, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, Sodian R, Visjager JF, Kolb SA, Grunenfelder J, Zund G, Turina MI. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation : I143–150, 2002. [PubMed] [Google Scholar]
- 150.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation : 2198–2202, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Hoogduijn MJ, Crop MJ, Peeters AM, Korevaar SS, Eijken M, Drabbels JJ, Roelen DL, Maat AP, Balk AH, Weimar W, Baan CC. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant : 222–230, 2009. [DOI] [PubMed] [Google Scholar]
- 152.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol : 539–540, 2012. [DOI] [PubMed] [Google Scholar]
- 153.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thoracic Cardiovasc Surg : 799–808, 2008. [DOI] [PubMed] [Google Scholar]
- 154.Huang CC, Tsai HW, Lee WY, Lin WW, Chen DY, Hung YW, Chen JW, Hwang SM, Chang Y, Sung HW. A translational approach in using cell sheet fragments of autologous bone marrow-derived mesenchymal stem cells for cellular cardiomyoplasty in a porcine model. Biomaterials : 4582–4591, 2013. [DOI] [PubMed] [Google Scholar]
- 155.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res : 1753–1762, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang L, Niu C, Willard B, Zhao W, Liu L, He W, Wu T, Yang S, Feng S, Mu Y, Zheng L, Li K. Proteomic analysis of porcine mesenchymal stem cells derived from bone marrow and umbilical cord: implication of the proteins involved in the higher migration capability of bone marrow mesenchymal stem cells. Stem Cell Res Ther : 77, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang SD, Liu XH, Bai CG, Lu FL, Yuan Y, Gong DJ, Xu ZY. Synergistic effect of fibronectin and hepatocyte growth factor on stable cell-matrix adhesion, re-endothelialization, and reconstitution in developing tissue-engineered heart valves. Heart Vessels : 116–122, 2007. [DOI] [PubMed] [Google Scholar]
- 158.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation : 2419–2429, 2010. [DOI] [PubMed] [Google Scholar]
- 159.Hwang HJ, Chang W, Song BW, Song H, Cha MJ, Kim IK, Lim S, Choi EJ, Ham O, Lee SY, Shim J, Joung B, Pak HN, Kim SS, Choi BR, Jang Y, Lee MH, Hwang KC. Antiarrhythmic potential of mesenchymal stem cell is modulated by hypoxic environment. J Am Coll Cardiol : 1698–1706, 2012. [DOI] [PubMed] [Google Scholar]
- 160.Ikhapoh IA, Pelham CJ, Agrawal DK. Sry-type HMG box 18 contributes to the differentiation of bone marrow-derived mesenchymal stem cells to endothelial cells. Differentiation : 87–96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.In'tAnker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FHJ, Willemze R, Fibbe WE, Kanhai HHH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood : 1548–1549, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife : e03696, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ishikane S, Yamahara K, Sada M, Harada K, Kodama M, Ishibashi-Ueda H, Hayakawa K, Mishima K, Iwasaki K, Fujiwara M, Kangawa K, Ikeda T. Allogeneic administration of fetal membrane-derived mesenchymal stem cells attenuates acute myocarditis in rats. J Mol Cell Cardiol : 753–761, 2010. [DOI] [PubMed] [Google Scholar]
- 164.Jackman CP, Shadrin IY, Carlson AL, Bursac N. Human cardiac tissue engineering: from pluripotent stem cells to heart repair. Curr Opin Chem Eng : 57–64, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem : 295–312, 1997. [PubMed] [Google Scholar]
- 166.Jameel MN, Li Q, Mansoor A, Qiang X, Sarver A, Wang X, Swingen C, Zhang J. Long-term functional improvement and gene expression changes after bone marrow-derived multipotent progenitor cell transplantation in myocardial infarction. Am J Physiol Heart Circ Physiol : H1348–H1356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer : 752–760, 2005. [DOI] [PubMed] [Google Scholar]
- 168.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation : 551–568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood : 4120–4126, 2005. [DOI] [PubMed] [Google Scholar]
- 170.Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ, Ramakrishna S. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomaterial : 2727–2738, 2014. [DOI] [PubMed] [Google Scholar]
- 171.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation : 1046–1052, 2001. [DOI] [PubMed] [Google Scholar]
- 172.Kantarcioglu M, Caliskan B, Demirci H, Karacalioglu O, Kekilli M, Polat Z, Gunal A, Akinci M, Uysal C, Eksert S, Gurel H, Celebi G, Avcu F, Ural AU, Bagci S. The efficacy of mesenchymal stem cell transplantation in caustic esophagus injury: an experimental study. Stem Cells Int : 939674, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol : H256–H270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res : 1302–1310, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J Am Coll Cardiol : 1990–1999, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kavanagh DP, Robinson J, Kalia N. Mesenchymal stem cell priming: fine-tuning adhesion and function. Stem Cell Rev : 587–599, 2014. [DOI] [PubMed] [Google Scholar]
- 177.Kelly ML, Wang M, Crisostomo PR, Abarbanell AM, Herrmann JL, Weil BR, Meldrum DR. TNF receptor 2, not TNF receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock : 602–607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell : 239–253, 2015. [DOI] [PubMed] [Google Scholar]
- 179.Kim BO, Tian H, Prasongsukarn K, Wu J, Angoulvant D, Wnendt S, Muhs A, Spitkovsky D, Li RK. Cell transplantation improves ventricular function after a myocardial infarction: a preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation : I96–104, 2005. [DOI] [PubMed] [Google Scholar]
- 180.Kim PJ, Mahmoudi M, Ge X, Matsuura Y, Toma I, Metzler S, Kooreman NG, Ramunas J, Holbrook C, McConnell MV, Blau H, Harnish P, Rulifson E, Yang PC. Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium. Circ Res : e40–e50, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res : 678–685, 2004. [DOI] [PubMed] [Google Scholar]
- 182.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation : 1543–1549, 2004. [DOI] [PubMed] [Google Scholar]
- 183.Kogler G, Radke TF, Lefort A, Sensken S, Fischer J, Sorg RV, Wernet P. Cytokine production and hematopoiesis supporting activity of cord blood-derived unrestricted somatic stem cells. Exp Hematol : 573–583, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Koninckx R, Hensen K, Daniels A, Moreels M, Lambrichts I, Jongen H, Clijsters C, Mees U, Steels P, Hendrikx M, Rummens JL. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy : 778–792, 2009. [DOI] [PubMed] [Google Scholar]
- 185.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA : 10711–10716, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kovacic JC, Fuster V. Cell therapy for patients with acute myocardial infarction: ACCRUEd evidence to date. Circ Res : 1287–1290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells : 386–398, 2006. [DOI] [PubMed] [Google Scholar]
- 188.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood : 59–66, 2005. [DOI] [PubMed] [Google Scholar]
- 189.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature Med : 962–969, 2007. [DOI] [PubMed] [Google Scholar]
- 190.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells : 734–742, 2010. [DOI] [PubMed] [Google Scholar]
- 191.Laflamme MA, Murry CE. Heart regeneration. Nature : 326–335, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lai PF, Panama BK, Masse S, Li G, Zhang Y, Kusha M, Farid TA, Asta J, Backx PH, Yau TM, Nanthakumar K. Mesenchymal stem cell transplantation mitigates electrophysiological remodeling in a rat model of myocardial infarction. J Cardiovasc Electrophysiol : 813–821, 2013. [DOI] [PubMed] [Google Scholar]
- 193.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res : 214–222, 2010. [DOI] [PubMed] [Google Scholar]
- 194.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics : 971907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ, Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS One : e47559, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol : 147–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Las G, Shirihai OS. Miro1: new wheels for transferring mitochondria. EMBO J : 939–941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O, Developmental Committee of the European Group for Bone Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet : 1579–1586, 2008. [DOI] [PubMed] [Google Scholar]
- 199.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant : 321–334, 2005. [DOI] [PubMed] [Google Scholar]
- 200.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA : 17438–17443, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol : 1604–1605, 2006. [DOI] [PubMed] [Google Scholar]
- 202.Leri A, Anversa P. Stem cells and myocardial regeneration: cooperation wins over competition. Circulation : 165–168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leri A, Rota M, Hosoda T, Goichberg P, Anversa P. Cardiac stem cell niches. Stem Cell Res : 631–646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S, Rogers AB, Montrose M, Houghton J. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res : 10889–10898, 2007. [DOI] [PubMed] [Google Scholar]
- 205.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol : H1772–H1781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest : 103–110, 2008. [DOI] [PubMed] [Google Scholar]
- 207.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells : 2118–2127, 2007. [DOI] [PubMed] [Google Scholar]
- 208.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol : 295–303, 2007. [DOI] [PubMed] [Google Scholar]
- 209.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol : 455–465, 2014. [DOI] [PubMed] [Google Scholar]
- 210.Li Z, Guo J, Chang Q, Zhang A. Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull : 1343–1346, 2009. [DOI] [PubMed] [Google Scholar]
- 211.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials : 9180–9187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nature Med : 1282–1286, 2000. [DOI] [PubMed] [Google Scholar]
- 213.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res : 530–542, 2006. [DOI] [PubMed] [Google Scholar]
- 214.Lin HY, Liou CW, Chen S, Hsu TY, Chuang JH, Wang PW, Huang ST, Tiao MM, Chen JB, Lin TK, Chuang YC. Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion : 31–44, 2015. [DOI] [PubMed] [Google Scholar]
- 215.Lindoso RS, Collino F, Bruno S, Araujo DS, Sant'Anna JF, Tetta C, Provero P, Quesenberry PJ, Vieyra A, Einicker-Lamas M, Camussi G. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev : 1809–1819, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells : 279–289, 2008. [DOI] [PubMed] [Google Scholar]
- 217.Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol : 67–75, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest : 3101–3113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell : 389–398, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nature Commun : 2307, 2013. [DOI] [PubMed] [Google Scholar]
- 221.Lutz M, Knaus P. Integration of the TGF-beta pathway into the cellular signalling network. Cell Signal : 977–988, 2002. [DOI] [PubMed] [Google Scholar]
- 222.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica : 516–525, 2005. [PubMed] [Google Scholar]
- 223.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng : 415–428, 1998. [DOI] [PubMed] [Google Scholar]
- 224.Mahmoud AI, O'Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, Burns CE, Burns CG, MacRae CA, Poss KD, Lee RT. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell : 387–399, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci : 228–241, 2003. [DOI] [PubMed] [Google Scholar]
- 226.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest : 697–705, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, Zheng J, Kar S, McClelan R, Miyamota T, Bick-Forrester J, Fishbein MC, Shah PK, Forrester JS, Sharifi B, Chen PS, Qayyum M. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther : 225–233, 2005. [DOI] [PubMed] [Google Scholar]
- 228.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol : 110–122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med : 191–209, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature Med : 1195–1201, 2003. [DOI] [PubMed] [Google Scholar]
- 231.Marelli D, Desrosiers C, el-Alfy M, Kao RL, Chiu RC. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant : 383–390, 1992. [DOI] [PubMed] [Google Scholar]
- 232.Markel TA, Crisostomo PR, Wang M, Herring CM, Meldrum DR. Activation of individual tumor necrosis factor receptors differentially affects stem cell growth factor and cytokine production. Am J Physiol Gastrointest Liver Physiol : G657–G662, 2007. [DOI] [PubMed] [Google Scholar]
- 233.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol : H2308–H2314, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol : 1455–1469, 2012. [DOI] [PubMed] [Google Scholar]
- 235.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology : 4456–4462, 1997. [DOI] [PubMed] [Google Scholar]
- 236.Matsuura K, Masuda S, Haraguchi Y, Yasuda N, Shimizu T, Hagiwara N, Zandstra PW, Okano T. Creation of mouse embryonic stem cell-derived cardiac cell sheets. Biomaterials : 7355–7362, 2011. [DOI] [PubMed] [Google Scholar]
- 237.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nature Clin Pract Cardiovasc Med Suppl 1: S21–S26, 2007. [DOI] [PubMed] [Google Scholar]
- 238.McMurray JJ, Pfeffer MA. Heart failure. Lancet : 1877–1889, 2005. [DOI] [PubMed] [Google Scholar]
- 239.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood : 4619–4621, 2004. [DOI] [PubMed] [Google Scholar]
- 240.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J : 2011–2017, 2015. [DOI] [PubMed] [Google Scholar]
- 241.Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A : 1735–1746, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells : 2734–2743, 2009. [DOI] [PubMed] [Google Scholar]
- 243.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA : 1643–1648, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Commun : 282, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Molina EJ, Palma J, Gupta D, Torres D, Gaughan JP, Houser S, Macha M. Reverse remodeling is associated with changes in extracellular matrix proteases and tissue inhibitors after mesenchymal stem cell (MSC) treatment of pressure overload hypertrophy. J Tissue Engineering Regenerative Med : 85–91, 2009. [DOI] [PubMed] [Google Scholar]
- 246.Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, Ferrone S, Barnaba V, Pistoia V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells : 1275–1287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol : 197–223, 2001. [DOI] [PubMed] [Google Scholar]
- 248.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association and Stroke Statistics Committee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation : e29–322, 2015. [DOI] [PubMed] [Google Scholar]
- 249.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol : 107–116, 2002. [DOI] [PubMed] [Google Scholar]
- 250.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation : 1128–1135, 2005. [DOI] [PubMed] [Google Scholar]
- 251.Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell : 392–403, 2008. [DOI] [PubMed] [Google Scholar]
- 252.Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res : 547–558, 2010. [DOI] [PubMed] [Google Scholar]
- 253.Niyaz M, Gurpinar OA, Oktar GL, Gunaydin S, Onur MA, Ozsin KK, Yener A. Effects of VEGF and MSCs on vascular regeneration in a trauma model in rats. Wound Repair Regen : 262–267, 2015. [DOI] [PubMed] [Google Scholar]
- 254.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther : 840–850, 2006. [DOI] [PubMed] [Google Scholar]
- 255.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ : g2688, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Med : 494–501, 2004. [DOI] [PubMed] [Google Scholar]
- 257.Oberwallner B, Brodarac A, Choi YH, Saric T, Anic P, Morawietz L, Stamm C. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J Biomed Mater Res Part A : 3263–3272, 2014. [DOI] [PubMed] [Google Scholar]
- 258.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol : 88–97, 2007. [DOI] [PubMed] [Google Scholar]
- 259.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity : 89–103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature : 701–705, 2001. [DOI] [PubMed] [Google Scholar]
- 261.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells : 377–384, 2004. [DOI] [PubMed] [Google Scholar]
- 262.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nature Med : 213–221, 2008. [DOI] [PubMed] [Google Scholar]
- 263.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PloS One : e941, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Park J, Kim B, Han J, Oh J, Park S, Ryu S, Jung S, Shin JY, Lee BS, Hong BH, Choi D, Kim BS. Graphene Oxide Flakes as a Cellular Adhesive: Prevention of Reactive Oxygen Species Mediated Death of Implanted Cells for Cardiac Repair. ACS Nano : 4987–4999, 2015. [DOI] [PubMed] [Google Scholar]
- 265.Pasolli HA. The hair follicle bulge: a niche for adult stem cells. Microscopy Microanalysis : 513–519, 2011. [DOI] [PubMed] [Google Scholar]
- 266.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med : 94, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Passipieri JA, Kasai-Brunswick TH, Suhett G, Martins AB, Brasil GV, Campos DB, Rocha NN, Ramos IP, Mello DB, Rodrigues DC, Christie BB, Silva-Mendes BJ, Balduino A, Sa RM, Lopes LM, Goldenberg RC, Campos de Carvalho AC, Carvalho AB. Improvement of cardiac function by placenta-derived mesenchymal stem cells does not require permanent engraftment and is independent of the insulin signaling pathway. Stem Cell Res Ther : 102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Patel AN, Genovese J. Potential clinical applications of adult human mesenchymal stem cell [Prochymal (R)] therapy. Stem Cells Cloning : 61–72, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood : 1662–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 270.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA : 4857–4861, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Perin EC, Sanz-Ruiz R, Sanchez PL, Lasso J, Perez-Cano R, Alonso-Farto JC, Perez-David E, Fernandez-Santos ME, Serruys PW, Duckers HJ, Kastrup J, Chamuleau S, Zheng Y, Silva GV, Willerson JT, Fernandez-Aviles F. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J : 88–95, 2014. [DOI] [PubMed] [Google Scholar]
- 272.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol : 486–495, 2008. [DOI] [PubMed] [Google Scholar]
- 273.Perin L, Sedrakyan S, Da Sacco S, De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol : 85–99, 2008. [DOI] [PubMed] [Google Scholar]
- 274.Petrini M, Pacini S, Trombi L, Fazzi R, Montali M, Ikehara S, Abraham NG. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev : 857–866, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood : 1422–1432, 2007. [DOI] [PubMed] [Google Scholar]
- 276.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation : 1161–1172, 1990. [DOI] [PubMed] [Google Scholar]
- 277.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science : 143–147, 1999. [DOI] [PubMed] [Google Scholar]
- 278.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P Jr,. Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT, Brink PR, Robinson RB, Cohen IS, Rosen MR. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation : 706–713, 2007. [DOI] [PubMed] [Google Scholar]
- 279.Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, Isaev NK, Sheval EV, Polyakov VY, Sukhikh GT, Zorov DB. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med : 1622–1631, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Poncelet AJ, Hiel AL, Vercruysse J, Hermans D, Zech F, Gianello P. Intracardiac allogeneic mesenchymal stem cell transplantation elicits neo-angiogenesis in a fully immunocompetent ischaemic swine model. Eur J Cardio-thoracic Surg : 781–787, 2010. [DOI] [PubMed] [Google Scholar]
- 281.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun : 419–422, 2008. [DOI] [PubMed] [Google Scholar]
- 282.Poynter JA, Herrmann JL, Manukyan MC, Wang Y, Abarbanell AM, Weil BR, Brewster BD, Meldrum DR. Intracoronary mesenchymal stem cells promote postischemic myocardial functional recovery, decrease inflammation, and reduce apoptosis via a signal transducer and activator of transcription 3 mechanism. J Am Coll Surg : 253–260, 2011. [DOI] [PubMed] [Google Scholar]
- 283.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant : 534–541, 2011. [DOI] [PubMed] [Google Scholar]
- 284.Premer C, Blum A, Bellio M, Schulman IH, Hurwitz B, Parker M, Dermarkarian C, DiFede DL, Balkan W, Hare JM. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine : 467–475, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol : 231–239, 2006. [DOI] [PubMed] [Google Scholar]
- 286.Qayyum AA, Haack-Sorensen M, Mathiasen AB, Jorgensen E, Ekblond A, Kastrup J. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): study design. Regen Med : 421–428, 2012. [DOI] [PubMed] [Google Scholar]
- 287.Qi CM, Ma GS, Liu NF, Shen CX, Chen Z, Liu XJ, Hu YP, Zhang XL, Teng GJ, Ju SH, Ma M, Tang YL. Transplantation of magnetically labeled mesenchymal stem cells improves cardiac function in a swine myocardial infarction model. Chin Med J : 544–550, 2008. [PubMed] [Google Scholar]
- 288.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature : 593–598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med 3 : 5–15, 2002. [DOI] [PubMed] [Google Scholar]
- 290.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA : 14022–14027, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R Jr, Gude NA, Sussman MA. Cardiac stem cell hybrids enhance myocardial repair. Circ Res : 695–706, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells : 151–162, 2008. [DOI] [PubMed] [Google Scholar]
- 293.Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J : 239–242, 2012. [PMC free article] [PubMed] [Google Scholar]
- 294.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation : 71–76, 2007. [DOI] [PubMed] [Google Scholar]
- 295.Rangappa S, Reddy VG, Bongso A, Lee EH, Sim EKW. Transformation of the adult human mesenchymal stem cells into cardiomyocyte-like cells in vivo. Cardiovasc Eng : 7–14, 2002. [Google Scholar]
- 296.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol : 336–343, 2007. [DOI] [PubMed] [Google Scholar]
- 297.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res : 33–41, 2005. [DOI] [PubMed] [Google Scholar]
- 298.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol : 887–893, 2007. [DOI] [PubMed] [Google Scholar]
- 299.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation : 193–202, 1999. [DOI] [PubMed] [Google Scholar]
- 300.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS One : e44092, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc : 2607–2611, 2009. [DOI] [PubMed] [Google Scholar]
- 302.Reuter S, Soonpaa MH, Firulli AB, Chang AN, Field LJ. Recombinant neuregulin 1 does not activate cardiomyocyte DNA synthesis in normal or infarcted adult mice. PloS One : e115871, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature Biotechnol : 1029–1034, 2001. [DOI] [PubMed] [Google Scholar]
- 304.Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res : 720–730, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rigol M, Solanes N, Farre J, Roura S, Roque M, Berruezo A, Bellera N, Novensa L, Tamborero D, Prat-Vidal C, Huzman MA, Batlle M, Hoefsloot M, Sitges M, Ramirez J, Dantas AP, Merino A, Sanz G, Brugada J, Bayes-Genis A, Heras M. Effects of adipose tissue-derived stem cell therapy after myocardial infarction: impact of the route of administration. J Cardiac Failure : 357–366, 2010. [DOI] [PubMed] [Google Scholar]
- 306.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation : 1390–1397, 2006. [DOI] [PubMed] [Google Scholar]
- 307.Roche ET, Hastings CL, Lewin SA, Shvartsman DE, Brudno Y, Vasilyev NV, O'Brien FJ, Walsh CJ, Duffy GP, Mooney DJ. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials : 6850–6858, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, Zwaginga JJ, Bax JJ, Schalij MJ, Beeres SL, Atsma DE. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res : 816–825, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rogers TB, Pati S, Gaa S, Riley D, Khakoo AY, Patel S, Wardlow RD 2nd Frederick CA, Hall G, He LP, Lederer WJ. Mesenchymal stem cells stimulate protective genetic reprogramming of injured cardiac ventricular myocytes. J Mol Cell Cardiol : 346–356, 2011. [DOI] [PubMed] [Google Scholar]
- 310.Rosca AM, Burlacu A. Effect of 5-azacytidine: evidence for alteration of the multipotent ability of mesenchymal stem cells. Stem Cells Dev : 1213–1221, 2011. [DOI] [PubMed] [Google Scholar]
- 311.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res : 5331–5339, 2009. [DOI] [PubMed] [Google Scholar]
- 312.Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev : 931–952, 2007. [DOI] [PubMed] [Google Scholar]
- 313.Roura S, Farre J, Hove-Madsen L, Prat-Vidal C, Soler-Botija C, Galvez-Monton C, Vilalta M, Bayes-Genis A. Exposure to cardiomyogenic stimuli fails to transdifferentiate human umbilical cord blood-derived mesenchymal stem cells. Basic Res Cardiol : 419–430, 2010. [DOI] [PubMed] [Google Scholar]
- 314.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood : 228–234, 2007. [DOI] [PubMed] [Google Scholar]
- 315.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood : 756–763, 2005. [DOI] [PubMed] [Google Scholar]
- 316.Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, Sakata Y, Hagiwara N, Kinugawa K, Miyagawa S. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J : 991–999, 2015. [DOI] [PubMed] [Google Scholar]
- 317.Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol : 43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators RA . Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med : 1210–1221, 2006. [DOI] [PubMed] [Google Scholar]
- 319.Schoolmeesters A, Eklund T, Leake D, Vermeulen A, Smith Q, Force Aldred S, Fedorov Y. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PloS One : e5605, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science : 108–111, 1998. [DOI] [PubMed] [Google Scholar]
- 321.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol : H2002–H2011, 2008. [DOI] [PubMed] [Google Scholar]
- 322.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J : 2722–2732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells : 212–222, 2008. [DOI] [PubMed] [Google Scholar]
- 324.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, Sache A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ : 952–961, 2010. [DOI] [PubMed] [Google Scholar]
- 325.Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol : 167–201, 2011. [DOI] [PubMed] [Google Scholar]
- 326.Shabbir A, Coz A, Rodriguez L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce the proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev : 1635–1647, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol : H1428–H1438, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol : H1888–H1897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thoracic Surg : 1919–1926, 2002. [DOI] [PubMed] [Google Scholar]
- 330.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell : 289–300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nature Clin Pract Cardiovasc Med Suppl 1: S57–S64, 2006. [DOI] [PubMed] [Google Scholar]
- 332.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun : 481–488, 2004. [DOI] [PubMed] [Google Scholar]
- 333.Shimizu T, Sekine H, Yamato M, Okano T. Cell sheet-based myocardial tissue engineering: new hope for damaged heart rescue. Curr Pharm Des : 2807–2814, 2009. [DOI] [PubMed] [Google Scholar]
- 334.Shu T, Zeng B, Ren X, Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell : 217–222, 2010. [DOI] [PubMed] [Google Scholar]
- 335.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation : 150–156, 2005. [DOI] [PubMed] [Google Scholar]
- 336.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood : 55–62, 1991. [PubMed] [Google Scholar]
- 337.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res : 302–311, 2014. [DOI] [PubMed] [Google Scholar]
- 338.Somers P, de Somer F, Cornelissen M, Thierens H, Van Nooten G. Bioactive porcine matrices in heart valve tissue engineering. J Heart Valve Dis : 535–543, 2012. [PubMed] [Google Scholar]
- 339.Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S. Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Scientific Reports : 7583, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature : 599–604, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells : 74–85, 2006. [DOI] [PubMed] [Google Scholar]
- 342.Souidi N, Stolk M, Seifert M. Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transplant : 34–43, 2013. [DOI] [PubMed] [Google Scholar]
- 343.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood : 1327–1333, 2008. [DOI] [PubMed] [Google Scholar]
- 344.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood : 1484–1490, 2006. [DOI] [PubMed] [Google Scholar]
- 345.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA : 1283–1288, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell : 484–492, 2008. [DOI] [PubMed] [Google Scholar]
- 347.Stern-Straeter J, Bonaterra GA, Juritz S, Birk R, Goessler UR, Bieback K, Bugert P, Schultz J, Hormann K, Kinscherf R, Faber A. Evaluation of the effects of different culture media on the myogenic differentiation potential of adipose tissue- or bone marrow-derived human mesenchymal stem cells. Int J Mol Med : 160–170, 2014. [DOI] [PubMed] [Google Scholar]
- 348.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit (+) cells in the heart are not cardiac stem cells. Nature Commun : 8701, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Suzuki G, Iyer V, Lee TC, Canty JM Jr. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res : 1044–1054, 2011. [DOI] [PubMed] [Google Scholar]
- 350.Syedain ZH, Bradee AR, Kren S, Taylor DA, Tranquillo RT. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A : 759–769, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, Zhang L, Huang Y. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Molecules Cells : 9–19, 2010. [DOI] [PubMed] [Google Scholar]
- 352.Tao B, Cui M, Wang C, Ma S, Wu F, Yi F, Qin X, Liu J, Wang H, Wang Z, Ma X, Tian J, Chen Y, Wang J, Cao F. Percutaneous intramyocardial delivery of mesenchymal stem cells induces superior improvement in regional left ventricular function compared with bone marrow mononuclear cells in porcine myocardial infarcted heart. Theranostics : 196–205, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Teng NY, Liu YS, Wu HH, Liu YA, Ho JH, Lee OK. Promotion of mesenchymal-to-epithelial transition by rac1 inhibition with small molecules accelerates hepatic differentiation of mesenchymal stromal cells. Tissue Eng Part A : 1444–1454, 2015. [DOI] [PubMed] [Google Scholar]
- 354.Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI, Paterno J, Neofytou E, Longaker MT, Gurtner GC. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells : 266–274, 2009. [DOI] [PubMed] [Google Scholar]
- 355.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature Rev Immunol : 569–579, 2002. [DOI] [PubMed] [Google Scholar]
- 356.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation : 93–98, 2002. [DOI] [PubMed] [Google Scholar]
- 357.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells : 99–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation : II247–256, 1999. [DOI] [PubMed] [Google Scholar]
- 359.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells : 562–569, 2008. [DOI] [PubMed] [Google Scholar]
- 360.Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod : 1450–1456, 2004. [DOI] [PubMed] [Google Scholar]
- 361.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation : 389–397, 2003. [DOI] [PubMed] [Google Scholar]
- 362.Tysnes BB, Bjerkvig R. Cancer initiation and progression: involvement of stem cells and the microenvironment. Biochim Biophys Acta : 283–297, 2007. [DOI] [PubMed] [Google Scholar]
- 363.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Rev Immunol : 726–736, 2008. [DOI] [PubMed] [Google Scholar]
- 364.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA : 9226–9231, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int : 340257, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol : 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 367.Vallabhaneni KC, Haller H, Dumler I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev : 3104–3113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-Kit+ cells minimally contribute cardiomyocytes to the heart. Nature : 337–341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation : 642–652, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation : S121–S129, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Vela DC, Silva GV, Assad JA, Sousa AL, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J Histochem Cytochem : 167–176, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Verma M, Lam TK, Hebert E, Divi RL. Extracellular vesicles: potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin Pathol : 6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Verweij FJ, van Eijndhoven MA, Middeldorp J, Pegtel DM. Analysis of viral microRNA exchange via exosomes in vitro and in vivo. Methods Mol Biol : 53–68, 2013. [DOI] [PubMed] [Google Scholar]
- 374.Vincentelli A, Wautot F, Juthier F, Fouquet O, Corseaux D, Marechaux S, Le Tourneau T, Fabre O, Susen S, Van Belle E, Mouquet F, Decoene C, Prat A, Jude B. In vivo autologous recellularization of a tissue-engineered heart valve: are bone marrow mesenchymal stem cells the best candidates? J Thoracic Cardiovasc Surg : 424–432, 2007. [DOI] [PubMed] [Google Scholar]
- 375.Vogel S, Trapp T, Borger V, Peters C, Lakbir D, Dilloo D, Sorg RV. Hepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and cardiomyocytic cells. Cell Mol Life Sci : 295–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Vunjak Novakovic G, Eschenhagen T, Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harb Perspect Med : 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wang DG, Zhang FX, Chen ML, Zhu HJ, Yang B, Cao KJ. Cx43 in mesenchymal stem cells promotes angiogenesis of the infarcted heart independent of gap junctions. Mol Med Rep : 1095–1102, 2014. [DOI] [PubMed] [Google Scholar]
- 378.Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thoracic Cardiovasc Surg : 999–1005, 2000. [DOI] [PubMed] [Google Scholar]
- 379.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nature Rev Mol Cell Biol : 643–655, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Wang Y, Crisostomo PR, Wang M, Weil B, Abarbanell A, Poynter J, Manukyan MC, Meldrum DR. Nitric oxide suppresses the secretion of vascular endothelial growth factor and hepatocyte growth factor from human mesenchymal stem cells. Shock : 527–531, 2008. [DOI] [PubMed] [Google Scholar]
- 381.Wang Y, Zhang D, Ashraf M, Zhao T, Huang W, Ashraf A, Balasubramaniam A. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol : H275–H286, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wei F, Wang TZ, Zhang J, Yuan ZY, Tian HY, Ni YJ, Zhuo XZ, Han K, Liu Y, Lu Q, Bai HY, Ma AQ. Mesenchymal stem cells neither fully acquire the electrophysiological properties of mature cardiomyocytes nor promote ventricular arrhythmias in infarcted rats. Basic Res Cardiol : 274, 2012. [DOI] [PubMed] [Google Scholar]
- 383.Wei HJ, Chen SC, Chang Y, Hwang SM, Lin WW, Lai PH, Chiang HK, Hsu LF, Yang HH, Sung HW. Porous acellular bovine pericardia seeded with mesenchymal stem cells as a patch to repair a myocardial defect in a syngeneic rat model. Biomaterials : 5409–5419, 2006. [DOI] [PubMed] [Google Scholar]
- 384.Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Mesenchymal stem cells attenuate myocardial functional depression and reduce systemic and myocardial inflammation during endotoxemia. Surgery : 444–452, 2010. [DOI] [PubMed] [Google Scholar]
- 385.Weil BR, Suzuki G, Leiker MM, Fallavollita JA, Canty JM Jr. Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ Res : 634–644, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Weisman HF, Bush DE, Mannisi JA, Weisfeldt ML, Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation : 186–201, 1988. [DOI] [PubMed] [Google Scholar]
- 387.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol : 565–571, 2007. [DOI] [PubMed] [Google Scholar]
- 388.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene : 19–39, 2004. [DOI] [PubMed] [Google Scholar]
- 389.White IA, Gordon J, Balkan W, Hare JM. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ Res : 990–994, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P, Bushway PJ, Lanier M, Walsh C, Kirchhausen T, Izpisua Belmonte JC, Cashman J, Mercola M. Small molecule-mediated TGF-beta type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell : 242–252, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res : 923–940, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation : 213–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res : 792–796, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther : 543–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res : 364–370, 2000. [DOI] [PubMed] [Google Scholar]
- 396.Wu JM, Hsueh YC, Ch'ang HJ, Luo CY, Wu LW, Nakauchi H, Hsieh PC. Circulating cells contribute to cardiomyocyte regeneration after injury. Circ Res : 633–641, 2015. [DOI] [PubMed] [Google Scholar]
- 397.Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy : 555–562, 2012. [DOI] [PubMed] [Google Scholar]
- 398.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells : 1556–1564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation : 2658–2665, 2004. [DOI] [PubMed] [Google Scholar]
- 400.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med : 623–631, 2004. [DOI] [PubMed] [Google Scholar]
- 401.Yamada Y, Yokoyama S, Fukuda N, Kidoya H, Huang XY, Naitoh H, Satoh N, Takakura N. A novel approach for myocardial regeneration with educated cord blood cells cocultured with cells from brown adipose tissue. Biochem Biophys Res Commun : 182–188, 2007. [DOI] [PubMed] [Google Scholar]
- 402.Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell : 661–664, 1998. [DOI] [PubMed] [Google Scholar]
- 403.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arteriosclerosis Thrombosis Vasc Biol : 2076–2082, 2009. [DOI] [PubMed] [Google Scholar]
- 404.Yao R, Wong JY. The effects of mechanical stimulation on controlling and maintaining marrow stromal cell differentiation into vascular smooth muscle cells. J Biomech Eng : 020907, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell : 750–761, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Delivery Rev : 336–341, 2013. [DOI] [PubMed] [Google Scholar]
- 407.Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci : 2513–2523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol : 277–284, 2005. [DOI] [PubMed] [Google Scholar]
- 409.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol : 349–360, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of microRNAs by embryonic stem cell microvesicles. PloS One : e4722, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood : 1755–1761, 2005. [DOI] [PubMed] [Google Scholar]
- 412.Zeng B, Ren X, Lin G, Zhu C, Chen H, Yin J, Jiang H, Yang B, Ding D. Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol Int : 1256–1264, 2008. [DOI] [PubMed] [Google Scholar]
- 413.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature : 836–841, 2003. [DOI] [PubMed] [Google Scholar]
- 414.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J : 3197–3207, 2007. [DOI] [PubMed] [Google Scholar]
- 415.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol : 907–921, 2001. [DOI] [PubMed] [Google Scholar]
- 416.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev : 263–271, 2004. [DOI] [PubMed] [Google Scholar]
- 417.Zhang X, Wei M, Zhu W, Han B. Combined transplantation of endothelial progenitor cells and mesenchymal stem cells into a rat model of isoproterenol-induced myocardial injury. Arch Cardiovasc Dis : 333–342, 2008. [DOI] [PubMed] [Google Scholar]
- 418.Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu Y, Su T, Sun D, Li W, Wang H, Liu B, Cao F. Selective inhibition of inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem cell engraftment and improves therapeutic efficacy for myocardial infarction. Basic Res Cardiol : 417, 2014. [DOI] [PubMed] [Google Scholar]
- 419.Zhao Y, Feric NT, Thavandiran N, Nunes SS, Radisic M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Can J Cardiol : 1307–1322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhou Y, Wang S, Yu Z, Hoyt RF Jr, Sachdev V, Vincent P, Arai AE, Kwak M, Burkett SS, Horvath KA. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem Biophys Res Commun : 902–907, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Med : 452–458, 2006. [DOI] [PubMed] [Google Scholar]
- 422.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integ Biol : 447–450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res : 477–488, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]