Abstract
This review proposes that physical inactivity could be considered a behavior selected by evolution for resting, and also selected to be reinforcing in life-threatening situations in which exercise would be dangerous. Underlying the notion are human twin studies and animal selective breeding studies, both of which provide indirect evidence for the existence of genes for physical inactivity. Approximately 86% of the 325 million in the United States (U.S.) population achieve less than the U.S. Government and World Health Organization guidelines for daily physical activity for health. Although underappreciated, physical inactivity is an actual contributing cause to at least 35 unhealthy conditions, including the majority of the 10 leading causes of death in the U.S. First, we introduce nine physical inactivity-related themes. Next, characteristics and models of physical inactivity are presented. Following next are individual examples of phenotypes, organ systems, and diseases that are impacted by physical inactivity, including behavior, central nervous system, cardiorespiratory fitness, metabolism, adipose tissue, skeletal muscle, bone, immunity, digestion, and cancer. Importantly, physical inactivity, itself, often plays an independent role as a direct cause of speeding the losses of cardiovascular and strength fitness, shortening of healthspan, and lowering of the age for the onset of the first chronic disease, which in turn decreases quality of life, increases health care costs, and accelerates mortality risk.
I. GENERAL INTRODUCTION: THEMES
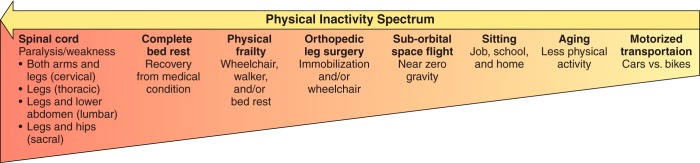
Multiple definitions of physical inactivity exist. For the purposes of the current review, physical inactivity is defined as the spectrum of any decrease in bodily movement that produces decreased energy expenditure toward basal level (FIGURE 1).
FIGURE 1.
Spectrum of the types of physical inactivity. Following the arrow from right (low intensity of physical inactivity) to left (high intensity of physical inactivity) shows our estimate of the intensity of physical inactivity per unit of time. Not shown is the volume (intensity × duration) of physical inactivity. For example, spinal cord severance is high intensity and health decrements appear within days. In opposite manner, sitting is low intensity, with long-term health effects not clinically apparent within days, but nonetheless unhealthy when first appearing after many years.
Our definition of physical inactivity is converse to the United States (U.S.) government’s definition of physical activity, which is “any bodily movement produced by the contraction of skeletal muscle that increases energy expenditure above a basal level” (82a). First, we will provide an overview of nine important themes and concepts about physical inactivity, followed by more in-depth consideration later in the review.
Theme 1: While the definitions of physical inactivity and physical activity are in essence the converse of each other, many of the underlying biochemical and molecular mechanisms of physical inactivity are not simply the converse of physical activity. Instead, mechanisms of physical inactivity in some cases employ totally different pathways than physical activity uses.
One explanation is that unidirectional steps often occur in biochemical pathways for anabolic and catabolic pathways (see sect. IXE). Importantly, potential consequences of some differing biochemical pathways between physical inactivity and physical activity suggest that 100% fidelity cannot be made for physical inactivity mechanisms merely by reversing directionality of known mechanisms for physical activity.
Theme 2: Epidemiological evidence exists that physical inactivity actually causes risk factors that, in turn, increase morbidity and mortality.
The U.S. Centers for Disease Control (CDC) published a series of papers in JAMA over the past quarter of a century on physical inactivity. In 1990, Hahn et al. (196) concluded that the risk factor of sedentary lifestyle contributed 23% to excess deaths from nine of the major chronic diseases. Mokdad et al. (337) titled their article and their descriptor of poor diet and physical inactivity as an “actual cause” of 15.2% of deaths in the U.S. In 2015, Carlson et al. (75) noted that 11.1% of all health care costs were associated with “inadequate” physical activity. Thus we contend that physical inactivity is an important component of the noncommunicable disease epidemic in the U.S., as well as worldwide (240, 305) (see sect. IIE).
Theme 3: Gene and environmental evidence exists for physical inactivity actually causes risk factors that, in turn, increase morbidity and mortality.
Our artificial breeding experiment determined if we could develop rats with the phenotype of low voluntary running distance in wheels, which provided indirect evidence for the existence of genes with functions for physical inactivity (422). Additional evidence is from twin studies [1,654 twins, 420 monozygotic and 352 dizygotic same-sex twin pairs, whose average age was 56 yr old, and body mass index (BMI) was 26.1 kg/m2] (113), that noted unique or nonshared environmental factors accounted for 55% of the variability for sedentary behavior, while additive genetic factors accounted for 31%. The remaining 15% was accounted for by common or shared environmental factors (see sect. IIG). Furthermore, Keller (242) has suggested replacement of the concept of the genome as a “static” collection of active genes with the “reactive genome.” Keller (242) contends that genome appears to function as “an exquisitely sensitive reaction (or response) mechanism–a device for regulating the production of specific proteins in response to the constantly changing signals it receives from its environment. . . .” Her concept would describe the genome as sensitive to physical inactivity. For example, in a 1968 study (443), maximal cardiac output, maximal stroke volume, and maximal oxygen consumption decreased 26, 29, and 28%, respectively, secondary to bed rest for 20 days in healthy young men. Additionally, rat gastrocnemius and soleus muscles atrophied 23 and 27%, respectively, within 1 wk when immobilized in a shortened position, the losses occurring as a first-order rate constant in a 1977 report (51). Taken together, the physical inactivity losses, if continued, without recovery, would increase the risk of chronic diseases and early mortality in later life (54, 55).
Theme 4: The incubation period for physical inactivity-developing pathologies to reach overt clinical symptoms is often long in duration and yet preclinically silent.
Goodman et al. in a CDC statement (183) offer 10 “Selected Definitions for Chronic Disease and Other Chronic Conditions” in their Table 1. Our selected first definition from the 10 is from the World Health Organization (WHO) (541), “Chronic diseases are diseases of long duration and generally slow progression.” Our selected second definition from the 10 is based upon McKenna and Collins in Goodman et al. (183); it provides greater specificity. “They are generally characterized by uncertain etiology, multiple risk factors, a long latency period, a prolonged course of illness, noncontagious origin, functional impairment or disability, and incurability.” A critical concept is that the roadmap from physical inactivity to overt type 2 diabetes (T2D), or most other chronic conditions, is that the process is “generally slow in progression” and a “long latency period.” Often the slow, natural progressions of chronic diseases require studies of aspects of the progression rather than the entire continuance (from physical inactivity → physiological dysfunction → concealed pathobiology → overt symptoms → diagnosis) (FIGURE 2). In summary, progression to more severe pathobiology during continuous physical inactivity is slow and long in duration.
FIGURE 2.
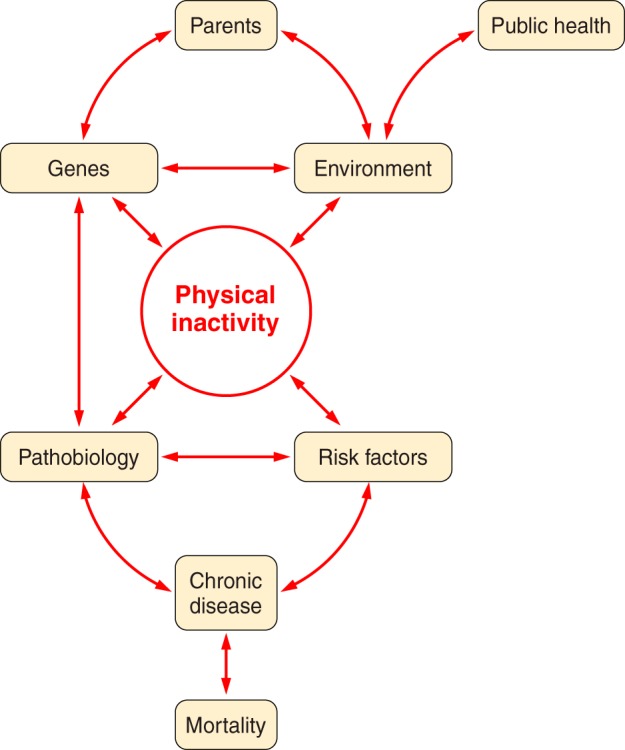
Parents provide their offspring with genes and environment, which both produce physical inactivity. Physical inactivity interacts with inherited gene predisposition of offspring to produce pathophysiology, which, in turn, interacts with risk factors to establish probability for chronic disease and mortality.
Theme 5: Continuous physical inactivity accelerates the lifelong decline in cardiovascular (maximal ability of the entire body to deliver and consume oxygen with all skeletal muscles in maximal rhythmic contraction) and strength (maximal force produced by a single contraction by a group of skeletal muscles) fitness.
Premature drops in either of the two aforementioned fitness levels accelerates the decline rate and the onset and prevalence of 1) morbidity and mortality and 2) endurance and strength frailty (see sects. VI and IX).
Theme 6: Selective breeding for the characteristic of low voluntary running distance provides evidence for the potential existence of genes having functions to produce physical inactivity.
For the first four generations of selective breeding, no decline in mean voluntary running distance was observed in offspring. However, the fifth generation produced offspring with low voluntary running distances less than in the founding population (422), suggesting that using artificial breeding that physical inactivity genes exist (see sect. IIH2).
Theme 7: Chronic disease genes and physical inactivity are both polygenic. Single gene variants correlated to any chronic disease prevalence offer insufficient predictability to be clinically relevant.
Bouchard et al. (59) were the initial pioneers of exercise genomic research with the Heritage Family Study. They studied in genetic variation in the adaptation to regular physical activity in terms of cardiorespiratory endurance and changes in cardiovascular disease and T2D risk factors. He provided a summary of the lack of progress in exercise genomics in a comprehensive review (58), where he noted that exercise genomics 1) has potential to make substantial contributions to an understanding of exercise biology; 2) has yet to deliver high-quality data; 3) “would benefit from a greater reliance on experimental studies and unbiased technologies to identify genomics, epigenomics and transcriptomics targets”; and 4) while worthy, translation is “highly premature” to advise fitness or athletic goals. Joyner (229) concurred, suggesting “many common diseases might have subtle genetic or DNA sequence variant based components but perhaps the best way to categorize most of them is ‘just barely’” (see sect. VB).
Theme 8: Adaptations to physical inactivity selected by physical inactivity during evolution enhanced survival by allowing for rapid transitions between endurance and strength phenotypes.
We speculate that genes for physical inactivity could have been advantageous for survival during natural selection, for example, for the intrinsic characteristic of rapid protein turnover. Most rate-limiting steps in biochemical pathways have short protein half-lives for rapid turnover (180), permitting protein concentrations to be able to change rapidly from one level to another, relative to longer protein turnover (222, 448). This notion may be explained by Darwinian (or evolutionary) medicine, which we define as the application of modern evolutionary theory to an understanding of health and disease (see sect. IIH).
Theme 9: The phenotype of physical inactivity behavior begins to become overt at, or near, puberty.
Supportive evidence to this concept for the existence of physical inactivity genes include decreases in voluntary wheel running, a subcategory of locomotor activity (174). Marck et al. (318) report that five species (Caenorhabditis elegans, Mus domesticus, Canis familiaris, Equus caballus, and Homo sapiens) have locomotion that has “an asymmetrical pattern throughout life” with its peak intersecting a rising developmental and declining phase (318). Indeed, maximal lifetime distance peaks early in life when rats voluntary run in wheels, thereafter falling with increasing age, at 8–9 wks of age in female rats (498) and at 6 wks of age in domestic mice (318). Gilbert defines aging as “the time-related deterioration of the physiological functions necessary for survival and fertility” (178). A conclusion from the above could be that biological aging of voluntary running is apparent around the age of puberty.
II. CHARACTERISTICS OF PHYSICAL INACTIVITY
A. History of Recognition of Physical Inactivity
Physical inactivity was recognized at least 2,500 yr ago. Physical inactivity has been based on health for millennia. In ∼600 BC, Susruta believed that regular moderate exercise offered resistance to disease(s) and “against physical decay” (497). In ∼400 BC, Hippocrates wrote “eating alone will not keep a man well; he must also take exercise, for food and exercise work together . . . to produce health” (35). Tipton (496) and Myers et al. (348) discuss a more complete history from Hippocrates to 50 yr ago. Paffenbarger, Blair, and Lee (376) recounted how Morris et al. (342) published in 1953 that London bus drivers, whose occupation was continuous sitting, had greater incidence of coronary heart disease twice that of physically active conductors in London double-decker buses. Taken together, physical inactivity has been historically defined based on its effect on health. To define “inactivity” in this review, we will base the definition on its impact on health.
Using the first U.S. Physical Activity Guidelines published in 2008 (511), we have set arbitrary time durations for physical activity based on public health criteria. The definition is <60 min/day of physical activity for ages of 17 yr old and under and <150 min of weekly physical activity for ages of 18 yr and older; these are minimum requirements for health (Table 1).
Table 1.
Definitions of physical inactivity for various age ranges
| Age Range | Frequency & Duration | Type of Physical Activity |
|---|---|---|
| 5–17 yr | <60 min/day | Including both moderate- or vigorous-intensity aerobic, including some muscle strengthening |
| >18 yr | <150 min/wk | <150 min/wk of either moderate- or vigorous-intensity aerobic, or <75 min/week of mixed moderate and high-intensity aerobic, preferably spread throughout the week; plus 2 days/wk of resistance training involving moderate to high intensities for all major muscle groups |
The U.S. definition of physical inactivity is similar to the WHO’s (541) definition. WHO divides physical inactivity into two classifications. Level 1 of physical inactivity (inactive) is defined as “doing no or very little physical activity at work, at home, for transport or in discretionary time.” Level 2 of physical inactivity (insufficiently active) is defined by WHO as “doing some physical activity, but less than 150 minutes of moderate-intensity physical activity or 60 minutes of vigorous-intensity physical activity a week accumulated across work, home, transport or discretionary domains (141). The current review considers the two WHO categories of physical inactivity together as a part of a continuum, as illustrated in FIGURE 2, providing a continuum of theme 4.
B. Physical Inactivity Has Increased in the Last Century
Societies today that do not employ power-driven machines and motorized transportation can provide estimates of what daily step count might have been centuries ago, allowing an educated guess as to the increase in physical inactivity seen today. Bassett et al. (24) provided one such estimate in The Old Order Amish in Canada who today refrain from using automobiles, electrical appliances, and other modern conveniences, and their occupation is labor-intensive farming. Men and women averaged ~18,000 and ~14,000 steps/day, reported 10 and 3 h/wk of vigorous physical activity, 43 and 40 h/wk of moderate physical activity, and 12 and 6 h/wk of walking, respectively. Many modern cultures have approximately one-third the number of daily steps as taken by Amish (23). On average, non-Amish adults report an average of 5,117 steps per day, and were separated into four groups: “very active” (6,805 steps/day); “somewhat active” (5,306 steps/day); “somewhat inactive” (4,140 steps/day); and “very inactive” (3,093 steps/day). Additionally, Church et al. (88) estimated that occupational energy expenditure decreased by >100 calories in both genders over the four decades. They concluded that a 100-kcal reduction in occupational energy expenditure would account for much of U.S. weight gain over the past half century. Myers, McAuley, Lavie, Despres, Arena, and Kokkinos (348), all experts in the field, support the high levels of physical inactivity in their quotation: “Current physical activity patterns are undeniably the lowest they have been in human history, with particularly marked declines in recent generations and future projections indicate further declines around the globe . . . Non-communicable health problems that afflict current societies are undeniably attributable to the fact that PA patterns are markedly different than those for which humans were genetically adapted” (8, 127, 356, 366).
C. Approximately 86% in the U.S. Do Not Meet Physical Activity Guidelines: Physical Inactivity Is Now Pandemic
Accelerometers to measure movement have superseded recall self-reporting and pedometers for validity recording of physical activity (509). We used accelerometer data in Troiano et al. (505) as a basis for estimating the prevalence of physical inactivity in the U.S. at ~86%, making it one of the highest, if not the highest, unhealthy condition in the U.S. With the U.S. population at ~325 million people and using the Troiano et al. (505) percentages of inactive humans, we estimate that >280 million in the U.S. are not meeting the U.S. physical activity guidelines for minimal physical activity to improve health.
It is unquestionable that physical inactivity has become a global health issue. Kohl et al. (254) concluded, “Physical inactivity is pandemic, a leading cause of death in the world, and clearly one of the top four pillars of a noncommunicable disease strategy. However, the role of physical activity continues to be undervalued despite evidence of its protective effects and the cost burden posed by present levels of physical inactivity globally.” Worldwide, percentages are similar to the U.S., as only 6 and 4% of English men and women, respectively, met requirements for 30 min of moderate or vigorous on at least 5 days/week with accumulated bouts of at least 10 min (86). Limited-available nonaccelerometer data suggest that >30% of the world’s population does not meet the minimum U.S. recommendations for physical activity (541). Therefore, >2.5 billion would be considered inactive by U.S. physical activity guideline standards. Lee et al. (287) estimate that 6–10% of worldwide deaths from noncommunicable diseases are due to physical inactivity. The incidence of physical inactivity is high, and unfortunately, current trends do not suggest a reversal is on the horizon. Taken together, the underappreciation of physical inactivity as a health threat could be described as “stealth” pandemic.
In the above context, the presentation of physical inactivity to alter behavior could use one of two generalized approaches. One proposal by Rose (431) is that structure (pertaining to social institutions and norms that shape the actions of individuals) may be more effective than from agency (pertaining to an individual's capacity to make the choice to act). Readers are directed to papers favoring the structure option (331). For example, Adams et al. (1) mention the example that if packaged foods had reduced salt content, then individuals would not have to “consciously engage with any information or actively change their behavior.” We speculate that an inactivity analogy could be providing safe bike paths (structure) instead of governmental policy recommendations to exercise 30 min most days of the week.
D. Annual Costs of Physical Inactivity in the U.S. Are Estimated Between $131 and $333 Billion and Are Rising
11.1% of aggregate health care expenditures in the U.S. during 2006–2011 were associated with physical inactivity according to Carlson et al. (75) at the CDC. They conservatively estimated the inactivity cost to be $131 billion. Carlson et al. (75) state that all previous cost estimates to U.S. health care were fivefold underestimates, implying the newest estimate is also an underestimate, and state that “this study did not estimate indirect costs, which include lost productivity from premature death and disability associated with illness, nor does it address the costs in the institutionalized population that may be associated with inadequate levels of physical activity. Other studies indicate that their estimates of physical inactivity are due to conservative methodologies (119). On the other hand, we estimated 2014 U.S. health care costs to be ~$333 billion (11.1% × $3 trillion of the total U.S. health care costs in 2014) (82b). Future studies that consider these additional costs may improve estimates of the economic burden of inadequate physical activity. Nevertheless, this study found that inadequate physical activity is associated with a significant percentage of health care expenditures in the U.S.” They also found health care expenditures were very similar for inactive adults in three independent studies. The costs were 26.6% for 51,000 U.S. adults who were 21 yr of age or older (75), 26.3% for 7,004 Australian women aged 50–55 yr old (68), and 23.5% for 5,689 individuals, 75% of whom were 40 yr or older in a Minnesota health plan (407). On the other hand, costs of physical inactivity in a total of 142 countries were “conservatively estimated” to be direct and indirect costs of $67.5 billion (international dollars) worldwide in 2013 (119), which is about one-half the estimate given above for the U.S. alone by Carlson et al. (75).
E. Evidence Exists That Physical Inactivity Actually Causes Risk Factors, Leading to Increased Morbidity and Mortality
This section continues theme 2 by providing detailed evidence of the link between physical inactivity, epidemiological evidence, and risk of morbidity and mortality. As mentioned, Morris et al. in 1953 (342) reported the novel observation that bus conductors in London double-deck buses, who had to continually climb stair to collect fares, had ~30% less coronary heart disease, were older when the disease was diagnosed, and had a lower death rate than bus drivers who sat on the same buses. In 2010, Blair et al. (42) wrote “the research field of exercise epidemiology that was initiated by Morris nearly 60 years earlier had grown to an impressive body of physical inactivity and low cardiorespiratory fitness (CRF) are major causes for increased physiological dysfunction, morbidity, and mortality. Blair et al. (41) noted in 40,000 subjects from the Aerobics Center Longitudinal Study that low CRF is a stronger predictor of mortality than any other risk factor.
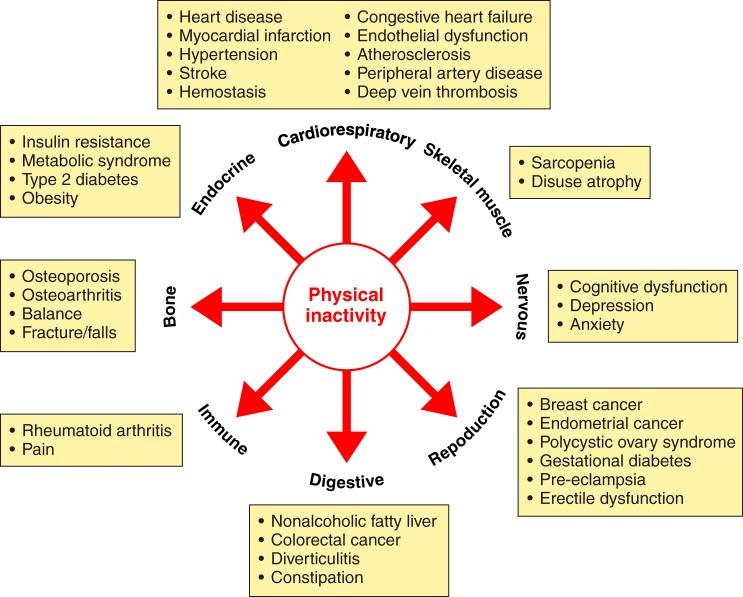
Physical exercise is not an actual causal mechanism of chronic diseases, but rather physical activity “protects” or is a therapy for diseases/conditions caused by physical inactivity (52). A 72-page review on prescribing exercise as a therapy for chronic diseases is available from Pedersen and Saltin (390). Physical inactivity, on the other hand, is one of numerous actual causes of 35 chronic diseases/conditions (55) (FIGURE 3).
FIGURE 3.
Physical inactivity increases 35 chronic diseases. See Booth et al. (55) for more details on how physical inactivity is a major cause of chronic diseases.
Many chronic diseases are polygenic, so it is not unexpected that more than a single mechanistic pathway may cause a polygenic disease. For some diseases, including six of the more prevalent chronic diseases discussed below, percent increases associated with physical inactivity range between 20 and 45%.
1. Cardiovascular diseases
Individuals performing no physical activity had 45% more cardiovascular diseases than those performing 41 MET·hr/wk (where 1 MET is the value of resting oxygen consumption).
2. T2D
Low activity groups had a 35 and 26% greater risk of T2D than in high activity groups in meta-analyses when total activity was determined in 14 cohort studies and leisure time activity was reported in a different 55 cohort studies, respectively (10).
3. Breast cancer
A 25% average increase in breast risk was present in the low physical activity groups compared with the high activity groups in the 51 studies that showed an increased risk. Case-control studies had a stronger effect (an average 30% increase) than cohort studies (a 20% increase) (310).
4. Colon cancer
The risk of proximal and distal colon cancers were increased by 27 and 26%, respectively, among the least active individuals in 21 meta-analyzed studies, as compared with the most physically active people (63).
5. Dementia
Beyboun et al. (37) noted that decreased physical activity was a strong predictor of incident Alzheimer’s disease based on an average of 27 studies that found an estimated population attributable risk percentage in dementia by physical activity to be 31.9%.
6. Depression
Meta-analysis of 25 studies (453) showed a large significant improvement of depression by exercise and that >1,000 studies with negative results would be required to reject the positive effects of exercise on depression, including larger effects for interventions in major depressive disorders. The effect size of exercise on depression is at a moderate level of 0.56 (533). Furthermore, it has been shown that exercise improves depressive symptoms to a comparable extent as pharmacotherapy and psychotherapy (48).
The CDC began categorizing physical inactivity as an actual cause of most chronic diseases only two decades ago. CDC evaluation of U.S. mortality from physical inactivity has provided varying results. Initially in 1993, McGinnis and Foege (328) published in JAMA that diet/activity pattern was an actual cause of ~300,000 deaths (or 13% of total deaths) in 1990. The CDC authors concluded “. . . the public health burden they [the major external (nongenetic) factors that contribute to death in the U.S.] impose is considerable and offers guidance for shaping health policy priorities.” Mokdad et al. (337) followed a decade later with estimates of 365,000 “actual” deaths (or 15.2%) from poor diet and physical inactivity. Another decade later, Murray et al. (345) estimated that ~230,000 (or 8.9%) of deaths were from physical inactivity and ~660,000 (or 25.4%) deaths from dietary risks occurred in the U.S. Interestingly, of the 2,596,993 deaths that occurred in the U.S. in 2013 (552), ~85% (>2,000,000) of those dying were directly or indirectly physically inactive during the majority of their lives, according to U.S. guidelines of 150 min/wk of moderate physical inactivity, or 75 min/wk of intense physical activity (505), yet physical inactivity was estimated by the CDC as responsible for only ~230,000 deaths. Thus we propose that the correct interpretation could be that physical inactivity makes at least some contribution to >2 million (or 86%) of U.S. deaths per year based on failure to reach 150 min/wk of moderate physical inactivity, or 75 min/wk of intense physical activity in ages >5 yr old.
Associations exist between physical inactivity and increased chronic disease and mortality. For example, inactive adolescents and young adults express a less healthy coronary risk profile, as compared with constantly active subjects (410). Pedersen (385) wrote, “Physical inactivity is an independent risk factor for abdominal obesity.” Manson et al. (219) noted, “both increased adiposity and reduced physical activity are significant and independent predictors of death.” Weinstein et al. (535) found, “BMI and physical inactivity are independent predictors of incident diabetes.” Blair’s group (534) stated, “Low cardiorespiratory fitness and physical inactivity are independent predictors of all-cause mortality in men with type 2 diabetes.” Later Blair’s group (286) further showed that the influence of physical inactivity on mortality is directed largely by CRF. However, physical inactivity, itself, decreases CRF (443).
Myers et al. (347) in 2004 reported that for each 1,000-kcal/wk loss decrease in physical activity, cardiovascular fitness fell one MET, and importantly, both were associated with a 20% increase in death rate. Myers et al. (347) also noted that age-adjusted mortality fell per each quartile increase in exercise capacity: hazard ratios of 1.0, 0.59, 0.46, and 0.28 for very low exercise capacity, low, moderate, and high quartiles, respectively. The same pattern existed for physical activity, but with less dramatic reductions compared with fitness: hazard ratios of 1.0, 0.63, 0.42, and 0.38 for very low physical activity, low, moderate and high quartiles, respectively. Myers et al. wrote, “. . . these two variables (aerobic fitness and physical activity quantity) were stronger predictors than established risk factors such as smoking, hypertension, obesity, and diabetes.” Warburton et al. (531) identified 254 articles with eligibility criteria for premature all-cause mortality. Women and men had ~45% average risk reductions for comparisons between high and low aerobic fitness categorizations. Furthermore, they found that high aerobic fitness also decreased mortality for seven clinical conditions: breast cancer, cardiovascular disease, colon cancer, hypertension, osteoporosis, stroke, and T2D.
Physical inactivity and poor diet are the second leading actual causes of death in the U.S. (337). The WHO report ranks physical inactivity as the fourth leading cause for global mortality, with responsibility for 6% deaths worldwide (287, 549a). Vita et al. (520) produced a metric to approximate delays with chronological aging producing chronic disease, described by Vita et al. (520) as the percentage of remaining lifespan after the onset of “cumulative disability.” A low percentage of remaining lifetime before cumulative health disabilities divided by the total lifespan would be “compression of morbidity.” Fries (167) has tested his concept with two longitudinal studies (29 and 31 yr in duration), comparing two groups: “ever runners” versus “never runners.” “Never runners” had initial cumulative disability from chronic diseases 16 yr earlier and died 3–4 yr younger, thus exhibiting low compression of morbidity, i.e., a longer percentage of life having at least one chronic disease. Fries (167) stated, “. . . the greatest effectiveness (on postponement of biological aging) may come from physical exercise, begun early, practiced hard, and continued for a lifetime.” Later, others presented an alternative terminology “healthspan,” which can be described as the percentage of life free before any chronic diseases. In summary, while physical inactivity causes hundreds of thousands of deaths, it is likely these estimates are underestimates of inactivity’s true contribution to death; however, knowing the relative contribution of individual factors is not easy to ascertain.
F. Why Extend Epidemiology to Inactivity-Induced Pathophysiology?
One simple answer is that despite all the outstanding epidemiological studies, they have not stopped the physical inactivity pandemic. The strong dogma that physical activity prevents chronic disease has not reversed the public health challenge of the physical inactivity pandemic. For example, <50 million out of 325 million in the U.S. population meet 2008 U.S. guidelines for minimal public health.
Pathophysiology is defined here as the structural and functional manifestations of a disease. One clinically significant factor is that structural changes are often irreversible. Thus, to prevent a chronic disease in the first place, i.e., primary prevention, structural changes must be prevented. Prevention of chronic physical inactivity is the primary preventer of chronic diseases. FIGURE 4 illustrates this concept.
FIGURE 4.
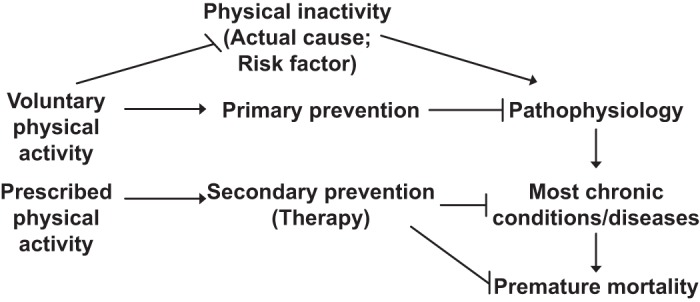
Chronic physical inactivity initiates a cascade of events. Physical inactivity is an actual cause of the numerous abnormal physiological values (physiological dysfunctions) that, in turn, cause usually permanent pathological changes (pathophysiology), which over time lead to overt diagnosed chronic diseases, that culminate as contributors to premature mortality. Two categories of physical activity are presented: voluntary physical activity, which commonly serves in primary prevention of pathophysiology, and prescribed physical activity, which is shown for common usage of secondary prevention of existing chronic disease.
As mentioned, the strong cycle of physical inactivity induces dysfunction and pathophysiology, causing at least 35 chronic diseases/conditions, which in turn results in greater levels of physical inactivity. The rapidity and severity of the response to physical inactivity is startling and is exemplified in the 1968 Dallas Bed Rest Study (443). Five healthy males underwent 20 days of continuous bed rest. The percentage declines in mean maximal physiological values for the subjects during maximal running on a treadmill were very significant for a 20-day period. V̇o2max (aerobic fitness) fell 27%. Underlying the decrease were decreases of 11% decrease in heart size, 26% decrease in maximal cardiac output, and 29% decrease in maximal stroke volume, but no significant changes in maximal heart rate or in mean arterial-venous O2 difference were noted. Taken together, the percentage size of the decrements in mass and function of the cardiopulmonary system in 20 days emphasizes the concept that the human body was built to rapidly adapt to a dysfunctional state with very short periods of physical inactivity. Remarkably, the transition from normal function for aerobic fitness in 20-yr-old, healthy men (physiological state) to low aerobic fitness for that age (physiological dysfunction) was equivalent to 30 yr of normal aging from 20 to 50 yr old. Twenty days of continuous bed rest places these individuals in a pathophysiological state leading closer to disease. These bed rest studies have been extended to present everyday living. For example, Pedersen et al. (263) had young Danish men reduce their daily step counts from 10,501 to 1,344 for a 2-wk period. A startling 6.6% decrease in V̇o2max suggests physical inactivity is a major environmental component. Unfortunately, the gene mechanisms underlying the decline in V̇o2max with aging and/or inactivity are virtually unknown. To further complicate this area of research is the fact that V̇o2max is regulated by multiple organ systems, each with unique contributions to V̇o2max.
Mechanisms of disease can be defined as defects in processes that trigger specific pathologies. Joyner and Green (230) comment that approximately half of the protective effects of physical activity are accounted for by traditional risk factors such as reductions in blood pressure and blood lipids. They suggest the missing one-half is due to the lack of knowledge to understand how the protective effects of physical activity are linked to health benefits, knowledge that is still lacking. We suggest that the missing link may be related to the concept that different molecular adaptations produce health-beneficial consequences of physical training and physical inactivity. In 2000, Booth et al. (52) proposed “the biochemical, molecular, and cellular mechanisms of physical inactivity will provide the scientific foundation for appropriate individual prescription of physical activity for health.” The topic is discussed in greater detail in Booth et al. (55).
G. General Disappointment in Gene Variants Becoming Predictive as Medicine Therapies to Prevent Chronic Diseases Caused by Physical Inactivity
This section provides the background for theme 3 regarding gene-environment evidence. Joyner and Pedersen’s review (231) notes disappointment that the promise that simple gene variances have not emerged for common diseases by suggesting “a second key example was the sequencing of the entire human genome announced in 2001 and the idea that a limited number of genetic variants would emerge and explain common diseases like cancer, hypertension, atherosclerosis, diabetes, etc.” For example, no significant genetic risk score to the incidence of total cardiovascular disease was observed for 101 single nucleotide polymorphisms in a prospective study of 19,000 initially healthy white women (383). Pedersen (392) commented that “findings such as those reported by Seshadri et al. (459) reinforce the futility of using individual genetic risk profiling for AD [Alzheimer’s disease] beyond collecting information on age, sex, family history, and APOE status.” A 2016 update by Talwar et al. (483) is, “therefore, these identified genetic markers individually or in combination have little or no clinical (predictive or diagnostic) utility in predicting AD [Alzheimer’s disease] risk.” The odds ratio for developing dementia in APOE ɛ4 non-carriers were twice as high in non-exercisers than in exercisers. However, APOE ɛ4 carriers found no difference in the odds ratio for dementia development was present between non-exercisers and exercisers (143). One conclusion in a 2013 issue of Diabetes Care for the status of genetic screening for T2D risk is summed by its statements, “however, available data to date do not yet provide convincing evidence to support use of genetic screening for the prediction of T2D . . . Genetic testing for the prediction of T2D in high risk individuals is currently of little value in clinical practice” (311). In addition, some outcomes of the Functional Single Nucleotide Polymorphisms Associated with Human Muscle Size and Strength study or FAMuSS were as follows: 1) “individual genetic variants explain a small portion of the variability in (511) muscle strength and size response to resistance training (393). 2) Genetic variants that were examined in the resistance training study only <1-12% of body composition and cardiometabolic markers in habitual physical activity levels, “suggesting these traits are highly polygenic with many loci contributing a very small proportion of the variation, and these phenotype-genotype associations were often sex specific” (393). Furthermore, in ~6,400 individuals of European descent and over 65 yr of age, no significant gene-variant associations were observed with lower body strength (325). The outcome contrasted with handgrip strength, which revealed an association with molecular targets in ~27,000 individuals >65 yr old, whose genes were of European descent (325). Taken together, the above verifies the quotation “simple genetic answers have not emerged for common diseases” (231).
Only one human gene variant has been identified that is related to physical inactivity. A gene variant in the FTO (fat mass and obesity-associated protein) gene only expresses its negative health effect of increased probability of obesity in the presence of physical inactivity. One FTO risk allele that is associated with obesity is 27% higher in physically inactive adults (243). Demerath et al. (111) stated that the FTO variant is the strongest common genetic susceptibility locus for obesity yet discovered (118, 161, 456). Thus we interpret that physical inactivity is a strong environmental stimulant of one gene variant in the FTO gene variant for obesity.
H. Polygenic Heritable Factors Regulate Sedentary Behavior
The existence of genes for sedentary behavior has indirect, strong support from 1) twin studies which identified heredity as a source of inactivity between pairs if twins (113), 2) selective breeding for the phenotype of physical inactivity in rats (423), and 3) comparisons between naturally low and high levels of voluntary running. FIGURE 5 provides an overview that evolution can be used as a foundation to speculate as to how physical inactivity could explain a genesis for observed interactions among genes, environment, and chronic diseases.
FIGURE 5.
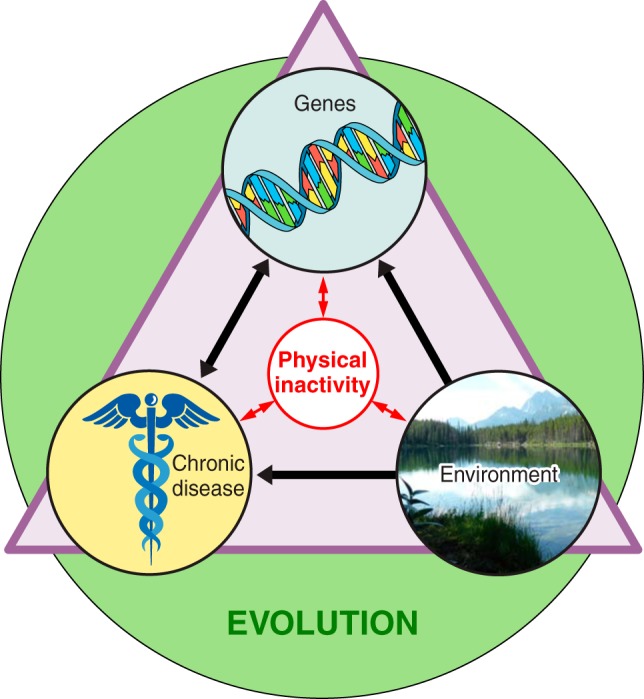
Overview of physical inactivity’s interactions. The three terms inside the triangle (chronic disease, genes, and environment) all interact directly with physical inactivity, and physical inactivity can directly influence them. The green circle indicates that evolution has and continues to play a role in shaping the interactions of all the terms inside the triangle.
1. Humans predisposed to physical inactivity
The median heritability of exercise participation was 62% in seven countries (Scandinavia, United Kingdom, and Australia) (475). Comparisons were made between 13,676 monozygotic twin pairs and 23,375 dizygotic twin pairs. Another later study of 1,654 twins (same-sex twins comprised 420 monozygotic and 352 dizygotic same-sex twin pairs) monitored by heart rate and accelerometers to time spent in moderate-to-vigorous intensity physical activity and sedentary behavior. Roos and co-workers (113) reported that sedentary behavior is moderately heritable in adults. Additive genetic factors (i.e., heritability) explained 31% of the time spent in sedentary behavior, with environmental and other factors explaining most of the remaining two-thirds. Moore-Harrison and Lightfoot (338) in their review of genomic locations associated with physical activity cite the Quebec Family Study as reporting different chromosomal linkages between human physical activity and physical inactivity.
2. Selective breeding
The question considered in the current section is as follows: Is there an evolutionary foundation for physical inactivity, and if so how much of physical inactivity was selected by evolution? One opinion to the question is from the anthropologist Lieberman (297). His contention was that limitations of the daily, caloric intake in hunter-gatherer populations drove a behavior of rest bouts during a part of the day to save calories to match calorie intake. Lieberman also contends, “selection never operated to cope with the long-term effects of chronic inactivity” (297). Thus he contends evolution never had an opportunity to develop protections against physical inactivity to producing chronic diseases.
Selective breeding can be defined as selecting one phenotype each generation to enrich genes underlying the phenotype. Our approach was modeled after two identical strategies that have provided significant insights into gene function: one by Garland (480) selectively bred mice for high distances of voluntary running distance, as compared with control mice, and another by Britton and Koch (251) from rats selectively bred for either high or low exercise capacity by forced running on motor-driven treadmills. Their publications led the Booth laboratory to selectively breed for the trait of low voluntary running by rats. The strategy was to determine if genes could be enriched to produce low behavior to voluntarily run. The selective breeding protocol uniquely produced rats with voluntary running distance behavior approaching zero for some rats (422). The founding population of females and males voluntarily ran 10.7 and 6.9 km/day, respectively. However, after nine generations of selective breeding for low voluntary running distance, females and males were running 1.4 and 1.1 km/day (422), translating to 87 and 84%, respectively, less compared with the founder population. To selectively breed for the phenotype of low voluntary running distance indicates the existence of genes to favor low voluntary running distance. While the genes, or gene variants, producing inherent physical inactivity are yet to be identified, complex transcriptomic responses direct physical inactivity. Taken together, the details above provide evidence for the role of selective breeding studies (theme 6).
Taken together, both studies provide inferential evidence that genes for physical inactivity exist and thus physical inactivity has an evolutionary basis. An enormous volume of literature exists on the artificial selection for the phenotype of high levels of voluntary running (173, 252, 298, 472). Since evidence indicates that physical activity has an evolutionary basis (31), one might ask why evolution would select the opposite, the need to be physically inactive?
3. Comparisons between mouse strains exhibiting naturally low and high levels of voluntary running
In contrast to studies of artificial breeding for voluntary running phenotypes that reveal gene identities, another type of model compares different mouse strains, which we designate as a “natural” model of voluntary running. Lightfoot and co-workers reported separate proteome signatures in the nucleus accumbens between naturally high- and low-physically active mice (145). Ferguson et al. (145) compared two strains of mice having an 8.9-fold difference in voluntary running distance. In sedentary mice of the higher voluntary running strain that were never allowed voluntary running, three proteins with metabolic functions were higher in the nucleus accumbens (creatine kinase B, succinyl-CoA ligase, and endophilin), as compared with the lowest natural running strain. The higher mouse strain voluntarily ran 10.7 km/day, as compared with the second mouse strain with a low voluntary running distance (1.2 km/day), which exhibited four different proteins (stress 70 mitochondrial protein, V-type proton ATPase catalytic subunit A, dihydropryimidinase, and transcription elongation factor A) in the nucleus accumbens of mice never exposed to running wheels. In skeletal muscle, Ferguson et al. (144) reported that transient knockdown of annexin A6 or calsequestrin 1 protein within hindlimb skeletal muscles of higher-active mouse strain was associated with reductions in voluntary running distance. They concluded that their data support a hypothesis that factors from skeletal muscle contribute to regulation of voluntary running. The finding reflects an earlier study using mice with sevenfold and threefold higher GLUT4 mRNA in hindlimb muscle and in heart, respectively (507); the GLUT4 overexpressing had a fourfold greater voluntary running distance than wild-type mice (508). The GLUT4 overexpressing mice are an early demonstration of muscles putatively “communicating” with brain regions regulating voluntary running distances.
Animal behavior is evidence for the existence of an evolutionary selection of “inactivity genes” in lower animals. Predators employ various foraging modes in nature. Two are ambush/sit-and-wait and active predation. They are considered to be the two extremes of the foraging mode spectrum (446). For example, copepods sit motionlessly in the water column to prevent detection by the prey (245), and sidewinder rattlesnakes sit and wait to ambush their prey (90). Thus some physical inactivity behaviors can be considered as inherent behavior.
In addition, transduction of physical inactivity is polygenic. An example of polygenic response to increasing physical activity is allowing pre-pubertal rats to perform natural voluntarily running in wheels, which mitigates growth of perirenal adipose tissue as body size increases, relative to peers without running wheels (268, 281). Running cessation (termed “wheel lock”) is associated with a rapid catch-up growth of abdominal adipose tissue to match the size of rats that never performed voluntary running. In a transcriptomic experiment, perirenal adipose tissue mass of 1-wk, wheel-locked rats were 78% greater than rats continued running; 646 known transcripts were differentially expressed between wheel-lock and continued wheel access groups in a pathway analysis of RNA-seq data (439). In wheel-locked rats, pathway analysis revealed increased transcripts for the functions of extracellular matrix, immunity, inflammation, and macrophage infiltration. These findings were interpreted to suggest polygenic responses in perirenal adipose tissue when the pre-pubertal rat became physically inactive following voluntary running. Together with the discussion on the polygenicity of chronic diseases in section IIG, these findings provide examples for theme 7.
I. Darwinian Medicine Application to Fitness
In our view, Darwinian medicine could suggest that survival of the species depends on rapid responses to new changes in the environment to increase the probability to survive, which was introduced earlier as theme 8. From this, we could suggest that a rapid transition between endurance and strength fitness might have been a survival advantage, as the two fitness phenotypes differ in function. We define endurance exercise as continuous submaximal contractions of large muscle groups, while strength exercise is defined as producing near-maximal forces for a short period (seconds) in any skeletal muscle group. Holloszy and Booth (217) noted that hypertrophied muscles from strength training have minimal, or no, increases in skeletal muscle mitochondrial concentration versus endurance-trained skeletal muscle having increased mitochondrial concentrations without hypertrophy. If one approximates the time for each contraction (repetition) against a near-maximal load to be ~3 s, then as an example, training a muscle for 3 sets with 8 repetitions per set would be ~72 s, and if trained 2 times/week, weekly duration of strength training would be ~2 min/wk. This duration is ~1% of the duration of 150 min/wk for endurance training in the U.S. physical activity guidelines. Taken together, the actual duration of muscular contraction may differ by 100-fold between two major modalities of exercise training for health adaptations to physical activity.
Along these lines, Coffey and Hawley (92) discuss the phenotype differences between endurance versus strength training, reporting that differing signaling pathways are not only producing the two phenotypes, but moreover that “it is likely that multiple integrated, rather than isolated, effectors or processes are required to generate the interference effect,” whereby maximal strength development is impaired in individuals who train using both strength and endurance workouts, as compared with strength training alone. Darwinian medicine could suggest that survival might have depended, in part, on the rapidity to transform from endurance to strength optimization, or vice versa. Such speculation could contribute to why physical inactivity is associated with rapid skeletal muscle atrophy (493) (for strength training) and rapid decline in mitochondrial concentrations (343) (for endurance training). Both genetically optimal skeletal muscle endurance and size/strength require differing molecular signals to produce different phenotypes.
While Darwinian Medicine concepts are applied to the gain in either endurance or strength fitness, the gain of one type of fitness is often associated with a decline in the other type of fitness because signaling pathways inducing both produce conflicting phenotypes. For example, endurance running requires small fiber diameters of skeletal muscle fibers to limit diffusion distance for optimal oxygen transport, while muscle strength is associated with large-diameter fibers to increase force per fiber. A speculative hypothesis would be that the rapid time required to increase endurance type of fitness for survival purposes in a new environment requiring endurance could be dependent on the rapid loss (increased degradation rate) of skeletal muscle diameter to obtain the short oxygen diffusion distance (see sect. IXB for detailed discussion).
J. Environmental Manipulation of Genes by Physical Inactivity and Possible Link to Epigenetics
Pima Indians provide an example of a human population highly predisposed to obesity and T2D. Although they share a common genetic background, they have come to reside in two geographical locations upon separation ~1,000 yr ago, with those residing in Arizona adopting a Western lifestyle of physical inactivity and diet (454). Arizona Pima Indians have developed one of the world’s highest prevalence of T2D (248). In contrast, the Mexican Pima Indian population maintained their historical, relatively low T2D prevalence (413), related to a physically active lifestyle that included wood milling, nonmechanized farming, livestock breeding, security guarding, construction, mining, and homemaking (136). Arizona Pima Indians were estimated to to expend ~500–600 kcal/day fewer than their Mexican counterparts (136). Although DNA sequences most likely did not change in the 1,000-yr separation, epigenetic changes likely occurred in the Arizona Pima population due to their lifestyle changes to less physical activity and a Western diet. Such a supposition could be based on what Noble’s reference (363) to Waddington who “demonstrated the inheritance of a characteristic acquired in a population in response to an environmental stimulus.” It is a reasonable notion that physical inactivity could induce changes in gene expression by epigenetic mechanisms. More recently, epigenetics was described as a molecular event that involves heritable changes in gene expression. Epigenetics encompasses alterations in gene expression without nucleotide alterations in the DNA coding sequence that are heritable through cell division. These modifications include histone modifications and DNA methylation, but new mechanisms suggest that other molecular events, such as noncoding RNAs, are implicated in several epigenetic mechanisms.” One example of this is Alibegovic et al. (6), who noted a trend toward greater DNA methylation of PPARGC1A in the vastus lateralis muscle after 10 days of bed rest, which could contribute to the impaired expression of PPARGC1A.
III. PHYSICAL INACTIVITY MODELS
As noted in FIGURE 1, the human physical inactivity continuum ranges from extreme (spinal cord injury) to limited inactivity (reduced stepping and sitting). Additionally, we present rodent preclinical models of physical inactivity.
A. Bed Rest
Bed rest represents an extreme level of physical inactivity. Typically, subjects participating in bed rest studies only move the upper limbs, while removing all weight bearing against gravity from the legs. In the early 1950s, the standard of care for a myocardial infarction was bed rest. However, President Eisenhower’s personal cardiologist, Paul Dudley White, argued against bed rest after President Eisenhower’s heart attack (277). He prescribed early ambulation, which later was credited for saving, or at least prolonging, the Presidents’ life. Interestingly, cardiac rehabilitation was developed around the same time after acute myocardial infarction, where before this, the standard of care was bedrest and inactivity. Interestingly, bed rest was used in early experiments to better understand the deleterious effects that occur during spaceflight, while on land. The strength of bed rest as physical inactivity model is that it permits a more homogeneous experimental treatment, i.e., the experimenter can control for subject variability and can limit amount of movement. The first human bed rest study with major impact was the Dallas bed rest study by Saltin et al. (443), described above. After only 20 days of continuous bed rest by healthy young men, remarkable decreases occurred in V̇o2max, maximal total heart volume, maximal stroke volume, and maximal cardiac output. One weakness of conducting bed rest studies is the expense of conducting these types of studies and the deleterious health outcomes common to the subjects involved. Furthermore, bed rest studies are clinically relevant to diseases/accidents that require bed rest to heal the primary disorder.
B. Spinal Cord Injury
Spinal cord injury approaches the most absolute form of physical inactivity. Within spinal cord injury, there are various forms and severities of spinal cord injury, such as paraplegia or quadriplegia (246). The location that the lesion or damage occurs within the spinal cord will determine the loss of function. If anything can be said positive about the tragic condition of quadriplegia, it is that this condition offers insights into the effects of inactivity in the absence of innervations. One experimental weakness of the condition is the heterogeneity in human subjects due to high variability in the severity of spinal cord injury. Several reviews on the model exist, including non-human primates for translational research to the human condition (365) and using the rat as the preclinical model (130).
Other types of skeletal muscle denervation are present. Aging is associated with loss of neuromuscular junctions (223, 438) in sarcopenia. Loss of motor unit numbers is slowed by life-long high-intensity physical activity (402), implying a role for inactivity in motor unit loss. A part of a recent review (93) considers molecular mechanisms during sarcopenia.
C. Spaceflight
Spaceflight is deleterious to many organ systems due to the lack of gravity (319). The uniqueness of the near-zero gravity form of physical inactivity in near-orbital flight is that it enlightens the role of lack of gravity in physical inactivity adaptation on Earth. Even though astronauts can perform physical activity in near-orbit in space under near zero gravity conditions, many of the positive physiological adaptations from exercise are not conferred due to the lack of Earth’s gravity. For example, running on a treadmill in near-zero gravity does not prevent bone loss by itself, as there is no mechanical stimulus developed from a weightless body on lower extremity bones on a treadmill belt in weightlessness. The skeletal system does not experience weight bearing and therefore undergoes bone decalcification (283, 518). The strength of spaceflight as a model of physical inactivity is due to being able to separate the factor of gravity from other exercise responses. Some weaknesses are that due to the limited numbers of individuals who go into space and the high demands placed on the astronauts in space on short missions, limited data are available on short-duration space flights for studying this form of inactivity. Additional, Morey-Horton and Globus (341) reviewed ground-based animal models of space flight.
D. Limb Immobilization
1. Humans
In 1948, Deitrick et al. (110) published a report on human immobilization that was imposed from the umbilicus to the toes in which nitrogen and calcium excretion increased. Some of the main concerns with human limb immobilization now are failure to recover lost bone strength and muscle mass post-limb immobilization in elderly. Recently, 2 wk of hindlimb immobilization on leg strength and work capacity of 23- and 68-yr-old men provides evidence of the impact of immobilization and retraining (519).
2. Animals
Several models of limb immobilizations have been applied to rodents in the elucidation of disuse atrophy and sarcopenia (50, 539). The strengths of limb immobilization are that it approximates a real-world model. A weakness is that it is not a model of whole body physical inactivity. An earlier review on rat hindlimb that provides basic information (50), and a recent article provides some mechanistic insight into effects of one-limb immobilization upon skeletal muscle atrophy (316).
E. Sitting
1. Human
In 1953, Morris et al. (342, 376) performed the aforementioned classic London double-level bus study, where drivers sat while conductors had to walk up and down stairs on double-level buses. Conductors, compared with drivers, had a 30% lower incidence rate of coronary heart disease. Furthermore, conductors were older when they developed the disease, which was less severe with lower fatality rates than the drivers (376). Follow-up research to the 1953 Morris study was relatively untested in humans until a 2007 study by Hamilton et al. (200). Sitting as a form of physical inactivity has had a recent surge of publications (going from 309 papers in 1995 to 513 papers in 2005 to 1,109 papers in 2015). Readers can refer to recent reviews for more in-depth analyses showing some evidence for a likely causal relationship between sedentary behavior and all-cause mortality (38, 132, 543). For example, van der Berg and co-workers (512, 543) noted using accelerometers on the thigh, that for each additional hour daily of sitting or prone position during waking hours, odds increased 22 and 39% for T2D and metabolic syndrome, respectively, and T2D outcomes were not related to sedentary breaks per day.
2. Animal
Hindlimb suspension mimics human sitting in regard to removal of weight-bearing on the legs. Several reviews on hindlimb suspension (unloading) exist (15, 87, 371), including Baldwin et al. (15) who reviewed molecular mechanisms underlying myosin heavy chain isoform switching during unloading of skeletal muscle by tail suspension.
F. Intermittent Breaks in Physical Inactivity
Edgerton’s laboratory hindlimb suspended rats for 1 wk such that soleus and gastrocnemius muscles became non-weightbearing (210). One group underwent the countermeasure of intermittent breaks from non-weightbearing, and the authors noted that high-load exercise at four intervals spaced over 12 h daily prevented about half of soleus muscle atrophy and hypertrophied the gastrocnemius muscle. In a subsequent study, the Edgerton laboratory (205) noted that non-weightbearing rats walking 40 min/day (10 min every 6 h) halved the amount of atrophy in the soleus muscle and mitigated atrophy in the gastrocnemius (185). In a separate study, the Booth laboratory (109) provided a non-weightbearing group 2 h daily centrifugation for 1 wk at one of three gravity levels. Soleus muscle masses were 48, 56, and 65% of control masses at 1, 1.5, or 2.6 G force, respectively, of the atrophy produced with continuous non-weightbearing. They followed up this noting that rats undergoing four 15-min periods of centrifugation at 1.2 G, spaced over a 12-h interval in the sleep period of the day, prevented 67% of soleus muscle atrophy (108).
G. Decrease in Daily Step Numbers
1. Human
Many humans obtained sufficient steps during earlier times historically simply by requirements for daily function and work productivity (farmer, construction, mining, blacksmith, etc.). Today, one of the simplest forms of physical activity, walking, has been largely engineered out of society. Indeed, motorized forms of transportation (automobiles, trains, boats, planes, public transportation, elevators, escalators, moving walkways, and modern walking machines) have replaced walking, carrying loads, and many occupational tasks such as production and farming use machinery. To provide a human model of physical inactivity mimicking the reduction in daily step numbers that has occurred in the last few decades, many investigators have use a reduced step model to investigate changes in real-world physical activity levels. Another approach is the 12-yr walkability study in Southern Ontario, where only the top quintile of walkable neighborhoods had associations with decreases in incidence of T2D prevalence (103).
2. Animal
The Booth laboratory has generated a model of rats that were selectively bred for low voluntary running. This is a unique rodent model that allows for the investigation of behavioral and physiological drivers of physical inactivity. Currently, this model is being used to understand central nervous system-related contributors to voluntary running (422, 424, 441). In addition, the Booth laboratory has used the previously described wheel-lock model of physical inactivity (95, 375, 414), in which access to voluntary running is permitted for several weeks followed by locking of the wheels, thereby ceasing normal activity and promoting inactivity. The acute effects of this inactivity can then be assessed in various tissues and organ systems.
IV. BEHAVIORAL INFLUENCE ON VOLUNTARY PHYSICAL INACTIVITY
It is well understood that an increase in physical inactivity increases chronic disease (287); however, the increase in physical inactivity with age is often nonlinear. Data support the notion that lifetime physical activity peaks in the pre-pubertal to pubertal ages, followed by a nonlinear decline in physical activity occurring thereafter. An innovation occurred when objective measurements of 24-h physical activity and sedentary times became available with the advent of accelerometer use (3). Accelerometers are purported to be superior in validity to recall questionnaires (289).
A. Accelerometer Data on U.S. Children and Adolescents
The data below from several studies indicate physical activity falling in childhood, implying the inverse that physical inactivity is increasing at the same time. Trost et al. (506) concluded that physical activity drops rapidly during childhood and adolescence, as evidenced by a 40% decline in moderate-vigorous physical activity going from grades 1–3 to 4–6 (FIGURE 6).
FIGURE 6.
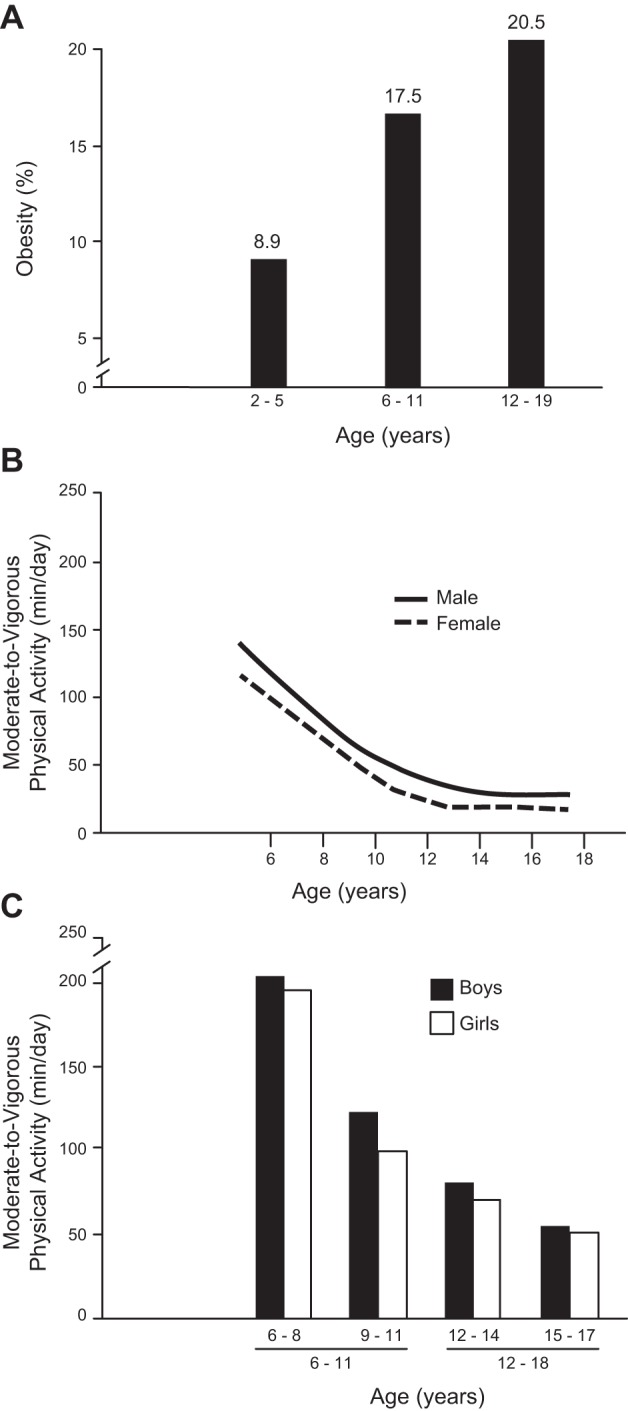
Increasing obesity and decreasing voluntary physical activity as a function of age in youth. A: percentage of overweight or obese (BMI for age grouping ≥85th percentile of the Centers for Disease Control Growth Charts) in the three age ranges increases from 2 to 5 yr (infants) to 6–11 yr (children) and 12–19 yr (adolescents) as originally presented in JAMA by Ogden et al. (369). B: best-fit lines for ages ascending from 6 to 19 yr old are descending curves that represent the 50th percentile of females and males. Accelerometer-determined moderate-to-vigorous physical activity decreases during 6–11 yr old ages and then plateaued during 12–18 yr old age range. [Modified from Wolff-Hughes et al. (547).] C: second confirming study to B that accelerometer-based moderate-to-vigorous physical activity decreased during 6–11 yr old age range and then began to asymptope during 12–18 yr old age range. [Redrawn from Trost et al. (506), with permission from Medicine and Science in Sports and Exercise.]
The U.S. Physical Activity Guidelines designate ≥60 min of daily moderate- or greater-intensity activity on 5 of 7 days/week is required for health. Both sexes also fell below the U.S. Guidelines for the chronological ages between the groups for grades 7–9 and 10–12. In addition, participation in 20-min continuous bouts of physical activity was low to nonexistent.
Troiano et al. (505) noted the majority of U.S. children and adolescents did not meet the U.S. Guidelines for physical activity. In 6–11 yr olds, 65 and 51% of females and males, respectively, were physically inactive by the U.S. Physical Activity Guidelines, while 12–19 yr olds exhibited an alarming 96 and 92% physically inactivity rates of females and males, respectively. More importantly, these percentages are high compared with other risk factors for cardiovascular disease. For comparison, 21% of children and adolescents exhibit at least one abnormal cholesterol measure [low high-density lipoprotein (HDL) cholesterol, high total cholesterol, or high non-HDL cholesterol] (357). The above comparison suggests that physical inactivity is an unappreciated risk factor for chronic disease.
Wolff-Hughes et al. (547) uniquely analyzed data from the US 2003–2006 National Health and Nutrition Examination Survey (NHANES). They employed wrist accelerometers on 3,700 U.S. youth (FIGURE 6). Moderate to vigorous physical activity decreased ~67 and ~60% in U.S. girls and boys, respectively, from the age of 6 to 19 yr old. After 8.7 and 10 yr of age, 50% of girls and of boys, respectively, engaged in <60 min of daily, intermittent moderate-vigorous physical activity. Rääsk et al. (409) also noted using accelerometry that pubertal boys started to be less active in their pubertal period. Taken together, the above four reports showed a significant decline accelerometer-detected physical activity in youth over time. Furthermore, more than half of U.S. youth did not perform sufficient daily physical activity, according to U.S. Physical Activity Guidelines. This has been extended to other countries, as Hallal et al. (197) reported that 80% of 13–15 yr olds in 105 countries averaged <60 min of moderate to vigorous, daily physical activity, with girls being less physically active than boys.
Despite the epidemic levels of physical inactivity, little is known about the underlying genetic and biological mechanisms that may contribute to the regulation of physical inactivity behavior. Some of what we know has emerged from physical activity studies. Garland and Carter (172) reviewed that physiologists have historically recognized that animals living in extreme environments show “clear examples of evolutionary adaption because of the presumably intense past selective pressures.” Swallow et al. (480) reported that an ~75% increase in distance run in voluntary running in wheels after 10 generations for high voluntary running. While cultural and social pressures definitely influence physical activity in humans, they do not regulate these behaviors 100%, and the estimated genetic component for physical inactivity has been estimated at between 20 and 80% in animal and human studies (247). We tested whether selective breeding would reveal the characteristic of low voluntary running. After nine generations, female and male rats selected for lowest distance of running in wheels exhibited an 87 and 84%, respectively, decrease in wheel running distance, as compared with wild-type rats (422). Our findings provide supporting evidence for a genetic component influencing sedentary behavior, that we have seen continue in future generations of rats selectively bred for the primary characteristic of low voluntary running distance (67, 422, 423, 425, 440, 441).
The above studies may also have evolutionary significance. The studies described above (409, 505, 506, 547) exhibit a similar age for declines in moderate to vigorous physical activity. Voluntary physical activity also reaches its lifetime highest value around the age of puberty in Wistar female rats bred to be high voluntary runners (498). Next, we will extend information from theme 9, introducing the topic of voluntary running behavior peaking early in the lifecourse. We speculate that evolution may have concurrently matched the ages, puberty, and maximum voluntary physical activity to increase the probability of successful mating to the next generation. Inherent gene regulation may trigger the initial decline from peak lifetime voluntary physical activity. As the clinical diagnosis of most inactivity-produced human chronic diseases occurs post-puberty, even in cases where chronic disease onset is pre-pubertal, reproductive viability is usually maintained into later adulthood. Thus reproduction will be successful in transferring genes to the next generation independent of the inactivity status of the youth. Consequently, it is unlikely that natural selection would extinguish inherited genes for physical inactivity.
B. Childhood Activity Sets the Stage for Adult Health
Some evidence exists to support the assertion that physical inactivity during youth is associated with a higher probability of lower CRF, bone strength, skeletal muscle mass/strength, and other cardiometabolic factors throughout the remainder of life (146). Furthermore, the likelihood of these factors being retained later in life increases through childhood to adolescence (158, 160).
For example, a 26-yr follow-up study of 1.1 million Swedish men who had mandatory military conscription and physicals noted that 18-yr-old recruits that has a combination of low exercise capacity and low muscle strength had 23% higher hazard ratios for vascular events 26 yr later (7).
Forrest and Riley (158) contend that the health of the population at later ages is affected by modifiable precursors of many common chronic disorders that arise during childhood. Some mechanistic evidence supports this contention. For example, voluntary wheel running after weaning reduced diet-induced obesity (381). Six-week exposure to early voluntary running exercise prevented diet-induced obesity in susceptible rats while they continued to consume an obesogenic diet, but not engaging in voluntary running. For hypothalamic peptides, the 6-wk voluntary running, 7-wk sedentary rats had a 55% greater mRNA expression of arcuate nucleus proopiomelanocortin, as compared with sedentary rats without voluntary running, suggesting a hypothalamic contribution to their sustained obesity resistance.
V. CENTRAL NERVOUS SYSTEM (COGNITION, MEMORY, AND MENTAL HEALTH)
Although some behavioral effects of physical inactivity are linked to the nervous system, it is also well established that participation in physical activity can enhance other nervous system functions, such as cognition and memory, as well as alleviate psychological conditions such as depression and anxiety (221, 326, 404, 514). While many studies have noted positive relationships between higher physical activity levels and brain health, based on our evolutionary contention that “normal” human behavior is highly dependent on physical activity, these data, by inference, argue that physical inactivity may be a factor causing declines in mental health. However, few studies have directly addressed how reductions in physical activity level influence mental health. We highlight findings linking decreases in physical activity with impaired brain function, as well as describe mechanisms by which physical activity could prevent declines in cognitive and mental health.
A. Physical Inactivity Increases Cognitive Dysfunction Throughout the Lifespan
Cognitive function is defined as the intellectual processes by which an individual becomes cognizant, perceptive, or understanding of ideas. The process engages all aspects of perception, reasoning, remembering, and thinking. Cognitive decline is associated with aging, including Alzheimer’s disease and other forms of dementia. Thus cognitive function is arbitrarily divided into two time frames of developing and then declining cognition. Cotman and co-workers (102, 221), Kramer et al. (404), van Pragg et al. (524), and others have noted that increased physical activity drives cognitive development of specific brain areas. On the other hand, physical inactivity is associated with reduced cognitive abilities. Low physical activity in older women increased risk of cognitive impairment, Alzheimer's disease, and any type of dementia by 72, 100, and 59%, respectively (279). Laurin et al. (279) concluded: “Regular physical activity could represent an important and potent protective factor for cognitive decline and dementia in elderly persons.”
Similar findings show physical activity improvements on cognitive health is prevalent across lifespan (214). Physically active Dutch men between 15 and 25 yr of age had a lower age-related decline in informational processing ability compared with individuals physically inactive over the same age range (115). Likewise, women had a lower likelihood of cognitive dysfunction later in life if they were physically active either early in life or became active after being teenagers (335). Importantly, physical activity during the teenage years appeared to strongly relate to improved cognitive function and to decreased cognitive impairment later in life (335). Increased physical activity level in children (age 4–18 yr) is strongly associated with increased achievement, developmental level/academic readiness, intelligence quotient, perceptual skills, and verbal and math test scores (137). Children and adolescents with low physical activity levels have lower cognitive performance as compared with physically active children (70, 81, 83a, 84, 122, 399), and physical activity may enhance children’s executive function, such as making decisions and prioritizing tasks, managing time efficiently, and organizing thoughts and activities (500).
Comparable relationships between cognitive function and physical inactivity have also been found in older adults. In ~18,000 women aged 71–80 yr, lower levels of long-term, regular exercise were related to decreased cognitive function and a 20% increased risk of cognitive impairment (538). Likewise, the incidence of dementia rose from 13.0 per 1,000 person-years to 19.7 per 1,000 person-years with greater physical inactivity (<3 bouts of exercise/week) in a >65 yr-old group (276). Physical activity levels were inversely related to dementia in men and women who were 65 yr old and older (398). Lower V̇o2peak was related negatively with preserved cognitive function during a 6-yr period in 349 subjects greater than 55 yr old (20). Similarly, 24 wk of resistance training positively affected multiple measures of cognitive function in 65–75 yr old males (80). However, whether the improvements in cognitive function may be dictated by physical activity or fitness is unclear. In a ~1,300 subject meta-analysis, Etnier et al. (137) concluded that cognitive performance is positively associated with physical activity, but that empirical evidence to support a relationship between cognitive performance and aerobic fitness was lacking. Other meta-analytic reviews have observed similar findings (94, 142). Conversely, Voss et al. (525) concluded “CRF is an important factor in moderating the adverse effects of aging on cognitively and clinically relevant functional brain networks.” They also caution that it is still necessary to measure both physical activity and CRF fitness because results for physical activity could be due to higher fitness among high responders to regular physical activity.
Minimal information is available for mechanisms by which physical inactivity initiates mechanisms causing cognitive dysfunction. However, physical activity could be used to build hypotheses for mechanisms by which exercise rescues cognitive dysfunction. A limitation of such a suggested approach would be that for some of the few known inactivity mechanisms, these signaling pathways are not always the reversal of exercise signaling pathways (470). A further limitation is “the underlying mechanisms for the positive effects of exercise on wellbeing remain poorly understood” (199). Thus only a limited number of exercise mechanisms are available to use in a strategy already limited by lack of inactivity mechanisms being a simple reversal of exercise mechanisms. For example, physical activity increases dentate gyrus neurogenesis, which reviews interpret as associating with cognitive preservation (312, 517). Other reviews (102, 313, 524) highlight that growth factors [brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-I)] transmit downstream exercise signaling to enhance hippocampal plasticity and related memory benefits (91, 135, 313, 521, 523).
We could hypothesize that physical inactivity with aging may lower V̇o2max to a level that may limit exercise intensity and reversibility of low neurogenesis, plasticity, cognition, BDNF, VEGF, and IGF-I in the dentate gyrus in old humans. For example, in older (60–77 yr), sedentary, healthy males and females, after 12 wk of progressive interval training, regional cerebral blood flow tended to increase in those near 60 yr old, but, in contrast, decreased in those in the 70–77 yr old age range (314). In addition, several correlations were reported, including three determinations (percent increase in hippocampal regional cerebral blood flow, percent increase in hippocampal volume, and percent increase in the Complex Figure Test, a measure of long-term memory), that all correlated with the increase in oxygen consumption obtained at anaerobic threshold. In addition, increase in hippocampal volume and Complex Figure Test were both correlated with the increase in hippocampal regional cerebral blood flow, and the increase in hippocampal regional cerebral blood flow was correlated with the increase in hippocampal volume (314). We speculate that as humans age from 60 to 77 yr old, age-associated declines in cardiovascular fitness will increase the relative work load intensity needed for the absolute oxygen consumption value that is required for the beneficial exercise effects on the hippocampal functions/structure, potentially decreasing the ability to work at the higher relative work intensities. In a second report (380) of older subjects (male and female subjects, age 60–72) with inadequate physical activity levels (<2–3 exercise events/month) and high levels of the pro-inflammatory biomarker IL-12p40 (one of two subunits of IL-12), at the end of a 6-yr period, the subjects had smaller volumes of hippocampus and lateral prefrontal cortex associated with greater declines in Mini-Mental State Examination test than two physically inactivity individuals with low values of IL-12p40 (380). The authors concluded that “these patterns of data suggested that inflammation was particularly detrimental in inactive older adults and may exacerbate the negative effects of physical inactivity on brain and cognition in old age.”
Others have hypothesized additional downstream mechanisms by which physical inactivity could produce cognitive decline. For example, sedentary rats had higher oxidative stress, as determined by protein carbonyls, and decreases in superoxide dismutase-1, glutathione peroxidase, as well as decreases in p-AMPK and PGC-1α proteins in hippocampus than endurance-trained rats by treadmill running (320). In humans, greater amyloid deposition in brain was found in sedentary, cognitively normal individuals (aged 55 to 88 yr old), as compared with those who exercised regularly (206), implying a negative role for physical inactivity in Alzheimer’s disease. The same investigators also tested subjects who were both cognitively normal and Apolipoprotein_E (APOE ϵ4)-positive individuals (aged 45 to 88 yr). Physically inactive subjects with APOE ϵ4 genotype also had greater amyloid than the exercised subjects (206). Furthermore, in a large epidemiological study (364), physical inactivity, as a risk factor for Alzheimer’s disease, exceeded each of six other modifiable risk factors, including depression, diabetes, low education, hypertension, obesity, and smoking.
B. A Lack of True Physical Inactivity Studies in Healthy Adults
While most reports associate cognitive benefits with increases in physical activity from a sedentary/baseline measure, very few reports have shown direct relationships between reductions in physical activity and cognitive function. One physical inactivity model previously discussed is spaceflight, and according to Strangeman (474), available evidence is inclusive of supporting or denying the existence of specific cognitive deficits during long-duration spaceflight. Cognitive effects of bed rest are also not conclusive and remain to be established (260, 302, 303). Furthermore, relatively few animal studies have analyzed how reductions in physical activity influence cognitive abilities. This paucity in research directly studying the mechanisms by which physical inactivity may promote decreases in cognitive function is an important research gap to fill, given the magnitude of its clinical consequences and how reductions in physical activity affect cognition.
C. Mechanistic Links Between Physical Inactivity and Cognitive Impairments
Seminal work by van Praag et al. (515) reported that voluntary wheel running (VWR) increased survival of nascent cells in dentate gyrus, a hippocampal region important for spatial recognition in 3-mo-old mice. Similar findings show that following 10 wk of voluntary wheel running, spatial pattern separation was strongly correlated with increased vasculature and neurogenesis in the dentate gyrus of 3-mo-old mice (104). Improvements in brain blood flow are also associated with improved cognitive performance. Underlying greater blood flow with higher brain angiogenesis that was associated with enhanced improvement in water maze time and retention of spatial-reference memory (516). VWR increases densification of blood vessel density, capillary perfusion, and blood flow in the motor cortex in rats (40, 479), potentially through increases in angiopoietin 1, endothelial proliferation, density of microvessels, and VEGF protein (120).
Many of the physical inactivity-related decreases in cognitive function have been associated with local and systemic expression of growth factors. For example, BDNF, particularly in the hippocampus, has been associated with many of the positive effects on cognitive enhancement (101, 353). Rodent studies demonstrate that BDNF protein levels increase progressively with regular VWR (32). Conversely, stopping VWR decreases hippocampal BDNF and BDNF/NT-3 growth factor receptor (TrkB) mRNAs (542). Additionally, BDNF promotes long-term potentiation by improving synaptic plasticity in the hippocampus of BDNF knockout mice (382), an analog of learning and memory. Improvements in long-term potentiation and synaptic plasticity (304, 513), as well as enhancements in dendritic arborization and synaptic plasticity in the hippocampus (114, 473), occur in response to physical activity.
Similarly, IGF-I is another critical growth factor for neuroprotection and brain health. Like BDNF, IGF-I levels are decreased in the circulation of sedentary compared with physically active animals (504). Both treadmill running and systemic infusion of IGF-I enhance the number and survivability of hippocampal BrdU+ cells (306, 504). Intracarotid infusion of IGF-I mimicked increases in neuronal c-fos and BDNF in the hippocampus observed with treadmill running, which was reversed by infusion of an anti-IGF-I antibody (76). Additionally, anti-IGF-I antibody treatment abrogated the protective effects of treadmill running on spatial memory in mice with hippocampal injury (77).
In the aforementioned studies on rats selectively bred for low voluntary wheel running (67, 422, 423, 425, 440, 441), after eight generations, selected low voluntary runners exhibited a 10-fold decrease in wheel running distance, as compared with rats simultaneously bred for high voluntary running, and roughly 4-fold less running distance than the outbred founding population (422). Genetically engineered rats for the phenotype of low voluntary running were linked to depressed function in the mesolimbic dopamine system, a system central to functions such as motivation, reward, and learning, and co-segregate with the selection for low voluntary running behavior (423, 425). Additionally, transcriptomic analysis of the nucleus accumbens, an important region of the brain containing the mesolimbic dopamine system, has identified inherent decrements in neuronal maturation in rats selected for low running behavior (425). Similar results were found in mice bred for high voluntary running and also implicate dopamine and certain midbrain structures as being important in the evolutionary regulation of physical activity (323, 332, 419). Although strong evidence exists to support a genetic contribution to physical activity regulation, other biological (nongenetic) and environmental factors must be investigated to completely understand the precise mechanisms regulating this complex and essential behavior.
Interestingly, mechanisms of activity may be linked to evolutionary changes. Ruben and Bennett (437) postulated in 1980 that the selection of burst-speed physical activity by animals might have contributed to the co-selection of “cephalization in protovertebrates and the appearance of vertebrates themselves.” They mention that adult invertebrate chordates have both a low degree of cephalization and are “relatively sedentary.” They then postulate that vertebrate cephalization might have developed during selection to fulfill the need for increased sensory and locomotor control as their more active lifestyle evolved. Overall, while epidemiological and mechanistic insight suggest that physical inactivity hastens the decline in cognitive function, this decline can be lessened, or even potentially reversed, by exercise. However, many questions remain unanswered concerning the best strategies to minimize these deficits.
In addition, it should be noted that physical inactivity in children in school can have a negative impact on cognitive ability and academic performance. Hillman and others have published numerous timely reviews in this emerging area of research (123, 213, 234).
D. Physical Inactivity Increases Risk of Depression and Anxiety
Depression is a leading cause of disability within developed nations (307), and by 2020 depression is predicted to be the second leading cause of human disability, next to cardiovascular disease (346). Depression has a lifetime prevalence of 16%. Furthermore, depression’s cost yearly in the U.S. is $210 billion and is growing (187). Similarly, the prevalence of anxiety is 10% in the general population, and it has many parallel symptoms and treatments as does depression. Both anxiety and depression are linked with many other disease risks. Recent research suggests that physical inactivity may be an actual cause of depression (385). Additionally, significant attention has been focused on the potential role of exercise in preventing and/or managing depression and depressive symptoms (378).
In general, data from observational and intervention studies hint that physical inactivity has similarities to depression and depressive systems (488). More than 100 population-based, observational experiments have been published since 1995. In analyzing these studies, the National Physical Activity Guidelines Report (511) concluded that inactive individuals were ~45% more likely to exhibit depressive symptoms than active individuals. Similarly, 28 prospective cohorts were examined to determine physical activity levels before the appearance of depression symptoms. Physical inactivity for 4 yr augmented the risk of depression by 49%, before any risk factor adjustments. After adjusting for the risk factors of age, alcohol use, chronic health conditions, education, income, race, sex, and smoking, 22% of depression was due to physical inactivity. Furthermore, in eight cohort studies containing the clinical diagnoses of depression symptoms, physically inactive individuals had 40% increased risk of depression diagnosis. Similar trends have been found in children. In children under the age of 15 in the United Kingdom, every hour of exercise reduced depressive symptoms by 8% reduction in depression symptoms (434).
The high association between physical inactivity and depression has made exercise a viable treatment of depression. As early as 1979, Greist et al. (188) found that running reduced depression symptoms similarly to time-limited/unlimited psychotherapy. The need for medication and percentage of relapses were reduced by exercise in depressed patients (11). Strikingly, the depressed patients had greater adherence to physical activity (66%) than to drug medication (40%). An exercise dose of walking roughly 12 miles/wk of walking for 12 wk, consistent with public health guidelines, lowered depressive symptoms by 47% (126). The antidepressive effects of physical activity can be seen as early as after only walking for 30 min/day for 10 days (117).
However, the precise mechanisms by which physical inactivity may cause and/or physical activity may prevent or treat depression remain largely unknown. Decreases in brain neurotransmitters and neurotrophic factors (e.g., dopamine, glutamate, serotonin, norepinephrine, BDNF, endorphins, and endocannabinoids) (209) accompany chronic physical inactivity and provide key hypotheses. Furthermore, many of these relationships have been determined in urine rather than the brain (128), and the precise relationship of physical inactivity with these neurotransmitters has not been studied. These factors are influenced by peripheral factors, which provide more potential explanations by which inactivity causes depression. Agudelo et al. (2) demonstrated that exercise training induces the activation of skeletal muscle PGC-1α1 and kynurenine aminotransferase, an enzyme whose activity is protected from stress-induced increases in depression.
Similarly, after examining cross-sectional studies of greater than 120,000 Americans, the National Physical Activity Guidelines Report (511) concluded that physical inactivity increases the odds for the development of an anxiety disorder. In particular, the National Comorbidity Survey noted that physical inactivity enhanced anxiety disorders by 1.75-fold using raw odds and by 1.38-fold after adjusting for sociodemographic and illness (184). Based on these population-based studies, the National Physical Activity Guidelines Report (511) concluded that moderate (>25 min/day) amounts of exercise (both aerobic and resistance types) lessened anxiety symptoms. Like depression, changes in monoamines and other circulating biomarkers are related to inactivity-induced augmentation in anxiety. Specifically, signaling and production of norepinephrine in the brain stem is reduced with physical inactivity, which is the origination of the only norepinephrine producing neurons serving the cerebellum, hippocampus, frontal cortex, and thalamus (121). Furthermore, chronic wheel running for as little as 30 min/day in rats lessened rises in norepinephrine levels in response to repeated stress (121). However, associations have not been studied among physical activity dosage, setting, and the likelihood of depression. Future studies must examine these relationships to provide additional evidence to support public health recommendations regarding the specific prescription of physical activity required to reduce the risk of depression.
VI. CARDIORESPIRATORY FITNESS
CRF has multiple synonymous and/or related terms, including maximal oxygen uptake/consumption (V̇o2max), peak oxygen consumption (V̇o2peak), aerobic fitness, aerobic capacity, and others; however, these subtle differences in the terminology will not be discussed here. The section gives evidence to support theme 5 on the impact of physical inactivity on fitness.
A. The Decline in V̇o2max With Aging Begins in Early Adulthood in Sedentary Humans: Impact of Aerobic Activity
CRF generally increases until late adolescence or early adulthood and then declines the remainder of life in sedentary humans (9, 116, 321, 430) The lifetime decline in V̇o2max is not trivial, as Schneider (450) found ~40% decline in healthy males and females (11 independent studies for both sexes) that spanned 20–70 yr of age. However, the percentage declines would have been greater if frailty levels were reached at 16–18 ml O2/kg body wt. Such a drop to physical frailty values would be 61 and 67% in females and males, respectively, from lifetime highest V̇o2max values (450). Two important factors contribute to decreases in CRF, biological aging beginning in the third decade of life, and physical inactivity which speeds the decline in V̇o2max at a given age. Furthermore, cross-sectional studies show the most physically active group has the same V̇o2max as a three-decade younger, less-physically active group (FIGURE 7).
FIGURE 7.
The age span shown for equivalent maximal oxygen consumption (V̇o2max) values is many decades later in life in a comparison of lifelong masters athletes (A), endurance-trained (B), or octogenarian endurance athletes (C) to V̇o2max values in younger, sedentary subjects. Data set 1: ~80-yr-old masters athletes’ V̇o2max was equivalent to lean untrained men, aged ~30 yr old, which is a >5 decades difference between trained and untrained humans (A). Similar decades’ difference for V̇o2max between lifelong trained and sedentary groups were published in two later publications. Data set 2: a >3 decades earlier in life equivalent V̇o2max was reported in younger sedentary as compared with the endurance-trained athletes (B). Data set 3: a >2–3 decades earlier in life for V̇o2max value was found in normative octogenarians who were lifelong, octogenarian athletes (C). Data in the panels were obtained by copying curvilinear lines from the original figure. Each line begins as early as the age of 20 yr old and ends at the oldest age group reported in each original figure. Superimposed upon each curvilinear line are dashed lines with arrows so to form a 3-sided-rectangle above each solid curved line. The vertical dashed line furthest to the right has an upward pointed arrow extending from the oldest age at which V̇o2max was determined, intercepting at the endurance-training V̇o2max curvilinear line. The second dashed line is horizontal and extends left to intercept the lower curvilinear line for the lesser V̇o2max. The final line in series of three dashed lines is a vertical drop-down from its interception point upon descending V̇o2max line. [A from Heath et al. (208). B from Tanaka and Seals (484). C from Trappe et al. (501).]
Aerobic training delays CRF’s decline with aging by two to four decades (FIGURE 7 shows 3 independent data sets). The physiological importance is that this important determinant of healthspan and mortality is not fixed by genes, but is modifiable by the level of lifelong physical activity. Healthspan and life expectancy are lengthened and shortened by aerobic training and physical inactivity, respectively.
In an attempt to better understand and elucidate molecular mechanisms governing the concept of lifetime-apex V̇o2peak and its initial decline, the Booth laboratory (498) studied rats that had access to voluntary running wheels or were sedentary. The initial hypothesis was that the active rats would experience the decline in CRF later in life compared with the sedentary rats; however, the only benefit that was conferred with activity was the ~20% increase V̇o2peak noted in the voluntary running group until 6 mo of age. Thus, when rats are sedentary during adolescence, their genetically highest peak CRF is not attenuated. Translating this to humans, if children and adolescents were physically inactive, they would have a lower CRF than their potential maximum.
B. Low CRF Is Associated With Increased Chronic Diseases and Increases Mortality Rate Three- to Fourfold
High levels of CRF are associated with reduced prevalence of several cardiometabolic risk factors including hypertension, hyperlipidemia, inflammation, and insulin resistance and lower incident rates of metabolic syndrome and T2D. Indeed, physical inactivity leads to a decrease in CRF, increasing the risk of numerous chronic diseases/conditions (25, 280, 530). In the Aerobics Center Longitudinal Study (19), the cumulative incidence rate of hypertension was highest in women with low CRF. Each successive 1-MET loss in CRF level was associated with increased hypertension risks of 19, 16, and 32% risk in all subjects, men, and women, respectively.
Mortality begins to increase when CRF falls below ~10 METs (FIGURE 8). Remarkably, when CRF falls from 10 to 4 METs, death rate increases ~4.5 times (4, 44, 256). With regard to the metabolic syndrome, Finnish men in the upper fourth of loadings on the metabolic syndrome factor were 2.3, 3.2, and 3.6 times more likely to die of any cause, cardiovascular disease, and coronary heart disease, respectively (272). In another study of 15,400 healthy men, and 3,700 men with the metabolic syndrome, the middle and lower tertiles for CRF had 2.08 and 3.48 times, respectively, the risk of death from cardiovascular disease than men in the upper tertile (241). The lowest third in V̇o2max from 676 Finnish women and 671 Finnish men, between 57 and 79 yr old, exhibited 10.8- and 10.2-fold higher risks, respectively, while those in the middle third demonstrated 4.7- and 2.9-fold higher risks, respectively, had the metabolic syndrome as compared with the highest V̇o2max after performing multivariable adjustments (204). In addition, men with high CRF display significantly lower levels of abdominal adipose tissue compared with those with low CRF (549). Overall, these examples clearly establish that CRF, which is decreased in part through increased physical inactivity, leads to increases in hallmark risk factors for metabolic diseases.
FIGURE 8.
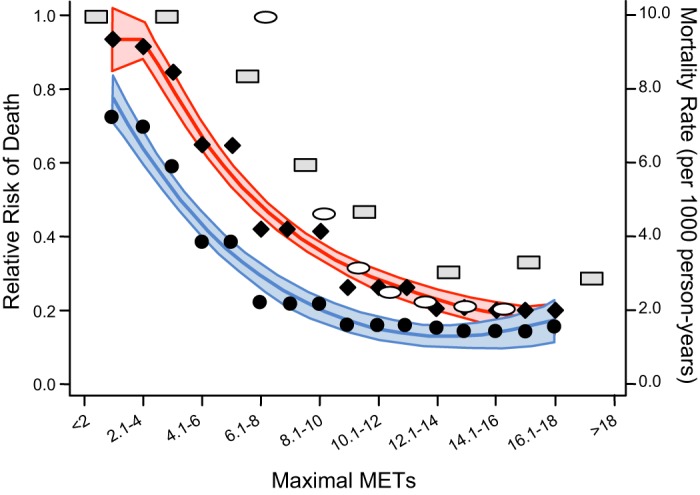
Relative risk of death for all MET values (x-axis) for 10 and greater are all similar during maximal aerobic-type exercise. When METs fall from ~10 to 4 with aging, risk of death increases 4-fold. Three studies are shown, with each from a different decade. Study 1: Blair et al.’s 1989 study (44) of relative risk of death (left y-axis) includes both male and female data from original Figure 4 in JAMA (shown within ovals). Study 2: Kokkinos’ 2008 study in Circulation (256) is relative risk of death in males (shown in rectangles). Study 3: Al-Mallah et al.’s 2016 publication in Mayo Clinic Proceedings (4) shows mortality rate (right y-axis) females (blue line and black circles) and males (red line and black diamonds) with the outer lines showing the 95% confidence intervals.
Regarding the impact of CRF on mortality, in the Aerobics Center Longitudinal Study, cardiovascular disease mortality increased 19% for every 1-MET loss in CRF in 14,345 men who were 44 yr old after an average 11.4-yr period (285). Low CRF had a 27% greater risk of cardiovascular disease mortality. Those who exhibited an increase CRF over the 11.4-yr period decreased risk of cardiovascular mortality by 39%.
In addition, a direct dose-response relationship exists between exercise volume (duration × intensity) and CRF (464, 481). Along the same lines, two landmark studies report a dose-response relationship between low fitness and increased mortality. Blair et al. (44) reported two fitness assessments performed on average 8 yr apart on 10,224 men and 3,124 women. From lowest to highest fitness quintile, age-adjusted all-cause mortality decreased 3.4-fold, falling from 64.0/10,000 yr in the least fit quintile to 18.6/10,000 yr in the highest fit quintile. A second report by Myers et al. (349) noted that for every one MET drop in maximal fitness, mortality increased 12%. Two cardiovascular fitness assessments performed on average 6 yr apart on 6,213 men averaging in their 7th decade of life. From lowest to highest fit quintile of cardiovascular fitness, age-adjusted all-cause mortality decreased 4.5-fold among normal subjects and 4.1-fold among patients with cardiovascular or pulmonary disease. Taken together, physical inactivity is a very powerful predictor of mortality once maximal cardiovascular fitness falls to less than ~10 METs (54).
C. CRF Is Not Fixed in Humans: Modifiability Throughout the Lifespan
Trappe et al. (503) mention that two men who had nearly the same CRF (V̇o2max) at the end of the 22 yr (no aging effect) when they continued the same high volume of endurance training volume (endurance volume is defined as duration × volume). They comment in their discussion (503), “thus it may be possible to augment the decline in aerobic capacity if running training is maintained at a very high level. Although running volume and intensity may drop off slightly, it appears that the reduction in aerobic capacity is significantly less compared with individuals who run less or not at all.” This indicates two points. First, the decline with normal aging between 30 and 50 yr of age could likely be almost 100% due to physical inactivity. An extreme extension of the notion that voluntary physical activity is one driver of the decline in CRF is to observe humans in an old-age facility. Most human lives at the end of a long life are spent sitting and lying down. The behavior of physical inactivity in old age is likely a driver of a negative cycle of inactivity begetting lower CRF (18, 436), which makes voluntary physical activity more fatiguing so less is performed, and so forth in a negative cycle.
In addition, low V̇o2max can be rescued by adding physical activity to inactive humans. Improvements in risks from co-morbidities and co-mortality are possible with the addition of physical training by inactive humans. A meta-analysis of 160 randomized clinical trials containing 7,487 women and men found that the inactive group did not improve in comparison to the healthy improvements by the exercise-trained group in CRF and in metabolic syndrome biomarkers for lipid and lipoprotein metabolism, glucose intolerance and insulin resistance, systemic inflammation, and hemostasis during the trial (300).
Regarding mortality, in a Cooper Clinic study, 9,777 men aged 20–82 yr old, who changed from unfit to fit over a 4.9-yr period, lowered their mortality risk by 44% (43). Mortality risk was lowered 7.9% for each minute increase in maximal treadmill time between measurements. In a Veterans Administration study of 5,314 male veterans aged 65–92 yr, Kokkinos et al. (255) noted that males who went from a low CRF to a high CRF decreased their risk of death by ~50% over an 8-yr period. The opposite occurred for fit men who became unfit in the two aforementioned studies (255), increasing mortality risk by ~50%. Taken together, physical inactivity can produce the loss of most cardiovascular fitness associated with physical activity.
D. Cardiovascular Fitness Is a Multi-organ Determined Phenotype
Nearly 100 yr ago, Nobel-Prize winner A.V. Hill discovered the concept of maximal oxygen (211, 212). To supply skeletal muscle with the oxygen, atmospheric air must first pass through the lung airways, where oxygen transport into pulmonary capillaries with hemoglobin that will carry 99% of oxygen. While the above may be taken for granted as they are not rate-limiting steps in oxygen flux from air to skeletal muscle mitochondria at sea level, this can become rate-limiting in specific disorders. Maximal cardiac output is thought to be a rate-limiting step (469), while capillarization and diffusion of oxygen to muscle mitochondria are generally not envisioned as rate-limiting in healthy humans. However, the concentration of muscle mitochondria is considered to be a second potential rate-limiting step, to limit V̇o2max. Even today, we are still trying to understand what limits V̇o2max (469). For example, Richardson’s group (177) has provided new data on whether maximal cardiac output or skeletal muscle mitochondria are rate-limiting for V̇o2max. In untrained subjects, V̇o2max is limited by working muscle’s demand for mitochondrial O2, with indication of adequate O2 supply, whereas in trained subjects, the exercise training-induced enlargement in mitochondrial concentration in skeletal muscle causes skeletal muscle V̇o2max to become limited by O2 supply. In addition, 7 days of bed rest reduced erythrocyte volume by 9% in healthy men who were 36–40 yr old (97).
E. Peripheral Circulation
One organ that epitomizes physical inactivity’s negative effects is the peripheral vasculature. The deleterious effects of inactivity on the vasculature depend on the type of inactivity and also on the specific region/location of the vasculature (491). For example, regional differences and severity of consequences between resistance and conduit vessels are with physical inactivity and the pathophysiological mechanisms underlying changes that occur are unique to the type of vessel (490). During physical activity and/or exercise, sheer stress and hemodynamic stimuli induce effects on the vasculature, promoting remodeling and an anti-atherogenic phenotype. In contrast, with inactivity and the absence of these physical stresses, endothelial dysfunction and arterial remodeling (186, 491, 528) have been postulated to initiate some of the negative phenotypic manifestations of inactivity on the vasculature.
Vascular responses to physical inactivity depend on the type of inactivity. We will begin with data collected from studies that are less extreme and more physiologically relevant, moving to vasculature consequences resulting from more extreme models of physical inactivity. Boyle et al. (62) used a simple model of reduced step count in recreationally active humans to determine whether reduced physical activity (<5,000 steps/day for 1, 3, and 5 days) altered flow-mediated dilation in the popliteal and brachial arteries. After 5 days, flow-mediated dilation was impaired in the popliteal artery (lower limb), but was not impaired in the brachial artery (upper limb) and increased circulating endothelial microparticles. These findings highlight the vulnerability of reduced physical activity on localized vasculature in a short time period. Along the same lines, a second study from the above research group looked at the impact of prolonged sitting on limb dilator function (417). A 6-h protocol of sitting was performed, and flow-mediated dilation was measured pre-sit, post-sit, and post-walk. The authors found that the impaired dilator function with sitting can be fully reversed after a short 10-min walk in the lower limbs, but not the upper-arm vasculature. In addition, several hindlimb unloading studies have been carried out in rodents examining the effects on the peripheral vasculature. For example, it has been shown that hindlimb unweighting reduces endothelium-dependent vasodilation and expression of endothelial nitric oxide synthase in isolated rat soleus skeletal muscle feed arteries (228, 452). This suggests that with 14 days of disuse, the vasculature’s ability to vasodilate via endothelium-dependent mechanisms is compromised.
Many studies to date examining vascular function in response to inactivity have chosen more extreme models of physical inactivity (39, 45, 46, 112, 198, 352). Bed rest typically does not restrict upper extremity movement; therefore, studying the effects on the vasculature in the lower limbs versus the upper limbs may yield different outcomes. Furthermore, unilateral lower limb immobilization only allows study of one leg and can increase the risk of deep vein thrombosis (33, 47, 170). For example, spinal cord injury patients exhibited increased femoral and carotid artery wall thicknesses after a 6-wk latent period (489). Distinct mechanisms regulate conduit artery wall thickness and diameter both above and below the spinal cord injury. Intriguingly, only the femoral artery diameter (below the spinal cord lesion) decreased over the 24-wk period post-spinal cord injury (489). Thus localized effects occur for arterial diameter (435). Spinal cord injury and 3 wk of unilateral limb immobilization patients (274) both exhibited reduced hyperemic flow, but spinal cord injury was diminished to a greater magnitude. In addition, intima-media thickness-to-lumen ratio was increased with both short- and long-term deconditioning (274).
Transcriptional adaptation to physical inactivity is one mechanistic factor that has been investigated. In the above study, Lammers et al. (274) noted downregulation of transcripts including HIF-2α, which binds the VEGFA and FLT1 promoter regions, VEGF co-receptor NRP, VEGF B, VEGF C, caveolin-1 (CAV1), nitric oxide synthase trafficker, soluble guanyyl cyclase, and the nitric oxide synthase. Several transcripts were upregulated including TGFB1, inhibiting angiogenesis and inducing arterial stiffening, and the authors concluded “thus, the VEGF signaling pathway, regulation through TGFB1 and involvement of extracellular matrix-related proteins seem to be important mechanisms after deconditioning, which may lie at the base of the associated vascular adaptations.”
F. Aerobic Capacities in Hunter-Gatherer Societies
Modern humans age 20–29 and 40–49 yr old have V̇o2max values of 40 and 35 ml·kg-1·min-1, respectively (100). V̇o2max values of various hunter-gatherer societies exceed those of modern human societies. For example, hunter-gatherer cultures, such as Eskimos living in the Canadian Arctic community of Igloolik, had V̇o2max values of 49–54 ml·kg-1·min-1 (428) and 62 and 42 ml·kg-1·min-1 for male and female, respectively, in 20–29 yr old Ache of Eastern Paraguay (526). V̇o2max values of 60–70 ml·kg-1·min-1 are noted in New Guinea Lufas, Mexican Indians, and Tanzanian Masai, and 50–60 ml·kg-1·min-1 in Venezuelan Indians and Finnish Laps (100). Cordain et al. (100) interpreted the V̇o2max values in modern humans as follows: “it should not be surprising that the limited physical activity typical of modern affluent humans generates mediocre aerobic fitness, nor that the aerobic fitness levels of recently-studied foragers are superior to those of Northern Americans.” The above taken together could support the notion that physical inactivity produced V̇o2max values that are 30% lower than their likely genetic potential.
G. Primary Artificial Selection for Low Endurance Capacity Co-selects for Low Aerobic Capacity
Britton, Koch, and Wisloff developed their working hypothesis: “variation in capacity for oxygen metabolism is the central mechanistic determinant between disease and health (aerobic hypothesis)” (252). As an unbiased test for their aerobic hypothesis, Wisloff et al. (545) employed selective breeding of rats, with the primary selection factor being the distance completed during forced running on the motor-driven treadmill. Two separate lines were artificially bred from the original founder line, with the primary selective factor for generation 1 being either low or high intrinsic endurance running distance (termed “endurance exercise capacity”). Compared with the distance run by the founder population, which was used to breed generation 1, rats selected for the low distance line ran 46% less distance and for the high distance line ran 140% further the distance that the founder runners had run 11 generations earlier. At the 11th generation, the primary selected phenotype (distance during a forced run) had co-selected the two phenotypes (aerobic capacity and risk factors for chronic diseases). V̇o2max was 58% lower in the low capacity runner (LCR) line and cardiovascular risk factors were worse for LCR, including 13% higher blood pressure, 7.8-fold more acetylcholine infusion required for one-half maximal vascular relaxation, fasting blood insulin and glucose 131% and 20% higher, respectively, lower mitochondrial protein concentrations in skeletal muscle, visceral adiposity/body weight ratio 63% higher, and blood triglycerides and free fatty acids 168% and 94% higher, respectively (545). A follow-up study reported that LCR rats had 28% shorter mean lifespan, with a calculated hazard ratio of 5.7 for death (253). The co-selections of aerobic capacity and chronic disease risk factors in artificial breeding imply some evidence for their co-selection could have occurred in natural selection during evolution and specific genetic differences based on endurance running capacity were developed between LCR and HCR lines. Koch and Britton (250) expressed their view by stating “the strong linkage of disease with low aerobic capacity is consistent with a pivotal role of oxygen in our evolutionary history. Nevertheless, even if these clues about the critical role of oxygen are correct, recognizing the mechanistic footprint of oxygen in our evolutionary path remains a challenge.”
H. Mechanisms
The cardiovascular disease risk factors produced by physical inactivity are mediated via multiple mechanisms. Mora et al. (340) categorized ∼60% of the risk factors for cardiovascular diseases that are found in physically inactive subjects. In rank order from highest to lowest, percentage contributions were inflammatory/hemostatic biomarkers, blood pressure, traditional blood lipids, and novel blood lipids. Remaining physical inactivity-induced risk factors for cardiovascular diseases are not known. A recent review (354) updates the risk factor “gap,” indicating “in fact, epidemiological evidence suggests that the protective effects of physical activity on cardiovascular disease are nearly double that which would be predicted based on changes in traditional risk factors,” suggesting that ~50% of the protection afforded by physical activity remains unexplained. Furthermore, the role of physical inactivity in the “gap” also remains unknown. Joyner and Green (230) suggest that the cardiovascular risk-factor gap producing cardiovascular disease is the attenuation of three positive physiological responses: 1) lower vagal tone so to increase heart rate variability via lessened peripheral baroreflex function and CNS cardiovascular regulation; 2) lower endothelial function so to decrease vascular compliance that diminishes vasodilatation and attenuates peripheral baroreflexes; and 3) heightened baseline sympathetic outflow on blood pressure by diminishing interactions between endothelial function and sympathetic outflow.
An example of different regulatory pathways for cardiovascular adaptations to exercise and deconditioning comes from Thijssen et al. (490), who reported that the vascular responses to deconditioning and exercise did not employ the same pathway in opposite directions. Specifically, they found that the cardioprotective effects of exercise were due to vasodilation by the nitric oxide pathway; however, deconditioning activated vasoconstrictor pathways. Taken together, the above findings provide evidence to support a viewpoint that exercise mechanisms sometimes cannot be simply reversed to explain physical inactivity mechanisms. One outcome from some separate regulatory mechanisms for exercise and inactivity could be that current emphasis on molecular transducers of physical activity as a treatment for modern sedentary chronic diseases/disorders will not reveal with 100% fidelity actual molecular causes of chronic diseases/disorders caused by physical inactivity.
I. “Phenotypic Knock-down” of Cardiovascular Fitness
We define “phenotypic ‘knock-down’” models of physical activity (53) as reductions in physical activity independent of transgenic methodologies. Physiological knockdowns produce physical inactivity relative to the baseline of initial physical activity. Thus “knocking down” physical activity is a primary act of environmental manipulation that causes secondary alterations in gene expression.
Two human models of endurance physical activity knockdown are presented. First, Saltin et al.’s (443) 1968 bed rest found that 20 days of complete bed rest by healthy 20-yr-old men had a 28% decrease in V̇o2max and a 26% decrease in maximal cardiac output (the latter being produced by a 29% decrease in maximal stroke volume with no change in maximal heart rate) (330). Of further interest is the five subjects exhibited a twofold range in their percentage decrease in V̇o2max, ranging from 46 to 20% to produce the mean decrease of 28%. After bed rest, the subjects underwent an approximate 8-wk period of endurance training for the aim of returning the subjects to pre-bed rest values, and V̇o2max increased from 2 to 52% in the retrained subjects. When the same subjects underwent V̇o2max testing 30 yr later at the age of 50, the percentage decline in V̇o2max had not decreased as much as it did after 20 days of bed rest when the subjects were 20 yr old. Second, Pedersen’s group (372) had 24-yr-old men reduce their daily step count from 10,501 to 1,344 steps/day for 2 wk. At the end of reduced stepping, plasma insulin increased 57 and 50% during oral-glucose tolerance test and oral-fat tolerance testing (OFTT), respectively, and plasma triglycerides increased 21% more during the OFTT. In addition, intra-abdominal fat mass increased 7% after 2 wk of reduced stepping. Both the Saltin et al. (443) and Olsen et al. (372) studies reported 26 and 7% percentage declines in human V̇o2max after only 3 and 2 wk of continuous bed rest and reduced daily stepping, respectively. The genes underlying the large decline in V̇o2max phenotype are currently unknown. In summary, the above two human experiments provide support that large increases in physical inactivity produce remarkably large percentage declines in physiological functions in very short periods.
As sitting is the most common form of inactivity and commonplace in today’s society, it is often reported that this has a significant impact per se, independent of fitness, on chronic disease risk. However, a shortcoming with this research is the lack of control for fitness. Nauman et al. (351) performed a cross-sectional study on 26,400 Norwegian adults and found that high levels of CRF protect against cardiovascular risk factors in men and women sitting >7 h/day, independent of whether or not these subjects were meeting U.S. weekly physical activity recommendations. Remarkably, high CRF (>43.3 ml·kg-1·min-1) abolished the odds for increased cardiovascular risk factor clustering (determined on the basis of the definition of the metabolic syndrome) in those subjects with high prolonged sitting times, independent of subjects not meeting current recommendations for physical activity (FIGURE 9). Furthermore, Myers et al. (347) reported that “no deaths were observed among subjects who were both fit (>10 METs) and active (>1,500 kcal/wk)” in their study of 842 males aged 59 yr old,” after subjects had a 5.5-yr follow-up period during which 89 deaths occurred.
FIGURE 9.
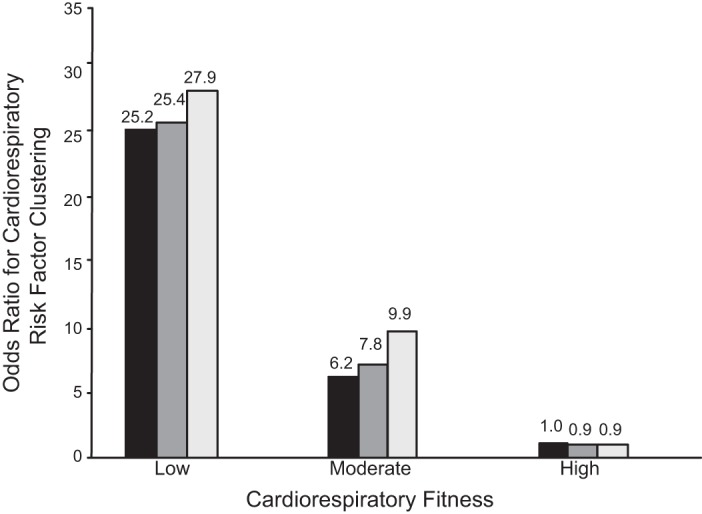
Adjusted odds ratio of clustering of cardiorespiratory risk factors in combined categories of fitness and sedentary time in men with decreasing cardiovascular fitness levels. Low, moderate, and high CRF levels were defined as the least fit 20%, the next fit 40%, and the most fit 40%, respectively, corresponding to <35.7 (low), 35.7–43.3 (moderate), and >43.3 ml·kg-1·min-1 in men. Sedentary time is reported as ≤4 h (black bars), 5–7 h (dark gray bars), and ≥7 h (light bars) *Significant difference (P < 0.05) from reference category. [Data from Nauman et al. (351), with permission from Medicine and Science in Sports and Exercise.]
VII. METABOLISM
A. Overview of Inactivity and Altered Metabolism
Whole body energy metabolism, substrate utilization, and trafficking are altered by numerous factors including nutrition, gender, age, and adiposity. But a largely underappreciated factor altering energy metabolism and substrate utilization is physical inactivity (85, 240). If Darwinian medicine is to be used as a guide, then metabolic pathways evolved under conditions in which physical activity and energy expenditure in most of human existence were performed for basic functions such as obtaining food through hunting and gathering or agriculture work. Such a defense mechanism has been noted in obese states. Rosenbaum and Leibel (432) suggest that physiological mechanisms exist for defense of body fat by acting to override maintenance of the reduced body weight in obese humans (149). To protect against weight loss, such as would occur in a weight loss program, an apparent evolutionary program of persistent hypometabolic and hyperphagic state emerges making it difficult to maintain a new, lower body mass. Their data suggest, “the hypometabolic state is characterized by declines in resting energy expenditure and activity energy expenditure (due to increased contractile efficiency of skeletal muscle), and is augmented by decreased activity of the hypothalamic-pituitary-thyroid axis, decreased sympathetic nervous system tone, and increased parasympathetic tone” (149).
As a part of efficient storage, glucose, which is limited in quantity (532), is utilized in tissues as dictated only by energy demands so as to protect limited circulating reserves of glucose (only 4 g of glucose in circulation and ~300–500 g of glycogen in muscle and liver) (532). Maintenance of glucose levels is essential for brain function and consciousness. The nervous system generally does not oxidize fatty acids except in long-term fasts (73) or in the absence of glucose intake. Fatty acids, which are a quite abundant energy source, are also preferentially shuttled towards energy stores or maintained in stores (adipose depots) if their use is reduced due to lower energy expenditure or constant feeding. However, as physical activity and energy demands increase, these depots of glucose (stored in muscle and liver glycogen) and fatty acids (stored in adipose tissue or within muscle and liver) can be rapidly mobilized to fuel muscle contraction and other essential processes until the work is done and food is procured. Again, Darwinian medicine would indicate that much like a car engine fills up with gas, the motor runs, and the tank must be filled up again, the human body was designed for cyclical periods of energy expenditure and energy storage. However, in the current physically inactive environment this cyclical pattern has theoretically been broken due to physical inactivity and a lack of contractile activity to drive energy demand (85), changes that putatively lay a foundation for subsequent metabolic dysfunction (495). Indeed, accumulating evidence strongly links inactivity to the development of obesity and insulin resistance (240). Although complex, a chronic positive energy balance results in weight gain, expanding adiposity, and obesity. Given that energy balance is dictated by energy intake and energy expenditure, chronic inactivity and associated reduced energy expenditure often leads to weight gain if energy intake levels are not lowered adequately. This concept is illustrated in FIGURE 10.
FIGURE 10.
Schematic of metabolic dysfunctions produced by physical inactivity in white adipose tissue and skeletal muscle.
Adult hunter-gatherers, for example, Tanzanian Hadza, do not hunt, or gather, all day. Rather when not hunting and gathering, energy intake was reduced sufficiently, when inactive to make daily, total energy usage unchanged. Pontzer et al. (401) suggested that their reduced energy observations were an apparent compensation to keep total daily energy usage unchanged. Pontzer et al. (400) recently modified their interpretation of their above data with the constrained total energy expenditure model. The theory suggests that total energy expenditure is maintained at a chronically tight homeostasis even if physical activity levels are changed over a chronic period of time. This is a controversial idea, because it suggests that physical activity or inactivity would not adjust total energy expenditure chronically. Even if true, there is no doubt that the percentage of total energy expenditure comprised of activity energy expenditure would be higher in an active state, and lower in an inactive state. Moreover, energy requirements in tissues and thus cyclical substrate utilization and storage patterns would be quite different between individuals who had the same daily total energy expenditure, but did so with robustly different activity levels within the same day (highly active vs. inactive). Therefore, the flux or lack of flux through these pathways could play a primary mechanistic role in metabolic dysfunction induced by inactivity.
B. Physical Inactivity Produces Rapid Skeletal Muscle Insulin Insensitivity
Although, largely underappreciated, the regulation of skeletal muscle insulin sensitivity (insulin-stimulated glucose transport) is driven by similar concepts as energy balance. Skeletal muscle is a critical glucose disposal site during postprandial conditions, especially if glycogen stores are not full and there is some level of energy demand due to contractile activity. If skeletal muscle is being regularly contracted, ATP demand is elevated, then there is a need for increased glucose to be actively transported into muscle cells (494). This can occur at even higher rates if glycogen is depleted (79, 171). Therefore, higher levels of activity and depletion of glycogen stores promote increased insulin sensitivity. However, if skeletal muscles are inactive, ATP demand is low, and glycogen is elevated, then the demand for glucose is low and thus insulin sensitivity decreases. Indeed, adiposity and physical inactivity contribute more to insulin insensitivity with aging than aging itself (275). Glucose is tightly regulated in this manner putatively because it is a substrate in limited supply [only ~4 g in circulation (532)] and is critical for brain metabolism.
C. Rat Wheel-lock Studies Link Physical Inactivity and Metabolic Changes
As discussed above, Booth and colleagues (267–269) conducted a series of experiments analyzing the effects of transitioning rats from daily wheeling running to cage only (sedentary conditions). Rats were provided with running wheel in their cages for weeks before the wheels being locked, effectively transitioning the rats to physical inactivity. Important aspects of VWR are that rodents run intermittently through the night and display mild improvements in hindlimb muscle mitochondrial adaptations (218, 415), compared with exercise training done on treadmills (154). Therefore, VWR is a model that can arguably emulate bouts of low to moderate intensity ambulatory activity of humans throughout the day. The model allows study of physical inactivity mimicking at least two human conditions, including 1) having an occurrence that temporarily stops daily physical activity, such as an illness, injury, etc.; and 2) a “fast-tracking” the aging portion of life.
Rats typically ran for 3–6 wk followed by being studied acutely after wheel-lock (from a few hours to 7 days post). Daily VWR increased insulin sensitivity in isolated skeletal muscle (267); however, wheel-lock lowered insulin-stimulated glucose transport to the level found in sedentary rats condition within 2 days. In a previous animal study, after a single day of hindlimb immobilization, mouse muscle exhibited decreased insulin responsiveness (457). Additionally, a prior human study observed that only 3 days of absolute bed rest in young healthy men caused significant reductions in peripheral glucose uptake that were similar to 14 days of bed rest, which was secondary to peripheral insulin resistance, rather than insulin deficiency (301). Furthermore, when endurance athletes stopped daily exercise, insulin sensitivity decreased to what was observed in sedentary, age-matched controls in only 2 days (71). Follow-up analysis revealed that reductions in insulin-stimulated glucose uptake into skeletal muscle tracked with reduced insulin-stimulated activation of the insulin-signaling pathway at both the levels of the insulin receptor and Akt, and also tracked reduced insulin binding to the insulin receptor (267). In addition, there was also a reduction in GLUT4 protein content, which tracks with insulin sensitivity levels. In addition, these changes were likely mediated in part by increases in the association of protein tyrosine phosphatase 1B (PTP1B) and protein kinase C (θ) with insulin receptors. PTP1B dephosphorylates the insulin receptor, while protein kinase C is a serine kinase, which putatively blocks tyrosine phosphorylation of the insulin receptor, and insulin receptor substrate, leading to downstream inactivation of the signaling pathway to translocate GLUT4 (444). Certainly, the induction of reduced insulin sensitivity in skeletal muscle after a transition to inactivity would not be considered “pathological.” Thus these changes were physiological adaptations to a condition of reduced energy expenditure within the skeletal muscle, and it appears that molecular mediators that have been implicated in the insulin resistance field played a role in feedback inhibition. However, it can be contended without the aforementioned physiological decrements that the likelihood of progression to the pathology of T2D is lessened. In other words, it would be rare for insulin resistance to develop in skeletal muscle if physical inactivity were eliminated on a daily basis (55).
D. Human Reduced Stepping Studies to Examine Links Between Physical Inactivity and Altered Metabolism
In the aforementioned study, Pedersen and co-workers (263, 372) took young men who were physically active with ~10,500 steps/day and had them change their lifestyle so that they approached 1,400 steps/day for 2 wk. After 2 wk, in response to an oral-glucose tolerance test (OGTT), the plasma insulin in the area under the curve increased 57%, coupled with a 17% decreased in glucose infusion rate during euglycemic clamp, while the area under the curve during an OFTT increased plasma insulin 50% and plasma triglycerides 21%. Additionally, intra-abdominal fat mass increased 7% and total fat-free mass decreased 2%. These studies were validated by studies in the Thyfault laboratory that demonstrated inducing a transition from >10,000 steps a day to <5,000 steps/day after only 3–5 days provided similar results in terms of elevated glucose, and insulin responses to an OGTT and elevated free-living postprandial glucose levels measured by continuous glucose monitors (336, 418). Thus, even with a shorter and smaller change in daily activity levels, insulin sensitivity was lowered. These studies also documented that there were greater swings in the peaks and nadirs of glucose levels measured by continuous glucose monitors worn during free living conditions (336, 418).
Knudsen et al. (249) went on to perform an additional inactivity study using reduced daily steps (from 10,000 to 1,500 steps/day), but also added the effects of hypercaloric burden through subjects eating an extra 1,500 kcal/day during the 2 wk of reducing stepping and found that inactivity, when paired with a 50% elevated energy intake, induced a 50% decrease in insulin sensitivity. A subsequent paper from Pedersen et al. (262) also examined the deleterious effects that a high caloric-intake diet would have over 2 wk on individuals who maintained activity greater than 10,000 steps/day or who reduced activity below 1,500 steps/day. Although both groups gained body mass, the inactive group gained more visceral fat, displayed worse glycemic control, and had greater increases in hepatic glucose production than the 10,000-step group. Stephens et al. (471) examined if 1 day of increased sedentary time would also lower insulin sensitivity and if this depended on energy balance. Subjects either performed a day of increased sitting with their normal caloric intake or a day of sitting in which caloric intake was lowered to appropriately match their lowered energy expenditure, and the authors noted that both conditions lowered insulin sensitivity, but the greatest effect occurred when energy intake was not lowered during the sedentary condition. Healy et al. (207) found interruptions in sedentary time were associated with healthier levels in metabolic risk variables. Increased numbers of breaks in sedentary time were related to smaller waist circumference, lower fasting serum triglycerides, and 2-h plasma glucose in OGTT. The differences were independent of both total sedentary time and moderate-to-vigorous intensity activity time, suggesting that the manner in which total daily sedentary time is accumulated may be important, rather than simply the total accumulated duration of sedentary time.
In addition, a recent study of 2,497 subjects age 40–75 yr old with either T2D, impaired glucose metabolism, or normal glucose metabolism noted that for each additional hour of physical inactivity, the odds of acquiring T2D and metabolic syndrome increased by 22 and 39%, respectively (512). The increased risk of T2D and the metabolic syndrome were independent of high-intensity physical activity. Furthermore, only weak associations with increased metabolic syndrome risks were observed with number of physical activity breaks, number of prolonged physical inactivity bouts, and average physical inactivity duration.
In summary, physical inactivity exerts powerful effects to alter substrate metabolism, including lowered insulin sensitivity, and alterations utilization of fatty acids. These mechanisms provide strong evidence that chronic inactivity plays a fundamental role in metabolic dysfunction and T2D. Without initial, chronic physiological dysfunction, dependent pathological and clinical maladies are less likely to occur (55).
VIII. ADIPOSE TISSUE
At one time, adipose tissue was said to be an “inert” tissue. Freidman’s studies contributed to ending that belief when he found adipose tissue was an endocrine organ that released an adipocyte hormone, leptin (166, 554), which signals adipocyte size to regulating neurons in the arcuate nucleus of the hypothalamus (308).
A. Physical Inactivity Contributes to Obesity
As discussed earlier, the Old Order Amish in Canada perform high levels of physical activity, averaging 18,000 and 14,000 steps/day (24). As mentioned, 2,252 individuals aged 13 yr and older had an average of 5,117 steps/day (23), while 325 women (average age 57 yr old) reported 4,944 steps/day (482). Additionally, data from NHANES (2005–2006) noted that 3,725 subjects had an average of 6,549 steps/day from self-reported accelerometers (455), and out of 3,744 adults, 84% were below 10,000 steps/day (36.1% had <5000 steps/day) as determined by accelerometers (463). Taken together, daily step numbers are less than half in the general population as compared with Amish adult men and women, equivalent to at least 300 kcal/day on average (>7,500 step difference ÷ 2,500 steps/100 kcal/mile). As body fat is largely a balance of fat calories expended and fat/sugar intake, it is also of note that Amish males report a greater energy intake (2,780 kcal) compared with non-Amish males (2,298 kcal) (107). In another study, average daily caloric intake for non-Amish men and women was 2,504 and 1,771 kcal, respectively (550). Together, the data suggest caloric intakes of Amish are greater than non-Amish. Some might suggest that Amish should then have higher percentages of body fat. However, Amish have very low levels of obesity, with 0% of the men and 9% of the women having a BMI ≥30 (24), compared with 35% in men and 40% in women adults (155), and 1.4% of the Amish youth being obese (22), compared with 17% of U.S. children (369). Taken together, both physical inactivity and caloric intake play shared roles in obesity.
Four other historical studies are of interest. One report contains BMI values of U.S. adults from 1882 to 1986 (257). The rise in U.S. BMI values peaked in the early 1920s, declining to a trough in 1945, and beginning to increase again by 1950. The declining period of BMI includes the years of the great depression (1929–1941) and World War II (1941–1945). Another comparison statistic is the number of automobiles. U.S. automobile number (in millions) were 8, 17, 23, 23, 27, 26, and 40 in the years 1920, 1925, 1930, 1935, 1940, 1945, and 1950 (368). Automobile numbers were relatively flat from 1930 to 1945. An association thus exists between percentage decline in BMI and an essential plateau in automobile number. A second study by the pioneer Jean Mayer concluded that within the sedentary activity range, decreasing physical activity was not associated with a decrease in food intake, but paradoxically was associated with an increase in food and body weight in mill workers in West Bengal (327). A third study covered the years following the 1991–1995 Cuban economic crisis. From 1995 to 2010, bicycling decreased from 80 to 55% of the total population concurrent with caloric intake increasing from 2,400 to 3,200 kcal (159). During this time, obesity rose 58%, from 33.5% in 1995 to 52.9% in 2010. A fourth paper reporting on 155,000 United Kingdom women and men reported higher BMI in car-only individuals when compared with mixed public and active transport commuters (156). Therefore, we caution as to whether physical inactivity or caloric intake is more important relative to the other in human obesity. We suggest that both have a participating role in weight gain, with each’s relative percentage dependent on the situation.
B. Overview for Current Physiological Regulation of Adiposity
Basal metabolic rate (BMR) is defined here as the minimal rate of energy expenditure needed to keep the human body alive with all of its vital functions at rest. While various BMR values occur for differences in age, gender, lean body mass, and body fat, among other factors. The storage of glucose is limited with the amount of glucose in blood and stored as glycogen on the same order of magnitude (532) as the daily BMR. Thus, to conserve glucose as a source of calories, fatty acids are important alternative sources of ATP. Importantly, neurons and erythrocytes normally oxidize glucose, although neurons can adapt to be able to oxidize fatty acids. Fat is very efficient per unit of its mass in storage of calories, as exemplified by a 200-pound human with 20% body fat approximating 140,000 stored fat calories, and storage of triglycerides is favored in adipose tissue energy to be available to substitute when food was not available. In the past, evolutionary pressures often limited fat stores, and Reed et al. (416) noted, “based on a dataset of 1,977 knockout strains, we found that that 31% of viable knockout mouse strains weighed less and an additional 3% weighed more than did controls.” Thus ~10 times greater molecules contribute to store triglycerides than to oxidize them.
C. Both Childhood Obesity and Inactivity Have Increased Dramatically in the Past Half Century: Links to Adult Obesity
The likely causes of childhood obesity include environmental changes by diet, physical inactivity, and/or epigenetic changes that induce changes in gene expression, versus new DNA nucleotide sequences. Obesity is defined as a BMI value at or above the 95th percentile for children. From the period of 1971–74 to 2013–14, the percentage of U.S. children (2–19 yr of age) who are “obese” increased from 5.1 to 17.0% (369, 370). Interestingly, from 2003-04 to 2013–14, obesity in youth did not change. These changes are associated with a large increase in physical inactivity. The mean minutes of moderate to vigorous physical activity per day (FIGURE 6) showed continuous yearly drops of ~67 and ~60% when the activity was plotted from 6 to 11 yr old, and then essentially leveling off each year from 12 to 19 yr of age (547). It seems reasonable to propose the notion that the 60% drop in physical activity from 6 to 11 yr old would increase body adiposity as well as numerous other cardiometabolic risk factors. The data beg the question of what the percentage of obese children have been if moderate to vigorous physical activity were to have been maintained at the 6-yr-old level until 11 yr of age? Also, what is the biological basis for the 60–67% drop in moderate to vigorous physical activity per day? The Wolff-Hughes et al. (547) data suggest that although other factors definitely contribute, this decrease in activity likely contributes to the increase in childhood obesity. Of course, a limitation is the lack of direct measures of calorie expenditure; however, while decreased physical activity does not provide absolute caloric values, the magnitude (60 and 67%) in the percentage decline in daily duration of moderate physical activity implies decreases in caloric expenditures in children.
A recent study (270) used accelerometers to estimate moderate to vigorous physical activity levels and DXA to determine body composition between 1998 and 2014. Only 9.5% of the “consistent active” subjects had entered the “becoming obese” group at age 19 yr old, while 23.8% of participants in the “decreasing active” group entered the “becoming obese group” and 88.3% in the “consistently inactive” or “decreasing activity” groups were in the “consistently obese” group. The authors (270) summarized that “adiposity development at age 13 or younger could be critical to determining obesity development in young adulthood. This finding sheds light on the importance of adiposity development during prepuberty and puberty, and supports current public health efforts to prevent obesity by focusing on early childhood.” Intriguingly, the lifetime peak of voluntary running in wheels by female rats discussed earlier occurs around the age of puberty (498).
A recent meta-analysis by Simmonds et al. (462) of the clinical importance of childhood obesity in increasing the odds of adult obesity was performed that included large prospective cohort studies. Overall, obese children and adolescents had 5.2 times the probability of becoming obese adults as compared with nonobese children and adolescents, ~55% of obese children retain being obese in adolescence, ~80% of obese adolescents maintain having obesity when in their 20s, and ~70% will be still be obese over age 30. The authors cautioned that ~70% of obese adults were not obese as children or adolescents, so what contributes to obesity in childhood should also be targeted in adults.
D. Physical Inactivity Causes Obesity
Importantly, the medical community largely believes that obesity, and particularly central obesity (abdominal or visceral) (129, 162), is a primary cause of insulin resistance; thus the role of physical inactivity can be largely ignored. However, an adipocentric hypothesis is not correct. The putative adipocentric mechanism is that obesity and in particular expansion of visceral adipose leads to increases in circulating fatty acids and inflammatory cytokines, which promote insulin resistance (460, 447). Insulin resistance, the reduced ability of insulin to promote glucose transport, is functionally simply “reduced insulin sensitivity” and is often used in terms of describing a pathological condition, although there is no clearly established clinical definition of insulin resistance. Indeed, experimental studies in humans, rodents, and muscle cells all support the hypothesis linking visceral adiposity and increased circulating factors that promote insulin resistance in skeletal muscle (34, 309). Free fatty acids and inflammatory cytokines such as tumor necrosis factor (TNF)-α reduce insulin sensitivity in all of these models (309, 444). However, the common feature in these studies may be inactivity or reduced energy expenditure that must likely be present for cytokines or free fatty acids to decrease insulin sensitivity. In other words, this only occurs when skeletal muscle energy demand is low, such as is found in physically inactive humans living in a modern environment or rodents in cages without access to running wheels.
Importantly, one opposition to the adipocentric hypothesis is that humans who are overfed calories or rodents that are hyperphagic or fed high-fat diets do not develop insulin resistance if they are active or exercising (190, 261, 249, 415, 536). For example, Haskell-Luevano et al. (203) found that voluntary running of melanocortin-4-receptor null mice prevented obesity and diabetic metabolic syndrome from developing, as had been reported in sedentary, melanocortin-4-receptor null mice.
The removal of wheel-running allows a rapid initial growth in adipose tissue mass following its retarded growth by wheel running in rats just post-weaning when they are growing (267, 281, 282, 439) or examining adipose tissue regrowth after a dietary restriction (477). Rats that had been exposed to VWR had reduced adiposity, body fat, and body weight compared with sedentary rats that did not have access to wheels. However, after only 2–7 days of wheel-lock, when rats were no longer undergoing voluntary running, total mass of various abdominal fat pad depots (epididymal, retroperitoneal, perirenal) and total body fat (measured by DXA) increased dramatically and matched levels found in sedentary animals (268, 282). Thus a transition to inactivity for only a few days caused an accrual of abdominal fat that occurred over multiple weeks in sedentary rats. Further analysis revealed that both the cell volume and lipid content of adipocytes within epididymal fat depots also increased following wheel-lock. In addition, Kump et al. (268) found that palmitate incorporation into triacylglycerol of adipocyte homogenates, an in vitro assessment of triacylglycerol synthesis, increased by ~3.5-fold from 5 h of wheel-lock compared with only 10 h of wheel-lock. Furthermore, palmitate incorporation into adipocyte triacylglycerol at 10 h after wheel-lock no longer cycled, but progressively continued to increase for 24 h/day at 1 and 2 days following wheel-lock, an effect described as an overshoot due to wheel lock-induced inactivity (53). These animal findings resemble a body of human evidence that physiological mechanisms exist to defend adiposity after weight loss programs (149). Furthermore, VWR rats consumed more food on a daily basis than age-matched, sedentary rats, and they continued to consume more food at a decreasing daily amount for 3 days following wheel-lock before matching food intake in age-matched, sedentary rats never exposed to VWR (282). Therefore, logic would indicate that the increased food consumption and associated positive energy balance might be driving the rapid increases in adiposity found during wheel-lock. However, Booth and colleagues (282) performed followup studies in which wheel-locked rats had food intake controlled by pair feeding to adjust food intake to what it should be in their new physically inactive state, and a rapid increase in abdominal adiposity and body fat after 7 days of wheel lock still occurred. The group went on to use transcriptomics analysis in the perirenal adipose tissue and detected an enrichment of transcripts having functions for proinflammation, extracellular matrix, macrophage infiltration, and immunity (439). Unloading of the rat hindlimbs had a delay of 4 h before a rapid fall in heparin-releasable lipoprotein lipase activity from its soleus muscle so that by the 10th hour of unloading, 95% of heparin-releasable lipoprotein lipase was no longer present (36).
Furthermore, in humans, daily physical activity is highly correlated to insulin sensitivity, and this is only modestly attenuated by adiposity (16). Therefore, inactivity generally must be present for insulin resistance to occur. Indeed, inactivity is a key etiological factor in the development of insulin resistance through two mechanisms: 1) inactivity, itself, lowers insulin sensitivity, and 2) inactivity provides a permissive environment whereby signaling molecules can impair insulin signaling processes and further reduce insulin sensitivity. Interestingly, the transition of rodents and humans from high to low levels of daily activity as a model to study inactivity leads to dampened insulin sensitivity and increased central adiposity that occur at the same time frame. Thus not only does inactivity promote insulin resistance through reduced utilization of glucose in muscle, but it may also promote the increased storage of glucose and free fatty acids into adipose tissue. Inactivity-induced reductions in fuel utilization of both glucose and fatty acids may divert these fuels to storage in adipose tissue. As such, adiposity and insulin resistance may develop at the same time through these processes. In addition, impaired insulin signaling was not found in muscle cells treated with TNF-α if they were contracted (273). In summary, the findings in this section, when taken together, suggest that acute changes in physical inactivity induce robust and rapid changes in metabolism.
IX. SKELETAL MUSCLE
A. Muscle Mass and Function Is Impacted by Activity and Inactivity Throughout Life
This section continues theme 5 that continuous physical inactivity accelerates the lifelong decline in skeletal muscle mass and functional ability for strength throughout life, from childhood to the elderly. For example, in youth, Behringer et al. (29) reported in a meta-analysis a progressive strength with age and increase for trainability of muscular strength age. Twin studies noted that consistently inactive twins had 20% lower knee extension forces than their active twins, and mid-thigh muscle cross-sectional area was 4% smaller in the inactive twin (294). In the aforementioned study from Krogh-Madsen et al. (263), reduced daily step counts led to reduced leg lean mass, a decrease in peripheral insulin sensitivity, as well as the insulin-stimulated ratio of pAktthr308/total Akt in the vastus lateralis muscle. In older subjects (mean age 72 yr), Breen et al. (65) produced a 76% reduction in daily step number to 1,413 steps/day for 14 days, and leg lean mass was reduced 3.9%. In the postprandial state, protein synthesis rates were attenuated 26%, and insulin sensitivity decreased 43%. Thus skeletal muscle is one of the most vital tissues impacted by the effects of physical inactivity (150).
B. Protein Synthesis Rates of Skeletal Muscle During Physical Inactivity: Links to Adaptive Remodeling
Early animal models demonstrated the importance of the effects of inactivity on protein synthesis. For example, in 1977 Goldspink (181) noted that protein synthesis in incubated soleus muscle decreased ∼20% after its removal from the rat at the sixth hour of the muscle’s immobilization in a shortened position. The Booth laboratory (57) noted that the fractional rate of soleus protein synthesis was decreased 37% in the first 6 h of hindlimb immobilization, which had the muscle fixed in a shortened position. The rapid changes in the onset of decreased protein synthesis of rat skeletal muscle are not unprecedented when considering hypertrophy studies in adult fowl (278). Thomason and Booth (493) showed that skeletal muscle protein synthesis significantly declined in the first 5 h of non-weight bearing (492), and then remained suppressed. You et al. (553) provide data that hindlimb-immobilization-induced muscle atrophy was associated with decreased protein synthesis independent of mTOR activity and cap-dependent-translation, as mTOR signaling was elevated, rather than suppressed. These earlier animal studies later led to confirmation in human studies. Phillips et al. (396) concluded, “it is now generally agreed that in humans, protein synthesis is downregulated (in skeletal muscle) as a result of uncomplicated (i.e., nonpathological) disuse.” Studies these authors cited reported human leg immobilization and included decreased protein synthesis rates of quadriceps muscles of 27, 25, and 23% after 14 days (179), 37 days (175), and 42 days (176), respectively. An additional study (148) found a 50% decrease in vastus lateralis muscle of men after 14 days of bed rest. Wall et al. (527) suggested that muscle protein synthesis rates are suppressed by 2–4 days in human leg models of physical inactivity and that they remained suppressed.
Often, increases in protein degradation are transient and related to adaptive remodeling to a new smaller size. Because valid methodologies for direct measurements of protein degradation are lacking, determination of protein degradation rates are made by indirect estimations from direct measurements in rates for muscle protein loss and changes in muscle protein synthesis rates. A transient rise in protein degradation would be consistent with Phillips et al. (396): “proposing that most of the loss of muscle mass during disuse atrophy can be accounted for by a depression in the rate of protein synthesis,” as a transient increase in degradation would be a minor percentage of total protein lost. Bodine and Baehr (49) noted: “animal and human data suggest that under conditions of disuse, MuRF1 and MAFbx RNA expression rapidly increases for a relatively short period of time, and thus the inability to measure elevated levels of MuRF1 and MAFbx after extended periods of unloading should not be interpreted to mean that these genes have not had a significant impact on the atrophy process.” Five days of unloading of rat soleus produced an increase in transcriptional activation of MuRF1, suggesting that NF-κB sites, not FoxO sites, were required for MuRF1 promoter activation in the unloaded soleus muscle (551). Baehr et al. (12) later observed defects in the proteostasis network in old skeletal muscles contributed to defects in remodeling of muscle fibers and functional recoveries of muscle mass and strength.
Goldberg and co-authors (397) provide a summary of comparative biology for skeletal muscle atrophy and hypertrophy among the diverse species of Drosophila, rodents, and humans. They indicate shortcomings of Drosophila for translational studies of exercise/inactivity are lack of satellite cells and expression of ubiquitin ligase MuRF, skeletal muscle growth only occurs during development, and no muscle atrophy was seen in flightless Drosophila mutants.
C. Sarcopenia
Rosenberg (433) proposed the term sarcopenia after the Greek sarx (514) and penia (loss) in 1988. Five years later, Evans and Campbell (140) defined sarcopenia as “the age-related loss in skeletal muscle mass, which results in decreased strength and aerobic capacity and thus functional capacity.” However, the consensus definition of sarcopenia is currently unsettled: “although sarcopenia has been researched for many years, currently there is a lack of consensus on its definition. Some studies define sarcopenia as low muscle mass alone, whereas other studies have recently combined low muscle mass, strength and physical performance suggested by the European Working Group on Sarcopenia in Older People, as well as the Asian Working Group for Sarcopenia. The arbitrary use of various available sarcopenia definitions within the literature can cause discrepancies in the prevalence and associated risk factors” (244). This is based on data to suggest that low skeletal muscle strength, rather than low mass, may be a better predictor of mortality (355). Grip strength, although it has limitations, is a simple measure of overall muscle strength (421), onset of sarcopenia (105), future disability (411, 420), physical health problems (99), cognitive decline (420), and both morbidity and mortality risk (292). The definition is evolving (105, 138, 152, 215, 317) and is likely to continue. McGregor et al. (329) suggested that “there is now evidence that not only changes in muscle mass but other factors underpinning muscle quality including composition, metabolism, aerobic capacity, insulin resistance, fat infiltration, fibrosis, and neural activation may also play a role in the decline in muscle function and impaired mobility associated with ageing.” Nevertheless, sarcopenia is a multifactorial geriatric syndrome that is affected by the endocrine system, growth factors, muscle protein turnover, behavior-mediated pathways, and inflammatory-mediated pathways and redox-related factors (106).
With the added years of life expectancy in the 20th century, a major clinical significance for a large number of individuals is the loss of mobility. The near elimination of most communicable diseases in the last century increased life expectancy by decades, but also increased chronic diseases like sarcopenia. The newly added physical frailty with increased life expectancy has increased the probability of falls, as well as an impaired ability to independently perform activities of daily living. Approximately 95% of hip fractures occur after falling (27).
This ultimately results in a lower quality of life in the extended end years of life (139). However, the origins of sarcopenia are complex. Nair (350) describes physical inactivity as inherent with aging. Additionally, aging is environmentally multifactorial in progression, and physical inactivity is only one of many environmental factors contributing to sarcopenia. Since chronic diseases are related to skeletal muscle mass, a delay in development of physical activity would slow aging when chronic disease occurs and when physical frailty first becomes clinically present.
Sarcopenia and inactivity amplify each other in a negative cycle leading to physical frailty. Fried et al. (163) proposed the concept of a negative cycle of frailty, which may begin with sarcopenia begetting lower strength and power, that, in turn, lowers walking speed, leading to lower levels of physical activity (inactivity), which then speeds sarcopenia. The cycle is continuous over the remaining period of life, unless intervened to slow down by resistance training. Thus sarcopenia and physical inactivity both lead to weaker skeletal muscle. Wolfe (546) noted: “elderly individuals, particularly women, are often too weak to perform the intensity of exercise necessary to induce the same magnitude of physiologic adaptations that occur in younger subjects.” Breen et al. (65) point out that older adults do not recover muscle mass and strength, even after heavy resistance exercise, as compared with younger individuals (220, 476). They also indicated that the failure to recover lost skeletal muscle because of inactivity would add to the progression of sarcopenia (64, 65), in agreement with animal data (540). That being said, a meta-analysis of resistance-trained subjects ≥50 yr of age reported percentage increases in strength of 29, 33, 24, and 25% for leg press, knee extension, chest press, and lat pulldown, respectively (394). In 1994, Fiatarone et al. (151) studied elderly men and women and noted that in trained subjects (combined groups with or without supplementation), muscle strength, gait velocity, stair climbing power increased by 113, 11, and 28%, respectively, but thigh muscle cross-sectional area increased only 3%. In another study, elderly males (mean age 82) gained strength, but not enlargement of fiber diameter with resistance training, as compared in an age-matched inactive group (465). The same trend occurred in elderly females (mean age 82), exhibiting a 26% increase in strength, but no increase in thigh muscle cross-sectional area and fiber diameter after 12 wk of resistance training (412). In contrast, resistance training in 74-yr-old men produced both increased strength and fiber diameter (502). In older adults, even endurance training may help preserve skeletal muscle mass. Men (202) and women (201) in their 70s who performed 12 wk of cycle-ergometer training had modest increases in quadriceps muscle volume and knee extensor power increased 20% (men) and 55% (women). We speculate that these subjects had so little physical activity that even endurance training was effective in producing muscle enlargement, which is usually not observed in endurance training studies of young adults. Regardless, Wolfe contends that “rather than initiate practices to reverse sarcopenia, it would be more effective to prevent its development” (546). He argues the need to intervene against sarcopenia should begin at middle age rather than later in life. Ortega et al. (374) noted that deaths from all causes, suicide, and cardiovascular diseases were 41, 70, and 46% higher, respectively, in the 1.1 million Swedish males with the lowest strength level 24 yr after a determination of their initial muscle strength at 16–19 yr olds. Nose’s group (322) found a 95% adherence to a 5-mo training program of interval walking training program produced a 15% increase in V̇o2peak and 20% decrease in lifestyle-related disease risk factors in 696 older men and women.
Lexall et al.’s 1988 paper (295) reported cross-sectionally that sarcopenia in men begins in as early as the third decade of their life, with acceleration after 50 yr of age to annual decreases in skeletal muscle mass rate of 1–2% and in muscle strength of 1.5% (522). Strength losses accelerate to ~3% a year after age 60. Sarcopenia was associated with a loss in total fiber number. Drey et al. (124) extended Lexall’s findings to include female and male Master’s athletes over the age of 65 yr old, who performed power-lifting. They had attenuated loss of muscle mass and motor units as compared with physically inactive and endurance-trained subjects of the same age.
The molecular mechanisms of sarcopenia caused by physical inactivity are not entirely clear, as suggested by Bowen et al. (60) who stated “the molecular mechanisms underlying how exercise prevents age-related loss of muscle mass are still poorly understood.” Extending this, even less is known regarding the molecular mechanisms by which physical inactivity causes the age-related loss of muscle mass. Two possible modes for physical inactivity accelerating sarcopenic progression exist, including a direct interaction with inherent aging genes that produce mechanisms that cause sarcopenia, and independent actions, such as physical inactivity, that modulate the rate of sarcopenia (FIGURE 11). However, lifelong resistance training by elite Master’s athletes can slow the absolute amount of muscle mass lost at a given chronological age with aging (384). Inherent genes for physical inactivity (498) and for aging (78) contribute to the loss of functional reserve by the decline of total body mass of skeletal muscle. Amino acids represent nutrient regulators of skeletal muscle anabolism, capable of enhancing lean mass accrual with resistance exercise, and also attenuating the loss of lean mass during periods of energy deficit, although factors such as protein dose, protein source, and timing of intake are likely important in mediating these effects (89). Glower et al. (179) concluded that much of the atrophy is due to a drop in postabsorptive protein synthesis rate caused by “anabolic resistance” to amino acids. An increased perfusion of amino acids diminished the reduction, but rates did not totally recover pre-atrophy rates. Furthermore, Phillips (395) suggests that clinical research is needed to test nutritional and supplement-based strategies with resistance training to prevent sarcopenia.
FIGURE 11.
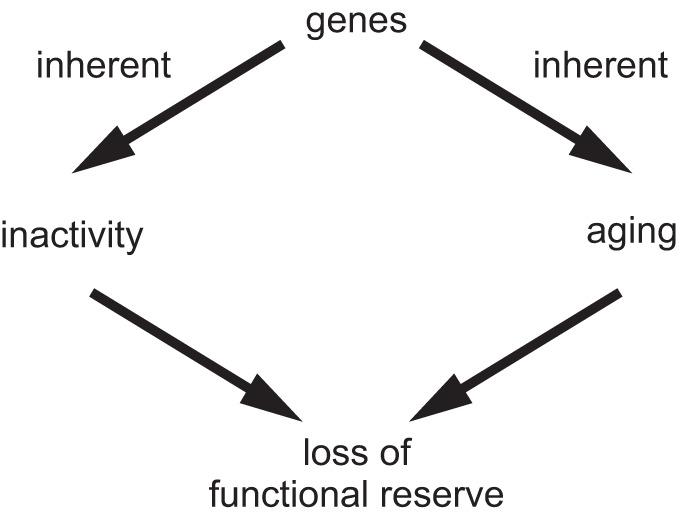
Schematic of two factors, physical inactivity and aging, that produce sarcopenia and its associated eventual loss of an important functional reserve.
D. Myokines
The term myokine was first defined by Pedersen et al. (386) as, “cytokines and other peptides that are produced, expressed, and released by muscle fibers and exert either paracrine or endocrine effects.” This provided the first evidence of skeletal muscle-secreting molecules having functional effects at a distant site from their origin of secretion, proving that skeletal muscle has an endocrine function. Interestingly, Goldstein (182) documented the idea nearly 60 yr ago. His working hypothesis in a 1961 Diabetes editorial (182) was that skeletal muscle fibers possessed a “humoral” factor that skeletal muscle contraction caused to be released into the circulation to be a message that muscle was consuming glucose to fuel the work. He experimentally had tested his hypothesis by transfer of the putative “humoral” factor by means of a cross-transfusion of both blood thoracic lymphatic fluid from the effluents from contracting skeletal muscle from one exercising animal into a second resting animal, which became hypoglycemic. Goldstein (182) noted that “with strenuous induced exercise one can enrich the body fluids of an animal with hypoglycemic properties which can then be transferred to a resting preparation without other concomitants of exercise such as pH shifts, hyperpnea, circulatory changes, etc.”
Pedersen and Goetz (389) detailed the intermittent history that led to the knowledge that skeletal muscle is an endocrine organ that influences metabolism in virtually all organs in the body (30, 451). One study design included transgenic mice that had GLUT4 overexpression of seven- and threefold in fast-twitch muscle fibers and heart, respectively, resulting in higher insulin-stimulated 2-deoxyglucose uptake and glycogen concentration (507). Remarkably, voluntarily running distance in wheels was four times greater in GLUT-4 overexpression mice than in wild-type mice (508), suggesting the potential for brain regulatory sites for voluntary running being at a “distant site” that had the ability to “sense” some unknown signal from the metabolic status of skeletal muscle.
Pedersen et al. (391) proposed that IL-6 met the earlier Goldstein criteria of: 1) produced and secreted from a tissue into the bloodstream in response to muscle demand for glucose to fuel its contractions and 2) exerted its effects at “distant” organs from the contracting skeletal muscle. They noted that the IL-6 gene is silent in resting skeletal muscle and is activated rapidly by muscle contractions with marked increases in muscle IL-6 mRNA. This IL-6 peptide is released in high amounts into the circulation from contracting skeletal muscle and exerts its effects at distance sites from the muscle, such as adipose tissue, where it induces lipolysis and gene transcription in abdominal subcutaneous fat, increasing whole body lipid oxidation (391). However, this concept (387) was not met with immediate approval because it came after the dogma that a continuous low level of increase in IL-6 is “pro-inflammatory.” Exercise-induced IL-6 produces a large transient rise in IL-6 which exerts anti-inflammatory effects. Pedersen and Febbrario (388) stated that “myokines may mediate protective effects of muscular exercise, with regard to diseases associated with physically inactive lifestyle.” In physically inactive humans, chronically low increases from basal plasma levels of IL-6 exist and closely associate with the metabolic syndrome (388). IL-6 was suggested to have systemic effects on liver and immune system, as well as to play a role in crosstalk between intestinal L cells and pancreatic islets. Skeletal muscle is now considered an endocrine organ (153, 239, 290, 403, 429). Taken together, acute exercise of sufficient intensity produces a large percentage rise in IL-6 that is transient, and importantly has an anti-inflammatory effect, while chronic physical inactivity produces a continuous, low-level increase in IL-6 that has pro-inflammatory effects (238). Additionally, Schnyder and Handschin (451) commented concerning myokines that, “What is missing is the response of the skeletal muscle system to physical inactivity. So far, only myostatin or ciliary neurotrophic factor (CNTF) might fit that description; however, it would be surprising if myostatin and CNTF were the only inactivity myokines. Overall, it has long been known that physical activity produces changes in levels of hormones from classical endocrine organs (169, 478, 487), and it is likely that skeletal muscle as a gene regulatory endocrine organ will help our understanding of the interaction of the role of skeletal muscle with inactivity-related chronic disease (239).
In addition, the release of IL-6 from contracting skeletal muscle induces the production of bioactive osteocalcin in bone, allowing its release into blood. A feedforward loop is formed as osteocalcin, in turn, further increases IL-6 release (56, 333). In contracting skeletal muscle, osteocalcin increases glycogen breakdown to glucose, GLUT4 translocation to the sarcolemma that increases glucose uptake into muscle, and fatty acids uptake and catabolism.
E. Signaling Mechanisms for Physical Inactivity to Produce Atrophy and for Physical Activity to Produce Hypertrophy Are Not Simply Reversal of the Same Pathways
Our notion is that the mechanistic continuum for physical activity to inactivity is not the same as going from inactivity to activity. For example, the mechanisms underlying muscle atrophy to muscle growth are not simply the reverse of a growth state to atrophy. The same could be said for 1,500 to 10,000 steps/day, compared with the reverse. Rather, separate cellular mechanisms for physical activity (82a) and physical inactivity (actual cause) often exist. Regarding the first example, with simple logic, one might assume that hypertrophy is simply the result of protein synthesis, while atrophy is exclusively due to protein degradation. However, data support the concept that protein degradation increases in both skeletal muscle hypertrophy and atrophy. Millward et al. (278) estimated that protein degradation rates of skeletal muscle increased during hypertrophy of chicken skeletal muscle. The unexpected increase in protein degradation was deduced from the increase in the rate of protein synthesis which exceeded the directly measured enlargement of skeletal muscle mass. They suggested that the protein synthesis rate was greater than needed because nascent proteins made were in excess of protein incorporation into muscle. They proposed that nascent protein not assembled into structure was rapidly degraded, termed “wastage” protein synthesis. Thus protein degradation of skeletal muscle is not only increased in atrophy, but also increases during hypertrophy. Stein and Bolster (470) provided more evidence for this concept and compared skeletal muscle transcripts between two treatments, one for muscle atrophy [Lecker and co-workers. (284, 470)] versus the other for muscle regrowth from atrophy [Fluck et al. (157)], and concluded “comparison of these two gene lists for atrophy and hypertrophy showed virtually no overlap. This is a common finding in biochemistry. Anabolic and catabolic pathways are usually separate.” This section, taken together, illustrates theme 1 on the dissociation of pathway reversibility for activity and inactivity.
X. BONE
A. Physical Inactivity Prevents Optimal Bone Maturation Early in Life: Relation to Lifetime Bone Mass Apex
Gunter et al. (193) provide a current viewpoint on the state of current information for bone health in children: “. . . Based on our work (191, 192, 194, 225–227) and that of others (361, 445), we hypothesize that engaging in regular and well-designed targeted physical activity in childhood is crucial to maintaining a healthy skeleton in adulthood. In fact, considering that 60% of the risk of developing osteoporosis can be explained by the amount of bone mass accrued by early adulthood (26), physical activity undertaken during or prior to puberty may have greater positive effects on bone mass than many pharmacological interventions undertaken by adults with osteoporosis.”
During growth and developmental years, nonphysically active youth will end up having 10–20% less peak bone mineral density compared with youth undergoing regular weight-loading physical activity during the growth phase (14, 21, 28, 195, 235, 236, 305, 499). Numerous studies document physically inactive adolescents not gaining various indexes of bone health, as compared with groups undergoing weight-bearing activities on bones. For example, less active 4- to 7-yr-old boys and girls had accelerometer-determined lifestyle physical activity levels, as well as proximal femoral measures of the neck, intertrochanteric, and shaft cross-sectional area (315) and section modulus (Z), indexes of axial and bending strength, and noted that boys and girls in the top third of vigorous activity had 7–9% higher CSA and 9–12% higher Z than the bottom third (224). Another study investigated 8-yr-old children that underwent 7 mo of non-weight-bearing (skeletal muscle stretching) or weight-bearing (jumping) physical training. Children in the stretching program had 4% less bone mineral content at their hip than those children who completed high-impact jumping exercises, and 8 yr later, when 16 yr old, the subjects in the stretching group had 1.4% less bone mineral content at their hip than the jumping group (191). A meta-analysis of 17 studies found that pre-pubertal non-gymnasts had smaller distal radius bone mass density and bone mineral content than pre-pubertal gymnasts (72). In addition, in 6- to 10-yr-old children, a non-jumping group had 4.5 and 3.1% lower femoral neck and lumbar spine bone mineral content, respectively, than high-intensity jumping group after 7 mo (168). Taken together, less physical activity during childhood leads to less bone strength during maturation. We speculate that those who enter adulthood with weaker bone strength have a greater probability of more bone fractures later on in life. Extending inactivity comparisons to young adults showed similar trends as the childhood data. For example, males (29–30 yr old) who were more physically inactive than when first measured between 8 and 15 yr of age had 13% lower adjusted torsional bone strength and 10% less adjusted tibial diaphysis density (125); females responded similarly. Thus inactive adolescents begin adulthood with a deficit in bone health. Others have speculated that optimizing peak bone mass in early life could be helpful to delaying osteoporosis later in life (28).
The lifetime apex for bone mass (i.e., peak bone mass) occurs at ~18–20 yr of age (26). By age 18, at least 90% of peak bone mass had been acquired, with the remaining 10% to be added later in the skeletal consolidation phase (14). The clinical importance of obtaining the genetically highest possible bone mass in youth is that it is difficult to add bone mass above that lifetime apex value after the second decade of life.
B. Bone Health After the Lifetime Apex
Bone mineral density is maintained at a maximum until ~30 yr of age, with loss of bone mineral thereafter (69). Bailey et al. (14) suggested the amount of bone mineral that is laid down in the 2-yr peak bone mass content velocity period during adolescent years is similar to the amount that most individuals end up losing during their later active lives (13). The Tromsø Study, a cross-sectional study (544), consisted of 7,273 subjects aged 24–84 yr old. The 80-yr-old, smoking, physically inactive subjects of both sexes were estimated to lose 25 and 38% more bone mineral density at female’s distal and ultradistal sites, respectively, and 39% more bone mineral density at male’s forearm sites.
C. Bone Loss in the Absence of Gravity
Some types of physical inactivity causing weak bones include skeletal muscle denervation/paralysis, space flight, bed rest, and aging (468). Hindlimb unloading inhibits bone formation, while bone resorption is enhanced or unchanged (258). In humans, spaceflight led to 20-fold greater bone loss in space than on Earth (518), at 1.8–2.0% bone loss per month. In addition, bones of astronauts and cosmonauts lost 11% (range 0–24%) of their total hip bone mass over the course of 4–6 mo in Skylab (518). Aerobic exercise in space did not inhibit head bone loss. Further support that endurance inactivity is not the primary factor for accelerated bone mass loss in near-zero gravity is that NASA found that treadmill running in space did not abate bone loss. So bone loss in spaceflight was due to the lack of gravity placing a mechanical stress on bones. Extrapolating this to Earth-based models, bed rest, which is often prescribed to patients for treatment of clinical maladies, and particularly in “retirement” living, lack of weight bearing could increase susceptibility to bone fractures.
D. Mortality Occurence Rates From Hip Fractures Predominantly Later in Life
Hip fractures are predicted to reach more than 6 million by 2050, compared with ~1.66 million in 1990 (98), with Asia accounting for 55% of fractures of all types worldwide (147, 233). Mortality progressively increases with postoperative duration after hip fracture. In one study, overall mortality was 10.5% 30 days post-surgery and increasing to 73.6% 7 yr post-surgery (379). Following surgery for femoral neck fractures in 1984–98, other studies found male fatality rates at 30 days increased from 4% at 64–69 yr old to 31% in those aged ≥90 yr (426), and reductions in quality of life due to broken hips are equivalent to reductions for multiple sclerosis or Parkinson’s disease (189). Interestingly, males had greater mortality than females, up to 5–10 yr after hip fracture (5, 82, 377).
E. Mechanisms for Physical Inactivity’s Effect on Bone
While it has been known for over 100 yr that mechanical loading of bone is necessary for optimal bone mass, mechanisms related to bone remodeling are not yet fully elucidated (468). Bone-mass quality and quantity are determined by osteoblasts, osteoclasts, and osteocytes. Osteocytes are the cell type that maintains the balance between bone formation and removal (406), and mice with ablated osteocytes are resistant to unloading-induced bone loss (485). Spatz et al. (468) interpreted the unloading insensitivity of osteocytes as providing evidence that osteocytes play some role in mechanosensation of forces within bone. Importantly, osteocytes transduce the sensed forces from mechanosignals to chemical signals, which, in turn, function to signal modulations of bone mass/strength. Taken together, we suggest that less mechanosensing from physically inactive bones is likely to be transduced to chemical signals that regulate the loss of bone structures. The next paragraph considers molecules that play a role from the mechanosensing process of osteocytes.
It is well established that the maintenance of bone mass over time is a balance between formation of new bone and resorption of old bone. Two important chemical modulators of bone mass in unloading are sclerostin (SOST), produced in osteocytes (96), and receptor activator of nuclear factor kappa-B ligand (RANKL). Sclerostin’s main function is to inhibit bone formation by directly reducing proliferation and differentiation of osteoblasts via its inhibition of the canonical Wnt signaling pathway (216), which enhances bone formation by controlling embryonic cartilage development and postnatal chondrogenesis, osteoblastogenesis, osteoclastogenesis, endochondral bone formation, and bone remodeling (315). Sclerostin mRNA in osteoclasts increases in human serum during bed rest (467), in plasma from patients with chronic spinal cord injury (344), and with hindlimb unloading of the mouse tibia (427). Sclerostin antibodies increased bone mass by increasing bone formation in hindlimb unloaded mice (466). Sclerostin also selectively suppressed Wnt/beta-catenin signaling, osteoblast activity, and viability of osteoblasts and osteocytes (299). Sost-deficient mice have increased bone formation and bone strength (296) and rescue mechanical unloading-induced bone loss (299, 362).
RANKL is a member of the TNF superfamily and is the ligand that binds to RANK, the osteoclast cell-surface receptor. RANKL/RANK signaling controls osteoclast replication, activation, and survival during normal bone modeling and remodeling, and also in conditions of increased bone turnover/remodeling (61). Upregulation of pyruvate dehydrogenase kinase 4 during bone unloading is a molecule that induces Rankl expression in osteoblasts (529). Moriishi and co-workers (529) performed a series of experiments in the femurs of wild-type mice that had undergone tail-suspension and noted that when the tibia bone was unloaded, osteoclastogenesis was enhanced and osteoblast function was inhibited, leading to bone loss in wild-type mice. They further observed that Rankl expression in osteoblasts in the unloaded tibia bone was increased along with increased Sost expression in osteocytes. Intriguingly, Moriishi and co-workers (529) found that overexpression of B cell lymphoma 2 (Bcl2) rescued the unloading phenotype in the wild-type mice described above. Femurs in tail-suspended Bcl2 transgenic mice had wild-type values for osteoblastic and osteoclastogenic functions, Sost expression in osteocytes, Rankl expression in osteoblasts and femur bone mass. Komori concluded: “osteocytes are responsible for bone loss in unloaded condition, and osteocytes augment their functions by further stimulating osteoclastogenesis and further inhibiting osteoblast function, at least partly, through the upregulation of receptor activator of nuclear factor-kappa B ligand (RANKL) in osteoblasts and Sost in osteocytes in unloaded condition” (259).
In addition, several subtypes of bone marrow cells support bone immune cell functions. One subtype, in particular, has a role as a primary lymphoid organ, supporting lymphoid development (334). Lescale et al. (293) published that mechanical unloading causes a decrease in B-cell progenitor populations and the generation of B lymphocytes in the bone marrow, which is concurrent with bone remodeling. The change was cell-type specific as no change occurred in hematopoietic stem cells or multipotent hematopoietic progenitor populations.
XI. IMMUNITY
Immune cells, including monocytes, granulocytes, and lymphocytes, become dysregulated in chronic disease (271, 442). Numerous papers go beyond the scope of this review and discuss exercise and immune system function (66, 134, 358, 360, 389, 510). However, few studies have been conducted on the immune system’s response to physical inactivity.
A. Physical Inactivity Depresses Some Components of the Immune System
Possibly more than any other body system, the immunity adheres to the U-shaped risk curve as it relates to physical activity. Highest immune suppression (i.e., greatest susceptibility to communicative diseases, such as upper respiratory tract infections) is at the top of both arms of “U” shape. Too little inactivity can elicit immune system suppression, while very high volume physical activity may also have immunosuppressive effects. Human data show that a regular, moderate amount of physical activity has best positive immune response on the bottom of “U”-shaped curve. Inactivity causes an increase in visceral adipose tissue (133, 486), which is associated with atherosclerosis, cancer, dyslipidemia, hypertension, mortality, and T2D, when compared with the healthier peripheral obesity (237, 388, 461). Importantly, some normal weight humans having a high ratio of central-to-peripheral fat have an increased prevalence of being insulin resistant (232). In a cohort study of 334,000 European men and women, the inactive group had 25 and 21% higher hazard ratios than the moderately inactive groups in both the abdominally lean and abdominally obese groups, respectively (133). Pedersen and Febbario (385, 388) proposed “. . . physical inactivity (leads to) accumulation of visceral adipose tissue and consequently to the activation of a network of inflammatory pathways, which promote development of insulin resistance, atherosclerosis, neurodegeneration and tumour growth and, thereby, promote the development of a cluster of chronic diseases.” Overall, physical inactivity leads to visceral adipose tissue accumulation, activating inflammatory pathways. Inflammation initiates the development of systemic insulin resistance, neurodegeneration, atherosclerosis, and tumor growth, thereby facilitating development of clusters of chronic diseases (FIGURE 3).
XII. DIGESTION
Historically, the digestive system has not been implicated in physical inactivity-induced negative major health outcomes. During endurance exercise, blood flow is shunted away from the gut and redirected to working skeletal muscle (408). What can be said about the gut response to physical inactivity? This is an area that has not received much attention (367); however, one area that is likely to receive more attention is the gut microbiome.
In the rapidly emerging field of the microbiome, no physical inactivity studies have been performed. Thus we will reference the control group as the inactive group and adjust some findings to indicate differences of the inactive group from the more physically active group. In a review by Campbell and Wisniewski (74), the inactive animals have less health promoting bacteria, less butyrate producing bacteria and colonic butyrate concentrations, and greater intestinal inflammation, than the exercising group of animals. Cerdá et al. (83) summarize exercise and intestinal microbiota research and provide potential mechanisms by which physical activity might alter intestinal microbiota. In addition, the increase in colon cancer by physical inactivity could be partially mediated by increasing colonic bile acid exposure, which could increase DNA mutagenesis, according to Wertheim et al. (537).
XIII. CANCER
Physical inactivity is associated with many site-specific cancers; however, the strength of the scientific evidence is variable (17, 131, 264, 266, 291). A recent report determined whether leisure-time physical activity, performed at an individual’s discretion and set as any activity performed to maintain or improve fitness over 3 METs, was associated with 26 cancer types in 1.44 million adults averaging 59 yr old revealed 186,932 cancer cases (339). When the highest (90th percentile of leisure-time physical activity) was compared with lowest (10th percentile), there was >20% reduction in esophageal adenocarcinoma, cancers of the endometrium, gastric cardia, kidney, liver, lung, and myeloid leukemia. 10–20% reductions were noted for colon cancer, head and neck cancer, rectal cancer, bladder cancer, and breast cancer. Limitations were that physical activity was self-reported and lack of dose-response data. The hazard ratio of malignant melanoma for leisure-time physical activity was 1.27 (339). Thus skin cancer can be increased by being physically in the skin, only if protective measures from the sun are not taken. Usage of ultraviolet-protecting lotions should decrease the hazard ratio for outdoor, daytime exercise.
For specific cancers, two that have been studied in detail are breast and colon cancer. For breast cancer, a meta-analysis of 79 studies puts the prevalence of breast cancer higher, at average increase of 25% (165). Friedenreich (164) indicates that given the multifactorial etiology of breast cancer, “it is likely that many interrelated pathways are involved in reducing breast cancer risk. It is also possible that certain mechanisms predominate with specific doses or types of physical activity or perhaps in select subgroups of women.” A dose-response effect of physical inactivity was also found in many of the reviewed 79 studies (165). Friedenreich (164) also noted potential biological mechanisms by which physical inactivity could produce breast cancer, including adiposity, sex hormones, insulin-related factors, adipokines, and inflammation. Thus the role of physical inactivity as a contributing causal factor to breast cancer is likely complex with multiple interacting mechanisms (265). For colon cancer, there was a significant 24% increased risk when comparing the least versus the most active individuals across 24 studies (548). Wolin et al. (548) summarized multiple mechanisms that might contribute to least active group having greater colon cancer, including insulin resistance and hyperinsulinemia and their associated growth factors, pro-inflammatory pathways, immune dysfunction, visceral obesity, increased stool transit time, increasing the exposure of the colon to carcinogens and lower vitamin D levels.
XIV. SUMMARY
Physical inactivity is an actual cause of over 35 chronic diseases/conditions. Some of these are major chronic conditions, for example, insulin resistance leading to T2D, aging leading to Alzheimer’s disease and other diseases, or high cardiovascular risk factors, leading to coronary artery disease. Overwhelming evidence from epidemiological studies proves that the physically inactive group has increased prevalence, that often range from 30 to 50%, for major causes of death, including cardiovascular disease, T2D, and Alzheimer’s disease. Consequently, evidence supports the notion that physical inactivity is an actual cause of both shorter healthspan and early mortality. An explanation is that physical inactivity speeds declines in important phenotypes, such as V̇o2max, skeletal muscle mass/strength, and cognition, but their molecular basis is uncertain.
Known molecular and biochemical mechanisms differ between exercise and physical inactivity in a sufficient number of examples to suggest caution. Consequently, it is tenuous to assume molecular transducers of one are a mere reversal of the other. We contend that knowing mechanisms of either physical activity or physical inactivity, without knowledge of the other, prevents assuming that the other is simply a reversal of molecular adaptations. For example, mechanisms of skeletal muscle atrophy are not simply the reversal of the same pathways for muscle hypertrophy. Furthermore, knowledge of molecular signatures for adaptations to physical inactivity provide the best accuracy in translation for exercise prescription, the best pharmacology, and gene targets for therapy. Thus extrapolation from a known mechanistic signal for an adaptation to exercise cannot be made when the reverse mechanism for physical inactivity is unknown. In addition, human twin studies and rat selective breeding studies provide indirect evidence of genes having functions for physical inactivity. However, studies on the molecular neurobiology for physical inactivity are only in their infancy.
Overall, physical inactivity is an underappreciated cause of almost all chronic diseases/conditions, whose outcome increases mortality and decreases healthspan. Remarkably, physical inactivity speeds biological aging, and physical inactivity gene identification will be a future challenge to help decrease inactivity-induced chronic disease and improve the health and well-being of our society.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-088940 and United States Department of Veterans Affairs Grant 1I01BX002567-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This paper is in honor of three of F. Booth’s teachers: Robert R. Haubrich who was his biology advisor at Denision University, Charles M. Tipton who was his PhD advisor at the University of Iowa, and John O. Holloszy who was his postdoctoral advisor at Washington University in St. Louis.
Address for reprint requests and other correspondence: F. Booth, Depts. of Biomedical Sciences, Medical Pharmacology and Physiology, and Nutrition and Exercise Physiology, Dalton Cardiovascular Research Center, Univ. of Missouri, Columbia, MO 65211 (e-mail: boothf@missouri.edu).
REFERENCES
- 1.Adams J, Mytton O, White M, Monsivais P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med : e1001990, 2016. doi: 10.1371/journal.pmed.1001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell : 33–45, 2014. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth B, Cahalin L, Buman M, Ross R. The current state of physical activity assessment tools. Prog Cardiovasc Dis : 387–395, 2015. doi: 10.1016/j.pcad.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mallah MH, Juraschek SP, Whelton S, Dardari ZA, Ehrman JK, Michos ED, Blumenthal RS, Nasir K, Qureshi WT, Brawner CA, Keteyian SJ, Blaha MJ. Sex differences in cardiorespiratory fitness and all-cause mortality: The Henry Ford ExercIse Testing (FIT) Project. Mayo Clin Proc : 755–762, 2016. doi: 10.1016/j.mayocp.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alegre-López J, Cordero-Guevara J, Alonso-Valdivielso JL, Fernández-Melón J. Factors associated with mortality and functional disability after hip fracture: an inception cohort study. Osteoporos Int : 729–736, 2005. doi: 10.1007/s00198-004-1740-0. [DOI] [PubMed] [Google Scholar]
- 6.Alibegovic AC, Sonne MP, Højbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab : E752–E763, 2010. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- 7.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundström J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ : h4543, 2015. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer E, Blair SN. Physical activity and the prevention of cardiovascular disease: from evolution to epidemiology. Prog Cardiovasc Dis : 387–396, 2011. doi: 10.1016/j.pcad.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Astrand PO. Human physical fitness with special reference to sex and age. Physiol Rev : 307–335, 1956. [DOI] [PubMed] [Google Scholar]
- 10.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol : 529–542, 2015. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 11.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med : 633–638, 2000. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) : 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med , Suppl 3: S191–S194, 1997. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 14.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res : 1672–1679, 1999. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin KM, Haddad F, Pandorf CE, Roy RR, Edgerton VR. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front Physiol : 284, 2013. doi: 10.3389/fphys.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E, EGIR-RISC Study Group . Physical activity and insulin sensitivity: the RISC study. Diabetes : 2613–2618, 2008. doi: 10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, George SM, Alfano CM, Schmitz K. Physical activity across the cancer continuum. Oncology (Williston Park) : 589–592, 2013. [PubMed] [Google Scholar]
- 18.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci : 262–266, 2006. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 19.Barlow CE, LaMonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol : 142–150, 2006. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 20.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc : 459–465, 2003. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 21.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res : 500–507, 1998. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 22.Bassett DR Jr, Tremblay MS, Esliger DW, Copeland JL, Barnes JD, Huntington GE. Physical activity and body mass index of children in an old order Amish community. Med Sci Sports Exerc : 410–415, 2007. doi: 10.1249/mss.0b013e31802d3aa7. [DOI] [PubMed] [Google Scholar]
- 23.Bassett DR Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc : 1819–1825, 2010. doi: 10.1249/MSS.0b013e3181dc2e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassett DR, Schneider PL, Huntington GE. Physical activity in an Old Order Amish community. Med Sci Sports Exerc : 79–85, 2004. doi: 10.1249/01.MSS.0000106184.71258.32. [DOI] [PubMed] [Google Scholar]
- 25.Bauman A, Merom D, Bull FC, Buchner DM, Fiatarone Singh MA. Updating the Evidence for Physical Activity: Summative Reviews of the Epidemiological Evidence, Prevalence, and Interventions to Promote “Active Aging”. Gerontologist , Suppl 2: S268–S280, 2016. doi: 10.1093/geront/gnw031. [DOI] [PubMed] [Google Scholar]
- 26.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res : 1729–1739, 2011. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 27.Beck BR, Daly RM, Singh MA, Taaffe DR. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport : 438–445, 2017. doi: 10.1016/j.jsams.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res : 467–478, 2014. doi: 10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 29.Behringer M, Vom Heede A, Yue Z, Mester J. Effects of resistance training in children and adolescents: a meta-analysis. Pediatrics : e1199–e1210, 2010. doi: 10.1542/peds.2010-0445. [DOI] [PubMed] [Google Scholar]
- 30.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol : 86–97, 2015. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 31.Bennett AF, Ruben JA. Endothermy and activity in vertebrates. Science : 649–654, 1979. doi: 10.1126/science.493968. [DOI] [PubMed] [Google Scholar]
- 32.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience : 853–861, 2005. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand : 63–70, 1996. doi: 10.1046/j.1365-201X.1996.476217000.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med , Suppl 1: S3–S8, 2007. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Berryman JW. The tradition of the “six things non-natural”: exercise and medicine from Hippocrates through ante-bellum America. Exerc Sport Sci Rev : 515–559, 1989. [PubMed] [Google Scholar]
- 36.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol : 673–682, 2003. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health : 643, 2014. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biddle SJ, Bennie JA, Bauman AE, Chau JY, Dunstan D, Owen N, Stamatakis E, van Uffelen JG. Too much sitting and all-cause mortality: is there a causal link? BMC Public Health : 635, 2016. doi: 10.1186/s12889-016-3307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birk GK, Dawson EA, Timothy Cable N, Green DJ, Thijssen DH. Effect of unilateral forearm inactivity on endothelium-dependent vasodilator function in humans. Eur J Appl Physiol : 933–940, 2013. doi: 10.1007/s00421-012-2505-7. [DOI] [PubMed] [Google Scholar]
- 40.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA : 5568–5572, 1990. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med : 1–2, 2009. [PubMed] [Google Scholar]
- 42.Blair SN, Davey Smith G, Lee IM, Fox K, Hillsdon M, McKeown RE, Haskell WL, Marmot M. A tribute to Professor Jeremiah Morris: the man who invented the field of physical activity epidemiology. Ann Epidemiol : 651–660, 2010. doi: 10.1016/j.annepidem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Blair SN, Kohl HW III, Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA : 1093–1098, 1995. doi: 10.1001/jama.1995.03520380029031. [DOI] [PubMed] [Google Scholar]
- 44.Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA : 2395–2401, 1989. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 45.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol : H1747–H1755, 2005. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- 46.Bleeker MW, De Groot PC, Rongen GA, Rittweger J, Felsenberg D, Smits P, Hopman MT. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol (1985) : 1293–1300, 2005. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- 47.Bleeker MW, Hopman MT, Rongen GA, Smits P. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol : R1176–R1177, 2004. doi: 10.1152/ajpregu.00718.2003. [DOI] [PubMed] [Google Scholar]
- 48.Blumenthal JA, Smith PJ, Hoffman BM. Is exercise a viable treatment for depression? ACSMs Health Fit J : 14–21, 2012. doi: 10.1249/01.FIT.0000416000.09526.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab : E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Booth FW. Effect of limb immobilization on skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol : 1113–1118, 1982. [DOI] [PubMed] [Google Scholar]
- 51.Booth FW. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol Respir Environ Exerc Physiol : 656–661, 1977. [DOI] [PubMed] [Google Scholar]
- 52.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol (1985) : 774–787, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol : 381–390, 2008. doi: 10.1007/s00421-007-0606-5. [DOI] [PubMed] [Google Scholar]
- 54.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) : 1497–1504, 2011. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 55.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol : 1143–1211, 2012. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Booth FW, Ruegsegger GN, Olver TD. Exercise has a bone to pick with skeletal muscle. Cell Metab : 961–962, 2016. doi: 10.1016/j.cmet.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 57.Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol Respir Environ Exerc Physiol : 974–977, 1979. [DOI] [PubMed] [Google Scholar]
- 58.Bouchard C. Exercise genomics–a paradigm shift is needed: a commentary. Br J Sports Med : 1492–1496, 2015. doi: 10.1136/bjsports-2015-095294. [DOI] [PubMed] [Google Scholar]
- 59.Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc : 721–729, 1995. doi: 10.1249/00005768-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle : 197–207, 2015. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys : 139–146, 2008. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) : 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst : 1548–1561, 2012. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 64.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutr Metab (Lond) : 68, 2011. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab : 2604–2612, 2013. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 66.Brines R, Hoffman-Goetz L, Pedersen BK. Can you exercise to make your immune system fitter? Immunol Today : 252–254, 1996. doi: 10.1016/0167-5699(96)80538-X. [DOI] [PubMed] [Google Scholar]
- 67.Brown JD, Green CL, Arthur IM, Booth FW, Miller DK. Cocaine-induced locomotor activity in rats selectively bred for low and high voluntary running behavior. Psychopharmacology (Berl) : 673–681, 2015. doi: 10.1007/s00213-014-3698-8. [DOI] [PubMed] [Google Scholar]
- 68.Brown WJ, Hockey R, Dobson AJ. Physical activity, body mass index and health care costs in mid-age Australian women. Aust N Z J Public Health : 150–155, 2008. doi: 10.1111/j.1753-6405.2008.00192.x. [DOI] [PubMed] [Google Scholar]
- 69.Browner WS, Seeley DG, Vogt TM, Cummings SR, Study of Osteoporotic Fractures Research Group . Non-trauma mortality in elderly women with low bone mineral density. Lancet : 355–358, 1991. doi: 10.1016/0140-6736(91)90489-C. [DOI] [PubMed] [Google Scholar]
- 70.Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to stroop task performance in preadolescent children. Med Sci Sports Exerc : 166–172, 2008. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- 71.Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes : 756–760, 1985. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 72.Burt LA, Greene DA, Ducher G, Naughton GA. Skeletal adaptations associated with pre-pubertal gymnastics participation as determined by DXA and pQCT: a systematic review and meta-analysis. J Sci Med Sport : 231–239, 2013. doi: 10.1016/j.jsams.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr : 1–22, 2006. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 74.Campbell SC, Wisniewski PJ II. Exercise is a novel promoter of intestinal health and microbial diversity. Exerc Sport Sci Rev : 41–47, 2017. doi: 10.1249/JES.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 75.Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis : 315–323, 2015. doi: 10.1016/j.pcad.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci : 2926–2933, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci : 5678–5684, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab : 1034–1047, 2016. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab : E494–E499, 1989. [DOI] [PubMed] [Google Scholar]
- 80.Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc : 1401–1407, 2007. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 81.Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. J Sport Exerc Psychol : 239–252, 2007. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- 82.Castronuovo E, Pezzotti P, Franzo A, Di Lallo D, Guasticchi G. Early and late mortality in elderly patients after hip fracture: a cohort study using administrative health databases in the Lazio region, Italy. BMC Geriatr : 37, 2011. doi: 10.1186/1471-2318-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82a.Centers for Disease Control and Prevention. Glossary of Terms https://www.cdc.gov/physicalactivity/basics/glossary/. 2016.
- 82b.Centers for Medicare and Medicaid Services. National Health Expenditure Data https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountshistorical.html. 2016.
- 83.Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Agnilera JF, Gonzàlez-Soltero R, Larrosa M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Excerise in Health? Front Physiol : 51, 2016. doi: 10.3389/fphys.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83a.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res : 172–183, 2010. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaddock L, Erickson KI, Prakash RS, VanPatter M, Voss MW, Pontifex MB, Raine LB, Hillman CH, Kramer AF. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev Neurosci : 249–256, 2010. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol (1985) : 3–10, 2004. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 86.Chaudhury M, Esliger D. Accelerometry in adults. In: Health Survey for England. Leeds, UK: NHS Information Centre for Health and Social Care, 2008, vol. 1, p. 59–88. [Google Scholar]
- 87.Cho SH, Kim JH, Song W. In vivo rodent models of skeletal muscle adaptation to decreased use. Endocrinol Metab (Seoul) : 31–37, 2016. doi: 10.3803/EnM.2016.31.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One : e19657, 2011. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Churchward-Venne TA, Murphy CH, Longland TM, Phillips SM. Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans. Amino Acids : 231–240, 2013. doi: 10.1007/s00726-013-1506-0. [DOI] [PubMed] [Google Scholar]
- 90.Clark RW, Dorr SW, Whitford MD, Freymiller GA, Putman BJ. Activity cycles and foraging behaviors of free-ranging sidewinder rattlesnakes (Crotalus cerastes): the ontogeny of hunting in a precocial vertebrate. Zoology (Jena) : 196–206, 2016. doi: 10.1016/j.zool.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduróz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr : 10–15, 2013. doi: 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 92.Coffey VG, Hawley JA. Concurrent exercise training: do opposites distract? J Physiol : 2883–2896, 2017. doi: 10.1113/JP272270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov : 58–74, 2015. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 94.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci : 125–130, 2003. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 95.Company JM, Roberts MD, Toedebusch RG, Cruthirds CL, Booth FW. Sudden decrease in physical activity evokes adipocyte hyperplasia in 70- to 77-day-old rats but not 49- to 56-day-old rats. Am J Physiol Regul Integr Comp Physiol : R1465–R1478, 2013. doi: 10.1152/ajpregu.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Compton JT, Lee FY. A review of osteocyte function and the emerging importance of sclerostin. J Bone Joint Surg Am : 1659–1668, 2014. doi: 10.2106/JBJS.M.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Convertino VA, Goldwater DJ, Sandler H. VO2 kinetics of constant-load exercise following bed-rest-induced deconditioning. J Appl Physiol Respir Environ Exerc Physiol : 1545–1550, 1984. [DOI] [PubMed] [Google Scholar]
- 98.Cooper C, Campion G, Melton LJ III. Hip fractures in the elderly: a world-wide projection. Osteoporos Int : 285–289, 1992. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 99.Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, Hardy R, FALCon and HALCyon Study Teams . Objective measures of physical capability and subsequent health: a systematic review. Age Ageing : 14–23, 2011. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cordain L, Gotshall RW, Eaton SB, Eaton SB III. Physical activity, energy expenditure and fitness: an evolutionary perspective. Int J Sports Med : 328–335, 1998. doi: 10.1055/s-2007-971926. [DOI] [PubMed] [Google Scholar]
- 101.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci : 295–301, 2002. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 102.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci : 464–472, 2007. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 103.Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, Matheson FI, Kaufman-Shriqui V, Rosella LC, Manuel DG, Booth GL. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA : 2211–2220, 2016. doi: 10.1001/jama.2016.5898. [DOI] [PubMed] [Google Scholar]
- 104.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA : 2367–2372, 2010. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing : 412–423, 2010. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curcio F, Ferro G, Basile C, Liguori I, Parrella P, Pirozzi F, Della-Morte D, Gargiulo G, Testa G, Tocchetti CG, Bonaduce D, Abete P. Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol : 1–8, 2016. doi: 10.1016/j.exger.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Cuyun Carter GB, Katz ML, Ferketich AK, Clinton SK, Grainger EM, Paskett ED, Bloomfield CD. Dietary intake, food processing, and cooking methods among Amish and non-Amish adults living in Ohio Appalachia: relevance to nutritional risk factors for cancer. Nutr Cancer : 1208–1217, 2011. doi: 10.1080/01635581.2011.607547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Aunno DS, Robinson RR, Smith GS, Thomason DB, Booth FW. Intermittent acceleration as a countermeasure to soleus muscle atrophy. J Appl Physiol (1985) : 428–433, 1992. [DOI] [PubMed] [Google Scholar]
- 109.D’Aunno DS, Thomason DB, Booth FW. Centrifugal intensity and duration as countermeasures to soleus muscle atrophy. J Appl Physiol (1985) : 1387–1389, 1990. [DOI] [PubMed] [Google Scholar]
- 110.Deitrick JE, Whedon GD, Shorr E. Effects of immobilization upon various metabolic and physiologic functions of normal men. Am J Med : 3–36, 1948. doi: 10.1016/0002-9343(48)90370-2. [DOI] [PubMed] [Google Scholar]
- 111.Demerath EW, Choh AC, Johnson W, Curran JE, Lee M, Bellis C, Dyer TD, Czerwinski SA, Blangero J, Towne B. The positive association of obesity variants with adulthood adiposity strengthens over an 80-year period: a gene-by-birth year interaction. Hum Hered : 175–185, 2013. doi: 10.1159/000351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA. WISE 2005: chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol : H3159–H3164, 2007. doi: 10.1152/ajpheart.00591.2007. [DOI] [PubMed] [Google Scholar]
- 113.Den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, Spector TD, Wareham NJ, Loos RJ. Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr : 1317–1325, 2013. doi: 10.3945/ajcn.113.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci : 10766–10771, 2008. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol : 643–653, 2003. doi: 10.1076/jcen.25.5.643.14583. [DOI] [PubMed] [Google Scholar]
- 116.Dill DB, Robinson S, Ross JC. A longitudinal study of 16 champion runners. J Sports Med Phys Fitness : 4–27, 1967. [PubMed] [Google Scholar]
- 117.Dimeo F, Bauer M, Varahram I, Proest G, Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med : 114–117, 2001. doi: 10.1136/bjsm.35.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi S, Chèvre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet : 724–726, 2007. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 119.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, Pratt M, Lancet Physical Activity Series 2 Executive Committee . The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet : 1311–1324, 2016. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 120.Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res : 15–23, 2006. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 121.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc : 63–74, 1997. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 122.Donnelly JE, Greene JL, Gibson CA, Smith BK, Washburn RA, Sullivan DK, DuBose K, Mayo MS, Schmelzle KH, Ryan JJ, Jacobsen DJ, Williams SL. Physical Activity Across the Curriculum (PAAC): a randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Prev Med : 336–341, 2009. doi: 10.1016/j.ypmed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Donnelly JE, Hillman CH, Castelli D, Etnier JL, Lee S, Tomporowski P, Lambourne K, Szabo-Reed AN. Physical activity, fitness, cognitive function, and academic achievement in children: a systematic review. Med Sci Sports Exerc : 1197–1222, 2016. doi: 10.1249/MSS.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Drey M, Sieber CC, Degens H, McPhee J, Korhonen MT, Müller K, Ganse B, Rittweger J. Relation between muscle mass, motor units and type of training in master athletes. Clin Physiol Funct Imaging : 70–76, 2016. doi: 10.1111/cpf.12195. [DOI] [PubMed] [Google Scholar]
- 125.Duckham RL, Baxter-Jones AD, Johnston JD, Vatanparast H, Cooper D, Kontulainen S. Does physical activity in adolescence have site-specific and sex-specific benefits on young adult bone size, content, and estimated strength? J Bone Miner Res : 479–486, 2014. doi: 10.1002/jbmr.2055. [DOI] [PubMed] [Google Scholar]
- 126.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med : 1–8, 2005. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Eaton SB, Eaton SB III. An evolutionary perspective on human physical activity: implications for health. Comp Biochem Physiol A Mol Integr Physiol : 153–159, 2003. doi: 10.1016/S1095-6433(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 128.Ebert MH, Post RM, Goodwin FK. Effect of physical activity on urinary M.H.P.G. excretion in depressed patients. Lancet : 766, 1972. doi: 10.1016/S0140-6736(72)92064-8. [DOI] [PubMed] [Google Scholar]
- 129.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet : 1415–1428, 2005. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 130.Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Brain Res Rev : 241–254, 2008. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA, Jemal A, Ward E, Plescia M, Ries LA, Edwards BK. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer : 2338–2366, 2012. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM, Lancet Physical Activity Series 2 Executive Committee, Lancet Sedentary Behaviour Working Group . Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet : 1302–1310, 2016. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 133.Ekelund U, Ward HA, Norat T, Luan J, May AM, Weiderpass E, Sharp SJ, Overvad K, Østergaard JN, Tjønneland A, Johnsen NF, Mesrine S, Fournier A, Fagherazzi G, Trichopoulou A, Lagiou P, Trichopoulos D, Li K, Kaaks R, Ferrari P, Licaj I, Jenab M, Bergmann M, Boeing H, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, Monnikhof E, Bueno-de-Mesquita HB, Quirós JR, Agudo A, Sánchez MJ, Huerta JM, Ardanaz E, Arriola L, Hedblad B, Wirfält E, Sund M, Johansson M, Key TJ, Travis RC, Khaw KT, Brage S, Wareham NJ, Riboli E. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am J Clin Nutr : 613–621, 2015. doi: 10.3945/ajcn.114.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elder SH, Nettles DL, Bumgardner JD. Synthesis and characterization of chitosan scaffolds for cartilage-tissue engineering. Methods Mol Biol : 41–48, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA : 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esparza J, Fox C, Harper IT, Bennett PH, Schulz LO, Valencia ME, Ravussin E. Daily energy expenditure in Mexican and USA Pima indians: low physical activity as a possible cause of obesity. Int J Obes Relat Metab Disord : 55–59, 2000. doi: 10.1038/sj.ijo.0801085. [DOI] [PubMed] [Google Scholar]
- 137.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Brain Res Rev : 119–130, 2006. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 138.Evans WJ. Sarcopenia should reflect the contribution of age-associated changes in skeletal muscle to risk of morbidity and mortality in elderly people. J Am Med Dir Assoc : 546–547, 2015. doi: 10.1016/j.jamda.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 139.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci , Special: 5–8, 1995. doi: 10.1093/gerona/50A.Special_Issue.5. [DOI] [PubMed] [Google Scholar]
- 140.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr , Suppl: 465–468, 1993. [DOI] [PubMed] [Google Scholar]
- 141.Ezzati M, Lopez AD, Rodgers AA, Murray CJL. Comparative Quantification of Health Risks. Geneva: World Health Organization, 2004. [Google Scholar]
- 142.Fedewa AL, Ahn S. The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes: a meta-analysis. Res Q Exerc Sport : 521–535, 2011. doi: 10.1080/02701367.2011.10599785. [DOI] [PubMed] [Google Scholar]
- 143.Fenesi B, Fang H, Kovacevic A, Oremus M, Raina P, Heisz JJ. Physical exercise moderates the relationship of apolipoprotein E (APOE) genotype and dementia risk: a population-based study. J Alzheimers Dis : 297–303, 2017. doi: 10.3233/JAD-160424. [DOI] [PubMed] [Google Scholar]
- 144.Ferguson DP, Dangott LJ, Schmitt EE, Vellers HL, Lightfoot JT. Differential skeletal muscle proteome of high- and low-active mice. J Appl Physiol (1985) : 1057–1067, 2014. doi: 10.1152/japplphysiol.00911.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ferguson DP, Dangott LJ, Vellers HL, Schmitt EE, Lightfoot JT. Differential protein expression in the nucleus accumbens of high and low active mice. Behav Brain Res : 283–288, 2015. doi: 10.1016/j.bbr.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 146.Fernandes RA, Coelho-E-Silva MJ, Spiguel Lima MC, Cayres SU, Codogno JS, Lira FS. Possible underestimation by sports medicine of the effects of early physical exercise practice on the prevention of diseases in adulthood. Curr Diabetes Rev : 201–205, 2015. doi: 10.2174/1573399811666150401104515. [DOI] [PubMed] [Google Scholar]
- 147.Fernandez MA, Griffin XL, Costa ML. Hip fracture surgery: improving the quality of the evidence base. Bone Joint J : 875–879, 2015. doi: 10.1302/0301-620X.97B7.35996. [DOI] [PubMed] [Google Scholar]
- 148.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab : E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 149.Ferrannini E, Rosenbaum M, Leibel RL. The threshold shift paradigm of obesity: evidence from surgically induced weight loss. Am J Clin Nutr : 996–1002, 2014. doi: 10.3945/ajcn.114.090167. [DOI] [PubMed] [Google Scholar]
- 150.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci : 1184–1194, 2016. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med : 1769–1775, 1994. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 152.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M, International Working Group on Sarcopenia . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc : 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol : 223–241, 2016. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 154.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol : 1029–1033, 1975. [DOI] [PubMed] [Google Scholar]
- 155.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA : 2284–2291, 2016. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Flint E, Cummins S. Active commuting and obesity in mid-life: cross-sectional, observational evidence from UK Biobank. Lancet Diabetes Endocrinol : 420–435, 2016. doi: 10.1016/S2213-8587(16)00053-X. [DOI] [PubMed] [Google Scholar]
- 157.Flück M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol : R4–R14, 2005. doi: 10.1152/ajpregu.00833.2004. [DOI] [PubMed] [Google Scholar]
- 158.Forrest CB, Riley AW. Childhood origins of adult health: a basis for life-course health policy. Health Aff (Millwood) : 155–164, 2004. doi: 10.1377/hlthaff.23.5.155. [DOI] [PubMed] [Google Scholar]
- 159.Franco M, Bilal U, Orduñez P, Benet M, Morejón A, Caballero B, Kennelly JF, Cooper RS. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980-2010: repeated cross sectional surveys and ecological comparison of secular trends. BMJ : f1515, 2013. doi: 10.1136/bmj.f1515. [DOI] [PubMed] [Google Scholar]
- 160.Franklin BA, Cushman M. Recent advances in preventive cardiology and lifestyle medicine: a themed series. Circulation : 2274–2283, 2011. doi: 10.1161/CIRCULATIONAHA.110.981613. [DOI] [PubMed] [Google Scholar]
- 161.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science : 889–894, 2007. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frayn KN. Visceral fat and insulin resistance–causative or correlative? Br J Nutr , Suppl 1: S71–S77, 2000. doi: 10.1017/S0007114500000982. [DOI] [PubMed] [Google Scholar]
- 163.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci : M146–M157, 2001. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 164.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res : 125–139, 2011. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 165.Friedenreich CM. The role of physical activity in breast cancer etiology. Semin Oncol : 297–302, 2010. doi: 10.1053/j.seminoncol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 166.Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol : T1–T8, 2014. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 167.Fries JF. Perspectives from masters in rheumatology and autoimmunity: perspectives on the conventional wisdom. Arthritis Rheumatol : 2806–2812, 2015. doi: 10.1002/art.39207. [DOI] [PubMed] [Google Scholar]
- 168.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res : 148–156, 2001. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 169.Galbo H, Richter EA, Hilsted J, Holst JJ, Christensen NJ, Henriksson J. Hormonal regulation during prolonged exercise. Ann NY Acad Sci : 72–80, 1977. doi: 10.1111/j.1749-6632.1977.tb38187.x. [DOI] [PubMed] [Google Scholar]
- 170.Gamrin L, Berg HE, Essén P, Tesch PA, Hultman E, Garlick PJ, McNurlan MA, Wernerman J. The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol Scand : 369–377, 1998. doi: 10.1046/j.1365-201X.1998.t01-1-00391.x. [DOI] [PubMed] [Google Scholar]
- 171.Garcia-Roves PM, Han DH, Song Z, Jones TE, Hucker KA, Holloszy JO. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol Endocrinol Metab : E729–E736, 2003. doi: 10.1152/ajpendo.00216.2003. [DOI] [PubMed] [Google Scholar]
- 172.Garland T Jr, Carter PA. Evolutionary physiology. Annu Rev Physiol : 579–621, 1994. doi: 10.1146/annurev.ph.56.030194.003051. [DOI] [PubMed] [Google Scholar]
- 173.Garland T Jr, Kelly SA. Phenotypic plasticity and experimental evolution. J Exp Biol : 2344–2361, 2006. doi: 10.1242/jeb.02244. [DOI] [PubMed] [Google Scholar]
- 174.Garland T Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol : 206–229, 2011. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) : 503–509, 1987. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 176.Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet : 767–770, 1988. doi: 10.1016/S0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- 177.Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ, Richardson RS. Symmorphosis and skeletal muscle V̇O2 max: in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol : 1741–1751, 2016. doi: 10.1113/JP271229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gilbert SF. Developmental Biology. Basingstoke, UK: Palgrave Macmillan, 2000. [Google Scholar]
- 179.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol : 6049–6061, 2008. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem : 835–869, 1974. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- 181.Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol : 267–282, 1977. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Goldstein MS. Humoral nature of the hypoglycemic factor of muscular work. Diabetes : 232–234, 1961. doi: 10.2337/diab.10.3.232. [DOI] [PubMed] [Google Scholar]
- 183.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis : 120239, 2013. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med : 698–703, 2003. doi: 10.1016/S0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 185.Graham SC, Roy RR, Hauschka EO, Edgerton VR. Effects of periodic weight support on medial gastrocnemius fibers of suspended rat. J Appl Physiol (1985) : 945–953, 1989. [DOI] [PubMed] [Google Scholar]
- 186.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol : 1–25, 2004. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry : 155–162, 2015. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 188.Greist JH, Klein MH, Eischens RR, Faris J, Gurman AS, Morgan WP. Running as treatment for depression. Compr Psychiatry : 41–54, 1979. doi: 10.1016/0010-440X(79)90058-0. [DOI] [PubMed] [Google Scholar]
- 189.Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML. Recovery of health-related quality of life in a United Kingdom hip fracture population. The Warwick Hip Trauma Evaluation–a prospective cohort study. Bone Joint J : 372–382, 2015. doi: 10.1302/0301-620X.97B3.35738. [DOI] [PubMed] [Google Scholar]
- 190.Gulli RA, Tishinsky JM, MacDonald T, Robinson LE, Wright DC, Dyck DJ. Exercise restores insulin, but not adiponectin, response in skeletal muscle of high-fat fed rodents. Am J Physiol Regul Integr Comp Physiol : R1062–R1070, 2012. doi: 10.1152/ajpregu.00176.2012. [DOI] [PubMed] [Google Scholar]
- 191.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuchs RK, Durski S, Snow C. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res : 986–993, 2008. doi: 10.1359/jbmr.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuller A, Durski S, Snow C. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone : 710–718, 2008. doi: 10.1016/j.bone.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 193.Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev : 13–21, 2012. doi: 10.1097/JES.0b013e318236e5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gunter KB, Kasianchuk A. . Examining the influence of participation in a community-based running program on skeletal health in growing girls. In: Osteoporosis International. London: Springer, 2011, p. S438–S438. [Google Scholar]
- 195.Gutin B, Kasper MJ. Can vigorous exercise play a role in osteoporosis prevention? A review. Osteoporos Int : 55–69, 1992. doi: 10.1007/BF01623838. [DOI] [PubMed] [Google Scholar]
- 196.Hahn RA, Teutsch SM, Rothenberg RB, Marks JS. Excess deaths from nine chronic diseases in the United States, 1986. JAMA : 2654–2659, 1990. doi: 10.1001/jama.1990.03450200062032. [DOI] [PubMed] [Google Scholar]
- 197.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, Lancet Physical Activity Series Working Group . Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet : 247–257, 2012. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 198.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol : 2650–2656, 2007. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hamer M, Endrighi R, Poole L. Physical activity, stress reduction, and mood: insight into immunological mechanisms. Methods Mol Biol : 89–102, 2012. doi: 10.1007/978-1-62703-071-7_5. [DOI] [PubMed] [Google Scholar]
- 200.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes : 2655–2667, 2007. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 201.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol : R1452–R1459, 2009. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985) : 1495–1504, 2012. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Haskell-Luevano C, Schaub JW, Andreasen A, Haskell KR, Moore MC, Koerper LM, Rouzaud F, Baker HV, Millard WJ, Walter G, Litherland SA, Xiang Z. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. FASEB J : 642–655, 2009. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hassinen M, Lakka TA, Savonen K, Litmanen H, Kiviaho L, Laaksonen DE, Komulainen P, Rauramaa R. Cardiorespiratory fitness as a feature of metabolic syndrome in older men and women: the Dose-Responses to Exercise Training study (DR’s EXTRA). Diabetes Care : 1242–1247, 2008. doi: 10.2337/dc07-2298. [DOI] [PubMed] [Google Scholar]
- 205.Hauschka EO, Roy RR, Edgerton VR. Periodic weight support effects on rat soleus fibers after hindlimb suspension. J Appl Physiol (1985) : 1231–1237, 1988. [DOI] [PubMed] [Google Scholar]
- 206.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol : 636–643, 2012. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care : 661–666, 2008. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 208.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol : 634–640, 1981. [DOI] [PubMed] [Google Scholar]
- 209.Heijnen S, Hommel B, Kibele A, Colzato LS. Neuromodulation of aerobic exercise–a review. Front Psychol : 1890, 2016. doi: 10.3389/fpsyg.2015.01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Herbert ME, Roy RR, Edgerton VR. Influence of one-week hindlimb suspension and intermittent high load exercise on rat muscles. Exp Neurol : 190–198, 1988. doi: 10.1016/0014-4886(88)90093-3. [DOI] [PubMed] [Google Scholar]
- 211.Hill A, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. QJM : 135–171, 1923. doi: 10.1093/qjmed/os-16.62.135. [DOI] [Google Scholar]
- 212.Hill AV, Long C, Lupton H. Muscular exercise, lactic acid, and the supply and utilisation of oxygen. Proc R Soc Lond, B : 84–138, 1924. doi: 10.1098/rspb.1924.0045. [DOI] [Google Scholar]
- 213.Hillman CH, Biggan JR. A review of childhood physical activity, brain, and cognition: perspectives on the future. Pediatr Exerc Sci : 170–176, 2017. doi: 10.1123/pes.2016-0125. [DOI] [PubMed] [Google Scholar]
- 214.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci : 58–65, 2008. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 215.Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc : 607–613, 2015. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 216.Holdsworth G, Slocombe P, Doyle C, Sweeney B, Veverka V, Le Riche K, Franklin RJ, Compson J, Brookings D, Turner J, Kennedy J, Garlish R, Shi J, Newnham L, McMillan D, Muzylak M, Carr MD, Henry AJ, Ceska T, Robinson MK. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem : 26464–26477, 2012. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol : 273–291, 1976. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 218.Houle-Leroy P, Garland T Jr, Swallow JG, Guderley H. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol (1985) : 1608–1616, 2000. [DOI] [PubMed] [Google Scholar]
- 219.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med : 2694–2703, 2004. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 220.Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ørtenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol (1985) : 1628–1634, 2010. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- 221.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis : 47–55, 2013. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 222.Ito Y. [Neoplasms and viruses (2). Problems related to human neoplasms]. Nihon Rinsho : 677, 1979. [PubMed] [Google Scholar]
- 223.Jang YC, Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp Gerontol : 193–198, 2011. doi: 10.1016/j.exger.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Janz KF, Burns TL, Levy SM, Torner JC, Willing MC, Beck TJ, Gilmore JM, Marshall TA. Everyday activity predicts bone geometry in children: the Iowa Bone Development study. Med Sci Sports Exerc : 1124–1131, 2004. doi: 10.1249/01.MSS.0000132275.65378.9D. [DOI] [PubMed] [Google Scholar]
- 225.Janz KF, Gilmore JM, Burns TL, Levy SM, Torner JC, Willing MC, Marshall TA. Physical activity augments bone mineral accrual in young children: The Iowa Bone Development study. J Pediatr : 793–799, 2006. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 226.Janz KF, Gilmore JM, Levy SM, Letuchy EM, Burns TL, Beck TJ. Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone : 216–222, 2007. doi: 10.1016/j.bone.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Torner JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc : 1072–1078, 2010. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol (1985) : 1476–1482, 1999. [DOI] [PubMed] [Google Scholar]
- 229.Joyner MJ. Precision medicine, cardiovascular disease and hunting elephants. Prog Cardiovasc Dis : 651–660, 2016. doi: 10.1016/j.pcad.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 230.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol : 5551–5558, 2009. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Joyner MJ, Pedersen BK. Ten questions about systems biology. J Physiol : 1017–1030, 2011. doi: 10.1113/jphysiol.2010.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte D Jr. Obesity, body fat distribution, insulin sensitivity and islet beta-cell function as explanations for metabolic diversity. J Nutr : 354S–360S, 2001. [DOI] [PubMed] [Google Scholar]
- 233.Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone , Suppl: 57S–63S, 1996. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 234.Kao SC, Westfall DR, Parks AC, Pontifex MB, Hillman CH. Muscular and aerobic fitness, working memory, and academic achievement in children. Med Sci Sports Exerc : 500–508, 2017. doi: 10.1249/MSS.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 235.Karlsson MK, Hasserius R, Obrant KJ. Bone mineral density in athletes during and after career: a comparison between loaded and unloaded skeletal regions. Calcif Tissue Int : 245–248, 1996. doi: 10.1007/s002239900117. [DOI] [PubMed] [Google Scholar]
- 236.Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E. Exercise during growth and bone mineral density and fractures in old age. Lancet : 469–470, 2000. doi: 10.1016/S0140-6736(99)05278-2. [DOI] [PubMed] [Google Scholar]
- 237.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol : 90–100, 2015. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 238.Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol : 146–150, 2016. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 239.Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care : 270–275, 2016. doi: 10.1097/MCO.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 240.Katzmarzyk PT. Physical activity, sedentary behavior, and health: paradigm paralysis or paradigm shift? Diabetes : 2717–2725, 2010. doi: 10.2337/db10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med : 1092–1097, 2004. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 242.Keller EF. From gene action to reactive genomes. J Physiol : 2423–2429, 2014. doi: 10.1113/jphysiol.2014.270991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, Ahmad T, Mora S, Kaakinen M, Sandholt CH, Holzapfel C, Autenrieth CS, Hyppönen E, Cauchi S, He M, Kutalik Z, Kumari M, Stančáková A, Meidtner K, Balkau B, Tan JT, Mangino M, Timpson NJ, Song Y, Zillikens MC, Jablonski KA, Garcia ME, Johansson S, Bragg-Gresham JL, Wu Y, van Vliet-Ostaptchouk JV, Onland-Moret NC, Zimmermann E, Rivera NV, Tanaka T, Stringham HM, Silbernagel G, Kanoni S, Feitosa MF, Snitker S, Ruiz JR, Metter J, Larrad MT, Atalay M, Hakanen M, Amin N, Cavalcanti-Proença C, Grøntved A, Hallmans G, Jansson JO, Kuusisto J, Kähönen M, Lutsey PL, Nolan JJ, Palla L, Pedersen O, Pérusse L, Renström F, Scott RA, Shungin D, Sovio U, Tammelin TH, Rönnemaa T, Lakka TA, Uusitupa M, Rios MS, Ferrucci L, Bouchard C, Meirhaeghe A, Fu M, Walker M, Borecki IB, Dedoussis GV, Fritsche A, Ohlsson C, Boehnke M, Bandinelli S, van Duijn CM, Ebrahim S, Lawlor DA, Gudnason V, Harris TB, Sørensen TI, Mohlke KL, Hofman A, Uitterlinden AG, Tuomilehto J, Lehtimäki T, Raitakari O, Isomaa B, Njølstad PR, Florez JC, Liu S, Ness A, Spector TD, Tai ES, Froguel P, Boeing H, Laakso M, Marmot M, Bergmann S, Power C, Khaw KT, Chasman D, Ridker P, Hansen T, Monda KL, Illig T, Järvelin MR, Wareham NJ, Hu FB, Groop LC, Orho-Melander M, Ekelund U, Franks PW, Loos RJ. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med : e1001116, 2011. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim H, Hirano H, Edahiro A, Ohara Y, Watanabe Y, Kojima N, Kim M, Hosoi E, Yoshida Y, Yoshida H, Shinkai S. Sarcopenia: prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr Gerontol Int , Suppl 1: 110–122, 2016. doi: 10.1111/ggi.12723. [DOI] [PubMed] [Google Scholar]
- 245.Kiørboe T, Jiang H, Colin SP. Danger of zooplankton feeding: the fluid signal generated by ambush-feeding copepods. Proc Biol Sci : 3229–3237, 2010. doi: 10.1098/rspb.2010.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kirshblum SC, Biering-Sørensen F, Betz R, Burns S, Donovan W, Graves DE, Johansen M, Jones L, Mulcahey MJ, Rodriguez GM, Schmidt-Read M, Steeves JD, Tansey K, Waring W. International standards for neurological classification of spinal cord injury: cases with classification challenges. Top Spinal Cord Inj Rehabil : 81–89, 2014. doi: 10.1310/sci2002-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci : 133–150, 2010. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev : 1–27, 1990. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- 249.Knudsen SH, Hansen LS, Pedersen M, Dejgaard T, Hansen J, Hall GV, Thomsen C, Solomon TP, Pedersen BK, Krogh-Madsen R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J Appl Physiol (1985) : 7–15, 2012. doi: 10.1152/japplphysiol.00189.2011. [DOI] [PubMed] [Google Scholar]
- 250.Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol : 83–95, 2008. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics : 45–52, 2001. [DOI] [PubMed] [Google Scholar]
- 252.Koch LG, Britton SL, Wisløff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med : 29–34, 2012. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisløff H, Høydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisløff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res : 1162–1172, 2011. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kohl HW III, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G, Kahlmeier S, Lancet Physical Activity Series Working Group . The pandemic of physical inactivity: global action for public health. Lancet : 294–305, 2012. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 255.Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, Papademetriou V, Fletcher R. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation : 790–797, 2010. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 256.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation : 614–622, 2008. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 257.Komlos J, Brabec M. The trend of BMI values of US adults by centiles, birth cohorts 1882–1986. National Bureau of Economic Research Working Paper Series, No. 16252, 2010.
- 258.Komori T. Animal models for osteoporosis. Eur J Pharmacol : 287–294, 2015. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 259.Komori T. Mouse models for the evaluation of osteocyte functions. J Bone Metab : 55–60, 2014. doi: 10.11005/jbm.2014.21.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Koppelmans V, Mulavara AP, Yuan P, Cassady KE, Cooke KA, Wood SJ, Reuter-Lorenz PA, De Dios YE, Stepanyan V, Szecsy DL, Gadd NE, Kofman I, Scott JM, Downs ME, Bloomberg JJ, Ploutz-Snyder L, Seidler RD. Exercise as potential countermeasure for the effects of 70 days of bed rest on cognitive and sensorimotor performance. Front Syst Neurosci : 121, 2015. doi: 10.3389/fnsys.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Krisan AD, Collins DE, Crain AM, Kwong CC, Singh MK, Bernard JR, Yaspelkis BB III. Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle. J Appl Physiol (1985) : 1691–1700, 2004. doi: 10.1152/japplphysiol.01054.2003. [DOI] [PubMed] [Google Scholar]
- 262.Krogh-Madsen R, Pedersen M, Solomon TP, Knudsen SH, Hansen LS, Karstoft K, Lehrskov-Schmidt L, Pedersen KK, Thomsen C, Holst JJ, Pedersen BK. Normal physical activity obliterates the deleterious effects of a high-caloric intake. J Appl Physiol (1985) : 231–239, 2014. doi: 10.1152/japplphysiol.00155.2013. [DOI] [PubMed] [Google Scholar]
- 263.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) : 1034–1040, 2010. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 264.Kruk J. Health and economic costs of physical inactivity. Asian Pac J Cancer Prev : 7499–7503, 2014. doi: 10.7314/APJCP.2014.15.18.7499. [DOI] [PubMed] [Google Scholar]
- 265.Kruk J. Lifestyle components and primary breast cancer prevention. Asian Pac J Cancer Prev : 10543–10555, 2014. doi: 10.7314/APJCP.2014.15.24.10543. [DOI] [PubMed] [Google Scholar]
- 266.Kruk J, Czerniak U. Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev : 3993–4003, 2013. doi: 10.7314/APJCP.2013.14.7.3993. [DOI] [PubMed] [Google Scholar]
- 267.Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol : 829–838, 2005. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol : 911–925, 2005. doi: 10.1113/jphysiol.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kump DS, Laye MJ, Booth FW. Increased mitochondrial glycerol-3-phosphate acyltransferase protein and enzyme activity in rat epididymal fat upon cessation of wheel running. Am J Physiol Endocrinol Metab : E480–E489, 2006. doi: 10.1152/ajpendo.00321.2005. [DOI] [PubMed] [Google Scholar]
- 270.Kwon S, Janz KF, Letuchy EM, Burns TL, Levy SM. Active lifestyle in childhood and adolescence prevents obesity development in young adulthood. Obesity (Silver Spring) : 2462–2469, 2015. doi: 10.1002/oby.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol : 15–28, 2016. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 272.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA : 2709–2716, 2002. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 273.Lambernd S, Taube A, Schober A, Platzbecker B, Görgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia : 1128–1139, 2012. doi: 10.1007/s00125-012-2454-z. [DOI] [PubMed] [Google Scholar]
- 274.Lammers G, van Duijnhoven NT, Hoenderop JG, Horstman AM, de Haan A, Janssen TW, de Graaf MJ, Pardoel EM, Verwiel ET, Thijssen DH, Hopman MT. The identification of genetic pathways involved in vascular adaptations after physical deconditioning versus exercise training in humans. Exp Physiol : 710–721, 2013. doi: 10.1113/expphysiol.2012.068726. [DOI] [PubMed] [Google Scholar]
- 275.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes : 2933–2942, 2008. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med : 73–81, 2006. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 277.Lasby CG. Eisenhower's Heart Attack: How Ike Beat Heart Disease and Held on to the Presidency. Lawrence, KS: Univ. of Kansas Press, 1997. [Google Scholar]
- 278.Laurent GJ, Sparrow MP, Millward DJ. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J : 407–417, 1978. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol : 498–504, 2001. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 280.Lavie CJ, Lee DC, Sui X, Arena R, O’Keefe JH, Church TS, Milani RV, Blair SN. Effects of running on chronic diseases and cardiovascular and all-cause mortality. Mayo Clin Proc : 1541–1552, 2015. doi: 10.1016/j.mayocp.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 281.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol (1985) : 161–168, 2009. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol (1985) : 1341–1347, 2007. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- 283.LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact : 157–160, 2000. [PubMed] [Google Scholar]
- 284.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J : 39–51, 2004. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 285.Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, Stanford FC, Kohl HW III, Blair SN. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation : 2483–2490, 2011. doi: 10.1161/CIRCULATIONAHA.111.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, Ekelund U, Katzmarzyk PT, Blair SN. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med : 504–510, 2011. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 287.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet : 219–229, 2012. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act : 115, 2011. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lee YS, Huynh TV, Lee SJ. Paracrine and endocrine modes of myostatin action. J Appl Physiol (1985) : 592–598, 2016. doi: 10.1152/japplphysiol.00874.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lemanne D, Cassileth B, Gubili J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology (Williston Park) : 580–585, 2013. [PubMed] [Google Scholar]
- 292.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S, Prospective Urban Rural Epidemiology (PURE) Study Investigators . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet : 266–273, 2015. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 293.Lescale C, Schenten V, Djeghloul D, Bennabi M, Gaignier F, Vandamme K, Strazielle C, Kuzniak I, Petite H, Dosquet C, Frippiat JP, Goodhardt M. Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J : 455–463, 2015. doi: 10.1096/fj.14-259770. [DOI] [PubMed] [Google Scholar]
- 294.Leskinen T, Sipilä S, Kaprio J, Kainulainen H, Alen M, Kujala UM. Physically active vs. inactive lifestyle, muscle properties, and glucose homeostasis in middle-aged and older twins. Age (Dordr) : 1917–1926, 2013. doi: 10.1007/s11357-012-9486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci : 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 296.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res : 860–869, 2008. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 297.Lieberman DE. Is exercise really medicine? an evolutionary perspective. Curr Sports Med Rep : 313–319, 2015. doi: 10.1249/JSR.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 298.Lightfoot JT. Why control activity? Evolutionary selection pressures affecting the development of physical activity genetic and biological regulation. BioMed Res Int : 821678, 2013. doi: 10.1155/2013/821678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res : 1651–1661, 2009. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 300.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc : e002014, 2015. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lipman RL, Raskin P, Love T, Triebwasser J, Lecocq FR, Schnure JJ. Glucose intolerance during decreased physical activity in man. Diabetes : 101–107, 1972. doi: 10.2337/diab.21.2.101. [DOI] [PubMed] [Google Scholar]
- 302.Lipnicki DM, Gunga HC. Physical inactivity and cognitive functioning: results from bed rest studies. Eur J Appl Physiol : 27–35, 2009. doi: 10.1007/s00421-008-0869-5. [DOI] [PubMed] [Google Scholar]
- 303.Lipnicki DM, Gunga HC, Belavý DL, Felsenberg D. Bed rest and cognition: effects on executive functioning and reaction time. Aviat Space Environ Med : 1018–1024, 2009. doi: 10.3357/ASEM.2581.2009. [DOI] [PubMed] [Google Scholar]
- 304.Liu HL, Zhao G, Cai K, Zhao HH, Shi LD. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res : 308–314, 2011. doi: 10.1016/j.bbr.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 305.Löfgren B, Dencker M, Nilsson JA, Karlsson MK. A 4-year exercise program in children increases bone mass without increasing fracture risk. Pediatrics : e1468–e1476, 2012. doi: 10.1542/peds.2011-2274. [DOI] [PubMed] [Google Scholar]
- 306.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA : 9833–9838, 2004. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Measuring the Global Burden of Disease and Risk Factors, 1990–2001. In: Global Burden of Disease and Risk Factors, edited by Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Washington, DC: World Bank The International Bank for Reconstruction and Development/The World Bank Group, 2006. doi: 10.1596/978-0-8213-6262-4. [DOI] [PubMed] [Google Scholar]
- 308.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature : 799–802, 1994. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 309.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest : 2111–2117, 2011. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res : 13–42, 2011. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 311.Lyssenko V, Laakso M. Genetic screening for the risk of type 2 diabetes: worthless or valuable? Diabetes Care , Suppl 2: S120–S126, 2013. doi: 10.2337/dcS13-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ma CL, Ma XT, Wang JJ, Liu H, Chen YF, Yang Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav Brain Res : 332–339, 2017. doi: 10.1016/j.bbr.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 313.Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lövdén M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Müller NG, Lessmann V, Sendtner M, Düzel E. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage : 142–154, 2016. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 314.Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövden M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Müller NG, Düzel E. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry : 585–593, 2015. doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- 315.Macsai CE, Foster BK, Xian CJ. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol : 578–587, 2008. doi: 10.1002/jcp.21342. [DOI] [PubMed] [Google Scholar]
- 316.Magne H, Savary-Auzeloux I, Migné C, Peyron MA, Combaret L, Rémond D, Dardevet D. Unilateral hindlimb casting induced a delayed generalized muscle atrophy during rehabilitation that is prevented by a whey or a high protein diet but not a free leucine-enriched diet. PLoS One : e70130, 2013. doi: 10.1371/journal.pone.0070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci : 28–40, 2012. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Marck A, Berthelot G, Foulonneau V, Marc A, Antero-Jacquemin J, Noirez P, Bronikowski AM, Morgan TJ, Garland T Jr, Carter PA, Hersen P, Di Meglio JM, Toussaint JF. Age-related changes in locomotor performance reveal a similar pattern for Caenorhabditis elegans, Mus domesticus, Canis familiaris, Equus caballus, and Homo sapiens. J Gerontol A Biol Sci Med Sci : 455–463, 2017. doi: 10.1093/gerona/glw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mark S, Scott GB, Donoviel DB, Leveton LB, Mahoney E, Charles JB, Siegel B. The impact of sex and gender on adaptation to space: executive summary. J Womens Health (Larchmt) : 941–947, 2014. doi: 10.1089/jwh.2014.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience : 21–28, 2012. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 321.Marti B, Howald H. Long-term effects of physical training on aerobic capacity: controlled study of former elite athletes. J Appl Physiol (1985) : 1451–1459, 1990. [DOI] [PubMed] [Google Scholar]
- 322.Masuki S, Mori M, Tabara Y, Sakurai A, Hashimoto S, Morikawa M, Miyagawa K, Sumiyoshi E, Miki T, Higuchi K, Nose H, Shinshu University Genetic Research Consortium . The factors affecting adherence to a long-term interval walking training program in middle-aged and older people. J Appl Physiol (1985) : 595–603, 2015. doi: 10.1152/japplphysiol.00819.2014. [DOI] [PubMed] [Google Scholar]
- 323.Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res : 155–163, 2010. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, Hara H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem : 572–576, 2008. doi: 10.1271/bbb.70474. [DOI] [PubMed] [Google Scholar]
- 325.Matteini AM, Tanaka T, Karasik D, Atzmon G, Chou WC, Eicher JD, Johnson AD, Arnold AM, Callisaya ML, Davies G, Evans DS, Holtfreter B, Lohman K, Lunetta KL, Mangino M, Smith AV, Smith JA, Teumer A, Yu L, Arking DE, Buchman AS, Chibinik LB, De Jager PL, Evans DA, Faul JD, Garcia ME, Gillham-Nasenya I, Gudnason V, Hofman A, Hsu YH, Ittermann T, Lahousse L, Liewald DC, Liu Y, Lopez L, Rivadeneira F, Rotter JI, Siggeirsdottir K, Starr JM, Thomson R, Tranah GJ, Uitterlinden AG, Völker U, Völzke H, Weir DR, Yaffe K, Zhao W, Zhuang WV, Zmuda JM, Bennett DA, Cummings SR, Deary IJ, Ferrucci L, Harris TB, Kardia SL, Kocher T, Kritchevsky SB, Psaty BM, Seshadri S, Spector TD, Srikanth VK, Windham BG, Zillikens MC, Newman AB, Walston JD, Kiel DP, Murabito JM. GWAS analysis of handgrip and lower body strength in older adults in the CHARGE consortium. Aging Cell : 792–800, 2016. doi: 10.1111/acel.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev : 37–45, 2015. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr : 169–175, 1956. [DOI] [PubMed] [Google Scholar]
- 328.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA : 2207–2212, 1993. doi: 10.1001/jama.1993.03510180077038. [DOI] [PubMed] [Google Scholar]
- 329.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan : 9, 2014. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation : 1350–1357, 2001. doi: 10.1161/hc3701.096099. [DOI] [PubMed] [Google Scholar]
- 331.McLaren L, McIntyre L, Kirkpatrick S. Rose’s population strategy of prevention need not increase social inequalities in health. Int J Epidemiol : 372–377, 2010. doi: 10.1093/ije/dyp315. [DOI] [PubMed] [Google Scholar]
- 332.Meek TH, Dlugosz EM, Vu KT, Garland T Jr. Effects of leptin treatment and Western diet on wheel running in selectively bred high runner mice. Physiol Behav : 252–258, 2012. doi: 10.1016/j.physbeh.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 333.Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab : 1078–1092, 2016. doi: 10.1016/j.cmet.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol : 49–60, 2011. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc : 1322–1326, 2010. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc : 225–231, 2012. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA : 1238–1245, 2004. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 338.Moore-Harrison T, Lightfoot JT. Driven to be inactive? The genetics of physical activity. Prog Mol Biol Transl Sci : 271–290, 2010. doi: 10.1016/B978-0-12-375003-7.00010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med : 816–825, 2016. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation : 2110–2118, 2007. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) : 1367–1377, 2002. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 342.Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet : 1053–1057, 1953. doi: 10.1016/S0140-6736(53)90665-5. [DOI] [PubMed] [Google Scholar]
- 343.Morrison PR, Montgomery JA, Wong TS, Booth FW. Cytochrome c protein-synthesis rates and mRNA contents during atrophy and recovery in skeletal muscle. Biochem J : 257–263, 1987. doi: 10.1042/bj2410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res : 352–359, 2012. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala, Jarlais, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami, Flaxman S, Flaxman AD, Gonzalez-Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KM, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray, U.S. Burden of Disease Collaborators . The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA : 591–608, 2013. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet : 1498–1504, 1997. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 347.Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, Froelicher V. Fitness versus physical activity patterns in predicting mortality in men. Am J Med : 912–918, 2004. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 348.Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis : 306–314, 2015. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 349.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med : 793–801, 2002. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 350.Nair KS. Aging muscle. Am J Clin Nutr : 953–963, 2005. [DOI] [PubMed] [Google Scholar]
- 351.Nauman J, Stensvold D, Coombes JS, Wisløff U. Cardiorespiratory fitness, sedentary time, and cardiovascular risk factor clustering. Med Sci Sports Exerc : 625–632, 2016. doi: 10.1249/MSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 352.Navasiolava NM, Dignat-George F, Sabatier F, Larina IM, Demiot C, Fortrat JO, Gauquelin-Koch G, Kozlovskaya IB, Custaud MA. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am J Physiol Heart Circ Physiol : H248–H256, 2010. doi: 10.1152/ajpheart.00152.2010. [DOI] [PubMed] [Google Scholar]
- 353.Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature : 109, 1995. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 354.Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab : 4–11, 2015. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 355.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci : 72–77, 2006. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 356.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev : 659–680, 2012. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Nguyen D, Kit B, Carroll M. Abnormal cholesterol among children and adolescents in the United States, 2011-2014. NCHS Data Brief : 1–8, 2015. [PubMed] [Google Scholar]
- 358.Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep : 239–242, 2003. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 359.Nieman DC. Risk of upper respiratory tract infection in athletes: an epidemiologic and immunologic perspective. J Athl Train : 344–349, 1997. [PMC free article] [PubMed] [Google Scholar]
- 360.Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Shannon M, Hjertman JM, Schmitt RL, Bolton MR, Austin MD, Schilling BK, Thorpe R. Immune function in female elite rowers and non-athletes. Br J Sports Med : 181–187, 2000. doi: 10.1136/bjsm.34.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med : 47, 2010. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Niziolek PJ, Farmer TL, Cui Y, Turner CH, Warman ML, Robling AG. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone : 1010–1019, 2011. doi: 10.1016/j.bone.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Noble D. Conrad Waddington and the origin of epigenetics. J Exp Biol : 816–818, 2015. doi: 10.1242/jeb.120071. [DOI] [PubMed] [Google Scholar]
- 364.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol : 788–794, 2014. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 365.Nout YS, Rosenzweig ES, Brock JH, Strand SC, Moseanko R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Courtine G, Ferguson AR, Edgerton VR, Beattie MS, Bresnahan JC, Tuszynski MH. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics : 380–392, 2012. doi: 10.1007/s13311-012-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.O’Keefe JH, Vogel R, Lavie CJ, Cordain L. Exercise like a hunter-gatherer: a prescription for organic physical fitness. Prog Cardiovasc Dis : 471–479, 2011. doi: 10.1016/j.pcad.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 367.O’Sullivan O, Cronin O, Clarke SF, Murphy EF, Molloy MG, Shanahan F, Cotter PD. Exercise and the microbiota. Gut Microbes : 131–136, 2015. doi: 10.1080/19490976.2015.1011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Office of Highway Information Management FHA. Highway Statistics Summary To 1995: Section II. Motor Vehicles. https://www.fhwa.dot.gov/ohim/summary95/. 2016.
- 369.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA : 2292–2299, 2016. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology : 2087–2102, 2007. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 371.Ohira T, Kawano F, Ohira T, Goto K, Ohira Y. Responses of skeletal muscles to gravitational unloading and/or reloading. J Physiol Sci : 293–310, 2015. doi: 10.1007/s12576-015-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA : 1261–1263, 2008. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 374.Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ : e7279, 2012. doi: 10.1136/bmj.e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Padilla J, Jenkins NT, Roberts MD, Arce-Esquivel AA, Martin JS, Laughlin MH, Booth FW. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp Physiol : 337–347, 2013. doi: 10.1113/expphysiol.2012.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Paffenbarger RS Jr, Blair SN, Lee IM. A history of physical activity, cardiovascular health and longevity: the scientific contributions of Jeremy N Morris, DSc, DPH, FRCP. Int J Epidemiol : 1184–1192, 2001. doi: 10.1093/ije/30.5.1184. [DOI] [PubMed] [Google Scholar]
- 377.Paksima N, Koval KJ, Aharanoff G, Walsh M, Kubiak EN, Zuckerman JD, Egol KA. Predictors of mortality after hip fracture: a 10-year prospective study. Bull NYU Hosp Jt Dis : 111–117, 2008. [PubMed] [Google Scholar]
- 378.Paluska SA, Schwenk TL. Physical activity and mental health: current concepts. Sports Med : 167–180, 2000. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- 379.Panula J, Pihlajamäki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, Kivelä SL. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord : 105, 2011. doi: 10.1186/1471-2474-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Papenberg G, Ferencz B, Mangialasche F, Mecocci P, Cecchetti R, Kalpouzos G, Fratiglioni L, Bäckman L. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum Brain Mapp : 3462–3473, 2016. doi: 10.1002/hbm.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol : R290–R301, 2008. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 382.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron : 1137–1145, 1996. doi: 10.1016/S0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 383.Paynter NP, Chasman DI, Paré G, Buring JE, Cook NR, Miletich JP, Ridker PM. Association between a literature-based genetic risk score and cardiovascular events in women. JAMA : 631–637, 2010. doi: 10.1001/jama.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, Harridge SD. Muscle function in elite master weightlifters. Med Sci Sports Exerc : 1199–1206, 2002. doi: 10.1097/00005768-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 385.Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol : 5559–5568, 2009. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) : 1093–1098, 2007. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 387.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev : 1379–1406, 2008. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 388.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol : 457–465, 2012. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 389.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev : 1055–1081, 2000. [DOI] [PubMed] [Google Scholar]
- 390.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports , Suppl 3: 1–72, 2015. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 391.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil : 113–119, 2003. doi: 10.1023/A:1026070911202. [DOI] [PubMed] [Google Scholar]
- 392.Pedersen NL. Reaching the limits of genome-wide significance in Alzheimer disease: back to the environment. JAMA : 1864–1865, 2010. doi: 10.1001/jama.2010.609. [DOI] [PubMed] [Google Scholar]
- 393.Pescatello LS, Devaney JM, Hubal MJ, Thompson PD, Hoffman EP. Highlights from the functional single nucleotide polymorphisms associated with human muscle size and strength or FAMuSS study. BioMed Res Int : 643575, 2013. doi: 10.1155/2013/643575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev : 226–237, 2010. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr : 452–460, 2015. doi: 10.3945/an.115.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) : 645–654, 2009. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- 397.Piccirillo R, Demontis F, Perrimon N, Goldberg AL. Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev Dyn : 201–215, 2014. doi: 10.1002/dvdy.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol : 639–651, 2005. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 399.Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci : 1332–1345, 2011. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- 400.Pontzer H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc Sport Sci Rev : 110–116, 2015. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 401.Pontzer H, Raichlen DA, Wood BM, Emery Thompson M, Racette SB, Mabulla AZ, Marlowe FW. Energy expenditure and activity among Hadza hunter-gatherers. Am J Hum Biol : 628–637, 2015. doi: 10.1002/ajhb.22711. [DOI] [PubMed] [Google Scholar]
- 402.Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: use it or lose it? Med Sci Sports Exerc : 1644–1650, 2010. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- 403.Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med : 208–217, 2016. doi: 10.1016/j.freeradbiomed.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol : 769–797, 2015. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 406.Prideaux M, Findlay DM, Atkins GJ. Osteocytes: the master cells in bone remodelling. Curr Opin Pharmacol : 24–30, 2016. doi: 10.1016/j.coph.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 407.Pronk NP, Goodman MJ, O’Connor PJ, Martinson BC. Relationship between modifiable health risks and short-term health care charges. JAMA : 2235–2239, 1999. doi: 10.1001/jama.282.23.2235. [DOI] [PubMed] [Google Scholar]
- 408.Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut : 583–587, 1987. doi: 10.1136/gut.28.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rääsk T, Konstabel K, Mäestu J, Lätt E, Jürimäe T, Jürimäe J. Tracking of physical activity in pubertal boys with different BMI over two-year period. J Sports Sci : 1649–1657, 2015. doi: 10.1080/02640414.2015.1012097. [DOI] [PubMed] [Google Scholar]
- 410.Raitakari OT, Porkka KV, Taimela S, Telama R, Räsänen L, Viikari JS. Effects of persistent physical activity and inactivity on coronary risk factors in children and young adults. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol : 195–205, 1994. doi: 10.1093/oxfordjournals.aje.a117239. [DOI] [PubMed] [Google Scholar]
- 411.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA : 558–560, 1999. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 412.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) : 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ravussin E, Valencia ME, Esparza J, Bennett PH, Schulz LO. Effects of a traditional lifestyle on obesity in Pima Indians. Diabetes Care : 1067–1074, 1994. doi: 10.2337/diacare.17.9.1067. [DOI] [PubMed] [Google Scholar]
- 414.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol : 4241–4249, 2008. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab : E1179–E1187, 2010. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genet : 4, 2008. doi: 10.1186/1471-2156-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol : 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Reynolds LJ, Credeur DP, Holwerda SW, Leidy HJ, Fadel PJ, Thyfault JP. Acute inactivity impairs glycemic control but not blood flow to glucose ingestion. Med Sci Sports Exerc : 1087–1094, 2015. doi: 10.1249/MSS.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rhodes JS, Gammie SC, Garland T Jr. Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol : 438–455, 2005. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- 420.Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int : 5–20, 2016. doi: 10.1111/ggi.12508. [DOI] [PubMed] [Google Scholar]
- 421.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing : 423–429, 2011. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 422.Roberts MD, Brown JD, Company JM, Oberle LP, Heese AJ, Toedebusch RG, Wells KD, Cruthirds CL, Knouse JA, Ferreira JA, Childs TE, Brown M, Booth FW. Phenotypic and molecular differences between rats selectively bred to voluntarily run high vs. low nightly distances. Am J Physiol Regul Integr Comp Physiol : R1024–R1035, 2013. doi: 10.1152/ajpregu.00581.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav : 661–668, 2012. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 424.Roberts MD, Toedebusch RG, Wells KD, Company JM, Brown JD, Cruthirds CL, Heese AJ, Zhu C, Rottinghaus GE, Childs TE, Booth FW. Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J Physiol : 2119–2135, 2014. doi: 10.1113/jphysiol.2013.268805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Roberts MD, Toedebusch RG, Wells KD, Company JM, Brown JD, Cruthirds CL, Heese AJ, Zhu C, Rottinghaus GE, Childs TE, Booth FW. Nucleus accumbens neuronal maturation differences in young rats bred for low versus high voluntary running behaviour. J Physiol : 2119–2135, 2014. doi: 10.1113/jphysiol.2013.268805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Roberts SE, Goldacre MJ. Time trends and demography of mortality after fractured neck of femur in an English population, 1968-98: database study. BMJ : 771–775, 2003. doi: 10.1136/bmj.327.7418.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem : 5866–5875, 2008. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 428.Rode A, Shephard RJ. Cardiorespiratory fitness of an Arctic community. J Appl Physiol : 519–526, 1971. [DOI] [PubMed] [Google Scholar]
- 429.Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf) : 362–381, 2017. doi: 10.1111/apha.12686. [DOI] [PubMed] [Google Scholar]
- 430.Rogers MA, Hagberg JM, Martin WH III, Ehsani AA, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol (1985) : 2195–2199, 1990. [DOI] [PubMed] [Google Scholar]
- 431.Rose G. The Strategy of Preventive Medicine. Oxford, UK: Oxford Univ. Press, 1992. [Google Scholar]
- 432.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol : T83–T96, 2014. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr , Suppl: 990S–991S, 1997. [DOI] [PubMed] [Google Scholar]
- 434.Rothon C, Edwards P, Bhui K, Viner RM, Taylor S, Stansfeld SA. Physical activity and depressive symptoms in adolescents: a prospective study. BMC Med : 32, 2010. doi: 10.1186/1741-7015-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Rowley NJ, Dawson EA, Hopman MT, George KP, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc : 844–849, 2012. doi: 10.1249/MSS.0b013e31823f6887. [DOI] [PubMed] [Google Scholar]
- 436.RoyChoudhury A, Dam TT, Varadhan R, Xue QL, Fried LP. Analyzing feed-forward loop relationship in aging phenotypes: physical activity and physical performance. Mech Ageing Dev : 5–11, 2014. doi: 10.1016/j.mad.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ruben JA, Bennett AF. Antiquity of the vertebrate pattern of activity metabolism and its possible relation to vertebrate origins. Nature : 886–888, 1980. doi: 10.1038/286886a0. [DOI] [PubMed] [Google Scholar]
- 438.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci : 99, 2014. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ruegsegger GN, Company JM, Toedebusch RG, Roberts CK, Roberts MD, Booth FW. Rapid alterations in perirenal adipose tissue transcriptomic networks with cessation of voluntary running. PLoS One : e0145229, 2015. doi: 10.1371/journal.pone.0145229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Ruegsegger GN, Toedebusch RG, Braselton JF, Roberts CK, Booth FW. Reduced metabolic disease risk profile by voluntary wheel running accompanying juvenile Western diet in rats bred for high and low voluntary exercise. Physiol Behav , Pt A: 47–55, 2015. doi: 10.1016/j.physbeh.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 441.Ruegsegger GN, Toedebusch RG, Will MJ, Booth FW. Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology : 171–181, 2015. doi: 10.1016/j.neuropharm.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 442.Saltiel AR. New therapeutic approaches for the treatment of obesity. Sci Transl Med : 323rv2, 2016. doi: 10.1126/scitranslmed.aad1811. [DOI] [PubMed] [Google Scholar]
- 443.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation , Suppl: VII1–VII78, 1968. [PubMed] [Google Scholar]
- 444.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet : 2267–2277, 2010. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int : 2205–2210, 2011. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Scharf I, Nulman E, Ovadia O, Bouskila A. Efficiency evaluation of two competing foraging modes under different conditions. Am Nat : 350–357, 2006. doi: 10.1086/506921. [DOI] [PubMed] [Google Scholar]
- 447.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest : 2992–3002, 2008. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Schimke RT. Regulation of protein degradation in mammalian tissues. In: Mammalian Protein Metabolism, edited by Munro HN, Allison JB. New York: Academic, 1970, vol. 4, p. 177–228. doi: 10.1016/B978-0-12-510604-7.50010-4. [DOI] [Google Scholar]
- 450.Schneider J. Age dependency of oxygen uptake and related parameters in exercise testing: an expert opinion on reference values suitable for adults. Lung : 449–458, 2013. doi: 10.1007/s00408-013-9483-3. [DOI] [PubMed] [Google Scholar]
- 451.Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone : 115–125, 2015. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol (1985) : 1483–1490, 2000. [DOI] [PubMed] [Google Scholar]
- 453.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res : 42–51, 2016. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 454.Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care : 1866–1871, 2006. doi: 10.2337/dc06-0138. [DOI] [PubMed] [Google Scholar]
- 455.Schuna JM Jr, Johnson WD, Tudor-Locke C. Adult self-reported and objectively monitored physical activity and sedentary behavior: NHANES 2005-2006. Int J Behav Nutr Phys Act : 126, 2013. doi: 10.1186/1479-5868-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet : e115, 2007. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Seider MJ, Nicholson WF, Booth FW. Insulin resistance for glucose metabolism in disused soleus muscle of mice. Am J Physiol Endocrinol Metab : E12–E18, 1982. [DOI] [PubMed] [Google Scholar]
- 459.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT Jr, Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM; CHARGE Consortium; GERAD1 Consortium; EADI1 Consortium . Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA : 1832–1840, 2010. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest : 1793–1801, 2006. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol : 1–10, 2012. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev : 95–107, 2016. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 463.Sisson SB, Camhi SM, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Characteristics of step-defined physical activity categories in U.S. adults. Am J Health Promot : 152–159, 2012. doi: 10.4278/ajhp.100326-QUAN-95. [DOI] [PubMed] [Google Scholar]
- 464.Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc : 539–545, 2009. doi: 10.1249/MSS.0b013e3181896c4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol : R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, Dwyer D, Stolina M, Ke HZ, Bouxsein ML. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res : 865–874, 2013. doi: 10.1002/jbmr.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab : E1736–E1740, 2012. doi: 10.1210/jc.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, Balcells-Camps M, Kronenberg HM, Babij P, Pajevic PD. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem : 16744–16758, 2015. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Spurway NC, Ekblom B, Noakes TD, Wagner PD. What limits VO(2max)? A symposium held at the BASES Conference, 6 September 2010. J Sports Sci : 517–531, 2012. doi: 10.1080/02640414.2011.642809. [DOI] [PubMed] [Google Scholar]
- 470.Stein TP, Bolster DR. Insights into muscle atrophy and recovery pathway based on genetic models. Curr Opin Clin Nutr Metab Care : 395–402, 2006. doi: 10.1097/01.mco.0000232899.51544.69. [DOI] [PubMed] [Google Scholar]
- 471.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism : 941–949, 2011. doi: 10.1016/j.metabol.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 472.Storz JF, Bridgham JT, Kelly SA, Garland T Jr. Genetic approaches in comparative and evolutionary physiology. Am J Physiol Regul Integr Comp Physiol : R197–R214, 2015. doi: 10.1152/ajpregu.00100.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus : 951–961, 2009. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Strangman GE, Sipes W, Beven G. Human cognitive performance in spaceflight and analogue environments. Aviat Space Environ Med : 1033–1048, 2014. doi: 10.3357/ASEM.3961.2014. [DOI] [PubMed] [Google Scholar]
- 475.Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, Harris JR, Pedersen NL, Hunkin J, Spector TD, de Geus EJ. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS One : e22, 2006. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) : 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 477.Summermatter S, Marcelino H, Arsenijevic D, Buchala A, Aprikian O, Assimacopoulos-Jeannet F, Seydoux J, Montani JP, Solinas G, Dulloo AG. Adipose tissue plasticity during catch-up fat driven by thrifty metabolism: relevance for muscle-adipose glucose redistribution during catch-up growth. Diabetes : 2228–2237, 2009. doi: 10.2337/db08-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Sutton JR, Young JD, Lazarus L, Hickie JB, Maksvytis J. The hormonal response to physical exercise. Australas Ann Med : 84–90, 1969. [DOI] [PubMed] [Google Scholar]
- 479.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience : 1037–1046, 2003. doi: 10.1016/S0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 480.Swallow JG, Carter PA, Garland T Jr. Artificial selection for increased wheel-running behavior in house mice. Behav Genet : 227–237, 1998. doi: 10.1023/A:1021479331779. [DOI] [PubMed] [Google Scholar]
- 481.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Johnson WD, Blair SN, Church TS, Newton RL Jr. Racial differences in the response of cardiorespiratory fitness to aerobic exercise training in Caucasian and African American postmenopausal women. J Appl Physiol (1985) : 1375–1382, 2013. doi: 10.1152/japplphysiol.01077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Swift DL, Johannsen NM, Tudor-Locke C, Earnest CP, Johnson WD, Blair SN, Sénéchal M, Church TS. Exercise training and habitual physical activity: a randomized controlled trial. Am J Prev Med : 629–635, 2012. doi: 10.1016/j.amepre.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Talwar P, Sinha J, Grover S, Rawat C, Kushwaha S, Agarwal R, Taneja V, Kukreti R. Dissecting complex and multifactorial nature of Alzheimer’s Disease pathogenesis: a clinical, genomic, and systems biology perspective. Mol Neurobiol : 4833–4864, 2016. doi: 10.1007/s12035-015-9390-0. [DOI] [PubMed] [Google Scholar]
- 484.Tanaka H, Seals DR. Invited Review: dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol (1985) : 2152–2162, 2003. doi: 10.1152/japplphysiol.00320.2003. [DOI] [PubMed] [Google Scholar]
- 485.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab : 464–475, 2007. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 486.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab : 644–656, 2013. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Terjung R. Endocrine response to exercise. Exerc Sport Sci Rev : 153–180, 1979. doi: 10.1249/00003677-197900070-00007. [DOI] [PubMed] [Google Scholar]
- 488.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med : 397–411, 2008. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 489.Thijssen DH, De Groot PC, van den Bogerd A, Veltmeijer M, Cable NT, Green DJ, Hopman MT. Time course of arterial remodelling in diameter and wall thickness above and below the lesion after a spinal cord injury. Eur J Appl Physiol : 4103–4109, 2012. doi: 10.1007/s00421-012-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Thijssen DH, Green DJ, Hopman MT. Blood vessel remodeling and physical inactivity in humans. J Appl Physiol (1985) : 1836–1845, 2011. doi: 10.1152/japplphysiol.00394.2011. [DOI] [PubMed] [Google Scholar]
- 491.Thijssen DH, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol : 845–875, 2010. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol : R300–R305, 1989. [DOI] [PubMed] [Google Scholar]
- 493.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol (1985) : 1–12, 1990. [DOI] [PubMed] [Google Scholar]
- 494.Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol : R1103–R1110, 2008. doi: 10.1152/ajpregu.00924.2007. [DOI] [PubMed] [Google Scholar]
- 495.Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) : 1218–1224, 2011. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 496.Tipton CM. Historical perspective: the antiquity of exercise, exercise physiology and the exercise prescription for health. World Rev Nutr Diet : 198–245, 2008. doi: 10.1159/000152988. [DOI] [PubMed] [Google Scholar]
- 497.Tipton CM. Susruta of India, an unrecognized contributor to the history of exercise physiology. J Appl Physiol (1985) : 1553–1556, 2008. doi: 10.1152/japplphysiol.00925.2007. [DOI] [PubMed] [Google Scholar]
- 498.Toedebusch RG, Ruegsegger GN, Braselton JF, Heese AJ, Hofheins JC, Childs TE, Thyfault JP, Booth FW. AMPK agonist AICAR delays the initial decline in lifetime-apex V̇o2 peak, while voluntary wheel running fails to delay its initial decline in female rats. Physiol Genomics : 101–115, 2016. doi: 10.1152/physiolgenomics.00078.2015. [DOI] [PubMed] [Google Scholar]
- 499.Tolonen S, Sievänen H, Mikkilä V, Telama R, Oikonen M, Laaksonen M, Viikari J, Kähönen M, Raitakari OT. Adolescence physical activity is associated with higher tibial pQCT bone values in adulthood after 28-years of follow-up–the Cardiovascular Risk in Young Finns Study. Bone : 77–83, 2015. doi: 10.1016/j.bone.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 500.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev : 111–131, 2008. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) : 3–10, 2013. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) : 143–152, 2000. [DOI] [PubMed] [Google Scholar]
- 503.Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol (1985) : 285–290, 1996. [DOI] [PubMed] [Google Scholar]
- 504.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci : 1628–1634, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc : 181–188, 2008. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 506.Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, Sirard J. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc : 350–355, 2002. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 507.Tsao TS, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes : 28–36, 1996. doi: 10.2337/diab.45.1.28. [DOI] [PubMed] [Google Scholar]
- 508.Tsao TS, Li J, Chang KS, Stenbit AE, Galuska D, Anderson JE, Zierath JR, McCarter RJ, Charron MJ. Metabolic adaptations in skeletal muscle overexpressing GLUT4: effects on muscle and physical activity. FASEB J : 958–969, 2001. doi: 10.1096/fj.00-0381. [DOI] [PubMed] [Google Scholar]
- 509.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med : 454–461, 2011. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 510.Tvede N, Heilmann C, Halkjaer-Kristensen J, Pedersen BK. Mechanisms of B-lymphocyte suppression induced by acute physical exercise. J Clin Lab Immunol : 169–173, 1989. [PubMed] [Google Scholar]
- 511.United States Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy! Washington, DC: U.S. Dept. of Health and Human Services, 2008, p. ix. [Google Scholar]
- 512.Van der Berg JD, Stehouwer CD, Bosma H, van der Velde JH, Willems PJ, Savelberg HH, Schram MT, Sep SJ, van der Kallen CJ, Henry RM, Dagnelie PC, Schaper NC, Koster A. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: The Maastricht Study. Diabetologia : 709–718, 2016. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA : 13427–13431, 1999. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Van Praag H, Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci : 15139–15149, 2014. doi: 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci : 266–270, 1999. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 516.Van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci : 5869–5878, 2007. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature : 1030–1034, 2002. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology : 157–166, 2010. doi: 10.1159/000252852. [DOI] [PubMed] [Google Scholar]
- 519.Vigelsø A, Gram M, Wiuff C, Andersen JL, Helge JW, Dela F. Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med : 552–560, 2015. doi: 10.2340/16501977-1961. [DOI] [PubMed] [Google Scholar]
- 520.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med : 1035–1041, 1998. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 521.Vital TM, Stein AM, de Melo Coelho FG, Arantes FJ, Teodorov E, Santos-Galduróz RF. Physical exercise and vascular endothelial growth factor (VEGF) in elderly: a systematic review. Arch Gerontol Geriatr : 234–239, 2014. doi: 10.1016/j.archger.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 522.Von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle : 129–133, 2010. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wójcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun : 90–99, 2013. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci : 525–544, 2013. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, Fanning J, Awick E, Gothe NP, Olson EA, McAuley E, Kramer AF. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage : 113–125, 2016. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Walker R, Hill K. Modeling growth and senescence in physical performance among the ache of eastern Paraguay. Am J Hum Biol : 196–208, 2003. doi: 10.1002/ajhb.10135. [DOI] [PubMed] [Google Scholar]
- 527.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev : 898–906, 2013. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 528.Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc Sport Sci Rev : 129–134, 2004. doi: 10.1097/00003677-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 529.Wang Y, Liu W, Masuyama R, Fukuyama R, Ito M, Zhang Q, Komori H, Murakami T, Moriishi T, Miyazaki T, Kitazawa R, Yoshida CA, Kawai Y, Izumi S, Komori T. Pyruvate dehydrogenase kinase 4 induces bone loss at unloading by promoting osteoclastogenesis. Bone : 409–419, 2012. doi: 10.1016/j.bone.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 530.Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend? Can J Cardiol : 495–504, 2016. doi: 10.1016/j.cjca.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 531.Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int J Behav Nutr Phys Act : 39, 2010. doi: 10.1186/1479-5868-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab : E11–E21, 2009. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta- analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets : 1002–1014, 2014. doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- 534.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med : 605–611, 2000. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 535.Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, Gaziano JM. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA : 1188–1194, 2004. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 536.Welly RJ, Liu TW, Zidon TM, Rowles JL III, Park YM, Smith TN, Swanson KS, Padilla J, Vieira-Potter VJ. Comparison of diet versus exercise on metabolic function and gut microbiota in obese rats. Med Sci Sports Exerc : 1688–1698, 2016. doi: 10.1249/MSS.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Wertheim BC, Martínez ME, Ashbeck EL, Roe DJ, Jacobs ET, Alberts DS, Thompson PA. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol Biomarkers Prev : 1591–1598, 2009. doi: 10.1158/1055-9965.EPI-08-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA : 1454–1461, 2004. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 539.Whedon GD. Disuse osteoporosis: physiological aspects. Calcif Tissue Int , Suppl 1: S146–S150, 1984. doi: 10.1007/BF02406148. [DOI] [PubMed] [Google Scholar]
- 540.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol : 17–32, 2015. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.WHO. Global health risks. Mortality and burden of disease attributable to selected major risks. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. 2015.
- 542.Widenfalk J, Olson L, Thorén P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res : 125–132, 1999. doi: 10.1016/S0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 543.Wijndaele K, Healy GN. Sitting and chronic disease: where do we go from here? Diabetologia : 688–691, 2016. doi: 10.1007/s00125-016-3886-7. [DOI] [PubMed] [Google Scholar]
- 544.Wilsgaard T, Emaus N, Ahmed LA, Grimnes G, Joakimsen RM, Omsland TK, Berntsen GR. Lifestyle impact on lifetime bone loss in women and men: the Tromsø Study. Am J Epidemiol : 877–886, 2009. doi: 10.1093/aje/kwn407. [DOI] [PubMed] [Google Scholar]
- 545.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science : 418–420, 2005. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 546.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr : 475–482, 2006. [DOI] [PubMed] [Google Scholar]
- 547.Wolff-Hughes DL, Bassett DR, Fitzhugh EC. Population-referenced percentiles for waist-worn accelerometer-derived total activity counts in U.S. youth: 2003–2006 NHANES. PLoS One : e115915, 2014. doi: 10.1371/journal.pone.0115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer : 611–616, 2009. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Wong SL, Katzmarzyk P, Nichaman MZ, Church TS, Blair SN, Ross R. Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc : 286–291, 2004. doi: 10.1249/01.MSS.0000113665.40775.35. [DOI] [PubMed] [Google Scholar]
- 549a.World Health Organization. Health Topics: Physical Activity. http://www.who.int/topics/physical_activity/en/. 2015.
- 550.Wright JD, Wang CY. Trends in intake of energy and macronutrients in adults from 1999-2000 through 2007-2008. NCHS Data Brief : 1–8, 2010. [PubMed] [Google Scholar]
- 551.Wu CL, Cornwell EW, Jackman RW, Kandarian SC. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol : C762–C767, 2014. doi: 10.1152/ajpcell.00361.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep : 1–119, 2016. [PubMed] [Google Scholar]
- 553.You JS, Anderson GB, Dooley MS, Hornberger TA. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis Model Mech : 1059–1069, 2015. doi: 10.1242/dmm.019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature : 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]